Abstract
Originating from ectodermal epithelium, radial glial cells (RGCs) retain apico-basolateral polarity and comprise a pseudostratified epithelial layer in the developing cerebral cortex. The apical endfeet of the RGCs faces the fluid-filled ventricles, while the basal processes extend across the entire cortical span towards the pial surface. RGC functions are largely dependent on this polarized structure and the molecular components that define it. In this review, we will dissect existing molecular evidence on RGC polarity establishment and during cerebral cortex development and provide our perspective on the remaining key questions.
Keywords: Radial glia, Embryonic neural stem cell, Cerebral cortex development, Neurogenesis, Epithelial polarity, Pseudostratified epithelium
Introduction
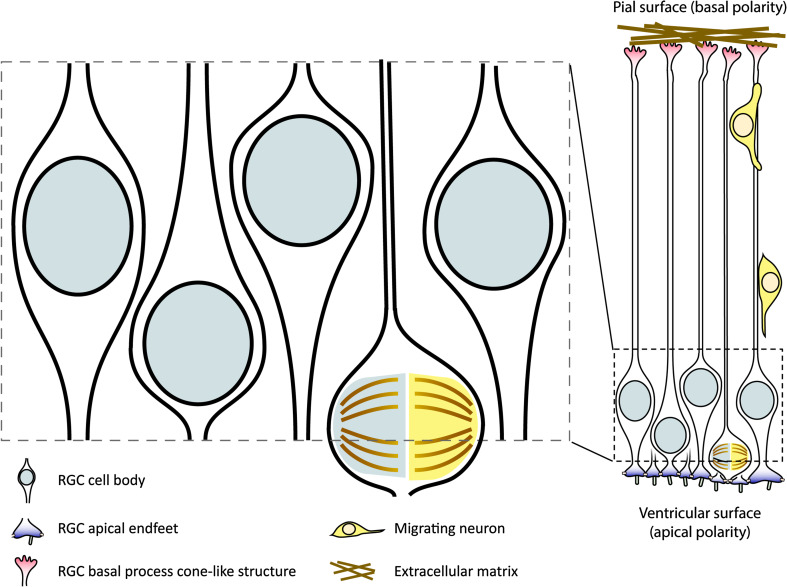
Cerebral cortex development begins upon closure of the neural tube. Initially, the neuroepithelial cells (NECs) lining the interior surface of the neural tube undergo symmetric cell division to expand their population. Subsequently, NECs transition into radial glial cells (RGCs) marks the onset of neurogenesis [1, 2]. RGCs serve as neural stem cells and undergo asymmetric cell division to produce neuronally committed postmitotic cells or intermediate progenitor cells. As neurogenesis approaches completion, RGCs begin to produce oligodendroglial lineage progenitors, which further give rise to oligodendrocytes. Around the time of birth, RGCs shift to producing ependymal cells and also transform themselves into astrocytes and adult neural stem cells [1]. In addition to their role as neural stem cells supplying specialized cells in the three neural lineages, RGCs also possess a scaffolding function for neuronal migration. In this role, the basal processes of RGCs serve as substrates for the postmitotic migrating neurons. The migratory distance of the neurons depends on their birth order, with early-born neurons migrating at a shorter distance and late-born neurons at a longer distance, forming an inside–out laminar structure in a mature brain. Overall, RGCs perform two fundamental functions during cortical development—cell division and scaffolding (Fig. 1).
Fig. 1.
Established roles of radial glial cells during cerebral cortex development. Radial glial cells (RGCs) comprise a pseudostratified epithelial layer lining the ventricular wall during cerebral cortex development. RGCs performs asymmetric neurogenic cell division to produce postmitotic neurons or neuronally committed intermediate progenitor cells. In addition, the membranous protrusions on the basal polarity provide scaffold and guidance for the postmitotic migrating neurons
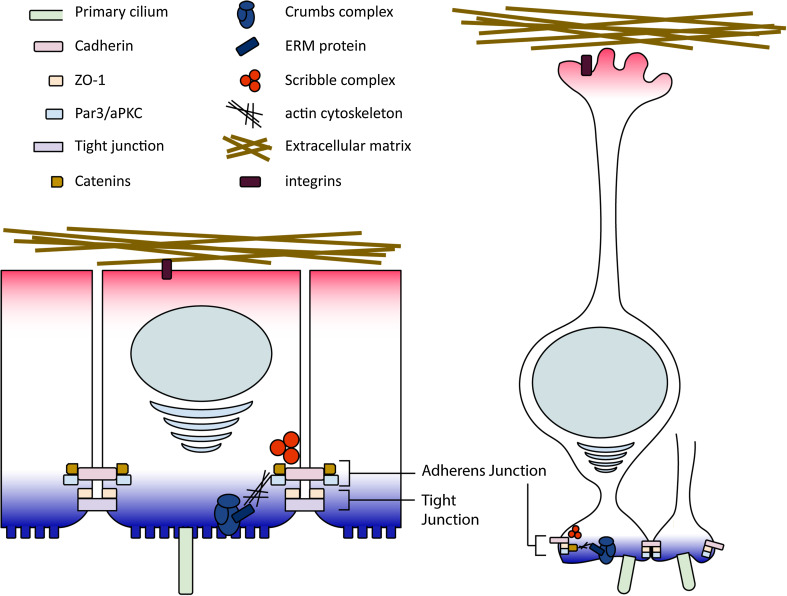
RGCs can be viewed as specialized epithelium with apico-basolateral polarity. Epithelium is the first tissue type to emerge during the development of a multicellular organism. In a recent review by Rodriguez-Boulan and Macara, the term “epithelial polarity program” was used to describe a network of protein and lipid regulators that are involved in the organization and execution of an apico-basolateral axis [3]. Traditionally, epithelium is categorized into simple, stratified, and pseudostratified types depending on the number of layers in the epithelial cells. Additionally, epithelial cells are further classified into squamous, cuboidal, and columnar types based on the height of their basolateral domains. External to the basal plasma membrane lies the laminin-enriched extracellular matrix. Laminin secretion by the epithelial cells downstream of β1 integrin-mediated activation of Rac1 on the basal plasma membrane also plays a critical role in epithelial polarity [4, 5]. On the apical surface, apical constriction mediated by apical actin cytoskeleton and molecular motor functioning facilitates tubulogenesis and tissue morphogenesis [6]. The actomyosin meshwork, Rho family GTPase regulators, as well as Twist and Snail transcription factors are involved in apical constriction [7, 8]. Much of the information regarding epithelial polarity is derived from studies of invertebrates, and the basic principles in polarity establishment and molecules involved are applicable to vertebral, including mammalian, epithelial cells [9]. An apparent benefit of epithelial polarity in a multicellular organism is to separate the interior environment from the outside world. Two characteristics unique to polarized epithelium are evolutionarily conserved to achieve this goal—polarized trafficking machinery and junctional complex formation.
Protein complexes involved in polarized trafficking machinery include the Crumbs–PALS1–PATJ polarity protein complex, the cdc42-Par6-aPKC-Par3 polarity module, and the Scribble–Lgl–Dlg polarity protein complex. These complexes are evolutionarily conserved across species from invertebrates to mammals [3, 10–12]. The Crumbs–PALS1–PATJ complex defines the apical domain, and the Scribble–Lgl–Dlg complex on the other hand defines the basolateral domain. The cdc42-Par6-aPKC-Par3 module plays a policing role in the trafficking control, making sure that respective proteins are localized in their domains. The interactions among polarity proteins are either through physical binding or through kinase-mediated phosphorylation. Polarity protein phosphorylation may lead to either retention of the protein in one polarity compartment or expulsion of the protein from the compartment. In addition to protein–protein interactions, phosphoinositide lipids are also involved early during epithelial polarity establishment [13–15]. Delicate interplay between polarity proteins and phospholipids through a polarized trafficking machinery is essential for the maintenance of the apico-basolateral polarity. A break in any component may disrupt the polarity that is already established.
Junctional complexes are membrane-bound structures located in the apico-lateral interface. Two major junction types are present in a polarized epithelial cell. Tight junctions (TJs, also called septate junctions in invertebrates) form a belt-like boundary around the cell at the apico-lateral boundary to block free movement of membrane-bound polarity proteins and lipids into the other domain, and also segregate the internal paracellular medium from the external environment. Adherens junctions (AJs), on the other hand, utilize calcium-dependent adhesion molecules (cadherins) to form a continuous adhesive belt. Junctional complex formation is initiated by actin aggregation and protrusions on the plasma membrane, followed by addition of adhesion proteins, including cadherins, catenins, and the TJ adaptor protein zonula occludins (ZO)-1. As the junctional complexes continue to mature, ZO-1 migrates away (apically in vertebrates, and basally in invertebrates) from the immature AJ and integrates with TJ proteins occludins and claudins to facilitate the assembly of mature TJs [16, 17].
