Abstract
Neurotrauma, a term referencing both traumatic brain and spinal cord injuries, is unique to neurodegeneration in that onset is clearly defined. From the perspective of matrix metalloproteinases (MMPs), there is opportunity to define their temporal participation in injury and recovery beginning at the level of the synapse. Here we examine the diverse roles of MMPs in the context of targeted insults (optic nerve lesion and hippocampal and olfactory bulb deafferentation), and clinically relevant focal models of traumatic brain and spinal cord injuries. Time-specific MMP postinjury signaling is critical to synaptic recovery after focal axonal injuries; members of the MMP family exhibit a signature temporal profile corresponding to axonal degeneration and regrowth, where they direct postinjury reorganization and synaptic stabilization. In both traumatic brain and spinal cord injuries, MMPs mediate early secondary pathogenesis including disruption of the blood–brain barrier, creating an environment that may be hostile to recovery. They are also critical players in wound healing including angiogenesis and the formation of an inhibitory glial scar. Experimental strategies to reduce their activity in the acute phase result in long-term neurological recovery after neurotrauma and have led to the first clinical trial in spinal cord injured pet dogs.
Keywords: Neuroinflammation, Angiogenesis, Deafferentation, Axonal plasticity, TBI, SCI
Introduction
In this review, we consider the diverse roles of matrix metalloproteinases (MMPs) in neurotrauma from axonal degeneration and synapse recovery in models of focal axonal injury and deafferentation, to disruption of the blood–brain/spinal cord barrier (BBB) after a traumatic injury. We address those proteases that facilitate wound healing, including angiogenesis and glial scar formation in a well-defined model of spinal cord injury (SCI) as well as studies of maladaptive and adaptive synaptic plasticity in the injured brain.
MMP expression following focal axonal insult and targeted deafferentation
The study of a targeted insult permits identification of postinjury axonal and/or synaptic reinnervation in a homologous population. Our understanding of the role of MMPs in central nervous system (CNS)-targeted axonal injury and deafferentation largely comes from optic nerve lesion, ablation of the entorhinal cortical input to the hippocampus, and lesion of the olfactory receptor neuron projection to the olfactory bulb. These models document time-dependent changes in a broad range of MMPs: collagenases (MMP-1,-13), gelatinases A and B (MMP-2 and MMP-9, respectively), stromelysin and matrilysin (MMP-3 and MMP-7, respectively), macrophage metalloelastase (MMP-12) and membrane-type matrix metalloproteinase 1 (MT1-MMP, also known as MMP-14), each linked to phases of the axon degeneration–synapse regeneration cycle. This cycle is initiated over the first 2 weeks after injury [1], with the induction of secreted MMPs (MMP-1, -2, -3, -7, -9, -12, -13), MT-MMPs (MT1-MMP, MT5-MMP, also known as MMP-24) and the family of a disintegrin and metalloproteinases (ADAMs) [ADAM thrombospondin (TS), ADAM10)] playing a prominent role. MMP substrates that direct postinjury reorganization appear dependent upon this timed enzyme induction [2]. Much of this enzyme/substrate interaction is involved with the management of cellular activity that removes degenerating axon terminals and reshapes synapses. These MMPs are often produced and secreted by local reactive glia. The following sections will review how recent studies reveal the timing of MMP postinjury signals, which are critical to synaptic recovery in different brain regions subjected to targeted axonal insult.
Optic nerve lesion
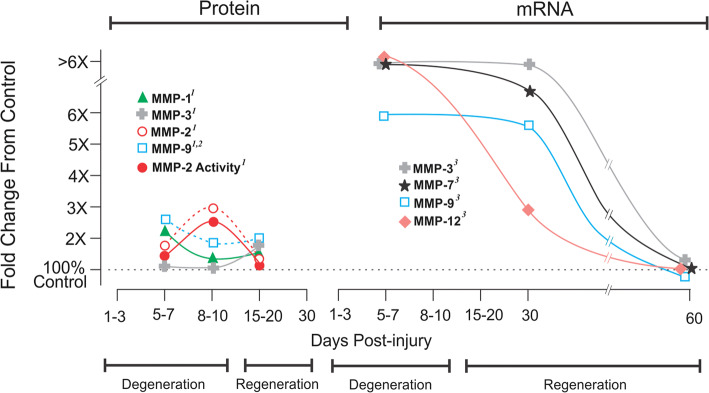
Both crush to the optic nerve or transection are applied to mammalian and non-mammalian species to map axonal degeneration and assess regeneration [3]. This process of repair occurs between 1 and 15 d (days) postinjury, followed by axon terminal regeneration (20–30 d) over a period of months [4–6]. Several MMP proteins have been compared at 6, 8 and 20 d after optic nerve crush by Ahmed and colleagues [4]. The active form of MMP-1 and -2 peaks at 6 and 8 d, respectively, but remains elevated up to 20 d after insult (Fig. 1 left panel). Zymography shows both pro and active enzymes are responsive to crush, with MMP-2 activity paralleling protein expression. This MMP response is attributed to reactive astrocytes, where both MMP-2 protein and activity are localized within GFAP+ astrocytes. Similarly, MMP-2 transcript is elevated 1 week post-lesion [7], with distinct time-dependent differences (Fig. 1 right panel). For example, there is an absence of acute (1–3 d) change in MMP-3 and -9 mRNA (not illustrated), but a six- to tenfold transcript increase over control at 7 d (see again Fig. 1 right panel). A ~ 24- and 45-fold rise in MMP-7 and MMP-12 mRNA at 7 d, extending to 30 d after crush, is also striking; likely due to vascular disruption accompanied by macrophage infiltration. Additionally, MMP-9 immunohistochemistry shows localization within reactive GFAP+ astrocytes, supporting MMP targeting of extracellular matrix (e.g., collagen, gelatin, laminin, fibronectin, elastin), as well as cell adhesion and guidance molecules (e.g., vitronectin, tenascin, proteoglycans). Notably, the major 20-d MMP-3 protein increase is time-delayed relative to its high 7-d transcript. By contrast, Liu et al. [6] reported postinjury MMP-9 immunolabeling, which did match mRNA induction (high at 7 d, persistent for 3 weeks). Recently, Lemmens and colleagues [8] examined MMP-2, -9, -13 and MT1-MMP after zebrafish optic crush, where degeneration/regeneration is accelerated relative to rodents and axon regrowth occurs by two weeks after injury (not illustrated). In this model, MMP-9 and MT1-MMP are induced during the acute postinjury phase, with MMP-9 showing a second 14-d peak during regeneration. By contrast, MT1-MMP falls to control level during this later phase of the recovery cycle, indicating its prominent role in the early injury response. Similar to MMP-9, active MMP-2 is associated with axonal regrowth, nearly twofold over control at 7 d and threefold by 10 d post-lesion. Finally, collagenase MMP-13 is most responsive, sixfold peak over control at 7 d, bracketed by fourfold rise at 4 and 10 d. At the 21-d mark of major axon regeneration, all four MMPs are near control expression. Together, studies in rodents and zebrafish support a time-dependent MMP response in the lesioned optic nerve, where enzymes are recruited as a function of tissue status during the degeneration/regeneration cycle. Most prominent is activation of MMP-2, -3, -7 and -9 at times marking both degeneration and onset of axon regrowth. Moreover, mRNA for MMPs, which are induced by vascular pathology (MMP-1 and -12) and provide membrane-targeted MMP activation (MT1-MMP), peak during regeneration.
Fig. 1.
Postinjury MMP protein and mRNA expression following optic nerve lesion. Rodent MMP expression after either optic nerve crush or transection insult. X-axis shows degeneration/regeneration time course. A 5–7-d increase in MMP protein levels (left panel) is matched by transcript elevation during early degeneration (right panel). Each MMP protein exhibits a different temporal pattern: MMP-9 increases earlier than MMP-2, whose activity maps concurrently; MMP-1 is biphasic, rising with degeneration and regeneration. MMP-3 is restricted to early regeneration. Transcript induction for each MMP is high into the regenerative phase, returning to control baseline over time. This time-dependent MMP response suggests cued role(s) to facilitate regeneration 1[4]; 2[6]; 3[7]
Hippocampal deafferentation
An early model of reactive synaptogenesis applied entorhinal cortical lesion (ECL) to deafferent the hippocampal dentate gyrus, where the time course of synapse replacement is mapped based on structural, molecular and functional parameters [1]. Local collateral axon sprouting and postsynaptic spine reorganization occurs over a 3-week period, with early degeneration (1–6 d) and subsequent regeneration (7–21 d). MMP-3 and ADAMTS upregulation [9, 10] is prominent at regenerative onset (Fig. 2a). MMP-3 mRNA and protein, as well as ADAMTS activity are all significantly elevated over controls at 7 d, the time point critical to the start of axonal sprouting and spine reformation. This 7-d peak in ADAMTS activity is bracketed by lower enzyme function at 2 and 15 d. Notably, immunohistochemistry for MMP-3 shows enzyme localizing to GFAP+ reactive astrocytes. Later, MMP-3 expression during axonal sprouting and synaptic replacement [11] exhibits dramatic MMP-3 increase within the deafferented dentate gyrus at 2 d post-lesion, a response attenuated and cell-focused at 7 and 15 d. In parallel, MMP-3 transcript returns to control level by 7 and 15 d post-lesion (Fig. 2a). MMP-3 protein is localized primarily within GFAP+ reactive astrocytes surrounding the affected synapses. Other studies probed 2–7-d MMP-2 and -9 activities as well [12]. Interestingly, enzyme function is significantly increased during acute degeneration (two- to fourfold). While ADAMTS activity profile peaks at 7 d, MMP-2 and -9 activities shift downward between 2 and 7 d after lesion. Clearly, both MMP-3 and gelatinases MMP-2 and -9 are recruited during acute synaptic damage. This occurs within local astrocytes responsive to the acute degeneration. It is likewise clear that MMP functional activity may be selectively engaged during the evolution of synaptic repair.
