Abstract
DNA methylation (CpG methylation) exerts an important role in normal differentiation and proliferation of hematopoietic stem cells and their differentiated progeny, while it has also the ability to regulate myeloid versus lymphoid fate. Mutations of the epigenetic machinery are observed in hematological malignancies including acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) resulting in hyper- or hypo-methylation affecting several different pathways. Enhancers are cis-regulatory elements which promote transcription activation and are characterized by histone marks including H3K27ac and H3K4me1/2. These gene subunits are target gene expression ‘fine-tuners’, are differentially used during the hematopoietic differentiation, and, in contrast to promoters, are not shared by the different hematopoietic cell types. Although the interaction between gene promoters and DNA methylation has extensively been studied, much less is known about the interplay between enhancers and DNA methylation. In hematopoiesis, DNA methylation at enhancers has the potential to discriminate between fetal and adult erythropoiesis, and also is a regulatory mechanism in granulopoiesis through repression of neutrophil-specific enhancers in progenitor cells during maturation. The interplay between DNA methylation at enhancers is disrupted in AML and MDS and mainly hyper-methylation at enhancers raising early during myeloid lineage commitment is acquired during malignant transformation. Interactions between mutated epigenetic drivers and other oncogenic mutations also affect enhancers’ activity with final result, myeloid differentiation block. In this review, we have assembled recent data regarding DNA methylation and enhancers’ activity in normal and mainly myeloid malignancies.
Keywords: Transcription factors, DNA methyltransferases, Histone marks, AML, MDS, Hematopoietic stem cells
DNA methylation
The process of DNA methylation (or CpG methylation) exerts a fundamental role in self-renewal and differentiation of hematopoietic stem cells (HSCs) and their downstream progeny through modulation of lineage-specific gene expression [1]. CpG methylation is relatively stable in fully differentiated cells, while undergoing changes during development, differentiation, aging, and disease progression [2]. Furthermore, CpG methylation controls myeloid versus lymphoid fate by silencing of genes from the alternative lineage through modulation of lineage-specific regions which are, by default, hypo-methylated [1, 3]. Myeloid commitment necessitates lower levels of DNA methylation than lymphoid commitment, and its seems that methylation profile of terminally differentiated cells from the myeloid lineage appears closely related, while lower levels of methylation are sufficient to maintain the lymphoid differentiation program once established [1, 4, 5]. Global DNA hypo-methylation leads to genomic instability, while focal hyper-methylation leads to gene silencing of tumour suppressor genes (TSGs) among others contributing to oncogenesis [6]. Mutations of the methylation machinery involve mainly the R882 position of the de novo methyltransferase DNMT3A and are found in acute myeloid leukemia (AML) cases, myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), chronic myelomonocytic leukemia (CMML), and in some types of T-cell lymphomas. These loss-of-function mutations, which might be observed early in a pre-leukemic phase, confer a selective advantage over the normal counterparts and might lead to haploinsufficiency predisposing to myeloid malignancies in vivo [7, 8]. On the contrary, the DNMT1 methyltransferase responsible for methylation maintenance might be involved in the pathogenesis of myeloid malignancies in a mutation-independent fashion as mutations are rarely observed in AML and MDS [9]. AML subgroups exhibit a very strong hyper-methylation signature compared to normal CD34+ cells and o classifies cytogenetically defined and mutation characterized AML, whereas the MDS genome is more extensively hyper-methylated than de novo AML. However, progression from MDS to secondary AML has been associated even more aberrant hyper-methylation, suggesting that hyper-methylation confers tumour clonal evolution [10, 11].
Enhancers’ biology
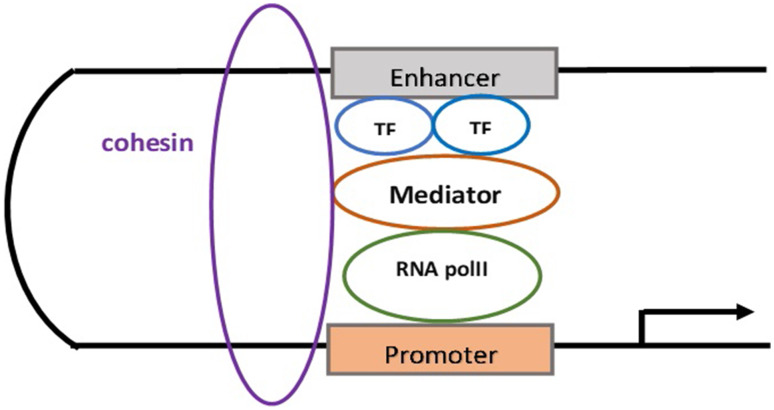
Enhancers are cis-regulatory DNA sequences capable of binding with combinations of transcription factors (TFs) and activate transcription irrespectively of their location, distance, or orientation of the related gene promoters, and are required for proper cell differentiation during development. Enhancers’ activation begins with the binding of TFs which interact with the Mediator complex recruiting RNA polymerase II (RNA pollII) at promoters in a gene-specific manner and local nucleosome remodeling [12, 13]. Subsequently, the Mediator complex undergoes conformational changes and binds to cohesin leading to gene activation [14]. Enhancers are marked by histone modifications such as methylation of histone H3 at lysine 4 (H3Kme1/2) and acetylation of Histone 3 at lysine 27 (H3K27ac) which are functionally active in a cell-type-specific fashion carrying epigenetic signals for gene induction and the differentiation history of cells [12]. They can be subclassified functionally as inactive, devoid of TF binding, and histone marks, primed, that is prior to activation characterized by H3K4me, hyper-mobile H3.3/H2a.z nucleosomes, DNA hypo-methylation, and hydroxylation (5hmC). Active enhancers are characterized by H3K4me, H3K79me3, H3K27ac, and RNA polII binding, and finally, poised enhancers are already in physical contact with their target genes before they become active, lack H3K27ac, and are marked by H3K4me and H3K27me3 [13, 15–19]. A subset of constituent enhancers arbitrarily defined as super enhancers (SEs) are typically marked by dense H3K27ac and H3K4me, Mediator and bromodomain containing protein 4 (BDR4), can be defined in any cell type, are associated with key cell-type specific TFs, control cell mammalian identity, and can be acquired by cancer cells at genes whose function is involved in oncogenesis [20–22]. Enhancers promote transcription activation through chromatin loops formation and physical interaction with core promoters located in cis on the same chromosome over hundreds of kb or even in different chromosomes [12] (Fig. 1). The interaction between enhancers and promoters is dynamic as a functional interchange between enhancers and promoters has been reported. A small fraction (2–3%) of gene promoters might act as enhancers, whereas intragenic enhancers might act as alternative promoters ensuring a more accurate regulation of gene expression [23, 24]. The same enhancer might regulate multiple genes simultaneously promoting transcriptional bursts, whereas a gene might be regulated by several enhancers and that joint effect of multiple enhancers should be taken in consideration while trying to identify target genes in normal and disease [25–27]. These enhancer–promoter interactions occur within topological association domains (TADs), established after pluripotency but before induction of terminal differentiation [28]. Target promoter identification by enhancers is a mechanism not completely elucidated and several mechanisms have been proposed trying to explain the enhancer–promoter recognition and interaction [29, 30]. Enhancers’ activity can be altered by mutated TFs by increasing their copy number amplifying their output, by promoting structural rearrangements which change the enhancer target, and by altering TFs binding creating de novo enhancers in a cancer-type specificity fashion. Furthermore, a genome-wide reprogramming of enhancers might lead to aberrant target gene expression. All these aberrations might impair cell differentiation and render a pre-cancerous state susceptible to additional genetic hits [31, 32].
Fig. 1.
