Abstract
The extracellular matrix (ECM) plays diverse roles in several physiological and pathological conditions. In the brain, the ECM is unique both in its composition and in functions. Furthermore, almost all the cells in the central nervous system contribute to different aspects of this intricate structure. Brain ECM, enriched with proteoglycans and other small proteins, aggregate into distinct structures around neurons and oligodendrocytes. These special structures have cardinal functions in the normal functioning of the brain, such as learning, memory, and synapse regulation. In this review, we have compiled the current knowledge about the structure and function of important ECM molecules in the brain and their proteolytic remodeling by matrix metalloproteinases and other enzymes, highlighting the special structures they form. In particular, the proteoglycans in brain ECM, which are essential for several vital functions, are emphasized in detail.
Keywords: Brain, Extracellular matrix, Matrix remodeling, Nodes of Ranvier, Perineuronal nets, Proteases, Synapses
Extracellular matrix
The extracellular matrix (ECM) encompasses all the secreted insoluble components that form a three-dimensional structure that scaffolds the cells [1, 2]. The ECM plays a vital role in maintaining the structural integrity of tissues and in transducing cellular communication by mediating signaling pathways. Several cell surface receptors, including integrins, cadherins, selectins, syndecans, and others are known to interact with the ECM molecules, thereby regulating vital processes such as migration, proliferation, and differentiation [3–7]. The ECM is primarily constructed of structural proteins, proteoglycans, glycoproteins, and matricellular proteins [2, 5]. The composition and characteristics of the ECM are constantly being modified during normal development and aging, and under pathological conditions, such as cancer [8–11]. The composition and modifications of the ECM dictate its mechanical properties; consequently, these properties largely control the biophysical, biochemical, and topological properties of different tissues [12–14]. The ECM components are regulated both at the transcriptional and translational levels. However, the most widely studied regulation is executed extracellularly, by different classes of proteolytic enzymes and their inhibitors, which maintain the homeostasis of the ECM deposition and degradation [2, 15]. In the current review, we discuss the composition, modifications, and structures of the ECM in the central nervous system (CNS). We focus on specialized ECM structures in the brain as well as proteolytic enzymes, such as matrix metalloproteinases (MMPs) that regulate the turnover, function, and architecture of the ECM.
Brain extracellular matrix
The ECM was initially referred to as a “ground substance” and was thought to be absent in the CNS [16, 17]. However, consistent efforts of cell and matrix biologists revealed not only the presence of ECM, but also its key role in the development and function of the brain. The total extracellular space, which is filled with interstitial fluid and matrix, is estimated to occupy 20% of the brain’s volume [18–20]. The adult brain has a unique ECM composition with almost negligible presence of collagen and other fibrillar ECM proteins, with the exception of the basement membrane and meningeal layers [19]. The ECM of the brain is enriched with non-fibrillar components such as proteoglycans, glycoproteins, small linker proteins, matricellular proteins, and importantly, enzymes that regulate the ECM deposition and degradation. The ECM in the brain can be broadly classified into interstitial ECM and specialized structures around neurons [18–21]. In this review, we discuss the structure and functions of perineuronal nets (PNNs), and the ECM around the nodes of Ranvier and synapses.
Chondroitin sulfate proteoglycans
A large portion of the ECM in the CNS consists of proteoglycans [22]. Proteoglycans are molecules with sugar moieties termed glycosaminoglycans (GAGs), which are covalently attached to core proteins [23, 24]. The most important proteoglycans found in the CNS are chondroitin sulfate proteoglycans (CSPGs), which are mostly secreted, and membrane-bound heparin sulfate proteoglycans (HSPGs) [25, 26]. Owing to their abundance, diversity, and key role in the assembly of special ECM structures in the brain, in this review we focus mainly on CSPGs. Several studies have shown that CSPGs play a crucial role in the development and normal maintenance of the CNS, and regarding abnormalities in their expression, leading to a variety of pathologies [25, 27–31]. Almost all cell types in the developing CNS secrete CSPGs and provide critical cues for neural patterning [32–34]. In the mature brain, CSPGs are the conspicuous components of a specialized structure termed perineuronal nets (PNNs) [19, 35]. CSPGs are composed of a core protein, which is attached to a long linear polysaccharide termed chondroitin sulfate GAG, through three sequential sugars [36]. The polymerization of GAGs to the growing chain is catalyzed by the enzyme chondroitin synthase in the Golgi apparatus; it can result in very large proteoglycans with over 100 repeating GAGs [37]. Chondroitin sulfotransferase enzymes add negatively charged sulfate groups to the sugar molecules at multiple sites, thus affecting the interaction of GAG chains with the positively charged amino acids in the core protein. These post-translational modifications change the interaction dynamics of the proteoglycans with other molecules [38, 39]. The position of the sulfation determines the five different types of CSPGs: CS-A (C4 of GalNAc), CS-C (C6 of GalNAc), CS-D (C6 of GalNAc and C2 of GlcUA), CS-E (C4 and C6 of GalNAc), and CS-B [39]. CS-B, also known as dermatan sulfate proteoglycan (DSPG), results from epimerization of GlcUA to iduronic acid (IdoA) and is classified as a separate molecule [37]. The most common CSPGs in the adult mouse brain are CS-A, CS-C, CS-D, and CS-E. These proteoglycans are distributed non-uniformly within the CNS and their functions vary widely, based on the core protein, its glycation, and the sulfation of the GAGs [40–42]. Specifically, CS-E is abundantly expressed in the cerebral cortex, whereas the cerebellum is enriched with CS-D and a few CS-E subunits [43] (Fig. 1).
Fig. 1.
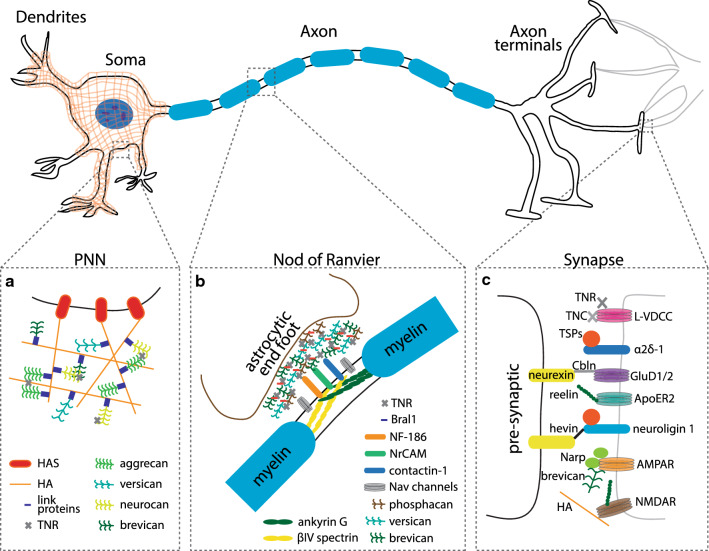
Diagrammatic sketch of the special ECM structures around the neurons. a The perineuronal net (PNN), which enwraps the soma and dendrites, is primarily made up of lecticans (aggrecan, versican, neurocan and brevican) that are bound to the hyaluronic acid (HA) backbone synthesized by the membrane bound enzyme hyaluronic acid synthetase (HAS). Lecticans are connected to HA with link proteins, which are crucial for the structure. Other molecules such as tenascin-R (TNR), which interact with PNN molecules, also play an important role in stabilizing the structure. b The gaps between the myelin sheaths, namely, the nods of Ranvier, are exposed to a myriad of ECM molecules through which it can interact with the adjacent astrocytes. These ECM molecules not only stabilize the nodes but also act as a regulator of neuron-glia communication. c Different ECM molecules present at pre-synaptic boutons and post-synaptic clefts interact dynamically. These molecular interactions regulate vital processes like synaptogenesis, neuronal migration and cell–cell communications
Lecticans: special CSPGs of the central nervous system
The most important and widely expressed CSPGs in the CNS are aggrecan, versican, neurocan and brevican, collectively termed lecticans [44]. They are also known as hyalectans for their ability to bind to hyaluronic acid (HA; hyaluronan) [45]. Structurally, lecticans can be divided into three segments: a core protein and two globular domains at the N- and C- terminals. The core protein links the N- and C-terminals and has structurally diverse features that serve as anchors for GAG chains to bind. The C-terminal (G3 domain) contains EGF and complement regulatory protein (CRP)-like domains, which flank the c-type lectin domain. On the other side, the N-terminal globular (G1) domain binds to HA and is homologous to other HA-binding proteins like CD44. The N terminal globular domain (G1 domain) consists of two distinct structures, an IgG-like loop, which is less conserved (40% identity) across the lectican family, and a link protein-like tandem repeat (60% identity), also referred to as a proteoglycan tandem repeat (PTR), which has structural similarities to hyaluronan and proteoglycan link proteins (HAPLNs) [46, 47]. Both the IgG-like loop and the PTR domains consist of conserved cysteine amino acids, which are important for the disulfide bonds that bridge the two domains of the N-terminal. Aggrecan, an exception of the lectican members, contains an additional domain in the N-terminal, termed the G2 domain. This domain contains only the PTR structure and is connected to the G1 domain with an interglobular domain of approximately 130 amino acids.
The core protein in lecticans varies in length and is the preferred site of glycosylation [44, 47]. The number of GAG attachment sites differs between the lecticans, with aggrecan having the most and brevican the least number of sites. Human aggrecan, in contrast to its rat and mouse counterparts, contains an additional subdomain downstream of the G2 domain, which acts as a binding region for keratan sulfate chains. The three subdomains in the G3 domain (EGF, CRP, and c-type lectin) exhibit a structural resemblance to the domain of the cell adhesion molecule, selectin. However, the molecular arrangements between lecticans and selectins differ; therefore, their interactions with other molecules vary [25, 26, 48].
Molecular interactions of CSPGs
The G1 domain in the N-terminal of aggrecan was shown to interact with HA and cartilage link protein 1 (also known as HAPLN1). The link protein is a 50 kDa glycoprotein crucial for stabilizing the structure between aggrecan and HA [49]. Other lectican molecules such as verscian and neurocan were also shown to bind HA [48]. Initially, studies on the C-terminal of lecticans showed that lecticans bind to sugar moieties and GAGs without understanding much about their physiological significance. However, later studies have shown that they can bind to more prominent and crucial molecules such as tenascin-R (TNR), which is a glycoprotein predominantly present in the CNS [50]. Versican was the first lectican found to bind to TNR [51]. Although most carbohydrate–protein-mediated interactions are calcium dependent, deglycosylation studies revealed that the fibronectin type III domains 3–5 of TNR are involved in protein–protein interactions [52]. Inspite the common notion that all lecticans bind to TNR, their molecular ultra-structural interactions are not fully understood, with the exception of brevican. Surface plasmon resonance studies showed that brevican has a tenfold stronger affinity than the other three lecticans [52]. Indeed brevican is found in the brain and interacts with TNR, as indicated by coimmunoprecipitation and immunohistological studies [52, 53]. Notably, brevican and TNR co-localize around the cell bodies and the proximal dendrites of large neurons. The interaction between brevican and TNR is interesting because of their presence in PNN (refer to the section “Perineuronal Nets” for a detailed description of the PNN structure and interactions of its components).
Much effort has been invested in identifying the carbohydrate ligands of the lectin domain of lecticans. In vitro, versican was shown to bind to heparin and heparin sulfate through its lectin domains, suggesting that HSPGs can be vital physiological partners of versican and other lecticans [54]. However, more studies are required to better comprehend these interactions. Additionally, it has been recently shown that C-type lectin domains of all four lecticans bind to sulfatides and HNK-1-reactive sulfoglucuronyl glycolipids (SGGLs) [55, 56]. Both of these cell surface glycolipids are abundant in the nervous system. Sulfatides are produced by the axon ensheathing oligodendrocytes, whereas SGGLs are enriched in both the embryonic cerebral cortex and the adult cerebellum [56, 57].
Neurocan and its interaction with other ECM molecules are one of the most extensively studied aspects of brain ECM. It has been shown to interact with tenascin-C (TNC), N-CAM, Ng-CAM/L1, Nr-CAM, contactin, TNR, TAG-1/axonin, heparin-binding growth-associated molecule (HBGAM), and amphoterin [58–61]. Rauch and his colleagues showed that all three domains of the neurocan C-terminal, including the EGF repeat, the C-type lectin domain, and the CRP-like domain, bind to the fibronectin domain of TNC [60]. Concurrently, fibrinogen-like domains in TNC interact with the core protein of neurocan [62].
