Abstract
Two decades ago, following a systematic screening of LOH regions on chromosome 8p22, TUSC3 has been identified as a candidate tumor suppressor gene in ovarian, prostate and pancreatic cancers. Since then, a growing body of evidence documented its clinical importance in various other types of cancers, and first initial insights into its molecular function and phenotypic effects have been gained, though the precise role of TUSC3 in different cancers remains unclear. As a part of the oligosaccharyltransferase complex, TUSC3 localizes to the endoplasmic reticulum and functions in final steps of N-glycosylation of proteins, while its loss evokes the unfolded protein response. We are still trying to figure out how this mechanistic function is reconcilable with its varied effects on cancer promotion. In this review, we focus on cancer-related effects of TUSC3 and envisage a possible role of TUSC3 beyond endoplasmic reticulum.
Keywords: TUSC3, Cancer, Tumor suppressor, Oncogene, Endoplasmic reticulum, N-Glycosylation, Immunoediting
Introduction
Development of cancer is a complex process involving stepwise series of molecular events that finally culminate in alterations of normal cellular phenotype towards malignancy. Genes that enhance or inhibit tumor growth—oncogenes and tumor suppressors—are usually assigned various aspects of cellular function, such as mitogenic and differentiation signaling, DNA damage repair, or apoptosis. The classical concept of carcinogenesis is based on impairment or activation of major oncogenes and tumor suppressors, making the development of malignant phenotype a cell-intrinsic and unambiguous process. Today we know, however, that tumor cell independent effects within the tumor microenvironment as well as immunoediting have significant impact on tumor development [1]. Screening of tumors and cancer cells by high-throughput omics technologies that allow global assessment of expression or mutational profiles revealed a high number of unapparent genes that possess a dual character in different cancers and lack a clear link to the classical molecular machineries driving cancer development. These minor drivers involved in general cell and tissue metabolism and homeostasis can switch from oncogene to tumor suppressor function depending on the cell and tissue context as well as the composition of the microenvironmental niche [2]. In this review, we discuss such dual role for TUmor Suppressor Candidate 3 (TUSC3) and envision its function in normal or cancerous tissues within specific contexts.
Tusc3 gene (N33) is localized on the short arm of chromosome 8 in a chromosomal band 8p22. It is encoded by 224 kbp genomic DNA and encompasses 11 exons, constituting three prototypical different transcripts [3]. The product of transcript 1 contains 11 exons encoding 348 amino acids with molecular weight of 39 kDa. Prototypically, it contains a thioredoxin domain with peptide-binding site and five predicted transmembrane domains, one of which is deleted in the final TUSC3 product. The transcript 2, lacking exon 10, and transcript 3, lacking three exons, encode 347 and 314 amino acids, respectively. Transcripts 1 and 2 are abundantly expressed in most non-lymphoid tissues [3, 4], while the transcript number 3 is predominantly expressed in placenta [4]. Interestingly, according to Ensembl gene annotation system (release 89—May 2017), 13 different transcripts or splice variants were predicted, but not all being translated [5].
Cellular functions of TUSC3: lessons from glycobiochemistry
Until recently, function of TUSC3 has only been assumed by its partial sequence homology with the yeast Ost3p subunit of the oligosaccharyltransferase (OST) complex within the endoplasmic reticulum (ER). In yeast, OST complex consists of eight subunits including Ost3p (Ost1p, Ost2p, Ost3p or Ost6p, Ost4p, Ost5p, Wbp1p, Swp1p and Stt3p) [6]. OST complex mediates a key step of protein N-glycosylation, the en bloc transfer of a preassembled oligosaccharide from a lipid-linked donor onto asparagine residues of nascent proteins entering the lumen of rough ER. The asparagine residues constitute the Asn-X-Ser/Thr (N-X-T/S) consensus sequence, where X can be any amino acid residue except proline that are read by OST and covalently modified [7]. Human OST complex contains seven subunits—catalytical core STT3A or STT3B, five non-catalytic subunits (ribophorin I, ribophorin II, OST48, DAD1 and OST4) and one of the isoform specific subunits KCP2 or DC2 for STT3A complex and MagT1 or TUSC3 for STT3B as documented for HeLa and Cho cells or using recombinant OST complex in vitro [7, 8].
In human cancer cells, TUSC3 indeed localizes to the rough ER as an intrinsic part of the OST complex directly binding to the STT3A or STT3B catalytic core [9, 10]. The STT3A OST complex is associated with the protein translocation channel and glycosylates the majority of acceptor sites of human glycoproteins as they enter the lumen of the ER, while the STT3B complex binds to and glycosylate acceptor sites that have been skipped by the STT3A complex [11, 12]. Thus, both complexes are essential for achievement of full N-glycoproteome [12].
Both the TUSC3 (yeast Ost3p) and MagT1 (yeast Ost6p) proteins bind to the STT3B complex in vitro and share 73% sequence identity. Both proteins are linked to the ER membrane by four transmembrane spanning segments and their N-terminal luminal domain consists of a thioredoxin fold with a peptide-binding site (CXXC motif) [11]. In yeast, neither Ost3p nor Ost6p are necessary for functional OST, as the single disruption causes only a moderate hypoglycosylation. However, the double knockdown of Ost3p and Ost6p resulted in a severe defect of N-glycosylation [13]. Such functional redundancy was also observed for MagT1 and TUSC3 in the human OST complex [12]. Similarly, the absence of both MagT1 and TUSC3 subunits or loss of STT3B causes a dramatic reduction of N-glycosylation [14].
TUSC3 seems to increase glycosylation efficiency for specific human glycoproteins by delaying oxidative substrate folding and increasing the probability of recognition of N-glycosylation sequon, envisaging the role for TUSC3 in substrate selection [7]. The direct targets of TUSC3 in OST remain unknown, except for integrin β1 [10]. Consequently, deregulation of TUSC3 probably does not impair the overall systemic rate of glycosylation, as documented by the absence of hypoglycosylation of total serum transferrin or alteration of the serum N-glycome analyzed by mass spectrometry [12, 15]. In prostate cancer cells, slightly reduced N-glycosylation efficiency was observed in TUSC3-silenced cells using the ER-localized luciferase reporter construct [16].
