Abstract
Cancers of the reproductive organs have a strong association with mitochondrial defects, and a deeper understanding of the role of this organelle in preneoplastic–neoplastic changes is important to determine the appropriate therapeutic intervention. Mitochondria are involved in events during cancer development, including metabolic and oxidative status, acquisition of metastatic potential, resistance to chemotherapy, apoptosis, and others. Because of their origin from melatonin-producing bacteria, mitochondria are speculated to produce melatonin and its derivatives at high levels; in addition, exogenously administered melatonin accumulates in the mitochondria against a concentration gradient. Melatonin is transported into tumor cell by GLUT/SLC2A and/or by the PEPT1/2 transporters, and plays beneficial roles in mitochondrial homeostasis, such as influencing oxidative phosphorylation and electron flux, ATP synthesis, bioenergetics, calcium influx, and mitochondrial permeability transition pore. Moreover, melatonin promotes mitochondrial homeostasis by regulating nuclear DNA and mtDNA transcriptional activities. This review focuses on the main functions of melatonin on mitochondrial processes, and reviews from a mechanistic standpoint, how mitochondrial crosstalk evolved in ovarian, endometrial, cervical, breast, and prostate cancers relative to melatonin’s known actions. We put emphasis on signaling pathways whereby melatonin interferes within cancer-cell mitochondria after its administration. Depending on subtype and intratumor metabolic heterogeneity, melatonin seems to be helpful in promoting apoptosis, anti-proliferation, pro-oxidation, metabolic shifting, inhibiting neovasculogenesis and controlling inflammation, and restoration of chemosensitivity. This results in attenuation of development, progression, and metastatic potential of reproductive cancers, in addition to lowering the risk of recurrence and improving the life quality of patients.
Keywords: Melatonin, Mitochondrial function, Ovarian cancer, Breast cancer, Endometrial cancer, Cervical cancer, Prostate cancer
Introduction: connecting melatonin, mitochondrial functions, and cancer
Overview of reproductive cancers: the starting point for mitochondria–melatonin relationship
The most common cancers that affect the reproductive system include the ovarian, cervical, endometrial, breast, and prostate cancers. Because women are often unaware of the development and symptoms associated with these female-related cancers, high rates of morbidity and mortality occur worldwide [1]. Therapies are still limited and many women relapse with a more aggressive cancer phenotype after acquisition of drug resistance [2]. For male reproductive cancers, prostate cancer has the highest incidence in elderly men. There are other common clinical features of prostate cancer, but age is considered to be a key element in terms of diagnosis and treatment decisions. To choose the proper intervention, tumors need to be evaluated to determine whether patients are at high or low risk for disease progression and invasion at the time of diagnosis [3]. For these cancers, there are vast numbers of regulatory factors responsible for cell proliferation and death and for modulating tumor microenvironment; most notably, these factors can act intrinsically or extrinsically to influence mitochondrial activity.
Mitochondrial dysfunction is known to offer survival advantages to these cancer cells by inducing resistance to apoptosis as a result of TP53 gene mutation, alterations in the proapoptotic factors (e.g., inhibition of Bax translocation), and reduced activities of the apoptotic-related gene products [4]. These changes contribute to a mitochondrial hyperfusion state following dynamin-related protein 1 (Drp1) dephosphorylation and inhibition of optic atrophy 1 (Opa1) processing. In addition to apoptosis resistance, a growing number of convincing data point to dysregulated mitochondrial dynamics as being a determinant factor of chemoresistance in gynecologic cancers [5–7]. To guide new approaches in this oncomolecular area, future studies involving the control of chemosensitivity and specific targets of mitochondria are expected. In this regard, the use of functional agents (e.g., melatonin) with antitumor activities represents additional opportunity for personalized therapies. Over the course of time, a plethora of evidence showed that melatonin is involved in complex signaling pathways and biological functions, which influence cancer at many levels, some of which will be discussed in this paper. Taken together, this evidence encourages some clinical trials, using melatonin as adjuvant or protective therapy. For instance, melatonin has been used as adjuvant therapy to increase the patients’ expectancy and quality of life, mostly enhancing appetite, body weight, and survival while improving tumor remission, in addition to attenuating the adverse effects of chemo and radiotherapy in a variety of cancers [8–15].
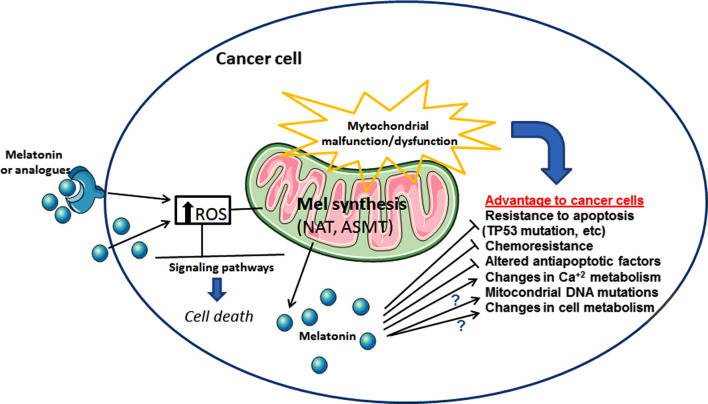
Melatonin and mitochondria have a documented ancient and well-established involvement [16], and its crosstalk is finely orchestrated depending on the cell status: melatonin protects mitochondria of healthy cells but does not have the same effect on cancer cells. Within the antioxidant context, melatonin and its derivatives interfere with mitochondrial processes through different systems and specific signaling pathways. The pharmacological significance of melatonin in regulating mitochondrial functions in cancer includes its ability to regulate the mitochondrial respiratory chain, thus reducing the highly glycolytic state of cells. Together with modulation of Ca2+ release, melatonin activates mitochondrial apoptotic effectors and enhances drug sensitization [17]. Based on the fact that melatonin has no systemic toxicity and is well tolerated, it is argued that melatonin represents a new promising perspective in the treatment of reproductive cancers. How melatonin promotes its mitochondrial effectiveness in regard to female and male reproductive cancer cells is the main subject of the current review. Figure 1 summarizes the potential actions of melatonin and its analogs (e.g., kynuramines) on multiple processes associated with mitochondrial dysfunctions, which were previously described for reproductive cancer cells. These processes include pro-oxidation and apoptosis-related mechanisms, and signaling pathways involved in chemoresistance to standard therapies. Ionic disturbances, mutations in mitochondrial DNA and metabolic shifting are further processes that may be possibly affected by melatonin.
Fig. 1.
Mitochondrial dysfunction is often present in cancer cells and, as a consequence, it may support cancer development. For instance, exogenous melatonin or its analogs may either activate membrane melatonin receptors or its intrinsic synthesis in mitochondria (via precursors and related enzymes), thus showing important effects in ROS production and cell death. Moreover, high melatonin levels in tumor cells are linked to inhibition of apoptosis resistance and chemoresistance, and stimulation of Ca2+ signaling. Although still not completely defined, other mechanisms by which melatonin contribute to reduce the advantage of cancer cells over normal cells may include mitochondrial DNA mutations and changes in metabolic processes. ROS reactive oxygen species, TP53 gene for tumor suppressor protein p53, Ca2+ calcium, DNA deoxyribonucleic acid, NAT N-acetyltransferase, ASMT acetylserotonin methyltransferase, ? uncertain mechanisms
Mitochondria: oxidative stress, signalling pathways, and the Warburg effect
Mitochondria are indispensable organelles in eukaryotic cells, mainly responsible for synthesize adenosine triphosphate (ATP), an energy source that sustains most biological activities of the cell. Functionally, mitochondria are essential for cellular metabolism, and further contribute to calcium metabolism and apoptotic processes [18–20]. There is a bidirectional communication between mitochondria and the different organelles of the cell, allowing an important pathway for signal transduction [21]. Metabolites generated from mitochondrial respiration send signals to other compartments of the cell. For example, citrate is cleaved by ATP citrate lyase in the cytosol to form oxaloacetate and acetyl-CoA, the latter being the substrate of histone acetyl transferase, which modifies the histone tails influencing the epigenetic state [22].
During ATP synthesis, electrons are carried by electron transporters to finally bind to oxygen and form water. These events occur in the inner membrane of the mitochondria, termed electron transport chain (ETC), which includes a set of electron carriers distributed into four enzyme complexes: complex I (NADH ubiquinone reductase), complex II (succinate ubiquinone reductase), complex III (ubiquinol–cytochrome c reductase), and complex IV (cytochrome c oxidase) [23]. Eventually, some electrons evade the ETC to reduce partially oxygen and generate free radicals [19]. Superoxide anion (O2·−) is essential for cell signaling functions [24], and its elevation is associated with oxidative damage and cell/tissue injury. O2·− is dismutated by the enzyme superoxide dismutase to form hydrogen peroxide (H2O2), which rapidly diffuses to other parts of the cell and thereby causing oxidative stress. When H2O2 is catalyzed, the hydroxyl radical (HO·), which is the most reactive-free radical, is generated; as a result, high levels of HO· lead to severe damage to proteins, DNA, carbohydrates, and lipids [25, 26].
These molecules (O2·−, H2O2, HO·) and other oxygen-related derivatives are classified into reactive oxygen species (ROS), while those related to nitrogen, such as nitric oxide (NO·) and peroxynitrite (ONOO−) are recognized as reactive nitrogen species (RNS). Oxidative stress induced by these reactive species is implicated in the etiology of many diseases, including diabetes [27], cardiovascular diseases [28], neurodegenerative diseases, and cancer [29]. An important mitochondrial component that is susceptible to oxidative attack by ROS is mitochondrial DNA (mtDNA); this DNA has higher mutation rates than nuclear DNA [30]. Overproduction of ROS may also arise from mutated nuclear DNA and mtDNA followed by impaired synthesis and activities of mitochondrial respiratory chain. During cell evolution, new mechanisms and strategies were naturally developed to protect cellular homeostasis against the oxidative processes induced by ROS and RNS [31]. By inducing DNA oxidation and genomic instability, altering gene expression and signaling pathways, ROS are considered as pivotal players in cancer development [32]. Interestingly, cells displaying mutant mtDNA appear to be protected from apoptosis; this phenomenon is likely due to the fact that oxidative phosphorylation (OXPHOS) pathway machinery is associated with reduced sensitivity to apoptosis [33].
Although mitochondria are strongly related to apoptosis, they are also relevant to other types of cell death including autophagy, necrosis, necroptosis, and pyroptosis [32, 34, 35]. Mitochondria are involved in the control of the intrinsic pathway by modulating the release of proapoptotic factors (e.g., cytochrome c and caspase activators). One of the most challenging mechanisms in the carcinogenesis that hampers a more effective therapy against cell proliferation and invasiveness is the resistance to apoptosis and chemotherapy. In this context, a number of compounds have been proposed to counteract these conditions: one approach is the use of agents to increase the conductance of the permeability pore resulting in the rupture of membrane and release of proapoptotic factors [36]; another approach is the use of Bcl-2 homology domain 3 (BH3) mimetics to trigger apoptosis by antagonizing the antiapoptotic Bcl-2/Bcl-xL and facilitating channel formation mediated by Bcl-2-associated X protein (BAX) and Bcl-2 antagonist/killer protein (BAK) [36, 37].
With regard to the pathophysiological settings, mitochondria have been associated with cancer initiation and progression [36]. The “so-called” Warburg effect occurs in most cancer cells by which glycolysis is highly upregulated to compensate its low ATP production as compared to OXPHOS, and might indicate mitochondrial malfunction [38]. Recently, this concept was revisited: numerous cancers present an enhanced aerobic glycolysis even exhibiting normal functioning mitochondria, and in cancer cells, OXPHOS continues to produce ATP in similar amounts as in normal cells [39]. It seems that cancer cells may proliferate after using intermediates of the glycolysis for anabolism. For instance, phosphoenolpyruvate (PEP) is dephosphorylated by pyruvate kinase (PK) to pyruvate; cancer cells use the M2 isoform (PKM2) in contrast to M1 (PKM1) to finally divert the metabolites upstream of pyruvate for the synthesis of lipids, amino acids, and nucleic acids [40]. In cancer cells, the bioenergetic profile is shifted between glycolysis and OXPHOS depending on tumor stage, nutrient and oxygen availability, and the activity of oncogenes [41]. Therefore, the microenvironment with glucose deprivation stimulates mitochondrial biogenesis and the OXPHOS system [42], while a microenvironment with low oxygen levels (hypoxia) leads to shift from OXPHOS to glycolysis [43]. A well-established metabolic change based on serial waves of gene set activation has been proposed for tumor development [44]. The first wave is marked by a partial glycolytic Warburg phenotype. Due to cell proliferation and hypoxia, the second wave of mitochondrial reprogramming potentiates glycolysis and suppresses OXPHOS. The contrasting condition between high-energy requirements and low availability of nutrients leads to the third wave of gene expression to ensure cell survival via conversion of glutamine into glutamate, also known as glutaminolysis.
Reproductive cancers: a link to mitochondrial DNA defects
Alterations in mitochondrial function have been widely documented to contribute to the development of various cancers, including breast, ovarian, and endometrial cancers [45]. Some of these alterations are related to mitochondrial DNA (mtDNA) because of its vulnerability to be mutated compared to nuclear DNA. Recent studies identified genetic substitutions with strand bias (C to T and A to G transversions) as indicative of mitochondrial G errors [46]. Mitochondrial mutations represent a challenge in terms of physiological consequences since mitochondria are numerous and harbor many copies of mtDNA; while homoplasmic mutations exist in all mtDNA copies and are relatively simple to detect, the heteroplasmic mutations share both the wild-type and mutant mtDNA and require special approaches to be identified [45]. As mitochondria play a central role in malignant tumor progression, treatments targeting mtDNA limits tumorigenesis [47].
In breast cancer (BC), the mutations are predominantly found in the D-loop region and also in the 16S rRNA, ND2, and ATPase genes [48]. Other studies identified mutations in the ND1, ND4, ND5, cytochrome b, and cytochrome c oxidase II mRNA genes [49]. In ovarian cancer (OC), most of the somatic mutations obtained from patients are restricted to the following regions called “mutational hotspots”: D-loop, 12 S rRNA, 16S rRNA, and cytochrome b [50]. In cervical cancer (CC), mtDNA content and human papillomavirus (HPV) infection are strongly associated with prognosis; high mtDNA content plus 10398A polymorphism represent a poor prognosis and may influence the predisposition to HPV infection [51]. Also, polymorphisms in C150T [52] and haplogroup B2 [53] showed an increased risk of HPV infection and CC progression, while no association with mtDNA copy number was detected. Notably, haplogroup B2 polymorphism is related to alterations in two mtDNA genes, namely mitochondrial aspartic acid tRNA (MT-TD) and mitochondrial lysine tRNA (MT-TK). In endometrial cancer (EC), scattered regions of mtDNA mutations are found in 50% of the EC samples and include D-loop region, the 2 rRNA genes, and 12 S rRNA gene [54, 55]. Semczuk et al. [56] sequenced small regions containing the nucleotides (135–433, 2986–3301, 4981–5500, 10,390–10,700, 12,005–12,386), and part of the D-loop region, 16S rRNA, and MT-ND4L gene. Notably, they reported no mutations in endometrial hyperplasia and in ECs co-existing with hyperplasia. In prostate cancer (PCa), intra-glandular and inter-patient mtDNA copy number variation is common; according to Kalsbeek et al. [57], PCa with increased mtDNA content is associated with poor prognosis including higher disease stage (PT2 vs. PT3), extracapsular extension, and increased Gleason score. Total tRNAs showed 26 mutations per kb, and following the larger regions, D-loop remains the most frequently mutated region, accompanied by CO3, RNR2, CO2 and CO1. The encoded proteins are located in the OXPHOS complex IV which is well conserved. Other affected regions after gene length correction include ATP8, ND6, and ATP6 [58], suggesting a negative selection for mutations during tumor development. Recently, Creed et al. [59] showed that deletion of 3.4 kb mtDNA is a reliable predictor for clinically significant PCa in men with prostate serum antigen (PSA) levels in the gray zone (< 10 ng/mL).
As a target of mtDNA, melatonin is reported to restore mtDNA content in a variety of conditions. A study by Feng et al. [60] showed that melatonin attenuated autophagy and mtDNA copy number reduction in opiate-addictive mice treated with morphine. Curiously, inhibition of mtDNA T8993G mutation-induced mitochondrial complex V is related to alterations in cardiolipin and mitochondrial dynamics as targets for the apoptotic stimulus; melatonin stabilized cardiolipin to prevent its oxidation and rescued mitochondrial dynamics-associated NARP-induced pathologies [61]. In tumor cells, melatonin exhibits oncostatic actions that are not related to nascent DNA synthesis [62]; however, when melatonin is added to cultured J774 macrophages, the mitochondrial respiration is suppressed together with development of DNA single-strand breakage [63]. In a wide set of analyses, melatonin prevents mtDNA from oxidative degradation and promotes a reduction of mtDNA transcripts in liver, skeletal muscle, heart, and brain [64, 65]. By reducing mtDNA and mitochondrial protein damage and improving ETC activity, melatonin is essential for the maintenance of mitochondrial functions. However, melatonin’s role in influencing mtDNA of tumor cells needs further investigation.
Melatonin and mitochondria: the most precise interaction
Melatonin (N-acetyl-5-methoxytryptamine) is an indolamine produced by the pineal gland in a circadian manner [66, 67]. In addition to the pineal gland, melatonin is synthesized in most (perhaps all) organs and tissues, but does not follow a circadian rhythm of secretion [68]. Some actions of melatonin have long been described as being mediated by the MT1 and MT2 membrane receptors [69], or possibly through nuclear receptors (RZR/ROR) which belong to the family of nuclear transcription factors [70], or via other regulatory effects by binding to intracellular proteins. Expression of melatonin receptors has already been documented in all reproductive tissues [71–73], thus suggesting that physiological functions of melatonin are also receptor mediated in these organs.
Melatonin plays an essential role in governing reproductive function by regulating the hypothalamus–pituitary–gonadal axis, and when melatonin levels decrease with aging, significant changes in gonadal function, pregnancy, and lipid metabolism take place [74]. Because of the low toxicity of melatonin, clinical trials are warranted in terms of protection of pathophysiological processes of the reproductive tissues [75]. Melatonin has long been successfully used as a potent agent to improve ovulation, and enhance luteal function and embryo implantation [75, 76]. Via its antioxidative actions, melatonin is highly produced by the ovary, possibly reducing oxidation and apoptosis while allowing preovulatory follicles to provide healthy oocytes for ovulation [74]. Melatonin is also useful to overcome low rates of fertilization and pregnancy during in vitro fertilization [76]. On the basis of ovarian aging, autophagy-related gene (LC3) and aging-related sirtuin gene (SIRT1) are upregulated by melatonin together with enhanced telomere length and free radical scavenging activities [77]. Also, Song et al. [78] reported that melatonin suppresses ovarian mitochondrial oxidative damage, inhibiting apoptosis and repressing the collapse of mitochondrial membrane potential (ΔΨmt). Melatonin decreases apoptosis in uterine tissue of rats exposed to constant light mainly via regulation of apoptotic-related genes [79]; this mechanism reinforces its protective effect against cell death. In prostate tissue, treatment with melatonin normalized glutathione-S-transferase activity and lipid peroxidation and increased GPx and CAT in diabetes-induced animals [80], emphasizing its antioxidant role in androgen-dependent organs.
Mitochondria were presumed to be a particular site for melatonin synthesis, since melatonin levels in this organelle are higher than that in the plasma of rodents [81–83]. In addition to this fact, products of the enzymes aralkylamine N-acetyltransferase/serotonin N-acetyltransferase (AANAT/SNAT) are present in the mitochondria of pinealocytes, while AANAT was found in the mitochondria of oocytes [84, 85], reinforcing the importance of melatonin to these organelles. Mitochondria have the ability to synthesize and metabolize melatonin, since its metabolites were detected inside the organelles. Not surprisingly, cytochrome c may actively participate in the metabolic processes of melatonin [86].