Transcription factor (TF) binding to enhancers leads to mediator recruitment which mediates the interaction between TFs and RNA polymerase II machinery (RNA polII). Besides this mediating function, Mediator interacts with cohesin in a cohesin–mediator complex forming a stable looping during the enhancer–promoter interaction
Enhancer DNA methylation
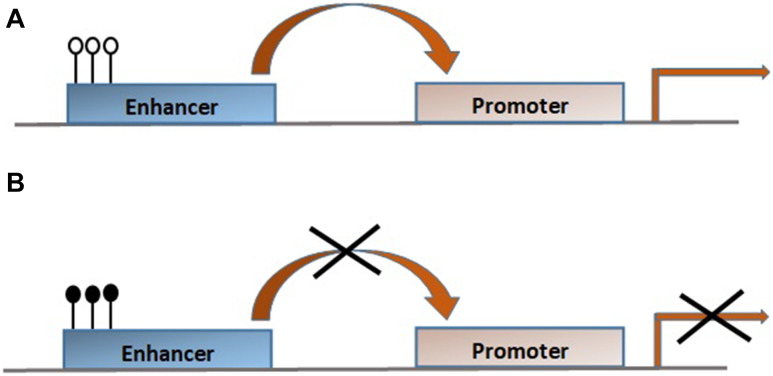
Enhancers exhibit the most dynamic but less stable DNA methylation changes and correlate negatively with enhancers’ activity and transcription of target genes. CpG methylation is regulated by DNA-binding TFs that interact with promoters and enhancers promoting the loss or acquisition of DNA methylation at these regulatory elements, very probably abnormal interference with TFs binding establishing an epigenetic signature at enhancers [33–36]. DNA methylation is essential for the deposition of specific histone modifications and specifically acts upstream of H3K27me3, H3K27ac, and H3K4me, histone marks with regulatory consequences at both gene promoters and enhancers, while H3K4me3 is deposited independently of DNA methylation. These data suggest that DNA methylation maintains and re-establishes silent or primed states at enhancers in a tissue-specific pattern and regulates H3K27ac at active enhancers through modulation of transcription factor binding [37]. Similar to DNA methylation, demethylation seems to be an early event in enhancers’ activation promoting cell differentiation and is enriched in cell-type-specific enhancers’ fashion [38, 39]. That effect is mediated by the TET2 protein as Tet2-only or Tet-TKO deletion causes abolishment of hydroxymethylation at enhancers accompanied by enhancer hyper-methylation, decreased enhancer activity, and delayed differentiation-involved gene expression detected at binding sites of core TFs such as Oct4, Nanog, and Sox2 [40, 41]. Active enhancers during development are hypo-methylated to be protected from silencing propagating this way epigenetic memory in adult tissues, inactive enhancers are hyper-methylated, and poised enhancers are more likely to be hyper-methylated [40, 42, 43]. However, recent data during early zebrafish development showed that primed enhancers are hypo-methylated and enriched close to TFs that act later in development, whereas active enhancers are hyper-methylated [44]. An explanation for these contradictory results between zebrafish and the mammalian system has not been reported and further studies should confirm these results, and if these findings are verified enhancer methylation pattern should be considered in a species-specific context (Fig. 2).
Fig. 2.
a Unmethylated CpGs (empty lollipops) activate enhancers which interact with cognate promoters resulting in gene transcription activation. b Methylated CpGs (filled lollipops) lead to gene transcription repression, differentiation block, increased proliferation, and malignant transformation
Several studies have established the role of aberrant enhancer methylation in oncogenesis [45, 46]. Aberrant enhancer CpG methylation is common in many cancer types, is more closely related to target genes expression changes than promoter methylation, and might occur even when the promoter is constantly unmethylated [47]. The interplay between DNA methylation and histone marks characterizing enhancers is able to affect chromatin structure of enhancers and enhancers’ priming, thus activating cancer key drivers including MES1, ESR1, MAP3K1, and Cyclin D1 within cancer risk loci in different cancer subtypes [48–50]. A disequilibrium in such crosstalk might have a role in the very early steps of tumorigenesis by disrupting the normal differentiation process at least in human epidermal stem cells (EpSCs). In vivo experiments show that loss of Dnmt3a renders the epidermis prone to damage situations by altering enhancers expression regulating genes involved in differentiation [51, 52]. It would be interest to verify if this is also true in pre-leukemic cells which harbor DNMT3A mutations, and would represent an important pathogenetic pathway [53]. Another mechanistic link between enhancers and DNA methylation in cancer occurrence is highlighted by the genome-wide reconfiguration of nucleosome-depleted regions (NDR) by TFs which is accompanied by enhancer hyper-methylation. The loss of NDR alone is sufficient to predict DNA methylation events in cancer cells, which in normal cells is cell-type-specific and preferentially found at enhancers [54]. Similar to classic enhancers, SEs also undergo CpGs methylation in several different cancer types mostly hypo-methylation and less hyper-methylation affecting the expression of the target genes [55].
Enhancers’ involvement in hematopoiesis
The generation of progenitor and terminally differentiated blood cells from HSCs requires the interplay between combinations of TFs, chromatin modifying enzymes and enhancers, and notably, the dynamic changes of enhancers could be more important than promoters in dictating cell fate [56]. This is highlighted by their differential use during each step of hematopoietic differentiation compared to promoters which are shared between HSCS, erythroid progenitors/precursors (EPP), and myeloid progenitors/precursors (MPP) [57]. Analysis of the interactome between promoters and enhancers in 17 human primary blood types showed that the enhancer–promoter interactions occur in a cell-type-specific pattern, while enhancer establishment is initiated early during lineage commitment in a lineage-specific manner, and can determine differentiation potential of progeny prior and more accurately of the RNA expression profile. A large fraction of these enhancers are maintained in the lineage in which they are initially marked in HSCs, while a smaller fraction of the enhancers are de novo lineage-specific enhancers established in the first progenitor of the myeloid lineage [58, 59]. However, in contrast to the previous data, Luyten et al. reported that myeloid enhancers are de novo established at each step of differentiation with only a limited fraction of enhancers being primed at earlier stages [60]. This discrepancy could be attributed to the different methodology used. Moreover, in B-cell lineage, early priming is a minor contributor for the generation of a dynamic enhancer landscape during differentiation. It rather seems that changes involve mainly establishment of novel enhancers where they are required to control target gene expression, and less closing and re-opening of pre-existing ones. These data indicate that most of the active enhancers in differentiated B-cell are not primed in earlier stages [61]. Enhancers are functionally active during the first steps of murine development in the endothelium of dorsal aorta and HSCs. For example, an enhancer located in the transcriptional regulator Hhex locus able to bind to the Gata2, Pu.1, among other lineage determining TFs (LDTFs) is involved in HSCs development and differentiation throughout all developmental stages of hematopoiesis from hematoendothelial precursors to their adult counterparts [62]. These LDTFs, functionally conserved within enhancers during lineage specification, cooperate with distinct stage-specific cofactors to modulate enhancers’ transition from an inactive to a primed or poised state during fetal and adult erythropoiesis [13]. Furthermore, Gata2-to-Gata1 switch drives erythroid enhancers’ commissioning and functions as a molecular driver of enhancer turnover acting as a maintenance mechanism of genes expression involved in differentiation in contrast to Gata2-only or Gata1-only enhancers. These findings suggest that distinct enhancers cooperate to optimally affect gene expression of target genes [63]. Similar to Gata2-to-Gata1 switch, PRC2 subunits EZH1 and EZH2 undergo expression switch during erythropoiesis. An erythroid-selective + 46 kb enhancer indispensable for the transcription of EZH1 controls the EZH1-to-EZH2 switch during erythropoiesis in a developmental stage-specific manner. That particular enhancer is located in EZH1 and occupied by the principal erythroid transcriptional regulators GATA1 and TAL1 mediates erythroid-selective transcription of EZH1. These results indicate that the GATA2-associated regulatory element negatively regulates EZH1 expression in stem/progenitor cells, whereas the switch to GATA1 expression and the activation of GATA1-associated EZH1 enhancer drive the transcriptional activation of EZH1 during erythropoiesis [64]. The cooperation between the GATA1 and TAL1 LDTFs with active enhancers is more a fine-tuning mechanism of target gene expression rather than a control mechanism of genes expression in an “on–off” pattern during erythropoiesis. This can be deduced by the presence of specific enhancers overlapping the same chromatin marks in both adult and fetal cells, fetal, or adult erythroid cells identified as “common”, “fetal-only”, and “adult-only” enhancers, respectively. Adult-only enhancers are mainly associated with genes upregulated in adult erythropoiesis, whereas fetal-only enhancers are highly associated with genes upregulated in fetal erythropoiesis. Finally, common enhancers are associated with both gene groups and might present little predictive value for gene expression changes [65]. Enhancers also control the macrophage expression program affecting innate immunity. Macrophages’ self-renewal does not involve the acquisition of dedicated lineage-independent self-renewal enhancers but rather activation of a subset of poised macrophage-specific enhancers [66]. That effect is mediated by TLR4, which affects the production of required cytokines, and activates signal-dependent TFs including NF-κB, IRFs, PU.1, C/EBPa, and STAT factors. These TFs directly select new enhancers during the monocyte-to-macrophage transition, while C/EBPa in particular promotes a myeloid expression program in pre-B cells by binding at de novo and pre-existing enhancers, as well, already occupied by PU.1. Probably pre-existing enhancers act as bona fide B-cell enhancers, and under the action of C/EBPa, they are converted into enhancers active in myeloid cells resulting in macrophage differentiation [67–70]. The fact that macrophages isolated from different tissues exhibit several macrophage-specific enhancers, whereas other enhancers are shared by the neutrophils, monocytes, and macrophages, suggests that tissue residing macrophages present distinct enhancer landscape reflecting their developmental origin and the residing microenvironment [71]. How signals act on macrophages genome to promote specialized tissue-specific phenotypes is not clear, but it seems that LDTFs select enhancers and enable binding of signal-dependent TFs [72, 73].
