Abstract
Protein misfolding and aggregation into fibrillar deposits is a common feature of a large group of degenerative diseases affecting the central nervous system or peripheral organs, termed protein misfolding disorders (PMDs). Despite their established toxic nature, clinical trials aiming to reduce misfolded aggregates have been unsuccessful in treating or curing PMDs. An interesting possibility for disease intervention is the regular intake of natural food or herbal extracts, which contain active molecules that inhibit aggregation or induce the disassembly of misfolded aggregates. Among natural compounds, phenolic molecules are of particular interest, since most have dual activity as amyloid aggregation inhibitors and antioxidants. In this article, we review many phenolic natural compounds which have been reported in diverse model systems to have the potential to delay or prevent the development of various PMDs, including Alzheimer’s and Parkinson’s diseases, prion diseases, amyotrophic lateral sclerosis, systemic amyloidosis, and type 2 diabetes. The lower toxicity of natural compounds compared to synthetic chemical molecules suggest that they could serve as a good starting point to discover protein misfolding inhibitors that might be useful for the treatment of various incurable diseases.
Keywords: Misfolded proteins, Aggregates, Amyloid inhibitors, Natural compounds, Polyphenols, Flavonoids, Amyloid beta, Tau, Alpha-synuclein, Amylin, Protein misfolding disorders, Alzheimer’s disease, Parkinson’s disease, Type 2 diabetes, Prion diseases
Introduction
Protein misfolding disorders (PMDs) or proteinopathies are a group of diseases characterized by alternative protein folding that leads to the formation, deposition and accumulation of toxic misfolded aggregates [1, 2]. Each type of protein aggregate is generally specific for one disease, whether occurring in the central nervous system (CNS) or the periphery. Amyloid beta (Aβ) and tau aggregates are the hallmarks of Alzheimer’s disease (AD) [3], whereas alpha-synuclein (α-syn) is the main protagonist in Parkinson’s disease (PD) [4]. All prion diseases or transmissible spongiform encephalopathies (TSE) are known to present aggregates of the misfolded prion protein (PrPSC) [5]. Likewise, amyotrophic lateral sclerosis (ALS) cases display aggregates of superoxide dismutase 1 (SOD1) and TAR DNA binding protein (TDP-43) [6]. Meanwhile, Huntington’s disease (HD) is characterized by the accumulation of the protein huntingtin (htt) that becomes prone to aggregate due to expansions of glutamine-rich regions (polyQ), accumulating in neuronal inclusion bodies [7]. Among the peripheral diseases, AA amyloidosis is characterized by abnormal deposition of the insoluble serum amyloid A protein (SAA) in the liver, spleen and kidney [8]. In type 2 diabetes (T2D), the islet amyloid polypeptide (IAPP or amylin) is deposited in the pancreatic beta cells of affected individuals [9]. For its part, transthyretin amyloidosis is a slowly progressive condition characterized by the accumulation of misfolded transthyretin (TTR) in the peripheral nervous system, resulting in a loss of sensation in the extremities or peripheral neuropathy [10]. Despite inducing different diseases, the progression of protein aggregation follows the same seeding–nucleation kinetic model from the formation of a soluble intermediate species to the generation of protofibrils and finally the accumulation of insoluble fibrillar aggregates [1, 11]. Regardless of their composition, protein aggregates display several common physicochemical features, including a β-sheet-rich structure, insolubility in strong solvents and detergents, protease resistance, and the ability to bind amyloid-specific dyes (i.e., Congo red and thioflavin) [12]. Accumulation of these amyloidogenic proteins causes cellular dysfunction and tissue damage leading to clinical onset in patients through the induction of inflammation, oxidative stress, and cell death [1]. Tissue damage progresses over many years before the appearance of clinical symptoms, often leading to irreversible organ dysfunction. Therefore, intervention maybe more successful through inhibiting aggregation pre-clinically by stimulating degradation, disrupting preformed aggregates, reducing cellular damage, or inducing the formation of non-toxic species. For this reason, interest in non-toxic natural compounds, derived from herbs and food, is rapidly growing. For instance, turmeric, grapes, parsley, spinach, and rosemary are sources for curcumin, resveratrol, apigenin, tannic acid, and rosmarinic acid, respectively, all molecules that, as described below, have been shown to reduce misfolded aggregates. The advantageous properties of these non-pharmacological molecules include world-wide availability, low cost, and low toxicity (especially at low concentrations). Their mechanisms of action are variable and, at least in some cases, seem to involve direct interaction with the aggregates which suggest they could be highly specific for this target [13, 14]. These inhibitors can interfere in amyloid aggregation by blocking the primary nucleation, inhibiting the formation of fibrillar aggregates, modifying the conformation of the cytotoxic species formed into non-toxic aggregates, or even disassembling preformed aggregates. In addition, some of them have also an effect on the disease pathology, such as reduction of oxidative stress, amelioration of the inflammatory process, or decrease in the levels of the misfolded protein by direct interaction with the disease-related enzymes. In this review, we will discuss the effect of some natural phenolic compounds on various misfolded aggregated proteins and their potential as a therapeutic agent to treat PMDs.
Role of misfolded proteins in disease and their mechanism of formation and toxicity
Misfolded aggregates are likely the main cause of the pathological events in PMDs [1, 2, 15, 16]. The conformational changes that a misfolded protein undergoes can result in a loss of its physiological function, thus causing a malfunction of the whole organ. Moreover, the resulting conformation can be toxic and initiate a cascade of events that can activate an inflammatory process or even conclude with the death of the cell. Furthermore, amyloid aggregates can impair and ultimately block the protein degradation machinery, resulting in the collapse of the protein quality control, compromising cellular function and survival. It is reported that aggregated β-sheet-rich proteins inhibit the proteasome system by jamming the entry site of the catalytic core [17–19]. Additionally, autophagy system is impaired in different models of PMDs as well as in inherited forms on neurodegenerative diseases, where accumulation of autophagic vacuoles has been observed [20–22]. All these processes can induce perturbations in the permeability of cellular membranes compromising its integrity, impair mitochondrial function, generate reactive oxygen species, induce inflammation, and disrupt protein homeostasis. Indirectly, aggregated misfolded proteins also expose “sticky” hydrophobic surfaces resulting in abnormal interactions with other proteins, which causes the sequestration of many normal functional proteins and their loss of function [1, 12, 23]. Together, all of these events contribute to the onset of the pathological alterations and clinical signs characteristic of PMDs [1].
The formation and accumulation of amyloidogenic misfolded aggregates are thought to follow a seeding–nucleation model [24, 25]. The limiting step in this process is the formation of small and soluble misfolded proteins acting as nuclei or seeds to propagate misfolding by recruiting more of the native proteins into the growing polymers. In this model, misfolded seeds are produced during the nucleation or lag phase, which can nucleate the aggregation of the same protein (homologous seeding) or another amyloidogenic protein (heterologous seeding or cross-seeding) [11, 26, 27]. The nucleation phase is rate limiting and thermodynamically unfavorable because correctly folded proteins are undergoing conformational change to unstable misfolded intermediates. The primary reason that this conformational transition occurs is because of the stabilization provided by oligomerization. In fact, the introduction of a preformed seed can accelerate the reaction by attenuating the nucleation phase. Upon reaching a conformation able to resist clearance, the misfolded oligomers enter the polymerization or exponential phase, where native proteins are rapidly recruited and misfolded. As a result, these protein aggregates develop into large, insoluble and proteolytically-resistant aggregates, thus resilient to cellular clearance [2, 16, 24].
To avoid the development of pathogenesis due to the accumulation of toxic misfolded aggregates, the use of amyloid aggregation inhibitors can be an effective therapeutic strategy for the prevention or treatment of PMDs. This can be achieved by reducing the toxicity of the aggregates, avoiding the formation of new aggregates, inducing the clearance of these species or even rescuing their original function. Amyloid inhibitors have been described to interact with amyloidogenic aggregates at different stages, by inhibiting, blocking, or impairing self-assembly or amyloid aggregation, or even clearing already formed aggregates [28–31] (Fig. 1). Therapeutically useful compounds can basically act at five different levels: (1) eliminating or reducing the production of the protein component of misfolded aggregates. These type of molecules may diminish or eliminate the synthesis of the protein or a post-translational modification (e.g., proteolysis, phosphorylation) required to render the protein susceptible to misfold. (2) Targeting directly the process of protein misfolding and aggregation. These compounds may block the early conformational changes stabilizing the functional monomeric protein, prevent the initial nucleation into oligomeric seeds, block the elongation of aggregates into large amyloid fibrils, and also the spreading of the seeds from cell-to-cell or tissue-to-tissue. (3) Inducing the elimination of misfolded aggregates. This class of compounds may act by disassembling preformed aggregates or enhancing the natural biological clearance mechanisms. (4) Targeting the abnormal consequences of protein aggregates. These molecules could attenuate the toxicity of the misfolded aggregates, convert the toxic structures into harmless species or attack the secondary cellular signaling pathways triggered by misfolded proteins (e.g., inflammation). (5) Affecting the intracellular trafficking or localization of aggregates. Some aggregates can be produced in different cellular compartments and specific small organic compounds are able to stabilize the location of the misfolded proteins, changing the deposition pattern [32–34]. Some of the natural phenolic compounds we review here can inhibit amyloid misfolding and aggregation by more than one of these mechanisms as described next (Table 1).
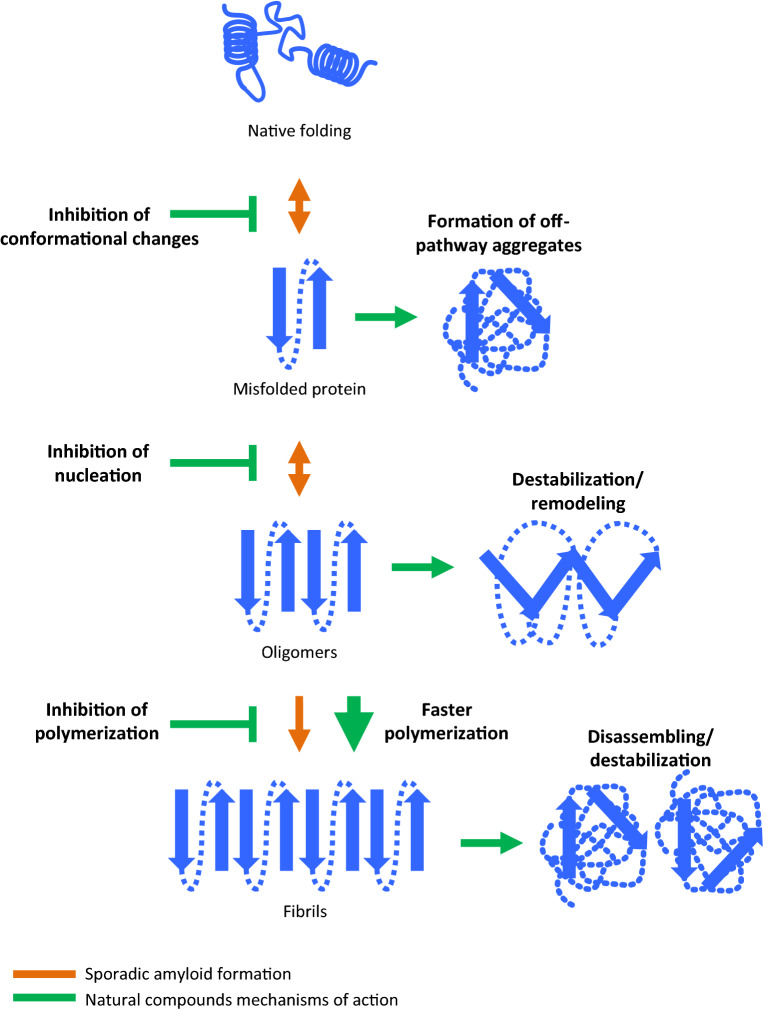
Fig. 1.
Stages of interaction of different amyloid inhibitors. The native conformation of a protein is in balance with many other possible conformations. Among them, partially folded forms can lead to alternative structures associated with PMDs. Formation of misfolded proteins is an unfavorable event and the intermediate species are not as stable as the native folded counterparts (double-headed orange arrows). Although cells have different mechanisms to get rid of the misfolded aggregates, when the first seeds are generated, the kinetic of the polymerization is faster, generating small oligomers, protofibers, and fibers (orange arrow). Inhibitors of amyloid misfolding and aggregation can interact at different stages of the polymerization process to block the formation of toxic species (green lines). They can act by inhibiting conformational changes from the native to the misfolded conformation; blocking or impairing self-assembly and production of oligomeric species; inhibiting the polymerization of small–intermediate into larger species; inducing the formation of off-pathway aggregates; and destabilizing, disassembling or remodeling already formed aggregates. In addition, some of them can trigger a faster polymerization of the misfolded conformation into larger fibrils, circumventing the formation of more toxic soluble oligomeric assemblies
Table 1.
