Abstract
In plants, mitogen-activated protein kinase (MAPK) cascades are involved in regulating many biological processes including immunity. They relay signals from membrane-residing immune receptors to downstream components for defense activation. Arabidopsis MPK3/6 and MPK4 are activated in two parallel MAPK cascades during PAMP-triggered immunity. MPK3/6 have been implicated in the activation of various immune responses and their inactivation leads to compromised defense against pathogens. On the other hand, the MEKK1-MKK1/2-MPK4 cascade plays critical roles in basal resistance. Disruption of this MAPK cascade results in constitutive defense responses mediated by the NB-LRR protein SUMM2. Interestingly, SUMM2 guards the MEKK1-MKK1/2-MPK4 cascade activity indirectly through monitoring the phosphorylation status of CRCK3, which is a substrate of MPK4. From the pathogens’ side, a number of effectors are shown to target various components of MAPK cascades in plants. Inactivation of MPK4 by the Pseudomonas effector HopAI1 triggers SUMM2-mediated immunity. Together, these findings suggest intricate interplays between PAMP-triggered immunity and effector-triggered immunity via MAPK signaling.
Keywords: Mitogen-activated protein kinases, MAPK cascade, MPK3, MPK6, MPK4, Effector-triggered immunity, PAMP-triggered immunity, SUMM2, CRCK3
Introduction
Plants are constantly under threat from numerous pathogens and have evolved complex defense mechanisms to recognize and respond against such attacks [1]. Successful recognition of pathogens is one of the most important aspects of plant immunity as it paves the way for defense initiation. Pattern recognition receptors (PRRs) recognize pathogen-associated molecular patterns (PAMPs) to activate PAMP-triggered immunity (PTI) [2, 3]. Pathogen attacks can also be sensed through recognition of effector proteins secreted by the pathogens. Such recognition is facilitated by intracellular or extracellular receptors, which activate effector-triggered immunity (ETI) [4, 5]. During PTI and ETI, defense signals are transmitted from these receptors through various signalling cascades, which ultimately induce the expression of genes that are responsible for resistance to pathogens.
MAPK cascades and PTI signaling
Unlike in animals where PAMPs are recognized by nucleotide binding and leucine-rich repeat containing receptors (NLRs), recognition of PAMPs in plants is facilitated by PRRs localized on the plasma membrane [2]. Most plant PRRs belong to the receptor-like kinase or receptor-like protein family. The most commonly studied plant PAMP and PRR pairs are flg22 and the flagellin receptor FLS2 [6, 7], elf18 and its receptor EFR [8, 9], and the fungal cell wall component chitin and its receptor CERK1 [10, 11]. Recognition of PAMPs by their receptors triggers a series of downstream defense responses such as reactive oxygen species (ROS) burst, ion influx, increased synthesis of the defense hormone salicylic acid (SA), activation of Mitogen-Activated Protein Kinases (MAPKs or MPKs) and up-regulation of defense genes [12].
MAPK cascades in PTI
MAPK cascades play diverse roles in plant immunity [13, 14]. They are three tiered consisting of MAPK Kinase Kinases (MAPKKKs/MEKKs), MAPK Kinases (MAPKKs/MKKs) and MAPKs/MPKs [15]. The signal is relayed and amplified through the cascades via phosphorylation of the next protein by the upstream protein kinase. MEKKs phosphorylate the MKKs, which in turn phosphorylate and activate MPKs. Active MPKs subsequently phosphorylate downstream components such as transcription factors and metabolic enzymes to regulate their activity [3, 16].
MAPK cascades have been shown to contribute to plant immune signaling. Early studies identified several stress-induced MPKs in plants such as WIPK (Wound-Induced Protein Kinase) and SIPK (Salicylic acid-Induced Protein Kinase) in tobacco and SIMK (Salt-stress Inducible MAP Kinase) and SAMK (Stress-Activated MAP Kinase) in alfalfa (Medicago sativa) [17–20]. SIMK and SAMK are also activated in response to different elicitors, suggesting that they may play a role in defense signaling [21]. The Arabidopsis orthologs of WIPK and SIPK, MPK3 and MPK6, were later shown to be activated upon the perception of PAMPs [22, 23]. In parsley (Petroselinum crispum), a MAPK cascade consisting of the MPKs PcMPK3a/b and PcMPK6 and the upstream PcMKK5 is involved in defensive responses in response to the Pep-13 elicitor [24]. Arabidopsis MPK3/6 and MKK4/5 form a MAPK cascade together with MAPKKK3/5 downstream of FLS2 [23, 25]. Another Arabidopsis MAPK cascade consisting of MEKK1, MKK1/2 and MPK4 is also activated during PTI [26, 27]. In this review, we focus our discussion on the last two well characterized MAPK cascades in Arabidopsis.
Roles of the MAPKKK3/5-MKK4/5 -MPK3/6 cascade in plant immunity
The Arabidopsis MAPKKK3/5-MKK4/5 -MPK3/6 cascade plays diverse roles in plant defense against pathogens [13, 25]. They are involved in the activation of ethylene, camalexin and indole glucosinolate biosynthetic pathways and are also required for stomatal immunity (Fig. 1).
Fig. 1.
