Abstract
Ischemic stroke is the most common cerebrovascular disease and considered as a worldwide leading cause of death. After cerebral ischemia, different pathophysiological processes including neuroinflammation, invasion and aggregation of inflammatory cells and up-regulation of cytokines occur simultaneously. In this respect, Toll-like receptors (TLRs) are the first identified important mediators for the activation of the innate immune system and are widely expressed in glial cells and neurons following brain trauma. TLRs are also able to interact with endogenous and exogenous molecules released during ischemia and can increase tissue damage. Particularly, TLR2 and TLR4 activate different downstream inflammatory signaling pathways. In addition, TLR signaling can alternatively play a role for endogenous neuroprotection. In this review, the gene and protein structures, common genetic polymorphisms of TLR2 and TLR4, TLR-related molecular pathways and their putative role after ischemic stroke are delineated. Furthermore, the relationship between neurosteroids and TLRs as neuroprotective mechanism is highlighted in the context of brain ischemia.
Keywords: Ischemic stroke, Neurosteroids, Toll-like receptors
Introduction
Stroke resulting from focal occlusion of a main artery in the brain is considered as the most important cause of disability in men and a leading cause of death worldwide. Initial damage after the primary ischemic event is followed by delayed metabolic alterations called secondary injury mechanisms which can increase excitatory neurotransmitter levels, neuronal apoptosis, immune-inflammatory activation as well as lipid degradation [1–3]. In this respect, systemic and local inflammatory responses play a main role in the pathophysiology of cerebral ischemia. The given responses can similarly not only increase the ischemic lesion but also protect injured tissue [4, 5]. Macrophages, neutrophils, and lymphocytes have equally distinct significant roles in different brain inflammatory events even though the underlying mechanisms leading to their activation are not fully clear. Toll-like receptors (TLRs) are activated in response to inflammation and are also recognized as innate immune receptors located on the cell surface or inside endosomes [4, 6]. Activation of TLRs leads to the initiation of different downstream inflammatory cascades. TLR expression can also be regulated in response to exogenous microorganisms as well as different types of cytokines (chemokines, interferons, and interleukins) which are secreted in the core and the penumbra region of ischemic brain tissue [7, 8]. Expression of these mediators depends on nuclear factor-kB (NF-κB) as the main transcription factor implicated in the TLR pathway [8]. Therefore, these receptors may have an important role mediating brain damage following ischemia and are thus assumed as clinical targets for preventing secondary injury after ischemic stroke [9, 10]. In this review article, we focus on the TLR family and their respective ligands, structures, and signaling pathways and also point at the role of TLRs in ischemic stroke emphasizing the interaction with neurosteroids. Moreover, gene and protein structures as well as common genetic polymorphisms of TLR2 and TLR4 molecules and their possible roles in stroke-induced inflammatory pathways are discussed. Finally, the relationship between neurosteroids and TLRs following an ischemic event in the brain is underlined as therapeutic targets for stroke therapy.
TLR family and expression
TLRs are a family of molecules playing a major role in the innate immune system [11]. TLR inflammatory responses and cytokine secretion typically increase in glial cells and leukocytes in response to microorganisms [12]. A total number of 13 TLRs have been identified until now, of which only 10 (TLR1-10) have been related to humans [13]. Generally, TLR localization and expression depends on the tissue or cell type. TLR1, 2, 4, 5, 6, and 10 are localized on the cell surface, whereas TLRs 3, 7, 8, and 9 are usually expressed exclusively in intracellular compartments such as endosomes and lysosomes [14, 15]. Based on their sequence homologies, human TLRs can be grouped into five subfamilies of TLR1/2/6/10, TLR3, TLR4, TLR5, TLR7/8/9 [16, 17]. TLRs have also been described as prototype pattern recognition receptors (PRRs) and are able to recognize danger-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) which are released from damaged tissue or microorganisms [18, 19]. It should be noted that DAMPs are released or modified and then bound to their respective receptors after an inflammatory response. DAMPs can also include extracellular matrix fibronectin, hyaluronan, heparin sulfate, and even molecules which are found in intracellular compartments such as ATP, heat-shock proteins, uric acid, and those of nuclear origin such as high mobility group box 1 protein (HMGB1), double-strand RNA (dsRNA), single-strand RNA (ssRNA), DNA, and microRNAs [19, 20]. PAMPs which are recognized by TLRs and derived from bacteria, viruses, parasites, and fungi include lipids, lipoproteins, proteins as well as nucleic acids [21]. In this regard, TLRs on the cell surface are capable to identify PAMPs as their ligands, e.g., TLR1/TLR2, TLR2/TLR6, and TLR2. TLR4 is considered as a receptor for lipopolysaccharide (LPS) and myeloid differentiation factor 2 (MD2), and TLR5 binds to flagellin. Intracellular TLRs include TLR3 which recognizes microbial nucleic acid including viral dsRNAs, TLR7, and TLR8 which can interact with ssRNAs, and TLR9 which binds to CpG-containing DNA [22]. TLRs are expressed in glial cells (microglia, astrocytes, oligodendrocytes), neurons in the central nervous system (CNS) and peripheral nervous system (PNS) [23–25]. Likewise, they are often found in circumventricular organs and meninges with direct access to the circulation. TLRs also exist on antigen-presenting cells (APCs) in the CNS such as dendritic cells, B cells, macrophages, microglia, monocytes, and endothelial cells [26, 27]. Thus, the presence of specific TLRs in microglia and astrocytes allows to respond in different manner. For instance, microglia can secrete vigorous chemokines and cytokines in response to TLR 2, 3, 4 stimulation, whereas astrocytes can secrete low IL-6 levels in response to a stimulation with TLR3 [26].
Structurally, TLRs are categorized as type I integral membrane glycoproteins that share a common domain structure consisting of an extracellular recognition domain, a single transmembrane domain (helix), as well as an intracellular Toll-interleukin 1 receptor (TIR) homology signaling domain [28, 29]. The extracellular domains encompass leucine-rich repeats and mediate the recognition of PAMPs, the transmembrane domain and the intracellular TIR domains are also essential for triggering downstream signaling pathways [15, 30]. The N-terminal ectodomains of TLRs are glycoproteins with 550–800 amino acids and are located in the extracellular region or appear in the lumen of endosomes, wherein they can recognize molecules secreted by invading pathogens [31].
Expression of TLR2 in innate immune cells appears differentially regulated by inflammatory mediators. TLR2 levels in monocytes are increased by IL-1, IL-10, and LPS, and decreased by TNF, IL-4, and IFN-g. TLR2 expression in purified granulocytes is also down-regulated by LPS and TNF, but strongly up-regulated by IL-10 [32]. Interestingly, studies investigating the expression of TLR2 in cultured mouse astrocytes were consistent in this respect, whereas other reports in other cell systems are contradictory. For example, Bsibsi et al. suggested the presence of TLR2 mRNA in human astrocytes, whereas Farina et al. were unable to discover this molecule [33, 34]. Moreover, cytokine- or LPS-activated mouse brains showed TLR2 expression in microglia but not in astrocytes [35, 36]. Besides, expression of TLR2 is found in primary human oligodendrocytes [33], though the functional importance of TLR2 in this cell type is unclear.
In vitro or in vivo expression of astrocytic TLR4 also appears controversial. Reports have revealed that cultured astrocytes require microglia to entirely respond to LPS in vitro. Furthermore, oxygen–glucose deprivation in mixed glial cultures comprising astrocytes and microglia induces expression of TLR4 in these cells. TLR4 expression was much lower in astrocytes than in microglia [37–39]. However, one study reported that TLR4 was expressed in astrocytes but at just a comparatively low ratio compared to microglia [38]. It was additionally examined whether pro-inflammatory agents including LPS or IL-1β could modify TLR4 expression in microglia and astrocytes. Neither LPS nor IL-1β significantly affected the expression of TLR4 in astrocytes. In contrast, TLR4 expression in microglia was up-regulated by IL-1β but unaffected by LPS [40].
