Abstract
Abstract
As an analgesic and antipyretic drug, acetaminophen (APAP) is commonly used and known to be safe at therapeutic doses. In many countries, the overuse of APAP provokes acute liver injury and even liver failure. APAP-induced liver injury (AILI) is the most used experimental model of drug-induced liver injury (DILI). Here, we have demonstrated elevated levels of growth arrest and DNA damage-inducible 45α (GADD45α) in the livers of patients with DILI/AILI, in APAP-injured mouse livers and in APAP-treated hepatocytes. GADD45α exhibited a protective effect against APAP-induced liver injury and alleviated the accumulation of small lipid droplets in vitro and in vivo. We found that GADD45α promoted the activation of AMP-activated protein kinase α and induced fatty acid beta-oxidation, tricarboxylic acid cycle (TCA) and glycogenolysis-related gene expression after APAP exposure. Liquid chromatography–mass spectrometry (LC–MS) analysis showed that GADD45α increased the levels of TCA cycle metabolites. Co-immunoprecipitation analysis showed that Ppp2cb, a catalytic subunit of protein phosphatase 2A, could interact directly with GADD45α. Our results indicate that hepatocyte GADD45α might represent a therapeutic target to prevent and rescue liver injury caused by APAP.
Graphical abstract
Keywords: Acetaminophen (APAP), Drug-induced liver injury (DILI), GADD45α, AMPK
Introduction
Drug-induced liver injury (DILI) is a significant clinical problem and a challenge to drug development worldwide [1]. Acetaminophen (N-acetyl-p-aminophenol, APAP) is commonly used as an analgesic and antipyretic drug and is known to be safe at therapeutic doses. However, APAP overdose has been one of the most common causes of acute liver injury and even liver failure in many countries [2, 3]. APAP-induced liver injury (AILI) is the most frequent drug hepatotoxicity and the most used experimental model of DILI.
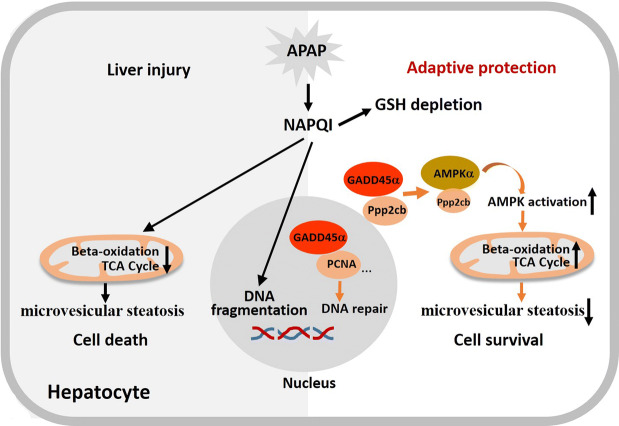
The accumulation of N-acetyl-p-benzoquinone imine (NAPQI), the reactive and toxic metabolite of APAP, is generally considered as the main cause of liver injury induced by an overdose of APAP [4]. The reaction between NAPQI and a protein containing sulfhydryl groups on its cysteine residues can trigger mitochondrial damage, oxidative stress, c-jun N-terminal kinase (JNK) activation, nuclear DNA fragmentation and cell death [5–8]. APAP-induced liver injury also exhibits microvesicular steatosis that is characterized by the accumulation of numerous small lipid droplets in the hepatocyte cytoplasm [9]. Microvesicular steatosis caused by APAP toxicity might be associated with the suppression of PPARα-regulated pathways and the inhibition of fatty acid β-oxidation induced by APAP treatment [10].
The growth arrest and DNA damage-inducible 45 (GADD45) family genes (Gadd45α, Gadd45β, Gadd45γ) are rapidly induced in response to endogenous and exogenous stress stimuli. The three small (18-kDa) proteins participate in genomic stability, cell cycle arrest, cell survival, apoptosis and DNA demethylation and repair [11–13]. One study has demonstrated that both agents that activate nuclear receptors (such as CAR and PXR) and partial hepatectomy (PH) can induce extremely high expression levels of Gadd45 [14]. Based on our own data, Gadd45α and Gadd45β levels rapidly increase in livers and hepatocytes that are subjected to APAP, while Gadd45γ does not increase. Gadd45α is the most elevated member of the Gadd45 family in response to APAP treatment.
Gadd45α is transcriptionally regulated by mitogen-activated kinase (MAPK) signaling, p53, BRCA1, FOXOA3, C/EBP and ATF4 [15–18]. Upon exposure to cellular stress stimuli, Gadd45α carries out its functions via protein–protein interactions. Gadd45α increases DNA repair by interacting and/or affecting various proteins, such as proliferating cell nuclear antigen (PCNA), APE and XPG [19–21]. Gadd45α also induces cell cycle arrest at both the S phase and G2/M phase by displacing PCNA from the cyclin D1 complex, binding to CDK1 and preventing the CDK1 association with cyclin B1 [22–24]. Protein partners also include histones, members of the p38/JNK stress-induced kinase pathway, p21, ER-1α, Bim, Nek2, Aurora-A, members of the β-catenin pathway and members of the mTOR pathway [25–27].
During the development of DILI, many aspects of hepatic metabolism are dysregulated, which influences the progression and prognosis of DILI [28]. AMP-activated protein kinase (AMPK) functions as a sensor of the cellular energy status and a master regulator of metabolism by controlling various metabolic pathways [29]. As a heterotrimeric enzyme, AMPK comprises catalytic α (α1 and α2) subunits, regulatory β (β1 and β2) subunits and γ (γ1, γ2 and γ3) subunits [30, 31]. In response to an energy deficit (low ATP/AMP), AMPK is activated and phosphorylates targets and then restores the energy balance by activating energy-producing pathways, such as glucose uptake, glycogen synthesis, glycolysis, fatty acid beta-oxidation and lipolysis while inhibiting energy-consuming processes, such as the de novo biosynthesis of fatty acids and cholesterol, protein synthesis and gluconeogenesis [32–35]. A recent study reported that salicylate, the base component and the unacetylated form of aspirin, can directly activate AMPK by binding to the β1 subunit [36]. However, until now, the effect of Gadd45α in the process of APAP-induced acute liver injury has not been well understood, and the relationship between Gadd45α and AMPK had not been previously explored.
In the present study, we investigated the role of Gadd45α in APAP overdose-induced acute liver injury in vitro and in vivo. Importantly, we demonstrated that the activation of AMPK was an underlying key mechanism through which Gadd45α ameliorated APAP-induced liver injury.
Materials and methods
Human sample collection
A total of nine DILI cases (four of them are APAP-induced DILI) were enrolled in this study to evaluate the expression of Gadd45α. Three healthy liver tissues (used as the control group) were collected from donors whose livers were subsequently used for liver transplantation. All patients provided written informed consent, and the protocol was approved by the Shanghai Jiao Tong University Ethics Committee.
Animals
C57BL/6J male mice, aged 6–8 weeks and weighing 18–22 g at the time of the experimental procedures, were obtained from the Shanghai Experimental Animal Centre of the Chinese Academy Sciences (Shanghai, China). All mice were maintained under controlled conditions (24 ± 2 °C and 50 ± 5% humidity) with free access to food and water under a 12-h light/dark cycle. All animal experiments were carried out in accordance with the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine and were approved by the National Research Council Guide for the Care and Use of Laboratory Animals.
Animal studies
Fresh acetaminophen (Sigma Aldrich, USA) solution was prepared for each experiment by dissolving acetaminophen in saline, which was warmed up to 55 °C and cooled down to 37 °C before use. Mice were fasted at 6:00 p.m. and then intraperitoneally (i.p.) injected with acetaminophen at a dose of 300 mg/kg (body weight) or with saline at 10:00 a.m. the following day. Mice were then killed after APAP intoxication, and the liver tissues were collected at the indicated time points (n = 4–6 per time point). Some mice were injected with saline as parallel controls at the same time points (n = 4–6 per time point). The livers were frozen at − 80 °C for later analysis.
Knockdown of GADD45α in vivo using siRNA
Cholesterol-conjugated GADD45α siRNA for in vivo RNA interference and its negative control were obtained from RiboBio (Guangzhou, China) [37, 38]. For in vivo transfection, 6- to 8-week-old C57BJ/6J mice were intravenously injected in the tail vein with 20 nmol siRNA of GADD45 α(GCTCGGAGTCAGCGCACCA) or the control siRNA in a total volume of 100 μl. 48 h later, mice were administered APAP (300 mg/kg) and killed after 24 h.
Histological analysis
For histological analyses, the livers were isolated and fixed in 4% paraformaldehyde solution for 24 h, or frozen in OCT embedding medium (Leica, Wetzlar, Germany). Paraffin embedded liver sections 5 μm in size were stained with hematoxylin and eosin (HE). Frozen sections of the liver were stained with Oil Red O. Images were captured directly using Leica DMI3000B Fluorescent microscope (Wetzlar, Germany).
