Abstract
To maintain physiological homeostasis, cell turnover occurs every day in the body via a form of programmed cell death called apoptosis. During apoptosis, cells undergo distinct morphological changes culminating in the disassembly of the dying cell into smaller fragments known as apoptotic bodies (ApoBDs). Dysregulation of apoptosis is associated with diseases including infection, cancer and atherosclerosis. Although the development of atherosclerosis is largely attributed to the accumulation of lipids and inflammatory debris in vessel walls, it is also associated with apoptosis of macrophages, smooth muscle cells (SMCs) and endothelial cells. During cellular activation and apoptosis, endothelial cells can release several types of membrane-bound extracellular vesicles (EVs) including exosomes, microvesicles (MVs)/microparticles and ApoBDs. Emerging evidence in the field suggests that these endothelial cell-derived EVs (EndoEVs) can contribute to intercellular communication during the development of atherosclerosis via the transfer of cellular contents such as protein and microRNA, which may prevent or promote disease progression depending on the context. This review provides an up-to-date overview of the known causes and consequences of endothelial cell death during atherosclerosis along with highlighting current methodological approaches to studying EndoEVs and the potential roles of EndoEVs in atherosclerosis development.
Keywords: Apoptotic bodies, Apoptotic cell disassembly, Atherosclerosis, Endothelial cells, Extracellular vesicles, Microparticles, Microvesicles
Apoptosis and the release of EVs during apoptosis
Apoptosis is a form of programmed cell death which occurs in multicellular organisms to maintain homeostasis [1]. Apoptosis is executed via two different biochemical pathways, namely the extrinsic and intrinsic pathways, which both converge at the downstream event of activating cysteine–aspartic proteases known as executioner caspases, leading to the proteolytic degradation of cellular proteins and ultimately cell death [2]. Briefly, the extrinsic apoptotic pathway is characterised by ligands binding to transmembrane death receptors [2]. As a result, recruitment of intracellular adaptor proteins occurs, which transmit the death signal by associating with and activating caspases to mediate cell death. Conversely, the intrinsic apoptotic pathway arises from various stimuli in the absence of ligand–receptor interactions and is mediated by increased mitochondrial membrane permeability. Intrinsic apoptotic stimuli like DNA damage or endoplasmic reticulum stress cause a change in the balance between pro-apoptotic and anti-apoptotic proteins of the Bcl-2 family. Subsequently, an increase in the permeability of the mitochondria leads to release of mitochondrial contents including cytochrome c, resulting in caspase activation and execution of cell death [3]. While the biochemical pathways leading to cell death have been well characterised and dysregulation of apoptosis has been implicated in autoimmune diseases [4], how apoptotic cells communicate with other cells, in particular via EVs, is not well understood.
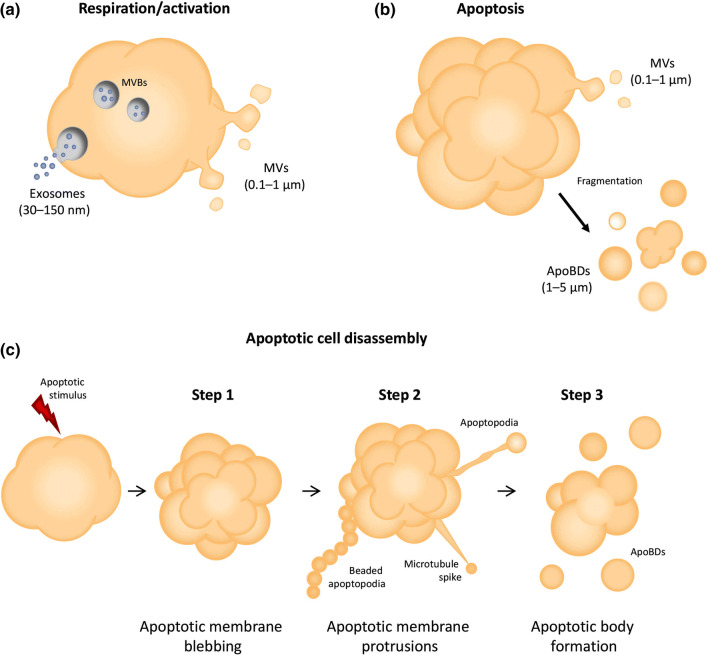
The term ‘EV’ refers to a membrane-bound vesicle released from a cell during normal cellular respiration, cellular activation or apoptosis. Three major subsets of EVs exist including exosomes, MVs and ApoBDs, which differ in size and mechanism of biogenesis [5] (Fig. 1a, b). Exosomes are the smallest type of EV ranging from 30 to 150 nm in diameter and are released after the fusion of multivesicular bodies with the plasma membrane via the endolysosomal pathway [6, 7]. Although a number of exosome markers exist (including ESCRT machinery proteins such as ALIX and TSG101, and tetraspanins CD63, CD81 and CD9), none of these are strictly specific to exosomes [8]. Therefore, detection of more than one type of these markers must occur in a given sample to confirm enrichment of exosomes. Additionally, the absence or depletion of intracellular components such as ER or Golgi proteins should also be demonstrated. A detailed description of the minimal experimental requirements of exosome characterisation can be found in Lotvall et al. [8]. Based on the complexities surrounding accurate exosomal classification, an increasingly preferred choice of nomenclature is to refer to EVs isolated via high-speed centrifugation (e.g. ≥ 100,000g) as ‘small EVs’ which may include both exosomes and small MVs [6]. MVs, also referred to as microparticles or ectosomes, are between 0.1 and 1 µm in diameter and arise during cellular activation or apoptosis [9]. Outward blebbing and pinching of the plasma membrane occurs due to phospholipid redistribution and Rho-kinase-mediated myosin light chain phosphorylation, facilitating budding and release of MVs [7]. Although no specific markers for MVs have been defined, proteins involved in plasma membrane shedding, such as ADP-ribosylation factor 6 (ARF6), are often found to be enriched in MV preparations. The role of ARF6 in the generation of EndoEVs has also been reported [10, 11]. In addition, the presence of membrane proteins from the cell of origin and exposure of phosphatidylserine (PtdSer) during the budding process have enabled the isolation and characterisation of MVs in inflammatory diseases including atherosclerosis [12]. Although apoptotic cells can generate MVs, ApoBDs represent the major type of EV released during apoptosis [13]. Importantly, while each EV subset is distinct and can exhibit different functional properties, EVs share the ability to mediate intercellular communication through transport of nucleic acids (RNA and DNA), proteins and lipids [13–15] (Table 1).
Fig. 1.
Modes of endothelial cell extracellular vesicle formation. a Exosomes and MVs are generated by endothelial cells under resting conditions as well as during activation. b ApoBDs (and MVs) are released by apoptotic endothelial cells. c The generation and release of ApoBDs during disassembly, illustrated as a three-step process
Table 1.
Basic characteristics of extracellular vesicle subtypes
| EV type | Exosomes | Microvesicles | Apoptotic bodies |
|---|---|---|---|
| Size range | 30–150 nm | 0.1–1 μm | 1–5 μm |
| Biogenesis | Of endosomal origin, secreted via fusion of multivesicular bodies with plasma membrane | Generated via outward budding of plasma membrane via actin–myosin contractions | Released from plasma membrane via blebbing and protrusion formation as late stage event in apoptotic cell disassembly |
| Known functional roles | Cell-to-cell communication | Cell-to-cell communication | Cell-to-cell communication, cell clearance |
| Known cargo | RNS, DNA, protein, lipids | ||
Apoptotic cell disassembly and clearance
Accompanying apoptosis is a set of distinct morphological steps governing the fragmentation of an apoptotic cell into ApoBDs that are approximately 1–5 µm in diameter [16]. The fragmentation process is known as apoptotic cell disassembly and is divided into three steps based on cellular morphology [13] (Fig. 1c). Step 1 of apoptotic cell disassembly is characterised by the formation of apoptotic membrane blebs. This dynamic process is regulated by a number of kinases including MLCK [17] and ROCK1 [18], which are activated upon caspase cleavage during apoptosis. The formation of apoptotic membrane protrusions following blebbing marks Step 2 of apoptotic cell disassembly. Apoptotic membrane protrusions extend from the membrane of a dying cell and can take several forms including microtubule spikes [19], apoptopodia [20] and beaded apoptopodia [21], depending on the cell type. The formation of apoptopodia and beaded apoptopodia is negatively regulated by the plasma membrane channel pannexin 1 in T cells and monocytes [20, 21], highlighting that cell disassembly is a carefully orchestrated process. Step 3 marks the final stage of apoptotic cell disassembly and involves fragmentation of the dying cell or apoptotic membrane protrusions to release distinct ApoBDs [13]. The formation of ApoBDs has been proposed to facilitate apoptotic cell clearance [22] and, like other EV subsets, ApoBDs have the potential to facilitate intercellular communication via transfer of cargo such as nucleic acids [23] or proteins [24].
