Abstract
Secondary damage following spinal cord injury leads to non-reversible lesions and hampering of the reparative process. The local production of pro-inflammatory cytokines such as TNF-α can exacerbate these events. Oligodendrocyte death also occurs, followed by progressive demyelination leading to significant tissue degeneration. Dental stem cells from human apical papilla (SCAP) can be easily obtained at the removal of an adult immature tooth. This offers a minimally invasive approach to re-use this tissue as a source of stem cells, as compared to biopsying neural tissue from a patient with a spinal cord injury. We assessed the potential of SCAP to exert neuroprotective effects by investigating two possible modes of action: modulation of neuro-inflammation and oligodendrocyte progenitor cell (OPC) differentiation. SCAP were co-cultured with LPS-activated microglia, LPS-activated rat spinal cord organotypic sections (SCOS), and LPS-activated co-cultures of SCOS and spinal cord adult OPC. We showed for the first time that SCAP can induce a reduction of TNF-α expression and secretion in inflamed spinal cord tissues and can stimulate OPC differentiation via activin-A secretion. This work underlines the potential therapeutic benefits of SCAP for spinal cord injury repair.
Electronic supplementary material
The online version of this article (10.1007/s00018-018-2764-5) contains supplementary material, which is available to authorized users.
Keywords: Spinal cord, Dental stem cells, Inflammation, Oligodendrocyte progenitor cells, Differentiation
Introduction
The cascade of secondary neuro-degenerative events following spinal cord injury (SCI) includes bleeding, necrotic or apoptotic processes, and lesion area enlargement [1, 2].
Among these, inflammation has been highlighted as a key regulator of degeneration and regeneration. Independent studies have shown that immunosuppression is permissive for central nervous system (CNS) tissue restoration [3, 4], while a beneficial role of inflammation on neuro-regeneration has also been described [5]. Both detrimental and favorable effects of the inflammatory response depend on the abundance of cell types (microglia and leukocytes), signaling molecules (chemokines and cytokines), and the post-traumatic timeline (acute, subacute, or chronic phase) [5]. Microglial activation is associated with both detrimental and beneficial neuro-regenerative outcomes depending on their activation state [5]. The presence of pro-inflammatory cytokines, such as TNF-α, may enhance cell death and intensify secondary damage in SCI [6, 7]. Furthermore, an increase in arginase-1 positive macrophages has already been associated with axon preservation, decrease of scar formation, increase in myelin sparing, and functional recovery after SCI [8].
Oligodendrocyte apoptosis that can be induced by inflammation, in and around the lesion site, leads to progressive demyelination, inducing axonal dysfunction and degeneration [9]. To restore the oligodendrocyte population, oligodendrocyte progenitor cell (OPC) proliferation and differentiation has to be activated. In the presence of pro-inflammatory cytokines and in the absence of growth promoting factors following SCI, remyelination is limited, among other factors, by impaired OPC differentiation [10]. Demyelination and inflammation are both crucial issues that must be addressed when developing therapeutic strategies aimed at the restoration of spinal cord function after injury.
Mesenchymal stem cell (MSC)-based therapies have proved to be promising strategies for spinal cord repair. Indeed, MSC therapy allows for multi-targeted and environmentally responsive benefits [11, 12]. The positive effects of MSC treatment for CNS diseases/disorders result from their ability to differentiate into neural cell lineages, secrete neurotrophic factors, reduce cell apoptosis, and modulate inflammation [13]. MSC improve outcomes after neural trauma by inhibiting the activation of pro-inflammatory microglia and by promoting their stimulation to an anti-inflammatory phenotype [14]. The pro-inflammatory phenotype of microglia has increased expression of pro-inflammatory cytokines such as TNF-α or IL-1β, whereas anti-inflammatory microglia releases neuroprotective factors including anti-inflammatory cytokines (IL-10, IL-1 receptor antagonist) as well as neurotrophic factors (nerve growth factor and transforming growth factor β) [15].
Human dental stem cells have been increasingly studied as an alternative source of MSC to bone marrow due to their accessibility, their neural crest origin and their high proliferation rate [16]. Indeed, dental stem cells can be easily isolated with a limited invasiveness and display a higher proliferation rate and expression of specific neural stem cell transcripts and proteins than bone-marrow-derived MSC [17, 18]. Although stem cells from human apical papilla (SCAP) are less studied and exploited than dental pulp stem cells (DPSC), they have a greater migratory and tissue regenerative capacity, as well as higher proliferative potential [19]. SCAP can regulate trigeminal nerve outgrowth in vitro and support an increased hind limp muscle strength in a rat spinal cord injury model [20]. We showed that implantation of SCAP embedded in their original niche (whole apical papilla tissue) into a rat hemi-section SCI model promoted functional recovery [21].
Activin-A is a member of the transforming growth factor β superfamily. It presents plethoric effects that have been extensively studied in various organs, but it was only recently that Miron et al. showed that activin-A stimulates OPC differentiation. In addition, activin-A has an anti-inflammatory effect and is produced by alternatively activated macrophages [22]. Jeong et al. showed that activin-A is constitutively expressed in healthy spinal cord and that levels increased up to 4 day post-injury to decrease again at day 7 [23]. Activin-A is synthesized by either neurons or inflammatory cells (possibly macrophages) or both and thus may participate in the protection of neuronal tissues after SCI. In addition, activin-A levels increased in astrocytes and oligodendrocytes at the peripheries of SCI lesions [23]. Thus, activin-A may protect glial cells, in addition to neurons, in either a paracrine or autocrine manner [23].
In this study, we examined the in vitro and ex vivo impacts of SCAP on microglia activation and OPC differentiation. We demonstrated that SCAP can reduce the expression and secretion of TNF-α in an LPS-activated microglial cell line and in LPS-activated spinal cord tissue. In addition, we showed that OPC differentiate when co-cultured with spinal cord tissue and SCAP, due, at least partially, to activin-A secretion. This study provides evidence that SCAP could support spinal cord repair by a neuroprotective action, driven by reduction of pro-inflammatory cytokines and the stimulation of OPC differentiation via activin-A secretion.
Methods
SCAP culture
Human SCAP were isolated from a healthy donor wisdom tooth and characterized [24]. SCAP were used between passages 5 and 8 [24, 25]. SCAP were cultured in minimum essential medium eagle (MEM, Sigma-Aldrich, St Louis, USA) supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine (ThermoFisher, Merelbeke, Belgium), and 1% Penicillin/Streptomycin (PEST) (ThermoFisher) (SCAP medium). Cells were either grown in normoxia (N) in 21% O2 and 5% CO2 or in hypoxia (H) in 1% O2 and 5% CO2 (InVivo2 400 hypoxia Workstation, Ruskinn, Bridgend, UK). SCAP medium was equilibrated at 1% O2 before medium change for SCAP grown in hypoxia.
BV-2 mouse microglial cell culture
BV-2 cells (ATCC) were maintained in Dulbecco’s modified eagle’s medium (DMEM) with Glutamax™ (ThermoFisher), supplemented with 10% FBS (Sigma-Aldrich) and 1% PEST (BV-2 medium) at 37 °C in 21% O2 and 5% CO2. BV-2 cells were seeded in 24-well plates at a density of 5 × 105 cells/well.
