Abstract
Pancreatic ductal adenocarcinoma (PDAC) is among the most devastating human malignancies, with approximately 20–30% of PDAC patients receiving the surgical resection with curative intent. Although many studies have focused on finding ideal “drug chaperones” that facilitate and/or potentiate the effects of gemcitabine (GEM) in pancreatic cancer, a significant benefit in overall survival could not be demonstrated for any of these combination therapies in PDAC. Given that pancreatic cancer is characterized by desmoplasia and the dual biological roles of stroma in pancreatic cancer, we reassess the importance of stroma in GEM-based therapeutic approaches in light of current findings. This review is focused on understanding the role of stromal components in the extrinsic resistance to GEM and whether anti-stroma therapies have a positive effect on the GEM delivery. This work contributes to the development of novel and promising combination GEM-based regimens that have achieved significant survival benefits for the patients with pancreatic cancer.
Keywords: Pancreatic cancer, Gemcitabine, Stroma, Drug delivery, Hyaluronan, Nab-paclitaxel
Introduction
Pancreatic ductal adenocarcinoma (PDAC) represents the most common form of pancreatic cancer [1]. It is characterized by a high propensity for local invasion and distant metastasis, which is associated with an extremely poor prognosis [2]. Surgical resection or surgery in combination with adjuvant therapy is the only curative therapy that improves the overall survival (OS) of pancreatic cancer patients [3]. However, only approximately 20% of PDAC patients qualify for surgical resection with curative intent [2]. Thus, chemotherapy is a particularly important treatment option to extend patient survival or reduce symptoms.
Since 1997, the nucleoside analogue gemcitabine (GEM) has been successfully established as the standard of care in first-line palliative therapy for advanced PDAC. GEM improves quality of life, but only a small number of patients responds to GEM [4, 5]. Thus, most research has focused on finding ideal “drug chaperones” that facilitate and/or potentiate the effect of GEM. Over the past decade, numerous trials have been conducted to improve the outcome of patients with metastatic disease using combination therapies with GEM as the backbone. However, despite a modest improvement in progression-free survival (PFS) in some trials, a significant benefit in OS is demonstrated for few of these combination therapies [2, 6]. Identifying the presence of resistance mechanisms and other determinants for GEM sensitivity in order to classify tumors into response categories has been an ongoing research effort.
Developing therapies for advanced PDAC is much more complicated than targeting only the cancer cells. Several defining features of PDAC influence its aggressive biology and resistance to multiple therapeutic modalities [1, 7]. Most notably, PDAC stroma is characterized by the development of extensive fibrosis termed desmoplasia [8]. This review is focused on understanding that stroma are instrumental in mediating the extrinsic GEM-resistant property of PDAC and whether anti-stroma therapies have positive effects on the delivery of GEM. The complex roles of stroma in PDAC have forced us to reassess various therapeutic approaches in light of current findings, contributing to the development of new promising combination GEM-based regimens that have achieved a significant survival benefit for pancreatic cancer.
Pancreatic cancer stroma: friend or foe
Stroma and its regulators in PDAC
The stromal microenvironment is a complex structure composed of cellular components such as cancer-associated fibroblasts (CAFs), immune cells and endothelial cells, acellular components such as collagens and laminin, cytokines and growth factors stored in the extracellular matrix (ECM) [9, 10]. Tumor stroma is a complex entity and functions as a dynamic interface between the tumor and normal host epithelial tissue [8]. Like all stroma across different tumor types, the formation of desmoplastic stroma in PDAC likely depends on a combination of instructive signals from tumor cells as well as site-dependent differences in resident stromal cells [10].
In PDAC, the major components of the dense stroma are a complex population of CAFs. CAFs have two different functional stages that can be clearly defined as the quiescent state and the activated state. In quiescence, CAFs store vitamin A droplets and are characterized by the presence of desmin and glial fibrillar acidic protein. Notably, upon activation, CAFs transform into a myofibroblast-like phenotype, which has emerged as an important event that accounts for the desmoplastic stroma production in PDAC [11, 12]. Activated CAFs secrete excessive amounts of structural matrix components, including proteoglycans, collagens and fibronectin, and secrete matrix metalloproteinases (MMPs) and their inhibitors, which are involved in the degradation, dynamic remodeling and turnover of ECM proteins in PDAC [10, 13–15]. CAFs have been shown to respond to extrinsic signals via autocrine and paracrine mechanisms, including cancer cell-derived growth factors, inflammatory cytokines or oxidative stress [7, 8, 10, 16–18].
At the molecular level, activation of multiple signaling cascades in cancer cells also contributes to the formation of desmoplastic stroma, linking tumor genotype to the fibrotic phenotype, which could identify the PDACs that would benefit from stroma-targeting therapies. The transforming growth factor (TGF-β)/SMAD4 pathway has been reported to be associated with collagen thickness and epithelial tension [19]. Moreover, sonic hedgehog (SHH) is overexpressed in pancreatic cancer cells [20], and activation of its paracrine downstream signaling in stromal cells promotes stromal desmoplasia [21, 22].
The dual roles of stroma in PDAC progression
Many efforts have been undertaken to elucidate the complex tumor–stroma interactions, and the results of these efforts provide interesting and fascinating insight into the stromal biology of PDAC [23]. Nearly 50 years have passed since Stoker’s pioneering studies of tumor–stroma interactions demonstrated that normal fibroblasts restrain the growth of transformed baby hamster kidney cells [24]. This “neighbor suppression” effect may be part of an evolved microenvironment surveillance against the development of preneoplasia [25]. Thus, the desmoplastic reaction is thought to represent a host defense mechanism, similar to wound healing and tissue regeneration, to repair or hopefully impede the conversion of a neoplastic lesion into invasive carcinoma [26, 27]. Nevertheless, various stromal elements have been reported that contribute to immune suppression, further supporting tumor survival and growth. These observations have led to the prevailing paradigm that stroma may in fact act as a “partner in crime” with tumor cells, promoting tumor progression [11, 28–30]. This concept has been bolstered by work on PDAC, a cancer with a particularly dense stroma. Due to the abundance of ECM proteins and CAFs in desmoplastic stroma, the stroma functions as a physical barrier to the tumor. Notably, this barrier not only restrains tumor growth and metastasis, but also increases interstitial fluid pressure (IFP) and impairs tumor vasculature. The latter limits the effective delivery of anti-cancer agents to pancreatic cancer cells [9, 17, 23].
