Abstract
Starting from their role exerted on osteoblast and osteoclast differentiation and activity pathways, microRNAs (miRNAs) have been recently identified as regulators of different processes in bone homeostasis. For this purpose, in a recent review, we highlighted, as deregulated miRNAs could be involved in different bone diseases such as osteoporosis. In addition, recent studies supported the concept that osteoporosis-induced bone alterations might offer a receptive site for cancer cells to form bone metastases, However, to date, no data on specific-shared miRNAs between osteoporosis and bone metastases have been considered and described to clarify the evidence of this link. The main goal of this review is to underline as deregulated miRNAs in osteoporosis may have specific roles in the development of bone metastases. The review showed that several circulating osteoporotic miRNAs could facilitate tumor progression and bone-metastasis formation in several tumor types, i.e., breast cancer, prostate cancer, non-small-cell lung cancer, esophageal squamous cell carcinoma, and multiple myeloma. In detail, serum up-regulation of pro-osteoporotic miRNAs, as well as serum down-regulation of anti-osteoporotic miRNAs are common features of all these tumors and are able to promote bone metastasis. These results are of key importance and could help researcher and clinicians to establish new therapeutic strategies connected with deregulation of circulating miRNAs and able to interfere with pathogenic processes of osteoporosis, tumor progressions, and bone-metastasis formation.
Keywords: miRNA, Osteoporosis, Osteolysis, Bone metastasis
Introduction
Bone homeostasis is regulated by different signals, as parathyroid hormone (PTH), vitamin D metabolites, etc. [1]. Bone performs its functions concerning electrolytes balance, energy metabolism and mechanical competence through a continuous homeostatic balance between modelling and remodeling processes carried out mainly by two cell populations, osteoblasts (OBs) and osteoclasts (OCs), which act bone formation and resorption, respectively. Bone-marrow mesenchymal stem cells (BMSCs) differentiate into OBs, and successively in osteocytes, in response to several microenvironment signals, as wingless-type MMTV integration site family members (Wnt), bone morphogenetic proteins (BMPs), transforming growth factor-beta (TGF-β), and other factors, synthesizing bone matrix [2–4]. Peripheral blood mononuclear cells (PBMCs) in response to the low ratio between osteoprotegerin (OPG) and receptor activator of nuclear factor kappa-B ligand (RANKL) give rise to OCs that degrade the bone matrix. Inflammation status and the synthesis of pro- and anti-inflammatory cytokines play important roles in the unbalance between bone resorption and formation, influencing the pathways of OC and OB differentiation. In fact, interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-17 enhance the expression of RANKL in immune system cells and OBs, inducing osteoclastogenesis. Furthermore, pro-inflammatory status induces IL-11 and ciliary neurotrophic factor (CNTF) expression in OBs, up-regulating OC differentiation, and activities [5]. The anti-inflammatory cytokines (IL-4, IL-10, and IL-13) are instead powerful activators of osteoblastogenesis and inhibitors of osteoclastogenesis [6, 7]. In particular, IL-10, high regulated cytokine at transcriptionally and posttranscriptionally levels [8, 9], has a key role in the regulation of inflammatory response. Its determine also a suppression of OC differentiation directly altering OPG, RANKL, and M-CSF-1 expressions in OBs, other than reducing NFAT-c1 expression in OCs [9]. Unbalance between bone resorption and formation, with an altered activation of OCs, can lead to low bone mineral density, which will cause osteopenia, osteoporosis, and other bone disorders. Osteoporosis is a great public health problem, associated with fragility fractures, due to the deregulation of bone homeostasis, with increased bone resorption and diminished bone synthesis. This disease is often associated with postmenopausal status in women and with aging in men (primary osteoporosis), or due to various pre-existing causes (nutritional factors, medical treatments, chronic inflammation diseases, and other pathologies) [10]. Minimizing the morbidity and economic impact related to osteoporosis would also be of key importance to limit the related complications. Although some evidences supported the concept that osteoporosis might offer a receptive site for cancer cells to form bone metastases [11–19], how bone microenvironment changes and/or alterations may affect the dissemination of cancer tumor cells to bone remains a poorly understood topic. Bone metastases are a serious complication of patients with tumor and represent the evidence of disseminated disease associated with a poor prognosis. It is a common finding in the natural history of several types of cancers and extensively contributes to morbidity and mortality in cancer patients.
Around 70% of patients with breast and prostate cancers and about 30–40% of patients with lung cancer can develop bone metastases, bringing to skeletal-related events (SREs) that cause hypercalcemia, pathological fractures, spinal compression and bone pain, leading to poor prognoses [20]. The tumor cells metastasizing in bone lead destructive osteolytic and/or bone-forming osteoblastic lesions and ‘teach’ this affected bone microenvironment to produce factors that stimulate tumor cell growth. Once cancer cells metastasize to bone, they interrupt the physiological bone metabolism coordinated by OBs and OCs, establishing a new cellular environment much more favorable to bone-metastasis progression, the “vicious cycle” [21]. In this cycle, OC stimulating factors released by metastatic cancer cells make active bone destruction, bringing to an increase secretion of bone-derived growth factors into the bone microenvironment. These factors act on cancer cells, causing more aggressive cancer phenotypes and further bone destruction, thus suggesting that OCs play key roles in bone-metastasis process. OCs play a pivotal role also in osteoporosis, but they are not the only shared actors between bone metastases and osteoporosis. In fact, the two pathological conditions share different alterations, such as alteration in the immune functions, disturbance in the balance between pro- and anti-inflammatory regulators, improvement in angiogenesis, platelets deregulation, thromboembolism events, extracellular matrix (ECM) components, and hormone changes [19]. The multiple factors implicated both in osteoporosis and in bone metastasis, the high incidence of the diseases, the prominent decline in quality of life, the huge costs, and high mortality underline how important would be to identify specific miRNAs involved in both osteoporosis and cancer bone metastases. However, to our knowledge, no studies investigated the presence of shared microRNAs (miRNAs) between osteoporosis and bone metastases, whose deregulated expression contributes to the onset and progression of both pathological conditions.
MiRNAs are small noncoding RNA molecules of about 15–25 nucleotides implicated in posttranscriptional gene expression regulation through targeting of mRNAs. The binding of miRNA to partially complementary sequences in mRNA targets inhibits their translation or enhances their degradation [22]. MiRNAs are generated by a precursor transcript, pre-miRNAs, located in an intron of a host gene, and can be transcripted independently through a miRNA specific promoter, or with the mRNA of host gene and subsequently matured by successive cleavages. miRNA maturation is carried out in the nucleus by Drosha and the Di George syndrome critical region gene 8 (DGCR8) complex, producing the pre-miRNA hairpin. In the cytoplasm, after export by Exportin 5, pre-miRNAs are subsequently processed by the ribonuclease Dicer, producing a double-stranded mature miRNA [23]. A strand of miRNA will be bound by Argonaut 2 (AGO2) protein that direct the binding of miRNA to 3′ Untranslated Region (3′UTR) of mRNA target, determining inhibition of expression of gene target [24].
Being aware of the strong bond and of the numerous shared factors between osteoporosis and bone metastasis [18, 19], in this review, deregulated miRNAs involved in primary osteoporosis were analysed for their possible role in favoring bone-metastasis formation and/or progression. Thus, to outline shared miRNAs between these two pathological conditions, a literature research was carried out in the MEDLINE database to analyse and verify whether it has been pointed out that the deregulated miRNAs in primary osteoporosis may have a specific role in the development of bone metastases. Results of this review highlighted how different circulating miRNAs, identified in primary osteoporosis, are implicated also in favoring the progression of bone metastases in different tumors, suggesting that these miRNAs might be triggering events able to increase bone homing of cancer cells. However, further preclinical and clinical studies carried out in specific experimental preclinical models and selected patient cohorts are mandatory to contribute to the existing knowledge on shared miRNAs between primary osteoporosis and bone metastases.
Research strategies
The following literature research was carried out in the MEDLINE database (PubMed research engine), using the string “(microRNA* OR miRNA* OR miR-*) AND osteoporosis” to identify miRNAs implicated in the regulation of bone formation, resorption, and osteoporosis. We included articles written in English (AND “English” [language]) and published after January 1, 2008 (AND (“2008/01/01″[Date-Entrez]: “2018/12/31″[Date-Entrez])). Four reviewers manually assessed the titles and abstracts of collected references and selected clinical studies that identify deregulated miRNAs in primary osteoporosis. Excluding review articles (NOT Review [Publication Type]), we retrieved 34 clinical studies.
Furthermore, another literature research using the following string—“((((((((((microRNA*) OR miRNA*) OR miR*) AND ((“tumor progression”) OR metastas*)))) AND ((serum) OR circulating))))”—was done in the MEDLINE database. Then, we manually selected all articles on circulating miRNAs, identified in primary osteoporosis, with specific activities in bone metastasis, such as tumor progression, metastasis formation and bone homing, discarding articles (a) on not-circulating miRNAs; (b) with a not-clear relationship between osteoporotic circulating miRNAs and tumor progression and metastasis formation; and (c) on osteoporotic miRNAs in tumor progression and bone metastasis, where miRNAs have opposite regulation with respect to osteoporotic studies. In this way, other 62 articles were retrieved (see Table 1). Finally, other 26 references were added, as they were considered of interest to up-grade information on some technical aspects, to have a major understanding of mechanisms acting in bone regulation and primary osteoporosis development. A detailed flux diagram of research strategies is reported in Fig. 1.
Table 1.
