Abstract
The crosstalk between prokaryotic bacteria and eukaryotic gut epithelial cells has opened a new field for research. Quorum sensing system (QS) molecules employed by gut microbiota may play an essential role in host–microbial symbioses of the gut. Recent studies on the gut microbiome will unveil evolved mechanisms of the host to affect bacterial QS and shape bacterial composition. Bacterial autoinducers (AIs) could talk to the host’s gut by eliciting proinflammatory effects and modulating the activities of T lymphocyte, macrophage, dendritic cells, and neutrophils. In addition, the gut mucosa could interfere with bacterial AIs by degrading them or secreting AI mimics. Moreover, bacterial AIs and gut hormones epinephrine and noradrenaline may be interchangeable in the crosstalk between the microbiota and human gut. Therefore, inter-kingdom signaling between gut microbiota and host may provide a novel target in the management of gut microbiota-related conditions or diseases in the future.
Keywords: Acylhomoserine lactone, Intestinal microflora, Mimic, Mucosa, Tumor immunity
Introduction
Microbiota contribute a lot to human beings, especially in the gut, where one hundred billion microorganisms reside [1]. In the gastrointestinal tract, the quantity of gut microbiota far exceeds the number of intestinal epithelium cells by one order of magnitude [2]. The commensal relationship between gut microbiota and intestinal cells is of extreme benefit to both partners [3]. Communication between microbiota and host is very important for homeostasis of host physiology, the responses to environmental change, and host survival [4]. There is growing evidence showing that changes in the composition and function of intestinal bacteria are associated with human disease [5], including obesity [6], diabetes [7], colorectal cancer (CRC) [8, 9], neurogenic disease like Alzheimer’s disease [10], and atherosclerotic cardiovascular disease [11].
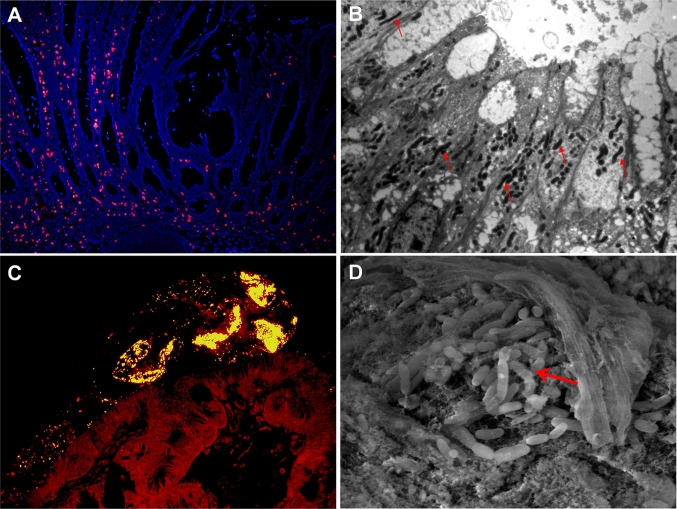
Gut microbiota can promote the maturation of the immune system [12], thereby playing a key role in establishing mucosal immunity [4, 13]. They have the ability to protect against colonization of pathogens by producing antimicrobial substances [14]. In addition, the metabolites derived from bacteria may act as important signals that mediate subsequent action of immune cells and the epithelial barrier in the intestine [15]. Some intestinal microbiota play an essential role in the initiation and development of gut tumors by invading the gut mucosa (Fig. 1a, b) [16]. However, the exact underlying mechanism of action between gut microbiota and the host intestine is currently not well understood.
Fig. 1.
Gut microbiota and colorectal tumor. Large number of invasive bacteria in a colon tumor detected by fluorescence in situ hybridization (FISH) (a) and transmission electron microscopy (TEM) (b). Bacterial biofilms on the surface of a colon adenoma detected by FISH (c) and scanning electron microscopy (SEM) (d). FISH, ×100 magnification; TEM, ×3000 magnification; SEM, ×7000 magnification
Modified from Ref. [35]
Prokaryotes signal through quorum sensing (QS), while eukaryotic cells communicate with each other through hormones [17]. Bacteria and host have co-evolved for billions of years, which have exposed prokaryotic bacteria to host hormones in the gut, and gut epithelial cells to the bacterial QS [2]. The crosstalk between prokaryotic bacteria and eukaryotic gut epithelial cells has opened a new field for research.