Apart from N-glycosylation, TUSC3 and MagT1 were proposed to be plasma membrane-associated magnesium transporters [17]. Expression of MagT1, but not TUSC3, was upregulated in yeast cultured in medium depleted of magnesium while overexpression of both MagT1 and TUSC3 resulted in modestly higher cellular MgII+ uptake [18]. However, unequivocal evidence for direct ion transporting has not been provided. Rather, TUSC3 and MagT1 influence the MgII+ homeostasis indirectly by N-glycosylation of a specific protein required for MgII+ transport.
TUSC3 in embryonic development
Expression of TUSC3 differs in individual tissues and stages of embryonic development [3, 15, 18] (Table 1) and what is of particular importance, its normal expression levels can be related with oncogenic or tumor-suppressive function in particular cancers. In contrast, the expression of human TUSC3 paralog, MagT1, is ubiquitous [3].
Table 1.
TUSC3 expression in human tissues
| High TUSC3 | Low or absent TUSC3 |
|---|---|
| Heart | Kidney |
| Cerebellum | Spinal cord |
| Fetal brain | Adult brain |
| Ovary | Trachea |
| Cervix | Lung |
| Placenta | Colon |
| Prostate | Small intestine |
| Testis | Spleen |
| Adipose tissue | Bone marrow |
| Thyroid gland | |
| Liver | |
| Thymus | |
| Peripheral lymphocytes | |
| Uterus |
The roles of TUSC3 and its paralog, MagT1, in general vertebrate development were investigated in zebrafish recently [18]. Zebrafish MagT1 and TUSC3 genes share 80 and 93% homology with their human counterparts, respectively. TUSC3 and MagT1 double knockdown severely reduced hatching of the embryos to only 5%, while a single knockdown only impaired the hatching rate if the expression was reduced at both maternal and zygotic levels. This confirms the requirement of TUSC3 and MagT1 for embryonic development of vertebrates and also their partial functional redundancy.
While the role of TUSC3 in histogenesis of most tissues is unknown, a pilot study has already been performed for rat testicular development, documenting its elevated expression in seminiferous tubules, including interstitial Leydig cells, and also in prostate epithelial cells [4]. In human trophoblast, TUSC3 is normally highly expressed, but downregulated in response to hypoxia exposure [19] which correlated with human placental pathologies including preeclampsia [20]. Indeed, TUSC3 promoter hypermethylation in trophoblasts was more frequent in patients with preeclampsia than without any metabolic pregnancy complications. TUSC3 hypermethylation was not observed in maternal blood or fetal tissues other than placenta [21].
TUSC3 is abundantly expressed in developing fetal brain, and truncating mutations as well as homozygous germline deletions were associated with autosomal recessive (AR) non-syndromic intellectual disability (NSID) [15, 22, 23]. Typically, AR-NSID affects about 1% of general population with substantial heterogeneity [24]. So far, approximately 40 genes correlating with AR-NSID were identified, including TUSC3 [25]. Molinari et al. performed autozygosity mapping in two siblings affected by intellectual disability born to first-cousin parents and identified a culprit region on 8p22–p23.1. Subsequent sequencing uncovered a 1 bp insertion in TUSC3 gene resulting in a premature stop codon and reduced amount of transcript mRNA [15]. Garshasbi et al. [22] identified a homozygous deletion including the first exon of TUSC3 in a large consanguineous Iranian family with AR-NSID. Third novel deletion mutation involving almost full TUSC3 gene was reported in a large consanguineous Pakistani family [28] and other reports of families with NSID additionally supported TUSC3 role in the development of cognitive functions [23, 26–31]. All affected patients exhibited mild to severe intellectual disability, while their parents were all heterogeneous for TUSC3 gene and exhibited intelligence in the normal range. Additionally, despite the fact that the TUSC3-induced intellectual disability is mostly referred as non-syndromic, some TUSC3-affected patients across the different studies exhibited similar minor anomalies including short stature, microcephaly, hypoplastic philtrum, hypertelorism and other moderate dysmorphic facial features. Interestingly, three patients studied by Molinari et al. [15] died due to cancer. A population study using common SNPs from 40 genes known to be associated with AR-NSID, including TUSC3, found no association between SNP distribution in these genes and normal range of intelligence, suggesting that intellectual disability is probably genetically divergent from the normal variation of intelligence differences [25]. Except for the localization of TUSC3 expression to the fetal brain and the genetic link to NSID there are currently no functional studies concerning TUSC3 role in developing neural tissue or cognitive functions either in impairing of intracellular signaling or altering N-glycosylation patterns.
Epigenetic regulation of TUSC3 expression
Sequence of TUSC3 gene contains multiple CpG islands spanning its promoter and first exon; hence in several cancer types its expression is frequently silenced by promoter hypermethylation. In particular, hypermethylation of TUSC3 promoter was observed in colorectal cancer (CRC) [32–34], glioblastoma multiforme (GBM) [35], non-small-cell lung cancer (NSCLC) [36], lung cancer [37] and ovarian cancer [38] (Table 2).
Table 2.
