Abstract
Abstract
mTORC1 signaling is the prototypical pathway regulating protein synthesis and cell proliferation. mTORC1 is active in stem cells located at the base of intestinal crypts but silenced as transit-amplifying cells differentiate into enterocytes or secretory cells along the epithelium. After an insult or injury, self-limiting and controlled activation of mTORC1 is critical for the renewal and repair of intestinal epithelium. mTORC1 promotes epithelial cell renewal by driving cryptic stem cell division, and epithelial cell repair by supporting the dedifferentiation and proliferation of enterocytes or secretory cells. Under repeated insult or injury, mTORC1 becomes constitutively active, triggering an irreversible return to stemness, cell division, proliferation, and inflammation among dedifferentiated epithelial cells. Epithelium-derived cytokines promulgate inflammation within the lamina propria, which in turn releases inflammatory factors that act back on the epithelium where undamaged intestinal epithelial cells participate in the pervading state of inflammation and become susceptible to tumorigenesis.
Graphical abstract
Keywords: Cell proliferation, Stem cell, Pro-inflammatory cytokines, Immunity, Colorectal cancer
Introduction
The intestinal epithelium is a single layer of specialized cells among which enterocytes absorb nutrients, goblet cells produce mucus, Paneth cells discharge antimicrobial peptides, and enteroendocrine cells secrete hormones [1, 2]. Owing to its multiple functions and active metabolism, the epithelium undergoes rapid turnover. As such intestinal epithelial cells (IEC) regenerate every 4–5 days [3]. Intestinal stem cells (ISC) residing at the base of the crypts give rise to all lineages of specialized epithelial cells and thus ensure the renewal of the epithelium and maintenance of intestinal homeostasis [4]. The intestinal epithelium constitutes the first line of defense by maintaining tight junctions, which seal the space between adjacent epithelial cells thus limiting the passage of solutes, molecules and microorganisms into the lamina propria.
Located at the interface with the lumen, the intestinal epithelium is continuously exposed to physical, chemical and biological insult or injury [5–9]. Strenuous physical activity, exposure to extreme temperatures, and radiation are a few examples of physical insults that increase damage susceptibility of the intestinal epithelium [7, 10–12]. Also, chemical toxins derived from alcohol such as acetaldehyde and ethanol, negatively impact the tight junctions and increase the permeability of the epithelial layer [10, 13]. Likewise, tobacco-specific nitrosamines, polycyclic aromatic hydrocarbons and heavy metals induce oxidative stress and have been associated increased risk of intestinal inflammation and tumorigenesis [14]. The long-term use of drugs such as non-steroidal anti-inflammatory drugs (NSAID), another form of chemical insult, can damage the intestinal layer [15]. Physical and chemical insults not only impair the epithelial barrier integrity but also cause dysbiosis, an imbalance in the gut microbiome [16, 17]. Thus, intestinal epithelium requires a rigorous repair mechanism owing to the frequent events of physical/chemical/dysbiotic insults.
The mechanistic target of rapamycin (mTORC1) promotes epithelial repair by stimulating stem cell division and migration from the crypts. Signals from nutrients, such as amino acids, and from commensal microbiota contribute significantly to mTORC1-mediated epithelial renewal [18–22]. But, the renewal of aged or damaged IEC—supported by the division of stem cells—is not the only means of maintaining homeostasis.
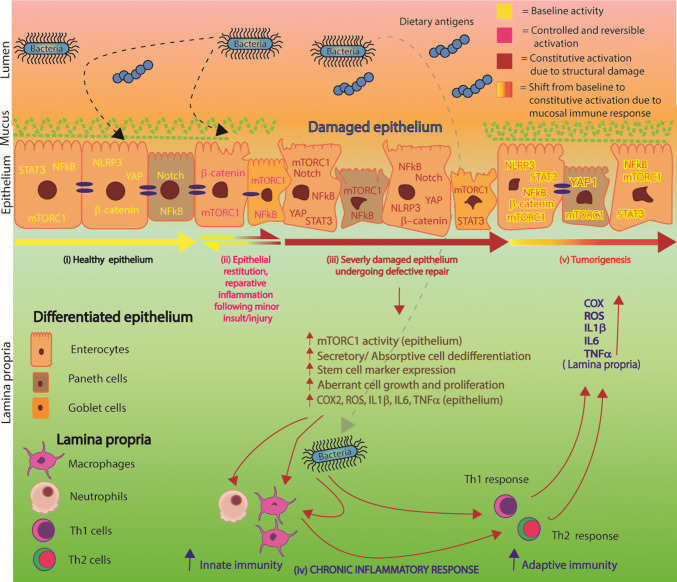
The epithelial restitution program is activated by a self-limiting inflammatory reaction, localized to the site of damage in which inflammatory cytokines participate in the repair and renewal of the intestinal barrier [23–25]. During epithelial restitution, differentiated cells located near the damaged area start to dedifferentiate, reorganize their cytoskeleton, migrate to the site of injury, and ultimately redifferentiate to heal the wounded area [24–26]. IEC dedifferentiation program is supported by mediators of proliferation, regeneration and repair, which include mTORC1, YAP1, Notch1 and WNT/β-catenin. In all, the combination of confined reparative inflammation, epithelial restitution, and cellular proliferation constitutes the basis by which the epithelium rectifies tissue damage.
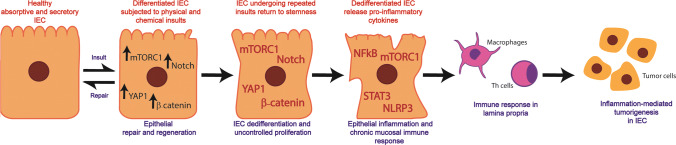
The Hippo-YAP, Notch1 and WNT/β-catenin pathways responsible for cell growth and proliferation become active in dedifferentiating IEC undergoing repair [27–34]. These pathways crosstalk with mTORC1, which regulates protein synthesis, IEC growth and proliferation [35–37]. Our work and that of others have shown that mTORC1 activity is silenced as IEC differentiate [30, 36, 38, 39]. As would be expected the YAP1, Notch1 and β-catenin pathways are also downregulated as epithelial cells attain the final stage of differentiation. However, this state is reversible and the reversal is inducible. The reactivation of mTORC1, Hippo-YAP, Notch1 and WNT signaling pathways is observed in the intestinal epithelium undergoing regeneration and repair secondarily to repeated dysbiotic events and physical/chemical insult [31, 33, 34, 40–44]. However, if unabated, the constitutive activation of mTORC1, YAP1, Notch1 and WNT provokes unwarranted IEC dedifferentiation and dedifferentiated IEC-derived inflammatory cytokine secretion, which collectively disrupt intestinal epithelial homeostasis permanently, as seen in individuals harboring single nucleotide polymorphisms in genes crucial for maintaining intestinal homeostasis (NOD2, STAT3 and ATG16L1) [45–47]. In this chain of events, mTORC1 is closely associated with the activation of inflammatory mediators, NFkB, STAT3, NLRP3, that cause chronic epithelial inflammation and an immune response as inflammatory cytokines elicit the infiltration of immune cells from the lamina propria to the epithelium [29, 48–52]. The accumulation of pro-inflammatory factors in the lamina propria also stimulates mediators of unwarranted proliferation and inflammation within differentiated IEC of undamaged regions of the epithelium as depicted in Fig. 1. Eventually, these events result in creating a tumorigenic microenvironment in the gut wall.
Fig. 1.
Role of mTORC1 in intestinal epithelial cell dedifferentiation, inflammation and consequent tumorigenesis. (i) In healthy epithelium, mTORC1 signaling, the mediators of proliferation (YAP1, Notch1 and WNT/β-catenin) and inflammation (NFkB, STAT3 and NLRP3) are downregulated. (ii) Upon minor damage, the epithelium undergoes localized repair promoted by mTORC1-dependent activation of mediators of cellular restitution, proliferation and inflammation. During this process, healthy epithelial cells located near the site of injury start to dedifferentiate, migrate to the site of damage, divide, and redifferentiate to seal the wounded area. (iii) Upon repeated dysbiotic, physical, or chemical insults, the epithelial repair process becomes dysregulated. As a result, mTORC1 becomes constitutively active in the IEC of the epithelium undergoing dysregulated repair leading to their aberrant dedifferentiation and proliferation, and to the excessive secretion of pro-inflammatory cytokines from dedifferentiated IEC. (iv) Pro-inflammatory cytokines released by dedifferentiated IEC induce an inflammatory response involving cells of the innate and adaptive immunity of the lamina propria. (v) The inflammatory response reaches healthy regions of the epithelium placing them at risk of developing tumors
This review examines the contribution of mTORC1 in post-insult/injury intestinal repair and tumorigenesis. The process of intestinal tumorigenesis is driven by transition between well-regulated mTORC1 signaling involved in intestinal tissue repair and regeneration to dysregulated constitutively active mTORC1 signaling, where epithelial cell dedifferentiation and inflammation serve as driving forces of tumorigenesis. We reviewed the role of mTORC1 in post-insult/injury renewal of the intestinal epithelium, in response to amino acids and microbiota-derived signals. The review also describes the molecular underpinnings of unwarranted epithelial dedifferentiation and ensuing development of the epithelial inflammatory profile observed in intestinal inflammatory disorders (e.g., inflammatory bowel disease, IBD) and inflammation-mediated tumorigenesis. We summarized the critical role of interactions of mTORC1 signaling with the Hippo-YAP, Notch and WNT/β-catenin signaling pathways in mediating IEC dedifferentiation and proliferation. Also included is the crosstalk between mTORC1 signaling with several mediators of inflammation (NFkB, STAT3 and NLRP3) which escalate the process of tumorigenesis in proliferative epithelium.
mTORC1 mediates intestinal epithelium renewal and regeneration following insult or injury
mTORC1 participates actively in the orderly renewal of epithelial cells from ISC and thus in intestinal homeostasis. By stimulating protein synthesis, mTORC1 supports ISC growth and division into progenitor cells of secretory cells (e.g., Paneth cells) and nutrient absorptive enterocytes. When intestinal mTOR gene is disrupted, mice exhibit small intestine shortening and epithelial morphology alterations [53]. mTORC1 also controls intestinal adaptation, a program deployed to compensate for the loss of cells during small bowel resection and maintain an adequate crypts-to-villi ratio [54]. The mTORC1 signaling is induced in the intestinal epithelium of mice subjected to various types of stress, such as DSS-initiated colitis, irradiation exposure, or ulcer healing, and contributes to epithelium repair post-injury [49, 53–57]. Tissue repair is mediated by reparative inflammation, epithelial restitution, and cellular proliferation, which may act individually or in coordination [26]. Reparative inflammation is orchestrated by cytokines, such as TGFβ, which drive epithelial restitution [24]. The Erk/mTOR signaling contributes to epithelial restitution by activating TGFβ-induced expression of FAK [57]. FAK is a tyrosine kinase within the focal adhesion complex; it mediates cell motility required during epithelial homeostasis and restitution [58]. FAK is activated in areas near the site of epithelial damage in mice having ulcers and in motile cells of expanding IEC-6 monolayers, suggesting that FAK helps in wound healing process post-insult/injury [59]. Blockade of Erk and mTORC1 by MAPK kinase inhibitor and rapamycin, respectively, impaired TGFβ-induced FAK protein synthesis and wound closure in IEC monolayers [57]. Following epithelial restitution, mTORC1 supports cell proliferation and prevents cell death by inhibiting apoptosis, thereby contributing to wound healing and re-establishment of epithelial barrier [18, 19, 60, 61].
Contribution of intestinal mTORC1 to epithelium renewal under amino acid-rich and amino acid-deficient conditions
Amino acids stimulate mTORC1 signaling through lysosomal RagGTPases (which are heterodimers of RagA, RagB, RagC and RagD) and Ragulator, a pentameric complex acting as lysosomal tether [62]. The availability of amino acids primes mTORC1 for activation by its translocation from cytosol to the lysosomal surface via GTP-bound Rag heterodimer. Rag heterodimers interact with Ragulator, which enables tethering of mTORC1 to lysosomes [63]. The lysosomal localization of mTORC1, places mTORC1 in the close proximity of Rheb, a GTP binding protein and activator of mTORC1 signaling [63]. Although the interplay between mTORC1 and amino acids has been described, the contribution of their interaction in intestinal epithelium renewal post-insult/injury is not well understood.
Amino acids, such as glutamine, effectively enhance enterocyte growth in intestinal porcine epithelial cells (IPEC-1) in an mTORC1-dependent manner [20]. Similarly, arginine and lysine induce mTORC1 signaling in rat IEC [64]. One mechanism by which dietary amino acids promote epithelial renewal and regeneration is through mTORC1’s coordination of polyamine synthesis [65]. Polyamines can be synthesized by decarboxylation of amino acids (proline, methionine, arginine and ornithine) [66–68] and utilized as energy source by actively dividing IEC [67]. The activity of ornithine decarboxylase (ODC), a rate-limiting enzyme in polyamine synthesis, is significantly increased in intestinal cells undergoing recovery and regeneration [67, 69]. Its inhibition depleted cellular polyamine stores and significantly reduced the rate of healing in IEC recovering from insults [69]. In this context, mTORC1 bolstered polyamine synthesis and cell proliferation by stimulating the translation of ODC mRNA [65]. Intestinal polyamine synthesis is also likely regulated by mTORC1-independent pathways since rapamycin partially inhibited cellular proliferation and ODC mRNA translation in IEC-6 cells [65].
Furthermore, treatment with amino acid-based oral rehydration solution (AA-ORS), consisting of valine, threonine, serine, tryptophan and tyrosine enhanced the number of actively proliferating crypt cells thus contributing to the restoration of adequate crypt/villus ratio in intestine of mice subjected to lethal dose of radiation [7]. AA-ORS administration increased the expression of cryptic stem cell marker Lgr5 and induced epithelial MAPK/PI3K signaling (p-Erk and p-Akt) thereby supporting the proliferation and migration of stem cells, increasing the number of functionally mature differentiated epithelial cells in villi, improving glucose absorption by enterocytes, and supporting the curtailment of apoptosis in irradiated intestines [7]. Consistent observations were made upon exogenous glucagon-like peptide 2 (GLP-2) treatment [70]. GLP-2 is secreted by enteroendocrine L-cells of the distal small intestine and proximal colon [71]. It promotes lysine transport in murine small intestine through mTORC1 [72]. GLP-2 treatment protected against intestinal mucosal atrophy by activating the EGFR/Erk signaling, which participates in epithelial cell renewal, and restoration of crypt depth and villus height [70]. Although mTORC1 activity markers were not determined in these studies, EGFR/Erk/mTORC1 and Akt/mTORC1 connections have been documented [73]. Moreover, there is evidence that Glp2r−/− mice exhibit impaired activation of mTORC1 signaling in response to oral protein supplementation in mice [72], suggesting a link between GLP2R and mTORC1 signaling.