TLR signaling pathways
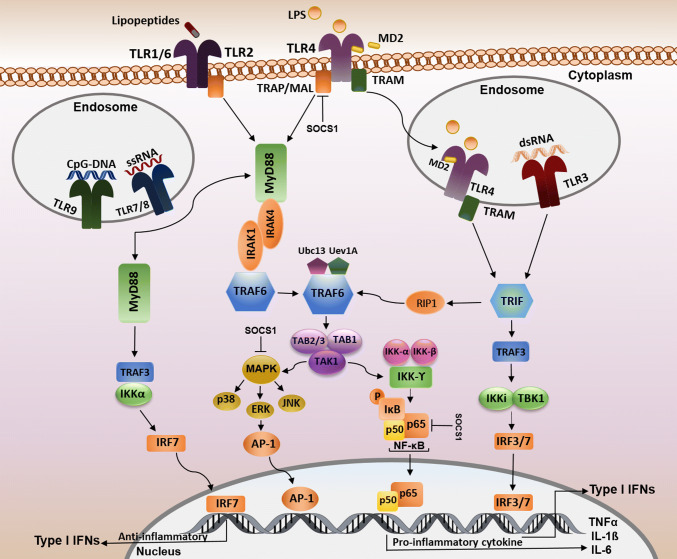
Pro-inflammatory cytokines and chemokines are considered as the final products of the TLR signaling pathway [41]. A total number of five different adaptor molecules are described for TLRs: the myeloid differentiation primary response gene 88 (MyD88), MyD88 adaptor-like protein (MAL), TIR-domain-containing adaptor protein including interferon-β-mediated transcription factor (TRIF), TRIF-related adaptor molecule (TRAM), and sterile-alpha and armadillo motif-containing protein (SARM) [42]. These adaptor molecules are considered as recruiters for the initiation and stimulation of downstream kinases and transcription factors that are involved in inflammation and antiviral responses [43]. TLRs and their respective adaptors can also interact with the homologous TIR domains present in both TLRs and adaptor molecules. Based on the recruitment of particular adaptors, TLRs can thus converge on two second messenger pathways: MyD88 dependent or MyD88 independent. Basically, MyD88 adaptor protein can activate signals of the MyD88-dependent pathway with all TLR family members involved with the exception of TLR3 [22, 44]. TLR1/2/4/6 is also bound to MyD88 after successfully assembling with TIRAP/Mal [45, 46] and then, MyD88 can activate the IL-1 receptor-associated kinase 4 (IRAK4). Moreover, MyD88 can recruit other members of the IRAK family, such as IRAK-1 that stimulates mitogen-activated protein kinase and the NF-κB pathway (Fig. 1) [47]. Formation of MyD88 and IRAKs complexes are likely to disassociate and react with tumor necrosis factor receptor-associated factor 6 (TRAF6). TRAF6 together with an ubiquitin-conjugating enzyme 13 (Ubc13) and ubiquitin-conjugating enzyme E2 variant 1 (Uev1A) can catalyze Lys-63 (K63)-linked polyubiquitination which initiates the activation of a complex consisting of transforming growth factor-β-activated kinase (TAK1), TAK1-binding protein 1 (TAB1), and TAB2 leading to phosphorylation of TAK1 and TAB2/3 [48, 49]. As well, TAK1 activates the inhibitor of NF-κB (IκB)–kinase complex (IKK) which is composed of IKK-α, IKK-β, and IKK-γ (Fig. 1). This complex phosphorylates IκB and causes NF-κB dissociation and translocation to the nucleus and the subsequent pro-inflammatory cytokine gene expression such as interleukin 1 β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) [22, 50]. NF-κB consists of p65 and p50 dimers that are inactive if present in the cytoplasm in association with IκB [51]. The second part of the TAK1 pathway is the activation of mitogen-activated protein kinases (MAPKs) which includes three families of p38 MAPKs, extracellular signal-regulated kinases (ERKs), and c-Jun-N-terminal kinases (JNKs). Finally, MAP kinase is responsible for the formation of AP-1, another transcription factor complex inducing cytokine gene expression (Fig. 1) [50, 52, 53].
Fig. 1.
TLR cell signaling pathways. TLR4 and the heterodimers of TLR2/TLR1 or TLR2/TLR6 located on the cell surface identify their respective ligands. TLR4 is the only member of the TLR family which is also located in endosomes. The intracellular TLRs, TLR3, TLR7, TLR8, TLR9 are also found in endosomes. Every TLR family member, except TLR3, uses the MyD88-dependent signaling pathway. TRAP/Mal adaptor proteins bridge MyD88 to TLR1/6/2/4 and promote their interactions with TRAF6. TRAF6 activates the complex of TAK1, TAB1, and TAB2/3. TAK1 activates both the IKK complex and MAPK. Complex of IKK which causes IκB phosphorylation resulting in the translocation of NF-κB to the nucleus where pro-inflammatory gene expression is triggered. p38, ERK, JNK induced by MAPKs cause the AP-1 nuclear translocation. TLR 7/8 and 9 can activate MyD88 through two pathways: one pathway causes the translocation of IRF7 transcription factor to the nucleus and leads to type I IFN expression; the other pathway induces NF-κB as a downstream signaling molecule. TRIF is another adaptor protein which is recruited by TLR3 and TLR4 independently from the MyD88 pathway and leads to IRF3/IRF7 dimer translocation to the nucleus resulting in the expression of type I IFN. Furthermore, TRIF can interact with TRAF6 through an effect on RIP-1 which is followed by NF-κB activation. SOCS1 can inhibit the TLR signaling pathway via impact on NF-κB and MAPK activity and phosphorylation of p65
TLR4 is the only member of the TLR family that is able to trigger both MyD88- and TRIF-dependent signaling pathway-related genes [44]. LPS is considered an important ligand which binds to TLR4 and leads to endosome formation resulting in TRAM translocation to cytosol and TRIF-dependent signaling pathway activation [50]. This pathway responds with TRAF3 expression and activation of the TANK-binding kinase/IκB kinase (TBK1/IKKi) complex which leads to the interferon regulatory factor (IRF), IRF3 and IRF7, phosphorylation. The phosphorylated IRF3 and IRF7 dimer will then translocate to the nucleus and finally induce IFN gene expression [22, 45, 50]. TRAF6 is another target after TRIF downstream activation pathway which is activated by receptor interacting protein 1 (RIP1) followed by TAK1 complex and NF-κB (Fig. 1) [46, 49].
As well, suppressor of cytokine signaling (SOCS1) is known as a protein induced by cytokines and can negatively regulate cytokine-signaling pathway and directly down-modulate the TLR-signaling pathway [54, 55]. SOCS1 also affects the TLR–NF-κB pathway and binds to the p65 subunit of NF-κB to help ubiquitylation of p65. SOCS1 might regulate stress-activated MAPKs and finally lead to the suppression of MAL-dependent p65 phosphorylation and NF-κB transactivation [56, 57].
Moreover, a molecule family termed triggering receptors expressed on myeloid cells (TREM) can influence the downstream signaling pathway of the TLRs. TREM molecules have both activating and inhibitory receptors as regulatory factors playing a role in TLR-mediated inflammatory responses [58]. TREM-1 and TREM-2 can act on immune cells involved in innate immune responses. It was reported that TREM-1 expression increases during an infection in vivo and following TLR stimulation in vitro [59, 60]. Silencing of TREM-1 influences chemokine and cytokine production by elevating access to downstream signaling molecules involved in acute inflammation. This silencing did not change TRIF-mediated expression of INF indicating that TREM-1 has no main role in reinforcing this TLR signaling pathway. However, the influence of TREM-1 silencing on CD14, MyD88, and other molecules in downstream signaling suggests reduced pro-inflammatory signaling. These authors also noted significant reductions in MyD88 and CD14 transcripts as well as in downstream molecules of the NF-κB pathway following LPS stimulation in the setting of TREM-1 silencing [61, 62]. The overexpression of surface-associated TREM-1, TLR2, and TLR4 in CD14hiCD16+ monocytes approves this pro-inflammatory role. However, simultaneous stimulation of TREM-1 and TLRs induced synergistically cytokine production and amplified inflammatory signals [63]. TREM-2 exists in naive primary astrocytes and was induced by exposure with LPS, OGD, or HMGB-1. Thus, the activation of TREM-2, overexpression of DAP12, and intracellular adaptor of TREM-2 could partly inhibit the activation of NF-κB induced by LPS in purified astrocytic cultures. Expression of TLR4 could also increase the sensitivity of astroglial cells to ligands, thereby facilitating an astrocyte shift towards a pro-inflammatory status. Then TREM-2 could moderate this response by decreasing downstream NF-κB activation. Therefore, TREM-2 and TLR4 expressions in astrocytes might control the sensitivity of astrocytes to TLR ligands by regulation of downstream NF-κB activation [39].
TLRs and stroke
There is good evidence that TLRs and their ligands play an important role after brain ischemic injury. The inflammatory responses in immune cells are mediated by TLRs suggesting that these receptors contribute to induce ischemic damage. It is evident that resident cells such as astrocytes and microglia initiate the inflammatory cascades after cerebral ischemia by recognizing injury-associated molecules. Subsequently, infiltration of immune cells such as macrophages and neutrophils to the damaged area take place and a massive release of inflammatory cytokines, proteolytic enzymes, and other cytotoxic mediators can be observed [7, 64, 65]. Several prospective studies including rodent models have investigated the function of TLRs in cerebral ischemia and addressed the basic question which TLR subpopulations are essential for the progression of ischemic damage in the brain [66].
TLR2 has been shown to be involved in sterile inflammation like ischemic brain damage [66, 67]. TLR2 can also act on glial cells to secrete inflammatory factors and pro-inflammatory mediators which further propagate brain damage [68]. Recent studies have indicated that leukocyte infiltration into the damaged area via a disrupted blood–brain barrier (BBB) and the subsequent induction of apoptosis in neurons can be induced by TLR2 and reduced by TLR2 suppression [69]. TLR2 mRNA levels in resident microglia are increased following brain ischemia in mice [66]. Besides, TLR2 can bind to endogenous ligands after ischemic stroke [70, 71]. HMGB1, considered as an essential DAMP in ischemic damage, is localized in the cell nucleus and translocated into the cytosol after ischemic injury to stimulate TLR2. It has been reported that HMGB1 neutralizing antibodies reduce the infarct volume after MCAO [72–74]. Peroxiredoxin (Prx) family protein which is expressed in the injured area is another member of DAMPs with a neuroprotective role [75, 76]. Neurons of TLR2-deficient mice are also protected against cell death induced by an ischemia-like energy exclusion model. In addition, less CNS damage has been seen in TLR2-knockout mice following focal cerebral ischemia [66, 77]. Mice lacking TLR2 gene have shown less brain damage and neurological deficits after MCAO compared to wild-type mice revealing an up-regulation of TLR2 gene in the non-ischemic brain hemisphere [68, 77]. An up-regulation of the TLR2 gene has been also reported in an in vitro ischemia/reperfusion model in cultured microglia [78]. It should be noted that the activation of microglia occurs by the release of toxic cytokines such as IL-23 and IL-17 during ischemia/reperfusion. The inhibition of this pathway in microglia can result in a protection after ischemia. Besides, inflammatory signaling of TLR2 in the post-ischemic brain requires the scavenger receptor CD36. The lack of this receptor can suppress inflammation. These findings suggest that the TLR2–CD36 complex could act as a sensor of ischemia at the onset of death signals and is vital for the inflammatory responses [79]. Therefore, TLR2 suppression could be potentially considered as a future treatment for ischemic stroke [69]. Other members of the TLR family, i.e., TLR3 or TLR9, also appear to be involved in brain ischemia [80–82]. For example, Alex et al. found that TLR3 signaling lowers the release of inflammatory cytokines by NF-κB pathway suppression in TLR3-knockout mice [82–85]. TLR4 is considered to play a pivotal role for the progression of infarct volume in the ischemic brain via its binding to the endogenous ligands like HMGB1 which then causes immune cell infiltration through BBB into the infarct area and its surrounding regions [70, 71, 73, 81, 86–88]. TLR4 gene expression is increased in neurons after cerebral ischemia accompanied by the enhancement of multiple inflammatory cytokines. Knockout of the TLR4 gene in neurons can thus improve their survival under glucose-deprived conditions [66]. This was also proven in TLR4-deficient mice which displayed less infarct volume compared to wild types [89–93]. Similarly, researchers revealed that TLR4-mutant mice had less expression of cyclooxygenase 2 (COX2), inducible nitric oxide synthase (iNOS), and IFN-g [89, 90, 92]. Moreover, it was suggested that LPS as a ligand of TLR4 could modulate this receptor [68, 94]. It should be noted that TLR7 and 8 seem to be directly involved in inflammatory damage following ischemic stroke [80].