Cell culture
The mouse hepatocyte AML-12 cell line was purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai) and cultured in Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12 (DMEM/F-12, Gibco-BRL, Gaithersburg, MD, USA) with 10% fetal bovine serum, penicillin, streptomycin and dexamethasone in a humidified incubator with 5% CO2 at 37 °C.
Isolation of hepatocytes from mice
Primary hepatocytes were purified from mice using the collagenase IV perfusion method [39, 40]. Briefly, the livers were perfused with 40 ml of EDTA solution (NaCl 137 mM, KCl 5.4 mM, NaH2PO4 0.64 mM, Na2HPO4 0.85 mM, HEPES 1 mM, NaHCO3 4.17 mM, EDTA 0.5 mM and glucose 5 mM, pH 7.4) at a flow rate of 5 ml/min and then further perfused with 25 ml of DMEM containing 1 mg/ml collagenase IV at the same rate. The digested liver sections were filtered through a 70-μm nylon mesh. The hepatocytes were collected after centrifugation at 50×g for 5 min. The cells were washed twice with DMEM and then resuspended in culture medium (William’s medium E, 10% FBS). Approximately, 4 × 105 cells were placed in the wells of six-well cell culture plates and incubated for 4 h with 5% CO2 at 37 °C to permit adhesion. The plates were then washed twice to remove dead or unattached cells and further incubated in William’s medium E with penicillin (100 U/ml) and streptomycin (100 mg/ml).
Infection of the adenovirus, and transfection of plasmid and siRNA in vitro
Mouse GADD45a adenovirus was obtained from Genechem Co., Ltd. (Shanghai, China). AML12 cells and primary mouse hepatocytes were infected with adenoviruses expressing GADD45a or with an adenovirus expressing EGFP used as a control for 48 h, followed by treatment with APAP for the indicated time. AML12 cells and primary mouse hepatocytes were transfected with small interfering RNA (siRNA) targeting murine Gadd45α, AMPKα (both AMPKα1 and AMPKα2) and Ppp2cb or control siRNA, and the overexpression plasmid of Ppp2cb was transfected into AML-12 cells using Lipofectamine 3000 (Life Technologies, Thermo Fisher Scientific) for 48 h, followed by treatment with APAP. The siRNA sequences were designed and synthesized by RiboBio. (Guangzhou, China) The siRNA sequence against Gadd45α was GCTCGGAGTCAGCGCACCA. The siRNA sequence against AMPKα1 (Prkaa1) was GCACACCCTGGATGAATTA. The siRNA sequence against AMPKα2 (Prkaa2) was CCAATTGACAGGCCATAAA. The siRNA sequence against Ppp2cb was GCAGATCACCAAGTGTAT.
Immunohistochemistry and immunofluorescence staining
Paraffin sections were dewaxed and rehydrated. Antigens were retrieved by heat-induced epitope retrieval. Endogenous peroxidase was quenched with 3% H2O2 in methanol. Sections were blocked and then incubated (4 °C) with anti-Gadd45α antibody (Cell Signaling Technology, USA), according to the manufacture’s guidelines. Based on the species origin of the primary antibody, an appropriate HRP-conjugated IgG was selected as the secondary antibody. After incubation with the secondary antibody for 90 min at 37 °C, the slices were washed with PBS three times at 3-min intervals. Finally, staining was visualized with DAB, and sections were counterstained with hematoxylin. Cell slices of AML12 were incubated with rabbit anti-Gadd45α (GENETEX, USA) followed by fluorescence staining with Cyanine Cy™3-conjugated donkey anti-rabbit IgG (H+L) (Jackson Immuno Research Laboratories, Inc., PA, USA). DAPI was applied to visualize the nuclei. Representative images were captured with a fluorescence microscope.
Co-immunoprecipitation and Western blotting
For immunoprecipitation, cells were lysed using NP-40 lysis buffer (Beyotime, China) supplemented with protease inhibitors. Lysates were incubated with appropriate monoclonal antibodies [GADD45α (GENETEX, USA), Ppp2cb (Proteintech, USA) and AMPKα (Cell Signaling Technology, USA)] or control IgG. After an overnight incubation at 4 °C, Protein G Sepharose beads (Bio-Rad) were added into the lysate mixtures, and then the lysates were incubated for 30 min at room temperature. The Sepharose beads were washed three times with 1 ml of ice-cold buffer. The protein precipitates were separated by SDS-PAGE, and Western blot analysis was performed.
Terminal dUTP nick-end labeling (TUNEL) staining
DNA fragmentation was detected with the TUNEL method using an in situ cell detection kit (Roche) for the detection. The staining of cell or tissue sections was performed according to the manufacturer’s instructions. Afterward, cells with positive nuclear staining were visualized, and images were captured with a microscope (Leica DFC310 FX).
Western blot analysis
Total protein was isolated from treated livers and cells using radioimmune precipitation assay (RIPA) lysis buffer. Equal amounts (30–50 μg) of protein samples from liver tissues or cells were loaded onto SDS-PAGE gels and resolved, and then the protein samples were transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membranes were incubated with primary antibodies against GADD45α (GENETEX, USA), P-AMPK, T-AMPK, P-S6, T-S6, P-ACC, T-ACC (Cell Signaling Technology, USA), Ppp2cb (Proteintech, USA) or β-actin (Proteintech, USA). After the membranes were incubated with the corresponding secondary antibodies, namely, horseradish peroxidase (HRP)-conjugated goat anti-mouse or goat anti-rabbit IgG, the signals were detected. The protein bands were visualized by enhanced chemiluminescence (ECL, Amersham Biosciences), and the membranes were then developed and quantified using an imaging system (Tanon, China).
Real-time quantitative PCR
Total RNA was isolated using TRIzol reagent (Life Technologies, Thermo Fisher Scientific), and cDNA synthesis was performed using the Prime Script RT reagent kit (Takara, Shiga, Japan) according to the manufacturer’s instructions. Quantitative PCR was performed with the SYBR Green PCR kit using 36B4 as an internal standard. The primers used for the gene expression studies are listed in Table 1.
Table 1.
Primer sets used for qPCR
| Gene name | Primer (5′–3′) | Accession number |
|---|---|---|
| GADD45α |
Forward: AGAAGACCGAAAGGATGGAC Reverse: CACGGATGAGGGTGAAATG |
NM_007836.1 |
| 36b4 |
Forward: TGAGATTCGGGATATGCTGTTGG Reverse: CGGGTCCTAGACCAGTGTTCT |
NM_001177352.1 |
| PCK1 |
Forward: CGCTGGATGTCGGAAGAG Reverse: CACCACATAGGGCGAGTCT |
NM_011044.2 |
| PCX |
Forward: AGAGGTGGTCCGCAAGAT Reverse: TAATAGGGAAGCCGTAGGTG |
NM_001162946.1 |
| Prkaa1 |
Forward: AGGTGGACATCTGGAGCA Reverse: AAAGGCTGATTACTGAAGGG |
NM_001013367.3 |
| Prkaa2 |
Forward: TCTCAACCGTTCTGTCGC Reverse: AGGGGTCTTCAGGAAATAGG |
NM_178143.2 |
| PYGM |
Forward: CAAATCAGCGTTCGTGGCTTA Reverse: CCACATTGCGATCCTTGACCA |
NM_011224.2 |
| PDK1 |
Forward: GGACTTCGGGTCAGTGAATGC Reverse: TCCTGAGAAGATTGTCGGGGA |
NM_001360002.1 |
| CPT1α |
Forward: GGAGAGAATTTCATCCACTTCCA Reverse: CTTCCCAAAGCGGTGTGAGT |
NM_013495.2 |
| PDHB |
Forward: AGTTGCCCAGTATGACGGTG Reverse: TCTGAGATGGGGGTGTCGAT |
NM_024221.3 |
| PCK2 |
Forward: ATGGCTGCTATGTACCTCCC Reverse: GCGCCACAAAGTCTCGAAC |
NM_028994.3 |
| PDK2 |
Forward: AGGGGCACCCAAGTACATC Reverse: TGCCGGAGGAAAGTGAATGAC |
NM_133667.2 |
| CROT |
Forward: AGAACGGACATTTCAGTACCAGG Reverse: TTCCAGCCAGTTTCGTTTTCC |
NM_023733.3 |
| ACOX |
Forward: TAACTTCCTCACTCGAAGCCA Reverse: AGTTCCATGACCCATCTCTGTC |
NM_001271898.1 |
| ACAA2 |
Forward: CTGCTACGAGGTGTGTTCATC Reverse: AGCTCTGCATGACATTGCCC |
NM_177470.3 |
| PPARα |
Forward: AACATCGAGTGTCGAATATGTGG Reverse: CCGAATAGTTCGCCGAAAGAA |
NM_001113418.1 |
| PKLR |
Forward: GAACATTGCACGACTCAACTTC Reverse: CAGTGCGTATCTCGGGACC |
NM_001099779.1 |
Statistical analysis
Data were expressed as the mean ± standard error of the mean. Two-tailed Student’s t tests were performed for two-sample comparisons. Multiple-group comparisons were examined using one-way ANOVA with Tukey’s post hoc test. P < 0.05 was considered as statistically significant. Calculations and graphs were prepared using GraphPad Prism software version 6.0 (San Diego, CA, USA).
