Abstract
Axons sense molecular cues in their environment to arrive at their post-synaptic targets. While many of the molecular cues have been identified, the mechanisms that regulate their spatiotemporal expression remain elusive. We examined here the transcriptional regulation of the guidance gene slit1 both in vitro and in vivo by specific fibroblast growth factor receptors (Fgfrs). We identified an Fgf-responsive 2.3 kb slit1 promoter sequence that recapitulates spatiotemporal endogenous expression in the neural tube and eye of Xenopus embryos. We found that signaling through Fgfr1 is the main regulator of slit1 expression both in vitro in A6 kidney epithelial cells, and in the Xenopus forebrain, even when other Fgfr subtypes are present in cells. These data argue that a specific signaling pathway downstream of Fgfr1 controls in a cell-autonomous manner slit1 forebrain expression and are novel in identifying a specific growth factor receptor for in vivo control of the expression of a key embryonic axon guidance cue.
Electronic supplementary material
The online version of this article (10.1007/s00018-018-2824-x) contains supplementary material, which is available to authorized users.
Keywords: Axon guidance, Forebrain, Promoter, Retinal ganglion cell, Signal transduction, Xenopus
Introduction
The establishment of a functional neural network requires that neurons make proper synaptic connections. Attractive and repulsive guidance cues in the milieu direct growing axons toward their appropriate post-synaptic targets. The molecular identity of these cues has been studied extensively [1–3], and a relatively small number of cues control the connections made by neurons throughout the nervous system. Thus, for an extensive number of specific connections to be made, the expression of individual cues and their receptors needs to be tightly regulated both spatially and temporally. The molecular mechanisms behind the spatiotemporal regulation of these cues during development, however, are poorly understood.
During development, four main families of guidance cues interact with their specific receptors to drive axons to their post-synaptic targets. Netrin, Ephrin, Slit, and Semaphorin ligands bind to receptors DCC/UNC5, Eph, Robo/EVA1C, and Plexins/Neuropilins, respectively [2–4]. Our knowledge of the transcriptional mechanisms that regulate these axon guidance molecules is based mostly on the tumorigenic context, where, for instance, the expression of members of the SLIT family (SLIT1–3) is downregulated by hypermethylation in human cancers [5–7]. Additionally, the EZH2 polycomb repressive complex binds and inhibits the SLIT2 promoter in prostate cancer [5, 8, 9].
Within a developmental context, we know more about the regulation of the expression of guidance receptors than guidance ligands, as the receptors are often regulated indirectly as part of the differentiation program of a neuron. For instance, the transcription factor Eve, and other stereotypical repressors such as Nkx6 and Engrailed likely exert indirect regulation of guidance receptor genes by de-repression of yet unknown factor(s) [10]. Further, there are examples of transcription factors binding to the cis-regulatory regions of receptor genes. Eve, Dbx1, and Isl2 bind to and exert direct transcriptional control of the axon guidance receptor genes unc5, ROBO3, and PLXNA4, respectively [10–12]. Additionally, COUP-TFII complexes with Sp1 to activate Neuropilin 2 (Nrp2) at specific Sp1 sites of the Nrp2 locus [13], and Fezf2 binds to the proximal promoter of Ephb1 [14].
Transcription factors become active downstream of cell extrinsic signaling molecules. However, current knowledge about the regulation of guidance genes in vivo by extrinsic signaling molecules is limited. We do know that hedgehog (Hh) signaling drives netrin1 (ntn1) in zebrafish [15] in a manner distinct from the patterning role of Hh signaling to indirectly control guidance genes by regulating cell identity [16, 17], i.e., Hh signaling drives ectopic ntn1 in determined cells that do not express ntn1. Fibroblast growth factor (Fgf) signaling is another example where a canonical morphogen has a non-canonical role in regulating guidance cue expression [18].
In mammals, there are 18 FGFs that bind four transmembrane FGF receptors (FGFR1–4) [19, 20]. Each FGFR has tyrosine kinase domains whose activation evokes intracellular signaling cascades [21]. FGFs initiate Semaphorin3f and EphrinA2/5 expression in the midbrain [22, 23]. They do so, however, as a result of tissue patterning. Thus, it is unclear whether FGF signaling can directly regulate the expression of axon guidance genes, or if FGFs do so as a by-product of altering tissue identity and/or cell proliferation.
In this regard, we found previously that Fgfs positively regulate slit1 and sema3a expression in the Xenopus forebrain, independently of a patterning role [18]. Slit1 and Sema3a, in conjunction, repel Xenopus optic tract axons out of the mid-diencephalon in the direction of their dorsal midbrain target, the optic tectum. Interestingly, ongoing Fgf signaling is required to maintain the expression of the guidance cues. Fgf8 overexpression expands the slit1 domain of expression, while slit1 is rapidly downregulated with pharmacological inhibition of Fgf signaling. Downregulation of slit1 and sema3a together, either directly by mRNA knockdown or indirectly via Fgfr inhibition, results in optic tract axons collecting aberrantly in the mid-diencephalon and failing to navigate past to the optic tectum.
Our previous pharmacological study [18] did not speak of potential functional diversity of the different Fgfr isoforms, nor did it address whether Fgfs could directly impact guidance cue transcription in a cell-autonomous manner. To understand the receptor specificity by which Fgfs regulate guidance gene expression, we investigated in Xenopus the Fgf-dependent transcriptional control of the slit1 gene in vitro and in vivo. We identified an Fgf-responsive 5’-regulatory region of slit1 that could recapitulate spatiotemporal expression of endogenous slit1 in the live embryo. Through expression analysis and molecular loss of function studies, we report that the slit1 promoter has differential responses to signaling via distinct Fgfrs in vitro and in the forebrain. In the embryonic forebrain, slit1 only depends on Fgfr1 activation. A similar dependence of slit1 transcription on Fgfr1 signaling is true in kidney epithelial cells, despite the presence of Fgfr2/3, indicating that Fgfr1 controls slit1 expression in vivo by activating a distinct signaling pathway from other Fgfrs.
Experimental procedures
X. laevis embryos
Eggs were collected from adult female X. laevis injected with human chorionic gonadotropin (Intervet) using established procedures [18]. The embryos were incubated at either 16 or 20 °C to control the rate of development [24]. All animal protocols were approved by the University of Calgary Animal Care Committee.
Identification of the slit1 promoter and plasmid constructs
PCR primers were designed to isolate the − 2285 to + 326 slit1 region (2.3 kb slit1 promoter, GenBank accession number KP322597.1) using hepatic genomic DNA from X. tropicalis as template. The isolated slit1 promoter was then cloned into the promoterless pGL3 (Promega) firefly luciferase reporter vector to yield − 2285 + 326slit1::luc and into the pcDNA3.1-GFP reporter vector to yield − 2285 + 326slit1::GFP. Deletion fragments were amplified by PCR from – 2285 + 326slit1::luc, using the primers in Supplementary Table S1. Deletion fragments were subcloned into pGL3. Plasmids were sequenced at the University of Calgary Core DNA facility.
The Xenopus constructs for dominant negative (DN) Fgfr1/2/4 and soluble Fgfr3 (sFgfr3) were pCS2-DNfgfr1 [25], pCS108-DNfgfr2, pCS108-sfgfr3, and pCS2MTC-DNfgfr4 [18, 26, 27]. The construct for Xenopus Fgf8 was pCS2-xfgf8 [18].
X. laevis blastomere injection
Embryos at the two-cell stage were transferred to 4% Ficoll (GE Healthcare) in 1× modified Barth’s saline (MBS; 0.7 mM CaCl2, 5 mM HEPES, 1 mM KCl, 1 mM MgSO4, 2.5 mM NaHCO3, 88 mM NaCl, pH 7.8). For luciferase assays, the DNA solution (80 ng/μL, 3:1:1 ratio by weight consisting of the luciferase construct, normalizing Renilla luciferase construct, and pCS2-GFP for screening injected embryos) was injected into both blastomeres of the embryo. For in vivo assessment of the expression of the slit1 reporter, − 2285 + 326slit1::GFP was injected alongside pCS108-tdTomato in a 2:1 w/w ratio, with tdTomato used to identify successful injections. A Picospritzer II (General Valve), equipped with a borosilicate needle, was used for injection. After injection, the embryos were transferred to 4% Ficoll in 0.1× MBS overnight at 16 °C and then into 0.1× Mark’s modified Ringer solution for development to the select stages.
Brain electroporation
Brain electroporation was performed according to established techniques [18, 28, 29]. A Picospritzer II (General Valve), equipped with a borosilicate needle, was used for injecting 1 μg/μL plasmid solution into the central forebrain ventricle of anesthetized Stage 27/28 embryos. The embryos were allowed to develop to Stage 32 to be analyzed quantitatively by qPCR or qualitatively by in situ hybridization.
Cell culture and transfection
A6 cells, X. laevis kidney epithelial cells [30] (provided by Dr. Lohka, University of Calgary) were cultured in 65% Leibovitz’s L-15 (Life Technologies) media supplemented with 10% fetal bovine serum (Life Technologies). Cells were kept at 28 °C.
