Abstract
Pumilio (PUM) proteins are RNA-binding proteins that posttranscriptionally regulate gene expression in many organisms. Their PUF domain recognizes specific PUM-binding elements (PBE) in the 3′ untranslated region of target mRNAs while engaging protein cofactors such as NANOS that repress the expression of target mRNAs through the recruitment of effector complexes. Although the general process whereby PUM recognizes individual mRNAs has been studied extensively, the particulars of the mechanism underlying PUM–NANOS cooperation in mRNA regulation and the functional overlap among PUM and NANOS paralogues in mammals have not been elucidated. Here, using the novel PUM1 and PUM2 mRNA target SIAH1 as a model, we show mechanistic differences between PUM1 and PUM2 and between NANOS1, 2, and 3 paralogues in the regulation of SIAH1. Specifically, unlike PUM2, PUM1 exhibited PBE-independent repression of SIAH1 3′UTR-dependent luciferase expression. Concordantly, the PUF domains of PUM1 and PUM2 showed different EMSA complex formation patterns with SIAH1 3′UTRs. Importantly, we show direct binding of NANOS3, but not NANOS2, to SIAH1 3′UTR, which did not require PBEs or the PUF domain. To the best of our knowledge, this is the first report, showing that an NANOS protein directly binds RNA. Finally, using NANOS1 and NANOS3 constructs carrying mutations identified in infertile patients, we show that these mutations disrupt repression of the SIAH1-luciferase reporter and that the central region in NANOS1 appears to contribute to the regulation of SIAH1. Our findings highlight the mechanistic versatility of the PUM/NANOS machinery in mammalian posttranscriptional regulation.
Electronic supplementary material
The online version of this article (10.1007/s00018-018-2926-5) contains supplementary material, which is available to authorized users.
Keywords: 3′UTR, RNA-binding proteins, Posttranscriptional gene regulation
Background
Pumilio (PUM) proteins are founding members of the PUF (PUM and fem-3 binding factor) family of eukaryotic RNA-binding proteins involved in posttranscriptional gene regulation during morphogenesis [1, 2], neurogenesis [3], and germ cell development [4, 5] in many eukaryotic organisms (for a review, see [6]). The conserved PUF RNA-binding domain consists of eight repeats, each recognizing and binding a single nucleotide within a specific short sequence in the 3′UTR (3′ untranslated region) of target mRNAs. These short sequences are called NANOS Response Elements (NREs), since it was initially thought that they were recognized by the NANOS protein, a partner of PUM, first studied in Drosophila [1, 2]. Each NRE is built of two short motifs: GUUGU (A) and AUUGUA (B), identified in PUM mRNA targets, such as hunchback (hb), cyclin B, and bicoid mRNAs [7–9]. Analysis of mRNA targets led to the identification of the PUF domain binding consensus UGUANAUA, which was called PUM-binding element (PBE) and partially overlaps the GUUGU and AUUGUA NRE motifs (UGU for A and UGUA for B, respectively). Besides the PUF domain, PUM contains three unique domains in the N-terminus with repressive activity, which can function autonomously from PUF domain [10]. While PUM is a unique protein in the fly, there are several paralogues in C. elegans, and six in yeast (for a review, see [6]). In mammals, including humans, there are two PUM paralogues, PUM1 and PUM2, containing the classical PUF domain. These two paralogues have a very similar structure, especially within the PUF domain with amino acid identities over 90%, and they are both expressed in many tissues [11]. The PUF–RNA interface is highly conserved among different organisms, to the extent that the PUF domain of the human PUM1 is able to recognize the NREs within the Drosophila hb mRNA with high affinity [9]. A global search for mRNA targets in human HeLa cells revealed that PUM1 and PUM2 proteins regulate up to 15% of the human transcriptome [12].
PUM proteins bind RNA and recruit protein cofactors, bringing about the formation of specific mRNA–protein complexes that determine variable biological outcomes for these mRNAs, such as translational activation, repression, and/or degradation (for a review, see [6, 13, 14]). NANOS is a well-established PUM cofactor. Only one gene encoding the Nanos protein exists in Drosophila, but three paralogues NANOS1, NANOS2, and NANOS3 are present in mammals, including humans. Their expression is germ cell specific, but they are also expressed in several cell lines (for a review, see [15]). NANOS proteins contain a highly conserved C-terminal zinc-finger domain (CCHC)2. By contrast, the N-terminal/central region is very divergent among NANOS paralogues, except for a conserved short 16 amino acid motif called NIM (CNOT1-interacting motif) located in the N-terminal region that interacts with the deadenylase complex during the repressor function of the complex [16]. Whether structural divergences, especially within the N-terminal region, influence the function of mammalian NANOS paralogues, and particularly in the case of the human NANOS paralogues, has not yet been elucidated. Moreover, although the general process used by PUM to recognize individual mRNAs has been studied extensively [17], the particulars of the mechanism underlying PUM–NANOS cooperation in mRNA regulation in mammals are not understood.
In this study, we aimed to study PUM–NANOS cooperation in mRNA regulation and the functional overlap among PUM and NANOS paralogues in mammals. To this end, we used the human PUM target mRNA SIAH1, since it contains two NREs with an arrangement very similar to that in the Drosophila hb mRNA, to which the human PUM1 binds in vitro [9], and which had been used to study PUM–NANOS cooperation during mRNA repression in Drosophila [2, 18]. We demonstrate that human PUM2 and NANOS3 cooperate and that PUM1 and PUM2 as well as NANOS1, NANOS2, and NANOS3 paralogues are functionally not redundant. In addition, we show that PUM1 and PUM2 paralogues differ in their in vitro recognition and binding of SIAH1 3′UTR as well as in their dependency on PBE for mRNA repression.
Methods
Bioinformatic search for PUM mRNA targets
NCBI GenBank human nucleotide and EST databases (700,000 and 10,000 sequences, respectively, at the time of analysis) were screened to find mRNAs containing NRE motifs (GUUGU and AUUGUA) in the arrangement present in D. melanogaster Pum targets bicoid (bc), hunchback (hb) and cyclin B (Fig. S1) using a custom script that used hb 3′UTR NREs previously found to be specifically recognized not only by D. melanogaster Pum but also by the human PUM1 PUF domain [8, 9].
Constructs
Full-length SIAH1 3′UTR and full-length 3′UTR of GAPDH mRNA (negative control) were amplified from human cDNA and were cloned into the psiCheck2 vector (Promega) in fusion with a Renilla luciferase open-reading frame (ORF) using primers described in Table S1. This vector also contained the firefly luciferase ORF used for normalization. The constructs encoding cDNAs for the PUF1 domain (from 769 up to 1186 amino acid position), or full-length NANOS2 and 3 for overexpression and protein purification from bacteria to be used in EMSA tests, were cloned in fusion with an INTEIN tag (N-terminal fusion) within the pTYB12 vector using the IMPACT-CN system (New England Biolabs) using primers described in Table S2. The construct encoding the PUF2 domain (from 649 up to 1065 amino acid position) was previously described [19]. All constructs encoding the wild-type NANOS and PUM proteins in the pCMV6-Entry vector used for overexpression in HEK293FT cells were purchased from OriGene.
Luciferase reporter assays
All experiments were conducted in HEK293FT cells, which were cultured in DMEM medium (Sigma-Aldrich) supplemented with 10% GlutaMAX (Gibco, Life Technologies), 10% MEM non-essential amino-acids (Sigma-Aldrich), 10% (v/v) fetal bovine serum (Sigma-Aldrich), and 1% (v/v) antibiotic antimycotic solution (Lonza). The cells were transfected with plasmids or siRNA using Neon Transfection System (Life Technologies), according to the manufacturer’s protocol followed by culture in the same medium without antibiotic and antimycotic solution. HEK293FT cells were co-transfected with a series of reporter constructs in psiCheck2 vector (Promega) (Table S1) and constructs for PUM and NANOS protein overexpression in a pCMV6-Entry vector (OriGene). Empty pCMV6-Entry vector was used as a negative control. These transfections were carried out in triplicate. After 48 h of culture, the cells were lysed, and luciferase luminescence was then measured using the Glomax-Multi Detection System and Dual-luciferase Reporter Kit (Promega). The mean ratio of Renilla to firefly luminescence for each sample from three repeated measurements in each experiment was presented as a % of the relative luciferase units (RLU) of the sample transfected with the reporter construct only, which was considered to be 100%. Standard deviations were calculated and shown as error bars, and the two-tailed t test was utilized to estimate statistical significance.
siRNA silencing
For transient silencing, 10 µM siRNA PUM1 (Santa Cruz Biotechnology, sc-62912), siRNA PUM2 (Santa Cruz Biotechnology, sc-44773), or control siRNA (Santa Cruz Biotechnology, sc-37007) was transfected into cells along with the reporter construct and NANOS1, 2, and 3 constructs or empty plasmid pCMV6-Entry, at the same concentrations as those used in the luciferase assays. Silencing efficiency was measured by western blot (WB) using the corresponding antibodies and anti-β-ACTIN antibody for normalization.
