Abstract
Mesenchymal stem cells (MSCs) are isolated from multiple biological tissues—adult bone marrow and adipose tissues and neonatal tissues such as umbilical cord and placenta. In vitro, MSCs show biological features of extensive proliferation ability and multipotency. Moreover, MSCs have trophic, homing/migration and immunosuppression functions that have been demonstrated both in vitro and in vivo. A number of clinical trials are using MSCs for therapeutic interventions in severe degenerative and/or inflammatory diseases, including Crohn’s disease and graft-versus-host disease, alone or in combination with other drugs. MSCs are promising for therapeutic applications given the ease in obtaining them, their genetic stability, their poor immunogenicity and their curative properties for tissue repair and immunomodulation. The success of MSC therapy in degenerative and/or inflammatory diseases might depend on the robustness of the biological functions of MSCs, which should be linked to their therapeutic potency. Here, we outline the fundamental and advanced concepts of MSC biological features and underline the biological functions of MSCs in their basic and translational aspects in therapy for degenerative and/or inflammatory diseases.
Keywords: Mesenchymal stem/stromal cells, Cell identity, Cell functions, Cell therapy
Introduction
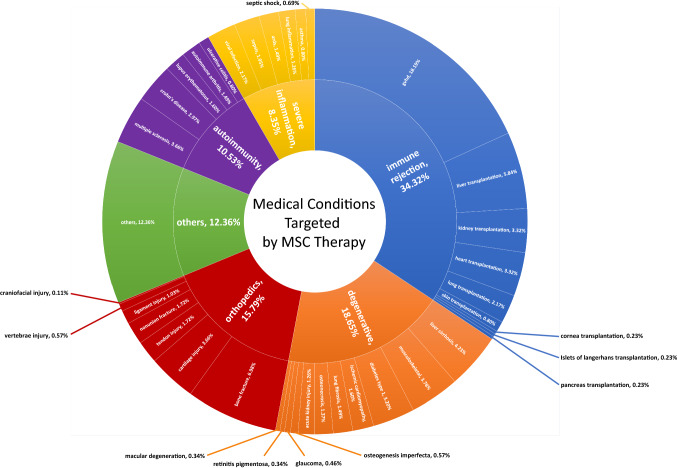
Hundreds of clinical trials are now using mesenchymal stem cells (MSCs) to test therapeutic interventions for numerous severe diseases, alone or in combination with other drugs [1–4]. These trials are designed mostly (Fig. 1) for treatment in (1) orthopedics (e.g., non-union bone fracture, craniofacial trauma); (2) degenerative diseases of the skeletal system (e.g., osteonecrosis, osteogenesis imperfecta), eyes (e.g., glaucoma, macular degeneration, retinitis pigmentosa), heart (e.g., ischemic cardiomyopathy), kidney (e.g., acute kidney injury), liver (e.g., liver cirrhosis), lung (e.g., pulmonary fibrosis) or multiple organs (e.g., diabetes complications); (3) autoimmunity affecting the skeletal system (e.g., osteoarthritis, rheumatoid arthritis), brain and spinal cord (e.g., multiple sclerosis), gastrointestinal tract (e.g., Crohn’s disease, ulcerative colitis), pancreas (e.g., diabetes type 1) or multiple organs (e.g., systemic lupus erythematosus); (4) inflammatory diseases of the lung (e.g., acute respiratory distress syndrome, chronic obstructive pulmonary disease) or multiple organs (e.g., sepsis); as well as (5) immune rejection in allogeneic transplantation [e.g., graft-versus-host disease (GvHD), solid organ rejection] [1–3, 5].
Fig. 1.
Medical conditions targeted by MSC therapy. Diagram of the conditions targeted by MSCs by proportion of trials. Data were obtained using a recurrent search of keywords for medical conditions (appearing in the diagram) associated with mesenchymal stem/stromal cells at ClinicalTrials.gov. The search was completed on April 2019. The group “medical conditions for degenerative disorders” represents about 18% of total trials, and inflammatory disorders combined (autoimmune diseases, transplant immune rejection, severe inflammatory diseases) represent about 45% of all trials and most of the clinical trials of MSCs for treatment
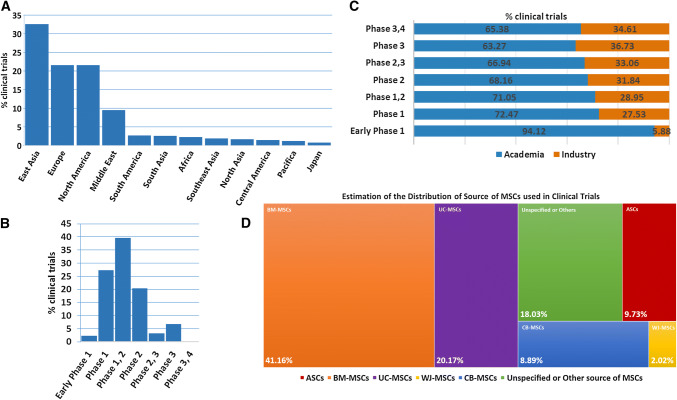
Most clinical studies are registered in the database of the US National Institutes of Health (NIH) (https://clinicaltrials.gov/). Such clinical assays are undertaken worldwide in university hospitals and biomedical institutions, principally in China, the European Union and the United States (Fig. 2a). Of note, most studies are in the early phases, typically phase 1 or 1/2; fewer are in phase 2 and even fewer in more advanced phases (Fig. 2b).
Fig. 2.
Worldwide usage and progress of MSC therapy. Proportion of clinical trials of MSCs by location. Data were obtained by a search of ClinicalTrials.gov completed on June 2018. a East Asia (mostly China) followed by Europe and North America (mostly United States) are the locations of 70% of all clinical trials investigating MSCs as treatment. b Phase 1 and 2 clinical trials of MSCs as treatment represent about 85% of the total number, whereas more advanced clinical trials in phases 3 and 4 represent less than 10%. c Increased proportion of pharmaceutical industry-sponsored clinical trials investigating MSCs as treatment progressing to the advanced phase. Academia-sponsored clinical trials represent most of the clinical trials of MSCs as treatment, about 60% at least. d Sources of MSCs by proportion of clinical trials. Data were obtained using a recurrent search of keywords for sources of MSCs in trials (appearing in the diagram) at ClinicalTrials.gov. The search was completed on June 2018. Bone marrow-derived MSCs (BM-MSCs) are investigated in about 40% of total trials, followed by umbilical cord-derived MSCs (UC-MSCs) at 20% and adipose tissue-derived MSCs (ASCs) at 10%
The available data support the safety of MSC therapy with both autologous and allogeneic MSCs, but actual data on the efficacy of MSC therapy are often preliminary [2]. However, MSCs embody a biological material for cell therapy that is safe, barely immunogenic and of immediate applicability in diseases [1–3, 5, 6]. Regardless, clinical practice requires better coordination of the characterization, production, and delivery of MSCs [2, 7–11]. Advanced-phase clinical trials expect to develop MSC therapy (Fig. 2c), which implies an increasing number of pharmaceutical biotechnologies [2, 12]. Yet, there are obstacles to the development of MSC therapy [1–4, 6, 8–11]; indeed, substantial clinical assays, with publicly disclosed results, have shown insufficient outcomes, with inconstant therapeutic benefits in diseases such as acute GvHD [1–3]. Recent research has progressed to further define MSC functions and modes of actions that should reflect therapeutic potentials of MSCs [13–17]. In this review, we attempt to outline the essential and advanced concepts in the biology of MSCs, especially MSC biological functions in their fundamental and translational aspects in degenerative and/or inflammatory diseases.
MSC biological concept
The Friedenstein group, in the 1960s and 1970s, demonstrated that only a marginal cell subset residing among rodent bone marrow cells had osteogenic abilities [18–23]. This bone marrow cell subset was defined as adherent colony-forming unit fibroblasts (CFU-Fs) in vitro in contrast to non-adherent hematopoietic CFU cells (CFU-Cs) [18, 21, 24–26]. CFU-Fs were initially considered to produce cells associated with skeletal tissue, that is, stem/progenitor cells [18, 22, 23, 27–29], but were also considered feeder cells for ex vivo culture of hematopoietic stem cells (HSCs), or stroma cells [19, 28–33]. The CFU-F designation then evolved into other terms that were supposed to best define the biology of the cells, based on cell functions, such as “osteogenic stem cells” or “bone-marrow stromal cells” [29, 34]. Furthermore, these biological concepts were substantiated in other species, including in humans [24, 27, 35–41]. Later, the general notion of adult “mesenchymal stem cells”, first proposed by Caplan et al., emerged by accommodating the concept of cells originating from the embryonic mesoderm [42, 43]. Of note, the appellation adult “mesenchymal stem cells” is still imprecise from strict biological opinion [13, 44, 45] but has endured and is widely used by scientists and clinicians [44–46]. However, the designation MSCs are often debated and/or further described with the terms “stem” and “stromal” combined, whereas the term “multipotent” is sometimes preferred to “mesenchymal”, for “mesenchymal stem/stromal cells” or “multipotent stem/stromal cells”. The hesitancy on strict denomination attests to the uncertainties of MSC identity and functions [13, 45, 47–49].
The concept of MSCs suggests the existence in vivo of stem and/or progenitor subsets within adult or neonatal tissues that sustain the homeostasis of other stem and/or progenitor cells while being able to provide de novo-specialized cells of mesodermal lineage [13, 48, 50]. MSC functions in vivo were believed to regulate the homeostasis of HSCs by producing trophic factors and favoring wound healing by differentiating into tissue-specific cells [13, 50–52]. In 1995, Lazarus et al. envisioned the use of MSCs as cell therapy similar to bone marrow transplant [48]. Later, in the 2000s, other studies drew attention to further MSC functions, namely homing/migration [53–62] and immunosuppression [63–68]. Thereafter, MSC therapy has been investigated extensively in both preclinical and clinical settings to evaluate its therapeutic effect in degenerative and/or inflammatory diseases lacking appropriate treatments [1–3, 5, 11, 69].
Here, we discuss the fundamental biology and translational advances regarding MSCs isolated from human adult or neonatal tissues, expanded in vitro and used as therapeutics directly after thawing MSCs from frozen batches or indirectly by harvesting “fresh” cells after continuous culture of MSCs that are delivered by topical or systemic adoptive transfer in autologous, allogeneic or xenogeneic contexts. First, we briefly consider elements of sources of human MSCs.
Adult and neonatal tissue source of MSCs
MSCs are typically obtained from adult bone marrow and adipose tissue (Fig. 2d); neonatal tissue such as umbilical cord is also commonly used to obtain MSCs [70]. MSCs in vivo may be confined to a marginal cell population that supposedly exists in all organs containing a perivascular niche because of the expression of stromal cell surface marker 1 (Stro-1) and/or α-smooth muscle actin (α-SMA) in all MSCs regardless of their source [71]. This population represents an estimated 0.00001% of bone marrow cells and up to 1% or more of adipose tissue cells [70, 72–82]. MSCs in umbilical cord likely represent a cell frequency comparable to or below that found among adult bone marrow cells but with better expandability in vitro as compared with their adult counterparts because of their fetal nature [80]. Still, bone marrow as a source of MSCs remains the most valued because this source is better documented and largely used in both preclinical and clinical research [83]. Therefore, MSCs derived from bone marrow (BM-MSCs) are considered a paragon of MSCs [72, 84].
BM-MSCs are isolated from total marrow obtained from the iliac crest of the pelvic bone of healthy donors. This is an invasive method that requires anesthesia and implies nosocomial infection hazards; it is now used mostly for BM-MSCs intended for clinical use [73, 81, 85, 86]. Total bone marrow from femoral heads obtained during orthopedic surgery with femur head and neck osteotomy is also a source of BM-MSCs but solely for preclinical research use [81, 85, 86]. Isolation of MSCs from total bone marrow involves density gradient centrifugation, with collection of the mononuclear cell fraction. To isolate MSCs by adherence and expansion, the mononuclear cells are seeded in culture dishes at low density, about 103–104 cells/cm2, but more commonly at 105 cells/cm2 and can reach up to 106 cells/cm2 [74, 81, 87].
MSCs from adipose tissue (ASCs) are isolated from tissue samples obtained after medical interventions involving liposuction or lipectomy. Adipose tissues are obtained from patients by aspirating or excising visceral or subcutaneous fat tissue located in the abdomen, brachium, femoral, or gluteal areas [88]. Furthermore, ASC isolation involves enzymatic digestion of fat tissue samples with collagenases, then red blood cell (RBC) removal with specific RBC lysis followed by cell filtration. Of note, methods for expanding ASCs are similar to those used for BM-MSCs. Today, adipose tissue is increasingly used as a source for MSCs, mostly because of its natural abundance of MSCs and also the less invasive surgical measures for obtaining adipose tissue as compared with bone marrow tissue and so is ideal for clinical use [72, 81, 82, 86, 87, 89].
MSCs may be isolated from neonatal tissues, especially umbilical cord, which is easily accessible. Whole umbilical cord or its individual biological compartments can be a source of MSCs [70, 90]. MSCs can be isolated from whole umbilical cord, containing conjunctive tissue, Wharton’s jelly tissue and vasculature. Conversely, they can be isolated from Wharton’s jelly after removal of blood vessels and residual conjunctive tissues from umbilical cord. Alternatively, MSCs can be isolated specifically from umbilical cord blood (i.e., fetal blood within umbilical vasculature [90]). Cell biology methods used for isolating MSCs from umbilical cord vary depending on the umbilical cord compartment chosen as a source. Typically, these methods may include enzymatic digestion of umbilical-cord tissue samples, RBC-specific lysis, cell filtration, and/or density gradient separation [74, 86, 90]. The procedures for isolation/expansion are similar for adult and neonatal MSCs. The existence of various umbilical compartments for sourcing MSCs suggests differences in MSC yield, and in fact whole umbilical cord and Wharton’s jelly tissue are superior to umbilical cord blood in terms of quantity of obtainable MSCs [70, 91].