Molecular phenotypes associated with TUSC3 in different types of cancer
| Cancer | Expression change OF TUSC3 | TUSC3-associated molecular phenotype | Integrated role | References |
|---|---|---|---|---|
| Ovary | Downregulation by promoter hypermethylation |
UPR EMT |
TSG | [10, 38, 61] |
| PROSTATE | Downregulation |
UPR N-Glycosylation Akt |
TSG | [9] |
| Glioblastoma multiforme | Downregulation by promoter hypermethylation | Akt | TSG | [35, 63] |
| Breast cancer | Downregulation by miRNA |
(1) SOX2 (2) miR-181a-5p (2) miR-30e-5p |
TSG | [72, 73] |
| Pancreatic cancer | Homozygous deletion | NF-κB | TSG | [48, 62] |
| Oral squamous cell carcinoma | Homozygous deletion | N/A | TSG | [74] |
| Hepatocellular carcinoma | Loss of heterozygosity | N/A | N/A | [46] |
| Small cell lung cancer | Downregulation | N/A | TSG | [75] |
| Larynx and pharynx carcinoma | Loss | N/A | TSG | [76] |
| Esophageal squamous cell carcinoma | Downregulation | N/A | TSG | [77] |
| Non-small-cell lung cancer | Downregulation by promoter hypermethylation | Wnt/β-catenin | TSG | [78] |
| Upregulation | Hedgehog | Oncogene | [64] | |
| Colorectal cancer | Upregulation |
MAPK PI3K/Akt Wnt/β-catenin |
Oncogene | [60] |
| Head and neck squamous carcinoma | Upregulation by amplification | N/A | Oncogene | [59] |
| Thyroid cancer | Upregulation (gain) | N/A | Oncogene | [57, 59] |
N/A not applicable (data unavailable)
In CRC, methylation of TUSC3 was elevated in tumor tissue compared to surrounding normal colon tissue or colonic mucosa of healthy donors [32, 39]. TUSC3 hypermethylation was also confirmed in adenomatous colorectal polyps and in colorectal mucosa of patients with ulcerative colitis which is among the common risk factors for developing CRC, suggesting that TUSC3 hypermethylation might be an early event in CRC [31, 40]. Nevertheless, in an independent cohort, TUSC3 methylation did not significantly differ between the normal mucosa of the non-cancerous patients and adenomatous polyps or tumor samples [34]. Furthermore, TUSC3 showed partial methylation in normal colorectal mucosa which was progressively increasing with age with frequency of around 15% in patients less than 20 years old and up to 58% in patients over 60 years of age [31, 33, 39]. Age-related TUSC3 hypermethylation was also observed in GBM [35], peripheral blood leukocyte DNA [31, 41], and in cases of NSCLC [42].
TUSC3 hypermethylation was found in 59% cases of GBM, and corresponding with methylation of the estrogen receptor (ESR) [35]. Methylation of TUSC3, estrogen receptor and MYOD1 were also found in colonic tissue of healthy individuals and in patients with ulcerative colitis [33, 40]. However, epigenetic patterns seem to be tissue specific, as the TUSC3 methylation is more frequent in the colon than in the liver, while methylation of ESR is higher in the liver [32]. Additionally, TUSC3 is methylated in both colonic epithelial and subepithelial connective tissues, whereas ESR shows higher methylation in the epithelium.
In DNA of peripheral white blood cells in a Chinese population with high risk of gastric cancer, TUSC3 methylation preceded approximately 5 years the clinical manifestation, suggesting that downregulation of TUSC3 can be a part of complex alterations as a biomarker for early diagnosis of gastric cancer [31]. Apart from that, TUSC3 methylation seems to be an effective prognostic marker in lung and ovarian cancers [37, 38]. The promoter of TUSC3 was frequently methylated in tumors, benign bronchi and alveolar lung tissues from lung cancer patients, but not in healthy individuals. No significant association was found between TUSC3 promoter methylation and age, grading, tumor histology, or lymph node or distant metastasis, but TUSC3 methylation correlated with smaller tumor size and longer overall survival of lung cancer patients [37]. In contrast, TUSC3 methylation and consequent low mRNA expression in ovarian cancer were associated with shorter progression-free and overall survival [38]. This correlation was independent of other risk factors including age, FIGO stage, histologic grade, or response to chemotherapy, making TUSC3 an independent candidate biomarker for ovarian cancer. In acute lymphoblastic leukemia (ALL), TUSC3 is hypermethylated in significantly higher frequency than in AML [43]. Microarray-based DNA methylation analysis covering 249 CpG islands of 57 genes was performed on mononuclear cells from patients with ALL and AML and TUSC3 methylation showed the highest divergence between AML and ALL.
Additionally, there is a potential shift towards increased methylation of TUSC3 promoter in men vs. women, and smokers vs. non-smokers [32, 41]. However, other studies found no correlation between TUSC3 methylation and gender, smoking or alcohol abuse [31, 33, 40, 44], suggesting the presence of complex epigenetic patterns of TUSC3 depending on investigated population, ethnical origin or clinical background. Rigorous population analyses of TUSC3 expression or epigenetic patterns are thus necessary to reveal the role of TUSC3 as a cancer-associated predictor.
TUSC3 in cancer
TUSC3 as tumor suppressor
First evidence for tumor-suppressive functions of TUSC3 came from systematic screenings of the chromosomal band 8p22 that is often lost in wide variety of epithelial cancers, including prostate [45], ovarian [46], breast [47], pancreatic [48], bladder [49], colorectal [46, 50], hepatocellular, NSCLC, or choriocarcinoma [51]. Particularly in prostate cancer, loss of the short arm of chromosome 8 is the most frequent genetic alteration occurring in over 60% of cases [52].
Substantial advance in searching for potential tumor suppressors in 8p22 was achieved by Bova [45], who constructed a deletion map of the 8p22 in prostate cancer and confined the common deletion interval to a 14 cM area. Next, a physical map of a 1–1.5 Mb deletion interval revealed six novel transcription units representing candidate tumor suppressor genes [53]. Pils et al. [54] then reported that five genes from the total of 22 genes located on the 8p22 band showed significantly reduced expression in ovarian tumors and two genes—TUSC3 and EFA6R—correlated with patient survival and offered prognostic information. Additionally, TUSC3 expression was lower in advanced ovarian cancer compared to low-grade tumors. At the same time, Bashyam et al. [48] described a TUSC3 homozygous deletion in pancreatic cancer by comparative genomic hybridization (CGH). Cooke et al. [55] used high-resolution array CGH in breast cancer cell lines and identified two putative tumor suppressors—TUSC3 and ARHGEF10—in the same chromosomal region.
