Abstract
Polarity is a fundamental feature of cells. Protein complexes, including the PAR3–PAR6–aPKC complex, have conserved roles in establishing polarity across a number of eukaryotic cell types. In neurons, polarity is evident as distinct axonal versus dendritic domains. The PAR3, PAR6, and aPKC proteins also play important roles in neuronal polarization. During this process, either aPKC kinase activity, the assembly of the PAR3–PAR6–aPKC complex or the localization of these proteins is regulated downstream of a number of signaling pathways. In turn, the PAR3, PAR6, and aPKC proteins control various effector molecules to establish neuronal polarity. Herein, we discuss the many signaling mechanisms and effector functions that have been linked to PAR3, PAR6, and aPKC during the establishment of neuronal polarity.
Keywords: PKMζ, Axon specification, Dendritogenesis, Signaling, Wnt, Crumbs, CDC42/RAC
Introduction
In the late 1800s, the enigma regarding the makeup of the brain had divided scientists. Joseph von Gerlach, Camillo Golgi, and the ‘reticularists’ saw the organization of the brain as a continuous network of fibers that defined a law for transmission of signals [1–3]. However, Santiago Ramon y Cajal, Heinrich von Waldeyer-Hartz, Albert Kolliker, Wilhelm His, and the ‘neuronists’ posited that individual ‘neurons’ made up the central nervous system. They proposed that each neuron consisted of three parts—a cell body, protoplasmic projections (dendrites), and an axis cylinder (axon). In an important distinction from the ‘reticularist’ view, the ‘neuronists’ postulated that the axon, albeit in close contact with dendrites from neighboring neurons, never fused with dendrites. They also correlated the structural specialization of the neuron to its function: discrete neuronal processes were engaged in reception, transmission, and distribution. Structural asymmetry, i.e., polarity was revealed as a fundamental feature of a neuron and neuronal circuits. Nerve impulses were found to propagate directionally, moving from dendrites, through the cell body and into the axon. These observations were later codified as the law of dynamic polarization [4]. The ‘neuronists’ also noted that neurons of the brain and spinal cord arise during embryonic development. New-born neurons are initially symmetrical. Axons arise first, followed by dendrites. The mature neuron is thus actively polarized into axonal and dendritic compartments.
Polarity is a biological leitmotiv
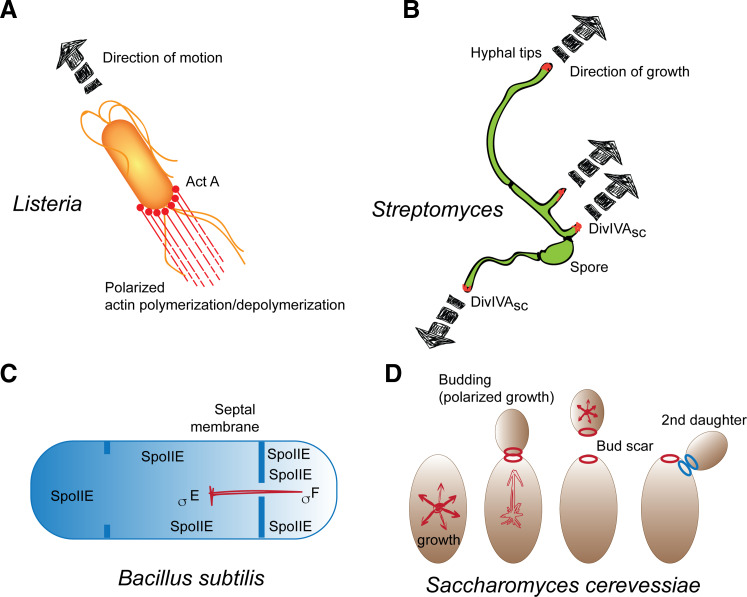
Polarity is a fundamental feature of most cells [5–8]. Morphologically, polarity is evident even in unicellular organisms such as bacteria or Saccharomyces cerevisiae [9–14]. The localization of flagella in bacteria is polar or subpolar. Listeria monocytogenes moves within the host cell by polymerizing actin at one end by the asymmetric localization of Act A protein (Fig. 1a). Shigella uses a similar strategy, albeit employing distinct proteins. Another bacterial structure, the pili, is similarly localized in a spatially asymmetric manner. Growth in the filamentous Streptomyces happens in a polarized manner (Fig. 1b). Sporulation in Bacillus subtilis also involves asymmetric division. During this process in B. subtilis, a septal membrane creates two unequally sized cytoplasmic compartments (Fig. 1c). Bud site selection in S. cerevisiae is a defined process where newly synthesized proteins and cell wall are directed to a ‘preselected’ membrane patch [15, 16]. During subsequent cell divisions, new bud sites are always excluded from the previous sites of budding, which are marked by bud scars in the mother yeast and birth scars in the daughter yeast. In haploids, new bud sites form adjacent to the scar. Mutants in which bud site selection is randomized or occurs at the previous bud site have reduced replicative lifespan and increased daughter cell death [17–19] (Fig. 1d). Eukaryal cells, including fertilized eggs, are also polarized, thus enabling division into asymmetric daughter cells, differentiation, and eventual organismal asymmetry. The orientation of the cytoskeleton including actin, microtubule (MT) polymers and the vectorial nature of vesicular trafficking underlie the spatial asymmetry within eukaryal cells.
Fig. 1.
Polarity is a fundamental feature of cells. Examples of directional movement in Listeria (a) and growth in Streptomyces (b). In Listeria, the Act A protein localizes at the base of the cell. In Streptomyces, the DivIVAsc protein localizes at hypal tips to direct regionalized growth. Similarly, cell division can be asymmetric. For example, in Bacillus subtilis during sporulation (c) or during S. cerevisiae budding (d). In B. subtilis, increased SpoIIE concentration within the smaller compartment signals through transcription factors to reinforce asymmetry. In S. cerevisiae, growth becomes asymmetric during budding. New buds form near the bud ‘scar’
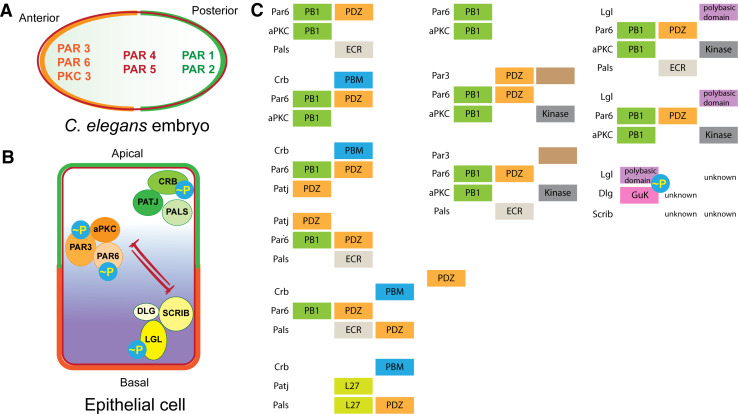
The mechanisms that drive polarity in bacteria or yeast differ widely [10–12], indicating, perhaps, convergent evolution to fulfill a fundamental need. Unsurprisingly, the mechanisms in eukaryal cells are also distinct from those seen in bacteria or in yeast. Nonetheless, within eukaryotes, certain cellular proteins play conserved roles across diverse cell types in polarizing the cell, i.e., specifying the axis along which cytoskeletal elements and cellular organelles are positioned. For example, the PAR3–PAR6–atypical protein kinase C (PAR3–PAR6–aPKC or simply PAR) complex has been ascribed an evolutionarily conserved function in the polarization of embryos [20, 21] (Fig. 2a). This system was first identified in a genetic screen for mutants that prevent embryonic development in Caenorhabditis elegans. Members of the Par genes Par3 and Par6 together with pkc-3 (C. elegans aPKC) play a fundamental role in the anterior–posterior polarization of the fertilized egg [21–23] (Fig. 2a). The loss of these proteins during embryonic polarization renders normally unequal and heterochronic cell divisions equal and/or synchronous [24]. As a consequence, the embryo fails to develop. In an ingenious screen, Kemphues et al. used egl-23 or lin-2 homozygous C. elegans that are defective in egg laying and, therefore, are consumed by their progenies. The authors screened for live animals and identified non-conditional maternal effect mutants that fail to develop as they cannot properly localize P granules and undergo abnormal early cleavages [24]. These genes were termed Partitioning defective.
Fig. 2.
Conserved proteins in eukaryotic cell polarity. The PAR proteins are conserved regulators of polarity across cell types in eukaryotic organisms. After fertilization, the C. elegans embryo becomes asymmetrical with different PAR proteins localizing to either the anterior or posterior end (a). Polarization along an apical–basal axis in epithelial cells is also regulated by the mutual exclusion of PAR3–PAR6–aPKC complex and DLG–LGL–SCRIB complex. While the PAR3–PAR6–aPKC and the CRB–PALS–PATJ complex localizes to the apical region, the DLG–LGL–SCRIB complex localizes to the basolateral region of the polarized cell (b). This is accomplished by interactions between members of the three complexes and phosphorylation. aPKC has been reported to phosphorylate Crb, Par6, Par3, and Lgl (b). Known protein–protein interactions including domains involved are listed in (c)
The PAR3–PAR6–aPKC complex segregates away from the sperm centrosome in the C. elegans embryo and defines the anterior end. This complex excludes LGL, PAR1, and PAR2 proteins that segregate to the posterior end of the embryo. PAR1, in turn, exclude MEX5/6. MEX5/6 excludes P granules and PIE-1 which, as a consequence, occupy the posterior region of the embryo. PAR2 can exclude PAR3–PAR6–aPKC. The function of lgl-1 in embryonic polarity is redundant with par2. Importantly, par2 lacks a mammalian homolog and is unique to C. elegans [25].
The kinase function intrinsic to the PAR complex is carried out by aPKC. This kinase phosphorylates components within the PAR3–PAR6–aPKC complex, such as PAR3 and PAR6, as well as components of other polarity complexes including LGL and CRB [21, 26–33]. PAR6 is an important scaffold that binds aPKC as well as PAR3, LGL, CRB, and PALS, thus recruiting aPKC substrates [21, 34]. aPKC can also phosphorylate substrates which, albeit not part of these three complexes, are involved in establishing polarity. For example, PAR1/MARK1-4 kinases are phosphorylated by aPKC [35–37].
In epithelial cells, the PAR complex, the SCRIB–LGL–DLG complex, and the CRB–PALS–PATJ complex segregate to distinct intracellular regions within epithelial cells to establish polarity [25] (Fig. 2b). This is accomplished, at least in part, by physical interaction between proteins of the three polarity complexes in a variety of combinations, as well as by phosphorylation/dephosphorylation (Fig. 2c). It is important to point out that polarizing events are often interdependent and connected by feedback and/or feed-forward loops. Thus, the initiating events, instructive versus permissive roles or the identity of the set of minimal components/interactions that are sufficient for the establishment of polarity are often not discernable. Notwithstanding, the PAR complex is central to the establishment of polarity in mammalian cells.
The PAR3–PAR6–aPKC module in axon specification
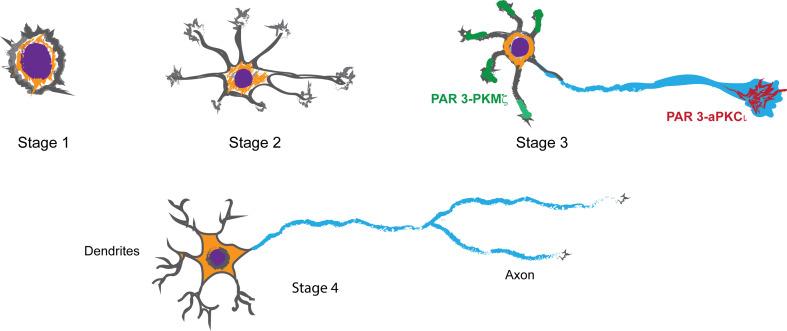
Unsurprisingly, proteins of the PAR3–PAR6–aPKC complex are important for the establishment of neuronal polarity. Pioneering work from Banker and colleagues in the 1970s demonstrated that mammalian embryonic hippocampal neurons can be dissociated and plated in culture where they first display a symmetric morphology exemplified by the extension of a number of MT-dependent cellular processes called neurites. Subsequently, and without any external directional cue, these neurons spontaneously and stochastically specify a single neurite for extended growth. This becomes the axon. The other neurites later form the dendrites [38–44] (Fig. 3a). Here, we review the function of the PAR3–PAR6–aPKC complex proteins and their interaction partners in the polarization of a new-born neuron into its axonal and dendritic domains.
Fig. 3.
Schematic representation of stages of neuronal polarization. At stage 1, neurites are not discernable and the neuron primarily has an actin-based cytoskeleton. At stage 2, microtubule-based neurites extend from the cell body of the neuron. The neurites are equipotent in terms of their capacity for axon specification. At stage 3, a single process has been specified as an axon. The axon continues to grow, while growth stalls at the other neurites (minor processes). At this stage, PAR3–PKMζ appear to segregate from PAR3–aPKCι. While PAR3–PKMζ is present in minor processes, PAR3–aPKCι localizes to the axon. At stage 4, minor processes start to grow and arborize into dendrites. Eventually, the neuron acquires its complex, fully mature phenotype (not shown)
Two mammalian genes, viz Pard3 and Pard3b, encode PAR3 (also known as PARD3) proteins. In neurons, the Pard3 transcript and the PARD3 protein are primarily derived from Pard3. At least 14 variant Pard3 transcripts are expressed in human cortex and hippocampus (the data used for analyses were obtained from the GTEx portal on 12/06/2017). In this review, we have used PAR3 and PARD3 interchangeably, with the mammalian protein referred to as PARD3 and protein from other species as PAR3. PARD3 protein domain organization consists of a conserved N-terminal region, three PDZ (PSD95-DLG1-ZO1) domains, and a C-terminal aPKC-binding region. The N-terminus of PARD3 mediates PARD3 oligomer formation [45–47]. It is also the region that binds MT. However, the C-terminus of PARD3 can inhibit this PARD3 function [48]. shRNA-mediated PARD3 knockout in in vitro cultured neurons (Banker culture) can disrupt hippocampal neuronal polarity. Furthermore, this defect can be rescued by expressing wild-type (WT) PARD3 but not PARD3 which is unable to bind MT [48]. The C-terminal of PARD3 is also where aPKC interacts with this scaffold. Conversely, the ectopically expressed PARD3, the N-terminal domain of PARD3 alone, or a C-terminal domain deletion mutant of PARD3 inhibits neuronal polarity [49]. These results suggest that allosteric changes in PARD3 regulate its microtubule interactions [48]. This C-terminal aPKC-binding region is, however, absent in some Pard3 transcripts and all Pard3b transcripts [50]. Therefore, these transcripts are not capable of aPKC binding. Shi et al. also reported that, in embryonic hippocampal neurons in culture, PARD3 localizes to the cell body and the axon, especially at the growth cone, and is excluded from the neurites destined to form dendrites [49, 51]. How does PARD3 localize to the growing axon? A kinesin called KIF-3A binds PARD3 and is required for the ‘plus-end’-directed movement of PARD3 and aPKC along the MTs within the axon [51, 52]. Interestingly, in embryonic dorsal root ganglia (DRG) neurons grown in a microfluidic chamber, Pard3 but not Pard6 or aPKC mRNA localized to growing axons when the axon was stimulated with Nerve Growth Factor (NGF) [53]. NGF and netrin-1 also induce local translation of PARD3 in the axon [53]. The PARD3–PARD6–aPKC complex and aPKC enzyme activity are required for NGF- and netrin1-stimulated growth of DRG and dorsal spinal commissure (DSC) neurons, respectively, but not for basal growth [53].
Mammalian homologs of Par6 are named Pard6a, Pard6b, and Pard6g. The most prevalent Pard6 gene product in mammalian neurons is Pard6b. Although structurally similar, PARD6 proteins differ in their spatiotemporal distribution upon expression in mammalian epithelial cells and show functional specialization [54]. In this review, we use PARD6 in reference to the mammalian protein while using PAR6 for other species. PARD6 contains a Phox/Bem1 (PB1) domain, a semi-CDC42/RAC interactive-binding (CRIB) domain and a PDZ domain. The PB1 domain of PARD6 binds other PB1 domain-containing proteins (including aPKC) [55]. The CRIB domain binds RAC-family small GTPases such as CDC42. The PDZ domain mediates an unusual PDZ–PDZ interaction with PARD3. In addition, PARD6 can interact with and forms complexes with CRUMBS, PALS, and PATJ [56]. In embryonic hippocampal neurons, the ectopic expression of PARD6 prevented neuronal polarization and axon specification [57].
There are two aPKC genes in mammals: Prkci and Prkcz. Prkci codes for PRKCI or aPKCι (formerly, the human protein was called aPKCι, while the mouse paralog was termed aPKCλ). Prkcz encodes two independent transcripts: transcript 1 (PRKCZ or aPKCζ) and transcript 2 (PKMζ). PRKCI and PRKCZ proteins have ~ 72% amino acid sequence identity overall. If just their kinase domains are compared, they are ~ 86% identical. Transcript 2 of Prkcz or PKMζ codes for a protein that is a truncated form of PRKCZ missing its regulatory domain but identical to the catalytic domain of aPKCζ. Free catalytic or kinase domains of PKCs are known as PKM. PKMζ can be generated by proteolytic cleavage, especially in invertebrates [58, 59]. However, in mammals, this form is also made as a specific transcript [60].
aPKCζ was first identified by Shi et al. as a critical regulator of hippocampal neuronal polarity [49]. This group used a membrane permeable peptide based on the pseudosubstrate sequence of aPKCζ (myr-ZIP) to conclude that the inhibition of the kinase activity of aPKCζ inhibited axon specification [49]. However, it was later discovered that only aPKCι and PKMζ proteins, but not aPKCζ, are expressed in rat hippocampal neurons [61]. myr-ZIP is not exclusively selective for aPKCζ and its actions on cultured cells or tissues have been recently questioned [62]. In fact, myr-ZIP, as well as a scrambled control peptide, can be expected to inhibit all aPKC proteins in vitro as the aPKC phosphorylation consensus is simply a sequence rich in basic amino acids [63]. The efforts to identify the correct aPKC involved in neuronal polarity typically suffer from ambiguous antibody and reagent specificity. Albeit that most studies implicate aPKCζ as the regulator of neuronal polarity, the aPKC in question that drives axon specification is likely to be aPKCι [61]. In light of the difficulties of correctly identifying the aPKCs, in this review, we will mostly ascribe reported functions of aPKCζ and aPKCι to aPKC generically. The high degree of amino acid identity is likely to lead to functional similarities between these two proteins. However, it should be noted that Prkcz knockout mice are grossly normal and viable, but Prkci knockout leads to the early embryonic lethality in mice. Whether this difference is due to expression (Prkci is expressed earlier or more broadly in the embryo than Prkcz) or due to non-overlapping functions remains to be determined. In case there are functional differences between aPKCζ and aPKCι, it is imperative that future studies dedicate more emphasis in correctly identifying the kinase involved.
