Abstract
The exact cause of Alzheimer’s disease (AD) is still unknown, but the deposition of amyloid-β (Aβ) plaques and chronic inflammation indicates that immune disturbances are involved in AD pathogenesis. Recent genetic studies have revealed that many candidate genes are expressed in both microglia and myeloid cells which infiltrate into the AD brains. Invading myeloid cells controls the functions of resident microglia in pathological conditions, such as AD pathology. AD is a neurologic disease with inflammatory component where the immune system is not able to eliminate the perpetrator, while, concurrently, it should prevent neuronal injuries induced by inflammation. Recent studies have indicated that AD brains are an immunosuppressive microenvironment, e.g., microglial cells are hyporesponsive to Aβ deposits and anti-inflammatory cytokines enhance Aβ deposition. Immunosuppression is a common element in pathological disorders involving chronic inflammation. Studies on cancer-associated inflammation have demonstrated that myeloid-derived suppressor cells (MDSCs) have a crucial role in the immune escape of tumor cells. Immunosuppression is not limited to tumors, since MDSCs can be recruited into chronically inflamed tissues where inflammatory mediators enhance the proliferation and activation of MDSCs. AD brains express a range of chemokines and cytokines which could recruit and expand MDSCs in inflamed AD brains and thus generate an immunosuppressive microenvironment. Several neuroinflammatory disorders, e.g., the early phase of AD pathology, have been associated with an increase in the level of circulating MDSCs. We will elucidate the immunosuppressive armament of MDSCs and present evidences in support of the crucial role of MDSCs in the pathogenesis of AD.
Keywords: Aging, Alzheimer, Hypoxia, Neuroinflammation, NF-κB, Senescence
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder involving the accumulation of amyloid-β (Aβ) plaques and tau-protein tangles in conjunction with the processes of both acute and chronic inflammation. Currently, it is a matter of debate whether the role of inflammation in the pathogenesis of AD is a cause or a consequence of AD pathology. The amyloid cascade hypothesis has dominated AD research for 25 years [1]. However, recent genetic studies on AD have revealed many candidate genes expressed in both microglia which are resident immune cells in the brain and also in infiltrating myeloid-derived cells which control the functions of microglia in pathological conditions, such as AD pathology. Microglial cells as well as infiltrating myeloid cells display extensive plasticity in their phenotypes and their responses to different insults. For instance, microglial cells can express either the pro-inflammatory M1 phenotype or anti-inflammatory M2 phenotype, or forms with the characteristics of both phenotypes [2, 3]. Moreover, invading myeloid cells can show a considerable adaptation and even differentiate into other cell types under the local microenvironmental pressure. The immune compartment of AD brains displays considerable flexibility, since both pro-inflammatory and anti-inflammatory responses occur concurrently with gradually escalating Aβ deposition [2, 4]. Given that inflammatory reactions are not able to eliminate the primary cause of AD pathology, e.g., excessive Aβ production, sporadic hypoxia, or virus infection, and thus, the immune system should attempt to fight against perpetrator while simultaneously protecting the integrity of neuronal networks. Several studies have demonstrated that AD brains reveal properties which are characteristic of an immunosuppressive milieu, e.g., microglial cells are hyporesponsive to Aβ deposits [5, 6]. In addition, there is clear evidence that anti-inflammatory cytokines enhance Aβ deposition. It is known that chronic inflammation in many pathological conditions stimulates the recruitment of myeloid cells which consequently switch on immunosuppressive milieu to protect the host tissue.
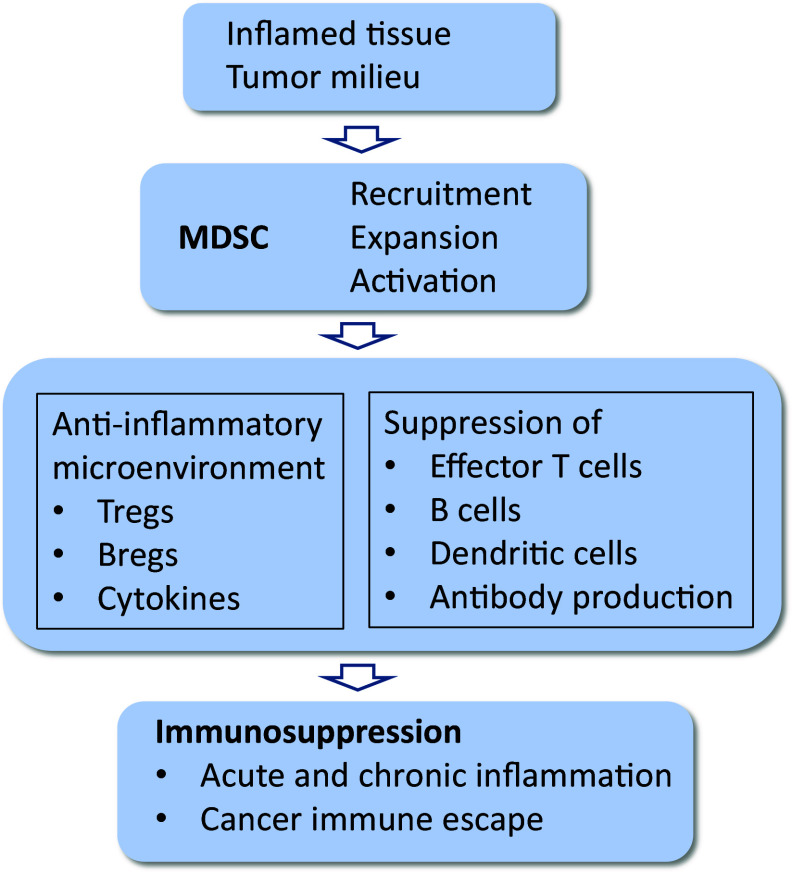
Myeloid-derived suppressor cells (MDSCs) are the major immunosuppressive cells which are recruited to inflamed tissues where they inhibit excessive inflammatory reactions and thus prevent tissues’ injuries [7, 8]. However, MDSCs are a double-edged sword, since the immune suppression induced by MDSCs has beneficial effects in acute inflammation, but MDSCs exert detrimental influences in conditions where the insult cannot be removed and inflammation turns to chronic phase. For instance, in inflamed tumors, MDSCs provide immune escape for cancer cells which can continue their proliferation, since MDSCs suppress effector T cells and also activate other suppressive cells, e.g., regulatory T cells (Tregs) (Fig. 1). Recent studies have revealed that MDSCs can also be recruited into inflamed normal tissues in different inflammatory disorders, e.g., in many neuroimmune diseases [9]. This means that the signals inducing the recruitment of MDSCs into tissues are dependent on inflammation and are not specific for cancer growth. It is known that several inflammatory mediators can either recruit MDSCs into tissues or enhance their proliferation and activation in inflamed tissues to facilitate immunosuppression. Given that MDSCs suppress the function of immune system, this is a major obstacle to the efficiency of vaccination therapies, e.g., in tumor immunotherapies [10], probably also for anti-amyloid therapies in AD. We will first elucidate the immune suppressive properties of MDSCs and then clarify the role of microglia and infiltrated myeloid cells in the generation of immunosuppressive microenvironment in AD. Finally, we will present evidences that support the crucial role of MDSCs in the pathogenesis of AD.
Fig. 1.
Functions of MDSCs in the generation of immunosuppression in inflamed tissues. Chemokines secreted by inflamed tissue recruit MDSCs into the tissue where MDSCs generate an anti-inflammatory microenvironment by activating Tregs and Bregs and producing immunosuppressive mediators. MDSCs suppress the functions of effector T cells as well as inhibit B cells and dendritic cells and thus reduce antibody production
Myeloid-derived suppressor cells (MDSCs)
Origin and trafficking of MDSCs
MDSCs are a heterogeneous population of immune suppressor cells which originate from common myeloid progenitor cells in the bone marrow, as earlier reviewed in detail [7, 11]. Chronic inflammatory mediators impair the differentiation of immature myeloid cells to macrophages, granulocytes, or dendritic cells. Subsequently, these cells will develop either monocytic or granulocytic MDSCs, which are potent immune suppressors in different pathological conditions. MDSCs, located in bone marrow and peripheral lymphoid organs, migrate through chemotaxis into inflamed tissues. Inflammatory cells release different chemokines which provoke the infiltration of MDSCs into the affected tissues where they will proliferate and become activated by diverse immunomodulators (Fig. 2). The two most important chemokines directing the chemotaxis of MDSCs are CC ligand 2 (CCL2), also called monocyte chemoattractant protein-1 (MCP-1), acting through the CC receptor 2 (CCR2) signaling [12] and CXC chemokine ligand 2 (CXCL2), previously called macrophage inflammatory protein-2 (MIP-2), signaling through the CXCL2/CXCR2 pathway [13]. There is a significant heterogeneity in the phenotype and immunosuppressive capacities of MDSCs [14, 15]. For instance, the phenotype and functional abilities of MDSC subsets are dependent on inflammatory conditions in pathological tissues. Bronte et al. [15] have recently proposed the nomenclature and identification markers of human and mouse MDSCs. The typical markers of human monocytic MDSCs are CD11b+, CD14+, CD33+, CD39+, and HLA-DRlow which appear in varying combinations in human diseases [8, 14]. In addition to flow cytometric markers, functional assays are required to identify those cells whether they are genuine MDSCs [15].
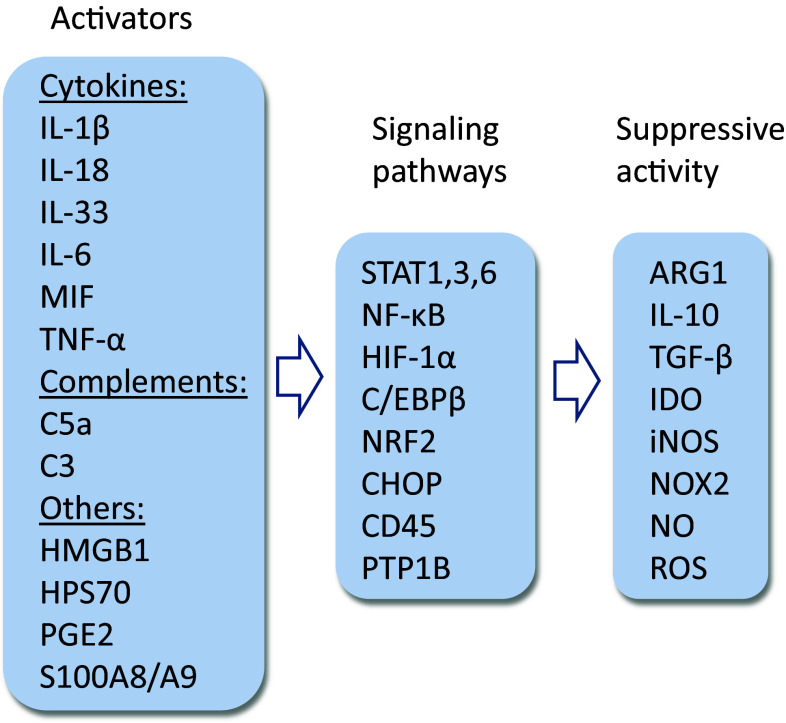
Fig. 2.
Activators and signaling pathways of MDSCs as well as the immunosuppressive mediators secreted by MDSCs. There are several activators of MDSC, mostly pro-inflammatory cytokines, complement factors, and many alarming factors. STAT, NF-κB, and C/EBPβ pathways are the key signaling mechanisms which activate the function of MDSCs. The major immunosuppressants include the cytokines IL-10 and TGF-β, the inducers of amino acid catabolism, i.e., ARG1 and IDO, and the activators of oxidative stress
Currently, there is debate whether MDSCs are differentiated from immature myeloid cells (IMC) in the bone marrow or whether IMCs are transported through circulation to the spleen and inflamed tissues where they are activated to MDSCs [11]. Inflamed tissues abundantly produce differentiation and activation factors both for IMCs and MDSCs. However, given that the numbers of circulating MDSCs increase in cancers and chronic inflammation [8, 16], it indicates that the differentiation of MDSCs has occurred in the spleen and the bone marrow via the signals mediated by circulating inflammatory mediators. It is likely that MDSCs are generated as a result of both the emergency myelopoiesis in the bone marrow and the extramedullary myelopoiesis in the spleen and inflamed tissues. Consequently, recruited MDSCs can proliferate and boost their immunosuppressive capacities in inflamed tissues.
Inflammatory mediators are major activators of MDSCs
There is a close connection between inflammation and cancer, i.e., chronic inflammation increases the risk of carcinogenesis, and it promotes the growth of tumors, angiogenesis, and metastasis [17]. The chronic inflammation present in many cancers enhances the infiltration, expansion, and activation of MDSCs which subsequently suppress immune surveillance and antitumor immunity [18, 19]. Unexpectedly, recent studies have revealed that not only cancers but also chronic inflammatory disorders can stimulate immunosuppression through the activation of MDSCs. This process is associated with many chronic inflammatory diseases, e.g., viral infections [20], hepatic inflammation and fibrosis [21], sepsis and trauma [16, 22], and several neuroimmune diseases [9]. It is known that infiltrated MDSCs have a beneficial role in the resolution of inflammation during the acute phase, e.g., through the secretion of anti-inflammatory cytokines and suppression of T cell proliferation [21–23]. However, in chronic inflammation, the MDSC-induced immunosuppression can prevent tissue injury, but, simultaneously, it disturbs the maintenance of immune homeostasis in tissues, e.g., suppressing functions of microglia/macrophages and effector T cells.
There is compelling evidence that several inflammatory mediators can stimulate the expansion and activation of MDSCs in inflamed tissues (Fig. 2). Given that the activation of inflammasomes has been reported to be associated with many chronic inflammatory diseases, it is not surprising that IL-1β and IL-18 cytokines can stimulate the function of MDSCs in several experimental conditions. For instance, Tu et al. [24] observed that the overexpression of IL-1β in the stomach of transgenic mice induced gastric inflammation associated with the early recruitment of MDSCs into the stomach. They also reported that IL-1β exposure-activated MDSCs via the IL-1R/NF-κB pathway. Lim et al. [25] revealed that the IL-18 treatment of myeloid progenitor cells induced the development of monocytic MDSCs which suppressed the functions of T cells in an NO-dependent manner. Furthermore, there are studies indicating that other cytokines, e.g., IL-6 [26], IL-33 [27], TNF-α [28], and MIF [29] also augmented the immunosuppressive activities of MDSCs (Fig. 2). It is also known that the activation of inflammation-associated complement system enhanced immunosuppression and consequently stimulated tumor growth. Markiewski et al. [30] demonstrated that complement C5a augmented the suppressive capacity of MDSCs in tumor microenvironment by increasing the production of reactive oxygen and nitrogen species by MDSCs. Hsieh et al. [31] reported that complement C3 also promoted the differentiation of MDSCs from hepatic stellate cells. These observations indicated that the complement system is also able to control inflammation through the activation of MDSCs (Fig. 2).