In addition, the mechanically poor perfusion creates a hypoxic and acidic microenvironment into PDAC [7]. This environmental stress leads to the activation of various genes by hypoxia-inducible factor 1α (HIF-1α) protein to promote cell survival, invasion, and metastasis of the epithelial cells [31]. These observations are in contrast to earlier views of the stroma functioning merely as a mechanical protective barrier for the benefit of the host [32]. Based on this paradigm, treating the primary tumor in pancreatic cancer is not enough, and the concept of anti-stromal therapies has emerged as a promising therapeutic approach [33, 34]. However, the majority of experimental and early clinical findings have failed when rigorous clinical phase II or III studies were conducted, and no approved anti-stromal therapy has actually entered the clinical routine [35, 36].
Recent experimental evidence has provided an explanation for the failure of some anti-stromal therapies in clinical trials. At least some stromal constituents can act to restrain, rather than promote, tumor progression. Stromal depletion approaches may favor tumor aggressiveness and spread [37] and thus have reignited the discussion of whether tumor stroma in PDAC is a ‘friend or foe’ [38]. The biophysical function of stroma, merely as a physical barrier, is neither favorable nor detrimental. This role is passively played, depending on the stroma–tumor balance and the microenvironment. Once the balance is broken, stroma assumes either a tumor-promoting role or a tumor-suppressive role. However, stroma can also actively play its biochemical roles through crosstalk between CAFs and cancer cells. Thus, the function of desmoplastic stroma is likely dynamic during cancer progression, and its heterogeneous cellular and acellular constituents change in relation to the evolving genetic landscape of cancer cells. The prognosis of patients with pancreatic cancer heavily depends on the stromal activity and ECM composition within the tumors. Remodeling the dense tumor stroma leads to both biophysical and biochemical modifications that may independently or collectively contribute to a favorable therapeutic response, and such information may guide the optimal translation of these preclinical findings to patients.
Stroma involved in chemoresistance and anti-stroma combination therapy
During the last few decades, the role of this stromal reaction has been largely neglected by the majority of research efforts, which have instead focused directly on tumor cells as the main determinant of drug resistance [32]. Stroma not only activates signaling pathways that limit the effect of chemotherapy [39, 40], but also limits the delivery of chemotherapy to cancer cells [33]. The role of the desmoplastic stroma as a ‘fortress’ fencing off tumor cells from drugs applied in the circulation has only very recently been recognized [17]. Improved knowledge of the genetic and molecular alterations not only occurring in tumor cells, but also in the surrounding stromal cells has recently resulted in the identification of stroma as an emerging attractive therapeutic target in PDAC. Various novel therapeutic approaches have been developed, specifically targeting profibrotic pathways, cytokines and growth factors involved in tumor desmoplasia and angiogenesis to control tumor growth and increase the cytotoxic effect of chemotherapeutics. Currently, several clinical studies using stroma-targeting agents combined with conventional chemotherapy have also been initiated and are entering clinical evaluation. In fact, the mechanisms and functional consequences of tumor–stroma crosstalk may be by far more complex than previously anticipated and should therefore be reassessed in an unbiased manner. Targeting tumor–stroma crosstalk may be an effective approach for improving chemotherapy efficacy—the seed and soil hypothesis proposes that tumor cells have the propensity to develop in specific microenvironment niches that preferentially support their growth. This points to the possibility of a novel type of therapeutic intervention [41].
Stroma confers extrinsic resistance to GEM in PDAC
Stroma impairs the delivery of GEM
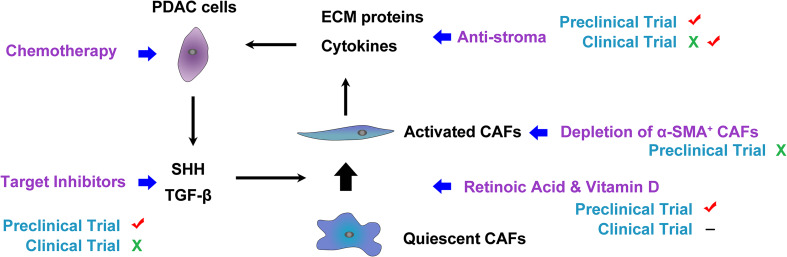
The determinants of extrinsic chemoresistance are theoretically more appealing to tamper with, especially in PDAC [42]. There is an increasing interest in targeting different components of PDAC stroma, and several preclinical studies using genetically engineered mouse models (GEMMs) of PDAC show promising results (Fig. 1) [33, 43, 44]. The most common explanation for the beneficial therapeutic effects of stromal elimination is that modulating vessel patency and density increased drug delivery in PDAC [33, 43, 44].
Fig. 1.
The SHH pathway involved in tumor–stroma crosstalk. SHH signaling may mediate the balance between the stroma (CAFs and type I collagen) and PDAC cells to form a steady state. Once the homeostasis is disrupted by targeting any constituent of this balance, unfavorable effects occur. This heterogeneous factor is one explanation for conflict results from clinical trials and preclinical trials, suggesting a need for remodeling the stroma, not depleting of stroma
GEM, similar to other drugs with intracellular targets, must traverse the vascular, extracellular, and cellular compartments of PDAC to ultimately have an effect. These different physical barriers span distances that vary by orders of magnitude (meters to angstroms), highlighting the significant challenge of drug delivery [45]. Thus, apart from complex biochemical cancer–stroma crosstalk within the tumor microenvironment, inefficient drug delivery through tumor tissue has been debated as one of the primary reasons for GEM resistance in PDAC [46]. PDAC exhibits distinct stromal architecture that can be considered as a ‘fortress-like’ barrier for effective drug delivery to the tumor bed. The architecture includes disorganized, leaky, and nonfunctional vasculature [44, 47]; characteristically dense stroma [17]; and deregulated cellular transport proteins [48]. Dense stroma leads to compression of existing capillaries and restricts the formation of new tumor vasculature to create high interstitial fluid pressure. The increased interstitial pressure and the impaired tumor vasculature prevent the movement of GEM from the vasculature to the extracellular compartment [33, 45]. Thus, this challenge sparked the quest for new therapeutic avenues capable of breaching this “stroma fortress” and easing chemotherapy access inside fibrotic pancreatic tumors [17].
CAFs affect the regional concentration of GEM
A recent study has reported that there is an ECM protein-independent mechanism for the extrinsic resistance to GEM in PDAC [49], an alternative explanation that challenges the paradigm of a biophysical stroma barrier for drug delivery. The extrinsic factor is fibroblast drug scavenging. The scavenging increases intratumoral GEM accumulation, entrapping active GEM within stromal cells and making it unavailable to tumor cells [50]. Therefore, metabolic targeting of CAFs may be a promising strategy to enhance the efficiency of GEM in PDAC [50]. However, CAFs accumulated GEM intracellularly and then were able to release it into the extracellular environment, becoming GEM-releasing-CAFs that inhibited the in vitro growth of PDAC [51]. This prompted the development of new therapeutic approaches that are based on the cell-based delivery of anti-cancer agents by CAFs. These also support our above corollary statement that CAFs are heterogeneous, exerting different effects on GEM efficacy.