In this review, we aim to discuss recent progress in the understanding of how apico-basolateral polarity affects RGC cellular functions during cerebral cortex development. Of note, a recently published review detailing the biochemical network in polarity establishment in neural stem cells and postmitotic neurons during cerebral cortex development may complement this article for interested readers [18]. Additionally, although it is now well established that RGCs are further subclassified into apical (or ventricular) RGCs and basal (or outer) RGCs depending on their respective localization and the presence or absence of apical endfeet on the ventricular surface in the developing cortex, our discussion, if not otherwise specified, will primarily focus on using apical RGCs as a stereotypical model, as these cells are the first cell type that is established upon a switch from NEC proliferation to neurogenesis, are present in abundant amount in cortices with or without gyrification (bRGCs forma prominent layer in species with cortical gyrification), and are the bona fide tissue-specific multipotent stem cells that give rise to other types of progenitor cells, including basal RGCs, neuronal and glial progenitors. A detailed discussion about the roles of the apical RGCs and their progenies during neurogenesis and corticogenesis is beyond the scope of this review. This topic is recently reviewed elsewhere [2, 19].
The apical polarity of the radial glial cells
As mentioned above, an intracellular polarized trafficking machinery is required for vectorial transportation of intracellular signals in order to facilitate compartmentalization of epithelial cells. The Crumbs-containing protein complex and the junctional complex are key determinants of the apical domain. Their roles in RGC polarity establishment have been studied extensively, and will be discussed here. In addition, two downstream activities of RGC polarity that take place on the ventricular surface—primary cilium formation and spindle orientation regulation—will also be discussed.
Apical polarity-defining proteins
Crumbs–PALS1–PATJ protein complex
The Crumbs–PALS1–PATJ polarity protein complex is apically localized in the epithelial cells and plays a key role in establishing and maintaining the apical polarity. Crumbs is a transmembranous protein, whereas PALS1 and PATJ are juxtamembranous and intracellular (Fig. 2). Crumbs proteins are highly conserved from invertebrates to mammals. Three homologs have been discovered in mammals: CRB1, CRB2, and CRB3. All three homologs have nonredundant roles in mice, as genetic ablation of any of the genes separately leads to a different set of phenotypes. Specifically, CRB1 knockout in mice leads to progressive defect in retinal AJs, and mutations in the CRB1 gene are associated with human retinal disease [20–22]. CRB2 knockout in mice results in gastrulation and brain developmental defects causing embryonic death at E12.5 [23]. Depletion of CRB3, a homolog with a short extracellular domain, in mice resulted in multiorgan developmental abnormalities, including the lungs, the kidneys, and the intestine. These mice die shortly after birth [24]. The Crumbs family proteins have been implicated in central nervous system development in zebrafish [25]. In mammals, it has been shown that CRB1 and CRB2 are upregulated, and CRB3 is downregulated, upon neural rosette differentiation from mouse embryonic stem cells in an in vitro neural differentiation system, and that CRB2 is localized to the ventricular surface of E12.5 mouse embryonic brain [26]. Furthermore, CRB2 knock-down results in cell death upon neuroectodermal differentiation, but has no effect on mesodermal or endodermal lineage differentiation. Mechanistically, CRB2 upregulation upon neural differentiation is also associated with increased phosphorylated aPKC and cdc42 protein levels, suggesting enhanced polarity [26]. Conversely, CRB2 global depletion led to a loss of columnar arrangement of NECs as well as discontinuous and dorsal appearance of basal lamina. In addition, there is mixing of NECs with the mesodermal layers at E9.5, suggesting complete loss of apicobasal polarity [23]. Surprisingly, the localization of other apical proteins such as ZO-1 or ezrin (actin cytoskeleton membrane anchorage protein, detailed below) was not affected, and the TJs were also present, indicating that the formation of junctional complexes and the assembly of cytoskeletal on the apical surface are regulated by a parallel pathway that does not involve the Crumbs protein complex. Of note, CRB1 and CRB3 global knockout did not reveal defects associated with cerebral cortex development, further emphasizing a non-redundant role of CRB2 in cerebral cortex development.
Fig. 2.
The apicobasal polarity and the subcellular structures of radial glial cells (RGCs) are reminiscent of stereotypical epithelial cells. Cartoon illustrations of a stereotypical epithelial cell (left) and a RGC (right)
Intracellular binding partners of Crumbs proteins include the FERM domain-containing Ezrin–Radixin–Moesin (ERM) family of proteins [25]. Specifically, Crumbs and Moesin (an ERM family protein) physically interact in zebrafish retina to form a complex on the apical surface of the retinal progenitors. Additionally, Moesin and Crumbs both localize to the apical surface of the NECs. The apical localization of both Moesin and Crumbs relies on the presence of each other [25]. As the ERM proteins serve as anchorage between actin cytoskeleton and the plasma membrane, they play a role in bridging the Crumbs complex to actin cytoskeleton. Moreover, the ERM proteins have also been found to mediate interaction between Crumbs and the Notch1 receptor to regulate the intensity of Notch signaling [27]. As mentioned above, apical localization of ezrin is not affected by loss of CRB2 in mouse embryos; however, Moesin relies on Crumbs for its apical attachment in zebrafish embryos. The difference may signify a redundant role of the mammalian CRB homologs when it comes to ERM protein binding. Interestingly, in human prenatal cerebral cortices, ezrin is abundantly expressed in the germinal matrix and in RGCs, although the protein does not seem to be restricted solely to the apical surface [28].
Apart from Crumbs, the role of PALS1 in RGC polarity and cerebral cortex development has also been elucidated in a recent study [29]. Using a conditional knockout approach, the study showed that loss of PALS1 leads to near-absence of cerebral cortex. It is associated with premature cell cycle exit and increased apoptotic cell death throughout the cortical wall. This is accompanied by a loss of AJs as well as basal misplacement or absence of β-catenin. These findings provide further evidence supporting a link between the Crumbs–PALS1–PATJ polarity complex and the AJs, and this link is likely mediated by actin cytoskeleton. It is further postulated that the loss of PALS1 and polarity in RGCs, and the resultant cell cycle exit may promote a neuronal cell fate, but these postmitotic cells quickly succumb to apoptosis via a pathway mediated by GSK3β. One possibility is that the β-catenin level, which is regulated by GSK3β, is critical for maintaining survival of the prematurely differentiated neurons. The mTOR pathway may also account for increased apoptosis [29].
Cdc42-Par6-aPKC-Par3 polarity module
In the addition to the ERM proteins and The Notch receptor, the Crumbs protein also interacts with Par6, a key player in the cdc42-Par6-aPKC-Par3 polarity module. Par6, rather than being an enzyme by itself, serves as an adaptor protein which allows multiple polarity proteins to interact upon activation by cdc42 [3, 10]. For one, Par6 draws aPKC to the apical domain and keeps aPKC in an active conformation by displacing its pseudosubstrate domain from binding to the enzymatic domain [30, 31]. Additionally, Par6 also brings Par3 and Lgl to the apical surface to be phosphorylated by aPKC during apical polarity establishment [32, 33].
In RGCs, Par3 was found to co-localize with N-cadherin, β-catenin, and ZO-1 at the AJs [34]. The dosage of Par3 in a dividing RGC may have an impact on the fates of the daughter cells. A reduction in Par3 levels will shift the dividing RGC from an asymmetric cell division to a symmetric neurogenic cell division [34]. Interestingly, unequal distribution of Par3 is observed during RGC asymmetric cell division, resulting in differential allocation of Par3 into each daughter cell. Although there has not been direct evidence suggesting a cell fate-determining role of Par3, it is possible that the Par3-inheriting daughter cell will continue to propagate via self-renewing asymmetric cell division, while the other daughter cell without inherited Par3 will switch to generate two neuronally committed cells at the subsequent round of cell division.
Similarly, depletion of cdc42 during the active stage of neurogenesis has been shown to be associated with misplacement of Par3 and aPKC, as well as loss of AJs, resulting in cortical delamination and premature neuronal differentiation [35]. Cdc42 depletion prior to RGC transitioning from NECs, on the other hand, resulted in a holoprosencephaly phenotype [36]. These findings further emphasize the crucial role of the cdc42-Par6-aPKC-Par3 polarity module as well as polarity establishment in cerebral cortex development.
Adherens junctions and cytoskeleton regulation
The formation of junctional complexes begins with clustering of transmembrane cadherins on the apical surface followed by addition of interacting proteins as the junction matures. In vertebrates, the TJs, marked by the presence of occludins and claudins, move apically during junctional complex formation, while the AJs move basally [3]. AJs contain transmembranous cadherin molecules. Whereas the extracellular domains of the cadherins from the neighboring cells interact with each other to form an epithelial sheet, the intracellular domains interact with the catenin molecules (α, β, and p120-catenin), which subsequently interact with actin cytoskeleton and other signaling molecules [37]. AJs serve as the interacting site for the neighboring epithelial cells, and more importantly define the interface between the apical and the basolateral domains. AJs exhibit a significant degree of plasticity through constant remodeling of the complex to accommodate for morphological changes of the tissue during development and as a result of an adaptive process. AJ remodeling is achieved by constant internalization of cadherins through Numb-mediated endocytosis, followed by recycling and reappearance of cadherins on the plasma membrane from the Rab11-positive endosomes [38–41].