The aPKC variant PKMζ is also highly expressed in neurons and functions in a manner contrasting to that of aPKCι [61]. PKMζ competes with aPKCι for PARD3 binding, and overexpression of PKMζ inhibits axon formation. Conversely, depletion of PKMζ results in supernumerary axons in cultured mammalian neurons [61]. These results indicate that PKMζ inhibits aPKCι function by sequestering PARD3 and preventing the formation of the aPKCι–PARD3 complex; while the aPKCι–PARD3 complex favors axon specification, PKMζ-PAR3 complex has the opposite effect and inhibits axon formation (Fig. 3b). Despite this report, the role of PKMζ in neuronal polarity remains poorly studied to date.
One notable exception to the axon-promoting effect of aPKCι generally observed during neuronal polarization was described in a study using an unbiased screen of kinases and phosphatases in the regulation of neuronal polarity [64]. In this study, the overexpression of aPKCι inhibited rather than promoted neurite growth and axon formation [64]. One potential explanation to reconcile this contrasting finding may be that overexpressed aPKCι, in this case, adopted a dominant-negative function as the stoichiometry of PARD3–aPKC and/or PARD6–aPKC complexes were disrupted. If overexpressed aPKCι can sequester PARD3 while failing to bind PARD6, it may function more like PKMζ. However, in the report from Parker et al., this appears not to be the case as in the experiments described therein, overexpressed aPKCι caused supernumerary axon formation [61]. The localization of overexpressed aPKCι in these experiments, or the effect of coexpressing PARD6 or concurrently silencing PARD6, remains to be carefully investigated.
Following its specification, the axon is ‘guided’ to its target through a series of interactions mediated by ‘guidance molecules’ [65–67]. aPKCs are also important in this process. Spinal cord commissural axons turn anteriorly after crossing the midline, and aPKC is required for this Wnt4-mediated anterior–posterior pathfinding event [68]. PI3-K, small G-proteins, and aPKC kinase activity are all required for this process [68].
Prkci global knockout is lethal [69]. Therefore, Imai et al. used a conditional deletion strategy using Nestin-driven Cre expression for neural-specific ablation of Prkci in mice [70]. This neural progenitor-specific ablation of Prkci led to the death of new-born mice within 1 month after birth. Closer examination identified the cause of death was due to hydrocephalous. The BrdU/phosphohistone H3+ and Ki67+ ventricular and subventricular zones in the developing brain were expanded and markedly disorganized in this cell-type specific Prkci knockout. Apical adherens junctions in radial glial cells were lost, resulting in the displacement of these cells beyond the progenitor zone cells and their intermingling with Tuj1+-differentiated neuronal zones. Regardless of this effect on neural stem cells, neurogenesis was not affected [70]. An almost identical conclusion was reached by overexpression of a membrane-targeted, active aPKCζ construct in avian neural progenitors [71]. These independent experiments again indicated that aPKCs are instructive for the polarized localization of N-cadherin, β-catenin, and ZO-1-at the end-feet or neuroepithelia–adherens’ junctions of the radial progenitors [71]. However, neuronal differentiation was again normal.
In contrast to the studies performed with Banker cultures, conditional genetic deletion of Prkci in neurons in vivo using Syn1 (synapsin) or Camk2a-driven Cre failed to reveal any overt phenotype [72]. While PRKCI, PARD6b, and LLGL1 cofractionated during gel filtration from mouse cortical lysates, the conditional ablation of Prkci led to a reduction of PARD6b in this complex and a change in its fractionation properties, but no change in the total amount of LLGL1. Dendrites, axons, Golgi localization, and axon-initial segments (AISs) were unaffected. However, these conditional knockout experiments were performed with Syn1 and Camk2a-driven Cres which are expressed in mature neurons. Therefore, this system is not identical to the Banker culture, wherein new-born, immature neurons polarize in culture before maturing. Overall, it can be concluded that PRKCI function is critical for mammalian neurodevelopment, but dispensable after neuronal maturation. Interestingly, aPKC as well as the Par3/Baz and Par6 genes appear dispensable for Drosophila neuron development and neuronal polarization [73]. Whether aPKC function is a requirement for mammalian neuronal polarity in vivo remains to be fully investigated.
Molecular interactions of aPKC–PAR proteins in neuronal polarization
Wnt signaling and aPKC
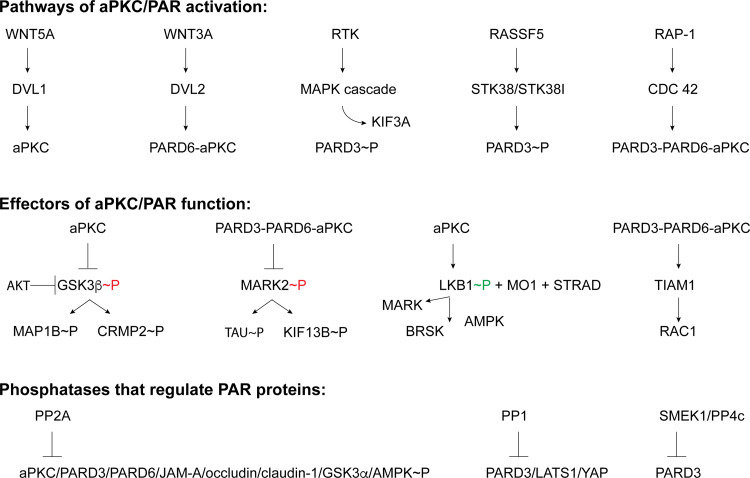
How is aPKC activated during the regulation of neuronal polarity? A number of potential upstream activators and downstream effectors of aPKC and PAR proteins have been identified to date. For example, the major developmental signaling pathway, WNT, targets aPKC for activation. WNT signals by binding LRP5/6 and the 7-pass transmembrane receptors of the Frizzled family. This leads to the recruitment of the cytoplasmic effector Dishevelled (DVL) and the associated complex to the WNT receptor. DVL preferentially accumulates at the tip of the growing axon [74]. Silencing DVL prevented axon outgrowth in cultured embryonic hippocampal neurons. Conversely, overexpression of DVL led to neurons with supernumerary neurons. Importantly, the Dvl siRNA-induced phenotype can be rescued by overexpressing aPKCζ. Although this is not the endogenous neuronal aPKC, presumably aPKCι has an identical function. As stated before, aPKCζ and aPKCι have 86% amino acid identity within the kinase domain and 72% amino acid identity overall. DVL binds the C-terminus of aPKCζ through a DEP-domain [75]. In the presence of aPKCζ, DVL associats with PARD3 and PARD6, implying that DVL associates with the PAR3–PAR6–aPKC complex. DVL enhances the catalytic activity of aPKC, including in hippocampal neurons wherein DVL expression increases the amount of aPKC phosphorylated on its autophosphorylation/mTORC2 site (Thr 410/403) which is regarded as a marker of aPKC activation (Fig. 4).
Fig. 4.
Representative examples of signaling upstream and downstream of the PAR complex. A few selected signaling pathways that lead to aPKC activation during neuronal polarization and examples of cascades downstream of aPKC/PAR complex that may establish axonal versus dendritic identity. For kinases, ~P in green indicates activating phosphorylation, whereas ~P in red indicates inactivating phosphorylation. Phosphatases can function to inhibit PAR complex proteins and can function as effectors of aPKC–PAR function
Importantly, this DVL-dependent aPKC activation was observed downstream of the non-canonical WNT, WNT-5A, but not the canonical WNT, WNT-3A, and did not affect GSK3β Ser 9 phosphorylation [74]. However, WNT-3A was reported to stimulate activating phosphorylations on aPKCι and lead to aPKCι binding to phosphorylated DVL2 (another member of the Dishevelled family) in another publication [76]. In this case, silencing aPKCι abolished WNT-3A-stimulated neurite outgrowth. The disruption of the aPKCι–PARD6 interaction with aurothiomalate similarly prevented neurite outgrowth in this model [76].
Given that GSK3β is a bona-fide target for aPKC-mediated inhibition [77], it is not clear why aPKC-dependent GSK3β phosphorylation was not observed in the Zhang et al. study. GSK3β Ser 9 phosphorylation has been linked to axon specification in many other studies. In canonical Wnt signaling, GSK3β is inhibited downstream of DVL, the human ortholog of Drosophila dishevelled (dsh) gene, allowing β-catenin stabilization, nuclear transport, and β-catenin-driven transcription. In NB2a neuroblastoma cells, the stability of MT was a function of Dvl-dependent inhibition of GSK3β, which in turn inhibited MAP1B phosphorylation and dissociation from MT, as well as increased JNK activity [78] (Fig. 4). In rat enteric neurons, the inhibition of aPKCζ with myr-ZIP or GSK3β inhibition with SB216763 or GSK3β-specific siRNA resulted in neurons with supernumerary axons [79].
Another kinase that phosphorylates GSK3β at Ser 9 is AKT (Fig. 4). AKT phosphorylation also inhibits GSK3 β activity. There are three AKT genes in mammalian cells—AKT1, AKT2, and AKT3. All three AKT paralogs were expressed in mouse hippocampal and cortical neurons [80]. In cortical neurons, AKT1 and 3 were almost uniformly expressed during neuronal development with a slight decrease in expression after axon specification followed by an increase to levels identical at initiation of culture. In contrast, AKT2 amounts rapidly decreased before axon specification. In hippocampal neurons, all three AKT amounts progressively, but minimally, decreased with development. Subcellular fractionation experiments indicated that AKT1 and 2 were primarily cytosolic. AKT3 was distributed equally between the cytosol and nucleus. Interestingly, there was very little AKT detected in the membrane fraction in this study. Thr 308 phosphorylation of AKT increased in both cortical and hippocampal neurons during axon growth in culture (post 4 days in vitro), while a concomitant increase in Ser 473 phosphorylation was seen only in hippocampal neurons [80]. Ser 473-phosphorylated AKT was preferentially detected at the growth tip of the axon in cultured embryonic hippocampal neurons [49]. How does AKT localize preferentially at the growth cone? A scaffold protein c-Jun N-terminal kinase interacting protein-1 (JIP1) was reported to be polarized to a single neurite in stage 2 cortical neurons in culture [81]. JIP1 knockdown resulted in the loss of AKT from the growth cone but did not affect the overall level of AKT in the cell body or in the axon shaft [82]. JIP1 silencing resulted in reduced axon length and branching [81]. JIP1 function required phosphorylation by c-Abl and interaction with kinesin-1 [81].
Hippocampal neurons transfected with a constitutively active, myristoylated AKT (AKT1myrΔPH [83] or myr-AKT [84]) or WT AKT exhibited the supernumerary axon phenotype in independent studies [83, 84]. The constitutively activated form of AKT (Myr-AKT) was reported to increase the length of the axon (as well as the total number of neurites), in cultured embryonic hippocampal neurons [84]. Minor differences between the studies can be noted, such as some neurons with AKT1myrΔPH were reported to fail to make tau1-positive axons [83] and WT AKT was sufficient to induce supernumerary axon formation in the report by Yoshimura et al. [83], but not in the Jiang study [84]. Regardless, AKT activity appears to be important for neuronal polarization. However, dominant-negative AKT did not disrupt normal neuronal polarization in these cells [83]. Similarly, the silencing of any one AKT (AKT1, AKT2, or AKT3) alone did not affect axon specification [80]. Coexpression of GSK3β S9A could partially reverse the effect of Myr-AKT [84]. Jiang et al. concluded that GSK3β was one of the many effectors of AKT downstream of PI3-K. However, experiments on mouse hippocampal neurons derived from the GSK3αβ S9A S21A double knock-in mouse (mutations that prevent GSK3β phosphorylation and consequent inactivation) failed to reproduce these results as these neurons polarized identically to those derived from the WT littermates [85]. Notwithstanding, the pharmacological inhibition of GSK3 still led to supernumerary axon formation in hippocampal neurons derived from GSK3αβ S9A S21A double knock-in mice [85].
The canonical Wnt, Wnt-7a, was reported to enhance axonal spreading and branching, but not extension, in cerebellar granular cells. Furthermore, the inhibition of GSK3β with lithium mimicked the effects of Wnt-7a [86]. The effect of Wnt is mediated through MT stabilization downstream of GSK3β inhibition. Wnt-7a-dependent inhibition of GSK3β leads to the dephosphorylation of the MT-binding protein MAP-1B. MAP-1B, when phosphorylated by GSK3β, binds tyrosinated, unstable MT [87, 88]. Phosphorylated MAP-1B prevents the detyrosination of MT and their conversion to stable MT [87]. Dephosphorylated MAP-1B dissociates from MT and allows axon specification [89]. The role of GSK3β in neuronal polarity is well established. In 1999, Leroy and Brion observed that GSK3β expression in the CNS peaks between E18 to P10 [90]. GSK3β is enriched in neurons, but not in astroglial cells [90]. Neuronal expression of GSK3β has been validated by recent RNAseq-based expression analyses in human and mouse cortical cell types [91, 92]. GSK3β2 is a neuron-specific splice variant of GSK3β [93]. The expression of this isoform correlates with neuronal differentiation, for example in SH-SY5Y neuroblastoma cells [94]. In cultured cortical neurons, GSK3β2 localizes to the cell body and neurites [93]. Inhibition of GSK3β leads to the development of supernumerary axons, while the ectopic expression of a CA-GSK3β mutant, GSK3β S9A, inhibits axon formation in cultured embryonic hippocampal neurons [84]. A series of reports from the Gordon-Weeks group had defined that GSK3β is activated downstream of NGF in PC12 cells, as well as in neurons from cultured superior cervical ganglion (SCG) and the dorsal root. NGF, acting through tropomyosin-related kinase A (TrkA) but not p75NTR in PC12 cells, recruits two distinct pathways: the mitogen-activated protein kinase (MAPK) and PI3-K pathways [95, 96]. It is important to indicate that these pathways intersect at GSK3β, with PI3-K inhibiting GSK3β, while the MAPK cascade activating GSK3β [88, 95]. NGF also stimulates growth of E13 DRG sensory neurons. In these neurons, NGF leads to the localized activation of PI3-K at the growth cone of the axon [97]. This corresponds to the spatial downregulation of GSK3β through Ser 9/Ser 21 phosphorylation [97].
A validated GSK3β target during neuronal polarization is the collapsin response mediator protein-2 (CRMP-2) (Fig. 4). CRMP-2 amounts are higher in growing axons and overexpression of CRMP-2 leads to the formation of supernumerary axons. Its C-terminal 24 amino acids appear crucial for this function [98]. GSK3β phosphorylates CRMP-2 [99]. Ectopic expression of CRMP-2 or non-phosphorylatable CRMP-2 leads to the formation of multiple axons [99]. Phosphorylated CRMP-2 shows reduced tubulin binding. NT-3 and BDNF-dependent AKT activation and GSK3β Ser 9 phosphorylation was determined to be the driver of CRMP-2 phosphorylation. A CRMP-2 which is unable to be phosphorylated counteracted the effects of CA-GSK3β in axon specification [99]. CRMP-2 function in axons also includes the regulation of Sra-1/WAVE1 complex and the actin cytoskeleton [100].
How is AKT activated during neuronal polarization? It is generally agreed that phosphoinositide 3-kinase (PI3-K) signaling is central to axon specification. PI3-K activation triggers the phosphorylation of the 3-OH group of inositol membrane lipids [101]. Class I PI3-Ks directly generate phosphatidylinositol(PtdIns)-3,4,5-triphosphate or PtdIns(3,4,5)P3 and indirectly generate PtdIns(3,4)P2. Class III and Class II PI3-Ks make PtdIns(3)P [101]. PI3-K signaling and the activation of PDK1 are important in neuronal development. The pleckstrin homology (PH) domain of proteins bind PtdIns(3,4,5)P3 and PtdIns(4,5)P2. PH domain-containing proteins, such as PDK1 and AKT, are recruited to PtdIns-enriched membranes after PI3-K activation. Many studies have independently demonstrated the activation of endogenous PI3-K in neurons during polarization using a reporter consisting of the PH domain of AKT fused in frame to a fluorescent protein such as GFP. In mice where a mutation disrupting the interaction of PDK1 PH domain with PtdIns (K465E) was introduced into the endogenous PDK1 locus resulted in reduced brain size and reduced brain-derived neurotrophic factor (BDNF)-dependent AKT activation [102]. More neuronal precursors failed to differentiate relative to PDK1+/+-derived precursors [102]. Cortical neurons isolated from PDK1K465E/K465E mice were shorter in length, but the number and branching of neurites were unaffected [102]. In hippocampal neurons, PDK1K465E/K465E alleles inhibited axon specification and reduced axon length [102]. AKT, once recruited to PtdIns(3,4,5)P3 and PtdIns(4,5)P2-rich membranes, is phosphorylated at Thr 308 by PDK1. A second phosphorylation at Ser 473 also occurs on AKT, as a result of mTORC2 activity. Consistent with these results, AKT Ser 473 phosphorylation has been described during neuronal polarization and the use of pharmacological inhibitors of PI3-K such as LY 294002 or wortmannin prevent neuronal polarization [49, 97, 99, 103, 104]. Furthermore, in rat hippocampal neurons, overexpression of PTEN prevented axon specification [84]. Conversely, silencing PTEN increased the number of axons [84].
In the peripheral nervous system (PNS), AKT functions downstream of NGF and Ras and promotes axon elongation in cooperation with the Raf–MEK axis [105]. DN-AKT or p85 or PI3-K (Δ p85), as well as DN-Ras (S17N Ha-Ras) or Raf inhibited NGF-mediated axon growth. However, CA-PI3-K (PI3-KCAAX) and AKT (myr-AKT) enhanced axon width and caliber in E13 DRG neurons. AKT activation also increased axon branching, whereas CA-Raf 1 (RafCAAX) caused an increase in axon length [105]. Another AKT target identified in PC12 cells is Nrf2/ARE signaling [106].
An alternative target of AKT is the TSC1–TSC2 complex. AKT-dependent phosphorylation dissociates this complex and relieves the negative regulation of Rheb and mTORC1 activity, promoting cap-dependent translation. Inhibition of AKT or mTORC1 reduced axon specification in hippocampal neurons, with TORC1 inhibition having the most dramatical reduction [102]. Independent of mTORC1, GSK3β-dependent phosphorylation of the translation initiation factor eIF2Be inhibits axon growth [107].
Kinase signaling and PAR proteins
Increased PARD6–aPKC complex formation, phosphorylation of PARD3/PARD6, and/or aPKC activation were also demonstrated to be downstream of several receptor tyrosine kinase-driven mechanisms that function in axon specification [108–110]. IGF1R silencing or IGF1R-blocking antibodies prevented axon specification [111]. Similarly, the addition of the pharmacological EGFR inhibitor AG1478 also inhibited axon specification [49]. ERK2 has been reported to directly bind and phosphorylate PARD3 [112]. In cultured embryonic sympathetic neurons, CaSR (extracellular calcium-sensing receptor) activation in the presence of NGF promoted neurite formation [113]. This effect was mediated by MAPK activation downstream from CaSR [113]. Grb2 adapter-dependent MAPK activation was also reported during NT-3-induced axon elongation in cultured hippocampal neurons [114]. Phosphorylated PARD3 localized to the growing tip of the axon [112]. Funahashi et al. demonstrated that ERK2 phosphorylation of PARD3 results in PARD3 dissociation from KIF3A, but not from the PARD6–aPKC complex [112] (Fig. 4). They proposed that this phosphorylation may be a mechanism of PARD3 unloading at the distal end of the axon, which results in PARD3 localization at the growing tip. Loss of MEK1/2 function resulted in defective corticospinal tract formation, and MAPK hyperactivation inhibited corticospinal axon elongation in CTIP2-positive layer 5 neurons in the cortex [115]. However, MAPK function was not required for axon formation in layer 2/3 callosal neurons, suggesting that there might be specificities intrinsic to neuronal types [115]. Activation of other members of MAPK signaling has also been linked to axon specification. JNK activity increased during cerebellar granule neuron maturation [116]. Active JNK is localized to axons, including at the tip of the major neurite during axon specification [117]. JNK inhibitor SP600125 prevented axon formation in cultured embryonic hippocampal neurons [117].