Other CSPGs
RPTP-β and phosphacan
Receptor-type protein-tyrosine phosphatase (RPTP) is a class of enzymes with at least eight sub-families [63, 64]. RPTP-β, also known as RPTP-ζ, is a variant expressed solely in the nervous system [65, 66]. It is involved in oligodendrite survival, recapitulation during demyelinating diseases, and in hippocampal memory formation. It is a membrane glycoprotein with two extracellular domains (ECDs) and two intracellular phosphate domains. The ECDs, a carbonic anhydrase-like domain and a fibronectin-type III-like domain, are highly variant in their sequences, with some of them sharing homology with cell adhesion molecules (CAMs) [63, 67, 68]. In fact, the sub-families are classified based on the sequence features of the ECDs. Digestion studies on RPTP-β with chondroitinase ABC, an enzyme that digests CSPGs, indicate that the core protein is heavily glycosylated [65]. Phosphacan, one of the products of the alternative splicing of RPTP-β, lacks cytoplasmic domains [69]. In 2003, Garwood et al. found a truncated form of phosphacan that they named phosphacan short isoform (PSI) [70]. PSI is a post-translationally modified protein corresponding to the N-terminal carbonic anhydrase-like and fibronectin type III-like domains and half of the spacer region. Although PSI follows the expression pattern of full-length phosphacan, it is not a proteoglycan [70]. Phosphacan can bind reversibly with a very high affinity to many CAMs (e.g., Ng-CAM/L1, NCAM, and TAG-1/axonin-1) and to TSC [71]. Both RPTP-β and phosphacan play important roles during embryonic development. Studies on mouse embryos revealed that RPTP-β proteins are expressed on the tangentially aligned neurons in the neocortex, cerebellum, and hippocampus. It was also suggested that the expression of RPTP-β proteins by neurons and PSI might modulate neurite outgrowth and synaptogenesis [72–74]. A detailed understanding of these functions in learning and memory consolidation is largely lacking. Additionally, considering their interactions with MMP-9 [75], investigating the role of RPTP-β in remodeling the PNN structures might reveal new functions for these proteins.
Neuroglycan C
Neuroglycan C (NGC), also known as CSPG5, is a transmembrane proteoglycan expressed only in the CNS [76–78]. It has four splice variants: NGC-I to IV with a core protein, the N-terminal domain decorated with chondroitin sulfate (CS) chains, an acidic domain, an EGF domain, a transmembrane segment, and a cytoplasmic domain responsible for the variants [78–81]. NGCs are developmentally regulated and are involved in synaptogenesis and neurite growth [82]. NGCs are present mostly in the cerebellum and retina as a proteoglycan but also in other regions as a protein without the CS chains [79].
Other prominent ECM molecules
Tenascins
The tenascin family in vertebrates comprises five members, which are characterized by typical motifs such as fibronectin type three (FNIII) domains, a cysteine-rich amino acid terminal, followed by EGF repeats, and finally a fibrinogen β-like carboxy terminus [83–85]. Among them, TNC and TNR are very relevant to the CNS [33, 83]. TNC proteins are found in developing mouse and chicken, and were initially termed J1-glycoproteins and contactins, respectively [86–88]. Both neurons and glia cells produce TNC, and it plays a key role in their interactions [89]. TNC domains are known for exhibiting both adhesive and anti-adhesive properties on neurons and other cell types [89]. The fibronectin domain is primarily involved in cell binding and neuronal migration, whereas the EGF repeats are attributed to its repulsive function [87, 89]. TNC forms a hexamer, which can be visualized through rotary shadowing electron microscopy. This highly symmetrical structure, termed hexabranchion, is composed of a central core from which six thin and rigid proximal arms emanate. The eight FNIII domains of TNC contain several alternate splice sites, which allow them to produce different isoforms with subtle structural and functional differences [90, 91]. The main binding partners of TNC are the G3 lectin domain of CSPGs, to which it binds through its fibronectin type III repeats 3–5 [90–92]. Importantly, studies with TNR knock-out (KO) mice proved this association to be essential for proper PNN assembly [93] (more detailed aspects of tenascins with respect to their functions are described in the PNN “functional attributes” section).
Hyaluronan and proteoglycan link proteins
Hyaluronan and proteoglycan link proteins (HAPLNs) are stabilizing proteins that non-covalently link the HA and G1 domains of lectins linking the structures that keep the PNN intact [94, 95]. Out of the four family members, three are found in the CNS: HAPLN1 (Crtl1), HAPLN2 (brain-specific hyaluronan-binding protein 1; Bral1), and HAPLN4 (Bral2) [96]. HAPLN1 and HAPLN4 are specifically present on neurons that bear PNN [97, 98]. They interact with CSPGs and HA in a tripartite complex, forming an exoskeleton framework in the PNN [99]. The PNN assembly around dendrites is strongly attenuated in mice lacking HAPLN1, as observed with wisteria floribunda agglutinin (WFA) staining [100]. Similarly, HAPLN4 reduction in the brain stem and cerebellum impairs PNN formation, along with downregulation of brevican and other PNN components [101–103]. HAPLN2, on the other hand, is produced by oligodendrocytes and is found around the nodes of Ranvier interacting with verscian V2 [104].
Hyaluronic acid
Hyaluronan, or hyaluronic acid (HA), is a GAG produced mostly in neurons by the enzyme hyaluronan synthases (HAS). Because HAS is a membrane-bound enzyme, it makes HA directly in the extracellular space by a process called extrusion [105]. Hitherto, three different isoforms of HAS have been identified, HAS1-3, each producing different lengths of HA at varying rates [106]. Transfection of HEK cells with HAS3 and HAPLN1 indicated that HAS3 alone is enough for synthesis of HA, whereas HAPLN1 is important for condensing the matrix to form a PNN-like structure (discussed in detail in the PNN section) [107]. Owing to the large size of the HA polymer, it can potentially bind to several proteins. In addition, HA can modulate the viscosity of the local ECM by adsorbing more water molecules [94, 108]. However, these important properties of HA and its role in maintaining the PNN morphology have not been investigated.
Perineuronal nets
Perineuronal nets (PNNs) are specialized ECM structures intimately enwrapping the cell body, soma, and dendrites of some neurons [35]. These honeycomb-like structures were first described in 1893 by Camillo Golgi in a nerve cell of the anterior horn of the cat spinal cord, and since then they have been identified in many animal species, including humans [35, 94, 109, 110]. The initial methods used to stain PNNs, such as methylene-blue staining, followed by ammonium molybdate fixation, were unreliable [109, 110]. Later, to stain PNNs, researchers started using lectins, which strongly bind to N-acetylglucosamine (a GAG), a prominent component in PNN [35, 111]. Initial theories suggested that PNNs consist of only a coagulation of soluble substances in pericellular space [35]. However, subsequent studies found PNNs to be much more complex structures intricately woven, not just by the neurons alone, but also by other cells such as microglia, astrocytes, and oligodendrocytes [112–115]. They are speculated to be involved in vital functions such as learning and memory by altering the neuronal connections [42, 94, 113, 116]. The chief components of PNN are hyaluronic acid, GAGs, lecticans, and link proteins, which connect them. The nets are established around the end of critical periods [42, 117], mainly in the cortex, hippocampus, thalamus, brainstem, and the spinal cord, at varying concentrations, and around different cell types [35, 94, 108]. The microenvironment of the PNN is crucial for its function and is very dynamic, since several ECM modulating enzymes are constantly secreted by the surrounding cells [94, 118, 119]. A great deal of structural diversity is exhibited in the PNN of different brain regions. In one study, Giamanco and his teammates performed a histological analysis on aggrecan KO mice and showed that there is a significant degree of molecular heterogeneity in these PNN molecules due to diversity in the glycosylation of aggrecan [120, 121].
Structural features: HLT model and further developments
In 1996, Ruoslahti proposed a concept in which the PNN is visualized as a supramolecular organization [122]. It was later designated as the “HLT (hyaluronan, lecticans, and TNR)” model by Yamaguchi [48]. This model is based on extensive studies on the recombinant G1 and G3 domains of the lecticans. The results indicated that the G1 domain of lecticans in the N-terminal is important for binding to HA, which in turn, binds to HAPLN, forming a tripartite complex [52, 90, 123]. Interestingly, the G1 domains of lecticans and HAPLN exhibit a high structural homology and share common binding properties, leading to a considerable degree of complexity in forming diverse quaternary structures [123–125]. Crystal structure analysis revealed that the C-type lectin domain of lectican and TNR forms a complex (the binding properties are discussed in the CSPG section) [52, 92]. Further electron microscopy studies on the TNR-aggrecan complex confirmed that the characteristic trimeric structure formed by TNR involves its N-terminal domain [92]. Although these observations are valid and highly useful, it is now apparent that the HLT model is incomplete and has been updated by numerous follow-up studies that unraveled the source, structure, and molecular interactions of the PNN components.
Some of the aforementioned ECM components are an integral part of PNN and are cardinal for its functions. Albeit its structure is not completely understood, the consensus is that lecticans interact with one another and bind to the hyaluronic acid backbone and other PNN molecules with link proteins bridging them. The most impressive aspect of the whole structure is that it can form “holes” in the network, providing a point of contact to the surrounding cells. In other words, PNNs can regulate the accessibility of cells by acting as a physical barrier, thereby controlling the cellular activity. Aggrecan is one of the most important CSPGs in PNN. Immunodetection experiments with WFA on mice lacking aggrecan showed a diminished reactivity to WFA. However, other PNN components are unaffected, indicating that this proteoglycan is necessary for maintaining PNN’s overall structural assembly [120].
Another important and the most studied molecule in PNN is HA. This GAG is synthesized by HAS on the membrane and forms the backbone of the whole network, interacting with multiple proteins and proteoglycans [42, 98, 126]. In contrast to most other PNN components, HA and aggrecan secretion are not dependent on glial cells. In fact, PNNs can still form in cultures in the absence of glial cells or glia-derived components, emphasizing the key role of the neuronal-secreted aggrecan and HA as basic units of PNN [112]. The link proteins, especially HAPLN1 and HAPLN4, connect the HA polymer with lecticans. Binding of tensacins to the C-terminal domains of lecticans completes the lattice structure of the PNN.
Functional attributes
Numerous studies have aspired to reveal the roles of PNNs. Most concluded that PNNs are important for the stabilization of synapses [115, 127–129] and have been proposed as the key elements underlying long-term memory consolidation [113]. In line with this notion, a reduction in the distribution of PNNs or individual PNN components was observed in many psychiatric diseases related to mitigated learning, memory, and information processing, including schizophrenia, autism spectrum disorders, Fragile-X syndrome, mood disorders, Alzheimer’s, and epilepsy (for a comprehensive review, see [129]). Intriguingly, contrary to these neuropathologies, subjects with Rett syndrome exhibit increased PNN labeling in the motor cortex [130]. A number of findings support the notion that PNNs play a key role in learning, memory, and information processing in health as well as disease.
First and foremost, PNNs are established towards the end of the critical period, primarily around parvalbumin (PV) interneurons, which are implicated as important mediators of the critical period [131]. In fact, because of their stabilizing effect, PNNs are thought to have a plasticity-impeding function [118, 131–133]. They act as a barrier, blocking the formation of new synapses [134]; as an obstacle, limiting receptor mobility [135]; and as a scaffold, interacting with molecules that can inhibit synaptic formation [136]. Indeed, removal of these structures with chondroitinase ABC restores a critical-period-like phenotype of the neuronal system, allowing remodeling and the formation of new synapses [133]. Thus, the synapse stabilizing role of PNNs seems to have a dual complementary function: preserving the existing synapses while restricting changes. Reduced plasticity is typically regarded as a disadvantageous feature, since it is important for learning [137]. However, the stability of cortical circuits is probably also valuable in maintaining the “erudite” neuronal connection [131]. In general, studies showing that removal of PNNs improves plasticity have focused on the immediate effect during the proximal period, but failed to examine the long-term and wide-range effects. Do individuals with Alzheimer’s or autism, and who have a decreased PNN distribution, possess improved learning abilities? Perhaps the extent and chronicity of reduced PNNs and sequential neuronal stability are detrimental.
The stabilizing effect of PNNs led Roger Tsien to argue that they are the best candidates responsible for holding long-term memories [138]. He based his hypothesis on the fact that ECM molecules in the PNN structure may have an exceptionally long protein turnover, as opposed to intrasynaptic proteins, which have a short turnover time (2–5 days) [139]. Importantly, although the turnover may be negligible, it does not indicate that PNNs cannot be rescued when degraded or absent. In fact, 9 days following the injection of the ECM-degrading enzyme hyaluronidase into one of the brain hemispheres of gerbils, PNNs reconstituted in the region, and by day 13, their numbers were comparable to that of the control hemisphere [140]. Furthermore, when embryonic PV neurons were transplanted into the visual cortex of adult mice, PNNs were deposited around them by day 21 following transplantation [141]. These findings imply that PNNs can be restored; hence, they might serve as a therapeutic target under pathological conditions. However, experiments directly linking PNN reconstitution to improved outcome should be conducted. In addition, the mechanism by which PNNs mediate plasticity needs to be better characterized.
In the cortex and hippocampus, two of the most relevant regions when considering learning, memory, and information processing, the majority of PNNs enwrap fast spiking parvalbumin(PV)-expressing interneurons [141–144]. The presence of PNN around PV interneurons was linked to lower excitability and to higher discharge frequency [145]. These “GABAergic” inhibitory neurons regulate the synchronous oscillatory output of pyramidal neuron assemblies [146]. Importantly, these gamma frequency band (30–80 Hz) oscillations were linked to various cognitive processes [146]. It is assumed that this gamma-band synchrony between neurons in higher and lower cortical areas is required for object representation, response selection, attention, and sensorimotor integration [147], as well as for memory [148]. PV cells are also essential for “ripple” oscillations (140–180 Hz) in the hippocampus, which occur during rest following learning phases and are thus associated with memory consolidation. Removal of the hyaluronic backbone of PNNs with hyaluronidase or CSPGs with chondroitinase ABC results in an increase in the frequency of these sharp wave ripples [149], emphasizing the potential role of PNNs in memory and learning. Unfortunately, it is not clear how the PNNs actually affect the activity of each neuron in the context of the neuronal system. To study this, one would have to record in vivo electrophysiological signals or image calcium influxes using Ca2+ indicators and differentiate between the cells enwrapped by PNNs and those that are not. However, currently no tools are available for in vivo staining of PNNs.