Disruption of enhancers’ interaction with pivotal TFs is observed in malignant hematopoiesis [74–77]. For example, compound heterozygous mutations of the GATA2-regulated + 9.5 kb enhancer which normally increases GATA2 expression in the hemogenic endothelium and HSPCs, and the − 77 kb enhancer essential for the myeloid-erythroid differentiation shows that both enhancers must reside on the same allele to induce megakaryocyte–erythrocyte progenitors (MEPs). Loss of the − 77 kb enhancer induces defective signaling and transcriptional circuitry required for erythroid maturation, and such deregulation finally leads to the onset of AML and MDS [78–80]. Moreover, the same enhancer may localize down- or upstream of EVI1 gene in inv(3)(q21;q26) or t(3;3)(q21;q26), respectively, and might misdirect EVI1 transcription in the translocated or inverted alleles which finally inhibits the activity of bound TFs, thus, blocking myeloid differentiation leading to AML and MDS [81, 82]. The murine C/ebpa gene contains a + 37 kb enhancer (homologous to the human + 42 kb) which is indispensable for neutrophilic maturation, is bound and activated by several TFs including Runx1, Gata2, C/ebpa, Pu.1 and Tal1, and the RUNX1-ETO and CBFB-MYH11 oncoproteins during normal and malignant progression of MPPs to CMPs and granulocyte macrophage progenitors (GMPs) [83–85]. Given that the + 37 kb enhancer acts autonomously to regulate C/ebpa expression in early HSPCs and later in CMPs and GMPs, it is possible that changes in enhancer function, possibly in combination with other enhancers, might have potential leukemogenic implications in humans [86]. The fact that deletion of this particular enhancer might promote pre-leukemic progenitor expansion and the fact that leukemic stem cells reside in GMPs the role of enhancers in the context of leukemic stem cells should be further evaluated [83, 84, 87].
Enhancer DNA methylation in normal and malignant hematopoiesis
Although there is an enormous body of data on methylation during normal and malignant hematopoiesis this mainly focus on methylation profile of genes promoters. Nevertheless, it now becomes evident that enhancer methylation has an important role in both normal and malignant hematopoiesis, as well.
DNA methylation at erythroid enhancers has the potential to underscore the transcriptional and developmental differences between fetal and adult erythropoiesis [88]. Dnmt1 is strongly recruited to the Gata1 methylation determining region (G1MDR) to maintain DNA methylation of the Gata1 locus in HSPCs, while deletion of G1MDR selectively promotes Gata1 repression associated with an increase of GATA2 occupancy in the GATA1 gene enhancer. That interplay between DNMT1–G1DMR–GATA1 enhancer has an essential role in HSPC maintenance, while demethylation promotes activation critical for subsequent GATA2-dependent Gata1 transcription initiation for erythroid commitment and differentiation [89, 90]. An increased variability of dynamic DNA methylation at enhancers especially near leukocyte differentiation-promoting genes transitioning between silenced and expressed state in neonatal cord blood HSPCs suggests that methylation is a regulatory mechanism in granulopoiesis in a fashion similar to erythropoiesis. That is achieved through repression of neutrophil-specific enhancers in HPCs and regulation of differentiation and lineage-specific enhancer activity during granulocytic maturation [91, 92]. DNA methylation at enhancers also has the ability to discriminate between myeloid and lymphoid progenitors. DNA methylation levels in 17 hematopoietic cell types are similar in HSCs and HPCs, while lower levels are observed in myeloid differentiated cells. Furthermore, enhancer DNA methylation levels are lower in myeloid progenitors than in lymphoid progenitors, and the same is also observed differentiated cells of the two lineages [93]. DNA methylation changes during B-cell differentiation control enhancers’ accessibility through transcriptional changes that correlate with cellular division and DNA demethylation in a specific methylation pattern at each stage [94, 95].
Enhancers’ aberrant DNA methylation, in concordance with TFs binding sites and expression, and gene expression in cancer has come in the spotlight [96–99]. Enhancers’ hyper-methylation is acquired during the malignant transformation process, representing a de novo reprogramming event in hematological malignancies with a methylation profile similar to stem cells as enhancers normally become demethylated during the transition of stem cells to terminally differentiated cells [100]. Enhancers present in embryonic stem cells (ESCs) and absent in HSCs are prone to methylation in cancer associated with changes in nearby genes expression suggest a potential mechanism of ESCs genes reactivation in cancer. Given that ESCs enhancers are present at the origin of all cell types, it could be supposed that they affect gene expression in various cancer subtypes.
Enhancer DNA methylation in AML and MDS
Recent data pave the path to our better understanding of enhancer DNA methylation in hematopoietic malignancies and especially myeloid neoplasms. Enhancers raising early during hematopoietic lineage commitment are more vulnerable to DNA methylation in hematological malignancies than enhancers appearing in more differentiated progeny and promoters, and this contribution could be probably an explanation for the origin of leukemic stem cells in AML and MDS in the GMPs fraction. Notably, HSCs enhancers are not vulnerable to hypo-methylation in hematopoietic cancers probably due to lineage-specific chromatin structures and activity state as a defense mechanism against the epigenome of cancer cells [101]. Oncogenic drivers may disrupt methylation at regions enriched in enhancers and TF-binding sites affecting their accessibility promoting both enhancer hypo- and hyper-methylation. In HSPCs, both active and poised enhancer-associated CpGs are located within introns and gene neighborhoods and exhibit aberrant methylation which is not equal in all AML clusters (characterized by cytogenetic, epigenetic, and molecular information). DNMT3A and IDH normally target distal and intronic regions, and promoters, respectively, and hyper- or hypo-methylate these distinct gene regions. In AML, however, co-occurring DNMT3A and IDH mutations exhibit a totally distinctive enhancer methylation pattern compared to the respective hypo-methylated or hyper-methylated profile. These double mutant cases exhibit very little enhancer methylation compared to IDH-only enhancer hyper-methylation and DNMT3A-only enhancer hypo-methylation, but there is a novel enrichment for TF-binding motifs not present in IDH or DNMT3A cases. That epigenetic antagonism in IDH/DNMT3A double mutants at enhancers might occur early in the pre-leukemic phase probably reflecting the origin of the transformation process, but also contribute in any way to the maintenance of the malignant phenotype [102]. These findings could explain the observation that DNMT3A-induced hyper-methylation is not a pathogenetic event in AML but rather DNMT3A-dependent methylation must co-localize with other epigenetic modifications, or there should be an epigenetic antagonism between hypo- and hyper--methylation in distinct gene regions [103].
Mouse models with Dnmt3a homozygous and heterozygous mutations co-expressing Flt3-ITD give rise lymphoid and myeloid leukemia, respectively, whereas double Dnmt3a/Flt3 mutants exhibit enhancer hypo-methylation as a primary transforming event at the HSCs level. That methylation disruption may deregulate transcription programs contributing to leukemogenesis, while the secondary event, Flt3-ITD in such case, determines disease specification and latency. Hence, Dnmt3a mutations in stem cells may prime a pre-leukemic clone to promote occurrence of secondary hits [104]. Hypo-methylation concurrently leads to the recruitment of active histone marks including H3K27ac- and DOT1L-dependent H3K79 at enhancers promoting binding of p300 at stemness genes including the Meis1–Mn1–Hoxa node in leukemic stem cells bearing the Dnmt3aR882H and N-Ras mutations [105].