A6 cells were transfected at 80% confluency in black 96-well plates (Greiner Bio-One) with Lipofectamine 2000 (Life Technologies) according to the manufacturer’s instructions. Briefly, 10 μL of Lipofectamine 2000 solution (10% solution diluted in serum-free L-15 media) was combined with 10 μL of DNA solution (100 ng of firefly luciferase reporter plasmid and 75 ng of Renilla). In the case of three-plasmid transfection, 50 ng of the third plasmid was included. Serum-free media was replaced with growing media 6 h after transfection and cells were harvested for luciferase assay at 48 h. For certain wells, 100 µM SU5402 (Sigma) was added 6 h after transfection.
Luciferase assay
The luciferase assay was conducted using the Dual-Glo Luciferase Assay System (Promega) according to the manufacturer’s instructions. Luciferase and Renilla activity was measured by adding 30 µL of the luciferase substrate and Stop & Glo reagent, respectively. Luminescence was measured using a FilterMax F5 Multi-Mode Microplate Reader (Molecular Devices). The luciferase activity was normalized against Renilla and expressed as relative lights units over the promoterless pGL3 basic.
RNA isolation and RT-PCR
A6 cells and Stage 32 X. laevis embryonic forebrains were lysed in TRIzol (Life Technologies). Total RNA was extracted using the GeneJET RNA Purification Kit (Thermo Fisher).
cDNA was reverse transcribed from template RNA and primed with oligodT (Thermo Fisher), using SuperScript II Reverse Transcriptase (Thermo Fisher) according to the manufacturer’s instructions.
For semi-quantitative RT-PCR, the genes of interest were amplified with PCR Master Mix (Thermo Scientific) with the primers in Supplementary Table S2.
qPCR was used to determine the relative gene expression using the CFX Connect Real-Time PCR Detection System (Bio-rad) and the CFX Manager Software v3.1 (Bio-rad). Primers are listed (Supplementary Table S3), along with their efficiencies and amplicon melt temperatures (Supplementary Table S4). qPCR was performed using the QuantiTect SYBR Green PCR Kit (Qiagen). Each 20 μL reaction contained 3 µL of reverse-transcribed cDNA solution, 2.5 μL of each forward and reverse primer (417 nM final concentration), 10 μL of SYBR Green QuantiTect RT-PCR master mix (Qiagen), and 2 μL water. The thermocycling parameters were: initial denaturing (95 °C, 10 min), denaturing (95 °C, 15 s), annealing (54 °C, 35 s), and elongation (72 °C, 30 s) for a total of 40 cycles. The melt curve was produced by heating the product to 95 °C in 0.5 °C increments. Gene expression was normalized to the reference genes beta-actin (actb), tubulin beta class I (tubb), and light chain dynein (dynll1) [31] using Relative Expression Software Tool 2009 V2.0.13 (Qiagen), which determined the statistical significance at p < 0.05.
Immunohistochemistry
Immunostaining was conducted according to [32]. Embryos were anesthetized in 0.01% tricaine, fixed overnight at 4 °C in 4% paraformaldehyde, immersed in 30% sucrose in PBS, mounted in OCT compound (Tissue Tek, Sakura Finetek, Inc., Torrance, CA, USA), and cryostat sectioned (12 µm thickness) prior to immunostaining. The primary antibody was rabbit anti-GFP IgG (1/1000 dilution, Molecular Probes Life Technologies A6455). The secondary antibody was goat anti-rabbit IgG conjugated to Alexa Fluor 488 (1/500 dilution, Molecular Probes Invitrogen R37116).
In situ hybridization (ISH)
Riboprobe synthesis and chromogenic ISH were conducted according to [32]. Anti-digoxigenin-AP Fab fragments (Roche 11 093 274 910) were used with BM Purple (Roche) for chromogenic visualization. For double fluorescent ISH, digoxigenin- and fluorescein- (Roche) labeled riboprobes were transcribed from linearized plasmid templates pBSK-xfgfr1, pBSK-xBek-ec, pBSK-xfgfr3, pBSK-xfgfr4, pJet-xspry1, and pCMV-SPORT6-slit1 [26, 27, 33] using SP6 or T7 polymerase (Roche). The specificity of the riboprobes was analyzed previously through sense controls by others for fgfrs [34], and by us for spry1 and slit1 (data not shown). Tissues were washed with anti-digoxigenin-POD (Roche 11 207 733 910) and anti-fluorescein-POD (Roche 11 426 346 910) and processed by the TSA Plus Fluorescein Evaluation Kit (PerkinElmer) and the TSA Cyanine 3 System (PerkinElmer) according to the manufacturer’s instructions. ISH staining was visualized by the AxioCam HRc software (Carl Zeiss) on the Stemi SVII stereomicroscope (Carl Zeiss) at ambient temperature. Digital images were processed for brightness and contrast by using Adobe Photoshop (2017.0.0 release). Quantification of mRNA co-expression after in situ hybridization was performed only in the “regions of interest” highlighted in yellow in the cartoons (Fig. 1).
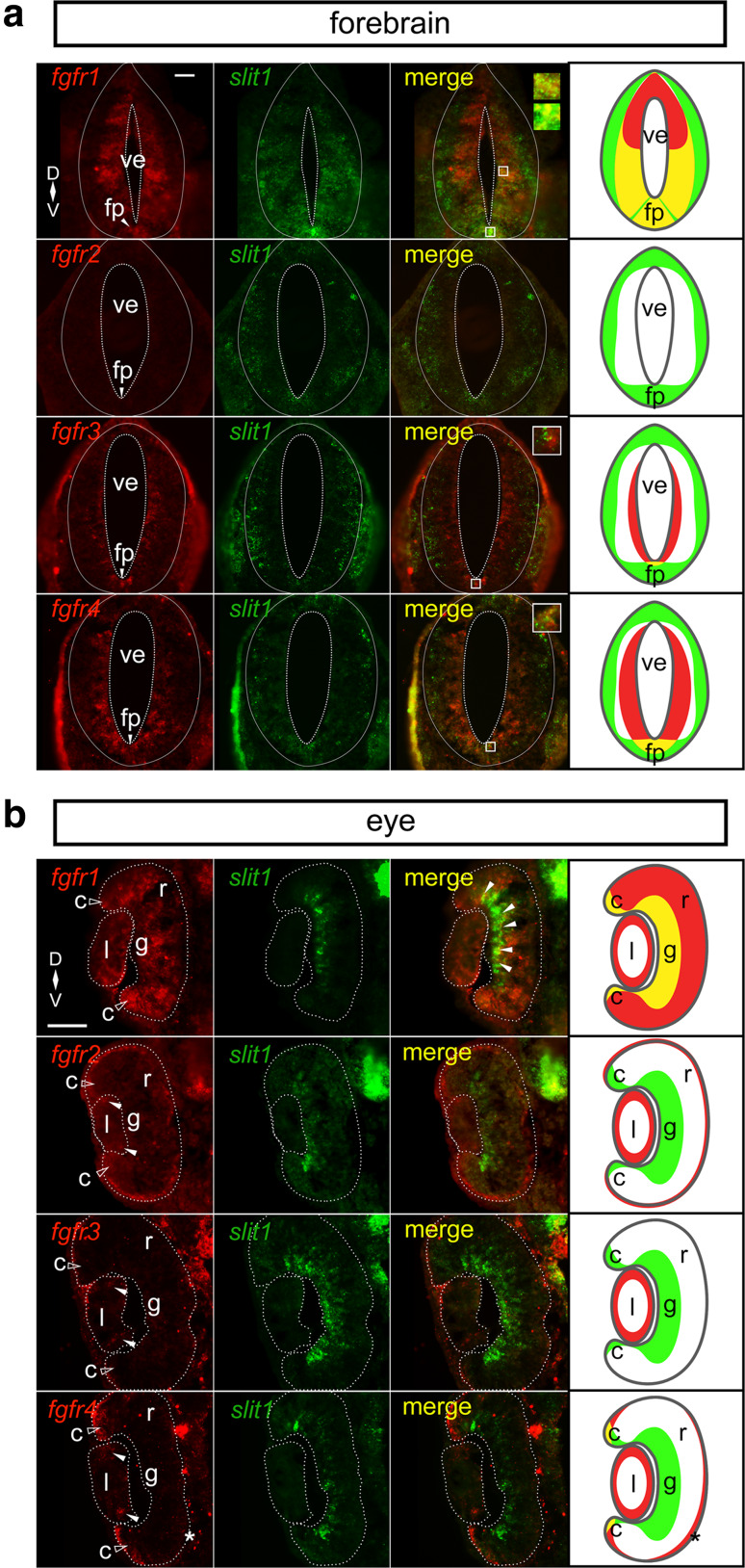
Fig. 1.
