Abstract
Oxygen constitutes a vital element for the survival of every single cell in multicellular aerobic organisms like mammals. A complex homeostatic oxygen-sensing system has evolved in these organisms, including detectors and effectors, to guarantee a proper supply of the element to every cell. The carotid body represents the most important peripheral arterial chemoreceptor organ in mammals and informs about hypoxemic situations to the effectors at the brainstem cardiorespiratory centers. To optimize organismal adaptation to maintained hypoxemic situations, the carotid body has evolved containing a niche of adult tissue-specific stem cells with the capacity to differentiate into both neuronal and vascular cell types in response to hypoxia. These neurogenic and angiogenic processes are finely regulated by the niche and by hypoxia itself. Our recent data on the cellular and molecular mechanisms underlying the functioning of this niche might help to comprehend a variety of different diseases coursing with carotid body failure, and might also improve our capacity to use these stem cells for the treatment of neurological disease. Herein, we review those data about the recent characterization of the carotid body niche, focusing on the study of the phenotype and behavior of multipotent stem cells within the organ, comparing them with other well-documented neural stem cells within the adult nervous system.
Keywords: Adult PNS stem cells, Carotid body, Hypoxia, Neural crest, Neurogenesis, Angiogenesis, Self-renewal, Differentiation, Plasticity, Glial phenotype, Glycolytic metabolism, Intermediate progenitors, Neuroblasts, Sympathetic over activation-related diseases, Neurological diseases
Introduction
The carotid body (CB) is a paired organ situated in the bifurcation of the carotid artery. The CB constitutes the main arterial chemoreceptor in mammals, and it sentinels for any significant changes in different chemical variables in the blood, such as oxygen or CO2 concentrations, pH, and glucose content. Especially important is the detection of oxygen levels (PO2), since this element is absolutely required for the survival of every single cell in mammalian pluricellular organisms [1]. The central role of oxygen as the final acceptor of electrons in the mitochondrial respiratory chain, thus making possible the production of energy by oxidative phosphorylation, has allowed the evolutionary radiation of aerobes. As a consequence, complex aerobic organisms like mammals have evolved incorporating a homeostatic oxygen-sensing system, including precise detectors of environmental oxygen levels and efficient effectors, to ensure appropriate supply of the element to every single cell [1]. The carotid body is a fundamental component of this system and constitutes the most important oxygen sensor in mammalian organisms [2]. This organ detects mild decreases in blood PO2 and informs the central nervous system through the glossopharyngeal nerve. This sensory information activates the cardiorespiratory centers within the brainstem, which in turn will increase breath and heart rates to recover oxygen levels arriving to cells [3].
The cellular structure of the carotid body was originally described by Fernando De Castro [4], using the same histological techniques as his mentor Santiago Ramón y Cajal [5]. De Castro morphologically identified the main chemoreceptor cell, termed as Type I or glomus cell, which was later corroborated as responsible for chemical variable detection and for communication to sensory nerves [6]. These glomus cells are organized in glomeruli, which are clusters of cells innervated by sensory fibers and separated by a profuse network of capillaries and connective tissue. Type I cells are enveloped by processes of type II or sustentacular cells, which express glial markers like the glial fibrillary acidic protein (GFAP) and were originally considered as supportive cells [7]. Carotid body glomus cells are neuron-like excitable cells that express voltage-dependent channels in their membranes and are loaded with exocytotic vesicles. A decrease in blood PO2 inhibits potassium channels in the glomus cell membrane, inducing a depolarization that will provoke voltage-dependent calcium channel opening, calcium entry, and exocytotic liberation of neurotransmitters to the chemoreceptive synapse [8, 9]. The first steps of oxygen detection and inhibition of potassium channels are recently being elucidated, with the description of the role of mitochondrial complex I in the process [10, 11]. Hence, the mechanism by which carotid body glomus cells detect an acute hypoxemic situation to trigger the counter-regulatory organismal response is recently being fully clarified [12]. Although mitochondrial complex I constitutes the strongest explanation for CB oxygen sensing, other theories, potentially complementary, still persist about the molecular nature of the CB O2 sensor [13].
In addition to its role detecting acute changes in blood PO2, the carotid body is also crucial during organismal adaptation to chronic hypoxia. When the human body is exposed to a maintained low level of oxygen, such as in high altitude dwellers or in patients with obstructive pulmonary diseases, the carotid body displays intense histochemical changes to increase the oxygen-sensing parenchyma, which in turn allows reinforcement of the respiratory drive from the brainstem and physiological adaptation [14, 15]. This way, the carotid body leads the respiratory and cardiovascular adjustments necessary to ensure survival in a maintained hypoxemic situation [16]. The adaptation process includes a clear hypertrophy of the organ in response to chronic hypoxia, involving an increase in the number of neuron-like glomus cells and a profound angiogenesis [15, 17]. The cellular events taking place in the carotid body growing parenchyma under chronic hypoxia have only recently been thoroughly studied. We have shown that the above-mentioned type II cells in the organ are truly neural crest-derived multipotent stem cells with the capacity to participate in both neurogenesis and angiogenesis by converting into new glomus and vascular cells in response to hypoxia [18, 19]. GFAP + sustentacular cells are quiescent in normoxic conditions and become activated under hypoxia, changing their phenotype to nestin +/GFAP- intermediate progenitors, which in turn will differentiate into new glomus or vascular cells [19]. CB stem cells (CBSCs), therefore, display a glial phenotype, similar to other populations of adult neural stem cells in both peripheral and central nervous systems. The discovery of these tissue-specific stem cells has allowed a better comprehension of the cellular events taking place in the CB during chronic hypoxia, thus permitting an improved understanding of organismal adaptation to low oxygen.
Herein, in this review we expose and analyze accumulating data regarding the nature and functioning of adult CBSCs, placing them in the context of other populations of adult neural stem cells and comparing them to the functioning of other germinal niches described in the adult nervous system.
Embryonic origin of carotid body stem cells
An interesting first aspect of adult carotid body stem cells that can be reviewed in the light of our recent data is their embryonic origin. Carotid body sustentacular cells are distinctly derived from the neural crest, as confirmed by cell fate mapping studies using Wnt-1-cre/R26R transgenic mice [19]. However, the specific neural crest cell lineage that gives rise to these cells during development has not unequivocally been identified. In order to shed some light into this hitch, existing literature about the embryonic origins of the organ will be reviewed.
The carotid body is a neuroendocrine derivative of the sympathoadrenal lineage of the trunk neural crest [20]. This particular lineage migrates during development of the neural crest, through the ventral pathway, from the apex of the neural tube to the vicinity of the dorsal aorta [21]. Bone morphogenetic protein (BMP) signaling from the aorta induces the segregation between sympathetic and neuroendocrine progenitors [22], and these cells complete migration to give rise to sympathetic ganglia and adrenal medulla respectively [23]. This sympathoadrenal specification occurs all along the trunk area of the embryo [24], although the adrenal medulla is formed at the caudal portion of this area. At the rostral end of the trunk area, right at the boundary with cranial cells, after aorta-induced specification, a group of sympathoadrenal progenitor cells arrive to the wall of the carotid artery, where sympathetic cells give rise to the superior cervical ganglion (SCG) while glomus cell progenitors move to the adventitia of the artery to engender the carotid body [25, 26]. Experimental procedures that compromise terminal differentiation or survival of sympathoadrenal neuronal cells result in the absence of SCG and CB [27–29], indicating the close association between a correct development of the SCG and an appropriate formation of the carotid body. However, the formation of the CB also depends on the presence of an earlier carotid artery mesenchymal anlage, of neural crest origin, that seems to influence the final specification of carotid body glomus cell progenitors [30]. It has been proposed that sustentacular cells might originate from neural crest-derived ectomesenchymal cells at the carotid artery wall. These cells would be responsible for the formation of the mesenchymal primordium prior to the arrival of sympathoadrenal progenitors [28]. However, it cannot be discarded that sustentacular cell precursors might arrive together with glomus cell precursors from the SCG and invade the mesenchymal primordium where they are finally transformed into sustentacular cells. In fact, expression of GFAP or S-100 is very weak during embryonic development, and no other specific staining has been performed to detect potential migration of undifferentiated multipotent progenitors from the SCG into the CB primordium, along with neuronal precursors. A sympathoadrenal origin and nature of adult CB stem cells might be more compatible with the severe specification for carotid body cell types, that is characteristic of these cells [19].
