Abstract
Progress in research on endocannabinoid signaling has greatly advanced our understanding of how it controls neural circuit excitability in health and disease. In general, endocannabinoid signaling at excitatory synapses suppresses seizures by inhibiting glutamate release. In contrast, endocannabinoid signaling promotes seizures by inhibiting GABA release at inhibitory synapses. The physiological distribution of endocannabinoid signaling molecules becomes disrupted with the development of epileptic focus in patients with mesial temporal lobe epilepsy and in animal models of experimentally induced epilepsy. Augmentation of endocannabinoid signaling can promote the development of epileptic focus at initial stages. However, at later stages, increased endocannabinoid signaling delays it and suppresses spontaneous seizures. Thus, the regulation of endocannabinoid signaling at specific synapses that cause hyperexcitability during particular stages of disease development may be effective for treating epilepsy and epileptogenesis.
Keywords: Endocannabinoid, 2-AG, CB1, Epilepsy, Epileptogenesis, Seizure
Introduction
Epilepsy is a chronic neurological disease with various causes, characterized by paroxysmal recurrent episodes of symptoms, termed epileptic seizures. The estimated proportion of the general population with epilepsy at any given time is between 3 and 7 per 1000 people [1] and its incidence is 50.4 per 100,000 people per year [2]. Treatment of epilepsy usually starts with medications to prevent the occurrence of seizures. Most conventional antiepileptic drugs such as valproate, carbamazepine, phenytoin, and benzodiazepines act via blockade of Na+ and/or Ca2+ channels, or activation of GABAA receptors [3]. However, approximately 30 percent of patients have uncontrollable episodes of seizures with current medications [4]. Hence, there is a critical unmet need for developing new anti-epileptic drugs with novel mechanisms of action.
Endocannabinoid signaling is a potential candidate for the development of a novel anti-epileptic treatment [5–7]. Endocannabinoids are a group of lipid substances that act as endogenous ligands for cannabinoid receptors to which active components of marijuana bind. Endocannabinoid signaling is known to regulate neuronal excitability via multiple mechanisms. In this review, we will first provide a brief overview of the physiological roles of endocannabinoid signaling in the control of synaptic transmission, synaptic plasticity, and neuronal excitability. We will then review and discuss how endocannabinoid signaling is involved in seizures and epileptogenesis.
Endocannabinoids
Marijuana is prepared from the plant Cannabis sativa and has been used for recreation as well as treatment of a variety of diseases for thousands of years. In 1964, Δ-9-tetrahydrocannabinol (Δ9-THC) was identified as the main psychoactive chemical component in marijuana [8]. Almost 3 decades after the discovery of THC, cannabinoid type 1 (CB1) and type 2 (CB2) receptors were cloned in 1990 [9] and 1993 [10], respectively. In parallel with these events, the endogenous ligands for cannabinoid receptors were sought. N-arachidonoyl ethanolamine (anandamide, AEA) and 2-arachidonoyl glycerol (2-AG) were eventually identified as the endogenous ligands for cannabinoid receptors in 1992 [11] and 1995 [12, 13], respectively.
Physiological roles of CB1 receptor-mediated endocannabinoid signaling
The CB1 receptor is a seven-transmembrane receptor coupled to Gi/o protein [9] and is mainly expressed in the central nervous system. Activation of the CB1 receptor is known to influence a wide range of effector molecules including voltage-gated Ca2+ channels, K+ channels, and protein kinase A [14–16]. In 2001, endocannabinoids were discovered to function as retrograde messengers that mediate signals from depolarized postsynaptic neurons to presynaptic CB1 receptors and causing a transient suppression of synaptic transmission [17–19]. Subsequently, endocannabinoid-mediated long-term suppression of synaptic transmission was reported [20–22]. Many studies thereafter have revealed detailed mechanisms of endocannabinoid-mediated retrograde suppression of inhibitory and excitatory synaptic transmission. In particular, the finding that endocannabinoid-mediated retrograde synaptic suppression is abolished in mice deficient in diacylglycerol lipase α (DGLα), a major enzyme that produces 2-AG from diacylglycerol [23], clarified that 2-AG is the retrograde messenger for synaptic suppression [24, 25]. Although the majority of short-term and long-term depression (STD and LTD, respectively) of synaptic transmission through the CB1 receptor is mediated by 2-AG [26, 27], AEA is reported to be necessary for LTD at several types of synapse [28, 29]. As the mechanism of endocannabinoid-mediated regulation of synaptic transmission has been described in detail in several excellent reviews [27, 30, 31], we will briefly describe the 2-AG- and AEA-mediated STD and LTD of excitatory and inhibitory synaptic transmission.
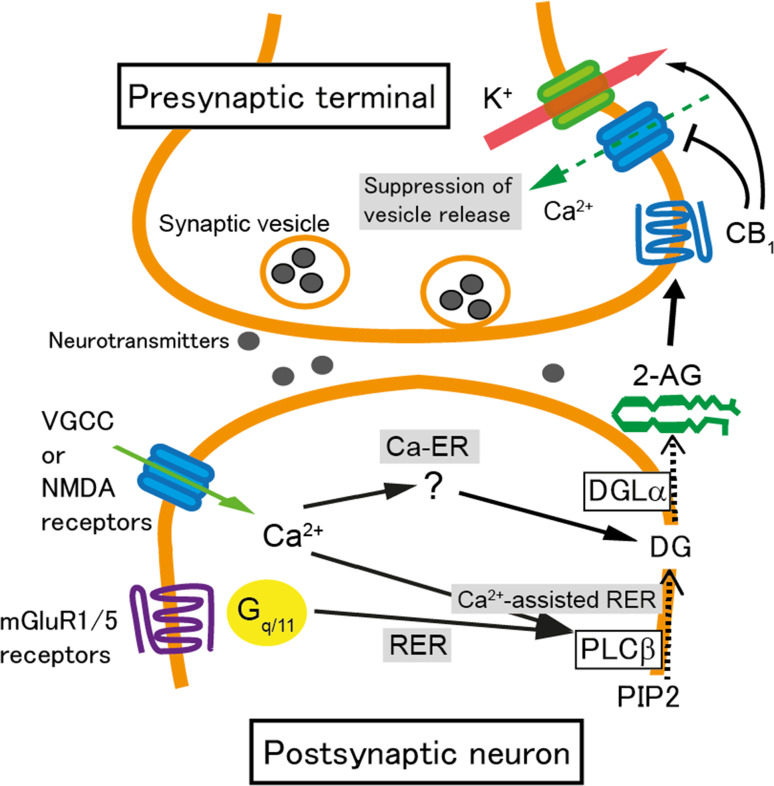
Production of 2-AG is induced by increased activity of postsynaptic neurons. 2-AG-mediated STD occurs when the postsynaptic neuron is depolarized and intracellular Ca2+ levels are elevated following Ca2+ influx through voltage-gated Ca2+ channels (Fig. 1). This form of 2-AG-mediated STD of excitatory or inhibitory synaptic transmission is called depolarization-induced suppression of excitation (DSE) or inhibition (DSI), respectively (Fig. 1). DSE/DSI is completely abolished in mice deficient in DGLα [24, 25]. Calcium influx into the postsynaptic neuron through NMDA receptors can also induce 2-AG-mediated STD [32] (Fig. 1). The increase in intracellular Ca2+ levels to several μM alone can trigger the biosynthesis of 2-AG, termed Ca2+-driven endocannabinoid release (Ca-ER) [33–35]. 2-AG then acts retrogradely upon CB1 receptors on presynaptic terminals, which suppresses neurotransmitter release mainly by inhibiting presynaptic voltage-gated Ca2+ channels (Fig. 1). Another pathway for 2-AG production is via Gq/11 protein-coupled receptors such as group I metabotropic glutamate receptors (mGluRs) and M1/M3 muscarinic acetylcholine receptors. This pathway is termed receptor-driven endocannabinoid release (RER) [34–37]. Activation of these receptors induces degradation of phosphatidylinositol into diacylglycerol by phospholipase Cβ, followed by production of 2-AG from diacylglycerol by DGLα (Fig. 1) [24, 25, 38]. The production of 2-AG is strongly elevated when the activation of Gq/11 protein-coupled receptors occurs simultaneously with the elevation of intracellular Ca2+ levels, termed Ca2+-assisted receptor-driven endocannabinoid release (Ca-RER) (Fig. 1) [33–35, 39]. 2-AG is thought to pass through the plasma membrane due to its lipophilic nature and diffuse towards presynaptic terminals. However, the precise details of these processes remain unknown. The amount of 2-AG may be regulated in postsynaptic neurons by 2-AG-hydrolyzing enzymes, namely α/β-hydrolase domain containing (ABHD) 6 and 12 [40], and by a 2-AG-oxygenizing enzyme, cyclooxygenase (COX)-2 [41].
Fig. 1.
Schematic illustration of the molecular mechanisms underpinning endocannabinoid-mediated short-term synaptic plasticity. When a large Ca2+ elevation is caused by the activation of voltage-gated Ca2+ channels (VGCC) or NMDA receptors, 2-arachidonoyl glycerol (2-AG) is generated in a diacylglycerol lipase (DGL) α-dependent manner. This process is termed Ca2+-driven endocannabinoid release (Ca-ER). The target enzyme, which is activated by Ca2+ elevation to produce diacylglycerol (DG), has yet to be identified. When phospholipase C (PLC) β is stimulated by the activation of metabotropic glutamate receptors (mGluR) 1/5 or other Gq/11-coupled receptors, DG is generated from phosphatidylinositol 4,5-bisphosphate (PIP2) and converted to 2-AG by DGLα (receptor-driven endocannabinoid release; RER). When the activation of Gq/11-coupled receptors and intracellular Ca2+ elevation occur simultaneously, the production of 2-AG is accelerated through PLCβ-dependent pathways (Ca2+-assisted receptor-driven endocannabinoid release; Ca2+-assisted RER). 2-AG is released from postsynaptic neurons, activates presynaptic CB1 receptors, and induces transient suppression of transmitter release through inhibition of voltage-gated Ca2+ channels and additionally through activation of K+ channels
At the presynaptic terminal, 2-AG binds to and activates CB1 receptors as a full agonist [42] and suppresses neurotransmitter release mainly by inhibiting voltage-gated Ca2+ channels [14, 16]. Furthermore, 2-AG suppresses neurotransmitter release through CB1 receptors by inhibiting the action of a presynaptic protein, Munc18-1, through phosphorylation by extracellular-regulated kinase (ERK) [43]. The actions of 2-AG are terminated upon degradation by monoacylglycerol lipase (MGL). MGL is densely expressed in the cytoplasm of subsets of presynaptic terminals and in astrocytes surrounding excitatory and inhibitory synapses [44–46]. Due to the highly heterogeneous expression pattern of MGL, 2-AG is thought to be degraded in a synapse non-specific manner [45].
In addition to STD, presynaptic forms of LTD are induced in several brain regions after activation of CB1 receptors by 2-AG derived from postsynaptic neurons. Production and release of 2-AG in LTD occur in a similar manner to that in STD, but prolonged 2-AG production and resultant CB1 receptor activation for several minutes are required for the induction of LTD. At inhibitory synapses in the hippocampus, LTD induction requires 5–10 min of continuous activation of CB1 receptors and presynaptic activity [47]. The activation of CB1 receptors mobilizes Gi/o protein, decreases cAMP production and PKA signaling, and eventually results in persistent reduction of the efficacy of neurotransmitter release [48]. Once this machinery is switched on, the suppression is sustained without further activation of CB1 receptors. Presynaptic proteins such as RIM1α [48], calcineurin [49], and potassium channels [50] are involved in the expression of 2-AG-mediated LTD. Furthermore, it has recently been shown that protein synthesis at presynaptic sites is necessary for the expression of CB1-mediated LTD at inhibitory synapses in the hippocampus [51].