fgfr1–4 and slit1 expression patterns in Stage 32 embryos. Double fluorescent in situ hybridization on transverse sections through the forebrain (a) and the eye (b) using specific antisense riboprobes against fgfr1–4 and slit1. a There is co-expression of slit1 and fgfr1 ventrally adjacent to the ventricle and in the floor plate, fgfr3 (floor plate), and fgfr4 (floor plate). The insets focus on the regions of co-expression. b In the neural retina, slit1 co-expresses with fgfr1 in the presumptive retinal ganglion cell layer (white arrowheads) and ciliary marginal zone (unfilled arrowheads). The ciliary marginal zone (unfilled arrow heads) and the lens express different combinations of fgfrs, but not in conjunction with slit1. fgfr2–4 expression in the lens is denoted by white arrowheads. The asterisk indicates fgfr4 expression in the retinal pigmented epithelium. The dotted lines outline the boundaries of the ventricle, forebrain, lens, and neural retina. The rightmost column in each composite is a cartoon of the fgfr (red) and slit1 (green) domains with co-expression in yellow. Scale bars 50 µm. c ciliary marginal zone, fp floor plate, g retinal ganglion cell layer, l lens, r neural retina, ve ventricle
Statistical analysis
For luciferase data, we classified datasets as parametric or non-parametric based on whether the datasets satisfied the assumptions of ANOVA. First, we assumed the independence of errors, meaning that each technical and biological replicate were mutually independent. Then, the Anderson–Darling algorithm was used to assess the normality of the data. The F test and Bartlett’s test were used to assess equal variances among treatment groups. We analyzed parametric data by Student’s t test and analysis of variance (ANOVA) and non-parametric data by Mann–Whitney and Kruskal–Wallis tests on GraphPad Prism 7. The parametric post hoc test was Dunnett’s test. Dunn’s test was the non-parametric post hoc test.
Results
Spatiotemporal expression of fgfr1–4 and slit1
We demonstrated previously in whole mount preparations that fgfr1–4 and slit1 are all expressed in the embryonic forebrain [18]. Not considered, however, was whether there was significant overlap at the cellular level between the expression of specific fgfr isoforms and slit1-positive forebrain domain. Here we address this issue, and whether the expression of fgfrs in the retina might also suggest a role for this signaling pathway in regulating retinal slit1 expression [35]. We compared the expression patterns by double fluorescent in situ hybridization (ISH) of fgfr1–4 and slit1 in transverse sections through the brain and eye at Stage 32, a time when we showed Fgfr signaling positively regulated slit1 forebrain expression [18]. At this stage, the first retinal ganglion cell (RGC) axons of the optic tract reach the optic chiasm and are en route to encounter Slit1 in the mid-diencephalon. In the retina, RGC and photoreceptor somata are starting to settle in their respective layers [36]. Proliferative cells are found dispersed throughout the neural retina, and progenitor populations are evident in the retinal periphery, as the ciliary marginal zone (CMZ), and at the periphery of the lens.
Only certain fgfrs were expressed by the cells that express slit1. We analyzed sections through the entire forebrain (Fig. 1a) and central retinal sections (Fig. 1b). Note that because of the distinct spatial expression patterns of the different fgfr isoforms in the forebrain, representative forebrain sections reflect those sections with maximal expression of the two genes being compared (i.e., may differ in the rostro-caudal axis), but assessments of co-expression were made from sections through the entire forebrain. In the forebrain, slit1-positive cells did not express fgfr2. Only the fgfr1 ISH label showed significant amounts of overlap with the slit1 signal. fgfr1 and slit1 were co-expressed ventrally by cells of the floor plate, and by neuroepithelial cells lining the ventricle, in particular at mid to ventral levels (co-expression shown in insets). In the region of interest, highlighted in yellow in the cartoon, virtually all (97.3%; 326/335, N = 3) slit1-expressing cells also express fgfr1. In contrast, slit1-positive cells that were presumably post-mitotic and located at the basal side of the forebrain epithelium no longer expressed fgfr1 mRNA, though Fgfr1 protein could still be present. fgfr4 and slit1 were mostly in separate populations of forebrain cells, though a small population of ventricular floor plate cells expressed both genes (co-expression shown in inset). fgfr3 expression was restricted to more posterior forebrain sections. Here, it was mainly present in cells sitting next to the ventricles, whereas slit1 was present in cells at the basal surface of the epithelium. Similar to fgfr4, fgfr3 mRNA was present in a few floor plate cells alongside slit1 (Fig. 1a; co-expression shown in inset). In these floor plate cells, all slit1-expressing cells express fgfr3 (58/58, N = 3) and fgfr4 (42/42, N = 3), respectively.
In the retina, slit1 was expressed by cells in the proliferative CMZ (higher expression in ventral than dorsal CMZ), post-mitotic RGCs, and in what were likely to be cells specified to become RGCs that were migrating to the RGC layer (Fig. 1b). fgfr1 mRNA was present in all of these populations. In the CMZ, all slit1-expressing cells also express fgfr1 (68/68, N = 3). In RGCs and cells migrating to the RGC layer, the vast majority of cells co-express slit1 and fgfr1 (96.5%; 165/171, N = 3). In contrast, with the exception of co-expression of slit1 and fgfr4 in the CMZ (95.1%; 39/41 slit1-expressing cells, N = 3), fgfr2–4-expressing cells of the eye did not express slit1. fgfr2–4 were expressed by the proliferative cells of the lens, fgfr2 and fgfr4 by the retinal pigmented epithelium outlining the neural retina, and fgfr4 by the CMZ (Fig. 1b). The presence of fgfr4 in the lens, retinal pigmented epithelium, and CMZ is consistent with that of zebrafish at 36 h post-fertilization [37, 38].
These data indicate that multiple Fgfrs are positioned to control slit1 expression by the floor plate. Only fgfr1, however, is significantly co-expressed by forebrain and retinal cells that express slit1. These data argue that Fgfr1 is the prime candidate to control Slit1 in a cell-autonomous manner in the forebrain. Nonetheless, in forebrain sections, fgfr3 and fgfr4-expressing cells are present alongside slit1-positive cells. Thus, we cannot exclude the possibility that the protein for the receptors persists in the basally located post-mitotic slit1-positive cells.
slit1 promoter sequence has neural tube- and eye-specific expression elements
To study the transcriptional regulation of slit1, we identified approximately 2.3 kb upstream of the slit1 transcription start site, from Xenopus tropicalis genomic DNA. We tested promoter activity in the 5′-flanking sequence by a luciferase reporter system. The luciferase reporter construct − 2285 + 326slit1::luc showed 20 times more activity than the promoterless pGL3 basic vector when injected into both cells of X. laevis blastomeres at the two-cell stage and analyzed at stage 12 (Fig. 2a, b).
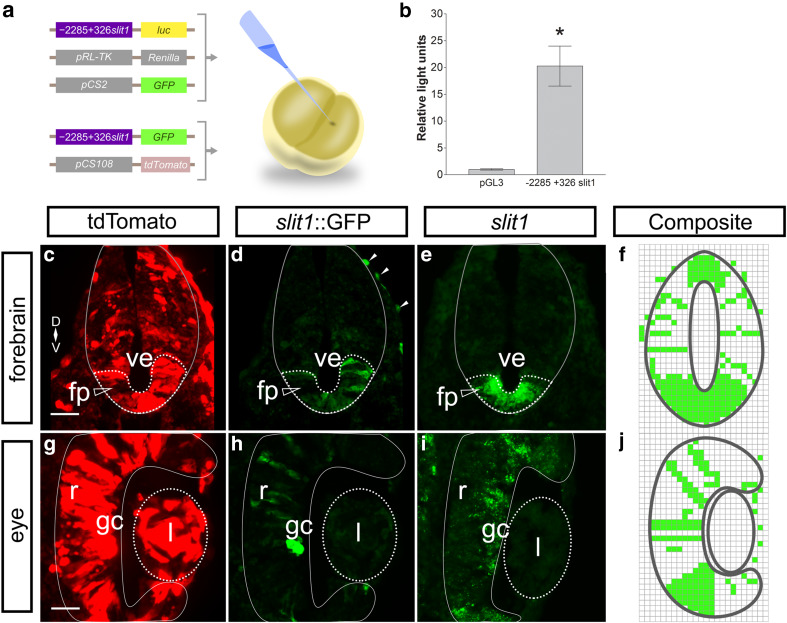
Fig. 2.