In human normal karyotype, AML (CN-AML) DNA demethylation may activate a subset of new and poised enhancers, while hyper-methylation silences enhancers mainly in GMPs and promyelocytes/myelocytes (PMCs). Differentially methylated CpGs (DMCs) are more hypo-methylated in more mature PMCs/neutrophils than in CMPs/GMPs and methylation changes seem to be similar between CN-AML and CMPs/GMPs. However, the larger fraction of DMCs represent leukemia-specific changes in CN-AML, as they do not occur during normal differentiation, while a subset of sites that change during normal myelopoiesis does not change in CN-AML. Furthermore, disrupted DNA methylation in the CN-AML is not simply related to the normal differentiation process but rather is part of complex networks with distinct regions that change during differentiation, others that retain the DNA methylation status of the parental epigenome, and others that act as cancer-specific epimutations. Hypo-methylated distal DMCs occur in regions that are inactive and poised in CD34+ cells, and activated in CN-AML, or are de novo CN-AML enhancers that have been established specifically in the CN-AML epigenome. Interestingly, methylation changes occurred in an IDH, DNMT3a, FLT3, NPM1 mutation-independent fashion, suggesting that the enhancers’ cytosine methylation landscape could be defined by other factors besides mutations [106]. An assumption could be a deregulation of factors recruiting DNA methyltransferases to their DNA substrate indispensable for their enzymatic activity such as UHRF1 and DNMT1, but this is to be verified [9]. However, not all hypo-methylated enhancers become active in CN-AML, as indicated by the absence of active histone marks and lack of chromatin accessibility, suggesting that DNA hypo-methylation is not per se sufficient to induce activation of a locus [106]. The above-described data strongly support the notion that deregulation of DNA methylation at enhancers potentially contributes to AML pathogenesis.
Data on enhancer methylation in myeloid compartment are obtained by studying TET proteins role and function. TET2-mutant AML and CMML exhibit hyper-methylation of hematopoietic-specific enhancers. However, similar to DNMT3A, TET2 mutations do not explain genome-wide differences in DNA methylation in CMML, and inconsistent differences at CpG islands (CGIs) between TET2-wild-type (TET2-WT) and TET2-mutant (TET2-MT) cases can be observed. In contrast, a large proportion of TET2-specific DMCs in non-CGIs are hyper-methylated in TET2-mutant cases, suggesting different biological effects of TET2 mutations at CGIs and non-CGIs which contain hematopoietic-specific enhancers and transcription factor-binding motifs. Thus, hyper-methylation at TET2-DMCs in non-CGIs disrupts gene expression through modulation of hematopoietic-specific TF-binding sites and enhancers, and probably has the potential to promote differentiation block observed in TET2-MT hematopoietic cells [107]. Furthermore, in mouse models that genetically resemble the human AML-ETO-induced AML, Tet2 mutations in pre-leukemic cells might induce widespread and progressive hyper-methylation in several enhancers, thus inactivating TSGs expression, and might promote stem cell proliferation. Increased methylation seems not to be unique to the AML-ETO fusion but also in other rearranged AML such as the MLL-AF9 fusion. However, Tet2-dependent enhancer hyper-methylation does not represent a feature exclusive of malignancy but also of normal hematopoiesis as it can be observed in in vivo isolated GMP cells. Thus, considering that enhancers’ hyper-methylation is observed in both malignant (AML-ETO and MLL-AF9 rearranged AML) and non-malignant cell types, it is likely that TET2 has a more general role in regulating DNA methylation of enhancers irrespective of the cell type. As enhancer hyper-methylation correlates negatively with H3K27ac levels, it seems that exerts its effect on enhancers’ activity probably by altering the chromatin structure and finally causing the downregulation of gene expression [108]. Similarly, epigenomic profiling of HPCs in Tet2-MT MDS mice following the loss of Ezh2 (Tet2KD/KDEzh2Δ/Δ) shows that the methylation difference is significantly higher in Tet2KD/KDEzh2Δ/Δ MDS cells compared with each single mutant, suggesting a synergistic action of combined loss of Tet2 and Ezh2 in altering the involved DMRs promoting DNA hyper-methylation and reprogramming the MDS epigenome. These data propose that enhancer DNA hyper-methylation propagation is a sequential process from a Tet2-insufficient state followed by the loss of Ezh2, and progresses to advanced MDS after serial transplantation [109].
Concluding the role of aberrant CpG methylation at enhancers has only recently emerged. Enhancer methylation seems to be more complex involving several TFs in different circuitries, affects differentially each cell type during lineage commitment, and co-operates with genetic and epigenetic marks to promote leukemogenesis. We have still a lot to learn about the interplay between enhancers and methylation regarding their role and their potential therapeutic targeting in myeloid malignancies.
Acknowledgements
We apologize to the authors whose papers have not been cited.
Author contributions
LB and GV collected data and wrote the paper.
Compliance with ethical standards
Conflict of interest
Authors declare no potential conflict of interest.
References
- 1.Bröske AM, Vockentanz L, Kharazi S, Huska MR, Mancini E, Scheller M, Kuhl C, Enns A, Prinz M, Jaenisch R, Nerlov C, Leutz A, Andrade-Navarro MA, Jacobsen SE, Rosenbauer F. DNA methylation protects hematopoietic stem cell multipotency from myeloerythroid restriction. Nat Genet. 2009;41:1207–1215. doi: 10.1038/ng.463. [DOI] [PubMed] [Google Scholar]
- 2.Gu J, Stevens M, Xing X, Li D, Zhang B, Payton JE, Oltz EM, Jarvis JN, Jiang K, Cicero T, Costello JF, Wang T. Mapping of variable DNA methylation across multiple cell types defines a dynamic regulatory landscape of the human genome. G3 (Bethesda) 2016;6:973–986. doi: 10.1534/g3.115.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodges E, Molaro A, Dos Santos CO, Thekkat P, Song Q, Uren PJ, Park J, Butler J, Rafii S, McCombie WR, Smith AD, Hannon GJ. Directional DNA methylation changes and complex intermediate states accompany lineage specificity in the adult hematopoietic compartment. Mol Cell. 2011;44:17–28. doi: 10.1016/j.molcel.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji H, Ehrlich LI, Seita J, Murakami P, Doi A, Lindau P, Lee H, Aryee MJ, Irizarry RA, Kim K, Rossi DJ, Inlay MA, Serwold T, Karsunky H, Ho L, Daley GQ, Weissman IL, Feinberg AP. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467:338–342. doi: 10.1038/nature09367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bocker MT, Hellwig I, Breiling A, Eckstein V, Ho AD, Lyko F. Genome-wide promoter DNA methylation dynamics of human hematopoietic progenitor cells during differentiation and aging. Blood. 2011;117:e182–e189. doi: 10.1182/blood-2011-01-331926. [DOI] [PubMed] [Google Scholar]
