Abstract
Gene therapy might represent a promising strategy for chondral and osteochondral defects repair by balancing the management of temporary joint mechanical incompetence with altered metabolic and inflammatory homeostasis. This review analysed preclinical and clinical studies on gene therapy for the repair of articular cartilage defects performed over the last 10 years, focussing on expression vectors (non-viral and viral), type of genes delivered and gene therapy procedures (direct or indirect). Plasmids (non-viral expression vectors) and adenovirus (viral vectors) were the most employed vectors in preclinical studies. Genes delivered encoded mainly for growth factors, followed by transcription factors, anti-inflammatory cytokines and, less frequently, by cell signalling proteins, matrix proteins and receptors. Direct injection of the expression vector was used less than indirect injection of cells, with or without scaffolds, transduced with genes of interest and then implanted into the lesion site. Clinical trials (phases I, II or III) on safety, biological activity, efficacy, toxicity or bio-distribution employed adenovirus viral vectors to deliver growth factors or anti-inflammatory cytokines, for the treatment of osteoarthritis or degenerative arthritis, and tumour necrosis factor receptor or interferon for the treatment of inflammatory arthritis.
Keywords: Regenerative medicine, Gene therapy procedures, Expression vectors, Cartilage repair, Osteoarthritis
Introduction
Various joint pathologies such as rheumatic disease, trauma, osteochondritis dissecans and osteonecrosis may lead to severe damage of articular cartilage and other joint structures, ranging from focal defects to osteoarthritis (OA) [1]. The critical actor in this pathophysiological process is the osteochondral unit. The activation of inflammatory cascades within the joint leads to a gradual deterioration of cartilaginous extracellular matrix (ECM) and activation of osteoclasts, with consequent degradation of subchondral bone [2]. Focal chondral and osteochondral defects are usually considered to have limited spontaneous healing or regenerative potential. This is due to cartilage characteristics, including low chondrocyte density, slow ECM production and cartilage aneural and avascular structure [2]. Synoviocytes intervene in repairing chondral defects, but their action is often inadequate and larger defects can arise [3].
An ideal treatment for these lesions should result in the regeneration of hyaline cartilage, well-integrated in the surrounding normal tissues and provided with mechanical competence. Various medical and surgical techniques, such as subchondral bone drilling, abrasion, microfractures, osteochondral autologous transfer, mosaicplasty, autologous chondrocytes implantation (ACI) and matrix-induced ACI (MACI) have been developed and applied to treat focal chondral and osteochondral lesions with important progresses. However, none of them has been able to provide regeneration of articular cartilage. In addition, these techniques show some drawbacks, such as donor site morbidity, graft rejections and further degenerative changes due to two-step surgery [4]. The treatment of generalized OA lesions is more complex. Progressively worsening of the joint microenvironment towards chronic inflammation limits the possibility of cartilage to repair or regenerate [2].
Recently, gene therapy has made some improvements in cartilage regeneration, especially for OA, and, might represent a future solution, despite the unsolved problems related to non-standardized procedures. In addition, the association of gene therapy to tissue engineering might represent a promising strategy for the treatment of chondral and osteochondral lesions, balancing the management of temporary joint mechanical incompetence with altered metabolic and inflammatory homeostasis [5].
Gene therapy usually aims at treating human diseases through gene transfer techniques that introduce genes or sequences in various cell types. Several aspects must be taken into account in the development of a gene therapy: (a) the expression vector to be used; (b) the identification of genes to be transferred; (c) the target cells; and (d) the in vivo delivery procedures [6, 7]. Compared with recombinant protein therapy, where factors have short half-lives, gene-based treatments potentially allow for a site-specific action, in a more physiologic manner and with long-term effects [8]. The concept of using gene therapy for cartilage repair originated from the idea that the expression of specific genes into the injury site could increase the regeneration process [9]. Recently, significant advances have been made in the basic science of gene transfer for articular cartilage, with both in vivo (directly delivered to a joint) and ex vivo (retrieved and explanted cells genetically modified in vitro and re-implanted into the joint with or without the use of a scaffold) procedures. In vitro cartilage regeneration is effectively promoted by delivering genes that increase cartilage differentiation, as well as by down regulating some negative factors [9].
The present review aimed at tackling the topic of gene therapy for the repair of articular cartilage during the past 10 years. After a literature search on this topic, we focused our attention on more relevant technical aspects, various approaches and different applications for chondral or osteochondral lesions and for OA damages.
Search strategies
The following literature research in the MEDLINE database (PubMed research engine) was carried out: “Genetic Therapy”[Mesh] AND (“Cartilage, Articular”[Mesh] OR ((osteochondral[All Fields] AND (lesion?[All Fields] OR defect?[All Fields])) OR (chondral[All Fields] AND (lesion?[All Fields] OR defect?[All Fields])))).
The search was limited to year of publication from 2006 to 2016 (“2006/01/01” [Date - Entrez]: “2016/12/31” [Date—Entrez]) and abstract availability in English. Two reviewers manually assessed the references of the retrieved studies and pertinent reviews to avoid papers regarding the following topics: tumour and autoimmune diseases. The number of unique papers from the electronic search and after abstract review was 80. Of these, 64 were included in the review, while 16 were discarded because it was impossible to collect the articles (8 studies), or they were already included in the cited reviews (8 studies). Further 39 studies were included in the final review to complete the introduction and conclusion section or to add information on some technical aspects.
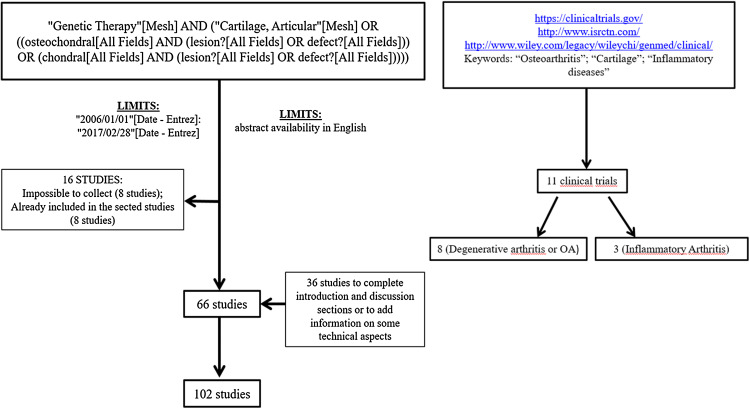
To find active or closed clinical trials on gene therapy for chondral and osteochondral defects from 2006 to 2016, we carried out a bibliographic research on the following database: US National Institutes of Health ClinicalTrials.gov database (https://clinicaltrials.gov/); International Standard Randomized Controlled Trial Number Register (http://www.isrctn.com/); and Wiley database on Gene Therapy Trials Worldwide (http://www.wiley.com/legacy/wileychi/genmed/clinical/). Since we did not find any clinical trials using the keywords ‘chondral’ and ‘osteochondral’, we decided to extend our research to the terms: ‘osteoarthritis’; ‘cartilage’; and ‘inflammatory diseases’. We found 11 clinical trials 8 of which are related to degenerative arthritis or OA and 3 to inflammatory arthritis (Fig. 1).
Fig. 1.
Diagram of the reference search strategy and selection
Gene delivery vectors
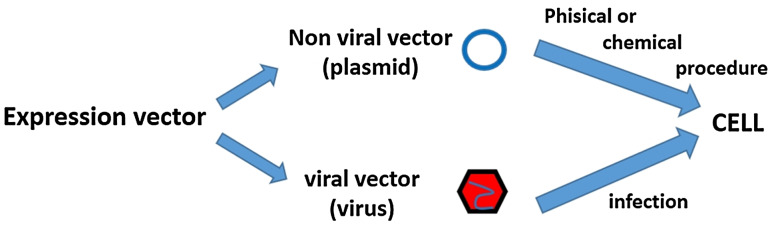
To allow the expression of a gene in a target cell, this must be inserted in an expression vector. Two classes of expression vectors exist, with different advantages and disadvantages: non-viral and viral (Figs. 2, 3).
Fig. 2.
Schematic drawing of classes of expression vector
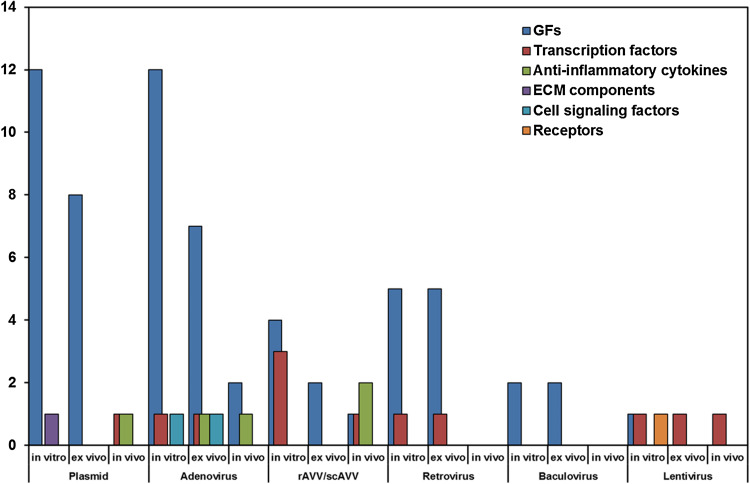
Fig. 3.
Barcharts of the number of preclinical in vitro, ex vivo and in vivo studies that employed non-viral and viral vectors
Non-viral vector
In non-viral vectors, the expression vector (usually a plasmid) is transferred in recipient cells with techniques that use physical procedures, such as in vivo electroporation and ultrasounds, or chemical transfection compounds. These procedures are safe, easy to handle and cost-effective, but they often result less efficient delivery compared to use of viral vectors, which allow the introduction of genes through a simple viral infection. Once introduced into the cells, non-viral vectors usually remain in the cytoplasm in episomal form, where they express the specific gene protein [10].
Electroporation is a physical transfection method that uses pulses of electrical field to open pores in the cellular membrane, allowing the introduction of small DNA molecules. This technique is used in intra-articular gene therapy, but the expression of the factor is often limited to synovial membrane [11]. In addition, it is reported that gene transfer into arthritic joints by electroporation, used to deliver anti-inflammatory cytokines, has a short duration of transgene expression, preventing its use for the treatment of arthritis [12].
Recently, ultrasound, which increases cell membrane permeability, has also been used to facilitate drug delivery or gene transfection into cells. In fact, ultrasound pulses determine cavitation (microbubbles) that allows the introduction of different molecules into the cell. This method is used for gene delivery in intervertebral discs, showing gene expression for up to 24 weeks. However, further studies are necessary to translate this technique to clinical application [13].
Chemical methods, employed in preclinical studies, form complexes with DNA and various macromolecules, including liposomes, cationic polysaccharide and non-liposomal lipid-based transfection [14–21]. They can deliver large genes and are easy to produce on a large scale. In addition, polymeric gene therapy is employed to delivery genes without transfecting cells, by complexing the plasmid with (a) a branched poly(ethylenimine)-hyaluronic acid (bPEI-HA) delivery vector, via a porous oligo-[poly(ethylene glycol) fumarate] hydrogel scaffold; (b) type I collagen gels, or (c) collagen-glycosaminoglycan scaffold in vivo by electrotransfer [12, 22–24].