The quorum sensing system of gut microbiota
Employed by large numbers of bacteria, QS is an important mechanism of intercellular communication among both intra- and interspecies [18], which enables bacteria to act as a group rather than an individual cell [19]. QS plays an indispensable role in bacterial biofilm formation, antibiotic production, virulence, symbiosis, bacterial cell density, and gene expression of those bacteria [20].
QS is mediated by small diffusible signal molecules termed autoinducers (AIs). Many Gram-negative bacteria use acylhomoserine lactones [AHL; also called AI-1, mainly including N-3-oxo-dodecanoyl-l-homoserine lactone (3O-C12-HSL)] for intraspecies communication. Gram-positive bacteria utilize small peptides for intraspecies communication. AI-2 has been described as being shared by both Gram-positive and Gram-negative bacteria [21]. It is a nonspecies-specific AI that mediates the communication among interspecies [22]. AI-2 is a family of molecules, and a major signal-type molecule of QS, which is a derivative of 4,5-dihydroxy 2,3-pentanedione synthesized by AI-2 synthetase (LuxS) enzymes [23]. Its levels modulate the abundance of major phyla of intestinal microbiota [24]. In the mammalian gastrointestinal tract, AI-2 mediated communication among bacteria, thereby shaping the structure of the microbial community [24]. In the last few years, accumulated evidence has suggested that the AI-2 system is closely related with the pathogenicity of various microorganisms [25].
Autoinducers of gut microbiota elicit proinflammatory effects
Recent studies have shown that QS molecules play an important role in microbiota–host interactions [25, 26]. QS molecules can elicit responses in mammalian cells [17]. AI-1 are fatty-acid-based signaling molecules, and many lipid-based hormones such as eicosanoid families, lipidic and steroid hormones, are involved in hundreds of biological functions in eukaryotes [17]. They are chemically analogous and it is supposed that bacterial AIs can enter the host cell, regulating gene transcription [3].
AIs can activate inflammatory pathways of host cells [23, 27, 28]. It is reported that AI-1 injection induced inflammation by stimulating the expression of a number of cytokines including IL-1α and IL-6 in mice [29]. In HCT-8 colon cancer cells, interleukin-8 expression was regulated by AI-2 [28]. AI-2 synthetase (LuxS) has the ability to affect the host’s proinflammatory responses to Porphyromonas gingivalis, an anaerobic oral pathogen [30]. In another study, it was demonstrated that the proliferation of gingival epithelial cells was enhanced after treatment with dihydroxy-2,3-pentanedione (DPD) (the precursor of AI-2) [27]. In addition, AI-2 was involved in the process of Avian pathogenic Escherichia coli-induced cellular damage of chicken type II pneumocytes [31]. Together, these findings indicate that AIs are involved in the inflammatory processes of host cells as a response to a challenge with bacterial pathogens.
In recent studies, it was demonstrated that QS contributed to the formation of bacterial biofilm [32]. Bacterial biofilms are widely present on the root surface of human teeth, respiratory epithelial cells, the epithelial surface of patients with inflammatory bowel disease, and colorectal tumors [30, 32–34]. Colon mucosal biofilms may be associated with an increased risk for sporadic CRC (Fig. 1c, d) [34, 35]. Thus, it would be interesting to hypothesize that AIs produced by gut microbiota contribute to the inflammation and carcinogenesis of intestinal mucosa.
Autoinducers talking to the gut immune system
Gut contains the majority of immune cells in the body. The mucosal immune system provides immune surveillance against foreign pathogens [36]. Gut microbiota and host immune systems co-evolve during host’s lifespans [37]. Bacterial QS signaling has been demonstrated to be involved in immune responses in the host’s tissue [38, 39]. AI-1 was found to have immunomodulatory activities on T lymphocyte, macrophage and antibody responses in mammalian cells [40]. AI-1 has immune suppressive activity by inhibiting cytokine production and lymphocyte proliferation [41, 42]. It is reported that 3O-C12-HSL entered mammalian immune cells by passive mechanism; and decreased the ability of bone marrow-derived dendritic cells (BM-DCs) to induce T-cell proliferation and activation in vitro, thereby impaired the adaptive immune against bacterial pathogen [43, 44]. Moreover, 3O-C12-HSL has critical roles in the modulation of the host immune system by inducing cytotoxicity in macrophages and neutrophils [38]. 3O-C12-HSL was shown to attenuate innate immune responses via disruption of NF-kB signaling, thereby potentially maintaining persistent infection in host [45].