Under normal physiological conditions, clearance of apoptotic cells occurs in the absence of immune activation and is mediated by various phagocytic cells [4, 25]. Dysregulation of apoptotic cell clearance can cause apoptotic cells to progress to secondary necrosis, resulting in the release of intracellular contents that could promote inflammation [25]. In particular, impaired clearance of apoptotic cells can promote the development of chronic inflammatory diseases like atherosclerosis [26]. This review will focus specifically on the effects of endothelial cell apoptosis and the potential roles of endothelial cell-derived ApoBDs and other EndoEVs on the development and progression of atherosclerosis.
Apoptosis in atherosclerosis
During the development of atherosclerotic plaques, apoptosis of macrophages within the vessel wall leads to impaired cell clearance and promotes inflammation [27]. Additionally, apoptosis of SMCs can compromise the structural integrity of the plaque, contributing to plaque rupture [28]. While the mechanisms by which macrophage and SMC apoptosis contribute to atherogenesis have been studied extensively, the role of endothelial cell apoptosis in atherosclerosis remains less well defined.
Endothelial cell death in atherosclerosis
Endothelial cells line the entire vascular network and are considered key players in the initiation and progression of atherosclerosis [29]. Forming a barrier that controls the passage of biomolecules and immune cells between the circulation and the tissues, the endothelium mediates several vital physiological functions. Under normal conditions, the endothelium regulates vascular tone, cell adhesion, SMC proliferation and maintains vascular homeostasis through anti-coagulant, anti-thrombotic and anti-inflammatory activity [30]. However, when pathological conditions arise causing apoptosis, the important biological functions maintained by the endothelium become compromised [31]. Due to impaired barrier function of the endothelium, infiltration of the vascular wall by circulating leukocytes in combination with increased low-density lipoprotein (LDL) storage initiates the development of atherosclerosis [32]. It is worth noting that increased endothelial cell turnover and apoptosis is observed in atherosclerosis-prone regions of the vasculature [33] and in the endothelium of human atherosclerotic plaques [34]. Taken together, these observations highlight an important role for endothelial cell death in the development of atherosclerosis.
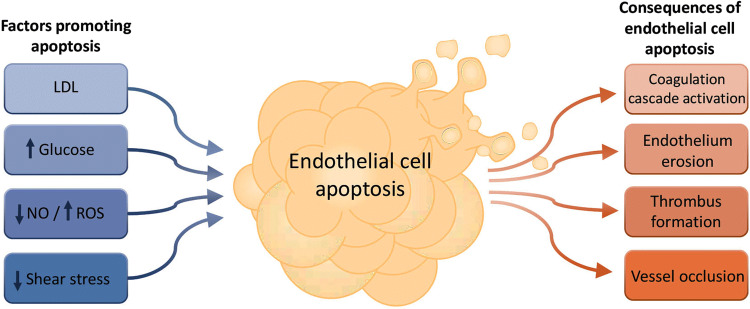
Apoptosis of endothelial cells has been associated with increased pro-coagulant properties through redistribution of PtdSer to the cell surface and loss of anti-coagulant surface components including thrombomodulin, heparan sulfate and the tissue factor pathway inhibitor [35]. Thrombus formation on an existing atherosclerotic plaque can either cause complete occlusion of the vessel lumen or through embolism, which can become lodged in microvessels leading to ischemic syndromes including unstable angina, heart attack and stroke [34]. Interestingly, the well-established pharmacological intervention of HMG-CoA-reductase inhibitor or ‘statin’ therapy for the prevention of cardiovascular disease (CVD) [36] can also mediate positive effects on the vasculature through preserving endothelial viability. Indeed, the pleiotropic effects of statins are well characterised [37]. In addition to reducing LDL and total serum cholesterol, which occurs via the inhibition of HMG-CoA reductase, statins also have the secondary cholesterol-independent effect of inhibiting isoprenoid synthesis, the downstream effects of which can modulate a variety of alternative cellular events and processes including cell signalling, inflammation, cell proliferation, migration and survival [37]. A number of studies have reported the anti-apoptotic effects of statins on endothelial cells, which may be due to the requirement of isoprenoids for the post-translational modification of Ras and Ras-like proteins that, in turn, mediate key pro-apoptotic signalling processes. For example, atorvastatin, a member of the statin drug class, prevented apoptosis in HUVECs through elevated BCL-2 expression and decreased BAX expression [38], while endothelial progenitor cells exhibited a reduction in homocysteine-induced apoptosis, mediated through reduced oxidative stress and downregulation of pro-apoptotic signalling in response to the drug [39]. Hence, although maintaining endothelial viability is a secondary effect of statin therapy, these observations highlight the importance of further investigation into endothelial cell death in atherosclerosis. Importantly, endothelial cell death can be attributed to several factors that contribute to atherosclerosis, as outlined below (Fig. 2).
Fig. 2.
Causes and consequences of endothelial cell apoptosis. Endothelial cells exposed to various environmental changes can undergo apoptosis, resulting in a range of detrimental events within the vasculature
Induction of endothelial cell apoptosis by low-density lipoprotein
LDL is a key contributor to the development of atherosclerosis and the plasma level of LDL is a strong predictor of coronary risk [32]. Oxidation of LDL renders it atherogenic by enabling its recognition and uptake through macrophage scavenger receptors, promoting the formation of macrophage foam cells that are central to the development and expansion of the necrotic core of atherosclerotic plaques [40]. Importantly, in addition to its effect on macrophages, oxidised LDL (oxLDL) also induces endothelial cell apoptosis in a Fas ligand-dependent manner [41], or through the generation of reactive oxygen species (ROS) [42]. Thus, LDL-induced endothelial cell apoptosis may contribute to the initiation of atherosclerotic lesions by compromising the endothelium.
Induction of endothelial cell apoptosis by elevated blood glucose
Hyperglycaemia is a major cause of vascular complications in diabetic patients, with a strong association between diabetes and CVD-related mortality [43]. Importantly, high glucose can induce apoptosis in endothelial cells. High glucose-induced apoptosis in human umbilical vein endothelial cells (HUVECs) is mediated by PI3 K/AKT signalling, which promotes NF-κB-dependent upregulation of COX-2 and triggers caspase activation [44]. Additionally, high glucose-induced overproduction of ROS promotes endothelial cell apoptosis in c-Jun N-terminal kinase activation-dependent [45] and NAD(P)H oxidase-dependent [46] mechanisms. These in vitro studies highlight that endothelial cell apoptosis due to hyperglycaemia in diabetes patients could manifest as vascular complications, including atherosclerosis. For newly diagnosed diabetes patients, pharmacological intervention to control elevated blood glucose levels commonly involves prescription of oral glucose-lowering medications such as metformin. Notably, metformin also prevents high glucose-induced endothelial cell death by inhibiting increased mitochondrial permeability and cytochrome c release [47]. Collectively, inhibition of endothelial cell death by regulating blood glucose levels in diabetic patients may ameliorate vascular damage that causes atherosclerosis.