Plasmids are principally used to transfer genes that encode for cartilage growth factors (GFs) into recipient cells. Such genes are insulin-like growth factor 1 (IGF-1) [15–18, 20, 23, 25], transforming growth factor β (TGF-β) [14, 26, 27], bone morphogenetic proteins (BMPs) [20, 22] and fibroblast growth factor 2 (FGF-2) [16, 18, 21]. Other genes belong to the transcription factors family, SRY-related HMG box (SOX) [24], the anti-inflammatory cytokine Interleukin 10 (IL10) [12] and cartilage oligomeric matrix protein (COMP), a ECM component [19] (Table 1).
Table 1.
List of gene families and genes delivered with specific vectors in in vitro, ex vivo and in vivo studies
| Gene family | Gene | Delivery system | Vector | Study | Cartilage defects | References |
|---|---|---|---|---|---|---|
| GFs | IGF1 | BMSCs+scaffold | Plasmid | In vitro and ex vivo | Knee osteochondral defect | [17] |
| (calcium alginate gel) | ||||||
| Chondrocytes | Plasmid | In vitro | [15] | |||
| Scaffold | Plasmid | In vitro | [23] | |||
| (COLL II-GAG) | ||||||
| Chondrocytes | Adenovirus | In vitro | [8] | |||
| Chondrocyte+scaffold (fibrinogen) | Adenovirus | Ex vivo | Knee full-thickness cartilage defect | [44] | ||
| Direct injection | Adenovirus | In vivo | Synovial tissues of metacarpophalange | [50] | ||
| BMSCs and chondrocytes | rAAV | In vitro and ex vivo | Knee osteochondral defect | [61] | ||
| Chondrocytes+scaffold (fibrinogen) | rAAV | Ex vivo | Femur full-thickness cartilage defect | [62] | ||
| Direct injection | rAAV | In vivo | Mechanically or collagen-induced knee arthritis | [63] | ||
| IGF-1+BMP2+BMP7 | Chondrocytes | Plasmid | In vitro | [20] | ||
| IGF-1+BMP2 | ADSCs | Plasmid (IGF-1); Adenovirus (BMP2) | In vitro | [25] | ||
| IGF-1+FGF-2 | Chondrocytes+scaffold (alginate) | Plasmid | In vitro and ex vivo | Knee osteochondral defect | [16] | |
| FBs+scaffold | Plasmid | In vitro and ex vivo | Knee osteochondral defect | [18] | ||
| (alginate) | ||||||
| ADSCs | Adenovirus | In vitro | [43] | |||
| TGF-β | BMSCs+scaffold | Plasmid | In vitro and ex vivo | Knee full-thickness defects | [26] | |
| (PLA) | ||||||
| BMSCs+scaffold | Plasmid | In vitro and ex vivo | Knee full-thickness defect | [14] | ||
| (PLGA/fibrin gel) | ||||||
| BMSCs+scaffold | Plasmid | Ex vivo | Knee full-thickness defect | [27] | ||
| (Gelatin+TCP sponge) | ||||||
| Chondrocytes+scaffold (Chitosan+gelatin matrix) | Plasmid | In vitro | [26] | |||
| BMSCs | Adenovirus | In vitro and ex vivo | Knee osteochondral defect | [40] | ||
| BMSCs clot | Adenovirus | Ex vivo | Knee partial-thickness defects | [41] | ||
| BMSCs+scaffold (PGA) | Adenovirus | In vitro and ex vivo | Subcutaneous tissue | [42] | ||
| Chondrocytes | Adenovirus | In vitro | [8] | |||
| BMSCs | rAAV | In vitro | [2] | |||
| Mixture of chondrocytes and irradiated TGF-β1 producing chondrocytes | Retrovirus | In vitro and ex vivo | Knee osteochondral defect | [70] | ||
| TGF-β+BMP2 | BMSCs+scaffold (DBM) | Adenovirus | In vitro and ex vivo | Knee full-thickness defect | [46] | |
| TGF-β+BMP6 | ADSCs+scaffold (PLGA) | Baculovirus | In vitro and ex vivo | Knee full-thickness defect | [82] | |
| BMP | BMSCs (vitro); | Plasmid | In vitro and ex vivo | Knee full-thickness defect | [22] | |
| COLL I sponge (vivo) | ||||||
| BMSCs | Adenovirus | In vitro | [45] | |||
| ACPC+scaffold (fibrin-PU) | Adenovirus | In vitro | [47] | |||
| Muscle and fat | Adenovirus | In vitro and ex vivo | Femora segmental defect or knee osteochondral defect | [48] | ||
| DCBM | Adenovirus | Ex vivo | Knee full-thickness defect | [52] | ||
| Direct injection | Adenovirus | In vivo | Knee osteochondral defect | [51] | ||
| BMSCs+scaffold (fibrin glue) | Retrovirus | In vitro and ex vivo | Knee full-thickness cartilage defect | [71] | ||
| Chondrocytes+scaffold (PLGA) | Baculovirus | In vitro and ex vivo | Knee osteochondral defect | [81] | ||
| Muscle-derived stem cells (MDSCs) | Retrovirus | In vitro and ex vivo | Knee osteochondral defect | [74, 75] | ||
| Chondrocytes | Lentivirus | In vitro | [74] | |||
| FGF-2 | Chondrocytes+scaffold (alginate) | Plasmid | In vitro and ex vivo | Knee osteochondral defects | [21] | |
| BMSCs | rAAV | In vitro | [65] | |||
| sFlt-1 | MDSCs | Retrovirus | In vitro and ex vivo | Knee osteochondral defects | [75] | |
| GDF-5 | ADSCs | Adenovirus | In vitro | [49] | ||
| sFlt-1 | BMSCs | Retrovirus | In vitro and ex vivo | Knee osteochondral defects | [72] | |
| Transcription factors | SOX | BMSCs+scaffold (PGA) | Adenovirus | In vitro and ex vivo | Knee full-thickness defect | [54] |
| BMSCs | rAAV | In vitro | [66] | |||
| BMSCs, chondrocytes+scaffold (alginate) (in vitro); Direct injection (in vivo) | rAAV | In vitro and in vivo | Knee osteochondral defect | [59] | ||
| SOX-5+SOX-6+SOX-9 | ADSCs+scaffold (fibrinogen) | Retrovirus | In vitro and ex vivo | Knee osteochondral defect; surgically-induced OA | [73] | |
| SOX-5+SOX-6+SOX-9+RUNX2 | Polymeric gene therapy | Plasmid | In vivo | Knee osteochondral defect | [24] | |
| SOX-9+TGF-β | BMSCs | rAAV | In vitro | [64] | ||
| ZNF145 | BMSCs | Lentivirus | In vitro and ex vivo | Knee osteochondral defect | [75] | |
| Anti-inflammatory cytokines | IL10 | Direct injection | Plasmid | In vivo | CIA | [12] |
| Direct injection | Lentivirus | In vivo | RA | [78] | ||
| IL1ra | Direct injection | rAAV | In vivo | Ankle chronic inflammatory arthropathy | [67] | |
| Direct injection | scAAV | In vivo | Middle carpal joint OA | [68] | ||
| IL1ra+IL10 | FBs | Adenovirus | Ex vivo | RA | [56] | |
| IL1ra+IGF-1 | Direct injection | Adenovirus | In vivo | Carpus and stifle full-thickness cartilage defect | [57] | |
| Cell signaling protein | iHH+BMP2 | BMSCs | Adenovirus | In vitro | [53] | |
| BMSCs | Adenovirus | Ex vivo | Knee osteochondral defect | [55] | ||
| ECM component | COMP | Chondrocytes and FBs | Plasmid | In vitro | [19] | |
| Receptors | Integrin β1 | Chondrocytes+scaffold (PGA) | Lentivirus | In vitro | [79] |
In the last few years, different studies have shown as exosomes, microvesicles (40-100 nm) produced by almost all cells, are important in cellular communications, both in physiologic and pathologic conditions [28–30]. Exosomes from different cell types (MSCs, immune cells, etc.) have shown high potential in cartilage and bone regeneration [31–34]. This have been used to engineer exosomes to target specific cells or tissues, and transport them a number of different molecules, including expression vectors. Engineered exosomes were up to now used for site-specific transport of chemotherapic agents (drugs, siRNA) to tumor cells [35–37], but this does not exclude their use in gene therapy in the next future. This allows avoidance of the use of viral proteins (capsid), whose immunogenicity is the main problem of the use of viral vectors [38].
Viral vector
Viral vectors are divided in different groups according to the type of virus used: adenovirus, recombinant adeno-associated viral (rAAV), retrovirus, and baculovirus [39]. Among the viral systems employed for gene therapy, adenoviruses are the most used because they have high transduction efficiencies and transgene expression in various types of cells, allowing in vivo approaches. More than 50 adenovirus serotypes are available for gene therapy and serotype 5 (Ad5) has been the mostly used in both in vitro and in vivo studies. Adenovirus is used to transfer GF genes (TGF-β, FGF-2, IGF-1, BMPs and Growth and differentiation factor 5, GDF-5) into cells [8, 20, 25, 40–49]. Genes, in encapsuled viral vector, can be injected directly in vivo [50, 51] or through decalcified cortical bone matrix (DCBM) as scaffold that contains the viral particles [52]. Indian hedgehog homolog (iHH) and SOXs genes employ adenovirus for transport into MSCs both in vitro [53, 54] and in vivo [55, 56]. Anti-inflammatory cytokines are introduced into Fibroblasts (FBs) or directly injected in vivo [56, 57] (Table 1).
There are serious concerns about the use of adenoviral vectors in clinical settings, due to the development of a strong host humoral and cellular immune response to the adenoviral gene products. A solution to this problem is to minimize the immune response, using a vector containing only the gene, the packaging sequence and the flanking viral terminal repeats. However, this requires the use of another virus (helper) for viral transduction (production of viral particles), which makes the system very complex [58]. Additionally, most individuals have a pre-existing immunity to adenovirus, which could neutralize the virus administered in vivo. Another problem is that the transgene expression is limited to 1–2 weeks. This is a result of the episomal state of the vector: the expression of a transgene persists only in non-dividing cells, while it gradually vanishes when modified cells are re-introduced in the tissue and the expression is diluted in a growing population [10].