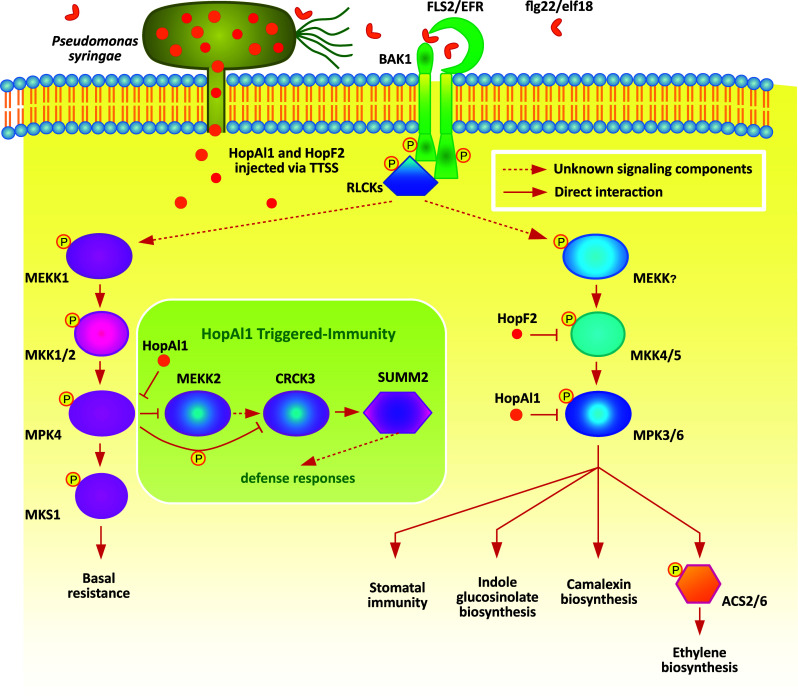
MAP kinase pathways downstream of pattern recognition receptors in Arabidopsis. Perception of PAMPs (e.g. flg22 and elf18) by their cognate receptors triggers activation of downstream MAPK cascades, which play diverse roles in promoting plant defense against pathogens. To suppress immune responses activated by MAPKs, pathogens secret effectors such as HopF2 and HopAI1 to inhibit MAPK signaling. Inhibition of MPK4 kinase activity by HopAI1 leads to reduced phosphorylation of CRCK3, resulting in the activation of SUMM2-mediated immunity. Dashed lines indicate unknown signalling events while solid lines illustrate demonstrated protein–protein interactions
Ethylene Biosynthesis
Ethylene is a phytohormone playing important roles in defense against necrotrophs [28]. It also contributes to resistance against Pseudomonas bacteria [29]. Ethylene biosynthesis involves two key steps: the conversion of S-adenosyl-L-Met to 1-aminocyclopropane-1-carboxylic acid (ACC) and the oxidative cleavage of ACC to form ethylene. ACC SYNTHASE (ACS) catalyzes the conversion of S-adenosyl-L-Met to ACC, which is the rate limiting step in ethylene biosynthesis [30]. Upon activation by the upstream MKKs, Arabidopsis MPK3/6 phosphorylates ACS2 and ACS6 at three conserved phosphorylation sites, leading to their stabilization and increased ethylene biosynthesis [31]. In mpk3 mpk6 double mutant plants, ethylene production in response to Botrytis cinerea infection is greatly reduced [32]. In addition to affecting the stability of ACS2 and ACS6, MPK3 and MPK6 also promote the expression of ACS2 and ACS6 through phosphorylation of the WRKY33 transcription factor, which positively regulates the transcription of these two genes [33].
Camalexin biosynthesis
Arabidopsis MPK3/6 also regulate the biosynthesis of camalexin, a low molecular weight antimicrobial compound produced by plants in response to pathogen attacks [34, 35]. Activation of MPK3/6 by the upstream MKKs leads to camalexin biosynthesis, whereas camalexin biosynthesis in response to B. cinerea infection is almost completely abolished in mpk3 mpk6 double mutant plants, demonstrating the essential role of MPK3/6 in the induction of camalexin accumulation. The downstream regulator in this process is WRKY33, which is phosphorylated by MPK3/6. The Phosphorylation of WRKY33 is required for its activity in promoting the transcription of camalexin biosynthetic genes [35]. MPK3 and MPK6 are also involved in the up-regulation of WRKY33 transcript levels in response to pathogen infection, as WRKY33 self-regulates its transcription through binding to its own promoter [35].
Indole glucosinolate biosynthesis
Glucosinolates are nitrogen and sulphur containing secondary metabolites that are commonly found in plants belonging to the Brassicaceae family [36]. Indole glucosinolates (IGS) have been shown to be critical in plant defense against pathogens [37, 38]. MPK3/6 regulate the expression of two MYB transcription factors involved in the activation of IGS biosynthesis, promoting the biosynthesis of indole-3-yl-methylglucosinolate (I3G) and its conversion to 4-methoxyindole-3-yl-methylglucosinolate (4MI3G) in responses to B. cinerea infection [39]. MPK3/6 also regulate the expression of genes encoding enzymes involved in the conversion of I3G to 4MI3G through their target transcription factor ETHYLENE RESPONSE FACTOR6 (ERF6) [39, 40].
Stomatal immunity
Restriction of pathogen entry through stomatal apertures is important in plant defense against bacterial pathogens. Early studies using Arabidopsis transgenic plants expressing an antisense construct of MPK3 driven by a guard cell specific promoter showed that MPK3 is required for stomatal closure in response to bacteria [41]. Recently it was showed that the MKK4/5 and MPK3/6 cascade plays essential roles in stomatal closure induced by PAMPs [42]. Loss of function of MKK4/5 or MPK3/6 abolishes PAMP and pathogen-induced stomatal closure, whereas constitutive activation of MPK3/6 induces stomatal closure. Interestingly, regulation of stomatal closure by MPK3/6 is independent of the phytohormone abscisic acid, but likely involves malate metabolism, as exogenously application of malate can reverse the stomatal closure induced by MPK3/6 activation and MKK4/5 and MPK3/6 are required for pathogen-induced malate metabolism [42].
