Abstract
G-protein-coupled receptors (GPCRs) can constitute complexes with non-GPCR integral membrane proteins, while such interaction has not been demonstrated at a single molecule level so far. We here investigated the potential interaction between the thyrotropin receptor (TSHR) and the monocarboxylate transporter 8 (MCT8), a member of the major facilitator superfamily (MFS), using fluorescence cross-correlation spectroscopy (FCCS). Both the proteins are expressed endogenously on the basolateral plasma membrane of the thyrocytes and are involved in stimulation of thyroid hormone production and release. Indeed, we demonstrate strong interaction between both the proteins which causes a suppressed activation of Gq/11 by TSH-stimulated TSHR. Thus, we provide not only evidence for a novel interaction between the TSHR and MCT8, but could also prove this interaction on a single molecule level. Moreover, this interaction forces biased signaling at the TSHR. These results are of general interest for both the GPCR and the MFS research fields.
Electronic supplementary material
The online version of this article (10.1007/s00018-017-2728-1) contains supplementary material, which is available to authorized users.
Keywords: GPCR, MCT8, MFS transporters, Oligomerization, TSHR
Introduction
GPCRs are known to interact with each other either as homo-oligomers or hetero-oligomers. Homo- or hetero-oligomerization between GPCRs is not a prerequisite for signaling, but is a feature that can modulate the signaling spectrum [1]. Moreover, recent reports demonstrated that GPCRs are in fact expressed as a mixture of monomers and homo-oligomers and it is suggested that these two forms interconvert dynamically in an equilibrium [2–4]. Assuming that the same holds true in the case of hetero-oligomerization, two GPCR monomers (x and y), two homo-oligomers (xx and yy) and a hetero-oligomer (x/y) may be present in a dynamic equilibrium which dramatically enhances the capacity for signal fine-tuning.
In addition to homo- and hetero-oligomerization, a few reports suggest that GPCRs also interact with non-GPCR integral membrane proteins which could be termed as “heteromeric interactions”. Macromolecular complexes have been reported for GPCRs and KIR-channels, or the voltage-gated calcium channel Cav 2.2 [5, 6]. GPCR/transporter interrelations have also been proposed for the beta-adrenergic receptor 2 (ADRB2) and the glucose transporter 4 (GLUT4) [7], the trace amine associated receptor 1 (TAAR1) and dopamine transporter [8], melatonin 1 receptor (MT1R) and dopamine transporter [6], the latter of which belongs to the major facilitator superfamily (MFS) of substrate transporters [9]. However, until now, there was no direct proof of a GPCR-transporter interaction at a single molecule level. The aims of our study are twofold. We wanted to test whether functionally relevant heteromers could be formed by two different proteins sharing the same physiological context namely the TSHR (a class A GPCR) and the monocarboxylate transporter 8 (MCT8). Moreover, we intended to analyze such an interaction at a single molecule level.
The TSHR is a key regulator of thyroid gland functions [10] and receptor activation by TSH induces thyroid hormone (TH) production [11]. The TSHR has the capacity for homo-oligomerization [12, 13]. Moreover, specific membrane-spanning transporter proteins are responsible for the transport of THs across the cell membrane. The MCT8 belongs to the MFS and translocates TH, particularly triiodothyronine [14, 15]. The MCT8 is co-localized with the TSHR at the basolateral plasma membrane of thyrocytes, therefore, we speculate that an interaction of TSHR and MCT8 might be possible [16, 17]. The ability to form oligomers could be a more general feature within the MFS, since homo-oligomeric complexes were reported, e.g., for the glycine transporter [18], the organic cation transporter-1 [19], or the multidrug-transporter EmrD [20]. Higher order complexes like dimers or oligomers may be functionally relevant such as those found for amphetamine transporters, for which influx and efflux occurs via separate but interacting transporter protomers [21].
To the best of our knowledge, a signaling relevant interaction of a GPCR with a structurally unrelated membrane protein has not been proven yet using a single molecule technique such as fluorescence cross-correlation spectroscopy (FCCS) or smTIRF measurements [2, 4, 22]. Here we applied the FCCS technique to analyze such an interaction between the TSHR and the MCT8. Finally, we confirmed our hypothesis that these co-expressed and functionally related integral membrane proteins form an interacting protein complex which also modulates functional properties.
Materials and methods
Plasmid construction
The cDNA of TSHR, the origin of all the TSHR constructs, and of rM3R (rat muscarinic receptor 3) was kindly provided by Prof. Torsten Schöneberg (University of Leipzig, Germany). The point mutation C636W was introduced into the cDNA of the TSHR using the QuikChange Site-Directed Mutagenesis protocol (Agilent Technologies, La Jolla, CA, USA) and confirmed by in-house direct sequencing. The YFP constructs used for YFP-BiFc were kindly provided by Hans-Peter Hauri (University of Basel, Switzerland). The insert was replaced by full-length cDNAs used in this study. Hemagglutinin tags were cloned at the amino terminus (HA, YPYDVPDYA), specifically after Met22 for the TSHR and Leu203 for the MCT8. FLAG tags (DYKDDDDK) were fused to the carboxyl terminus. Untagged constructs were used to study the signaling properties of TSHR. The vector pEGFP-N1 and pmCherry-N1 were obtained from Clontech (Mountain View, USA). For the FCCS experiments, the cDNA of the TSHR was cloned into pmCherry-N1, thereby creating a C-terminally mCherry-tagged TSHR (construct TSHR.mCherry). The cDNA of MCT8 was cloned into vector plasmid pEGFP-N1, thereby creating a C-terminally GFP-tagged MCT8 (construct MCT8.GFP). As positive and negative controls for the FCCS experiments, the previously described constructs CRF1R.GFP/CRF1R.mCherry and CRF2(a)R.GFP/CRF2(a)R.mCherry were used, respectively [4].
Cell culture and transfection
COS-7 (African green monkey kidney) and HEK293 (human embryonic kidney) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Biochrom) and Earl’s minimum essential medium (MEM, Biochrom), supplemented with 5 or 10% fetal bovine serum (PAA Laboratories), 100 U/mL penicillin, 100 µg/mL streptomycin (Biochrom) and 2 mM l-glutamine (Invitrogen) at 37 °C and 5% CO2.
For BiFc approaches, HEK293 cells were seeded in 60 mm diam. dishes (8 × 105 cells/dish) and transfected with 1.9 µg DNA and 5.9 µl MetafecteneTM/dish (Biontex, Germany). For cAMP accumulation, COS-7 cells were seeded into 96-well plates (0.9 × 104 cells/well) and transfected with 41.6 ng DNA and 0.47 µl MetafecteneTM/well; IP3 formation in HEK293 cells was examined in 96-well plates (1.3 × 104 cells/well) coated with poly-l-lysine (Biochrom, Germany). Transfection of HEK293 cells was performed with 45 ng plasmid DNA/well as well as 41.7 ng reporter construct/well and 0.45 µl MetafecteneTM/well.
For cell-surface expression studies, COS-7 cells were seeded into 48-well plates (2 × 104 cells/well) and transfected with 167 ng DNA and 1 µl MetafecteneTM/well. Total expression studies via sandwich ELISA were performed with COS-7 cells seeded in 6 cm dishes (6 × 105 cells/dish) and transfected with 3 µg DNA and 8 µl MetafecteneTM/dish. Co-expression of TSHR and MCT8 was always performed in equimolar amounts of plasmid DNA.
For the FCCS experiments, transiently transfected HEK293 cells were used. Cells were grown in DMEM containing 10% (v/v) fetal calf serum. Cells were cultured at 37 °C and 5% CO2. Transfection of the cells was carried out 24 h after seeding in 35 mm diam. wells using 0.2 µg plasmid DNA (for each labeled protein) and 0.8 µl PEI (Merck-Millipore, Darmstadt, Germany). Measurements were performed 48 h after transfection.
Microscopy
For co-localization studies with the plasma membrane marker CellMask Deep Red (Molecular Probes, Darmstadt, Germany), cells were incubated for 5 min at 37 °C with the stain (5 µg/2 mL in DMEM). After washing three times with PBS, the mCherry-tagged receptors were visualized using a laser scanning microscope LSM510 system (Carl Zeiss MicroImaging GmbH, Jena, Germany) with a 63x/1.4 oil objective. The mCherry-tagged receptors were detected on one channel (HeNe laser λexc = 543 nm, 560–615 nm band pass filter), CellMask Deep Red fluorescence was recorded on a second channel (HeNe laser, λexc = 633 nm, 650–740 nm band pass filter) and the overlay was computed. The spectral parts were split using a MBS 488/543 for channel one and a MBS 488/543/633 for channel two. Images were analyzed using the ZEN 2010 software (Carl Zeiss MicroImaging GmbH, Germany).