In addition to their role in modulating synapse formation and stability, PNNs may have an indirect effect on neuronal activity and cognitive function. These dense ECM structures have been shown to protect neurons from oxidative stress [143] and from attacks by activated microglia [150], minimizing the adverse neurological outcome of pathological conditions.
ECM at the synapse
Thrombospondins (TSPs) are a family of five extracellular calcium-binding glycoproteins (TSP1-5) that interact with the neuronal receptors α2δ-1 (Cacna2d1) and neuroligin 1 (NL1) and bind different components of the ECM [151]. These astrocyte-secreted factors are expressed mainly during the early postnatal period, when synapses between dendrites and axons form [152]. Importantly, they were shown to induce synapse formation both in vitro and in vivo [153]. Removal of TSPs from cultures [153], or knocking down endogenous NL1 [154], inhibited TSP1-induced synaptogenesis, whereas the addition of TSP1 and TSP2 to cultured neurons resulted in an increase in the number of synapses [153]. In accordance, TSP1/2 double KO mice have fewer synapses [153]. In line with their proposed role in synaptogenesis, TSPs are upregulated following spinal cord injury [155] and stroke [156, 157], and their inhibition hinders structural plasticity following injury in the cortex [158]. In particular, TSPs induce the formation of ultrastructurally normal synapses, but for activation of the excitatory postsynaptic sites, insertion of AMPA receptors (AMPARs) is required [153]. Interestingly, it was recently shown that brevican, a PNN-related protein, which is also secreted by astrocytes, controls interneuron plasticity by regulating the localization of potassium channels and AMPARs [159]. Indeed, brevican-deficient animals display impaired long-term potentiation (LTP) in the hippocampal CA1 region [160]. In another study, neuronal activity-regulated pentraxin (Narp or NP2) was also shown to recruit AMPARs to PV interneurons at excitatory synapses, consequently regulating the excitation/inhibition of homeostasis [161]. Knockout of Narp or its receptor resulted in enhanced epileptic activity and impaired hippocampal-dependent working memory [162]. Notably, Narp accumulation around PV interneurons is significantly enhanced by the existence of PNNs, pointing to an important indirect role of PNNs in maintaining the homeostasis of neuronal activity. In line with this finding, the expression of Narp is reduced in Alzheimer’s disease and is correlated with cognitive performance [163].
Bridging the gap between the presynaptic and postsynaptic neurons (i.e., the synaptic cleft) is also important for synaptogenesis and maturation of synapses, and it relies on ECM molecules. For example, hevin (or SC1), an astrocyte-secreted protein, bonds presynaptic neurexins and postsynaptic neuroligins [164]. SPARC, a homolog of hevin, plays a contradictory role, hampering the activity of hevin and synaptogenesis [165]. Cerebellins (Cbln1–4) are another family of trans-synaptic linkers, bridging between neurexins (Cbln1-4) [166] or “deleted in colorectal cancer” (DCC; Cbln4) [167] and the postsynaptic delta-type glutamate (GluD1 and GluD2) receptors. For example, Cbln1 is secreted from presynaptic terminals in granular cells and is essential for stabilizing Purkinje cell synapses in the cerebellum, and loss of Cbln1 results in ataxia and diminished motor learning [168, 169]. In contrast with the cerebellum, the thalamic axons of Cbln1-null mice exhibited an increase in synaptic spine density instead of synapse loss [170]. Mutations in cerebellins or their neurexin receptors have been associated with neurodevelopmental disorders such as ASDs, Tourette, and schizophrenia (reviewed in [171]).
Reelin is a key regulator of neuronal layering and migration in the cortex, hippocampus, and cerebellum during development (reviewed in [172]). Reelin is also secreted by GABAergic interneurons and it surrounds dendritic spines of pyramidal neurons, thereby modulating synaptic signaling pathways and regulating synaptic plasticity and axonal and dendritic outgrowth [172–174]. In accordance, reelin-deficient mice exhibited reduced dendritic branching and lower spine density in vitro and in vivo [175]. Furthermore, factors downstream of reelin [176, 177] and reelin’s ApoER2 receptor [178] were shown to regulate spinogenesis and spine morphology. Additionally, reelin also increases LTP [178, 179] by enhancing N-methyl-D-aspartate receptor (NMDAR)-mediated Ca2+ conductance and phosphorylation of cAMP-response element-binding protein (CREB) [180], and by controlling the maturation of NMDARs [181] and the insertion of AMPARs into synaptic membranes [182]. Importantly, a deficiency involving reelin’s receptors results in diminished hippocampus-dependent contextual fear memory [179]. Accordingly, reduced reelin expression has been associated with neurological disorders, including ASD, schizophrenia, Alzheimer’s, and with mood disorders such as depression and bipolar disorder (reviewed in [178, 183, 184]).
Tenascins, another important family of ECM molecules, are linked to synaptic plasticity, specifically TNR and TNC, which are predominantly expressed in the CNS [185, 186]. TNR, a major component of the PNN, is necessary for synaptic transmission and plasticity, and consequently for behavior. TNR deficiency in mice did not affect long-term depression (LTD) in the hippocampal CA1 area, but led to impaired LTP and increased basal synaptic transmission at this location, accompanied by anxiety and motor impairments [187–190]. TNR deficiency also resulted in a reduced number of active zones in perisomatic inhibitory synapses in the CA1 pyramidal cell layer, suggesting that TNR may play a crucial role in regulating the architecture of perisomatic inhibitory synapses [191]. In contrast to TNR, TNC is predominantly expressed during development [192]. However, although its levels are significantly decreased thereafter, LTP induces transient TNC expression in the adult brain, suggesting that it plays a role in synaptic plasticity [193]. Indeed, a deficiency in TNC leads to a reduction in L-type voltage-dependent Ca2+ channel (L-VDCC)-dependent LTP and abolished LTD in the CA1 region of the hippocampus. Moreover, gamma oscillations increased in TNC-deficient mice in the cortex and in CA1 (but not in other hippocampal regions). These animals also exhibited an impaired extinction of conditioned fear responses, with normal learning and memory in the contextual fear paradigm [194].
While the paramount role of hyaluronic acid as the backbone of PNN is well acknowledged, it was also shown to play a role in synapse maturation and LTP. Synapse stabilization (and reduced plasticity) is partially due to a shift in the NMDARs' (a subtype of the ionotropic glutamate receptors') composition, switching the subunit GluN2B to GluN2A. This shift seems to be mediated by hyaluronic acid, since its removal with hyaluronidase induces an increase in the surface expression of GluN2B in neuronal cultures and acute hippocampal slices [195]. A similar treatment of hippocampal slices also suppressed postsynaptic L-type voltage-dependent calcium channel (L-VDCC)-mediated signals and subsequent LTP, and in vivo removal of HA resulted in impaired contextual fear conditioning [196].
ECM around the nodes of Ranvier
The nodes of Ranvier are gaps between myelin sheaths enwrapping axons. These gaps are rich in voltage-gated sodium (i.e., Nav) channels, allowing propagation of action potentials. Notably, in these gaps the axons are exposed to the ECM, which plays an important role in the stability of the nodes and, hence their efficacy [197]. The ECM around the nodes of Ranvier is rich in brevican, versican, phosphacan, and TNR [198–200]. Interestingly, in wild-type animals, TNR and phosphacan seem to appear only in large-diameter axons, whereas in brevican-deficient animals they are found in nodes of both small- and large-diameter axons [102]. The specialized ECM complex around the node binds to the cell adhesion molecules neurofascin-186 (NF-186), neuron–glia-related CAM (NrCAM), and contactin-1, which interact with the neuronal cytoskeletal proteins ankyrin G and βIV spectrin at the node, bridging between the node and the perinodal astrocyte processes [200]. In addition, the hyaluronan-binding, brain-specific link protein Bral1 also co-localizes with brevican and versican in the nodal ECM [201], and in a subset of CNS nodes Bral1 localization depends on them [198], whereas in others it seems to be independent of brevican. Mice lacking paranodal junctions and versican, brevican, or Bral1 have fewer NaV channel clusters. Furthermore, animals deficient in paranodal junctions and either versican or brevican have profound motor dysfunction compared to animals lacking only paranodal junctions [198].
The immediate roles of ECM around the nodes of Ranvier regarding plasticity and learning have not been clearly characterized. However, they are important for the propagation of action potentials, resulting in activity, which is key for instigating new synapses and their maintenance, and for controlling their strength [197]. Moreover, recent evidence points to activity-dependent myelination as a central mechanism for plasticity [202]. Hence, the efficiency of the nodes, which is partially dependent on the proximal ECM assembly, is arguably key for learning and memory [202].
Extracellular matrix remodeling enzymes in the brain
Matrix metalloproteinase-9
Matrix metalloproteinases (MMPs) are a large family of zinc-containing endopeptidases with pivotal functions in ECM remodeling. There are at least 25 different MMPs identified so far and they can be subdivided into multiple groups based on their structure and function [203]. MMP-9 belongs to the gelatinase family and is implicated in numerous physiological and pathological processes [204]. It has been shown that MMP-9 protein levels and its proteolytic activity were rapidly increased by stimuli that induce long-lasting LTP [205]. A deficiency in MMP-9, or its pharmacological blockage with broad-spectrum MMP inhibitors, antisense oligonucleotides, or neutralizing antibodies results in altered LTP in the hippocampus (summarized in [206]). Furthermore, multiple studies have shown that the same LTP-inducing stimuli also evoke local MMP-9 release, resulting in dendritic spine enlargement [207–210], whereas specific blocking of MMP-9 in slices prevented late LTP [211]. Similarly, LTP elicited in hippocampal cultures has also been demonstrated to depend on MMP activity and to involve enhanced MMP-9 levels [212–214]. Interestingly, LTP-evoking stimuli in the prefrontal cortex of rats resulted in overexpression of the endogenous tissue inhibitor of MMPs (TIMP)-1, an intrinsic inhibitor of several MMPs, including MMP-9; perhaps acting as a homeostatic modulator [211, 215].
Upregulation of MMP-9 expression in the hippocampus was also found following exposure to the enriched environment paradigm [216]. This paradigm, in which animals are housed in cages with excessive sensory and motor stimuli, is known to increase synaptic plasticity [217]. Induced seizures, however, cause upregulation of TIMP-1 [215, 218, 219] and hippocampal spine loss that is blocked in MMP-9-deficient mice. In line with its role in mediating hippocampal LTP, MMP-9 deficiency was associated with poor memory in contextual fear conditioning and appetitive learning [205, 220–222]. In a different study, spatial learning was found to elevate MMP-3 and MMP-9 levels. Importantly, spatial learning was also found to depend on these MMPs, evidently through their ability to activate NMDA receptors [223].
As in the hippocampus, MMP-9 deficiency also reduced experience-dependent plasticity in the barrel cortex [224]. In contrast, in the visual cortex, non-specific MMP inhibition did not affect homeostatic plasticity; however, it did prevent an increase in dendritic spine density evident one week following monocular deprivation [225]. Emphasizing the location-dependent role of MMP-9 in plasticity, disruption of MMP-9 activity abolished late-phase LTP in the basolateral and central nucleus of the amygdala, but did not affect LTP in the cortical pathway leading to the lateral amygdala [226]. Furthermore, MMP-9 deficiency did not affect amygdala-related tasks, such as discrete cue conditioning or aversive learning [205, 220].
There are also a few indirect indications that MMP-9 mediates plasticity. For example, activator protein 1 (AP-1), a transcription factor associated with plasticity, learning, and memory, regulates MMP-9 and TIMP-1 [219, 220, 227, 228]. Interestingly, local dendritic translation of MMP-9 mRNA was found to be controlled by the fragile X mental retardation protein, FMRP, which is silenced in subjects with Fragile-X syndrome (FXS) [229, 230]. Indeed, animals with FXS have increased MMP-9 expression, coinciding with longer and thinner spines and abnormal spine turnover, which are normalized by treatment with various MMP-9 inhibitors [207, 231, 232].
Although the molecular chain of events is still vague, a number of mediators downstream of MMP-9 activity have been suggested, including β-dystroglycan, ICAM-5, neuroligin-1, and integrins, especially β1 integrins [205, 233–236]. Another hypothesis regarding how MMP-9 contributes to enhanced plasticity concerns its ability to cleave pro-BDNF to BDNF, a key regulator of synaptic structure and function [237]. Notably, MMP-9 mRNA, protein, and enzymatic activity are present at the dendritic spines of excitatory synapses, whereas they are absent in inhibitory synapses [222, 238–240]. Although MMP-9 has been the main focus of brain metalloproteinase research, MMP-3 is emerging as a key player, since it may act upstream and activate MMP-9 [241]. Unravelling the substrates of MMP-9 in the brain is also important in the context of PNN integrity. For instance, Fmr-1 KO mice exhibit elevated MMP-9 levels in the brain, and a genetic reduction of MMP-9 expression promotes the formation of PNNs [142]. This finding is intriguing, since it remains unclear how MMP-9 affects PNN formation or degradation, given that none of the PNN elements was shown to be a substrate of MMP-9 [242–244].