OPC isolation and culture
All animal-related experiments were approved by the local ethical committee for animal care (2016/UCL/MD/011). OPC were isolated and plated as previously described [26]. Briefly, adult Wistar rat spinal cords were isolated and dissociated into single cells through enzymatic digestion with trypsin–EDTA (ThermoFisher). The cell suspension was then strained, centrifuged and the resulting pellet re-suspended in DMEM/F12 containing 1% PEST, 10 mM HEPES buffer (ThermoFisher), 10 ng/ml FGF2 (PeproTech, Rocky Hill, USA), 10 ng/ml platelet-derived growth factor-AA (PDGFAA) (PeproTech), and 10 ng/ml IGF-I (PeproTech) and plated in uncoated culture flasks. Fresh growth factors were added every other day, and media were changed completely after 7 days of culture. Free floating OPC spheres were collected and passaged following dissociation with Accutase® (ThermoFisher). OPC (fourth passage) were seeded as single cells onto poly-d-lysine (PDL) (Sigma-Aldrich) coated 13 mm glass coverslips at a density of 10,000 cells/cm2 following dissociation of spheres with Accutase®. For seeding, cells were maintained in their growth media with the addition of 0.5% FBS for 60 min to allow the cells to adhere. Seeded coverslips were then transferred to a 30 mm petri dish (3 coverslips per dish) and maintained in SCAP medium.
Spinal cord organotypic section (SCOS) preparation and culture
Spinal cords of P7 Wistar rats were extracted, dissected as previously described [27] and 350 µm sections were transversally cut with a tissue chopper (McIlWain Tissue Chopper, The Mickle Laboratory Engineering Co. Ltd., Surrey, UK). Four sections were placed on type I collagen-coated (10 μg/cm2, Sigma-Aldrich) Millicell® Organotypic Cell Culture Inserts (Merck Millipore, Billerica, USA). One ml of medium consisting of 48.37% MEM, 24.18% horse serum, and 24.18% HBSS (ThermoFisher) supplemented with 2% d-glucose, 0.97% Glutamax™, 0.97% PEST, 1.21% HEPES, and 0.12% amphotericin B (Thermofisher) (pH 7.4, SCOS medium) was added in the lower compartment of each insert. SCOS were maintained at 37 °C in a humidified incubator for 2 weeks before use. Medium was changed 24 h after the dissection and then twice a week until SCOS were used for the experiments.
BV-2 cells–SCAP co-culture and activation by LPS
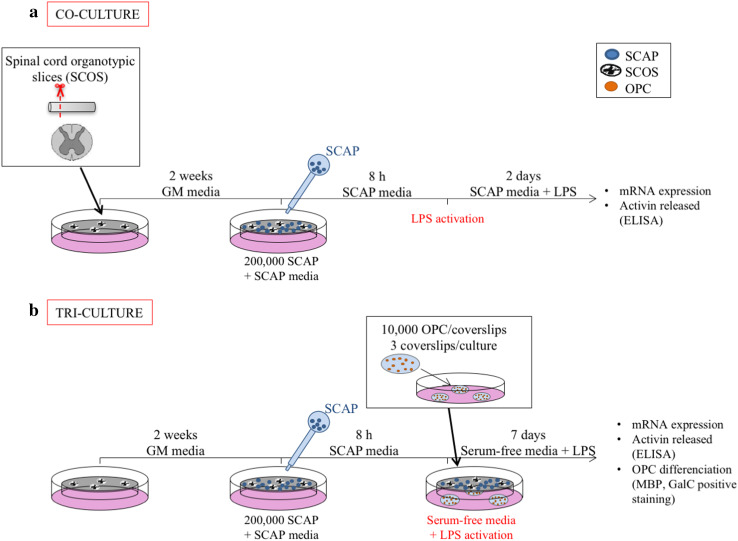
BV-2 cells (5 × 105 cells/well) were seeded in 24 well plates. Eight hours later, resting cells were stimulated by incubation with 100 ng/ml of lipopolysaccharides from Escherichia coli (LPS, O55:B5) (Sigma-Aldrich) for 16 h. SCAP previously grown in normoxia or hypoxia were then added to the LPS-activated BV-2 cells at the same density (5 × 105 cells/well). The BV-2 cells–SCAP co-cultures were maintained in SCAP medium supplemented with 100 ng/ml of LPS at 37 °C in normoxia for 48 h. LPS-activated BV-2 cells cultured without SCAP and non-activated BV-2 cells were used as controls (N = 3, n = 4).
SCOS–SCAP co-culture and activation by LPS
SCAP grown in normoxia and hypoxia (2 × 105 SCAP/insert in 200 µl of SCAP media) were co-cultured with SCOS by seeding them on the upper side of inserts (Fig. 1a). Medium was changed from SCOS medium to SCAP medium and cells were allowed to attach on the inserts for 8 h. We checked that SCAP attached properly on the inserts by bright field microscopy. SCAP attached and spread on the inserts with the same morphology as when grown in culture flasks (data not shown). The medium was then changed for SCAP medium containing LPS (100 ng/ml). Co-cultures were maintained for 48 h before analysis. LPS-activated SCOS cultured without SCAP and non-activated SCOS were used as controls (N = 3; n = 4–5).
Fig. 1.
Graphical experimental plan. a Co-culture model of SCOS with SCAP on insert. b Tri-culture model of SCOS with SCAP on inserts and with OPC isolated from adult rat spinal cord seeded on coverslips. Groups without SCAP are presented in white, groups with SCAP grown in normoxia (N) in light grey and groups with SCAP grown in hypoxia (H) in dark grey
SCOS–SCAP–OPC tri-culture and activation by LPS
SCAP cultured in either normoxia or hypoxia were seeded on the inserts as described above. Coverslips seeded with adult rat OPC were placed under seeded inserts (three coverslips per insert). Medium was then changed for serum-free SCAP medium supplemented with LPS (100 ng/ml) (Fig. 1b). Cultures were maintained for 7 days before analysis. OPC without SCOS or without SCAP were used as controls (N = 3, n = 3–4).
Impact of activin-A inhibition on OPC differentiation
SCOS–SCAP–OPC were tri-cultured as described above. Follistatin, a natural activin-A inhibitor [28], was added in the lower compartment of SCOS–SCAP–OPC cultures [50 ng/ml for 48 h and then 10 ng/ml for 3 days (R&D system, Minneapolis, USA)]. Cultures were maintained for 7 days before analysis. Cultures of SCAP alone, OPC alone, SCAP with OPC, and SCOS with OPC were used as controls. OPC alone, SCOS–OPC, and SCOS–SCAP–OPC were supplemented with exogenous recombinant activin-A at the same concentration as detected using enzyme-linked immunosorbent assay (ELISA) in SCOS–SCAP supernatants (10 ng/ml) and were used as positive controls (N = 2, n = 3).