Induction of endothelial cell apoptosis by decreased nitric oxide and oxidative stress
The endothelium plays an important role in maintaining vascular tone via the synthesis and secretion of vasoactive mediators such as nitric oxide (NO). Synthesis of NO by endothelial NO synthase (eNOS) is stimulated by shear stress created by blood streaming across the endothelium [48]. In response to shear stress, the endothelium continually synthesises NO, which is crucial in mediating vasodilation, preventing platelet aggregation, inhibiting the adhesion of neutrophils and the expression of macrophage chemotactic proteins [49]. Additionally, NO plays an important role in maintaining endothelial cell viability by inhibiting apoptosis via cyclic GMP-dependent and independent mechanisms [50], or via inhibition of IL-1α-converting enzyme-like and cysteine protease protein-32-like proteases [51]. Decreased NO is associated with endothelial cell dysfunction, which can be caused by impaired production of NO by eNOS or increased inactivation of NO by ROS produced by several cell types involved in atherogenesis [52]. While ROS can lead to endothelial dysfunction through inactivation of NO, endothelial cell apoptosis can also occur in response to exogenous ROS such as hydrogen peroxide or superoxide [53]. Furthermore, pro-atherosclerotic factors including oxLDL [54] and high blood glucose [55], as well as pro-inflammatory mediators such as TNFα [56], are known to induce ROS synthesis by endothelial cells. Notably, ROS production occurs before the formation of atherosclerotic plaques [57], highlighting the potential of ROS-induced endothelial cell apoptosis as an initiating event in atherogenesis.
Induction of endothelial cell apoptosis by low shear stress
An important relationship exists between shear stress exerted by blood flow and the function of the endothelium. In areas of laminar blood flow or high shear stress (HSS), endothelial cells are maintained in a quiescent state with the ability to efficiently contribute to vascular homeostasis. At bifurcations or arterial branching, however, blood flow becomes turbulent, exhibiting low shear stress (LSS) on the endothelium and as a result endothelial cell dysfunction and apoptosis arises. Several studies have demonstrated that HSS protects endothelial cells from apoptosis through upregulation of superoxide dismutase [58], downregulation of Fas receptor [59], activation of ERK5-Nrf2 signalling [60], AKT-mediated activation of eNOS and subsequent inhibition of the caspase cascade [61]. Conversely, apoptosis of endothelial cells is observed when subjected to LSS by promoting the release of cytochrome c from the mitochondria [62], increasing AKT signalling [63] and ROS levels [64]. Taken together with observations of preferential atherosclerotic plaque development in areas of the vasculature subjected to LSS, endothelial cell apoptosis induced by LSS may be an important initiating factor in atherosclerosis. The effect of LSS on the endothelium has also been shown to contribute to later stages of disease progression and may be a major determinant of plaque erosion and thrombosis [65]. As pro-atherogenic lipids and necrotic immune cells accumulate within the vessel wall, the SMC cap provides stability for the growing plaque. Over time, however, plaque rupture is influenced by shear stress. Shear stress exerted on atherosclerotic plaques varies dramatically upstream compared to downstream, with laminar flow exhibited upstream and turbulent flow observed downstream. Endothelial cell apoptosis caused by LSS in distal regions, downstream of atherosclerotic plaques, results in endothelial cell erosion and subsequent exposure of underlying necrotic debris [34]. As a result, initiation of the coagulation cascade leads to platelet activation, thrombus formation and the potential for vessel occlusion resulting in the presentation of cardiovascular events [66].
Characterising EndoEVs in atherosclerosis
While it is evident that factors involved in atherogenesis can compromise the function of the endothelium through the induction of endothelial cell apoptosis, the release of EVs from activated and apoptotic endothelial cells can also contribute to atherosclerotic disease progression (Fig. 3). The term endothelial microvesicles (EMVs, also called endothelial microparticles or EMPs) refer to MVs that arise from endothelial cells during cellular activation, whereas apoptotic EMVs arise during apoptosis and endothelial cell ApoBDs are derived from cell fragmentation during apoptotic cell disassembly. Characterising the roles of EndoEVs has become an important focus area in the past decade with several studies demonstrating a correlation between the levels of EMVs in the blood and CVD [67, 68]. These discoveries have led to the suggestion that EMVs could be used as biomarkers for CVD development and represent a rapid method for disease detection and monitoring. However, our understanding about the precise roles of EndoEVs in CVD is limited due to experimental variation in generation techniques, isolation and characterisation, as outlined below.
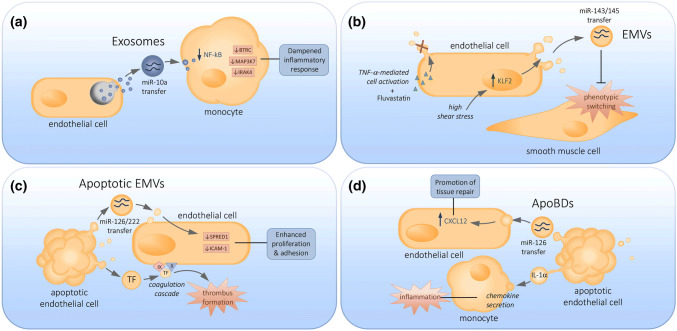
Fig. 3.
Endothelial cell-derived extracellular vesicles and their known functions. The transfer of cargo-bearing a endothelial exosomes, b EMVs, c apoptotic EMVs and d endothelial ApoBDs to neighboring cells within the vasculature can have either atheroprotective or atherogenic consequences
Methods of promoting EndoEV generation
Insult to the endothelium can result in cellular activation or apoptosis, leading to the generation of EMVs, apoptotic EMVs and endothelial cell ApoBDs. These EndoEVs have been identified in several disease states including coronary artery disease [31], multiple sclerosis [69] and type 2 diabetes [70]. When investigating the functional properties of EMVs generated under in vitro conditions, various stimuli have been reported to induce cellular activation including lipopolysaccharide [71], IL-1β or phorbol-myristate-acetate [72]. Activation of endothelial cells and formation of EMVs in vitro can also be achieved by treatment with TNFα at a relatively low concentration (10 ng/mL) [73], and this approach has been used in several studies to characterise the generation and content of EMVs [9, 74]. Other studies have used a tenfold higher concentration of TNFα to induce EMV formation, a concentration which is known to induce apoptosis in endothelial cells in other settings [75]. Interestingly, in studies using relatively high concentrations of TNFα (100 ng/mL) to generate EMVs, apoptosis was not monitored [72, 76]. This has important implications for downstream functional analysis, as different methods used to generate EMVs may alter the contents in EMVs. For example, EMVs generated by untreated, viable cells exhibit significantly reduced levels of the microRNA miR-126 compared to EMVs generated upon high glucose treatment [77]. Likewise, various methods of promoting the release of EVs from apoptotic cells have been reported. Thrombin has been shown to induce EMV release from both activated [71, 72] and apoptotic [78] endothelial cells. Other studies characterising EMVs generated during apoptosis rely on growth factor deprivation to induce cell death [9, 79, 80], or treatment with camptothecin [81] or mitomycin [73]. Furthermore, endothelial cell ApoBDs can be generated upon growth factor deprivation [23, 82] and upon growth factor deprivation in combination with TNFα stimulation [24].
Methods of EndoEV isolation
To characterise the roles of EndoEVs in disease settings like atherosclerosis, EndoEV isolation from cell culture or patient samples is required. Until now, approaches for the isolation of EndoEVs have varied greatly due to the lack of standardised methods. Some of the commonly used EV isolation methods (including for EndoEVs) are differential centrifugation (DC), density gradient centrifugation, size exclusion chromatography and ultrafiltration. Although each of these methods has its advantages, they vary considerably with regard to time and labour required, and degree of purity achieved. Two recent position papers by Coumans et al. and Sluijter et al. describe these and other commonly used EndoEV isolation methods and their associated advantages and pitfalls, and may be referred to for in depth descriptions of all methods [83, 84].