TLR2 and TLR4 are known to be more essential than other TLRs in the pathological development of ischemic brain damage [10]. Nalamolu et al. described the neuroprotective impact of simultaneous TLR2/TLR4 suppression under ischemic stroke conditions probably mediated by mitigating the induction of the pro-inflammatory cytokines TNF, IL-1, and IL-6 [95]. Therefore, TLR2 and TLR4 can be considered as interesting targets for stroke treatment. A better knowledge about the molecular structure, genetic variations, and modulation by several reagents of TLRs can help in future managing stroke prevalence and treatments.
TLR gene polymorphisms and stroke risk
There are reportedly several polymorphisms in TLR2 and TLR4 genes which may possibly change the risk for stroke. According to the National Center for Biotechnology Information (NCBI) database, many single nucleotide polymorphisms (SNPs) exist in TLR2 and TLR4 genes including rs5743708, rs1927911, rs4986790, and TLR4-C119A [96, 97]. TLR2 is located on chromosome 4 (4q31.3) with 5 exons and rs5743708 transition is situated on exon 5 [98]. This polymorphism, a missense mutation, can lead to a glutamine-to-arginine substitution at codon 735 (p.Arg753Gln). Besides, the rs1927911 variant is located on intron 1 of the TLR4 gene on chromosome 9 (9q33.1) [99]. Considered as a missense SNP, the rs4986790 is situated on exon 4 of TLR4 with the substitution of glycine to aspartate at codon 299 (p.Asp299Gly). In addition, the TLR4–C119A transversion is located on intron 1 of the TLR4 gene. Results from a previous study revealed that allele and genotype frequencies of rs1927911 were significantly different between stroke and control groups, while rs5743708 was not reported to be different between these groups. For rs1927911, it was shown that fasting blood glucose, blood pressure, and levels of serum lipids were not significantly different among the various genotypes in stroke and control groups [96]. Lin et al. reported a significant association between A119C polymorphism and stroke risk and demonstrated that the TLR4-119A allele might be a risk factor for ischemic stroke and it was also concluded that rs4986790 was a rare variation in the given study population [97].
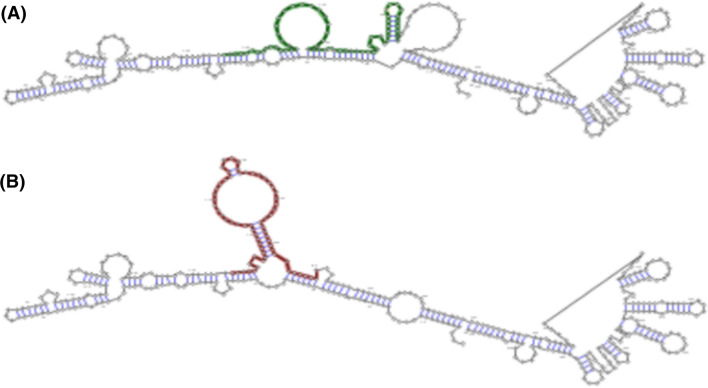
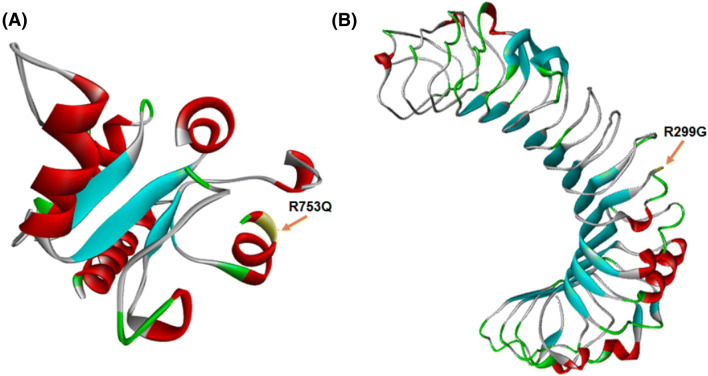
Pathogenic effects of gene polymorphisms can be variable and depend on the position of SNP on the gene, RNA, and protein sequences [100, 101]. The four aforementioned SNPs may also influence molecular aspects of TLR2 and TLR4. As mentioned in (Table 1) and according to the RNAsnp database, only the rs4986790 polymorphism appeared to be deleterious for the TLR4 RNA structure [102]. This polymorphism could reduce the minimum free energy of RNA, change the expression of interested gene, and consequently change the expression of the genes of interest (Fig. 2) [103–105]. It should be noted that rs5743708 and rs4986790 polymorphisms are non-synonymous SNPs. Generally, non-synonymous polymorphisms cause amino acid substitutions in the protein sequence [106, 107]. According to the SNPeffect database, rs5743708 transition is located in a helix region of TLR2, whereas the rs4986790 SNP is situated in an extended region of TLR4 protein (Fig. 3). In addition, the database records have predicted both rs5743708 and rs4986790 SNPs as harmful polymorphisms that are likely to reduce protein stability [108]. Other databases including PolyPhen-2 [109], SNAP [110], SIFT [111], and SNPeffect [112] can also provide evidence that rs5743708 transition is deleterious. Despite the fact that PolyPhen-2, SIFT, PhD-SNP, and SNPs&GO [113] databases have predicted rs4986790 transition as a benign SNP for TLR4 molecule (Table 2), it could be concluded that damaging effects of rs4986790 could arise from alterations in TLR4 RNA structure, while harmful effects of rs5743708 were likely to be caused by changes in the protein structure of TLR2.
Table 1.
Effects of SNPs on RNA structure of TLR2 and TLR4
| SNP | Gene | Folding window | Local region | Distance | p value |
|---|---|---|---|---|---|
| rs5743708 | TLR2 | 3085–3485 | 3261–3316 | 0.0009 | 0.9718 |
| rs1927911 | TLR4 | 8395–8795 | 8566–8615 | 0.0188 | 0.6715 |
| rs4986790 | TLR4 | 827–1227 | 972–1036 | 0.1510 | 0.1203 |
| C119A | TLR4 | 5208–5608 | 5399–5606 | 0.0422 | 0.5465 |
p values less than 0.2 are considered to be significant
Fig. 2.
Secondary structure of RNA of human TLR4 before and after rs4986790 transition. Optimal secondary structure of global sequence (highlighted from 827 to 1227 nt) in the wild type with minimum free energy = − 129.20 kcal/mol (a) and mutant with minimum free energy = − 74.60 kcal/mol (b).
The figure was modified from rnaSNP
Fig. 3.
Partial three-dimensional structures of TLR2 and TLR4. The rs5743708 and rs4986790 substitutions are a helix domain of TLR2 (a) and an extend domain of TLR4 (b), respectively
Table 2.
Effects of rs5743708 and rs4986790 SNPs on protein structure
| SNP | Gene | Motif position | PolyPhen-2 | SNAP | SIFT | SNPeffect | PhD-SNP | SNPs&GO |
|---|---|---|---|---|---|---|---|---|
| rs5743708 | TLR2 | Helix | + | + | + | + | + | − |
| rs4986790 | TLR4 | Extend | − | + | − | + | − | − |
The “+” and “−” symbols show deleterious and benign effects, respectively
Neurosteroids as TLR modulators
Neurosteroids can be synthesized by both neurons and glial cells and act within CNS [114]. Neurosteroids synthesized by nervous tissue can act through their intracellular nuclear receptors thereby regulating gene expression [115]. Thus, they are able to protect the brain via an inhibition of TLR-related pathways from damage [116]. The risk of stroke in women typically increases at post-menopause stage indicating the protective potentials of female sex hormones, i.e., estrogen and progesterone (EP) [117]. Generally, both steroids are neuroprotective in the CNS under acute neurodegenerative and neuropathological conditions [118–121]. Animal and human studies have also shown that EP decreases post-stroke ischemic injury [122–125]. Vitamin D and its metabolites are also recognized as important neurosteroids that are capable to guard neurons following ischemia [126–128].