List of miRNAs identified in osteoporosis and implicated in bone metastasis, and reported in clinical studies
| Circulating miRNA | Up- or down-in cancer | References on osteoporosis | Effects on bone cells | Tumor | Effects in microenvironment and tumor cells | Number of patients Age range (Mean age) |
Sample type | Reference on bone metastasis |
|---|---|---|---|---|---|---|---|---|
| Let-7b | Down | [40] | Let-7b down-regulation enhances matrix degradation and bone turnover | PC | Down-regulation of let-7b of PC patients is related to tumor progression |
72 – (76.5) |
Blood samples | [85] |
| MM | Down-regulation of let-7b in blood and bone marrow of MM patients is related to tumor progression and osteolytic lesion formation |
20 47–80 (64) |
Blood samples | [120] | ||||
| Let-7f | Down | [52] | Let-7b down-regulation inhibit OB differentiation | NSCLC | Down-regulation of let-7b in blood is related to tumor progression and associated with poor outcome |
106 – – |
Blood samples | [102] |
| miR-7 | Down | [40] | Down-regulation promote OC differentiation | ESCC | miR-7 represent a non-invasive biomarker of diagnosis and predicting treatment responses to concurrent chemoradiation therapy |
105 – – |
Blood samples | [113] |
| miR-10b | Up | [37, 52] | miR-10b up-regulation inhibit OB differentiation | BC | Serum miR-10b level is significantly higher in patients with bone metastases than in patients without bone metastases or control subjects |
23 – – |
Primary and metastatic BC samples | [76] |
|
61 – – |
Blood samples; primary BC samples | [63] | ||||||
|
122 26–77 52 |
Blood samples | [77] | ||||||
| ESCC | Serum miR-10b level is up-regulated in serum e tumoral tissue of ESCC patients and its level in ESCC is revealed as independent prognostic marker of the overall survival rates of patients |
195 – – |
Blood samples; primary ESCC samples | [117] | ||||
| miR-16 | Up | [40, 56] | miR-16 down-regulation improve OB differentiation and inhibits OC differentiation | BC | Metastatic miR-16 up-regulation improve osteolytic lesion formation |
38 – – |
Blood samples; primary and metastatic BC samples | [59] |
| miR-16 level is related positively with tumor progression. |
130 33–76 (56) |
Blood samples | [60] | |||||
| miR-21 | Up | [31, 37, 46, 52] | miR-21 up-regulation inhibits OB and enhances OC differentiation | BC |
Over-expression of miR-21 in the plasma of patients with BC is related to tumor progression Circulating miR-21 concentrations is able to distinguish patients with breast cancer from healthy females and further distinguish patients with distant metastases from those with locoregional disease |
197 – – |
Blood samples; primary BC samples | [64] |
|
15 – – |
Blood samples; primary BC samples | [65] | ||||||
|
326 27–65 (46) |
Blood samples; primary BC samples | [66] | ||||||
|
68 – – |
Blood samples; primary BC samples | [61] | ||||||
|
102 – – |
Blood samples, primary BC samples | [62] | ||||||
|
100 – – |
Blood samples, primary BC samples | [69] | ||||||
|
61 – – |
Blood samples; primary BC samples | [63] | ||||||
|
54 25–78 – |
Blood samples; primary BC samples | [67] | ||||||
|
118 32.1–76.6 (45.6) |
Blood samples | [68] | ||||||
| PC | miR-21 level is correlated to the progression of tumor and metastasis formation and to the resistance to docetaxel-based chemotherapy |
56 – (70.9) |
Blood samples | [86] | ||||
| Up-regulation of serum miR-21 is related PC progression |
25 51–82 (67) |
Blood samples | [87] | |||||
| miR-21 up-regulation improve Tumor Growth, migration phenotype, bone colonization and metastasis formation |
160 – – |
Primary PC samples | [88] | |||||
| NSCLC | miR-21 up-regulation is related to malignant phenotype |
221 – (66.3) |
Blood samples | [98] | ||||
| miR-21 is up-regulated in serum of NSCLC and is correlated with tumor progression |
63 36–83 – |
Blood samples | [97] | |||||
| ESCC | miR-21 is up-regulated in serum of ESCC patient, permit discrimination between malign and benign disease, and response to treatment |
99 27–83 (56.4) |
Blood samples | [118] | ||||
| miR-24 | Up | [31, 46] | miR-24 up-regulation inhibits OB differentiation | BC | Up-regulation of serum miR-24 is related to the relapse of early BC and metastasis formation |
133 37–84 (61.5) |
Blood samples | [78] |
| NSCLC | Serum miR-24 level is related NSCLC progression |
400 – – |
Blood samples | [108] | ||||
| ESCC | Serum miR-24 level is related ESCC progression |
105 – – |
Blood samples | [116] | ||||
| miR-25 | Up | [31] | miR-25 is up-regulated in osteoporotic patients | NSCLC | Up-regulation of serum miR-25 is related NSCLC progression |
400 – – |
Blood samples | [108] |
| miR-25 level is high in NSCLC patients with metastasis respect to patient with non-metastatic tumors and healthy controls |
438 – – |
Blood samples | [100] | |||||
| ESCC | miR-25 is related to tumor progression and can considered as potential biomarkers for predicting the prognosis of ESCC patients |
98 – (62) |
Blood samples | [110] | ||||
|
194 33–81 (61.4) |
Blood samples | [111] | ||||||
|
99 27–83 (56.4) |
Blood samples | [118] | ||||||
| miR-27a | Down | [55] | Up regulation inhibits OB differentiation | BC | Up-regulation of miR-27a is related to tumor progression |
54 25–78 – |
Blood samples | [67] |
| miR-29b | Down | [40, 45, 50] | miR-29b down-regulation inhibit OB differentiation | PC | Down-regulation of miR-29b is related to cancer cell migration and invasion of PC cells |
27 63–88 (73.7) |
Primary PC samples | [91] |
| Down-regulation of miR-29b is related to metastasis formation |
187 – – |
Primary PC samples | [92] | |||||
| MM | Up-regulation of miR-29b inhibits proliferation and induces apoptosis |
9 – – |
Primary MM patient samples | [122] | ||||
| miR-30e | Down | [40] | miR-30e down-regulation inhibit OB differentiation | BC | miR-30e down-regulation is related to cancer cell invasion, osteomimicry, and bone destruction |
109 – – |
Primary BC cells | [79] |
| NSCLC | Down-regulation of miR-30e in blood is related to tumor progression and associated with poor outcome |
106 – – |
Blood samples | [102] | ||||
| miR-34a | Down | [35] | miR-34a down-regulation inhibits OB and enhances OC differentiation | BC | miR-34a low expressions were associated with worse prognosis |
84 > 50 – |
Blood samples | [75] |
| miR-34a is moderately expressed in serum of BC patients, while is strongly downregulated in serum of patients with bone metastasis. |
10 – – |
Primary and metastatic BC samples | [74] | |||||
| PC | miR-34a is downregulated in Ras activated PC cells and is related to tumor growth and invasive phenotype. |
170 – – |
Primary and metastatic PC samples | [89] | ||||
| NSCLC | miR-34a high expression in plasma and tumor tissue were correlated with prolonged survival. |
196 27–82 (58) |
Blood samples; primary NSCLC samples | [99] | ||||
| ESCC | miR-34a down-expressions were associated with ESCC tumor progression and invasive phenotypes |
111 – – |
Primary ESCC samples | [114] | ||||
| miR-34a down-expressions were associated with ESCC tumor progression |
148 – – |
Primary ESCC samples | [115] | |||||
| miR-34c | Down | [40] | miR-34c down-regulation inhibits OB differentiation | BC | miR-34c low expressions were associated with worse prognosis |
84 > 50 – |
Blood samples | [75] |
| miR-34c is down-regulated in serum of BC patients |
15 – – |
Blood samples; primary BC samples | [65] | |||||
| NSCLC | miR-34c high expression in plasma and tumor tissue were both correlated with prolonged survival |
196 27–82 (58) |
Blood samples; primary NSCLC samples | [99] | ||||
| miR-96 | Up | [51] | miR-96 up-regulation inhibits OB differentiation | BC | Over-expression of miR-96 in the plasma of patients with BC is related to tumor progression |
197 – – |
Blood samples; primary BC samples | [64] |
| PC | miR-96 levels were significantly higher in the blood of PC patients and is related to poor prognosis |
26 > 55 – |
Blood samples; primary PC samples | [93] | ||||
| Up-regulation of miR-96 induce tumor progression by suppression of ETV6 expression |
69 – – |
Primary PC samples | [94] | |||||
| Up-regulation of miR-96 induce tumor progression by inducing mTOR activities |
170 – – |
Primary PC samples | [95] | |||||
| NSCLC | High levels of circulating miR-96 in NSCLC patients is related to tumor progression |
70 – – |
Blood samples; primary NSCLC samples | [103] | ||||
|
57 – – |
Primary NSCLC samples | [104] | ||||||
| miR-100 | Up | [31, 46] | miR-100 up-regulation inhibits OB differentiation | NSCLC | miR-100 levels were significantly higher in the blood of NSCLC patients with short survival |
184 – – |
Pleural effusion samples | [105] |
| ESCC | miR-100 is related to tumor progression and can considered as potential biomarkers for predicting the prognosis of ESCC patients |
98 – (62) |
Blood samples | [110] | ||||
| miR-125b | Up | [30, 31, 46] | miR-125b up-regulation inhibits OB differentiation | BC | Over-expression of miR-125b in the plasma of patients with BC is related to tumor progression |
197 – – |
Blood samples; primary BC samples | [64] |
| Over-expression of miR-21 in the plasma of patients with BC is related to tumor progression |
118 32.1–76.6 (45.6) |
Blood samples | [68] | |||||
| Higher circulating miR-125b level is correlated to more advanced ductal carcinoma of the breast and resistance for adjuvant chemotherapy |
56 36–78 (55) |
Blood samples | [70] | |||||
| Over-expression of miR-125b in the blood of patients with BC is related to tumor progression |
61 – – |
Blood samples; primary BC samples | [63] | |||||
| PC | High level of circulating miR-125b is related to aggressive phenotypes of PC cells |
82 – – |
Blood samples | [96] | ||||
| NSCLC | Circulating miR-125b level positively with cancer stage and associated with poor prognosis |
193 – – |
Blood samples | [106] | ||||
| Circulating miR-125b level is associated positively with cancer stage, tumor differentiation status and response to therapeutic treatment. |
260 – (56.45) |
Blood samples | [107] | |||||
| miR-130 | down | [40, 43] | miR-130 is down-regulated in osteoporotic patients | BC | Circulating miR-130 down-regulation is related to aggressive phenotype of BC |
40 – – |
Blood samples; Primary BC samples | [80] |
| miR-133 | Up | [27, 32, 36, 37, 44] | miR-133 down-regulation inhibits OB and enhances OC differentiation | BC | miR-133 serum levels are significantly increased in BC patients |
132 25–83 (53.8) |
Blood samples; primary BC samples | [71] |
| ESCC | miR-133 serum levels are significantly increased in ESCC patients |
290 52–70 (61.3) |
Blood samples | [109] | ||||
| miR-144 | Down | [53] | miR-144 is down-regulated in osteoporotic patients | BC | miR-144 down-regulation is related to BC tumor progression |
237 – – |
Blood samples | [81] |
| miR-148 | Up | [31, 41, 46] | miR-148 down-regulation inhibits OB and enhances OC differentiation | ESCC | miR-148 serum levels are significantly increased in ESCC patients |
290 52–70 (61.3) |
Blood samples | [109] |
| miR-152 | Up | [40] | miR-152 is up-regulated in osteoporotic patients | PC | Up-regulation of serum miR-152 is related PC progression |
25 51–82 (67) |
Blood samples | [87] |
| NSCLC | Up-regulation of serum miR-152 is related NSCLC progression |
400 – – |
Blood samples | [108] | ||||
| miR-214 | Up | [28, 52] | miR-214 up-regulation inhibit OB differentiation and promote OC differentiation | BC | miR-214 level is related to tumor progression and lytic bone lesion formations |
134 30–82 (60) |
Blood samples | [73] |
|
37 – (53) |
Primary BC samples | [72] | ||||||
| PC | Increased expression of miR-214 are associated with tumor malignancy in PC patients through inhibition of PTEN expression |
75 55–86 (72) |
Blood samples | [90] | ||||
| NSCLC | miR-214 level is high in NSCLC patients with metastasis respect to patient with only primary tumours and healthy controls |
438 – – |
Blood samples | [100] | ||||
| MM | miR-214 level is related to tumor progression and lytic bone lesion formations |
260 – – |
Blood samples | [121] | ||||
| miR-215 | down | [40] | miR-25 is down-regulated in osteoporotic patients | BC | miR-215 level is related to BC tumor progression |
75 – – |
Blood samples | [82] |
|
237 – – |
Blood samples | [81] | ||||||
| miR-320 | Up | [39, 40] | miR-320 up-regulation inhibit OB differentiation | NSCLC | Up-regulation of serum miR-320 is related NSCLC progression |
400 – – |
Blood samples | [108] |
| miR-335 | Down | [40, 48] | miR-335 down-regulation inhibit OB and promote OC differentiation | BC | miR-335 down-regulation is related to BC tumor progression |
68 – – |
Blood samples; primary BC samples | [61] |
| miR-365 | Down | [40] | miR-365 down-regulation promote OC differentiation | NSCLC | Circulating miR-365 level in NSCLC patients is correlated with poor differentiation tumor cells, advanced tumor stage and presence of metastasis, as well as short patients’ survival |
100 30–88 60.18 |
Blood samples; primary NSCLC samples | [101] |
| miR-451 | Up | [52] | miR-451 up-regulation inhibits OB differentiation | BC | High level of serum miR-451is associated to tumor grade |
240 – – |
Blood samples; primary BC samples | [83] |
| ESCC | High level of serum miR-451 is associated to aggressive phenotype |
65 – (62.77) |
Blood samples; Primary ESCC samples | [112] | ||||
| miR-483 | Up | [39] | miR-483 up-regulation inhibits OB differentiation | NSCLC | miR-483 level is high in NSCLC patients with metastasis respect to patient with only primary tumours and healthy controls |
438 – – |
Blood samples | [100] |
Fig. 1.
