Abstract
Extracellular vesicles (EVs) include a variety of nanosized vesicles released to the extracellular microenvironment by the vast majority of cells transferring bioactive lipids, proteins, mRNA, miRNA or non-coding RNA, as means of intercellular communication. Remarkably, among other fields of research, their use has become promising for immunomodulation, tissue repair and as source for novel disease-specific molecular signatures or biomarkers. However, a major challenge is to define accurate, reliable and easily implemented techniques for EV isolation due to their nanoscale size and high heterogeneity. In this context, differential ultracentrifugation (dUC) has been the most widely used laboratory methodology, but alternative procedures have emerged to allow purer EV preparations with easy implementation. Here, we present and discuss the most used of the different EV isolation methods, focusing on the increasing impact of size exclusion chromatography (SEC) on the resulting EV preparations from in vitro cultured cells-conditioned medium and biological fluids. Comparatively, low protein content and cryo-electron microscopy analysis show that SEC removes most of the overabundant soluble plasma proteins, which are not discarded using dUC or precipitating agents, while being more user friendly and less time-consuming than gradient-based EV isolation. Also, SEC highly maintains the major EVs’ characteristics, including vesicular structure and content, which guarantee forthcoming applications. In sum, together with scaling-up possibilities to increase EV recovery and manufacturing following high-quality standards, SEC could be easily adapted to most laboratories to assist EV-associated biomarker discovery and to deliver innovative cell-free immunomodulatory and pro-regenerative therapies.
Keywords: Exosomes, Purification, Isolation methods, Nanomedicine, Theranostics
Introduction
The term extracellular vesicles (EVs) refers to the broad range of membrane nanovesicles that cells can generate for cell communication, containing endosomal, cytosolic and membrane molecules from the secreting cell. EV contents are enveloped with a lipid bilayer, which encapsulates and protects them from degradation. EV composition includes functional membrane-associated and luminal proteins, lipids, metabolites and nucleic acids, specially mRNAs and microRNAs, as a source of physiological and pathological information that can act paracrine and systemically [1]. In recent times, the field of research on EVs has prominently attracted the attention of both pre-clinical and clinical researchers to reveal the pathophysiological role of EVs in body fluids and potential associations of their presence, levels and/or differential cargo with many human conditions or diseases, and use them as source for novel biomarkers discovery in liquid biopsy [2]. EVs have also appeared on stage as an attractive option to deliver new pro-regenerative cell-free therapeutic approaches [3, 4].
At present, EVs are being envisioned as potential non-invasive sources of biomarkers and also as cell-free therapeutic products. Amongst their advantages to cells as therapeutic agents are that they would be unchanged by the microenvironment once administered, with no concern about embolism or differentiation, better biodistribution, ease of handling and storing, and being sterilizable by filtration. For the future, accomplishing high-quality standards in the production and testing of EVs is crucial. An unsolved issue that may explain (at least in part) the diversity of the experimental results on the EV field is the lack of a consensus method for their isolation. Here, we provide an overview of the existing EV isolation methods with the focus on the potential of size exclusion chromatography (SEC), which results in well-purified and defined EV preparations from both in vitro cultured cells-conditioned medium and multiple biological fluids, including plasma or serum, saliva, urine, breast milk, amniotic fluid, peritoneal dialysis efflux and exudate.
EV heterogeneity
Multiple names have been used to refer to EVs, according to their origin and features or functions, and many still use the popular word “exosomes” to refer to them. Nowadays, the knowledge of EV biology and biogenesis is much deeper and is yet constantly expanding. The current consensus distinguishes EVs in two different groups depending on their biogenesis: exosomes and microvesicles (MVs). Nevertheless, the presence of apoptotic bodies cannot be excluded from EV preparations.
Exosomes are specifically those nanovesicles with a size varying between 30 and 150 nm of intracellular origin generated by most prokaryotic and eukaryotic cells. In multicellular organisms, EVs have been found in all types of biofluids [1]. They are formed along the endocytic pathway through inward budding of the endosomal membrane, forming the intraluminal vesicles (ILVs) that constitute multivesicular bodies (MVB). Exosomes are released upon MVB fusion with the plasma membrane, instead of ending in the lysosomal route of degradation (Fig. 1) [5, 6]. In light of the growing knowledge on exosome biogenesis, there is more evidence on the heterogeneity of EV preparations [7], and at the same time, it provides exosome-enriched markers for their definition. To name a few, these can be hallmark molecules of early/late endosomes or MVBs such as the tetraspanin CD63, lysosomal-associated membrane proteins LAMP1 and LAMP2; proteins associated with the ESCRT machinery such as TSG101 (ESCRT-I component), ALIX, syndecan and syntenin (ESCRT-III-associated); ESCRT-independent tetraspanins (CD9, CD63, CD81) or flotillin; mechanisms for inclusion of soluble cytosolic proteins into exosomes like ubiquitinated proteins and chaperones (HSC70, HSP70); Rab proteins (RAB2B, RAB5A, RAB7, RAB9A, RAB11, RAB27A/B, RAB35) or SNARE proteins (VAMP7, YKT6).
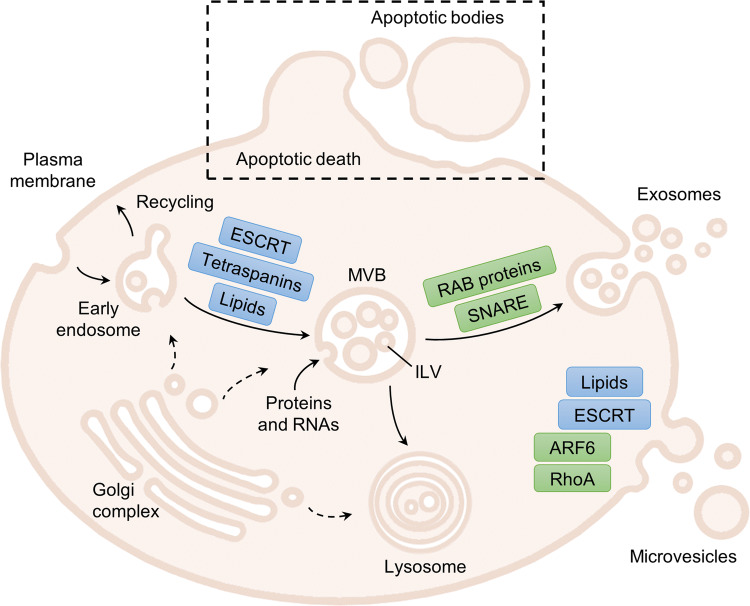
Fig. 1.