A disintegrin and metalloproteinase with thrombospondin motifs
A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) are another family of extracellular matrix remodeling enzymes with multiple domains. ADAMTS-1 and ADAMTS-4, which belong to a subgroup called aggrecanases or proteoglycanases, were found to be upregulated following induced seizures in rats. Their expression leads to proteolysis of brevican, which is associated with a reduction in synaptic density in the dentate gyrus of the hippocampus [245]. Following spinal cord injury, local ADAMTS-4 administration resulted in enhanced axonal regeneration/sprouting, significantly promoting motor function recovery [246]. In vitro, ADAMTS-4 was also found to induce neurite elongation, which can explain the increase in synaptic density [247]. Similarly, the expression of synaptic markers, such as synaptosomal nerve-associated protein 25 (SNAP-25) and post-synaptic density (PSD) -95, was lower in ADAMTS-1 null female mice. Interestingly, this was not the case in male animals, suggesting a sexual dimorphism of ADAMTS-1 involvement in synaptic density. Nonetheless, these alterations in the expression of synaptic proteins were not found to cause deficits in learning and memory; therefore, their significance is unclear [248].
Recently, it was reported that cortical fast-spiking PV interneurons enwrapped in PNN express the metallopeptidases ADAMTS8, ADAMTS15, and Neprilysin [145]. Notably, aggrecan and versican, CSPGs of the PNN, are substrates of ADAMTS-8 and ADAMTS-15. Thus, the expression of these proteases in PNN-enwrapped cells might reflect their involvement in the local regulation of its structure and function [145].
The tissue plasminogen activator
Traditionally referred to as a dissolver of clots, tissue plasminogen activator (tPA), a member of the serine proteinase family, has drawn attention as a possible mediator of neuronal plasticity. It was found to be an important protease associated with various aspects of neuronal plasticity, learning, memory, and emotion [249–251]. In fact, its expression is induced in the hippocampus following various modes of neuronal activation such as seizures, kindling, or LTP [252]. tPA-deficient mice exhibit an impairment in spatial navigation tasks, cerebellar motor learning, fear conditioning, and passive avoidance [250, 253–255]. tPA deficiency concurrently results in reduced LTP [250, 256], and overexpression of tPA, results in elevated LTP [257]. Zhuo et al. [258] found that the lipoprotein receptor-related protein (LRP), a receptor of tPA, is abundantly expressed in hippocampal neurons and is essential for the effects of tPA on hippocampal LTP. Proteolytic mechanisms that mediate plasticity have also been described, such as conversion of pro-BDNF to BDNF by tPA [259], or activation of plasmin, which can cleave ECM components such as fibronectin or laminin [206]. The activity of tPA is spatially and temporally controlled by serine protease inhibitors (i.e., serpins), such as plasminogen activator inhibitor-1 or neuroserpin [206]. Interestingly, transgenic expression of urokinase plasminogen activator in the brain increased the longevity and reduced body weight in mice [260, 261]. However, they performed poorly in the cortex and limbic system-associated learnings [262]. More studies are required to completely delineate this enzyme’s potential in not only memory and learning but also in other diseases and afflictions like cancer and obesity.
MMP inhibitors in brain disorders
Multiple studies have corroborated the important and diverse functions of MMPs in the health and pathology of CNS, including in development, vascular integrity and function, neuronal activity, and cancer progression, pointing to MMP inhibitors as a potential “game-changer” in the treatment modalities [263–265]. Indeed, such inhibitors are studied rigorously in various pathologies, with some promising results. For example, Ro31-9730 and minocycline, non-specific MMP inhibitors, have been shown to neutralize the unwarranted MMP activity and improved outcomes in experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis [266, 267]. In rodent models of stroke, the broad specific inhibitors GM6001 and BB-94 showed encouraging results when given immediately following stroke induction [268, 269]. However, in a different study, prolonged treatment for a week with the MMP inhibitors FN-439 or BB-94 hindered recovery from stroke [270], implying that MMP inhibition can have a contradictory impact on stroke outcome. Hence, a delicate balance between MMP activity and their inhibition must be maintained. Remarkably, the field has revived interest in blocking MMP-9 and MMP-2 in stroke owing to the design of SB-3CT, a thiirane-based gelatinase inhibitor, by Shahriar et al. [271, 272]. Administration of SB-3CT in a mouse model of stroke resulted in protection from brain damage, compared with mice that did not receive the treatment. Inhibition of gelatinases by SB-3CT was also shown to protect neurovasculature from embolic focal cerebral ischemia [273]. Importantly, it was argued that selective MMP inhibitors will benefit only during the acute phase of the injury, suggesting that the timing of the use of the MMP inhibitors in stroke is critical [274].
The use of protease inhibitors, specifically MMP inhibitors, has also been tested in human clinical trials for other pathological conditions. In a study on 60 patients with acute ischemic stroke, a combined treatment of tPA and minocycline was more effective compared with tPA alone [275]. In another study on a small cohort of multiple sclerosis patients (n = 16), doxycycline was given together with interferon-β-1a for 4 months, resulting in a better score in the expanded disability status scale (EDSS), with negligible toxicity [276]. However, the study concluded that in spite of the safe and effective therapeutic potential of these molecules, a larger study should be performed. A combinatorial treatment of glioma and recurrent glioblastoma patients with marimastat and temozolomide increased their progression-free survival (PFS) [277, 278]. However, other studies had only discouraging outcomes [279, 280]. Vandenbrouke and Libert summarized several reasons for the failure of the trials. This includes metabolically unstable molecules, poor oral bioavailability, and lack of a complete understanding of MMPs [281].
One of the recent developments in treatments based on MMP inhibitors lies in the field of fragile X syndrome (FXS). FXS has been shown to have elevated serum MMP-9 levels in both humans and mouse models. Numerous studies have shown compelling results supporting the involvement of MMP-9 in this neurodevelopmental disorder. Follow-up studies on MMP-9 inhibition and genetic KO in rodents indicated that they rescued the characteristic phenotypes in the neurons and that the rodents displayed enhanced learning in behavioral tasks. The broad specific antibiotic, minocycline, with its already proven abilities to inhibit MMPs, was tested extensively in mouse models and later in a human clinical study, and showed marked improvements. The study concluded that further long-term studies are required. Very recently, other molecules such as metformin [282], lovastatin, along with minocycline are being clinically investigated on human subjects [283]. Although studies like this are important and encouraging for finding a drug for FXS, the lessons that can be learned from the MMP inhibition-based trials should be prioritized and implemented. MMP biology is highly enigmatic; thus, a higher degree of comprehension is required. More importantly, novel approaches such as use of a highly specific antibody or protein-based inhibitors is essential for producing tangible MMP inhibitors for treating brain disorders [284].
Summary and future perspectives
The field of ECM biology has taken an unprecedented journey from mere speculation of its presence to its undeniably vital role in several brain functions including learning and memory. The ECM in the brain forms unique structures, which perform a plethora of cellular functions. A special class of CSPGs, termed lecticans, dominates both the interstitial ECM and special structures like PNN. Although the composition of PNNs and the importance of each constitutive element to the development of the nets have been characterized in numerous studies [128, 132, 285–287], a number of questions remain open regarding the significance of the structure of the net. How does the density of the net affect its function? Is it important how large the holes of the net are, or the extent to which the net enwraps the dendrites? In addition, it has been shown that differences exist in the molecular composition of the nets between different locations in the CNS [288, 289]. However, the variance within each population is not clear, and the impact of such differences. Although attempts to rescue phenotypes in behavioral disorders like FXS by modulating ECM-regulating proteases are actively being pursued, a complete understanding of the role of ECM in attaining tangible targets for treatment is still a distant goal. Additionally, lack of specific inhibitors to suppress the unwanted protease activity impedes progress in comprehending the disease phenotype, at least in conditions like FXS. A new class of novel inhibitors and specific antibodies for inhibiting MMPs are being developed, and this might pave the way for treating diseases like FXS where the protease levels and activity are unwarranted (Reviewed in [284, 290]). In Toto, the full potential of the brain ECM in several physiological and pathological processes remains to be deciphered completely.
Acknowledgements
Irit Sagi is an Incumbent of the Maurizio Pontecorvo Professorial Chair and has received funding from the Israeli Science Foundation (1226/13), the European Research Council AdG (THZCALORIMETRY—DLV-695437), and the USA-Israel Binational Science Foundation (712506-01). She is grateful for the Azrieli Foundation for its generous grant to conduct research on the extracellular matrix of the brain.
Abbreviations
- AMPAR
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- AP-1
Activator protein 1
- ASD
Autism spectrum disorders
- Bral
Brain-specific hyaluronan-binding protein
- CAM
Cell adhesion molecule
- Cbln
Cerebellin
- CNS
Central nervous system
- CREB
cAMP-response element-binding
- CRP
Complement regulatory protein
- CSPG
Chondroitin sulfate proteoglycan
- DCC
Deleted in colorectal cancer
- ECD
Extracellular domain
- ECM
Extracellular matrix
- GAG
Glycosaminoglycan
- HA
Hyaluronic acid
- HAPLN1
Hyaluronan and proteoglycan link protein 1
- HAS
Hyaluronan synthase
- HSPG
Heparin sulfate proteoglycan
- LTD
Long-term depression
- LTP
Long-term potentiation
- L-VDCC
l-type voltage-dependent Ca2+ channels
- MMP
Matrix metalloproteinase
- NMDAR
N-methyl-d-aspartate receptor
- Narp
Neuronal activity-regulated pentraxin
- NF-186
Neurofascin-186
- NGC
Neuroglycan C
- NrCAM
Neuron–glia-related cell adhesion molecule
- PNN
Perineuronal net
- PSI
Phosphacan short isoform
- PTR
Proteoglycan tandem repeat
- RPTP
Receptor-type protein-tyrosine phosphatase
- SGGL
Sulfoglucuronyl glycolipid
- SNAP-25
Synaptosomal nerve-associated protein 25
- TIMP
Tissue inhibitor of MMPs
- TNC
Tenascin-C
- TNR
Tenascin-R
- tPA
Tissue plasminogen activator
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Venkat Raghavan Krishnaswamy and Amit Benbenishty contributed equally.