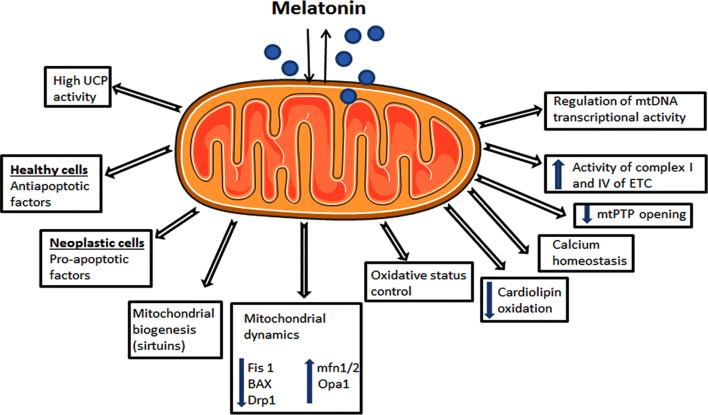
In recent years, melatonin has been proposed as a promising agent that plays an effective role in promoting mitochondrial homeostasis, such as regulating oxidative phosphorylation and electron flux, ATP synthesis, bioenergetics, calcium influx, and mitochondrial permeability transition pore (mtPTP) opening [87–89]. Moreover, melatonin was also proven to exert mitochondrial homeostasis by regulating nuclear DNA and mtDNA transcriptional activities [90]. These activities certainly help the cell in preventing DNA mutations and might shed some light on the mechanisms responsible for cancer initiation, drug resistance, and disease progression. Figure 2 describes some mitochondrial functions whereby melatonin may have a direct or indirect influence.
Fig. 2.
Possible mitochondrial processes by which melatonin influences tumor growth depending on the cell status. Since melatonin can be both taken up and synthesized in mitochondria, a more direct and close relationship is expected to finely orchestrate these functions. UCP uncoupling proteins, Fis1 mitochondrial fission 1 protein, BAX Bcl-2-associated X protein, Drp1 dynamin-related protein 1, mfn1/2 mitofusins 1 and 2, Opa1 optic atrophy 1, mtPTP mitochondrial permeability transition pore, ETC electron transport chain, mtDNA mitochondrial DNA
A wide variety of in vitro and in vivo studies have documented that melatonin reduces oxidative stress [91–95], and as a result, it decreases apoptosis and improves metabolic status and survival rate of cells [19, 96]. There is an endless series of evidence that mentions melatonin as a powerful scavenger of oxidative products (ROS and RNS) in cells, mostly acting on mitochondria. Melatonin has also been reported to protect mitochondria from damage, especially by preventing cardiolipin oxidation, an essential phospholipid involved in several bioenergetics processes and in mitochondrial-related events of apoptosis [97]. Consistently, melatonin also promotes mitochondrial biogenesis through upregulation of sirtuins [98], a class of proteins associated with aging, apoptosis, inflammation, and control of the circadian clocks.
Mitochondrial functions are rhythmically controlled through the day. In addition to the basic functions, mitochondrial dynamics including biogenesis, fission, fusion, and mitophagy [99] are believed to follow daily oscillations of the clock proteins Period1 and Period2 (PER1/2). PER1/2 are centrally and peripherally upregulated by melatonin [100]; consistent with this, melatonin is responsible for coordinating daily morphological changes of mitochondria [101], eliminating damaged and old mitochondria, while preserving young and healthy ones [19]. In pinealocytes, melatonin regulates mitochondrial morphology from fission to fusion stages, the latter being correlated with high melatonin levels in healthy cells [19]. From a mechanistic standpoint, melatonin attenuates mitochondrial fission by downregulating mitochondrial fission 1 protein (Fis 1), BAX, and Drp1. On the contrary, melatonin stimulates mitochondrial fusion by upregulating mitofusins 1 and 2 (Mfn1/2) and Opa1. The regulatory mechanisms by which melatonin coordinates the mitochondrial dynamics are complex [19, 102].
Mitochondrial biogenesis is associated with mitophagy. Mitophagy is a specialized process of autophagy that selectively degrades non-repairable or damaged mitochondria [103]. Together, biogenesis and mitophagy regulate mitochondrial function and quality; defective mitochondria produce the “eat me” signal involving phosphorylation and ubiquitination of proteins, and reduced cellular energy activates biogenesis via adenosine 5′-monophosphate-activated protein kinase (AMPK). Consistently, melatonin seems to enhance the process of mitophagy by activating (AMPK), while suppressing mTOR signaling. Activation of AMPK also stimulates biogenesis via Sirt1 dependence on deacetylation of peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) or its expression [104, 105].
Loss of the ΔΨmt promotes a profound state of mitochondrial malfunction and can be associated with induction of apoptosis or mitophagy. In addition, the frequency and duration of the mtPTP opening seem to be decisive and, when the opening duration is shortened, apoptosis does not occur. In this regard, melatonin is reported not to prevent mtPTP opening, but to significantly reduce the duration, thereby keeping the permeability transition at a minimum and avoiding apoptosis [106]. With regard to the ΔΨmt are the uncoupling proteins (UCPs), a group of proteins capable of accelerating the proton gradient from NADH-powered pumping into the mitochondrial intermembrane space, thus reducing ROS and cellular oxidative damage [107, 108]. Melatonin has been reported to increase the activity of UCPs without compromising ATP production; these effects on UCPs may occur either by upregulation of specific genes or direct regulation of UCPs activities [109, 110].
In mitochondria, there are many potential sites where ROS could be produced, especially related to the ETC. Both respiratory complex I and complex III leak electrons between donor and receptor molecules [111], and while ROS generated by complex I are restricted to the matrix, ROS arising from the complex III are located within the matrix and within the intermembrane space [112]. Melatonin has already demonstrated an ability to modulate mitochondrial complex activities and ROS formation. Experimental studies with mitochondria isolated from rat brain and liver tissues showed that melatonin, at doses of 10 mg/kg, rapidly increases the activities of complex I and IV of the mitochondrial ETC, whereas no stimulation was observed in complex II and III [113]. Other relevant in vitro studies also corroborate these effects [81, 87].
In vitro and in vivo studies demonstrated that melatonin has a stabilizing effect on the ΔΨmt through decreasing the O2 consumption and phase 3 mitochondrial respiration, thereby regulating the respiratory control index (ICR = V3/V4), and interfering with the participation of a reducing substrate in the TCA cycle [114, 115]. Also, Zhang et al. [116] and Fu et al. [117] reported that pharmacological doses of melatonin induce a large production of ROS, suggesting melatonin binds to the site Qi of complex III like antimycin A, thereby causing allosteric modulation of the enzyme. The activity of theETC, which is stimulated by melatonin and repressed by antimycin A, was more evident in cancer cells than in normal cells, confirming the strong relation between the electron flux and oxygen consumption by neoplastic cells [117]. Presumably, melatonin’s role on ETC complexes is related to electron donor–acceptor transfer, which may increase and facilitate the electron flow and ATP synthesis while decreasing ROS generation. In cancer cells, melatonin is known for its ability to alter the redox state, thus increasing ROS production and triggering activation of the pro-apoptotic program. Via receptor-independent pathway, melatonin interacts with calmodulin and the PI3K/AKT/ERK signalling to modulate Sirt1, ROS balance, activation of pro-apoptotic molecules (Bax, Bak, Bam) and inhibition of anti-apoptotic proteins (Bcl-2 and Bcl-xl) [118]. Despite this, the link between ROS production and pro-apoptotic effectors is not completely unravelled; these events seem to benefit the effects of other compounds during cancer therapy [119]. Finally, induced mitochondrial ROS production in tumor cell death correlates with the high efficiency to chemotherapy [120]; in this case, melatonin could be highly useful in limiting tumor growth and enhancing cancer therapy.
Because mitochondria have been closely associated with cancer development, new anticancer agents targeting mitochondria are potential therapies. The major obstacle for these agents is their inability to exhibit mitochondrial permeability as, in most cases, transmembrane transporters are required. Two synthetic agents possessing these features include mitochondrial-targeted coenzyme Q10 (MitoQ) and mitochondrial-targeted vitamin E (MitoE); they can accumulate within the mitochondrial matrix at high levels [121]. After comparing these artificially produced compounds with melatonin in a septic shock mouse model, a more effective response regarding cellular protection was observed with melatonin [122]. Some transporters have been proposed for carrying melatonin into cells, such as the glucose transporter 1 (GLUT1) in the presence of high glucose levels [123]; more recently, Huo et al. [124] found that melatonin and its metabolites can potentially be transported into cancer cell mitochondria (PC3 and U118 cell lines) after binding to oligopeptide transporters, PEPT 1/2, against a gradient concentration. Although melatonin uptake was linear, its uptake through PEPT1/2 was saturable after prolonged incubation. Whether the mechanism can be applied to other cell types remains unknown; these transporters might be useful to improve the therapeutic effects of melatonin during cancer management.
Mitochondria, melatonin, and their functional mechanisms in reproductive cancers
Ovarian cancer: interplay between mitochondria and melatonin
Ovarian cancer (OC) represents the most common lethal gynecologic malignancy; it has poor prognosis when diagnosed in advanced stages of the disease [125]. About 90% of the OC subtypes evolve from ovarian surface epithelium or fallopian tube fimbriae and they are classified as epithelial ovarian cancer (EOC), and ~ 70% of the EOC present with aggressive phenotype and widespread metastasis [125]. The EOC can be subclassified in serous carcinoma, mucinous, clear cell, and endometrioid, all of them showing a particular physiology, genetic background, and molecular components [126]. Chemotherapy with platinum derivatives or taxanes followed by debulking are often the “gold standard” choice to achieve no residual disease. However, many women develop chemoresistance to treatments and tumor relapse with a malignant potential to metastasize. Normally, these chemoresistant OC cells have a high threshold for apoptosis activation, most likely due to the overexpression of antiapoptotic genes [127, 128]; searching for novel chemotherapeutic adjuvants to overcome drug-resistance and induce chemosensitivity in human OC may hold great promise.
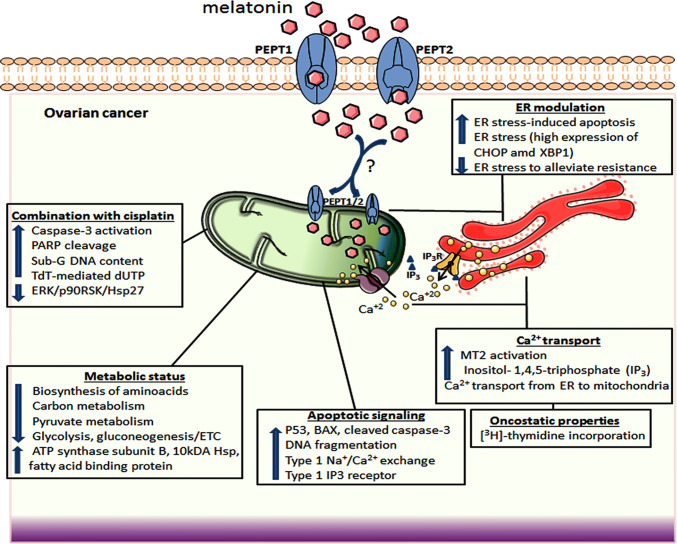
Mitochondrial function is strictly related to OC treatment and prognosis. Numerous studies have focused on molecular mechanisms displayed by the OC cell mitochondria to get better outcomes during tumor management. These approaches are typically tested in both in vivo and in vitro assays and include modulation of fundamental metabolic pathways [129], calcium homeostasis [130], resistance to cisplatin and apoptosis [131], ROS generation and DNA damage [132]. The knowledge that a new compound can specifically target the mitochondria to induce apoptosis or even overcome chemoresistance associated with mitochondrial dynamics is of significant value for OC treatment. Through specific receptors or PEPT1/2 transporters, melatonin can be moved into mitochondria influencing a number of mitochondrial responses in OC (Fig. 3). These activities include induction of apoptosis by the engagement of pro-apoptotic effectors and ion exchanges, in addition to changes in cell metabolism and reduction of chemoresistance by the ER modulation and Ca2+ signaling.
Fig. 3.
A summary of the effects of melatonin on mitochondria of ovarian cancer cells. In addition to internal production by mitochondria, melatonin can be transported into mitochondria possibly via PEPT1/2, thus promoting effective responses on apoptosis, cellular energy metabolism, ER-stress modulation, and stimulating the action of other chemotherapeutics (e.g., cisplatin). Upon MT2 activation, Ca2+ is transported from ER to mitochondria triggering apoptosis and alleviating chemoresistance. P53 tumor suppressor protein p53, Ca2+ calcium, DNA deoxyribonucleic acid, MT2 melatonin receptor 2, IP3 inositol-1,4,5-triphosphate, IP3R inositol-1,4,5-triphosphate receptor, ERK extracellular signal-regulated kinase, p90RSK dephosphorylation of 90-kDa ribosomal S6 kinase, Hsp27 heat shock protein 27, ER endoplasmic reticulum, BAX bcl-2-like protein 4, ETC electron transport chain, PARP poly(ADP-ribose) polymerase, PEPT1/2 human oligotransporters 1 and 2, CHOP Cruxhalorhodopsin-1, XBP1 X-box binding protein 1, ? uncertain actions for OC
Melatonin has already shown an ability to exert oncostatic properties in ovarian cancer BG-1 cell line. In addition to its antiproliferative activity, melatonin, at concentrations of 10−9 and 10−7 M, promoted reduction in [3H]-thymidine incorporation without increasing cell death and altering the cytosolic-free Ca2+ levels [133]. As melatonin receptor (MT2) activation is related to an increase in phosphoinositide hydrolysis to produce inositol-1,4,5-triphosphate (IP3), the pivotal player involved in Ca2+ release from the endoplasmic reticulum (ER) to the cytosol, BG-1 cells were loaded with fura-2 AM to monitor Ca2+ levels. Under these conditions, acute administration of melatonin did not alter Ca2+ release by these OC cells. In fact, Ca2+ signaling is altered in cancer cells and might result in disarrangement of mitochondrial bioenergetics, cell proliferation, apoptosis, migration, and survival [134]. Several proto-oncogenes and tumor suppressors interfere with intracellular Ca2+ transport from ER to the mitochondria which are critical for both cell death and survival [135, 136]. Ca2+ overload can trigger cardiolipin oxidation, leading to the disassembly of the succinate dehydrogenase complex and, subsequently, producing ROS [137]. For instance, ROS stimulates the opening of the mPTP and then mitochondrial outer membrane permeabilization.
The anti-apoptotic Bcl-2 members regulate Ca2+ signaling between the ER and mitochondria, which participate in the so-called mitochondria-associated ER membranes (MAMs). Normally, Bcl-2 promotes Ca2+ oscillations mediated by the IP3R, thereby enhancing pro-survival effects with high mitochondrial energy production and cell proliferation [138, 139], while it inhibits pro-apoptotic events associated with Ca2+ release [140]. With regard to chemotherapeutic approaches, new evidence revealed that cisplatin causes a stress to mitochondria, ER, and the cytosol, and only a small amount of the agent can indeed penetrate into the nucleus to bind DNA [141]. In addition to this, Bcl-2 is thought to block cisplatin-mediated apoptosis via Ca2+ regulation in a variety of cancer cells, and its overexpression may lead to cisplatin resistance in OC [142]. Although the exact mechanism responsible for its inhibition is not well characterized, Xu et al. [141] reported that Bcl-2 attenuated cisplatin-induced Ca2+ release from the ER to cytoplasm and mitochondria, thus reducing the ER stress, and consequently, apoptosis via mitochondrial pathway; this phenomenon was markedly accompanied by a drop in the number of ER–mitochondrial contact sites in SKOV-3 human ovarian cancer cells. In addition, the expression of cyt c, Bax/Bcl-2 ratio, and cleaved caspase-9 and -3 was significantly reduced after Bcl-2 overexpression, possibly indicating inhibition of the mitochondrial apoptotic activation cascade. Recent studies involving long-term treatment with melatonin in an in vivo model of OC have shown numerous findings related to apoptosis of serous OC cells [125, 143]. In this rat model, the levels of p53, BAX, total caspase-3, and cleaved caspase-3 were downregulated in OC tissue; whereas, Bcl-2 and survivin were upregulated. Conversely, upregulation of p53, BAX, and cleaved caspase-3 was achieved in OC cells after melatonin therapy (200 mg melatonin/100 g BW) for 60 days; it was also reported that melatonin reduced tumor sizes and masses by enhancing DNA fragmentation during the apoptotic process.
As a double-edge sword, melatonin might act to promote ER stress-induced apoptosis or protect cells from ER stress to alleviate chemotherapy-related side effects and resistance. To examine whether melatonin is capable of regulating ER stress, apoptosis, and the oxidative status, ovarian cancer A2780 cells were incubated with increasing melatonin doses of 0.1 µM, 1 µM, and 10 µM for 24 h; surprisingly, melatonin induced apoptosis via activation of type 1 sodium/calcium exchanger and type 1 IP3 receptor associated with decreased levels of cytosolic Ca2+. In addition, melatonin promoted ER stress as evidenced by the increase in the expression of biomarkers CHOP and XBP1in a concentration-dependent manner, while decreasing ROS levels [144]. Differentially targeting the Ca2+ transport system may indicate a key mechanism by which melatonin exerts its anticancer activities in OC.
On the basis of proteomic analysis, we recently showed that melatonin therapy promoted down-regulation in several proteins involved in important signaling pathways in a model of OC [143]. The main set of proteins belonged to the class of mitochondrial processes, such as biosynthesis of amino acids, carbon metabolism, pyruvate metabolism, glycolysis, gluconeogenesis, and the ETC (e.g., glyceraldehyde-3-phosphate dehydrogenase, fructose-bisphosphate aldolase A, pyruvate kinase isozymes M1/M2, malate dehydrogenase, l-lactate dehydrogenase (LDH) A chain, creatine kinase B type, ATP synthase subunit α, peptidyl-prolyl cis–trans isomerase A, peroxiredoxin-5, α1 antiproteinase, superoxide dismutase (Cu–Zn), thioredoxin, serotransferrin, hemopexin, and hemoglobin subunits β1/2 and α1/2). Other molecules that are significantly reduced by melatonin are associated with ER stress and include Hsp70, 78-kDa glucose-regulated protein (GRP78), and protein disulfide isomerase (PDI) A3 and A6. Interestingly, Hsp 70 and GPR78 are the major stress-induced chaperones in ER and serve as Ca2+ buffers associated with the mitochondrial apoptotic program. Only a small portion of overexpressed proteins included ATP synthase subunit β, fatty acid-binding protein, and 10-kDa heat shock protein. Taken together, these results provide new insights into the metabolic regulation of the OC and suggest melatonin as an additional therapeutic strategy for this cancer type.
To investigate melatonin’s ability to increase the effectiveness of cisplatin treatment in OC, SKOV-3 cells were co-treated with 2 nM melatonin and 80 µM cisplatin for 24 h [145]. When melatonin and cisplatin are combined, a significant increase in sub-G1 DNA contents and TdT-mediated dUTP nick end-labeling (TUNEL) is observed in SKOV-3 cells compared to cisplatin alone. Moreover, co-administration also induced caspase-3 activation along with enhanced PARP cleavage; this may be a result of the synergistic role of melatonin in inhibiting the phosphorylation of extracellular signal-regulated kinase (ERK) and dephosphorylation of 90-kDa ribosomal S6 kinase (p90RSK) and Hsp27 induced by cisplatin. The findings by Kim et al. [145] indicated that melatonin synergistically increases the cisplatin-induced apoptosis via the inactivation of ERK/p90RSK/Hsp27 system in SKOV-3 cells.
