Abstract
Store-operated Ca2+ entry is a pathway that is remodelled in a variety of cancers, and altered expression of the components of store-operated Ca2+ entry is a feature of breast cancer cells of the basal molecular subtype. Studies of store-operated Ca2+ entry in breast cancer cells have used non-specific pharmacological inhibitors, complete depletion of intracellular Ca2+ stores and have mostly focused on MDA-MB-231 cells (a basal B breast cancer cell line). These studies compared the effects of the selective store-operated Ca2+ entry inhibitors Synta66 and YM58483 (also known as BTP2) on global cytosolic free Ca2+ ([Ca2+]CYT) changes induced by physiological stimuli in a different breast cancer basal cell line model, MDA-MB-468. The effects of these agents on proliferation as well as serum and epidermal growth factor (EGF) induced migration were also assessed. Activation with the purinergic receptor activator adenosine triphosphate, produced a sustained increase in [Ca2+]CYT that was entirely dependent on store-operated Ca2+ entry. The protease activated receptor 2 activator, trypsin, and EGF also produced Ca2+ influx that was sensitive to both Synta66 and YM58483. Serum-activated migration of MDA-MB-468 breast cancer cells was sensitive to both store-operated Ca2+ inhibitors. However, proliferation and EGF-activated migration was differentially affected by Synta66 and YM58483. These studies highlight the need to define the exact mechanisms of action of different store-operated calcium entry inhibitors and the impact of such differences in the control of tumour progression pathways.
Electronic supplementary material
The online version of this article (10.1007/s00018-018-2904-y) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Orai1, Store-operated Ca2+ entry, Synta66, YM58483
Introduction
The existence of a mechanism by which the depletion of intracellular Ca2+ stores triggers a Ca2+ influx pathway that is then used to refill Ca2+ stores was proposed by Putney in 1986 [1], with store-operated Ca2+ entry, one of the terms used to describe this phenomenon. The molecular components that drive Ca2+ influx to refill the endoplasmic reticulum (ER) intracellular Ca2+ stores after depletion, were identified a little over a decade ago [2]. Since the original finding, the process of store-operated Ca2+ entry (SOCE) has been defined further, with a variety of regulators identified [3]. Store-operated Ca2+ entry is typically initiated by the sensing of reduced levels of Ca2+ in the ER by stromal interaction molecule 1 (STIM1). Subsequently, STIM1 is oligomerised and locates to sites of the ER that are in close proximity to the plasma membrane. STIM1 then interacts with the hexamers of the plasma membrane protein and Ca2+ channel component Orai1 to promote Ca2+ influx [4–6].
Although the refilling of Ca2+ stores could be seen as a simple “house-keeping” function, it is clear that Orai1-mediated Ca2+ influx is more than this, given its important role in key cellular processes, including specific immune system events, lactation and male fertility [7–9]. Indeed, decoding of Ca2+ changes in cellular compartments allows Orai1-mediated Ca2+ influx to differentially regulate transcription by NFAT1 and NFAT4 [10]. These characteristics of the store-operated Ca2+ influx pathway have defined it as a legitimate pharmacological target for drug discovery programs to improve human health. The conditions where store-operated Ca2+ entry inhibitors have been proposed to potentially have therapeutic benefit are varied, and range from vascular thrombosis to inflammatory bowel disease [11] and pancreatitis [12] to renal fibrosis [13].
Another disease state where Orai1-mediated Ca2+ influx has been recently investigated is cancer [14]. Remodelling of the expression of components of store-operated Ca2+ entry is a feature of many cancers including those of the colon [15], liver [16] and oesophagus [17]. However, the potential remodelling of Orai1-mediated Ca2+ influx is not ubiquitous, as reflected in the apparent switch from store-operated Ca2+ entry to more Orai3-mediated Ca2+ influx in some prostate cancers [18]. Differences may even be evident between breast cancer subtypes, which differ significantly in their prognosis and therapy. For example, Orai1 levels are higher in basal compared to non-basal breast cancers [19], and cell line data suggest that Orai3 is more important than Orai1 in store-operated Ca2+ entry in estrogen receptor-positive breast cancer cells compared to estrogen receptor-negative breast cancer cells [20]. In the specific context of Orai1-mediated Ca2+ influx in basal breast cancer, which has a significant overlap with triple negative breast cancers, where the need for new therapies has been consistently highlighted [21], Orai1 silencing reduces the proliferation and invasiveness of MDA-MB-231 triple negative breast cancer cells [19, 22]. This combined with the association between high STIM1 and low STIM2 with poorer prognosis in basal breast cancers [19], has highlighted the potential importance of Orai1 in this breast cancer subtype.
Future therapies for basal/triple negative breast cancer that seek to exploit the remodelling of Orai1-mediated Ca2+ influx will most likely involve pharmacological inhibitors of this Ca2+ influx pathway. Although encouraging, studies assessing the effects of store-operated Ca2+ entry inhibitors in triple negative breast cancer cell lines in vitro or in vivo have had some limitations [19, 22]. First, studies have often focused on the most common triple negative breast cancer cell line model (MDA-MB-231 cells) and have not explored other triple negative breast cancer cell lines. Effects on Ca2+ influx have involved pronounced Ca2+ store depletion in the absence of extracellular Ca2+ and have not assessed or compared the contribution of store-operated Ca2+ influx with different physiological stimuli under more biologically relevant conditions. Finally, the store-operated Ca2+ channel inhibitors used in previous studies (2-APB and SKF96365) were not those that are more selective for this Ca2+ influx pathway [23]. Hence, in these studies, we sought to compare the nature of Ca2+ influx induced by different stimuli under more physiological conditions and the sensitivity of this Ca2+ influx to the store-operated Ca2+ entry inhibitors Synta66 and YM58483, in a different basal breast cancer cell model, MDA-MB-468 triple negative breast cancer cells. MDA-MB-468 cells are PTEN mutant with high levels of EGFR expression [24–26], two features that are also associated with metastasis and poor patient survival [27, 28]. These cells also possess epithelial–mesenchymal plasticity features [29], a characteristic that is a critical regulator of cancer progression [30]. This work provides new insights into how different stimuli may engage store-operated Ca2+ influx and point to differences between Synta66 and YM58483 in the regulation of specific aspects important in breast cancer progression.
