Abstract
The PPARγ coactivator 1α (PGC-1α) is a transcriptional regulator of mitochondrial biogenesis and oxidative metabolism. Recent studies have highlighted a fundamental role of PGC-1α in promoting breast cancer progression and metastasis, but the physiological role of this coactivator in the development of mammary glands is still unknown. First, we show that PGC-1α is highly expressed during puberty and involution, but nearly disappeared in pregnancy and lactation. Then, taking advantage of a newly generated transgenic mouse model with a stable and specific overexpression of PGC-1α in mammary glands, we demonstrate that the re-expression of this coactivator during the lactation stage leads to a precocious regression of the mammary glands. Thus, we propose that PGC-1α action is non-essential during pregnancy and lactation, whereas it is indispensable during involution. The rapid preadipocyte–adipocyte transition, together with an increased rate of apoptosis promotes a premature mammary glands involution that cause lactation defects and pup growth retardation. Overall, we provide new insights in the comprehension of female reproductive cycles and lactation deficiency, thus opening new roads for mothers that cannot breastfeed.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03160-y) contains supplementary material, which is available to authorized users.
Keywords: Mammary glands, Nuclear receptor, Coactivator, Development, Adipocytes, Involution
Introduction
Development of the mammary gland is initiated during embryogenesis, although it is not until the onset of puberty that ductal elongation takes place and fat becomes filled with a system of epithelial ducts. The mammary glands undergo substantial changes in morphology during reproduction, being one of the most active metabolic tissues. In response to pregnancy, mammary epithelial cells develop into mature alveoli in concert with de-differentiation of the adipocytes of the fat-pad [1]. Parturition induces copious milk production in response to diminished level of progesterone resulting from placenta loss and increased secretion of prolactin by pituitary gland [2]. During lactation, the mammary glands display a high synthesis of triglycerides, utilizing both fatty acids obtained from bloodstream, as well as by de novo lipogenesis [3], thus providing sufficient energy to fulfil the developmental demands of the offspring. In humans, the lactating mammary glands secrete 800 mL of milk per day containing almost 32 g of fat [4]. The mammary glands of a lactating mouse secrete 5 mL of milk per day containing 30% of lipids, accounting for approximately 30 g of milk lipids over the course of 20 days, equivalent to the entire body weight of the mother [5].
Upon weaning, milk accumulation induces apoptosis of the secretory epithelial cells and their shedding in the lumen. This first phase of involution is reversible and lactation can recommence if suckling is resumed within 48 h. If the gland remains unemptied for an extended period of time, the mammary gland undergoes changes to regenerate the fat pad, characterized by tissue remodelling and re-differentiation of adipocytes [1, 6].
Until recently, the leading hypothesis of mammary gland development during pregnancy involved transdifferentiating subcutaneous white adipocytes into “pink adipocytes”, the mammary gland alveolar epithelial cells responsible for the milk production and secretion [7]. Upon interruption of lactation, pink adipocytes transdifferentiate into brown adipocytes, establishing a pregnancy–lactation adipocyte-to-epithelium-to-adipocyte circle [7, 8]. Notably, this hypothesis has been recently challenged by the new evidence showing that over multiple pregnancies, a cycle of adipocytes de-differentiation into preadipocyte and fibroblast-like cells during pregnancy and lactation occurred, and de-differentiated fibroblasts proliferated and re-differentiated into adipocytes upon weaning [9].
The peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC-1α) is a transcriptional coactivator, playing a central role in metabolism. Together with the other family members, PGC-1β and PRC, PGC-1α promotes mitochondrial biogenesis and respiration [10, 11]. Typically expressed at low levels under normal conditions, PGC-1α is highly expressed in tissues with high oxidative capacity, such as brown adipose tissue during thermogenesis [12], skeletal muscle in fiber type switching [13] and in liver during fasting to promote fatty acids β-oxidation and gluconeogenesis [14, 15]. Despite recent progress in understanding PGC-1α contribution to mammalian development [13, 16–18], the role of this coactivator in mammary glands is still not well defined. Indeed, although different studies have pointed out a crucial role for PGC-1α in promoting breast cancer tumor growth and metastasis [19–21], its physiological role in mammary glands development remains fairly unexplored.
In this study, we investigate the contribution of PGC-1α coactivator in mammary glands development, taking advantage of a newly generated in vivo mouse model in which PGC-1α is selectively and specifically overexpressed in mammary tissue. The constitutive PGC-1α overexpression throughout all the stages of mammary glands evolution resulted in a precocious involution, characterized by re-differentiation into adipocytes and promotion of apoptosis, which ultimately mediated lactation and growth retardation defects.
Materials and methods
Animals
The mmtvPGC1α transgenic mice were generated by injecting the mmtv-SV40-PGC1α plasmid digested with HpaI into the pronuclei of the fertilized eggs of the FVB/N mice. To generate the mmtv-SV40-PGC1α, first hPGC1α (2.4 kb) fragment was generated by PCR from pcDNA4-His-PGC1α plasmid (Addgene, USA). Then the fragment was subcloned at the HindIII and EcoRI restriction sites downstream from the mmtv promoter region of the mmtv-SV40-Bssk plasmid (Addgene, USA). Mice carrying the transgene were identified by PCR of genomic DNA to confirm the presence of an hPGC1α coding sequence. Kidney, uterus, ovary, salivary glands, hypophysis and heart of transgenic mice were dissected to evaluate the specific mammary glands expression of transgene under the mmtv promoter control. For mammary gland analyses during pregnancy, lactation and involution, 8–16-week-old nulliparous female mmtvPGC-1α and littermates’ mice were bred to wild-type FVB/N male mice. For weight gaining-pups experiments, on day 1 from birth litter size was normalized to seven pups. For foster mother experiments, two pairs of mmtvPGC1α and control wild-type female mice were bred with wild-type FVB/N male mice and delivered pups on the same day were used. On P2, the litter size was normalized to seven pups. Five pups were switched between the mmtvPGC1α mother and the wild-type mother, and two pups were left with the original mother as controls. The fostered pups and original pups were distinguished by ear punching. For milk analysis, milk was isolated from mammary tissue at day 10 of lactation after cervical dislocation of the mice. While pressure was applied to the tissue, the milk was harvested using a Pasteur pipette put onto the nipple. Milk was then transferred into a microcentrifuge tube and stored at − 80 °C.
All mice were housed with a standard diet provided ad libitum and examined daily. Genotyping was performed using DNA extracted from tail biopsies of 4-week-old pups, and new breeding harems of 8-week-old mice were established to expand the population. The ethics committee of the University of Bari approved this experimental setup, which was also certified by the Italian Ministry of Health in accordance with internationally accepted guidelines for animal care.
Whole mount and histological analysis
For whole mount analysis the first inguinal gland were dissected at the indicated ages and were spread on a glass slide. After fixation with Carnoy’s fixative for 2–4 h, the tissues were hydrated and stained in Carmine alum overnight as previously described [22]. Samples were then dehydrated, cleared with xylene and mounted.