In OC cells, most of the melatonin’ effects are mediated by its MT1 receptor, so that this expression is reduced with the grade of tumor aggressiveness [146, 147]; the lowest level was detected in OVCAR-3 cell line, a poorly differentiated OC subtype [148]. Recently, the influence of pharmacological doses of melatonin (1 mM and 2 mM) in combination with cisplatin (2.5 µg/mL) showed a dose-dependent reduction in OC cell survival, even though no substantial involvement of MT1 receptors was observed [149]. Thus, melatonin and cisplatin revealed synergistic actions regardless of MT1 activation; these results suggest additional confirmation on how melatonin elicits its cytostatic effects in OC. To assure melatonin as the cisplatin-adjuvant chemotherapeutic agent, further studies correlating the intrinsic mitochondrial pathway of melatonin synthesis in the presence or absence of its receptors, together with their involvement with apoptotic molecules, must be considered in cisplatin-treated OC cell.
Endometrial cancer: interplay between mitochondria and melatonin
Endometrial cancer (EC) is a gynecological disease, and represents one of the most common cancers in North America and Europe [150]. About 75% of patients are diagnosed in the early stages (stages I and II), while ~ 25% are diagnosed in advanced stage (stages III or IV) with a low survival rate. EC comprises a clinically, morphologically, and genetically heterogeneous groups of tumors. According to Suarez et al. [151], ECs are divided into types I or II; type I cancers are estrogen dependent, and most of these are low-grade endometrioid tumors being associated with obesity and other factors of the metabolic syndrome and type II cancers are estrogen-independent high-grade non-endometrioid tumors, being associated with endometrial atrophy and aggressiveness. With regard to the histopathological features, these cancers are subtyped serous carcinoma, endometrioid carcinoma, carcinosarcoma, and clear-cell carcinomas [152].
Although not so effective, the prognosis for EC still relies on histological type and grade, and myometrial or lymphovascular space invasion [153]. There are some molecular biomarkers (e.g., PTEN, TP53, K-ras, MSH2, and MSH6) that may help in assisting diagnosis and better outcomes regarding EC [154]. The unopposed estrogenic stimulation is responsible for slow-growing EC, and estrogens may have direct or indirect impact on mitochondrial functions via differential expressions of its receptors [155]. Estrogen is, therefore, associated with mtDNA mutations in EC presumably by increasing ROS production and stimulating mitochondrial biogenesis [156]. In response to ROS, antioxidant proteins, namely peroxiredoxin 3 (Prx3) and 6 (Prx6), thioredoxin, and SOD are upregulated in EC in addition to the expression of augmenter of liver regeneration (ALR); since Prx3 and ALR are suggested to have a protective response against ROS increase, they might represent special targets for the development of new therapeutic strategies for patients with EC [157].
Mitochondrial biogenesis is associated with energy requirements in EC [156]. In addition to a twofold increase in mtDNA content, a significant rise in activities of citrate synthase, PGC-1α, nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2), and mitochondrial transcription factor A (TFAM) was documented in type I EC [158, 159]. Furthermore, changes in respiratory complex I and in respiratory subunits NDFUA9, SDHA, SDHB, Core II, and MnSOD are found in a number of EC patients and may be a result of mtDNA and nuclear DNA mutations [160]; these altered oncocytic foci are related to mitochondrial biogenesis in 72% of EC patients. Higher antioxidant activities and mitochondrial biogenesis seem to counteract the bioenergetic deficit and ROS generated from complex I [161, 162].
Mitochondrial homeostasis is strongly related to EC, and new agents that alter mitochondrial fission and mitophagy may have significant potential. The mammalian sterile 20-like kinase 1 (Mst1) is an important inhibitor of cell proliferation and inducer of apoptosis, highly involved in tumorigenesis, differentiation and organ growth [163]. Importantly, Mst1 is downregulated in ectopic endometrium and its recovery is closely associated with the inability of stromal cells to migrate and proliferate [164]; functionally, Mst1 enhanced Drp1 post-transcriptional phosphorylation at Ser616 and suppressed Parkin activity via p53, resulting in mitochondrial fission and mitophagy inhibition besides evoking oxidative stress, Ca2+ overload and caspase-9 activity. Mst1 was also reported to be a negative regulator of TGF-B and EGF signaling in a transient Mst1-transfected HEC-1-A-endometrial cancer cell, thus demonstrating a significant inhibition of cell invasiveness, migration and proliferation with involvement of E-cadherin and without activation of Smad2 [165].
EC has long been correlated with low melatonin levels, and this decrease may be considered as a possible risk factor. The mean value for the cancer-patient group was 6.1 pg/mL, whereas the mean value for cancer-control group was 33.2 pg/mL; in this study, no differences in menopausal status or age were noted [166]. In addition to this evidence, molecular analysis of the EC-related melatonin receptor genes was performed in 37 patients samples (grades 1–3) using microarray HG-U133A and qRT-PCR and the results were relevant as additional diagnostic and prognostic tools. A total of 18 ID mRNAs were differentially expressed in grade G2-ASMTL, GNA 11, PER2, PTGDS and in grade G3-GNA12, GNA 11 showing that regulation of melatonin receptors activity was dependent on the histopathological grade; down-regulation of genes involved in melatonin biosynthetic pathway (ASMTL) and melatonin signal transmitters (RTGS, GNA 11, GNA 12) were the most representative [167].
A recent study using Ramelteon, a selective agonist for melatonin receptors (MT1 and MT2), showed that drug treatment with 10−8 M for 96 h efficiently suppressed the proliferation and invasiveness of the estrogen receptor (ER)-positive EC cell line (HHUA), thereby eliciting a similar activity as melatonin; to prove this specific action, luzindole, a MT1 and MT2 receptor antagonist, was added to the culture medium and completely abolished these effects [168]. Watanabe et al. [169] reported that MT1 receptor, but not MT2, is expressed in Ishikawa cells, an estrogen receptor-positive EC cell line. They confirmed that the cytostatic effect of melatonin (10−9 M) in reducing the ER-α expression by the cells is mediated by MT1.
Treatment with melatonin has often been investigated in reference to EC cell growth [170]. SNG-II and Ishikawa cell lines were studied with regard to their ER status, and their responsiveness of melatonin. While physiological concentrations of melatonin (10−9 M) exhibit no effect on growth inhibition of ER-negative SNG-II cells, melatonin at all cell densities and after 96-h incubation, significantly inhibited the growth of ER-positive Ishikawa cells. This anti-proliferative action was achieved at 10−9 M concentration, compared with supra (10−6, 10−8 M) or subphysiological concentrations (10−10, 10−12 M); these actions seem to be mediated by a steroid receptor (e.g., ER) and a melatonin receptor. Thereafter, Kobayashi et al. [170] suggested that MT2 receptor is expressed on the surface of Ishikawa cells, and the antiproliferative action of melatonin is dependent on MT2 receptor; no effect has been documented in estrogen-unresponsive cell lines. Experimental studies have shown that the addition of melatonin to estrogen replacement is effective in decreasing the endometrial proliferation index, intra-retroperitoneal fat, and promoting metabolic changes [171]. Whether melatonin modulates some mitochondrial processes to influence proliferation and apoptosis in EC, during its development, progression and metastasis, remains to be determined.
Cervical cancer: interplay between mitochondria and melatonin
Cervical cancer (CC) is the second most common cancer accounting for ~ 8% of total cancer deaths in women [172]. CC is associated with infection of high-risk human papillomavirus (HPV) subtypes, and conventional methods of treatments include surgery followed by radiotherapy and cisplatin (CIS)-mediated chemotherapy [173]. Importantly, resistance to chemotherapy is one of the main causes of tumor recurrence and enhancement of the CIS-induced apoptosis may help the efficiency of chemotherapy [174]. CIS can affect mitochondria by promoting the release of pro-apoptotic factors such as cytochrome c and smac molecules, thereby activating caspase-9 and -3 activities [175]. More recently, SH2 domain-containing protein tyrosine phosphatase-2 (SHP-2) was associated with malignant transformation in HPV-infected CC patients. Overexpression of SHP-2 suppressed apoptosis induced by 5-fluorouracil (5-FU) through activation of autophagy to degrade damaged mitochondria via ubiquitin ligase function of Parkin [176].
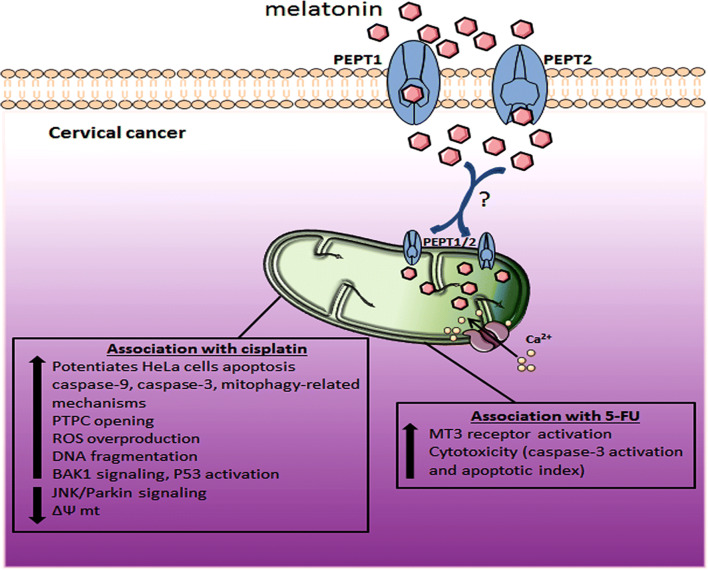
Through its pro-apoptotic and pro-oxidant effects in tumor cells, melatonin has been proposed to potentiate the effect of CIS on HeLa cell apoptosis, mainly via caspase-9 pathway and a mitophagy-mediated anti-apoptotic mechanism. The combination of CIS with melatonin increased apoptosis and mitochondrial damage by stimulating pro-apoptotic caspase-9 and ROS production, while lowering mitochondria membrane potential (Fig. 4). In the same study, melatonin further inhibited the anti-apoptotic mitophagy by blocking the JNK/Parkin signaling pathway [177]. Another recent study reported that association of melatonin with chemotherapeutic agents, namely CIS, 5-fluorouracil (5-FU), and doxorubicin, reduced HeLa cell viability [178]. Notably, co-stimulation of HeLa cells with these agents in the presence of melatonin led to increased caspase-3 activation: the combination of melatonin with CIS enhanced HeLa cells apoptosis via ROS overproduction related to more extensive DNA fragmentation (Fig. 4). The combination of melatonin with either 5-FU or doxorubicin only produced moderate chemosensitizing effects on HeLa cells, as evidenced by a low percentage of endogenous ROS-stimulated cells. In this context, the authors believe that longer exposure times (48, 72, and 96 h) would be more efficient for melatonin in sensitizing HeLa cells [178]. More recently, Pariente et al. [179] showed that melatonin (1 mM) significantly increased the cytotoxic effect of 5-FU in HeLa cells, after 48-h exposure, by elevating caspase-3 activation and the apoptotic index. In these pharmacological studies, the effectiveness of melatonin on HeLa apoptosis seems to be receptor mediated. The involvement of melatonin with 5-FU may result in cytotoxicity and apoptosis via melatonin receptor MT3 (Fig. 4), whereas MT1 and MT2 receptors were not apparently involved with these functions.
Fig. 4.
The effects of melatonin on cervical cancer mitochondria. Melatonin can be transported into mitochondria possibly via PEPT1/2 and the most ameliorative effects occur in association with other chemotherapies (e.g., cisplatin and 5-fluorouracil) to induce apoptosis and overcome chemoresistance. PTPC mitochondrial permeability transition pore, ROS reactive oxygen species, DNA deoxyribonucleic acid, BAK1 Bcl-2 homologous antagonist/killer gene, P53 tumor suppressor protein p53, 5-FU 5-fluorouracil, Ca2+ calcium, JNK c-Jun N-terminal kinase, ΔΨ mt mitochondrial membrane potential, MT3 melatonin receptor 3, PEPT1/2 human oligotransporters 1 and 2, ? uncertain actions for CC
The CIS-related mitochondrial signalling may be linked to higher ROS and NO levels which favor the opening of the PTPC, transduction of mitochondrial outer membrane signals via BAK1, and activation of cytoplasmic p53 [180]. Supporting the role of CIS, melatonin is proven to stimulate endogenous ROS, and subsequent PTPC opening while inducing activation of pro-apoptotic proteins like Bax to the mitochondrial outer membrane, and enhancing p53 function [118]. Therefore, melatonin and CIS not only induce pro-apoptotic signals at the mitochondrial level, but also stimulate a more rapid apoptotic process.
Melatonin levels are inversely associated with CC aggressiveness [181]; whether reduced melatonin levels are related to its decreased secretion or increased utilization remains to be uncovered. Because melatonin concentration is significantly reduced in advanced stages (3 and 4) of CC in women, especially at night time (24:00, 02:00, and 04:00 h), supplementation with melatonin may be helpful in controlling tumor progression or even promoting better health benefits for patients. With respect to the cervical carcinogenesis, melatonin (20 mg/L), given in drinking water at night time, abolished tumor development by reducing the mutagenicity of 7,12-dimethylbenz[a]anthracene (DMBA) in experimental mice. To verify the in vitro effects of melatonin, the strains TA 97 and TA 98 of Salmonella typhimurium, and the adult Chinese hamster ovary cells (CHOK1) were tested for DMBA mutagenicity: a significant protective effect of melatonin (antimutagenic and anticlastogenic activities) was observed at concentrations varying from 0.1 to 100 nM [182].
It has been documented that elevation in GSH levels in cancer cells is associated with resistance to chemotherapy and radiotherapy. Past strategies trying to change the availability of GSH in the culture medium of human cervical cancer cells (ME-180) did not find any significant variation in the sensitivity of the cells to melatonin’s anti-growth effects; in this experiment, only the 2-mM melatonin dose effectively reduced the proliferation rate of ME-180 cells [183].
Breast cancer: interplay between mitochondria and melatonin
Despite the remarkable progress in the treatment of breast cancer (BC) in recent decades, this disease is still one of the leading causes of death among women [184], and is, certainly, a public health issue accounting for 25% of all cancers in females worldwide [185]. Although the mortality rates are falling in most European countries, as well as in South and North America [186], the American Cancer Society estimated 40,610 deaths with 252,710 new cases of invasive and 63,410 in situ BC in 2017. Such as for other cancer types, the best way to achieve success in the BC treatment is early diagnosis; several criteria are used for identification of risk factors. BC is indeed a heterogeneous and complex disease and the related risk factors and alternative chemotherapeutics are still open for debate [187].
Melatonin is an endogenous molecule which has been intensively studied because its positive effects against BC. A plethora of data has shown that melatonin acts via MT receptor-dependent and -independent pathways to prevent circadian disruption while inhibiting metastasis, angiogenesis and telomerase activity; in addition, it acts as a modulator of cell cycle/apoptosis pathways, thereby regulating the expression and transactivation of ER and influencing the local synthesis of estrogens [188–190]. In fact, it has been consistently shown that circadian melatonin levels regulate cell signaling and metabolic activity, and it inhibits BC initiation, promotion and progression [190–193]. Figure 5 describes the most important mechanisms whereby melatonin exerts significant actions against BC; they include the participation of melatonin in Ca2+-related signaling, metabolic shifting, apoptotic signaling, drug-delivery systems, in addition to serving as a chemopreventive antioxidant molecule.
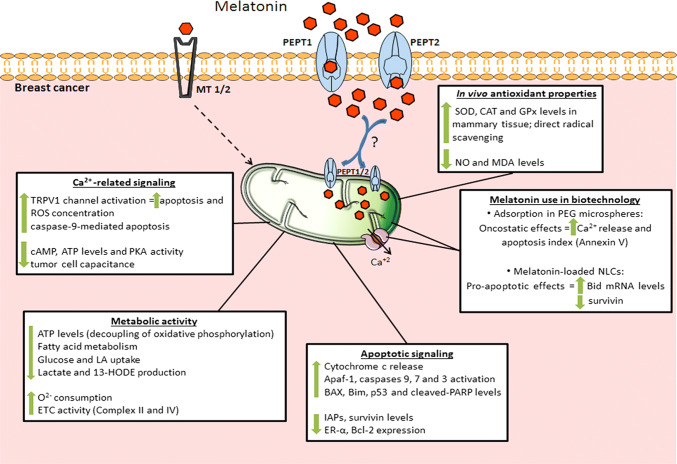
Fig. 5.
Melatonin effects on mitochondrial function in breast cancer cells may be mediated by MT1 and MT2 receptors or via PEPT1/2 transporters. Melatonin might be transported into the organelle through PEPT1/2, and in addition to the intra-organelle production, triggers apoptosis through different pathways, such as caspases activation, upregulation of pro-apoptotic proteins, and ROS generation. In association with nanoparticles, melatonin stimulates Ca2+ release, which enhances the apoptotic process. Moreover, melatonin efficiently restores aerobic metabolism and promotes alterations in ATP metabolism. Also, melatonin has important protective effects against oxidative stress, by stimulating antioxidant activities and repressing pro-oxidative processes in healthy mammary cells. 13-HODE 13-hydroxyoctadecadienoic acid, Apaf1 apoptotic protease activating factor 1, ATP adenosine triphosphate, BAX bcl-2-like protein 4, Bcl-2 B-cell lymphoma 2, Bid pro-apoptotic member of the Bcl-2 family, Bim Bcl-2-like protein 11, Ca2+ calcium, CAT catalase, cAMP cyclic adenosine monophosphate, ER-α estrogen receptor alpha, ETC electron transport chain, GPx glutathione peroxidase, IAPs inhibitors of apoptosis proteins family, LA linoleic acid, MDA malondialdehyde, MT1/2 melatonin receptors 1 and 2, mRNA messenger ribonucleic acid, NLCs nanostructured lipid carriers, NO nitric oxide, O2− oxygen, p53 tumor suppressor protein p53, PARP poly(ADP-ribose) polymerase, PEG polyethylene glycol, PEPT1/2 human oligotransporters 1 and 2, PKA protein kinase A, ROS reactive oxygen species, SOD superoxide dismutase, TRPV1 transient receptor potential vanilloid 1, ? uncertain actions for BC
Mitochondria play fundamental roles in physiological and pathological contexts [194, 195], and are involved not solely with cell energy production, via OXPHOS, but also with Ca2+ handling, ROS signaling, and apoptosis. Additionally, mitochondrial morphology and biogenesis are also closely related to the breast carcinogenesis process [196–198]. The relationship between BC and mitochondria was already demonstrated in MCF-7 cells, which are estrogen-responsive human BC cells [199]. A more recent study has shown that ERα, but not ERβ, participate in the mitochondrial morphological alterations found in this cell line [198]. Curiously, Scott et al. [62] had already observed that melatonin (100 nM) altered the mitochondrial ultrastructure in MCF-7 BC cells, resulting in dissolution of the organelle outer membrane and degenerating mitochondrial cristae. Oo et al. [198] also demonstrated Drp1 phosphorylation to be involved with estrogen-related effects on mitochondria ultrastructure. Mitochondria are constantly changing size and intracellular location and, these events rely upon fission- and fusion-controlled processes [200].