A third embryonic origin for CB sustentacular cells has been recently hypothesized. It has been demonstrated that there are three elements required for the correct formation and development of the carotid body. In addition to the two elements already mentioned, i. e. the ectomesenchymal anlage at the carotid artery wall and the cellular migration from the SCG, the carotid sinus nerve innervation has also been described as necessary [31]. When the innervation of the area by the carotid sinus nerve, a sensory branch of the glossopharyngeal nerve, is compromised, the CB primordium is either not formed or not invaded by progenitors from the SCG [31]. Interestingly, recent findings introduced the possibility of multipotent Schwann cell progenitors migrating along the nerves and giving rise to not only peripheral glia but also neuronal subtypes in the different innervation-target tissues [32–34]. In fact, in the case of adrenal medulla, a tissue directly related to the carotid body, it has been described that up to 80% of total chromaffin cells can be derived from Schwann cell precursors arriving through the nerves [35]. In the case of CB, recent data indicate that at least part of the developing CB glomus cell population derives from progenitor cells with a glial phenotype (Plp1+), although the origin of those glia-like progenitors has not been unequivocally established [36]. Therefore, it cannot be discarded that CB sustentacular cells, and thus all their glomus cell derivatives, might be derived from Schwann cell precursors arriving through the sinus nerve.
In summary, further experimentation is necessary to elucidate the specific embryonic origin of CB stem cells. Cell fate mapping experiments using specific transgenic mice should solve the issue as to whether CBSCs are sympathoadrenal-, ectomesenchymal-, or Schwann cell precursor derived. Obviously, a possible answer to this issue might be that CB sustentacular cells constitute a mixed population of neural crest-derived cells originated from different embryonic lineages.
Glial nature of adult neural stem cells
Accumulating evidence indicates that stem cells within the nervous system acquire a glial phenotype, mainly in adult tissues and especially when they are quiescent. Adult neural stem cells express glial proteins, such as GFAP or S-100, within their niches, and this characteristic has classically induced researchers to confound them with differentiated glia. Stem cells within the adult carotid body germinal niche are not an exception to this rule. Herein, we now review existing data about the glial nature of neural stem cells both in central and peripheral nervous systems to highlight the similarities existing between carotid body and other neurogenic niches.
Stem cells within the central nervous system acquire a glial phenotype early during development, and consolidate this phenotype in postnatal life. Early neuroepithelial cells within the ectoderm rapidly elongate and get converted into radial glial cells as the epithelium thickens [37]. Radial glial cells express glial proteins and span a prolongation throughout the whole epithelium thickness [37]. After birth, radial glial cells within the subventricular zone (SVZ) are converted into astrocyte-like cells that no longer expand through the whole brain parenchyma but still have prolongations making contact mainly with blood vessels [38, 39]. These adult SVZ astrocyte-like stem cells are usually quiescent, and when they get activated they acquire expression of filament protein nestin as they lose expression of GFAP, becoming nestin +/GFAP- transit-amplifying progenitors [39, 40]. A nestin + activated stem cell can revert its phenotype back into quiescence again if the activating stimuli are terminated [40]. These data suggest that adult neural stem cells modify their cytoskeletal dynamics when switching from quiescence to activation and vice versa, expressing different filamentous proteins depending on their stage, GFAP in quiescence and nestin during activation [40]. We have reported a very similar situation in carotid body stem cells, which also switch from GFAP to nestin when being activated, and back to GFAP when the hypoxic stimulus is resumed [19].
Neural stem cells in the peripheral nervous system, called neural crest-derived stem cells (NCSCs) after their embryonic origin, also acquire a glial phenotype and can persist into adulthood, similar to their central counterparts. Due to their glial phenotype, NCSCs within adult ganglia have classically been overlooked and confused with mature peripheral glia. In fact, we still experience a lack of appropriate markers that can unambiguously distinguish between NCSCs and differentiated glia [41]. The current strategy includes studying their biology and assaying their behavior to accurately determine their nature. What we classically considered as peripheral glia comprises a heterogeneous group of neural crest-derived cells including autonomic and sensory satellite glial cells, enteric glia, and non-myelinating and myelinating Schwann cells. Some of these cell types have been described to behave as multipotent stem cells in adult tissues. For instance, behavior of satellite glial cells as multipotent NCSCs has been exposed in the adult dorsal root ganglia (DRG) [42, 43]. These cells seem to participate in neurogenesis upon nerve injury, although it is not clear whether they undergo multipotent differentiation in vivo [42]. In the enteric nervous system, NCSCs have been cultured from adult ganglia [44]. These enteric stem cells have also been described to display a glial phenotype in vivo [45] and have been reported to participate in tissue dynamics and maintenance [46], in addition to their activation upon tissue injury [47]. Other types of neural crest-target tissues have also been shown to contain stem cells persisting into adulthood, such as bone marrow [43], cornea [48], heart [49], or skin [50], although their behavior and phenotype are not yet fully documented.
Regarding sympathetic nervous system, adult satellite glial cells within sympathetic tissues share some characteristics, such as expression of GFAP, their disposition forming a cover with neuronal somata, or the use of purinergic signaling as a way of communicating with nearby neurons [51]. However, not all satellite glial cells in adult sympathetic tissues have been described to retain multipotent capacity and behave as NCSCs. Some sort of adult stem cell activity in these glial cells has only been described in neuroendocrine derivatives of the sympathoadrenal lineage, both in the adrenal medulla [52, 53] and in the carotid body [19]. However, to the best of our knowledge, no report exists about such a stem cell activity in adult sympathetic ganglia. Glia-like sustentacular cells, which belong to the more general family of satellite glial cells and are present in both CB and adrenal medulla, have been shown to behave as adult NCSCs both in vitro and in vivo. In the adrenal medulla, these glial cells express nestin to some extent, form neurospheres in culture, and undergo multipotent differentiation in vitro [52]. Moreover, in addition to their role in the adaptation of the organ to physiological needs, they seem to participate in the organismal response to stress [52, 54]. On the other hand, we have described that adult CB sustentacular cells are NCSCs that get activated under hypoxia, contributing to physiological adaptation of the organ [19]. In the case of CB, the glial phenotype is acquired by stem cells early postnatally, as shown by genetic studies using the GFAP promoter demonstrating that these GFAP+ cells are already present and participate in the postnatal maturation of the organ [55].
In summary, glia-like progenitor cells within the adult nervous system participate in neurogenesis in their resident tissues in response to physiological adaptation, stress, or injury. Mounting data confirm that numerous glial cell types in the adult peripheral nervous system are truly multipotent neural crest stem cells that have evolved acquiring a glial phenotype during development or in adult tissues.
Glycolytic metabolism of central and peripheral neural stem cells
Nervous system-specific stem cells, just like most somatic stem cells, rely on a glycolytic metabolism to maintain themselves during quiescence and when self-renewing [56, 57]. This strategy might be forced by the hypoxic environment of germinal niches and the stabilization of Hypoxia Inducible Factor (HIF), which stimulates the expression of glycolytic enzymes [57, 58]. Quiescent and low-proliferating stem cells and progenitors benefit from glycolysis to obtain the necessary energy while minimizing the production of damaging Reactive Oxygen Species (ROS), characteristic of oxidative phosphorylation at the mitochondria [58]. Upon differentiation, a series of metabolic changes take place, making differentiating cells to depend more on oxidative phosphorylation for energy production, with consequent modulation of mitochondria biogenesis and increase of ROS production [59, 60]. These observations, however, do not exclude mitochondria as important players for stem cell homeostasis both in quiescence and during activation. Accumulating evidence suggests that mitochondria play a crucial role in shaping stem cell fate through the modulation of bioenergetics, redox balance, calcium buffering, and epigenetics [59, 61, 62]. Nevertheless, in general, neural stem cells are mostly glycolytic within their neurogenic niches, a feature that makes them resist reasonably well the hypoxic situation, much better than their differentiated progeny, which, in the case of neurons, are extremely dependent on an intact mitochondrial oxidative phosphorylation [63].
The carotid body constitutes an interesting niche where to study cellular dependence on aerobic or anaerobic metabolisms, due to its crucial role as sensor of moderate hypoxia. Similar to central neurons, carotid body glomus cells perish shortly after compromising oxidative phosphorylation, by for example genetic elimination of complex II from mitochondrial electron transport chain [27]. However, when we performed the same genetic strategy but using the CBSC-specific GFAP promoter, no major effect was observed in neurosphere formation or growth, despite death of the affected neuronal progeny [55]. During this study, we confirmed that central nervous system stem cells were also resistant to the lack of a functional oxidative phosphorylation [55], suggesting that both central and peripheral neural stem cells relay on glycolytic metabolism for their normal function.