Differential centrifugation: a popular yet limited isolation technique
Of these common methods, DC has long-remained the most widely used approach for EndoEV isolation. Despite this, DC has several drawbacks including poor reproducibility, low relative purity and the potential for aggregation [83, 84]. Also of concern is the high degree of variation in both the speeds and durations used for DC, resulting in a lack of comparable finings across individual studies. For in vitro studies of EMVs derived from cultured cells, an initial centrifugation step is utilised to remove cells and large cellular debris, including ApoBDs. Reported speeds of this initial centrifugation step vary considerably, ranging from 2700 [81] to 17,571g [74]. Subsequent centrifugation speeds used to pellet EMVs for analysis are also extremely variable, ranging from 19,800 [81] to 100,000g [85]. Furthermore, alternative steps are required for the isolation of EMVs from patient samples. For blood samples, an initial centrifugation step is employed to remove cells including platelets and ApoBDs with reported speeds ranging from 160 to 11,000g to achieve this [68, 86, 87]. The subsequent centrifugation step to pellet patient-derived EMVs also differs greatly between studies, with some studies centrifuging for just 2 min at 13,000g [88], while other groups centrifuge at 100,000g for up to 90 min [72, 73, 85]. The methods of endothelial cell ApoBD isolation also vary between studies. For in vitro studies, cells are removed first by centrifugation at 300–800g, which is followed by a 4500–16,000g centrifugation step to obtain ApoBDs [23, 24, 82]. Importantly, as EV subsets exhibit differences in density, centrifugation at different speeds observed between studies could lead to the isolation of different EV subsets and likely variation in functional properties. Duration of centrifugation is also a factor to consider. For example, a comparative study on the influence of different experimental parameters on exosome yield and purity from blood plasma reported that exosome samples isolated via DC exhibited variable levels of CD63 expression at time points spanning 1, 3, 6 and 14 h. Also in this study, average EV sizes varied depending on the duration of centrifugation, with longer centrifugation time recovering smaller EV sizes on average [89], thereby suggesting that the degree of heterogeneity of different EV subtypes could be influenced by the duration of centrifugation. Moreover, the type of centrifugal rotor (fixed angle or swinging bucket) is also reported to have a significant impact on the yield and purity of EVs isolated using differential centrifugation [90]. Another important concern with DC is its potential for co-isolation of non-EV components, such as growth factors, cytokines or other circulating proteins [89, 91]. Such contaminants could affect the ability to accurately report on EndoEV-specific functions. For example, co-isolation of albumin with exosomes from blood plasma was shown to occur differentially depending on centrifugation duration, suggesting that this factor alone could influence the levels of reported protein yields [89]. An effector protein of miRNA-mediated silencing, Arogonaute2 (Ago2), has also been shown to co-purify with plasma-derived EVs via DC. Argo2 is known to associate with miRNA extracellularly [91] and could potentially interact with miRNA within EVs, thereby leading to disparities between the reported miRNA profiles of the EV samples [92].
Therefore, there remains a strong need for standardised guidelines for isolation of EVs including EndoEVs by DC, and caution must be taken when reporting on functional properties of EndoEVs, given the challenges faced in determining sample purity. Recent advancements in approaches used to isolate EVs may improve sample purity. For example, the use of a fluorescence-activated cell sorting approach, which exploits the properties of ApoBDs such as PtdSer exposure, size and granularity, has enabled the specific isolation of highly pure ApoBDs from several cell types including endothelial cells. Additionally, employment of alternative methods of EndoEV isolation such as ultrafiltration may increase in the future. Ultracentrifugation has been reported to enable high sample purity whilst maintaining high concentration [83, 93], which could become particularly appealing as the field of EndoEV analysis evolves and demands more rigorous isolation techniques.
Methods for EndoEVs characterisation
Following isolation of EndoEVs, several methods are employed to verify vesicle identity. Analysis of surface antigens and protein content, as well as vesicle diameter, assists in the confirmation of the cell origin and type of EV isolated. For endothelial exosomes, immunoblotting of EV markers CD63, ALIX, TSG101 and CD9 has been reported [94]. As described above, to characterise exosomes specifically, these markers must be used in combination, rather than in isolation [8]. Since PtdSer exposure occurs during MV biogenesis, it is also common to confirm the presence of PtdSer on the surface of EMVs by annexin A5 (A5) staining through flow cytometry analysis. Importantly, MVs also bear surface antigens from the cell of origin [12]. Therefore, in combination with A5 staining, analysis of cell type-specific antigens on EVs allows further confirmation of EMV status.
Confirming cell type-specific surface antigens is imperative when isolating EMVs from patient samples due to the presence of MVs from other cell types, such as platelets and leukocytes. Using a combination of markers including endothelial cell marker CD31, platelet markers CD41 and CD42, and leukocyte marker CD45 enables the distinction of CD31+/CD41−/CD42−/CD45− EMVs from MVs generated by other cell types present in the blood [87]. It should be noted that, similar to EMVs, ApoBDs generated from endothelial cells can also be characterised based on PtdSer exposure and the presence of surface antigens from the cell of origin [95]. Therefore, to distinguish EMVs or apoptotic EMVs from ApoBDs, it is important to monitor vesicle size. As outlined above, the expected size of EMVs and apoptotic EMVs is between 0.1 and 1 µm in diameter, whereas ApoBDs are generally between 1 and 5 µm in diameter. Although size is an important feature to distinguish each EV subset, when characterising EMVs in cell culture and disease settings, several studies have neglected to examine the size of the vesicles isolated for investigation [86, 96, 97]; therefore, it is unclear what type of EVs were investigated. ApoBDs can readily be viewed under a light microscope [16], making the measurement of size relatively simple. In contrast, to validate the submicron size of smaller EVs, other approaches are used including electron microscopy [98, 99], flow cytometry [72, 73, 77] and nanoparticle tracking analysis [100].
Function of EndoEVs released during activation and apoptosis in atherosclerosis
The ability of EVs to mediate intercellular communication is a well-described process. Through the transfer of cellular contents including miRNA, proteins and other biomolecules, exosomes, MVs and ApoBDs have been proposed to contribute to the pathogenesis of several disease states including atherosclerosis [101]. Due to differences in size, biogenesis and content of the different EndoEV subsets, they could exhibit different functional properties in atherosclerosis, as outlined below (see Table 2 for examples of known EndoEV cargoes).
Table 2.
Cargos of EndoEVs and their effects on atherosclerosis
| Cargo | Type | Ev carrier | Role in atherosclerosis | References |
|---|---|---|---|---|
| miRNA | miR-10a | Exosome | Reduced inflammation through downregulation of proinflammatory genes BTRC, MAP3K7, IRAK4 | [94] |
| miR-126 | Apoptotic EMV | Transfer to HCAECs down regulates SPRED1 expression, mediating vascular homeostasis | [77] | |
| miR-126 | ApoBD | Promotion of recruitment of progenitor cells via chemokine signalling, regulating in plaque stability and limiting apoptosis | [23] | |
| miR-143/145 | EMV | Reduced in atherosclerotic lesion formation in KLF2-expressing endothelial cells | [99] | |
| miR-222 | Apoptotic EMV | Transfer to HCAECs downregulates ICAM-1 expression, mediating vascular homeostasis | [88] | |
| Protein | HSP70 | Exosome | Induction of monocyte activation and endothelial cell adhesion | [102] |
| Tissue Factor | Apoptotic EMV | Promotion of thrombus formation through procoagulant effects | [72] | |
| Activated protein C | EMV | Anticoagulant activity through protein C pathway signalling | [121] | |
| Cytokines e.g. IL-1α | ApoBD | Introduction of chemokine production in monocytes, contributing to inflammation | [24] | |
| Adhesion molecules e.g. ICAM-1, E-Selectin | Apoptotic EMV | Promotion of adhesion to vasculature, procoagulant activity | [72] | |
| Lipid | Oxidised phospholipid | Apoptotic EMV | Stimulation of monocyte adhesion to endothelial cells, promoting atherogenesis | [114] |
| PtdSer | EMV | Associated with impaired coronary vasorelaxation, thereby dysregulating endothelial function | [31] |
Endothelial exosomes in atherosclerosis
A fundamental process in the pathogenesis of atherosclerosis is the trans-endothelial migration of monocytes into the vessel wall where differentiation into macrophages and uptake of oxLDL drives foam cell formation, initiating atherosclerotic lesion development [32]. Notably, endothelial exosomes can modulate inflammation and regulate monocyte activation and migration. EVs released by quiescent HUVECs, described to be between 50 and 300 nm in diameter and most likely to be exosomes, have demonstrated anti-inflammatory effects [94]. Transfer of the microRNA miR-10a by these EVs from endothelial cells to monocytes is associated with the downregulation of NF-κB signalling in monocytes and suppresses the activation of pro-inflammatory genes including BTRC, MAP3K7 and IRAK4, dampening the overall inflammatory response [94]. Further evidence of intercellular communication between endothelial cells and monocytes via endothelial exosomes exists with the transfer of heat shock protein 70 (HSP70). HSP70 contained in endothelial exosomes can induce monocyte activation and adhesion to endothelial cells [102].