Drp1 phosphorylation at serine 616-residue induces its activity and is related to BC progression [201]. Kashatus et al. [202] demonstrated the expression of Ras oncogene and its related MAPK pathway to be responsible for Drp1 phosphorylation; these signaling pathways are associated with tumor growth. Moreover, BC cells with a faster migration rate exhibit higher Drp1 levels [203]. Controlling the mitochondrial fission/fusion machinery seems to be a promising therapeutic approach, at least, for BC metastasis. Generally, increased melatonin concentrations are responsible to reduced mitochondrial fission and elevated mitochondrial fusion [19, 204, 205]. In fact, melatonin promotes both the translocation of proteins involved in mitochondrial fission, such as Drp1, Fis1, Bax, and the expression of mitochondrial fusion proteins Opa1 and mitofusins 1 and 2 [19]. The indole suppresses mitochondrial fission by attenuating the translocation of Drp1 and Fis1 to the mitochondrial outer membrane [19, 206]. Zhou et al. [207] recently reported a growth reduction of both in vivo and in vitro nasopharyngeal carcinoma by inhibition of Drp1 activity through mitochondrial COX-2 suppression. On the other hand, the role of melatonin in mitochondrial fusion remains unclear, with studies suggesting that it could downregulate Mfn1 and Opa1 [208] or upregulate Notch1 signaling pathway to increase Mfn2 levels [102]. In association with sorafenib, melatonin induces a late increase in Mfn2 expression in hepatocellular carcinoma, which in association with elevated BAX and PARP cleavage, could lead to apoptosis [209]. With regard to BC, no study has evaluated the link between the melatonin’s effect and mitochondrial dynamics.
Cancer cells have also evolved to avoid or minimize the apoptosis process, in such a way that several anti-cancer agents act by augmenting the apoptosis rate of malignant cells [197]. Some ERs have been described to be located in the mitochondrion matrix of MCF-7 cells, mainly the ERβ; in response to E2, these cells avoid apoptosis by upregulating manganese SOD activity. This is an exclusively antioxidant mitochondrial enzyme and appears to be related to E2-mediated ROS inhibition, thus preventing ROS formation and, consequently, cell death [210]. In combination with lycopene, melatonin (2.5 mg/kg) exhibited a protective effect against DMBA-induced oxidative stress in Sprague–Dawley female rats. This combination efficiently increased mammary tissue levels of SOD, catalase, and glutathione peroxidase (GPx), while decreasing malondialdehyde and nitric oxide serum levels [211]. Kim et al. [197] showed that MDA-MD-231 cells treated with natural anti-cancer agents had a reduced viability and it was associated with elevated ROS levels which, in turn, activated both intrinsic and extrinsic apoptotic pathways leading to ΔΨmt loss and increasing the release of cytochrome c together with high BAX/Bcl-2 ratio.
It is well known that melatonin triggers apoptosis, at least in BC cells, through two distinct pathways: an early process independent of caspases and TGFβ1, and a later apoptotic process dependent of TGFβ1 and caspases activity [212]. The transcriptional factor Apaf-1 is a key target of p53 in mitochondrial-associated apoptosis, being essential for caspase 9 and 3 activation and apoptosome assembly [213–215]. Apaf-1 seems to be inactive in cancer cells but its overexpression restores the likelihood of apoptosis mediated by chemotherapy [216, 217]. Wang et al. [217] reported that melatonin (1.0 mM) increases the release of cytochrome c from the mitochondrial intermembrane space to the cytoplasm of BC cells. Additionally, this augmented release recruits and activates cytosolic Apaf-1, favoring apoptosome assembly via activation of caspases 9 and 3, and finally inducing apoptosis.
It was previously documented that melatonin-treated BC cells presented decreased Bcl-2/BAX ratio with upregulation of activated caspases 9 and 7, and cleaved PARP, which characterizes the activation of late apoptosis pathway [212]. Also, melatonin-loaded nanostructured lipid carriers markedly increased apoptosis, demonstrated by decreased levels of survivin and increased mRNA levels of the pro-apoptotic Bid [218]. Combining anticancer agents and melatonin appears to be an effective strategy to treat BC, since the indole improves their effect. Interestingly, Kosar et al. [219] showed that melatonin (0.3 mM) increased doxorubicin-induced apoptosis in BC cells. This result is consistent with an earlier report which observed that a melatonin agonist, termed S23478-1, downregulated the estrogen-signaling pathway, resulting in decreased ER-α and Bcl-2 expression and enhanced Bax levels [220]. In pharmacological concentrations, melatonin and arsenic-trioxide (ATO) synergistically induce apoptosis via ROS generation in MDA-MB-231 BC cell line [221]. Mechanistically, melatonin plus ATO therapy upregulated p53 expression, thereby significantly reducing Bcl-2/BAX index coupled with downregulation of survivin, a potent inhibitor member of the apoptosis proteins (IAPs) family [222]. Additionally, melatonin plus tunicamycin increased Bim levels, a key factor that suppresses the anti-apoptotic protein Bcl-2, thus positively modulating the apoptotic process [223].
Free Ca2+ plays crucial roles in the maintenance of cell functions, acting as an important second messenger, promoting muscle contraction, participating in the neurotransmitter release, proliferation, and apoptosis, in addition to other functions in different cells including cancer cells [224, 225]. Other authors, however, pointed to the release of Ca2+ from organelles may be responsible for cell migration [226], and, in this case, mitochondria may be involved [227]. Breast cells are intrinsically dependent on their Ca2+ cellular content, which plays important roles during breast growth and lactation. The E2 responsiveness (non-genomic pathway) is particularly dependent on Ca2+ concentration through activation of MAPK signaling [225, 228–230]. Many Ca2+ channels, ATPase pumps, and transporters markedly influence BC cell viability through mitochondria-related Ca2+ transporters such as Na+/Ca2+ exchanger (NCX) and mitochondrial Ca2+ uniporter (MCU) [225, 231, 232].
MCU is responsible for the rapid Ca2+ uptake by mitochondria and these ions are pumped back to the cytosol by NCX [233, 234]. These two processes in malignant cells contribute to their ability to avoid cell death [235]. Curry et al. [236] studying MDA-MB-231 BC cells demonstrated the elevated cytoplasmic Ca2+ loading to be related with the augmentation of necrotic cell death. VanHouten et al. [237] showed lower levels of cytoplasmic Ca2+ prevent apoptosis via calpain activation in T47D BC cells, and associated these findings with increased expression of plasma membrane calcium-ATPase 2 (PMCA2). In addition, PMCA2 overexpression was also related to poorer prognosis of BC patients. Notably, MCU mRNA levels are generally elevated in ER-negative BC [238], and MCU gene silencing potentiates caspase-independent cell death in MDA-MB-231 cells [239]. Regulating mitochondrial Ca2+ homeostasis, therefore, may be a strategy for BC therapeutics approaches.
França et al. [240] recently used melatonin adsorbed into Polyethylene Glycol (PEG) microspheres to show that the melatonin’s oncostatic effects in MCF-7 BC cells may be related to enhanced intracellular Ca2+ release and an augmented apoptosis index (annexin V). It is well known that doxorubicin efficiently induces an overload of intracellular Ca2+, thus inducing apoptosis and elevated ROS concentrations through mitochondrial membrane depolarization; this causes DNA damage and impairment of mitochondrial machinery, such as the respiratory chain [241, 242]. Melatonin seems to have a protective effect against ROS production in normal cells exposed to doxorubicin through a variety of redox systems, including those involving NADPH oxidase and mitochondria oxidation [243, 244]; the indole is reported to induce ROS concentration and then trigger apoptosis in tumor cells [243, 245].
Kosar et al. [219] recently reported that the doxorubicin-induced elevation in cytosolic Ca2+ is induced by activation of the transient receptor potential vanilloid 1 (TRPV1), a member of the TRP calcium-permeable channels family, which may activate apoptosis and ROS production. In the same study, melatonin synergistically upregulated caspase-9-mediated apoptosis. In MCF-7 BC cells, the proliferative potential may be sustained by both an increased membrane depolarization and voltage-dependent K+ and Ca2+ flux. In these cells, melatonin treatment reduced cell viability and proliferation by modulation of the voltage-dependent ion channels, particularly by inhibiting the characteristic rise of tumor cell capacitance [246]. In addition, melatonin impairs the functionality of MCF-7 cells by depletion of ATP levels, documenting the importance of Ca2+ channels. Melatonin causes a decrease in cAMP levels, which, in turn, leads to Ca2+ channel phosphorylation and decreased protein kinase A activity, revealing a potential mechanism through which melatonin affects Ca2+ currents [247, 248]. At the mitochondrial level, melatonin increases both oxygen consumption and ETC activity. This latter effect was demonstrated by the rise in the activity of succinate dehydrogenase (complex II) and cytochrome c oxidase (complex IV). Because melatonin-treated cells exhibited low levels of cellular ATP content, authors suggested that one of the melatonin cytotoxic mechanisms is likely due to uncoupling of oxidative phosphorylation [62].
Since Otto Warburg demonstrated the aerobic glycolytic pathway of tumor cells [249, 250], several authors have mentioned that cancer is an energetic metabolism-related disease. The Warburg effect operates continuously in many cancer cells allowing them to adapt to low-oxygen microenvironment and to avoid apoptosis. Interestingly, but not surprisingly, Vaupel et al. [251] noted the median PO2 in BC to be 6.5 lower than in normal breast tissue. In BRCA1-mutated BC cells, the movement of HSP60 into mitochondria acts as an anti-apoptotic signal [196]. These same authors have also shown hypoxia-inducible factor-1α (HIF-1α) to be elevated in BC cells, which were accompanied by overproduction of fatty acid synthase and up-regulation of adenylate kinase A4 (AK4). Taken together, these data corroborate the reprogrammed expression of genes and their products to a glycolytic phenotype, whereby HIF-1α plays a pivotal role (for a more detailed discussion see Semenza [252]).
Cancer cells also exhibit different metabolic profiles related to the source of nutrients needed to ensure their biomass. For instance, the amino acids including serine and glycine as well as their metabolism play important roles in tumor biology [253, 254]. Using in vivo negative-selection RNAi, Possemato et al. [255] pointed out the phosphoglycerate dehydrogenase (PHGDH) levels to be elevated in 70% of the aggressive BC cells. This enzyme is compromised with serine synthesis pathway and seems to be responsible for the intermediate input for the TCA cycle, mainly α-ketoglutarate. Also, suppression of PHGDH expression promoted a drastic reduction in the cell number and death in MDA-MDB-468, BT-20, and HCC70 cell lines, demonstrating the relevance of this particular pathway in ER-negative BCs. Furthermore, since l-serine biosynthetic pathway is dysregulated in BC cells [256], its underlying mechanisms and control emerge as novel therapeutic interventions.
Recent evidence supports the role of melatonin in the regulation of glycolytic metabolism in tumor cells through inhibition of HIF-1α, down-regulating its levels in prostate and oral carcinoma [257, 258]. Despite the lack of reports elucidating the effects of melatonin targeting the Warburg effect in BC cells, an important study by Blask et al. [259], using a xenograft BC model, brings some perspective into tumor growth biomarkers: fatty acid levels, glucose and linoleic acid (LA) uptake, and lactate production were inversely correlated with melatonin levels during the 12:12 light:dark cycle. Melatonin also inhibited 13-hydroxyoctadecadienoic acid (HODE) formation and its ability to activate AKT; perfusion of BC xenografts with rat blood melatonin (1 nM) promoted downregulation in total and phospho(p)-AKT proteins most likely via MT1 receptor activation. On the contrary, when the circadian rhythms are disrupted by altering the light:dark cycle, the sustained low levels of melatonin during the 24-h period allowed the persistent increased of those markers, which was associated with high proliferation and growth-related activity of BC cells.
Prostate cancer: interplay between mitochondria and melatonin
Prostate cancer (PCa) is the second major cause of cancer death in Western men [260], and age, lifestyle, race and family history are major risk factors associated with PCa [261, 262]. The tests for detecting PCa include circulating prostate-specific antigen (PSA) levels, digital rectal examination and prostate biopsy to confirm the diagnosis [263]. Recently, Xiao et al. [264] described a novel mitochondrial-encoded peptide as a potential biomarker for PCa risk termed small humanin-like peptide-2 (SHLP2); they reported that lower SHLP2 levels are associated with increased PCa risk in white men but not in black men, suggesting a role in the development and racial disparity of PCa in addition to the involvement in aging process. The Gleason score classifies the tumor differentiation degree (1–10 scores) and can be used to compose the five PCa graded groups. TNM system is used to determine PCa stages based on tumor extension, affected lymph nodes, metastasized focus, PSA levels and Grade group [263]. The main strategy in the treatment of androgen-dependent PCa is hormonal castration; however, tumors often become androgen independent during its development and progression [265]. Although there are diagnostic tools available such as PSA, no effective therapies for late-stage PCa (e.g., hormone refractory) exist. The first chemotherapeutic agents approved by the United States Food and Drug Administration (FDA) were the combination of mitoxantrone with prednisone [266]. Other agents used in PCa chemotherapy are docetaxel, estramustine, mitoxantrone and cabazitaxel, docetaxel and estramustine. These are the most common chemotherapeutic agents currently used, and their combination with prednisone shows benefits but also includes some side effects [267].
Hormones related to the circadian cycle, such as melatonin and cortisol, have an oncostatic and immunomodulatory role in PCa. Tai et al. [268] compared the concentration of PSA and the levels of melatonin and cortisol in patients with or without PCa and, curiously, patients with PCa presented higher levels of PSA and lower urinary levels of 6-sulfatoxymelatonin (aMT6s) and cortisol than men without the disease. The urinary secretion of melatonin and cortisol could be a potential biomarker for PCa, and together with PSA, might help in the diagnosis and staging of PCa [268].
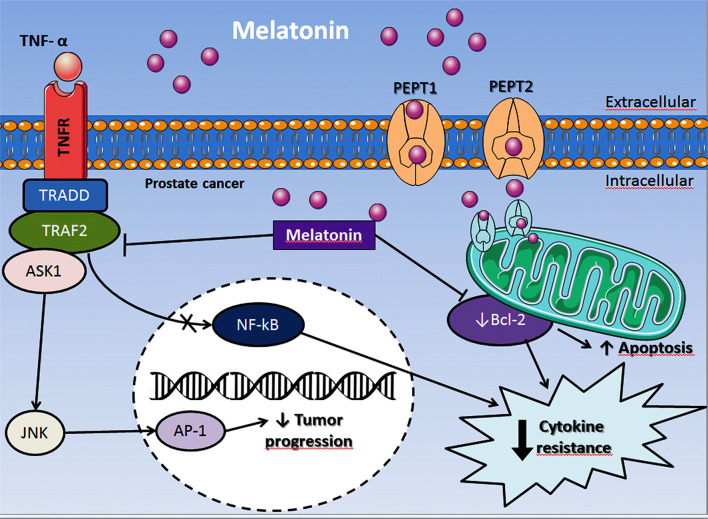
Melatonin exerts an oncostatic role in PCa through the interaction with its membrane receptors MT1 and MT2. These are G protein-coupled receptors and, specifically, the MT1 receptor has been documented to be involved with antitumor activity in prostate tumor cell lines [269], animals [270] and patient samples [271]. When the LNCaP cells were treated with 2-iodomelatonin, an analog of melatonin displaying high affinity for the MT1, they had a decrease in cell proliferation [271, 272]. Moreover, the effects of 2-iodomelatonin were reduced in the presence of luzindole, a non-selective antagonist for MT1 and MT2 receptors, demonstrating the importance of these receptors in PCa cell cycle signaling [273–275]. In addition to MT1/2 receptors, melatonin can be directly taken up by prostatic tumor cells through the GLUT1 transporter [123, 276]; the influx of melatonin appears to compete with glucose uptake [123]. More recently, PEPT1/2 transporters were first localized in the mitochondrial membrane of androgen-independent PC3 cells, and mainly the PEPT1 was proven to facilitate the transport of melatonin into mitochondria. Treatments with different concentrations of melatonin showed dose-dependent caspase-3 activation, increased BAX/Bcl-2 ratio, and release of cytochrome c to the cytosol [124]. Furthermore, melatonin induced higher ROS production and strongly reduced the ΔΨmt in PC3 cells (Fig. 6).
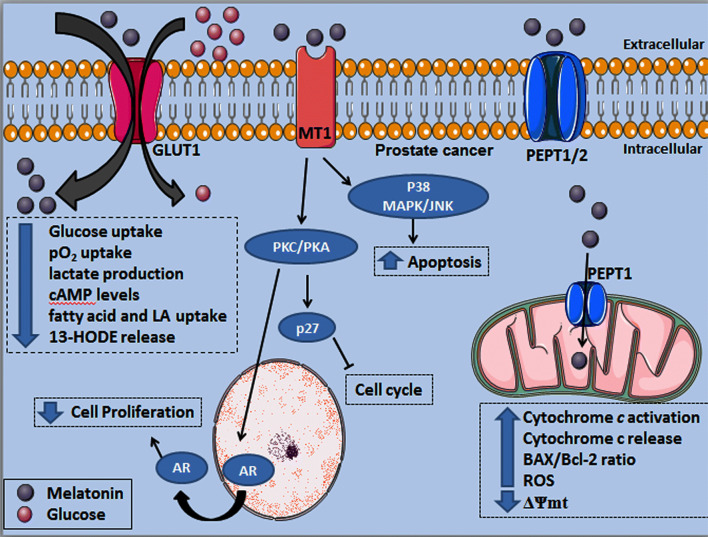
Fig. 6.
Melatonin exerts differential effects in prostate cancer cells including signaling via MT1 receptor or after being transported into the cell by GLUT1 and PEPT1/2. Melatonin competes with glucose uptake by the GLUT1, which reduces the PCa metabolic activity associated to the Warburg effect. Upon melatonin binding to the MT1 receptor, PKC and PKA are activated, resulting in AR translocation from nucleus to the cytoplasm while upregulating the p27 protein, a cell cycle inhibitor. In addition, MT1 activation significantly increases the p38MAPK/JNK activity finally resulting in elevation of apoptosis rate. Melatonin transport into PCa cell mitochondria is likely through PEPT1 increasing cytochrome c release to the cytoplasm, in association with higher BAX/Bcl-2 ratio and ROS production; these functional alterations are accompanied by a reduced ΔΨmt, which favor the induction of apoptosis. GLUT1 glucose transporter 1, MT1 melatonin receptor 1, ROS reactive oxygen species, ΔΨmt mitochondrial membrane potential, BAX Bcl-2-associated X protein, Bcl-2 B-cell lymphoma 2, PEPT1/2 human peptide transporter 1 and 2, cAMP cyclic adenosine monophosphate, pO2 oxygen pressure, LA linoleic acid, 13-HODE 13-hydroxyoctadecadienoic acid, AR androgen receptor, p27 p27 cell cycle protein, P38 MAPK P38 mitogen-activated protein kinase, JNK Jun N-terminal kinases, PKC protein kinase C, PKA protein kinase A
Melatonin is capable of interacting with the androgen receptor (AR), a key molecule involved with PCa growth and progression [277]. Activation of MT1 receptors by melatonin signals via protein kinase C (PKC) [278, 279], resulting in AR translocation from the nucleus to cytoplasm and also stimulating the overexpression of cell cycle inhibitory proteins, such as p27 [273, 274]. LNCaP and 22Rv1 cells exhibit a depressed cell proliferation rate after melatonin exposure, and such alteration occurred via MT1 activation and, consequently, activation of PKA/PKC, and up-regulation of the p27 protein [280]. Other mechanisms of melatonin’s action were observed in LNCaP cells, where the activation of p38 kinase and JNK contributed to the apoptotic processes induced by melatonin [281]. Xi et al. [272] reported the involvement of MT1 receptor in the reduction of androgen-induced Ca2+ influx, thereby promoting an anti-proliferative action in LNCap cells and decreasing detectable PSA levels. This antiproliferative effect may occur through Ca2+-binding proteins, such as calmodulin and PKC, which are highly responsive to variations in pharmacological concentrations of melatonin (from 5 × 10−10 to 5 × 10−5). Furthermore, its anti-proliferative effect on tumor cells was found to be androgen dependent [269, 272]. The inhibition of proliferation by melatonin occurs through its action on the induction of apoptosis [243] or through cell-cycle arrest [282]. Joo and Yoo [281] showed that when LNCaP cells are exposed to melatonin, a higher rate of apoptosis mediated by the JNK and p38 MAPK signaling pathways is observed, with increased expression of mitochondrial BAX and cytochrome c (Fig. 6).
