Abstract
Mitochondria are cellular organelles of crucial importance, playing roles in cellular life and death. In certain cell types, such as neurons, mitochondria must travel long distances so as to meet metabolic demands of the cell. Mitochondrial movement is essentially microtubule (MT) based and is executed by two main motor proteins, Dynein and Kinesin. The organization of the cellular MT network and the identity of motors dictate mitochondrial transport. Tight coupling between MTs, motors, and the mitochondria is needed for the organelle precise localization. Two adaptor proteins are involved directly in mitochondria-motor coupling, namely Milton known also as TRAK, which is the motor adaptor, and Miro, which is the mitochondrial protein. Here, we discuss the active mitochondria transport process, as well as motor–mitochondria coupling in the context of MT organization in different cell types. We focus on mitochondrial trafficking in different cell types, specifically neurons, migrating cells, and polarized epithelial cells.
Keywords: Dynein, Kinesin, Microtubules, Milton, Miro, Mitochondria, Neurons
Introduction
Mitochondria are cellular organelles that serve essential functions in all eukaryotic cells. Most importantly, mitochondria supply energy for most cellular processes. Yet, beyond their primary role as the cellular “energy plant”, mitochondria are crucial for a diverse range of cellular processes, including proliferation [2], stress response [98], apoptosis [26], and Ca2+ homeostasis [62, 93, 102]. As such, precise mitochondrial positioning is extremely important for cells to properly meet their energy demands throughout the cellular life cycle and in response to changing environment conditions. Accordingly, mitochondrial localization must be tightly regulated in response to highly variable physiological cues in all cell types. Mitochondria are dynamic organelles that are subject to active directed transport [41, 43], states of pausing or active docking [48], and morphological rearrangements via the processes of fission (division) and fusion [52, 61, 76, 93]. Mitochondrial immobilization at the level of a local energy-demanding cell region is called mitochondrial docking or retention [48, 93]. Basically, the docking event can occur on actin or microtubule (MT) filaments. In budding yeast, as in other fungi and plants, mitochondrial transport is mainly actin based [31], although in higher eukaryotes, the role of the actin cytoskeleton remains unclear. At the same time, myosin motors are implicated in short-range mitochondrial movement and docking in actin-rich regions [39, 78, 93].
The long-distance transport of mitochondria relies on MT filaments and is executed by MT-based motor proteins, together with a myriad of diverse motor- and mitochondria-associated adaptor proteins. The direction of mitochondrial movement is basically dictated by the intrinsic polarity of the MT tracks and the motor proteins attached. In this review, we discuss long-range mitochondrial transport in the context of cell type-specific MT network organization.
MT organization in different cell types
Introduction to the MTs
MT polymers are composed of alpha- and beta-tubulin subunits attached in a head-to-tail fashion. This characteristic monomer organization gives rise to MT fiber polarity, reflected as plus- and minus-end orientation. MTs are known to undergo polymerization and rapid depolymerization events preferentially at their plus ends, a phenomenon known as dynamic instability [44, 63, 69]. In most cell types, MT nucleation takes place in the centrosome, which serves as a major MT-organizing center (MTOC) located near the nucleus [20]. There, the minus end of the elongating MT is “hidden” and stabilized, while the dynamic plus ends extend towards the cell periphery, resulting in a radial MT organization [8]. Thus, centrosome localization relative to nucleus establishes the intracellular polarity. MT nucleation relies on γ-tubulin, a homologue of α- and β-tubulin that is not incorporated into the growing MT fiber. γ-tubulin is found as a part of multi-subunit protein complex called γ-tubulin ring complex (γTuRC), which is enriched in the centrosome and provides a structural template for MT nucleation. Other than the centrosome, alternative MT nucleation sites are known. MT nucleation and anchoring can occur in association with non-centrosomal cellular structures, such as the Golgi apparatus, nucleus, and pre-existing MTs [99]. These alternative MT nucleation sites can co-exist with the prototypical centrosomal MT array, thus producing sub-sets of MT populations within the cell, each possessing different dynamic behaviors. Moreover, the formation of non-centrosomal arrays was shown to involve MT release from the centrosome and transport to the final cellular compartment, de novo polymerization at non-centrosomal nucleation sites or severing of existing MTs [58].
MT organization in neurons
The unique metabolic requirements of polarized cells depend on the developmental stage, as well as on their function. Morphologically complex neurons, responsible for signal conduction over long distances, are extremely vulnerable to even slight defects in mitochondrial dynamics, resulting in a wide spectrum of neurodegenerative diseases in mammals. Neurons present precisely defined cellular compartments, namely the cell body or soma, the axon, and dendrites (Fig. 1e, f). Each neuronal compartment has its own energetic and biosynthetic demands, such that precise mitochondrial positioning is fundamental. In young non-differentiated neurons, the centrosome acts as a MTOC, while later in development, the MTOC loses its function and MT nucleation becomes acentrosomal, instead relying on γ-tubulin and calmodulin-regulated spectrin-associated protein 2 (CAMSAP2), which stabilizes MT-free minus ends [19, 95, 116]. The elongating neurite growth cones contain MT bundles and a dense actin meshwork, both of which produce pulling forces that mediate membrane extension at the growth cone. Tight interactions exist between the peripheral MTs and the actin network that facilitates MT bundling in the axon shaft [32]. In the mature axon, most MTs are stable and are bundled such that they are organized in a parallel manner, with this MT population being resistant to MT-destabilizing drugs [5, 49]. Indeed, this increased MT stability is thought to be the basis for the establishment of preferential transport tracks and a point of initiation for structural differences between axon and dendrite [49]. In addition to highly stable populations, dynamic MTs are also present in neurons. The two populations are not, however, fully separated, as dynamic plus-end MTs may exist on a stable MT fiber, whereas the rest of the MT “wire” would be immune to depolymerization [49].
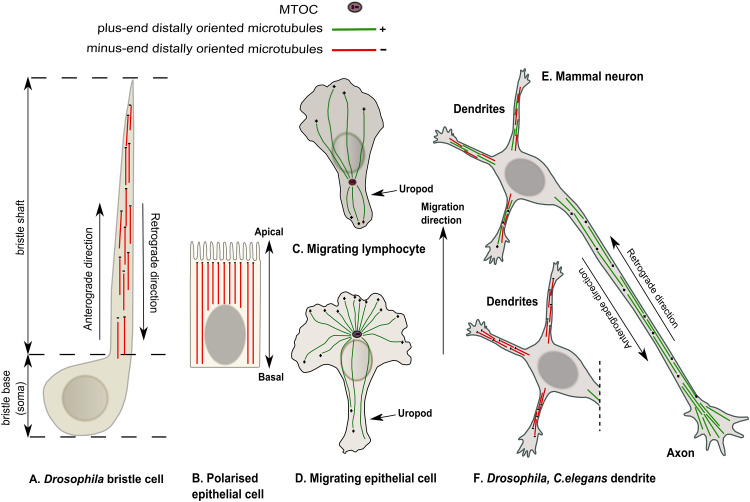
Fig. 1.
Microtubule organization in different cell types. a Drosophila bristle cell, b polarized epithelial cell, c migrating lymphocyte, d migrating epithelial cell, e mammalian neuron, f Drosophila and C. elegans dendrite. Plus- and minus-end MTs are marked in green and red, respectively. The Drosophila bristle contains a stable MT array oriented with the minus ends out within the bristle shaft (a). Epithelial cells contain an acentrosomal MT array with the minus ends oriented apically (b). Migrating lymphocytes exhibit a centrosome located at the rear edge of the cell (the uropod) (c). In migrating epithelial cells, centrosomal MT organization is seen, with the centrosome oriented towards the migration axis and MT plus ends facing the cell periphery (d). In mammalian, Drosophila, and C. elegans neurons, the axon displays an acentrosomal MT array oriented with the plus-ends out (i.e., away from soma) (e). However, whereas mammalian dendrites exhibit mixed MT polarity (e), in Drosophila and C. elegans, the dendritic MT network is uniformly oriented with the minus ends pointed distally (f)
Differentiated axons and dendrites exhibit different MT polarity patterns. In vertebrate axons, MTs are uniformly oriented with their plus ends facing out (i.e., with minus ends facing the soma), while dendrites contain MTs of mixed polarity, with equal numbers of plus- and minus-end-oriented MTs [49, 74, 115]. It was hypothesized that selective organization of uniformly oriented MT arrays in axons is critical for establishing neural polarization [106, 115]. In invertebrates, such as Drosophila and C. elegans, axons display uniform plus-end-out MT polarity similar to vertebrates, whereas in dendrites, MTs are organized exclusively with their minus ends being distal [96].