EMVs in atherosclerosis
In this review, EMVs have been defined as MVs released by endothelial cells during cellular activation. Endothelial cell activation can be initiated by several factors including pro-inflammatory cytokines TNFα and IL-6, and is also influenced by shear stress. Laminar blood flow creates HSS which confers vascular protection [103]. The transcription factor kruppel-like factor 2 (KLF2) is integral for the maintenance of the atheroprotective endothelial phenotype observed in response to HSS [104]. HSS-mediated activation (20 dynes/cm2) of KLF2 in HUVECs was associated with the release of EVs of less than 1 µm in diameter, most likely to be EMVs, which were found to contain miR-143/145 [99]. Transfer of these microRNAs to SMCs in vitro prevents phenotypic switching [99], which is a key event in atherosclerotic plaque development. Additionally, intravenous administration of EVs from KLF2-expressing endothelial cells reduced atherosclerotic lesion development in atherosclerosis-prone ApoE−/− mice [99]. Taken together, EMVs derived from cellular activation can modulate key atherogenic events such as SMC proliferation and migration, altering atherosclerotic lesion development.
Although protective effects of EMVs in atherosclerosis have been described, it has also been suggested that EMVs could play detrimental roles in disease progression. Prior to the discovery that the transfer of microRNAs may be the mechanism linking EMVs to atherogenesis, an association between elevated plasma levels of EMVs and clinical atherosclerosis had already been observed [67]. These observations led to the investigation of the possible influence of statins on EMV levels. As described above, the use of statins to lower plasma LDL levels is associated with a number of pleiotropic effects. In the context of CVD, these effects contribute to a significant reduction in CVD morbidity and mortality [105]. Interestingly, in addition to the observed decrease in endothelial cell apoptosis, a decrease in EMV levels have also been reported in response to statin therapies [74, 106, 107]. For example, Atorvastatin induced a significant decrease in the number of circulating EMVs in ischaemic cardiomyopathy patient serum samples [107], whilst fluvastatin treatment of TNFα-activated human coronary artery endothelial cells (HCAECs) in vitro also inhibited EMV release [74]. It was suggested that the inhibitory effect of statins on EMV generation by TNFα-activated HCAECs may be mediated by the suppression of rho-mediated cytoskeletal reorganisation [74]. Therefore, in combination with LDL lowering effects, statin use may modulate the development of atherosclerosis by regulating the formation of EMVs.
Apoptotic EMVs in atherosclerosis
In addition to cellular activation, MVs from endothelial cells can also be generated during apoptosis [9, 78, 81], referred to as apoptotic EMVs. As outlined above, exposure of PtdSer on the surface of MVs in combination with the presence of endothelial cell type-specific antigens enables their detection in cell culture and patient samples. Using this approach, analysis of atherosclerotic plaques revealed the presence of PtdSer+ MVs from different cell types including endothelial cells [98]. Additionally, coronary artery disease patients exhibit high numbers of circulating PtdSer+/CD31+ EMVs which are associated with impaired coronary vasorelaxation [31], a key physiological function regulated by the endothelium. A positive correlation between apoptotic EMVs and impaired functioning of the endothelium highlights the importance of endothelial cell apoptosis in the initiation of atherosclerosis [31]. It is worth noting that EMVs and apoptotic EMVs share similarities in PtdSer exposure and endothelial cell-specific markers. Therefore, while EVs analysed in these studies [31, 98] are defined as apoptotic EMVs, whether these EVs are derived from endothelial cell activation or apoptosis is unclear.
Apoptotic EMVs can also be generated in response to LSS. After the induction of disturbed blood flow, elevated apoptotic EMVs can be identified in the blood of patients [108], suggesting that even following acute changes in shear stress, endothelial apoptosis and the release of apoptotic EMVs can be detected in patient samples. The release of apoptotic EMVs into the circulation in response to LSS may contribute to atherosclerosis by promoting IL-6-mediated inflammation [109] and the level of apoptotic EMVs in patient samples could be used as a biomarker for CVD. The application of EndoEVs as therapeutic biomarkers will be discussed further below.
Apoptotic EMVs have also been described to regulate vascular homeostasis through microRNAs. Transfer of miR-126 [77] and miR-222 [88] via apoptotic EMVs to recipient HCAECs can result in the downregulation of target proteins SPRED1 and ICAM-1, respectively. Downregulation of these target proteins is associated with enhanced endothelial cell proliferation and migration [77], along with the inhibition of monocyte adhesion to endothelial cells [88], mediating vascular homeostasis. It is interesting to note that levels of miR-126 and miR-222 in circulating EMVs were reduced in diabetic patients with coronary artery disease [77, 88]. Since diabetes is associated with accelerated atherosclerosis [43], the reduced levels of atheroprotective miR-126 and miR-222 in circulating EMVs of diabetic patients further supports the notion that apoptotic EMVs are important in maintaining vascular homeostasis.
Although apoptotic EMVs may exhibit atheroprotective properties through the transfer of microRNAs, trafficking procoagulant proteins like tissue factor (TF) via apoptotic EMVs may exert detrimental effects in atherosclerotic disease progression. Coagulation is an important consideration in atherosclerosis, whereby formation of a thrombus in response to plaque rupture could result in occlusion of the vessel lumen. Through activation of factors X and IX, TF initiates the coagulation cascade leading to thrombin generation and fibrin formation [110]. HUVECs treated with thrombin and TNFα generate apoptotic EMVs that bear TF and induce coagulation via the TF pathway [72]. In addition to TF, exposure of PtdSer on apoptotic EMVs may also promote coagulation by assembling coagulation factors on the surface of apoptotic EMVs, leading to thrombin formation [111]. High levels of procoagulant apoptotic EMVs have also been identified in the circulation of patients with acute coronary syndromes [112]. In addition to the procoagulant effects mediated by TF-bearing EMVs, the ability of apoptotic EMVs to promote atherosclerosis through the transport of oxidated phospholipids has also been observed. As described above, oxidised phospholipids in the form of LDLs are detrimental to endothelial function through their ability to promote plaque formation. Apoptotic EMVs bearing biologically active oxidised phospholipids have been shown to induce monocyte binding in vitro, suggesting that they may act in a similar way to LDLs to promote the formation of atherogenic foam cells [113, 114], further highlighting the potential for apoptotic EMV-mediated detrimental effects in atherosclerosis.
Endothelial ApoBDs in atherosclerosis
Besides apoptotic EMVs, ApoBDs are a major EV subset released during endothelial cell apoptosis and have been proposed to regulate the progression atherosclerosis. The pro-inflammatory cytokine IL-1α was identified in endothelial ApoBDs and these ApoBDs were able to induced chemokine secretion by monocytes in vitro and neutrophil-mediated inflammation in vivo [24]. Importantly, since the clearance of apoptotic debris is impaired in atherosclerotic plaques [26], the persistence of endothelial ApoBDs containing IL-1α could further contribute to inflammation associated with atherosclerosis. While endothelial ApoBDs may exacerbate inflammation and accelerate atherosclerosis, they may also mediate repair of the vasculature through recruitment of endothelial progenitor cells [82]. In vitro findings suggest that the uptake of endothelial ApoBDs by endothelial progenitor cells can promote cell proliferation and differentiation [82]. Interestingly, miR-126 can be found in endothelial ApoBDs and transfer of miR-126 to recipient vascular cells via endothelial ApoBDs can promote the production of chemokine CXCL12 and recruitment of progenitor cells to mediate tissue repair [23]. Although these studies [23, 82] suggest endothelial ApoBDs could exhibit atheroprotective properties, the methods of ApoBDs isolation and characterisation used must be taken into consideration. To isolate endothelial ApoBDs, these studies first performed a centrifugation step on cell culture supernatant at 800g to remove whole cells. However, at this centrifugation step, ApoBDs could be removed from the supernatant [115] and as a result a significant proportion of endothelial ApoBDs may have been excluded from analysis. Additionally, a second centrifugation step of 16,000g was performed to pellet what was considered to be ApoBDs [23, 82]. However, this centrifugation step is also sufficient to pellet other EV subsets, in particular EMVs and apoptotic EMVs. Furthermore, these studies [23, 82] did not confirm the vesicle size of the isolated ApoBDs, which is a key characteristic used to distinguish EMVs from ApoBDs. Therefore, to accurately characterise the role of endothelial ApoBDs in atherosclerotic disease development, accurate, reproducible and standardised techniques for isolation and characterisation of ApoBDs are required.