For co-localization studies with the plasma membrane marker trypan blue (Merck-Millipore, Darmstadt, Germany), cells were incubated for 1 min at 37 °C with the stain (0.05% in PBS). The GFP-tagged receptors were visualized using a laser scanning microscope LSM510 system (Carl Zeiss MicroImaging GmbH, Jena, Germany) system with a 63x/1.4 oil objective. The GFP-tagged receptors were detected on one channel (argon laser, λexc = 488 nm, 505–530 nm band pass filter), trypan blue fluorescence was recorded on a second channel (HeNe laser, λexc = 543 nm, 560 long pass filter) and the overlay was computed. The spectral parts were split using a MBS 488/543 for channel one and a LP 560 for channel two. Images were analyzed using the ZEN 2010 software (Carl Zeiss MicroImaging GmbH, Germany).
Interaction studies via BiFc
Direct interaction between the two defined proteins was determined in a BiFc as described by Nyfeler et al. [23]. In this assay, YFP fragments fused to the proteins of interest reconstitute fluorescent YFP only in case of dimer formation. HEK293 cells were harvested with PBS/EDTA 48 h after transfection. YFP-fluorescent cells were measured using the FACS Canto II at the Berlin-Brandenburg Center for Regenerative Therapies (BCRT, Charité Berlin) and were analyzed with FlowJo 8.8.6 (Tree Star Inc., Ashland, OR, USA).
Fluorescence cross-correlation spectroscopy
The principles of FCCS technique, its use with LSM systems [24–26] and its application to monitor GPCR interactions [4, 27] have been described previously. Briefly, the GFP-tagged MCT8 and mCherry-tagged TSHR constructs were expressed in transiently transfected HEK293 cells and FCCS measurements were performed at room temperature at the basal plasma membrane using a LSM710-ConfoCor3 system. GFP and mCherry fluorescence signals were analyzed using a 40x/1.2 water objective (GFP: argon laser, λexc = 488 nm, 505-540 nm band pass filter; mCherry: diode-pumped solid state laser, λexc = 561 nm, 580 nm long pass filter) and a main beam splitter 488/561 and a dichroic mirror 565 respectively. Cross-talk between the GFP and mCherry signals contributed up to 8% under these conditions. The fluctuations of the fluorescence intensities were recorded for 5 s and 10 repetitions. The calculation of the average auto- and cross-correlation curves was performed using the LSM710 software ZEN 2010 (Carl Zeiss Microscopy GmbH, Jena Germany) and convergent curves were used for averaging. The auto-correlation function is defined as (Eq. 1).
| 1 |
where indicates the average time-varying signal and the fluctuations around the mean intensity. Correlation curves were derived using a two-component model of free diffusion in two dimensions with triplet fraction and offset for membrane-associated proteins using the ZEN 2010 software [25]. A two-component model was used for the two-dimensional fits to obtain satisfactory results. The analytical function is described by the following equation:
| 2 |
where is the offset from 1. N and T represent the total number of particles and the triplet fraction. respectively. and represent free diffusion times (the subscripts indicate the different molecule species). is the triplet time, f and 1 − f are the fractions of species 1 and 2 and is the correlation time.
Protein expression studies
Total- and cell-surface expression studies were performed in COS-7 cells 48 h after transfection using an ELISA system that detects HA-tags, as described previously [28]. Untagged constructs were used as negative controls. The detection of total receptor expression requires HA- and FLAG-double tagged constructs. For total expression studies, the same protein amounts of transfected cells were incubated after solubilization in anti-FLAG (Sigma) coated 96-well plates and probed with anti-HA antibody (Roche). To investigate cell surface expression, cells were paraformaldehyde-fixed and prepared for HA detection.
Functional studies on TSHR signaling pathways
For the Gs-signaling pathway, intracellular cAMP accumulation was measured in COS-7 cells 48 h after transfection using the AlphaScreen technology (PerkinElmer). Stimulation with various concentrations of bovine TSH (bTSH, Sigma) in serum-free medium containing 1 mM 3-isobutyl-1-methylxanthine (IBMX, Sigma) was performed for 45 min at 37 °C. Cell lysis (50 µL/well lysis buffer) and cAMP measurement were conducted as described elsewhere [29] and according to the manufacturer´s protocol (PerkinElmer).
Intracellular IP3 formation was measured via NFAT reporter gene assay [30] and analyzed in HEK293 cells 48 h after transfection using a luciferase reporter gene assay. This method requires the co-transfection of equal amounts of the reporter constructs pGL4.30[luc2P/NFAT-RE/Hygro] (Promega) for the determination of IP3 formation containing a response element and firefly luciferase gene under the control of the nuclear factor of activated T-cells (NFAT). Equal amounts of transfected plasmid DNA was achieved by co-transfection of a non-interacting control (rM3R or MC4R [31]). Transfected cells were stimulated with bTSH for six h at 37 °C and lysed using 50 µl/well 1x passive Lysis Buffer (Promega). The measurement of the luciferase activity was performed according to the manufacturer’s protocol (Promega).
Thyroid samples
Thyroid samples were obtained from patients undergoing thyroid surgery for nodular thyroid disease or thyroid cancer. For analysis of MCT8 and TSHR expression, frozen tissues of eight follicular thyroid carcinoma (FTC), eight papillary thyroid carcinoma (PTC), eight Graves disease (GD) tissues and eight normal surrounding thyroid tissues were studied. Classification of the thyroid nodules was performed by pathologists according to the World Health Organization (WHO) criteria.
Real-time PCR
For MCT8 and TSHR expression, total RNA of the human thyroid tissues was isolated using the RNeasy Kit (Qiagen, Germany) and reverse transcribed into cDNA (Life Technologies, Germany). Quantitative real-time PCR was performed as described previously [32]. Sequences of oligonucleotides are provided in Suppl. Table 1. The following housekeeping genes were used: 18S (18S ribosomal RNA), PPIA (peptidylprolyl isomerase A, cyclophilin A) and ACTB (actin beta). The stability of housekeeping genes was determined by calculation of the coefficient of variation on the normalized relative quantities and by calculation of the geNorm M value. The geNorm M value determines the most stable housekeeping genes and calculates the gene expression normalization factor based on the geometric mean of the housekeeping genes. The genomic average of the “best” three housekeeping genes (best keeper index) was calculated by repeated pairwise correlation analysis. Target genes were correlated to the calculated index (best keeper index). Shown is the relative fold-change expression (log 2) of TSHR over MCT8. To calculate relative fold-change expression (log 2) of TSHR over MCT8 Ct-values ≤ 35 were used [33, 34].
Statistical analysis
Data on mRNA expression in human tissue samples are shown as relative fold-change expression (log 2) of TSHR over MCT8. Statistical analysis (unpaired t test) was performed using GraphPad Prism 5 software. Values of *p < 0.05, **p < 0.01 and ***p < 0.001 were considered statistically significant.
Functional data are represented as means + (SEM) of at least three independent experiments performed in triplicate. Statistical analyses (two-tailed student’s t-test with Welch’s correction in case of different variances) were performed using the statistical tools implemented in Graph Pad Prism, version 5 (GraphPad Software, San Diego, CA, USA).
