Abstract
Multicopper oxidases (MCOs) are a pervasive family of enzymes that oxidize a wide range of phenolic and nonphenolic aromatic substrates, concomitantly with the reduction of dioxygen to water. MCOs are usually divided into two functional classes: metalloxidases and laccases. Given their broad substrate specificity and eco-friendliness (molecular oxygen from air as is used as the final electron acceptor and they only release water as byproduct), laccases are regarded as promising biological green tools for an array of applications. Among these laccases, those of bacterial origin have attracted research attention because of their notable advantages, including broad substrate spectrum, wide pH range, high thermostability, and tolerance to alkaline environments. This review aims to summarize the significant research efforts on the properties, mechanisms and structures, laccase-mediator systems, genetic engineering, immobilization, and biotechnological applications of the bacteria-source laccases and laccase-like enzymes, which principally include Bacillus laccases, actinomycetic laccases and some other species of bacterial laccases. In addition, these enzymes may offer tremendous potential for environmental and industrial applications.
Keywords: Laccase, Green catalyst, Mediator, Engineering, Immobilization, Decolorization, Degradation, Delignification
Introduction
Laccases (benzenediol:oxygen oxidoreductases, EC 1.10.3.2) belong to a family of blue multicopper enzymes that oxidize a range of substrates, such as phenols (–OH), anilines (–NH2), arylamines, ascorbic acid, and certain inorganic compounds, coupled to the four-electron reduction of dioxygen to water [1–3]. Laccases are widely distributed in nature. The first laccase was extracted from the latex of the Japanese lacquer tree Rhus vernicifera in the late 19th century [4]. To date, laccases are widely distributed in almost all wood rotting fungi [5]. Laccases have also been identified in several higher plant species [4, 6–8], lichens [9], and sponges [10]. Moreover, polyphenol oxidases with laccase-like activity have also been found in oysters [11], insects [12–14], metagenome libraries of bovine rumen [15], and acidic bog soil metagenome [16]. Among these laccases, fungal laccases have been widely studied. Several studies on the application of Trametes versicolor laccase have been conducted in recent years [17–27], but fungal laccases generally fail to work in extreme environments during industrial operations. Laccases are usually suitable under mesophilic and acidic reaction conditions [28]. Therefore, bacterial laccases have attracted research attention [29–42]. Here, we summarized bacterial laccases, mainly including Bacillus and actinomycetic laccases.
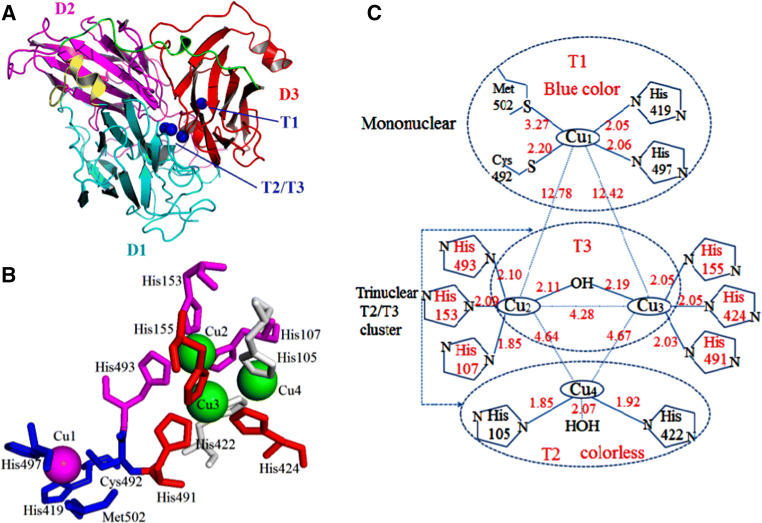
Laccases are monomeric, dimeric, or tetrameric glycoproteins. First and foremost, bacteria are the sources of prokaryotic laccase. To our knowledge, the molecular structure of typical bacterial laccase usually contains three types of copper ions according to their magnetic and spectroscopic properties, namely, type 1 (T1), type 2 (T2), and double type 3 (T3) copper ions [43, 44]. The center of T1 copper, which is in charge of electron transfer, is responsible for substrate oxidation. T1 copper exhibits strong electronic absorbance around 610 nm, and electro-paramagnetic resonance (EPR) can be detected. The T2 site is a mono-nuclear center formed by T2 copper; the site is colorless, and EPR is also detectable. The T3 site is composed of two strongly coupled T3 coppers, which provide a weak absorbance near the UV spectrum (330 nm); here, EPR is not detectable [43]. The trinuclear T2/T3 cluster is composed of one T2 copper ion and two T3 copper ions, which bind and reduce molecular oxygen to water [45, 46]. The copper centers are shown using Bacillus subtilis CotA-laccase, a fully characterized and studied laccase (Fig. 1).
Fig. 1.
Overall structure and copper centers of B. subtilis CotA-laccase (PDB code 1GSK). a Domains, T1, and T2/T3 copper (domains1, 2, and 3 represented in cyan, magenta, and red, respectively). D2 acts as a bridge between D1 and D3. A short α-helical fragment shown in yellow connects D1 and D2. A large loop segment shows D2 and D3 in green links). b Conservative amino acids around the copper center. Molecular representations were generated using PyMOL [190]. c Schematic of T1 and T2/T3 centers, including interatomic distances among all relevant atoms [89]
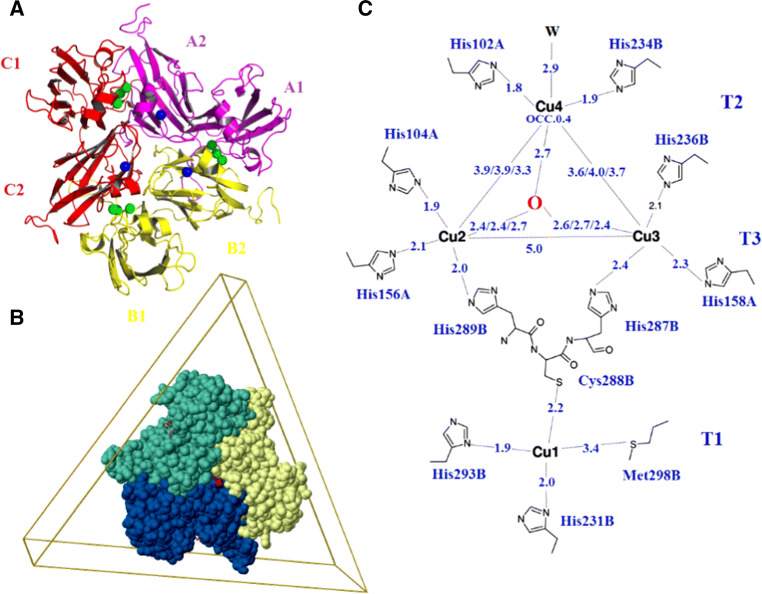
The other significant bacterial laccases are from actinomycetes, which belong to broad bacteriology. Streptomyces laccases of actinomycetes are the most identified and characterized and are known as extracellular enzymes. A variety of laccases have been identified in species including S. cyaneus [30, 47, 48], S. coelicolor [49, 50], S. bikiniensis [51], and S. ipomoea [52]. Among these laccases, S. coelicolor laccase is the most extensively characterized. Different from the typical three-domain bacterial laccases, Streptomyces laccases are usually two-domain laccases, such as the so-called small laccase (SLAC) from S. coelicolor (Fig. 2). Compared with common three-domain laccases, SLAC is composed of only two domains without domain 2. Domain 2 is responsible for the connection and positioning of domains 1 and 3 in three-domain laccase, which is essential for the formation of trinuclear cluster at the interface of domains 1 and 3 [28]. However, to form the trinuclear cluster and intact catalytic site, the homotrimer structure is formed to overcome the lacking domain 2 in two-domain laccases [50, 53]. SLAC contains 12 copper ions that form three pseudo-symmetrically related active units. Each SLAC monomer consists of two domains (Fig. 2a, domain 1, which includes A1, B1, and C1, and domain 2, which is composed of A2, B2, and C2). Each copper center is formed by two neighboring chains organized in a head-to-tail manner (A1–B2, B1–C2, and C1–A2) and contains four copper ions (Fig. 2c). The three copper ions of type 1 are localized near the surface of the central part of the trimer. Three trinuclear copper clusters are placed between domains 1 and 2 of each of the two neighbor chains of the trimer, contributing strongly to the stability of the trimer [50]. However, the spectroscopic and kinetic properties remained similar to those of the common three-domain laccases [54].
Fig. 2.
Structure of the two-domain laccase SLAC from S. coelicolor (PDB: 3CG8). a SLAC forms homotrimers and monomers are colored in magenta, yellow, and red. T1Cu is shown in blue, and T2/T3Cu is shown in green. b 3D view of SLAC. c Copper binding scheme in SLAC. Three active sites in the SLAC trimer at domain interfaces A1–B2, B1–C2, and C1–A2. The chain notation corresponds to the interface A1–B2 [50]
In recent years, bacterial laccases from different microorganisms have been isolated and characterized. The strategies of upgrading the production of laccases have also been summarized [55]. Metagenomic analysis [16] has become the most useful and powerful technological tool for determining potential laccase from natural microorganisms, especially from the genes of uncultured and non-cultivable microbes. Immobilization and genetic engineering technologies remain in demand [39, 56–60]. Nanomaterials and ultrafiltration membranes have been extensively researched as vectors for laccase [18, 25, 61–65]. The applications of bacterial laccases, such as degradation of textile dyes, pollutant degradation, bio-sensor, and paper industry, are increasing due to their notable features in extreme industrial environment. Compared with fungal laccases, bacterial laccases exhibit the most significant advantages of high thermostability, wide pH range, and tolerance to alkaline conditions. Bacterial laccases are regarded as promising biological green tools for industrial applications.
Species and properties
Bacterial laccase was first isolated from Azospirillum sp. in 1993 from rice rhizosphere [66]. Then, laccases were gradually discovered from numerous other bacteria of different genera, such as Gram-positive bacteria, including Bacillus, Streptomyces, Geobacterial, Staphylococcus, Lysinibacillus, and Aquisalibacillus, and Gram-negative bacteria, including Pseudomonas, Delfia, Enterobacter, Proteobacterium, and Alteromonas [28]. In addition, an increasing number of laccase genes from the metagenome libraries of soil sludge and water are recombinantly expressed [16, 67, 68]. The most characterized CotA-laccases are from Bacillus, such as B. subtilis [57, 69, 70], B. pumilus [42, 44], B. licheniformis [71], B. halodurans [72], Bacillus sp. HR03 [73], B. vallismortis [74, 75], B. tequilensis [76], B. amyloliquefaciens [77], Bacillus sp. ADR [78], B. sphaericus [79], B. clausii [80], B. altitudinis [42], B. safensis [81], and B. cereus [82]. Streptomyces laccases also exist in a variety of species, such as S. coelicolor [49, 83], S. cyaneus [48], S. griseus [84], S. lavendulae [85], S. psammoticus [86], S. ipomoea [52], S. sviceus [53], S. bikiniensis [51], S. violaceusniger, S. lividans, and S. viridosporus [87, 88]. Several novel, special sources of laccases are depicted in Table 1.
Table 1.
Bacterial laccases and laccase-like from different environmental sources and their properties reported in recent years
| No. | Laccase source | Expression host | Molecular weight (kDa) | Substrates used in enzyme assay | Optimum temperature (°C) | Thermal stability (°C) | Optimum pH | pH stability | kcat/Km (s−1 mM−1) | Activity and function | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Klebsiella pneumoniae | E. coli | ~ 55.6 | ABTS, 2,6-DMP |
35 (ABTS) 70 (2,6-DMP) |
30–70 |
4.0 (ABTS) 8.0 (2,6-DMP) |
5.0–9.0 |
0.19 (ABTS) 2.36 (2,6-DMP) |
Dyes decolorization | [183] |
| 2 | Acidic bog soil metagenome | E. coli | ~ 50.3 |
ABTS, 2,6-DMP l-DOPA Vanillic acid Syringaldazine Pyrogallol Pyrocatechol |
50 (ABTS) | 40–60 |
4.0 (ABTS) 5.0 (2,6-DMP) |
– |
8.45 (ABTS) 6.42 (2,6-DMP) 173.6 (Pyrogallol) |
Decolored azo and triphenylmethane dyes | [16] |
| 3 | Metagenome of chemical plant sludge (CueO-G276R) | E. coli | ~ 60 |
ABTS Benzo[α]pyrene |
60 (ABTS) | – | 3.5 (ABTS) | – | 123 (ABTS) | Oxidize carcinogen benzo[α]pyrene and biological remediation | [68] |
| 4 | Proteus hauseri ZMd44 (MCO) | E. coli | – | ABTS | 55 (ABTS) | – | 2.2 (ABTS) | – | 73.03 (ABTS) | Au adsorption, as a biosensor and bioremediation of electronic waste | [182] |
| 5 | Bacillus lichniformis LS04 | P. pastoris | – | ABTS |
70 [WT] 70 [D500G] |
70 °C/1.8 h [D500G] |
4.2 [WT] 4.6 [D500G] |
– |
127.27 [WT] 115.12 [D500G] |
Dyes decolorization | [103] |
| 6 | Bacillus subtilis cjp3 | E. coli | ~ 58.5 |
ABTS SGZ |
80 (ABTS) | 20–80 | 5.0 (ABTS) | 9.0/10 h | – | Treating waste water containing synthetic dyes | [70] |
| 7 | Streptomyces cyaneus CECT 3335 | E. coli | ~ 69.5 |
DMP ABTS Guaiacol |
30–90 (DMP) | 60 °C/1 h/50% activity | 5.5 (DMP) | 5.5/24 h/40% |
4.737 (DMP) 1.72 (ABTS) 5.17 (Guaiacol) |
Biomass degradation | [30] |
| 8 | Chromohalobacter salexigens | – | – |
2,6-DMP, ABTS l-Tyrosine, SGZ catechol l-DOPA, guaiacol Gallic acid, tannic acid, pyrogallol Resorcinol |
– | 25–55 °C/80% activity | – | 6.0–9.0/80% | – | Cellulose fibres extraction, delignification of ligin and ligin-derived industrial wastes | [171] |
| 9 | Bacillus vallismortis fmb-103 | E. coli | ~ 70 |
ABTS SGZ Acetosyringone |
84 [H/AS] 85 [Ni–NTA] (ABTS) |
70 °C/10 h/> 50% activity |
4.8 (ABTS) 7.4 (SGZ) |
8.0/10 d/90%[H/AS] 8.0/10 d/80%[Ni–NTA] |
Km = 35.79 μM [1] Km = 24.4 μM [2] (ABTS) Km = 379.12 μM[1] Km = 302.4 μM [2] (SGZ) |
Malachite green degradation | [74] |
| 10 | Setosphaeria turcica | E. coli | ~ 71.5 | ABTS | 60 (ABTS) | – | 4.0 (ABTS) | – | – | Industrial effluents application | [184] |
| 11 | Aquisalibacillus elongatus | – | ~ 75 | 2,6-DMP | 40 | 25–55 °C/6 h/> 80% activity |
8.0 (2,6-DMP) 6.0 (ABTS) 7.0 (SGZ) |
5.0–10.0/6 h/> 40% | 1.23 × 105 | Delignification of sugar beet pulp | [170] |
| 12 | Thermus thermophilus | E. coli | ~ 53 |
ABTS, SGZ 4-fluoro-2-methylp-henol |
90 (ABTS) 80 (SGZ) [short duration] 65 (ABTS) [longer duration] |
– |
6.0 (ABTS) 7.0 (SGZ) 8.0 (4-fluoro-2-methylphenol) |
– |
1.99 (ABTS) [60 °C] 673.20 (SGZ) [40 °C] |
Decolourization of industrial dyes | [154] |
| 13 | Pediococcus Acidilactici CECT 5930 | E. coli | ~ 60 | ABTS | 28 (ABTS) | 28–60 °C/10 min/> 80% activity |
4.0 (ABTS) 9.5 (Tyramine) |
– |
Km = 1.7 mM (ABTS) |
Degrade tyramine in food | [175] |
| 14 | Anoxybacillus sp. UARK-01 | E. coli | – | Congo Red | 90 | – | 9 | – | – | Dyes decolorization | [152] |
| 15 | Geobacillus thermopakista-niensis | E. coli | ~ 60 | Syringaldazine | 60 (SGZ) | – | 7–7.5 (SGZ) | – | 1179 (SGZ) | Bioremediation of colored wastewater | [185] |
| 16 | Spirulina platensis CFTRI | E. coli | ~ 66 | ABTS | 30 (ABTS) | 30 °C/1 h/100%, 50 °C/1.5 h/80% | 3.0 (ABTS) | 8.0/1 h/100% | – | Decolorize synthetic dyes and treat waste water | [153] |
| 17 | A marine microbial metagenome library Lac15 | E. coli | ~ 43 to 52 [NaCl concentration] |
ABTS SGZ 2,6-DMP Guaiacol Dopamine hydrochloride, Potassium ferrocyanide trihyrate |
– | – | – | – |
260 [0 mM NaCl] 240 [200 mM NaCl] 270 [500 mM NaCl] 290 [2000 mM NaCl] 310 [2500 mM NaCl] (SGZ) |
Biodegradation of organic pollutants and synthesis of novel drugs | [67] |
| 18 | Bacillus pumilus W3 | E. coli | ~ 65 | ABTS | 80 (ABTS) [WT] |
50 °C/6 h/ >50% |
3.6 (ABTS) | Stable at alkaline pH | 157.86 [WT] | Dye decolorization | [102] |
The molecular weight of the majority of bacterial laccases is predicted to be in the range of 50–70 kDa according to various experimental reports. For example, the molecular weights of B. subtilis, B. pumilus, and Bacillus sp. HR03 CotAs are ~ 65 kDa [44, 73, 89], whereas that of CotAs from B. subtilis are ~ 67.5 and ~ 66 kDa [41, 90]. SDS-PAGE analysis revealed that the molecular mass of S. cyaneus CECT 3335 is ~ 69.5 kDa, and Mrlac from Meiothermus ruber DSM 1279 possesses a molecular weight of ~ 50 kDa [30, 33]. However, a special extracellular thermo-alkali-stable laccase, in which laccase is a monomeric protein with a molecular weight of ~ 32 kDa, has been identified from B. tequilensis SN4 [76]. The molecular weights of several other bacterial laccases are shown in Table 1. In CotA from B. subtilis, the ~ 65 kDa form represents the fully denatured protein, and the fast migrating ~ 30 kDa represents a partially unfolded form of the enzyme [69]. In B. pumilus MK001, CotA was boiled for 10 min, showing a band at ~ 65 kDa; without boiling, a band at ~ 35 kDa was observed [35].