Once the nature and phenotype of CBSCs have been reviewed and placed in the context of other neural stem cells, we will now present our current understanding of the functioning of the carotid body niche, drawing parallelisms again with other germinal niches in the nervous system.
Functioning of the adult CB germinal niche
Neuronal activity-dependent regulation of stem cell activation
The more we know about the behavior and functioning of carotid body stem cells, the more we can trace parallelisms with other populations of neural stem cells in the organism. A clear example is the activation of CBSCs in response to neuronal activity. Adult neural stem cells live in germinal niches, where they are surrounded by cellular and non-cellular components that regulate their behavior both in physiological and pathological situations [64, 65]. Neurogenic niches in the adult central nervous system are well characterized and they typically include neuronal axons among their components [66]. This innervation of neurogenic niches suggests a regulation of neural stem cell behavior by neuronal activity. In fact, there is strong evidence showing that neural stem cells survey surrounding neuronal firing to allow the production of new neurons to be conditioned by brain activity and experience. We will now briefly review the evidence for this, in order to contextualize our recent observations on the regulation of CBSCs by neuronal activity.
The importance of neuronal innervation for stem cell activity is particularly evident in the subventricular zone (SVZ), a very well-characterized neurogenic niche lining the walls of the brain lateral ventricles. In this adult niche, astrocyte-like neural stem cells make contact with the cerebrospinal fluid inside the ventricles through a signaling cilium and, on the other side, they expand a cellular extension to contact blood vessels [67, 68]. Two different types of innervation of SVZ stem cells have been described: a basal innervation running in parallel to blood vessels [66], and an apical innervation that makes synapses with neural stem cell apical processes [69]. Basal innervation activity is detected through neurotransmitter spillover and allows the connection of neural stem cell activity with the dopaminergic mesostriatal pathway, involved in reward, addiction, and movement [70–74]. Regarding apical innervation, serotonergic axons at the surface of the lateral ventricle wall present varicosities with synaptic vesicles and form synapses with the apical prolongation of neural stem cells [69]. Neuronal activity in these axons is related to circuitries involved in different aspects of behavior such as mood, sleep, appetite, or cognition [75]. Ablation of this apical innervation results in decreased SVZ stem cell proliferation [76, 77], suggesting a connection between mood disorders and SVZ stem cell behavior.
The subgranular zone (SGZ) of the hippocampus also encloses a population of adult neural stem cells resembling astrocytes. These stem cells give rise to intermediate progenitor cells and migrating neuroblasts, which in turn differentiate into new neurons that will supply the granular layer of the hippocampal Dentate Gyrus (DG) [78]. Incorporation of new neurons to hippocampal circuits has functional effects in the activities of learning and memory [79]. In this case, all newly generated hippocampal neurons stay at the DG, where neuronal activity-dependent regulation has been reported [80]. For example, excitatory activity exerts an increase in neuronal differentiation from SGZ stem cells, diminishing glial differentiation and not affecting proliferation [81]. On the contrary, serotonergic innervation has been shown to enhance NSC proliferation in the SGZ [82]. Moreover, neuronal activity regulation in the SGZ also depends on local innervations, such as the GABAergic regulation of stem cells by granule cell layer interneurons [83, 84]. More recent evidence has shown that this GABA, released from interneurons, supports the maintenance of quiescence in SGZ radial glia-like stem cells [85].
Classical ultrastructural studies on the carotid body have described sustentacular cells engulfing or surrounding neuron-like cells in the organ [7, 86]. Glomeruli of glomus cells are usually covered by sustentacular cell prolongations that intimately attach to the membrane, resulting in an apposition of the two cells with an intermembrane distance no thicker than a synaptic space [87] (Fig. 1). This structure is particularly revealing of its function, since glomus cells are specialized neurons with synaptic vesicles distributed all around their membranes, with only some specific synaptic areas described in front of other glomus cells [88]. Therefore, this anatomical relationship between neuron-like cells and stem cells within the carotid body is suggestive of a close crosstalk between the two cell types. In fact, in vitro co-culture assays have demonstrated an intimate communication between type I and type II cells that might condition the physiology of neuron-like cells [89]. We ourselves tested this communication in the context of stem cell activity and proliferation by exposing neurosphere-forming sustentacular cells to media containing different neurotransmitters and neuromodulators described as abundant in glomus cell vesicles. From all substances examined, endothelin-1 (ET-1) displayed the clearest activating effect on neurosphere growth and size [87]. Although ET-1 is a cytokine classically described as secreted by vascular cells [90], in the case of the carotid body, glomus cells have been demonstrated to release ET-1 in response to the hypoxic stimulus [87, 91–93]. Moreover, we have shown that CB progenitor cells express receptors for ET-1 both in quiescence and upon activation [87]. Abrogation of glomus cell exocytosis by means of genetic manipulation or through pharmacological inhibition in vivo decreases the percentage of CBSCs activated by hypoxia [87], confirming the importance of neuronal activity as a trigger for stem cell proliferation and organ growth. ET-1 is a potent proliferative agent that has been described to regulate the behavior of neural crest progenitors during development [94–96]. Hence, our results are compatible with the neural crest origin of CBSCs and offer a new molecular and cellular mechanism by which these progenitors respond to physiological hypoxia, through neuronal activity.
Fig. 1.
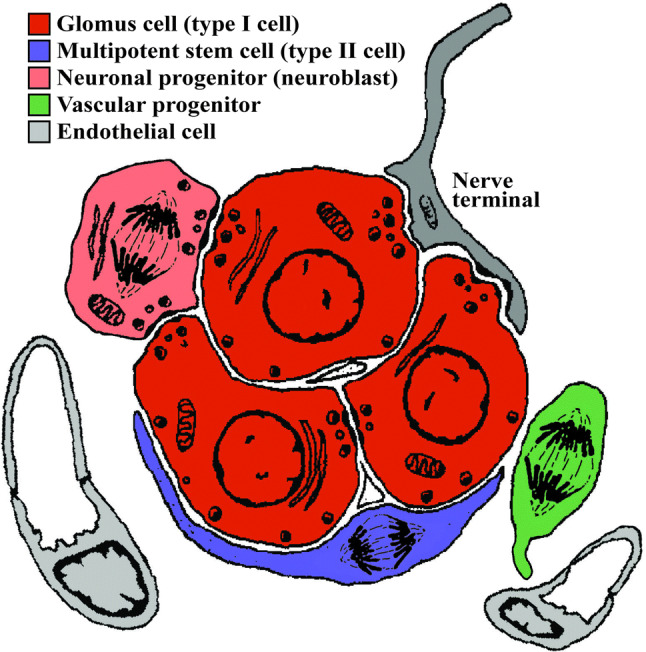
Diagram representing the most relevant cell types present in an adult carotid body glomerulus. Cells with the capacity to proliferate are depicted with metaphase separating chromosomes
An important question raised during our studies on CBSC activation course was if these cells are intrinsically sensitive to hypoxia itself, a process that could complement their surveillance of neuronal activity. Stabilization of Hypoxia Inducible Factor (HIF) by low oxygen has been reported to regulate self-renewal, proliferation, and differentiation of stem cells, including neural stem cells [97–99]. In general, with regard to stem cells in the nervous system, physiological low oxygen (2–5% O2) promotes self-renewal and proliferation, while extreme hypoxia (< 1% O2) or hyperoxia (ambient 20% O2) induces terminal differentiation and apoptosis [98, 100]. However, in the case of CB stem cells, exposure to hypoxia in vitro (1–3% O2) does not have any effect on neurosphere formation and growth [87]. In fact, mimicking hypoxia in vivo by pharmacological inhibition of prolyl hydroxylases (PHDs), the enzymes responsible for tagging HIF for degradation in normoxia, does not affect CBSC proliferation [87], while it affects general proliferation in the organ [101]. These results confirm that CBSCs are not intrinsically sensitive to hypoxia and that their activation process is niche dependent, mainly through scrutiny of the chemoreceptor activity of neuron-like cells.
The process of neurogenesis in the carotid body
Neural stem cells contribute to the plasticity of the adult nervous system by giving rise to new neurons that integrate into the existing circuits [66]. Neurogenesis in both CNS niches occurs from glia-like stem cells giving rise to proliferating intermediate progenitors first. Subsequently, these progenitors originate committed neuroblasts which proliferate, migrate, and initiate a slow maturation process into new neuronal cells [39, 102, 103]. The whole progression typically takes from days to even months. In the SVZ, for example, neuroblasts proliferate and migrate through the rostral migratory stream all the way to the olfactory bulb, where they complete maturation into new interneurons. The complete sequence of events defining SVZ neurogenesis takes about 2 weeks to be finalized [39, 104]. Moreover, an even longer delay has been described for the case of hippocampal DG neurogenesis [102].