Schematic representation of EV biogenesis and secretion. The proteins or family of proteins implicated in the formation (in blue) and release (in green) of EVs are shown. Exosomes originate within the endocytic pathway, by invagination of the endosomal membrane, forming intraluminal vesicles (ILVs) in a multivesicular body (MVB). ILV generation relies on the ESCRT machinery, tetraspanins and/or lipid-mediated membrane curvature, and are loaded with proteins and RNAs (miRNA, mRNA…) from the originating cell and/or the endocytic pathway. Then, the RAB proteins mediate the trafficking through microtubules, docking to sub-membrane actin, and the SNARE proteins cause the fusion of MVBs with the plasma membrane, to release exosomes. Alternatively, early endosomes can recycle back to the plasma membrane, and MVBs can end up in the lysosome or autophagosome to degrade and recycle its cargo. Microvesicles are instead shed directly from the plasma membrane. Although a clear mechanism has not been fully defined, the loss of lipid asymmetry is important for the curvature of plasma membrane, while components of the ESCRT and SNARE machineries have been also related to the outward budding of the plasma membrane. Then, cytoskeletal remodelling through cleavage or depolymerization of cytoskeletal proteins (ARF6, RhoA) is needed for microvesicle release. The third type of EVs that can be found, apoptotic bodies, are generated upon apoptotic cell death. They are generally bigger than exosomes and microvesicles and carry “eat-me” and DAMP signals like damaged DNA. ARF6 ADP-ribosylation factor 6, DAMP damage-associated molecular pattern, ESCRT endosomal sorting complex required for transport, ILV intraluminal vesicle, MVB multivesicular body, RAB Ras-related proteins in brain (member of the superfamily of GTPases), SNARE soluble N-ethylmaleimide-sensitive fusion attachment protein (SNAP) receptors, RhoA Ras-homologue family member A GTPase. Original graphical artwork
On the other hand, MVs bud directly from the plasma membrane and have a wider size range than exosomes, with a diameter from 50 nm to up to 1 µm (Fig. 1). MV release is quickly induced after stimuli such as calcium ionophores, but the exact mechanism for MV formation and shedding remains not fully deciphered. The major mechanisms described so far for MV release relies on a rise in intracellular calcium that modulates lipid metabolism enzymes for lipid raft formation and local loss of membrane lipid asymmetry, which induces membrane curvature for MV budding. Some have also described the use of the ESCRT and SNARE machineries (TSG101, VPS4 or RAB22A, but independently of ESCRT-0) for the outward budding of MVs [8, 9]. Finally, cytoskeleton remodelling through cleavage or depolymerization of cytoskeletal proteins (ARF6, RhoA) releases MVs [10]. Although some claim MVs-enriched markers are thus phosphatidylserine-enriched membranes and annexins, MVs are indistinguishable from exosomes because they are enriched in classic exosome markers such as CD63, CD81 and TSG101 and share similar size and density.
Moreover, EV preparations can include apoptotic bodies, which originate throughout the membrane blebbing process occurring after apoptotic cell death (Fig. 1). Apoptotic bodies exhibit a diameter ranging from 1 to 5 µm, contain condensed DNA (which is absent in most reported EV studies), are rich in phosphatidylserine in the outer leaflet of the membrane and have a density between 1.16 and 1.8 g/ml, which partly overlaps with that of exosomes and MVs [11]. Therefore, apoptosis induction must be controlled—especially in in vitro EV production—to avoid artefacts coming from apoptotic bodies’ contamination instead of physiological EVs.
Methods for EV isolation
The high heterogeneity of EV populations found in a biofluid in terms of sizes, cargo content and EV markers reflects their different cell and subcellular origins [7, 12, 13]. Besides, there are different EV isolation methods, relying either on the physical or molecular characteristics of EVs for their harvest [14, 15], but due to their small size, the purification of EVs is challenging. An agreement on a gold-standard method is still missing, since every EV isolation protocol might have a certain bias towards subsets of EVs or contamination of non-vesicular particles or protein aggregates. Here, we critically review the most widespread EV isolation methodologies (Fig. 2).
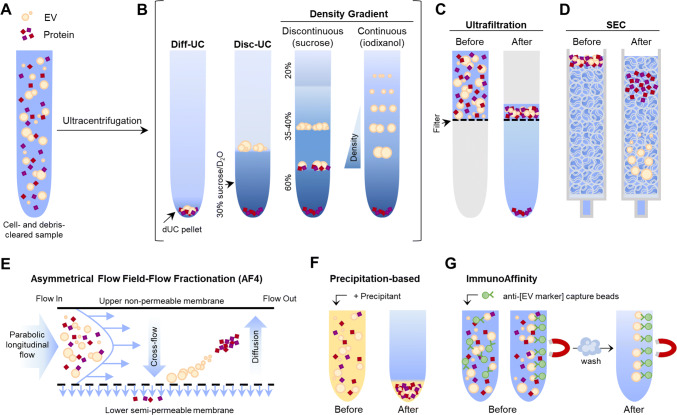
Fig. 2.
Graphical summary of mainly used EV isolation methods. a The starting sample is a cell- and debris-cleared biofluid containing EVs and proteins in suspension. b Ultracentrifugation renders an EV pellet that also contains proteins (dUC pellet), which can be further purified by discontinuous ultracentrifugation (disc-UC), like floatation in a sucrose cushion, or by density gradient (DG) ultracentrifugation: in a discontinuous gradient using different sucrose solutions or in a continuous, self-making gradient using solutions of iodixanol (optiprep). This way, proteins and the different EV populations are separated by their density. Ultrafiltration (c), size-exclusion chromatography (SEC; d) and asymmetrical flow filed-flow fractionation (AF4; e) separate molecules by their hydrodynamic radius (size). c Ultrafiltration is a dead-end filtration system that allows the separation of molecules according to the molecular weight cutoff (size) of the filter pore used. It renders a mixed sample of EVs and proteins, but allows great sample volume reduction. d In SEC, the first to elute are the molecules bigger than the matrix pores (EVs), while smaller particles within the fractionation range (proteins) get slowed down by entering the matrix bead pores and so elute later on. e In AF4, a cross-flow (field) perpendicular to the longitudinal laminar flow forces particles towards the semipermeable membrane. Particles smaller than the membrane pore are removed through the membrane. Retained ones migrate away due to diffusion and flow in the equilibrium position of the two forces (field and diffusion) according to their size. The velocity of the longitudinal flow increases parabolically, thus smaller particles, in the centre of the flow, are carried faster and elute before bigger ones. This way, proteins and differently sized EV populations are separated. f Precipitation-based isolation relies on the addition of water-excluding precipitants like PEG to concentrate all particles in one pellet. g Immunoaffinity isolation is based on EV capture using a specific antibody that recognizes an EV-specific marker, coupled to beads that can be separated by centrifugation or magnetically (like depicted). Given the lack of pan-EV markers, not all EV populations are isolated. Original graphical artwork
Differential ultracentrifugation
Differential ultracentrifugation (dUC), based on sedimentation of solutes including EVs at a high centrifugation force, was the first extended technique for EV isolation [16] and thus most commonly found in the literature [17]. However, this method is relatively time-consuming, operator sensitive, requiring prior training and a specialized ultracentrifuge, which limits EV process and subsequent scaling up to the clinical setting. There are plenty of protocols using different rotor types, washing steps, centrifugal g-forces, time and clearing factors (k-factor) that need to be adapted to the volume and viscosity of the sample [18, 19] and thus can influence the yield and purity of vesicles with distinct sedimentation coefficients [14, 20, 21]. In particular, the k-factor should determine the sufficient time of centrifugation, which changes depending on the maximum speed and rotor used, and sedimentation efficiency, which decreases with an increase in the viscosity of the sample. An approximate consensus is to use two first rounds of 400×g and 2000×g to eliminate cells and debris, respectively, with an optional centrifugation at 10,000×g to obtain apoptotic bodies and larger vesicles, and then a UC of 100,000×g for 1–2 h to obtain an EV pellet that can be further resuspended and washed in a final UC of 100,000×g for 1–2 h [16]. Nevertheless, it has been shown that added rounds of UC yield extra amounts of EVs, indicating the inefficient, low recovery of the method [21, 22]. Given that the sedimentation efficiency parameters are not standardized, the downstream analyses of these varying preparations can yield discordant results and misleading conclusions. Moreover, there are growing evidences in the field showing that centrifuging at such high speeds can negatively affect the intactness of EVs: dUC might lead to aggregation and co-precipitation with soluble proteins present in the biofluid, as albumin in plasma, or even cause vesicle rupture or fusion with contaminants and other proteins, affecting the physical properties of the exosomes and the downstream analysis of the preparation [11, 21, 23].