The tissues described above for sources of MSCs seem somewhat disparate, but they are not entirely unlike each other because bone marrow, adipose tissue, and umbilical cord share biological similarities [80]. To exemplify, bone marrow resembles adipose tissue because in adults, bone marrow consists of nearly 30–70% adipose tissue, known as yellow marrow or marrow adipose tissue (MAT), but adipose tissue in other anatomical areas typically consists of white adipose tissue (WAT) [92]. The function of MAT is not yet definitively established. However, although MAT and WAT have unique specificities [92], MAT exhibits certain WAT properties such as lipid-storage and endocrine functions [92]. Moreover, MSCs are derived from both bone marrow (containing MAT) and WAT, and both derived MSCs fit ISCT criteria. Thus, MAT and WAT could be considered tissues sharing certain biological features and likely cellular contents, including MSCs. Moreover, bone marrow, adipose tissue, and umbilical cord consist of connective tissues with perivascular niches where MSCs are thought to reside [93].
Furthermore, MSCs have been isolated from multiple other adult tissue including bursa [94], dental pulp [77], dermis [95], gingival tissue [96], ligaments [97], peripheral blood [98], and synovium [99] as well as other neonatal tissue such as placenta [100]. Of note, MSCs may be obtained via in vitro differentiation of human induced pluripotent stem cells [101]. However, these last MSC sources are not much used for MSC therapy.
MSC culture methods, cryopreservation and standardization needs
Unrelated to sourcing, MSCs are produced in vitro with rather similar culture methods. Mononuclear cells isolated from biological tissues are suspended in derivatives of Eagle’s medium (α-MEM or DMEM) supplemented with fetal bovine serum (FBS)- or human-derived supplement such as serum AB or platelet derivatives such as platelet lysates or platelet-rich plasma with or without additional factors. Of note, the composition of serum and their derivatives are ill defined and, therefore, these products must often be screened for their efficacy in MSC culture, for sustaining and promoting cell proliferation of MSCs without affecting their undifferentiated state [102].
Importantly, to meet standards of good manufacturing production and to satisfy demands for the highest safety, quality and quantity of MSCs, MSC culture systems must be optimized and standardized [2, 5, 103]. Especially, attempts to improve MSC culture include (1) privileging advanced stringent aseptic methods, (2) using hypoxic conditions mimicking the native microenvironment of MSCs, (3) avoiding non-human products (i.e., xenogeneic-free medium) and (4) restraining undefined medium composition (e.g., by promoting the use of serum-free medium) [2, 87, 104–108]. Furthermore, MSCs are subcultured using standard cell culture systems or by large-scale bioprocessing with high-capacity bioreactors intended for extensive cell production yield to meet therapeutic demands [86, 104, 105, 109–112]. Especially, bioprocesses with large-scale bioreactors are culture methods used for producing MSCs with pharmaceutical biotechnologies conferring improved yield and reduced production costs [103, 106, 112]. Yet, this large-scale production of MSCs needs to be standardized for reliability and require even more stringent post-production quality controls of MSC products for consistency, efficacy, and safety [103, 106].
In addition, various cryopreservation procedures for MSCs used in academic and pharmaceutical laboratories require further optimization and standardization [113]. Concerns exist about the therapeutic capabilities of extemporaneous freeze–thawed MSCs and MSCs harvested from continuous cultures [8]. Thus, immediately thawed MSC products might show attenuated therapeutic effects as compared with freshly cultured MSCs [8]. Currently, no clear consensus has emerged for the MSCs used in therapy [114], but the trend is to use immediately thawed MSC products in the clinic because of the ease of use and readiness of frozen off-the-shelf MSC products [2, 115].
Practices in culture, cryopreservation and clinical usage of MSC products require standards because current practices among laboratories remain inconsistent, in both preclinical and clinical settings, and these fluctuating practices may affect the identity and functions of MSCs [2, 8].
MSC identity and functions
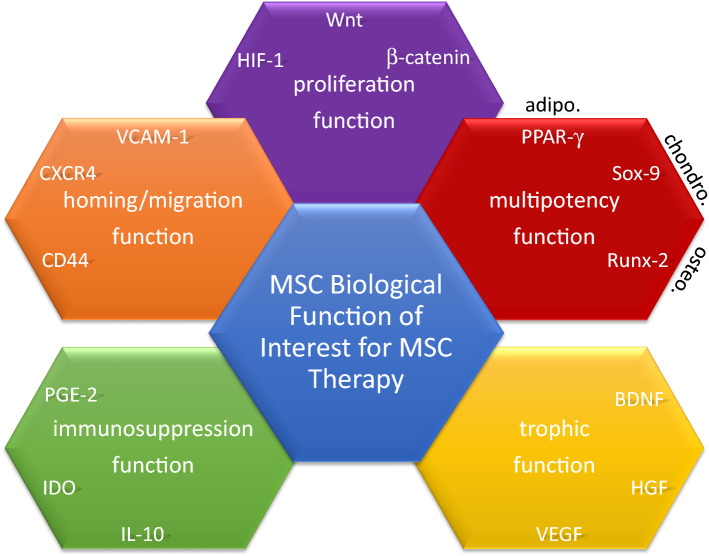
MSCs in culture are thought to contain diverse cell subsets resulting from intrinsic and extrinsic influences in addition to inherent disparities related to different sources and donors [16, 116–119]. Cell heterogeneity is expected in MSC cultures for use in preclinical and clinical settings [116, 118–123]. MSCs in culture include multipotent cells and/or diverse but coherent committed progenitors [21]. MSCs from different sources may not be all alike, but MSC cultures might share common features in agreement with the International Society of Cell Therapy (ISCT) criteria established in 2006 [11, 119, 124]. Of note, ISCT proposed minimal criteria to define MSCs: (1) MSCs must be adherent cells showing a spindle-shape morphology in standard culture conditions; (2) MSCs must show cell surface expression of cluster of differentiation (CD)105, CD73 and CD90 but not CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR antigens; and (3) MSCs must differentiate to osteoblasts, adipocytes and chondroblasts in vitro following a definite stimulation [124]. Originally, these nominal principles were intended to homogenize the depiction of MSCs among research laboratories. Today, these criteria require modification with new knowledge of MSCs [1, 10, 49, 125]. Particularly, surface antigens that identify MSCs in vivo and/or in vitro remain to be elucidated. Indeed, surface antigens that conceivably relate to stemness, including Stro-1, stage-specific embryonic antigen (SSEA)-1, SSEA-4, CD271, and CD146, have been examined but are not satisfactory because of the wide variation in expression of these antigens depending on the source of MSCs [75, 126]. Meanwhile, thorough transcriptomic and functional analysis of MSCs from different biological sources have revealed transcriptional signatures that differ among cultures of MSCs from different sources, with close but nonetheless different differentiation abilities [11, 16, 119–123, 127]. The identity of MSCs is not yet clear; thus, defining the biological functions of MSCs that would support identification of MSCs with therapeutic interests is vital. Hereafter, we discuss MSC functions (Fig. 3) including proliferation, multipotency, trophic ability, homing/migration and immunosuppression in fundamental aspects and their clinical implications.
Fig. 3.
Biological functions of MSCs. The five biological functions of MSCs with interest in therapeutics: (1) proliferation function, (2) multipotency function, (3) homing/migration function, (4) trophic function, and (5) immunosuppression function. The diagram gives three representative molecules involved in each of these functions. HIF-1 hypoxia-inducible factor 1, Wnt wingless type, PPAR-γ2 peroxisome proliferator-activated receptor 2, SOX9 sex-determining region of the Y chromosome-box 9, RUNX-2 runt-related transcription factor 2, Adipo adipocyte, Chondro chondroblast, Osteo osteoblast, BDNF brain-derived neurotrophic factor, HGF hepatocytes growth factor, VEGF vascular endothelial growth factor, IL interleukin, IDO indoleamine 2,3 dioxygenase, PGE2 prostaglandin E2, CD cluster of differentiation, CXCR CXC chemokine receptor, VCAM-1 vascular cell adhesion molecular 1
Mesenchymal stem cell proliferation function
A cell must be able to proliferate for self-renewal and cell expansion, which is related to “stemness” [108, 128]. MSCs are proliferative in vitro but retain a fibroblast-like morphology. Early in culture, the proliferation function of MSCs (Table 1) seems tightly controlled under low activity of wingless type (Wnt)/β-catenin signaling [129]. Moreover, the availability of O2 regulates MSC proliferation by modulating the transcription factor (TF) hypoxia-inducible factor 1, which permits the expression of genes controlling cell cycle progression [130, 131]. Hence, hypoxic culture conditions in vitro (O2 < 10%) improve MSC proliferation by mirroring the usual O2 strain conditions in vivo. Furthermore, in vivo hypoxic conditions protect mitochondria physiology by decreasing the oxidative metabolism needs of MSCs in contrast to atmospheric normoxia (O2 > 20%), with in vitro-expanded MSCs subjected to elevated oxidative stress, thereby impeding MSC proliferation [131].
Table 1.
Pathways of MSC proliferation function
MSCs are proliferative in vitro, but their proliferation ability decreases with time of culture along with a lack of telomerase activities and modifications in cell morphology [132, 133]. Decreased cell proliferation abilities of MSCs appear as an archetypical cell senescence, with progressive loss of proliferation and cell cycle arrest [134]. Even with senescence, the phenotype of MSCs remains rather unchanged and with virtually no genetic disturbance or chromosomal instability [133]. Yet, senescence-associated DNA methylations are identified on specific CpG sites and seem to be the typical epigenetic signature of senescent MSCs [135]. MSCs exposed to overwhelming stimuli, including metabolic stress and/or attempts to repair genomic DNA damage during in vitro expansion, might promote senescence, which is likely a defense against cell death or genetic subversion [133, 135]. Of note, the frequency of senescence occurring in MSCs can be intrinsically influenced by the origin of MSCs from different tissues and different donors [133, 135]. Other than the expansion concerns of MSCs, the significance of senescence in terms of further upheaval of other MSC functions and therapeutic potency of MSCs are unclear but are drawing increasing interest [136].
Mesenchymal stem cell multipotency function
MSCs differentiate into adipocytes, chondroblasts and osteoblasts under a definite stimulation in vitro (Table 2); the differentiation is perceived morphologically and/or with specific expression of biomarkers [73, 124, 137]. MSCs undergo an overhaul of intracellular signaling and transcriptional modifications, possibly in vivo depending on the biological conditions or during in vitro manipulations [15]. Manipulation of MSCs in vitro includes use of diverse molecules such as chemicals, cytokines, hormones, vitamins and/or mechanical/physical supports by means of scaffold biomaterials [40, 73, 124, 137, 138].
Table 2.
Pathways of MSC multipotency function
| MSC multipotency function | Adipocyte | Chondroblast | Osteoblast | References |
|---|---|---|---|---|
| Upstream molecular pathways | ||||
| BMP-2, -4, -6 | − | + | + | Sekiya et al. [151], Friedman et al. [149] |
| EGF | − | − | + | Kratchmarova et al. [303], Platt et al. [304] |
| FGF-2 | − | + | + | Chiou et al. [143], Miraoui et al. [305] |
| IGF-1 | + | + | − | Scavo et al. [306], Indrawattana et al. [148] |
| TGF-β1, -β3 | − | + | − | Roelen et al. [307], Maeda et al. [308] |
| Intracellular signaling pathways | ||||
| HH | − | + | + | Fontaine et al. [156], Oliveira et al. [309] |
| MAPK | − | + | + | Chang et al. [310], Celil et al. [152] |
| Notch | − | − | + | Oldershaw et al. [157], Ugarte et al. [314] |
| Smad-3, -4 | − | + | + | Furumatsu et al. [147], Zhou et al. [312] |
| Wnt-3a, -7a/β-catenin | + | + | + | Tuli et al. [142], De Boer et al. [129] |
| Downstream transcription factor | ||||
| C/EBP-α/β | + | − | − | Qian et al. [313], Cristancho et al. [140] |
| Osterix/Sp7 | − | − | + | Celil et al. [152], Zhu et al. [317] |
| PPAR-γ2 | + | − | − | Cristancho et al. [140], Yu et al. [316] |
| RUNX-2 | − | − | + | Xu et al. [315], Thiagarajan et al. [314] |
| SOX9 | − | + | − | Indrawattana et al. [148], Furumatsu et al. [147] |
The activity status of most critical upstream molecular pathways, intracellular signaling and downstream transcription factors essential for the multipotency function of MSCs differentiating into adipocytes, chondroblasts and osteoblasts. Data are summarized from references [129, 140, 142, 143, 147–149, 151, 152, 156, 157, 303–317]
EGF epidermal growth factor, FGF-2 fibroblast growth factor 2, IGF-1 insulin-like growth factor 1, TGF-β1 or -β3 transforming growth factor β1 or β3, HH hedgehog, MAPK mitogen-activated protein kinase, Wnt-3a or -7a wingless type 3a or 7a, PPAR-γ2 peroxisome proliferator-activated receptor 2, C/EBP-α/β CCAAT/enhancer-binding protein α, RUNX-2 runt-related transcription factor 2, SOX9 sex-determining region of the Y chromosome-box 9
Adipogenesis is typically achieved by stimulating MSCs with dexamethasone, insulin, isobutylmethylxanthine, and indomethacin in vitro [73, 139]. MSCs differentiating into adipocytes is revealed by lipid vacuoles that gradually form a single large vacuole in terminally differentiated adipocytes [73, 139]. Furthermore, adipogenesis can be assessed by enzyme expression and/or activity of lipoprotein lipase and the accumulation of fatty acid-binding protein adipocyte P2 in mature adipocytes [73, 139]. Mainly, the action of Wnt/β-catenin signaling is required for commitment of MSCs into preadipocytes, whereas in later stages, the inactivation of the Wnt/β-catenin pathway seems necessary to complete the maturation of adipocytes [139]. Thus, the Wnt/β-catenin pathway affects the downstream action of specific TFs such as CCAAT/enhancer-binding protein α/β (C/EBP-α/β) and peroxisome proliferator-activated receptor γ2 (PPAR-γ2). Both C/EBP-α/β and PPAR-γ2 activities are essential during early and late stages of adipogenesis [139, 140].