Materials and methods
Cell culture
The MDA-MB-468 cell line was obtained from The Brisbane Breast Bank, The University of Queensland Centre for Clinical Research (UQCCR), Brisbane, QLD, Australia. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 1000 mg/L of glucose (D6546, Sigma-Aldrich, St Louise, MO, USA), supplemented with 10% foetal bovine serum (FBS) and 4 mM l-glutamine, and maintained in a humidified incubator at 37 °C in a 5% CO2 atmosphere. Cells were tested every 6 months for mycoplasma using MycoAlert kit (Lonza, Basel, Switzerland), and authenticated by short tandem repeat (STR) profiling using the GenePrint 10 System (Promega, Madison, WA, USA) at QIMR Berghofer Medical Research Institute, Brisbane, QLD, Australia.
Measurement of intracellular Ca2+
Measurement of cytosolic free Ca2+ ([Ca2+]CYT) was performed using a fluorometric Imaging Plate Reader (FLIPRTETRA, Molecular Devices) with either a PBX no-wash Ca2+ Assay Kit (640175, BD Biosciences) in 384-well black plates (in case of initial characterisation of agonist-induced Ca2+ increases shown in Fig. 1) as previously described [23, 31], or Fluo-4 loaded cells in 96-well black plates (in case of Ca2+ measurements in Figs. 2, 3) as previously described [32]. Data were analysed using ScreenWorks Software (v2.0.0.27, molecular devices), and response over baseline was assessed as a relative measure of [Ca2+]CYT.
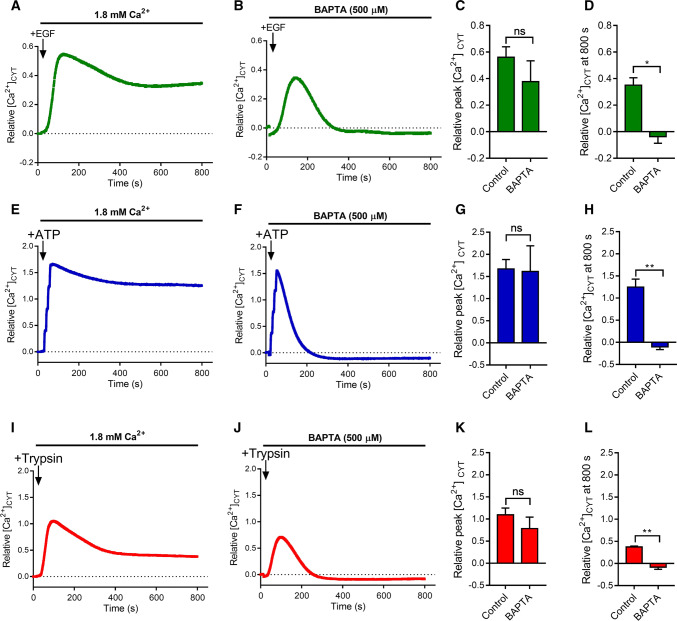
Fig. 1.
Characterisation of EGF, ATP and Trypsin-induced Ca2+ increases in MDA-MB-468 breast cancer cells. Mean [Ca2+]CYT levels during addition of EGF (50 ng/mL), ATP (100 µM) and Trypsin (10 nM) in the presence of 1.8 mM extracellular Ca2+ (a, e, i) or the extracellular Ca2+ chelator, BAPTA (500 µM) in the absence of added extracellular Ca2+ (b, f, j). Quantification of relative peak [Ca2+]CYT (c, g, k), and relative [Ca2+]CYT at 800 s (d, h, l) is shown for each treatment group (mean ± SD, n = 3). ns not significant (P > 0.05), *P < 0.05, **P < 0.01 (paired t test)
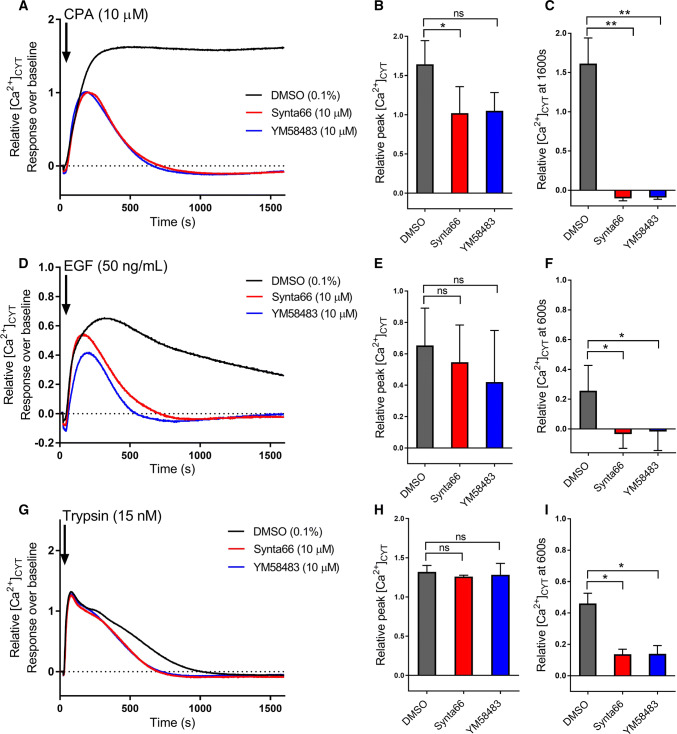
Fig. 2.
Inhibition of CPA, EGF and trypsin-induced store-operated Ca2+ entry by Synta66 and YM58483. Mean relative [Ca2+]CYT levels and quantification of relative peak [Ca2+]CYT and relative [Ca2+]CYT at 1600 s during addition of CPA (10 µM) (a–c), and at 600 s during addition of EGF (50 ng/mL) (d–f) and Trypsin (15 nM) (g–i) in the presence of 10 µM Synta66 or YM58483 compared with the DMSO control (0.1%). Cells were pre-treated with Synta66, YM58483 or DMSO control for 15 min prior to addition of agonists (containing the same concentration of Synta66, YM58483 and DMSO control). All the Ca2+ traces and bar graphs presented are the mean of three independent experiments ± SD and for bar graphs, ns not significant (P > 0.05), *P < 0.05, **P < 0.01 (paired t test compared to the DMSO control)
Fig. 3.