To date, the best vector that could represent an adequate candidate for gene therapy is the rAAV vector family or self-complementary AAV (scAAV). rAAV vectors are based on the non-pathogenic parvovirus AAV, which has a single-stranded DNA genome and does not provoke potent host immune responses. These vectors are obtained by complete removal of the viral gene coding sequences, to make them less immunogenic and less toxic. Furthermore, rAAV vectors do not require cell division or vector integration for gene expression, and their delivered genes are expressed with very high efficiency for a long time (months to years) through the stabilization of episomal DNA by the formation of concatemers [59]. On the other hand, sc-AAV produces higher levels of protein more quickly and, for this reason, it could become the preferred choice for gene therapy trials [60]. rAAV is used for the delivery of IGF-1 [61–63], TGF-β [2, 64], FGF-2 [65], SOXs [59, 64, 66] and IL1ra genes [67]. scAVV is used for the delivery of IL1ra [68] (Table 1).
Retroviruses have the advantage integrating their DNA into the host genome, allowing to maintain gene expression for longer periods of time [69]. In comparison to the previous mentioned vectors, fewer studies employed retrovirus for the delivery of GFs, such as TGF-β, BMPs or VEGF inhibitor, as sFlt-1, and SOXs, both in vitro and in vivo) [70–75] (Table 1). The main problem is insertional mutagenesis and the potential activation of oncogenes. In fact, retroviruses show a preferential integration near highly expressed genes, causing leukaemia in some patients with X-linked severe combined immunodeficiency. In addition, retroviruses can transduce only dividing cells with a restricted host range and a low efficacy. To overcome this last disadvantage, lentiviruses, belonging to a subclass of retrovirus family derived from the human immunodeficiency virus (HIV), have been proposed because they can transduce in non-dividing cells [76]. Lentivirus were employed for the delivery of sequence that codifies for BMPs [76], zinc-finger protein 145 (ZNF145) [77], IL10 [78] and Integrin-β [79] (Table 1). However, lentiviral vectors, similarly to retroviruses, favour gene integration, which might determine an insertional transformation and, subsequently, tumour formation. Furthermore, there are still concerns associated to the psychological problems of introducing genetic material of HIV sequences in vivo [80]. For these reasons, retroviruses have been used only for ex vivo gene delivery procedures, adopting the strategy to select cultured cells through a selection marker (i.e. antibiotic resistance) and consequently irradiate them to prevent further in vivo growth and minimize tumour development [70, 81].
Finally, the viral vector least employed is baculovirus, a virus that infects insect cells, but that is also able to transduce numerous mammalian cells, including chondrocytes and ADSCs for TGF-β [82] and BMPs [82, 83] in vitro and in vivo. However, this virus is not able to replicate and integrate its DNAs into the chromosomes of transduced mammalian cells, determining a transient transgene expression (<7 days). For these properties, baculoviruses have attracted research interests although their application is not allowed in cases requiring continuous expression.
Delivered genes
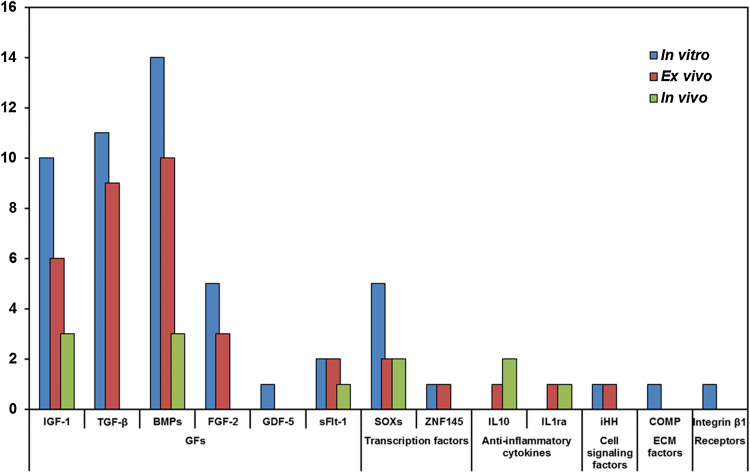
It is important to identify the most important genes delivered into cells or directly injected into a joint affected by cartilaginous pathologies, whose overexpression can increase the production of different proteins useful to improve cartilage healing. Gene therapy has not the aim of replacing or repairing abnormal genes that causes the disease, but it induces the overexpression of therapeutic factors, such as GFs and ECM proteins, or the suppression of genes (through miRNA synthesis) involved in joint degeneration. The selection of genes to be delivered through specific expression vectors is important. This is because for the repair of chondral and osteochondral defects it is necessary to overcome some pathophysiological processes that often determine tissue degeneration worsening. Genes belong to different groups: (1) GFs; (2) TFs; (3) anti-inflammatory cytokines; (4) cell signalling proteins involved in chondrocyte differentiation, proliferation and maturation; (5) ECM proteins; and (6) receptors (Fig. 4).
Fig. 4.
Barcharts of the number of groups of genes evaluated in preclinical, in vitro, ex vivo and in vivo studies
Growth factors
The largest group includes GF genes, such as IGF-1 [8, 15–18, 20, 23, 25, 43, 44, 50, 57, 61–63], TGF-β [2, 8, 14, 26, 27, 39–42, 46, 64, 70, 82], BMPs [20, 22, 25, 45, 47, 48, 51–53, 55, 71, 76, 83], FGF-2 [16, 18, 21, 43, 65], GDF-5 [49] and VEGF antagonist [72] (Table 1). TGF-β1 plays a key role in cell growth, cartilaginous tissue differentiation and ECM protein synthesis. IGF-1 has anabolic effects, increases large aggregating proteoglycans and collagen II synthesis and inhibits their degradation. FGF-2 increases chondrogenesis and cartilage matrix formation. BMPs are potent inducers of cartilage repair, but they develop chondrocyte hypertrophy and endochondral ossifications. GDF-5 is considered an initiator of chondrogenesis of MSCs and induces COLL II and glycosaminoglycans production. The transfection of these genes stimulates MSC chondrogenesis and chondrocyte proliferation and reduces MSC osteogenic markers in vitro [2, 8, 14, 15, 17, 21–23, 25, 26, 31, 42, 45, 47–49, 61, 65, 70, 71, 76, 83]. Conversely, in vivo it improves osteochondral or full-thickness defects [14, 17, 21, 22, 27, 40–42, 44, 46, 48, 51, 52, 61, 62, 70, 71, 83, 85] and joints affected by OA [63]. VEGF, transfected into MSCs, induces arthritic-like changes in the joints and the co-transfection with sFlt-1 ameliorated this condition in osteochondral defects [72]. The introduction of two or more GFs into the cells, induces more cartilage regeneration in comparison with a single gene, with a synergic effect. This is observed for IGF-1, co-transfected with FGF-2, in osteochondral defects [16, 18] and in MSCs [43], or with BMP2 and BMP7 in chondrocytes [20]. In addition, TGF-β, co-transfected with BMP2 or BMP6, is used to treat full-thickness defects through MSC infection [46, 82].
Transcription factors
The second important group of genes includes TFs such as SOX genes (5, 6 and 9) and ZNF145 (Table 1). SOXs regulate chondrocyte differentiation, improve ECM production and reduce the levels of hypertrophy and osteogenic/adipogenic markers. ZNF145 regulates the differentiation of 3-lineages of MSCs, as observed in in vitro studies [54, 59, 65, 77], while in vivo it increases osteochondral and full-thickness defects healing [54, 59, 75]. The co-infection of SOX9 and TGF-β potentiates chondrogenesis of MSCs in vitro, in comparison to their separate use [64]. The trio-co-transduction of SOXs (SOX-5, -6 and -9) increases osteochondral defect and OA healing in vivo and chondrogenesis of MSCs in vitro [73], and their combination with RUNX2 gene further improves osteochondral defect regeneration [24].
Anti-inflammatory cytokines
The third group of genes includes anti-inflammatory cytokines such as IL10 [12, 78] and IL1ra [67, 68] (Table 1). They contrast pro-inflammatory cytokines and can be overexpressed in target cells determining a chondroprotective effect. Furthermore, gene therapy strategy, using genes that downregulate some negative factors, promotes chondrocyte differentiation and ECM production. These genes were principally employed in vivo for the reduction of arthritis or OA knee pathologies. The co-transfection of two anti-inflammatory cytokines decreases cartilage destruction, in joints affected by RA, more than their separate use [56], while the co-infection of IL1ra and a GF (IGF-1) improves in vivo repair of full-thickness cartilage defects in association with microfractures [57].
miRNA
Recently, some miRNAs have been found to be regulators of chondrogenic differentiation that can be used in gene therapy. So far, they have only been used in in vitro studies. MiR-23b, and miR-140 have proven to have a pro-chondrogenic effect when expressed in MSCs [85, 86]. In addition, some miRNA, which are negative modulators of cartilage development, can be down-regulated. MiR-181b, whose targets are different proteins of hippo pathways [87] implicated in chondrocyte regulation, is a negative regulator of chondrocyte differentiation and cartilage development and its synthesis upregulates MMPs increasing the degradation of ECM [88]. MiR221 silencing shows a pro-chondrogenic role in vivo [89] inducing an increase of chondrogenic markers (e.g. collagen type II), and of positive chondrogenic transcription factor Sox9 and Tricho-rhino-phalangeal syndrome 1 protein (TRPS1). In fact, MiR-221 targeting of MDM2 mRNA, whose product regulates proteasomal degradation of Slug, is implicated in the maintenance of the undifferentiated status of MSCs [90]. miR-145 and miR-335-5p have Sox9 as target and suppress chondrogenesis, anti-miR molecules (through synthetic shRNA genes) can contrast the effects of these miRNA and induce the expression of Col2a1, aggrecan and avoid cartilage degeneration [91, 92].
Other factors
Less is known about genes encoding for the cell signalling protein iHH [53, 55], for ECM component (COMP) [19] and for integrin β1 [79] (Table 1). COMP is a multidomain homo-pentameric protein, which is an important regulator of collagen fiber assembly, interacting with other matrix proteins and inducing chondrocyte proliferation in vitro. Integrin β1 is the main receptor implicated in mechanosensing and induction of chondrocyte differentiation and matrix synthesis. The co-infection of iHH with BMP-2, in vitro and in osteochondral lesions in vivo, shows that iHH mitigates the hypertrophy of MSCs induced by BMP2 [53, 55].
