Abstract
Glycinergic neurotransmission has long been known for its role in spinal motor control. During the last two decades, additional functions have become increasingly recognized—among them is a critical contribution to spinal pain processing. Studies in rodent pain models provide proof-of-concept evidence that enhancing inhibitory glycinergic neurotransmission reduces chronic pain symptoms. Apparent strategies for pharmacological intervention include positive allosteric modulators of glycine receptors and modulators or inhibitors of the glial and neuronal glycine transporters GlyT1 and GlyT2. These prospects have led to drug discovery efforts in academia and in industry aiming at compounds that target glycinergic neurotransmission with high specificity. Available data show promising analgesic efficacy. Less is currently known about potential unwanted effects but the presence of glycinergic innervation in CNS areas outside the nociceptive system prompts for a careful evaluation not only of motor function, but also of potential respiratory impairment and addictive properties.
Keywords: Pain, Analgesia, Neuropathy, Drug discovery, Disinhibition, Central inhibition, Adverse effect, Therapeutic application
Chronic pain is an unmet clinical need
World-wide, chronic pain affects about one in five individuals [1]. In particular chronic neuropathic pain is a difficult-to-treat medical condition. The most widely used analgesics, the cyclooxygenase inhibitors, which include aspirin, ibuprofen or diclofenac, and paracetamol or metamizole, are poorly effective against neuropathic pain. Opioids, the second widely used class of analgesics, are effective, but their use in patients with chronic non-malignant pain is highly problematic [2] and has caused major health problems in the US. Chronic inflammatory pain responds typically well to cyclooxygenase inhibitors [non-selective non-steroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase-2-specific coxibs], but severe and potentially life-threatening adverse events such as gastrointestinal bleeds and myocardial infarction severely limit their long-term use [3]. Drugs that are frequently used in chronic neuropathic pain conditions include gabapentin and pregabalin, which target voltage-gated calcium channels, in addition to compounds such as duloxetine that inhibit the re-uptake of noradrenaline and serotonin and thereby enhance endogenous pain control. Although clinical trials have clearly documented the therapeutic efficacy of these drugs, typically only about one out of seven patients shows clear benefits [4]. Chronic pain, thus, remains a major therapeutic challenge prompting for renewed drug discovery efforts.
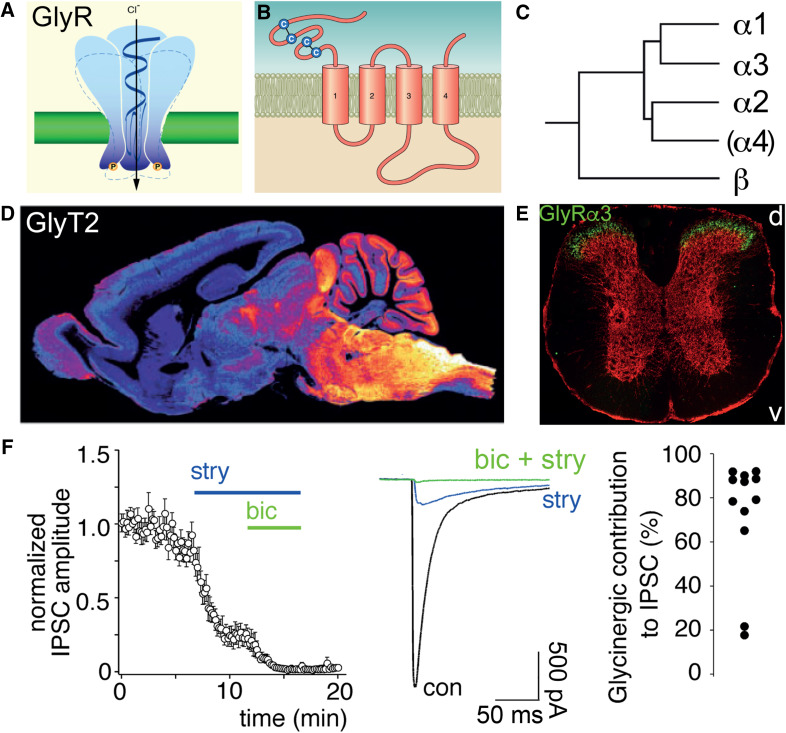
Symptoms of chronic pain include hyperalgesia, allodynia and spontaneous pain [5]. Hyperalgesia defines an increased sensitivity to noxious (painful) sensory stimuli, such as heat, cold, or intense mechanical stress. It results from a sensitization either of peripheral nociceptors (i.e., of nerve fibers and cells that are only activated by painful stimuli) and/or of central neuronal pathways, which can occur via enhanced transmission from nociceptors to second-order neurons located in the spinal dorsal horn [6]. A second frequent symptom of chronic pain is allodynia, which describes painful sensation evoked by stimulation of non-nociceptive nerve fibers, in particular of touch-sensitive fibers. Allodynia is, therefore, always of central origin. A third characteristic of chronic pain is spontaneous pain, occurring in the absence of any sensory input. Its physiological correlate is likely the spontaneous discharge of peripheral and/or central neurons in the pain pathway(s). A plethora of molecular mechanisms have been identified that can contribute to these symptoms [7], but their specific contribution to defined human patient pain states still remains obscure. It is, however, remarkable that all three key symptoms occur when synaptic inhibition in the spinal dorsal horn is compromised [8]. Not surprisingly, one of the newly emerging targets against chronic pain is the spinal glycinergic system, which together with the GABAergic system exerts a powerful inhibitory control over spinal nociception. Figure 1 illustrates key elements of the spinal glycinergic system.
Fig. 1.
The glycinergic system. a Inhibitory (strychnine-sensitive) glycine receptors are pentameric chloride-permeable ion channels. b Each subunit has four transmembrane segments and two cys-loops in the extracellular domain. c Five genes encode for glycine receptor subunits. The α4 subunit gene (GLRA4) is a pseudogene in humans. d Sagittal section through the mouse brain stained with an antibody against GlyT2 illustrates the distribution of glycinergic axon terminals (modified from ref. [10]). e Distribution of the glycine receptor α3 subunit (green) in the mouse spinal cord (modified from ref. [14]). Red, distribution of gephyrin. d and v indicate dorsal and ventral direction, respectively. f Contribution of glycine receptors to the total evoked IPSC in excitatory neurons of the superficial mouse spinal dorsal horn. In the majority of neurons, the contribution of glycine receptors to the IPSC amplitude dominates over that of GABA.
Modified from ref. [8]
Glycine and GABA in central pain control
GABA and glycine are the two fast inhibitory neurotransmitters in the adult spinal cord and brainstem. Neurons that synaptically release glycine or GABA (i.e., glycinergic or GABAergic neurons) are found both in the ventral and the dorsal part of the spinal cord. Glycinergic neurons of the dorsal horn are concentrated in the deep dorsal horn (lamina III and deeper), which is mainly innervated by myelinated low-threshold (tactile) sensory nerve fibers [9, 10]. Some glycinergic neurons are located in lamina I, which receives sensory input mainly from non-myelinated high threshold (nociceptive) sensory nerve fibers. Lamina II contains only few glycinergic neurons. Most spinal glycinergic neurons co-release glycine together with GABA from the same presynaptic vesicles [11–13]. Despite the relative absence of glycinergic somata in lamina II, neurons located in this lamina still express glycine receptors at high density [14] and also receive prominent glycinergic input, likely through innervation from neighboring laminae and axons descending from the rostral ventromedial medulla [8, 15–17].
Early work from the 1980s indicated a major role of inhibitory glycine receptors in the spinal control of nociception [18–20]. These studies used intrathecal injections of the competitive glycine receptor antagonist strychnine in rats to show that blockade of spinal glycine receptors induces a profound agitation of the injected animals and a sensitization to tactile stimuli. Although subconvulsive doses of strychnine were used, such studies did not permit to restrict the actions of strychnine to the sensory (dorsal) spinal cord and spare ventral horn glycine receptors. More recent work has employed virus-based methods to locally and specifically ablate, silence, or activate glycinergic neurons of the mouse spinal dorsal horn [8]. These studies revealed that impaired inhibition by glycinergic dorsal horn neurons rendered mice hypersensitive to a broad range of noxious sensory stimuli including heat, cold and mechanical stimuli and induced signs of spontaneous discomfort. Conversely, sensitivity to these stimuli was strongly reduced when dorsal horn glycinergic neurons were activated using a chemogenetic approach. Other evidence indicates that glycinergic neurons become activated under acute and even more pronounced under chronic pain conditions [21]. Furthermore, one of the typical and most bothersome symptoms of neuropathic pain is allodynia, or touch-evoked pain [5]. Allodynia is likely due to the activation of nociceptive projection neurons by polysynaptic input from touch-sensitive (non-nociceptive) sensory fibers. Increasing evidence suggests that inhibitory neurons in the deep dorsal horn normally prevent this activation and that their activity is compromised in neuropathic and, possibly equally, in inflammatory pain states [22]. Restoring this inhibition would hence target a major symptom of chronic pain.
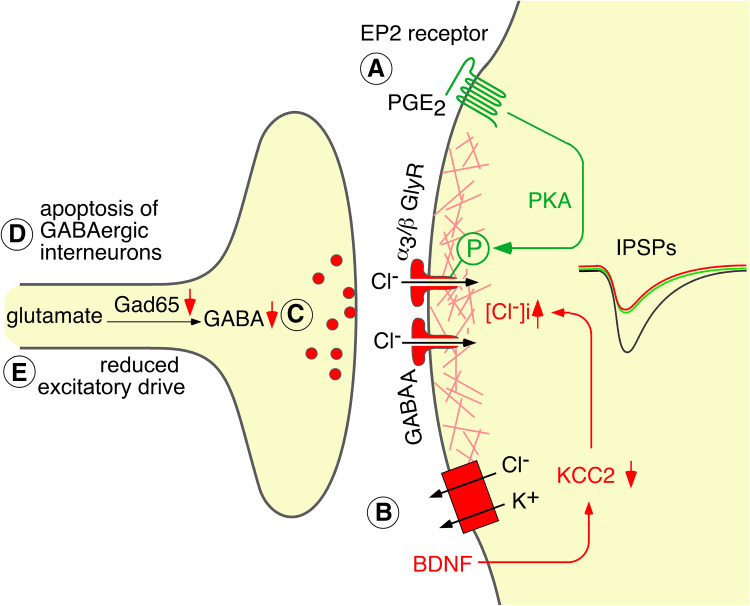
Several groups have demonstrated that synaptic inhibition becomes diminished in rodent models of chronic inflammatory or neuropathic pain [23–25] (Fig. 2). This reduction in inhibition occurs apparently through multiple mechanisms, of which some affect both GABAergic and glycinergic inhibition, whereas others are specific for the glycinergic component (for a review on these mechanisms see [26]). In the superficial dorsal horn, responsiveness of glycine receptors to their physiological agonist glycine is reduced by the inflammatory cytokine prostaglandin E2 (PGE2). PGE2 induces a specific protein kinase A-dependent phosphorylation and inhibition of glycine receptors that contain α3 subunits (α3 glycine receptors) [14, 27–29]. In lamina I of the spinal cord, peripheral neuropathy induces a down-regulation of the cell membrane potassium and chloride exporter KCC2 leading to an intracellular chloride accumulation and a subsequent decrease in the inhibitory effect of GABAergic and glycinergic input [23]. In addition, several presynaptic mechanisms have been demonstrated. Epigenetic down-regulation of glutamate decarboxylase 65 (GAD65), which is one of the two GABA producing enzymes, reduces GABAergic inhibition [25] and peripheral nerve damage has been reported to induce apoptosis of GABAergic interneurons of the dorsal horn [30, 31]. A recent study demonstrated in addition reduced glycinergic but unchanged GABAergic innervation of a certain type of excitatory dorsal horn neurons (radial cells) after peripheral nerve damage [32].
Fig. 2.
