Abstract
Toll-like receptors (TLR)s are central in immune response by recognizing pathogen-associated molecular patterns (PAMP)s. If they are essential to eliminate pathogens in earlier stages of infection, they also might play a role in homeostasis and tissue repair. TLR versatility parallels the plasticity of monocytes, which represent an heterogeneous population of immune cells. They are rapidly recruited to sites of infection and involved in clearance of pathogens and in tissue healing. This review underlines how TLRs have proved to be an interesting tool to study the properties of monocytes and why different therapeutic strategies exploring monocyte plasticity may be relevant in the context of chronic inflammatory disorders.
Keywords: Monocytes, TLR, Mal/TIRAP, IRAK4, Inflammation
Introduction
Toll-Like Receptor (TLR) discovery was crucial for immunology and was recognized as such with the awarding of the 2011 Nobel Prize in Physiology or Medicine to Jules Hoffmann, Bruce Beutler, and Ralph Steimann [1]. These receptors were described in the first line of host defense against microbe infections (bacteria, virus, fungi, and protozoan) to mount specific responses like cytokine production after recognizing several classes of components from microorganisms, the pathogen-associated molecular patterns (PAMPs) [2–5]. TLRs also promote maturation of antigen presenting cells such as dendritic cells (DC)s, which in turn direct the induction of adaptive immune response. For this reason, TLR agonists are being exploited as vaccine adjuvants for infectious diseases and as therapeutics against tumors. The cooperation between TLRs during infection is a notion that is well accepted and probably explains the broad range of TLR actions on immune cells [6, 7]. Over the past years, the development of new therapeutics based on TLR field has focused on three main areas: the identification of new ligands including putative endogenous, the further elucidation of components of individual TLR signalling, and in vivo studies to understand the collaborative function of TLRs with other receptors of innate immunity in the resistance to infection. Recently, it has been proposed that TLRs might play a prominent role in tissue repairing [8].
In parallel, intensive research efforts have unraveled important functional characteristics of monocytes. In this regard, the remarkable multi-potency of monocytes has been described in inflammatory environment [9–12]. Monocytes have an essential role in antimicrobial immune defense [13] and promote tissue healing [14], but, like a double-edged sword, they also contribute to tissue destruction during some infections and chronic inflammatory diseases. In mice, there are two major monocyte subsets based on their expression of Ly6C, Ly6Chigh (classical) with proinflammatory function and Ly6Clow (non-classical) with patrolling behavior and healing function [15]. At least, three human monocyte subsets were identified: CD14++CD16− (classical), which resemble Ly6Chigh monocytes; CD14++CD16+ (intermediate), with proinflammatory roles; and CD14+CD16++ monocytes with patrolling behavior, which resemble Ly6Clow monocytes [16]. Each cell subset responds to different chemotactics that explains a different localization in homeostasis, one patrolling in blood while the other residing in tissue. The networks of cell information and signal amplification allowing pro- or antiinflammatory cytokine production by monocytes are not well known.
Here, the review focuses on the role of TLR as a marker of monocyte plasticity. Based on this, different therapeutic strategies exploring monocyte plasticity are discussed that concern inflammatory disorders in general since different monocyte actions share common themes during inflammation.
The orchestra of TLRs in innate immune response
Up to now, 10 TLRs have been reported in humans and 12 in mice. Generally, their localization is in accord with the type of pathogen recognized. TLRs frequently expressed on cell surface (1, 2, 4, 5, 6, and 10) recognize microbial membrane components such as lipids, lipoproteins, and proteins [17, 18] while TLRs expressed in endosomes (3, 7, 8 and 9) recognize nucleic acids derived from bacteria, viruses, parasites and also recognize self-nucleic acids in disease conditions such as autoimmunity [19]. TLRs form heterodimers or homodimers as a means of triggering a signal. Most TLRs form homodimers, with a few exceptions. For example, TLR2 forms heterodimers with TLR1 or TLR6, which enables differential recognition of lipopeptides [20]. In addition, to ensure proper detection of PAMPs and discrimination between self- and non-self, specific accessory proteins or cofactors are involved in final response to TLRs [21].
TLR signalling events have been the subject of intense investigation and are reviewed extensively elsewhere [22–24]. The individual response to pathogens is mediated through a family of adapter molecules comprising MyD88 (myeloid differentiation primary response 88), Mal (MyD88 adapter-like)/TIRAP (TIR-domain containing adapter protein), TRIF (Toll-receptor-associated activator of interferon), and TRAM (TRIF-related adapter molecule) [25]. The fifth adapter SARM (sterile α- and armadillo-motif-containing protein) is functionally unique, suppressing immune signalling instead of promoting it.
With the exception of TLR3, all TLRs initiate a MyD88-dependent signalling pathway. The signal adaptor protein MyD88 contains two main conserved protein domains: a C-terminal TIR and a N-terminal death domain [26]. Upon TLR activation and dimerisation, MyD88 is recruited to the TIR domain of the activated TLR via TIR–TIR interaction. Through its death domain adaptor region, MyD88 recruits the kinases IRAK1 and IRAK4 into the signalling complex via death domains [27]. The IRAK and other kinase families are responsible for the propagation of signal downstream of protein adapter and consequently cell responses [23, 24] (Fig. 1). IRAK4 plays a crucial role in MyD88-dependent response interacting with TRAF6 and IRAK1, leading to IKK and MAPK phosphorylation, and culminating in transcription factor activation, mainly NF-κB [22, 28, 29]. Generally, proinflammatory cytokines such as IL-12, TNF-α, and IFN-γ were clearly associated with TLR-MyD88-NF-κB signal [30, 31]. This explains the central role of MyD88 in protozoan infections, where generally a Th1 response has been shown to be protective [18]. Besides NF-κB, TLR pathway activation involves the use of three families of transcription factor that will take into account the diversity of TLR response: AP1 (activator protein 1 Jun/Fos proteins), CREB (cAMP response element-binding protein), and IRF (Interferon Regulatory Factor). In general, the activation of NF-κB and AP1 has been linked to a Th1 profile [32], whereas the concomitant activation with CREB leads to IL-10 synthesis and a Th2 pattern [33, 34]. The activation of IRFs is closely linked to internalized TLR4 and nucleic acids’ recognition by endosomal TLRs [35, 36].