High mobility group box 1 (HMGB1/HMG1) is a multifunctional nuclear protein which is involved in several cellular functions, e.g., control of inflammatory responses [32]. HMGB1 is a common host defence signal released from cells under stress, which, consequently, alerts the immune system. HMGB1 stimulates immune cells through the Toll-like receptors (TLR2, TLR4, and TLR9) and receptor for advanced glycation end-products (RAGE). HMGB1 is a major innate alarmin which triggers sterile inflammation by activating resident immune cells. Activated immune cells further secrete HMGB1 which in turn can recruit MDSCs into inflamed tissues, thus potentiating the generation of chronic inflammation (Fig. 2). Parker et al. [33] demonstrated that HMGB1 exposure stimulated the differentiation of MDSCs from myeloid-derived progenitor cells in mice. They also reported that HMGB1 increased the production of IL-10 by MDSCs as well as decreased their capacity to suppress antigen-driven T-cell activation. Recently, Li et al. [34] revealed that HMGB1 increased the proliferation of MDSCs and thus promoted the immune escape of renal carcinoma cells in mice. In addition to the cancer-associated inflammation, there are observations that HMGB1 augmented immunosuppression after tissue trauma through the increased functions of MDSCs and regulatory T cells [35]. Two other alarmins, S100A8 and A9, which are calcium-binding proteins, are crucial pro-inflammatory mediators in both acute and chronic inflammation [36]. These proteins are expressed mainly in myeloid-derived cells, especially in inflamed tissue, where they increase cytokine expression via the activation of NF-κB signaling and inflammasome function. S100A8/A9 proteins are highly expressed in and secreted from MDSCs [37]. Sinha et al. [37] demonstrated that S100 proteins are important mediators in the accumulation of MDSCs into inflamed tissues. The alarmins S100A8 and A9 signal through RAGE and TLR4 receptors and subsequently activate the STAT3-dependent pathway in MDSCs. Sinha et al. [37] proposed that S100 proteins act in the autocrine loop which enhances and maintains the function of MDSCs in inflammatory conditions.
Prostaglandins (PGs) are potent regulators of inflammation in both acute and chronic pathological conditions [38]. PGs are synthesized from arachidonic acid through the action of cyclooxygenase (COX) isoenzymes and they can either promote inflammation or enhance its resolution in a prostaglandin subtype-specific manner. For instance, PGE2 can control inflammatory responses through four different prostanoid receptor subtypes EP1–EP4 [38]. Sinha et al. [39] demonstrated that PGE2 and EP receptor agonists induced MDSC differentiation from bone marrow stem cells, whereas EP antagonists blocked this process. Subsequent studies revealed that EP2 and EP4 receptors are involved in the PGE2-induced development of MDSCs [39, 40]. Obermajer et al. [41] revealed that there was a close positive feedback loop between COX-2 and PGE2 which sustained local PGE2 production and consequently induced the differentiation of monocytes into immature MDSC cells in the inflammatory environment of human cancers. An increased level of PGE2 inhibited the differentiation of dendritic cells redirecting their development toward monocytic MDSCs. Moreover, inhibition of COX-2 enzyme prevented the differentiation and accumulation of MDSCs into inflamed cancer tissues [39, 41]. It is known that an increased PGE2 exposure enhanced the trafficking of MDSCs into tumors by stimulating the function of CXCL12/CXCR4 homing axis as well as inducing the expression of immune suppressive factors in MDSCs [40]. Obermajer et al. [40] have reviewed, in detail, the PGE2-driven regulation of MDSC functions. In conclusion, it seems that inflammatory mediators can control various functions of MDSCs in different phases of inflammation, i.e., enhancing the resolution of inflammation at the acute phase, but, since MDSCs can generate immunosuppression, they have harmful effects during the chronic phase of inflammation.
Diverse signaling pathways are involved in the activation of MDSCs
Given that several inflammatory mediators can control the differentiation, trafficking, expansion, and activation of MDSCs, a diverse network of signaling pathways is required to regulate the genes involved in the generation of immune suppressive milieu in inflamed tissues. This topic has been examined in detailed reviews elsewhere [42, 43]. The JAK-STAT pathway mediates signals from many cytokine receptors subsequently activating the expression of distinct immunosuppressive factors which are secreted from MDSCs (Fig. 2). The STAT factor family contains seven members which have specific upstream connections, e.g., IFN-γ activates STAT1, IL-6 and IL-10 stimulate STAT3, and IL-4 triggers STAT6 [42, 43]. STAT3 is a key factor in the MDSC-induced immunosuppression and immune escape of tumors, since it can activate Tregs, Th17, and MDSCs [44]. STAT3 controls the expression of arginase 1 (ARG1) which is a marker enzyme for MDSCs and a crucial inducer of immunosuppression [45]. The NF-κB system is another signaling pathway working in conjunction with the JAK-STAT axis to activate the function of MDSCs in inflamed tissue (Fig. 2). There is substantial evidence that the STAT3 and NF-κB pathways have a close interaction in the regulation of inflammation and cancer progression. For instance, Yu et al. [46] demonstrated that the STAT3-induced expression of indoleamine-pyrrole 2,3-dioxygenase (IDO) was mediated through the non-canonical activation of NF-κB in MDSCs. The NF-κB system is the master regulator of acute inflammatory responses, but it can also augment immune suppression and cancer progression in chronic inflammation through the expansion and activation of MDSCs [24, 33, 46]. In addition to many cytokines, a variety of insults can activate NF-κB signaling through the TLR pathways, e.g., LPS, HMGB1, and HSP70 [47]. There is compelling evidence, indicating that the activation of NF-κB signaling pathway can have both damaging and neuroprotective effects in neurodegenerative diseases [48].
Tissue hypoxia, associated with cancer growth and inflammatory disorders, stimulates the expression of hypoxia-inducible factor-1α (HIF-1α) as a host defence response in affected tissues [49]. In fact, hypoxia has an important role in the control of acute inflammatory effects as well as chronic reactions by inducing immunosuppression; this occurs in both the cancer milieu and inflamed normal tissues [50, 51]. Corzo et al. [52] demonstrated that an increase in the expression of HIF-1α stimulated the expression of arg1 and inos genes in mouse MDSCs and subsequently directed their differentiation toward the phenotype of tumor-associated macrophages in tumor microenvironment. HIF-1α expression also greatly upregulated the production of NO in MDSCs and thus suppressed the proliferation of nonspecific T cells. Noman et al. [53] reported that the hypoxia-induced HIF-1α expression in mouse MDSCs increased the secretion of immunosuppressive IL-10 cytokine as well as the expression of programmed death-ligand 1 (PD-L1), an inhibitor of effector T cells. These studies clearly indicated that hypoxia increased the immunosuppressive capacity of MDSCs through HIF-1α signaling. There is also mounting evidence that the hypoxia/HIF-1α signaling can directly control the functions of macrophages, dendritic cells, and T cells in inflamed tissues [50, 51]. Adenosine is a potent immunomodulator acting through the adenosine receptors, e.g., A2A and A2B receptors. Ryzhov et al. [54] observed that adenosinergic regulation increased the proliferation and immunosuppressive activity of mouse MDSCs. They also reported that the stimulation of A2B receptor, but not other receptor subtypes, promoted the expansion of MDSCs, preferentially that of granulocytic MDSCs. Sitkovsky [50] proposed that the hypoxia-adenosinergic activation of Tregs-induced immunosuppression and activated tissue-protecting mechanism in hypoxic-inflamed tissues.
Marigo et al. [55] demonstrated that CCAAT/enhancer-binding protein β (C/EBPβ) is a crucial transcription factor for the activation of cytokine-induced immunosuppressive program in both bone marrow-derived and tumor-induced MDSCs. They reported that the deficiency of C/EBPβ protein in MDSCs caused a significant decrease in the expression of ARG1 and NOS2 enzymes and thus reduced the suppression of T-cell activation. Moreover, the C/EBPβ-deficient MDSCs could not inhibit inflammation at the early sepsis and were not immunosuppressive at the late phase of sepsis [56]. C/EBPβ is an important inflammatory mediator, e.g., through its crosstalk with NF-κB signaling. For instance, C/EBPβ is a potent inducer of COX-2 transactivation [57] and thus stimulates PGE2 production and subsequently the activation of MDSCs. There are also observations that stress-related transcription factors, C/EBP-homologous protein (CHOP) [58], and nuclear factor-erythroid 2-related factor 2 (NRF2/NFE2L2) [59] can stimulate the accumulation, survival, and activity of MDSCs (Fig. 2).
The signaling networks regulating the expansion and activation of MDSCs also contain several protein phosphatases and micro-RNAs [60–62]. For instance, Kumar et al. [62] reported that hypoxia induced the upregulation of CD45, a protein tyrosine phosphatase, which inhibited the STAT3 signaling in mouse MDSCs (Fig. 2). Protein tyrosine phosphatase 1B (PTP1B) also targets STAT3, since a lack of PTP1B enhanced the proliferation of MDSCs by increasing the activity of STAT3 [60]. There is mounting number of micro-RNAs (miRs) which are able to activate or suppress the functions of MDSCs by targeting different components of signaling pathways, e.g., miR-9, mir-17-5p, miR-20a, miR-34a, miR-210, and miR-494 [61, 63]. Huang et al. [63] demonstrated that miR-34a was able to increase the expansion of mouse MDSCs by inhibiting the apoptosis of these cells. In conclusion, a multifaceted signaling network controls the immunosuppressive functions of MDSCs which means that there might be multiple targets to ameliorate the functions of immune system in both cancers and chronic inflammatory diseases.
MDSC-induced immunosuppression
MDSCs are the major inhibitory cells of immune system, suppressing both adaptive and innate immunity reactions [7, 64, 65]. The immunosuppressive capacities of MDSCs have been clarified especially in cancer growth, since MDSCs have a key role in tumor escape from immune surveillance. Currently, it is known that MDSCs generate anti-inflammatory and immunosuppressive effects by controlling the functions of regulatory and effector T cells, macrophages, and dendritic cells. There is a diverse set of mechanisms through which MDSCs can inhibit the functions of T lymphocytes (Fig. 2). For instance, MDSCs secrete reactive oxygen species (ROS), e.g., nitric oxide (NO), which nitrates the tyrosine residues of T-cell receptor and thus renders T cells unresponsive to antigen-specific stimulation [66]. The activation of MDSCs stimulates the expression of inducible nitric oxide synthase (iNOS) and NADPH oxidase 2 (NOX2) which generate NO and other ROS compounds [67]. However, increased ROS production can also enhance inflammatory disorders, e.g., by causing endothelial dysfunction which promotes the migration of myeloid cells across the BBB into inflamed tissues [68]. Activated MDSCs also express an elevated level of ARG1 which consumes l-arginine, an essential amino acid, thus leading to arginine starvation in inflammatory microenvironments [69, 70] (Fig. 2). NO is synthesized from l-arginine, and thus, its production augments the shortage of l-arginine during the activation of MDSCs. l-arginine deficiency stimulates GCN2 kinase which phosphorylates the translation initiation factor eIF2α and thus inhibits protein synthesis. Arginine deprivation represses the proliferation and activation of T cells as well as other inflammatory cells, thus enhancing immunosuppression. MDSCs also display a high expression of IDO enzyme which depletes extracellular tryptophan levels and subsequently inhibits the proliferation of T cells, triggering their apoptosis [71]. There are observations that MDSCs can also suppress B-cell proliferation and thus repress antibody production, probably through NO secretion and arginine deprivation [72] (Fig. 1).
Activated MDSCs as well as other immune-suppressive cells are abundant sources of secreted anti-inflammatory cytokines in inflammatory microenvironments (Fig. 2). IL-10 and TGF-β are the two major cytokines mediating a variety of immunosuppressive functions via many signaling pathways [73, 74]. IL-10 stimulates mainly the JAK-STAT pathways, e.g., activating MDSCs, whereas TGF-β triggers the SMAD-driven gene expression. In general, IL-10 and TGF-β suppress the expression of pro-inflammatory cytokines/chemokines, and thus, they maintain immune homeostasis and protect tissues from excessive inflammatory responses. For instance, IL-10 and TGF-β1 can skew pro-inflammatory M1 macrophages to the anti-inflammatory M2 phenotype [73, 75]. TGF-β inhibits the proliferation of T cells and represses their differentiation into CD8+ and Th1/2 cells, while it stimulates the expansion of immunosuppressive FoxP3+ Tregs [73]. It is also known that TGF-β inhibits the proliferation and activation of B cells, and it can induce the apoptosis of immature and resting B cells. There is substantial evidence that TGF-β also inhibits the expression of proteins involved in phagocytosis and antigen presentation of macrophages [73]. IL-10 also impedes the maturation of dendritic cells and thus impairs their antigen presentation process [76]. These observations clearly indicate that MDSCs can inhibit the functions of different myeloid-derived cells through the secretion of immunosuppressive cytokines and thus inhibit immune responses in both acute and chronic inflammation.
MDSCs can also induce immunosuppression by stimulating the differentiation of Tregs and regulatory B cells (Bregs) [65, 77] (Figs. 1, 3). Tregs have an important role in the maintenance of immune homeostasis, since they can suppress immune responses by secreting IL-10 and TGF-β cytokines as well as having cell–cell contacts with effector T cells [78]. Tregs can suppress the proliferation and activation of T cells in different pathological conditions. MDSCs and Tregs can also benefit the membrane-bound PD-L1 protein to inhibit T-cell activation through the binding of PD-L1 to the PD-1 receptor of T cells [79, 80]. The PD-L1/PD-1 connections maintain T-cell tolerance and prevent immune-mediated damages in inflamed tissues [79]. In addition to T cells, many other immune cells, e.g., B cells, dendritic cells, monocytes, and macrophages, express proteins involved in the PD-L1/PD-1 system [79]. Forkhead box P3 (FOXP3) transcription factor is a phenotypic marker of Tregs and a crucial regulator of immune tolerance [81]. IL-2 and TGF-β are potent inducers of FOXP3 expression and consequently control the activity of FOXP3+ Tregs. MDSCs and Tregs form an immunosuppressive network, since Tregs can reciprocally regulate the proliferation and activity of MDSCs in the inflammatory milieu [65]. Recently, it was observed that MDSCs stimulated the expansion of regulatory B cells (Bregs) and ameliorated autoimmunity in systemic lupus erythematosus [77]. Bregs, immature B cells, are immunosuppressive cells impairing the functions of Th1 and Th17 cells, CD8+ cytotoxic T cells, dendritic cells, and monocytes, whereas they stimulate the expansion of Tregs [82]. Bregs control other immune cells through the secretion of IL-10, IL-35, and TGF-β cytokines. It seems that immune homeostasis is regulated by flexible interactions between MDSCs and different T- and B-cell populations, dendritic cells, and macrophages. However, this regulation is highly specific for pathological processes and tissue microenvironments.