Western blotting and antibodies
Western blots were performed at the standard conditions by resolving protein extracts by SDS-PAGE and transferring to a nitrocellulose membrane, followed by incubation with primary antibodies (listed below) and horseradish peroxidase (HRP)-conjugated secondary antibodies. The chemiluminescent signal was detected using Clarity™ Western ECL Substrate for HRP (Bio-Rad). Protein expression was normalized to β-ACTIN and semi-quantitative measurements were performed using the ImageLab 5.1 software (Bio-Rad). The anti-DDK antibody (OriGene Technologies, TA50011) for the detection of PUM and NANOS proteins in the pCMV6-Entry vector was used for WB at a dilution of 1:1000, anti-β-ACTIN (Sigma-Aldrich, A2066) at 1:10,000, anti-PUM1 (Abcam, Ab3717) at 1:5000 or 5 µg per 100 µl for immunoprecipitation reactions, anti-PUM2 (Santa Cruz Biotechnology, sc-31535) for WB at 1:250 or 5 µg per 100 µl for immunoprecipitation reactions, and anti-NANOS1 (Everest Biotech, EB06680) for WB at 1:2000. The SIAH1 expression level under PUM1 or PUM2 overexpression was measured by WB using a primary anti-SIAH1 antibody (Santa Cruz Biotechnology sc-5505) at 1:250 and its expression was normalized to that of β-ACTIN.
RIP and quantitative real-time RT-qPCR
RNA immunoprecipitation (RIP) was carried out using the Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Merck Millipore) and anti-PUM1 or anti-PUM2 antibodies, or goat IgG (Sigma-Aldrich, I5256) for the negative RIP control. RNA was isolated from immunoprecipitates using RNeasy® Plus Micro Kit (Qiagen). For quantitative real-time RT-qPCR, RNA was treated with DNase I (Sigma-Aldrich) and reverse-transcribed using a Maxima First Strand cDNA Synthesis Kit (Thermo Scientific). Total cDNA was subsequently used as a template for quantitative PCR product amplification using Sybr Green (Sigma-Aldrich) and specific primers (Table S3). For real-time RT-qPCR analysis of SIAH1 mRNA (RIP) content, β-ACTIN and GAPDH mRNAs were used for normalization. For statistical analysis, this experiment was performed in three replicates.
EMSA
Constructs encoding the PUF1 or PUF2 RNA-binding domains of human PUM1 and PUM2 proteins, respectively, or a full-length NANOS2 or NANOS3 protein fused to INTEIN within the pTYB12 vector (IMPACT-CN system New England Biolabs) were used for protein expression in bacteria. Purification, including INTEIN tag removal by DTT treatment, was carried out as described earlier [19]. The 3′UTR of SIAH1 mRNA was amplified in 15 overlapping fragments (Fr) that were ~ 100 nt in length; a CDKN1B 3′UTR fragment of 103 nt (positive control) was PCR-amplified from human DNA. Each upstream primer contained the T7 polymerase promoter sequence, as shown in Table S4. The SIAH1 3′UTR Fr 6, which contains no PBE motifs and binds neither the PUF1 domain nor the PUF2 domain, was used as a nonspecific RNA competitor. In vitro transcription was performed in the presence of 32P-UTP. Radioactive transcripts were polyacrylamide-gel-purified and added to a reaction mixture-containing 10–250-nM PUF1 or PUF2 domain protein preparations for PUF domain binding or 100-nM preparations to test for cooperation with NANOS proteins, the latter being used in a 5–15-µM preparation. The same buffer composition for binding reactions, including 15-mM NaCl, was utilized for each EMSA experiment. In addition, to ensure PUF domain/NANOS cooperation, the EMSA experiments were repeated at the higher concentration of 100-mM NaCl. Reaction mixtures were incubated on ice for 1 h and run on a 4% native polyacrylamide gel-containing 2.5% glycerol at 4 °C as described earlier [19]. Gels were subject to autoradiography at -80 °C.
Site-directed mutagenesis
Six PBEs (− 2 up to 4-like motif) were mutated in the SIAH1 3′UTR by nucleotide substitutions using the QuickChange II XL Site-Directed Mutagenesis kit (Agilent). The sequences of all the primers used for this purpose are listed in Table S5.
Site-directed mutagenesis was also used to generate amino acid substitutions into the NIM region of NANOS1, NANOS2, and NANOS3 as previously described [16] to test the importance of the NIM region for SIAH1 mRNA regulation. In addition, NANOS1 constructs encoding mutations previously identified in infertile male patients [20] and NANOS3 constructs containing mutations described in female patients manifesting premature ovarian failure [21, 22] were generated. The primers used for all mutated NANOS constructs are listed in Table S6. Wild-type and mutated luciferase reporter constructs carrying the SIAH1 3′UTR or wild-type GAPDH 3′UTR, together with mutated NANOS constructs, were entirely verified by DNA sequencing.
PUM1 and PUM2 co-immunoprecipitation
For PUM1 and PUM2 co-immunoprecipitation, ~ 2 × 106 cells were transfected with 10 µg of PUM1 in pCMV6-entry or PUM2 in pCMV6-entry or empty pCMV6-entry vector, as described above, and were grown in 10-cm plates. After 48 h and reaching ~ 70% confluency cells were lysed 30 min on ice with 1 ml of lysis buffer supplemented with protease inhibitors. Lysates were centrifuged at 15,000×g at 4 °C for 15 min, followed by 2-h incubation with anti-FLAG M2 Magnetic Beads (Sigma-Aldrich). Beads were washed three times with TBS buffer, resuspended in SDS-PAGE sample buffer, and analysed by western blot using anti-DDK, anti-PUM1, or anti-PUM2 antibodies. Cells transfected with empty pCMV6-Entry were used as a negative control.
Results
Identification of SIAH1 as a potential PUM target
To study cooperation between human PUM and NANOS proteins on a target mRNA, we first performed a search for human mRNAs with NREs distribution similar to the NREs’ distribution in the Drosophila hb mRNA. We chose this distribution first, because it had been previously used as a model to study cooperation between Pumilio and Nanos in Drosophila [2, 18, 23], and second, because the PUF domain of human PUM1 protein has been shown to specifically recognize hb NREs in vitro [9]. To identify PUM targets carrying hb-like NREs with the conserved elements A and B, we screened the NCBI GenBank and EST databases using the strategy outlined in Fig. S1a. Among the bioinformatically selected PUM candidate targets, we identified an mRNA-encoding SIAH1, a ubiquitously expressed E3 ubiquitin ligase-mediating 26S proteasome-dependent protein degradation [24]. The SIAH1 mRNA was selected, since the distribution of the two 3′UTR NREs (Fig. S1b, NRE1 and NRE2), in terms of distance, was similar to that of the 3′UTR NREs of hb in D. melanogaster (Fig. S1c). Importantly, individual A (GUUGU) and B (AUUGUA) motifs in SIAH1 NRE1 and NRE2 (Fig. S1b A and B motifs are in bold) contained a UGUA tetranucleotide, representing the core of UGUANAUA which is now under consideration to be designated as a PBE consensus sequence [12, 25]. We, therefore, refer to these motifs as PBE-like motifs (Fig. S1b). Altogether, we identified PBE1- and PBE2-like motifs in NRE1, PBE3-, and PBE4-like motifs in NRE2, and upstream from NREs, we also identified −PBE2-like and −PBE1-consensus (Fig. S1b). The 3′UTR of SIAH1 mRNA is highly conserved between humans and mice with their overall identity reaching 75% (Fig. S2). Moreover, SIAH1 was among the PUM-binding mRNAs that were identified in genome-wide studies [12, 25]. In view of all the above, SIAH1 appears to be a likely candidate for regulation by PUM.