Fig. 2.
Postinjury MMP response in hippocampus and olfactory bulb following deafferentation. a Rodent MMP expression after entorhinal cortical lesion (ECL) deafferentation. X-axis shows degeneration/regeneration time course. MMP-3 transcript and protein increase acutely, in tandem with membrane-type MT5-MMP and ADAM10 protein (left panel). MMP-3 remains elevated throughout synapse regeneration, while the membrane-type enzymes return to baseline with synapse maturation at 15 d. Inducible activity of pro-enzyme is similarly elevated 1–2 d postlesion, with MMP-9 rapidly reduced by 7 d (right panel). By contrast, ADAMTS activity is below baseline at 1–2 d, but peaks at 7-d onset of terminal sprouting. The hippocampus shows time-dependent MMP response during reactive synaptogenesis. MMPs are prominent during acute degeneration and during the onset of synapse regeneration 1[1]; 2[10]; 3[26]; 4[13]; 5[12]. b Rodent MMP expression after olfactory bulb deafferentation (nerve transection [TX] or sensory neuron nasal chemical lesion [CL]). X-axis shows degeneration/regeneration time course. Rapid acute MMP-9 upregulation, peaks in degenerative phase, with a delayed MMP-2 induction at onset of synaptic repair. Nerve transection vs. sensory neuron lesion exhibits different response. MMP-9 rise is delayed after chemical lesion and peaks much higher. MMP-2 shows the opposite temporal pattern, where chemical lesion produces a modest 5-d peak relative vs. > 5X rise at 10-d rise with nerve transection. Olfactory bulb shows a time-dependent MMP response with deafferentation-induced synaptic plasticity and illustrates how injury modality alters cued MMP response 1[15]; 2[14]; 3[17]
Entorhinal cortex lesion (ECL) deafferentation is also used to map membrane-associated MMPs during reactive synaptogenesis (Fig. 2a). These metalloproteases target proteolysis at cell surfaces, as well as recruit and activate secreted MMPs for local activity. Warren and colleagues [13] mapped hippocampal MT5-MMP and ADAM10 after ECL. Like MMP-2, -3, -9, these enzymes are elevated during the acute degenerative phase (2 d), an effect maintained into 7-d regenerative onset. With synapse reformation at 15 d, MT5-MMP and ADAM10 return to control expression. Both are also predominant in reactive GFAP+ astrocytes; however, glial cell bodies had punctate MT5-MMP, while ADAM10 is distributed throughout the cells. Like MMP-2, -3, -9 and ADAMTS, both MT5-MMP and ADAM10 are acutely induced after ECL, with similar persistent elevation during axonal sprouting and onset of spine reformation. However, MT5-MMP and ADAM10 return to baseline during synaptic stabilization at 15 d, pointing to distinct roles for secreted vs membrane-type MMPs during synapse recovery.
Olfactory bulb deafferentation
A third CNS model of deafferentation used to map postinjury MMP response is the lesion of olfactory receptor axon input to the olfactory bulb (OB) by either knife transection near the bulb [14, 15], or a nasal chemical lesion of receptor cells [16, 17]. The period for synapse replacement in these models is longer given the distance required for regenerating olfactory axons to travel compared with local collateral sprouting after ECL. Morrison and Costanzo [18] found that olfactory nerve transection induces OB reinnervation, but requires 30 d for significant recovery. The OB exhibits a rapid, acute peak of two- to threefold in MMP-9 protein at 1 d after transection [15], followed by a gradual decrease over time, approaching control value at 35 d (Fig. 2b). MMP-2 protein exhibits a different pattern of bulb induction, with a delayed peak at 7 d post-lesion, surging to tenfold over control. Afterward, MMP-2 expression drops rapidly toward baseline between 10 and 15 d. During OB reinnervation, MMP-2 remains at or near the control baseline. By contrast, nasal chemical lesion of olfactory neurons generates a different pattern of gelatinase expression. Peak MMP-9 protein is delayed until 5 d after injury, with higher enzyme levels than after knife lesion of the olfactory nerve (Fig. 2b). This gelatinase peak gradually decreases over 5–10 d postinjury, remaining stable for 2 weeks, then returning to baseline at 35–40 d. Overall, MMP-2 response to chemical lesion is attenuated (twofold rise versus > fivefold with knife cut), hovering near baseline for the balance of the recovery time course.
It is interesting that the timing and extent of MMP response is shifted as a function of OB deafferentation method. With transection, a more rapid MMP-9 induction occurs, separate from MMP-2 peak at regeneration onset. This may result from more traumatic injury, rapidly recruiting vascular-associated MMP production, whereas chemical death of sensory neurons innervating the OB generates a partial, delayed deafferentation, with moderation of MMP response. Notably, the intensity and timing of MMP-2 response is altered. With chemical lesion, response is earlier (5 d) and significantly lower (twofold versus > fivefold). Given the greater burden of tissue recovery after transection, a higher level of MMP-2 is likely required to facilitate matrix reorganization and regeneration. It is noteworthy that rapid gelatinase activation (2–5 d postinjury) is reproduced across all three types of CNS deafferentation discussed here.
MMP substrates critical to axon and synapse recovery
MMP activation during axon regeneration and synaptogenesis serves as a switch to remove or modify a local matrix substrate, while attenuated MMP lysis permits substrate re-emergence. These substrates act to reshape synaptic structure and promote active growth. Whether repair involves axon regeneration, as with optic nerve lesion models, or a complex reconstruction of the synaptic junction, the spectrum of targeted substrates is similar. Overall, these substrates can be grouped into three categories: matrix proteins, growth factors and membrane proteins. In the case of ECL [1], they mark time-dependent phases of synapse degeneration, regeneration, and stabilization. Early lysis of collagen, agrin, laminin, brevican, tenascin and N-cadherin permits synaptic junction dissociation, preparing the local environment for the removal of dying axon terminals. MMP-2, -3, -9 and MT5-MMP, which target these proteins, are elevated within 1 d after injury. As an example, acute ADAM10 elevation correlates with reduction of N-cadherin in the dentate gyrus after ECL, consistent with a breakdown of synaptic junction partners for degeneration clearance [13]. Recently, a cell signaling osteopontin (OPN) fragment, cleaved by acute induction of MMP-2 and -9 activities, was reported in the ECL model [12]. RGD integrin-binding zones within OPN are exposed, providing both autocrine and paracrine signals for local glial activation [12, 19], while also fostering cell migration [20] and neuroprotection [21]. MMPs go on to degrade the RGD peptide to reduce OPN/integrin interaction [22, 23], which could further facilitate synapse fluidity.
MMP substrates are also critical for the onset and progress of synapse re-establishment. Sprouting axonal environment is conditioned with growth factor and cytokine signals (e.g., FGF, BDNF, NGF, TNF-α, OPN, IL-1β), as well as guidance proteins (e.g., agrin, proteoglycans, phosphacans). A complex of membrane-associated and secreted MMPs cleave growth factors to release active forms [24]. MT5-MMP and ADAM10 support this pattern after ECL; they are elevated at the 7 d after injury, a time point which coincides with the onset of axonal sprouting. Secreted MMP-2 and -9 lyse axonal stabilizers like neurofascin [1] and generate cytokine signals including OPN and TNF-α to stimulate local cell reactivity to support regeneration [1, 12, 25]. The visible shift toward protein reduction and enzyme activity between 7 and 15 d after injury indicates that as MMP response declines, targeted matrix guidance proteins re-emerge. For example, after ECL, a 7-d increase of MMP-3 cleaved agrin correlates with its role as a boundary marker between zones of intact and recovering synapses [26]. Similarly, MMP-mediated generation of phosphacan increases within the deafferented zone during 7-d synapse reorganization [1, 27]. In the final stage of synaptogenesis (15–21 d postinjury), synaptic growth peaks and pruning of new junctions occurs to stabilize the reinnervation pattern. As MMP expression shifts toward baseline at 15 d, expression of targeted substrates changes. Phosphacan protein, generated by the rise of MMP-2, -3 and -9 [28], returns to control level at a time when its role in axon guidance is essentially complete [27]. By contrast, membrane-tethering N-cadherin protein re-emerges at 15 d, in tandem with ADAM10 reduction and synapse stabilization [13].
In summary, there is evidence for time-dependent MMP induction following CNS deafferentation of multiple types. These MMPs target and process substrates critical to the specific phases of axon and synapse regeneration. There is a prominent role for matrilysin/stromelysin, gelatinase B and collagenase family members (MMP-12 and -13) in the acute degenerative phase, with MMP-2, MT-MMPs and ADAMs more pronounced with onset and execution of synapse regeneration. It is likely that these patterns reflect a MMP proteolytic switch, providing control of target substrates during the specific phases of recovery.
Central nervous system (CNS) trauma: MMPs and the barrier
The blood–brain barrier (BBB) is located at the level of the neurovascular unit, which consists of endothelial cells and the adjacent basal lamina, pericytes, glia, and neurons. Endothelial cells in the CNS are unique in that they lack fenestrations, have increased expression of tight junctional complexes that adjoin adjacent endothelial cells, show greater pericyte coverage per endothelial cell, and increased mitochondrial content compared to their peripheral counterparts [29–31].