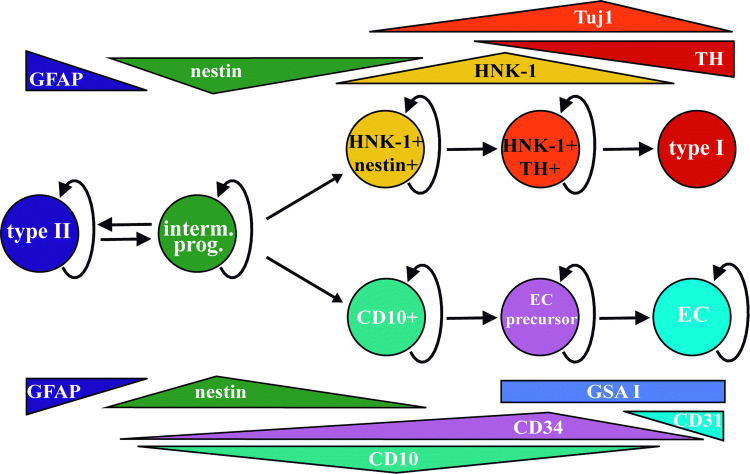
We have recently described two different types of neurogenic processes within the adult CB parenchyma in response to the hypoxic stimulus. On one hand, cell fate mapping studies using transgenic mice revealed the capacity of multipotent CBSCs to convert into new glomus cells. Glia-like sustentacular cells respond to hypoxia by getting activated, switching to nestin + proliferative progenitors, and eventually transforming into new neuron-like cells [19], among other cell types. BrdU incorporation studies in mice determined that 5–7 days under stimulation are necessary for the first stem cell-derived glomus cells to appear [19]. However, we have very recently described a second, much faster, process of adult neurogenesis in the CB. We found a subpopulation of immature neuroblasts that are quiescent in normoxia and get activated during hypoxia, to rapidly convert into mature glomus cells [105] (Fig. 1). This ‘fast’ neurogenesis is completed in 24–48 h since the beginning of the stimulus and might facilitate an immediate adaptation of the organism to the hypoxemic situation. CB neuroblasts express some markers typical of neural crest cells, such as HNK-1 [106, 107], in addition to general neuroblast markers like Tuj1 [105] (Fig. 2). These CB immature neuron-like cells already express ion channels in their membranes, contain some secretory vesicles, and respond to some physiological stimuli. However, they are still insensitive to an acute hypoxic stimulus themselves, so they cannot participate in low PO2 chemoreception yet [105]. Despite their insensitivity to acute hypoxia, CB neuroblasts seem to get activated in chronic hypoxia by self-detecting the stimulus, likely through HIF2alpha stabilization [105]. A recent report confirms the HIF2alpha-dependence of this TH + cell proliferation and suggests that in the case of mice this neurogenic mechanism might be much stronger than the stem cell-dependent production of new neuron-like cells [108]. To the best of our knowledge, the existence of quiescent immature neuroblasts has no clear parallel in any other adult nervous system niche, which argues about the importance of the CB germinal niche when guaranteeing a rapid organismal adaptation to the lack of oxygen.
Fig. 2.
Balls and arrows diagram symbolizing the cellular lineages characterized in the adult carotid body from multipotent stem cells. Expression of the different markers spanning the different cells is depicted in triangles close to the corresponding circles. Cells with proliferative potential have curved arrows pointing to themselves. GFAP+ multipotent type II cells, and nestin+ multipotent progenitors (prior to the expression of lineage-specific markers), are common for both lineages and interchangeable depending on the presence of stimuli. The neuronal lineage gives rise to TH+ mature glomus cells, while the ectomesenchymal lineage gives rise to either CD31+ endothelial cells (EC) or SMA+ smooth muscle cells and perycytes (not included in the diagram)
CBSCs contribute to angiogenesis in addition to neurogenesis
During CB adaptation to hypoxia, a profound angiogenic process takes place in addition to the growth of neural parenchyma [15, 109]. The new vessels ensure proper supply to the growing neuronal glomeruli and guarantee the chemoreceptive function of the organ. Since CBSCs are derived from the neural crest, and neural crest stem cells have mesectodermal differentiation potential [110], the question rapidly arises as to whether stem cells in the CB might also contribute to angiogenesis by differentiating into vascular cells. Conversion of neural stem cells into vascular cell types has been reported in vitro [111, 112], and in pathological situations in vivo such as tumor growth [113–116]. However, participation of nervous system stem cells in the production of vascular cells in a physiological setting in vivo had only been described in cephalic and cardiac neural crest during development. Cephalic NCSCs give rise to pericytes and smooth muscle cells in capillaries of the face and forebrain during development [117], while cardiac NCSCs contribute to the developing heart valves and to the walls of cardiac outflow arteries [118, 119]. We, therefore, tested the possibility of CBSCs being able to convert into vascular cells, using cell fate mapping approaches with transgenic mice [18]. We found that these tissue-specific stem cells do retain multipotent differentiation capacity in vivo, being able to contribute to both neurogenesis and angiogenesis in response to the hypoxic stimulus. CBSCs are able to convert into smooth muscle and endothelial cells, the main components of blood vessels [18, 120]. Differentiation of CBSCs into endothelial cells is favored by different vascular cytokines such as erythropoietin or VEGF, and it is also activated by hypoxia itself in a HIF2alpha-dependent manner [18]. Moreover, we even identified a subpopulation of mesectodermal-committed intermediate progenitors (Fig. 1), positive for the membrane marker CD10, present in quiescence in the normoxic CB and prepared for conversion into both smooth muscle and endothelial cells upon hypoxic stimulation [120] (see Fig. 2 for other cellular makers for this vascular lineage of CBSCs). To the best of our knowledge, CBSCs are the only neural stem cells described so far as capable of differentiating into both neural and vascular cell types in an adult physiological situation in vivo. Our findings stress out again the importance of this germinal niche for the correct homeostasis of oxygen and hence for the survival of the organism in changing environmental situations.
Specification and plasticity of adult CBSCs
A classical discussion in the neural crest stem cell field refers to whether NCSCs lose multipotentiality as they are specified for a particular target tissue [121, 122]. Experiments in avian embryos early showed that pre-migratory and migratory NCSCs are mostly multipotent [123] and that they become more restricted in response to local cues along migratory routes and at target tissues [124]. Postmigratory trunk NCSCs isolated from different regions are intrinsically different, which allows the production of appropriate cell types on each location [125, 126]. However, this local specification is not achieved at the expense of multipotentiality. Different NCSCs giving rise to completely different neuronal subtypes are still able to differentiate into smooth muscle and glial cells in vitro [127]. Our studies on the CB confirm this hypothesis by showing that CBSCs retain the ability to differentiate into vascular derivatives despite their high specification for dopaminergic differentiation. We have shown that CBSCs differentiate specifically into CB glomus cells, with typical functional and electrical properties and are not able to convert into other neuronal subtypes [19], while they are still able to convert into vascular cells [18]. Recent experiments of cell fate mapping using confetti technique have come to the same conclusion that NCSCs can be specified to a particular tissue while preserving multipotency [128]. Hence, altogether the data suggest that neural crest specification takes place without completely losing multipotentiality [41] and that plasticity seems to be preserved depending on the tolerant nature of the local environment.
Clinical relevance of the adult CB germinal niche
The CB seems to be involved in a number of different pathologies coursing with sympathetic over activation, generally affecting the cardiorespiratory system, such as hypertension, sleep apnea, chronic heart failure, some forms of chronic kidney disease, or even in diabetes [129, 130]. In some other pathologies, like asthma or obstructive pulmonary disease, a direct and relevant participation of the CB has been described [131, 132]. In the majority of these illnesses, an increase in CB size has been documented [129], corroborating over activation of the organ during disease progression. Currently, the CB constitutes a principal target in the treatment of most of these diseases. In fact, resection of the CB is being examined to try to ameliorate chronic heart failure and hypertension symptoms [133–135]. Our work on the functioning of the CB germinal niche and on the biology and behavior of CBSCs might offer new targets and strategies for the treatment of this variety of diseases.