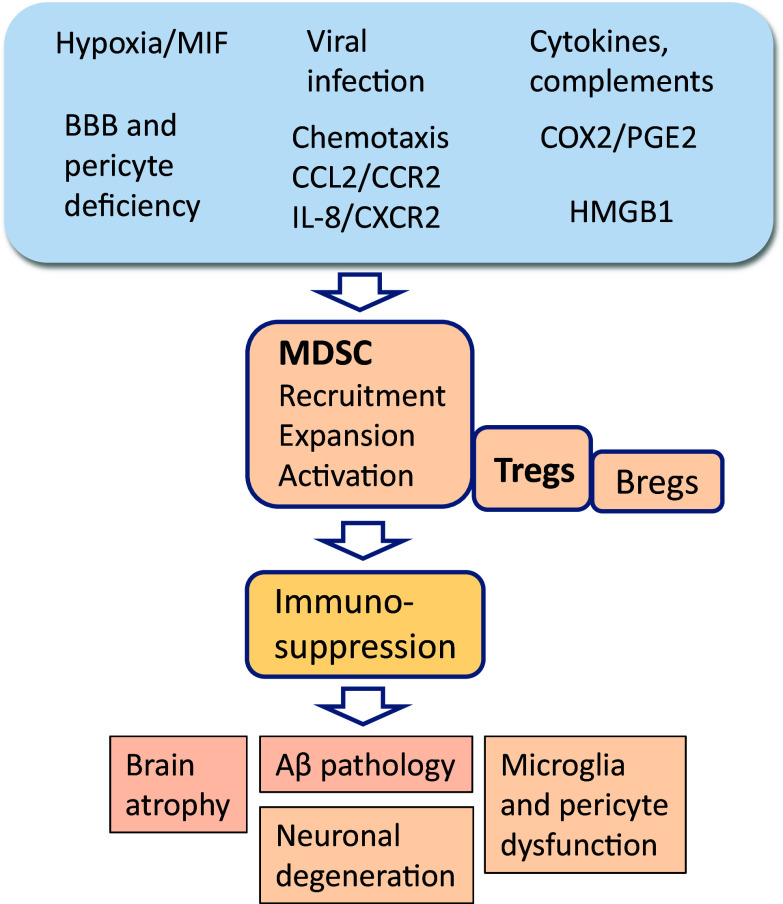
Fig. 3.
Potential mechanisms which could promote immunosuppression and maintain chronic inflammation in AD brains through the recruitment and activation of MDSCs. Hypoxia and viral infections, probable causes of AD, are strong inducers of the accumulation of MDSCs into the brain. It is known that inflamed AD brains secrete several chemokines which trigger the chemotaxis of MDSCs into the brain. Consequently, several cytokines and complements as well as prostaglandin PGE2 and alarmin HMGB1 stimulate the immunosuppressive functions of MDSCs in AD brains. Sustained MDSC activity provokes chronic inflammation involving the deposition of Aβ, increases brain atrophy, and, finally, leads to neuronal degeneration
MDSC-induced immunosuppression in gliomas and neuroinflammatory disorders
Gliomas are primary brain tumors originating from proliferating glial cells, either from astrocytes, oligodendrocytes, or ependymal cells. Although there seem to be multiple causes for gliomas, the infiltration and expansion of MDSCs have a crucial role in the growth of gliomas [12, 83]. Several studies have indicated that cancerous glial cells and stem cells are able to manipulate brain microglia/macrophages and affect their immune functions, e.g., increasing their immunosuppressive capacities and enhancing the chemotaxis of myeloid cells [84]. It seems that the glioma-associated, alternatively activated M2 microglia/macrophages (GAMs) recruit MDSCs into gliomas, and consequently, GAMs and MDSCs act in collaboration to create the immunosuppressive milieu present in gliomas [83, 84]. Chang et al. [12] demonstrated that CCL2 was a critical chemokine in the recruitment of monocytic MDSCs and Tregs into murine gliomas. Immunosuppressive cells secrete IL-4, IL-10, and TGF-β cytokines which activate MDSCs and Tregs, inhibit the functions of T cells and increase their apoptosis, reduce the phagocytic capacity of macrophages, and stimulate angiogenesis [83]. Fujita et al. [85] demonstrated that the blockade of COX-2/PGE2 axis suppressed the growth of gliomas which indicates that MDSCs have a significant role in gliomagenesis. Gliomas provide an interesting model to understand the adaptability of microglia and their crosstalk with cancerous glial cells and infiltrated myeloid cells.
Acute and chronic inflammatory responses have been associated with diverse neurological disorders and neurodegenerative diseases. It is known that the brain is a target of different myeloid-derived cells which possess both anti-inflammatory and immunosuppressive capacities (see below). In the brain, the role of MDSCs in autoimmune diseases has been mostly studied in experimental autoimmune encephalomyelitis in mice [9] but to a lesser extent in human multiple sclerosis [86]. There is an abundant literature to demonstrate that immune-suppressive MDSCs can inhibit the damaging responses of T cells in autoimmune brain diseases, enhance the resolution of inflammation, and thus probably trigger the remission phase of multiple sclerosis [86, 87]. Given that Tregs prevent the functions of myelin-specific autoreactive T cells, it seems that there are dysfunctions in Tregs in multiple sclerosis [88] which might also affect the responses of MDSCs. Neuronal demyelination induced by Theiler’s virus provoked the infiltration of MDSCs into mouse brain where they suppressed virus-specific CD4+ and CD8+ T cells [89]. In this case, MDSCs extended the demyelination phase and augmented the disease. Correspondingly, the depletion of MDSCs reduced the virus-induced inflammation and demyelination injuries in mouse brain.
There is mounting evidence that MDSCs are involved in the inflammatory processes associated with injuries in central nervous system (CNS), e.g., brain trauma, stroke, and spinal cord injuries. Acute CNS lesions stimulate an early immune activation in both CNS and peripheral immune system which triggers a profound systemic immunosuppression [22, 90, 91]. Liesz et al. [91] demonstrated that the release of HMGB1 from mouse brain in the acute phase after stroke not only induced a pro-inflammatory cytokine response but also enhanced the release of immature monocytes from the bone marrow and stimulated a great expansion of MDSCs in mouse spleen. An increased proliferation of MDSCs in the spleen was abrogated by anti-HMGB1 treatment, indicating that HMGB1-RAGE signaling was involved in the activation of MDSCs. The splenic MDSCs, isolated 3 days after a stroke, strongly suppressed the proliferation of T cells. A significant increase in the number of MDSCs, with a phenotype of CD11b+Ly-6C+, was also observed in the blood of human stroke patients. Moreover, Liesz et al. [91] revealed that the HMGB1-RAGE signaling in collaboration with catecholamines was involved in the post-stroke immune suppression in mice. For instance, norepinephrine can stimulate the proliferation of MDSCs and block the function of T cells [92]. It is likely that both cytokine and hormonal regulation mechanisms control the expansion of MDSCs to generate immunosuppression after brain injuries. Saiwai et al. [22] demonstrated that, in mouse spinal cord injury, MDSCs enhanced the resolution of acute inflammation and reduced tissue damages. Consequently, MDSCs promoted angiogenesis in spinal cord and improved the functional recovery of mice.
Chronic inflammation is involved in progressive neurodegenerative diseases, e.g., Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), Parkinson’s disease, and Huntington’s disease. Currently, the exact role of immune responses in the pathogenesis of these diseases still needs to be clarified. Vaknin et al. [93] reported a twofold increase in the number of MDSCs in the blood samples of sporadic ALS patients as compared to healthy controls. Moreover, a clear increase has been observed in the number of circulating MDSCs in Parkinson’s disease patients [94]. Henkel et al. [95] observed that the number of Tregs was significantly reduced in the blood of ALS patients during the rapid progression phase, indicating that immune-suppressive capacity might be impaired in that stage and thus leading to enhanced inflammatory damages. In Alzheimer’s disease, Le Page et al. [96] detected a significant twofold increase of circulating monocytic and granulocytic MDSCs in amnestic mild cognitive impairment (aMCI) which is an early phase of AD pathology. However, this upregulation was not present in blood during the later AD phase. Currently, there are no studies on the levels of MDSCs in the brain tissues of MCI and AD patients or transgenic AD mice. Saresella et al. [97] reported that the number of different types of Tregs was significantly increased in the blood of both MCI and AD patients as compared to controls. Especially, the PD-1− pool of Tregs was increased in MCI patients indicating a strong immune-suppressive activity during the early phase of AD. In functional assays, the immunosuppression induced by circulating Tregs was more efficient in MCI than in AD patients. Baruch et al. [98] demonstrated in 5xFAD transgenic mice that the transient depletion of Tregs or their pharmacological inhibition alleviated the phenotype linked to AD pathology, e.g., Aβ accumulation, neuroinflammation, and cognitive impairment. We will discuss below more thoroughly on the role MDSCs, Tregs, and immunosuppression in the pathogenesis of AD.
Immune suppression in AD brain
Chronic neuroinflammation in AD
Chronic inflammation is one of the hallmarks of AD pathology [4]. AD is a neurodegenerative disease, although, currently, it is not clear whether inflammation is a cause of AD pathology, through neurotoxic inflammatory mediators, or an immune consequence of neuronal damages. The histopathology of AD reveals an increased number of glial cells, both microglia and astrocytes, in the affected areas of AD brains. Entorhinal cortex and hippocampus are the most vulnerable regions of AD-related pathology. The amyloid cascade hypothesis, proposed by Hardy and Allsop [1], is the most commonly accepted theory on the origin of AD pathology. This hypothesis is supported not only by the accumulation of Aβ peptides in brain but also substantial genetic evidence which indicates that distinct variants in the genes regulating APP processing, i.e., APP and presenilin PSEN1/2 genes, can lead to the deposition of amyloid plaques. Interestingly, recent studies on the AD risk genes have revealed that many candidate genes, e.g., CD33 and TREM2, are expressed in microglia and regulate phagocytosis in these cells [99, 100]. Moreover, Huang et al. [101] demonstrated that the genetic down-regulation of PU.1 (SPI-1) expression, a major transcription factor of several microglial genes affecting the expression levels of CD33 and TREM2 proteins, delayed the onset of AD. However, PU.1, CD33, and TREM2 are also expressed in other myeloid cells, e.g., CD33 protein is the molecular marker of human monocytic and granulocytic MDSCs [14]. In addition, Fahrenhold et al. [102] demonstrated that TREM2 protein seems to be located in recruited monocytes rather than microglia in the brain samples of AD patients. Currently, the role of CD33 and TREM2 proteins in the regulation of immune functions by MDSCs and other myeloid-derived cells needs to be clarified.
Microglia are resident cells in the brain which are originally derived from the yolk sac macrophages during embryonal development [103]. Microglial cells act as tissue sentinel cells in the same way as macrophages in peripheral tissues, eliminating microbes, dead cells, and extracellular protein aggregates, i.e., they are the professional phagocytes in the brain [104, 105]. Microglia can also control neurogenesis and synaptic plasticity as well as conferring protection against excitotoxicity. Microglial cells are immune cells which express a variety of pattern-recognition receptors detecting both pathogen-associated and damage-associated molecular patterns (PAMPs/DAMPs). For instance, Aβ oligomers trigger innate immunity defence by activating several pattern-recognition receptors [106]. Moreover, microglia contain a wide range of other immune receptors, e.g., cytokine and chemokine receptors and receptors for immune complexes as well as receptors for many neurotransmitters [104]. At rest, microglia show a ramified phenotype actively surveying their microenvironment [107]. The activation of microglia triggers changes in their morphology and stimulates the expression of a set of pro-inflammatory genes, e.g., involving cytokines, chemokines, and innate immunity receptors. However, recent phenotyping and transcriptome studies have revealed the biological complexity of microglial responses in health and diseases [105]. There is extensive heterogeneity between microglial cells at the level of gene expression and their functional responses to distinct insults [105, 108]. Microglial cells are major guardians of brain homeostasis, and thus, it is reasonable that their properties and reactions are controlled by the crosstalk with other cells, e.g., astrocytes and infiltrated myeloid cells. In pathological conditions, astrocytes secrete chemokines, e.g., CCL2, CCL5, CXCL2, and CXCL12 [109], potential chemoattractants to MDSCs. Astrocytes and microglia also have close interaction in Aβ clearance and neuroinflammation in AD pathology [4], e.g., the activation of microglia can induce reactive astrocytosis, abundantly present in AD brain [110].
AD is a progressive neurodegenerative disease involving simultaneously acute pro-inflammatory and chronic anti-inflammatory reactions. This can be seen in the upregulation of distinct cytokines and chemokines normally associated with either acute or chronic inflammation (see below). Macrophages and microglia can display remarkable plasticity, since they are able to change their phenotype from pro-inflammatory to anti-inflammatory subtype, or vice versa, as a response to pathological alterations. This process is called the macrophage/microglia polarization [3, 75]. Briefly, the pro-inflammatory M1 phenotype is present in acute inflammatory conditions, whereas the M2 polarization, an alternative activation, has been switched on to facilitate resolution of acute inflammation. In addition, the M2 phenotype, both that of macrophages and microglia, can be categorized to M2a, M2b, and M2c subtypes using distinct markers [2, 3, 75]. Some cytokines, e.g., IL-4, IL-10, IL-13, and TGF-β, are the inducers of the anti-inflammatory M2 phenotype. Correspondingly, alternatively activated M2 macrophages/microglia secrete anti-inflammatory cytokines, e.g., IL-10 and TGF-β, as well as several chemokines and growth factors which facilitate the maintenance of anti-inflammatory milieu and enhance the tissue repair process. Interestingly, IL-10 and TGF-β, two potent inducers and maintenance factors of M2 phenotype, are also major immune suppressors secreted by MDSCs (Fig. 2). Currently, the paradigm of M1 and M2 phenotypes has been critically debated due to the large functional heterogeneity of cells including in the M1 and M2 groups [111]. It seems that the phenotypes of microglia form a continuum of different activation states. Studies on AD have revealed controversial results on the role of microglia in the pathogenesis of AD. For instance, Weitz and Town [112] proposed that the role of microglia would be widely context-dependent, i.e., they provoke either beneficial or deleterious actions during the progression of AD pathology. Microglia are not only versatile effector cells but also sensitive sensors which can respond to local conditions in cooperation with astrocytes and infiltrated myeloid cells [113]. Microglial cells are also able to adapt to long-term environmental changes, e.g., inflammatory disorders, aging process, and growth of glioma [114].
Recruitment of myeloid-derived cells into brain in AD
The brain is not completely immune-privileged tissue, since circulating, bone marrow-derived myeloid, and lymphoid cells can infiltrate into the brain where they form an immune compartment with tissue-resident microglial cells [115, 116]. Hematopoietic stem cells differentiate into the myeloid and lymphoid progenitor cells which, consequently, can be committed to specific lineages [117]. The subsequent maturation of these progenitor cells can occur in myeloid tissues, peripheral immune organs, e.g., spleen and thymus, or even in inflamed and cancerous tissues after the penetration of immature cells. The immature myeloid cells can differentiate into monocytes, MDSCs, dendritic cells, or neutrophils. Consequently, monocytes and MDSCs are able to maturate to tissue macrophages. The lymphoid progenitor lineage generates B and T lymphocytes and natural killer cells. The T helper cells, i.e., Th1, Th2, and Th17, and Tregs originate from naïve CD4+ T cells. Colony stimulating factors, chemokines, and cytokines direct the differentiation process of myeloid-derived cells. There is substantial evidence that the distinct subsets of myeloid and lymphoid cells can infiltrate into the brain, especially in gliomas and inflammatory disorders [115, 118, 119]. The recruitment process of immune cells into the CNS is guided by several chemokines and their corresponding chemokine receptors in immune cells. For example, monocytes, MDSCs, T cells, and Tregs can invade into the inflamed brain. The choroid plexus, an interface between blood and cerebrospinal fluid, is an important entry site for immune cells [120], although it seems that immune cells can also migrate across the blood–brain barrier (BBB) elsewhere in the brain [119]. Specific penetration mechanisms have been earlier described in detail [115].