Floatation-related methods
There are then more stringent methods, such as floatation in a density barrier or in a density gradient after dUC, which allows improvement of EV purity and classification of EV subtypes based on exosomes having densities between 1.15 and 1.19 g/ml, while vesicles purified from the endoplasmic reticulum float at 1.18–1.25 g/ml, the ones from the Golgi at 1.05–1.12 g/ml [16] and protein at 1.35–1.41 g/ml [24]. The floatation in a sucrose cushion allows the separation of EVs from contaminating protein aggregates, based on their differential floatability on a 30% sucrose–D2O solution. This way, non-EV material like protein or protein–RNA aggregates that would be co-purified in the dUC pellet is removed [25]. For a more refined purification or fractionation of the different subpopulations of EVs, the density gradient (DG) floatation method can be employed. DG separates according to their physical characteristics (size, shape and density), in which EVs migrate to their equilibrium buoyant density in a continuous or discontinuous gradient. The first is self-generated by ultracentrifugation of 5–60% iodixanol layers [e.g. 60% (w/v) aqueous iodixanol, density: 1.320 ± 0.001 g/ml], while the second maintains the density separation between 20 and 60% sucrose layers, with EV finding equilibrium at the 35–40% sucrose layer as already described in the early definition of exosomes [26]. The sample loading can be performed for a bottom-up or a top-down migration, and the gradient ranges adjusted for the distinction of EV subpopulations according to the viscous solution used to form the gradient, considering to always wash it off for EV functional analysis to reduce osmotic pressure, toxicity and potential methodological artefacts. These methods are mainly used in research studies addressing the basic science of EV heterogeneity and biology, as these can differentiate exosomes from other EV types [12, 27–29]. However, these methods are surely difficult to translate into the clinic given their more expensive and time-consuming costs, their low and operator-dependent yield, the need of an ultracentrifuge and lack of automatization [25].
Precipitation-based protocols
On the other side, there are the precipitation-based protocols, which are user friendly, cheap and easy to implement as a common practice in the laboratory, firstly described to attain the highest EV recovery. The majority are based on the polyethylene glycol (PEG)-based volume exclusion precipitation; thus, recovery can be high, but with not much purity, as it basically precipitates all soluble particles, including EVs, to a pellet. Variations of the PEG method can be found in isolation kits available from different companies (e.g. ExoQuick, TEI), containing volume-excluding polymers (e.g. PEG, dextrans or polyvinyls). The precipitating agent is hardly removed from the final preparation, and as the pellet is obtained by high-speed centrifugation it is commonly contaminated with off-target protein aggregates [30, 31]. Nevertheless, these methods, with high translational potential, given their manageable standardization and scalability, are being studied and compared with dUC to switch to an easier EV isolation method.
In terms of recovery and purity of the EV sample, dUC yields a lower recovery rate but higher purity compared to commercial kits based on polymeric precipitation, although contaminating proteins are still found in the EV pellet [32, 33]. Depending on the principle and affinity of the isolation method, preparations vary enormously, including subpopulations with different protein contents, enriched in different EV protein markers [34]. In addition, their combination with different RNA extraction methods changes RNA yield and purity, with discordant small RNA profiles and miRNA content [32], and RNA levels might not even correlate to sample size [33]. Therefore, although they are easy to apply, precipitation-based isolation might not be the recommended method of choice for descriptive or functional analysis [35] of bona fide EVs.
Immunoaffinity isolation
Besides the methods that rely on the physical characteristics of EVs for their isolation, there are also techniques based on the analysis and/or separation of specific EVs according to their surface protein expression. The study of the EV subpopulations that DG allows and the research on their biogenesis have contributed to increase our knowledge of markers enriched in EVs, which can be used for their immunoaffinity isolation. Immunoaffinity isolation can be used in small volumes, in an analytics (e.g. ELISA-like) approach but also can be coupled to magnetic isolation, which renders an EV preparation that can be used for content or even functional downstream analysis [36]. However, a pan-EV marker has not been found yet, so the EV preparations obtained in this case are always a biased EV subpopulation expressing the marker(s) used for isolation. There are different kits already available in the market using mainly antibodies against the tetraspanins CD9, CD63 and CD81 alone or in combination for “total” EV fishing from a biofluid [37]. With the aim of including as many EV populations as possible, other affinity purifications have been envisioned, using broad EV-binding molecules such as heparin, heat shock protein- or phosphatidylserine-binding peptides (Vn96 and Tim4) or membrane curvature sensor peptides [38–41]. Other approaches use cell/tissue-specific markers to isolate or detect only the EVs coming from a target cell/tissue of interest, which is a quite attractive option in a pathological context, such as cardiovascular diseases or cancer. Although promising, this technique still lacks specific and fully deterministic EV markers, which biases EV biology study and biomarker screening and also, being still the most expensive method, deters its wide use in the clinics.
Flow cytometry
There are relentless efforts towards detection and isolation of EVs by flow cytometry, as it would allow high-throughput, multi-parametric analysis and separation of single EVs based on their surface composition. Their submicron size and low refraction index are, however, major drawbacks in using this technique [42]. The first issue is that particles of < 600 nm (EVs) fall below the detection limit of the forward/side scattered (FSC/SSC) light detectors, with scattered light signals overlapping with the buffer’s and electrical noise in the current sensitivity of flow cytometers. This can be solved by working with fluorescently labelled EVs, which allows using a fluorescence rather than the FSC or SSC trigger channel for EV detection. The second problem is to find proper size-standardization beads of adequate refractive index to better correlate scatter units to EV diameter and signals between instruments [43]. The third problem of their small size is swarm detection in current fluidics systems. This means that more than one particle is analysed at once, something easily avoided in cell analysis with the doublet exclusion gating, but not possible in EV analysis due to the negligible differences between the FSC-A/HW and SCC-A/H/W signals and interference of non-vesicular particles in the light scatter and fluorescence parameters. Technically, this can be improved by serially diluting the EV sample to work on the EV concentration that has a linear correlation with EV detection numbers [44]. There are also modified high-resolution flow cytometers for the specialized analysis and sorting of EVs, with dedicated fluidics (i.e. varying nozzle size and sheath pressure), but still relying on stained EV preparations that thus need proper controls to minimize dye or antibody-related artefacts [45–48]. Indeed, this powerful technique still needs optimization and standardization for its full work on the EV field.
Alternative methods have been established in recent years to replace traditional EV isolation techniques in search of more user- and EV-friendly procedures. There are methods gaining interest that separate EVs according to their size relying on the correlation between elution volume or diffusion coefficient and the molecule’s hydrodynamic radius, without the need of ultrahigh centrifugations or content-based selection of EV subpopulations. These are size-exclusion chromatography (SEC, also referred to as gel filtration) [49, 50], ultrafiltration and field-flow fractionation (FFF), and its lately evolved version, the asymmetrical flow FFF (AF4).
Ultrafiltration
EV fractionation by ultrafiltration (UF) allows for separation using semipermeable membranes with defined pore size or molecular weight cutoffs [51, 52]. Nevertheless, the deviation/range of particles going through a given pore size does not allow the fractionation of the different EV types or assure protein separation. It is nonetheless a quick, easy and valid method to reduce sample volume with minimal EV interference, which has been proven to yield a better recovery than concentrating EVs by UC [22, 53].