Inhibition of ATP-induced store-operated Ca2+ entry by Synta66 and YM58483. Mean [Ca2+]CYT levels (a, b) and quantification of relative peak [Ca2+]CYT and relative [Ca2+]CYT at 600 s during addition of 0.5 and 10 µM ATP (c, d) in the presence of 10 µM Synta66 or YM58483 compared with the DMSO control (0.1%). Cells were pre-treated with Synta66, YM58483 or DMSO control for 15 min prior to addition of ATP solution (containing the same concentration of compounds and DMSO control). All the Ca2+ traces and bar graphs presented are the mean of three independent experiments and for bar graphs ± SD, ns not significant (P > 0.05), **P < 0.01 (paired t test compared to the DMSO control)
Cell migration assay
Cell motility was measured using a collagen-based single cell migration assay and a live cell imaging system. 96-well plates were coated with 50 µL of collagen solution containing sterile-filtered 10× phosphate-buffered saline (PBS) (8% v/v), cell culture media (24% v/v), type I collagen from bovine skin, buffered to physiological pH with 1 M NaOH. After incubating plates at 37 °C and 5% CO2 in humidified incubator for 1 h to allow collagen gel to solidify, cells were plated on top of the gel (500 cells, 100 µL volume per well). Post seeding (24 h), cell culture media were replaced with serum-reduced media (SRM, 0.5% FBS) containing Synta66 (10 µM) or YM58483 (10 µM) or DMSO control (0.1%) for 24 h. Media were replaced with either complete media (CM, 10% FBS) or SRM with EGF (50 ng/mL), containing DMSO control, Synta66 or YM58483. Plates were then placed in a JuLi™ Stage Live Cell Imaging System (NanoEnTek Inc. Seoul, South Korea) housed in a 37 °C and 5% CO2 humidified incubator for 24 h with bright-field images acquired every 15 min. Single cell tracking was performed on images acquired 12 h after addition of EGF or complete media for a period of 12 h. ImageJ 1.49q software (NIH, Bethesda, MD, https://imagej.nih.gov/ij/) was used to track cells. Accumulated distance travelled by each cell was calculated and illustrated by Chemotaxis and Migration Tool V2.0 (Ibidi, Munich, Germany). Strict criteria were employed regarding exclusion of cells from analysis. Cells which died (loss of plasma membrane integrity or formation of apoptotic bodies), or moved into or out of the field of view during the period of migration assessment was excluded from analysis. Cells which clumped together were also excluded as only single cell motility was assessed.
Cell proliferation assay
Cells were seeded at a density of 6 × 103 per well in 96-well plates. Post seeding (24 h), cells were treated with vehicle (0.1% DMSO) or 10 µM Synta66 or YM58483 for 72 h. Fresh DMSO control or SOCE inhibitor containing media was added every 24 h. Cell proliferation was assessed using the colorimetric MTS assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay; Promega, Madison, WA, USA). CellTiter 96® AQueous One Solution Reagent (20 µL) was added into each well containing 100 µL of culture medium. Plates were incubated at 37 °C and 5% CO2 in a humidified incubator for 2 h. Subsequently, absorbance was measured at 490 nm using a model 550 microplate Reader (Bio-Rad, Hercules, CA, USA) and numbers subtracted from the background “no cell” absorbance.
siRNA-mediated silencing
MDA-MB-468 cells were seeded at 15 × 103 cells per well in 96-well plates. 24 h post seeding, DharmaFECT4 Transfection Reagent (0.1 μL per well) and Dharmacon ON-TARGETplus SMARTpool siRNAs (Dharmacon Inc, USA), were used for silencing Orai1 (L-014998-00) and TRPC3 (L-006509-00). Non-targeting siRNA (siNT; D-001810-10) was used as control treatment. RNA was isolated 48 h post-transfection and silencing efficiency was confirmed using real-time RT-PCR.
Real-time RT-PCR
RNA was isolated and purified using the Qiagen RNeasy Mini Kit (Qiagen, Hilden, Germany). cDNA was synthesized from isolated RNA using the Omniscript Reverse Transcription Kits (Qiagen) and amplified using the TaqMan Fast Universal PCR Master Mix (Applied Biosystems, CA, USA). Real-time PCR were performed using a StepOne Plus Real-Time PCR System (Applied Biosystems) under universal cycling conditions. TaqMan gene expression assay IDs used were: ORAI1 (Hs03046013_m1) and TRPC3 (Hs00162985_m1). The comparative CT method was used to quantify the relative expression of assessed genes normalised to 18 s ribosomal RNA gene.
Statistical analysis
Statistical analyses were performed using GraphPad Prism Version 7.00 for Windows, GraphPad Software (La Jolla, CA, USA, www.graphpad.com). Specific statistical tests for each experiment are stated in the corresponding figure legend.
Results
To compare the degree of sustained Ca2+ influx in MDA-MB-468 triple negative breast cancer cells initiated by different stimuli, we compared [Ca2+]CYT transients in the presence and absence of extracellular free Ca2+ initiated by epidermal growth factor (EGF) (50 ng/mL), adenosine triphosphate (ATP) (100 µM) and trypsin (10 nM) (Fig. 1). EGF is an activator of the EGF receptor (EGFR), a tyrosine kinase receptor that can couple to phospholipase C (PLC)β, and induce Ca2+ store release via generation of inositol 1,4,5-trisphosphate (IP3) [33]. As can be seen in Fig. 1a–d, and consistent with the pronounced expression of EGFR in MDA-MB-468 cells [24], EGFR produced a gradual increase in [Ca2+]CYT with a sustained phase of elevated [Ca2+]CYT that was abolished by the removal of extracellular Ca2+. ATP is an activator of various P2Y G-protein coupled receptors, which can couple to PLCβ to generate IP3 and then cause intracellular Ca2+ store release [34]. ATP can also activate various P2X receptors that are ligand gated Ca2+ permeable ion channels [34]. ATP produced a rapid increase in [Ca2+]CYT with a very pronounced sustained phase of [Ca2+]CYT that was entirely dependent of Ca2+ influx (Fig. 1e–h). Similarly, the G-protein coupled receptor protease-activated receptor 2 (PAR2) activator, trypsin, produced Ca2+ store release but with a less sustained phase of [Ca2+]CYT elevation, although this sustained phase was also abolished by the removal of extracellular Ca2+ (Fig. 1i–l). Hence, all of the activators assessed had two components of increase in [Ca2+]CYT in MDA-MB-468 breast cancer cells. The first phase was Ca2+ store release, which as expected, was not dependent on Ca2+ influx (Fig. 1c, g, k), and a second sustained phase of elevated [Ca2+]CYT, which was due to the influx of extracellular Ca2+ (Fig. 1d, h, l). We then sought to assess the sensitivity of this Ca2+ influx to two pharmacological inhibitors of store-operated Ca2+ entry, Synta66 and YM58483 at 10 µM, a concentration selected from previous studies that showed this concentration is above the IC50 in cell line models [23, 35–37].