Chondrogenesis in vitro ensues usually with MSCs placed in aggregate or pellet cultures and stimulated with transforming growth factor-β1 or -β3 (TGF-β1 or -β3), and/or insulin-like growth factor 1 (IGF-1), fibroblast growth factor 2 (FGF-2), or bone morphogenic protein 2 (BMP-2) [73, 141–145]. FGF-2 facilitates chondrogenesis of aggregated MSCs stimulated with TGF-β1 or -β3 and/or IGF-1 [143, 144]. Particularly, FGF-2 alone does not induce chondrogenesis; rather, FGF-2 enables chondrogenesis by upregulating FGF-R2 and transcription factor SOX9 [143]. During chondrogenesis, progenies gradually produce sulfated proteoglycans such as aggrecan and type II and IX collagen, with the development of a distinctive chondroblast cell morphology [73, 141]. MSC differentiation into chondroblasts is regulated by molecular pathways including Wnt/β-catenin, TGF-βs, hedgehog (HH), BMPs, and FGFs [139, 146]. Together, activation of these upstream signaling pathways converges to dictate the proper action of TFs belonging to the sex-determining region of the Y chromosome-box (SOX) family, notably not only SOX9, but also SOX5 and SOX6, which are required for completion of chondrogenesis [146–148].
Osteogenesis can be achieved in vitro by stimulating MSCs with ascorbic acid, β-glycerophosphate, vitamin D3 and/or BMP-2, -4, -6 and -7 [73, 149]. MSCs commit to osteoblast progenies with increasing activity of alkaline phosphatase L (ALPL) isoform (also known as tissue non-specific isoform or liver, kidney, or bone isoform) and calcium deposition, progressively assuming the morphology and phenotype of osteoblasts [73, 150, 151]. Osteogenic differentiation of MSCs implies multiple signaling pathways, which ultimately depends mostly on the action of the TF runt-related transcription factor 2 (RUNX-2) associated with other specific TFs such as Osterix/SP7 [152–154]. RUNX-2 acts to modify transcription in favor of bone-related gene expression and is regulated by upstream pathways, especially Wnt/β-catenin, HH, Notch and BMPs [142, 151, 155–157].
Other studies suggested that MSCs may also differentiate into endothelial progenitors and myoblasts as well as specialized cells beyond the mesoderm lineage, notably neuroblasts [158–160]. Such differentiation potential of MSCs remains not well substantiated, and findings of their signaling pathways are still lacking, especially if they are to be considered events of cell transdifferentiation [161].
The MSC multipotency function was long thought to be therapeutically practical for tissue regeneration with the adoptive transfer of MSCs to improve conditions in degenerative disorders [162]. Early initiatives evaluated the therapeutic effects of MSC adoptive transfer in patients with osteogenesis imperfecta (OI), a congenital disease with altered expression of collagen genes leading to skeletal malfunctions. Some results from preliminary clinical studies showed bone tissue reinforcements after adoptive transfer of MSCs in children with severe OI symptoms, including recovery of skeletal growth and strength [163]. However, MSCs in the host bone tissue accounted for less than 1% of the total MSCs given to these patients, which suggests that the multipotency function is probably not essential in ameliorating OI symptoms [163, 164]. Likewise, suggestions that MSCs could differentiate into neurons led some investigators to consider MSC therapy in patients with eye diseases such as glaucoma, macular degeneration and retinitis pigmentosa [165]. Preclinical studies have shown a certain therapeutic benefit of MSC adoptive transfer in improving conditions in experimental models of retina diseases [165, 166], yet evidence showing MSC engraftment into the retina has not been clearly established [166]. Similarly, clinical studies showing reduced symptoms of retinal degeneration in patients after MSC adoptive transfer [167–169] supported that the beneficial effects are not likely related to MSC engraftment and that the therapeutic effect results from a transient presence of MSCs into damaged tissue [170, 171]. Furthermore, amelioration of disease after MSC adoptive transfer has been substantiated in experimental models of acute kidney injury [172], cardiomyopathy [173], diabetes complications [174], and liver cirrhosis [175]. Yet again, any permanent engraftment of MSCs into these diseased tissues has not been verified, which suggests that the multipotency function does not likely explain the therapeutic ability of MSCs in degenerative diseases [164, 176]. Overall, both preclinical and clinical studies have provided indications supporting MSC therapy in degenerative diseases, but the mechanism leading to the observed therapeutic effects is considered solely executed via a brief “hit-and-run” mode of action of MSCs [17, 164, 177, 178].
By contrast, the multipotency function of MSCs may be exploited in tissue engineering for therapeutic needs in trauma and/or in malfunctioning or loss of an organ [179, 180]. Particularly, tissue engineering based on MSCs may be used in orthopedics to attempt to improve the formation of organs related to the skeletal system that are deficient [179]. Tissue engineering combining innovative biomaterials with MSCs offers interesting alternatives that allow for producing advanced prosthetics to align biological functionality and mechanical compliance [181]. MSCs seeded on 3-D biomaterial scaffolds facilitate cell differentiation toward the formation of skeletal-related tissues [181, 182]. Hence, 3-D culture systems have been found to augment cell–cell interactions, favoring organized tissue formation while being compliant for transplantation [182, 183]. Biomimetic porous materials resembling the composition of bones, including hydroxyapatite and β-tricalcium phosphate, or biodegradable polymers such as polylactic acid, are regularly used as scaffolds for MSCs for tissue-engineering organs related to the skeletal system [183]. As well, pioneering research is focusing on advanced 3-D microfluidic bioprinting technologies based on MSCs and are currently being developed for clinical practice [184]. Tissue engineering using MSCs is rapidly evolving and is still in its infancy. Nonetheless, some clinical studies have shown therapeutic benefits with use of tissue engineering based on MSCs, remarkably in healing of femoral osteonecrosis [185] as well as for aiding the functional restoration of mandibles in severely atrophied mandibular defects [186].
Mesenchymal stem cell trophic function
MSCs are considered to regulate homeostasis within hematopoietic niches in vivo by supporting the maintenance, expansion and/or differentiation of HSCs. Also, MSCs may support the in vivo homeostasis of other progenitors [67, 179, 187, 188]. The MSC trophic function toward HSCs could be attributed to MSCs producing: (1) growth factors such as stem cell factor, platelet-derived growth factor, macrophage-colony stimulating factor (M-CSF), granulocyte-CSF (G-CSF), FMS-like tyrosine kinase-3 ligand, thrombopoietin, erythropoietin (EPO), angiopoietin 1 (Ang-1); (2) chemokines such as CXCL12, also known as stromal-derived factor 1, and CCL5, also known as regulated on activation normal T cell expressed and secreted (RANTES); (3) interleukins (ILs) including IL-3 and IL-6; and (4) extracellular matrix molecules such as hyaluronans [47, 189–191]. Hence, HSCs co-transplanted with MSCs enable HSC transplant success in vivo, which was revealed in a phase 1/2 clinical trial showing both amelioration of HSC engraftment in bone marrow and improvement in hematopoietic function recovery [192].
Beyond the ability to sustain homeostasis of HSCs and bone tissue, the MSC trophic function (Table 3) might be beneficial in favoring tissue healing and regeneration in different organs [47, 69]. To exemplify, Ang-1 and CXCL12 produced by MSCs have a significant impact on angiogenesis by recruiting adjacent endothelial progenitor cells in vivo [193, 194], whereas brain-derived neurotrophic factor (BDNF) or neurotrophin-3 (NT-3) released by MSCs acts on neural progenitors in the lesion area, thereby improving neurogenesis [195, 196]. Hence, the therapeutic effects of MSCs in preclinical models of neurodegenerative diseases were revealed particularly in amyotrophic lateral sclerosis (ALS), Huntington disease, multiple sclerosis, Parkinson disease, and spinal cord injury [197]. The benefits of MSC therapy are alleged to occur via neurotrophic factors produced by MSCs, such as BDNF, ciliary neurotrophic factor, glial cell-derived neurotrophic factor (GDNF), nerve growth factor, and NT-3 [196–202]. Furthermore, in experimental models of neurodegenerative diseases, MSC production of other growth factors including vascular endothelial growth factor (VEGF) acted synergistically with neurotrophic factors to improve conditions [197, 203]. Furthermore, in a clinical study of 37 patients with ALS, organ improvements were correlated with the paracrine actions of the neurotrophic factor BDNF and growth factors including VEGF [204]. Consistently, in a clinical trial of ten patients with secondary progressive MS treated with MSC adoptive transfer, the benefits were assessed by functional and physiological amelioration, including some visual endpoints that suggested neuroprotection [171].
Table 3.
Fundamental molecules of MSC trophic function
| Growth factors | Biologic effect on progenitors | References |
|---|---|---|
| MSC trophic function | ||
| Ang-1 | Angiogenesis | Pedersen et al. [318], Kingham et al. [319] |
| EGF | Pleiotropic | Li et al. [320], Ding et al. [321] |
| EPO | Angiogenesis | Zwezdaryk et al. [322], Hu et al. [323] |
| FGF-1, -18 | Pleiotropic | Wu et al. [324], Zhang et al. [325] |
| GDNF | Neurogenesis | Horita et al. [326], Ding et al. [327] |
| BDNF | Neurogenesis | Jeong et al. [328], Pollock et al. [195] |
| HGF | Pleiotropic | Neuss et al. [329], Kennelly et al. [220] |
| IGF-1 | Neurogenesis | Zhang et al. [330], Tfilin et al. [331] |
| KGF | Epithelialization | Casey et al. [332], Zhu et al. [333] |
| PDGF-AB | Pleiotropic | Ding et al. [334], Osborne et al. [335] |
| SDF-1 (CXCL12) | Angiogenesis, neurogenesis | Mishra et al. [336], Lin et al. [200] |
| VEGF | Angiogenesis | Mayer et al. [337], Beckermann et al. [338] |
The most critical molecules produced and secreted by MSCs for their trophic function. Data are summarized from references [195, 200, 220, 318–338]
Ang-1 angiopoietin-1, EGF epidermal growth factor, EPO erythropoietin, FGF-1 or -18 fibroblast growth factor 1 or 18, GDNF glial cell line-derived neurotrophic factor, BDNF brain-derived neurotrophic factor, HGF hepatocyte growth factor, IGF-1 insulin-like growth factor 1, KGF keratinocyte growth factor, PDGF-AB platelet-derived growth factor AB, SDF-1 stromal cell-derived factor 1, VEGF vascular endothelial growth factor
The clinical use of culture-conditioned or genetically engineered MSCs with enhanced aptitude to produce neurotrophic factors has been considered to improve the efficacy of MSC therapy in neurodegenerative disorders [195, 197]. For instance, in a phase 1/2 clinical trial, investigators examined MSCs overexpressing neurotrophic factors (MSC-NTFs) induced by a culture stimulation method before adoptive transfer in patients with ALS [205]. The results suggested that intratracheal and intramuscular adoptive transfer of MSC-NTFs in patients with ALS is safe, with significant enhancement of clinical benefits, to be confirmed in an upcoming phase 3 clinical trial (ClinicalTrials.gov: NCT03280056).
The MSC trophic function has been assessed in experimental models of degenerative diseases affecting the kidney (acute kidney injury), liver (liver cirrhosis), or multiple organs (diabetes complications) [172, 206, 207]. Organ condition and/or function improvements with MSC therapy in those diseases are associated with MSC production of growth factors, notably hepatocyte growth factor (HGF), IGF-1, and sometimes growth factors related to angiogenesis and neurogenesis such as VEGF, EPO, and GDNF [208–212]. Likewise, in degenerative diseases affecting the heart such as myocardial infarction, preclinical and clinical findings support the therapeutic benefit via a paracrine action of various growth factors after MSC adoptive transfer, to improve heart condition and function [213–215]. Remarkably, often this amelioration is associated with neovascularization into damaged myocardium, which suggests a role for growth factors associated with angiogenesis in the therapeutic effect of MSCs [216–218]. Other investigators suggested a broader role for growth factors involved in myocardium remodeling, including IGF-1 and HGF, produced by MSCs [218]. Similarly, preclinical studies associated the beneficial therapeutic effects of MSCs in lung emphysema and chronic obstructive pulmonary disease (COPD) with production of HGF and VEGF by MSCs [219, 220]. Consistently, in phase 1 clinical trials, adoptive transfer of MSCs in patients with COPD facilitated functional recovery of respiratory capacities, and the therapeutic benefits of MSCs may involve trophic factors [221, 222]. Moreover, a clinical assay undertaken on a compassionate basis involving two patients with severe acute respiratory distress syndrome (ARDS) receiving MSC adoptive transfer reported some positive outcomes with recovery of respiratory capacities concomitant to a lessening in lung tissue damage [223]. Especially, the investigators suggested that the MSC mode of action in ARDS may involve at least in part the action of a number of growth factors, and a further phase 1/2 clinical trial is ongoing [223].
Mesenchymal stem cell homing/migration function
The MSC fate resulting from systemic adoptive transfer might occur with (1) passage/location in non-specific tissues, (2) homing into native niches or (3) migration into damaged and/or diseased tissues [224]. Whatever the MSC fate within a short time after systemic adoptive transfer, how and under which conditions MSCs might survive or be eliminated from the host is not well established [177, 225]. This situation has critical significance both in terms of pharmacokinetics and pharmacodynamics of a given MSC therapy [226, 227]. The MSC function of homing/migration (Table 4) has been documented in preclinical studies, but the actual biodistribution of MSCs after systemic adoptive transfer in humans is just being revealed [227, 228]. Of note, some preclinical studies gave clues to the prospective mode of action of MSC homing/migration including for chemotaxis, rolling/adhesion, diapedeses and interstitial migration [53, 55, 56, 58, 60–62, 229, 230]. After adoptive transfer, MSCs may move along blood vessels, pass through the endothelial wall, and home into niches where they naturally reside or further migrate into tissues that are damaged and/or diseased [231, 232]. The expression and functionality of adhesion molecules, chemokine receptors, and enzymes belonging to the molecular class of metalloproteinases (MMPs) are indispensable for enabling trafficking of MSCs from peripheral blood toward specific target organs [231, 233].