Direct vascular damage and breakdown of the BBB are universal consequences of CNS trauma. This disruption exposes the brain to neurotoxic products that can contribute to neurological impairments [30]. Vascular tracers, including horseradish peroxidase, Evans blue albumin, luciferase, and fluorescently-tagged dextrans, have been used to map and quantify the temporal pattern of disruption. Leakage to exogenously administered proteins after a SCI, resulting from a contusion, occurs within minutes post-injury [32, 33] and extends along the axis of the cord [33]. A lower level of abnormal permeability is evident by 1 d, peaking at 2–3 d postinjury [33] and may continue for up to 1–2 months [34, 35]. Such an extended time course may be due to ongoing neuroinflammation, neurodegeneration, and formation of nascent vessels that exhibit a leaky phenotype.
In contrast to SCI, where the dominant preclinical model is a contusion, there are multiple, clinically relevant preclinical models of TBI, such as controlled cortical impact, closed skull impact, and fluid percussion injury all of which reflect the heterogeneity that is seen in the clinical population. The disruption of the barrier after a TBI is, in part, dependent upon the severity and type of injury. For example, a fluid percussion brain injury (FPI), which is typically characterized by diffuse axonal injury, results in widespread barrier disruption throughout both hemispheres [36]. In contrast, barrier disruption after a focal cortical contusion is primarily restricted to the ipsilateral hemisphere [37]. Similar to SCI, there is biphasic permeability to circulating proteins with pronounced leakage within minutes to hours after a TBI [36–41] followed by a 2nd peak at 3–7 days postinjury [38, 41, 42]. It is thought that the early vascular leakage, reported in both TBI and SCI preclinical models, likely reflects direct mechanical damage to vessels [43]. The second and lower peak at 3–7 d postinjury is characterized, in part, by barrier leakage to circulating plasma proteins that may coincide with increased caveolae and pronounced transcytotic activity in endothelial cells [44]. It is noteworthy that TBI may result in delayed discrete opening of the BBB in the white matter that is associated with microbleeds at 3 months postinjury [45]. Such findings raise increasing awareness of the parallels between TBI and other neurodegenerative disorders, including chronic traumatic encephalopathy and Alzheimer’s disease [46, 47].
MMPs are integral to barrier disruption after neurotrauma and, in general, blockade of those expressed acutely postinjury result in an improvement in neurological recovery. MMP-9 was the first of these proteases to be studied [48, 49]. Genetic deletion of MMP-9 or pharmacological inhibition of MMPs result in a reduction in barrier disruption after SCI and TBI [48, 50, 51]. It is now known that a number of other MMPs contribute to barrier disruption in neurotrauma including MMP-2, -3, -7, -8 (neutrophil collagenase), -9 and -12 [52–54]. These MMPs target the tight junction proteins occludin and claudin-5 and basal lamina proteins fibronectin, laminin, collagen, and heparan sulfate as well as adhesion molecules, receptors, myelin, axons, chemokines and growth factors [50, 51, 54–57]. Their temporal pattern of upregulation coincides with barrier disruption occurring within the acute to subacute phase (MMP-1, -3, -8, -9, -12) as well as permeability associated with angiogenesis during wound healing (MMP-2, -9 and -12) (Fig. 3). Infiltrating leukocytes are early sources of these MMPs whereas newly forming blood vessels and glia, associated with formation of a fibrotic scar, serve as sources during wound healing.
Fig. 3.
Summary of the temporal pattern of blood–brain barrier (BBB) disruption after spinal cord injury, relative to the expression of MMPs. Note the prominent leakage that occurs immediately after the injury, which likely reflects direct damage to the neurovascular unit (NVU). This is followed by a second phase of permeability, which peaks by about 2–3 d postinjury, with a reduction in permeability thereafter, corresponding to the angiogenic phase. The mechanism(s) driving extended permeability beyond about a month has yet to be identified
Infiltrating leukocytes and MMPs
While endothelial cells, neurons, and glia within the injured CNS express MMPs, infiltrating leukocytes are a major source of MMPs in the acutely injured spinal cord and brain (Fig. 3) [51, 52]. Here we focus on SCI, where the roles of MMPs and inflammation have been well established. Other reviews also address this relationship in TBI [52, 58]. MMP-8 and MMP-9, which are present in specific granules and in secondary and tertiary granules in neutrophils, respectively, are elevated in the injured cord at 1 to 3 d postinjury [49, 59]. This increase in MMP expression coincides with degradation of barrier-related tight junction proteins, neutrophil recruitment and expression of inflammatory cytokines. MMP-3 activity is increased from 8 h to 1 d postinjury, where it co-localizes in myeloperoxidase (MPO)-positive leukocytes, presumed to be neutrophils, and also in blood vessels. Studies of the MMP-3 knockout as well as direct administration of MMP-3 siRNA into the spinal cord have suggested its direct involvement in leukocyte recruitment and barrier disruption as well as indirectly, through its ability to activate MMP-9 [60].
Macrophage recruitment is likewise influenced by MMPs. MMP-12 is expressed by cells of the mononuclear phagocyte lineage. Transcripts for MMP-12 are elevated between 5 and 21 d postinjury. Spinal cord-injured MMP-12 knockout animals show reduced microglia/macrophage density, greater stabilization of the barrier and enhanced neurological recovery [61, 62]. MMP-9 mobilizes blood-borne monocytes to the injured cord. It does so through synergism with stromal cell-derived factor-1, expressed in the injured cord and acting as a chemoattractant for CXCR4+ monocytes [63]. More recent studies have revealed that MMP-9 is elevated in the lumbar spinal cord within the first week after a thoracic SCI where it is thought to contribute to microglial activation and impede activity-based training and recovery [64]. Subsequent studies demonstrated the dependency of MMP-9 on recruitment of myeloid cells into locomotor networks in the lumbar spinal cord after SCI [65]. These studies highlight infiltrating myeloid cells as modifiers of activity-dependent plasticity.
MMPs and vascular remodeling after CNS injury
Focal injuries to the brain and spinal cord result in primary mechanical damage to the cortical mantle and pericentral gray matter, respectively, with injury severity being a key factor in defining the extent of this primary injury. In the case of SCI, vulnerability is most notable in the pericentral gray matter where there is a secondary progression of hemorrhage in the central gray matter that expands into the pericentral white matter, a process that has been referred to as “progressive hemorrhagic necrosis”. One of the key mechanisms in this pathological event is the early destruction of capillaries [66]. The resulting hypoxia compounds toxicity, imposed by local hemorrhage, creating a hostile environment that is toxic to those cells that survived the initial insult. Hence, understanding the molecular players that regulate vascular function and stability after a traumatic event is essential for understanding early pathogenesis and the extent to which these acute events influence long-term wound healing.
Angiogenesis, which accompanies wound healing after a traumatic injury, is defined as the growth of vasculature from existing vessels. This involves distinct steps including the digestion of the extracellular matrix (ECM), proliferation and migration of endothelial cells, regression of vessels, and maturation as defined by the deposition of basal lamina and pericyte investment. Endothelial cell loss and subsequent angiogenesis, hallmarks of SCI and TBI, are evident within the first week after injury [29, 44], peaking between 4 and 7 d after injury. There is a progressive increase in vascular density by 7 d followed by normalization of vascular density [51]. These nascent vessels, emerging within the first week postinjury, do not express the glucose-1 transporter, lack key elements of the neurovascular unit (neurons, astrocytes or pericytes) and display vascular permeability to plasma proteins [33, 51]. Thus, prolonged permeability during wound healing reflects the unique phenotype of this regenerating vasculature. In the injured spinal cord, angiogenesis is prominent within a central, macrophage rich zone that is separated from the residual cord tissue by a glial scar. Hence, it is likely that this local environment, dominated by macrophages and nonneuronal cells, defines the leaky phenotype of these endothelial cells [33]. Newly forming vessels that reside outside of this glial scar undergo pruning by 2 weeks postinjury. However, angiogenesis may continue over several months postinjury and, as such, may also contribute to a low level of permeability until vessels assume a mature phenotype [29].
MMPs play a crucial role in vascular remodeling after a traumatic injury. ADAM8, expressed by cells near the tips of endothelial cells in the injured spinal cord, co-localizes with isolectin B4+ vessels and the proliferation marker, Ki67. Based on its temporal and spatial expression pattern, ADAM8 has been implicated in angiogenesis after injury [67]. MMP-2 likewise supports wound healing events such as angiogenesis and glial scar formation [68, 69]. MMPs, expressed at low levels in the naïve spinal cord and brain, increase in response to injury with unique temporal profiles for each of the proteases [51, 70]. While MMP-9 is elevated acutely after injury, MMP-2 is expressed during the subacute phase where this gelatinase is localized within blood vessels [51, 70, 71].
MMP-2 and -9 are critical to angiogenesis during wound healing after CNS trauma [72]. The digestion of the ECM and basal lamina allows for the migration and proliferation of not only endothelial cells but also progenitor cells that support vessel formation [52]. MMPs are involved in these events, as well as breakdown of the pericyte–endothelial interaction [69], release of angiogenic growth factors, promoting cell migration and exposing angiogenesis-inducing receptors [57].
In contrast to MMP-9 that is expressed acutely after CNS trauma, the active form of MMP-2 is expressed during the wound healing phase [51]. Inhibition of MMPs during the sub-acute and chronic phases has been shown to reduce neuronal plasticity, impair angiogenesis, and increase secondary tissue damage [51]. A recent study addressed the role of gelatinases in angiogenesis after SCI [69]. While MMP-2 is required for endothelial cell proliferation, formation of vessels in vivo after SCI and in in vitro assays proceeds independently of MMP-2 in the presence of MMP-9. These findings suggest redundant roles of these gelatinases in supporting angiogenesis after SCI [69]. One of the mechanisms by which MMP-2 may regulate angiogenesis is through binding to αVβ3 receptor and activation of P13K/AKT pathway to induce expression of VEGF, a known proangiogenic factor [73]. Activation of αVβ3 integrin via an integrin-binding peptide has been shown to rescue angiogenesis after SCI and improve white matter sparing and locomotor function [74].