Estrogen
This hormone exerts its biological actions mainly through three different estrogen receptors (ERs) including ERα, ERβ, and G protein-coupled receptor 30 (GPR30) which are all expressed in the brain and linked to various molecular pathways such as membrane-linked receptors and nuclear receptors [129, 130]. The modulation of neuroinflammation is considered as one the most important neuroprotective mechanisms of estrogens after brain ischemia [131]. Accordingly, studies have shown that estrogen decreases toxic damage to neurons and regulates neuro-inflammatory processes via the modulation of glial cell function, and consequently reduces infarct volume when it is given after permanent or transient brain ischemia [11, 132–136]. 17β-Estradiol (E2) has been reported to suppress the release of cytokines and pro-inflammatory factors such as TNF-α, IFN-γ, and IL-17. Additionally, E2 can promote the release of the anti-inflammatory factors such as transforming growth factor beta (TGF-β) and IL-10 [137, 138]. Generally, E2 plays an important role for the TLR/NF-κB signaling pathway. It has been demonstrated that TLR4 is down-regulated by E2. Moreover, TLR2 is significantly down-regulated in ERα mutant ovariectomized mice [139, 140]. Additional studies have indicated the neuroprotective effects of ERα following experimental ischemic damage through the interaction of ERα with IL-6 and NF-κB [141–145].
As mentioned above, E2 can act via ERα independent of its nuclear function by preventing NF-κB nuclear translocation. This demonstrates that E2–ERα signaling pathways play an important role in immediate-early inflammatory response (Fig. 4a) [146]. E2 levels elevated in obesity and during pregnancy could also increase the expression of IκBα as an anti-inflammatory molecule. On the other hand, ERβ could block nuclear translocation of NF-κB in cerebral ischemia [147]. Elevated levels of TLR2, TLR4, and MyD88 mRNA, activated NF-κB, and also phosphorylated p65 in ovariectomized mice may be reversed by ERβ agonists (Fig. 4a) [139]. Moreover, estrogen treatment could reduce the expression of TNF-α after cerebral ischemia, although it has been reported that chronic administration of E2 stimulates TNF-α and pro-inflammatory cytokine release [148–150].
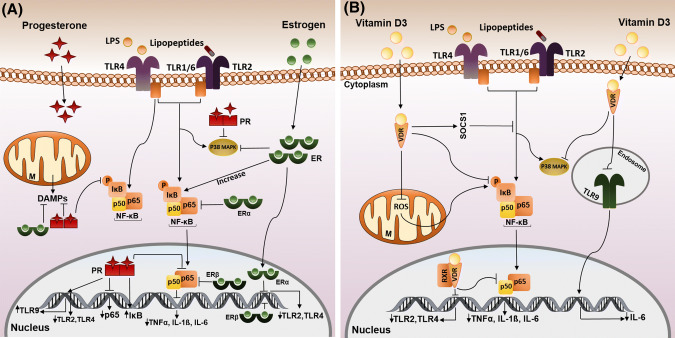
Fig. 4.
Effects of neurosteroids on TLR signaling pathways under ischemic conditions. a Estrogen/ER could increase IκB, also ERα could block NF-κB nuclear translocation. In the nucleus, ERα/β inhibits the expression of TLR2/4, and ERβ suppresses the p65 subunit of NF-κB. Progesterone suppresses LPS-induced NF-kB activation by prevention of the phosphorylation of IκB and the p65 subunit. Progesterone also blocks the expression of TLR2/4, increases the expression of IκB, and TLR9, and suppresses the transcription of the p65 gene. Estrogen/progesterone promote mitochondrial function by blocking of DAMP action and suppressing p38 MAPK activation. b Vitamin D3 binding to VDR leads to anti-inflammatory effects by suppressing phosphorylation of IκB, p38 MAPK and NF-κB translocation to the nucleus and also by stimulation of SOCS1 which inhibits the TLR pathway. VD3 exerts a negative regulation of the phosphorylation of IκB by blocking ROS activity and suppressing the expression of TLR2/4 and NF-κB activity. Pro-inflammatory effects of VD3 occur via the down-regulation of TLR9 and by blocking of IL-6 expression. Up- and down-regulation of genes are depicted by ↑ and ↓ symbols
Pre-treatment of male rats with E2 in a MCAO model also showed a significant down-regulation of IL-1β and a lowering of infarct volume compared to non-treated males [151]. There are other reports showing IL-6 inhibition and control of inflammation after E2 treatment in ischemic stroke [152]. Furthermore, E2 could boost the MAPK/ERK pathway which mediated neuroprotection and deprivation of this hormone modulates activation and expression of the p38 MAPK family members in the brain [153, 154]. In this respect, Koerner et al. revealed that estrogen replacement in ovariectomized mice could lead to temporal anti-inflammatory responses that were likely to induce immunosuppression [140]. Following brain ischemia, estrogen can also promote the formation of the resting microglia and astrocyte phenotype which reduce their activation, proliferation, and migration towards the damaged region and may be assumed responsible for the inhibition of glial-derived pro-inflammatory factors [122].
Progesterone
This neurosteroid appears to be neuroprotective in a variety of species including human particularly in neurodegenerative diseases, stroke, traumatic brain injury (TBI), and spinal cord injury. Generally, this hormone decreases neuronal loss and brain edema by down-regulating distinct inflammatory pathways [155–162]. Progesterone typically exerts its actions through progesterone receptors (PRs) such as classical isoforms of PR-A and PR-B which are found in the nucleus and operate as transcription factors via binding to progesterone response elements in the promoter of gene. In addition, progesterone membrane receptors also exist in the brain which transmit a wide variety of progesterone effects [162–165]. Both nuclear and membrane PRs mediate progesterone-regulated responses in the brain affecting directly microglia, astrocytes, and neurons [163]. This hormone can reduce inflammation after cerebral ischemia and TBI via modulating TLR signaling [116, 166]. Hua et al. showed that progesterone did not suppress the activation of TLR2 but rather promoted the expression of TLR9 [94, 116]. Furthermore, progesterone and its metabolites regulate TLR4 and NF-κB signal transduction pathways after subarachnoid hemorrhage and ischemic brain damage [166, 167]. There is more information available concerning the progesterone-mediated control of protective signaling cascades such as inhibition of LPS-induced NF-κB activation by blocking IκBα and suppressing p65 subunit phosphorylation, down-regulation of phosphorylation of the p38, JNK and ERK MAPKs in microglia [168], and attenuation of TLR2/4 expression and antagonizing NF-κB activity by increasing transcription of IκBα gene or suppression of p65 gene (Fig. 4a) [169, 170]. It has also reported that progesterone controls IL-1β and TNF-α after TBI [171] and MCAO [172]. However, another report described that progesterone has no influence on the expression of IL-1β and TNF-α following brain injury [173].
In general, data suggest that a co-treatment of E2 with progesterone is more effective in the treatment of neurodegeneration than exposure to single steroids possibly due to their synergistic effects [174]. A combined EP treatment of cerebral cortex neurons appears to be more effective in preventing glucose serum deprivation (CGSD) and might be the result of direct and indirect interactions with microglia [175–177]. EP treatment also promotes mitochondrial function by attenuating DAMP activity and blocking the TLR4/NF-κB pathway [176, 178–181].
The negative results of both clinical trials called SyNAPSE and ProTECT III, which used neurosteroids and their derivatives as a treatment regimen created a lot of frustration in the field of steroid-mediated neuroprotection in the brain. The method for administration of progesterone might be one reason for the failure. In the SyNAPSe III and ProTECT trials, natural progesterone has been used by continuous intravenous infusion to patients with TBI. However, none of the preclinical TBI trainings has used this method but instead subcutaneous or intraperitoneal injections [182]. In addition, large doses of progesterone were given in the clinical trials throughout. Only a few animal studies have tried dose–response relationships of progesterone and suggested that high doses of progesterone might be less effective or even damaging after TBI [183]. Furthermore, the abrupt discontinuation of the intravenous progesterone administration and long-term treatment with progesterone after experimental TBI are more effective than a short-term treatment [184, 185]. Therefore, the design of the SyNAPSe and ProTECT III trials could imply some ideal options that were not always supported by existing preclinical outcomes [182].
Vitamin D
Vitamin D3 (cholecalciferol) is mainly synthesized in the skin or is intestinally absorbed. Cholecalciferol can be initially metabolized in the liver into calcifediol (25-hydroxycholecalciferol) and then in the kidney into calcitriol (1,25(OH)2D3). Calcitriol is considered the most important biologically active metabolite of vitamin D. It has also been identified as a neuroprotective factor for stroke [126, 186–188]. Vitamin D is structurally similar to classical steroid hormones and mainly acts via nuclear receptors thereby regulating inflammatory genes expressed in macrophages and T lymphocytes [189–191]. Vitamin D can also inhibit inflammatory responses and may elicit anti-inflammatory pathways by acting through specific vitamin D receptors (VDR) [192] which are found in immune cells [193]. There are additional functions of vitamin D along with its influence on immune cells which can be beneficial under pathological conditions including regulation of apoptosis and cell cycle [188]. Calcitriol can also modulate TLR signaling through SOCS1 stimulation, prevent the phosphorylation of p38 MAPK, and inhibit NF-κB activity (Fig. 4b) [193–197]. IkB phosphorylation is a result of mitochondrial dysfunction which occurs as a result of an elevation of reactive oxygen species (ROS) in mitochondria of injured cells after hypoxia. It has been suggested that calcitriol/VDR can directly interfere with the IkB/NF-κB complex or IKKβ, thus preventing the translocation of p65 into the cell nucleus [198, 199]. Treatment of human monocytes and macrophages with vitamin D3 can also reduce the expression of TLR2/4 and block NF-κB within the nucleus (Fig. 4b) [200, 201]. Vitamin D3 can further minimize the release of TNF-α, IL-6, and IL-1b in human/murine macrophages and monocytes following LPS stimulation [201]. Interestingly, TLR2 can promote VDR expression and TLR agonists can elevate the conversion of calcifediol into calcitriol by regulating the expression of 1α-hydroxylase (1αOHase) [202, 203]. Recent evidence from animal studies and clinical trials suggest that vitamin D deficiency is correlated with several inflammatory diseases and increased infarct volumes in stroke [193, 204, 205]. Moreover, treatment with vitamin D has been reported to cause TLR9 down-regulation in monocytes and subsequently lower IL-6 release [206]. As well, it has been revealed that co-treatment of vitamin D and progesterone in TBI, an ischemic in vitro neuronal model, and a cerebral ischemia animal model results in better outcomes by modulation of the TLR4/NF-κB signaling cascade [207, 208].