Mechanisms of diminished inhibitory neurotransmission in inflammatory and neuropathic pain. a and b postsynaptic mechanisms. a The inflammatory cytokine PGE2 triggers a PKA-dependent phosphorylation of α3 glycine receptors rendering these receptors less responsive to glycine. b Peripheral nerve injury causes a brain-derived neurotrophic factor dependent down-regulation of the potassium chloride co-exporter KCC2 in the plasma membrane of superficial dorsal horn neurons. This leads to a reduced chloride extrusion capacity and an increase in intracellular chloride, which eventually renders glycinergic and GABAergic input less inhibitory. c and d presynaptic mechanisms. c Epigenetic down-regulation of the GABA producing enzyme GAD65 reduces the synaptic release of GABA. d Peripheral nerve damage may also lead to a loss of GABAergic (GAD65 positive) neurons of the spinal dorsal horn [30, 31] but see also [165–167]
These findings suggest that restoring proper central pain control normally provided by GABAergic and glycinergic neurons should be a highly rational approach to the treatment of chronic pain syndromes. Work with subtype-selective GABA A receptor modulators has shown that this strategy is effective at least in preclinical models of chronic pain [29, 33–35]. Similar evidence for selective pharmacological modulators of glycinergic neurotransmission is still sparse but, given the strong glycinergic innervation of the superficial dorsal horn and its known role in regulation of inflammatory pain, it appears likely that glycinergic modulators should have similar or even stronger effects. With respect to side effects of pharmacological intervention, the glycinergic system may have substantial advantages over that of GABA, due to its profound caudo-rostral gradient with strong innervation of the spinal cord and hindbrain and weaker innervation of the midbrain and forebrain [10]. In the forebrain, glycine receptors and glycinergic axon terminals are much less abundant than GABAA receptors and GABAergic terminals [10]. The relative sparsity of glycinergic forebrain innervation may help avoiding possible central side effects that typically limit the use of GABAA receptor modulators for many indications. Furthermore, about 75% of the inhibitory input onto excitatory neurons of the superficial dorsal horn is glycinergic and only 25% GABAergic [8]. So, a certain degree of potentiation of glycine receptors will, in comparison, lead to a much larger increase in central inhibition, when compared to that of GABAA receptors. Finally, since glycine and GABA are very often co-released from the same presynaptic terminals, a potentiation of glycine receptor function should restore inhibition even if the inhibitory loss occurred specifically for GABAA receptors.
Molecular targets within the dorsal horn glycinergic system
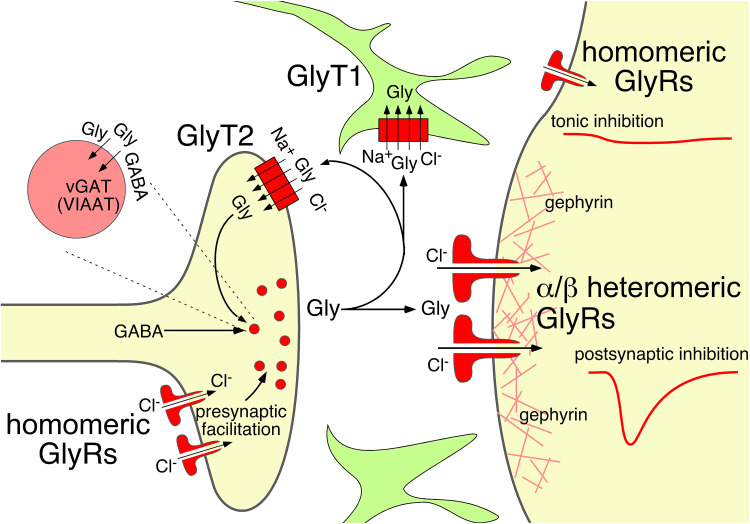
Enhanced efficacy of glycinergic inhibition may be achieved through compounds that target glycine receptors to enhance their activity or glycine transporters to interfere with glycine re-uptake (Fig. 3).
Fig. 3.
Druggable targets within the glycinergic system. Inhibition of the astrocytic glycine transporter GlyT1 prolongs the presence of glycine in the synaptic cleft. GlyT2 also contributes to the removal of glycine after synaptic release but is primarily needed for the loading of presynaptic terminal of glycinergic neurons with glycine. Its long-term impairment depletes glycinergic neurons from glycine. Postsynaptic glycinergic inhibition is mediated by α/β heteromeric glycine receptors, which cluster at subsynaptic membranes through their binding to the postsynaptic scaffold protein gephyrin. Positive allosteric modulators of these receptors enhance postsynaptic (phasic) inhibition. Extrasynaptic glycine receptors are homomeric glycine receptors lacking the β subunits. High levels of ambient glycine activate these receptors to cause tonic glycinergic inhibition. Homomeric glycine receptors are also expressed in presynaptic terminals of glycinergic neurons where they enhance glycine release. Their facilitation should enhance inhibition through an increased release of glycine
Glycine receptors
Glycine receptors are pentameric anion-permeable ion channels. Together with GABAA, nicotinic acetylcholine and 5-HT3 receptors, they form the cys-loop superfamily of ion channels. The human genome harbors four genes (GLRA1, GLRA2, GLRA3, GLRB) that encode for the glycine receptor subunits α1, α2, α3, and β. The genomes of other mammals, including rodents and non-human primates, contain a fourth gene (glra4) encoding the α4 subunit. In humans, functional subunits cannot be expressed from the GLRA4 gene because of several amino acid exchanges and a premature STOP codon [36]. Functional glycine receptors can either be composed only of α subunits (homomeric) or can be heteromeric receptors composed of α and β subunits, most likely in a 2:3 stoichiometry [37]. Most adult glycine receptors are heteropentameric complexes containing α1 and β subunits. The glycine receptor subunit α3 is also contained in adult glycine receptors but its distribution is not as wide-spread as that of α1. In the spinal cord, α3 expression is concentrated in the superficial dorsal horn layers [14]. The α2 subunit is most strongly expressed in the developing nervous system, but has also been shown to be present in areas in which glycinergic innervation is very sparse in the adult such as the cerebral cortex [38]. In some brain areas, such as the striatum, α2 subunits remain expressed at rather high levels into adulthood [39–41]. Additional evidence suggests that α2 subunits play a role in cortical development [42–44] and mutations in GLRA2 have been linked to autism spectrum disorders [45]. Unlike the different α glycine receptor subunits, the β subunit alone cannot form functional glycine receptors. However, the β subunits are required for postsynaptic clustering of glycine receptors via their interaction with gephyrin [46, 47] and in addition contribute to the glycine binding site in heteromeric receptors [37]. Glycine receptors devoid of β subunits (homomeric glycine receptors) cannot interact with gephyrin and are thus not enriched at postsynaptic sites. At extrasynaptic and presynaptic sites, they may still be important as mediators of tonic inhibition and as enhancers of neurotransmitter release in different CNS areas [48, 49]. As discussed below in detail, some glycine receptor modulators target preferentially or exclusively homomeric glycine receptors and thus may regulate tonic inhibition and presynaptic transmitter release rather than postsynaptic inhibition. All three processes would serve the aim of restoring a proper inhibitory tone in the spinal dorsal horn.
Several recent studies provide good evidence that positive allosteric modulation of glycine receptors reduces hyperalgesia in rodent models of chronic pain [29, 50–53]. One glycine receptor subtype (α3 glycine receptors) has attracted particular interest as a potential target for pain therapy. In the spinal dorsal horn (and in the trigeminal nucleus caudalis), these α3 glycine receptors are highly enriched to the superficial layers, where nociceptive sensory afferents terminate [14]. Although glycine receptors are expressed on presynaptic terminals of central neurons, they are absent from primary sensory neurons and their terminals ([54, 55] but see also [56]). Primary afferent depolarization and presynaptic inhibition of nociceptive input are not mediated by glycine. Inhibition of dorsal horn α3 glycine receptors by spinally produced PGE2 is a key mechanism of the central component of inflammatory hyperalgesia. Mice lacking α3 glycine receptors are protected from the pronociceptive effects of spinally injected PGE2 and recover much faster than their wild-type littermate from peripheral inflammation induced hyperalgesia [14, 28]. Since prostaglandins are largely irrelevant for the induction and maintenance of neuropathic pain, this inhibition does not occur in neuropathic pain models. Mice lacking α3 glycine receptors are, therefore, not protected from hyperalgesia or allodynia arising from neuropathic conditions [14, 57]. However, this does not implicate that α3 glycine receptors are irrelevant for neuropathic pain. It only indicates that the PGE2-mediated inhibition of α3 glycine receptors is not a relevant mechanism of sensitization in neuropathic pain (see also [29, 51, 53]).
Glycine transporters
Glycine is taken up from the extracellular space by two distinct cell membrane transporter proteins; GlyT1 (SLC6A9) and GlyT2 (SLC6A5). GlyT1 is widely expressed throughout the brain and spinal cord. It is mainly found in astrocytes [58–60] but has also been reported in neurons [61–63]. At glycinergic synapses, GlyT1 plays a major role in the removal of glycine from the synaptic cleft following its release from presynaptic terminals. Mice lacking GlyT1 exhibit a hyperglycinergic phenotype with reduced muscle tone and respiratory deficits [64]. These mice are perinatal lethal. The expression of GlyT1 is, however, not limited to sites of glycinergic innervation. GlyT1 is also densely expressed in the forebrain where it may regulate NMDA receptor function by controlling the glycine concentration at their glycine binding site (also known as glycineB site). glycine binding site. Because GlyT1 uses a stoichiometry of 2Na+/1Cl−/1glycine [65], glycine transport by GlyT1 may reverse direction under certain conditions to promote calcium-independent glycine release from astrocytes [66]. Triggered by the idea that inhibition by GlyT1 might correct a deficit in NMDA receptor activity that underlies psychotic symptoms in schizophrenic patients, GlyT1 has attracted significant interest as a possible target for the development of antipsychotic (anti-schizophrenic) medications [67] but results of clinical trials were rather disappointing [68, 69].
GlyT2 is exclusively expressed by glycinergic neurons, where it serves two functions. It (1) transports glycine from the synaptic cleft back into the cytoplasm of glycinergic neurons to terminate the synaptic signal and (2) accumulates glycine to the level required for loading of presynaptic vesicles via the vesicular GABA transporter (vGAT), also known as vesicular inhibitory amino acid transporter (VIAAT) [70, 71]. Expression of GlyT2 is an indispensible prerequisite for a glycinergic neuronal phenotype. Mice lacking GlyT2 display a hypoglycinergic phenotype with spasticity, tremor, and an inability to right [72]. They die roughly at 2 weeks of age from neuromotor deficiency. However, acute or incomplete block of GlyT2 delays the decay of glycinergic postsynaptic currents via the extended presence of glycine in the synaptic cleft [73–75] and promotes tonic glycinergic currents [73, 75, 76]. Data from different laboratories strongly suggest an analgesic effect of both GlyT1 [77–80] and GlyT2 [77, 78, 80–87] inhibitors (for three recent reviews see refs. [88–90]).
What potential side effects need to be considered?
Glycine receptor modulators
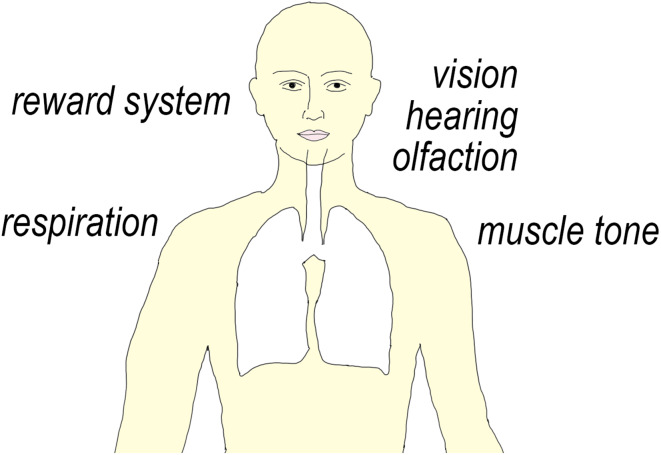
Potential target-related (“on-target”) side effects may in principle originate from all processes that are regulated by glycine receptors. Obvious functions include, besides somatosensory processing, control of muscle strength, motor coordination, respiratory control, visual perception, hearing, and olfaction. Some evidence also points to a role of glycine in midbrain reward circuits (Fig. 4). Such potential side effects of glycine receptor activators/modulators have not yet been systematically assessed in pharmacological experiments. Most data discussed here are from morphological or electrophysiological experiments or report the phenotypes of knock-out mice. Below, we discuss potential side effects arising from CNS areas with significant glycinergic innervation and whether selective targeting of glycine receptor subtypes might help reducing unwanted drug effects.
Fig. 4.