MMP-2 acts not only as a regulator of angiogenesis (as defined by cell proliferation) but is also important for vascular stabilization after SCI. Nonetheless, prolonged imbalance in the expression of these gelatinases (extended expression of MMP-9 in the absence of MMP-2) results in vascular destabilization through the detachment of pericytes, followed by subsequent loss of vascular integrity and the emergence of vascular regression [69] (Fig. 4). MMP-9 may also induce vascular regression via the generation of anti-angiogenic factors that are substrates for MMP-9 [75].
Fig. 4.
Role of MMP-2 and MMP-9 on angiogenesis is time and context dependent. MMP-2 modulates endothelial proliferation and tube formation, whereas MMP-9 transiently supports only the latter. However, prolonged exposure to MMP-9 leads to pericyte detachment and vascular regression. Data represented from [69]. Image adapted from [105]
Pericytes play a critical role in the formation, maturation, and maintenance of the BBB [76, 77] and promoting vascular stability in existing blood vessels [78]. Interaction between pericytes and endothelial cells is important for vascular stability and is one of the last steps of angiogenesis [79]. Vascular stability is conferred through the deposition of ECM and also by release of factors that may promote endothelial cell differentiation and quiescence. PDGF production by endothelial tip cells is required for the recruitment of pericytes that express PDGFR-B and induces the upregulation of MMP-2 expression [80]. Detachment of pericytes from endothelial cells has been shown to lead to vascular destabilization, as characterized by increased permeability, loss of vascular integrity and regression [81].
In summary, the disruption of vessels after SCI and TBI and the hypoxic environment are triggers for angiogenesis. MMPs show a temporal response with regard to their contributions to angiogenesis. Early in the angiogenic cycle they are required for the digestion of the basal lamina and to promote migration of tip cells and progenitor cells. In the later phase, they are involved in stabilizing the vasculature.
MMPs and the glial scar in the injured spinal cord
One of the stages in wound healing after SCI is the formation of fibrotic glial scar [82]. This phase of tissue remodeling begins at the end of the first week. The glial scar is made up of resident glia, astrocytes, and oligodendrocyte progenitor cells [83]. The glial scar separates a macrophage-rich non-CNS environment from the spared cord tissue. The astrocytic scar serves as a protective barrier, segregating the inflammatory cells at the epicenter and immature blood vessels [51]. During scar formation, there is proliferation and migration of astrocytes towards the lesion margin and profound remodeling of the ECM with the deposition of CSPG, fibronectin, collagen and laminin in the lesion epicenter. This scar formation not only stabilizes the spread of secondary injury but also acts as a barrier that prevents axonal regeneration.
MMPs are implicated in the formation of the glial scar. MMP-1 is induced in the injured spinal cord as early as 12 h postinjury and continues to be upregulated till day 14 in neurons and astrocytes. MMP-1 expression is co-localized with PCNA in astrocytes, and activated caspase 3-expressing neurons, suggesting its role in proliferation of astrocytes and death of neurons after SCI. MMP-1-driven neuronal apoptosis occurs via the caspase-3-dependent, Bcl-2/Bax pathway [84]. Both MMP-2 and MMP-9 are also elevated during this period of wound healing, where they are each expressed in astrocytes. Early studies suggested that MMP-2 is involved in glial scarring in the injured spinal cord [85]. These findings were based upon experiments in the MMP-2 knockout, where it was later learned that there was a compensatory increase in MMP-9 in the MMP-2 knockout mice. Subsequent studies confirmed the involvement of MMP-9 in migration of astrocytes both in in vitro and during glial formation and in the injured spinal cord [68].
While the above findings argue for a dominant role of MMP-9 in glial scarring, recent studies highlight a novel contribution of MMP-2 to this event [71]. New evidence suggests that signal transducer and activator of transcription-3 (STAT3), a transcription factor expressed by astrocytes, regulates MMP-2 expression. Astrocytes, isolated from STAT3 knockout mice, have decreased expression and activity of MMP-2 in the absence of any change in MMP-9. Importantly, this reduction in MMP-2 is associated with impaired migration of astrocytes through Matrigel. Similar results are seen with the MMP-2 KO mice, suggesting a role for MMP-2 in proteolysis-dependent astrocyte migration [71]. Earlier studies using MMP-2- and MMP-9-specific inhibitors showed MMP-2-dependent migration of astrocytes in 2D culture models and colocalization of MMP-2 to the leading edge of migrating astrocytes [86].
In addition to enhancing the migration of astrocytes, MMPs are able to digest inhibitory molecules associated with the glial scar [68]. NG2, a chondroitin sulfate proteoglycan and a major component of the glial scar, is a target of MMP-9. Other inhibitory molecules, such as neurocan, versican, tenascin-C, brevican, neurocan, NG2, and phosphacan are substrates for MMP-2 and MMP-3.
MMPs as determinants of adaptive synaptic plasticity after injury
While the phases of adaptive synaptic recovery are cued by MMP activation, this plasticity becomes maladaptive in the presence of added pathologies. Excessive neuroexcitation is often co-expressed with axotomy, affecting time course and efficiency of synaptic repair. The exploration of whether MMP response changes during recovery can be studied in a maladaptive plasticity model [1]. When ECL is combined with diffuse fluid percussion injury (FPI + ECL), the recovery cycle is attenuated and synaptic plasticity is undermined [1]. These differences are apparent in structural, molecular and physiological features, closely associated with persistent, elevated MMP response. It is posited that such enzyme asynchrony underlies the aberrant pattern of synaptic regeneration and MMP manipulation would shift the outcome to adaptive plasticity.
Early work exploring FPI + ECL maladaptive plasticity reveals exacerbated postinjury increase of MMPs. This increase is first reported for MMP-2 transcript and elevated MMP-2 activity relative to adaptive ECL plasticity [87]. The enzyme also shows a broader tissue distribution over the deafferented region after the combined insult. When MMP-3 is mapped after FPI + ECL [9], it too shows increased transcription (Fig. 5a) and protein localization in deafferented sites. Interestingly, administration of MK-801 to block glutamate excitation in the combined insult is not only neuroprotective, but also reduces MMP-3 expression, suggesting that high MMP-3 contributes to the transformation of maladaptive into adaptive plasticity. For instance, as MMP-3 transcript increases under maladaptive conditions, expression of its substrate agrin is severely attenuated (Fig. 5a). Since agrin can organize postsynaptic sites for reinnervation, lysis by MMP-3 would contribute to the synaptic disruption seen with maladaptive plasticity. Similarly, FPI + ECL can change membrane type MMP response, affecting postsynaptic junction structure, cell surface protein shedding, as well as the local activation of secreted MMPs. MT5-MMP and ADAM10 are two membrane-associated metalloproteinases, linked to axon growth and spine formation [88, 89], which can also facilitate neurite outgrowth and synapse formation induced by adult pathology [90, 91]. During deafferentation-induced plasticity, these MMPs exhibit multi-fold increases; however, after FPI + ECL, ADAM10 increase was 30% greater than ECL alone [13]. Unlike MT5-MMP, this elevated response persists into the period of synapse stabilization and is correlated with a suppression of N-cadherin (Fig. 5a). Absence of N-cadherin upregulation in the maladaptive plasticity model results in the formation of weak synaptic junctions relative to those generated with adaptive synaptic recovery. Earlier FPI + ECL studies suggest that blocking such excessive MMP activity can attenuate maladaptive synaptic reorganization and improve functional outcome. Indeed, Falo and colleagues [11] report that reduction of MMP-3 transcription with postinjury MK-801 treatment improves cognitive recovery. The same authors go on to show that application of FN-439, a high-affinity MMP-3 inhibitor, reduces Morris Water Maze cognitive deficits after FPI + ECL (Fig. 5b). This effect is correlated with recovery of normal hippocampal synapse structure, suggesting that optimizing MMP induction encourages successful postinjury plasticity.
Fig. 5.
Effect of maladaptive plasticity on metalloproteinase response and functional outcome. a Paired metalloproteinase and substrate data showing exacerbated MMP-3/ADAM10 response with maladaptive combined fluid percussion injury and entorhinal cortical lesion (FPI + ECL) vs. adaptive ECL. Maladaptive increase in metalloproteinase expression (mRNA or protein) attenuates Agrin and N-cadherin level at the onset of reinnervation (7 d) and synapse maturation (15 d). Acute postinjury MMP inhibition reverses both reversal of cognitive deficits (b) and restores capacity for LTP induction (c) in the maladaptive FPI + ECL model. d Working model of MMP interaction with signaling proteins LCN2 and OPN to affect glial mediation of synaptic plasticity. Infiltrating leukocytes secrete LCN2 and OPN, which are processed by MMPs to activate local glia (left box). These glia can release leukocyte chemoattractant MCP-1 (blue) to enhance infiltration. MMP/signal protein amplification can further mediate cell response during acute degeneration and synapse regeneration (right boxes). GF growth factor. Data in a–c are taken from [11, 13, 26]
Interestingly, MT-MMPs are not equally sensitive to enzyme inhibition in the combined insult model. Warren and colleagues [13] also probed the effects of 6–7 d postinjury MMP inhibition using broad-spectrum inhibitor GM6001. Following FPI + ECL, GM6001 not only reduces 15 d ADAM10 expression to baseline, but shows a commensurate elevation of N-cadherin, equivalent to that of the adaptive ECL response. Notably, the drug fails to affect MT5-MMP expression. When hippocampal long-term potentiation (LTP) induction is tested at 7 d after FPI + ECL, field excitatory postsynaptic potential (fEPSP) is suppressed 15 min following high-frequency stimulation. However, this acute LTP suppression is no longer detected after GM6001 treatment, which reduces ADAM10 and increases N-cadherin expression (Fig. 5c). Thus, synapse integrity is strengthened with MMP/N-cadherin manipulation in the maladaptive model, generating structurally stable synapses capable of reinstating LTP. This would be consistent with the reported MMP/adhesion protein regulation of altered synaptic efficacy and LTP [92, 93]. These results provide evidence that poor structural and functional plasticity evoked with FPI + ECL can be manipulated by targeting MMPs, essentially moving a maladaptive condition toward adaptive recovery.