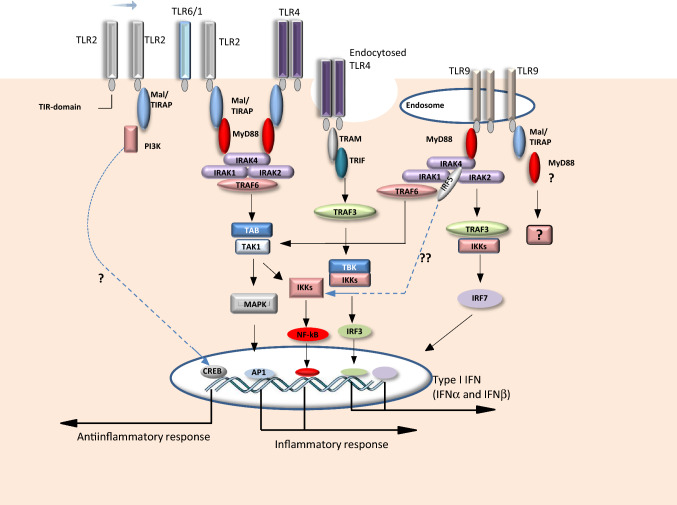
Fig. 1.
TLR pathway is exemplified by the esquematic representation of TLR2, 4, and 9 signalling pathways (canonical and non-canonical). After dimerization of receptors initiated by recognize of ligands, the Toll–IL-1-resistance (TIR) domains of TLRs engage TIR domain-containing adaptor proteins (either myeloid differentiation primary-response protein 88 (MyD88) and MyD88-adaptor-like protein (Mal)/TIRAP, or TIR domain-containing adaptor protein inducing IFNβ (TRIF) and TRIF-related adaptor molecule (TRAM)). Generally, Mal/TIRAP is not involved in TLR9 pathway, but Bonham et al. [39] and our group [41] have described a Mal/TIRAP-dependent TLR9 response. Other peculiarity in TLR functions is the MyD88-independent TLR2 pathway leading to an antiinflammatory response where the phosphatidylinositol-3-kinase (PI3K) might play a role. In a second step, the engagement of the signalling adaptor molecules stimulates downstream signalling pathways that involve IRAK family and the adaptor molecules TNF receptor-associated factors (TRAFs) and lead to the activation of the mitogen-activated protein kinases (MAPKs) and transcription factors. Two important families of transcription factors that are activated downstream of TLR signalling are the nuclear factor-κB (NF-κB) and interferon-regulatory factor (IRF) families, but other transcription factors, such as cyclic AMP-responsive element-binding protein (CREB) and activator protein 1 (AP1), also play a role in TLR responses. Generally, the activation of TLR pathways leads to proinflammatory cytokine production in a MyD88-dependent way, to type I interferon production when endosomal TLRs are engaged and to IL-10 release often linked to Mal/TIRAP pathway. IKK inhibitor of NF-κB kinase, TAB TAK1-binding protein, TAK TGFβ-activated kinase, TBK1 TANK-binding kinase 1
Originally, it has been stated that the MyD88-dependent pathway could be initiated by TLR5 and TLR7, 8, 9 using the adaptor MyD88 alone, while the adaptor protein Mal/TIRAP would be required with MyD88 to initiate signalling downstream of TLR2 and TLR4. However, different studies have challenged this picture. While Mal/TIRAP was initially excluded as an adaptor for endosomal TLRs because Mal/TIRAP-deficient cells retain the ability to respond to synthetic TLR7 and TLR9 ligands [37, 38], Bonham et al. have suggested that Mal/TIRAP is necessary for TLR9 signalling in natural situations such as HSV-1 infection [39]. Indeed, the requirement of Mal/TIRAP may be bypassed when high concentrations of synthetic TLR agonists are used that is often the case for in vitro studies. Further, Mal/TIRAP has been described as dispensable when the interactions between TLR2 and its agonist are prolonged or enhanced [40]. This parallels a study using a model of infection with the protozoan parasite Trypanosoma cruzi, the agent of Chagas disease. In this work, Mal/TIRAP has been shown to be dispensable for TLR2 response in Ly6Chigh monocytes but crucial for cytokine production by Ly6Clow monocytes in response to TLR9 agonist [41] (Fig. 1). Adding to the complexity of the field, it has been suggested that Mal/TIRAP plays a role out of TLR system [42]. According to the authors, Mal/TIRAP has a TLR-independent function in IFN-γ receptor signalling what they have shown in cells infected with Mycobacterium tuberculosis. Concerning TRIF, this adapter is recruited by TLR3 and TLR4 and promotes an alternative pathway that leads to the activation of IRF3, NF-κB, and MAPKs for induction of type I IFN and inflammatory cytokine genes. TRAM is selectively recruited to TLR4 but not TLR3 to link between TRIF and TLR4 since TLR3 directly interacts with TRIF [25].
Recently, Mal/TIRAP and TRAM earned the name of “sorting adapter” in opposition to the “signalling adapter” Myd88 or TRIF [43]. Mal/TIRAP and TRAM are localized to specific organelles at steady state and they are probably the first to detect activated TLRs and sequentially, recruit signalling proteins to their site of residence to initiate signal transduction. Importantly, the mislocalization of sorting adapters to the cytosol results in a deficient signalling response. The change in the localization of Mal/TIRAP impedes the MyD88-dependent signal transduction to occur [44]. In a same way, forcing TRAM to be located at the cell surface or in the cytosol instead of being located on endosomes diminishes the ability of TLR4 to induce type I IFN expression [45]. These findings highlight the role of sorting adapters in the subtle regulation of TLR activity that might vary according to cell type and TLR combinations.