References
- 1.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014;23:S20–S23. doi: 10.1097/IJG.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichardt L. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci. 1991;14:531–570. doi: 10.1146/annurev.neuro.14.1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naba A, Clauser KR, Ding H, et al. The extracellular matrix: tools and insights for the “omics” era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15:771–785. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afratis NA, Nikitovic D, Multhaupt HAB, et al. Syndecans—key regulators of cell signaling and biological functions. FEBS J. 2017;284:27–41. doi: 10.1111/febs.13940. [DOI] [PubMed] [Google Scholar]
- 7.O’Toole EA. Extracellular matrix and keratinocyte migration. Clin Exp Dermatol. 2001;26:525–530. doi: 10.1046/j.1365-2230.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 8.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15(12):1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker C, Mojares E, del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018;19:3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afratis N, Gialeli C, Nikitovic D, et al. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012;279:1177–1197. doi: 10.1111/j.1742-4658.2012.08529.x. [DOI] [PubMed] [Google Scholar]
- 11.Druso JE, Fischbach C. Biophysical properties of extracellular matrix: linking obesity and cancer. Trends Cancer. 2018;4:271–273. doi: 10.1016/j.trecan.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urciuolo F, Garziano A, Imparato G, et al. Biophysical properties of dermal building-blocks affect extra cellular matrix assembly in 3D endogenous macrotissue. Biofabrication. 2016;8:015010. doi: 10.1088/1758-5090/8/1/015010. [DOI] [PubMed] [Google Scholar]
- 13.Koláčná L, Bakešová J, Varga F, et al. Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol Res. 2007;56:51–60. doi: 10.33549/physiolres.931302. [DOI] [PubMed] [Google Scholar]
- 14.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubmacher D, Apte SS. The biology of the extracellular matrix: novel insights. Curr Opin Rheumatol. 2013;25:65. doi: 10.1097/BOR.0b013e32835b137b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolman M. Symposium on the ground substance of the central nervous system. Introduction. World Neurol. 1962;3:97. [PubMed] [Google Scholar]
- 17.Hess A. The ground substance of the central nervous system revealed by histochemical staining. J Comp Neurol. 1953;98:69–91. doi: 10.1002/cne.900980106. [DOI] [PubMed] [Google Scholar]
- 18.Lei Y, Han H, Yuan F, et al. The brain interstitial system: anatomy, modeling, in vivo measurement, and applications. Prog Neurobiol. 2017;157:230–246. doi: 10.1016/j.pneurobio.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–653. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 20.Cragg B. Brain extracellular space fixed for electron microscopy. Neurosci Lett. 1979;15:301–306. doi: 10.1016/0304-3940(79)96130-5. [DOI] [PubMed] [Google Scholar]
- 21.Rasband MN, Peles E. The nodes of Ranvier: molecular assembly and maintenance. Cold Spring Harb Perspect Biol. 2016;8:1–16. doi: 10.1101/cshperspect.a020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- 23.Hook M, Kjellen L, Johansson S, Robinson J. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz NB, Domowicz MS. Proteoglycans in brain development and pathogenesis. FEBS Lett. 2018 doi: 10.1002/1873-3468.13026. [DOI] [PubMed] [Google Scholar]
- 25.Aono S, Oohira A. Chondroitin sulfate proteoglycans in the brain. Adv Pharmacol. 2006;53:323–336. doi: 10.1016/S1054-3589(05)53015-1. [DOI] [PubMed] [Google Scholar]
- 26.Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;4:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Dyck SM, Karimi-Abdolrezaee S (2015) Chondroitin sulfate proteoglycans: key modulators in the developing and pathologic central nervous system. Exp Neurol 269:169–187. 10.1016/j.expneurol.2015.04.006 [DOI] [PubMed]
- 28.Pantazopoulos H, Woo TUW, Lim MP, et al. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010 doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berretta S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonneh-Barkay D, Wiley CA. Brain extracellular matrix in neurodegeneration. Brain Pathol. 2009;19:573–585. doi: 10.1111/j.1750-3639.2008.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avram S, Shaposhnikov S, Buiu C, Mernea M. Chondroitin sulfate proteoglycans: structure-function relationship with implication in neural development and brain disorders. Biomed Res Int. 2014 doi: 10.1155/2014/642798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brittis PA, Canning DR, Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992 doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- 33.Laabs T, Carulli D, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Sugahara K, Mikami T. Chondroitin/dermatan sulfate in the central nervous system. Curr Opin Struct Biol. 2007;17:536–545. doi: 10.1016/j.sbi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–515. doi: 10.1016/S0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- 36.Kjellen L. Proteoglycans: structures and interactions. Annu Rev Biochem. 2002 doi: 10.1146/annurev.biochem.60.1.443. [DOI] [PubMed] [Google Scholar]
- 37.Silbert JE, Sugumaran G. Biosynthesis of chondroitin/dermatan sulfate. IUBMB life (International Union Biochem Mol Biol Life) 2002;54:177–186. doi: 10.1080/15216540214923. [DOI] [PubMed] [Google Scholar]
- 38.Akita K, von Holst A, Furukawa Y, et al. Expression of multiple chondroitin/dermatan sulfotransferases in the neurogenic regions of the embryonic and adult central nervous system implies that complex chondroitin sulfates have a role in neural stem cell maintenance. Stem Cells. 2008;26:798–809. doi: 10.1634/stemcells.2007-0448. [DOI] [PubMed] [Google Scholar]
- 39.Gama CI, Tully SE, Sotogaku N, et al. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 40.Sherman LS, Back SA. A “GAG” reflex prevents repair of the damaged CNS. Trends Neurosci. 2008;31:44–52. doi: 10.1016/j.tins.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Beller JA, Kulengowski B, Kobraei EM, et al. Comparison of sensory neuron growth cone and filopodial responses to structurally diverse aggrecan variants, in vitro. Exp Neurol. 2013;247:143–157. doi: 10.1016/J.EXPNEUROL.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyata S, Nishimura Y, Hayashi N, Oohira A. Construction of perineuronal net-like structure by cortical neurons in culture. Neuroscience. 2005;136:95–104. doi: 10.1016/j.neuroscience.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 43.Maeda N. Structural variation of chondroitin sulfate and its roles in the central nervous system. Cent Nerv Syst Agents Med Chem. 2010;10:22–31. doi: 10.2174/187152410790780136. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 46.Sandy JD, Flannery CR, Boynton RE, Neame PJ. Isolation and characterization of disulfide-bonded peptides from the three globular domains of aggregating cartilage proteoglycan. J Biol Chem. 1990;265:21108–21113. doi: 10.1109/CEIDP.1994.592029. [DOI] [PubMed] [Google Scholar]
- 47.Neame PJ, Christner JE, Baker JR. Cartilage proteoglycan aggregates. The link protein and proteoglycan amino-terminal globular domains have similar structures. J Biol Chem. 1987;262:17768–17778. [PubMed] [Google Scholar]
- 48.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Binette F, Cravens J, Kahoussi B, et al. Link protein is ubiquitously expressed in non-cartilaginous tissues where it enhances and stabilizes the interaction of proteoglycans with hyaluronic acid. J Biol Chem. 1994;269:19116–19122. [PubMed] [Google Scholar]
- 50.Faissner A. The tenascin gene family in axon growth and guidance. Cell Tissue Res. 1997;290:331–341. doi: 10.1007/s004410050938. [DOI] [PubMed] [Google Scholar]
- 51.Aspberg A, Binkert C, Ruoslahti E. The versican C-type lectin domain recognizes the adhesion protein tenascin-R. Proc Natl Acad Sci USA. 1995;92:10590–10594. doi: 10.1073/pnas.92.23.10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aspberg A, Miura R, Bourdoulous S, et al. The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc Natl Acad Sci USA. 1997;94:10116–10121. doi: 10.1073/pnas.94.19.10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagihara K, Miura R, Kosaki R, et al. Immunohistochemical evidence for the brevican-tenascin-R interaction: colocalization in perineuronal nets suggests a physiological role for the interaction in the adult rat brain. J Comp Neurol. 1999;410:256–264. doi: 10.1002/(SICI)1096-9861(19990726)410:2<256::AID-CNE7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 54.Ujita M, Shinomura T, Ito K, et al. Expression and binding activity of the carboxyl-terminal portion of the core protein of PG-M, a large chondroitin sulfate proteoglycan. J Biol Chem. 1994;269:27603–27609. [PubMed] [Google Scholar]
- 55.Miura R, Aspberg A, Ethell IM, et al. The proteoglycan lectin domain binds sulfated cell surface glycolipids and promotes cell adhesion. J Biol Chem. 1999;274:11431–11438. doi: 10.1074/jbc.274.16.11431. [DOI] [PubMed] [Google Scholar]
- 56.Jungalwala FB. Expression and biological functions of sulfoglucuronyl glycolipids (SGGLs) in the nervous system—a review. Neurochem Res. 1994;19:945–957. doi: 10.1007/BF00968704. [DOI] [PubMed] [Google Scholar]
- 57.Poduslo S, Miller K. Levels of sulfatide synthesis distinguish oligodendroglia in different stages of maturation. Neurochem Res. 1985;10:1285–1297. doi: 10.1007/BF00964847. [DOI] [PubMed] [Google Scholar]
- 58.Grumet M, Milev P, Sakurai T, et al. Interactions with tenascin and differential effects on cell adhesion of neurocan and phosphacan, two major chondroitin sulfate proteoglycans of nervous tissue. J Biol Chem. 1994;269:12142–12146. [PubMed] [Google Scholar]
- 59.Grumet M, Flaccus A, Margolis RU. Functional characterization of chondroitin sulfate proteoglycans of brain: interactions with neurons and neural cell adhesion molecules. New York: Rockefeller University Press; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rauch U, Clement A, Retzler C, et al. Mapping of a defined neurocan binding site to distinct domains of tenascin-C. J Biol Chem. 1997;272:26905–26912. doi: 10.1074/jbc.272.43.26905. [DOI] [PubMed] [Google Scholar]
- 61.Friedlander DR, Milev P, Karthikeyan L, et al. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milev P, Fischer D, Haring M, et al. The fibrinogen-like globe of tenascin-C mediates its interactions with neurocan and phosphacan/protein-tyrosine phosphatase-ζ/β. J Biol Chem. 1997;272:15501–15509. doi: 10.1074/jbc.272.24.15501. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y, Fisher GJ. Receptor type protein tyrosine phosphatases (RPTPs)—roles in signal transduction and human disease. Signal: J. Cell Commun; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischer EH, Charbonneau H, Tonks NK. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991;253(80):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- 65.Shitara K, Yamada H, Watanabe K, et al. Brain-specific receptor-type protein-tyrosine phosphatase RPTPβ is a chondroitin sulfate proteoglycan in vivo. J Biol Chem. 1994;269:20189–20193. doi: 10.1111/bjc.12074. [DOI] [PubMed] [Google Scholar]
- 66.Krueger NX, Saito H. A human transmembrane protein-tyrosine-phosphatase, PTP zeta, is expressed in brain and has an N-terminal receptor domain homologous to carbonic anhydrases. Proc Natl Acad Sci USA. 1992;89:7417–7421. doi: 10.1073/pnas.89.16.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maeda N, Noda M. Involvement of receptor-like protein tyrosine phosphatase ζ/RPTPβ and its ligand pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) in neuronal migration. J Cell Biol. 1998;142:203–216. doi: 10.1083/jcb.142.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saito H. Structural diversity of eukaryotic protein tyrosine phosphatases: functional and evolutionary implications. Semin Cell Dev Biol. 1993 doi: 10.1006/scel.1993.1045. [DOI] [PubMed] [Google Scholar]
- 69.Milev P, Friedlander DR, Sakurai T, et al. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol. 1994;127:1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garwood J, Heck N, Reichardt F, Faissner A. Phosphacan short isoform, a novel non-proteoglycan variant of phosphacan/receptor protein tyrosine phosphatase-β, interacts with neuronal receptors and promotes neurite outgrowth. J Biol Chem. 2003;278:24164–24173. doi: 10.1074/jbc.M211721200. [DOI] [PubMed] [Google Scholar]
- 71.Peles E, Schlessinger J, Grumet M. Multi-ligand interactions with receptor-like protein tyrosine phosphatase β: implications for intercellular signaling. Trends Biochem Sci. 1998;23:121–124. doi: 10.1016/S0968-0004(98)01195-5. [DOI] [PubMed] [Google Scholar]
- 72.Ohyama K, Ikeda E, Kawamura K, et al. Receptor-like protein tyrosine phosphatase ζ/RPTP β is expressed on tangentially aligned neurons in early mouse neocortex. Dev Brain Res. 2004;148:121–127. doi: 10.1016/j.devbrainres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 73.Hayashi N, Miyata S, Yamada M, et al. Neuronal expression of the chondroitin sulfate proteoglycans receptor-type protein-tyrosine phosphatase β and phosphacan. Neuroscience. 2005;131:331–348. doi: 10.1016/j.neuroscience.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 74.Dobbertin A, Rhodes KE, Garwood J, et al. Regulation of RPTPβ/phosphacan expression and glycosaminoglycan epitopes in injured brain and cytokine-treated glia. Mol Cell Neurosci. 2003;24:951–971. doi: 10.1016/S1044-7431(03)00257-4. [DOI] [PubMed] [Google Scholar]
- 75.Chow JPH, Fujikawa A, Shimizu H, et al. Metalloproteinase- and γ-secretase-mediated cleavage of protein-tyrosine phosphatase receptor type Z. J Biol Chem. 2008 doi: 10.1074/jbc.M802976200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watanabe E, Maeda N, Matsui F, et al. Neuroglycan C, a novel membrane-spanning chondroitin sulfate proteoglycan that is restricted to the brain. J Biol Chem. 1995;270:26876–26882. doi: 10.1074/jbc.270.45.26876. [DOI] [PubMed] [Google Scholar]
- 77.Yasuda Y, Tokita Y, Aono S, et al. Cloning and chromosomal mapping of the human gene of neuroglycan C (NGC), a neural transmembrane chondroitin sulfate proteoglycan with an EGF module. Neurosci Res. 1998;32:313–322. doi: 10.1016/S0168-0102(98)00098-4. [DOI] [PubMed] [Google Scholar]
- 78.Aono S, Tokita Y, Yasuda Y, et al. Expression and identification of a new splice variant of neuroglycan C, a transmembrane chondroitin sulfate proteoglycan, in the human brain. J Neurosci Res. 2006;83:110–118. doi: 10.2337/dc13-0560. [DOI] [PubMed] [Google Scholar]