RNA extraction and real-time qPCR
For mRNA analysis, media were removed and TriPure reagent (Roche, Basel, Switzerland) was added to each well of BV2 cells or co-/tri-cultures at the end of the incubation period. Regarding SCOS, each section was carefully picked up and placed in a 0.5 ml tube with TriPure. The plates were then stored at − 80 °C for later assessment. Total RNA was extracted using the TriPure reagent according to the manufacturer’s instructions. cDNA was synthesized using a reverse transcription kit (Promega corporation, Leiden, The Netherlands) from 1 μg of total RNA. qPCR was performed with a STEP one PLUS instrument and software (Applied Biosystems, Foster City, CA, USA) as previously described [29]. Data were normalized to the 60S ribosomal protein L19 (RPL19) mRNA expression for the BV-2 cells, and to the 60S ribosomal protein L13 (RPL13) mRNA expression for the SCAP and SCOS. For each experiment, the absence of treatment effect on reference gene expression was verified. Primer sequences and accession numbers are listed in Table 1. Primers were designed to study the gene expression of activin-A. Activin-A mRNA sequence shows substantial homology between rodents and humans; however, the mRNA sequence of human Activin-A is longer. Thus, primers were designed specifically for human activin-A (human-specific primers), while it was not possible to design primers specific for rat activin-A. Therefore, the designed primers were able to recognize human, rat, and mouse activin-A (universal primers).
Table 1.
Primer sequences
| Gene name | Accession number | Cell type | Forward primer (5′–3′) | Reverse primer (3′–5′) | Figure |
|---|---|---|---|---|---|
| Rat RPL13 | NM_173340.2 | SCOS | GGCTGAAGCCTACCAGAAAG | CTTTGCCTTTTCCTTCCGTT | Figures 2d, f, 3d |
| Rat arginase | NM_017134.3 | SCOS | GAAGGTCTCTACATCACAGAAGAAA | CAAGGTCAACGCCACTGC | Figure 2f |
| Rat TNF-α | NM_012675.3 | SCOS | AGTGACAAGCCCGTAGCC | TTGAAGAGAACCTGGGAGTAGA | Figure 2d |
| Mouse RPL19 | NM_009078.2 | BV-2 cells | GAAGGTCAAAGGGAATGTGTTCA | CCTTGTCTGCCTTCAGCTTGT | Figure 2a, c |
| Mouse Arginase | NM_007482.3 | BV-2 cells | GGTTCTGGGAGGCCTATCTT | TGAAAGGAGCCCTGTCTTGT | Figure 2c |
| Mouse TNF-α | NM_013693.3 | BV-2 cells | AGCCCCCAGTCTGTATCCTT | GGTCACTGTCCCAGCATCTT | Figure 2a |
| Human RPL13 | NM_012423.3 | SCAP | CATAGGAAGCTGGGAGCAAG | GCCCTCCAATCAGTCTTCTG | Figure 3c |
| Human Activin | NM_002192.3 | SCAP | TCCCTTGTGAGCCTTGAATC | CCTGGGTAATTGGGTAGGAAAG | Figure 3c |
| UNIVERSAL Activin | NM_002192.3 (Human) NM_017128.2 (Rat) NM_008380.2 (Mouse) | SCOS and SCAP | TCATCACCTTTGCCGAGTCA | CTGGTTCTGTTAGCCTTGGG | Figure 3d |
When several variants exist, the primers were designed to recognize all variants
TNF-α and activin-A quantification by ELISA
TNF-α and activin-A were quantified in undiluted cell supernatants using a Murine TNF-α Standard TMB ELISA Development Kit (PeproTech) and a DuoSet ELISA kit (R&D System, Abingdon, UK), respectively. The TNF-α ELISA kit has been tested against human TNF-α by the manufacturer and did not show cross reactivity with human TNF-α. ELISA assays were performed as recommended by the manufacturer. TNF-α was quantified 48 h after treatment in BV-2 cells–SCAP and SCOS–SCAP co-cultures. Activin-A was quantified 48 h after treatment in SCOS–SCAP co-cultures and in SCOS–SCAP–OPC tri-cultures (N = 3, n = 3–5). All measurements were performed in duplicate.
Immunofluorescence
SCOS were stained for Myelin Basic Protein (MBP), chemokine (C-C motif) ligand 1 (CCL1), and neural/glial antigen 2 (NG2) after co-culture with SCAP. SCOS were fixed in 4% paraformaldehyde (PFA) for 1 h and rinsed twice in PBS. Samples were blocked for 1 h at room temperature in 2.5% horse serum containing 0.03% triton X100 and incubated with primary antibodies at room temperature for 1 h (Table 2). Sections were then washed in PBS and incubated for 2 h at room temperature with the secondary antibodies (Table 2). Native OPC were identified by NG2 immunoreactivity and mature oligodendrocytes by MBP immunoreactivity.
Table 2.
List of antibodies used for immunofluorescence analysis
| Primary antibody | Dilution | Supplier | Secondary antibody | Dilution | Supplier |
|---|---|---|---|---|---|
| MBP | 1:250 | BioRada | Anti-rat IgG2a Alexa 555 (mouse) | 1:1000 | Life Technologiesd |
| CC1 | 1:100 | Abcamb | Anti-mouse Alexa 647 | 1:1000 | Life Technologiesd |
| NG2 | 1:200 | Merck Milliporec | Anti-rabbit IgG Alexa 488 (goat) | 1:1000 | Life Technologiesd |
| GalC | 1:800 | Merck Milliporec | Anti-mouse IgG3 Alexa 594 (goat) | 1:400 | Life Technologiesd |
aBioRad, Hercules, USA
bAbcam, Cambridge, UK
cMerck Millipore, Temecula, CA
dLife Technologies, Eugene, OR, USA
OPC derived from adult rat spinal cord were stained for GalC after tri-culture with SCOS and SCAP. OPC were fixed in 4% PFA for 10 min, washed with PBS, and incubated in blocking solution (2% bovine serum albumin, 10% normal goat serum, and 0.03% Triton X100 in PBS) for 1 h. Samples were incubated with primary antibodies diluted in blocking solution (Table 2) for 1 h at room temperature. After being washed in PBS, coverslips were incubated with secondary antibodies (diluted in PBS containing 0.5% BSA, Table 2) for 1 h at room temperature. Negative controls were performed by omitting primary antibodies. Vectashield hardset [containing 4′, 6-diamidino-2-phenylindole, dihydrochloride (DAPI)] (Vectorlabs, Burlingame, CA) was used as mounting medium. Quantification of positive cell number and area was performed on three random images per coverslips. MBP and GalC positive staining was quantified using Volocity (Perkin Elmer, Coventry, UK) and ImageJ (1.43u, National Institutes of Health, Bethesda, MD, USA), respectively.
Statistical analysis
Statistical analyses were performed using GraphPad PRISM (GraphPad Software, San Diego, CA, USA). One-way analysis of variance (ANOVA) with an appropriate post hoc test was performed. Paired t test was performed for MBP quantification. Error bars represent the standard error of the mean in all figures. Statistical significance was accepted at the 5% level (p < 0.05). Conditions not related by the same letter are significantly different. “N” and “n” indicate number of independent experiments and number of biological replicates, respectively. Between 3 and 5 sections per condition were analyzed depending of the total number of sections isolated from rat spinal cords.
Results
SCAP modulate the expression of inflammatory markers
The impact of SCAP on TNF-α and arginase-1 expression was investigated in LPS-activated BV-2 cells (microglia). Spinal cord lesions present a hypoxic environment that could impact MSC behavior and its secretome [30]. We previously demonstrated that hypoxia induced an up-regulation of SCAP gene expression of neurotrophic factors [25]. Thus, we assessed the impact of SCAP hypoxia-preconditioning treatment on their immunomodulatory properties.