EndoEVs as biomarkers for disease
As discussed above, elevated levels of circulating EVs in patient samples are often observed in CVD patient samples. Recent advances in the previously discussed EV isolation methods and the ability to characterise EVs based on analysis of cell type-specific antigens have given rise to the potential use of circulating EVs as biomarkers for disease [116, 117]. EndoEVs have been investigated in a number of studies for their potential value as therapeutic biomarkers for atherosclerosis and related diseases. For example, a cohort study of 844 patients with a history of CVD revealed positive correlation between EMV levels and risk of triglyceride levels, hypertension and metabolic syndrome [118]. In a study investigating CVD risk in menopausal, asymptomatic women, elevated levels of EMVs contributed to assessment of overall CVD risk through showing positive correlation with high blood pressure [119]. In addition, high levels of EMVs were positively associated with angiographic lesions in patients undergoing coronary angiography, suggesting EMVs could be a useful marker for risk of acute coronary syndrome [120]. Although these, and several other studies reviewed previously [116, 117], demonstrate the value in pursuing the use of EMVs as biomarkers, variation in EV isolation methods between studies will remain a major limitation and further reflects the need for standardisation of laboratory techniques.
Concluding remarks
A better understanding of the mechanisms underlying the development and progression of atherosclerosis is required to develop new treatments to prevent associated CVDs, which remain the number one cause of morbidity and mortality worldwide. Endothelial cell apoptosis can occur in response to risk factors for atherosclerosis including elevated plasma LDL and glucose levels, or natural physiological processes such as shear stress and ROS. These observations highlight the importance of further investigating the consequences of endothelial cell death in atherosclerosis. Furthermore, endothelial cells can release distinct EV subsets during activation and apoptosis including exosomes, MVs, apoptotic MVs and ApoBDs. To date, several studies have suggested that through the transfer of cellular contents, EndoEVs contribute to intercellular communication throughout the development of atherosclerosis. However, the characterisation of the roles of EndoEVs in atherosclerosis has been limited due to ambiguity in methods of EV generation, isolation and characterisation. The development of standardised and reproducible techniques is required to determine the precise functions of EndoEVs in atherosclerosis and to elucidate whether EndoEVs can be used as biomarkers for disease progression.
Funding
This work was supported by grants from the National Health and Medical Research Council of Australia (GNT1125033) to I.K.H.P. and (GNT1141732) to A.A.B., and Australian Research Council (DP170103790) to I.K.H.P.
Compliance with ethical standards
Conflict of interest
The authors declare that they do not have anything to disclose.
Footnotes
Stephanie Paone and Amy A. Baxter have contributed equally to this work.
References
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wajant H (2002) The fas signaling pathway: more than a paradigm. Science (80-) 296:1635–1636 [DOI] [PubMed]
- 3.Tait SWG, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 4.Nagata S. Apoptosis and autoimmune diseases. Ann N Y Acad Sci. 2010;1209:10–16. doi: 10.1111/j.1749-6632.2010.05749.x. [DOI] [PubMed] [Google Scholar]
- 5.Kalra H, Drummen GPC, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tricarico C, Clancy J, D’Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8:220–232. doi: 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014 doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jimenez JJ, Jy W, Mauro LM, et al. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003;109:175–180. doi: 10.1016/S0049-3848(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 10.Pasquier J, Al Thawadi H, Ghiabi P, et al. Microparticles mediated cross-talk between tumoral and endothelial cells promote the constitution of a pro-metastatic vascular niche through Arf6 up regulation. Cancer Microenviron. 2014;7:41–59. doi: 10.1007/s12307-013-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews AM, Lutton EM, Merkel SF, et al. Mechanical injury induces brain endothelial-derived microvesicle release: implications for cerebral vascular injury during traumatic brain injury. Front Cell Neurosci. 2016;10:43. doi: 10.3389/fncel.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallat Z, Hugel B, Ohan J, et al. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–353. doi: 10.1161/01.CIR.99.3.348. [DOI] [PubMed] [Google Scholar]
- 13.Atkin-Smith GK, Poon IKH. Disassembly of the dying: mechanisms and functions. Trends Cell Biol. 2017;27:151–162. doi: 10.1016/j.tcb.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 15.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 16.Tixeira R, Caruso S, Paone S, et al. Defining the morphologic features and products of cell disassembly during apoptosis. Apoptosis. 2017;22:475–477. doi: 10.1007/s10495-017-1345-7. [DOI] [PubMed] [Google Scholar]
- 17.Petrache I, Birukov K, Zaiman AL, et al. Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Faseb J. 2003;17:407–416. doi: 10.1096/fj.02-0672com. [DOI] [PubMed] [Google Scholar]
- 18.Coleman ML, Sahai EA, Yeo M, et al. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- 19.Moss DK, Betin VM, Malesinski SD, Lane JD. A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J Cell Sci. 2006;119:2362–2374. doi: 10.1242/jcs.02959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon IKH, Chiu YH, Armstrong AJ, et al. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature. 2014;507:329–334. doi: 10.1038/nature13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkin-Smith GK, Tixeira R, Paone S, et al. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun. 2015;6:7439. doi: 10.1038/ncomms8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orlando KA, Stone NL, Pittman RN. Rho kinase regulates fragmentation and phagocytosis of apoptotic cells. Exp Cell Res. 2006;312:5–15. doi: 10.1016/j.yexcr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Zernecke A, Bidzhekov K, Noels H, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 24.Berda-Haddad Y, Robert S, Salers P, et al. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1alpha. Proc Natl Acad Sci USA. 2011;108:20684–20689. doi: 10.1073/pnas.1116848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 2010;17:381–397. doi: 10.1038/cdd.2009.195. [DOI] [PubMed] [Google Scholar]
- 26.Schrijvers DM, De Meyer GR, Kockx MM, et al. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 27.Kockx MM, Herman AG. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc Res. 2000;45:736–746. doi: 10.1016/S0008-6363(99)00235-7. [DOI] [PubMed] [Google Scholar]
- 28.Bennett MR, Evan G, Schwartz SM (1995) Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques [DOI] [PMC free article] [PubMed]
- 29.Gimbrone MAJ, Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajendran P, Rengarajan T, Thangavel J, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werner N, Wassmann S, Ahlers P, et al. Circulating CD31 +/annexin V + apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:112–116. doi: 10.1161/01.atv.0000191634.13057.15. [DOI] [PubMed] [Google Scholar]
- 32.Libby P, Okamoto Y, Rocha V, Folco E. Inflammation in atherosclerosis. Circ J. 2010;74:213–220. doi: 10.1253/circj.CJ-09-0706. [DOI] [PubMed] [Google Scholar]
- 33.Gerrity RG, Richardson M, Somer JB, et al. Endothelial cell morphology in areas of in vivo evans blue uptake in the aorta of young pigs: ultrastructure of the intima in areas of differing permeability to proteins. Am J Pathol. 1977;89:313–334. [PMC free article] [PubMed] [Google Scholar]
- 34.Tricot O, Mallat Z, Heymes C, et al. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101:2450–2453. doi: 10.1161/01.CIR.101.21.2450. [DOI] [PubMed] [Google Scholar]
- 35.Bombeli T, Karsan A, Tait JF, Harlan JM. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89:2429–2442. [PubMed] [Google Scholar]