As described above, phasic suppression of synaptic transmission at excitatory and inhibitory synapses may occur when the activity of postsynaptic neurons is elevated. In addition, endocannabinoid signaling is reported to mediate tonic suppression of synaptic transmission at inhibitory synapses in the CA3 area of the hippocampus [52]. In this report, the authors tested the fidelity of synaptic transmission from cholecystokinin-positive inhibitory interneurons onto CA3 pyramidal cells, which usually show extremely low fidelity of synaptic transmission, in acute hippocampal slices treated with a CB1 antagonist (AM251; 10 µM). They demonstrated that the CB1 antagonist raised the fidelity of inhibitory synaptic transmission, suggesting that persistently active cannabinoid receptors mute the output of cholecystokinin-positive inhibitory interneurons in the hippocampal CA3 area.
In addition to 2-AG, the other major endocannabinoid AEA is crucial for the induction of CB1-mediated LTD at inhibitory synapses in the striatum [28] and amygdala [53]. In addition, AEA-mediated LTD through the vanilloid receptor TRPV1 was reported at perforant path-granule cell synapses in the dentate gyrus [54] and in dopamine D2 receptor-positive medium spiny neurons in the nucleus accumbens [55]. Biochemical pathways for the synthesis of AEA are not fully understood, but a relevant signaling cascade involves the synthesis of N-acylphosphatidylethanolamine (NAPE) from phospholipid by N-acyltransferase [56] and the lysis of NAPE by NAPE-specific phospholipase D (NAPE-PLD) into AEA [57, 58]. AEA is cleaved by fatty acid amide hydrolase (FAAH) into arachidonic acid [59]. Similar to 2-AG, AEA is also degraded by COX-2 at postsynaptic sites [60].
CB2 receptor-mediated signaling
The CB2 receptor is Gi/o coupled and is the other canonical cannabinoid receptor that is involved in 2-AG-mediated signaling. This receptor is mainly expressed in immune cells such as T cells, B cells, and monocytes, and is responsible for the anti-inflammatory effects of cannabis. However, recent reports describe CB2 receptor mRNA expression in neurons using fluorescent in situ hybridization [61, 62]. Although the expression of CB2 receptor protein in neurons remains to be confirmed, several studies using CB2 knockout mice demonstrated that CB2 receptors were involved in the regulation of neuronal excitability and synaptic transmission. Li and Kim [63] demonstrated that the amplitude of field excitatory post-synaptic potentials and the magnitude of LTP at Schaffer collateral-CA1 pyramidal cell synapses were reduced in CB2 knockout mice. In contrast, there was no difference in paired-pulse facilitation at the same synapses between CB2 knockout and wild-type littermates, suggesting that the decrease in excitatory synaptic transmission is of postsynaptic origin. The authors claim that CB2 receptor signaling is required for maintaining excitatory synaptic transmission. In contrast, Stempel et al. compared CB2 knockout mice to DGLα knockout mice and reported that CB2 receptor signaling mediated activity-dependent long-lasting hyperpolarization and inhibition of CA3 pyramidal cells [62]. This study suggests that CB2 receptor signaling has inhibitory actions on the excitability of hippocampal CA3 pyramidal neurons. In line with this study, excitability of dopamine neurons in the ventral tegmental area was reported to decrease through activation of CB2 receptors [64]. Thus, the effects of CB2 receptor signaling on neuronal excitability may differ in different brain areas and cell types.
Because of the suppressive effect of CB1 receptor signaling on synaptic transmission and the potential regulation of neuronal excitability by CB2 receptors, many studies have been conducted regarding the roles of endocannabinoid signaling in the control of neuronal hyperexcitability, seizures, and epilepsy. Next, we provide an overview of studies on human epileptic patients with respect to endocannabinoid signaling.
Endocannabinoid signaling and epilepsy
Possible involvement of endocannabinoid signaling in epileptic patients
The brains of patients with mesial temporal lobe epilepsy (mTLE) with hippocampal sclerosis show reduced expression of DGLα mRNA compared to specimens from a non-epileptic control group [65] (Fig. 2). In contrast, no differences were found between control and epileptic patients in the expression levels of NAPE-PLD, MGL, or FAAH mRNA. These results suggest that there may be a selective reduction in 2-AG production, whereas 2-AG degradation, AEA production, and AEA degradation remain normal in the hippocampi of patients with mTLE. On the other hand, the concentration of AEA in the cerebrospinal fluid is reduced in untreated patients with mTLE, whereas the level of 2-AG was similar between untreated patients and control subjects [66]. In this report, however, the concentrations of 2-AG and AEA were measured in the non-ictal state. It is, therefore, possible that the concentrations may differ during ictal activity when the production of endocannabinoids may be enhanced.
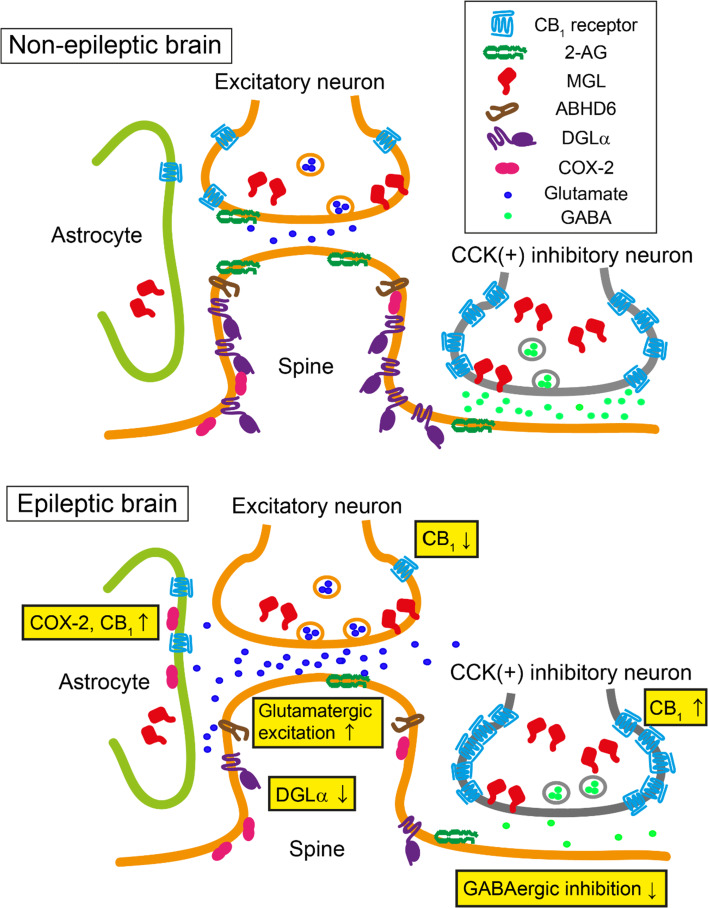
Fig. 2.
Schematic illustration of changes in 2-AG and CB1 receptor signaling observed in epileptic patients and animal models of epilepsy. Localization and expression of molecules involved in endocannabinoid production and degradation around synapses in a non-epileptic control brain (upper panel) and in an epileptic brain (lower panel). A significant decrease in the expression of DGLα may result in decreased production of 2-AG during the ictal phase. The expression of CB1 receptors decreases at excitatory synapses, whereas it increases at axon terminals of cholecystokinin-positive (CCK+) interneurons. The expression of CB1 receptors is detectable in hippocampal astrocytes in epileptic patients, whereas it is hardly detectable in non-epileptic control tissue. The expression of monoacylglycerol lipase (MGL) and α/β-hydrolase domain containing 6 (ABHD6) remains unchanged. These changes are thought to underlie the excessive glutamatergic transmission and scarce GABAergic inhibition in the epileptic brain. The expression of cyclooxygenase (COX)-2 increases in hippocampal astrocytes in severe epileptic patients, which may reduce CB1 receptor signaling in the astrocytes of these patients
Ludányi et al. reported that the mRNA level of CB1 receptors in the hippocampus was reduced in patients with mTLE compared to control subjects [65]. This reduction was even more significant in mTLE patients with hippocampal sclerosis, suggesting a negative correlation between mRNA levels and the severity of disease. Immunohistochemical analysis of CB1 receptor expression in the inner molecular layer of the dentate gyrus demonstrated a significant reduction in mTLE patients, and the expression had almost completely disappeared in sclerotic hippocampal samples [65]. Further analysis with electron microscopy revealed that in addition to a decrease in the total number of excitatory synapses, the loss of CB1 receptors in excitatory axon terminals was responsible for the reduced expression of CB1 receptors in the inner molecular layer (Fig. 2) [65]. No changes were observed in the expression levels of CB1 receptors at inhibitory axon terminals or in the number of inhibitory axon terminals in the dentate gyrus. In the hippocampus proper of mTLE patients with hippocampal sclerosis, an increase in the expression of CB1 receptors was observed in the stratum oriens of the hippocampal CA2 and CA3 regions [65].
In contrast to the report described above, Maglóczky et al. found an increase in the expression of CB1 receptors in the hippocampal dentate gyrus in patients with mTLE [67]. A previous study [68] using the same antibody against CB1 receptors reported that the antibody exclusively labeled CB1 receptors at inhibitory presynaptic terminals that expressed a high density of CB1 receptors. Therefore, it is reasonable to assume that the expression of CB1 receptors at inhibitory synapses may be increased in patients with mTLE (Fig. 2). However, it was unclear in the report by Maglóczky et al. [67] whether CB1 receptors were expressed at excitatory synapses in the dentate gyrus of mTLE patients. As mentioned previously, increased CB1 receptor expression at inhibitory synapses potentially causes disinhibition of postsynaptic neurons in a tonic and phasic manner. Consistent with the report by Maglóczky et al. [67], another study using positron emission tomography showed that in mTLE patients with hippocampal sclerosis, the availability of CB1 receptors in the ipsilateral temporal lobe was increased compared to healthy controls [69]. Based on these results, the disruption or strengthening of endocannabinoid signaling at excitatory synapses or inhibitory synapses, respectively, may potentially underpin the increased excitability of neural circuits which underlies the chronic susceptibility to seizures observed in epileptic patients.
Recently, it was reported that over half of the hippocampal specimens taken from epileptic patients expressed CB1 receptors in astrocytes. In contrast, there was minimal expression of CB1 receptors in hippocampal astrocytes in non-epileptic control subjects (Fig. 2) [70]. In mice, activation of astrocytic CB1 receptors caused glutamate release from astrocytes [71], suggesting that the elevated expression of CB1 receptors in astrocytes observed in epileptic patients may increase the excitability of neural circuits. However, the influence of increased astrocytic CB1 receptor expression may be small in patients with hippocampal sclerosis, since the expression of an endocannabinoid degradation enzyme, COX-2, is elevated in the hippocampal astrocytes of these patients [72].
Studies on genetic mutations in human epileptic patients may reveal causal relationships between the changes in endocannabinoid signaling and epilepsy. It is known that patients with mTLE often have histories of febrile seizures. When the frequency of single nucleotide polymorphisms (SNPs) in CNR1 gene was compared between patients who experience febrile seizures and normal control subjects, no significant differences were observed [73], suggesting that SNPs in CNR1 gene are not the cause of febrile seizures. Recently, a paper investigating the contribution of human genetic variations in DAGLA and CNR1 to disease phenotypes was published [74]. In this study, a high incidence of seizures was observed in people with mutations in DAGLA, suggesting that the disruption of 2-AG-mediated endocannabinoid signaling in humans may cause epilepsy. The DAGLA mutation observed in this study may also contribute to the decrease in mRNA reported by Ludányi et al. [65].
In the next section, we review how alterations in endocannabinoid signaling are implicated in animal models of epilepsy.
Epileptogenesis
Epilepsy is caused by various factors, ranging from hereditary genetic mutations to traumatic brain injuries in adults. A group of patients with epilepsy have a specific episode such as febrile seizures, central nervous system infections, or traumatic brain injuries several years before the onset of paroxysmal, recurrent episode of seizures. The period between the initial insult and the onset of epileptic seizures is called the latent period. During the latent period, it is hypothesized that anatomical and physiological changes gradually occur, resulting in the development of epileptic foci from which seizures arise. This process is known as epileptogenesis.