For histology and immunohistochemistry, inguinal mammary glands were dissected and fixed in 10% (vol/vol) formalin for 24 h, dehydrated and paraffin embedded and then 5 μm-thick sections were obtained. Standard histology (H&E staining) and immunohistochemical procedures were performed. Briefly, samples were treated with 3% (vol/vol) hydrogen peroxide for 5 min to quench endogenous peroxidase and were subjected to antigen retrieval by boiling the slides in an antigen-unmasking solution (Vector Laboratories, California, USA) for 20 min according to the manufacturer’s instructions. Sections were sequentially incubated for 1 h at room temperature in 50% (vol/vol) non immune serum (from the host animal in which the secondary antibody was raised) in PBS (to avoid unspecific signal) and overnight at 4 °C with primary antibodies rabbit peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) (in-house antibody), uncoupling protein 1 (UCP1, ab10983, Abcam, Cambridge, UK) and cytochrome c oxidase 1 (COX1, LS-C343872, LifeSpan Biosciences, Washington, USA). Sections were washed for 10 min in PBS and incubated for 30 min at room temperature with the secondary biotinylated antibody (Vector Laboratories, California, USA). After three 5-min washing steps with PBS, sections were incubated with the avidin–biotin complex (Vector Laboratories, California, USA) for 30 min at room temperature. After washing in PBS, the peroxidase reaction was developed by incubation with 3,3-diaminobenzidine (Sigma-Aldrich, Missouri, USA). Counterstaining was carried out with methylene blue (Sigma-Aldrich, Missouri, USA). For negative controls, the primary antibodies were replaced by 1% non-immune serum in PBS.
For immunofluorescence, 4 μm-thick mammary gland sections were double stained with primary antibody for Wheat Germ Agglutinin, Alexa Fluor 488 Conjugate (WGA, W11261, Thermo Fisher Scientific, Massachusetts, USA) and Adipophilin, Alexa Fluor 594 conjugate (ADFP, ab206356, Abcam, Cambridge, UK). Specifically, the slides were incubated whit 10% goat serum for 1 h at room temperature. Slides were incubated overnight at 4 °C with WGA 5 µl/ml and ADFP 1:50 mix. All sections were counterstained with TO-PRO-3 (Thermo Fisher Scientific, Massachusetts, USA). Negative controls were prepared with irrelevant antibody. The sections were analysed using the Leica TCS SP2 (Leica, Wetzlar, Germany) confocal laser-scanning microscope.
Image processing was performed using ImageScope software (Leica, Wetzlar, Germany). For each sample, 15 representative images were taken and the percentage of stained area/total area was measured. Values from all consecutive images for each sample were averaged and displayed as mean ± SEM.
Apoptotic assay
For the TUNEL assay in vivo, tissue specimens were fixed in 10% formalin for 12–24 h, dehydrated, and paraffin embedded. Detection of apoptosis at the single-cell level based on the labelling of DNA strand breaks was performed using the in-situ cell detection kit (Roche, Basel, Switzerland) following the manufacturer’s instructions.
RNA extraction and quantitative real-time PCR analysis
Inguinal mammary glands tissues were snap frozen in liquid nitrogen and stored at − 80 °C. Total RNA was isolated by RNeasy lipid tissue kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. To avoid possible DNA contamination, RNA was treated with DNase (Thermo Fisher Scientific, Massachusetts, USA). RNA purity also was checked by spectrophotometer, and RNA integrity was checked by examination on agarose gel electrophoresis. For RT-qPCR analysis of pregnancy and lactation stages, cDNA was synthesized by retro-transcribing 1 μg of total RNA in a total volume of 100 μL utilizing the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Massachusetts, USA) according to the manufacturer’s instructions. PCR assays were performed in 96-well optical reaction plates using a Quantum5 machine (Thermo Fisher Scientific, Massachusetts, USA). PCR assays were conducted in triplicate wells for each sample. The following reaction mixture was used in each well: 10μL Power SYBR Green (Thermo Fisher Scientific, Massachusetts, USA), 2.4 μL of primers at a final concentration of 150 nM each, 1.6 μL RNase-free water, and 3 μL cDNA. The following PCR conditions were used: denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and then at 60 °C for 60 s. For RT-qPCR analysis of different developmental stages, cDNA was synthesized by utilizing the High-Capacity RNA to cDNA Kit (Thermo Fisher Scientific, Massachusetts, USA) according to the manufacturer’s instructions. PCR assays were performed in triplicate wells for each sample, in 96-well optical reaction plates using a Quantum5 machine (Thermo Fisher Scientific, Massachusetts, USA). PCR assays were conducted utilizing TaqMan Gene Expression Assays and TaqMan Universal PCR Master Mix with UNG following the manufacturer’s instructions. The following PCR conditions were used: incubation at 50 °C for 2 min, denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, and then at 60 °C for 60 s. For all the experiments, quantitative normalization of cDNA in each sample was performed using TBP mRNA as an internal control. Relative quantification was done using the ΔΔCT method.
Gel electrophoresis
For immunoblot analysis, tissue samples were homogenized in RIPA buffer supplemented with protease and phosphatase inhibitors. Equal amount of protein lysates (50 µg), quantified using Bradford-based assay (BioRad Laboratories, Hercules, CA, USA), were denatured with Laemmli sample buffer, separated by 10% or 12.5% SDS-PAGE and then transferred onto a nitrocellulose membrane (Protran, Whatman). Membranes were blocked with 5% bovine serum albumin in Tris-buffered saline/0.01% Tween 20 and probed with specific antibodies against PGC-1α (in-house antibody) or UCP1 (ab10983, Abcam, Cambridge, UK). Nuclear encoded β-actin (ab8229, Abcam, Cambridge, UK) were used as loading control where possible. Membranes were finally incubated with horseradish peroxidase–conjugated secondary antibodies and developed with a chemiluminescent reagent (Bio-Rad Laboratories, Hercules, CA, USA).
For milk quality analyses, equivalent amounts of protein from milk collected on day 10 of lactation from each mouse genotype were analysed by 12% SDS-PAGE. Post electrophoresis, the gel was fixed, stained with Coomassie Brilliant Blue, destained and kept in preservation solution.
Fatty acids analysis
Fatty acids were analysed as fatty acid methyl esters (FAMEs). The milk was transmethylated with 1 mL of BF3 in methanol (1:20, v/v) for 60 min at 80 °C, evaporated to dryness, and the FAMEs extracted with hexane/water (3:1). The organic phase was evaporated to dryness and dissolved in 50 µL ethyl acetate. One microliter of FAMEs was analysed by gas–liquid chromatography on a 5890 Hewlett-Packard system (Hewlett-Packard, Palo Alto, CA, USA) using a Famewax fused-silica capillary column (30 m, 0.32 mm internal diameter, 0.25 mm film thickness; Restek, Belfast, UK). The oven temperature was programmed from 110 to 220 °C at a rate of 2 °C/min, with hydrogen as the carrier gas (0.5 bar). The injector and detector were at 225 and 245 °C, respectively. Heptadecanoic acid (Sigma) was used as an external standard for fatty acid methyl esters.
Statistical analysis
All results are expressed as mean ± SEM. Data distribution and gene expression statistical analysis were performed with GraphPad Prism software (v5.0; GraphPad Software Inc., La Jolla, CA, USA). Comparisons of two groups were performed using Mann–Whitney U test. Comparison of four groups were performed using Kruskal–Wallis test. A value of p < 0.05 was considered as statistically significant.