Amino acid-mediated activation of intestinal mTORC1 participates in maintaining epithelial barrier function, which becomes disrupted in the event of exogenous microbial infection or dysbiosis [19, 74]. Barrier disruption stems from an inflammatory reaction carried out mainly by mucosal immune cells, which are attracted to the site of insult by chemokines released by IEC that have been colonized or invaded [74]. Infectious agents such as Pseudomonas entomophila repressed translation and consequently epithelial renewal by downregulation of mTORC1 signaling in drosophila gut [5]. Conversely, glutamate-mediated activation of mTOR signaling enhanced the renewal of mucosal epithelium (increase in villus/crypt ratio) and expression of tight junction protein (claudin-1), increased the expression of markers of enterocyte differentiation (sucrase, maltase and lactase), and inhibited markers of epithelial inflammation (TLR4, MyD88, IRAK1, TRAF6 and NOD2) in intestinal epithelium of LPS-challenged pigs [18].
There is evidence that mTORC1 inhibition protects from the detrimental effects of NEAA (non-essential amino acids) deprivation on the epithelial barrier function in IPEC-1 cells [75]. NEAA deprivation compromised transepithelial resistance, lowered the expression of tight junction proteins (claudin-1 and ZO-1) and thus increased epithelial permeability. In this context, rapamycin ameliorated NEAA-induced barrier dysfunction through protective autophagy, which counteracted the apoptotic events responsible for compromising the epithelial barrier function [75].
Role of intestinal mTORC1 in microbiota-mediated epithelium homeostasis
Commensal microbiota and their metabolites are key extrinsic factors regulating epithelial turnover and contributing to intestinal homeostasis. The comparison of intestinal homeostasis in germ-free versus conventional mice revealed that commensal gut bacteria are critically important for maintaining intestinal stem cell niche. Germ-free mice exhibit lower mitotic index, fewer dividing cells, and smaller crypt regions as compared to conventional mice [76, 77]. Conventionalized mice showed altered expression of proliferation genes (Lgr5, Gata4, Gata6 and Bmp4) in ISC [78]. This suggests that epithelium renewal is diminished in germ-free mice or antibiotic-treated specific pathogen free (SPF) mice [22, 76, 77, 79, 80]. However, treatment with metabolites of gut bacteria or the conventionalization of germ-free mice can restore epithelial turnover [22, 81]. Although mTORC1 is implicated in the regulation of crypt stem cell mitosis, the nature and extent by which the mTORC1 signaling integrates signals from commensal bacteria to promote epithelium renewal lacks clarity.
MAPK/mTORC1 signaling is involved in the restoration of epithelium renewal in antibiotic-treated SPF mice. Treatment of SPF mice with antibiotics negatively impacted Gram-positive microbiota in the gut of the treated mice leading to reduced cellular proliferation and migration along the villus [22]. This coincided with decreased cyclin D and reduced number of Ki67+ cells in the crypt region of the ileum. In addition, antibiotic treatment led to significant reduction in the activity of MAPK kinase such as p-Erk. The common substrate of MAPK and mTORC1 pathways, ribosomal S6 protein, also showed a decrease in expression, although it did not reach statistical significance. The proliferation of crypt cells and intestinal organoids was re-established (via induction of p-Erk) upon short-chain fatty acids (SCFA) supplementation [22]. Although, there is insufficient evidence to suggest that mTORC1 and SCFA coordinately support epithelium renewal, a strong link between the MAPK and mTORC1 pathways justifies further studies.
Paradoxically, SCFAs have been shown to inhibit the growth of actively dividing cryptic stem cells and colon cancer cell lines [82, 83]. Evidence showed that butyrate and propionate can lower the ATP/AMP ratio thereby upregulating AMP-activated protein kinase (AMPK), a negative regulator of mTORC1 signaling [84]. In doing so, butyrate and propionate inhibit mTOR, and induce protective autophagy in human colon cancer cells [84]. The inhibition of cell growth and proliferation-promoting kinase, such as mTOR, explains the negative effect of SCFA on the expansion of cryptic stem cells.
Dysbiotic events also disrupt the epithelial homeostasis by negatively affecting the epithelial barrier function, anti-microbial peptide and mucus secretion; ultimately leading to the induction of mucosal inflammatory response [74, 85]. Activation of mTORC1 signaling improves endotoxin-induced amelioration of epithelial barrier function and epithelial renewal by enhancing proliferation and reducing IEC death [18, 60, 61]. Additionally, mTORC1 protects epithelial layer from microbial invasion by upregulating anti-microbial peptide synthesis in Paneth cells. Knockdown of Mtor and Stat3 perturbates SCFA-mediated induction of anti-microbial peptide synthesis in Paneth cells [86]. Thus, mTORC1 activity in various IEC lineages is differentially regulated by SCFA. More studies are needed to better understand the contribution of mTORC1 in intestinal renewal in response to commensal microbiota and their metabolites.
mTORC1 hyperactivation intensifies epithelium dedifferentiation, gut inflammation, and drives tumorigenesis
Owing to its role in epithelium repair and regeneration, the unabated activation of intestinal mTORC1 brought about by repeated insult or injury to the mucosa has been linked to abnormal cell dedifferentiation, escalation of gut inflammatory response, and tumorigenesis [9, 30, 49–52, 56, 87, 88]. While the pathophysiology of neoplasm derived from actively dividing stem cells is well established, tumors of the liver and pancreas—organs lacking reservoir of stem cells and exhibiting slower turnover—suggest that neoplasia can also arise from mature epithelial cells undergoing dedifferentiation [89]. This is seen in the intestine where differentiated IEC, under stress or while harboring genetic defects, acquire stem cell-like characteristics [29, 89, 90]. The re-entry in cell-division cycle can activate these mutations and lock these cells in an irreversible state of proliferation, disposing them to become neoplastic [91]. The degree of IEC differentiation dictates the prognosis of colorectal adenocarcinoma. Hence, the 5-year survival rate is markedly lower (31%) for poorly differentiated adenocarcinoma cells as compared to that of well-differentiated adenocarcinoma cells (72%) [92]. Conversely, induction of IEC differentiation imposes a tumor suppressive effect by repressing cell proliferation, migration and invasion in colon cancer cells and murine colon tumors [93–96].
The mTORC1 pathway is activated in cells bearing genetic mutations in tumor suppressor genes such as Apc, Kras, P53 and Rb [55, 88, 97–99]. Mutations in Apc, Kras and P53 act cooperatively to progress adenomas into invasive intestine carcinomas. The progression of P53 and KRAS mutant CRC remains largely dependent on Apc mutation [100]. It stems from the robust control of Apc over cell fate in murine small intestine [32, 101, 102]. In Apc-deficient intestine, the crypt–villus structures become unidentifiable, the crypt regions appear extended, and the epithelium is repopulated with crypt-like cells [32, 102]. This is accompanied by declining expression of enterocyte markers (alkaline phosphatase and villin), goblet cell marker (Ulex europaeus agglutinin type 1), and enteroendocrine lineage marker (chromogranin) [100, 102]. The rate of proliferation and expression of Mdm2 was enhanced in pre-malignant crypt-like cells [32]. Conversely, Apc restoration stimulated IEC differentiation, which was marked by the reappearance of secretory cells and enterocytes expressing differentiation-associated markers; and resulted in regression of colorectal adenocarcinoma [100]. Loss of Apc often coincides with activation of intestinal mTORC1 [55, 97, 98]. In contrast, rapamycin repressed translation elongation in Apc mutant mice and consequently decreased polyp formation, indicating the oncogenicity of Apc mutants hinges on mTORC1 signaling [97]. The constitutive activation of mTORC1 achieved by TSC2 knockdown (repressor of mTORC1 signaling) also results in the loss of goblet and Paneth cell differentiation in vivo and in vitro [50, 103]. mTORC1 hyperactivation repressed the expression of MUC2, a marker of goblet cell differentiation [103, 104]. MUC2 downregulation correlated with poor prognosis in colon cancer patients [105]. Mechanistically, MUC2 depletion enhanced the secretion of proinflammatory cytokine IL6 by the epithelium; IL6 promotes cell proliferation and migration in colon cancer cell line [105]. This supports the role of mTORC1 hyperactivation in driving dedifferentiation-mediated tumorigenesis either alone or in coordination with mutated tumor suppressor genes, Apc, P53 and Kras.
Another implication of mTORC1-mediated IEC dedifferentiation is elevated inflammatory profile of the intestinal epithelium (Fig. 1) [50]. The rising levels of pro-inflammatory cytokines derived from dedifferentiated IEC, prompt macrophage and neutrophil infiltration from the lamina propria into the epithelium and further the inflammatory responses in intestine [49–51]. Placed under inflammatory stress, epithelial cells acquire stem cell-like properties allowing them to divide and migrate along the epithelium [6, 29, 90, 106, 107].
Recently, the constitutive activation of mTORC1 was shown to upregulate epithelial secretion of COX-2, IL6, IL1 and IL17; and consequently enhance Th17 immune cell infiltration in the epithelium [49]. mTOR promoted epithelial inflammation by physically interacting with NLRP3 inflammasome in IEC [48]. Moreover, mTORC1 and STAT-3 activation coincided with higher expression of inflammatory cytokines (IL-6, TNFα, IL12α, IFNγ and IL10) and formation of polyps in the colon of mice with colitis associated cancer (CAC) [87]. Conversely, the administration of mTORC1 inhibitors, RAD001 and Everolimus, improved epithelial inflammation, polyp and tumor formation in the colon tissue of colitis mice harboring constitutively active JAK-STAT [56] or Il10 deletion [51]. Thus, strong evidence links mTORC1 hyperactivation with IBD and inflammation-induced CRC [9, 49, 51, 56, 87, 88], suggesting mTORC1-mediated epithelial inflammation predisposes an organism to tumorigenesis. Moreover, patients with inflamed colon are at higher risk of developing colon cancer than control subjects [108, 109]. The molecular mechanisms involving intestinal mTORC1 in IEC dedifferentiation, inflammation and tumorigenesis are reviewed next.
mTORC1 drives IEC dedifferentiation, epithelium inflammation and consequent tumorigenesis through interactions with the YAP1, Notch1 and WNT proliferation-promoting pathways
Epithelial mTORC1 interacts with the YAP1, Notch1 and WNT pathways to enhance cell dedifferentiation and give rise to chronic intestinal inflammation and inflammation-mediated tumorigenesis (Fig. 2). This section describes the mechanistic basis of these interactions and their implications for intestinal tumorigenesis following epithelial dedifferentiation and inflammation.
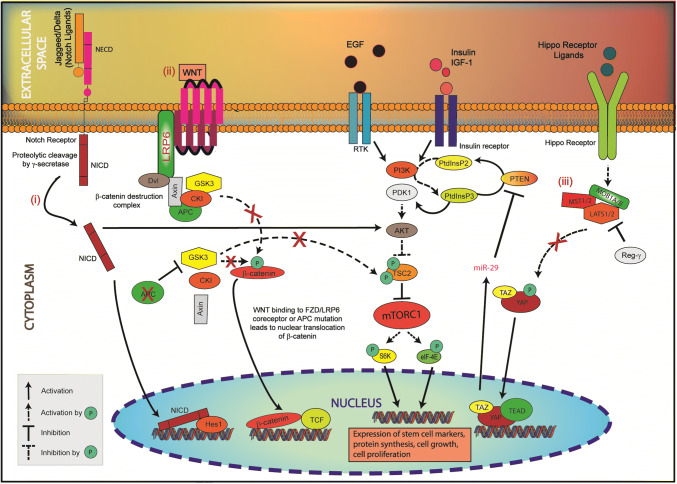
Fig. 2.
mTORC1 hyperactivation promotes IEC dedifferentiation and multiplication by integrating signals from proliferation-promoting pathways, YAP1, Notch1 and WNT. (i) In Notch1 signaling, NICD activates AKT/mTORC1. (ii) Upon WNT binding to FZD/LRP6 coreceptor or APC mutation, the kinase activity of GSK3 is impaired. As a result, GSK3 is unable to phosphorylate TSC2 (Ser1337, Ser1341), thus TSC2 is destabilized and cannot repress mTORC1 signaling. (iii) When LATS1/2 is degraded by Regγ or when MST/LATS kinases are inhibited, YAP/TAZ is not phosphorylated, and thus is active and able to translocate to the nucleus. Nuclear YAP1 stimulates the PI3K/AKT/mTORC1 signaling via miR-29-mediated degradation of PTEN. These interactions stimulate mTORC1, which induces the expression of stem cell markers, upregulates protein synthesis, promotes cell growth and cell proliferation causing the dedifferentiation of IEC
YAP1 and mTORC1
YAP1 is an oncogene conserved from yeast to humans and crucial component of the Hippo pathway [110]. Signal transduction through the Hippo pathway leads to the phosphorylation-dependent activation of various kinases including MST1/2 (mammalian STE20-like serine/threonine kinase1/2). Once phosphorylated, MST1/2 associates with hMOB1 (human Mps one binder1) and LATS1/2 (large tumor suppressor serine/threonine kinase1/2) forming a complex that phosphorylates and in turn represses proto-oncogenes YAP1 (Yes associated protein 1) and TAZ (transcription co-activator with PDZ-binding motif) (Fig. 2) [43, 111].
YAP1 protein, whose expression is mostly restricted to intestinal crypts, facilitates epithelium renewal and repair by promoting the proliferation of stem cells residing at the base of the crypts [31]. However, epithelial YAP1 is associated with reduced number of mature goblet and Paneth cells in the small intestine and conversely with enhanced growth of undifferentiated progenitor cells. This is because YAP1 overexpression not only promotes the expression of stem cell markers in cryptic stem cells but also in otherwise differentiated intestinal epithelium causing IEC to dedifferentiate and proliferate [31, 112]. As a result, mice with YAP1 overexpression exhibit markedly lower intestinal alkaline phosphatase activity and overall poor tissue differentiation in YAP1-positive villi [112]. In contrast, differentiated enterocytes, goblet and Paneth cells reappeared once YAP1 protein expression returned to baseline in these mice.