Just as MMP manipulation has the potential to shift maladaptive into adaptive synaptic plasticity, there is also evidence that the opposite can occur. Two ECL studies illustrate this adaptive to maladaptive shift. Reeves and colleagues [94] describe the effect of MMP inhibition on electrophysiological properties of reinnervated synapses. Postinjury FN-439 is delivered to ECL animals in a dose, which effectively targeted MMP-3 and -9. In contrast to the positive effects of GM6001 for FPI + ECL, FN-439 MMP inhibition prevents the normal reemergence of LTP in the adaptive plasticity model. Further, using current source density analysis, the pattern of recovering presynaptic input can be mapped along granule cell dendrites. With ECL + vehicle, the predicted loss and gain of input along affected dendrites is observed, consistent with adaptive synaptic reinnervation. When MMP-3 and -9 are inhibited, this pattern is markedly disrupted. Input from regenerative axons is very limited and focused to a narrow zone on the affected dendrites. Ultrastructural analysis of vehicle and FN-439-treated cases confirms that MMP inhibition delayed debris clearance and correlate with poor synapse recovery.
More recently, the potential for MMP-9 to affect inflammatory signals during reactive synaptogenesis was also explored. These studies are based on the fact that MMP cleavage of the cytokine OPN exposes integrin-binding sites. This interaction is particularly relevant to neurotrauma since vascular disruption permits entry of leukocytes into the CNS, cells which are potent sources of OPN and MMPs. In this case, ECL animals are treated postinjury with minocycline, an anti-inflammatory antibiotic that reduces acute MMP-9 activity [12]. With MMP-9 inhibition, OPN processing is reduced, compromising glial activation and migration, as well as the removal of degenerating terminals. It is concluded that MMP-9 serves as a switch to mediate membrane signaling for local cellular support of synaptic recovery (Fig. 5d). Disruption of this time-dependent cellular response generates a maladaptive outcome. To further test the validity of this MMP-9/OPN interaction, Chan and colleagues [12] probed for the effect of OPN loss on MMP-9 activity. Since OPN indirectly influences MMP-9 activation and MMP-9 can be regulated by injury-induced expression of Lipocalin-2 (LCN2), both enzyme activity and LCN2 expression were measured in OPN knockout (OPNKO) mice subjected to ECL. Like OPN, LCN2 is increased with vascular disruption due to its siderophore-binding properties. Importantly, ECL upregulates the LCN2 form which binds and persistently activates MMP-9. When MMP-9 activity and the LCN2 protein are compared in C57BL/6 wild-type (WT) and OPNKO-injured cases, there is a 50% reduction in both enzyme activity and LCN2 expression for the KO relative to WT. Moreover, in OPNKO cases there is a reduced clearance of degenerating presynaptic terminals, shifting adaptive toward a maladaptive form. Thus, MMP-9 and LCN2 expression appear dependent on OPN, illustrating the complex regulatory role of the three proteins in support of adaptive synaptic repair after neurotrauma.
In summary, MMP mediation of synaptic recovery extends beyond modifying extracellular matrix structure, including the regulation of cell signaling critical to successful neuroplasticity. Processing of substrates such as agrin, N-cadherin and OPN support this regulation. Models of maladaptive plasticity exhibit aberrant MMP expression, often during postinjury intervals essential to adaptive synaptic recovery. Moreover, persistent MMP elevation results in the inefficient management of molecules critical to that successful reinnervation. Targeted inhibition of MMP activity in the maladaptive condition shows reversal of postinjury functional deficits and structural disruption. It appears that the posited ‘MMP switch’ exerts profound influence on the progress of brain plasticity after neurotrauma.
Role of MMPs in acute corpus callosum injury and recovery
As with SCI, the corpus callosum also shows MMP induction after injury. Early gelatin zymography confirms qualitative upregulation of MMP-2 and -9 within the injured callosum; however, the two gelatinases respond differently than after hippocampal deafferentation [95]. MMP-2 activity is higher in hippocampal gray matter, while MMP-9 response predominates within callosal white matter. Subsequent quantitative assay for MMP-9 activity and tissue distribution in rat callosum subjected to moderate FPI shows time-dependent increase in MMP-9 activity [96]. At 1 d postinjury, enzyme activity is 60% over paired sham-injured controls, but returns to baseline by 3 d. MMP-9 signal is co-localized within reactive microglia interposed along axons in the injured callosum fibers. By contrast, MMP-3 is elevated within the white matter tract at 3 d after FPI. Immunoelectron microscopy reveals MMP-3 distribution around damaged unmyelinated fibers and within disrupted myelin sheaths. These results show that white matter injury also stimulates a time-dependent and glial-associated induction of MMPs. The pattern of MMP-3 and -9 response is consistent with rapid transcript induction reported after optic nerve lesion [7] (Fig. 1 right panel), and the time frame of enzyme protein increase after gray matter insult.
In a second approach, Reeves and colleagues [97] took advantage of the fact that callosal myelinated and unmyelinated fibers show a differential response to injury, with unmyelinated axons being the most vulnerable. Compound action potentials (CAPs) of each fiber type were measured in FPI subjects exposed to either minocycline or the MMP inhibitor FN-439. While both drugs attenuate postinjury MMP activity [12, 94], minocycline broadly reduces MMP-associated inflammatory response, and FN-439 shows high-affinity MMP-3 inhibition. Drugs were delivered independently in the acute postinjury period, and CAPs assayed at 1-d and 3-d survival. Neither MMP manipulation affects myelinated axon functional activity, however, both compounds reverse the injury-induced depression of unmyelinated fiber CAP amplitude [96]. For minocycline, this CAP reversal is most likely related to drug effect on MMP-9 since it occurs at 1 d, but not at 3 d postinjury. These data are consistent with callosal MMP-9 zymography results. By contrast, FN-439 restoration of CAP signal occurs at 3 d postinjury, where MMP-3 was pervasive among damaged axons. Thus, CNS white matter shows a rapid MMP response to diffuse injury, exemplified by induced enzyme activity, increased glial localization and major effects on axon function. From these data, it can be posited that the differential effect on unmyelinated fibers is due to the increased exposure of their surface membranes to pathological signals and injury-induced MMPs.
Targeting MMPs to support recovery after SCI: the first “Clinical Trial”
Preclinical studies of both TBI and SCI have demonstrated that MMP-9, expressed within the first 3 d postinjury, is a key determinant of early barrier disruption [48, 49] and degradation of myelin [48, 98], and that these early changes correspond to a long-term loss of white matter sparing and neurological impairments [49]. Consistent with that position, the broad-spectrum MMP inhibitor, GM6001, given within the first 3 d after SCI, when MMP-9 is maximally expressed, results in decreased degradation of myelin in the acutely injured spinal cord and greater sparing of white matter coincident with improved neurological function by 6 weeks postinjury [49].
These early findings served as the basis for a clinical trial, conducted in pet dogs that sustained naturally SCIs from intervertebral disc herniation (IVDH) [99]. IVDH creates a combined, acute contusive/compressive event that recapitulates facets of human SCI such as upregulation of pro-inflammatory cytokines, microglial activation, central necrosis with cavitation, and ultimately long-tract degeneration [100, 101]. Standard of care therapy for SCI is also similar between humans and dogs. For example, dogs require neuro-imaging for diagnosis, have decompressive surgery, and post-operatively undergo pain management and rehabilitation. There are also numerous validated outcome measures that can be deployed in clinical trials including ordinal gait scores, kinematics and kinetics, cystometry, and electrophysiology. Importantly, these injuries occur at a high frequency, and recent estimates suggest over 20,000 cases a year are presented to veterinary specialists for diagnostic evaluation [101]. Clinical trials relying on dogs with naturally occurring disease represent a unique win–win opportunity, where clients, pets, and science all potentially benefit.
MMPs likely play a key role in canine SCI. In the acutely injured dog spinal cord, MMP-2 mRNA expression is downregulated and MMP-9 mRNA expression is upregulated compared to healthy spinal cord tissue [102]. Moreover, the activity of MMP-9 is increased in the CSF and serum of dogs with acute SCI compared to healthy dogs [103]. Two randomized, double-blinded, placebo-controlled clinical trials were conducted to assess the efficacy of GM6001 in dogs with naturally occurring SCIs associated with IVDH. While favorable safety, pharmacokinetics, and pharmacodynamics of this approach were demonstrated, acute inhibition of MMPs did not improve locomotor recovery when compared to controls [99]. While such a finding may indicate that MMPs, expressed in the acutely injured cord are not a determinant of long-term neurological recovery, it should also be noted that a majority of these dogs showed a natural recovery of locomotor function and as such, this may have limited sensitivity to detect a change in locomotor function.