Glycinergic functions in the CNS that may potentially cause undesired drug effects. Potential addictive properties may arise from glycine receptors located in the Nucleus accumbens or the ventral tegmental area (VTA) where enhanced glycinergic inhibition may lead to a disinhibition of dopamine release. Central respiratory rhythms depend on a well-balanced glycinergic inhibition in the pre-Bötzinger complex. Glycine receptors are also found in parts of the CNS involved in primary sensory processing such as the retina, the olfactory bulb and in cochlear synapses. Potentiation of glycinergic inhibition of motoneurons reduces muscle tone and may interfere with muscle strength. It should be kept in mind that the presence of glycine receptors in areas outside the pain system may also hint at potential additional indications such as impaired hearing or impaired respiratory drive
Muscle tone
Glycine exerts a powerful feed-back and feed-forward control over motoneuron activity. Convulsions are the most obvious symptom of glycine receptor blockade by strychnine. Motoneurons express α1 and β glycine receptor subunits but lack α3 glycine [14]. Given the extremely weak muscle tone observed in GlyT1-deficient mice [64], it is likely that positive modulators of α1/β glycine receptors will cause muscle relaxation at sufficiently high doses. However, this does not necessarily mean that muscle relaxation will be a limiting side effect, as studies with GlyT1 inhibitors have found antinociceptive effects in mice at doses that did not cause movement problems [81]. Along the same lines, muscle relaxation by benzodiazepines, which also potentiate inhibitory input onto motoneurons [35, 91], is not a relevant concern as long as the patients’ muscle strength is not otherwise compromised. In fact, a certain degree of muscle relaxation might actually be beneficial in pain patients especially when pain conditions are accompanied by heightened muscle tone such as in low back pain. If muscle weakness turns out to be a problem of non-selective glycine receptor modulators, compounds devoid of activity at α1 (and α1/β) subtypes might offer a therapeutic solution.
Respiration
Impaired respiration is of major concern in opioid use and abuse. The acute life-threatening toxicity of opioids is due to respiratory depression. Respiratory failure can occur as a result of impaired neuronal breathing drive (as in opioids) or come from impaired neuromuscular function. Although not fully excluded, the latter is rather unlikely to occur with glycine receptor modulators. Impaired central respiratory control is, however, a relevant concern. Several lines of evidence indicate that well-balanced glycinergic activity is critical for respiratory rhythm generation. Mice lacking GlyT1 show highly reduced and irregular breathing activity and die within the first day after birth. Blockade of glycine receptors with strychnine injected into the pre-Bötzinger complex severely reduced respiratory activity [92]. Optogenetic stimulation of glycinergic neurons of the pre-Bötzinger complex prematurely terminated inspiratory activity and prolonged the interval until the next inspiratory phase [93]. Babies born with genetic defects in the glycinergic system have an increased risk of sudden infant death [94, 95]. These data are further supported by the expression of α1, α2, and α3 glycine receptors on neurons of the pre-Bötzinger complex, a key area of central respiratory control [96, 97]. Two studies have addressed the relative contribution of α1 and α3 glycine receptors to respiratory control in α1 and α3 subunit deficient mice. Mice lacking the glycine receptor α3 subunit survive and show no obvious deficits [14]. However, more-in-depth analyses have revealed subtle respiratory abnormalities such as a rather variable post-inspiratory phase [96]. The glycine receptor mutant mouse oscillator, which carries a frame-shift mutation in the glra1 gene preventing the expression of a functional α1 subunit protein, dies at about 3 weeks after birth [98] and exhibits greater respiratory abnormalities [99]. Taking these studies together, it appears that both increased and decreased glycinergic inhibition in the pre-Bötzinger complex compromise respiratory activity. Whether this poses a risk to the use of drugs enhancing glycinergic inhibition remains to be determined. In case of benzodiazepines, which are also linked to a certain degree of respiratory depression, therapeutic use is not curtailed unless when used in combination with other sedative drugs or in patients with otherwise impaired respiratory drive. Furthermore, under conditions of impaired respiratory drive, potentiation of glycine receptors in the pre-Boetzinger complex might also be beneficial [96].
Reward system
Addiction is a major concern severely limiting the use of opioid analgesic in pain patients with chronic non-malignant pain. Addiction is also a significant problem of chronic benzodiazepine use indicating that drugs that enhance inhibition (in this case via GABAA receptor modulation) can have a significant addictive potential [100]. A common denominator of most, if not all, drugs with liability to addiction is their ability to trigger dopamine release in the brain’s reward system, where drugs such as morphine excite (or more precisely disinhibit) dopaminergic neurons in the ventral tegmental area (VTA) that project to the Nucleus accumbens (NAc) [101]. Significant evidence suggests that glycinergic signaling in the VTA or NAc is involved in reward and addiction circuits. Glycine infused into the NAc causes dopamine release via its interaction with strychnine-sensitive glycine receptors [102]. Acute blockade of GlyT1 with Org 25935 inhibits ethanol intake and preference in rats [103]. At a first glance, it appears surprising that an inhibitory neurotransmitter increases dopamine release. However, a disinhibitory circuit in which enhanced glycinergic inhibition of GABAergic neurons relieves dopamine release from GABAergic inhibition is a likely explanation. A disinhibitory circuit also explains benzodiazepine-induced dopamine release from VTA neurons [104]. Dopamine release triggered by a number of addictive drugs (ethanol, tetrahydrocannabinol (THC) and nicotine [105]) is blocked by strychnine suggesting that glycine receptors are an element of the reward circuit of these drugs. In case of ethanol, this process probably involves the direct potentiation of glycine receptors by ethanol. Such a potentiation has been demonstrated not only in heterologous expression systems [106, 107], but also in acutely dissociated VTA neurons whose glycine receptors are potentiated by ethanol concentrations reached in the brain after “social” drinking (10–20 mM; [108]) [109]. More over, a recent report has shown that medium spiny neurons of the VTA, which are GABAergic and inhibit dopamine release, express ethanol-sensitive α1 glycine receptors at high density [110]. These data strongly support the concept of a disinhibitory action of ethanol and glycine receptors in the VTA/NAc reward circuits.
Drugs that activate and/or potentiate the action of glycine may thus have rewarding properties and may carry a risk for addiction. Even if this turns out to be the case, it might still be possible to avoid addiction by targeting specific glycine receptor subtypes. Work on the mechanisms of benzodiazepine addiction has shown that their addictive properties can be avoided with compounds that lack efficacy at α1 GABAA receptors (such as L-838’417) [104]. With the presently available data, a potential liability to addiction cannot be attributed to a specific glycine receptor subtype. All glycine receptor α subunits (α1, α2, and α3) are expressed in the NAc and VTA [40, 41]. A recent study that compared ethanol effects in wild-type and α2 or α3 glycine receptor deficient mice found that rewarding properties were more strongly affected by deletion of α2 than of α3 glycine receptors [111]. α3 glycine receptors even appeared to have an anti-ethanol seeking effect. On the other hand, a genetic association study in African Americans found a statistically significant link between alcoholism and single nucleotide polymorphisms (SNPs) in the GLRA3 gene [112]. It is at present not known whether these SNPs are accompanied by increased or decreased glycinergic activity. In any case, drug discovery programs should carefully evaluate potential liability to addition of glycine receptor modulators.
Vision, hearing and olfaction
Glycine receptors are also expressed in the retina, the auditory and olfactory systems. Studies in glycine receptor knock-out mice have found phenotypes in tests of acoustic or visual function [113–115]. Whether modulators of glycine receptor would affect sensory function is currently unknown. Early work in humans from the 1960s reported minor effects of non-convulsive doses of strychnine on sensory systems with small and statistically insignificant increases in visual acuity and speech perception [116, 117]. On the other hand, some recent evidence points to a potential use of glycine receptor modulators in patients with tinnitus [118, 119] or impaired hearing [115].
Potential excitatory effects of glycine in neuropathic conditions
A potentiation of glycine receptor activation can compensate for diminished inhibition caused by partial glycine receptor blockade or diminished glycine release. However, in cases when a down-regulation of the potassium chloride exporter KCC2 increases intracellular chloride concentration and turns glycinergic inhibition into excitation, facilitation of glycinergic input may actually promote pain [23]. Several lines of evidence indicate that drugs which increase GABAergic or glycinergic spinal inhibition do not enhance nociceptive responses even after peripheral nerve damage, the condition for which a down-regulation of KCC2 has been demonstrated [23]. First, spinally applied benzodiazepines reduce both inflammatory and neuropathic hyperalgesia in mice, although experiments with GABAA receptor point mutated mice have unambiguously shown that this is a genuine antihyperalgesic effect occurring through a facilitation of GABAA receptors in the spinal dorsal horn [33, 35, 120]. Chemogenetic activation of glycinergic dorsal horn neurons reduces not only acute pain responses but also nerve injury-induced hyperalgesia [8]. Finally, experiments with several positive allosteric glycine receptor modulators (DH-CBD, LT-01-25, 2,6-DTBP, AM-1488, for details see Table 1) revealed clear antihyperalgesic effects in a rodent model of neuropathic pain [29, 51–53]. That does, however, not exclude that a disturbed chloride homeostasis would limit analgesic effects of glycine or GABAA receptor potentiators. The increase in chloride influx caused by glycine receptor modulators may overcharge the neurons’ chloride extrusion system, which is compromised in neuropathic pain conditions [23]. Down-regulation of KCC2 may hence potentially reduce the therapeutic efficacy of glycine receptor modulators. Combinations of glycine receptor modulators with compounds that can correct impaired chloride homeostasis such as KCC2 modulators or carboanhydrase inhibitors might be an interesting and complementary option [121].
Table 1.
GlyR modulators
| Modulator | Group | Potency, μM (EC50) | GlyR selectivity | Techniques employed | Additional targets | Pain models | References |
|---|---|---|---|---|---|---|---|
| Cannabidiol (CBD), dehydroxyl-cannabidiol (DH-CBD) | Cannabinoid ligand | ≈3–5 | α1 ≈ α3 > α2 | Electrophysiology |
GPR55, CB2, TRPV1, PLA2, 5-HT1A |
CFA; spinal nerve ligation | [50, 169, 170] |
| 2,6-Di-tert-butylphenol (2,6-DTBP) | Propofol analog | ≈20–25 | α3 > α1 | Electrophysiology | HCN1 | Zymosan A, CFA; chronic constriction injury | [29, 137] |
| Propofol analog | ≈0.001 | α1 > α3 = α2 | Electrophysiology | ND | Partial nerve ligation, streptozocin-induced diabetic neuropathy | [52] | |
| Gelsemine | Natural alkaloid | ≈0.6–49a |
α2 ≈ α3 (inhibition) α1 (biphasic modulation) |
Electrophysiology,3H-strychnine binding | ND | Formalin-induced pain, bone cancer, spinal nerve ligation | [156, 157] |
| AM-1488/AM-3607 | Tricyclic sulfonamide | ≈0.016–0.066 | α1 ≈ α2 ≈ α3 | Fluorescence-based membrane potential assays | ND | Spared nerve injury | [53, 163] |
| Compound 19 and (ent2)-20 |
Azetidine sulfonamides aminothiazole sulfone |
0.7 (at α3/β) 2.2 (at α3/β) |
α1 = α3 ND |
Fluorescence-based chloride flux measurement | ND | ND | [164, 171] |
| Compound 2 (4-fluoro-N-(2-(quinolin-8-yloxy)ethyl) benzenesulfonamide) | Sulfonamide | ≈3.9–8.0 | α1 = α3 | Fluorescence-based membrane potential assays, electrophysiology (ion flux HT assay and manual patch-clamp) | ND | ND | [162] |
| Compounds 3 and 6 | sesterterpene glycinyl-lactams | ≈8.5 (compound 3) |
Compound 3: α1 > α3 Compound 6: α3 > α1 |
Electrophysiology | ND | ND | [159–161] |
| Tropisetron (ICS-205,930) | tropeines |
nM >10 µM fmolar µmolar |
α1/β, α2/β potentiation inhibition α1 potentiation α2 inhibition |
Electrophysiology Electrophysiology |
5-HT3 5-HT3 |
ND ND |
[144] [146] |
ND not determined, CFA complete Freund’s adjuvant
aBiphasic modulation
Potential side effects of glycine transport modulation
Both data obtained from GlyT1-deficient mice [64] and with GlyT1 inhibitors [122] demonstrate that GlyT1 plays a dominant role in the removal of glycine from the synaptic cleft. In addition, GlyT1 may limit the activity of extrasynaptic glycine receptors and thereby regulate tonic glycinergic inhibition. Blocking GlyT1 should, therefore, allow a fine-tuned enhancement of neuronal inhibition. Problems may, however, arise from increases in local glycine in the vicinity of NMDA receptors which require the binding of glycine (or d-serine) to the so called glycine B site for full activation [123]. Enhanced NMDA receptor activation by increased glycine concentrations may be particularly relevant in the spinal cord where spill-over of synaptically released glycine to neighboring NMDA receptors has been demonstrated [124]. Because NMDA receptor activation is typically linked to pain perception and the generation of hyperalgesia, this effect may counteract the analgesic effect of increased activation of inhibitory glycine receptors [77, 79]. NMDA receptor-related side effects might also occur at other sites in particular in the forebrain, which is rich in GlyT1 protein and NMDA receptors. In these brain areas, which are basically devoid of glycinergic innervation, GlyT1 still regulates NMDA receptor activity by controlling the concentration of ambient extracellular glycine at the glycine B site [125, 126]. The role of GlyT1 in this process has actually made GlyT1 blockers candidates for anti-schizophrenic treatment. Some recently developed GlyT1 inhibitors have been promoted to clinical trials in schizophrenic patients. These clinical trials yielded disappointing results with respect to improvement of psychotic symptoms [68, 69] but severe side effects were not reported. Whether these compounds possess analgesic efficacy in human patients with chronic pain is currently unknown.