Flowchart of the research strategy and selection of bibliographic references
Osteoporotic-deregulated miRNAs in bone metastases
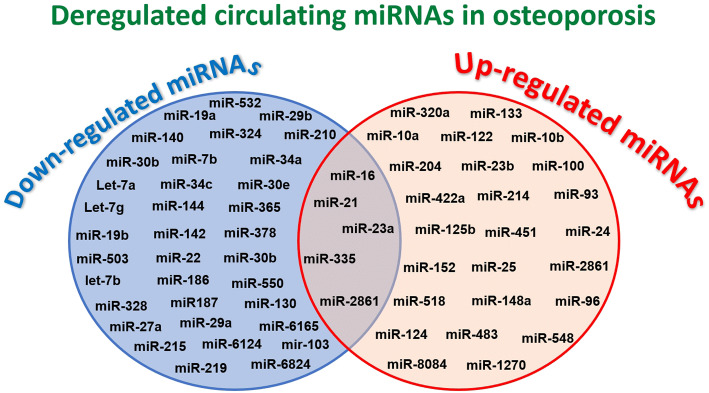
In our previous review, we reported as the deregulation of miRNAs implicated in osteogenesis and osteoclastogenesis, or both, determines profound alterations in bone homeostasis that could lead to the development of bone diseases [25]. The results of collected preclinical studies showed how a deregulation of only one miRNA has effects on OB and OC differentiations and activities, unbalancing bone formation, and resorption that lead to osteoporosis [25]. On the other hand, high-throughput RNA screening in clinical studies on osteoporosis highlighted altered serum levels only for some of these miRNAs related to bone metabolism and supposed to be implicated in osteoporosis pathogenesis [26–56]. To identify deregulated circulating miRNAs in osteoporotic patients, these clinical studies were carried out in two steps: (1) a ‘discovery step’ using RNA-sequencing or miRNA-array analysis to identify deregulated miRNAs in small cohorts patients and (2) a ‘replication or validation step’ using quantitative real-time polymerase chain reaction (qRT-PCR) to validate miRNAs in larger cohort patients [26–28, 30–48, 50–57]. By analysing the results of these clinical studies, 64 miRNAs were identified and validated, showing to be deregulated in osteoporotic patients (Fig. 2). In most studies, miR-124, miR-125b, miR-133, and miR-148a showed to be up-regulated in osteoporotic patients, while miR-29b was found to be down-regulated [27, 30–32, 36, 37, 40, 41, 44–46, 50]. On the contrary, miR-21 and miR-23a reported as deregulated in different clinical studies, had contrasting results on their up- or down-regulation probably due to the small-analysed cohorts of patients, and second to the selected control cohorts, where the control individuals frequently present other bone pathologies, as, for example, osteoarthritis, contributing to the contrasting results [29, 31, 32, 37, 45, 46, 49].
Fig. 2.
Venn diagram of down- and up-regulated miRNA in osteoporosis
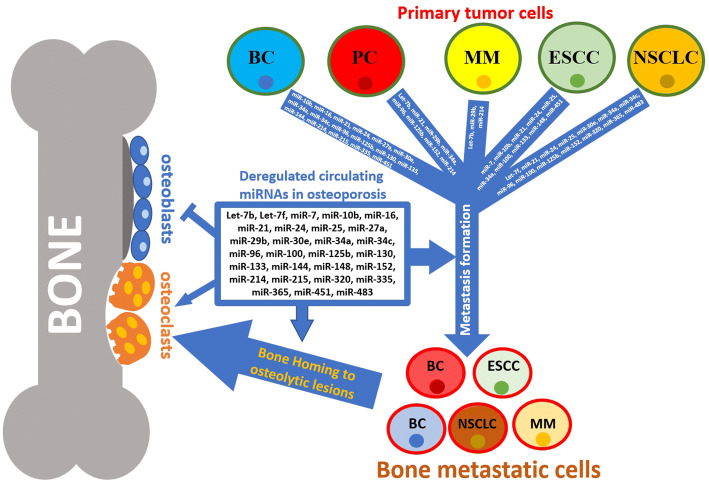
Since the progression of different tumors and metastasis formation is related to the serum level modifications of specific miRNA produced by tumor cells or other cells conditioned in tumor microenvironment [58], we screened clinical literature on bone metastases to achieve information on those circulating miRNAs identified in osteoporotic patients and implicated in bone homing and metastasis formation (Table 1 and Fig. 3).
Fig. 3.
Schematic draw of miRNA actions in bone homeostasis, and metastatic process and cell homing in bone
Table 1 reports, in alphabetically order, circulating miRNAs altered in osteoporotic patients (diagnosed with dual-energy X-ray absorptiometry—DXA), and involved in tumor progression and/or metastasis formation. Almost all studies were performed in two investigative steps. In the first step, indicated as ‘discovery step’, circulating miRNAs were screened through RNA-sequencing or miRNA-array analysis in small cohorts of osteoporotic and healthy patients. In a second step, miRNAs were validated in a larger study cohort.
Deregulated miRNAs in breast cancer
Breast cancer (BC) metastases are commonly osteolytic, characterized by enhanced OC activities due to tumor cell interactions with microenvironment. Intercommunication of BC with OCs and OBs through circulating miRNAs in the microenvironment is crucial for osteolytic bone-metastasis formation and development. In BC microenvironment, it was found that up-regulation of miR-16 [59, 60], miR-21 [61–69], miR-125b [63, 64, 68, 70], miR-133 [71], and miR-214 [72, 73] as well as down-regulation of miR-34a [74] and miR-335 [61] in serum was implicated both in augmented OC and diminished OB differentiation and activities, determining osteolytic bone lesion formation. All these miRNAs are also implicated in BC progression and bone-metastasis formations [59–74]. In particular, high serum level of miR-16 was associated with tumor progression and metastasis formation [59, 60]. Although several articles reported as miR-16 is an oncogene, it is often used as housekeeping in different studies, due to its high levels of expression in serum and cells. The use of deregulated miRNAs as housekeeping could have been determine errors in PCR data of the studies, leading to wrong conclusions, as indicated by Stückrath et al. [60]. Serum miR-21 up-regulation is commonly present in patients with BC and its serum level increase depending on tumor grade and metastasis abilities [61–69]. The first clinical study of Wang et al. showed as miR-21 is up-regulated in BC tumor samples and parallelly in patients serum [61]. Successively, Asaga et al. in a pilot study (10 healthy donors and 40 pathologic BC patients with stage I–stage IV) showed that miR-21 is correlated with BC tumor grade; the data are confirmed in a validation cohort of 62 BC patients, indicating a correlation between miR-21 levels and tumor grade and metastasis formation [62]. In addition, Mar-Aguilar et al. confirmed as miR-21 level could be used as potential BC biomarker with 94% and 80% of sensitivity and specificity, respectively [63]. Other studies, discovering deregulated serum miRNAs in BC patients, showed as seral levels of miR-21 reflect the presence of breast tumors and as its deregulation could be related to tumor progression and metastasis occurrence [64, 66–68]. Discovery strategy showed also as the high serum level of miR-125b, implicated in tumor progression of primary tumor through E2F3 targeting, is related to chemo-resistant phenotype [63, 64, 68, 70]. Chan et al. identified the up-regulation of miR-133 in serum of 32 BC patient, and validated it in a cohort of 132 patients, indicating its possible use as non-invasive biomarker [71]. Analysis of circulating miRNAs in serum of patients with malignant BC or benign breast disease evidenced as miR-214 up-regulation is implicated in tumor progression and in particular in osteolytic lesion, through TRAF3 mRNA targeting and through the stimulation of OC differentiation and activities [72, 73]. In the analysis of circulating miR-34 family members in BC, Zeng et al. showed as miR-34a down-regulation is related to patients with worse prognosis [75]. This is probably due to the fact that down-regulation of miR-34a is inversely related to MET expression, an oncogene expressed in metastasis formation, and associated with tumor grade and poor patients prognosis [74].
Deregulation of circulating miR-10b [63, 76, 77], miR-24 [78], miR-27a [67], miR-30e [79], miR-34c [65, 75], and miR-96 [64] that impair OB differentiation are also common features of BC progression and bone-metastasis formations. The first studies indicating a role for miR-10b in the development of BC metastasis is that of Zhao et al. in 2012, which supposed that circulating miR-10b could be used as discriminant between healthy and BC patients, and between BC patients with and without metastasis [77]. Expression of miR-10b is responsible for high mobility and invasive phenotype of Twist activity, through inhibition of HoxD10 transcription factor expression. Inhibition of HoxD10 results in enhanced expression of a pro-metastatic RHOC gene and invasive phenotype and is related to tumor progression and metastasis formation in BC patients [76, 77]. Liu et al. reported the correlation of a well-known miR-21 upregulation with the down-regulation of miR-34c; miR-21 up-regulation through the targeting of PTEN determines the up-regulation of AKT activities, which inactivates the FOXO3 transcription factor, inhibiting miR-34c expression, representing a common feature of BC tumor progression [65, 67].