Most studies assessing store-operated Ca2+ entry have used a protocol involving removal of extracellular Ca2+ followed by pronounced Ca2+ store depletion with sarco/endoplasmic reticulum Ca2+-ATPase inhibitors [e.g. cyclopiazonic acid (CPA)], and then the re-addition of extracellular Ca2+. Consistent with the promotion of store-operated Ca2+ entry induced by CPA in MDA-MB-468 cells under these conditions [38], CPA induced a Ca2+ influx phase that was sensitive to the store-operated Ca2+ entry inhibitors Synta66 and YM58483 (15 min pre-incubation) (Fig. 2a–c), with only a modest effect on maximal [Ca2+]CYT, due to concurrent Ca2+ influx during the peak, which was a statistically significant effect for Synta66. The [Ca2+]CYT transient initiated by EGF was totally remodelled by Synta66 and YM58483 (Fig. 2d), with no effect on the peak [Ca2+]CYT initiated by Ca2+ store release (Fig. 2e), but a complete attenuation of the very sustained phase of Ca2+ influx, which was observed after 600 s of EGF addition (Fig. 2f). Although trypsin (15 nM), did not produce the same degree of sustained Ca2+ influx as EGF (Fig. 2g), it similarly had a peak [Ca2+]CYT increase that was insensitive to Synta66 and YM58483 (Fig. 2h), but a sustained Ca2+ influx that was sensitive to these store-operated Ca2+ entry inhibitors (Fig. 2i). Assessment of increases in [Ca2+]CYT initiated by ATP under these conditions indicated that the contribution of store-operated Ca2+ entry varied with the concentration of ATP (Fig. 3a, b). Although peak [Ca2+]CYT initiated by both submaximal (0.5 µM) and maximal (100 µM) ATP were insensitive to Synta66 and YM58483 (15 min pre-incubation) (Fig. 3c), the sustained phase of [Ca2+]CYT elevation with 100 µM ATP was far more sensitive to Synta66 and YM58483 (Fig. 3d). These studies collectively indicate that many stimuli can promote store-operated Ca2+ entry that is sensitive to Synta66 and YM58483 in MDA-MB-468 breast cancer cells. We, therefore, assessed the effects of these agents on the migration and proliferation [19, 22] of this breast cancer cell line.
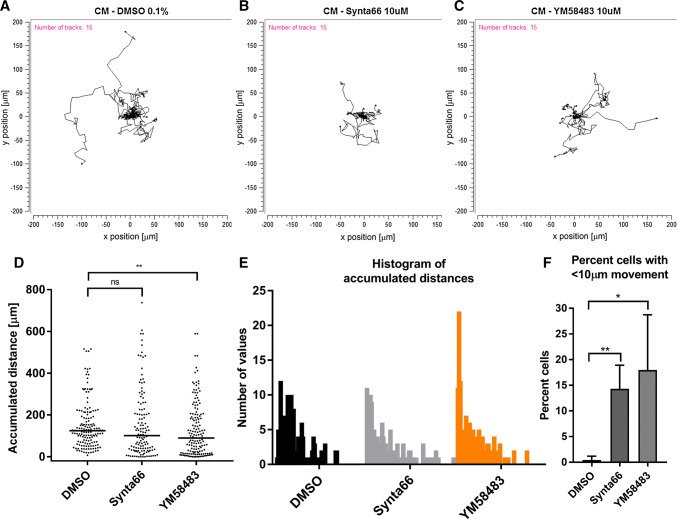
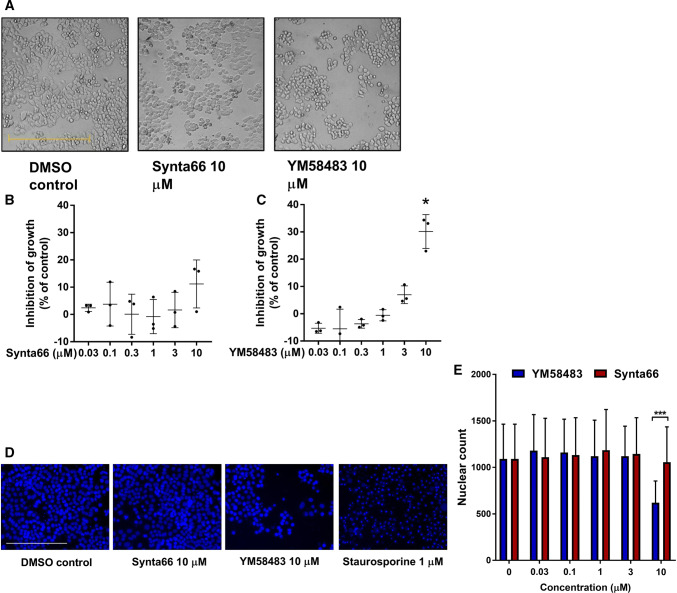
Cell migration of MDA-MB-468 breast cancer cells was assessed in control, Synta66 and YM58483 treated wells using live cell imaging. In the case of cell migration induced by the addition of complete media (including 10% FBS), single cell analysis (Fig. 4a–c) demonstrated that there was no significant effect of Synta66 on mean accumulated distance travelled during the assessed period, but YM58483 significantly reduced mean accumulated distance travelled (Fig. 4d). However, it was clear that the proportion of cells with little to no movement was increased by both Synta66 and YM58483 (Fig. 4e) and this was significant for both inhibitors (Fig. 4f). EGF was also assessed (Fig. 5a–c), and EGF was a more effective stimulus for MDA-MB-468 breast cancer cell migration, with a mean accumulated distance of around 270 µm compared to that for complete medium-induced migration at 160 µm (Fig. 4d vs 5d, and also Fig. S3). YM58483, but not Synta66, significantly reduced the mean accumulated distance of cell migration observed in the presence of EGF (Fig. 5d). In contrast to complete media, there was no promotion of the population of cells that have little to no migration, as neither Synta66 nor YM58483 had a significant effect on this population of cells (Fig. 5e, f). The differential effects of Synta66 and YM58483 were also apparent when proliferation was compared between MDA-MB-468 breast cancer cells cultured in the presence and absence of YM58483 and Synta66 for 3 days, where YM58483, but not Synta66, had a significant effect (Fig. 6a, b). Although further study is required, this difference could be related to the nature of inhibition of these two agents, as evident by the clear difference in time dependence of SOCE inhibition, where prolonging incubation with the inhibitor (24 h vs 15 min) promoted SOCE inhibition more with YM58483 than Synta66 (Fig. S1). The anti-proliferative effects of YM58483 were evident in MTS metabolism assays and decreased nuclei numbers (using Hoechst 33342 staining) (Fig. 6d, e). These decreases were not due to cell death as indicated by the absence of apoptotic morphology in bright field imaging (Fig. 6a) and apoptotic/condensed nuclei (Fig. 6d).
Fig. 4.
