Abstract
To successfully feed, ticks inject pharmacoactive molecules into the vertebrate host including cystatin cysteine protease inhibitors. However, the molecular and cellular events modulated by tick saliva remain largely unknown. Here, we describe and characterize a novel immunomodulatory cystatin, Iristatin, which is upregulated in the salivary glands of feeding Ixodes ricinus ticks. We present the crystal structure of Iristatin at 1.76 Å resolution. Purified recombinant Iristatin inhibited the proteolytic activity of cathepsins L and C and diminished IL-2, IL-4, IL-9, and IFN-γ production by different T-cell populations, IL-6 and IL-9 production by mast cells, and nitric oxide production by macrophages. Furthermore, Iristatin inhibited OVA antigen-induced CD4+ T-cell proliferation and leukocyte recruitment in vivo and in vitro. Our results indicate that Iristatin affects wide range of anti-tick immune responses in the vertebrate host and may be exploitable as an immunotherapeutic.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03034-3) contains supplementary material, which is available to authorized users.
Keywords: Cathepsin, Crystal structure, Immune responses, Ixodes ricinus, Saliva
Introduction
Ticks are obligatory ectoparasites that feed on the blood of their vertebrate hosts. Hard ticks (family Ixodidae) feed continuously for days to weeks during each life stage, so must overcome specific host antigen-specific immune responses, non-specific innate responses, and hemostasis to successfully finish their blood meal [1]. The hard tick Ixodes ricinus is an important arthropod disease vector of several pathogens in Europe [1]. To counteract vertebrate host anti-tick responses, I. ricinus secretes saliva rich in biomolecules that facilitate tick feeding and pathogen transmission [2].
Tick saliva affects blood coagulation, complement activation, and immune reaction in terms of immune cell recruitment, cytokine production, and cell maturation [2]. It also facilitates the transmission of Borrelia, Anaplasma, Rickettsia, and various viruses to the vertebrate host [2]. Protease inhibitors are an important group of tick salivary effectors that are divided according to specificity into serine and cysteine protease inhibitors, and according to structure into Kunitz domain inhibitors, serpins, cystatins, and other less abundant families [1]. Kunitz inhibitors are thought to be mainly anti-hemostatic, serpins both anti-hemostatic and immunomodulatory, and cystatins mainly anti-inflammatory and immunosuppressive [1, 3].
Cystatins are tight binding, reversible legumain, and papain-like cysteine protease inhibitors [4]. Cystatins are subdivided into three subfamilies according to the MEROPS nomenclature: I25A (type 1, stefins), I25B (type 2 and type 3, kininogens), and I25C (type 4, fetuins) [5]. Only type 1 and type 2 cystatins have, thus, far been identified in ticks [6]. Type 1 cystatins lack a signaling peptide for secretion and are, therefore, thought to regulate intracellular blood digestion in the tick midgut, while type 2 cystatins are secreted and expressed in both tick salivary glands and the midgut, and are, therefore, thought to play pleiotropic roles in both ticks and vertebrate hosts [6]. For example, sialostatin L, a cystatin identified in the hard tick Ixodes scapularis, inhibits cathepsins C, L, S, V, X, and papain, modulates cytokine production by lymphocytes, dendritic cells, and mast cells, and impairs T-cell proliferation [7, 8]. A similar cystatin in I. scapularis, sialostatin L2, inhibits cathepsins C, L, S, and V [9], diminishes IL-1β and IL-18 secretion by macrophages, and inhibits caspase-1 maturation [10]. Furthermore, both sialostatins alter dendritic cell signaling [11], and inhibition of sialostatin by RNA interference and immunization of guinea pigs impairs tick feeding [9, 12, 13]. Therefore, salivary cystatins may be useful targets for anti-tick vaccines.
However, until the first I. ricinus genome was released [14], tick genomic and proteomic studies have been hampered by a lack of full genomic sequences. Here, we report the structural and functional characterization of a novel type 2 cystatin in the hard tick I. ricinus, which we name Iristatin. We present the crystal structure of Iristatin, which inhibits the vertebrate cathepsins C and L. Furthermore, we report the anti-inflammatory and immunomodulatory activities of Iristatin. Rather than being target specific, Iristatin appears to affect many immune mechanisms and is a broad-spectrum immunosuppressor that may be useful in the treatment of immune-mediated diseases.
Materials and methods
Quantitative real-time PCR
Female I. ricinus ticks were fed on rabbits for 1, 2, 4, 6, or 7 days. Tick salivary glands were dissected, and total RNA isolated and transcribed to cDNA for quantitative analysis of Iristatin by qRT-PCR. Expression profiles were normalized to ferritin mRNA, the levels of which are independent of blood feeding [15]. Detailed methods can be found in the supplement, and the primer and probe sequences are in Supplementary Table S3.
Crystallization, data collection, and structure determination
Screening for crystallization conditions was performed using the JCSG-plus kit (Molecular Dimensions Ltd., Newmarket, UK) and the sitting drop vapor diffusion technique. Preliminary crystals were obtained in 0.1 M Bis–Tris, pH 5.5, 1 M ammonium sulfate, 1% PEG 3350. Optimal Iristatin crystals were prepared at 18 °C using the hanging drop vapor diffusion technique in 15-well NeXtal plates (Qiagen, Hilden, Germany). Experiments, crystal parameters, data collection statistics, and structure determination are detailed in the Supplementary Methods and in Supplementary Table S2.
Enzyme assays
Iristatin inhibition constants against various proteases were determined by measuring the loss of enzymatic activity in the presence of increasing Iristatin concentrations, the corresponding enzyme and a fluorogenic substrate. The enzymes tested were: human liver cathepsin B (BiomolGmBH, Hamburg, Germany); human recombinant cathepsins C, L, S (Calbiochem, Merck Millipore, Burlington, MA, USA); human cathepsin G (Molecular Innovations Inc., Novi, MI, USA); and human factor Xa (Calbiochem). I. ricinus legumain IrAE [16] was kindly provided by Daniel Sojka, Ph.D. Experimental details are provided in the Supplementary Methods.