It is reasonable that eukaryotic hosts have receptors that sense bacterial AI molecules; however, the receptors remain to be identified [21]. Some AI peptides can signal through the epidermal growth factor receptor (EGFR), activating intracellular signaling cascade and leading to tumor metastasis [46]. It is hypothesized that the eukaryotic receptor for AI-1 is a nuclear hormone receptor (NHR), such as peroxisome proliferators-activated receptor (PPAR), a transcription factor-modulating the expression of proinflammatory genes [47]. Identifying the exact receptors and associated signaling cascade in the host gut responding to microbiota AIs will enhance our understanding of crosstalk mechanism between microbiota and their host gut.
Host responses to gut microbiota autoinducers
During long-term interaction between microorganisms and the host, a series of reactions of the host to inhabiting microorganisms may occur. The host’s immune system has control over the localization and community of resident microbiota by secreting antibacterial substance such as α-defensin [48]. What’s more, the host’s intestinal microenvironment could affect AI-2 activity, including pH, bile acid, temperature, osmotic pressure, and starvation [49]. This means that changes in the host intestinal conditions can affect AIs’ activity, and influence the density and structure of gut microbiota, thereby affecting the health of the host in return.
Moreover, host cells could have developed the abilities to disrupt QS signaling, fighting against bacterial infection [2]. For example, human airway epithelia can fight back AIs by degrading them by the production of paraoxonases [50, 51]. Interestingly, plants have mastered the art of secreting bacterial AI mimics and thus have the potential to lead to disrupt QS in associated bacteria [52]. It has recently been reported that mammalian epithelial cells of tumor produced AI-2 mimics, which have AI-2 activity [26]. Communication between host cells and bacteria occurs via generating AI-2 mimics by the epithelium, which affects gene expression of bacteria through the AI-2 pathway in return [26].
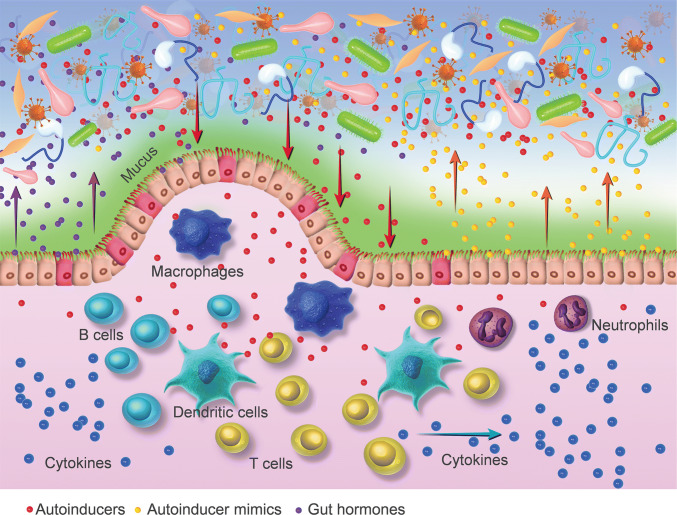
In addition, human hormones epinephrine and noradrenaline (NE) are abundantly present within the human gastrointestinal tract [53]. NE has been shown to promote bacterial growth [54]. AIs and hormones may be interchangeable in the crosstalk between prokaryotes and eukaryotes [55]. It is reported that AI-3 and epinephrine may signal through the same pathway. Epinephrine can mimic the activity of AI-3, and this effect is inhibited by adrenergic antagonists [56]. A recent study suggested that the mammalian stress hormone dynorphin released from the mice intestinal mucosa activated the QS signaling in an opportunistic pathogen P. aeruginosa, and enhanced its virulence [57]. Taken together, these findings provide evidences of crosstalk between host hormone signaling and bacterial QS system in the gut (Table 1; Fig. 2).
Table 1.