In addition, it was demonstrated that down-regulation of miR-30e [79] and miR-34a [74, 75] in serum and BC cells is also related to epithelium–mesenchymal transition (EMT), and invasion. In fact, down-regulation of the tumor suppressor miR-30e is related to the expression of osteo-mimetic genes as CDH11, Cx43, and DKK1, osteoclastogenic interleukins, IL-8 and IL-11, connective tissue growth factor (CTGF), and integrins ITGA5 and ITGB3, stimulating motility, and invasive phenotype [79]. As already descripted, BC metastasis formation is also favored by down-regulation of miR-34a that is determined by hypermethylation of its promotor, favoring pro-metastatic oncogene Met and EMT process [74].
Other miRNAs deregulated in osteoporotic patient and found to have activities in tumor progression and metastasis formation are miR-130 [80], miR-144 [81], miR-215 [81, 82], and miR-451 [83]. In fact, miR-130a is down-regulated in human BC tissues and circulating exosomes. This miRNA inhibits cellular proliferation, migration, and invasion by regulation of RAB5B in BC stem cell-like cells [80]. Van Schooneveld et al., through microarray analyses, investigated miRNA levels in tumor and blood samples of patients with breast adenocarcinoma, evidencing a strong down-regulation of circulating miR-215 in comparison with healthy volunteers [81]. Madhavan et al., exploring circulating miRNAs in serum of BC patients, showed as miR-144 and miR-215 were significantly down-regulated and as this aspect was related to poor patients prognosis, evidenced by Kaplan–Meier curves [81]. Overexpression of miR-451 was related to cancer proliferation, and serum levels were sensibly higher with respect to healthy controls [83]. Osteolytic lacunas are described to promote bone homing of metastatic tumor cells; then, all these miRNAs are indirectly related to bone homing. However, miR-16 [59, 60] and miR-24 [78] have an active function in bone homing of metastatic cells. In fact, expression of miR-16 is related to soluble intracellular adhesion molecule (sICAM1) production, used as physical anchors that permit to metastatic cells to establish interaction with bone [59]. In BC, miR-24 transcription is inversely related to prosaposin expression, an anti-metastatic protein. Prosaposin overexpression is able to inhibit metastasis formation of different high metastatic BC cell lines, as well as reverts metastatic phenotype induced by miR-24 overexpression [78].
This BC microenvironment with these deregulated miRNAs promotes osteolytic lesions as well as tumor progression and bone-metastasis formation. In this optic, a pre-existent osteoporotic microenvironment, where these miRNAs are already deregulated, could accelerate BC tumor progression and bone-metastasis formation.
Deregulated miRNAs in prostate cancer
Like BC, prostate cancer (PC) patients frequently exhibit pathological complications related to bone metastasis; however, contrary to BC, the disease is frequently characterized by osteoblastic bone lesions [84]. In addition, in PC, tumor-circulating miRNAs influence bone cell differentiation and activities. In particular, different clinical studies showed as deregulated let-7b [85], miR-21 [86–88], miR-34a [89], and miR-214 [96] are able to influence positively OC and negatively OB differentiation. Zhang et al. demonstrated, as serum miR-21 level was correlated with serum prostate-specific antigen (PSA) level in patients with androgen-dependent prostate cancer (ADPC) and with hormone-refractory prostate cancer (HRPC); it was found that serum miR-21 level was related also to resistance of HRPC. In fact, circulating miR-21 level is higher in patients resistant to docetaxel-based chemotherapy, compared to those sensitive to chemotherapy, indicating miR-21 level as possible predictor for this chemotherapy [86, 87]. Serum up-regulation of miR-21 increases the expression of matrix metalloproteinase (MMP)2, MMP9, and MMP13, inducing extracellular matrix remodeling and facilitating EMT, thus favoring tumor cell dissemination [86–88]. Furthermore, miR-21 activates TGF-β signaling and promotes bone-marrow homing and osteolytic lesion formations in PC [88].
Similarly, down-regulation of Let-7b [85] and miR-34a [89] in PC patients is related to tumor progression, EMT, and bone-metastasis formation through different mechanisms. Guo et al. analysing serum miRNAs found that Let-7b is significantly down-regulated, and this down-regulation is related to PSA level (lower if PSA > 4 mg/ml), tumor stage (lower in T3/T4 with respect to T1/T2), and lower in androgen-resistant with respect to androgen-dependent PC cells [85]. More recently, Fang et al. using a mini-invasive approach carried out a serum miRNA analysis to found possible biomarker for discriminate between PC patients with bone metastasis, non-metastatic PC patients, patients with benign prostate hypertrophy, and healthy patients. This study evidenced the serum and tissue up-regulation of miR-214 in PC patients and this is associated with more invasive phenotype of metastatic cells. In fact, miR-214 has Pten mRNA target that determines EMT, invasive phenotype, and bone homing of tumoral cells, enhancing bone-metastasis formation [90].
Deregulated circulating miR-29b [91, 92], miR-96 [93–95], and miR-125 are common features in PC tumor progressions and they are implicated in the inhibition of OB differentiation. In fact, serum miR-29 family (miR-29a/b/c) is significantly down-regulated in PC metastases; its members’ down-regulation is responsible for the change of protein expression of focal adhesion pathways, determining the improvement of migration and bone homing of PC cells [91, 92]. Furthermore, the study carried out by Zhu et al. demonstrated that miR-29b has laminin γ1 (LAMC1) mRNA as target and is able to inhibit cancer cell migration and invasion of PC cells through the modification laminin expression, implicated in EMT [92]. In Xu et al.’s article, the authors showed as miR-96 was up-regulated in PC tissue with respect to adjacent non-cancerous tissues. Serum levels of this miRNA reflect this up-regulation showing high levels. Regarding the activity of this miRNA, the expression of miR-96 has been found inversely related to metastasis suppressor protein 1 (MTSS1) [93], ETS variant gene 6 (ETV6) [94], and AKT1S1 [95] levels, which are tumor suppressors in PC, resulting in tumor progression with activation of survival and proliferation signaling, EMT, and metastasis formation.
Using a microarray analysis, Singh et al. identify the up-regulation of miR-125b in 14 PC patients and validated it by qRT-PCR in 78 PC patients [96]. Elevated expression of miR-125 significantly predicts increased probability of biochemical progression and metastasis formation [96].
In Watahiki et al.’s study, analysing circulating miRNAs overexpressions than miR-21, it was found out that miR-152, deregulated in osteoporosis [40], seems to discriminate a sub-group of PC patients with a higher probability (> 90%) of recurrence after prostatectomy [87].
Pre-existent microenvironment with these deregulated miRNAs accelerates tumor progression and EMT process of PC cells, leading to metastasis formation.
Deregulated miRNA in non-small-cell lung cancer (NSCLC)
Non-small-cell lung cancer (NSCLC) is the most prevalent lung cancer (about 80%) with a third that develops bone metastases. In addition, for NSCLC, the intercommunication with tumor microenvironment, and in particular with circulating miRNAs, is very important for tumor progression and bone-metastasis formation.
Deregulation of miR-21 [97, 98], miR34a [99], miR-214 [100], and miR-365 [101] in NSCLC microenvironment determines an improving of OC differentiation and osteolytic lesion formation. Similarly, different deregulated miRNAs as let-7f [102], miR-24 [100], miR-30e [102], miR-34c [99], miR-96 [103, 104], miR100 [105], miR-125b [106, 107], miR320 [108], and miR483 [100] are able to impair OB differentiation and promote metastatic transformation. In addition, deregulated osteoporotic miR-25 [100, 108] and miR152 [108] are common feature of NSCLC tumor progression and bone-metastasis formation.
In Silva et al.’s study, the analysis of circulating miRNAs in NSCLC patients revealed the down-regulation of Let-7f and miR-30e, which are important tumor suppressors implicated in proliferation and tumor progression [102]. In this study, Kaplan–Meier analysis showed as levels of circulating miR-30e-3p and let-7f were associated with short disease-free survival and overall survival, respectively [102].
Zhu et al. evaluated the level of miR-183 family members that include miR-96, miR-182, and miR-183, in serum of NSCLC patients. They found as overexpression of circulating miR-96 promoted cell migration and invasion of NSCLC [103]. In fact, miR-96 acts through down-regulation of glypican 3 (GPC3), a proteoglycan implicated in the binding of extracellular matrix and regulation of heparin-binding growth factor and in different intracellular signaling pathways, promoting metastasis formation [104].
Wei et al. investigated if circulating miR-21 could be used as a biomarker for the early detection of NSCLC and its clinicopathologic features and chemotherapy sensitivity. The study effectively showed that the expression of circulating miR-21 in serum of patients with NSCLC with bone metastasis was significantly higher, representing a prognostic marker of platinum-based chemosensivity of the tumor [97]. In addition, in Shen et al., high level of miR-21 is associated with malignant phenotype with respect to benign primary tumor and this with respect to healthy controls, indicating a relation of miR-21 level with tumor progression [98].
Wang et al. showed, as miR-100 is up-regulated in pleural effusion of NSCLC patients and Kaplan–Meier curve reveals, as the up-regulation of miR-100 level in lung cancer patients was associated with short overall survival [105].
Yuxia et al. analysed circulating miR-125b in a large cohort of NSCLC patients and found that high miR-125b expression displayed a significantly poorer prognosis compared with patients with low expression of this miRNA [106]. Successively, Cui et al. confirm these data and show as high level of miR-125b is related to resistance to cisplatin-based chemotherapy treatment, as indicated by Kaplan–Meier plot. This is due to the fact that miR-125b affects cell apoptosis and proliferation, and its up-regulation determines marked inhibition of cisplatin-induced apoptosis and then the resistance to cisplatin [107].
Chen et al. carried out the analysis of 96 circulating miRNAs in serum of NSCLC patients. The study analysed the miRNA extracted from serum of 200 NSCLC patients as discovery step, and validated the miRNAs identified in the serum of other 200 NSCLC patients, identifying 10 miRNAs as possible biomarkers. Among them, miR-24, miR-25, miR-152, and miR-320 were up-regulated in osteoporotic patients. All these miRNAs are implicated in tumor progression and metastasis formation as indicated by Chen et al. [108].