Results
APAP overdose triggers the upregulated expression of GADD45α
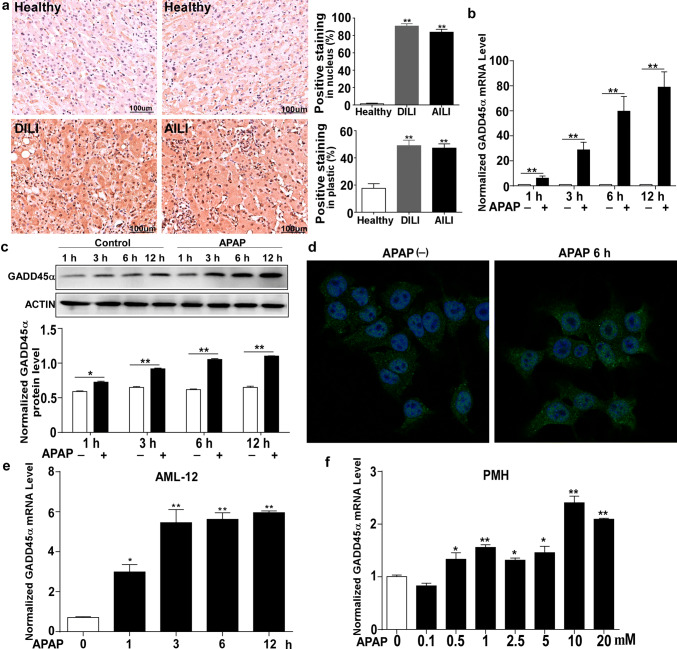
To determine the expression level of GADD45α in DILI, we conducted a series of immunohistological analyses to examine GADD45α expression. As shown in Fig. 1a, our results revealed a dramatic increase in both nuclear and cytoplasmic GADD45α accumulation in the human liver tissues of DILI, and particularly GADD45α also presented an obvious increase in APAP-induced DILI both in the nucleus and cytoplasm. Then, we generated a model of liver injury by injecting C57BL/6J mice with acetaminophen (APAP) at a dose of 300 mg/kg. In line with the observation in Fig. 1a, the GADD45α mRNA and protein expression levels showed dramatic increases in the liver as early as 1 h after the APAP treatment compared with after the saline treatment (Fig. 1b, c). To further examine the levels of GADD45α during the process of APAP-induced liver injury, both AML-12 cells and primary mouse hepatocytes were treated with APAP [APAP was dissolved in DMEM/F12 or DMEM without FBS and filter-sterilized (0.22 μm Millipore filter, Millipore, USA)]. As shown in Fig. 1d, using immunofluorescence, we found that GADD45α was also remarkably increased after APAP administration for 6 h and that GADD45α expression was obvious in both the nucleus and cytoplasm. Moreover, the mRNA expression of GADD45α after APAP exposure was elevated in both the AML-12 cells and primary mouse hepatocytes (Fig. 1e, f). These results indicated that GADD45α significantly increased when the liver was subjected to an APAP overdose.
Fig. 1.
GADD45α is upregulated during the process of APAP-induced liver injury. a GADD45α expression in the liver was determined by immunohistochemistry and quantified. Human samples from healthy control subjects (Healthy) (n = 3) and from patients with drug-induced liver injury (DILI) (n = 9, four of them APAP-induced DILI). b, c qRT-PCR analysis of the mRNA levels and Western blot analysis of the protein levels of GADD45α in livers from C57BL/6J mice after 300 mg/kg APAP treatment (n = 5 per time point). d Representative images of immunofluorescence staining for GADD45α in AML-12 cells. e qRT-PCR analysis of GADD45α in AML-12 cells after APAP treatment at different time points. f qRT-PCR analysis of GADD45α in primary mouse hepatocytes after different doses of APAP for 6 h. The mRNA levels were normalized to 36B4 and subsequently normalized to the control group. Data are expressed as the mean ± standard error of the mean. *P < 0.05, **P < 0.01
GADD45α plays a critical role in preventing APAP-induced hepatotoxicity
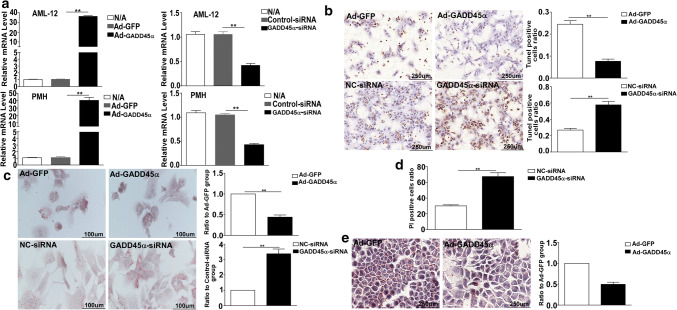
To investigate the role of GADD45α in APAP-induced hepatotoxicity, we used primary mouse hepatocytes and AML-12 cells to examine the direct effect of a challenge with APAP in vitro. We first used Ad-GADD45a infection to induce GADD45α overexpression and siRNA to reduce GADD45α expression. The expression levels of GADD45α are shown in Fig. 1a. DNA fragmentation, which is a specific feature of APAP-induced hepatocyte death [41], was detected by TUNEL staining. As shown in Fig. 2b, the TUNEL staining of hepatocytes confirmed that GADD45α directly protected hepatocytes against APAP-induced DNA fragmentation. The accumulation of small lipid droplets was also decreased in the hepatocytes overexpressing GADD45α, and when GADD45α loss of function occurred, a significant increase in small lipid droplets was evident (Fig. 2c). The PI staining which can verify the cell death when exposed to APAP treatment also demonstrated that a more serious hepatocytes’ death occurred followed by the reduced expression of GADD45α (Fig. 2d). A similar increase in small lipid droplets was observed in GADD45α-overexpressing AML-12 cells (Fig. 2e). Taken together, these data demonstrate that GADD45α plays a significant protective role against APAP-induced hepatotoxicity.
Fig. 2.