Table 4.
Fundamental molecules of MSC homing/migration function
| Molecule class | MSC homing/migration function | References |
|---|---|---|
| Adhesion molecules | CD44 | Herrera et al. [339], Sackstein et al. [59] |
| Integrin α1 | Popov et al. [340] | |
| Integrin α3 | Frith et al. [341] | |
| Integrin α4 | Semon et al. [237] | |
| Integrin α5 | Veevers-Lowe [342] | |
| Integrin β1 | Semon et al. [237] | |
| Chemokine receptors | CCR2 | Ringe et al. [238] |
| CCR7 | Sordi et al. [61] | |
| CCR10 | Von Lüttichau et al. [343] | |
| CXCR4 | Ringe et al. [238], Baek et al. [344] | |
| CXCR5 | Baek et al. [344] | |
| CXCR6 | Baek et al. [344], Jung et al. [345] | |
| Metalloproteinases | MT1-MMP | Lu et al. [346] |
| MMP-1 | Ho et al. [245] | |
| MMP-9 | Kim et al. [246] | |
| Protease inhibitors | TIMP-1 | Egea et al. [347] |
| TIMP-2 | Ries et al. [244] | |
| TIMP-4 | Chelluboina et al. [348] |
The most critical molecules—adhesion molecules, chemokine receptors and metalloproteinases—involved in the MSC homing/migration function. Data are summarized from references [59, 61, 237, 238, 244–246, 339–348]
CD cluster of differentiation, CCR CC chemokine receptor, CXCR CXC chemokine receptor, MMP matrix metalloproteinase, MT1-MMP membrane type 1-MMP, TIMP tissue inhibitor of metalloproteinases
The interaction of MSCs with endothelial cells (ECs) requires adhesion molecules, most being integrins. However, recent findings indicate that MSCs show a deficit in expression and/or functionality of adhesion molecules implicated in homing/migration as compared with HSCs or leukocytes [234]. Hence, P-selectin (CD62P) glycoprotein ligand 1 (PSGL-1) is not found to be expressed on MSCs [59]. Yet, adhesion of MSCs onto microvasculature seems to remain fully dependent on a CD62P receptor expressed on ECs. Actually, MSCs interact with CD62P but via at least another glycoform ligand of CD62P that is dissimilar to the natural ligand PSGL-1 [59, 229]. In addition, firm adhesion of MSCs with ECs is mediated by integrins, including heterodimeric integrin α4β1, also known as very late antigen-4 (VLA-4), which interacts with its receptor vascular cell adhesion molecule 1 (VCAM-1) expressed on ECs [229, 235–237]. Both CD62P and VCAM-1 expressed on ECs are required for MSCs in rolling/adhesion processes, which has been verified in vivo in preclinical studies [230, 231]. Similarly, MSCs showed a weak aptitude to interact with E-selectin (CD62E) [230]. CD62E is expressed constitutively by cells of blood vessels irrigating bone marrow and is expressed on any ECs when activated by inflammatory cytokines [230, 231]. Although MSCs express CD44, an adhesion molecule that interacts with selectins, CD44 on MSCs lack proper posttranslational glycosylation modifications, so it is unable to interact well with CD62E [59, 230, 235]. Especially cell homing into bone marrow requires the expression of a ligand to CD62L/CD62E, also known as hematopoietic cell E-/L-selectin ligand (HCELL), a competent glycoform of CD44, and although HSCs express HCELL, MSCs do not [59, 230]. Thus, modifying CD44 expressed on MSCs in vitro to enhance in vivo trafficking of MSCs to bone marrow has been considered [59, 230]. A preclinical study showed that converting the native CD44 glycoform on MSCs into molecules resembling HCELL using enzymatic glycosylation procedures could significantly improve MSC homing to bone marrow [59].
Chemokine receptors that are expressed variably on the cell surface of MSCs include CCR2, 3, 4, 7, 10, and CXCR4, 5, and 6 [238–240]. Notably, CXCR4 is an important molecule regulating homing/migration of HSCs and is likely involved in MSC homing/migration as well [240]. However, CXCR4 is expressed sporadically on the cell surface of MSCs as compared with HSCs [240]. Still, the expression of CXCR4 on MSCs could be upregulated with inflammatory cytokine stimulation such as tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β) [241, 242]. Moreover, MSCs with enhanced production of CXCR4 enabled by genetic engineering showed better homing/migration in vivo in preclinical studies [243].
The activity of proteolytic enzymes belonging to the molecular class of MMPs enables diapedeses and interstitial migration of MSCs toward tissues [231]. Notably, MMP-2 and its activator membrane type-1-MMP and tissue inhibitor of metalloproteinases 2 have been found critical for diapedesis of MSCs [244]. Other MMP pathways with various collagenase activities such as MMP-1, -3 and -9 were also found positively associated with MSC homing/migration [245, 246].
Research of the MSC homing/migration function has involved mostly preclinical models, but in certain aspects, some results agree with clinical findings, especially regarding the biodistribution of MSC post-adoptive transfer [192, 231, 234, 247]. Thus, modification of MSCs by biological manipulations with enzymatic procedures or genetic methods to reinforce MSC homing/migration is envisaged to improve MSC therapy [231, 234].
Considered routes for adoptive transfer of MSCs may be topical into damaged/diseased tissue or systemic into peripheral blood. In this context, preclinical studies compared the therapeutic effects of MSCs with topical or systemic delivery of MSCs [164, 225, 231]. When adoptive transfer of MSCs is topical, that is, by intra-articular, intracoronary, intramuscular, intrathecal, or intratracheal routes, the therapeutic effects of MSCs are expected to be exerted locally. Therefore, MSC homing/migration is rather less indispensable, except from essential interstitial migration of MSCs within damaged/diseased tissue itself [164]. If delivery of MSCs is systemic, using intravascular adoptive transfer via intraarterial or intravenous routes, MSC homing/migration is absolutely required for MSCs to reach their target [164, 231]. Of note, MSC homing/migration may be also affected by whether adoptive transfer of MSCs is by intra-arterial or intravenous means [164, 225, 231]. MSCs delivered via the intraarterial route may avoid the lung first-pass effect inherent to any intravenous injection that considerably reduces the pharmacodynamics of MSCs. After an adoptive transfer of MSCs via an intra-arterial route, MSC entrapment into the lung is virtually absent as compared with the intravenous route [248]. However, intra-arterial injection is a complex procedure that requires medical expertise and has health risks [248, 249]. Therefore, intravenous delivery of MSCs, despite its disadvantages, is regularly used in both preclinical and clinical settings.
Further investigations are needed to properly define MSC homing/migration in vivo in humans and to determine whether findings from preclinical studies could be valuable to enhance the efficacy of MSC therapy.
Mesenchymal stem cell immunosuppression function
MSCs expanded in vitro are rather hypoimmunogenic because they do not express HLA-class II molecules or costimulatory molecules including CD40, CD80, CD83, CD86 and CD154. Yet, MSCs express HLA-class I molecules, and MSCs stimulated with interferon γ (IFN-γ), IL-1β, and/or TNF-α showed upregulated HLA-class I molecules and promoted the expression of HLA-class II and adhesion molecules [14, 68, 250]. Under these conditions, MSCs remain unable to express costimulatory molecules, but the capacity for co-stimulation is critical for activating T lymphocytes [68, 250]. Counterintuitively, inflammation enhances the immunosuppression function of MSCs. Indeed, inflammation upregulates HLA-class II molecules on MSCs and thus their interaction abilities with T lymphocytes. The absence of costimulatory molecules on MSCs, despite the inflammatory signals, will result in suboptimal activation of T lymphocytes and clonal anergy [251–253]. Of note, MSCs do not induce allogeneic proliferation of T lymphocytes in vitro, even when HLA class II molecules are upregulated on MSCs and co-stimulatory signaling is delivered by an anti-CD28 monoclonal antibody [68]. This finding strongly suggests an active MSC immunosuppression function, whereas enhancement of this function would require a “licensing” signal delivered by inflammatory factors [14, 251]. Hence, some investigators attributed failures in MSC therapy to inappropriate “licensing” of the MSCs used, whereas others suggested optimizing MSC therapy with a preconditioning treatment, that is, a prior in vitro stimulation of MSCs with appropriate inflammatory factors, to obtain optimal therapeutic effects in vivo [254, 255].
The MSC immunosuppression function was interpreted mostly by preclinical studies, both in vitro and in vivo, but substantial results are also sustained in clinical findings [256]. MSCs suppress a broad range of immune cells, including T, B, and natural killer (NK) lymphocytes, and affect functions of myeloid cells such as monocytes, dendritic cells (DCs) and macrophages [68, 257–262]. MSCs modulate both innate and adaptive immune cells by disrupting their activation, proliferation, maturation, cytokine production, cytolytic activity, or antibody production [256]. Specifically, MSCs impede effector T-lymphocyte functions such as T helper 17 (Th17) cytokine production [263, 264] while favoring tolerogenic CD4+ Th2 lymphocyte differentiation, at the expense of immunity mediated by CD4+ Th1 lymphocytes [265]. Furthermore, MSCs obstruct B lymphocytes from further differentiating into plasma cells and impede their ability to secrete immunoglobulins [257]. MSCs inhibit the cytotoxic potential of NK lymphocytes as well as their ability to secrete INF-γ [258, 259]. Moreover, MSCs prevent the differentiation of CD14+ monocytes and CD34+ progenitors into mature DCs [266]. Also, MSCs can diminish the DC ability to express HLA-class II as well as CD80 and CD86 costimulatory molecules [260, 266]. Notably, MSCs promote the emergence and/or recruitment of regulatory/suppressive immune subsets, including CD4+CD25+FOXP3+ T lymphocytes [258, 267], CD8+CD28− T lymphocytes [268], IL-10-producing B lymphocytes [269], IL-10-producing DCs [270], and alternatively activated M2-macrophages [262, 271]. Such MSC abilities facilitate the amplification of their immunosuppression effects by reinforcing the host’s own regulatory/immunosuppressive immune subsets [256].
The MSC immunosuppression function (Table 5) is mostly executed via production of soluble factors and their paracrine actions on immune cells. Direct cell–cell contacts between MSCs and immune cells are also involved, although seemingly occasional as compared with actions obtained by soluble factors [14, 256, 272]. MSCs produce and release various soluble factors that are accountable for the immunosuppression function; among them are a diverse class of molecules comprising ILs, such as IL-6, leukemia inhibitory factor, IL-10, TGF-β, and TNF-stimulated gene 6 (TSG-6) but also metabolic enzymes including heme oxygenase 1 (HO-1), indoleamine 2,3 dioxygenase (IDO), and inducible nitric oxide synthase (iNOS) as well as pleiotropic hormones such as prostaglandin E2 (PGE2) and other proteins such as galectin-1, non-classical HLA-class Ib HLA-G, and semaphorin-3A. Relating to membrane-bound molecules expressed on MSCs and implicated in their immunosuppressive function are immunoregulatory B7 family member proteins such as B7-H4, also known as V-set domain-containing T cell activation inhibitor 1; but also B7-H1 and B7-DC, also known as programmed death-ligand 1 (PD-L1) and PD-L2; and TNF family member protein fas ligand, also known as CD95L; as well as intercellular adhesion molecule 1, also known as CD54; and VCAM-1, also known as CD106 [4, 14, 66, 256, 272, 273]. Hence, MSCs are multiarmed for immunosuppression, which, therefore, validates an assessment of their therapeutic value in various immune disorders [256].
Table 5.