As shown in Fig. 2, YAP1 stimulates mTORC1 signaling through miR-29-mediated downregulation of PTEN protein; PTEN negatively regulates the PI3K/mTORC1 signaling [113]. Indeed, YAP1 plays a rate-limiting role in PI3K/mTORC1-mediated cellular proliferation and tissue growth [114, 115]. In addition, YAP1 positively regulated cell proliferation and mTORC1 activity by increasing the expression of amino acid transporters, SLC38A1 and SLC7A5, in hepatocellular carcinoma (HCC) tumors [115].
Conversely, mTORC1 stimulates YAP1. Constitutive activation of mTORC1, following the deletion of tuberous sclerosis complex 2 (TSC2, a conserved inhibitor of mTORC1), led to YAP1-mediated aberrant proliferation and survival of TSC2 deficient cells [116]. These cells had high levels of YAP1, in part, due to the repression of autophagy by mTORC1. The inhibition of mTORC1 restored autophagy which in turn led to autophagy-mediated YAP1 degradation, repressed cell proliferation and enhanced apoptosis [116]. The inhibition of mTORC1 by rapamycin also led to the regression of YAP1-positive HCC tumors in mice with constitutive YAP1 expression [115]. This shows that YAP1-mediated tumorigenesis is, in part, regulated by mTORC1. Overall, YAP1 and mTORC1 co-regulate cell size, tissue development and hyperplasia [113].
The sustained activation of YAP1 and mTORC1 is associated with intestinal inflammation and cancer. Abnormally high YAP1 expression is observed in colon biopsies of Crohn’s disease (CD) patients and colitis mice treated with DSS and TNBS (2,4,6-trinitrobenzenesulfonic acid) [44, 117, 118]. Apc, one of the most commonly mutated gene in CRC, leads to the activation of YAP1 [28] and mTORC1 [55, 97] and in turn results in the loss of differentiated secretory/enterocyte cells, aberrant IEC proliferation, and intestinal tumorigenesis [27, 31, 32, 56, 100]. However, different research groups reported that the inhibition of YAP1 or mTORC1 in Apc mutant mice can help mitigate intestinal cancer by decreasing the number and multiplicity of tumors, and by restoration of differentiated cells in the epithelium [27, 28, 31, 35, 50].
Evidence indicates that YAP1 regulates the pro-inflammatory NFkB signaling. Indeed, repressors of YAP1, such as LATS1/2, suppress inflammation by downregulating NFkB expression. Mechanistically, LATS2 competes with TAK1 for interaction with IKKβ thereby blocking the formation of active TAK1-IKKβ complex and the phosphorylation of transcription-competent NFkB p65 [119]. Consequently, LATS2-mediated inhibition of YAP1 and NFkB signaling downregulates the expression of pro-inflammatory cytokines and their tumorigenic effects. However, the protection afforded by LATS1 lessens when the 11S REGγ, which is overexpressed in the gut of CRC individuals, degrades LATS1 via the proteasome. In the end, high expression of REGγ, YAP1 and NFkB concomitantly occurs in CRC and correlates with a low survival rate in CRC patients [120].
Intestinal YAP1 and mTORC1 are known to mediate the effects of IL-6 family of cytokines on gut homeostasis. Recent study revealed that transgenic mice with mutated epithelial gp130 receptor (IL-6 family cytokine receptor) harboring constitutively active JAK-STAT signaling exhibit aberrant IEC proliferation characterized by deeper crypts, and loss of differentiation marked by low alkaline phosphatase activity and low number of secretory cells [27, 28]. The colonic mucosa also exhibited immune cell infiltration and elevated gene expression of pro-inflammatory cytokines. In these mice, the inhibition of YAP1 or Notch signaling, but not MEK or PI3K/mTORC1 signaling, lessened IEC proliferation and restored the abundance of secretory cells. This suggests that YAP1 and Notch1 are upstream drivers of IL-6 mediated intestinal dysfunction [27, 28]. However, Thiem et al. showed that the targeted inhibition of mTORC1 was sufficient to reduce the number, size and multiplicity of inflammation-derived gastrointestinal tumors in mice with gp130 constitutive activation [56]. The discrepancy may stem from the different approaches adopted to attain constitutive activation of gp130-JAK-STAT3 signaling in the two mouse models. In frame deletion of S187-Y190 amino acids in IL6ST gene encoding gp130 coreceptor was used by Taniguchi et al.; whereas, Thiem et al. used knock-in mutation to disrupt the negative feedback loop mediated by SOCS3 to hyperactivate gp130 receptor-dependent STAT3 signaling in transgenic mice [27, 28, 56, 121].
In a co-culture model of colon epithelial cells and monocytes designed to study the effects of a pro-inflammatory microenvironment on tumor growth, Yen et al. found that the differentiation of monocytes to pro-inflammatory M2 macrophages was hindered if YAP1 was silenced in colon epithelial cells [122]. YAP1-silenced colon epithelial HCT-116 and DLD-1 cells were also less responsive to IL-6. The silencing of YAP1 in these colonocytes led to the downregulation of oncogenic markers (GTPase KRAS and mTOR), pro-M2 polarization markers (IL-4 and IL-13) and mesenchymal marker (vimentin) [122]. Furthermore, key characteristics of tumorigenesis such as cell migration, invasion and colony formation were weakened in cells subjected to YAP1 silencing. Other approaches aimed at lowering inflammation-derived tumorigenesis through the inhibition of YAP1 have been described, such as the use of phytochemical extract or microRNA technology [122]. For instance, YAP1, mTOR and β-catenin inhibition by Ovatodiolide, a bioactive phytochemical purified from Indian catmint (Anisomeles indica), reduced tumor growth in mouse xenograft model of CT26 mouse colon cancer cells [122]. CRC and colitis were alleviated in CRC cells, colon of CRC xenograft mouse model and TNBS-induced colitis mice by miR-590-5P-mediated degradation of YAP1 upon transfection of mi-590-5P mimics to CRC cells and colitis mice [118, 123]. Paradoxically, miR-590-5P was shown to activate PI3K/AKT/mTORC1 via degradation of PTEN and thus to promote cell proliferation [124].This shows that, in certain situations, YAP1 and mTORC1 function independently of each other. It is also worth mentioning that even though overexpression of YAP1 and mTORC1 is associated with colitis and CRC, the processes of intestinal regeneration and tissue repair largely depend on their expression [27, 28, 35, 51, 112, 120], thus their constitutive inhibition in intestinal tissue will interfere with post-insult/injury regeneration of the intestine [42, 111]. However, the targeted inhibition of YAP1 and mTORC1 in tumors is a conceivable strategy to treat YAP1/mTORC1 mediated inflammation and tumors without interfering with tissue regeneration and repair.
Notch1 and mTORC1
The Notch signaling plays a central role in determining the fate of intestinal epithelial cells [33], in particular, it contributes to expanding the pool of progenitor cells while inhibiting cell differentiation and lineage commitment [34]. Ligands, such as Delta and Jagged, activate the Notch signaling by catalyzing a series of proteolytic cleavages in the Notch receptor thereby liberating the Notch intracellular domain (NICD), which goes on to induce the transcription of various Notch target genes including HES1 and C-MYC (Fig. 2). The simultaneous activation of Notch and mTORC1 signaling has been shown to block the differentiation and function of goblet and Paneth cells in the intestinal epithelium [103]. The exact crosstalk between mTORC1 and Notch signaling needs to be elucidated. However, NICD overexpression or stimulation has been shown to activate the Akt–mTORC1 signaling by an unknown mechanism (Fig. 2) [125–127]. Conversely, mTORC1 stimulates the Notch signaling. Specifically, the expression of Notch transactivator NICD and Hes1, a Notch-specific transcription factor [40], were induced upon Tsc2 inactivation [103]. In contrast, rapamycin inhibited mTORC1 and Notch1 signaling in the intestinal epithelium of Tsc2 mutant transgenic mice. This resulted in attenuation of mTORC1–Notch1 induced dedifferentiation of goblet and Paneth cells in murine intestine, and higher count of MUC2 and lysozyme expressing cells in these mice [103]. Thus, the downregulation of mTORC1 and Notch1 activity is associated with the differentiation of intestinal goblet and Paneth cells [103]. Moreover, downregulation of MUC2 positively correlated with elevated IL6 proinflammatory signaling in human CRC cells, ultimately promoting colon cancer metastasis [105]. Therefore, mTORC1/Notch1-mediated IEC dedifferentiation and inflammation contribute to progression of colon cancer.
Induction of colitis is associated with an increase in the expression of HES1, which blocks the expression of goblet cell lineage commitment marker, MATH1 and HATH1, in mouse and human intestine, respectively [128, 129]. Decrease in HATH1 expression and goblet cell count were observed in the gut of ulcerative colitis (UC) patients. This effect was attributed to the aberrant expression of HES1 and disappearance of causal type homeobox 2 (CDX2), a gene associated with IEC differentiation [129]. Blocking Notch signaling in the initial phase of colitis by dibenzazepine (DBZ) prevented the reduction in goblet cells. This initial observation was supported by the upregulation of goblet cell markers, Math1 and Muc2, in the epithelium of DSS/DBZ-treated mice [128]. Moreover, the expression of pro-inflammatory cytokines (IL1β, IL6) and metalloproteinases (MMP)-3 and 9 were reduced in DSS/DBZ-treated versus DSS only-treated mice. Overall, inhibition of Notch signaling enabled colon healing and inhibited intestinal inflammation by preserving goblet cell differentiation in DSS-induced colitis [128].
Currently, there is no evidence to suggest that mTORC1 hyperactivation inhibits MATH1 or HATH1; however, rapamycin inhibits HES1, and HES1 inhibits HATH1 [103, 128, 129]. Therefore, it is expected that mTORC1 and Notch signaling promote inflammation by downregulating goblet cell differentiation and mucin secretion, in part, via suppression of MATH1/HATH1. It is worth mentioning that the expression levels of HES1 and HATH1 vary depending upon the severity and type of gut inflammatory disorder [130]. Biopsies from IBD colons revealed that HATH1 expression were higher in CD patients than UC patients [130]. Gersemann et al. reported that HATH1 expression was positively correlated with that of HES1, MUC1, MUC2 and MUC4 in control and IBD patients [130]. This is explained by the compensatory mechanism intended to induce the differentiation of goblet cells to overcome continuous loss of IEC in the inflamed colon of CD and UC patients. However, goblet cells exhibit defective differentiation under inflammatory conditions ultimately rendering the compensatory mechanism futile.
Regarding GI health, growing evidence indicates that cell signaling between mTORC1 and Notch1 is implicated in intestinal inflammation and inflammation-mediated CRC. Notch1 overexpression drives the activation of mTORC1 signaling in colon cancer cell lines [127]. In contrast, the mTORC1/2 inhibitor, Torin1, downregulated Notch1 and other markers of proliferation and stemness (Ki67, DLL1, DLL4, Lgr5, and CD44), which collectively slowed down the growth of CRC xenografts in nude mice [131]. Studies in CRC cell lines indicated that apoptosis is a possible mechanism by which the simultaneous inhibition of Notch1 and mTORC1 signaling leads to cancer regression [127, 131]. Furthermore, curtailment of Notch1/2/3 and target gene Hey1 also led to regression in growth and migration of colon cancer cells. Downregulation of Notch signaling was accompanied by increased apoptosis of cancer cells. Inhibition of Notch signaling led to re-expression of tumor suppressor miR-200 family, which were previously downregulated in colon cancer cells [132].
Although increased expression of colonic mTORC1, Notch1, Jagged1 and NICD was observed in DSS and TNBS models of acute colitis, Notch1 also functions independently of mTORC1 [37, 42, 87, 128, 133, 134]. DSS treatment led to the activation of Notch1 in a transgenic mouse model of claudin-1 (Cldn1) overexpression, associated with IEC dedifferentiation and colorectal cancer. Upon DSS treatment, activation of Notch1 signaling resulted in the decline of MUC2 expression and loss of goblet cell differentiation in Cldn1 transgenic mice [135]. These events intensified the susceptibility of the transgenic mice to develop inflammation-driven colon cancer [135]. Similarly, activation of Notch signaling coincided with the loss of goblet cell function in jejunum and colon; and reduced Paneth cells in jejunum in mice [136]. The defective lineage commitment disrupted the epithelial barrier function, which in turn caused inflammatory markers to rise in the intestinal mucosa [136]. Notch signaling is also induced by inflammatory factors acting through Toll-like receptor 5 (TLR5) resulting in activation of NFkB signaling and consequent IL-6 synthesis. In this context, inhibition of the Notch signaling ameliorated IL-6 production and improved disease features, such as colon length, mouse body weight, and colitis histological markers [37].
However, long-term inhibition of the Notch signaling can disrupt intestinal homeostasis. Studies in azoxymethane (AOM) + DSS-treated mice showed that Notch1 inhibition drives CAC by inhibiting the expression of MMP-9. MMP-9 has been shown to positively control the expression of cell cycle regulators (p53, Bax-1, p21WAF1/Cip1) in mice intestine [134, 137]. Consequently, Notch1 inhibition by γ-secretase increased the number of polyps and tumors, infiltration of neutrophils, and dysplasia in colon tissue of DSS-treated mice [134, 137].
WNT and mTORC1
The WNT/β-catenin pathway is one of the main signaling pathways regulating the proliferation and differentiation of IEC [32, 55, 97, 138]. The pathway is upregulated in undifferentiated and dividing cells. High expression of WNT and β-catenin, but low expression of alkaline phosphatase, are observed in M2 macrophage cocultured Caco-2 and mucosa of UC patients [139, 140]. mTORC1, which is positioned downstream of WNT/β-catenin, is active in the colon of UC patients [141]. WNT signaling is negatively regulated by APC, a gene encoding an essential scaffolding protein of β-catenin destruction complex. The β-catenin destruction complex directs β-catenin toward proteasomal degradation thereby preventing its nuclear translocation. In contrast, loss-of-function mutation in APC, as seen in most cases of intestinal cancer, disrupts the formation of the β-catenin destruction complex and thus stabilizes β-catenin (Fig. 2) [101]. This aids in the formation of β-catenin/TCF complex in the nucleus, and in turn, in the constitutive transcription of WNT target genes involved in cell dedifferentiation and proliferation, such as C-MYC and CYCLIN D1 [32, 100, 101]. Constitutive activation of WNT signaling also promotes inflammation due to signal transduction through the WNT, JAK/STAT and NFkB pathways [29, 142–144]. The combination of WNT-driven dedifferentiation and inflammation has been shown to lower the abundance of absorptive/secretory IEC and drive tumorigenesis [29].