Assessment of the effect of Synta66 and YM58483 on a complete media (CM)-model of cell migration. Cells were serum-deprived (0.5% FBS) for 24 h prior to addition of complete media (10% FBS) containing DMSO (0.1%), Synta66 (10 µM) or YM58483 (10 µM) for 24 h. Cell migration was analysed from 12 h to 24 h after addition of complete media. Representative spatial plots of DMSO control (a), Synta66 (b) and YM58483 (c) of 15 randomised cells from one experiment. d Quantitative analysis of the median accumulated distance travelled by all the cells from three independent experiment (duplicate wells) treated with DMSO control (total 152 cells), Synta66 (total 126 cells) or YM58483 (total number of 156 cells). ns not significant (P > 0.05), **P < 0.01 (nonparametric Mann–Whitney test). e Histograms of accumulated distances travelled by cells binned at 10 µm intervals. f Percent of cells with accumulated distance travelled of less than 10 µm. Data are the mean ± SD from three independent experiments (n = 3). ns not significant (P > 0.05), *P < 0.05, **P < 0.01 (unpaired t test compared to the DMSO control)
Fig. 5.
Assessment of the effect of Synta66 and YM58483 on an EGF-model of cell migration. Cells were serum-deprived (0.5% FBS) for 24 h prior to addition of EGF (50 ng/mL) containing DMSO (0.1%), Synta66 (10 µM) or YM58483 (10 µM) for 24 h. Cell migration was analysed from 12 h to 24 h after EGF addition. Representative spatial plots of DMSO control (a), Synta66 (b) and YM58483 (c) of 15 randomised cells from one experiment. d Quantitative analysis of the median accumulated distance travelled by cells from three independent experiment (duplicate wells) treated with DMSO control (total number of 185 cells), Synta66 (total number of 176 cells) or YM58483 (total number of 198 cells). ns not significant (P > 0.05), ****P < 0.0001 (nonparametric Mann–Whitney test). e Histograms of accumulated distances travelled by cells binned at 10 µm intervals. f Percent of cells with accumulated distance travelled of less than 10 µm. Data are the mean ± SD from three independent experiments (n = 3). ns not significant (P > 0.05), ***P < 0.0001 (unpaired t test compared to the DMSO control)
Fig. 6.
Assessment of cell viability with Synta66 and YM58483 treatment. a Representative pictures of MDA-MB-468 breast cancer cells after treatment with DMSO control (0.1%) or 10 µM Synta66 or YM58483 for 72 h. b, c Percent inhibition of growth of MDA-MB-468 cells treated with different concentrations of Synta66 or YM58483 (0.03, 0.1, 0.3, 1, 3, 10 µM) for 72 h normalised to the DMSO control group. Data are the mean ± SD from three independent experiments (n = 3). *P < 0.01 (unpaired t test compared to the DMSO control). Scale bar = 500 µm. d Representative images from cell staining with Hoechst 33342 in cells treated with Synta66 or YM58483 for 72 h and a 24 h treatment with staurosporine (1 µM) as a positive control for apoptotic nuclei (4 regions per well, in triplicate wells). Scale bar = 250 µM. e Nucleus number of cells treated with DMSO control, or different concentrations of Synta66 or YM58483 (0.03, 0.1, 1, 3, 10 µM) for 72 h (n = 3, ± SEM). *** P < 0.001 (two-way ANOVA with Bonferroni’s multiple comparison)
Discussion
The remodelling of Orai1 mediated Ca2+ influx in breast cancer is particularly associated with breast cancers of the basal molecular phenotype, which are associated with a poorer prognosis. Basal breast cancers have higher levels of Orai1 than non-basal breast cancers [19]. Moreover, breast cancers of the basal molecular phenotype that have high STIM1 but low STIM2, are associated with a poor prognosis [19]. This association is not evident in breast cancers that are not of the basal molecular subtype [19]. The importance of Orai1 in basal breast cancers is also reflected in the anti-proliferative and anti-migratory effects of Orai1 silencing in the MDA-MB-231 basal breast cancer cell line [19, 22]. MDA-MB-231 breast cancer cells are of the Basal B molecular classification [39]. By comparison there has been limited study of breast cancer cell lines of the basal A subtype such as MDA-MB-468 cells, which are of particular interest because of their cellular plasticity and sensitivity to undergo epithelial–mesenchymal transition, a process important in breast cancer progression [40, 41]. Although Orai1-mediated store-operated Ca2+ entry has been demonstrated using Orai1 silencing in MDA-MB-468 breast cancer cells [42], these like most studies in cancer cells, have assessed store-operated Ca2+ entry under conditions that promote substantial and sustained Ca2+ store depletion, i.e. by including experimental periods where extracellular Ca2+ was excluded. Other limitations in the study of store-operated Ca2+ entry in basal breast cancer cells include using pharmacological modulators to inhibit store-operated Ca2+ entry, that we now know have significant off target effects [11, 19, 22, 23]. The research conducted here, therefore, sought to address some of these knowledge gaps.
MDA-MB-468 basal A breast cancer cells are clearly capable of sustained Ca2+ influx as exemplified by our comparison of the [Ca2+]CYT changes elicited by ATP, EGF and trypsin. However, the magnitude of this Ca2+ influx phase was dependent on the stimuli, with ATP (100 µM) resulting in the most pronounced Ca2+ influx phase. The store-operated Ca2+ entry inhibitors Synta66 and YM58483 had a dramatic effect on [Ca2+]CYT changes associated with the SERCA inhibitor, CPA. The abolition of the CPA-induced sustained phase of Ca2+ influx by Synta66 and YM58483 is consistent with Orai1 silencing studies in this cell line [42] and indicative that this Ca2+ influx pathway is important in this basal A breast cancer cell line. In contrast to the other activators assessed in these studies, there was a modest, and in the case of Synta66, a significant effect of store-operated Ca2+ entry inhibitors on peak [Ca2+]CYT levels induced by CPA. This is likely because the other agents assessed in this study cause reductions in endoplasmic reticulum Ca2+ levels (the driving force of store-operated Ca2+ influx), via the rapid generation of IP3 and release of Ca2+ via IP3 gated endoplasmic Ca2+ channels, whereas SERCA inhibition by CPA depletes endoplasmic Ca2+ levels, via stopping the re-sequestration of Ca2+ slowly leaking from the endoplasmic reticulum [43]. Hence, in the case of CPA, store-operated Ca2+ entry is activated more gradually, and therefore, it would also contribute to the maximal increase in [Ca2+]CYT seen with CPA. EGF and trypsin also produced Ca2+ influx that was sensitive to store-operated Ca2+ entry inhibitors.