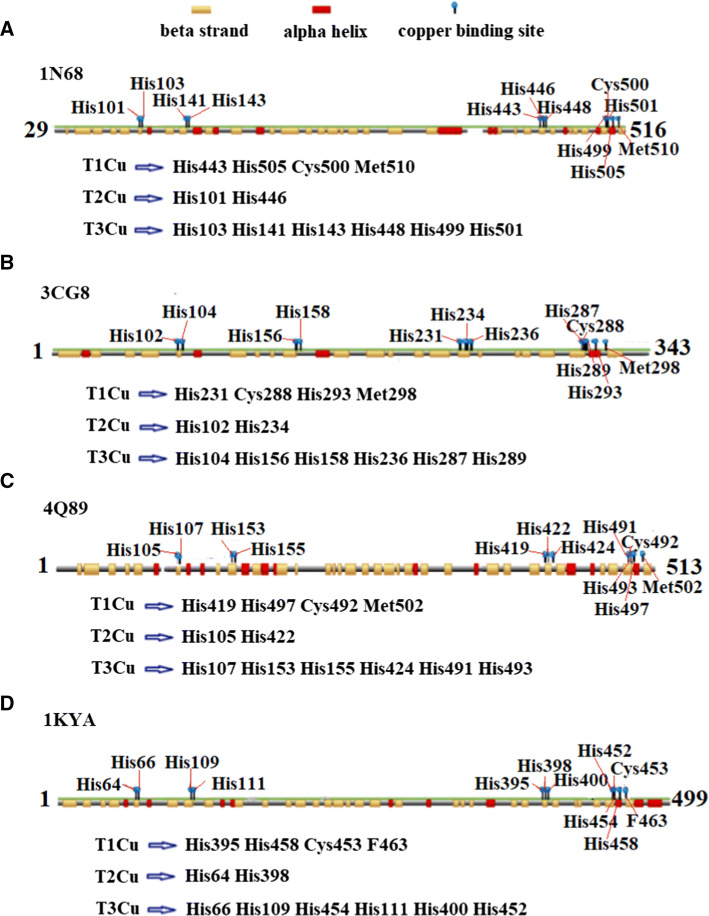
The most significant biochemical properties of bacterial laccases are their stability under various pH, high temperature, organic solvents, and metal ion conditions. Thermostable laccases have been isolated from S. lavendulae with a half-life of 100 min at 70 °C and from B. subtilis for 112 min at 80 °C [43]. The CotA laccases from B. pumilus MK001 and B. pumilus W3 exhibit a half-life of 1 and 1.14 h, respectively, at 80 °C [35, 45]. The spore-bound laccase of B. subtilis WD23 exhibited a high thermal and pH stability with a temperature half-life of 2.5 h at 80 °C, and its pH half-life is more than 6 months at pH 6.8 and 15 days at pH 9.0 [91]. The laccase also exhibits high tolerance to acetone, petroleum ether, ethyl acetate, and chloroform. The pure CotA laccase is 157% activated by Cu2+ and remains stable toward Fe2+ [90]. Moreover, special thermophilic and alkaliphilic bacterial strains that contain laccase-like multicopper oxidases genes exist. TtMCO from the thermophilic bacterium Thermobaculum terrenum is extremely thermophilic with an inactivation half-life of 2.24 days at 70 °C and 350 min at 80 °C at pH 7.0 [92]. ALRh from an alkaliphilic bacterial strain Thioalkalivibrio sp. is a pH-tolerant laccase that is stable in the pH from 2.1 to 9.9 at 20 °C [93]. CotA also exhibited a considerably higher H2O2 tolerance than fungal laccases from T. versicolor and T. trogii [42]. Several laccases and laccase-like bacterial species from different environment sources and their properties were studied in recent years (Table 1). Several factors, including hydrogen bonds and salt bridges, distribution of charged residues on the surface, protein packing, and acid composition [94], contribute to the stability of enzymes. The proline content is apparently associated with increasing protein thermostability [95, 96]. The introduction or increase of proline number is believed to be conducive for improving protein thermostability among many mesophilic bacteria and hyperthermophiles [95]. The percentage of proline residues from B. pumilus W3 CotA is 9.0% (46 pro residues and 513 total residues), and those of other laccases of known structure are 8% for TvLa, 8.2% for MaLa, 7.5% for CcLa, and 6.2% for CueO [89]. Copper ions also play key roles in the stability of MCOs [54]. Laccases possess a secondary structure that displays high β-strand (Fig. 3), which is a special characteristic that may explain their high stability.
Fig. 3.
Secondary structure of four laccases and conservative copper binding site of four typical laccases. a E. coli laccase (CueO, PDB: 1N68, 488 residues). b S. coelicolor laccase (SLAC, PDB: 3CG8, 343 residues, SLAC forms homotrimers). c B. subtilis laccase (CotA, PDB: 4Q89, 513 residues). d T. versicolor laccase (fungal laccase, PDB: 1KYA, 499 residues; the 1KYA structure contains a total of 4 chains and is represented by one chain)
Substrates and mediators
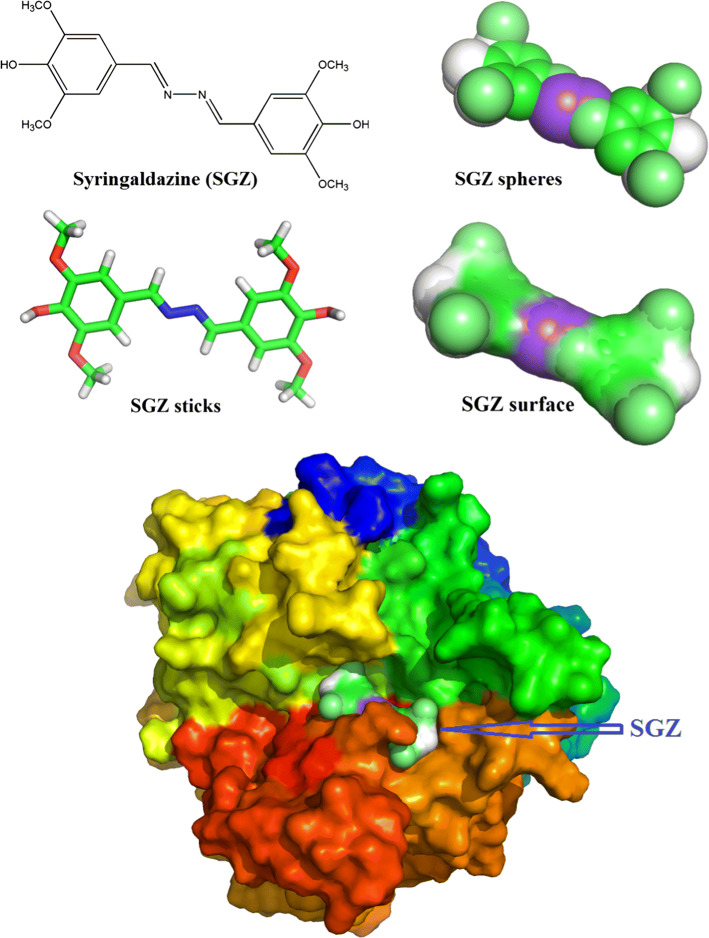
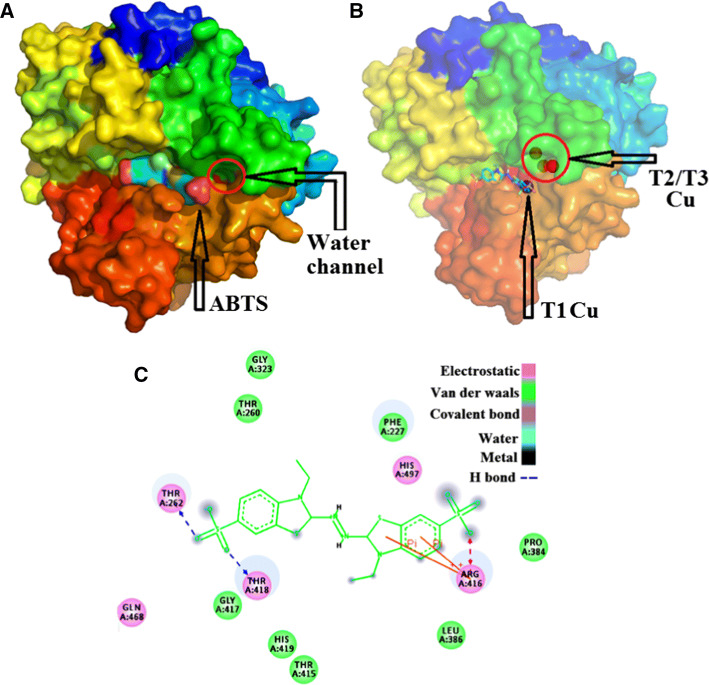
Laccase is a notably substrate-specific enzyme that oxidizes a wide range of substrates. It can catalyze the synthesis and the breakdown reaction of various organic and aromatic compounds. The breakdown of environmentally harmful pollutants contributes to an eco-friendly environment, and the synthesis of complex compounds by producing non-toxic substances leads to bioremediation [97]. Substrates such as 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,6-dimethylphenol (2,6-DMP), syringaldazine (SGZ), acetosyringone (ACS), guaiacol, and L-DOPA are extensively used, and substrated ABTS, SGZ, and 2,6-DMP are the most used substrates for enzyme assays (Table 1). The oxidation reactions of ABTS, SGZ, and 2,6-DMP usually occur at 420 (ε = 36,000 M−1 cm−1), 525 (ε = 65,000 M−1 cm−1), and 468 nm (ε = 37,500 M−1 cm−1), respectively [93, 98]. Substrate binding with protein by using SGZ is exhibited in Fig. 4. A large number of substrates with large size or high redox potential, such as several azo and anthraquinonic dyes, cannot be oxidized directly by laccase. These substrates require an “electron shuttle,” called mediator, between them and laccase [99]. Mediators are low-molecular weight laccase substrates whose enzymatic oxidation causes stable high-potential intermediates; these substrates chemically react with other compounds that cannot be oxidized by laccase alone [100]. ABTS is the first synthetic mediator that was identified to serve as a laccase substrate mediator that enhanced enzyme action [101]. ABTS binds to the enzyme’s “pocket” mainly through H-bonds, vander Waals forces, and electrostatic force. The substrate binding pocket and interaction force between ABTS with the amines of laccase are shown in Fig. 5. In recent years, ABTS and especially ACS have been widely used as mediators in dye decolorization because of their high efficiency [70, 74, 102–104]. Several N-heterocycles bearing N–OH, such as violuric acid, N-hydroxyl-N-phenyl acetamide, and N-hydroxybenzotriazole, are effective mediators [43].
Fig. 4.
Structure of syringaldazine (SGZ) and docking model of laccase from B. pumilus W3 (homology modeling using CotA from B. subtilis PDB code: 1GSK) with substrate using Autodock Vina [191]
Fig. 5.
Binding pocket of substrate (ABTS as an example) near T1Cu and the interaction force between ABTS with ammines of laccase. Docking using AutoDock Vina and Pymol software. Interaction force is shown using Discovery Studio 3.5 Visualizer [192]. a ABTS binds to the enzyme’s “pocket”, b Showed by 40% transparency, c The substrate binding pocket and interaction force between ABTS with the amines of laccase
Laccase-mediator systems (LMSs) involve three main categories of mediators, namely synthetic mediators, natural mediators, and polyoxometalates (POM). An increasing number of synthetic mediators, such as ABTS, 1-hydroxybenzotriazole (HBT), violuric acid, and 2,2′,6,6′-tetramethylpiperidinyl-1-oxy (TEMPO), have been identified. The chemical structures of several synthetic mediators are shown in Fig. 6b. The synthetic mediators can be used in lignin degradation, dye decolorization, and polycyclic aromatic hydrocarbon (PAH) oxidation. However, three major drawbacks of synthetic mediators are as follows: (1) high cost for application at industrial scale; (2) possible formation of toxic derivatives that inactivate laccases [105]; and (3) poor regeneration capacity and high ratio of mediator/substrate is high [106].
Fig. 6.
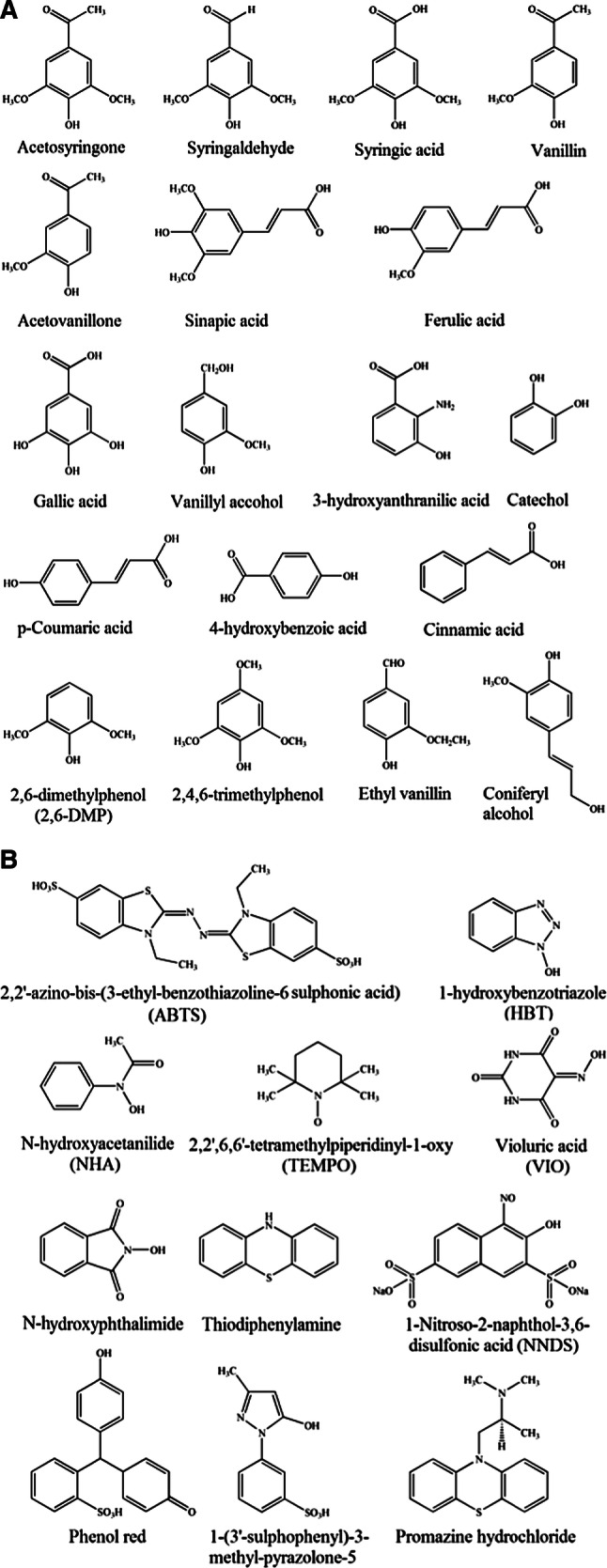
Chemical structures of several natural and synthetic mediators. a Natural mediators; b Synthetic mediators
Inexpensive and eco-friendly natural mediators from plants and industrial by-products [107–109] are available. Most natural mediators are a series of phenol compounds with low chemical potential that can be rapidly oxidized by laccases. By analyzing the interactions between CotA and sinapic acid (SA), the presence of methoxy groups in the ortho-position of the phenolic structure is crucial for substrate recognition by CotA-laccase [110]. Acetylacetone (AA) can be used to form a laccase-AA system and enhance the stability of laccase [111]. Moreover, SA, AS, and p-coumaric acid (p-PCA) can improve the stability of laccase during the degradation of flax pulp [112]. The chemical structures of certain natural mediators are shown in Fig. 6a.