Conclusion
This review article focused on aspects of TLR signaling during acute neuropathological challenges in the brain and highlighted the potential neuroprotective capacity of estrogen, progesterone and vitamin D3 and their putative interactions with TLR signaling pathways after cerebral ischemia and TBI. In particular, TLR2 and TLR4 signaling appeared to be pivotal for controlling pathogenic immune responses following stroke. All three hormones were able to modulate TLR2 and TLR4 signal transduction. Thus, these steroids and the vitamin can be considered as therapeutic options for stroke therapy.
Acknowledgements
This work was supported by a grant (IR.KAUMS.REC. 1395. Grant no. 95086) from Kashan University of Medical Sciences.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Gentile NT, McIntosh TK. Antagonists of excitatory amino acids and endogenous opioid peptides in the treatment of experimental central nervous system injury. Ann Emerg Med. 1993;22:1028–1034. doi: 10.1016/S0196-0644(05)82746-5. [DOI] [PubMed] [Google Scholar]
- 2.Moghadam SE, Tameh AA, Vahidinia Z, Atlasi MA, Bafrani HH, Naderian H. Neuroprotective effects of oxytocin hormone after an experimental stroke model and the possible role of Calpain-1. J Stroke Cerebrovasc Dis. 2018;27(3):724–732. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Yakovlev AG, Knoblach SM, Fan L, Fox GB, Goodnight R, Faden AI. Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J Neurosci. 1997;17:7415–7424. doi: 10.1523/JNEUROSCI.17-19-07415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10:471–480. doi: 10.1016/S1474-4422(11)70066-7. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y-C, Lin S, Yang Q-W. Toll-like receptors in cerebral ischemic inflammatory injury. J Neuroinflamm. 2011;8:134. doi: 10.1186/1742-2094-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crack PJ, Bray PJ. Toll-like receptors in the brain and their potential roles in neuropathology. Immunol Cell Biol. 2007;85:476–480. doi: 10.1038/sj.icb.7100103. [DOI] [PubMed] [Google Scholar]
- 9.Kaczorowski DJ, Mollen KP, Edmonds R, Billiar TR. Early events in the recognition of danger signals after tissue injury. J Leukoc Biol. 2008;83:546–552. doi: 10.1189/jlb.0607374. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Ge P, Zhu Y. TLR2 and TLR4 in the brain injury caused by cerebral ischemia and reperfusion. Mediat Inflamm. 2013;2013:124614. doi: 10.1155/2013/124614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kipp M, Norkute A, Johann S, Lorenz L, Braun A, Hieble A, et al. Brain-region-specific astroglial responses in vitro after LPS exposure. J Mol Neurosci. 2008;35:235–243. doi: 10.1007/s12031-008-9057-7. [DOI] [PubMed] [Google Scholar]
- 12.Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 13.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdörfer B, Giese T, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 16.Matsushima N, Tanaka T, Enkhbayar P, Mikami T, Taga M, Yamada K, et al. Comparative sequence analysis of leucine-rich repeats (LRRs) within vertebrate toll-like receptors. BMC Genom. 2007;8:124. doi: 10.1186/1471-2164-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, et al. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W, Xiong Y, Li Q, Yang H. Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front Physiol. 2017;8:508. doi: 10.3389/fphys.2017.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shichita T, Sakaguchi R, Suzuki M, Yoshimura A. Post-ischemic inflammation in the brain. Front Immunol. 2012;3:132. doi: 10.3389/fimmu.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccinini A, Midwood K. DAMPening inflammation by modulating TLR signalling. Mediat Inflamm. 2010;2010:672395. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gürtler C, Bowie AG. Innate immune detection of microbial nucleic acids. Trends Microbiol. 2013;21:413–420. doi: 10.1016/j.tim.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem. 2010;114:13–27. doi: 10.1111/j.1471-4159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 26.Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009;158:1007–1020. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carty M, Bowie AG. Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem Pharmacol. 2011;81:825–837. doi: 10.1016/j.bcp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Bell JK, Mullen GE, Leifer CA, Mazzoni A, Davies DR, Segal DM. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003;24:528–533. doi: 10.1016/S1471-4906(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 29.Brodsky I, Medzhitov R. Two modes of ligand recognition by TLRs. Cell. 2007;130:979–981. doi: 10.1016/j.cell.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Uematsu S, Akira S. Toll-Like receptors (TLRs) and their ligands. Handb Exp Pharmacol. 2008;183:1–20. doi: 10.1007/978-3-540-72167-3_1. [DOI] [PubMed] [Google Scholar]
- 31.Botos I, Segal DM, Davies DR. The structural biology of Toll-like receptors. Structure. 2011;19:447–459. doi: 10.1016/j.str.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flo TH, Halaas O, Torp S, Ryan L, et al. Differential expression of Toll-like receptor 2 in human cells. J Leukoc Biol. 2001;69:474–481. [PubMed] [Google Scholar]
- 33.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 34.Farina C, Krumbholz M, Giese T, Hartmann G, et al. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–19. doi: 10.1016/S0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 36.Owens T, Babcock AA, Millward JM, Toft-Hansen H. Cytokine and chemokine inter-regulation in the inflamed or injured CNS. Brain Res Rev. 2005;48:178–184. doi: 10.1016/j.brainresrev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jack CS, Arbour N, Manusow J, Montgrain V, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 39.Rosciszewski G, Cadena V, Murta V, et al. Toll-like receptor 4 (TLR4) and triggering receptor expressed on myeloid cells-2 (TREM-2) activation balance astrocyte polarization into a proinflammatory phenotype. Mol Neurobiol. 2018;55:3875–3888. doi: 10.1007/s12035-017-0618-z. [DOI] [PubMed] [Google Scholar]
- 40.Crocker SJ, et al. A novel method to establish microglia-free astrocyte cultures: comparison of matrix metalloproteinase expression profiles in pure cultures of astrocytes and microglia. Glia. 2008;56:1187–1198. doi: 10.1002/glia.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 42.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–564. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 43.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 44.Takeda K, Akira S (eds) (2004) TLR signaling pathways. Semin Immunol 16:3–9 [DOI] [PubMed]
- 45.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Ostuni R, Zanoni I, Granucci F. Deciphering the complexity of Toll-like receptor signaling. Cell Mol Life Sci. 2010;67:4109–4134. doi: 10.1007/s00018-010-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9:684–691. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- 48.Xia Z-P, Sun L, Chen X, Pineda G, Jiang X, Adhikari A, et al. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature. 2009;461:114–119. doi: 10.1038/nature08247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medzhitov R. Toll-like receptors and innate immunity. Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 51.Guijarro C, Egido J. Transcription factor-κB (NF-κB) and renal disease. Kidney Int. 2001;59:415–424. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
- 52.Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol. 2001;23:1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- 53.Nito C, Kamada H, Endo H, Narasimhan P, Lee Y-S, Chan PH. Involvement of mitogen-activated protein kinase pathways in expression of the water channel protein aquaporin-4 after ischemia in rat cortical astrocytes. J Neurotrauma. 2012;29:2404–2412. doi: 10.1089/neu.2012.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 55.Nakagawa R, Naka T, Tsutsui H, Fujimoto M, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/S1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 57.Starczynowski DT, Karsan A. Innate immune signaling in the myelodysplastic syndromes. Hematol Oncol Clin N Am. 2010;24:343–359. doi: 10.1016/j.hoc.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 59.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 60.Nathan C, Ding A. TREM-1: a new regulator of innate immunity in sepsis syndrome. Nat Med. 2001;7:530. doi: 10.1038/87846. [DOI] [PubMed] [Google Scholar]
- 61.Klesney-Tait J, Colonna M. Uncovering the TREM-1-TLR connection. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1374–L1376. doi: 10.1152/ajplung.00415.2007. [DOI] [PubMed] [Google Scholar]
- 62.Ornatowska M, Azim AC, Wang X, Christman JW, et al. Functional genomics of silencing TREM-1 on TLR4 signaling in macrophages. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1377–L1384. doi: 10.1152/ajplung.00140.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Golovkin A, Matveeva VG, Kudryavtsev IV, Chernova MN, et al. Perioperative dynamics of TLR2, TLR4, and TREM-1 expression in monocyte subpopulations in the setting of on-pump coronary artery bypass surgery. ISRN Inflamm. 2013;2013:817901. doi: 10.1155/2013/817901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marsh BJ, Stenzel-Poore MP. Toll-like receptors: novel pharmacological targets for the treatment of neurological diseases. Curr Opin Pharmacol. 2008;8:8–13. doi: 10.1016/j.coph.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marsh BJ, Stevens SL, Hunter B, Stenzel-Poore MP. Inflammation and the emerging role of the toll-like receptor system in acute brain ischemia. Stroke. 2009;40:S34–S37. doi: 10.1161/STROKEAHA.108.534917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang S-C, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen S, Wong MH, Schulte DJ, Arditi M, Michelsen KS. Differential expression of Toll-like receptor 2 (TLR2) and responses to TLR2 ligands between human and murine vascular endothelial cells. J Endotoxin Res. 2007;13:281–296. doi: 10.1177/0968051907085096. [DOI] [PubMed] [Google Scholar]