The dilemma of anti-stromal therapy in PDAC
The confusing results of targeting the SHH pathway
Sonic hedgehog, a secreted hedgehog ligand that is essential during embryonic pancreatic development, is normally absent in the adult pancreas. Reactivation of the developmental SHH pathway has been identified as one mediator that contributes to stromal desmoplasia during PDAC progression [22]. Canonical SHH signaling in PDAC is likely to occur in a paracrine fashion that exclusively acts on CAFs, whose activation in turn promotes the malignant behavior of pancreatic cancer cells [22, 52]. Numerous strategies have been developed to interrupt the SHH signaling pathway as a therapeutic means of ablating stroma.
In 2009, Olive et al. examined in a landmark study the impact of targeting SHH signaling as one major pathway known to stimulate stromal reaction [33]. In the GEMM of PDAC, inhibition of the SHH signaling pathway by IPI-926, an inhibitor of SHH pathway, resulted in a significant depletion of tumor-associated stroma paralleled by an increase in intratumoral vascular density. Although stroma depletion alone had no immediate antitumor effect in this experimental setting, systemic co-administration of GEM and IPI-926 resulted in markedly enhancing active intracellular metabolite of GEM, transiently stabilizing the disease and significantly prolonging the survival time. However, the pronounced stromal reaction ultimately returned, suggesting that tumors can adapt to chronic SHH inhibition in the GEMM [33]. Unfortunately, these results could not be replicated in clinical trials, despite the fact that the block of the SHH pathway in the preclinical study achieved great efficacy [53]. There are mechanisms independent of the SHH pathway that affect the efficacy of GEM [37, 54] (Fig. 1). Vismodegib, another hedgehog pathway inhibitor, showed no clinical benefit after adding to GEM in metastatic pancreatic cancer in a phase Ib/II study [55]. Recently, an interim analysis has been presented at ASCO 2014 of a phase II study which describes a median overall survival of 10 months by the addition of vismodegib to gemcitabine and nab-paclitaxel to patients with pancreatic cancer [56]. The final results are still awaited and will need to be further interpreted.
Type I collagen and CAFs are dispensable for GEM delivery
Type I collagen is one element of the physical barriers within the pancreatic tumor matrix that is regulated by SHH pathways. Collagen cross-linking is mediated by lysyl oxidase (LOX) family members, LOXL1 and LOXL2. In PDAC, increased fibrillar collagen along with increased LOX family activity decreased GEM intratumoral diffusion, conferring chemoresistance [57, 58]. CAFs contribute to the production of type I collagen. Targeting CAFs leads to extensive remodeling of the tumor ECM, with a significant decrease in tumor tissue stiffness and total collagen content, improving the therapeutic delivery in desmoplastic tumors [59]. However, the significant reduction in collagen content via CAF depletion did not alter vessel permeability and perfusion or improve the efficacy of GEM. Therefore, CAFs and type I collagen do not appear to serve as physical barriers to the exposure of cancer cells to GEM in PDAC [44, 60]. These failed anti-stromal approaches imply that a matrix molecule from a non-myofibroblast source might affect GEM efficacy (Fig. 1).
The promising anti-stromal therapies increase GEM efficacy
Hyaluronan is expected to be a target for improving GEM delivery
Hyaluronan (HA) is identified as another primary matrix determinant of the physical barriers within the pancreatic tumor and is derived from a non-myofibroblast source, as shown by the lack of change in HA content after CAF depletion [43, 44, 60]. Reportedly, HA contributes to a very high IFP, leading to vascular compression and hypoperfusion. Targeting HA also induced fenestrations and interendothelial junctional gaps in the endothelia of tumor vessels, which promoted a tumor-specific increase in macromolecular permeability [61]. This suggests that in addition to normalization of the IFP, the response to cytotoxic treatment was improved by modulating the tumor vessel permeability induced by ultrastructural changes in the endothelium [61]. Thus, the degradation of HA has been hypothesized to enhance drug delivery to tumors [44].
Indeed, one of the most exciting and anticipated stroma-directed approaches in PDAC has been the targeting of HA. Administration of PEGPH20, a pegylated hyaluronidase, was highly effective in ablating stromal HA in a spontaneous murine PDAC model and remodeling the tumor microenvironment [43, 44]. Moreover, administration of PEGPH20 before GEM led to increased intratumoral concentration of the active GEM metabolite in the tumor [61]. Following enhanced drug delivery, combination therapy consistently achieved objective tumor responses, resulting in significantly diminished tumor growth and prolonged survival of the mice [44, 61]. These promising results are particularly encouraging as enhancing intratumoral drug concentrations is a continual battle in the effort to maximize both the efficacy and pharmacokinetic properties of drugs. This effort represents an approach, independent of the properties of any particular drug, which may enhance overall efficacy in a variety of conditions in patients. In this regard, the potential role of HA, independent of type I collagen, in determining IFP in PDAC tissue needs further mechanistic unraveling, and clinical trials with PEGPH20 will offer more insights in the future [44].
The researchers also noted increased expression of HA in metastatic sites of disease, which suggests that the stroma has a critical role and that this therapeutic approach might also be of benefit in metastatic PDAC [44]. Similar results were observed in a phase Ib/II clinical trial (NCT01453153) when PEGPH20 was combined with GEM as a first-line treatment among patients with advanced pancreatic cancer. In an exploratory analysis, tumor biopsies were evaluated for pretreatment tissue HA levels. The median PFS and OS for patients with high intratumoral HA content (HA-high) were 7.2 and 13.0 months, respectively, but were 3.5 and 5.7 months for patients with low intratumoral HA content (HA-low). Thus, PEGPH20 in combination with GEM shows promising clinical activity in advanced pancreatic cancer, especially in HA-high tumors [62]. Subsequently, a randomized, phase II trial is evaluating PEGPH20 in combination with nanoparticle albumin-bound paclitaxel (nab-paclitaxel) and GEM in metastatic pancreatic cancer patients (NCT01839487). The interim results have shown a high response rate and PFS when PEGPH20 is added to nab-paclitaxel/GEM to treat advanced pancreatic cancer with HA-high tumors. The latest analysis has shown that across 279 patients in the study, the median PFS was 6.0 months in the PEGPH20 arm versus 5.3 months for nab-paclitaxel/GEM. In those with HA-high expression, the median PFS was 9.2 months with PEGPH20 compared with 5.2 months in the control arm. As the median PFS is a notable increase over the current standard of care, the clinically important progress in the treatment of PDAC confidently supports continued exploration in the current phase III study (NCT02715804).