Polyoxometalates, a class of polymetal oxygen cluster compounds formed by the transition metal ions through oxygen linkages, is a kind of bifunctional catalyst characterized by redox and catalysis [113]. POM can be used as mediator of laccase because of its highly stable structure and catalytic activity. It has also been used for lignin degradation and dye bleaching [114–116]. In recent years, LMSs mixed with other enzymes have been widely used in reaction systems. For example, the alkalophilic bacterial xylanase, mannanese, and LMS were combined for biobleaching of mixed wood kraft pulp, and the results demonstrated a reduction of 30% chlorine and 44.4% H2O2 consumption after the triple enzyme treatment, thereby revealing an eco-friendly alternative for total chemical bleaching [117].
Mechanisms of laccase and LMS
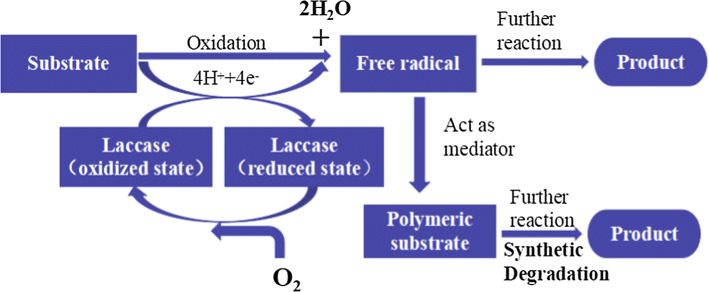
Polyphenols can be oxidized by certain enzymes, such as laccase (EC 1.10.3.2), catechol oxidase (EC 1.10.3.1), and cresolase (EC 1.18.14.1) [118], that exhibit oxidase activities. As shown in Fig. 1, laccase contains four copper atoms that form the active center. The binding pocket of the substrate is near the T1Cu center (Fig. 5b). T2/T3 trinuclear cluster is responsible for the reduction of oxygen to water. Laccase catalysis is believed to involve three steps. First, T1Cu is reduced by a reducing substrate. Then, internal electron transferring from T1Cu to T3Cu and T2Cu occurs through a Cys–His pathway that is highly conserved among multicopper oxidases. Finally, oxygen is reduced to water at the T2/T3 trinuclear cluster center [28, 43]. The reaction mechanism of O2 to H2O is shown in Fig. 7. The reaction of fully reduced enzyme with O2 occurs in two two-electron steps. Thus, the reaction is a four-electron process. According to Solomon et al. [119], the first step is slower than the second step; thus, the first step is the rate determining step, which is driven by the presence of an anionic Asp residue near the T2Cu. The second step indicates the large driving force for the two-electron reduction of peroxide combining with the trinuclear center, which presents a triangular topology. The reaction of the fully reduced enzyme with O2 can generate the native intermediate (NI). Without substrates, the NI undergoes a gradual decay into a resting enzyme form. NI and resting form are both fully oxidized forms, but with a difference: type 2 and coupled-binuclear type 3 Cu are isolated in the resting enzyme whereas these are all bridged by μ3-oxo (O–Cu–O) ligand in NI. Moreover, the T2 OH− ligand and T3 OH− bridge are maintained in the resting enzyme [120].
Fig. 7.
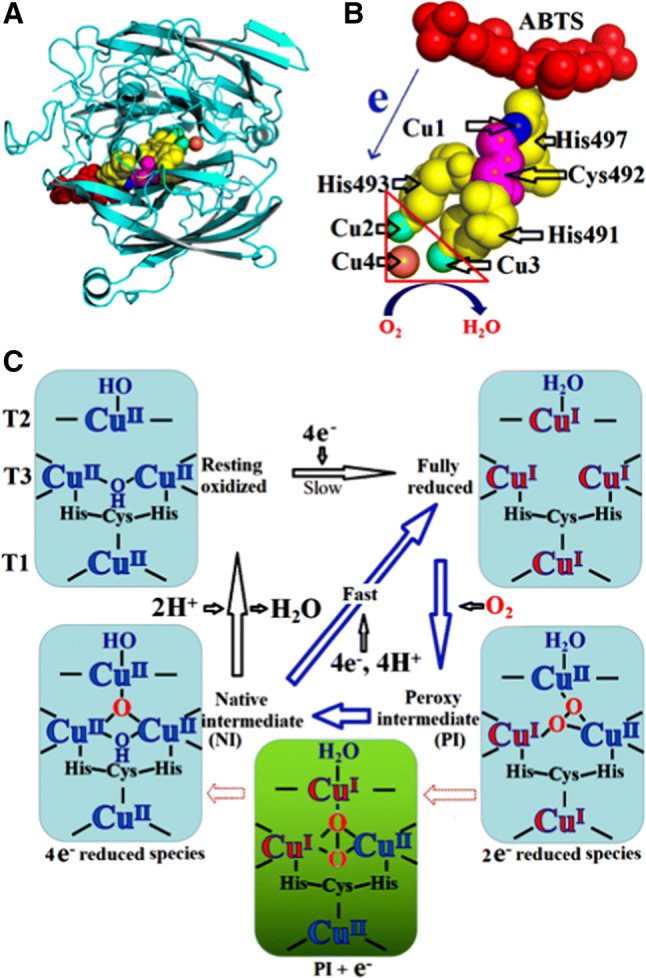
Reaction mechanism of O2 to H2O of bacterial laccases. a The model of ABTS with Cu center in laccase. b The abstracted electron moves from the T1Cu center to the trinuclear cluster through a Cu–Cys–His pathway. c Mechanism of O2 reduction to water. Blue arrows indicate the steps in the catalytic cycle of the laccases. Black arrows indicate the steps that are not part of the catalytic cycle but can be experimentally observed. The green box indicates the transfer of an electron from T1Cu to the T2Cu to yield PI + e−, which occurs in the transfer from PI to NI but is not experimentally observed [119]
The LMS can enhance reaction efficiency and enlarge the range of reaction. Three mechanisms govern the function of mediators in LMS: (1) electron transfer (ET); (2) hydrogen atom transfer (HAT); and (3) ionic mechanism type (IM). The cation radical is significant for LMS. For example, ABTS is a commonly used substrate for bioassay and mediator for oxidizing other substrates, and ABTS2+ is the most useful radical. ABTS isoxidized in two stages (Fig. 8). The first stage is the formation of ABTS+∙ cation radial, followed by slow oxidation of ABTS+∙ to ABTS2+ [100]. According to Morozova et al. [100], ABTS+∙ can interact only with lignin phenolic groups, and ABTS2+ is required for the degradation of nonphenolic lignin structures. Anisyl alcohol and benzylalcohol can be better oxidized by ABTS+∙ than by ABTS2+ [43]. The redox states of ABTS are stable and reversible with formal redox potentials of 0.472 V for ABTS/ABTS+∙ couple and 0.885 V for ABTS+∙/ABTS2+ according to cyclic voltammetry studies [100]. In the ET mechanism, the Cɑ–Cβ bonds of nonphenolic aromatic substrates are disconnected by a cation radical and generate H–Cɑ=O as the final product (Fig. 9a). The oxidization of nonphenolic lignin structures by this mechanism includes ABTS and coordinated transition metals. The HAT mechanism is usually generated with a mediator that contains N–OH or phenoxy-group, such as HBT, PCA, VIO, and NHA. In these mediators containing N–OH, the formation of N–O∙ is significant. The mechanism of HAT is preferable for the formation of O=Cɑ–Cβ group, such as the oxidation of nonphenolic lignin model compounds (4-methoxybenzylalcohol) by LMS (Fig. 9) [100]. The IM mechanism that is independent of the redox features of the substrate is suggested with the laccase/TEMPO system. TEMPO can be oxidized by laccase to form oxoammonium ion, and then the oxoammonium ion oxidizes the alcoholic substances to form carbonyl product or hydroxylamine. The oxidation of alcohols by laccase/TEMPO is shown in Fig. 10 [121].
Fig. 8.
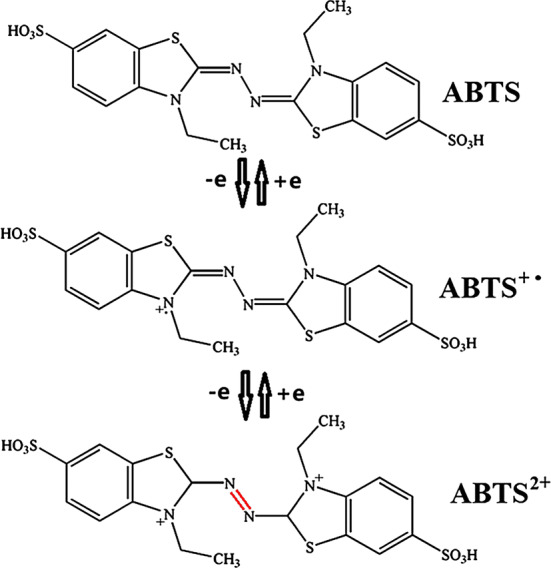
Oxidation of ABTS to ABTS2+catalyzed by laccase
Fig. 9.
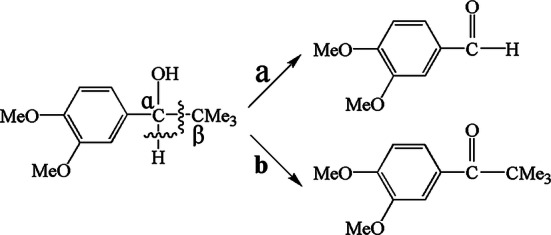
Oxidation of nonphenolic lignin model compounds (4-methoxybenzylalcohol) by laccase mediators. a Oxidation mechanism of ET; b Oxidation mechanism of HAT
Fig. 10.
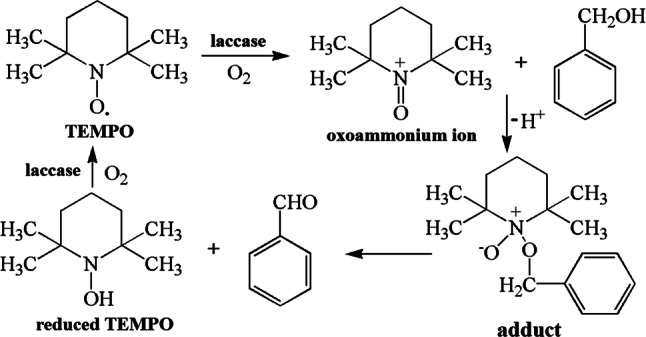
Oxidation of alcohols with laccase/TEMPO
Polycyclic aromatic hydrocarbons can be oxidized by LMS, but different mediators show distinctions. Taking the oxidation of benzo[ɑ]pyrene for example, laccase/ABTS, laccase/PCA, and laccase/HBT all oxidize benzo[ɑ]pyrene-generated quinine. However, only the accumulation of 6-benzo[ɑ]pyrene acetate intermediate in the laccase/ABTS system is observed (Fig. 11). ABTS-mediated reaction follows an ET route, whereas HBT (nitroxyl) radicals oxidized the aromatic substrate by HAT route, and PCA phenoxyl radicals act similarly to nitroxyl radicals [122, 123]. Laccases not only catalyze the synthetic reaction but also degradation. The formation of C–O, C–N, C–S, and C–C bonds and the reaction of O–O can be attained by oxidative reaction of laccase. Five examples are shown in Fig. 12 [99, 124, 125]. Using a bacterial laccase, the lignin model syringylglycerol β-guaiacylether is successfully coupled using the phenolic compound tyramine as substrate [126], and indole is trimerized to form 2,2-bis(3′-indolyl)indoxyl [127]. The mechanism of degradation, that is, the degradation of perfluorooctanoic acid, is complicated and can form approximately 10 byproducts from ECOHRs in the mineral buffer and Cu2+ solution [128]. The catalytic process of laccases is exhibited in Fig. 13.
Fig. 11.
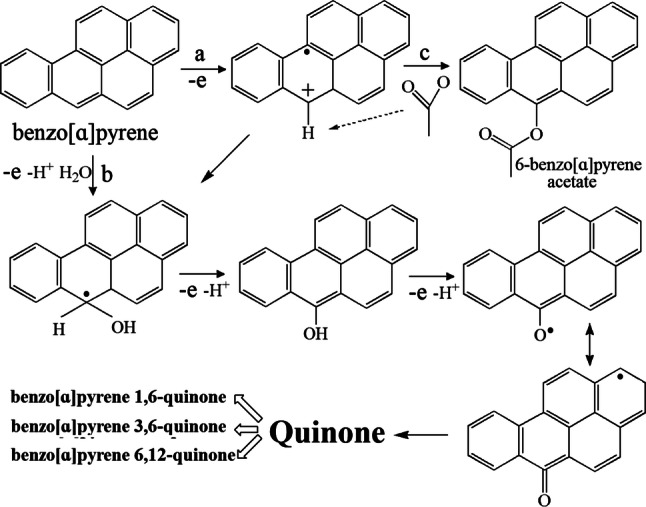
Three oxidation routes of benzo[ɑ]pyrene by laccase-mediator system. a ET route by laccase/ABTS; b HAT route by laccase/PCA or laccase/HBT; c Nucleophilic attack of the acetate ions of the reaction mixture toward the benzo[ɑ]pyrene radicals generated by laccase/ABTS system. Three different quinines are the final products
Fig. 12.
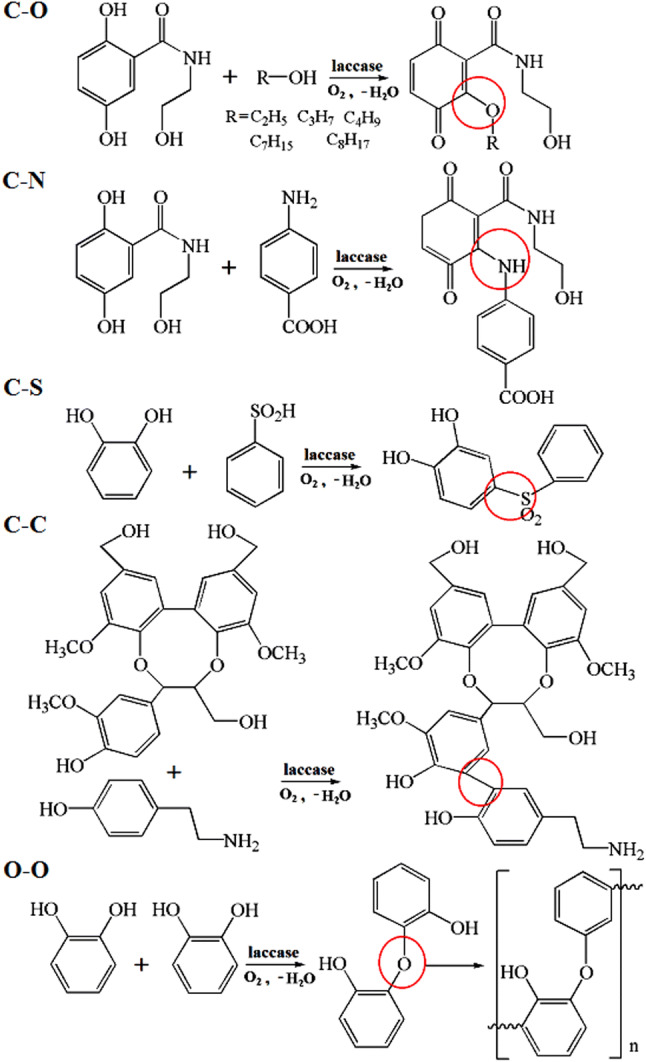
Formation of C–O, C–N, C–S, and C–C bonds, as well as reaction of O–O, can be attained by oxidation of laccase
Fig. 13.