Identifying a functional slit1 promoter in X. laevis embryos. a The slit1 5′-flanking region was inserted upstream of the firefly luciferase (luc) construct in the pGL3 basic vector, − 2285 + 326 slit1::luc, and co-injected into X. laevis embryos with the Renilla luciferase vector. b The firefly luciferase luminescence was normalized against the Renilla luciferase luminescence and expressed with respect to the promoterless luciferase vector pGL3 basic. Bars reflect mean ± SEM from 29 embryos in five independent experiments. *p < 0.05, Mann–Whitney test compared to pGL3 basic. a The − 2285 + 326slit1::GFP reporter and pCS108-tdTomato were co-injected into X. laevis blastomeres. Transverse sections through the Stage 32 forebrain (c) and eye (g) show near ubiquitous expression of tdTomato, but restricted expression of GFP (d and h, white arrowheads denote ectopic GFP expression). GFP expression was comparable to the slit1 in situ hybridization (e and i) in the floor plate and the presumptive retinal ganglion cell layer of the neural retina. The solid outline marks the boundary of the neural tube (c–e) and the neural retina (g–i). The dotted outline encircles the floor plate (c–e, unfilled arrowhead) and the lens (g–i). The mosaic GFP expression from − 2285 + 326slit1::GFP embryos, n = 17 from three independent experiments, was combined into composites (f and j). Scale bars 50 µm. fp floor plate, gc retinal ganglion cell layer, l lens, r neural retina, ve ventricle
To analyze whether tissue-specific regulatory elements were present in the identified slit1 promoter sequence, we inserted the 2.3 kb slit1 promoter upstream of GFP cDNA to generate the − 2285 + 326slit1::GFP reporter construct. This GFP reporter was injected into both blastomeres of the X. laevis two-cell embryo. The embryos were allowed to develop to Stage 32, at which time we compared the expression of the reporter GFP, assessed immunohistochemically, with endogenous expression of slit1 mRNA. Of note, the ISH for endogenous mRNA captures expression within a short time period, while GFP represents summed expression over time due to the considerably longer half-life of GFP protein compared to slit1 mRNA. Further, while mRNA is generally localized to the nucleus and cell body, GFP labels the entire cytoplasmic extent of cells. Finally, GFP expression driven by the slit1 promoter in plasmid-injected embryos would be mosaic in nature, as is the case for cDNA plasmids injected into blastomeres of the two-cell embryo.
We co-injected into X. laevis blastomeres − 2285 + 326slit1::GFP along with pCS108-tdTomato, driven by the ubiquitous CMV promoter (Fig. 2a). tdTomato protein was expressed throughout the forebrain and the eye (Fig. 2c, g), exhibiting no tissue specificity. In contrast, the GFP-positive domains made up a subset of the tdTomato expressing cells, indicating that the slit1 promoter has tissue specificity (Fig. 2d, h). The GFP-immunostained sections revealed expression that was similar to that of slit1 mRNA (Fig. 2e, i). The identified slit1 promoter was active in characteristic slit1 mRNA expression domains, i.e., the floor plate in the forebrain and the cells that radially span the neural retina (presumptive RGCs).
Out of a total of 22 injected embryos, 17 expressed mosaic GFP in the neural tube and eye similar to the example in Fig. 2d, h. The mosaic expression patterns of the 17 embryos were combined into composites (Fig. 2f, j). These results suggest that important cis-regulatory elements controlling slit1 expression in the neural tube and retina are present in the 2.3 kb slit1 promoter. Since the slit1 promoter was cloned from X. tropicalis DNA, these data also indicate that the regulatory regions of the slit1 gene are conserved between X. laevis and X. tropicalis.
Characterization of the slit1 promoter
Knowing that the identified slit1 promoter drives tissue-specific expression similar to the endogenous mRNA, we then further characterized the promoter and tested its ability to respond to Fgf signaling in a cell-autonomous manner in vitro. To identify regulatory elements in the slit1 promoter, we performed a bidirectional deletion analysis of the 2.3 kb slit1 sequence (Fig. 3a) by using the A6 epithelial cell line from adult frog kidney [30]. First, we verified by RT-PCR that A6 cells endogenously express slit1 (Fig. 3b), which would argue that the cells have the proper regulatory mechanisms for studying slit1 transcriptional control.
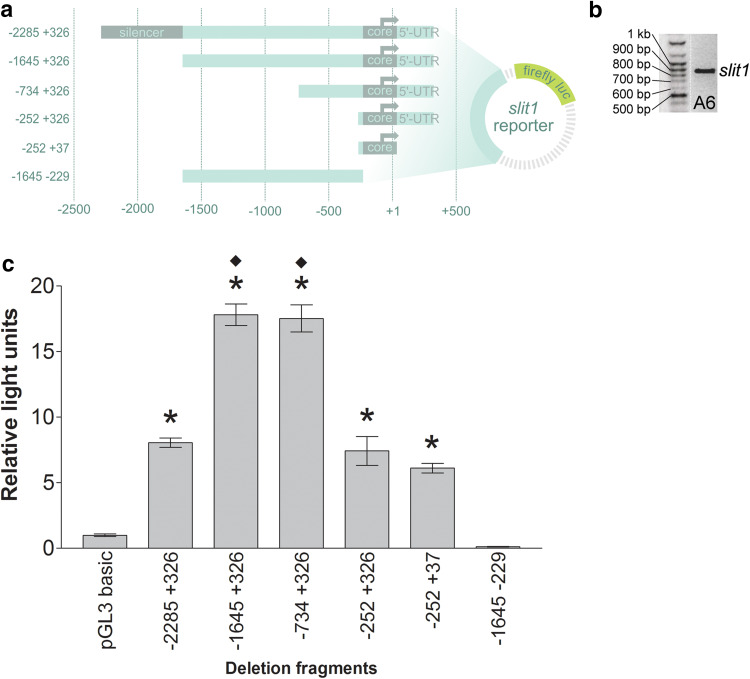
Fig. 3.
Deletion analysis of the identified slit1 promoter in A6 cells. a Schematic representation of the deletion fragments from the slit1 5′-flanking region cloned upstream of the reporter firefly luciferase (luc) cDNA in the pGL3 basic vector. b Endogenous expression of slit1 in A6 cells was confirmed by RT-PCR. c A6 cells were co-transfected transiently with the slit1::luc deletion constructs and the Renilla luciferase plasmid to normalize transfection efficiency. Cells were harvested 48 h after transfection. Promoter activity was calculated as luciferase activity over Renilla activity and expressed as the ratio over that of pGL3 basic promoterless vector (relative lights units). Bars reflect mean ± SEM from n = 236 wells from 13 independent experiments. Statistical significance was determined by Kruskal–Wallis test followed by *p < 0.01 comparison with pGL3 basic (Dunn’s post hoc test) and ♦p < 0.001 comparison with − 2285 + 326slit1::luc (Dunn’s post hoc test)
The slit1 deletion fragments driving reporter luciferase were co-transfected with the Renilla luciferase construct. The Renilla luciferase construct, driven by the ubiquitous tyrosine kinase promoter, served to normalize transfection efficiency. Promoter activity was quantified by luciferase assay. A 3′-deletion of the slit1 reporter showed that the minimal promoter region (core promoter) was located between nucleotides − 229 and +37, since the − 252 to +37 construct remained functional while the region − 1645 to − 229 did not (Fig. 3c). From the 5′-end, the slit1 regulatory region has silencing activity between nucleotides − 2285 and − 1645, as deletion of this region increased promoter activity approximately twofold (compare constructs − 2285 + 326slit1::luc vs. − 1645 + 326slit1::luc and − 734 + 326slit1::luc). Therefore, the 2.3 kb slit1 promoter contains a core promoter and a silencer.
Fgf signaling transcriptionally regulates slit1
We next focused on the possible regulation of slit1 transcription by Fgf signaling. Our previous work showed that Fgf signaling maintains slit1 expression in the X. laevis forebrain [18], but did not establish whether Fgf regulated slit1 transcription in a cell-autonomous manner, and which Fgfrs were involved. We took advantage of the luciferase assay in A6 cells to address these issues. First, we established by RT-PCR that all fgfrs were detected in A6 cells, including two forms for receptors 1 and 4 (1a–b and 4a–b) (Fig. 4a). All PCR amplicons of approximately 900 bp corresponded to the indicated fgfr, as confirmed by sequencing, with the exception of fgfr1b amplicons smaller than 900 bp, which might have been alternatively spliced transcripts [39], but were not sequenced.
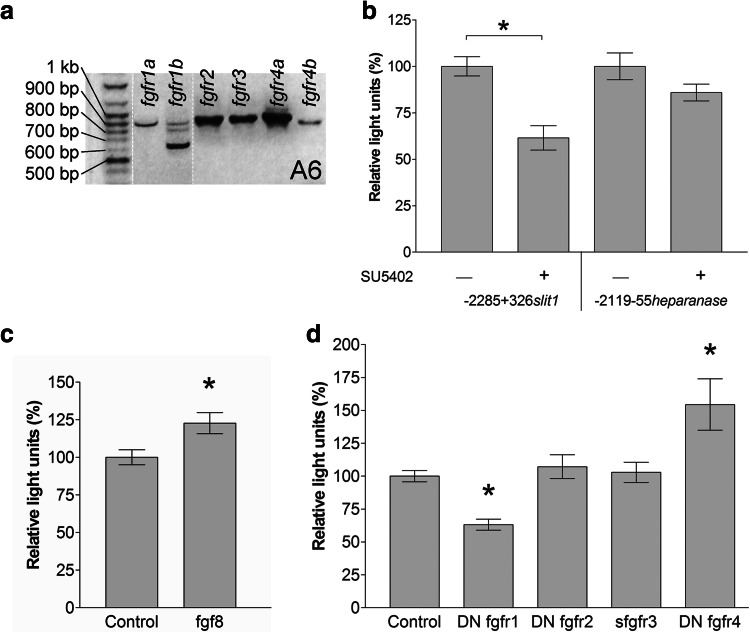
Fig. 4.