Results
PGC-1α is highly expressed in mammary gland during puberty and involution
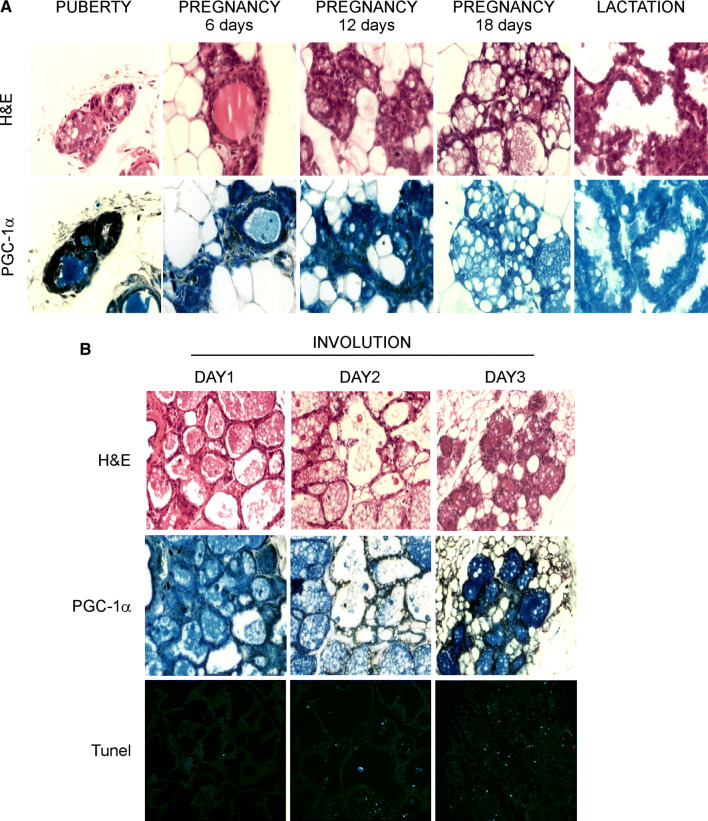
As the expression and localization of coactivator PGC-1α in the mammary gland is still unknown, we first investigated the expression of endogenous PGC-1α throughout the stages of mammary glands development. Immunohistochemistry analysis on wild type female mice revealed that PGC-1α is expressed in normal mammary epithelium, being upregulated during puberty and within the first 12 days of pregnancy. In contrast, PGC-1α expression starts to decrease by the 18th day of pregnancy and is wholly absent during lactation (Fig. 1a).
Fig. 1.
Dynamic expression of PGC-1α protein levels during mammary glands development and involution. Highly expressed during puberty and in the first 12 days of pregnancy, the PGC-1α levels slightly disappear in the last days of pregnancy and during lactation. On the first day after cessation of lactation, PGC-1α starts to be re-expressed, reaching its steady state on day 3 of involution. a Staining of mammary glands sections from WT mice at different development stages with H&E (upper panel) and PGC-1α immunohistochemistry (lower panel) (n = 3 mice per stage; magnification ×200). b Mammary glands prepared from WT mice on the indicated days of involution and stained for H&E, PGC-1α immunohistochemistry, and TUNEL (n = 3 mice per stage; magnification ×100)
At weaning, involution of mammary glands occurs. This process takes place just after suckling interruption and it is associated with programmed cell death of secretory epithelium cells, redevelopment of mammary adipose tissue together with a remodelling of the lobular-alveolar structure [23]. Histological analysis on mammary glands on the first days after weaning displayed ductal structure surrounded by adipocytes. Moreover, as involution proceeded, we observed an increase in PGC-1α expression, which becomes extremely evident on the 3rd day (Fig. 1b). The involuted mammary glands displayed PGC-1α expression similar to that of virgin mammary glands (i.e., puberty), with prominent nuclear PGC-1α staining, surrounded by increasing accumulation of adipocytes. Concomitantly to the PGC-1α gain expression, TUNEL analysis showed a high apoptosis rate on day 3, thus suggesting that the high levels of PGC-1α actively correlates with the induction of apoptosis (Fig. 1b).
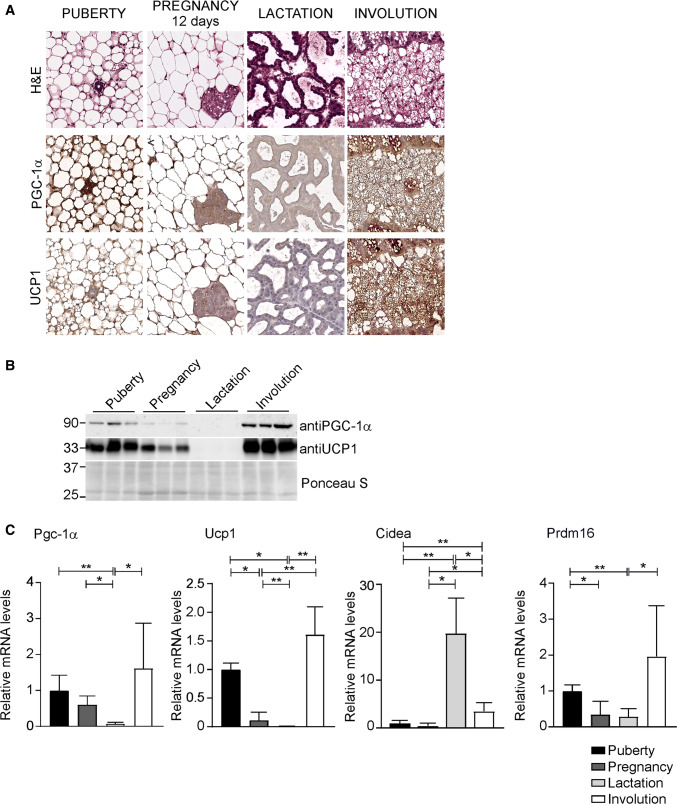
Intriguingly, the re-expression of PGC-1α during the involution stage is associated with browning markers induction. In particular, the expression of uncoupling protein 1 (Ucp1) and PR domain containing 16 (Prdm16) nearly disappeared during pregnancy and lactation, but they are promptly induced during involution (Fig. 2a–c). Differently, cell death-inducing DNA fragmentation factor alpha-like effector A (Cidea) levels are low during puberty and pregnancy, but importantly increased throughout the lactation stage. At involution, Cidea expression is turned down, but still significantly induced compared to puberty and pregnancy (Fig. 2c). Indeed, beside its expression in brown and beige adipocytes in mouse, Cidea is also an essential transcriptional coactivator capable of regulating mammary gland secretion of milk lipids [24–26].
Fig. 2.
PGC-1α expression in mammary glands is associated with UCP1 induction. a Staining of mammary glands sections from WT mice at different development stages with H&E (upper panel) and PGC-1α (middle panel) and UCP1 immunohistochemistry (lower panel) (n = 5 mice per stage; magnification ×200). b Western blot analysis of PGC-1α and UCP1 on mammary glands samples throughout different developmental stages. Ponceau stain was used as loading control. c Relative expression of PGC-1α and fat-browning related genes, Ucp1, Cidea and Prdm16, at different stages of mammary glands development evaluated by real time qPCR. TBP was used as housekeeping gene. Comparison of different groups (n = 6, 7) was performed using Kruskal–Wallis test. Data are expressed as mean ± SEM (*p < 0.05; **p < 0.01)
The dynamic changes observed in PGC-1α expression during the mammary gland development suggest that this coactivator is dispensable during lactation, but its induction is crucial during involution.
