Abstract
Prior to the cytokinesis, the cell–matrix interactions should be disrupted, and the mitotic cells round up. Prerequisite of mitosis, the centrosomes duplicate, spindle fibers are generated and move away from each other to opposite sides of the cells marking the cell poles. Later, an invagination in the plasma membrane is formed a few minutes after anaphase. This furrow ingression is driven by a contractile actomyosin ring, whose assembly is regulated by RhoA GTPase. At the completion of cytokinesis, the two daughter cells are still connected by a thin intercellular bridge, which is subjected to abscission, as the terminal step of cytokinesis. Here, it is overviewed, how syndecan-4, a transmembrane, heparan sulfate proteoglycan, can contribute to these processes in a phosphorylation-dependent manner.
Keywords: Mitotic rounding, Small GTPases, Microtubules, Midbody, Polyploidy, Protein phosphorylation, Tiam1, Dynamin, ESCRT
Syndecans belong to heparan sulfate proteoglycans (HSPGs)
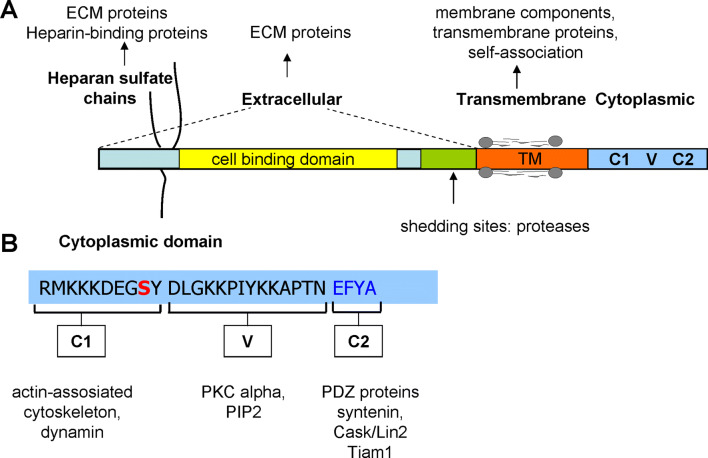
The syndecan family belongs to the type I transmembrane proteoglycans, bearing 3–5 heparan sulfate (HS) and chondroitin sulfate chains on the core proteins. In vertebrates, there are four members syndecan-1–4. The expression of syndecan-1–3 is tissue specific, meanwhile that of syndecan-4 is universal. Syndecan-1 is expressed mostly on epithelial and plasma cells; syndecan-2 is characteristic for endothelial cells and fibroblasts; syndecan-3 is found predominantly in the nervous system [8, 13]. They share similar structure: an N-terminal ectodomain, a conserved short, one span transmembrane domain (TM—25 amino acids) and a conserved approximately 30 amino acid length cytoplasmic domain (CD) (Fig. 1). The divergent N-terminal extracellular domains (ectodomains) contain the glycosaminoglycan (GAG) attachment sites for heparin sulfate (HS) near the N-terminus and can be decorated with chondroitin sulfate at the juxtamembrane region. The ectodomain of syndecan-4 comprises a cell-binding domain (CBD) mediating cell–cell, cell–matrix attachment sites (Fig. 1) [42, 43]. Syndecan-4 incorporates into focal contacts and matured adhesions, interacts with several focal adhesion components e.g. alpha-actinin, paxillin and integrins [13, 16, 46, 49]. Syndecans are engaged as co-receptors with integrins to regulate adhesion to extracellular matrix (ECM), syndecan-4 cooperates with β1-integrin to promote attachment to fibronectin, collagen or laminin of ECM [5, 45, 49].
Fig. 1.
Functional domains of syndecan-4 and their potential interactions. The transmembrane and cytoplasmic domains of the core protein display high degree conservation across species. A schematic view of the structure of syndecan-4 shows the different domains (a), and the amino acid sequence of the conserved cytoplasmic domain (b). The potential interactions are indicated. ECM extracellular matrix, PKC protein kinase C, TM transmembrane domain, C1 and C2 conserved regions, V the variable region of the cytoplasmic domain
The phosphorylation of Ser179 in the C1 conserved region of syndecan-4 CD (Fig. 1) is extensively studied, and considered as a molecular switch in signal transduction [33–35]. However, syndecan-4 is phosphorylated periodically during the cell cycle. Robust phosphorylation of syndecan-4 starts in the late G2 phase (from 3 h upon release of hydroxyurea block) and reaches the peak between 6 and 9 h and decreases to the basic level after 18 h [33].