To investigate epileptogenesis, two different strategies are typically employed in animal models. One strategy involves the application of severe initial insults such as sustained, generalized tonic–clonic seizures called status epilepticus (SE), prolonged febrile seizures, and traumatic brain injury. These severe insults trigger epileptogenic changes in neural circuits and usually result in spontaneous seizures several weeks after the initial insults. Spontaneous seizures in these models are also useful for the evaluation of ictogenesis in the epileptic brain. The other strategy is kindling, in which sub-convulsive stimuli are initially applied in an intermittent fashion instead of strong initial insults. This makes it possible to observe the process of epileptogenesis in a stepwise manner [75]. In conventional kindling, electrical stimulation to the lateral amygdala or to the perforant path is used as a model of mTLE. Other protocols for kindling include transcorneal electrical stimulation and chemical kindling with pentylenetetrazole (PTZ) or picrotoxin. PTZ and picrotoxin are GABAA receptor antagonists and are considered to evoke seizures by strong disinhibition. All of the kindling protocols fulfill the condition of “seizure beget seizure”, meaning that a seizure promotes epileptogenesis.
The disruption of long-term synaptic plasticity via blockade of NMDA receptor or mGluR1/5 receptor signaling affects epileptogenesis [76–78]. Moreover, electrical stimulation protocols used for the induction of LTD delay kindling epileptogenesis [79, 80]. As endocannabinoid signaling mediates long-term synaptic plasticity, it is possible that the initial insult strongly mobilizes 2-AG and AEA, which may induce long-term synaptic plasticity and affect the process of epileptogenesis. In addition, intense and prolonged activation of CB1 receptors by 2-AG can cause downregulation of cannabinoid receptors [81–83], possibly resulting in the disruption of 2-AG signaling. Furthermore, endocannabinoids are known to exert protective effects on neurons [84]. Given that neuronal damage caused by brain injury, infection, seizures, or hypoxia can trigger epileptogenesis, neuroprotection by endocannabinoid signaling may prevent epileptogenesis.
In this section, we provide an overview of studies on the involvement of endocannabinoid signaling in epileptogenesis.
Involvement of 2-AG and AEA (Fig. 2, Table 1)
Table 1.
Epileptogenesis and ictogenesis modulated by manipulations of endocannabinoid signaling
| Epileptogenesis | Ictogenesis | |||||
|---|---|---|---|---|---|---|
| Kainate Pilocarpine Febrile seizure Trauma Spontaneous |
Kindling | Kainate Pilocarpine |
Pentylenetetrazole Bicuculine |
Electrical stim. | Spontaneous seizure |
|
| 2-AG↑ | × [95] | ○ [90, 92, 93] | ○ [90] | ○ [117], ≈ [93] | ○ [90, 92, 93] | ○ [90, 117] |
| 2-AG↓ | ○ [95] | × [90] | × [90], ≈ [114] | ? | × [90] | ? |
| AEA↑ | ○ [100], × [101] | ≈ [99] | ○ [86, 122], × [101] | ○ [123], × [101] | ○ [121] | ? |
| CB1↑ | ○ [111], × [101] | ○ [99] | ○ [130, 131] | × [123], ○ [136] | ○ [134, 135] | ○ [85] |
| CB1↓ |
× [113] |
≈ [90, 112] |
× Excitatory [129] ≈ Inhibitory [129] |
≈ [117], × [123] |
× Excitatory [112] ○ Inhibitory [112] |
× [85, 90] |
| CB2↑ | ? | ? | ? | × [139] | ? | ? |
| CB2↓ | × [113] | ≈ [90] | × [90] |
× Kindled rat[138] × [117] |
≈ [137] × [90] |
? |
Cells in the leftmost column indicate the manipulations and those in the first and second rows denote the types of animal models. The observed effects are represented as follows: ○: suppresses ictogenesis or epileptogenesis, ≈: no change, ×: promotes ictogenesis or epileptogenesis, ?: unknown. Excitatory: results observed in mice with excitatory neuron-specific deletion of cannabinoid type 1 (CB1) receptors; inhibitory: results observed in mice with inhibitory neuron-specific deletion of CB1 receptors
The activation of mGluR1/5 receptors and the increase in intracellular Ca2+ concentration induce the production of 2-AG. It is highly likely that this process occurs during SE or febrile seizures. Indeed, the production of 2-AG is enhanced by seizures [85, 86]. Intraperitoneal (i.p.) administration of pilocarpine, a muscarinic acetylcholine receptor agonist, causes SE. When the concentration of 2-AG was assessed in rat hippocampi 15 min after the initiation of pilocarpine-induced SE (375 mg/kg, i.p.) with high-performance liquid chromatography, the level of 2-AG exhibited an approximately 1.5-fold increase compared to that of control [85]. Elevation of 2-AG levels was also observed in the brains of animals with kainate-induced epilepsy [86]. Kainate is an agonist of the kainate-type ionotropic glutamate receptor and triggers SE when administered intraperitoneally in a manner similar to pilocarpine. In the aforementioned report, the authors measured the level of 2-AG in brain tissue 100 min after kainate (30 mg/kg, i.p.) or vehicle injection in 8-week-old rats and observed that the concentration was 1.5-fold higher in the kainate-treated group than in the control group [86]. They also measured the enzymatic activity of DGLα, MGL, NAPE-PLD, and FAAH under the same schedule and found that the enzymatic activity of DGLα increased, whereas that of NAPE-PLD decreased after kainate administration. In contrast, the activity of MGL and FAAH was the same between the kainate and vehicle-treated groups. Interestingly, the changes in endocannabinoid levels showed the opposite trend in 14-day-old rats, whereby 2-AG decreased whereas AEA increased significantly after kainate treatment [86], suggesting that the changes may depend on the age of animal.
In contrast to these reports, other studies have failed to report changes in 2-AG levels in kainate-induced epilepsy. Marsicano et al. used the kainate-induced epilepsy model and measured the concentration of 2-AG and AEA [87]. They reported that the concentration of 2-AG in the hippocampi of adult mice 20 min after kainate injection (30 mg/kg, i.p.) did not change, while that of AEA showed a significant rise up to 300% of pre-treatment levels [87]. The concentration of AEA returned to baseline within 1 h after kainate injection [87]. A recent study [88] assessed the levels of various lipid substances 1 h after kainate injection (30 mg/kg, i.p.) and reported that the levels of AEA and 2-AG remained unchanged in the cerebral cortex, striatum, hippocampus, thalamus, hypothalamus, and cerebellum compared to those of the vehicle-treated group [88]. The reasons for these variable results regarding 2-AG levels in the brain after kainate application remain unclear. In contrast, the increase in brain AEA levels after kainate application may reflect the production of AEA through activation of the postsynaptic kainate receptor, GluK2. Lourenço et al. applied kainate (3 μM) to hippocampal slices for 100 s and observed a significant increase in AEA levels with no change in 2-AG levels [89]. This increase was abolished in GluK2 knockout mice or by manipulating the chelation of Ca2+ in postsynaptic neurons [89], suggesting that AEA was mobilized by GluK2 activation and elevation of postsynaptic Ca2+ concentration.
The enzymes involved in 2-AG production and degradation undergo changes in expression during epileptogenesis. We recently reported a significant decrease in the expression of DGLα in the hippocampal CA1 and dentate gyrus of mice that received unilateral intrahippocampal kainate injections (0.2 µg) 4 weeks before immunohistochemical analysis [90]. The decrease in DGLα in the hippocampus is consistent with the findings observed in the hippocampi of mTLE patients, which may result in insufficient 2-AG production when it is required for retrograde suppression of excitatory synaptic transmission. On the other hand, there were no changes in 2-AG and AEA levels in the hippocampus after 4 weeks of PTZ kindling [91], suggesting that basal levels of 2-AG and AEA during the inter-ictal period were maintained during the course of epileptogenesis.
Decreased expression of DGLα in patients with mTLE and animal models of kainate-induced epilepsy provide potential explanatory mechanisms for the process of epileptogenesis. Recently, we demonstrated that kindling epileptogenesis with electrical stimulation of the perforant path was significantly promoted in DGLα knockout mice compared to wild-type littermates [90]. In line with these results, manipulations that increase 2-AG levels suppress kindling epileptogenesis (Table 1). For example, the MGL inhibitor JZL184 (4 mg/kg, i.p., twice/day) significantly delayed epileptogenesis induced by perforant path kindling in wild-type mice [90]. This result is consistent with the effect of JZL184 (8 mg/kg/day, i.p.) on the amygdala kindling model [92] and with that of the MGL inhibitor SAR127303 [30 mg/kg/day, per os (p.o.)] on the corneal kindling model [93]. Furthermore, in cultured hippocampal slices with intense activation of NMDA receptors (50 μM NMDA for 4 h), the increase in 2-AG levels by JZL184 (1 μM) prevented cell death in the CA1 region [94]. These results indicate that 2-AG prevents epileptogenesis by kindling procedures, and imply that elevation of 2-AG levels can delay epileptogenesis and prevent cell death.
However, contrasting results regarding the effect of 2-AG have been reported in epileptogenesis after SE. When DGLα was blocked by RHC80267 (1.3 µmol, intracerebroventricular; i.c.v.) for 7 days commencing immediately after the termination of pilocarpine-induced SE, the frequency of spontaneous seizures and the occurrence of cell death decreased [95], suggesting that 2-AG promotes SE-induced epileptogenesis (Table 1). The pro-epileptogenic effect of 2-AG during the initial 7 days after SE may be ascribed to downregulation of CB1 receptor signaling. In rat organotypic cultured hippocampal slices, the concentration of 2-AG increased 24–72 h after NMDA treatment (50 μM) [94]. Moreover, 5 days of 2-AG upregulation by JZL treatment (16 mg/kg, i.p.) was sufficient to induce downregulation of CB1 receptor signaling in non-epileptic wild-type mice [83]. It is, therefore, likely that downregulation of CB1 receptor signaling occurs due to a sustained increase in 2-AG concentration in the initial stage of SE-induced epileptogenesis.
In an in vitro model of epilepsy [96–98], continuous epileptiform high-frequency bursts were induced in primary cultures of hippocampal neurons by the application of low Mg2+ artificial cerebrospinal fluid (aCSF) for 3 h. After restoration of normal Mg2+ concentration, spontaneous recurrent burst activity appeared within 5 min and persisted for more than 24 h. Application of a synthetic cannabinoid agonist, WIN55212-2, for 24 h after restoration of Mg2+ significantly increased neuronal burst activity and decreased the expression of CB1 receptors [96]. These results are consistent with CB1 receptor activation after initial insults downregulate CB1 receptor expression.
In contrast to 2-AG, an increase in AEA by the FAAH inhibitor URB597 (3 mg/kg, i.p.) did not change kindling epileptogenesis [99]. However, when kainate-induced seizures were mild, elevation of AEA levels by i.cv. administration of URB597 (10 μg/μl for 10 days beginning 24 h after kainate administration) was able to reduce kainate-induced cell death in the hippocampal CA1 [100]. In contrast, when the concentration of AEA was further elevated by the deletion of FAAH combined with AEA administration (50 mg/kg, i.p.), severe neuronal cell death was observed in the hippocampus after administration of kainate (15 mg/kg, i.p.) or bicuculline (4 mg/kg, i.p.) [101]. Surprisingly, neuronal cell death after bicuculline administration was prevented by injection of a CB1 antagonist, SR141716A (3 mg/kg, i.p.) [101]. These results suggest that the effects of AEA on epileptogenesis may be concentration-dependent.