MT organization in migrating cells
Cell migration corresponds to the ability of a cell to move certain distances, and is an essential feature of many cellular types, such as neurons during the development, epithelial cells, white blood cells in an immune response, and cancer cells during metastasis and tissue invasion [91, 110]. In migrating cells, MTs form centrosomal arrays, resulting in radial MT organization with free plus ends facing the cellular membrane (Fig. 1c, d). The dynamic growth edge of the actively migrating cell is filled with a dense actin network, which is responsible for force generation during cell movement [15]. In the most cell types, MTs are excluded from the leading edge of this actin-enriched cellular protrusion [27, 65]. However, in migrating astrocytes and neurons, a few pioneer microtubules do enter cell protrusions, such as lamellipodia or filopodia. During migration, the cell needs to reorganize into defined sub-cellular compartments, i.e., the leading edge and the rear edge. The polarization of the MT cytoskeleton in migrating cells is crucial for directed movement and for defining sub-cellular compartments. During movement of epithelial cells and fibroblasts, the centrosome localizes towards the cellular migration axis in front of the nucleus, establishing network with high MT density oriented towards the leading edge [7]. In contrast, in leukocytes, the centrosome is localized to the cell rear (the uropod) and re-localizes rapidly together with the Golgi apparatus upon interaction with antigen-presenting cell towards the immunological synapse [7]. Plus-end polarization with respect to the axis of movement, together with unequal MT density, produces a strong bias in directional intracellular transport, reflected in the differential roles for minus- and plus-end-directed molecular motors.
MT organization in other non-neuronal polarized cells
In polarized epithelial cells, MTs are organized in acentrosomal parallel array aligned along the apico-basal axis with minus ends facing the apical membrane (Fig. 1b) [103]. This characteristic MT organization is conserved through different epithelial cell types, although the mechanism of the array formation varies in terms of organism and developmental stage [103]. In some polarized epithelial cells (e.g., Madin Darby canine kidney—MDCK cells), in addition to apical–basal linear MT array, distinct networks of short non-centrosomal MTs exist at the basal cortex that exhibit mixed polarity and dynamic growth patterns [83].
Another highly polarized non-neuronal cell model is the Drosophila bristle, which elongates to a length of nearly 450 μm during pupal development. Live-imaging in this cell is possible over long periods without any need for anesthesia; therefore, it is a valuable model for understanding polarized cell morphology development and long-distance transport. Bristle MTs contain two populations, with one being stable and uniformly oriented with minus ends pointing distally (Fig. 1a) (i.e., towards the bristle growing edge), and the second being dynamic and exhibiting mixed polarity [9].
MT-based mitochondrial transport machinery
MT plus-end-directed motors
The kinesin superfamily consists of three major groups, divided according to the motor domain being localized to the N-terminal, middle or C-terminal region [41, 42]. N-terminal KIFs are MT plus-end-directed motors, while C-terminal KIFs translocate cargo towards the MT minus end. To date, kinesin-1, KIF1Bα, and KLP6 are kinesin motors found to be directly involved in the transport of mitochondria.
The major MT plus-end-oriented motor protein involved in the transport of mitochondria is kinesin-1, also called conventional kinesin. Kinesin-1 appears as a heterotetrameric complex of ~380 kDa and comprises two kinesin heavy chains (KHC) and two kinesin light chains (KLC) [70]. Mammals possess three kinesin-1 genes (KIF5A, KIF5B, KIF5C). KIF5B is expressed ubiquitously, while KIF5A and KIF5B are predominantly expressed in neuronal tissues [47, 101]. Drosophila and C. elegans bear a single kinesin-1-encoding gene [46, 94].
KIF1B is a member of the kinesin-3 family and was first identified as a mitochondria-associated motor, being predominantly enriched in mouse brain and heart tissues [73]. The single KIF1B gene is expressed as two main splice variants, KIF1Bα and KIF1Bβ, which encode a common N-terminal sequence of 660 amino acids and completely different tail domains [34, 41, 112]. The motor domain of KIF1B is located in the N-terminal region and the motor is a MT plus-end-directed kinesin [41]. In addition, kinesin-like protein (KLP6), a member of the kinesin-3 family, was identified as being associated with mitochondria in C. elegans and subsequently in rat [100]. The mechano-chemical properties of kinesin-3 proteins are still not well characterized. KIF1B was initially identified as being monomeric but recent studies proposed that only dimers can undergo processive movement on MTs. Despite accumulating data on kinesin-3 motor activity, the actual mode of action remains controversial [109]. In this review we will thus focus solely on the role of kinesin-1 in terms of mitochondrial movement.
MT minus-end-directed motor in mitochondria transport
Dynein produces force toward the minus end of MTs [85, 105]. In vertebrates, approximately 15 forms of dyneins are found, most of which are axonemal and are involved in ciliary and flagellar movements; only two cytoplasmic forms are known [1, 18, 105]. Cytoplasmic dynein 1 is responsible for the majority of minus-end-directed MT-based movement. Each cytoplasmic dynein complex consists of two dynein heavy chains (DHC), two dynein intermediate chains (DIC), two dynein light–intermediate chains (DLIC), and a variable number of dynein light chains (DLC) [105, 113]. Dynactin is a large multi-subunit complex comprising at least seven polypeptide chains of 22–150 kDa in size, and is believed to be essential for most dynein functions. The largest dynactin subunit, p150/Glued, participates in motor binding and increasing dynein processivity; however, it must be bound to other dynactin subunits for proper function [92].
Motor adaptors
There are several motor–mitochondria adaptor proteins involved in the transport of mitochondria. In this review, we will focus mainly on the two well-characterized adaptors, namely Milton and Mitochondrial Rho-GTPase (Miro). Milton is a well-characterized kinesin-1 adaptor protein implicated in neuronal mitochondrial movement in Drosophila. Milton forms a complex with KHC and does not rely on KLC-dependent cargo sorting or binding [33, 84]. Milton is, moreover, linked indirectly to the mitochondria through an interaction with Miro, a mitochondrial Ca2+-binding membrane protein. Mammals contain two Milton orthologues (TRAK1 and TRAK2) and two ubiquitously expressed Miro orthologues, Miro1 and Miro2 [30, 93].
Long-distance mitochondrial transport
Mitochondrial transport in neurons
Mitochondria motility events can be basically divided into certain categories, such as active processive movement towards the MT plus- or minus-end characterized by long run lengths, the paused state, when mitochondria remain stationary for a certain period, short solitary movements or movements observed due to mitochondrial fission and fusion events. Mitochondria net movement from soma towards the axon/dendrite periphery is defined as anterograde movement, while the movement back to soma is defined as retrograde movement. Mitochondria in cultured hippocampal neurons show high organelle density in the soma but lower density in dendrites and axons. In both axons and dendrites, the mitochondria showed elongated tubular morphology parallel to the axis of the neuronal process. 3D reconstructions of electron microscope micrographs of thin serial sections of rat and squirrel neurons showed dendritic mitochondria extending up to 36 μm, although in axons, more discrete mitochondria extending to a length of 3 μm were found [80]. Additionally, only some 25–50% of mitochondria are motile in axons and dendrites [53]. While the overall net mitochondrial flow direction is similar in axons and in dendrites, axonal mitochondria show more dynamic motility pattern with longer run lengths than do those in the dendritic fraction [77]. The roles of MT motors and adaptor proteins in neuronal mitochondrial distribution and/or flux and/or movement are summarized in Tables 1, 2, and 3.
Table 1.