The MEKK1-MKK1/2 -MPK4 Cascade
The Arabidopsis MEKK1-MKK1/2-MPK4 cascade is one of the best studied MAPK cascades in plant immunity (Fig. 1). Initial observations of MKK1/2 interacting with both MEKK1 and MPK4 in yeast two-hybrid assays suggest that MEKK1, MKK1/2 and MPK4 may form a MAPK cascade [43, 44]. Both MKK1 and MKK2 were shown to activate MPK4 in vitro and in vivo [45–47]. In addition, MEKK1 and MKK1/2 have been shown to be essential for flg22-mediated activation of MPK4 [26, 27, 48, 49]. Bimolecular fluorescence complementation assays revealed that MEKK1 interacts with MKK1/2 on the plasma membrane whereas MKK1/2 interact with MPK4 on the plasma membrane as well as in the nucleus [26], suggesting the MEKK1-MKK1/2-MPK4 cascade transduces external signals perceived at the cell membrane to the nucleus.
Disruption of the MEKK1-MKK1/2-MPK4 cascade causes autoimmunity. Arabidopsis mpk4 mutant plants exhibit a dwarf morphology, accumulate high levels of salicylic acid, constitutively express defense marker genes and exhibit enhanced resistance to the virulent bacterial pathogen Pseudomonas syringae pv. tomato DC3000 (P.s.t. DC3000) and oomycete pathogen Hyaloperonospora arabidopsidis (H.a.) Noco2 [50]. The mkk1 and mkk2 single mutants do not show any autoimmune phenotypes, but the mkk1 mkk2 double mutants are extremely dwarfed and display constitutive defense responses [26, 27]. Similar to mpk4 and mkk1 mkk2 mutants, knockout mutants of mekk1 have extensive cell death and accumulate high levels of H2O2 [48, 49, 51]. However, mekk1 plants show a more severe phenotype than mpk4 and mkk1/2 mutants, suggesting that other MKKs and MPKs may partially compensate the loss of MKK1/2 or MPK4 [14].
One unexpected finding is that kinase dead MEKK1 (MEKK K361 M) is able to rescue the autoimmune phenotypes of mekk1 and restore flg22-induced activation of MPK4, suggesting that the kinase activity of MEKK1 is dispensable in the MEKK1-MKK1/2-MPK4 pathway [49]. It is possible that MEKK1 acts as a scaffold for MKK1/2 and MPK4 interaction and has a structural rather than a functional role in the MAPK cascade.
MAPK signaling and ETI
To overcome PTI, pathogens secrete effector proteins to evade recognition by PRRs, disrupt plant immune signalling or promote access to nutrients. For example, AvrPto and AvrPtoB target early signaling components of PTI such as the PRRs FLS2 and EFR and their co-receptor BAK1 to block PTI [52, 53]. Unlike PAMPs that are common for a variety of microbes that can be both pathogenic and non-pathogenic, effector proteins are often not conserved and only present in specific pathogens. To combat effectors, plants have evolved a large number of NLRs to sense these proteins and initiate ETI [4]. Activation of ETI often leads to ROS production, increased SA accumulation and defense gene expression as well as programmed cell death [12].
Plant immune receptors can recognize pathogen effectors either through direct protein–protein interactions or by sensing changes to host proteins that are targeted by the effectors [1]. There are multiple examples of direct interactions between NLRs and their cognate effectors from bacterial or fungal pathogens. For example, direct interaction was observed between NLR Pi-ta and Avr-Pita in rice, which activates ETI in response to infection by the blast fungus [54]. Direct interactions between NLRs and rust effectors were also observed in NLR-mediated resistance against flax rust [55]. PopP2, an effector protein from the bacterial wilt pathogen Ralstonia solanacearum, also directly interacts with RRS1-R, the corresponding NLR receptor in Arabidopsis [56].
Although recognition of pathogen effectors via direct interaction is straightforward, such mechanism requires a large number of R proteins to recognize effectors from different pathogens. It also makes it easy for the pathogen to evolve a new effector to overcome recognition. Indirect recognition of pathogen effectors, however, allows the detection of different effectors by a single immune receptor, enabling plants to defend against a variety of pathogens without having to maintain a large repertoire of R proteins [1]. This is facilitated by host proteins directly targeted or indirectly affected by the activities of pathogen effectors. The host protein monitored by the immune receptor is designated as a “guardee” or “decoy”. A “guardee”, but not “decoy”, usually has another role in immunity in addition to facilitating the recognition of pathogen effectors [57, 58]. One of the best studied examples of “guardee” is RIN4, which is guarded by two NLRs, RPM1 and RPS2 [59–61]. Three Pseudomonas effectors, AvrB, AvrRpm1 and AvrRpt2, interact with RIN4. Alteration of RIN4 phosphorylation induced by AvrB and AvrRPM1 triggers RPM1-mediated immune responses [62, 63]. Cleavage of RIN4 by AvrRpt2 activates RPS2-mediated immunity [59, 60]. In the RIN4 example, RIN4 is the point of convergence of all three effectors, allowing different effectors to be recognized by a single NLR.
Disruption of the MEKK1-MKK1/2-MPK4 cascade activates SUMM2-mediated defense responses
To understand the molecular basis of autoimmunity in mekk1, mkk1 mkk2 and mpk4 mutants, a suppressor screen was carried out in the mkk1 mkk2 mutant background. Suppressor of mkk1 mkk2 2 (summ2) mutants were found to fully suppress the dwarf morphology and constitutive immune responses of mkk1 mkk2 and mekk1 [64]. The dwarfism and elevated PR gene expression in mpk4 are largely suppressed by mutations in SUMM2, but the expression levels of PR genes in the mpk4 summ2 double mutant are still higher than in wild type, suggesting that MPK4 also regulates SUMM2-independent plant immunity [64]. SUMM2 encodes a coiled coil (CC) type of NLR. Substituting Val with Asp in the MHD motif of SUMM2 causes cell death when transiently expressed in N. benthamiana, suggesting that SUMM2 is a typical NLR receptor with ATPase activity. The identification of SUMM2 suggests that the MEKK1-MKK1/2-MPK4 cascade is under the surveillance of SUMM2. Disruption or interference of the MAPK cascade can result in activation of SUMM2-mediated immune responses.