Overexpression of PUM1 and PUM2 results in decreased expression of endogenous SIAH1 protein
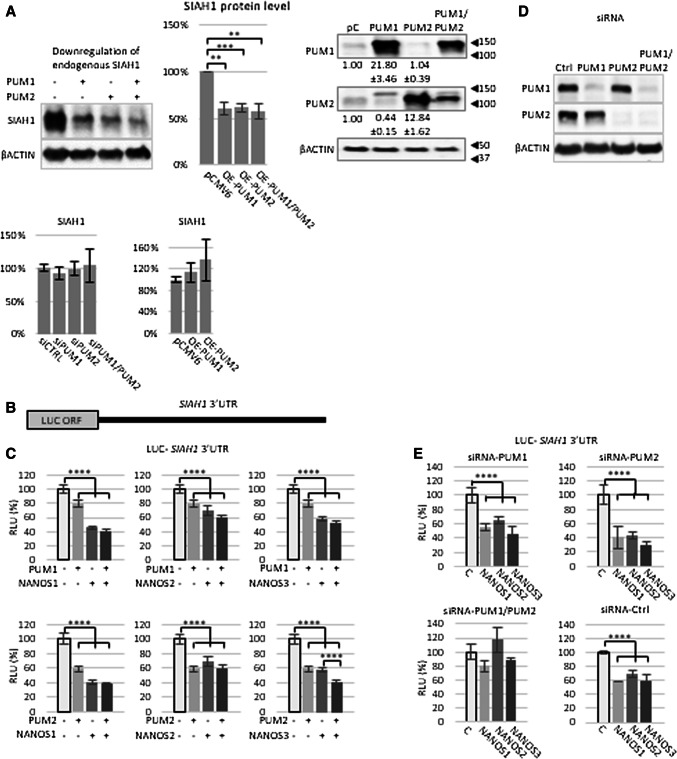
Since PUM-mediated regulation of SIAH1 has not been studied so far, we decided to first investigate whether endogenous SIAH1 is regulated by PUM. Upon transient overexpression of PUM1 or PUM2 in HEK293FT cells, the protein expression of endogenous SIAH1 was significantly decreased (Fig. 1a), suggesting that SIAH1 is regulated by PUM. However, neither upon silencing nor overexpression of PUM1 and PUM2, the level of SIAH1 mRNA has been changed (Fig. 1a).
Fig. 1.
SIAH1 mRNA is under PUM and NANOS repression. a Protein extracts from HEK293FT cells transfected with plasmids expressing PUM1 or PUM2 were resolved by SDS-PAGE followed by western blot with the indicated antibodies. β-ACTIN was used as loading control. All values (quantification of the bands) were significantly higher than the negative control (cells transfected with empty vector) (P < 0.01, P < 0.005). The image on the left is representative of three independent experiments, while the middle panel is a statistical assessment of those three experiments. Western blot analysis showing overexpression of PUM1 and PUM2 compared to endogenous PUM1 and PUM2 protein level is on the right. The lower panel represents analysis of SIAH1 mRNA level upon PUM siRNA silencing and overexpression. b Schematic diagram of luciferase reporter construct carrying the 3′UTR of SIAH1 mRNA. c Luciferase reporter repression under overexpression of single PUM or NANOS proteins or PUM/NANOS combination. All values (RLU%) were significantly lower than the negative control (luciferase reporter construct only) (P < 0.001). Luminescence values (RLU relative luciferase units) are expressed as % of the RLU of samples transfected with reporter construct only, which was set to 100%. Renilla luciferase values were normalized using firefly luciferase measurements. d Control western blot showing effect of single (P1 or P2) or double (P1/P2) siRNA downregulation of PUM protein expression compared to β-ACTIN. e Effects of PUM1 and PUM2 siRNA silencing on luciferase reporter repression mediated by NANOS1, NANOS2, and NANOS3. All values (RLU%) were significantly changed (P < 0.001) compared to the negative control (luciferase reporter construct only), C transfected with control siRNA. Each group of experiments in a, c, and e was performed three times, and on each occasion, they were performed in triplicate. Error bars denote standard deviation (n = 9)
PUM represses SIAH1 3′UTR-dependent luciferase expression
To further investigate the regulation of SIAH1 mRNA by PUM proteins, we co-transfected a construct containing a luciferase reporter upstream of the SIAH1 3′UTR (Fig. 1b) along with constructs expressing PUM1 or PUM2. Overexpression of either PUM1 or PUM2 (Fig. S3a) significantly repressed SIAH1 3′UTR-dependent luciferase expression (Fig. 1c). In addition, the repression of luciferase expression with PUM2 (40%) was much higher than that seen with PUM1 (20%). The relative repressive effect of PUM2 could, in fact, be even higher given that its expression levels were 0.6 times lower than PUM1 levels in comparison to their endogenous level, as shown in Fig. 1a right panel.
Repression of SIAH1 3′UTR-dependent luciferase activity is enhanced when PUM2 and NANOS3 are co-expressed
Next, we investigated the role of PUM–NANOS cooperation in regulating SIAH1 by overexpressing NANOS paralogues in combination with PUM paralogues. Apart from NANOS1, the endogenous expression of other NANOS proteins in HEK293FT cells is negligible (Human Protein Atlas http://www.proteinatlas.org). Since the exogenous expression of NANOS1 strongly exceeded its endogenous expression (Fig. S3b), any observed effects on the luciferase reporter would be mainly due to ectopic NANOS protein expression, which was roughly similar between the paralogues (Fig. S3c–e). Interestingly, all the NANOS paralogues repressed SIAH1 3′UTR-dependent luciferase expression when overexpressed individually (Fig. 1c). However, the repression observed with the PUM2/NANOS3 combination was significantly greater than the repression observed with either PUM2 or NANOS3 alone (Fig. 1c, right bottom panel). This combined repressive effect may occur as a result of interplay between PUM2 and NANOS3 or may represent an additive effect from independent actions. No changes in luciferase expression were observed upon co-transfection of a luciferase reporter construct carrying the complete 3′UTR of GAPDH mRNA which contains no PBEs and PUM-expressing constructs (Fig. S4).
PUM proteins are required for NANOS-mediated regulation of SIAH1
Since repression of SIAH1 3′UTR-dependent luciferase activity was observed with NANOS alone (Fig. 1c), we tested whether PUM is required for NANOS-mediated effects on SIAH1. To this end, we performed siRNA-mediated knockdown of PUM1 and/or PUM2 (Fig. 1d). Knockdown of PUM1 or PUM2 individually did not affect repression of luciferase expression by NANOS1, NANOS2, or NANOS3 (Fig. 1e). However, knockdown of both PUM1 and PUM2 eliminated NANOS-mediated repression of luciferase expression (Fig. 1e), indicating that PUM1 and PUM2 play critical and redundant roles in NANOS-mediated regulation of SIAH1.
PUM1 and PUM2 co-immunoprecipitate with SIAH1 mRNA in HEK293FT cells
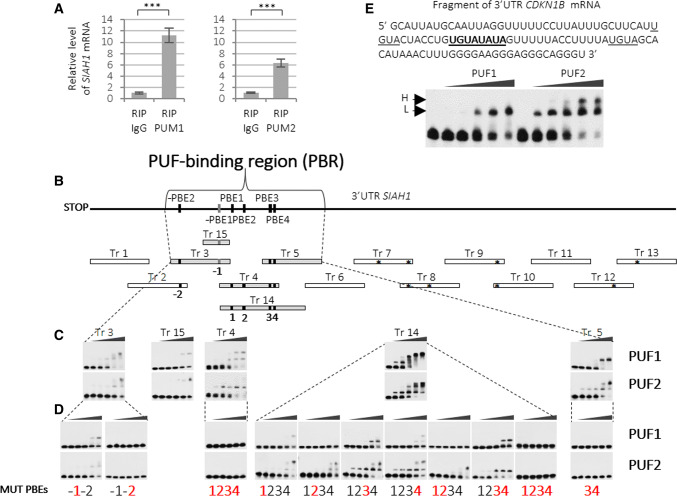
To assess whether PUM-mediated regulation of SIAH1 occurred via binding to the mRNA, we tested for the enrichment of SIAH1 mRNA in anti-PUM1 and anti-PUM2 immunoprecipitates from HEK293FT cell lysates by RIP (RNA immunoprecipitation) assay (Fig. S5). As measured by quantitative real-time RT-PCR, we found that SIAH1 mRNA was significantly enriched in both anti-PUM1 and anti-PUM2 immunoprecipitates (Fig. 2a). These results confirm that both PUM1 and PUM2 associate with and may bind directly to SIAH1 mRNA.
Fig. 2.