Recent studies have revealed that pericytes, i.e., perivascular cells surrounding endothelial cells in capillaries, control inflammation, and immune cell trafficking across capillary wall [121–123]. There are significant changes in the structure and functions of pericytes in chronic neuroinflammation. Pericytes also have many properties of immune cells, e.g., they respond to inflammatory stimuli by secreting cytokines and chemokines as well as they can polarize microglia and display phagocytic ability. Stark et al. [121] demonstrated that inflammatory mediators stimulated the expression of several chemokines and adhesion molecule ICAM-1 in human NG2-positive pericytes. ICAM-1 mediated the attachment of monocytes and neutrophils to pericytes. In mouse inflamed skin, arteriolar and capillary NG2 pericytes induced the chemotaxis of myeloid leukocytes and subsequently interacted with extravasated immune cells. Pericytes directed the chemotaxis of immune cells by releasing chemoattractants, e.g., MIF, CCL2, and IL-8, the level being subject to the intensity of inflammatory stimulus. In particular, MIF, a hypoxia-inducible chemokine, induced a potent chemotaxis of monocytes. Hypoperfusion and cerebral amyloid angiopathy are typical disorders of AD brain [124–126]. Interestingly, it is known that pericytes have an essential role in the regulation of capillary blood flow into the brain [127, 128]. Impaired pericyte function in AD might enhance the recruitment of myeloid cells into the AD brain.
There is substantial evidence that AD is associated with both structural and functional disturbances in the BBB [129, 130]. It seems that a reduced integrity of BBB and the disorders of pericyte functions in inflamed AD brain enhance the infiltration of immune cells into the brain parenchyma. In addition to parenchymal resident microglia, healthy brain also contains myeloid-derived perivascular and meningeal macrophages [116]. In AD, the infiltrated blood-derived cells represent a diverse population of immune cells ranging from myeloid monocytes to lymphoid T cells [131–133]. For instance, circulating monocytes are versatile immune cells, since, after recruitment into AD brains, they can secrete inflammatory modulators and mature to macrophages (also called monocyte-derived macrophages). There is compelling evidence that the penetration of T lymphocytes is increased into the brains of AD patients and transgenic AD mice [97, 133, 134]. For instance, Tregs and effector T cells, e.g., Th1 and Th2 helper cells, can invade AD brains and control inflammation and subsequently affect neurodegenerative processes.
Secreted chemokines and their receptors in invading immune cells have a key role in the recruitment of myeloid-derived cells into the brain during the inflammatory processes of AD [135, 136]. There is abundant evidence that the CCL2/CCR2 chemotactic axis is the major mechanism which recruits blood CCR2+ monocytes into the inflamed brains including AD pathology [136, 137]. Galimberti et al. [138] observed that the serum level of CCL2/MCP-1 was clearly elevated in MCI and mild AD patients but not in severe AD patients. Correspondingly, it has been reported that the expression level of CCR2 was significantly increased in blood mononuclear cells, especially in CD4+ cells, in AD patients [135]. CCL2 is commonly expressed in brain, e.g., astrocytes, microglia, and endothelial cells, can secrete CCL2 chemokine which is a potent chemoattractant to monocyte/macrophage. Selenica et al. [139] induced the overexpression of CCL2 in mouse brain through the intracranial injection of the rAAV viral construct expressing CCL2 gene. They observed that viral induction dramatically stimulated the expression of CCL2 in mouse hippocampus and cortex regions. The overexpression of CCL2 induced an abundant extravasation of myeloid-derived CD11b+ cells into the CNS parenchyma. Moreover, the virally expressed CCL2 activated the proliferation of microglia and polarized them to either the M1 or M2 states. Interestingly, CCL2 is the most important chemoattractant for MDSCs, and in glioma, CCL2 stimulated the recruitment of MDSCs and Tregs into the brain [12]. There are several other chemokines and receptors which could augment the chemotaxis of immune cells into the brain, e.g., CCL5, CXCL2, CXCL8, and CXCL12 [109, 136]. For instance, Liu et al. [140] revealed that the expression of CXCR2 was increased in the blood T cells of AD patients which increased their transendothelial migration capacity. They also reported that the injection of Aβ into rat hippocampus increased the expression of IL-8/CXCL8 (ligand for CXCR2) in circulating T cells and brain endothelial cells. It seems that pro-inflammatory responses precede the chemotaxis of T cells, since the inhibition of microglial TNF-α production suppressed the CXCL8/CXCR2-mediated recruitment of T cells into the brain [140]. Interestingly, it was reported that CXCL8 secreted by tumors stimulated the recruitment of MDSCs through the CXCL8/CXCR2 chemotaxis, thus inducing immune escape of tumors [13].
Immunosuppressive microenvironment in AD
The common hallmarks of AD are the deposition of extracellular Aβ plaques and intracellular tau-protein tangles being accompanied by chronic inflammation. This progressive pathological process can be initiated about 10–20 years before the appearance of first dementia disorders. Currently, the role of inflammation in the pathogenesis of AD still needs to be clarified. Many early epidemiological studies indicated that a lifelong use of NSAIDs could prevent the appearance of AD pathology and cognitive impairment, but, consequently, clinical studies with AD patients have failed to show any beneficial effects of NSAID therapy [141]. It has been postulated that inflammation has a detrimental role in the early phase, and thus, NSAIDs could prevent the onset of pathological processes, even before the appearance of amyloid plaques [142]. This assumption has received experimental support from the studies with transgenic AD mice and rats (McGill-R-Thy1-APP). These studies demonstrated that the levels of several inflammatory markers were already increased before the appearance of Aβ plaques [143, 144]. For instance, microglial activation and astrogliosis preceded the accumulation of Aβ deposits in cerebral cortex and hippocampus. Moreover, inflammatory changes seemed to be associated with the intraneuronal increase of Aβ-oligomers which might have alerted innate immunity system through the secretion of danger-associated molecules. These observations emphasized the fact that inflammation process, both its cellular players and inflammatory reactions, may fluctuate during the evolution of AD pathology. Many studies have indicated that the brain immune system is immunosuppressed rather than activated during the progression of AD which might augment AD pathology. For instance, it seems that microglial cells are hyporesponsive to Aβ and they are not able to phagocytose accumulating Aβ deposits which further disturb the maintenance of homeostasis [5, 6, 145]. There is compelling evidence that the function of microglial cells is impaired in both AD patients and transgenic AD mice, thus exacerbating AD pathology [6, 145, 146]. However, the overexpression of pro-inflammatory cytokines, e.g., IL-1β and TNF-α [147, 148], or the injection of lipopolysaccharide (LPS) [149] significantly reduced the Aβ load in the brains of transgenic AD mice. This implies that the AD-related microglial immunosuppression can be mitigated by increasing pro-inflammatory stimulus. Accordingly, it is known that the exposure of anti-inflammatory cytokines increase the burden of Aβ accumulation in transgenic AD mice [150–152]. It seems that the anti-inflammatory M2 phenotype promotes the resolution of inflammation and improves neuronal survival rather than supports the clearance of Aβ plaques.
The screening of cytokine levels has revealed that the profiles of inflammatory cytokines express both pro-inflammatory and anti-inflammatory modifications in a context-dependent manner, indicating that there are significant changes in the functions of microglia and astrocytes, another source of cytokines, during the progression of AD pathology [153–155]. Different research approaches have indicated that especially two anti-inflammatory cytokines, IL-10 and TGF-β, have a crucial role in the suppression of immune functions in AD brains [150, 156, 157]. Zheng et al. [155] have recently reviewed thoroughly the changes observed in the levels of cytokines in AD patients and transgenic AD mice. However, there are considerable inconsistencies in the results between different human studies, probably indicating that AD pathology contains fluctuating processes in different phases of AD pathogenesis. It seems that the pro- and anti-inflammatory processes are occurring simultaneously, i.e., pro-inflammatory responses take care of Aβ clearance but might impair neuronal homeostasis, whereas anti-inflammatory activity will protect neurons from the detrimental effects of chronic inflammation by inhibiting microglial activity, but, simultaneously, it augments Aβ deposition.
Currently, it is difficult to corroborate the distinct role of infiltrated myeloid and lymphoid cells in the clearance of Aβ deposits or in the control of immunosuppressive functions which is attributable to several puzzling results. There are many studies, indicating that myeloid-derived cells can phagocytose Aβ aggregates and thus reduce Aβ deposition in the brains of AD mice [131, 158, 159]. Prokop et al. [160] demonstrated that when they conditionally depleted the resident microglial population in the brains of AD mice and replaced that by circulating myeloid cells, they observed that the blood-derived myeloid cells were not clustered around Aβ plaques and Aβ pathology was not alleviated in transgenic AD mice. Moreover, Spangenberg et al. [161] revealed that the chronic elimination of microglia in the brains of AD mice rescued dendritic spines and prevented neuronal loss but did not affect the accumulation of Aβ plaques. Varvel et al. [162] also reported that the replacement of microglia with blood monocytes did not reduce Aβ burden, although monocytes gradually clustered around Aβ plaques if the repopulation time was extended up to 6 months. It is known that the brain infiltrating myeloid cells represent a heterogeneous population of cells which are not only able to differentiate in the brain, but they also undergo specific crosstalk with microglia and astrocytes in a context-dependent manner. Given that myeloid cells, e.g., monocytes, are extremely plastic cells, it is difficult to interpret results due to difficulties in characterizing the invaded/injected cells after the experiment. However, the microglia-depletion experiments clearly indicated that infiltrated myeloid cells are immunosuppressive cells which can rescue neurons but are clearly tolerant to Aβ deposits [160–162].
It is known that recruited myeloid cells trigger immunosuppression in brain in many pathological conditions where MDSCs and Tregs are involved in the protection of neurons against excessive inflammation in the context-dependent crosstalk with resident microglia and recruited T cells and monocytes/macrophages. An increased level of Treg cells has been detected in the brain samples of both human MCI and AD patients [97]. In immune defence, Treg cells can suppress effector T cells, but they can also inhibit the function of resident microglia [163, 164]. Xie et al. [164] demonstrated that cerebral Tregs inhibited the LPS-induced expression of pro-inflammatory cytokines in microglia through the secretion of anti-inflammatory IL-10 cytokine. IL-10 is a potent enhancer of Aβ deposition in transgenic AD mouse (see below). Tiemessen et al. [165] demonstrated that human Tregs can induce the M2 polarization of monocytes/macrophages either through cell contacts or via the secretion of anti-inflammatory cytokines. On the other hand, Ebner et al. [166] reported that the distinct type of microglial cells could induce the activation of CD4+FoxP3+ Tregs which subsequently inhibited the activity of effector T cells. Recently, Baruch et al. [98] demonstrated that the transient depletion of FoxP3+ Treg cells or their pharmacological inhibition enhanced the clearance of Aβ plaques and mitigated the cognitive impairment of transgenic 5xFAD mice. They also reported that the systemic increase in the level of Tregs with all-trans retinoic acid augmented the deposition of Aβ plaques and increased cerebral astrogliosis in AD mice. However, Dansokho et al. [167] reported that the early transient depletion of Tregs did not affect the level of Aβ deposition but accelerated the onset of cognitive impairment in transgenic APP/PS1 mice. It seems that differences in mouse AD models as well as the Treg depletion methods and timing in the phase of AD pathology might affect the responses in mice.
There is also an interesting question about how the activation of innate immunity affects the function of Tregs in AD pathology. It is known that TLR ligands, especially those of TLR2, can induce the proliferation of mouse Tregs in a MyD88-dependent signaling, both in vitro and in vivo [168]. The expanded Treg population was functionally intact displaying increased immunosuppressive activity. Interestingly, TLR2 is a primary receptor for Aβ42 oligomeric aggregates in mouse microglia [169]. This means double-edged responses, since TLR2 agonists can trigger inflammation through microglia, whereas, by activating Tregs, they suppress inflammation and support its resolution. There is also clear evidence that TLR agonists can control the differentiation and activation of MDSCs, thus enhancing immunosuppression [170]. In addition, many pro-inflammatory cytokines present in AD brain are potent activators of MDSCs which, consequently, can stimulate Tregs and thus boost immunosuppression (Fig. 2).
Recent immune therapies targeting Aβ peptides have provided a compelling proof-of-concept, indicating that there is immune suppression in AD brains [171]. Unfortunately, human studies have not been as successful as those conducted in transgenic AD mice, which might be attributable to the nature of mouse AD models or the functional differences in immune systems between humans and mice. For instance, there are considerable differences in the phenotypic markers between mouse and human MDSCs [14], although it seems that functional differences are minor. Given that MDSCs and Tregs can inhibit the function of dendritic cells and B lymphocytes, thus, they are able to suppress the production of anti-Aβ antibodies in AD. However, there are surprisingly few studies on the role of dendritic cells and B lymphocytes in the pathogenesis of AD. Butovsky et al. [172] reported that the selective depletion of bone marrow-derived dendritic cells augmented the accumulation of Aβ plaques in the brains of AD mice. There are also findings, indicating that the percentage of myeloid-derived dendritic cells was decreased in the blood of AD patients and the decline was correlated with the severity of AD-related symptoms [173]. These observations might indicate that antigen presentation is impaired in AD. Moreover, Ethell et al. [174] demonstrated that the infusion of Aβ-specific T cells from Aβ-vaccinated mice reduced the level of soluble Aβ in the hippocampus of APP/PS1 mice as well as reversed the memory impairment of these mice. Currently, it is not known whether changes in memory B cells and plasma cells are involved in the pathogenesis of AD. It seems that there is a multifaceted contribution of both innate and adaptive immunity behind the immunosuppression of AD pathology.
Anti-inflammatory cytokines enhance amyloid-β deposition
There is mounting evidence that several anti-inflammatory cytokines, e.g., IL-4, IL-10, and TGF-β, control the accumulation of Aβ into the immunosuppressive microenvironment of AD brain [150–152, 157, 175]. The families of IL-10 and TGF-β cytokines are the key anti-inflammatory cytokines which control not only the functions of immune cells but also affect other cells of inflamed tissues via specific receptors [73, 74]. For instance, IL-10 and TGF-β suppress the function of macrophages/microglia, inhibits effector T cells, and suppress antigen presentation by dendritic cells. The suppression of inflammatory response is a double-edged sword, since, in the case of pathogen infection, e.g., viruses and bacteria, the clearance of a pathogen is blocked, or even can lead to pathogen growth. The main purpose is to stimulate the resolution of detrimental inflammation and protect the host tissue. Interestingly, MDSCs and Tregs are the potent producers of IL-10 and TGF-β, and these anti-inflammatory cytokines are included in their major armaments of immunosuppression (Figs. 2, 3). In the case of tumors, the protection of the host leads to the growth of tumors, whereas, in tissue transplantation, the suppression of inflammation is a vital response protecting the graft.