Mechanisms of action of phenolic compounds on amyloid aggregate inhibition
| Compound | Amyloid target | Mechanism of action | Therapeutic effectiveness |
|---|---|---|---|
| Apigenin | Aβ, α-syn, TTR, insulin | Prevention of conformational changes | Reduces Aβ burden in mice |
| Brazilin | Aβ, PAP | Inhibition of fibrillogenesis | Not tested |
| Curcumin | Aβ, α-syn, PrP, TTR |
Prevention of conformational changes Inhibition of fibrillogenesis Disaggregation of preformed aggregates |
Reduces plaque burden in AD mice Reduces of p-α-syn in PD mice Reduction IAPP aggregation in mice No effect in PrPSc infected hamsters Variety of outcomes in clinical trials for AD and T2D |
| Epigallocatechin-3-gallate | Aβ, tau, α-syn, IAPP, TTR, Htt |
Prevention of conformational changes Inhibition of seeding Disaggregation of preformed aggregates |
Reduces TTR and IAPP deposition in mice Amelioration of symptoms in AL patients that drink green tea |
| Fisetin | Aβ, Htt | Inhibition of fibrillogenesis |
Prevents memory impairment in mice Suppresses HD pathology in flies |
| Gallic acid, gallotannins | Aβ, α-syn, insulin, IAPP |
Formation of off-pathway aggregates Inhibition of fibrillogenesis Disaggregation of preformed aggregates |
PGG reduces the burden of Aβ deposition |
| Isoquinolines | Aβ |
Inhibition of fibrillogenesis Disaggregation of preformed aggregates |
Not tested |
| Kaempferol | Aβ, α-syn | Prevention of conformational changes | Inhibition of α-syn aggregation in mice. |
| Morin | Aβ, IAPP, insulin |
Prevention of conformational changes Formation of off-pathway aggregates Disaggregation of preformed aggregates |
Reduced Aβ load in mice. |
| Myricetin | Aβ, α-syn, IAPP |
Prevention of conformational changes Inhibition of fibrillogenesis |
Not tested |
| Oleocanthal | Aβ, tau |
Prevention of conformational changes Inhibition of fibrillogenesis |
Reduces Aβ burden in mice |
| Oleuropein | Aβ, tau |
Prevention of conformational changes Inhibition of fibrillogenesis |
Not tested |
| Oleuropein aglycon | Aβ, tau, α-syn, IAPP, TTR |
Formation of off-pathway aggregates Inhibition of fibrillogenesis Disaggregation of preformed aggregates |
Reduces Aβ deposition in AD mice. |
| Orcein, O4 | Aβ, α-syn, IAPP |
Inhibition of seeding Formation of off-pathway aggregates Induction of faster polymerization |
Not tested |
| Quercitin | Aβ, tau, α-syn |
Prevention of conformational changes Inhibition of fibrillogenesis |
Reduces NFTs and levels of Aβ in mice |
| Resveratrol | Aβ, IAPP, Htt, TTR |
Formation of off-pathway aggregates Inhibition of fibrillogenesis Disaggregation of preformed aggregates |
Reduces Aβ burden in mice Decreases IAPP formation in mice Ameliorates memory impairment in MCI patients |
| Rosmarinic acid | Aβ, α-syn, HEWL, IAPP, insulin |
Prevention of conformational changes Inhibition of fibrillogenesis Disaggregation of preformed aggregates |
Reduces Aβ oligomerization and plaque burden in mice |
| Tannic acid | Aβ, α-syn, PrP | Inhibition of fibrillogenesis Disaggregation of preformed aggregates | Negative results in CWD-infected mule deer |
| Tanshinones | Aβ, α-syn |
Prevention of conformational changes Inhibition of fibrillogenesis Disaggregation of preformed aggregates |
Reduction of associated pathology in mice |
This table summarizes the phenolic compounds reviewed, the amyloidogenic proteins that have been reported to have an effect on, the mechanisms of action by which the inhibitor is able to block the misfolding and aggregation of the amyloid, and available data on effectiveness of the compound in vivo. In most of the cases, one single inhibitor has more than one target and can block the aggregation by more than one mechanism determined by in vitro assays
α-syn alpha-synuclein, Aβ amyloid beta, AL amyloid light chain amyloidosis, CWD chronic wasting disease, HEWL hen egg white lysozyme, Htt huntingtin, IAPP islet amyloid polypeptide, PAP prostatic acidic phosphatase, PrP prion protein, TTR transthyretin
Natural phenolic compounds as amyloid inhibitors
Due to the recognized importance of misfolded aggregates in the pathology and the incurable nature of most PMDs, many different substances have been tested as amyloid inhibitors with very variable results. They include peptides, antibodies, antibiotics, nanoparticles, small synthetic molecules and natural compounds. Although numerous compounds have been shown to be active in the test tube, their effectiveness in vivo, particularly in the real disease in humans, is limited [35–38]. Some of the molecules tested have serious side effects, including toxicity and induction of inflammation [39]. Some others are difficult to synthetize, have a very short half-life, or are not even able to cross the blood–brain barrier (BBB), indispensable in the case of brain disorders. During the last decade, much investigation has focused on small natural molecules, rich in aromatic groups (like polyphenols). The attractiveness of these compounds resides on the fact that they are normal biological molecules found in food or herbal extracts, and thus usually exhibit high availability, stability, low side effects, and convenience. Phenolic compounds can be found in fruits, vegetables and other types of food such as wine, tea, and spices. More than 8000 phenolic compounds have been identified ranging from simple phenolic acids to high-polymerized structures [40, 41]. Polyphenols are secondary metabolites synthesized by plants, fungi, and microorganisms involved in defense responses to different stress triggers such as pathogens and UV radiation. They contribute to the organoleptic quality of foods derived from plants including color, astringency, aroma, and bitterness. Their qualitative and quantitative distribution in the plant varies according to species, organs, tissues, and the stage of development. Polyphenols are characterized by the presence of phenolic groups in their structure, i.e., presence of one or more hydroxyl groups on a benzene ring. They are categorized according to the chemical structures of the aglycones in flavonoids and non-flavonoids. Flavonoid group is the largest one with more than 5.000 compounds that contain two phenolic rings in their backbone. They are classified as flavonols, flavones, isoflavones, flavanones, flavanols, and anthocyanidins with regard to the hydroxylation pattern in the chromane ring [40, 41]. The majority of phenolic compounds have a high antioxidant potential which allows them to inhibit the oxidation of hydrophobic residues in the peptide sequence. In general, flavonoids have limited absorption and a low bioavailability in the brain [42, 43]; however, they seem to be involved in neurogenesis and neuronal regeneration, therefore, considered neuroprotective and able to potentiate cognitive function, probably also due to their antioxidant potential [44, 45]. In addition to flavonoids, there are some non-flavonoid polyphenols that have been attributed with anti-amyloidogenic effect and most of them are also considered potent antioxidant compounds. In the past, polyphenols were not considered to have any substantial nutritional value; however, in recent decades, great interest has been raised due to their potential to prevent many diseases such as atherosclerosis, cancer, T2D, cardiovascular and neurodegenerative diseases.
Flavonoids
Apigenin
Apigenin (Fig. 2a) is a natural non-mutagenic flavone with low intrinsic toxicity, which is found in parsley, celery, chamomile tea, onions, artichoke, and orange peels. Apigenin can inhibit a broad spectrum of amyloidogenic misfolded proteins of the CNS (Aβ, α-syn) and the periphery (TTR, insulin), by extending the lag phase and reducing amyloid formation [46–50]. This function has been primarily attributed to its ability to stabilize unfolded structures via hydrophobic interactions and hydrogen bonding, resulting in an increase in the lag time of the fibrillation process and a reduction in the amount of fibrillar structures produced [46–50]. However, it seems not to have the ability to destabilize preformed amyloid fibrils nor inhibit the oligomerization process once it has started or it does it very weakly, as in the case of α-syn oligomers [48, 50, 51]. Administration of apigenin effectively reduced the Aβ burden and improved learning and memory deficits in the APP/PS1 transgenic mice model for AD [52]. Although a promising candidate, we are not aware of the existence of any clinical trial with apigenin in PMDs.
Fig. 2.
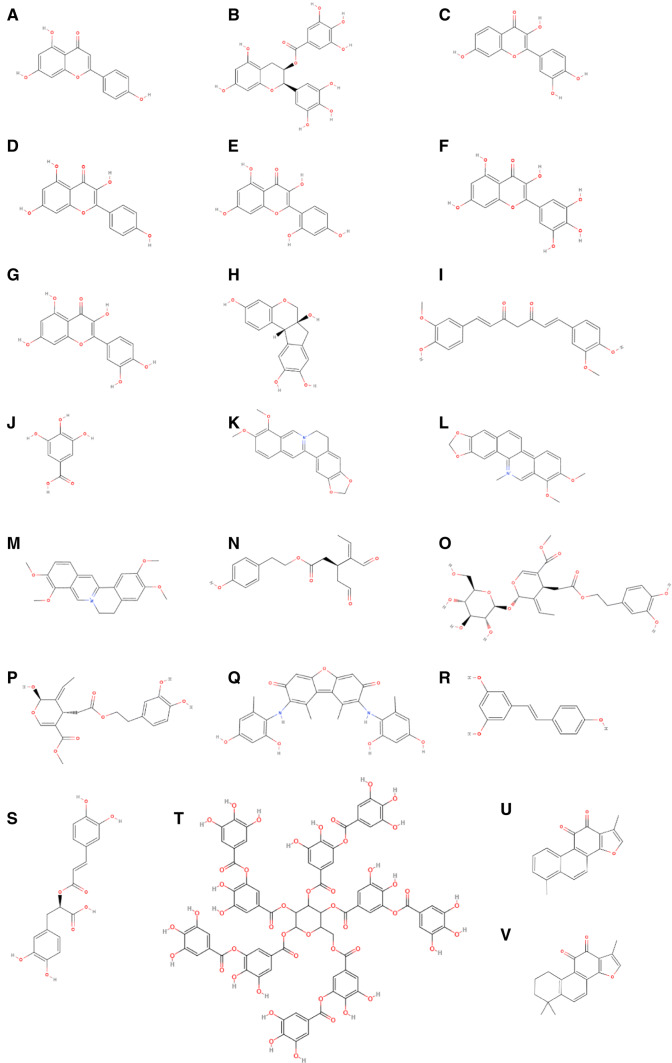
Chemical structure of reviewed phenolic compounds able to inhibit amyloid misfolding and aggregation. a Apigenin, b epigallocatechin-3-gallate, c fisetin, d kaempferol, e morin, f myricetin, d quercetin, h brazilin, i curcumin, j gallic acid, k berberine, l chelerythrine, m palmatine, n oleocanthal, o oleuropein, p oleuropein aglycone, q orcein, r resveratrol, s rosmarinic acid, t tannic acid, u tanshinone, i, v tanshinone IIA
Epigallocatechin-3-gallate
Epigallocatechin-3-gallate (EGCG) (Fig. 2b), one of the main active components of green tea leaves [53], is a flavanol that has attracted a lot of interest due to its antioxidant, antitumor, antibacterial and neuroprotective activity both in vitro and in vivo [54, 55]. EGCG has been reported to have promising effects on the reduction of aggregation and toxicity of a large number of proteins that are involved in PMDs; however, the mechanism of action remains unclear and seems to be through the modulation of multiple pathways. Recent reports suggest that this flavanol may directly interact, in a non-sequence-specific manner, with misfolded proteins during very early stages of the aggregation cascade [56]. A proposed mechanism is through the modulation of toxic oligomer formation by binding and stabilizing unfolded species of α-syn and Aβ, reducing fibrillation and redirecting the aggregation pathway to form off-pathway, amorphous non-toxic aggregates, blocking seeding and further conformational changes that may result in aggregation and cytotoxicity [57]. EGCG seems to interact in a different way with tau, probably by binding to a partially misfolded intermediate, preventing seeding and rescuing cells from tau-induced toxicity [58]. EGCG also binds monomers, intermediates, and mature fibers of IAPP by hydrogen-bond interactions, inhibiting amyloid formation [59]. EGCG can modulate TTR aggregation in vitro, maintaining the protein in its soluble form and disassembling preformed fibrils into amorphous aggregates [60, 61]. Similarly, EGCG suppresses the formation of oligomeric htt in a dose-dependent manner by inducing the formation of larger forms [62]. In vivo, subchronic administration of EGCG in the drinking water to transgenic mouse models overexpressing human TTR demonstrated that EGCG diminishes TTR aggregation and deposition in several mouse tissues [63], whereas in hIAPP mice decreases the amount of IAPP fibrils in the pancreas although the clinical signs were not altered [64]. In humans, patients with amyloid light chain (AL) amyloidosis—a bone marrow disorder where misfolded immunoglobulins deposit in different organs, including the heart—show an amelioration of their cardiac condition after regular consumption of green tea compared with patients that do not drink it [65], suggesting a beneficial effect of this EGCG-rich beverage. The multiple mechanisms of action discovered for EGCG to inhibit amyloid aggregation and the apparent effect of EGCG administration in AD patients position this flavonoid as a one of the candidates to be used in the early stage of the diseases or individuals at high risk.