Side effects observed in preclinical tests with sarcosine-like irreversible GlyT1 inhibitors (NFPS, ORG 25935 and RG1678) were partially reminiscent of the phenotypes of GlyT1-deficient mice and related to over-stimulation of inhibitory glycine receptors. Such problems might not occur with more recently developed competitive inhibitors, as their effects would be self-limiting. This concept may be helpful when increased activation of NMDA receptors is the desired effect (such as in the schizophrenia indication). However, when inhibitory glycinergic transmission shall be enhanced with competitive GlyT1 blockers, nearly full activation of the glycine B site at NMDA receptors will occur with potential negative consequences on hyperalgesia. An alternative and, in our opinion more promising, approach might be the development of reversible allosteric GlyT1 inhibitors that cause only incomplete blockade even at saturating concentrations. Competitive inhibitors may also offer opportunities, if they have low affinities (to prevent saturation of NMDA receptors) but slow dissociation kinetics to prevent fast displacement by synaptically released glycine.
GlyT2 inhibitors are less likely to cause NMDA receptor-related side effects although it cannot be completely excluded that reduced reuptake of glycine via GlyT2 at sites with glycinergic innervation also fosters glycine spill-over to NMDA receptors. Problems might, however, arise from the dual role of GlyT2 at glycinergic synapses. The primary function of GlyT2 is very likely pro-glycinergic by providing glycine to presynaptic glycinergic terminals for synaptic release. Blocking GlyT2 function will, therefore, have to be closely balanced to yield the desired prolongation of glycine presence in synapses without reducing glycine filling of presynaptic glycinergic terminals. Org 25543, one of the earliest GlyT2 inhibitors, indeed prolongs glycinergic IPSCs after acute application in spinal cord slices but glycinergic synaptic events become greatly diminished after prolonged exposure [73]. It acts as a nearly irreversible inhibitor and induces lethal convulsions at doses only ten-times higher than those required for half-maximal analgesia (suppression of flinches in the formalin test) and still below full target (GlyT2) exposure [85]. These side effects are reminiscent of the phenotype of GlyT2-deficient mice and likely caused by a deficit in glycinergic inhibition. Such side effects may be avoided by more recently developed competitive (reversible) inhibitors (compound 1 in [85]), which did not induce convulsions after acute treatment. However, sedation was observed with this compound at high doses.
Drug-like modulators of glycine receptors
Ligands with modulator activity at inhibitory glycine receptors but with low selectivity have been known for longer. Such compounds include bivalent cations, mono- and disaccharides, glutamic acid, ginkgolic acids, ivermectins, synthetic neurosteroids, and n-alcohols. Their actions on glycine receptors have been reviewed previously (e.g. [127, 128]). Given the presumed therapeutic potential of glycine receptor modulators, a need for molecules with drug-like properties was evident. Here, we focus on glycine receptor modulators that have already been tested in pain models and on molecules, which have been discovered recently through unbiased high throughput screen (HTS) of large libraries of synthetic or natural compound libraries. Table 1 provides an overview about these compounds and Fig. 5 illustrates the current knowledge about their respective binding sites on the mammalian crystal structure of glycine receptors.
Fig. 5.
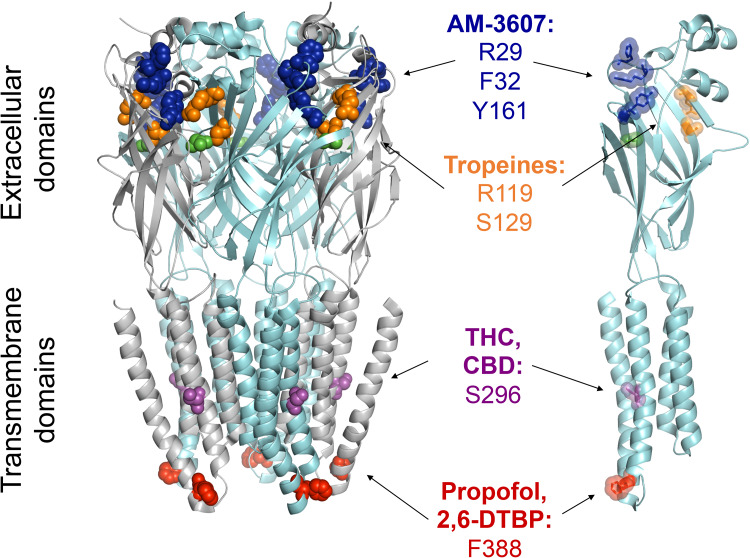
Modulatory sites at glycine receptors. The most relevant amino acids (highlighted in colored spheres) are shown in a single α3 subunit (right) and for a homopentameric α3 glycine receptor (left). The structural data (PDB ID:5TIO) were taken from [53]. For the compound AM-3607, which binds to an interface of two adjacent subunits, only the residues of the (+) subunit are shown [53]. The residues relevant for the actions of tropeines on glycine receptors have not yet been identified for α3 glycine receptors. The sites indicated are the homologous sites identified originally for the α1 glycine receptor subunit [143–146]. The sites for THC, CBD, propofol and 2,6-DTBP were identified and characterized in [29, 50, 148, 168]. Glycine molecules bound to the orthosteric sites are shown in green
Derivatives of existing drugs
Propofol derivatives
The intravenous anesthetic propofol (2,6-diisopropylphenol) primarily targets GABAA receptors but also modulates glycine receptors [129, 130]. Due to a ~tenfold lower potency at glycine receptors relative to GABAA receptors, it is unlikely that these receptors contribute significantly to propofol anesthesia ([131, 132], see, however [133]), but halogenated propofol derivatives (4-fluoro propofol, 4-bromo propofol, and 4-chloropropofol) have increased potencies at glycine receptors [134, 135] reaching values comparable to those at α1β3γ2 GABAA receptors [136]. A large series of propofol derivatives has been synthesized in an attempt to correlate the anesthetic properties of propofol with its GABAA receptor modulatory effects [137]. Among these compounds was 2,6-DTBP which is completely devoid of anesthetic actions and lacks modulatory and agonistic activity at α1β3γ2 GABAA receptors. Subsequent work verified the absence of modulatory properties at GABAA receptors [138] but demonstrated activity at glycine receptors [139]. The actions of 2,6-DTBP on glycine receptors and on hyperalgesia have been extensively investigated in a recent study [29]. 2,6-DTBP turned out to be an allosteric modulator of recombinant homomeric α1 and α3 glycine receptors with no activity at recombinant and native spinal cord GABAA receptors. Critical for its interaction with glycine receptors is a phenylalanine residue (F388, in α3) in the so-called membrane-associated stretch of the intracellular loop between transmembrane segment TM3 and TM4. This residue is also critical for the interaction of the channel with propofol. Interestingly, recombinant heteromeric α3β and synaptic glycine receptors were potentiated only in a phosphorylated configuration. The relevant phosphorylation site is serine 346, which becomes phosphorylated in vivo in inflammatory pain conditions and in response to PGE2 [14], a pivotal inflammatory mediator which is produced in the spinal cord in response to peripheral inflammation [140]. Posttranslational modifications such as phosphorylation may add another level of specificity to glycine receptor modulation. Consistent with this dependence on phosphorylation, 2,6-DTBP exerted analgesic effects only in models of chronic pain. Although 2,6-DTBP had no activity at GABAA receptors, it still targets other ion channels including HCN1 channels [141]. This lack of general specificity and the high doses (90 mg/kg, i.p.) required for in vivo effects make 2,6-DTBP a poor drug candidate. However, a patent search revealed new propofol derivatives (such as 4-(benzyloxy)-3,5-diisopropylbenzoyl chloride; LT-01-25) with potency for glycine receptor modulation in the sub-nanomolar range [52]. In rodent experiments, LT-01-25 normalized mechanical and cold allodynia in the streptozotocin model of painful diabetic neuropathy at doses of 10–20 mg/kg p.o.
Tropeines
Tropeines are potent 5-HT3 receptor antagonists. They are clinically used as anti-emetic drugs in patients with chemotherapy induced nausea and vomiting. Tropisetron, also known as ICS-205,930, is one of the best known members of this group. Two tropeines, MDL-72222 and tropisetron, in addition potentiate glycine receptor currents in nanomolar concentrations, while higher micromolar concentrations cause inhibition [142]. Tropeines appear to bind to an interface formed by two α or one α and one β subunit in the extracellular domain of glycine receptors close to the glycine binding site [143–146]. Tropisetron displays some subunit-specificity with potentiation of homomeric α1 but inhibition of homomeric α2 glycine receptors. Co-expression of β subunits significantly increased the sensitivity of α1 glycine receptors to potentiation and turned inhibition of α2 glycine receptors into potentiation [144]. The high affinity binding and the remarkable sensitivity of GlyRs to tropeines makes this group of compounds one of the most promising candidates for the development of specific drugs targeting glycine receptors. However, despite of some differences between the chemical requirement for tropeine binding to glycine and 5-HT3 receptors, most tropeines still bind and modulate 5-HT3 receptors with high affinity [145]. A better understanding of the structural basis of the interaction of glycine receptor subtypes with tropeines may perhaps lead to new tropeine derivatives acting as specific enhancers of glycinergic inhibition.
Natural ligands
Plant-derived cannabinoids
Although G protein-coupled CB1 and CB2 receptors constitute the main targets of cannabinoids, several of these molecules, Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD) and its synthetic derivative dehydroxyl-cannabidiol (DH-CBD), also modulate glycine receptors [50, 51, 147–149].
THC was the first phytocannabinoid to be shown to potentiate glycine receptor responses [147]. In an effort to produce derivatives that more specifically interact with glycine receptors, 5-desoxy THC (identical to dehydroxyl-cannabidiol, DH-CBD), 1-desoxy THC and di-desoxy THC (identical to di-dehydroxyl-cannabidiol, DD-CBD) were generated [50]. While THC exerts effects on both G protein-coupled CB1 and CB2 receptors and on glycine receptors, DH-CBD has strongly reduced affinity to CB1 and CB2 receptors but still potentiates glycine receptors with unaltered potency and efficacy. DD-CBD does no longer bind to CB1 receptors and is an antagonist at the THC binding site of glycine receptors.
In case of CBD and DH-CBD, potentiation occurs through an increase in the apparent affinity for glycine [51, 150]. THC exhibits some preferential activity at α1 and α3 glycine receptors, which are potentiated by THC with similar potency and efficacy, while α2 glycine receptors exhibit a significantly lower sensitivity [50]. The differences between subunits allowed the identification of structural requirements of glycine receptors for modulation by THC. Sequence comparisons of the highly sensitive α1 and α3 with low sensitivity α2 glycine receptors suggested that a conserved serine residue within the transmembrane domain 3 (TM3) (i.e. S296 in α1 and S307 in α3GlyR) confers high sensitivity to THC [50]. Substitution of this amino acid with the corresponding alanine residue present in α2 glycine receptors (A302) significantly decreased the sensitivity of α1 and α3 glycine receptors. Subsequent NMR analyses with purified transmembrane glycine receptor domains together with molecular modeling revealed that these serine residues are directly interacting with THC and DH-CBD [50, 148].
The action of glycine receptor modulators is in several cases different for homomeric and α/β heteromeric receptors. This has been specifically shown for 2,6-DTBP [29] and applies also to DH-CBD/5-desoxy THC [50, 149]. Such a difference may be relevant for potential in vivo applications because glycine receptors at postsynaptic sites are α/β heteromeric receptors [151–153], while presynaptic glycine receptors are considered a homomeric receptors. Specific modulation of homomeric or heteromeric receptors may thus restrict activity to presynaptic or postsynaptic sites and may have specific therapeutic indications [149].
Consistent with this in vivo glycine receptor modulation THC, CBD and DH-CBD reduce hyperalgesia in inflammatory and/or neuropathic rodent pain models [50, 51]. Analgesic actions of THC in acute pain models are well established and part of the classical tetrad of in vivo CB1 receptor-mediated effects. Acute analgesic effects of THC were accordingly blocked by a CB1 receptor antagonist (AM-251) and absent in CB1 receptor (CB1−/−) deficient mice. However, the antihyperalgesic effects of THC in chronic pain models persisted in CB1−/− mice and were blocked by di-desoxy THC/DD-CBD [50]. A subsequent study demonstrated that DH-CBD also exerts antihyperalgesic effects that were blocked by di-desoxy THC/DD-CBD [51]. Interestingly, the antihyperalgesic actions of all three compounds (THC, DH-CBD and CBD) depended on the expression of α3 glycine receptors [50, 51]. These encouraging results together with the availability of structural information from crystallized α1 glycine receptors have stimulated virtual screening efforts which have identified 12 previously unknown glycine receptor modulators among 1549 FDA approved drugs [154]. These compounds have not yet been tested in vivo for potential analgesic efficacy, but their discovery may foster such studies in the future.