- 6.Baylin SB, Jones PA. Epigenetic determinants of cancer. Cold Spring Harb Perspect Biol. 2016;8:a019505. doi: 10.1101/cshperspect.a019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Rau R, Goodell MA. DNMT3A in haematological malignancies. Nat Rev Cancer. 2015;15:152–165. doi: 10.1038/nrc3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole CB, Russler-Germain DA, Ketkar S, Verdoni AM, Smith AM, Bangert CV, Helton NM, Guo M, Klco JM, O’Laughlin S, Fronick C, Fulton R, Chang GS, Petti AA, Miller CA, Ley TJ. Haploinsufficiency for DNA methyltransferase 3A predisposes hematopoietic cells to myeloid malignancies. J Clin Investig. 2017;127:3657–3674. doi: 10.1172/JCI93041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benetatos L, Vartholomatos G. On the potential role of DNMT1 in acute myeloid leukemia and myelodysplastic syndromes: not another mutated epigenetic driver. Ann Hematol. 2016;95:1571–1582. doi: 10.1007/s00277-016-2636-8. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa ME, Skrabanek L, Li Y, Jiemjit A, Fandy TE, Paietta E, Fernandez H, Tallman MS, Greally JM, Carraway H, Licht JD, Gore SD, Melnick A. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood. 2009;114:3448–3458. doi: 10.1182/blood-2009-01-200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Y, Dunbar A, Gondek LP, Mohan S, Rataul M, O’Keefe C, Sekeres M, Saunthararajah Y, Maciejewski JP. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood. 2009;113:1315–1325. doi: 10.1182/blood-2008-06-163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengupta S, George RE. Super-Enhancer-Driven Transcriptional Dependencies in Cancer. Trends Cancer. 2017;3:269–281. doi: 10.1016/j.trecan.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinz S, Romanoski CE, Benner C, Glass CK. The selection and function of cell type-specific enhancers. Nat Rev Mol Cell Biol. 2015;16:144–154. doi: 10.1038/nrm3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, Taatjes DJ, Dekker J, Young RA. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spitz F, Furlong EE. Transcription factors: from enhancer binding to developmental control. Nat Rev Genet. 2012;13:613–626. doi: 10.1038/nrg3207. [DOI] [PubMed] [Google Scholar]
- 16.Calo E, Wysocka J. Modification of enhancer chromatin: what, how, and why? Mol Cell. 2013;49:825–837. doi: 10.1016/j.molcel.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, Boyer LA, Young RA, Jaenisch R. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci USA. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz-Molina S, Respuela P, Tebartz C, Kolovos P, Nikolic M, Fueyo R, van Ijcken WFJ, Grosveld F, Frommolt P, Bazzi H, Rada-Iglesias A. PRC2 facilitates the regulatory topology required for poised enhancer function during pluripotent stem cell differentiation. Cell Stem Cell. 2017;20(689–705):e9. doi: 10.1016/j.stem.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-André V, Sigova AA, Hoke HA, Young RA. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niederriter AR, Varshney A, Parker SC, Martin DM. Super enhancers in cancers, complex disease, and developmental disorders. Genes (Basel) 2015;6:1183–1200. doi: 10.3390/genes6041183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowalczyk MS, Hughes JR, Garrick D, Lynch MD, Sharpe JA, Sloane-Stanley JA, McGowan SJ, De Gobbi M, Hosseini M, Vernimmen D, Brown JM, Gray NE, Collavin L, Gibbons RJ, Flint J, Taylor S, Buckle VJ, Milne TA, Wood WG, Higgs DR. Intragenic enhancers act as alternative promoters. Mol Cell. 2012;45:447–458. doi: 10.1016/j.molcel.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Dao LTM, Galindo-Albarrán AO, Castro-Mondragon JA, Andrieu-Soler C, Medina-Rivera A, Souaid C, Charbonnier G, Griffon A, Vanhille L, Stephen T, Alomairi J, Martin D, Torres M, Fernandez N, Soler E, van Helden J, Puthier D, Spicuglia S. Genome-wide characterization of mammalian promoters with distal enhancer functions. Nat Genet. 2017;49:1073–1081. doi: 10.1038/ng.3884. [DOI] [PubMed] [Google Scholar]
- 25.Cao Q, Anyansi C, Hu X, Xu L, Xiong L, Tang W, Mok MTS, Cheng C, Fan X, Gerstein M, Cheng ASL, Yip KY. Reconstruction of enhancer-target networks in 935 samples of human primary cells, tissues and cell lines. Nat Genet. 2017;49:1428–1436. doi: 10.1038/ng.3950. [DOI] [PubMed] [Google Scholar]
- 26.Fulco CP, Munschauer M, Anyoha R, Munson G, Grossman SR, Perez EM, Kane M, Cleary B, Lander ES, Engreitz JM. Systematic mapping of functional enhancer-promoter connections with CRISPR interference. Science. 2016;354:769–773. doi: 10.1126/science.aag2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukaya T, Lim B, Levine M. Enhancer control of transcriptional bursting. Cell. 2016;166:358–368. doi: 10.1016/j.cell.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin AJ, Barajas BC, Furlan-Magaril M, Lopez-Pajares V, Mumbach MR, Howard I, Kim DS, Boxer LD, Cairns J, Spivakov M, Wingett SW, Shi M, Zhao Z, Greenleaf WJ, Kundaje A, Snyder M, Chang HY, Fraser P, Khavari PA. Lineage-specific dynamic and pre-established enhancer-promoter contacts cooperate in terminal differentiation. Nat Genet. 2017;49:1522–1528. doi: 10.1038/ng.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matharu N, Ahituv N. Minor loops in major folds: enhancer-promoter looping, chromatin restructuring, and their association with transcriptional regulation and disease. PLoS Genet. 2015;11:e1005640. doi: 10.1371/journal.pgen.1005640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zabidi MA, Arnold CD, Schernhuber K, Pagani M, Rath M, Frank O, Stark A. Enhancer-core-promoter specificity separates developmental and housekeeping gene regulation. Nature. 2015;518(7540):556–559. doi: 10.1038/nature13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teppo S, Laukkanen S, Liuksiala T, Nordlund J, Oittinen M, Teittinen K, Grönroos T, St-Onge P, Sinnett D, Syvänen AC, Nykter M, Viiri K, Heinäniemi M, Lohi O. Genome-wide repression of eRNA and target gene loci by the ETV6-RUNX1 fusion in acute leukemia. Genome Res. 2016;26:1468–1477. doi: 10.1101/gr.193649.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ooi WF, Xing M, Xu C, Yao X, Ramlee MK, Lim MC, Cao F, Lim K, Babu D, Poon LF, Lin Suling J, Qamra A, Irwanto A, Qu Zhengzhong J, Nandi T, Lee-Lim AP, Chan YS, Tay ST, Lee MH, Davies JO, Wong WK, Soo KC, Chan WH, Ong HS, Chow P, Wong CY, Rha SY, Liu J, Hillmer AM, Hughes JR, Rozen S, Teh BT, Fullwood MJ, Li S, Tan P. Epigenomic profiling of primary gastric adenocarcinoma reveals super-enhancer heterogeneity. Nat Commun. 2016;7:12983. doi: 10.1038/ncomms12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawkins RD, Hon GC, Lee LK, Ngo Q, Lister R, Pelizzola M, Edsall LE, Kuan S, Luu Y, Klugman S, Antosiewicz-Bourget J, Ye Z, Espinoza C, Agarwahl S, Shen L, Ruotti V, Wang W, Stewart R, Thomson JA, Ecker JR, Ren B. Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell. 2010;6:479–491. doi: 10.1016/j.stem.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiench M, John S, Baek S, Johnson TA, Sung MH, Escobar T, Simmons CA, Pearce KH, Biddie SC, Sabo PJ, Thurman RE, Stamatoyannopoulos JA, Hager GL. DNA methylation status predicts cell type-specific enhancer activity. EMBO J. 2011;30:3028–3039. doi: 10.1038/emboj.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petell CJ, Alabdi L, He M, San Miguel P, Rose R, Gowher H. An epigenetic switch regulates de novo DNA methylation at a subset of pluripotency gene enhancers during embryonic stem cell differentiation. Nucleic Acids Res. 2016;44:7605–7617. doi: 10.1093/nar/gkw426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blattler A, Pj Farnham. Cross-talk between site-specific transcription factors and DNA methylation states. J Biol Chem. 2013;288:34287–34294. doi: 10.1074/jbc.R113.512517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King AD, Huang K, Rubbi L, Liu S, Wang CY, Wang Y, Pellegrini M, Fan G. Reversible regulation of promoter and enhancer histone landscape by DNA methylation in mouse embryonic stem cells. Cell Rep. 2016;17:289–302. doi: 10.1016/j.celrep.2016.