- 68.Ziegler G, Harhausen D, Schepers C, Hoffmann O, Röhr C, Prinz V, et al. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun. 2007;359:574–579. doi: 10.1016/j.bbrc.2007.05.157. [DOI] [PubMed] [Google Scholar]
- 69.Ziegler G, Freyer D, Harhausen D, Khojasteh U, Nietfeld W, Trendelenburg G. Blocking TLR2 in vivo protects against accumulation of inflammatory cells and neuronal injury in experimental stroke. J Cereb Blood Flow Metab. 2011;31:757–766. doi: 10.1038/jcbfm.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Q-W, Lu F-L, Zhou Y, Wang L, Zhong Q, Lin S, et al. HMBG1 mediates ischemia—reperfusion injury by TRIF-adaptor independent toll-like receptor 4 signaling. J Cereb Blood Flow Metab. 2011;31:593–605. doi: 10.1038/jcbfm.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Q-W, Wang J-Z, Li J-C, Zhou Y, Qi-Zhong LuF-L, et al. High-mobility group protein box-1 and its relevance to cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:243–254. doi: 10.1038/jcbfm.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 73.Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, et al. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28:12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21:3904–3916. doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- 75.Patenaude A, Murthy M, Mirault M-E. Emerging roles of thioredoxin cycle enzymes in the central nervous system. Cell Mol Life Sci CMLS. 2005;62:1063–1080. doi: 10.1007/s00018-005-4541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rashidian J, Rousseaux MW, Venderova K, Qu D, Callaghan SM, Phillips M, et al. Essential role of cytoplasmic cdk5 and Prx2 in multiple ischemic injury models, in vivo. J Neurosci. 2009;29:12497–12505. doi: 10.1523/JNEUROSCI.3892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, et al. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 78.Lv M, Liu Y, Zhang J, Sun L, Liu Z, Zhang S, et al. Roles of inflammation response in microglia cell through Toll-like receptors 2/interleukin-23/interleukin-17 pathway in cerebral ischemia/reperfusion injury. Neuroscience. 2011;176:162–172. doi: 10.1016/j.neuroscience.2010.11.066. [DOI] [PubMed] [Google Scholar]
- 79.Abe T, Shimamura M, Jackman K, Kurinami H, Anrather J, Zhou P, et al. Key role of CD36 in Toll-like receptor 2 signaling in cerebral ischemia. Stroke. 2010;41:898–904. doi: 10.1161/STROKEAHA.109.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brea D, Sobrino T, Rodríguez-Yáñez M, Ramos-Cabrer P, Agulla J, Rodríguez-González R, et al. Toll-like receptors 7 and 8 expression is associated with poor outcome and greater inflammatory response in acute ischemic stroke. Clin Immunol. 2011;139:193–198. doi: 10.1016/j.clim.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 81.Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Tanaka H, Uematsu S, et al. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171:258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 82.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 83.Cameron JS, Alexopoulou L, Sloane JA, DiBernardo AB, Ma Y, Kosaras B, et al. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J Neurosci. 2007;27:13033–13041. doi: 10.1523/JNEUROSCI.4290-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okun E, Griffioen K, Barak B, Roberts NJ, Castro K, Pita MA, et al. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proc Natl Acad Sci. 2010;107:15625–15630. doi: 10.1073/pnas.1005807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang P-F, Xiong X-Y, Chen J, Wang Y-C, Duan W, Yang Q-W. Function and mechanism of toll-like receptors in cerebral ischemic tolerance: from preconditioning to treatment. J Neuroinflamm. 2015;12:80. doi: 10.1186/s12974-015-0301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann N Y Acad Sci. 2010;1207:50–57. doi: 10.1111/j.1749-6632.2010.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe C-U, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Maruo T, et al. Anti-high mobility group box-1 monoclonal antibody protects the blood–brain barrier from ischemia-induced disruption in rats. Stroke. 2011;42:1420–1428. doi: 10.1161/STROKEAHA.110.598334. [DOI] [PubMed] [Google Scholar]
- 89.C-x Cao, Q-w Yang, F-l Lv, Cui J, H-b Fu, J-z Wang. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 90.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 91.Tang S-C, Lathia JD, Selvaraj PK, Jo D-G, Mughal MR, Cheng A, et al. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid β-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp Neurol. 2008;213:114–121. doi: 10.1016/j.expneurol.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caso JR, Pradillo JM, Hurtado O, Leza JC, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in subacute stress–induced neuroinflammation and in the worsening of experimental stroke. Stroke. 2008;39:1314–1320. doi: 10.1161/STROKEAHA.107.498212. [DOI] [PubMed] [Google Scholar]
- 93.Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 94.Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule J-L, et al. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nalamolu KR, Smith NJ, Chelluboina B, Klopfenstein JD, et al. Prevention of the severity of post-ischemic inflammation and brain damage by simultaneous knockdown of Toll-like receptors 2 and 4. Neuroscience. 2018;373:82–91. doi: 10.1016/j.neuroscience.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 96.Song Y, Liu H, Long L, Zhang N, Liu Y. TLR4 rs1927911, but Not TLR2 rs5743708, is associated with atherosclerotic cerebral infarction in the Southern Han population: a case–control study. Medicine. 2015;94:e381. doi: 10.1097/MD.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin Y-C, Chang Y-M, Yu J-M, Yen J-H, Chang J-G, Hu C-J. Toll-like receptor 4 gene C119A but not Asp299Gly polymorphism is associated with ischemic stroke among ethnic Chinese in Taiwan. Atherosclerosis. 2005;180:305–309. doi: 10.1016/j.atherosclerosis.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 98.Ioana M, Ferwerda B, Plantinga T, Stappers M, Oosting M, McCall M, et al. Different patterns of Toll-like receptor 2 polymorphisms in populations of various ethnic and geographic origins. Infect Immun. 2012;80:1917–1922. doi: 10.1128/IAI.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou L, Zheng D, Wang S, Zhu J, Jia Y, Sun D, et al. Genetic association of Toll-like receptor 4 gene and coronary artery disease in a Chinese Han population. SpringerPlus. 2016;5:1533. doi: 10.1186/s40064-016-3177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ebrahimi A, Colagar AH, Karimian M. Association of human methionine synthase-A2756G transition with prostate cancer: a case–control study and in silico analysis. Acta Med Iran. 2017;55:297–303. [PubMed] [Google Scholar]
- 101.Zamani-Badi T, Nikzad H, Karimian M. IL-1RA VNTR and IL-1α 4845G > T polymorphisms and risk of idiopathic male infertility in Iranian men: a case–control study and an in silico analysis. Andrologia. 2018;3:e13081. doi: 10.1111/and.13081. [DOI] [PubMed] [Google Scholar]
- 102.Sabarinathan R, Tafer H, Seemann SE, Hofacker IL, Stadler PF, Gorodkin J. RNAsnp: efficient detection of local RNA secondary structure changes induced by SNPs. Hum Mutat. 2013;34:546–556. doi: 10.1002/humu.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zamani-Badi T, Karimian M, Azami-Tameh A, Nikzad H. Association of C3953T transition in interleukin 1β gene with idiopathic male infertility in an Iranian population. Hum Fertil. 2017;3:1–7. doi: 10.1080/14647273.2017.1384857. [DOI] [PubMed] [Google Scholar]
- 104.Karimian M, Aftabi Y, Mazoochi T, Babaei F, et al. Survivin polymorphisms and susceptibility to prostate cancer: a genetic association study and an in silico analysis. EXCLI J. 2018;17:479. doi: 10.17179/excli2018-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salimi S, Keshavarzi F, Mohammadpour-Gharehbagh A, Moodi M, et al. Polymorphisms of the folate metabolizing enzymes: association with SLE susceptibility and in silico analysis. Gene. 2017;637:161–172. doi: 10.1016/j.gene.2017.09.037. [DOI] [PubMed] [Google Scholar]
- 106.Tameh AA, Karimian M, Zare-Dehghanani Z, Aftabi Y, et al. Role of steroid therapy after ischemic stroke by N-methyl-d-aspartate receptor gene regulation. J Stroke Cerebrovasc Dis. 2018;27:3066–3075. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 107.Nejati M, Atlasi MA, Karimian M, Nikzad H, et al. Lipoprotein lipase gene polymorphisms as risk factors for stroke: a computational and meta-analysis. Iran J Basic Med Sci. 2018;21:701–708. doi: 10.22038/IJBMS.2018.29009.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reumers J, Schymkowitz J, Ferkinghoff-Borg J, Stricher F, Serrano L, Rousseau F. SNPeffect: a database mapping molecular phenotypic effects of human non-synonymous coding SNPs. Nucleic Acids Res. 2005;33:D527–D532. doi: 10.1093/nar/gki086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bromberg Y, Rost B. SNAP: predict effect of non-synonymous polymorphisms on function. Nucleic Acids Res. 2007;35:3823–3835. doi: 10.1093/nar/gkm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 112.De Baets G, Van Durme J, Reumers J, Maurer-Stroh S, Vanhee P, Dopazo J, et al. SNPeffect 4.0: on-line prediction of molecular and structural effects of protein-coding variants. Nucleic Acids Res. 2011;40:D935–D939. doi: 10.1093/nar/gkr996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Capriotti E, Calabrese R, Fariselli P, Martelli PL, Altman RB, Casadio R. WS-SNPs&GO: a web server for predicting the deleterious effect of human protein variants using functional annotation. BMC Genom. 2013;14:S6. doi: 10.1186/1471-2164-14-S3-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 115.Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/S0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- 116.Hua F, Wang J, Ishrat T, Wei W, Atif F, Sayeed I, et al. Genomic profile of Toll-like receptor pathways in traumatically brain-injured mice: effect of exogenous progesterone. J Neuroinflamm. 2011;8:42. doi: 10.1186/1742-2094-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lobo RA, Pinkerton JV, Gass ML, Dorin MH, Ronkin S, Pickar JH, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009;92:1025–1038. doi: 10.1016/j.fertnstert.2009.03.113. [DOI] [PubMed] [Google Scholar]