Failure in mitotic rounding and its consequences thereof
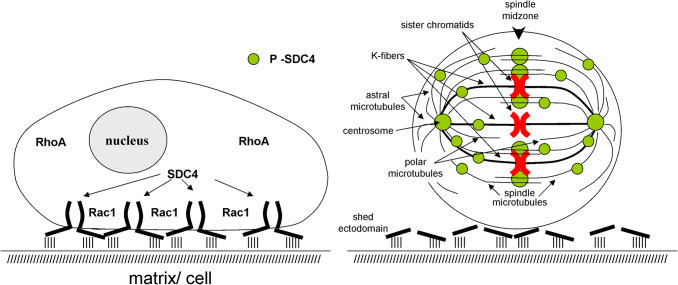
Cell rounding prior to mitosis is the process, in which cells change their shape to become spherical. Cell rounding starts in the late G2 followed by chromosome condensation and nuclear envelope breakdown. The spherical shape gives the basis of the correct mitotic spindle assembly providing an appropriate space, in which the condensed chromosomes are captured by the spindles (Fig. 2). Due to the incomplete detachment, the mitotic cells remain elongated; failures are increased in spindle assembly, pole splitting, and in mitotic progression. These errors can be rescued by increasing lengths of microtubules that appears to be a direct consequence of the limited reach of mitotic microtubules [39]. The overexpression of non-phosphorylatable Ser179Ala syndecan-4 frequently originates giant, multinucleated cells [33]. Mitotic rounding provides an appropriate space, where the K-fibers can capture chromosomes (Fig. 2). If the cells remain flat, well spread during mitosis, there is a tendency to form multipolar spindles [17, 38]. In the case of Ser179Ala syndecan-4, the periodic shedding could not be detected and giant cells occur frequently. The interactions between cells and the surrounding matrix or adjacent cells can remain intact, the mitotic rounding is not completed and the cells have tendency to stay flat, well spread (Fig. 2). Appearance of the giant cells is a consequence of the expression of the non-phosphorylatable Ser179Ala syndecan-4, and the cells due to their size are predestinated for mitotic failure such as polyploidy [33].
Fig. 2.
Localization of syndecan-4 in interphase cells and in dividing cells during mitosis. a The cell surface syndecan-4 can connect the cells to the surrounding matrix and/or neighbor cells through the sulfonated sugar side chains and the cell-binding domain of the extracellular segment of syndecan-4. b Prior to mitosis syndecan-4 is periodically shed, i.e., the ectodomain is cleaved off, helping so the mitotic rounding. The phosphorylated shed remnants and phospho-syndecan-4 are retrieved from the plasma membrane and co-localize with the duplicated centrosomes, the mitotic spindles, and the K-fibers. Later, it accumulates in the spindle midzone. Syndecan-4 can be observed only with the mitotic machinery. It cannot be detected with MTOC, or microtubules of interphase cells
Failure in mitosis and cytokinesis may lead to missegregation of chromosomes resulting in aneuploidy/euploidy/polyploidy and most probably chromosomal instability that is a challenge for cell viability. Polyploidization constitutes early events in the development of many types of cancer [55, 59].
Mitotic cell rounding is accompanied by changes in actin organization
The somatic cells keep tight contact with their environment, the surrounding extracellular matrix (ECM). In contrast, the proliferating cells, prior to mitosis, lose their adhesive interactions with the ECM, change their morphology as they round up; following cytokinesis, they reattach again and spread (Fig. 2) [38, 40, 56].
This dramatic change in the shape of any animal cell is the consequence of RhoA-dependent reorganization of the actin cytoskeleton [39, 40]. In the interphase, cellular actin forms a cortical meshwork, adherent actin belt, and stress fibers that span the cytoplasm ending in focal adhesions. Upon entry into mitosis, the stress fibers and the adherent actin belts are reorganized to cortical meshwork. At the same time, the adherent cells abrogate their adhesive interactions with the ECM; the focal adhesions are disassembled (Fig. 2). However, not all interactions are abolished; the mitotic cells remain tethered to the substratum and the neighboring cells with thin tubular strands called retraction fibers. These are rich in actin filaments and contain ERM proteins. Phase-dense nodules can be detected on the fibers, which move inward the mitotic cell on the fiber [21, 33, 39, 44].