Unexpectedly, although SUMM2 guards the MEKK1-MKK1/2-MPK4 cascade, it does not interact with MPK4. Instead, it directly interacts with Calmodulin-Binding Receptor-like Kinase 3 (CRCK3/SUMM3), another protein identified from the same suppressor screen of mkk1 mkk2 [65]. CRCK3 is required for the autoimmune phenotypes of mekk1, mpk4 and mkk1 mkk2. MPK4 interacts with and phosphorylates CRCK3. In mpk4 mutant plants, there is reduced phosphorylation of CRCK3. It is, therefore, hypothesized that CRCK3 is mainly present in its phosphorylated form in wild type plants, which binds to SUMM2, but is unable to activate SUMM2-mediate immunity. When MPK4 activity is blocked, reduced CRCK3 phosphorylation causes a conformational change of SUMM2, leading to SUMM2 activation. Such intricate mechanism of guarding a key immune regulating MAPK cascade through a MPK substrate provides more versatility compared with guarding individual proteins in the pathway.
Another SUMM gene identified from the mkk1 mkk2 suppressor screen encodes MEKK2/SUMM1, an MAPKKK closely related to MEKK1 [66]. Mutations in MEKK2 fully suppress the dwarf morphology and constitutive defense responses of mekk1 and mkk1 mkk2 mutants. The autoimmune phenotype of mpk4 is also largely dependent on MEKK2 [66, 67]. MEKK2 interacts with MPK4 via its N-terminal domain and it can be phosphorylated by MPK4 [66]. Additionally, the expression level of MEKK2 is elevated in mpk4 plants and overexpression of MEKK2 triggers SUMM2-dependent defense responses, suggesting that up-regulation of MEKK2 contributes to the defense activation in mpk4 mutants [67]. However, unlike CRCK3, MEKK2 does not interact with SUMM2. It remains to be determined how MEKK2 contributes to immunity mediated by SUMM2.
The mRNA decay factor PAT1 is another substrate of MPK4 that is also involved in SUMM2-mediated immunity [68]. pat1 knockout mutants show elevated defense gene expression and enhanced pathogen resistance, but do not exhibit severe dwarfism as seen in mekk1, mkk1 mkk2 and mpk4 plants. Interestingly, SUMM2 is required for the elevated defense responses in pat1. How the loss of PAT1 leads to SUMM2-mediated defense responses is an interesting question to be addressed in the future.
The MEKK1-MKK1/MKK2-MPK4 cascade contributes to basal resistance
While MPK4 is activated upon PAMP-treatment, it has been difficult to determine whether MPK4 is required for resistance against pathogens because of the constitutive defense phenotype in mpk4 mutants. The identification of SUMM2 made it possible to analyze the contribution of the MEKK1-MKK1/MKK2-MPK4 cascade to plant immunity without the interference of SUMM2-mediated autoimmunity. When challenged with virulent pathogens P.s.t. DC3000 and H.a. Noco2, summ2 mekk1 and summ2 mkk1 mkk2 plants exhibit increased susceptibility than summ2 and wild type plants, suggesting that MEKK1 and MKK1/2 are required for basal defense and the MEKK1-MKK1/2 -MPK4 cascade promotes plant defense against pathogens [64], which puts to rest the notion that this cascade was a negative regulator of immunity.
Currently it is unclear how the MEKK1-MKK1/2 -MPK4 cascade contributes to basal defense. Analysis of crck3 mutant plants showed that they do not exhibit enhanced disease susceptibility [65], suggesting that MPK4 likely regulates basal resistance through other target proteins such as MAP KINASE SUBSTRATE 1 (MKS1), which is phosphorylated by MPK4 both in vitro and in vivo [69]. Transgenic plants overexpressing MKS1 have increased SA levels and PR1 expression and exhibit enhanced resistance to P.s.t. DC3000 [69]. On the other hand, basal resistance against P.s.t. DC3000 and H.a. Noco2 is compromised in mks1 [70].
Consistent with the positive role in basal defense, MPK4 is required for the induction of about half of the flg22-responsive genes [71]. Interestingly, MPK4 was also found to be involved in the negative regulation of flg22-induced gene expression. ASR3 is a transcriptional repressor that negatively regulates a large subset of flg22-induced genes [72]. MPK4 phosphorylates ASR3 and enhances its DNA binding activity. In addition, transgenic plants expressing a constitutively active MPK4 mutant protein accumulate less SA following pathogen infection and exhibit enhanced susceptibility to a number of pathogens [73]. The opposing roles of MPK4 in regulating immune output are likely dependent on its diverse substrates of different functions.
Plant immune MAPK cascades are targeted by pathogen effectors
Several Pseudomonas effectors have been shown to disrupt MAPK cascades downstream of PAMP receptors. HopF2 targets MKKs to inhibit flg22-induced MPK activation and defense responses [74]. HopF2 has ADP-ribosyltransferase activity and has been shown to ADP-ribosylate MKK5 in vitro. HopAI1 directly interacts with MPK3 and MPK6 and suppresses PAMP-induced gene expression and callose deposition [75]. It inactivates MPK3/6 by removing the phosphate group from phosphothreonine via its phosphothreonine lyase activity. Another Pseudomonas effector AvrRpt2 does not affect activation of MPK3 and MPK6 in protoplast transient expression system, but it blocks flg22-induced activation of MPK4 and MPK11, which is associated with reduced defense gene expression and enhanced pathogen infection in transgenic plants expressing AvrRpt2 [76]. How AvrRpt2 affects the activation of MPK4 and MPK11 remains to be determined.