Maternal PGC-1α overexpression in mammary glands results in growth retardation defects
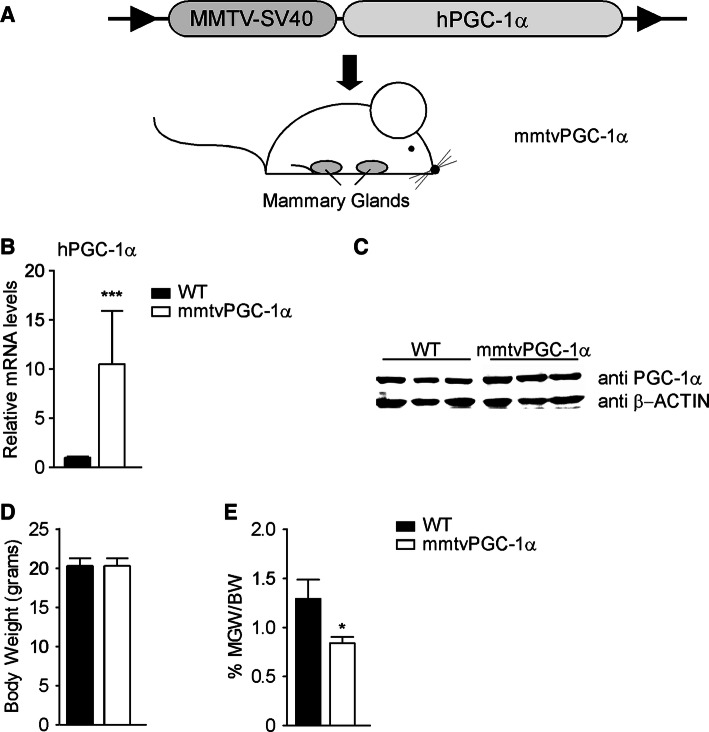
To dissect the role of PGC-1α in mammary glands development, we generated a transgenic mouse model with mammary specific gain of function of PGC-1α (mmtvPGC-1α). To this end, the sequence coding for human PGC-1α was subcloned to the mmtv promoter, thereby allowing a tissue-specific expression of hPGC-1α in the mammary gland epithelium (Fig. 3a). Successful overexpression was confirmed by mRNA and protein analysis of 8-week-old virgin mice mammary glands (Fig. 3b, c). The exclusive overexpression of hPGC-1α in mammary glands was subsequently confirmed by comparison with other tissues examined by real-time qPCR (Supplementary Fig. 1A).
Fig. 3.
Generation of mouse model with stable specific overexpression of human PGC-1α in mammary glands. A transgenic mouse model, mmtvPGC-1α, was generated by cloning the human sequence of PGC-1α downstream the mmtv promoter. Analysis of 8 weeks-old wild type and mmtvPGC-1α virgin mice shows a specific PGC-1α overexpression in the mammary glands of transgenic mice. a Scheme of the mmtvPGC-1α transgenic mouse model generated: the hPGC-1α coding sequence was cloned downstream of the mmtv promoter of mmtv-SV40-Bssk plasmid and then injected into the pronuclei of the fertilized eggs of the FVB/N mice. b Relative mRNA expression of hPGC-1α in mammary glands isolated from mmtvPGC-1α and WT control mice measured by real time qPCR, using TBP as housekeeping gene and WT mice as calibrator. c Western blot analysis of PGC-1α on mammary glands samples isolated from transgenic and WT mice. d Body weight and e inguinal mammary glands weight/body weight ratio (MGW/BW) of wild type and mmtvPGC-1α mice. Results are expressed as mean ± SEM (***p < 0.001, *p < 0.05). Comparison of wild type and transgenic mice (n = 6, 7) was performed using Mann–Whitney U test
Despite similar body weight between wild type and mmtvPGC-1α mice (Fig. 3d), mammary glands harvested from 8-week-old transgenic virgin mice were significantly smaller compared to wild type ones as indicated by inguinal mammary glands weight/body weight ratio (MGW/BW) (Fig. 3e), thus suggesting that high levels of PGC-1α may disrupt a proper mammary gland development.
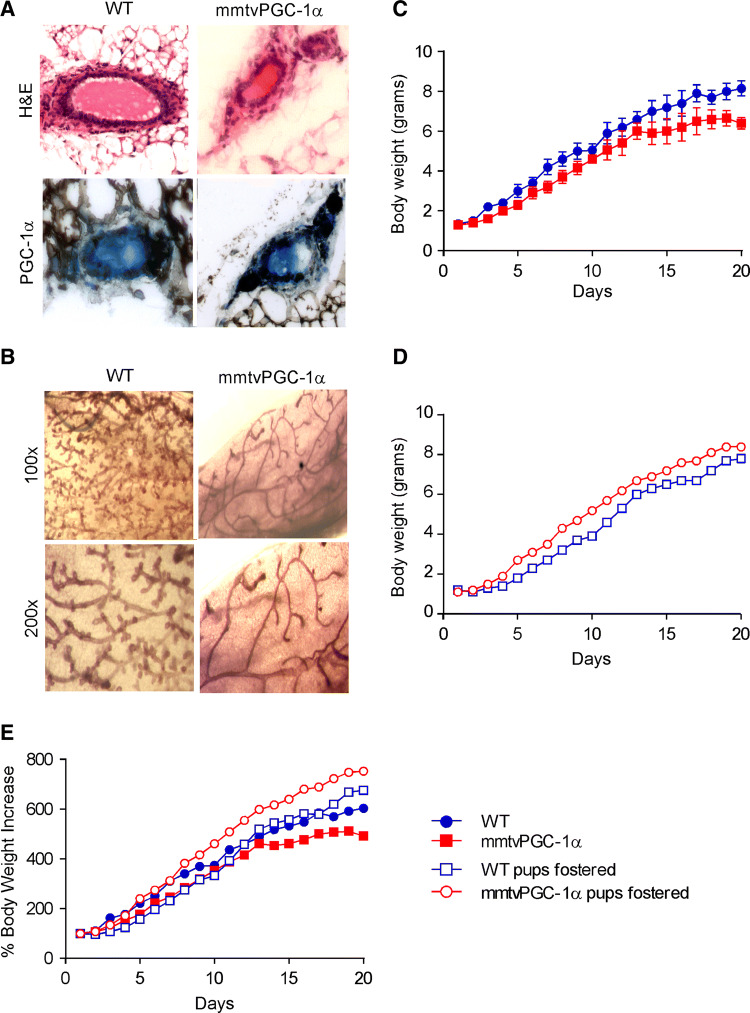
Examination of wild type and mmtvPGC-1α mammary glands from 8-week-old virgin mice by paraffin section (Fig. 4a) and whole mount (Fig. 4b) revealed a difference in terminal end bud formation, ductal growth and ductal branching. Indeed, transgenic-derived mammary glands showed thinner ductal branches, lower alveolar bud-like structures and a less branched ductal network than wild type mice (Fig. 4b).
Fig. 4.
Maternal PGC-1α overexpression decreases lobulogenesis in mammary glands during early development and results in growth retardation in the nursing neonates. Examination of WT and mmtvPGC-1α mammary glands from 8 weeks-old virgin mice reveals that PGC-1α overexpression in transgenic mice is associated with an impairment of ductal growth and ductal branching. a H&E staining and PGC-1α immunohistochemistry of mammary glands sections from WT and mmtvPGC-1α mice (magnification ×200). b Whole mount of the inguinal mammary glands in a virgin WT and a mmtvPGC-1α mouse. Developmental time line is depicted as the average body weight (± SEM) of pups from each of four independent litters on each postnatal day, nursed by the c biological mother and the d foster one. e Comparison of the four different group depicted as percentage of body weight increase. Blue color indicates WT pups, whereas red one specifies mmtvPGC-1α newborns. Circle symbol is used for WT breastfeeding mother and square one for mmtvPGC-1α breastfeeding mother. Closed circle indicates that pups are fed by biological mother, while open circle is used to denote pups fed by foster mother
Then, we crossed mmtvPGC-1α female with male of same genotype and we did the same for wild type mice. We monitored pups’ weight from birth until the time of weaning (21 days). On the 2nd day, the litter size was normalized to seven pups. Daily measurements of pup body weight from female mmtvPGC-1α revealed growth retardation (Fig. 4c–e, red closed square) and, in rare cases (5%), hair cycling defects and gout, compared to wild type littermates (blue closed circle). At the time of weaning (postnatal day 21), these pups exhibited low dimensions and body weights (Supplementary Fig. 1B). Interestingly, weight gain resumed immediately when the pups were placed on a standard chow diet (data not shown). Afterwards, pups maintained a normal phenotype that was completely dependent on maternal genotype (mmtvPGC-1α).