Gene therapy procedures
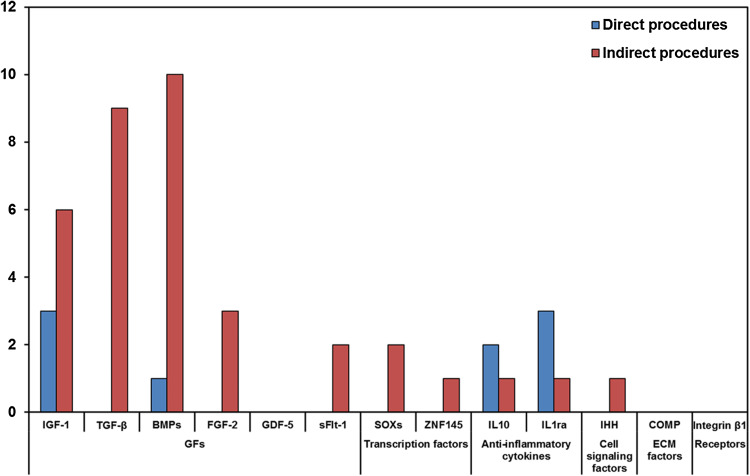
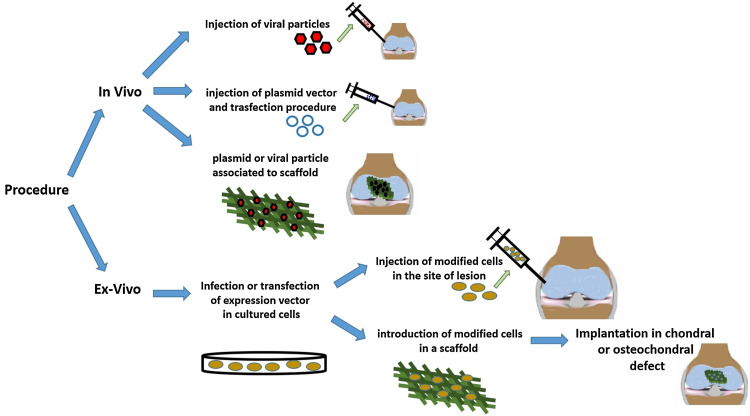
Two procedures can be used to deliver genes into cartilaginous defects according to the modality of administration of the selected vector: a ‘direct’ procedure, when the vector is administered in target organs or anatomical regions of the patients with or without a scaffold (in vivo) [12, 50, 51, 57, 63, 67, 68, 74, 75, 81], or an ‘indirect’ procedure, when the vector is previously inserted into target cells and subsequently implanted in the injury site with or without a scaffold (ex vivo) [14, 16–18, 21, 22, 27, 40–42, 44, 46, 48, 52, 54–56, 59, 61, 62, 70–73, 77, 82–84] (Figs. 5, 6).
Fig. 5.
Barcharts of the number of procedures (direct and indirect) to bring genes into cartilaginous defects according to the modality of administration of the selected vector
Fig. 6.
Schematic drawing of different modalities of administration of the expression vector
Direct procedures
In most studies, the direct procedure has the advantage that vector exposure is restricted to the intra-articular space, avoiding systemic and too long local effects. Therefore, transgene expression can be present in the joint even for up to 4 months after the injection. However, with a direct procedure cartilage defects cannot be the specific target of the injected vector, because the expression of the gene is often limited to the synovial membrane, which is largely available and more easily accessible inside the joint. In fact, synoviocytes have been used as target cells for the expression of anti-inflammatory molecules very important to counteract OA disease. For this reason, in comparison to the indirect procedure, the direct injection of a vector into a lesion site is less applied in cartilaginous defects. Direct injection is mainly employed for the delivery of anti-inflammatory cytokines such as IL10 and IL1ra into a joint affected by rheumatoid arthritis, OA and full-thickness defects [12, 57, 67, 68, 78]. Even GFs, such as IGF1 and BMPs are delivered with this modality in arthritis, full-thickness and osteochondral defects [50, 51, 57, 63]. In the direct procedures, the most employed vectors are adenovirus, rAAV or scAAV [50, 51, 57, 63, 67, 68], followed by plasmid [12] and Lentivirus [78] (Table 1).
Indirect procedures
The indirect procedure, where transfected cells are delivered into cartilage defects, is the most used (Fig. 7). The cell vehicles, with and without scaffolds, are MSCs of different origin [14, 17, 26, 27, 40–42, 46, 54, 55, 59, 61, 71–75, 77, 82], chondrocytes [16, 21, 44, 59, 61, 62, 70, 83] and, to a lesser extent, fibroblasts [18, 56]. In fewer cases vectors are implanted into scaffolds without cells as vehicles: the plasmid vector is supported by a collagen I sponge or complexed with bPEI–HA via a porous oligo[poly(ethylene glycol) fumarate] hydrogel scaffold [22, 24] and adenoviral vector by DCBM [51] (Table 1).
Fig. 7.
Barcharts of the number of indirect procedure studies that employed different techniques for gene therapy
Ex vivo gene therapy for cartilage repair involves three phases: removal of cells from healthy tissue of the patient, modification/expansion of these cells, and their reintroduction into the damaged area. This procedure has been thwarted by the high costs of two-step autologous therapy, since the target cell population must be collected and the gene must be transfected and expanded (for at least 4 weeks) before being re-implanted. The complexity and costs would be reduced with the use of allograft cells by donors that could allow the implantation of modified cells directly in one surgery step [16–18, 21, 26, 40, 42, 44, 56, 61, 70–72, 74, 75, 77, 82, 83]. To translate ex vivo approaches to clinical application, all procedures must be performed according to good manufacture practice (GMP) in a well-organized structure (cell factory) to avoid the introduction of contaminants. All working spaces should be organised to separate the different processes of modification and expansion of the cells and avoid cross-contaminations [93].
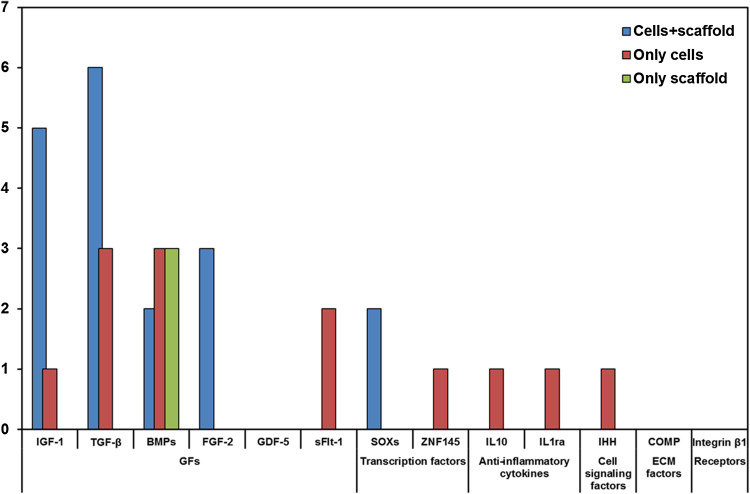
Use of scaffold in gene therapy procedures
The main problem of gene therapy for focal defects using ex vivo procedures, is the dilution of modified cells after intra-articular injection. To avoid this dilution, a possibility is to deliver these modified cells with different scaffolds used as carrier. When the scaffold is degraded, the encapsulated expression vector is adsorbed locally by the cells. The combination of gene therapy and scaffolds seems to greatly enhance both the efficiency and duration of transfected genes, leading to systems able to promote bone, cartilage, and osteochondral regeneration [22, 23, 26]. Scaffolds can be natural such as DBM, gelatine, alginate, fibrinogen and collagen based [16–18, 21, 27, 46, 52, 62, 71, 73, 84], or synthetic such as polyglycolic acid (PGA), polylactic acid (PLA) and poly(lactic-co-glycolide) (PLGA) [14, 42, 54, 82–84] (Table 1). Besides favouring cell engineering within the scaffold, their design can be used as guide for the correct integration and directional localization of cells in the defect. Although there have been important advances in the creation of these systems, different factors, such as kinetic of release, type of interaction of the vector with the scaffold, or interactions between scaffolds and microenvironment, can still be improved [22, 23, 84].
Preclinical and clinical studies on: safety, toxicity, biodistribution, efficacy, biological activity
Before translating any new innovative treatment into clinical practice, clinical trials must be carried out to achieve pre- (phases 0–III) and post-marketing (phase IV) data on safety and efficacy. It is very difficult to translate gene therapy from pre-clinical studies into clinical practice. After having achieved enough data on a new gene therapy from preclinical evaluations on safety, biodistribution and efficacy (proof-of-concept), it is mandatory to establish the safety of the developed genetic treatment in selected patients through various phases I or phase I/II clinical trials, considering that these treatments could be deleterious for the patient. Usually, recruited patients present a severe degree of joint pathology and are candidates to future joint replacement. Other important aspects investigated in phase I and II clinical trials, are the level of transgene expression in the targeted joint as well as at systemic level, and the development of humoral or cellular immune response against proteins or vectors.
Preclinical studies
Preclinical studies of gene therapy for chondral and osteochondral regeneration reported in literature were on safety and efficacy of intra-articular administration of: (a) a 3:1 mixture of normal human chondrocytes and genetically modified human chondrocytes expressing TGF-β1 (TG-C, TissueGene, Inc., Rockville, USA) [81]; (b) a rAAV vector containing the cDNA for a human tumor necrosis factor receptor (TNFR) and immunoglobulin (IgG1) Fc fragment (TNFR:Fc) fusion gene (tgAAC94, Targeted Genetics Corporation, Seattle-Washington, USA) [98]; (c) genetically modified synovial cells (self-complementing recombinant AAV vector delivered in vitro) that express IL-1 Ra (sc-rAAV2.5IL-1Ra, Mayo Clinic, Rochester, Minnesota, USA). [60, 97]; and (d) rAAV vector expressing human interferon-β (ART-I02, Arthrogen, Amsterdam, The Netherlands) [99, 100].
Regarding preclinical evaluation of TG-C, three different studies were carried out to evaluate its biodistribution in SCID mice, and its safety and efficacy in knee articular damage in rabbit and goat models at short, mid and long experimental times [81]. TG-C was completely cleared from tissues by day 15 and from lung by day 30; in addition, PCR analysis of TG-C DNA performed on blood and other tissues retrieved by rabbits and goats at 30 days and 8 weeks, respectively, did not show any signs [81]. 3 and 6-months safety and efficacy study of TG-C to treat mono-laterally partial cartilage defect (patellar groove) in rabbits highlighted a relationship between intra-articular TG-C administered dose and speed of cartilage regeneration. Higher dose administered (9 × 106 cells/animal) fast the cartilage repair respect to control group. However, no cartilage regeneration was associated with the lowest dose administered (1.8 × 106 cells/animal). Eight-week pilot and 1-year safety and efficacy study of TG-C to treat bilaterally single full-thickness cartilage defects (patellar groove) in goats showed in comparison to control group (vehicle) that: (1) hyaline cartilage was present in defects, without relationship to exercise restriction at 8 weeks from TG-C intraarticular administration (1 × 106 or 1 × 107 cells); and (2) a positive effect on the joint cartilage may be present at 6 months and diminish at 12 months after TG-C intraarticular administration (3 × 107 cells) [81].
Preclinical studies on experimental arthritis models in rats (streptococcal cell wall-induced arthritis) and on healthy monkeys, showed that intraarticular administration of rAAv vector containing a fusion gene, where TNFR gene was fused with gene of Fc fragment of immunoglobulin (IgG) (tgAAC94), was well tolerated, as evidenced by the lack of inflammation or joint swelling, and could suppress arthritis after a single dose of 1 × 1012–1013 DNase-resistant particles (DRP)/ml of joint volume. In fact, while the joints from control arthritic animals showed an intense inflammatory cellular infiltrates, pannus, and cartilage and bone degradation, the intraarticular injection of rAAV–TNFR:Fc decreased the cell infiltrates, reducing pro-inflammatory cytokines without detectable signs of cartilage degradation. TNFR:Fc RNA expression was detectable in joint tissue for a period of 3 months (monkey) to 1 year (rat) [98].