Fisetin
Fisetin (Fig. 2c) is a flavone that can be found in many fruits and vegetables, such as strawberries, apples, grapes, onions and cucumbers. Fisetin, as other flavonoids such as quercetin, sturdily inhibits aggregation of Aβ in vitro by inhibiting its fibrillogenesis [66]. Although the flavonol C-3 hydroxyl group seems to be responsible for a high inhibitory activity in fibril formation, the lack of a hydroxyl group at C-5 in fisetin compared to quercetin at C-5 has a significant effect on its inhibitory activity [66]. Moreover, the 3′,4′-dihydroxyl group is indispensable for the anti-fibrillogenic activity of fisetin [67]. Intraperitoneal treatment with fisetin in Aβ-injected animals reduces Aβ accumulation, tau phosphorylation, and associated brain pathology including synaptic impairment, inflammation, and cell death [68], whereas in Drosophila models of HD the administration of fisetin is able to suppress HD-related pathology [69]. Oral administration of fisetin to APP/PS1 AD mice prevents the development of learning and memory deficits [70]. Currently, there is a clinical trial recruiting patients with mild cognitive impairment (MCI) to test the effect of fisetin in cognitive function (http://www.clinicaltrials.gov).
Kaempferol
Kaempferol (Fig. 2d) is a flavonol phytoestrogen present in apples, grapes, Brussels sprouts, tea, broccoli, grapefruit, and tomatoes. Regarding its activity in protein aggregation inhibition, it has been reported that kaempferol provides protection against cytotoxicity induced by Aβ42 in a concentration-dependent manner. The apparent mechanism of anti-fibrillogenic activity is by preventing conformational changes through interaction with hydrophobic regions of the proteins [71, 72]. In mice, kaempferol reduces Aβ-induced neurotoxicity and memory impaired performance [73, 74], as well as in AD flies [75]. Similar compounds sharing kaempferol backbone, such as YCF2, can also block Aβ42 polymerization and generate non-toxic species [76]. With a proposed similar mechanism, kaempferol 3-O-rutinoside, one of the main components of safflower flavonoid extract, has been confirmed to inhibit α-syn aggregation in PD mice [77]. Whether the effect of kaempferol is due to direct aggregation inhibition or just reducing associated neurotoxicity remains to be determined.
Morin
Morin (Fig. 2e) is a flavonol present in wine and several fruits and vegetables, including guava, mock orange, and dyer’s mulberry. In vitro studies have shown that morin exerts neuroprotective effects by inhibition of Aβ aggregation through a mechanism that involves destabilization of fibrils and production of off-pathway intermediates [78]. Morin is also effective in the inhibition of the formation of IAPP aggregates, as well as the disaggregation of preformed fibrils, expanding its potential for the treatment of other non-neurological PMDs [79]. Even more, morin binds to monomeric, oligomeric and fibrillary insulin, preventing conformational changes and avoiding the release of oligomeric species [80]. The treatment of APP/PS1 AD transgenic mice with morin reduces the levels of soluble and insoluble Aβ production, the formation of amyloid plaques, and rescues the cognitive impairment observed in these animals. Furthermore, morin efficiently blocks tau phosphorylation, making it an interesting therapeutic agent for the treatment of AD and other tauopathies [81]. In summary, morin is able to block fibril formation and promote their disassembling. Due to its ability to inhibit IAPP and insulin aggregation, this flavonol should be considered as a potential treatment for T2D.
Myricetin
Myricetin (Fig. 2f) is a natural flavonol present in vegetables, fruits, and green tea. Several studies have reported the pharmacological potential of myricetin as an inhibitor of amyloid formation. It has been suggested that this flavonol has the ability of binding to hydrophobic moieties of amyloid oligomers preventing their aggregation [82]. In vitro, it blocks tau aggregation by interfering with the elongation phase of fibril assembly [83] and inhibits the oligomerization of Aβ42 [51]. It also inhibits the formation of oligomers and fibrils of α-syn by preventing its conformational change into β-sheet and the disruption of the fibrils, in a dose-dependent manner [50, 84]. Myricetin destabilizes preformed α-syn and blocks fibrillization in a dose-dependent manner [85]. IAPP is another amyloidogenic protein that has been tested using myricetin as an amyloid inhibitor. The report shows that it is able to reduce IAPP fiber formation, therefore, decreasing its cytotoxicity in vitro [86]. The inhibitory activity of myricetin has been also tested against the aggregation of hen egg white lysozyme, a non-disease-related amyloidogenic protein. In this case, the polyphenol produces a reduction in the population of partially unfolded intermediates, decreasing amyloid formation [87]. To our knowledge, no in vivo studies have been performed using myricetin to treat PMDs.
Quercetin
Quercetin (Fig. 2g) is a flavonol widely distributed in fruits, vegetables, especially abundant in beans and capers, and red wine. In vitro, quercetin has been proved to be an effective inhibitor against several aggregation-prone proteins, such as Aβ, α-syn, and tau [88–90]. The proposed mechanisms of inhibition imply the direct interaction with the misfolded proteins causing the stabilization of oligomeric species and inhibition of fibril growth. Quercetin has been also shown to covalently bind α-syn, increasing its hydrophilicity and, therefore, inhibiting the aggregation [89]. Additionally, several lines of evidence suggest that quercetin exerts cellular protective effects against amyloids both in vitro and in vivo, potentiating the importance of this compound as a hit for drug development. For instance, quercetin was found to be a protective molecule in cells exposed to Aβ [91]. In a triple-transgenic mouse model of AD, the administration of quercetin induced the reduction of NFTs and levels of Aβ, as well as an amelioration in neuroinflammatory process and learning and memory impairment [92]. Quercetin3-O-glucuronide, a glycosylated derivative form of quercetin, is a potent inhibitor of Aβ aggregation [93]. In summary, quercetin represents a great candidate to treat neurological disorders associated with protein misfolding and aggregation, especially due to its ability to cross the BBB [94]. However, no clinical trials have tested its efficacy in humans with any PMD.
Non-flavonoids
Brazilin
Brazilin (Fig. 2h) is a polyphenolic compound present in the legume Caesalpinia sappan. A recent study using virtual screening, biophysical, biochemical, and cellular models has shown that brazilin is a strong inhibitor of Aβ42 fibrillogenesis, disfavoring the formation of on-pathway fibrils. It also has the ability to remodel mature fibrils and protect cultured cells from Aβ42-induced cytotoxicity [95]. The suggested mechanism is by direct hydrophobic interactions and hydrogen bonding between brazilin and fibrillar structures. Based on pharmacokinetic studies in vivo, brazilin could be considered a good therapeutic candidate due to its high stability, long half-life after oral ingestion and intravenous inoculation, and its ability to reach the brain [96–98]. In comparison to other polyphenols, brazilin has superior potency against Aβ aggregation with an IC50 of 1.5 µM, respectively, compared to 2.4 and 13.3 µM for EGCG and curcumin. Moreover, brazilin can inhibit fibrillization of other disease-related amyloids such as prostatic acidic phosphatase (PAP) polymerization [99]. Although brazilin is more potent inhibiting fibrillization than other natural compounds, it has not been tested in vivo yet, so its efficacy and ability to cross the BBB is unknown.
Curcumin
Curcumin (Fig. 2i) is a non-flavonoid biphenolic compound found in Curcuma longa (the Indian turmeric or curry spice) well known for its anti-inflammatory and antioxidant properties [100, 101]. Curcumin is a general inhibitor of amyloid aggregation because of its beneficial activity against various PMDs, including AD, PD, T2D and prion diseases [100, 102, 103], and it has been taken to clinical trial for AD and T2D already. In the case of AD, it has been reported that curcumin can directly bind and inhibit Aβ aggregation in vitro [104] and in vivo [105]. When Aβ40 is incubated in the presence of curcumin, its aggregation is significantly prevented. In addition, curcumin can disaggregate preformed aggregates and limit fibril and oligomer formation. In vivo administration of curcumin significantly reduces plaque burden in APP transgenic mice [105]. To explain these activities, docking studies revealed that curcumin prevents the stacking interaction of the KLVFFA region of Aβ, inhibiting the formation of steric-zipper needed for oligomer formation [106]. Chronic administration of curcumin also improves cognition in a rat model intracerebrally injected with Aβ42 aggregates. In clinical trials, however, curcumin was not able to reduce Aβ40 levels in plasma or ameliorate impaired cognition [107].
Curcumin has also been tested as an inhibitor of α-syn aggregation in vitro. Curcumin efficiently destabilizes preformed α-syn and avoids fibril formation in a dose-dependent manner [85]. In cellular models, it disrupts aggregation by increasing the amount of soluble monomeric α-syn species [108]. In a fruit fly model of PD, curcumin was able to ameliorate PD-related symptoms and favor brain cellular survival [109]. Moreover, when curcumin was used to treat a mouse model overexpressing human α-syn, there was an improvement in animal’s locomotion due to α-syn phosphorylation modulation [103]. In the case of IAPP, in vitro studies suggested that curcumin can diminish aggregate formation and protect cells against amyloid-induced toxicity [110]. This effect is due to the fact that curcumin retards IAPP aggregation by modifying its conformation [111].
The anti-amyloid potential of curcumin has also been explored in the case of prion diseases. Curcumin has been shown to reduce PrP-res accumulation in a cellular model and partially inhibit the aggregation of PrP in a cell-free assay [112]. However, no effect was observed when curcumin was orally administered to hamsters intracerebrally inoculated with scrapie. Some other studies suggested that it binds in vitro to PrP intermediates and fibrils, selectively to the non-native β-forms and α-helical intermediates of PrP, preventing further conformational arrangements that could result in aggregation [113].
In the case of transthyretin amyloidosis, curcumin stabilizes TTR tetramers leading to a successfully abolishment of TTR fibrillization by generating non-toxic off-pathway oligomers [61]. It is reported that TTR inhibitors do not affect stability of the TTR tetramer but bind to intermediate species, hindering unfolding and aggregation [114]. Oral treatment with curcumin reduces TTR aggregation and deposition and increases plasma TTR tetramer stabilization [115].
In summary, curcumin is able to inhibit protein misfolding and aggregation and even disaggregate fibrils both in vitro and in vivo of a wide variety of aggregated proteins. It also possesses a good safety profile and has the ability to cross the BBB, which has encouraged its application to in vivo PMD models. However, it has a poor bioavailability and several derivatives have been synthetized to overcome this issue [100, 116]. This might be the reason why the multiple clinical trials studying curcumin as a treatment for PMDs have not provided the expected results.
Gallic acid
Gallic acid (Fig. 2j) is a structurally simple phenolic acid present in grape seeds, pomegranate, and black tea [117]. It has been reported that is able to inhibit aggregation of several proteins implicated in PMDs, such as Aβ, α-syn, and insulin [118]. It seems that the particular moiety of gallic acid is fundamental for its anti-amyloid activity, as it forms part of more complex structures such as EGCG and some tannins that have also been shown to be inhibitors of aggregation. Regarding Aβ aggregation in vitro, gallic acid is able to inhibit mature fibrillar Aβ aggregation [118]. Gallic acid also prevents the formation of amyloids of an aggregation-prone form of α-syn, inhibiting the production of mature fibrils and favoring the formation of short amorphous aggregates. The mechanism of action is thought to be through a transient and direct interaction of the compound with unfolded monomeric species, stabilizing them [119]. This polyphenol also inhibits insulin conformational transition to β-sheet structure. However, no change in lag time was observed, suggesting gallic acid interacts and stabilizes folded insulin, probably by disruption of aromatic interactions or creation of hydrogen bonding [120].
Some gallic acid derivatives, such as galloylated esters, are flavonoids found in green and black tea with anti-aggregation activity. Gallotannins are hydrolysable tannins derived from gallic acid bound to a polyol carbohydrate. PGG (1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose) is a high molecular weight gallotannin that can be isolated from Paeonia suffruticosa, a traditional herbal medicine with anti-pyretic and anti-inflammatory activity. PGG inhibits Aβ fibril formation and destabilizes preformed Aβ fibrils in a dose-dependent manner in vivo and in vitro [121]. When administered to AD mice by oral route, it reduced the burden of Aβ40 and Aβ42 deposition in the brain without any toxic or adverse effect [121]. On the other hand, PGG is able to inhibit IAPP aggregation in vitro, preventing the cytotoxic effect of IAPP oligomers when tested in cell culture [122]. However, its high molecular weight and hydrophilicity may make complicated its brain delivery due to a low BBB permeability. Therefore, it could be considered a good candidate to treat systemic misfolding disorders such as T2D rather than AD.