Identification of PUF1 and PUF2-binding region in SIAH1 3′UTR. a Results of real-time RT-qPCR showing enrichment of endogenous SIAH1 mRNA in anti-PUM1 or anti-PUM2 immunoprecipitates in HEK293FT cells. β-ACTIN and GAPDH mRNAs were used for normalization and represent 0 level. Nonimmune serum (IgG) was used as a negative control (RIP RNA immunoprecipitation). Error bars denote standard deviation (n = 9, P < 0.005). Western blot for RIP reaction is shown in Fig. S5. b Scheme of strategy used for the identification of specific PUF1 and PUF2-binding regions in SIAH1 3′UTR. Transcripts (Tr) identified as specifically binding PUF1 and PUF2 domains are shown in grey bars. EMSA-tested transcripts are marked at the top with asterisks denoting the presence of UGUA tetranucleotide (considered to be the core of the PBE motif). Motifs of PUM-binding elements −PBE2 and PBE2–4 on the diagram of SIAH1 3′UTR are shown in black squares, whereas, −PBE1, the only one identical to the PBE UGUANAUA consensus, is shown in dark grey. All PBEs are numbered (− 1 to 4) below each specifically binding fragment. c EMSA results of PUF1 and PUF2 domains specifically binding SIAH1 3′UTR fragments (PUF1—upper panels; PUF2—lower panels). The first lane in each EMSA result contains the transcript only and is followed by lanes containing increasing concentrations of PUF1 or PUF2-domains d EMSA analysis of transcripts with mutated PBE-like motifs (upper panels—PUF1 added; lower panels—PUF2 added). Mutated PBE motifs numbered in red bold. e EMSA analysis showing PUF1 and PUF2 domains binding the CDKN1B 3′UTR. The same protein concentration was used for both domains. Sequence of CDKN1B 3′UTR transcript used for EMSA with both PUF1 and PUF2 domains is presented at the top. PBE motif is in bold and underlined, and two core consensus sequences UGUA are underlined. Lower (L) and higher (H) complexes formed are indicated. The first lane of each EMSA panel indicates naked transcript, whereas the other lanes denote increasing concentrations of PUF1 or PUF2 domains added to the binding reaction (20, 50, 100, 300, and 500 nM)
PUF1 and PUF2 domains exhibit different recognition patterns for SIAH1 PBE motifs
Since both PUM paralogues downregulated endogenous SIAH1 expression and SIAH1 3′UTR-dependent luciferase expression, we used electrophoretic mobility shit analysis (EMSA) to examine if the PUF1 and PUF2 domains of PUM1 and PUM2, respectively, could bind to the 3′UTR of SIAH1. To the best of our knowledge, no study has compared the binding of PUF1 and PUF2 domains to the same mRNA target so far. Given that the whole 3′UTR of SIAH1 contains the following motifs: −PBE2-like, −PBE1-consensus, PBE1-like, PBE2-like, PBE3-like, and PBE4-like, we subjected all of the motifs to EMSA to delimit specific PUF1- and PUF2-binding regions. For this purpose, we generated 15 overlapping transcripts (Tr), encompassing the 3′UTR of SIAH1, each approximately 100 nt in length (Fig. S6), and analysed them for PUF1- and PUF2-domain binding using identical reaction conditions. Any Tr that demonstrated a mobility shift at PUF domain concentrations of up to 200 nM was further confirmed using the EMSA competition assay. Using this approach, we defined a PUF-binding region (PBR) localized between 144 and 379 nt of 3′UTR, comprising Tr 3, Tr 4, and Tr 5 and overlapping with Tr 14 and Tr 15 (Fig. 2b, grey boxes).
Although the PBR of SIAH1 was bound by both PUF domains, the number of complexes formed with the three Tr was different between PUF1 and PUF2, as indicated by the number of shifted bands for Tr 4 (PUF1—two; PUF2—one), Tr 5 (PUF1—one; PUF2—two), and Tr 14 (PUF1—one; PUF2—two, but only at higher protein concentrations) (Fig. 2c). Furthermore, we analysed a series of substitution mutants of different PBE/PBE-like motifs (Table S1; Fig. S7) to identify which PBE/PBE-like motifs were essential for PUF1 and PUF2 binding, and to identify any differences between PUF1 and PUF2. We found that, unexpectedly, the PBE-like motifs that were recognized by PUF1 were different from those recognized by PUF2. In particular, the analysis of Tr 14 demonstrated that each of the PBE1–4-like motifs, when removed by substitutions, caused a stronger decrease on PUF1- than on PUF2-domain binding (PBE1, PBE3, and PBE4) or a lack of only PUF1-domain binding (PBE2) (Fig. 2d).
We then sought to test whether the differences in SIAH1 PBE recognition between PUF1 and PUF2 also existed for another known PUM mRNA target, CDKN1B, which has been described as being regulated by both PUM1 and PUM2 [26]. Notably, direct binding of the PUF1 and PUF2 domains to CDKN1B mRNA has not been demonstrated so far. For this purpose, we used a CDKN1B 3′UTR fragment-containing only one classical PBE motif (UGUAUAUA) [12] and found that, even at a high PUF domain concentration, PUF1 formed only one complex with this target, whereas PUF2 formed two complexes (Fig. 2e). It is noteworthy, however, that mutation of the −PBE1-consensus motif in Tr3 (Fig. 2d, bottom left) appears to change the gel shift from two bands to one for both PUF1 and PUF2. Similarly, both PUF1 and PUF2 showed no difference in binding SIAH1 PBR Tr 15 (Fig. 2c), which contains −PBE1 consensus (Fig. 2b, grey motif), identical to the above CDKN1B PBE (UGUAUAUA) (Fig. S1b; Fig. 2e). Thus, given that the Tr 15 and the CDKN1B 3′UTR fragments used for EMSA only differed in the regions flanking the UGUAUAUA motif, it seems likely that these flanking regions contribute to recognition specificity, which may differ for the PUF1 and PUF2 domains. Taken together, these results indicate that the mode of recognition and binding preferences to SIAH1 3′UTR differ between PUF1 and PUF2 domains.
PUM2-mediated but not PUM1-mediated regulation of SIAH1 is dependent on PBE-like motifs
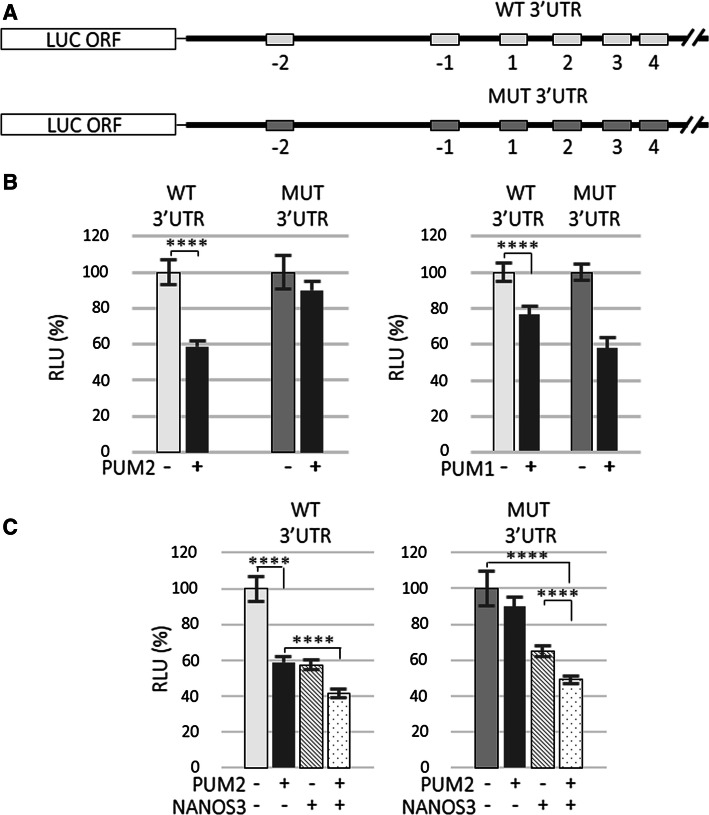
Having established that PUF domains demonstrate a certain degree of specificity in recognizing PBE motifs on the 3′UTR of SIAH1 (Fig. 2), the next step would be to examine how the PUF–PBE interaction influences the regulation of SIAH1. It is well established that the specific recognition of PBEs by the PUF domain is a prerequisite for posttranscriptional regulation by PUM proteins [17]. To investigate the importance of such PBE recognition for the regulation of SIAH1, we used a luciferase reporter upstream of SIAH1 3′UTR with wild-type or mutated PBEs generated by site-directed mutagenesis, exactly the same as in EMSA (Table S5; Fig. S7). We generated reporter construct harbouring mutated PBEs, MUT (Fig. 3a). In the MUT construct, all identified PBE-like motifs, −PBE2 (which exclusively contains a UGUA core) and PBEs − 1 up to 4 plus −PBE1 consensus (Fig. S1b and S7) were mutated. SIAH1 3′UTR-dependent luciferase expression in wild-type and mutant PBE-harbouring constructs was examined upon PUM1 or PUM2 overexpression in HEK293FT cells. As expected, PUM2-mediated repression of luciferase expression was completely eliminated with the MUT construct, but, unexpectedly, PUM1-mediated repression was not relieved (Fig. 3b). These results provide strong in vitro evidence that PUM2 regulates SIAH1 in a PBE-dependent manner, whereas PUM1 regulates it independently of the identified six PBE/PBE-like (−PBE2 to PBE4) motifs.