The contribution of store-operated Ca2+ entry to global sustained increases in [Ca2+]CYT was dependent (as would be predicted), on the degree of Ca2+ store depletion, as exemplified by a major effect of Synta66 and YM58483 on a maximal but not a submaximal concentration of ATP. However, the absence of pronounced effects on global [Ca2+]CYT at lower concentrations of activators does not discount a potentially important role for store-operated Ca2+ entry during these more modest stimuli. Local increases around Orai1 channels are a regulator of NFAT1-mediated gene transcription; indeed Orai1 has been described as having a private line of communication to NFAT1 [10]. Hence more modest activation of Orai1 may remodel signalling and processes in breast cancer cells using this or similar mechanisms. Despite the presence of purinergic ATP activated Ca2+ permeable ion channels (P2X channels) in MDA-MB-468 breast cancer cells [42, 44], the sustained phase of Ca2+ influx induced by ATP (100 µM) was completely abolished by inhibitors of store-operated Ca2+ entry. This defines store-operated Ca2+ entry as either the major trigger and/or the major mediator of Ca2+ influx induced by some stimuli in MDA-MB-468 basal A breast cancer cells. In this context, the consequences of pharmacological inhibitors on two key processes in breast cancer progression (migration and proliferation) was assessed.
Consistent with studies using Orai1 silencing and less specific pharmacological inhibitors in MDA-MB-231 basal B breast cancer cells, Synta66 and YM58483 reduced the migration of MDA-MB-468 basal A breast cancer cells, as reflected in a significant increase in the percentage of cells with levels of migration less than 10 µm with complete media re-addition. However, effects on migration were not universal. The effects of Synta66 and YM58483 were not as evident when migration was induced by EGF. The degree of migration by EGF was greater than complete media as reflected in a higher mean accumulated distance, and this may indicate that other pathways that are initiated by EGF are less dependent on store-operated Ca2+ entry. EGF activated pathways in this cell line include STAT3, AKT, ERK1/2 [45, 46], which may be not be completely dependent on store-operated Ca2+ entry. However, under these conditions YM58483, but not Synta66, did significantly reduce accumulated distance observed with EGF stimulated migration. This suggests that Synta66 and YM58483 may not be totally equivalent and not interchangeable despite their similar effects on store-operated Ca2+ entry in various model systems [11, 23]. Differences were also clear in our studies on proliferation with YM58483, but not Synta66, having a significant effect on this parameter. Future studies could explore the potential impact of SOCE inhibitors on the modification of Ca2+ signals induced by ATP and trypsin and on other cellular processes in this model.
The differences between Synta66 and YM58483 in EGF-induced migration and cellular proliferation could be due to a variety of factors. These factors include differential degrees of store-operated Ca2+ entry inhibition, different effects on other ion channels and different mechanisms of action that may influence the nature of Orai1 inhibition. In the context of different degrees of store-operated Ca2+ entry inhibition, this was not evident in our Ca2+ assays using a variety of stimuli. Distinctive off targets effects may contribute to differential effects, indeed YM58483 inhibits TRPC3 [47, 48], which is expressed in MDA-MB-468 cells [38]. However, this is unlikely given the consistent reports of the inhibitory effects of Orai1 silencing on the proliferation and migration of many cancer cell lines [19, 22, 49, 50], and the lack of inhibition of SOCE with TRPC3 silencing in the MDA-MB-468 cells used in this study (Fig. S2). Our previous studies also showed that silencing of TRPC1, that is also expressed in MDA-MB-468 cells, did not inhibit SOCE in these cells [38]. Differential mechanisms of inhibition may have a greater impact on the effectiveness of store-operated Ca2+ entry inhibitors in different models; this could be through the duration of channel inhibition or what stimuli may be most affected by these inhibitors. Although YM58483 has been postulated to act via an extracellular site [51], this has not been fully explored and the mechanism of Synta66 is still unknown or at least unpublished [11]. As studies begin to further define the mechanism of action for these agents, the reasons for the differential effects of YM58483 and Synta66 observed in this study may become clearer. Unfortunately, very few studies have directly compared different store-operated Ca2+ entry inhibitors in the same model system as has been done in these studies. Our experiments did demonstrate that the SOCE inhibition mediated by YM58483 is enhanced to a much greater extent with increased pre-incubation time (as previously reported [52]) than Synta66, which to our knowledge has not been previously described (Fig. S1). Our work, therefore, demonstrates the importance of using multiple SOCE inhibitors in pharmacological studies. Such an approach should be used more widely at least until the mechanisms of these and other store-operated Ca2+ entry inhibitors are better understood.
In conclusion, these studies implicate store-operated Ca2+ entry as a differential contributor to global sustained increases in [Ca2+]CYT induced by different stimuli in MDA-MB-468 basal A breast cancer cells. The use of more selective pharmacological inhibitors of store-operated Ca2+ entry than previously used in MDA-MB-231 basal B breast cancer cells, supports a role for such agents in the control of breast cancer cell migration in MDA-MB-468 breast cancer with some stimuli. However, these studies have also identified differences between YM58483 and Synta66 in EGF-induced migration and proliferation that require further investigation in other basal breast cancer cell lines and other model systems.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was partially supported by the National Health and Medical Research Council (NHMRC; project Grant 1079672). G.R.M. is supported by the Mater Foundation. The Translational Research Institute is supported by a Grant from the Australian Government.
Abbreviations
- ATP
Adenosine triphosphate
- CM
Complete media
- CPA
Cyclopiazonic acid
- DMEM
Dulbecco’s modified Eagle’s medium
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- ER
Endoplasmic reticulum
- FBS
Foetal bovine serum
- IP3
Inositol 1,4,5-trisphosphate
- PAR2
Protease-activated receptor 2
- PBS
Phosphate-buffered saline
- PLC
Phospholipase C
- SOCE
Store-operated Ca2+ entry
- SRM
Serum-reduced media
- STIM1
Stromal interaction molecule 1
- STR
Short tandem repeat
Compliance with ethical standards
Conflict of interest
GR.M. and S.J.R.-T. are associated with QUE Oncology Inc.