Signals and biological functions involved in crosstalk between bacteria and mammalian hosts
| Prokaryotic signals | Eukaryotic cell | Eukaryotic function | References | Eukaryotic signals | Prokaryotic function | References |
|---|---|---|---|---|---|---|
| 3O-C12-HSL | Skin cells | Stimulates the expression of cytokines including IL-1α and IL-6 | [29] | α-Defensin | Controls bacterial localization | [48] |
| 3O-C12-HSL | Macrophage, B cells, T cells, spleen cells | Modulates cell activities and antibody responses | [40] | Bile acid | Affects AI activity | [49] |
| 3O-C12-HSL | Lymphocytes | Inhibits cytokine production and lymphocyte proliferation | [41, 42] | Paraoxonases | Degrading AIs | [50, 51] |
| 3O-C12-HSL | BM-DCs | Decreases the ability of BM-DCs to induce T-cell proliferation and activation | [43, 44] | AI-2 mimics | Affect gene expression of bacteria | [26] |
| 3O-C12-HSL | Macrophages, neutrophils | Cytotoxicity | [38] | Noradrenaline | Promotes bacterial growth | [54] |
| 3O-C12-HSL | BM-DMs | Disruption of NF-kB signaling | [45] | Epinephrine | Mimic the activity of AI-3 | [56] |
| AI-2 | Colon cancer cells | Regulates Interleukin-8 expression | [28] | Dynorphin | Activates QS signaling and enhanced bacterial virulence | [57] |
| AI-2 | Pneumocytes | Cellular damage | [31] | |||
| DPD | Gingival epithelial cells | Enhances cell proliferation | [27] | |||
| AI peptides | Colon cancer cells | Tumor metastasis | [46] | |||
| AI-3 | HeLa cells | Produces AE lesions | [56] |
AE attaching and effacing, AI autoinducer, BM-DCs bone marrow-derived dendritic cells, BM-DMs bone marrow-derived macrophages, DPD dihydroxy-2,3-pentanedione (precursor of AI-2), QS quorum sensing
Fig. 2.
Inter-kingdom signaling between gut microbiota and their host. Autoinducers of gut microbiota talk to the host’s gut by eliciting proinflammatory effects and modulating the activities of T lymphocyte, macrophage, dendritic cells and neutrophils. The gut mucosa interferes with bacterial autoinducers by secreting autoinducer mimics. Bacterial autoinducers and gut hormones epinephrine and noradrenaline may be interchangeable in the crosstalk between the microbiota and human gut
Personal experience
We have demonstrated that Fusobacterium nucleatum (Fn) was enriched within CRC tissues and metastatic lymphnodes [35]. Furthermore, we have revealed that high abundance of Fn in CRC tissue is associated with a lower density of CD4+ T cells [58]. In addition, Fn had an immunosuppressive effect by promoting M2 polarization of macrophages in Fn-related CRCs [59].
Concluding remarks
Gut microbiota has recently been found to play an important role in many human diseases. QS is an important mechanism of intercellular communication among both intra- and interspecies of the gut microbiota. Coevolution of gut microbiota and host has exposed prokaryotic bacteria to host hormones, and gut epithelial cells to the bacterial AIs. The crosstalk between prokaryotic bacteria and eukaryotic gut cells has opened a new field for research. AIs of gut microbiota could elicit proinflammatory effects by stimulating the expression of a number of cytokines, and inducing cellular proliferation or damage of the host. Moreover, AIs could talk to the gut immune system by modulating the activities of T lymphocyte, macrophage, dendritic cells and neutrophils. However, the exact receptors of bacterial AIs and associated signaling cascade in the host gut remain to be unraveled. In addition, the epithelium and immune cells of the gut could fight back bacterial AIs by degrading them or secreting AI mimics. Interestingly, bacterial AIs and gut hormones may be interchangeable in the crosstalk between prokaryotes and eukaryotes. Therefore, inter-kingdom signaling between gut microbiota and host may provide a novel target in the management of gut microbiota-related conditions or diseases.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interests for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qing Li and Yixing Ren contributed equally to this work.