Shortly after TUSC3 was identified as a possible tumor suppressor candidate at 8p22, the evidence for its involvement in pathogenesis of various cancer entities widened. The most common genetic aberration of TUSC3 gene in tumors is homozygous deletion, which was revealed in various human cancers (Table 2). Loss of TUSC3 was also found in cases of canine osteosarcoma [56]. In lung, esophageal, pancreatic, larynx and pharynx cancers, association between low TUSC3 expression and lymph node or distant metastasis formation was described. DNA amplification or elevated expressions of TUSC3 were found in papillary thyroid cancer [57, 58], head and neck squamous carcinoma [59], and CRC [60].
In vitro studies revealed that downregulation of TUSC3 in ovarian cancer cells resulted in increased proliferation, migration and adhesion to extracellular matrix [10, 38]. Similar results were found in prostate cancer [9]. Moreover, knockdown of TUSC3 was accompanied with ER rearrangements and alterations in unfolded protein response (UPR). Additionally, in PTEN negative PC3 cell line, loss of TUSC3 promoted Akt activity in serum starved cells. In ovarian cancer, cells lacking TUSC3 displayed dilated ER cisternae and activation of PERK-mediated UPR pathway [61]. Loss of TUSC3 was accompanied by decreased expression of epithelial markers together with upregulation of mesenchymal markers and epithelial-to-mesenchymal transition (EMT) transcription factors resulting in enhanced cell migration. Moreover, these effects were enhanced under ER-stress conditions. Interestingly, silencing of TUSC3 augmented formation of 3D cell clusters under low-adhesion conditions, mimicking spreading of ovarian cancer through peritoneum.
The in vivo data based on subcutaneous xenografts were provided for ovarian [61], prostate [9] and pancreatic cancers [62] where cancer cells lacking TUSC3 generally developed larger tumors and in case of ovarian cancer also massively disseminated through peritoneum [61]. In GBM, decreased expression of TUSC3 due to promoter hypermethylation protected GBM cells from apoptosis, while TUSC3 overexpression induced caspase-3 activity. GBM cells lacking TUSC3 had also a high rate of proliferation dependent on Akt signaling [63], similar to the Akt-dependent phenotype observed in prostate cancer [9]. In pancreatic cancer, TUSC3 downregulation corresponded with higher tumor stage and worse patient survival, while in vitro, TUSC3 knockdown in three different cell lines negatively influenced NF-κB activity [62]. Pancreatic cancer cell lines with decreased TUSC3 also exhibited enhanced proliferation, migration and invasion capability and resulted in more aggressive phenotype with more frequent liver metastases in mouse models [62].
TUSC3 as oncogene
In colorectal carcinoma, TUSC3 levels were found upregulated in tumor tissue when compared to surrounding non-malignant stroma [60]. Interestingly, when the CRC cell lines LS174T and HCT116 were transduced with lentiviral vectors coding for TUSC3, the non-invasive epithelial phenotype was converted to invasive mesenchymal phenotype, followed by downregulation of E-cadherin and upregulation of vimentin. Downregulation of TUSC3 in HT29 and SW480 CRC cell lines repressed the proliferation and migration in vitro and led to a decreased growth of xenografts in vivo. On the molecular level, WNT/β-catenin and MAPK signaling were activated in TUSC3-overexpressing CRC lines, while Akt and ERK1 phosphorylation was attenuated. Interestingly, physical interaction between TUSC3 and β-catenin was revealed by co-immunoprecipitation by Gu et al. [60]. Similarly, in NSCLC, overexpression of TUSC3 increased proliferation and suppressed apoptosis of NSCLC H322 model cell line. In tumor tissues, staining for TUSC3 positively correlated with the TNM stage and presence of distant metastases, while in normal lung tissue the TUSC3 expression was maintained low or below detection limits [64]. Further screening for molecular pathways affected by TUSC3 in lung cancer identified Hedgehog (HH) signaling axis to be involved in TUSC3-dependent manner. Using A549 cells, levels of proteins downstream to HH signaling, such as GLI1, SMO, PTCH1, and PTCH2 were increased in TUSC3 expressing cells. Physical interaction between TUSC3 and GLI1 was also detected, suggesting so far unknown roles of TUSC3 beyond ER. Missense mutations in TUSC3 have been reported in cases of relapsing ALL [65], further suggesting its role in ER-stress or N glycosylation besides solid cancers. Interestingly, activation of UPR upon induction of ER-stress led to enhanced activity of major leukemic drivers, such as PML-RARα [66] or inactivation of differentiation factors, such as C/EBPα [67] in AML or BCR-Abl [68] in B cell ALL.