References
- 1.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 2.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 3.Lopez JJ, Albarran L, Gomez LJ, Smani T, Salido GM, Rosado JA. Molecular modulators of store-operated calcium entry. Bba-Mol Cell Res 1863. 2016;8:2037–2043. doi: 10.1016/j.bbamcr.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Cai XY, Zhou YD, Nwokonko RM, Loktionova NA, Wang XM, Xin P, Trebak M, Wang YJ, Gill DL. The Orai1 store-operated calcium channel functions as a hexamer. J Biol Chem. 2016;291(50):25764–25775. doi: 10.1074/jbc.M116.758813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putney JW, Steinckwich-Besancon N, Numaga-Tomita T, Davis FM, Desai PN, D’Agostin DM, Wu SL, Bird GS. The functions of store-operated calcium channels. Bba-Mol Cell Res 1864 . 2017;6:900–906. doi: 10.1016/j.bbamcr.2016.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahrner M, Schindl R, Muik M, Derler I, Romanin C. The STIM-Orai pathway: the interactions between STIM and Orai. Adv Exp Med Biol. 2017;993:59–81. doi: 10.1007/978-3-319-57732-6_4. [DOI] [PubMed] [Google Scholar]
- 7.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis FM, Janoshazi A, Janardhan KS, Steinckwich N, D’Agostin DM, Petranka JG, Desai PN, Roberts-Thomson SJ, Bird GS, Tucker DK, Fenton SE, Feske S, Monteith GR, Putney JW. Essential role of Orai1 store-operated calcium channels in lactation. Proc Natl Acad Sci USA. 2015;112(18):5827–5832. doi: 10.1073/pnas.1502264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis FM, Goulding EH, D’Agostin DM, Janardhan KS, Cummings CA, Bird GS, Eddy EM, Putney JW. Male infertility in mice lacking the store-operated Ca2+ channel Orai1. Cell Calcium. 2016;59(4):189–197. doi: 10.1016/j.ceca.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kar P, Parekh AB. Distinct spatial Ca2+ signatures selectively activate different NFAT transcription factor isoforms. Acta Physiol. 2015;58(2):232–243. doi: 10.1016/j.molcel.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev. 2015;95(4):1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen L, Voronina S, Javed MA, Awais M, Szatmary P, Latawiec D, Chvanov M, Collier D, Huang W, Barrett J, Begg M, Stauderman K, Roos J, Grigoryev S, Ramos S, Rogers E, Whitten J, Velicelebi G, Dunn M, Tepikin AV, Criddle DN, Sutton R. Inhibitors of ORAI1 prevent cytosolic calcium-associated injury of human pancreatic acinar cells and acute pancreatitis in 3 mouse models. Gastroenterology. 2015;149(2):481–492. doi: 10.1053/j.gastro.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mai XY, Shang JY, Liang SJ, Yu BX, Yuan JN, Lin Y, Luo RF, Zhang FR, Liu YY, Lv XF, Li CL, Liang XL, Wang WD, Zhou JG. Blockade of Orai1 store-operated calcium entry protects against renal fibrosis. J Am Soc Nephrol. 2016;27(10):3063–3078. doi: 10.1681/ASN.2015080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jardin I. Rosado JA (2016) STIM and calcium channel complexes in cancer. Bba-Mol Cell Res. 1863;6:1418–1426. doi: 10.1016/j.bbamcr.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Sobradillo D, Hernandez-Morales M, Ubierna D, Moyer MP, Nunez L, Villalobos C. A reciprocal shift in transient receptor potential channel 1 (TRPC1) and stromal interaction molecule 2 (STIM2) contributes to Ca2+ remodeling and cancer hallmarks in colorectal carcinoma cells. J Biol Chem. 2014;289(42):28765–28782. doi: 10.1074/jbc.M114.581678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang BD, Xia X, Lv XF, Yu BX, Yuan JN, Mai XY, Shang JY, Zhou JG, Liang SJ, Pang RP. Inhibition of Orai1-mediated Ca2+ entry enhances chemosensitivity of HepG2 hepatocarcinoma cells to 5-fluorouracil. J Cell Mol Med. 2017;21(5):904–915. doi: 10.1111/jcmm.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H, Zhang H, Jin F, Fang MZ, Huang M, Yang CS, Chen T, Fu LW, Pan Z. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget. 2014;5(11):3455–3471. doi: 10.18632/oncotarget.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubois C, Vanden AF, Lehen’kyi V, Gkika D, Guarmit B, Lepage G, Slomianny C, Borowiec AS, Bidaux G, Benahmed M, Shuba Y, Prevarskaya N. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell. 2014;26(1):19–32. doi: 10.1016/j.ccr.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 19.McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, Smart CE, Brown MA, Kenny PA, Roberts-Thomson SJ, Monteith GR. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther. 2011;10(3):448–460. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- 20.Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem. 2010;285(25):19173–19183. doi: 10.1074/jbc.M110.102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalimutho M, Parsons K, Mittal D, Lopez JA, Srihari S, Khanna KK. Targeted therapies for triple-negative breast cancer: combating a stubborn disease. Trends Pharmacol Sci. 2015;36(12):822–846. doi: 10.1016/j.tips.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15(2):124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Azimi I, Flanagan JU, Stevenson RJ, Inserra M, Vetter I, Monteith GR, Denny WA. Evaluation of known and novel inhibitors of Orai1-mediated store operated Ca2+ entry in MDA-MB-231 breast cancer cells using a fluorescence imaging plate reader assay. Bioorg Med Chem. 2017;25(1):440–449. doi: 10.1016/j.bmc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 24.deFazio A, Chiew YE, Sini RL, Janes PW, Sutherland RL. Expression of c-erbB receptors, heregulin and oestrogen receptor in human breast cell lines. Int J Cancer. 2000;87(4):487–498. doi: 10.1002/1097-0215(20000815)87:4<487::AID-IJC5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 25.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, Carey M, Hu Z, Guan Y, Sahin A, Symmans WF, Pusztai L, Nolden LK, Horlings H, Berns K, Hung MC, van de Vijver MJ, Valero V, Gray JW, Bernards R, Mills GB, Hennessy BT. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68(15):6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azimi I, Petersen RM, Thompson EW, Roberts-Thomson SJ, Monteith GR. Hypoxia-induced reactive oxygen species mediate N-cadherin and SERPINE1 expression, EGFR signalling and motility in MDA-MB-468 breast cancer cells. Sci Rep. 2017;7(1):15140. doi: 10.1038/s41598-017-15474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phuah SY, Looi LM, Hassan N, Rhodes A, Dean S, Taib NA, Yip CH, Teo SH. Triple-negative breast cancer and PTEN (phosphatase and tensin homologue) loss are predictors of BRCA1 germline mutations in women with early-onset and familial breast cancer, but not in women with isolated late-onset breast cancer. Breast Cancer Res. 2012;14(6):R142. doi: 10.1186/bcr3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Changavi AA, Shashikala A, Ramji AS. Epidermal growth factor receptor expression in triple negative and nontriple negative breast carcinomas. J Lab Phys. 2015;7(2):79–83. doi: 10.4103/0974-2727.163129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cursons J, Leuchowius KJ, Waltham M, Tomaskovic-Crook E, Foroutan M, Bracken CP, Redfern A, Crampin EJ, Street I, Davis MJ, Thompson EW. Stimulus-dependent differences in signalling regulate epithelial-mesenchymal plasticity and change the effects of drugs in breast cancer cell lines. Cell Commun Signal. 2015;13:26. doi: 10.1186/s12964-015-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye X, Weinberg RA. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol. 2015;25(11):675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson RJ, Azimi I, Flanagan JU, Inserra M, Vetter I, Monteith GR, Denny WA. An SAR study of hydroxy-trifluoromethylpyrazolines as inhibitors of Orai1-mediated store operated Ca2+ entry in MDA-MB-231 breast cancer cells using a convenient Fluorescence Imaging Plate Reader assay. Bioorg Med Chem. 2018;26(12):3406–3413. doi: 10.1016/j.bmc.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Wu TTL, Peters AA, Tan PT, Roberts-Thomson SJ, Monteith GR. Consequences of activating the calcium-permeable ion channel TRPV1 in breast cancer cells with regulated TRPV1 expression. Cell Calcium. 2014;56(2):59–67. doi: 10.1016/j.ceca.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Kolch W, Pitt A. Functional proteomics to dissect tyrosine kinase signalling pathways in cancer. Nat Rev Cancer. 2010;10(9):618–629. doi: 10.1038/nrc2900. [DOI] [PubMed] [Google Scholar]
- 34.Burnstock G, Di VF. Purinergic signalling and cancer. Purinerg. Signal. 2013;9(4):491–540. doi: 10.1007/s11302-013-9372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takezawa R, Cheng H, Beck A, Ishikawa J, Launay P, Kubota H, Kinet JP, Fleig A, Yamada T, Penner R. A pyrazole derivative potently inhibits lymphocyte Ca2+ influx and cytokine production by facilitating transient receptor potential melastatin 4 channel activity. Mol Pharmacol. 2006;69(4):1413–1420. doi: 10.1124/mol.105.021154. [DOI] [PubMed] [Google Scholar]
- 36.Ng SW, di Capite J, Singaravelu K, Parekh AB. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J Biol Chem. 2008;283(46):31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 37.Di Sabatino A, Rovedatti L, Kaur R, Spencer JP, Brown JT, Morisset VD, Biancheri P, Leakey NA, Wilde JI, Scott L, Corazza GR, Lee K, Sengupta N, Knowles CH, Gunthorpe MJ, McLean PG, MacDonald TT, Kruidenier L. Targeting gut T cell Ca2+ release-activated Ca2+ channels inhibits T cell cytokine production and T-box transcription factor T-bet in inflammatory bowel disease. J Immunol. 2009;183(5):3454–3462. doi: 10.4049/jimmunol.0802887. [DOI] [PubMed] [Google Scholar]
- 38.Azimi I, Milevskiy MJG, Kaemmerer E, Turner D, Yapa KTDS, Brown MA, Thompson EW, Roberts-Thomson SJ, Monteith GR. TRPC1 is a differential regulator of hypoxia-mediated events and Akt signalling in PTEN-deficient breast cancer cells. J Cell Sci. 2017;130(14):2292–2305. doi: 10.1242/jcs.196659. [DOI] [PubMed] [Google Scholar]
- 39.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 41.Azimi I, Monteith GR. Plasma membrane ion channels and epithelial to mesenchymal transition in cancer cells. Endocr Relat Cancer. 2016;23(11):R517–R525. doi: 10.1530/ERC-16-0334. [DOI] [PubMed] [Google Scholar]
- 42.Davis FM, Peters AA, Grice DM, Cabot PJ, Parat MO, Roberts-Thomson SJ, Monteith GR. Non-stimulated, agonist-stimulated and store-operated Ca2+ influx in MDA-MB-468 breast cancer cells and the effect of EGF-induced EMT on calcium entry. PLoS One. 2012;7(5):e36923. doi: 10.1371/journal.pone.0036923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, VanBreemen C. Agonist- and CPA-induced elevation of cytoplasmic free Ca2+ in intact valvular endothelium from rabbits. Am J Physiol-Heart C. 1996;270(3):H837–H848. doi: 10.1152/ajpheart.1996.270.3.H837. [DOI] [PubMed] [Google Scholar]
- 44.Azimi I, Beilby H, Davis FM, Marcial DL, Kenny PA, Thompson EW, Roberts-Thomson SJ, Monteith GR. Altered purinergic receptor-Ca signaling associated with hypoxia-induced epithelial-mesenchymal transition in breast cancer cells. Mol Oncol. 2015;10(1):166–178. doi: 10.1016/j.molonc.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartkowiak K, Effenberger KE, Harder S, Andreas A, Buck F, Peter-Katalinic J, Pantel K, Brandt BH. Discovery of a novel unfolded protein response phenotype of cancer stem/progenitor cells from the bone marrow of breast cancer patients. J Proteome Res. 2010;9(6):3158–3168. doi: 10.1021/pr100039d. [DOI] [PubMed] [Google Scholar]
- 46.Davis FM, Azimi I, Faville RA, Peters AA, Jalink K, Putney JW, Jr, Goodhill GJ, Thompson EW, Roberts-Thomson SJ, Monteith GR. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33(18):2307–2316. doi: 10.1038/onc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He LP, Hewavitharana T, Soboloff J, Spassova MA, Gill DL. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J Biol Chem. 2005;280(12):10997–11006. doi: 10.1074/jbc.M411797200. [DOI] [PubMed] [Google Scholar]
- 48.Putney JW. Pharmacology of store-operated calcium channels. Mol Interv. 2010;10(4):209–218. doi: 10.1124/mi.10.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motiani RK, Hyzinski-Garcia MC, Zhang XX, Henkel MM, Abdullaev IF, Kuo YH, Matrougui K, Mongin AA, Trebak M. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflug Arch Eur J Physiol. 2013;465(9):1249–1260. doi: 10.1007/s00424-013-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhan ZY, Zhong LX, Feng M, Wang JF, Liu DB, Xiong JP. Over-expression of Orai1 mediates cell proliferation and associates with poor prognosis in human non-small cell lung carcinoma. Int J Clin Exp Pathol. 2015;8(5):5080–5088. [PMC free article] [PubMed] [Google Scholar]
- 51.Jairaman A, Prakriya M. Molecular pharmacology of store-operated CRAC channels. Channels. 2013;7(5):402–414. doi: 10.4161/chan.25292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zitt C, Strauss B, Schwarz EC, Spaeth N, Rast G, Hatzelmann A, Hoth M. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem. 2004;279(13):12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.