A second trial was conducted in spinal cord-injured dogs, designed to assess urological outcomes [104]. Bladder dysfunction resulting from a SCI results from loss of control of central micturition reflexes, combined with neurogenic bladder overactivity and reflex dyssynergia. The bladder undergoes remarkable remodeling that culminates in hypertrophy and hyperplasia and low bladder compliance. While this second trial likewise revealed no differences in long-term locomotor recovery between controls and GM6001-treated groups when given acutely postinjury, early treatment resulted in an increase in bladder compliance by 6 weeks postinjury. From a clinical point of view, these findings are important as reduced compliance is a major factor contributing to urinary tract infection, pyelonephritis, and vesicoureteral reflux after SCI. From a biological perspective, early blockade of MMPs may result in improved compliance by reducing damage to descending pathways that control micturition, acting locally in the spinal cord circuitry that controls bladder function, or changing the mechanotransductive properties of the distended bladder. Future studies are warranted to further investigate the direct actions of MMPs in the bladder wall after SCI as well as their potential involvement in altered signaling pathways in the injured spinal cord.
Conclusions and future possibilities
There is a common pattern of rapid MMP responders after neurotrauma (MMP-9, MMP-8, MMP-3, MMP-12), serving to direct acute cell activation and infiltration, as well as support inflammation cues to assist in the isolation, breakdown and clearance of pathology (be it vascular, axonal or synaptic). A second phase of tissue regeneration is fostered by MMP-2, ADAM8 and 10, and MMP-1, which serve to initiate and conduct cell processes required for repair. Glial cells are common parenchymal mediators of these cued roles across injury models; however, cells affected by vascular pathology itself serve as a nexus of MMP expression, prominently endothelial cells and associated infiltrating leukocytes. MMP-9 has the strongest association with these reactive cells, beginning in the immediate postinjury period. SCI, TBI and CNS deafferentation all employ this cued pattern of MMP recruitment. In addition, the progression of SCI angiogenesis is similar to that of synaptogenesis after TBI or CNS deafferentation, where MMPs can act as regulatory switches. Their timed expression is critical for the success of tissue plasticity, whether supporting the regeneration and stabilization of new vasculature or reinnervation of damaged synapses. Importantly, the temporal on–off cycle of individual MMP appears to coordinate transition from nascent to stable vasculature during this recovery. Most evident across neurotrauma models is the balance of MMP-2 and -9 activities, which guides this transition through cytokine binding to integrin receptors that stimulate growth factors for tissue regeneration. With the time-dependent mapping of MMP role in plasticity after neurotrauma, future studies targeting these enzymes hold great promise. Manipulation of postinjury enzyme function will likely change the efficacy of repair and improve outcome, particularly valuable for the more onerous conditions such as the neuropathic pain of SCI. Assaying MMP response with different injury combinations will determine how unique pathologies employ these enzymes to affect outcome. Lastly, understanding how cells that pervade the injured CNS from peripheral sites contribute to MMP response will reveal an aspect of neurotrauma plasticity regulation presently unrecognized and uncontrolled.
Acknowledgements
We would like to acknowledge the following grant support for this work: Department of Defense grant DOD SCIRP W81XWH-11-1-79 to LJN-H, and National Institutes of Health Grants NS39278 to LJN-H, NS56247 to LP and NS57758 to TR.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Phillips LL, Chan JL, Doperalski AE, Reeves TM. Time dependent integration of matrix metalloproteinases and their targeted substrates directs axonal sprouting and synaptogenesis following central nervous system injury. Neural Regener Res. 2014;9(4):362–376. doi: 10.4103/1673-5374.128237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andries L, Van Hove I, Moons L, De Groef L. Matrix metalloproteinases during axonal regeneration, a multifactorial role from start to finish. Mol Neurobiol. 2017;54(3):2114–2125. doi: 10.1007/s12035-016-9801-x. [DOI] [PubMed] [Google Scholar]
- 3.Fischer D, Harvey AR, Pernet V, Lemmon VP, Park KK. Optic nerve regeneration in mammals: regenerated or spared axons? Exp Neurol. 2017;296:83–88. doi: 10.1016/j.expneurol.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed Z, Dent RG, Leadbeater WE, Smith C, Berry M, Logan A. Matrix metalloproteases: degradation of the inhibitory environment of the transected optic nerve and the scar by regenerating axons. Mol Cell Neurosci. 2005;28(1):64–78. doi: 10.1016/j.mcn.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Diekmann H, Kalbhen P, Fischer D. Characterization of optic nerve regeneration using transgenic zebrafish. Front Cell Neurosci. 2015;9:118. doi: 10.3389/fncel.2015.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu LY, Zheng H, Xiao HL, She ZJ, Zhao SM, Chen ZL, Zhou GM. Comparison of blood-nerve barrier disruption and matrix metalloprotease-9 expression in injured central and peripheral nerves in mice. Neurosci Lett. 2008;434(2):155–159. doi: 10.1016/j.neulet.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 7.Hughes PM, Wells GM, Perry VH, Brown MC, Miller KM. Comparison of matrix metalloproteinase expression during Wallerian degeneration in the central and peripheral nervous systems. Neuroscience. 2002;113(2):273–287. doi: 10.1016/S0306-4522(02)00183-5. [DOI] [PubMed] [Google Scholar]
- 8.Lemmens K, Bollaerts I, Bhumika S, de Groef L, Van Houcke J, Darras VM, Van Hove I, Moons L. Matrix metalloproteinases as promising regulators of axonal regrowth in the injured adult zebrafish retinotectal system. J Comp Neurol. 2016;524(7):1472–1493. doi: 10.1002/cne.23920. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Fillmore HL, Reeves TM, Phillips LL. Elevation of hippocampal MMP-3 expression and activity during trauma-induced synaptogenesis. Exp Neurol. 2005;192(1):60–72. doi: 10.1016/j.expneurol.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Mayer J, Hamel MG, Gottschall PE. Evidence for proteolytic cleavage of brevican by the ADAMTSs in the dentate gyrus after excitotoxic lesion of the mouse entorhinal cortex. BMC Neurosci. 2005;6:52. doi: 10.1186/1471-2202-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falo MC, Fillmore HL, Reeves TM, Phillips LL. Matrix metalloproteinase-3 expression profile differentiates adaptive and maladaptive synaptic plasticity induced by traumatic brain injury. J Neurosci Res. 2006;84(4):768–781. doi: 10.1002/jnr.20986. [DOI] [PubMed] [Google Scholar]
- 12.Chan JL, Reeves TM, Phillips LL. Osteopontin expression in acute immune response mediates hippocampal synaptogenesis and adaptive outcome following cortical brain injury. Exp Neurol. 2014;261:757–771. doi: 10.1016/j.expneurol.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren KM, Reeves TM, Phillips LL. MT5-MMP, ADAM-10, and N-cadherin act in concert to facilitate synapse reorganization after traumatic brain injury. J Neurotrauma. 2012;29(10):1922–1940. doi: 10.1089/neu.2012.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costanzo RM, Perrino LA. Peak in matrix metalloproteinases-2 levels observed during recovery from olfactory nerve injury. NeuroReport. 2008;19(3):327–331. doi: 10.1097/WNR.0b013e3282f50c7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costanzo RM, Perrino LA, Kobayashi M. Response of matrix metalloproteinase-9 to olfactory nerve injury. NeuroReport. 2006;17(17):1787–1791. doi: 10.1097/WNR.0b013e32800fef87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakos SR, Costanzo RM. Matrix metalloproteinase-9 is associated with acute inflammation after olfactory injury. NeuroReport. 2011;22(11):539–543. doi: 10.1097/WNR.0b013e328348ab94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakos SR, Schwob JE, Costanzo RM. Matrix metalloproteinase-9 and -2 expression in the olfactory bulb following methyl bromide gas exposure. Chem Senses. 2010;35(8):655–661. doi: 10.1093/chemse/bjq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison EE, Costanzo RM. Regeneration of olfactory sensory neurons and reconnection in the aging hamster central nervous system. Neurosci Lett. 1995;198(3):213–217. doi: 10.1016/0304-3940(95)11943-Q. [DOI] [PubMed] [Google Scholar]
- 19.Ladwig A, Walter HL, Hucklenbroich J, Willuweit A, Langen KJ, Fink GR, Rueger MA, Schroeter M. Osteopontin augments M2 microglia response and separates M1- and M2-polarized microglial activation in permanent focal cerebral ischemia. Mediat Inflamm. 2017;2017:7189421. doi: 10.1155/2017/7189421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong M, Munoz N, Valdivia A, Alvarez A, Herrera-Molina R, Cardenas A, Schneider P, Burridge K, Quest AF. Leyton L (2013) Thy-1-mediated cell-cell contact induces astrocyte migration through the engagement of alphaVbeta3 integrin and syndecan-4. Biochem Biophys Acta. 1833;6:1409–1420. doi: 10.1016/j.bbamcr.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peluffo H, Gonzalez P, Aris A, Acarin L, Saura J, Villaverde A, Castellano B, Gonzalez B. RGD domains neuroprotect the immature brain by a glial-dependent mechanism. Ann Neurol. 2007;62(3):251–261. doi: 10.1002/ana.21170. [DOI] [PubMed] [Google Scholar]