The patterns of IL-1Ra transgene expression through sc-rAAV vector was previously evaluated in normal and inflamed knee joints of rabbit. Inflammation status was achieved at 10–15 days after parapatellar injection of 5 × 104 HIG-82 cells retrovirally transfected with IL1β gene [60]. Sc-rAAV vector provided a 25-fold enhancement compared to conventional vector (rAAV), without differences in levels or duration of transgenic IL-1Ra expression between normal and inflamed joints. However, the transgene production of IL-1Ra determined a decrease of leukocytic infiltration in inflamed joints [60]. Subsequently, another in vivo study was carried out in a model of OA induced by mono-iodoacetate (MIA) intra-articular injection in rats to evaluate the local and systemic safety and biodistribution of sc-rAAV2.5IL-1Ra [97]. No adverse effects were found following sc-rAAV2.5IL-1Ra injection in MIA-induced osteoarthrosis rats. IL-1Ra expression persisted in the treated knees up to a year post-injection, slowing the rate of cartilage loss, without being present in other sites [97].
Preclinical in vivo studies on mice, rats, rabbits and monkeys [99, 100], with collagen II—induced arthritis, showed that ART-I02 determined an overexpression of human IFN-β in targeted joints, determining the up regulation of anti-inflammatory cytokine IL-1Ra and the down regulation of metalloproteinase 3 (MMP3) and pro-inflammatory cytokines IL-8 and IL-6. These in situ modulations determined a reduction of synovial inflammation and bone erosion with limited biodistribution to other peripheral organs. In monkey studies, it was demonstrated that intraarticular injection of ART-I02 was safety with no adverse effects. Biodistribution investigations highlighted that high copy numbers of the ART-I02 genome were found only in the injected joints, and no persistence of ART-I02 sequences were detected in other tissues. ART-I02 treated joints showed the inhibition of arthritis development with the highest dose used (0.2 × 1013 viral genomes injected) [100].
Clinical studies
Our search found eight clinical trials on OA or degenerative arthritis and 3 on inflammatory arthritis (Table 2). Seven of the clinical trials on gene therapy applied to degenerative OA are on cell therapy involving genetically modified allogenic human chondrocytes (with retrovirus vector delivered in vitro) to produce TGF-β1 and associated (ratio 1:3) to normal allogenic human chondrocytes (TG-C, TissueGene, Inc., Rockville, USA). The 2 chondrocytes fractions were administered together to provide elements critical to regenerate hyaline cartilage matrix, such as chondrocytes, TGF- β1 protein, type II collagen and glycosaminoglycans [81]. 6 of the 7 clinical trials on TG-C completed phase 1 or 2 evaluation on the safety and efficacy of the use of different modified chondrocytes concentrations in patients with grade IV degenerative arthritis of the knee joint (ICRS evaluation criteria from the MRI scan) with different lesion size:
-
Phase I: NCT00599248-2007 (lesion: >2 cm2, 12 patients aged ≥18 years, completed);
NCT02341391-2007 (lesion: 2–6 cm2, 12 patients aged ≥45 years, completed);
-
Phase II: NCT02341378-2009 (lesion: <6 cm2, 28 patients aged ≥45 years, completed);
NCT01221441-2010 (Kellgren and Lawrence–KS Grade 3, 102 patients aged 18–70 years, completed);
NCT01671072-2011 (KS Grade 2–3, lesion: >6 cm2, 54 patients aged ≥18 years, completed);
NCT01825811-2012 (lesion: 2–10 cm2, 18 patients aged ≥18 years, completed).
Table 2.
Genes delivered in preclinical and clinical studies
| Gene | Delivery system | Vector | Study | Cartilage defects | Evaluations | References |
|---|---|---|---|---|---|---|
| TG-C | Retrovirus | A phase I dose-escalating clinical trial | Knee advanced OA | Safety and biological activity | NCT00599248-2007 [94] | |
| Retrovirus | A dose-escalating, single-center, phase I clinical trial | Knee degenerative arthritis | Safety and biological efficacy | NCT02341391-2007 | ||
| Retrovirus | A multicenter, single-blind, phase II A clinical trial | Knee degenerative arthritis | Efficacy and safety | NCT02341378-2009 | ||
| Retrovirus | A double-blind, randomized, parallel-group, phase II study | Knee OA grade III | Efficacy and safety | NCT01221441-2010 [95] | ||
| Retrovirus | A placebo controlled, single-blind, randomized, multicenter phase IIB study | Knee degenerative arthritis | Efficacy and safety | NCT01671072-2011 | ||
| Retrovirus | A single-blind, randomized, parallel-group, multicenter phase II study | Knee degenerative arthritis | Efficacy and Safety | NCT01825811-2012 | ||
| Retrovirus | A placebo controlled, double-blind, randomized, parallel group, multicenter phase III study | Knee degenerative arthritis | Efficacy and Safety | NCT02072070-2013 | ||
| TGF-β | Mix (3:1) of unmodified chondrocytes+TG-C | Retrovirus | Ex vivo | Partial cartilage defect or full-thickness knee cartilage defects | Safety, efficacy, toxicity, biodistribution | [81] |
| IL1ra | Direct injection | scrAAV | A phase I study | Knee moderate OA | Local and systemic safety | NCT02790723-2016 |
| FBs | Adenovirus or scAAV | In vitro and ex vivo | Naïve knees or with IL-1β-induced arthritis | Transgenic expression and persistence, infiltrating leukocytes | [60] | |
| Direct injection | scAAV | In vivo | MIA-induced OA | Safety and biodistribution | [97] | |
| TNFR:Fc | Direct injection | rAAV | A double-blind, randomized, parallel group, phase 1 dose escalation study | Inflammatory arthritis | Safety and tolerability | NCT00617032-2004 [98] |
| rAAV | A double-blind, randomized, parallel group, phase I/II study | Inflammatory arthritis | Safety | NCT00126724-2005 | ||
| IFN-β | Direct injection | rAAV | A non-randomized, parallel-group, open-label, pahe I study | RA | Safety | NCT02727764-2016 |
| FLS (in vitro); direct injection (in vivo) | rAAV | In vitro and in vivo | Collagen-induced arthritis | Biodistribution | [99] | |
| Direct injection | rAAV | In vivo | Collagen-induced arthritis | Safety, biodistribution, and efficacy | [100] |
Starting from positive pre-clinical results on safety and efficacy (cartilage proliferation) [81], a phase I clinical trial performed in 12 patients (NCT00599248-2007) showed an improvement in knee OA symptoms and minimal adverse effects [94]. The results of one of the phase II clinical trials carried out on 102 patients (NCT01221441-2010) at 1 year follow-up, showed that TG-C had positive effects on pain levels determining a decrease of analgesics intake in treated patients compared to placebo [95].
The seventh clinical trial on TG-C is a phase 3 study (NCT02072070-2013), started in 2013 and still in progress. It compares, in 156 patients aged ≥19 years, a single TG-C intra-articular injection to the damaged knee joint (ICRS grade lesion III-IV <6 cm2) at a dose of 1.8 × 107cells vs. placebo at 28 and 52 weeks. The primary outcomes of this trial are the evaluation of symptoms, sport activities, and function as well as pain according to the International Knee Documentation Committee (IKDC) and visual analogue scale (VAS), respectively [96].
The clinical trial on gene therapy applied for degenerative arthritis (NCT02790723-2016) will evaluate, in patients aged 18–65 years with moderate knee OA, the local and systemic safety (phase I) of 10 ml intra-articular injection of three different doses (low—1011, medium—1012 and high—1013) of genetically modified synovial cells (self-complementing recombinant AAV vector delivered in vitro) that express IL-1 Ra (sc-rAAV2.5IL-1Ra, Mayo Clinic, Rochester, Minnesota, USA).
Clinical trials on gene therapy applied for inflammatory arthritis have been performed to evaluate the safety of intra-articular delivery of: (1) a rAAV serotype 2 genetically engineered vector containing the cDNA for a human tumor necrosis factor receptor (TNFR) immunoglobulin (IgG1) Fc fusion (TNFR:Fc) gene (tgAAC94, Targeted Genetics Corporation, Seattle-Washington, US) to treat patients not responding to TNFα antagonist therapy; and (2) a rAAV serotype 5 vector expressing human interferon-β (ART-I02, Arthrogen, Amsterdam, The Netherlands) to treat patients with rheumatoid arthritis.
A phase I clinical trial on tgAAC94 (NCT00617032-2004, 15 patients aged ≥18 years, completed) showed that its intra-articular injection at doses up to 1 × 1011 DRP/ml joint volume appear to be safe and well tolerated in subjects not taking TNFα antagonists, with a limited systemic biodistribution (3 days) and no joint swelling and tenderness [98]. A phase I/II clinical trial (NCT00126724) on 120 patients aged 18–75 years is still in progress; its primary outcomes are the safety (serious and very serious adverse events) and efficacy of higher and repeated doses of tgAAC94 (1 × 1011 DRP/ml or 1 × 1012 DRP/ml or 1 × 1013 DRP/ml as single dose or in 2 doses administered 12 weeks apart). The secondary outcomes are the change in tenderness and swelling of target joint, the reduction in disease activity confirmed by MRI the levels of TNFR:Fc protein in synovial fluid and anti-rAAV2 capsid neutralizing antibodies in serum.
The phase I clinical trial on ART-I02 (NCT02727764-2016, 15 female patients aged ≥18 years, underway) will evaluate the safety (treatment emergent serious adverse effects) and tolerability of its single intra-articular administration in patients with rheumatoid arthritis according to three experimental cohorts: (I) 2.4 × 1012 vector genomes (VG) single injection (n = 3 patients); (II) 2.4 × 1013 VG single injection (n = 3 patients); (III) single injection of maximum tolerated dose assessed in cohort I and II (n = 9 patients). The primary outcomes of this clinical trial will be to identify any change from baseline: clinical signs and symptoms, function of the target joint, pain, synovitis and osteitis in the injected joint, as well as any induction of immune responses against AAV5 and hIFN-β.
Conclusion
Despite numerous advances on articular cartilage regeneration, this process remains a great challenge for clinical translation. The integration of newly formed cartilage and the restoration of all cartilage-specific local structures are crucial for articular cartilage function, but so far, no technique has been able to form a native cartilage structure of in the joints [53].
Gene therapy might have a good potential for cartilage repair and OA treatment, but several problems remain to be solved. For example, the induction of Stem Cells to chondrogenesis is often followed by osteogenesis and hypertrophy, and the number of chondrocytes or chondral progenitors that can be obtained is limited [101]. Recent works suggest that the combination of appropriate delivery vectors, genes, target cells and scaffolds might offer the possibility to obtain hyaline cartilage, even though the type of lesion (size, localization, structure) determines a variation of the combination of these factors [82].