In transgenic AD mice, the immunohistochemical stainings of IL-10 and TGF-β are strongly increased in glial cells, especially in reactive astroglia surrounding Aβ deposits [176]. Chakrabarty et al. [152] demonstrated that the virally induced IL-10 expression in transgenic AD mice exacerbated the burden of Aβ deposits in the hippocampus and cortex, but it did not affect APP processing. They also reported that IL-10 overexpression decreased the level of synaptic proteins and impaired cognitive capacities. Accordingly, Guillot-Sestier et al. [157] revealed that the genetic depletion of IL-10 in APP/PS1 mice promoted Aβ clearance and mitigated the amyloidosis in AD mice. The reduced Aβ deposition was associated with an activation of innate immunity, e.g., microglial capacity to phagocytose Aβ aggregates was clearly improved. They also reported that the markers of IL-10 signaling were elevated in the post-mortem samples of AD patients, e.g., the expression of IL-10 receptor α (IL-10Rα) was significantly increased in both Western blot and immunohistochemical assays. The levels of phospho-JAK1 and phospho-STAT3 were also upregulated. Park-Min et al. [177] demonstrated that the IL-10 signaling inhibited the transcription of TREM2 gene. TREM2 protein is a trigger protein of phagocytosis, and thus, its inhibition could enhance the accumulation of Aβ plaques.
TGF-β regulates many functions of T lymphocytes, especially it stimulates Tregs via SMAD3 pathway, while, simultaneously, it inhibits Th1 and Th2 cells [73]. TGF-β also suppresses the proliferation of B cells and triggers the apoptosis of immature and resting B cells. Consequently, Tregs as well as MDSCs are potent producers of TGF-β, thus enhancing and maintaining its context-dependent responses in tissues. However, the data on the effects of TGF-β on the functions of macrophage/microglia are inconsistent, although it is known that TGF-β induced anti-inflammatory M2 polarization of macrophages [178] as well as it provoked disturbances in microglial chemotaxis [179]. Lesne et al. [180] demonstrated that TGF-β stimulated the expression of APP in human and murine astrocytes but not in neurons and subsequently increased the production of amyloidogenic Aβ. There are also studies, indicating that TGF-β induced the expression of clusterin [181]. Clusterin (ApoJ) is a versatile chaperone which binds to Aβ peptides and controls their fibrillization, either preventing it or enhancing the aggregation process [182]. Clusterin also acts as an Aβ peptide transporter at the BBB. It is known that the protein level of clusterin was significantly increased in AD brains, and it was abundantly localized in Aβ plaques and amyloid deposits in the cerebrovascular walls in AD patients [182]. Wyss-Coray et al. [150] revealed that the overexpression of TGF-β in transgenic hAPP mice substantially increased Aβ deposition in cerebral blood vessels rather than common parenchymal sites. Several studies have confirmed that increased TGF-β expression, either in normal or transgenic AD mice, induced the development of cerebrovascular amyloidosis and microvascular degeneration. Accordingly, the blockade of the TGF-β signaling alleviated AD pathology in mice [175]. Vascular Aβ deposition may be caused by the TGF-β-induced disturbances in pericytes [123] or its capacity to generate fibrosis [183]. These studies on vascular Aβ deposition indicated that TGF-β might be involved in the development of CAA in AD pathology.
IL-4 is the third anti-inflammatory cytokine which can enhance immunosuppression in the AD brain by affecting the functions of microglia. For instance, IL-4 exposure provoked the alternatively activated M2 phenotype in microglia [151, 184, 185]. Pepe et al. [185] observed that the central administration of IL-4 in mice induced M2a polarization only in one specific subset of brain microglia. Fenn et al. [184] reported that neuroinflammation upregulated the expression of IL-4 receptor α (IL-4Rα) in microglia. This induced the IL-4-mediated reprogramming of microglia, i.e., it robustly increased the expression of ARG1, IL-1β, and CCL2. Subsequently, this unique M2 microglia phenotype stimulated the recruitment of immunosuppressive CCR2+/IL-4Rα+/ARG1+ macrophages into the brain and enhanced neurite outgrowth. Chakrabarty et al. [151] demonstrated that the transient virally mediated hippocampal overexpression of IL-4 in transgenic APP mice induced the M2 phenotype of microglia and clearly exacerbated Aβ deposition, especially Aβ42 peptides, into the hippocampus. In addition to the well-known anti-inflammatory cytokines, i.e., IL-4, IL-10, and TGF-β, there are some other cytokines, e.g., IL-18 and IL-33, which can stimulate immunosuppression in a context-dependent manner. For instance, IL-18 augmented immune suppression in cancer [186]. Accordingly, IL-33 contributed to the sepsis-induced immunosuppression [187]. Interestingly, both IL-18 and IL-33 are able to stimulate the function of MDSCs (Fig. 2).
MDSC hypothesis of AD pathogenesis
Currently, the amyloid hypothesis on the pathogenesis of AD is the most commonly accepted theory, although it does not explain the mechanisms of the accumulation of Aβ plaques and tau tangles [1]. Recent studies have emphasized the role of microglia and immune system in the accumulation of Aβ plaques which could be induced by either increased generation of Aβ peptides or disturbances in their clearance. In 2009, we proposed that Aβ plaques could be masked by sialylated sphingolipids, e.g., gangliosides, which can suppress the function of microglia through the inhibitory siglec receptors, e.g., siglec-3 (CD33) receptors [188]. There is convincing evidence that the variation of CD33 gene is associated with a risk for AD [189]. Moreover, CD33 inhibits Aβ clearance by microglia [99]. Recently, Kaya et al. [190] revealed using MALDI mass spectrometry that Aβ plaques in transgenic AD mice contained several species of gangliosides, e.g., GM2 and GM3, which could activate CD33 receptors through their sialic acid groups. Moreover, Bernardo et al. [191] demonstrated that the elimination of GD3 ganglioside synthase in transgenic APP/PS1 mice robustly reduced the accumulation of Aβ plaques and rescued cognitive impairment. However, this hypothesis has not received adequate experimental confirmation, and currently, it is accepted that the deficiency in Aβ clearance is attributable to the immune suppression of microglia and/or invaded myeloid cells.
As described above, both microglia and infiltrated myeloid cells demonstrate an extensive plasticity in their phenotypes. For instance, invaded monocytes can differentiate to brain macrophages and subsequently represent the M1 or M2 functional phenotypes, as well as resident microglia. Since it is evident that the anti-inflammatory cytokines, secreted by M2 microglia/macrophages, trigger inflammatory resolution but simultaneously can disturb Aβ clearance, it seems likely that the regulation of the M1/M2 balance controls the pathological processes in AD. In fact, there are three subtypes in the M2 group, i.e., M2a, M2b, and M2c, which have very different activation mechanisms and functions in inflammation [2, 75, 192, 193]. Briefly, IL-4 and some infections induce the M2a shift, after which the cells secrete ARG1, TGF-β, and IL-1 receptor antagonist. The M2b subtype is induced by LPS and immune complexes, and this subtype is a mixed type secreting both pro- and anti-inflammatory cytokines. The third M2 subtype, M2c, a so-called acquired deactivation subtype, is induced by IL-10, TGF-β, and glucocorticoids, and subsequently, it is a potent source of IL-10 and TGF-β secretion in inflamed tissues. In AD patients, it seems that the early phases of pathogenesis are associated with increases in M1 and M2a polarization, whereas the later stages of AD display M1, M2a, and M2c markers [194, 195]. It should be emphasized that these results were analysed by gene expression markers and, thus, may also contain invaded macrophages and MDSCs. The key question is how the polarization of these specific M2 subtypes is provoked in the inflamed AD brain. For instance, the switch to M2c requires anti-inflammatory cytokines, but their source in inflamed tissue is unknown. However, it is known that MDSCs are a rich source of IL-10 and TGF-β (Fig. 2), and thus, they could induce the polarization to anti-inflammatory M2 phenotypes. In neurodegenerative diseases, such as AD, where the cause of inflammation has not been removed, MDSCs could maintain immunosuppressive microenvironment, thus minimizing direct injuries to neurons, although, simultaneously, this would challenge the clearance of Aβ deposits and cell debris in the brain (Fig. 3).
In addition to the accumulation of Aβ plaques, the presence of MDSCs in AD brain might also enhance the appearance of many other hallmarks in AD pathology. The function of MDSCs generates immunosuppression through (1) the secretion of anti-inflammatory cytokines, e.g., IL-10 and TGF-β, (2) the induction of the expression of ARG1 and IDO1 which suppress the proliferation of inflammatory cells, and (3) the generation of NO and other ROS compounds (Fig. 2). MDSCs induce the deprivation of arginine and tryptophan in inflamed tissues through the increased secretion of ARG1 and IDO1 enzymes (Fig. 2). An amino acid deficiency stimulates GCN2 kinase which phosphorylates eIF2α factor and thus inhibits protein synthesis [196]. Deprivation not only blocks the proliferation of inflammatory cells, but it might provoke homeostatic disturbances and atrophy in neighbouring neurons (Fig. 3). Arginine metabolism is interesting, since l-arginine is the substrate of NOS and ARG1 enzymes. It is known that M1 macrophages activate NOS, whereas M2 stimulates ARG1 expression and its secretion [197], i.e., both MDSCs and M2 cells are capable to ARG1 secretion. However, recently, Greenhalgh et al. [198] demonstrated that only infiltrated myeloid cells expressed ARG1 but not microglia in autoimmune encephalomyelitis and spinal cord injury. It is known that arginine metabolism is disturbed in the brains of AD patients and this might aggravate the pathogenesis [199]. For instance, experimental arginine deprivation induced immunosuppression and AD-like changes in mice [200]. There are also studies, indicating that the IDO-activated kynurenine pathway, most active in immune cells, was clearly induced in the brains of AD patients and transgenic AD mice [201].
There is mounting evidence that the inflamed microenvironment of AD brain recruits myeloid cells into the brain. Interestingly, the chemokines secreted by AD brain, e.g., CCL2, CXCL2, and CXCL8 (IL-8), are also chemotactic factors for MDSCs (see above). Actually, the same chemokines can also recruit MDSCs into gliomas. For instance, Chang et al. [12] demonstrated that, in glioma milieu, the major source of CCL2 was macrophages and microglia rather than tumor cells which recruited MDSCs and Tregs into the mouse glioma. Given that MDSCs are a heterogeneous population of immature myeloid cells with several marker sets, it is believed that there exist different subgroups of MDSCs, probably related to different diseases [202]. Considering the plasticity of MDSCs, it is likely that MDSCs can adopt distinct properties influenced by microenvironment, thus causing changes in their phenotypes in inflamed tissues after infiltration. There is also a considerable difference between human and mouse markers of MDSCs, e.g., human MDSCs do not express Gr-1 markers [14]. Human monocytic MDSCs are CD14 positive, whereas granulocytic MDSCs express CD15 protein. MDSCs display several myeloid lineage markers, e.g., CD11b+ and CD33+, which appear in human macrophages, microglia, and MDSCs. Bronte et al. [15] have presented the criteria for the characterization of different MDSC subsets for human and mouse MDSCs. Currently, the fluorescence-activated cell sorting (FACS) provides a reliable technique to identify and quantitate different subsets of MDSCs and subsequently to characterize their immunosuppressive capacities. Le Page et al. [96] exploited this technique in their seminal study and observed that the number and percentage of circulating monocytic CD33+HLA-DR− and granulocytic CD33+HLA-DR−CD11b+CD15+ subsets of MDSCs were at a significantly higher level in subjects with amnestic mild cognitive impairment than in AD patients or healthy control individuals. This indicates that MDSCs can be mobilized during the early phase of AD pathogenesis. Moreover, it is known that MDSCs can be maturated and, consequently, proliferate and become activated in inflamed tissues. Unfortunately, the reliable histochemical characterization and quantification of MDSCs in post-mortem AD samples is not possible at this moment.
AD pathology involves an increased expression of many inflammatory mediators known to induce the expansion and activation of MDSCs in inflamed tissues (Fig. 2). For instance, IL-1β and IL-18 cytokines, the products of inflammasome processing, induce the proliferation and activation of MDSCs and generate immunosuppression in inflamed tissues. Currently, there is substantial evidence that inflammasomes are activated in AD pathology [203, 204]. Ojala et al. [205] demonstrated that the expression of IL-18 was significantly increased in microglia, astrocytes, and even in neurons in the brains of AD patients. It is known that IL-18 could promote the differentiation of monocytic MDSCs and thus enhance immunosuppression in tumor milieu [25]. Moreover, PGE2 is also a potent inducer of MDSC accumulation into inflamed tissues (Figs. 2, 3). There are studies, indicating that PGE2 exposure suppressed Aβ clearance in transgenic AD mice [206], and correspondingly, the deletion of EP2 receptor of PGE2 reduced the Aβ burden in the brains of AD mice [207]. Furthermore, COX-2 inhibitors can inhibit the expression of CCL2 [208] or CCR2 [209] and thus could suppress the recruitment of MDSCs into gliomas [12]. The inhibition of MDSC chemotaxis by COX-2 inhibitors might explain why epidemiological studies have revealed the protection of NSAIDs against AD pathology but not clinical trials with AD patients (see above).
There are speculations that AD pathology could be caused by pathogens, especially herpes simplex virus type 1 (HSV1) [210–213] (Fig. 3). Briefly, several experimental studies have revealed that the HSV1 infection of mice induced a typical AD pathology involving chronic inflammation, accumulation of Aβ, and the phosphorylation of neuronal tau protein. In humans, HSV1 DNA is present as a latent form in the brains of elderly people (about 60% of population), mostly in the regions affected by AD. Wozniak et al. [210] demonstrated that HSV1 DNA was present in the plaques of AD patients. In addition, Letenneur et al. [214] revealed a strong correlation between the appearance of antibodies against HSV1 in serum and the incidence of AD dementia in a population-based longitudinal study. These observations suggest that there might exist a chronic HSV1 infection which sporadically reactivates and provokes a progressive AD pathology. Currently, it is not known how HSV1 could evade host immunity and how it could be reactivated in AD brains. Interestingly, several studies have reported that MDSCs suppress antiviral immunity by inhibiting the functions of T cells, antigen presenting cells, and natural killer cells [20]. An expansion of MDSCs has been observed in chronic infections caused by several viruses including hepatitis C virus, human immunodeficiency virus, vesicular stomatitis virus, as well as vaccinia and adenoviruses, which like HSV1 are also DNA viruses [20]. Wang et al. [215] demonstrated that the infection of Japanese encephalitis virus (JEV) expanded the MDSC and Treg populations and IL-10 production in mouse brain. MDSCs suppressed the function of CD4+ T cells, especially the activity of T follicular helper cells was reduced which decreased the levels of splenic B cells and plasma cells and thus impaired the production of neutralizing antibodies against JEVs.