The roles of motor proteins on mitochondria transport in neurons
| Neuron compartment | Motor | Motor subunit | Organism | Cell type | Mobility (distribution/flux/movement) | Polarized sorting | References |
|---|---|---|---|---|---|---|---|
| Axon | Kinesin | KHC | Drosophila | Motor neuron | Reduced anterograde/retrograde flux | NT | [79] |
| KHC | Drosophila | Da neurons of dorsal clusters | Reduced distribution | NT | [89, 90] | ||
| KHC | Drosophila | Wing nerve | Reduced anterograde/retrograde movement | NT | [104] | ||
| KHC | Mammals (Mouse) | Embryonic motor neuron | No defects | NT | [50] | ||
| KHC | Mammals (Rat) | Primary hippocampal neurons | Reduced distribution/reduce anterograde movement | NT | [11] | ||
| KHC | Mammals (Rat) | Primary hippocampus neuron | Reduced anterograde/retrograde movement | Yes | [107] | ||
| KHC | Zebrafish | Peripheral sensory neurons | Reduced distribution | NT | [16] | ||
| KHC | Zebrafish | Retinal ganglion | Reduce distribution/increase in retrograde movement | NT | [4] | ||
| KHC | C. elegans | PHC sensory neurons | Reduced distribution/absent | No | [82, 114] | ||
| Dynein | DHC | Drosophila | Motor neuron | Reduced retrograde flux | NT | [79] | |
| DHC | Drosophila | Wing nerve | Reduced anterograde/retrograde movement | NT | [104] | ||
| Dynactin | Glued | Drosophila | Motor neuron | No defects | NT | [79] | |
| Arp | Drosophila | Larval segmental nerve | Reduced anterograde/retrograde movement | NT | [37] | ||
| P150 | Mammals | Primary hippocampus neuron | Reduced anterograde/retrograde movement | Yes | [107] | ||
| Actr10 | Zebrafish | Peripheral sensory | Reduced retrograde movement | NT | [25] | ||
| P150a/b | Zebrafish | Peripheral sensory | Reduced anterograde/retrograde movement | NT | [25] | ||
| Dendrite | Kinesin | KHC | Drosophila | da neurons of dorsal clusters/ | No defects | NT | [89, 90] |
| KHC | C. elegans | PHC sensory neurons | Absent | No | [114] | ||
| KHC | Mammals (Rat) | Hippocampal neuron | No defect | No | [107] | ||
| Dynein | Dlic | Drosophila | da neurons of dorsal clusters | Reduced distribution | NT | [89, 90] | |
| Dynactin | P150 | Mammals (rat) | Primary hippocampus neuron | Reduced anterograde/retrograde movement | Yes | [107] |
NT not tested
Table 2.
The roles of motor adaptor Milton/TRAK on neuronal mitochondrial transport
| Neuron compartment | Milton/TRAKs | Organism | Cell type | Associated motor | Distribution/flux/movement | Velocity | References |
|---|---|---|---|---|---|---|---|
| Axon | Milton | Drosophila | Photoreceptor axons | Kinesin-1 (KHC) | Terminals lack mitochondria | NT | [35, 97] |
| Milton | Drosophila | Motor and sensory axons | Kinesin-1 (KHC) | Absence of mitochondria | NT | [33] | |
| Milton | Drosophila | Wing axon | Kinesin-1 (KHC) | Absence of mitochondria | NT | [28] | |
| Milton | Drosophila | Segmental nerves | Kinesin-1 (KHC) | Reduction of moving mitochondria in retrograde direction | Reduced retrograde velocities | [118] | |
| TRAK2 | Mammals (rat) | Cultured hippocampal neurons | KIF5C | Reduction of moving mitochondria in antero- and retrograde direction | No defects | [10] | |
| TRAK1 | Mammals (rat) | Cultured hippocampal neurons | KIF5C | Reduction of moving mitochondria in antero- and retrograde direction | No defects | [10] | |
| TRAK2 | Mammals (rat) | Cultured hippocampal neurons | KIF5 dynein/dynactin via p150Glued | No effect | NT | [107] | |
| TRAK1 | Mammals (rat) | Cultured hippocampal neurons | KIF5 dynein/dynactin via p150Glued | Reduction of moving mitochondria | NT | [107] | |
| TRAK1 | Mammal (rat) | Cortical/hippocampal neurons | NT | Reduction of moving mitochondria with no effect on antero- and retrograde direction (affects throughout neuronal development) | No defects | [57] | |
| TRAK2 | Mammal (rat) | Cortical/hippocampal neurons | NT | Reduction of moving mitochondria with no effect on antero- and retrograde direction (mostly affects at early stages of neuronal development) | No defects | [57] | |
| Dendrite | TRAK1 | Mammals (rat) | Cultured hippocampal neurons | KIF5 (KHC) dynein/dynactin via p150Glued | Reduction of moving mitochondria in antero- and retrograde direction | NT | [107] |
| TRAK2 | Mammals (rat) | Cultured hippocampal neurons | KIF5 (KHC) dynein/dynactin via p150Glued | Reduction of moving mitochondria in antero- and retrograde direction | NT | [107] | |
| TRAK2 | Mammals (mice) | Pyramidal neurons | NT | Reduction of mitochondria density? | NT | [51] | |
| TRAK1 | Mammals (rat) | Cortical/hippocampal neurons | NT | Reduction of moving mitochondria with no effect on antero- and retrograde direction (mostly affects at early stages of neuronal development) | No defects | [57] | |
| TRAK2 | Mammals (rat) | Cortical/hippocampal neurons | NT | Reduction of moving mitochondria with no effect on antero- and retrograde direction (affects throughout neuronal development) | No defects | [57] |
Table 3.
The roles of mitochondrial adaptor protein Miro in neuronal transport of mitochondria
| Neuron compartment | Miro | Organism | Cell type | Distribution/flux/movement | Velocity | References |
|---|---|---|---|---|---|---|
| Axon | Miro | Drosophila | Larval segmental nerves, NMJ | Absence of mitochondria | NT | [36] |
| Miro | Drosophila | Larval motor neurons | Reduced distribution, decreased antero- and retrograde mitochondrial flux | Reduction in net velocity of antero- and retrograde movement | [86] | |
| Miro | Drosophila | Larval motor neurons | Reduced distribution, decreased antero- and retrograde mitochondrial flux | Reduction in net velocity of antero- and retrograde movement | [54] | |
| Miro | Drosophila | Larval motor neurons | Absence of mitochondria | Reduction in net velocity of antero- and retrograde movement | [6] | |
| Miro1 | Mammals (mouse) | Cortical neurons | Reduced distribution | Reduction in retrograde velocity | [75] | |
| Miro1 | Mammals (mouse) | Hippocampal neurons | Reduction of moving mitochondria in antero- and retrograde direction | Unaltered | [55] | |
| Miro1/2 | C. elegans | GABA neurons | Reduced mitochondrial density | NT | [38] | |
| Dendrite | Miro | Drosophila | Larval segmental nerves, NMJ | Absence of mitochondria | NT | [36] |
| Miro | Drosophila | Larval motor neurons | Absence of mitochondria | NT | [86] | |
| Miro1 | Mammals (rat) | Hippocampal neurons | Reduction of moving mitochondria | No defects | [59] | |
| Miro1 | Mammals (mouse) | Hippocampal neurons | Reduction of moving mitochondria in antero- and retrograde direction | Reduced by ~50% in the anterograde direction | [55] | |
| Miro2 | Mammals (mouse) | Hippocampal neurons | No significant affect | No significant affect | [55] |
MT motors involved in axonal mitochondrial transport
A role for kinesin-1 in axonal mitochondrial movement
Several studies revealed that altered kinesin-1 activity reduced the distribution of mitochondria in axons [4, 11, 16, 89, 114] (Table 1). More specifically, kinesin-1 affects both anterograde and retrograde mitochondrial movement in Drosophila motor and wing neurons [79, 104], rat primary hippocampal neurons [107]. In contrast, it was reported that kinesin-1 is required only for anterograde mitochondrial movement in primary hippocampal neurons [11]. Another report showed that interfering with kinesin-1 activity led to an increase in retrograde mitochondrial movement in zebrafish retinal ganglion axons [4]. As described above, differentiated axons contain MTs of uniform polarity, oriented with their plus ends out. In agreement with axon MT polarization and the results summarized above, kinesin-1 is widely considered to be the main motor for anterograde mitochondrial axonal transport. The fact that mutations in kinesin-1 also affect retrograde axonal mitochondrial transport [79, 104, 107] can be attributed to the influence of kinesin-1 on dynein-driven retrograde transport [79].