A number of different tumorigenic growths appear in sympathoadrenal tissues, such as pheochromocytomas in the adult adrenal medulla, paragangliomas in the adult CB, or neuroblastomas in the developing sympathetic ganglia. It has been shown for example that paragangliomas resemble the CB of individuals exposed to chronic hypoxia [14, 136]. In fact, the incidence of this type of benign tumors increases in high altitude residents [137–139]. However, it has not been thoroughly studied whether there could be any potential relationship between the proliferative capability of CB or adrenal medulla germinal niches and the pathophysiology of these diseases. Future studies should determine whether transformation of multipotent progenitors into cancer stem cells, or hyperproliferation of uncontrolled neuroblasts, might be behind the etiology of some of these tumors.
CB cell aggregates have been successfully transplanted into the brain for the amelioration of Parkinson’s disease symptoms, in animal models [140, 141] and in patients [142]. Transplanted CB glomus cells release trophic factors, especially glial-derived neurotrophic factor (GDNF), to recover dopaminergic Substantia Nigra neurons from degeneration [143]. CBSC cultures have been proposed as a strategy to optimize glomus cell transplantation [144]. Our knowledge on the functioning of the CB stem cell niche might help to obtain better yields of glomus cell production in vitro and hence to increase the efficiency of this type of transplants against Parkinson’s disease. Moreover, CB grafts are characterized by a remarkable longevity within the brain parenchyma, with some of them lasting the whole life of receptor animals [145]. A striking possibility is that CBSCs are contributing to vascularization of the transplant, facilitating blood flow and consequently survival of trophic factor-producing CB glomus cells. Future experimental work should elucidate whether these CB-specific stem cells maintain their mesenchymal potential and give rise to blood vessels upon transplantation into parkinsonian brains, facilitating survival of the transplant and recovery of disease symptoms.
Concluding remarks
In summary, the CB constitutes a remarkable chemoreceptor organ in the adult mammalian peripheral nervous system. Thanks to the existence of a stunning germinal niche within the glomus parenchyma, the CB is able to contribute to physiological adaptation of the organism to chronic hypoxemic situations, guaranteeing survival. A neat population of neural crest-derived adult multipotent stem cells with a glial phenotype remains in the organ throughout adult life, giving rise to new neuron-like cells and vascular cells in response to physiological demand. Our growing understanding of the molecular and cellular mechanisms underlying the functioning of this niche will very likely improve our comprehension of different types of pathologies, and our capacity to use these stem cells in cell therapy against neurological disorders.
Acknowledgements
This work was supported by grants from the Spanish Ministry of Economy and Competitiveness (SAF2016-80412-P, co-funded by FEDER funds) and the European Research Council (ERC Starting Grant to RP). We apologize to our peers whose work is not cited due to space constraints.
References
- 1.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353(19):2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Barneo J, Gonzalez-Rodriguez P, Gao L, Fernandez-Aguera MC, Pardal R, Ortega-Saenz P. Oxygen sensing by the carotid body: mechanisms and role in adaptation to hypoxia. Am J Physiol Cell Physiol. 2016;310(8):C629–642. doi: 10.1152/ajpcell.00265.2015. [DOI] [PubMed] [Google Scholar]
- 3.López-Barneo J, Pardal R, Ortega-Sáenz P. Cellular mechanisms of oxygen sensing. Annu Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- 4.De Castro F. Sur la structure et l’innervation de la glande intercarotidienne (glomus caroticum) de l’homme et des mammifères, et sur un nouveau système d’innervation autonome du nerf glossopharyngien. Trab Lab Invest Biol Univ Madrid. 1926;24(365432):13. [Google Scholar]
- 5.Ramón S, de Castro F. Elementos de técnica micrográfica del sistema nervioso. Tipografía artística. 1933;XII:409. [Google Scholar]
- 6.Heymans C, Bouckaert J, Dautrebande L. Sinus carotidien et réflexes respiratoires. II. Influences respiratoires reflexes de l’acidose, de l’alcalose, de l’anhydride carbonique, de l’ion hydrogene et de l’anoxémie: sinus carotidiens et échanges respiratoires dans les poumons et au dela des poumons. Arch Int Pharmacodyn Ther. 1930;39(2):400–408. [Google Scholar]
- 7.Kameda Y. Immunoelectron microscopic localization of vimentin in sustentacular cells of the carotid body and the adrenal medulla of guinea pigs. J Histochem Cytochem. 1996;44(12):1439–1449. doi: 10.1177/44.12.8985136. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K + current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241(4865):580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- 9.Urena J, Fernandez-Chacon R, Benot AR, Alvarez de Toledo GA, Lopez-Barneo J. Hypoxia induces voltage-dependent Ca2 + entry and quantal dopamine secretion in carotid body glomus cells. Proc Natl Acad Sci USA. 1994;91(21):10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arias-Mayenco I, Gonzalez-Rodriguez P, Torres-Torrelo H, Gao L, Fernandez-Aguera MC, Bonilla-Henao V, Ortega-Saenz P, Lopez-Barneo J. Acute O2 sensing: role of coenzyme QH2/Q ratio and mitochondrial ROS compartmentalization. Cell Metab. 2018;28(1):145–158. doi: 10.1016/j.cmet.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Aguera MC, Gao L, Gonzalez-Rodriguez P, Pintado CO, Arias-Mayenco I, Garcia-Flores P, Garcia-Perganeda A, Pascual A, Ortega-Saenz P, Lopez-Barneo J. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab. 2015;22(5):825–837. doi: 10.1016/j.cmet.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Gonzalez-Rodriguez P, Ortega-Saenz P, Lopez-Barneo J. Redox signaling in acute oxygen sensing. Redox biology. 2017;12:908–915. doi: 10.1016/j.redox.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakoczy RJ, Wyatt CN. Acute oxygen sensing by the carotid body: a rattlebag of molecular mechanisms. J Physiol. 2018;596(15):2969–2976. doi: 10.1113/JP274351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arias-Stella J, Valcarcel J. Chief cell hyperplasia in the human carotid body at high altitudes; physiologic and pathologic significance. Hum Pathol. 1976;7(4):361–373. doi: 10.1016/S0046-8177(76)80052-4. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZY, Bisgard GE. Chronic hypoxia-induced morphological and neurochemical changes in the carotid body. Microsc Res Tech. 2002;59(3):168–177. doi: 10.1002/jemt.10191. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Barneo J, Ortega-Saenz P, Gonzalez-Rodriguez P, Fernandez-Aguera MC, Macias D, Pardal R, Gao L. Oxygen-sensing by arterial chemoreceptors: mechanisms and medical translation. Mol Aspects Med. 2016;47–48:90–108. doi: 10.1016/j.mam.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Dhillon DP, Barer GR, Walsh M. The enlarged carotid body of the chronically hypoxic and chronically hypoxic and hypercapnic rat: a morphometric analysis. Q J Exp Physiol. 1984;69(2):301–317. doi: 10.1113/expphysiol.1984.sp002807. [DOI] [PubMed] [Google Scholar]
- 18.Annese V, Navarro-Guerrero E, Rodriguez-Prieto I, Pardal R. Physiological plasticity of neural-crest-derived stem cells in the adult mammalian carotid body. Cell Rep. 2017;19(3):471–478. doi: 10.1016/j.celrep.2017.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardal R, Ortega-Saenz P, Duran R, Lopez-Barneo J. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell. 2007;131(2):364–377. doi: 10.1016/j.cell.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 20.Pearse AG, Polak JM, Rost FW, Fontaine J, Le Lievre C, Le Douarin N. Demonstration of the neural crest origin of type I (APUD) cells in the avian carotid body, using a cytochemical marker system. Histochemie. 1973;34(3):191–203. doi: 10.1007/BF00303435. [DOI] [PubMed] [Google Scholar]
- 21.Anderson DJ. Molecular control of cell fate in the neural crest: the sympathoadrenal lineage. Annu Rev Neurosci. 1993;16:129–158. doi: 10.1146/annurev.ne.16.030193.001021. [DOI] [PubMed] [Google Scholar]
- 22.Saito D, Takase Y, Murai H, Takahashi Y. The dorsal aorta initiates a molecular cascade that instructs sympatho-adrenal specification. Science. 2012;336(6088):1578–1581. doi: 10.1126/science.1222369. [DOI] [PubMed] [Google Scholar]