As the mitosis progresses at anaphase, the actin cytoskeleton is remodeled to a contractile actomyosin ring at the equatorial cell cortex. Contraction of the actomyosin ring generates furrow ingression. Remodeling the actin cytoskeleton and the contraction is driven mostly by RhoA activation [6, 40]. However the detachment of cells is independent of RhoA activity [40]. Importantly, syndecan-4 can mediate cell–cell and cell–matrix interactions [42, 43]. Syndecan-4 undergoes a periodical shedding in G2 phases, loosing the extracellular domain that serves as a connection to the surroundings. Shedding reaches the peak in the late G2 phase or in the beginning of M phase promoting, thus, the mitotic rounding [33]. Shedding and syndecan-4 phosphorylation take place in parallel. The phosphorylation of syndecan-4 promotes RhoA activation [34], which in turn manages the rearrangement of the actin meshwork and the release of the connection of the extracellular space.
Implication of syndecan-4 in mitosis
The centrosome replicates during the S phase of the cell cycle. The two centrosomes remain together until the end of G2. As mitosis is initiated, the two centrosomes migrate to opposite poles of the cell serving as a nucleation template for the bipolar mitotic spindles that form between the two centrosomes. The chromosomes condense and a dynamic, bipolar, microtubule-based mitotic spindle is assembled forming antiparallel microtubule bundles or some couple to the chromosomes on the kinetochores called K-fiber (kinetochor fiber). Captured chromosomes are then brought to the spindle midzone to form a metaphase plate (Fig. 2) [53].
The central spindle is largely composed of antiparallel non-kinetochore spindle microtubules (Fig. 2). The assembly of the central spindle is a very important event, as it defines the division plane and provides template for the midbody, a targeting platform for abscission factors. At the central spindle, the centralspindlin complex is assembled comprising a kinesin-6 motor protein MKLP1, and a Rho GTPase-activating protein (RhoGAP), MgcRacGAP/CYK4 [60].
The bridging fibers are associated with and connect to K-fibers of sister kinetochores. Kinesin motor proteins can crosslink the antiparallel microtubules and walk to the plus ends generating counteracting forces, which slide apart the microtubules and push the sister K-fibers toward the poles of the cell [31, 53, 58].
Syndecan-4 is distributed along the antiparallel, interpolar (polar) microtubule bundles and astral microtubules concentrating in the overlapping segments of the interpolar microtubules at the equator region (Fig. 2) [33]. As mitosis progresses from pro-metaphase, the phosphorylated syndecan-4 localizes with the centrosomes, and along the mitotic spindle accumulated in the spindle midzone. Importantly, syndecan-4 cannot be detected with the microtubule-organizing centres (MTOC), and along the microtubules of interphase cells at all [33]. Importantly, syndecan-4 can associate only with mitotic spindles, and not with interphase microtubules; therefore, its binding to the microtubules must be mediated by some spindle-associated protein(s). Woodcock et al. [61] reported that Tiam1 and Rac1 localize to centrosomes during prophase and prometaphase, and both are involved in the regulation of centrosome separation; in the cases of missing Rac1 activity and/or Tiam1 activity, the interchromosomal distance is increased by 40%. The extended length of mitotic spindles can lead frequently to chromosome congression errors [38, 61].
Immunocytochemical analysis revealed in syndecan-4 expressing MCF7 cells that phospho-syndecan-4 has enhanced affinity with centrosomes [33], and further, syndecan-4 is capable of reducing Rac1 activity by blocking Tiam1 [34]. It is a plausible presumption that syndecan-4 can take part in centrosome separation and chromosome congression-regulating Rac1 activity through Tiam1.
RhoA–Rac1 activity in the cleavage furrow
The best-known members of the Rho family of small GTPases RhoA, Rac1 and Cdc42 are the major regulators of the cytoskeleton, formation of the cell shape and cell polarity, migration and differentiation [10]. They are molecular switches cycling between an active GTP-bound and inactive GDP-bound conformations. RhoA activity is responsible mostly for the assembly, reorganization and dynamics of the actin meshwork. Cdc42 and Rac1 take part in the cell shape, polarity and migration. Their activation cycle is mostly regulated by the guanine nucleotide exchange factors (GEFs), which catalyze the exchange of GDP for GTP and by GTPase-activating proteins (GAPs) accelerating their inactivation [9]. There is a cross talk among the activity of family members influencing their individual activities, which supposes a coordinated action among GEFs and GAPs. For example, the activity of Rac1 can suppress the activity of RhoA and influence the relative activity of Cdc42; at the same time, the Rac1 activity is regulated by RhoG, etc. [32, 34, 37, 66].