Cooperation between TLRs contributes to the versatility of TLRs during infection
The pathogens possess several PAMPs able to activate different TLRs that appears as a prerequisite for the induction of effective innate immune responses as evaluated in mouse models of infection. Indeed, TLR–TLR crosstalk enables the innate immune system to orchestrate immediate local and global response. Besides, the concomitant activation of several TLRs represents a way to reduce the chance of false detection. In vivo studies confirm that in many models of infectious disease, deficiencies of multiple TLRs cause a greater reduction in host resistance than single TLR deficiencies [46–48]. The effects of TLR cooperation are complex, often involving multiple effector cells and responses. TLRs might interact at the level of the same cell or at the level of multiple cell types [7]. The final result of the triggering of multiple receptors can be either the enhancement of a single effector function or the coordinated induction of distinct responses, which together mediate more effective control of pathogen growth. This is perfectly illustrated in the mouse model of infection with T. cruzi from which GPI-anchored mucin-like glycoprotein (tGPI-mucin) from membrane and specific sequences into genome (unmethylated CpG DNA motifs) were characterized as TLR2 and TLR9 agonist, respectively [49–51]. In this infection model characterized by an immunoregulatory response, TLR2 and TLR9 play differential functions (complementary or antagonist role) according to immune cell type to promote an efficient response without damage to the host [41, 52, 53].
At first, cooperation between TLRs has been described as a way to modulate cytokine production by synergism or antagonism in a same cell. Different pair-wise combinations of TLRs are possible. For example, it was reported that simultaneous stimulation with MALP2 and LPS (TLR2 and TLR4 ligand, respectively) results in the production of TNF-α at levels much higher than that observed for each of the ligands alone [54]. Furthermore, TLR4 and TLR9 were shown to synergize in the production of TNF-α in mouse macrophages [55] associated with enhanced MAPK signalling. It has been proposed that the effects of different combinations of TLR ligands in vitro have occurred between ligands that trigger distinct signalling pathways (such as the MyD88- and TRIF-dependent signalling pathways). In regard to this, different groups [56, 57] working with DCs have found other pair-wise combinations of TLR ligands which are able to cause the greater-than-additive production of IL-12p70 as well as other cytokines, leading to a Th1 polarizing phenotype. The main results of the first study indicate that in both human and mouse DCs the simultaneous activation of a TRIF-coupled receptor (TLR3 or TLR4) together with an endosomal receptor (TLR8 in humans and TLR7 or TLR9 in mice) leads to a potent synergistic activation of IL-12p70 production. In the second study, Zhu et al. have reported that in human and mouse DCs TLR3 or TLR4 potently synergized with TLR7, 8, and 9 in the induction of a selected set of genes in contrast to TLR2 agonist which could not.
Interestingly, specific TLR association appeared recurrently in vivo during pathogen infection like the combinatorial action of TLR2 and TLR9. Dunggan et al. have reported that the combination of TLR2 and TLR9 agonists promoted intrapulmonary pathogen killing and survival of infectious challenges to an extent that far surpassed most other tested TLR ligand doublets [58]. This is consistent with several observations found in the literature indicating that the absence of TLR2 and TLR9 affects the course of infection. In a Mycobacterium tuberculosis model, TLR2/9−/− mice displayed markedly enhanced susceptibility to infection in association with combined defects in proinflammatory cytokine production in vitro and altered pulmonary pathology compared to the single TLR-deficient animals [47]. The authors have shown that DCs from TLR2/9−/− mice showed greater impairment in their mycobacteria-induced IL-12 responses than did the equivalent populations from each of the single TLR-deficient animals. In a similar way, the cooperation between TLR2 and TLR9 is important to maximize the host response including cytokine production by DCs in a mouse model of infection with Herpes simplex [59].
But, TLR9 and TLR2 may play opposed role during infection. We can cite mouse models of infection with bacteria and parasite to illustrate the complexity of these cooperative systems. While TLR9−/− mice are characterized by a shortened survival, an increased cytokine production, and more severe Salmonella hepatitis than wild-type mice, TLR2−/− mice exhibited the inversed phenomenon in the same conditions [60]. In a mouse model of infection with T. cruzi—of which Y strain infection is characterized by a balanced immune response [49–51]—the cooperation effect of TLR2 and TLR9 was supported by the fact that TLR2/9−/− mice showed a greater susceptibility to T. cruzi than animals single deficient for TLR2 or TLR9 [48]. However, it has been demonstrated that TLR9, but not TLR2, was crucial for the establishment of a Th1 response and consequently for mice survival; further, it appeared that TLR2 plays an immunoregulatory role during the infection [41, 52, 53]. The study at cell level indicates a complementary use of TLR2 and TLR9 by immune cells [53]. TLR2 was associated with a proinflammatory response of macrophages by preferentially inducing TNF-α release, whereas splenic DCs appeared to be committed with IL-12 production through TLR9 in this mouse model of T.cruzi infection. Interestingly, TLR2 can down-regulate TLR9 signalling through MAPKs and transcription factors in splenic DCs leading to a decreased IL-12 production. This latter infection model underlines the complexity of TLR cooperation and illustrates why a decoding of these multiple receptor interactions is necessary.
Cooperation between TLRs may greatly influence the dynamic of monocyte functions. In the next section, monocyte activation will be examined through the prism of TLRs.
Different TLR signalling pathways for different subsets of monocytes
Monocytes demonstrate extensive plasticity and heterogeneity and adjust their functional phenotype in response to the context [12, 13]. These cells, after the egress from bone marrow, are rapidly recruited to tissues during infection and inflammation [61]. As resolution of inflammation requires balanced pro- and antiinflammatory responses, a distribution of tasks between classical and non-classical subsets is critical since they possess distinct functional properties. Inflammatory monocytes depend on the chemokine receptor CCR2 for their localization to injured tissue [62]. First, the cells react to the chemokine MCP-1 (monocyte chemoattractant protein 1), which binds CCR2 (chemokine receptor), to depart into bone marrow sinusoids, and thus leave hematopoietic tissue. Once in circulation, inflammatory monocytes continue to rely on CCR2 for recruitment into tissue. Infection with diverse pathogens (including bacteria, parasites, fungi, and viruses) induces the recruitment of Ly6Chigh monocytes at sites of infection, where they restrict further microbial growth and invasion, but in most cases of infection, like a double-edged sword, they also contribute to tissue destruction during some infections and inflammatory diseases. Indeed, exacerbated monocyte activity in tissue damage after infectious process has been commonly reported [61]. In this regard, blocking Ly6Chigh monocyte recruitment has been tested by silencing CCR2, to reduce Ly6Chigh monocyte numbers in the heart and improve outcome during myocarditis or atherosclerosis [63, 64].