Receptor serine-threonine kinase TGFβ is an important modulator of axon identity. TβR1 and TβR2 receptors are highly expressed in the mouse neocortex at E14–E15 [118]. TβR2 receptors also colocalize with TAG1-positive axons. The immunoglobulin (Ig)-superfamily protein TAG-1 containing 6 Ig-domains and 4 fibronectin-type III-like domains is a GPI-anchored membrane protein identified from an E13 rat spinal cord cDNA library [119] involved in homophilic cell–cell adhesion. TAG-1-coated substrate promoted neurite outgrowth of embryonic DRG neurons which expressed TAG-1, but not SCG sympathetic neurons that lacked TAG-1 expression, indicating a homophilic, extracellular cell adhesion function [119]. TAG-1 can also engage in heterophilic interactions with L1-like molecules and β1 integrins [120]. TAG-1 was identified to mediate the migration of GABAergic cortical interneurons along TAG-1 expressing corticofugal axon fibers [121]. Using sequential electroporation of different fluorescent proteins in vivo, Namba et al. observed that late-born neurons form axons that contact the early born axons. Identical results were observed in in vitro cocultures where neurons were allowed to form axons before a second set of neurons were plated on the same dish. The neurons plated later formed axons nearly parallel to preexisting axons, suggesting that axon specification was guided by contacting preexisting axons. Importantly, this contact of a neurite from the multipolar, late-born neuron with the pioneering axon of the early born neuron was mediated by TAG-1. Silencing TAG-1 locked more neurons into a multipolar phenotype, indicating that this molecule was crucial for the transition into a bipolar phenotype. However, it is important to point out that the TAG-1 knockout mouse does not show axon specification defects [122].
In immature E14.5 neurons of the SVZ (subventricular zone), TβR2 immunostaining was observed at the trailing process that specifies into the axon, but not in the leading process. TβR2 immunostaining was also concentrated at the proximal region within the axon. Similar results were observed in cultured embryonic hippocampal neurons where TβR2 was diffusely distributed over the neuronal surface at stage 2 neurons, but localized to the axon in stage 3 neurons. Cre-mediated excision of Tgfbr2 resulted in failure of axon specification. Cultured neurons also failed to specify the tau-positive axon, while MAP2-positive neurites were unaffected. Identical results were obtained with the pharmacological inhibitor of TβR1, SB-431542, or by transfection of kinase-inactive TβR2. Conversely, overexpression of TβR2 led to neurons with multiple axons, even when TβR2 was transfected after neuronal polarization and axon specification. Exposure of hippocampal neurons in culture to TGF-β-conjugated beads led to growth of the neurite in close proximity to the bead, indicating that local TGF-β signal through most likely the TβR2 receptor leads to axon specification and growth. Importantly, TβR1 coimmunoprecipitated with PARD6 and PARD3 from E18 rat cortex. PARD6 is a direct downstream phosphorylation target of TGF-β signaling. Coexpression of phosphorylation-defective Par6 (Par6-S345A) prevented TβR2-driven supernumerary axon formation, while a phosphomimic Par6 (Par6-S345E) rescued axon specification in TβR2-KO neurons [118]. PARD6 S345 phosphorylation has been demonstrated to lead to the recruitment of the ubiquitin ligase Smurf-1 and the consequent degradation of RhoA in epithelial cells [123]. TGF-β signaling in Oryzias latipes (medaka fish) during retinal axon specification activates miR-181a/b, which in turn inhibits MAPK signaling [124, 125]. This signaling also leads to the degradation of RhoA [124].
A number of substrates for aPKC phosphorylation have been identified. The Luo group demonstrated that aPKC, in complex with PARD3 and PARD6, phosphorylates and negatively regulates the activity of MARK kinase, MARK2 (Fig. 4). Active MARK2 phosphorylates the KXGS motifs of the MT-binding proteins, including tau. Phosphorylation of these motifs decreases tau binding to MT, or the dissociation of hyper-phosphorylated tau from MT, and results in MT destabilization [126, 127]. aPKC phosphorylation of MARK2 inhibits its activity, preventing MARK2-dependent phosphorylation of the KXGS motifs of tau [35, 126]. Thus, aPKC-dependent inhibitory phosphorylations on MARK2 promote MT stability and axon growth. This inhibition can result in neurons with supernumerary axons, while ectopic expression of MARK2 leads to unpolarized neurons that fail to specify the axon.
Another evolutionarily conserved substrate of MARK2 is KIF13B. KIF13B is enriched in axons and overexpression of this kinesin motor protein induces supernumerary axons. Silencing KIF13B expression prevented axon specification [128]. MARK2 phosphorylation of KIF13B is required for the negative regulation of this kinesin and the prevention of the multi-axon phenotype, suggesting that MARK2 inactivation downstream of PI3-K and aPKC in the major process allows for accumulation of KIF13B and axon specification [128]. It was also demonstrated that MARK inhibitors that suppressed the activity of these kinases in cultured rat hippocampal neurons, as detected by activity biosensors, terminated axon elongation [129]. Phosphorylation of MARK by aPKC leads to recruitment of 14-3-3 proteins and sequestering of MARK to the cytosol [37, 130]. The mammalian YWHA family encodes 14-3-3 proteins including the Par5 ortholog YWHAS.
MARK kinases are also a substrate of LKB1. Except for MELK, all members of the AMPK (AMP-activated protein kinase) family are phosphorylated in their T-loop by LKB1 in a complex with the pseudokinase STRAD and the scaffold protein MO25 [131] (Fig. 4). Cell-type-specific genetic ablation of LKB1 by the use of Emx1cre+ Lkb1 fl/fl mice by Barnes et al. revealed that cortical axons in general were significantly reduced in the absence of this kinase [132]. Axon growth ex vivo in organotypic slice cultures from Emx1cre+/− Lkb1 fl/fl mouse was also reduced, as well as in cultured cortical neurons. Coexpression of LKB1 and Stradα induced the supernumerary axon phenotype. Consistent with a role in enabling LKB1 function, the overexpression of multiple splice variants of STRADα and the full-length variant of STRADβ together with LKB1 induced supernumerary axons [133]. These genes function redundantly as either STRADα or STRADβ loss-of-function alone do not affect axon specification. However, deletion of both together resulted in loss of TAG1-positive corticofugal projection axons in vivo and axon specification in cultured cortical neurons in vitro [133]. Interestingly, LKB1 itself is phosphorylated by aPKC [134, 135] (Fig. 4).
Other substrates of LKB1 are the SAD/BRSK kinases. SAD1 kinase regulates neuronal polarity in C. elegans by restricting synaptic vesicle proteins to the axon [136]. In sad-1 mutants, sensory axons fail to terminate, while overexpression of this kinase results in the premature termination of axons [137]. Genetic ablation of both the mammalian orthologs BRSK1(SAD-A) and BRSK2(SAD-B), but not each one individually, resulted in live birth. However, the pups showed a little spontaneous movement and were not responsive to tactile stimulation [138]. These animals die within 2 h of birth [138]. While all major neural subtypes were present in mutant mice, cortical neurons had processes that extended diagonally or tangentially rather than radially, unlike the WT neurons. WT neurons had a single basal axon, but axons were not distinguishable in BRSK1−/−BRSK2−/− neurons [138]. Cultured embryonic hippocampal and cortical neurons also failed to specify an axon [138]. Loss of BRSK1 and BRSK2 correlated with reduced tau phosphorylation [138]. Surprisingly, these kinases are dispensable for axon specification in neurons outside the hippocampus and cortex [139].
LKB1 is also the key upstream activator of AMPK. AMPK, a major metabolic sensor, can affect neuronal polarization. Pharmacological activation of AMPK or ectopic expression of CA-AMPK inhibits the polarization of cultured embryonic hippocampal neurons [103, 140]. Kinase-inactive AMPK (AMPKD157A) allows the establishment of neuronal polarity even in the presence of AICAR [103]. Surprisingly, PI3-K acted downstream of AMPK-increased pharmacological activation of AMPK in these neurons resulted in loss of axon-specific localization of PI3-K, phosphorylated AKT, and PARD3. The kinesin KIF5 is involved in the transport of PI3-K to the tip of the growing axon, and the kinesin light chain (KLC)2 was phosphorylated by AMPK [141]. Finally, in organotypic slice cultures, pharmacological activation of AMPK with AICAR or ischemic challenge resulted in the AMPK-dependent loss of neuronal polarization [103]. Importantly, Emx1cre+ AMPKα1−/− AMPKα2 fl/fl (AMPKα dKO) mice survived to adulthood [140]. No differences in axons or dendrites were observed in these mice in comparison with WT littermates [140]. However, neurons lacking AMPK were resistant to AICAR treatment and polarized to the same extent as in DMSO. In contrast, AICAR disrupted the polarization of the WT littermates [140]. Metformin treatment inhibited the polarization of WT as well as AMPK-deleted neurons to a similar extent [140]. The LKB1–AMPK axis regulates the mTOR pathway. There are two mTOR-containing complexes in eukaryotic cells: mTORC1 and mTORC2. The mTORC1 complex, including mTOR, RAPTOR, MLST8, PRAS40, and DEPTOR, controls protein translation by recruiting 4EBP1 and the ribosomal p70S6K1 kinase. The mTORC2 complex, which includes mTOR, RICTOR, GβL, mSIN1, DEPTOR, and others, regulates the cytoskeleton and glucose homeostasis. In neurons, AMPK activation leads to enhanced phosphorylation of RAPTOR but reduced phosphorylation of mTOR, p70S6 K, and 4EBP1 [140]. In addition, knockdown of TSC1 or TSC2 rescues the AICAR-induced neuronal polarization defect [140]. This result suggests that AMPK-dependent activation of the GAP activity of TSC1/TSC2 complex results in the inhibition of the TSC1/TSC2 target Rheb (Ras homolog enriched in brain) GTPase. Rheb has intrinsically low GTPase activity and exists in an activated (GTP-bound) state. Rheb is negatively regulated by the GAP activity of the TSC1–TSC2 complex. Rheb∙GTP functions to activate mTOR. Therefore, the downregulation of Rheb activity, as well as the inhibition of RAPTOR, resulted in reduced mTORC1 activity and the consequent blockade of protein translation. These results are consistent with the report that while TSC2 is distributed uniformly throughout hippocampal neurons in culture, threonine 1462 phosphorylated (and thus inactivated) TSC2 is enriched in the axon [142]. TSC2 inactivation led to enhanced mTORC1 activity in the axon as determined by increased phosphop70S6K signal. In the same study, the authors also demonstrated that overexpression of TSC1 and TSC2, or the pharmacological inhibition of mTOR with rapamycin, inhibited axon specification, while silencing TSC2 resulted in neurons with supernumerary axons. Furthermore, the genetic ablation of Tsc1 similarly enhanced the number of neurons with supernumerary axons [142]. Syn1cre+ Tsc1 fl/fl mice suffer from neurological symptoms including seizures and die between 3 and 5 weeks after birth [143]. These mice were reported to have enlarged neurons, thicker dendritic arbors, and ectopic axons throughout the cortex, including in the cortical plate, with the axons in a state of permanent growth [142, 143].
Another group of kinases implicated in neuronal polarization include the Stk38 (Ndr1) and Stk38l (Ndr2) kinases [144]. The STK38 and STK38l kinases are distributed at all neurites prior to neuronal polarization, but accumulate at the axon after its specification in cultured hippocampal neurons. Simultaneous knockdown of both kinases, but not individual knockdowns, resulted in neurons with supernumerary axons. One of the phosphorylation targets of STK38 and STK38l is PARD3. Furthermore, a PARD3 phosphomimic mutant (PARD3-S383D) was sufficient to rescue the loss-of-function of STK38/STK38l and RASSF5 [144].
The STK38 and STK38l kinases function downstream of the tumor suppressor Rassf5 in this pathway. RASSF5 associates with the GTP-activated forms of RAS, RAP1, and other small GTPases. In cultured hippocampal neurons, RASSF5 shows a distribution similar to STK8 and STK38l at stage 2 and stage 3. STK38/STK38l phosphorylation of PARD3 at Ser 383 is downstream of Rassf5 (Fig. 4). This phosphorylation of PARD3 inhibits its interaction with dynein and leads to its localization at the axon. Phosphomimic PARD3-S383D is more abundant in the axon relative to minor neurites, while non-phosphorylatable PARD3-S383A is distributed in the cell body and minor processes. Knockdown of RASSF5 results in supernumerary axons, and this phenotype can be rescued by coexpression of a non-phosphorylatable STK38l-T442A. Knockdown of Rassf5 in E14.5 cortical neurons by ex vivo electroporation and examination of slice cultures after 36 h reveal a defect in migration of neurons from the intermediate zone to the cortical plate. Many intermediate zone neurons display multiple processes (multipolar) instead of a bipolar morphology. Cultured neurons obtained from Rassf5−/− mouse hippocampus recapitulate the knockdown results. However, Rassf5 mice are viable with no gross changes in cortical layers or hippocampal structures.
A third STK, STK25, is a modifier of the Reelin-Dab1 signaling pathway [145]. An LKB1–STK25–GM130 complex regulates Golgi morphology and neuronal polarity [145]. Overexpression of Stk25 results in Golgi condensation and supernumerary axon formation [145]. Reelin1 can rescue these phenotypes [145]. Conversely, STK25 overexpression inhibits the Reelin-induced Golgi extension into apical dendrites [145]. Thus, Reelin1-Dab1 and LKB1–Stk25–GM130 have opposing functions which regulate Golgi localization, axon specification, and dendrite morphogenesis.
Molecular interactions with other polarity complexes
The mammalian CRB, PALS, and PATJ family of proteins form the eponymous CRB–PALS–PATJ complex. The CRB paralogs CRB1-3 and the MPP paralogs MPP1-4 are considered to be the mammalian orthologs of D. melanogaster crb (crumbs) [25]. Mammalian orthologs of D. melanogaster sdt (stardust), i.e., PALS, include the genes MPP5-7 in humans [25]. Similarly, the mammalian PATJ are encoded by INADL and MPDZ. CRB, PALS, and PATJ transcripts are expressed in mammalian cortical neurons. CRB1 and MPP1 are expressed in human cortical neurons, while Crb1 and Mpp2 are detected in mouse cortical neurons [91, 92]. Similarly, MPP5 and MPP6, as well as MPDZ, are detected in both human and mouse cortical neurons [91, 92]. Loss of Mpdz results in postnatal lethality at approximately 20 days [146]. From P5, Mpdz−/− mice show growth and neurological defects, including decreased alertness and apathy. These mice have macrocephaly and cerebrospinal fluid accumulation in the lateral ventricles (hydrocephaly) due to aqueductal stenosis and severe defects in the ependymal layer. Ependymal loss in Mpdz−/− correlated with reduced expression and mislocalization of MPP5 [146].
The PAR complex interacts with the CRB–PALS–PATJ complex during the formation of Drosophila photoreceptors. The arthropod photoreceptors are specialized neurons. Drosophila photoreceptors and their mammalian counterparts share structural and functional similarities [147]. For example, the light sensitive outer segment of the mammalian photoreceptor resembles the rhabdomere of the Drosophila photoreceptor. Axons from each of the eight photoreceptors that form an ommatidium of the complex eye in insects fasciculate and project as a single bundle towards the optic lobes of the brain [148]. Vertebrate retinal ganglion cells directly inherit their apicobasal polarity from their precursors—the retinal neuroepithelial cells [149–153]. The development of the Drosophila photoreceptor is essentially a two-step process in terms of acquisition of polarity: (1) acquisition of apical-basal polarity and (2) regionalization of the apical membrane into the stalk and the rhabdomere [154]. During pupal development, the apical region of each photoreceptor cell within an ommatidium is involuted 90° laterally, with the apical side now facing the center of the cell cluster [155]. This surface develops into the rhabdomere. The rhabdomere and the stalk (inner segment) are separated from the basolateral membrane by the photoreceptor zonula adherens or adherens’ junctions (ZA) [148, 156, 157]. The ZA connects adjacent cells through homotypic, extracellular interactions of E-cadherin, which is a transmembrane protein. The cytoplasmic tail of E-cadherin interacts with multiple binding partners, including β-catenin/ARM (armadillo) [158]. The proper localization of the CRB–PALS–PATJ complex, as well as of PAR6 and PAR3/BAZ, is essential for ZA assembly and rhabdomere development [159–161]. PAR3 is required for the early apical-basal polarity and the apical domain is not formed in its absence [154]. The CRB, PALS, and PATJ proteins colocalize at the apical domain of undifferentiated cells and in developing photoreceptor clusters. After involution, these proteins can be detected in the rhabdomere stalk [154, 161]. Similar localization is seen for PAR6 and aPKC in the rhabdomere stalk [147, 162]; however, PAR3/BAZ localizes to the ZA along with β-catenin/ARM [154, 161]. CRB, together with SDT/PALS and PATJ, are required for rhabdomere extension and ZA, although CRB is dispensable for apical-basal polarity [161, 163–166].
In mammalian photoreceptors, CRB1, CRB2, CRB3, MPP4, PALS1, MUPP1, and MPDZ are localized at the subapical region along with aPKC [167]. The ZA contains β-catenin, p120 catenin, ZO-1, and ZO-2 [167]. The retina in Crb−/− mice is normal at birth and up to 2 months of age [167]. By 3 months, however, these mice develop retinal degeneration. The outer limiting membrane is disrupted and the stratification of the photoreceptor cells is lost, with frequent double layers or half-rosette structures. This phenotype was exacerbated with age or by light exposure [167].
The PAR complex is required for the basolateral segregation of the SCRIB–DLG–LGL complex in epithelial cells. LLGL1 phosphorylation by aPKC removes it from the apical membrane domain and allows segregation of the PAR3–PAR6–aPKC and SCRIB–DLG–LGL complexes [30, 168, 169]. However, protein–protein interactions within the SCRIB–DLG–LGL complex and its dynamics remain mostly unknown. Members of the SCRIB–DLG–LGL complex have been described and also functionally implicated in neurodevelopment. The human ortholog of Drosophila scribble (scrib) is known as Vartul. The protein encoded by scrib contains 16 leucine-rich repeats (LRRs), LAP-specific domains (LAPSDs)a/b and four PDZ domains [170, 171]. All of the N-terminal LRRs and four PDZ domains remain conserved in mammalian Scrib [172]. The PDZ domains of the mammalian homolog bind βPIX, which is a GEF for RAC and CDC42. The C-terminal of SCRIB contains three β-spectrin-binding motifs [173]. Both SCRIB and βPIX localize to presynaptic compartments in cerebellar cell cultures [174]. Scrib is also expressed in mouse cortical cells [92]. Apart from its role in apical-basal polarity, SCRIB also functions in the regulation of planar cell polarity (PCP). PCP signaling is important in axon guidance and scrib’s function in the anterior guidance of commissural axons in zebrafish (Danio rerio) has been described [175]. Sun et al. demonstrated the role of SCRIB in cultured mouse embryonic hippocampal neurons in the clustering of synaptic vesicles [176]. SCRIB colocalizes with and coimmunoprecipitates with β-catenin and, in the absence of SCRIB, synaptic vesicles were diffusely distributed along the axon and were defective in recycling back to the presynaptic active zone. While β-catenin was required for the proper localization of SCRIB, the opposite was not true—β-catenin localization was unaffected in the absence of SCRIB [176].