In addition to MPK3 and MPK6, HopAI1 also interacts with MPK4. Expression of HopAI1 in Arabidopsis blocks flg22-induced MPK4 activation [64]. Transgenic lines expressing HopAI1 in wild type, but not the summ2-8 background, exhibit dwarf morphology and constitutive defense responses, suggesting that HopAI1 activates SUMM2-mediated immunity [64]. Activation of SUMM2-mediated defense responses by HopAI1 suggests that SUMM2 was evolved to sense the disturbance of MAPK signaling by pathogens. In the absence of pathogen attack, CRCK3 is phosphorylated by basal levels of MPK4 activity and phosphorylated CRCK3 does not activate SUMM2-mediated immunity [65]. Inhibition of the MPK4 kinase activity by pathogen effectors such as HopAI1 results in reduced phosphorylation of CRCK3 and accumulation of unphosphorylated CRCK3, which leads to activation of SUMM2-mediated defense responses (Fig. 1). In this case, the host protein recognized by SUMM2 is not a direct target of the effectors. Such indirect sensing allows a single immune receptor to guard the entire MEKK1-MKK1/2-MPK4 kinase cascade, enabling it to sense a variety of effectors targeting MAPK signaling at different levels.
MAPK signaling and programed cell death in ETI
MAPKs are activated not only in PTI, but also in ETI. For example, following activation of N gene-mediated immunity, both WIPK and SIPK are activated in tobacco plants [77]. WIPK and SIPK are also activated in Cf9-expressing tobacco cells in response to Avr9 from Cladosporium fulvum [78]. In Arabidopsis plants, activation of RPS2-mediated immunity by AvrRpt2 results in prolonged activation of MPK3 and MPK6 [79]. However, no obvious activation of MPK3 and MPK6 was observed in Arabidopsis protoplasts transiently expressing AvrRpt2 [76].
Activation of MAPKs is often associated with cell death in plants. Expression of a constitutively active form of the MAPKK NtMEK2 activates the downstream WIPK and SIPK and causes cell death in tobacco [80, 81]. Similarly, expression of constitutive forms of Arabidopsis MKK4 and MKK5 also results in cell death in tobacco and Arabidopsis [81]. In addition, overexpression of LeMAPKKKα leads to activation of cell death in Nicotiana (N.) benthamiana and tomato [82]. Recently it was shown that transient overexpression of Arabidopsis MAPKKK5 in N. benthamiana also results in cell death [83].
Several components of MAPK cascades have been shown to play important roles in NLR-mediated immunity. Silencing Nicotiana Protein Kinase 1 (NPK1), which encodes a MAPKKK in tobacco, attenuates cell death triggered by NLRs such as N, Bs2 and Rx [84]. Silencing the tobacco MAPK NTF6 and MAPKK MEK1 also compromises N-mediated resistance to tobacco mosaic virus [85]. Cell death mediated by Pto is reduced when NbMAPKKKα or its downstream MAPKKs and MAPKs are silencing in N. benthamiana [82]. In tomato, silencing of MAPKKs LeMEK1/LeMEK2 and MAPKs LeNTF6 and LeWIPK also attenuates Pto-mediated immunity [86]. These findings suggest that MAPK signaling contributes to the activation of programed cell death and defense responses during ETI.
Concluding remarks
Genetic and biochemical analyses of MAPK cascades revealed diverse functions of these pathways in regulating plant immunity. Arabidopsis MPK3/4/6 all play critical roles in positive regulation of PTI, whereas MPK4 also contributes to negative control of immune output. The MEKK1/MKK1/2-MPK4 cascade is critical to basal resistance and is guarded by the NLR SUMM2, which monitors the phosphorylation status of the MPK4 substrate CRCK3. Disruption of this MAPK cascade by the Pseudomonas effector HopAI1 results in activation of SUMM2-mediated immunity, suggesting intricate interplays between PTI and ETI via MAPK signalling. It remains to be determined whether there are additional NLRs involved in guarding plant immune signaling MAPK cascades.
Acknowledgement
The authors would like to thank financial supports from CFI and NSERC-Discovery program.
Abbreviations
- FLS2
FLAGELLIN-SENSITIVE2
- EFR
Elongation factor (EF)-TU receptor
- BAK1
BRI1-associated receptor kinase1
- RLCKs
Receptor-like cytoplasmic kinases
- MEKK
MAPK/ERK kinase kinase
- MKK
MAP kinase kinase
- MPK
MAP kinase
- CRCK3
Calmodulin-binding receptor-like cytoplasmic kinase 3
- SUMM2
Suppressor of mkk1 mkk2 2
- MKS1
MAP kinase substrate 1
- WRKY33
WRKY DNA-binding protein 33
- ACS
1-Amino-cyclopropane-1-carboxylate synthase
References
- 1.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 2.Monaghan J, Zipfel C. Plant pattern recognition receptor complexes at the plasma membrane. Curr Opin Plant Biol. 2012;15(4):349–357. doi: 10.1016/j.pbi.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Bigeard J, Colcombet J, Hirt H. Signaling mechanisms in pattern-triggered immunity (PTI) Mol Plant. 2015;8(4):521–539. doi: 10.1016/j.molp.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Kapos P, Zhang Y. NLRs in plants. Curr Opin Immunol. 2015;32:114–121. doi: 10.1016/j.coi.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Cui H, Tsuda K, Parker JE. Effector-triggered immunity: from pathogen perception to robust defense. Annu Rev Plant Biol. 2015;66:487–511. doi: 10.1146/annurev-arplant-050213-040012. [DOI] [PubMed] [Google Scholar]
- 6.Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18(3):265–276. doi: 10.1046/j.1365-313X.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Gomez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5(6):1003–1011. doi: 10.1016/S1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 8.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125(4):749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell. 2004;16(12):3496–3507. doi: 10.1105/tpc.104.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA. 2007;104(49):19613–19618. doi: 10.1073/pnas.0705147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, Stacey MG, Stacey G. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell. 2008;20(2):471–481. doi: 10.1105/tpc.107.056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng Y, Wersch Rv, Zhang Y (2017) Convergent and divergent signaling in PAMP-triggered immunity and Effector-triggered immunity. Molecular Plant-Microbe Interactions (ja) [DOI] [PubMed]