The role of TLRs in migration of monocytes is not well documented but TLR2 has been involved in their transmigration through a Rac/PI3K pathway during infection [65]. This may be put in parallel with a role for TLR2 in the expansion of hematopoietic stem cells (HSCs) as reported [66], noting that HSCs are responsible for the continuous replenishment of monocytes at sites of inflammation. In addition, another group has demonstrated that while monocyte recruitment to the site of bacterial infection is MyD88-independent during Listeria monocytogenes infection, monocyte activation is MyD88-dependent [67]. Interestingly, during the course of T. cruzi infection, a differential impact of TLRs on Ly6Chigh and Ly6Clow monocyte migration has been detected in mice [41]. Indeed, we have observed that TLR2 and TLR9 influence the migration to spleen of Ly6Chigh, but not of Ly6Clow monocytes. This is an important point to consider since it suggests that Ly6Clow monocyte population emerges independently of Ly6Chigh monocytes and addresses the existence of specific progenitor that can give rise to Ly6Clow cells. In a same way, another group has shown the selective impairment of Ly6Chigh monocytes in IRF8 transcription factor mutant mice revealing an independent developmental pathway for Ly6Clow monocytes [68]. This contradicts the widespread model where Ly6Chigh monocytes convert into antiinflammatory Ly6Clow monocytes [69–71].
According to different studies, TLR2 may serve as an inflammatory marker and be responsible for the immunopathogenesis in different situations. An up-regulation of TLR2 expression in mouse or human monocytes has been frequently reported in inflammatory diseases [72, 73]. An increase of TLR2 expression on blood monocytes has been associated with Kawasaki disease. The higher levels of TLR2 and TLR9 detected on different monocyte subsets from rheumatoid arthritis patients were in line with the increased cytokine production by monocytes in response to stimulation with TLR2 and TLR9 ligands [74].
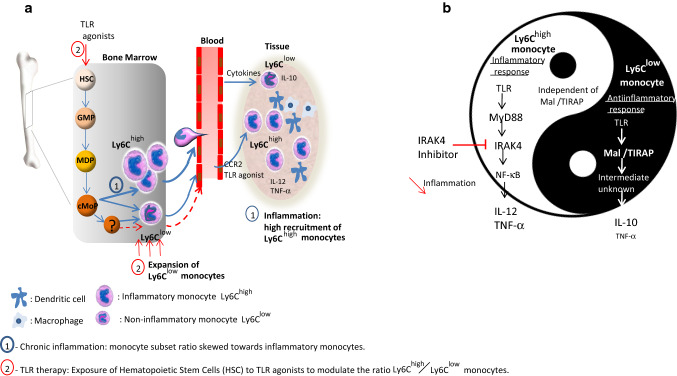
When the study includes a comparative analysis of monocyte subpopulations in balanced inflammatory model—like T. cruzi infection with the Y strain—an increased TLR2 expression was observed in both monocyte subsets that correlated with an increased pro- and antiinflammatory response of Ly6Chigh and Ly6Clow monocytes, respectively, after triggering TLR2 and TLR9 [41]. One of the scenarios that we believe plausible to explain the TLR2-TLR9 cross-talk in monocytes involves a sequential activation of TLRs: TLR2 may generate the first signal following recognition of TLR9 agonist. Importantly, the use of adapter would be different in the distinct monocyte subpopulations since the absence of Mal/TIRAP abolished the capacity of Ly6Clow cells to respond to TLR agonists without altering the activity of Ly6Chigh monocytes [41]. By referring to previous studies [44, 45], one could hypothesize that the intracellular localization of Mal/TIRAP is a determining factor in the different functions of monocytes, but further studies are needed to determine with certainly a correlation between both events. Whatever the existence or not of such correlation, these unrevealed differences in the use of TLR signalling pathway by monocytes complement the yin and yang model of monocyte activation where both monocyte populations are responsive to a same trigger event leading to complementary responses during infection (Fig. 2b). This may constitute a basis for new therapeutic strategies to modulate the inflammation as developed in the next section. Further, these observations reinforce the idea that monocytes are conditioned for specialized programs after the egress from bone marrow. In the same line, it has been reported that a specific human monocyte subset would be specialized in the detection of viruses and nucleic acids through TLR7, 8 [75]. These monocytes that lack CD14 and express CD16 (CD14dim) produce the proinflammatory cytokines TNF-α and IL-1β in response to virus and nucleic acids via a unique MyD88/MEK/ERK pathway that differs from that of the other monocyte populations. In the same study, the authors have suggested that CD14dim monocytes would be responsible for a sustained inflammatory state in autoimmune disease such as systemic lupus erythematosus, a disease associated with autoantibodies to nucleosome and ribonucleoproteins, and immune complex deposition in several organs. CD14dim monocytes could represent a potentially useful cellular therapeutic target in selected inflammatory diseases.
Fig. 2.