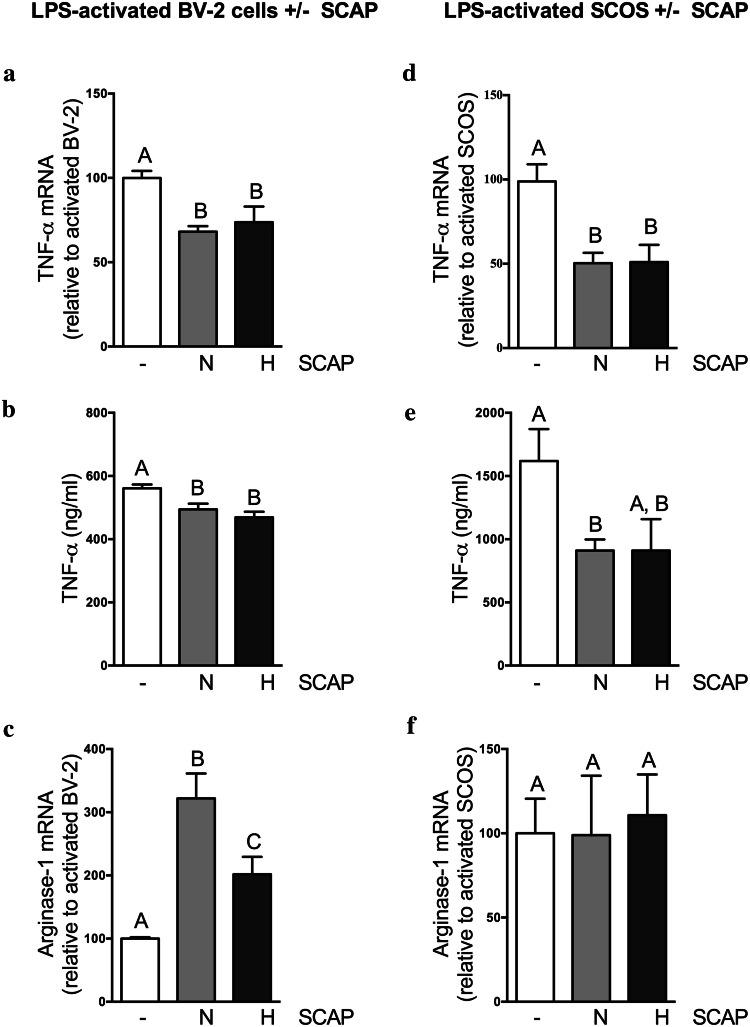
LPS treatment induced an increased expression of TNF-α while decreasing the expression of arginase-1 in comparison with non-activated BV-2 cells (supplementary data a and b). Co-culture of SCAP with LPS-treated BV-2 cells induced a significant decrease of TNF-α mRNA expression (Fig. 2a) and secretion (Fig. 2b) compared to BV-2 cells cultured alone. There was no influence of SCAP culture condition (i.e., hypoxia or normoxia). Arginase-1 mRNA expression was increased in BV-2 cells when co-cultured with SCAP relative to BV-2 mono-cultures. This effect was more pronounced when BV-2 cells were cultured with SCAP grown in normoxia than when cultured with SCAP grown in hypoxia (Fig. 2c).
Fig. 2.
SCAP modulate the inflammatory markers gene expression and secretion. a–c SCAP impact on LPS-activated BV-2 cells, 48 h after incubation. a SCAP impact on TNF-α gene expression of BV-2 cells evaluated by RT-qPCR (N = 2, n = 4). b Impact of SCAP co-cultured with BV-2 cells on BV-2 cells TNF-α production in culture media (ELISA) (N = 2, n = 4). c SCAP impact on arginase-1 gene expression of BV-2 cells (N = 2, n = 4). d–f SCAP impact on LPS-activated SCOS, 48 h after incubation. d Impact of SCAP on SCOS TNF-α gene expression (N = 2, n = 4). E: Impact of SCAP co-cultured with SCOS on TNF-α production in culture media (ELISA) (N = 2, n = 4). f Impact of SCAP on SCOS arginase gene expression (N = 2, n = 4). Conditions not related by the same letter are significantly different. Groups without SCAP are presented in white, groups with SCAP grown in normoxia (N) in light grey and groups with SCAP grown in hypoxia (H) in dark grey
The same experiment was performed to evaluate the effect of SCAP on TNF-α and arginase-1 mRNA expression by LPS-activated SCOS. This culture model enables the preservation of the basic cytoarchitecture and neuronal–glial interactions [31], while allowing us to assess responses of both endogenous and exogenous cells as well as their interactions [32]. SCAP, whether grown in normoxia or hypoxia, were able to adhere to inserts and displayed a normal cell morphology compared to cells grown in flasks (data not shown). LPS treatment of SCOS induced an increased expression of TNF-α mRNA compared with gene expression of non-activated SCOS (supplementary data c). Co-culture of SCAP (grown in normoxia or hypoxia) with LPS-treated SCOS significantly decreased TNF-α expression (Fig. 2d) compared to SCOS cultured alone. A significant decrease of TNF-α secretion was observed in the supernatants of SCOS co-cultured with SCAP in comparison with SCOS alone (Fig. 2e). However, SCAP co-culture with SCOS did not influence arginase-1 gene expression (Fig. 2f).
SCOS–SCAP co-culture increased MBP staining in SCOS
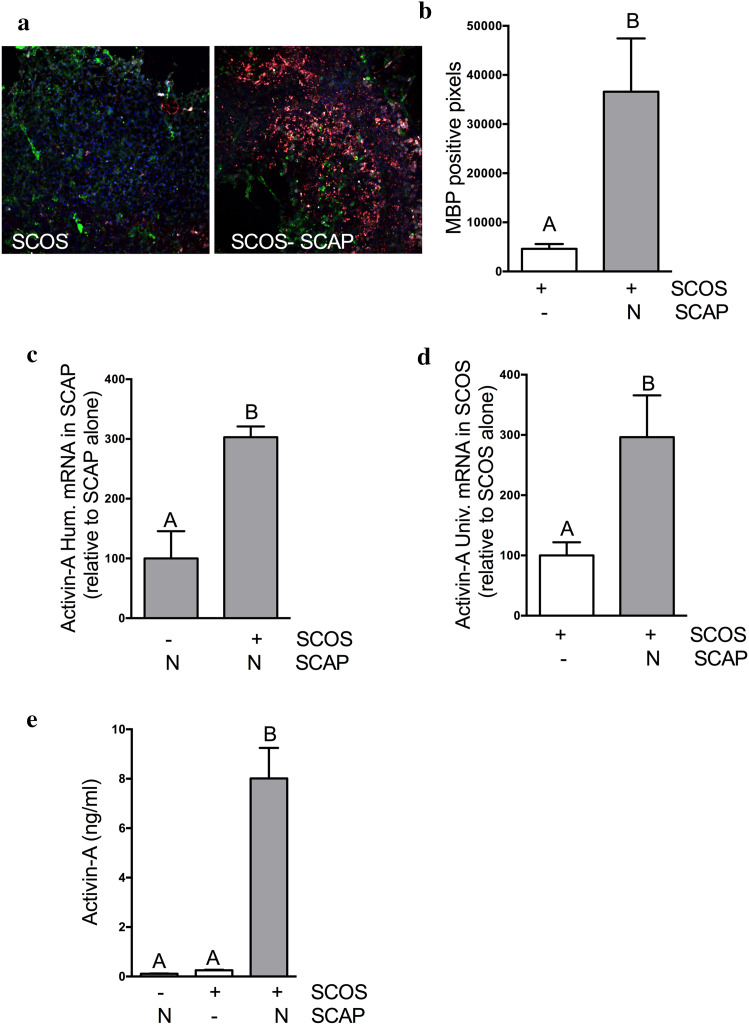
Remyelination is a key regenerative process, promoting functional recovery following SCI [33–35]. Therefore, proliferation and differentiation of OPC are crucial to restore and maintain myelin. Co-culture of SCOS with SCAP showed an increase in staining for mature oligodendrocyte markers (MBP and CC1 positive cells) in SCOS compared to SCOS alone (Fig. 3a). Pixel quantification showed that significantly more cells were positive for MBP in SCOS–SCAP co-cultures than in SCOS alone (Fig. 3b).