Mammalian orthologs of Drosophila l(2)gl are Llgl1 and Llgl2. Of the two Lgl genes, only Llgl1 is expressed in the brain [177]. Mammalian LGL and its yeast ortholog contain two β-propeller domains that each contains seven WD40 repeats [178]. LGL has been reported to contain a polybasic (PB) plasma membrane-targeting domain between the 10th and 11th WD40 repeats of the second β-propeller domain [179]. This region also has a series of phosphorylation sites including conserved aPKC and aurora kinase phosphorylation sites. Phosphorylation of LGL by aPKC is important for the establishment of polarity [28–30]. Phosphorylated LGL binds the GUK domain of DLG [169]. Silencing Llgl1 in rat hippocampal neuronal cultures markedly reduced axonogenesis and axon growth. In contrast, overexpressed Llgl1 resulted in supernumerary axons and increased axon length [180]. Llgl1−/− mice show defective brain development by E12.5 and die 24 days after birth from severe hydrocephalous [177]. In Llgl1−/− mice, neuroepithelial cells lose polarity, probably due to disorganization of apical junctional complexes and loss of apical membrane domain, and the neuroepithelia expands into a differentiated cell zone to form aberrant neuroblastic rosette-like structures. The progenitor cell population is expanded with a concomitant reduction in differentiated neurons [177].
Phosphatases and PAR proteins
Phosphatase function, in coordination with kinase activation, controls the output of signaling circuits. In Drosophila photoreceptors, PAR3/Baz localization at the ZA is enabled by Protein Phosphatase 2A (PP2A). While PAR-1/MARK phosphorylation of PAR3/Baz prevents its localization at the ZA, PP2A reverses this effect by dephosphorylating PAR3/Baz [181]. PP2A also functions as an antagonist of aPKC as it dephosphorylates JAM-A at the site phosphorylated by aPKC during epithelial cell polarization [182]. Other PP2A targets include the tight junction (TJ) proteins ZO-1, occluding, and claudin-1 [183] (Fig. 4). Expression of the catalytic subunit of PP2A inhibits TJ assembly in epithelial cells, whereas aPKC can induce TJ formation. In fact, PP2A associates with and can directly regulate aPKC function during TJ formation [183]. Whether similar mechanisms are in effect during neuronal polarization remains unknown. However, PP2A dephosphorylated Par6 at its Aurora A phosphorylation site and thus suppresses aPKC signaling in Drosophila neuroblasts [184].
Protein Phosphatase 1A (PP1A) has also been implicated in the regulation of PARD3-PARD6-aPKC function. In epithelial cells, the PARD3 scaffold can interact with the mammalian hippo signaling pathway components LATS1 and YAP, as well as PP1A. This leads to the dephosphorylation of YAP, its subsequent nuclear translocation, and hippo signaling [185]. Albeit that the function of hippo signaling in axon specification remains unknown, this signaling pathway is important in neural stem cell self-renewal, neural progenitor proliferation, and differentiation [186–188]. In addition, proteins of the polarity complex, such as DLG5, or proteins that interact with components of the polarity complex, such as the PARD3-interacting protein AMOT, can provide an interface between polarity and hippo signaling. DLG5 can also form complexes with hippo signaling components, including MST1/2 and YAP/TAZ [189]. Interaction of DLG5 with MST1/2 favors the association of this hippo pathway protein with MARK3, rather than with LATS1/2, and inhibits hippo pathway activation [189]. AMOT can colocalize with Pals1, Patj, and PARD3 [190] and also regulate YAP/TAZ activation [191]. AMOT has been implicated in dendritic spine maturation [192]. More importantly, PP1 can also directly dephosphorylate PARD3 (Fig. 4) and regulate the kinetics of TJ formation [193]. The regulatory subunit SMEK1 and the corresponding catalytic subunit Protein Phosphatase 4c (PP4c) of PP4 dephosphorylates and inactivates PARD3 [194] (Fig. 4). This suppresses the proliferation of mammalian neuronal progenitor cells and favors their differentiation [194]. Phosphatases also regulate aPKC substrates and downstream components such as GSK3α/β (PP2A and PP1, respectively) and AMPK (PP2A) [195, 196].
Small G-proteins’ signaling
aPKC activity is regulated by small G-proteins. The founding members of small G-proteins, the RAS oncoproteins, have been implicated in neuronal polarity. Overexpression of H-Ras or CA-H-Ras (H-Ras G12V) induced multiple axon formation in hippocampal neurons, while dominant-negative H-Ras (H-Ras S17N) inhibited axon specification [83]. aPKC interacts directly with H-RAS and functions in the H-RAS signal transduction cascade [197, 198].
A branch of the RAS subfamily, Ras-Proximal or RAP, functions upstream of aPKC–PAR complex. In the absence of Rap1 in Drosophila during cellularization, PAR3 and ZA are mispositioned [199]. In mammalian stage 2 neurons, RAP1B was described to localize to nascent axons [57]. In this study, RAP1B localization preceded CDC42 localization, which in turn occurred before PARD3–PARD6–aPKC localization in the axon of stage 3 neurons (Fig. 4). Rap1B silencing was rescued by a fast-cycling CDC42 mutant, Cdc42F28L. CDC42 was reported to be required for axon formation [57]. However, CDC42 silencing was not rescued by CA-Rap1B. Therefore, Rap1B and CDC42 functioned sequentially and hierarchically in specifying an axon. Importantly, localization of RAP1B to a single axon required the activation of PI3-K. In addition, CA-Rap1B (Rap1BG12V) and Cdc42F28L induced multiple axons. However, in a study by Fu et al., where the authors transfected WT, CA- and DN-Rap1 or Rap2 in hippocampal neurons, the results were markedly different [200]. Spiny but not aspiny neurons transfected with CA-Rap2 (Rap2G12V) had reduced axon length and showed profuse branching at the termini. The dendrites were also shorter and thicker. In the same study, CA-Rap1 (Rap1G12V) did not alter neuronal morphology. An important difference between the studies was that, in the later study, transfections were carried out at 10 days in culture as compared to 2 h after plating in the former study. It is likely that RAP1 functions early during neuronal development, while RAP2 has a later role.
AFADIN (also called AF6) functions as an effector in RAP1-mediated cell adhesion [201, 202]. This protein has important functions in dendrites and at synapses [203–205]. Silencing afadin inhibits axon branching, but does not affect axon length. In the absence of RAP1 and AFADIN in Drosophila during cellularization, PAR3 and the ZA are also mispositioned [199]. Interestingly, PAR3 and aPKC can regulate AFADIN localization [199]. AFADIN binds to the Ca2+-independent cell adhesion protein nectins, as well as to F-actin [206]. Another protein that coimmunoprecipitates with AFADIN during axon formation in cortical neurons is RRas [207]. Overexpression of RRas or CA-RRas (RRasQ87L) resulted in supernumerary axons, while RRas silencing or expression of membrane-targeted RRas GAP domain (Myr-RRasGAP) abolished axon specification. RRas inhibits GSK3β in an ILK-PI3-K-dependent manner, which in turn prevents CRMP-2 phosphorylation [208].
The Ras-like (RAL) branch function in neuronal polarity is represented by RAL A. In a study by Lalli, 48 h after plating, 75% of cortical neurons specified an axon [209]. Silencing RalA enhanced the percentage of non-polarized neurons in culture by twofold [209]. Neurites were threefold longer. In contrast, RalB silencing had no effect. CA-RalA (RalA Q72L), DN-RalA (RalA S28N), or Rlf-CAAX, a membrane-targeted, CA-Ral GEF, similarly inhibited neuron polarization. However, a CA-RalA mutant that failed to bind to the exocyst complex (RalA Q72LD49E) did not disrupt neuronal polarization [209]. Consistent with a role of the exocyst in neuronal polarity and axon specification, depletion of Sec6, Sec8, or Exo84 resulted in impairment of axon specification. Neurites were longer and lacked tau-1. In the case that axons were specified, branching was reduced. PAR3 and aPKC coimmunoprecipitate with exocyst proteins, and silencing RalA or exocyst components reduced PAR3 localization at neurites [209]. Thus, RAL A and exocyst function is important for the localization of the PAR3–PAR6–aPKC complex to the axon. RAL A also promotes neurite branching in cortical and sympathetic SCG neurons [210]. Rlf-CAAX (constitutively active Ral GEF), CA-RalA, and CA-RalB (RalB G23V) increased neurite branching, while DN-RalA and RalB (RalB S28N) decreased neurite branching in SCG and cortical neurons. CA- and DN-RalA but not RalB or Rlf-CAAX affected axon length. CA-RalA increased, while DN-RalA reduced, axon length [210].
Many other small G-proteins interact with members of the PARD3–PARD6–aPKC complex. RIT1 and RIT2 interact with PARD6 [211, 212]. RIT1 signals regulate SCG sympathetic and hippocampal neuron development. RIT1 is distributed in the cell body and at neurites in stage 2 hippocampal neurons, as well as in the axons and minor neurites in stage 3 neurons [213]. DN-Rit1 (Rit1 S35N) reduced the number of polarized neurons and decreased axon length in the polarized neurons. CA-Rit1 (Rit1 Q79L) did not change the number of axon in neurons but increased axon length [213].
Apart from the RAS subfamily, the RHO subfamily of small G-proteins has essential roles in neuronal polarization. DN-RhoA, DN-Rac1, and DN-Cdc42 all inhibited axonal growth in pyramidal neurons [214]. In Xenopus retinal ganglion cells (RGC), CA-RhoA, Rac1, and Cdc42 nearly eliminated axon formation [215]. In contrast, WT-RhoA, Rac1 or Cdc42, or DN-RhoA or Cdc42 did not affect axonogenesis [215]. The PARD3–PARD6–aPKC complex binds CDC42 and is subsequently activated [216–221]. One model proposes that aPKC kinase activity is kept constrained by PARD6 binding. This inhibition is released when CDC42-GTP binds PARD3–PARD6–aPKC [217]. Another model proposes that CDC42 is involved in recruitment or localization of the PAR–aPKC complex at the membrane and favors aPKC activity by relieving PARD6 inhibition [221]. This model was later refined to indicate that PARD6 interaction actually promoted aPKC activation [220]. A similar conclusion was reached by Kusne et al., where enhanced aPKC–PARD6 complex formation downstream of EGFR signaling was construed as increased aPKC activation [108]. Other small GTPases such as ECT2 might function in a similar fashion [222].
RAC1 is a downstream target of aPKC signaling (Fig. 4). RAC1 localizes to the axonal growth cone and the overexpression of its GEF TIAM1 resulted in multiple, long, axon-like processes in hippocampal neurons [223]. TIAM1 also localizes at the growth cone, and silencing TIAM1 in neurons resulted in the failure of axon specification [223]. Interestingly, TIAM1 directly binds PAR3 [224] and TIAM1 phosphorylation by aPKC is required for its activity [225]. TC10 interacts with the aPKCs [216, 226]. CA-TC10 (TC10Q75L), but not inactive TC10 (TC10T31N), targeted aPKC to the plasma membrane via the PARD6–PARD3 complex [226]. Insulin, as well as CA-TC10, stimulated aPKC phosphorylation. Silencing of TC10 or expression of DN-TC10 (TC10 S23N) prevented axon specification [227].
The RAS superfamily GTPase Ran has also been implicated in neuronal polarity. TPX2 is a target of Ran. Ran-dependent TPX2 activation promotes MT nucleation from the acentrosomal microtubule organizing center (MTOC) in neurons during neurite extension [228]. TPX2 also regulates MT nucleation from the centrosome/MTOC via the aPKC-Aurora A-NDEL1 pathway in DRG neurons. aPKC phosphorylates and activates Aurora A, which in turn interacts with TPX2 and phosphorylates NDEL1. Inhibition of aPKC with myr-ZIP, expression of a kinase-dead aPKC, siRNA knockdown of Aurora A, expression of kinase-dead Aurora A, siRNA knockdown of TPX2, or genetic ablation of Ndel1 all reduced neurite length [229]. Importantly, NDEL1 is a binding partner of LIS1. LIS1 is a gene mutated in lissencephaly, a condition which leads to microcephaly and severe developmental delays in children [230].
aPKC and PAR proteins interact with and/or their localization is dependent on a number of Rabs, such as RAB2, RAB5, RAB11, and RAB14. RAB2 is a cis-Golgi-associated protein required for ER-to-Golgi protein transport. RAB2 recruits aPKCι to vesicular tubular clusters (stations in the ER-to-Golgi transport pathway) [231]. Introduction of the RAB2 protein into E15 rat midbrain neurons by trituration increased the growth of dendrite-like neurites on poly-ornithine [232].
In the C. elegans embryo, depleting RAB5 resulted in the loss of cortical localization of PAR6 [233]. RAB4 and RAB5 function in plasma membrane recycling of endosomes in Xenopus RGC axons. DN-RAB4 (RAB4N121I), morpholino-induced knockdown of RAB4, and CA-Rab5c (Rab5cQ79L) decreased the extension rate in Xenopus RGC. In contrast, this rate was unaffected when RGCs expressed WT RAB4 or CA-RAB4 (Rab4Q67L) [234]. Rab5 knockdown in mouse hippocampal neurons inhibited axonogenesis as well as dendritic morphogenesis [235]. Rabex-5 functions in targeting RAB5, as well as RAB21, to both the axon and dendrites [236]. Rabex-5 knockdown resulted in a marked reduction in the length of both axons and dendrites as well as in axon branching, but did not affect dendritic branching [235].
In epithelial cells, RAB11A forms a complex with aPKC–PAR3 and Huntingtin. Huntingtin is required for the MT-dependent trafficking of apical vesicles carrying aPKC–PAR3 and for the formation of lumen during epithelial morphogenesis [237]. Mutant HTT can alter the thickening of the cortical layer in mouse models [238]. The expression of WT Rab11 or CA-Rab11A (Rab11AQ70L) enhanced axonogenesis [239]. However, expression of CA-Rab11 (Rab11Q70L) inhibited neurite formation in PC12 cells, while DN-Rab11 (Rab11S25N) promotes neuritogenesis [240]. In neuronal polarity, RAB11 function has been studied in the context of the Aatk/Lmtk1 gene which encodes a serine/threonine kinase that functions in endosomal recycling. AATK, in turn, is phosphorylated by Cdk5 during axon growth and colocalizes with RAB11A [239]. Expression of the un-phosphorylatable mutant of AATK (AATK S34A) silences this kinase and similarly enhanced axonogenesis. Consistent with these results, cultured cortical neurons from Aatk−/− mice had significantly longer axons. Notwithstanding, the cerebral cortex was grossly histologically normal [239]. In contrast, silencing RAB11A decreased axon length. The effect of the AATK S34A mutant in enhancing axon growth was reversed by coexpressing DN-Rab11A (Rab11AS25N) [239]. The RAB11 adaptor Nuclear Fallout (Nuf) is also phosphorylated by aPKC, and loss of aPKC altered recycling endosome trafficking [241]. Whether these functions are important in neuronal polarization remains to be investigated.
Rab11 is also associated with TGN and TGN-to-plasma membrane trafficking, apart from its function in recycling endosomes. Hereditary spastic paraplegias are neurological disorders associated with > 50 genetic loci [242]. Common loci are genes that function in ER network formation—SPG3A encodes atlastin-1, SPG4 encodes spastin, SPG12 encodes reticulon 2 and SPG31 encodes receptor expression-enhancing protein1. An SPG gene, SPG33, encodes Protrudin [242]. This protein, involved in tubular ER network formation, also colocalizes with and binds Rab11 in its GDP-bound state [240, 242]. In rat hippocampal neurons, expression of exogenous protrudin promoted neurite extension in the neurons [240]. Conversely, knock down of protrudin in PC12 cells led to inhibition of NGF-induced neurite outgrowth.
Ubiquitin ligases and PAR proteins
Another protein posttranslational modification with an essential role in axon specification is ubiquitylation and the subsequent proteasomal degradation of ubiquitylated proteins. For example, treatment of hippocampal neurons with the proteasome inhibitor MG132, lactacystin, or a dominant-negative ubiquitin (DN-Ub; Ub K48R) resulted in the formation of supernumerary axons [243]. Similarly, DN-Ub decreased terminal branching in Xenopus RGCs [244]. Of note, many of the proteins implicated in neuronal polarity, including PARD6 and aPKC, are stabilized in hippocampal neurons treated with MG132 [245].
E3 ubiquitin ligases confer substrate specificity to the process of ubiquitylation. Axon specification in hippocampal neurons is stimulated by BDNF [246]. BDNF or a cAMP analog increased the half-life of PARD6 and LKB1 while decreasing the half-life of RHO A [245]. Interestingly, both PARD6 and RHO A are substrates for the E3 ligase Smurf1. During BDNF signaling, activation of PKA phosphorylates Smurf1, thus changing its substrate preference from PARD6 to RHO A [245]. Thus, localized activation of PKA at a single neurite eventually leads to RHO A turnover at this site and specifies axon initiation [245]. Similar results were obtained with neurons in mice electroporated in utero with a phosphomimic Smurf1 (Smurf T306D). These neurons migrated normally to the cortical plate at P4, but were multipolar. In contrast, WT neurons were polarized normally with dendrites oriented towards the pia, and the axon projected radially towards the cortical plate and horizontally near the intermediate zone (IZ) and SVZ [245]. Interestingly, in rat enteric neurons, silencing Smurf1 resulted in failure of axon specification but did not increase the supernumerary axon phenotype [79]. This is despite the likely association of Smurf1 in a complex with PARD6, PARD3, aPKC, and GSK3β in these neurons [79].
Other molecules that interact with PAR proteins
The nuclear pore protein (nucleoporin) Nup358 or RanBP2 is distributed throughout the neuronal cell body, as well as in neurites, at stage 2 and stage 3 rat hippocampal neurons, but appears to function in specifying the axon [247]. Silencing Nup358 led to supernumerary axons, while overexpression of the leucine-rich repeat-containing N-terminal fragment of Nup358 impaired axon specification. Nup358-depletion-induced supernumerary axon phenotype could be inhibited by silencing Dvl or inhibiting aPKC kinase activity, indicating that this protein functions upstream of DVL–aPKC axis [247]. Nup358 was recently identified as an SUMO E3 ligase [248]. Nup358-dependent SUMOylation of aPKC activates aPKC. Depletion of Nup38 reduced aPKC SUMOylation and aPKC activation.
aPKC kinase activity was also reported to be reduced when PARD3 was deacetylated by SIRT-2 (Sir-two-homolog-2) [249]. There might be other regulators of the PAR3–PAR6–aPKC complex. PARD3 directly interacts with the lipid phosphatase PTEN [250–252] and phosphoinositides [253]. Another regulator of the PAR3–PAR6–aPKC complex is netrin-G-ligand-2 (NGL-2). Its association through binding PARD6 resulted in local stabilization of MT in the axon [254].