Numerous studies have shed lights on an essential role of AJs in RGC polarity and functions. Complementary reading on this topic is available from another recently published review article [42]. It is worth mentioning that junctional complexes evolve during the transitioning from NECs to RGCs at the beginning of neurogenesis. Whereas NECs contain both TJs and AJs, RGCs only contain AJs [43]. The TJ protein ZO-1, an adaptor molecule that has been proposed to connect TJs, AJs, and actin cytoskeleton, is incorporated into the AJs in RGCs [37]. The significance of the loss of TJs in RGCs has not been elucidated in the literature. We speculate that the occluding roles of TJs may prevent the establishment of a paracellular signal gradient, such as the Notch signal gradient, required during RGC neurogenesis, while such paracellular gradient may be unnecessary or even detrimental to the symmetrically dividing NECs.
The importance of AJs during cerebral cortex development has been exemplified by a knockout study of N-cadherin (the major cadherin type in the nervous system) showing loss of AJs resulting in cortical delamination [44, 45]. Furthermore, by using an N-cadherin knock-down approach, researchers have shown that N-cadherin prevents β-catenin from being degraded prematurely. Moreover, the presence of N-cadherin increases β-catenin activity in RGCs via the Akt pathway to keep the cells from premature departure from the ventricular zone and to prevent premature differentiation [46]. Direct depletion of β-catenin leads to the same phenotypic changes [47]. These findings further strengthen a link between AJs and cell fate, and also identify β-catenin as the mediator. Interestingly, ectopic expression of stabilized β-catenin leads to increased neural progenitor proliferation and the appearance of gyri in mouse brains, with preservation of cortical lamination [48]. These findings suggest that transcription-associated roles of β-catenin may only occur after it has been saturated on the AJ as a structural protein.
Strikingly, depletion of α-catenin is also associated with loss of AJ and cortical delamination, presumably due to its negative effect on AJ integrity and RGC polarity maintenance [49, 50]. On the other hand, α-catenin depletion delays normal cell cycle exit timing and causes cortical hyperplasia, likely due to disassembly of the catenin complex and increased availability of β-catenin for nuclear translocation and transcriptional activity to promote cell proliferation [49].
It has not been systemically studied whether E-cadherin also has a role in AJ formation and RGC polarity and functions, but evidence has suggested expression of E-cadherin in the ventricular zone in fetal ferret brains [51]. Whether the expression of E-cadherin is associated with “cadherin switch” to facilitate transitioning of newly generated neuronally committed progenitor cells from an apico-basolateral polarity to a multi-polar or uni-polar morphology merits further studies. Interestingly, E-cadherin in the developing ferret brains was found to be downregulated transiently in the later phase of neurogenesis prior to birth, and this downregulation has been proposed to promote the production of basal RGCs (bRGCs), which are a type of RGCs that are derived from apical RGCs and are localized to the subventricular zone (SVZ), suggesting that E-cadherin may actually have a significant role in the attachment of the RGC apical endfeet to the ventricular surface [51].
As mentioned earlier, cadherins are constantly internalized and recycled to maintain plasticity of the AJs. Indeed, multiple studies have demonstrated the critical roles of the Numb and the Numb-like (Numbl) endocytic adaptor proteins in cerebral cortex development [52–54]. Numb and Numbl are present both in the AJ protein complex and in the Rab11 recycling endosomes, and are required for the maintenance of AJs and polarity in RGCs via a cadherin-dependent pathway. As expected, loss of the endocytic adaptor proteins leads to progenitor dispersion and a disorganized cortical lamination [54]. As the Arp2/3 actin cytoskeleton branching nucleator and actin cytoskeleton are also involved in endocytosis, Arp2/3 depletion in RGCs has been shown to lead to loss of AJs and the apical endfeet, resulting in cortical delamination and premature neuronal differentiation of the RGCs [55, 56]. Consistently, Rap1, a GTPase required for endocytosis, and its guanine nucleotide exchange factors Rapgef2 and Rapgef6, have been shown to be essential for apico-basal polarity establishment in RGCs during cerebral cortex development. Their depletion also leads to complete loss of AJs [57, 58].
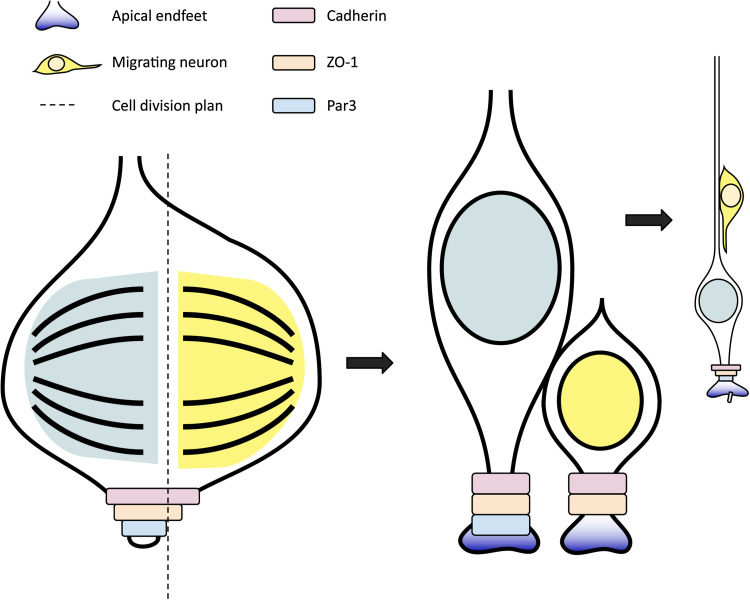
A recurring theme here is that, the RGCs require AJs to keep their apico-basal polarity in check and to prevent their premature differentiation into neurons. How is the AJ’s belt-like structure involved in cell fate decision? To answer this question, the structure of the AJ has been further analyzed using confocal microscopy with three-dimensional reconstruction, from which three distinct domains have been recognized [59]. The ZO-1-containing domain is positioned centrally, the Par3/aPKC module is enriched in the apical domain, and the structural protein N-cadherin is located in the basal domain. The cone-shaped apical endfeet makes it possible to have the entire apically placed Par3/aPKC domain segregated into one daughter cell during asymmetric division while splitting the other two domains into both daughter cells depending on the spindle orientation (Fig. 3). Such setup puts Par3 and aPKC in the category of cell fate determinants in addition to being polarity proteins.
Fig. 3.
Asymmetric inheritance of apical cell fate determinants leads to radial glial cell (RGC) neurogenic cell division. Three belt-like domains have been characterized in the adherens junction complex on the RGC apical endfeet, with the cadherin-containing domain (purple) residing on the basal side, and the Par3-containing domain (blue) on the apical side. The cone-shaped apical endfeet makes differential inheritance of each domain possible depending on spindle orientation. The apically placed Par3 domain is more likely to be segregated only into one daughter cell, resulting in different cell fates of the daughter cells. In the illustration, The green-shaded daughter cell is the self-renewing RGC and the yellow-shaded daughter cell becomes a migrating neuron as a result of preferential inheritance of Par3 cell fate determinant by the daughter RGC
The role of primary cilium in RGC polarity and cerebral cortex development
In vertebrates, an ultramicroscopic structure called primary cilium is formed on the apical surface of a non-dividing epithelial cell [3]. Primary cilium consists of a 9 + 0 axonemal microtubule doublet structure as a continuum from the mother centriole of the centrosome, which docks on the apical membrane following cell cycle exit. Primary cilium is initially formed as a result of fusion of the mother centriole with a Golgi-derived vesicle. The growing cilium further fuses with additional vesicles while migrating to the cell surface and is eventually exposed on the apical plasma membrane into the extracellular medium [60]. A mature primary cilium contains an intraflagellar transport system for transporting proteins in and out of the ciliary body. Although primary cilium does not contain motor units like a motile cilium does, it bends passively in response to environmental fluid flow or by mechanical force. The bending triggers calcium influx and signal transduction. The sonic hedgehog (Shh) signaling pathway, which is required for epithelial differentiation, is dependent on the presence of primary cilium on the apical surface. Shh signaling is regulated by the regulatory GTPase Arl13b that is also localized in the primary cilium [61, 62]. Primary cilium can be viewed as a sensory organelle of the epithelial cell. Over the past decade, vigorous research has been undertaken to delineate the exact function of primary cilium in epithelium. It is now widely accepted that primary cilium is involved in the development of various tissue types [63–67]. Additionally, the critical role of this apical organelle has also been implicated by its link to numerous clinical disorders, including polycystic liver and kidney diseases, situs inversus, Bardet–Biedl syndrome, Meckel syndrome, and Type C Niemann-Pick Disease, among others [68–72].