Measurement of cytokine production
T cells were differentiated as follows. For Th1 T cells, naïve CD4+ T cells from BALB/c mice were stimulated with anti-CD3/CD28 (4 µg/ml each) under Th1-skewing conditions (IL-12, anti-IL-4). For Th2 cells, naïve CD4+ T cells were stimulated with anti-CD3/CD28 (4 µg/ml each) under Th2-skewing conditions (IFN-γ, anti-IL-4). For Th9 cells, naïve CD4+ T cells from BALB/c mice were stimulated with anti-CD3/CD28 (4 µg/ml each) under Th9-skewing conditions (IL-4, TGF-β, anti-IFN-γ). Fully differentiated cells were then re-stimulated solely with plate-bound CD3 mAb for 48 h in the presence or absence of 6 µM LPS-free Iristatin. IL-2, IL-4, IL-9, and IFN-γ production were determined by Ready-SET-Go! ELISA (eBioscience, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
Bone marrow-derived mast cells (BMMCs) were stimulated with ionomycin (Iono, 0.75 μM) in the presence or absence of 6 μM Iristatin. IL-4, IL-6, and IL-9 production was determined by ELISA after 48 h of stimulation.
For viability screening, naïve CD4+ T cells from BALB/c mice were stimulated with anti-CD3/CD28 (4 µg/ml each) in the presence or absence of different concentrations of LPS-free Iristatin (6, 3, 1.5, and 0.75 µM) under Th9-skewing conditions for 72 h. T cells were stained with a fixable viability dye and cell viability determined by flow cytometry.
Nitric oxide (NO) measurement
Macrophages of the PMJ2-R cell line were preincubated for 4 h with 3 and 6 μM Iristatin and then stimulated by adding LPS to final concentration 100 ng/ml and IFN-γ to final concentration 5 ng/ml. NO concentration was assessed 24 h after stimulation with modified Griess reagent (Sigma-Aldrich, St. Louis, MO, USA).
OVA antigen-induced proliferation of CD4+ splenocytes
OT-II mouse spleens were disintegrated through a 70 μm cell strainer to obtain a single-cell suspension, and red blood cells were removed using RBC lysis buffer (eBioscience). Splenocytes were then stained with eFluor™ 670 cell proliferation dye (eBioscience). Stained splenocytes were seeded in a 96-well plate (5 × 105 cells in 200 µl complete RPMI) and preincubated for 2 h in the presence or absence of 3 µM Iristatin. Cells were then stimulated using ovalbumin (OVA) peptide 323–339 (100 ng/ml; Sigma-Aldrich), and splenocytes were incubated for 72 h at 37 °C in 5% CO2. Cells were then stained with FITC-labeled anti-CD4 antibody and propidium iodide and analyzed by flow cytometry on a BD FACSCanto™ II using BD FACSDiva™ Software v. 6.1.3.
Thioglycollate-induced peritonitis
Female C57BL/6N mice were purchased from Velaz (Prague, Czech Republic). Mice were housed in individually ventilated cages maintained in a 12 h light/dark cycle and given a standard pellet diet and water ad libitum. All animals were used at 8–12 weeks of age. All experiments were approved by the local ethical committee and the Ministry of Education and Sports in accordance with law 246/1992 Sb (ethical approval number MSMT-19085/2015-3).
Control group mice were injected intraperitoneally (i.p.) with saline (10 ml/kg of body weight) and, 1 h later, acute peritonitis was induced by i.p. injection of 200 μl 3% sterile, fully oxidized Difco™ thioglycollate medium (BD Biosciences, Franklin Lakes, NJ, USA). Mice in the experimental group were first injected i.p. with Iristatin (2 mg/kg of body weight in saline). One hour later, mice were treated i.p. with Iristatin (2 mg/kg of body weight) together with 200 μl of 3% thioglycollate medium.
Four hours after thioglycollate medium injection, mice were killed by cervical dislocation, and peritoneal cavities were washed with 10 ml cold PBS to harvest cells. Red blood cells were lysed with RBC lysis buffer (eBioscience). Collected peritoneal cells were counted using a hemocytometer and light microscope. The percentage of live myeloid cells (CD11b+), neutrophils (CD11b+Ly-6g+), monocytes (CD11b+Ly-6c+), and eosinophils (CD11b+Siglec-F+) was assessed by flow cytometry (see Supplementary Methods for details). Absolute cell counts were obtained by combining flow cytometry data with cell counting under a light microscope.
Neutrophil in vitro migration assay
Neutrophils were obtained from the bone marrow of C57BL/6J mice by magnetic separation using a Neutrophil Isolation Kit (Miltenyi Biotec). Isolated neutrophils were preincubated in the RPMI medium-containing 0.5% BSA in the presence or absence of 3 µM Iristatin for 1 h at 37 °C and 5% CO2. Cells were than seeded into the upper inserts of 3 μm pore Corning® Transwell® chambers (24-well format; Sigma-Aldrich, St. Louis, MO, USA) and allowed to migrate towards 1 µM fMLP in RPMI with 0.5% BSA in the lower chamber. After incubation for 1 h at 37 °C and 5% CO2, cell migration was determined by counting cells in the lower chamber using a hemocytometer.