TUSC3 as a cancer-related gene
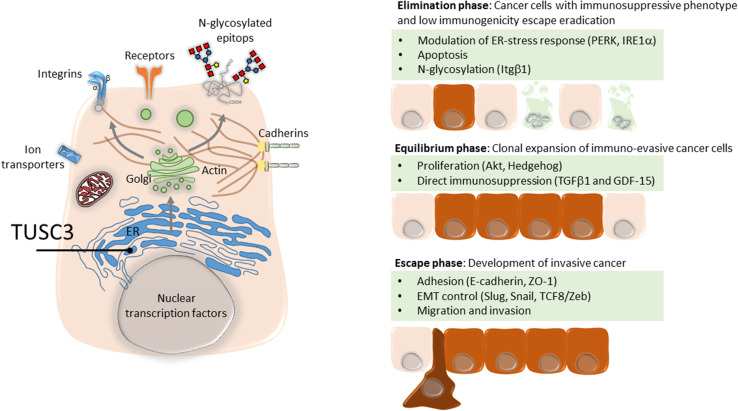
Experiments performed indicate a convergence on several signaling pathways altered in distinct manner in TUSC3-deregulated cancer cells, such as Akt, suggesting that TUSC3 is involved in selection of particular substrates that directly or indirectly affect the molecular signaling pathways (Table 2). Addressing potential physical interactions of TUSC3 with major components of oncogenic signaling pathways, such as MTOR/Akt, Wnt/β-catenin or Hedgehog will raise a question of non-canonical roles of TUSC3 next to ER-localized N-glycosylation. However, TUSC3-driven substrate specificity of OST can alter expression of surface receptors or molecules mediating cell–cell or cell–matrix interactions, as documented for E-cadherin or Integrin β1 [10, 61]. Portfolio of potential targets is probably extremely broad considering that approximately up to one-fifth of the proteome can be glycosylated in the ER [69]. Due to TUSC3 association with high-grade cancer, it is likely to be involved in the immunoediting of cancer cells by immune system (Fig. 1) [70], providing the hypothetic platform for explaining the molecular or cellular context where TUSC3 acts either as a tumor suppressor or an oncogene. Thus, in addition to cell-intrinsic phenotypic conversions, such as EMT, or reduced sensitivity to apoptosis, subtle alterations of N-glycosylation patterns in TUSC3-deregulated cancer cells enable survival during the elimination phase and immune escape and drive the expansion of non-immunogenic cancer clones [71]. Indeed, we documented that immunosuppressive cytokines TGFβ1 and GDF-15 are upregulated in TUSC3-silenced cells [61], raising the possibility of backwards autocrine signaling or paracrine modulation of tumor cells, tumor-associated cells or immune cells.
Fig. 1.
Immunoediting mechanisms associated with TUSC3. TUSC3 residing in the ER enzymatic machinery determines substrates for N-glycosylation by oligosaccharyl transferase (OST) complex. Depending on the availability of proteins entering the ER and given the gatekeeper function of the OST complex, cancer cells can exhibit various patterns of glycosylated surface or secreted proteins with altered functional domains. Regulation of the OST complex through TUSC3 can potentially impact all major classes of signaling molecules involved in immunoediting
Conclusions
Since its initial discovery, many reports emerged documenting TUSC3 as a genuine tumor suppressor in various cancers. However, the variety of molecular machineries affected by TUSC3 deregulation, due to its principal localization within final steps of N-glycosylation machinery in ER, is remarkably diverse. Global signaling alterations due to differential TUSC3 activity integrate on common phenotypic changes and determine whether TUSC3 acts as an oncogene or a tumor suppressor gene. In summary, there is more than one dedicated role for TUSC3 in the life of a cell, but its pathogenic effects are rather mediated through indirect modulation of N-glycosylation of available downstream proteins than through a direct override of cellular homeostasis.
Author contributions
KV drafted the manuscript, PH and PV conceptualized, wrote and revised the manuscript.
Compliance with ethical standards
Funding
Grant Agency of Masaryk University (MUNI/A/1369/2016), European Regional Development Fund (Center for Analysis and Modeling of Tissues and Organs, CZ.1.07/2.3.00/20.0185), the National Program of Sustainability II (Project no. LQ1605, MEYS CR).
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.He Q, He Q, Liu X, et al. Genome-wide prediction of cancer driver genes based on SNP and cancer SNV data. Am J Cancer Res. 2014;4(4):394–410. [PMC free article] [PubMed] [Google Scholar]
- 3.MacGrogan D, Levy A, Bova GS, Isaacs WB, Bookstein R. Structure and methylation-associated silencing of a gene within a homozygously deleted region of human chromosome band 8p22. Genomics. 1996;35:55–65. doi: 10.1006/geno.1996.0322. [DOI] [PubMed] [Google Scholar]
- 4.Khalid AM, Asano A, Hosaka YZ, Takeuchi T, Yamano Y. Tumor suppressor candidate TUSC3 expression during rat testis maturation. Biosci Biotechnol Biochem. 2013;77:2019–2024. doi: 10.1271/bbb.130327. [DOI] [PubMed] [Google Scholar]
- 5.Aken BL, Ayling S, Barrell D, et al. The Ensembl gene annotation system. Database (Oxford) 2016;2016:baw093. doi: 10.1093/database/baw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelleher DJ, Gilmore R. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology. 2006;16:47–62. doi: 10.1093/glycob/cwj066. [DOI] [PubMed] [Google Scholar]
- 7.Mohorko E, Owen RL, Malojčić G, Brozzo MS, Aebi M, Glockshuber R. Structural basis of substrate specificity of human oligosaccharyl transferase subunit N33/Tusc3 and its role in regulating protein N-glycosylation. Structure. 2014;22:590–601. doi: 10.1016/j.str.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Shrimal S, Cherepanova NA, Gilmore R. Cotranslational and posttranslocational N-glycosylation of proteins in the endoplasmic reticulum. Semin Cell Dev Biol. 2015;41:71–78. doi: 10.1016/j.semcdb.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horak P, Tomasich E, Vaňhara P, et al. TUSC3 loss alters the ER stress response and accelerates prostate cancer growth in vivo. Sci Rep. 2014;4:3739. doi: 10.1038/srep03739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaňhara P, Horak P, Pils D, et al. Loss of the oligosaccharyl transferase subunit TUSC3 promotes proliferation and migration of ovarian cancer cells. Int J Oncol. 2013;42:1383–1389. doi: 10.3892/ijo.2013.1824. [DOI] [PubMed] [Google Scholar]