A role for dynein in axonal mitochondrial transport
Mutations in the DHC affect retrograde mitochondrial movement in Drosophila motor neuron axons [79], and in the axons of zebrafish peripheral sensory neurons [25]. However, in Drosophila wing nerve axons, dynein is required for both anterograde and retrograde mitochondrial movement [104]. In agreement with axon MT polarization and results cited here (Table 1), it seems that dynein drives the retrograde transport of mitochondria in axons.
A role for dynactin in axonal mitochondrial movement
In Drosophila and zebrafish, mutations in various dynactin subunits showed different defects in motor neurons. For instance, whereas mutation in Drosophila p150/Glued had no effect on mitochondrial mobility [79], mutations in another dynactin subunit, arp1, reduced both axonal anterograde and retrograde mitochondrial movement [37]. In zebrafish peripheral sensory axons, mutation in Actr10 reduced retrograde movement; however, mutations in p150a/b resulted in decreases in both axonal anterograde and retrograde mitochondrial movement [25]. There is only one report on the role of dynactin in mammals, showing that mutation in p150 led to decreases in both anterograde and retrograde movement in rat hippocampal neuron axons [107]. These observations raise several questions, such as why in Drosophila is there a difference between the functions of dynactin complex and dynein [79] and how different dynactin subunits influence axonal mitochondrial transport. Further studies are needed to elucidate the exact mechanism of regulation of the dynactin complex in mitochondrial movement.
Motor proteins involved in dendritic mitochondrial transport
There is sparse information on the role of MT motors in mitochondrial movement in dendrites. In rat hippocampal neurons, dynactin but not kinesin is required for dendritic anterograde and retrograde mitochondrial movement [107]. In Drosophila, mutations in dynein (DLIC) but not in kinesin-1 affect mitochondrial distribution [90]. However, in C. elegans, mutations in kinesin-1 led to a reduction in mitochondrial distribution, probably due to an effect on MT polarity [114]. Mammalian dendrites present mixed MT arrays, which are organized in an anti-parallel manner, suggesting that dynein/dynactin is responsible for both anterograde and retrograde mitochondrial transport. However, in Drosophila, the fact that MTs within the dendrite are organized minus-end-out suggests that dynein is only involved in anterograde mitochondrial movement, although the identity of the motor involved in retrograde transport is still unknown.
Mitochondrial sorting into axons and dendrites
How sorting of non-polarized cargo as mitochondria into the axons and dendrites is accomplished? The differential MT polarization and the identity of motor/adaptor steers mitochondria to the precise cellular compartment. In rat hippocampal neurons, altered kinesin and dynactin activity severely affects the steering of mitochondria into the axon, with mutations in dynactin but not in kinesin affecting mitochondrial sorting into dendrites [107]. Thus, dynein is responsible for dendritic sorting of mitochondria and kinesin is responsible for axonal sorting of mitochondria.
Axonal and dendritic motor adaptor proteins
Milton/TRAKs
In Drosophila Milton mutants, the absence of mitochondria in photoreceptors, wing nerves, and motor axons suggests that Milton is required for the polarized sorting of mitochondria [28, 35, 97]. Moreover, it was shown in Drosophila axons that milton knockdown caused significant reduction in retrograde velocity, with no effect on anterograde parameters [118].
TRAK1 and TRAK2, the mammalian Milton homologues, are differentially expressed in axons and dendrites in cultured rat hippocampal neurons, with TRAK1 being predominantly localized to axons and TRAK2 being preferentially distributed in dendrites [56, 107]. In agreement, TRAK1 disruption resulted in an axon-exclusive decrease in mitochondria mobility, while defects in TRAK2 led to reduced movement of mitochondria in dendrites but not in axons, with no changes in velocity or run length of moving mitochondria [51, 107]. Interestingly, early in neuronal development, both TRAKs contribute similarly to both axonal and dendritic mitochondrial movement [57]. Thus, the current model that describes polarized mitochondria trafficking suggests that kinesin-1 and dynein being controlled by TRAK1 drives mitochondria into axons, while dynein controlled by TRAK2 exclusively steers mitochondria to dendrites [107]. All the relevant effects of Milton/TRAK downregulation are summarized in Table 2.
Miro
Similar to Milton mutants, mutations in Miro show aberrant mitochondrial aggregation in the soma of Drosophila larval nerves [6, 36, 54, 86] and in C. elegans GABA neurons [38]. The loss of Miro1 in mouse models leads to disrupted axonal retrograde transport [75], as well as in rat hippocampal neurons dendrites [59]. A more recent study showed that depletion of Miro1 but not Miro2 resulted in decreases in anterograde and retrograde mitochondrial trafficking in hippocampal neurons’ dendrites, while no defects in axonal mitochondrial transport were observed [55]. All the relevant effects of Miro downregulation are summarized in Table 3.
Mitochondrial trafficking in migrating cells
Lymphocytes
In lymphoid cells of the immune system, the leading edge contains the actin polymerization machinery, while the uropod (i.e., the rear edge) contains the MTOC and the majority of cytoplasm and organelles [87]. It was shown that during directed lymphocyte migration, mitochondria specifically re-localize and accumulate in the uropod [17]. However, during the interaction between leukocytes and endothelial cells, re-localization of mitochondria to the contact zone occurs, with the MTOC moving together with mitochondria towards the contact zone [71] or towards the immunological synapse in a dynein-dependent manner [64]. Given MT organization in lymphocytes, it is expected that kinesin-1 would drive the anterograde transport of mitochondria towards the cell periphery, and that dynein should be responsible for retrograde motility [88]. Indeed, disruption of kinesin-1 in T lymphocytes resulted in complete blockage of anterograde mitochondrial transport towards the plasma membrane [81]. The role of dynein in mitochondrial movement in lymphocyte cells was not addressed, although dynein is also involved in MTOC translocation during the cell migration and immunological synapse establishment [64]. This suggests that dynein can indirectly influence anterograde translocation of mitochondria as part of MT organization. Accordingly, it was shown that Miro-1 links mitochondria to DHC to regulate mitochondrial distribution [71]. At the same time, the function of Track is still unknown.
Migrating cancer cells
Cancer cells must rearrange mitochondrial network dynamics to support cell motility, invasion, and metastasis formation. In contrast to lymphocytes, migrating epithelial cells re-localize mitochondria in the anterior direction (i.e., towards the leading edge) [23, 24]. Mitochondrial relocation to the leading edge of migrating epithelial cancer cells is MT dependent [22]. Knockdown of Miro-1 in epithelial cancer cells resulted in mislocalized mitochondria and slower cell migration rates [24]. Suppressing KIF5B, Miro1, and Miro2 expression by siRNA inhibited the movement of mitochondria to the cell periphery [13]. Thus, trafficking mitochondria to the cell cortical area is undertaken by Miro1/2 and KIF5B, whereas the functions of dynein and TRAK remain to be defined.
Mitochondrial transport in non-neuronal polarized cells
Polarized epithelial cells
In epithelial cells, mitochondria form an evenly distributed tubular network within the cytoplasm. Mitochondrial transport by motor proteins and their adaptors in polarized epithelial cells is not well characterized. In polarized epithelial cells of the Drosophila oocyte follicle, it was shown that Milton and KHC are responsible for proper localization of mitochondria during some but not all stages of ovarian follicle development [21]. Thus, as with migrating cells, the identity and exact role of the motor proteins and their adaptors require investigation.
Other cell types
Little data on mitochondrial movement in non-neuronal cells are currently available. In HeLa cells, disruption of dynein by overexpression of dynactin subunit p50 unexpectedly resulted in mitochondrial clustering near the nucleus [108]. Mutations in Miro1 in COS7 and NIH3T3 cells resulted in perinuclear clustering of mitochondria and induced apoptosis [30]. Knockdown of Miro1 and Miro2 in HeLa cells led to accumulation of mitochondria near the nucleus [54]. Thus, in non-neuronal cells, the same mitochondrial trafficking machinery seems to be responsible for the localization of this organelle, although no evidence for the involvement of TRAK/Milton has appeared.