- 23.Huber K. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev Biol. 2006;298(2):335–343. doi: 10.1016/j.ydbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131(19):4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- 25.Kameda Y. Carotid body and glomus cells distributed in the wall of the common carotid artery in the bird. Microsc Res Tech. 2002;59(3):196–206. doi: 10.1002/jemt.10194. [DOI] [PubMed] [Google Scholar]
- 26.Korkala O, Hervonen A. Origin and development of the catecholamine-storing cells of the human fetal carotid body. Histochemie. 1973;37(4):287–297. doi: 10.1007/BF00274965. [DOI] [PubMed] [Google Scholar]
- 27.Diaz-Castro B, Pintado CO, Garcia-Flores P, Lopez-Barneo J, Piruat JI. Differential impairment of catecholaminergic cell maturation and survival by genetic mitochondrial complex II dysfunction. Mol Cell Biol. 2012;32(16):3347–3357. doi: 10.1128/MCB.00128-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kameda Y. Mash1 is required for glomus cell formation in the mouse carotid body. Dev Biol. 2005;283(1):128–139. doi: 10.1016/j.ydbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Kameda Y, Saitoh T, Nemoto N, Katoh T, Iseki S. Hes1 is required for the development of the superior cervical ganglion of sympathetic trunk and the carotid body. Dev Dyn. 2012;241(8):1289–1300. doi: 10.1002/dvdy.23819. [DOI] [PubMed] [Google Scholar]
- 30.Kameda Y, Nishimaki T, Takeichi M, Chisaka O. Homeobox gene hoxa3 is essential for the formation of the carotid body in the mouse embryos. Dev Biol. 2002;247(1):197–209. doi: 10.1006/dbio.2002.0689. [DOI] [PubMed] [Google Scholar]
- 31.Kameda Y, Ito M, Nishimaki T, Gotoh N. FRS2 alpha 2F/2F mice lack carotid body and exhibit abnormalities of the superior cervical sympathetic ganglion and carotid sinus nerve. Dev Biol. 2008;314(1):236–247. doi: 10.1016/j.ydbio.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Furlan A, Adameyko I. Schwann cell precursor: a neural crest cell in disguise? Dev Biol. 2018 doi: 10.1016/j.ydbio.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Kastriti ME, Adameyko I. Specification, plasticity and evolutionary origin of peripheral glial cells. Curr Opin Neurobiol. 2017;47:196–202. doi: 10.1016/j.conb.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Uesaka T, Nagashimada M, Enomoto H. Neuronal differentiation in schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J Neurosci. 2015;35(27):9879–9888. doi: 10.1523/JNEUROSCI.1239-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furlan A, Dyachuk V, Kastriti ME, Calvo-Enrique L, Abdo H, Hadjab S, Chontorotzea T, Akkuratova N, Usoskin D, Kamenev D, Petersen J, Sunadome K, Memic F, Marklund U, Fried K, Topilko P, Lallemend F, Kharchenko PV, Ernfors P, Adameyko I. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science. 2017;357:6346. doi: 10.1126/science.aal3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hockman D, Adameyko I, Kaucka M, Barraud P, Otani T, Hunt A, Hartwig AC, Sock E, Waithe D, Franck MCM, Ernfors P, Ehinger S, Howard MJ, Brown N, Reese J, Baker CVH. Striking parallels between carotid body glomus cell and adrenal chromaffin cell development. Dev Biol. 2018 doi: 10.1016/j.ydbio.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6(11):1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 39.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/S0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 40.Codega P, Silva-Vargas V, Paul A, Maldonado-Soto AR, Deleo AM, Pastrana E, Doetsch F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron. 2014;82(3):545–559. doi: 10.1016/j.neuron.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zurkirchen L, Sommer L. Quo vadis: tracing the fate of neural crest cells. Curr Opin Neurobiol. 2017;47:16–23. doi: 10.1016/j.conb.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Li HY, Say EH, Zhou XF. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 2007;25(8):2053–2065. doi: 10.1634/stemcells.2007-0080. [DOI] [PubMed] [Google Scholar]
- 43.Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Morikawa S, Okada Y, Mabuchi Y, Katoh H, Okada S, Fukuda K, Suda T, Matsuzaki Y, Toyama Y, Okano H. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2(4):392–403. doi: 10.1016/j.stem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35(4):657–669. doi: 10.1016/S0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph NM, He S, Quintana E, Kim YG, Nunez G, Morrison SJ. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest. 2011;121(9):3398–3411. doi: 10.1172/JCI58186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulkarni S, Micci MA, Leser J, Shin C, Tang SC, Fu YY, Liu L, Li Q, Saha M, Li C, Enikolopov G, Becker L, Rakhilin N, Anderson M, Shen X, Dong X, Butte MJ, Song H, Southard-Smith EM, Kapur RP, Bogunovic M, Pasricha PJ. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci USA. 2017;114(18):E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P, Pachnis V. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011;121(9):3412–3424. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida S, Shimmura S, Nagoshi N, Fukuda K, Matsuzaki Y, Okano H, Tsubota K. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24(12):2714–2722. doi: 10.1634/stemcells.2006-0156. [DOI] [PubMed] [Google Scholar]
- 49.Tomita Y, Matsumura K, Wakamatsu Y, Matsuzaki Y, Shibuya I, Kawaguchi H, Ieda M, Kanakubo S, Shimazaki T, Ogawa S, Osumi N, Okano H, Fukuda K. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J Cell Biol. 2005;170(7):1135–1146. doi: 10.1083/jcb.200504061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong CE, Paratore C, Dours-Zimmermann MT, Rochat A, Pietri T, Suter U, Zimmermann DR, Dufour S, Thiery JP, Meijer D, Beermann F, Barrandon Y, Sommer L. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175(6):1005–1015. doi: 10.1083/jcb.200606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanani M. Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res Rev. 2010;64(2):304–327. doi: 10.1016/j.brainresrev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Rubin de Celis MF, Garcia-Martin R, Wittig D, Valencia GD, Enikolopov G, Funk RH, Chavakis T, Bornstein SR, Androutsellis-Theotokis A, Ehrhart-Bornstein M. Multipotent glia-like stem cells mediate stress adaptation. Stem Cells. 2015;33(6):2037–2051. doi: 10.1002/stem.2002. [DOI] [PubMed] [Google Scholar]
- 53.Santana MM, Chung KF, Vukicevic V, Rosmaninho-Salgado J, Kanczkowski W, Cortez V, Hackmann K, Bastos CA, Mota A, Schrock E, Bornstein SR, Cavadas C, Ehrhart-Bornstein M. Isolation, characterization, and differentiation of progenitor cells from human adult adrenal medulla. Stem Cells Transl Med. 2012;1(11):783–791. doi: 10.5966/sctm.2012-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vukicevic V, Rubin de Celis MF, Pellegata NS, Bornstein SR, Androutsellis-Theotokis A, Ehrhart-Bornstein M. Adrenomedullary progenitor cells: isolation and characterization of a multi-potent progenitor cell population. Mol Cell Endocrinol. 2015;408:178–184. doi: 10.1016/j.mce.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 55.Diaz-Castro B, Pardal R, Garcia-Flores P, Sobrino V, Duran R, Piruat JI, Lopez-Barneo J. Resistance of glia-like central and peripheral neural stem cells to genetically induced mitochondrial dysfunction–differential effects on neurogenesis. EMBO Rep. 2015;16(11):1511–1519. doi: 10.15252/embr.201540982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lange C, Turrero Garcia M, Decimo I, Bifari F, Eelen G, Quaegebeur A, Boon R, Zhao H, Boeckx B, Chang J, Wu C, Le Noble F, Lambrechts D, Dewerchin M, Kuo CJ, Huttner WB, Carmeliet P. Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. EMBO J. 2016;35(9):924–941. doi: 10.15252/embj.201592372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rafalski VA, Mancini E, Brunet A. Energy metabolism and energy-sensing pathways in mammalian embryonic and adult stem cell fate. J Cell Sci. 2012;125(Pt 23):5597–5608. doi: 10.1242/jcs.114827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burgess RJ, Agathocleous M, Morrison SJ. Metabolic regulation of stem cell function. J Intern Med. 2014;276(1):12–24. doi: 10.1111/joim.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lisowski P, Kannan P, Mlody B, Prigione A. Mitochondria and the dynamic control of stem cell homeostasis. EMBO Rep. 2018;19:5. doi: 10.15252/embr.201745432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wanet A, Arnould T, Najimi M, Renard P. Connecting mitochondria, metabolism, and stem cell fate. Stem Cells Dev. 2015;24(17):1957–1971. doi: 10.1089/scd.2015.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H, Menzies KJ, Auwerx J. The role of mitochondria in stem cell fate and aging. Development. 