Atypical PKC–PAR proteins in dendritogenesis
Following growth of the axon, the minor neurites grow and branch to form the dendrites. In neurons, the axon is often considered the equivalent of the apical domain, while the dendrites are analogous to the basolateral domain. The polarity complex proteins also partake in the regulation of dendritic growth. The PAR3–PAR6–aPKC complex localizes to glutamatergic synapses in Drosophila, and aPKC regulates bouton formation by regulating MT dynamics [255]. At presynapses, MAP1B-related Futsch protein and aPKC associated with and stabilize MT [255]. Even the postsynaptic peribouton is altered in dapkc (Drosophila aPKC) loss-of-function mutants [255]. aPKC has been implicated in dendritogenesis in D. rerio. Purkinje cells in the D. rerio cerebellum initially extend multiple dendrites. Subsequently, these are retracted leaving behind the primary dendrite. The Golgi localizes to the base of this primary dendrite, even at the multipolar stage [256]. The heart and soul mutant, which has a premature stop codon in the Prkci gene, retains multiple dendrites [256]. This phenotype coincides with the disruption of Golgi localization within the neuron [256].
PARD3, also, is required for dendritic spine formation. Silencing PARD3 in cultured hippocampal neurons resulted in long, thin filopodia-like protrusions, but a failure of spine morphogenesis [257]. This phenotype could be rescued by coexpression of DN-RAC1, indicating that RAC1 activity at ectopic sites, such as in the dendritic shaft, results in this abnormal dendritic growth [257]. Normally, the interaction of PARD3 with TIAM1 restricts RAC1 activation to the spine [257]. However, this function of PARD3 was reported to be aPKC- and PARD3-independent [257]. In contrast, the aPKC–PARD6 complex has a distinct function in spine formation. Silencing PARD6 reduced spine formation, while overexpression of PARD6 resulted in an increased number of spines in an aPKC-dependent manner [258]. The aPKC–PARD6 complex functions upstream of p190RHO-GAP and RHO A in spine morphogenesis [258].
The truncated aPKCζ isoform, PKMζ, has also been implicated in spine growth. Notably, this aPKCζ isoform cannot bind PARD6. Transfection of cortical neurons in culture with PKMζ resulted in reduced spine length and altered morphology but did not affect spine density or dendritic branching [259]. Miniature excitatory postsynaptic current (mEPSC) amplitude was altered in PKMζ-overexpressing neurons, but not the frequency or kinetics [259]. In Aplysia, PKMζ generated by calpain-dependent cleavage of aPKCζ is required for associative and non-associative long-term facilitation in sensory and L7 motor neurons [260]. Thus, PKMζ was suggested to be essential for long-term synaptic plasticity [260].
PKMζ also functions as an inhibitor of axon formation during hippocampal neuron polarization in culture [61]. The absence/reduction of PKMζ through siRNA-mediated knockdown promoted the specification and growth of supernumerary axons, while overexpression of PKMζ prevented axon formation. Whether PKMζ promotes dendritic identity remains to be investigated.
Apart from its role in axon specification, WNT signaling is important in dendrite formation and organization. In the previous discussion on axon specification, we described the DVL-dependent activation of aPKC downstream of non-canonical Wnts. Treating mouse hippocampal neurons with Wnt7b conditioned media led to neurons with more (and longer) dendrites compared to controls [261]. Similarly, neurons that overexpress DVL had dendrites that were longer and more branched than neurons without DVL. Furthermore, DVL interacts with RAC1 in neurons and increases its activity. Neurons expressing DVL and DN-Rac1 had shorter and poorly branched dendrites compared to neurons expressing DVL alone. Expression of DN-Rac resulted in an identical phenotype. JNK is a downstream target of RAC, and inhibiting JNK led to neurons with shorter and less elaborated dendrites [261].
Other polarity complex proteins in dendritogenesis
mRNA and/or protein for the cell polarity regulator SCRIB were detected in the soma and dendrites of adult hippocampal pyramidal neurons, dentate gyrus granule cells and interneurons, and in neocortical pyramidal cells and interneurons. Mice heterozygous for the spontaneous circletail mutation in the Scrib gene (Scribcrc/+) show a 50% decrease in SCRIB protein levels. These mice had weaker synaptic strength and plasticity compared with WT. Scribcrc/+ mice also demonstrated improved hippocampal-dependent learning ability compared with WT, but they were impaired in social behavior. At the level of the dendritic spines, mutant mice exhibited changes in postsynaptic density morphology, increased dendritic arborization, and a decrease in spine number [262].
Drosophila dlg1 codes for a protein containing an N-terminal L27 domain, three PDZ domains, an SH3 domainn and a GUK domain at the C-terminus. Between the SH3 and the GUK domain is a HOOK domain [263]. Hypomorphic dlg mutations in Drosophila result in poorly developed sub-synaptic reticulum, which forms normally but fails to expand with the growth of the target muscle [264–266]. The mammalian orthologs that share the characteristic MAGUK structural domains found in Drosophila dlg1 include Dlg1, Dlg2, Dlg3, Dlg4n and Dlg5. While mammalian DLG1 contains same domains as Drosophila DLG1, mammalian DLG2-4 lack the N-terminal L27 domain. Mammalian DLG5 has an N-terminal CARD (caspase recruitment domain), a domain of unknown function (DUF domain), a coiled-coil domain, four PDZ domains, SH3 domainn and GUK domain [267]. Many of the Dlg paralogs are expressed in mouse and human cortical neurons. Dlg2 is abundantly expressed in forebrain and dorsal horn of the spinal cord, but only weakly in ventral horn and DRG [268]. Dlg2 knockout mice have reduced cell surface NR2A and NR2B NMDAR subunit expression. These mice also have defects in NMDAR-mediated postsynaptic function [268]. For example, excitatory postsynaptic current and potential is reduced in these animals. Consistent with the expression pattern of Dlg2, knockout mice demonstrated blunted NMDAR-mediated persistent pain in a peripheral nerve injury model or in an inflammatory pain model (injection of Complete Freund’s Adjuvant). Acute nociceptive transmission (to thermal or mechanical stimuli) was unaffected. Furthermore, this defect is limited to NMDAR expression and function as AMPAR expression and function are not affected. Dlg3 knockout mice have defects in synaptic plasticity, although basal synaptic transmission is not affected [269]. These mice have significant deficits in spatial learning and reference memory. Knockout mice use suboptimal strategies for learning spatial tasks. However, Dlg3 knockout mice can overcome their spatial learning impairments with continued training, despite the failure to optimize their strategies. In Dlg4 knockout mice, neither the synaptic localization of NMDAR nor synaptic transmission were grossly altered [270]. Synaptic plasticity, however, was enhanced in the mutant mice. High-frequency stimulation (100 Hz) to induce NMDAR-dependent LTP produced larger potentiation in Dlg4−/− brain slices. Even trains (900 pulses) of intermediate (10 or 20 Hz)- or low (5 Hz)-frequency stimulation produced near saturating potentiation of synaptic transmission. Dlg4−/− mice displayed marked inability in spatial learning tasks. Dlg5−/− mice have a ~ 40% reduction in spine density [271]. Spine morphology, total dendritic length, and basal dendrite complexity were unchanged. These defects were recapitulated in a Dlg5 mutant in the SH3 domain (Dlg5L1642P), where β-catenin localization at vGLUT1+ synapses, but not DLG5-β-catenin interaction, is altered. Dlg5L1642P mice also show a defect in N-cadherin trafficking to the neuronal surface.
Small G-proteins in dendritogenesis
Small GTPases are also important for dendritic growth. In cortical neurons, RRas3 silencing inhibited MAPK activation and impaired dendritic outgrowth and branching [272]. M-Ras expression is enhanced in cortical neurons during dendritic development. Silencing M-Ras, but not R-Ras, reduced dendrite growth and branching, while CA-M-Ras (M-RasQ71L) enhanced dendritic outgrowth and branching [272]. CA-Rit1 significantly reduced dendrite numbers and lengths in a MAPK-dependent manner [213]. Similarly, in sympathetic neurons, CA-Rit1 decreased NGF and BMP7-induced dendritic growth [213]. Activation of RHO A decreased dendritic growth and branching, whereas activation of RAC1 increased dendrite branching [215, 273, 274]. In cortical pyramidal neurons, DN-Rac1, DN-Cdc42, membrane-targeted RhoGAP (myr-RhoGAP), and Rho inhibitor C3 transferase, all reduced the number of primary dendrites in non-pyramidal neurons and the number of basal dendrites in pyramidal neurons [214]. In contrast, CA-Rac1, CA-Cdc42, and CA-RhoA increased the number of primary dendrites [214]. In Xenopus retinal ganglion cells, dendritogenesis required RAC1 and CDC42 activity, but CA-Rho abolished nearly all dendrites [215]. In Xenopus tectal neurons, the findings were essentially identical. Enhanced RAC1 and, to a lesser extent CDC42 activity, increased dendritic branching and remodeling, while increased RHO A activity decreased branch extension [274]. Similarly, in chick RGC, CA-Rac1 reduced secondary and tertiary branching, but increased the number of short terminal processes [275]. DN-Rac1 or CA-RhoA expressing cells, on the other hand, exhibited secondary and tertiary branching with a reduction in the number of short terminal processes, but showed decreased dendritic motility [275]. In Drosophila mushroom body neurons, RhoA function was dispensable for axon growth, but neurons overextended their dendrites [273]. Expression of CA-RhoA reduced dendritic complexity [273]. Finally, in mature pyramidal neurons, DN-Rac1 expression resulted in spine elimination. CA-Rac1 (Rac1Q61L or Rac1G12V) also led to defective spine development. CA-RhoA reduced the complexity of both apical and basal dendritic arborization, although DN-RhoA did not affect dendritic morphology [276]. This CA-RhoA-dependent effect required ROCK activity, as the phenotype was rescued by the kinase inhibitor Y-27632 [276]. The function of aPKC in regulating small GTPase signaling, and additionally its role as an effector of small GTPase signaling, is well known. Nonetheless, the direct involvement of either aPKC or PAR proteins in small GTPase functions in dendritogenesis is not well described except for the examples discussed above.
Amongst the Rabs, RAB17 silencing reduces total dendritic length as well as dendritic branching [235]. During neuronal development RAB17 redistributes from the cell body to dendrites. At the early stages, when WT Rab17 is primarily localized in cell body, CA-Rab17 (Rab17Q77L) preferentially localizes at the dendrites [235]. Rabex-5 is required for dendritic translocation of RAB17 [236]. As described earlier, Rab5 silencing reduced length and branching of both axons and dendrites. While Rabex-5 silencing similarly reduced axon and dendrite length and axonal branching, it had no effect on dendritic branching [235]. Rabex-5 is also required for the axonal and dendritic localization of RAB5 and RAB21 [236]. RAB3A is localized to synaptic vesicles and regulates the Ca2+-triggered docking of transport vesicles to the target membrane [277]. In hippocampal neurons, RAB8 is localized preferentially in the somatodendritic domain and not the axon [278]. Antisense knockdown of RAB8 inhibited cell body-to-dendrite transport but did not affect axonal transport [278]. In a C. elegans screen, Rab10 was required for dendritic morphogenesis and arborization of PVD neurons [279]. Furthermore, loss of function of Rab10 demonstrated that the loss of exocyst components, such as exoc-8 and sec-8, in causes proximal dendritic arborization defects [279]. In exoc-8 and sec-8 mutants, Rab10 accumulated in intracellular vesicles. Although aPKCs and PARs interact with Rabs, the specific interactions involved in dendritogenesis remain to be characterized.
Other aPKC-interacting proteins in dendritogenesis
The aPKC–AFADIN interaction was described above in the context of axon formation. Genetic ablation of afadin in excitatory neurons also resulted in a striking reduction in synaptic density and lamination defects in CA1 and 3, likely due to mislocalization of pyramidal neurons, although dendritic growth and branching were unchanged [203]. While overall N-cadherin and β-, αN- or p120-catenin amounts were unaffected, clustering of these molecules was reduced in the absence of AFADIN [203]. AFADIN also links nectins with the cadherin junctional complex by binding p120-catenin and α-catenin [203]. aPKC functions in establishing N-cadherin junctions in neural progenitors [70, 71]. The protein AFADIN also binds several EphB receptors and neurexins, which promote synaptogenesis [203].
Concluding comments
A preponderance of causal and associative evidence indicates that aPKC and PAR proteins are involved in the regulation of neuronal polarity. Notwithstanding, the mechanisms leading to aPKC activation or to the formation of the PAR3–PAR6–aPKC complex remain poorly defined. WNT, TGFβ, and receptor tyrosine kinases can activate aPKC and cause allosteric changes in, and alter posttranslational modifications of protein–protein interactions of the PAR proteins. One common theme appears to be the activation of PI3-K and changes in the composition of membrane PtdIns. Similarly, small G-proteins can lead to aPKC activation and perhaps altered membrane-recruitment or localization of proteins of the PAR–aPKC complex. How these signaling pathways are precisely integrated to activate aPKC and PAR proteins in directing neuronal polarization remains unsolved. It is possible that different pathways are used in response to specific signals, at unique domains (axon versus dendrites) or in unique subsets of neurons.
Moreover, the specific aPKC protein functioning in axon versus dendritic polarization is still controversial. Most publications report aPKCζ as the primary neuronal aPKC, but this transcript is usually not identifiable in mammalian CNS neurons. The truncated PKMζ is uniquely neuronal, but most studies on aPKC function in neurons ignore this Prkcz isoform. We have proposed an activator–inhibitor scenario involving molecular competition between aPKCι and PKMζ [61]. However, additional investigation is required to fully establish this paradigm. Another outstanding question is whether aPKC and PAR proteins function identically in contexts where no external cues are available for symmetry breaking, such as in dissociated cultured neurons, versus situations where nearby cells and morphogen gradients are present, such as in vivo.
Finally, the targets of aPKC and PAR proteins in neuronal polarization need to be comprehensively described. In axon specification, it appears that the ultimate targets of this pathway are microtubules. This occurs through a number of MT-motor proteins or MT-binding proteins such as kinesins or Tau. Effects on sorting and trafficking proteins appear to be important as well, both in the spatial localization of aPKC and PAR proteins and for the recruitment of effectors. Yet, the complete list, including critical components, overlaps, and redundancies, remains to be fully described. Moreover, the knowledge of target specificity of aPKC and PAR proteins in axon versus dendrite specification remains incomplete. In conclusion, aPKC and PARs are undoubtedly critical mediators of neuronal polarization, yet the complete understanding of their function remains an area of active pursuit.
Acknowledgements
The authors would like to thank Edward K. Mandel for the discussions. CVR is a HHMI Faculty Scholar (Grant 55108561).