- 13.Meng X, Zhang S. MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol. 2013;51:245–266. doi: 10.1146/annurev-phyto-082712-102314. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez MC, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol. 2010;61:621–649. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- 15.MAPK-Group Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci. 2002;7(7):301–308. doi: 10.1016/S1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Eschen-Lippold L, Lassowskat I, Böttcher C, Scheel D. Cellular reprogramming through mitogen-activated protein kinases. Frontiers Plant Sci. 2015;6:940. doi: 10.3389/fpls.2015.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science. 1995;270(5244):1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Klessig DF. Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell Online. 1997;9(5):809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiegerl S, Cardinale F, Siligan C, Gross A, Baudouin E, Liwosz A, Eklöf S, Till S, Bögre L, Hirt H. SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress–induced MAPK, SIMK. Plant Cell. 2000;12(11):2247–2258. doi: 10.1105/tpc.12.11.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonak C, Nakagami H, Hirt H. Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 2004;136(2):3276–3283. doi: 10.1104/pp.104.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardinale F, Jonak C, Ligterink W, Niehaus K, Boller T, Hirt H. Differential activation of four specific MAPK pathways by distinct elicitors. J Biol Chem. 2000;275(47):36734–36740. doi: 10.1074/jbc.M007418200. [DOI] [PubMed] [Google Scholar]
- 22.Nuhse TS, Peck SC, Hirt H, Boller T. Microbial elicitors induce activation and dual phosphorylation of the Arabidopsis thaliana MAPK 6. J Biol Chem. 2000;275(11):7521–7526. doi: 10.1074/jbc.275.11.7521. [DOI] [PubMed] [Google Scholar]
- 23.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415(6875):977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 24.Kroj T, Rudd JJ, Nürnberger T, Gäbler Y, Lee J, Scheel D. Mitogen-activated protein kinases play an essential role in oxidative burst-independent expression of pathogenesis-related genes in parsley. J Biol Chem. 2003;278(4):2256–2264. doi: 10.1074/jbc.M208200200. [DOI] [PubMed] [Google Scholar]
- 25.Sun T, Nitta Y, Zhang Q, Wu D, Tian H, Lee JK, Zhang Y (2018) Antagonistic interactions between two MAP Kinase cascades in plant development and immune signaling. EMBO Rep (in press) [DOI] [PMC free article] [PubMed]
- 26.Gao M, Liu J, Bi D, Zhang Z, Cheng F, Chen S, Zhang Y. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 2008;18(12):1190–1198. doi: 10.1038/cr.2008.300. [DOI] [PubMed] [Google Scholar]
- 27.Qiu JL, Zhou L, Yun BW, Nielsen HB, Fiil BK, Petersen K, Mackinlay J, Loake GJ, Mundy J, Morris PC. Arabidopsis mitogen-activated protein kinase kinases MKK1 and MKK2 have overlapping functions in defense signaling mediated by MEKK1, MPK4, and MKS1. Plant Physiol. 2008;148(1):212–222. doi: 10.1104/pp.108.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 29.Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F. Network properties of robust immunity in plants. PLoS Genet. 2009;5(12):e1000772. doi: 10.1371/journal.pgen.1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Ann Rev Plant Physiol. 1984;35(1):155–189. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- 31.Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16(12):3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han L, Li GJ, Yang KY, Mao G, Wang R, Liu Y, Zhang S. Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J. 2010;64(1):114–127. doi: 10.1111/j.1365-313X.2010.04318.x. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Meng X, Wang R, Mao G, Han L, Liu Y, Zhang S. Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet. 2012;8(6):e1002767. doi: 10.1371/journal.pgen.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren D, Liu Y, Yang KY, Han L, Mao G, Glazebrook J, Zhang S. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA. 2008;105(14):5638–5643. doi: 10.1073/pnas.0711301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell. 2011;23(4):1639–1653. doi: 10.1105/tpc.111.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radojčić Redovniković I, Glivetić T, Delonga K, Vorkapić-Furač J. Glucosinolates and their potential role in plant. Period Biol. 2008;110(4):297–309. [Google Scholar]
- 37.Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science. 2009;323(5910):95–101. doi: 10.1126/science.1164627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bednarek P, Piślewska-Bednarek M, Svatoš A, Schneider B, Doubský J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;323(5910):101–106. doi: 10.1126/science.1163732. [DOI] [PubMed] [Google Scholar]
- 39.Xu J, Meng J, Meng X, Zhao Y, Liu J, Sun T, Liu Y, Wang Q, Zhang S. Pathogen-responsive MPK3 and MPK6 reprogram the biosynthesis of indole glucosinolates and their derivatives in Arabidopsis immunity. Plant Cell. 2016;28(5):1144–1162. doi: 10.1105/tpc.15.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng X, Xu J, He Y, Yang KY, Mordorski B, Liu Y, Zhang S. Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell. 2013;25(3):1126–1142. doi: 10.1105/tpc.112.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gudesblat GE, Iusem ND, Morris PC. Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol. 2007;173(4):713–721. doi: 10.1111/j.1469-8137.2006.01953.x. [DOI] [PubMed] [Google Scholar]