Fig. 3.
SCOS–SCAP co-culture increased the expression of MBP in SCOS and induces activin-A secretion. a OPC were identified in SCOS by immunofluorescence using NG2 (green) staining and oligodendrocytes were identified using MBP (red) and CC1 (white) staining. b MBP positive pixels were quantified in SCOS cultured with and without SCAP (N = 2, n = 3). c Activin-A gene in SCAP 48 h after incubation with SCOS (N = 2, n = 3). d Activin-A gene expression in SCOS 48 h after incubation with SCAP (N = 2, n = 3). e Activin-A quantification in culture media using ELISA (N = 3, n = 3). Conditions not related by the same letter are significantly different. Groups without SCAP are presented in white, groups with SCAP grown in normoxia (N) in light grey and groups with SCAP grown in hypoxia (H) in dark grey
SCOS–SCAP co-culture induces activin-A expression and secretion
Since SCAP co-culture with SCOS increased OPC to oligodendrocyte differentiation, we assessed the expression and secretion of activin-A in co-cultures in the presence of LPS. SCAP and SCOS cultured alone expressed activin-A (Fig. 3c, d). A significant increase of human activin-A gene expression was observed when SCAP were co-cultured with SCOS compared to SCAP alone (Fig. 3c). Using primers that recognized both human and rat activin-A, we observed that co-culture of SCAP with SCOS also showed a significant increase of activin-A gene expression (Fig. 3d). Importantly, SCAP co-culture with SCOS induced a higher concentration of activin-A in supernatants compared to SCAP or SCOS alone (Fig. 3e). No effect of hypoxia was observed on activin-A expression (Supplementary data d), so only SCAP grown in normoxia were used for the following experiments.
SCOS–SCAP promotes adult OPC differentiation
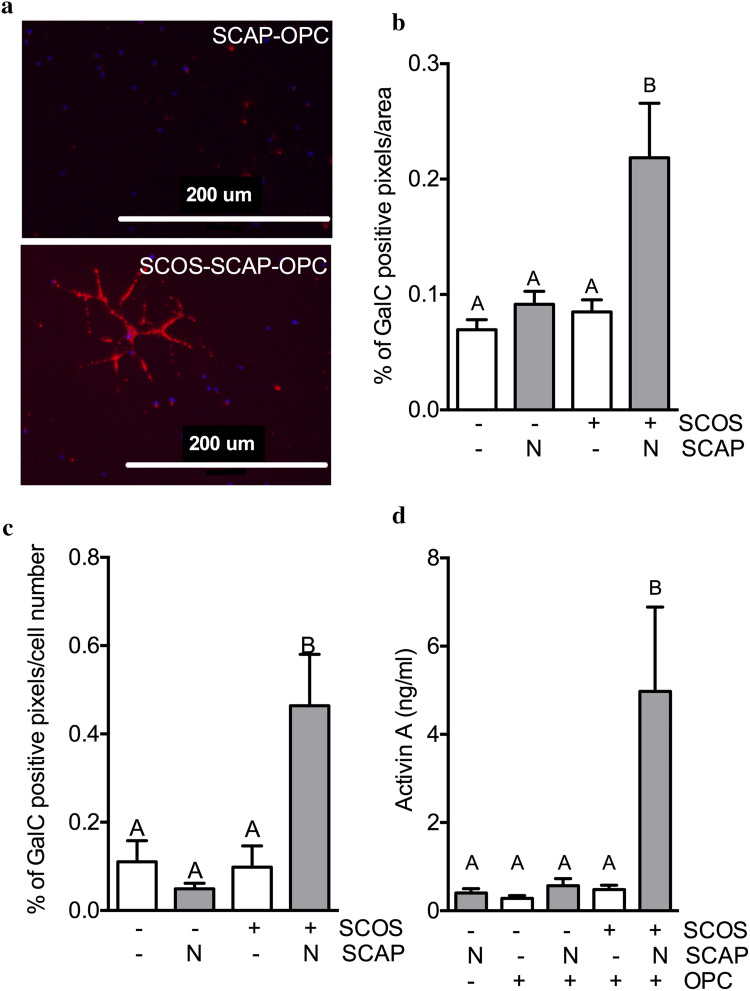
To confirm the impact of SCAP on adult spinal cord OPC differentiation, we developed an original tri-culture model, where SCAP and SCOS were co-cultured with OPC isolated from adult rat spinal cord (Fig. 1b). The area of GalC+ staining was significantly higher, as well as the percentage of positive cells, when OPC were co-cultured with both SCOS and SCAP relative to OPC alone, SCAP and OPC or SCOS and OPC (Fig. 4b, c, respectively). Consistent with the hypothesis that activin-A promotes OPC differentiation, a higher activin-A concentration was detected only in culture media of SCOS cultured with SCAP and OPC compared to the other conditions (Fig. 4d).
Fig. 4.
SCOS–SCAP promotes adult OPC differentiation and induces activin-A secretion. a Oligodendrocytes were identified by immunofluorescence using GalC staining 7 days after incubation of adult rat spinal cord OPC with SCOS and SCAP. b–c GalC positive pixels were quantified by ImageJ software per area (b) and cell numer (c) [N = 2, n = 3 (3 pictures/coverslips)]. d Activin-A quantification in culture media after 48 h of incubation (ELISA) (N = 3, n = 3). Conditions not related by the same letter are significantly different. Groups without SCAP are presented in white, groups with SCAP grown in normoxia (N) in light grey and groups with SCAP grown in hypoxia (H) in dark grey
Activin-A produced by SCAP and SCOS co-culture induces OPC differentiation
We next investigated whether activin-A was responsible for OPC differentiation by treating the cultures with the activin-A-sequestering protein follistatin, which prevents its action and its detection by ELISA. Follistatin impact on activin-A concentration in culture media was first evaluated, and we observed that follistatin was able to reduce the amount of activin-A detected in the medium of the tri-cultures (Fig. 5a). We observed a similar effect of follistatin when exogenous activin-A was added to the cultures (Fig. 5a, grey bars), confirming activin-A inhibition by follistatin.
Fig. 5.
Activin-A produced by SCAP and SCOS induces OPC differentiation. a Activin-A quantification in culture media (ELISA) 48 h after incubation (N = 2, n = 3). b Oligodendrocytes were identified by immunofluorescence using GalC staining 7 days after incubation. c–d GalC positive pixels were quantified by ImageJ software per area (c) and cell number (d) [N = 2, n = 3 (3 pictures/coverslips)]. Conditions not related by the same letter are significantly different. Groups without SCAP are presented in white, groups with SCAP grown in normoxia (N) in light grey and groups with SCAP grown in hypoxia (H) in dark grey
When treated with exogenous activin-A, the percentage of GalC+ cells and area tended to increase in OPC–SCOS cultures (Fig. 5c, d). The percentage of surface area significantly increased only when OPC were co-cultured with SCOS and SCAP (Fig. 5c), as the percentage of positive cells was not significantly different between activin-A-treated OPC–SCOS cultures and OPC–SCOS–SCAP tri-cultures (Fig. 5d). When treated with follistatin, the percentage of adult rat OPC that were GalC+ decreased significantly in the tri-cultures (Fig. 5b, d).