β-Catenin/TCF expression negatively correlates with the degree of differentiation and promoter activity of gene markers of cell differentiation, such as alkaline phosphatase and fatty acid-binding protein in Caco-2 cells [143]. The expression of differentiation-inducing gene CDX2 was induced upon inhibition of WNT/β-catenin and restoration of wild-type APC in CRC cells [138]. Germline deletion of Tcf4 in transgenic mice provoked the differentiation of stem cells in intestinal crypts and decline of dividing cells in the gut [142]. In contrast, the constitutive expression of β-catenin/TCF promoted the rapid expansion of cryptic stem cells in mice [29, 142]. In all, the evidence shows that the WNT/β-catenin/TCF pathway supports the proliferation of stem cells while repressing their differentiation [29, 143].
The expression of WNT target genes, C-MYC and CYCLIN D1, is upregulated in undifferentiated cryptic stem cells and intestinal tumors, and conversely, downregulated in differentiated IEC [29, 32, 143–145]. This suggests that constitutively activated WNT signaling promotes the dedifferentiation of epithelial cells, rendering them susceptible to tumorigenesis. Indeed, the evidence shows that β-catenin silencing led to the downregulation of crypt stem cell markers (EPHB2, LGR5 and BMP4) and upregulation of differentiation markers (MUC2, CA2 and TM4SF4) in colorectal cancer cells. Xenograft transplantation of cancer cells exhibiting doxycycline-induced β-catenin silencing showed compromised proliferation due to cell-cycle arrest and differentiation of transplanted cancer cells. However, withdrawal of doxycycline led to reactivation of β-catenin and potential dedifferentiation of transplanted cancer cells [93]. Thus, evidence substantiates tumorigenic potential of WNT signaling, which often coincides with activation of mTORC1 signaling [55, 97, 98]. Conversely, repression of WNT/mTORC1 signaling by inhibitors or by dietary bioactive compounds was shown to benefit colon cancer [146, 147].
Mechanistically, signaling through WNT leads to mTORC1 activation by preventing GSK3β-mediated stabilization of TSC2. GSK3α/β cooperate with AMPK to stabilize TSC2 via phosphorylation of Ser1341 and Ser1337 residues [148]. When WNT binds LRP6/FZD co-receptor, GSK3β is prevented from mediating the stabilization of TSC2. This may be because WNT negatively regulates the kinase activity of GSK3 bound to the β-catenin destruction complex [149]. However, in cases of Apc mutation, the β-catenin destruction complex cannot form. Polyps from Apc mutant mice show reduced GSK3 activity and repressed phosphorylation of its substrate, glycogen synthase, as compared to wild-type mice [98, 150]. Thus, Apc mutation activates mTORC1 signaling by releasing the negative control of GSK3 (stabilization of TSC2) over mTORC1 signaling (Fig. 2) [55, 97]. In these mice, mTORC1 drives cell proliferation and tumor growth. The critical role of mTORC1 was revealed by the use of rapamycin, which improved the survival of Apc mutant mice, in part, by reducing polyp formation, tumor growth, and angiogenesis [55, 97]. Rapamycin benefited these mice by inhibiting translation elongation via mTORC1/S6K/eEF2K/eEF2 axis in adenomatous cells and thus protein synthesis [35].
Intestine-specific constitutive stimulation of the WNT/β-catenin signaling is linked to the activation of NFkB, the induction of dedifferentiation, the acquisition of stem cell-like characteristics in Lgr5 negative non-stem cells, and higher mortality in mice [29]. The mechanism involves WNT, the oncogenic protein Kras, and NFkB. WNT and Kras signaling cooperate to dedifferentiate IEC into adenomatous crypt-like cells in an NFkB-dependent manner [29]. NFkB physically interacts with β-catenin and CREB-binding protein (CBP) allowing the formation of a nuclear complex with transcription factors of the TCF/LEF-1 family. This transcriptional complex supports a genetic program aiming to dedifferentiate IEC into proliferative crypt-like stem cells. However, this process was successfully halted following the inhibition of NFkB or deletion of Tnf in mice with a constitutively active WNT signaling. As a result, mouse survival improved and differentiated IEC were restored in the gut epithelium [29]. This suggests that dedifferentiated and proliferative epithelial cells undergo genetic reprograming that activates mediators of inflammation primarily in the epithelium, which further lamina propria’s inflammatory response (Fig. 1). Although the study did not assess the contribution of mTORC1 in the events leading to IEC dedifferentiation and epithelial inflammation, the interactions that exist between mTORC1, WNT and NFkB support the view that mTORC1 is stimulated downstream of WNT and NFkB signaling [55, 98, 151]. Constitutively active WNT also stimulates mTORC1 via the RAC1 signaling [152]. RAC1 signaling drives tumorigenesis secondarily to promoting cell proliferation and inflammation via activation of NFkB, mTOR and p-JNK, and the production of ROS [152, 153].
Collectively, the evidence indicates that mTORC1 supports tumorigenesis and inflammation downstream of WNT [55, 97] and gp130/JAK/STAT3 [56]. In contrast, inhibition of the gp130/JAK/STAT3 pathway prevents WNT-mediated tumor growth in Apc mutant mice and human CRC cell xenografts [144]. This suggests that blocking mTORC1 may be beneficial in mice harboring these mutations. Indeed, results showed that mTORC1 inhibition remediated the progression of WNT/APC-dependent tumors and gp130-dependent inflammation-mediated tumorigenesis in mice [55, 56, 97, 98].
mTORC1 stimulates the synthesis of pro-inflammatory cytokines and consequent tumorigenesis in IEC by activating NFkB, STAT3 and NLR
As discussed in the previous section, mTORC1 interacts with pathways promoting cell growth and proliferation to drive IEC dedifferentiation and proliferation. Further evidence shows that the constitutive activation of mTORC1 mediates the synthesis of inflammatory cytokines in dedifferentiated epithelial cells by activating the NFkB, STAT3 and NLR pathways (Fig. 3). Epithelial cells in which the Hippo/YAP, Notch and WNT/β-catenin signaling pathways are upregulated often exhibit activated NFkB, STAT3 and NLRP3 signaling, and elevated inflammatory cytokine production [27–29, 49, 122, 145, 154–156]. This section delineates the interactions between epithelial mTORC1 and inflammatory factors (NFkB, STAT3 and NLR) in the setting of gut inflammation.
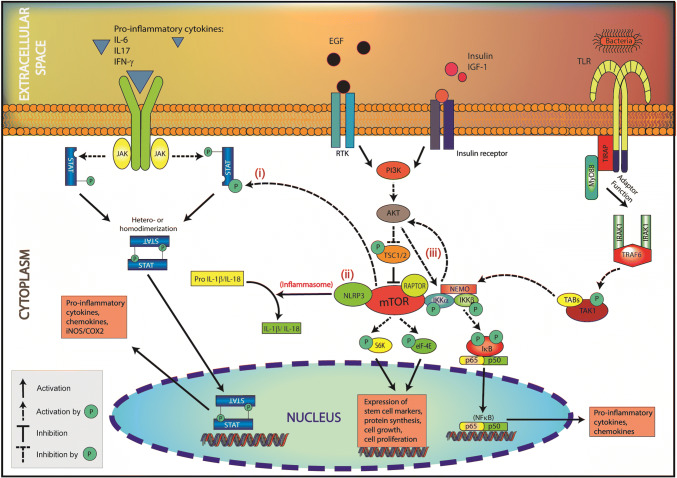
Fig. 3.
In dedifferentiated IEC, mTORC1 interacts with inflammatory mediators, NFkB, STAT3 and NLRP3, to initiate epithelial inflammation. (i) mTOR phosphorylates STAT3 (Tyr705, Ser727) causing its dimerization and nuclear translocation. (ii) mTORC1 interacts with and thus stimulates the NLRP3 inflammasome causing caspase-1-mediated activation of IL1β and IL18. (iii) mTORC1 interacts with and thus activates IKK leading to the nuclear translocation of NFkB. AKT activates NFkB signaling by phosphorylating IKK (Thr23). Activation of these pathways induces the production of pro-inflammatory cytokines by damaged IEC. In turn, the epithelial inflammation elicits an innate and adaptive immune response in the lamina propria
NFkB and mTORC1
NFkB is the quintessential pathway in states of inflammation and inflammation-mediated CRC. Activation of NFkB is observed in the colon of DSS and TNBS colitis mice, and in biopsies from inflammatory bowel disease (IBD) patients [157–159]. NFkB regulates the transcription of many genes whose products, such as cytokines, chemokines and adhesion molecules, induce cell proliferation, tissue inflammation and infiltration of macrophages and neutrophils into the epithelium [29, 52]. Markers of neutrophil infiltration (e.g., myeloperoxidase activity) and lipid peroxidation (e.g., malondialdehyde concentration) also become elevated in inflamed gut mucosa [158]. In this context, symptoms of colitis are improved upon NFkB inhibition. Several pharmaceutical agents and dietary bioactive compounds have been shown to ameliorate colitis by downregulating the NFkB signaling [158–160]. For instance, curcumin and resveratrol improve inflammation by inhibiting mTORC1 and NFkB [36, 161]. However, the nature and extent with which epithelial mTORC1 and NFkB interact to promote intestinal inflammation and tumorigenesis is not well understood.
The crosstalk between NFkB and mTORC1 is mostly mediated by AKT, an upstream positive modulator of mTORC1. AKT phosphorylates IKKα at Thr23; a critical event in the activation of NFkB signaling [162]. AKT constitutive activation as in PTEN mutant cells leads to the formation of a mTORC1–IKK complex [151]. Conversely, inhibition of the PI3K/AKT pathway by Wortmannin leads to inhibition of (Thr23) IKKα phosphorylation and, in turn, of NFkB nuclear translocation [162]. The activity of IKK is negatively affected by inhibition of mTOR, suggesting mTOR may regulate IKK in an AKT-dependent manner. While additional mechanistic studies are needed to fully understand the nature of the activation of IKK by mTOR, the possibility exists that mTOR phosphorylates specific amino acid residue(s) in the activation loop of IKK. Raptor, an essential scaffolding component of mTORC1, plays a critical role in mTOR–IKK interaction. Indeed, approaches to repress mTORC1 activity, such as Raptor knockdown and rapamycin treatment, disrupted the association between mTOR and IKK, and as a result repressed NFkB activity and expression of NFkB target genes by 50% [151]. It is worth noting that mTORC1 inhibition eases the negative feedback of p70S6K1 on the IRS/PI3K/AKT pathway [163], and in doing so stimulates the NFkB signaling in an AKT-dependent manner. Thus, mTORC1 inhibition and NFkB activation can coincide. This has been observed in monocytes and macrophages but not yet in IEC [164, 165]. Studies also reported that AKT is regulated downstream of NFkB. In this scenario, the degradation of IkBα (a NFkB repressor) and nuclear translocation of NFkB p65 subunit precede the activation of AKT (Fig. 3). Furthermore, the inhibition of AKT by PI3K/AKT inhibitors did not affect signaling through NFkB, whereas the overexpression of IkBα or the use of pharmacological inhibitors of NFkB suppressed AKT phosphorylation at Ser473 [166].
Epithelial mesenchymal transition (EMT) is the most common event in the process of tumorigenesis. Cells undergoing EMT acquire hallmark features of tumors, such as expression of stem cell markers, excessive proliferation, migration, and invasiveness upon dedifferentiation of epithelial cells into the mesenchymal phenotype [6, 106, 107, 167]. The contribution of mTORC1 and NFkB signaling in EMT and metastasis in CRC cells has been established [168, 169]. mTORC1 and mTORC2 act as upstream regulators of GTPases (RhoA and Rac1) which drive actin-mediated cytoskeleton rearrangement and cell migration [168]. NFkB signaling is also found to be activated upon Rac1 overexpression in CRC [169]. Collectively, the evidence supports the notion that the mTORC1 and NFkB signaling pathways contribute to the propagation of colon cancer. In further support, the presence of a functional mTORC1 was required for TNFα expression (a NFkB target gene) in human colorectal adenocarcinoma HT-29 cells [36]. Inhibiting mTORC1 activity in these cells with phytochemicals, curcumin and piperine, lowered TNFα mRNA levels and TNFα-induced COX-2 expression. Moreover, the co-activation of mTOR and NFkB signaling promoted epithelial cell proliferation and immune cell infiltration in mice deficient in epithelial NLRC3 tumor suppressor gene, suggesting that epithelial mTOR/NFkB interaction drives inflammation by upregulating pro-inflammatory mediators in colonic mucosa [52, 156]. Other groups validated this assertion by reporting that epithelial mTORC1 hyperactivation drove colitis by upregulating the STAT3-mediated synthesis of COX-2 and pro-inflammatory cytokines in IEC [49]. Mechanistically, STAT3 and NFkB physically interact with one another [170]. In contrast, blocking PI3K/AKT decreased STAT3 and NFkB activity in cells and mice with lymphoma [170]. Thus, the crosstalk between PI3K/STAT3/NFkB signaling explains the induction of proinflammatory cytokine synthesis upon activation of mTORC1/STAT3 signaling in the intestinal mucosa [49].
NFkB and mTORC1 pathways also counteract each other. Activation of mTORC1 is thought to trigger a compensatory mechanism that prevents the deleterious effects of inflammation on the survival of healthy IEC. Accordingly, the induction of mTORC1 by arginine protected against LPS-mediated cell death in IPEC-1 cells [61]. Upon activation of mTORC1, cell survival and growth improved due to increased protein synthesis and decreased protein degradation in arginine + LPS treated IPEC-1 cells. mTORC1 activation coincided with the curtailment of proinflammatory signaling, namely a significant decrease in the expression of TLR4 and level of phospho-Ser536 NFkB signaling was observed in arginine + LPS treated IPEC-1 cells [61]. In contrast, NFkB activity counterbalanced the detrimental consequences of mTORC1 stimulation during colitis. NFkB-mediated ROS accumulation repressed STAT3 and mTOR activity in inflamed colon of transgenic mice. The decrease in STAT3 and mTOR activity in colon was associated with lower blood levels of IL6 and G-CSF in DSS colitis mice and resulted in a macrophage polarization shift from pro-inflammatory M1 to anti-inflammatory M2 [171]. The anti-inflammatory potential of M2 macrophages was attributable to the rise in NFkB and ROS activity in macrophages and colon tissue in these mice [171].