Sporadic hypoxia is another plausible cause which could augment the progression of late-onset AD in association with increased cerebrovascular dysfunctions. Recent neuroimaging studies have revealed that cerebral blood flow is clearly reduced in the brains of AD patients, especially microvascular hypoperfusion is an early event in the evolution of AD pathology [125, 126]. Kisler et al. [128] demonstrated that the degeneration of pericytes in the loss-of-function pericyte-deficient mice reduced capillary blood flow which induced hypoxia and metabolic stress in the brain. Interestingly, Sagare et al. [216] demonstrated that the deficiency of pericytes accelerated amyloid angiopathy and induced Alzheimer-like neurodegeneration in transgenic AD mice. Hypoxia stimulates the activity of β- and γ-secretases which might increase the production and subsequent deposition of Aβ into AD brains, also into the capillary walls [217]. Hypoxia is also a potent inducer of immunosuppression [218] which might inhibit the clearance of Aβ in AD brains. Many studies have demonstrated that hypoxia, especially the activation of HIF-1α signaling, promoted the recruitment of MDSCs into tumor microenvironments where they were activated and differentiated into immunosuppressive macrophages [52]. Hypoxia-inducible MIF, a chemokine-like factor, has a critical role in the recruitment and activation of MDSCs in hypoxic conditions [29, 219]. There are observations that the level of MIF was clearly increased in the cerebrospinal fluid of MCI and AD patients [220]. These studies indicate that the HIF-1α/MIF axis could be an important pathway in the recruitment of MDSCs into AD brains.
Conclusions and future perspectives
The gradual accumulation of Aβ deposits into AD brains concurrently with chronic inflammatory processes has been a paradox in AD research for years. Microglia as well as invading myeloid cells can display a great plasticity in their properties, but how this is connected to the deposition of Aβ has remained unclear. However, recent studies, mostly with transgenic AD mice, have revealed that the accumulation of Aβ is associated with immunosuppressive microenvironment in AD brains. The suppression of immune responses in AD has its benefits in the prevention of neuronal injuries provoked by inflammatory mediators. Immunosuppression is commonly involved in chronic inflammatory disorders in different tissues. Studies on inflamed tumors have revealed that infiltrating MDSCs and Tregs are the major immune cells which can suppress the functions of both innate and adaptive immunity. Currently, it is known that MDSCs are recruited into inflamed tissues to enhance the resolution of acute inflammation and prevent excessive inflammatory responses during chronic inflammatory disorders. There is abundant evidence that AD brains secrete chemokines which are required for the recruitment of MDSCs into the inflamed CNS, e.g., in glioma, multiple sclerosis, and spinal cord injury. Seminal studies have revealed that the number of circulating MDSCs significantly increased in MCI patients, indicating that the maturation and expansion of MDSC populations might occur in AD pathogenesis.
As far as we know, there are no direct studies on the recruitment or accumulation of MDSCs into the brains of AD patients, not even in the brains of transgenic AD mice. In future, isolated myeloid cells from the brains of transgenic AD mice should be identified as well as MDSCs quantified with flow cytometry. For instance, the cell sorting technique has been used in the analysis of immune cells in ischemic mouse brain [221] and MDSCs in metastatic mouse brain [222]. However, transgenic AD mice are “an amyloid plaque model” and do not necessary represent the actual AD in patients. In the brains of AD patients, the analysis of MDSCs will be more difficult, since cell sorting techniques are not suitable for post-mortem brains, e.g., freezing induces a significant loss of granulocytic MDSCs [223]. Moreover, immunohistochemical techniques have not been recommended for the analysis of MDSCs due to the problems in phenotypic characterization of myeloid cells, especially in human samples [15]. However, there is an intensive search for the specific antigens of MDSCs which could be targeted in cancer immunotherapies. For instance, Dominguez et al. [224] presented promising results with the TRAIL-R2 antibody. The lack of specific antibodies for the identification of MDSCs is not the only difficulty, since there are several subtypes of MDSCs, and in addition, the plasticity of MDSCs may disturb the studies on immunosuppressive MDSCs.
Acknowledgements
This study was financially supported by the Grants from the Terveyden Tutkimuksen Toimikunta of Academy of Finland (AK297267, AK307341, and KK296840), the Kuopio University Hospital VTR Grant (KK5503743), the Emil Aaltonen Foundation, the Finnish Cultural Foundation, and the Finnish Eye Foundation. The authors thank Dr. Ewen MacDonald for checking the language of the manuscript.
Abbreviations
- Aβ
Amyloid-β
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- ARG1
Arginase 1
- Breg
Regulatory B cell
- CAA
Cerebral amyloid angiopathy
- C/EBPβ
CCAAT/enhancer-binding protein β
- CHOP
C/EBP-homologous protein
- FOXP3
Forkhead box P3
- GCN2
General control nonderepressible 2 kinase
- HIF-1α
Hypoxia-inducible factor-1α
- HMGB1
High mobility group box 1
- HSV1
Herpes simplex virus type 1
- IDO
Indoleamine-pyrrole 2,3-dioxygenase
- MCI
Mild cognitive impairment
- MDSC
Myeloid-derived suppressor cell
- MIF
Macrophage migration inhibitory factor
- NF-κB
Nuclear factor-κB
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- NOX2
NADPH2 oxidase 2
- NRF2
Nuclear factor-erythroid 2-related factor 2
- NSAID
Non-steroidal anti-inflammatory drug
- PD-L1
Programmed death-ligand 1
- PGE2
Prostaglandin E2
- STAT
Signal transducer and activator of transcription
- TGF-β
Transforming growth factor-β
- TNF-α
Tumor necrosis factor-α
- Treg
Regulatory T cell
- TREM2
Triggering receptor expressed on myeloid cells 2
Compliance with ethical standards
Conflict of interest
The authors state that there are no personal or institutional conflicts of interest.
References
- 1.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-V. [DOI] [PubMed] [Google Scholar]
- 2.Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch Immunol Ther Exp (Warsz) 2012;60:251–266. doi: 10.1007/s00005-012-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131(65–86):138. doi: 10.1016/j.pneurobio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monsonego A, Maron R, Zota V, Selkoe DJ, Weiner HL. Immune hyporesponsiveness to amyloid β-peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:10273–10278. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melero-Jerez C, Ortega MC, Moline-Velazquez V, Clemente D. Myeloid derived suppressor cells in inflammatory conditions of the central nervous system. Biochim Biophys Acta. 2016;1862:368–380. doi: 10.1016/j.bbadis.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Draghiciu O, Lubbers J, Nijman HW, Daemen T. Myeloid derived suppressor cells—an overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. 2015;4:e954829. doi: 10.4161/21624011.2014.954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millrud CR, Bergenfelz C, Leandersson K. On the origin of myeloid-derived suppressor cells. Oncotarget. 2017;8:3649–3665. doi: 10.18632/oncotarget.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang AL, Miska J, Wainwright DA, et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016;76:5671–5682. doi: 10.1158/0008-5472.CAN-16-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Wu T, Shao S, Shi B, Zhao Y. Phenotype, development, and biological function of myeloid-derived suppressor cells. Oncoimmunology. 2015;5:e1004983. doi: 10.1080/2162402X.2015.1004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuenca AG, Delano MJ, Kelly-Scumpia KM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med. 2011;17:281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 18.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 19.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh C, Narayanan S, Hahn YS. Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunol Rev. 2013;255:210–221. doi: 10.1111/imr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammerich L, Tacke F. Emerging roles of myeloid derived suppressor cells in hepatic inflammation and fibrosis. World J Gastrointest Pathophysiol. 2015;6:43–50. doi: 10.4291/wjgp.v6.i3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saiwai H, Kumamaru H, Ohkawa Y, et al. Ly6C+ Ly6G− myeloid-derived suppressor cells play a critical role in the resolution of acute inflammation and the subsequent tissue repair process after spinal cord injury. J Neurochem. 2013;125:74–88. doi: 10.1111/jnc.12135. [DOI] [PubMed] [Google Scholar]
- 23.Fullerton JN, O’Brien AJ, Gilroy DW. Pathways mediating resolution of inflammation: when enough is too much. J Pathol. 2013;231:8–20. doi: 10.1002/path.4232. [DOI] [PubMed] [Google Scholar]
- 24.Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin-1β induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim HX, Hong HJ, Cho D, Kim TS. IL-18 enhances immunosuppressive responses by promoting differentiation into monocytic myeloid-derived suppressor cells. J Immunol. 2014;193:5453–5460. doi: 10.4049/jimmunol.1401282. [DOI] [PubMed] [Google Scholar]
- 26.Cheng L, Wang J, Li X, Xing Q, Du P, Su L, Wang S. Interleukin-6 induces Gr-1+CD11b+ myeloid cells to suppress CD8+ T cell-mediated liver injury in mice. PLoS One. 2011;6:e17631. doi: 10.1371/journal.pone.0017631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao P, Wan X, Cui B, et al. Interleukin 33 in tumor microenvironment is crucial for the accumulation and function of myeloid-derived suppressor cells. Oncoimmunology. 2015;5:e1063772. doi: 10.1080/2162402X.2015.1063772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sade-Feldman M, Kanterman J, Ish-Shalom E, Elnekave M, Horwitz E, Baniyash M. Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity. 2013;38:541–554. doi: 10.1016/j.immuni.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Simpson KD, Templeton DJ, Cross JV. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J Immunol. 2012;189:5533–5540. doi: 10.4049/jimmunol.1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh CC, Chou HS, Yang HR, Lin F, Bhatt S, Qin J, Wang L, Fung JJ, Qian S, Lu L. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood. 2013;121:1760–1768. doi: 10.1182/blood-2012-06-440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker KH, Sinha P, Horn LA, Clements VK, Yang H, Li J, Tracey KJ, Ostrand-Rosenberg S. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014;74:5723–5733. doi: 10.1158/0008-5472.CAN-13-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Sun J, Rong R, Li L, Shang W, Song D, Feng G, Luo F. HMGB1 promotes myeloid-derived suppressor cells and renal cell carcinoma immune escape. Oncotarget. 2017;8:63290–63298. doi: 10.18632/oncotarget.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan X, Darwiche SS, Cai C, Scott MJ, Pape HC, Billiar TR. Anti-HMGB1 monoclonal antibody ameliorates immunosuppression after peripheral tissue trauma: attenuated T-lymphocyte response and increased splenic CD11b+ Gr-1+ myeloid-derived suppressor cells require HMGB1. Mediat Inflamm. 2015;2015:458626. doi: 10.1155/2015/458626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simard JC, Cesaro A, Chapeton-Montes J, Tardif M, Antoine F, Girard D, Tessier PA. S100A8 and S100A9 induce cytokine expression and regulate the NLRP3 inflammasome via ROS-dependent activation of NF-κB. PLoS One. 2013;8:e72138. doi: 10.1371/journal.pone.0072138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aoki T, Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol Sci. 2012;33:304–311. doi: 10.1016/j.tips.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 40.Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P. PGE2-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Investig. 2012;41:635–657. doi: 10.3109/08820139.2012.695417. [DOI] [PubMed] [Google Scholar]
- 41.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118:5498–5505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ko HJ, Kim YJ. Signal transducer and activator of transcription proteins: regulators of myeloid-derived suppressor cell-mediated immunosuppression in cancer. Arch Pharm Res. 2016;39:1597–1608. doi: 10.1007/s12272-016-0822-9. [DOI] [PubMed] [Google Scholar]
- 44.Rebe C, Vegran F, Berger H, Ghiringhelli F. STAT3 activation: a key factor in tumor immunoescape. JAKSTAT. 2013;2:e23010. doi: 10.4161/jkst.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasquez-Dunddel D, Pan F, Zeng Q, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Investig. 2013;123:1580–1589. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu J, Wang Y, Yan F, Zhang P, Li H, Zhao H, Yan C, Yan F, Ren X. Noncanonical NF-κB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer. J Immunol. 2014;193:2574–2586. doi: 10.4049/jimmunol.1400833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vallejo JG. Role of toll-like receptors in cardiovascular diseases. Clin Sci (Lond) 2011;121:1–10. doi: 10.1042/CS20100539. [DOI] [PubMed] [Google Scholar]
- 48.Kaltschmidt B, Kaltschmidt C. NF-κB in the nervous system. Cold Spring Harb Perspect Biol. 2009;1:a001271. doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corzo CA, Condamine T, Lu L, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marigo I, Bosio E, Solito S, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 56.McPeak MB, Youssef D, Williams DA, Pritchett CL, Yao ZQ, McCall CE, El Gazzar M. Myeloid cell-specific deletion of Cebpb decreases sepsis-induced immunosuppression in mice. J Leukoc Biol. 2017;102:191–200. doi: 10.1189/jlb.4HI1216-537R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu KK, Liou JY, Cieslik K. Transcriptional control of COX-2 via C/EBPβ. Arterioscler Thromb Vasc Biol. 2005;25:679–685. doi: 10.1161/01.ATV.0000157899.35660.61. [DOI] [PubMed] [Google Scholar]
- 58.Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, Ochoa AC, Cui Y, Del Valle L, Rodriguez PC. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41:389–401. doi: 10.1016/j.immuni.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beury DW, Carter KA, Nelson C, Sinha P, Hanson E, Nyandjo M, Fitzgerald PJ, Majeed A, Wali N, Ostrand-Rosenberg S. Myeloid-derived suppressor cell survival and function are regulated by the transcription factor Nrf2. J Immunol. 2016;196:3470–3478. doi: 10.4049/jimmunol.1501785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Wang B, Zhang W, Wei Y, Bian Z, Zhang CY, Li L, Zen K. Protein tyrosine phosphatase 1B deficiency ameliorates murine experimental colitis via the expansion of myeloid-derived suppressor cells. PLoS One. 2013;8:e70828. doi: 10.1371/journal.pone.0070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Gazzar M. microRNAs as potential regulators of myeloid-derived suppressor cell expansion. Innate Immun. 2014;20:227–238. doi: 10.1177/1753425913489850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar V, Cheng P, Condamine T, et al. CD45 phosphatase inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity. 2016;44:303–315. doi: 10.1016/j.immuni.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang A, Zhang H, Chen S, Xia F, Yang Y, Dong F, Sun D, Xiong S, Zhang J. miR-34a expands myeloid-derived suppressor cells via apoptosis inhibition. Exp Cell Res. 2014;326:259–266. doi: 10.1016/j.yexcr.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 64.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–281. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. l-Arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–306. doi: 10.1016/S1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 70.Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–651. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 72.Crook KR, Jin M, Weeks MF, et al. Myeloid-derived suppressor cells regulate T cell and B cell responses during autoimmune disease. J Leukoc Biol. 2015;97:573–582. doi: 10.1189/jlb.4A0314-139R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 74.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 75.Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. 2015;34:22–27. doi: 10.1016/j.coi.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park MJ, Lee SH, Kim EK, Lee EJ, Park SH, Kwok SK, Cho ML. Myeloid-derived suppressor cells induce the expansion of regulatory B cells and ameliorate autoimmunity in the Sanroque mouse model of systemic lupus erythematosus. Arthritis Rheumatol. 2016;68:2717–2727. doi: 10.1002/art.39767. [DOI] [PubMed] [Google Scholar]