Wang et al. [100] evidenced deregulated miRNAs in a multicentric and multiethnic cohort of NSCLC patients (China and America). In this study, authors have previously carried out miRNA array, containing 754 miRNAs, in a small cohort (31) of China NSCLC patients, identifying 16 miRNAs as possible biomarkers. These were tested by qRT-PCR in another cohort (19) of NSCLC patients recruited in another hospital, selecting only 9 deregulated miRNAs in this cohort. Successively, identified miRNA are validated by qRT-PCR in a three different cohorts of Chinese NSCLC patients of different hospitals (overall 63 NSCLC patients), identifying only 5 miRNAs. Finally, selected miRNA are tested in a large cohort of American NSCLC patients, including malignant NSCLC, benign nodules, and healthy controls (of 108, 56, and 48 individuals, respectively) confirming the 5 miRNA as possible biomarkers for NSCLC [100]. Among identified circulating miRNAs, miR-25, miR-214, and miR-483 are also deregulated in osteoporotic patients, where miR-25 and miR-483 are tumor cell growth/cycle-related miRNAs, while miR-214 is an immune response-related miRNA found in intercommunication between tumor cells and microenvironment [100]. Liu et al. found that tumor suppressor miR-365 is down-regulated in serum of NSCLC patients. This down-regulation determines increased concentration of serum protein TTF-1. Kaplan–Meier survival curves of NSCLC patients showed the poorest overall survival in the patient group with low miR-365 expression or high TTF-1 expression, and notably, overall survival curve for patients with miR-365-high/TTF-1-low was dramatically discrepant from those of the miR-365-low/TTF-1-high group. Patients with miR-365-high/TTF-1-high or miR-365-low/TTF-1-low show intermediate results. These results indicate that the deregulation of miR-365/TTF-1 axis contributes to tumor progression of human NSCLC [101].
Zhao et al. evaluate the activities of circulating miR-34a and miR-34c in serum of a large cohort of NSCLC patients. Serum miR-34a and miR-34c levels were positively associated with their cellular levels in NSCLCs; they were strongly down-regulated in the tumor and their levels were associated with metastatic formations. Kaplan–Meier curves indicate that high serum expression of these miRNAs was significantly correlated with prolonged disease-free survival and overall survival, indicating their tumor suppressor activities [99].
All these altered circulating miRNAs in tumor microenvironment represent a step of metastasis promoting process and could be used as possible indicators of tumor state and its progression.
Deregulated miRNA in esophageal squamous cell carcinoma (ESCC)
Esophageal squamous cell carcinoma (ESCC) is a disease with high morbidity and mortality and lack of sensitive and specific biomarkers for its early detection.
Zhang et al. carried out a screening of miRNAs, through Solexa sequencing, in serum of patients with ESCC [109]. The validation results obtained by qRT-PCR, indicated that among 25 miRNAs selected in discovery analysis, only 7 are deregulated in serum of ESCC patients. Among these 7 miRNAs, the up-regulation of miR-148 and miR-133, which represents common features of ESCC and characterizes its EMT and metastasis formation, was related to inhibited osteogenesis and improved OC differentiation. These phenomena promote osteolytic lesion formation and bone colonization by ESCC cells [109].
Wu et al.’s study reported the screening of 754 serum miRNA using Taq man microarray to identify possible biomarker for early detection of ESCC [110]. One hundred 11 ESCC patients were enrolled: 28 of them were used for the discovery step and all the others for the validation step, identifying 7 circulating miRNAs useful as possible biomarkers. Serum up-regulation of miR-25 was significantly high in ESCC patients and correlated with TNM stage and the presence of metastases [110], but function of this up-regulation must to be investigated, given that up-regulation in the tumor inhibits proliferation and progression [111]. Although it has a tumor suppressor activity in ESCC cells, serum miR-100 up-regulation seems related to more invasive phenotype [110].
In ESCC, miR-34a overexpression significantly suppressed cell proliferation and growth, promoted apoptosis, and inhibited cell migration, acting as a tumor suppressor; its down-regulation represents an important step in the process of ESCC metastasis formation [114, 115]. Concerning miR-7, it was seen that its serum level is significantly correlated with the dimension of tumor and with the presence of metastases. Serum miR-7 level is significantly lower in patients with bigger tumors compared with patients with smaller ones. Furthermore, miR-7 level is also related to response to chemoradiotherapy, probably due to targeting of EGFR mRNA, indicating possible use as response predictive marker [113].
The analysis of serum miR-24 reveals its potential as diagnostic factor and predictive biomarker for ESCC. It is significantly up-regulated in patient with ESCC with respect to healthy controls, and evidenced a correlation with miR-24 level with the response to chemoradiotherapy. In fact, ROC curve indicates high sensitivity (81.9%) and specificity (83.3%) with respect to controls [116]. In a recent study, Lu et al. evidenced the up-regulation of serum e tumoral miR-10b due to hypomethylation of its promotor in ESCC cells. Furthermore, miR-10b level in ESCC is revealed as independent prognostic marker of the overall survival rates of patients, due to Foxo3a mRNA targeting. Foxo3A targeting by miR-10b contributes to proliferation of ESCC cells and metastasis formation [117].
Khazaei et al. evaluated miR-451 overexpression in serum and down-expression in tissue samples of ESCC patients in comparison with healthy controls and normal tissue, respectively [112]. The authors demonstrated, as miR-451 is synthetized in tumoral cells but exported through exosomes, indicating a miR-451 function in tumor microenvironment [112].
Finally, Wang et al. investigated the feasibility of using serum microRNAs as biomarkers for ESCC. They found that the up-regulation of miR-21 and miR-25 in serum of ESCC patients and assumed that it could be used as biomarkers for ESCC diagnosis, discriminating benign diseases to ESCC, and also therapeutic efficiency of chemotherapeutic treatments. Particularly, miR-21 with its high and stable sensitivity and specificity for diagnostic and monitoring treatment efficacy could be to represent an ideal tumor markers [118].
Deregulated miRNA in multiple myeloma (MM)
It is known that conditioned medium of multiple myeloma (MM) tumor cells and its exosomes fraction are able to induce OC differentiation producing osteolytic lesions [119]. Wang et al. evidenced a specific miRNA profile of bone-marrow microenvironment of MM patients, stating that the progression of MM requires the careful coordination of miRNA-regulatory pathways [120]. Different miRNAs with anti-proliferative and pro-apoptotic activities, such as let-7b, were found to be down-regulated in bone marrow and serum; this event was associated with tumor progression of MM and osteolytic lesion formation [120]. As in BC, the high expression of miR-214 [121] as well as the down-regulation of miR-29b [122] in bone marrow and serum were related to MM progression. In Hao et al.’s study [121], the analysis of serum miRNAs of 108 MM patients evidenced the up-regulation of miR-214 with respect to healthy controls, which highly correlated with osteolytic disease of these patients; thus, high miR-214 level is considered a predictor for poor prognosis of MM patients [121]. Using miRNA array and qRT-PCR, Zhang et al. evidenced, as miR-29b was down-regulated in MM patients. They demonstrated in an in vitro model using MM cell lines, as the overexpression of miR-29b was able to induce apoptosis and elevated caspase-3 activation. This effect is due to myeloid-cell-leukemia 1(Mcl-1) mRNA targeting, antagonizing IL-6-dependent Mcl-1 induction in myeloma cells [122]. MiR-29b and miR-214 are implicated in both osteoblastogenesis and osteoclastogenesis, inhibiting the first and promoting the second, determining osteolytic lesion formation and colonization of tumor cells [121, 122].
Conclusion
Aging is accompanied to a progressively decrease of OB differentiation abilities and to a preferential differentiation in adipocytes of BMSCs. The increased adipose tissue produces different factors that inhibit OB differentiation. In postmenopausal women, as well as in aged men, the diminished sex-related hormone production induces bone loss. In both cases, in aging population increase osteoporotic fracture risk.
The current review was focused on clinical studies regarding circulating deregulated miRNAs in osteoporosis and as these could favor bone-metastasis dissemination, promoting tumor progression and/or osteoclastic lesion formations. We observed previously how different circulating miRNAs, identified in osteoporosis are implicated also in favoring the onset and progression of metastatic bone disease in different tumors. This evidence suggests the possibility that these deregulated miRNAs might be triggering events that increase bone homing of cancer cells [18]. A recent literature review supports this hypothesis [19] and using miRNAs, as adjuvant tools in bone metastases targets might be promising, but further preclinical and clinical studies carried out in specific experimental models and selected patient cohorts, respectively, are mandatory.
The analysis of the reported clinical studies on miRNAs implicated in breast, prostate, lung, esophageal, and multiple myeloma bone metastases indicates a role played by specific miRNAs, previously identified also in osteoporosis, as factors implicated in bone homing and osteolytic lesion formations through interactions with bone cells (OB, OC, stem cells and others) and their microenvironments. The principal identified miRNA deregulated in osteoporosis is also deregulated in different types of tumors, where they exert their effects in bone homing and tumor dissemination. In particular, serum up-regulation of pro-osteoporotic miR-21, as well as serum down-regulation of anti-osteoporotic miR-34a, that induce osteoclastic differentiation, are common features of almost all tumors (BC, PC, NSCLC, and ESCC) and are able to promote osteolytic metastasis. In addition, current review evidenced also as deregulated miRNAs in primary osteoporosis might play a critical role in bone metastatic niche preparation in the tumor microenvironments, acting directly to tumor cells, where miRNAs interfere in different pathways, as well as inducing osteolytic lesions, or where miRNAs act enhancing OC and inhibiting OB differentiation in metastatic niches. This dual vision, bone-metastasis-inducing osteoporosis, and osteoporosis-inducing bone metastasis could help to establish new therapeutic strategy interfering in both pathogenic processes, osteoporosis, tumor progressions, and bone-metastasis formation, closely interconnected with deregulation of circulating miRNAs.
Implementing the biological knowledge and both preclinical and clinical studies on miRNAs involved in osteoporosis and bone metastasis could help researchers and clinicians from different disciplines (oncologists, general and orthopedic surgeons, endocrinologists, pathologists, and nuclear medicine physicians) to better understand the importance of pre- and postmenopausal bone microenvironment and its clinical implications for the development and establishment of a personalized and precision medicine approach in cancer patients. However, the use of these miRNAs in routine clinical practice still requires further research and prospective clinical trials with a larger number of patients with multicenter cross validation. The limits of the clinical studies considered in the current review are partially responsible for the need for these further studies. Among them, the most frequently reported limits are: (1) small sample size or patient cohorts limited to some ethnic populations; (2) the necessity to verify achieved results in different chemotherapy regimens; and (3) the need to characterize marker proteins that allow enrichment of tumor-derived exosomes over healthy exosomes [64, 66, 68, 99, 104].