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsagaratou A, Äijö T, Lio CW, Yue X, Huang Y, Jacobsen SE, Lähdesmäki H, Rao A. Dissecting the dynamic changes of 5-hydroxymethylcytosine in T-cell development and differentiation. Proc Natl Acad Sci USA. 2014;111:E3306–E3315. doi: 10.1073/pnas.1412327111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sérandour AA, Avner S, Oger F, Bizot M, Percevault F, Lucchetti-Miganeh C, Palierne G, Gheeraert C, Barloy-Hubler F, Péron CL, Madigou T, Durand E, Froguel P, Staels B, Lefebvre P, Métivier R, Eeckhoute J, Salbert G. Dynamic hydroxymethylation of deoxyribonucleic acid marks differentiation-associated enhancers. Nucleic Acids Res. 2012;40:8255–8265. doi: 10.1093/nar/gks595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hon GC, Song CX, Du T, Jin F, Selvaraj S, Lee AY, Yen CA, Ye Z, Mao SQ, Wang BA, Kuan S, Edsall LE, Zhao BS, Xu GL, He C, Ren B. mC oxidation by Tet2 modulates enhancer activity and timing of transcriptome reprogramming during differentiation. Mol Cell. 2014;56:286–297. doi: 10.1016/j.molcel.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu F, Liu Y, Jiang L, Yamaguchi S, Zhang Y. Role of Tet proteins in enhancer activity and telomere elongation. Genes Dev. 2014;28:2103–2119. doi: 10.1101/gad.248005.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hon GC, Rajagopal N, Shen Y, McCleary DF, Yue F, Dang MD, Ren B. Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues. Nat Genet. 2013;45:1198–1206. doi: 10.1038/ng.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Pope SD, Jazirehi AR, Attema JL, Papathanasiou P, Watts JA, Zaret KS, Weissman IL, Smale ST. Pioneer factor interactions and unmethylated CpG dinucleotides mark silent tissue-specific enhancers in embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:12377–12382. doi: 10.1073/pnas.0704579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaaij LJ, Mokry M, Zhou M, Musheev M, Geeven G, Melquiond AS, de Jesus Domingues AM, de Laat W, Niehrs C, Smith AD, Ketting RF. Enhancers reside in a unique epigenetic environment during early zebrafish development. Genome Biol. 2016;17:146. doi: 10.1186/s13059-016-1013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou S, Treloar AE, Lupien M. Emergence of the noncoding cancer genome: a target of genetic and epigenetic alterations. Cancer Discov. 2016;6:1215–1229. doi: 10.1158/2159-8290.CD-16-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell RE, Golan T, Sheinboim D, Malcov H, Amar D, Salamon A, Liron T, Gelfman S, Gabet Y, Shamir R, Levy C. Enhancer methylation dynamics contribute to cancer plasticity and patient mortality. Genome Res. 2016;26:601–611. doi: 10.1101/gr.197194.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aran D, Sabato S, Hellman A. DNA methylation of distal regulatory sites characterizes dysregulation of cancer genes. Genome Biol. 2013;14:R21. doi: 10.1186/gb-2013-14-3-r21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahé EA, Madigou T, Sérandour AA, Bizot M, Avner S, Chalmel F, Palierne G, Métivier R, Salbert G. Cytosine modifications modulate the chromatin architecture of transcriptional enhancers. Genome Res. 2017;27:947–958. doi: 10.1101/gr.211466.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aran D, Hellman A. DNA methylation of transcriptional enhancers and cancer predisposition. Cell. 2013;154:11–13. doi: 10.1016/j.cell.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 50.Jeong KW, Andreu-Vieyra C, You JS, Jones PA, Stallcup MR. Establishment of active chromatin structure at enhancer elements by mixed-lineage leukemia 1 to initiate estrogen-dependent gene expression. Nucleic Acids Res. 2014;42:2245–2256. doi: 10.1093/nar/gkt1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinaldi L, Datta D, Serrat J, Morey L, Solanas G, Avgustinova A, Blanco E, Pons JI, Matallanas D, Von Kriegsheim A, Di Croce L, Benitah SA. Dnmt3a and Dnmt3b associate with enhancers to regulate human epidermal stem cell homeostasis. Cell Stem Cell. 2016;19:491–501. doi: 10.1016/j.stem.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 52.Rinaldi L, Avgustinova A, Martín M, Datta D, Solanas G, Prats N, Benitah SA. Loss of Dnmt3a and Dnmt3b does not affect epidermal homeostasis but promotes squamous transformation through PPAR-γ. Elife. 2017;6:e21697. doi: 10.7554/eLife.21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW, McLeod JL, Doedens M, Medeiros JJ, Marke R, Kim HJ, Lee K, McPherson JD, Hudson TJ, Brown AM, HALT Pan-Leukemia Gene Panel Consortium. Yousif F, Trinh QM, Stein LD, Minden MD, Wang JC, Dick JE. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taberlay PC, Statham AL, Kelly TK, Clark SJ, Jones PA. Reconfiguration of nucleosome-depleted regions at distal regulatory elements accompanies DNA methylation of enhancers and insulators in cancer. Genome Res. 2014;24:1421–1432. doi: 10.1101/gr.163485.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heyn H, Vidal E, Ferreira HJ, Vizoso M, Sayols S, Gomez A, Moran S, Boque-Sastre R, Guil S, Martinez-Cardus A, Lin CY, Royo R, Sanchez-Mut JV, Martinez R, Gut M, Torrents D, Orozco M, Gut I, Young RA, Esteller M. Epigenomic analysis detects aberrant super-enhancer DNA methylation in human cancer. Genome Biol. 2016;17:11. doi: 10.1186/s13059-016-0879-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cico A, Andrieu-Soler C, Soler E. Enhancers and their dynamics during hematopoietic differentiation and emerging strategies for therapeutic action. FEBS Lett. 2016;590:4084–4104. doi: 10.1002/1873-3468.12424. [DOI] [PubMed] [Google Scholar]
- 57.Romano O, Peano C, Tagliazucchi GM, Petiti L, Poletti V, Cocchiarella F, Rizzi E, Severgnini M, Cavazza A, Rossi C, Pagliaro P, Ambrosi A, Ferrari G, Bicciato S, De Bellis G, Mavilio F, Miccio A. Transcriptional, epigenetic and retroviral signatures identify regulatory regions involved in hematopoietic lineage commitment. Sci Rep. 2016;6:24724. doi: 10.1038/srep24724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Javierre BM, Burren OS, Wilder SP, Kreuzhuber R, Hill SM, Sewitz S, Cairns J, Wingett SW, Várnai C, Thiecke MJ, Burden F, Farrow S, Cutler AJ, Rehnström K, Downes K, Grassi L, Kostadima M, Freire-Pritchett P, Wang F, BLUEPRINT Consortium. Stunnenberg HG, Todd JA, Zerbino DR, Stegle O, Ouwehand WH, Frontini M, Wallace C, Spivakov M, Fraser P. Lineage-specific genome architecture links enhancers and non-coding disease variants to target gene promoters. Cell. 2016;167:1369–1384.e19. doi: 10.1016/j.cell.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lara-Astiaso D, Weiner A, Lorenzo-Vivas E, Zaretsky I, Jaitin DA, David E, Keren-Shaul H, Mildner A, Winter D, Jung S, Friedman N, Amit I. Immunogenetics. Chromatin state dynamics during blood formation. Science. 2014;345:943–949. doi: 10.1126/science.1256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luyten A, Zang C, Liu XS, Shivdasani RA. Active enhancers are delineated de novo during hematopoiesis, with limited lineage fidelity among specified primary blood cells. Genes Dev. 2014;28:1827–1839. doi: 10.1101/gad.240101.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choukrallah MA, Song S, Rolink AG, Burger L, Matthias P. Enhancer repertoires are reshaped independently of early priming and heterochromatin dynamics during B cell differentiation. Nat Commun. 2015;6:8324. doi: 10.1038/ncomms9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Migueles RP, Shaw L, Rodrigues NP, May G, Henseleit K, Anderson KG, Goker H, Jones CM, de Bruijn MF, Brickman JM, Enver T. Transcriptional regulation of Hhex in hematopoiesis and hematopoietic stem cell ontogeny. Dev Biol. 2017;424:236–245. doi: 10.1016/j.ydbio.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 63.Huang J, Liu X, Li D, Shao Z, Cao H, Zhang Y, Trompouki E, Bowman TV, Zon LI, Yuan GC, Orkin SH, Xu J. Dynamic control of enhancer repertoires drives lineage and stage-specific transcription during hematopoiesis. Dev Cell. 2016;36:9–23. doi: 10.1016/j.devcel.