Like dendrites, Drosophila bristles contain a minus-end-out MT array. Work done in our lab showed that dynein is the major motor for mitochondrial movement, as both the antero- and retrograde velocities of moving mitochondria were significantly decreased in “slow” dynein mutants. Intriguingly, disruption of dynactin in bristles showed no effect on mitochondrial localization patterns or on the velocity of movement in both directions. Surprisingly, kinesin-1 knockdown by RNAi resulted in a slight increase in the velocity of mitochondrial movement in both directions, suggesting that kinesin-1 acts as an opposing force for dynein-driven movement [67]. We noted different defects upon Milton and Miro knockdown. In Milton RNAi expressing bristles, mitochondria entry into the bristle shaft was severely decreased, with no defects in velocity-related parameters being seen. Miro mutants showed normal mitochondrial distribution, yet presented defective retrograde transport parameters, such as net retrograde velocity and the proportion of mitochondria moving in the retrograde direction both being significantly decreased. Thus, we suggest that Milton is responsible for anterograde movement or for polarized mitochondrial sorting in the bristle and that Miro is the key regulator of the retrograde directional switch of mitochondria trafficking exclusively driven by dynein [66].
Mitochondria transport impairment and disease
In this review, we described recent progress in our understanding of a basic cellular process, i.e., mitochondrial active transport, and dissected and summarized the roles of the components directly involved in the context of cell type and microtubule (MT) organization. A recently discovered mitochondria-specific transport complex consisting of kinesin/dynein/Milton/Miro has attracted great attention, mainly in the neuronal field. However, data on the players involved in non-neuronal cell types remain lacking, as described in this review. It was previously noted that Miro/Milton is the key player in mitochondrial transport in white blood cells, affecting migration rates and mitochondrial positioning during an immune response, although data on pathological conditions in human or mouse models are not yet available. In epithelial cells displaying acentrosomal MT organization, mitochondria form dense network with no visible preferred location. Moreover, work performed in Drosophila follicle cells as mentioned here highlights the involvement of the transport complex in proper mitochondria patterning. Still, far less data on mammal epithelia in this context have been reported.
Due to the complex morphology and physically long distances traveled by mitochondria in neurons, both in axons and dendrites, neuronal cells are extremely sensitive to even mild alterations in mitochondrial dynamics. Damaged or aged mitochondria must be removed from distal neuronal compartments. To date, it is still controversial whether mitochondria undergo onset degradation by the process of mitophagy, or whether they must be removed to the soma and are degraded there. In both cases, the kinesin/dynein/Milton/Miro complex is involved. Thus, the complex affects not only basic energy supply to the growing or mature cellular extension but also affects maintenance and turnover of the organelle. As such, coupling between proper mitochondrial transport and removal of damaged mitochondria must be tightly regulated under normal conditions, with impairment in one of the components leading to pathologies. Evidence for local mitochondrial degradation by autolysosomes engulfment has been presented [29, 111]. On the other hand, it was shown that damaged mitochondria accumulated at somatodendritic regions [12], with mitochondrial transport direction depending on the mitochondrial metabolic state [68]. Recently, it was shown that local mitophagy takes place in distal regions of rat hippocampal neurons, through the recruitment of PINK1 (PTEN-induced putative kinase 1) and Parkin (E3-ubiquitin ligase) [3, 54], two Parkinson’s disease (PD)-associated proteins. The PINK1/Parkin pathway is involved in Miro degradation and release of mitochondria from the associated motors [111], thus leading to mitochondrial immobilization and further elimination. Although, mutations in Miro have yet to be found in PD patients, a recent study showed elevated accumulation of Miro in mitochondria due to defective Miro degradation pathways involving PINK1/Parkin and LRRK2 (leucine-rich repeat kinase 2). It was thus concluded that Miro is resistant to degradation in PD patients. Interestingly, partial reduction of Miro levels in PD neurons had a neuroprotective effect against mitochondrial stress [45]. In a mouse model, loss of Miro1 leads to postnatal death due to the failure to breathe as a result of specific respiratory neural circuit loss. Indeed, Miro1 neuron-specific KO mice showed upper motor neuron disease phenotypes [75].
The progressive accumulation of damaged mitochondria is a characteristic feature of other neurological condition, namely Alzheimer’s disease (AD) [117]. In AD neurons, induced localization of Parkin to depolarized mitochondria favors Parkin-mediated mitophagy [117]. Neurons from AD mouse models show decreased damaged mitochondria anterograde transport [14], and increased retrograde transport [117]. Parkin-targeted depolarized mitochondria are restricted to somatic areas in both human and mouse AD models, with induced Parkin recruitment possibly influencing turnover of Miro on the mitochondrial surface, in turn affecting transport dynamics [117]. In a mouse model of amyotrophic lateral sclerosis (ALS), significant decreases in the proportion of retrograde mitochondrial transport were detected with no changes in anterograde transport parameters at early developmental stages, accompanied by a decrease in the anterograde direction later in development. Overall, a decrease in mitochondrial density in neural terminals was seen [60]. Miro1 levels are dramatically reduced in ALS patients, albeit in the spinal cord and not in the brain [119]. An additional aspect of Miro1 involvement in ALS comes through the interaction of Miro with vesicle-associated membrane protein-associated protein B (VAPB). VAPB is an integral endoplasmic reticulum (ER) protein whose function is not fully understood. VAPB is, however, mutated in familial cases of ALS. Intriguingly, mutated VAPB causes decreases in anterograde mitochondrial transport, with a reduction in velocity of the observed movement. Such defects were connected to a decrease in the amount of tubulin bound to the kinesin/Miro/TRAK complex. Expression of Ca2+-insensitive Miro rescued the observed defects in mitochondrial transport parameters [72].
These observations make Miro a promising target for the development of neuropathological treatment strategies. As for the involvement of Milton/TRAK in neurodegenerative diseases, it would be of interest to investigate its role in mitochondrial transport in neural and non-neural pathologies.
Concluding remarks
The majority of our knowledge about active mitochondrial movement comes from studies of neuronal systems, mostly axons. This is due the fact that highly specialized and extremely elongated neurons must maintain “healthy” mitochondrial dynamics and transport. Defects in long-distance neuronal transport result in neurodegenerative diseases in mammals. In this review, we focused on key mitochondrial transport players in different cell types with respect to MT organization. MTs correspond to the main tracks upon which mitochondrial movement relies, while kinesin-1 and dynein serve as the main mitochondrial transport motors, acting through the adaptor proteins Milton/TRAKs and mitochondrial Miro1/2. In the case of neurons, dendrites and axons adopt different transport strategies. In axons, kinesin and dynein act together on uni-polarized MT tracks to establish mitochondrial distribution. The interplay between the two opposing motors remains an open question, as the simple domination of one motor cannot explain the patterns of mitochondrial movement seen in axons.
Although mitochondrial transport in axons has been extensively studied, relatively little is known of mitochondrial trafficking in dendrites. Mammalian dendrites rely exclusively on dynein-driven mitochondrial transport, which solely drives dendritic cargo in both directions. On the other hand, in Drosophila dendrites, where MTs are organized minus-end-out, dynein is only responsible for anterograde mitochondrial transport. The identity of the retrograde mitochondrial motor proteins, however, remains unknown. As in dendrites, Drosophila bristle cells contain a MT array with a stable minus-end-out arrangement. During the extensive elongation process, the bristle encounters engineering challenges similar to those met by dendrites, but here only internal cues guide the process. Unlike what occurs in dendrite, in the bristle, dynein was found to exclusively drive mitochondrial transport in both directions, even though the mechanisms of such transport have yet to be defined.