Results
Bimolecular fluorescence complementation (BiFc) and fluorescence cross-correlation spectroscopic (FCCS) measurements demonstrate a close spatial proximity and interaction between TSHR and MCT8
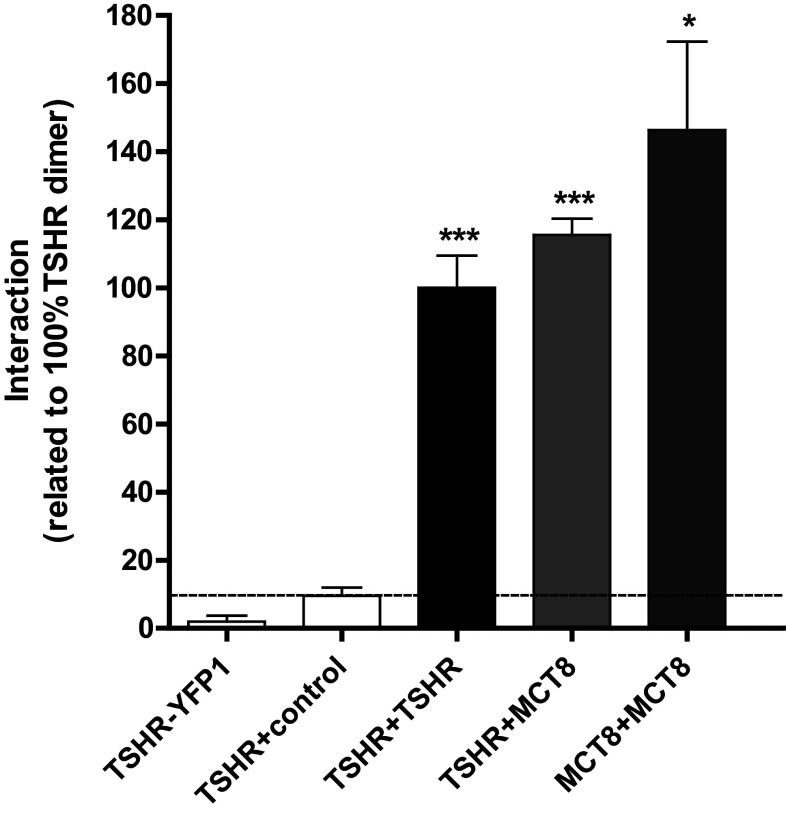
A potential MCT8/TSHR association was first tested by applying a BiFc approach. In comparison to negative controls, we could demonstrate a profound capacity for TSHR/MCT8 interaction which was as strong as TSHR and MCT8 homomerization, respectively (Fig. 1). To prove this interaction unambiguously, we used FCCS measurements. This method allows the detection of fluorescence signals in live cells at a single molecule level in both the plasma membrane and intracellular compartments. If two different diffusing fluorescent molecules pass a very small confocal volume, auto- and cross-correlation analyses can be performed to analyze whether the two fluorescent partners diffuse independently or together. A significant cross-correlation on the basis of mathematical transformations (see the “Materials and methods” section) not only demonstrates co-diffusion of the two fluorescent molecules, but also proves their interaction. Independent diffusion and a lack of cross-correlation on the other side exclude association of the molecules. We carried out FCCS measurements using the C-terminally mCherry-tagged TSHR (TSHR.mCherry) and the C-terminally GFP-tagged MCT8 (MCT8.GFP). We first analyzed whether fusion of the fluorescent proteins might interfere with protein trafficking. A co-localization study of TSHR.mCherry and MCT8.GFP using the respective plasma membrane markers CellMask Deep Red and trypan blue showed that both the fusions are readily expressed in the plasma membrane of transiently transfected HEK293 cells (Fig. 2). For the setup and reliability of the FCCS measurements, the following criteria were defined:
To obtain correct data, the amount of TSHR.mCherry and MCT8.GFP in the plasma membrane must be equal. Since this is normally not the case upon co-transfection, we preselected cells expressing the fusions in equivalent amounts. As a control for a 50/50 ratio of expression, we used the previously described tandem construct GFP-mCherry [4], which represents a covalent fusion of GFP and mCherry and thus leads necessarily to equal amounts of the signals in the measurements. The settings derived from this construct were used to preselect cells expressing matching amounts of TSHR.mCherry and MCT8.GFP and these adjustments were not changed during the experiments. We also preselected cells expressing a low (but equal) amount of TSHR.GFP and MCT8.mCherry, since this is a prerequisite for single molecule analyses (protein density = 60 entities/µm2). The additional advantage is that such cells reflect more closely endogenous membrane protein expression. Under the settings described above, the count rates for all the detected constructs were in the range of 50–200 kHz.
The dynamic range of our measurements was determined previously [4]. It is defined by the above-mentioned GFP-mCherry tandem construct, yielding maximal cross-correlation (60%) and GFP and mCherry proteins expressed separately, yielding minimal cross-correlation (8%). The linear relation between maximal and minimal cross-correlation values was also confirmed previously [4]. These controls allowed accurate rescaling of the dynamic range of the cross-correlation values of TSHR.mCherry and MCT8.GFP.
We preclude that the observed cross-correlation amplitudes at the plasma membrane are impaired by receptors which only co-diffuse within the same microdomain. Such a co-diffusion would lead to a strong increase in diffusion time which was not observed.
FCCS measurements are not only suitable to prove protein/protein interactions at a single molecule level, the cross-correlation values also allow the portion of interacting and non-interacting molecules to be determined. We preclude that endogenous, non-fluorescent TSHR molecules impair these measurements, because this receptor is not expressed in HEK293 cells (data not shown). However, we cannot preclude that endogenous MCT8 affects these measurements. In this case, however, the portion of interacting molecules would be slightly underestimated rather than overestimated.
As positive and negative controls, we used the previously described construct pairs CRF1R.mCherry/CRF1R.GFP and CRF2(a)R.GFP/CRF2(a)R.mCherry, respectively. The CRF1R forms an equilibrium of monomers and homodimers in the plasma membrane, whereas the CRF2(a)R is an exclusive monomeric GPCR [4]. These controls are also suitable to check the accuracy of the analyses. Actual measurements are reliable, if the same cross-correlation values were observed for these constructs as described previously [4].