Different therapeutic strategies to control the inflammatory activity of monocytes based on a mouse model. During infection and chronic inflammation, Ly6Chigh monocytes that derived from granulocyte-monocyte progenitor (GMPs), monocyte-dendritic cell progenitor (MDP) and common monocyte progenitor (cMoP), egress from the bone marrow and selectively traffic to sites of inflammation. The Ly6Chigh monocyte egression requires expression of the chemokine receptor CCR2. The migration of Ly6Chigh monocyte to tissue where they can retain their own properties without differentiating into macrophage or dendritic cell is prominent and robust. Ly6Chigh monocytes can theoretically convert into Ly6Clow cells although this is debatable due to the possible existence of Ly6Clow progenitors not yet identified [68]. When the antiinflammatory activity of Ly6Clow monocytes is not able to counteract the inflammatory mediator release, the inflammatory state persists causing tissue damage. In this context, a therapeutic intervention is necessary to return to homeostasis. a One strategy may consist in the manipulation of the hematopoietic stem cell (HSC) development by systemic administration of TLR agonist (alone or in association) that may change the ratio Ly6Chigh versus Ly6Clow monocyte number by promoting the expansion, egress, and activation of Ly6Clow monocytes. b The second strategy is based on the differential use of signalling pathways by monocyte subpopulations according to Gravina et al. [41] allowing to specifically target inflammatory monocytes. In a context where proinflammatory cytokines are released by Ly6Chigh monocytes in a MyD88/IRAK4-dependent way and antiinflammatory cytokines are produced by Ly6Clow monocyte in a Mal/TIRAP- dependent way, IRAK4 inhibitors appear relevant to treat exacerbated inflammatory disorders. This may allow to specifically reduce inflammatory mediators released by Ly6Chigh monocytes without altering repair functions of other monocyte populations
If additional evidence of monocyte adaptability was still needed, it was given by the interdependence between both monocyte subsets and the influence of microenvironment on them during T. cruzi infection model. Indeed, in the absence of Mal/TIRAP that is involved in the antiinflammatory response of Ly6Clow monocytes, a reduction of the number of splenic inflammatory monocytes was detected while the lack of IL-12 reduced the number of Ly6Clow cells [41].
In an in vitro study using intravital confocal microscopy, the dynamics of Ly6Clow monocytes from mice have been compared in response to several TLR agonists that were applied onto the mesenteric vessels to trigger inflammation. Agonists for TLR2 and TLR9 induced a strong early recruitment of monocytes within 30 min., whereas TLR3 and TLR4 promote a late accumulation (around 3 h) [76]. Further, TLR2 and TLR9 are the strongest inducers in these experimental settings, leading to about nine to tenfold more patrolling monocytes after 3 h. In a study where the objective was to elucidate the molecular, migratory, and functional phenotypes of patrolling monocytes in large arteries in healthy, hyperlipidemic, and atherosclerotic conditions in mouse, the authors observed that the number of patrolling monocytes was increased ninefold by applying topical TLR7/8 agonist [77]. It thus seems that vascular activation by most TLRs suffices to intensify local surveillance by patrolling monocytes.
New perspectives in antiinflammatory therapy
In the context of chronic disease, the ratio of the monocyte subsets may often be skewed toward inflammatory monocytes, inducing a bias to prolonged or exaggerated inflammatory responses. Indeed, this scenario is reported in pathological conditions such as sepsis [78], tuberculosis [79], rheumatoid arthritis [80] as well as coronary heart disease [81]. A role for inflammatory monocytes has also been described in the chronic phase of Chagas disease caused by T. cruzi [82, 83]. Indeed, the analysis of the expression of immunoregulatory cytokines showed that while monocytes from indeterminate-disease patients are committed to IL-10 expression, a higher percentage of monocytes from cardiac-disease patients express TNF-α after exposure to live parasites. In this context, Chagas disease has been assimilated to a chronic inflammatory disease with all the hallmarks of such disorder [84]. This is also the case for diabetes where Ly6Chigh monocytes drive chronic inflammation and impair wound healing indicating that a selective targeting of inflammatory monocytes is a viable therapeutic strategy in diabetic wounds [85]. In atherosclerosis, the lipid-rich atherosclerotic plaques are infiltrated by inflammatory cells, including monocytes. Atherosclerosis induces profound expansion of Ly6Chigh monocytes into the blood. Interrupting monocyte migration from the blood to the atherosclerotic plaque is a promising therapeutic option currently being investigated [86, 87]. On the other hand, it has been pointed out that TLRs play a role in different clinical situations involving monocytes. It has been claimed that the incidence and outcome of human sepsis is influenced by the expression of TLRs on monocytes and particularly by TLR2 expression [88]. In another paper, the authors have investigated whether peripheral monocyte TLR expression was associated with the atrial fibrillation that is the most common sustained arrhythmia [89]. In this study, a correlation has been made between the enhanced peripheral monocyte TLR2 and TLR4 expression and the proinflammatory status in the heart. As previously cited, a relationship has been established between cell inflammatory activity and expression level of TLR2 and TLR9 on monocyte subsets of active rheumatoid arthritis patients [74].
Monocytosis could theoretically be regulated at different stages: monocyte production in the hematopoietic niche, release from the bone marrow, and recruitment to sites of inflammation and/or cell polarization. Thus, manipulating the abundance and biological activity of monocyte subsets may have utility in moderating pathogenesis.
The interest in having a better understanding of the molecular mechanisms of patrolling in vasculature is growing [90]. Indeed, the unique ability of non-classical monocytes to actively patrol the vasculature and to resolve inflammation makes them attractive targets for disease therapy. Generally, non-classical monocytes are thought to be involved in the resolution of inflammation and differentiate into resident macrophage populations that work to heal wounds and to resolve the inflammation. One strategy to control the abundance and activity of monocyte subsets may consist in interfering with development of HSCs, the progenitors of monocytes (Fig. 2a). The systemic use of TLR agonist (alone or in association) may constitute an interesting alternative to influence the myeloid development of HSCs and the migration of cells that derived from them to tissue. This is workable since it has been shown that injected LPS rapidly diffuses into the bone marrow cavity and engages the receptors of stem cells and progenitors [91]. Further, a systemic exposure of mice to a TLR2 agonist has led to an expansion of HSCs indicating that HSCs possess functional TLR signalling pathways [66]. In a same way, the transient exposure of both mouse and human progenitor cells to TLR2 agonist prior to differentiation would be sufficient to suppress the inflammatory cytokine response of macrophages subsequently derived from them [92] that is in accord with the study done by Megias et al. [93]. The concept of TLR directly stimulating HSCs to interfere on myeloid cell fate is certainly attractive in therapy of Chagas disease and other inflammatory disorders. This is why a better knowledge of TLR signalling in HSCs appears highly relevant to predict TLR influence on bone marrow microenvironment and consequently on monocyte subset equilibrium.