References
- 1.Wu Y, Wu J, Chen T, Li Q, Peng W, Li H, Tang X, Fu X. Fusobacterium nucleatum potentiates intestinal tumorigenesis in mice via a toll-like receptor 4/p21-activated kinase 1 cascade. Dig Dis Sci. 2018;63(5):1210–1218. doi: 10.1007/s10620-018-4999-2. [DOI] [PubMed] [Google Scholar]
- 2.Pacheco AR, Sperandio V. Inter-kingdom signaling: chemical language between bacteria and host. Curr Opin Microbiol. 2009;12(2):192–198. doi: 10.1016/j.mib.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiner EK, Rumbaugh KP, Williams SC. Inter-kingdom signaling: deciphering the language of acyl homoserine lactones. FEMS Microbiol Rev. 2005;29(5):935–947. doi: 10.1016/j.femsre.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Dzutsev A, Badger JH, Perez-Chanona E, Roy S, Salcedo R, Smith CK, Trinchieri G. Microbes and cancer. Annu Rev Immunol. 2017;35:199–228. doi: 10.1146/annurev-immunol-051116-052133. [DOI] [PubMed] [Google Scholar]
- 5.Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. 2017;18(11):690–699. doi: 10.1038/nrg.2017.63. [DOI] [PubMed] [Google Scholar]
- 6.Maruvada P, Leone V, Kaplan LM, Chang EB. The human microbiome and obesity: moving beyond associations. Cell Host Microbe. 2017;22(5):589–599. doi: 10.1016/j.chom.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott TA, Quintaneiro LM, Norvaisas P, Lui PP, Wilson MP, Leung KY, Herrera-Dominguez L, Sudiwala S, Pessia A, Clayton PT, et al. Host–microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell. 2017;169(3):442 e418–456 e418. doi: 10.1016/j.cell.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Peng Y, Yu J, Chen T, Wu Y, Shi L, Li Q, Wu J, Fu X. Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget. 2017;8(19):31802–31814. doi: 10.18632/oncotarget.15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ost KS, Round JL. A few good commensals: gut microbes use IFN-gamma to fight salmonella. Immunity. 2017;46(6):977–979. doi: 10.1016/j.immuni.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rook G, Bäckhed F, Levin BR, McFall-Ngai MJ, McLean AR. Evolution, human–microbe interactions, and life history plasticity. Lancet. 2017;390(10093):521–530. doi: 10.1016/S0140-6736(17)30566-4. [DOI] [PubMed] [Google Scholar]
- 15.Postler TS, Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 2017;26(1):110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williams HF, Rhodes JM. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology. 2004;127(1):80–93. doi: 10.1053/j.gastro.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 17.Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol. 2008;6(2):111–120. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiteley M, Diggle SP, Greenberg EP. Progress in and promise of bacterial quorum sensing research. Nature. 2017;551(7680):313–320. doi: 10.1038/nature24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaper JB, Sperandio V. Bacterial cell-to-cell signaling in the gastrointestinal tract. Infect Immun. 2005;73(6):3197–3209. doi: 10.1128/IAI.73.6.3197-3209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Gamez S, Sorg RA, Domenech A, Kjos M, Weissing FJ, van Doorn GS, Veening JW. Quorum sensing integrates environmental cues, cell density and cell history to control bacterial competence. Nat Commun. 2017;8(1):854. doi: 10.1038/s41467-017-00903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sintim HO, Smith JA, Wang J, Nakayama S, Yan L. Paradigm shift in discovering next-generation anti-infective agents: targeting quorum sensing, c-di-GMP signaling and biofilm formation in bacteria with small molecules. Fut Med Chem. 2010;2(6):1005–1035. doi: 10.4155/fmc.10.185. [DOI] [PubMed] [Google Scholar]
- 22.Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR. Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol. 2005;3(5):383–396. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- 23.Xavier KB, Bassler BL. Interference with AI-2-mediated bacterial cell–cell communication. Nature. 2005;437(7059):750–753. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson JA, Oliveira RA, Djukovic A, Ubeda C, Xavier KB. Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated gut microbiota. Cell Rep. 2015;10(11):1861–1871. doi: 10.1016/j.celrep.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 25.Galloway WR, Hodgkinson JT, Bowden SD, Welch M, Spring DR. Quorum sensing in Gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem Rev. 2011;111(1):28–67. doi: 10.1021/cr100109t. [DOI] [PubMed] [Google Scholar]