Different genes have been transfected into MSCs, chondrocytes or FBs to improve or modify their phenotypic properties [5]. Usually, the strategy developed for cartilage gene therapy has been to deliver genes which mainly codify for GFs, inducing a chondrogenic differentiation. In addition, transcription factors and anti-inflammatory cytokines, counteracting the progression of inflammatory disease, are the most employed. The preferred viral vectors are adenovirus and its recombinant vectors for all gene categories and cartilage diseases. The combination of two or more GFs genes, transcription factors or anti-inflammatory cytokines increases the effects of the single gene in reducing inflammation or improving the healing process.
Nevertheless, the clinical use of gene therapy seems to be still a distant reality because several safety and efficacy evaluations are needed before it can be applied in the clinic. Moreover, given the non-lethal nature of cartilage diseases, possible side effects are of particular interest [53].
Clinical trials are beginning to highlight the weaknesses that still exist in this type of treatment. Furthermore, the use of viral vectors is still hampered by the perception that viral vectors are not safe, especially after the occurrence of serious side effects such as leukaemia (after retrovirus treatment of X-linked SCID) and death (after the intra-articular rAAV-2 injection for the delivery of a TNF-α antagonist gene in patient affected by RA) [102]. Even though this death was not related to viral exposure, it highlighted the need to adopt further monitoring procedures during these types of clinical trials [102].
Phase I and II clinical trials on safety, biological activity and efficacy, toxicity and biodistribution are conducted mainly with GFs, such as TGF-β delivered by retroviruses into chondrocytes. In addition, anti-inflammatory cytokines are also used, including IL1ra (conveyed by FBs transfected with adenoviruses, or directly injected into the lesions), TNF receptor and immunomodulatory cytokine (IFN-β) (directly into the injury site). Retroviruses and adenoviruses or scAVV are the only vectors that have been tested in clinical trials so far.
Finally, to design highly efficient gene therapy methods, that minimize negative effects, such as host immune responses, and improve indirect procedures, it is mandatory to carry out interdisciplinary and translational studies on molecular and cellular biology, immunology and virology aspects as well as on osteochondral scaffold improvement. All efforts should aim at identifying key interactions between scaffold, cells and microenvironment of the implant, as these data will give the information necessary to the success of future gene therapy techniques for cartilage repair.
Acknowledgements
 This study has been developed with the contribution of the National Operational Programme for Research and Competitiveness 2007–2013—PONa03_00011 “Potenziamento strutturale di una rete di eccellenza per la ricerca clinica sulla terapia personalizzata in oncologia e in medicina rigenerativa”.
This study has been developed with the contribution of the National Operational Programme for Research and Competitiveness 2007–2013—PONa03_00011 “Potenziamento strutturale di una rete di eccellenza per la ricerca clinica sulla terapia personalizzata in oncologia e in medicina rigenerativa”.
Abbreviations
- ACI
Autologous chondrocytes implantation
- ACPC
Articular cartilage progenitor cells
- ADSCs
Adipose derived mesenchymal stem cells
- BMP
Bone morphogenetic protein
- BMSCs
Bone Marrow Derived Mesenchymal Stem Cells
- bPEI-HA
Branched poly(ethylenimine)-hyaluronic acid
- CIA
Collagen induced arthritis
- COLL I
Collagen I
- COLL II
Collagen II
- Col2a1
Collagen 2 alpha 1
- COMP
Cartilage oligomeric matrix protein
- DBM
Demineralized bone matrix
- DCBM
Decalcified cortical bone matrix
- DRP
DNase-resistant particles
- ECM
Extracellular matrix
- FBs
Fibroblasts
- FGF-2
Fibroblast growth factor 2
- FLS
Fibroblast-like synoviocytes
- GAG
Glycosaminoglycans
- GDF-5
Growth and differentiation factor 5
- GFs
Growth factor
- GMP
Good manufacture practice
- HIV
Human immunodeficiency virus
- IFN-β
Interferon-β
- IGF-1
Insulin-like growth factor 1
- iHH
Indian hedgehog homolog
- IKDC
International Knee Documentation Committee
- IL10
Interleukin 10
- IL1ra
Interleukin 1 receptor antagonist
- MACI
Matrix-induced autologous chondrocytes implantation
- MDSCs
Muscle-derived stem cells
- MMP
Metalloproteinase
- MSCs
MESENCHYMAL Stem cells
- OA
Osteoarthritis
- PGA
Polyglycolic acid
- PLA
Polylactic acid
- PLGA
Poly(lactic-co-glycolide)
- PU
Polyurethane
- RA
Rheumatoid arthritis
- rAAV
Recombinant adeno-associated viral vector
- RUNX2
Runt-related transcription factor 2
- scAAV
Self-complementary AAV
- sFlt-1
Soluble Fms-related tyrosine kinase 1
- SOX
Sex-determining Region Y -related High Mobility Group box
- TCP
Tricalcium phosphate
- TFs
Transcription factors
- TGF-β
Transforming growth factor β
- TNFR:Fc
Human tumor necrosis factor receptor immunoglobulin (IgG1) Fc fusion
- VAS
Visual analogue scale
- VEGF
Vascular endothelial growth factor
- ZNF145
Zinc-finger protein 145
Compliance with ethical standards
Conflict of interest
All authors have no conflict of interest.
References
- 1.Rey-Rico A, Frisch J, Venkatesan JK, Schmitt G, Rial-Hermida I, Taboada P, et al. PEO-PPO-PEO carriers for rAAV-mediated transduction of human articular chondrocytes in vitro and in a human osteochondral defect model. ACS Appl Mater Interfaces. 2016;8:20600–20613. doi: 10.1021/acsami.6b06509. [DOI] [PubMed] [Google Scholar]
- 2.Frisch J, Orth P, Venkatesan JK, Rey-Rico A, Schmitt G, Kohn D, et al. Genetic modification of human peripheral blood aspirates using recombinant adeno-associated viral vectors for articular cartilage repair with a focus on chondrogenic transforming growth factor-β gene delivery. Stem Cells Transl Med. 2017;6:249–260. doi: 10.5966/sctm.2016-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi F, Giavaresi G, Tschon M, Borsari V, Nicoli Aldini N, Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cells Dev. 2013;22:181–192. doi: 10.1089/scd.2012.0373. [DOI] [PubMed] [Google Scholar]
- 5.Ondrésik M, Azevedo Maia FR, da Silva Morais A, Gertrudes AC, Dias Bacelar AH, Correia C et al (2016) Management of knee osteoarthritis. Current status and future trends. Biotechnol Bioeng (epub ahead of print) [DOI] [PubMed]
- 6.Heiligenstein S, Cucchiarini M, Laschke MW, Bohle RM, Kohn D, Menger MD, et al. Evaluation of nonbiomedical and biomedical grade alginates for the transplantation of genetically modified articular chondrocytes to cartilage defects in a large animal model in vivo. J Gene Med. 2011;13:230–242. doi: 10.1002/jgm.1557. [DOI] [PubMed] [Google Scholar]
- 7.Shui W, Yin L, Luo J, Li R, Zhang W, Zhang J, et al. Characterization of chondrocyte scaffold carriers for cell-based gene therapy in articular cartilage repair. J Biomed Mater Res A. 2013;101:3542–3550. doi: 10.1002/jbm.a.34661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S, Chan AG, Mercer S, Eckert GJ, Trippel SB. Endogenous versus exogenous growth factor regulation of articular chondrocytes. J Orthop Res. 2014;32:54–60. doi: 10.1002/jor.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li KC, Hu YC. Cartilage tissue engineering: recent advances and perspectives from gene regulation/therapy. Adv Healthc Mater. 2015;4:948–968. doi: 10.1002/adhm.201400773. [DOI] [PubMed] [Google Scholar]
- 10.Hardee CL, Arévalo-Soliz LM, Hornstein BD, Zechiedrich L. Advances in non-viral DNA vectors for gene therapy. Genes (Basel) 2017;8:65. doi: 10.3390/genes8020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohashi S, Kubo T, Kishida T, Ikeda T, Takahashi K, Arai Y, et al. Successful genetic transduction in vivo into synovium by means of electroporation. Biochem Biophys Res Commun. 2002;293:1530–1535. doi: 10.1016/S0006-291X(02)00386-8. [DOI] [PubMed] [Google Scholar]
- 12.Khoury M, Bigey P, Louis-Plence P, Noel D, Rhinn H, Scherman D, et al. A comparative study on intra-articular versus systemic gene electrotransfer in experimental arthritis. J Gene Med. 2006;8:1027–1036. doi: 10.1002/jgm.922. [DOI] [PubMed] [Google Scholar]
- 13.Nishida K, Doita M, Takada T, Kakutani K, Miyamoto H, Shimomura T, et al. Sustained transgene expression in intervertebral disc cells in vivo mediated by microbubble-enhanced ultrasound gene therapy. Spine (Phila Pa 1976) 2006;31:1415–1419. doi: 10.1097/01.brs.0000219945.70675.dd. [DOI] [PubMed] [Google Scholar]
- 14.Li B, Li F, Ma L, Yang J, Wang C, Wang D, et al. Poly(lactide-co-glycolide)/fibrin gel construct as a 3D model to evaluate gene therapy of cartilage in vivo. Mol Pharm. 2014;11:2062–2070. doi: 10.1021/mp5000136. [DOI] [PubMed] [Google Scholar]
- 15.Zhang SK, Liu Y, Song ZM, Fu CF, Xu XX. Green fluorescent protein as marker in chondrocytes overexpressing human insulin-like growth factor-1 for repair of articular cartilage defects in rabbits. Chin J Traumatol. 2007;10:10–17. [PubMed] [Google Scholar]
- 16.Orth P, Kaul G, Cucchiarini M, Zurakowski D, Menger MD, Kohn D, et al. Transplanted articular chondrocytes co-overexpressing IGF-I and FGF-2 stimulate cartilage repair in vivo. Knee Surg Sports Traumatol Arthrosc. 2011;19:2119–2130. doi: 10.1007/s00167-011-1448-6. [DOI] [PubMed] [Google Scholar]
- 17.Leng P, Ding CR, Zhang HN, Wang YZ. Reconstruct large osteochondral defects of the knee with hIGF-1 gene enhanced Mosaicplasty. Knee. 2012;19:804–811. doi: 10.1016/j.knee.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Madry H, Orth P, Kaul G, Zurakowski D, Menger MD, Kohn D, et al. Acceleration of articular cartilage repair by combined gene transfer of human insulin-like growth factor I and fibroblast growth factor-2 in vivo. Arch Orthop Trauma Surg. 2010;130:1311–1322. doi: 10.1007/s00402-010-1130-3. [DOI] [PubMed] [Google Scholar]
- 19.Alcorn JL, Merritt TM, Farach-Carson MC, Wang HH, Hecht JT. Ribozyme-mediated reduction of wild-type and mutant cartilage oligomeric matrix protein (COMP) mRNA and protein. RNA. 2009;15:686–695. doi: 10.1261/rna.1335909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi S, Mercer S, Eckert GJ, Trippel SB. Regulation of articular chondrocyte aggrecan and collagen gene expression by multiple growth factor gene transfer. J Orthop Res. 2012;30:1026–1031. doi: 10.1002/jor.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaul G, Cucchiarini M, Arntzen D, Zurakowski D, Menger MD, Kohn D, et al. Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overexpressing human fibroblast growth factor 2 (FGF-2) in vivo. J Gene Med. 2006;8:100–111. doi: 10.1002/jgm.819. [DOI] [PubMed] [Google Scholar]