Fig. 3.
Mutation of PBE motifs in 3′UTR causes reversal of PUM2- but not PUM1-mediated repression of SIAH1 mRNA. a Schematic diagram of the luciferase constructs used. Grey-shaded boxes refer to mutated motifs (−PBE2 and −PBE1 consensus; PBE1, PBE2, PBE3, and PBE4). b Effects of PUM1 and PUM2 overexpression on reporter luciferase construct carrying wild-type (WT) or (MUT) lacking six PBE/PBEs-like in SIAH1 full-length 3′UTR. Derepression of the SIAH1 3′UTR reporter carrying mutated PBE occurs only under PUM2 overexpression. c Effects of PUM2, NANOS3, or PUM2/NANOS3 overexpression on luciferase reporter carrying wild-type or mutated full-length SIAH1 3′UTR (****P < 0.001). Error bars denote standard deviation (n = 9). Luminescence values (RLU relative luciferase units) are presented as % of the RLU of the sample transfected with reporter construct only which was set to 100%. Renilla luciferase values were normalized using firefly luciferase measurements
Since PUM2 and NANOS3 may cooperate in regulating SIAH1 (Fig. 1b), we sought to test whether SIAH1 regulation in the presence of PUM2 and NANOS3 is PBE-dependent. In contrast to the complete derepression of MUT luciferase expression seen when PUM2 was expressed alone, only partial derepression was observed when NANOS3 was expressed alone or in combination with PUM2 (Fig. 3c). Since PUM2-mediated regulation of SIAH1 is dependent on PBE binding, these results indicate that NANOS3-mediated regulation of SIAH1 is partially independent of PUM2 and may involve endogenous PUM1 which could compensate for the loss of PUM2 functions (Fig. 1d).
NANOS3 directly binds SIAH1 RNA and forms ribonucleoprotein complexes with PUF1 and PUF2 domains in vitro
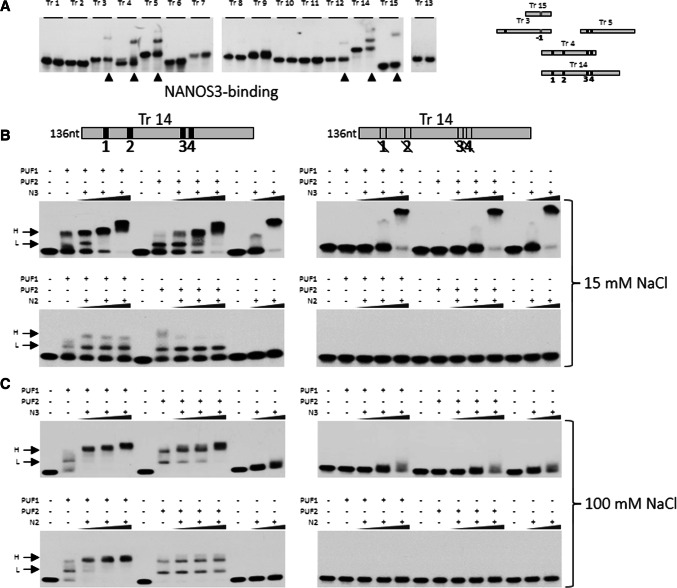
Although the functional relationship between PUM and NANOS is reasonably well established in organisms such as D. melanogaster [2, 18], the mechanisms underlying PUM–NANOS cooperation are poorly understood in mammals. In addition, although, in Drosophila, Nanos has been suggested to bind to the 3′UTR of hb [27], this had not been experimentally confirmed [23], except a simple filter-binding assay [27]. Since NANOS3 and PUM2 may cooperate in regulating SIAH1 (Fig. 1c, lower right graph), we used EMSA to investigate the binding of NANOS3 to all 15 SIAH1 3′UTR transcript fragments (Fig. 2b). We found that NANOS3 could bind to the 3′UTR of SIAH1 around PBE1–4 represented by Tr 14 which overlaps with NREs and this binding was PUF-domain-independent and limited to the PBR region (Tr 3, 4, 5, 14, and 15) (Fig. 4a). However, a faint retardation band was also seen with Tr 12 that is outside of the PBR, but not with any other tested Tr fragments (Fig. 4a). NANOS3 binding to SIAH1 RNA, however, required a high (10 μM) protein concentration (see “Methods” section) and was stronger at 15 mM than at 100 mM salt concentrations (Fig. 4b, c, upper left panels). To test for dependence of NANOS on PBE for binding, we chose Tr 14, since it contains the highest number of PBE motifs and significantly overlaps with Tr 4 and Tr 5, which also bind NANOS3 (Fig. 4a). A mutated Tr 14 lacking all four PBEs completely disabled PUF1 and PUF2 domain binding, only slightly disabled binding of NANOS3 at a lower concentration and had no effect on binding of NANOS3 at a higher concentration (Fig. 4b, upper right panel). This result shows some limited dependence of NANOS3 on PBE for binding and thus limited sequence specificity. Importantly, NANOS3 boosted higher complex (H) formation with PUF1 and PUF2 domains when bound to Tr 14 at both 15 mM and 100 mM salt concentrations (Fig. 4b, c, upper left panels).
Fig. 4.
NANOS2 and NANOS3 bind to SIAH1 3′UTR and form complexes with PUF domains. a EMSA analysis of all the SIAH1 transcripts for testing binding with NANOS3. Numbers correspond to SIAH1 transcripts; the left lane represents migration of the naked RNA of each fragment, whereas the right lane represents its migration in the presence of NANOS3 protein. Lanes showing retardation bands are indicated with an arrow. b EMSA analysis of SIAH1 3′UTR transcript 14 (Tr 14, wild-type or mutated) for binding PUF1 or PUF2 domain alone or in combination with NANOS3 or NANOS2 in 15 mM NaCl. NANOS proteins were used at a concentration of 5–15 μM, whereas PUF1 and PUF2 were used at 100 nM. Wild-type Tr 14 is on the left and mutated Tr 14 is on the right. c EMSA analysis according to the same scheme as b, but performed at a higher NaCl concentration (100 mM). H higher complex; L lower complex
Interestingly, we also found that, unlike NANOS3, NANOS2 does not bind to Tr 14 alone, neither at 15 mM nor 100 mM salt concentrations. Moreover, the boosting of EMSA higher complex (H) formation with PUF1 and PUF2 induced by NANOS3 was observed for NANOS2 with PUF1 but not with PUF2 (Fig. 4b, c, lower left panels). Due to technical difficulties in obtaining good-quality NANOS1 fusion protein by bacterial overexpression, EMSA analysis could not be performed for NANOS1.