Fig. 1.
TSHR in interaction with MCT8. The analysis was performed via YFP-based bimolecular fluorescence complementation assay (YFP-BiFc) in transiently transfected HEK293 cells. The expression of TSHR-YFP1 and the co-expression of TSHR/control (rM3R) (white bars) served as negative control, homodimers of TSHR and MCT8, respectively, served as positive controls. The bars represent results from the pooled co-expression values from vice versa assays (f.e. TSHR+MCT8: TSHR-YFP1/MCT8-YFP2 and TSHR-YFP2/MCT8-YFP1). Data were assessed from a minimum of three independent experiments (50.000 cells counted per experiment) and calculated to 100% TSHR dimer. Values represent mean + SEM. Significances are shown compared to the co-expression of TSHR/control. *p ≤ 0.05, ***p ≤ 0.001 (unpaired t test, two-tailed)
Fig. 2.
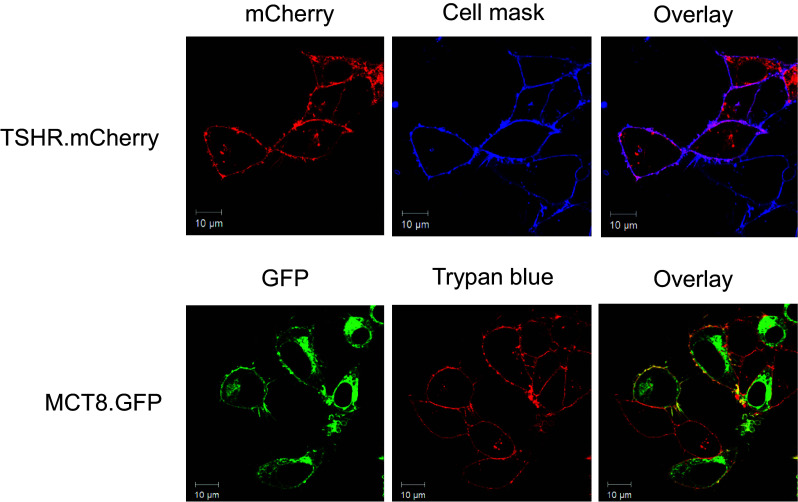
Localization of TSHR.mCherry and MCT8.GFP in the plasma membrane of transiently transfected HEK293 cells. The fluorescence signals of TSHR.mCherry (red, upper left panel) were co-localized with those of the plasma membrane stain CellMask Deep Red (blue, upper central panel). Purple color in the overlay (upper right panel) demonstrates that TSHR.mCherry is readily expressed in the plasma membrane. The fluorescence signals of MCT8.GFP (green, lower left panel) were co-localized with those of the plasma membrane stain trypan blue (red, lower central panel). Yellow color in the overlay (lower right panel) demonstrates that MCT8.GFP is expressed in the plasma membrane. Note that GFP and mCherry signals are only detectable in transfected cells whereas the dyes trypan blue and CellMask Deep Red stain each cell in the field of view. The scans show representative cells. Scale bar 10 μm. Similar data were obtained in four independent experiments
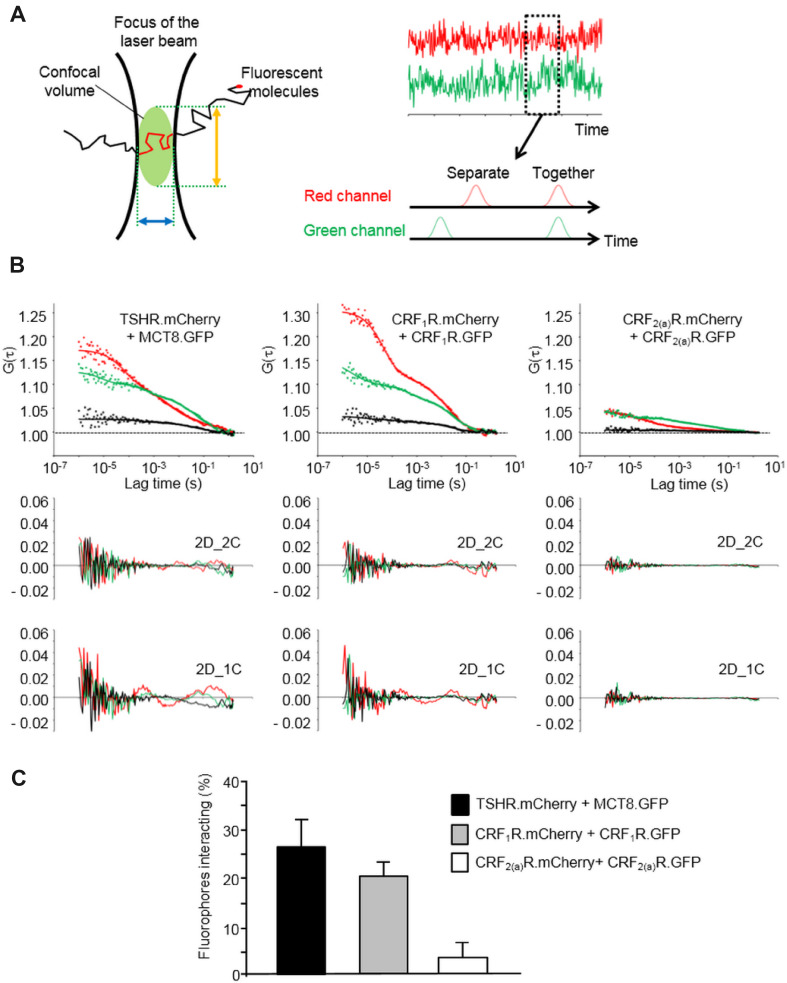
Taking the criteria outlined above into account, we performed the FCCS analyses using transiently transfected HEK293 cells co-expressing TSHR.mCherry and MCT8.GFP and the above-mentioned control constructs. The principle of the FCCS methodology is shown in Fig. 3a, representative auto- and cross-correlation curves of TSHR.mCherry/MCT8.GFP and the control constructs are shown in Fig. 3b, the cross-correlation values and diffusion parameters are given in Table 1. Cross-correlation amplitudes were observed for CRF1R.mCherry/CRF1R.GFP, but not for CRF2(a)R.GFP/CRF2(a)R.mCherry confirming our previous results [4]. A significant cross-correlation amplitude could also be derived for TSHR.mCherry and MCT8.GFP. We then rescaled the raw cross-correlation values according to the criteria outlined above. The results for CRF1R.mCherry/CRF1R.GFP (20% interacting molecules) were very similar to what was described previously (22% interacting molecules) [4] confirming the accuracy of the measurements (Fig. 3). In the case of TSHR.mCherry and MCT8.GFP, the rescaled values show that 26% of the fluorophores present in the plasma membrane form heteromeric complexes (Fig. 3).
Fig. 3.
FCCS analysis of the TSHR.mCherry and MCT8.GFP interaction. a Principles of the FCCS methodology. A single fluorescent molecule diffusing through a very small confocal volume is recorded (left panel). The confocal volume is defined by the diameter of the microscopic lens (blue arrow) and axial structural parameter of the microscope objective (orange arrow). The focus of the laser beam is set to the plasma membrane. If two different fluorescent molecules were used, the fluctuations of molecules are recorded in two channels over time (right panel, red and green) and auto- and cross-correlation analyses can be performed to analyze whether the two fluorescent partners diffuse independently or together. b FCCS measurements using transiently co-transfected HEK293 cells expressing TSHR.mCherry and MCT8.GFP. The constructs CRF1R.mCherry/CRF1.GFP and CRF2(a)R.mCherry/CRF2(a)R.GFP were used as positive and negative controls respectively. The laser beam was focused at the plasma membrane. Representative auto- and cross-correlation data points and fitted curves for the analyzed constructs are shown. Auto-correlation data are depicted in red (mCherry) and green (GFP), cross-correlation data are shown in black. Curves were obtained by two-dimensional fits with two components (2D_2C) which gave the best results. Below each diagram, the residuals of this fitting are shown (auto-correlation data: GFP = green, mCherry = red). In addition, the residuals of suboptimal fits with 2 dimensions and 1 component (2D_1C) are depicted to demonstrate the necessity of 2D_2C fits. c Quantification of the TSHR.mCherry/MCT8.GFP interaction. The diagram shows the rescaled and distribution-corrected data of the FCCS experiments and indicates the portion of interacting TSHR.mCherry/MCT8.GFP fluorophores in the plasma membrane. In the case of CRF1R.mCherry/CRF1.GFP (positive control) and CRF2(a)R.mCherry/CRF2(a)R.GFP (negative control), the columns represent the portion of homodimers. Columns represent mean values ± SD of a total of 68 (TSHR.mCherry/MCT8.GFP), 51 (CRF1R.mCherry/CRF1.GFP) and 68 cells (CRF2(a)R.mCherry/CRF2(a)R.GFP). The total number of cells was collected in 5 independent experiments