- 118.Carwile E, Wagner AK, Crago E, Alexander SA. Estrogen and stroke: a review of the current literature. J Neurosci Nurs. 2009;41:18–25. doi: 10.1097/JNN.0b013e31819345f8. [DOI] [PubMed] [Google Scholar]
- 119.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- 120.Baulieu E-E, Schumacher M. Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Steroids. 2000;65:605–612. doi: 10.1016/S0039-128X(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 121.Johann S, Beyer C. Neuroprotection by gonadal steroid hormones in acute brain damage requires cooperation with astroglia and microglia. J Steroid Biochem Mol Biol. 2013;137:71–81. doi: 10.1016/j.jsbmb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 122.Ritzel RM, Capozzi LA, McCullough LD. Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke. Horm Behav. 2013;63:238–253. doi: 10.1016/j.yhbeh.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Q-G, Raz L, Wang R, Han D, De Sevilla L, Yang F, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor α-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Semin Reprod Med. 2009;27:229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fu J, Xue R, Gu J, Xiao Y, Zhong H, Pan X, et al. Neuroprotective effect of calcitriol on ischemic/reperfusion injury through the NR3A/CREB pathways in the rat hippocampus. Mol Med Rep. 2013;8:1708–1714. doi: 10.3892/mmr.2013.1734. [DOI] [PubMed] [Google Scholar]
- 127.Brewer LD, Thibault V, Chen K-C, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21:98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kalueff A, Eremin K, Tuohimaa P. Mechanisms of neuroprotective action of vitamin D3. Biochemistry (Moscow) 2004;69:738–741. doi: 10.1023/B:BIRY.0000040196.65686.2f. [DOI] [PubMed] [Google Scholar]
- 129.Manthey D, Behl C. From structural biochemistry to expression profiling: neuroprotective activities of estrogen. Neuroscience. 2006;138:845–850. doi: 10.1016/j.neuroscience.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 130.Strom JO, Theodorsson A, Theodorsson E. Dose-related neuroprotective versus neurodamaging effects of estrogens in rat cerebral ischemia: a systematic analysis. J Cereb Blood Flow Metab. 2009;29:1359–1372. doi: 10.1038/jcbfm.2009.66. [DOI] [PubMed] [Google Scholar]
- 131.Strom JO, Theodorsson A, Theodorsson E. Mechanisms of estrogens’ dose-dependent neuroprotective and neurodamaging effects in experimental models of cerebral ischemia. Int J Mol Sci. 2011;12:1533–1562. doi: 10.3390/ijms12031533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kipp M, Karakaya S, Pawlak J, Araujo-Wright G, Arnold S, Beyer C. Estrogen and the development and protection of nigrostriatal dopaminergic neurons: concerted action of a multitude of signals, protective molecules, and growth factors. Front Neuroendocrinol. 2006;27:376–390. doi: 10.1016/j.yfrne.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 133.Zhang Q-G, Wang R, Tang H, Dong Y, Chan A, Sareddy GR, et al. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol Cell Endocrinol. 2014;389:84–91. doi: 10.1016/j.mce.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jover-Mengual T, Castelló-Ruiz M, Burguete MC, Jorques M, López-Morales MA, Aliena-Valero A, et al. Molecular mechanisms mediating the neuroprotective role of the selective estrogen receptor modulator, bazedoxifene, in acute ischemic stroke: a comparative study with 17β-estradiol. J Steroid Biochem Mol Biol. 2017;171:296–304. doi: 10.1016/j.jsbmb.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 135.Gibson CL, Gray LJ, Murphy SP, Bath PM. Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab. 2006;26:1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- 136.Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marks MA, Gravitt PE, Burk RD, Studentsov Y, Farzadegan H, Klein SL. Progesterone and 17β-estradiol enhance regulatory responses to human papillomavirus type 16 virus-like particles in peripheral blood mononuclear cells from healthy women. Clin Vaccine Immunol. 2010;17:609–617. doi: 10.1128/CVI.00441-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu Y, Sheng H, Bao Q, Wang Y, Lu J, Ni X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression-and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun. 2016;56:175–186. doi: 10.1016/j.bbi.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 140.Cordeau P, Lalancette-Hébert M, Weng YC, Kriz J. Estrogen receptors alpha mediates postischemic inflammation in chronically estrogen-deprived mice. Neurobiol Aging. 2016;40:50–60. doi: 10.1016/j.neurobiolaging.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 141.Behl C. Oestrogen as a neuroprotective hormone. Nat Rev Neurosci. 2002;3:433–442. doi: 10.1038/nrn846. [DOI] [PubMed] [Google Scholar]
- 142.Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, et al. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERα in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- 143.Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, et al. 17β-Estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.STR.30.8.1665. [DOI] [PubMed] [Google Scholar]
- 144.Kurebayashi S, Miyashita Y, Hirose T, Kasayama S, Akira S, Kishimoto T. Characterization of mechanisms of interleukin-6 gene repression by estrogen receptor. J Steroid Biochem Mol Biol. 1997;60:11–17. doi: 10.1016/S0960-0760(96)00175-6. [DOI] [PubMed] [Google Scholar]
- 145.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995;15:4971–4979. doi: 10.1128/MCB.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ghisletti S, Meda C, Maggi A, Vegeto E. 17β-estradiol inhibits inflammatory gene expression by controlling NF-κB intracellular localization. Mol Cell Biol. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Transl Stroke Res. 2013;4:390–401. doi: 10.1007/s12975-012-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liao SL, Chen WY, Chen CJ. Estrogen attenuates tumor necrosis factor-α expression to provide ischemic neuroprotection in female rats. Neurosci Lett. 2002;330:159–162. doi: 10.1016/S0304-3940(02)00754-1. [DOI] [PubMed] [Google Scholar]
- 149.Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, et al. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Res. 2004;1008:147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 150.Calippe B, Douin-Echinard V, Laffargue M, Laurell H, Rana-Poussine V, Pipy B, et al. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol. 2008;180:7980–7988. doi: 10.4049/jimmunol.180.12.7980. [DOI] [PubMed] [Google Scholar]
- 151.Chiappetta O, Gliozzi M, Siviglia E, Amantea D, Morrone LA, Berliocchi L, et al. Evidence to implicate early modulation of interleukin-1β expression in the neuroprotection afforded by 17β-estradiol in male rats undergone transient middle cerebral artery occlusion. Int Rev Neurobiol. 2007;82:357–372. doi: 10.1016/S0074-7742(07)82019-8. [DOI] [PubMed] [Google Scholar]
- 152.Petrone AB, Simpkins JW, Barr TL. 17β-Estradiol and inflammation: implications for ischemic stroke. Aging Dis. 2014;5:340–345. doi: 10.14336/AD.2014.0500340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dominguez R, Liu R, Baudry M. 17-β-Estradiol-mediated activation of extracellular-signal regulated kinase, phosphatidylinositol 3-kinase/protein kinase B-Akt and N-methyl-d-aspartate receptor phosphorylation in cortical synaptoneurosomes. J Neurochem. 2007;101:232–240. doi: 10.1111/j.1471-4159.2006.04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pinceti E. Consequences of estrogen receptor beta phosphorylation in the aged female brain and heart. Chicago: Loyola University Chicago; 2016. [Google Scholar]
- 155.Stein DG. A clinical/translational perspective: can a developmental hormone play a role in the treatment of traumatic brain injury? Horm Behav. 2013;63:291–300. doi: 10.1016/j.yhbeh.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 156.Wright DW, Bauer ME, Hoffman SW, Stein DG. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J Neurotrauma. 2001;18:901–909. doi: 10.1089/089771501750451820. [DOI] [PubMed] [Google Scholar]
- 157.O’Connor CA, Cernak I, Vink R. Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 2005;1062:171–174. doi: 10.1016/j.brainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 158.Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049:112–119. doi: 10.1016/j.brainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 159.Guo Q, Sayeed I, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp Neurol. 2006;198:469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 160.Leonelli E, Bianchi R, Cavaletti G, Caruso D, Crippa D, Garcia-Segura L, et al. Progesterone and its derivatives are neuroprotective agents in experimental diabetic neuropathy: a multimodal analysis. Neuroscience. 2007;144:1293–1304. doi: 10.1016/j.neuroscience.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 161.VanLandingham JW, Cekic M, Cutler SM, Hoffman SW, Washington ER, Johnson SJ, et al. Progesterone and its metabolite allopregnanolone differentially regulate hemostatic proteins after traumatic brain injury. J Cereb Blood Flow Metab. 2008;28:1786–1794. doi: 10.1038/jcbfm.2008.73. [DOI] [PubMed] [Google Scholar]