During mitosis, the activity of RhoA is elevated [40]. The strict regulation of the activity of Rho GTPases in specific areas within the cell is essential to coordinate cell functions. Following the separation of the mitotic chromosomes, the active form of RhoA accumulates at the equatorial of the cells. RhoA-GTP is necessary and sufficient for the assembly and constriction of the actomyosin ring, which manages the furrow ingression at the division plane [6]. The contractile ring is composed of F-actin and myosin II, where the actin filaments are nucleated by formins [24, 29]. The diaphanous-like formins are RhoA effectors; promote actin polymerization and microtubule organization [4, 28, 51]. Myosin II is a major motor protein that generates the constriction forces for the cytokinesis [23]. For the generation and stabilization of the actomyosin ring in the furrow, contribution of dynamin is necessary [41]. Dynamins belong to the group of large GTPases responsible for diverse cellular processes in eukaryotic cells, including the release of transport vesicles, associate with the spindle midzone and are required for cytokinesis maintaining proper myosin II organization in the cleavage furrow stabilizing, thus, the actin filaments [27, 41, 57]. Syndecan-4 can connect to dynamin II through the C1 conserved region of syndecan-4 cytoplasmic domain (Fig. 2) [63] and most probably guide it. Dynamin II is periodically phosphorylated by Cdk1–cyclin B1 prior to mitosis and dephosphorylated by calcineurin during cytokinesis. The absence of dephosphorylation caused multinucleation of HeLa cells [12, 30].
In the central spindle, the RhoA activity is regulated mainly by ECT2 Rho-GEF [48] and MgcRacGAP [15, 60]. The exact role of MgcRacGAP is subject of debate. Some claim that its function is to promote RhoA activation [6, 64], whereas others suggest that it is necessary to inactivate Rac1 [66]. However, it is not a contradiction, because the activity of RhoA and Rac1 is antagonistic. Suppression of Rac1 activity increases the RhoA activity. Phospho-syndecan-4 accumulates in the furrow ingression, distributed with the central spindle and later it is concentrated at the intercellular bridge (ICB) and in the midbody. Phospho-syndecan-4 is able to suppress Rac1 activity, at the same time elevating RhoA activity (Fig. 3) [33, 34].
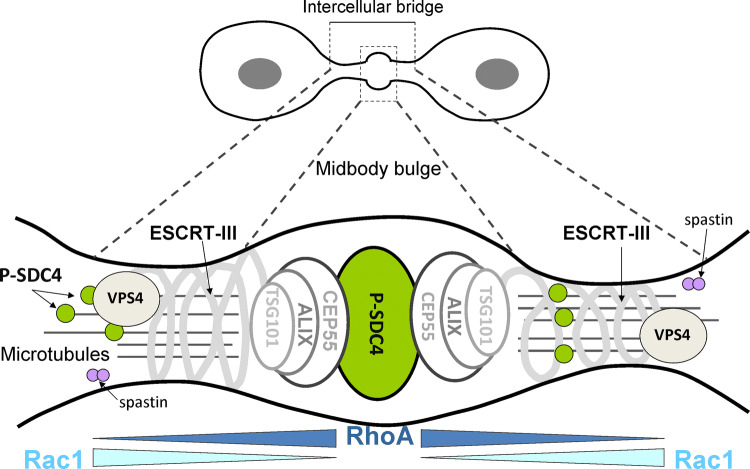
Fig. 3.
Schematic representation of animal cytokinesis. Progress of furrow ingression results in a thin plasma bridge termed intercellular bridge (ICB). At the middle of ICB, there is an electron dense structure called midbody (Flemming body). The centralspindlin components recruit among others phospho-syndecan-4 (green) and CEP55 to the midbody. CEP55 interacts with the adaptor protein ALIX and the ESCRT-I protein TSG101, which are in turn proposed to recruit ESCRT-III components (CHMP2,-3,-4,-5,-6) with the microtubule-severing enzyme spastin [47]. The cortical constriction of the membrane is driven by VPS4, which leads to abscission
Overexpression of Ser179Glu syndecan-4 delays abscission
Constriction of the actomyosin ring creates cleavage furrow [1, 2]. Cleavage furrow ingression eventually narrows a thin ICB that connects the dividing cells and midbody (Flemming body) formed at the center of the ICB. Some midbody components are present at furrow ingression; others are delivered, including membrane components, endocytic factors, and secretory vesicles and their associated fusion machinery such as endosomal-sorting complex required for transport (ESCRT) complexes [1, 2, 20]. The first step towards abscission is the connection of CEP55 to centralspindlin [47, 65]. The dimeric coiled-coil CEP55 forms a bridge among centralspindlin and ALIX (PDCD6IP) and the TSG101 subunit of the ESCRT-I subunit. These components recruit, in turn, the ESCRT-III complex and VPS4, which is probably directly responsible for abscission (Fig. 3) [2, 60].