Nevertheless, clinical applications using RNA-based techniques have been hindered by high RNA fragility, which should be delivered avoiding degradation to preserve their therapeutic efficacy. Our recent review highlights the rapidly evolution of engineered exosomes for miRNAs’ delivery in various research fields such as regenerative medicine (miRNAs in osteoporosis or osteoarthritis) and oncology (for siRNA and chemotherapeutic agents), representing ideal vehicle for these molecules in a in vivo procedure [123].
Acknowledgements
Dr. Daniele Bellavia, Dr. Angela De Luca, Dr. Valeria Carina, Dr. Viviana Costa, and Dr. Lavinia Raimondi contributed to the manuscript by working at the Technology Platform of Tissue Engineering, Theranostics and Oncology (Lab.Manager Dr. Gianluca Giavaresi), a laboratory started-up by the Rizzoli Orthopedic Institute in Palermo (Italy) with the grants also of National Operative Program projects (PON, MIUR).
Abbreviations
- 3′UTR
3′ Untranslated region
- AGO2
Argonaut 2
- BC
Breast cancer
- BMSCs
Bone-marrow mesenchymal stem cells
- BMPs
Bone morphogenetic proteins
- CNTF
Ciliary neurotrophic factor
- DGCR8
Di George syndrome critical region gene 8
- ESCC
Esophageal squamous cell carcinoma
- GPC3
Glypican 3
- IL
Interleukin
- MM
Multiple myeloma
- MMP
Matrix metalloproteinase
- MTSS1
Metastasis suppressor protein 1
- MiRNAs
MicroRNAs
- NSCLC
Non-small-cell lung cancer
- OB
Osteoblast
- OC
Osteoclast
- OPG
Osteoprotegerin
- PC
Prostate cancer
- PTH
Parathyroid hormone
- PBMCs
Peripheral blood mononuclear cells
- qRT-PCR
Quantitative real-time polymerase chain reaction
- RANKL
Receptor activator of nuclear factor kappa-B ligand
- SREs
Skeletal-related events
- TGFβ
Transforming growth factor-beta
- TNFα
Tumor necrosis factor alpha
- Wnt
Wingless-type MMTV integration site family
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests. No benefits in any form were received or will be received from a commercial party related directly or indirectly to the subject of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 2015;2015:421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011;112:750–755. doi: 10.1002/jcb.22994. [DOI] [PubMed] [Google Scholar]
- 3.Guntur AR, Rosen CJ. IGF-1 regulation of key signaling pathways in bone. Bonekey Rep. 2013;2:437. doi: 10.1038/bonekey.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larousserie F, et al. Frontline science: human bone cells as a source of IL-27 under inflammatory conditions: role of TLRs and cytokines. J Leukoc Biol. 2017;101:1289–1300. doi: 10.1189/jlb.3HI0616-280R. [DOI] [PubMed] [Google Scholar]
- 6.Yamada A, et al. Interleukin-4 inhibition of osteoclast differentiation is stronger than that of interleukin-13 and they are equivalent for induction of osteoprotegerin production from osteoblasts. Immunology. 2007;120:573–579. doi: 10.1111/j.1365-2567.2006.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silfverswärd CJ, Penno H, Frost A, Nilsson O, Ljunggren O. Expression of markers of activity in cultured human osteoblasts: effects of interleukin-4 and interleukin-13. Scand J Clin Lab Investig. 2010;70:338–342. doi: 10.3109/00365513.2010.488698. [DOI] [PubMed] [Google Scholar]
- 8.Forte GI, et al. Characterization of two alternative Interleukin(IL)-10 5′UTR mRNA sequences, induced by lipopolysaccharide (LPS) stimulation of peripheral blood mononuclear cells. Mol Immunol. 2009;46:2161–2166. doi: 10.1016/j.molimm.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Chen B, Yan F, Guo J, Zhu X, Ma S, Yang W. Interleukin-10 inhibits bone resorption: a potential therapeutic strategy in periodontitis and other bone loss diseases. Biomed Res Int. 2014;2014:284836. doi: 10.1155/2014/284836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellavia D, Costa V, De Luca A, Maglio M, Pagani S, Fini M, Giavaresi G. Vitamin D level between calcium-phosphorus homeostasis and immune system: new perspective in osteoporosis. Curr Osteoporos Rep. 2016 doi: 10.1007/s11914-016-0331-2. [DOI] [PubMed] [Google Scholar]
- 11.Kraemer B, Rothmund R, Banys M, Krawczyk N, Solomayer EF, Mack C, Wallwiener D, Fehm T. Impaired bone microenvironment: correlation between bone density and presence of disseminated tumor cells. Anticancer Res. 2011;31:4423–4428. [PubMed] [Google Scholar]
- 12.Coleman R, et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15:997–1006. doi: 10.1016/S1470-2045(14)70302-X. [DOI] [PubMed] [Google Scholar]
- 13.Ottewell PD, Wang N, Brown HK, Reeves KJ, Fowles CA, Croucher PI, Eaton CL, Holen I. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin Cancer Res. 2014;20:2922–2932. doi: 10.1158/1078-0432.CCR-13-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagani S, Fini M, Giavaresi G, Salamanna F, Borsari V. The active role of osteoporosis in the interaction between osteoblasts and bone metastases. Bone. 2015;79:176–182. doi: 10.1016/j.bone.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Salamanna F, et al. An in vitro 3D bone metastasis model by using a human bone tissue culture and human sex-related cancer cells. Oncotarget. 2016;7:76966–76983. doi: 10.18632/oncotarget.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salamanna F, Contartese D, Maglio M, Fini M. A systematic review on in vitro 3D bone metastases models: a new horizon to recapitulate the native clinical scenario? Oncotarget. 2016;7:44803–44820. doi: 10.18632/oncotarget.8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salamanna F, Pagani S, Maglio M, Borsari V, Giavaresi G, Martelli AM, Buontempo F, Fini M. Estrogen-deficient osteoporosis enhances the recruitment and activity of osteoclasts by breast cancer cells. Histol Histopathol. 2016;31:83–93. doi: 10.14670/HH-11-651. [DOI] [PubMed] [Google Scholar]
- 18.Salamanna F, Borsari V, Brogini S, Torricelli P, Cepollaro S, Cadossi M, Fini M. A human 3D in vitro model to assess the relationship between osteoporosis and dissemination to bone of breast cancer tumor cells. J Cell Physiol. 2017;232:1826–1834. doi: 10.1002/jcp.25708. [DOI] [PubMed] [Google Scholar]
- 19.Salamanna F, Borsari V, Contartese D, Nicoli Aldini N, Fini M. Link between estrogen deficiency osteoporosis and susceptibility to bone metastases: a way towards precision medicine in cancer patients. Breast. 2018;41:42–50. doi: 10.1016/j.breast.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 21.David Roodman G, Silbermann R. Mechanisms of osteolytic and osteoblastic skeletal lesions. Bonekey Rep. 2015;4:753. doi: 10.1038/bonekey.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landgraf P, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizoguchi F, et al. Osteoclast-specific Dicer gene deficiency suppresses osteoclastic bone resorption. J Cell Biochem. 2010;109:866–875. doi: 10.1002/jcb.22228. [DOI] [PubMed] [Google Scholar]
- 24.Bartel DP. Metazoan microRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellavia D, et al. Deregulated miRNAs in bone health: epigenetic roles in osteoporosis. Bone. 2019;122:52–75. doi: 10.1016/j.bone.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Li H, et al. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Investig. 2009;119:3666–3677. doi: 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Li L, Moore BT, Peng XH, Fang X, Lappe JM, Recker RR, Xiao P. MiR-133a in human circulating monocytes: a potential biomarker associated with postmenopausal osteoporosis. PLoS One. 2012;7:e34641. doi: 10.1371/journal.pone.0034641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, et al. miR-214 targets ATF4 to inhibit bone formation. Nat Med. 2013;19:93–100. doi: 10.1038/nm.3026. [DOI] [PubMed] [Google Scholar]
- 29.Yang N, et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res. 2013;28:559–573. doi: 10.1002/jbmr.1798. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, et al. MicroRNA-125b suppresses the proliferation and osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Mol Med Rep. 2014;9:1820–1826. doi: 10.3892/mmr.2014.2024. [DOI] [PubMed] [Google Scholar]
- 31.Seeliger C, Karpinski K, Haug AT, Vester H, Schmitt A, Bauer JS, van Griensven M. Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J Bone Miner Res. 2014;29:1718–1728. doi: 10.1002/jbmr.2175. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Wang Z, Fu Q, Zhang J. Plasma miRNA levels correlate with sensitivity to bone mineral density in postmenopausal osteoporosis patients. Biomarkers. 2014;19:553–556. doi: 10.3109/1354750X.2014.935957. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Cheng P, Xie H, Zhou HD, Wu XP, Liao EY, Luo XH. MiR-503 regulates osteoclastogenesis via targeting RANK. J Bone Miner Res. 2014;29:338–347. doi: 10.1002/jbmr.2032. [DOI] [PubMed] [Google Scholar]
- 34.Cao Z, Moore BT, Wang Y, Peng XH, Lappe JM, Recker RR, Xiao P. MiR-422a as a potential cellular microRNA biomarker for postmenopausal osteoporosis. PLoS One. 2014;9:e97098. doi: 10.1371/journal.pone.0097098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krzeszinski JY, et al. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature. 2014;512:431–435. doi: 10.1038/nature13375. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Lv H, Sun Y, Zhang Y. MiR-133 is involved in estrogen deficiency-induced osteoporosis through modulating osteogenic differentiation of mesenchymal stem cells. Med Sci Monit. 2015;21:1527–1534. doi: 10.12659/MSM.894323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weilner S, et al. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone. 2015;79:43–51. doi: 10.1016/j.bone.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 38.Garmilla-Ezquerra P, Sañudo C, Delgado-Calle J, Pérez-Nuñez MI, Sumillera M, Riancho JA. Analysis of the bone microRNome in osteoporotic fractures. Calcif Tissue Int. 2015;96:30–37. doi: 10.1007/s00223-014-9935-7. [DOI] [PubMed] [Google Scholar]
- 39.De-Ugarte L, et al. MiRNA profiling of whole trabecular bone: identification of osteoporosis-related changes in MiRNAs in human hip bones. BMC Med Genom. 2015;8:75. doi: 10.1186/s12920-015-0149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kocijan R, et al. Circulating microRNA signatures in patients with idiopathic and postmenopausal osteoporosis and fragility fractures. J Clin Endocrinol Metab. 2016;101:4125–4134. doi: 10.1210/jc.2016-2365. [DOI] [PubMed] [Google Scholar]