Table 1.
Original FCCS fitting parameters (± S.E.) for the GFP- and mCherry-tagged constructs in the plasma membrane of transiently transfected HEK293 cells
| Constructs | τD,1 (%) | τD,1 (ms) | τD,2 (%) | τD,2 (ms) | N | G(0)x/G(0)min |
|---|---|---|---|---|---|---|
| MCT8-GFP | 36.8 ± 6.7 | 0.45 ± 0.02 | 63.2 ± 0.8 | 44.9 ± 2.2 | 69 | 20.8 ± 0.3 |
| TSH-mCherry | 50.0 ± 2.5 | 0.26 ± 0.04 | 50.0 ± 2.4 | 26.4 ± 7.3 | ||
| CRF1-GFP | 38.5 ± 0.8 | 0.45 ± 0.03 | 61.5 ± 0.8 | 40.8 ± 2.5 | 51 | 17.8 ± 0.2 |
| CRF1-mCherry | 60.0 ± 1.8 | 0.24 ± 0.04 | 40.0 ± 1.8 | 20.2 ± 2.1 | ||
| CRF2a-GFP | 40.6 ± 1.1 | 0.37 ± 0.02 | 59.4 ± 1.1 | 36.5 ± 1.4 | 63 | 9.4 ± 0.3 |
| CRF2a-mCherry | 60.5 ± 1.4 | 0.19 ± 0.04 | 39.5 ± 1.4 | 22.7 ± 1.9 |
Decay times [τD,1 (ms); τD,2 (ms)], fractions [(τD,1 (%); τD,2 (%)], and CC values [G(0)x/G(0)min] are listed. The best fits were obtained assuming free diffusion in two dimensions and two components. Triplet fractions [TF (%)] ranged from 8.34–40.83% and triplet times (τD,F) from 5.00 to 159.97 µs
Expression levels of TSHR and MCT8
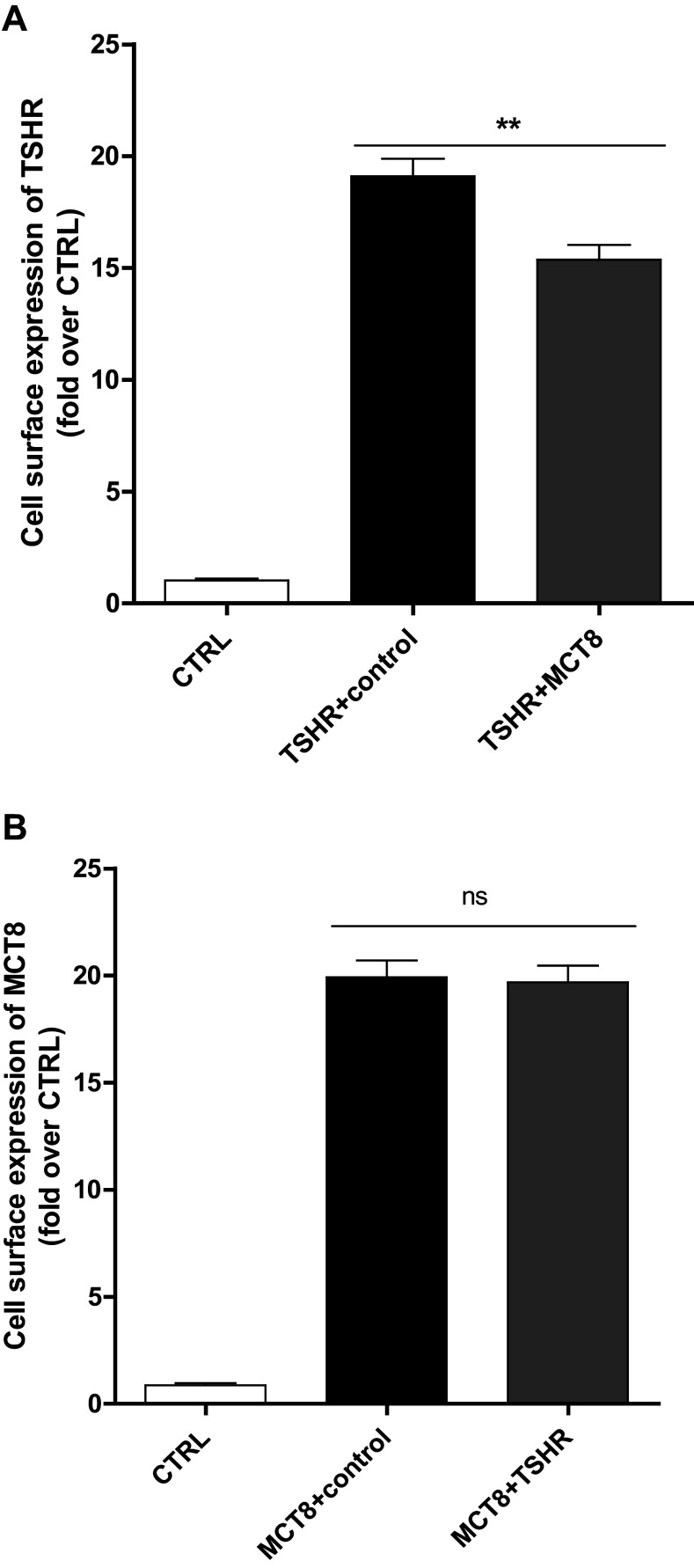
To gain insight into a potential functional significance of the TSHR/MCT8 interaction, we first analyzed protein expression as total- and cell-surface expression of the two proteins upon co-expression after transfection of equimolar amounts of plasmids. Total expression of TSHR in the presence of MCT8 is slightly up-regulated to 120% compared to TSHR co-expression with the rat muscarinic receptor (rM3R) as a non-interacting control (Figure S1). Total expression of MCT8 is also up-regulated to 120% in the presence of the TSHR (Figure S1), whereas cell-surface expression is comparable to wild type (wt) (Fig. 4). Cell-surface expression of the TSHR, however, is slightly decreased by around 20-30% in the presence of MCT8 (Fig. 4).
Fig. 4.
Cell-surface expression of TSHR co-expressed with MCT8 or vice versa. Cell-surface expression was determined in an ELISA system via a N-terminal HA-tag. Co-expressions with the non-interactive rM3R (control) were used to ensure equal DNA amount. Data were assessed from a minimum of three independent experiments, performed in a minimum of triplicates, and calculated x-fold to negative control (CTRL: co-expression of untagged protein of interest and rM3R). Values represent mean + SEM. ns not significant, **p ≤ 0.01(unpaired t test, two-tailed)
Modified TSHR signaling caused by co-expression with MCT8
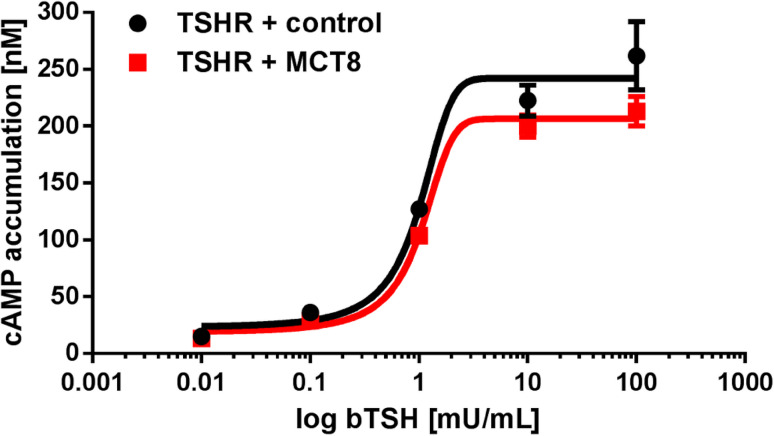
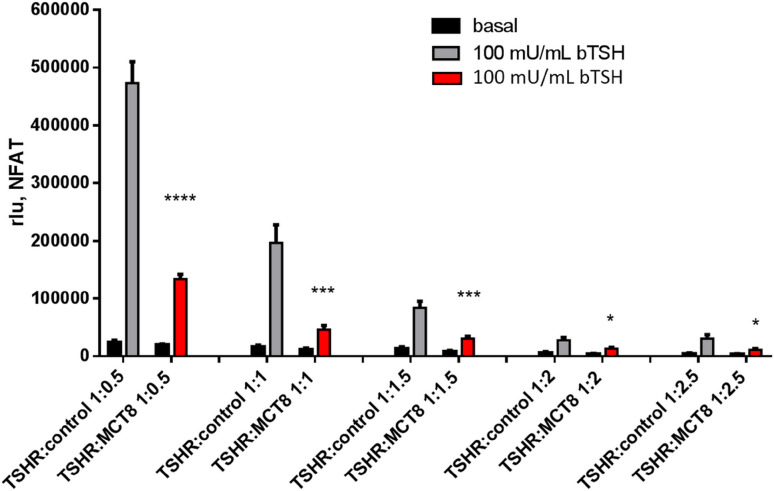
The TSHR signaling capacity in terms of cyclic AMP (cAMP) accumulation and IP3 formation upon co-expression with MCT8 was tested after transfection of equal amounts of plasmids. cAMP accumulation of bovine TSH (bTSH) is slightly reduced, but changes in potency and efficacy were not significant (Fig. 5). However, a profound decrease in efficacy by approximately 60–70% was detectable in IP3 formation (Fig. 6) measured via NFAT reporter gene assay [30]. To test if this reduction is concentration-dependent, our intention was to compare co-transfection of TSHR + control with TSHR + MCT8 at different ratios with equimolar amounts of TSHR and MCT8 as starting point of transfected plasmid DNA. Plasmid amounts of MCT8 and control DNA were increased while keeping TSHR plasmid constant. This increase in transfected MCT8 plasmid DNA did not further decrease the measured 60-70% reduction in signaling (Fig. 6).
Fig. 5.
Gs-signaling of TSHR co-expressed with MCT8. Gs-activity (cAMP) was measured with the AlphaScreen technology in transiently transfected COS-7 cells after stimulation with bTSH (range 0.01–100 mU/mL bTSH). The co-expression of TSHR with the non-interactive rM3R (control) was used to ensure equal DNA amount. Cells transfected with empty vector showed no response in cAMP accumulation to bTSH (data not shown). Values represent raw data mean + SEM from at least three independent experiments, performed in triplicates. Statistical analysis was performed using two-way ANOVA which did not reveal any statistical differences between the concentration response curves of TSHR + control and TSHR + MCT8
Fig. 6.