Isoquinolines
A variety of natural compounds contain the isoquinoline group, known by their dual ability to both inhibit enzymatic activity and Aβ aggregation. Berberine (Fig. 2k) is usually found in the roots, rhizomes, stems, and bark of several plants including tree turmeric and barberry. Chelerythrine (Fig. 2l) is found in several herbaceous plants that belong to the poppy family, whereas palmatine (Fig. 2m) can be extracted from a number of plants used in the Chinese traditional medicine. These isoquinolines are able to both inhibit Aβ aggregation as well as disaggregate preformed Aβ40 aggregates in the micromolar range, chelerythrine being the most powerful of them (chelerythrine > berberine > palmatine) [123]. In fact, chelerythrine demonstrated its ability to also inhibit the formation of Aβ fibrils. However, chelerythrine is a well-known selective protein kinase C (PKC) inhibitor [124]. PKC reduces the accumulation of aggregated Aβ by activating the α-secretase activity; therefore, promoting the non-amyloidogenic pathway [125]. In fact, some studies have demonstrated that when chelerythrine was added to neuronal culture it was able to increase Aβ-induced cytotoxicity [126, 127]. Therefore, this cannot be considered as a candidate to treat AD although s its efficacy inhibiting other misfolded proteins is not known.
Olive oil derivatives
Olive oil is obtained from the fruit of the Mediterranean Olea europaea tree. It contains high levels of phenolic compounds, which have received considerable attention due to its association with lower risk of cardiovascular and cancer development, and delayed cognitive decline. Oleocanthal, oleuropein, and oleuropein aglycone are the most investigated olive oil derivative phenols due to their ability to neutralize amyloid aggregation and toxicity.
Oleocanthal (Fig. 2n) is a phenolic secoiridoid component of extra-virgin olive oil known for its anti-inflammatory and anticancer features. Oleocanthal interacts with soluble oligomeric Aβ species, altering Aβ oligomerization and modifying its conformation. The amyloidogenic species formed in the presence of this polyphenol display a decreased synaptic binding in vitro, conferring less synaptotoxicity to the aggregates [128]. In regard to tau aggregation, oleocanthal has been proven to inhibit tau polymerization and, therefore, the formation of tau fibrils in vitro by stabilizing the peptide into the unfolded state, without interfering with tau-microtubule interaction [129]. When TgSwDI AD transgenic mice were treated with oleocanthal, a reduction in brain Aβ burden was observed, as well as a reduction in astrocyte activation [130].
Oleuropein (Fig. 2o) is a phenylethanoid tyrosol found in olive leaves and unprocessed olive drupes. Oleuropein is able to interact with Aβ, and it is possible that this interaction may prevent its aggregation [131]. In culture cells, oleuropein reduces Aβ oligomers’ production by promoting the non-amyloidogenic pathway [132]. In addition, this polyphenol interacts in vitro with tau aggregates with an IC50 of 4.1 μM, inhibiting its aggregation. In the presence of oleuropein, tau fibers appear as shorter rods instead of long twisted fibers [133]. However, it does not seem to penetrate cell monolayers, so its potential to reach the brain and enter into neurons appears to be very unlikely.
In extra-virgin olive oil, the tyrosol oleuropein aglycone (Fig. 2p) is the most abundant derivative of oleuropein, formed during olive ripening by enzymatic glucose removal. It is been reported that oleuropein aglycone delays Aβ42 and pyroglutamylated-3 Aβ (pE3-Aβ) aggregation, reducing the formation of oligomeric forms towards the development of non-toxic protofibrils during the aggregation process, decreasing its cytotoxicity [134, 135]. Oleuropein aglycone inhibits tau fibrillization more efficiently (IC50 = 1.4 μM) than its parent compound oleuropein, reducing also the size of the fibrils [133]. In addition, it is more lipophilic than oleuropein, so it may have higher bioavailability. AD worms fed with oleuropein aglycone showed less Aβ oligomeric species and decreased deposition, ameliorated motor impairment and increased their lifespan, presumably due to the reduction of toxic Aβ forms [136]. It prevents Aβ42 and pE3-Aβ deposition and disassembles deposited Aβ plaques in the transgenic AD mouse model TgCRND8 [135, 137]. As a consequence, plaques look less dense, cell proliferation was increased, the inflammatory process was reduced, and the cognitive impairment was improved [138]. In the case of T2D, oleuropein aglycone interferes with IAPP aggregation by reducing its cytotoxicity in cell culture due to the generation of structurally different non-toxic aggregates, favoring off-pathway species [139]. Recently, it has been shown to inhibit the growth of α-syn oligomers into bigger misfolded species [140]. Furthermore, oleuropein aglycone blocks the formation of mature fibers and induces disaggregation of TTR fibrils [141]. In summary, this olive oil derivative does not prevent misfolded protein aggregation but it rather inhibits the formation of fibers and disassembles aggregates, an important feature when treating patients with already abundant aggregate deposition. Currently, a phase 2 clinical trial is open to study the effect of extra-virgin olive oil on cognitive impairment in MCI and AD patients.
Orcein
Orcein (Fig. 2q) is a mixture of substances that share a phenoxazine backbone. It is extracted from lichens (Roccella tinctoria) and used as natural purple dye for both food coloring and histological stain. Orcein successfully accelerates spontaneous Aβ42 aggregation in vitro, converting toxic oligomeric forms into non-toxic fibrillary species [142]. The blue dye O4 is orcein-related small molecule that has been identified as a potent Aβ42 aggregation accelerator by reducing polymerization lag phase without altering fibril conformation, stabilizing the aggregates, and generating seeding-competent aggregates [142]. Although O4 is able to increase the amount of mature fibrils, they seem to be less toxic for neurons in culture. The rapid increase in the fibrillar forms may decrease the concentration of soluble Aβ42 oligomers, producing a non-cytotoxic enriched scenario [142]. O4 is also able to reduce the toxicity of α-syn aggregates in vitro. While O4 does not change the aggregation kinetic of α-syn, the compound reduces the formation of oligomers towards the generation of large fibrils that appear to be less toxic in neuronal cultures and have a reduced seeding ability [143]. In regard to IAPP, it has been recently published that O4 strongly inhibits the formation of mature fibrils, producing instead globular, amorphous off-pathway assemblies [144]. The idea that small soluble aggregates are more toxic than mature fibrils is reinforced by the mechanism of action of this molecule to accelerate the aggregation process and produce a more mature species that is less harmful.
Resveratrol
Resveratrol (Fig. 2r) is a natural phytoalexin stilbenoid polyphenolic compound produced naturally by several plants in response to injury or when the plant is attacked by pathogens (bacteria or fungi). It is abundant in grapes, berries, soy beans, peanuts, and red wine. Several promising features have been associated with resveratrol and its derivatives, being of special interest its anti-aggregation capability against different amyloids. For instance, resveratrol inhibits the aggregation of Aβ by selectively transforming oligomers and converting them into off-pathway species unable to aggregate, reducing Aβ cytotoxicity [145]. Resveratrol is able to bind to different conformations such as monomeric Aβ42 and Aβ40, and even fibrillar Aβ, although in a weaker manner [146]. While resveratrol can disaggregate Aβ42 fibrils, it has been reported that it cannot prevent formation of oligomeric species [147]. In addition, the presence of this compound in the culture medium diminishes tau hyperphosphorylation [148]. In AD murine models, resveratrol treatment reduces amyloid plaque deposition without affecting APP processing [149, 150], ameliorates cognitive impairment [151, 152], protects the integrity of the BBB [153], decreases microgliosis, and reactive oxygen species [154, 155]. Although resveratrol is rapidly metabolized and its bioavailability would not be sufficient to exert its action, its conjugation with nanoparticles for brain delivery shows promising results [156]. Recently, it has been reported that people affected by MCI showed ameliorated memory impairment after resveratrol supplementation [157]. However, it did not show a significant effect in Aβ plasma or CSF levels when administered to Alzheimer’s patients in a controlled clinical trial [158].
Resveratrol has been also studied for its potential to inhibit the aggregation of IAPP and mitigate its toxicity [159]. Molecular simulations suggest a direct interaction of the polyphenolic compound with IAPP oligomeric species, causing conformational changes that ultimately lead to inhibition of fibril formation but without disaggregating preformed IAPP amyloid fibrils [160–162]. This may be due to its ability to stabilize oligomeric forms and to inhibit IAPP oligomerization [163, 164]. It has been proposed that it is also able to inhibit stacking of IAPP oligomers, avoiding its aggregation and deposition [165]. In vitro cell survival studies demonstrate that resveratrol produces off-pathway non-toxic conformations, suggesting that its neuroprotective effect is not just due to its antioxidant activity [166]. In vivo, resveratrol derivatives abolish amyloid growth and associated damage [167].
Regarding other proteinopathies, resveratrol has also been shown to decrease the toxicity associated with polyQ aggregates in neuronal culture and C. elegans Huntington’s models [168]. Even more, resveratrol inhibits TTR amyloid fibril formation and reduces the cytotoxicity induced by TTR in human chondrocyte cultures [169, 170]. Therefore, resveratrol is an attractive molecule that could be used to reduce the burden of aggregated proteins that occur in PMDs and with anti-oxidant benefits that have shown some promising results in recent clinical trials in AD and PD patients and that is currently under evaluation to treat MCI and T2D.
Rosmarinic acid
Rosmarinic acid (Fig. 2s) is a polyphenolic compound present in species used commonly as culinary herbs such as sage, oregano, basil, thyme, and peppermint. This curcumin analog inhibits, in a dose-dependent manner, the formation and extension of oligomeric and fibrillar Aβ40 and Aβ42 and it destabilizes preformed aggregates in vitro [171]. This effect is through direct interaction with the oligomeric forms, reducing Aβ-associated toxicity, tau hyperphosphorylation, and inducing neuroprotection [172–174]. In vivo data demonstrate that oral administration of rosmarinic acid exerts beneficial effects on cognition in mouse models of AD and cerebral amyloid angiopathy (CAA) and prevents the development of AD-related neuropathology by reducing Aβ oligomerization and plaque burden [172, 175, 176].
In addition, rosmarinic acid blocks the formation and induces the destabilization of α-syn fibrils. It also inhibits the new generation of small oligomers through a mechanism involving the inhibition of the β-sheet conversion, reducing associated synaptotoxicity [84, 85]. This is particularly important as small soluble oligomeric species are thought to be highly toxic. Furthermore, this polyphenol is also able to hinder fibril formation and disrupt preformed aggregates of hen egg white lysozyme (HEWL), reducing its cytotoxicity in cell culture [177, 178]. It has been reported that several naturally occurring lysozyme mutants are associated with familial non-neuropathic systemic amyloidosis; therefore, this amyloidogenic protein is widely used to study aggregation associated with these amyloidosis.
Regarding T2D, rosmarinic acid has been proven to repress the formation of IAPP amyloidogenic aggregates, probably by opening the beta-sheet conformation, reducing IAPP-associated toxicity [179]. It is also able to bind to the insulin monomer, blocking its ability to form amyloid fibrils [180]. The pharmacological activity of rosmarinic acid makes it a promising compound to treat several proteinopathies, especially due to its ability to inhibit both oligomeric and fibrillary conformations. Nevertheless, its anti-amyloidogenic effect is not dose dependent and it is less efficient than curcumin. So far, it has not been tested in any clinical trial for PMDs.
Tannic acid
Tannins are polyphenolic molecules present in fruits, nuts, legumes, chocolate, tea, and red wine and are well known by their astringent potential. Tannic acid (Fig. 2t) is a naturally occurring plant tannin composed of a central glucose molecule with one or more galloyl residues. In vitro, tannic acid is able to inhibit Aβ40 and Aβ42 fibrillation and extension and destabilize preformed Aβ fibrils and the polymerization of Aβ peptides in dose-dependent manner [181] and is able to inhibit the formation of oligomeric and fibrillar α-syn, and destabilize and disaggregate preformed oligomeric and fibrillar α-syn [50, 85]. In prion diseases, tannic acid is considered a potent inhibitor of both murine and ovine the protease resistant form of prion protein (PrP-res) formation in the test tube [182]. In addition, it was able to prevent prion aggregation when evaluated in a solid-phase cell-free hamster PrP conversion assay [183]. However, tannic acid failed to extend the incubation period of chronic wasting disease (CWD) when it was assayed as a therapeutic intervention to prevent prion infection in experimentally challenged mule deer [184].
Tanshinones
Tanshinones (Fig. 2u, v) are both lipophilic and hydrophilic diterpenes isolated from the rhizomes of Salvia miltiorrhiza, also named Danshen, commonly used in the traditional Chinese medicine as a remedy for several diseases. To date, more than 90 tanshinones or structurally related compounds have been identified [185]. In vitro, tanshinones I and IIA (TS1, and TS2, respectively), the major components of Danshen, are able to extend Aβ aggregation lag phase in an equimolar range, TS1 being the most potent compound to inhibit formation of fibrillary species. In addition, both molecules disassemble fibrillar Aβ into smaller arrangements by inducing structural disruption of the β-sheets in the structure [186]. Cryptotanshinone, another active constituent of S. miltiorrhiza, has been shown to inhibit Aβ oligomerization in vitro [187]. Regarding PD, TS1 and TS2 can inhibit α-syn aggregation increasing the nucleation phase, induce the formation of alternative conformations, decrease the formation of oligomers and fibrils, and disassemble preformed fibrillary species in the test tube [188]. Tanshinones have been poorly studied in vivo to determine their effectiveness to treat PMDs; however, an amelioration in the neuroinflammatory process associated with Aβ has been observed in AD mice, indicating that their mechanism of action in vivo could be a downregulation of the pro-inflammatory reaction [189].