GADD45α shows an important capability of protecting against APAP-induced hepatocytes’ death and decreasing the APAP-induced accumulation of small lipid droplets. a The mRNA expression levels of the GADD45α after overexpression and siRNA treatment. b Representative images of TUNEL staining in primary mouse hepatocytes after the overexpression or knockdown of GADD45α at 6 h after APAP treatment and the quantification of the number of TUNEL-positive cells. c Oil Red O staining of primary mouse hepatocytes after the overexpression or knockdown of GADD45α at 6 h after APAP treatment and the quantification of the ratio relative to the control group. d PI staining of AML-12 cells after knockdown of GADD45α at 24 h after APAP treatment. e Oil Red O staining of AML-12 cells from the overexpression of GADD45α at 6 h after APAP treatment and the quantification of the ratio relative to the control group. Data are expressed as the mean ± standard error of the mean. *P < 0.05, **P < 0.01. LPF, low power field
The AMPK pathway is activated after exposure to APAP
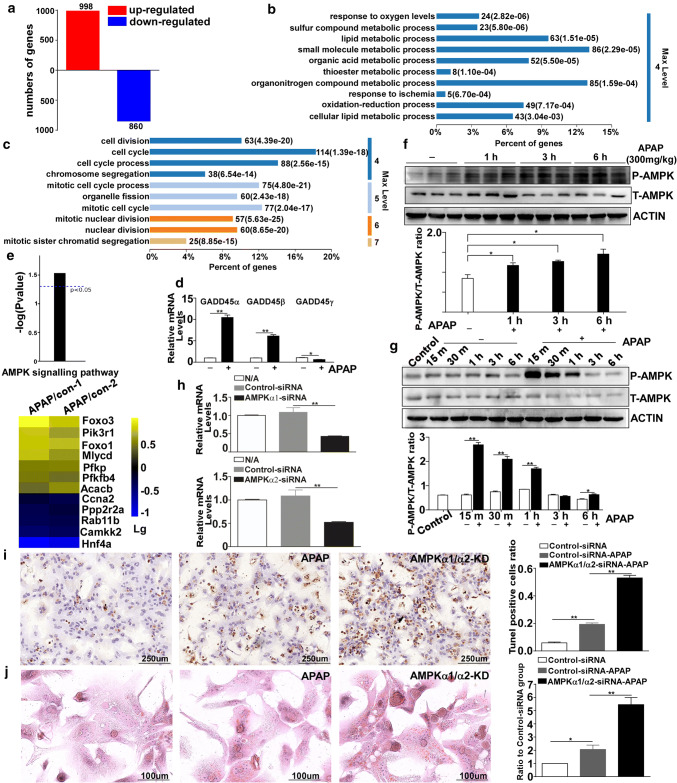
Using cell samples from AML-12 cells treated with APAP, global gene expression was analyzed by RNA-seq and bioinformatics analysis. As shown in Fig. 3a, 998 and 860 genes were upregulated and downregulated, respectively, (over twofold, P < 0.05) in the DILI group compared with the control group. We then conducted gene ontology (GO) analysis using the default parameters of DAVID. We observed that the 998 genes upregulated in the DILI group were mainly enriched in the areas of “response to oxygen levels”, “lipid metabolic process” and “small molecule metabolic process” (Fig. 3b), suggesting that multiple metabolic processes were affected in response to APAP overdose. However, as shown in Fig. 3c, the genes downregulated in the DILI group were all enriched in biological processes, including “cell division”, “cell cycle” and “cell cycle process”. Based on the RNA-seq data, we found that in response to APAP treatment, the expression levels of GADD45 family genes (Gadd45α, Gadd45β and Gadd45γ) were altered; Gadd45α and Gadd45β were increased after APAP treatment compared with the control group, and Gadd45γ was reduced. We also found that GADD45α showed the most significant increase in response to APAP exposure (Fig. 3d). GO analysis suggested that APAP overdose upregulated the transcription of genes involved in metabolic progression. Using KEGG pathway analysis, we further found that the AMPK pathway was activated in APAP-induced hepatotoxicity (Fig. 3e), and the heat map in Fig. 3e represents the log(10) fold change in the expression of the AMPK pathway genes in APAP-induced hepatotoxicity compared with the control group. The protein levels of P-AMPK in APAP-injured mouse livers were analyzed by Western blotting (Fig. 3f). The results showed that P-AMPK levels were enhanced. Furthermore, P-AMPK was also activated as early as 15 min in the AML-12 cells (Fig. 3g). To investigate whether AMPK activation played a critical role during the process of APAP-induced liver injury, we isolated primary mouse hepatocytes, which were transfected with either 100 nM murine AMPKα1 siRNA and AMPKα2 siRNA or control siRNA. As shown in Fig. 3h, the expression levels of AMPKα1 and AMPKα2 were obviously knocked down. TUNEL staining revealed that after APAP exposure, DNA fragmentation was more aggravated when AMPKα was knocked down compared to the control group (Fig. 3i). We further conducted Oil Red O staining to observe the accumulation of small lipid droplets in the hepatocytes, and as shown in Fig. 3j, when we knocked down AMPKα expression, the number of small lipid droplets was increased compared with the control siRNA group after exposure to APAP. Together, these findings suggest that APAP exposure-induced cytotoxicity promotes AMPK activation, which may play a vital role in alleviating APAP-induced acute liver injury.
Fig. 3.
The AMPK pathway is activated after exposure to APAP. a Diagram illustrating the genes that were upregulated and downregulated at 6 h. b, c Significantly enriched biological process category in the gene ontology (GO) analysis concerning the genes that were upregulated or downregulated at 6 h after APAP treatment. d The mRNA expression levels of the GADD45 family members (GADD45α, GADD45β and GADD45γ). e Diagram displaying the activation of the AMPK signaling pathway and a heat map representing the log(10) fold change for the genes relating to the AMPK signaling pathway in the APAP treatment group compared with the control group. f Western blot analysis of P-AMPK and T-AMPK in the livers of C57BL/6J mice after treatment with 300 mg/kg APAP for different times. g The protein levels of P-AMPK and T-AMPK in AML-12 cells after APAP treatment for different times. h The mRNA expression levels of AMPKα1 and AMPKα2 in primary mouse hepatocytes after transfections with siRNA targeting AMPKα, confirming the knocking down of AMPKα. (i) TUNEL staining in primary mouse hepatocytes with AMPKα knockdown at 6 h after APAP treatment and the quantification of the number of TUNEL-positive cells. j Oil Red O staining of primary mouse hepatocytes with AMPKα knockdown at 6 h after APAP treatment and the quantification of the ratio relative to the control group. Data are expressed as the mean ± standard error of the mean. *P < 0.05, **P < 0.01
GADD45α promotes AMPK activation in APAP-induced hepatotoxicity
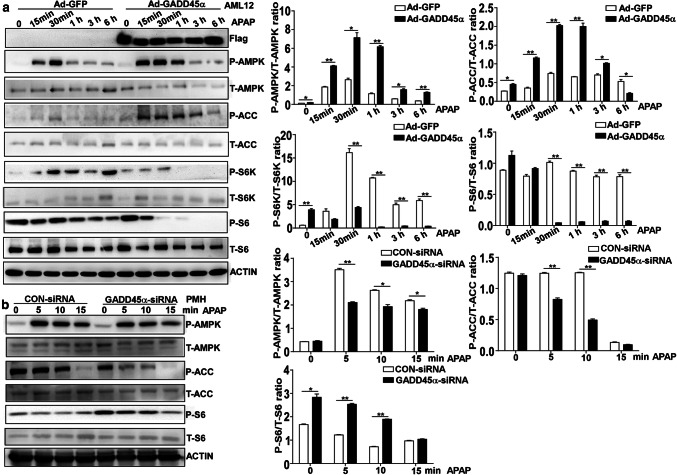
As evident from the above results, GADD45α plays an important protective role against APAP-induced liver injury and can reduce the accumulation of small lipid droplets in the hepatocyte cytoplasm. Furthermore, AMPK activation is increased during APAP-induced liver injury. We next investigated whether GADD45α prevented APAP-induced hepatotoxicity by promoting AMPK activation. We used Ad-GADD45α infection to induce the overexpression of GADD45α in AML-12 cells. Cell lysates were collected at different time points (15 min, 30 min, 1 h, 3 h, 6 h) after APAP treatment, and P-AMPK and P-ACC levels were further measured in AML-12 cells from the Ad-GADD45α and Ad-GFP groups by Western blotting. As shown in Fig. 4a, we found that P-AMPK was increased significantly in the AML-12 cells overexpressing GADD45α as early as 15 min after APAP exposure compared with the control group. Similarly, the levels of P-ACC, a downstream protein of the AMPK pathway, were higher in the GADD45α-overexpressing group than in the control group. Moreover, the levels of P-S6K and P-S6, which are downstream proteins of the AMPK pathway, were consistent with the levels of P-AMPK. To further confirm these findings, we isolated primary mouse hepatocytes from C57BL/6J mice as described in “Materials and methods” and reduced the expression of GADD45α using siRNA. The hepatocytes were exposed to APAP for 5 min, 10 min and 15 min, and the results showed that when GADD45α was knocked down, the protein levels of P-AMPK and P-ACC in the hepatocytes were decreased compared with the levels in the control siRNA group, and P-S6 was dramatically increased in line with the reduced levels of P-AMPK (Fig. 4b). Collectively, these results indicate that GADD45α can promote AMPK activation during the process of APAP-induced hepatotoxicity.
Fig. 4.