- 42.Su J, Zhang M, Zhang L, Sun T, Liu Y, Lukowitz W, Xu J, Zhang S. Regulation of stomatal immunity by interdependent functions of a pathogen-responsive MPK3/MPK6 cascade and abscisic acid. Plant Cell. 2017;29(3):526–542. doi: 10.1105/tpc.16.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizoguchi T, Ichimura K, Irie K, Morris P, Giraudat J, Matsumoto K, Shinozaki K. Identification of a possible MAP kinase cascade in Arabidopsis thaliana based on pairwise yeast two-hybrid analysis and functional complementation tests of yeast mutants. FEBS Lett. 1998;437(1–2):56–60. doi: 10.1016/S0014-5793(98)01197-1. [DOI] [PubMed] [Google Scholar]
- 44.Ichimura K, Mizoguchi T, Irie K, Morris P, Giraudat J, Matsumoto K, Shinozaki K. Isolation of ATMEKK1 (a MAP kinase kinase kinase)-interacting proteins and analysis of a MAP kinase cascade in Arabidopsis. Biochem Biophys Res Commun. 1998;253(2):532–543. doi: 10.1006/bbrc.1998.9796. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y, Li H, Gupta R, Morris PC, Luan S, Kieber JJ. ATMPK4, an Arabidopsis homolog of mitogen-activated protein kinase, is activated in vitro by AtMEK1 through threonine phosphorylation. Plant Physiol. 2000;122(4):1301–1310. doi: 10.1104/pp.122.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuoka D, Nanmori T, Ki Sato, Fukami Y, Kikkawa U, Yasuda T. Activation of AtMEK1, an Arabidopsis mitogen-activated protein kinase kinase, in vitro and in vivo: analysis of active mutants expressed in E. coli and generation of the active form in stress response in seedlings. Plant J. 2002;29(5):637–647. doi: 10.1046/j.0960-7412.2001.01246.x. [DOI] [PubMed] [Google Scholar]
- 47.Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell. 2004;15(1):141–152. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 48.Ichimura K, Casais C, Peck SC, Shinozaki K, Shirasu K. MEKK1 is required for MPK4 activation and regulates tissue-specific and temperature-dependent cell death in Arabidopsis. J Biol Chem. 2006;281(48):36969–36976. doi: 10.1074/jbc.M605319200. [DOI] [PubMed] [Google Scholar]
- 49.Suarez-Rodriguez MC, Adams-Phillips L, Liu Y, Wang H, Su SH, Jester PJ, Zhang S, Bent AF, Krysan PJ. MEKK1 is required for flg22-induced MPK4 activation in Arabidopsis plants. Plant Physiol. 2007;143(2):661–669. doi: 10.1104/pp.106.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, Sharma SB, Klessig DF, Martienssen R, Mattsson O, Jensen AB, Mundy J. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103(7):1111–1120. doi: 10.1016/S0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 51.Nakagami H, Soukupova H, Schikora A, Zarsky V, Hirt H. A Mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem. 2006;281(50):38697–38704. doi: 10.1074/jbc.M605293200. [DOI] [PubMed] [Google Scholar]
- 52.Shan L, He P, Li J, Heese A, Peck SC, Nurnberger T, Martin GB, Sheen J. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe. 2008;4(1):17–27. doi: 10.1016/j.chom.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang TT, Zong N, Zou Y, Wu Y, Zhang J, Xing WM, Li Y, Tang XY, Zhu LH, Chai JJ, Zhou JM. Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr Biol. 2008;18(1):74–80. doi: 10.1016/j.cub.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 54.Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 2000;19(15):4004–4014. doi: 10.1093/emboj/19.15.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dodds PN, Lawrence GJ, Catanzariti AM, Teh T, Wang CI, Ayliffe MA, Kobe B, Ellis JG. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc Natl Acad Sci USA. 2006;103(23):8888–8893. doi: 10.1073/pnas.0602577103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deslandes L, Olivier J, Peeters N, Feng DX, Khounlotham M, Boucher C, Somssich I, Genin S, Marco Y. Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc Natl Acad Sci USA. 2003;100(13):8024–8029. doi: 10.1073/pnas.1230660100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van der Biezen EA, Jones JD. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem Sci. 1998;23(12):454–456. doi: 10.1016/S0968-0004(98)01311-5. [DOI] [PubMed] [Google Scholar]
- 58.van der Hoorn RAL, Kamoun S. From Guard to Decoy: a new model for perception of plant pathogen effectors. Plant Cell. 2008;20(8):2009–2017. doi: 10.1105/tpc.108.060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Axtell MJ, Staskawicz BJ. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell. 2003;112(3):369–377. doi: 10.1016/S0092-8674(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 60.Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell. 2003;112(3):379–389. doi: 10.1016/S0092-8674(03)00040-0. [DOI] [PubMed] [Google Scholar]
- 61.Mackey D, Holt BF, Wiig A, Dangl JL. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell. 2002;108(6):743–754. doi: 10.1016/S0092-8674(02)00661-X. [DOI] [PubMed] [Google Scholar]
- 62.Liu J, Elmore JM, Lin ZJ, Coaker G. A receptor-like cytoplasmic kinase phosphorylates the host target RIN4, leading to the activation of a plant innate immune receptor. Cell Host Microbe. 2011;9(2):137–146. doi: 10.1016/j.chom.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung EH, da Cunha L, Wu AJ, Gao Z, Cherkis K, Afzal AJ, Mackey D, Dangl JL. Specific threonine phosphorylation of a host target by two unrelated type III effectors activates a host innate immune receptor in plants. Cell Host Microbe. 2011;9(2):125–136. doi: 10.1016/j.chom.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z, Wu Y, Gao M, Zhang J, Kong Q, Liu Y, Ba H, Zhou J, Zhang Y. Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe. 2012;11(3):253–263. doi: 10.1016/j.chom.