Gelsemine-derived alkaloids
The alkaloid gelsemine has been recently shown to have activity at glycine receptors. Gelsemine is one of the principal alkaloids produced by the Gelsemium genus plants [155]. Gelsemine elicits anti-nociceptive effects in rat models of acute chemical and neuropathic pain after intrathecal injection but not after local peripheral or intracerebroventricular injection [156]. The antineuropathic pain effects were blocked by strychnine and after si-RNA mediated knock-down of spinal α3 glycine receptors but not of α1 glycine receptors. Subsequent characterization of gelsemine action on glycine receptor function revealed rather complex effects [157]. The authors observed a bi-phasic action on homomeric α1 glycine receptors with potentiation at low concentrations and inhibition at higher concentrations, while all other glycine receptors (α1/β heteromeric receptors, and α2 and α3 homomeric and α2/β and α3/β heteromeric receptors) were inhibited. Native glycine (and GABAA) receptors in cultured spinal cord neurons were inhibited by gelsemine. In spinal cord slices, the frequencies of spontaneous glycine and glutamate mediated mIPSCs and mEPSCS were reduced while amplitudes were not affected. It remains at present unclear how these complex cellular effects relate to the in vivo antinociceptive effects of gelsemine.
New lead structures revealed based on molecules obtained from marine sponges
The screening of natural compound libraries is a highly timely approach to the discovery of new chemical entities acting on an identified target. Libraries obtained from marine organisms appear to be particularly rich in hitherto unknown biologically active molecules [158]. One such screen at the University of Queensland, Australia, was performed on a library of more than 2500 compounds extracted from southern Australian and Antarctic marine invertebrates and algae. It led to the identification of three Irciniidae sponges that contained sesterterpene glycinyl-lactam compounds with activity at glycine receptors [159–161]. Following an initial screen based on a fluorescent chloride sensitive protein, detailed chemical characterization and electrophysiological analyses identified two novel glycine receptor modulators from Psammocinia sponges (a genus of the Irciniidae family) with considerable specificity for either α1 or α3 glycine receptors. Compound 3 in ref. [160] specifically potentiated responses in α3 glycine receptors with virtually absent activity in α1 glycine receptors, and compound 6 potentiated α1 glycine receptors while moderately blocking α3 glycine receptors. Potential effects of these compounds on native receptors or in vivo actions have not been reported, likely because due to the highly complex structure of both compounds chemical synthesis of larger quantities is a major challenge. Nevertheless, the discovery of the glycinyl-lactam structure as a new pharmacophore for subtype-specific glycine receptor modulation should constitute an interesting starting point for new drug discovery programs.
New chemical scaffolds
Up until recently, published glycine receptor modulators suffered greatly from low potency and insufficient selectivity over other cys-loop channel subtypes and/or unfavorable pharmacokinetic properties limited their suitability for in vivo studies. This triggered a large industry effort to identify chemical matter that would alleviate these limitations and address more specifically the desired therapeutic application. Drug companies have published some of their efforts on new glycine receptor modulators that were discovered from unbiased screens of large chemical libraries [53, 162–164].
The screen done by Neusentis (a former research unit of Pfizer) aimed specifically at positive modulators of α3 glycine receptors [162]. This group employed fluorescence membrane potential assays for a first selection, which yielded 147 hits from more than 55,000 compounds. Medium throughput electrophysiology verified four compounds and a subsequent additional membrane potential screen of 1986 compounds identified another 31 hits, with nine compounds verified in electrophysiology. Seven compounds were evaluated in manual patch-clamp experiments. 4-fluoro-N-(2-(quinolin-8yloxy)ethyl)benzene sulfonamide (compound 2) showed an EC50 for positive allosteric modulation of α3 glycine receptors of around 5–6 μM. Although identified in a screen for the α3 glycine receptor subtype, it had also modulatory activity at α1 glycine receptors. It did not activate glycine receptors directly and did not elicit any functional effects at α1β3γ2 or α3β3γ2 GABAA receptors. So far, no in vivo data have been published on these hits.
Amgen [163] utilized a similar FLIPR-based HTS strategy targeting human α3β glycine receptors which resulted in the discovery of a novel class of tricyclic sulfonamides. These hits were further improved into high quality proof-of-concept and co-crystallization molecules further supporting the role of glycine receptors and their potential in being used as therapeutic analgesics. Indeed, Huang and co-workers [53, 163] demonstrated how a rather low potency first hit was subsequently optimized for potency and tissue penetration sufficient for ex vivo tests of the compound on native mouse dorsal horn glycine receptors. Further optimization led to a compound (AM-1488) that was metabolically stable enough for in vivo testing. At an oral dose of 20 mg/kg, AM-1488 reduced allodynia in mice with spared nerve injury-induced neuropathic pain to a degree similar to pregabalin (30 mg/kg). AM-1488 did not significantly impair movement in naïve mice and had no detectable off-target effects on other Cys-loop receptors or membrane proteins in in vitro screens suggesting that the observed anti-allodynia effect was due to its action on glycine receptors. This compound was then further optimized to yield the high potency/affinity compound AM-3607 [163]. Although the initial screen targeted α3 glycine receptors, both compounds enhanced the activity of homomeric and heteromeric α1 and α3 glycine receptors (EC50 in ranges from 0.016 to 0.066 μM). The affinity of AM-3607 to α3 glycine receptors was amenable for co-crystallization with human α3 glycine receptors. X-ray analyses at 2.6-Å resolution revealed a new inter-subunit allosteric binding site located in the extracellular domain and approximately 10 Å away from the orthosteric glycine binding site (Fig. 5). The close association of the AM-3607 site with the orthosteric site and further biochemical experiments is consistent with a stabilizing action of AM-3607 on glycine binding and a subsequent increases the apparent affinity for glycine and channel activity. This binding pocket is conserved between α1 and α3 subunits explaining why AM-1488 does not differentiate between α1 and α3 glycine receptors. More recently, Chakka et al. [164] reported two novel classes of glycine receptor potentiators using similarity- and property-guided scaffold hopping enabled by parallel synthesis and pharmacophore-based virtual screening strategies. These included azetidine sulfonamides and aminothiazole sulfone leads that have been reported to bind in the previously described AM-3607 glycine receptor pocket. These novel scaffolds combine potencies in the low micromolar and submicromolar range with selectivity profiles that favour glycine receptors over other cys-loop family receptors and with ADME-like properties. They therefore significantly expand the current tools available for the analysis of glycine receptor function. In combination with the recently solved crystal structure of a mammalian glycine receptor, they are also anticipated to foster the discovery and development of novel pain therapeutics.
Conclusions
Available data clearly support on a pivotal role of glycinergic inhibition in spinal nociception. Positive allosteric modulation of glycine receptors and inhibition of glycine re-uptake appear as attractive opportunities to restore proper synaptic inhibition in spinal nociceptive circuits. Antinociceptive effects of glycine receptors modulators or glycine transport inhibitors have already been demonstrated in preclinical models of chronic pain. Open questions relate to the therapeutic value of this target in chronic pain in human patients and at least equally relevant to possible unwanted effects. GlyT1 inhibitors, which have already been in large-scale clinical trials for indications different from pain, are well tolerated. Much less is known in case of GlyT2 inhibitors and especially in the emerging field of glycine receptor modulators. It is likely that such compounds will be devoid of many side effects of centrally acting analgesics and of drugs that target the GABAergic system, but solid evidence is still missing. Future studies on drug candidates should carefully evaluate potential adverse effects arising from impaired muscle strength or respiratory drive. Potential liability addiction due to activation of glycine receptors in the brain’s reward circuit is another potential concern. Closely related to this question is whether therapeutically used glycine receptor modulators will need to show subunit-specificity (either α1 or α3). Subtype-specific modulators may be less prone to side effects but may also have lower analgesic efficacy. With last year’s arrival of first compounds suitable for in vivo preclinical testing, we will hopefully soon know more about the potential that this new target carries for the treatment of patients in chronic pain.
Acknowledgements
The authors thank Dr. Carlos F. Burgos, University of Concepción, Chile) for help with the glycine receptor model shown in Fig. 5. The authors’ work on glycine receptors has been supported by grants from the Swiss National Science Foundation (116064 and 131093) to HUZ and the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1170252) to GEY. MAA and GEY have partially been supported through fellowships from the rare disease initiative Zurich (RADIZ) (MAA) and by the Forschungskredit of the University of Zurich (GEY).
References
- 1.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Nuesch E, Rutjes AW, Husni E, Welch V, Juni P (2009) Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev CD003115 [DOI] [PMC free article] [PubMed]
- 3.Zeilhofer HU, Brune K. Cyclooxygenase inhibitors: basic aspects. In: McMahon SB, Koltzenburg M, Tracey I, Turk D, editors. Wall and Melzack’s textbook of pain. 6. New South Wales: Saunders; 2013. pp. 444–454. [Google Scholar]
- 4.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brix Finnerup N, Hein Sindrup S, Staehelin Jensen T. Management of painful neuropathies. Handb Clin Neurol. 2013;115:279–290. doi: 10.1016/B978-0-444-52902-2.00017-5. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jäger T, Sandkühler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- 7.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 8.Foster E, Wildner H, Tudeau L, Haueter S, Ralvenius WT, Jegen M, Johannssen H, Hösli L, Haenraets K, Ghanem A, Conzelmann KK, Bösl M, Zeilhofer HU. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron. 2015;85:1289–1304. doi: 10.1016/j.neuron.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todd AJ, Sullivan AC. Light microscope study of the coexistence of gaba-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990;296:496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- 10.Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bösl MR, Fritschy JM. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol. 2005;482:123–141. doi: 10.1002/cne.20349. [DOI] [PubMed] [Google Scholar]