Field-flow fractionation
Another technique that separates EVs with minimal interaction is field-flow fractionation (FFF). Briefly, this technology relies on separation of particles in a channel with parabolic longitudinal flow combined with an external gradient or “field”. The field can be generated through the application of thermal energy (thermal FFF), centrifugal force (sedimentation FFF), electrostatic force (electrical FFF) or cross/tangential flow applied through one (asymmetric; AF4) or two semipermeable membranes (cross or tangential flow filtration; FFFF/TFF). This way, depending on the interaction of the field with the particles, they separate into different layers. At the same time, a longitudinal flow (perpendicular to the generated field) carries particles through the channel and, given the different velocities of the flow streamlines, particles running in different field-induced layers are separated [54]. This technology has been lately adopted for EV separation, fractionating EVs according to their distinct electrophoretic mobility by electrical FFF or hydrodynamic diameter (size/molecular weight) by FFFF/TFF/AF4 [13, 55–58]. For instance, AF4 manages a high-resolution EV subpopulation separation, with 10 nm accuracy, once crucial experimental parameters such as cross-flow velocity, membrane cutoff (commonly 10-kDa) and channel thickness are optimized [57]. It can also be coupled to multidetection systems such as UV detectors (260 and 280 nm) to monitor particle elution [55], multi-angle light scattering (MALS) [56, 57] and dynamic light scattering (DLS) to predict the root mean square radius (best for 10–500 nm particles) and the hydrodynamic radius of the particles (0.5–200 nm particles), respectively [56]. On the other hand, thanks to the moderate negative charge of EVs [zeta (ζ) potential of − 16 mV in PBS buffer] [59, 60], EV can also be separated by electrical FFF, when diluted in buffers with low ionic strength [58].
While its use is still at its infancy in the EV field, lack of a static phase and label-free isolation permits the absence of interactions with the sample given, yielding untouched EV preparations for large-scale production.
Microfluidics technology
Microfluidics technology and on-chip biosensors are also very promising technologies for high-throughput analysis using a minimal sample volume and reagent consumption in integrated miniaturized devices, separating EVs according to size, external markers or innovative sorting mechanisms such as acoustic, electrophoretic or electromagnetic fields. Thus, these new methods are subject of ongoing research and not yet developed enough for standardized, broad use for EV isolation but with great perspectives [61–63].
Alternatively, in the following section, we describe the basis and advantages of SEC as an increasingly used isolation method for EVs.
Size-exclusion chromatography
It was back in 2014 when Böing et al. resumed the use of SEC to isolate EVs from a biofluid [50]. In fact, this methodology was actually already employed in the beginning of the EV field to demonstrate the existence of EV-enclosed proteins aside from the rest of soluble molecules [26]. Particularly, the isolation by SEC is based on the differential elution profiles of particles of different sizes running through a porous polymer, constituting the stationary phase—also known as gel filtration matrix or resin—and carried through the mobile phase of the SEC column. Small particles, such as proteins, are slowed down by entering the pores of the polymer, so they elute later than EVs. EVs, being bigger than the polymer’s pores, travel quicker along the column and so elute first, right after the column’s void volume [49].
Methodologically, there are different stationary phases that can be used (Table 1). Sepharose CL-2B is the most employed matrix for successful EV isolation, yielding better purity and recovery than alternative procedures such as dUC [50, 64]. There are some studies arguing the use of other polymers with smaller intra-pores such as Sepharose 6B as initially described [26], Superdex 200, used by some to avoid disrupted EVs co-isolated after dUC [65] and Sepharose CL-4B or Sephacryl S-400 [21, 66], which allow a more refined separation of EV from serum and plasma, to reduce co-elution with common contaminants such as albumin or lipoproteins, found specifically in this body fluid [21]. At the same time, it can be combined with other methodologies to best suit the experimental approach and purposes. For instance, while SEC allows the separation of EV from HDL particles found in a plasma sample, that otherwise co-precipitate when using dUC [50], it cannot fully exclude other lipoproteins like chylomicrons (75–1200 nm), LDL (25 nm) or VLDL (30–80 nm) [67, 68]. It can be then combined with floatation in a density cushion to eliminate them [68], or use a combination of SEC with bind–elute chromatography, which uses hydrophobic, positively charged octylamine ligands within the core of the matrix particles that enhance entrapment of small soluble proteins and impurities (kDa < resin’s exclusion limit) found in plasma [69, 70]. Also, given the dilution of the EV sample after SEC elution, SEC can be downstream processed with ultrafiltration to concentrate the EV pooled fractions.
Table 1.
Specifications of the different stationary phase polymer types used as SEC matrix for EV isolation
| SEC matrix | Polymer type | Particle size (μm)a | Fractionation range (kDa) | Exclusion limit (kDa)b | Exclusion limit (nm)d | %e | |
|---|---|---|---|---|---|---|---|
| Protein Mbr | Dextrans Mcp | ||||||
| Sepharose CL-2B | 2% cross-linked agarose | 60–200 | 70–40,000 | 100–20,000 | 40,000 | 75 | 45.8 |
| Sepharose CL-4B | 4% cross-linked agarose | 45–165 | 60–20,000 | 30–5000 | 20,000 | 42 | 10.4 |
| Sepharose CL-6B | 6% cross-linked agarose | 40–165 | 10–4000 | 10–1000 | 4000 | 24 | 2.1 |
| Superose 6 | Cross-linked agarose | 8.6 | 5–5000 | 1–300 | 40,000 | 29 | 6.3 |
| Superdex 200 | Cross-linked agarose and dextran | 8.6 | 10–600 | 1–100 | 1300 | 13 | 16.7 |
| Sephacryl S-200 HR | Cross-linked allyl dextran and N,N′-methylene bisacrylamide | 25–75 | 5–250 | 1–80 | 250 | 7.7 | 2.1 |
| Sephacryl S-300 HR | 25–75 | 10–1500 | 2–400 | 2000 | 13 | 0 | |
| Sephacryl S-400 HR | 25–75 | 20–8000 | 10–2000 | 8000 | 31 | 6.3 | |
| Sephacryl S-500 HR | 25–75 | – | 40–20,000 | 20,000 | 42 | 6.3 | |
| Sephacryl S-1000 SF | Spherical allyl dextran and N,N′-methylene bisacrylamide | 40–105 | – | 500–100,000 | 100,000 | 400 | 4.2 |
The SEC matrix types enumerated are available from different manufacturers both as bulk chromatography resin or as already-made columns for bench-top use and also compatible with automatic chromatography systems, e.g. FPLC systems
aDiameter of the matrix particles
bMolecular weight of globular proteins
cPeak molecular mass of dextrans
a,b,cValues according to manufacturers
dMatrix pore sizes according to [90], unless stated by manufacturer
ePercentage of works using the corresponding SEC resin out of the total scientific publications using SEC for EV isolation (EV-Track database available in January 2019; n = 48)
An adaptation of a previously published protocol by Böing and co-workers [50] has been used in our laboratory for the optimization of EV isolation from different biofluids, including urine, plasma, peritoneal dialysis effluent and cell culture-conditioned medium [71–74]. Of note, we were able to use this method to efficiently distinguish EV-associated proteins from rather soluble proteins [75], which can misleadingly be associated with the EV preparation using other less stringent methods such as precipitation-based or ultracentrifugation methods. Also, the fact that SEC enables a more accurate EV purification allows novel biomarker screening that is not hindered by masking with the highly abundant soluble proteins [74, 76, 77]. Moreover, in search of novel biomarkers of disease progression, we have used SEC to describe the distinct proteomic signature between peripheral EVs derived from patients afflicted by dilated cardiomyopathy and those from healthy subjects [75, 77].
This also applies when trying to unravel EV’s genuinely functional capacities. For instance, our results have shown the use of SEC to be greatly efficient to prepare well-purified and defined preparations of mesenchymal stem cell-derived EVs with immunomodulatory potential, as determined in polyclonal T cell proliferation assays and after analysis of cytokine induction [71]. Indeed, SEC allowed the purification of EVs that mimicked MSC’s immunosuppressive functionality, while dUC did not.