- 41.Bedene A, et al. MiR-148a the epigenetic regulator of bone homeostasis is increased in plasma of osteoporotic postmenopausal women. Wien Klin Wochenschr. 2016;128:519–526. doi: 10.1007/s00508-016-1141-3. [DOI] [PubMed] [Google Scholar]
- 42.You L, Pan L, Chen L, Gu W, Chen J. MiR-27a is essential for the shift from osteogenic differentiation to adipogenic differentiation of mesenchymal stem cells in postmenopausal osteoporosis. Cell Physiol Biochem. 2016;39:253–265. doi: 10.1159/000445621. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, et al. Identification of suitable reference gene and biomarkers of serum miRNAs for osteoporosis. Sci Rep. 2016;6:36347. doi: 10.1038/srep36347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, et al. LncRNA MEG3 inhibited osteogenic differentiation of bone marrow mesenchymal stem cells from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed Pharmacother. 2017;89:1178–1186. doi: 10.1016/j.biopha.2017.02.090. [DOI] [PubMed] [Google Scholar]
- 45.Yavropoulou MP, Anastasilakis AD, Makras P, Tsalikakis DG, Grammatiki M, Yovos JG. Expression of microRNAs that regulate bone turnover in the serum of postmenopausal women with low bone mass and vertebral fractures. Eur J Endocrinol. 2017;176:169–176. doi: 10.1530/EJE-16-0583. [DOI] [PubMed] [Google Scholar]
- 46.Kelch S, Balmayor ER, Seeliger C, Vester H, Kirschke JS, van Griensven M. miRNAs in bone tissue correlate to bone mineral density and circulating miRNAs are gender independent in osteoporotic patients. Sci Rep. 2017;7:15861. doi: 10.1038/s41598-017-16113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiménez-Ortega RF, et al. Identification of microRNAs in human circulating monocytes of postmenopausal osteoporotic Mexican-Mestizo women: a pilot study. Exp Ther Med. 2017;14:5464–5472. doi: 10.3892/etm.2017.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YJ, Chang WA, Huang MS, Chen CH, Wang KY, Hsu YL, Kuo PL. Identification of novel genes in aging osteoblasts using next-generation sequencing and bioinformatics. Oncotarget. 2017;8:113598–113613. doi: 10.18632/oncotarget.22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu CH, Sui BD, Du FY, Shuai Y, Zheng CX, Zhao P, Yu XR, Jin Y. miR-21 deficiency inhibits osteoclast function and prevents bone loss in mice. Sci Rep. 2017;7:43191. doi: 10.1038/srep43191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feichtinger X, et al. Bone-related circulating microRNAs miR-29b-3p, miR-550a-3p, and miR-324-3p and their association to bone microstructure and histomorphometry. Sci Rep. 2018;8:4867. doi: 10.1038/s41598-018-22844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu H, Liu Q, Wu XP, He HB, Fu L. MiR-96 regulates bone metabolism by targeting osterix. Clin Exp Pharmacol Physiol. 2018;45:602–613. doi: 10.1111/1440-1681.12912. [DOI] [PubMed] [Google Scholar]
- 52.Ren H, et al. miRNA-seq analysis of human vertebrae provides insight into the mechanism underlying GIOP. Bone. 2018;120:371–386. doi: 10.1016/j.bone.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 53.Wang C, He H, Wang L, Jiang Y, Xu Y. Reduced miR-144-3p expression in serum and bone mediates osteoporosis pathogenesis by targeting RANK. Biochem Cell Biol. 2018;96:627–635. doi: 10.1139/bcb-2017-0243. [DOI] [PubMed] [Google Scholar]
- 54.Ramírez-Salazar EG, et al. Serum miRNAs miR-140-3p and miR-23b-3p as potential biomarkers for osteoporosis and osteoporotic fracture in postmenopausal Mexican-Mestizo women. Gene. 2018;679:19–27. doi: 10.1016/j.gene.2018.08.074. [DOI] [PubMed] [Google Scholar]
- 55.Aquino-Martinez R, et al. miR-219a-5p regulates Rorβ during osteoblast differentiation and in age-related bone loss. J Bone Miner Res. 2018;34:135–144. doi: 10.1002/jbmr.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mencía Castaño I, Curtin CM, Duffy GP, O'Brien FJ. Harnessing a novel inhibitory role of miR-16 in osteogenesis by human mesenchymal stem cells for advanced scaffold-based bone tissue engineering. Tissue Eng Part A. 2018;25:24–33. doi: 10.1089/ten.TEA.2017.0460. [DOI] [PubMed] [Google Scholar]
- 57.Mäkitie RE, Hackl M, Niinimäki R, Kakko S, Grillari J, Mäkitie O. Altered microRNA profile in osteoporosis caused by impaired WNT signaling. J Clin Endocrinol Metab. 2018;103:1985–1996. doi: 10.1210/jc.2017-02585. [DOI] [PubMed] [Google Scholar]
- 58.Haider MT, Taipaleenmäki H. Targeting the metastatic bone microenvironment by MicroRNAs. Front Endocrinol (Lausanne) 2018;9:202. doi: 10.3389/fendo.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ell B, Mercatali L, Ibrahim T, Campbell N, Schwarzenbach H, Pantel K, Amadori D, Kang Y. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell. 2013;24:542–556. doi: 10.1016/j.ccr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stückrath I, Rack B, Janni W, Jäger B, Pantel K, Schwarzenbach H. Aberrant plasma levels of circulating miR-16, miR-107, miR-130a and miR-146a are associated with lymph node metastasis and receptor status of breast cancer patients. Oncotarget. 2015;6:13387–13401. doi: 10.18632/oncotarget.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang F, Zheng Z, Guo J, Ding X. Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol Oncol. 2010;119:586–593. doi: 10.1016/j.ygyno.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 62.Asaga S, Kuo C, Nguyen T, Terpenning M, Giuliano AE, Hoon DS. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011;57:84–91. doi: 10.1373/clinchem.2010.151845. [DOI] [PubMed] [Google Scholar]
- 63.Mar-Aguilar F, Mendoza-Ramírez JA, Malagón-Santiago I, Espino-Silva PK, Santuario-Facio SK, Ruiz-Flores P, Rodríguez-Padilla C, Reséndez-Pérez D. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis Markers. 2013;34:163–169. doi: 10.1155/2013/259454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matamala N, et al. Tumor microRNA expression profiling identifies circulating microRNAs for early breast cancer detection. Clin Chem. 2015;61:1098–1106. doi: 10.1373/clinchem.2015.238691. [DOI] [PubMed] [Google Scholar]
- 65.Liu X, Feng J, Tang L, Liao L, Xu Q, Zhu S. The regulation and function of miR-21-FOXO3a-miR-34b/c signaling in breast cancer. Int J Mol Sci. 2015;16:3148–3162. doi: 10.3390/ijms16023148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang G, Wang L, Sun S, Wu J, Wang Q. Quantitative measurement of serum microRNA-21 expression in relation to breast cancer metastasis in Chinese females. Ann Lab Med. 2015;35:226–232. doi: 10.3343/alm.2015.35.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jurkovicova D, et al. Evaluation of expression profiles of microRNAs and two target genes, FOXO3a and RUNX2, effectively supports diagnostics and therapy predictions in breast cancer. Neoplasma. 2016;63:941–951. doi: 10.4149/neo_2016_613. [DOI] [PubMed] [Google Scholar]
- 68.Liu B, et al. Serum miR-21 and miR-125b as markers predicting neoadjuvant chemotherapy response and prognosis in stage II/III breast cancer. Hum Pathol. 2017;64:44–52. doi: 10.1016/j.humpath.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Si H, Sun X, Chen Y, Cao Y, Chen S, Wang H, Hu C. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J Cancer Res Clin Oncol. 2013;139:223–229. doi: 10.1007/s00432-012-1315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, Tan G, Dong L, Cheng L, Li K, Wang Z, Luo H. Circulating MiR-125b as a marker predicting chemoresistance in breast cancer. PLoS One. 2012;7:e34210. doi: 10.1371/journal.pone.0034210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan M, et al. Identification of circulating microRNA signatures for breast cancer detection. Clin Cancer Res. 2013;19:4477–4487. doi: 10.1158/1078-0432.CCR-12-3401. [DOI] [PubMed] [Google Scholar]
- 72.Liu J, et al. Osteoclastic miR-214 targets TRAF3 to contribute to osteolytic bone metastasis of breast cancer. Sci Rep. 2017;7:40487. doi: 10.1038/srep40487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwarzenbach H, Milde-Langosch K, Steinbach B, Müller V, Pantel K. Diagnostic potential of PTEN-targeting miR-214 in the blood of breast cancer patients. Breast Cancer Res Treat. 2012;134:933–941. doi: 10.1007/s10549-012-1988-6. [DOI] [PubMed] [Google Scholar]
- 74.Maroni P, Puglisi R, Mattia G, Carè A, Matteucci E, Bendinelli P, Desiderio MA. In bone metastasis miR-34a-5p absence inversely correlates with Met expression, while Met oncogene is unaffected by miR-34a-5p in non-metastatic and metastatic breast carcinomas. Carcinogenesis. 2017;38:492–503. doi: 10.1093/carcin/bgx027. [DOI] [PubMed] [Google Scholar]
- 75.Zeng Z, Chen X, Zhu D, Luo Z, Yang M. Low expression of circulating microRNA-34c is associated with poor prognosis in triple-negative breast cancer. Yonsei Med J. 2017;58:697–702. doi: 10.3349/ymj.2017.58.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 77.Zhao FL, Hu GD, Wang XF, Zhang XH, Zhang YK, Yu ZS. Serum overexpression of microRNA-10b in patients with bone metastatic primary breast cancer. J Int Med Res. 2012;40:859–866. doi: 10.1177/147323001204000304. [DOI] [PubMed] [Google Scholar]
- 78.Bašová P, Pešta M, Sochor M, Stopka T. Prediction potential of Serum miR-155 and miR-24 for relapsing early breast cancer. Int J Mol Sci. 2017;18:1–10. doi: 10.3390/ijms18102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Croset M, et al. miRNA-30 Family members inhibit breast cancer invasion, osteomimicry, and bone destruction by directly targeting multiple bone metastasis-associated genes. Cancer Res. 2018;78:5259–5273. doi: 10.1158/0008-5472.CAN-17-3058. [DOI] [PubMed] [Google Scholar]
- 80.Kong X, Zhang J, Li J, Shao J, Fang L. MiR-130a-3p inhibits migration and invasion by regulating RAB5B in human breast cancer stem cell-like cells. Biochem Biophys Res Commun. 2018;501:486–493. doi: 10.1016/j.bbrc.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 81.Madhavan D, et al. Circulating miRNAs with prognostic value in metastatic breast cancer and for early detection of metastasis. Carcinogenesis. 2016;37:461–470. doi: 10.1093/carcin/bgw008. [DOI] [PubMed] [Google Scholar]