Catalytic process of laccases
Engineering of bacterial laccases
At present, bacterial laccases are not widely used in industries because of their low expression levels and catalytic activity compared with fungal laccases. To meet the industrial demands for high expression level, high catalytic activity, stability, and reduced production cost, many researchers paid attention to bacterial laccase engineering. In recent years, heterologous functional expression and rational, semi-rational, or directed evolution approaches have been used to convert bacterial laccases into high value-added biocatalysts (Table 2).
Table 2.
Several typical bacterial laccases engineering
| Source | Expression host | Modification | Main results | References |
|---|---|---|---|---|
| Bacillus subtilis | E. coli |
M502L M502F |
Redox potential increased 100 mv; but catalytic activity is compromised; T1 copper depletion determine thermodynamic stability | [132] |
| Bacillus subtilis | E. coli |
L386A I494A |
Both redox potentials downward; and catalytic efficiency decreased | [134] |
| Bacillus licheniformis | E. coli |
K316N D500G K316N/D500G |
11.4-fold higher expression level; high dimerization of phenolic and decolorization of industrial dyes | [46] |
| Shigella dyesenteriae | E. coli |
E106F Deletion α-helix |
Promoting both enzymatic activity and thermostability | [138] |
| Bacillus subtilis | E. coli |
E498D E498T E498L |
Catalytic impairment; decreased affinity; Glu498 plays a key role in the protonation events | [186] |
| Bacillus subtilis | B. subtilis | T260L | 120-fold more specific for ABTS | [129] |
| Bacillus sp. HR03 | E. coli |
E188K E188R E188A |
Thermal stability increased; kcat/Km of E188R enhancement | [137] |
| Streptomyces coelicolor | E. coli |
Y108A Y108F |
Tyr108 does form an integral part of the active site and affects enzyme kinetics | [187] |
| Bacillus subtilis | E. coli |
D116A D116N D116E |
Catalytic properties severely compromised; the position 116 in CotA is important for catalysis | [188] |
| Streptomyces coelicolor | E. coli |
Y229A Y230A |
Over 10-fold increase in activity | [49] |
| Streptomyces coelicolor | – |
M168G M168A Y199W M266A M266W M168G/M266A M168G/M266W |
Enhanced kinetic parameters with a phenolic substrate; enhancement of the ability to decolorize indigo carmine in the presence of commercial mediators (methylsyringate and TEMPO) | [133] |
| Bacillus sp. HR03 | E. coli |
D500E D500G D500S G-insertion |
Increase in the expression level up to threefold (D500G) | [135] |
| Bacillus subtilis | P. pastoris | – | 76-fold increase in laccase activity through sorbitol addition and pH adjustment in P. pastoris | [104] |
| Bacillus subtilis | E. coli |
T415A R416A |
Arg416 is crucial in the oxidation of ABTS and SGZ | [110] |
| Aquifex aeolicus | E. coli |
M449T, I441L K245R, R471G P58S, I199T Y172C, V19A F55S |
M449T, K245R, I441L, Y172C, V19A, F55S, and in particular I199T have likely contributed individually or synergistically to improve oxidation for aromatic substrates; R471G and V19A is significant in the stabilization | [189] |
| Bacillus subtilis | E. coli |
R146K, R429K R476K, L431F A478F, T480A T480F |
The residues Arg146, Arg429, and Arg476 are essential for the oxidation of ABTS and syringaldazine. T480F was identified to be more specific for ABTS than syringaldazine | [155] |
| Bacillus subtilis | B. subtilis 1S101 |
E29V, L343S E498G, T480A T480A/E498G E29V/L343S/E498G |
An organic-tolerant and acid-stable variant T480A/E498G had a t1/2 62.1 times increased than wt-CotA | [57] |
| Bacillus amyloliquefaciens | E. coli | D501G | Better stability and catalytic efficiency | [39] |
| Bacillus subtilis | E. coli | ɑ-Hemolysin secretion system and YebF secretion system | A simpler approach for extracellular production of recombinant CotA laccase in E. coli | [40] |
| Bacillus licheniformis | P. pastoris | D500G | 2.1-fold higher activity expressed in P. pastoris and 9.3-folds higher expression than wild-type enzyme | [103] |
| Bacillus pumilus | E. coli |
L386W, G417L L386W/G417L L386W/G417L/G57F |
Higher catalytic efficiency for ABTS and decolorization of four industrial dyes. Higher thermostability and resistance to alkaline environment | [130] |
| Bacillus pumilus | E. coli |
K317N/WLF D501G/WLF K317N/D501G/WLF |
Enhanced functional expression and remained high thermostability, high decolorization of azo, anthraquinonic and triphenylmethane dyes | [102] |
Improvement of catalytic activity and substrate specificity
With the resolution of the crystal structure of several laccases, the laccase structure–function relationships have been elucidated. The “substrate binding pocket” or in the vicinity of the catalytic coppers affected the activity. A useful “platform” from B. subtilis spores is available for directed evolution studies to broaden substrate specificities. The ABTS-bound structure shows the substrate surrounded by 23 amino acids (Pro226, Ala227, Phe228, Cys229, Thr260, Arg261, Thr262, Gly321, Cys322, Gly323, Gly324, Gln378, Gly382, Pro384, Leu386, Thr415, Arg416, Gly417, Thr418, His419, Ile494, His497, and Met502). Among these amino acids, His419, His497, and Cys492 are coordinated with T1Cu, and a disulfide bond is formed between Cys229 and Cys322. The five residues are not altered [129]. The residues of the substrate binding pocket of CotA are randomly modified by saturated mutagenesis to increase the specificity of the enzyme, and the mutant (L386W/G417L) is 132 times more specific for ABTS over SGZ [129]. This finding is also observed in B. pumilus CotA, and the catalytic efficiency of its mutant L386W/G417L for ABTS is 4.3-fold higher than that of WT CotA-laccase [130]. A CotA mutant T260L from B. subtilis spore is 120-fold more specific for ABTS compared with the baseline [131]. According to Xie et al. [110], Arg416 in CotA-laccase plays an important role in substrate oxidation, and the flexibility of Arg416 facilitates the binding of various substrates. Several other site mutations in Bacillus laccases enhance catalytic activity (Table 2). In addition to positive mutations, unalterable conservative sites, such as Met502 of CotA-laccase, occur and act as the axial ligands of T1Cu according to several studies. Catalytic activities decrease strongly after substitution of this residue [132]. The substrate binding pocket of SLAC from S. coelicolor has also been redesigned through site-directed mutagenesis to improve its activity toward compounds of interest as redox mediators. The substitution of the two Met of the pocket by small residues (Ala and Gly) substantially increased the catalytic efficiency with DMP and the decolorization of indigo carmine mediated by methylsyringate and TEMPO [133]. According to site-directed mutagenesis by Sherif et al. [49], 17 amino acid residues, including 10 His involved in copper coordination, are crucial for SLAC activity. In particular, the Y229A and Y230A mutants showed over 10-fold increase in inactivity compared with WT SLAC. In S. sviceus, several Met residues (Met195, Met220, Met293, and Met295) located in the putative substrate binding site of the enzyme are substituted with Leu. Moreover, a truncated mutant without 17 residues corresponding to the C-terminus of the laccase was evaluated. All variants exhibited increased redox potentials ranging from 16 and 81 mV over the WT enzyme [53]. According to Durão et al. [134], the changes in amino acid residues in direct contact with metal center (including ligands) significantly affect the properties of the T1 copper sites of laccase and suggested that the redox potential may be modulated without compromising the overall reactivity through changes in residues away from the immediate contact shell. The mechanisms influencing the catalytic efficiency through mutation is the change in interaction between the enzyme and substrate, as well as the variety of redox potential in the T1Cu site. Moreover, the quantity and distance of H-bonds may also be changed between laccase pocket residues with substrate.
Enhancement of expression level
E. coli, B. subtilis, and P. pastoris are typically used as expression hosts. CotA-laccase mutants are more easily expressed in E. coli compared with other hosts, but isolation of high enzyme quantities from its cell extracts in practical industrial applications is expensive. The advantage of B. subtilis and P. pastoris is their exocrine expression, but mutation is more easily conducted successfully in E. coli. In recent years, site-directed mutagenesis technology has been used to improve functional expression and achieve success in B. licheniformis, Bacillus sp. HR03, and B. pumilus. The soluble expression in E. coli of endospore laccase from B. licheniformis, which is similar to B. subtilis CotA, is enhanced 11.4-fold by a combination of random and site-directed mutagenesis [46]. One of the selected mutations (D500G) is found to be adjacent to the axial Met of T1Cu, which is responsible for an eightfold increase in soluble expression. After sequence alignment, an Asp residue in this position has only been observed in laccases from Bacillus genus, whereas other bacterial and fungal laccases present Gly. This mutation is subsequently introduced in laccase from Bacillus sp. HR03, which also caused a threefold increase in the expression in E. coli [135] and in B. pumilus (D501G/WLF), thereby inducing a 4.48-fold increase in the expression level compared with WLF [102]. The position Asp501 (equivalent to Asp500 in B. licheniformis) is located in the C-terminal segment and close to the T1Cu center that lies in a conserved region (Fig. 14). One to three amino residues (X) in the conserved region (His–Cys–His–XXX–His–XXXX–Met) connected with copper ligands are acidic in numerous multicopper oxidases. Asp501 in B. subtilis CotA-laccase is found at the surface of a water channel [136].
Fig. 14.
C-terminal sequence alignment of bacterial and fungal laccase genes. The conserved regions are represented by the red background. The box shows the different residues in the purpose position
In addition to site-directed mutagenesis, different expression hosts also enhance expression level. According to Wang et al. [104], protease-deficient P. pastoris strain SMD1168H is selected for the heterologous expression of the CotA-laccase from B. subtilis. The enzyme production phase is prolonged, and the expression level of rCotA is effectively improved (sorbitol together with pH adjustment enhanced the rCotA production by 76-fold). A D500G mutant of B. licheniformis LS04 laccase, which is constructed by site-directed mutagenesis, demonstrates 2.1-fold higher activity when expressed in P. pastoris, and the protein yield under the optimized conditions is approximately 59 mg L−1, which is 9.3-fold higher than that of WT enzyme [103]. Moreover, an interesting experiment on CotA-laccase extracellular production in E. coli was conducted by a simple strategy. Two secretion systems (ɑ-hemolysin and YebF secretion systems) were used to achieve the secretion of recombinant CotA into the culture medium. The uropathogenic E. coli ɑ-hemolysin (HlyA) secretion system is the most used secretion system for recombinant protein production. Meanwhile, by optimizing the induction parameters, the extracellular yield of recombinant CotA-laccase was improved by 15-fold (157.4 to 2401.3 U L−1) [40]. The enhancement of expression level renders laccases an increasingly effective catalyst for industrial applications.
Improvement of laccase stability
For industrial application of laccases, highly stable robust enzymes that are active under harsh operational conditions are required. According to a study by Mollania et al. [137], the introduction of positive charge in a connecting loop between domains 1 and 2 promotes the thermal stability of laccase from Bacillus sp. HR03. They further demonstrate that not only the reduction of negative charges but also the size of newly created positive residues can affect laccase stability. Another mutant T480A obtained from B. subtilis is screened for organic solvent resistance. Then, a T480A/E498G variant is constructed, and the t1/2 is 62.1 times larger than that of WT-CotA [57]. In addition to substitution, fragment deletion can enhance thermostability. For example, deletion of helix-5 creates a WlacD that is more thermostable than wild-type Wlac. Other factors that improve protein thermostability include deletion of surface loop, increased hydrophobic residues with branched side chains, and increased proportions of charged residues [138]. According to Enguita et al. [89], additional proline content and increasingly tightly packed residues in the interface region between domains I and II are apparently associated with enhanced protein thermostability in a bacterial multicopper CotA. These methods can be referenced by other unstable enzymes.
Applications of bacterial laccases
Biocatalysis is regarded as a key component for the development of a sustainable bio-economy. The application of enzymes as biocatalysts is becoming increasingly popular in numerous industries. Laccases are promising biological green tools that work in air and generate water as the only by-product. Hence, laccases, especially fungal laccases, are widely applied in different areas (Fig. 15), such as decolorization of dyes, degradation of toxic pollutants, biosensors, effluent treatment, textiles, food industry, paper and pulp production, organic synthesis (phenolic compounds, alkaloids, antibiotics, and bioactive polymers), cosmetics, paints, furniture, and nanobiotechnology [99, 125, 139, 140]. As environmentally friendly enzymes of prokaryotic laccases, bacterial laccases are being increasing researches investigated in terms of their applications. In the present study, we summarize the main application studies on bacterial laccases conducted in recent years.
Fig. 15.
Application areas of laccases
Laccase immobilization
Immobilized enzyme is a new technology that was developed in the 1960s. Immobilized enzymes are water-soluble enzymes that are physically or chemically treated to render them water-insoluble but still enzymatically active. Enzyme immobilization methods can be roughly classified into physical and chemical methods. Physical methods include adsorption and embedding, whereas chemical methods include coupling and cross-linking. Each method presents specific advantages and disadvantages depending on the application purpose. In general, immobilization methods are developed to facilitate enzyme recovery and reusability and to increase enzymatic stability. Materials for laccase immobilization include alginate gel, gelatin, polyacrylamide, hybrid nafion/sol-set silicate film, chitosan film, silica spheres, and other magnetically separable particles [43, 141].
The utilization of laccases for practical application is usually limited due to their high production costs. The use of enzyme immobilization technology in industrial processes, in comparison with the use of soluble enzymes, could reduce process costs by reducing the quantity of enzyme required, since the immobilized biocatalyst can be recovered at the end of a reaction cycle and reused, as long as the enzyme remains active. In addition, immobilization technology can be applied in order to improve enzyme properties such as activity and selectivity as well as stability, because the major advantages of laccase immobilization are the enhancement in the thermostability of the enzyme and its resistance to extreme conditions and chemical reagents. There are many useful methods of laccase immobilization described in the research review paper [142].
Recently, a spore laccase from B. pumilus W3 was efficiently immobilized on amino-functionalized celite. The immobilized spore laccase removed 84.15% of methyl green and 69.70% of acid red 1 after 48 h of treatment. Moreover, the immobilized spore laccase retained 87.04% of its initial decolorization activity after six cycles in the decolorization of acid red 1. This laccase can be a useful biocatalyst in the treatment of textile wastewater [60]. Fan et al. [143] loaded hollow microspheres obtained from Ganoderma lucidum spores with Fe3O4 nanoparticles to prepare novel magnetic spore microspheres. The magnetic microspheres loaded with CotA-laccase, which can be easily and quickly recovered by an external magnetic field, were used for dye decolorization. As a result, 99% of indigo carmine was removed using 10 mg of microspheres after 1 h, and the immobilized CotA retained 75% of activity after 10 consecutive cycles. The magnetic spore microspheres are regarded as good support materials for enzyme immobilization. Laccase–Cu3(PO4)2 hybrid microspheres with hierarchical structure are successfully prepared and loaded on a treated copper foil surface. Furthermore, the microspheres exhibit higher decoloration efficiency and decoloration rate (nearly 3.6 times) on Congo red dye solution after 3 h compared with free laccase [144]. In addition, numerous new materials, including different bio-polymers, such as agar–agar, polyacrylamide, and gelatin, were utilized as bolster materials for immobilization of fungal laccase (T. versicolor) and commercialization [145]. Laccase immobilized on nanocopper-incorporated electrospun fibrous membrane successfully removed 2,4,6-trichlorophenol [146]. Poly (glycidyl methacrylate) (PGMA) microspheres can act as ideal supports for enzyme immobilization [147]. These materials and methods can be referenced to affix bacterial laccases.
Decolorization of dyes
Effluents produced in textile industries are always strongly colored; consequently, their disposal into the receiving waters reduces light penetration and subsequently disrupts the photosynthetic activity of aquatic plants [148]. The affected wastewater poses a severe environmental risk and may be mutagenic or carcinogenic because of the presence of metals, chlorides, and dye breakdown products [149, 150]. At present, treatment of textile effluents by expensive physicochemical methods that generally fail to degrade the pollutants but only cause dye accumulation as sludge results in disposal problems. Enhanced microbial decolorization may provide a cost-effective and environment-friendly method [151, 149].