- 22.Di Cesare PE, Frenkel SR, Carlson CS, Fang C, Liu C. Regional gene therapy for full-thickness articular cartilage lesions using naked DNA with a collagen matrix. J Orthop Res. 2006;24:1118–1127. doi: 10.1002/jor.20143. [DOI] [PubMed] [Google Scholar]
- 23.Capito RM, Spector M. Collagen scaffolds for nonviral IGF-1 gene delivery in articular cartilage tissue engineering. Gene Ther. 2007;14:721–732. doi: 10.1038/sj.gt.3302918. [DOI] [PubMed] [Google Scholar]
- 24.Needham CJ, Shah SR, Dahlin RL, Kinard LA, Lam J, Watson BM, et al. Osteochondral tissue regeneration through polymeric delivery of DNA encoding for the SOX trio and RUNX2. Acta Biomater. 2014;10:4103–4112. doi: 10.1016/j.actbio.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An C, Cheng Y, Yuan Q, Li J. IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann Biomed Eng. 2010;38:1647–1654. doi: 10.1007/s10439-009-9892-x. [DOI] [PubMed] [Google Scholar]
- 26.Guo X, Zheng Q, Yang S, Shao Z, Yuan Q, Pan Z, et al. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor beta1 gene. Biomed Mater. 2006;1:206–215. doi: 10.1088/1748-6041/1/4/006. [DOI] [PubMed] [Google Scholar]
- 27.He CX, Zhang TY, Miao PH, Hu ZJ, Han M, Tabata Y, et al. TGF-beta1 gene-engineered mesenchymal stem cells induce rat cartilage regeneration using nonviral gene vector. Biotechnol Appl Biochem. 2012;59:163–169. doi: 10.1002/bab.1001. [DOI] [PubMed] [Google Scholar]
- 28.Raimondi L, De Luca A, Amodio N, Manno M, Raccosta S, Taverna S, Bellavia D, Naselli F, Fontana S, Schillaci O, Giardino R, Fini M, Tassone P, Santoro A, De Leo G, Giavaresi G, Alessandro R. Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget. 2015;6:13772–13789. doi: 10.18632/oncotarget.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tkach M, Théry C. Communication by Extracellular Vesicles: where We Are and Where We Need to Go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 30.Zappulli V, Friis KP, Fitzpatrick Z, Maguire CA, Breakefield XO. Extracellular vesicles and intercellular communication within the nervous system. J Clin Invest. 2016;126:1198–1207. doi: 10.1172/JCI81134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartil. 2016;24:2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, Gu HF, Hong JF, Cao L, Chen Y, et al. Altered microrna expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2014;9:e114627. doi: 10.1371/journal.pone.0114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui Y, Luan J, Li H, Zhou X, Han J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microrna expression. FEBS Lett. 2016;590:185–192. doi: 10.1002/1873-3468.12024. [DOI] [PubMed] [Google Scholar]
- 34.Ekstrom K, Omar O, Graneli C, Wang X, Vazirisani F, Thomsen P. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS One. 2013;8:e75227. doi: 10.1371/journal.pone.0075227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, Xiao ZD. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289:22258–22267. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, Memeo L, Manno M, Raccosta S, Diana P, Cirrincione G, Giavaresi G, Monteleone F, Fontana S, De Leo G, Alessandro R. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics. 2017;7:1333–1345. doi: 10.7150/thno.17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW, Jay SM. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2015;21:45–54. doi: 10.1089/ten.teb.2014.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ames RS, Lu Q. Viral-mediated gene delivery for cell-based assays in drug discovery. Expert Opin Drug Discov. 2009;4:243–256. doi: 10.1517/17460440902751599. [DOI] [PubMed] [Google Scholar]
- 40.Pagnotto MR, Wang Z, Karpie JC, Ferretti M, Xiao X, Chu CR. Adeno-associated viral gene transfer of transforming growth factor beta 1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14:804–813. doi: 10.1038/sj.gt.3302938. [DOI] [PubMed] [Google Scholar]
- 41.Ivkovic A, Pascher A, Hudetz D, Maticic D, Jelic M, Dickinson S, et al. Articular cartilage repair by genetically modified bone marrow aspirate in sheep. Gene Ther. 2010;17:779–789. doi: 10.1038/gt.2010.16. [DOI] [PubMed] [Google Scholar]
- 42.Xia W, Jin YQ, Kretlow JD, Liu W, Ding W, Sun H, et al. Adenoviral transduction of hTGF-beta1 enhances the chondrogenesis of bone marrow derived stromal cells. Biotechnol Lett. 2009;31:639–646. doi: 10.1007/s10529-009-9930-7. [DOI] [PubMed] [Google Scholar]
- 43.Garza-Veloz I, Romero-Diaz VJ, Martinez-Fierro ML, Marino-Martinez IA, Gonzalez-Rodriguez M, Martinez-Rodriguez HG, et al. Analyses of chondrogenic induction of adipose mesenchymal stem cells by combined co-stimulation mediated by adenoviral gene transfer. Arthritis Res Ther. 2013;15:R80. doi: 10.1186/ar4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Jt Surg Br. 2007;89:672–685. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- 45.Park J, Gelse K, Frank S, von der Mark K, Aigner T, Schneider H. Transgene-activated mesenchymal cells for articular cartilage repair: a comparison of primary bone marrow-, perichondrium/periosteum- and fat-derived cells. J Gene Med. 2006;8:112–125. doi: 10.1002/jgm.826. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Li Y, Han R, He C, Wang G, Wang J, et al. Demineralized bone matrix combined bone marrow mesenchymal stem cells, bone morphogenetic protein-2 and transforming growth factor-β3 gene promoted pig cartilage defect repair. PLoS One. 2014;9:e116061. doi: 10.1371/journal.pone.0116061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neumann AJ, Gardner OF, Williams R, Alini M, Archer CW, Stoddart MJ. Human Articular Cartilage Progenitor Cells Are Responsive to Mechanical Stimulation and Adenoviral-Mediated Overexpression of Bone-Morphogenetic Protein 2. PLoS One. 2015;10:e0136229. doi: 10.1371/journal.pone.0136229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans CH, Liu FJ, Glatt V, Hoyland JA, Kirker-Head C, Walsh A, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater. 2009;18:96–111. doi: 10.22203/eCM.v018a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng G, Wan Y, Balian G, Laurencin CT, Li X. Adenovirus-mediated expression of growth and differentiation factor-5 promotes chondrogenesis of adipose stem cells. Growth Factors. 2008;26:132–142. doi: 10.1080/08977190802105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodrich LR, Brower-Toland BD, Warnick L, Robbins PD, Evans CH, Nixon AJ. Direct adenovirus-mediated IGF-I gene transduction of synovium induces persisting synovial fluid IGF-I ligand elevations. Gene Ther. 2006;13:1253–1262. doi: 10.1038/sj.gt.3302757. [DOI] [PubMed] [Google Scholar]
- 51.Menendez MI, Clark DJ, Carlton M, Flanigan DC, Jia G, Sammet S, et al. Direct delayed human adenoviral BMP-2 or BMP-6 gene therapy for bone and cartilage regeneration in a pony osteochondral model. Osteoarthr Cartil. 2011;19:1066–1075. doi: 10.1016/j.joca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Zheng Z, Liu P, Ma Y, Lin L, Lang N, et al. The synergistic effects of microfracture, perforated decalcified cortical bone matrix and adenovirus-bone morphogenetic protein-4 in cartilage defect repair. Biomaterials. 2008;29:4616–4629. doi: 10.1016/j.biomaterials.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 53.Steinert AF, Weissenberger M, Kunz M, Gilbert F, Ghivizzani SC, Göbel S, et al. Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthritis Res Ther. 2012;14:R168. doi: 10.1186/ar3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao L, Yang F, Liu G, Yu D, Li H, Fan Q, et al. The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials. 2011;32:3910–3920. doi: 10.1016/j.biomaterials.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 55.Sieker JT, Kunz M, Weißenberger M, Gilbert F, Frey S, Rudert M, et al. Direct bone morphogenetic protein 2 and Indian hedgehog gene transfer for articular cartilage repair using bone marrow coagulates. Osteoarthr Cartil. 2015;23:433–442. doi: 10.1016/j.joca.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Knedla A, Riepl B, Lefèvre S, Kistella S, Grifka J, Straub RH, et al. The therapeutic use of osmotic minipumps in the severe combined immunodeficiency (SCID) mouse model for rheumatoid arthritis. Ann Rheum Dis. 2009;68:124–129. doi: 10.1136/ard.2007.086116. [DOI] [PubMed] [Google Scholar]
- 57.Morisset S, Frisbie DD, Robbins PD, Nixon AJ, McIlwraith CW. IL-1ra/IGF-1 gene therapy modulates repair of microfractured chondral defects. Clin Orthop Relat Res. 2007;462:221–228. doi: 10.1097/BLO.0b013e3180dca05f. [DOI] [PubMed] [Google Scholar]
- 58.Clément N, Grieger JC. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol Ther Methods Clin Dev. 2016;3:16002. doi: 10.1038/mtm.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cucchiarini M, Orth P, Madry H. Direct rAAV SOX9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J Mol Med (Berl) 2013;91:625–636. doi: 10.1007/s00109-012-0978-9. [DOI] [PubMed] [Google Scholar]
- 60.Kay JD, Gouze E, Oligino TJ, Gouze JN, Watson RS, Levings PP, et al. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J Gene Med. 2009;11:605–614. doi: 10.1002/jgm.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cucchiarini M, Madry H. Overexpression of human IGF-I via direct rAAV-mediated gene transfer improves the early repair of articular cartilage defects in vivo. Gene Ther. 2014;21:811–819. doi: 10.1038/gt.2014.58. [DOI] [PubMed] [Google Scholar]
- 62.Griffin DJ, Ortved KF, Nixon AJ, Bonassar LJ. Mechanical properties and structure-function relationships in articular cartilage repaired using IGF-I gene-enhanced chondrocytes. J Orthop Res. 2016;34:149–153. doi: 10.1002/jor.23038. [DOI] [PubMed] [Google Scholar]
- 63.Izal I, Acosta CA, Ripalda P, Zaratiegui M, Ruiz J, Forriol F. IGF-1 gene therapy to protect articular cartilage in a rat model of joint damage. Arch Orthop Trauma Surg. 2008;128:239–247. doi: 10.1007/s00402-007-0407-7. [DOI] [PubMed] [Google Scholar]