Nab-paclitaxel synergistically improves GEM efficacy
In PDAC, nab-paclitaxel was the first successful drug hypothesized to target the stroma. Preclinical data indicated that co-administration of nab-paclitaxel plus GEM resulted in a higher tumor regression than either agent alone in patient-derived pancreatic cancer xenograft murine models receiving nab-paclitaxel plus GEM, nab-paclitaxel alone and GEM alone. Moreover, treatment with nab-paclitaxel alone was also more effective than GEM alone [6]. Phase I/II study (NCT02382263) data also suggest that a high level of antitumor activity can be achieved with this combination in pancreatic cancer [6]. These clinical findings were recently validated in a phase III MPACT clinical trial (NCT00844649), which showed that nab-paclitaxel plus GEM significantly improved OS and PFS versus GEM alone for first-line treatment of patients with metastatic pancreatic cancer [63]. Moreover, this regimen was also of benefit as neoadjuvant therapy and evaluated by several clinical trials [64].
The promising efficacy might be associated with stromal depletion induced by nab-paclitaxel because there is growing evidence from preclinical studies to show that nab-paclitaxel targets both the tumor and stroma in pancreatic cancer. Indeed, in GEM-resistant xenografts, a profuse desmoplastic stroma remained after treatment with vehicle or GEM alone, whereas the administration of nab-paclitaxel resulted in a significant reduction in stromal content. In contrast, nab-paclitaxel treatment specially collapsed the PDAC stroma accompanied by a marked distortion of the collagen and tumor vascularization, which were particularly prominent in the combination therapy cohort. These effects translated into diminished tumor stiffness as measured by endoscopic ultrasonography elastography [65]. The reduction in tumor stroma and the accompanying increase in vascularization facilitated the delivery of GEM to these tumors. Importantly, the intratumoral concentration of GEM was approximately 2.8 times higher than that observed with monotherapy [6].
In fact, similar results showing that co-treatment with nab-paclitaxel and GEM resulted in an increase in intratumoral GEM concentration have been obtained in subsequent GEMMs of PDAC and smaller patients studies using nab-paclitaxel [65, 66]. However, others have not observed stromal reduction by nab-paclitaxel [66–68], and the increased GEM concentrations were explained by inactivation of the cytidine deaminase in cancer cells through induction of reactive oxygen species-mediated degradation [66]. This suggests that the nab-paclitaxel-mediated increase in the efficacy of GEM may instead be due to enhancement of GEM stabilization [66]. This discrepancy in the data may be a result of differences in CAF sensitivity to nab-paclitaxel in each tumor model. If CAFs are effectively killed by nab-paclitaxel, tumor stroma density is decreased, and consequently the vascular stress of tumors is reduced. This leads to relief from the compression on tumor vessels, increased blood perfusion, and in turn improved drug delivery. However, a significant decrease in CAF content was observed in only 58% of pancreatic cancer patients after nab-paclitaxel plus GEM therapy [65]. If CAFs are resistant to nab-paclitaxel, the tumor stroma may be only slightly affected by nab-paclitaxel therapy, as would be the delivery of the drug to the tumor.
Cytoplasmic SPARC may be a predictive factor for efficacy of GEM
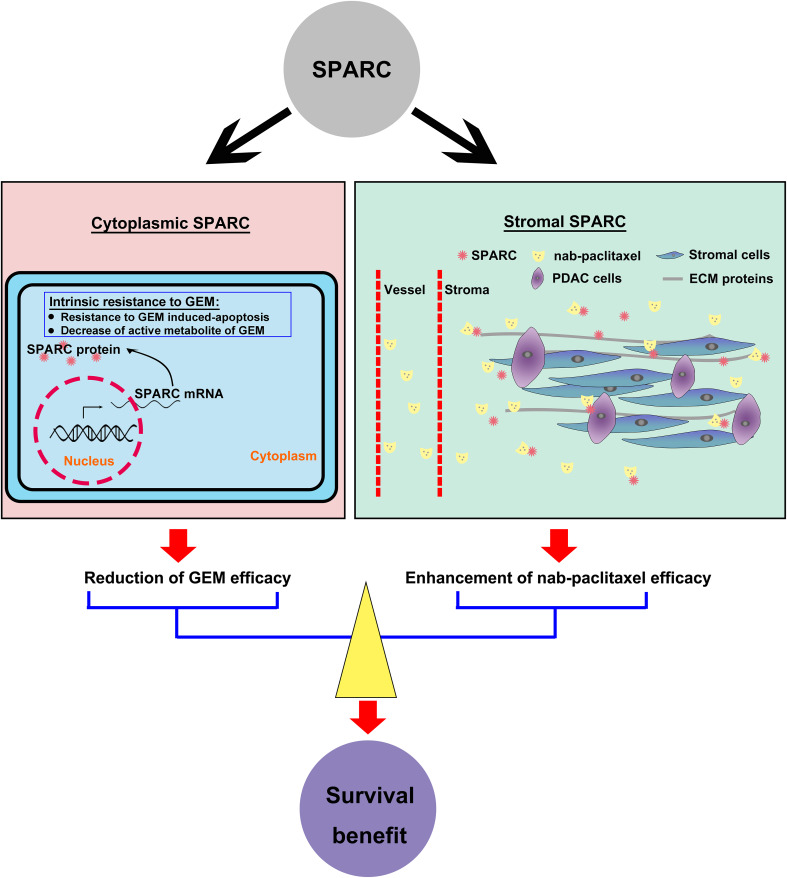
Secreted protein acidic and rich in cysteine (SPARC) was associated with the therapeutic mechanism of nab-paclitaxel. SPARC has recently gained increased attention as it interacts with and modulates the ECM and influences cell migration as well as angiogenesis and tissue remodeling [69]. In most PDACs, SPARC is an abundant extracellular protein expressed by CAF-derived stroma. Nab-paclitaxel is an albumin-based nanoparticle that does not require solvent for resuspension. SPARC has high binding affinity for albumin via the albumin receptor (gp60)–caveolin-1–caveolae pathway. Of interest, the distribution pattern of human serum albumin in tumor tissue was consistent with that of tumor stroma [70]. Thus, the promising antitumor activity of nab-paclitaxel may, in part, be explained by stromal SPARC-mediated enrichment of the concentration of nab-paclitaxel to boost its delivery [71–73] (Fig. 2). Indeed, a potential link between stromal SPARC expression and efficacy was observed in an exploratory analysis of a single-arm phase I/II study of patients with metastatic pancreatic cancer treated with nab-paclitaxel plus GEM [6]. Moreover, recent studies have indicated there is another, SPARC independent, mechanism of nab-paclitaxel delivery into pancreatic tumors [67, 68], and it is debatable whether SPARC is correlated with improved efficacy of nab-paclitaxel-based therapy [74]. Thus, the preclinical and clinical translational significance of SPARC is worth further study and discussion.