GADD45α promotes AMPK activation in APAP-induced hepatocyte injury. a Western blot analysis for P-AMPK, T-AMPK, P-ACC, T-ACC, P-S6K, T-S6K, P-S6 and T-S6 expression in AML-12 cells with overexpression of GADD45α in response to APAP treatment for 0, 0.25, 0.5, 1, 3 and 6 h, followed with quantified protein levels of the above genes. b Western blot analysis for P-AMPK, T-AMPK, P-ACC, T-ACC, P-S6 and T-S6 expression in primary mouse hepatocytes transfected with NC-siRNA or GADD45α-siRNA in response to APAP treatment for 0, 5, 10 and 15 min, followed by quantified protein levels of the above genes. Data are expressed as the mean ± standard error of the mean, *P < 0.05, **P < 0.01
Enhanced expression of GADD45α affects fatty acid beta-oxidation-, tricarboxylic acid cycle- and glycogenolysis-related gene expression after APAP exposure
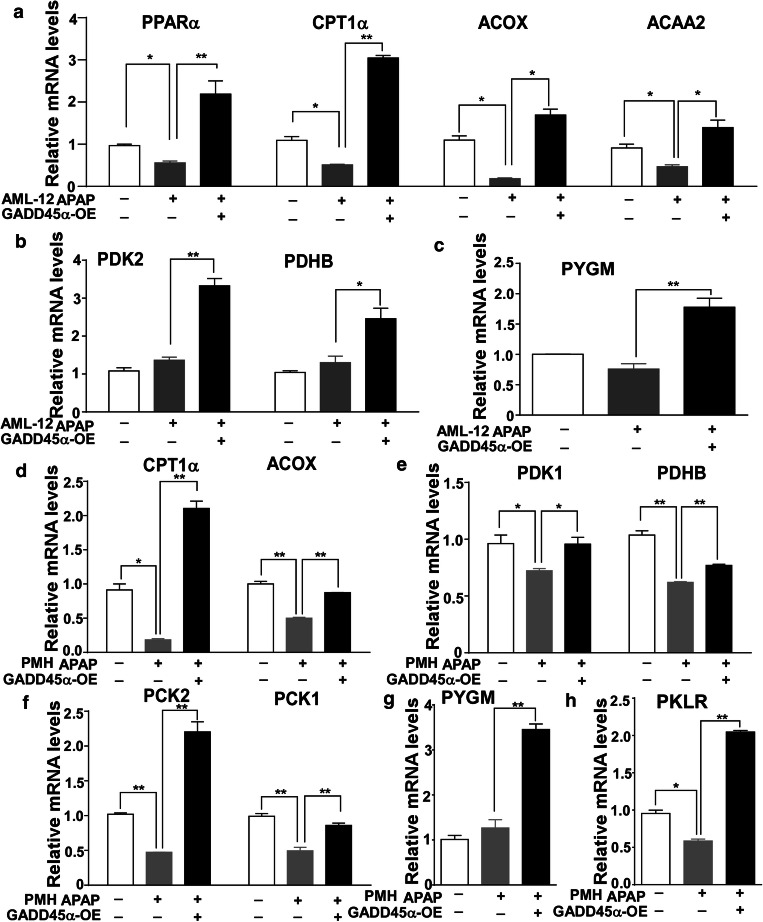
Previous studies have shown that AMPK functions as a sensor of the cellular energy status and as a master regulator of metabolism by controlling various metabolic pathways [29]. We have already verified that GADD45α can promote AMPK activation. To confirm whether GADD45α could affect metabolic events by promoting AMPK activation during the process of APAP-induced liver injury, we measured the mRNA expression levels of genes from various metabolic pathways, including fatty acid beta-oxidation, the tricarboxylic acid (TCA) cycle and glycogenolysis, in AML-12 cells with stably overexpressed GADD45α after APAP treatment. As shown in Fig. 5a, after the exposure to APAP, the cells overexpressing GADD45α showed strikingly enhanced mRNA levels of PPARα compared with the Ad-GFP group. Similar results were observed for the expression of CPT1α, ACOX and ACAA2, which are all involved in fatty acid beta-oxidation. The mRNA levels of PDK2 and PDHB, which are associated with the TCA cycle, and PYGM, which is associated with glycogenolysis, also showed similar patterns (Fig. 5b, c). Moreover, when we measured the mRNA expression levels of the genes involved in the above metabolic pathways in primary mouse hepatocytes after the forced expression of GADD45α, we found that the expression levels of genes involved in fatty acid beta-oxidation were decreased after exposure to APAP, while the forced expression of GADD45α enhanced the fatty acid beta-oxidation pathway (Fig. 5d). The levels of genes associated with TCA cycle and gluconeogenesis were significantly increased after GADD45α overexpression compared to the control group (Fig. 5e, f). The mRNA levels of PYGM and PKLR showed similar dynamics (Fig. 5g, h). Together, these data demonstrate that GADD45α affects the metabolic events associated with fatty acid beta-oxidation, the TCA cycle and glycogenolysis by promoting AMPK activation after APAP exposure.
Fig. 5.
Enhanced expression of GADD45α affects fatty acid beta-oxidation-, tricarboxylic acid cycle- and glycogenolysis-related gene expression after APAP treatment. a–c The mRNA levels of genes involved in fatty acid beta-oxidation, tricarboxylic acid cycle and glycogenolysis in AML-12 cells with the enhanced expression of GADD45α after APAP treatment for 6 h. d–h The mRNA levels of genes involved in fatty acid beta-oxidation, tricarboxylic acid cycle and glycogenolysis in primary mouse hepatocytes with overexpression of GADD45α after APAP treatment for 6 h. The relative mRNA levels were normalized to 36B4 and subsequently normalized to the control group. Data are expressed as the mean ± standard error of the mean, *P < 0.05, **P < 0.01
APAP-induced tricarboxylic acid (TCA) cycle metabolites are enhanced in GADD45α-overexpressing AML-12 cells
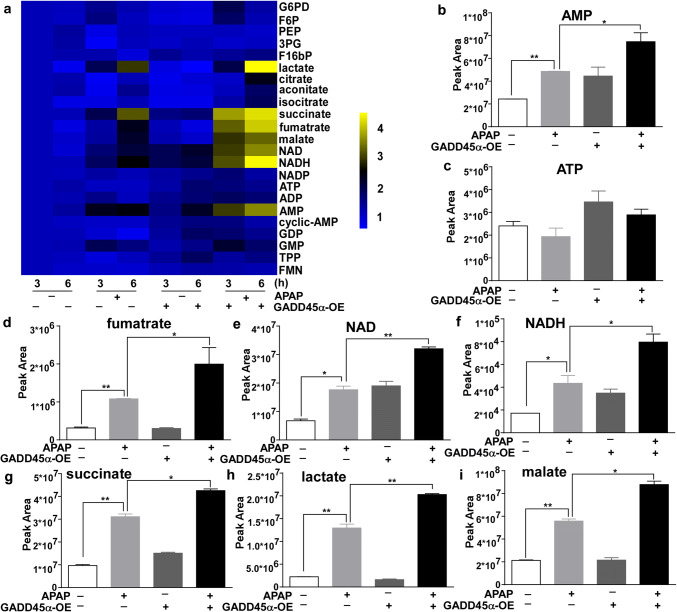
To confirm the contribution of enhanced GADD45α expression to these metabolic pathways during the process of APAP-induced liver injury, we applied liquid chromatography–mass spectrometry (LC–MS) to analyze the levels of several metabolites in APAP-treated AML-12 cell samples. As shown in Fig. 6a, the heat map demonstrated changes in the levels of 23 metabolites after APAP treatment. Moreover, our results showed that the levels of the metabolites involved in the TCA cycle (such as NAD, NADH, succinate and fumarate), lactate and AMP were significantly increased after APAP treatment, with more marked increases in the cells with the forced expression of GADD45α compared to the control group (Fig. 6b–i). Together, these data demonstrate that the overexpression of GADD45α can increase the levels of TCA cycle metabolites after APAP treatment.
Fig. 6.
Overexpressed GADD45α can increase the levels of tricarboxylic acid (TCA) cycle metabolites after APAP treatment. a The heat map shows the changes in the levels of 23 metabolites after APAP treatment. b–i The relative peak areas of the metabolites involved in the TCA cycle. Data are expressed as the mean ± standard error of the mean. *P < 0.05, **P < 0.01
Ppp2cb interacts with GADD45α in hepatocytes
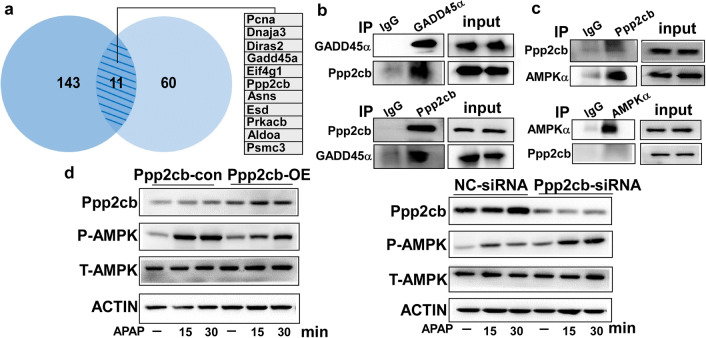
To investigate the underlying molecular mechanism through which GADD45α promoted AMPK activation, we collected the cell lysates of AML-12 cells with forced expression of GADD45α after APAP treatment and then conducted co-immunoprecipitation followed by mass spectrometry analysis. As shown in Fig. 7a, a total of 11 proteins (Pcna, Dnaja3, Diras2, GADD45α, Eif4g1, Ppp2cb, Asns, Esd, Prkacb, Aldoa and Psmc3) were identified in two independent experiments. Protein phosphatase 2A (PP2A), which comprises two regulatory subunits, namely, A and B, and one C catalytic subunit, including Ppp2ca and Ppp2cb, is an important serine/threonine phosphatase [42]. Thus, we further conducted a series of co-immunoprecipitations to determine whether GADD45α interacted directly with Ppp2cb. As shown in Fig. 7b, GADD45α interacted directly with Ppp2cb. We further confirmed the direct interaction between Ppp2cb and AMPKα by co-immunoprecipitation (Fig. 7c). Then, we overexpressed and knocked down the expression of Ppp2cb in AML-12 cells. As shown in Fig. 7d, the overexpression of Ppp2cb could suppress APAP-induced AMPK activation; however, when we knocked down the expression of Ppp2cb, APAP-induced AMPK activation was obviously enhanced in hepatocytes. Taken together, these results indicated that GADD45α could interact with Ppp2cb, which might influence AMPK activation induced by APAP.
Fig. 7.