Involvement of CB1 and CB2 receptors (Fig. 2, Table 1)
Similar to DGLα, the expression of CB1 receptors is affected by epileptogenic processes. Pilocarpine-induced SE (375 mg/kg, i.p.) induced a time-dependent redistribution of hippocampal CB1 receptors in rats [102, 103]. Within 1 week after SE, there was a pronounced loss of CB1 receptor expression throughout the hippocampus. By 2 weeks after SE, the decreased CB1 receptor expression was mostly restored in the CA1 but not in the dentate gyrus. By 1 month after SE, pilocarpine-treated rats began to show spontaneous seizures. Concurrently, a characteristic redistribution of CB1 receptors was observed in chronic epileptic rats, whereby CB1 receptor immunoreactivity in the dentate gyrus inner molecular layer and the CA1 pyramidal cell layer was specifically decreased; conversely, that in the strata oriens and radiatum of the CA1–3 was increased [103]. This unique redistribution of CB1 receptors persisted for up to 6 months in chronic epileptic rats, which is consistent with the distribution of CB1 receptors in the hippocampi of chronic mTLE patients [65, 67]. As described, we also investigated the distribution of CB1 receptors 4 weeks after kainate-induced SE in mice [90]. The pattern of CB1 receptor distribution was similar to that observed in rat 7 days after pilocarpine-induced SE: i.e., a pronounced loss in CB1 receptor expression throughout the hippocampus. Since we were able to observe spontaneous seizures 4 weeks after kainate-induced SE, we propose that the decrease in CB1 receptors in the inner molecular layer of the dentate gyrus and the pyramidal cell layer of the hippocampal CA1 are responsible for the occurrence of spontaneous seizures, rather than a successive increase of CB1 receptors in the strata oriens and radiatum of the CA1–3.
Chen et al. [104] used a rat model of mTLE induced by febrile seizures and observed changes in CB1 receptor expression. On postnatal day 10, the body temperature of rat pups was raised to 41–42 °C to evoke febrile seizures for approximately 20 min. Febrile seizures induced persistent enhancement of DSI in hippocampal CA1 pyramidal cells for up to 5 weeks [104], which resulted from an increase in CB1 receptors associated with inhibitory inputs from cholecystokinin-positive interneurons. Following the period of febrile seizures, no significant effects were observed either on DSE in CA1 pyramidal neurons at 5 weeks or on 2-AG and anandamide levels at 1 week. These results suggest that febrile seizures induce changes in CB1 receptor expression, which may disinhibit hippocampal neural circuits thus contributing to the development of epileptic foci. In this model, decreased CB1 receptors in the inner molecular layer of the dentate gyrus and pyramidal cell layer of the hippocampal CA1 was not observed. Therefore, decreased suppression of excitatory synaptic transmission is unlikely to be the cause of increased seizure susceptibility in this model. Instead, disinhibition due to increased CB1 receptor expression could be the major cause of heightened seizure susceptibility.
Chen et al. monitored DSI following a single injection of a CB1 antagonist, SR141716A (1 mg/kg, i.p.) into rat pups 1 h before the start of febrile seizures [105]. Although the biological half-life of SR141716A measured in blood samples is 4.95 h in mice [106] and 6–9 days in humans [107], the acute injection of SR141716A blocked febrile seizure-induced enhancement of DSI, increase in CB1 receptors, and worsening of acute kainate-induced seizures 6 weeks after the febrile seizure [105]. These results underscore the pertinence of the immediate anatomical and physiological changes in the endocannabinoid system after febrile seizures for elucidating key epileptogenic processes as well as the development of novel therapeutics.
As discussed previously, prolonged activation of CB1 receptors causes their downregulation, which accounts for the decrease in CB1 receptors at the initial stage of SE-induced epileptogenesis. However, at later stages of SE- or febrile seizure-induced epileptogenesis, CB1 receptor expression is increased. Notably, this effect can be blocked by CB1 receptor antagonists in the febrile seizure model. Therefore, heightened activation of CB1 receptors at the initial stage of epileptogenesis may trigger the elevation of CB1 receptor expression at later stages as well as the decrease in receptor expression at the initial stage. The detailed molecular mechanisms underlying these phenomena are unclear. It has been reported that an increase in interleukin β was observed up to 12 h after febrile seizures and administration of an interleukin 1 receptor antagonist (100 ng, i.c.v.) prevented the increase in CB1 receptors after febrile seizures in rats [108].
Almost 10% of patients with traumatic brain injury later acquire epilepsy during their lifetime [109]. In animal models, brain injury also induces persistent hyperexcitability of neural circuits. In a rat model of traumatic brain injury, Echegoyen et al. demonstrated that treatment with a CB1 antagonist, SR141716A (10 mg/kg, i.p.), immediately after injury to the cerebral cortex prevented the long-term increase in seizure susceptibility induced by kainate (5 mg/kg, i.p.) [110]. This result is consistent with the findings in the febrile seizure model described above,and indicates that short-term blockade of CB1 receptors may be broadly applicable for the prevention of epileptogenesis.
The evidence presented suggests that epileptogenesis may be promoted when CB1 receptor signaling is activated for several hours immediately after the initial insult. In contrast, epileptogenesis may be suppressed when CB1 receptor activation is commenced sub-acutely and continues for longer periods (Table 1). Di Maio et al. reported that treatment with a CB1 agonist, WIN55212-2 (2 mg/kg/day, i.p.), for 2 weeks starting 1 day after pilocarpine-induced SE (360 mg/kg i.p.) significantly reduced the number of seizures observed 1–6 months after SE [111].
CB1 receptor expression was increased by approximately three- to fourfold in the hippocampal CA1 and dentate gyrus after amygdala kindling [112]. The role of CB1 receptors in kindling epileptogenesis has also been examined using knockout mice or pharmacological interventions (Table 1). Examination of amygdala kindling in conditional CB1 receptor knockout mice in forebrain principal neurons revealed that CB1 receptors expressed in these neurons decreased the duration of afterdischarges by amygdala stimulation [112]. However, there was no difference between conditional CB1 knockout mice and their control littermates in the number of kindling stimuli necessary for the development of generalized tonic–clonic seizures [112]. Thus, the lack of CB1 receptor signaling in principal neurons does not affect kindling epileptogenesis. The role of CB1 receptors in inhibitory neurons has also been investigated by crossing Dlx5-Cre mice and CB1-floxed mice. In these crossed transgenic mice, the specific deletion of CB1 receptors in inhibitory terminals shortened the duration of afterdischarge without affecting the development of the kindling response. These findings are in contrast with the results obtained from DGLα knockout mice that present faster kindling development [90]. In addition, both WIN 55212-2 (4 mg/kg, i.p.) [99] and 2-AG augmentation [90, 92, 93] exert suppressive effects on kindling epileptogenesis. The reasons for the phenotypic differences between mouse models with genetic manipulation of CB1 receptor expression and those of 2-AG levels remain unclear. However, our recent results suggest that CB2 receptors may be involved in kindling development [90]. We reported that blockade of CB1 or CB2 receptors alone with AM251 (20 mg/kg, i.p., twice/day) or AM630 (2 mg/kg, i.p., twice/day), respectively, did not promote epileptogenesis in the perforant path kindling model [90]. In contrast, blockade of both CB1 and CB2 receptors significantly reduced the number of perforant path stimuli required to develop generalized tonic–clonic seizures, which is consistent with the results obtained in DGLα knockout mice [90]. A recent study demonstrated that spontaneous seizures were present in CB1 and CB2 double knockout mice [113]. Importantly, CB1 or CB2 single knockout mice did not show spontaneous seizures, demonstrating complementary roles played by CB1 and CB2 receptors on the prevention of epileptogenesis [113].
Collectively, these results suggest that CB2 and CB1 receptor signaling act in concert to block epileptogenesis. Further studies using CB2 receptor knockout mice and/or CB1 and CB2 receptor double knockout mice will be required to elucidate the precise role of each receptor in epileptogenesis.
Ictogenesis
Ictogenesis is the process of transition from the non-ictal state to a seizure. This process is often investigated in animals showing spontaneous seizures or reduced seizure thresholds. Ictogenesis is also investigated by applying chemical convulsants or electrical stimulation to non-epileptic animals or tissues.
Involvement of 2-AG (Table 1)
To clarify how 2-AG signaling influences ictogenesis, we used mouse models of acute kainate administration or acute electrical stimulation [90]. After administration of kainate (30 mg/kg, i.p.), DGLα knockout mice exhibited faster development of tonic–clonic seizures and a higher mortality rate than wild-type mice. Furthermore, afterdischarges in the dentate gyrus evoked by perforant path stimulation were longer in DGLα knockout mice than in wild-type mice. Combined administration of the CB1 blocker AM251 (20 mg/kg, i.p.) and the CB2 blocker AM630 (2 mg/kg, i.p.) abolished the difference in the duration of afterdischarge between DGLα knockout and wild-type mice. These results indicate that 2-AG reduces the duration of afterdischarge through both CB1 and CB2 receptor-dependent mechanisms. Taken together, 2-AG seems to be crucial for the suppression of seizures [90].
Contrasting results have been reported in mice with virus-mediated overexpression of MGL in excitatory neurons of the dentate hilus, CA1, and CA3 [114]. In these mice, DSE was abolished in pyramidal cells, although DSI showed no difference compared to wild-type mice. Therefore, 2-AG signaling in these mice was functionally comparable to that in mice with conditional deletion of CB1 receptors at hippocampal excitatory synapses. In mice with MGL overexpression, no differences were observed in the severity of kainate-induced seizures (35 mg/kg i.p.), which contrasts with the aforementioned results in DGLα knockout mice [90]. It may be possible that the difference in seizure susceptibility between the mice with MGL overexpression and DGLα knockout mice is due to different concentrations of 2-AG in hippocampal tissue. In the hippocampi of MGL-overexpressed mice, the level of 2-AG was approximately half that of control mice, while that in DGLα knockout mice was reduced to approximately 1/10 that of wild-type mice [24]. In hippocampal homogenate samples, the efficiency of G protein activation by CB1 receptors was higher at glutamatergic synapses than at inhibitory synapses [115]. However, it has been clearly shown that the cannabinoid sensitivity of excitatory presynaptic terminals is considerably lower than that of inhibitory synaptic terminals in hippocampal slices and cultures. Therefore, DSE requires a substantially higher transient elevation of endocannabinoid levels than DSI [116]. Thus, even if DSE is absent in the hippocampus of mice with MGL overexpression, the occurrence of kainate-induced seizures may be alleviated by the remaining levels of 2-AG, which are presumably sufficient to trigger normal DSI.
The augmentation of 2-AG concentration above physiological levels can ameliorate seizures. Administration of the MGL inhibitor JZL184 (40 mg/kg, i.p.) to wild-type mice prior to kainate injection significantly reduced the incidence of tonic–clonic seizures [90]. Moreover, administration of JZL184 (40 mg/kg, i.p.) shortened the duration of afterdischarge and reduced the minimal current intensity required to elicit afterdischarges in response to electrical stimulation of the amygdala or perforant path [90, 92]. A different MGL inhibitor, SAR127303 (30 mg/kg, p.o.), suppressed seizures caused by transcorneal electrical stimulation [93]. In mice with PTZ-induced seizures (50 mg/kg, i.p.), the severity of seizures was reduced by administration of WWL123 (10 mg/kg, i.p.) [117], an inhibitor of a postsynaptic 2-AG degrading enzyme, ABHD6. This indicates that augmented 2-AG effectively suppresses seizures evoked by disinhibition of neural circuit activity by the GABAA receptor blocker PTZ. Surprisingly, as this effect was observed in CB1 and CB2 knockout mice, the suppressive effect on seizures seems to be independent of CB1 or CB2 receptor-mediated mechanisms. It has been reported that 2-AG acted directly on GABAA receptors and enhanced the inhibitory effect of GABA [117]. The blockade of ABHD6 and the resulting increase in postsynaptic 2-AG levels seem to be crucial for 2-AG-mediated enhancement of GABAA receptor signaling. Conversely, increased 2-AG levels in presynaptic terminals by the application of the MGL inhibitor SAR127303 (30 mg/kg, p.o.) did not change the minimal dose of PTZ required to induce seizures [93].