Fig. 2.
The clinical value of SPARC in pancreatic cancer. Cytoplasmic SPARC may be involved in the intrinsic resistance to GEM, whereas stromal SPARC can enrich the concentration of nab-paclitaxel to enhance its efficacy. The survival benefit of PDAC patients treated with GEM plus nab-paclitaxel is likely affected by SPARC expression
Abundant studies have determined the prognostic significance of stromal SPARC and cytoplasmic SPARC (expressed in the tumor epithelium) in PDAC [75–78] (Table 1). First, stromal SPARC and cytoplasmic SPARC are two independent elements that are not correlated with each other [74, 75]. Next, SPARC, especially in the stroma, seems to be independently correlated with a worse prognosis in resected pancreatic cancer after curative-intended resection [79–81]. Moreover, the unfavorable prognostic impact was restricted to patients treated with adjuvant GEM, suggesting both stromal SPARC and cytoplasmic SPARC were potential negative predictive markers for response to GEM, as shown in a translational analysis from a prospective phase III CONKO-001 study [79]. Notably, the patients with strong stromal or cytoplasmic SPARC expression in the GEM group had a poor OS compared similar to the patients of the observation group. These findings imply that SPARC expression might be associated with GEM resistance in pancreatic cancer. Stromal SPARC expression was increased in dense desmoplastic stroma, and therefore, stromal SPARC impedes the uptake of GEM by altered diffusion in differentially composed tumor stroma, in accordance with the hypothesis that the stroma is a treatment barrier in PDAC [44]. However, to date, several studies on SPARC expression in metastatic PDAC have been published, with controversial data on the impact of SPARC expression on patient outcome [6, 74, 75]. The analyses from the phase III MPACT study [74] and Ormanns et al. study [75] agree that the stromal SPARC level has no significant effect on prognosis or on the efficacy of GEM-based chemotherapy in metastatic PDAC patients [74, 75]. The predictive role of stromal SPARC expression in advanced PDAC was lost, likely due to the abundance of dense stroma, leading to impairment of GEM delivery affected by other stromal elements. However, for patients with resected PDAC, the primary tumor was removed, and the residual pancreas harbored little tumor-related stroma. Thus, both cytoplasmic and stromal SPARC examined in resected specimens are the important independent prognostic factors and predict the efficiency of GEM in resected PDAC patients. Ormanns et al. also demonstrated that cytoplasmic SPARC expression in the primary tumor serves as an independent biomarker associated with inferior PFS and OS in advanced PDAC and a negative predictive biomarker for the efficacy of GEM-based chemotherapy [75]. This result was in accordance with the data from CONKO-001 in resected PDAC. This effect of cytoplasmic SPARC might result from SPARC inducing intrinsic resistance to GEM in pancreatic cancer cells (Fig. 2). Recent reports have shown that SPARC might be involved in epithelial–mesenchymal transition [82], mediating GEM resistance in PDAC [83]. However, this result is in contrast to the translational MPACT results. The data discrepancy may be a result of the difference in methodology concerning protein detection and staining evaluation. In fact, Ormanns et al. reassessed the cytoplasmic SPARC expression using a scoring system with the same stringent cutoff for SPARC positivity as the MPACT investigators used. As expected, a similarly low percentage of cytoplasmic SPARC expression was determined, which was not associated with OS [75]. Interestingly, the predictive and prognostic roles of SPARC changed in metastatic PDAC treated with nab-paclitaxel plus GEM. In fact, a high SPARC level in the stroma was significantly correlated with improved OS of patients with advanced pancreatic cancer in the phase I/II clinical trial of nab-paclitaxel plus GEM [6]. This is particularly important because, historically, stromal SPARC expression has been associated with poor survival [80, 84], suggesting that a unique mechanism of action of the present regimen may play a role in this reverse outcome. Although the number of samples in this study was fairly small and the data need further conformation and interpretation, the results imply that stromal SPARC expression may be an important marker of early activity of GEM plus nab-paclitaxel combination regimens in advanced pancreatic cancer. High stromal SPARC expression enriched the concentration of nab-paclitaxel. In addition to intrinsic antitumor effects against the cancer cells, nab-paclitaxel softened the dense stroma to ease the other restrictive factors for GEM delivery within PDAC stroma. This might make the predictive role of stroma SPARC expression in GEM efficiency show up. Taken together, high stromal SPARC expression not only predicts the nab-paclitaxel efficiency, but also predicts GEM resistance, forming two opposite effects. Comprehensively, the result of a phase I/II clinical trial might be obtained. However, once the two effects offset each other, it seems reasonable that stromal SPARC expression did not predict the efficacy of nab-paclitaxel plus GEM for metastatic pancreatic cancer in the exploratory analysis of the phase III MPACT trial (Table 1). Moreover, cytoplasmic SPARC expression might be irrelevant to nab-paclitaxel delivery, and nab-paclitaxel has been shown to synergize with GEM. The comprehensive result was dependent on the intrinsic GEM resistance induced by cytoplasmic SPARC (Fig. 2). Thus, eliminating the influence of the stringent scoring system for SPARC in the MPACT study, we hypothesize that cytoplasmic SPARC can also predict an unfavorable efficacy of nab-paclitaxel plus GEM for metastatic pancreatic cancer, based on the results from Ormanns et al. [75].
Table 1.