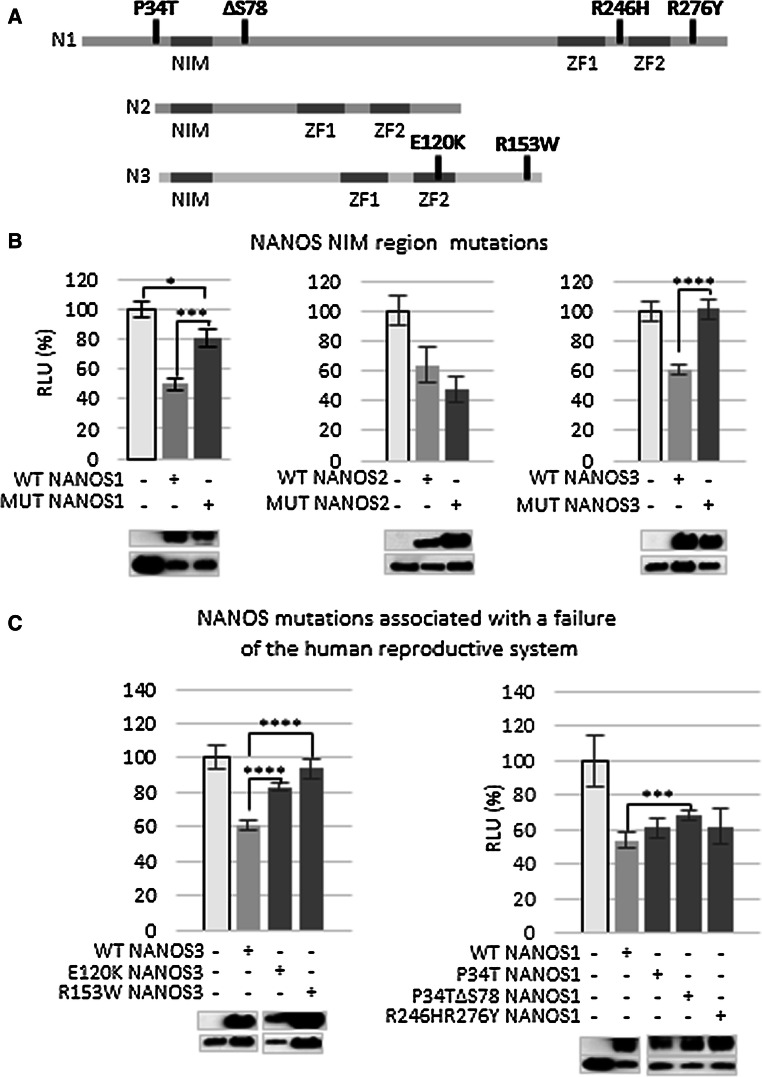
NIM region is important in NANOS1- and NANOS3-mediated SIAH1 repression
Since NIM has been identified to be essential for human NANOS functions [16], we examined the importance of NIM in NANOS-mediated regulation of SIAH1. The NIM region is located in N-terminal region of NANOS proteins (Fig. 5a). The NIM region was found to be sufficient for some functions of NANOS2 and 3, whereas, in NANOS1, additional residues are required [16]. Therefore, it was of interest to check whether a difference between NANOS1 and other paralogues in NIM requirements for SIAH1 regulation existed. To examine the NIM region’s role in each NANOS paralogue for SIAH1 mRNA repression, we substituted key amino acid residues within NIM (three for NANOS1 and NANOS3 and two for NANOS2), as previously described [16] (Table S6). These substitutions have been reported to disrupt the binding of the NIM region to NOT1, a CCR4-NOT deadenylase complex component [16]. The effect of the NIM disruptions on a luciferase reporter upstream of the SIAH1 3′UTR was examined in HEK293FT cells (Fig. 5b; Fig. S8). We found that NIM disruption of NANOS3 caused complete derepression (Fig. 5b, right panel) of the SIAH1 3′UTR-dependent luciferase expression, which is in concordance with the previous observations [16]. NIM disruption of NANOS1 resulted in strong, but incomplete derepression of the reporter (Fig. 5b, left panel), which is also in agreement with previously published data [16], showing the importance of N-terminal residues beyond NIM for repression by this paralogue. Unexpectedly, NIM disruption in the NANOS2 NIM mutant had no significant effect on the repression of the SIAH1-luciferase reporter (Fig. 5b, middle panel).
Fig. 5.
Influence of NANOS gene mutations on SIAH1 mRNA regulation. a Mutations related to human infertility in NANOS proteins are schematically depicted. NIM and zinc-finger domains are indicated as dark rectangles, and mutations identified in patients manifesting reproductive system failure are presented in bold. b Influence of NANOS NIM region mutations on expression of luciferase reporter carrying SIAH1 3′UTR. c Effects of mutated NANOS proteins (from patients diagnosed with failure of the reproductive system) on expression of luciferase reporter carrying SIAH1 3′UTR (P < 0.05, P < 0.005, P < 0.001). Error bars denote standard deviation (n = 9). Luminescence values (RLU relative luciferase units) are presented as % relative to the sample transfected with the reporter construct only which was set to 100%
NANOS mutations associated with human infertility point to NANOS protein regions that are important for repression
As NANOS proteins play important roles in the reproduction of many organisms up to mammals [4, 28, 29], including humans [30], we sought to use a panel of human NANOS mutations (Fig. S8) previously described as being associated with infertility [20–22, 31–33] to probe for regions other than NIM that may be important in regulation of SIAH1. It is important to note that an SIAH1 3′UTR-based reporter was used in this study primarily as a model for functional analysis of NANOS; we do not intend to propose a putative role for SIAH1 in human reproduction, although such an involvement could be addressed in the future.
First, we tested the NANOS3 protein carrying an E120K amino acid substitution located in the second zinc finger (ZF2) of the RNA-binding domain (Fig. 5a). The glutamic acid at position 120 of NANOS3 protein is conserved among mammals, and this mutation has been associated with premature ovarian failure [21]. We found that this amino acid substitution caused a significant derepression of the SIAH1-luciferase reporter (Fig. 5c, left panel), confirming the importance of the ZF2 domain for NANOS3-mediated regulation and presumably in vivo functionality.
We next examined the effect of an R153W amino acid substitution located at the very end of the NANOS3 C-terminal region (Fig. 5a). This mutation destabilizes protein structure and promotes aggregate formation, thus making the NANOS3 protein less soluble [22]. The R153W mutation was identified in association with premature ovarian failure in a patient who was a heterozygote, i.e., carried a second wild-type allele. We observed full derepression of the reporter with this construct (Fig. 5c, left panel) [22].
For NANOS1, we examined the influence of the ΔS78 mutation that is located in the central region, but beyond NIM (Fig. 5a). This mutation, which was found in a patient with a lack of germ cells in his seminiferous tubules, coexists on the same allele with P34T which is considered to be a neutral mutation as it is frequently found in the general population [20]. Interestingly, we demonstrated that an NANOS1 construct carrying the P34T/ΔS78 allele caused partial derepression, whereas an NANOS1 construct carrying only P34T did not affect repression of the SIAH1-luciferase reporter (Fig. 5c, right panel). Thus, the NANOS1 central region, which is located a short distance downstream from NIM, appears to contribute to the regulation of SIAH1.
Finally, we examined an NANOS1 protein carrying a double R246H/R276Y substitution. The R246H substitution is located between the first and second zinc finger, and R276Y is located beyond the second zinc finger; both substitutions are located towards the C-terminal (Fig. 5a). The R246H/R276Y allele was identified in an infertile man carrying a second wild-type allele [20]. As shown here, however, this substitution had no effect on SIAH1-luciferase reporter expression (Fig. 5c, right panel). These mutations, which are located at sites flanking the ZF1 and ZF2 region, may be less important for NANOS1 function, or may be important in the regulation of other mRNAs.
Discussion
Although the general process whereby PUM recognizes individual mRNAs has been studied extensively, the particulars of the mechanism underlying PUM–NANOS cooperation in mRNA regulation and the functional overlap among PUM and NANOS paralogues in mammals have not been elucidated. It was only shown that NANOS1 co-immunoprecipitates with PUM2 in transfected cells [30] and the murine PUM2 binds NANOS3 in vitro [34]. To the best of our knowledge, this is the first study that aimed to reveal the differences between the repression mechanisms of PUM1 and PUM2 in the context of one specific human mRNA target. Given their very high structural similarity, it has been accepted that mammalian PUM1 and PUM2 are equivalent in vivo in terms of mRNA recognition and regulation, leading to functional redundancy, despite the fact that structural alignments of PUM1 and PUM2 PUF domains reveal a subtle difference in their overall curvatures [17]. The previous studies demonstrated PUM1 and PUM2 redundancy using a short artificial luciferase reporter-containing an array of three PBEs which was then confirmed by EMSA [35]. By contrast, in the present study, we used a 1-kb full-length SIAH1 3′UTR that is likely to be more physiologically relevant, and found that PUM1 and PUM2 act differently in RNA binding and regulation. Specifically, we showed that the EMSA pattern of SIAH1 3′UTR recognition was reproducibly different for the PUF1 and PUF2 domains even with several independent protein preparations (Fig. 2c, d). Moreover, dependence on PBEs, as tested in cells using luciferase reporters, was an important discriminating factor that was observed for only PUM2-, but not PUM1-mediated repression (Fig. 3b, right panel). This finding indicates that, at least in the case of SIAH1 regulation, PUM1 has repressive ability that does not require the presence of PBEs in the target 3′UTR, whereas PUM2 does not have this ability (Fig. 3c). This leads us to speculate that the N-terminal region of PUM1, which is known to have repressive activity in the absence of the PUF domain, although not shown to bind RNA [10], may be involved in the regulation of SIAH1.