Ppp2cb interacts with GADD45α in hepatocytes. a The diagram represents a total of 11 proteins that were found in two independent co-immunoprecipitation experiments followed by mass spectrometry analysis. b GADD45α (Ppp2cb) was immunoprecipitated from AML-12 cell lysates with enhanced GADD45α expression. Then, the precipitate was evaluated for the presence of Ppp2cb (GADD45α) by Western blot. c AML-12 cells were treated with APAP for 6 h. Then, Ppp2cb (AMPKα) was immunoprecipitated with anti-Ppp2cb (anti-AMPKα), and the presence of AMPKα (Ppp2cb) in the precipitate was evaluated with an anti-AMPKα (anti-Ppp2cb) antibody. d The protein levels of Ppp2cb, P-AMPK and T-AMPK in AML-12 cells were analyzed by Western blot
Knockdown of GADD45α aggravated APAP-induced liver injury in vivo
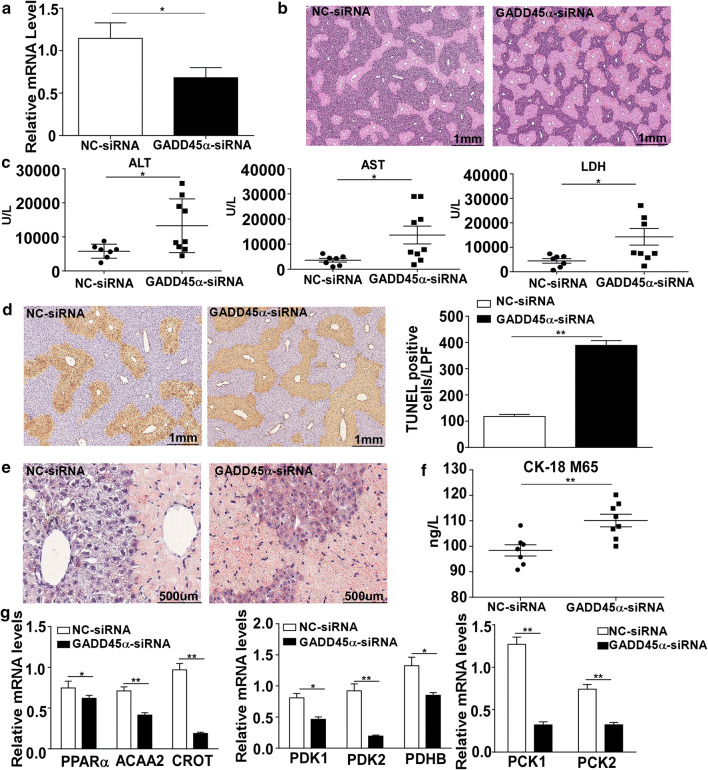
Since GADD45α performs the hepatoprotective effect in AML-12 cells and primary mouse hepatocytes, we next further confirmed the protective effect of GADD45α on APAP-induced liver injury in vivo. As shown in Fig. 8a, we successfully reduced GADD45α expression in the mice liver. As shown in Fig. 8b, in response to APAP treatment, liver histological observation demonstrated that there was more severe liver damage in the GADD45α-siRNA treated mice, accompanied by a dramatic elevation of serum levels of ALT, AST and LDH (Fig. 8c) compared to the control group. The TUNEL staining showed that DNA fragmentation in the mice liver was obviously aggravated by GADD45α knocking down (Fig. 8d). Moreover, the Oil Red O staining also showed that small lipid droplets were markedly increased after GADD45α-siRNA treatment compared to the control group (Fig. 8e). As CK18-M65 is considered a sensitive marker of cell necrosis, we determined the serum levels of CK-18 M65 by ELISA assays. As shown in Fig. 8f, upon stimulation with APAP, the level of CK18-M65 in the GADD45α-siRNA group was increased obviously compared with the control group. To further verify whether GADD45α can affect the metabolite pathway via promoting AMPK activation during the process of APAP-induced liver injury in mice, we further detected the mRNA levels of genes participating in a number of metabolic pathways, including fatty acid beta-oxidation, tricarboxylic acid cycle and gluconeogenesis. As shown in Fig. 8g, the expression levels of genes involved in fatty acid beta-oxidation, tricarboxylic acid cycle and gluconeogenesis were decreased when the expression of GADD45α was reduced.
Fig. 8.
Knockdown of GADD45α aggravated APAP-induced liver injury in vivo. a The mRNA expression levels of the GADD45α after GADD45α-siRNA transfection in mice. b Liver histological examination with H&E; typical images were chosen from each experimental group. c Serum was harvested at 24 h after APAP injection for the measurement of ALT, AST and LDH in the mice of GADD45α-siRNA and control groups. d Representative images of TUNEL staining after the knockdown of GADD45α in mice at 24 h after APAP treatment and the quantification of the number of TUNEL-positive cells. e Oil Red O staining after the knockdown of GADD45α in mice at 24 h after APAP treatment. f Serum levels of CK18 M65, in the GADD45α-siRNA or control groups. g The mRNA levels of genes involved in fatty acid beta-oxidation, tricarboxylic acid cycle and glycogenolysis in the mice with knockdown of GADD45α after APAP treatment for 24 h. The relative mRNA levels were normalized to 36B4 and subsequently normalized to the control group. Data are expressed as the mean ± standard error of the mean, *P < 0.05, **P < 0.01
Discussion
Acetaminophen overdose-induced liver injury is the most frequent cause of acute liver failure in many developed countries and threatens human health and drug development [43]. The identification of the molecular mechanisms of APAP-induced liver injury will provide promising therapeutic avenues for the treatment of APAP overdose [44, 45]. In the current study, we have demonstrated, for the first time, that hepatocyte GADD45α is crucial for protecting against APAP-induced hepatotoxicity and functions by promoting AMPK activation.
Gadd45 proteins, a family of p53-regulated and DNA damage-inducible proteins, have been shown to regulate cell growth and apoptosis [12, 46]. Additionally, several studies have demonstrated an essential role for GADD45α in various kinds of cancer, with GADD45α having anti-tumor properties [47, 48]. Recently, GADD45α was shown to play an important role in liver disease. A recent study reported that GADD45α could counteract hepatic fibrosis by regulating the activation of HSCs via the inhibition of the TGF-β/Smad signaling pathway [49]. Another recent study revealed a protective role for GADD45α in non-alcoholic steatohepatitis, a common liver disease worldwide [50]. A recent report demonstrated that GADD45α, as a downstream gene regulated by Egr1, played an important role in preventing APAP-induced liver injury [51]. In line with these findings, our own study demonstrated that GADD45α had an important role in alleviating cell necrosis and accumulation of lipid droplets in APAP-induced hepatotoxicity in vitro. Importantly, when we reduced the expression of GADD45α in mice liver using siRNA injection through the tail vein, we also observed enhanced liver injury and accumulation of lipid droplets after APAP treatment.
GADD45α functions as a stress sensor in response to various physiological or environmental stressors, including hypoxia, ionizing radiation, UV radiation, growth factor withdrawal and medium depletion, finally resulting in cell cycle arrest, DNA repair, genomic stability and apoptosis [11, 13, 27]. However, studies related to the underlying relationship between GADD45α and metabolic mechanisms in disease are few. Until now, no reports have shown that GADD45α participates in some metabolic processes to protect against various physiological or environmental stressors. In our study, we have provided new insights into how GADD45α exhibits a protective effect against APAP-induced liver injury. Our data have demonstrated that the activation of AMPK is promoted by GADD45α in hepatocytes, following with the mRNA levels of genes associated with a number of metabolic pathways presented the consistent change in vivo and vitro. Hence, our current data indicate for the first time that GADD45α is not only a stress sensor, but also a hepatoprotectant protein in the context of APAP-induced liver injury by promoting AMPK activation.
AMPK is a key regulator of energy homeostasis [52, 53]. Abundant studies have confirmed that AMPK not only regulates energy homeostasis, but also carries out cytoprotective functions in hepatocytes by inhibiting apoptosis, protecting against mitochondrial injury and activating autophagy [54–56]. A recent study has shown that AMPK plays a critical role in APAP cytotoxicity by regulating autophagy [57]. Another study revealed that the pharmacological activation of AMPK can obviously ameliorate APAP-induced liver injury [58]. A large number of studies have indicated that AMPK is responsible for regulating hepatic lipid metabolic pathways, including lipid biogenesis and uptake, fatty acid β-oxidation and the TCA cycle [59, 60]. Furthermore, the enhancement of hepatic lipid metabolism involving fatty acid β-oxidation and the TCA cycle via the activation of AMPK have been shown to play an important role in alleviating liver injury. A recent study has demonstrated that demethyleneberberine (DMB), a natural product that exists in Chinese herbs, can serve as an AMPK activator to regulate hepatic lipid metabolism by reducing lipid synthesis and inducing fatty acid β-oxidation, with the subsequent attenuation of non-alcoholic fatty liver disease [61]. Another report has confirmed that CYP2J2 overexpression obviously decreases lipid accumulation and increases fatty acid oxidation mostly by activating the AMPK pathway, with a subsequent amelioration of HFD-induced hyperlipidemia [62]. Similarly, in our present study, qRT-PCR and LC–MS analysis revealed that the significantly increased fatty acid β-oxidation and TCA cycle components corresponded to the activation of the AMPK pathway promoted by the overexpression of GADD45α.