ALIX and the core ESCRT-III proteins accumulate on mature midbodies, although they are not always co-recruited [7] indicating that additional regulatory mechanisms are at play [60]. Syndecan-4 can bind ALIX via syntenin, a PDZ protein characterized as syndecan-4 interactor [3, 25, 50], although a direct interaction cannot be ruled out between syndecan and ALIX because a syndecan mutant devoid of the PDZ binding site has not been tested. Most probably, the phospho-syndecan-4 is positioned at the middle of midbody connected to CEP55 and ALIX (Fig. 3). Once all of these components are properly assembled and activated, abscission occurs and the two daughter cells separate completely [20, 52].
Membrane traffic during cytokinesis and the fate of the midbody remnant after abscission
Cytokinesis requires highly regulated and extensive membrane vesicle trafficking both to, and from the cleavage furrow. Membrane trafficking is required for midzone delivery of cytoskeletal and regulatory molecules (e.g., Rho GTPases and their interacting partners), as well as components of the membrane abscission machinery [11, 54]. Exocytic secretory vesicles move towards the midbody either in an asymmetric [22] or a symmetric [26] manner from the forming daughter cells, which in turn can influence the symmetric or asymmetric nature of abscission [18]. Immunocytochemical analysis of phospho-syndecan-4, and overexpressed syndecan-4-GFP revealed an asymmetric arrangement of secretory vesicles marked by syndecan-4 cargoes [33]; unfortunately, other midbody components were not studied at this publication.
The fate of the midbody remnant is of interest and actively studied phenomenon because it might have crucial consequences for cell differentiation and tumorigenesis [14, 19, 36]. The accumulation of remnants is associated with increased proliferation, induced pluripotent stem cell colony formation, asymmetrical cell divisions in several tissues. The stem cells retract the midbody remnant into their cytoplasm to maintain the stem cell status [36]. The symmetrical cleavage of midbody (Fig. 3) is associated with normal cell differentiation [19]. Therefore, it should be important, if there is any effect of the extended ICBs due to the delayed or postponed abscission in the proliferating cells. A further question is whether the accumulation of the phospho/or phosphomimetic syndecan-4 in the midbody can interfere with the symmetrical/asymmetrical nature of abscission.
Conclusions
Taken together, it can be stated that syndecan-4 takes part in the mitosis and the subsequent cytokinesis. Its direct roles in the mitosis should be clarified in the future. Syndecan-4 is detectable from the very beginning of the mitosis associating with the duplicated centrosomes, and the mitotic spindles. Later, it is present in the developing furrow ingression and ICB including midbody, too. It can be implicated in the elevation of the active RhoA, and the phosphomimetic form can delay the abscission. Recently, heparanase was shown to localize with centrosomes. Silencing the heparanase expression disrupts mitotic spindles, resulting in chromosome instability, mis-segregation and increased micronuclei formation [62]. Considering this, we can assume that for the correct cell division, the heparan sulfate chains of syndecan-4 or other proteoglycans should be degraded. However, the exact mechanism should be the subject of future investigations.
Acknowledgements
We thank Dr. Ferenc Deak for his critical reading of the manuscript. This work was supported by the Hungarian National Research, Development and Innovation Office (GINOP-2.3.2-15-2016-00058). B. U. received a fellowship from the Bolyai János Research Foundation of the Hungarian Academy of Science and the UNKP-18-4 New National Excellence Program of the Hungarian Ministry of Human Capacities.