- 82.van Schooneveld E, et al. Expression profiling of cancerous and normal breast tissues identifies microRNAs that are differentially expressed in serum from patients with (metastatic) breast cancer and healthy volunteers. Breast Cancer Res. 2012;14:R34. doi: 10.1186/bcr3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ng EK, et al. Circulating microRNAs as specific biomarkers for breast cancer detection. PLoS One. 2013;8:e53141. doi: 10.1371/journal.pone.0053141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin SC, Yu-Lee LY, Lin SH. Osteoblastic factors in prostate cancer bone metastasis. Curr Osteoporos Rep. 2018;16:642–647. doi: 10.1007/s11914-018-0480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo X, Han T, Hu P, Zhu C, Wang Y, Chang S. Five microRNAs in serum as potential biomarkers for prostate cancer risk assessment and therapeutic intervention. Int Urol Nephrol. 2018;50:2193–2200. doi: 10.1007/s11255-018-2009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang HL, et al. Serum miRNA-21: elevated levels in patients with metastatic hormone-refractory prostate cancer and potential predictive factor for the efficacy of docetaxel-based chemotherapy. Prostate. 2011;71:326–331. doi: 10.1002/pros.21246. [DOI] [PubMed] [Google Scholar]
- 87.Watahiki A, Macfarlane RJ, Gleave ME, Crea F, Wang Y, Helgason CD, Chi KN. Plasma miRNAs as biomarkers to identify patients with castration-resistant metastatic prostate cancer. Int J Mol Sci. 2013;14:7757–7770. doi: 10.3390/ijms14047757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonci D, et al. A microRNA code for prostate cancer metastasis. Oncogene. 2016;35:1180–1192. doi: 10.1038/onc.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen WY, et al. MicroRNA-34a regulates WNT/TCF7 signaling and inhibits bone metastasis in Ras-activated prostate cancer. Oncotarget. 2015;6:441–457. doi: 10.18632/oncotarget.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fang Y, Qiu J, Jiang ZB, Xu SR, Zhou ZH, He RL. Increased serum levels of miR-214 in patients with PCa with bone metastasis may serve as a potential biomarker by targeting PTEN. Oncol Lett. 2019;17:398–405. doi: 10.3892/ol.2018.9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishikawa R, et al. Tumor-suppressive microRNA-29s inhibit cancer cell migration and invasion via targeting LAMC1 in prostate cancer. Int J Oncol. 2014;45:401–410. doi: 10.3892/ijo.2014.2437. [DOI] [PubMed] [Google Scholar]
- 92.Zhu C, Hou X, Zhu J, Jiang C, Wei W. Expression of miR-30c and miR-29b in prostate cancer and its diagnostic significance. Oncol Lett. 2018;16:3140–3144. doi: 10.3892/ol.2018.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu L, Zhong J, Guo B, Zhu Q, Liang H, Wen N, Yun W, Zhang L. miR-96 promotes the growth of prostate carcinoma cells by suppressing MTSS1. Tumour Biol. 2016;37:12023–12032. doi: 10.1007/s13277-016-5058-2. [DOI] [PubMed] [Google Scholar]
- 94.Tsai YC, Chen WY, Siu MK, Tsai HY, Yin JJ, Huang J, Liu YN. Epidermal growth factor receptor signaling promotes metastatic prostate cancer through microRNA-96-mediated downregulation of the tumor suppressor ETV6. Cancer Lett. 2017;384:1–8. doi: 10.1016/j.canlet.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 95.Siu MK, Tsai YC, Chang YS, Yin JJ, Suau F, Chen WY, Liu YN. Transforming growth factor-β promotes prostate bone metastasis through induction of microRNA-96 and activation of the mTOR pathway. Oncogene. 2015;34:4767–4776. doi: 10.1038/onc.2014.414. [DOI] [PubMed] [Google Scholar]
- 96.Singh PK, et al. Serum microRNA expression patterns that predict early treatment failure in prostate cancer patients. Oncotarget. 2014;5:824–840. doi: 10.18632/oncotarget.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei J, Gao W, Zhu CJ, Liu YQ, Mei Z, Cheng T, Shu YQ. Identification of plasma microRNA-21 as a biomarker for early detection and chemosensitivity of non-small cell lung cancer. Chin J Cancer. 2011;30:407–414. doi: 10.5732/cjc.010.10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen J, et al. Diagnosis of lung cancer in individuals with solitary pulmonary nodules by plasma microRNA biomarkers. BMC Cancer. 2011;11:374. doi: 10.1186/1471-2407-11-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao K, Cheng J, Chen B, Liu Q, Xu D, Zhang Y. Circulating microRNA-34 family low expression correlates with poor prognosis in patients with non-small cell lung cancer. J Thorac Dis. 2017;9:3735–3746. doi: 10.21037/jtd.2017.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang C, et al. A five-miRNA panel identified from a multicentric case-control study serves as a novel diagnostic tool for ethnically diverse non-small-cell lung cancer patients. EBioMedicine. 2015;2:1377–1385. doi: 10.1016/j.ebiom.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, Zhang G, Li H, Han L, Fu A, Zhang N, Zheng Y. Serum microRNA-365 in combination with its target gene TTF-1 as a non-invasive prognostic marker for non-small cell lung cancer. Biomed Pharmacother. 2015;75:185–190. doi: 10.1016/j.biopha.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 102.Silva J, et al. Vesicle-related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival. Eur Respir J. 2011;37:617–623. doi: 10.1183/09031936.00029610. [DOI] [PubMed] [Google Scholar]
- 103.Zhu W, Liu X, He J, Chen D, Hunag Y, Zhang YK. Overexpression of members of the microRNA-183 family is a risk factor for lung cancer: a case control study. BMC Cancer. 2011;11:393. doi: 10.1186/1471-2407-11-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fei X, Zhang J, Zhao Y, Sun M, Zhao H, Li S. miR-96 promotes invasion and metastasis by targeting GPC3 in non-small cell lung cancer cells. Oncol Lett. 2018;15:9081–9086. doi: 10.3892/ol.2018.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang T, et al. Cell-free microRNA expression profiles in malignant effusion associated with patient survival in non-small cell lung cancer. PLoS One. 2012;7:e43268. doi: 10.1371/journal.pone.0043268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuxia M, Zhennan T, Wei Z. Circulating miR-125b is a novel biomarker for screening non-small-cell lung cancer and predicts poor prognosis. J Cancer Res Clin Oncol. 2012;138:2045–2050. doi: 10.1007/s00432-012-1285-0. [DOI] [PubMed] [Google Scholar]
- 107.Cui EH, Li HJ, Hua F, Wang B, Mao W, Feng XR, Li JY, Wang X. Serum microRNA 125b as a diagnostic or prognostic biomarker for advanced NSCLC patients receiving cisplatin-based chemotherapy. Acta Pharmacol Sin. 2013;34:309–313. doi: 10.1038/aps.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen X, et al. Identification of ten serum microRNAs from a genome-wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. Int J Cancer. 2012;130:1620–1628. doi: 10.1002/ijc.26177. [DOI] [PubMed] [Google Scholar]
- 109.Zhang C, et al. Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin Chem. 2010;56:1871–1879. doi: 10.1373/clinchem.2010.147553. [DOI] [PubMed] [Google Scholar]
- 110.Wu C, et al. Diagnostic and prognostic implications of a serum miRNA panel in oesophageal squamous cell carcinoma. PLoS One. 2014;9:e92292. doi: 10.1371/journal.pone.0092292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu C, Li M, Hu C, Duan H. Clinical significance of serum miR-223, miR-25 and miR-375 in patients with esophageal squamous cell carcinoma. Mol Biol Rep. 2014;41:1257–1266. doi: 10.1007/s11033-013-2970-z. [DOI] [PubMed] [Google Scholar]
- 112.Khazaei S, Nouraee N, Moradi A, Mowla SJ. A novel signaling role for miR-451 in esophageal tumor microenvironment and its contribution to tumor progression. Clin Transl Oncol. 2017;19:633–640. doi: 10.1007/s12094-016-1575-0. [DOI] [PubMed] [Google Scholar]
- 113.Dong W, Li B, Wang J, Song Y, Zhang Z, Fu C, Zhang P. Diagnostic and predictive significance of serum microRNA-7 in esophageal squamous cell carcinoma. Oncol Rep. 2016;35:1449–1456. doi: 10.3892/or.2015.4499. [DOI] [PubMed] [Google Scholar]
- 114.Lin X, Xu XY, Chen QS, Huang C. Clinical significance of microRNA-34a in esophageal squamous cell carcinoma. Genet Mol Res. 2015;14:17684–17691. doi: 10.4238/2015.December.21.41. [DOI] [PubMed] [Google Scholar]
- 115.Cui XB, et al. MicroRNA-34a functions as a tumor suppressor by directly targeting oncogenic PLCE1 in Kazakh esophageal squamous cell carcinoma. Oncotarget. 2017;8:92454–92469. doi: 10.18632/oncotarget.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dong W, Li B, Wang Z, Zhang Z, Wang J. Clinical significance of microRNA-24 expression in esophageal squamous cell carcinoma. Neoplasma. 2015;62:250–258. doi: 10.4149/neo_2015_030. [DOI] [PubMed] [Google Scholar]
- 117.Lu YF, et al. Promoter hypomethylation mediated upregulation of MicroRNA-10b-3p targets FOXO3 to promote the progression of esophageal squamous cell carcinoma (ESCC) J Exp Clin Cancer Res. 2018;37:301. doi: 10.1186/s13046-018-0966-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang K, Chen D, Meng Y, Xu J, Zhang Q. Clinical evaluation of 4 types of microRNA in serum as biomarkers of esophageal squamous cell carcinoma. Oncol Lett. 2018;16:1196–1204. doi: 10.3892/ol.2018.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Raimondi L, et al. Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget. 2015;6:13772–13789. doi: 10.18632/oncotarget.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang W, et al. Aberrant levels of miRNAs in bone marrow microenvironment and peripheral blood of myeloma patients and disease progression. J Mol Diagn. 2015;17:669–678. doi: 10.1016/j.jmoldx.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hao M, et al. Serum high expression of miR-214 and miR-135b as novel predictor for myeloma bone disease development and prognosis. Oncotarget. 2016;7:19589–19600. doi: 10.18632/oncotarget.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang YK, et al. Overexpression of microRNA-29b induces apoptosis of multiple myeloma cells through down regulating Mcl-1. Biochem Biophys Res Commun. 2011;414:233–239. doi: 10.1016/j.bbrc.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 123.Bellavia D, et al. Engineered exosomes: a new promise for the management of musculoskeletal diseases. Biochim Biophys Acta. 2018;1862:1893–1901. doi: 10.1016/j.bbagen.2018.06.003. [DOI] [PubMed] [Google Scholar]