Discussion
Stem cells can stimulate tissue repair primarily via two mechanisms: cell replacement and secretion of bioactive molecules. MSC have been described to have immunomodulatory properties [13], but little information is available on dental stem cells, and in particular SCAP, a promising source of MSC for CNS repair. In this study, we demonstrate that SCAP impact microglial function and stimulate OPC differentiation in the presence of pro-inflammatory stimuli. We also showed for the first time that SCAP express activin-A and that this expression was increased in the presence of inflamed spinal cord tissue. We established a link between activin-A secretion by SCAP–SCOS and the differentiation of adult spinal cord OPC into mature oligodendrocytes.
LPS treatment stimulated TNF-α expression and secretion by both BV-2 cells and SCOS. The impact of LPS on BV-2 cells has been extensively described by others [36, 37], but this is the first time that spinal cord organotypic cultures have been used to study the impact of stem cells on the activation of spinal cord tissue. We showed that when SCOS were treated with 100 ng/ml of LPS, gene expression for TNF-α significantly increased. Thus, SCOS reactivity to LPS makes this ex vivo model a useful tool to study the effect of new therapies on neuro-inflammation.
We demonstrated that SCAP induced a decrease in TNF-α expression and secretion in LPS-activated BV-2 cells and SCOS. The ability of two types of dental stem cells (dental pulp stem cells and stem cells from human exfoliated deciduous teeth) to reduce secretion of pro-inflammatory cytokines like TNF-α has already been described [38, 39], but little is known about SCAP. SCAP could have an anti-inflammatory action by secretion of active molecules such as HGF, TGF-β, IDO, PGE2, and cytokines like IL-10 and IL-4 [40, 41]. MSC could also act via extracellular vesicles containing miRNA (i.e., miRNA 146 and 155) or via an EV surface molecule like PD-L1 or galectin-1 [42]. Another possible explanation could be the production of TNF-stimulated gene 6 protein (TSG-6), a protein that inhibits the NF-kB and MAPK activation pathway, by SCAP activated by LPS-treated microglial cells. Expression of pro-inflammatory cytokines such as TNF-α increases during the acute phase (first hours) of SCI and exacerbates secondary tissue degeneration [43, 44]. TNF-α induces neuron and oligodendrocyte apoptosis [43], lesion area enlargement and promotes Wallerian degeneration [9]. Down-regulation of TNF-α has also been linked with greater neuronal survival and reduced apoptosis [7]. Furthermore, MSC have been reported to reduce BV-2 cell proliferation and TNF-α expression following LPS stimulation [37, 45]. Similarly, our data demonstrate the impact of SCAP on pro-inflammatory cytokine expression. The transplantation of MSC after SCI has already been correlated with an increase of alternatively activated macrophages (i.e., arginase-1 positive cells) that were associated with functional recovery [8]. Stem cells from human exfoliated deciduous teeth have been reported to be associated with the induction of an anti-inflammatory macrophage phenotype when injected into the spinal cord after injury. This effect was attributed to the secreted proteins MCP-1 and ED-Siglec-9 [46]. Thus, SCAP could protect the spinal cord after injury by acting on macrophages and microglia, limiting damage that is associated with prolonged inflammation.
We observed that culturing SCAP in hypoxia did not offer any advantage, but was not detrimental to their immunomodulation properties nor to their activin-A expression (supplementary data d). Although Jiang and colleagues reported that MSC can be grown in hypoxia to enhance their immunomodulatory properties, migration, proliferation, and survival [47], these effects depend on the origin of the MSC, the serum used [48] and O2 tension under which the study was performed [49]. Since SCAP properties are not limited by hypoxia, we may expect that local hypoxic conditions in spinal cord lesion should not affect their immunomodulation properties while supporting neurotrophic factor production.
It has already been demonstrated that dental stem cells could support remyelination in vitro and in vivo [50, 51]. In addition, other dental stem cells (dental pulp stem cells and stem cells from human exfoliated deciduous teeth) demonstrate neuro-regenerative properties, which they exert via different mechanisms, including the prevention of neural apoptosis, the blocking of axon growth inhibitors, and the replacement of dead oligodendrocytes [51]. These findings suggest that dental stem cells could promote remyelination after SCI. Our objective was thus to evaluate the impact of SCAP on adult OPC differentiation via paracrine actions. To that end, we developed an innovative tri-culture model composed of SCAP, SCOS and isolated adult OPC from rat spinal cord. This model can be used to investigate and optimize treatments for SCI before resorting to the more challenging in vivo SCI models. We showed that SCAP, when associated with spinal cord tissue, promoted the differentiation of adult OPC into mature oligodendrocytes. MSC have been described to support remyelination, reduce demyelination and cell loss by decreasing the astroglial response and apoptosis in a cuprizone model [52]. The mechanisms involved are not clearly elucidated yet, but the positive action of MSC is attributed to the protection of damaged axons and immunomodulation.
This study reports, for the first time, that activin-A is expressed by SCAP and SCOS. Moreover, we showed that activin-A was expressed by both SCAP and SCOS separately, but that the interaction of SCAP and SCOS induced a higher degree of activin-A expression and secretion. Djouad and colleagues observed that activin-A secretion by MSC, as well as the activin-A:follistatin ratio, depends on their origin [53]. Activin-A expression is required for the maintenance of stemness and the regulation of MSC functions. Activin-A also plays a functional role in the suppression of inflammatory or immune processes [54]. A correlation between activin-A and MSC-mediated immunosuppression has been made and suggested that lower concentrations of activin-A produced by tonsil-derived MSC (compared with other MSC types), corresponded to their significantly lower immunosuppressive potential [53]. Activin-A produced by human umbilical cord-derived MSC suppressed interferon gamma (INF-γ) production by natural killer cells [55]. An up-regulation of activin-A has been observed in the early stage of SCI [23]. This higher level of activin-A was correlated with neuroprotection and immunomodulation, potentially by stimulating proliferation of alternatively activated macrophages [23]. In the present study, a significant increase of activin-A concentration in the medium of SCAP–SCOS co-cultures correlated with increased MBP staining in SCOS. Recently, it has been shown that activin-A stimulates OPC differentiation to MBP positive oligodendrocytes [22]. In support of the earlier report, we demonstrated that OPC differentiation was correlated with increased activin-A production, which was enhanced by the presence of SCAP. Interestingly, we observed that the presence of spinal tissue was indispensable for the increased activin-A secretion and OPC differentiation. Our findings suggest the presence of a potential synergy between SCAP and spinal tissue that leads to an increase of activin-A production and OPC differentiation.