Literature also bears evidence supporting the beneficial effects of NFkB and mTOR coactivation in intestinal cells and tissues. Activation of mTOR and NFkB can induce oxidative stress and repress colon cancer progression by inducing cancer cell apoptosis. mTOR-NFkB-mediated ROS synthesis led to cell cycle arrest in CRC cells and reduced tumor growth in CRC xenograft-implanted mice [172]. In contrast, the inhibition of mTORC1 by rapamycin repressed NFkB activity and ROS production, which led to cancer cell survival by allowing interaction of CDK2 with cyclin E, and CDK4 with cyclin D1 [172]. Of note, baseline NFkB also regulates epithelial integrity and immune homeostasis in intestinal epithelium. IKK ablation in intestinal epithelium resulted in the dysregulation of the TNFαR1-MyD88 signaling. As a result, immune cell infiltration and elevated synthesis of proinflammatory cytokines were observed in the intestinal epithelium [173]. Thus, it is critically important to evaluate the source and consequence of inflammation before targeting the NFkB pathway in treatment of intestinal inflammation. Since NFkB supports inflammatory events that contribute to tissue repair and regeneration, the long-term inhibition of intestinal epithelium NFkB may be detrimental.
STAT3 and mTORC1
STAT3 is a crucial transcription factor, activated by several cytokines including IL6, IL11 and IL22 [56, 174]. STAT3 and mTOR signaling help maintain intestinal homeostasis by promoting SCFA-induced production of antimicrobial peptides such as RegIIIγ and β-defensin 1, 3 and 4 [86]. STAT3 serves a dual role in intestinal epithelium. Its expression is required for post-injury recovery and regeneration of intestinal epithelium [41, 42]. Indeed, STAT3 facilitates epithelial repair by regulating the transcription of genes implicated in cell survival, cell proliferation, and reparative inflammation [46]. However, the constitutive expression of epithelial STAT3 is associated with the synthesis of pro-inflammatory cytokines in chronic gut inflammation [49, 51]. STAT3-driven epithelial inflammation propagates into lamina propria and induces infiltration of CD8+ lymphocytes into the epithelium while inhibiting the recruitment of anti-inflammatory regulatory T-cells (Tregs) [175]. In this way, constitutive STAT3 activation contributes to intestinal inflammation.
The activation of mTORC1 frequently coincides with the induction of STAT3 signaling in colonic epithelium. Strong evidence links constitutive epithelial STAT3 and mTORC1 signaling with intestinal inflammation [49, 51, 56]. As evidence, upregulated expression of epithelial STAT3 and mTOR proteins is observed in inflamed colon tissues collected from animal models of chronic colitis and active IBD patients [51]. Elevated expressions of mTORC1 and STAT3 are also observed in mice with UC [49]. Initial mechanistic studies showed that mTORC1 phosphorylates STAT3 at Ser727 and Tyr705 (Fig. 3) [176]. Hyperactive epithelial mTORC1 is implicated in the synthesis of pro-inflammatory cytokines through the stimulation of STAT3-dependent COX-2 signaling [49], which exacerbates the infiltration of Th17 cells in intestinal mucosa. In these mice, the inhibition of epithelial mTORC1 by Raptor knockdown reversed inflammation by downregulating STAT3 and consequently protected against colitis [49]. Similarly, treatment with metformin, a type-2 diabetes drug with anti-inflammatory properties led to the repression of colonic p-STAT3 and p-mTOR, lowered the expression of pro-inflammatory cytokines and relieved the symptoms of IBD in colitis mice [177]. Thus, intestinal inflammation can be improved by inhibiting epithelial mTORC1 and STAT3 signaling since their elevated expression contributes to intestinal inflammation.
Intestinal inflammation led by constitutively active mTORC1/STAT3 also entails intestinal tumorigenesis [51, 56]. The activation of mTORC1/STAT3 signaling by cytokines of the IL6 family via their common receptor gp130 has been shown to drive tumorigenesis in gastrointestinal epithelium [56]. Upon reception of pro-inflammatory stimuli, gp130 receptor is phosphorylated by kinases JAK1/2. This event prompts STAT3 dimerization followed by STAT3 nuclear translocation and transcription of STAT3 target genes involved in inflammation and cell-proliferation. STAT3 signaling is regulated in part by the negative feedback inhibition of SOCS3, which represses JAK1/2 and inhibits nuclear translocation of STAT3 dimer [178, 179]. Thiem et al. employed knock-in mutation to disrupt the SOCS3 negative feedback and thus activate gp130 receptor-dependent STAT3 signaling in mice to study the role of mTORC1/STAT3 in intestinal tumorigenesis [56, 121]. In these mice, the selective inhibition of mTORC1 by RAD001 led to a significant decrease in overall tumor mass; primarily reducing early tumorigenesis [56]. RAD001 showed no effect on STAT3 expression. This indicates that even when STAT3 is constitutively active, mTORC1 activity weighs heavily on inflammation-mediated tumorigenesis.
Furthermore, mTOR and STAT3 were found to be simultaneously activated in C57BL/6 mice treated with AOM + DSS to induce CAC [87]. Rapamycin treatment led to a decrease in mTORC1 and STAT3 activity in colon tissue of DSS-treated mice, which in turn ameliorated inflammation through downregulation of cytokine production (TNFα, IL6, IL12α, IL10 and IFN-γ). As a result, rapamycin treatment decreased tumor load and multiplicity in colon tissues of mice treated with AOM + DSS [87]. Repression of colonic STAT3 by rapamycin suggested that STAT3 is placed downstream of mTORC1 signaling [87]. The role of mTORC1 in mediating CAC was further substantiated by the lower abundance of Ki67+ proliferative cells in the inflamed colon upon treatment with everolimus, a rapalog that also repressed p-STAT3 expression [51]. Overall, the current evidence indicates that mTORC1 inhibition can remediate intestinal inflammation and inflammation-mediated tumorigenesis under STAT3 constitutive activation.
NLR and mTORC1
Nod like receptors (NLR) are among the many pattern recognition receptors (PPR) expressed by IECs serving to activate the innate and adaptive immune responses and maintain the intestinal homeostasis following a pathogenic insult. Each NLR is composed of three components; the central nucleotide binding domain, C-terminal leucine-rich repeat domain, and N-terminal variable domain [180]. Based on the presence of a pyrin or caspase recruitment domain in the N-terminus, NLR are divided in two broad categories, NLRP and NLRC. NLR serve as a crucial structural component of inflammasomes, multi-component complexes composed of NLRP or NLRC, an adaptor protein apoptosis-associated speck like protein (ASC), and procaspase-1 [180]. Inflammasomes can be activated by several factors such as pro-inflammatory cytokines, ATP, LPS and ROS. The inflammasome contributes to intestinal inflammation by caspase-1 mediated maturation of pro-IL1β and pro-IL18 cytokines [180].
Upon microbial invasion observed in colitis, the inflammasome components (NLRP, ASC and procaspase-1) oligomerize into active NLRP3 inflammasome. For this reason, NLRP3 expression is elevated in intestinal mucosa of CD patients and in DSS colitis mice [9, 48]. However, the activation of inflammasome can be inhibited by autophagy, a process deployed to degrade damaged macromolecules and organelles, and prevent ROS generation [180]. One plausible mechanism by which mTORC1 contributes to NLRP3 inflammasome-dependent intestinal inflammation is by opposing the activation of autophagy through the repression of Unc-51 like autophagy activating kinase (ULK1) and autophagy-related gene 13 (ATG13) [181, 182]. In contrast, stimulation of autophagy upon mTORC1 inhibition has been shown to repress NLRP3 inflammasome activation, resulting in protection against pro-inflammatory response in colitis [48].
mTOR was identified as a binding partner of NLRP3 in IEC [48], and their interaction promoted the epithelial secretion of pro-inflammatory cytokines (Fig. 3). NLRP3 and mTOR expression were concomitantly upregulated in the colon mucosa of DSS-treated mice and Il10 deficient mice [48]. In contrast, the disruption of NLRP3–mTOR interaction by HIF1α repressed NLRP3, induced autophagy, and protected against colitis via downregulation of TNFα, IL6 and IL1β expression in colon biopsies of DSS-treated mice [48]. There is no information on the regulation of IL18 by mTORC1, and thus, should be matter of further investigation.
Paradoxically, studies pointed out that activation of NLRP3 inflammasome and IL18 synthesis protected against DSS- and TNBS-colitis [183, 184]. This is conceivable since baseline NLRP3 activity helps in maintaining epithelial integrity and protects against inflammation-mediated CRC by decreasing microbial penetration into the lamina propria [183, 184]. Owing to the reduced production of IL18, mice deficient in Nlrp3 and Caspase 1 were more susceptible to colitis than wild-type mice. The susceptibility of these mice to colitis was attributable to the increased permeability of the epithelium, the infiltration of leukocytes, and the elevated production of chemokines in the colon [184]. Similarly, the colon of Il18−/− mice administered AOM + DSS showed enhanced epithelial damage, leukocyte inflammation and hyperplasia compared to AOM + DSS-treated wild-type mice [183]. Mechanistically, IL-18 afforded protection by activating IFNγ synthesis, which stimulated the expression of tumor suppressor STAT1. Expression of STAT1 was abrogated upon Nlrp3 inactivation in colon rendering the mice prone to colitis-induced tumorigenesis [183, 184]. Administration of recombinant IL18 reversed body weight loss in DSS-treated Caspase 1−/− mice. IL18 and IFNγ treatment also restored STAT1 levels in Caspase 1−/− mice [183, 184]. In sum, NLRP3 plays an important role in epithelial healing by supporting reparative inflammation. In contrast, the permanent loss of NLRP3 impairs the restoration of barrier function. Discrepancies surround the role of NLRP3–mTORC1 interaction during epithelial healing. Their interaction can be protective; however, their long-lasting interaction supports an excessive pro-inflammatory response.
NLRC3, a recently identified member of the NLR family, functions as tumor and inflammation suppressor. mTORC1 has been shown to stimulate cell proliferation and inflammation upon selective deletion of Nlrc3 in intestinal epithelium. NLRC3 acts as a negative regulator of (i) Toll-like receptor-mediated TRAF-6 signaling [185] and (ii) innate immune response upon exposure to PAMPs or viruses [156]. NLRC3 serves as an inflammation checkpoint, in part, by negatively regulating mTORC1 signaling in IEC [52]. Mechanistically, NLRC3 inhibits mTOR signaling by disrupting the interaction between the p85 and p110α subunits of PI3K, and as a result, the activity of p85 is repressed [52]. In addition, NLRC3 inhibits the mTORC1 signaling by sequestration of TRAF6, which is required for lysosomal translocation and subsequent activation of mTOR by the TRAF6-p62 complex [155, 186].
Accordingly, mTORC1 signaling is found to be activated upon intestinal knockout of Nlrc3, thereby making Nlrc3−/− mice highly susceptible to colitis and colorectal tumorigenesis [52]. Upon AOM injection, organoids collected from Nlrc3−/− mice showed higher expression of mTORC1 signaling components such as ribosomal S6 and 4EBP1. Higher mTORC1 activity translated into higher rate of proliferation in organoids derived from Nlrc3−/− mice as compared to organoids derived from wild-type mice [52]. Moreover, increased protein expression of Ki67, PCNA, c-MYC and CYCLIN D was observed in intestinal crypts of Nlrc3−/− mice, supporting enhanced proliferation of these crypt cells. The tumorigenicity of these cells was confirmed by the decreased expression of FoxO proteins, FoxO3a and FoxO1, which are recognized for their role as tumor suppressors [155]. In Nlrc3−/− mice, NFkB signaling was activated secondarily to PI3K/mTORC1 signaling. This is consistent with the ability of AKT to induce NFkB via mTORC1-IKK interaction [151]. Activation of NFkB led to higher expression of proinflammatory cytokines (IL17 and IL22) in IEC responsible for the infiltration of macrophages, natural killer cells and neutrophils into colonic epithelium of Nlrc3−/− mice. This effect was most pronounced when Nlrc3 was knocked down in the intestinal epithelium versus hematopoietic cells. In all, the contribution of mTORC1 signaling is central to intestinal inflammation upon Nlrc3 deletion in IEC [52].
Future directions: is mTORC1 inhibition a solution to epithelium dedifferentiation, inflammation and consequent tumorigenesis?
On the basis that sustained mTORC1 activity is associated with epithelium repair dysregulation, which in turn promotes unwarranted cellular dedifferentiation and inflammation in the epithelium (Fig. 1), therapeutic strategies centered on the inhibition of intestinal mTORC1 have been considered. But the long-term inhibition of mTORC1 is not without concern.
Recent evidence in experimental colitis cautions against long-term mTORC1 inhibition as it interferes with IEC renewal, induces cell death and consequently disturbs the state of homeostasis in intestinal epithelium [42]. Excessive cell death and tissue damage triggers reparative inflammation where the mucosal immunity attempts to compensate for lost cells by inducing wound healing through cell proliferation [23, 55]. However, a detrimental shift from self-limiting reparative inflammation to sustained and aggressive inflammatory response can take place when the processes involved in repair and regeneration are dysregulated [23, 55]. Indeed, inhibition of mTORC1 has been implicated in IL6-mediated uncontrolled inflammatory response which promotes unrestrained cryptic cell proliferation, unwarranted tissue regeneration and induces chromosomal instability. These factors collectively contribute to spontaneous CRC development [55]. Long-term rapamycin treatment is also associated with higher mortality rate, slow tissue regeneration and post-injury recovery in DSS-treated mice. In contrast, mTORC1 reactivation by amino acids provided protection against colitis and increased survival of Rheb−/− mice by activating IL6-induced STAT3 signaling, critical for tissue regeneration and repair [42, 174]. Henceforth, the long-term use of rapalogs can complicate the pathogenesis of the intestinal inflammation and tumorigenesis.