The slit1 promoter responds to Fgfr manipulation in A6 cells. a Expression of fgfrs in A6 cells was assessed by RT-PCR. b The − 2285 + 326 slit1::luc and − 2119 − 55heparanase::luc reporters were each transfected into A6 cells and incubated with 100 µM of the Fgfr inhibitor, SU5402, for 42 h. Luminescence is expressed as a percentage of the control (DMSO) without SU5402. Bars represent the mean ± SEM for n = 123 wells from six independent experiments. *p < 0.05 ANOVA followed by Dunnett’s post hoc test compared with control. The −2285 + 326 slit1::luc reporter was co-transfected with pCS2-xfgf8 (c) or constructs for truncated Fgfrs (d). Luminescence is expressed as a percentage of the control (pCS2-GFP). Bars represent the mean ± SEM for n = 16 wells from four independent experiments (c) and n = 98 wells from nine independent experiments (d). *p < 0.05 two-tailed Student’s t test compared with control (c) and p < 0.05 Kruskal–Wallis test followed by Dunn’s post hoc test compared with control (d)
It was important to verify that the 2.3 kb regulatory sequence we had identified was responsive to Fgf signaling. To do so, we treated A6 cells with a well-characterized Fgfr inhibitor, SU5402 [40] (Fig. 4b). SU5402 at 100 µM significantly decreased the promoter activity of the slit1 reporter construct, but did not affect the heparanase promoter construct, − 2119 − 55heparanase::luc, used as the negative control [41]. Further, co-transfection of pCS2-xfgf8 with − 2285 + 326slit1::luc significantly increased promoter induction (Fig. 4c). Thus, the slit1 regulatory sequence contained the necessary elements to respond to Fgf signals.
To identify the specific Fgfrs that regulate slit1 expression, we took a molecular approach to inhibit Fgfrs. We used truncated Fgfrs: dominant negative Fgfr1/2/4 (DN Fgfr1/2/4) and soluble Fgfr3 (sFgfr3), all from Xenopus, which inhibit Fgf signaling through their wild-type counterparts [18, 25–27]. The dominant negative receptors contain the extracellular and intramembrane domains, but lack the catalytic intracellular domain. The soluble Fgfr3 has only the extracellular domain.
The effects of each truncated Fgfr at blocking slit1 induction were analyzed by co-transfection of A6 cells with each one of the DN fgfr1/2/4 and sfgfr3 expression constructs together with the 2.3 kb slit1 luciferase reporter construct (Fig. 4d). The promoter activity of slit1 was not affected by co-transfection with the DN fgfr2 or sfgfr3. Interestingly, blockade of Fgfr1 and Fgfr4 reduced and stimulated slit1 promoter induction, respectively. These data suggest that Fgf signaling regulates slit1 transcription through select Fgfr pathways. Moreover, slit1 expression appears to be regulated differentially by the particular receptor that is activated.
Transcriptional regulation of forebrain slit1 is mediated by Fgfr1 signaling
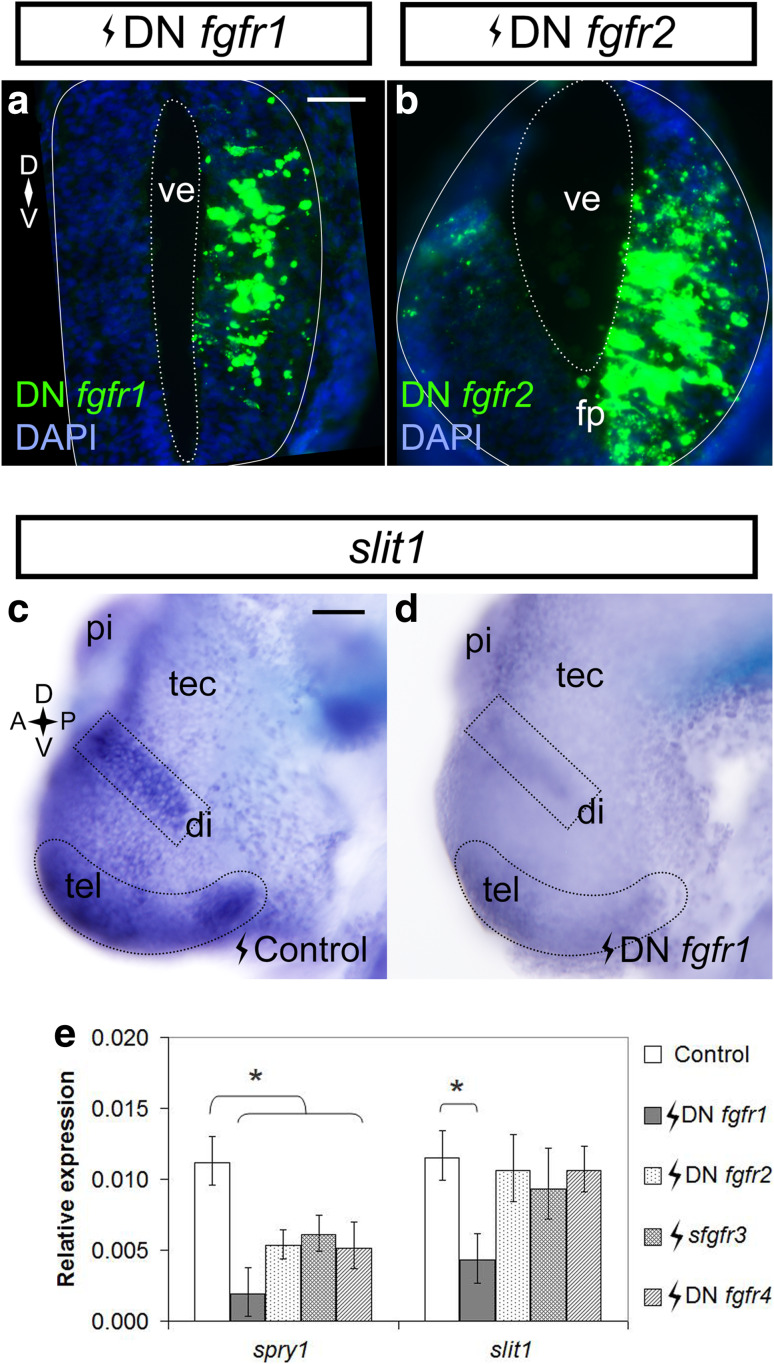
To identify in vivo the Fgfrs that control slit1 expression in the embryonic forebrain, the same truncated Fgfrs used in vitro were electroporated into the right half of the forebrain of X. laevis embryos at Stage 27/28. The pCS2-GFP plasmid served as electroporation control. The majority of cells in the electroporated side of the forebrain expressed each of the truncated fgfrs, as assessed by ISH (Fig. 5a, b). We first assessed in a qualitative manner slit1 expression in DN fgfr-electroporated embryos by ISH. Only expression of the DN Fgfr1 affected slit1 expression, with slit1 signal being noticeably decreased in multiple domains of the diencephalon and telencephalon as compared to the control (Fig. 5c, d). The other DN Fgfr constructs did not impact slit1 expression. These data agree with the in vitro A6 cell data where DN Fgfr1 decreased slit1 promoter activity.
Fig. 5.
Fgfr1 inhibition downregulates slit1 in the forebrain. Stage 27/28 embryos were electroporated in the forebrain with pCS2-DNfgfr1 (a) and pCS108-DNfgfr2 (b) and processed in transverse sections for fgfr1/2 in situ hybridization at Stage 32 to reveal the expression of the constructs. In a and b, the solid outline encircles the neural tube, and the dotted outline borders the ventricle. Stage 27/28 embryos were electroporated with control pCS2-GFP (c) (n = 13 embryos from two independent experiments) or pCS2-DNfgfr1 (d) (n = 12 embryos from two independent experiments; 10/12 brains showed downregulation) and processed for slit1 expression by whole mount in situ hybridization at Stage 32. The dotted outlines in c and d indicate the slit1 domains of interest. e The slit1 expression in the brains of embryos electroporated with truncated fgfrs was measured by qPCR. spry1 was a readout of Fgfr inhibition. Bars represent mean ± SEM for n = 137 embryos from four independent experiments. *p < 0.05 statistical significance versus the control was determined using the REST 2009 algorithm. Scale bars 50 µm. di diencephalon, fp floor plate, pi pineal gland, tec optic tectum, tel telencephalon, ve ventricle
To verify the ISH data, we quantified changes in slit1 expression by qPCR. Importantly, spry1, the negative feedback regulator of Fgf signaling, was reduced with each one of the fgfr inhibition constructs [18, 42] (Supplementary Fig. S1; Fig. 5e), arguing that the constructs effectively blocked Fgf signaling. In agreement with the ISH data, qPCR revealed that slit1 expression was significantly reduced by blocking signaling by Fgfr1, but not Fgfr2–4. Therefore, in both A6 epithelial cells and the developing forebrain, it seems that Fgfr1 is a key regulator of slit1.