Similar to stereotypical epithelial cells, the apical surface of a polarized RGC serves as the docking site for the mother centriole following mitosis. In vertebrates, the apically located mother centriole becomes the basal body, from which the primary cilium grows into the fluid-filled cerebral ventricles. Although in continuum with the cytoplasm, substance transport in and out of primary cilium is restricted, making it a distinct organelle [60]. Indeed, primary cilium communicates with the “external” environment of the cerebrospinal fluid (CSF), where proliferation-promoting signaling molecules, such as Igf2, have been identified [73]. Different from the traditional view that primary cilium is disassembled prior to mitosis, it has been shown that primary cilium is internalized upon entry into the metaphase of the mitotic cycle, but the ciliary membrane persists throughout the mitotic cycle, in early embryonic murine brains (E12.5) [74, 75]. The percentage of dividing cells with persistent ciliary membrane decreases as the embryonic brain develops. The daughter cell that inherits the mother centriole with ciliary membrane from the mother RGC quickly docks the mother centriole onto the apical surface to reassemble the primary cilium. This daughter cell usually is the same cell that inherits the basal process from the mother RGC and is also the very cell that retains the RGC characteristics. On the other hand, the daughter cell that fails to inherit primary cilium will assemble from the centriole and the Golgi-derived vesicles. The docking site of the newly formed centriole on either the apical or the basolateral surface may depend on the type of cell division (symmetric vs asymmetric, self-renewing vs differentiating) that the mother RGC undergoes [74]. The signaling pathway that regulates the docking orientation of the newly formed centriole and the primary cilium remains to be elucidated.
Primary cilium exists only in vertebral epithelial cells, and has been found to be required for Sonic hedgehog (Shh) signaling. Most components of the Shh pathway, including the receptor (Patched1) and the transducer (Smoothened), are located in primary cilium [76, 77]. Moreover, the downstream effector (the Gli proteins) and the negative regulator of Shh signaling, Suppressor of fused (SuFu), are transported in and out of primary cilium in response to Shh signaling activation [78]. Although it has not been reported specifically whether Shh signaling plays a role in RGC morphogenesis and polarity establishment, the patterning nature of the Shh signaling pathway has been shown to play an essential role in neural tube formation and forebrain development [79]. In an in vitro mouse fibroblast model, the Shh signaling pathway has been shown to be regulated by Arl13b, a regulatory GTPase that is found in the primary cilium [62]. In the developing brain, Arl13b is located at the apical surface in the NECs and the RGCs where the primary cilium is expected to be present [80]. Strikingly, using an Arl13b-null mouse model induced by ENU with defective cilial architecture and Shh signaling, a study showed that the orientation of cortical lamination was completely reversed, with RGCs aberrantly lining the pial surface, and the differentiated neuronal cells found close to the ventricular surface. The reversal of cell type distribution apparently results from misorientation of the basal process scaffold. Consistently, apically localized proteins such as N-cadherin, β-catenin, and Numb were disrupted, although the extracellular matrix glycoprotein Reelin that is normally present in the marginal zone on the pial surface continued to be found on the pial surface in the mutant mouse embryonic brains. Interestingly, the ability of the RGCs to generate postmitotic neurons is also not compromised in the absence of Arl13b and an intact primary cilium. It was further shown that Arl13b was only required during RGC production from NECs; once the RGC is produced and the polarity as well as the basal process scaffold is established, loss of Arl13b has no impact on cerebral cortex development. Given that the basal process is retracted during symmetric NEC division but not during asymmetric RGC division (see below for detail), it is possible that the orientation for basal process reestablishment requires the presence of primary cilium on the opposite (apical) direction. Once neurogenesis has begun, RGC basal process tends to persist through the cell cycle, making primary cilium dispensable.
Of note, apart from serving as a signaling entry site for the Shh signaling pathway, primary cilium has also been shown to play a role in Notch signaling and the commitment of progenitors to differentiate during skin development. By contrast, Shh signaling defect occurs later in ciliary mutants, and only plays a role in hair follicle morphogenesis in the skin [81]. Whether distinct spatiotemporal functions of primary cilium are present in RGC and cerebral cortical development remains to be investigated.
Asymmetric cell division in polarized RGCs
Polarized epithelial cells divide asymmetrically to produce daughter cells that assume different cell fates as a result of unequal distribution of polarity proteins and cell fate determinants followed by placement of the mitotic spindle under tight regulations. Generally speaking, spindle orientation that is parallel to the apical membrane (planar spindle orientation and vertical division) results in symmetric division with both daughter cells inheriting equal share of the apical membrane. Conversely, spindle orientation that is perpendicular to the apical membrane (vertical spindle orientation and planar division) leads to preferential inheritance of the apical membrane, the apical polarity proteins, and AJs by the apical daughter cell, as well as preferential inheritance of the basolateral domain and basement membrane (BM) by the basal daughter cell, resulting in asymmetric division and a split in the fates of the daughter cells. If the spindle orientation is oblique, both daughter cells inherit unequal share of the apical membrane and the polarity domains. The cell fate of the daughter cells may be difficult to predict, and likely depends on the positioning and segregation of cell fate determinants.
The Pins/Mud/Gαi (or LGN/NuMA/Gαi in mammals) core ternary complex has been identified as a key player in spindle orientation for its ability to anchor between the plasma membrane, the spindle motor units, and the microtubules [82, 83]. Furthermore, Inscuteable/mINSC links Pins/LGN to the Par6/aPKC/Par3 polarity complex. Phosphorylation of Pins/LGN by apically placed aPKC leads to its removal from the apical surface, favoring planar spindle orientation and vertical cell division to allow the retention of apico-basal polarity in both daughter cells [82, 84]. Furthermore, LGN has recently been shown to interact with lateral polarity protein Dlg upon dissociation of Lgl from Dlg following Aurora A-mediated phosphorylation [85]. Of note, the relationship between spindle orientation, asymmetric vs symmetric cell division, and cell fate decision is not as straightforward as once thought, as it is now known that junctional complexes, basal lamina, primary cilium, as well as additional external cues all play a critical role in guiding cell fate decision. Multiple reviews on the cell biology perspective of this topic are available for interested readers [83, 86, 87].
Following asymmetric cell division, one of the daughter cells may lose epithelial attachment and assume a polarized yet motile mesenchymal cell type, a process called epithelial–mesenchymal transition (EMT) [3, 88]. EMT occurs during organogenesis and in cancer metastasis. Upon EMT initiation, a phenomenon called “cadherin switch” has been observed, in which E-cadherin is downregulated and N-cadherin is upregulated [89]. Cadherin switch leads to weaker binding between the extracellular domains of the cadherins and results in loosening of AJs between the neighboring cells. In addition, junctional complex proteins are downregulated, leading to detachment of the cell from the epithelial sheet. Moreover, the expression of Crumbs, the aforementioned scaffold protein in the Crumbs-PALS1-PATJ complex that defines the apical polarity, is also repressed. Twist and Snail transcription factor families are well-established molecular switches for EMT. Additional transcription factors, such as the Forkhead box and the zinc-finger E-box-binding (ZEB) transcription factors are also involved in the process. It has also been shown that the transforming growth factor-beta (TGFβ) signaling pathway plays an essential role in instructing the onset of EMT. Additional pathways, such as Shh, Wnt/β-catenin, Notch, integrin-mediated pathways, as well as inflammation and hypoxia all contribute to EMT, at least in the context of cancer metastasis. Importantly, the Rho GTPase actin cytoskeleton regulators promote actin cytoskeleton rearrangement during EMT. Along with redistribution of polarity proteins, the cells are able to re-establish a front–rear polarity axis to facilitate directional migration.
In this section, we will discuss current understanding of the roles of spindle orientation regulation and EMT during cerebral cortex development.
Regulation of spindle orientation in neurogenic RGCs
One major feature of the emerging apical RGCs during neurogenesis is to perform asymmetric cell division to produce a self-renewing RGC and a neuronally committed progenitor or postmitotic cell [90, 91]. In a stereotypical epithelial cell, symmetric cell division is associated with vertical orientation of the mitotic spindle, whereas asymmetric cell division is associated with oblique or planar orientation of the spindle. Intriguingly, most studies have now agreed that RGC neurogenic asymmetric division is vertical (or close to vertical) [2, 91–95]. Mitotic spindle orientation is dynamic prior to RGC cell division [2, 96]. Mitotic spindle misorientation has been observed in various disorders of cerebral cortex development, including mutations of the Lis1 gene that is associated with lissencephaly and subcortical band heterotopia [86, 97]. A recent study using a genetic knockout approach to deplete the LGN/NuMA/Gαi spindle orientation complex revealed loss of planar mitotic spindle orientation during RGC neurogenesis, resulting in equal distribution of spindle orientation from planar to vertical orientation [93]. As a result of an increase in a vertical spindle orientation, an increase in the frequency of Pax6-positive RGCs outside the ventricular zone due to loss of apical contact was observed, suggesting inheritance of RGC characteristics in the basal daughter cells. Strikingly, loss of apical placement did not change cell fate distribution in the progeny cells. On the other hand, the apical daughter cell on the ventricular surface that failed to inherit the basal process quickly became postmitotic, reminiscent of epidermal differentiation where more differentiated postmitotic cells are located apically to the stem and progenitor cells. These findings raised a question of whether basal process inheritance downstream of spindle orientation placement upon cell division, instead of apical endfoot inheritance, is key to the retention of progenitor characteristics in the daughter cell (please refer to the section entitled “Basal process inheritance in RGC cell division” below). Alternatively, it may also be possible that spindle orientation is upstream of cell fate determinant distribution during mitosis, and is uncoupled from distribution of non-cell-fate-determinant polarity proteins, which to some extent was implicated in the study, as their findings showed no impact on the daughter cell fate distribution following a shift in the distribution of spindle orientation. This concept is similar to the distinction between cell fate determinants and non-cell fate determinants among polarity proteins in the AJs, and may actually be interconnected [59]. It remains to be determined whether loss of the spindle orientation complex leads to cortical delamination as a result of misplaced progenitor cells and postmitotic neurons.