The clinical roles of SPARC in PDAC based on recent studies
| IHC analysis | Authors of study | Year of published | Poor prognosis | Prediction | Stage of PDAC | |
|---|---|---|---|---|---|---|
| GEM | n-P + GEM | |||||
| Stromal SPARC | Ormanns et al. | 2016 | ns | ns | / | Metastatic PDAC |
| Hidalgo et al. | 2015 | ns | ns | ns | Metastatic PDAC | |
| Sinn et al. | 2014 | + | – | / | Resected PDAC | |
| Von Hoff et al. | 2011 | – | / | + | Metastatic PDAC | |
| Gundewar et al. | 2015 | + | / | / | Resected PDAC | |
| Infante et al. | 2007 | + | / | / | Resected PDAC | |
| Mantoni et al. | 2008 | + | / | / | LAPC | |
| Cytoplasmic SPARC | Ormanns et al. | 2016 | + | – | / | Metastatic PDAC |
| Hidalgo et al. | 2015 | ns | ns | ns | Metastatic PDAC | |
| Sinn et al. | 2014 | + | – | / | Resected PDAC | |
| Von Hoff et al. | 2011 | ns | / | ns | Metastatic PDAC | |
| Mao et al. | 2014 | – | / | / | Resected PDAC | |
| Infante et al. | 2007 | ns | / | / | Resected PDAC | |
| Mantoni et al. | 2008 | + | / | / | LAPC | |
LAPC locally advanced pancreatic cancer, + positive correlation, – negative correlation, ns no correlation,/ undetected, IHC immunohistochemistry, n-P nab-paclitaxel
These hypotheses were based on the idea that nab-paclitaxel was concentrated in the PDAC stroma and depleted the stroma in a SPARC-dependent manner. Thus, external validation of these hypotheses is necessary. If other groups confirmed these hypotheses, a possible biomarker for treatment decisions regarding GEM-based first-line regimens based on cytoplasmic SPARC expression in advanced PDAC patients could be obtained. Specifically, the role of cytoplasmic SPARC expression in resected PDAC should be re-investigated within the multicenter APACT trial (NCT01964430), a phase III randomized study of nab-paclitaxel plus GEM versus GEM alone as an adjuvant therapy.
Discussion
Although anti-stromal therapy in PDAC patients has led to many frustrations that have cast doubt on its potential, several promising anti-stroma drugs for advanced PDAC are worthy of attention. These drugs include nab-paclitaxel (NCT00844649, completed), PEGPH20 (NCT02715804, recruiting), and GDC-0449 (an inhibitor of SHH pathway; NCT01088815, active, not recruiting; NCT00878163, active, not recruiting). Improvements to current treatment methods and the development of more effective novel therapies based on known stromal features are clearly and urgently needed.
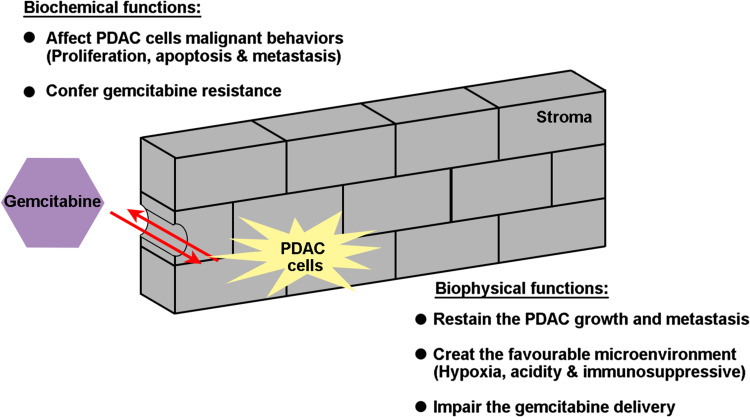
However, there is a mass of confusing and conflicting results in regard to anti-stroma therapy, from both preclinical and clinical studies. These conflicting results suggest that the role of stroma in PDAC is complicated, and stromal properties cannot be absolutely defined as favorable or unfavorable. The tumor stroma acts like a rampart, segregating PDAC with tumor epithelial cells inside the stroma and chemotherapy drugs outside the stroma. The strength of the determinants inside and outside the rampart should be considerate to determine whether anti-stroma therapy is valuable to prompt them to meet each other. Once the gate is opened by punching holes in the stromal barrier, there is a strong counteraction between the factors inside and outside the stroma. If cancer cells are sensitive to the drug, anti-stroma therapy exhibits beneficial effects; if cancer cells are resistant to the drug, this therapy provides the tumor new space to grow and the probability metastasis. This is the biophysical function of tumor stroma. However, the idea of the stroma as a mere treatment barrier in PDAC may be too simplistic because it does not reflect the complex signaling interaction between the stromal and the epithelial compartments. The malignant potential of cancer cells largely determines the inherent drug resistance. However, the biochemical function of stroma is another determinant involved in the transformation of intrinsic drug resistance. This has a strong correlation with the broad intertumoral heterogeneity and intratumoral heterogeneity that consists of tumor cell heterogeneity and stromal heterogeneity (Fig. 3).
Fig. 3.
The complex interaction between the stromal and the epithelial compartment within PDAC during treatment with GEM. The stroma acts as a biophysical rampart, segregating PDAC into tumor epithelial cells inside the stroma and chemotherapy drugs outside the stroma, mediating the extrinsic resistance to GEM. The biochemical function of stroma is another determinant involved in transformation of intrinsic drug resistance
Therefore, we should define some indicators in PDAC at every stage to identify subpopulations of patients for anti-stroma therapy. This typing to guide therapy selection is extremely important for individualized treatment. Considering the heterogeneity of PDAC, some patients can be stratified for timely treatment with the appropriate anti-stroma therapy, which could significantly improve the awkward situation of PDAC treatment. Despite the slow progress made in the past decade, we are making progress in deciphering the heterogeneity within pancreatic cancers. The integration of conventional and anti-stroma therapy will hopefully be the key to effective treatment of this deadly disease.
Acknowledgements
This study was jointly funded by the National Science Foundation for Distinguished Young Scholars of China (No. 81625016), the National Natural Science Foundation of China (No. 81372651, 81502031) and the Shanghai Sailing Program (No. 17YF1402500).
Footnotes
Chen Liang, Si Shi and Qingcai Meng contributed equally to this work.