- 11.Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 12.Keller AF, Coull JA, Chery N, Poisbeau P, De Koninck Y. Region-specific developmental specialization of gaba-glycine cosynapses in laminas I–II of the rat spinal dorsal horn. J Neurosci. 2001;21:7871–7880. doi: 10.1523/JNEUROSCI.21-20-07871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell EA, Gentet LJ, Dempster J, Belelli D. GABAA and glycine receptor-mediated transmission in rat lamina II neurones: relevance to the analgesic actions of neuroactive steroids. J Physiol. 2007;583:1021–1040. doi: 10.1113/jphysiol.2007.134445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey RJ, Depner UB, Wässle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schütz B, Abo-Salem OM, Zimmer A, Poisbeau P, Welzl H, Wolfer DP, Betz H, Zeilhofer HU, Müller U. GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- 15.Antal M, Petko M, Polgar E, Heizmann CW, Storm-Mathisen J. Direct evidence of an extensive gabaergic innervation of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. Neuroscience. 1996;73:509–518. doi: 10.1016/0306-4522(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 16.Kato G, Yasaka T, Katafuchi T, Furue H, Mizuno M, Iwamoto Y, Yoshimura M. Direct gabaergic and glycinergic inhibition of the substantia gelatinosa from the rostral ventromedial medulla revealed by in vivo patch-clamp analysis in rats. J Neurosci. 2006;26:1787–1794. doi: 10.1523/JNEUROSCI.4856-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hossaini M, Goos JA, Kohli SK, Holstege JC. Distribution of glycine/GABA neurons in the ventromedial medulla with descending spinal projections and evidence for an ascending glycine/GABA projection. PLoS ONE. 2012;7:e35293. doi: 10.1371/journal.pone.0035293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyer C, Roberts LA, Komisaruk BR. Hyperalgesia induced by altered glycinergic activity at the spinal cord. Life Sci. 1985;37:875–882. doi: 10.1016/0024-3205(85)90523-5. [DOI] [PubMed] [Google Scholar]
- 19.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa T, Marsala M, Sakabe T, Yaksh TL. Characterization of spinal amino acid release and touch-evoked allodynia produced by spinal glycine or GABAA receptor antagonist. Neuroscience. 2000;95:781–786. doi: 10.1016/S0306-4522(99)00461-3. [DOI] [PubMed] [Google Scholar]
- 21.Hossaini M, Duraku LS, Sarac C, Jongen JL, Holstege JC. Differential distribution of activated spinal neurons containing glycine and/or GABA and expressing c-fos in acute and chronic pain models. Pain. 2010;151:356–365. doi: 10.1016/j.pain.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Peirs C, Williams SP, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, Seal RP. Dorsal horn circuits for persistent mechanical pain. Neuron. 2015;87:797–812. doi: 10.1016/j.neuron.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 24.Müller F, Heinke B, Sandkühler J. Reduction of glycine receptor-mediated miniature inhibitory postsynaptic currents in rat spinal lamina I neurons after peripheral inflammation. Neuroscience. 2003;122:799–805. doi: 10.1016/j.neuroscience.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ. Epigenetic suppression of gad65 expression mediates persistent pain. Nat Med. 2011;17:1448–1455. doi: 10.1038/nm.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeilhofer HU, Wildner H, Yévenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmadi S, Lippross S, Neuhuber WL, Zeilhofer HU. PGE2 selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat Neurosci. 2002;5:34–40. doi: 10.1038/nn778. [DOI] [PubMed] [Google Scholar]
- 28.Reinold H, Ahmadi S, Depner UB, Layh B, Heindl C, Hamza M, Pahl A, Brune K, Narumiya S, Müller U, Zeilhofer HU. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. J Clin Invest. 2005;115:673–679. doi: 10.1172/JCI23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acuña MA, Yévenes GE, Ralvenius WT, Benke D, Di Lio A, Lara CO, Muñoz B, Burgos CF, Moraga-Cid G, Corringer PJ, Zeilhofer HU. Phosphorylation state-dependent modulation of spinal glycine receptors alleviates inflammatory pain. J Clin Invest. 2016;126:2547–2560. doi: 10.1172/JCI83817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imlach WL, Bhola RF, Mohammadi SA, Christie MJ. Glycinergic dysfunction in a subpopulation of dorsal horn interneurons in a rat model of neuropathic pain. Sci Rep. 2016;6:37104. doi: 10.1038/srep37104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy J-M, Rudolph U, Möhler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 34.Di Lio A, Benke D, Besson M, Desmeules J, Daali Y, Wang ZJ, Edwankar R, Cook JM, Zeilhofer HU. HZ166, a novel GABAA receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacology. 2011;60:626–632. doi: 10.1016/j.neuropharm.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ralvenius WT, Benke D, Acuña MA, Rudolph U, Zeilhofer HU. Analgesia and unwanted benzodiazepine effects in point-mutated mice expressing only one benzodiazepine-sensitive GABAA receptor subtype. Nat Commun. 2015;6:6803. doi: 10.1038/ncomms7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA. Analysis of the set of GABAA receptor genes in the human genome. J Biol Chem. 2004;279:41422–41435. doi: 10.1074/jbc.M401354200. [DOI] [PubMed] [Google Scholar]
- 37.Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. The & #x03B2; subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 38.Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mrnas in the adult and developing rat brain. EMBO J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haverkamp S, Müller U, Zeilhofer HU, Harvey RJ, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the α2 subunit. J Comp Neurol. 2004;477:399–411. doi: 10.1002/cne.20267. [DOI] [PubMed] [Google Scholar]
- 40.Jonsson S, Kerekes N, Hyytia P, Ericson M, Soderpalm B. Glycine receptor expression in the forebrain of male AA/ANA rats. Brain Res. 2009;1305(Suppl):S27–S36. doi: 10.1016/j.brainres.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 41.Jonsson S, Morud J, Pickering C, Adermark L, Ericson M, Soderpalm B. Changes in glycine receptor subunit expression in forebrain regions of the wistar rat over development. Brain Res. 2012;1446:12–21. doi: 10.1016/j.brainres.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 42.Young-Pearse TL, Ivic L, Kriegstein AR, Cepko CL. Characterization of mice with targeted deletion of glycine receptor α2. Mol Cell Biol. 2006;26:5728–5734. doi: 10.1128/MCB.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avila A, Vidal PM, Dear TN, Harvey RJ, Rigo JM, Nguyen L. Glycine receptor α2 subunit activation promotes cortical interneuron migration. Cell Rep. 2013;4:738–750. doi: 10.1016/j.celrep.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morelli G, Avila A, Ravanidis S, Aourz N, Neve RL, Smolders I, Harvey RJ, Rigo JM, Nguyen L, Brone B. Cerebral cortical circuitry formation requires functional glycine receptors. Cereb Cortex. 2017;27:1863–1877. doi: 10.1093/cercor/bhw025. [DOI] [PubMed] [Google Scholar]
- 45.Pilorge M, Fassier C, Le Corronc H, Potey A, Bai J, De Gois S, Delaby E, Assouline B, Guinchat V, Devillard F, Delorme R, Nygren G, Rastam M, Meier JC, Otani S, Cheval H, James VM, Topf M, Dear TN, Gillberg C, Leboyer M, Giros B, Gautron S, Hazan J, Harvey RJ, Legendre P, Betancur C. Genetic and functional analyses demonstrate a role for abnormal glycinergic signaling in autism. Mol Psychiatry. 2016;21:936–945. doi: 10.1038/mp.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor β subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 47.Sola M, Bavro VN, Timmins J, Franz T, Ricard-Blum S, Schoehn G, Ruigrok RW, Paarmann I, Saiyed T, O’Sullivan GA, Schmitt B, Betz H, Weissenhorn W. Structural basis of dynamic glycine receptor clustering by gephyrin. EMBO J. 2004;23:2510–2519. doi: 10.1038/sj.emboj.7600256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- 49.Jeong HJ, Jang IS, Moorhouse AJ, Akaike N. Activation of presynaptic glycine receptors facilitates glycine release from presynaptic terminals synapsing onto rat spinal sacral dorsal commissural nucleus neurons. J Physiol. 2003;550:373–383. doi: 10.1113/jphysiol.2003.041053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong W, Cheng K, Cui T, Godlewski G, Rice KC, Xu Y, Zhang L. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat Chem Biol. 2011;7:296–303. doi: 10.1038/nchembio.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong W, Cui T, Cheng K, Yang F, Chen SR, Willenbring D, Guan Y, Pan HL, Ren K, Xu Y, Zhang L. Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J Exp Med. 2012;209:1121–1134. doi: 10.1084/jem.20120242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leuwer M, O’Neill P, Berry N, Pidathala C, Wells A (2016) Pharmacologically active compounds. In: Patent application number: PCT/GB2014/053838
- 53.Huang X, Shaffer PL, Ayube S, Bregman H, Chen H, Lehto SG, Luther JA, Matson DJ, McDonough SI, Michelsen K, Plant MH, Schneider S, Simard JR, Teffera Y, Yi S, Zhang M, DiMauro EF, Gingras J. Crystal structures of human glycine receptor α3 bound to a novel class of analgesic potentiators. Nat Struct Mol Biol. 2017;24:108–113. doi: 10.1038/nsmb.3329. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell K, Spike RC, Todd AJ. An immunocytochemical study of glycine receptor and GABA in laminae I–III of rat spinal dorsal horn. J Neurosci. 1993;13:2371–2381. doi: 10.1523/JNEUROSCI.13-06-02371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell rna sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 56.Bae JY, Mah W, Rah JC, Park SK, Bae YC. Expression of glycine receptor α3 in the rat trigeminal neurons and central boutons in the brainstem. Brain Struct Funct. 2016;221:4601–4613. doi: 10.1007/s00429-016-1190-4. [DOI] [PubMed] [Google Scholar]
- 57.Hösl K, Reinold H, Harvey RJ, Müller U, Narumiya S, Zeilhofer HU. Spinal prostaglandin e receptors of the EP2 subtype and the glycine receptor α3 subunit, which mediate central inflammatory hyperalgesia, do not contribute to pain after peripheral nerve injury or formalin injection. Pain. 2006;126:46–53. doi: 10.1016/j.pain.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Adams RH, Sato K, Shimada S, Tohyama M, Puschel AW, Betz H. Gene structure and glial expression of the glycine transporter GlyT1 in embryonic and adult rodents. J Neurosci. 1995;15:2524–2532. doi: 10.1523/JNEUROSCI.15-03-02524.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J. Glycine transporters are differentially expressed among cns cells. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zafra F, Gomeza J, Olivares L, Aragon C, Gimenez C. Regional distribution and developmental variation of the glycine transporters GlyT1 and GlyT2 in the rat cns. Eur J Neurosci. 1995;7:1342–1352. doi: 10.1111/j.1460-9568.1995.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 61.Cubelos B, Gimenez C, Zafra F. Localization of the GlyT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb Cortex. 2005;15:448–459. doi: 10.1093/cercor/bhh147. [DOI] [PubMed] [Google Scholar]
- 62.Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, Knuesel I, Benke D, Feldon J, Möhler H, Boison D. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J Neurosci. 2006;26:3169–3181. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eulenburg V, Retiounskaia M, Papadopoulos T, Gomeza J, Betz H. Glial glycine transporter 1 function is essential for early postnatal survival but dispensable in adult mice. Glia. 2010;58:1066–1073. doi: 10.1002/glia.20987. [DOI] [PubMed] [Google Scholar]
- 64.Gomeza J, Hülsmann S, Ohno K, Eulenburg V, Szoke K, Richter D, Betz H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron. 2003;40:785–796. doi: 10.1016/S0896-6273(03)00672-X. [DOI] [PubMed] [Google Scholar]
- 65.Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/S0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 66.Hanuska A, Szenasi G, Albert M, Koles L, Varga A, Szabo A, Matyus P, Harsing LG., Jr Some operational characteristics of glycine release in rat retina: the role of reverse mode operation of glycine transporter type-1 (GlyT-1) in ischemic conditions. Neurochem Res. 2016;41:73–85. doi: 10.1007/s11064-015-1713-z. [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto K. Glycine transporter-1: a new potential therapeutic target for schizophrenia. Curr Pharm Des. 2011;17:112–120. doi: 10.2174/138161211795049598. [DOI] [PubMed] [Google Scholar]
- 68.Singer P, Dubroqua S, Yee BK. Inhibition of glycine transporter 1: the yellow brick road to new schizophrenia therapy? Curr Pharm Des. 2015;21:3771–3787. doi: 10.2174/1381612821666150724100952. [DOI] [PubMed] [Google Scholar]
- 69.Bugarski-Kirola D, Iwata N, Sameljak S, Reid C, Blaettler T, Millar L, Marques TR, Garibaldi G, Kapur S. Efficacy and safety of adjunctive bitopertin versus placebo in patients with suboptimally controlled symptoms of schizophrenia treated with antipsychotics: results from three phase 3, randomised, double-blind, parallel-group, placebo-controlled, multicentre studies in the searchlyte clinical trial programme. Lancet Psychiatry. 2016;3:1115–1128. doi: 10.1016/S2215-0366(16)30344-3. [DOI] [PubMed] [Google Scholar]
- 70.Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, vGAT, localizes to synaptic vesicles in sets of glycinergic as well as gabaergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 72.Gomeza J, Ohno K, Hülsmann S, Armsen W, Eulenburg V, Richter DW, Laube B, Betz H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron. 2003;40:797–806. doi: 10.1016/S0896-6273(03)00673-1. [DOI] [PubMed] [Google Scholar]