Furthermore, we compared SEC to precipitating-based methods, demonstrating that it minimally alters the EV preparation [35]. Only SEC allowed the detection of the EV-specific markers CD9, CD63 and CD81, LGALS3BP and CD5L, suggesting a putative interference of the precipitating agents in the inherent structure and composition of the EVs recovered [35]. Furthermore, when added to cultured cells, PEG and PRotein Organic Solvent PRecipitation (PROSPR)-based EV isolation resulted in reduced cell viability in in vitro assays [35].
Regarding adaptation to most laboratories, SEC is scaling up to be more widely used for EV fractionation, as it offers a simpler, quicker, purer and more functional untouched EV product than traditional methods. SEC would be easily implemented since its bench-top use is relatively cheap compared to other methods, the columns can be re-used after washing steps and also autoclaved [78]. Furthermore, SEC can be automated, in which case it would require special equipment, e.g. FLPC systems, but also improve product consistency and data reproducibility by reducing hands-on time and thus user influence. Samples to be fractionated can be concentrated before loading to SEC, by means of dead-end ultrafiltration for small volumes (≤ 500 ml) or TFF, which is scalable for larger volumes and avoids membrane saturation, using different pore sizes (10–100 kDa).
In terms of worldwide practice, the EV field is experiencing a subtle transition in the EV isolation method of choice. Besides our study, many other works demonstrated better performance in EV recovery and purity of SEC compared to dUC [14, 21, 30], while others showed the influence of dUC in deficient standardization on efficiency [18] and there is also evidence that dUC could modify EV intactness [37]. Thus, although dUC (without density gradient floatation) is still the most commonly used technique [17], SEC is increasingly being adopted and more widespread when articles published from 2017 are considered (Fig. 3). In particular, it is mainly employed as a combination method, as we described, with initial centrifugation steps to remove cells and debris and ultrafiltration to manage sample volume. SEC can be applied to multiple biofluids and, depending on the starting sample, the pre-processing might be more demanding [78]. For instance, plasma has to be depleted of platelets to avoid contamination with activated platelet-derived EVs, but it does not need concentration prior to SEC loading to obtain enough recovery for downstream analysis [73, 77]. On the other hand, urine requires pre-processing to disaggregate the Tamm–Horsfall protein tangles—to release entrapped EV—and concentration to improve EV yield. This is also the case when working with conditioned culture medium and also peritoneal dialysis effluent, which are best concentrated by means of ultrafiltration or TFF to be loaded to an SEC column [71, 76, 79, 80].
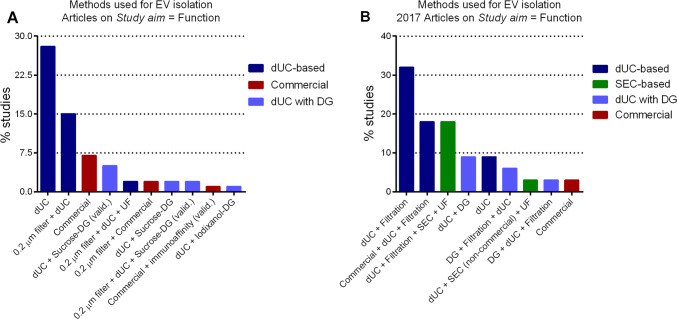
Fig. 3.
The methodology used for EV isolation is shifting towards a broader use of SEC. EV isolation protocols used in articles published and registered in the EV-TRACK database with the study aim on the function of EVs (a, n = 937) or only accounting articles published in 2017 (b, n = 34). dUC is by far the most used EV isolation method and used also in combination with other methods as starting steps to get rid of cell debris and large vesicles. Commercial methods mainly refer to proprietary methods based on precipitating agents and appear overall as the second most used after dUC. DG is mostly used as a validation technique (valid.) or in combination with dUC—to get rid of the gradient-forming solution—for downstream functional assays. While SEC is absent as one of the methods of choice when looking at all the literature (a), it appears as the second method of choice for EV isolation when only articles published in 2017 are considered (b), always as a combination method as the one described in Sect. 2. DG density gradient, dUC differential ultrafiltration, SEC size exclusion chromatography, UF ultrafiltration.
Data source: EV-TRACK database, August 2018 [89]
Towards clinical EV-based platforms: large-scale and quality manufacturing
With an interest in studying their promising applications into clinics, in which large amounts of safe and multifunctional EVs will be potentially required, numerous investigations focus on ensuring a standardized, large-scale preparation of EVs [81–84]. In this context, compared to alternative cell-based products, the use of EVs seems to be advantageous in terms of safety and low toxicity, biocompatibility, ease of handling and storage, biological permeability, high immunomodulatory ability and efficacy in both autologous and allogeneic therapeutic scenarios [85, 86].
As described above, each isolation method has intrinsic benefits and restrictions in terms of complexity, cost-efficacy, yield, purity and functionality of the EV recovered. This is especially of paramount importance, since the EVs’ field evolves towards a controlled manufacturing at the appropriate scale to develop distinct EV-based products as novel tools in a vast array of clinical scenarios. However, regardless the isolation technique, the yield of EVs is still a limiting factor. Thus, next-generation approaches that replace currently used methods for purifying EVs and guarantee significantly higher EV yields are mandatory. Indeed, the inclusion of sequential steps comprising filtration-aided concentration procedures followed by SEC should be a technical advancement for EV manufacturing. This would lead to lower volume, easier to process compared to the vast quantities of conditioned medium that are presently recovered from large-scale cell cultures, and to more defined products without compromising the EV identity. Also, large-scale EV production will require a variety of specialized facilities, skilled personnel and sufficient financial resources to generate stable and effective EVs consistently, from batch to batch, in compliance with the complex regulatory recommendations. To that end, the maintenance of well-controlled master and post-production cell banks without animal-derived growth supplements, together with the use of bioreactors allowing high cell density or growth surface, media recirculation and repeated EV recovery are currently under scrutiny to meet clinical standards. Furthermore, instead of using large volumes of media from standard two-dimensional plastic tissue culture dishes or flasks—that does not really mimic the in vivo environment—innovative hollow-fibre bioreactors containing semipermeable fibres allow to increase surface area for cell growth and provides more trustworthy three-dimensional living conditions [82, 87]. In addition, routine quality control tests such as those ensuring sterility and no presence of bacterial endotoxins as well as EV potency are crucial for the development of EV-based therapeutic platforms. With no standardized techniques for EV enumeration, overcoming batch-to-batch variation in degree of efficacy and determination of optimal EV dose are also challenging, thus urging the development of reliable potency assays [86].
Conclusions and perspectives
EVs constitute powerful biological agents of intercellular communication due to their capability of transferring proteins, lipids and nucleic acids, thereby influencing pathophysiologically both recipient and parent cells. While the role of EVs in different pathological processes such as cancer, autoimmune and cardiovascular diseases is under intensive investigation, some researchers are paving the way for potential EV-based clinical applications, including cell-free therapy and disease-specific diagnosis and/or prognosis (Fig. 4). For that, it would be mandatory to develop reliable automated production platforms that ensure the scale-up to clinical manufacturing as quality assurance, and to obtain highly purified EV fractions for further analyses in biomarker discovery. SEC-based isolation of EVs would rapidly be translated to high-quality facilities given the already established use of chromatography for clinical-grade GLP procedures such as isolation of antibodies for immunotherapy infusion [88].
Fig. 4.