- 22.Lindsey ML, Zouein FA, Tian Y, Padmanabhan Iyer R, de Castro Bras LE. Osteopontin is proteolytically processed by matrix metalloproteinase 9. Can J Physiol Pharmacol. 2015;93(10):879–886. doi: 10.1139/cjpp-2015-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27(11):2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 24.Gaublomme D, Buyens T, De Groef L, Stakenborg M, Janssens E, Ingvarsen S, Porse A, Behrendt N, Moons L. Matrix metalloproteinase 2 and membrane type 1 matrix metalloproteinase co-regulate axonal outgrowth of mouse retinal ganglion cells. J Neurochem. 2014;129(6):966–979. doi: 10.1111/jnc.12703. [DOI] [PubMed] [Google Scholar]
- 25.Lagos-Cabre R, Alvarez A, Kong M, Burgos-Bravo F, Cardenas A, Rojas-Mancilla E, Perez-Nunez R, Herrera-Molina R, Rojas F, Schneider P, Herrera-Marschitz M, Quest AFG, van Zundert B, Leyton L. alphaVbeta3 Integrin regulates astrocyte reactivity. J Neuroinflamm. 2017;14(1):194. doi: 10.1186/s12974-017-0968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falo MC, Reeves TM, Phillips LL. Agrin expression during synaptogenesis induced by traumatic brain injury. J Neurotrauma. 2008;25(7):769–783. doi: 10.1089/neu.2008.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris JL, Reeves TM, Phillips LL. Injury modality, survival interval, and sample region are critical determinants of qRT-PCR reference gene selection during long-term recovery from brain trauma. J Neurotrauma. 2009;26(10):1669–1681. doi: 10.1089/neu.2009-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, Ellis C, Fawcett JW, Rogers JH. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res. 2002;100(1–2):103–117. doi: 10.1016/S0169-328X(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 29.Ng MT, Stammers AT, Kwon BK. Vascular disruption and the role of angiogenic proteins after spinal cord injury. Transl Stroke Res. 2011;2(4):474–491. doi: 10.1007/s12975-011-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood–brain barrier: from physiology to disease and back. Physiol Rev. 2019;99(1):21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7(4):452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maikos JT, Shreiber DI. Immediate damage to the blood-spinal cord barrier due to mechanical trauma. J Neurotrauma. 2007;24(3):492–507. doi: 10.1089/neu.2006.0149. [DOI] [PubMed] [Google Scholar]
- 33.Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003;74(2):227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu SJ, Narayana PA. Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed. 2009;22(3):332–341. doi: 10.1002/nbm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp Neurol. 1996;142(2):258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- 36.Tanno H, Nockels RP, Pitts LH, Noble LJ. Breakdown of the blood–brain barrier after fluid percussive brain injury in the rat. Part 1: Distribution and time course of protein extravasation. J Neurotrauma. 1992;9(1):21–32. doi: 10.1089/neu.1992.9.21. [DOI] [PubMed] [Google Scholar]
- 37.Dhillon HS, Donaldson D, Dempsey RJ, Prasad MR. Regional levels of free fatty acids and Evans blue extravasation after experimental brain injury. J Neurotrauma. 1994;11(4):405–415. doi: 10.1089/neu.1994.11.405. [DOI] [PubMed] [Google Scholar]
- 38.Baskaya MK, Rao AM, Dogan A, Donaldson D, Dempsey RJ. The biphasic opening of the blood–brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett. 1997;226(1):33–36. doi: 10.1016/S0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- 39.Cortez SC, McIntosh TK, Noble LJ. Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res. 1989;482(2):271–282. doi: 10.1016/0006-8993(89)91190-6. [DOI] [PubMed] [Google Scholar]
- 40.Fukuda K, Tanno H, Okimura Y, Nakamura M, Yamaura A. The blood–brain barrier disruption to circulating proteins in the early period after fluid percussion brain injury in rats. J Neurotrauma. 1995;12(3):315–324. doi: 10.1089/neu.1995.12.315. [DOI] [PubMed] [Google Scholar]
- 41.Habgood MD, Bye N, Dziegielewska KM, Ek CJ, Lane MA, Potter A, Morganti-Kossmann C, Saunders NR. Changes in blood–brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur J Neurosci. 2007;25(1):231–238. doi: 10.1111/j.1460-9568.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- 42.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood–brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2(4):492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saunders NR, Dziegielewska KM, Mollgard K, Habgood MD. Markers for blood–brain barrier integrity: how appropriate is Evans blue in the twenty-first century and what are the alternatives? Front Neurosci. 2015;9:385. doi: 10.3389/fnins.2015.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prakash R, Carmichael ST. Blood–brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr Opin Neurol. 2015;28(6):556–564. doi: 10.1097/WCO.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glushakova OY, Johnson D, Hayes RL. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood–brain barrier disruption, and progressive white matter damage. J Neurotrauma. 2014;31(13):1180–1193. doi: 10.1089/neu.2013.3080. [DOI] [PubMed] [Google Scholar]
- 46.Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer’s disease: innocent observation or key player? Brain J Neurol. 2011;134(Pt 2):335–344. doi: 10.1093/brain/awq321. [DOI] [PubMed] [Google Scholar]
- 47.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cerebral Blood Flow Metabol. 2000;20(12):1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22(17):7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trivedi A, Lee SM, Zhang H, Linda J, Noble-Haeusslein L. Neutrophils as determinants of vascular stability in the injured spinal cord. In: Eng H, Lo JL, MingMing N, Michael JW, editors. Vascular mechanisms in CNS trauma. Springer Series in Translational Stroke Research. New York: Springer; 2014. pp. 285–302. [Google Scholar]
- 51.Zhang H, Adwanikar H, Werb Z, Noble-Haeusslein LJ. Matrix metalloproteinases and neurotrauma: evolving roles in injury and reparative processes. Neurosci. 2010;16(2):156–170. doi: 10.1177/1073858409355830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdul-Muneer PM, Pfister BJ, Haorah J, Chandra N. Role of matrix metalloproteinases in the pathogenesis of traumatic brain injury. Mol Neurobiol. 2016;53(9):6106–6123. doi: 10.1007/s12035-015-9520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jha RM, Kochanek PM, Simard JM. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology. 2018 doi: 10.1016/j.neuropharm.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar H, Ropper AE, Lee SH, Han I. Propitious therapeutic modulators to prevent blood-spinal cord barrier disruption in spinal cord injury. Mol Neurobiol. 2017;54(5):3578–3590. doi: 10.1007/s12035-016-9910-6. [DOI] [PubMed] [Google Scholar]
- 55.Van Hove I, Lemmens K, Van de Velde S, Verslegers M, Moons L. Matrix metalloproteinase-3 in the central nervous system: a look on the bright side. J Neurochem. 2012;123(2):203–216. doi: 10.1111/j.1471-4159.2012.07900.x. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi M, Jadhav V, Obenaus A, Colohan A, Zhang JH. Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgically-induced brain injury. Neurosurgery. 2007;61(5):1067–1075. doi: 10.1227/01.neu.0000303203.07866.18. [DOI] [PubMed] [Google Scholar]
- 57.Yang Y, Rosenberg GA. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015;1623:30–38. doi: 10.1016/j.brainres.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sulhan S, Lyon KA, Shapiro LA, Huang JH. Neuroinflammation and blood–brain barrier disruption following traumatic brain injury: pathophysiology and potential therapeutic targets. J Neurosci Res. 2018 doi: 10.1002/jnr.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar H, Jo MJ, Choi H, Muttigi MS, Shon S, Kim BJ, Lee SH, Han IB. Matrix metalloproteinase-8 inhibition prevents disruption of blood-spinal cord barrier and attenuates inflammation in rat model of spinal cord injury. Mol Neurobiol. 2018;55(3):2577–2590. doi: 10.1007/s12035-017-0509-3. [DOI] [PubMed] [Google Scholar]
- 60.Lee JY, Choi HY, Ahn HJ, Ju BG, Yune TY. Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury. Am J Pathol. 2014;184(11):2985–3000. doi: 10.1016/j.ajpath.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 61.Chelluboina B, Nalamolu KR, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK. MMP-12, a promising therapeutic target for neurological diseases. Mol Neurobiol. 2018;55(2):1405–1409. doi: 10.1007/s12035-017-0418-5. [DOI] [PubMed] [Google Scholar]