- 11.Cherepanova N, Shrimal S, Gilmore R. N-Linked glycosylation and homeostasis of the endoplasmic reticulum. Curr Opin Cell Biol. 2016;41:57–65. doi: 10.1016/j.ceb.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherepanova NA, Shrimal S, Gilmore R. Oxidoreductase activity is necessary for N-glycosylation of cysteine-proximal acceptor sites in glycoproteins. J Cell Biol. 2014;206:525–539. doi: 10.1083/jcb.201404083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz M, Knauer R, Lehle L. Yeast oligosaccharyltransferase consists of two functionally distinct sub-complexes, specified by either the Ost3p or Ost6p subunit. FEBS Lett. 2005;579:6564–6568. doi: 10.1016/j.febslet.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 14.Cherepanova NA, Gilmore R. Mammalian cells lacking either the cotranslational or posttranslocational oligosaccharyltransferase complex display substrate-dependent defects in asparagine linked glycosylation. Sci Rep. 2016;6:20946. doi: 10.1038/srep20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molinari F, Foulquier F, Tarpey PS, et al. Oligosaccharyltransferase-subunit mutations in nonsyndromic mental retardation. Am J Hum Gen. 2015;82:1150–1157. doi: 10.1016/j.ajhg.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contessa JN, Bhojani MS, Freeze HH, Ross BD, Rehemtulla A, Lawrence TS. Molecular imaging of N-linked glycosylation suggests glycan biosynthesis is a novel target for cancer therapy. Clin Cancer Res. 2010;16(12):3205–3214. doi: 10.1158/1078-0432.CCR-09-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goytain A, Quamme GA. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics. 2005;1:48. doi: 10.1186/1471-2164-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc Natl Acad Sci USA. 2009;106:15750–15755. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pak BJ, Park H, Chang ER, Pang SC, Graham CH. Differential expression display in first analysis trimester of oxygen-mediated human trophoblast changes cells in gene. Placenta. 1998;19:483–488. doi: 10.1016/S0143-4004(98)91041-4. [DOI] [PubMed] [Google Scholar]
- 20.Soleymanlou N, Jurisica I, Nevo O, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90(7):4299–4308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuen RKC, Avila L, Peñaherrera MS, et al. Human placental-specific epipolymorphism and its association with adverse pregnancy outcomes. PLoS ONE. 2009;4:1–11. doi: 10.1371/journal.pone.0007389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garshasbi M, Hadavi V, Habibi H, et al. Report a defect in the TUSC3 gene is associated with autosomal recessive mental retardation. Am J Hum Genet. 2008;2008:1158–1164. doi: 10.1016/j.ajhg.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garshasbi M, Kahrizi K, Hosseini M, et al. Clinical report a novel nonsense mutation in TUSC3 is responsible for non-syndromic autosomal recessive mental retardation in a consanguineous Iranian family. Am J Med Genet. 2011;2011:1976–1980. doi: 10.1002/ajmg.a.34077. [DOI] [PubMed] [Google Scholar]
- 24.Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies: Research in developmental disabilities. Res Dev Dis. 2011;32(2):419–436. doi: 10.1016/j.ridd.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Hill WD, Davies G, Liewald DC, et al. Examining non-syndromic autosomal recessive intellectual disability (NS-ARID) genes for an enriched association with intelligence differences. Intelligence. 2016;54:80–89. doi: 10.1016/j.intell.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan MA, Rafiq MA, Noor A, et al. A novel deletion mutation in the TUSC3 gene in a consanguineous Pakistani family with autosomal recessive nonsyndromic intellectual disability. BMC Med Genet. 2011;12:56. doi: 10.1186/1471-2350-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Amri A, Saegh AA, Al-Mamari W, et al. Homozygous single base deletion in TUSC3 causes intellectual disability with developmental delay in an Omani family. Am J Med Genet. 2016;170:1826–1831. doi: 10.1002/ajmg.a.37690. [DOI] [PubMed] [Google Scholar]
- 28.Loddo S, Parisi V, Doccini V, et al. Homozygous deletion in TUSC3 causing syndromic intellectual disability: a new patient. Am J Med Genet. 2013;161:2084–2087. doi: 10.1002/ajmg.a.36028. [DOI] [PubMed] [Google Scholar]
- 29.Mosrati MA, Schrauwen I, Kamoun H, et al. Genome wide analysis in a family with sensorineural hearing loss, autism and mental retardation. Gene. 2012;510(2):102–106. doi: 10.1016/j.gene.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Piovani G, Savio G, Traversa M, et al. De novo 1 Mb interstitial deletion of 8p22 in a patient with slight mental retardation and speech delay. Mol Cytogenet. 2014;7:25. doi: 10.1186/1755-8166-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Su HJ, Pan KF, et al. Methylation status of blood leukocyte DNA and risk of gastric cancer in a high-risk Chinese population. Cancer Epidemiol Biomark Prev. 2014;23:2019–2026. doi: 10.1158/1055-9965.EPI-13-0994. [DOI] [PubMed] [Google Scholar]
- 32.Ahuja N, Li Q, Mohan AL, Baylin SB. Issa J-PJ. Aging and DNA methylation in colorectal mucose and cancer. Cancer Res. 1998;58:5489–5494. [PubMed] [Google Scholar]