Statistical analysis
All experiments were performed in biological triplicates. Data are presented as mean ± standard error of mean (SEM) in all graphs. Student’s t test or one-way ANOVA was used to calculate statistical differences between two or more groups, respectively. Statistically significant results were marked: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
Results
Expression and functional and structural analyses of Iristatin, a novel tick cystatin
Consistent with the other proteins that play important roles in the tick lifecycle [9], Iristatin mRNA expression increased significantly over time in the salivary glands of I. ricinus ticks fed on rabbits: 15–20-fold over the first 1–4 days; 50-fold between days 4 and 6, and 80-fold by day 7, when compared with unfed ticks (Supplementary Figure S1). Iristatin (GenBank accession number KY348759) BLAST search revealed three genomic contigs (Genbank accession numbers JXMZ02144755.1; JXMZ02161024.1, and JXMZ02194599.1) [17] with 92.6%, 90.3%, and 96.4% nucleotide identity, respectively, each representing a unique exon of a single gene. Furthermore, the search in available I. ricinus transcriptomes revealed 98.8% identity of Iristatin nucleotide sequence with its best match (Genbank accession number GFVZ01039973.1). Sequence analysis showed that Iristatin belongs to the cystatin superfamily [7–9, 13], specifically to a clade-containing only tick cystatins from the genus Ixodes (Fig. 1a). For structural and functional analyses, Iristatin was overexpressed in a prokaryotic system [7] to produce > 95% pure recombinant protein with 119 amino acids, molecular weight 13.8 kDa, and a pI of 7.67, as predicted (Supplementary Figure S2 and S3).
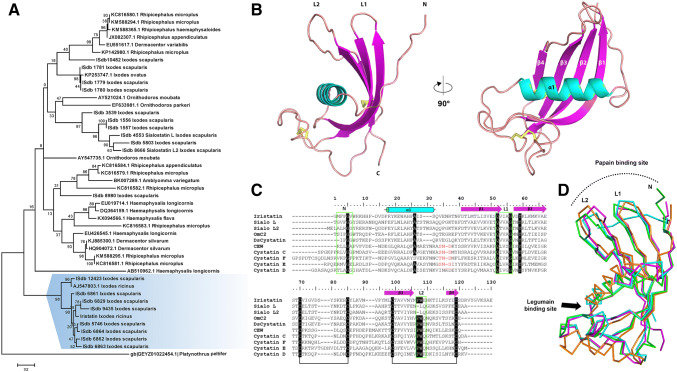
Fig. 1.
Crystal structure of Iristatin and its comparison with the other family 2 cystatins. a Molecular phylogenetic analysis (maximum-likelihood model) of secreted tick cystatins. Iristatin clusters with the other cystatins in the genus Ixodes (highlighted in blue). Platynothrus cystatin was used as an outgroup. The tree with the highest log likelihood (− 4870, 9711) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. b The three-dimensional structure of Iristatin is shown as a cartoon representation colored by secondary structure elements (α1, cyan; β1-4, magenta). The N- and C-termini and two disulfide bridges, Cys64–Cys76 and Cys87–Cys107 (yellow sticks), are indicated. The hairpin loops L1 and L2 and the N-terminus of cystatins are involved in the binding of papain-type peptidases. c Structure-based sequence alignment of Iristatin (Iris) with OmC2 (from the soft tick O. moubata), sialostatins L and L2 (from the hard tick I. scapularis), DsCystatin (from the hard tick D. silvarum), chicken egg-white cystatin (CEW), and representative human members of family 2 cystatins (cystatins D, C, E/M, and F). Residues identical to those of Iristatin are shaded black. The secondary structure elements of Iristatin are depicted as in b (magenta for strands, cyan for helices). The conserved disulfide bridges are indicated by the connecting black lines. Three regions involved in the interaction of cystatins with papain-type peptidases are boxed in green and labeled; the region size was selected based on predominant binding residues in the available complex structures. The putative legumain-binding site in four cystatins is highlighted in red. Mature sequences (i.e., without signal peptide) were used in the alignment; residue numbering is according to Iristatin. d A superposition of Cα traces of Iristatin with three other cystatin structures. The tick salivary cystatins Iristatin (PDB code 5O46), OmC2 (3L0R), and sialostatin L2 (3LH4) are colored magenta, green, and cyan, respectively. Chicken egg-white cystatin (1CEW) is shown in orange. Positions of the binding sites for papain-type peptidases and legumains are indicated
The crystal structure of Iristatin was determined by molecular replacement using the soft tick Ornithodoros moubata cystatin OmC2 structure as a search model and refined using data to 1.76 Å resolution (Supplementary Table S2). The orthorhombic crystal form contained two molecules in the asymmetric unit with a solvent content of 50.8%. All protein residues could be modeled into a well-defined electron density map with the exception of the first two N-terminal residues (Gly1 and Met2) and the last four C-terminal residues (Lys116 to Glu119). The final model consisted of two Iristatin molecules, each containing 114 amino acid residues. The root-mean-square deviation (RMSD) for superposition of the Cα atoms of the two molecules was 0.52 Å, a value within the range observed for different crystal structures of identical proteins. Minor structural changes were localized to loop regions exposed to solvent and/or involved in crystal contacts (residues 1–2, 47–48, 77–80, 95–96, and 111–114).
Figure 1b shows the overall structure of Iristatin. The molecule adopts a typical cystatin fold similar to that of the other homologs, characterized by a twisted antiparallel β-sheet wrapped around an α-helix. However, the Iristatin β-sheet is four-stranded instead of five-stranded, lacking the N-terminal β-strand. Iristatin contains two conserved disulfide bridges connecting Cys64 with Cys76 and Cys87 with Cys107. The structure-based sequence alignment demonstrated that Iristatin displays all the characteristics of family 2 cystatins, including disulfide pattern, the Gln-Xaa-Xaa-Xaa-Gly motif, and a secretion signal removed from the mature protein (Fig. 1c) [18].
The closest structural homologs of Iristatin were salivary cystatins OmC2 from the soft tick O. moubata and sialostatin L2 from the hard tick I. scapularis; the RMSDs for Cα were 1.10 Å and 1.18 Å (without flexible N-termini) and sequence identity was 42% and 36%, respectively (Fig. 1d). Lower structural similarity was found with vertebrate family 2 cystatins, namely human cystatins C, D, F and E/M and chicken egg-white cystatin (RMSDs from 1.53 to 2.21 Å); their sequence identity with Iristatin was between 16 and 24%.