The first report on dye degradation involved an alkali-tolerant bacterial laccase from ɤ-proteobacterium JB that degraded indigo carmine at pH 9.0 at 55 °C by using syringaldehyde, p-hydroxybenzoic acid, and vanillin as mediators. According to Guan et al. [148], CotA gene of B. pumilus W3 achieved efficient secretory expression in B. subtilis WB600 by screening a suitable signal peptide. The toxicity of the CotA–ACS-treated effluent is markedly lower than that of the crude effluent. B. pumilus W3 CotA-laccase mutant is also used to decolorize five industrial dyes (acid red 1, acid blue 129, methyl green, malachite green, and methyl blue), and these variants maintain high decolorization rates [102]. In the presence of ACS as a mediator, the CotA from B. subtilis cjp3 can decolorize all tested dyes (reactive black 5, indigo carmine, and reactive blue 19), but it cannot decolorize the dyes without mediators [70]. Similarly, laccase from B. vallismortis fmb-103 with ABTS, ACS, or syringaldehyde can efficiently degrade malachite [74]. In addition, certain new bacterial laccases can decolorize different dyes without mediators. For example, a new thermophilic soil bacterium with hyperthermostable alkaline laccase activity named Anoxybacillus sp. UARK-01 can decolorize approximately 1.64 nM of Congo Red per microgram protein in 30 min at 90 °C and pH 9 [152]. Pure Spirulina platensis CFTRI laccase alone can efficiently decolorize anthraquinonic dye reactive blue 4 in 4 h without any mediators [153]. Pure TthLAC (T. thermophilus laccase) decolorized green dye, orange dye, and acid red dye by itself [154]. Except for the thermostable and pH-stable laccase from K. pneumoniae, both bacterial laccase-like enzymes in an acidic bog soil metagenome can efficiently decolor/oxidize sundry dyes in the absence of redox mediators [16, 153]. Studies on the decolorization mechanism of different dyes by bacterial laccases are limited and thus may be a new research topic.
Paper and pulp industry
Bacterial laccases have successfully showed effectiveness for biobleaching and kraft pulps. The capability of laccases to oxidize nonphenolic compound, such as veratryl alcohol, in the presence of ABTS provides new possibilities for the use of bacterial laccases in the pulp and paper industry. The laccase from S. cyaneus CECT 3335 with ABTS as mediator in the biobleaching of eucalyptus kraft pulps resulted in a significant decrease in the kappa number (2.3 U) and a significant increase in the brightness of the pulps [47]. Laccase from S. ipomoea CECT 3341, with ACS as natural mediator, was also used to enhance the conventional chemical bleaching process of an industrial eucalyptus kraft pulp [52]. In addition, a recombinant laccase from hyperthermophilic T. thermophilus was applied for the biobleaching of wheat straw pulp. With the ABTS (5 mM) as mediator, pulp brightness considerably increased by 1.5% ISO [156]. According to Sondhi et al. [157], an extracellular thermo-alkali stable laccase from B. tequilensis SN4 can be used for pulp biobleaching. An increase in brightness by 7.6% and decrease in lignin content by 28% are retained without N-hydroxy-benzotriazole as mediators, whereas 12% improvement in brightness and 47% decrease in lignin content were observed in the presence of a mediator. An effective method for deinking and biobleaching involves the co-production of thermo-alkali stable ligninolytic and hemicellulolytic enzymes by growing two different Bacillus spp. in the same solid-state fermentation medium. The combination of laccase and xylanase reveals a synergistic effect for enhancing pulp properties. The dual cultivation not only improves the utilization rate of substrates but also enhances enzyme yield and the inhibitory effect on the growth of non-desirable microorganisms [28, 158].
Biomass delignification and degradation
Lignin degradation and delignification by laccases are important both in the environment (lignocelluosic bio-waste) and in commercial biofuel production [159, 160]. Laccase digestion provides a mild, clean, and efficient treatment method for bio-delignification of lignocellulose without damaging the cellulose [161, 162]. However, according to Rocha-Martin et al. [163], laccases are supplemented to the enzymatic hydrolysis resulting in contradictory results depending on the pretreatment and substrate used. The addition of laccase on the hydrolysis of softwood increased the glucose conversion, while an inhibitory effect was observed during the hydrolysis of hardwood or agricultural residues like wheat straw. They used two strategies: simultaneous laccase treat enzymatic hydrolysis of pretreated sugar cane straw and corn stover (strategy 1); and a previous laccase treatment and a subsequent hydrolysis step of both pre-processing substrates (strategy 2). Significant reduction of the glucose concentration after enzymatic hydrolysis was found when any of the two strategies were used. The results do not support the use of laccases to detoxify pretreated lignocellulosic materials for improving the bioethanol production [163]. In addition laccase-derived compounds affect negatively the enzymatic hydrolysis being the level of inhibition dependent on the type of phenol, besides phenoxyl radicals and oligomeric phenols [164]. Laccase enzymes are promising detoxifying agents during lignocellulosic bioethanol production from wheat straw. However, they affect the enzymatic hydrolysis of this material by lowering the glucose recovery yields. This work revealed that a grafting process of phenoxy radicals onto the ligin fiber could be the cause of diminished accessibility of cellulases to cellulases in pretreated wheat straw [165].
According to Singh et al. [166], an SLAC from Amycolatopsis sp. 75iv3 enhances the delignification of steam-pretreated poplar. Their study established the lignolytic activity of SLAC on woody biomass and highlighted the biocatalytic potential of bacterial enzymes. In addition, numerous agricultural residues are efficiently degraded by various bacterial laccases, such as wheat straw by S. ipomoeae laccase [167, 168], peanut shell bio-waste by Aquisalibacillus elongatus laccase [169], sugar beet pulp by A. elongatus laccase [170], almond shell bio-waste by Chromohalobacter salexigens laccase [171], and paddy straw by S. griseorubens ssr38 [172]. Moreover, co-expressed mixture methods are used for lignocellulose degradation. Fonseca-Maldonado et al. [173] demonstrated that synergism occurs between an endoxylanase and laccase with milled sugar cane bagasse as a lignocellulose substrate. As examples, the three genes of bacterial laccase (cotA), pectate lyase (pel), and endoxylanase (xyl) are simultaneously cloned in single vector E. coli. This enzyme cocktail is important in the pretreatment of lignocellulosic residues for biofuel production [174].
Pollutant degradation
Polycyclic aromatic hydrocarbons and biogenic amines are pollutants that are widely distributed in natural environments, such as soil, air, or aquatic environment. Most of these pollutants and their intermediates are toxic for living beings [28]. To date, increasing reports have shown that bacterial laccase can degrade xenobiotic compounds. Recently, several bacterial laccase CueO mutations from the metagenome of chemical plant sludge displayed that the mutants G276R, G276N, G276Y, and G276K oxidize carcinogen benzo[ɑ]pyrene more efficiently than the WT-enzyme [68]. According to Callejón et al. [175], recombinant laccase from Pediococcus acidilactici can degrade the biogenic amine tyramine at pH 9.5 and 4.0 with or without ABTS as mediator. Tyramine degradation by laccases can solve the problems generated in food due to the presence of this toxic compound. In addition, gallic acid, syringaldehyde, vanillin, and catechol can be degraded by bacterial-derived laccase [176]. Several micropollutants, such as BPA (bisphenol A), inflammatory drug DFC (diclofenac), and MFA (mefenamic acid), can also be oxidized by laccases [177, 178]. Thus, bacterial laccases are potential biological green tools for the environment.
Other applications
In addition to the above-mentioned applications, laccases can be used in polymer production [36], synthesis of indo dyes [179], detection [180, 181], biosensor, and bioremediation [182]. In a word, laccases have become important industrially relevant enzymes as promising biological green tools for the environment and in industries.
Concluding remarks
The current review provides a detailed and comprehensive study of bacterial laccases, their species and properties, and substrates and mediators, as well as the mechanisms of laccase and LMS. Moreover, the strategies for further enhancement of the catalytic activity and substrate specificity, expression level, and laccase stability by genetic engineering are summarized. Bacterial laccases exhibit a wide range of applications for decolorization of dyes, effluent treatment, degradation of toxic pollutants, and other fields (textile, food, and paper industries). Biological enzyme engineering (Fig. 16) is a powerful and useful technical area for environment-friendly and industrial production. However, the main obstacles for the large-scale application of bacterial laccases include low expression level, high price of mediator, and inadequate capacity to produce large volumes of highly active enzyme at low cost. Future research should focus on the following areas: (1) expansion of the production scale using exocrine strain as expression vector; (2) separation and screening of novel laccases or transformation of current laccases to new enzymes, which offer high application potential in the absence of mediators, to reduce the cost; (3) development of immobilized laccases using new environmentally friendly nanoscale material that can be easily reused and recycled; (4) reaction engineering to optimize the synthesis of specifically desired products of economic value; and (5) potential of laccases for Au adsorption which may become a novel application for bioremediation in the future. In addition, investigations on dye degradation–detoxification mechanism by laccases should be conducted. Hopefully, these questions will attract researchers’ attention in the future.
Fig. 16.
Biological enzyme engineering
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31472003), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the 111 Project (No. 111-2-06). Thanks for Jing Xia, Ying-Nan Li, Yuan Guo, Bi-Ying Wang, Wei Jiang, and Yun-Ya Li’s kind advice.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Zheng-Bing Guan and Quan Luo contributed equally to this work.
References
- 1.Morozova OV, Shumakovich GP, Gorbacheva MA, Shleev SV, Yaropolov AI. “Blue” laccases. Biochem (Mosc) 2007;72:1136–1150. doi: 10.1134/S0006297907100112. [DOI] [PubMed] [Google Scholar]
- 2.Xu F, Shin W, Brown SH, Wahleithner JA, Sundaram UM, Solomon EI. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta. 1996;1292:303–311. doi: 10.1016/0167-4838(95)00210-3. [DOI] [PubMed] [Google Scholar]
- 3.Yaropolov AI, Skorobogat’Ko OV, Vartanov SS, Varfolomeyev SD. Laccase: properties, catalytic mechanism, and applicability. Appl Biochem Biotechnol. 1994;49:257–280. doi: 10.1007/BF02783061. [DOI] [Google Scholar]
- 4.Yoshida H. LXIII.-Chemistry of lacquer (Urushi). Part I. Communication from the chemical cociety of Tokio. J Chem Soc Trans. 1883;43:472–486. doi: 10.1039/CT8834300472. [DOI] [Google Scholar]
- 5.Brijwani K, Rigdon A, Vadlani PV. Fungal laccases: production, function, and applications in food processing. Enzyme Res. 2010;2010:149748–149758. doi: 10.4061/2010/149748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryan AC, Jawdy S, Gunter L, Gjersing E, Sykes R, Hinchee MA, Winkeler KA, Collins CM, Engle N, Tschaplinski TJ, Yang X, Tuskan GA, Muchero W, Chen JG. Knockdown of a laccase in Populus deltoides confers altered cell wall chemistry and increased sugar release. Plant Biotechnol J. 2016;14:2010–2020. doi: 10.1111/pbi.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Q, Luo L, Wang X, Shen Z, Zheng L. Comprehensive analysis of rice laccase gene (OsLAC) family and ectopic expression of OsLAC10 enhances tolerance to copper stress in Arabidopsis. Int J Mol Sci. 2017;18:209–225. doi: 10.3390/ijms18020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Xie Y, Yi M, Zhang S, Sun X. Isolation, expression and single nucleotide polymorphisms (SNPs) analysis of laccase gene (LkLAC8) from Japanese larch (Larix kaempferi) J For Res. 2017;28:891–901. doi: 10.1007/s11676-016-0360-9. [DOI] [Google Scholar]
- 9.Laufer Z, Beckett RP, Minibayeva FV, Lüthje S, Böttger M. Diversity of laccases from lichens in suborder Peltigerineae. Bryologist. 2009;112:418–426. doi: 10.1639/0007-2745-112.2.418. [DOI] [Google Scholar]
- 10.Li Q, Wang X, Korzhev M, Schröder HC, Link T, Tahir MN, Diehl-Seifert B, Müller WE. Potential biological role of laccase from the sponge Suberites domuncula as an antibacterial defense component. BBA Gen Subj. 2015;1850:118–128. doi: 10.1016/j.bbagen.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Luna-Acosta A, Rosenfeld E, Amari M, Fruitier-Arnaudin I, Bustamante P, Thomas-Guyon H. First evidence of laccase activity in the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2010;28:719–726. doi: 10.1016/j.fsi.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Dittmer NT, Suderman RJ, Jiang H, Zhu YC, Gorman MJ, Kramer KJ, Kanost MR. Characterization of cDNAs encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae. Insect Biochem Mol Biol. 2004;34:29–41. doi: 10.1016/j.ibmb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Hoegger PJ, Kilaru S, James TY, Thacker JR, Kües U. Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J. 2006;273:2308–2326. doi: 10.1111/j.1742-4658.2006.05247.x. [DOI] [PubMed] [Google Scholar]
- 14.Lang M, Kanost MR, Gorman MJ. Multicopper oxidase-3 is a laccase associated with the peritrophic matrix of Anopheles gambiae. PLoS One. 2012;7:e33985. doi: 10.1371/journal.pone.0033985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beloqui A, Pita M, Polaina J, Martínez-Arias A, Golyshina OV, Zumárraga M, Yakimov MM, García-Arellano H, Alcalde M, Fernández VM, Elborough K, Andreu JM, Ballesteros A, Plou FJ, Timmis KN, Ferrer M, Golyshin PN. Novel polyphenol oxidase mined from a metagenome expression library of bovine rumen: biochemical properties, structural analysis, and phylogenetic relationships. J Biol Chem. 2006;281:22933–22942. doi: 10.1074/jbc.M600577200. [DOI] [PubMed] [Google Scholar]
- 16.Ausec L, Berini F, Casciello C, Cretoiu MS, van Elsas JD, Marinelli F, Mandic-Mulec I. The first acidobacterial laccase-like multicopper oxidase revealed by metagenomics shows high salt and thermo-tolerance. Appl Microbiol Biotechnol. 2017;101:6261–6276. doi: 10.1007/s00253-017-8345-y. [DOI] [PubMed] [Google Scholar]
- 17.Tušek AJ, Šalić A, Zelić B. Catechol removal from aqueous media using laccase immobilized in different macro- and microreactor systems. Appl Biochem Biotechnol. 2017;182:1575–1590. doi: 10.1007/s12010-017-2419-2. [DOI] [PubMed] [Google Scholar]