- 73.Bradaia A, Schlichter R, Trouslard J. Role of glial and neuronal glycine transporters in the control of glycinergic and glutamatergic synaptic transmission in lamina X of the rat spinal cord. J Physiol. 2004;559:169–186. doi: 10.1113/jphysiol.2004.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanabe M, Takasu K, Yamaguchi S, Kodama D, Ono H. Glycine transporter inhibitors as a potential therapeutic strategy for chronic pain with memory impairment. Anesthesiology. 2008;108:929–937. doi: 10.1097/ALN.0b013e31816c9044. [DOI] [PubMed] [Google Scholar]
- 75.Jeong HJ, Vandenberg RJ, Vaughan CW. N-Arachidonyl-glycine modulates synaptic transmission in superficial dorsal horn. Br J Pharmacol. 2010;161:925–935. doi: 10.1111/j.1476-5381.2010.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eckle VS, Antkowiak B. ALX 1393 inhibits spontaneous network activity by inducing glycinergic tonic currents in the spinal ventral horn. Neuroscience. 2013;253:165–171. doi: 10.1016/j.neuroscience.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 77.Hermanns H, Muth-Selbach U, Williams R, Krug S, Lipfert P, Werdehausen R, Braun S, Bauer I. Differential effects of spinally applied glycine transporter inhibitors on nociception in a rat model of neuropathic pain. Neurosci Lett. 2008;445:214–219. doi: 10.1016/j.neulet.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 78.Dohi T, Morita K, Kitayama T, Motoyama N, Morioka N. Glycine transporter inhibitors as a novel drug discovery strategy for neuropathic pain. Pharmacol Ther. 2009;123:54–79. doi: 10.1016/j.pharmthera.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 79.Barthel F, Urban A, Schlosser L, Eulenburg V, Werdehausen R, Brandenburger T, Aragon C, Bauer I, Hermanns H. Long-term application of glycine transporter inhibitors acts antineuropathic and modulates spinal N-methyl-d-aspartate receptor subunit NR-1 expression in rats. Anesthesiology. 2014;121:160–169. doi: 10.1097/ALN.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 80.Motoyama N, Morita K, Shiraishi S, Kitayama T, Kanematsu T, Uezono Y, Dohi T. Relief of cancer pain by glycine transporter inhibitors. Anesth Analg. 2014;119:988–995. doi: 10.1213/ANE.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 81.Morita K, Motoyama N, Kitayama T, Morioka N, Kifune K, Dohi T. Spinal antiallodynia action of glycine transporter inhibitors in neuropathic pain models in mice. J Pharmacol Exp Ther. 2008;326:633–645. doi: 10.1124/jpet.108.136267. [DOI] [PubMed] [Google Scholar]
- 82.Haranishi Y, Hara K, Terada T, Nakamura S, Sata T. The antinociceptive effect of intrathecal administration of glycine transporter-2 inhibitor ALX1393 in a rat acute pain model. Anesth Analg. 2010;110:615–621. doi: 10.1213/ANE.0b013e3181c7ebbb. [DOI] [PubMed] [Google Scholar]
- 83.Nishikawa Y, Sasaki A, Kuraishi Y. Blockade of glycine transporter (GlyT) 2, but not GlyT1, ameliorates dynamic and static mechanical allodynia in mice with herpetic or postherpetic pain. J Pharmacol Sci. 2010;112:352–360. doi: 10.1254/jphs.09351FP. [DOI] [PubMed] [Google Scholar]
- 84.Yoshikawa S, Oguchi T, Funahashi Y, de Groat WC, Yoshimura N. Glycine transporter type 2 (GlyT2) inhibitor ameliorates bladder overactivity and nociceptive behavior in rats. Eur Urol. 2012;62:704–712. doi: 10.1016/j.eururo.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mingorance-Le Meur A, Ghisdal P, Mullier B, De Ron P, Downey P, Van Der Perren C, Declercq V, Cornelis S, Famelart M, Van Asperen J, Jnoff E, Courade JP. Reversible inhibition of the glycine transporter GlyT2 circumvents acute toxicity while preserving efficacy in the treatment of pain. Br J Pharmacol. 2013;170:1053–1063. doi: 10.1111/bph.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Omori Y, Nakajima M, Nishimura K, Takahashi E, Arai T, Akahira M, Suzuki T, Kainoh M. Analgesic effect of GT-0198, a structurally novel glycine transporter 2 inhibitor, in a mouse model of neuropathic pain. J Pharmacol Sci. 2015;127:377–381. doi: 10.1016/j.jphs.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi Y, Hara K, Haranishi Y, Terada T, Obara G, Sata T. Antinociceptive effect of intracerebroventricular administration of glycine transporter-2 inhibitor ALX1393 in rat models of inflammatory and neuropathic pain. Pharmacol Biochem Behav. 2015;130:46–52. doi: 10.1016/j.pbb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Harvey RJ, Yee BK. Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nat Rev Drug Discov. 2013;12:866–885. doi: 10.1038/nrd3893. [DOI] [PubMed] [Google Scholar]
- 89.Carland JE, Handford CA, Ryan RM, Vandenberg RJ. Lipid inhibitors of high affinity glycine transporters: identification of a novel class of analgesics. Neurochem Int. 2014;73:211–216. doi: 10.1016/j.neuint.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 90.Vandenberg RJ, Ryan RM, Carland JE, Imlach WL, Christie MJ. Glycine transport inhibitors for the treatment of pain. Trends Pharmacol Sci. 2014;35:423–430. doi: 10.1016/j.tips.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 91.Crestani F, Low K, Keist R, Mandelli M, Möhler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- 92.Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Bötzinger complex in the cat suppresses respiratory rhythm generation in vivo. J Physiol. 1998;509(Pt 1):245–254. doi: 10.1111/j.1469-7793.1998.245bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sherman D, Worrell JW, Cui Y, Feldman JL. Optogenetic perturbation of prebotzinger complex inhibitory neurons modulates respiratory pattern. Nat Neurosci. 2015;18:408–414. doi: 10.1038/nn.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nigro MA, Lim HC. Hyperekplexia and sudden neonatal death. Pediatr Neurol. 1992;8:221–225. doi: 10.1016/0887-8994(92)90073-8. [DOI] [PubMed] [Google Scholar]
- 95.Giacoia GP, Ryan SG. Hyperekplexia associated with apnea and sudden infant death syndrome. Arch Pediatr Adolesc Med. 1994;148:540–543. doi: 10.1001/archpedi.1994.02170050098025. [DOI] [PubMed] [Google Scholar]
- 96.Manzke T, Niebert M, Koch UR, Caley A, Vogelgesang S, Hulsmann S, Ponimaskin E, Müller U, Smart TG, Harvey RJ, Richter DW. Serotonin receptor 1A-modulated phosphorylation of glycine receptor α3 controls breathing in mice. J Clin Invest. 2010;120:4118–4128. doi: 10.1172/JCI43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Q, Wong-Riley MT. Postnatal development of glycine receptor subunits α1, α2, α3, and β immunoreactivity in multiple brain stem respiratory-related nuclear groups of the rat. Brain Res. 2013;1538:1–16. doi: 10.1016/j.brainres.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buckwalter MS, Cook SA, Davisson MT, White WF, Camper SA. A frameshift mutation in the mouse α1 glycine receptor gene (glra1) results in progressive neurological symptoms and juvenile death. Hum Mol Genet. 1994;3:2025–2030. doi: 10.1093/hmg/3.11.2025. [DOI] [PubMed] [Google Scholar]
- 99.Büsselberg D, Bischoff AM, Becker K, Becker CM, Richter DW. The respiratory rhythm in mutant oscillator mice. Neurosci Lett. 2001;316:99–102. doi: 10.1016/S0304-3940(01)02382-5. [DOI] [PubMed] [Google Scholar]
- 100.Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44:151–347. [PubMed] [Google Scholar]
- 101.Lüscher C. Drug-evoked synaptic plasticity causing addictive behavior. J Neurosci. 2013;33:17641–17646. doi: 10.1523/JNEUROSCI.3406-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Molander A, Lof E, Stomberg R, Ericson M, Soderpalm B. Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcohol Clin Exp Res. 2005;29:38–45. doi: 10.1097/01.ALC.0000150009.78622.E0. [DOI] [PubMed] [Google Scholar]
- 103.Molander A, Lido HH, Lof E, Ericson M, Soderpalm B. The glycine reuptake inhibitor ORG 25935 decreases ethanol intake and preference in male wistar rats. Alcohol Alcohol. 2007;42:11–18. doi: 10.1093/alcalc/agl085. [DOI] [PubMed] [Google Scholar]
- 104.Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jonsson S, Adermark L, Ericson M, Soderpalm B. The involvement of accumbal glycine receptors in the dopamine-elevating effects of addictive drugs. Neuropharmacology. 2014;82:69–75. doi: 10.1016/j.neuropharm.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 106.Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol. 1996;50:402–406. [PubMed] [Google Scholar]
- 107.Valenzuela CF, Cardoso RA, Wick MJ, Weiner JL, Dunwiddie TV, Harris RA. Effects of ethanol on recombinant glycine receptors expressed in mammalian cell lines. Alcohol Clin Exp Res. 1998;22:1132–1136. doi: 10.1111/j.1530-0277.1998.tb03712.x. [DOI] [PubMed] [Google Scholar]
- 108.Cui C, Koob GF. Titrating tipsy targets: the neurobiology of low-dose alcohol. Trends Pharmacol Sci. 2017;38:556–568. doi: 10.1016/j.tips.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ye JH, Tao L, Ren J, Schaefer R, Krnjevic K, Liu PL, Schiller DA, McArdle JJ. Ethanol potentiation of glycine-induced responses in dissociated neurons of rat ventral tegmental area. J Pharmacol Exp Ther. 2001;296:77–83. [PubMed] [Google Scholar]
- 110.Forstera B, Muñoz B, Lobo MK, Chandra R, Lovinger DM, Aguayo LG. Presence of ethanol sensitive glycine receptors in medium spiny neurons in the mouse nucleus accumbens. J Physiol. 2017;595:5285–5300. doi: 10.1113/JP273767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blednov YA, Benavidez JM, Black M, Leiter CR, Osterndorff-Kahanek E, Harris RA. Glycine receptors containing & #x03B1;2 or & #x03B1;3 subunits regulate specific ethanol-mediated behaviors. J Pharmacol Exp Ther. 2015;353:181–191. doi: 10.1124/jpet.114.221895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Han S, Gelernter J, Kranzler HR, Yang BZ. Ordered subset linkage analysis based on admixture proportion identifies new linkage evidence for alcohol dependence in african-americans. Hum Genet. 2013;132:397–403. doi: 10.1007/s00439-012-1255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang C, Nobles RD, McCall MA. GlyRα2, not GlyRα3, modulates the receptive field surround of off retinal ganglion cells. Vis Neurosci. 2015;32:E026. doi: 10.1017/S0952523815000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dlugaiczyk J, Hecker D, Neubert C, Buerbank S, Campanelli D, Becker CM, Betz H, Knipper M, Ruttiger L, Schick B. Loss of glycine receptors containing the α3 subunit compromises auditory nerve activity, but not outer hair cell function. Hear Res. 2016;337:25–34. doi: 10.1016/j.heares.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 115.Tziridis K, Buerbank S, Eulenburg V, Dlugaiczyk J, Schulze H. Deficit in acoustic signal-in-noise detection in glycine receptor α3 subunit knockout mice. Eur J Neurosci. 2017;45:581–586. doi: 10.1111/ejn.13489. [DOI] [PubMed] [Google Scholar]
- 116.Oskarsson V. The effect of strychnine on visual acuity. Acta Pharmacol Toxicol. 1962;19:16–22. doi: 10.1111/j.1600-0773.1962.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 117.Preibisch-Effenberger R, Knothe J. Der Einfluß zentral wirkender Pharmaka auf die Wortverständlichkeit des Normalhörenden bei binauraler Testsprache. Z Laryngol Rhinol Otol Ihre Grenzgeb. 1965;44:619–630. [PubMed] [Google Scholar]
- 118.Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brozoski TJ, Caspary DM, Bauer CA, Richardson BD. The effect of supplemental dietary taurine on tinnitus and auditory discrimination in an animal model. Hear Res. 2010;270:71–80. doi: 10.1016/j.heares.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paul J, Yévenes GE, Benke D, Di Lio A, Ralvenius WT, Witschi R, Scheurer L, Cook JM, Rudolph U, Fritschy JM, Zeilhofer HU. Antihyperalgesia by α2-GABAA receptors occurs via a genuine spinal action and does not involve supraspinal sites. Neuropsychopharmacology. 2014;39:477–487. doi: 10.1038/npp.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Asiedu M, Ossipov MH, Kaila K, Price TJ. Acetazolamide and midazolam act synergistically to inhibit neuropathic pain. Pain. 2010;148:302–308. doi: 10.1016/j.pain.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Herdon HJ, Godfrey FM, Brown AM, Coulton S, Evans JR, Cairns WJ. Pharmacological assessment of the role of the glycine transporter GlyT-1 in mediating high-affinity glycine uptake by rat cerebral cortex and cerebellum synaptosomes. Neuropharmacology. 2001;41:88–96. doi: 10.1016/S0028-3908(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 123.Johnson JW, Ascher P. Glycine potentiates the nmda response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 124.Ahmadi S, Muth-Selbach U, Lauterbach A, Lipfert P, Neuhuber WL, Zeilhofer HU. Facilitation of spinal nmda receptor currents by spillover of synaptically released glycine. Science. 2003;300:2094–2097. doi: 10.1126/science.1083970. [DOI] [PubMed] [Google Scholar]