Determination of Gq/11 signaling via NFAT reporter gene assay of TSHR and MCT8 co-expressed in different ratios. HEK293 cells were co-transfected with TSHR and MCT8, for control, TSHR was co-transfected with a non-interacting control (MC4R, [31]) in the indicated ratios with a starting point of equimolar amounts of both plasmids as it is used for Figs. 1 and 4. Two days after transfection, IP3 formation (NFAT-Luc, via reporter gene assay) was analyzed in the absence or presence of 100 mU/mL bTSH, stimulation of TSHR:MCT8 is indicated in red bars. Data of four independent experiments performed in triplicates are shown. Comparison was performed between TSHR + control versus TSHR + MCT8. Values represent mean + SEM. *p ≤ 0.05, ***p ≤ 0.001, ****p ≤ 0.0001 (unpaired t test with Welch’s correction)
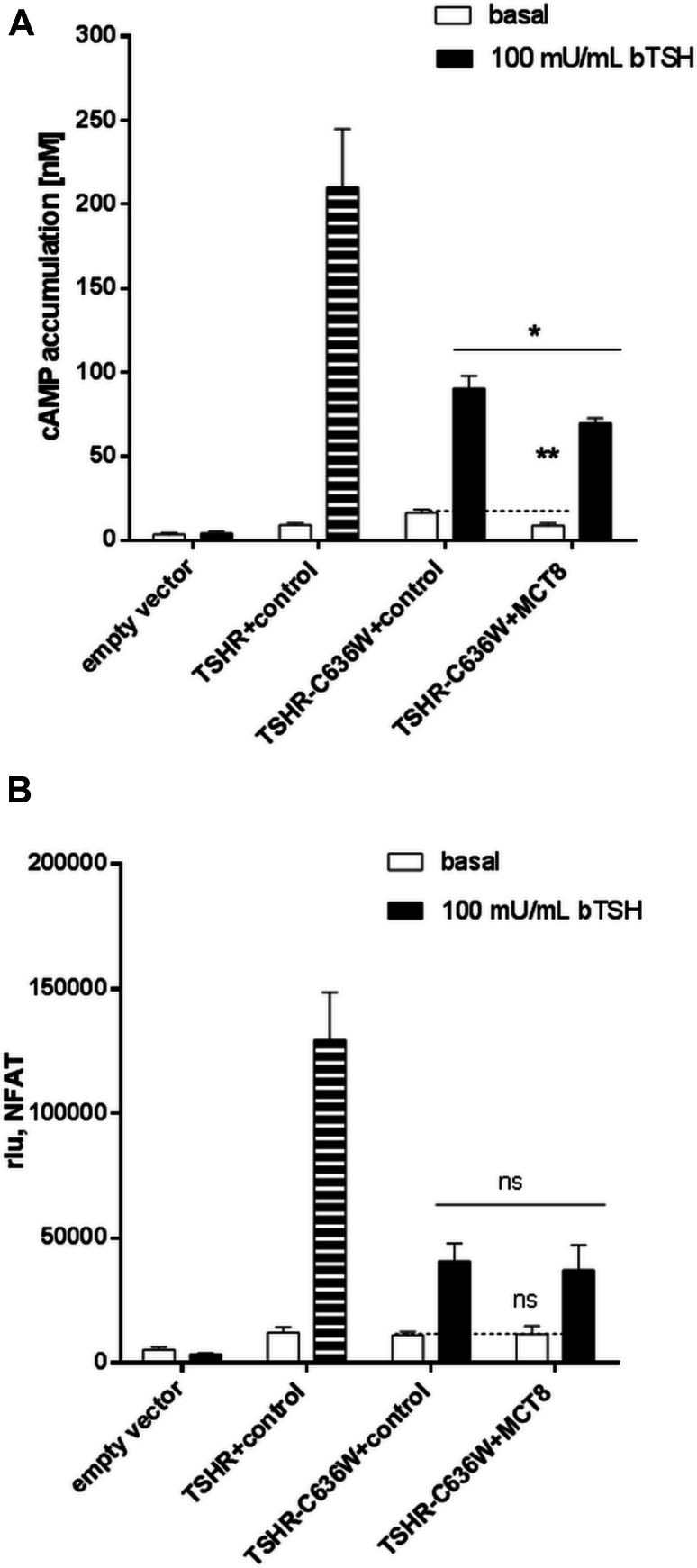
Furthermore, a pathogenic TSHR variant (C636W) with a reduced capacity for Gq/11 activation and an increased constitutive basal cAMP signaling [30] was co-expressed with wt MCT8 and functionally characterized. This mutation was identified in a patient with non-autoimmune hyperthyroidism yet lacking goiter, although basal Gs signaling was enhanced. In this patient, TSH is suppressed, however, TH concentrations are mildly elevated (weak phenotype), which is in contrast to the other reported activating TSHR mutations [35]. We here found, that this TSHR mutation did not alter interaction with MCT8 (Figure S2). Total expression of this variant co-expressed with MCT8 is unchanged (Figure S1), whereas cell-surface expression is reduced in the absence of MCT8 and even more in the presence of MCT8 (Figure S3). Activation of Gq/11 of TSHR-C636W is reduced and is not further affected in the presence of MCT8 (Fig. 7). Most intriguingly, the constitutive activation of the TSHR mutation in terms of Gs/adenylyl cyclase signaling is significantly reduced compared to wt-TSHR levels in the presence of MCT8 transfected in equimolar amounts (Fig. 7). At this point, we could only speculate that TSHR and MCT8 might be expressed in comparable amounts, however, our data indicate that the presence of MCT8 had the capacity to suppress the constitutive signaling of the C636W mutation of the patient. To our knowledge, these are the first functional data that point to a modulating effect of proteins that are expressed in the same compartment, here the basolateral membrane, as the TSHR. That dual signaling bias of MCT8 on the TSHR by only affecting Gq/11 signaling of the wt-TSHR, but influencing basal Gs signaling in case of a constitutive mutation might be one reason for the phenotypical variability of TSHR mutation carriers.
Fig. 7.
Functional characterization of TSHR mutant C636W in the presence of MCT8. Functional studies of TSHR mutants co-expressed with MCT8 by determination of the a) Gs-signaling pathway (cAMP accumulation, via AlphaScreen technology in COS-7 cells) and b) of IP3 formation (NFAT-Luc, via reporter gene assay in HEK293 cells). The wt-TSHR stimulated with 100 mU/mL bTSH served as control for comparison with the TSHR mutation C636W and is indicated in stripped bars. Cells were stimulated with 100 mU/mL bTSH. To ensure equal amounts of DNA in each transfection, TSHRs were co-transfected with the non-interactive rM3R (control). Data were assessed from at least three independent experiments, performed in triplicates. Values represent mean + SEM. Significances are shown compared to the co-expression of TSHR-C636W/control basal or stimulated, ns not significant, *p ≤ 0.05 (unpaired t test, two-tailed)
TSHR and MCT8 expression levels in different thyroid pathologies
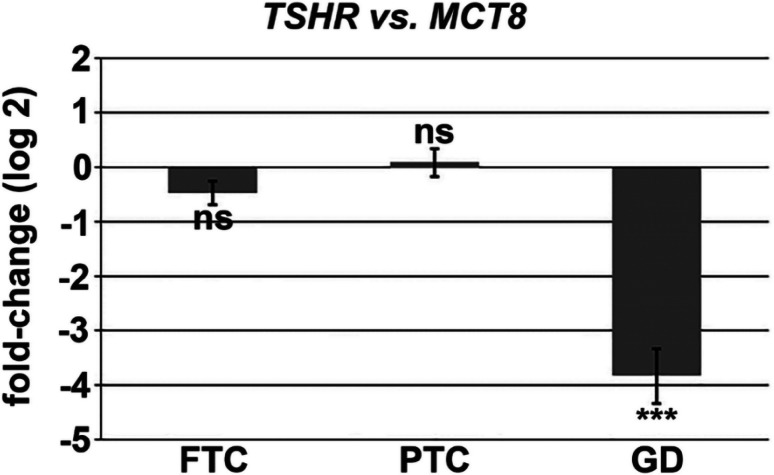
Next, we were interested in the ratio of TSHR and MCT8 expression in different thyroid pathologies. To investigate MCT8 and TSHR expression in human thyroid tissues, quantitative RT-PCR was performed and the relative fold-change expression (log 2) of TSHR over MCT8 was calculated. The relative fold-change expression (log 2) of MCT8 over TSHR in human thyroid tissues revealed significantly lower TSHR mRNA level in Graves’ disease (GD, high TH concentration due to autoimmune activation of the TSHR) as compared to MCT8 mRNA level. Otherwise, in follicular thyroid carcinoma (FTC) and papillary thyroid carcinoma (PTC), TSHR and MCT8 were at the same mRNA expression level (Fig. 8).
Fig. 8.
Relative fold-change expression (log 2) of TSHR over MCT8 to compare MCT8 and TSHR gene expression levels in human thyroid tissues. TSHR and MCT8 genes were correlated to the “best” three housekeeping genes (best keeper index), calculated by the repeated pairwise correlation analysis. 18S (18S ribosomal RNA), PPIA (peptidylprolyl isomerase A, cyclophilin A) and ACTB (actin beta) were used as housekeeping genes. Comparable TSHR and MCT8 expression levels are shown in follicular thyroid carcinoma (FTC, second most frequent subtype of differentiated thyroid carcinoma) and papillary thyroid carcinoma (PTC, most frequent subtype of differentiated thyroid carcinoma). TSHR expression is significantly lower in human thyroid tissues of Grave’s disease (GD, high TH concentration due to autoimmune activation of the TSHR) as compared to MCT8 expression. Shown is the relative fold-change expression (log2) of TSHR over MCT8, n = 8, t test, ns = not significant, ***p < 0.001
Discussion
The intention of the present study was the investigation of a putative interplay of two large membrane-spanning proteins, a GPCR and a substrate transporter from the MFS. We here investigated the TSHR and the MCT8, because both proteins are expressed on the basolateral plasma membrane of thyrocytes [16, 17] and function in a physiological context: TSHR activation leads to TH production and MCT8 is responsible for releasing TH from the thyrocytes.