The second strategy is based on the observation that monocyte subsets use different signalling pathways to assume their functions [41, 75] (Fig. 2b). Recently, it has been reported that inhibitors of IRAK4 kinase activity should have therapeutic value to treat inflammation disorders due to their involvement in MyD88 pathway. There exist potent IRAK4 inhibitors that have been tested in various in vivo disease models [94]. Some of these compounds have proven to be capable of reducing cytokine production induced by injection of several different TLR agonists, including those for TLR2, TLR7, and TLR9 [95]. In their study, Cushing et al. have shown that the effect of inhibition of IRAK4 activity in inflammatory monocyte leads to a diminution of proinflammatory cytokines through inhibition of IRF5 phosphorylation without affecting NF-κB activity [96], which is a benefit given the pivotal role of NF-κB in host defense against infections. This gives hope for targeted therapy in inflammatory disorders.
Conclusion
Due to the close relationship between TLRs and monocytes in all aspects of cell life from maturation, replenishment, expansion, migration to inflammatory activity, TLR targeted therapy appears as a therapy of choice in chronic inflammatory disease. Exploring the impact of TLR therapy that includes systemic administration of TLR agonists or selective inhibitor of TLR pathway is relevant to manipulate number and function of monocytes in chronic inflammatory disease. This opens new perspectives in antiinflammatory therapy.
Compliance with ethical standards
Conflict of interest
The author declares that there is no conflict of interest.
References
- 1.Steinman R, Hoffmann J, Beutler B. Editorial: nobel Prize to immunology. Nat Rev Immunol. 2011;11:714. doi: 10.1038/nri3103. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill LAJ, Golenbock D, Bowie AG. The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol. 2013;13(6):453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 3.Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity. 2008;29:182–191. doi: 10.1016/j.immuni.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–635. doi: 10.1016/S1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Ozinsky A, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 8.Farrar CA, et al. Inhibition of TLR2 promotes graft function in a murine model of renal transplant ischemia-reperfusion injury. FASEB J. 2012;26:799–807. doi: 10.1096/fj.11-195396. [DOI] [PubMed] [Google Scholar]
- 9.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 10.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 12.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 14.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 15.Ingersoll MA, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol. 2013;4(23):1–5. doi: 10.3389/fimmu.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi O, et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 18.Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol. 2006;6:895–906. doi: 10.1038/nri1978. [DOI] [PubMed] [Google Scholar]
- 19.Blasius AL, Beutler B. Intracellular toll-like receptors. J Immunol. 2010;32(3):305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 20.van Bergenhenegouwen J, Plantinga TS, Joosten LAB, Netea MG, Folkerts G, Kraneveld AD, Garssen J, Vos AP. TLR2 & Co: a critical analysis of the complex interactions between TLR2 and coreceptors. J Leuk Biol. 2013;94(5):885–902. doi: 10.1189/jlb.0113003. [DOI] [PubMed] [Google Scholar]
- 21.Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their functions. Nat Rev Immunol. 2012;12:168–179. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawasaki T, Kawai T. Toll-like receptors signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flannery S, Bowie AG. The interleukin-1 receptor-associated kinases: critical regulators of innate immune signalling. Biochem Pharmacol. 2010;80:1981–1991. doi: 10.1016/j.bcp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Cohen P. The TLR and IL-1 signalling network at a glance. J Cell Sci. 2014;127:2383–2390. doi: 10.1242/jcs.149831/-/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Neill LAJ, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 26.Deguine J, Barton GM (2014) MyD88: a central player in innate immune signaling. F1000 Prime Re 6:97. 10.12703/P6-97 [DOI] [PMC free article] [PubMed]
- 27.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/S1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 28.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465(7300):885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawai T, Akira S. Signaling to NF-kB by Toll-like receptors. Trends Mol Med. 2007;13(11):460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Com. 2009;388(4):621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 31.Verstak B, Nagpal K, Bottomley SP, Golenbock DT, Hertzog PJ, Mansell A. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. J Biol Chem. 2009;284(36):24192–24203. doi: 10.1074/jbc.M109.023044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 33.Mellett M, Atzei P, Jackson R, O’Neill LA, Moynagh PN. Mal mediates TLR-induced activation of CREB and expression of IL-10. J Immunol. 2011;186(8):4925–4935. doi: 10.4049/jimmunol.1002739. [DOI] [PubMed] [Google Scholar]
- 34.Sanin DE, Prendergast CT, Mountford AP. IL-10 production in macrophages is regulated by a TLR-driven CREB-mediated mechanism that is linked to gene involved in cell metabolism. J Immunol. 2015;195(3):1218–1232. doi: 10.4049/jimmunol.1500146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colonna M. TLR pathways and IFN-regulatory factors: to each its own. Eur J Immunol. 2007;37(2):306–309. doi: 10.1002/eji.200637009. [DOI] [PubMed] [Google Scholar]
- 36.Honda K, Taniguchi T. Toll-like receptor signaling and IRF transcription factors. IUBMB Life. 2006;58(5–6):290–295. doi: 10.1080/15216540600702206. [DOI] [PubMed] [Google Scholar]