- 36.Enomoto S, Sata M, Fukuda D, et al. Rosuvastatin prevents endothelial cell death and reduces atherosclerotic lesion formation in ApoE-deficient mice. Biomed Pharmacother. 2009;63:19–26. doi: 10.1016/j.biopha.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Oesterle A, Laufs U, Liao JK (2017) Pleiotropic effects of statins on the cardiovascular system. 10.1161/CIRCRESAHA.116.308537 [DOI] [PMC free article] [PubMed]
- 38.Li Y, Liu H, Wu Y, Zhu M. Effect of atorvastatin on the apoptosis of human umbilical vein endothelial cells and its drug mechanism. Pak J Pharm Sci. 2018;31:1761–1766. [PubMed] [Google Scholar]
- 39.Bao X, Wu C, Lu G. Atorvastatin inhibits homocysteine-induced dysfunction and apoptosis in endothelial progenitor cells. Acta Pharmacol Sin. 2010;31:476–484. doi: 10.1038/aps.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ball RY, Stowers EC, Burton JH, et al. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54. doi: 10.1016/0021-9150(94)05463-S. [DOI] [PubMed] [Google Scholar]
- 41.Sata M, Walsh K. Oxidized LDL activates Fas-mediated endothelial cell apoptosis. J Clin Invest. 1998;102:1682–1689. doi: 10.1172/JCI3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harada-Shiba M, Kinoshita M, Kamido H, Shimokado K. Oxidized low density lipoprotein induces apoptosis in cultured human umbilical vein endothelial cells by common and unique mechanisms. J Biol Chem Biol Chem. 1998;273:9681–9687. doi: 10.1074/jbc.273.16.9681. [DOI] [PubMed] [Google Scholar]
- 43.Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2:120–126. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- 44.Sheu ML, Ho FM, Sen Yang R, et al. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol. 2005;25:539–545. doi: 10.1161/01.ATV.0000155462.24263.e4. [DOI] [PubMed] [Google Scholar]
- 45.Ho FM, Liu SH, Liau CS, et al. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH(2)-terminal kinase and caspase-3. Circulation. 2000;101:2618–2624. doi: 10.1161/01.CIR.101.22.2618. [DOI] [PubMed] [Google Scholar]
- 46.Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 47.Detaille D, Guigas B, Chauvin C, et al. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 48.Korenaga R, Ando J, Tsuboi H, et al. Laminar flow stimulates ATP- and shear stress-dependent nitric oxide production in cultured bovine endothelial cells. Biochem Biophys Res Commun. 1994;198:213–219. doi: 10.1006/bbrc.1994.1030. [DOI] [PubMed] [Google Scholar]
- 49.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polte T, Oberle S, Schroder H. The nitric oxide donor SIN-1 protects endothelial cells from tumor necrosis factor-alpha-mediated cytotoxicity: possible role for cyclic GMP and heme oxygenase. J Mol Cell Cardiol. 1997;29:3305–3310. doi: 10.1006/jmcc.1997.0565. [DOI] [PubMed] [Google Scholar]
- 51.Dimmeler S, Rippmann V, Weiland U, et al. Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ Res. 1997;81:970–976. doi: 10.1161/01.RES.81.6.970. [DOI] [PubMed] [Google Scholar]
- 52.Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179–183. doi: 10.1161/01.RES.87.3.179. [DOI] [PubMed] [Google Scholar]
- 53.Hermann C, Zeiher AM, Dimmeler S (1997) Shear stress inhibits H2O2-induced apoptosis of human endothelial cells by modulation of the glutathione redox cycle and nitric oxide synthase. Arterioscler Thromb Vasc Biol 17:3588 LP-3592 [DOI] [PubMed]
- 54.Cominacini L, Garbin U, Pasini AF, et al. Oxidized low-density lipoprotein increases the production of intracellular reactive oxygen species in endothelial cells: inhibitory effect of lacidipine. J Hypertens. 1998;16:1913–1919. doi: 10.1097/00004872-199816121-00010. [DOI] [PubMed] [Google Scholar]
- 55.Inoguchi T, Li P, Umeda F et al (2000) High glucose level and free fatty acid stimulate protein kinase c-dependent sctivation of NAD(P)H oxidase in cultured vascular cells. 1939–1945 [DOI] [PubMed]
- 56.Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2009;296:422–432. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sima AV, Stancu CS, Simionescu M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2008;335:191. doi: 10.1007/s00441-008-0678-5. [DOI] [PubMed] [Google Scholar]
- 58.Dimmeler S, Haendeler J, Rippmann V, et al. Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett. 1996;399:71–74. doi: 10.1016/S0014-5793(96)01289-6. [DOI] [PubMed] [Google Scholar]
- 59.Bartling B, Tostlebe H, Darmer D, et al. Shear stress-dependent expression of apoptosis-regulating genes in endothelial cells. Biochem Biophys Res Commun. 2000;278:740–746. doi: 10.1006/bbrc.2000.3873. [DOI] [PubMed] [Google Scholar]
- 60.Kim M, Kim S, Lim JH, et al. Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation. J Biol Chem. 2012;287:40722–40731. doi: 10.1074/jbc.M112.381509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dimmeler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 62.Hu YL, Hur SS, Lei L, et al. Shear stress induces apoptosis via cytochrome c release from dynamic mitochondria in endothelial cells. FASEB J. 2017;31:689.14. doi: 10.1096/fj.201600961RR. [DOI] [Google Scholar]
- 63.Junxia Z, Zhimei W, Guangfeng ZUO, et al. Low shear stress induces human vascular endothelial cell apoptosis by activating Akt signal and increasing reactive oxygen species. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:313–317. doi: 10.3969/j.issn.1673-4254.2013.03.01. [DOI] [PubMed] [Google Scholar]
- 64.Dong G, Yang S, Cao X, et al. Low shear stress-induced autophagy alleviates cell apoptosis in HUVECs. Mol Med Rep. 2017;15:3076–3082. doi: 10.3892/mmr.2017.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu F, Sun Y, Chen Y, et al. Endothelial cell apoptosis is responsible for the formation of coronary thrombotic atherosclerotic plaques. Tohoku J Exp Med. 2009;218:25–33. doi: 10.1620/tjem.218.25. [DOI] [PubMed] [Google Scholar]
- 66.Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276:618–632. doi: 10.1111/joim.12296. [DOI] [PubMed] [Google Scholar]
- 67.Bernal-Mizrachi L, Jy W, Jimenez JJ, et al. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–970. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 68.Amabile N, Guerin AP, Leroyer A, et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 69.Minagar A, Jy W, Jimenez JJ et al (2001) Elevated plasma endothelial microparticles in multiple sclerosis. Neurology 56:1319 LP-1324 [DOI] [PubMed]
- 70.Koga H, Sugiyama S, Kugiyama K, et al. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;45:1622–1630. doi: 10.1016/j.jacc.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 71.Kagawa H, Nomura S, Miyake T, et al. Expression of prothrombinase activity and CD9 antigen on the surface of small vesicles from stimulated human endothelial cells. Thromb Res. 1995;80:451–460. doi: 10.1016/0049-3848(95)00200-6. [DOI] [PubMed] [Google Scholar]
- 72.Combes V, Simon A-C, Grau G-E, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jimenez JJ, Jy W, Mauro LM, et al. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol. 2001;112:81–90. doi: 10.1046/j.1365-2141.2001.02516.x. [DOI] [PubMed] [Google Scholar]
- 74.Tramontano AF, O’Leary J, Black AD, et al. Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the Rho-kinase pathway. Biochem Biophys Res Commun. 2004;320:34–38. doi: 10.1016/j.bbrc.2004.05.127. [DOI] [PubMed] [Google Scholar]
- 75.Rastogi S, Rizwani W, Joshi B, et al. TNF-alpha response of vascular endothelial and vascular smooth muscle cells involve differential utilization of ASK1 kinase and p73. Cell Death Differ. 2012;19:274–283. doi: 10.1038/cdd.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pihusch V, Rank A, Steber R, et al. Endothelial cell-derived microparticles in allogeneic hematopoietic stem cell recipients. Transplantation. 2006;81:1405–1409. doi: 10.1097/01.tp.0000209218.24916.ba. [DOI] [PubMed] [Google Scholar]
- 77.Jansen F, Yang X, Hoelscher M, et al. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128:2026–2038. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- 78.Sapet C, Simoncini S, Loriod B, et al. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868–1876. doi: 10.1182/blood-2006-04-014175. [DOI] [PubMed] [Google Scholar]
- 79.Hogg N, Browning J, Howard T, et al. Apoptosis in vascular endothelial cells caused by serum deprivation, oxidative stress and transforming growth factor-beta. Endothelium. 1999;7:35–49. doi: 10.3109/10623329909165310. [DOI] [PubMed] [Google Scholar]