MCT8 and TSHR form heteromers
Here we provide the first report showing the interaction of a GPCR and a MFS transporter at the single molecule level. Using FCCS, we could show that 26% of the total fluorescence-labeled proteins present in the plasma membrane interacted (Fig. 3). It should be stressed, however, that this value represents only a snapshot taken under the conditions used in these experiments (low but equal expression levels of the TSHR and MCT8) and may be influenced by many factors. First of all, this value may even be underestimated due to the presence of endogenous, non-GFP-tagged MCT8 expressed in HEK293 cells which may form complexes with TSHR.mCherry. We did not assess the amount of endogenous MCT8 in HEK293 cells. According to RNA expression analyses (http://www.proteinatlas.org/ENSG00000147100-SLC16A2/cell), MCT8 expression seems to be low in these cells.
Moreover, it is not known whether the TSHR/MCT8 interaction is stable or undergoes dynamic changes. If the latter was the case and assuming statistic interactions, the expression levels of TSHR and MCT8 should influence the amount of interacting molecules according to the law of mass order which could be determined in the future by titration experiments. It should be stressed, however, that the amount of interacting molecules may not only simply be defined by molecule numbers. In case of a functional interaction, receptor activation and/or transporter influx or efflux stimulation may also influence the amount of associating molecules due to conformational changes of the interaction partners (see below). However, it will be difficult to address these interesting hypotheses experimentally, since THs are required in the cell culture medium for cell survival meaning that MCT8 is always present in a more or less active (transport) state.
In addition, it can be assumed that a TSHR/MCT8 interaction not only occurs at the plasma membrane, but also in the ER and during vesicular transport to the cell surface. If so, the interaction may also influence or even regulate the plasma membrane expression of the interaction partners. Indeed, we could detect decreases in TSHR expression at the cell-surface when co-expressed with MCT8 (Fig. 4).
Finally, it should be noted that we know neither which oligomeric form of the TSHR and MCT8 (monomeric or oligomeric) interact (this cannot be resolved by FCCS) nor do we know the interaction interface(s). We could recently show that the TSHR is expressed as a mixture of monomers and homodimers in the plasma membrane [4] and the same may be true for MCT8. In this case, interaction of two monomers, two dimers or even of monomers and dimers are possible. If higher order oligomers were present for the TSHR and MCT8 (e.g., TSHR or MCT8 trimers), the resulting scenario would be even more complex. These interesting questions may be addressed in future studies by single molecule total internal reflection microscopy (smTIRF) experiments.
Functional consequences of TSHR/MCT8 interaction
In this study, we show that co-expression of TSHR and MCT8 profoundly reduces the capacity of TSH to induce Gq/11 mediated signaling at TSHR (Fig. 6), while Gs-mediated cAMP accumulation is only barely affected (Fig. 5). This finding is interesting, because in addition to the important effect of Gs/adenylyl cyclase activation for thyroid growth and TH production [10], TSHR-mediated Gq/11 activation was also found to contribute to these effects [36]. Our findings may also have implications with respect to the pathophysiological condition of Graves’ disease, where MCT8 expression is higher in comparison to TSHR (Fig. 8), which is in agreement to a recent publication describing an upregulation of MCT8 in human tissues of Graves’ disease [37]. We found that the ratio of overexpression of MCT8 only slightly affects the degree of reduction in TSHR-mediated Gq signaling (Fig. 6). However, the here measured reduced Gq/11 signaling by TSHR in the presence of MCT8 should be related to different observations:
A decreased cell surface expression level of TSHR (around 20–30%) when co-expressed with MCT8, whereby this hypothesis is not supported by the fact that the decrease in cell-surface expression is moderate compared to the drastic effect on IP3 formation.
Another possibility is based on observations of TSHR dimer properties in relation to Gq/11 mediated signaling. It was suggested that TSHR coupling to phosphoinositide signaling depends on binding of two TSH molecules to a TSHR homodimer, allowing activation of Gq/11 [38]. This indicates that the homo-dimer of TSHR should be relevant for IP3 formation. It may, therefore, be postulated that heteromerization between TSHR and MCT8 could decrease the capacity for IP3 formation by a hampered TSHR homo-dimerization.
Anyhow, our studies imply that the formation of heteromeric TSHR/MCT8 complexes could directly modulate the signaling capacity and pathway selectivity of the TSHR. According to our data, MCT8/TSHR interaction leads to biased signaling characterized by a profound loss of Gq signaling. A forced biased signaling by interacting proteins such as TSHR/MCT8 (Figure S4) might also contribute to the high degree of phenotypical variability of TSHR mutation carriers [35]. We here tested exemplarily the pathogenic TSHR mutation C636W found in a patient with only mild effects of non-autoimmune hyperthyroidism [30]. The TSHR C636W variant is characterized by a specific signaling pattern of enhanced basal Gs/adenylyl cyclase activation in combination with a profound reduction in TSH induced Gq/11 signaling. This reduced Gq/11 signaling was suggested to participate potentially in the molecular mechanism responsible for the lack of goiter [30]. Our findings in this current study lead to the assumption that co-expression of TSHR-C636W and MCT8 may reduce constitutive Gs/adenylyl cyclase signaling of the TSHR variant (Fig. 7). At this point, we do not know the ratio of MCT8 to TSHR-C636W expression in the patient and we could only speculate that this ratio might influence the phenotype. However, as the patient has elevated TH concentration, we surmise that MCT8 co-expression cannot completely reduce the elevated basal Gs signaling of the TSHR mutation.
Final conclusions
The interaction of the TSHR and MCT8 leads to a biased signaling which may be relevant in thyrocytes or in non-thyroid tissues where the TSHR is expressed. This first description of a TSHR-interacting protein that has the capacity to modify signaling properties of the TSHR raises the question of whether this is also true for other TSHR-interacting proteins. Moreover, our results open the possibility that further GPCRs are influenced by MFS binding, too. We suggest that such interactions act as fine-tuning elements for signaling, related to the diverse sets of proteins expressed in different tissues. Of note, the expression of GPCRs and transporters is dependent on many factors, such as biological rhythms (e.g., circadian, annual, life-span), gender, and genetic predisposition. In conclusion, a full understanding and estimation of either particular GPCR or transporter function under physiological or disease condition is enormously complex, especially considering the impact of the individual protein “interactome”, which is hard to simulate under in vitro conditions. Future experiments should address at least three aspects: the exact heteromeric nature of the TSHR/MCT8 complex, its dynamics and finally its physiological and pathophysiological consequences.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG), projects BI 893/6-3, KL 2334/2-2, in the framework of the SPP 1629 “Thyroid Trans Act” BI893/5-2, BR 1308/11-2, KR1710/5-1, ZW 221/2-1, FU356/3-3 and FU356/7-2. We would like to acknowledge the assistance of the BCRT Flow Cytometry Lab (Charité-Universitätsmedizin Berlin). We would also like to thank Sabine Jyrch, Cigdem Cetindag and Jenny Eichhorst for excellent technical assistance, Vahid Asimi for independent reproduction of the FCCS data and Martin Lehmann for useful discussions.
Abbreviations
- BiFc
Bimolecular fluorescence complementation
- FCCS
Fluorescence cross-correlation spectroscopy
- MCT8
Mono-carboxylate transporter 8
- TSHR
Thyrotropin receptor
- GPCR
G-protein-coupled receptor
- MFS
Major facilitator superfamily
- smTIRF
Single molecule total internal reflection microscopy
- AT1R
Angiotensin-1-receptor (AT1R)
- TRPC3
Transient receptor potential cation channel subfamily C3
- rM3R
Muscarinic acetylcholine receptor 3 (rat)
- MC4R
Melanocortin receptor 4
- ELISA
Enzyme linked immunosorbent assay
- cAMP
Cyclic adenosine mono phosphate
- IP3
Inositol triphosphate
- T3
3,3,5-triiodo-l-thyronine
- TH
Thyroid hormone
- TSH
Thyroid stimulating hormone
- YFP
Yellow fluorescent protein
- GD
Graves disease
- FA
Follicular adenoma
- FTC
Follicular thyroid carcinoma
- PTC
Papillary thyroid carcinoma
Footnotes
Jana Fischer, Gunnar Kleinau denotes equal contributions.