- 18.Canbolat MF, Savas HB, Gultekin F. Enzymatic behavior of laccase following interaction with γ-CD and immobilization into PCL nanofibers. Anal Biochem. 2017;528:13–18. doi: 10.1016/j.ab.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Chan CKY, Zeeb B, McClements DJ, Weiss J. Impact of laccase on the colour stability of structured oil-in-water emulsions. Food Res Int. 2017;97:223–230. doi: 10.1016/j.foodres.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Jia W, Wang Q, Fan X, Dong A, Yu Y, Wang P. Laccase-mediated in situ oxidation of dopa for bio-inspired coloration of silk fabric. Rsc Adv. 2017;7:12977–12983. doi: 10.1039/C6RA25533G. [DOI] [Google Scholar]
- 21.Jiang J, Ye W, Liu L, Wang Z, Fan Y, Saito T, Isogai A. Cellulose nanofibers prepared using the TEMPO/laccase/O2 system. Biomacromol. 2017;18:288–294. doi: 10.1021/acs.biomac.6b01682. [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Lai Q, Liu Y, Chen S, Le X, Zhou X. Laccase—methacrylyol functionalized magnetic particles: highly immobilized, reusable, and efficacious for methyl red decolourization. Int J Biol Macromol. 2017;102:144–152. doi: 10.1016/j.ijbiomac.2017.03.169. [DOI] [PubMed] [Google Scholar]
- 23.Lonappan L, Rouissi T, Laadila MA, Brar SK, Hernándezgalán L, Verma M, Rao YS. Agro-industrial produced laccase for degradation of diclofenac and identification of transformation products. Acs Sustain Chem Eng. 2017;5:5772–5781. doi: 10.1021/acssuschemeng.7b00390. [DOI] [Google Scholar]
- 24.Munk L, Andersen ML, Meyer AS. Direct rate assessment of laccase catalysed radical formation in lignin by electron paramagnetic resonance spectroscopy. Enzyme Microb Technol. 2017;106:88–96. doi: 10.1016/j.enzmictec.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Naghdi M, Taheran M, Brar SK, Kermanshahi-Pour A, Verma M, Surampalli RY. Immobilized laccase on oxygen functionalized nanobiochars through mineral acids treatment for removal of carbamazepine. Sci Total Environ. 2017;584–585:393–401. doi: 10.1016/j.scitotenv.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Vishnu D, Neeraj G, Swaroopini R, Shobana R, Kumar VV, Cabana H. Synergetic integration of laccase and versatile peroxidase with magnetic silica microspheres towards remediation of biorefinery wastewater. Environ Sci Pollut Res. 2017;24:17993–18009. doi: 10.1007/s11356-017-9318-5. [DOI] [PubMed] [Google Scholar]
- 27.Wu G, Gao Y, Zhao D, Ling P, Gao F. A methanol/oxygen enzymatic biofuel cell using laccase and NAD+-dependent dehydrogenase cascades as biocatalysts on carbon nanodots electrodes. ACS Appl Mater Interfaces. 2017;9:40978–40986. doi: 10.1021/acsami.7b12295. [DOI] [PubMed] [Google Scholar]
- 28.Chauhan PS, Goradia B, Saxena A. Bacterial laccase: recent update on production, properties and industrial applications. 3 Biotech. 2017;7:323–343. doi: 10.1007/s13205-017-0955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butterfield CN, Tebo BM. Substrate specificity and copper loading of the manganese-oxidizing multicopper oxidase Mnx from Bacillus sp. PL-12. Metallomics. 2017;9:183–191. doi: 10.1039/C6MT00239K. [DOI] [PubMed] [Google Scholar]
- 30.Ece S, Lambertz C, Fischer R, Commandeur U. Heterologous expression of a Streptomyces cyaneus laccase for biomass modification applications. AMB Express. 2017;7:86–98. doi: 10.1186/s13568-017-0387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonçalves I, Nunes C, Mendes S, Martins LO, Ferreira P, Coimbra MA. CotA laccase-ABTS/hydrogen peroxide system: an efficient approach to produce active and decolorized chitosan-genipin films. Carbohydr Polym. 2017;175:628–635. doi: 10.1016/j.carbpol.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Gounel S, Rouhana J, Stineschaumeil C, Cadet M, Mano N. Increasing the catalytic activity of Bilirubin oxidase from Bacillus pumilus: importance of host strain and chaperones proteins. J Biotechnol. 2016;230:19–25. doi: 10.1016/j.jbiotec.2016.04.035. [DOI] [PubMed] [Google Scholar]
- 33.Kalyani DC, Munk L, Mikkelsen JD, Meyer AS. Molecular and biochemical characterization of a new thermostable bacterial laccase from Meiothermus ruber DSM 1279. Rsc Adv. 2015;6:3910–3918. doi: 10.1039/C5RA24374B. [DOI] [Google Scholar]
- 34.Koschorreck K, Wahrendorff F, Biemann S, Jesse A, Urlacher VB. Cell thermolysis—a simple and fast approach for isolation of bacterial laccases with potential to decolorize industrial dyes. Process Biochem. 2017;56:171–176. doi: 10.1016/j.procbio.2017.02.015. [DOI] [Google Scholar]
- 35.Kumar S, Jain KK, Rani S, Bhardwaj KN, Goel M, Kuhad RC. In-vitro refolding and characterization of recombinant laccase (CotA) from Bacillus pumilus MK001 and its potential for phenolics degradation. Mol Biotechnol. 2016;58:789–800. doi: 10.1007/s12033-016-9978-2. [DOI] [PubMed] [Google Scholar]
- 36.Lončar N, Božić N, Vujčić Z. Expression and characterization of a thermostable organic solvent-tolerant laccase from Bacillus licheniformis ATCC 9945a. J Mol Catal B Enzym. 2016;134:390–395. doi: 10.1016/j.molcatb.2016.06.005. [DOI] [Google Scholar]
- 37.Safary A, Moniri R, Hamzeh-Mivehroud M, Dastmalchi S. A strategy for soluble overexpression and biochemical characterization of halo-thermotolerant Bacillus laccase in modified E. coli. J Biotechnol. 2016;227:56–63. doi: 10.1016/j.jbiotec.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Safary A, Moniri R, Hamzeh-Mivehroud M, Dastmalchi S. Identification and molecular characterization of genes coding pharmaceutically important enzymes from halo-thermo tolerant Bacillus. Adv Pharm Bull. 2016;6:551–561. doi: 10.15171/apb.2016.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Lu L, Feng F. Improving the indigo carmine decolorization ability of a Bacillus amyloliquefaciens laccase by site-directed mutagenesis. Catalysts. 2017;7:1–10. [Google Scholar]
- 40.Wang TN, Zhao M. A simple strategy for extracellular production of CotA laccase in Escherichia coli and decolorization of simulated textile effluent by recombinant laccase. Appl Microbiol Biotechnol. 2017;101:685–696. doi: 10.1007/s00253-016-7897-6. [DOI] [PubMed] [Google Scholar]
- 41.Zeng J, Zhu Q, Wu Y, Lin X. Oxidation of polycyclic aromatic hydrocarbons using Bacillus subtilis CotA with high laccase activity and copper independence. Chemosphere. 2016;148:1–7. doi: 10.1016/j.chemosphere.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Li X, Hao Z, Xi R, Cai Y, Liao X. Hydrogen peroxide-resistant CotA and YjqC of Bacillus altitudinis spores are a promising biocatalyst for catalyzing reduction of sinapic acid and sinapine in rapeseed meal. PLoS One. 2016;11:e0158351. doi: 10.1371/journal.pone.0158351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandra R, Chowdhary P. Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ Sci Process Impacts. 2015;17:326–342. doi: 10.1039/C4EM00627E. [DOI] [PubMed] [Google Scholar]
- 44.Guan ZB, Song CM, Zhang N, Zhou W, Xu CW, Zhou LX, Zhao H, Cai YJ, Liao XR. Overexpression, characterization, and dye-decolorizing ability of a thermostable, pH-stable, and organic solvent-tolerant laccase from Bacillus pumilus W3. J Mol Catal B Enzym. 2014;101:1–6. doi: 10.1016/j.molcatb.2013.11.009. [DOI] [Google Scholar]
- 45.Chen Y, Luo Q, Zhou W, Xie Z, Cai YJ, Liao XR, Guan ZB. Improving the catalytic efficiency of Bacillus pumilus CotA-laccase by site-directed mutagenesis. Appl Microbiol Biotechnol. 2017;101:1–10. doi: 10.1007/s00253-016-7972-z. [DOI] [PubMed] [Google Scholar]
- 46.Koschorreck K, Schmid RD, Urlacher VB. Improving the functional expression of a Bacillus licheniformis laccase by random and site-directed mutagenesis. BMC Biotechnol. 2009;9:12–22. doi: 10.1186/1472-6750-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arias ME, Arenas M, Rodríguez J, Soliveri J, Ball AS, Hernández M. Kraft pulp biobleaching and mediated oxidation of a nonphenolic substrate by laccase from Streptomyces cyaneus CECT 3335. Appl Environ Microbiol. 2003;69:1953–1958. doi: 10.1128/AEM.69.4.1953-1958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moya R, Hernández M, García-Martín AB, Ball AS, Arias ME. Contributions to a better comprehension of redox-mediated decolouration and detoxification of azo dyes by a laccase produced by Streptomyces cyaneus CECT 3335. Bioresour Technol. 2010;101:2224–2229. doi: 10.1016/j.biortech.2009.11.061. [DOI] [PubMed] [Google Scholar]
- 49.Sherif M, Waung D, Korbeci B, Mavisakalyan V, Flick R, Brown G, Abouzaid M, Yakunin AF, Master ER. Biochemical studies of the multicopper oxidase (small laccase) from Streptomyces coelicolor using bioactive phytochemicals and site-directed mutagenesis. Microb Biotechnol. 2013;6:588–597. doi: 10.1111/1751-7915.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skálová T, Dohnálek J, Østergaard LH, Østergaard PR, Kolenko P, Dusková J, Stepánková A, Hasek J. The structure of the small laccase from Streptomyces coelicolor reveals a link between laccases and nitrite reductases. J Mol Biol. 2009;385:1165–1178. doi: 10.1016/j.jmb.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 51.Kandasamy S, Devi P, Chendrayan Uthandi S. Laccase producing Streptomyces bikiniensis CSC12 isolated from compost. J Microbiol Biotechnol Food Sci. 2017;6:794–798. [Google Scholar]
- 52.Eugenio ME, Hrenández M, Moya R, Martínsampedro R. Evaluation of a new laccase produced by Streptomyces ipomoea on biobleaching and ageing of kraft pilps. BioResources. 2011;6:3231–3241. [Google Scholar]
- 53.Gunne M, Höppner A, Hagedoorn PL, Urlacher VB. Structural and redox properties of the small laccase Ssl1 from Streptomyces sviceus. FEBS J. 2014;281:4307–4318. doi: 10.1111/febs.12755. [DOI] [PubMed] [Google Scholar]
- 54.Martins LO, Durão P, Brissos V, Lindley PF. Laccases of prokaryotic origin: enzymes at the interface of protein science and protein technology. Cell Mol Life Sci. 2015;72:911–922. doi: 10.1007/s00018-014-1822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertrand B, Martínez-Morales F, Trejo-Hernández MR. Upgrading laccase production and biochemical properties: strategies and challenges. Biotechnol Progr. 2017;33:1015–1034. doi: 10.1002/btpr.2482. [DOI] [PubMed] [Google Scholar]
- 56.Mate DM, Alcalde M. Laccase engineering: from rational design to directed evolution. Biotechnol Adv. 2015;33:25–40. doi: 10.1016/j.biotechadv.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Sheng S, Jia H, Topiol S, Farinas ET. Engineering CotA laccase for acidic pH stability using Bacillus subtilis spore display. J Microb Biotechnol. 2017;27:507–513. doi: 10.4014/jmb.1608.08026. [DOI] [PubMed] [Google Scholar]
- 58.Torrinha Á, Montenegro MCBSM, Araújo AN. Implementation of a simple nanostructured bio-electrode with immobilized Rhus Vernicifera laccase for oxygen sensing applications. Electroanalysis. 2017;29:1566–1572. doi: 10.1002/elan.201600738. [DOI] [Google Scholar]
- 59.Wan J, Sun X, Liu C, Tang M, Li L, Ni H. Decolorization of textile dye RB19 using volcanic rock matrix immobilized Bacillus thuringiensis cells with surface displayed laccase. World J Microbiol Biotechnol. 2017;33:123–135. doi: 10.1007/s11274-017-2290-x. [DOI] [PubMed] [Google Scholar]
- 60.Zhou W, Guan ZB, Chen Y, Zhang F, Cai YJ, Xu CW, Chen XS, Liao XR. Production of spore laccase from Bacillus pumilus W3 and its application in dye decolorization after immobilization. Water Sci Technol. 2017;76(1–2):147–154. doi: 10.2166/wst.2017.192. [DOI] [PubMed] [Google Scholar]
- 61.Dagys M, Laurynėnas A, Ratautas D, Kulys J, Vidžiūnaitė R, Talaikis M, Niaura G, Marcinkevičienė L, Meškys R, Shleev S. Oxygen electroreduction catalysed by laccase wired to gold nanoparticles via the trinuclear copper cluster. Energy Environ Sci. 2017;10:498–502. doi: 10.1039/C6EE02232D. [DOI] [Google Scholar]
- 62.Fernandes RA, Daniel-Da-Silva AL, Tavares APM, Xavier AMRB. EDTA-Cu (II) chelating magnetic nanoparticles as a support for laccase immobilization. Chem Eng Sci. 2017;158:599–605. doi: 10.1016/j.ces.2016.11.011. [DOI] [Google Scholar]
- 63.Fortes CCS, Daniel-Da-Silva AL, Xavier AMRB, Tavares APM. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem Eng Process Process Intensif. 2017;117:1–8. doi: 10.1016/j.cep.2017.03.009. [DOI] [Google Scholar]
- 64.Lloret L, Eibes G, Feijoo G, Moreira MT, Lema JM. Application of response surface methodology to study the removal of estrogens in a laccase-mediated continuous membrane reactor. Biocatalysis. 2013;31:197–207. doi: 10.3109/10242422.2013.815745. [DOI] [Google Scholar]
- 65.Lloret L, Eibes G, Moreira MT, Feijoo G, Lema JM. Removal of estrogenic compounds from filtered secondary wastewater effluent in a continuous enzymatic membrane reactor. Identification of biotransformation products. Environ Sci Technol. 2013;47:4536–4543. doi: 10.1021/es304783k. [DOI] [PubMed] [Google Scholar]
- 66.Givaudan A, Effosse A, Faure D, Potier P, Bouillant ML, Bally R. Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiol Lett. 1993;108:205–210. doi: 10.1111/j.1574-6968.1993.tb06100.x. [DOI] [Google Scholar]
- 67.Li J, Xie Y, Wang R, Fang Z, Fang W, Zhang X, Xiao Y. Mechanism of salt-induced activity enhancement of a marine-derived laccase, Lac15. Eur Biophys J. 2018;47:225–236. doi: 10.1007/s00249-017-1251-5. [DOI] [PubMed] [Google Scholar]
- 68.Yue Q, Yang Y, Zhao J, Zhang L, Xu L, Chu X, Liu X, Tian J, Wu N. Identification of bacterial laccase cueO mutation from the metagenome of chemical plant sludge. Bioresour Bioprocess. 2017;4:48–56. doi: 10.1186/s40643-017-0178-0. [DOI] [Google Scholar]
- 69.Martins LGO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem. 2002;277:18849–18859. doi: 10.1074/jbc.M200827200. [DOI] [PubMed] [Google Scholar]
- 70.Qiao W, Chu J, Ding S, Song X, Yu L. Characterization of a thermo-alkali-stable laccase from Bacillus subtilis cjp3 and its application in dyes decolorization. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2017;52:710–717. doi: 10.1080/10934529.2017.1301747. [DOI] [PubMed] [Google Scholar]
- 71.Koschorreck K, Richter SM, Ene AB, Roduner E, Schmid RD, Urlacher VB. Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl Microbiol Biotechnol. 2008;79:217–224. doi: 10.1007/s00253-008-1417-2. [DOI] [PubMed] [Google Scholar]
- 72.Ruijssenaars HJ, Hartmans S. A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity. Appl Microbiol Biotechnol. 2004;65:177–182. doi: 10.1007/s00253-004-1571-0. [DOI] [PubMed] [Google Scholar]
- 73.Mohammadian M, Fathiroudsari M, Mollania N, Badoeidalfard A, Khajeh K. Enhanced expression of a recombinant bacterial laccase at low temperature and microaerobic conditions: purification and biochemical characterization. J Ind Microbiol Biotechnol. 2010;37:863–869. doi: 10.1007/s10295-010-0734-5. [DOI] [PubMed] [Google Scholar]
- 74.Sun J, Zheng M, Lu Z, Lu F, Zhang C. Heterologous production of a temperature and pH-stable laccase from Bacillus vallismortis fmb-103 in Escherichia coli and its application. Process Biochem. 2017;55:77–84. doi: 10.1016/j.procbio.2017.01.030. [DOI] [Google Scholar]