- 80.Jimenez JJ, Jy W, Mauro LM, et al. Endothelial microparticles released in thrombotic thrombocytopenic purpura express von Willebrand factor and markers of endothelial activation. Br J Haematol. 2003;123:896–902. doi: 10.1046/j.1365-2141.2003.04716.x. [DOI] [PubMed] [Google Scholar]
- 81.Simak J, Holada K, Vostal JG. Release of annexin V-binding membrane microparticles from cultured human umbilical vein endothelial cells after treatment with camptothecin. BMC Cell Biol. 2002;3:11. doi: 10.1186/1471-2121-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 83.Coumans FAW, Brisson AR, Buzas EI, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120:1632–1648. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- 84.Sluijter JPG, Davidson SM, Boulanger CM, et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2018;114:19–34. doi: 10.1093/cvr/cvx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heloire F, Weill B, Weber S, Batteux F. Aggregates of endothelial microparticles and platelets circulate in peripheral blood. Variations during stable coronary disease and acute myocardial infarction. Thromb Res. 2003;110:173–180. doi: 10.1016/S0049-3848(03)00297-4. [DOI] [PubMed] [Google Scholar]
- 86.Arteaga RB, Chirinos JA, Soriano AO, et al. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol. 2006;98:70–74. doi: 10.1016/j.amjcard.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 87.Boulanger CM, Amabile N, Guerin AP, et al. In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension. 2007;49:902–908. doi: 10.1161/01.HYP.0000259667.22309.df. [DOI] [PubMed] [Google Scholar]
- 88.Jansen F, Yang X, Baumann K, et al. Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism. J Cell Mol Med. 2015;19:2202–2214. doi: 10.1111/jcmm.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baranyai T, Herczeg K, Onódi Z, et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. 2015;10:e0145686. doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cvjetkovic A, Lötvall J, Lässer C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles. 2014;3:23111. doi: 10.3402/jev.v3.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arroyo JD, Chevillet JR, Kroh EM, et al (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci 108:5003 LP-5008 [DOI] [PMC free article] [PubMed]
- 92.Van Deun J, Mestdagh P, Sormunen R, et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell vesicles. 2014 doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Njock M-S, Cheng HS, Dang LT, et al. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing anti-inflammatory microRNAs. Blood. 2015;125:3202–3212. doi: 10.1182/blood-2014-11-611046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang L, Paone S, Caruso S, et al. Determining the contents and cell origins of apoptotic bodies by flow cytometry. Sci Rep. 2017;7:14444. doi: 10.1038/s41598-017-14305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boulanger CM, Scoazec A, Ebrahimian T, et al. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001;104:2649–2652. doi: 10.1161/hc4701.100516. [DOI] [PubMed] [Google Scholar]
- 97.VanWijk MJ, Nieuwland R, Boer K, et al. Microparticle subpopulations are increased in preeclampsia: possible involvement in vascular dysfunction? Am J Obstet Gynecol. 2002;187:450–456. doi: 10.1067/mob.2002.124279. [DOI] [PubMed] [Google Scholar]
- 98.Leroyer AS, Isobe H, Leseche G, et al. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol. 2007;49:772–777. doi: 10.1016/j.jacc.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 99.Hergenreider E, Heydt S, Treguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 100.Holnthoner W, Bonstingl C, Hromada C, et al. Endothelial cell-derived extracellular vesicles size-dependently exert procoagulant activity detected by thromboelastometry. Sci Rep. 2017;7:3707. doi: 10.1038/s41598-017-03159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boulanger CM, Loyer X, Rautou P-E, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259–272. doi: 10.1038/nrcardio.2017.7. [DOI] [PubMed] [Google Scholar]
- 102.Zhan R, Leng X, Liu X, et al. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem Biophys Res Commun. 2009;387:229–233. doi: 10.1016/j.bbrc.2009.06.095. [DOI] [PubMed] [Google Scholar]
- 103.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Investig. 2004;85:9. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 104.Boon RA, Leyen TA, Fontijn RD, et al. KLF2-induced actin shear fibers control both alignment to flow and JNK signaling in vascular endothelium. Blood. 2010;115:2533–2542. doi: 10.1182/blood-2009-06-228726. [DOI] [PubMed] [Google Scholar]
- 105.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 106.Suades R, Padró T, Alonso R, et al. Lipid-lowering therapy with statins reduces microparticle shedding from endothelium, platelets and inflammatory cells. Thromb Haemost. 2013;110:366–377. doi: 10.1160/TH13-03-0238. [DOI] [PubMed] [Google Scholar]
- 107.Huang B, Cheng Y, Xie Q, et al. Effect of 40 mg versus 10 mg of atorvastatin on oxidized low-density lipoprotein, high-sensitivity C-reactive protein, circulating endothelial-derived microparticles, and endothelial progenitor cells in patients with ischemic cardiomyopathy. Clin Cardiol. 2012;35:125–130. doi: 10.1002/clc.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jenkins NT, Padilla J, Boyle LJ, et al. Disturbed blood flow acutely induces activation and apoptosis of the human vascular endothelium. Hypertension. 2013;61:615–621. doi: 10.1161/HYPERTENSIONAHA.111.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chirinos JA, Zambrano JP, Virani SS, et al. Correlation between apoptotic endothelial microparticles and serum interleukin-6 and c-reactive protein in healthy men. Am J Cardiol. 2005;95:1258–1260. doi: 10.1016/j.amjcard.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 110.Komiyama Y, Pedersen AH, Kisiel W. Proteolytic activation of human factors IX and X by recombinant human factor VIIa: effects of calcium, phospholipids, and tissue factor. Biochemistry. 1990;29:9418–9425. doi: 10.1021/bi00492a016. [DOI] [PubMed] [Google Scholar]
- 111.Owens AP, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mallat Z, Benamer H, Hugel B, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.CIR.101.8.841. [DOI] [PubMed] [Google Scholar]
- 113.Keyel PA, Tkacheva OA, Larregina AT, Salter RD. Coordinate stimulation of macrophages by microparticles and TLR ligands induces foam cell formation. J Immunol. 2012;189:4621–4629. doi: 10.4049/jimmunol.1200828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huber J, Vales A, Mitulovic G, et al. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 115.Atkin-Smith GK, Paone S, Zanker DJ, et al. Isolation of cell type-specific apoptotic bodies by fluorescence-activated cell sorting. Sci Rep. 2017;7:39846. doi: 10.1038/srep39846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dickhout A, Koenen RR. Extracellular Vesicles as Biomarkers in Cardiovascular Disease. Chances and Risks. Front Cardiovasc Med. 2018;5:113. doi: 10.3389/fcvm.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schiro A, Wilkinson FL, Weston R, et al. Endothelial microparticles as conveyors of information in atherosclerotic disease. Atherosclerosis. 2014;234:295–302. doi: 10.1016/j.atherosclerosis.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 118.Amabile N, Cheng S, Renard JM, et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur Heart J. 2014;35:2972–2979. doi: 10.1093/eurheartj/ehu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jayachandran M, Litwiller RD, Lahr BD, et al. Alterations in platelet function and cell-derived microvesicles in recently menopausal women: relationship to metabolic syndrome and atherogenic risk. J Cardiovasc Transl Res. 2011;4:811–822. doi: 10.1007/s12265-011-9296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bernal-Mizrachi L, Jy W, Fierro C, et al. Endothelial microparticles correlate with high-risk angiographic lesions in acute coronary syndromes. Int J Cardiol. 2004;97:439–446. doi: 10.1016/j.ijcard.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 121.Pérez-Casal M, Downey C, Cutillas-Moreno B, et al. Microparticle-associated endothelial protein C receptor and the induction of cytoprotective and anti-inflammatory effects. Haematologica. 2009;94:387–394. doi: 10.3324/haematol.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]