- 37.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420(6913):329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto M, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 39.Bonham KS, Orzalli MH, Hayashi K, Wolf AI, Glanemann C, Weninger W, Iwasaki A, Knipe DM, Kagan JC. A promiscuous lipid-binding protein diversifies the subcellular sites of Toll-like Receptor signal transduction. Cell. 2014;156(4):705–716. doi: 10.1016/j.cell.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenny EF, Talbot S, Gong M, Golenbock DT, Bryant CE, O’Neill LA. MyD88 adaptor-like is not essential for TLR2 signaling and inhibits signaling by TLR3. J Immunol. 2009;183:3642–3651. doi: 10.4049/jimmunol.0901140. [DOI] [PubMed] [Google Scholar]
- 41.Gravina HD, Goes AM, Murta SMF, Ropert C. MyD88 adapter-like (Mal)/TIRAP is required for cytokine pro duction by splenic Ly6CloTLR2hi but not by Ly6ChiTLR2hi monocytes during infection. J Biol Chem. 2016;291:23832–23841. doi: 10.1074/jbc.M116.729509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ní Cheallaigh C, et al. A common variant in the adaptor mal regulates interferon gamma signaling. Immunity. 2016;44:368–379. doi: 10.1016/j.immuni.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kagan JC. Defining the subcellular sites of innate immune signal Transduction. Trends Immunol. 2012;33(9):442–448. doi: 10.1016/j.cell.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125(5):943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 45.Rowe DC, McGettrick AF, Latz E, Monks BG, Gay NJ, Yamamoto M, Akira S, O’Neill LA, Fitzgerald KA, Golenbock DT. The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc Natl Acad Sci USA. 2006;103(16):6299–6304. doi: 10.1073/pnas.0510041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss DS, Raupach B, Takeda K, Akira S, Zychlinsky A. Toll-like receptors are temporally involved in host defense. J Immunol. 2004;172:4463–4469. doi: 10.4049/jimmunol.172.7.4463. [DOI] [PubMed] [Google Scholar]
- 47.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates TH1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bafica A, Santiago HC, Goldszmid R, Ropert C, Gazzinelli RT, Sher A. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol. 2006;177:3515–3519. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- 49.Ropert C, Almeida IC, Closel M, Luiz R, Ferguson MAJ, Cohen P, Gazzinelli T. Requirement of mitogen-activated protein kinases and iκb phosphorylation for induction of proinflammatory cytokines synthesis by macrophages indicates functional similarity of receptors triggered by glycosylphosphatidylinositol anchors from parasitic protozoan. J Immunol. 2001;166:3423–3431. doi: 10.4049/jimmunol.166.5.3423. [DOI] [PubMed] [Google Scholar]
- 50.Campos MA, Almeida IC, Takeuchi O, Akira S, Valente EP, Procópio DO, Travassos LR, Smith JA, Golenbock DT, Gazzinelli RT. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J Immunol. 2001;167(1):416–423. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- 51.Bartholomeu DC, et al. Recruitment and endo-lysosomal activation of TLR9 in dendritic cells infected with Trypanosoma cruzi. J Immunol. 2008;181:1333–1344. doi: 10.4049/jimmunol.181.2.1333. [DOI] [PubMed] [Google Scholar]
- 52.Ropert C, Gazzinelli RT. Regulatory role of Toll-like receptor 2 during infection with Trypanosoma cruzi. J Endotoxin Res. 2004;10:425–430. doi: 10.1177/09680519040100060801. [DOI] [PubMed] [Google Scholar]
- 53.Gravina HD, Antonelli L, Gazzinelli RT, Ropert C. Differential Use of TLR2 and TLR9 in the Regulation of Immune Responses during the Infection with Trypanosoma cruzi. PLoS One. 2013;8(5):e63100. doi: 10.1371/journal.pone.0063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sato S, Nomura F, Kawai T, Takeuchi O, Mühlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165(12):7096–7101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 55.De Nardo D, De Nardo CM, Nguyen T, Hamilton JA, Scholz GM. Signaling crosstalk during sequential TLR4 and TLR9 activation amplifies the inflammatory response of mouse macrophages. J Immunol. 2009;183(12):8110–8118. doi: 10.4049/jimmunol.0901031. [DOI] [PubMed] [Google Scholar]
- 56.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Q, Egelston C, Vivekanandhan A, Uematsu S, Akira S, Klinman DM, Belyakov IM, Berzofsky JA. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proc Natl Acad Sci USA. 2008;105(42):16260–16265. doi: 10.1073/pnas.0805325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duggan JM, You D, Cleaver JO, Larson DT, Garza RJ, Guzmán Pruneda FA, Tuvim MJ, Zhang J, Dickey BF, Evans SE. Synergistic interactions of TLR2/6 and TLR9 induce a high level of resistance to lung. J Immunol. 2011;186(10):5916–5926. doi: 10.4049/jimmunol.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci USA. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhan R, Han Q, Zhang C, Tian Z, Zhang J. Toll-like receptor 2 (TLR2) and TLR9 play opposing roles in host innate immunity against Salmonella enterica serovar typhimurium infection. Infect Immun. 2015;83:1641–1649. doi: 10.1128/IAI.02870-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2014;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 63.Majmudar MD, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127(20):2038–2041. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leuschner F, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29(11):1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harokopakis E, Albzreh MH, Martin MH, Hajishengallis G. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3 K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J Immunol. 2006;176(12):7645–7656. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- 66.Herman AC, Monlish DA, Romine MP, Bhatt ST, Zippel S, Schuettpelz LG. Systemic TLR2 agonist exposure regulates hematopoietic stem cells via cell-autonomous and cell-non-autonomous mechanisms. Blood Cancer J. 2016;6:e437. doi: 10.1038/bcj.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19(6):891–901. doi: 10.1016/S1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 68.Kurotaki D, et al. Essential role of the IRF8-KLF4 transcription factor cascade in murine monocyte differentiation. Blood. 2013;121(10):1839–1849. doi: 10.1182/blood-2012-06-437863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sunderkötter C, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 70.Ancuta P, Liu KY, Misra V, Wacleche VS, Gosselin A, Zhou X, Gabuzda D. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16− monocyte subsets. BMC Genom. 2009;10:403. doi: 10.1186/1471-2164-10-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenkranz ME, Schulte DJ, Agle LM, Wong MH, Zhang W, et al. TLR2 and MyD88 contribute to Lactobacillus casei extract-induced focal coronary arteritis in a mouse model of Kawasaki disease. Circulation. 2005;112:2966–2973. doi: 10.1161/CIRCULATIONAHA.105.537530. [DOI] [PubMed] [Google Scholar]