- 75.Zhang C, Diao H, Lu F, Bie X, Wang Y, Lu Z. Degradation of triphenylmethane dyes using a temperature and pH stable spore laccase from a novel strain of Bacillus vallismortis. Bioresour Technol. 2012;126:80–86. doi: 10.1016/j.biortech.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 76.Sondhi S, Sharma P, Saini S, Puri N, Gupta N. Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS One. 2014;9:e96951. doi: 10.1371/journal.pone.0096951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li J, Li GF, Lu L, Pan JB, Zhao M, Wang TN, Xu TF, Wang JY. Application of spore laccase from Bacillus amyloliquefaciens in dye decolorization. J Beijing For Univer. 2013;35:125–129. [Google Scholar]
- 78.Telke AA, Ghodake GS, Kalyani DC, Dhanve RS, Govindwar SP. Biochemical characteristics of a textile dye degrading extracellular laccase from a Bacillus sp. ADR. Bioresour Technol. 2011;102:1752–1756. doi: 10.1016/j.biortech.2010.08.086. [DOI] [PubMed] [Google Scholar]
- 79.Claus H, Filip Z. The evidence of a laccase-like enzyme activity in a Bacillus sphaericus strain. Microbiol Res. 1997;152:209–216. doi: 10.1016/S0944-5013(97)80014-6. [DOI] [Google Scholar]
- 80.Brander S, Mikkelsen JD, Kepp KP. Characterization of an alkali- and halide-resistant laccase expressed in E. coli: CotA from Bacillus clausii. PLoS One. 2014;9:e99402. doi: 10.1371/journal.pone.0099402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh D, Sharma KK, Jacob S, Gakhar SK. Molecular docking of laccase protein from Bacillus Safensis DSKK5 isolated from earthworm gut: a novel method to study dye decolorization potential. Water Air Soil Pollut. 2014;225:2175–2187. doi: 10.1007/s11270-014-2175-7. [DOI] [Google Scholar]
- 82.Rajeswari Vennila, Bhuvaneswari V. Optimization of laccase production media by Bacilllus cereus TSS1 using Box–Behnken design. Int J Chem Pharm Sci. 2015;6:95–101. [Google Scholar]
- 83.Machczynski MC, Vijgenboom E, Samyn B, Canters GW. Characterization of SLAC: a small laccase from Streptomyces coelicolor with unprecedented activity. Protein Sci. 2004;13:2388–2397. doi: 10.1110/ps.04759104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Endo K, Hayashi Y, Hibi T, Hosono K, Beppu T, Ueda K. Enzymological characterization of EpoA, a laccase-like phenol oxidase produced by Streptomyces griseus. J Biochem. 2003;133:671–677. doi: 10.1093/jb/mvg086. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki T, Endo K, Ito M, Tsujibo H, Miyamoto K, Inamori Y. A thermostable laccase from Streptomyces lavendulae REN-7: purification, characterization, nucleotide sequence, and expression. Biosci Biotechnol Biochem. 2003;67:2167–2175. doi: 10.1271/bbb.67.2167. [DOI] [PubMed] [Google Scholar]
- 86.Niladevi KN, Sheejadevi PS, Prema P. Strategies for enhancing laccase yield from Streptomyces psammoticus and its role in mediator-based decolorization of azo dyes. Appl Biochem Biotechnol. 2008;151:9–19. doi: 10.1007/s12010-008-8175-6. [DOI] [PubMed] [Google Scholar]
- 87.Lu L, Zeng G, Fan C, Zhang J, Chen A, Chen M, Jiang M, Yuan Y, Wu H, Lai M. Diversity of two-domain laccase-like multicopper oxidase genes in Streptomyces spp.: identification of genes potentially involved in extracellular activities and lignocellulose degradation during composting of agricultural waste. Appl Environ Microbiol. 2014;80:3305–3314. doi: 10.1128/AEM.00223-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Majumdar S, Lukk T, Solbiati JO, Bauer S, Nair SK, Cronan JE, Gerlt JA. Roles of small laccases from Streptomyces in lignin degradation. Biochemistry. 2014;53:4047–4058. doi: 10.1021/bi500285t. [DOI] [PubMed] [Google Scholar]
- 89.Enguita FJ, Martins LO, Henriques AO, Carrondo MA. Crystal Structure of a bacterial endospore coat component a laccase with enhanced thermostability properties. J Biol Chem. 2003;278:19416–19425. doi: 10.1074/jbc.M301251200. [DOI] [PubMed] [Google Scholar]
- 90.Wang C, Cui D, Lu L, Zhang N, Yang H, Zhao M, Dai S. Cloning and characterization of CotA laccase from Bacillus subtilis WD23 decoloring dyes. Ann Microbiol. 2016;66:461–467. doi: 10.1007/s13213-015-1128-8. [DOI] [Google Scholar]
- 91.Wang CL, Zhao M, Wei XD, Li TL, Lu L. Characteristics of spore-bound laccase from Bacillus subtilis WD23 and its use in dye decolorization. Adv Mater Res. 2011;113–116:226–230. [Google Scholar]
- 92.Brander S, Mikkelsen JD, Kepp KP. TtMCO: a highly thermostable laccase-like multicopper oxidase from the thermophilic Thermobaculum terrenum. J Mol Catal B Enzym. 2015;112:59–65. doi: 10.1016/j.molcatb.2014.12.002. [DOI] [Google Scholar]
- 93.Ausec L, Črnigoj M, Šnajder M, Ulrih NP, Mandic-Mulec I. Characterization of a novel high-pH-tolerant laccase-like multicopper oxidase and its sequence diversity in Thioalkalivibrio sp. Appl Microbiol Biotechnol. 2015;99:9987–9999. doi: 10.1007/s00253-015-6843-3. [DOI] [PubMed] [Google Scholar]
- 94.Kumar S, Nussinov R. How do thermophilic proteins deal with heat? Cell Mol Life Sci. 2001;58:1216–1233. doi: 10.1007/PL00000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Modarres HP, Mofrad MR, Sanatinezhad A. Protein thermostability engineering. Rsc Adv. 2016;6:115252–115270. doi: 10.1039/C6RA16992A. [DOI] [Google Scholar]
- 96.Pack SP, Yoo YJ. Protein thermostability: structure-based difference of amino acid between thermophilic and mesophilic proteins. J Biotechnol. 2004;111:269–277. doi: 10.1016/j.jbiotec.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 97.Cañas AI, Camarero S. Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv. 2010;28:694–705. doi: 10.1016/j.biotechadv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 98.Reiss R, Ihssen J, Thöny-Meyer L. Bacillus pumilus laccase: a heat stable enzyme with a wide substrate spectrum. BMC Biotechnol. 2011;11:9–20. doi: 10.1186/1472-6750-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Forootanfar H, Faramarzi MA. Insights into laccase producing organisms, fermentation states, purification strategies, and biotechnological applications. Biotechnol Progr. 2015;31:1443–1463. doi: 10.1002/btpr.2173. [DOI] [PubMed] [Google Scholar]
- 100.Morozova OV, Shumakovich GP, Shleev SV, Iaropolov AI. Laccase-mediator systems and their applications: a review. Prikl Biokhim Mikrobiol. 2007;43:583–597. [PubMed] [Google Scholar]
- 101.Bourbonnais R, Paice MG. Oxidation of non-phenolic substrates. FEBS Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-W. [DOI] [PubMed] [Google Scholar]
- 102.Luo Q, Chen Y, Xia J, Wang KQ, Cai YJ, Liao XR, Guan ZB. Functional expression enhancement of Bacillus pumilus CotA-laccase mutant WLF through site-directed mutagenesis. Enzyme Microb Technol. 2018;109:11–19. doi: 10.1016/j.enzmictec.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 103.Wang J, Lu L, Feng F. Combined strategies for improving production of a thermo-alkali stable laccase in Pichia pastoris. Electron J Biotechnol. 2017;28:7–13. doi: 10.1016/j.ejbt.2017.04.002. [DOI] [Google Scholar]
- 104.Wang TN, Lu L, Wang JY, Xu TF, Li J, Zhao M. Enhanced expression of an industry applicable CotA laccase from Bacillus subtilis in Pichia pastoris by non-repressing carbon sources together with pH adjustment: recombinant enzyme characterization and dye decolorization. Process Biochem. 2015;50:97–103. doi: 10.1016/j.procbio.2014.10.009. [DOI] [Google Scholar]
- 105.Kudanga T, Roes-Hill ML. Laccase applications in biofuels production: current status and future prospects. Appl Microbiol Biotechnol. 2014;98:6525–6542. doi: 10.1007/s00253-014-5810-8. [DOI] [PubMed] [Google Scholar]
- 106.D’Acunzo F, Galli C. First evidence of catalytic mediation by phenolic compounds in the laccase-induced oxidation of lignin models. Eur J Biochem. 2003;270:3634–3640. doi: 10.1046/j.1432-1033.2003.03752.x. [DOI] [PubMed] [Google Scholar]
- 107.Luo XL, Zhan HY, Fu SY, Zhan Y. Separation and GC-MS analysis of low-molecular-weight phenolic compounds from black liquor of Eucalyptus urophylla kraft pulping. Chem Ind For Prod. 2009;29:53–58. [Google Scholar]
- 108.Moldes D, Díaz M, Tzanov T, Vidal T. Comparative study of the efficiency of synthetic and natural mediators in laccase-assisted bleaching of Eucalyptus kraft pulp. Bioresour Technol. 2008;99:7959–7965. doi: 10.1016/j.biortech.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 109.Vila C, Barneto AG, Fillat A, Vidal T, Ariza J. Use of thermogravimetric analysis to monitor the effects of natural laccase mediators on flax pulp. Bioresour Technol. 2011;102:6554–6561. doi: 10.1016/j.biortech.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 110.Xie T, Liu Z, Liu Q, Wang G. Structural insight into the oxidation of sinapic acid by CotA laccase. J Struct Bio. 2015;190:155–161. doi: 10.1016/j.jsb.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 111.Yang H, Sun H, Zhang S, Wu B, Pan B. Potential of acetylacetone as a mediator for Trametes versicolor laccase in enzymatic transformation of organic pollutants. Environ Sci Pollut Res Int. 2015;22:10882–10889. doi: 10.1007/s11356-015-4312-2. [DOI] [PubMed] [Google Scholar]
- 112.Fillat A, Colom JF, Vidal T. A new approach to the biobleaching of flax pulp with laccase using natural mediators. Bioresour Technol. 2010;101:4104–4110. doi: 10.1016/j.biortech.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 113.Tavares APM, Gamelas JAF, Gaspar AR, Evtuguin DV, Xavier AMRB. A novel approach for the oxidative catalysis employing polyoxometalate–laccase system: application to the oxygen bleaching of kraft pulp. Catal Commun. 2004;5:485–489. doi: 10.1016/j.catcom.2004.06.002. [DOI] [Google Scholar]
- 114.Carneiro A, Abreu A, Evtuguin DV, Neto CP, Guebitz G, Paulo AC. Polyoxometalates as promoters of laccase-assisted reactions. J Mol Catal B Enzym. 2000;9:293–295. doi: 10.1016/S1381-1177(00)00006-0. [DOI] [Google Scholar]
- 115.Gamelas JAF, Pontes ASN, Evtuguin DV, Xavier AMRB, Esculcas AP. New polyoxometalate–laccase integrated system for kraft pulp delignification. Biochem Eng J. 2007;33:141–147. doi: 10.1016/j.bej.2006.10.014. [DOI] [Google Scholar]
- 116.Gamelas JAF, Tavares APM, Evtuguin DV, Xavier AMB. Oxygen bleaching of kraft pulp with polyoxometalates and laccase applying a novel multi-stage process. J Mol Catal B Enzym. 2005;33:57–64. doi: 10.1016/j.molcatb.2005.03.001. [DOI] [Google Scholar]
- 117.Woldesenbet F, Virk AP, Gupta N, Sharma P. Biobleaching of mixed wood kraft pulp with alkalophilic bacterial xylanase, mannanase and laccase-mediator system. J Microbiol Biotechnol. 2013;3:32–41. [Google Scholar]
- 118.Mathews SL, Smithson CE, Grunden AM. Purification and characterization of a recombinant laccase-like multi-copper oxidase from Paenibacillus glucanolyticus SLM1. J Appl Microbiol. 2016;121:1335–1345. doi: 10.1111/jam.13241. [DOI] [PubMed] [Google Scholar]
- 119.Solomon EI, Augustine AJ, Yoon J. O2 reduction to H2O by the multicopper oxidases. Dalton Trans. 2008;252:3921–3932. doi: 10.1039/b800799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoon J, Liboiron BD, Sarangi R, Hodgson KO, Hedman B, Solomon EI. The two oxidized forms of the trinuclear Cu cluster in the multicopper oxidases and mechanism for the decay of the native intermediate. Proc Nati Acad Sci USA. 2007;104:13609–13614. doi: 10.1073/pnas.0705137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.D’Acunzo F, Baiocco P, Fabbrini M, Galli C, Gentili P. A mechanistic survey of the oxidation of alcohols and ethers with the enzyme laccase and its mediation by TEMPO. Eur J Org Chem. 2002;2002:4195–4201. doi: 10.1002/1099-0690(200212)2002:24<4195::AID-EJOC4195>3.0.CO;2-X. [DOI] [Google Scholar]
- 122.Cañas AI, Alcalde M, Plou F, Martínez MJ, Martínez AT, Camarero S. Transformation of polycyclic aromatic hydrocarbons by laccase is strongly enhanced by phenolic compounds present in soil. Environ Sci Technol. 2007;41:2964–2971. doi: 10.1021/es062328j. [DOI] [PubMed] [Google Scholar]
- 123.Luo S, Xie T, Liu Z, Wang G. Laccase-mediator system: a reviewcase-mediator system: a review. Chin J Appl Environ Biol. 2015;21:987–995. [Google Scholar]
- 124.Cannatelli MD, Ragauskas AJ. Conversion of lignin into value-added materials and chemicals via laccase-assisted copolymerization. Appl Microbiol Biotechnol. 2016;100:8685–8691. doi: 10.1007/s00253-016-7820-1. [DOI] [PubMed] [Google Scholar]
- 125.Kudanga T, Nemadziva B, Roes-Hill ML. Laccase catalysis for the synthesis of bioactive compounds. Appl Microbiol Biotechnol. 2017;101:13–33. doi: 10.1007/s00253-016-7987-5. [DOI] [PubMed] [Google Scholar]
- 126.Kudanga T, Prasetyo EN, Sipilä J, Eberl A, Nyanhongo GS, Guebitz GM. Coupling of aromatic amines onto syringylglycerol β-guaiacylether using Bacillus SF spore laccase: a model for functionalization of lignin-based materials. J Mol Catal B Enzym. 2009;61:143–149. doi: 10.1016/j.molcatb.2009.06.003. [DOI] [Google Scholar]
- 127.Ganachaud C, Garfagnoli V, Tron T, Iacazio G. Trimerisation of indole through laccase catalysis. Tetrahedron Lett. 2008;49:2476–2478. doi: 10.1016/j.tetlet.2008.02.021. [DOI] [Google Scholar]
- 128.Luo Q, Wang Z, Feng M, Chiang D, Woodward D, Liang S, Lu J, Huang Q. Factors controlling the rate of perfluorooctanoic acid degradation in laccase-mediator systems: the impact of metal ions. Environ Pollut. 2017;224:649–657. doi: 10.1016/j.envpol.2017.02.050. [DOI] [PubMed] [Google Scholar]
- 129.Gupta N, Lee FS, Farinas ET. Laboratory evolution of laccase for substrate specificity. J Mol Catal B Enzym. 2010;62:230–234. doi: 10.1016/j.molcatb.2009.10.012. [DOI] [Google Scholar]
- 130.Yu C, Quan L, Wen Z, Zeng X, Cai YJ, Liao XR, Guan ZB. Improving the catalytic efficiency of Bacillus pumilus CotA-laccase by site-directed mutagenesis. Appl Microbiol Biotechnol. 2017;101:1935–1944. doi: 10.1007/s00253-016-7962-1. [DOI] [PubMed] [Google Scholar]
- 131.Gupta N, Farinas ET. Directed evolution of CotA laccase for increased substrate specificity using Bacillus subtilis spores. Protein Eng Des Sel. 2010;23:679–682. doi: 10.1093/protein/gzq036. [DOI] [PubMed] [Google Scholar]
- 132.Durão P, Bento I, Fernandes AT, Melo EP, Lindley PF, Martins LO. Perturbations of the T1 copper site in the CotA laccase from Bacillus subtilis: structural, biochemical, enzymatic and stability studies. J Biol Inorg Chem. 2006;11:514–526. doi: 10.1007/s00775-006-0102-0. [DOI] [PubMed] [Google Scholar]
- 133.Toscano MD, De ML, Lobedanz S, Ostergaard LH. Optimization of a small laccase by active-site redesign. ChemBioChem. 2013;14:1209–1211. doi: 10.1002/cbic.201300256. [DOI] [PubMed] [Google Scholar]
- 134.Durão P, Chen Z, Silva CS, Soares CM, Pereira MM, Todorovic S, Hildebrandt P, Bento I, Lindley PF, Martins LO. Proximal mutations at the type 1 copper site of CotA laccase: spectroscopic, redox, kinetic and structural characterization of I494A and L386A mutants. Biochem J. 2008;412:339–346. doi: 10.1042/BJ20080166. [DOI] [PubMed] [Google Scholar]