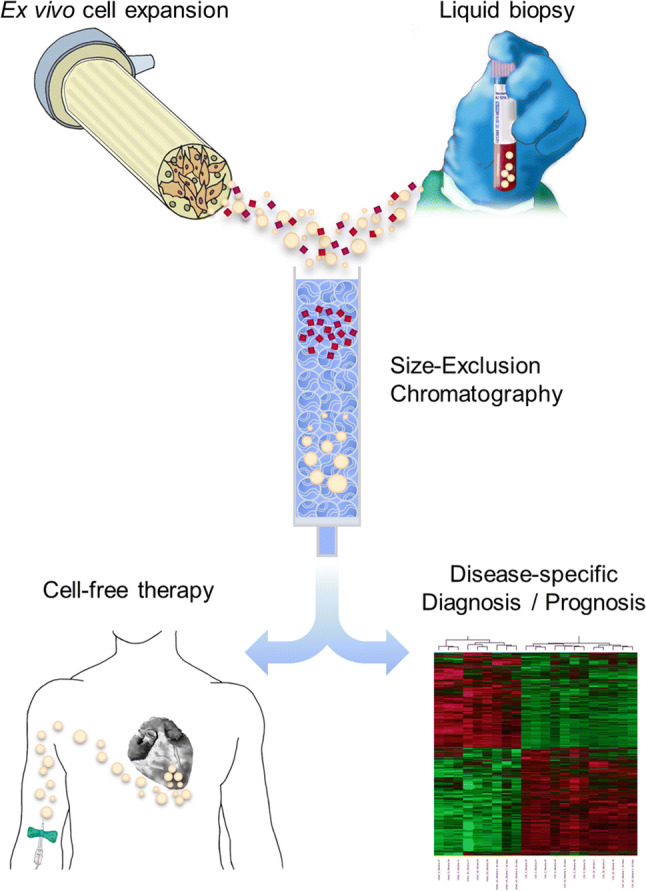
Potential EV-based clinical applications. Highly purified EV preparations extracted from both ex vivo cultured cells-conditioned medium and body fluids by using SEC are greatly expected to be part of emerging cell-free therapies and source of novel biomarkers for disease-specific diagnosis and/or prognosis, respectively. In brief, donor cells seeded in specialized bioreactors designed to highly increase cell growth area and better reproduce three-dimensional conditions may produce safe and multifunctional EVs for potential therapeutic administration in standardized, large-scale and high-quality context. In addition, SEC obtains very purified EV preparations derived from almost all body fluids, including peripheral blood samples, for further multiple screening with disease-specific markers by using e.g. omics technologies
In this context, each EV isolation method has its own strengths and pitfalls, and thus depending on the type of initial sample to be processed and purpose/downstream use of the EVs, a specific method would be advised (Table 2).
Table 2.
Qualitative summary of the different EV isolation techniques
| Diff-UC | Disc-UC | DG | Precip | IA | FC | SEC | UF | FFF | MF | |
|---|---|---|---|---|---|---|---|---|---|---|
| Puritya | 1 | 3 | 3 | 1 | 3 | 2 | 3 | 1 | 3 | 3 |
| Resolutiona | 1 | 2 | 3 | 1 | 3 | 2 | 2 | 1 | 3 | 3 |
| Recoverya | 2 | 1 | 1 | 3 | 2 | 1 | 3 | 2 | 3 | 1 |
| Functional studiesb | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 1 |
| Ability to concentrateb | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 2 |
| Ease of usea | 2 | 1 | 1 | 3 | 2 | 1 | 3 | 3 | 1 | 2 |
| Time commitmenta | 2 | 1 | 1 | 3 | 1 | 2 | 3 | 3 | 2 | 2 |
| Scalabilitya | 2 | 1 | 1 | 3 | 2 | 1 | 3 | 2 | 3 | 1 |
| Specialized equipmentb | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 |
| Commercialb | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 1 |
| Automatizationb | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Costc | 3 | 2 | 1 | 3 | 1 | 2 | 3 | 2 | 3 | 2 |
The score in Table 2 has been given according to the degree of demonstrated completion of the indicated features. The user should correlate this abbreviated description with the specific considerations for each method, sample type and downstream application, as discussed in the text. Purity: separation of EV from the bulk of protein and other contaminants. Resolution: ability to separate different EV populations, either in terms of size, density or marker-defined populations. Recovery: efficiency in terms of the EV yield. Functional studies: possibility to use the processed sample for functional studies without further refinement. Ease of use: according to technical difficulty and need of specific training to perform the procedure. Time: experimental time expressly spent for the processing of one sample. Scalability: possibility to scale-up sample processing without sensibly increasing time, expensive equipment or personnel needed. Specialized equipment: need of expensive, specialized non-consumable equipment to process the sample. Commercial: availability of commercial kits to perform the method. Automatization: ease of an automated sample processing conversion
Cost consumables spent in the processing of one sample, UC ultracentrifugation, diff-UC differential UC, disc-UC discontinuous (cushion) UC, DG density gradient UC, Precip precipitation-based isolation, IA immunoaffinity isolation, FC flow cytometry, SEC size exclusion chromatography, UF ultrafiltration, FFF field-flow fractionation, MF microfluidics technology
aQualitatively, from 1(low) to 3(high)
b1(no)–2(yes)
c1(expensive) to 3(cheap) cost per sample
In terms of functional activity of isolated EVs, it can be actually modified depending on the isolation method used. For instance, as we lately reported, precipitation-based isolation of EVs may affect cell viability of target cells (Gámez-Valero et al. [35]), while SEC-isolated EVs maintain the genuine EV functions compared to dUC [71, 80]. SEC also shows technical advantages such as it can remove most of the overabundant soluble plasma proteins which are not discarded using dUC or precipitating agents, allowing the recovery of purer EV preparations. Indeed, SEC is more user friendly and less time-consuming than other EV isolation methods such as those based on gradient establishment and highly maintains EVs’ properties to guarantee forthcoming applications.
In sum, together with scaling-up possibilities to increase the amount of EVs recovered, SEC could be easily adapted to most laboratories to assist EV-based research and clinical applications in the incoming years.
Acknowledgements
This work was supported in part by Fundació La Marató de TV3 (201516-10, 201502-30), SGR programme of Generalitat de Catalunya (2017-SGR-301 REMAR Group, and 2017-SGR-483 ICREC Group), ISCIII-REDinREN (RD16/0009 Feder Funds) and Instituto Carlos III (PI17/00336), grants from the Spanish Ministry of Economy and Competitiveness—MINECO (SAF2017-84324-C2-1-R), Instituto de Salud Carlos III (PI17/01487, PIC18/00014), Red de Terapia Celular-TerCel (RD16/00111/0006), CIBER Cardiovascular (CB16/11/00403) projects, as part of the Plan Nacional de I + D+I, and co-funded by ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER). MMT is sponsored by the PERIS (SLT002/16/00234) from the Generalitat de Catalunya, “la Caixa” Banking Foundation, and Societat Catalana de Cardiologia. This work has been developed in the context of AdvanceCat with the support of ACCIÓ (Catalonia Trade & Investment; Generalitat de Catalunya) under the Catalonian ERDF (European Regional Development Fund) operational program 2014–2020; FEB is a researcher from Fundació Institut de Recerca en Ciències de la Salut Germans Trias i Pujol, supported by the Health Department of the Catalan Government (Generalitat de Catalunya).
Author contributions
MM-T, SR and FB wrote the manuscript; MM-T, CG-M, AB-G, SR and FB: critical reading and final approval of the manuscript. All authors reviewed the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Santiago Roura, Phone: (+34) 93 497 8670, Email: sroura@igtp.cat.