Fundamental molecules of MSC immunosuppression function
| Molecule | Molecule class | MSC immunosuppression function | References |
|---|---|---|---|
| Soluble | Interleukins | IL-6 | Najar et al. [349] |
| IL-10 | Beyth et al. [350], Rasmusson et al. [351] | ||
| LIF | Nasef et al. [352] | ||
| TGF-β | Sotiropoulo et al. [353], Patel et al. [354] | ||
| TSG-6 | Choi et al. [355] | ||
| Enzymes | HO-1 | Mougiakakos et al. [356] | |
| IDO | Francois et al. [357] | ||
| iNOS | Ren et al. [358] | ||
| Hormones | PGE2 | Spaggiari et al. [259, 359] | |
| Others | Galectin-1 | Gieseke et al. [360] | |
| HLA-G | Selmani et al. [258] | ||
| Semaphorin-3A | Lepelletier et al. [361] | ||
| Membrane bound | Adhesion molecules | ICAM-1 | Espagnolle et al. [362] |
| VCAM-1 | Yang et al. [363] | ||
| B7-family members | B7-DC | Davies et al. [364] | |
| B7-H1 | Tipnis et al. [365] | ||
| B7-H4 | Xue et al. [366] | ||
| TNF-family members | FasL | Gu et al. [367] |
The most critical soluble and membrane-bound molecules involved in the MSC immunosuppression function. Data are summarized from references [258, 259, 349–367]. Inducible nitric oxide synthase (iNOS) is shown in this table because it can be expressed in human MSCs but seems less active than indoleamine 2,3 dioxygenase (IDO) in the immunosuppression function of human MSCs [358]
IL interleukin, TGF transforming growth factor, TSG-6 TNF-stimulated gene 6, HO-1 heme oxygenase 1, PGE2 prostaglandin E2, HLA-G human leucocyte antigen G, ICAM-1 intercellular adhesion molecule 1, VCAM-1 vascular cell adhesion molecular 1, B7-DC also known as programmed death-ligand 2, B7-H1 also known as programmed death-ligand 1, B7-H4 also known as V-set domain-containing T-cell activation inhibitor 1, FasL Fas ligand, TNF tumor necrosis factor
MSC therapy is used for immunomodulation mostly in immune rejection and autoimmunity, including conditions such as in HSC transplantation, solid organ transplantation, Crohn’s disease (CD), rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) [256, 274]. Nevertheless, the mode of action of the MSC immunosuppression function in vivo is still not known in all aspects [2, 256]. Typically, in experimental models, the efficacy of MSC therapy is associated with the action of soluble factors such as IL-10, IDO, PGE2, TGF-β, TSG-6 and expansion of CD4+CD25+FOXP3+ regulatory T cells [256]. The first translational attempts used MSCs for immunomodulation purposes, especially for severe GvHD in patients unresponsive to available treatments [66, 273, 275, 276]. The proof-of-concept was shown in a pilot study reporting the practicability of adoptive transfer of MSCs in a 9-year-old patient with acute GvHD grade IV [276]. Adoptive transfer of MSCs in this patient resulted in overall improvement of his condition, with outcomes observed within few days and lasting for at least up to 1 year post-treatment [276]. Later, the investigators reported promising results for MSC therapy in a population of 55 patients with acute GvHD refractory to steroid treatments [275]. Notably, patients responded to MSCs with higher survival rate, which reached 52% at 2 years post-treatment, as compared with just 10% in control patients who did not receive MSCs [275]. Altogether, in this phase 2 clinical study, the authors interpreted their results positively and suggested that adoptive transfer of MSCs might be a suitable therapeutic approach in patients with acute GvHD [275]. Meanwhile, a phase 3 clinical trial (ClinicalTrials.gov: NCT00366145), explored an MSC product (Prochymal®) produced on an industrial scale by Osiris Therapeutics and tested for treatment of steroid-resistant GvHD in 240 patients [8]. However, the company reported non-positive outcomes for Prochymal®, with failure to ameliorate the clinical conditions of GvHD as compared with placebo [8]. This non-success was found to contrast with results observed in a clinical assay led by academic institutions, with use of adoptive transfer of MSCs from different donors and sources [8, 275, 276]. MSC product uncertainties including the quality of MSCs produced with industrial-scale methods and cryopreservation have been discussed as possible causes for the failure of Prochymal® [8]. However, an amended type of Prochymal, namely MSC-100-IV (Remestemcel-L®), produced by Mesoblast, has been tested in children with severe GvHD resistant to steroids in a clinical trial (ClinicalTrials.gov: NCT02336230). Early in 2018, data from a press release on this clinical trial described significant therapeutic benefits in pediatric steroid-resistant GvHD, with an overall response of up to 69% at day 28 after MSC therapy with Remestemcel-L® as compared with the protocol-defined control rate of 45% [2]. Furthermore, in solid organ transplantation, clinical assays to evaluate therapeutic efficacy of MSCs in acute allogeneic rejection in kidney transplantation and liver transplantation showed that MSC therapy combined with low-dose anticalcineurin reduced kidney or liver allograft pathology, with decreased rejection episodes [277–284]. Moreover, biological analyses in clinical studies have shown a link between observed therapeutic benefits and an increase in regulatory/suppressive subsets in peripheral blood, particularly CD4+CD25+FOXP3+ regulatory T cells, which is consistent with most preclinical findings [277, 278, 280–284].
The benefits of MSC therapy have been evaluated in clinical studies for autoimmune diseases, including CD, RA, and SLE [285–293]. A phase-2 clinical trial aiming to assess both the safety and efficacy of adoptive transfer of MSCs for patients with luminal CD has been undertaken [288]. Promisingly, results showed that MSC therapy diminished both the CD Activity Index and CD Endoscopic Index of Severity in patients unresponsive to any available treatment [288]. Furthermore, an MSC product (Cx601) from Tigenix (Alofisel®) recently demonstrated long-term therapeutic efficacy in CD perianal fistula complications in 212 patients in a phase 3 clinical trial, with remission in 51.5% of patients given Cx601 versus 35.6% given placebo [292]. Similarly, in patients with RA, MSC therapy seems to be pertinent to modulate inflammation and ameliorate conditions of RA [290, 291]. A phase 1/2 clinical trial evaluated another MSC product (Cx611) from Tigenix: results showed a predisposition for therapeutic efficacy of Cx611 in patients with RA that needs further assessment [289]. In addition, a 5-year follow-up study of MSC therapy in 81 patients with severe SLE lacking other therapeutic options showed a remarkable alleviation of conditions with MSC therapy [293]. The survival rate was 84% (n = 68/81) after MSC therapy; 34% achieved long-term clinical remission with better outcomes than controls [293].
Conclusion
MSC identity is still being questioned, and practices in culture, cryopreservation and clinical use of MSCs require standardization, both in preclinical and clinical settings, because these unknowns certainly affect the functions of MSCs used in cell therapy. Here, we attempted to delineate MSC functions that are essential in therapeutic effects of MSCs, including (1) proliferation, (2) multipotency, (3) trophic ability, (4) homing/migration, (5) and immunosuppression. Each of these functions may alone and/or when combined remain essential to the therapeutic ability of MSCs for various diseases.
MSC proliferation function (Table 1) is vital for MSC therapy, because the number of MSCs obtainable after isolation from biological sources is scarce, but an elevated number of MSCs is often required in clinical settings. Typically, a dose for MSC therapy represents about 1–10 × 106 MSCs/kg body weight [162]. The ability of MSCs to expand vigorously in vitro to yield a significant number of MSCs is critical to ease the development of MSC therapy. Thus, considerable efforts are made both in academia and industry to better address MSC proliferation function to improve their production while preserving the safety and therapeutic potency of MSC products [162].
Furthermore, permanent engraftment of MSCs into diseased tissues does not seem to occur, so the multipotency function of MSCs is not likely involved in the therapeutic ability of MSCs in degenerative diseases [164, 176]. Although current research has provided indications supporting MSC therapy in degenerative diseases, the modes of actions are considered to occur by a brief “hit-and-run” mechanism via the MSC trophic function and/or immunosuppression function [17, 164, 177, 178, 294]. However, the multipotency function of MSCs (Table 2) is of particular interest in tissue engineering for rebuilding organs to correct malfunctioning or replace lost organs following disease or trauma, especially in orthopedics [179, 180].
By contrast, the MSC trophic function appears to have a critical role in mediating the beneficial effect of MSC therapy for degenerative and/or inflammatory diseases and is currently a matter of intensive research [47, 125, 190]. Furthermore, the MSC production of trophic factors (Table 3) at lesions close to tissue damage in degenerative diseases could be enforced via a paracrine action targeted to specific tissue-resident progenitor cells and/or parenchymal cells by enhancing their own ability to metabolize, proliferate, differentiate and/or migrate, thus limiting further tissue damage [47, 69, 179].
To allow MSCs to exert their therapeutic effects after systemic adoptive transfer, the MSC homing/migration function (Table 4) is absolutely required. The MSC function of homing has been envisaged as comparable to the HSC ability to home into bone marrow after the adoptive transfer of HSCs into peripheral blood [54, 57, 224, 228, 231]. MSCs may also migrate into damaged and/or diseased tissues, where allegedly they can deploy their therapeutic effects [224, 228, 231]. The migration function of MSCs has been considered to have similarities to the migration function of leukocytes in diseases but, in contrast to leukocytes, MSCs migrate for a longer time, with specific modes of action [224, 228, 231]. Here, we considered together the function of homing/migration of MSCs because the fate of homing or migration of MSCs is solely determined by in vivo pathophysiological conditions [231, 295]. MSC homing/migration is required in therapeutics but is not sufficient to ensure that MSCs would reach damaged and/or diseased organs. Indeed, administration routes for adoptive transfer of MSCs may also significantly affect MSC homing/migration abilities [164]. Hence, selection of MSC delivery routes is critical because it ultimately affects the pharmacokinetics and pharmacodynamics of a given MSC therapy [164, 225, 231].
Most importantly, the MSC immunosuppression function (Table 5) is thought to mediate most of the therapeutic effects in the treatment of severe inflammatory diseases with limited medical options, including GvHD, immune rejection in allogeneic solid organ transplantation, sepsis, ARDS, CD, RA, and SLE [10, 69, 256, 258, 272, 273]. Significantly, the immunosuppression function of MSCs currently serves in functional in vitro assay approaches as release criterion for MSC products intended for clinical use [9]. The ISCT has proposed novel guidance for therapeutic potency assessment of MSCs intended for clinical release on the basis of their immunosuppression function in vitro [1, 10, 115, 125]. The recommendation is motivated by the need to improve consistency and effectiveness of MSC therapy and is based on the assumption that ensuring in vitro robustness of the immunosuppression function of MSCs would be associated with the therapeutic effect in vivo [2, 8–11]. Thus, use of easy-to-implement in vitro potency assays imply co-culture of MSCs with activated peripheral blood leukocytes in conditions under which lymphocyte proliferation inhibition is measurable concurrent with the secretome and transcriptome dynamic response of MSCs [223]. These in vitro immunopotency assay matrices are now being developed and the first clinical results seem relevant to help define release criterion for MSC products, with anticipation to bring more stringent consistency in MSC therapy [2, 10].
However, the biological functions of MSCs might remain incompletely defined. Indeed, the trophic function and the immunosuppression function could be associated with their function to modulate uncontrolled cell death occurring during diseases [6, 187, 296–302]. Therefore, further investigations are needed to understand the biological functions of MSCs, to improve and facilitate the use of MSC therapy in the clinic.
Acknowledgements
Support for research was provided by Suzuken Memorial Foundation (Japan) and the Japanese Society for the Promotion of Science (JSPS), Young B KAKENHI (Grant No. 17K15729) to A.N.
Author contributions
AN and NS contributed to establishing the concept convoyed in the manuscript. AN wrote the manuscript and designed figures and tables. ME, BF, FD, NRF and NS contributed to the writing and editing of the manuscript. All authors reviewed the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 2.Galipeau J, Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Naji A, et al. Concise review: combining human leukocyte antigen G and mesenchymal stem cells for immunosuppressant biotherapy. Stem Cells. 2013;31:2296–2303. doi: 10.1002/stem.1494. [DOI] [PubMed] [Google Scholar]
- 5.Wei X, et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naji A, et al. Rationale for determining the functional potency of mesenchymal stem cells in preventing regulated cell death for therapeutic use. Stem Cells Transl Med. 2017;6:713–719. doi: 10.5966/sctm.2016-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinnadurai R, et al. Cryopreserved mesenchymal stromal cells are susceptible to T-cell mediated apoptosis which is partly rescued by IFNgamma licensing. Stem Cells. 2016;34:2429–2442. doi: 10.1002/stem.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galipeau J. The mesenchymal stromal cells dilemma: does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Galipeau J, Krampera M. The challenge of defining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy. 2015;17:125–127. doi: 10.1016/j.jcyt.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Galipeau J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phinney DG, et al. MSCs: science and trials. Nat Med. 2013;19:812. doi: 10.1038/nm.3220. [DOI] [PubMed] [Google Scholar]
- 12.Sheridan C. First off-the-shelf mesenchymal stem cell therapy nears European approval. Nat Biotechnol. 2018;36:212–214. doi: 10.1038/nbt0318-212a. [DOI] [PubMed] [Google Scholar]
- 13.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma S, et al. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacchetti B, et al. No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep. 2016;6:897–913. doi: 10.1016/j.stemcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7:125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 19.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Friedenstein AJ, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 21.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 22.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. doi: 10.1097/00007890-196803000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 24.Castro-Malaspina H, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 25.Jinnai I, Bessho M, Murohashi I, Nara N, Hirashima K. Relationship between fibroblastoid colony-forming units (CFU-f) and hemopoietic precursor cells in normal human bone marrow. Int J Cell Cloning. 1984;2:341–347. doi: 10.1002/stem.5530020602. [DOI] [PubMed] [Google Scholar]
- 26.Nara N, Jinnai I, Imai Y, Bessho M, Hirashima K. Reduction of granulocyte-macrophage progenitor cells (CFU-C) and fibroblastoid colony-forming units (CFU-F) by leukemic cells in human and murine leukemia. Acta Haematol. 1984;72:171–180. doi: 10.1159/000206383. [DOI] [PubMed] [Google Scholar]
- 27.Dennis JE, Haynesworth SE, Young RG, Caplan AI. Osteogenesis in marrow-derived mesenchymal cell porous ceramic composites transplanted subcutaneously: effect of fibronectin and laminin on cell retention and rate of osteogenic expression. Cell Transplant. 1992;1:23–32. doi: 10.1177/096368979200100106. [DOI] [PubMed] [Google Scholar]
- 28.Owen M. Marrow stromal stem cells. J Cell Sci Suppl. 1988;10:63–76. doi: 10.1242/jcs.1988.Supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 29.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 30.Chang J, Allen TD, Dexter TM. Long-term bone marrow cultures: their use in autologous marrow transplantation. Cancer Cells. 1989;1:17–24. [PubMed] [Google Scholar]
- 31.Dexter TM. Stromal cell associated haemopoiesis. J Cell Physiol Suppl. 1982;1:87–94. doi: 10.1002/jcp.1041130414. [DOI] [PubMed] [Google Scholar]
- 32.Dexter TM, Spooncer E. Growth and differentiation in the hemopoietic system. Annu Rev Cell Biol. 1987;3:423–441. doi: 10.1146/annurev.cb.03.110187.002231. [DOI] [PubMed] [Google Scholar]