References
- 1.Ying H, Dey P, Yao W, Kimmelman AC, Draetta GF, Maitra A, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2016;30:355–385. doi: 10.1101/gad.275776.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 3.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 4.Sun C, Ansari D, Andersson R, Wu DQ. Does gemcitabine-based combination therapy improve the prognosis of unresectable pancreatic cancer? World J Gastroenterol. 2012;18:4944–4958. doi: 10.3748/wjg.v18.i35.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson R, Aho U, Nilsson BI, Peters GJ, Pastor-Anglada M, Rasch W, et al. Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44:782–786. doi: 10.1080/00365520902745039. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu W, et al. Metabolic plasticity in heterogeneous pancreatic ductal adenocarcinoma. Biochim Biophys Acta. 2016;1866:177–188. doi: 10.1016/j.bbcan.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Mahadevan D, Von Hoff DD. Tumor–stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 9.Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101:887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 10.Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194:S84–S86. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 13.Schneiderhan W, Diaz F, Fundelvzv M, Zhou S, Siech M, Hasel C, et al. Pancreatic stellate cells are an important source of MMP in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci. 2007;120:512–519. doi: 10.1242/jcs.03347. [DOI] [PubMed] [Google Scholar]
- 14.Jones L, Ghaneh P, Humphreys M, Neoptolemos JP. The matrix metalloproteinases and their inhibitors in the treatment of pancreatic cancer. Ann N Y Acad Sci. 1999;880:288–307. doi: 10.1111/j.1749-6632.1999.tb09533.x. [DOI] [PubMed] [Google Scholar]
- 15.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ide T, Kitajima Y, Miyoshi A, Ohtsuka T, Mitsuno M, Ohtaka K, et al. Tumor–stromal cell interaction under hypoxia increases the invasiveness of pancreatic cancer cells through the hepatocyte growth factor/c-Met pathway. Int J Cancer. 2006;119:2750–2759. doi: 10.1002/ijc.22178. [DOI] [PubMed] [Google Scholar]
- 17.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 18.Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, et al. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid Redox Signal. 2012;16:1264–1284. doi: 10.1089/ars.2011.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laklai H, Miroshnikova YA, Pickup MW, Collisson EA, Kim GE, Barrett AS, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med. 2016;22:497–505. doi: 10.1038/nm.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhardwaj A, Srivastava SK, Singh S, Tyagi N, Arora S, Carter JE, et al. MYB promotes desmoplasia in pancreatic cancer through direct transcriptional up-regulation and cooperative action of sonic hedgehog and adrenomedullin. J Biol Chem. 2016;291:16263–16270. doi: 10.1074/jbc.M116.732651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 24.Stoker MG, Shearer M, O’Neill C. Growth inhibition of polyoma-transformed cells by contact with static normal fibroblasts. J Cell Sci. 1966;1:297–310. doi: 10.1242/jcs.1.3.297. [DOI] [PubMed] [Google Scholar]
- 25.Klein G. Evolutionary aspects of cancer resistance. Semin Cancer Biol. 2014;25:10–14. doi: 10.1016/j.semcancer.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikenaga N, Ohuchida K, Mizumoto K, Cui L, Kayashima T, Morimatsu K, et al. CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer. Gastroenterology. 2010;139:1041–1051. doi: 10.1053/j.gastro.2010.05.084. [DOI] [PubMed] [Google Scholar]
- 29.Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012;11:1282–1290. doi: 10.4161/cc.19679. [DOI] [PubMed] [Google Scholar]
- 30.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Erkan M, Reiser-Erkan C, Michalski CW, Kleeff J. Tumor microenvironment and progression of pancreatic cancer. Exp Oncol. 2010;32:128–131. [PubMed] [Google Scholar]
- 32.Luo G, Long J, Zhang B, Liu C, Xu J, Ni Q, et al. Stroma and pancreatic ductal adenocarcinoma: an interaction loop. Biochim Biophys Acta. 2012;1826:170–178. doi: 10.1016/j.bbcan.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engels B, Rowley DA, Schreiber H. Targeting stroma to treat cancers. Semin Cancer Biol. 2012;22:41–49. doi: 10.1016/j.semcancer.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA, Marimastat Pancreatic Cancer Study G Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol. 2001;19:3447–3455. doi: 10.1200/JCO.2001.19.15.3447. [DOI] [PubMed] [Google Scholar]
- 36.Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296–3302. doi: 10.1200/JCO.2003.02.098. [DOI] [PubMed] [Google Scholar]
- 37.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gore J, Korc M. Pancreatic cancer stroma: friend or foe? Cancer Cell. 2014;25:711–712. doi: 10.1016/j.ccr.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dangi-Garimella S, Krantz SB, Barron MR, Shields MA, Heiferman MJ, Grippo PJ, et al. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res. 2011;71:1019–1028. doi: 10.1158/0008-5472.CAN-10-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dangi-Garimella S, Sahai V, Ebine K, Kumar K, Munshi HG. Three-dimensional collagen I promotes gemcitabine resistance in vitro in pancreatic cancer cells through HMGA2-dependent histone acetyltransferase expression. PLoS One. 2013;8:e64566. doi: 10.1371/journal.pone.0064566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kozono S, Ohuchida K, Eguchi D, Ikenaga N, Fujiwara K, Cui L, et al. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res. 2013;73:2345–2356. doi: 10.1158/0008-5472.CAN-12-3180. [DOI] [PubMed] [Google Scholar]
- 42.Binenbaum Y, Na’ara S, Gil Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist Updates. 2015;23:55–68. doi: 10.1016/j.drup.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koay EJ, Truty MJ, Cristini V, Thomas RM, Chen R, Chatterjee D, et al. Transport properties of pancreatic cancer describe gemcitabine delivery and response. J Clin Invest. 2014;124:1525–1536. doi: 10.1172/JCI73455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tannock IF, Lee CM, Tunggal JK, Cowan DS, Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res. 2002;8:878–884. [PubMed] [Google Scholar]
- 47.Pries AR, Hopfner M, le Noble F, Dewhirst MW, Secomb TW. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer. 2010;10:587–593. doi: 10.1038/nrc2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–195. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 49.Nagathihalli NS, Castellanos JA, Shi C, Beesetty Y, Reyzer ML, Caprioli R, et al. Signal transducer and activator of transcription 3, mediated remodeling of the tumor microenvironment results in enhanced tumor drug delivery in a mouse model of pancreatic cancer. Gastroenterology. 2015;149(1932–1943):e1939. doi: 10.1053/j.gastro.2015.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hessmann E, Patzak MS, Klein L, Chen N, Kari V, Ramu I, et al. Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer. Gut. 2017 doi: 10.1136/gutjnl-2016-311954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonomi A, Sordi V, Dugnani E, Ceserani V, Dossena M, Cocce V, et al. Gemcitabine-releasing mesenchymal stromal cells inhibit in vitro proliferation of human pancreatic carcinoma cells. Cytotherapy. 2015;17:1687–1695. doi: 10.1016/j.jcyt.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 53.Anonymous (January 27, 2012) Press release: Infinity reports update from phase 2 study of saridegib plus gemcitabine in patients with metastatic pancreatic cancer. Available at: http://www.businesswire.com/news/home/20120127005146/en/Infinity-Reports-Update-Phase-2-Study-Saridegib#.UxAvFfRdVxV. Accessed 2 July 2014