Interaction of family 2 cystatins with papain-type peptidases is mediated by three regions, the N-terminal segment and two hairpin loops L1 and L2, which form a tripartite wedge-shaped edge that binds to the enzyme active site cleft (Fig. 1d). In Iristatin, the first part of the binding site is formed by the N-terminal segment around a conserved Gly5 residue, the orientation suggesting conformational flexibility, as with the other cystatins: Gly5 can function as a hinge that allows the flexible N-terminal segment to adopt an optimal conformation for target enzyme binding. The L1 loop of Iristatin (between β1 and β2) is similar in conformation to the other cystatins, only with an isoleucine instead of valine in the conserved sequence motif Gln–Xaa–Val–Xaa–Gly (Fig. 1c). The L2 loop (between β3 and β4) is characterized in Iristatin and other cystatins, except sialostatins, by the presence of conservative Pro-Trp residues.
We next analyzed which relevant representative proteases recombinant Iristatin inhibited. As predicted by the crystal structure and the absence of the legumain-binding site localized at a critical Asn residue in Iristatin (Fig. 1c, d), there was no activity against legumain. Among papain-type peptidases, cathepsins B and S were not significantly inhibited under given experimental conditions (Supplementary Table S3). Iristatin was active only against two tested enzymes, cathepsins C and L, displaying similar sub-micromolar affinity as the I. scapularis cystatins sialostatins L and L2 to cathepsin C but much lower micromolar affinity to cathepsin L (Supplementary Figure S4). We did not observe any effect of Iristatin against two representative serine peptidases—cathepsin G and factor Xa (Supplementary Table S3).
Iristatin affects cytokine production by T cells and mast cells
Tick salivary cystatins are known to inhibit T-cell cytokine production [3]. To elucidate Iristatin’s influence on host immunity and inflammation, we activated different immune cell populations and measured the effect of Iristatin on the production of characteristic cytokines for a given subpopulation. As predicted, Iristatin was a potent inhibitor of T-cell-derived cytokines (Fig. 2). In cell cultures, recombinant Iristatin inhibited the production of pro-inflammatory cytokines IFN-γ and IL-2 by polyclonally stimulated [CD3/CD28 monoclonal antibody (mAB)] Th1 cells after 48 h of incubation (Fig. 2a, b). Iristatin also suppressed the production of the anti-inflammatory cytokine IL-4 by Th2 cells (Fig. 2c) and IL-2 and IL-9 by Th9 cells (Fig. 2d, e). The inhibition of IL-9 may be an indirect effect, because IL-9 production is known to be IL-2 dependent [19]. Iristatin had no effect on IL-17 production by Th17 cells (Fig. 2f).
Fig. 2.
The effect of Iristatin on T-cell and mast cell cytokine production. a–f Different subpopulations of T-helper cells were preincubated with 6 μM Iristatin and polyclonally stimulated with a combination of CD3 and CD28 mAbs. Cytokine levels were determined 48 h after stimulation. Th cells activated in the absence of Iristatin were used as controls and set as 100% in all experiments; all other values are expressed as percentages of these controls. Iristatin inhibited IFN-γ (a) and IL-2 (b) production by Th1 cells. c Iristatin strongly reduced IL-4 production by Th2 cells. Iristatin inhibited IL-2 (d) and IL-9 (e) production by Th9 cells. f The inhibition of IL-17 production by Th-17 cells was not significant. g–i Mast cells were pretreated with Iristatin and stimulated with ionomycin. Cytokine levels were measured 48 h after stimulation. g IL-4 production was not affected by Iristatin treatment. h 6 μM Iristatin inhibited IL-6 production by mast cells. i Iristatin strongly inhibited IL-9 production by mast cells. The mean of three independent experiments (± SEM) is shown in all graphs. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; n.s. not significant
Mast cell numbers are positively correlated with resistance to tick feeding [2]. The only tick cystatin reported to have a direct effect on mast cells is I. scapularis sialostatin L, which indirectly reduced IL-9 expression [20]. We stimulated Iristatin pretreated mast cells with ionomycin in cell cultures and measured their cytokine production. While IL-4 production by mast cells was not affected (Fig. 2g), IL-6 levels decreased significantly (Fig. 2h) and IL-9 production was almost blocked by preincubation with 6 µM Iristatin (Fig. 2i).
Iristatin inhibits antigen-specific CD4+ T-cell proliferation and impairs leukocyte recruitment in vitro and in vivo
Saliva, salivary gland extract, or even individual salivary molecules from many tick species inhibit CD4+ T-cell proliferation [1]. Cystatins from I. scapularis [7] and O. moubata [21] are known inhibitors of T lymphocyte proliferation. Accordingly, we investigated whether Iristatin has similar properties in the OVA antigen-specific CD4+ T-cell proliferation model using splenocytes isolated from OT-II mice. Iristatin significantly decreased dendritic cell-dependent CD4+ T-cell proliferation upon OVA treatment from 88% in PBS-treated controls to 72% in the Iristatin-treated group (Fig. 3a; p ≤ 0.05) without affecting cell viability (Fig. 3b).
Fig. 3.