Concluding remarks
Misfolding and aggregation of amyloidogenic proteins are the main culprits in the pathogenesis of a large group of diverse diseases affecting the brain and various systemic organs. Currently, there is not an efficient therapy for curing or slowing progression of the majority of PMDs. Since amyloidogenesis is a multi-step process, there are several targets where therapeutic intervention is possible. Although many molecules have been shown to reduce protein misfolding and its negative consequences in in vitro or in vivo models, the few molecules that have been tested in human clinical trials have shown disappointing results. Recently, special attention has been paid to natural compounds due to the ease of modifying their structure, their druggability features, and especially their low toxicity which make them good candidates for a safe treatment that can be started at the pre-clinical stage of the disease. A growing body of evidence indicates that natural phenolic compounds, especially polyphenols, are potent inhibitors of amyloidogenesis. This diverse group of compounds acts at various different levels and often has multiple beneficial activities. Some polyphenols have been shown to target directly the process of protein misfolding and aggregation of various amyloidogenic proteins. Others have shown to act downstream of protein aggregation to prevent the toxic consequences of the accumulation of misfolded proteins. Yet, others may target the secondary processes induced by the accumulation of misfolded proteins, such as inflammation, oxidative stress, and protein homeostasis. Interestingly, many polyphenols have been shown to have simultaneously two or more beneficial activities in combating PMDs. Among all phenolic compounds tested for their amyloid inhibitory activity, curcumin and resveratrol are be the best studied, with confirmed targets including Aβ, α-syn, IAPP, PrPSc, and TTR. Since both are recognized as anti-aggregation molecules and antioxidants, the combination of these two activities may be necessary to obtain a potent effect against the progression of proteinopathies. However, despite extensive testing in in vitro and in vivo models, polyphenols are yet to be used as a treatment for PMDs, a reluctance attributed to their poor metabolic stability and low bioavailability at the needed pharmacological concentrations. This is especially important to treat brain disorders, where the drugs need to cross the BBB and reach the affected area in sufficient quantities to produce an effect. Phenolic compounds should be further considered as a prophylactic intervention due to their easy availability and accessibility, and especially because their relatively low toxicity permits to begin treatment before the onset of clinical symptoms. More research is necessary to fully take advantage of the promising possibilities opened by the use of polyphenolic natural compounds for the treatment and prevention of some of today’s most debilitating and chronic diseases.
References
- 1.Moreno-Gonzalez I, Soto C. Misfolded protein aggregates: mechanisms, structures and potential for disease transmission. Semin Cell Dev Biol. 2011;22:482–487. doi: 10.1016/j.semcdb.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 3.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 4.Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 5.Hetz C, Soto C. Protein misfolding and disease: the case of prion disorders. Cell Mol Life Sci. 2003;60:133–143. doi: 10.1007/s000180300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor JP, Brown RH, Cleveland DW. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 8.Westermark GT, Fändrich M, Westermark P. AA amyloidosis: pathogenesis and targeted therapy. Annu Rev Pathol Mech Dis. 2015;10:321–344. doi: 10.1146/annurev-pathol-020712-163913. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee A, Morales-Scheihing D, Butler PC, Soto C. Type 2 diabetes as a protein misfolding disease. Trends Mol Med. 2015;21:439–449. doi: 10.1016/j.molmed.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekijima Y. Transthyretin (ATTR) amyloidosis: clinical spectrum, molecular pathogenesis and disease-modifying treatments. J Neurol Neurosurg Psychiatry. 2015;86:1036–1043. doi: 10.1136/jnnp-2014-308724. [DOI] [PubMed] [Google Scholar]
- 11.Morales R, Moreno-Gonzalez I, Soto C. Cross-seeding of misfolded proteins: implications for etiology and pathogenesis of protein misfolding diseases. PLoS Pathog. 2013 doi: 10.1371/journal.ppat.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson R, Sawaya MR, Balbirnie M, Madsen AØ, Riekel C, Grothe R, Eisenberg D. Structure of the cross-beta spine of amyloid-like fibrils. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefani M. Protein misfolding and aggregation: new examples in medicine and biology of the dark side of the protein world. Biochim Biophys Acta Mol Basis Dis. 2004;1739:5–25. doi: 10.1016/j.bbadis.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Monsellier E, Chiti F. Prevention of amyloid-like aggregation as a driving force of protein evolution. EMBO Rep. 2007;8:737–742. doi: 10.1038/sj.embor.7401034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–332. doi: 10.1016/S0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 16.Duran-Aniotz C, Moreno-Gonzalez I, Morales R. Amyloid aggregates: role in protein misfolding disorders. Rev Med Chile. 2013;141:495–505. doi: 10.4067/S0034-98872013000400011. [DOI] [PubMed] [Google Scholar]
- 17.Andre R, Tabrizi SJ. Misfolded PrP and a novel mechanism of proteasome inhibition. Prion. 2012;6:32–36. doi: 10.4161/pri.6.1.18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol. 2012;181:1426–1435. doi: 10.1016/j.ajpath.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuanalo-Contreras K, Mukherjee A, Soto C. Role of protein misfolding and proteostasis deficiency in protein misfolding diseases and aging. Int J Cell Biol. 2013;2013:638083. doi: 10.1155/2013/638083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nixon RA, Yang DS, Lee JH. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy. 2008;4:590–599. doi: 10.4161/auto.6259. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Varo R, Trujillo-Estrada L, Sanchez-Mejias E, Torres M, Baglietto-Vargas D, Moreno-Gonzalez I, De Castro V, Jimenez S, Ruano D, Vizuete M, Davila JC, Garcia-Verdugo JM, Jimenez AJ, Vitorica J, Gutierrez A. Abnormal accumulation of autophagic vesicles correlates with axonal and synaptic pathology in young Alzheimer’s mice hippocampus. Acta Neuropathol. 2012;123:53–70. doi: 10.1007/s00401-011-0896-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly JW. Alternative conformations of amyloidogenic proteins govern their behavior. Curr Opin Struct Biol. 1996;6:11–17. doi: 10.1016/S0959-440X(96)80089-3. [DOI] [PubMed] [Google Scholar]
- 24.Soto C, Estrada L, Castilla J. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci. 2006;31:150–155. doi: 10.1016/j.tibs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Gonzalez I, Edwards G, III, Salvadores N, Shahnawaz M, Diaz-Espinoza R, Soto C. Molecular interaction between type 2 diabetes and Alzheimer’s disease through cross-seeding of protein misfolding. Mol Psychiatry. 2017;22:1327–1334. doi: 10.1038/mp.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales R, Estrada LD, Diaz-Espinoza R, Morales-Scheihing D, Jara MC, Castilla J, Soto C. Molecular cross talk between misfolded proteins in animal models of Alzheimer’s and prion diseases. J Neurosci. 2010;30:4528–4535. doi: 10.1523/JNEUROSCI.5924-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soto C. Protein misfolding and disease; protein refolding and therapy. FEBS Lett. 2001;498:204–207. doi: 10.1016/S0014-5793(01)02486-3. [DOI] [PubMed] [Google Scholar]
- 29.Estrada LD, Soto C. Inhibition of protein misfolding and aggregation by small rationally-designed peptides. Curr Pharm Des. 2006;12:2557–2567. doi: 10.2174/138161206777698792. [DOI] [PubMed] [Google Scholar]
- 30.Soto C, Sigurdsson EM, Morelli L, Kumar RA, Castaño EM, Frangione B. Beta-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer’s therapy. Nat Med. 1998;4:822–826. doi: 10.1038/nm0798-822. [DOI] [PubMed] [Google Scholar]
- 31.Ladiwala ARA, Dordick JS, Tessier PM. Aromatic small molecules remodel toxic soluble oligomers of amyloid beta through three independent pathways. J Biol Chem. 2011;286:3209–3218. doi: 10.1074/jbc.M110.173856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goold R, Rabbanian S, Sutton L, Andre R, Arora P, Moonga J, Clarke AR, Schiavo G, Jat P, Collinge J, Tabrizi SJ. Rapid cell-surface prion protein conversion revealed using a novel cell system. Nat Commun. 2011;2:281. doi: 10.1038/ncomms1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marijanovic Z, Caputo A, Campana V, Zurzolo C. Identification of an intracellular site of prion conversion. PLoS Pathog. 2009;5:e1000426. doi: 10.1371/journal.ppat.1000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarnataro D, Pepe A, Altamura G, De Simone I, Pesapane A, Nitsch L, Montuori N, Lavecchia A, Zurzolo C. The 37/67 kDa laminin receptor (LR) inhibitor, NSC47924, affects 37/67 kDa LR cell surface localization and interaction with the cellular prion protein. Sci Rep. 2016;6:24457. doi: 10.1038/srep24457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valera E, Masliah E. Immunotherapy for neurodegenerative diseases: focus on α-synucleinopathies. Pharmacol Ther. 2013;138:311–322. doi: 10.1016/j.pharmthera.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wisniewski T, Goñi F. Immunotherapeutic approaches for Alzheimer’s disease. Neuron. 2015;85:1162–1176. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer’s disease. Trends Mol Med. 2015;21:394–402. doi: 10.1016/j.molmed.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Rosenblum WI. Why Alzheimer trials fail: removing soluble oligomeric beta amyloid is essential, inconsistent, and difficult. Neurobiol Aging. 2014;35:969–974. doi: 10.1016/j.neurobiolaging.2013.10.085. [DOI] [PubMed] [Google Scholar]
- 39.Van Dyck CH. Anti-amyloid-β monoclonal antibodies for Alzheimer’s disease: pitfalls and promise. Biol Psychiatry. 2018;83:311–319. doi: 10.1016/j.biopsych.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 42.D’Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R. Bioavailability of the polyphenols: status and controversies. Int J Mol Sci. 2010;11:1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Asp Med. 2010;31:446–467. doi: 10.1016/j.mam.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Maruszak A, Pilarski A, Murphy T, Branch N, Thuret S. Hippocampal neurogenesis in Alzheimer’s disease: is there a role for dietary modulation? J Alzheimer’s Dis. 2014;38:11–38. doi: 10.3233/JAD-131004. [DOI] [PubMed] [Google Scholar]
- 45.Dias GP, Cavegn N, Nix A, Do Nascimento Bevilaqua MC, Stangl D, Zainuddin MSA, Nardi AE, Gardino PF, Thuret S. The role of dietary polyphenols on adult hippocampal neurogenesis: molecular mechanisms and behavioural effects on depression and anxiety. Oxid Med Cell Longev. 2012;2012:541971. doi: 10.1155/2012/541971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao L, Hou L, Sun H, Yan X, Sun X, Li J, Bian Y, Chu Y, Liu Q. Apigenin isolated from the medicinal plant Elsholtzia rugulosa prevents β-Amyloid 25–35-induces toxicity in rat cerebral microvascular endothelial cells. Molecules. 2011;16:4005–4019. doi: 10.3390/molecules16054005. [DOI] [Google Scholar]
- 47.Florio P, Folli C, Cianci M, Del Rio D, Zanotti G, Berni R. Transthyretin binding heterogeneity and anti-amyloidogenic activity of natural polyphenols and their metabolites. J Biol Chem. 2015;290:29769–29780. doi: 10.1074/jbc.M115.690172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amini R, Yazdanparast R, Bahramikia S. Apigenin reduces human insulin fibrillation in vitro andprotects SK-N-Mc cellsagainstinsulinamyloids. Int J Biol Macromol. 2013;60:334–340. doi: 10.1016/j.ijbiomac.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Thapa A, Woo ER, Chi EY, Sharoar MG, Jin HG, Shin SY, Park IS. Biflavonoids are superior to monoflavonoids in inhibiting amyloid beta toxicity and fibrillogenesis via accumulation of nontoxic oligomer-like structures. Biochemistry. 2011;50:2445–2455. doi: 10.1021/bi101731d. [DOI] [PubMed] [Google Scholar]