Bioactive natural compounds and their synthetic derivatives may potentiate the effect of chemotherapeutic agents in the treatment of cancer [283]. Calastretti et al. [284] showed the oncostatic efficiency of UCM 1037, a melatonin analog, in androgen-sensitive (LNCaP and 22Rv1) and -insensitive (PC3 and DU145) PCa cells. Cells were exposed to UCM 1037 at 10−4 M for 24, 48 and 72 h, and showed antiproliferative and cytotoxicity effects in LNCaP and 22Rv1 cells. However, androgen-insensitive cells, such as PC3 and DU145, presented low susceptibility to UCM 1037, documenting a more effective role of melatonin in hormone-dependent PCa cells.
During tumor progression, metabolic changes are acquired [285], and organs with differential metabolic features, such as the prostate, modify their metabolism across the stages of development. In differentiated prostate cells, citrate is the end product of glucose metabolism, as part of the seminal fluid. The increase in citrate levels is related to the rise in the glycolytic rate and low levels of OXPHOS [286]. In early stages of PCa, cells produce ATP through OXPHOS, and following tumor progression, cells become highly glycolytic. So, the Warburg effect is more pronounced during the advanced PCa stage (e.g., metastasis or stages of resistance to hormonal ablation) [286]; unfortunately, this peculiar metabolic feature of PCa limits PET-scan tests for the detection of tumors at an early stage [287]. Alternatively, melatonin therapy reduces the growth of PCa by changing the glucose uptake by the cells [288]. Recently, this group hypothesized that melatonin alters glucose uptake and metabolic pathways in PCa. To achieve these results, mitochondrial metabolites from LNCaP and PC-3 cells were evaluated after exposure to melatonin through enrichment with a stable isotope of 13C-glucose; the levels of ATP/AMP and lactate dehydrogenase or pentose phosphate pathways were also investigated. Interestingly, melatonin, at dose of 1 mM for 24 h, limited glucose uptake by the PCa cells, slowing down the TCA cycle and glucose-6-phosphate dehydrogenase activity, possibly reducing the pentose phosphate pathway; this effect confirms that the reduction in glucose absorption is a significant target of melatonin in PCa [288].
Lactate is a molecule present in energetic metabolic tissues with two isomeric forms: l-lactate and d-lactate [289]. The d-lactato isomer is the end product of methylglyoxal pathway and may cause changes in tumor cells, such as in PCa [290]. Past studies have shown that d-lactate produced by prostatic tumor cells was twofold higher than in normal cells, and its increase was associated with malate transit, possibly resulting in the synthesis of fatty acids and glutathione reductase with ROS scavenging activity [291]. Since oral administration of melatonin leads to an increase in lactate production [292], the indole could act as a chemopreventive agent for PCa development.
Using PC3 xenografts in the nude rat (Crl: NIH-Foxn1rnu strain), Dauchy et al. [293] reported that animals maintained in blue-tinted rodent cages with exposure to blue spectrum light (462–484 nm) during the daytime presented a change in the circadian rhythms accompanied by an exaggerated increase in nocturnal melatonin levels (968.3 ± 102.2 pg/mL at 24:00 h) compared to animals in clear (control) rodent cages (1.50 ± 0.40 pg/mL at 08:00 h). This large increase in melatonin levels resulted in the inhibition of metabolism (Warburg effect), signaling activities, and PCa growth; total and phosphorylated forms of GSK3β, AKT, CREB, ERK 1/2, NF-κB and PDK1 were only higher when the melatonin levels were lowest (12:00 h) in animals in blue-tinted cages than in both cages at different periods. Also, rats in blue cages had a reduction in tumor glucose, pO2 uptake, lactate production, cAMP levels, total fatty acid and LA uptake, and 13-HODE release, compared with those in clear cages (Fig. 6).
Melatonin changes the phenotype to sensitize PCa cells to apoptosis via TNF-alpha and TNF-related apoptosis-inducing ligand (TRAIL) [294]. Chronic administration of melatonin (> 0.5 nm for 6 days), with the aim of increasing the sensitivity of androgen-dependent PCa cells, resulted in promotion of neuroendocrine biomarkers (NSE and SYN), making these cells more sensitive to cytokines. In addition to AR depletion, melatonin promoted neuroendocrine differentiation, with increased sensitivity to TNF-alpha and TRAIL in LNCaP and PC3 cells, which are dependent and independent of androgens, respectively [294].
NF-κB is a protein aggregate responsible for controlling the transcriptional processes of DNA; it is related to cell survival and cytokine production [295]. Activation of NF-κB together with the action of Bcl-2 and IAPs promotes cytokine resistance by PCa cells [294]. The TNF receptor (TNF-R)-associated factor 2 (TRAF2), a biomarker related to PCa prognosis [296], participates in the NF-κB activation and regulation of apoptosis signal-regulating kinase 1 (ASK1) after TNF-alpha stimulation. According to Rodriguez-Garcia et al. [294], melatonin administration reduced the TRAF2 levels in a time-dependent manner, leading to the NF-κB decrease and favoring the ASK1 pathway, which is responsible for the AP-1 recruitment, the precursor of the glutathione S-transferase [297]. The AP-1 protein together with p38 MAPK opposed the growth of LNCaP cells, and NF-κB activity was decreased by melatonin; this finally resulted in TNF-alpha-induced apoptosis mediated by Bcl-2 and survivin reduction (Fig. 7).
Fig. 7.
Melatonin enters the prostate cancer cell cytoplasm and mitochondria through the PEPT1 and PEPT2 transporters and promotes the inhibition of Bcl-2, thereby increasing apoptosis and attenuating cytokine resistance. By inhibiting TRAF2, melatonin also reduces the nuclear translocation of NF-κB, resulting in a decline in cytokine resistance and favoring the ASK1 pathway; the latter activates the JNK and p38 and, as a consequence, they stimulate the nuclear translocation of AP-1 reducing the tumor progression. PEPT1/2 human peptide transporter 1 and 2, Bcl-2 B-cell lymphoma 2, TRAF2 TNF receptor-associated factor 2, TRADD tumor necrosis factor receptor type 1-associated DEATH domain protein, NF-κB nuclear factor kappa B, ASK1 apoptosis signal-regulating kinase 1, JNK Jun N-terminal kinases, AP-1 activating protein, TNFR tumor necrosis factor receptor, TNF-α tumor necrosis factor alpha
According to Rodriguez-Garcia et al. [283], an in vitro study involving melatonin extracted from edible plants showed a significant reduction of ROS, indicated by low levels of mitochondrial O2−, and a preventive action against the oxidation of thioredoxin (TRX1) system in PCa cells. TRX1 is formed by the interaction between thioredoxin reductase (TRXR) and NADPH, and presents several biological functions, such as ROS scavenging, apoptosis regulation and redox signaling [298]. Because TRX1 can be translocated from cytoplasm to the nucleus in response to a variety of cellular events (e.g., oxidative stress, NF-κB activation, and TNF-alpha treatment) some PCa cells might develop chemoresistance. For instance, melatonin associated with silibinin reduced nuclear TRX1, thereby inhibiting the growth and sensitizing the LNCaP cells without inducing apoptosis [283].
Concluding remarks and perspective
Since mitochondria and melatonin have an intricate relationship, every organ or cell can be influenced by this molecule including tumor cells. As summarized in this report, melatonin has a broad range of physiological and molecular facets that make it efficacious in inhibiting or stimulating key cellular elements or signaling pathways associated with development and aggressiveness of reproductive cancers. Depending on reproductive cancer type and stage of development, melatonin might exert one or multiple mitochondrial functions; these biological effects have been consistently demonstrated to counteract cell proliferation, apoptosis and chemotherapy resistance, metabolism shifting, and cellular transformation, while inducing potentiation of chemotherapeutics, reducing chemotherapy side effects, and even tumor growth restriction in a variety of in vivo and in vitro reproductive cancer models. In terms of mechanisms, melatonin exerts in part its actions through its receptors or binding to other cellular substrates, and more recently, it was proven to be transported into mitochondria of tumor cells by PEPT1/2 transporters. What is the most intriguing and interesting question is the fact that melatonin paradoxically signals with divergent functions on cancer cells compared to a normal cell from the ovary, uterus, breast, or prostate tissue; for these reproductive cancers, melatonin differentially regulates important signaling pathways to produce harmful effects (e.g., cytostatic and cytotoxic effect) against tumor development. Whether there is a physical or chemical molecular arrangement inside or outside these tumor cells which is perceived by melatonin and changes its interaction with them remains to be demonstrated.
Because melatonin works as a multitasking molecule in cells [299] as well as in the mitochondria of reproductive cancer cells, it is difficult to estimate which specific event is the most effective in eliminating cancer cells and promoting an increased quality of life for patients. Melatonin surely provides versatile and beneficial protection for patients; it is not only an endogenously produced molecule but also can be safely taken as a dietary supplement. Certainly, more attention has been directed to the melatonin’s action in terms of usefulness and natural adjuvants for the treatment of hormone-dependent or -independent reproductive cancers. At pharmacological doses, melatonin is mostly active in inducing cancer cell death through the intrinsic pathway of apoptosis; in regard to these reproductive neoplastic cells, the melatonin’s role on ETC, ion channels, mitochondrial metabolomics, and sensitivity to O2 levels should emerge as new in vivo and in vitro mechanistic paradigms. In addition to its ability to reduce tumor growth, melatonin also has important actions in limiting cancer metastases which contribute to its usefulness as an important oncostatic agent [300, 301].
Acknowledgements
We are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP—Grant number 2016/03993-9) for providing financial support.
Author contributions
LGAC, LALJ, HSS, FRFS, and MSC: concept and design of the review and drafted the manuscript. RR: critical revision of the manuscript. All authors significantly contributed with compilation of the literature and approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Weiderpass E, Labrèche F. Malignant tumors of the female reproductive system. Saf Health Work. 2012;3:166–180. doi: 10.5491/SHAW.2012.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuffa LG, Lupi-Júnior LA, Costa AB, Amorim JP, Seiva FR. The role of sex hormones and steroid receptors on female reproductive cancers. Steroids. 2017;118:93–108. doi: 10.1016/j.steroids.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Stangelberger A, Waldert M, Djavan B. Prostate cancer in elderly men. Rev Urol. 2008;10:111–119. [PMC free article] [PubMed] [Google Scholar]
- 4.Kong B, Tsuyoshi H, Orisaka M, Shieh DB, Yoshida Y, Tsang BK. Mitochondrial dynamics regulating chemoresistance in gynecological cancers. Ann N Y Acad Sci. 2015;1350:1–16. doi: 10.1111/nyas.12883. [DOI] [PubMed] [Google Scholar]
- 5.Dan HC, Sun M, Kaneko S, et al. Akt phosphorylation and stabilization of x linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 6.Abedini MR, Muller EJ, Brun J, Bergeron R, Gray DA, Tsang BK. CDDP induces p53-dependent FLICE-like inhibitory protein ubiquitination in ovarian cancer cells. Cancer Res. 2008;68:4511–4517. doi: 10.1158/0008-5472.CAN-08-0673. [DOI] [PubMed] [Google Scholar]
- 7.Woo MG, Xue K, Liu JY, McBride H, Tsang BK. Calpain-mediated processing of p53 associated, Parkin-like cytoplasmic protein (PARC) affects chemosensitivity of human ovarian cancer cells by promoting p53 subcellular trafficking. J Biol Chem. 2012;287:3963–3975. doi: 10.1074/jbc.M111.314765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lissoni P, Rovelli F, Meregalli S, et al. Melatonin as a new possible anti-inflammatory agent. J Biol Regul Homeost Agents. 1997;11:157–159. [PubMed] [Google Scholar]
- 9.Vijayalaxmi Thomas CR, Jr, Reiter RJ, Herman TS. Melatonin: from basic research to cancer treatment clinics. J Clin Oncol. 2002;20:2575–2601. doi: 10.1200/JCO.2002.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Seely D, Wu P, Fritz H, et al. Melatonin as adjuvant cancer care with and without chemotherapy: a systematic review and meta-analysis of randomized trials. Integr Cancer Ther. 2012;11:293–303. doi: 10.1177/1534735411425484. [DOI] [PubMed] [Google Scholar]
- 11.Wang YM, Jin BZ, Ai F, et al. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials. Cancer Chemother Pharmacol. 2012;169:1213–1220. doi: 10.1007/s00280-012-1828-8. [DOI] [PubMed] [Google Scholar]
- 12.Del Fabbro E, Dev R, Hui D, Palmer L, et al. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: a double-blind placebo-controlled trial. J Clin Oncol. 2013;31:1271–1276. doi: 10.1200/JCO.2012.43.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sookprasert A, Johns NP, Phunmanee A, et al. Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial. Anticancer Res. 2014;34:7327–7337. [PubMed] [Google Scholar]
- 14.Ben-David MA, Elkayam R, Gelernter I, et al. Melatonin for prevention of breast radiation dermatitis: a phase II, prospective, double-blind randomized trial. Isr Med Assoc J. 2016;18:188–192. [PubMed] [Google Scholar]
- 15.Onseng K, Johns NP, Khuayjarernpanishk T, et al. Beneficial effects of adjuvant melatonin in minimizing oral mucositis complications in head and neck cancer patients receiving concurrent chemoradiation. J Altern Complement Med. 2017;12:957–963. doi: 10.1089/acm.2017.0081. [DOI] [PubMed] [Google Scholar]
- 16.Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell Mol Life Sci. 2017;74:3863–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proietti S, Cucina A, Minini M, Bizzarri M. Melatonin, mitochondria, and the cancer cell. Cell Mol Life Sci. 2017;74:4015–4025. doi: 10.1007/s00018-017-2612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cedikova M, Kripnerova M, Dvorakova J, et al. Mitochondria in white, brown, and beige adipocytes. Stem Cells Int. 2016;2016:6067349. doi: 10.1155/2016/6067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan DX, Manchester LC, Qin L, Reiter RJ. Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci. 2016;17:E2124. doi: 10.3390/ijms17122124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.TeSlaa T, Setoguchi K, Teitell MA. Mitochondria in human pluripotent stem cell apoptosis. Semin Cell Dev Biol. 2016;52:76–83. doi: 10.1016/j.semcdb.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandel NS. Mitochondria as signaling organelles. BMC Biol. 2014;12:34. doi: 10.1186/1741-7007-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaelin WG, Jr, McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153:56–69. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenaz G, Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid Redox Signal. 2010;12:961–1008. doi: 10.1089/ars.2009.2704. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed KA, Sawa T, Ihara H, Shigemoto F, Hozumi M, Takaaki A. Regulation by mitochondrial superoxide and NADPH oxidase of cellular formation of nitrated cyclic GMP: potential implications for ROS signalling. Biochem J. 2012;441:719–730. doi: 10.1042/BJ20111130. [DOI] [PubMed] [Google Scholar]
- 25.Obata T. Role of hydroxyl radical formation in neurotoxicity as revealed by in vivo free radical trapping. Toxicol Lett. 2002;132:83–93. doi: 10.1016/S0378-4274(02)00076-0. [DOI] [PubMed] [Google Scholar]
- 26.Lipinski B. Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev. 2011;2011:809696. doi: 10.1155/2011/809696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baharvand-Ahmadi B, Bahmani M, Tajeddini P, Nasrollah N, Mahmoud RK. An ethno-medicinal study of medicinal plants used for the treatment of diabetes. J Nephropathol. 2016;5:44–50. doi: 10.15171/jnp.2016.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadeghi M, Khosravi-Boroujeni H, Sarrafzadegan N, et al. Cheese consumption in relation to cardiovascular risk factors among Iranian adults-IHHP study. Nutr Res Pract. 2014;8:336–341. doi: 10.4162/nrp.2014.8.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharafati-Chaleshtori R, Shirzad H, Rafieian-Kopaei M, Soltani A. Melatonin and human mitochondrial diseases. J Res Med Sci. 2017;22:2. doi: 10.4103/1735-1995.199092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penta JS, Johnson FM, Wachsman JT, Copeland WC. Mitochondrial DNA in human malignancy. Mutat Res. 2001;488:119–133. doi: 10.1016/S1383-5742(01)00053-9. [DOI] [PubMed] [Google Scholar]
- 31.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Karakhanova S, Hartwig W, D’Haese JG, Philippov PP, Werner J, Bazhin AV. Mitochondria and mitochondrial ROS in cancer: novel targets for anticancer therapy. J Cell Physiol. 2016;231:2570–2581. doi: 10.1002/jcp.25349. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki S, Naito A, Asano T, Teresa TE, Shrikanth AG, Masahiro H. Constitutive activation of AKT pathway inhibits TNF-induced apoptosis in mitochondrial DNA-deficient human myelogenous leukemia ML-1a. Cancer Lett. 2008;268:31–37. doi: 10.1016/j.canlet.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 35.Yu J, Nagasu H, Murakami T, Hoang H, Broderick L, Hoffman HM, Horng T. Inflammasome activation leads to caspase-1 dependent mitochondrial damage and block of mitophagy. Proc Natl Acad Sci USA. 2014;111:15514–15519. doi: 10.1073/pnas.1414859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 37.Zhang E, Zhang C, Su Y, Cheng T, Shi C. Newly developed strategies for multifunctional mitochondria-targeted agents in cancer therapy. Drug Discov Today. 2011;16:140–146. doi: 10.1016/j.drudis.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Qian Y, Wu S. The Warburg effect: evolving interpretations of an established concept. Free Radic Biol Med. 2015;79:253–263. doi: 10.1016/j.freeradbiomed.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim Biophys Acta. 2011;1807:552–561. doi: 10.1016/j.bbabio.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Smolkova K, Bellance N, Scandurra F, et al. Mitochondrial bioenergetic adaptations of breast cancer cells to a glycemia and hypoxia. J Bioenerg Biomembr. 2010;42:55–67. doi: 10.1007/s10863-009-9267-x. [DOI] [PubMed] [Google Scholar]