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu J, Shao Z, Li D, Xie H, Kim W, Huang J, Taylor JE, Pinello L, Glass K, Jaffe JD, Yuan GC, Orkin SH. Developmental control of polycomb subunit composition by GATA factors mediates a switch to non-canonical functions. Mol Cell. 2015;57:304–316. doi: 10.1016/j.molcel.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu J, Shao Z, Glass K, Bauer DE, Pinello L, Van Handel B, Hou S, Stamatoyannopoulos JA, Mikkola HK, Yuan GC, Orkin SH. Combinatorial assembly of developmental stage-specific enhancers controls gene expression programs during human erythropoiesis. Dev Cell. 2012;23:796–811. doi: 10.1016/j.devcel.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soucie EL, Weng Z, Geirsdóttir L, Molawi K, Maurizio J, Fenouil R, Mossadegh-Keller N, Gimenez G, VanHille L, Beniazza M, Favret J, Berruyer C, Perrin P, Hacohen N, Andrau JC, Ferrier P, Dubreuil P, Sidow A, Sieweke MH. Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells. Science. 2016;351:aad5510. doi: 10.1126/science.aad5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Oevelen C, Collombet S, Vicent G, Hoogenkamp M, Lepoivre C, Badeaux A, Bussmann L, Sardina JL, Thieffry D, Beato M, Shi Y, Bonifer C, Graf T. C/EBPα activates pre-existing and de novo macrophage enhancers during induced pre-B cell transdifferentiation and myelopoiesis. Stem Cell Rep. 2015;5:232–247. doi: 10.1016/j.stemcr.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaikkonen MU, Spann NJ, Heinz S, Romanoski CE, Allison KA, Stender JD, Chun HB, Tough DF, Prinjha RK, Benner C, Glass CK. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell. 2013;51:310–325. doi: 10.1016/j.molcel.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pham TH, Benner C, Lichtinger M, Schwarzfischer L, Hu Y, Andreesen R, Chen W, Rehli M. Dynamic epigenetic enhancer signatures reveal key transcription factors associated with monocytic differentiation states. Blood. 2012;119:e161–e171. doi: 10.1182/blood-2012-01-402453. [DOI] [PubMed] [Google Scholar]
- 70.Tagore M, McAndrew MJ, Gjidoda A, Floer M. The lineage-specific transcription factor PU.1 prevents polycomb-mediated heterochromatin formation at macrophage-specific genes. Mol Cell Biol. 2015;35:2610–2625. doi: 10.1128/MCB.00027-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heinz S, Romanoski CE, Benner C, Allison KA, Kaikkonen MU, Orozco LD, Glass CK. Effect of natural genetic variation on enhancer selection and function. Nature. 2013;503:487–492. doi: 10.1038/nature12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, Glass CK. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang Y, Sitwala K, Bronstein J, Sanders D, Dandekar M, Collins C, Robertson G, MacDonald J, Cezard T, Bilenky M, Thiessen N, Zhao Y, Zeng T, Hirst M, Hero A, Jones S, Hess JL. Identification and characterization of Hoxa9 binding sites in hematopoietic cells. Blood. 2012;119:388–398. doi: 10.1182/blood-2011-03-341081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McKeown MR, Corces MR, Eaton ML, Fiore C, Lee E, Lopez JT, Chen MW, Smith D, Chan SM, Koenig JL, Austgen K, Guenther MG, Orlando DA, Lovén J, Fritz CC, Majeti R. Superenhancer analysis defines novel epigenomic subtypes of non-APL AML, including an RARα dependency targetable by SY-1425, a potent and selective RARα agonist. Cancer Discov. 2017;7:1136–1153. doi: 10.1158/2159-8290.CD-17-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang E, Aifantis I. Targeting the noncoding genome: superenhancers meet their kryptonite. Cancer Discov. 2017;7:1065–1066. doi: 10.1158/2159-8290.CD-17-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu J, Li L, Xiong J, denDekker A, Ye A, Karatas H, Liu L, Wang H, Qin ZS, Wang S, Dou Y. MLL1 and MLL1 fusion proteins have distinct functions in regulating leukemic transcription program. Cell Discov. 2016;2:16008. doi: 10.1038/celldisc.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katsumura KR, Ong IM, DeVilbiss AW, Sanalkumar R, Bresnick EH. GATA factor-dependent positive-feedback circuit in acute myeloid leukemia cells. Cell Rep. 2016;16:2428–2441. doi: 10.1016/j.celrep.2016.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hewitt KJ, Katsumura KR, Matson DR, Devadas P, Tanimura N, Hebert AS, Coon JJ, Kim JS, Dewey CN, Keles S, Hao S, Paulson RF, Bresnick EH. GATA factor-regulated Samd14 enhancer confers red blood cell regeneration and survival in severe anemia. Dev Cell. 2017;42(213–225):e4. doi: 10.1016/j.devcel.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mehta C, Johnson KD, Gao X, Ong IM, Katsumura KR, McIver SC, Ranheim EA, Bresnick EH. Integrating enhancer mechanisms to establish a hierarchical blood development program. Cell Rep. 2017;20:2966–2979. doi: 10.1016/j.celrep.2017.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamazaki H, Suzuki M, Otsuki A, Shimizu R, Bresnick EH, Engel JD, Yamamoto M. A remote GATA2 hematopoietic enhancer drives leukemogenesis in inv(3)(q21;q26) by activating EVI1 expression. Cancer Cell. 2014;25:415–427. doi: 10.1016/j.ccr.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gröschel S, Sanders MA, Hoogenboezem R, de Wit E, Bouwman BAM, Erpelinck C, van der Velden VHJ, Havermans M, Avellino R, van Lom K, Rombouts EJ, van Duin M, Döhner K, Beverloo HB, Bradner JE, Döhner H, Löwenberg B, Valk PJM, Bindels EMJ, de Laat W, Delwel R. A single oncogenic enhancer rearrangement causes concomitant EVI1 and GATA2 deregulation in leukemia. Cell. 2014;157:369–381. doi: 10.1016/j.cell.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 83.Guo H, Cooper S, Friedman AD. In vivo deletion of the Cebpa +37 kb enhancer markedly reduces Cebpa mRNA in myeloid progenitors but not in non-hematopoietic tissues to impair granulopoiesis. PLoS One. 2016;11:e0150809. doi: 10.1371/journal.pone.0150809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cooper S, Guo H, Friedman AD. The +37 kb Cebpa enhancer is critical for Cebpa myeloid gene expression and contains functional sites that bind SCL, GATA2, C/EBPα, PU.1, and additional Ets factors. PLoS One. 2015;10:e0126385. doi: 10.1371/journal.pone.0126385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Avellino R, Havermans M, Erpelinck C, Sanders MA, Hoogenboezem R, van de Werken HJ, Rombouts E, van Lom K, van Strien PM, Gebhard C, Rehli M, Pimanda J, Beck D, Erkeland S, Kuiken T, de Looper H, Gröschel S, Touw I, Bindels E, Delwel R. An autonomous CEBPA enhancer specific for myeloid-lineage priming and neutrophilic differentiation. Blood. 2016;127:2991–3003. doi: 10.1182/blood-2016-01-695759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Avellino R, Delwel R. Expression and regulation of C/EBPα in normal myelopoiesis and in malignant transformation. Blood. 2017;129:2083–2091. doi: 10.1182/blood-2016-09-687822. [DOI] [PubMed] [Google Scholar]
- 87.Goardon N, Marchi E, Atzberger A, Quek L, Schuh A, Soneji S, Woll P, Mead A, Alford KA, Rout R, Chaudhury S, Gilkes A, Knapper S, Beldjord K, Begum S, Rose S, Geddes N, Griffiths M, Standen G, Sternberg A, Cavenagh J, Hunter H, Bowen D, Killick S, Robinson L, Price A, Macintyre E, Virgo P, Burnett A, Craddock C, Enver T, Jacobsen SE, Porcher C, Vyas P. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell. 2011;19:138–152. doi: 10.1016/j.ccr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 88.Lessard S, Beaudoin M, Benkirane K, Lettre G. Comparison of DNA methylation profiles in human fetal and adult red blood cell progenitors. Genome Med. 2015;7:1. doi: 10.1186/s13073-014-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takai J, Moriguchi T, Suzuki M, Yu L, Ohneda K, Yamamoto M. The Gata1 5′ region harbors distinct cis-regulatory modules that direct gene activation in erythroid cells and gene inactivation in HSCs. Blood. 2013;122:3450–3460. doi: 10.1182/blood-2013-01-476911. [DOI] [PubMed] [Google Scholar]