Treatment strategies that transiently repress an otherwise continuously elevated epithelial mTORC1 signaling without compromising the regenerative potential of the epithelium may prove beneficial to individuals with epithelial mTORC1-driven intestinal inflammation or inflammation-mediated tumorigenesis. Dietary bioactive compounds, such as resveratrol or curcumin, have been evaluated against inflammation and tumors in colon cancer cells and tissues [36, 160, 161, 187]. The fact that their absorbability across the intestinal epithelial layer is low and their metabolism in enterocytes is extensive implies that their bioactivity is comparatively limited and their inhibition of intestinal mTORC1 transient [188, 189]. Despite a growing interest in resveratrol, curcumin and other phytochemicals, the consequences of their long-term use on intestinal regeneration is currently unknown and should be matter of further studies.
Abbreviations
- AKT
Protein kinase B (PKB)
- AMPK
AMP-activated protein kinase
- ATG16L1
Autophagy related gene16L1
- ATP
Adenosine triphosphate
- BMP4
Bone morphogenetic protein 4
- CA2
Carbonic anhydrase 2
- CDK
Cyclin dependent kinases
- CDX2
Homeobox protein CDX2
- CKI
Casein kinase I
- COX2
Cyclooxygenase
- DLL
Delta like canonical notch ligand
- eEF2
Eukaryotic elongation factor 2
- eEF2K
Eukaryotic elongation factor 2 kinase
- eIF-4e
Eukaryotic translation initiation factor 4E
- EGF
Epidermal growth factor
- ERK
Extracellular signal regulated kinase
- EPHB2
Ephrin type-B receptor 2
- FAK
Focal adhesion kinase
- GATA
GATA binding protein
- G-CSF
Granulocyte-colony stimulating factor
- GSK3
Glycogen synthase kinase 3
- hATH1 and mATH1
Human and mouse atonal homolog 1
- HES1
Hairy/enhancer of split 1
- HEY1
Hairy/enhancer-of-split related with YRPW mortif 1
- IFNγ
Interferon γ
- IGF-1
Insulin growth factor 1
- IkBα
Inhibitor of kappa B
- IKK
IkBα kinase
- IRAK1
Interleukin-1 receptor associated kinase
- IRS
Insulin receptor substrate
- JAK
Janus kinase
- JNK
c-Jun N-terminal kinases
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- LEF1
Lymphoid enhancer binding factor 1
- LRP5/6
Low density lipoprotein receptor-related protein 5/6
- MEK
Mitogen activated protein/extracellular signal regulated kinase kinase
- MUC
Mucin
- MMP
Matrix metalloproteinase
- NECD
Notch extra-cellular domain
- NFkB
Nuclear factor kappa B
- NOD2
Nucleotide-binding oligomerization domain-containing protein 2
- NLRP3
Nod-like receptor family pyrin domain containing 3
- PAMP
Pathogen associated molecular pattern
- PDK1
Phosphoinositide dependent kinase 1
- PtdIns
Phosphatidylinositide
- PI3K
Phosphoinositide 3-kinase
- PTEN
Phosphatase and tensin homolog deleted on chromosome 10
- RAC1
Ras-related C3 botulinum toxin substrate 1
- REGγ
Regenerating gene gamma
- RTK
Receptor tyrosine kinase
- SIRT1
Sirtuin 1
- SOCS3
Suppressor of cytokine signaling 3
- STAT3
Signal transducer and activator of transcription 3
- TAB
TAK1 binding proteins
- TAK1
Transforming growth factor beta-activated kinase 1
- TEAD
Transcription enhancer factor TEF-1
- TCF
T cell factor transcription factor
- TLR
Toll-like receptor
- TM4SF4
Transmembrane 4 L six family member 4
- TNFαR
Tumor necrosis α receptor
- TRAF
TNFαR associated factor
Author contributions
HK conducted the literature search, drafted and revised the manuscript. RM critically reviewed and revised the manuscript.
Funding
Support was received from the Agriculture and Food Research Initiative (Award Number 2016-67017-24431) and from the USDA National Institute of Food and Agriculture (Program: Food Safety, Nutrition, and Health—A1341).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 2.Pelaseyed T, Bergstrom JH, Gustafsson JK, Ermund A, Birchenough GM, Schutte A, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 4.Noah TK, Donahue B, Shroyer NF. Intestinal development and differentiation. Exp Cell Res. 2011;317:2702–2710. doi: 10.1016/j.yexcr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakrabarti S, Liehl P, Buchon N, Lemaitre B. Infection-induced host translational blockage inhibits immune responses and epithelial renewal in the Drosophila gut. Cell Host Microbe. 2012;12:60–70. doi: 10.1016/j.chom.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 6.García-Arrarás JE, Valentín-Tirado G, Flores JE, Rosa RJ, Rivera-Cruz A, San Miguel-Ruiz JE, et al. Cell dedifferentiation and epithelial to mesenchymal transitions during intestinal regeneration in H. glaberrima. BMC Dev Biol. 2011;11:61. doi: 10.1186/1471-213X-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidyasagar LY, Reshu G, Lauren V, Astrid G, Paul O, Sadasivan V. An amino acid-based oral rehydration solution (AA-ORS) enhanced intestinal epithelial proliferation in mice exposed to radiation. Sci Rep. 2016;6:37220. doi: 10.1038/srep37220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousefi M, Nakauka-Ddamba A, Berry CT, Li N, Schoenberger J, Simeonov KP, et al. Calorie restriction governs intestinal epithelial regeneration through cell-autonomous regulation of mTORC1 in reserve stem cells. Stem Cell Rep. 2018;10:703–711. doi: 10.1016/j.stemcr.2018.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 10.Wilson GS, George J. Physical and chemical insults induce inflammation and gastrointestinal cancers. Cancer Lett. 2014;345:190–195. doi: 10.1016/j.canlet.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Yi G, Li L, Luo M, He X, Zou Z, Gu Z, et al. Heat stress induces intestinal injury through lysosome-and mitochondria-dependent pathway in vivo and in vitro. Oncotarget. 2017;8:40741–40755. doi: 10.18632/oncotarget.16580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa R, Snipe R, Kitic C, Gibson P. Systematic review: exercise-induced gastrointestinal syndrome—implications for health and intestinal disease. Aliment Pharmacol Ther. 2017;46:246–265. doi: 10.1111/apt.14157. [DOI] [PubMed] [Google Scholar]
- 13.Elamin E, Jonkers D, Juuti-Uusitalo K, van IJzendoorn S, Troost F, Duimel H, et al. Effects of ethanol and acetaldehyde on tight junction integrity: in vitro study in a three dimensional intestinal epithelial cell culture model. PLoS One. 2012;7:e35008. doi: 10.1371/journal.pone.0035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Chan R, Lu L, Shen J, Zhang L, Wu W, et al. Cigarette smoking and gastrointestinal diseases: the causal relationship and underlying molecular mechanisms. Int J Mol Med. 2014;34:372–380. doi: 10.3892/ijmm.2014.1786. [DOI] [PubMed] [Google Scholar]
- 15.Maiden L, Thjodleifsson B, Seigal A, Bjarnason II, Scott D, Birgisson S, et al. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol. 2007;5:1040–1045. doi: 10.1016/j.cgh.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld CS. Gut dysbiosis in animals due to environmental chemical exposures. Front Cell Infect Microbiol. 2017;7:396. doi: 10.3389/fcimb.2017.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earley ZM, Akhtar S, Green SJ, Naqib A, Khan O, Cannon AR, et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS One. 2015;10:e0129996. doi: 10.1371/journal.pone.0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin Q, Xu X, Wang X, Wu H, Zhu H, Hou Y, et al. Glutamate alleviates intestinal injury, maintains mTOR and suppresses TLR4 and NOD signaling pathways in weanling pigs challenged with lipopolysaccharide. Sci Rep. 2018;8:15124. doi: 10.1038/s41598-018-33345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ewaschuk JB, Murdoch GK, Johnson IR, Madsen KL, Field CJ. Glutamine supplementation improves intestinal barrier function in a weaned piglet model of Escherichia coli infection. Br J Nutr. 2011;106:870–877. doi: 10.1017/S0007114511001152. [DOI] [PubMed] [Google Scholar]
- 20.Yi D, Hou Y, Wang L, Ouyang W, Long M, Zhao D, et al. l-Glutamine enhances enterocyte growth via activation of the mTOR signaling pathway independently of AMPK. Amino Acids. 2015;47:65–78. doi: 10.1007/s00726-014-1842-8. [DOI] [PubMed] [Google Scholar]
- 21.Zhou W, Li W, Zheng X-H, Rong X, Huang L-G. Glutamine downregulates TLR-2 and TLR-4 expression and protects intestinal tract in preterm neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2014;49:1057–1063. doi: 10.1016/j.jpedsurg.2014.02.078. [DOI] [PubMed] [Google Scholar]
- 22.Park J-H, Kotani T, Konno T, Setiawan J, Kitamura Y, Imada S, et al. Promotion of intestinal epithelial cell turnover by commensal bacteria: role of short-chain fatty acids. PLoS One. 2016;11:e0156334. doi: 10.1371/journal.pone.0156334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor β. Gastroenterology. 1993;105:1323–1332. doi: 10.1016/0016-5085(93)90136-Z. [DOI] [PubMed] [Google Scholar]
- 25.Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol WJG. 2008;14:348–353. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto R, Watanabe M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J Gastroenterol. 2016;51:11–21. doi: 10.1007/s00535-015-1098-4. [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi K, Wu L-W, Grivennikov SI, De Jong PR, Lian I, Yu F-X, et al. A gp130–Src–YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taniguchi K, Moroishi T, de Jong PR, Krawczyk M, Grebbin BM, Luo H, et al. YAP–IL-6ST autoregulatory loop activated on APC loss controls colonic tumorigenesis. Proc Natl Acad Sci. 2017;114:1643–1648. doi: 10.1073/pnas.1620290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Rychahou P, Wang Q, Weiss HL, Evers BM. TSC2/mTORC1 signaling controls Paneth and goblet cell differentiation in the intestinal epithelium. Cell Death Dis. 2015;6:e1631. doi: 10.1038/cddis.2014.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 32.Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura T, Tsuchiya K, Watanabe M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol. 2007;42:705–710. doi: 10.1007/s00535-007-2087-z. [DOI] [PubMed] [Google Scholar]
- 34.Artavanis-Tsakonas SF, Mathilde H, Philippos M, Sylvie R, Daniel L. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 35.Faller WJ, Jackson TJ, Knight JR, Ridgway RA, Jamieson T, Karim SA, et al. mTORC1-mediated translational elongation limits intestinal tumour initiation and growth. Nature. 2015;517:497–500. doi: 10.1038/nature13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaur H, He B, Zhang C, Rodriguez E, Hage DS, Moreau R. Piperine potentiates curcumin-mediated repression of mTORC1 signaling in human intestinal epithelial cells: implications for the inhibition of protein synthesis and TNFalpha signaling. J Nutr Biochem. 2018;57:276–286. doi: 10.1016/j.jnutbio.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Aziz M, Ishihara S, Ansary MU, Sonoyama H, Tada Y, Oka A, et al. Crosstalk between TLR5 and Notch1 signaling in epithelial cells during intestinal inflammation. Int J Mol Med. 2018;32:1051–1062. doi: 10.3892/ijmm.2013.1501. [DOI] [PubMed] [Google Scholar]
- 38.Kapuria S, Karpac J, Biteau B, Hwangbo D, Jasper H. Notch-mediated suppression of TSC2 expression regulates cell differentiation in the Drosophila intestinal stem cell lineage. PLoS Genet. 2012;8:e1003045. doi: 10.1371/journal.pgen.1003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu Z, Kim J, Vidrich A, Sturgill TW, Cohn SM. Intestinal cell kinase, a MAP kinase-related kinase, regulates proliferation and G1 cell cycle progression of intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2009;297:G632–G640. doi: 10.1152/ajpgi.00066.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 41.Grivennikov S, Karin E, Terzic J, Mucida D, Yu G-Y, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan Y, Zhang L, Li X, Zhang X, Liu S, Gao N, et al. Repression of mammalian target of rapamycin complex 1 inhibits intestinal regeneration in acute inflammatory bowel disease models. J Immunol (Baltimore, Md: 1950) 2015;195:339–346. doi: 10.4049/jimmunol.1303356. [DOI] [PubMed] [Google Scholar]
- 43.Zhao B, Tumaneng K, Guan K-L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng F, Peng L, Li Z, Tan G, Liang E, Chen S, et al. YAP triggers the Wnt/β-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury. Cell Death Dis. 2018;9:153. doi: 10.1038/s41419-017-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res. 2011;71:7226–7237. doi: 10.1158/0008-5472.CAN-10-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, Collette C, et al. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat Genet. 2009;41:71–76. doi: 10.1038/ng.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cosin-Roger J, Simmen S, Melhem H, Atrott K, Frey-Wagner I, Hausmann M, et al. Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation. Nat Commun. 2017;8:98. doi: 10.1038/s41467-017-00213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin X, Sun Q, Zhou L, He M, Dong X, Lai M, et al. Colonic epithelial mTORC1 promotes ulcerative colitis through COX-2-mediated Th17 responses. Mucosal Immunol. 2018;11:1663–1673. doi: 10.1038/s41385-018-0018-3. [DOI] [PubMed] [Google Scholar]