Abbreviations
- AIS
Axon-initial segment
- AMPK
AMP-activated protein kinase
- aPKC
Atypical protein kinase C
- BDNF
Brain-derived neurotrophic factor
- CaSR
Extracellular calcium-sensing receptor
- CRIB
CDC42/RAC interactive binding
- CRMP-2
Collapsin response mediator protein-2
- DRG
Dorsal root ganglia
- DSC
Dorsal spinal commissure
- Ig
Immunoglobulin
- IZ
Intermediate zone
- JIP-1
c-Jun N-terminal kinase interacting protein-1
- KLC
Kinesin light chain
- MAPK
Mitogen-activated protein kinase
- mEPSC
Miniature excitatory postsynaptic current
- MTOC
Microtubule organizing center
- MTs
Microtubules
- Myr
Myristoylated
- NGF
Nerve growth factor
- NGL-2
Netrin-G-ligand-2
- PB1
Phox/Bem1
- PDZ
PSD95-DLG1-ZO1
- PH
Pleckstrin homology
- PI3-K
Phosphoinositide 3-kinase
- PNS
Peripheral nervous system
- PtdIns
Phosphatidylinositol
- RAL
Ras-like
- RGC
Retinal ganglion cells
- Rheb
Ras homolog enriched in brain
- SCG
Superior cervical ganglion
- SIRT-2
Sir-two-homolog-2
- SVZ
Subventricular zone
- TJ
Tight junction
- TrkA
Tropomyosin-related kinase A
- WT
Wild type
References
- 1.Guillery RW. Observations of synaptic structures: origins of the neuron doctrine and its current status. Philos Trans R Soc Lond B Biol Sci. 2005;360(1458):1281–1307. doi: 10.1098/rstb.2003.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Carlos JA, Borrell J. A historical reflection of the contributions of Cajal and Golgi to the foundations of neuroscience. Brain Res Rev. 2007;55(1):8–16. doi: 10.1016/j.brainresrev.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Glickstein M. Golgi and Cajal: the neuron doctrine and the 100th anniversary of the 1906 Nobel Prize. Curr Biol. 2006;16(5):R147–R151. doi: 10.1016/j.cub.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 4.Berlucchi G. Some aspects of the history of the law of dynamic polarization of the neuron. From William James to Sherrington, from Cajal and van Gehuchten to Golgi. J Hist Neurosci. 1999;8(2):191–201. doi: 10.1076/jhin.8.2.191.1844. [DOI] [PubMed] [Google Scholar]
- 5.Thompson BJ. Cell polarity: models and mechanisms from yeast, worms and flies. Development. 2013;140(1):13–21. doi: 10.1242/dev.083634. [DOI] [PubMed] [Google Scholar]
- 6.Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84(3):335–344. doi: 10.1016/S0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 7.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422(6933):766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macara IG, Mili S. Polarity and differential inheritance—universal attributes of life? Cell. 2008;135(5):801–812. doi: 10.1016/j.cell.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebersbach G, Jacobs-Wagner C. Exploration into the spatial and temporal mechanisms of bacterial polarity. Trends Microbiol. 2007;15(3):101–108. doi: 10.1016/j.tim.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Bowman GR, Lyuksyutova AI, Shapiro L. Bacterial polarity. Curr Opin Cell Biol. 2011;23(1):71–77. doi: 10.1016/j.ceb.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro L, McAdams HH, Losick R. Generating and exploiting polarity in bacteria. Science. 2002;298(5600):1942–1946. doi: 10.1126/science.1072163. [DOI] [PubMed] [Google Scholar]
- 12.Treuner-Lange A, Sogaard-Andersen L. Regulation of cell polarity in bacteria. J Cell Biol. 2014;206(1):7–17. doi: 10.1083/jcb.201403136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiou JG, Balasubramanian MK, Lew DJ. Cell polarity in yeast. Annu Rev Cell Dev Biol. 2017;33:77–101. doi: 10.1146/annurev-cellbio-100616-060856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin SG, Arkowitz RA. Cell polarization in budding and fission yeasts. FEMS Microbiol Rev. 2014;38(2):228–253. doi: 10.1111/1574-6976.12055. [DOI] [PubMed] [Google Scholar]
- 15.Drubin DG. Development of cell polarity in budding yeast. Cell. 1991;65(7):1093–1096. doi: 10.1016/0092-8674(91)90001-F. [DOI] [PubMed] [Google Scholar]
- 16.Irazoqui JE, Lew DJ. Polarity establishment in yeast. J Cell Sci. 2004;117(Pt 11):2169–2171. doi: 10.1242/jcs.00953. [DOI] [PubMed] [Google Scholar]
- 17.Wedlich-Soldner R. A longer life for yeast with good memory. Dev Cell. 2014;31(4):391–392. doi: 10.1016/j.devcel.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Meitinger F, Khmelinskii A, Morlot S, Kurtulmus B, Palani S, Andres-Pons A, Hub B, Knop M, Charvin G, Pereira G. A memory system of negative polarity cues prevents replicative aging. Cell. 2014;159(5):1056–1069. doi: 10.1016/j.cell.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Meitinger F, Richter H, Heisel S, Hub B, Seufert W, Pereira G. A safeguard mechanism regulates Rho GTPases to coordinate cytokinesis with the establishment of cell polarity. PLoS Biol. 2013;11(2):e1001495. doi: 10.1371/journal.pbio.1001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13(5):609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141(5):757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Motegi F, Seydoux G. The PAR network: redundancy and robustness in a symmetry-breaking system. Philos Trans R Soc Lond B Biol Sci. 2013;368(1629):20130010. doi: 10.1098/rstb.2013.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose L, Gonczy P (2014) Polarity establishment, asymmetric division and segregation of fate determinants in early C. elegans embryos. WormBook, pp 1–43. 10.1895/wormbook.1.30.2 [DOI] [PubMed]
- 24.Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52(3):311–320. doi: 10.1016/S0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 25.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778(3):614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 26.Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S. Regulated protein–protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells. 2002;7(11):1161–1171. doi: 10.1046/j.1365-2443.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- 27.Hurd TW, Fan S, Liu CJ, Kweon HK, Hakansson K, Margolis B. Phosphorylation-dependent binding of 14-3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr Biol. 2003;13(23):2082–2090. doi: 10.1016/j.cub.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol. 2003;5(4):301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- 29.Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422(6929):326–330. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- 30.Hutterer A, Betschinger J, Petronczki M, Knoblich JA. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell. 2004;6(6):845–854. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Gunaratne A, Di Guglielmo GM. Par6 is phosphorylated by aPKC to facilitate EMT. Cell Adhes Migr. 2013;7(4):357–361. doi: 10.4161/cam.25651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunaratne A, Thai BL, Di Guglielmo GM. Atypical protein kinase C phosphorylates Par6 and facilitates transforming growth factor beta-induced epithelial-to-mesenchymal transition. Mol Cell Biol. 2013;33(5):874–886. doi: 10.1128/MCB.00837-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sotillos S, Diaz-Meco MT, Caminero E, Moscat J, Campuzano S. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J Cell Biol. 2004;166(4):549–557. doi: 10.1083/jcb.200311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bose R, Wrana JL. Regulation of Par6 by extracellular signals. Curr Opin Cell Biol. 2006;18(2):206–212. doi: 10.1016/j.ceb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Chen YM, Wang QJ, Hu HS, Yu PC, Zhu J, Drewes G, Piwnica-Worms H, Luo ZG. Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/atypical PKC complex in regulating hippocampal neuronal polarity. Proc Natl Acad Sci USA. 2006;103(22):8534–8539. doi: 10.1073/pnas.0509955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol. 2004;14(8):736–741. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, Miwa Y, Ohno S. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol. 2004;14(16):1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126(3):397–442. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- 39.Bartlett WP, Banker GA. An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I. Cells which develop without intercellular contacts. J Neurosci. 1984;4(8):1944–1953. doi: 10.1523/JNEUROSCI.04-08-01944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8(4):1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goslin K, Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol. 1989;108(4):1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Craig AM, Jareb M, Banker G. Neuronal polarity. Curr Opin Neurobiol. 1992;2(5):602–606. doi: 10.1016/0959-4388(92)90025-G. [DOI] [PubMed] [Google Scholar]
- 43.Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 44.Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330(6145):254–256. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- 45.Feng W, Wu H, Chan LN, Zhang M. The Par-3 NTD adopts a PB1-like structure required for Par-3 oligomerization and membrane localization. EMBO J. 2007;26(11):2786–2796. doi: 10.1038/sj.emboj.7601702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Wang W, Chen J, Zhang K, Gao F, Gao B, Zhang S, Dong M, Besenbacher F, Gong W, Zhang M, Sun F, Feng W. Structural insights into the intrinsic self-assembly of Par-3 N-terminal domain. Structure. 2013;21(6):997–1006. doi: 10.1016/j.str.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Mizuno K, Suzuki A, Hirose T, Kitamura K, Kutsuzawa K, Futaki M, Amano Y, Ohno S. Self-association of PAR-3-mediated by the conserved N-terminal domain contributes to the development of epithelial tight junctions. J Biol Chem. 2003;278(33):31240–31250. doi: 10.1074/jbc.M303593200. [DOI] [PubMed] [Google Scholar]
- 48.Chen S, Chen J, Shi H, Wei M, Castaneda-Castellanos DR, Bultje RS, Pei X, Kriegstein AR, Zhang M, Shi SH. Regulation of microtubule stability and organization by mammalian Par3 in specifying neuronal polarity. Dev Cell. 2013;24(1):26–40. doi: 10.1016/j.devcel.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112(1):63–75. doi: 10.1016/S0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- 50.Gao L, Macara IG, Joberty G. Multiple splice variants of Par3 and of a novel related gene, Par3L, produce proteins with different binding properties. Gene. 2002;294(1–2):99–107. doi: 10.1016/S0378-1119(02)00681-9. [DOI] [PubMed] [Google Scholar]
- 51.Shi SH, Cheng T, Jan LY, Jan YN. APC and GSK-3beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol. 2004;14(22):2025–2032. doi: 10.1016/j.cub.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol. 2004;6(4):328–334. doi: 10.1038/ncb1118. [DOI] [PubMed] [Google Scholar]
- 53.Hengst U, Deglincerti A, Kim HJ, Jeon NL, Jaffrey SR. Axonal elongation triggered by stimulus-induced local translation of a polarity complex protein. Nat Cell Biol. 2009;11(8):1024–1030. doi: 10.1038/ncb1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao L, Macara IG. Isoforms of the polarity protein par6 have distinct functions. J Biol Chem. 2004;279(40):41557–41562. doi: 10.1074/jbc.M403723200. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119(Pt 6):979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 56.Bulgakova NA, Knust E. The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J Cell Sci. 2009;122(Pt 15):2587–2596. doi: 10.1242/jcs.023648. [DOI] [PubMed] [Google Scholar]
- 57.Schwamborn JC, Puschel AW. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci. 2004;7(9):923–929. doi: 10.1038/nn1295. [DOI] [PubMed] [Google Scholar]
- 58.Smith L, Smith JB. Lack of constitutive activity of the free kinase domain of protein kinase C zeta. Dependence on transphosphorylation of the activation loop. J Biol Chem. 2002;277(48):45866–45873. doi: 10.1074/jbc.M206420200. [DOI] [PubMed] [Google Scholar]
- 59.Bougie JK, Cai D, Hastings M, Farah CA, Chen S, Fan X, McCamphill PK, Glanzman DL, Sossin WS. Serotonin-induced cleavage of the atypical protein kinase C Apl III in Aplysia. J Neurosci. 2012;32(42):14630–14640. doi: 10.1523/JNEUROSCI.3026-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC. Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem. 2003;278(41):40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- 61.Parker SS, Mandell EK, Hapak SM, Maskaykina IY, Kusne Y, Kim JY, Moy JK, St John PA, Wilson JM, Gothard KM, Price TJ, Ghosh S. Competing molecular interactions of aPKC isoforms regulate neuronal polarity. Proc Natl Acad Sci USA. 2013;110(35):14450–14455. doi: 10.1073/pnas.1301588110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu-Zhang AX, Schramm CL, Nabavi S, Malinow R, Newton AC. Cellular pharmacology of protein kinase Mzeta (PKMzeta) contrasts with its in vitro profile: implications for PKMzeta as a mediator of memory. J Biol Chem. 2012;287(16):12879–12885. doi: 10.1074/jbc.M112.357244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujii K, Zhu G, Liu Y, Hallam J, Chen L, Herrero J, Shaw S. Kinase peptide specificity: improved determination and relevance to protein phosphorylation. Proc Natl Acad Sci USA. 2004;101(38):13744–13749. doi: 10.1073/pnas.0401881101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buchser WJ, Slepak TI, Gutierrez-Arenas O, Bixby JL, Lemmon VP. Kinase/phosphatase overexpression reveals pathways regulating hippocampal neuron morphology. Mol Syst Biol. 2010;6:391. doi: 10.1038/msb.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tessier-Lavigne M. Wiring the brain: the logic and molecular mechanisms of axon guidance and regeneration. Harvey Lect. 2002;98:103–143. [PubMed] [Google Scholar]
- 67.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274(5290):1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 68.Wolf AM, Lyuksyutova AI, Fenstermaker AG, Shafer B, Lo CG, Zou Y. Phosphatidylinositol-3-kinase-atypical protein kinase C signaling is required for Wnt attraction and anterior-posterior axon guidance. J Neurosci. 2008;28(13):3456–3467. doi: 10.1523/JNEUROSCI.0029-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soloff RS, Katayama C, Lin MY, Feramisco JR, Hedrick SM. Targeted deletion of protein kinase C lambda reveals a distribution of functions between the two atypical protein kinase C isoforms. J Immunol. 2004;173(5):3250–3260. doi: 10.4049/jimmunol.173.5.3250. [DOI] [PubMed] [Google Scholar]
- 70.Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133(9):1735–1744. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- 71.Ghosh S, Marquardt T, Thaler JP, Carter N, Andrews SE, Pfaff SL, Hunter T. Instructive role of aPKCzeta subcellular localization in the assembly of adherens junctions in neural progenitors. Proc Natl Acad Sci USA. 2008;105(1):335–340. doi: 10.1073/pnas.0705713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamanaka T, Tosaki A, Kurosawa M, Akimoto K, Hirose T, Ohno S, Hattori N, Nukina N. Loss of aPKClambda in differentiated neurons disrupts the polarity complex but does not induce obvious neuronal loss or disorientation in mouse brains. PLoS One. 2013;8(12):e84036. doi: 10.1371/journal.pone.0084036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rolls MM, Doe CQ. Baz, Par-6 and aPKC are not required for axon or dendrite specification in Drosophila. Nat Neurosci. 2004;7(12):1293–1295. doi: 10.1038/nn1346. [DOI] [PubMed] [Google Scholar]
- 74.Zhang X, Zhu J, Yang GY, Wang QJ, Qian L, Chen YM, Chen F, Tao Y, Hu HS, Wang T, Luo ZG. Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat Cell Biol. 2007;9(7):743–754. doi: 10.1038/ncb1603. [DOI] [PubMed] [Google Scholar]
- 75.Consonni SV, Maurice MM, Bos JL. DEP domains: structurally similar but functionally different. Nat Rev Mol Cell Biol. 2014;15(5):357–362. doi: 10.1038/nrm3791. [DOI] [PubMed] [Google Scholar]
- 76.Greer YE, Fields AP, Brown AM, Rubin JS. Atypical protein kinase Ciota is required for Wnt3a-dependent neurite outgrowth and binds to phosphorylated dishevelled 2. J Biol Chem. 2013;288(13):9438–9446. doi: 10.1074/jbc.M112.448282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421(6924):753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 78.Ciani L, Salinas PC. c-Jun N-terminal kinase (JNK) cooperates with Gsk3beta to regulate Dishevelled-mediated microtubule stability. BMC Cell Biol. 2007;8:27. doi: 10.1186/1471-2121-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vohra BP, Fu M, Heuckeroth RO. Protein kinase Czeta and glycogen synthase kinase-3beta control neuronal polarity in developing rodent enteric neurons, whereas SMAD specific E3 ubiquitin protein ligase 1 promotes neurite growth but does not influence polarity. J Neurosci. 2007;27(35):9458–9468. doi: 10.1523/JNEUROSCI.0870-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diez H, Garrido JJ, Wandosell F. Specific roles of Akt iso forms in apoptosis and axon growth regulation in neurons. PLoS One. 2012;7(4):e32715. doi: 10.1371/journal.pone.0032715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dajas-Bailador F, Jones EV, Whitmarsh AJ. The JIP1 scaffold protein regulates axonal development in cortical neurons. Curr Biol. 2008;18(3):221–226. doi: 10.1016/j.cub.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dajas-Bailador F, Bantounas I, Jones EV, Whitmarsh AJ. Regulation of axon growth by the JIP1–AKT axis. J Cell Sci. 2014;127(Pt 1):230–239. doi: 10.1242/jcs.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoshimura T, Arimura N, Kawano Y, Kawabata S, Wang S, Kaibuchi K. Ras regulates neuronal polarity via the PI3-kinase/Akt/GSK-3beta/CRMP-2 pathway. Biochem Biophys Res Commun. 2006;340(1):62–68. doi: 10.1016/j.bbrc.2005.11.147. [DOI] [PubMed] [Google Scholar]
- 84.Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120(1):123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 85.Gartner A, Huang X, Hall A. Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3beta) independently of Akt/PKB serine phosphorylation. J Cell Sci. 2006;119(Pt 19):3927–3934. doi: 10.1242/jcs.03159. [DOI] [PubMed] [Google Scholar]
- 86.Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol. 1997;192(1):31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- 87.Goold RG, Owen R, Gordon-Weeks PR. Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J Cell Sci. 1999;112(Pt 19):3373–3384. doi: 10.1242/jcs.112.19.3373. [DOI] [PubMed] [Google Scholar]
- 88.Goold RG, Gordon-Weeks PR. The MAP kinase pathway is upstream of the activation of GSK3beta that enables it to phosphorylate MAP1B and contributes to the stimulation of axon growth. Mol Cell Neurosci. 2005;28(3):524–534. doi: 10.1016/j.mcn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 89.Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J Cell Sci. 1998;111(Pt 10):1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- 90.Leroy K, Brion JP. Developmental expression and localization of glycogen synthase kinase-3beta in rat brain. J Chem Neuroanat. 1999;16(4):279–293. doi: 10.1016/S0891-0618(99)00012-5. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA, 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89(1):37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34(36):11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wood-Kaczmar A, Kraus M, Ishiguro K, Philpott KL, Gordon-Weeks PR. An alternatively spliced form of glycogen synthase kinase-3beta is targeted to growing neurites and growth cones. Mol Cell Neurosci. 2009;42(3):184–194. doi: 10.1016/j.mcn.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Castano Z, Gordon-Weeks PR, Kypta RM. The neuron-specific isoform of glycogen synthase kinase-3beta is required for axon growth. J Neurochem. 2010;113(1):117–130. doi: 10.1111/j.1471-4159.2010.06581.x. [DOI] [PubMed] [Google Scholar]
- 95.Goold RG, Gordon-Weeks PR. Glycogen synthase kinase 3beta and the regulation of axon growth. Biochem Soc Trans. 2004;32(Pt 5):809–811. doi: 10.1042/BST0320809. [DOI] [PubMed] [Google Scholar]
- 96.Goold RG, Gordon-Weeks PR. NGF activates the phosphorylation of MAP1B by GSK3beta through the TrkA receptor and not the p75(NTR) receptor. J Neurochem. 2003;87(4):935–946. doi: 10.1046/j.1471-4159.2003.02062.x. [DOI] [PubMed] [Google Scholar]