- 90.Yu L, Takai J, Otsuki A, Katsuoka F, Suzuki M, Katayama S, Nezu M, Engel JD, Moriguchi T, Yamamoto M. Derepression of the DNA methylation machinery of the Gata1 gene triggers the differentiation cue for erythropoiesis. Mol Cell Biol. 2017;37:e00592-16. doi: 10.1128/MCB.00592-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wijetunga NA, Delahaye F, Zhao YM, Golden A, Mar JC, Einstein FH, Greally JM. The meta-epigenomic structure of purified human stem cell populations is defined at cis-regulatory sequences. Nat Commun. 2014;5:5195. doi: 10.1038/ncomms6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rönnerblad M, Andersson R, Olofsson T, Douagi I, Karimi M, Lehmann S, Hoof I, de Hoon M, Itoh M, Nagao-Sato S, Kawaji H, Lassmann T, Carninci P, Hayashizaki Y, Forrest AR, Sandelin A, Ekwall K, Arner E, Lennartsson A, FANTOM Consortium Analysis of the DNA methylome and transcriptome in granulopoiesis reveals timed changes and dynamic enhancer methylation. Blood. 2014;123:e79–89. doi: 10.1182/blood-2013-02-482893. [DOI] [PubMed] [Google Scholar]
- 93.Farlik M, Halbritter F, Müller F, Choudry FA, Ebert P, Klughammer J, Farrow S, Santoro A, Ciaurro V, Mathur A, Uppal R, Stunnenberg HG, Ouwehand WH, Laurenti E, Lengauer T, Frontini M, Bock C. DNA methylation dynamics of human hematopoietic stem cell differentiation. Cell Stem Cell. 2016;19:808–822. doi: 10.1016/j.stem.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kulis M, Merkel A, Heath S, Queirós AC, Schuyler RP, Castellano G, Beekman R, Raineri E, Esteve A, Clot G, Verdaguer-Dot N, Duran-Ferrer Russiñol N, Vilarrasa-Blasi R, Ecker S, Pancaldi V, Rico D, Agueda L, Blanc J, Richardson D, Clarke L, Datta A, Pascual M, Agirre X, Prosper F, Alignani D, Paiva B, Caron G, Fest T, Muench MO, Fomin ME, Lee ST, Wiemels JL, Valencia A, Gut M, Flicek P, Stunnenberg HG, Siebert R, Küppers R, Gut IG, Campo E, Martín-Subero JI. Whole-genome fingerprint of the DNA methylome during human B cell differentiation. Nat Genet. 2015;47:746–756. doi: 10.1038/ng.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barwick BG, Scharer CD, Bally APR, Boss JM. Plasma cell differentiation is coupled to division-dependent DNA hypomethylation and gene regulation. Nat Immunol. 2016;17:1216–1225. doi: 10.1038/ni.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fleischer T, Tekpli X, Mathelier A, Wang S, Nebdal D, Dhakal HP, Sahlberg KK, Schlichting E, Oslo Breast Cancer Research Consortium (OSBREAC) Børresen-Dale AL, Borgen E, Naume B, Eskeland R, Frigessi A, Tost J, Hurtado A, Kristensen VN. DNA methylation at enhancers identifies distinct breast cancer lineages. Nat Commun. 2017;8:1379. doi: 10.1038/s41467-017-00510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wahlberg P, Lundmark A, Nordlund J, Busche S, Raine A, Tandre K, Rönnblom L, Sinnett D, Forestier E, Pastinen T, Lönnerholm G, Syvänen AC. DNA methylome analysis of acute lymphoblastic leukemia cells reveals stochastic de novo DNA methylation in CpG islands. Epigenomics. 2016;8:1367–1387. doi: 10.2217/epi-2016-0052. [DOI] [PubMed] [Google Scholar]
- 98.Bergmann AK, Castellano G, Alten J, Ammerpohl O, Kolarova J, Nordlund J, Martin-Subero JI, Schrappe M, Siebert R (2017) DNA methylation profiling of pediatric B-cell lymphoblastic leukemia with KMT2A rearrangement identifies hypomethylation at enhancer sites. Pediatr Blood Cancer 64(3). 10.1002/pbc.26251 [DOI] [PubMed]
- 99.Burda P, Vargova J, Curik N, Salek C, Papadopoulos GL, Strouboulis J, Stopka T. GATA-1 inhibits PU.1 gene via DNA and histone H3K9 methylation of its distal enhancer in erythroleukemia. PLoS One. 2016;11:e0152234. doi: 10.1371/journal.pone.0152234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agirre X, Castellano G, Pascual M, Heath S, Kulis M, Segura V, Bergmann A, Esteve A, Merkel A, Raineri E, Agueda L, Blanc J, Richardson D, Clarke L, Datta A, Russiñol N, Queirós AC, Beekman R, Rodríguez-Madoz JR, San José-Enériz E, Fang F, Gutiérrez NC, García-Verdugo JM, Robson MI, Schirmer EC, Guruceaga E, Martens JH, Gut M, Calasanz MJ, Flicek P, Siebert R, Campo E, Miguel JF, Melnick A, Stunnenberg HG, Gut IG, Prosper F, Martín-Subero JI. Whole-epigenome analysis in multiple myeloma reveals DNA hypermethylation of B cell-specific enhancers. Genome Res. 2015;25:478–487. doi: 10.1101/gr.180240.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aran D, Abu-Remaileh M, Levy R, Meron N, Toperoff G, Edrei Y, Bergman Y, Hellman A. Embryonic stem cell (ES)-specific enhancers specify the expression potential of ES genes in cancer. PLoS Genet. 2016;12:e1005840. doi: 10.1371/journal.pgen.1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Glass JL, Hassane D, Wouters BJ, Kunimoto H, Avellino R, Garrett-Bakelman FE, Guryanova OA, Bowman R, Redlich S, Intlekofer AM, Meydan C, Qin T, Fall M, Alonso A, Guzman ML, Valk PJM, Thompson CB, Levine R, Elemento O, Delwel R, Melnick A, Figueroa ME. Epigenetic identity in AML depends on disruption of nonpromoter regulatory elements and is affected by antagonistic effects of mutations in epigenetic modifiers. Cancer Discov. 2017;7:868–883. doi: 10.1158/2159-8290.CD-16-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spencer DH, Russler-Germain DA, Ketkar S, Helton NM, Lamprecht TL, Fulton RS, Fronick CC, O’Laughlin M, Heath SE, Shinawi M, Westervelt P, Payton JE, Wartman LD, Welch JS, Wilson RK, Walter MJ, Link DC, DiPersio JF, Ley TJ. CpG island hypermethylation mediated by DNMT3A is a consequence of AML progression. Cell. 2017;168(801–816):e13. doi: 10.1016/j.cell.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang L, Rodriguez B, Mayle A, Park HJ, Lin X, Luo M, Jeong M, Curry CV, Kim SB, Ruau D, Zhang X, Zhou T, Zhou M, Rebel VI, Challen GA, Gottgens B, Lee JS, Rau R, Li W, Goodell MA. DNMT3A loss drives enhancer hypomethylation in FLT3-ITD-associated leukemias. Cancer Cell. 2016;29:922–934. doi: 10.1016/j.ccell.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu R, Wang P, Parton T, Zhou Y, Chrysovergis K, Rockowitz S, Chen WY, Abdel-Wahab O, Wade PA, Zheng D, Wang GG. Epigenetic perturbations by Arg882-mutated DNMT3A potentiate aberrant stem cell gene-expression program and acute leukemia development. Cancer Cell. 2016;30:92–107. doi: 10.1016/j.ccell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qu Y, Siggens L, Cordeddu L, Gaidzik VI, Karlsson K, Bullinger L, Döhner K, Ekwall K, Lehmann S, Lennartsson A. Cancer-specific changes in DNA methylation reveal aberrant silencing and activation of enhancers in leukemia. Blood. 2017;129:e13–e25. doi: 10.1182/blood-2016-07-726877. [DOI] [PubMed] [Google Scholar]
- 107.Yamazaki J, Jelinek J, Lu Y, Cesaroni M, Madzo J, Neumann F, He R, Taby R, Vasanthakumar A, Macrae Ostler KR, Kantarjian HM, Liang S, Estecio MR, Godley LA, Issa JP. TET2 mutations affect non-CpG island DNA methylation at enhancers and transcription factor-binding sites in chronic myelomonocytic leukemia. Cancer Res. 2015;75:2833–2843. doi: 10.1158/0008-5472.CAN-14-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rasmussen KD, Jia G, Johansen JV, Pedersen MT, Rapin N, Bagger FO, Porse BT, Bernard OA, Christensen J, Helin K. Loss of TET2 in hematopoietic cells leads to DNA hypermethylation of active enhancers and induction of leukemogenesis. Genes Dev. 2015;29:910–922. doi: 10.1101/gad.260174.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hasegawa N, Oshima M, Sashida G, Matsui H, Koide S, Saraya A, Wang C, Muto T, Takane K, Kaneda A, Shimoda K, Nakaseko C, Yokote K, Iwama A. Impact of combinatorial dysfunctions of Tet2 and Ezh2 on the epigenome in the pathogenesis of myelodysplastic syndrome. Leukemia. 2017;31:861–871. doi: 10.1038/leu.2016.268. [DOI] [PubMed] [Google Scholar]