To further confirm that PGC-1α expression in lactating mammary glands are indeed responsible for the observed pups’ phenotype, we subjected the mice to a cross-fostering experiment. Fostering is the movement of pups from the birth (donor) dam to a recipient (foster) dam. We fostered pups within 48 h of birth to recipient dams that had delivered age-matched litters. We completely replaced the recipient dam’s litter with fostered neonates, to maintain a controlled number of mice (7 mice per litter) and to fully characterize the mutant newborns. As expected, mmtvPGC-1α pups nursed by wild type female completely lost their phenotype (Fig. 4d, e, red open circle) and did not show any growth retardation. On postnatal day 19, wild type-fostered mmtvPGC-1α pups reached a weight gain similar to the one observed for wild type pups nursed by wild type mother (8.4 g ± 0.13 versus 8.2 g ± 0.38) (Fig. 4d, e). On the other side, wild type pups nursed by mmtvPGC-1α dam (blue open square) showed a considerable delay on body growth, that became less consistent around weaning time when switched from maternal milk to cage food. Notably, on postnatal day 19th, wild type-fostered mmtvPGC-1α pups weighted over one gram more than the wild type pups nursed by mmtvPGC-1α foster mother (8.4 g ± 0.13 versus 7.2 g ± 0.16) (Fig. 4d, e, Supplementary Fig. 1C).
Collectively, these data suggest that PGC-1α is physiologically turned off during lactation to totally fulfil the metabolic needs for offspring development and its re-expression during lactation—as we observed with our transgenic model—may interfere to delay a complete development of newborns. However, as the transgenic phenotype was rescued at weaning and during cross-fostering experiments, it is not improbable to hypothesize that nutritional defects due to milk quality or quantity from mmtvPGC-1α mothers may mediate the growth retardation observed in offspring.
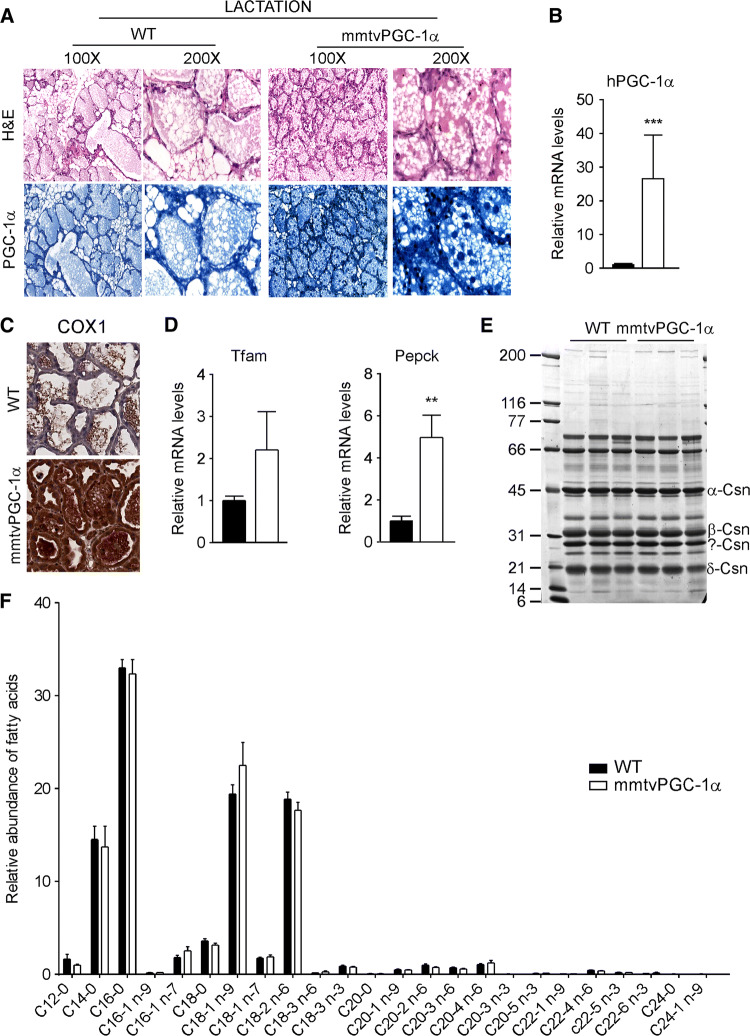
PGC-1α overexpression during lactation does not affect milk quality
To explore whether pup’s growth defects could be ascribable to the ectopic PGC-1α overexpression, we analysed if stable induction of PGC-1α during lactation stage could affect milk production. As shown by immunohistochemical (Fig. 5a) and gene expression data (Fig. 5b), the overexpression of ectopic hPGC-1α remained unchanged during lactation. Moreover, mmtvPGC-1α displayed a net increase in COX-1 staining compared to wild type mice, indicating that the overexpression of PGC-1α is closely related with the activation of mitochondria metabolism (Fig. 5c). In line with this data, mice overexpressing PGC-1α in the mammary glands exhibited the induction of mitochondrial transcription factor A (Tfam) and phosphoenolpyruvate carboxykinase (Pepck), two PGC-1α target genes involved in mitochondrial biogenesis and gluconeogenesis, respectively (Fig. 5d). To further determine the consequences of PGC-1α expression during lactation stage, we quantified the milk composition to find out if any anomalies occurred. The proteins (Fig. 5e) and fatty acids (Fig. 5f) profiles were comparable in the milk harvested from both wild type and mmtvPGC-1α mice. Overall, these data indicate that the stable PGC-1α expression during lactation stage is able to boost mitochondria respiration without overt effects on milk quality.
Fig. 5.