To determine if activin-A secreted by SCAP/SCOS was solely responsible for OPC differentiation, we treated the tri-cultures with follistatin. This protein inhibits activin-A actions by forming a complex consisting of one activin-A dimer and two follistatin molecules, thus preventing activin-A from binding to its receptor [28]. Activin-A was no longer detected by ELISA when culture supernatants were supplemented with follistatin, confirming that the majority of activin-A had been sequestered and inactivated. In addition, blocking activin-A with follistatin in tri-cultures suppressed OPC differentiation, which was not rescued when exogenous activin-A was added in the absence of SCAP. As the percentage of GalC+ area was higher in the tri-cultures than in cultures supplemented with exogenous activin-A, and as this percentage was reduced nonetheless at the same level by follistatin than in exogenous activin-A supplemented cultures, we concluded that activin-A was required, but did not induce OPC differentiation alone. Supporting this hypothesis is the fact that as follistatin is mostly known to inhibit activin-A, it also inhibits factors from the TGF superfamily such as the bone morphogenic proteins [56]. It may be hypothesized that SCAP produce other essential bioactive molecules that stimulate OPC differentiation along with activin-A, the actions of which are also inhibited by follistatin. Several other related factors, such as growth factors (e.g., PDGF, IGF-1) or cytokines (e.g., TGF-β1, CXCL12), have been reported to induce OPC differentiation [57] and could, therefore, be involved in this phenomenon.
This study highlights the neuroprotective potential of SCAP via two mechanisms: modulation of neuro-inflammation and the promotion of OPC differentiation to mature oligodendrocytes. For the first time, we demonstrate that SCAP, in association with spinal tissue, produce activin-A and that this protein supports OPC differentiation. Our data demonstrate that SCAP may provide therapeutic benefits for treating acute SCI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Results obtained for LPS-treated samples are presented in this manuscript, while results for non-LPS-treated samples are in supplementary data.
Acknowledgements
Anne des Rieux is a Research Associate, Mireille Alhouayek is a Post-doctoral Researcher and Pauline Bottemanne is a FRIA Doctoral Researcher at the FRS-FNRS (Fonds de la Recherche Scientifique). The authors acknowledge Prof. O. Feron (UCL) for the access to hypoxia incubator and Daniel Soong (EdU) for his help with MBP quantification as well as Loïc Germain (UCL) for his support in the development of the tri-cultures. We are also grateful to Université Catholique de Louvain (FSR) and International Foundation for Research in Paraplegia (IRP) for the financial support. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Footnotes
Giulio G. Muccioli and Anne des Rieux equally contributed to the work.
Change history
3/22/2018
In the original publication, sixth author’s surname was incorrectly published as “Llyod” instead of “Lloyd”. The correct name should read as “Amy Lloyd”.
References
- 1.Bianco J, De Berdt P, Deumens R, des Rieux A. Taking a bite out of spinal cord injury: do dental stem cells have the teeth for it? Cell Mol Life Sci. 2016;73(7):1413–1437. doi: 10.1007/s00018-015-2126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1(1):80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209(2):294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyritsis N, Kizil C, Brand M. Neuroinflammation and central nervous system regeneration in vertebrates. Trends Cell Biol. 2014;24(2):128–135. doi: 10.1016/j.tcb.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Held KS, Lane TE. Spinal cord injury, immunodepression, and antigenic challenge. Semin Immunol. 2014;26(5):415–420. doi: 10.1016/j.smim.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue H, Zhang XY, Liu JM, Song Y, Liu TT, Chen D. NDGA reduces secondary damage after spinal cord injury in rats via anti-inflammatory effects. Brain Res. 2013;1516:83–92. doi: 10.1016/j.brainres.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29(8):1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004;10(12):580–583. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Myelin damage and repair in pathologic CNS: challenges and prospects. Front Mol Neurosci. 2015;8:35. doi: 10.3389/fnmol.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badner A, Siddiqui AM, Fehlings MG. Spinal cord injuries: how could cell therapy help? Expert Opin Biol Ther. 2017 doi: 10.1080/14712598.2017.1308481. [DOI] [PubMed] [Google Scholar]
- 12.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez AM, Goulart CO, Ramalho Bdos S, Oliveira JT, Almeida FM. Neurotrauma and mesenchymal stem cells treatment: from experimental studies to clinical trials. World J Stem Cells. 2014;6(2):179–194. doi: 10.4252/wjsc.v6.i2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsuan YC, Lin CH, Chang CP, Lin MT. Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke. Brain Behav. 2016;6(10):e00526. doi: 10.1002/brb3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chio CC, Lin MT, Chang CP. Microglial activation as a compelling target for treating acute traumatic brain injury. Curr Med Chem. 2015;22(6):759–770. doi: 10.2174/0929867321666141106124657. [DOI] [PubMed] [Google Scholar]
- 16.Giuliani A, Manescu A, Langer M, Rustichelli F, Desiderio V, Paino F, De Rosa A, Laino L, d’Aquino R, Tirino V, Papaccio G. Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: biological and clinical implications. Stem Cells Transl Med. 2013;2(4):316–324. doi: 10.5966/sctm.2012-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaoz E, Demircan PC, Saglam O, Aksoy A, Kaymaz F, Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol. 2011;136(4):455–473. doi: 10.1007/s00418-011-0858-3. [DOI] [PubMed] [Google Scholar]
- 19.Pomerat CM, Contino RM. The cultivation of dental tissues. Oral Surg Oral Med Oral Pathol. 1965;19:628–632. doi: 10.1016/0030-4220(65)90408-1. [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Li X, Sun L, Guo W, Tian W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J Neural Eng. 2017;14(2):026005. doi: 10.1088/1741-2552/aa596b. [DOI] [PubMed] [Google Scholar]
- 21.De Berdt P, Vanacker J, Ucakar B, Elens L, Diogenes A, Leprince JG, Deumens R, des Rieux A. Dental apical papilla as therapy for spinal cord injury. J Dent Res. 2015;94(11):1575–1581. doi: 10.1177/0022034515604612. [DOI] [PubMed] [Google Scholar]
- 22.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong J, Ahn M, Sim KB, Moon C, Shin T. Immunohistochemical analysis of activin A expression in spinal cords of rats with clip compression injuries. Acta Histochem. 2014;116(5):747–752. doi: 10.1016/j.acthis.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Ruparel NB, de Almeida JF, Henry MA, Diogenes A. Characterization of a stem cell of apical papilla cell line: effect of passage on cellular phenotype. J Endod. 2013;39(3):357–363. doi: 10.1016/j.joen.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Vanacker J, Viswanath A, De Berdt P, Everard A, Cani PD, Bouzin C, Feron O, Diogenes A, Leprince JG, des Rieux A. Hypoxia modulates the differentiation potential of stem cells of the apical papilla. J Endod. 2014;40(9):1410–1418. doi: 10.1016/j.joen.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Bianco J, Carradori D, Deumens R, des Rieux A. Rapid serum-free isolation of oligodendrocyte progenitor cells from adult rat spinal cord. Stem Cell Rev. 2017;13(4):499–512. doi: 10.1007/s12015-017-9742-4. [DOI] [PubMed] [Google Scholar]