- 78.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 79.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu C, Redd PS, Lee JR, Savage N, Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology. 2016;5:e1247135. doi: 10.1080/2162402X.2016.1247135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol. 2017;17:703–717. doi: 10.1038/nri.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 83.Wurdinger T, Deumelandt K, van der Vliet HJ, Wesseling P, de Gruijl TD. Mechanisms of intimate and long-distance cross-talk between glioma and myeloid cells: how to break a vicious cycle. Biochim Biophys Acta. 2014;1846:560–575. doi: 10.1016/j.bbcan.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, Sawaya R, Heimberger AB. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ioannou M, Alissafi T, Lazaridis I, et al. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol. 2012;188:1136–1146. doi: 10.4049/jimmunol.1101816. [DOI] [PubMed] [Google Scholar]
- 87.Moline-Velazquez V, Vila-Del Sol V, de Castro F, Clemente D. Myeloid cell distribution and activity in multiple sclerosis. Histol Histopathol. 2016;31:357–370. doi: 10.14670/HH-11-699. [DOI] [PubMed] [Google Scholar]
- 88.Danikowski KM, Jayaraman S, Prabhakar BS. Regulatory T cells in multiple sclerosis and myasthenia gravis. J Neuroinflamm. 2017;14:117. doi: 10.1186/s12974-017-0892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bowen JL, Olson JK. Innate immune CD11b+Gr-1+ cells, suppressor cells, affect the immune response during Theiler’s virus-induced demyelinating disease. J Immunol. 2009;183:6971–6980. doi: 10.4049/jimmunol.0902193. [DOI] [PubMed] [Google Scholar]
- 90.Hazeldine J, Lord JM, Belli A. Traumatic brain injury and peripheral immune suppression: primer and prospectus. Front Neurol. 2015;6:235. doi: 10.3389/fneur.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liesz A, Dalpke A, Mracsko E, et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci. 2015;35:583–598. doi: 10.1523/JNEUROSCI.2439-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Y, Wei J, Guo G, Zhou J. Norepinephrine-induced myeloid-derived suppressor cells block T-cell responses via generation of reactive oxygen species. Immunopharmacol Immunotoxicol. 2015;37:359–365. doi: 10.3109/08923973.2015.1059442. [DOI] [PubMed] [Google Scholar]
- 93.Vaknin I, Kunis G, Miller O, Butovsky O, Bukshpan S, Beers DR, Henkel JS, Yoles E, Appel SH, Schwartz M. Excess circulating alternatively activated myeloid (M2) cells accelerate ALS progression while inhibiting experimental autoimmune encephalomyelitis. PLoS One. 2011;6:e26921. doi: 10.1371/journal.pone.0026921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen S, Liu Y, Niu Y, Xu Y, Zhou Q, Xu X, Wang J, Yu M. Increased abundance of myeloid-derived suppressor cells and Th17 cells in peripheral blood of newly-diagnosed Parkinson’s disease patients. Neurosci Lett. 2017;648:21–25. doi: 10.1016/j.neulet.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 95.Henkel JS, Beers DR, Wen S, Rivera AL, Toennis KM, Appel JE, Zhao W, Moore DH, Powell SZ, Appel SH. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med. 2013;5:64–79. doi: 10.1002/emmm.201201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Le Page A, Garneau H, Dupuis G, Frost EH, Larbi A, Witkowski JM, Pawelec G, Fülöp T. Differential phenotypes of myeloid-derived suppressor and T regulatory cells and cytokine levels in amnestic mild cognitive impairment subjects compared to mild Alzheimer diseased patients. Front Immunol. 2017;8:783. doi: 10.3389/fimmu.2017.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saresella M, Calabrese E, Marventano I, Piancone F, Gatti A, Calvo MG, Nemni R, Clerici M. PD1 negative and PD1 positive CD4+ T regulatory cells in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2010;21:927–938. doi: 10.3233/JAD-2010-091696. [DOI] [PubMed] [Google Scholar]
- 98.Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, Tsitsou-Kampeli A, Sarel A, Cahalon L, Schwartz M. Breaking immune tolerance by targeting Foxp3+ regulatory T cells mitigates Alzheimer’s disease pathology. Nat Commun. 2015;6:7967. doi: 10.1038/ncomms8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid β. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ulrich JD, Ulland TK, Colonna M, Holtzman DM. Elucidating the role of TREM2 in Alzheimer’s disease. Neuron. 2017;94:237–248. doi: 10.1016/j.neuron.2017.02.042. [DOI] [PubMed] [Google Scholar]
- 101.Huang KL, Marcora E, Pimenova AA, et al. A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nat Neurosci. 2017;20:1052–1061. doi: 10.1038/nn.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fahrenhold M, Rakic S, Classey J, Brayne C, Ince PG, Nicoll JAR, Boche D. TREM2 expression in the human brain: a marker of monocyte recruitment? Brain Pathol. 2017 doi: 10.1111/bpa.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ginhoux F, Prinz M. Origin of microglia: current concepts and past controversies. Cold Spring Harb Perspect Biol. 2015;7:a020537. doi: 10.1101/cshperspect.a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- 106.Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer’s disease: amyloid-β oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87:181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 107.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 108.Gertig U, Hanisch UK. Microglial diversity by responses and responders. Front Cell Neurosci. 2014;8:101. doi: 10.3389/fncel.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 110.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 112.Weitz TM, Town T. Microglia in Alzheimer’s disease: it’s all about context. Int J Alzheimers Dis. 2012;2012:314185. doi: 10.1155/2012/314185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 114.Eggen BJ, Raj D, Hanisch UK, Boddeke HW. Microglial phenotype and adaptation. J Neuroimmune Pharmacol. 2013;8:807–823. doi: 10.1007/s11481-013-9490-4. [DOI] [PubMed] [Google Scholar]
- 115.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Investig. 2010;120:1368–1379. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Herz J, Filiano AJ, Smith A, Yogev N, Kipnis J. Myeloid cells in the central nervous system. Immunity. 2017;46:943–956. doi: 10.1016/j.immuni.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kondo M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol Rev. 2010;238:37–46. doi: 10.1111/j.1600-065X.2010.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sallusto F, Impellizzieri D, Basso C, Laroni A, Uccelli A, Lanzavecchia A, Engelhardt B. T-cell trafficking in the central nervous system. Immunol Rev. 2012;248:216–227. doi: 10.1111/j.1600-065X.2012.01140.x. [DOI] [PubMed] [Google Scholar]
- 119.Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, Lyck R, Schwaninger M, Engelhardt B, de Vries HE. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta. 2016;1862:461–471. doi: 10.1016/j.bbadis.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 120.Meeker RB, Williams K, Killebrew DA, Hudson LC. Cell trafficking through the choroid plexus. Cell Adhes Migr. 2012;6:390–396. doi: 10.4161/cam.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stark K, Eckart A, Haidari S, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 122.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rustenhoven J, Aalderink M, Scotter EL, Oldfield RL, Bergin PS, Mee EW, Graham ES, Faull RL, Curtis MA, Park TI, Dragunow M. TGF-β1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflamm. 2016;13:37. doi: 10.1186/s12974-016-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jeynes B, Provias J. The possible role of capillary cerebral amyloid angiopathy in Alzheimer lesion development: a regional comparison. Acta Neuropathol. 2006;112:417–427. doi: 10.1007/s00401-006-0099-z. [DOI] [PubMed] [Google Scholar]
- 125.Montagne A, Nation DA, Pa J, Sweeney MD, Toga AW, Zlokovic BV. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol. 2016;131:687–707. doi: 10.1007/s00401-016-1570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nielsen RB, Egefjord L, Angleys H, et al. Capillary dysfunction is associated with symptom severity and neurodegeneration in Alzheimer’s disease. Alzheimers Dement. 2017;13:1143–1153. doi: 10.1016/j.jalz.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 127.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kisler K, Nelson AR, Rege SV, Ramanathan A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, Boas DA, Sakadzic S, Zlokovic BV. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci. 2017;20:406–416. doi: 10.1038/nn.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Montagne A, Zhao Z, Zlokovic BV. Alzheimer’s disease: a matter of blood–brain barrier dysfunction? J Exp Med. 2017;214:3151–3169. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 132.Saresella M, Marventano I, Calabrese E, Piancone F, Rainone V, Gatti A, Alberoni M, Nemni R, Clerici M. A complex proinflammatory role for peripheral monocytes in Alzheimer’s disease. J Alzheimers Dis. 2014;38:403–413. doi: 10.3233/JAD-131160. [DOI] [PubMed] [Google Scholar]
- 133.Bryson KJ, Lynch MA. Linking T cells to Alzheimer’s disease: from neurodegeneration to neurorepair. Curr Opin Pharmacol. 2016;26:67–73. doi: 10.1016/j.coph.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 134.Gonzalez H, Pacheco R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J Neuroinflamm. 2014;11:201. doi: 10.1186/s12974-014-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reale M, Iarlori C, Feliciani C, Gambi D. Peripheral chemokine receptors, their ligands, cytokines and Alzheimer’s disease. J Alzheimers Dis. 2008;14:147–159. doi: 10.3233/JAD-2008-14203. [DOI] [PubMed] [Google Scholar]
- 136.Azizi G, Khannazer N, Mirshafiey A. The potential role of chemokines in Alzheimer’s disease pathogenesis. Am J Alzheimers Dis Other Dement. 2014;29:415–425. doi: 10.1177/1533317513518651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010;224:93–100. doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 138.Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corra B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol Aging. 2006;27:1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 139.Selenica ML, Alvarez JA, Nash KR, Lee DC, Cao C, Lin X, Reid P, Mouton PR, Morgan D, Gordon MN. Diverse activation of microglia by chemokine (C–C motif) ligand 2 overexpression in brain. J Neuroinflamm. 2013;10:86. doi: 10.1186/1742-2094-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu YJ, Guo DW, Tian L, Shang DS, Zhao WD, Li B, Fang WG, Zhu L, Chen YH. Peripheral T cells derived from Alzheimer’s disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-α-dependent. Neurobiol Aging. 2010;31:175–188. doi: 10.1016/j.neurobiolaging.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 141.Miguel-Alvarez M, Santos-Lozano A, Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Garatachea N, Lucia A. Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer’s disease: a systematic review and meta-analysis of treatment effect. Drugs Aging. 2015;32:139–147. doi: 10.1007/s40266-015-0239-z. [DOI] [PubMed] [Google Scholar]
- 142.Cuello AC. Early and late CNS inflammation in Alzheimer’s disease: two extremes of a continuum? Trends Pharmacol Sci. 2017;38:956–966. doi: 10.1016/j.tips.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 143.Ferretti MT, Bruno MA, Ducatenzeiler A, Klein WL, Cuello AC. Intracellular Aβ-oligomers and early inflammation in a model of Alzheimer’s disease. Neurobiol Aging. 2012;33:1329–1342. doi: 10.1016/j.neurobiolaging.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 144.Hanzel CE, Pichet-Binette A, Pimentel LS, Iulita MF, Allard Ducatenzeiler A, Do Carmo S, Cuello AC. Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer’s disease. Neurobiol Aging. 2014;35:2249–2262. doi: 10.1016/j.neurobiolaging.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 145.Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U, Miller KR, Prokop S, Kettenmann H, Heppner FL. Functional impairment of microglia coincides with β-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8:e60921. doi: 10.1371/journal.pone.0060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baron R, Babcock AA, Nemirovsky A, Finsen B, Monsonego A. Accelerated microglial pathology is associated with Aβ plaques in mouse models of Alzheimer’s disease. Aging Cell. 2014;13:584–595. doi: 10.1111/acel.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O’Banion MK. Sustained hippocampal IL-1β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Investig. 2007;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chakrabarty P, Herring A, Ceballos-Diaz C, Das P, Golde TE. Hippocampal expression of murine TNFα results in attenuation of amyloid deposition in vivo. Mol Neurodegener. 2011;6:16. doi: 10.1186/1750-1326-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.DiCarlo G, Wilcock D, Henderson D, Gordon M, Morgan D. Intrahippocampal LPS injections reduce Aβ load in APP + PS1 transgenic mice. Neurobiol Aging. 2001;22:1007–1012. doi: 10.1016/S0197-4580(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 150.Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, Mucke L. Amyloidogenic role of cytokine TGF-β1 in transgenic mice and in Alzheimer’s disease. Nature. 1997;389:603–606. doi: 10.1038/39321. [DOI] [PubMed] [Google Scholar]
- 151.Chakrabarty P, Tianbai L, Herring A, Ceballos-Diaz C, Das P, Golde TE. Hippocampal expression of murine IL-4 results in exacerbation of amyloid deposition. Mol Neurodegener. 2012;7:36. doi: 10.1186/1750-1326-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chakrabarty P, Li A, Ceballos-Diaz C, et al. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron. 2015;85:519–533. doi: 10.1016/j.neuron.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Azizi G, Mirshafiey A. The potential role of proinflammatory and antiinflammatory cytokines in Alzheimer disease pathogenesis. Immunopharmacol Immunotoxicol. 2012;34:881–895. doi: 10.3109/08923973.2012.705292. [DOI] [PubMed] [Google Scholar]
- 154.Goldeck D, Witkowski JM, Fülop T, Pawelec G. Peripheral immune signatures in Alzheimer disease. Curr Alzheimer Res. 2016;13:739–749. doi: 10.2174/1567205013666160222112444. [DOI] [PubMed] [Google Scholar]