Iristatin inhibits CD4+ lymphocyte proliferation. a Splenocytes from OT-II mice were preincubated with Iristatin and subsequently stimulated with OVA peptide. The percentage of proliferating cells was evaluated after 72 h. Incubation with 3 μM Iristatin decreased the percentage of proliferating cells from 88% in the control group to 72% in the experimental group. The mean of three independent experiments (± SEM) is shown. *P ≤ 0.05 (two-tailed, unpaired t test). b Iristatin had no effect on cell viability
We next investigated whether Iristatin alters inflammatory responses in vivo in a mouse model of thioglycollate-induced peritonitis. Iristatin significantly impaired recruitment of total immune cells to the peritoneum (Fig. 4a) without affecting the proportion of living cells (Fig. 4b), excluding the possibility of Iristatin cytotoxicity. When individual cell populations were examined, Iristatin significantly inhibited the migration of myeloid cells and neutrophils, and showed a trend to decreasing monocyte and eosinophil migration. Consistent with these in vivo findings, the migration of neutrophils pretreated with Iristatin was significantly less than untreated controls towards an fMLP gradient (8.4% vs. 12.1%, p ≤ 0.05; Fig. 4g).
Fig. 4.
Iristatin’s effect on leukocyte recruitment and migration and macrophage production of NO. a–f Mice were injected with 3% TGM with saline or Iristatin. After 4 h, peritoneal lavage was performed and infiltrating cells analyzed by flow cytometry. In all figures, mice injected with TGM and saline are marked as Untreated, while all mice injected with TGM and Iristatin (2 mg/kg of mouse weight) are labeled as Iristatin. a Total number of cells in the peritoneum in tested mice. b Percentage of all living cells in mouse peritoneum. c–f Total number of all living CD11b+ cells, neutrophils, monocytes, or eosinophils, respectively. g Mouse bone marrow neutrophils were preincubated with 3 μM Iristatin and subjected to migration towards fMLP in a Boyden chamber. h Iristatin inhibited NO production by PMJ2-R macrophages in a dose-dependent manner. Macrophages were preincubated with 3 and 6 μM Iristatin, stimulated with LPS and IFN-γ, and NO concentration was assessed after 24 h. The mean of three independent experiments (± SEM) is shown. *P ≤ 0.05; **P ≤ 0.01; n.s. not significant
Finally, macrophages play an important role in the interaction between the host immune system, ticks, and transmitted pathogens. Activated macrophages are crucially involved in immune cell recruitment to sites of inflammation or towards pathogens by secreting signaling molecules such as chemokines or nitric oxide (NO) [22]. Saliva (or salivary gland extracts) from different tick species has been shown to reduce NO production by macrophages [23]. Accordingly, the incubation of monocyte/macrophage PMJ2-R cells with Iristatin reduced in vitro production of NO in a dose-dependent manner to nearly 40% of controls in the presence of 6 µM Iristatin (Fig. 4h), suggesting a considerable suppression of macrophage activation, perhaps, explaining the reduced recruitment of other immune cell types.
Discussion
Hard ticks feed for several days on their vertebrate host. To feed successfully, ticks control and evade the host immune response and maintain blood flow by secreting saliva into the feeding cavity. As I. scapularis cystatins are known to be strong immunomodulators [2], we focused on cloning a cystatin from the closely related tick I. ricinus, which we named Iristatin. At the time of this project initiation, I. ricinus genome has not yet been sequenced, so the traditional cloning procedures were needed to reveal this first immunomodulatory cystatin from this important disease vector.
The three-dimensional structural analysis of Iristatin provided useful insights into its biochemistry and function. Three conserved cystatin domains mediate their specificity to papain-like proteases [4, 24], including the N-terminal domain and two hairpin loops L1 and L2. Iristatin differed by over 50% in the N-terminal domain sequence but by only one amino acid in hairpin L1 (Ile50 instead of Val) or L2 (Glu99 instead of Gln) compared to the most structurally similar tick cystatin OmC2. This hairpin loop similarity, perhaps, explains why both cystatins inhibit cathepsins C and L. Iristatin showed a major difference in affinity to cathepsin L compared to sialostatins L and L2, in which target specificity is attributed to the lack of a conserved Pro-Trp motif in hairpin L2, at least with regard to the lower affinity to cathepsin B and no increased inhibition of cathepsin L [7, 9, 25]. While the only difference between Iristatin and the sialostatins in hairpin L1 is a Val/Ile substitution, we speculate that the significant difference in cathepsin L affinity can be explained by the different N-termini of these cystatins or in structures outside the conserved domains. Similarly, both Iristatin and DsCystatin [26] inhibited cathepsin L, but differed in their affinity to cathepsins B and C. While both of these cystatins are almost identical in their L1 and L2 hairpin sequences, the difference in their inhibitory specificity probably originates in the N-terminal region or outside the conserved domains. Similar to sialostatin L, we speculate that inhibition of cathepsins C and L by Iristatin impairs the maturation of other proteases from their proenzymes by blocking the cleavage of their N-terminal propeptides by cathepsins C and L [7]. This effect could reduce granzyme activity in cytotoxic T lymphocytes and natural killer cells, tryptase and chymase in mast cells, cathepsin G, proteinase 3, and elastase in neutrophils, or impair the maturation of cathepsins D and B in various cell types [2].
Iristatin suppressed immune responses both in vitro and in vivo. I. ricinus saliva which has previously been shown to polarize immune responses towards the Th2 pathway [27], although saliva-driven inhibition of both Th1 and Th2 pathways has been observed in dendritic cells [28]. However, Iristatin appears to have a more general effect on vertebrate immunity. Inhibition of the production of pro-inflammatory cytokines TNF and IL-12 by the tick cystatin OmC2 or IL-1β, IFN-γ, TNF, and IL6 by DsCystatin has been described in dendritic cells [21] and macrophages [26]. Moreover, impaired T-cell production of IFN-γ by sialostatin L has also been reported [8] together with a decrease in IL-17 production, which was not observed with Iristatin. Similar to sialostatin L, Iristatin suppressed IL-2 and IL-9 production by Th9 and mast cells, respectively, suggesting that Iristatin could also have a similar inhibitory effect on experimental asthma [29]. To our knowledge, no secreted tick cystatin has been reported to inhibit both Th1 and Th2 cytokines as much as Iristatin. It has been observed in ticks and other blood-feeding parasites that whole saliva can have general immunosuppressive effects, with individual proteins inhibiting specific elements. Saliva from the Aedes aegypti mosquito has been reported to suppress both Th1 and Th2 cytokines [30]. In contrast, a single A. aegypti salivary protein, SAAG-4, potently polarized a Th2 immune response by reducing expression of the Th1 cytokine IFN-γ and upregulating the Th2 cytokine IL-4 [31]. Such immunomodulation is not limited to arthropods, as the parasitic trematode Schistosoma japonicum also polarized the vertebrate immune response towards Th2 upon infection, with rSjCystatin increasing IL-4 production by splenocytes [32].