Early in development, Drosophila dendritic MTs display mixed polarity similar to what is seen in the mammalian system. Later in development, MTs switch to present a uni-polar MT array. Interestingly, mitochondria and the dendrite-specific marker APC were found to enter elongating dendrites before the uni-polarized minus-end orientation of the MT network is established [40]. Thus, the initial sorting of cellular components cannot rely only on differences in MT polarity. In this context, differential functions of TRAK1 in axon and TRAK2 in dendrite may provide explanations for the alternative targeting of mitochondria to different compartments in mammals. In Drosophila, however, only a single TRAK orthologue, Milton, exists, albeit with two splice variants. As such, understanding how polarized sorting transpires in Drosophila neurons remains unclear.
Although it is accepted that Milton/TRAKs and Miro function as a complex, in Drosophila bristles different roles for Milton and Miro have been shown [66]. Whereas Milton is being implicated in the initiation of dynein-driven mitochondria movement, Miro is involved in the retrograde switch of the direction of movement. In addition, the function of these two adaptors in other cell types has yet to be fully characterized. Thus, further detailed studies are needed to reveal the role of MT adaptor proteins in mitochondrial movement.
Abbreviations
- Miro
Mitochondrial Rho-GTPase
- MT
Microtubules
- MTOC
Microtubule-organizing center
- TRAK
Trafficking kinesin protein
- γTuRC
γ-Tubulin ring complex
References
- 1.Allan VJ. Cytoplasmic dynein. Biochem Soc Trans. 2011;39:1169–1178. doi: 10.1042/BST0391169. [DOI] [PubMed] [Google Scholar]
- 2.Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC. Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal. 2012;16:1150–1180. doi: 10.1089/ars.2011.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auer TO, Xiao T, Bercier V, Gebhardt C, Duroure K, Concordet JP, Wyart C, Suster M, Kawakami K, Wittbrodt J, Baier H, Del Bene F (2015) Deletion of a kinesin I motor unmasks a mechanism of homeostatic branching control by neurotrophin-3. eLife 4:e05061. doi:10.7554/eLife.05061 [DOI] [PMC free article] [PubMed]
- 5.Baas PW, Ahmad FJ. Beyond taxol: microtubule-based treatment of disease and injury of the nervous system. Brain. 2013;136:2937–2951. doi: 10.1093/brain/awt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babic M, Russo GJ, Wellington AJ, Sangston RM, Gonzalez M, Zinsmaier KE. Miro’s N-terminal GTPase domain is required for transport of mitochondria into axons and dendrites. J Neurosci. 2015;35:5754–5771. doi: 10.1523/JNEUROSCI.1035-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker AR, McIntosh KV, Dawe HR. Centrosome positioning in non-dividing cells. Protoplasma. 2016;253:1007–1021. doi: 10.1007/s00709-015-0883-5. [DOI] [PubMed] [Google Scholar]
- 8.Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8:451–463. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- 9.Bitan A, Rosenbaum I, Abdu U. Stable and dynamic microtubules coordinately determine and maintain Drosophila bristle shape. Development. 2012;139:1987–1996. doi: 10.1242/dev.076893. [DOI] [PubMed] [Google Scholar]
- 10.Brickley K, Stephenson FA. Trafficking kinesin protein (TRAK)-mediated transport of mitochondria in axons of hippocampal neurons. J Biol Chem. 2011;286:18079–18092. doi: 10.1074/jbc.M111.236018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Q, Gerwin C, Sheng ZH. Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J Cell Biol. 2005;170:959–969. doi: 10.1083/jcb.200506042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Q, Zakaria HM, Simone A, Sheng ZH. Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr Biol . 2012;22:545–552. doi: 10.1016/j.cub.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caino MC, Seo JH, Aguinaldo A, Wait E, Bryant KG, Kossenkov AV, Hayden JE, Vaira V, Morotti A, Ferrero S, Bosari S, Gabrilovich DI, Languino LR, Cohen AR, Altieri DC. A neuronal network of mitochondrial dynamics regulates metastasis. Nat Commun. 2016;7:13730. doi: 10.1038/ncomms13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callan-Jones AC, Voituriez R. Actin flows in cell migration: from locomotion and polarity to trajectories. Curr Opin Cell Biol. 2016;38:12–17. doi: 10.1016/j.ceb.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Campbell PD, Shen K, Sapio MR, Glenn TD, Talbot WS, Marlow FL. Unique function of Kinesin Kif5A in localization of mitochondria in axons. J Neurosci. 2014;34:14717–14732. doi: 10.1523/JNEUROSCI.2770-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campello S, Lacalle RA, Bettella M, Manes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203:2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho C, Vale RD. The mechanism of dynein motility: insight from crystal structures of the motor domain. Biochem Biophys Acta. 2012;1823:182–191. doi: 10.1016/j.bbamcr.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 20.Conduit PT, Wainman A, Raff JW. Centrosome function and assembly in animal cells. Nat Rev Mol Cell Biol. 2015;16:611–624. doi: 10.1038/nrm4062. [DOI] [PubMed] [Google Scholar]
- 21.Cox RT, Spradling AC. Milton controls the early acquisition of mitochondria by Drosophila oocytes. Development. 2006;133:3371–3377. doi: 10.1242/dev.02514. [DOI] [PubMed] [Google Scholar]
- 22.Cunniff B, McKenzie AJ, Heintz NH, Howe AK. AMPK activity regulates trafficking of mitochondria to the leading edge during cell migration and matrix invasion. Mol Biol Cell. 2016;27:2662–2674. doi: 10.1091/mbc.E16-05-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Silva AF, Mariotti FR, Maximo V, Campello S. Mitochondria dynamism: of shape, transport and cell migration. Cell Mol Life Sci. 2014;71:2313–2324. doi: 10.1007/s00018-014-1557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai SP, Bhatia SN, Toner M, Irimia D. Mitochondrial localization and the persistent migration of epithelial cancer cells. Biophys J. 2013;104:2077–2088. doi: 10.1016/j.bpj.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drerup CM, Herbert AL, Monk KR, Nechiporuk AV (2017) Regulation of mitochondria-dynactin interaction and mitochondrial retrograde transport in axons. eLife 6:e22234. doi:10.7554/eLife.22234 [DOI] [PMC free article] [PubMed]
- 26.Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol. 2012;942:157–183. doi: 10.1007/978-94-007-2869-1_7. [DOI] [PubMed] [Google Scholar]
- 27.Etienne-Manneville S. Microtubules in cell migration. Annu Rev Cell Dev Biol. 2013;29:471–499. doi: 10.1146/annurev-cellbio-101011-155711. [DOI] [PubMed] [Google Scholar]
- 28.Fang Y, Soares L, Teng X, Geary M, Bonini NM. A novel Drosophila model of nerve injury reveals an essential role of Nmnat in maintaining axonal integrity. Curr Biol. 2012;22:590–595. doi: 10.1016/j.cub.2012.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferree AW, Trudeau K, Zik E, Benador IY, Twig G, Gottlieb RA, Shirihai OS. MitoTimer probe reveals the impact of autophagy, fusion, and motility on subcellular distribution of young and old mitochondrial protein and on relative mitochondrial protein age. Autophagy. 2013;9:1887–1896. doi: 10.4161/auto.26503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fransson A, Ruusala A, Aspenstrom P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- 31.Frederick RL, Shaw JM. Moving mitochondria: establishing distribution of an essential organelle. Traffic. 2007;8:1668–1675. doi: 10.1111/j.1600-0854.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. J Cell Sci. 2009;122:3595–3604. doi: 10.1242/jcs.042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong TW, Winnicki RS, Kohrman DC, Lomax MI. A novel mouse kinesin of the UNC-104/KIF1 subfamily encoded by the Kif1b gene. Gene. 1999;239:117–127. doi: 10.1016/S0378-1119(99)00370-4. [DOI] [PubMed] [Google Scholar]
- 35.Gorska-Andrzejak J, Stowers RS, Borycz J, Kostyleva R, Schwarz TL, Meinertzhagen IA. Mitochondria are redistributed in Drosophila photoreceptors lacking milton, a kinesin-associated protein. J Comp Neurol. 2003;463:372–388. doi: 10.1002/cne.10750. [DOI] [PubMed] [Google Scholar]