RGCs in the embryonic lateral ganglionic eminence (LGE) initially undergo self-renewing asymmetric cell division during embryonic brain development. At the end of neurogenesis, these RGCs transform into adult-type neural stem cells (aNSC) and reside in the SVZ. A recent report using dominant-negative LGN and spindle orientation regulator INSC showed that spindle orientation and the cell division plane in the LGE are a major determinant of the embryonic progenitor pool size, as well as the frequency of aNSC later on in adult mice. Specifically, disruption of the spindle complex resulted in an increased frequency of symmetric neurogenic cell division, resulting in premature exhaustion of the self-renewing RGCs and a decrease in the aNSC pool size. Interestingly, spindle complex manipulation at a postnatal age did not result in the same phenotype, suggesting a developmentally critical window for the regulation of aNSC frequency by spindle orientation [98].
RGC neurogenesis as an EMT process
In RGC asymmetric cell division, one of the daughter cells becomes either a neuronally committed postmitotic cell or an intermediate progenitor cell (IPC). In either case, these daughter cells quickly lose their apico-basal polarity. A front–rear polarity is subsequently established through redistribution of polarity proteins and rearrangement of actin cytoskeleton to prepare for radial migration from their original apical location towards the cortical plate, a process that is reminiscent of EMT [3, 88, 91]. As mentioned above, multiple transcription factors have been shown to be involved in the activation of the EMT program, including the Snail family of transcription factors [88]. Interestingly, it has recently been shown that Scratch 1 and 2, members of the Snail superfamily, are required for apical detachment and initiation of radial migration of the neuronally committed cells following neurogenesis, suggesting that an EMT-like program is involved in transforming a polarized epithelial RGC into a migratory neuron [99]. Activation of the EMT program is largely regulated by TGFβ signaling in development and in cancer metastasis. In the developing cerebral cortex, although both chemical-induced activation and inhibition of TGFβ signaling are associated with increased presence of neurons throughout the cortex (in addition to promoting astrocytic differentiation of the RGC), the roles of TGFβ signaling in the transitioning of the postmitotic neurons into a migratory phenotype following RGC asymmetric division have not been investigated specifically [100]. Future studies should focus on understanding the connection between cell fate determinants and the activation of the EMT-like program in the migrating neurons, as well as how the EMT-like program is involved in the crosstalk between the migrating neurons and the radial glia scaffold to determine their final location in the cortical plate.
The basolateral polarity of the radial glial cells
Unlike epithelial tissues in other organ systems, the basolateral domain of the RGC epithelium assumes an elongated structure composed of a basal process shaft and a growth cone-like structure at the most basal location of the process (Fig. 1). The roles of these subcellular structures and the extracellular matrix in the immediate vicinity during cerebral cortex development will be discussed here.
Basolateral polarity-defining proteins
Scribble–Lgl–Dgl protein complex
The Scribble–Lgl–Dgl protein complex is located basolaterally in a polarized epithelial cell and defines the basolateral domain. Two Lgl homologs have been identified in mice, Lgl1 and Llgl2, with Llgl1 being the predominant form in the brain [101]. Llgl1 global knockout leads to neonatal death within 24 h after birth, and visible hemorrhage in the cerebral ventricles are readily identifiable in the E12.5 embryos [101]. Further investigation found ectopic Nestin-positive rosette formation as well as cortical delamination and neural progenitor expansion, suggesting a loss of apico-basolateral polarity in the RGCs and ectopic localization. Moreover, electron microscopy showed disruptions of the AJ complex in the ventricular zone of the striatum area, which the authors further speculated to be responsible for Numb misplacement and increased Notch signaling activity as well as a change in RGC cell fate favoring self-renewing symmetric cell division [34, 101]. To more closely study the contribution of Llgl1 in Nestin-expressing RGC polarity establishment and the observed phenotypes, a recent study conditionally knocked out Llgl1 in Nestin-expressing cells. In addition to the above-mentioned phenotype, periventricular heterotopia was also observed, with gray matters flanking the white matter in the cortex instead of dorsal to the white matter as seen in the wildtype brain. The misplacement of the gray matter is thought to be due to a disruption of apico-basolateral polarity in the RGCs as a result of Llgl1 loss [102]. It was also found that Llgl1 as a basolateral polarity protein plays a critical role in maintaining AJ integrity by keeping N-Cadherin in a polarized distribution in the apical domain. This is achieved by direct sequestering of N-Cadherin by Llgl1. This sequestering interaction is restricted to the basolateral domain. In a sense, the Scripple–Lgl–Dgl protein complex serves as a negative regulator to prevent basolateral invasion of the apical domain. Without well-established polarity, the basal processes of the RGCs cannot properly guide neuronal migration, and cerebral heterotopia ensues.
Other than Llgl1, opportunities continue to exist for studies regarding the roles of the other proteins of the basolateral domain-defining Scribble–Lgl–Dgl protein complex in RGC polarity and functions as well as in the context of mammalian cortex development.
Basal processes
Role of actin cytoskeleton in the growth cone-like structure
Whereas the cell bodies of the NECs and the RGCs reside in close proximity to the apical surface facing the CSF, the basal polarity contains an elongated radial process that spans the entire thickness of the growing cortex. The basal process is reminiscent of the axon of a maturing neuron. The shaft is filled with intermediate filaments Nestin and microtubules; towards the tip, a growth cone-like structure with dense actin cytoskeleton and lamellipodia formation has been described [55, 103]. Using Dil fluorescent lipophilic dye, earlier observations suggest that the tip of the basal process is stably attached to the pial surface. As the cortex thickens, the process stretches and a biophysical tension is developed [104]. On the other hand, a more recent study combined in utero electroporation for VZ cell labeling and time-lapse imaging techniques and demonstrated that RGC basal processes are highly dynamic structures, with the protruding membranes constantly interacting with one another and with the microenvironment on the pial surface [103]. It is possible that different cell labeling approaches identified different RGC subpopulations. Interestingly, a recent study using human fetal brain slices demonstrated that the RGC basal process length is significantly longer than the NEC basal process length in the same tissue, and is longer than the cortical thickness with an oblique orientation in the marginal zone [95]. These findings would also suggest that the length of the basal processes is not passively determined by the cortical span. The RGC basal process is a unique subcellular structure with a unique function not present in other types of epithelial cells. The process is required for interactions with the pial surface and the postmitotic neurons to maintain the proper functions of the RGCs and to ensure cortical lamination [105–107]. Whether the extension of the basal process is under tension as a result of the increasing cortical thickness or an active process that precedes deposition of neuronal cells, or both, remains to be further delineated.
Recent work showed that the Arp2/3 actin cytoskeleton actin branching complex and its regulator Cdc42 are present abundantly in the growth cone-like structure on the basal endfeet [55, 56, 103]. Depletion of cdc42 or Apr2/3 in the RGC leads to shorter basal processes and defective scaffolding to guide neuronal migration [55, 56, 103]. Interestingly, depletion of an Arp2/3 subunit is associated with loss of the growth cone-like structure, as well as rapid and uncontrolled extension/retraction of the basal processes, suggesting that actin cytoskeleton meshwork plays a critical role in stabilizing the intermediate filament-filled cytoskeleton in the shaft and preventing any uncontrolled growth and sudden collapse [55]. It merits further studies to determine the molecular mechanism by which actin cytoskeleton contributes to the steady growth of the intermediate filament-filled process shafts.