- 43.Goto M, Miwa H, Suganuma K, et al. Adaptation of leukemia cells to hypoxic condition through switching the energy metabolism or avoiding the oxidative stress. BMC Cancer. 2014;14:76. doi: 10.1186/1471-2407-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smolkova K, Plecita-Hlavata L, Bellance N, Benard G, Rossignol R, Ježek P. Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells. Int J Biochem Cell Biol. 2011;43:950–968. doi: 10.1016/j.biocel.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Carew JS, Huang P. Mitochondrial defects in cancer. Mol Cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ju YS, Alexandrov LB, Gerstung M, et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. Elife. 2014;1:3. doi: 10.7554/eLife.02935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zong WX, Rabinowitz JD, White E. Mitochondria and cancer. Mol Cell. 2016;61:667–676. doi: 10.1016/j.molcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan DJ, Bai RK, Wong LJ. Comprehensive scanning of somatic mitochondrial DNA mutations in breast cancer. Cancer Res. 2002;62:972–976. [PubMed] [Google Scholar]
- 49.Sharp MG, Adams SM, Walker RA, Brammar WJ, Varley JM. Differential expression of the mitochondrial gene cytochrome oxidase II in benign and malignant breast tissue. J Pathol. 1992;168:163–168. doi: 10.1002/path.1711680203. [DOI] [PubMed] [Google Scholar]
- 50.Liu VW, Shi HH, Cheung AN, et al. High incidence of somatic mitochondrial DNAmutations in human ovarian carcinomas. Cancer Res. 2001;61:5998–6001. [PubMed] [Google Scholar]
- 51.Feng D, Xu H, Li X, et al. An association analysis between mitochondrial DNA content, G10398A polymorphism, HPV infection, and the prognosis of cervical cancer in the chinese han population. Tumour Biol. 2016;37:5599–5607. doi: 10.1007/s13277-015-4429-4. [DOI] [PubMed] [Google Scholar]
- 52.Zhai K, Chang L, Zhang Q, et al. Mitochondrial C150T polymorphism increases the risk of cervical cancer and HPV infection. Mitochondrion. 2011;4:559–563. doi: 10.1016/j.mito.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Guardado-Estrada M, Medina-Martínez I, Juárez-Torres E, et al. The amerindian mtDNA haplogroup B2 enhances the risk of HPV for cervical cancer: de-regulation of mitochondrial genes may be involved. J Hum Genet. 2012;57:269–276. doi: 10.1038/jhg.2012.17. [DOI] [PubMed] [Google Scholar]
- 54.Liu VW, Yang HJ, Wang Y, et al. High frequency of mitochondrial genome instability in human endometrial carcinomas. Br J Cancer. 2003;89:697–701. doi: 10.1038/sj.bjc.6601110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Liu VW, Tsang PC, Chui PM, Cheung ANY, Khoo US, Nagley P. Microsatellite instability in mitochondrial genome of common female cancers. Int J Gynecol Cancer. 2006;1:259–266. doi: 10.1136/ijgc-00009577-200602001-00042. [DOI] [PubMed] [Google Scholar]
- 56.Semczuk A, Lorenc A, Putowski L, Bartnik E. Clinic prognostical features of endometrial cancer patients with somatic mtDNA mutations. Oncol Rep. 2006;16:1041–1045. [PubMed] [Google Scholar]
- 57.Kalsbeek AMF, Chan EKF, Grogan J, et al. Altered mitochondrial genome content signals worse pathology and prognosis in prostate cancer. Prostate. 2018;78:25–31. doi: 10.1002/pros.23440. [DOI] [PubMed] [Google Scholar]
- 58.Kalsbeek AMF, Chan EKF, Corcoran NM, Hovens CM, Hayes VM. Mitochondrial genome variation and prostate cancer: a review of the mutational landscape and application to clinical management. Oncotarget. 2017;8:71342–71357. doi: 10.18632/oncotarget.19926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Creed J, Klotz L, Harbottle A, Maggrah A, Reguly B, George A, Gnanapragasm V. A single mitochondrial DNA deletion accurately detects significant prostate cancer in men in the PSA ‘grey zone’. World J Urol. 2018;36:341–348. doi: 10.1007/s00345-017-2152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng YM, Jia YF, Su LY, Wang D, Lv L, Xu L, Yao YG. Decreased mitochondrial DNA copy number in the hippocampus and peripheral blood during opiate addiction is mediated by autophagy and can be salvaged by melatonin. Autophagy. 2013;9:1395–1406. doi: 10.4161/auto.25468. [DOI] [PubMed] [Google Scholar]
- 61.Peng TI, Hsiao CW, Reiter RJ, Tanaka M, Lai YK, Jou MJ. mtDNA T8993G mutation-induced mitochondrial complex V inhibition augments cardiolipin-dependent alterations in mitochondrial dynamics during oxidative, Ca(2+), and lipid insults in NARP cybrids: a potential therapeutic target for melatonin. J Pineal Res. 2012;52:93–106. doi: 10.1111/j.1600-079X.2011.00923.x. [DOI] [PubMed] [Google Scholar]
- 62.Scott AE, Cosma GN, Frank AA, Wells RL, Gardner HS. Disruption of mitochondrial respiration by melatonin in MCF-7 cells. Toxicol Appl Pharmacol. 2001;171:149–156. doi: 10.1006/taap.2000.9115. [DOI] [PubMed] [Google Scholar]
- 63.Gilad E, Cuzzocrea S, Zingarelli B, Salzman AL, Szabó C. Melatonin is a scavenger of peroxynitrite. Life Sci. 1997;60:169–174. doi: 10.1016/S0024-3205(97)00008-8. [DOI] [PubMed] [Google Scholar]
- 64.Mansouri A, Gaou I, De Kerguenec C, et al. An alcoholic binge causes massive degradation of hepatic mitochondrial DNA in mice. Gastroenterology. 1999;117:181–190. doi: 10.1016/S0016-5085(99)70566-4. [DOI] [PubMed] [Google Scholar]
- 65.Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial dna in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J Pharmacol Exp Ther. 2001;298:737–743. [PubMed] [Google Scholar]
- 66.Lerner AB, Case JD, Takahashy Y, et al. Structure of melatonin and 5 methoxy-indole 3-acetic acid from bovine pineal gland. J Biol Chem. 1960;235:1992–1997. [PubMed] [Google Scholar]
- 67.Champney TH, Holtorf AP, Steger RW, Reiter RJ. Concurrent determination of enzymatic activities and substrate concentrations in the melatonin synthetic pathway within the same rat pineal gland. J Neurosci Res. 1984;11:59–66. doi: 10.1002/jnr.490110107. [DOI] [PubMed] [Google Scholar]
- 68.Acuña-Castroviejo D, Rahim I, Acuña-Fernández C, et al. Melatonin, clockgenes and mitochondria in sepsis. Cell Mol Life Sci. 2017;74:3965–3987. doi: 10.1007/s00018-017-2610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jockers R, Delagrange P, Dubocovich ML, et al. Update on melatonin receptors: IUPHAR review. Br J Pharmacol. 2016;173:2702–2725. doi: 10.1111/bph.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Becker-Andre M, Wiesenberg I, Schaeren-Wiemers N, André E, Missbach M, Saurat JH, Carlberg C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J Biol Chem. 1994;269:28531–28534. [PubMed] [Google Scholar]
- 71.Chuffa LG, Seiva FR, Fávaro WJ, et al. Melatonin reduces LH, 17 beta-estradiol and induces differential regulation of sex steroid receptors in reproductive tissues during rat ovulation. Reprod Biol Endocrinol. 2011;9:108. doi: 10.1186/1477-7827-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chuffa LG, Seiva FR, Fávaro WJ, et al. Melatonin and ethanol intake exert opposite effects on circulating estradiol and progesterone and differentially regulate sex steroid receptors in the ovaries, oviducts, and uteri of adult rats. Reprod Toxicol. 2013;39:40–49. doi: 10.1016/j.reprotox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 74.Tamura H, Takasaki A, Taketani T, et al. Melatonin and female reproduction. J Obstet Gynaecol Res. 2014;40:1–11. doi: 10.1111/jog.12177. [DOI] [PubMed] [Google Scholar]
- 75.Reiter RJ, Tan DX, Manchester LC, et al. Melatonin and reproduction revisited. Biol Reprod. 2009;81:445–456. doi: 10.1095/biolreprod.108.075655. [DOI] [PubMed] [Google Scholar]
- 76.Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 77.Tamura H, Kawamoto M, Sato S, et al. Long-term melatonin treatment delays ovarian aging. J Pineal Res. 2017;62:e12381. doi: 10.1111/jpi.12381. [DOI] [PubMed] [Google Scholar]
- 78.Song C, Peng W, Yin S, et al. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci Rep. 2016;6:35165. doi: 10.1038/srep35165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferreira CS, Carvalho KC, Maganhin CC, et al. Does melatonin influence the apoptosis in rat uterus of animals exposed to continuous light? Apoptosis. 2016;21:155–162. doi: 10.1007/s10495-015-1195-0. [DOI] [PubMed] [Google Scholar]
- 80.Gobbo MG, Costa CF, Silva DG, de Almeida EA, Góes RM. Effect of melatonin intake on oxidative stress biomarkers in male reproductive organs of rats under experimental diabetes. Oxid Med Cell Longev. 2015;2015:614579. doi: 10.1155/2015/614579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martín M, Macias M, Escames G, León J, Acuña-Castroviejo D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000;14:1677–1679. doi: 10.1096/fj.99-0865fje. [DOI] [PubMed] [Google Scholar]
- 82.Venegas C, García JA, Escames G, et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52:217–227. doi: 10.1111/j.1600-079X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 83.Acuña-Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71:2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He C, Wang J, Zhang Z, et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int J Mol Sci. 2016;17:E939. doi: 10.3390/ijms17060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suofu Y, Li W, Jean-Alphonse FG, et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci USA. 2017;114:7997–8006. doi: 10.1073/pnas.1705768114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Semak I, Naumova M, Korik E, Terekhovich V, Wortsman J, Slominski A. A novel metabolic pathway of melatonin: oxidation by cytochrome c. Biochemistry. 2005;44:9300–9307. doi: 10.1021/bi050202d. [DOI] [PubMed] [Google Scholar]
- 87.Martín M, Macías M, León J, Escames G, Khaldy H, Acuña-Castroviejo D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int J Biochem Cell Biol. 2002;34:348–357. doi: 10.1016/S1357-2725(01)00138-8. [DOI] [PubMed] [Google Scholar]
- 88.Leon J, Acuña-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ. Melatonin and mitochondrial function. Life Sci. 2004;75:765–790. doi: 10.1016/j.lfs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 89.López A, García JA, Escames G, Venegas C, Ortiz F, López LC, Acuña-Castroviejo D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res. 2009;46:188–198. doi: 10.1111/j.1600-079X.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- 90.Acuña-Castroviejo D, Lopez LC, Escames G, López A, García JA, Reiter RJ. Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem. 2011;11:221–240. doi: 10.2174/156802611794863517. [DOI] [PubMed] [Google Scholar]
- 91.Acuña-Castroviejo D, Escames G, Carazo A, Reiter RJ. Melatonin, mitochondrial homeostasis and mitochondrial-related diseases. Curr Top Med Chem. 2002;2:133–151. doi: 10.2174/1568026023394344. [DOI] [PubMed] [Google Scholar]
- 92.Jou MJ, Peng TI, Yu PZ, et al. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J Pineal Res. 2007;43:389–403. doi: 10.1111/j.1600-079X.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 93.Bonnefont-Rousselot D. Obesity and oxidative stress: potential roles of melatonin as antioxidant and metabolic regulator. Endocr Metab Immune Disord Drug Targets. 2014;14:159–168. doi: 10.2174/1871530314666140604151452. [DOI] [PubMed] [Google Scholar]
- 94.Galano A, Tan DX, Reiter RJ. Melatonin: a versatile protector against oxidative DNA damage. Molecules. 2018;23:E530. doi: 10.3390/molecules23030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galano A, Reiter RJ. Melatonin and its metabolites vs oxidative stress: from individual actions to collective protection. J Pineal Res. 2018;65:e12514. doi: 10.1111/jpi.12514. [DOI] [PubMed] [Google Scholar]
- 96.Munoz-Casares FC, Padillo FJ, Briceno J, et al. Melatonin reduces apoptosis and necrosis induced by ischemia/reperfusion injury of the pancreas. J Pineal Res. 2006;40:195–203. doi: 10.1111/j.1600-079X.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 97.Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res. 2010;48:297–310. doi: 10.1111/j.1600-079X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 98.Mayo JC, Sainz RM, Gonzalez MP, Cepas V, Tan DX, Reiter RJ. Melatonin and sirtuins: a “not-so unexpected” relationship. J Pineal Res. 2017;62:e12391. doi: 10.1111/jpi.12391. [DOI] [PubMed] [Google Scholar]
- 99.Horbay R, Bilyy R. Mitochondrial dynamics during cell cycling. Apoptosis. 2016;21:1327–1335. doi: 10.1007/s10495-016-1295-5. [DOI] [PubMed] [Google Scholar]
- 100.Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52:139–166. doi: 10.1111/j.1600-079X.2011.00934.x. [DOI] [PubMed] [Google Scholar]
- 101.Swietoslawski J, Karasek M. Day-night changes in the ultrastructure of pinealocytes in the Syrian hamster: a quantitative study. Endokrynol Pol. 1993;44:81–87. [PubMed] [Google Scholar]
- 102.Pei H, Du J, Song X, et al. Melatonin prevents adverse myocardial infarction remodeling via Notch1/Mfn2 pathway. Free Radic Biol Med. 2016;97:408–417. doi: 10.1016/j.freeradbiomed.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 103.Randow F, Youle RJ. Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe. 2014;15:403–411. doi: 10.1016/j.chom.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang JW, Hong JM, Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res. 2016;60:383–393. doi: 10.1111/jpi.12319. [DOI] [PubMed] [Google Scholar]
- 105.Lin C, Chao H, Li Z, et al. Melatonin attenuates traumatic brain injury-induced inflammation: a possible role for mitophagy. J Pineal Res. 2016;61:177–186. doi: 10.1111/jpi.12337. [DOI] [PubMed] [Google Scholar]
- 106.Jou MJ. Melatonin preserves the transient mitochondrial permeability transition for protection during mitochondrial Ca2+ stress in astrocyte. J Pineal Res. 2011;50:427–435. doi: 10.1111/j.1600-079X.2011.00861.x. [DOI] [PubMed] [Google Scholar]
- 107.Akhmedov AT, Rybin V, Marin-Garcia J. Mitochondrial oxidative metabolism and uncoupling proteins in the failing heart. Heart Fail Rev. 2015;20:227–249. doi: 10.1007/s10741-014-9457-4. [DOI] [PubMed] [Google Scholar]
- 108.Vitale SG, Rossetti P, Corrado F, et al. How to achieve high-quality oocytes? The key role of Myo-Inositol and melatonin. Int J Endocrinol. 2016;2016:4987436. doi: 10.1155/2016/4987436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tan DX, Manchester LC, Fuentes-Broto L, Paredes SD, Reiter RJ. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes Rev. 2011;12:167–188. doi: 10.1111/j.1467-789X.2010.00756.x. [DOI] [PubMed] [Google Scholar]
- 110.Jimenez-Aranda A, Fernandez-Vazquez G, Campos D, et al. Melatonin induces browning of inguinal white adipose tissue in zucker diabetic fatty rats. J Pineal Res. 2013;55:416–423. doi: 10.1111/jpi.12089. [DOI] [PubMed] [Google Scholar]
- 111.Reiter RJ, Tan DX, Rosales-Corral S, Bing X. Mitochondria: central organelles for melatonin’s antioxidant and anti-aging actions. Molecules. 2018;23:509. doi: 10.3390/molecules23020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Muller FL, Liu Y, Van Rammen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 113.Martín M, Macías M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, Acuña-Castroviejo D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res. 2000;28:242–248. doi: 10.1034/j.1600-079X.2000.280407.x. [DOI] [PubMed] [Google Scholar]
- 114.Reyes-Toso CF, Ricci CR, de Mignone IR, et al. In vitro effect of melatonin on oxygen consumption in liver mitochondria of rats. Neuro Endocrinol Lett. 2003;24:341–344. [PubMed] [Google Scholar]
- 115.Pacini N, Borziani F. Oncostatic-cytoprotective effect of melatonin and other bioactive molecules: a common target in mitochondrial respiration. Int J Mol Sci. 2016;17:341. doi: 10.3390/ijms17030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang H, Zhang HM, Wu LP, et al. Impaired mitochondrial complex III and melatonin responsive reactive oxygen species generation in kidney mitochondria of db/db mice. J Pineal Res. 2011;51:338–344. doi: 10.1111/j.1600-079X.2011.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fu JL, Zhang HM, Zhang H, Kamat A, Yeh CK, Zhang BX. A melatonin-based fluorescence method for the measurement of mitochondrial complex III function in intact cells. J Pineal Res. 2013;55:364–370. doi: 10.1111/jpi.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bizzarri M, Proietti S, Cucina A, Reiter RJ. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: a review. Expert Opin Ther Targets. 2013;17:1483–1496. doi: 10.1517/14728222.2013.834890. [DOI] [PubMed] [Google Scholar]
- 119.Sánchez-Hidalgo M, Guerrero JM, Villegas I, Packham G, de la Lastra CA. Melatonin, a natural programmed cell death inducer in cancer. Curr Med Chem. 2012;19:3805–3821. doi: 10.2174/092986712801661013. [DOI] [PubMed] [Google Scholar]
- 120.Yun J, Mullarky E, Lu C, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oyewole AO, Birch-Machin MA. Mitochondria-targeted antioxidants. FASEB J. 2015;29:4766–4771. doi: 10.1096/fj.15-275404. [DOI] [PubMed] [Google Scholar]
- 122.Lowes DA, Webster NR, Murphy MP, Galleyet HF. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth. 2013;110:472–480. doi: 10.1093/bja/aes577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hevia D, Gonzalez-Menendez P, Quiros-Gonzalez I, et al. Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J Pineal Res. 2015;58:234–250. doi: 10.1111/jpi.12210. [DOI] [PubMed] [Google Scholar]
- 124.Huo X, Wang C, Yu Z, et al. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: an implication of the therapeutic potential. J Pineal Res. 2017;62:e12390. doi: 10.1111/jpi.12390. [DOI] [PubMed] [Google Scholar]
- 125.Chuffa LG, Alves MS, Martinez M, et al. Apoptosis is triggered by melatonin in an in vivo model of ovarian carcinoma. Endocr Relat Cancer. 2016;23:65–76. doi: 10.1530/ERC-15-0463. [DOI] [PubMed] [Google Scholar]
- 126.Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;60:237–249. doi: 10.1007/s00428-012-1203-5. [DOI] [PubMed] [Google Scholar]
- 127.Karaca B, Atmaca H, Bozkurt E, et al. Combination of AT-101/cisplatin overcomes chemoresistance by inducing apoptosis and modulating epigenetics in human ovarian cancer cells. Mol Biol Rep. 2013;40:3925–3933. doi: 10.1007/s11033-012-2469-z. [DOI] [PubMed] [Google Scholar]
- 128.Togashi K, Okada M, Yamamoto M, et al. A small-molecule kinase inhibitor, CEP-1347, inhibits survivin expression and sensitizes ovarian cancer stem cells to paclitaxel. Anticancer Res. 2018;38:4535–4542. doi: 10.21873/anticanres.12757. [DOI] [PubMed] [Google Scholar]
- 129.Ornelas A, McCullough CR, Lu Z, et al. Induction of autophagy by ARHI (DIRAS3) alters fundamental metabolic pathways in ovarian cancer models. BMC Cancer. 2016;16:824. doi: 10.1186/s12885-016-2850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xie Q, Su J, Jiao B, et al. ABT737 reverses cisplatin resistance by regulating ER-mitochondria Ca2+ signal transduction in human ovarian cancer cells. Int J Oncol. 2016;49:2507–2519. doi: 10.3892/ijo.2016.3733. [DOI] [PubMed] [Google Scholar]