- 36.Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Haghnia M, Cavalli V, Shah SB, Schimmelpfeng K, Brusch R, Yang G, Herrera C, Pilling A, Goldstein LS. Dynactin is required for coordinated bidirectional motility, but not for dynein membrane attachment. Mol Biol Cell. 2007;18:2081–2089. doi: 10.1091/mbc.E06-08-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han SM, Baig HS, Hammarlund M. Mitochondria localize to injured axons to support regeneration. Neuron. 2016;92:1308–1323. doi: 10.1016/j.neuron.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatch AL, Gurel PS, Higgs HN. Novel roles for actin in mitochondrial fission. J Cell Sci. 2014;127:4549–4560. doi: 10.1242/jcs.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill SE, Parmar M, Gheres KW, Guignet MA, Huang Y, Jackson FR, Rolls MM. Development of dendrite polarity in Drosophila neurons. Neural Dev. 2012;7:34. doi: 10.1186/1749-8104-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 42.Hirokawa N, Tanaka Y. Kinesin superfamily proteins (KIFs): various functions and their relevance for important phenomena in life and diseases. Exp Cell Res. 2015;334:16–25. doi: 10.1016/j.yexcr.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Front Biosci. 1996;1:d91–d102. doi: 10.2741/A118. [DOI] [PubMed] [Google Scholar]
- 44.Howard J, Hyman AA. Growth, fluctuation and switching at microtubule plus ends. Nat Rev Mol Cell Biol. 2009;10:569–574. doi: 10.1038/nrm2713. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh CH, Shaltouki A, Gonzalez AE, Bettencourt da Cruz A, Burbulla LF, St Lawrence E, Schule B, Krainc D, Palmer TD, Wang X. Functional impairment in miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson’s disease. Cell Stem Cell. 2016;19:709–724. doi: 10.1016/j.stem.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurd DD, Saxton WM. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila . Genetics. 1996;144:1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanai Y, Okada Y, Tanaka Y, Harada A, Terada S, Hirokawa N. KIF5C, a novel neuronal kinesin enriched in motor neurons. J Neurosci. 2000;20:6374–6384. doi: 10.1523/JNEUROSCI.20-17-06374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kapitein LC, Hoogenraad CC. Building the neuronal microtubule cytoskeleton. Neuron. 2015;87:492–506. doi: 10.1016/j.neuron.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 50.Karle KN, Mockel D, Reid E, Schols L. Axonal transport deficit in a KIF5A(-/-) mouse model. Neurogenetics. 2012;13:169–179. doi: 10.1007/s10048-012-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimura T, Murakami F. Evidence that dendritic mitochondria negatively regulate dendritic branching in pyramidal neurons in the neocortex. J Neurosci. 2014;34:6938–6951. doi: 10.1523/JNEUROSCI.5095-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee H, Yoon Y. Mitochondrial fission: regulation and ER connection. Mol Cells. 2014;37:89–94. doi: 10.14348/molcells.2014.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ligon LA, Steward O. Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:340–350. doi: 10.1002/1096-9861(20001120)427:3<340::AID-CNE2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 54.Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P, Millan I, Shen A, Saxton W, Kanao T, Takahashi R, Hattori N, Imai Y, Lu B. Parkinson’s disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez-Domenech G, Higgs NF, Vaccaro V, Ros H, Arancibia-Carcamo IL, MacAskill AF, Kittler JT. Loss of dendritic complexity precedes neurodegeneration in a mouse model with disrupted mitochondrial distribution in mature dendrites. Cell Rep. 2016;17:317–327. doi: 10.1016/j.celrep.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loss O, Stephenson FA. Localization of the kinesin adaptor proteins trafficking kinesin proteins 1 and 2 in primary cultures of hippocampal pyramidal and cortical neurons. J Neurosci Res. 2015;93:1056–1066. doi: 10.1002/jnr.23549. [DOI] [PubMed] [Google Scholar]
- 57.Loss O, Stephenson FA. Developmental changes in TRAK-mediated mitochondrial transport in neurons. Mol Cell Neurosci. 2017;80:134–147. doi: 10.1016/j.mcn.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luders J, Stearns T. Microtubule-organizing centres: a re-evaluation. Nat Rev Mol Cell Biol. 2007;8:161–167. doi: 10.1038/nrm2100. [DOI] [PubMed] [Google Scholar]
- 59.Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magrane J, Cortez C, Gan WB, Manfredi G. Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum Mol Genet. 2014;23:1413–1424. doi: 10.1093/hmg/ddt528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mannella CA. Structural diversity of mitochondria: functional implications. Ann N Y Acad Sci. 2008;1147:171–179. doi: 10.1196/annals.1427.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchi S, Patergnani S, Missiroli S, Morciano G, Rimessi A, Wieckowski MR, Giorgi C, Pinton P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2017 doi: 10.1016/j.ceca.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Margolin G, Gregoretti IV, Cickovski TM, Li C, Shi W, Alber MS, Goodson HV. The mechanisms of microtubule catastrophe and rescue: implications from analysis of a dimer-scale computational model. Mol Biol Cell. 2012;23:642–656. doi: 10.1091/mbc.E11-08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin-Cofreces NB, Robles-Valero J, Cabrero JR, Mittelbrunn M, Gordon-Alonso M, Sung CH, Alarcon B, Vazquez J, Sanchez-Madrid F. MTOC translocation modulates IS formation and controls sustained T cell signaling. J Cell Biol. 2008;182:951–962. doi: 10.1083/jcb.200801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 66.Melkov A, Baskar R, Alcalay Y, Abdu U. A new mode of mitochondrial transport and polarized sorting regulated by Dynein, Milton and Miro. Development. 2016;143:4203–4213. doi: 10.1242/dev.138289. [DOI] [PubMed] [Google Scholar]
- 67.Melkov A, Simchoni Y, Alcalay Y, Abdu U. Dynamic microtubule organization and mitochondrial transport are regulated by distinct Kinesin-1 pathways. Biol Open. 2015;4:1696–1706. doi: 10.1242/bio.015206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 69.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 70.Morfini G, Schmidt N, Weissmann C, Pigino G, Kins S. Conventional kinesin: biochemical heterogeneity and functional implications in health and disease. Brain Res Bull. 2016;126:347–353. doi: 10.1016/j.brainresbull.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 71.Morlino G, Barreiro O, Baixauli F, Robles-Valero J, Gonzalez-Granado JM, Villa-Bellosta R, Cuenca J, Sanchez-Sorzano CO, Veiga E, Martin-Cofreces NB, Sanchez-Madrid F. Miro-1 links mitochondria and microtubule Dynein motors to control lymphocyte migration and polarity. Mol Cell Biol. 2014;34:1412–1426. doi: 10.1128/MCB.01177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morotz GM, De Vos KJ, Vagnoni A, Ackerley S, Shaw CE, Miller CC. Amyotrophic lateral sclerosis-associated mutant VAPBP56S perturbs calcium homeostasis to disrupt axonal transport of mitochondria. Hum Mol Genet. 2012;21:1979–1988. doi: 10.1093/hmg/dds011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen MM, Stone MC, Rolls MM. Microtubules are organized independently of the centrosome in Drosophila neurons. Neural Dev. 2011;6:38. doi: 10.1186/1749-8104-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen TT, Oh SS, Weaver D, Lewandowska A, Maxfield D, Schuler MH, Smith NK, Macfarlane J, Saunders G, Palmer CA, Debattisti V, Koshiba T, Pulst S, Feldman EL, Hajnoczky G, Shaw JM. Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc Natl Acad Sci USA. 2014;111:E3631–E3640. doi: 10.1073/pnas.1402449111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochem Biophys Acta. 2013;1833:1256–1268. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 77.Overly CC, Rieff HI, Hollenbeck PJ. Organelle motility and metabolism in axons vs dendrites of cultured hippocampal neurons. J Cell Sci. 1996;109(Pt 5):971–980. doi: 10.1242/jcs.109.5.971. [DOI] [PubMed] [Google Scholar]