Basal process inheritance in RGC cell division
Whereas the apical endfeet and the AJs are inherited by the daughter cells regardless of their cell fates, it is debatable how the basal process is inherited [78, 107]. All three scenarios, namely equal inheritance by splitting, inheritance by the self-renewing progenitor, and inheritance by the neuronally committed cell, have all been reported [92, 105, 111, 112]. The more prevalent view is that, the basal process is inherited by the self-renewing progenitors [81, 92]. The retention of the basal processes may actually be critical for keeping the RGC daughter cells in a progenitor state. This is supported by an observation made in a genetic model that was used to manipulate spindle complex orientation showing that basal process-retaining RGCs relocated to the SVZ following loss of their apical endfeet (as a result of spindle orientation and cell division plane change) after cell division continued to remain in a progenitor state and are capable of undergoing subsequent rounds of self-renewing asymmetric cell division [93]. It is possible that the basal process, through its unique morphology and its interactions with the environment, is required for the establishment and the re-establishment of signals required for RGC self-renewal (e.g., Notch signaling) regardless of where these cells reside [58, 108, 113] Interestingly, one recurring observation in the literature is that, during transition from the G2 to the M phase, there is apparent downward flow of plasma membrane and varicosities in the basal process with gradual thinning of the shaft until the cells enter the telophase [95, 108]. It would be interesting to investigate whether centripetal delivery of basally located cell fate determinants accounts for these observations.
Roles of the basal process shafts in RGC functions
In addition to intermediate filaments, microtubules have also been shown to fill the basal process shafts. The plus end growth of the microtubules is regulated by adenomatous polyposis coli (APC), which is localized to the growth cone-like structure of the basal process. Genetic depletion of APC in RGCs resulted in failure of basal process extension and a defect in cortical lamination [109]. Given the involvement of APC in the Wnt/β-catenin pathway, it is possible that basal process extension is regulated by β-catenin levels and the AJs. Alternatively, regulation of basal process formation may also have implications in cell fate decision as seen in the regulation of AJ formation.
Intriguingly, basal process abnormalities are associated with reduced proliferative potential of the VZ cells in the above-mentioned APC depletion model, suggesting that, in addition to its scaffolding function, basal processes may indeed contribute to promoting RGC self-renewal to further expand its pool size during cerebral cortex development [109]. Similarly, a recent report showed that, in a polarized RGC, the cyclin D2 mRNA was transported in the basal process towards the basal endfeet, where translation occurred. The transport signal has been identified in the 3′ UTR of the cyclin D2 mRNA [110]. Localization of cyclin D2 and asymmetric inheritance of the basal process (see below) may explain how the integrity of the basal processes impacts on RGC cell division and cell fate determination. A recent publication conducted an even more detailed analysis and identified a local transcriptome in the RGC basal endfeet that is highly regulated, with gene products highly implicated in autism and neurogenesis [111]. These findings shed lights on the roles of the basal processes not only in providing scaffolds for neuronal migration but also in the regulation of RGC cell fate determination.
The basal processes of the RGCs play a third role during cerebral cortex development. As mentioned above, the RGCs are organized into a pseudostratified epithelial structure, in which the nuclei move along the basal process during cell cycle progression, a process called interkinetic nuclear migration (IKNM). Specifically, the nuclei of the RGCs migrate away from the CSF-filled ventricle following mitotic cell division, and then travel back towards the ventricular surface just prior to the next round of cell division. Many speculations have been made to understand the benefit for the neural progenitors to adapt to a pseudostratified structure with IKNM. Although extensive discussion about this topic is beyond the scope of this review, it is prudent to point out that, this migratory process is propelled by actomyosin and microtubule motor proteins in the basal process, and that the basal process-inherited daughter RGCs are able to have their nuclei migrate outwards more quickly to avoid crowdedness in the VZ and to provide room for the process-non-inherited daughter cells to transition into an IPC or a postmitotic neuron. Interested readers may refer to recently published review articles on this topic [112, 113].
Taken together, the RGC basal processes are essential for both of their fundamental functions—proliferation and scaffolding.
Basement membrane and extracellular matrix contact
External to the tip of the basal process lies a thin layer of extracellular matrix that is composed of members of the laminin family proteins and other glycoproteins secreted by the epithelial RGCs to form the BM. In a stereotypical epithelial cell, β1-integrin on the basal plasma membrane provides an inside–out signal to promote laminin secretion [3]. In the developing brain, earlier studies showed that targeted deletion of either β1 or α6 integrin abolishes laminin deposition in the extracellular matrix and attachment of RGC basal endfeet to the BM, causing features of migrational defect of the postmitotic neurons, including cortical dysplasia and cortical ectopia [114–116].
Similarly, genetic depletion of focal adhesion kinase (FAK) in the dorsal forebrain RGCs by Emx1-driven Cre recombinase causes disruption of the cortical basement membrane and a disorganized cortical lamination. This is associated with RGC basal process retraction and disorganization, as well as areas of ectopic neurons [116]. As FAK is a non-receptor tyrosine kinase which is activated following integrin activation and binding to extracellular matrix, these findings suggest that the interaction between the basal processes and the basal microenvironment may play a role in maintaining the scaffolding shafts of the RGCs and is critical for neuronal migration and cortical lamination.
Earlier studies also showed that the BM is essential for the RGC scaffolding function by instructing migration of postmitotic neurons during cortical lamination, but is dispensable for the RGC proliferative function. Loss of the pial surface is associated with dysregulation of neuronal migration, resulting in cortical ectopia [116–119]. A biophysical phenomenon may account for the phenotype [104]. On the other hand, biochemical interactions between RGC basal processes and the BM microenvironment may also be crucial for the precise neuronal positioning [120–124].
Conclusion
RGCs as specialized epithelium exhibit multiple characteristics of polarized epithelial cells. The subcellular structures that are critical for apico-basal polarity are also instructive of their functions as neural stem cells. Much attention has been paid towards understanding the structure and functions of the apical endfeet. The roles of the basal process, on the other hand, are now beginning to be elucidated. An area for future research may include further exploring the biological significance of the basal processes in apico-basal polarity, cell fate decision, and neuronal migration. Moreover, while forced disruption of the apico-basal polarity by depleting essential components of the polarity program has repeatedly proven the critical roles of structure-based functions in RGC, it remains largely unknown how the microenvironment instructs such polarity program spatiotemporally, and how an impending loss of polarity can be rescued by RGC–environmental interactions. As clinical migrational defects have huge implications in neurodevelopmental outcome and prognosis, it is prudent to further elucidate the critical molecular pathways that are involved in RGC biology during cerebral cortex development.
Acknowledgements
This work is supported by Children’s Mercy-Kansas City Children’s Research Institute. The authors would also like to acknowledge the editing services of the Medical Writing Center at Children’s Mercy-Kansas City for reviewing the manuscript.