- 33.Dexter TM, Whetton AD, Spooncer E, Heyworth C, Simmons P. The role of stromal cells and growth factors in haemopoiesis and modulation of their effects by the src oncogene. J Cell Sci Suppl. 1985;3:83–95. doi: 10.1242/jcs.1985.Supplement_3.9. [DOI] [PubMed] [Google Scholar]
- 34.Beresford JN. Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop Relat Res. 1989;240:270–280. [PubMed] [Google Scholar]
- 35.Castro-Malaspina H, et al. Characteristics of bone marrow fibroblast colony-forming cells (CFU-F) and their progeny in patients with myeloproliferative disorders. Blood. 1982;59:1046–1054. [PubMed] [Google Scholar]
- 36.Castro-Malaspina H, Ebell W, Wang S. Human bone marrow fibroblast colony-forming units (CFU-F) Prog Clin Biol Res. 1984;154:209–236. [PubMed] [Google Scholar]
- 37.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 38.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 40.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. doi: 10.1002/(SICI)1097-4644(199702)64:2<295::AID-JCB12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 41.Vilamitjana-Amedee J, Bareille R, Rouais F, Caplan AI, Harmand MF. Human bone marrow stromal cells express an osteoblastic phenotype in culture. Vitro Cell Dev Biol Anim. 1993;29A:699–707. doi: 10.1007/BF02631426. [DOI] [PubMed] [Google Scholar]
- 42.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 43.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianco P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol. 2014;30:677–704. doi: 10.1146/annurev-cellbio-100913-013132. [DOI] [PubMed] [Google Scholar]
- 45.Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6:1445–1451. doi: 10.1002/sctm.17-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhartiya D. The need to revisit the definition of mesenchymal and adult stem cells based on their functional attributes. Stem Cell Res Ther. 2018;9:78. doi: 10.1186/s13287-018-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 48.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- 49.Horwitz EM, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 50.Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21:1045–1056. doi: 10.1089/hum.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delorme B, Chateauvieux S, Charbord P. The concept of mesenchymal stem cells. Regen Med. 2006;1:497–509. doi: 10.2217/17460751.1.4.497. [DOI] [PubMed] [Google Scholar]
- 52.Phinney DG, Sensebe L. Mesenchymal stromal cells: misconceptions and evolving concepts. Cytotherapy. 2013;15:140–145. doi: 10.1016/j.jcyt.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR53 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7:19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 55.Cheng Z, et al. Targeted migration of mesenchymal stem cells modified with CXCR55 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 56.De Becker A, et al. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440–449. doi: 10.3324/haematol.10475. [DOI] [PubMed] [Google Scholar]
- 57.Henschler R, Deak E, Seifried E. Homing of mesenchymal stem cells. Transfus Med Hemother. 2008;35:306–312. doi: 10.1159/000143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–427. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- 59.Sackstein R, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 60.Son BR, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR60 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 61.Sordi V, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 62.Wu GD, et al. Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation. 2003;75:679–685. doi: 10.1097/01.TP.0000048488.35010.95. [DOI] [PubMed] [Google Scholar]
- 63.Devine SM, Peter S, Martin BJ, Barry F, McIntosh KR. Mesenchymal stem cells: stealth and suppression. Cancer J. 2001;7(Suppl 2):S76–S82. [PubMed] [Google Scholar]
- 64.Djouad F, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 65.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 66.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 67.Maitra B, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 68.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 69.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maria AT, et al. Adipose-derived mesenchymal stem cells in autoimmune disorders: state of the art and perspectives for systemic sclerosis. Clin Rev Allergy Immunol. 2017;52:234–259. doi: 10.1007/s12016-016-8552-9. [DOI] [PubMed] [Google Scholar]
- 73.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 74.Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;174:249–282. doi: 10.1007/978-3-540-77855-4_11. [DOI] [PubMed] [Google Scholar]
- 75.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 76.Gronthos S, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 77.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 78.Gronthos S, Zannettino AC. A method to isolate and purify human bone marrow stromal stem cells. Methods Mol Biol. 2008;449:45–57. doi: 10.1007/978-1-60327-169-1_3. [DOI] [PubMed] [Google Scholar]
- 79.Zannettino AC, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 80.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 81.Lee RH, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 82.Zuk PA, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.e02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yin JQ, Zhu J, Ankrum JA. Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng. 2019;3:90–104. doi: 10.1038/s41551-018-0325-8. [DOI] [PubMed] [Google Scholar]
- 84.Wang LT, et al. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23:76. doi: 10.1186/s12929-016-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee HS, et al. Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells. 2003;21:190–199. doi: 10.1634/stemcells.21-2-190. [DOI] [PubMed] [Google Scholar]
- 86.Wagey R, Short B. Isolation, enumeration, and expansion of human mesenchymal stem cells in culture. Methods Mol Biol. 2013;946:315–334. doi: 10.1007/978-1-62703-128-8_20. [DOI] [PubMed] [Google Scholar]
- 87.Sensebe L. Clinical grade production of mesenchymal stem cells. Biomed Mater Eng. 2008;18:S3–S10. [PubMed] [Google Scholar]
- 88.Varghese J, Griffin M, Mosahebi A, Butler P. Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res Ther. 2017;8:45. doi: 10.1186/s13287-017-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zuk PA, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 90.Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6:195–202. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: from biology to cell therapy. World J Stem Cells. 2010;2:81–92. doi: 10.4252/wjsc.v2.i4.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow adipose tissue: trimming the fat. Trends Endocrinol Metab. 2016;27:392–403. doi: 10.1016/j.tem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corselli M, Chen CW, Crisan M, Lazzari L, Peault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–1109. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- 94.Song N, Armstrong AD, Li F, Ouyang H, Niyibizi C. Multipotent mesenchymal stem cells from human subacromial bursa: potential for cell based tendon tissue engineering. Tissue Eng Part A. 2014;20:239–249. doi: 10.1089/ten.TEA.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vaculik C, et al. Human dermis harbors distinct mesenchymal stromal cell subsets. J Invest Dermatol. 2012;132:563–574. doi: 10.1038/jid.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fournier BP, et al. Multipotent progenitor cells in gingival connective tissue. Tissue Eng Part A. 2010;16:2891–2899. doi: 10.1089/ten.TEA.2009.0796. [DOI] [PubMed] [Google Scholar]
- 97.Cheng MT, Yang HW, Chen TH, Lee OK. Isolation and characterization of multipotent stem cells from human cruciate ligaments. Cell Prolif. 2009;42:448–460. doi: 10.1111/j.1365-2184.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chong PP, Selvaratnam L, Abbas AA, Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2012;30:634–642. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 99.Fan J, Varshney RR, Ren L, Cai D, Wang DA. Synovium-derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng Part B Rev. 2009;15:75–86. doi: 10.1089/ten.teb.2008.0586. [DOI] [PubMed] [Google Scholar]
- 100.Miao Z, et al. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 101.Wang LT, et al. Differentiation of mesenchymal stem cells from human induced pluripotent stem cells results in downregulation of c-Myc and DNA replication pathways with immunomodulation toward CD4 and CD8 cells. Stem Cells. 2018;36:903–914. doi: 10.1002/stem.2795. [DOI] [PubMed] [Google Scholar]
- 102.Lennon DP, Haynesworth SE, Bruder SP, Jaiswal N, Caplan AI. Human and animal mesenchymal progenitor cells from bone marrow: identification of serum for optimal selection and proliferation. Vitro Cell Dev Biol Anim. 1996;32:602–611. doi: 10.1007/BF02724045. [DOI] [Google Scholar]
- 103.Rojewski MT, et al. GMP-compliant isolation and expansion of bone marrow-derived MSCs in the closed, automated device quantum cell expansion system. Cell Transplant. 2013;22:1981–2000. doi: 10.3727/096368912X657990. [DOI] [PubMed] [Google Scholar]
- 104.Corotchi MC, et al. Isolation method and xeno-free culture conditions influence multipotent differentiation capacity of human Wharton’s jelly-derived mesenchymal stem cells. Stem Cell Res Ther. 2013;4:81. doi: 10.1186/scrt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dos Santos F, et al. A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. Biotechnol Bioeng. 2014;111:1116–1127. doi: 10.1002/bit.25187. [DOI] [PubMed] [Google Scholar]
- 106.Sensebe L, Gadelorge M, Fleury-Cappellesso S. Production of mesenchymal stromal/stem cells according to good manufacturing practices: a review. Stem Cell Res Ther. 2013;4:66. doi: 10.1186/scrt217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dos Santos F, et al. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223:27–35. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- 108.Fotia C, Massa A, Boriani F, Baldini N, Granchi D. Hypoxia enhances proliferation and stemness of human adipose-derived mesenchymal stem cells. Cytotechnology. 2015;67:1073–1084. doi: 10.1007/s10616-014-9731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eaker S, et al. Concise review: guidance in developing commercializable autologous/patient-specific cell therapy manufacturing. Stem Cells Transl Med. 2013;2:871–883. doi: 10.5966/sctm.2013-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blazquez-Prunera A, Almeida CR, Barbosa MA. Human bone marrow mesenchymal stem/stromal cells preserve their immunomodulatory and chemotactic properties when expanded in a human plasma derived xeno-free medium. Stem Cells Int. 2017;2017:2185351. doi: 10.1155/2017/2185351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blazquez-Prunera A, Diez JM, Gajardo R, Grancha S. Human mesenchymal stem cells maintain their phenotype, multipotentiality, and genetic stability when cultured using a defined xeno-free human plasma fraction. Stem Cell Res Ther. 2017;8:103. doi: 10.1186/s13287-017-0552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rafiq QA, Brosnan KM, Coopman K, Nienow AW, Hewitt CJ. Culture of human mesenchymal stem cells on microcarriers in a 5 l stirred-tank bioreactor. Biotechnol Lett. 2013;35:1233–1245. doi: 10.1007/s10529-013-1211-9. [DOI] [PubMed] [Google Scholar]
- 113.Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JA. Mesenchymal stromal cells derived from various tissues: biological, clinical and cryopreservation aspects. Cryobiology. 2015;71:181–197. doi: 10.1016/j.cryobiol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 114.Moll G, et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32:2430–2442. doi: 10.1002/stem.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lechanteur C, et al. Clinical-scale expansion of mesenchymal stromal cells: a large banking experience. J Transl Med. 2016;14:145. doi: 10.1186/s12967-016-0892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wagner W, et al. The heterogeneity of human mesenchymal stem cell preparations—evidence from simultaneous analysis of proteomes and transcriptomes. Exp Hematol. 2006;34:536–548. doi: 10.1016/j.exphem.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 117.Pevsner-Fischer M, Levin S, Zipori D. The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev. 2011;7:560–568. doi: 10.1007/s12015-011-9229-7. [DOI] [PubMed] [Google Scholar]
- 118.Han ZC, Du WJ, Han ZB, Liang L. New insights into the heterogeneity and functional diversity of human mesenchymal stem cells. Biomed Mater Eng. 2017;28:S29–S45. doi: 10.3233/BME-171622. [DOI] [PubMed] [Google Scholar]
- 119.Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113:2806–2812. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 120.Mindaye ST, Lo Surdo J, Bauer SR, Alterman MA. The proteomic dataset for bone marrow derived human mesenchymal stromal cells: effect of in vitro passaging. Data Brief. 2015;5:864–870. doi: 10.1016/j.dib.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mindaye ST, Ra M, Lo Surdo J, Bauer SR, Alterman MA. Improved proteomic profiling of the cell surface of culture-expanded human bone marrow multipotent stromal cells. J Proteomics. 2013;78:1–14. doi: 10.1016/j.jprot.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 122.Mindaye ST, Ra M, Lo Surdo JL, Bauer SR, Alterman MA. Global proteomic signature of undifferentiated human bone marrow stromal cells: evidence for donor-to-donor proteome heterogeneity. Stem Cell Res. 2013;11:793–805. doi: 10.1016/j.scr.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 123.Mindaye ST, Surdo JL, Bauer SR, Alterman MA. System-wide survey of proteomic responses of human bone marrow stromal cells (hBMSCs) to in vitro cultivation. Stem Cell Res. 2015;15:655–664. doi: 10.1016/j.scr.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 124.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 125.Samsonraj RM, et al. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015;33:1878–1891. doi: 10.1002/stem.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 127.Rennerfeldt DA, Van Vliet KJ. Concise review: when colonies are not clones: evidence and implications of intracolony heterogeneity in mesenchymal stem cells. Stem Cells. 2016;34:1135–1141. doi: 10.1002/stem.2296. [DOI] [PubMed] [Google Scholar]
- 128.Cai J, Weiss ML, Rao MS. In search of “stemness”. Exp Hematol. 2004;32:585–598. doi: 10.1016/j.exphem.2004.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Boer J, Wang HJ, Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10:393–401. doi: 10.1089/107632704323061753. [DOI] [PubMed] [Google Scholar]
- 130.Fehrer C, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 131.Estrada JC, et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ. 2012;19:743–755. doi: 10.1038/cdd.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zimmermann S, et al. Lack of telomerase activity in human mesenchymal stem cells. Leukemia. 2003;17:1146–1149. doi: 10.1038/sj.leu.2402962. [DOI] [PubMed] [Google Scholar]
- 133.Bernardo ME, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 134.Wagner W, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bork S, et al. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell. 2010;9:54–63. doi: 10.1111/j.1474-9726.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Turinetto V, Vitale E, Giachino C. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci. 2016;17:1164. doi: 10.3390/ijms17071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jaiswal RK, et al. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 138.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158–5166. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 139.Chen Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23:1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl 1):S58–S65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 142.Tuli R, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 143.Chiou M, Xu Y, Longaker MT. Mitogenic and chondrogenic effects of fibroblast growth factor-2 in adipose-derived mesenchymal cells. Biochem Biophys Res Commun. 2006;343:644–652. doi: 10.1016/j.bbrc.2006.02.171. [DOI] [PubMed] [Google Scholar]
- 144.Longobardi L, et al. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006;21:626–636. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 145.Schmitt B, et al. BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation. 2003;71:567–577. doi: 10.1111/j.1432-0436.2003.07109003.x. [DOI] [PubMed] [Google Scholar]
- 146.Leung VY, et al. SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet. 2011;7:e1002356. doi: 10.1371/journal.pgen.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2005;280:8343–8350. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 148.Indrawattana N, et al. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 149.Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem. 2006;98:538–554. doi: 10.1002/jcb.20719. [DOI] [PubMed] [Google Scholar]