Discussion
Axon guidance cues are expressed at strategic times and locations to direct growing axons toward their post-synaptic targets. For instance, Slit1/2 guides optic tract development [43, 44], controls the development of RGC dendrites [45], and facilitates commissural and longitudinal axon pathfinding in the forebrain [46–48]. Currently, not much is known about the transcriptional regulation of guidance cues during embryogenesis. In Xenopus, Fgf signaling is required to maintain slit1 expression throughout the anterior brain [18]. Here, we build on our previous work to identify for the first time in vivo a specific growth factor receptor which controls the expression of a guidance cue, providing novel insight into how extrinsic signals function in the developing brain to control the map of molecular cues that guide growing axons. Specifically, we identify: (1) that Fgf signaling regulates slit1 expression transcriptionally and most likely in a cell-autonomous manner; (2) the slit1 promoter recapitulates endogenous expression in the forebrain and the eye and contains the necessary elements to respond to Fgf signals, and (3) via expression analysis and in vitro and in vivo molecular loss of function studies that the Fgf signal is specifically mediated by Fgfr1.
The observation that the slit1 promoter sequence obtained from X. tropicalis recapitulates the endogenous spatiotemporal mRNA expression in X. laevis argues for conservation in transcriptional regulatory activity between Xenopus species. Interestingly, the expression pattern we observe in X. laevis for slit1 is similar to that observed in rat and chick, especially the characteristic slit1 domain in the floor plate [49, 50]. These data suggest slit1 expression is conserved among multiple species even though the Xenopus slit1 promoter sequence does not show high homology by alignment of the sequences from human, rat, or chick (NCBI BLAST and ConBind Motif-Aware Phylogenetic Footprinting, data not shown).
Of note, while the X. tropicalis slit1 promoter recapitulates endogenous X. laevis mRNA expression patterns, the sequence also drives ectopic reporter GFP expression in some skin cells. Thus, the 2.3 kb slit1 promoter sequence likely does not fully capture the domains that repress expression in the skin, but is sufficient for expression in the neural tube and retina. Importantly, for our studies, the sequence also contains the domains necessary for responsiveness to Fgf signals.
We found previously that Fgfr signaling maintains the expression of slit1 in the Xenopus forebrain [18], in that, transient in vivo pharmacological inhibition of Fgfrs by SU5402 rapidly downregulates slit1 transcripts in the embryonic forebrain within hours. Unclear, however, was whether Fgf-dependent regulation of slit1 expression occurred in a cell-autonomous manner, or whether Fgfs stimulated the production and secretion of an extrinsic factor that then controlled slit1 mRNA levels in neighboring cells. The rapid speed of the downregulation of slit1 (6 h or less) with pharmacological Fgfr inhibition [18] argues for a more direct regulation of expression by Fgfr signaling, as does the fact that forebrain cells and A6 cells co-express fgfrs and slit1. Moreover, molecular inhibition of Fgfr signaling in A6 cells downregulates slit1 promoter activity, despite the fact that the efficiency of transfection is sufficiently low (around 30% for all constructs, data not shown) that the large non-transfected population of A6 cells would continue to provide any key secreted signal. Thus, the data support cell-autonomous regulation of slit1 gene expression by an Fgf signaling pathway.
Intriguingly, we find both in vitro and in vivo that signaling downstream of only certain Fgfrs regulates slit1 expression. Only fgfr1, and, to a much lesser extent fgfr3/4 in the floor plate, is co-expressed along with slit1 by forebrain neuroepithelial cells, indicating that Fgfr1 could be particularly key in controlling slit1 transcription. In agreement, manipulating Fgfr1 function revealed that Fgfr1 in both A6 cells and the Xenopus forebrain positively regulated slit1 expression, while inhibition of Fgfr2/3 had no impact on slit1 levels, even though the mRNA levels of the spry1 Fgfr signaling feedback mediator [42] were downregulated by the expression of Fgfr2/3 truncated receptors. The mRNA expression data indicate that possibly the failure of Fgfr2/3 to control slit1 levels in slit1-expressing forebrain neuroepithelial cells is because the receptors are largely absent from the cells. The A6 cell data, however, argue that signaling through Fgfr2/3 is simply unable to regulate expression. A6 cells express all of the fgfrs, but only Fgfr1/4 inhibition impacts slit1 promoter activity. Thus, the signaling pathway that functions downstream of Fgfr1 is potentially sufficiently distinct from those that are active downstream of Fgfr2/3 [51], such that only the former can control slit1 gene activity.
The upregulation of the slit1 promoter in A6 cells transfected with DN Fgfr4 suggests that Fgfr4 may also control slit1 expression. However, the data show that Fgfr4 signaling normally inhibits rather than promotes slit1 promoter activity. A possible explanation for this discrepancy is the strength of the response generated by Fgfr1 and Fgfr4 [52], in that although Fgfr1 and Fgfr4 compete for Fgf ligands, downstream Fgf signaling is evoked more strongly by Fgfr1 than Fgfr4 activation. Such is the case for the effect of Fgfr1/4 signaling on pax2 expression at the Xenopus midbrain–hindbrain boundary [52, 53]. Thus, in A6 cells, the DN Fgfr4 and DN Fgfr1 might both exert their effects through modulation of Fgfr1 signaling pathway: the DN Fgfr1 would effectively inhibit signaling through the wild-type Fgfr1, while the DN Fgfr4, by eliminating the Fgfr4 signaling pathway, would allow the stronger Fgfr1 signaling pathway to prevail, and would be seen as activation of Fgfr signaling. The result would be induction of slit1 promoter activity.
Whether Fgfr4 also normally inhibits forebrain slit1 transcription is debatable. First, while fgfr1 is co-expressed by a significant number of slit1-expressing forebrain cells, fgfr4 and slit1 are co-expressed by only a subset of floor plate cells. Nevertheless, we cannot exclude the possibility that a control of slit1 forebrain mRNA levels by Fgfr4 was not revealed in our experiments either because we failed to target the small domain of fgfr4/slit1 co-expression with the truncated receptor, or that changes in slit1 mRNA levels in this small domain contributed only a small degree to the amount of slit1 in the anterior brain as measured by qPCR. In combination, however, the functional and expression data argue that Fgfr4 is not a significant regulator of slit1 transcription.
The fact that we observed regulation of slit1 transcription by Fgfr1 and not Fgfr2/3 in both mature kidney epithelial cells and embryonic forebrain argues for the generalizability of Fgfr1-dependent slit1 expression. Testing whether Fgfr1 controls slit1 expression by RGCs would help explore this idea further. Also, since Fgfr1 is expressed by progenitors lining the ventricle of the mouse forebrain [54], similar to our fgfr1 mRNA domain in the Xenopus forebrain, Slit1 may similarly be controlled by FGF signaling in mammals. In rats, however, forebrain progenitor cells also express Fgfr2, but not Fgfr3/4 [55]. Thus, unlike in Xenopus where Fgfr2 appears to play no role in controlling slit1 transcription, in mammals Fgfr2 is a potential participant. Nonetheless, Fgfr2 may not do so, if, as we find in Xenopus A6 cells, the downstream signaling pathways do not link Fgfr2 to control of the slit1 gene.
Interestingly, slit1 and fgfr1 mRNA are co-expressed likely by the neural progenitor cells of the ventricular zone of the forebrain, while slit1 is expressed toward the basal face where post-mitotic cells reside. Thus, it appears that slit1 is positively regulated in progenitors in an fgfr1-dependent fashion, and slit1 continues to be expressed as neurons are born and move away from the ventricular zone, even as fgfr1 expression turns off. Presumably, the Fgfr1 protein continues to be expressed in the post-mitotic cells for a period, and we propose that it maintains high slit1 levels. Eventually, however, slit1 must become independent of Fgf signaling, given that we find slit1 continues to be expressed by the Xenopus forebrain into the larval period (data not shown).
The involvement of guidance cues and their receptors in cancer has been well studied [2, 56]. Here, the dysregulation of guidance molecule expression is correlated with tumorigenesis and prognosis. For instance, dysregulation of Slit2 levels stimulates angiogenesis and cell motility [57–59], and increased Slit-Robo signaling can promote cancer [60–62]. Slit and Robo, however, also have tumor-suppressive roles [54, 61, 62]. In various cancers, SLIT1–3 are epigenetically silenced by hypermethylation of the promoter [5, 6, 63, 64], and in neuroblastomas, NeuroD1 directly binds to the SLIT2 promoter to repress transcription [65]. Yet, for both SLIT and other axon guidance cue genes, the signaling pathways and the transcription factors that control gene transcription in cancer, just as in development, are largely unknown. Interestingly, Slit can control its own expression. For instance, as cells extrude aberrantly from the main epithelial disc of Drosophila, c-Jun N-terminal kinase, Slit, and Robo2 form a positive feedback loop to increase Slit expression [66]. It is noteworthy that Slits, Semaphorins, and Ephrins, among other guidance molecules, are upregulated at the site of injury in the central nervous system, though the signals involved are largely unknown [67, 68].