PGC-1α overexpression during lactation does not affect milk quality. Analysis of WT and mmtvPGC-1α mammary glands from lactating females on day 10 of lactation shows that PGC-1α overexpression is preserved in transgenic mice during lactation. a H&E staining (left) and PGC-1α immunohistochemistry (right) of mammary glands sections from WT and mmtvPGC-1α female mice on 10th day of lactation. b Relative expression of human PGC-1α in lactating mammary glands evaluated by real time qPCR demonstrating that the transgene is not lost during lactation. TBP was used as housekeeping gene to normalize data and WT mice was used as calibrators c COX1 immunohistochemistry of mammary glands sections from WT and mmtvPGC-1α female mice on 10th day of lactation. d Relative expression of PGC-1α target genes, Tfam and Pepck, in lactating mammary glands evaluated by real-time qPCR. TBP was used as housekeeping gene. e A 12% SDS–polyacrylamide gel analysis of different types of casein in milk derived from lactating WT and mmtvPGC-1α mice. The sizes of the protein molecular weight markers are indicated in lane 1. f Fatty acids composition of milk harvested from lactating WT and mmtvPGC-1α females. Fatty acids were analyzed as fatty acid methyl esters (FAMEs) by gas–liquid chromatography. Comparison of WT and transgenic mice (n = 6, 7) was performed using Mann–Whitney U test. Results are expressed as mean ± SEM (*p < 0.05; ***p < 0.001)
Mammary PGC-1α overexpression during lactation leads to a rapid mammary glands involution
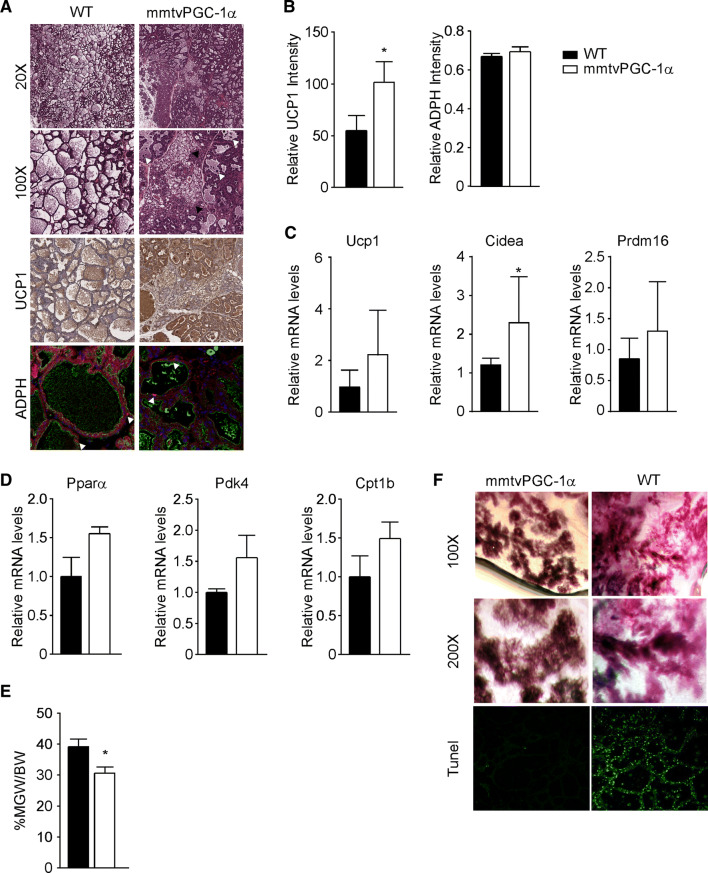
Since we did not observe any substantial differences between wild type and PGC-1α mice regarding milk composition, we wondered if the ectopic expression of PGC-1α could have any consequences on milk quantity. At 10 days of lactation, mammary glands of transgenic mice displayed smaller cytoplasmic lipids droplets and, consequently, reduced lumina, as compared with wild type (Fig. 6a). However, the analysis of lipid droplets accumulation using a specific antibody against adipophilin (ADPH), a protein found in cytoplasmic and secreted-milk lipid droplets coat [27, 28], did not reveal eminent differences between the two groups (Fig. 6a, b). Surprisingly, H&E staining of mammary glands sections revealed the presence of small cells, containing multilocular cytoplasmic lipid droplets, resembling brown adipocytes (Fig. 6a). To confirm our hypothesis, we tested mammary glands immunoreactivity for UCP1, a mitochondrial thermogenic protein uniquely expressed in brown adipose tissue [29] and we found marked areas of UCP1 staining in mmtvPGC-1α mammary glands compared to wild type (Fig. 6a, b). At the same time, gene expression analysis revealed a trend toward induction of fat-browning related genes, such as Ucp1, Cidea and Prdm16 (Fig. 6c). Interestingly, we observed high concentration of UCP1 also in alveolar epithelial cells, thus pointing out that the expression of PGC-1α during lactation promotes an early changing in mammary gland architecture, becoming repopulated with adipocytes that rapidly differentiate into brown ones. Brown adipose cells are usually densely packed with small lipid droplets together and abundant mitochondria. Indeed, we observed that mmtvPGC-1α mammary glands displayed a higher induction of mitochondrial metabolism compared to wild type ones (Fig. 5c, d). Since fatty acid β-oxidation is critically required for the thermogenic function of BAT, to further confirm our results, we examined the expression of genes involved in fatty acid β-oxidation (Peroxisome proliferator-activated receptor α, Pyruvate dehydrogenase kinase 4, and Carnitine Palmitoyltransferase 1B) and we found a trend towards increase of those genes in mammary gland harvested from transgenic mice compared to wild type ones (Fig. 6d).
Fig. 6.
Stable overexpression of PGC-1α during lactation promotes apoptosis and mammary glands regression. a Mammary tissue sections from 10 days lactating WT and mmtvPGC-1α females mice stained with H&E (different magnification), with UCP1 immunohistochemistry, and ADPH immunofluorescence. In ×100 H&E, white arrows indicate the shedding of epithelial cells into the alveolar lumen; and black arrows represent brown adipocytes. Immunolocalization of ADPH was performed using Alexa 594-conjugated antibodies against the N-terminus of mouse ADPH (red, arrowed). Luminal borders of mammary alveoli were identified by staining with Alexa 488-conjugated WGA (green). Nuclei were stained with TO-PRO-3 (blue). b Quantification of UCP1 and ADPH immunostaining. Relative expression of c fat-browning related genes, Ucp1, Cidea and Prdm16, and d β-oxidation genes, Pparα, Pdk4 and Cpt1b, in lactating mammary glands evaluated by real time qPCR. TBP was used as housekeeping gene. e Inguinal mammary glands weight to body weight ratio (MGW/BW) of WT and mmtvPGC-1α lactating mice. f Low and high magnification of whole mount staining together with TUNEL staining of mammary glands isolated from lactating mmtvPGC-1α and WT mice. Comparison of wild-type and transgenic mice (n = 6, 7) was performed using Mann–Whitney U test. Data are expressed as mean ± SEM (*p < 0.05; **p < 0.01)
To understand whether the overexpression of PGC-1α is involved in the modulation of signals mediating the regression of mammary glands development, we determined the ratio between inguinal mammary gland and total mice weight on day 10 of lactation, revealing a marked decrease in mmtvPGC-1α mice compared to wild type mice (Fig. 6e). Concurrently, analysis on paraffin section and whole mount confirmed that the overexpression of PGC-1α in mammary glands was highly correlated with a hypoplastic phenotype and reduced alveolar structures (Fig. 6a–f). Moreover, we observed a marked increment of apoptosis rate, as indicated by TUNEL assay and the shedding of epithelial cells into the alveolar lumen (Fig. 6a).
Overall, our results depict a unique scenario in which high levels of PGC-1α during lactation prevent mammary glands development and maturation by both inducing an early apoptosis and promoting a rapid modification of mammary gland architecture, characterized by differentiation into brown adipocytes. Taken together, all these transformations collectively accelerate the involution of tissue, and finally lead to a decrease of milk production, hence affecting normal pups’ growth and development.
Discussion
The fundamental function of the mammary glands is to provide nourishment to newborns. Milk represents the food for young mammals, being one essential constituents of mammalian life. Human breastfeeding conveys established benefits for both maternal and child health, as maternal milk supplies calories from lipids and essential fatty acids together with signalling molecules. However, one out of two mothers stop breastfeeding before the recommended timespan, mostly due to the perception that milk quantity is insufficient to address the need of infants [30, 31].
In our in vivo study, we used integrated approaches of molecular genetics, biochemistry and metabolomic to demonstrate that PGC-1α is dispensable during pregnancy and lactation, but specifically required during involution, where the coactivator causes regression and apoptosis of mammary epithelial cells. The timing of the observed phenotype is clearly related to the temporal expression of PGC-1α, as highlighted by immunohistochemical analysis (Fig. 1a).
Using a transgenic mouse model, we showed that the stable and specific expression of PGC-1α in mammary gland is associated with lactation defects and retarded pups’ growth. Indeed, the specific overexpression of PGC-1α resulted in an altered mammary glands morphogenesis, as well as a precocious mammary glands regression. Surprisingly, this regression occurred during the lactation stage, through the development of abnormal alveolar structures that contributed to lactation deficiency. In line with our experiments, it has been reported that several transgenic and knockout mouse models exhibit lactation defects as a result of insufficient development of the alveolar structures during pregnancy [32–34] and it would be interesting if those genes could be related to the PGC-1α coactivator activity.
Normally, mammary gland involution is a two-step process which encompasses the apoptosis of secretory epithelial cells followed by re-differentiation of the adipocytes [6, 9]. In the present study, we show that PGC-1α is physiologically involved in the promotion of apoptosis during involution, and its expression throughout the lactation stage contributes to the regression of mammary glands, thus limiting the availability of milk.