- 50.Caruana M, Högen T, Levin J, Hillmer A, Giese A, Vassallo N. Inhibition and disaggregation of α-synuclein oligomers by natural polyphenolic compounds. FEBS Lett. 2011;585:1113–1120. doi: 10.1016/j.febslet.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 51.Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- 52.Zhao L, Wang JL, Liu R, Li XX, Li JF, Zhang L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules. 2013;18:9949–9965. doi: 10.3390/molecules18089949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De la Luz Cádiz-Gurrea M, Fernández-Arroyo S, Segura-Carretero A. Pine bark and green tea concentrated extracts: antioxidant activity and comprehensive characterization of bioactive compounds by HPLC–ESI-QTOF-MS. Int J Mol Sci. 2014;15:20382–20402. doi: 10.3390/ijms151120382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Betts JW, Sharili AS, Phee LM, Wareham DW. In vitro activity of epigallocatechin gallate and quercetin alone and in combination versus clinical isolates of methicillin-resistant Staphylococcus aureus [J] J Nat Prod. 2015;78:2145–2148. doi: 10.1021/acs.jnatprod.5b00471. [DOI] [PubMed] [Google Scholar]
- 55.BituPinto N, DaSilvaAlexandre B, Neves KRT, Silva AH, Leal LKAM, Viana GSB. Neuroprotective properties of the standardized extract from Camellia sinensis (green tea) and its main bioactive components, epicatechin and epigallocatechin gallate, in the 6-OHDA model of Parkinson’s disease. Evid Based Complement Altern Med. 2015 doi: 10.1155/2015/161092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc Natl Acad Sci. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 58.Wobst HJ, Sharma A, Diamond MI, Wanker EE, Bieschke J. The green tea polyphenol (−)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015;589:77–83. doi: 10.1016/j.febslet.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao P, Raleigh DP. Analysis of the inhibition and remodeling of islet amyloid polypeptide amyloid fibers by flavanols. Biochemistry. 2012;51:2670–2683. doi: 10.1021/bi2015162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira N, Saraiva MJ, Almeida MR. Natural polyphenols as modulators of TTR amyloidogenesis: in vitro and in vivo evidences towards therapy. Amyloid. 2012;19(Suppl 1):39–42. doi: 10.3109/13506129.2012.668502. [DOI] [PubMed] [Google Scholar]
- 61.Ferreira N, Saraiva MJ, Almeida MR. Natural polyphenols inhibit different steps of the process of transthyretin (TTR) amyloid fibril formation. FEBS Lett. 2011;585:2424–2430. doi: 10.1016/j.febslet.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 62.Ehrnhoefer DE, Duennwald M, Markovic P, Wacker JL, Engemann S, Roark M, Legleiter J, Marsh JL, Thompson LM, Lindquist S, Muchowski PJ, Wanker EE. Green tea (−)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum Mol Genet. 2006;15:2743–2751. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- 63.Mereles D, Hunstein W. Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises? Int J Mol Sci. 2011;12:5592–5603. doi: 10.3390/ijms12095592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franko A, Rodriguez Camargo DC, Böddrich A, Garg D, Rodriguez Camargo A, Rathkolb B, Janik D, Aichler M, Feuchtinger A, Neff F, Fuchs H, Wanker EE, Reif B, Häring HU, Peter A, Hrabě De Angelis M. Epigallocatechin gallate (EGCG) reduces the intensity of pancreatic amyloid fibrils in human islet amyloid polypeptide (hIAPP) transgenic mice. Sci Rep. 2018;8:1116. doi: 10.1038/s41598-017-18807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mereles D, Buss SJ, Hardt SE, Hunstein W, Katus HA. Effects of the main green tea polyphenol epigallocatechin-3-gallate on cardiac involvement in patients with AL amyloidosis. Clin Res Cardiol. 2010;99:483–490. doi: 10.1007/s00392-010-0142-x. [DOI] [PubMed] [Google Scholar]
- 66.Kim H, Park BS, Lee KG, Cheol YC, Sung SJ, Kim YH, Lee SE. Effects of naturally occurring compounds on fibril formation and oxidative stress of β-amyloid. J Agric Food Chem. 2005;53:8537–8541. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 67.Akaishi T, Morimoto T, Shibao M, Watanabe S, Sakai-Kato K, Utsunomiya-Tate N, Abe K. Structural requirements for the flavonoid fisetin in inhibiting fibril formation of amyloid beta protein. Neurosci Lett. 2008;444:280–285. doi: 10.1016/j.neulet.2008.08.052. [DOI] [PubMed] [Google Scholar]
- 68.Ahmad A, Ali T, Park HY, Badshah H, Rehman SU, Kim MO. Neuroprotective effect of fisetin against amyloid-beta-induced cognitive/synaptic dysfunction, neuroinflammation, and neurodegeneration in adult mice. Mol Neurobiol. 2017;54:2269–2285. doi: 10.1007/s12035-016-9795-4. [DOI] [PubMed] [Google Scholar]
- 69.Maher P, Dargusch R, Bodai L, Gerard PE, Purcell JM, Lawrence Marsh J. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Hum Mol Genet. 2011;20:261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Currais A, Prior M, Dargusch R, Armando A, Ehren J, Schubert D, Quehenberger O, Maher P. Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in Alzheimer’s disease transgenic mice. Aging Cell. 2014;13:379–390. doi: 10.1111/acel.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhat WF, Bhat SA, Bano B. Evaluation of polyphenols as possible therapeutics for amyloidoses: comparative analysis of Kaempferol and Catechin. Int J Biol Macromol. 2015;81:60–68. doi: 10.1016/j.ijbiomac.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 72.Roth A, Schaffner W, Hertel C. Phytoestrogen kaempferol (3,4′,5,7-tetrahydroxyflavone) protects PC12 and T47D cells from β-amyloid–induced toxicity. J Neurosci Res. 1999;57:399–404. doi: 10.1002/(SICI)1097-4547(19990801)57:3<399::AID-JNR12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 73.Kim JK, Shin E-C, Kim CR, Park GG, Choi SJ, Park S-G, Shin D-H. Effects of brussels sprouts and their phytochemical components on oxidative stress-induced neuronal damages in PC12 cells and ICR mice. J Med Food. 2013;16:1057–1061. doi: 10.1089/jmf.2012.0280. [DOI] [PubMed] [Google Scholar]
- 74.Kim JK, Choi SJ, Cho HY, Hwang H-J, Kim YJ, Lim ST, Kim C-J, Kim HK, Peterson S, Shin D-H. Protective effects of kaempferol (3,4’,5,7-tetrahydroxyflavone) against amyloid beta peptide (Abeta)-induced neurotoxicity in ICR mice. Biosci Biotechnol Biochem. 2010;74:397–401. doi: 10.1271/bbb.90585. [DOI] [PubMed] [Google Scholar]
- 75.Beg T, Jyoti S, Naz F, Rahul X, Ali F, Ali SK, Reyad AM, Siddique YH. Protective effect of kaempferol on the transgenic Drosophila model of Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2018 doi: 10.2174/1871527317666180508123050. [DOI] [PubMed] [Google Scholar]
- 76.Yang S, Liu W, Lu S, Tian YZ, Wang WY, Ling TJ, Liu RT. A novel multifunctional compound Camellikaempferoside B decreases Aβ production, interferes with Aβ aggregation, and prohibits Aβ-mediated neurotoxicity and neuroinflammation. ACS Chem Neurosci. 2016;7:505–518. doi: 10.1021/acschemneuro.6b00091. [DOI] [PubMed] [Google Scholar]
- 77.Ren R, Shi C, Cao J, Sun Y, Zhao X, Guo Y, Wang C, Lei H, Jiang H, Ablat N, Xu J, Li W, Ma Y, Qi X, Ye M, Pu X, Han H. Neuroprotective effects of a standardized flavonoid extract of safflower against neurotoxin-induced cellular and animal models of Parkinson’s disease. Sci Rep. 2016;6:22135. doi: 10.1038/srep22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lemkul JA, Bevan DR. Morin inhibits the early stages of amyloid β-peptide aggregation by altering tertiary and quaternary interactions to produce “off-pathway” structures. Biochemistry. 2012;51:5990–6009. doi: 10.1021/bi300113x. [DOI] [PubMed] [Google Scholar]
- 79.Noor H, Cao P, Raleigh DP. Morin hydrate inhibits amyloid formation by islet amyloid polypeptide and disaggregates amyloid fibers. Protein Sci. 2012;21:373–382. doi: 10.1002/pro.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel P, Parmar K, Das M. Inhibition of insulin amyloid fibrillation by morin hydrate. Int J Biol Macromol. 2018;108:225–239. doi: 10.1016/j.ijbiomac.2017.11.168. [DOI] [PubMed] [Google Scholar]
- 81.Du Y, Qu J, Zhang W, Bai M, Zhou Q, Zhang Z, Li Z, Miao J. Morin reverses neuropathological and cognitive impairments in APPswe/PS1dE9 mice by targeting multiple pathogenic mechanisms. Neuropharmacology. 2016;108:1–13. doi: 10.1016/j.neuropharm.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 82.Das S, Stark L, Musgrave IF, Pukala T, Smid SD. Bioactive polyphenol interactions with β amyloid: a comparison of binding modelling, effects on fibril and aggregate formation and neuroprotective capacity. Food Funct. 2016;7:1138–1146. doi: 10.1039/C5FO01281C. [DOI] [PubMed] [Google Scholar]
- 83.Taniguchi S, Suzuki N, Masuda M, Hisanaga SI, Iwatsubo T, Goedert M, Hasegawa M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J Biol Chem. 2005;280:7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi R, Ono K, Takamura Y, Mizuguchi M, Ikeda T, Nishijo H, Yamada M. Phenolic compounds prevent the oligomerization of α-synuclein and reduce synaptic toxicity. J Neurochem. 2015;134:943–955. doi: 10.1111/jnc.13180. [DOI] [PubMed] [Google Scholar]
- 85.Ono K, Yamada M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. J Neurochem. 2006;97:105–115. doi: 10.1111/j.1471-4159.2006.03707.x. [DOI] [PubMed] [Google Scholar]
- 86.Zelus C, Fox A, Calciano A, Faridian BS, Nogaj LA, Moffet DA. Myricetin inhibits islet amyloid polypeptide (IAPP) aggregation and rescues living mammalian cells from IAPP toxicity. Open Biochem J. 2012;6:66–70. doi: 10.2174/1874091X01206010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He J, Wang Y, Chang AK, Xu L, Wang N, Chong X, Li H, Zhang B, Jones GW, Song Y. Myricetin prevents fibrillogenesis of hen egg white lysozyme. J Agric Food Chem. 2014;62:9442–9449. doi: 10.1021/jf5025449. [DOI] [PubMed] [Google Scholar]
- 88.Matsuzaki K, Noguch T, Wakabayashi M, Ikeda K, Okada T, Ohashi Y, Hoshino M, Naiki H. Inhibitors of amyloid β-protein aggregation mediated by GM1-containing raft-like membranes. Biochim Biophys Acta. 2007;1768:122–130. doi: 10.1016/j.bbamem.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 89.Zhu M, Han S, Fink AL. Oxidized quercetin inhibits α-synuclein fibrillization. Biochim Biophys Acta. 2013;1830:2872–2881. doi: 10.1016/j.bbagen.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 90.Jiang W, Luo T, Li S, Zhou Y, Shen XY, He F, Xu J, Wang HQ. Quercetin protects against okadaic acid-induced injury via MAPK and PI3K/Akt/GSK3β signaling pathways in HT22 hippocampal neurons. PLoS One. 2016;11:e0152371. doi: 10.1371/journal.pone.0152371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, Zhou S, Li J, Sun Y, Hasimu H, Liu R, Zhang T. Quercetin protects human brain microvascular endothelial cells from fibrillar β-amyloid1-40-induced toxicity. Acta Pharm Sin B. 2015;5:47–54. doi: 10.1016/j.apsb.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, Cardona-Gómez GP. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93:134–145. doi: 10.1016/j.neuropharm.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ho L, Ferruzzi MG, Janle EM, Wang J, Gong B, Chen TY, Lobo J, Cooper B, Wu QL, Talcott ST, Percival SS, Simon JE, Pasinetti GM. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013;27:769–781. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ishisaka A, Ichikawa S, Sakakibara H, Piskula MK, Nakamura T, Kato Y, Ito M, Miyamoto KI, Tsuji A, Kawai Y, Terao J. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic Biol Med. 2011;51:1329–1336. doi: 10.1016/j.freeradbiomed.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 95.Du WJ, Guo JJ, Gao MT, Hu SQ, Dong XY, Han YF, Liu FF, Jiang S, Sun Y. Brazilin inhibits amyloid β-protein fibrillogenesis, remodels amyloid fibrils and reduces amyloid cytotoxicity. Sci Rep. 2015;5:7992. doi: 10.1038/srep07992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deng Z, Wang X, Zhao H, Cui S, Yao Q, Bai H. A validated LC-MS/MS method for rapid determination of brazilin in rat plasma and its application to a pharmacokinetic study. Biomed Chromatogr. 2013;27:802–806. doi: 10.1002/bmc.2863. [DOI] [PubMed] [Google Scholar]