Our data identify an extrinsic signaling pathway involving Fgfr1 that appears to work in a cell-autonomous fashion to control slit1 expression in the developing forebrain. In future, we can use our experimentally accessible in vivo Xenopus forebrain model to ask whether other axon guidance cues are similarly controlled by Fgfs, and specifically Fgfr1, and the identity of the signal transduction pathways and transcription factors that act downstream of Fgfr1 to control slit1 expression. In summary, our work provides a basis to explore how guidance cues may be regulated in development, cancer, and injury.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (TIFF 12428 kb)
Acknowledgements
This research was funded by an operating grant from the Canadian Institutes of Health Research (CIHR), bridge funding from Alberta Innovates-Health Solutions (AI-HS), salary awards from the CIHR Training Program in Genetic Determinants of Maternal and Child Health to JLJY, and from AI-HS to SM. We extend our thanks to M. Servetnick for fgfr2–4 probes and dominant negative constructs. We appreciate the feedback on the data from Drs. Schuurmans and Nguyen and guidance in qPCR from Dr. Visser.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.McFarlane S, Lom B. The Xenopus retinal ganglion cell as a model neuron to study the establishment of neuronal connectivity. Dev Neurobiol. 2012;72(4):520–536. doi: 10.1002/dneu.20928. [DOI] [PubMed] [Google Scholar]
- 2.Chédotal A, Kerjan G, Moreau-Fauvarque C. The brain within the tumor: new roles for axon guidance molecules in cancers. Cell Death Differ. 2005;12:1044–1056. doi: 10.1038/sj.cdd.4401707. [DOI] [PubMed] [Google Scholar]
- 3.Dickson B. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 4.James G, Foster SR, Key B, Beverdam A. The expression pattern of EVA1C, a novel slit receptor, is consistent with an axon guidance role in the mouse nervous system. PLoS One. 2013;8(9):e74115.1–e74115.10. doi: 10.1371/journal.pone.0074115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Cao Q, Yu J, Wu L, Dallol A, Li J, Chen G, Grasso C, Cao X, Lonigro R, Varambally S, Mehra R, Palanisamy N, Wu J, Latif F, Chinnaiyan A. The neuronal repellent SLIT2 is a target for repression by EZH2 in prostate cancer. Oncogene. 2010;29(39):5370–5380. doi: 10.1038/onc.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickinson RE, Dallol A, Bieche I, Krex D, Morton D, Maher E, Latif F. Epigenetic inactivation of SLIT3 and SLIT1 genes in human cancers. Br J Cancer. 2004;91:2071–2078. doi: 10.1038/sj.bjc.6602222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayan G, Goparaju C, Arias-Pulido H, Kaufmann AM, Schneider A, Dürst M, Mansukhani M, Pothuri B, Murty VV. Promoter hypermethylation-mediated inactivation of multiple Slit-Robo pathway genes in cervical cancer progression. Mol Cancer. 2006;5:16. doi: 10.1186/1476-4598-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldiri I, Moore KB, Hutcheson KA, Zhang J, Vetter ML. Polycomb repressive complex PRC2 regulates Xenopus retina development downstream of Wnt/β-catenin signaling. Development. 2013;140:2867–2878. doi: 10.1242/dev.088096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldiri I, Vetter ML. PRC2 during vertebrate organogenesis: a complex in transition. Dev Biol. 2012;367(2):91–99. doi: 10.1016/j.ydbio.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarin AA, Asadzadeh J, Labrador JP. Transcriptional regulation of guidance at the midline and in motor circuits. Cell Mol Life Sci. 2014;71:419–432. doi: 10.1007/s00018-013-1434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inamata Y, Shirasaki R. Dbx1 triggers crucial molecular programs required for midline crossing by midbrain commissural axons. Development. 2014;141:1260–1271. doi: 10.1242/dev.102327. [DOI] [PubMed] [Google Scholar]
- 12.Miyashita T, Yeo S, Hirate Y, Segawa H, Wada H, Little M, Yamada T, Takahashi N, Okamoto H. PlexinA4 is necessary as a downstream target of Islet2 to mediate Slit signaling for promotion of sensory axon branching. Development. 2004;131:3705–3715. doi: 10.1242/dev.01228. [DOI] [PubMed] [Google Scholar]
- 13.Tang K, Rubenstein JL, Tsai SY, Tsai MJ. COUP-TFII controls amygdala patterning by regulating neuropilin expression. Development. 2012;139:1630–1639. doi: 10.1242/dev.075564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodato S, Molyneaux BJ, Zuccaro E, Goff LA, Chen HH, Yuan W, Meleski A, Takahashi E, Mahony S, Rinn JL, Gifford DK, Arlotta P. Gene co-regulation by Fezf2 selects neurotransmitter identity and connectivity of corticospinal neurons. Nat Neurosci. 2014;17:1046–1054. doi: 10.1038/nn.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strähle U, Fischer N, Blader P. Expression and regulation of a netrin homologue in the zebrafish embryo. Mech Dev. 1997;62:147–160. doi: 10.1016/S0925-4773(97)00657-6. [DOI] [PubMed] [Google Scholar]
- 16.Hörndli CS, Chien CB. Sonic hedgehog is indirectly required for intraretinal axon pathfinding by regulating chemokine expression in the optic stalk. Development. 2012;139:2604–2613. doi: 10.1242/dev.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barresi MJF, Hutson LD, Chien CB, Karlstrom RO. Hedgehog regulated Slit expression determines commissure and glial cell position in the zebrafish forebrain. Development. 2005;132:3643–3656. doi: 10.1242/dev.01929. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson-Leadbeater K, Bertolesi GE, Hehr CL, Webber CA, Cechmanek PB, McFarlane S. Dynamic expression of axon guidance cues required for optic tract development is controlled by fibroblast growth factor signaling. J Neurosci. 2010;30:685–693. doi: 10.1523/JNEUROSCI.4165-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai PS, Brooks LR, Rochester JR, Kavanaugh SI, Chung WCJ. Fibroblast growth factor signaling in the developing neuroendocrine hypothalamus. Front Neuroendocrinol. 2011;32(1):95–107. doi: 10.1016/j.yfrne.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner N, Grose R. Fibroblast growth factor signaling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 21.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi K, Mizushima S, Tamada A, Yamamoto N, Takashima S, Murakami F. FGF8 signaling regulates growth of midbrain dopaminergic axons by inducing semaphorin 3F. J Neurosci. 2009;29(13):4044–4055. doi: 10.1523/JNEUROSCI.4794-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamim H, Mahmood R, Logan C, Doherty P, Lumsden A, Mason I. Sequential roles for Fgf4, En1 and Fgf8 in specification and regionalisation of the midbrain. Development. 1999;126:945–959. doi: 10.1242/dev.126.5.945. [DOI] [PubMed] [Google Scholar]
- 24.Faber J, Nieuwkoop P. Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. New York: Garland Publishing; 1994. [Google Scholar]
- 25.Ueno H, Gunn M, Dell K, Tseng A, Williams L. A truncated form of fibroblast growth factor receptor 1 inhibits signal transduction by multiple types of fibroblast growth factor receptor. J Biol Chem. 1992;267:1470–1476. [PubMed] [Google Scholar]
- 26.Atkinson-Leadbeater K, Hehr C, McFarlane S. Fgfr signaling is required as the early eye field forms to promote later patterning and morphogenesis of the eye. Dev Dyn. 2014;243:663–675. doi: 10.1002/dvdy.24113. [DOI] [PubMed] [Google Scholar]
- 27.Golub R, Adelman Z, Clementi J, Weiss R, Bonasera J, Servetnick M. Evolutionarily conserved and divergent expression of members of the FGF receptor family among vertebrate embryos, as revealed by FGFR expression patterns in Xenopus. Dev Genes Evol. 2000;210:345–357. doi: 10.1007/s004270000076. [DOI] [PubMed] [Google Scholar]
- 28.Chen YY, Hehr CL, Atkinson-Leadbeater K, Hocking JC, McFarlane S. Targeting of retinal axons requires the metalloproteinase ADAM10. J Neurosci. 2007;27:8448–8456. doi: 10.1523/JNEUROSCI.1841-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas K, Jensen K, Sin WC, Foa L, Cline HT. Targeted electroporation in Xenopus tadpoles in vivo: from single cells to the entire brain. Differentiation. 2002;70:148–154. doi: 10.1046/j.1432-0436.2002.700404.x. [DOI] [PubMed] [Google Scholar]
- 30.Rafferty KJ, Sherwin RW. The length of secondary chromosomal constrictions in normal individuals and in a nucleolar mutant of Xenopus laevis. Cytogenetics. 1969;8:427–438. doi: 10.1159/000130054. [DOI] [PubMed] [Google Scholar]
- 31.Brunsdon HR (2015) An analysis of FGF-regulated genes during Xenopus neural development. Dissertation, University of York
- 32.Sive H, Grainger RM, Harland RM. Early development of Xenopus laevis: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 33.Atkinson-Leadbeater K, Bertolesi GE, Johnston JA, Hehr CL, McFarlane S. FGF receptor dependent regulation of Lhx9 expression in the developing nervous system. Dev Dyn. 2009;238:367–375. doi: 10.1002/dvdy.21850. [DOI] [PubMed] [Google Scholar]