- 150.Marupanthorn K, Tantrawatpan C, Kheolamai P, Tantikanlayaporn D, Manochantr S. Bone morphogenetic protein-2 enhances the osteogenic differentiation capacity of mesenchymal stromal cells derived from human bone marrow and umbilical cord. Int J Mol Med. 2017;39:654–662. doi: 10.3892/ijmm.2017.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320:269–276. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 152.Celil AB, Campbell PG. BMP-2 and insulin-like growth factor-I mediate Osterix (Osx) expression in human mesenchymal stem cells via the MAPK and protein kinase D signaling pathways. J Biol Chem. 2005;280:31353–31359. doi: 10.1074/jbc.M503845200. [DOI] [PubMed] [Google Scholar]
- 153.Nakashima K, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 154.Nishio Y, et al. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene. 2006;372:62–70. doi: 10.1016/j.gene.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 155.Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112:3491–3501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fontaine C, Cousin W, Plaisant M, Dani C, Peraldi P. Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells. 2008;26:1037–1046. doi: 10.1634/stemcells.2007-0974. [DOI] [PubMed] [Google Scholar]
- 157.Oldershaw RA, et al. Notch signaling through Jagged-1 is necessary to initiate chondrogenesis in human bone marrow stromal cells but must be switched off to complete chondrogenesis. Stem Cells. 2008;26:666–674. doi: 10.1634/stemcells.2007-0806. [DOI] [PubMed] [Google Scholar]
- 158.Janeczek Portalska K, et al. Endothelial differentiation of mesenchymal stromal cells. PLoS One. 2012;7:e46842. doi: 10.1371/journal.pone.0046842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells—current trends and future prospective. Biosci Rep. 2015;35:e00191. doi: 10.1042/bsr20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Beier JP, et al. Myogenic differentiation of mesenchymal stem cells co-cultured with primary myoblasts. Cell Biol Int. 2011;35:397–406. doi: 10.1042/CBI20100417. [DOI] [PubMed] [Google Scholar]
- 161.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 162.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Horwitz EM, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013;2013:732742. doi: 10.1155/2013/732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tomita M, et al. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells. 2002;20:279–283. doi: 10.1634/stemcells.20-4-279. [DOI] [PubMed] [Google Scholar]
- 166.Inoue Y, et al. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res. 2007;85:234–241. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 167.Oner A, Gonen ZB, Sinim N, Cetin M, Ozkul Y. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: a phase I clinical safety study. Stem Cell Res Ther. 2016;7:178. doi: 10.1186/s13287-016-0432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Satarian L, et al. Intravitreal injection of bone marrow mesenchymal stem cells in patients with advanced retinitis pigmentosa; a safety study. J Ophthalmic Vis Res. 2017;12:58–64. doi: 10.4103/2008-322X.200164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Limoli PG, Limoli C, Vingolo EM, Scalinci SZ, Nebbioso M. Cell surgery and growth factors in dry age-related macular degeneration: visual prognosis and morphological study. Oncotarget. 2016;7:46913–46923. doi: 10.18632/oncotarget.10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10:649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- 171.Connick P, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 173.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–1430. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Concise review: mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. 2011;29:5–10. doi: 10.1002/stem.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kuo TK, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Muller I, Lymperi S, Dazzi F. Mesenchymal stem cell therapy for degenerative inflammatory disorders. Curr Opin Organ Transplant. 2008;13:639–644. doi: 10.1097/MOT.0b013e328317a462. [DOI] [PubMed] [Google Scholar]
- 177.von Bahr L, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- 178.Kurtz A. Mesenchymal stem cell delivery routes and fate. Int J Stem Cells. 2008;1:1–7. doi: 10.15283/ijsc.2008.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 180.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Leach JK, Whitehead J. Materials-directed differentiation of mesenchymal stem cells for tissue engineering and regeneration. ACS Biomater Sci Eng. 2018;4:1115–1127. doi: 10.1021/acsbiomaterials.6b00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. 2013;19:485–502. doi: 10.1089/ten.TEB.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sheikh Z, et al. Biodegradable materials for bone repair and tissue engineering applications. Materials (Basel) 2015;8:5744–5794. doi: 10.3390/ma8095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Costantini M, et al. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication. 2016;8:035002. doi: 10.1088/1758-5090/8/3/035002. [DOI] [PubMed] [Google Scholar]
- 185.Aoyama T, et al. An exploratory clinical trial for idiopathic osteonecrosis of femoral head by cultured autologous multipotent mesenchymal stromal cells augmented with vascularized bone grafts. Tissue Eng Part B Rev. 2014;20:233–242. doi: 10.1089/ten.TEB.2014.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gjerde C, et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res Ther. 2018;9:213. doi: 10.1186/s13287-018-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Benvenuto F, et al. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007;25:1753–1760. doi: 10.1634/stemcells.2007-0068. [DOI] [PubMed] [Google Scholar]
- 188.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–848. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 190.Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther. 2016;7:131. doi: 10.1186/s13287-016-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Greenbaum A, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koc ON, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 193.Watt SM, et al. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull. 2013;108:25–53. doi: 10.1093/bmb/ldt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 195.Pollock K, et al. Human mesenchymal stem cells genetically engineered to overexpress brain-derived neurotrophic factor improve outcomes in huntington’s disease mouse models. Mol Ther. 2016;24:965–977. doi: 10.1038/mt.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA. 2005;102:18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Joyce N, et al. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 199.McCoy MK, et al. Autologous transplants of Adipose-Derived Adult Stromal (ADAS) cells afford dopaminergic neuroprotection in a model of Parkinson’s disease. Exp Neurol. 2008;210:14–29. doi: 10.1016/j.expneurol.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lin YT, et al. Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington’s disease mouse models. PLoS One. 2011;6:e22924. doi: 10.1371/journal.pone.0022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wakabayashi K, et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88:1017–1025. doi: 10.1002/jnr.22279. [DOI] [PubMed] [Google Scholar]
- 202.Wilkins A, et al. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 203.Kim KS, et al. Transplantation of human adipose tissue-derived stem cells delays clinical onset and prolongs life span in ALS mouse model. Cell Transplant. 2014;23:1585–1597. doi: 10.3727/096368913X673450. [DOI] [PubMed] [Google Scholar]
- 204.Kim HY, et al. Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: an investigator-initiated trial and in vivo study. Stem Cells. 2014;32:2724–2731. doi: 10.1002/stem.1770. [DOI] [PubMed] [Google Scholar]
- 205.Petrou P, et al. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: results of phase 1/2 and 2a clinical trials. JAMA Neurol. 2016;73:337–344. doi: 10.1001/jamaneurol.2015.4321. [DOI] [PubMed] [Google Scholar]
- 206.Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M. Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32:2818–2823. doi: 10.1002/stem.1818. [DOI] [PubMed] [Google Scholar]
- 207.Lee RH, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Morigi M, Benigni A. Mesenchymal stem cells and kidney repair. Nephrol Dial Transplant. 2013;28:788–793. doi: 10.1093/ndt/gfs556. [DOI] [PubMed] [Google Scholar]
- 209.Morigi M, Rota C, Remuzzi G. Mesenchymal stem cells in kidney repair. Methods Mol Biol. 2016;1416:89–107. doi: 10.1007/978-1-4939-3584-0_5. [DOI] [PubMed] [Google Scholar]
- 210.Zhang Y, Li Y, Zhang L, Li J, Zhu C. Mesenchymal stem cells: potential application for the treatment of hepatic cirrhosis. Stem Cell Res Ther. 2018;9:59. doi: 10.1186/s13287-018-0814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yeung TY, et al. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One. 2012;7:e38189. doi: 10.1371/journal.pone.0038189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Park KS, et al. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation. 2010;89:509–517. doi: 10.1097/TP.0b013e3181c7dc99. [DOI] [PubMed] [Google Scholar]
- 213.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen SL, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 215.Amado LC, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kinnaird T, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 217.Kinnaird T, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 218.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Guan XJ, et al. Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF-VEGF receptors. J Cell Biochem. 2013;114:323–335. doi: 10.1002/jcb.24377. [DOI] [PubMed] [Google Scholar]
- 220.Kennelly H, Mahon BP, English K. Human mesenchymal stromal cells exert HGF dependent cytoprotective effects in a human relevant pre-clinical model of COPD. Sci Rep. 2016;6:38207. doi: 10.1038/srep38207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stessuk T, et al. Phase I clinical trial of cell therapy in patients with advanced chronic obstructive pulmonary disease: follow-up of up to 3 years. Rev Bras Hematol Hemoter. 2013;35:352–357. doi: 10.5581/1516-8484.20130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.de Oliveira HG, et al. Combined bone marrow-derived mesenchymal stromal cell therapy and one-way endobronchial valve placement in patients with pulmonary emphysema: a phase I clinical trial. Stem Cells Transl Med. 2017;6:962–969. doi: 10.1002/sctm.16-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Simonson OE, et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl Med. 2016;5:845. doi: 10.5966/sctm.2015-0021erratum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 225.Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li M, et al. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther. 2016;7:160. doi: 10.1186/s13287-016-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Brooks A, et al. Concise review: quantitative detection and modeling the in vivo kinetics of therapeutic mesenchymal stem/stromal cells. Stem Cells Transl Med. 2018;7:78–86. doi: 10.1002/sctm.17-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. doi: 10.1155/2013/130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ruster B, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 230.Thankamony SP, Sackstein R. Enforced hematopoietic cell E- and L-selectin ligand (HCELL) expression primes transendothelial migration of human mesenchymal stem cells. Proc Natl Acad Sci USA. 2011;108:2258–2263. doi: 10.1073/pnas.1018064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nitzsche F, et al. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells. 2017;35:1446–1460. doi: 10.1002/stem.2614. [DOI] [PubMed] [Google Scholar]
- 232.Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137:491–502. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- 233.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 234.Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. doi: 10.1186/s13287-015-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Aldridge V, et al. Human mesenchymal stem cells are recruited to injured liver in a beta1-integrin and CD44 dependent manner. Hepatology. 2012;56:1063–1073. doi: 10.1002/hep.25716. [DOI] [PubMed] [Google Scholar]
- 236.Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007;21:3917–3927. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- 237.Semon JA, et al. Integrin expression and integrin-mediated adhesion in vitro of human multipotent stromal cells (MSCs) to endothelial cells from various blood vessels. Cell Tissue Res. 2010;341:147–158. doi: 10.1007/s00441-010-0994-4. [DOI] [PubMed] [Google Scholar]
- 238.Ringe J, et al. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR238, CXCR238 and CCR238, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 239.Ponte AL, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 240.Wynn RF, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR240 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 241.Ziaei R, Ayatollahi M, Yaghobi R, Sahraeian Z, Zarghami N. Involvement of TNF-alpha in differential gene expression pattern of CXCR241 on human marrow-derived mesenchymal stem cells. Mol Biol Rep. 2014;41:1059–1066. doi: 10.1007/s11033-013-2951-2. [DOI] [PubMed] [Google Scholar]
- 242.Fan H, et al. Pre-treatment with IL-1beta enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473–481. doi: 10.1038/cmi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Park SA, et al. CXCR243-transfected human umbilical cord blood-derived mesenchymal stem cells exhibit enhanced migratory capacity toward gliomas. Int J Oncol. 2011;38:97–103. [PubMed] [Google Scholar]
- 244.Ries C, et al. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 245.Ho IA, et al. Matrix metalloproteinase 1 is necessary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma. Stem Cells. 2009;27:1366–1375. doi: 10.1002/stem.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kim Y, et al. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007;20:867–876. doi: 10.1159/000110447. [DOI] [PubMed] [Google Scholar]
- 247.Gholamrezanezhad A, et al. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 2011;38:961–967. doi: 10.1016/j.nucmedbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 248.Fischer UM, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Argibay B, et al. Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Sci Rep. 2017;7:40758. doi: 10.1038/srep40758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Di Nicola M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 251.Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO-independent suppression of T cell effector function by IFN-gamma-licensed human mesenchymal stromal cells. J Immunol. 2014;192:1491–1501. doi: 10.4049/jimmunol.1301828. [DOI] [PubMed] [Google Scholar]
- 252.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 253.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Boland L, et al. IFN-gamma and TNF-alpha pre-licensing protects mesenchymal stromal cells from the pro-inflammatory effects of palmitate. Mol Ther. 2018;26:860–873. doi: 10.1016/j.ymthe.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Krampera M. Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia. 2011;25:1408–1414. doi: 10.1038/leu.2011.108. [DOI] [PubMed] [Google Scholar]
- 256.Gao F, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Corcione A, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 258.Selmani Z, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+ CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 259.Spaggiari GM, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 260.Jiang XX, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 261.Ramasamy R, et al. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 262.Ylostalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30:2283–2296. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ghannam S, Pene J, Moquet-Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 264.Rozenberg A, et al. Human mesenchymal stem cells impact Th17 and Th1 responses through a prostaglandin E2 and myeloid-dependent mechanism. Stem Cells Transl Med. 2016;5:1506–1514. doi: 10.5966/sctm.2015-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bai L, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 267.Luz-Crawford P, et al. Mesenchymal stem cells generate a CD4+ CD25+ Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liu Q, et al. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8(+)CD28(−) regulatory T cells. Cell Mol Immunol. 2015;12:708–718. doi: 10.1038/cmi.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cho KA, et al. Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cell Mol Immunol. 2017;14:895. doi: 10.1038/cmi.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Liu X, et al. Mesenchymal stem/stromal cells induce the generation of novel IL-10-dependent regulatory dendritic cells by SOCS3 activation. J Immunol. 2012;189:1182–1192. doi: 10.4049/jimmunol.1102996. [DOI] [PubMed] [Google Scholar]