The effects of 2-AG signaling on the occurrence of spontaneous seizures in the epileptic brain have been investigated. As discussed in the previous section, expression of DGLα and CB1 receptors may have been altered from physiological levels during the onset of spontaneous seizures. Therefore, it is necessary to investigate whether 2-AG signaling exerts similar suppressive effects on seizures in the epileptic brain. We have recently reported that the MGL inhibitor JZL184 (4 mg/kg, i.p.) has a suppressive effect on the frequency of spontaneous seizures after kainate-induced SE (0.2 μg in 100 nl saline, intra-hippocampus) [90]. Naydenov et al. (2014) investigated the R6/2 mouse strain [117], which has spontaneous seizures without any preceding treatment. Although the etiology of these seizures in the R6/2 mouse strain is unknown, they demonstrated that pharmacological blockade of ABHD6 with WWL123 (10 mg/kg, i.p.) completely suppressed the occurrence of spontaneous seizures. These results suggest that augmentation of 2-AG signaling effectively suppresses ictogenesis in the epileptic brain.
The mechanisms underlying the suppressive effect of 2-AG signaling on ictogenesis can be classified into cannabinoid receptor-dependent and -independent mechanisms. Cannabinoid receptor-dependent mechanisms consist of CB1 receptor-mediated suppression of synaptic transmission, as well as mechanisms involving CB2 receptor signaling. The CB1 receptor-dependent mechanism is considered to be dominant relative to CB2 receptor-dependent mechanisms, since the deletion of CB1 receptors can explain the majority of the epileptic phenotypes in DGLα knockout mice [90]. The contribution of each cannabinoid receptor to seizures will be discussed in the following section. On the other hand, cannabinoid receptor-independent mechanisms include enhancement of GABAA receptor-mediated currents by 2-AG [117, 118]. In Xenopus oocytes, Sigel et al. showed that 2-AG increased GABA (1 µM)-induced currents through interaction with β2 subunits [118], indicating that 2-AG exerts allosteric modulation of GABAergic inhibition. However, this effect was not corroborated at synaptic GABAA receptors [118]. A possible explanation for the cannabinoid receptor-independent suppressive effect of 2-AG on ictogenesis is the modulation of AMPA receptor currents via the postsynaptic degradation enzyme, ABHD6. ABHD6 is integrated into the AMPA receptor channel complex as an auxiliary protein [119]. Inactivation of ABHD6 by short hairpin RNAs increased the frequency of AMPA receptor-mediated miniature excitatory post-synaptic currents (EPSCs) in hippocampal neurons [120]. Therefore, dampening AMPA receptor signaling by the binding of 2-AG to ABHD6 protein during seizures may be beneficial in epilepsy. However, application of WWL70 (10 μM), a specific inhibitor of ABHD6-mediated degradation of 2-AG, onto hippocampal slices did not affect the suppressive effect of ABHD6 on AMPA receptor-mediated miniature EPSCs [120], suggesting that 2-AG binding to ABHD6 may not alter AMPA receptor currents.
Involvement of AEA (Table 1)
The threshold of acute seizures induced by transcorneal electrical stimulation increased when AEA (300 mg/kg, i.p.) was directly administered or its degradation was blocked by the FAAH inhibitor O-1812 (5 mg/kg, i.p.) before the induction of seizures [121]. Since these suppressive effects were no longer observed when the CB1 antagonist SR141716A (10 mg/kg, i.p.) was co-administered, the suppressive effect of AEA on seizure seems to be mediated by CB1 receptors. Moreover, subsequent studies in rats showed that an FAAH inhibitor, AM374 (8 mg/kg, i.p.), alleviated acute seizures induced by kainate (10 mg/kg, i.p.) [122], and a different FAAH inhibitor, URB-597 (0.3–3 mg/kg, i.p.), reduced the dose of PTZ required to induce seizures [123].
Disparate results regarding the effect of AEA on seizures in FAAH knockout mice have been reported. Degradation of AEA was slower and AEA levels in the cortex, hippocampus, and cerebellum were 10 times higher in FAAH knockout mice than in wild-type mice. However, seizures induced by intraperitoneal administration of 30 mg/kg of kainate were more severe in FAAH knockout mice than in wild-type mice [101]. Interestingly, seizures did not worsen when the GABAA receptor antagonist bicuculline (4 mg/kg, i.p.) was administered to naïve FAAH knockout mice. When AEA (25 mg/kg, i.p.) was administered to FAAH knockout mice to further increase the concentration of AEA, seizures induced by intraperitoneal administration of 15 mg/kg of kainate or 4 mg/kg of bicuculline worsened. Furthermore, kainate-induced cell death was enhanced by pre-treatment with AEA. These results indicate that AEA aggravates seizures, presumably through suppression of GABA release from inhibitory presynaptic terminals. In support of this, kainate suppresses evoked inhibitory postsynaptic currents in the hippocampal CA1 region through activation of CB1 or GABAB receptors [89]. Suppression of inhibitory postsynaptic currents by kainate (3 µM) was not blocked by the DGLα inhibitor THL (5 µM) or the MGL inhibitor JZL184 (1 μM). However, the FAAH inhibitor URB597 (1 μM) significantly prolonged the effect of kainate, suggesting that AEA causes disinhibition of hippocampal CA1 neurons after administration of kainate.
AEA acts as a partial agonist of the CB1 receptor [42] as well as a full agonist of the TRPV1 receptor [124], a cation channel that depolarizes cells when activated. TRPV1 activation suppressed 2-AG synthesis and increased tonic inhibition through the reduction of tonic endocannabinoid signaling in CA1 pyramidal cells [125]. Moreover, it increased AMPA receptor endocytosis at excitatory synapses and decreased excitatory synaptic transmission in dentate granule cells [54]. These results suggest that TRPV1 activation suppresses activity of CA1 and dentate granule cells. However, the overall effect of TRPV1 activation seems to be pro-convulsive, since i.c.v. application of the TRPV1 agonist, capsaicin (10 or 100 μg, i.c.v.), aggravated seizures [126].
Involvement of CB1 receptors (Table 1)
Early studies reported suppressive effects of Δ9-THC on ictogenesis [127, 128]. However, Marsicano et al. were the first to clearly demonstrate a protective effect of CB1 receptor signaling against acute seizures [87]. Administration of kainate (30 mg/kg, i.p.) exacerbated acute seizures in CB1 knockout mice compared to wild-type mice. Moreover, acute seizures induced by kainate (30 mg/kg i.p.) were more severe in mice carrying CaMKII-positive neuron-specific CB1 receptor deletion, as well as in virus-mediated conditional knockout mice carrying CB1 deletion specifically in hippocampal excitatory neurons compared to control mice [129]. Nevertheless, there was no observable difference compared to control mice when CB1 receptors were knocked out specifically in inhibitory neurons [129]. This indicates that CB1 receptors in hippocampal excitatory neurons are necessary to suppress kainate-induced seizures. Overexpression of CB1 receptors in pyramidal cells of the hippocampal CA1, CA2, and CA3 regions; and in hilar mossy cells in the dentate gyrus significantly alleviated kainate-induced SE (30 mg/kg, i.p.) [130], suggesting that CB1 receptors at these excitatory synaptic terminals are involved in the suppression of ictogenesis. Furthermore, expression of CB1 receptors in excitatory neurons of the cerebral cortex, hippocampus, and amygdala of global CB1 receptor knockout mice prevented the exacerbation of seizures induced by kainate (30 mg/kg, i.p.) [131]. Taken together, CB1 receptors at excitatory synaptic terminals in the hippocampus are crucial for the suppression of kainate-induced seizures.
Seizure and cell death induced by administration of pilocarpine (250 mg/kg, i.p.) were more severe in CB1 knockout mice than in wild-type littermates [132]. Moreover, pretreatment of wild-type mice with the CB1 antagonist SR141716 (10 mg/kg) exacerbated pilocarpine-induced acute seizures [132]. In contrast, administration of the CB1 agonist WIN55212-2 (10 mg/kg, i.p.) ameliorated pilocarpine-induced seizures (350 mg/kg, i.p.) [133]. These results indicate that endocannabinoid signaling mediated by CB1 receptors effectively suppresses seizures induced by kainate receptor activation and those induced by activation of muscarinic acetylcholine receptors with pilocarpine.
Seizures induced by electrical stimulation of the amygdala were also more severe in excitatory neuron-specific CB1 knockout mice than in wild-type mice [112]. Conversely, seizures induced by amygdala stimulation were milder in inhibitory neuron-specific CB1 knockout mice than in wild-type mice [112]. Moreover, augmentation of CB1 receptor signaling effectively suppressed seizures evoked by electrical stimulation [134, 135]. These results indicate that the activation of CB1 receptors at excitatory synapses prevent, whereas those at inhibitory synapses exacerbate, seizures induced by electrical stimulation.
In marked contrast to seizures induced by kainate, pilocarpine, or electrical stimulation, no significant difference was found between CB1 receptor knockout mice and wild-type littermates in the severity of disinhibition-induced seizures by administration of PTZ (70 mg/kg, i.p.) [117]. This suggests that PTZ-induced seizures do not involve CB1 receptor signaling. Other reports have shown conflicting effects of CB1 receptor modulation on PTZ-induced seizures. For instance, administration of a CB1 agonist, WIN55212-2 (1 mg/kg, i.p.) or a CB1 antagonist, ACEA (4 mg/kg i.p.) [123] exacerbated PTZ-induced seizures, whereas WIN55212-2 (10 μg, i.c.v.) was also shown to ameliorate PTZ-induced seizures [136]. At present, it is difficult to conclude whether CB1 receptor signaling has anti- or pro-convulsive effects on PTZ-induced seizures. Future studies should aim to clarify these issues.
As discussed in the previous section, 2-AG signaling suppresses the occurrence of spontaneous seizures in the epileptic brain. In rats with pilocarpine-induced SE, the number of spontaneous seizures was significantly decreased by the endocannabinoid agonist WIN55212-2 (5 mg/kg, i.p.) and was significantly increased by the CB1 receptor antagonist SR141716A (10 mg/kg, i.p.) [85]. In SE induced by intra-hippocampal injection of kainate (0.2 μg), we showed that CB1 receptor signaling played an important role in the suppression of ictogenesis [90]. We administered a CB1 receptor antagonist, AM251 (20 mg/kg, i.p.), and counted the number of spontaneous seizures that occurred 2–3 weeks after SE. We observed a significant increase in the number of spontaneous seizures in AM251-treated mice compared to vehicle-treated mice. These results suggest that CB1 receptor signaling in the epileptic brain suppresses the occurrence of spontaneous seizures.
Involvement of CB2 receptors (Table 1)
In situ hybridization studies have reported CB2 mRNA expression in granule cells and inhibitory interneurons of the dentate gyrus, pyramidal cells in CA3, and pyramidal cells and inhibitory interneurons in the CA1 region [61]. Acute administration of AM630 (2 mg/kg, i.p.), a CB2 receptor antagonist, had no impact on seizures in the hippocampal dentate gyrus after electrical stimulation of the perforant path in anesthetized rats [137]. We also observed that AM630 (2 mg/kg, i.p.) alone had no effect on kainate-induced acute seizures (30 mg/kg, i.p.) in wild-type mice [90]. However, when AM630 was administered to wild-type mice pretreated with the CB1 receptor antagonist AM251 (20 mg/kg, i.p.), or to CB1 receptor knockout mice, kainate-induced acute seizures became more severe. These results suggest that the suppressive effect of CB2 receptor signaling on ictogenesis may become obvious in neural circuits with increased excitability caused by the disruption of CB1 receptor signaling. This notion is consistent with the results of a study that investigated the effects of AM630 on evoked seizures in kindled rats. Repeated PTZ administration increases susceptibility to seizures, a process termed PTZ kindling. Intraventricular administration of the CB2 receptor antagonist AM630 (5 μg/kg) to fully kindled rats resulted in longer PTZ-induced (37.5 mg/kg, i.p.) seizures [138]. Taken together, these results suggest that CB2 receptor signaling may be more potent at alleviating seizures when the excitability of neural circuits is higher.