- 125.Lim R, Hoang P, Berger AJ. Blockade of glycine transporter-1 (GlyT-1) potentiates nmda receptor-mediated synaptic transmission in hypoglossal motorneurons. J Neurophysiol. 2004;92:2530–2537. doi: 10.1152/jn.01123.2003. [DOI] [PubMed] [Google Scholar]
- 126.Imamura Y, Ma CL, Pabba M, Bergeron R. Sustained saturating level of glycine induces changes in NR2B-containing-NMDA receptor localization in the CA1 region of the hippocampus. J Neurochem. 2008;105:2454–2465. doi: 10.1111/j.1471-4159.2008.05324.x. [DOI] [PubMed] [Google Scholar]
- 127.Yévenes GE, Zeilhofer HU. Allosteric modulation of glycine receptors. Br J Pharmacol. 2011;164:224–236. doi: 10.1111/j.1476-5381.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zeilhofer HU, Benke D, Yévenes GE. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annu Rev Pharmacol Toxicol. 2012;52:111–133. doi: 10.1146/annurev-pharmtox-010611-134636. [DOI] [PubMed] [Google Scholar]
- 129.Pistis M, Belelli D, Peters JA, Lambert JJ. The interaction of general anaesthetics with recombinant GABAA and glycine receptors expressed in xenopus laevis oocytes: a comparative study. Br J Pharmacol. 1997;122:1707–1719. doi: 10.1038/sj.bjp.0701563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;55:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sonner JM, Zhang Y, Stabernack C, Abaigar W, Xing Y, Laster MJ. GABAA receptor blockade antagonizes the immobilizing action of propofol but not ketamine or isoflurane in a dose-related manner. Anesth Analg. 2003;96:706–712. doi: 10.1213/01.ANE.0000048821.23225.3A. [DOI] [PubMed] [Google Scholar]
- 132.Grasshoff C, Antkowiak B. Propofol and sevoflurane depress spinal neurons in vitro via different molecular targets. Anesthesiology. 2004;101:1167–1176. doi: 10.1097/00000542-200411000-00017. [DOI] [PubMed] [Google Scholar]
- 133.Nguyen HT, Li KY, daGraca RL, Delphin E, Xiong M, Ye JH. Behavior and cellular evidence for propofol-induced hypnosis involving brain glycine receptors. Anesthesiology. 2009;110:326–332. doi: 10.1097/ALN.0b013e3181942b5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de la Roche J, Leuwer M, Krampfl K, Haeseler G, Dengler R, Buchholz V, Ahrens J. 4-Chloropropofol enhances chloride currents in human hyperekplexic and artificial mutated glycine receptors. BMC neurology. 2012;12:104. doi: 10.1186/1471-2377-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eckle VS, Grasshoff C, Mirakaj V, O’Neill PM, Berry NG, Leuwer M, Antkowiak B. 4-Bromopropofol decreases action potential generation in spinal neurons by inducing a glycine receptor-mediated tonic conductance. Br J Pharmacol. 2014;171:5790–5801. doi: 10.1111/bph.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Germann AL, Shin DJ, Manion BD, Edge CJ, Smith EH, Franks NP, Evers AS, Akk G. Activation and modulation of recombinant glycine and GABAA receptors by 4-halogenated analogues of propofol. Br J Pharmacol. 2016;173:3110–3120. doi: 10.1111/bph.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Krasowski MD, Jenkins A, Flood P, Kung AY, Hopfinger AJ, Harrison NL. General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of γ-aminobutyric acid GABA current at the GABAA receptor but not with lipid solubility. J Pharmacol Exp Ther. 2001;297:338–351. [PubMed] [Google Scholar]
- 138.Ahrens J, Leuwer M, de la Roche J, Foadi N, Krampfl K, Haeseler G. The non-anaesthetic propofol analogue 2,6-di-tert-butylphenol fails to modulate GABAA receptor function. Pharmacology. 2009;83:95–98. doi: 10.1159/000180125. [DOI] [PubMed] [Google Scholar]
- 139.Ahrens J, Haeseler G, Leuwer M, Mohammadi B, Krampfl K, Dengler R, Bufler J. 2,6 Di-tert-butylphenol, a nonanesthetic propofol analog, modulates α1β glycine receptor function in a manner distinct from propofol. Anesth Analg. 2004;99:91–96. doi: 10.1213/01.ANE.0000120083.10269.54. [DOI] [PubMed] [Google Scholar]
- 140.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1β-mediated induction of COX-2 in the cns contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 141.Tibbs GR, Rowley TJ, Sanford RL, Herold KF, Proekt A, Hemmings HC, Jr, Andersen OS, Goldstein PA, Flood PD. HCN1 channels as targets for anesthetic and nonanesthetic propofol analogs in the amelioration of mechanical and thermal hyperalgesia in a mouse model of neuropathic pain. J Pharmacol Exp Ther. 2013;345:363–373. doi: 10.1124/jpet.113.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chesnoy-Marchais D. Potentiation of chloride responses to glycine by three 5-HT3 antagonists in rat spinal neurones. Br J Pharmacol. 1996;118:2115–2125. doi: 10.1111/j.1476-5381.1996.tb15651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maksay G. Bidirectional allosteric modulation of strychnine-sensitive glycine receptors by tropeines and 5-HT3 serotonin receptor ligands. Neuropharmacology. 1998;37:1633–1641. doi: 10.1016/S0028-3908(98)00127-0. [DOI] [PubMed] [Google Scholar]
- 144.Supplisson S, Chesnoy-Marchais D. Glycine receptor β subunits play a critical role in potentiation of glycine responses by ICS-205,930. Mol Pharmacol. 2000;58:763–770. doi: 10.1124/mol.58.4.763. [DOI] [PubMed] [Google Scholar]
- 145.Maksay G, Nemes P, Biro T. Synthesis of tropeines and allosteric modulation of ionotropic glycine receptors. J Med Chem. 2004;47:6384–6391. doi: 10.1021/jm040814g. [DOI] [PubMed] [Google Scholar]
- 146.Yang Z, Ney A, Cromer BA, Ng HL, Parker MW, Lynch JW. Tropisetron modulation of the glycine receptor: femtomolar potentiation and a molecular determinant of inhibition. J Neurochem. 2007;100:758–769. doi: 10.1111/j.1471-4159.2006.04242.x. [DOI] [PubMed] [Google Scholar]
- 147.Hejazi N, Zhou C, Oz M, Sun H, Ye JH, Zhang L. Δ9-tetrahydrocannabinol and endogenous cannabinoid anandamide directly potentiate the function of glycine receptors. Mol Pharmacol. 2006;69:991–997. doi: 10.1124/mol.105.019174. [DOI] [PubMed] [Google Scholar]
- 148.Xiong W, Wu X, Li F, Cheng K, Rice KC, Lovinger DM, Zhang L. A common molecular basis for exogenous and endogenous cannabinoid potentiation of glycine receptors. J Neurosci. 2012;32:5200–5208. doi: 10.1523/JNEUROSCI.6347-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xiong W, Chen SR, He L, Cheng K, Zhao YL, Chen H, Li DP, Homanics GE, Peever J, Rice KC, Wu LG, Pan HL, Zhang L. Presynaptic glycine receptors as a potential therapeutic target for hyperekplexia disease. Nat Neurosci. 2014;17:232–239. doi: 10.1038/nn.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ahrens J, Demir R, Leuwer M, de la Roche J, Krampfl K, Foadi N, Karst M, Haeseler G. The nonpsychotropic cannabinoid cannabidiol modulates and directly activates α-1 and α-1-β glycine receptor function. Pharmacology. 2009;83:217–222. doi: 10.1159/000201556. [DOI] [PubMed] [Google Scholar]
- 151.Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci. 2001;58:760–793. doi: 10.1007/PL00000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- 153.Hruskova B, Trojanova J, Kulik A, Kralikova M, Pysanenko K, Bures Z, Syka J, Trussell LO, Turecek R. Differential distribution of glycine receptor subtypes at the rat calyx of held synapse. J Neurosci. 2012;32:17012–17024. doi: 10.1523/JNEUROSCI.1547-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wells MM, Tillman TS, Mowrey DD, Sun T, Xu Y, Tang P. Ensemble-based virtual screening for cannabinoid-like potentiators of the human glycine receptor α1 for the treatment of pain. J Med Chem. 2015;58:2958–2966. doi: 10.1021/jm501873p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jin GL, Su YP, Liu M, Xu Y, Yang J, Liao KJ, Yu CX. Medicinal plants of the genus gelsemium (gelsemiaceae, gentianales)—a review of their phytochemistry, pharmacology, toxicology and traditional use. J Ethnopharmacol. 2014;152:33–52. doi: 10.1016/j.jep.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 156.Zhang JY, Gong N, Huang JL, Guo LC, Wang YX. Gelsemine, a principal alkaloid from gelsemium sempervirens Ait., exhibits potent and specific antinociception in chronic pain by acting at spinal α3 glycine receptors. Pain. 2013;154:2452–2462. doi: 10.1016/j.pain.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 157.Lara CO, Murath P, Munoz B, Marileo AM, Martin LS, San Martin VP, Burgos CF, Mariqueo TA, Aguayo LG, Fuentealba J, Godoy P, Guzman L, Yévenes GE. Functional modulation of glycine receptors by the alkaloid gelsemine. Br J Pharmacol. 2016;173:2263–2277. doi: 10.1111/bph.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kiuru P, D’Auria MV, Muller CD, Tammela P, Vuorela H, Yli-Kauhaluoma J. Exploring marine resources for bioactive compounds. Planta Med. 2014;80:1234–1246. doi: 10.1055/s-0034-1383001. [DOI] [PubMed] [Google Scholar]
- 159.Balansa W, Islam R, Fontaine F, Piggott AM, Zhang H, Webb TI, Gilbert DF, Lynch JW, Capon RJ. Ircinialactams: subunit-selective glycine receptor modulators from Australian sponges of the family irciniidae. Bioorg Med Chem. 2010;18:2912–2919. doi: 10.1016/j.bmc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 160.Balansa W, Islam R, Fontaine F, Piggott AM, Zhang H, Xiao X, Webb TI, Gilbert DF, Lynch JW, Capon RJ. Sesterterpene glycinyl-lactams: a new class of glycine receptor modulator from australian marine sponges of the genus psammocinia. Org Biomol Chem. 2013;11:4695–4701. doi: 10.1039/c3ob40861b. [DOI] [PubMed] [Google Scholar]
- 161.Balansa W, Islam R, Gilbert DF, Fontaine F, Xiao X, Zhang H, Piggott AM, Lynch JW, Capon RJ. Australian marine sponge alkaloids as a new class of glycine-gated chloride channel receptor modulator. Bioorg Med Chem. 2013;21:4420–4425. doi: 10.1016/j.bmc.2013.04.061. [DOI] [PubMed] [Google Scholar]
- 162.Stead C, Brown A, Adams C, Nickolls SJ, Young G, Kammonen J, Pryde D, Cawkill D. Identification of positive allosteric modulators of glycine receptors from a high-throughput screen using a fluorescent membrane potential assay. J Biomol Screen. 2016;21:1042–1053. doi: 10.1177/1087057116657779. [DOI] [PubMed] [Google Scholar]
- 163.Bregman H, Simard JR, Andrews KL, Ayube S, Chen H, Gunaydin H, Guzman-Perez A, Hu J, Huang L, Huang X, Krolikowski PH, Lehto SG, Lewis RT, Michelsen K, Pegman P, Plant MH, Shaffer PL, Teffera Y, Yi S, Zhang M, Gingras J, DiMauro EF. The discovery and hit-to-lead optimization of tricyclic sulfonamides as potent and efficacious potentiators of glycine receptors. J Med Chem. 2017;60:1105–1125. doi: 10.1021/acs.jmedchem.6b01496. [DOI] [PubMed] [Google Scholar]
- 164.Chakka N, Andrews K, Berry L, Bregman H, Gunaydin H, Huang L, Guzman-Perez A, Plant MH, Simard J, Gingras J, Dimauro EF. Applications of parallel synthetic lead hopping and pharmacophore-based virtual screening in the discovery of efficient glycine receptor potentiators. Eur J Med Chem. 2017;137:63–75. doi: 10.1016/j.ejmech.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 165.Polgár E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal gabaergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/S0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 166.Polgár E, Gray S, Riddell JS, Todd AJ. Lack of evidence for significant neuronal loss in laminae I–III of the spinal dorsal horn of the rat in the chronic constriction injury model. Pain. 2004;111:144–150. doi: 10.1016/j.pain.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 167.Polgár E, Hughes DI, Arham AZ, Todd AJ. Loss of neurons from laminas I–III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci. 2005;25:6658–6666. doi: 10.1523/JNEUROSCI.1490-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moraga-Cid G, Yévenes GE, Schmalzing G, Peoples RW, Aguayo LG A single phenylalanine residue in the main intracellular loop of α1 γ-aminobutyric acid type A and glycine receptors influences their sensitivity to propofol. Anesthesiology. 2011;115:464–473. doi: 10.1097/ALN.0b013e31822550f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Espejo-Porras F, Fernandez-Ruiz J, Pertwee RG, Mechoulam R, Garcia C. Motor effects of the non-psychotropic phytocannabinoid cannabidiol that are mediated by 5-HT1A receptors. Neuropharmacology. 2013;75:155–163. doi: 10.1016/j.neuropharm.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 171.Sparling BA, DiMauro EF. Progress in the discovery of small molecule modulators of the cys-lool superfamily receptors. Bioorg Med Chem Lett. 2017;27:3207–3218. doi: 10.1016/j.bmcl.2017.04.073. [DOI] [PubMed] [Google Scholar]