- 78.Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J Neurosci. 2010;30:8984–8992. doi: 10.1523/JNEUROSCI.1621-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Popov V, Medvedev NI, Davies HA, Stewart MG. Mitochondria form a filamentous reticular network in hippocampal dendrites but are present as discrete bodies in axons: a three-dimensional ultrastructural study. J Comp Neurol. 2005;492:50–65. doi: 10.1002/cne.20682. [DOI] [PubMed] [Google Scholar]
- 81.Quintana A, Schwarz EC, Schwindling C, Lipp P, Kaestner L, Hoth M. Sustained activity of calcium release-activated calcium channels requires translocation of mitochondria to the plasma membrane. J Biol Chem. 2006;281:40302–40309. doi: 10.1074/jbc.M607896200. [DOI] [PubMed] [Google Scholar]
- 82.Rawson RL, Yam L, Weimer RM, Bend EG, Hartwieg E, Horvitz HR, Clark SG, Jorgensen EM. Axons degenerate in the absence of mitochondria in C. elegans. Curr Biol: CB. 2014;24:760–765. doi: 10.1016/j.cub.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reilein A, Yamada S, Nelson WJ. Self-organization of an acentrosomal microtubule network at the basal cortex of polarized epithelial cells. J Cell Biol. 2005;171:845–855. doi: 10.1083/jcb.200505071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rice SE, Gelfand VI. Paradigm lost: milton connects kinesin heavy chain to miro on mitochondria. J Cell Biol. 2006;173:459–461. doi: 10.1083/jcb.200604071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roberts AJ, Kon T, Knight PJ, Sutoh K, Burgess SA. Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Biol. 2013;14:713–726. doi: 10.1038/nrm3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Russo GJ, Louie K, Wellington A, Macleod GT, Hu F, Panchumarthi S, Zinsmaier KE. Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J Neurosci. 2009;29:5443–5455. doi: 10.1523/JNEUROSCI.5417-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanchez-Madrid F, Serrador JM. Mitochondrial redistribution: adding new players to the chemotaxis game. Trends Immunol. 2007;28:193–196. doi: 10.1016/j.it.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 89.Satoh AK, Li BX, Xia H, Ready DF. Calcium-activated Myosin V closes the Drosophila pupil. Curr Biol . 2008;18:951–955. doi: 10.1016/j.cub.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Satoh D, Sato D, Tsuyama T, Saito M, Ohkura H, Rolls MM, Ishikawa F, Uemura T. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat Cell Biol. 2008;10:1164–1171. doi: 10.1038/ncb1776. [DOI] [PubMed] [Google Scholar]
- 91.Scarpa E, Mayor R. Collective cell migration in development. J Cell Biol. 2016;212:143–155. doi: 10.1083/jcb.201508047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 93.Sheng ZH, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13:77–93. doi: 10.1038/nrg3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siddiqui SS. Metazoan motor models: kinesin superfamily in C. elegans . Traffic. 2002;3:20–28. doi: 10.1034/j.1600-0854.2002.30104.x. [DOI] [PubMed] [Google Scholar]
- 95.Stiess M, Maghelli N, Kapitein LC, Gomis-Ruth S, Wilsch-Brauninger M, Hoogenraad CC, Tolic-Norrelykke IM, Bradke F. Axon extension occurs independently of centrosomal microtubule nucleation. Science. 2010;327:704–707. doi: 10.1126/science.1182179. [DOI] [PubMed] [Google Scholar]
- 96.Stone MC, Roegiers F, Rolls MM. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol Biol Cell. 2008;19:4122–4129. doi: 10.1091/mbc.E07-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on Milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/S0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- 98.Suhm T, Ott M. Mitochondrial translation and cellular stress response. Cell Tissue Res. 2017;367:21–31. doi: 10.1007/s00441-016-2460-4. [DOI] [PubMed] [Google Scholar]
- 99.Sulimenko V, Hajkova Z, Klebanovych A, Draber P. Regulation of microtubule nucleation mediated by gamma-tubulin complexes. Protoplasma. 2017;254:1187–1199. doi: 10.1007/s00709-016-1070-z. [DOI] [PubMed] [Google Scholar]
- 100.Tanaka K, Sugiura Y, Ichishita R, Mihara K, Oka T. KLP6: a newly identified kinesin that regulates the morphology and transport of mitochondria in neuronal cells. J Cell Sci. 2011;124:2457–2465. doi: 10.1242/jcs.086470. [DOI] [PubMed] [Google Scholar]
- 101.Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/S0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- 102.Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/S0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- 103.Toya M, Takeichi M. Organization of non-centrosomal microtubules in epithelial cells. Cell Struct Funct. 2016;41:127–135. doi: 10.1247/csf.16015. [DOI] [PubMed] [Google Scholar]
- 104.Vagnoni A, Hoffmann PC, Bullock SL. Reducing Lissencephaly-1 levels augments mitochondrial transport and has a protective effect in adult Drosophila neurons. J Cell Sci. 2016;129:178–190. doi: 10.1242/jcs.179184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: an ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- 106.van Beuningen SF, Hoogenraad CC. Neuronal polarity: remodeling microtubule organization. Curr Opin Neurobiol. 2016;39:1–7. doi: 10.1016/j.conb.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 107.van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, Wulf PS, Keijzer N, Demmers J, Kapitein LC, Jaarsma D, Gerritsen HC, Akhmanova A, Hoogenraad CC. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron. 2013;77:485–502. doi: 10.1016/j.neuron.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 108.Varadi A, Johnson-Cadwell LI, Cirulli V, Yoon Y, Allan VJ, Rutter GA. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J Cell Sci. 2004;117:4389–4400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- 109.Verhey KJ, Kaul N, Soppina V. Kinesin assembly and movement in cells. Ann Rev Biophys. 2011;40:267–288. doi: 10.1146/annurev-biophys-042910-155310. [DOI] [PubMed] [Google Scholar]
- 110.Vinogradova T, Miller PM, Kaverina I. Microtubule network asymmetry in motile cells: role of Golgi-derived array. Cell Cycle. 2009;8:2168–2174. doi: 10.4161/cc.8.14.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wozniak MJ, Melzer M, Dorner C, Haring HU, Lammers R. The novel protein KBP regulates mitochondria localization by interaction with a kinesin-like protein. BMC cell Biol. 2005;6:35. doi: 10.1186/1471-2121-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yagi T. Bioinformatic approaches to dynein heavy chain classification. Methods Cell Biol. 2009;92:1–9. doi: 10.1016/S0091-679X(08)92001-X. [DOI] [PubMed] [Google Scholar]
- 114.Yan J, Chao DL, Toba S, Koyasako K, Yasunaga T, Hirotsune S, Shen K. Kinesin-1 regulates dendrite microtubule polarity in Caenorhabditis elegans. eLife. 2013;2:e00133. doi: 10.7554/eLife.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yau KW, Schatzle P, Tortosa E, Pages S, Holtmaat A, Kapitein LC, Hoogenraad CC. Dendrites in vitro and in vivo contain microtubules of opposite polarity and axon formation correlates with uniform plus-end-out microtubule orientation. J Neurosci. 2016;36:1071–1085. doi: 10.1523/JNEUROSCI.2430-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yau KW, van Beuningen SF, Cunha-Ferreira I, Cloin BM, van Battum EY, Will L, Schatzle P, Tas RP, van Krugten J, Katrukha EA, Jiang K, Wulf PS, Mikhaylova M, Harterink M, Pasterkamp RJ, Akhmanova A, Kapitein LC, Hoogenraad CC. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron. 2014;82:1058–1073. doi: 10.1016/j.neuron.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 117.Ye X, Sun X, Starovoytov V, Cai Q. Parkin-mediated mitophagy in mutant hAPP neurons and Alzheimer’s disease patient brains. Hum Mol Genet. 2015;24:2938–2951. doi: 10.1093/hmg/ddv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu Y, Lee HC, Chen KC, Suhan J, Qiu M, Ba Q, Yang G. Inner membrane fusion mediates spatial distribution of axonal mitochondria. Sci Rep. 2016;6:18981. doi: 10.1038/srep18981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang F, Wang W, Siedlak SL, Liu Y, Liu J, Jiang K, Perry G, Zhu X, Wang X. Miro1 deficiency in amyotrophic lateral sclerosis. Front Aging Neurosci. 2015;7:100. doi: 10.3389/fnagi.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]