- 27.Gerardo-Nava J, Hodde D, Katona I, Bozkurt A, Grehl T, Steinbusch HW, Weis J, Brook GA. Spinal cord organotypic slice cultures for the study of regenerating motor axon interactions with 3D scaffolds. Biomaterials. 2014;35(14):4288–4296. doi: 10.1016/j.biomaterials.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Refaat B. Role of activins in embryo implantation and diagnosis of ectopic pregnancy: a review. Reprod Biol Endocrinol. 2014;12:116. doi: 10.1186/1477-7827-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alhouayek M, Masquelier J, Cani PD, Lambert DM, Muccioli GG. Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6. Proc Natl Acad Sci USA. 2013;110(43):17558–17563. doi: 10.1073/pnas.1314017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouanet C, Reges D, Rocha E, Gagliardi V, Silva GS. Traumatic spinal cord injury: current concepts and treatment update. Arq Neuropsiquiatr. 2017;75(6):387–393. doi: 10.1590/0004-282x20170048. [DOI] [PubMed] [Google Scholar]
- 31.Gerardo-Nava J, Mayorenko II, Grehl T, Steinbusch HW, Weis J, Brook GA. Differential pattern of neuroprotection in lumbar, cervical and thoracic spinal cord segments in an organotypic rat model of glutamate-induced excitotoxicity. J Chem Neuroanat. 2013;53:11–17. doi: 10.1016/j.jchemneu.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Daviaud N, Garbayo E, Schiller PC, Perez-Pinzon M, Montero-Menei CN. Organotypic cultures as tools for optimizing central nervous system cell therapies. Exp Neurol. 2013;248:429–440. doi: 10.1016/j.expneurol.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25(30):6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8(3):346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 35.Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, Tetzlaff W. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol. 2014;117:54–72. doi: 10.1016/j.pneurobio.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Jeong YH, Park JS, Kim DH, Kang JL, Kim HS. Anti-inflammatory mechanism of lonchocarpine in LPS- or poly(I:C)-induced neuroinflammation. Pharmacol Res. 2017;119:431–442. doi: 10.1016/j.phrs.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 37.Jose S, Tan SW, Ooi YY, Ramasamy R, Vidyadaran S. Mesenchymal stem cells exert anti-proliferative effect on lipopolysaccharide-stimulated BV2 microglia by reducing tumour necrosis factor-alpha levels. J Neuroinflammation. 2014;11:149. doi: 10.1186/s12974-014-0149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demircan PC, Sariboyaci AE, Unal ZS, Gacar G, Subasi C, Karaoz E. Immunoregulatory effects of human dental pulp-derived stem cells on T cells: comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy. 2011;13(10):1205–1220. doi: 10.3109/14653249.2011.605351. [DOI] [PubMed] [Google Scholar]
- 39.Yamagata M, Yamamoto A, Kako E, Kaneko N, Matsubara K, Sakai K, Sawamoto K, Ueda M. Human dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal mice. Stroke. 2013;44(2):551–554. doi: 10.1161/STROKEAHA.112.676759. [DOI] [PubMed] [Google Scholar]
- 40.Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6(5):552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zachar L, Bacenkova D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res. 2016;9:231–240. doi: 10.2147/JIR.S121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dostert G, Mesure B, Menu P, Velot E. How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication? Front Cell Dev Biol. 2017;5:6. doi: 10.3389/fcell.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee YB, Yune TY, Baik SY, Shin YH, Du S, Rhim H, Lee EB, Kim YC, Shin ML, Markelonis GJ, Oh TH. Role of tumor necrosis factor-alpha in neuronal and glial apoptosis after spinal cord injury. Exp Neurol. 2000;166(1):190–195. doi: 10.1006/exnr.2000.7494. [DOI] [PubMed] [Google Scholar]
- 44.Amini Pishva A, Akbari M, Farahabadi A, Arabkheradmand A, Beyer C, Dashti N, Moradi F, Hassanzadeh G. Effect of estrogen therapy on TNF-alpha and iNOS gene expression in spinal cord injury model. Acta Med Iran. 2016;54(5):296–301. [PubMed] [Google Scholar]
- 45.Liu Y, Zhang R, Yan K, Chen F, Huang W, Lv B, Sun C, Xu L, Li F, Jiang X. Mesenchymal stem cells inhibit lipopolysaccharide-induced inflammatory responses of BV2 microglial cells through TSG-6. J Neuroinflammation. 2014;11:135. doi: 10.1186/1742-2094-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsubara K, Matsushita Y, Sakai K, Kano F, Kondo M, Noda M, Hashimoto N, Imagama S, Ishiguro N, Suzumura A, Ueda M, Furukawa K, Yamamoto A. Secreted ectodomain of sialic acid-binding Ig-like lectin-9 and monocyte chemoattractant protein-1 promote recovery after rat spinal cord injury by altering macrophage polarity. J Neurosci. 2015;35(6):2452–2464. doi: 10.1523/JNEUROSCI.4088-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang CM, Liu J, Zhao JY, Xiao L, An S, Gou YC, Quan HX, Cheng Q, Zhang YL, He W, Wang YT, Yu WJ, Huang YF, Yi YT, Chen Y, Wang J. Effects of hypoxia on the immunomodulatory properties of human gingiva-derived mesenchymal stem cells. J Dent Res. 2015;94(1):69–77. doi: 10.1177/0022034514557671. [DOI] [PubMed] [Google Scholar]
- 48.Page P, DeJong J, Bandstra A, Boomsma RA. Effect of serum and oxygen concentration on gene expression and secretion of paracrine factors by mesenchymal stem cells. Int J Cell Biol. 2014;2014:601063. doi: 10.1155/2014/601063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paquet J, Deschepper M, Moya A, Logeart-Avramoglou D, Boisson-Vidal C, Petite H. oxygen tension regulates human mesenchymal stem cell paracrine functions. Stem Cells Transl Med. 2015;4(7):809–821. doi: 10.5966/sctm.2014-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014;28(4):1634–1643. doi: 10.1096/fj.13-243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Investig. 2012;122(1):80–90. doi: 10.1172/JCI59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Akabawy G, Rashed LA. Beneficial effects of bone marrow-derived mesenchymal stem cell transplantation in a non-immune model of demyelination. Ann Anat. 2015;198:11–20. doi: 10.1016/j.aanat.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Djouad F, Jackson WM, Bobick BE, Janjanin S, Song Y, Huang GT, Tuan RS. Activin A expression regulates multipotency of mesenchymal progenitor cells. Stem Cell Res Ther. 2010;1(2):11. doi: 10.1186/scrt11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abe M, Shintani Y, Eto Y, Harada K, Kosaka M, Matsumoto T. Potent induction of activin A secretion from monocytes and bone marrow stromal fibroblasts by cognate interaction with activated T cells. J Leukoc Biol. 2002;72(2):347–352. [PubMed] [Google Scholar]
- 55.Chatterjee D, Marquardt N, Tufa DM, Hatlapatka T, Hass R, Kasper C, von Kaisenberg C, Schmidt RE, Jacobs R. Human umbilical cord-derived mesenchymal stem cells utilize activin-A to suppress interferon-gamma production by natural killer cells. Front Immunol. 2014;5:662. doi: 10.3389/fimmu.2014.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fodor J, Gomba-Toth A, Olah T, Almassy J, Zador E, Csernoch L. Follistatin treatment suppresses SERCA1b levels independently of other players of calcium homeostasis in C2C12 myotubes. J Muscle Res Cell Motil. 2017 doi: 10.1007/s10974-017-9474-8. [DOI] [PubMed] [Google Scholar]
- 57.Boulanger JJ, Messier C. From precursors to myelinating oligodendrocytes: contribution of intrinsic and extrinsic factors to white matter plasticity in the adult brain. Neuroscience. 2014;269:343–366. doi: 10.1016/j.neuroscience.2014.03.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.