In contrast, the CB2 receptor agonist AM1241 (1 and 10 μg) increased the duration of PTZ-induced acute seizures (70 mg/kg, i.p.). This effect was blocked by pretreatment with the CB2 receptor antagonist AM630 (1 mg/kg, i.p.), suggesting a pro-convulsant effect of CB2 receptor signaling [139]. In this report, the incidence of generalized seizures was lower and the latency to generalized tonic–clonic seizures was longer than those of PTZ-induced seizures in other reports [117, 138]. It is, therefore, possible that CB2 receptor signaling may be pro-epileptic in the mildly epileptic brain. However, in the latter PTZ-induced seizure models, the number of generalized tonic–clonic seizures evoked by PTZ (70 mg/kg, i.p.) was significantly higher in CB2 knockout mice than in their wild-type littermates [117]. It is, therefore, conceivable that CB2 receptor signaling has suppressive effects on ictogenesis in the severely epileptic brain.
Medical marijuana
There has been interest in the medical use of cannabinoids as potential antiepileptic treatments. However, evidence for the use of marijuana in the treatment of epilepsy is still insufficient. There are several excellent reviews on the medical use of marijuana and cannabinoids for epilepsy, and we direct readers to them [140, 141]. Here, we briefly summarize the possible interaction of two major cannabinoids, Δ9-THC and cannabidiol (CBD), with endocannabinoid signaling during epileptogenesis and ictogenesis. Acute treatment with Δ9-THC (0.25 mg/kg in cats, 20–100 mg/kg in rats, i.p.) suppresses ictogenesis [127, 142, 143]. However, sub-chronic treatment with Δ9-THC for several days is sufficient to induce tolerance and a loss of suppressive effects on seizures [142, 143]. Tolerance may be caused by decreased number [144] and/or desensitization of CB1 receptors [145] in a dose-dependent manner [146], and may reduce the suppressive effect of endocannabinoids on epileptogenesis and ictogenesis.
On the other hand, CBD has been reported to have antiepileptic effects without generating tolerance [143]. Recently, a series of randomized, double-blind, placebo-controlled trials of CBD have been published [147, 148]. These trials demonstrated that CBD was significantly more effective than placebo in the treatment of seizures in Dravet syndrome [147] and Lennox-Gastaut syndrome [148]. However, the mechanism by which CBD suppressed seizures in these patients remains unknown. CBD has very low affinity for CB1 and CB2 receptors [149] and acts as an inverse agonist of these receptors when it binds to them [150]. However, CBD blocks the activity of FAAH [151] and increases serum levels of AEA in humans (200–800 mg of CBD per day) [152]. Furthermore, injection of CBD (3 nmol) into the periaqueductal gray in rats increases the concentration of 2-AG at the injection site up to 2.6-fold higher than that of vehicle-injected rats [153]. Therefore, CBD may exert its suppressive effect on seizures by increasing endocannabinoids.
Concluding remarks
Endocannabinoid signaling exerts multifaceted effects on epileptogenesis and ictogenesis. Endocannabinoid signaling promotes epileptogenesis in the initial stage and suppresses ictogenesis in already established epileptic foci as well as non-epileptic brains. The roles of endocannabinoid signaling change from pro-epileptic to anti-epileptic during the course of epileptogenesis. Homeostatic downregulation of endocannabinoid signaling molecules such as DGLα and CB1 receptors appears to be induced by increased production of AEA acutely, and 2-AG subacutely, after initial insults. Suppression of epileptogenesis by antagonists of DGLα or CB1 receptors during the initial period of epileptogenesis may provide novel therapeutic targets. In kindling models, presumably due to the mild seizure phenotypes compared to SE models, augmentation of 2-AG or CB1 receptor signaling can suppress epileptogenesis. However, it should be noted that the deletion of DGLα worsens kindling epileptogenesis, while deletion of CB1 receptors does not, suggesting possible involvement of other signaling molecules such as CB2 and/or GABAA receptors. AEA seems to have less contribution to epileptogenesis compared to 2-AG.
As for the regulation of ictogenesis, endocannabinoid signaling at excitatory synapses has important roles in suppressing seizures. In particular, 2-AG suppresses seizures through multiple downstream signaling pathways including CB1, and possibly CB2 receptors. In contrast, activation of CB1 receptors at inhibitory synapses promotes ictogenesis. On the other hand, because of its partial agonist activity at CB1 receptors and full agonist activity at TRPV1 receptors, AEA may have a smaller suppressive effect, or may potentially even promote ictogenesis. After animals become epileptic and exhibit spontaneous seizures, augmentation of 2-AG or CB1 receptor signaling may prove to be an effective treatment, although excessive activation of these signaling pathways may cause downregulation of CB1 receptors and worsen seizures.
Future perspectives
Three important issues remain unresolved. First, are CB2 receptors involved in ictogenesis and epileptogenesis; and if so, how? CB2 receptor knockout mice showed more severe PTZ-induced seizures than wild-type mice. However, it is necessary to investigate global or conditional CB2 receptor knockout mice in several different models of ictogenesis and epileptogenesis to determine their precise roles. Moreover, there is a paucity of specific CB2 antibodies that reliably reflect their cellular and subcellular localizations in immunohistochemical analyses.
Second, how does the expression of CB1 receptors increase during the course of epileptogenesis? It is unlikely that the increased expression of CB1 receptors is a compensatory response for increased excitability, as it seems to occur predominantly at inhibitory synapses, which leads to the reduction of tonic and phasic suppression of inhibitory synaptic transmission. Although involvement of CB1 receptor signaling and interleukin β signaling in this process has been reported [108], possible interactions between these two molecular pathways are unclear.
Third, what molecules are involved in epileptogenesis and ictogenesis? Since CB1 receptor-mediated STD and LTD use different molecular machinery, the molecules involved in epileptogenesis may differ from those in ictogenesis. Understanding the molecular processes of epileptogenesis and ictogenesis related to endocannabinoid signaling will contribute to developing novel anti-epileptic and anti-epileptogenic drugs.
References
- 1.Forsgren L, Beghi E, Oun A, Sillanpää M. The epidemiology of epilepsy in Europe—a systematic review. Eur J Neurol. 2005;12:245–253. doi: 10.1111/j.1468-1331.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 2.Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology. 2011;77:1005–1012. doi: 10.1212/WNL.0b013e31822cfc90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 4.Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–1554. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013;29:574–577. doi: 10.1016/j.yebeh.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soltesz I, Alger BE, Kano M, Lee SH, Lovinger DM, Ohno-Shosaku T, Watanabe M. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci. 2015;16:264–277. doi: 10.1038/nrn3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- 8.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. doi: 10.1021/ja01062a046. [DOI] [Google Scholar]
- 9.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 10.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 11.Devane WA, Hanuš L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 12.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 13.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-D. [DOI] [PubMed] [Google Scholar]
- 14.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/S0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 15.Daniel H, Crepel F. Control of Ca2+ influx by cannabinoid and metabotropic glutamate receptors in rat cerebellar cortex requires K+ channels. J Physiol. 2001;537:793–800. doi: 10.1111/j.1469-7793.2001.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/S0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 19.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/S0896-6273(01)00246-X. [DOI] [PubMed] [Google Scholar]
- 20.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 21.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 23.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, Sakimura K, Kano M. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase α mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, Mark L, Piesla MJ, Deng K, Kouranova EV, Ring RH, Whiteside GT, Bates B, Walsh FS, Williams G, Pangalos MN, Samad TA, Doherty P. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luchicchi A, Pistis M. Anandamide and 2-arachidonoylglycerol: pharmacological properties, functional features, and emerging specificities of the two major endocannabinoids. Mol Neurobiol. 2012;46:374–392. doi: 10.1007/s12035-012-8299-0. [DOI] [PubMed] [Google Scholar]
- 27.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 28.Mathur BN, Tanahira C, Tamamaki N, Lovinger DM. Voltage drives diverse endocannabinoid signals to mediate striatal microcircuit-specific plasticity. Nat Neurosci. 2013;16:1275–1283. doi: 10.1038/nn.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khlaifia A, Farah H, Gackiere F, Tell F. Anandamide, cannabinoid type 1 receptor, and NMDA receptor activation mediate non-Hebbian presynaptically expressed long-term depression at the first central synapse for visceral afferent fibers. J Neurosci. 2013;33:12627–12637. doi: 10.1523/JNEUROSCI.1028-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohno-Shosaku T, Hashimotodani Y, Ano M, Takeda S, Tsubokawa H, Kano M. Endocannabinoid signalling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J Physiol. 2007;584:407–418. doi: 10.1113/jphysiol.2007.137505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cβ4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimotodani Y, Ohno-Shosaku T, Kano M. Ca2+-assisted receptor-driven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities. Curr Opin Neurobiol. 2007;17:360–365. doi: 10.1016/j.conb.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Ohno-Shosaku T, Tanimura A, Hashimotodani Y, Kano M. Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist. 2012;18:119–132. doi: 10.1177/1073858410397377. [DOI] [PubMed] [Google Scholar]
- 36.Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/S0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 37.Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- 38.Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- 39.Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonoylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 42.Hillard CJ. Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat. 2000;61:3–18. doi: 10.1016/S0090-6980(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz SK, King C, Kortleven C, Huson V, Kroon T, Kevenaar JT, Schut D, Saarloos I, Hoetjes JP, de Wit H, Stiedl O, Spijker S, Li KW, Mansvelder HD, Smit AB, Cornelisse LN, Verhage M, Toonen RF. Presynaptic inhibition upon CB1 or mGlu2/3 receptor activation requires ERK/MAPK phosphorylation of Munc18-1. EMBO J. 2016;35:1236–1250. doi: 10.15252/embj.201592244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu QS. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31:13420–13430. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanimura A, Uchigashima M, Yamazaki M, Uesaka N, Mikuni T, Abe M, Hashimoto K, Watanabe M, Sakimura K, Kano M. Synapse type-independent degradation of the endocannabinoid 2-arachidonoylglycerol after retrograde synaptic suppression. Proc Natl Acad Sci USA. 2012;109:12195–12200. doi: 10.1073/pnas.1204404109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchigashima M, Yamazaki M, Yamasaki M, Tanimura A, Sakimura K, Kano M, Watanabe M. Molecular and morphological configuration for 2-arachidonoylglycerol-mediated retrograde signaling at mossy cell-granule cell synapses in the dentate gyrus. J Neurosci. 2011;31:7700–7714. doi: 10.1523/JNEUROSCI.5665-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/S0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 48.Chevaleyre V, Heifets BD, Kaeser PS, Südhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1α. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heifets BD, Chevaleyre V, Castillo PE. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc Natl Acad Sci USA. 2008;105:10250–10255. doi: 10.1073/pnas.0711880105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yasuda H, Huang Y, Tsumoto T. Regulation of excitability and plasticity by endocannabinoids and PKA in developing hippocampus. Proc Natl Acad Sci USA. 2008;105:3106–3111. doi: 10.1073/pnas.0708349105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Younts TJ, Monday HR, Dudok B, Klein ME, Jordan BA, Katona I, Castillo PE. Presynaptic protein synthesis is required for long-term plasticity of GABA release. Neuron. 2016;92:479–492. doi: 10.1016/j.neuron.2016.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Losonczy A, Biró AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci USA. 2004;101:1362–1367. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgänsberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chávez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17:1226–1242. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- 58.Leishman E, Mackie K, Luquet S, Bradshaw HB. Lipidomics profile of a NAPE-PLD KO mouse provides evidence of a broader role of this enzyme in lipid metabolism in the brain. Biochim Biophys Acta. 2016;1861:491–500. doi: 10.1016/j.bbalip.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 60.Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Kim J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience. 2015;311:253–267. doi: 10.1016/j.neuroscience.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stempel AV, Stumpf A, Zhang HY, Özdoğan T, Pannasch U, Theis AK, Otte DM, Wojtalla A, Rácz I, Ponomarenko A, Xi ZX, Zimmer A, Schmitz D. Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron. 2016;90:795–809. doi: 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Kim J. Deletion of CB2 cannabinoid receptors reduces synaptic transmission and long-term potentiation in the mouse hippocampus. Hippocampus. 2016;26:275–281. doi: 10.1002/hipo.22558. [DOI] [PubMed] [Google Scholar]
- 64.Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, Gardner EL, Wu J, Xi ZX. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci USA. 2014;111:E5007–E5015. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ludányi A, Erőss L, Czirják S, Vajda J, Halász P, Watanabe M, Palkovits M, Maglóczky Z, Freund TF, Katona I. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci. 2008;28:2976–2990. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romigi A, Bari M, Placidi F, Marciani MG, Malaponti M, Torelli F, Izzi F, Prosperetti C, Zannino S, Corte F, Chiaramonte C, Maccarrone M. Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia. 2010;51:768–772. doi: 10.1111/j.1528-1167.2009.02334.x. [DOI] [PubMed] [Google Scholar]
- 67.Maglóczky Z, Tóth K, Karlócai R, Nagy S, Erőss L, Czirják S, Vajda J, Rásonyi G, Kelemen A, Juhos V, Halász P, Mackie K, Freund TF. Dynamic changes of CB1-receptor expression in hippocampi of epileptic mice and humans. Epilepsia. 2010;51(Suppl 3):115–120. doi: 10.1111/j.1528-1167.2010.02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katona I, Sperlágh B, Maglóczky Z, Sántha E, Köfalvi A, Czirják S, Mackie K, Vizi ES, Freund TF. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/S0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- 69.Goffin K, Van Paesschen W, Van Laere K. In vivo activation of endocannabinoid system in temporal lobe epilepsy with hippocampal sclerosis. Brain. 2011;134:1033–1040. doi: 10.1093/brain/awq385. [DOI] [PubMed] [Google Scholar]
- 70.Meng XD, Wei D, Li J, Kang JJ, Wu C, Ma L, Yang F, Zhu GM, Ou-Yang TP, Liu YY, Jiang W. Astrocytic expression of cannabinoid type 1 receptor in rat and human sclerotic hippocampi. Int J Clin Exp Pathol. 2014;7:2825–2837. [PMC free article] [PubMed] [Google Scholar]
- 71.Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous DN, Mendizabal-Zubiaga J, Grandes P, Liu Q, Bai G, Wang W, Xiong L, Ren W, Marsicano G, Zhang X. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 72.Desjardins P, Sauvageau A, Bouthillier A, Navarro D, Hazell AS, Rose C, Butterworth RF. Induction of astrocytic cyclooxygenase-2 in epileptic patients with hippocampal sclerosis. Neurochem Int. 2003;42:299–303. doi: 10.1016/S0197-0186(02)00101-8. [DOI] [PubMed] [Google Scholar]
- 73.Kira R, Ishizaki Y, Torisu H, Sanefuji M, Takemoto M, Sakamoto K, Matsumoto S, Yamaguchi Y, Yukaya N, Sakai Y, Gondo K, Hara T. Genetic susceptibility to febrile seizures: case–control association studies. Brain Dev. 2010;32:57–63. doi: 10.1016/j.braindev.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 74.Smith DR, Stanley CM, Foss T, Boles RG, McKernan K. Rare genetic variants in the endocannabinoid system genes CNR1 and DAGLA are associated with neurological phenotypes in humans. PLoS One. 2017;12:e0187926. doi: 10.1371/journal.pone.0187926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214:1020–1021. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- 76.Morimoto K, Katayama K, Inoue K, Sato K. Effects of competitive and noncompetitive NMDA receptor antagonists on kindling and LTP. Pharmacol Biochem Behav. 1991;40:893–899. doi: 10.1016/0091-3057(91)90103-9. [DOI] [PubMed] [Google Scholar]
- 77.Pan YZ, Rutecki PA. Enhanced excitatory synaptic network activity following transient group I metabotropic glutamate activation. Neuroscience. 2014;275:22–32. doi: 10.1016/j.neuroscience.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 78.Renaud J, Emond M, Meilleur S, Psarropoulou C, Carmant L. AIDA, a class I metabotropic glutamate-receptor antagonist limits kainate-induced hippocampal dysfunction. Epilepsia. 2002;43:1306–1317. doi: 10.1046/j.1528-1157.2002.10402.x. [DOI] [PubMed] [Google Scholar]
- 79.Weiss SR, Li XL, Rosen JB, Li H, Heynen T, Post RM. Quenching: inhibition of development and expression of amygdala kindled seizures with low frequency stimulation. Neuroreport. 1995;6:2171–2176. doi: 10.1097/00001756-199511000-00018. [DOI] [PubMed] [Google Scholar]
- 80.Jalilifar M, Yadollahpour A, Moazedi AA, Ghotbeddin Z. Low frequency electrical stimulation either prior to or after rapid kindling stimulation inhibits the kindling-induced epileptogenesis. Biomed Res Int. 2017;2017:8623743. doi: 10.1155/2017/8623743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, Selley DE. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol. 2006;70:986–996. doi: 10.1124/mol.105.019612. [DOI] [PubMed] [Google Scholar]
- 82.Schlosburg JE, Kinsey SG, Ignatowska-Jankowska B, Ramesh D, Abdullah RA, Tao Q, Long JZ, Selley DE, Cravatt BF, Lichtman AH. Prolonged monoacylglycerol lipase blockade causes equivalent cannabinoid receptor type 1 receptor-mediated adaptations in fatty acid amide hydrolase wild-type and knockout mice. J Pharmacol Exp Ther. 2014;350:196–204. doi: 10.1124/jpet.114.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, Lichtman AH. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther. 2013;345:492–501. doi: 10.1124/jpet.112.201426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sánchez AJ, García-Merino A. Neuroprotective agents: cannabinoids. Clin Immunol. 2012;142:57–67. doi: 10.1016/j.clim.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 85.Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther. 2003;307:129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- 86.Fezza F, Marrone MC, Avvisati R, Di Tommaso M, Lanuti M, Rapino C, Mercuri NB, Maccarrone M, Marinelli S. Distinct modulation of the endocannabinoid system upon kainic acid-induced in vivo seizures and in vitro epileptiform bursting. Mol Cell Neurosci. 2014;62:1–9. doi: 10.1016/j.mcn.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, López-Rodriguez ML, Casanova E, Schütz G, Zieglgänsberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 88.Lerner R, Post J, Loch S, Lutz B, Bindila L. Targeting brain and peripheral plasticity of the lipidome in acute kainic acid-induced epileptic seizures in mice via quantitative mass spectrometry. Biochim Biophys Acta. 2017;1862:255–267. doi: 10.1016/j.bbalip.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 89.Lourenço J, Matias I, Marsicano G, Mulle C. Pharmacological activation of kainate receptors drives endocannabinoid mobilization. J Neurosci. 2011;31:3243–3248. doi: 10.1523/JNEUROSCI.3512-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sugaya Y, Yamazaki M, Uchigashima M, Kobayashi K, Watanabe M, Sakimura K, Kano M. Crucial roles of the endocannabinoid 2-arachidonoylglycerol in the suppression of epileptic seizures. Cell Rep. 2016;16:1405–1415. doi: 10.1016/j.celrep.2016.06.083. [DOI] [PubMed] [Google Scholar]
- 91.Hansen SL, Nielsen AH, Knudsen KE, Artmann A, Petersen G, Kristiansen U, Hansen SH, Hansen HS. Ketogenic diet is antiepileptogenic in pentylenetetrazole kindled mice and decrease levels of N-acylethanolamines in hippocampus. Neurochem Int. 2009;54:199–204. doi: 10.1016/j.neuint.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 92.von Rüden EL, Bogdanovic RM, Wotjak CT, Potschka H. Inhibition of monoacylglycerol lipase mediates a cannabinoid 1-receptor dependent delay of kindling progression in mice. Neurobiol Dis. 2015;77:238–245. doi: 10.1016/j.nbd.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 93.Griebel G, Pichat P, Beeské S, Leroy T, Redon N, Jacquet A, Françon D, Bert L, Even L, Lopez-Grancha M, Tolstykh T, Sun F, Yu Q, Brittain S, Arlt H, He T, Zhang B, Wiederschain D, Bertrand T, Houtmann J, Rak A, Vallée F, Michot N, Augé F, Menet V, Bergis OE, George P, Avenet P, Mikol V, Didier M, Escoubet J. Selective blockade of the hydrolysis of the endocannabinoid 2-arachidonoylglycerol impairs learning and memory performance while producing antinociceptive activity in rodents. Sci Rep. 2015;5:7642. doi: 10.1038/srep07642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kallendrusch S, Hobusch C, Ehrlich A, Nowicki M, Ziebell S, Bechmann I, Geisslinger G, Koch M, Dehghani F. Intrinsic up-regulation of 2-AG favors an area specific neuronal survival in different in vitro models of neuronal damage. PLoS One. 2012;7:e51208. doi: 10.1371/journal.pone.0051208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma L, Wang L, Yang F, Meng XD, Wu C, Ma H, Jiang W. Disease-modifying effects of RHC80267 and JZL184 in a pilocarpine mouse model of temporal lobe epilepsy. CNS Neurosci Ther. 2014;20:905–915. doi: 10.1111/cns.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blair RE, Deshpande LS, Sombati S, Elphick MR, Martin BR, DeLorenzo RJ. Prolonged exposure to WIN55, 212-2 causes downregulation of the CB1 receptor and the development of tolerance to its anticonvulsant effects in the hippocampal neuronal culture model of acquired epilepsy. Neuropharmacology. 2009;57:208–218. doi: 10.1016/j.neuropharm.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blair RE, Deshpande LS, Sombati S, Falenski KW, Martin BR, DeLorenzo RJ. Activation of the cannabinoid type-1 receptor mediates the anticonvulsant properties of cannabinoids in the hippocampal neuronal culture models of acquired epilepsy and status epilepticus. J Pharmacol Exp Ther. 2006;317:1072–1078. doi: 10.1124/jpet.105.100354. [DOI] [PubMed] [Google Scholar]
- 98.Deshpande LS, Sombati S, Blair RE, Carter DS, Martin BR, DeLorenzo RJ. Cannabinoid CB1 receptor antagonists cause status epilepticus-like activity in the hippocampal neuronal culture model of acquired epilepsy. Neurosci Lett. 2007;411:11–16. doi: 10.1016/j.neulet.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wendt H, Soerensen J, Wotjak CT, Potschka H. Targeting the endocannabinoid system in the amygdala kindling model of temporal lobe epilepsy in mice. Epilepsia. 2011;52:e62–e65. doi: 10.1111/j.1528-1167.2011.03079.x. [DOI] [PubMed] [Google Scholar]