- 33.Hanks J, Ayed I, Kukreja N, et al. The association between mthfr 677C>T genotype and folate status and genomic and gene-specific dna methylation in the colon of individuals without colorectal neoplasia. Am J Clin Nutr. 2013;98:1564–1574. doi: 10.3945/ajcn.113.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu XL, Yu J, Zhang HY, et al. Methylation profile of the promoter CpG islands of 31 genes that may contribute to colorectal carcinogenesis. World J Gastroenterol. 2004;10:3441–3454. doi: 10.3748/wjg.v10.i23.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q, Jedlicka A, Ahuja N, et al. Concordant methylation of the ER and N33 genes in glioblastoma multiforme. Oncogene. 1998;16:3197–3202. doi: 10.1038/sj.onc.1201831. [DOI] [PubMed] [Google Scholar]
- 36.Zemlyakova VV, Zhevlova AI, Zborovskaya IB, et al. Methylation profile of several tumor suppressor genes in non-small-cell lung cancer. Mol Biol. 2003;37:836–840. doi: 10.1023/B:MBIL.0000008351.36435.d6. [DOI] [Google Scholar]
- 37.Duppel U, Woenckhaus M, Schulz C, Merk J, Dietmaier W. Quantitative detection of TUSC3 promoter methylation—a potential biomarker for prognosis in lung cancer. Oncol Lett. 2016;2016:3004–3012. doi: 10.3892/ol.2016.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pils D, Horak P, Vanhara P, et al. Methylation status of TUSC3 is a prognostic factor in ovarian cancer. Cancer. 2013;119(5):946–954. doi: 10.1002/cncr.27850. [DOI] [PubMed] [Google Scholar]
- 39.Belshaw NJ, Elliott GO, Foxall RJ, et al. Profiling CpG island field methylation in both morphologically normal and neoplastic human colonic mucosa. Br J Cancer. 2008;99:136–142. doi: 10.1038/sj.bjc.6604432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arasaradnam RP, Khoo K, Bradburn M, Mathers J, Kelly S. DNA methylation of ESR-1 and N-33 in colorectal mucosa of patients with ulcerative colitis (UC) Epigenetics. 2010;5:422–426. doi: 10.4161/epi.5.5.11959. [DOI] [PubMed] [Google Scholar]
- 41.Yuasa Y, Nagasaki H, Oze I, et al. Insulin-like growth factor 2 hypomethylation of blood leukocyte DNA is associated with gastric cancer risk. Int J Cancer. 2012;131:2596–2603. doi: 10.1002/ijc.27554. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, He RQ, Dang YW, et al. Comprehensive analysis of the long noncoding RNA HOXA11-AS gene interaction regulatory network in NSCLC cells. Cancer Cell Int. 2016;16:89. doi: 10.1186/s12935-016-0366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholz C, Nimmrich I, Burger M, et al. Distinction of acute lymphoblastic leukemia from acute myeloid leukemia through microarray-based DNA methylation analysis. Ann Hematol. 2005;84:236–244. doi: 10.1007/s00277-004-0969-1. [DOI] [PubMed] [Google Scholar]
- 44.Conway K, Edmiston SN, Tse C-K, et al. Racial variation in breast tumor promoter methylation in the Carolina Breast Cancer Study. Cancer Epidemiol Biomark Prev. 2015;24:921–930. doi: 10.1158/1055-9965.EPI-14-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bova GS, Carter BS, Bussemakers MJG, et al. Homozygous deletion and frequent allelic loss of chromosome 8p22 loci in human prostate cancer. Cancer Res. 1993;1993:3869–3873. [PubMed] [Google Scholar]
- 46.Emi M, Fujiwara Y, Nakajima T, Cancer C, Cancer L. Frequent loss of heterozygosity for loci on chromosome 8p in hepatocellular carcinoma, colorectal cancer, and lung cancer. Cancer Res. 1992;2:5368–5372. [PubMed] [Google Scholar]
- 47.Tirkkonen M, Tanner M, Karhu R, Kallioniemi A, Isola J, Kallioniemi OP. Molecular cytogenetics of primary breast cancer by CGH. Genes Chromosome Cancer. 1998;21(3):177–184. doi: 10.1002/(SICI)1098-2264(199803)21:3<177::AID-GCC1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 48.Bashyam MD, Bair R, Kim YH, et al. Array-based comparative genomic hybridization identifies localized DNA amplifications and homozygous deletions in pancreatic cancer. Neoplasia. 2005;7:556–562. doi: 10.1593/neo.04586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takle LA, Knowles MA. Deletion mapping implicates two tumor suppressor genes on chromosome 8p in the development of bladder cancer. Oncogene. 1996;12(5):1083–1087. [PubMed] [Google Scholar]
- 50.Fujiwara Y, Emi M, Ohata H, et al. Evidence for the presence of two tumor suppressor genes on chromosome 8p for colorectal carcinoma. Cancer Res. 1993;53(5):1172–1174. [PubMed] [Google Scholar]
- 51.Ahmed MN, Kim K, Haddad B, Berchuck A, Qumsiyeh MB. Comparative genomic hybridization studies in hydatidiform moles and choriocarcinoma: amplification of 7q21–q31 and loss of 8p12–p21 in choriocarcinoma. Cancer Genet Cytogenet. 2000;116:10–15. doi: 10.1016/S0165-4608(99)00103-X. [DOI] [PubMed] [Google Scholar]
- 52.Cunningham JM, Shan A, Wick MJ, et al. Allelic imbalance and microsatellite instability in prostatic adenocarcinoma. Cancer Res. 1996;56(19):4475–4482. [PubMed] [Google Scholar]
- 53.Arbieva ZH, Banerjee K, Kim SY, et al. High-resolution physical map and transcript identification of a prostate cancer deletion interval on 8p22. Genome Res. 2000;10:244–257. doi: 10.1101/gr.10.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pils D, Horak P, Gleiss A, et al. Five genes from chromosomal band 8p22 are significantly down-regulated in ovarian carcinoma: N33 and EFA6R have a potential impact on overall survival. Cancer. 2005;104(11):2417–2429. doi: 10.1002/cncr.21538. [DOI] [PubMed] [Google Scholar]
- 55.Cooke SL, Pole JCM, Chin S-F, Ellis IO, Caldas C, Edwards PAW. High-resolution array CGH clarifies events occurring on 8p in carcinogenesis. BMC Cancer. 2008;8:288. doi: 10.1186/1471-2407-8-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angstadt AY, Motsinger-Reif A, Thomas R, et al. Characterization of canine osteosarcoma by array comparative genomic hybridization and RT-qPCR: signatures of genomic imbalance in canine osteosarcoma parallel the human counterpart. Genes Chromosome Cancer. 2011;50:859–874. doi: 10.1002/gcc.20908. [DOI] [PubMed] [Google Scholar]