PP2A is one of four major serine/threonine phosphatases. PP2A consists of a common heteromeric core enzyme, which comprises two regulatory subunits, namely, A and B, and one C catalytic subunit, including Ppp2ca and Ppp2cb [42]. Several studies have shown that a group of protein phosphatases, which includes PP2A, PP1 and PP2C, can dephosphorylate AMPK in either cell culture or in vitro kinase dephosphorylation assays [63–66]. Moreover, a previous report has shown that Ppp2ca, one of the catalytic subunits of PP2C, can interact directly with AMPKα in co-immunoprecipitation assays, leading to the dephosphorylation of AMPK. This study also showed that after the expression levels of Ppp2ca or Ppp2cb were reduced, the Thr-172 residue of AMPK showed a significant increase in phosphorylation; moreover, an elevation in Acc1 phosphorylation was evident [67]. These findings suggest that Ppp2cb plays a vital role in regulating the dephosphorylation of AMPK. In our current study, we found that overexpression of Ppp2cb could suppress APAP-induced AMPK activation, but knockdown of Ppp2cb could enhance APAP-induced AMPK activation in hepatocytes. GADD45α could interact with Ppp2cb, which might influence AMPK activation induced by APAP. However, how GADD45α-Ppp2cb interaction affects AMPK activation might need further investigation in future.
In conclusion, our present study demonstrates for the first time that GADD45α has a protective effect against APAP-induced liver injury. The protective effects of GADD45α on the APAP overdose-induced liver injury can be attributed to its activation of AMPKα. Our encouraging results indicate that hepatocyte GADD45α may be a potential therapeutic target for APAP-induced liver injury and may be helpful in the development of a potential hepatoprotective agent for remedying APAP-induced liver injury.
Acknowledgements
This work was supported by the Major Project of National Twelfth Five Plan (2012ZX09303-001), the Major Project of National Thirteenth Five Plan (2017ZX09304016), the National Natural Science Foundation of China (NSFC 81670524, NSFC 31771308), the Shanghai Municipal Natural Science Foundation (17ZR1401800) and the Clinical Research Center at Shanghai Jiao Tong University School of Medicine. The funders had no role in the study design, data collection and analysis, decision to publish or manuscript preparation.
Footnotes
Chunmin Li and Yanan Ming contributed equally.
Contributor Information
Xiaobo Li, Email: xbli@fudan.edu.cn.
Yimin Mao, Email: maoym11968@163.com.
References
- 1.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144(7):1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Lin CC, Tsai P, Sun HY, Hsu MC, Lee JC, Wu IC, Tsao CW, Chang TT, Young KC. Apolipoprotein J, a glucose-upregulated molecular chaperone, stabilizes core and NS5A to promote infectious hepatitis C virus virion production. J Hepatol. 2014;61(5):984–993. doi: 10.1016/j.jhep.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 3.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376(9736):190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity—a clinically relevant model to test the efficacy of natural products. Life Sci. 2011;88(17–18):737–745. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42(1):110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Ye D, Wang Y, Li H, Jia W, Man K, Lo CM, Wang Y, Lam KS, Xu A. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1alpha-mediated antioxidant capacity in mice. Hepatology. 2014;60(3):977–989. doi: 10.1002/hep.27060. [DOI] [PubMed] [Google Scholar]
- 7.Yuan L, Kaplowitz N. Mechanisms of drug-induced liver injury. Clin Liver Dis. 2013;17(4):507–518. doi: 10.1016/j.cld.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begriche K, Massart J, Robin MA, Borgne-Sanchez A, Fromenty B. Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J Hepatol. 2011;54(4):773–794. doi: 10.1016/j.jhep.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Ouyang J, Thung SN. Histopathologic manifestations of drug-induced hepatotoxicity. Clin Liver Dis. 2013;17(4):547–564. doi: 10.1016/j.cld.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Krausz KW, Shah YM, Idle JR, Gonzalez FJ. Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2009;22(4):699–707. doi: 10.1021/tx800464q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvador JM, Brown-Clay JD, Fornace AJ., Jr Gadd45 in stress signaling, cell cycle control, and apoptosis. Adv Exp Med Biol. 2013;793:1–19. doi: 10.1007/978-1-4614-8289-5_1. [DOI] [PubMed] [Google Scholar]
- 12.Schafer A. Gadd45 proteins: key players of repair-mediated DNA demethylation. Adv Exp Med Biol. 2013;793:35–50. doi: 10.1007/978-1-4614-8289-5_3. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Yang Z, Liu Y. GADD45 proteins: roles in cellular senescence and tumor development. Exp Biol Med. 2014;239(7):773–778. doi: 10.1177/1535370214531879. [DOI] [PubMed] [Google Scholar]
- 14.Tian J, Locker J. Gadd45 in the liver: signal transduction and transcriptional mechanisms. Adv Exp Med Biol. 2013;793:69–80. doi: 10.1007/978-1-4614-8289-5_5. [DOI] [PubMed] [Google Scholar]
- 15.Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 16.Oh-Hashi K, Maruyama W, Isobe K. Peroxynitrite induces GADD34, 45, and 153 VIA p38 MAPK in human neuroblastoma SH-SY5Y cells. Free Radic Biol Med. 2001;30(2):213–221. doi: 10.1016/S0891-5849(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 17.Harkin DP, Bean JM, Miklos D, Song YH, Truong VB, Englert C, Christians FC, Ellisen LW, Maheswaran S, Oliner JD, Haber DA. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97(5):575–586. doi: 10.1016/S0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- 18.Gomis RR, Alarcon C, He W, Wang Q, Seoane J, Lash A, Massague J. A FoxO–Smad synexpression group in human keratinocytes. Proc Natl Acad Sci USA. 2006;103(34):12747–12752. doi: 10.1073/pnas.0605333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith ML, Chen IT, Zhan Q, Bae I, Chen CY, Gilmer TM, Kastan MB, O’Connor PM, Fornace AJ., Jr Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994;266(5189):1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 20.Vairapandi M, Azam N, Balliet AG, Hoffman B, Liebermann DA. Characterization of MyD118, Gadd45, and proliferating cell nuclear antigen (PCNA) interacting domains. PCNA impedes MyD118 AND Gadd45-mediated negative growth control. J Biol Chem. 2000;275(22):16810–16819. doi: 10.1074/jbc.275.22.16810. [DOI] [PubMed] [Google Scholar]
- 21.Barreto G, Schafer A, Marhold J, Stach D, Swaminathan SK, Handa V, Doderlein G, Maltry N, Wu W, Lyko F, Niehrs C. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445(7128):671–675. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- 22.Wang XW, Zhan Q, Coursen JD, Khan MA, Kontny HU, Yu L, Hollander MC, O’Connor PM, Fornace AJ, Jr, Harris CC. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci USA. 1999;96(7):3706–3711. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan Q, Antinore MJ, Wang XW, Carrier F, Smith ML, Harris CC, Fornace AJ., Jr Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene. 1999;18(18):2892–2900. doi: 10.1038/sj.onc.1202667. [DOI] [PubMed] [Google Scholar]
- 24.Maeda T, Hanna AN, Sim AB, Chua PP, Chong MT, Tron VA. GADD45 regulates G2/M arrest, DNA repair, and cell death in keratinocytes following ultraviolet exposure. J Investig Dermatol. 2002;119(1):22–26. doi: 10.1046/j.1523-1747.2002.01781.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhu N, Shao Y, Xu L, Yu L, Sun L. Gadd45-alpha and Gadd45-gamma utilize p38 and JNK signaling pathways to induce cell cycle G2/M arrest in Hep-G2 hepatoma cells. Mol Biol Rep. 2009;36(8):2075–2085. doi: 10.1007/s11033-008-9419-9. [DOI] [PubMed] [Google Scholar]
- 26.Tong T, Ji J, Jin S, Li X, Fan W, Song Y, Wang M, Liu Z, Wu M, Zhan Q. Gadd45a expression induces Bim dissociation from the cytoskeleton and translocation to mitochondria. Mol Cell Biol. 2005;25(11):4488–4500. doi: 10.1128/MCB.25.11.4488-4500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hildesheim J, Bulavin DV, Anver MR, Alvord WG, Hollander MC, Vardanian L, Fornace AJ., Jr Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Can Res. 2002;62(24):7305–7315. [PubMed] [Google Scholar]