- 26.Ismail AS, Valastyan JS, Bassler BL. A host-produced autoinducer-2 mimic activates bacterial quorum sensing. Cell Host Microbe. 2016;19(4):470–480. doi: 10.1016/j.chom.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elmanfi S, Ma X, Sintim HO, Kononen E, Syrjanen S, Gursoy UK. Quorum-sensing molecule dihydroxy-2,3-pentanedione and its analogs as regulators of epithelial integrity. J Periodontal Res. 2018;53(3):414–421. doi: 10.1111/jre.12528. [DOI] [PubMed] [Google Scholar]
- 28.Zargar A, Quan DN, Carter KK, Guo M, Sintim HO, Payne GF, Bentley WE. Bacterial secretions of nonpathogenic Escherichia coli elicit inflammatory pathways: a closer investigation of interkingdom signaling. mBio. 2015;6(2):e00025. doi: 10.1128/mBio.00025-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith RS, Harris SG, Phipps R, Iglewski B. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J Bacteriol. 2002;184(4):1132–1139. doi: 10.1128/jb.184.4.1132-1139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheres N, Lamont RJ, Crielaard W, Krom BP. LuxS signaling in Porphyromonas gingivalis-host interactions. Anaerobe. 2015;35(Pt A):3–9. doi: 10.1016/j.anaerobe.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Cui ZQ, Wu ZM, Fu YX, Xu DX, Guo X, Shen HQ, Wei XB, Yi PF, Fu BD. Autoinducer-2 of quorum sensing is involved in cell damage caused by avian pathogenic Escherichia coli. Microb Pathog. 2016;99:247–252. doi: 10.1016/j.micpath.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 32.Vidal JE, Howery KE, Ludewick HP, Nava P, Klugman KP. Quorum-sensing systems LuxS/autoinducer 2 and Com regulate Streptococcus pneumoniae biofilms in a bioreactor with living cultures of human respiratory cells. Infect Immun. 2013;81(4):1341–1353. doi: 10.1128/IAI.01096-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trier JS. Mucosal flora in inflammatory bowel disease: intraepithelial bacteria or endocrine epithelial cell secretory granules? Gastroenterology. 2002;123(3):955. doi: 10.1016/S0016-5085(02)70059-0. [DOI] [PubMed] [Google Scholar]
- 34.Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC, Borisy GG, Lazarev M, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci USA. 2014;111(51):18321–18326. doi: 10.1073/pnas.1406199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Chen Y, Fu X, Zhou X, Peng Y, Shi L, Chen T, Wu Y. Invasive Fusobacterium nucleatum may play a role in the carcinogenesis of proximal colon cancer through the serrated neoplasia pathway. Int J Cancer. 2016;139(6):1318–1326. doi: 10.1002/ijc.30168. [DOI] [PubMed] [Google Scholar]
- 36.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28(4):384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere JC, Standiford TJ, Ishiguro M, Yamaguchi K. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun. 2003;71(10):5785–5793. doi: 10.1128/IAI.71.10.5785-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiner EK, Terentyev D, Bryan A, Sennoune S, Martinez-Zaguilan R, Li G, Gyorke S, Williams SC, Rumbaugh KP. Pseudomonas aeruginosa autoinducer modulates host cell responses through calcium signalling. Cell Microbiol. 2006;8(10):1601–1610. doi: 10.1111/j.1462-5822.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 40.Telford G, Wheeler D, Williams P, Tomkins PT, Appleby P, Sewell H, Stewart GS, Bycroft BW, Pritchard DI. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect Immun. 1998;66(1):36–42. doi: 10.1128/iai.66.1.36-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritchie AJ, Yam AO, Tanabe KM, Rice SA, Cooley MA. Modification of in vivo and in vitro T- and B-cell-mediated immune responses by the Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone. Infect Immun. 2003;71(8):4421–4431. doi: 10.1128/IAI.71.8.4421-4431.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chhabra SR, Harty C, Hooi DS, Daykin M, Williams P, Telford G, Pritchard DI, Bycroft BW. Synthetic analogues of the bacterial signal (quorum sensing) molecule N-(3-oxododecanoyl)-l-homoserine lactone as immune modulators. J Med Chem. 2003;46(1):97–104. doi: 10.1021/jm020909n. [DOI] [PubMed] [Google Scholar]