- 57.Griffith OL, Melck A, Jones SJ, Wiseman SM. Meta-analysis and meta-review of thyroid cancer gene expression profiling studies identifies important diagnostic biomarkers. J Clin Oncol. 2006;24(31):5043–5051. doi: 10.1200/JCO.2006.06.7330. [DOI] [PubMed] [Google Scholar]
- 58.Chung K-W, Kim SW, Kim SW. Gene expression profiling of papillary thyroid carcinomas in Korean patients by oligonucleotide microarrays. J Korean Surg Soc. 2012;82:271–280. doi: 10.4174/jkss.2012.82.5.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gutierrez VF, Marcos CA, Llorente JL, et al. Genetic profile of second primary tumors and recurrences in head and neck squamous cell carcinomas. Head Neck. 2012;34(6):830–839. doi: 10.1002/hed.21824. [DOI] [PubMed] [Google Scholar]
- 60.Gu Y, Wang Q, Guo K, et al. TUSC3 promotes colorectal cancer progression and epithelial-mesenchymal transition (EMT) through WNT/beta-catenin and MAPK signalling. J Pathol. 2016;239:60–71. doi: 10.1002/path.4697. [DOI] [PubMed] [Google Scholar]
- 61.Kratochvílová K, Horak P, Ešner M, et al. Tumor suppressor candidate 3 (TUSC3) prevents the epithelial-to-mesenchymal transition and inhibits tumor growth by modulating the endoplasmic reticulum stress response in ovarian cancer cells. Int J Cancer. 2015;137(6):1330–1340. doi: 10.1002/ijc.29502. [DOI] [PubMed] [Google Scholar]
- 62.Fan X, Zhang X, Shen J, et al. Decreased TUSC3 promotes pancreatic cancer proliferation, invasion and metastasis. PLoS ONE. 2016;11(2):e0149028. doi: 10.1371/journal.pone.0149028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang Z, Guo M, Zhang X, et al. TUSC3 suppresses glioblastoma development by inhibiting Akt signaling. Tumor Biol. 2016;37:12039–12047. doi: 10.1007/s13277-016-5072-4. [DOI] [PubMed] [Google Scholar]
- 64.Gu Y, Pei X, Ren Y, et al. Oncogenic function of TUSC3 in non-small cell lung cancer is associated with Hedgehog signalling pathway. Biochim Biophys Acta. 2017;1863(7):1749–1760. doi: 10.1016/j.bbadis.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Mullighan CG, Zhang J, Kasper LH, et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature. 2011;471(7337):235–239. doi: 10.1038/nature09727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan MM, Nomura T, Chiba T, et al. The fusion oncoprotein PML-RARalpha induces endoplasmic reticulum (ER)-associated degradation of N-CoR and ER stress. J Biol Chem. 2004;279(12):11814–11824. doi: 10.1074/jbc.M312121200. [DOI] [PubMed] [Google Scholar]
- 67.Schardt JA, Eyholzer M, Timchenko NA, Mueller BU, Pabst T. Unfolded protein response suppresses CEBPA by induction of calreticulin in acute myeloid leukaemia. J Cell Mol Med. 2010;14(6B):1509–1519. doi: 10.1111/j.1582-4934.2009.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kharabi Masouleh B, Geng H, Hurtz C, et al. Mechanistic rationale for targeting the unfolded protein response in pre-B acute lymphoblastic leukemia. Proc Nat Acad Sci USA. 2014;111(21):E2219–E2228. doi: 10.1073/pnas.1400958111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khoury GA, Baliban RC, Floudas CA. Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Sci Rep. 2011;1:90. doi: 10.1038/srep00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li CW, Lim SO, Xia W, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lauc G, Huffman JE, Pucic M, et al. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 2013;9(1):e1003225. doi: 10.1371/journal.pgen.1003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu K, Xie F, Gao A, et al. SOX2 regulates multiple malignant processes of breast cancer development through the SOX2/miR-181a-5p, miR-30e-5p/TUSC3 axis. Mol Cancer. 2017;16(1):62. doi: 10.1186/s12943-017-0632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pole JCM, Courtay-Cahen C, Garcia MJ, et al. High-resolution analysis of chromosome rearrangements on 8p in breast, colon and pancreatic cancer reveals a complex pattern of loss, gain and translocation. Oncogene. 2006;25:5693–5706. doi: 10.1038/sj.onc.1209570. [DOI] [PubMed] [Google Scholar]
- 74.Ribeiro IP, Marques F, Caramelo F, et al. Genetic gains and losses in oral squamous cell carcinoma: impact on clinical management. Cell Oncol. 2014;37:29–39. doi: 10.1007/s13402-013-0161-5. [DOI] [PubMed] [Google Scholar]
- 75.Yu X, Zhang K, Liu F, et al. Tumor suppressor candidate 3 as a novel predictor for lymph node metastasis in lung cancer patients. Oncol Lett. 2016;2016:5099–5105. doi: 10.3892/ol.2016.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guervos MA, Marcos CA, Hermsen M, Nuno AS, Suarez C, Llorente JL. Deletions of N33, STK11 and TP53 are involved in the development of lymph node metastasis in larynx and pharynx carcinomas. Cell Oncol. 2007;29(4):327–334. doi: 10.1155/2007/635962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu X, Zhang J, Zhong H, et al. Decreased tumor suppressor candidate 3 predicts poor prognosis of patients with esophageal squamous cell carcinoma. Int J Med Sci. 2016;13:963–969. doi: 10.7150/ijms.16381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng Y, Cao J, Yao XY, Wang JX, Zhong MZ, Gan PP, Li JH. TUSC3 induces autophagy in human non-small cell lung cancer cells through Wnt/β-catenin signaling. Oncotarget. 2017;8:52960–52974. doi: 10.18632/oncotarget.17674. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]