- 64.Tao K, Frisch J, Rey-Rico A, Venkatesan JK, Schmitt G, Madry H, et al. Co-overexpression of TGF-β and SOX9 via rAAV gene transfer modulates the metabolic and chondrogenic activities of human bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2016;7:20. doi: 10.1186/s13287-016-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cucchiarini M, Ekici M, Schetting S, Kohn D, Madry H. Metabolic activities and chondrogenic differentiation of human mesenchymal stem cells following recombinant adeno-associated virus-mediated gene transfer and overexpression of fibroblast growth factor 2. Tissue Eng Part A. 2011;17:1921–1933. doi: 10.1089/ten.tea.2011.0018. [DOI] [PubMed] [Google Scholar]
- 66.Venkatesan JK, Ekici M, Madry H, Schmitt G, Kohn D, Cucchiarini M. SOX9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells. Stem Cell Res Ther. 2012;3:22–36. doi: 10.1186/scrt113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hur W, Cho ML, Yoon SK, Kim SY, Ju JH, Jhun JY, et al. Adenoviral delivery of IL-1 receptor antagonist abrogates disease activity during the development of autoimmune arthritis in IL-1 receptor antagonist-deficient mice. Immunol Lett. 2006;106:154–162. doi: 10.1016/j.imlet.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Goodrich LR, Grieger JC, Phillips JN, Khan N, Gray SJ, McIlwraith CW, et al. scAAV IL-1ra dosing trial in a large animal model and validation of long-term expression with repeat administration for osteoarthritis therapy. Gene Ther. 2015;22:536–545. doi: 10.1038/gt.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ueblacker P, Wagner B, Vogt S, Salzmann G, Wexel G, Krüger A, et al. In vivo analysis of retroviral gene transfer to chondrocytes within collagen scaffolds for the treatment of osteochondral defects. Biomaterials. 2007;28:4480–4487. doi: 10.1016/j.biomaterials.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 70.Yoon HJ, Kim SB, Somaiya D, Noh MJ, Choi KB, Lim CL, et al. Type II collagen and glycosaminoglycan expression induction in primary human chondrocyte by TGF-β1. BMC Musculoskelet Disord. 2015;16:141. doi: 10.1186/s12891-015-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuroda R, Usas A, Kubo S, Corsi K, Peng H, Rose T, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54:433–442. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 72.Kubo S, Cooper GM, Matsumoto T, Phillippi JA, Corsi KA, Usas A, et al. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis Rheum. 2009;60:155–165. doi: 10.1002/art.24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee JM, Im GI. SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials. 2012;33:2016–2024. doi: 10.1016/j.biomaterials.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 74.Li H, Lu A, Tang Y, Beckman S, Nakayama N, Poddar M, Hogan MV, Huard J. The superior regenerative potential of muscle-derived stem cells for articular cartilage repair is attributed to high cell survival and chondrogenic potential. Mol Ther Methods Clin Dev. 2016;3:16065. doi: 10.1038/mtm.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, Fu FH, Huard J. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60:1390–1405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wübbenhorst D, Dumler K, Wagner B, Wexel G, Imhoff A, Gansbacher B, et al. Tetracycline-regulated bone morphogenetic protein 2 gene expression in lentivirally transduced primary rabbit chondrocytes for treatment of cartilage defects. Arthritis Rheum. 2010;62:2037–2046. doi: 10.1002/art.27461. [DOI] [PubMed] [Google Scholar]
- 77.Liu TM, Guo XM, Tan HS, Hui JH, Lim B, Lee EH. Zinc-finger protein 145, acting as an upstream regulator of SOX9, improves the differentiation potential of human mesenchymal stem cells for cartilage regeneration and repair. Arthritis Rheum. 2011;63:2711–2720. doi: 10.1002/art.30430. [DOI] [PubMed] [Google Scholar]
- 78.Vermeij EA, Broeren MG, Bennink MB, Arntz OJ, Gjertsson I, van Lent PL, et al. Disease-regulated local IL-10 gene therapy diminishes synovitis and cartilage proteoglycan depletion in experimental arthritis. Ann Rheum Dis. 2015;74:2084–2091. doi: 10.1136/annrheumdis-2014-205223. [DOI] [PubMed] [Google Scholar]
- 79.Liang W, Zhu C, Liu F, Cui W, Wang Q, Chen Z, et al. Integrin β1 gene therapy enhances in vitro creation of tissue-engineered cartilage under periodic mechanical stress. Cell Physiol Biochem. 2015;37:1301–1314. doi: 10.1159/000430253. [DOI] [PubMed] [Google Scholar]
- 80.Gouze E, Pawliuk R, Pilapil C, Gouze JN, Fleet C, Palmer GD, et al. In vivo gene delivery to synovium by lentiviral vectors. Mol Ther. 2002;5:397–404. doi: 10.1006/mthe.2002.0562. [DOI] [PubMed] [Google Scholar]
- 81.Noh MJ, Copeland RO, Yi Y, Choi KB, Meschter C, Hwang S, et al. Pre-clinical studies of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 (TG-C) Cytotherapy. 2010;12:384–393. doi: 10.3109/14653240903470639. [DOI] [PubMed] [Google Scholar]
- 82.Lu CH, Yeh TS, Yeh CL, Fang YH, Sung LY, Lin SY, et al. Regenerating cartilages by engineered ASCs: prolonged TGF-β3/BMP-6 expression improved articular cartilage formation and restored zonal structure. Mol Ther. 2014;22:186–195. doi: 10.1038/mt.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen HC, Chang YH, Chuang CK, Lin CY, Sung LY, Wang YH, et al. The repair of osteochondral defects using baculovirus-mediated gene transfer with de-differentiated chondrocytes in bioreactor culture. Biomaterials. 2009;30:674–681. doi: 10.1016/j.biomaterials.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 84.Guo T, Zeng X, Hong H, Diao H, Wangrui R, Zhao J, et al. Gene-activated matrices for cartilage defect reparation. Int J Artif Organs. 2006;29:612–621. doi: 10.1177/039139880602900611. [DOI] [PubMed] [Google Scholar]
- 85.Ham O, Lee CY, Song BW, Lee SY, Kim R, Park JH, et al. Upregulation of miR-23b enhances the autologous therapeutic potential for degenerative arthritis by targeting PRKACB in synovial fluid-derived mesenchymal stem cells from patients. Mol Cells. 2014;37:449–456. doi: 10.14348/molcells.2014.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang Y, Duan L, Xiong J, Zhu W, Liu Q, Wang D, et al. E2 regulates MMP-13 via targeting miR-140 in IL-1β-induced extracellular matrix degradation in human chondrocytes. Arthritis Res Ther. 2016;18:105. doi: 10.1186/s13075-016-0997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Z, Shi H, Sun S, Xu H, Cao D, Luo J. MicroRNA-181b suppresses TAG via target IRS2 and regulating multiple genes in the Hippo pathway. Exp Cell Res. 2016;348:66–74. doi: 10.1016/j.yexcr.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 88.Song J, Lee M, Kim D, Han J, Chun CH, Jin EJ. MicroRNA-181b regulates articular chondrocytes differentiation and cartilage integrity. Biochem Biophys Res Commun. 2013;431:210–214. doi: 10.1016/j.bbrc.2012.12.133. [DOI] [PubMed] [Google Scholar]
- 89.Lolli A, Narcisi R, Lambertini E, Penolazzi L, Angelozzi M, Kops N, et al. Silencing of anti-chondrogenic MicroRNA-221 in human mesenchymal stem cells promotes cartilage repair in vivo. Stem Cells. 2016;34:1801–1811. doi: 10.1002/stem.2350. [DOI] [PubMed] [Google Scholar]
- 90.Kim D, Song J, Jin EJ. MicroRNA-221 regulates chondrogenic differentiation through promoting proteosomal degradation of slug by targeting Mdm2. J Biol Chem. 2010;285:26900–26907. doi: 10.1074/jbc.M110.115105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Martinez-Sanchez A, Dudek KA, Murphy CL. Regulation of human chondrocyte function through direct inhibition of cartilage master regulator SOX9 by microRNA-145 (miRNA-145) J Biol Chem. 2012;287:916–924. doi: 10.1074/jbc.M111.302430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin X, Wu L, Zhang Z, Yang R, Guan Q, Hou X, et al. MiR-335-5p promotes chondrogenesis in mouse mesenchymal stem cells and is regulated through two positive feedback loops. J Bone Miner Res. 2014;29:1575–1585. doi: 10.1002/jbmr.2163. [DOI] [PubMed] [Google Scholar]
- 93.Montemurro T, Viganò M, Budelli S, Montelatici E, Lavazza C, Marino L, et al. How we make cell therapy in Italy. Drug Des Devel Ther. 2015;9:4825–4834. doi: 10.2147/DDDT.S80403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ha CW, Noh MJ, Choi KB, Lee KH. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy. 2012;14:247–256. doi: 10.3109/14653249.2011.629645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cherian JJ, Parvizi J, Bramlet D, Lee KH, Romness DW, Mont MA. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthr Cartil. 2015;23:2109–2118. doi: 10.1016/j.joca.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 96.Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC), Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS) Arthritis Care Res (Hoboken) 2011;63:S208–S228. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang G, Evans CH, Benson JM, Hutt JA, Seagrave J, Wilder JA, et al. Safety and biodistribution assessment of sc-rAAV2.5IL-1Ra administered via intra-articular injection in a mono-iodoacetate-induced osteoarthritis rat model. Mol Ther Methods Clin Dev. 2016;3:15052. doi: 10.1038/mtm.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mease PJ, Hobbs K, Chalmers A, El-Gabalawy H, Bookman A, Keystone E, et al. Local delivery of a recombinant adenoassociated vector containing a tumour necrosis factor alpha antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Ann Rheum Dis. 2009;68:1247–1254. doi: 10.1136/ard.2008.089375. [DOI] [PubMed] [Google Scholar]
- 99.Aalbers CJ, Bevaart L, Loiler S, de Cortie K, Wright JF, Mingozzi F, et al. Preclinical potency and biodistribution studies of an AAV 5 vector expressing human interferon-β (ART-I02) for local treatment of patients with rheumatoid arthritis. PLoS One. 2015;10:e0130612. doi: 10.1371/journal.pone.0130612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bevaart L, Aalbers CJ, Vierboom MP, Broekstra N, Kondova I, Breedveld E, et al. Safety, biodistribution, and efficacy of an AAV-5 vector encoding human interferon-beta (ART-I02) delivered via intra-articular injection in rhesus monkeys with collagen-induced arthritis. Hum Gene Ther Clin Dev. 2015;26:103–112. doi: 10.1089/humc.2015.009. [DOI] [PubMed] [Google Scholar]
- 101.Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014;20:596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frank KM, Hogarth DK, Miller JL, Mandal S, Mease PJ, Samulski RJ, et al. Investigation of the cause of death in a gene-therapy trial. N Engl J Med. 2009;361:161–169. doi: 10.1056/NEJMoa0801066. [DOI] [PubMed] [Google Scholar]