Macrophages play a crucial role in inflammatory responses through high cytokine and NO production and represent known cystatin targets [26]. Most cystatins tend to increase macrophage NO production [33]. Conversely, rSjCystatin from S. japonicum [34] and Iristatin decreased macrophage NO levels. Therefore, Iristatin has a rather unique effect on NO production, which is consistent with the previous studies, showing that I. ricinus saliva inhibits NO production [23]. Since NO is an important regulator of many processes in various immune and inflammatory cell types, we propose that its reduction by Iristatin leads to further immunosuppression. NO can be associated with decreased T-cell proliferation [35]. Moreover, the dose-dependent differences in NO-mediated polarization of immune response have been described. Low NO levels selectively enhanced Th1 polarization, while Th1 differentiation was suppressed at higher NO levels [36]. In contrast to these studies, we observed that Iristatin decreased NO production by macrophages and also reduced T-cell proliferation; however, since both experiments were performed separately in vitro, the in vivo milieu may be different. Furthermore, we used whole splenocytes in the proliferation assays, so it is unclear whether the observed lower CD4+ cell proliferation was a direct or an indirect effect via APCs.
Finally, Iristatin suppressed immune cell recruitment to the site of inflammation. Several parasitic nematode cystatins have been reported to affect inflammatory cell migration while polarizing towards Th2 immune responses and recruiting IL-10-producing macrophages or T cells [37–39]. Conversely, cystatin C inhibited T-cell and monocyte transmigration [40]. Until now, only one tick cystatin, DsCystatin, has been reported to have an impact on immune cell recruitment in a mouse arthritis model [26]. Another tick cystatin, RHcyst-1 from Rhipicephalus haemaphysaloides, suppressed tumor cell migration and invasion [41]. We speculate that the Iristatin effect on cell recruitment can partially be mediated by the suppression of macrophage activation [22] as shown by NO inhibition.
In conclusion, Iristatin is a potent immunomodulator of the host immune system. Iristatin is a specific cathepsin C and L inhibitor, as evidenced by both structure and function. Iristatin attenuated both Th1 and Th2 vertebrate host immune responses and inhibited T-cell proliferation and leukocyte recruitment. Our data clearly demonstrate that individual molecules contribute differentially to the overall effect of arthropod saliva on blood feeding. Our model for the action of tick salivary immunomodulators [42] stresses the importance of pluripotency and redundancy in their action; specifically, the function of each tick salivary immunomodulator may overlap with that of another one and the same tick salivary immunomodulator may simultaneously affect different vertebrate immune system functions [42]. We speculate that synergy exists between individual salivary proteins that might increase the activity of tick saliva; however, there is currently no direct evidence for such an effect. Pluripotency and redundancy seem to be essential for potent immunomodulation by tick saliva, and Iristatin displays both of these features. These structural and functional data further increase our understanding of vertebrate host immunomodulation by tick saliva. Furthermore, these properties could potentially be exploited for the development of novel immune-related disease drugs or vaccines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Professor Jose MC Ribeiro and Drs. Petr Kopáček and Daniel Sojka for constructive discussions, Dr. Daniel Sojka for kindly providing published reagent, ARVYS Proteins for service provision, Dr. Andrezza Campos-Chagas and Mr. Jan Erhart for technical assistance and Nextgenediting for editorial assistance. We also thank the anonymous reviewers for constructive comments about the draft.
Author contributions
JKot designed and performed experiments, performed the analyses, and wrote the manuscript; NS, MB, AC, ZB, PŘ, HL, and AS designed and performed experiments; JC and MK designed experiments, performed analyses, and edited the manuscript; MM and ES designed experiments and revised the manuscript; EC and JKop revised the manuscript.
Funding
This work was supported by the Grant Agency of the Czech Republic (Grant 19-07247S to MK and Grant 16-07117Y to JC), by the Grant Agency of the University of South Bohemia (Grant 038/2016/P to JKot), and by ERD Funds, project CePaViP OPVVV (No. CZ.02.1.01/0.0/0.0/16_019/0000759 to MK) and institutional project RVO 60077344 to MK. MB, MM, and PŘ were supported by project ChemBioDrug (No. CZ.02.1.01/0.0/0.0/16_019/0000729) from ERD Funds and institutional project RVO 61388963.