References
- 1.Agromayor M, Martin-Serrano J. Knowing when to cut and run: mechanisms that control cytokinetic abscission. Trends Cell Biol. 2013;23:433–441. doi: 10.1016/j.tcb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 2.D’Avino PP, Giansanti MG, Petronczki M. Cytokinesis in animal cells. Cold Spring Harb Perspect Biol. 2015;7(4):a015834. doi: 10.1101/cshperspect.a015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 4.Bartolini F, Gundersen GG. Formins and microtubules. Biochim Biophys Acta. 2010;1803(2):164–173. doi: 10.1016/j.bbamcr.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bass MD, Morgan MR, Roach KA, Settleman J, Goryachev AB, Humphries MJ. p190RhoGAP is the convergence point of adhesion signals from alpha 5 beta 1 integrin and syndecan-4. J Cell Biol. 2008;181:1013–1026. doi: 10.1083/jcb.200711129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basant A, Glotzer M. A GAP that divides. F1000 Res. 2017;6:1788. doi: 10.12688/f1000research.12064.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastos RN, Barr FA. Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission. J Cell Biol. 2010;191:751–760. doi: 10.1083/jcb.201008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 9.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caballe A, Martin-Serrano J. ESCRT machinery and cytokinesis: the road to daughter cell separation. Traffic. 2011;12:1318–1326. doi: 10.1111/j.1600-0854.2011.01244.x. [DOI] [PubMed] [Google Scholar]
- 12.Chircop M, Sarcevic B, Larsen MR, Malladi CS, Chau N, Zavortink M, Smith CM, Quan A, Anggono V, Hains PG, Graham ME, Robinson PJ. Phosphorylation of dynamin II at serine-764 is associated with cytokinesis. Biochim Biophys Acta. 2011;1813:1689–1699. doi: 10.1016/j.bbamcr.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol. 2003;4:926–937. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- 14.Crowell EF, Gaffuri AL, Gayraud-Morel B, Tajbakhsh S, Echard A. Engulfment of the midbody remnant after cytokinesis in mammalian cells. J Cell Sci. 2014;127:3840–3851. doi: 10.1242/jcs.154732. [DOI] [PubMed] [Google Scholar]
- 15.Davies T, Kodera N, Kaminski Schierle GS, Rees E, Erdelyi M, Kaminski CF, Ando T, Mishima M. CYK4 promotes antiparallel microtubule bundling by optimizing MKLP1 neck conformation. PLoS Biol. 2015;13(4):e1002121. doi: 10.1371/journal.pbio.1002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denhez F, Wilcox-Adelman SA, Baciu PC, Saoncella S, Lee S, French B, Neveu W, Goetinck PF. Syndesmos, a syndecan-4 cytoplasmic domain interactor, binds to the focal adhesion adaptor proteins paxillin and Hic-5. J Biol Chem. 2002;277:12270–12277. doi: 10.1074/jbc.M110291200. [DOI] [PubMed] [Google Scholar]
- 17.Dinarina A, Pugieux C, Corral MM, Loose M, Spatz J, Karsenti E, Nédélec F. Chromatin shapes the mitotic spindle. Cell. 2009;138(3):502–513. doi: 10.1016/j.cell.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Dionne LK, Wang XJ, Prekeris R. Midbody: from cellular junk to regulator of cell polarity and cell fate. Curr Opin Cell Biol. 2015;35:51–58. doi: 10.1016/j.ceb.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettinger AW, Wilsch-Brauninger M, Marzesco AM, Bickle M, Lohmann A, Maliga Z, Karbanova J, Corbeil D, Hyman AA, Huttner WB. Proliferating versus differentiating stem and cancer cells exhibit distinct midbody-release behaviour. Nat Commun. 2011;2:503. doi: 10.1038/ncomms1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fededa JP, Gerlich DW. Molecular control of animal cell cytokinesis. Nat Cell Biol. 2012;14:440–447. doi: 10.1038/ncb2482. [DOI] [PubMed] [Google Scholar]
- 21.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 24.Green RA, Paluch E, Oegema K. Cytokinesis in animal cells. Annu Rev Cell Dev Biol. 2012;28:29–58. doi: 10.1146/annurev-cellbio-101011-155718. [DOI] [PubMed] [Google Scholar]