Contributor Information
Ralf Schülein, Phone: +49 30 94 793 255, Email: schuelein@fmp-berlin.de.
Heike Biebermann, Phone: +49 30 450 559 828, Email: heike.biebermann@charite.de.
References
- 1.George SR, O’Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 2.Calebiro D, et al. Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc Natl Acad Sci USA. 2013;110:743–748. doi: 10.1073/pnas.1205798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert NA. GPCR dimers fall apart. Sci Signal. 2010;3:pe12. doi: 10.1126/scisignal.3115pe12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teichmann A, et al. The specific monomer/dimer equilibrium of the corticotropin-releasing factor receptor type 1 is established in the endoplasmic reticulum. J Biol Chem. 2014;289:24250–24262. doi: 10.1074/jbc.M114.553644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doupnik CA. GPCR-Kir channel signaling complexes: defining rules of engagement. J Recept Signal Transduct Res. 2008;28:83–91. doi: 10.1080/10799890801941970. [DOI] [PubMed] [Google Scholar]
- 6.Benleulmi-Chaachoua A, et al. Protein interactome mining defines melatonin MT1 receptors as integral component of presynaptic protein complexes of neurons. J Pineal Res. 2016;60:95–108. doi: 10.1111/jpi.12294. [DOI] [PubMed] [Google Scholar]
- 7.Dehvari N, et al. beta(2)-Adrenoceptors increase translocation of GLUT4 via GPCR kinase sites in the receptor C-terminal tail. Br J Pharmacol. 2012;165:1442–1456. doi: 10.1111/j.1476-5381.2011.01647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie Z, Westmoreland SV, Miller GM. Modulation of monoamine transporters by common biogenic amines via trace amine-associated receptor 1 and monoamine autoreceptors in human embryonic kidney 293 cells and brain synaptosomes. J Pharmacol Exp Ther. 2008;325:629–640. doi: 10.1124/jpet.107.135079. [DOI] [PubMed] [Google Scholar]
- 9.Law CJ, Maloney PC, Wang DN. Ins and outs of major facilitator superfamily antiporters. Annu Rev Microbiol. 2008;62:289–305. doi: 10.1146/annurev.micro.61.080706.093329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- 11.Biebermann H, Winkler F, Kleinau G. Genetic defects, thyroid growth and malfunctions of the TSHR in pediatric patients. Front Biosci. 2010;15:913–933. doi: 10.2741/3654. [DOI] [PubMed] [Google Scholar]
- 12.Latif R, Graves P, Davies TF. Oligomerization of the human thyrotropin receptor: fluorescent protein-tagged hTSHR reveals post-translational complexes. J Biol Chem. 2001;276:45217–45224. doi: 10.1074/jbc.M103727200. [DOI] [PubMed] [Google Scholar]
- 13.Davies T, Marians R, Latif R. The TSH receptor reveals itself. J Clin Invest. 2002;110:161–164. doi: 10.1172/JCI0216234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278:40128–40135. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- 15.Kinne A, Kleinau G, Hoefig CS, Gruters A, Kohrle J, Krause G, Schweizer U. Essential molecular determinants for thyroid hormone transport and first structural implications for monocarboxylate transporter 8. J Biol Chem. 2010;285:28054–28063. doi: 10.1074/jbc.M110.129577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss RE, Refetoff S. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest. 2010;120:3377–3388. doi: 10.1172/JCI42113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sequeira M, Jasani B, Fuhrer D, Wheeler M, Ludgate M. Demonstration of reduced in vivo surface expression of activating mutant thyrotrophin receptors in thyroid sections. Eur J Endocrinol. 2002;146:163–171. doi: 10.1530/eje.0.1460163. [DOI] [PubMed] [Google Scholar]
- 18.Bartholomaus I, et al. Glycine transporter dimers: evidence for occurrence in the plasma membrane. J Biol Chem. 2008;283:10978–10991. doi: 10.1074/jbc.M800622200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller T, et al. The large extracellular loop of organic cation transporter 1 influences substrate affinity and is pivotal for oligomerization. J Biol Chem. 2011;286:37874–37886. doi: 10.1074/jbc.M111.289330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin Y, He X, Szewczyk P, Nguyen T, Chang G. Structure of the multidrug transporter EmrD from Escherichia coli. Science. 2006;312:741–744. doi: 10.1126/science.1125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidel S, et al. Amphetamines take two to tango: an oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol Pharmacol. 2005;67:140–151. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- 22.Kasai RS, Suzuki KG, Prossnitz ER, Koyama-Honda I, Nakada C, Fujiwara TK, Kusumi A. Full characterization of GPCR monomer-dimer dynamic equilibrium by single molecule imaging. J Cell Biol. 2011;192:463–480. doi: 10.1083/jcb.201009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyfeler B, Michnick SW, Hauri HP. Capturing protein interactions in the secretory pathway of living cells. Proc Natl Acad Sci USA. 2005;102:6350–6355. doi: 10.1073/pnas.0501976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elson EL. Fluorescence correlation spectroscopy measures molecular transport in cells. Traffic. 2001;2:789–796. doi: 10.1034/j.1600-0854.2001.21107.x. [DOI] [PubMed] [Google Scholar]
- 25.Schwille P, Korlach J, Webb WW. Fluorescence correlation spectroscopy with single-molecule sensitivity on cell and model membranes. Cytometry. 1999;36:176–182. doi: 10.1002/(SICI)1097-0320(19990701)36:3<176::AID-CYTO5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Haustein E, Schwille P. Ultrasensitive investigations of biological systems by fluorescence correlation spectroscopy. Methods. 2003;29:153–166. doi: 10.1016/S1046-2023(02)00306-7. [DOI] [PubMed] [Google Scholar]
- 27.Teichmann A, Rutz C, Kreuchwig A, Krause G, Wiesner B, Schulein R. The Pseudo signal peptide of the corticotropin-releasing factor receptor type 2A prevents receptor oligomerization. J Biol Chem. 2012;287:27265–27274. doi: 10.1074/jbc.M112.360594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer J, et al. Modulation of monocarboxylate transporter 8 oligomerization by specific pathogenic mutations. J Mol Endocrinol. 2015;54:39–50. doi: 10.1530/JME-14-0272. [DOI] [PubMed] [Google Scholar]
- 29.Muller A, et al. G-protein coupled receptor 83 (GPR83) signaling determined by constitutive and zinc(II)-induced activity. PLoS One. 2013;8:e53347. doi: 10.1371/journal.pone.0053347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winkler F, et al. A new phenotype of nongoitrous and nonautoimmune hyperthyroidism caused by a heterozygous thyrotropin receptor mutation in transmembrane helix 6. J Clin Endocrinol Metab. 2010;95:3605–3610. doi: 10.1210/jc.2010-0112. [DOI] [PubMed] [Google Scholar]
- 31.Biebermann H, Krude H, Elsner A, Chubanov V, Gudermann T, Gruters A. Autosomal-dominant mode of inheritance of a melanocortin-4 receptor mutation in a patient with severe early-onset obesity is due to a dominant-negative effect caused by receptor dimerization. Diabetes. 2003;52:2984–2988. doi: 10.2337/diabetes.52.12.2984. [DOI] [PubMed] [Google Scholar]
- 32.Zwanziger D, Rakov H, Engels K, Moeller LC, Fuhrer D. Sex-dependent claudin-1 expression in the liver of euthyroid and hypothyroid mice. Eur Thyroid J. 2015;4:67–73. doi: 10.1159/000431316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 35.Kleinau G, Neumann S, Gruters A, Krude H, Biebermann H. Novel insights on thyroid-stimulating hormone receptor signal transduction. Endocr Rev. 2013;34:691–724. doi: 10.1210/er.2012-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kero J, Ahmed K, Wettschureck N, Tunaru S, Wintermantel T, Greiner E, Schutz G, Offermanns S. Thyrocyte-specific Gq/G11 deficiency impairs thyroid function and prevents goiter development. J Clin Invest. 2007;117:2399–2407. doi: 10.1172/JCI30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badziong J, et al. Differential regulation of monocarboxylate transporter 8 expression in thyroid cancer and hyperthyroidism. Eur J Endocrinol. 2017;177:243–250. doi: 10.1530/EJE-17-0279. [DOI] [PubMed] [Google Scholar]
- 38.Allen MD, Neumann S, Gershengorn MC. Occupancy of both sites on the thyrotropin (TSH) receptor dimer is necessary for phosphoinositide signaling. FASEB J. 2011;25:3687–3694. doi: 10.1096/fj.11-188961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.