- 43.Skindersoe ME, Zeuthen LH, Brix S, Fink LN, Lazenby J, Whittall C, Williams P, Diggle SP, Froekiaer H, Cooley M, et al. Pseudomonas aeruginosa quorum-sensing signal molecules interfere with dendritic cell-induced T-cell proliferation. FEMS Immunol Med Microbiol. 2009;55(3):335–345. doi: 10.1111/j.1574-695X.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- 44.Ritchie AJ, Whittall C, Lazenby JJ, Chhabra SR, Pritchard DI, Cooley MA. The immunomodulatory Pseudomonas aeruginosa signalling molecule N-(3-oxododecanoyl)-l-homoserine lactone enters mammalian cells in an unregulated fashion. Immunol Cell Biol. 2007;85(8):596–602. doi: 10.1038/sj.icb.7100090. [DOI] [PubMed] [Google Scholar]
- 45.Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Grauer DC, Lehmann M, Meijler MM, Janda KD, Ulevitch RJ. Modulation of gene expression via disruption of NF-kappaB signaling by a bacterial small molecule. Science. 2008;321(5886):259–263. doi: 10.1126/science.1156499. [DOI] [PubMed] [Google Scholar]
- 46.Wynendaele E, Verbeke F, D’Hondt M, Hendrix A, Van De Wiele C, Burvenich C, Peremans K, De Wever O, Bracke M, De Spiegeleer B. Crosstalk between the microbiome and cancer cells by quorum sensing peptides. Peptides. 2015;64:40–48. doi: 10.1016/j.peptides.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Jahoor A, Patel R, Bryan A, Do C, Krier J, Watters C, Wahli W, Li G, Williams SC, Rumbaugh KP. Peroxisome proliferator-activated receptors mediate host cell proinflammatory responses to Pseudomonas aeruginosa autoinducer. J Bacteriol. 2008;190(13):4408–4415. doi: 10.1128/JB.01444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeo S, Park H, Ji Y, Park S, Yang J, Lee J, Mathara JM, Shin H, Holzapfel W. Influence of gastrointestinal stress on autoinducer-2 activity of two Lactobacillus species. FEMS Microbiol Ecol. 2015;91(7):fiv065. doi: 10.1093/femsec/fiv065. [DOI] [PubMed] [Google Scholar]
- 50.Chun CK, Ozer EA, Welsh MJ, Zabner J, Greenberg EP. Inactivation of a Pseudomonas aeruginosa quorum-sensing signal by human airway epithelia. Proc Natl Acad Sci USA. 2004;101(10):3587–3590. doi: 10.1073/pnas.0308750101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teiber JF, Horke S, Haines DC, Chowdhary PK, Xiao J, Kramer GL, Haley RW, Draganov DI. Dominant role of paraoxonases in inactivation of the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-l-homoserine lactone. Infect Immun. 2008;76(6):2512–2519. doi: 10.1128/IAI.01606-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anolles G, Rolfe BG, Bauer WD. Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc Natl Acad Sci USA. 2003;100(3):1444–1449. doi: 10.1073/pnas.262672599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eisenhofer G, Aneman A, Friberg P, Hooper D, Fandriks L, Lonroth H, Hunyady B, Mezey E. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab. 1997;82(11):3864–3871. doi: 10.1210/jcem.82.11.4339. [DOI] [PubMed] [Google Scholar]
- 54.Lyte M, Frank CD, Green BT. Production of an autoinducer of growth by norepinephrine cultured Escherichia coli O157:H7. FEMS Microbiol Lett. 1996;139(2–3):155–159. doi: 10.1111/j.1574-6968.1996.tb08196.x. [DOI] [PubMed] [Google Scholar]
- 55.Williams SC, Patterson EK, Carty NL, Griswold JA, Hamood AN, Rumbaugh KP. Pseudomonas aeruginosa autoinducer enters and functions in mammalian cells. J Bacteriol. 2004;186(8):2281–2287. doi: 10.1128/JB.186.8.2281-2287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. Bacteria-host communication: the language of hormones. Proc Natl Acad Sci USA. 2003;100(15):8951–8956. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaborina O, Lepine F, Xiao G, Valuckaite V, Chen Y, Li T, Ciancio M, Zaborin A, Petrof EO, Turner JR, et al. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog. 2007;3(3):e35. doi: 10.1371/journal.ppat.0030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen T, Li Q, Zhang X, Long R, Wu Y, Wu J, Fu X. TOX expression decreases with progression of colorectal cancers and is associated with CD4 T-cell density and Fusobacterium nucleatum infection. Hum Pathol. 2018;79:93–101. doi: 10.1016/j.humpath.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Chen T, Li Q, Wu J, Wu Y, Peng W, Li H, Wang J, Tang X, Peng Y, Fu X. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol Immunother CII. 2018;67(10):1635–1646. doi: 10.1007/s00262-018-2233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]