- 135.Nasoohi N, Khajeh K, Mohammadian M, Ranjbar B. Enhancement of catalysis and functional expression of a bacterial laccase by single amino acid replacement. Int J Biol Macromol. 2013;60:56–61. doi: 10.1016/j.ijbiomac.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 136.Enguita FJ, Marçal D, Martins LO, Grenha R, Henriques AO, Lindley PF, Carrondo MA. Substrate and dioxygen binding to the endospore coat laccase from Bacillus subtilis. J Biol Chem. 2004;279:23472–23476. doi: 10.1074/jbc.M314000200. [DOI] [PubMed] [Google Scholar]
- 137.Mollania N, Khajeh K, Ranjbar B, Hosseinkhani S. Enhancement of a bacterial laccase thermostability through directed mutagenesis of a surface loop. Enzyme Microb Technol. 2011;49:446–452. doi: 10.1016/j.enzmictec.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 138.Shao X, Gao Y, Jiang M, Li L. Deletion and site-directed mutagenesis of laccase from Shigella dysenteriae results in enhanced enzymatic activity and thermostability. Enzyme Microb Technol. 2009;44:274–280. doi: 10.1016/j.enzmictec.2008.12.013. [DOI] [Google Scholar]
- 139.Rodríguez Couto S, Toca Herrera JL. Industrial and biotechnological applications of laccases: a review. Biotechnol Adv. 2006;24:500–513. doi: 10.1016/j.biotechadv.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 140.Mate DM, Alcalde M. Laccase: a multi-purpose biocatalyst at the forefront of biotechnology. Microb Biotechnol. 2016;10:1457–1467. doi: 10.1111/1751-7915.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gasser CA, Ammann EM, Shahgaldian P, Corvini FX. Laccases to take on the challenge of emerging organic contaminants in wastewater. Appl Microbiol Biotechnol. 2014;98:9931–9952. doi: 10.1007/s00253-014-6177-6. [DOI] [PubMed] [Google Scholar]
- 142.Fernández-Fernández M, Sanromán MÁ, Moldes D. Recent developments and applications of immobilized laccase. Biotechnol Adv. 2013;31:1808–1825. doi: 10.1016/j.biotechadv.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 143.Fan L, Wang Y, Zhao M, Song J, Wang J, Jin Z. Magnetic Ganoderma lucidum spore microspheres: a novel material to immobilize CotA multicopper oxidase for dye decolorization. J Hazard Mater. 2016;313:122–129. doi: 10.1016/j.jhazmat.2016.03.083. [DOI] [PubMed] [Google Scholar]
- 144.Rong J, Zhang T, Qiu F, Zhu Y. Preparation of efficient, stable and reusable laccase-Cu3(PO4)2 hybrid microspheres based on copper foil for decolouration of congo red. Acs Sustain Chem Eng. 2017;5:4468–4477. doi: 10.1021/acssuschemeng.7b00820. [DOI] [Google Scholar]
- 145.Asgher M, Noreen S, Bilal M. Enhancement of catalytic, reusability, and long-term stability features of Trametes versicolor IBL-04 laccase immobilized on different polymers. Int J Biol Macromol. 2017;95:54–62. doi: 10.1016/j.ijbiomac.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 146.Xu R, Cui JY, Tang RZ, Li F, Zhang B. Removal of 2,4,6-trichlorophenol by laccase immobilized on nano-copper incorporated electrospun fibrous membrane-high efficiency, stability and reusability. Chem Eng J. 2017;326:647–655. doi: 10.1016/j.cej.2017.05.083. [DOI] [Google Scholar]
- 147.Vera M, Rivas BL. Immobilization of Trametes versicolor laccase on different PGMA-based polymeric microspheres using response surface methodology: optimization of conditions. J Appl Polym Sci. 2017;134:45249–45259. doi: 10.1002/app.45249. [DOI] [Google Scholar]
- 148.Guan ZB, Shui Y, Song CM, Zhang N, Cai YJ, Liao XR. Efficient secretory production of CotA-laccase and its application in the decolorization and detoxification of industrial textile wastewater. Environ Sci Pollut Res. 2015;22:9515–9523. doi: 10.1007/s11356-015-4426-6. [DOI] [PubMed] [Google Scholar]
- 149.Khlifi R, Belbahri L, Woodward S, Ellouz M, Dhouib A, Sayadi S, Mechichi T. Decolourization and detoxification of textile industry wastewater by the laccase-mediator system. J Hazard Mater. 2010;175:802–808. doi: 10.1016/j.jhazmat.2009.10.079. [DOI] [PubMed] [Google Scholar]
- 150.Singh G, Bhalla A, Kaur P, Capalash N, Sharma P. Laccase from prokaryotes: a new source for an old enzyme. Rev Environ Sci Biotechnol. 2011;10:309–326. doi: 10.1007/s11157-011-9257-4. [DOI] [Google Scholar]
- 151.Mendes S, Farinha A, Ramos CG, Leitão JH, Viegas CA, Martins LO. Synergistic action of azoreductase and laccase leads to maximal decolourization and detoxification of model dye-containing wastewaters. Bioresour Technol. 2011;102:9852–9859. doi: 10.1016/j.biortech.2011.07.108. [DOI] [PubMed] [Google Scholar]
- 152.Al-Kahem Al-Balawi TH, Wood AL, Solis A, Cooper T, Barabote RD. Anoxybacillus sp. strain UARK-01, a new thermophilic soil bacterium with hyperthermostable alkaline laccase activity. Curr Microbiol. 2017;74:1–10. doi: 10.1007/s00284-017-1239-5. [DOI] [PubMed] [Google Scholar]
- 153.Afreen S, Shamsi TN, Baig MA, Ahmad N, Fatima S, Qureshi MI, Hassan MI, Fatma T. A novel multicopper oxidase (laccase) from cyanobacteria: purification, characterization with potential in the decolorization of anthraquinonic dye. PLoS One. 2017;12:e0175144. doi: 10.1371/journal.pone.0175144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kumari A, Kishor N, Guptasarma P. Characterization of a mildly alkalophilic and thermostable recombinant Thermus thermophilus laccase with applications in decolourization of dyes. Biotechnol Lett. 2018;40:285–295. doi: 10.1007/s10529-017-2461-8. [DOI] [PubMed] [Google Scholar]
- 155.Liu Z, Tian X, Zhong Q, Wang G. Crystal structure of CotA laccase complexed with 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate) at a novel binding site. Acta Crystallogr A. 2016;72:328–335. doi: 10.1107/S2053273316096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zheng Z, Li H, Li L, Shao W. Biobleaching of wheat straw pulp with recombinant laccase from the hyperthermophilic Thermus thermophilus. Biotechnol Lett. 2012;34:541–547. doi: 10.1007/s10529-011-0796-0. [DOI] [PubMed] [Google Scholar]
- 157.Sondhi S, Sharma P, George N, Chauhan PS, Puri N, Gupta N. An extracellular thermo-alkali-stable laccase from Bacillus tequilensis SN4, with a potential to biobleach softwood pulp. 3 Biotech. 2015;5:175–185. doi: 10.1007/s13205-014-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gupta V, Garg S, Capalash N, Gupta N, Sharma P. Production of thermo-alkali-stable laccase and xylanase by co-culturing of Bacillus sp. and B. halodurans for biobleaching of kraft pulp and deinking of waste paper. Bioprocess Biosyst Eng. 2015;38:947–956. doi: 10.1007/s00449-014-1340-0. [DOI] [PubMed] [Google Scholar]
- 159.Virk AP, Sharma P, Capalash N. Use of laccase in pulp and paper industry. Biotechnol Progr. 2012;28:21–32. doi: 10.1002/btpr.727. [DOI] [PubMed] [Google Scholar]
- 160.Woolridge EM. Mixed enzyme systems for delignification of lignocellulosic biomass. Catalysts. 2014;4:1–35. doi: 10.3390/catal4010001. [DOI] [Google Scholar]
- 161.Asina F, Brzonova I, Voeller K, Kozliak E, Kubátová A, Yao B, Ji Y. Biodegradation of lignin by fungi, bacteria and laccases. Bioresour Technol. 2016;220:414–424. doi: 10.1016/j.biortech.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 162.Sindhu R, Binod P, Pandey A. Biological pretreatment of lignocellulosic biomass–an overview. Bioresour Technol. 2016;199:76–82. doi: 10.1016/j.biortech.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 163.Rocha-Martín J, Martínez-Bernal C, Zamorano LS, Reyes-Sosa FM, García BD. Inhibition of enzymatic hydrolysis of pretreated corn stover and sugar cane straw by laccases. Process Biochem. 2018;67:88–91. doi: 10.1016/j.procbio.2018.01.021. [DOI] [Google Scholar]
- 164.Oliva-Taravilla A, Tomás-Pejó E, Demuez M, González-Fernández C, Ballesteros M. Inhibition of cellulose enzymatic hydrolysis by laccase-derived compounds from phenols. Biotechnol Prog. 2015;31:700–706. doi: 10.1002/btpr.2068. [DOI] [PubMed] [Google Scholar]
- 165.Oliva-Taravilla A, Moreno AD, Demuez M, Ibarra D, Tomás-Pejó E, González-Fernández C, Ballesteros M. Unraveling the effects of laccase treatment on enzymatic hydrolysis of steam-exploded wheat straw. Bioresour Technol. 2015;175:209–215. doi: 10.1016/j.biortech.2014.10.086. [DOI] [PubMed] [Google Scholar]
- 166.Singh R, Hu J, Regner MR, Round JW, Ralph J, Saddler JN, Eltis LD. Enhanced delignification of steam-pretreated poplar by a bacterial laccase. Sci Rep. 2017;7:42121–42134. doi: 10.1038/srep42121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Arias ME, Blánquez A, Hernández M, Rodríguez J, Ball AS, Jiménez-Morillo NT, González-Vila FJ, González-Pérez JA. Role of a thermostable laccase produced by Streptomyces ipomoeae in the degradation of wheat straw lignin in solid state fermentation. J Anal Appl Pyrol. 2016;122:202–208. doi: 10.1016/j.jaap.2016.09.023. [DOI] [Google Scholar]
- 168.Blánquez A, Ball AS, González-Pérez JA, Jiménez-Morillo NT, González-Vila F, Arias ME, Hernández M. Laccase SilA from Streptomyces ipomoeae CECT 3341, a key enzyme for the degradation of lignin from agricultural residues? PLoS One. 2017;12:e0187649. doi: 10.1371/journal.pone.0187649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rezaie R, Rezaei S, Jafari N, Forootanfar H, Khoshayand MR, Faramarzi MA. Delignification and detoxification of peanut shell bio-waste using an extremely halophilic laccase from an Aquisalibacillus elongatus isolate. Extremophiles. 2017;21:993–1004. doi: 10.1007/s00792-017-0958-7. [DOI] [PubMed] [Google Scholar]
- 170.Rezaei S, Shahverdi AR, Faramarzi MA. Isolation, one-step affinity purification, and characterization of a polyextremotolerant laccase from the halophilic bacterium Aquisalibacillus elongatus and its application in the delignification of sugar beet pulp. Bioresour Technol. 2017;230:67–75. doi: 10.1016/j.biortech.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 171.Jafari N, Rezaei S, Rezaie R, Dilmaghani H, Khoshayand MR, Faramarzi MA. Improved production and characterization of a highly stable laccase from the halophilic bacterium Chromohalobacter salexigens for the efficient delignification of almond shell bio-waste. Int J Biol Macromol. 2017;105:489–498. doi: 10.1016/j.ijbiomac.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 172.Saritha M, Arora A, Singh S, Nain L. Streptomyces griseorubens mediated delignification of paddy straw for improved enzymatic saccharification yields. Bioresour Technol. 2013;135:12–17. doi: 10.1016/j.biortech.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 173.Fonseca-Maldonado R, Ribeiro LF, Furtado GP, Arruda LM, Meleiro LP, Alponti JS, Botelho-Machado C, Vieira DS, Bonneil E, Furriel RDPM. Synergistic action of co-expressed xylanase/laccase mixtures against milled sugar cane bagasse. Process Biochem. 2014;49:1152–1161. doi: 10.1016/j.procbio.2014.03.027. [DOI] [Google Scholar]
- 174.Kumar S, Jain KK, Bhardwaj KN, Chakraborty S, Kuhad RC. Multiple genes in a single host: cost-effective production of bacterial laccase (cotA), pectatel yase (pel), and endoxylanase (xyl) by simultaneous expression and cloning in single vector in E. coli. PLoS One. 2015;10:e0144379. doi: 10.1371/journal.pone.0144379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Callejón S, Sendra R, Ferrer S, Pardo I. Recombinant laccase from Pediococcus acidilactici CECT 5930 with ability to degrade tyramine. PLoS One. 2017;12:e0186019. doi: 10.1371/journal.pone.0186019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ajao O, Hir ML, Rahni M, Chadjaa H, Marinova M. Comparative biocatalytic degradation of kraft prehydrolysate phenolic fermentation inhibitors using bacteria-derived laccase. Wood Sci Technol. 2017;51:585–599. doi: 10.1007/s00226-016-0879-0. [DOI] [Google Scholar]
- 177.Margot J, Bennati-Granier C, Maillard J, Blánquez P, Barry DA, Holliger C. Bacterial versus fungal laccase: potential for micropollutant degradation. AMB Express. 2013;3:63–77. doi: 10.1186/2191-0855-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Murugesan R, Vembu B. Production of extracellular laccase from the newly isolated Bacillus sp. PK4. Afr J Biotechnol. 2016;15:1813–1826. doi: 10.5897/AJB2016.15509. [DOI] [Google Scholar]
- 179.Sousa AC, Piedade MFMD, Martins LO, Robalo MPA. Eco-friendly synthesis of indo dyes mediated by a bacterial laccase. Green Chem. 2016;18:6063–6070. doi: 10.1039/C6GC02050J. [DOI] [Google Scholar]
- 180.Jia L, Fei R, Zhang X, Tang H, Hu Y. Sustainable endospore-based microreactor system for antioxidant capacity assay. Anal Chem. 2014;86:11578–11585. doi: 10.1021/ac500866r. [DOI] [PubMed] [Google Scholar]
- 181.Zhiming Z, Longjian T, Zheng L, Lina J, Xinya Z, Miaomiao X, Yonggang H. Whole-cell method for phenol detection based on the color reaction of phenol with 4-aminoantipyrine catalyzed by CotA laccase on endospore surfaces. Biosens Bioelectron. 2015;69:162–166. doi: 10.1016/j.bios.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 182.Tan SI, Ng IS, Yu YJ. Heterologous expression of an acidophilic multicopper oxidase in Escherichia coli and its applications in biorecovery of gold. Bioresour Bioprocess. 2017;4:20–30. doi: 10.1186/s40643-017-0150-z. [DOI] [Google Scholar]
- 183.Liu Y, Huang L, Guo W, Jia L, Fu Y, Gui S, Lu F. Cloning, expression, and characterization of a thermostable and pH-stable laccase from Klebsiella pneumoniae and its application to dye decolorization. Process Biochem. 2017;53:125–134. doi: 10.1016/j.procbio.2016.11.015. [DOI] [Google Scholar]
- 184.Ma S, Liu N, Jia H, Dai D, Zang J, Cao Z, Dong J. Expression, purification, and characterization of a novel laccase from Setosphaeria turcica in Eschericha coli. J Basic Microbiol. 2018;58:68–75. doi: 10.1002/jobm.201700212. [DOI] [PubMed] [Google Scholar]
- 185.Basheer S, Rashid N, Ashraf R, Akram MS, Siddiqui MA, Imanaka T, Akhtar M. Identification of a novel copper-activated and halide-tolerant laccase in Geobacillus thermopakistaniensis. Extremophiles. 2017;21:563–571. doi: 10.1007/s00792-017-0925-3. [DOI] [PubMed] [Google Scholar]
- 186.Chen Z, Durão P, Silva CS, Pereira MM, Todorovic S, Hildebrandt P, Bento I, Lindley PF, Martins LO. The role of Glu498 in the dioxygen reactivity of CotA-laccase from Bacillus subtilis. Dalton Trans. 2010;39:2875–2882. doi: 10.1039/b922734b. [DOI] [PubMed] [Google Scholar]
- 187.Gupta A, Nederlof I, Sottini S, Tepper AWJW, Groenen EJJ, Thomassen EAJ, Canters GW. Involvement of Tyr108 in the enzyme mechanism of the small laccase from Streptomyces coelicolor. J Am Chem Soc. 2012;134:18213–18216. doi: 10.1021/ja3088604. [DOI] [PubMed] [Google Scholar]
- 188.Silva CS, Damas JM, Chen Z, Brissos V, Martins LO, Soares CM, Lindley PF, Bento I. The role of Asp116 in the reductive cleavage of dioxygen to water in CotA laccase: assistance during the proton-transfer mechanism. Acta Crystallogr A. 2012;68:186–193. doi: 10.1107/S0108767312099230. [DOI] [PubMed] [Google Scholar]
- 189.Brissos V, Ferreira M, Grass G, Martins LO. Turning a hyperthermostable metallo-oxidase into a laccase by directed evolution. Acs Catal. 2015;5:4932–4941. doi: 10.1021/acscatal.5b00771. [DOI] [Google Scholar]
- 190.Mooers BH. Simplifying and enhancing the use of PyMOL with horizontal scripts. Protein Sci. 2016;25:1873–1882. doi: 10.1002/pro.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Discovery Studio Visualizer (2012) Accelrys Inc, San Diego, CA, USA