- 131.Matsuura K, Huang NJ, Cocce K, Zhang L, Kornbluth S. Downregulation of the proapoptotic protein MOAP-1 by the UBR5 ubiquitin ligase and its role in ovarian cancer resistance to cisplatin. Oncogene. 2017;36:1698–1706. doi: 10.1038/onc.2016.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang J, Zhao X, Tang M, et al. The role of ROS and subsequent DNA-damage response in PUMA-induced apoptosis of ovarian cancer cells. Oncotarget. 2017;8:23492–23506. doi: 10.18632/oncotarget.15626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Petranka J, Baldwin W, Biermann J, Jayadev S, Barrett JC, Murphy E. The oncostatic action of melatonin in an ovarian carcinoma cell line. J Pineal Res. 1999;26:129–136. doi: 10.1111/j.1600-079X.1999.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 134.Vervliet T, Clerix E, Seitaj B, Ivanova H, Monaco G, Bultynck G. Modulation of Ca(2+) signaling by anti-apoptotic B-Cell lymphoma 2 proteins at the endoplasmic reticulum-mitochondrial interface. Front Oncol. 2017;7:75. doi: 10.3389/fonc.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Akl H, Bultynck G. Altered Ca2+ signaling in cancer cells: proto-oncogenes and tumor suppressors targeting IP3 receptors. Biochim Biophys Acta. 2013;1835:180–193. doi: 10.1016/j.bbcan.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 136.Giorgi C, Missiroli S, Patergnani S, Duszynski J, Wieckowski MR, Pinton P. Mitochondria-associated membranes: composition, molecular mechanisms, and physiopathological implications. Antioxid Redox Signal. 2015;22:995–1019. doi: 10.1089/ars.2014.6223. [DOI] [PubMed] [Google Scholar]
- 137.Hwang MS, Schwall CT, Pazarentzos E, Datler C, Alder NN, Grimm S. Mitochondrial Ca2+ influx targets cardiolipin to disintegrate respiratory chain complex II for cell death induction. Cell Death Differ. 2014;21:1733–1745. doi: 10.1038/cdd.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eckenrode EF, Yang J, Velmurugan GV, Foskett JK, White C. Apoptosis protec-tion by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem. 2010;285:13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Monaco G, Beckers M, Ivanova H, Missiaen L, Parys JB, De Smedt H, Bultynck G. Profiling of the Bcl-2/Bcl-XL-binding sites on type 1 IP3 receptor. Biochem Biophys Res Commun. 2012;428:31–35. doi: 10.1016/j.bbrc.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 140.Hanson CJ, Bootman MD, Distelhorst CW, Wojcikiewicz RJ, Roderick HL. Bcl-2 suppresses Ca2+ release through inositol 1,4,5-trisphosphate receptors and inhibits Ca2+ uptake by mitochondria without affecting ER calcium store content. Cell Calcium. 2008;44:324–338. doi: 10.1016/j.ceca.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 141.Xu L, Xie Q, Qi L, et al. Bcl-2 overexpression reduces cisplatin cytotoxicity by decreasing ER-mitochondrial Ca2+ signaling in SKOV3 cells. Oncol Rep. 2018;39:985–992. doi: 10.3892/or.2017.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dai Y, Jin S, Li X, Wang D. The involvement of Bcl-2 family proteins in AKT regulated cell survival in cisplatin resistant epithelial ovarian cancer. Oncotarget. 2017;8:354–1368. doi: 10.18632/oncotarget.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chuffa LG, Lupi Júnior LA, Seiva FR, et al. Quantitative proteomic profiling reveals that diverse metabolic pathways are influenced by melatonin in an in vivo model of ovarian carcinoma. J Proteome Res. 2016;15:3872–3882. doi: 10.1021/acs.jproteome.6b00713. [DOI] [PubMed] [Google Scholar]
- 144.Chovancova B, Hudecova S, Lencesova L, et al. Melatonin-induced changes in cytosolic calcium might be responsible for apoptosis induction in tumour cells. Cell Physiol Biochem. 2017;44:763–777. doi: 10.1159/000485290. [DOI] [PubMed] [Google Scholar]
- 145.Kim JH, Jeong SJ, Kim B, Yun SM, Choi DY, Kim SH. Melatonin synergistically enhances cisplatin-induced apoptosis via the dephosphorylation of ERK/p90 ribosomal S6 kinase/heat shock protein 27 in SK-OV-3cells. J Pineal Res. 2012;52:244–252. doi: 10.1111/j.1600-079X.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- 146.Collins A, Yuan L, Kiefer TL, Cheng Q, Lai L, Hill SM. Overexpression of the mt1 melatonin receptor in mcf-7 human breast cancer cells inhibit mammary tumor formation in nude mice. Cancer Lett. 2003;189:49–57. doi: 10.1016/S0304-3835(02)00502-5. [DOI] [PubMed] [Google Scholar]
- 147.Nakamura E, Kozaki K, Tsuda H, et al. Frequent silencing of a putative tumor suppressor gene melatonin receptor 1 a (mtnr1a) in oral squamous-cell carcinoma. Cancer Sci. 2008;99:1390–1400. doi: 10.1111/j.1349-7006.2008.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jablonska K, Pula B, Zemla A, et al. Expression of the mt1 melatonin receptor in ovarian cancer cells. Int J Mol Sci. 2014;15:23074–23089. doi: 10.3390/ijms151223074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zemła A, Grzegorek I, Dzięgiel P, Jabłońska K. Melatonin synergizes the chemotherapeutic effect of cisplatin in ovarian cancer cells independently of MT1 melatonin receptors. Vivo. 2017;31:801–809. doi: 10.21873/invivo.11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 151.Suarez AA, Felix AS, Cohn DE. Bokhman Redux: endometrial cancer “types” in the 21st century. Gynecol Oncol. 2017;144:243–249. doi: 10.1016/j.ygyno.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 152.Silverberg SG, Kurman RJ, Nogales F, et al. Epithelial tumours and related lesions. In: Tavassoli FA, Devilee P, et al., editors. World Health Organization classification of tumours: pathology and genetics—tumours of the breast and female genital organs. Lyon: IARC Press; 2003. p. 232123. [Google Scholar]
- 153.Doghri R, Chaabouni S, Houcine Y, et al. Evaluation of tumor-free distance and depth of myometrial invasion as prognostic factors in endometrial cancer. Mol Clin Oncol. 2018;9:87–91. doi: 10.3892/mco.2018.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matias-Guiu X, Davidson B. Prognostic biomarkers in endometrial and ovarian carcinoma. Virchows Arch. 2014;464:315–331. doi: 10.1007/s00428-013-1509-y. [DOI] [PubMed] [Google Scholar]
- 155.Klinge CM. Estrogens regulate life and death in mitochondria. J Bioenerg Biomembr. 2017;49:307–324. doi: 10.1007/s10863-017-9704-1. [DOI] [PubMed] [Google Scholar]
- 156.Cormio A, Cormio G, Musicco C, Sardanelli AM, Gasparre G, Gadaleta MN. Mitochondrial changes in endometrial carcinoma: possible role in tumor diagnosis and prognosis (review) Oncol Rep. 2015;33:1011–1018. doi: 10.3892/or.2014.3690. [DOI] [PubMed] [Google Scholar]
- 157.Musicco C, Cormio G, Pesce V, et al. Mitochondrial dysfunctions in type I endometrial carcinoma: exploring their role in oncogenesis and tumor progression. Int J Mol Sci. 2018;19:E2076. doi: 10.3390/ijms19072076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Y, Liu VW, Xue WC, Tsang PC, Cheung AN, Ngan HY. The increase of mitochondrial DNA content in endometrial adenocarcinoma cells: a quantitative study using laser-captured microdissected tissues. Gynecol Oncol. 2005;98:104–110. doi: 10.1016/j.ygyno.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 159.Cormio A, Guerra F, Cormio G, Pesce V, et al. The PGC-1alpha–dependent pathway of mitochondrial biogenesis is upregulated in type I endometrial cancer. Biochem Biophys Res Commun. 2009;390:1182–1185. doi: 10.1016/j.bbrc.2009.10.114. [DOI] [PubMed] [Google Scholar]
- 160.Guerra F, Kurelac I, Cormio A, et al. Placing mitochondrial DNA mutations within the progression model of type I endometrial carcinoma. Hum Mol Genet. 2011;20:2394–2405. doi: 10.1093/hmg/ddr146. [DOI] [PubMed] [Google Scholar]
- 161.Murphy MP. How mitochondria produce reactive oxygen species. Biochemistry. 2009;J417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Papa S, De Rasmo D, Technikova-Dobrova Z, et al. Respiratory chain complex I, a main regulatory target of the cAMP/PKA pathway is defective in different human diseases. FEBS Lett. 2012;586:568–577. doi: 10.1016/j.febslet.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 163.Cinar B, Collak FK, Lopez D, et al. MST1 is a multifunctional caspase-independent inhibitor of androgenic signaling. Cancer Res. 2011;71:4303–4313. doi: 10.1158/0008-5472.CAN-10-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao Q, Ye M, Yang W, et al. Effect of Mst1 on endometriosis apoptosis and migration: role of Drp1-related mitochondrial fission and parkin-required mitophagy. Cell Physiol Biochem. 2018;45:1172–1190. doi: 10.1159/000487450. [DOI] [PubMed] [Google Scholar]
- 165.Attarha S, Andersson S, Mints M, Souchelnytskyi S. Mammalian sterile-like1 kinase inhibits TGFβ and EGF-dependent regulation of invasiveness, migration and proliferation of HEC-1-endometrial cancer cells. Int J Oncol. 2014;45:853–860. doi: 10.3892/ijo.2014.2447. [DOI] [PubMed] [Google Scholar]
- 166.Grin W, Grünberger W. A significant correlation between melatonin deficiency and endometrial cancer. Gynecol Obstet Investig. 1998;45:62–65. doi: 10.1159/000009926. [DOI] [PubMed] [Google Scholar]
- 167.Witek A, Jęda A, Baliś M, et al. Expression of melatonin receptors genes and genes associated with regulation of their activity in endometrial cancer. Ginekol Pol. 2015;86:248–255. doi: 10.17772/gp/2069. [DOI] [PubMed] [Google Scholar]
- 168.Osanai K, Kobayashi Y, Otsu M, Izawa T, Sakai K, Iwashita M. Ramelteon, a selective MT1/MT2 receptor agonist, suppresses the proliferation and invasiveness of endometrial cancer cells. Hum Cell. 2017;30:209–215. doi: 10.1007/s13577-017-0169-7. [DOI] [PubMed] [Google Scholar]
- 169.Watanabe M, Kobayashi Y, Takahashi N, Kiguchi K, Ishizuka B. Expression of melatonin receptor (MT1) and interaction between melatonin and estrogen in endometrial cancer cell line. J Obstet Gynaecol Res. 2008;34:567–573. doi: 10.1111/j.1447-0756.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- 170.Kobayashi Y, Itoh MT, Kondo H, et al. Melatonin binding sites in estrogen receptor-positive cells derived from human endometrial cancer. J Pineal Res. 2003;35:71–74. [PubMed] [Google Scholar]
- 171.Ciortea R, Costin N, Braicu I, et al. Effect of melatonin on intra-abdominal fat in correlation with endometrial proliferation in ovariectomized rats. Anticancer Res. 2011;31:2637–2643. [PubMed] [Google Scholar]
- 172.Bentivegna E, Gouy S, Maulard A, Chargari C, Leary A, Morice P. Oncological outcomes after fertility-sparing surgery for cervical cancer: a systematic review. Lancet Oncol. 2016;17:240–253. doi: 10.1016/S1470-2045(16)30032-8. [DOI] [PubMed] [Google Scholar]
- 173.Lai JC, Chou YJ, Huang N, et al. Survival analysis of stage IIA1 and IIA2 cervical cancer patients. Taiwan J Obstet Gynecol. 2013;52:33–38. doi: 10.1016/j.tjog.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 174.Kuzuya K. Chemoradiotherapy for uterine cancer: current status and perspectives. Int J Clin Oncol. 2004;9:458–470. doi: 10.1007/s10147-004-0452-y. [DOI] [PubMed] [Google Scholar]
- 175.Kakimoto PA, Kowaltowski AJ. Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biol. 2016;8:216–225. doi: 10.1016/j.redox.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yan D, Zhu D, Zhao X, Su J. SHP-2 restricts apoptosis induced by chemotherapeutic agents via Parkin-dependent autophagy in cervical cancer. Cancer Cell Int. 2018;18:8. doi: 10.1186/s12935-018-0505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen L, Liu L, Li Y, Gao J. Melatonin increases human cervical cancer HeLa cells apoptosis induced by cisplatin via inhibition of JNK/Parkin/mitophagy axis. In Vitro Cell Dev Biol Anim. 2018;54:1–10. doi: 10.1007/s11626-017-0200-z. [DOI] [PubMed] [Google Scholar]
- 178.Pariente R, Pariente JA, Rodríguez AB, Espino J. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J Pineal Res. 2016;60:55–64. doi: 10.1111/jpi.12288. [DOI] [PubMed] [Google Scholar]
- 179.Pariente R, Bejarano I, Espino J, Rodríguez AB, Pariente JA. Participation of MT3 melatonin receptors in the synergistic effect of melatonin on cytotoxic and apoptotic actions evoked by chemotherapeutics. Cancer Chemother Pharmacol. 2017;80:985–998. doi: 10.1007/s00280-017-3441-3. [DOI] [PubMed] [Google Scholar]
- 180.Galluzzi L, Vitale I, Michels J, et al. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis. 2014;5:1257. doi: 10.1038/cddis.2013.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Karasek M, Kowalski AJ, Suzin J, Zylinska K, Swietoslawski J. Serum melatonin circadian profiles in women suffering from cervical cancer. J Pineal Res. 2005;39:73–76. doi: 10.1111/j.1600-079X.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 182.Anisimov VN, Zabezhinski MA, Popovich IG, et al. Inhibitory effect of melatonin on 7, 12-dimethylbenz[a]anthracene-induced carcinogenesis of the uterine cervix and vagina in mice and mutagenesis in vitro. Cancer Lett. 2000;156:199–205. doi: 10.1016/S0304-3835(00)00463-8. [DOI] [PubMed] [Google Scholar]
- 183.Chen LD, Leal BZ, Reiter RJ, et al. Melatonin’s inhibitory effect on growth of ME-180 human cervical cancer cells is not related to intracellular glutathione concentrations. Cancer Lett. 1995;91:153–159. doi: 10.1016/0304-3835(95)03745-I. [DOI] [PubMed] [Google Scholar]
- 184.Fourkala EO, Blyuss O, Field H, et al. Sex hormone measurements using mass spectrometry and sensitive extraction radioimmunoassay and risk of estrogen receptor negative and positive breast cancer: case control study in uk collaborative cancer trial of ovarian cancer screening (UKCTOCS) Steroids. 2016;110:62–69. doi: 10.1016/j.steroids.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 185.Omoto Y, Iwase H. Clinical significance of estrogen receptor β in breast and prostate cancer from biological aspects. Cancer Sci. 2015;106:337–343. doi: 10.1111/cas.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Veronesi U, Boyle P, Goldhirsch A, Orecchia R, Viale G. Breast cancer. Lancet. 2005;365:1727–1741. doi: 10.1016/S0140-6736(05)66546-4. [DOI] [PubMed] [Google Scholar]
- 187.Collaborative Group on Hormonal Factors in Breast Cancer Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118.964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mediavilla MD, Sanchez-Barcelo EJ, Tan DX, Manchester L, Reiter RJ. Basic mechanisms involved in the anti-cancer effects of melatonin. Curr Med Chem. 2010;17:4462–4481. doi: 10.2174/092986710794183015. [DOI] [PubMed] [Google Scholar]
- 189.Sanchez-Barcelo EJ, Mediavilla MD, Alonso-Gonzalez C, Reiter RJ. Melatonin uses in oncology: breast cancer prevention and reduction of the side effects of chemotherapy and radiation. Expert Opin Investig Drugs. 2012;21:819–831. doi: 10.1517/13543784.2012.681045. [DOI] [PubMed] [Google Scholar]
- 190.Hill SM, Belancio VP, Dauchy RT, et al. Melatonin: an inhibitor of breast cancer. Endocr Relat Cancer. 2015;22:183–204. doi: 10.1530/ERC-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Grant SG, Melan MA, Latimer JJ, Witt-Enderby PA. Melatonin and breast cancer: cellular mechanisms, clinical studies and future perspectives. Expert Rev Mol Med. 2009;11:1–15. doi: 10.1017/S1462399409000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hill SM, Blask DE, Xiang S, et al. Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J Mammary Gland Biol Neoplasia. 2011;16:235–245. doi: 10.1007/s10911-011-9222-4. [DOI] [PubMed] [Google Scholar]
- 193.Proietti S, Cucina A, Reiter R, Bizzarri M. Molecular mechanisms of melatonin’s inhibitory actions on breast cancer. Cell Mol Life Sci. 2013;70:2139–2157. doi: 10.1007/s00018-012-1161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:313–339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 195.Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28:285–296. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- 196.Concolino A, Olivo E, Tamme L, et al. Proteomics analysis to assess the role of mitochondria in brca1-mediated breast tumorigenesis. Proteomes. 2018;6:E16. doi: 10.3390/proteomes6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim MY, Choi EO, Hwang Bo H, et al. Reactive oxygen species-dependent apoptosis induction by water extract of citrus unshiu peel in MDA-MB-231 human breast carcinoma cells. Nutr Res Pract. 2018;12:129–134. doi: 10.4162/nrp.2018.12.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Oo PS, Yamaguchi Y, Sawaguchi A, et al. Estrogen regulates mitochondrial morphology through phosphorylation of dynamin-related protein 1 in mcf7 human breast cancer cells. Acta Histochem Cytochem. 2018;51:21–31. doi: 10.1267/ahc.17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vic P, Vignon F, Derocq D, Rochefort H. Effect of estradiol on the ultrastructure of the MCF7 human breast cancer cells in culture. Cancer Res. 1982;42:667–673. [PubMed] [Google Scholar]
- 200.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 201.Trotta AP, Chipuk JE. Mitochondrial dynamics as regulators of cancer biology. Cell Mol Life Sci. 2017;74:1999–2017. doi: 10.1007/s00018-016-2451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kashatus JA, Nascimento A, Myers LJ, et al. Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Mol Cell. 2015;57:537–551. doi: 10.1016/j.molcel.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhao J, Zhang J, Yu M, et al. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene. 2013;32:4814–4824. doi: 10.1038/onc.2012.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chuang JI, Pan IL, Hsieh CY, et al. Melatonin prevents the Drp1-dependent mitochondrial fission and oxidative insult in the cortical neurons after MPP treatment. J Pineal Res. 2016;61:230–240. doi: 10.1111/jpi.12343. [DOI] [PubMed] [Google Scholar]
- 205.Xu S, Pi H, Zhang L, et al. Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J Pineal Res. 2016;60:291–302. doi: 10.1111/jpi.12310. [DOI] [PubMed] [Google Scholar]
- 206.Parameyong A, Charngkaew K, Govitrapong P, et al. Melatonin attenuates methamphetamine induced disturbances in mitochondrial dynamics and degeneration in neuroblastoma SH-SY5Y cells. J Pineal Res. 2013;55:313–323. doi: 10.1111/jpi.12078. [DOI] [PubMed] [Google Scholar]
- 207.Zhou TJ, Zhang SL, He CY, et al. Downregulation of mitochondrial cyclooxygenase-2 inhibits the stemness of nasopharyngeal carcinoma by decreasing the activity of dynamin-related protein 1. Theranostics. 2017;7:1389–1406. doi: 10.7150/thno.17647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Suwanjang W, Abramov AY, Charngkaew K, Govitrapong P, Chetsawang B. Melatonin prevents cytosolic calcium overload, mitochondrial damage and cell death due to toxically high doses of dexamethasone-induced oxidative stress in human neuroblastoma SH-SY5Y cells. Neurochem Int. 2016;97:34–41. doi: 10.1016/j.neuint.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 209.Prieto-Domínguez N, Ordóñez R, Fernández A, et al. Melatonin-induced increase in sensitivity of human hepatocellular carcinoma cells to sorafenib is associated with reactive oxygen species production and mitophagy. J Pineal Res. 2016;61:396–407. doi: 10.1111/jpi.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pedram A, Razandi M, Wallace DC, Levin ER. Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell. 2006;17:2125–2137. doi: 10.1091/mbc.e05-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Moselhy SS, Al Mslmani MA. Chemopreventive effect of lycopene alone or with melatonin against the genesis of oxidative stress and mammary tumors induced by 7,12 dimethyl(a)benzanthracene in Sprague–Dawley female rats. Mol Cell Biochem. 2008;319:175–180. doi: 10.1007/s11010-008-9890-6. [DOI] [PubMed] [Google Scholar]