- 25.Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Durr J, David G. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Nat Acad Sci. 1997;94:13683–13688. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goss JW, Toomre DK. Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. J Cell Biol. 2008;181:1047–1054. doi: 10.1083/jcb.200712137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heymann JA, Hinshaw JE. Dynamins at a glance. J Cell Sci. 2009;122:3427–3431. doi: 10.1242/jcs.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishizaki T, Morishima Y, Okamoto M, Furuyashiki T, Kato T, Narumiya S. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat Cell Biol. 2001;3:8–14. doi: 10.1038/35050598. [DOI] [PubMed] [Google Scholar]
- 29.Jordan SN, Davies T, Zhuravlev Y, Dumont J, Shirasu-Hiza M, Canman JC. Cortical PAR polarity proteins promote robust cytokinesis during asymmetric cell division. J Cell Biol. 2016;212:39–49. doi: 10.1083/jcb.201510063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi S, Perera S, Gilbert J, Smith CM, Mariana A, Gordon CP, Sakoff JA, McCluskey A, Robinson PJ, Braithwaite AW, Chircop M. The dynamin inhibitors MiTMAB and OcTMAB induce cytokinesis failure and inhibit cell proliferation in human cancer cells. Mol Cancer Ther. 2010;9:1995–2006. doi: 10.1158/1535-7163.MCT-10-0161. [DOI] [PubMed] [Google Scholar]
- 31.Kapitein LC, Kwok BH, Weinger JS, Schmidt CF, Kapoor TM, Peterman EJ. Microtubule cross-linking triggers the directional motility of kinesin-5. J Cell Biol. 2008;182:421–428. doi: 10.1083/jcb.200801145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–464. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- 33.Keller-Pinter A, Bottka S, Timar J, Kulka J, Katona R, Dux L, Deak F, Szilak L. Syndecan-4 promotes cytokinesis in a phosphorylation-dependent manner. Cell Mol Life Sci. 2010;67:1881–1894. doi: 10.1007/s00018-010-0298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller-Pinter A, Ughy B, Domoki M, Pettko-Szandtner A, Letoha T, Tovari J, Timar J, Szilak L. The phosphomimetic mutation of syndecan-4 binds and inhibits Tiam1 modulating Rac1 activity in PDZ interaction-dependent manner. PLoS One. 2017;12:e0187094. doi: 10.1371/journal.pone.0187094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koo BK, Jung YS, Shin J, Han I, Mortier E, Zimmermann P, Whiteford JR, Couchman JR, Oh ES, Lee W. Structural basis of syndecan-4 phosphorylation as a molecular switch to regulate signaling. J Mol Biol. 2006;355:651–663. doi: 10.1016/j.jmb.2005.09.087. [DOI] [PubMed] [Google Scholar]
- 36.Kuo TC, Chen CT, Baron D, Onder TT, Loewer S, Almeida S, Weismann CM, Xu P, Houghton JM, Gao FB. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat Cell Biol. 2011;13:1214–1223. doi: 10.1038/ncb2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, Collard JG, Der CJ. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002;4:621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 38.Lancaster OM, Le Berre M, Dimitracopoulos A, Bonazzi D, Zlotek-Zlotkiewicz E, Picone R, Duke T, Piel M, Baum B. Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation. Dev Cell. 2013;25:270–283. doi: 10.1016/j.devcel.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Lancaster OM, Baum B. Shaping up to divide: coordinating actin and microtubule cytoskeletal remodelling during mitosis. Semin Cell Dev Biol. 2014;34:109–115. doi: 10.1016/j.semcdb.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Maddox AS, Burridge K. RhoA is required for cortical retraction and rigidity during mitotic cell rounding. J Cell Biol. 2003;160:255–265. doi: 10.1083/jcb.200207130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masud Rana AY, Tsujioka M, Miyagishima S, Ueda M, Yumura S. Dynamin contributes to cytokinesis by stabilizing actin filaments in the contractile ring. Genes Cells. 2013;18:621–635. doi: 10.1111/gtc.12060. [DOI] [PubMed] [Google Scholar]
- 42.McFall AJ, Rapraeger AC. Identification of an adhesion site within the syndecan-4 extracellular protein domain. J Biol Chem. 1997;272:12901–12904. doi: 10.1074/jbc.272.20.12901. [DOI] [PubMed] [Google Scholar]
- 43.McFall AJ, Rapraeger AC. Characterization of the high affinity cell-binding domain in the cell surface proteoglycan syndecan-4. J Biol Chem. 1998;273:28270–28276. doi: 10.1074/jbc.273.43.28270. [DOI] [PubMed] [Google Scholar]
- 44.Mitchison TJ. Actin based motility on retraction fibers in mitotic PtK2 cells. Cell Motil Cytoskelet. 1992;22(2):135–151. doi: 10.1002/cm.970220207. [DOI] [PubMed] [Google Scholar]