- 162.Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, et al. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mani S. Progestin receptor subtypes in the brain: the known and the unknown. Endocrinology. 2008;149:2750–2756. doi: 10.1210/en.2008-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Singh M, Su C. Progesterone and neuroprotection. Horm Behav. 2013;63:284–290. doi: 10.1016/j.yhbeh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li X, Zhang J, Zhu X, Wang P, Wang X, Li D. Progesterone reduces inflammation and apoptosis in neonatal rats with hypoxic ischemic brain damage through the PI3K/Akt pathway. Int J Clin Exp Med. 2015;8:8197–8203. [PMC free article] [PubMed] [Google Scholar]
- 167.Wang Z, Zuo G, Shi X-Y, Zhang J, Fang Q, Chen G. Progesterone administration modulates cortical TLR4/NF-κB signaling pathway after subarachnoid hemorrhage in male rats. Mediat Inflamm. 2011;2011:848309. doi: 10.1155/2011/848309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lei B, Mace B, Dawson HN, Warner DS, Laskowitz DT, James ML. Anti-inflammatory effects of progesterone in lipopolysaccharide-stimulated BV-2 microglia. PLoS One. 2014;9:e103969. doi: 10.1371/journal.pone.0103969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen G, Shi J, Jin W, Wang L, Xie W, Sun J, et al. Progesterone administration modulates TLRS/NF-κB signaling pathway in rat brain after cortical contusion. Ann Clin Lab Sci. 2008;38:65–74. [PubMed] [Google Scholar]
- 170.Hardy DB, Janowski BA, Chen C-C, Mendelson CR. Progesterone receptor inhibits aromatase and inflammatory response pathways in breast cancer cells via ligand-dependent and ligand-independent mechanisms. Mol Endocrinol. 2008;22:1812–1824. doi: 10.1210/me.2007-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.He J, Evans C-O, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 172.Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone and allopregnanolone attenuate blood–brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol. 2010;226:183–190. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jones NC, Constantin D, Prior MJ, Morris PG, Marsden CA, Murphy S. The neuroprotective effect of progesterone after traumatic brain injury in male mice is independent of both the inflammatory response and growth factor expression. Eur J Neurosci. 2005;21:1547–1554. doi: 10.1111/j.1460-9568.2005.03995.x. [DOI] [PubMed] [Google Scholar]
- 174.Toung TJ, Chen T-Y, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24:1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- 175.Lorenz L, Dang J, Misiak M, Tameh Abolfazl A, Beyer C, Kipp M. Combined 17β-oestradiol and progesterone treatment prevents neuronal cell injury in cortical but not midbrain neurones or neuroblastoma cells. J Neuroendocrinol. 2009;21:841–849. doi: 10.1111/j.1365-2826.2009.01903.x. [DOI] [PubMed] [Google Scholar]
- 176.Habib P, Dang J, Slowik A, Victor M, Beyer C. Hypoxia-induced gene expression of aquaporin-4, cyclooxygenase-2 and hypoxia-inducible factor 1α in rat cortical astroglia is inhibited by 17β-estradiol and progesterone. Neuroendocrinology. 2014;99:156–167. doi: 10.1159/000362279. [DOI] [PubMed] [Google Scholar]
- 177.Dang J, Mitkari B, Kipp M, Beyer C. Gonadal steroids prevent cell damage and stimulate behavioral recovery after transient middle cerebral artery occlusion in male and female rats. Brain Behav Immun. 2011;25:715–726. doi: 10.1016/j.bbi.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 178.Vahidinia Z, Alipour N, Atlasi MA, Naderian H, Beyer C, Azami Tameh A. Gonadal steroids block the calpain-1-dependent intrinsic pathway of apoptosis in an experimental rat stroke model. Neurol Res. 2017;39:54–64. doi: 10.1080/01616412.2016.1250459. [DOI] [PubMed] [Google Scholar]
- 179.Slowik A, Beyer C. Inflammasomes are neuroprotective targets for sex steroids. J Steroid Biochem Mol Biol. 2015;153:135–143. doi: 10.1016/j.jsbmb.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 180.Lammerding L, Slowik A, Johann S, Beyer C, Zendedel A. Poststroke inflammasome expression and regulation in the peri-infarct area by gonadal steroids after transient focal ischemia in the rat brain. Neuroendocrinology. 2016;103:460–475. doi: 10.1159/000439435. [DOI] [PubMed] [Google Scholar]
- 181.Hoffmann S, Beyer C, Zendedel A. Comparative analysis of gonadal steroid-mediated neuroprotection after transient focal ischemia in rats: route of application and substrate composition. J Mol Neurosci. 2015;56:12–16. doi: 10.1007/s12031-014-0462-9. [DOI] [PubMed] [Google Scholar]
- 182.Schumacher M, Denier C, Oudinet JP, Adams D, et al. Progesterone neuroprotection: the background of clinical trial failure. J Steroid Biochem Mol Biol. 2016;160:53–66. doi: 10.1016/j.jsbmb.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 183.Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: a progesterone dose-response study. Pharmacol Biochem Behav. 2003;76:231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 184.Galani R, Hoffman SW, Stein DG. Effects of the duration of progesterone treatment on the resolution of cerebral edema induced by cortical contusions in rats. Restor Neurol Neurosci. 2001;18:161–166. [PubMed] [Google Scholar]
- 185.Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- 186.Eelen G, Verlinden L, Van Camp M, Van Hummelen P, Marchal K, De Moor B, et al. The effects of 1α, 25-dihydroxyvitamin D3 on the expression of DNA replication genes. J Bone Miner Res. 2004;19:133–146. doi: 10.1359/jbmr.0301204. [DOI] [PubMed] [Google Scholar]
- 187.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 188.Yuan J, Guo X, Liu Z, Zhao X, Feng Y, Song S, et al. Vitamin D receptor activation influences the ERK pathway and protects against neurological deficits and neuronal death. Int J Mol Med. 2018;41:364–372. doi: 10.3892/ijmm.2017.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Farach-Carson MC, Nemere I. Membrane receptors for vitamin D steroid hormones: potential new drug targets. Curr Drug Targets. 2003;4:67–76. doi: 10.2174/1389450033347118. [DOI] [PubMed] [Google Scholar]
- 190.De Bosscher K, Vanden Berghe W, Haegeman G. Cross-talk between nuclear receptors and nuclear factor kappaB. Oncogene. 2006;25:6868–6886. doi: 10.1038/sj.onc.1209935. [DOI] [PubMed] [Google Scholar]
- 191.Norman AW, Nemere I, Zhou L-X, Bishop JE, Lowe KE, Maiyar AC, et al. 1,25 (OH) 2-vitamin D3, a steroid hormone that produces biologic effects via both genomic and nongenomic pathways. J Steroid Biochem Mol Biol. 1992;41:231–240. doi: 10.1016/0960-0760(92)90349-N. [DOI] [PubMed] [Google Scholar]
- 192.Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. 2015;10:e0141770. doi: 10.1371/journal.pone.0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J Biol Chem. 2013;288:19450–19458. doi: 10.1074/jbc.M113.467670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vuolo L, Di Somma C, Faggiano A, Colao A. Vitamin D and cancer. Front Endocrinol (Lausanne) 2012;3:58. doi: 10.3389/fendo.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sanchez-Niño M-D, Bozic M, Córdoba-Lanús E, Valcheva P, Gracia O, Ibarz M, et al. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2011;302:F647–F657. doi: 10.1152/ajprenal.00090.2011. [DOI] [PubMed] [Google Scholar]
- 197.Mutt SJ, Hyppönen E, Saarnio J, Järvelin M-R, Herzig K-H. Vitamin D and adipose tissue—more than storage. Front Physiol. 2014;5:228. doi: 10.3389/fphys.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Won S, Sayeed I, Peterson BL, Wali B, Kahn JS, Stein DG. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS One. 2015;10:e0122821. doi: 10.1371/journal.pone.0122821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen Y, Liu W, Sun T, Huang Y, Wang Y, Deb DK, et al. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting MicroRNA-155–SOCS1 in macrophages. J Immunol. 2013;190:3687–3695. doi: 10.4049/jimmunol.1203273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 201.Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, Malaguarnera L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. 2012;280:36–43. doi: 10.1016/j.cellimm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 202.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med. 2010;88:441–450. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kawai T, Akira S (eds) (2007) TLR signaling. Semin Immunol 19:24–32 [DOI] [PubMed]
- 204.Turetsky A, Goddeau RP, Henninger N. Low serum vitamin D is independently associated with larger lesion volumes after ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24:1555–1563. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 205.Balden R, Selvamani A, Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology. 2012;153:2420–2435. doi: 10.1210/en.2011-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dickie LJ, Church LD, Coulthard LR, Mathews RJ, Emery P, McDermott MF. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology. 2010;49:1466–1471. doi: 10.1093/rheumatology/keq124. [DOI] [PubMed] [Google Scholar]
- 207.Tang H, Hua F, Wang J, Yousuf S, Atif F, Sayeed I, et al. Progesterone and vitamin D combination therapy modulates inflammatory response after traumatic brain injury. Brain Inj. 2015;17:1–10. doi: 10.3109/02699052.2015.1035330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Atif F, Yousuf S, Sayeed I, Ishrat T, Hua F, Stein DG. Combination treatment with progesterone and vitamin D hormone is more effective than monotherapy in ischemic stroke: the role of BDNF/TrkB/Erk1/2 signaling in neuroprotection. Neuropharmacology. 2013;67:78–87. doi: 10.1016/j.neuropharm.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]