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z, Liu Y, Huang H, Gao M, Wu D, Kong Q, Zhang Y. The NLR protein SUMM2 senses the disruption of an immune signaling MAP kinase cascade via CRCK3. EMBO Rep. 2017;18(2):292–302. doi: 10.15252/embr.201642704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong Q, Qu N, Gao M, Zhang Z, Ding X, Yang F, Li Y, Dong OX, Chen S, Li X, Zhang Y. The MEKK1-MKK1/MKK2-MPK4 kinase cascade negatively regulates immunity mediated by a mitogen-activated protein kinase kinase kinase in Arabidopsis. Plant Cell. 2012;24(5):2225–2236. doi: 10.1105/tpc.112.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su SH, Bush SM, Zaman N, Stecker K, Sussman MR, Krysan P. Deletion of a tandem gene family in Arabidopsis: increased MEKK2 abundance triggers autoimmunity when the MEKK1-MKK1/2-MPK4 signaling cascade is disrupted. Plant Cell. 2013;25(5):1895–1910. doi: 10.1105/tpc.113.112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roux ME, Rasmussen MW, Palma K, Lolle S, Regue AM, Bethke G, Glazebrook J, Zhang W, Sieburth L, Larsen MR, Mundy J, Petersen M. The mRNA decay factor PAT1 functions in a pathway including MAP kinase 4 and immune receptor SUMM2. EMBO J. 2015;34(5):593–608. doi: 10.15252/embj.201488645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andreasson E, Jenkins T, Brodersen P, Thorgrimsen S, Petersen NH, Zhu S, Qiu JL, Micheelsen P, Rocher A, Petersen M, Newman MA, Bjorn Nielsen H, Hirt H, Somssich I, Mattsson O, Mundy J. The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J. 2005;24(14):2579–2589. doi: 10.1038/sj.emboj.7600737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petersen K, Qiu J-L, Lütje J, Fiil BK, Hansen S, Mundy J, Petersen M. Arabidopsis MKS1 is involved in basal immunity and requires an intact N-terminal domain for proper function. PLoS One. 2010;5(12):e14364. doi: 10.1371/journal.pone.0014364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frei dit Frey N, Garcia AV, Bigeard J, Zaag R, Bueso E, Garmier M, Pateyron S, de Tauzia-Moreau ML, Brunaud V, Balzergue S, Colcombet J, Aubourg S, Martin-Magniette ML, Hirt H. Functional analysis of Arabidopsis immune-related MAPKs uncovers a role for MPK3 as negative regulator of inducible defences. Genome Biol. 2014;15(6):R87. doi: 10.1186/gb-2014-15-6-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li B, Jiang S, Yu X, Cheng C, Chen S, Cheng Y, Yuan JS, Jiang D, He P, Shan L. Phosphorylation of trihelix transcriptional repressor ASR3 by MAP KINASE4 negatively regulates Arabidopsis immunity. Plant Cell. 2015;27(3):839–856. doi: 10.1105/tpc.114.134809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berriri S, Garcia AV, Frei dit Frey N, Rozhon W, Pateyron S, Leonhardt N, Montillet JL, Leung J, Hirt H, Colcombet J. Constitutively active mitogen-activated protein kinase versions reveal functions of Arabidopsis MPK4 in pathogen defense signaling. Plant Cell. 2012;24(10):4281–4293. doi: 10.1105/tpc.112.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Li J, Hou S, Wang X, Li Y, Ren D, Chen S, Tang X, Zhou JM. A Pseudomonas syringae ADP-ribosyltransferase inhibits Arabidopsis mitogen-activated protein kinase kinases. Plant Cell. 2010;22(6):2033–2044. doi: 10.1105/tpc.110.075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, Chen S, Tang X, Zhou JM. A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe. 2007;1(3):175–185. doi: 10.1016/j.chom.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 76.Eschen-Lippold L, Jiang X, Elmore JM, Mackey D, Shan L, Coaker G, Scheel D, Lee J. Bacterial AvrRpt2-like cysteine proteases block activation of the arabidopsis mitogen-activated protein kinases, MPK4 and MPK11. Plant Physiol. 2016;171(3):2223–2238. doi: 10.1104/pp.16.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang S, Klessig DF. Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc Natl Acad Sci. 1998;95(13):7433–7438. doi: 10.1073/pnas.95.13.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JD. Rapid Avr9-and Cf-9–dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell. 1999;11(2):273–287. doi: 10.1105/tpc.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsuda K, Mine A, Bethke G, Igarashi D, Botanga CJ, Tsuda Y, Glazebrook J, Sato M, Katagiri F. Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genet. 2013;9(12):e1004015. doi: 10.1371/journal.pgen.1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang K-Y, Liu Y, Zhang S. Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci. 2001;98(2):741–746. doi: 10.1073/pnas.98.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ren D, Yang H, Zhang S. Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem. 2002;277(1):559–565. doi: 10.1074/jbc.M109495200. [DOI] [PubMed] [Google Scholar]
- 82.del Pozo O, Pedley KF, Martin GB. MAPKKKα is a positive regulator of cell death associated with both plant immunity and disease. EMBO J. 2004;23(15):3072–3082. doi: 10.1038/sj.emboj.7600283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamada K, Yamaguchi K, Shirakawa T, Nakagami H, Mine A, Ishikawa K, Fujiwara M, Narusaka M, Narusaka Y, Ichimura K. The Arabidopsis CERK1-associated kinase PBL27 connects chitin perception to MAPK activation. EMBO J. 2016;35(22):2468–2483. doi: 10.15252/embj.201694248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jin H, Axtell MJ, Dahlbeck D, Ekwenna O, Zhang S, Staskawicz B, Baker B. NPK1, an MEKK1-like mitogen-activated protein kinase kinase kinase, regulates innate immunity and development in plants. Dev Cell. 2002;3(2):291–297. doi: 10.1016/S1534-5807(02)00205-8. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y, Schiff M, Dinesh-Kumar S. Involvement of MEK1 MAPKK, NTF6 MAPK, WRKY/MYB transcription factors, COI1 and CTR1 in N-mediated resistance to tobacco mosaic virus. Plant J. 2004;38(5):800–809. doi: 10.1111/j.1365-313X.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- 86.Ekengren SK, Liu Y, Schiff M, Dinesh-Kumar S, Martin GB. Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J. 2003;36(6):905–917. doi: 10.1046/j.1365-313X.2003.01944.x. [DOI] [PubMed] [Google Scholar]