- 155.Zheng C, Zhou XW, Wang JZ. The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl Neurodegener. 2016;5:7. doi: 10.1186/s40035-016-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ongali B, Nicolakakis N, Lecrux C, Aboulkassim T, Rosa-Neto P, Papadopoulos P, Tong XK, Hamel E. Transgenic mice overexpressing APP and transforming growth factor-β1 feature cognitive and vascular hallmarks of Alzheimer’s disease. Am J Pathol. 2010;177:3071–3080. doi: 10.2353/ajpath.2010.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guillot-Sestier MV, Doty KR, Gate D, Rodriguez J, Jr, Leung BP, Rezai-Zadeh K, Town T. IL10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015;85:534–548. doi: 10.1016/j.neuron.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Malm TM, Koistinaho M, Pärepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to β-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 159.Hohsfield LA, Humpel C. Migration of blood cells to β-amyloid plaques in Alzheimer’s disease. Exp Gerontol. 2015;65:8–15. doi: 10.1016/j.exger.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Prokop S, Miller KR, Drost N, Handrick S, Mathur V, Luo J, Wegner A, Wyss-Coray T, Heppner FL. Impact of peripheral myeloid cells on amyloid-β pathology in Alzheimer’s disease-like mice. J Exp Med. 2015;212:1811–1818. doi: 10.1084/jem.20150479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Spangenberg EE, Lee RJ, Najafi AR, Rice RA, Elmore MR, Blurton-Jones M, West BL, Green KN. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β pathology. Brain. 2016;139:1265–1281. doi: 10.1093/brain/aww016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Varvel NH, Grathwohl SA, Degenhardt K, Resch C, Bosch A, Jucker M, Neher JJ. Replacement of brain-resident myeloid cells does not alter cerebral amyloid-β deposition in mouse models of Alzheimer’s disease. J Exp Med. 2015;212:1803–1809. doi: 10.1084/jem.20150478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yang Z, Yu A, Liu Y, Shen H, Lin C, Lin L, Wang S, Yuan B. Regulatory T cells inhibit microglia activation and protect against inflammatory injury in intracerebral hemorrhage. Int Immunopharmacol. 2014;22:522–525. doi: 10.1016/j.intimp.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 164.Xie L, Choudhury GR, Winters A, Yang SH, Jin K. Cerebral regulatory T cells restrain microglia/macrophage-mediated inflammatory responses via IL-10. Eur J Immunol. 2015;45:180–191. doi: 10.1002/eji.201444823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ebner F, Brandt C, Thiele P, Richter D, Schliesser U, Siffrin V, Schueler J, Stubbe T, Ellinghaus A, Meisel C, Sawitzki B, Nitsch R. Microglial activation milieu controls regulatory T cell responses. J Immunol. 2013;191:5594–5602. doi: 10.4049/jimmunol.1203331. [DOI] [PubMed] [Google Scholar]
- 167.Dansokho C, Ait Ahmed D, Aid S, et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain. 2016;139:1237–1251. doi: 10.1093/brain/awv408. [DOI] [PubMed] [Google Scholar]
- 168.Sutmuller RP, den Brok MH, Kramer M, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Investig. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu S, Liu Y, Hao W, et al. TLR2 is a primary receptor for Alzheimer’s amyloid β peptide to trigger neuroinflammatory activation. J Immunol. 2012;188:1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 170.Wang J, Shirota Y, Bayik D, Shirota H, Tross D, Gulley JL, Wood LV, Berzofsky JA, Klinman DM. Effect of TLR agonists on the differentiation and function of human monocytic myeloid-derived suppressor cells. J Immunol. 2015;194:4215–4221. doi: 10.4049/jimmunol.1402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wisniewski T, Goni F. Immunotherapeutic approaches for Alzheimer’s disease. Neuron. 2015;85:1162–1176. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Butovsky O, Kunis G, Koronyo-Hamaoui M, Schwartz M. Selective ablation of bone marrow-derived dendritic cells increases amyloid plaques in a mouse Alzheimer’s disease model. Eur J Neurosci. 2007;26:413–416. doi: 10.1111/j.1460-9568.2007.05652.x. [DOI] [PubMed] [Google Scholar]
- 173.Ciaramella A, Salani F, Bizzoni F, Orfei MD, Caltagirone C, Spalletta G, Bossu P. Myeloid dendritic cells are decreased in peripheral blood of Alzheimer’s disease patients in association with disease progression and severity of depressive symptoms. J Neuroinflamm. 2016;13:18. doi: 10.1186/s12974-016-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ethell DW, Shippy D, Cao C, Cracchiolo JR, Runfeldt M, Blake B, Arendash GW. Aβ-specific T-cells reverse cognitive decline and synaptic loss in Alzheimer’s mice. Neurobiol Dis. 2006;23:351–361. doi: 10.1016/j.nbd.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 175.Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA. Blocking TGF-β-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Apelt J, Schliebs R. β-Amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894:21–30. doi: 10.1016/S0006-8993(00)03176-0. [DOI] [PubMed] [Google Scholar]
- 177.Park-Min KH, Ji JD, Antoniv T, Reid AC, Silver RB, Humphrey MB, Nakamura M, Ivashkiv LB. IL-10 suppresses calcium-mediated costimulation of receptor activator NF-κB signaling during human osteoclast differentiation by inhibiting TREM-2 expression. J Immunol. 2009;183:2444–2455. doi: 10.4049/jimmunol.0804165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gong D, Shi W, Yi SJ, Chen H, Groffen J, Heisterkamp N. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13:31. doi: 10.1186/1471-2172-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huang WC, Yen FC, Shie FS, Pan CM, Shiao YJ, Yang CN, Huang FL, Sung YJ, Tsay HJ. TGF-β1 blockade of microglial chemotaxis toward Aβ aggregates involves SMAD signaling and down-regulation of CCL5. J Neuroinflamm. 2010;7:28. doi: 10.1186/1742-2094-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lesne S, Docagne F, Gabriel C, et al. Transforming growth factor-β1 potentiates amyloid-β generation in astrocytes and in transgenic mice. J Biol Chem. 2003;278:18408–18418. doi: 10.1074/jbc.M300819200. [DOI] [PubMed] [Google Scholar]
- 181.Jin G, Howe PH. Regulation of clusterin gene expression by transforming growth factor β. J Biol Chem. 1997;272:26620–26626. doi: 10.1074/jbc.272.42.26620. [DOI] [PubMed] [Google Scholar]
- 182.Nuutinen T, Suuronen T, Kauppinen A, Salminen A. Clusterin: a forgotten player in Alzheimer’s disease. Brain Res Rev. 2009;61:89–104. doi: 10.1016/j.brainresrev.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 183.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 184.Fenn AM, Hall JC, Gensel JC, Popovich PG, Godbout JP. IL-4 signaling drives a unique arginase+/IL-1β+ microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Rα after traumatic spinal cord injury. J Neurosci. 2014;34:8904–8917. doi: 10.1523/JNEUROSCI.1146-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pepe G, Calderazzi G, De Maglie M, Villa AM, Vegeto E. Heterogeneous induction of microglia M2a phenotype by central administration of interleukin-4. J Neuroinflamm. 2014;11:211. doi: 10.1186/s12974-014-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Terme M, Ullrich E, Aymeric L, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71:5393–5399. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 187.Nascimento DC, Melo PH, Pineros AR, et al. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat Commun. 2017;8:14919. doi: 10.1038/ncomms14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Salminen A, Kaarniranta K. Siglec receptors and hiding plaques in Alzheimer’s disease. J Mol Med (Berl) 2009;87:697–701. doi: 10.1007/s00109-009-0472-1. [DOI] [PubMed] [Google Scholar]
- 189.Hollingworth P, Harold D, Sims R, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kaya I, Brinet D, Michno W, Syvänen S, Sehlin D, Zetterberg H, Blennow K, Hanrieder J. Delineating amyloid plaque associated neuronal sphingolipids in transgenic Alzheimer’s disease mice (tgArcSwe) using MALDI imaging mass spectrometry. ACS Chem Neurosci. 2017;8:347–355. doi: 10.1021/acschemneuro.6b00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bernardo A, Harrison FE, McCord M, et al. Elimination of GD3 synthase improves memory and reduces amyloid-β plaque load in transgenic mice. Neurobiol Aging. 2009;30:1777–1791. doi: 10.1016/j.neurobiolaging.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 192.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 193.Walker DG, Lue LF. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther. 2015;7:56. doi: 10.1186/s13195-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sudduth TL, Schmitt FA, Nelson PT, Wilcock DM. Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiol Aging. 2013;34:1051–1059. doi: 10.1016/j.neurobiolaging.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wilcock DM, Hurban J, Helman AM, Sudduth TL, McCarty KL, Beckett TL, Ferrell JC, Murphy MP, Abner EL, Schmitt FA, Head E. Down syndrome individuals with Alzheimer’s disease have a distinct neuroinflammatory phenotype compared to sporadic Alzheimer’s disease. Neurobiol Aging. 2015;36:2468–2474. doi: 10.1016/j.neurobiolaging.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Castilho BA, Shanmugam R, Silva RC, Ramesh R, Himme BM, Sattlegger E. Keeping the eIF2α kinase Gcn2 in check. Biochim Biophys Acta. 2014;1843:1948–1968. doi: 10.1016/j.bbamcr.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 197.Rath M, Müller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Greenhalgh AD, Passos Dos Santos R, Zarruk JG, Salmon CK, Kroner A, David S. Arginase-1 is expressed exclusively by infiltrating myeloid cells in CNS injury and disease. Brain Behav Immun. 2016;56:61–67. doi: 10.1016/j.bbi.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 199.Liu P, Fleete MS, Jing Y, Collie ND, Curtis MA, Waldvogel HJ, Faull RL, Abraham WC, Zhang H. Altered arginine metabolism in Alzheimer’s disease brains. Neurobiol Aging. 2014;35:1992–2003. doi: 10.1016/j.neurobiolaging.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 200.Kan MJ, Lee JE, Wilson JG, Everhart AL, Brown CM, Hoofnagle AN, Jansen M, Vitek MP, Gunn MD, Colton CA. Arginine deprivation and immune suppression in a mouse model of Alzheimer’s disease. J Neurosci. 2015;35:5969–5982. doi: 10.1523/JNEUROSCI.4668-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wu W, Nicolazzo JA, Wen L, Chung R, Stankovic R, Bao SS, Lim CK, Brew BJ, Cullen KM, Guillemin GJ. Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS One. 2013;8:e59749. doi: 10.1371/journal.pone.0059749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Damuzzo V, Pinton L, Desantis G, Solito S, Marigo I, Bronte V, Mandruzzato S. Complexity and challenges in defining myeloid-derived suppressor cells. Cytom B Clin Cytom. 2015;88:77–91. doi: 10.1002/cytob.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Saresella M, La Rosa F, Piancone F, Zoppis M, Marventano I, Calabrese E, Rainone V, Nemni R, Mancuso R, Clerici M. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol Neurodegener. 2016;11:23. doi: 10.1186/s13024-016-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ojala J, Alafuzoff I, Herukka SK, van Groen T, Tanila H, Pirttilä T. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol Aging. 2009;30:198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 206.Johansson JU, Woodling NS, Wang Q, Panchal M, Liang X, Trueba-Saiz A, Brown HD, Mhatre SD, Loui T, Andreasson KI. Prostaglandin signaling suppresses beneficial microglial function in Alzheimer’s disease models. J Clin Investig. 2015;125:350–364. doi: 10.1172/JCI77487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liang X, Wang Q, Hand T, Wu L, Breyer RM, Montine TJ, Andreasson K. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J Neurosci. 2005;25:10180–10187. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kaur J, Sanyal SN. Diclofenac, a selective COX-2 inhibitor, inhibits DMH-induced colon tumorigenesis through suppression of MCP-1, MIP-1α and VEGF. Mol Carcinog. 2011;50:707–718. doi: 10.1002/mc.20736. [DOI] [PubMed] [Google Scholar]
- 209.Futagami S, Hamamoto T, Shimpuku M, Nagoya H, Kawagoe T, Horie A, Shindo T, Gudis K, Sakamoto C. Celecoxib inhibits CD133-positive cell migration via reduction of CCR2 in Helicobacter pylori-infected Mongolian gerbils. Digestion. 2010;81:193–203. doi: 10.1159/000252790. [DOI] [PubMed] [Google Scholar]
- 210.Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. J Pathol. 2009;217:131–138. doi: 10.1002/path.2449. [DOI] [PubMed] [Google Scholar]
- 211.Agostini S, Clerici M, Mancuso R. How plausible is a link between HSV-1 infection and Alzheimer’s disease? Expert Rev Antiinfect Ther. 2014;12:275–278. doi: 10.1586/14787210.2014.887442. [DOI] [PubMed] [Google Scholar]
- 212.Itzhaki RF. Herpes and Alzheimer’s disease: subversion in the central nervous system and how it might be halted. J Alzheimers Dis. 2016;54:1273–1281. doi: 10.3233/JAD-160607. [DOI] [PubMed] [Google Scholar]
- 213.Costa AS, Agostini S, Guerini FR, Mancuso R, Zanzottera M, Ripamonti E, Racca V, Nemni R, Clerici M. Modulation of immune responses to herpes simplex virus type 1 by IFNL3 and IRF7 polymorphisms: a study in Alzheimer’s disease. J Alzheimers Dis. 2017;60:1055–1063. doi: 10.3233/JAD-170520. [DOI] [PubMed] [Google Scholar]
- 214.Letenneur L, Peres K, Fleury H, Garrigue I, Barberger-Gateau P, Helmer C, Orgogozo JM, Gauthier S, Dartigues JF. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer’s disease: a population-based cohort study. PLoS One. 2008;3:e3637. doi: 10.1371/journal.pone.0003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang C, Zhang N, Qi L, et al. Myeloid-derived suppressor cells inhibit T follicular helper cell immune response in Japanese encephalitis virus infection. J Immunol. 2017;199:3094–3105. doi: 10.4049/jimmunol.1700671. [DOI] [PubMed] [Google Scholar]
- 216.Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 217.Salminen A, Kauppinen A, Kaarniranta K. Hypoxia/ischemia activate processing of Amyloid Precursor Protein: impact of vascular dysfunction in the pathogenesis of Alzheimer’s disease. J Neurochem. 2017;140:536–549. doi: 10.1111/jnc.13932. [DOI] [PubMed] [Google Scholar]
- 218.Westendorf AM, Skibbe K, Adamczyk A, Buer J, Geffers R, Hansen W, Pastille E, Jendrossek V. Hypoxia enhances immunosuppression by inhibiting CD4+ effector T cell function and promoting Treg activity. Cell Physiol Biochem. 2017;41:1271–1284. doi: 10.1159/000464429. [DOI] [PubMed] [Google Scholar]
- 219.Zhang H, Ye YL, Li MX, et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene. 2017;36:2095–2104. doi: 10.1038/onc.2016.367. [DOI] [PubMed] [Google Scholar]
- 220.Popp J, Bacher M, Kölsch H, Noelker C, Deuster O, Dodel R, Jessen F. Macrophage migration inhibitory factor in mild cognitive impairment and Alzheimer’s disease. J Psychiatr Res. 2009;43:749–753. doi: 10.1016/j.jpsychires.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 221.Pösel C, Möller K, Boltze J, Wagner DC, Weise G. Isolation and flow cytometric analysis of immune cells from the ischemic mouse brain. J Vis Exp. 2016;108:53658. doi: 10.3791/53658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu Y, Kosaka A, Ikeura M, Kohanbash G, Fellows-Mayle W, Snyder LA, Okada H. Premetastatic soil and prevention of breast cancer brain metastasis. Neurooncology. 2013;15:891–903. doi: 10.1093/neuonc/not031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Trellakis S, Bruderek K, Hütte J, Elian M, Hoffmann TK, Lang S, Brandau S. Granulocytic myeloid-derived suppressor cells are cryosensitive and their frequency does not correlate with serum concentrations of colony-stimulating factors in head and neck cancer. Innate Immun. 2013;19:328–336. doi: 10.1177/1753425912463618. [DOI] [PubMed] [Google Scholar]
- 224.Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, Forero A, Bendell J, Witt R, Hockstein N, Kumar P, Gabrilovich DI. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin Cancer Res. 2017;23:2942–2950. doi: 10.1158/1078-0432.CCR-16-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]