- 50.Lyons J, Ghazi PC, Starchenko A, Tovaglieri A, Baldwin KR, Poulin EJ, et al. The colonic epithelium plays an active role in promoting colitis by shaping the tissue cytokine profile. PLoS Biol. 2018;16:e2002417. doi: 10.1371/journal.pbio.2002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng L, Zhou J-F, Sellers RS, Li J-F, Nguyen AV, Wang Y, et al. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am J Pathol. 2010;176:952–967. doi: 10.2353/ajpath.2010.090622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanneganti RK, Si Ming M, Malireddi RKS, Sannula K, Qifan Z, Amanda RB, et al. NLRC3 is an inhibitory sensor of PI3K–mTOR pathways in cancer. Nature. 2016;540:583. doi: 10.1038/nature20597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sampson LL, Davis AK, Grogg MW, Zheng Y. mTOR disruption causes intestinal epithelial cell defects and intestinal atrophy postinjury in mice. FASEB J. 2015;30:1263–1275. doi: 10.1096/fj.15-278606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barron L, Sun RC, Aladegbami B, Erwin CR, Warner BW, Guo J. Intestinal epithelial-specific mTORC1 activation enhances intestinal adaptation after small bowel resection. Cell Mol Gastroenterol Hepatol. 2017;3:231–244. doi: 10.1016/j.jcmgh.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandt M, Grazioso TP, Fawal MA, Tummala KS, Torres-Ruiz R, Rodriguez-Perales S, et al. mTORC1 inactivation promotes colitis-induced colorectal cancer but protects from APC loss-dependent tumorigenesis. Cell Metab. 2018;27(118–35):e8. doi: 10.1016/j.cmet.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Thiem S, Pierce TP, Palmieri M, Putoczki TL, Buchert M, Preaudet A, et al. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J Clin Invest. 2013;123:767–781. doi: 10.1172/JCI65086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suer S, Ampasala D, Walsh MF, Basson MD. Role of ERK/mTOR signaling in TGFβ-modulated focal adhesion kinase mRNA stability and protein synthesis in cultured rat IEC-6 intestinal epithelial cells. Cell Tissue Res. 2009;336:213–223. doi: 10.1007/s00441-009-0776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Owen KA, Abshire MY, Tilghman RW, Casanova JE, Bouton AH. FAK regulates intestinal epithelial cell survival and proliferation during mucosal wound healing. PLoS One. 2011;6:e23123. doi: 10.1371/journal.pone.0023123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walsh MF, Ampasala DR, Hatfield J, Vander Heide R, Suer S, Rishi AK, et al. Transforming growth factor-β stimulates intestinal epithelial focal adhesion kinase synthesis via Smad-and p38-dependent mechanisms. Am J Pathol. 2008;173:385–399. doi: 10.2353/ajpath.2008.070729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan B, Xiao H, Xiong X, Wang J, Li G, Yin Y, et al. l-arginine improves DNA synthesis in LPS-challenged enterocytes. Front Biosci (Landmark ed) 2015;20:989–1003. doi: 10.2741/4352. [DOI] [PubMed] [Google Scholar]
- 61.Tan B, Yin Y, Kong X, Li P, Li X, Gao H, et al. l-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids. 2010;38:1227–1235. doi: 10.1007/s00726-009-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014;24:400–406. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, et al. Arginine and leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med. 2004;13:537–543. [PubMed] [Google Scholar]
- 65.Seidel E, Ragan V. Inhibition by rapamycin of ornithine decarboxylase and epithelial cell proliferation in intestinal IEC-6 cells in culture. Br J Pharmacol. 1997;120:571–574. doi: 10.1038/sj.bjp.0700936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY) 2011;3:716–732. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Timmons J, Chang ET, Wang JY, Rao JN. Polyamines and gut mucosal homeostasis. J Gastrointest Digest. 2012;2:001. [PMC free article] [PubMed] [Google Scholar]
- 68.Wu G, Flynn NE, Knabe DA. Enhanced intestinal synthesis of polyamines from proline in cortisol-treated piglets. Am J Physiol Endocrinol Metab. 2000;279:E395–E402. doi: 10.1152/ajpendo.2000.279.2.E395. [DOI] [PubMed] [Google Scholar]
- 69.Wang J-Y, Johnson LR. Polyamines and ornithine decarboxylase during repair of duodenal mucosa after stress in rats. Gastroenterology. 1991;100:333–343. doi: 10.1016/0016-5085(91)90200-5. [DOI] [PubMed] [Google Scholar]
- 70.Feng Y, Demehri FR, Xiao W, Tsai Y-H, Jones JC, Brindley CD, et al. Interdependency of EGF and GLP-2 signaling in attenuating mucosal atrophy in a mouse model of parenteral nutrition. Cell Mol Gastroenterol Hepatol. 2017;3:447–468. doi: 10.1016/j.jcmgh.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brubaker PL, Izzo A, Hill M, Drucker DJ. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol Endocrinol Metab. 1997;272:E1050–E1058. doi: 10.1152/ajpendo.1997.272.6.E1050. [DOI] [PubMed] [Google Scholar]
- 72.Lee J, Koehler J, Yusta B, Bahrami J, Matthews D, Rafii M, et al. Enteroendocrine-derived glucagon-like peptide-2 controls intestinal amino acid transport. Mol Metab. 2017;6:245–255. doi: 10.1016/j.molmet.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rossi O, van Baarlen P, Wells JM. Host-recognition of pathogens and commensals in the mammalian intestine. Curr Top Microbiol Immunol. 2011;358:291–321. doi: 10.1007/82_2011_191. [DOI] [PubMed] [Google Scholar]
- 75.Yang Y, Li W, Sun Y, Han F, Hu C-AA, Wu Z. Amino acid deprivation disrupts barrier function and induces protective autophagy in intestinal porcine epithelial cells. Amino acids. 2015;47:2177–2184. doi: 10.1007/s00726-014-1844-6. [DOI] [PubMed] [Google Scholar]
- 76.Nowacki M. Cell proliferation in colonic crypts of germ-free and conventional mice—preliminary report. Folia Histochem Cytobiol. 1993;31:77–81. [PubMed] [Google Scholar]
- 77.Alam M, Midtvedt T, Uribe A. Differential cell kinetics in the ileum and colon of germfree rats. Scand J Gastroenterol. 1994;29:445–451. doi: 10.3109/00365529409096836. [DOI] [PubMed] [Google Scholar]
- 78.Peck BC, Mah AT, Pitman WA, Ding S, Lund PK, Sethupathy P. Functional transcriptomics in diverse intestinal epithelial cell types reveals robust microRNA sensitivity in intestinal stem cells to microbial status. J Biol Chem. 2017;292:2586–2600. doi: 10.1074/jbc.M116.770099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hooper LV, Gordon JI. Commensal host–bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 80.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 81.Khoury KA, Floch MH, Hersh T. Small intestinal mucosal cell proliferation and bacterial flora in the conventionalization of the germfree mouse. J Exp Med. 1969;130:659–670. doi: 10.1084/jem.130.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, et al. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell. 2016;165:1708–1720. doi: 10.1016/j.cell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J, Yi M, Zha L, Chen S, Li Z, Li C, et al. Sodium butyrate induces endoplasmic reticulum stress and autophagy in colorectal cells: implications for apoptosis. PLoS One. 2016;11:e0147218. doi: 10.1371/journal.pone.0147218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang Y, Chen Y, Jiang H, Nie D. Short-chain fatty acids induced autophagy serves as an adaptive strategy for retarding mitochondria-mediated apoptotic cell death. Cell Death Differ. 2011;18:602–618. doi: 10.1038/cdd.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martini E, Krug SM, Siegmund B, Neurath MF, Becker C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2017;4:33–46. doi: 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao Y, Chen F, Sun M, Bilotta AJ, Yao S, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11:752–762. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He Z, He X, Chen Z, Ke J, He X, Yuan R, et al. Activation of the mTORC1 and STAT3 pathways promotes the malignant transformation of colitis in mice. Oncol Rep. 2014;32:1873–1880. doi: 10.3892/or.2014.3421. [DOI] [PubMed] [Google Scholar]
- 88.Ro S-H, Xue X, Ramakrishnan SK, Cho C-S, Namkoong S, Jang I, et al. Tumor suppressive role of sestrin2 during colitis and colon carcinogenesis. Elife. 2016;5:e12204. doi: 10.7554/eLife.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krah NM, Murtaugh LC. Differentiation and inflammation:‘Best Enemies’ in gastrointestinal carcinogenesis. Trends Cancer. 2016;2:723–735. doi: 10.1016/j.trecan.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jilkine A, Gutenkunst RN. Effect of dedifferentiation on time to mutation acquisition in stem cell-driven cancers. PLoS Comput Biol. 2014;10:e1003481. doi: 10.1371/journal.pcbi.1003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mills JC, Sansom OJ. Reserve stem cells: differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal. 2015;8:re8. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sugao Y, Yao T, Kubo C, Tsuneyoshi M. Improved prognosis of solid-type poorly differentiated colorectal adenocarcinoma: a clinicopathological and immunohistochemical study. Histopathology. 1997;31:123–133. doi: 10.1046/j.1365-2559.1997.2320843.x. [DOI] [PubMed] [Google Scholar]
- 93.Scholer-Dahirel A, Schlabach MR, Loo A, Bagdasarian L, Meyer R, Guo R, et al. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/β-catenin signaling. Proc Natl Acad Sci USA. 2011;108:17135–17140. doi: 10.1073/pnas.1104182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu H, Zhang H, Ge W, Liu X, Loera S, Chu P, et al. Secreted protein acidic and rich in cysteines-like 1 suppresses aggressiveness and predicts better survival in colorectal cancers. Clin Cancer Res. 2012;18:5438–5448. doi: 10.1158/1078-0432.CCR-12-0124. [DOI] [PubMed] [Google Scholar]
- 95.Hatano Y, Semi K, Hashimoto K, Lee MS, Hirata A, Tomita H, et al. Reducing DNA methylation suppresses colon carcinogenesis by inducing tumor cell differentiation. Carcinogenesis. 2015;36:719–729. doi: 10.1093/carcin/bgv060. [DOI] [PubMed] [Google Scholar]
- 96.Yang B, Cao L, Liu B, McCaig CD, Pu J. The transition from proliferation to differentiation in colorectal cancer is regulated by the calcium activated chloride channel A1. PLoS One. 2013;8:e60861. doi: 10.1371/journal.pone.0060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcΔ716 mice. Proc Natl Acad Sci. 2008;105:13544–13549. doi: 10.1073/pnas.0800041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Valvezan AJ, Huang J, Lengner CJ, Pack M, Klein PS. Oncogenic mutations in adenomatous polyposis coli (Apc) activate mechanistic target of rapamycin complex 1 (mTORC1) in mice and zebrafish. Dis Models Mech. 2014;7:63–71. doi: 10.1242/dmm.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang W, Ding M-L, Zhang J-N, Qiu J-R, Shen Y-H, Ding X-Y, et al. mTORC1 maintains the tumorigenicity of SSEA-4 + high-grade osteosarcoma. Sci Rep. 2015;5:9604. doi: 10.1038/srep09604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dow LE, O’Rourke KP, Simon J, Tschaharganeh DF, van Es JH, Clevers H, et al. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell. 2015;161:1539–1552. doi: 10.1016/j.cell.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schneikert J, Behrens J. The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut. 2007;56:417–425. doi: 10.1136/gut.2006.093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–1451. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 103.Zhou Y, Rychahou P, Wang Q, Weiss HL, Evers BM. TSC2/mTORC1 signaling controls Paneth and goblet cell differentiation in the intestinal epithelium. Cell Death Dis. 2015;6:e1631. doi: 10.1038/cddis.2014.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou Y, Wang Q, Guo Z, Weiss HL, Evers BM. Nuclear factor of activated T-cell c3 inhibition of mammalian target of rapamycin signaling through induction of regulated in development and DNA damage response 1 in human intestinal cells. Mol Biol Cell. 2012;23:2963–2972. doi: 10.1091/mbc.e12-01-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsu H-P, Lai M-D, Lee J-C, Yen M-C, Weng T-Y, Chen W-C, et al. Mucin 2 silencing promotes colon cancer metastasis through interleukin-6 signaling. Sci Rep. 2017;7:5823. doi: 10.1038/s41598-017-04952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gavert N, Ben-Ze’ev A. Epithelial–mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;14:199–209. doi: 10.1016/j.molmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 107.Friedmann-Morvinski D, Verma IM. Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO Rep. 2014;15:244–253. doi: 10.1002/embr.201338254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karvellas CJ, Fedorak RN, Hanson J, Wong CK. Increased risk of colorectal cancer in ulcerative colitis patients diagnosed after 40 years of age. Can J Gastroenterol Hepatol. 2007;21:443–446. doi: 10.1155/2007/136406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsianos EV. Risk of cancer in inflammatory bowel disease (IBD) Eur J Intern Med. 2000;11:75–78. doi: 10.1016/S0953-6205(00)00061-3. [DOI] [PubMed] [Google Scholar]
- 110.Hergovich A, Stegert MR, Schmitz D, Hemmings BA. NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol. 2006;7:253–264. doi: 10.1038/nrm1891. [DOI] [PubMed] [Google Scholar]
- 111.Cai J, Zhang N, Zheng Y, De Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 113.Tumaneng K, Schlegelmilch K, Russell R, Yimlamai D, Basnet H, Mahadevan N, et al. YAP mediates crosstalk between the Hippo and PI3K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Straßburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol. 2012;367:187–196. doi: 10.1016/j.ydbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 115.Park YY, Sohn BH, Johnson RL, Kang MH, Kim SB, Shim JJ, et al. YAP1 and TAZ Activates mTORC1 pathway by regulating amino acid transporters in hepatocellular carcinoma. Hepatology. 2016;63:159–172. doi: 10.1002/hep.28223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liang N, Zhang C, Dill P, Panasyuk G, Pion D, Koka V, et al. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med. 2014;211:2249–2263. doi: 10.1084/jem.20140341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim H-B, Kim M, Park Y-S, Park I, Kim T, Yang S-Y, et al. Prostaglandin E2 activates YAP and a positive-signaling loop to promote colon regeneration after colitis but also carcinogenesis in mice. Gastroenterology. 2017;152:616–630. doi: 10.1053/j.gastro.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu M, Luo Y, Cong Z, Mu Y, Qiu Y, Zhong M. MicroRNA-590-5p inhibits intestinal inflammation by targeting YAP. J Crohns Colitis. 2018;12:993–1004. doi: 10.1093/ecco-jcc/jjy046. [DOI] [PubMed] [Google Scholar]
- 119.Yao F, Zhou W, Zhong C, Fang W. LATS2 inhibits the activity of NF-κ B signaling by disrupting the interaction between TAK1 and IKKβ. Tumor Biol. 2015;36:7873–7879. doi: 10.1007/s13277-015-3362-x. [DOI] [PubMed] [Google Scholar]
- 120.Wang Q, Gao X, Yu T, Yuan L, Dai J, Wang W, et al. REGγ controls Hippo signaling and reciprocal NF-κB–YAP regulation to promote colon cancer. Clin Cancer Res. 2018;24:2015–2025. doi: 10.1158/1078-0432.CCR-17-2986. [DOI] [PubMed] [Google Scholar]