- 97.Yan-Yan J, Yan L, Ying S, Jinyi Z, Fang D, Yuan S, Ai-Dong WJ. A simple high-performance liquid chromatographic method for the determination of brazilin and its application to a pharmacokinetic study in rats. J Ethnopharmacol. 2014;151:108–113. doi: 10.1016/j.jep.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 98.Jia Y, Wang H, Song Y, Liu K, Dou F, Lu C, Ge J, Chi N, Ding Y, Hai W, Wen A. Application of a liquid chromatography-tandem mass spectrometry method to the pharmacokinetics, tissue distribution and excretion studies of Brazilin in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;931:61–67. doi: 10.1016/j.jchromb.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 99.Li M, Dong X, Liu Y, Sun Y. Brazilin inhibits prostatic acidic phosphatase fibrillogenesis and decreases its cytotoxicity. Chem Asian J. 2017;12:1062–1068. doi: 10.1002/asia.201700058. [DOI] [PubMed] [Google Scholar]
- 100.Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol. 2015;83:111–124. doi: 10.1016/j.fct.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 101.He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20:9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin C-F, Yu K-H, Jheng C-P, Chung R, Lee C-I. Curcumin reduces amyloid fibrillation of prion protein and decreases reactive oxidative stress. Pathogens. 2013;2:506–519. doi: 10.3390/pathogens2030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spinelli KJ, Osterberg VR, Meshul CK, Soumyanath A, Unni VK. Curcumin treatment improves motor behavior in α-synuclein transgenic mice. PLoS One. 2015;10:e0128510. doi: 10.1371/journal.pone.0128510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yanagisawa D, Shirai N, Amatsubo T, Taguchi H, Hirao K, Urushitani M, Morikawa S, Inubushi T, Kato M, Kato F, Morino K, Kimura H, Nakano I, Yoshida C, Okada T, Sano M, Wada Y, Wada K, Yamamoto A, Tooyama I. Relationship between the tautomeric structures of curcumin derivatives and their Abeta-binding activities in the context of therapies for Alzheimer’s disease. Biomaterials. 2010;31:4179–4185. doi: 10.1016/j.biomaterials.2010.01.142. [DOI] [PubMed] [Google Scholar]
- 105.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen P, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 106.Rao PPN, Mohamed T, Teckwani K, Tin G. Curcumin binding to beta amyloid: a computational study. Chem Biol Drug Des. 2015;86:813–820. doi: 10.1111/cbdd.12552. [DOI] [PubMed] [Google Scholar]
- 107.Baum L, Lam CWK, Cheung SK-K, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu HFK, Goggins WB, Zee BCY, Cheng KF, Fong CYS, Wong A, Mok H, Chow MSS, Ho PC, Ip SP, Ho CS, Yu XW, Lai CYL, Chan M-H, Szeto S, Chan IHS, Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 108.Pandey N, Strider J, Nolan WC, Yan SX, Galvin JE. Curcumin inhibits aggregation of alpha-synuclein. Acta Neuropathol. 2008;115:479–489. doi: 10.1007/s00401-007-0332-4. [DOI] [PubMed] [Google Scholar]
- 109.Siddique YH, Naz F, Jyoti S. Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson’s disease. Biomed Res Int. 2014;2014:606928. doi: 10.1155/2014/606928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Daval M, Bedrood S, Gurlo T, Huang C-J, Costes S, Butler PC, Langen R. The effect of curcumin on human islet amyloid polypeptide misfolding and toxicity. Amyloid. 2010;17:118–128. doi: 10.3109/13506129.2010.530008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sparks S, Liu G, Robbins KJ, Lazo ND. Curcumin modulates the self-assembly of the islet amyloid polypeptide by disassembling α-helix. Biochem Biophys Res Commun. 2012;422:551–555. doi: 10.1016/j.bbrc.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 112.Caughey B, Raymond LD, Raymond GJ, Maxson L, Silveira J, Baron GS. Inhibition of protease-resistant prion protein accumulation in vitro by curcumin. J Virol. 2003;77:5499–5502. doi: 10.1128/JVI.77.9.5499-5502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hafner-Bratkovic I, Gaspersic J, Smid LM, Bresjanac M, Jerala R. Curcumin binds to the alpha-helical intermediate and to the amyloid form of prion protein: a new mechanism for the inhibition of PrP(Sc) accumulation. J Neurochem. 2008;104:1553–1564. doi: 10.1111/j.1471-4159.2007.05105.x. [DOI] [PubMed] [Google Scholar]
- 114.Saelices L, Johnson LM, Liang WY, Sawaya MR, Cascio D, Ruchala P, Whitelegge J, Jiang L, Riek R, Eisenberg DS. Uncovering the mechanism of aggregation of human transthyretin. J Biol Chem. 2015;290:28932–28943. doi: 10.1074/jbc.M115.659912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ferreira N, Santos SAO, Domingues MRM, Saraiva MJ, Almeida MR. Dietary curcumin counteracts extracellular transthyretin deposition: insights on the mechanism of amyloid inhibition. Biochim Biophys Acta Mol Basis Dis. 2013;1832:39–45. doi: 10.1016/j.bbadis.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 116.Ahsan N, Mishra S, Jain MK, Surolia A, Gupta S. Curcumin pyrazole and its derivative (N-(3-nitrophenylpyrazole) curcumin inhibit aggregation, disrupt fibrils and modulate toxicity of wild type and mutant α-synuclein. Sci Rep. 2015;5:9862. doi: 10.1038/srep09862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Del Rio D, Stewart AJ, Mullen W, Burns J, Lean MEJ, Brighenti F, Crozier A. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J Agric Food Chem. 2004;52:2807–2815. doi: 10.1021/jf0354848. [DOI] [PubMed] [Google Scholar]
- 118.Liu Y, Pukala TL, Musgrave IF, Williams DM, Dehle FC, Carver JA. Gallic acid is the major component of grape seed extract that inhibits amyloid fibril formation. Bioorg Med Chem Lett. 2013;23:6336–6340. doi: 10.1016/j.bmcl.2013.09.071. [DOI] [PubMed] [Google Scholar]
- 119.Liu Y, Carver JA, Calabrese AN, Pukala TL. Gallic acid interacts with α-synuclein to prevent the structural collapse necessary for its aggregation. Biochim Biophys Acta. 2014;1844:1481–1485. doi: 10.1016/j.bbapap.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 120.Jayamani J, Shanmugam G. Gallic acid, one of the components in many plant tissues, is a potential inhibitor for insulin amyloid fibril formation. Eur J Med Chem. 2014;85:352–358. doi: 10.1016/j.ejmech.2014.07.111. [DOI] [PubMed] [Google Scholar]
- 121.Fujiwara H, Tabuchi M, Yamaguchi T, Iwasaki K, Furukawa K, Sekiguchi K, Ikarashi Y, Kudo Y, Higuchi M, Saido TC, Maeda S, Takashima A, Hara M, Yaegashi N, Kase Y, Arai H. A traditional medicinal herb Paeonia suffruticosa and its active constituent 1,2,3,4,6-penta-O-galloyl-beta-d-glucopyranose have potent anti-aggregation effects on Alzheimer’s amyloid beta proteins in vitro and in vivo. J Neurochem. 2009;109:1648–1657. doi: 10.1111/j.1471-4159.2009.06069.x. [DOI] [PubMed] [Google Scholar]
- 122.Bruno E, Pereira C, Roman KP, Takiguchi M, Kao P-Y, Nogaj LA, Moffet DA. IAPP aggregation and cellular toxicity are inhibited by 1,2,3,4,6-penta-O-galloyl-β-d-glucose. Amyloid. 2013;20:34–38. doi: 10.3109/13506129.2012.762761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brunhofer G, Fallarero A, Karlsson D, Batista-Gonzalez A, Shinde P, Gopi Mohan C, Vuorela P. Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: the case of chelerythrine. Bioorgan Med Chem. 2012;20:6669–6679. doi: 10.1016/j.bmc.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 124.Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291X(90)91544-3. [DOI] [PubMed] [Google Scholar]
- 125.Kim T, Hinton DJ, Choi D. Protein kinase C-regulated Aβ production and clearance. Int J Alzheimers Dis. 2011;2011:857368. doi: 10.4061/2011/857368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ma W, Zheng WH, Belanger S, Kar S, Quirion R. Effects of amyloid peptides on cell viability and expression of neuropeptides in cultured rat dorsal root ganglion neurons: a role for free radicals and protein kinase C. Eur J Neurosci. 2001;13:1125–1135. doi: 10.1046/j.1460-9568.2001.01475.x. [DOI] [PubMed] [Google Scholar]
- 127.Ba F, Pang PKT, Benishin CG. The establishment of a reliable cytotoxic system with SK-N-SH neuroblastoma cell culture. J Neurosci Methods. 2003;123:11–22. doi: 10.1016/S0165-0270(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 128.Pitt J, Roth W, Lacor P, Smith AB, Blankenship M, Velasco P, De Felice F, Breslin P, Klein WL. Alzheimer’s-associated Aβ oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol Appl Pharmacol. 2009;240:189–197. doi: 10.1016/j.taap.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li W, Sperry JB, Crowe A, Trojanowski JQ, Smith AB, Lee VMY. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. J Neurochem. 2009;110:1339–1351. doi: 10.1111/j.1471-4159.2009.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qosa H, Batarseh YS, Mohyeldin MM, El Sayed KA, Keller JN, Kaddoumi A. Oleocanthal enhances amyloid-β clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS Chem Neurosci. 2015;6:1849–1859. doi: 10.1021/acschemneuro.5b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Galanakis PA, Bazoti FN, Bergquist J, Markides K, Spyroulias GA, Tsarbopoulos A. Study of the interaction between the amyloid beta peptide (1–40) and antioxidant compounds by nuclear magnetic resonance spectroscopy. Biopolymers. 2011;96:316–327. doi: 10.1002/bip.21558. [DOI] [PubMed] [Google Scholar]
- 132.Kostomoiri M, Fragkouli A, Sagnou M, Skaltsounis LA, Pelecanou M, Tsilibary EC, Tzinia AK. Oleuropein, an anti-oxidant polyphenol constituent of olive promotes α-secretase cleavage of the amyloid precursor protein (AβPP) Cell Mol Neurobiol. 2013;33:147–154. doi: 10.1007/s10571-012-9880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Daccache A, Lion C, Sibille N, Gerard M, Slomianny C, Lippens G, Cotelle P. Oleuropein and derivatives from olives as Tau aggregation inhibitors. Neurochem Int. 2011;58:700–707. doi: 10.1016/j.neuint.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 134.Rigacci S, Guidotti V, Bucciantini M, Nichino D, Relini A, Berti A, Stefani M. Aβ(1-42) aggregates into non-toxic amyloid assemblies in the presence of the natural polyphenol oleuropein aglycon. Curr Alzheimer Res. 2011;8:841–852. doi: 10.2174/156720511798192682. [DOI] [PubMed] [Google Scholar]
- 135.Luccarini I, Grossi C, Rigacci S, Coppi E, Pugliese AM, Pantano D, la Marca G, Ed Dami T, Berti A, Stefani M, Casamenti F. Oleuropein aglycone protects against pyroglutamylated-3 amyloid-Δ toxicity: biochemical, epigenetic and functional correlates. Neurobiol Aging. 2015;36:648–663. doi: 10.1016/j.neurobiolaging.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 136.Diomede L, Rigacci S, Romeo M, Stefani M, Salmona M. Oleuropein aglycone protects transgenic C. elegans strains expressing Aβ42 by reducing plaque load and motor deficit. PLoS One. 2013 doi: 10.1371/journal.pone.0058893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grossi C, Rigacci S, Ambrosini S, Ed Dami T, Luccarini I, Traini C, Failli P, Berti A, Casamenti F, Stefani M. The polyphenol oleuropein aglycone protects TgCRND8 mice against Aß plaque pathology. PLoS One. 2013;8:e71702. doi: 10.1371/journal.pone.0071702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grossi C, EdDami T, Rigacci S, Stefani M, Luccarini I, Casamenti F. Employing Alzheimer disease animal models for translational research: focus on dietary components. Neurodegener Dis. 2014;13:131–134. doi: 10.1159/000355461. [DOI] [PubMed] [Google Scholar]
- 139.Rigacci S, Guidotti V, Bucciantini M, Parri M, Nediani C, Cerbai E, Stefani M, Berti A. Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J Nutr Biochem. 2010;21:726–735. doi: 10.1016/j.jnutbio.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 140.Palazzi L, Bruzzone E, Bisello G, Leri M, Stefani M, Bucciantini M, Polverino de Laureto P. Oleuropein aglycone stabilizes the monomeric α-synuclein and favours the growth of non-toxic aggregates. Sci Rep. 2018;8:8337. doi: 10.1038/s41598-018-26645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Leri M, Nosi D, Natalello A, Porcari R, Ramazzotti M, Chiti F, Bellotti V, Doglia SM, Stefani M, Bucciantini M. The polyphenol oleuropein aglycone hinders the growth of toxic transthyretin amyloid assemblies. J Nutr Biochem. 2016;30:153–166. doi: 10.1016/j.jnutbio.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 142.Bieschke J, Herbst M, Wiglenda T, Friedrich RP, Boeddrich A, Schiele F, Kleckers D, Lopez Del Amo JM, Grüning BA, Wang Q, Schmidt MR, Lurz R, Anwyl R, Schnoegl S, Fändrich M, Frank RF, Reif B, Günther S, Walsh DM, Wanker EE. Small-molecule conversion of toxic oligomers to nontoxic β-sheet-rich amyloid fibrils. Nat Chem Biol. 2011;8:93–101. doi: 10.1038/nchembio.719. [DOI] [PubMed] [Google Scholar]