- 212.Cucina A, Proietti S, D’Anselmi F, Coluccia P, Dinicola S, Frati L, Bizzarri M. Evidence for a biphasic apoptotic pathway induced by melatonin in MCF-7 breast cancer cells. J Pineal Res. 2009;46:172–180. doi: 10.1111/j.1600-079X.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- 213.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, et al. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–957. doi: 10.1016/S1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 214.Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- 215.Moroni MC, Hickman ES, Lazzerini DE, et al. Apaf-1 is a transcriptional target for E2F and p53. Nat Cell Biol. 2001;3:552–558. doi: 10.1038/35078527. [DOI] [PubMed] [Google Scholar]
- 216.Riedl SJ. Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature. 2005;434:926–933. doi: 10.1038/nature03465. [DOI] [PubMed] [Google Scholar]
- 217.Wang J, Xiao X, Zhang Y, et al. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res. 2012;53:77–90. doi: 10.1111/j.1600-079X.2012.00973.x. [DOI] [PubMed] [Google Scholar]
- 218.Sabzichi M, Samadi N, Mohammadian J, Hamishehkar H, Akbarzadeh M, Molavi O. Sustained release of melatonin: a novel approach in elevating efficacy of tamoxifen in breast cancer treatment. Colloids Surf B Biointerfaces. 2016;145:64–71. doi: 10.1016/j.colsurfb.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 219.Koşar PA, Nazıroğlu M, Övey İS, Çiğ B. Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells: involvement of TRPV1 channels. J Membr Biol. 2016;249:129–140. doi: 10.1007/s00232-015-9855-0. [DOI] [PubMed] [Google Scholar]
- 220.Mao L, Cheng Q, Guardiola-Lemaître B, et al. In vitro and in vivo antitumor activity of melatonin receptor agonists. J Pineal Res. 2010;49:210–221. doi: 10.1111/j.1600-079X.2010.00781.x. [DOI] [PubMed] [Google Scholar]
- 221.Yun SM, Woo SH, St Oh, et al. Melatonin enhances arsenic trioxide-induced cell death via sustained upregulation of Redd1 expression in breast cancer cells. Mol Cell Endocrinol. 2016;422:64–73. doi: 10.1016/j.mce.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 222.Nooshinfar E, Bashash D, Safaroghli-Azar A, Bayati S, Rezaei-Tavirani M, Ghaffari SH, Akbari ME. Melatonin promotes ATO-induced apoptosis in MCF-7 cells: proposing novel therapeutic potential for breast cancer. Biomed Pharmacother. 2016;83:456–465. doi: 10.1016/j.biopha.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 223.Woo SM, Min KJ, Kwon TK. Melatonin-mediated Bim up-regulation and cyclooxygenase-2 (COX-2) down-regulation enhances tunicamycin-induced apoptosis in MDA-MB-231 cells. J Pineal Res. 2015;58:310–320. doi: 10.1111/jpi.12217. [DOI] [PubMed] [Google Scholar]
- 224.Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer. 2007;7:519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 225.Tajbakhsh A, Pasdar A, Rezaee M. The current status and perspectives regarding the clinical implication of intracellular calcium in breast cancer. J Cell Physiol. 2017;233:5623–5641. doi: 10.1002/jcp.26277. [DOI] [PubMed] [Google Scholar]
- 226.Xu HT, Yuan XB, Guan CB, Duan S, Wu CP, Feng L. Calcium signaling in chemorepellant Slit2-dependent regulation of neuronal migration. Proc Natl Acad Sci USA. 2004;12:4296–4301. doi: 10.1073/pnas.0303893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nita LI, Hershfinkel M, Sekler I. Life after the birth of the mitochondrial Na+/Ca2+ exchanger, NCLX. Sci China Life Sci. 2015;58:59–65. doi: 10.1007/s11427-014-4789-9. [DOI] [PubMed] [Google Scholar]
- 228.Barnes DM, Millis RR, Gillett CE, Ryder K, Skilton D, Fentiman IS, Rubens RD. The interaction of oestrogen receptor status and pathological features with adjuvant treatment in relation to survival in patients with operable breast cancer: a retrospective study of 2660 patients. Endocr Relat Cancer. 2004;11:85–96. doi: 10.1677/erc.0.0110085. [DOI] [PubMed] [Google Scholar]
- 229.Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Ver. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- 230.Watson CS, Alyea RA, Jeng YJ, Kochukov MY. Nongenomic actions of low concentration estrogens and xenoestrogens on multiple tissues. Mol Cell Endocrinol. 2007;274:1–7. doi: 10.1016/j.mce.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Busselberg D, Florea AM. Targeting Intracellular calcium signaling ([Ca(2+)]i) to overcome acquired multidrug resistance of cancer cells: a mini-overview. Cancers (Basel) 2017;9:48. doi: 10.3390/cancers9050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cui C, Merritt R, Fu L, Zui P. Targeting calcium signaling in cancer therapy. Acta Pharm Sin. 2017;7:3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Brookes PS. Mitochondrial nitric oxide synthase. Mitochondrion. 2004;3:187–204. doi: 10.1016/j.mito.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 234.Csordas G, Golenar T, Seifert EL, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter. Cell Metab. 2013;7:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Curry MC, Luk NA, Kenny PA, Roberts-Thomson SJ, Monteith GR. Distinct regulation of cytoplasmic calcium signals and cell death pathways by different plasma membrane calcium ATPase isoforms in MDA-MB-231 breast cancer cells. J Biol Chem. 2012;287:28598–28608. doi: 10.1074/jbc.M112.364737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.VanHouten J, Sullivan C, Bazinet C, et al. PMCA2 regulates apoptosis during mammary gland involution and predicts outcome in breast cancer. Proc Natl Acad Sci USA. 2010;25:11405–11410. doi: 10.1073/pnas.0911186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Curry MC, Peters AA, Kenny PA, Roberts-Thomson SJ, Monteith GR. Mitochondrial calcium uniporter silencing potentiates caspase-independent cell death in MDA-MB-231 breast cancer cells. Biochem Biophys Res Commun. 2013;434:695–700. doi: 10.1016/j.bbrc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 240.França EL, Honorio-França AC, Fernandes RT, Marins CM, Pereira CC, Varotti Fde P. The effect of melatonin adsorbed to polyethylene glycol microspheres on the survival of MCF-7 cells. Neuro Immunomodulation. 2016;23:27–32. doi: 10.1159/000439277. [DOI] [PubMed] [Google Scholar]
- 241.Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress: a review. J Biomed Sci. 2000;7:444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 242.Naziroglu M, Karaoğlu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004;195:221–230. doi: 10.1016/j.tox.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 243.Uguz AC, Cig B, Espino J, et al. Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J Pineal Res. 2012;53:91–98. doi: 10.1111/j.1600-079X.2012.00974.x. [DOI] [PubMed] [Google Scholar]
- 244.Sag CM, Wagner S, Maier LS. Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes. Free Radic Biol Med. 2013;63:338–349. doi: 10.1016/j.freeradbiomed.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 245.Bejarano I, Espino J, Barriga C, Reiter RJ, Pariente JA, Rodríguez AB. Pro-oxidant effect of melatonin in tumour leucocytes: relation with its cytotoxic and pro-apoptotic effects. Basic Clin Pharmacol Toxicol. 2011;108:14–20. doi: 10.1111/j.1742-7843.2010.00619.x. [DOI] [PubMed] [Google Scholar]
- 246.Squecco R, Tani A, Zecchi-Orlandini S, Formigli L, Francini F. Melatonin affects voltage-dependent calcium and potassium currents in MCF-7 cell line cultured either in growth or differentiation medium. Eur J Pharmacol. 2015;758:40–52. doi: 10.1016/j.ejphar.2015.03.068. [DOI] [PubMed] [Google Scholar]
- 247.Mahapatra S, Marcantoni A, Zuccotti A, Carabelli V, Carbone E. Equal sensitivity of cav1.2 and cav1.3 channels to the opposing modulations of PKA and PKG in mouse chromaffin cells. J Physiol. 2012;590:5053–5073. doi: 10.1113/jphysiol.2012.236729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Margheri M, Pacini N, Tani A, et al. Combined effects of melatonin and all-trans retinoic acid and somatostatin on breast cancer cell proliferation and death: molecular basis for the anticancer effect of these molecules. Eur J Pharmacol. 2012;681:34–43. doi: 10.1016/j.ejphar.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 249.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 251.Vaupel P, Mayer A, Höckel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004;381:335–354. doi: 10.1016/S0076-6879(04)81023-1. [DOI] [PubMed] [Google Scholar]
- 252.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Locasale JW, Grassian AR, Melman T, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Keibler MA, Wasylenko TM, Kelleher JK, Iliopoulos O, Vander Heiden MG, Stephanopoulos G. Metabolic requirements for cancer cell proliferation. Cancer Metab. 2016;4:16. doi: 10.1186/s40170-016-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Possemato R, Marks KM, Shaul YD, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pollari S, Käkönen SM, Edgren H, et al. Enhanced serine production by bine metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res Treat. 2011;125:421–430. doi: 10.1007/s10549-010-0848-5. [DOI] [PubMed] [Google Scholar]
- 257.Goncalves Ndo N, Rodrigues RV, Jardim-Perassi BV, Moschetta MG, Lopes JR, Colombo J, Zuccari DA. Molecular markers of angiogenesis and metastasis in lines of oral carcinoma after treatment with melatonin. Anticancer Agents Med Chem. 2014;14:1302–1311. doi: 10.2174/1871520614666140812110246. [DOI] [PubMed] [Google Scholar]
- 258.Sohn EJ, Won G, Lee J, Lee S, Kim SH. Upregulation of miRNA3195 and miRNA374b mediates the anti-angiogenic properties of melatonin in hypoxic PC-3 prostate cancer cells. J Cancer. 2015;6:19–28. doi: 10.7150/jca.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Blask DE, Dauchy RT, Dauchy EM, et al. Light exposure at night disrupts host/cancer circadian regulatory dynamics: impact on the warburg effect, lipid signaling and tumor growth prevention. PLoS One. 2014;9:102776. doi: 10.1371/journal.pone.0102776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Das R, Gregory PA, Hollier BG, Tilley WD, Selth LA. Epithelial plasticity in prostate cancer: principles and clinical perspectives. Trends Mol Med. 2014;20:643–655. doi: 10.1016/j.molmed.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 261.Glass AS, Cary KC. Risk-based prostate cancer screening: who and how? Curr Urol Rep. 2013;14:192–198. doi: 10.1007/s11934-013-0319-8. [DOI] [PubMed] [Google Scholar]
- 262.Gathirua-Mwang WG, Zhang J. Dietary factors and risk for advanced prostate cancer. Eur J Cancer Prev. 2014;23:96–109. doi: 10.1097/CEJ.0b013e3283647394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.American Cancer Society. (2017) Information and resources about cancer: breast, colon, lung, prostate, skin. https://www.cancer.org/about-us.html. Accessed 26 June 2018
- 264.Xiao J, Howard L, Wan J. Low circulating levels of the mitochondrial-peptide hormone SHLP2: novel biomarker for prostate cancer risk. Oncotarget. 2017;8:94900–94909. doi: 10.18632/oncotarget.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sainz RM, Mayo JC, Tan D, León J, Manchester L, Reiter RJ. Melatonin reduces prostate cancer cell growth leading to neuroendocrine differentiation via a receptor and PKA independent mechanism. Prostate. 2005;63:29–43. doi: 10.1002/pros.20155. [DOI] [PubMed] [Google Scholar]
- 266.Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 267.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 268.Tai SY, Huang SP, Bao BY, Wu MT. Melatonin-sulfate/cortisol ratio and the presence of prostate cancer: a case–control study. Sci Rep. 2016;6:29606. doi: 10.1038/srep29606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Marelli MM, Limonta P, Maggi R, Motta M, Moretti RM. Growth-inhibitory activity of melatonin on human androgen-independent DU 145 prostate cancer cells. Prostate. 2000;45:238–344. doi: 10.1002/1097-0045(20001101)45:3<238::AID-PROS6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 270.Xi SC, Siu SW, Fong SW, Shiu SY. Inhibition of androgen-sensitive LNCaP prostate cancer growth in vivo by melatonin: association of antiproliferative action of the pineal hormone with mt1 receptor protein expression. Prostate. 2001;46:52–61. doi: 10.1002/1097-0045(200101)46:1<52::AID-PROS1008>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 271.Shiu SY, Law IC, Lau KW, Tam PC, Yip AW, Ng WT. Melatonin slowed the early biochemical progression of hormone-refractory prostate cancer in a patient whose prostate tumor tissue expressed MT1 receptor subtype. J Pineal Res. 2003;35:177–182. doi: 10.1034/j.1600-079X.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 272.Xi SC, Tam PC, Brown GM, Pang SF, Shiu SY. Potential involvement of mt1 receptor and attenuated sex steroid-induced calcium influxin the direct anti-proliferative action of melatonin on androgen-responsive LNCaP human prostate cancer cells. J Pineal Res. 2000;29:172–183. doi: 10.1034/j.1600-079X.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 273.Tam CW, Mo CW, Yao KM, Shiu SY. Signaling mechanisms of melatonin in antiproliferation of hormone-refractory 22Rv1 human prostate cancer cells: implications for prostate cancer chemoprevention. J Pineal Res. 2007;42:191–202. doi: 10.1111/j.1600-079X.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 274.Tam CW, Chan KW, Liu VW, et al. Melatonin as a negative mitogenic hormonal regulator of human prostate epithelial cell growth: potential mechanisms and clinical significance. J Pineal Res. 2008;45:403–412. doi: 10.1111/j.1600-079X.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 275.Shiu SY, Pang B, Tam CW, Yao KM. Signal transduction of receptor-mediated antiproliferative action of melatonin on human prostate epithelial cells involves dual activation of galpha(s) and galpha(q) proteins. J Pineal Res. 2010;49:301–311. doi: 10.1111/j.1600-079X.2010.00795.x. [DOI] [PubMed] [Google Scholar]
- 276.Bazwinsky-Wutschke I, Bieseke L, Mühlbauer E, Peschke E. Influence of melatonin receptor signalling on parameters involved in blood glucose regulation. J Pineal Res. 2014;56:82–96. doi: 10.1111/jpi.12100. [DOI] [PubMed] [Google Scholar]
- 277.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinog. 2011;10:20. doi: 10.4103/1477-3163.83937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lupowitz Z, Rimler A, Zisapel N. Evaluation of signal transduction pathways mediating the nuclear exclusion of the androgen receptor by melatonin. Cell Mol Life Sci. 2001;58:2129–2135. doi: 10.1007/PL00000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rimler A, Culig Z, Levy-Rimler G, et al. Melatonin elicits nuclear exclusion of the human androgen receptor and attenuates its activity. Prostate. 2001;49:145–154. doi: 10.1002/pros.1129. [DOI] [PubMed] [Google Scholar]
- 280.Tam CW, Shiu S. Functional interplay between Melatonin receptor-mediated antiproliferative signaling and androgen receptor signaling in human prostate epithelial cells: potential implications for therapeutic strategies against prostate cancer. J Pineal Res. 2011;51:297–312. doi: 10.1111/j.1600-079X.2011.00890.x. [DOI] [PubMed] [Google Scholar]
- 281.Joo SS, Yoo YM. Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res. 2009;47:8–14. doi: 10.1111/j.1600-079X.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- 282.Moretti RM, Marelli MM, Maggi R, et al. Antiproliferative action of melatonin on human prostate cancer LNCaP cells. Oncol Rep. 2000;7:347–351. [PubMed] [Google Scholar]
- 283.Rodriguez-Garcia A, Hevia D, Mayo JC, et al. Thioredoxin 1 modulates apoptosis induced by bioactive compounds in prostate cancer cells. Redox Biol. 2017;12:634–647. doi: 10.1016/j.redox.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Calastretti A, Gatti G, Lucini V, Dugnani S, Canti G, Scaglione F, Bevilacqua A. Melatonin analogue antiproliferative and cytotoxic effects on human prostate cancer cells. Int J Mol Sci. 2018;18:19. doi: 10.3390/ijms19051505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Cutruzzolà F, Giardina G, Marani M, et al. Glucose metabolism in the progression of prostate cancer. Front Physiol. 2017;8:97. doi: 10.3389/fphys.2017.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Schöder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med. 2004;34:274–292. doi: 10.1053/j.semnuclmed.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 288.Hevia D, Gonzalez-Menendez P, Fernandez-Fernandez M, et al. Melatonin decreases glucose metabolism in prostate cancer cells: a 13c stable isotope-resolved metabolomic study. Int J Mol Sci. 2017;26:18. doi: 10.3390/ijms18081620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.John RP, Nampoothiri KM, Pandey A. Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl Microbiol Biotechnol. 2007;74:524–534. doi: 10.1007/s00253-006-0779-6. [DOI] [PubMed] [Google Scholar]
- 290.Rulli A, Carli L, Romani R, Baroni T, Giovannini E, Rosi G, Talesa V. Expression of glyoxalase I and II in normal and breast cancer tissues. Breast Cancer Res. 2001;66:67–72. doi: 10.1023/A:1010632919129. [DOI] [PubMed] [Google Scholar]
- 291.de Bari L, Moro L, Passarella S. Prostate cancer cells metabolize d-lactate inside mitochondria via a d-lactate dehydrogenase which is more active and highly expressed than in normal cells. FEBS Lett. 2013;587:467–473. doi: 10.1016/j.febslet.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 292.Sayed RKA, Fernández-Ortiz M, Diaz-Casado ME, et al. The protective effect of melatonin against age-associated sarcopenia-dependent tubular aggregates formation, lactate depletion and mitochondrial changes. J Gerontol A Biol Sci Med Sci. 2018;73:1330–1338. doi: 10.1093/gerona/gly059. [DOI] [PubMed] [Google Scholar]
- 293.Dauchy RT, Hoffman AE, Wren-Dail MA, et al. Daytime blue light enhances the nighttime circadian melatonin inhibition of human prostate cancer growth. Comp Med. 2015;65:473–485. [PMC free article] [PubMed] [Google Scholar]
- 294.Rodriguez-Garcia A, Mayo JC, Hevia D, Quiros-Gonzalez I, Navarro M, Sainz RM. Phenotypic changes caused by melatonin increased sensitivity of prostate cancer cells to cytokine-induced apoptosis. J Pineal Res. 2013;54:33–45. doi: 10.1111/j.1600-079X.2012.01017.x. [DOI] [PubMed] [Google Scholar]
- 295.Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 296.Wei B, Liang J, Hu J, et al. TRAF2 is a valuable prognostic biomarker in patients with prostate cancer. Med Sci Monit. 2017;23:4192–4204. doi: 10.12659/MSM.903500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Aggarwal BB. Signaling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 298.Muri J, Heer S, Matsushita M, et al. The thioredoxin-1 system is essential for fueling DNA synthesis during T-cell metabolic reprogramming and proliferation. Nat Commun. 2018;9:1851. doi: 10.1038/s41467-018-04274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Reiter RJ, Tan DX, Fuentes-Broto L. Melatonin: a multitasking molecule. Prog Brain Res. 2010;181:127–151. doi: 10.1016/S0079-6123(08)81008-4. [DOI] [PubMed] [Google Scholar]
- 300.Su SC, Hsieh MJ, Yang WE, Chung WH, Reiter RJ, Yang SF. Cancer metastasis: mechanisms of inhibition by melatonin. J Pineal Res. 2017;62:e12370. doi: 10.1111/jpi.12370. [DOI] [PubMed] [Google Scholar]
- 301.Reiter RJ, Rosales-Corral SA, Tan DX, Acuna-Castroviejo D, Qin L, Yang SF, Xu K. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci. 2017;18:843. doi: 10.3390/ijms18040843. [DOI] [PMC free article] [PubMed] [Google Scholar]