- 121.Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med. 2005;11:845–852. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- 122.Huang YJ, Yang CK, Wei PL, Huynh T-T, Whang-Peng J, Meng T-C, et al. Ovatodiolide suppresses colon tumorigenesis and prevents polarization of M2 tumor-associated macrophages through YAP oncogenic pathways. J Hematol Oncol. 2017;10:60. doi: 10.1186/s13045-017-0421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ou C, Sun Z, Li X, Ren W, Qin Z, Zhang X, et al. MiR-590-5p, a density-sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Lett. 2017;399:53–63. doi: 10.1016/j.canlet.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 124.Zhang G-J, San Y-Z, Zhang H-Q, Zhang J-F, Yang Z, Yu Z-F, et al. MiR-590-5p as potential oncogenic microRNA of human colorectal cancer cells by targeting PTEN. Int J Clin Exp Pathol. 2017;10:1322–1330. [Google Scholar]
- 125.Meng RD, Shelton CC, Li Y-M, Qin L-X, Notterman D, Paty PB, et al. γ-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Can Res. 2009;69:573–582. doi: 10.1158/0008-5472.CAN-08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang X, Chen T, Zhang J, Mao Q, Li S, Xiong W, et al. Notch1 promotes glioma cell migration and invasion by stimulating β-catenin and NF-κB signaling via AKT activation. Cancer Sci. 2012;103:181–190. doi: 10.1111/j.1349-7006.2011.02154.x. [DOI] [PubMed] [Google Scholar]
- 127.Koduru S, Kumar R, Srinivasan S, Evers MB, Damodaran C. Notch-1 inhibition by Withaferin-A: a therapeutic target against colon carcinogenesis. Mol Cancer Ther. 2010;9:202–210. doi: 10.1158/1535-7163.MCT-09-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shinoda M, Shin-Ya M, Naito Y, Kishida T, Ito R, Suzuki N, et al. Early-stage blocking of Notch signaling inhibits the depletion of goblet cells in dextran sodium sulfate-induced colitis in mice. J Gastroenterol. 2010;45:608–617. doi: 10.1007/s00535-010-0210-z. [DOI] [PubMed] [Google Scholar]
- 129.Zheng X, Tsuchiya K, Okamoto R, Iwasaki M, Kano Y, Sakamoto N, et al. Suppression of hath1 gene expression directly regulated by hes1 via notch signaling is associated with goblet cell depletion in ulcerative colitis. Inflamm Bowel Dis. 2011;17:2251–2260. doi: 10.1002/ibd.21611. [DOI] [PubMed] [Google Scholar]
- 130.Gersemann M, Becker S, Kübler I, Koslowski M, Wang G, Herrlinger KR, et al. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation. 2009;77:84–94. doi: 10.1016/j.diff.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 131.Francipane MG, Lagasse E. Selective targeting of human colon cancer stem-like cells by the mTOR inhibitor Torin-1. Oncotarget. 2013;4:1948–1962. doi: 10.18632/oncotarget.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Suliman MA, Zhang Z, Na H, Ribeiro AL, Zhang Y, Niang B, et al. Niclosamide inhibits colon cancer progression through downregulation of the Notch pathway and upregulation of the tumor suppressor miR-200 family. Int J Mol Med. 2016;38:776–784. doi: 10.3892/ijmm.2016.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dahan S, Rabinowitz KM, Martin AP, Berin MC, Unkeless JC, Mayer L. Notch-1 signaling regulates intestinal epithelial barrier function, through interaction with CD4 + T cells, in mice and humans. Gastroenterology. 2011;140:550–559. doi: 10.1053/j.gastro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garg P, Jeppsson S, Dalmasso G, Ghaleb AM, McConnell BB, Yang VW, et al. Notch1 regulates the effects of matrix metalloproteinase-9 on colitis-associated cancer in mice. Gastroenterology. 2011;141:1381–1392. doi: 10.1053/j.gastro.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pope JL, Bhat AA, Sharma A, Ahmad R, Krishnan M, Washington MK, et al. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut. 2014;63:622–634. doi: 10.1136/gutjnl-2012-304241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Turgeon N, Blais M, Gagne JM, Tardif V, Boudreau F, Perreault N, et al. HDAC1 and HDAC2 restrain the intestinal inflammatory response by regulating intestinal epithelial cell differentiation. PLoS One. 2013;8:e73785. doi: 10.1371/journal.pone.0073785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Garg P, Jeppsson S, Yang VW, Gewirtz AT, Merlin D, Sitaraman SV. MMP-9 mediates colitis associated cancer in mice through Notch-1 via p53 activation O-21. Inflamm Bowel Dis. 2011;17:S9. doi: 10.1097/00054725-201112002-00023. [DOI] [Google Scholar]
- 138.Da Costa LT, He T-C, Yu J, Sparks AB, Morin PJ, Polyak K, et al. CDX2 is mutated in a colorectal cancer with normal APC/β-catenin signaling. Oncogene. 1999;18:5010–5014. doi: 10.1038/sj.onc.1202872. [DOI] [PubMed] [Google Scholar]
- 139.Cosín-Roger J, Ortiz-Masiá D, Calatayud S, Hernández C, Álvarez A, Hinojosa J, et al. M2 macrophages activate WNT signaling pathway in epithelial cells: relevance in ulcerative colitis. PLoS One. 2013;8:e78128. doi: 10.1371/journal.pone.0078128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mikami T, Mitomi H, Hara A, Yanagisawa N, Yoshida T, Tsuruta O, et al. Decreased expression of CD44, alpha-catenin, and deleted colon carcinoma and altered expression of beta-catenin in ulcerative colitis-associated dysplasia and carcinoma, as compared with sporadic colon neoplasms. Cancer Interdiscip Int J Am Cancer Soc. 2000;89:733–740. doi: 10.1002/1097-0142(20000815)89:4<733::aid-cncr3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 141.Khare V, Dammann K, Asboth M, Krnjic A, Jambrich M, Gasche C. Overexpression of PAK1 promotes cell survival in inflammatory bowel diseases and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:287–296. doi: 10.1097/MIB.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 143.Mariadason JM, Bordonaro M, Aslam F, Shi L, Kuraguchi M, Velcich A, et al. Down-regulation of β-catenin TCF signaling is linked to colonic epithelial cell differentiation. Can Res. 2001;61:3465–3471. [PubMed] [Google Scholar]
- 144.Phesse TJ, Buchert M, Stuart E, Flanagan DJ, Faux M, Afshar-Sterle S, et al. Partial inhibition of gp130-Jak-Stat3 signaling prevents Wnt–β-catenin–mediated intestinal tumor growth and regeneration. Sci Signal. 2014;7:ra92. doi: 10.1126/scisignal.2005411. [DOI] [PubMed] [Google Scholar]
- 145.Xing Y, Chen X, Cao Y, Huang J, Xie X, Wei Y. Expression of Wnt and Notch signaling pathways in inflammatory bowel disease treated with mesenchymal stem cell transplantation: evaluation in a rat model. Stem Cell Res Ther. 2015;6:101. doi: 10.1186/s13287-015-0092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wee LH, Morad NA, Aan GJ, Makpol S, Ngah WZW, Yusof YAM. Mechanism of chemoprevention against colon cancer cells using combined Gelam honey and Ginger extract via mTOR and Wnt/β-catenin pathways. Asian Pac J Cancer Prev. 2015;16:6549–6556. doi: 10.7314/APJCP.2015.16.15.6549. [DOI] [PubMed] [Google Scholar]
- 147.Mashima T, Taneda Y, Jang M-K, Mizutani A, Muramatsu Y, Yoshida H, et al. mTOR signaling mediates resistance to tankyrase inhibitors in Wnt-driven colorectal cancer. Oncotarget. 2017;8:47902–47915. doi: 10.18632/oncotarget.18146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 149.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Valvezan AJ, Zhang F, Diehl JA, Klein PS. Adenomatous polyposis coli (APC) regulates multiple signaling pathways by enhancing glycogen synthase kinase-3 (GSK-3) activity. J Biol Chem. 2012;287:3823–3832. doi: 10.1074/jbc.M111.323337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP-Y, Baldwin AS. Akt-dependent regulation of NF-κB is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Myant KB, Cammareri P, McGhee EJ, Ridgway RA, Huels DJ, Cordero JB, et al. ROS production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 2013;12:761–773. doi: 10.1016/j.stem.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ellenbroek SI, Collard JG. Rho GTPases: functions and association with cancer. Clin Exp Metas. 2007;24:657–672. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 154.Huang C-F, Chen L, Li Y-C, Wu L, Yu G-T, Zhang W-F, et al. NLRP3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36:116. doi: 10.1186/s13046-017-0589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Karki R, Malireddi RS, Zhu Q, Kanneganti T-D. NLRC3 regulates cellular proliferation and apoptosis to attenuate the development of colorectal cancer. Cell Cycle. 2017;16:1243–1251. doi: 10.1080/15384101.2017.1317414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang L, Mo J, Swanson KV, Wen H, Petrucelli A, Gregory SM, et al. NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity. 2014;40:329–341. doi: 10.1016/j.immuni.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Andresen L, Jørgensen V, Perner A, Hansen A, Eugen-Olsen J, Rask-Madsen J. Activation of nuclear factor κB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut. 2005;54:503–509. doi: 10.1136/gut.2003.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kazi HA, Qian Z. Crocetin reduces TNBS-induced experimental colitis in mice by downregulation of NFkB. Saudi J Gastroenterol. 2009;15:181–187. doi: 10.4103/1319-3767.54750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sánchez-Fidalgo S, Villegas I, Rosillo MÁ, Aparicio-Soto M, de la Lastra CA. Dietary squalene supplementation improves DSS-induced acute colitis by downregulating p38 MAPK and NFkB signaling pathways. Mol Nutr Food Res. 2015;59:284–292. doi: 10.1002/mnfr.201400518. [DOI] [PubMed] [Google Scholar]
- 160.Lubbad A, Oriowo M, Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem. 2009;322:127–135. doi: 10.1007/s11010-008-9949-4. [DOI] [PubMed] [Google Scholar]
- 161.Zhu X, Liu Q, Wang M, Liang M, Yang X, Xu X, et al. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS One. 2011;6:e27081. doi: 10.1371/journal.pone.0027081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine–threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 163.Rozengurt E, Soares HP, Sinnet-Smith J. Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: an unintended consequence leading to drug resistance. Mol Cancer Ther. 2014;13:2477–2488. doi: 10.1158/1535-7163.MCT-14-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Byles V, Covarrubias AJ, Ben-Sahra I, Lamming DW, Sabatini DM, Manning BD, et al. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834. doi: 10.1038/ncomms3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 166.Meng F, Liu L, Chin PC, D’Mello SR. Akt is a downstream target of NF-κB. J Biol Chem. 2002;277:29674–29680. doi: 10.1074/jbc.M112464200. [DOI] [PubMed] [Google Scholar]
- 167.Cao H, Xu E, Liu H, Wan L, Lai M. Epithelial–mesenchymal transition in colorectal cancer metastasis: a system review. Pathol Res Pract. 2015;211:557–569. doi: 10.1016/j.prp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 168.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Matos P, Jordan P. Rac1, but not Rac1B, stimulates RelB-mediated gene transcription in colorectal cancer cells. J Biol Chem. 2006;281:13724–13732. doi: 10.1074/jbc.M513243200. [DOI] [PubMed] [Google Scholar]
- 170.Han S-S, Yun H, Son D-J, Tompkins VS, Peng L, Chung S-T, et al. NF-κB/STAT3/PI3K signaling crosstalk in iMyc Eμ B lymphoma. Mol Cancer. 2010;9:97. doi: 10.1186/1476-4598-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Formentini L, Santacatterina F, de Arenas CN, Stamatakis K, López-Martínez D, Logan A, et al. Mitochondrial ROS production protects the intestine from inflammation through functional M2 macrophage polarization. Cell Rep. 2017;19:1202–1213. doi: 10.1016/j.celrep.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 172.Lu C-C, Huang W-S, Lee K-F, Lee K-C, Hsieh M-C, Huang C-Y, et al. Inhibitory effect of Erinacines A on the growth of DLD-1 colorectal cancer cells is induced by generation of reactive oxygen species and activation of p70S6K and p21. J Funct Foods. 2016;21:474–484. doi: 10.1016/j.jff.2015.12.031. [DOI] [Google Scholar]
- 173.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 174.Nguyen PM, Putoczki TL, Ernst M. STAT3-Activating Cytokines: a therapeutic opportunity for inflammatory bowel disease? J Interferon Cytokine Res. 2015;35:340–350. doi: 10.1089/jir.2014.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nguyen AV, Wu YY, Liu Q, Wang D, Nguyen S, Loh R, et al. STAT3 in epithelial cells regulates inflammation and tumor progression to malignant state in Colon1. Neoplasia. 2013;15:998–1008. doi: 10.1593/neo.13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee SY, Lee SH, Yang EJ, Kim EK, Kim JK, Shin DY, et al. Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the between Th17/Treg balance. PLoS One. 2015;10:e0135858. doi: 10.1371/journal.pone.0135858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Croker BA, Krebs DL, Zhang J-G, Wormald S, Willson TA, Stanley EG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 179.Heinrich PC, Behrmann I, Serge H, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/bj20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Takahama M, Akira S, Saitoh T. Autophagy limits activation of the inflammasomes. Immunol Rev. 2018;281:62–73. doi: 10.1111/imr.12613. [DOI] [PubMed] [Google Scholar]
- 181.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, et al. Nutrient-dependent mTORC1 association with the ULK1–Atg13–FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.e08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ganley IG, Lam DH, Wang J, Ding X, Chen S, Jiang X. ULK1·ATG13·FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti T-D. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol. 2010;185:4912–4920. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti T-D. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schneider M, Zimmermann AG, Roberts RA, Zhang L, Swanson KV, Wen H, et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat Immunol. 2012;13:823–831. doi: 10.1038/ni.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol Cell. 2013;51:283–296. doi: 10.1016/j.molcel.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park D, Jeong H, Lee MN, Koh A, Kwon O, Yang YR, et al. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci Rep. 2016;6:21772. doi: 10.1038/srep21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 189.Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]