- 143.Lam HT, Graber MC, Gentry KA, Bieschke J. Stabilization of α-synuclein fibril clusters prevents fragmentation and reduces seeding activity and toxicity. Biochemistry. 2016;55:675–685. doi: 10.1021/acs.biochem.5b01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gao M, Estel K, Seeliger J, Friedrich RP, Dogan S, Wanker EE, Winter R, Ebbinghaus S. Modulation of human IAPP fibrillation: cosolutes, crowders and chaperones. Phys Chem Chem Phys. 2015;17:8338–8348. doi: 10.1039/C4CP04682J. [DOI] [PubMed] [Google Scholar]
- 145.Ladiwala ARA, Lin JC, Bale SS, Marcelino-Cruz AM, Bhattacharya M, Dordick JS, Tessier PM. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid Abeta into off-pathway conformers. J Biol Chem. 2010;285:24228–24237. doi: 10.1074/jbc.M110.133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ge JF, Qiao JP, Qi CC, Wang CW, Zhou JN. The binding of resveratrol to monomer and fibril amyloid beta. Neurochem Int. 2012;61:1192–1201. doi: 10.1016/j.neuint.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 147.Feng Y, Wang XP, Yang SG, Wang YJ, Zhang X, Du XT, Sun XX, Zhao M, Huang L, Liu RT. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology. 2009;30:986–995. doi: 10.1016/j.neuro.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 148.He X, Li Z, Rizak JD, Wu S, Wang Z, He R, Su M, Qin D, Wang J, Hu X. Resveratrol attenuates formaldehyde induced hyperphosphorylation of tau protein and cytotoxicity in N2a cells. Front: Neuroscience; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 151.Wang R, Zhang Y, Li J, Zhang C. Resveratrol ameliorates spatial learning memory impairment induced by Aβ1-42 in rats. Neuroscience. 2017;344:39–47. doi: 10.1016/j.neuroscience.2016.08.051. [DOI] [PubMed] [Google Scholar]
- 152.Porquet D, Griñán-Ferré C, Ferrer I, Camins A, Sanfeliu C, Del Valle J, Pallàs M. Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer’s disease. J Alzheimers Dis. 2014;42:1209–1220. doi: 10.3233/JAD-140444. [DOI] [PubMed] [Google Scholar]
- 153.Zhao HF, Li N, Wang Q, Cheng XJ, Li XM, Liu TT. Resveratrol decreases the insoluble Aβ1-42 level in hippocampus and protects the integrity of the blood-brain barrier in AD rats. Neuroscience. 2015;310:641–649. doi: 10.1016/j.neuroscience.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 154.Yao Y, Li J, Niu Y, Yu J, Yan L, Miao Z, Zhao X, Li Y, Yao W, Zheng P, Li W. Resveratrol inhibits oligomeric Abeta-induced microglial activation via NADPH oxidase. Mol: Med Rep; 2015. [DOI] [PubMed] [Google Scholar]
- 155.Huang TC, Lu KT, Wo YYP, Wu YJ, Yang YL. Resveratrol protects rats from Aβ-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS One. 2011;6:e29102. doi: 10.1371/journal.pone.0029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Loureiro JA, Andrade S, Duarte A, Neves AR, Queiroz JF, Nunes C, Sevin E, Fenart L, Gosselet F, Coelho MAN, Pereira MC, Latruffe N. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules. 2017;22:E277. doi: 10.3390/molecules22020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Köbe T, Witte AV, Schnelle A, Tesky VA, Pantel J, Schuchardt JP, Hahn A, Bohlken J, Grittner U, Flöel A. Impact of resveratrol on glucose control, hippocampal structure and connectivity, and memory performance in patients with mild cognitive impairment. Front Neurosci. 2017 doi: 10.3389/fnins.2017.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Turner RS, Thomas RG, Craft S, Van Dyck CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA, Raman R, Aisen PS. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pithadia A, Brender JR, Fierke CA, Ramamoorthy A. Inhibition of IAPP aggregation and toxicity by natural products and derivatives. J Diabetes Res. 2016;2016:2046327. doi: 10.1155/2016/2046327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Q, Ning L, Niu Y, Liu H, Yao X. Molecular mechanism of the inhibition and remodeling of human islet amyloid polypeptide (hIAPP(1–37)) oligomer by resveratrol from molecular dynamics simulation. J Phys Chem B. 2015;119:15–24. doi: 10.1021/jp507529f. [DOI] [PubMed] [Google Scholar]
- 161.Lolicato F, Raudino A, Milardi D, La Rosa C. Resveratrol interferes with the aggregation of membrane-bound human-IAPP: a molecular dynamics study. Eur J Med Chem. 2015;92:876–881. doi: 10.1016/j.ejmech.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 162.Tu LH, Young LM, Wong AG, Ashcroft AE, Radford SE, Raleigh DP. Mutational analysis of the ability of resveratrol to inhibit amyloid formation by islet amyloid polypeptide: critical evaluation of the importance of aromatic-inhibitor and histidine-inhibitor interactions. Biochemistry. 2015;54:666–676. doi: 10.1021/bi501016r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nedumpully-Govindan P, Kakinen A, Pilkington EH, Davis TP, Ke PC, Ding F. Stabilizing off-pathway oligomers by polyphenol nanoassemblies for IAPP aggregation inhibition. Sci Rep. 2016;6:19463. doi: 10.1038/srep19463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wei L, Jiang P, Xu W, Li H, Zhang H, Yan L, Chan-Park MB, Liu XW, Tang K, Mu Y, Pervushin K. The molecular basis of distinct aggregation pathways of islet amyloid polypeptide. J Biol Chem. 2011;286:6291–6300. doi: 10.1074/jbc.M110.166678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiang P, Li W, Shea JE, Mu Y. Resveratrol inhibits the formation of multiple-layered β-Sheet oligomers of the human islet amyloid polypeptide segment 22–27. Biophys J. 2011;100:1550–1558. doi: 10.1016/j.bpj.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mishra R, Sellin D, Radovan D, Gohlke A, Winter R. Inhibiting islet amyloid polypeptide fibril formation by the red wine compound resveratrol. ChemBioChem. 2009;10:445–449. doi: 10.1002/cbic.200800762. [DOI] [PubMed] [Google Scholar]
- 167.Sciacca MFM, Chillemi R, Sciuto S, Greco V, Messineo C, Kotler SA, Lee DK, Brender JR, Ramamoorthy A, La Rosa C, Milardi D. A blend of two resveratrol derivatives abolishes hIAPP amyloid growth and membrane damage. Biochim Biophys Acta Biomembr. 2018 doi: 10.1016/j.bbamem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 168.Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Néri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- 169.Klabunde T, Petrassi HM, Oza VB, Raman P, Kelly JW, Sacchettini JC. Rational design of potent human transthyretin amyloid disease inhibitors. Nat Struct Biol. 2000;7:312–321. doi: 10.1038/74082. [DOI] [PubMed] [Google Scholar]
- 170.Akasaki Y, Reixach N, Matsuzaki T, Alvarez-Garcia O, Olmer M, Iwamoto Y, Buxbaum JN, Lotz MK. Transthyretin deposition in articular cartilage: a novel mechanism in the pathogenesis of osteoarthritis. Arthritis Rheumatol. 2015;67:2097–2107. doi: 10.1002/art.39178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 172.Yamada M, Ono K, Hamaguchi T, Noguchi-Shinohara M. Natural phenolic compounds as therapeutic and preventive agents for cerebral amyloidosis. Adv Exp Med Biol. 2015;863:79–95. doi: 10.1007/978-3-319-18365-7_4. [DOI] [PubMed] [Google Scholar]
- 173.Ono K, Li L, Takamura Y, Yoshiike Y, Zhu L, Han F, Mao X, Ikeda T, Takasaki JI, Nishijo H, Takashima A, Teplow DB, Zagorski MG, Yamada M. Phenolic compounds prevent amyloid β-protein oligomerization and synaptic dysfunction by site specific binding. J Biol Chem. 2012;287:14631–14643. doi: 10.1074/jbc.M111.325456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Iuvone T, De Filippis D, Esposito G, Amico AD, Izzo AA, D’Amico A. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-beta peptide-induced neurotoxicity. J Pharmacol Exp Ther. 2006;317:1143–1149. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- 175.Lee AY, Hwang BR, Lee MH, Lee S, Cho EJ. Perilla frutescens var. japonica and rosmarinic acid improve amyloid-β25–35 induced impairment of cognition and memory function. Nutr Res Pract. 2016;10:274–281. doi: 10.4162/nrp.2016.10.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hamaguchi T, Ono K, Murase A, Yamada M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-beta aggregation pathway. Am J Pathol. 2009;175:2557–2565. doi: 10.2353/ajpath.2009.090417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ramazzotti M, Melani F, Marchi L, Mulinacci N, Gestri S, Tiribilli B, Degl’Innocenti D. Mechanisms for the inhibition of amyloid aggregation by small ligands. Biosci Rep. 2016;36:e00385. doi: 10.1042/BSR20160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shariatizi S, Meratan AA, Ghasemi A, Nemat-Gorgani M. Inhibition of amyloid fibrillation and cytotoxicity of lysozyme fibrillation products by polyphenols. Int J Biol Macromol. 2015;80:95–106. doi: 10.1016/j.ijbiomac.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 179.Rashidi AA, Mirhashemi SM, Taghizadeh M, Sarkhail P. Iranian medicinal plants for diabetes mellitus: a systematic review. Pakistan J Biol Sci PJBS. 2013;16:401–411. doi: 10.3923/pjbs.2013.401.411. [DOI] [PubMed] [Google Scholar]
- 180.Zheng Q, Lazo LD. Mechanistic studies of the inhibition of insulin fibril formation by rosmarinic acid. J Phys Chem B. 2018;122:2323–2331. doi: 10.1021/acs.jpcb.8b00689. [DOI] [PubMed] [Google Scholar]
- 181.Ono K, Hasegawa K, Naiki H, Yamada M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s beta-amyloid fibrils in vitro. Biochim Biophys Acta. 2004;1690:193–202. doi: 10.1016/j.bbadis.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 182.Kocisko DA, Engel AL, Harbuck K, Arnold KM, Olsen EA, Raymond LD, Vilette D, Caughey B. Comparison of protease-resistant prion protein inhibitors in cell cultures infected with two strains of mouse and sheep scrapie. Neurosci Lett. 2005;388:106–111. doi: 10.1016/j.neulet.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 183.Kocisko DA, Baron GS, Rubenstein R, Chen J, Kuizon S, Caughey B. New inhibitors of scrapie-associated prion protein formation in a Library of 2,000 drugs and natural products. J Virol. 2003;77:10288–10294. doi: 10.1128/JVI.77.19.10288-10294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wolfe LL, Kocisko DA, Caughey B, Miller MW. Assessment of prospective preventive therapies for chronic wasting disease in Mule Deer. J Wildl Dis. 2012;48:530–533. doi: 10.7589/0090-3558-48.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang Y, Jiang P, Ye M, Kim SH, Jiang C, Lü J. Tanshinones: sources, pharmacokinetics and anti-cancer activities. Int J Mol Sci. 2012;13:13621–13666. doi: 10.3390/ijms131013621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang Q, Yu X, Patal K, Hu R, Chuang S, Zhang G, Zheng J. Tanshinones inhibit amyloid aggregation by amyloid-β peptide, disaggregate amyloid fibrils, and protect cultured cells. ACS Chem Neurosci. 2013;4:1004–1015. doi: 10.1021/cn400051e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mei Z, Yan P, Situ B, Mou Y, Liu P. Cryptotanshinione inhibits β-amyloid aggregation and protects damage from β-amyloid in SH-SY5Y cells. Neurochem Res. 2012;37:622–628. doi: 10.1007/s11064-011-0652-6. [DOI] [PubMed] [Google Scholar]
- 188.Ji K, Zhao Y, Yu T, Wang Z, Gong H, Yang X, Liu Y, Huang K. Inhibition effects of tanshinone on the aggregation of α-synuclein. Food Funct. 2016;7:409–416. doi: 10.1039/C5FO00664C. [DOI] [PubMed] [Google Scholar]
- 189.Maione F, Piccolo M, De Vita S, Chini MG, Cristiano C, De Caro C, Lippiello P, Miniaci MC, Santamaria R, Irace C, De Feo V, Calignano A, Mascolo N, Bifulco G. Down regulation of pro-inflammatory pathways by tanshinone IIA and cryptotanshinone in a non-genetic mouse model of Alzheimer’s disease. Pharmacol Res. 2018;129:482–490. doi: 10.1016/j.phrs.2017.11.018. [DOI] [PubMed] [Google Scholar]