Data availability
The data set supporting the conclusions of this article is available in GenBank, accession number KY348759; PDB code 5O46.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci (Landmark Ed) 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotal J, Langhansova H, Lieskovska J, Andersen JF, Francischetti IM, Chavakis T, Kopecky J, Pedra JH, Kotsyfakis M, Chmelar J. Modulation of host immunity by tick saliva. J Proteomics. 2015;128:58–68. doi: 10.1016/j.jprot.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chmelar J, Kotal J, Langhansova H, Kotsyfakis M. Protease inhibitors in tick saliva: the role of serpins and cystatins in tick–host–pathogen interaction. Front Cell Infect Microbiol. 2017;7:216. doi: 10.3389/fcimb.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turk V, Bode W. The cystatins: protein inhibitors of cysteine proteinases. FEBS Lett. 1991;285(2):213–219. doi: 10.1016/0014-5793(91)80804-C. [DOI] [PubMed] [Google Scholar]
- 5.Rawlings ND, Waller M, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014;42(Database issue):D503–D509. doi: 10.1093/nar/gkt953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz A, Valdes JJ, Kotsyfakis M. The role of cystatins in tick physiology and blood feeding. Ticks Tick Borne Dis. 2012;3(3):117–127. doi: 10.1016/j.ttbdis.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotsyfakis M, Sa-Nunes A, Francischetti IM, Mather TN, Andersen JF, Ribeiro JM. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J Biol Chem. 2006;281(36):26298–26307. doi: 10.1074/jbc.M513010200. [DOI] [PubMed] [Google Scholar]
- 8.Sa-Nunes A, Bafica A, Antonelli LR, Choi EY, Francischetti IM, Andersen JF, Shi GP, Chavakis T, Ribeiro JM, Kotsyfakis M. The immunomodulatory action of sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity. J Immunol. 2009;182(12):7422–7429. doi: 10.4049/jimmunol.0900075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotsyfakis M, Karim S, Andersen JF, Mather TN, Ribeiro JM. Selective cysteine protease inhibition contributes to blood-feeding success of the tick Ixodes scapularis. J Biol Chem. 2007;282(40):29256–29263. doi: 10.1074/jbc.M703143200. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Wang X, Severo MS, Sakhon OS, Sohail M, Brown LJ, Sircar M, Snyder GA, Sundberg EJ, Ulland TK, Olivier AK, Andersen JF, Zhou Y, Shi GP, Sutterwala FS, Kotsyfakis M, Pedra JH. The tick salivary protein sialostatin L2 inhibits caspase-1-mediated inflammation during Anaplasma phagocytophilum infection. Infect Immun. 2014;82(6):2553–2564. doi: 10.1128/IAI.01679-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieskovska J, Palenikova J, Langhansova H, Campos Chagas A, Calvo E, Kotsyfakis M, Kopecky J. Tick sialostatins L and L2 differentially influence dendritic cell responses to Borrelia spirochetes. Parasit Vectors. 2015;8:275. doi: 10.1186/s13071-015-0887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotsyfakis M, Horka H, Salat J, Andersen JF. The crystal structures of two salivary cystatins from the tick Ixodes scapularis and the effect of these inhibitors on the establishment of Borrelia burgdorferi infection in a murine model. Mol Microbiol. 2010;77(2):456–470. doi: 10.1111/j.1365-2958.2010.07220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotsyfakis M, Anderson JM, Andersen JF, Calvo E, Francischetti IM, Mather TN, Valenzuela JG, Ribeiro JM. Cutting edge: immunity against a “silent” salivary antigen of the Lyme vector Ixodes scapularis impairs its ability to feed. J Immunol. 2008;181(8):5209–5212. doi: 10.4049/jimmunol.181.8.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramaro WJ, Revets D, Hunewald OE, Sinner R, Reye AL, Muller CP. Integration of Ixodes ricinus genome sequencing with transcriptome and proteome annotation of the naive midgut. BMC Genomics. 2015;16:871. doi: 10.1186/s12864-015-1981-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopacek P, Zdychova J, Yoshiga T, Weise C, Rudenko N, Law JH. Molecular cloning, expression and isolation of ferritins from two tick species—Ornithodoros moubata and Ixodes ricinus. Insect Biochem Mol Biol. 2003;33(1):103–113. doi: 10.1016/S0965-1748(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 16.Sojka D, Hajdusek O, Dvorak J, Sajid M, Franta Z, Schneider EL, Craik CS, Vancova M, Buresova V, Bogyo M, Sexton KB, McKerrow JH, Caffrey CR, Kopacek P. IrAE: an asparaginyl endopeptidase (legumain) in the gut of the hard tick Ixodes ricinus. Int J Parasitol. 2007;37(7):713–724. doi: 10.1016/j.ijpara.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cramaro WJ, Hunewald OE, Bell-Sakyi L, Muller CP. Genome scaffolding and annotation for the pathogen vector Ixodes ricinus by ultra-long single molecule sequencing. Parasites Vectors. 2017;10(1):71. doi: 10.1186/s13071-017-2008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vray B, Hartmann S, Hoebeke J. Immunomodulatory properties of cystatins. Cell Mol Life Sci. 2002;59(9):1503–1512. doi: 10.1007/s00018-002-8525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kuhn R, Muller W, Palm N, Rude E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153(9):3989–3996. [PubMed] [Google Scholar]