- 54.Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci USA. 2014;111:E3091–E3100. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a Hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Jesus-Acosta A, O’Dwyer PJ, Ramanathan RK, Von Hoff DD, Maitra A, Rasheed Z et al (2014) A phase II study of vismodegib, a hedgehog (Hh) pathway inhibitor, combined with GEM and nab-paclitaxel (nab-P) in patients (pts) with untreated metastatic pancreatic ductal adenocarcinoma (PDA). J Clin Oncol 32(Suppl 3;abstr 257)
- 57.Le Calve B, Griveau A, Vindrieux D, Marechal R, Wiel C, Svrcek M, et al. Lysyl oxidase family activity promotes resistance of pancreatic ductal adenocarcinoma to chemotherapy by limiting the intratumoral anticancer drug distribution. Oncotarget. 2016;7:32100–32112. doi: 10.18632/oncotarget.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller BW, Morton JP, Pinese M, Saturno G, Jamieson NB, McGhee E, et al. Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol Med. 2015;7:1063–1076. doi: 10.15252/emmm.201404827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miao L, Liu Q, Lin CM, Luo C, Wang Y, Liu L, et al. Targeting tumor-associated fibroblasts for therapeutic delivery in desmoplastic tumors. Cancer Res. 2017;77:719–731. doi: 10.1158/0008-5472.CAN-16-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bapiro TE, Richards FM, Goldgraben MA, Olive KP, Madhu B, Frese KK, et al. A novel method for quantification of gemcitabine and its metabolites 2′,2′-difluorodeoxyuridine and gemcitabine triphosphate in tumour tissue by LC-MS/MS: comparison with (19)F NMR spectroscopy. Cancer Chemother Pharmacol. 2011;68:1243–1253. doi: 10.1007/s00280-011-1613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res. 2016;22:2848–2854. doi: 10.1158/1078-0432.CCR-15-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heinemann V, Reni M, Ychou M, Richel DJ, Macarulla T, Ducreux M. Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies. Cancer Treat Rev. 2014;40:118–128. doi: 10.1016/j.ctrv.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109:926–933. doi: 10.1038/bjc.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, et al. Nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2:260–269. doi: 10.1158/2159-8290.CD-11-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim H, Samuel S, Lopez-Casas P, Grizzle W, Hidalgo M, Kovar J, et al. SPARC-Independent delivery of nab-paclitaxel without depleting tumor stroma in patient-derived pancreatic cancer xenografts. Mol Cancer Ther. 2016;15:680–688. doi: 10.1158/1535-7163.MCT-15-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neesse A, Frese KK, Chan DS, Bapiro TE, Howat WJ, Richards FM, et al. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2014;63:974–983. doi: 10.1136/gutjnl-2013-305559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagaraju GP, Dontula R, El-Rayes BF, Lakka SS. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis. 2014;35:967–973. doi: 10.1093/carcin/bgu072. [DOI] [PubMed] [Google Scholar]
- 70.Kiessling F, Fink C, Hansen M, Bock M, Sinn H, Schrenk HH, et al. Magnetic resonance imaging of nude mice with heterotransplanted high-grade squamous cell carcinomas: use of a low-loaded, covalently bound Gd-Hsa conjugate as contrast agent with high tumor affinity. Invest Radiol. 2002;37:193–198. doi: 10.1097/00004424-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Schnitzer JE, Oh P. Antibodies to SPARC inhibit albumin binding to SPARC, gp60, and microvascular endothelium. Am J Physiol. 1992;263:H1872–H1879. doi: 10.1152/ajpheart.1992.263.6.H1872. [DOI] [PubMed] [Google Scholar]
- 72.Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl Oncol. 2009;2:59–64. doi: 10.1593/tlo.09109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 74.Hidalgo M, Plaza C, Musteanu M, Illei P, Brachmann CB, Heise C, et al. SPARC expression did not predict efficacy of nab-paclitaxel plus gemcitabine or gemcitabine alone for metastatic pancreatic cancer in an exploratory analysis of the phase III MPACT Trial. Clin Cancer Res. 2015;21:4811–4818. doi: 10.1158/1078-0432.CCR-14-3222. [DOI] [PubMed] [Google Scholar]
- 75.Ormanns S, Haas M, Baechmann S, Altendorf-Hofmann A, Remold A, Quietzsch D, et al. Impact of SPARC expression on outcome in patients with advanced pancreatic cancer not receiving nab-paclitaxel: a pooled analysis from prospective clinical and translational trials. Br J Cancer. 2016;115:1520–1529. doi: 10.1038/bjc.2016.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mantoni TS, Schendel RR, Rodel F, Niedobitek G, Al-Assar O, Masamune A, et al. Stromal SPARC expression and patient survival after chemoradiation for non-resectable pancreatic adenocarcinoma. Cancer Biol Ther. 2008;7:1806–1815. doi: 10.4161/cbt.7.11.6846. [DOI] [PubMed] [Google Scholar]
- 77.Gundewar C, Sasor A, Hilmersson KS, Andersson R, Ansari D. The role of SPARC expression in pancreatic cancer progression and patient survival. Scand J Gastroenterol. 2015;50:1170–1174. doi: 10.3109/00365521.2015.1024281. [DOI] [PubMed] [Google Scholar]
- 78.Mao Z, Ma X, Fan X, Cui L, Zhu T, Qu J, et al. Secreted protein acidic and rich in cysteine inhibits the growth of human pancreatic cancer cells with G1 arrest induction. Tumour Biol. 2014;35:10185–10193. doi: 10.1007/s13277-014-2315-0. [DOI] [PubMed] [Google Scholar]
- 79.Sinn M, Sinn BV, Striefler JK, Lindner JL, Stieler JM, Lohneis P, et al. SPARC expression in resected pancreatic cancer patients treated with gemcitabine: results from the CONKO-001 study. Ann Oncol. 2014;25:1025–1032. doi: 10.1093/annonc/mdu084. [DOI] [PubMed] [Google Scholar]
- 80.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 81.Miyoshi K, Sato N, Ohuchida K, Mizumoto K, Tanaka M. SPARC mRNA expression as a prognostic marker for pancreatic adenocarcinoma patients. Anticancer Res. 2010;30:867–871. [PubMed] [Google Scholar]
- 82.Heeg S, Das KK, Reichert M, Bakir B, Takano S, Caspers J, et al. ETS-transcription factor ETV1 regulates stromal expansion and metastasis in pancreatic cancer. Gastroenterology. 2016;151(540–553):e514. doi: 10.1053/j.gastro.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fan X, Mao Z, Ma X, Cui L, Qu J, Lv L, et al. Secreted protein acidic and rich in cysteine enhances the chemosensitivity of pancreatic cancer cells to gemcitabine. Tumour Biol. 2016;37:2267–2273. doi: 10.1007/s13277-015-4044-4. [DOI] [PubMed] [Google Scholar]
- 84.Guweidhi A, Kleeff J, Adwan H, Giese NA, Wente MN, Giese T, et al. Osteonectin influences growth and invasion of pancreatic cancer cells. Ann Surg. 2005;242:224–234. doi: 10.1097/01.sla.0000171866.45848.68. [DOI] [PMC free article] [PubMed] [Google Scholar]