- 271.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 273.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 274.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 275.Le Blanc K, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 276.Le Blanc K, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 277.Tan J, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 278.Peng Y, et al. Donor-derived mesenchymal stem cells combined with low-dose tacrolimus prevent acute rejection after renal transplantation: a clinical pilot study. Transplantation. 2013;95:161–168. doi: 10.1097/TP.0b013e3182754c53. [DOI] [PubMed] [Google Scholar]
- 279.Hartleif S, et al. Safety and tolerance of donor-derived mesenchymal stem cells in pediatric living-donor liver transplantation: the MYSTEP1 study. Stem Cells Int. 2017;2017:2352954. doi: 10.1155/2017/2352954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Perico N, et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6:412–422. doi: 10.2215/CJN.04950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Reinders ME, et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl Med. 2013;2:107–111. doi: 10.5966/sctm.2012-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pan GH, et al. Low-dose tacrolimus combined with donor-derived mesenchymal stem cells after renal transplantation: a prospective, non-randomized study. Oncotarget. 2016;7:12089–12101. doi: 10.18632/oncotarget.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Detry O, et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: a phase I–II, open-label, clinical study. J Hepatol. 2017;67:47–55. doi: 10.1016/j.jhep.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 284.Soeder Y, et al. First-in-human case study: multipotent adult progenitor cells for immunomodulation after liver transplantation. Stem Cells Transl Med. 2015;4:899–904. doi: 10.5966/sctm.2015-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mao F, et al. Mesenchymal stem cells and their therapeutic applications in inflammatory bowel disease. Oncotarget. 2017;8:38008–38021. doi: 10.18632/oncotarget.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Duijvestein M, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 287.Ciccocioppo R, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 288.Forbes GM, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 289.Alvaro-Gracia JM, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2017;76:196–202. doi: 10.1136/annrheumdis-2015-208918. [DOI] [PubMed] [Google Scholar]
- 290.Djouad F, Bouffi C, Ghannam S, Noel D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5:392–399. doi: 10.1038/nrrheum.2009.104. [DOI] [PubMed] [Google Scholar]
- 291.Gonzalez-Rey E, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 292.Panes J, et al. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2018;154:1334–1342 e1334. doi: 10.1053/j.gastro.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 293.Wang D, et al. A long-term follow-up study of allogeneic mesenchymal stem/stromal cell transplantation in patients with drug-resistant systemic lupus erythematosus. Stem Cell Rep. 2018;10:933–941. doi: 10.1016/j.stemcr.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Roddy GW, et al. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 295.De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem Cells. 2016;8:73–87. doi: 10.4252/wjsc.v8.i3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Cselenyak A, Pankotai E, Horvath EM, Kiss L, Lacza Z. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 2010;11:29. doi: 10.1186/1471-2121-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kong D, et al. Mesenchymal stem cells protect neurons against hypoxic-ischemic injury via inhibiting parthanatos, necroptosis, and apoptosis, but not autophagy. Cell Mol Neurobiol. 2017;37:303–313. doi: 10.1007/s10571-016-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Mahrouf-Yorgov M, et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017;24:1224–1238. doi: 10.1038/cdd.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Naji A, et al. Endocytosis of indium-tin-oxide nanoparticles by macrophages provokes pyroptosis requiring NLRP3-ASC-Caspase1 axis that can be prevented by mesenchymal stem cells. Sci Rep. 2016;6:26162. doi: 10.1038/srep26162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Scheibe F, Klein O, Klose J, Priller J. Mesenchymal stromal cells rescue cortical neurons from apoptotic cell death in an in vitro model of cerebral ischemia. Cell Mol Neurobiol. 2012;32:567–576. doi: 10.1007/s10571-012-9798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zhao K, et al. Bone marrow-derived mesenchymal stem cells ameliorate chronic high glucose-induced beta-cell injury through modulation of autophagy. Cell Death Dis. 2015;6:e1885. doi: 10.1038/cddis.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Zilka N, et al. Mesenchymal stem cells rescue the Alzheimer’s disease cell model from cell death induced by misfolded truncated tau. Neuroscience. 2011;193:330–337. doi: 10.1016/j.neuroscience.2011.06.088. [DOI] [PubMed] [Google Scholar]
- 303.Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308:1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 304.Platt MO, Roman AJ, Wells A, Lauffenburger DA, Griffith LG. Sustained epidermal growth factor receptor levels and activation by tethered ligand binding enhances osteogenic differentiation of multi-potent marrow stromal cells. J Cell Physiol. 2009;221:306–317. doi: 10.1002/jcp.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Miraoui H, et al. Fibroblast growth factor receptor 2 promotes osteogenic differentiation in mesenchymal cells via ERK1/2 and protein kinase C signaling. J Biol Chem. 2009;284:4897–4904. doi: 10.1074/jbc.M805432200. [DOI] [PubMed] [Google Scholar]
- 306.Scavo LM, Karas M, Murray M, Leroith D. Insulin-like growth factor-I stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes. J Clin Endocrinol Metab. 2004;89:3543–3553. doi: 10.1210/jc.2003-031682. [DOI] [PubMed] [Google Scholar]
- 307.Roelen BA, Dijke P. Controlling mesenchymal stem cell differentiation by TGFBeta family members. J Orthop Sci. 2003;8:740–748. doi: 10.1007/s00776-003-0702-2. [DOI] [PubMed] [Google Scholar]
- 308.Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 2004;23:552–563. doi: 10.1038/sj.emboj.7600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Oliveira FS, et al. Hedgehog signaling and osteoblast gene expression are regulated by purmorphamine in human mesenchymal stem cells. J Cell Biochem. 2012;113:204–208. doi: 10.1002/jcb.23345. [DOI] [PubMed] [Google Scholar]
- 310.Chang J, et al. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007;282:30938–30948. doi: 10.1074/jbc.M702391200. [DOI] [PubMed] [Google Scholar]
- 311.Ugarte F, et al. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp Hematol. 2009;37:867–875 e861. doi: 10.1016/j.exphem.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 312.Zhou S. TGF-beta regulates beta-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J Cell Biochem. 2011;112:1651–1660. doi: 10.1002/jcb.23079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Qian SW, et al. Characterization of adipocyte differentiation from human mesenchymal stem cells in bone marrow. BMC Dev Biol. 2010;10:47. doi: 10.1186/1471-213X-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Thiagarajan L, Abu-Awwad HAM, Dixon JE. Osteogenic programming of human mesenchymal stem cells with highly efficient intracellular delivery of RUNX2. Stem Cells Transl Med. 2017;6:2146–2159. doi: 10.1002/sctm.17-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Xu J, Li Z, Hou Y, Fang W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am J Transl Res. 2015;7:2527–2535. [PMC free article] [PubMed] [Google Scholar]
- 316.Yu WH, et al. PPARgamma suppression inhibits adipogenesis but does not promote osteogenesis of human mesenchymal stem cells. Int J Biochem Cell Biol. 2012;44:377–384. doi: 10.1016/j.biocel.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 317.Zhu F, Friedman MS, Luo W, Woolf P, Hankenson KD. The transcription factor osterix (SP7) regulates BMP6-induced human osteoblast differentiation. J Cell Physiol. 2012;227:2677–2685. doi: 10.1002/jcp.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Pedersen TO, et al. Mesenchymal stem cells induce endothelial cell quiescence and promote capillary formation. Stem Cell Res Ther. 2014;5:23. doi: 10.1186/scrt412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014;23:741–754. doi: 10.1089/scd.2013.0396. [DOI] [PubMed] [Google Scholar]
- 320.Li D, et al. Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res Ther. 2013;4:103. doi: 10.1186/scrt314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ding C, et al. Human amniotic mesenchymal stem cells improve ovarian function in natural aging through secreting hepatocyte growth factor and epidermal growth factor. Stem Cell Res Ther. 2018;9:55. doi: 10.1186/s13287-018-0781-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Zwezdaryk KJ, et al. Erythropoietin, a hypoxia-regulated factor, elicits a pro-angiogenic program in human mesenchymal stem cells. Exp Hematol. 2007;35:640–652. doi: 10.1016/j.exphem.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 323.Hu X, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 324.Wu L, Leijten J, van Blitterswijk CA, Karperien M. Fibroblast growth factor-1 is a mesenchymal stromal cell-secreted factor stimulating proliferation of osteoarthritic chondrocytes in co-culture. Stem Cells Dev. 2013;22:2356–2367. doi: 10.1089/scd.2013.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhang Z, Wang Y, Li M, Li J, Wu J. Fibroblast growth factor 18 increases the trophic effects of bone marrow mesenchymal stem cells on chondrocytes isolated from late stage osteoarthritic patients. Stem Cells Int. 2014;2014:125643. doi: 10.1155/2014/125683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Horita Y, et al. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res. 2006;84:1495–1504. doi: 10.1002/jnr.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ding D-C, et al. Enhancement of neuroplasticity through upregulation of β1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27:339–353. doi: 10.1016/j.nbd.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 328.Jeong CH, et al. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed Res Int. 2014;2014:129145. doi: 10.1155/2014/129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Neuss S, Becher E, Woltje M, Tietze L, Jahnen-Dechent W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405–414. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- 330.Zhang J, et al. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004;1030:19–27. doi: 10.1016/j.brainres.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 331.Tfilin M, et al. Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Mol Psychiatry. 2010;15:1164–1175. doi: 10.1038/mp.2009.110. [DOI] [PubMed] [Google Scholar]
- 332.Casey ML, MacDonald PC. Keratinocyte growth factor expression in the mesenchymal cells of human amnion. J Clin Endocrinol Metab. 1997;82:3319–3323. doi: 10.1210/jcem.82.10.4294. [DOI] [PubMed] [Google Scholar]
- 333.Zhu YG, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Ding W, et al. Platelet-derived growth factor (PDGF)–PDGF receptor interaction activates bone marrow–derived mesenchymal stromal cells derived from chronic lymphocytic leukemia: implications for an angiogenic switch. Blood. 2010;116:2984–2993. doi: 10.1182/blood-2010-02-269894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Osborne A, Sanderson J, Martin KR. Neuroprotective effects of human mesenchymal stem cells and platelet-derived growth factor on human retinal ganglion cells. Stem Cells. 2018;36:65–78. doi: 10.1002/stem.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Mishra PJ, et al. Carcinoma-associated fibroblast–like differentiation of human mesenchymal stem cells. Can Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mayer H, et al. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem. 2005;95:827–839. doi: 10.1002/jcb.20462. [DOI] [PubMed] [Google Scholar]
- 338.Beckermann BM, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622–631. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Herrera MB, et al. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430–441. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 340.Popov C, et al. Integrins alpha2beta1 and alpha11beta1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2011;2:e186. doi: 10.1038/cddis.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Frith JE, Mills RJ, Hudson JE, Cooper-White JJ. Tailored integrin–extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev. 2012;21:2442–2456. doi: 10.1089/scd.2011.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Veevers-Lowe J, Ball SG, Shuttleworth A, Kielty CM. Mesenchymal stem cell migration is regulated by fibronectin through α5β1-integrin-mediated activation of PDGFR-β and potentiation of growth factor signals. J Cell Sci. 2011;124:1288–1300. doi: 10.1242/jcs.076935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lüttichau IV, et al. Human adult CD34− progenitor cells functionally express the chemokine receptors CCR344, CCR344, CCR344, CXCR344, and CCR344 but not CXCR344. Stem cells and development. 2005;14:329–336. doi: 10.1089/scd.2005.14.329. [DOI] [PubMed] [Google Scholar]
- 344.Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011;43:596. doi: 10.3858/emm.2011.43.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Jung Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Lu C, Li X-Y, Hu Y, Rowe RG, Weiss SJ. MT1-MMP controls human mesenchymal stem cell trafficking and differentiation. Blood. 2010;115:221–229. doi: 10.1182/blood-2009-06-228494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Egea V, et al. Tissue inhibitor of metalloproteinase-1 (TIMP-1) regulates mesenchymal stem cells through let-7f microRNA and Wnt/β-catenin signaling. Proc Natl Acad Sci. 2012;109:E309–E316. doi: 10.1073/pnas.1115083109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Chelluboina B, et al. Mesenchymal stem cell treatment prevents post-stroke dysregulation of matrix metalloproteinases and tissue inhibitors of metalloproteinases. Cell Physiol Biochem. 2017;44:1360–1369. doi: 10.1159/000485533. [DOI] [PubMed] [Google Scholar]
- 349.Najar M, et al. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: the importance of low cell ratio and role of interleukin-6. Cytotherapy. 2009;11:570–583. doi: 10.1080/14653240903079377. [DOI] [PubMed] [Google Scholar]
- 350.Beyth S, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 351.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 352.Nasef A, et al. Leukemia inhibitory factor: role in human mesenchymal stem cells mediated immunosuppression. Cell Immunol. 2008;253:16–22. doi: 10.1016/j.cellimm.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 353.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 354.Patel SA, et al. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-β. J Immunol. 2010;184:5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- 355.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Mougiakakos D, et al. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117:4826–4835. doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- 357.Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 358.Ren G, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 359.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 360.Gieseke F, et al. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;116:3770–3779. doi: 10.1182/blood-2010-02-270777. [DOI] [PubMed] [Google Scholar]
- 361.Lepelletier Y, et al. Galectin-1 and semaphorin-3A are two soluble factors conferring T-cell immunosuppression to bone marrow mesenchymal stem cell. Stem Cells Dev. 2010;19:1075–1079. doi: 10.1089/scd.2009.0212. [DOI] [PubMed] [Google Scholar]
- 362.Espagnolle N, Balguerie A, Arnaud E, Sensebe L, Varin A. CD54-mediated interaction with pro-inflammatory macrophages increases the immunosuppressive function of human mesenchymal stromal cells. Stem Cell Reports. 2017;8:961–976. doi: 10.1016/j.stemcr.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yang ZX, et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One. 2013;8:e59354. doi: 10.1371/journal.pone.0059354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells. 2017;35:766–776. doi: 10.1002/stem.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Tipnis S, Viswanathan C, Majumdar AS. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol. 2010;88:795–806. doi: 10.1038/icb.2010.47. [DOI] [PubMed] [Google Scholar]
- 366.Xue Q, et al. The negative co-signaling molecule b7-h4 is expressed by human bone marrow-derived mesenchymal stem cells and mediates its T-cell modulatory activity. Stem Cells Dev. 2010;19:27–38. doi: 10.1089/scd.2009.0076. [DOI] [PubMed] [Google Scholar]
- 367.Gu YZ, et al. Different roles of PD-L1 and FasL in immunomodulation mediated by human placenta-derived mesenchymal stem cells. Hum Immunol. 2013;74:267–276. doi: 10.1016/j.humimm.2012.12.011. [DOI] [PubMed] [Google Scholar]