Francesc E. Borràs, Phone: (+34) 93 497 8676, Email: feborras@igtp.cat
References
- 1.Yáñez-Mó M, Siljander PR-M, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379:958–966. doi: 10.1056/NEJMra1704286. [DOI] [PubMed] [Google Scholar]
- 3.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fais S, O’Driscoll L, Borras FE, et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano. 2016 doi: 10.1021/acsnano.5b08015. [DOI] [PubMed] [Google Scholar]
- 5.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 7.Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 8.Booth AM, Fang Y, Fallon JK, et al. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang T, Gilkes DM, Takano N, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci. 2014;111:E3234–E3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hugel B, Martínez MC, Kunzelmann C, Freyssinet J-M. Membrane microparticles: two sides of the coin. Physiology. 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 11.van der Pol E, Böing AN, Harrison P, et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 12.Willms E, Johansson HJ, Mäger I, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6:22519. doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Freitas D, Kim HS, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20:332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Willms E, Cabañas C, Mäger I, et al. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9:738. doi: 10.3389/fimmu.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Théry C, Clayton A, Amigorena S, Raposo G. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;2006:3.22.1–3.22.29. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 17.Gardiner C, Di Vizio D, Sahoo S, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell vesicles. 2016;5:32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Momen-Heravi F, Balaj L, Alian S, et al. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol. 2012;3:162. doi: 10.3389/fphys.2012.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livshits MA, Khomyakova E, Evtushenko EG, et al. Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319. doi: 10.1038/srep17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cvjetkovic A, Lötvall J, Lässer C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles. 2014;3:23111. doi: 10.3402/jev.v3.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baranyai T, Herczeg K, Onódi Z, et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. 2015;10:e0145686. doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welton JL, Webber JP, Botos L-A, et al. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J Extracell vesicles. 2015;4:27269. doi: 10.3402/jev.v4.27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linares R, Tan S, Gounou C, et al. High-speed centrifugation induces aggregation of extracellular vesicles. Routledge: Taylor & Francis; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer H, Polikarpov I, Craievich AF. Average protein density is a molecular-weight-dependent function. Protein Sci. 2004;13:2825–2828. doi: 10.1110/ps.04688204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles. 2013;2:19861. doi: 10.3402/jev.v2i0.19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 27.Bobrie A, Colombo M, Krumeich S, et al. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1:18397. doi: 10.3402/jev.v1i0.18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aalberts M, van Dissel-Emiliani FMF, van Adrichem NPH, et al. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol Reprod. 2012;86:82. doi: 10.1095/biolreprod.111.095760. [DOI] [PubMed] [Google Scholar]
- 29.Jeppesen DK, Hvam ML, Primdahl-Bengtson B, et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J Extracell Vesicles. 2014;3:25011. doi: 10.3402/jev.v3.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Int: Biomed Res; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Y-T, Huang Y-Y, Zheng L, et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. 2017;40:834–844. doi: 10.3892/ijmm.2017.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helwa I, Cai J, Drewry MD, et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS One. 2017;12:e0170628. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Royo F, Zuñiga-Garcia P, Sanchez-Mosquera P, et al. Different EV enrichment methods suitable for clinical settings yield different subpopulations of urinary extracellular vesicles from human samples. J Extracell Vesicles. 2016;5:29497. doi: 10.3402/jev.v5.29497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, et al. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci Rep. 2016;6:33641. doi: 10.1038/srep33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koliha N, Wiencek Y, Heider U, et al. A novel multiplex bead-based platform highlights the diversity of extracellular vesicles. J Extracell vesicles. 2016;5:29975. doi: 10.3402/jev.v5.29975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiklander OPB, Bostancioglu RB, Welsh JA, et al. Systematic methodological evaluation of a multiplex bead-based flow cytometry assay for detection of extracellular vesicle surface signatures. Front Immunol. 2018;9:1326. doi: 10.3389/fimmu.2018.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balaj L, Atai NA, Chen W, et al. Heparin affinity purification of extracellular vesicles. Sci Rep. 2015 doi: 10.1038/srep10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh A, Davey M, Chute IC, et al. Rapid isolation of extracellular vesicles from cell culture and biological fluids using a synthetic peptide with specific affinity for heat shock proteins. PLoS One. 2014;9:e110443. doi: 10.1371/journal.pone.0110443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakai W, Yoshida T, Diez D, et al. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep. 2016;6:33935. doi: 10.1038/srep33935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morton LA. Identifying peptide sensors for highly curved membranes and lipid components. Boulder: University of Colorado; 2013. [Google Scholar]
- 42.Welsh JA, Holloway JA, Wilkinson JS, Englyst NA. Extracellular vesicle flow cytometry analysis and standardization. Front Cell Dev Biol. 2017;5:78. doi: 10.3389/fcell.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Pol E, Sturk A, van Leeuwen T, et al. Standardization of extracellular vesicle measurements by flow cytometry through vesicle diameter approximation. J Thromb Haemost. 2018;16:1236–1245. doi: 10.1111/jth.13972. [DOI] [PubMed] [Google Scholar]
- 44.Libregts SFWM, Arkesteijn GJA, Németh A, et al. Flow cytometric analysis of extracellular vesicle subsets in plasma: impact of swarm by particles of non-interest. J Thromb Haemost. 2018 doi: 10.1111/jth.14154. [DOI] [PubMed] [Google Scholar]
- 45.Nolte-’t Hoen ENM, van der Vlist EJ, Aalberts M, et al. Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles. Nanomedicine. 2012;8:712–720. doi: 10.1016/j.nano.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kormelink TG, Arkesteijn GJA, Nauwelaers FA, et al. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytom Part A. 2016;89:135–147. doi: 10.1002/cyto.a.22644. [DOI] [PubMed] [Google Scholar]
- 47.Higginbotham JN, Zhang Q, Jeppesen DK, et al. Identification and characterization of EGF receptor in individual exosomes by fluorescence-activated vesicle sorting. J Extracell vesicles. 2016;5:29254. doi: 10.3402/jev.v5.29254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morales-Kastresana A, Telford B, Musich TA, et al. Labeling extracellular vesicles for nanoscale flow cytometry. Sci Rep. 2017;7:1878. doi: 10.1038/s41598-017-01731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grubisic Z, Rempp P, Benoit H. A universal calibration for gel permeation chromatography. J Polym Sci Part B Polym Lett. 1967;5:753–759. doi: 10.1002/pol.1967.110050903. [DOI] [Google Scholar]
- 50.Böing AN, van der Pol E, Grootemaat AE, et al. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell vesicles. 2014 doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu R, Greening DW, Rai A, et al. Highly-purified exosomes and shed microvesicles isolated from the human colon cancer cell line LIM1863 by sequential centrifugal ultrafiltration are biochemically and functionally distinct. Methods. 2015;87:11–25. doi: 10.1016/j.ymeth.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Xu R, Simpson RJ, Greening DW (2017) A protocol for isolation and proteomic characterization of distinct extracellular vesicle subtypes by sequential centrifugal ultrafiltration. In: Methods in molecular biology (Clifton, N.J.). pp 91–116 [DOI] [PubMed]