2018;145:8. doi: 10.1242/dev.143420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beckervordersandforth R, Ebert B, Schaffner I, Moss J, Fiebig C, Shin J, Moore DL, Ghosh L, Trinchero MF, Stockburger C, Friedland K, Steib K, von Wittgenstein J, Keiner S, Redecker C, Holter SM, Xiang W, Wurst W, Jagasia R, Schinder AF, Ming GL, Toni N, Jessberger S, Song H, Lie DC. Role of mitochondrial metabolism in the control of early lineage progression and aging phenotypes in adult hippocampal neurogenesis. Neuron. 2017;93(3):560–573e566. doi: 10.1016/j.neuron.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.d’Anglemont de Tassigny X, Sirerol-Piquer MS, Gomez-Pinedo U, Pardal R, Bonilla S, Capilla-Gonzalez V, Lopez-Lopez I, De la Torre-Laviana FJ, Garcia-Verdugo JM, Lopez-Barneo J. Resistance of subventricular neural stem cells to chronic hypoxemia despite structural disorganization of the germinal center and impairment of neuronal and oligodendrocyte survival. Hypoxia (Auckl) 2015;3:15–33. doi: 10.2147/HP.S78248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–686. doi: 10.1016/S0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 65.Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10(6):698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bond AM, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17(4):385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3(3):289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tong CK, Chen J, Cebrian-Silla A, Mirzadeh Z, Obernier K, Guinto CD, Tecott LH, Garcia-Verdugo JM, Kriegstein A, Alvarez-Buylla A. Axonal control of the adult neural stem cell niche. Cell Stem Cell. 2014;14(4):500–511. doi: 10.1016/j.stem.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20(2):575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- 71.Freundlieb N, Francois C, Tande D, Oertel WH, Hirsch EC, Hoglinger GU. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci. 2006;26(8):2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7(7):726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 73.Lennington JB, Pope S, Goodheart AE, Drozdowicz L, Daniels SB, Salamone JD, Conover JC. Midbrain dopamine neurons associated with reward processing innervate the neurogenic subventricular zone. J Neurosci. 2011;31(37):13078–13087. doi: 10.1523/JNEUROSCI.1197-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Keeffe GC, Barker RA, Caldwell MA. Dopaminergic modulation of neurogenesis in the subventricular zone of the adult brain. Cell Cycle. 2009;8(18):2888–2894. doi: 10.4161/cc.8.18.9512. [DOI] [PubMed] [Google Scholar]
- 75.Young SZ, Taylor MM, Bordey A. Neurotransmitters couple brain activity to subventricular zone neurogenesis. Eur J Neurosci. 2011;33(6):1123–1132. doi: 10.1111/j.1460-9568.2011.07611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29(3):450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- 77.Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89(4):999–1002. doi: 10.1016/S0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- 78.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gu Y, Janoschka S, Ge S. Neurogenesis and hippocampal plasticity in adult brain. Curr Top Behav Neurosci. 2013;15:31–48. doi: 10.1007/7854_2012_217. [DOI] [PubMed] [Google Scholar]
- 80.Zaben MJ, Gray WP. Neuropeptides and hippocampal neurogenesis. Neuropeptides. 2013;47(6):431–438. doi: 10.1016/j.npep.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 81.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42(4):535–552. doi: 10.1016/S0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 82.Brezun JM, Daszuta A. Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur J Neurosci. 2000;12(1):391–396. doi: 10.1046/j.1460-9568.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- 83.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47(6):803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 84.Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29(2):181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 85.Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, Deisseroth K, Luscher B, Christian KM, Ming GL, Song H. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012;489(7414):150–154. doi: 10.1038/nature11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biscoe TJ, Stehbens WE. Ultrastructure of the carotid body. J Cell Biol. 1966;30(3):563–578. doi: 10.1083/jcb.30.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Platero-Luengo A, Gonzalez-Granero S, Duran R, Diaz-Castro B, Piruat JI, Garcia-Verdugo JM, Pardal R, Lopez-Barneo J. An O2-sensitive glomus cell-stem cell synapse induces carotid body growth in chronic hypoxia. Cell. 2014;156(1–2):291–303. doi: 10.1016/j.cell.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 88.McDonald DM, Mitchell RA. The innervation of glomus cells, ganglion cells and blood vessels in the rat carotid body: a quantitative ultrastructural analysis. J Neurocytol. 1975;4(2):177–230. doi: 10.1007/BF01098781. [DOI] [Google Scholar]
- 89.Murali S, Nurse CA. Purinergic signalling mediates bidirectional crosstalk between chemoreceptor type I and glial-like type II cells of the rat carotid body. J Physiol. 2016;594(2):391–406. doi: 10.1113/JP271494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Faller DV. Endothelial cell responses to hypoxic stress. Clin Exp Pharmacol Physiol. 1999;26(1):74–84. doi: 10.1046/j.1440-1681.1999.02992.x. [DOI] [PubMed] [Google Scholar]
- 91.Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002;282(6):L1314–1323. doi: 10.1152/ajplung.00454.2001. [DOI] [PubMed] [Google Scholar]
- 92.McQueen DS, Dashwood MR, Cobb VJ, Bond SM, Marr CG, Spyer KM. Endothelins and rat carotid body: autoradiographic and functional pharmacological studies. J Auton Nerv Syst. 1995;53(2–3):115–125. doi: 10.1016/0165-1838(94)00179-N. [DOI] [PubMed] [Google Scholar]
- 93.Paciga M, Vollmer C, Nurse C. Role of ET-1 in hypoxia-induced mitosis of cultured rat carotid body chemoreceptors. NeuroReport. 1999;10(18):3739–3744. doi: 10.1097/00001756-199912160-00003. [DOI] [PubMed] [Google Scholar]
- 94.Bonano M, Tribulo C, De Calisto J, Marchant L, Sanchez SS, Mayor R, Aybar MJ. A new role for the Endothelin-1/Endothelin-A receptor signaling during early neural crest specification. Dev Biol. 2008;323(1):114–129. doi: 10.1016/j.ydbio.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 95.Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79(7):1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 96.Shin MK, Levorse JM, Ingram RS, Tilghman SM. The temporal requirement for endothelin receptor-B signalling during neural crest development. Nature. 1999;402(6761):496–501. doi: 10.1038/990040. [DOI] [PubMed] [Google Scholar]
- 97.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 98.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220(3):562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 99.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9(4):298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 100.De Filippis L, Delia D. Hypoxia in the regulation of neural stem cells. Cell Mol Life Sci. 2011;68(17):2831–2844. doi: 10.1007/s00018-011-0723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bishop T, Talbot NP, Turner PJ, Nicholls LG, Pascual A, Hodson EJ, Douglas G, Fielding JW, Smith TG, Demetriades M, Schofield CJ, Robbins PA, Pugh CW, Buckler KJ, Ratcliffe PJ. Carotid body hyperplasia and enhanced ventilatory responses to hypoxia in mice with heterozygous deficiency of PHD2. J Physiol. 2013;591(14):3565–3577. doi: 10.1113/jphysiol.2012.247254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9(6):723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 103.Kohler SJ, Williams NI, Stanton GB, Cameron JL, Greenough WT. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc Natl Acad Sci USA. 2011;108(25):10326–10331. doi: 10.1073/pnas.1017099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22(7):2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sobrino V, Gonzalez-Rodriguez P, Annese V, Lopez-Barneo J, Pardal R. Fast neurogenesis from carotid body quiescent neuroblasts accelerates adaptation to hypoxia. EMBO Rep. 2018;19:3. doi: 10.15252/embr.201744598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev Biol. 1986;115(1):44–55. doi: 10.1016/0012-1606(86)90226-5. [DOI] [PubMed] [Google Scholar]