References
- 1.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paridaen JTML, Huttner WB. Neurogenesis during development of the vertebrate central nervous system. EMBO Rep. 2014;15:351–364. doi: 10.1002/embr.201438447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15:225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien LE, Jou TS, Pollack AL, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 5.Yu W, Datta A, Leroy P, et al. β1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes VM, McCormack K, Lewellyn L, Verheyen EM. Integrins regulate apical constriction via microtubule stabilization in the drosophila eye disc epithelium. Cell Rep. 2014;9:2043–2055. doi: 10.1016/j.celrep.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 7.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin–myosin network drive apical constriction. Nature. 2009;457:495. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawyer JM, Harrell JR, Shemer G, et al. Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol. 2010;341:5–19. doi: 10.1016/j.ydbio.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campanale JP, Sun TY, Montell DJ. Development and dynamics of cell polarity at a glance. J Cell Sci. 2017;130:1201–1207. doi: 10.1242/jcs.188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assémat E, Bazellières E, Pallesi-Pocachard E, et al. Polarity complex proteins. Biochim et Biophys Acta (BBA) Biomembr. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 11.Bulgakova NA, Knust E. The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J Cell Sci. 2009;122:2587–2596. doi: 10.1242/jcs.023648. [DOI] [PubMed] [Google Scholar]
- 12.Su W-H, Mruk DD, Wong EWP, et al. Polarity protein complex scribble/Lgl/Dlg and epithelial cell barriers. Adv Exp Med Biol. 2012;763:149–170. doi: 10.1007/978-1-4614-4711-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gassama-Diagne A, Yu W, ter Beest M, et al. Phosphatidylinositol-3, 4, 5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol. 2006;8:963–970. doi: 10.1038/ncb1461. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Belmonte F, Gassama A, Datta A, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chartier FJ-M, Hardy ÉJ-L, Laprise P. Crumbs controls epithelial integrity by inhibiting Rac1 and PI3K. J Cell Sci. 2011;124:3393–3398. doi: 10.1242/jcs.092601. [DOI] [PubMed] [Google Scholar]
- 16.Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132:451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell HK, Maiers JL, DeMali KA. Interplay between tight junctions & adherens junctions. Exp Cell Res. 2017 doi: 10.1016/j.yexcr.2017.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen AH, Duellberg C, Mieck C, et al. Cell polarity in cerebral cortex development-cellular architecture shaped by biochemical networks. Front Cell Neurosci. 2017;11:176. doi: 10.3389/fncel.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dwyer ND, Chen B, Chou S-J, et al. Neural stem cells to cerebral cortex: emerging mechanisms regulating progenitor behavior and productivity. J Neurosci. 2016;36:11394–11401. doi: 10.1523/JNEUROSCI.2359-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Den Hollander AI, Ten Brink JB, De Kok YJM, et al. Mutations in a human homologue of drosophila crumbs cause retinitis pigmentosa (RP12) Nat Genet. 1999;23:217–221. doi: 10.1038/13848. [DOI] [PubMed] [Google Scholar]
- 21.den Hollander AI, Heckenlively JR, van den Born LI, et al. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet. 2001;69:198–203. doi: 10.1086/321263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Pavert SA, Kantardzhieva A, Malysheva A, et al. Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J Cell Sci. 2004;117:4169–4177. doi: 10.1242/jcs.01301. [DOI] [PubMed] [Google Scholar]
- 23.Xiao Z, Patrakka J, Nukui M, et al. Deficiency in crumbs homolog 2 (Crb2) affects gastrulation and results in embryonic lethality in mice. Dev Dyn. 2011;240:2646–2656. doi: 10.1002/dvdy.22778. [DOI] [PubMed] [Google Scholar]
- 24.Whiteman EL, Fan S, Harder JL, et al. Crumbs3 is essential for proper epithelial development and viability. Mol Cell Biol. 2014;34:43–56. doi: 10.1128/MCB.00999-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu Y-C, Willoughby JJ, Christensen AK, Jensen AM. Mosaic eyes is a novel component of the crumbs complex and negatively regulates photoreceptor apical size. Development. 2006;133:4849–4859. doi: 10.1242/dev.02685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boroviak T, Rashbass P. The apical polarity determinant crumbs 2 is a novel regulator of ESC-derived neural progenitors. Stem Cells. 2011;29:193–205. doi: 10.1002/stem.567. [DOI] [PubMed] [Google Scholar]
- 27.Ohata S, Aoki R, Kinoshita S, et al. Dual roles of notch in regulation of apically restricted mitosis and apicobasal polarity of neuroepithelial cells. Neuron. 2011;69:215–230. doi: 10.1016/j.neuron.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 28.Johnson MW, Miyata H, Vinters HV. Ezrin and moesin expression within the developing human cerebrum and tuberous sclerosis-associated cortical tubers. Acta Neuropathol. 2002;104:188–196. doi: 10.1007/s00401-002-0540-x. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Lehtinen MK, Sessa A, et al. The apical complex couples cell fate and cell survival to cerebral cortical development. Neuron. 2010;66:69–84. doi: 10.1016/j.neuron.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graybill C, Wee B, Atwood SX, Prehoda KE. Partitioning-defective protein 6 (Par-6) activates atypical protein kinase C (aPKC) by pseudosubstrate displacement. J Biol Chem. 2012;287:21003–21011. doi: 10.1074/jbc.M112.360495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobias IS, Newton AC. Protein scaffolds control localized protein kinase Cζ activity. J Biol Chem. 2016;291:13809–13822. doi: 10.1074/jbc.M116.729483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin D, Edwards AS, Fawcett JP, et al. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 33.Plant PJ, Fawcett JP, Lin DCC, et al. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol. 2003;5:301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- 34.Bultje RS, Castaneda-Castellanos DR, Jan LY, et al. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cappello S, Attardo A, Wu X, et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–1107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Liao G, Yang L, et al. Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc Natl Acad Sci USA. 2006;103:16520–16525. doi: 10.1073/pnas.0603533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Classen A-K, Anderson KI, Marois E, Eaton S. Hexagonal packing of drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Desclozeaux M, Venturato J, Wylie FG, et al. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am J Physiol Cell Physiol. 2008;295:C545–C556. doi: 10.1152/ajpcell.00097.2008. [DOI] [PubMed] [Google Scholar]
- 40.Sato K, Watanabe T, Wang S, et al. Numb controls E-cadherin endocytosis through p120 catenin with aPKC. Mol Biol Cell. 2011;22:3103–3119. doi: 10.1091/mbc.E11-03-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brüser L, Bogdan S. Adherens Junctions on the move-membrane trafficking of E-Cadherin. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stocker AM, Chenn A. The role of adherens junctions in the developing neocortex. Cell Adh Migr. 2015;9:167–174. doi: 10.1080/19336918.2015.1027478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malatesta P, Appolloni I, Calzolari F. Radial glia and neural stem cells. Cell Tissue Res. 2008;331:165–178. doi: 10.1007/s00441-007-0481-8. [DOI] [PubMed] [Google Scholar]
- 44.Gänzler-Odenthal SI, Redies C. Blocking N-cadherin function disrupts the epithelial structure of differentiating neural tissue in the embryonic chicken brain. J Neurosci. 1998;18:5415–5425. doi: 10.1523/JNEUROSCI.18-14-05415.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadowaki M, Nakamura S, Machon O, et al. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Woodhead GJ, Swaminathan SK, et al. Cortical neural precursors inhibit their own differentiation via N-cadherin maintenance of β-catenin signaling. Dev Cell. 2010;18:472–479. doi: 10.1016/j.devcel.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodhead GJ, Mutch CA, Olson EC, Chenn A. Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci. 2006;26:12620–12630. doi: 10.1523/JNEUROSCI.3180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 49.Lien W-H, Klezovitch O, Fernandez TE, et al. alphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006;311:1609–1612. doi: 10.1126/science.1121449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmid M-T, Weinandy F, Wilsch-Bräuninger M, et al. The role of α-E-catenin in cerebral cortex development: radial glia specific effect on neuronal migration. Front Cell Neurosci. 2014;8:215. doi: 10.3389/fncel.2014.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martínez-Martínez MÁ, De Juan Romero C, Fernández V, et al. A restricted period for formation of outer subventricular zone defined by Cdh1 and Trnp1 levels. Nat Commun. 2016;7:11812. doi: 10.1038/ncomms11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen PH, Zou K, Hwang JK, et al. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- 53.Li HS, Wang D, Shen Q, et al. Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron. 2003;40:1105–1118. doi: 10.1016/S0896-6273(03)00755-4. [DOI] [PubMed] [Google Scholar]
- 54.Rašin M-R, Gazula V-R, Breunig JJ, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- 55.Wang P-S, Chou F-S, Ramachandran S, et al. Crucial roles of the Arp2/3 complex during mammalian corticogenesis. Development. 2016;143:2741–2752. doi: 10.1242/dev.130542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chou F-S, Wang P-S. The Arp2/3 complex is essential at multiple stages of neural development. Neurogenesis. 2016;3:e1261653. doi: 10.1080/23262133.2016.1261653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah B, Lutter D, Tsytsyura Y, et al. Rap1 GTPases are master regulators of neural cell polarity in the developing neocortex. Cereb Cortex. 2016 doi: 10.1093/cercor/bhv341. [DOI] [PubMed] [Google Scholar]
- 58.Maeta K, Edamatsu H, Nishihara K, et al. Crucial role of Rapgef2 and Rapgef6, a family of guanine nucleotide exchange factors for Rap1 small GTPase, in formation of apical surface adherens junctions and neural progenitor development in the mouse cerebral cortex. eNeuro. 2016 doi: 10.1523/ENEURO.0142-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marthiens V, ffrench-Constant C. Adherens junction domains are split by asymmetric division of embryonic neural stem cells. EMBO Rep. 2009;10:515–520. doi: 10.1038/embor.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedersen LB, Veland IR, Schrøder JM, Christensen ST. Assembly of primary cilia. Dev Dyn. 2008;237:1993–2006. doi: 10.1002/dvdy.21521. [DOI] [PubMed] [Google Scholar]