- 28.Pessayre D, Fromenty B, Berson A, Robin MA, Letteron P, Moreau R, Mansouri A. Central role of mitochondria in drug-induced liver injury. Drug Metab Rev. 2012;44(1):34–87. doi: 10.3109/03602532.2011.604086. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89(3):1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 30.Stephenne X, Foretz M, Taleux N, van der Zon GC, Sokal E, Hue L, Viollet B, Guigas B. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia. 2011;54(12):3101–3110. doi: 10.1007/s00125-011-2311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzamko N, van Denderen BJ, Hevener AL, Jorgensen SB, Honeyman J, Galic S, Chen ZP, Watt MJ, Campbell DJ, Steinberg GR, Kemp BE. AMPK beta1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem. 2010;285(1):115–122. doi: 10.1074/jbc.M109.056762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Hardie DG. AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu Rev Nutr. 2014;34:31–55. doi: 10.1146/annurev-nutr-071812-161148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990;9(8):2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao B, Sanders MJ, Carmena D, Bright NJ, Haire LF, Underwood E, Patel BR, Heath RB, Walker PA, Hallen S, Giordanetto F, Martin SR, Carling D, Gamblin SJ. Structural basis of AMPK regulation by small molecule activators. Nat Commun. 2013;4:3017. doi: 10.1038/ncomms4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, Kemp BE, Sakamoto K, Steinberg GR, Hardie DG. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336(6083):918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou J, Zhou Y, Zheng Y, Fan J, Zhou W, Ng IO, Sun H, Qin L, Qiu S, Lee JM, Lo CM, Man K, Yang Y, Yang Y, Yang Y, Zhang Q, Zhu X, Li N, Wang Z, Ding G, Zhuang SM, Zheng L, Luo X, Xie Y, Liang A, Wang Z, Zhang M, Xia Q, Liang T, Yu Y, Cao X. Hepatic RIG-I predicts survival and interferon-alpha therapeutic response in hepatocellular carcinoma. Cancer Cell. 2014;25(1):49–63. doi: 10.1016/j.ccr.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Han Q, Zhang C, Zhang J, Tian Z. Reversal of hepatitis B virus-induced immune tolerance by an immunostimulatory 3p-HBx-siRNAs in a retinoic acid inducible gene I-dependent manner. Hepatology. 2011;54(4):1179–1189. doi: 10.1002/hep.24505. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Yang X, Chen L, Zhi X, Lu H, Ning Y, Yeong J, Chen S, Yin L, Wang X, Li X. Upregulation of hydroxysteroid sulfotransferase 2B1b promotes hepatic oval cell proliferation by modulating oxysterol-induced LXR activation in a mouse model of liver injury. Arch Toxicol. 2017;91(1):271–287. doi: 10.1007/s00204-016-1693-z. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Hylemon P, Pandak WM, Ren S. Enzyme activity assay for cholesterol 27-hydroxylase in mitochondria. J Lipid Res. 2006;47(7):1507–1512. doi: 10.1194/jlr.M600117-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Investig. 2012;122(4):1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen PT, Brewis ND, Hughes V, Mann DJ. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 1990;268(2):355–359. doi: 10.1016/0014-5793(90)81285-V. [DOI] [PubMed] [Google Scholar]
- 43.de Achaval S, Suarez-Almazor M. Acetaminophen overdose: a little recognized public health threat. Pharmacoepidemiol Drug Saf. 2011;20(8):827–829. doi: 10.1002/pds.2162. [DOI] [PubMed] [Google Scholar]
- 44.Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int Off J Int Assoc Study Liver. 2012;32(1):8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SP, Apte U. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol. 2014;184(11):3013–3025. doi: 10.1016/j.ajpath.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin S, Antinore MJ, Lung FD, Dong X, Zhao H, Fan F, Colchagie AB, Blanck P, Roller PP, Fornace AJ, Jr, Zhan Q. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J Biol Chem. 2000;275(22):16602–16608. doi: 10.1074/jbc.M000284200. [DOI] [PubMed] [Google Scholar]
- 47.Su LY, Xin HY, Liu YL, Zhang JL, Xin HW, Su XL. Anticancer bioactive peptide (ACBP) inhibits gastric cancer cells by upregulating growth arrest and DNA damage-inducible gene 45A (GADD45A) Tumour Biol J Int Soc Oncodev Biol Med. 2014;35(10):10051–10056. doi: 10.1007/s13277-014-2272-7. [DOI] [PubMed] [Google Scholar]
- 48.Maekawa T, Sano Y, Shinagawa T, Rahman Z, Sakuma T, Nomura S, Licht JD, Ishii S. ATF-2 controls transcription of Maspin and GADD45 alpha genes independently from p53 to suppress mammary tumors. Oncogene. 2008;27(8):1045–1054. doi: 10.1038/sj.onc.1210727. [DOI] [PubMed] [Google Scholar]
- 49.Hong L, Sun QF, Xu TY, Wu YH, Zhang H, Fu RQ, Cai FJ, Zhou QQ, Zhou K, Du QW, Zhang D, Xu S, Ding JG. New role and molecular mechanism of Gadd45a in hepatic fi brosis. World J Gastroenterol. 2016;22(9):2779–2788. doi: 10.3748/wjg.v22.i9.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka N, Takahashi S, Hu X, Lu Y, Fujimori N, Golla S, Fang ZZ, Aoyama T, Krausz KW, Gonzalez FJ. Growth arrest and DNA damage-inducible 45alpha protects against nonalcoholic steatohepatitis induced by methionine- and choline-deficient diet. Biochem Biophys Acta 1863. 2017;12:3170–3182. doi: 10.1016/j.bbadis.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pang C, Shi L, Sheng Y, Zheng Z, Wei H, Wang Z, Ji L. Caffeic acid attenuated acetaminophen-induced hepatotoxicity by inhibiting ERK1/2-mediated early growth response-1 transcriptional activation. Chem Biol Interact. 2016;260:186–195. doi: 10.1016/j.cbi.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100(3):328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 53.Yang YM, Han CY, Kim YJ, Kim SG. AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. World J Gastroenterol. 2010;16(30):3731–3742. doi: 10.3748/wjg.v16.i30.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468(7324):653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi SH, Kim YW, Kim SG. AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem Pharmacol. 2010;79(9):1352–1362. doi: 10.1016/j.bcp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 56.Hoyer-Hansen M, Jaattela M. AMP-activated protein kinase: a universal regulator of autophagy? Autophagy. 2007;3(4):381–383. doi: 10.4161/auto.4240. [DOI] [PubMed] [Google Scholar]
- 57.Saberi B, Ybanez MD, Johnson HS, Gaarde WA, Han D, Kaplowitz N. Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and -independent signaling pathways. Hepatology. 2014;59(4):1543–1554. doi: 10.1002/hep.26625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang JH, Kim YH, Noh JR, Choi DH, Kim KS, Lee CH. Enhanced production of adenosine triphosphate by pharmacological activation of adenosine monophosphate-activated Protein kinase ameliorates acetaminophen-induced liver injury. Mol Cells. 2015;38(10):843–850. doi: 10.14348/molcells.2015.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes. 2008;32(Suppl 4):S7–12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- 60.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Qiang X, Xu L, Zhang M, Zhang P, Wang Y, Wang Y, Zhao Z, Chen H, Liu X, Zhang Y. Demethyleneberberine attenuates non-alcoholic fatty liver disease with activation of AMPK and inhibition of oxidative stress. Biochem Biophys Res Commun. 2016;472(4):603–609. doi: 10.1016/j.bbrc.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 62.Zhang S, Chen G, Li N, Dai M, Chen C, Wang P, Tang H, Hoopes SL, Zeldin DC, Wang DW, Xu X. CYP2J2 overexpression ameliorates hyperlipidemia via increased fatty acid oxidation mediated by the AMPK pathway. Obesity. 2015;23(7):1401–1413. doi: 10.1002/oby.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamura S, Tsuiki S. Purification and subunit structure of rat-liver phosphoprotein phosphatase, whose molecular weight is 260000 by gel filtration (phosphatase IB) Eur J Biochem. 1980;111(1):217–224. doi: 10.1111/j.1432-1033.1980.tb06096.x. [DOI] [PubMed] [Google Scholar]
- 64.Tamura S, Kikuchi H, Kikuchi K, Hiraga A, Tsuiki S. Purification and subunit structure of a high-molecular-weight phosphoprotein phosphatase (phosphatase II) from rat liver. Eur J Biochem. 1980;104(2):347–355. doi: 10.1111/j.1432-1033.1980.tb04435.x. [DOI] [PubMed] [Google Scholar]
- 65.Moore F, Weekes J, Hardie DG. Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur J Biochem. 1991;199(3):691–697. doi: 10.1111/j.1432-1033.1991.tb16172.x. [DOI] [PubMed] [Google Scholar]
- 66.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403(1):139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joseph BK, Liu HY, Francisco J, Pandya D, Donigan M, Gallo-Ebert C, Giordano C, Bata A, Nickels JT., Jr Inhibition of AMP Kinase by the Protein Phosphatase 2A Heterotrimer, PP2APpp2r2d. J Biol Chem. 2015;290(17):10588–10598. doi: 10.1074/jbc.M114.626259. [DOI] [PMC free article] [PubMed] [Google Scholar]