- 97.Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42(6):897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 98.Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4(8):781–782. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- 99.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120(1):137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 100.Kawano Y, Yoshimura T, Tsuboi D, Kawabata S, Kaneko-Kawano T, Shirataki H, Takenawa T, Kaibuchi K. CRMP-2 is involved in kinesin-1-dependent transport of the Sra-1/WAVE1 complex and axon formation. Mol Cell Biol. 2005;25(22):9920–9935. doi: 10.1128/MCB.25.22.9920-9935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13(3):195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 102.Zurashvili T, Cordon-Barris L, Ruiz-Babot G, Zhou X, Lizcano JM, Gomez N, Gimenez-Llort L, Bayascas JR. Interaction of PDK1 with phosphoinositides is essential for neuronal differentiation but dispensable for neuronal survival. Mol Cell Biol. 2013;33(5):1027–1040. doi: 10.1128/MCB.01052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Amato S, Liu X, Zheng B, Cantley L, Rakic P, Man HY. AMP-activated protein kinase regulates neuronal polarization by interfering with PI 3-kinase localization. Science. 2011;332(6026):247–251. doi: 10.1126/science.1201678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Menager C, Arimura N, Fukata Y, Kaibuchi K. PIP3 is involved in neuronal polarization and axon formation. J Neurochem. 2004;89(1):109–118. doi: 10.1046/j.1471-4159.2004.02302.x. [DOI] [PubMed] [Google Scholar]
- 105.Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35(1):65–76. doi: 10.1016/S0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- 106.Xia B, Liu H, Xie J, Wu R, Li Y. Akt enhances nerve growth factor-induced axon growth via activating the Nrf2/ARE pathway. Int J Mol Med. 2015;36(5):1426–1432. doi: 10.3892/ijmm.2015.2329. [DOI] [PubMed] [Google Scholar]
- 107.Guo X, Snider WD, Chen B. GSK3beta regulates AKT-induced central nervous system axon regeneration via an eIF2Bepsilon-dependent, mTORC1-independent pathway. Elife. 2016;5:e11903. doi: 10.7554/eLife.11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kusne Y, Carrera-Silva EA, Perry AS, Rushing EJ, Mandell EK, Dietrich JD, Errasti AE, Gibbs D, Berens ME, Loftus JC, Hulme C, Yang W, Lu Z, Aldape K, Sanai N, Rothlin CV, Ghosh S. Targeting aPKC disables oncogenic signaling by both the EGFR and the proinflammatory cytokine TNFalpha in glioblastoma. Sci Signal. 2014;7(338):ra75. doi: 10.1126/scisignal.2005196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol. 2011;47(1):R1–R10. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- 110.Farese RV, Sajan MP, Standaert ML. Atypical protein kinase C in insulin action and insulin resistance. Biochem Soc Trans. 2005;33(Pt 2):350–353. doi: 10.1042/BST0330350. [DOI] [PubMed] [Google Scholar]
- 111.Sosa L, Dupraz S, Laurino L, Bollati F, Bisbal M, Caceres A, Pfenninger KH, Quiroga S. IGF-1 receptor is essential for the establishment of hippocampal neuronal polarity. Nat Neurosci. 2006;9(8):993–995. doi: 10.1038/nn1742. [DOI] [PubMed] [Google Scholar]
- 112.Funahashi Y, Namba T, Fujisue S, Itoh N, Nakamuta S, Kato K, Shimada A, Xu C, Shan W, Nishioka T, Kaibuchi K. ERK2-mediated phosphorylation of Par3 regulates neuronal polarization. J Neurosci. 2013;33(33):13270–13285. doi: 10.1523/JNEUROSCI.4210-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vizard TN, Newton M, Howard L, Wyatt S, Davies AM. ERK signaling mediates CaSR-promoted axon growth. Neurosci Lett. 2015;603:77–83. doi: 10.1016/j.neulet.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shinoda T, Taya S, Tsuboi D, Hikita T, Matsuzawa R, Kuroda S, Iwamatsu A, Kaibuchi K. DISC1 regulates neurotrophin-induced axon elongation via interaction with Grb2. J Neurosci. 2007;27(1):4–14. doi: 10.1523/JNEUROSCI.3825-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xing L, Larsen RS, Bjorklund GR, Li X, Wu Y, Philpot BD, Snider WD, Newbern JM. Layer specific and general requirements for ERK/MAPK signaling in the developing neocortex. Elife. 2016 doi: 10.7554/eLife.11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coffey ET, Hongisto V, Dickens M, Davis RJ, Courtney MJ. Dual roles for c-Jun N-terminal kinase in developmental and stress responses in cerebellar granule neurons. J Neurosci. 2000;20(20):7602–7613. doi: 10.1523/JNEUROSCI.20-20-07602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oliva AA, Jr, Atkins CM, Copenagle L, Banker GA. Activated c-Jun N-terminal kinase is required for axon formation. J Neurosci. 2006;26(37):9462–9470. doi: 10.1523/JNEUROSCI.2625-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yi JJ, Barnes AP, Hand R, Polleux F, Ehlers MD. TGF-beta signaling specifies axons during brain development. Cell. 2010;142(1):144–157. doi: 10.1016/j.cell.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990;61(1):157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- 120.Felsenfeld DP, Hynes MA, Skoler KM, Furley AJ, Jessell TM. TAG-1 can mediate homophilic binding, but neurite outgrowth on TAG-1 requires an L1-like molecule and beta 1 integrins. Neuron. 1994;12(3):675–690. doi: 10.1016/0896-6273(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 121.Denaxa M, Chan CH, Schachner M, Parnavelas JG, Karagogeos D. The adhesion molecule TAG-1 mediates the migration of cortical interneurons from the ganglionic eminence along the corticofugal fiber system. Development. 2001;128(22):4635–4644. doi: 10.1242/dev.128.22.4635. [DOI] [PubMed] [Google Scholar]
- 122.Denaxa M, Kyriakopoulou K, Theodorakis K, Trichas G, Vidaki M, Takeda Y, Watanabe K, Karagogeos D. The adhesion molecule TAG-1 is required for proper migration of the superficial migratory stream in the medulla but not of cortical interneurons. Dev Biol. 2005;288(1):87–99. doi: 10.1016/j.ydbio.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 123.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307(5715):1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 124.Carrella S, Barbato S, D’Agostino Y, Salierno FG, Manfredi A, Banfi S, Conte I. TGF-beta controls miR-181/ERK regulatory network during retinal axon specification and growth. PLoS One. 2015;10(12):e0144129. doi: 10.1371/journal.pone.0144129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carrella S, D’Agostino Y, Barbato S, Huber-Reggi SP, Salierno FG, Manfredi A, Neuhauss SC, Banfi S, Conte I. miR-181a/b control the assembly of visual circuitry by regulating retinal axon specification and growth. Dev Neurobiol. 2015;75(11):1252–1267. doi: 10.1002/dneu.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matenia D, Mandelkow EM. The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem Sci. 2009;34(7):332–342. doi: 10.1016/j.tibs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 127.Reiner O, Sapir T. Mark/Par-1 marking the polarity of migrating neurons. Adv Exp Med Biol. 2014;800:97–111. doi: 10.1007/978-94-007-7687-6_6. [DOI] [PubMed] [Google Scholar]
- 128.Yoshimura Y, Terabayashi T, Miki H. Par1b/MARK2 phosphorylates kinesin-like motor protein GAKIN/KIF13B to regulate axon formation. Mol Cell Biol. 2010;30(9):2206–2219. doi: 10.1128/MCB.01181-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Timm T, von Kries JP, Li X, Zempel H, Mandelkow E, Mandelkow EM. Microtubule affinity regulating kinase activity in living neurons was examined by a genetically encoded fluorescence resonance energy transfer/fluorescence lifetime imaging-based biosensor: inhibitors with therapeutic potential. J Biol Chem. 2011;286(48):41711–41722. doi: 10.1074/jbc.M111.257865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Watkins JL, Lewandowski KT, Meek SE, Storz P, Toker A, Piwnica-Worms H. Phosphorylation of the Par-1 polarity kinase by protein kinase D regulates 14-3-3 binding and membrane association. Proc Natl Acad Sci USA. 2008;105(47):18378–18383. doi: 10.1073/pnas.0809661105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23(4):833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129(3):549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 133.Veleva-Rotse BO, Smart JL, Baas AF, Edmonds B, Zhao ZM, Brown A, Klug LR, Hansen K, Reilly G, Gardner AP, Subbiah K, Gaucher EA, Clevers H, Barnes AP. STRAD pseudokinases regulate axogenesis and LKB1 stability. Neural Dev. 2014;9:5. doi: 10.1186/1749-8104-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xie Z, Dong Y, Zhang J, Scholz R, Neumann D, Zou MH. Identification of the serine 307 of LKB1 as a novel phosphorylation site essential for its nucleocytoplasmic transport and endothelial cell angiogenesis. Mol Cell Biol. 2009;29(13):3582–3596. doi: 10.1128/MCB.01417-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu H, Moriasi CM, Zhang M, Zhao Y, Zou MH. Phosphorylation of serine 399 in LKB1 protein short form by protein kinase Czeta is required for its nucleocytoplasmic transport and consequent AMP-activated protein kinase (AMPK) activation. J Biol Chem. 2013;288(23):16495–16505. doi: 10.1074/jbc.M112.443580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim JS, Hung W, Zhen M. The long and the short of SAD-1 kinase. Commun Integr Biol. 2010;3(3):251–255. doi: 10.4161/cib.3.3.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crump JG, Zhen M, Jin Y, Bargmann CI. The SAD-1 kinase regulates presynaptic vesicle clustering and axon termination. Neuron. 2001;29(1):115–129. doi: 10.1016/S0896-6273(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 138.Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307(5711):929–932. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- 139.Lilley BN, Krishnaswamy A, Wang Z, Kishi M, Frank E, Sanes JR. SAD kinases control the maturation of nerve terminals in the mammalian peripheral and central nervous systems. Proc Natl Acad Sci USA. 2014;111(3):1138–1143. doi: 10.1073/pnas.1321990111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Williams T, Courchet J, Viollet B, Brenman JE, Polleux F. AMP-activated protein kinase (AMPK) activity is not required for neuronal development but regulates axogenesis during metabolic stress. Proc Natl Acad Sci USA. 2011;108(14):5849–5854. doi: 10.1073/pnas.1013660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Amato S, Man HY. AMPK links cellular bioenergy status to the decision making of axon initiation in neurons. Cell Logist. 2011;1(3):103–105. doi: 10.4161/cl.1.3.16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi YJ, Di Nardo A, Kramvis I, Meikle L, Kwiatkowski DJ, Sahin M, He X. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008;22(18):2485–2495. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27(21):5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang R, Kong E, Jin J, Hergovich A, Puschel AW. Rassf5 and Ndr kinases regulate neuronal polarity through Par3 phosphorylation in a novel pathway. J Cell Sci. 2014;127(Pt 16):3463–3476. doi: 10.1242/jcs.146696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matsuki T, Matthews RT, Cooper JA, van der Brug MP, Cookson MR, Hardy JA, Olson EC, Howell BW. Reelin and stk25 have opposing roles in neuronal polarization and dendritic Golgi deployment. Cell. 2010;143(5):826–836. doi: 10.1016/j.cell.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Feldner A, Adam MG, Tetzlaff F, Moll I, Komljenovic D, Sahm F, Bauerle T, Ishikawa H, Schroten H, Korff T, Hofmann I, Wolburg H, von Deimling A, Fischer A. Loss of Mpdz impairs ependymal cell integrity leading to perinatal-onset hydrocephalus in mice. EMBO Mol Med. 2017 doi: 10.15252/emmm.201606430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pichaud F, Desplan C. Cell biology: a new view of photoreceptors. Nature. 2002;416(6877):139–140. doi: 10.1038/416139a. [DOI] [PubMed] [Google Scholar]
- 148.Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53(2):217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- 149.Zolessi FR, Poggi L, Wilkinson CJ, Chien CB, Harris WA. Polarization and orientation of retinal ganglion cells in vivo. Neural Dev. 2006;1:2. doi: 10.1186/1749-8104-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Randlett O, Norden C, Harris WA. The vertebrate retina: a model for neuronal polarization in vivo. Dev Neurobiol. 2011;71(6):567–583. doi: 10.1002/dneu.20841. [DOI] [PubMed] [Google Scholar]
- 151.Randlett O, Poggi L, Zolessi FR, Harris WA. The oriented emergence of axons from retinal ganglion cells is directed by laminin contact in vivo. Neuron. 2011;70(2):266–280. doi: 10.1016/j.neuron.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Namba T, Funahashi Y, Nakamuta S, Xu C, Takano T, Kaibuchi K. Extracellular and intracellular signaling for neuronal polarity. Physiol Rev. 2015;95(3):995–1024. doi: 10.1152/physrev.00025.2014. [DOI] [PubMed] [Google Scholar]
- 154.Hong Y, Ackerman L, Jan LY, Jan YN. Distinct roles of Bazooka and Stardust in the specification of Drosophila photoreceptor membrane architecture. Proc Natl Acad Sci USA. 2003;100(22):12712–12717. doi: 10.1073/pnas.2135347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Longley RL, Jr, Ready DF. Integrins and the development of three-dimensional structure in the Drosophila compound eye. Dev Biol. 1995;171(2):415–433. doi: 10.1006/dbio.1995.1292. [DOI] [PubMed] [Google Scholar]
- 156.Robertson F, Pinal N, Fichelson P, Pichaud F. Atonal and EGFR signalling orchestrate rok- and Drak-dependent adherens junction remodelling during ommatidia morphogenesis. Development. 2012;139(18):3432–3441. doi: 10.1242/dev.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991;113(3):841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- 158.Coopman P, Djiane A. Adherens Junction and E-Cadherin complex regulation by epithelial polarity. Cell Mol Life Sci. 2016;73(18):3535–3553. doi: 10.1007/s00018-016-2260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416(6877):178–183. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- 160.Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, Ready DF, Tepass U. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416(6877):143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- 161.Nam SC, Choi KW. Interaction of Par-6 and Crumbs complexes is essential for photoreceptor morphogenesis in Drosophila. Development. 2003;130(18):4363–4372. doi: 10.1242/dev.00648. [DOI] [PubMed] [Google Scholar]
- 162.Walther RF, Pichaud F. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr Biol. 2010;20(12):1065–1074. doi: 10.1016/j.cub.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 163.Bachmann A, Schneider M, Theilenberg E, Grawe F, Knust E. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 2001;414(6864):638–643. doi: 10.1038/414638a. [DOI] [PubMed] [Google Scholar]
- 164.Hong Y, Stronach B, Perrimon N, Jan LY, Jan YN. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 2001;414(6864):634–638. doi: 10.1038/414634a. [DOI] [PubMed] [Google Scholar]
- 165.Nam SC, Choi KW. Domain-specific early and late function of Dpatj in Drosophila photoreceptor cells. Dev Dyn. 2006;235(6):1501–1507. doi: 10.1002/dvdy.20726. [DOI] [PubMed] [Google Scholar]
- 166.Richard M, Grawe F, Knust E. DPATJ plays a role in retinal morphogenesis and protects against light-dependent degeneration of photoreceptor cells in the Drosophila eye. Dev Dyn. 2006;235(4):895–907. doi: 10.1002/dvdy.20595. [DOI] [PubMed] [Google Scholar]
- 167.van de Pavert SA, Kantardzhieva A, Malysheva A, Meuleman J, Versteeg I, Levelt C, Klooster J, Geiger S, Seeliger MW, Rashbass P, Le Bivic A, Wijnholds J. Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J Cell Sci. 2004;117(Pt 18):4169–4177. doi: 10.1242/jcs.01301. [DOI] [PubMed] [Google Scholar]
- 168.Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439(7076):594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- 169.Zhu J, Shang Y, Wan Q, Xia Y, Chen J, Du Q, Zhang M. Phosphorylation-dependent interaction between tumor suppressors Dlg and Lgl. Cell Res. 2014;24(4):451–463. doi: 10.1038/cr.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Albertson R, Chabu C, Sheehan A, Doe CQ. Scribble protein domain mapping reveals a multistep localization mechanism and domains necessary for establishing cortical polarity. J Cell Sci. 2004;117(Pt 25):6061–6070. doi: 10.1242/jcs.01525. [DOI] [PubMed] [Google Scholar]
- 171.Zeitler J, Hsu CP, Dionne H, Bilder D. Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J Cell Biol. 2004;167(6):1137–1146. doi: 10.1083/jcb.200407158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Elsum I, Yates L, Humbert PO, Richardson HE. The Scribble-Dlg-Lgl polarity module in development and cancer: from flies to man. Essays Biochem. 2012;53:141–168. doi: 10.1042/bse0530141. [DOI] [PubMed] [Google Scholar]
- 173.Boeda B, Etienne-Manneville S. Spectrin binding motifs regulate Scribble cortical dynamics and polarity function. Elife. 2015 doi: 10.7554/eLife.04726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lecine P, Bellaiche Y, Dupont JL, Premont RT, Sempere C, Strub JM, Van Dorsselaer A, Vitale N, Borg JP. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol. 2004;14(11):987–995. doi: 10.1016/j.cub.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 175.Sun SD, Purdy AM, Walsh GS. Planar cell polarity genes Frizzled3a, Vangl2, and Scribble are required for spinal commissural axon guidance. BMC Neurosci. 2016;17(1):83. doi: 10.1186/s12868-016-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sun Y, Aiga M, Yoshida E, Humbert PO, Bamji SX. Scribble interacts with beta-catenin to localize synaptic vesicles to synapses. Mol Biol Cell. 2009;20(14):3390–3400. doi: 10.1091/mbc.E08-12-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18(5):559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hattendorf DA, Andreeva A, Gangar A, Brennwald PJ, Weis WI. Structure of the yeast polarity protein Sro7 reveals a SNARE regulatory mechanism. Nature. 2007;446(7135):567–571. doi: 10.1038/nature05635. [DOI] [PubMed] [Google Scholar]
- 179.Dong W, Zhang X, Liu W, Chen YJ, Huang J, Austin E, Celotto AM, Jiang WZ, Palladino MJ, Jiang Y, Hammond GR, Hong Y. A conserved polybasic domain mediates plasma membrane targeting of Lgl and its regulation by hypoxia. J Cell Biol. 2015;211(2):273–286. doi: 10.1083/jcb.201503067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang T, Liu Y, Xu XH, Deng CY, Wu KY, Zhu J, Fu XQ, He M, Luo ZG. Lgl1 activation of rab10 promotes axonal membrane trafficking underlying neuronal polarization. Dev Cell. 2011;21(3):431–444. doi: 10.1016/j.devcel.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 181.Choi KW, Nam SC, Mukhopadhyay B. Par-1 and PP2A: Yin-Yang of Bazooka localization. Fly (Austin) 2007;1(4):235–237. doi: 10.4161/fly.4954. [DOI] [PubMed] [Google Scholar]
- 182.Iden S, Misselwitz S, Peddibhotla SS, Tuncay H, Rehder D, Gerke V, Robenek H, Suzuki A, Ebnet K. aPKC phosphorylates JAM-A at Ser285 to promote cell contact maturation and tight junction formation. J Cell Biol. 2012;196(5):623–639. doi: 10.1083/jcb.201104143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, 3rd, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158(5):967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ogawa H, Ohta N, Moon W, Matsuzaki F. Protein phosphatase 2A negatively regulates aPKC signaling by modulating phosphorylation of Par-6 in Drosophila neuroblast asymmetric divisions. J Cell Sci. 2009;122(Pt 18):3242–3249. doi: 10.1242/jcs.050955. [DOI] [PubMed] [Google Scholar]
- 185.Zhang P, Wang S, Wang S, Qiao J, Zhang L, Zhang Z, Chen Z. Dual function of partitioning-defective 3 in the regulation of YAP phosphorylation and activation. Cell Discov. 2016;2:16021. doi: 10.1038/celldisc.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Poon CL, Mitchell KA, Kondo S, Cheng LY, Harvey KF. The Hippo pathway regulates neuroblasts and brain size in Drosophila melanogaster. Curr Biol. 2016;26(8):1034–1042. doi: 10.1016/j.cub.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 187.Kawamori H, Tai M, Sato M, Yasugi T, Tabata T. Fat/Hippo pathway regulates the progress of neural differentiation signaling in the Drosophila optic lobe. Dev Growth Differ. 2011;53(5):653–667. doi: 10.1111/j.1440-169X.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- 188.Emoto K. The growing role of the Hippo–NDR kinase signalling in neuronal development and disease. J Biochem. 2011;150(2):133–141. doi: 10.1093/jb/mvr080. [DOI] [PubMed] [Google Scholar]