We also provide evidence here that the flanking regions strongly contribute to differences in RNA recognition between the PUF1 and PUF2 domains, as shown by EMSA. Indeed, the only difference between Tr 15 and the CDKN1B 3′UTR fragments is the regions flanking the UGUAUAUA motif; hence, they seem to be responsible for differences in EMSA complexes between PUF1 and PUF2 (Fig. 2c). Therefore, as one of the possible explanations, it seems plausible that flanking regions, in addition to PBEs, may play a more significant role for PUM1-mediated recognition and repression of this target RNA, which would be consistent with the strikingly different effects of PUM1 (no derepression) and PUM2 (full derepression) on the PBE-mutated SIAH1 luciferase reporter (Fig. 3b).
To the best of our knowledge, this is also the first study that aimed to uncover mechanistic differences between the three NANOS mammalian paralogues. Although mouse genetic models have revealed that NANOS3 is required for the early development of primordial germ cells and NANOS2 is required for spermatogonial development [29], the underlying mechanistic reasons for the functional differences have not been elucidated [29]. First, although cooperation between NANOS and PUM in mRNA repression was studied extensively in Drosophila and potential NANOS RNA targets were published [36], it has not been demonstrated that NANOS directly binds RNA in vitro, except a simple filter-binding assay [27]. Here, using a more precise assay as EMSA, we demonstrate, for the first time, that NANOS3, indeed, directly binds RNA, although high protein concentration was required for binding (Fig. 4a, left upper panel). Under the same conditions, NANOS2 was unable to bind to RNA on its own. According to the studies in D. melanogaster, NANOS binding to RNA if present was considered to be nonspecific [27]. Although binding specificity was not directly tested in this study, we found that NANOS3 binding was limited to mainly the PBR region (Fig. 4a, right panel). Given that, at higher protein concentrations, NANOS3 binding to PBR was PBE-independent (Fig. 4b, upper left versus right EMSA panels), there are possibly other motifs and/or structures in the PBR region that account for NANOS3 binding. Moreover, the fact that repression of SIAH1 3′UTR-dependent luciferase expression was stronger under PUM2/NANOS3 co-expression than under PUM2 or NANOS3 expression points to their combined effect in the repression of SIAH1 mRNA. This combined effect of PUM2/NANOS3 co-expression on PBE-mutated reporters also points to the NANOS3 repressive effect being mostly independent of the PBE-binding requirement of PUM2 (Fig. 3c), whereas, in regard to receiving indispensable cooperation from one of the PUMs (Fig. 1c), NANOS3 likely relies on a PUM2 interaction with endogenous PUM1 (Fig. S9), which retains its repressive capacity on the PBE-mutated reporter (Fig. 3b). Such an additive effect, which was absent with any other co-expression, especially co-expression of PUM2 with NANOS1 or NANOS2, also indicates functional differences between NANOS3 and its paralogues. The weak derepression of the luciferase reporter with mutated PBEs in the presence of NANOS3 confirms that NANOS3-induced repression is mostly independent of NANOS3 binding to PBEs (Fig. 3c). Since we were unable to silence NANOS1, the only one among NANOS paralogues that is expressed in HEK293FT cells, we could not discriminate whether PUM proteins require the presence of NANOS protein to repress SIAH1 target.
Second, it has been recently reported that mammalian NANOS-induced repression is followed by the deadenylation and degradation of target mRNA. This mechanism involves recruitment by NANOS of the CNOT1 component of CCR4-NOT deadenylase complex [37]. In our study, however, repression of SIAH1 mRNA does not lead to its degradation (Fig. 1a) which is in line with some previous reports, e.g., by Chekulaeva and coauthors 2011 that CNOT1-mediated repression may not result in a decrease in mRNA level [38]. More recently, it was reported that many translationally repressed mRNAs are stored within P-bodies and do not undergo a decay [39]. The recruitment of CNOT1 is mediated by the conserved NIM region of each mammalian and human NANOS1, NANOS2 and NANOS3 that serves as an interface for binding the CNOT1 component of the CCR4–NOT deadenylase complex [16]. Importantly, the NIM region of mouse NANOS1, 2, and 3 and that of human NANOS1, 2, and 3 have been shown to be necessary for the repression of a beta-globin reporter-containing six MS2-binding sites in the 3′ untranslated region tethered to MS2-tagged NANOS protein; this reporter construct enabled the study of NANOS independently of PUM [16]. Our results show that, in the context of SIAH1 mRNA, when NANOS is not tethered to the RNA reporter, the NIM region is, similarly, important for NANOS1- and NANOS3-mediated repression (Fig. 5b). While there was no statistically significant effect on SIAH1 mRNA repression in response to mutations in the NIM region of NANOS2, NANOS proteins could interact with additional protein cofactors, including other RBPs to mediate NIM-independent mRNA repression.
Conclusions
The findings presented in this paper highlight the mechanistic versatility of the PUM/NANOS machinery in posttranscriptional regulation as tested in the context of SIAH1 mRNA regulation. The PUM paralogues use different mechanisms, as evidenced by the different recognition profiles of the PUF1 and PUF2 domains in RNA binding (as shown in vitro) and different requirements for PBEs (as shown in cells). This versatility reflects the very divergent structure of the N-terminal/central region among NANOS paralogues (beyond NIM), which contrasts with the highly conserved zinc-finger C-terminal domain. It is possible that this structural variability enables the interaction of NANOS paralogues with different sets of protein cofactors to fine tune the repression of their targets or to provide alternative mechanisms of regulation (e.g., NIM-independent mechanism in the case of NANOS2). Importantly, this study indicates that the PUM/NANOS system may have some bearing on human reproductive health, as the NANOS1 and NANOS3 mutations associated with infertility altered repression of SIAH1; thus, it would be exciting to investigate how such a versatile system globally orchestrates the expression of multiple mRNAs across different stages of germ cell development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 20813 kb)
Acknowledgements
This work was supported by the National Science Center Poland (Grant no. 2011/01/B/NZ2/04833 to BGM and ETIUDA scholarship no. 2014/12/T/NZ1/00497 to MS) and Ministry of Science and Higher Education (Grant no. N N401318439 to JJ). We thank Dr. Witold Filipowicz, Dr. Thomas Tuschl, Dr. Damian Brauze, Christine Rickards-Rostworowska, and Dr. Miroslawa Siatecka for the helpful discussions and for commenting on the manuscript.
Author contributions
MS conducted the experiments and prepared the figures, DMJ conducted the experiments and prepared the figures, MJS conducted the experiments, BGM analysed the results, AS conducted the experiments, SO performed the bioinformatic search for PUM mRNA targets, EI conducted experiments, KKZ conducted the experiments, MK conducted the experiments and prepared the manuscript, JB designed software to bioinformatically select PUM mRNA targets, and JJ conceived the experiments and prepared the manuscript. All authors reviewed the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interest.
Contributor Information
Barbara Ginter-Matuszewska, Email: bginter@man.poznan.pl.
Jadwiga Jaruzelska, Email: jadwiga.jaruzelska@igcz.poznan.pl.