- 107.Yagi H, Yanagisawa M, Suzuki Y, Nakatani Y, Ariga T, Kato K, Yu RK. HNK-1 epitope-carrying tenascin-C spliced variant regulates the proliferation of mouse embryonic neural stem cells. J Biol Chem. 2010;285(48):37293–37301. doi: 10.1074/jbc.M110.157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fielding JW, Hodson EJ, Cheng X, Ferguson DJP, Eckardt L, Adam J, Lip P, Maton-Howarth M, Ratnayaka I, Pugh CW, Buckler KJ, Ratcliffe PJ, Bishop T. PHD2 inactivation in Type I cells drives HIF-2alpha-dependent multilineage hyperplasia and the formation of paraganglioma-like carotid bodies. J Physiol. 2018;596(18):4393–4412. doi: 10.1113/JP275996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen J, He L, Liu X, Dinger B, Stensaas L, Fidone S. Effect of the endothelin receptor antagonist bosentan on chronic hypoxia-induced morphological and physiological changes in rat carotid body. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1257–1262. doi: 10.1152/ajplung.00419.2006. [DOI] [PubMed] [Google Scholar]
- 110.Le Douarin NM, Calloni GW, Dupin E. The stem cells of the neural crest. Cell Cycle. 2008;7(8):1013–1019. doi: 10.4161/cc.7.8.5641. [DOI] [PubMed] [Google Scholar]
- 111.Ii M, Nishimura H, Sekiguchi H, Kamei N, Yokoyama A, Horii M, Asahara T. Concurrent vasculogenesis and neurogenesis from adult neural stem cells. Circ Res. 2009;105(9):860–868. doi: 10.1161/CIRCRESAHA.109.199299. [DOI] [PubMed] [Google Scholar]
- 112.Wurmser AE, Nakashima K, Summers RG, Toni N, D’Amour KA, Lie DC, Gage FH. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430(6997):350–356. doi: 10.1038/nature02604. [DOI] [PubMed] [Google Scholar]
- 113.Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, Min W, McLendon RE, Rich JN, Bao S. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153(1):139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pezzolo A, Parodi F, Marimpietri D, Raffaghello L, Cocco C, Pistorio A, Mosconi M, Gambini C, Cilli M, Deaglio S, Malavasi F, Pistoia V. Oct-4 +/tenascin C + neuroblastoma cells serve as progenitors of tumor-derived endothelial cells. Cell Res. 2011;21(10):1470–1486. doi: 10.1038/cr.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468(7325):824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 116.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468(7325):829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 117.Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128(7):1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- 118.Brown CB, Baldwin HS. Neural crest contribution to the cardiovascular system. Adv Exp Med Biol. 2006;589:134–154. doi: 10.1007/978-0-387-46954-6_8. [DOI] [PubMed] [Google Scholar]
- 119.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127(8):1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 120.Navarro-Guerrero E, Platero-Luengo A, Linares-Clemente P, Cases I, Lopez-Barneo J, Pardal R. Gene expression profiling supports the neural crest origin of adult rodent carotid body stem cells and identifies CD10 as a marker for mesectoderm-committed progenitors. Stem Cells. 2016;34(6):1637–1650. doi: 10.1002/stem.2331. [DOI] [PubMed] [Google Scholar]
- 121.Anderson DJ. Cell and molecular biology of neural crest cell lineage diversification. Curr Opin Neurobiol. 1993;3(1):8–13. doi: 10.1016/0959-4388(93)90028-W. [DOI] [PubMed] [Google Scholar]
- 122.Bronner ME, LeDouarin NM. Development and evolution of the neural crest: an overview. Dev Biol. 2012;366(1):2–9. doi: 10.1016/j.ydbio.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fraser SE, Bronner-Fraser M. Migrating neural crest cells in the trunk of the avian embryo are multipotent. Development. 1991;112(4):913–920. doi: 10.1242/dev.112.4.913. [DOI] [PubMed] [Google Scholar]
- 124.Dupin E, Sommer L. Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol. 2012;366(1):83–95. doi: 10.1016/j.ydbio.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 125.Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35(4):643–656. doi: 10.1016/S0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 126.Mosher JT, Yeager KJ, Kruger GM, Joseph NM, Hutchin ME, Dlugosz AA, Morrison SJ. Intrinsic differences among spatially distinct neural crest stem cells in terms of migratory properties, fate determination, and ability to colonize the enteric nervous system. Dev Biol. 2007;303(1):1–15. doi: 10.1016/j.ydbio.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.White PM, Morrison SJ, Orimoto K, Kubu CJ, Verdi JM, Anderson DJ. Neural crest stem cells undergo cell-intrinsic developmental changes in sensitivity to instructive differentiation signals. Neuron. 2001;29(1):57–71. doi: 10.1016/S0896-6273(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 128.Bronner M. Confetti clarifies controversy: neural crest stem cells are multipotent. Cell Stem Cell. 2015;16(3):217–218. doi: 10.1016/j.stem.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 129.Cramer JA, Wiggins RH, Fudim M, Engelman ZJ, Sobotka PA, Shah LM. Carotid body size on CTA: correlation with comorbidities. Clin Radiol. 2014;69(1):e33–36. doi: 10.1016/j.crad.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 130.Paton JFR, Sobotka PA, Fudim M, Engleman ZJ, Hart ECJ, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013 doi: 10.1161/hypertensionaha.111.00064. [DOI] [PubMed] [Google Scholar]
- 131.Bencini C, Pulera N. The carotid bodies in bronchial asthma. Histopathology. 1991;18(3):195–200. doi: 10.1111/j.1365-2559.1991.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 132.Iturriaga R, Del Rio R, Idiaquez J, Somers VK. Carotid body chemoreceptors, sympathetic neural activation, and cardiometabolic disease. Biol Res. 2016;49:13. doi: 10.1186/s40659-016-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol. 2013;62(25):2422–2430. doi: 10.1016/j.jacc.2013.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Narkiewicz K, Ratcliffe LE, Hart EC, Briant LJ, Chrostowska M, Wolf J, Szyndler A, Hering D, Abdala AP, Manghat N, Burchell AE, Durant C, Lobo MD, Sobotka PA, Patel NK, Leiter JC, Engelman ZJ, Nightingale AK, Paton JF. Unilateral carotid body resection in resistant hypertension: a safety and feasibility trial. JACC Basic Transl Sci. 2016;1(5):313–324. doi: 10.1016/j.jacbts.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes. 2013;62(8):2905–2916. doi: 10.2337/db12-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kliewer KE, Wen DR, Cancilla PA, Cochran AJ. Paragangliomas: assessment of prognosis by histologic, immunohistochemical, and ultrastructural techniques. Hum Pathol. 1989;20(1):29–39. doi: 10.1016/0046-8177(89)90199-8. [DOI] [PubMed] [Google Scholar]
- 137.Arias-Stella J, Bustos F. Chronic hypoxia and chemodectomas in bovines at high altitudes. Arch Pathol Lab Med. 1976;100(12):636–639. [PubMed] [Google Scholar]
- 138.Astrom K, Cohen JE, Willett-Brozick JE, Aston CE, Baysal BE. Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum Genet. 2003;113(3):228–237. doi: 10.1007/s00439-003-0969-6. [DOI] [PubMed] [Google Scholar]
- 139.Saldana MJ, Salem LE, Travezan R. High altitude hypoxia and chemodectomas. Hum Pathol. 1973;4(2):251–263. doi: 10.1016/S0046-8177(73)80012-7. [DOI] [PubMed] [Google Scholar]
- 140.Espejo EF, Montoro RJ, Armengol JA, Lopez-Barneo J. Cellular and functional recovery of Parkinsonian rats after intrastriatal transplantation of carotid body cell aggregates. Neuron. 1998;20(2):197–206. doi: 10.1016/S0896-6273(00)80449-3. [DOI] [PubMed] [Google Scholar]
- 141.Luquin MR, Montoro RJ, Guillen J, Saldise L, Insausti R, Del Rio J, Lopez-Barneo J. Recovery of chronic parkinsonian monkeys by autotransplants of carotid body cell aggregates into putamen. Neuron. 1999;22(4):743–750. doi: 10.1016/S0896-6273(00)80733-3. [DOI] [PubMed] [Google Scholar]
- 142.Arjona V, Mínguez-Castellanos A, Montoro RJ, Ortega A, Escamilla F, Toledo-Aral JJ, Pardal R, Méndez-Ferrer S, Martin JM, Pérez M, Katati MJ, Valencia E, García T, López- Barneo J. Autotransplantation of human carotid body cell aggregates for treatment of Parkinson's disease. Neurosurgery. 2003;53(2):321–328. doi: 10.1227/01.NEU.0000073315.88827.72. [DOI] [PubMed] [Google Scholar]
- 143.Villadiego J, Mendez-Ferrer S, Valdes-Sanchez T, Silos-Santiago I, Farinas I, Lopez-Barneo J, Toledo-Aral JJ. Selective glial cell line-derived neurotrophic factor production in adult dopaminergic carotid body cells in situ and after intrastriatal transplantation. J Neurosci. 2005;25(16):4091–4098. doi: 10.1523/JNEUROSCI.4312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pardal R, Lopez-Barneo J. Neural stem cells and transplantation studies in Parkinson’s disease. Adv Exp Med Biol. 2012;741:206–216. doi: 10.1007/978-1-4614-2098-9_14. [DOI] [PubMed] [Google Scholar]
- 145.Toledo-Aral JJ, Méndez-Ferrer S, Pardal R, Echevarría M, López-Barneo J. Trophic restoration of the nigrostriatal dopaminergic pathway in long-term carotid bodygrafted parkinsonian rats. J Neurosci. 2003;23(1):141–148. doi: 10.1523/JNEUROSCI.23-01-00141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]