Recently, it has been observed that mammary adipocytes fully de-differentiates into preadipocytes during lactation and promptly re-differentiate during involution [7, 9]. Interestingly, several lines of evidence indicate that white adipocytes may transdifferentiate into brown adipocytes (a process known as “browning” or “brightening” of WAT), characterized by UCP1 expression coupled with a multilocular lipid droplets morphology [35, 36]. Whether this process occurs as a transdifferentiation of pre-existing white adipocytes or by de novo adipogenesis from a subgroup of precursor cells is still unknown [35, 37, 38]. Our study revealed that re-expression of PGC-1α during lactation promotes an early adipocytes transdifferentiation of mammary glands, consequently affecting milk production. Indeed, mmtvPGC-1α lactating mammary glands display large area of resembling brown adipocytes, with increased mitochondrial UCP1 expression associated with the reduction of cytoplasmic lipid droplets. It is not unfair to hypothesize that the uncoupling of the oxidative phosphorylation would release the feedback control exerted by the mitochondrial membrane potential on the respiratory NADH oxidation, thus allowing the cells to reach maximum capacity of fatty acids β-oxidation. The indication of re-expression of PGC-1α during lactation is sufficient to induce UCP1 expression in the mammary gland is consistent with the described regulation of the brown fat in the interscapular fat pad, as well as the induction of brown-fat-like regions within white adipose depots [39, 40]. Interestingly, it has been recently described that during mammary glands involution some milk secreting epithelial cells in the anterior subcutaneous depot (pink adipocytes) may transdifferentiate to brown adipocytes [8, 41]. Although this data may contradict the “cycle of adipocyte-pre-adipocyte transition” postulated by Wang and colleagues [9], our results depict the possibility that re-expression of PGC-1α during mammary gland involution may contribute to browning of de-differentiated mammary adipocytes.
Intriguingly, several studies have indicated that brown adipocytes are a component of the mammary fatty stroma during postnatal development or in virgin mice exposed to cold, and that PGC-1α and genes involved in fatty acids β-oxidation are coordinately upregulated in this tissue [42, 43]. Moreover, multilocular brown adipocytes have been detected in the adult mammary gland of Brca1 mutant mice, which develop high-grade undifferentiated adenocarcinoma in a similar way to human BRCA-1 mutated breast cancer [44]. Noteworthy, a high expression of PGC-1α in cell culture and in tissue specimens isolated from established human breast cancer correlates with low survival and metastasis [19, 21, 45]. It would, therefore, be intriguing to explore whether the expression of this coactivator is essential to promote tumor onset and progression. Recent observations regarding the increased risk of breast cancer in women who do not breastfeed [46–48] highlight once more the importance of PGC-1α in the pathophysiology of mammary glands, suggesting that turning off the expression of this coactivator during lactation could exert protective actions against cancer development.
All together, these data infer that PGC-1α is indeed necessary in the post-lactation stage, as it is involved in both steps of mammary glands involution by promoting apoptosis and remodelling of mammary gland architecture. Therefore, its expression is physiologically turned off during late pregnancy and lactation to allow the full development of secretory epithelial cells and milk production. The overall effects exerted by enhanced PGC-1α expression, including the precocious regression of the glands suggest a possible role of PGC-1α in mastitis and, consequently, milk loss. Premature mammary glands involution promoted by PGC-1α could be mediated through NF-κB, a transcription factor that has been involved in mastitis, where it sustains the rapid loss of milk and secretory structures [49]. However, due to its fundamental role in host defence, the therapeutic intervention to block NF-κB is not recommended. Alternatively, a strategy aimed to inhibit PGC-1α could be designed to provide beneficial effects for nursing mothers.
The limitation of the present study is the lack of a loss of function model. However, it is rather possible that the effects of specific PGC-1α ablation from the mammary glands would not be easy to identify, since different coping mechanisms may occur to counteract the loss of this coactivator. PGC-1β expression could represent one of the possible compensatory strategies. Indeed, PGC-1β and PGC-1α exert similar functions in many tissues, and are both considered as master regulators of oxidative metabolism due to their capacity to promote mitochondrial biogenesis [50]. In mammary glands, both coactivators are significantly downregulated during pregnancy and lactation, and are target of nutri-regulated miRNA [51, 52]. At the same time, oestrogens are able to induce only PGC-1β expression in the breast, thereby supporting mitochondrial biogenesis and oxidative phosphorylation in this tissue [53]. On the other hand, at variance of PGC-1α that do not affect milk composition, PGC-1β modulation interferes with lipogenic process, thus altering the quantity and the quality of fatty acids incorporated into milk. Precisely, the negative regulation exerted by miR-25 on PGC-1β activity results in a reduced triglyceride synthesis and lipid droplets accumulation [54]. Moreover, fat-dietary type and physiological stimuli, such as the increased amount of polyunsaturated fatty acids (PUFAs) in lactating mammary glands, diminish PGC-1β expression and promotion of lipogenesis in mammary glands [55]. Thereby, it is plausible that a compensatory adaptive increase of PGC-1β under suitable stimuli would result in aberrant milk production which may impact in normal growth and development of the newborns.
Overall, taking advantage of this newly generated transgenic mouse model, we depicted a direct involvement of PGC-1α in the physiological homeostasis of mammary glands, where it actively promotes involution. Our model provides a hypothetical underlying mechanism controlling the process of adipocyte de-differentiation and re-differentiation during a female reproductive cycle. However, high coactivator levels during lactation lead to a precocious re-differentiation into adipocytes together with increased apoptosis rates consequently hasten tissue involution, thus preventing a complete development of mammary glands. Our results provide new insights in the comprehension of lactation deficiency, opening new possibilities for mothers that, for several reasons, have to face an early stop in breastfeeding and offering future perspective for prevention of breast cancer onset.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 PGC-1α overexpression affects pups development. (A) hPGC-1α (white bars) and mPGC-1α (black bar) relative mRNA expression in different tissue specimens isolated from transgenic and wild type mice (n = 6) by real-time qPCR. TBP was used as housekeeping gene to normalize data and wild type mice was used as calibrators. Results are expressed as mean ± SEM. (B) Picture of wild-type and mmtvPGC-1α newborns at 21 days after birth. (C) Picture of wild type and mmtvPGC-1α newborns at 19 days after birth fostered by mmtvPGC-1α and wild-type mother, respectively. (TIFF 5283 kb)
Acknowledgements
We thank L. Salvatore and G. Di Tullio, for their invaluable help during the study, and J-M. Lobaccaro and J. Hardfeldth, for their help with the manuscript. A. Moschetta is funded by Italian Association for Cancer Research (AIRC, IG 18987), NR-NET FP7 Marie Curie People ITN and EU-JPI FATMAL 2017.
Abbreviations
- PGC-1
Peroxisome proliferator-activated receptor gamma coactivator 1
- UCP1
Uncoupling protein 1
- TFAM
Mitochondrial transcription factor A
- Mmtv
Mouse mammary tumor virus
Author contributions
EP contributed to study design, performed experiments, analysed data and wrote the paper; AM contributed to study design, performed experiments and data analysis; CP, AC, JBM, MA and HG performed experiments; GV contributed to paper writing; AM designed the study, supervised the project and paper writing.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Ethics statement
The Ethical Committee of the Consorzio Mario Negri Sud and the University of Bari approved this experimental set-up, which also was certified by the Italian Ministry of Health in accordance with internationally accepted guidelines for animal care.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elena Piccinin and Annalisa Morgano contributed equally.