- 20.Klein M, Bruhl TJ, Staudt V, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Bohn T, Ulges A, Stergiou N, de Graaf J, Lower M, Taube C, Becker M, Hain T, Dietzen S, Stassen M, Huber M, Lohoff M, Campos Chagas A, Andersen J, Kotal J, Langhansova H, Kopecky J, Schild H, Kotsyfakis M, Schmitt E, Bopp T. Tick salivary sialostatin L represses the initiation of immune responses by targeting IRF4-dependent transcription in murine mast cells. J Immunol. 2015;195(2):621–631. doi: 10.4049/jimmunol.1401823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salat J, Paesen GC, Rezacova P, Kotsyfakis M, Kovarova Z, Sanda M, Majtan J, Grunclova L, Horka H, Andersen JF, Brynda J, Horn M, Nunn MA, Kopacek P, Kopecky J, Mares M. Crystal structure and functional characterization of an immunomodulatory salivary cystatin from the soft tick Ornithodoros moubata. Biochem J. 2010;429(1):103–112. doi: 10.1042/BJ20100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laroux FS, Pavlick KP, Hines IN, Kawachi S, Harada H, Bharwani S, Hoffman JM, Grisham MB. Role of nitric oxide in inflammation. Acta Physiol Scand. 2001;173(1):113–118. doi: 10.1046/j.1365-201X.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 23.Kyckova K, Kopecky J. Effect of tick saliva on mechanisms of innate immune response against Borrelia afzelii. J Med Entomol. 2006;43(6):1208–1214. doi: 10.1093/jmedent/43.6.1208. [DOI] [PubMed] [Google Scholar]
- 24.Bode W, Huber R. Structural basis of the endoproteinase-protein inhibitor interaction. Biochim Biophys Acta. 2000;1477(1–2):241–252. doi: 10.1016/S0167-4838(99)00276-9. [DOI] [PubMed] [Google Scholar]
- 25.Bjork I, Brieditis I, Raub-Segall E, Pol E, Hakansson K, Abrahamson M. The importance of the second hairpin loop of cystatin C for proteinase binding. Characterization of the interaction of Trp-106 variants of the inhibitor with cysteine proteinases. Biochemistry. 1996;35(33):10720–10726. doi: 10.1021/bi960420u. [DOI] [PubMed] [Google Scholar]
- 26.Sun T, Wang F, Pan W, Wu Q, Wang J, Dai J. An immunosuppressive tick salivary gland protein DsCystatin interferes with toll-like receptor signaling by downregulating TRAF6. Front Immunol. 2018;9:1245. doi: 10.3389/fimmu.2018.01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skallova A, Iezzi G, Ampenberger F, Kopf M, Kopecky J. Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J Immunol. 2008;180(9):6186–6192. doi: 10.4049/jimmunol.180.9.6186. [DOI] [PubMed] [Google Scholar]
- 28.Slamova M, Skallova A, Palenikova J, Kopecky J. Effect of tick saliva on immune interactions between Borrelia afzelii and murine dendritic cells. Parasite Immunol. 2011;33(12):654–660. doi: 10.1111/j.1365-3024.2011.01332.x. [DOI] [PubMed] [Google Scholar]
- 29.Horka H, Staudt V, Klein M, Taube C, Reuter S, Dehzad N, Andersen JF, Kopecky J, Schild H, Kotsyfakis M, Hoffmann M, Gerlitzki B, Stassen M, Bopp T, Schmitt E. The tick salivary protein sialostatin L inhibits the Th9-derived production of the asthma-promoting cytokine IL-9 and is effective in the prevention of experimental asthma. J Immunol. 2012;188(6):2669–2676. doi: 10.4049/jimmunol.1100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasserman HA, Singh S, Champagne DE. Saliva of the Yellow Fever mosquito, Aedes aegypti, modulates murine lymphocyte function. Parasite Immunol. 2004;26(6–7):295–306. doi: 10.1111/j.0141-9838.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 31.Boppana VD, Thangamani S, Adler AJ, Wikel SK. SAAG-4 is a novel mosquito salivary protein that programmes host CD4 T cells to express IL-4. Parasite Immunol. 2009;31(6):287–295. doi: 10.1111/j.1365-3024.2009.01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, He B, Hou W, He L. Cysteine protease inhibitor of Schistosoma japonicum—a parasite-derived negative immunoregulatory factor. Parasitol Res. 2017;116(3):901–908. doi: 10.1007/s00436-016-5363-0. [DOI] [PubMed] [Google Scholar]
- 33.Verdot L, Lalmanach G, Vercruysse V, Hartmann S, Lucius R, Hoebeke J, Gauthier F, Vray B. Cystatins up-regulate nitric oxide release from interferon-gamma-activated mouse peritoneal macrophages. J Biol Chem. 1996;271(45):28077–28081. doi: 10.1074/jbc.271.45.28077. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Liu J, Yue Y, Chen W, Song M, Zhan X, Wu Z. Cloning, expression and characterisation of a type II cystatin from Schistosoma japonicum, which could regulate macrophage activation. Parasitol Res. 2014;113(11):3985–3992. doi: 10.1007/s00436-014-4064-9. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 36.Niedbala W, Cai B, Liew FY. Role of nitric oxide in the regulation of T cell functions. Ann Rheum Dis. 2006;65(Suppl 3):iii37–iii40. doi: 10.1136/ard.2006.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuijs MJ, Hartmann S, Selkirk ME, Roberts LB, Openshaw PJ, Schnoeller C. The helminth-derived immunomodulator AvCystatin reduces virus enhanced inflammation by induction of regulatory IL-10+ T cells. PLoS One. 2016;11(8):e0161885. doi: 10.1371/journal.pone.0161885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danilowicz-Luebert E, Steinfelder S, Kuhl AA, Drozdenko G, Lucius R, Worm M, Hamelmann E, Hartmann S. A nematode immunomodulator suppresses grass pollen-specific allergic responses by controlling excessive Th2 inflammation. Int J Parasitol. 2013;43(3–4):201–210. doi: 10.1016/j.ijpara.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, Hamann A, Hamelmann E, Lucius R, Hartmann S. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol. 2008;180(6):4265–4272. doi: 10.4049/jimmunol.180.6.4265. [DOI] [PubMed] [Google Scholar]
- 40.Staun-Ram E, Miller A. Cathepsins (S and B) and their inhibitor Cystatin C in immune cells: modulation by interferon-beta and role played in cell migration. J Neuroimmunol. 2011;232(1–2):200–206. doi: 10.1016/j.jneuroim.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Wei N, Lin Z, Xu Z, Cao J, Zhou Y, Zhang H, Gong H, Zhou J, Li G. A tick cysteine protease inhibitor RHcyst-1 exhibits antitumor potential. Cell Physiol Biochem. 2018;46(6):2385–2400. doi: 10.1159/000489645. [DOI] [PubMed] [Google Scholar]
- 42.Chmelar J, Kotal J, Kopecky J, Pedra JHF, Kotsyfakis M. All for one and one for all on the tick-host battlefield. Trends Parasitol. 2016;32(5):368–377. doi: 10.1016/j.pt.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set supporting the conclusions of this article is available in GenBank, accession number KY348759; PDB code 5O46.