- 61.Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mariani LE, Bijlsma MF, Ivanova AI, et al. Arl13b regulates Shh signaling from both inside and outside the cilium. Mol Biol Cell. 2016 doi: 10.1091/mbc.E16-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhattacharyya S, Rainey MA, Arya P, et al. Endocytic recycling protein EHD1 regulates primary cilia morphogenesis and SHH signaling during neural tube development. Sci Rep. 2016;6:20727. doi: 10.1038/srep20727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi H, Shin JH, Kim ES, et al. Primary cilia negatively regulate melanogenesis in melanocytes and pigmentation in a human skin model. PLoS One. 2016;11:e0168025. doi: 10.1371/journal.pone.0168025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaku M, Komatsu Y. Functional diversity of ciliary proteins in bone development and disease. Curr Osteoporos Rep. 2017 doi: 10.1007/s11914-017-0351-6. [DOI] [PubMed] [Google Scholar]
- 66.Snedeker J, Schock EN, Struve JN, et al. Unique spatiotemporal requirements for intraflagellar transport genes during forebrain development. PLoS One. 2017;12:e0173258. doi: 10.1371/journal.pone.0173258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Millington G, Elliott KH, Chang Y-T, et al. Cilia-dependent GLI processing in neural crest cells is required for tongue development. Dev Biol. 2017 doi: 10.1016/j.ydbio.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wheatley DN. Landmarks in the first hundred years of primary (9 + 0) cilium research. Cell Biol Int. 2005;29:333–339. doi: 10.1016/j.cellbi.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 70.Wong MY, McCaughan GW, Strasser SI. An update on the pathophysiology and management of polycystic liver disease. Expert Rev Gastroenterol Hepatol. 2017 doi: 10.1080/17474124.2017.1309280. [DOI] [PubMed] [Google Scholar]
- 71.Ma M, Gallagher A-R, Somlo S. Ciliary mechanisms of cyst formation in polycystic kidney disease. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goetz SC, Bangs F, Barrington CL, et al. The Meckel syndrome—associated protein MKS1 functionally interacts with components of the BBSome and IFT complexes to mediate ciliary trafficking and hedgehog signaling. PLoS One. 2017;12:e0173399. doi: 10.1371/journal.pone.0173399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lehtinen MK, Zappaterra MW, Chen X, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paridaen JTML, Wilsch-Bräuninger M, Huttner WB. Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell. 2013;155:333–344. doi: 10.1016/j.cell.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 75.Wilsch-Bräuninger M, Florio M, Huttner WB. Neocortex expansion in development and evolution—from cell biology to single genes. Curr Opin Neurobiol. 2016;39:122–132. doi: 10.1016/j.conb.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Huangfu D, Liu A, Rakeman AS, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 77.Corbit KC, Aanstad P, Singla V, et al. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 78.Haycraft CJ, Banizs B, Aydin-Son Y, et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma Y, Erkner A, Gong R, et al. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell. 2002;111:63–75. doi: 10.1016/S0092-8674(02)00977-7. [DOI] [PubMed] [Google Scholar]
- 80.Higginbotham H, Guo J, Yokota Y, et al. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci. 2013;16:1000–1007. doi: 10.1038/nn.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ezratty EJ, Stokes N, Chai S, et al. A role for the primary cilium in notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–1141. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bergstralh DT, Haack T, St Johnston D. Epithelial polarity and spindle orientation: intersecting pathways. Philos Trans R Soc Lond B Biol Sci. 2013;368:20130291. doi: 10.1098/rstb.2013.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vorhagen S, Niessen CM. Mammalian aPKC/Par polarity complex mediated regulation of epithelial division orientation and cell fate. Exp Cell Res. 2014;328:296–302. doi: 10.1016/j.yexcr.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 84.Durgan J, Kaji N, Jin D, Hall A. Par6B and atypical PKC regulate mitotic spindle orientation during epithelial morphogenesis. J Biol Chem. 2011;286:12461–12474. doi: 10.1074/jbc.M110.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carvalho CA, Moreira S, Ventura G, et al. Aurora A triggers Lgl cortical release during symmetric division to control planar spindle orientation. Curr Biol. 2015;25:53–60. doi: 10.1016/j.cub.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 86.Lancaster MA, Knoblich JA. Spindle orientation in mammalian cerebral cortical development. Curr Opin Neurobiol. 2012;22:737–746. doi: 10.1016/j.conb.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.di Pietro F, Echard A, Morin X. Regulation of mitotic spindle orientation: an integrated view. EMBO Rep. 2016;17:1106–1130. doi: 10.15252/embr.201642292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wheelock MJ, Shintani Y, Maeda M, et al. Cadherin switching. J Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 90.Noctor SC, Flint AC, Weissman TA, et al. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 91.Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 92.Noctor SC, Martínez-Cerdeño V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508:28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Konno D, Shioi G, Shitamukai A, et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- 94.Shitamukai A, Konno D, Matsuzaki F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci. 2011;31:3683–3695. doi: 10.1523/JNEUROSCI.4773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Subramanian L, Bershteyn M, Paredes MF, Kriegstein AR. Dynamic behaviour of human neuroepithelial cells in the developing forebrain. Nat Commun. 2017;8:14167. doi: 10.1038/ncomms14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haydar TF, Ang E, Jr, Rakic P. Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc Natl Acad Sci USA. 2003;100:2890–2895. doi: 10.1073/pnas.0437969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yingling J, Youn YH, Darling D, et al. Neuroepithelial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell. 2008;132:474–486. doi: 10.1016/j.cell.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Falk S, Bugeon S, Ninkovic J, et al. Time-specific effects of spindle positioning on embryonic progenitor pool composition and adult neural stem cell seeding. Neuron. 2017;93(777–791):e3. doi: 10.1016/j.neuron.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Itoh Y, Moriyama Y, Hasegawa T, et al. Scratch regulates neuronal migration onset via an epithelial-mesenchymal transition-like mechanism. Nat Neurosci. 2013;16:416–425. doi: 10.1038/nn.3336. [DOI] [PubMed] [Google Scholar]
- 100.Stipursky J, Francis D, Dezonne RS, et al. TGF-β1 promotes cerebral cortex radial glia-astrocyte differentiation in vivo. Front Cell Neurosci. 2014;8:393. doi: 10.3389/fncel.2014.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jossin Y, Lee M, Klezovitch O, et al. Llgl1 connects cell polarity with cell–cell adhesion in embryonic neural stem cells. Dev Cell. 2017 doi: 10.1016/j.devcel.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yokota Y, Eom T-Y, Stanco A, et al. Cdc42 and Gsk3 modulate the dynamics of radial glial growth, inter-radial glial interactions and polarity in the developing cerebral cortex. Development. 2010;137:4101–4110. doi: 10.1242/dev.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miyata T, Ogawa M. Twisting of neocortical progenitor cells underlies a spring-like mechanism for daughter-cell migration. Curr Biol. 2007;17:146–151. doi: 10.1016/j.cub.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 105.Siegenthaler JA, Ashique AM, Zarbalis K, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seuntjens E, Nityanandam A, Miquelajauregui A, et al. Sip1 regulates sequential fate decisions by feedback signaling from postmitotic neurons to progenitors. Nat Neurosci. 2009;12:1373–1380. doi: 10.1038/nn.2409. [DOI] [PubMed] [Google Scholar]
- 107.Griveau A, Borello U, Causeret F, et al. A novel role for Dbx1-derived Cajal-Retzius cells in early regionalization of the cerebral cortical neuroepithelium. PLoS Biol. 2010;8:e1000440. doi: 10.1371/journal.pbio.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/S0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 109.Yokota Y, Kim W-Y, Chen Y, et al. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron. 2009;61:42–56. doi: 10.1016/j.neuron.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsunekawa Y, Britto JM, Takahashi M, et al. Cyclin D2 in the basal process of neural progenitors is linked to non-equivalent cell fates. EMBO J. 2012;31:1879–1892. doi: 10.1038/emboj.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pilaz L-J, Lennox AL, Rouanet JP, Silver DL. Dynamic mRNA transport and local translation in radial glial progenitors of the developing brain. Curr Biol. 2016;26:3383–3392. doi: 10.1016/j.cub.2016.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taverna E, Huttner WB. Neural progenitor nuclei IN motion. Neuron. 2010;67:906–914. doi: 10.1016/j.neuron.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 113.Miyata T, Okamoto M, Shinoda T, Kawaguchi A. Interkinetic nuclear migration generates and opposes ventricular-zone crowding: insight into tissue mechanics. Front Cell Neurosci. 2014;8:473. doi: 10.3389/fncel.2014.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Georges-Labouesse E, Mark M, Messaddeq N, Gansmüller A. Essential role of α6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/S0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 115.Graus-Porta D, Blaess S, Senften M, et al. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/S0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 116.Beggs HE, Schahin-Reed D, Zang K, et al. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/S0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Halfter W, Dong S, Yip Y-P, et al. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Haubst N, Georges-Labouesse E, De Arcangelis A, et al. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development. 2006;133:3245–3254. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- 119.Zarbalis K, Siegenthaler JA, Choe Y, et al. Cortical dysplasia and skull defects in mice with a Foxc1 allele reveal the role of meningeal differentiation in regulating cortical development. Proc Natl Acad Sci USA. 2007;104:14002–14007. doi: 10.1073/pnas.0702618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng C, Heintz N, Hatten ME. CNS gene encoding astrotactin, which supports neuronal migration along glial fibers. Science. 1996;272:417–419. doi: 10.1126/science.272.5260.417. [DOI] [PubMed] [Google Scholar]
- 121.Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- 122.Schmid RS, McGrath B, Berechid BE, et al. Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc Natl Acad Sci. 2003;100:4251–4256. doi: 10.1073/pnas.0630496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Patten BA, Peyrin JM, Weinmaster G, Corfas G. Sequential signaling through Notch1 and erbB receptors mediates radial glia differentiation. J Neurosci. 2003;23:6132–6140. doi: 10.1523/JNEUROSCI.23-14-06132.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anthony TE, Mason HA, Gridley T, et al. Brain lipid-binding protein is a direct target of notch signaling in radial glial cells. Genes Dev. 2005;19:1028–1033. doi: 10.1101/gad.1302105. [DOI] [PMC free article] [PubMed] [Google Scholar]