- 79.Aono S, Keino H, Ono T, et al. Genomic organization and expression pattern of mouse neuroglycan C in the cerebellar development. J Biol Chem. 2000;275:337–342. doi: 10.1074/jbc.275.1.337. [DOI] [PubMed] [Google Scholar]
- 80.Aono S, Tokita Y, Shuo T, et al. Glycosylation site for chondroitin sulfate on the neural part-time proteoglycan, neuroglycan C. J Biol Chem. 2004;279:46536–46541. doi: 10.1074/jbc.M403263200. [DOI] [PubMed] [Google Scholar]
- 81.Kinugasa Y, Ishiguro H, Tokita Y, et al. Neuroglycan C, a novel member of the neuregulin family. Biochem Biophys Res Commun. 2004;321:1045–1049. doi: 10.1016/j.bbrc.2004.07.066. [DOI] [PubMed] [Google Scholar]
- 82.Nakanishi K, Aono S, Hirano K, et al. Identification of neurite outgrowth-promoting domains of neuroglycan C, a brain-specific chondroitin sulfate proteoglycan, and involvement of phosphatidylinositol 3-kinase and protein kinase C signaling pathways in neuritogenesis. J Biol Chem. 2006 doi: 10.1074/jbc.M601498200. [DOI] [PubMed] [Google Scholar]
- 83.Nörenberg U, Hubert M, Rathjen FG. Structural and functional characterization of tenascin-R (restrictin), an extracellular matrix glycoprotein of glial cells and neurons. Int J Dev Neurosci. 1996;14:217–231. doi: 10.1016/0736-5748(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 84.Kammerer RA, Schulthess T, Landwehr R, et al. Tenascin-C hexabrachion assembly is a sequential two-step process initiated by coiled-coil α-helices. J Biol Chem. 1998;273:10602–10608. doi: 10.1074/jbc.273.17.10602. [DOI] [PubMed] [Google Scholar]
- 85.Valcourt U, Alcaraz LB, Exposito JY, et al. Tenascin-X: beyond the architectural function. Cell Adhes Migr. 2015;9:154–165. doi: 10.4161/19336918.2014.994893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faissner A, Kruse J, Chiquet-Ehrismann R, Mackie E. The high-molecular-weight J1 glycoproteins are immunochemically related to tenascin. Differentiation. 1988;37:104–114. doi: 10.1111/j.1432-0436.1988.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 87.Chiquet-Ehrismann R, Tucker RP. Tenascins and the importance of adhesion modulation. Cold Spring Harb Perspect Biol. 2011;3:1–19. doi: 10.1101/cshperspect.a004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tucker RP, Drabikowski K, Hess JF, et al. Phylogenetic analysis of the tenascin gene family: evidence of origin early in the chordate lineage. BMC Evol Biol. 2006;6:60. doi: 10.1186/1471-2148-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Götz B, Scholze A, Clement A, et al. Tenascin-C contains distinct adhesive, anti-adhesive, and neurite outgrowth promoting sites for neurons. J Cell Biol. 1996 doi: 10.1083/jcb.132.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Joester A, Faissner A. The structure and function of tenascins in the nervous system. Matrix Biol. 2001;20:13–22. doi: 10.1016/S0945-053X(00)00136-0. [DOI] [PubMed] [Google Scholar]
- 91.Pas J, Wyszko E, Rolle K, et al. Analysis of structure and function of tenascin-C. Int J Biochem Cell Biol. 2006;38:1594–1602. doi: 10.1016/j.biocel.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 92.Lundell A, Olin AI, Mörgelin M, et al. Structural basis for interactions between tenascins and lectican C-type lectin domains: evidence for a crosslinking role for tenascins. Structure. 2004;12:1495–1506. doi: 10.1016/j.str.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 93.Brückner G, Grosche J, Schmidt S, et al. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol. 2000;428:616–629. doi: 10.1002/1096-9861(20001225)428:4<616::AID-CNE3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 94.Testa D, Prochiantz A, Di Nardo AA. Perineuronal nets in brain physiology and disease. Semin Cell Dev Biol. 2018 doi: 10.1016/j.semcdb.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 95.Spicer AP, Joo A, Bowling RA. A hyaluronan binding link protein gene family whose members are physically linked adjacent to chrondroitin sulfate proteoglycan core protein genes. The missing links. J Biol Chem. 2003;278:21083–21091. doi: 10.1074/jbc.M213100200. [DOI] [PubMed] [Google Scholar]
- 96.Oohashi T, Edamatsu M, Bekku Y, Carulli D. The hyaluronan and proteoglycan link proteins: organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp Neurol. 2015;274:134–144. doi: 10.1016/j.expneurol.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 97.Bekku Y, Su WD, Hirakawa S, et al. Molecular cloning of Bral2, a novel brain-specific link protein, and immunohistochemical colocalization with brevican in perineuronal nets. Mol Cell Neurosci. 2003 doi: 10.1016/S1044-7431(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 98.Deepa SS, Carulli D, Galtrey C, et al. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 99.Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 2011;21:353–359. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carulli D, Pizzorusso T, Kwok JCF, et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–2347. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- 101.Bekku Y, Saito M, Moser M, et al. Bral2 is indispensable for the proper localization of brevican and the structural integrity of the perineuronal net in the brainstem and cerebellum. J Comp Neurol. 2012;520:1721–1736. doi: 10.1002/cne.23009. [DOI] [PubMed] [Google Scholar]
- 102.Bekku Y, Rauch U, Ninomiya Y, Oohashi T. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J Neurochem. 2009;108:1266–1276. doi: 10.1111/j.1471-4159.2009.05873.x. [DOI] [PubMed] [Google Scholar]
- 103.Bekku Y, Vargova L, Goto Y, et al. Bral1: its role in diffusion barrier formation and conduction velocity in the CNS. J Neurosci. 2010;30:3113–3123. doi: 10.1523/JNEUROSCI.5598-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oohashi T, Hirakawa S, Bekku Y, et al. Bral1, a brain-specific link protein, colocalizing with the versican V2 isoform at the nodes of Ranvier in developing and adult mouse central nervous systems. Mol Cell Neurosci. 2002 doi: 10.1006/mcne.2001.1061. [DOI] [PubMed] [Google Scholar]
- 105.Brückner G, Brauer K, Härtig W, et al. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8:183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- 106.Itano N, Sawai T, Yoshida M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 107.Kwok JCF, Carulli D, Fawcett JW. In vitro modeling of perineuronal nets: hyaluronan synthase and link protein are necessary for their formation and integrity. J Neurochem. 2010;114:1447–1459. doi: 10.1111/j.1471-4159.2010.06878.x. [DOI] [PubMed] [Google Scholar]
- 108.Carulli D, Rhodes KE, Brown DJ, et al. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–577. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- 109.Golgi C. Intorno all'origine del quarto nervo cerebrale (patetico o trocleare) e di una questione di Isto - fisiologia generale che a questo argomento si ricollega. Rend Mat Acc Lincei. 1893;s.5, 2(1):379–389. [Google Scholar]
- 110.Golgi C. Intorno alla struttura delle cellule nervose. XXV. Sulla struttura delle cellule nervose dei ganglin spinali. Boll Soc Med Chirurgica di Pavia. 1898;1:655–665. [Google Scholar]
- 111.Härtig W, Brauer K, Brückner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. NeuroReport. 1992 doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- 112.Giamanco KAA, Matthews RTT. Deconstructing the perineuronal net: cellular contributions and molecular composition of the neuronal extracellular matrix. Neuroscience. 2012;218:367–384. doi: 10.1016/j.neuroscience.2012.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsien RY. Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc Natl Acad Sci. 2013 doi: 10.1073/pnas.1310158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lorenzo Bozzelli P, Alaiyed S, Kim E, et al. Proteolytic remodeling of perineuronal nets: effects on synaptic plasticity and neuronal population dynamics. Neural Plast. 2018 doi: 10.1155/2018/5735789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sorg BA, Berretta S, Blacktop JM, et al. Casting a wide net: role of perineuronal nets in neural plasticity. J Neurosci. 2016 doi: 10.1523/JNEUROSCI.2351-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lasek AW, Chen H, Chen WY. Releasing addiction memories trapped in perineuronal nets. Trends Genet. 2017;34:197–208. doi: 10.1016/j.tig.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 118.Lorenzo Bozzelli P, Alaiyed S, Kim E, et al. Proteolytic remodeling of perineuronal nets: effects on synaptic plasticity and neuronal population dynamics. Neural Plast. 2018;2018:1–13. doi: 10.1155/2018/5735789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wen TH, Binder DK, Ethell IM, Razak KA. The Perineuronal ‘Safety’ Net? Perineuronal net abnormalities in neurological disorders. Front Mol Neurosci. 2018 doi: 10.3389/fnmol.2018.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giamanco KA, Morawski M, Matthews RT. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience. 2010;170:1314–1327. doi: 10.1016/j.neuroscience.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 121.Matthews RT, Kelly GM, Zerillo CA, et al. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22:7536–7547. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ruoslahti E. Brain extracellular matrix. Glycobiology. 1996;6:489–492. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- 123.Matsumoto K, Shionyu M, Go M, et al. Distinct interaction of versican/PG-M with hyaluronan and link protein. J Biol Chem. 2003;278:41205–41212. doi: 10.1074/jbc.M305060200. [DOI] [PubMed] [Google Scholar]
- 124.Blundell CD, Almond A, Mahoney DJ, et al. Towards a structure for a TSG-6·hyaluronan complex by modeling and NMR, spectroscopy: insights into other members of the link module superfamily. J Biol Chem. 2005;280:18189–18201. doi: 10.1074/jbc.M414343200. [DOI] [PubMed] [Google Scholar]
- 125.Seyfried NT, McVey GF, Almond A, et al. Expression and purification of functionally active hyaluronan-binding domains from human cartilage link protein, aggrecan and versican: formation of ternary complexes with defined hyaluronan oligosaccharides. J Biol Chem. 2005;280:5435–5448. doi: 10.1074/jbc.M411297200. [DOI] [PubMed] [Google Scholar]
- 126.Rutka JT, Apodaca G, Stern R, Rosenblum M. The extracellular matrix of the central and peripheral nervous systems: structure and function. J Neurosurg. 1988;69:155–170. doi: 10.3171/jns.1988.69.2.0155. [DOI] [PubMed] [Google Scholar]
- 127.van’t Spijker HM, Kwok JCF. A sweet talk: the molecular systems of perineuronal nets in controlling neuronal communication. Front Integr Neurosci. 2017 doi: 10.3389/fnint.2017.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karetko M, Skangiel-Kramska J. Diverse functions of perineuronal nets. Acta Neurobiol Exp (Wars) 2009;69:564–577. doi: 10.55782/ane-2009-1766. [DOI] [PubMed] [Google Scholar]
- 129.Pantazopoulos H, Berretta S. In sickness and in health: perineuronal nets and synaptic plasticity in psychiatric disorders. Neural Plast. 2016;2016:9847696. doi: 10.1155/2016/9847696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Belichenko PV, Hagberg B, Dahlström A. Morphological study of neocortical areas in Rett syndrome. Acta Neuropathol. 1997;93:50–61. doi: 10.1007/s004010050582. [DOI] [PubMed] [Google Scholar]
- 131.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 132.Sorg BA, Berretta S, Blacktop JM, et al. Casting a wide net: role of perineuronal nets in neural plasticity. J Neurosci. 2016;36:11459–11468. doi: 10.1523/JNEUROSCI.2351-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lensjø KK, Lepperød ME, Dick G, et al. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J Neurosci. 2017;37:1269–1283. doi: 10.1523/JNEUROSCI.2504-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van’t Spijker HM, Kwok JCF. A sweet talk: the molecular systems of perineuronal nets in controlling neuronal communication. Front Integr Neurosci. 2017;11:33. doi: 10.3389/fnint.2017.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Frischknecht R, Heine M, Perrais D, et al. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 136.Deepa SS, Umehara Y, Higashiyama S, et al. Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin-binding growth factors: implications as a physiological binding partner in the brain and other tissues. J Biol Chem. 2002;277:43707–43716. doi: 10.1074/jbc.M207105200. [DOI] [PubMed] [Google Scholar]
- 137.Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012;13:478–490. doi: 10.1038/nrn3258. [DOI] [PubMed] [Google Scholar]
- 138.Tsien RY. Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc Natl Acad Sci. 2013;110:12456–12461. doi: 10.1073/pnas.1310158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cohen LD, Zuchman R, Sorokina O, et al. Metabolic turnover of synaptic proteins: kinetics, interdependencies and implications for synaptic maintenance. PLoS One. 2013;8:e63191. doi: 10.1371/journal.pone.0063191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Happel MFK, Niekisch H, Castiblanco Rivera LL, et al. Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proc Natl Acad Sci. 2014;111:2800–2805. doi: 10.1073/pnas.1310272111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bradshaw KP, Figueroa Velez DX, Habeeb M, Gandhi SP. Precocious deposition of perineuronal nets on Parvalbumin inhibitory neurons transplanted into adult visual cortex. Sci Rep. 2018;8:7480. doi: 10.1038/s41598-018-25735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wen TH, Afroz S, Reinhard SM, et al. Genetic reduction of matrix metalloproteinase-9 promotes formation of perineuronal nets around parvalbumin-expressing interneurons and normalizes auditory cortex responses in developing fmr1 knock-out mice. Cereb Cortex. 2017 doi: 10.1093/cercor/bhx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cabungcal J-H, Steullet P, Morishita H, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci. 2013;110:9130–9135. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Balmer TS. Perineuronal nets enhance the excitability of fast-spiking neurons. eNeuro. 2016 doi: 10.1523/ENEURO.0112-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rossier J, Bernard A, Cabungcal JH, et al. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol Psychiatry. 2015;20:154–161. doi: 10.1038/mp.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dupret D, Pleydell-Bouverie B, Csicsvari J. Inhibitory interneurons and network oscillations. Proc Natl Acad Sci. 2008;105:18079–18080. doi: 10.1073/pnas.0810064105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top–down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 148.Howard MW, Rizzuto DS, Caplan JB, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 149.Sun ZY, Bozzelli PL, Caccavano A, et al. Disruption of perineuronal nets increases the frequency of sharp wave ripple events. Hippocampus. 2018;28:42–52. doi: 10.1002/hipo.22804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schüppel K, Brauer K, Härtig W, et al. Perineuronal nets of extracellular matrix around hippocampal interneurons resist destruction by activated microglia in trimethyltin-treated rats. Brain Res. 2002;958:448–453. doi: 10.1016/S0006-8993(02)03569-2. [DOI] [PubMed] [Google Scholar]