- 62.Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, Yong VW. An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci. 2003;23(31):10107–10115. doi: 10.1523/JNEUROSCI.23-31-10107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H, Trivedi A, Lee JU, Lohela M, Lee SM, Fandel TM, Werb Z, Noble-Haeusslein LJ. Matrix metalloproteinase-9 and stromal cell-derived factor-1 act synergistically to support migration of blood-borne monocytes into the injured spinal cord. J Neurosci. 2011;31(44):15894–15903. doi: 10.1523/JNEUROSCI.3943-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hansen CN, Fisher LC, Deibert RJ, Jakeman LB, Zhang H, Noble-Haeusslein L, White S, Basso DM. Elevated MMP-9 in the lumbar cord early after thoracic spinal cord injury impedes motor relearning in mice. J Neurosci. 2013;33(32):13101–13111. doi: 10.1523/JNEUROSCI.1576-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansen CN, Norden DM, Faw TD, Deibert R, Wohleb ES, Sheridan JF, Godbout JP, Basso DM. Lumbar myeloid cell trafficking into locomotor networks after thoracic spinal cord injury. Exp Neurol. 2016;282:86–98. doi: 10.1016/j.expneurol.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simard JM, Woo SK, Aarabi B, Gerzanich V. The Sur1-Trpm4 channel in spinal cord injury. J Spine. 2013 doi: 10.4172/2165-7939.s4-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahoney ET, Benton RL, Maddie MA, Whittemore SR, Hagg T. ADAM8 is selectively up-regulated in endothelial cells and is associated with angiogenesis after spinal cord injury in adult mice. J Comp Neurol. 2009;512(2):243–255. doi: 10.1002/cne.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsu JY, Bourguignon LY, Adams CM, Peyrollier K, Zhang H, Fandel T, Cun CL, Werb Z, Noble-Haeusslein LJ. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J Neurosci. 2008;28(50):13467–13477. doi: 10.1523/JNEUROSCI.2287-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trivedi A, Zhang H, Ekeledo A, Lee S, Werb Z, Plant GW, Noble-Haeusslein LJ. Deficiency in matrix metalloproteinase-2 results in long-term vascular instability and regression in the injured mouse spinal cord. Exp Neurol. 2016;284(Pt A):50–62. doi: 10.1016/j.expneurol.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Xiong Y, Mahmood A, Zhang ZG, Chopp M. Angiogenesis and functional recovery after traumatic brain injury. In: Eng H, Lo JL, MingMing N, Michael JW, editors. Vascular mechanisms. Springer Series in Translational Stroke Research. New York: Springer; 2014. pp. 141–156. [Google Scholar]
- 71.Renault-Mihara F, Mukaino M, Shinozaki M, Kumamaru H, Kawase S, Baudoux M, Ishibashi T, Kawabata S, Nishiyama Y, Sugai K, Yasutake K, Okada S, Nakamura M, Okano H. Regulation of RhoA by STAT3 coordinates glial scar formation. J Cell Biol. 2017;216(8):2533–2550. doi: 10.1083/jcb.201610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verslegers M, Lemmens K, Van Hove I, Moons L. Matrix metalloproteinase-2 and -9 as promising benefactors in development, plasticity and repair of the nervous system. Prog Neurobiol. 2013;105:60–78. doi: 10.1016/j.pneurobio.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 73.Chetty C, Lakka SS, Bhoopathi P, Rao JS. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer. 2010;127(5):1081–1095. doi: 10.1002/ijc.25134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han S, Arnold SA, Sithu SD, Mahoney ET, Geralds JT, Tran P, Benton RL, Maddie MA, D’Souza SE, Whittemore SR, Hagg T. Rescuing vasculature with intravenous angiopoietin-1 and alpha v beta 3 integrin peptide is protective after spinal cord injury. Brain J Neurol. 2010;133(Pt 4):1026–1042. doi: 10.1093/brain/awq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007;74(2–3):85–89. doi: 10.1016/j.mvr.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cerebral Blood Flow Metabol. 2012;32(10):1841–1852. doi: 10.1038/jcbfm.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geevarghese A, Herman IM. Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl Res. 2014;163(4):296–306. doi: 10.1016/j.trsl.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teichert M, Milde L, Holm A, Stanicek L, Gengenbacher N, Savant S, Ruckdeschel T, Hasanov Z, Srivastava K, Hu J, Hertel S, Bartol A, Schlereth K, Augustin HG. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat Commun. 2017;8:16106. doi: 10.1038/ncomms16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Risinger GM, Jr, Updike DL, Bullen EC, Tomasek JJ, Howard EW. TGF-beta suppresses the upregulation of MMP-2 by vascular smooth muscle cells in response to PDGF-BB. Am J Physiol Cell Physiol. 2010;298(1):C191–C201. doi: 10.1152/ajpcell.00417.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simonavicius N, Ashenden M, van Weverwijk A, Lax S, Huso DL, Buckley CD, Huijbers IJ, Yarwood H, Isacke CM. Pericytes promote selective vessel regression to regulate vascular patterning. Blood. 2012;120(7):1516–1527. doi: 10.1182/blood-2011-01-332338. [DOI] [PubMed] [Google Scholar]
- 82.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol Rev. 2018;98(2):881–917. doi: 10.1152/physrev.00017.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou Y, Cui Z, Xia X, Liu C, Zhu X, Cao J, Wu Y, Zhou L, Ben Z, Song Y, Zhang H, Zhang D. Matrix metalloproteinase-1 (MMP-1) expression in rat spinal cord injury model. Cell Mol Neurobiol. 2014;34(8):1151–1163. doi: 10.1007/s10571-014-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, Noble-Haeusslein LJ. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 2006;26(39):9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ogier C, Bernard A, Chollet AM, Le Diguardher T, Hanessian S, Charton G, Khrestchatisky M, Rivera S. Matrix metalloproteinase-2 (MMP-2) regulates astrocyte motility in connection with the actin cytoskeleton and integrins. Glia. 2006;54(4):272–284. doi: 10.1002/glia.20349. [DOI] [PubMed] [Google Scholar]
- 87.Phillips LL, Reeves TM. Interactive pathology following traumatic brain injury modifies hippocampal plasticity. Restor Neurol Neurosci. 2001;19(3–4):213–235. [PubMed] [Google Scholar]
- 88.Hayashita-Kinoh H, Kinoh H, Okada A, Komori K, Itoh Y, Chiba T, Kajita M, Yana I, Seiki M. Membrane-type 5 matrix metalloproteinase is expressed in differentiated neurons and regulates axonal growth. Cell Growth Differen. 2001;12(11):573–580. [PubMed] [Google Scholar]
- 89.Kieseier BC, Pischel H, Neuen-Jacob E, Tourtellotte WW, Hartung HP. ADAM-10 and ADAM-17 in the inflamed human CNS. Glia. 2003;42(4):398–405. doi: 10.1002/glia.10226. [DOI] [PubMed] [Google Scholar]
- 90.Bernstein HG, Bukowska A, Krell D, Bogerts B, Ansorge S, Lendeckel U. Comparative localization of ADAMs 10 and 15 in human cerebral cortex normal aging, Alzheimer disease and Down syndrome. J Neurocytol. 2003;32(2):153–160. doi: 10.1023/B:NEUR.0000005600.61844.a6. [DOI] [PubMed] [Google Scholar]
- 91.Ortiz RM, Karkkainen I, Huovila AP, Honkaniemi J. ADAM9, ADAM10, and ADAM15 mRNA levels in the rat brain after kainic acid-induced status epilepticus. Brain Res Mol Brain Res. 2005;137(1–2):272–275. doi: 10.1016/j.molbrainres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 92.Conant K, Wang Y, Szklarczyk A, Dudak A, Mattson MP, Lim ST. Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience. 2010;166(2):508–521. doi: 10.1016/j.neuroscience.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci USA. 2008;105(49):19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reeves TM, Prins ML, Zhu J, Povlishock JT, Phillips LL. Matrix metalloproteinase inhibition alters functional and structural correlates of deafferentation-induced sprouting in the dentate gyrus. J Neurosci. 2003;23(32):10182–10189. doi: 10.1523/JNEUROSCI.23-32-10182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harris LKBR, Reeves TM, Phillips LL. Application of zymographic methods to study matrix enzymes following traumatic brain injury. In: Chen JXZ, Xu X-M, Zheng J, editors. Animal models of acute neurological injuries II: injury and mechanistic assessments. New York: Humana Press; 2012. [Google Scholar]
- 96.DA Reeves TM, Phillips LL. Unmyelinated and myelinated axons exhibit differential injury and treatment responses following traumatic injury. In: Baltan SCS, Matute C, Xi G, Zhang JH, editors. White matter injury in stroke and CNS diseases. New York: Springer Press; 2013. [Google Scholar]
- 97.Reeves TM, Phillips LL, Povlishock JT. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol. 2005;196(1):126–137. doi: 10.1016/j.expneurol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 98.Trivedi AHJ, Lin Y, Goussev S, Gan J, Topp KS, Noble-Haeusslein LJ. The effects of acute and extended inhibition of matrix metalloproteinases on demyelination and functional recovery after spinal cord injury. Int J Neuroprotect Neuroregen. 2005;2:30–38. [Google Scholar]
- 99.Levine JM, Cohen ND, Heller M, Fajt VR, Levine GJ, Kerwin SC, Trivedi AA, Fandel TM, Werb Z, Modestino A, Noble-Haeusslein LJ. Efficacy of a metalloproteinase inhibitor in spinal cord injured dogs. PLoS One. 2014;9(5):e96408. doi: 10.1371/journal.pone.0096408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Levine JM, Levine GJ, Porter BF, Topp K, Noble-Haeusslein LJ. Naturally occurring disk herniation in dogs: an opportunity for pre-clinical spinal cord injury research. J Neurotrauma. 2011;28(4):675–688. doi: 10.1089/neu.2010.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moore SA, Granger N, Olby NJ, Spitzbarth I, Jeffery ND, Tipold A, Nout-Lomas YS, da Costa RC, Stein VM, Noble-Haeusslein LJ, Blight AR, Grossman RG, Basso DM, Levine JM. Targeting translational successes through CANSORT-SCI: using pet dogs to identify effective treatments for spinal cord injury. J Neurotrauma. 2017;34(12):2007–2018. doi: 10.1089/neu.2016.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bock P, Spitzbarth I, Haist V, Stein VM, Tipold A, Puff C, Beineke A, Baumgartner W. Spatio-temporal development of axonopathy in canine intervertebral disc disease as a translational large animal model for nonexperimental spinal cord injury. Brain Pathol. 2013;23(1):82–99. doi: 10.1111/j.1750-3639.2012.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Levine JM, Ruaux CG, Bergman RL, Coates JR, Steiner JM, Williams DA. Matrix metalloproteinase-9 activity in the cerebrospinal fluid and serum of dogs with acute spinal cord trauma from intervertebral disk disease. Am J Vet Res. 2006;67(2):283–287. doi: 10.2460/ajvr.67.2.283. [DOI] [PubMed] [Google Scholar]
- 104.Levine JM, Cohen ND, Fandel TM, Levine GJ, Mankin J, Griffin JF, Kerwin SC, Boudreau CE, Trivedi A, Noble-Haeusslein LJ. Early Blockade of matrix metalloproteinases in spinal-cord-injured dogs results in a long-term increase in bladder compliance. J Neurotrauma. 2017;34(18):2656–2667. doi: 10.1089/neu.2017.5001. [DOI] [PubMed] [Google Scholar]
- 105.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]