- 45.Mostafavi-Pour Z, Askari JA, Parkinson SJ, Parker PJ, Ng TT, Humphries MJ. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J Cell Biol. 2003;161:155–167. doi: 10.1083/jcb.200210176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Multhaupt HA, Yoneda A, Whiteford JR, Oh ES, Lee W, Couchman JR. Syndecan signaling: when, where and why? J Physiol Pharmacol. 2009;60(Suppl 4):31–38. [PubMed] [Google Scholar]
- 47.Neto H, Gould GW. The regulation of abscission by multi-protein complexes. J Cell Sci. 2011;124(Pt 19):3199–3207. doi: 10.1242/jcs.083949. [DOI] [PubMed] [Google Scholar]
- 48.Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci. 2006;119(Pt 1):104–114. doi: 10.1242/jcs.02737. [DOI] [PubMed] [Google Scholar]
- 49.Roper JA, Williamson RC, Bass MD. Syndecan and integrin interactomes: large complexes in small spaces. Curr Opin Struct Biol. 2012;22:583–590. doi: 10.1016/j.sbi.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roucourt B, Meeussen S, Bao J, Zimmermann P, David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015;25:412–428. doi: 10.1038/cr.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002;4:408–415. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt O, Teis D. The ESCRT machinery. Curr Biol. 2012;22:R116–R120. doi: 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simunić J, Tolić IM. Mitotic spindle assembly: building the bridge between sister K-fibers. Trends Biochem Sci. 2016;41:824–833. doi: 10.1016/j.tibs.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Tang BL. Membrane trafficking components in cytokinesis. Cell Physiol Biochem. 2012;30:1097–1108. doi: 10.1159/000343301. [DOI] [PubMed] [Google Scholar]
- 55.Telentschak S, Soliwoda M, Nohroudi K, Addicks K, Klinz FJ. Cytokinesis failure and successful multipolar mitoses drive aneuploidy in glioblastoma cells. Oncol Rep. 2015;33:2001–2008. doi: 10.3892/or.2015.3751. [DOI] [PubMed] [Google Scholar]
- 56.Thery M, Bornens M. Get round and stiff for mitosis. HFSP J. 2008;2:65–71. doi: 10.2976/1.2895661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson HM, Skop AR, Euteneuer U, Meyer BJ, McNiven MA. The large GTPase dynamin associates with the spindle midzone and is required for cytokinesis. Curr Biol. 2002;12:2111–2117. doi: 10.1016/S0960-9822(02)01390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tolić IM. Mitotic spindle: kinetochore fibers hold on tight to interpolar bundles. Eur Biophys J. 2018;47:191–203. doi: 10.1007/s00249-017-1244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006;18:658–667. doi: 10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 60.White EA, Glotzer M. Centralspindlin: at the heart of cytokinesis. Cytoskeleton. 2012;69:882–892. doi: 10.1002/cm.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodcock SA, Rushton HJ, Castañeda-Saucedo E, Myant K, White GR, Blyth K, Sansom OJ, Malliri A. Tiam1-Rac signaling counteracts Eg5 during bipolar spindle assembly to facilitate chromosome congression. Curr Biol. 2010;20:669–675. doi: 10.1016/j.cub.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang S, Liao Y, Zhao Q, Xie Y, Zheng A, Wan H. Heparanase is a critical regulator of mitotic spindles required for maintaining chromosome stability. DNA Cell Biol. 2018;37:291–297. doi: 10.1089/dna.2017.3990. [DOI] [PubMed] [Google Scholar]
- 63.Yoo J, Jeong MJ, Cho HJ, Oh ES, Han MY. Dynamin II interacts with syndecan-4, a regulator of focal adhesion and stress-fiber formation. Biochem Biophys Res Commun. 2005;328:424–431. doi: 10.1016/j.bbrc.2004.12.179. [DOI] [PubMed] [Google Scholar]
- 64.Zhang D, Glotzer M. The RhoGAP activity of CYK-4/MgcRacGAP functions non-canonically by promoting RhoA activation during cytokinesis. eLife. 2015;7:4. doi: 10.7554/eLife.08898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao W, Seki A, Fang G. Cep55, a microtubule-bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis. Mol Biol Cell. 2006;17:3881–3896. doi: 10.1091/mbc.e06-01-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhuravlev Y, Hirsch SM, Jordan SN, Dumont J, Shirasu-Hiza M, Canman JC. CYK-4 regulates Rac, but not Rho, during cytokinesis. Mol Biol Cell. 2017;28:1258–1270. doi: 10.1091/mbc.e17-01-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]