- 151.Ferrer-Ferrer M, Dityatev A. Shaping synapses by the neural extracellular matrix. Front Neuroanat. 2018;12:40. doi: 10.3389/fnana.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Eroglu C. The role of astrocyte-secreted matricellular proteins in central nervous system development and function. J Cell Commun Signal. 2009;3:167–176. doi: 10.1007/s12079-009-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Christopherson KS, Ullian EM, Stokes CCA, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 154.Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci. 2010;13:22–24. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
- 155.Benton RL, Maddie MA, Worth CA, et al. Transcriptomic screening of microvascular endothelial cells implicates novel molecular regulators of vascular dysfunction after spinal cord injury. J Cereb Blood Flow Metab. 2008;28:1771–1785. doi: 10.1038/jcbfm.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lin T-N, Kim G-M, Chen J-J, et al. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34:177–186. doi: 10.1161/01.STR.0000047100.84604.BA. [DOI] [PubMed] [Google Scholar]
- 157.Liauw J, Hoang S, Choi M, et al. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab. 2008;28:1722–1732. doi: 10.1038/jcbfm.2008.65. [DOI] [PubMed] [Google Scholar]
- 158.Eroglu C, Allen NJ, Susman MW, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Favuzzi E, Marques-Smith A, Deogracias R, et al. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron. 2017;95:639–655. doi: 10.1016/J.NEURON.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 160.Brakebusch C, Seidenbecher CI, Asztely F, et al. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol. 2002;22:7417–7427. doi: 10.1128/mcb.22.21.7417-7427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chang MC, Park JM, Pelkey KA, et al. Narp regulates homeostatic scaling of excitatory synapses on Parvalbumin interneurons. Nat Neurosci. 2010;13:1090–1097. doi: 10.1038/nn.2621.Narp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pelkey KA, Barksdale E, Craig MT, et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron. 2015;85:1257–1272. doi: 10.1016/j.neuron.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xiao MF, Xu D, Craig MT, et al. NPTX2 and cognitive dysfunction in Alzheimer’s Disease. Elife. 2017;6:1–27. doi: 10.7554/eLife.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baudouin S, Scheiffele P. SnapShot: neuroligin-neurexin complexes. Cell. 2010;141:908. doi: 10.1016/j.cell.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 165.Kucukdereli H, Allen NJ, Lee AT, et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci. 2011;108:E440–E449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wei P, Pattarini R, Rong Y, et al. The Cbln family of proteins interact with multiple signaling pathways. J Neurochem. 2012;121:717–729. doi: 10.1111/j.1471-4159.2012.07648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haddick PCG, Tom I, Luis E, et al. Defining the ligand specificity of the deleted in colorectal cancer (DCC) receptor. PLoS One. 2014;9:e84823. doi: 10.1371/journal.pone.0084823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rong Y, Wei P, Parris J, et al. Comparison of Cbln1 and Cbln2 functions using transgenic and knockout mice. J Neurochem. 2012;120:528–540. doi: 10.1111/j.1471-4159.2011.07604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hirai H, Pang Z, Bao D, et al. Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat Neurosci. 2005;8:1534–1541. doi: 10.1038/nn1576. [DOI] [PubMed] [Google Scholar]
- 170.Kusnoor SV, Parris J, Muly EC, et al. Extracerebellar role for cerebellin 1: modulation of dendritic spine density and synapses in striatal medium spiny neurons. J Comp Neurol. 2010;518:2525–2537. doi: 10.1002/cne.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Knight D, Xie W, Boulianne GL. Neurexins and neuroligins: recent insights from invertebrates. Mol Neurobiol. 2011;44:426. doi: 10.1007/S12035-011-8213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bock HH, May P. Canonical and non-canonical Reelin signaling. Front Cell Neurosci. 2016;10:166. doi: 10.3389/fncel.2016.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Leemhuis J, Bouché E, Frotscher M, et al. Reelin signals through apolipoprotein E receptor 2 and Cdc42 to increase growth cone motility and filopodia formation. J Neurosci. 2010;30:14759–14772. doi: 10.1523/JNEUROSCI.4036-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee GH, D’Arcangelo G. New insights into Reelin-mediated signaling pathways. Front Cell Neurosci. 2016;10:122. doi: 10.3389/fncel.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Niu S, Renfro A, Quattrocchi CC, et al. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004;41:71–84. doi: 10.1016/S0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- 176.Niu S, Yabut O, D’Arcangelo G. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J Neurosci. 2008;28:10339–10348. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bosch C, Masachs N, Exposito-Alonso D, et al. Reelin regulates the maturation of dendritic spines, synaptogenesis and glial ensheathment of newborn granule cells. Cereb Cortex. 2016;26:4282–4298. doi: 10.1093/cercor/bhw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lane-Donovan C, Philips GT, Herz J. More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration. Neuron. 2014;83:771–787. doi: 10.1016/J.NEURON.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Weeber EJ, Beffert U, Jones C, et al. Reelin and apoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277:39944–39952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- 180.Chen Y, Beffert U, Ertunc M, et al. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25:8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Campo CG, Sinagra M, Verrier D, et al. Reelin secreted by GABAergic neurons regulates glutamate receptor homeostasis. PLoS One. 2009;4:e5505. doi: 10.1371/journal.pone.0005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci. 2006;26:12943–12955. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Knuesel I. Reelin-mediated signaling in neuropsychiatric and neurodegenerative diseases. Prog Neurobiol. 2010;91:257–274. doi: 10.1016/j.pneurobio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 184.Folsom TD, Fatemi SH. The involvement of Reelin in neurodevelopmental disorders. Neuropharmacology. 2013;68:122–135. doi: 10.1016/j.neuropharm.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oohashi T, Edamatsu M, Bekku Y, Carulli D. The hyaluronan and proteoglycan link proteins: organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp Neurol. 2015;274:134–144. doi: 10.1016/j.expneurol.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 186.Vecino E, Kwok JCF (2016) The extracellular matrix in the nervous system: the good and the bad aspects. In: Travascio F (ed) Composition and function of the extracellular matrix in the human body. InTech
- 187.Bukalo O, Schachner M, Dityatev A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience. 2001;104:359–369. doi: 10.1016/S0306-4522(01)00082-3. [DOI] [PubMed] [Google Scholar]
- 188.Saghatelyan AK, Dityatev A, Schmidt S, et al. Reduced perisomatic inhibition, increased excitatory transmission, and impaired long-term potentiation in mice deficient for the extracellular matrix glycoprotein tenascin-R. Mol Cell Neurosci. 2001;17:226–240. doi: 10.1006/MCNE.2000.0922. [DOI] [PubMed] [Google Scholar]
- 189.Freitag S, Schachner M, Morellini F. Behavioral alterations in mice deficient for the extracellular matrix glycoprotein tenascin-R. Behav Brain Res. 2003;145:189–207. doi: 10.1016/S0166-4328(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 190.Gurevicius K, Gureviciene I, Valjakka A, et al. Enhanced cortical and hippocampal neuronal excitability in mice deficient in the extracellular matrix glycoprotein tenascin-R. Mol Cell Neurosci. 2004;25:515–523. doi: 10.1016/J.MCN.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 191.Nikonenko A, Schmidt S, Skibo G, et al. Tenascin-R-deficient mice show structural alterations of symmetric perisomatic synapses in the CA1 region of the hippocampus. J Comp Neurol. 2003;456:338–349. doi: 10.1002/cne.10537. [DOI] [PubMed] [Google Scholar]
- 192.Ferhat L, Chevassus Au Louis N, Jorquera I, et al. Transient increase of tenascin-C in immature hippocampus: Astroglial and neuronal expression. J Neurocytol. 1996;25:53–66. doi: 10.1007/BF02284785. [DOI] [PubMed] [Google Scholar]
- 193.Nakic M, Manahan-Vaughan D, Reymann KG, Schachner M. Long-term potentiation in vivo increases rat hippocampal tenascin-C expression. J Neurobiol. 1998;37:393–404. doi: 10.1002/(SICI)1097-4695(19981115)37:3<393::AID-NEU5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 194.Morellini F, Malyshev A, Volgushev M, et al. Impaired fear extinction due to a deficit in Ca2+ influx through L-type voltage-gated Ca2+ channels in mice deficient for tenascin-C. Front Integr Neurosci. 2017;11:16. doi: 10.3389/fnint.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schweitzer B, Singh J, Fejtova A, et al. Hyaluronic acid based extracellular matrix regulates surface expression of GluN2B containing NMDA receptors. Sci Rep. 2017;7:10991. doi: 10.1038/s41598-017-07003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kochlamazashvili G, Henneberger C, Bukalo O, et al. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca2+ channels. Neuron. 2010;67:116–128. doi: 10.1016/j.neuron.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Arancibia-Carcamo IL, Attwell D. The node of Ranvier in CNS pathology. Acta Neuropathol. 2014;128:161–175. doi: 10.1007/s00401-014-1305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Susuki K, Chang K-J, Zollinger DR, et al. Three mechanisms assemble central nervous system nodes of Ranvier. Neuron. 2013;78:469–482. doi: 10.1016/j.neuron.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Susuki K, Rasband MN. Molecular mechanisms of node of Ranvier formation. Curr Opin Cell Biol. 2008;20:616–623. doi: 10.1016/j.ceb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Faivre-Sarrailh C, Devaux JJ. Neuro-glial interactions at the nodes of Ranvier: implication in health and diseases. Front Cell Neurosci. 2013 doi: 10.3389/fncel.2013.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Oohashi T, Hirakawa S, Bekku Y, et al. Bral1, a brain-specific link protein, colocalizing with the versican V2 isoform at the nodes of Ranvier in developing and adult mouse central nervous systems. Mol Cell Neurosci. 2002;19:43–57. doi: 10.1006/mcne.2001.1061. [DOI] [PubMed] [Google Scholar]
- 202.Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci. 2015;16:756–767. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Löffek S, Schilling O, Franzke C-W. Biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011;38:191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- 204.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nagy V. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tsilibary E, Tzinia A, Radenovic L, et al. Neural ECM proteases in learning and synaptic plasticity. 1. Amsterdam: Elsevier B.V; 2014. [DOI] [PubMed] [Google Scholar]
- 207.Bilousova TV, Dansie L, Ngo M, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2008;46:94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- 208.Michaluk P, Wawrzyniak M, Alot P, et al. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J Cell Sci. 2011;124:3369–3380. doi: 10.1242/jcs.090852. [DOI] [PubMed] [Google Scholar]
- 209.Szepesi Z, Hosy E, Ruszczycki B, et al. Synaptically released matrix metalloproteinase activity in control of structural plasticity and the cell surface distribution of GluA1-AMPA receptors. PLoS One. 2014;9:e98274. doi: 10.1371/journal.pone.0098274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang X, Bozdagi O, Nikitczuk JS, et al. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Okulski P, Jay TM, Jaworski J, et al. TIMP-1 abolishes MMP-9-dependent long-lasting long-term potentiation in the prefrontal cortex. Biol Psychiatry. 2007;62:359–362. doi: 10.1016/j.biopsych.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 212.Niedringhaus M, Chen X, Dzakpasu R, Conant K. MMPs and soluble ICAM-5 increase neuronal excitability within in vitro networks of hippocampal neurons. PLoS One. 2012;7:e42631. doi: 10.1371/journal.pone.0042631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Szepesi Z, Bijata M, Ruszczycki B, et al. Matrix metalloproteinases regulate the formation of dendritic spine head protrusions during chemically induced long-term potentiation. PLoS One. 2013;8:e63314. doi: 10.1371/journal.pone.0063314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lonskaya I, Partridge J, Lalchandani RR, et al. Soluble ICAM-5, a product of activity dependent proteolysis, increases mEPSC frequency and dendritic expression of GluA1. PLoS One. 2013;8:e69136. doi: 10.1371/journal.pone.0069136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nedivi E, Hevroni D, Naot D, et al. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- 216.Cao W, Duan J, Wang X, et al. Early enriched environment induces an increased conversion of proBDNF to BDNF in the adult rat’s hippocampus. Behav Brain Res. 2014;265:76–83. doi: 10.1016/j.bbr.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 217.Bayat M, Sharifi MD, Haghani M, Shabani M. Enriched environment improves synaptic plasticity and cognitive deficiency in chronic cerebral hypoperfused rats. Brain Res Bull. 2015;119:34–40. doi: 10.1016/j.brainresbull.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 218.Rivera S, Tremblay E, Timsit S, et al. Tissue inhibitor of metalloproteinases-1 (TIMP-1) is differentially induced in neurons and astrocytes after seizures: evidence for developmental, immediate early gene, and lesion response. J Neurosci. 1997;17:4223–4235. doi: 10.1523/JNEUROSCI.17-11-04223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jaworski J, Biedermann IW, Lapinska J, et al. Neuronal excitation-driven and AP-1-dependent activation of tissue inhibitor of metalloproteinases-1 gene expression in rodent hippocampus. J Biol Chem. 1999;274:28106–28112. doi: 10.1074/jbc.274.40.28106. [DOI] [PubMed] [Google Scholar]