References
- 1.Murata Y, Wharton RP. Binding of pumilio to maternal hunchback mRNA is required for posterior patterning in Drosophila embryos. Cell. 1995;80:747–756. doi: 10.1016/0092-8674(95)90353-4. [DOI] [PubMed] [Google Scholar]
- 2.Wharton RP, Struhl G. RNA regulatory elements mediate control of Drosophila body pattern by the posterior morphogen nanos. Cell. 1991;67:955–967. doi: 10.1016/0092-8674(91)90368-9. [DOI] [PubMed] [Google Scholar]
- 3.Ye B, Petritsch C, Clark IE, Gavis ER, Jan LY, Jan YN. Nanos and Pumilio are essential for dendrite morphogenesis in Drosophila peripheral neurons. Curr Biol. 2004;14:314–321. doi: 10.1016/j.cub.2004.01.052. [DOI] [PubMed] [Google Scholar]
- 4.Asaoka-Taguchi M, Yamada M, Nakamura A, Hanyu K, Kobayashi S. Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nat Cell Biol. 1999;1:431–437. doi: 10.1038/15666. [DOI] [PubMed] [Google Scholar]
- 5.Kadyrova LY, Habara Y, Lee TH, Wharton RP. Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline. Development. 2007;134:1519–1527. doi: 10.1242/dev.002212. [DOI] [PubMed] [Google Scholar]
- 6.Wickens M, Bernstein DS, Kimble J, Parker R. A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet. 2002;18:150–157. doi: 10.1016/S0168-9525(01)02616-6. [DOI] [PubMed] [Google Scholar]
- 7.Dalby B, Glover DM. Discrete sequence elements control posterior pole accumulation and translational repression of maternal cyclin B RNA in Drosophila. EMBO J. 1993;12:1219–1227. doi: 10.1002/j.1460-2075.1993.tb05763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamore PD, Bartel DP, Lehmann R, Williamson JR. The PUMILIO-RNA interaction: a single RNA-binding domain monomer recognizes a bipartite target sequence. Biochemistry. 1999;38:596–604. doi: 10.1021/bi982264s. [DOI] [PubMed] [Google Scholar]
- 9.Zamore PD, Williamson JR, Lehmann R. The Pumilio protein binds RNA through a conserved domain that defines a new class of RNA-binding proteins. RNA. 1997;3:1421–1433. [PMC free article] [PubMed] [Google Scholar]
- 10.Weidmann CA, Goldstrohm AC. Drosophila Pumilio protein contains multiple autonomous repression domains that regulate mRNAs independently of Nanos and brain tumor. Mol Cell Biol. 2012;32:527–540. doi: 10.1128/MCB.06052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spassov DS, Jurecic R. The PUF family of RNA-binding proteins: does evolutionarily conserved structure equal conserved function? IUBMB Life. 2003;55:359–366. doi: 10.1080/15216540310001603093. [DOI] [PubMed] [Google Scholar]
- 12.Galgano A, Forrer M, Jaskiewicz L, Kanitz A, Zavolan M, Gerber AP. Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PLoS One. 2008;3:e3164. doi: 10.1371/journal.pone.0003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet. 2014;15:829–845. doi: 10.1038/nrg3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quenault T, Lithgow T, Traven A. PUF proteins: repression, activation and mRNA localization. Trends Cell Biol. 2011;21:104–112. doi: 10.1016/j.tcb.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 15.De Keuckelaere E, Hulpiau P, Saeys Y, Berx G, van Roy F. Nanos genes and their role in development and beyond. Cell Mol Life Sci. 2018;75:1929–1946. doi: 10.1007/s00018-018-2766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhandari D, Raisch T, Weichenrieder O, Jonas S, Izaurralde E. Structural basis for the Nanos-mediated recruitment of the CCR4–NOT complex and translational repression. Genes Dev. 2014;28:888–901. doi: 10.1101/gad.237289.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu G, Hall TM. Alternate modes of cognate RNA recognition by human PUMILIO proteins. Structure. 2011;19:361–367. doi: 10.1016/j.str.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonoda J, Wharton RP. Recruitment of Nanos to hunchback mRNA by Pumilio. Genes Dev. 1999;13:2704–2712. doi: 10.1101/gad.13.20.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spik A, Oczkowski S, Olszak A, Formanowicz P, Blazewicz J, Jaruzelska J. Human fertility protein PUMILIO2 interacts in vitro with testis mRNA encoding Cdc42 effector 3 (CEP3) Reprod Biol. 2006;6:103–113. [PubMed] [Google Scholar]
- 20.Kusz-Zamelczyk K, Sajek M, Spik A, Glazar R, Jedrzejczak P, Latos-Bielenska A, Kotecki M, Pawelczyk L, Jaruzelska J. Mutations of NANOS1, a human homologue of the Drosophila morphogen, are associated with a lack of germ cells in testes or severe oligo-astheno-teratozoospermia. J Med Genet. 2013;50:187–193. doi: 10.1136/jmedgenet-2012-101230. [DOI] [PubMed] [Google Scholar]
- 21.Santos MG, Machado AZ, Martins CN, Domenice S, Costa EM, Nishi MY, Ferraz-de-Souza B, Jorge SA, Pereira CA, Soardi FC, de Mello MP, Maciel-Guerra AT, Guerra-Junior G, Mendonca BB. Homozygous inactivating mutation in NANOS3 in two sisters with primary ovarian insufficiency. Biomed Res Int. 2014;2014:787465. doi: 10.1155/2014/787465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Wang B, Dong Z, Zhou S, Liu Z, Shi G, Cao Y, Xu Y. A NANOS3 mutation linked to protein degradation causes premature ovarian insufficiency. Cell Death Dis. 2013;4:e825. doi: 10.1038/cddis.2013.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weidmann CA, Qiu C, Arvola RM, Lou TF, Killingsworth J, Campbell ZT, Tanaka Hall TM, Goldstrohm AC. Drosophila Nanos acts as a molecular clamp that modulates the RNA-binding and repression activities of Pumilio. Elife. 2016 doi: 10.7554/eLife.17096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorick KL, Jensen JP, Fang S, Ong AM, Hatakeyama S, Weissman AM. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci USA. 1999;96:11364–11369. doi: 10.1073/pnas.96.20.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris AR, Mukherjee N, Keene JD. Ribonomic analysis of human Pum1 reveals cis-trans conservation across species despite evolution of diverse mRNA target sets. Mol Cell Biol. 2008;28:4093–4103. doi: 10.1128/MCB.00155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kedde M, van Kouwenhove M, Zwart W, Oude Vrielink JA, Elkon R, Agami R. A Pumilio-induced RNA structure switch in p27-3′ UTR controls miR-221 and miR-222 accessibility. Nat Cell Biol. 2010;12:1014–1020. doi: 10.1038/ncb2105. [DOI] [PubMed] [Google Scholar]
- 27.Curtis D, Treiber DK, Tao F, Zamore PD, Williamson JR, Lehmann R. A CCHC metal-binding domain in Nanos is essential for translational regulation. EMBO J. 1997;16:834–843. doi: 10.1093/emboj/16.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraemer B, Crittenden S, Gallegos M, Moulder G, Barstead R, Kimble J, Wickens M. NANOS-3 and FBF proteins physically interact to control the sperm-oocyte switch in Caenorhabditis elegans. Curr Biol. 1999;9:1009–1018. doi: 10.1016/S0960-9822(99)80449-7. [DOI] [PubMed] [Google Scholar]
- 29.Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y. Conserved role of nanos proteins in germ cell development. Science. 2003;301:1239–1241. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- 30.Jaruzelska J, Kotecki M, Kusz K, Spik A, Firpo M, Reijo Pera RA. Conservation of a Pumilio-Nanos complex from Drosophila germ plasm to human germ cells. Dev Genes Evol. 2003;213:120–126. doi: 10.1007/s00427-003-0303-2. [DOI] [PubMed] [Google Scholar]
- 31.Kusz K, Tomczyk L, Spik A, Latos-Bielenska A, Jedrzejczak P, Pawelczyk L, Jaruzelska J. NANOS3 gene mutations in men with isolated sterility phenotype. Mol Reprod Dev. 2009;76:804. doi: 10.1002/mrd.21070. [DOI] [PubMed] [Google Scholar]
- 32.Kusz KM, Tomczyk L, Sajek M, Spik A, Latos-Bielenska A, Jedrzejczak P, Pawelczyk L, Jaruzelska J. The highly conserved NANOS2 protein: testis-specific expression and significance for the human male reproduction. Mol Hum Reprod. 2009;15:165–171. doi: 10.1093/molehr/gap003. [DOI] [PubMed] [Google Scholar]
- 33.Julaton VT, Reijo Pera RA. NANOS3 function in human germ cell development. Hum Mol Genet. 2011;20:2238–2250. doi: 10.1093/hmg/ddr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lolicato F, Marino R, Paronetto MP, Pellegrini M, Dolci S, Geremia R, Grimaldi P. Potential role of Nanos3 in maintaining the undifferentiated spermatogonia population. Dev Biol. 2008;313:725–738. doi: 10.1016/j.ydbio.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Van Etten J, Schagat TL, Hrit J, Weidmann CA, Brumbaugh J, Coon JJ, Goldstrohm AC. Human Pumilio proteins recruit multiple deadenylases to efficiently repress messenger RNAs. J Biol Chem. 2012;287:36370–36383. doi: 10.1074/jbc.M112.373522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato Y, Katsuki T, Kokubo H, Masuda A, Saga Y. Dazl is a target RNA suppressed by mammalian NANOS2 in sexually differentiating male germ cells. Nat Commun. 2016;7:11272. doi: 10.1038/ncomms11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki A, Igarashi K, Aisaki K, Kanno J, Saga Y. NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc Natl Acad Sci USA. 2010;107:3594–3599. doi: 10.1073/pnas.0908664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chekulaeva M, Mathys H, Zipprich JT, Attig J, Colic M, Parker R, Filipowicz W. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat Struct Mol Biol. 2011;18:1218–1226. doi: 10.1038/nsmb.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hubstenberger A, Courel M, Benard M, Souquere S, Ernoult-Lange M, Chouaib R, Yi Z, Morlot JB, Munier A, Fradet M, Daunesse M, Bertrand E, Pierron G, Mozziconacci J, Kress M, Weil D. P-body purification reveals the condensation of repressed mRNA regulons. Mol Cell. 2017;68:144–157 e5. doi: 10.1016/j.molcel.2017.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 20813 kb)