- 189.Kwan J, Sczaniecka A, Heidary Arash E, Nguyen L, Chen CC, Ratkovic S, Klezovitch O, Attisano L, McNeill H, Emili A, Vasioukhin V. DLG5 connects cell polarity and Hippo signaling protein networks by linking PAR-1 with MST1/2. Genes Dev. 2016;30(24):2696–2709. doi: 10.1101/gad.284539.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, Colwill K, Starostine A, Metalnikov P, Pawson T. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 2006;125(3):535–548. doi: 10.1016/j.cell.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 191.Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, Guan KL. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25(1):51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wigerius M, Quinn D, Diab A, Clattenburg L, Kolar A, Qi J, Krueger SR, Fawcett JP. The polarity protein Angiomotin p130 controls dendritic spine maturation. J Cell Biol. 2018;217(2):715–730. doi: 10.1083/jcb.201705184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Traweger A, Wiggin G, Taylor L, Tate SA, Metalnikov P, Pawson T. Protein phosphatase 1 regulates the phosphorylation state of the polarity scaffold Par-3. Proc Natl Acad Sci USA. 2008;105(30):10402–10407. doi: 10.1073/pnas.0804102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lyu J, Kim HR, Yamamoto V, Choi SH, Wei Z, Joo CK, Lu W. Protein phosphatase 4 and Smek complex negatively regulate Par3 and promote neuronal differentiation of neural stem/progenitor cells. Cell Rep. 2013;5(3):593–600. doi: 10.1016/j.celrep.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hernandez F, Langa E, Cuadros R, Avila J, Villanueva N. Regulation of GSK3 isoforms by phosphatases PP1 and PP2A. Mol Cell Biochem. 2010;344(1–2):211–215. doi: 10.1007/s11010-010-0544-0. [DOI] [PubMed] [Google Scholar]
- 196.Joseph BK, Liu HY, Francisco J, Pandya D, Donigan M, Gallo-Ebert C, Giordano C, Bata A, Nickels JT., Jr Inhibition of AMP kinase by the protein phosphatase 2A heterotrimer, PP2APpp2r2d. J Biol Chem. 2015;290(17):10588–10598. doi: 10.1074/jbc.M114.626259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Diaz-Meco MT, Lozano J, Municio MM, Berra E, Frutos S, Sanz L, Moscat J. Evidence for the in vitro and in vivo interaction of Ras with protein kinase C zeta. J Biol Chem. 1994;269(50):31706–31710. [PubMed] [Google Scholar]
- 198.Uberall F, Hellbert K, Kampfer S, Maly K, Villunger A, Spitaler M, Mwanjewe J, Baier-Bitterlich G, Baier G, Grunicke HH. Evidence that atypical protein kinase C-lambda and atypical protein kinase C-zeta participate in Ras-mediated reorganization of the F-actin cytoskeleton. J Cell Biol. 1999;144(3):413–425. doi: 10.1083/jcb.144.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Choi W, Harris NJ, Sumigray KD, Peifer M. Rap1 and Canoe/afadin are essential for establishment of apical-basal polarity in the Drosophila embryo. Mol Biol Cell. 2013;24(7):945–963. doi: 10.1091/mbc.E12-10-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fu Z, Lee SH, Simonetta A, Hansen J, Sheng M, Pak DT. Differential roles of Rap1 and Rap2 small GTPases in neurite retraction and synapse elimination in hippocampal spiny neurons. J Neurochem. 2007;100(1):118–131. doi: 10.1111/j.1471-4159.2006.04195.x. [DOI] [PubMed] [Google Scholar]
- 201.Tawa H, Rikitake Y, Takahashi M, Amano H, Miyata M, Satomi-Kobayashi S, Kinugasa M, Nagamatsu Y, Majima T, Ogita H, Miyoshi J, Hirata K, Takai Y. Role of afadin in vascular endothelial growth factor- and sphingosine 1-phosphate-induced angiogenesis. Circ Res. 2010;106(11):1731–1742. doi: 10.1161/CIRCRESAHA.110.216747. [DOI] [PubMed] [Google Scholar]
- 202.Birukova AA, Fu P, Wu T, Dubrovskyi O, Sarich N, Poroyko V, Birukov KG. Afadin controls p120-catenin-ZO-1 interactions leading to endothelial barrier enhancement by oxidized phospholipids. J Cell Physiol. 2012;227(5):1883–1890. doi: 10.1002/jcp.22916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Beaudoin GM, 3rd, Schofield CM, Nuwal T, Zang K, Ullian EM, Huang B, Reichardt LF. Afadin, a Ras/Rap effector that controls cadherin function, promotes spine and excitatory synapse density in the hippocampus. J Neurosci. 2012;32(1):99–110. doi: 10.1523/JNEUROSCI.4565-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Miyata M, Maruo T, Kaito A, Wang S, Yamamoto H, Fujiwara T, Mizoguchi A, Mandai K, Takai Y. Roles of afadin in the formation of the cellular architecture of the mouse hippocampus and dentate gyrus. Mol Cell Neurosci. 2016;79:34–44. doi: 10.1016/j.mcn.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 205.Toyoshima D, Mandai K, Maruo T, Supriyanto I, Togashi H, Inoue T, Mori M, Takai Y. Afadin regulates puncta adherentia junction formation and presynaptic differentiation in hippocampal neurons. PLoS One. 2014;9(2):e89763. doi: 10.1371/journal.pone.0089763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116(Pt 1):17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- 207.Iwasawa N, Negishi M, Oinuma I. R-Ras controls axon branching through afadin in cortical neurons. Mol Biol Cell. 2012;23(14):2793–2804. doi: 10.1091/mbc.E12-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Oinuma I, Katoh H, Negishi M. R-Ras controls axon specification upstream of glycogen synthase kinase-3beta through integrin-linked kinase. J Biol Chem. 2007;282(1):303–318. doi: 10.1074/jbc.M607979200. [DOI] [PubMed] [Google Scholar]
- 209.Lalli G. RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J Cell Sci. 2009;122(Pt 10):1499–1506. doi: 10.1242/jcs.044339. [DOI] [PubMed] [Google Scholar]
- 210.Lalli G, Hall A. Ral GTPases regulate neurite branching through GAP-43 and the exocyst complex. J Cell Biol. 2005;171(5):857–869. doi: 10.1083/jcb.200507061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rudolph JL, Shi GX, Erdogan E, Fields AP, Andres DA. Rit mutants confirm role of MEK/ERK signaling in neuronal differentiation and reveal novel Par6 interaction. Biochim Biophys Acta. 2007;1773(12):1793–1800. doi: 10.1016/j.bbamcr.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hoshino M, Yoshimori T, Nakamura S. Small GTPase proteins Rin and Rit Bind to PAR6 GTP-dependently and regulate cell transformation. J Biol Chem. 2005;280(24):22868–22874. doi: 10.1074/jbc.M411592200. [DOI] [PubMed] [Google Scholar]
- 213.Lein PJ, Guo X, Shi GX, Moholt-Siebert M, Bruun D, Andres DA. The novel GTPase Rit differentially regulates axonal and dendritic growth. J Neurosci. 2007;27(17):4725–4736. doi: 10.1523/JNEUROSCI.5633-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19(3):625–634. doi: 10.1016/S0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 215.Ruchhoeft ML, Ohnuma S, McNeill L, Holt CE, Harris WA. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J Neurosci. 1999;19(19):8454–8463. doi: 10.1523/JNEUROSCI.19-19-08454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2(8):531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 217.Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, Ohno S. PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells. 2001;6(8):721–731. doi: 10.1046/j.1365-2443.2001.00453.x. [DOI] [PubMed] [Google Scholar]
- 218.Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol. 2001;152(6):1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2(8):540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 220.Graybill C, Wee B, Atwood SX, Prehoda KE. Partitioning-defective protein 6 (Par-6) activates atypical protein kinase C (aPKC) by pseudosubstrate displacement. J Biol Chem. 2012;287(25):21003–21011. doi: 10.1074/jbc.M112.360495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Atwood SX, Chabu C, Penkert RR, Doe CQ, Prehoda KE. Cdc42 acts downstream of Bazooka to regulate neuroblast polarity through Par-6 aPKC. J Cell Sci. 2007;120(Pt 18):3200–3206. doi: 10.1242/jcs.014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu XF, Ishida H, Raziuddin R, Miki T. Nucleotide exchange factor ECT2 interacts with the polarity protein complex Par6/Par3/protein kinase Czeta (PKCzeta) and regulates PKCzeta activity. Mol Cell Biol. 2004;24(15):6665–6675. doi: 10.1128/MCB.24.15.6665-6675.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kunda P, Paglini G, Quiroga S, Kosik K, Caceres A. Evidence for the involvement of Tiam1 in axon formation. J Neurosci. 2001;21(7):2361–2372. doi: 10.1523/JNEUROSCI.21-07-02361.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7(3):262–269. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- 225.Matsuzawa K, Akita H, Watanabe T, Kakeno M, Matsui T, Wang S, Kaibuchi K. PAR3–aPKC regulates Tiam1 by modulating suppressive internal interactions. Mol Biol Cell. 2016;27(9):1511–1523. doi: 10.1091/mbc.E15-09-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kanzaki M, Mora S, Hwang JB, Saltiel AR, Pessin JE. Atypical protein kinase C (PKCzeta/lambda) is a convergent downstream target of the insulin-stimulated phosphatidylinositol 3-kinase and TC10 signaling pathways. J Cell Biol. 2004;164(2):279–290. doi: 10.1083/jcb.200306152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dupraz S, Grassi D, Bernis ME, Sosa L, Bisbal M, Gastaldi L, Jausoro I, Caceres A, Pfenninger KH, Quiroga S. The TC10-Exo70 complex is essential for membrane expansion and axonal specification in developing neurons. J Neurosci. 2009;29(42):13292–13301. doi: 10.1523/JNEUROSCI.3907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chen WS, Chen YJ, Huang YA, Hsieh BY, Chiu HC, Kao PY, Chao CY, Hwang E. Ran-dependent TPX2 activation promotes acentrosomal microtubule nucleation in neurons. Sci Rep. 2017;7:42297. doi: 10.1038/srep42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mori D, Yamada M, Mimori-Kiyosue Y, Shirai Y, Suzuki A, Ohno S, Saya H, Wynshaw-Boris A, Hirotsune S. An essential role of the aPKC-Aurora A-NDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat Cell Biol. 2009;11(9):1057–1068. doi: 10.1038/ncb1919. [DOI] [PubMed] [Google Scholar]
- 230.Yamada M, Hirotsune S, Wynshaw-Boris A. The essential role of LIS1, NDEL1 and Aurora-A in polarity formation and microtubule organization during neurogensis. Cell Adhes Migr. 2010;4(2):180–184. doi: 10.4161/cam.4.2.10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tisdale EJ. Rab2 interacts directly with atypical protein kinase C (aPKC) iota/lambda and inhibits aPKCiota/lambda-dependent glyceraldehyde-3-phosphate dehydrogenase phosphorylation. J Biol Chem. 2003;278(52):52524–52530. doi: 10.1074/jbc.M309343200. [DOI] [PubMed] [Google Scholar]
- 232.Ayala J, Touchot N, Zahraoui A, Tavitian A, Prochiantz A. The product of rab2, a small GTP binding protein, increases neuronal adhesion, and neurite growth in vitro. Neuron. 1990;4(5):797–805. doi: 10.1016/0896-6273(90)90206-U. [DOI] [PubMed] [Google Scholar]
- 233.Hyenne V, Tremblay-Boudreault T, Velmurugan R, Grant BD, Loerke D, Labbe JC. RAB-5 controls the cortical organization and dynamics of PAR proteins to maintain C. elegans early embryonic polarity. PLoS One. 2012;7(4):e35286. doi: 10.1371/journal.pone.0035286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Falk J, Konopacki FA, Zivraj KH, Holt CE. Rab5 and Rab4 regulate axon elongation in the Xenopus visual system. J Neurosci. 2014;34(2):373–391. doi: 10.1523/JNEUROSCI.0876-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mori Y, Matsui T, Fukuda M. Rabex-5 protein regulates dendritic localization of small GTPase Rab17 and neurite morphogenesis in hippocampal neurons. J Biol Chem. 2013;288(14):9835–9847. doi: 10.1074/jbc.M112.427591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mori Y, Fukuda M. Rabex-5 determines the neurite localization of its downstream Rab proteins in hippocampal neurons. Commun Integr Biol. 2013;6(5):e25433. doi: 10.4161/cib.25433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Elias S, McGuire JR, Yu H, Humbert S. Huntingtin is required for epithelial polarity through RAB11A-mediated apical trafficking of PAR3–aPKC. PLoS Biol. 2015;13(5):e1002142. doi: 10.1371/journal.pbio.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Molina-Calavita M, Barnat M, Elias S, Aparicio E, Piel M, Humbert S. Mutant huntingtin affects cortical progenitor cell division and development of the mouse neocortex. J Neurosci. 2014;34(30):10034–10040. doi: 10.1523/JNEUROSCI.0715-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Takano T, Tomomura M, Yoshioka N, Tsutsumi K, Terasawa Y, Saito T, Kawano H, Kamiguchi H, Fukuda M, Hisanaga S. LMTK1/AATYK1 is a novel regulator of axonal outgrowth that acts via Rab11 in a Cdk5-dependent manner. J Neurosci. 2012;32(19):6587–6599. doi: 10.1523/JNEUROSCI.5317-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shirane M, Nakayama KI. Protrudin induces neurite formation by directional membrane trafficking. Science. 2006;314(5800):818–821. doi: 10.1126/science.1134027. [DOI] [PubMed] [Google Scholar]
- 241.Calero-Cuenca FJ, Espinosa-Vazquez JM, Reina-Campos M, Diaz-Meco MT, Moscat J, Sotillos S. Nuclear fallout provides a new link between aPKC and polarized cell trafficking. BMC Biol. 2016;14:32. doi: 10.1186/s12915-016-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chang J, Lee S, Blackstone C. Protrudin binds atlastins and endoplasmic reticulum-shaping proteins and regulates network formation. Proc Natl Acad Sci USA. 2013;110(37):14954–14959. doi: 10.1073/pnas.1307391110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yan D, Guo L, Wang Y. Requirement of dendritic Akt degradation by the ubiquitin-proteasome system for neuronal polarity. J Cell Biol. 2006;174(3):415–424. doi: 10.1083/jcb.200511028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Drinjakovic J, Jung H, Campbell DS, Strochlic L, Dwivedy A, Holt CE. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron. 2010;65(3):341–357. doi: 10.1016/j.neuron.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cheng PL, Lu H, Shelly M, Gao H, Poo MM. Phosphorylation of E3 ligase Smurf1 switches its substrate preference in support of axon development. Neuron. 2011;69(2):231–243. doi: 10.1016/j.neuron.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 246.Shelly M, Lim BK, Cancedda L, Heilshorn SC, Gao H, Poo MM. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327(5965):547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- 247.Vyas P, Singh A, Murawala P, Joseph J. Nup358 interacts with Dishevelled and aPKC to regulate neuronal polarity. Biol Open. 2013;2(11):1270–1278. doi: 10.1242/bio.20135363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yadav SK, Magre I, Singh A, Khuperkar D, Joseph J. Regulation of aPKC activity by Nup358 dependent SUMO modification. Sci Rep. 2016;6:34100. doi: 10.1038/srep34100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Beirowski B, Gustin J, Armour SM, Yamamoto H, Viader A, North BJ, Michan S, Baloh RH, Golden JP, Schmidt RE, Sinclair DA, Auwerx J, Milbrandt J. Sir-two-homolog 2 (Sirt2) modulates peripheral myelination through polarity protein Par-3/atypical protein kinase C (aPKC) signaling. Proc Natl Acad Sci USA. 2011;108(43):E952–E961. doi: 10.1073/pnas.1104969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.von Stein W, Ramrath A, Grimm A, Muller-Borg M, Wodarz A. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development. 2005;132(7):1675–1686. doi: 10.1242/dev.01720. [DOI] [PubMed] [Google Scholar]
- 251.Feng W, Wu H, Chan LN, Zhang M. Par-3-mediated junctional localization of the lipid phosphatase PTEN is required for cell polarity establishment. J Biol Chem. 2008;283(34):23440–23449. doi: 10.1074/jbc.M802482200. [DOI] [PubMed] [Google Scholar]
- 252.Itoh N, Nakayama M, Nishimura T, Fujisue S, Nishioka T, Watanabe T, Kaibuchi K. Identification of focal adhesion kinase (FAK) and phosphatidylinositol 3-kinase (PI3-kinase) as Par3 partners by proteomic analysis. Cytoskeleton (Hoboken) 2010;67(5):297–308. doi: 10.1002/cm.20444. [DOI] [PubMed] [Google Scholar]
- 253.Krahn MP, Klopfenstein DR, Fischer N, Wodarz A. Membrane targeting of Bazooka/PAR-3 is mediated by direct binding to phosphoinositide lipids. Curr Biol. 2010;20(7):636–642. doi: 10.1016/j.cub.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 254.Xu G, Wang R, Wang Z, Lei Q, Yu Z, Liu C, Li P, Yang Z, Cheng X, Li G, Wu M. NGL-2 is a new partner of PAR complex in axon differentiation. J Neurosci. 2015;35(18):7153–7164. doi: 10.1523/JNEUROSCI.4726-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ruiz-Canada C, Ashley J, Moeckel-Cole S, Drier E, Yin J, Budnik V. New synaptic bouton formation is disrupted by misregulation of microtubule stability in aPKC mutants. Neuron. 2004;42(4):567–580. doi: 10.1016/S0896-6273(04)00255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tanabe K, Kani S, Shimizu T, Bae YK, Abe T, Hibi M. Atypical protein kinase C regulates primary dendrite specification of cerebellar Purkinje cells by localizing Golgi apparatus. J Neurosci. 2010;30(50):16983–16992. doi: 10.1523/JNEUROSCI.3352-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang H, Macara IG. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol. 2006;8(3):227–237. doi: 10.1038/ncb1368. [DOI] [PubMed] [Google Scholar]
- 258.Zhang H, Macara IG. The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev Cell. 2008;14(2):216–226. doi: 10.1016/j.devcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ron S, Dudai Y, Segal M. Overexpression of PKMzeta alters morphology and function of dendritic spines in cultured cortical neurons. Cereb Cortex. 2012;22(11):2519–2528. doi: 10.1093/cercor/bhr323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hu J, Adler K, Farah CA, Hastings MH, Sossin WS, Schacher S. Cell-specific PKM isoforms contribute to the maintenance of different forms of persistent long-term synaptic plasticity. J Neurosci. 2017;37(10):2746–2763. doi: 10.1523/JNEUROSCI.2805-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8(1):34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 262.Moreau MM, Piguel N, Papouin T, Koehl M, Durand CM, Rubio ME, Loll F, Richard EM, Mazzocco C, Racca C, Oliet SH, Abrous DN, Montcouquiol M, Sans N. The planar polarity protein Scribble1 is essential for neuronal plasticity and brain function. J Neurosci. 2010;30(29):9738–9752. doi: 10.1523/JNEUROSCI.6007-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 2008;13:6693–6707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- 264.Lahey T, Gorczyca M, Jia XX, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13(4):823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17(4):627–640. doi: 10.1016/S0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Guan B, Hartmann B, Kho YH, Gorczyca M, Budnik V. The Drosophila tumor suppressor gene, dlg, is involved in structural plasticity at a glutamatergic synapse. Curr Biol. 1996;6(6):695–706. doi: 10.1016/S0960-9822(09)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zhou Z, Guo Y, Liu Y, Zhang F, Wang Y, Shen B, Qin Y, Qiu J. Methylation-mediated silencing of Dlg5 facilitates bladder cancer metastasis. Exp Cell Res. 2015;331(2):399–407. doi: 10.1016/j.yexcr.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 268.Tao YX, Rumbaugh G, Wang GD, Petralia RS, Zhao C, Kauer FW, Tao F, Zhuo M, Wenthold RJ, Raja SN, Huganir RL, Bredt DS, Johns RA. Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein. J Neurosci. 2003;23(17):6703–6712. doi: 10.1523/JNEUROSCI.23-17-06703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cuthbert PC, Stanford LE, Coba MP, Ainge JA, Fink AE, Opazo P, Delgado JY, Komiyama NH, O’Dell TJ, Grant SG. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci. 2007;27(10):2673–2682. doi: 10.1523/JNEUROSCI.4457-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396(6710):433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 271.Wang SH, Celic I, Choi SY, Riccomagno M, Wang Q, Sun LO, Mitchell SP, Vasioukhin V, Huganir RL, Kolodkin AL. Dlg5 regulates dendritic spine formation and synaptogenesis by controlling subcellular N-cadherin localization. J Neurosci. 2014;34(38):12745–12761. doi: 10.1523/JNEUROSCI.1280-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Saito Y, Oinuma I, Fujimoto S, Negishi M. Plexin-B1 is a GTPase activating protein for M-Ras, remodelling dendrite morphology. EMBO Rep. 2009;10(6):614–621. doi: 10.1038/embor.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25(2):307–316. doi: 10.1016/S0896-6273(00)80896-X. [DOI] [PubMed] [Google Scholar]
- 274.Li Z, Van Aelst L, Cline HT. Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nat Neurosci. 2000;3(3):217–225. doi: 10.1038/72920. [DOI] [PubMed] [Google Scholar]
- 275.Wong WT, Faulkner-Jones BE, Sanes JR, Wong RO. Rapid dendritic remodeling in the developing retina: dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20(13):5024–5036. doi: 10.1523/JNEUROSCI.20-13-05024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20(14):5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Geppert M, Goda Y, Stevens CF, Sudhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387(6635):810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- 278.Huber LA, de Hoop MJ, Dupree P, Zerial M, Simons K, Dotti C. Protein transport to the dendritic plasma membrane of cultured neurons is regulated by rab8p. J Cell Biol. 1993;123(1):47–55. doi: 10.1083/jcb.123.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zou W, Yadav S, DeVault L, Nung Jan Y, Sherwood DR. RAB-10-dependent membrane transport is required for dendrite arborization. PLoS Genet. 2015;11(9):e1005484. doi: 10.1371/journal.pgen.1005484. [DOI] [PMC free article] [PubMed] [Google Scholar]