- 100.Mikheeva IB, Shubina L, Matveeva N, Pavlik LL, Kitchigina VF. Fatty acid amide hydrolase inhibitor URB597 may protect against kainic acid-induced damage to hippocampal neurons: dependence on the degree of injury. Epilepsy Res. 2017;137:84–94. doi: 10.1016/j.eplepsyres.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 101.Clement AB, Hawkins EG, Lichtman AH, Cravatt BF. Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase. J Neurosci. 2003;23:3916–3923. doi: 10.1523/JNEUROSCI.23-09-03916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Falenski KW, Blair RE, Sim-Selley LJ, Martin BR, DeLorenzo RJ. Status epilepticus causes a long-lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat pilocarpine model of acquired epilepsy. Neuroscience. 2007;146:1232–1244. doi: 10.1016/j.neuroscience.2007.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Falenski KW, Carter DS, Harrison AJ, Martin BR, Blair RE, DeLorenzo RJ. Temporal characterization of changes in hippocampal cannabinoid CB1 receptor expression following pilocarpine-induced status epilepticus. Brain Res. 2009;1262:64–72. doi: 10.1016/j.brainres.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen K, Ratzliff A, Hilgenberg L, Gulyás A, Freund TF, Smith M, Dinh TP, Piomelli D, Mackie K, Soltesz I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/S0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- 105.Chen K, Neu A, Howard AL, Földy C, Echegoyen J, Hilgenberg L, Smith M, Mackie K, Soltesz I. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci. 2007;27:46–58. doi: 10.1523/JNEUROSCI.3966-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Son MH, Kim HD, Chae YN, Kim MK, Shin CY, Ahn GJ, Choi SH, Yang EK, Park KJ, Chae HW, Moon HS, Kim SH, Shin YG, Yoon SH. Peripherally acting CB1-receptor antagonist: the relative importance of central and peripheral CB1 receptors in adiposity control. Int J Obes (Lond) 2010;34:547–556. doi: 10.1038/ijo.2009.253. [DOI] [PubMed] [Google Scholar]
- 107.Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 108.Feng B, Tang Y, Chen B, Xu C, Wang Y, Dai Y, Wu D, Zhu J, Wang S, Zhou Y, Shi L, Hu W, Zhang X, Chen Z. Transient increase of interleukin-1β after prolonged febrile seizures promotes adult epileptogenesis through long-lasting upregulating endocannabinoid signaling. Sci Rep. 2016;6:21931. doi: 10.1038/srep21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373:1105–1110. doi: 10.1016/S0140-6736(09)60214-2. [DOI] [PubMed] [Google Scholar]
- 110.Echegoyen J, Armstrong C, Morgan RJ, Soltesz I. Single application of a CB1 receptor antagonist rapidly following head injury prevents long-term hyperexcitability in a rat model. Epilepsy Res. 2009;85:123–127. doi: 10.1016/j.eplepsyres.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Di Maio R, Cannon JR, Greenamyre JT. Post-status epilepticus treatment with the cannabinoid agonist WIN 55,212-2 prevents chronic epileptic hippocampal damage in rats. Neurobiol Dis. 2015;73:356–365. doi: 10.1016/j.nbd.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 112.von Rüden EL, Jafari M, Bogdanovic RM, Wotjak CT, Potschka H. Analysis in conditional cannabinoid 1 receptor-knockout mice reveals neuronal subpopulation-specific effects on epileptogenesis in the kindling paradigm. Neurobiol Dis. 2015;73:334–347. doi: 10.1016/j.nbd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 113.Rowley S, Sun X, Lima IV, Tavenier A, de Oliveira ACP, Dey SK, Danzer SC. Cannabinoid receptor 1/2 double-knockout mice develop epilepsy. Epilepsia. 2017;58:e162–e166. doi: 10.1111/epi.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guggenhuber S, Romo-Parra H, Bindila L, Leschik J, Lomazzo E, Remmers F, Zimmermann T, Lerner R, Klugmann M, Pape HC, Lutz B. Impaired 2-AG signaling in hippocampal glutamatergic neurons: aggravation of anxiety-like behavior and unaltered seizure susceptibility. Int J Neuropsychopharmacol. 2015;19:1–13. doi: 10.1093/ijnp/pyv091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Steindel F, Lerner R, Häring M, Ruehle S, Marsicano G, Lutz B, Monory K. Neuron-type specific cannabinoid-mediated G protein signalling in mouse hippocampus. J Neurochem. 2013;124:795–807. doi: 10.1111/jnc.12137. [DOI] [PubMed] [Google Scholar]
- 116.Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Marsicano G, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Naydenov AV, Horne EA, Cheah CS, Swinney K, Hsu KL, Cao JK, Marrs WR, Blankman JL, Tu S, Cherry AE, Fung S, Wen A, Li W, Saporito MS, Selley DE, Cravatt BF, Oakley JC, Stella N. ABHD6 blockade exerts antiepileptic activity in PTZ-induced seizures and in spontaneous seizures in R6/2 mice. Neuron. 2014;83:361–371. doi: 10.1016/j.neuron.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sigel E, Baur R, Rácz I, Marazzi J, Smart TG, Zimmer A, Gertsch J. The major central endocannabinoid directly acts at GABAA receptors. Proc Natl Acad Sci USA. 2011;108:18150–18155. doi: 10.1073/pnas.1113444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, Müller CS, Bildl W, Baehrens D, Hüber B, Kulik A, Klöcker N, Schulte U, Fakler B. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron. 2012;74:621–633. doi: 10.1016/j.neuron.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 120.Wei M, Zhang J, Jia M, Yang C, Pan Y, Li S, Luo Y, Zheng J, Ji J, Chen J, Hu X, Xiong J, Shi Y, Zhang C. α/β-Hydrolase domain-containing 6 (ABHD6) negatively regulates the surface delivery and synaptic function of AMPA receptors. Proc Natl Acad Sci USA. 2016;113:E2695–E2704. doi: 10.1073/pnas.1524589113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wallace MJ, Martin BR, DeLorenzo RJ. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol. 2002;452:295–301. doi: 10.1016/S0014-2999(02)02331-2. [DOI] [PubMed] [Google Scholar]
- 122.Karanian DA, Karim SL, Wood JT, Williams JS, Lin S, Makriyannis A, Bahr BA. Endocannabinoid enhancement protects against kainic acid-induced seizures and associated brain damage. J Pharmacol Exp Ther. 2007;322:1059–1066. doi: 10.1124/jpet.107.120147. [DOI] [PubMed] [Google Scholar]
- 123.Vilela LR, Medeiros DC, Rezende GH, de Oliveira AC, Moraes MF, Moreira FA. Effects of cannabinoids and endocannabinoid hydrolysis inhibition on pentylenetetrazole-induced seizure and electroencephalographic activity in rats. Epilepsy Res. 2013;104:195–202. doi: 10.1016/j.eplepsyres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 124.Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee SH, Ledri M, Tóth B, Marchionni I, Henstridge CM, Dudok B, Kenesei K, Barna L, Szabó SI, Renkecz T, Oberoi M, Watanabe M, Limoli CL, Horvai G, Soltesz I, Katona I. Multiple forms of endocannabinoid and endovanilloid signaling regulate the tonic control of GABA release. J Neurosci. 2015;35:10039–10057. doi: 10.1523/JNEUROSCI.4112-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Manna SS, Umathe SN. Involvement of transient receptor potential vanilloid type 1 channels in the pro-convulsant effect of anandamide in pentylenetetrazole-induced seizures. Epilepsy Res. 2012;100:113–124. doi: 10.1016/j.eplepsyres.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 127.Wada JA, Wake A, Sato M, Corcoran ME. Antiepileptic and prophylactic effects of tetrahydrocannabinols in amygdaloid kindled cats. Epilepsia. 1975;16:503–510. doi: 10.1111/j.1528-1157.1975.tb06080.x. [DOI] [PubMed] [Google Scholar]
- 128.Boggan WO, Steele RA, Freedman DX. Δ9-tetrahydrocannabinol effect on audiogenic seizure susceptibility. Psychopharmacologia. 1973;29:101–106. doi: 10.1007/BF00422641. [DOI] [PubMed] [Google Scholar]
- 129.Monory K, Massa F, Egertová M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wölfel B, Dodt HU, Zieglgänsberger W, Wotjak CT, Mackie K, Elphick MR, Marsicano G, Lutz B. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guggenhuber S, Monory K, Lutz B, Klugmann M. AAV vector-mediated overexpression of CB1 cannabinoid receptor in pyramidal neurons of the hippocampus protects against seizure-induced excitoxicity. PLoS One. 2010;5:e15707. doi: 10.1371/journal.pone.0015707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ruehle S, Remmers F, Romo-Parra H, Massa F, Wickert M, Wörtge S, Häring M, Kaiser N, Marsicano G, Pape HC, Lutz B. Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J Neurosci. 2013;33:10264–10277. doi: 10.1523/JNEUROSCI.4171-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kow RL, Jiang K, Naydenov AV, Le JH, Stella N, Nathanson NM. Modulation of pilocarpine-induced seizures by cannabinoid receptor 1. PLoS One. 2014;9:e95922. doi: 10.1371/journal.pone.0095922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carletti F, Gambino G, Rizzo V, Ferraro G, Sardo P. Cannabinoid and nitric oxide signaling interplay in the modulation of hippocampal hyperexcitability: study on electrophysiological and behavioral models of temporal lobe epilepsy in the rat. Neuroscience. 2015;303:149–159. doi: 10.1016/j.neuroscience.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 134.Rizzo V, Ferraro G, Carletti F, Lonobile G, Cannizzaro C, Sardo P. Evidences of cannabinoids-induced modulation of paroxysmal events in an experimental model of partial epilepsy in the rat. Neurosci Lett. 2009;462:135–139. doi: 10.1016/j.neulet.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 135.Wallace MJ, Wiley JL, Martin BR, DeLorenzo RJ. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol. 2001;428:51–57. doi: 10.1016/S0014-2999(01)01243-2. [DOI] [PubMed] [Google Scholar]
- 136.Naderi N, Ahmad-Molaei L, Aziz Ahari F, Motamedi F. Modulation of anticonvulsant effects of cannabinoid compounds by GABAA receptor agonist in acute pentylenetetrazole model of seizure in rat. Neurochem Res. 2011;36:1520–1525. doi: 10.1007/s11064-011-0479-1. [DOI] [PubMed] [Google Scholar]
- 137.Rizzo V, Carletti F, Gambino G, Schiera G, Cannizzaro C, Ferraro G, Sardo P. Role of CB2 receptors and cGMP pathway on the cannabinoid-dependent antiepileptic effects in an in vivo model of partial epilepsy. Epilepsy Res. 2014;108:1711–1718. doi: 10.1016/j.eplepsyres.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 138.Aghaei I, Rostampour M, Shabani M, Naderi N, Motamedi F, Babaei P, Khakpour-Taleghani B. Palmitoylethanolamide attenuates PTZ-induced seizures through CB1 and CB2 receptors. Epilepsy Res. 2015;117:23–28. doi: 10.1016/j.eplepsyres.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 139.de Carvalho CR, Hoeller AA, Franco PL, Martini AP, Soares FM, Lin K, Prediger RD, Whalley BJ, Walz R. The cannabinoid CB2 receptor-specific agonist AM1241 increases pentylenetetrazole-induced seizure severity in Wistar rats. Epilepsy Res. 2016;127:160–167. doi: 10.1016/j.eplepsyres.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 140.Rosenberg EC, Tsien RW, Whalley BJ, Devinsky O. Cannabinoids and epilepsy. Neurotherapeutics. 2015;12:747–768. doi: 10.1007/s13311-015-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Friedman D, Devinsky O. Cannabinoids in the treatment of epilepsy. N Engl J Med. 2015;373:1048–1058. doi: 10.1056/NEJMra1407304. [DOI] [PubMed] [Google Scholar]
- 142.Colasanti BK, Lindamood C, III, Craig CR. Effects of marihuana cannabinoids on seizure activity in cobalt-epileptic rats. Pharmacol Biochem Behav. 1982;16:573–578. doi: 10.1016/0091-3057(82)90418-X. [DOI] [PubMed] [Google Scholar]
- 143.Karler R, Turkanis SA. Subacute cannabinoid treatment: anticonvulsant activity and withdrawal excitability in mice. Br J Pharmacol. 1980;68:479–484. doi: 10.1111/j.1476-5381.1980.tb14562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Romero J, Berrendero F, Manzanares J, Pérez A, Corchero J, Fuentes JA, Fernández-Ruiz JJ, Ramos JA (1998) Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to Δ9-tetrahydrocannabinol. Synapse 30:298–308. https://doi.org/10.1002/(sici)1098-2396(199811)30:3<298::aid-syn7>3.0.co;2-6 [DOI] [PubMed]
- 145.Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with Δ9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPγS autoradiography in rat brain. J Neurosci. 1996;16:8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, Sim-Selley LJ. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2008;324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA, Wright S, Cannabidiol in Dravet Syndrome Study G Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–2020. doi: 10.1056/nejmoa1611618. [DOI] [PubMed] [Google Scholar]
- 148.Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, Lyons PD, Taylor A, Roberts C, Sommerville K, Group GS. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:1085–1096. doi: 10.1016/s0140-6736(18)30136-3. [DOI] [PubMed] [Google Scholar]
- 149.Petitet F, Jeantaud B, Reibaud M, Imperato A, Dubroeucq MC. Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of Δ9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998;63:PL1–PL6. doi: 10.1016/S0024-3205(98)00238-0. [DOI] [PubMed] [Google Scholar]
- 150.Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bisogno T, Hanuš L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, Klosterkötter J, Hellmich M, Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maione S, Piscitelli F, Gatta L, Vita D, De Petrocellis L, Palazzo E, de Novellis V, Di Marzo V. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br J Pharmacol. 2011;162:584–596. doi: 10.1111/j.1476-5381.2010.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]