- 53.Musante L, Tataruch D, Gu D, et al. A simplified method to recover urinary vesicles for clinical applications, and sample banking. Sci Rep. 2015;4:7532. doi: 10.1038/srep07532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giddings JC, Myers MN. Flow-field-flow fractionation: a versatile new separation method. Science (80-) 1976;193:1244–1245. doi: 10.1126/science.959835. [DOI] [PubMed] [Google Scholar]
- 55.Kang D, Oh S, Ahn S-M, et al. Proteomic analysis of exosomes from human neural stem cells by flow field-flow fractionation and nanoflow liquid chromatography–tandem mass spectrometry. J Proteome Res. 2008;7:3475–3480. doi: 10.1021/pr800225z. [DOI] [PubMed] [Google Scholar]
- 56.Petersen KE, Manangon E, Hood JL, et al. A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal Bioanal Chem. 2014;406:7855–7866. doi: 10.1007/s00216-014-8040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sitar S, Kejžar A, Pahovnik D, et al. Size characterization and quantification of exosomes by asymmetrical-flow field-flow fractionation. Anal Chem. 2015;87:9225–9233. doi: 10.1021/acs.analchem.5b01636. [DOI] [PubMed] [Google Scholar]
- 58.Petersen KE, Shiri F, White T, et al. Exosome isolation: cyclical electrical field flow fractionation in low-ionic-strength fluids. Anal Chem. 2018;90:12783–12790. doi: 10.1021/acs.analchem.8b03146. [DOI] [PubMed] [Google Scholar]
- 59.Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation. Anal Biochem. 2014;448:41–49. doi: 10.1016/j.ab.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akagi T, Kato K, Hanamura N, et al. Evaluation of desialylation effect on zeta potential of extracellular vesicles secreted from human prostate cancer cells by on-chip microcapillary electrophoresis. Jpn J Appl Phys. 2014;53:06JL01. doi: 10.7567/JJAP.53.06JL01. [DOI] [Google Scholar]
- 61.Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14:1891–1900. doi: 10.1039/C4LC00136B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gholizadeh S, Shehata Draz M, Zarghooni M, et al. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: current status and future directions. Biosens Bioelectron. 2017;91:588–605. doi: 10.1016/j.bios.2016.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo S-C, Tao S-C, Dawn H. Microfluidics-based on-a-chip systems for isolating and analysing extracellular vesicles. J Extracell Vesicles. 2018;7:1508271. doi: 10.1080/20013078.2018.1508271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muller L, Hong C-S, Stolz DB, et al. Isolation of biologically-active exosomes from human plasma. J Immunol Methods. 2014 doi: 10.1016/j.jim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ronquist GK, Larsson A, Stavreus-Evers A, Ronquist G. Prostasomes are heterogeneous regarding size and appearance but affiliated to one DNA-containing exosome family. Prostate. 2012;72:1736–1745. doi: 10.1002/pros.22526. [DOI] [PubMed] [Google Scholar]
- 66.Lane RE, Korbie D, Trau M, Hill MM. Optimising size exclusion chromatography for extracellular vesicle enrichment and proteomic analysis from clinically relevant samples. Proteomics. 2019 doi: 10.1002/pmic.201800156. [DOI] [PubMed] [Google Scholar]
- 67.Sódar BW, Kittel Á, Pálóczi K, et al. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci Rep. 2016;6:24316. doi: 10.1038/srep24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karimi N, Cvjetkovic A, Jang SC, et al. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell Mol Life Sci. 2018;75:2873–2886. doi: 10.1007/s00018-018-2773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corso G, Mäger I, Lee Y, et al. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci Rep. 2017;7:11561. doi: 10.1038/s41598-017-10646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Onódi Z, Pelyhe C, Terézia Nagy C, et al. Isolation of high-purity extracellular vesicles by the combination of iodixanol density gradient ultracentrifugation and bind-elute chromatography from blood plasma. Front Physiol. 2018;9:1479. doi: 10.3389/fphys.2018.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monguió-Tortajada M, Roura S, Gálvez-Montón C, et al. Nanosized UCMSC-derived extracellular vesicles but not conditioned medium exclusively inhibit the inflammatory response of stimulated T cells: implications for nanomedicine. Theranostics. 2017;7:270–284. doi: 10.7150/thno.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lozano-Ramos I, Bancu I, Oliveira-Tercero A, et al. Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. J Extracell vesicles. 2015;4:27369. doi: 10.3402/jev.v4.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Menezes-Neto A, Sáez MJF, Lozano-Ramos I, et al. Size-exclusion chromatography as a stand-alone methodology identifies novel markers in mass spectrometry analyses of plasma-derived vesicles from healthy individuals. J Extracell vesicles. 2015;4:27378. doi: 10.3402/jev.v4.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carreras-Planella L, Soler-Majoral J, Rubio-Esteve C, et al. Characterization and proteomic profile of extracellular vesicles from peritoneal dialysis efflux. PLoS One. 2017;12:e0176987. doi: 10.1371/journal.pone.0176987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roura S, Gálvez-Montón C, de Gonzalo-Calvo D, et al. Extracellular vesicles do not contribute to higher circulating levels of soluble LRP1 in idiopathic dilated cardiomyopathy. J Cell Mol Med. 2017;21:3000–3009. doi: 10.1111/jcmm.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lozano-Ramos SI, Bancu I, Carreras-Planella L, et al. Molecular profile of urine extracellular vesicles from normo-functional kidneys reveal minimal differences between living and deceased donors. BMC Nephrol. 2018;19:189. doi: 10.1186/s12882-018-0985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roura S, Gámez-Valero A, Lupón J, et al. Proteomic signature of circulating extracellular vesicles in dilated cardiomyopathy. Lab Investig. 2018 doi: 10.1038/s41374-018-0044-5. [DOI] [PubMed] [Google Scholar]
- 78.Monguió-Tortajada M, Morón-Font M, Gámez-Valero A, et al. Extracellular-vesicle isolation from different biological fluids by size-exclusion chromatography. Curr Protoc Stem Cell Biol. 2019 doi: 10.1002/cpsc.82. [DOI] [PubMed] [Google Scholar]
- 79.Nordin JZ, Lee Y, Vader P, et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed Nanotechnol Biol Med. 2015;11:879–883. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Mol EA, Goumans M-J, Doevendans PA, et al. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomed Nanotechnol Biol Med. 2017;13:2061–2065. doi: 10.1016/j.nano.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 81.Montaner-Tarbes S, Borrás FE, Montoya M, et al. Serum-derived exosomes from non-viremic animals previously exposed to the porcine respiratory and reproductive virus contain antigenic viral proteins. Vet Res. 2016;47:59. doi: 10.1186/s13567-016-0345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watson DC, Bayik D, Srivatsan A, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pachler K, Lener T, Streif D, et al. A good manufacturing practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy. 2017;19:458–472. doi: 10.1016/j.jcyt.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Andriolo G, Provasi E, Lo Cicero V, et al. Exosomes from human cardiac progenitor cells for therapeutic applications: development of a GMP-grade manufacturing method. Front Physiol. 2018;9:1169. doi: 10.3389/fphys.2018.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heldring N, Mäger I, Wood MJA, et al. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum Gene Ther. 2015;26:506–517. doi: 10.1089/hum.2015.072. [DOI] [PubMed] [Google Scholar]
- 86.Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J Extracell vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing exosomes: a promising therapeutic platform. Trends Mol Med. 2018;24:242–256. doi: 10.1016/j.molmed.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 88.Horenstein AL, Durelli I, Malavasi F. Purification of clinical-grade monoclonal antibodies by chromatographic methods. Methods Mol Biol. 2005;308:191–208. doi: 10.1385/1-59259-922-2:191. [DOI] [PubMed] [Google Scholar]
- 89.Van Deun J, Mestdagh P, Agostinis P, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14:228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 90.Hagel L, Östberg M, Andersson T. Apparent pore size distributions of chromatography media. J Chromatogr A. 1996;743:33–42. doi: 10.1016/0021-9673(96)00130-6. [DOI] [Google Scholar]