- 73.Lin IC, et al. Augmented TLR2 expression on monocytes in both human kawasaki disease and a mouse model of coronary arteritis. PLoS One. 2012;7(6):e38635. doi: 10.1371/journal.pone.0038635.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lacerte P, Brunet A, Egarnes B, Duchêne B, Brown JP, Gosselin J. Overexpression of TLR2 and TLR9 on monocyte subsets of active rheumatoid arthritis patients contributes to enhance responsiveness to TLR agonists. Arthritis Res Ther. 2016;18:10. doi: 10.1186/s13075-015-0901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33(3):375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imhof BA, Jemelin S, Emre Y. Toll-like receptors elicit different recruitment kinetics of monocytes and neutrophils in mouse acute inflammation. Eur J Immunol. 2017;47(6):1002–1008. doi: 10.1002/eji.201746983. [DOI] [PubMed] [Google Scholar]
- 77.Quintar A, McArdle S, Wolf D, Marki A, Ehinger E, Vassallo M, Miller J, Mikulski Z, Ley K, Buscher K. Endothelial protective monocyte patrolling in large arteries intensified by western diet and atherosclerosis. Circ Res. 2017;120(11):1789–1799. doi: 10.1161/CIRCRESAHA.117.310739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fingerle G, Pforte A, Passlick B, Blumenstein M, Ströbel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82(10):3170–3176. [PubMed] [Google Scholar]
- 79.Brilha S, Wysoczanski R, Whittington AM, Friedland JS, Porter JC. Monocyte adhesion, migration, and extracellular matrix breakdown are regulated by integrin αVβ3 in Mycobacterium tuberculosis infection. J Immunol. 2017;199(3):982–999. doi: 10.4049/jimmunol.1700128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, Lackner KJ, Iz M, Meyer J, Darius H, Rupprecht HJ. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-α levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 81.Shahid F, Lip GYH, Shantsila E. Role of monocyte in Heart failure and Atrial fibrillation. J Am Heart Assoc. 2018;7:e007849. doi: 10.1161/JAHA.117.007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Souza PE, Rocha MO, Rocha-Vieira E, Menezes CA, Chaves AC, Gollob KJ, Dutra WO. Monocytes from patients with indeterminate and cardiac forms of chagas’ disease display distinct phenotypic and functional characteristics associated with morbidity. Infect Immun. 2004;72:5283–5291. doi: 10.1128/IAI.72.9.5283-5291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pinto BF, Medeiros NI, Teixeira-Carvalho A, Eloi-Santos SM, Fontes-Cal TCM, Rocha DA, Dutra WO, Correa-Oliveira R, Gomes JAS. CD86 expression by monocytes influences an immunomodulatory profile in asymptomatic patients with chronic chagas disease. Front Immunol. 2018;9:454. doi: 10.3389/fimmu.2018.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cruz JS, Machado FS, Ropert C, Roman-Campos D. Molecular mechanisms of cardiac electromechanical remodeling during Chagas disease: role of TNF and TGF-β. Trends Cardiovasc Med. 2017;27(2):81–91. doi: 10.1016/j.tcm.2016.08.00383. [DOI] [PubMed] [Google Scholar]
- 85.Kimball A, Schaller M, Joshi A, Davis FM, denDekker A, Boniakowski A, Bermick J, Obi A, Moore B, Henke PK, Kunkel SL, Gallagher KA. Ly6CHi blood monocyte/macrophage drive chronic inflammation and impair wound healing in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2018;38(5):1102–1114. doi: 10.1161/atvbaha.118.310703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, vanRooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Armstrong L, Medford AR, Hunter KJ, Uppington KM. Millar AB (2004) Differential expression of Toll-like receptor (TLR)-2 and TLR-4 on monocytes in human sepsis. Clin Exp Immunol. 2004;136(2):312–319. doi: 10.1111/j.1365-2249.2004.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gurses KM, Kocyigit D, Yalcin MU, Canpinar H, Yorgun H, Sahiner ML, Kaya EB, Oto MA, Ozer N, Guc D, Aytemir K. Monocyte toll-like receptor expression in patients with atrial fibrillation. Am J Cardiol. 2016;117(9):1463–1467. doi: 10.1016/j.amjcard.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 90.Konrad B, Marcovecchio P, Hedrick CC, Ley K. Patrolling mechanics of non-classical monocytes in vascular inflammation. Front Cardiovasc Med. 2017;4:80. doi: 10.3389/fcvm.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24(6):801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yáñez A, Goodridge HS, Gozalbo D, Gil ML. TLRs control hematopoiesis during infection. Eur J Immunol. 2013;43(10):2526–2533. doi: 10.1002/eji.201343833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Megías J, Yáñez A, Moriano S, O’Connor JE, Gozalbo D, Gil ML. Direct Toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem cells. 2012;30(7):1486–1495. doi: 10.1002/stem.1110. [DOI] [PubMed] [Google Scholar]
- 94.Seganish WM. Inhibitors of interleukin-1 receptor-associated kinase 4 (IRAK4): a patent review (2012-2015) Expert Opin Ther Pat. 2016;26(8):917–932. doi: 10.1080/13543776.2016.1202926. [DOI] [PubMed] [Google Scholar]
- 95.Dudhgaonkar S, et al. Selective IRAK4 inhibition attenuates disease in murine lupus models and demonstrates steroid sparing activity. J Immunol. 2017;198(3):1308–1319. doi: 10.4049/jimmunol.1600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cushing L, Winkler A, Jelinsky SA, Lee K, Korver W, Hawtin R, Rao VR, Fleming M, Lin LL. IRAK4 kinase activity controls Toll-like receptor-induced inflammation through the transcription factor IRF5 in primary human monocytes. J Biol Chem. 2017;292(45):18689–18698. doi: 10.1074/jbc.M117.796912. [DOI] [PMC free article] [PubMed] [Google Scholar]