- 34.Hongo I, Kengaku M, Okamoto H. FGF signaling and the anterior neural induction in Xenopus. Dev Biol. 1999;216:561–581. doi: 10.1006/dbio.1999.9515. [DOI] [PubMed] [Google Scholar]
- 35.Hocking JC, Hehr CL, Bertolesi GE, Wu JY, McFarlane S. Distinct roles for Robo2 in the regulation of axon and dendrite growth by retinal ganglion cells. Mech Dev. 2010;127(1–2):36–48. doi: 10.1016/j.mod.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Holt CE, Bertsch TW, Ellis HM, Harris WA. Cellular determination in the Xenopus retina is independent of linear and birth date. Neuron. 1988;1:15–26. doi: 10.1016/0896-6273(88)90205-X. [DOI] [PubMed] [Google Scholar]
- 37.Russell C. The roles of hedgehogs and fibroblast growth factors in eye development and retinal cell rescue. Vis Res. 2002;43:899–912. doi: 10.1016/S0042-6989(02)00416-9. [DOI] [PubMed] [Google Scholar]
- 38.Sleptsova-Friedrich I, Li Y, Emelyanov A, Ekker M, Korzh V, Ge R. fgfr3 and regionalization of anterior neural tube in zebrafish. Mech Dev. 2001;102(1–2):213–217. doi: 10.1016/S0925-4773(01)00280-5. [DOI] [PubMed] [Google Scholar]
- 39.Friesel R, Dawid I. cDNA cloning and developmental expression of fibroblast growth factor receptors from Xenopus laevis. Mol Cell Biol. 1991;11:2481–2488. doi: 10.1128/MCB.11.5.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh B, Hubbard S, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- 41.Bertolesi GE, Su HY, Michaiel G, Dueck SM, Hehr CL, McFarlane S. Two promoters with distinct activities in different tissues drive the expression of heparanase in Xenopus. Dev Dyn. 2011;240:2657–2672. doi: 10.1002/dvdy.22770. [DOI] [PubMed] [Google Scholar]
- 42.Hacohen N, Kramer S, Sutherland S, Hiromi Y, Krasnow MA. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92(2):253–263. doi: 10.1016/S0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- 43.Plump A, Erskine L, Sabatier C, Brose K, Epstein C, Goodman C, Mason C, Tessier-Lavigne M. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33:219–232. doi: 10.1016/S0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 44.Erskine L, Williams S, Brose K, Kidd T, Rachel R, Goodman C, Tessier-Lavigne M, Mason C. Retinal ganglion cell axon guidance in the mouse optic chiasm: expression and function of robos and slits. J Neurosci. 2000;20:4975–4982. doi: 10.1523/JNEUROSCI.20-13-04975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zolessi F, Poggi L, Wilkinson C, Chien C, Harris W. Polarization and orientation of retinal ganglion cells in vivo. Neural Dev. 2006;1:1–21. doi: 10.1186/1749-8104-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tosa Y, Tsukano K, Itoyama T, Fukagawa M, Nii Y, Ishikawa R, Suzuki KT, Fukui M, Kawaguchi M, Murakami Y. Involvement of Slit-Robo signaling in the development of the posterior commissure and concomitant swimming behavior in Xenopus laevis. Zool Lett. 2015;1:28. doi: 10.1186/s40851-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hofmeister W, Devine CA, Rothnagel JA, Key B. Frizzled-3a and slit2 genetically interact to modulate midline axon crossing in the telencephalon. Mech Dev. 2012;129(5–8):109–124. doi: 10.1016/j.mod.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Devine CA, Key B. Robo-Slit interactions regulate longitudinal axon pathfinding in the embryonic vertebrate brain. Dev Biol. 2008;313(1):371–383. doi: 10.1016/j.ydbio.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 49.Philipp M, Niederkofler V, Debrunner M, Alther T, Kunz B, Stoeckli ET. RabGDI controls axonal midline crossing by regulating Robo1 surface expression. Neural Dev. 2012;7:36. doi: 10.1186/1749-8104-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brose K, Bland K, Wang K, Arnott D, Henzel W, Goodman C, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/S0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 51.Chung WCJ, Moyle SS, Tsai PS. Fibroblast growth factor 8 signaling through FGF receptor 1 is required for gonadotropin-releasing hormone neuronal development in mice. Endocrinology. 2008;149(10):4997–5003. doi: 10.1210/en.2007-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamagishi M, Okamoto H. Competition for ligands between FGFR1 and FGFR4 regulates Xenopus neural development. Int J Dev Biol. 2010;54:93–104. doi: 10.1387/ijdb.092849my. [DOI] [PubMed] [Google Scholar]
- 53.Heller N, Brändli A. Xenopus Pax-2/5/8 orthologues: novel insights into Pax gene evolution and identification of Pax-8 as the earliest marker for otic and pronephric cell lineages. Dev Genet. 1992;24:208–219. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<208::AID-DVG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 54.Choubey L, Collette JC, Smith KM. Quantitative assessment of fibroblast growth factor receptor 1 expression in neurons and glia. PeerJ. 2017;5:e3173. doi: 10.7717/peerj.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frinchi M, Bonomo A, Trovato-Salinaro A, Condorelli DF, Fuxe K, Spampinato MG, Mudo G. Fibroblast growth factor-2 and its receptor expression in proliferating precursor cells of the subventricular zone in the adult rat brain. Neurosci Lett. 2008;447(1):20–25. doi: 10.1016/j.neulet.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 56.Mehlen P, Delloye-Bourgeois C, Chédotal A. Novel roles for Slits and netrins: axon guidance cues as anticancer targets? Nat Rev Cancer. 2011;11:188–197. doi: 10.1038/nrc3005. [DOI] [PubMed] [Google Scholar]
- 57.Amodeo V, Deli A, Betts J, Bartesaghi S, Zhang Y, Richard-Londt A, Ellis M, Roshani R, Vouri M, Galavotti S, Oberndorfer S, Leite AP, Mackay A, Lampada A, Stratford E, Li N, Dinsdale D, Grimwade D, Jones C, Nicotera P, Michod D, Brandner S, Salomoni P. A PML/Slit axis controls physiological cell migration and cancer invasion in the CNS. Cell Rep. 2017;20:411–426. doi: 10.1016/j.celrep.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 58.Ballard MS, Hinck L. A roundabout way to cancer. Adv Cancer Res. 2012;114:187–235. doi: 10.1016/B978-0-12-386503-8.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou WJ, Geng ZH, Chi S, Zhang W, Niu XF, Lan SJ, Ma L, Yang X, Wang LJ, Ding YQ, Geng JG. Slit-Robo signaling induces malignant transformation through Hakai-mediated E-cadherin degradation during colorectal epithelial cell carcinogenesis. Cell Res. 2011;21(4):609–626. doi: 10.1038/cr.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang XM, Han HX, Sui F, Dai YM, Chen M, Geng JG. Slit-Robo signaling mediates lymphangiogenesis and promotes tumor lymphatic metastasis. Biochem Biophys Res Commun. 2010;396(2):571–577. doi: 10.1016/j.bbrc.2010.04.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang B, Dietrich UM, Geng JG, Bicknell R, Esko JD, Wang L. Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood. 2009;114:4300–4309. doi: 10.1182/blood-2008-12-193326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang B, Xiao Y, Ding BB, Zhang N, Yuan XB, Gui L, Qian KX, Duan S, Chen Z, Rao Y, Geng JG. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell. 2003;4(1):19–29. doi: 10.1016/S1535-6108(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 63.Kong R, Yi F, Wen P, Liu J, Chen X, Ren J, Li X, Shang Y, Nie Y, Wu K, Fan D, Zhu L, Feng W, Wu JY. Myo9b is a key player in SLIT/ROBO-mediated lung tumor suppression. J Clin Invest. 2015;125(12):4407–4420. doi: 10.1172/JCI81673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunwell TL, Dickinson RE, Stankovic T, Dallol A, Weston V, Austen D, Catchpoole D, Maher ER, Latif F. Frequent epigenetic inactivation of the SLIT2 gene in chronic and acute lymphocytic leukemia. Epigenetics. 2009;4(4):265–269. doi: 10.4161/epi.9137. [DOI] [PubMed] [Google Scholar]
- 65.Huang P, Kishida S, Cao D, Murakami-Tonami Y, Mu P. The neuronal differentiation factor NeuroD1 downregulates the neuronal repellent factor Slit2 expression and promotes cell motility and tumor formation of neuroblastoma. Cancer Res. 2011;71(8):2938–2948. doi: 10.1158/0008-5472.CAN-10-3524. [DOI] [PubMed] [Google Scholar]
- 66.Vaughen J, Igaki T. Slit-Robo repulsive signaling extrudes tumorigenic cells from epithelia. Dev Cell. 2016;39:683–695. doi: 10.1016/j.devcel.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobi A, Schmalz A, Bareyre FM. Abundant expression of guidance and synaptogenic molecules in the injured spinal cord. PLoS One. 2014;9(2):e88449. doi: 10.1371/journal.pone.0088449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giger RJ, Hollis ER, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010;2(7):a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 2 (TIFF 12428 kb)