- 220.Knapska E, Lioudyno V, Kiryk A, et al. Reward learning requires activity of matrix metalloproteinase-9 in the central amygdala. J Neurosci. 2013;33:14591–14600. doi: 10.1523/JNEUROSCI.5239-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Brown TE, Forquer MR, Cocking DL, et al. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wilczynski GM, Konopacki FA, Wilczek E, et al. Important role of matrix metalloproteinase 9 in epileptogenesis. J Cell Biol. 2008;180:1021–1035. doi: 10.1083/jcb.200708213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Meighan SE, Meighan PC, Choudhury P, et al. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96:1227–1241. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- 224.Kaliszewska A, Bijata M, Kaczmarek L, Kossut M. Experience-dependent plasticity of the barrel cortex in mice observed with 2-DG brain mapping and c-Fos: effects of MMP-9 KO. Cereb Cortex. 2012;22:2160–2170. doi: 10.1093/cercor/bhr303. [DOI] [PubMed] [Google Scholar]
- 225.Spolidoro M, Putignano E, Munaf C, et al. Inhibition of matrix metalloproteinases prevents the potentiation of nondeprived-eye responses after monocular deprivation in juvenile rats. Cereb Cortex. 2012;22:725–734. doi: 10.1093/cercor/bhr158. [DOI] [PubMed] [Google Scholar]
- 226.Gorkiewicz T, Balcerzyk M, Kaczmarek L, Knapska E. Matrix metalloproteinase 9 (MMP-9) is indispensable for long term potentiation in the central and basal but not in the lateral nucleus of the amygdala. Front Cell Neurosci. 2015;9:73. doi: 10.3389/fncel.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kaczmarek L, Lapinska-Dzwonek J, Szymczak S. Matrix metalloproteinases in the adult brain physiology: a link between c-Fos, AP-1 and remodeling of neuronal connections? EMBO J. 2002;21:6643–6648. doi: 10.1093/emboj/cdf676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ganguly K, Rejmak E, Mikosz M, et al. Matrix metalloproteinase (MMP) 9 transcription in mouse brain induced by fear learning. J Biol Chem. 2013;288:20978–20991. doi: 10.1074/jbc.M113.457903. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 229.Dziembowska M, Milek J, Janusz A, et al. Activity-dependent local translation of matrix metalloproteinase-9. J Neurosci. 2012;32:14538–14547. doi: 10.1523/JNEUROSCI.6028-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Janusz A, Milek J, Perycz M, et al. The fragile X mental retardation protein regulates matrix metalloproteinase 9 mRNA at synapses. J Neurosci. 2013;33:18234–18241. doi: 10.1523/JNEUROSCI.2207-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nagaoka A, Takehara H, Hayashi-Takagi A, et al. Abnormal intrinsic dynamics of dendritic spines in a fragile X syndrome mouse model in vivo. Sci Rep. 2016;6:26651. doi: 10.1038/srep26651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gantois I, Khoutorsky A, Popic J, et al. Metformin ameliorates core deficits in a mouse model of fragile X syndrome. Nat Med. 2017;23:674–677. doi: 10.1038/nm.4335. [DOI] [PubMed] [Google Scholar]
- 233.Conant K, Wang Y, Szklarczyk A, et al. Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience. 2010;166:508–521. doi: 10.1016/j.neuroscience.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Michaluk P, Mikasova L, Groc L, et al. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin _1 signaling. J Neurosci. 2009;29:6007–6012. doi: 10.1523/JNEUROSCI.5346-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Michaluk P, Kaczmarek L. Matrix metalloproteinase-9 in glutamate-dependent adult brain function and dysfunction. Cell Death Differ. 2007;14:1255–1258. doi: 10.1038/sj.cdd.4402141. [DOI] [PubMed] [Google Scholar]
- 236.Tian L, Stefanidakis M, Ning L, et al. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol. 2007;178:687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2015;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- 238.Gawlak M, Górkiewicz T, Gorlewicz A, et al. High resolution in situ zymography reveals matrix metalloproteinase activity at glutamatergic synapses. Neuroscience. 2009;158:167–176. doi: 10.1016/j.neuroscience.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 239.Konopacki FA, Rylski M, Wilczek E, et al. Synaptic localization of seizure-induced matrix metalloproteinase-9 mRNA. Neuroscience. 2007;150:31–39. doi: 10.1016/j.neuroscience.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 240.Sbai O, Ferhat L, Bernard A, et al. Vesicular trafficking and secretion of matrix metalloproteinases-2, -9 and tissue inhibitor of metalloproteinases-1 in neuronal cells. Mol Cell Neurosci. 2008;39:549–568. doi: 10.1016/J.MCN.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 241.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–3584. doi: 10.1108/20423891211271728. [DOI] [PubMed] [Google Scholar]
- 242.Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. doi: 10.1002/jnr. [DOI] [PubMed] [Google Scholar]
- 243.Overall CM. Molecular determinants of metalloproteinase substrate specificity: matrix metalloproteinase substrate binding domains, modules, and exosites. Mol Biotechnol. 2002;22:051–086. doi: 10.1385/MB:22:1:051. [DOI] [PubMed] [Google Scholar]
- 244.Cua RC, Lau LW, Keough MB, et al. Overcoming neurite-inhibitory chondroitin sulfate proteoglycans in the astrocyte matrix. Glia. 2013;61:972–984. doi: 10.1002/glia.22489. [DOI] [PubMed] [Google Scholar]
- 245.Yuan W, Matthews RT, Sandy JD, Gottschall PE. Association between protease-specific proteolytic cleavage of brevican and synaptic loss in the dentate gyrus of kainate-treated rats. Neuroscience. 2002;114:1091–1101. doi: 10.1016/S0306-4522(02)00347-0. [DOI] [PubMed] [Google Scholar]
- 246.Tauchi R, Imagama S, Natori T, et al. The endogenous proteoglycan-degrading enzyme ADAMTS-4 promotes functional recovery after spinal cord injury. J Neuroinflammation. 2012;9:53. doi: 10.1186/1742-2094-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hamel MG, Ajmo JM, Leonardo CC, et al. Multimodal signaling by the ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs) promotes neurite extension. Exp Neurol. 2008;210:428–440. doi: 10.1016/j.expneurol.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Howell MD, Torres-Collado AX, Iruela-Arispe ML, Gottschall PE. Selective decline of synaptic protein levels in the frontal cortex of cemale mice deficient in the extracellular metalloproteinase ADAMTS1. PLoS One. 2012;7:e47226. doi: 10.1371/journal.pone.0047226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Calabresi P, Napolitano M, Centonze D, et al. Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur J Neurosci. 2000;12:1002–1012. doi: 10.1046/j.1460-9568.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- 250.Huang YY, Bach ME, Lipp HP, et al. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc Natl Acad Sci. 1996;93:8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Samson AL, Medcalf RL. Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron. 2006;50:673–678. doi: 10.1016/j.neuron.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 252.Qian Z, Gilbert ME, Colicos MA, et al. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- 253.Norris EH, Strickland S. Modulation of NR2B-regulated contextual fear in the hippocampus by the tissue plasminogen activator system. Proc Natl Acad Sci. 2007;104:13473–13478. doi: 10.1002/asna.18440211403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pawlak R, Nagai N, Urano T, et al. Rapid, specific and active site-catalyzed effect of tissue-plasminogen activator on hippocampus-dependent learning in mice. Neuroscience. 2002;113:995–1001. doi: 10.1016/S0306-4522(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 255.Seeds NW, Basham ME, Haffke SP. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proc Natl Acad Sci. 1999;96:14118–14123. doi: 10.1073/pnas.96.24.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Frey U, Müller M, Kuhl D. A different form of long-lasting potentiation revealed in tissue plasminogen activator mutant mice. J Neurosci. 1996;16:2057–2063. doi: 10.1523/JNEUROSCI.16-06-02057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Madani R, Hulo S, Toni N, et al. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J. 1999;18:3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhuo M, Holtzman DM, Li Y, et al. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J Neurosci. 2000;20:542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pang PT, Teng HK, Zaitsev E, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306(80):487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 260.Tirosh O, Schwartz B, Zusman I, et al. Long-lived αMUPA transgenic mice exhibit increased mitochondrion-mediated apoptotic capacity. Ann N Y Acad Sci. 2004;1019:439–442. doi: 10.1196/annals.1297.080. [DOI] [PubMed] [Google Scholar]
- 261.Miskin R, Masos T. Transgenic mice overexpressing urokinase-type plasminogen activator in the brain exhibit reduced food consumption, body weight and size, and increased longevity. J Gerontol Ser A Biol Sci Med Sci. 1997;52:B118–B124. doi: 10.1093/gerona/52A.2.B118. [DOI] [PubMed] [Google Scholar]
- 262.Meiri N, Masos T, Rosenblum K, et al. Overexpression of urokinase-type plasminogen activator in transgenic mice is correlated with impaired learning. Proc Natl Acad Sci USA. 1994;91:3196–3200. doi: 10.1073/pnas.91.8.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rivera S, Khrestchatisky M, Kaczmarek L, et al. Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J Neurosci. 2010;30:15337–15357. doi: 10.1523/JNEUROSCI.3467-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Baranger K, Rivera S, Liechti FD, Grandgirard D, Bigas J, Seco J, Tarrago T, Leib SL, Khrestchatisky M. Endogenous and synthetic MMP inhibitors in CNS physiopathology. Prog Brain Res. 2014;214:313–351. doi: 10.1016/B978-0-444-63486-3.00014-1. [DOI] [PubMed] [Google Scholar]
- 265.Agrawal S, Lau L, Yong V. MMPs in the central nervous system: where the good guys go bad. Semin Cell Dev Biol. 2008;19:42–51. doi: 10.1016/j.semcdb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 266.Hewson AK, Smith T, Leonard JP, Cuzner ML. Suppression of experimental allergic encephalomyelitis in the Lewis rat by the matrix metalloproteinase inhibitor Ro31-9790. Inflamm Res. 1995;44:345–349. doi: 10.1007/BF01796266. [DOI] [PubMed] [Google Scholar]
- 267.Brundula V, Rewcastle NB, Metz LM, et al. Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain. 2002;125:1297–1308. doi: 10.1093/BRAIN/AWF133. [DOI] [PubMed] [Google Scholar]
- 268.Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36:1481–1507. doi: 10.1177/0271678X16655551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Suzuki Y, Nagai N, Umemura K, et al. Stromelysin-1 (MMP-3) is critical for intracranial bleeding after t-PA treatment of stroke in mice. J Thromb Haemost. 2007;5:1732–1739. doi: 10.1111/j.1538-7836.2007.02628.x. [DOI] [PubMed] [Google Scholar]
- 270.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 271.Testero SA, Lee M, Staran RT, et al. Sulfonate-containing thiiranes as selective gelatinase inhibitors. ACS Med Chem Lett. 2011;2:177–181. doi: 10.1021/ml100254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gu Z, Cui J, Brown S, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25:6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cui J, Chen S, Zhang C, et al. Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol Neurodegener. 2012;7:21. doi: 10.1186/1750-1326-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zhao B-Q, Wang S, Kim H-Y, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 275.Fagan SC, Waller JL, Nichols FT, et al. Minocycline to improve neurologic outcome in stroke (MINOS) Stroke. 2010;41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Minagar A, Alexander JS, Schwendimann RN, et al. Combination therapy with interferon beta-1a and doxycycline in multiple sclerosis. Arch Neurol. 2008;65:199–204. doi: 10.1001/archneurol.2007.41. [DOI] [PubMed] [Google Scholar]
- 277.Groves MD, Puduvalli VK, Conrad CA, et al. Phase II trial of temozolomide plus marimastat for recurrent anaplastic gliomas: a relationship among efficacy, joint toxicity and anticonvulsant status. J Neurooncol. 2006;80:83–90. doi: 10.1007/s11060-006-9160-y. [DOI] [PubMed] [Google Scholar]
- 278.Groves MD, Puduvalli VK, Hess KR, et al. Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme. J Clin Oncol. 2002;20:1383–1388. doi: 10.1200/JCO.2002.20.5.1383. [DOI] [PubMed] [Google Scholar]
- 279.Prinomastat plus temozolomide following radiation therapy in treating patients with newly diagnosed glioblastoma multiforme—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00004200. Accessed 8 Apr 2019
- 280.Rudek MA, New P, Mikkelsen T, et al. Phase I and pharmacokinetic study of COL-3 in patients with recurrent high-grade gliomas. J Neurooncol. 2011;105:375–381. doi: 10.1007/s11060-011-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov. 2014;13:904–927. doi: 10.1038/nrd4390. [DOI] [PubMed] [Google Scholar]
- 282.Gantois I, Khoutorsky A, Popic J, et al. Metformin ameliorates core deficits in a mouse model of fragile X syndrome. Nat Med. 2017;23:674–677. doi: 10.1038/nm.4335. [DOI] [PubMed] [Google Scholar]
- 283.Combined treatment of minocycline and lovastatin to treat individuals with fragile × syndrome—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02680379. Accessed 8 Apr 2019
- 284.Levin M, Udi Y, Solomonov I, Sagi I. Next generation matrix metalloproteinase inhibitors—novel strategies bring new prospects. Biochim Biophys Acta Mol Cell Res. 2017;1864:1927–1939. doi: 10.1016/j.bbamcr.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 285.Pantazopoulos H, Berretta S. In sickness and in health: perineuronal nets and synaptic plasticity in psychiatric disorders. Neural Plast. 2016;2016:9847696. doi: 10.1155/2016/9847696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Suttkus A, Rohn S, Weigel S, et al. Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death Dis. 2014;5:e1119. doi: 10.1038/cddis.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Morawski M, Dityatev A, Hartlage-Rübsamen M, et al. Tenascin-R promotes assembly of the extracellular matrix of perineuronal nets via clustering of aggrecan. Philos Trans R Soc B Biol Sci. 2014;369:20140046. doi: 10.1098/rstb.2014.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Vitellaro-Zuccarello L, Bosisio P, Mazzetti S, et al. Differential expression of several molecules of the extracellular matrix in functionally and developmentally distinct regions of rat spinal cord. Cell Tissue Res. 2007;327:433–447. doi: 10.1007/s00441-006-0289-y. [DOI] [PubMed] [Google Scholar]
- 289.Matthews RT, Kelly GM, Zerillo CA, et al. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22:7536–7547. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Mohan V, Talmi-Frank D, Arkadash V, Papo NSI. Matrix metalloproteinase protein inhibitors: highlighting a new beginning for metalloproteinases in medicine. Met Med. 2016;3:31–47. doi: 10.2147/MNM.S119588. [DOI] [Google Scholar]