References
- 1.Watson CJ, Kreuzaler PA. Remodeling mechanisms of the mammary gland during involution. Int J Dev Biol. 2011;55:757–762. doi: 10.1387/ijdb.113414cw. [DOI] [PubMed] [Google Scholar]
- 2.Neville MC. Physiology of lactation. Clin Perinatol. 1999;26(251–79):v. [PubMed] [Google Scholar]
- 3.Neville MC, Picciano MF. Regulation of milk lipid secretion and composition. Annu Rev Nutr. 1997;17:159–183. doi: 10.1146/annurev.nutr.17.1.159. [DOI] [PubMed] [Google Scholar]
- 4.Allen JC, Keller RP, Archer P, Neville MC. Studies in human lactation: milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr. 1991;54:69–80. doi: 10.1093/ajcn/54.1.69. [DOI] [PubMed] [Google Scholar]
- 5.Schwertfeger KL, McManaman JL, Palmer CA, Neville MC, Anderson SM. Expression of constitutively activated Akt in the mammary gland leads to excess lipid synthesis during pregnancy and lactation. J Lipid Res. 2003;44:1100–1112. doi: 10.1194/jlr.M300045-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Watson CJ. Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res. 2006;8:203. doi: 10.1186/bcr1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morroni M, Giordano A, Zingaretti MC, Boiani R, De MR, Kahn BB, Nisoli E, Tonello C, Pisoschi C, Luchetti MM, Marelli M, Cinti S. Reversible transdifferentiation of secretory epithelial cells into adipocytes in the mammary gland. Proc Natl Acad Sci USA. 2004;101:16801–16806. doi: 10.1073/pnas.0407647101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giordano A, Perugini J, Kristensen DM, Sartini L, Frontini A, Kajimura S, Kristiansen K, Cinti S. Mammary alveolar epithelial cells convert to brown adipocytes in post-lactating mice. J Cell Physiol. 2017;232:2923–2928. doi: 10.1002/jcp.25858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang QA, Song A, Chen W, Schwalie PC, Zhang F, Vishvanath L, Jiang L, Ye R, Shao M, Tao C, Gupta RK, Deplancke B, Scherer PE. Reversible de-differentiation of mature white adipocytes into preadipocyte-like precursors during lactation. Cell Metab. 2018;28:282–288. doi: 10.1016/j.cmet.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 11.Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 2002;1576:1–14. doi: 10.1016/s0167-4781(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 14.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 15.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 16.Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 17.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 18.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 19.McGuirk S, Gravel SP, Deblois G, Papadopoli DJ, Faubert B, Wegner A, Hiller K, Avizonis D, Akavia UD, Jones RG, Giguere V, St-Pierre J. PGC-1alpha supports glutamine metabolism in breast cancer. Cancer Metab. 2013;1:22. doi: 10.1186/2049-3002-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Audet-Walsh E, Papadopoli DJ, Gravel SP, Yee T, Bridon G, Caron M, Bourque G, Giguere V, St-Pierre J. The PGC-1alpha/ERRalpha axis represses one-carbon metabolism and promotes sensitivity to anti-folate therapy in breast cancer. Cell Rep. 2016;14:920–931. doi: 10.1016/j.celrep.2015.12.086. [DOI] [PubMed] [Google Scholar]
- 21.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM, Asara JM, Kalluri R. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol. 1995;168:47–61. doi: 10.1006/dbio.1995.1060. [DOI] [PubMed] [Google Scholar]
- 23.Stein T, Salomonis N, Gusterson BA. Mammary gland involution as a multi-step process. J Mammary Gland Biol Neoplasia. 2007;12:25–35. doi: 10.1007/s10911-007-9035-7. [DOI] [PubMed] [Google Scholar]
- 24.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De MR, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298:E1244–E1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Lv N, Zhang S, Shui G, Qian H, Zhang J, Chen Y, Ye J, Xie Y, Shen Y, Wenk MR, Li P. Cidea is an essential transcriptional coactivator regulating mammary gland secretion of milk lipids. Nat Med. 2012;18:235–243. doi: 10.1038/nm.2614. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Z, Yon TS, Chen Z, Guo K, Ng CP, Ponniah S, Lin SC, Hong W, Li P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
- 27.Russell TD, Palmer CA, Orlicky DJ, Fischer A, Rudolph MC, Neville MC, McManaman JL. Cytoplasmic lipid droplet accumulation in developing mammary epithelial cells: roles of adipophilin and lipid metabolism. J Lipid Res. 2007;48:1463–1475. doi: 10.1194/jlr.M600474-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Straub BK, Gyoengyoesi B, Koenig M, Hashani M, Pawella LM, Herpel E, Mueller W, Macher-Goeppinger S, Heid H, Schirmacher P. Adipophilin/perilipin-2 as a lipid droplet-specific marker for metabolically active cells and diseases associated with metabolic dysregulation. Histopathology. 2013;62:617–631. doi: 10.1111/his.12038. [DOI] [PubMed] [Google Scholar]
- 29.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 30.Ahluwalia IB, Morrow B, Hsia J. Why do women stop breastfeeding? Findings from the pregnancy risk assessment and monitoring system. Pediatrics. 2005;116:1408–1412. doi: 10.1542/peds.2005-0013. [DOI] [PubMed] [Google Scholar]
- 31.Lewallen LP, Dick MJ, Flowers J, Powell W, Zickefoose KT, Wall YG, Price ZM. Breastfeeding support and early cessation. J Obstet Gynecol Neonatal Nurs. 2006;35:166–172. doi: 10.1111/j.1552-6909.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- 32.Seagroves TN, Hadsell D, McManaman J, Palmer C, Liao D, McNulty W, Welm B, Wagner KU, Neville M, Johnson RS. HIF1alpha is a critical regulator of secretory differentiation and activation, but not vascular expansion, in the mouse mammary gland. Development. 2003;130:1713–1724. doi: 10.1242/dev.00403. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 34.Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 35.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 38.Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab. 2009;297:E977–E986. doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- 39.Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z, Gingras AC, Katsume A, Elchebly M, Spiegelman BM, Harper ME, Tremblay ML, Sonenberg N. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med. 2001;7:1128–1132. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- 40.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giordano A, Smorlesi A, Frontini A, Barbatelli G, Cinti S. White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur J Endocrinol. 2014;170:R159–R171. doi: 10.1530/EJE-13-0945. [DOI] [PubMed] [Google Scholar]
- 42.Gouon-Evans V, Pollard JW. Unexpected deposition of brown fat in mammary gland during postnatal development. Mol Endocrinol. 2002;16:2618–2627. doi: 10.1210/me.2001-0337. [DOI] [PubMed] [Google Scholar]
- 43.Master SR, Hartman JL, D’Cruz CM, Moody SE, Keiper EA, Ha SI, Cox JD, Belka GK, Chodosh LA. Functional microarray analysis of mammary organogenesis reveals a developmental role in adaptive thermogenesis. Mol Endocrinol. 2002;16:1185–1203. doi: 10.1210/mend.16.6.0865. [DOI] [PubMed] [Google Scholar]
- 44.Jones LP, Buelto D, Tago E, Owusu-Boaitey KE (2011) Abnormal mammary adipose tissue environment of Brca1 mutant mice show a persistent deposition of highly vascularized multilocular adipocytes. J Cancer Sci Ther (Suppl 2):004 [DOI] [PMC free article] [PubMed]
- 45.Klimcakova E, Chenard V, McGuirk S, Germain D, Avizonis D, Muller WJ, St-Pierre J. PGC-1alpha promotes the growth of ErbB2/Neu-induced mammary tumors by regulating nutrient supply. Cancer Res. 2012;72:1538–1546. doi: 10.1158/0008-5472.CAN-11-2967. [DOI] [PubMed] [Google Scholar]
- 46.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Reproductive and hormonal risk factors for postmenopausal luminal, HER-2-overexpressing, and triple-negative breast cancer. Cancer. 2008;113:1521–1526. doi: 10.1002/cncr.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lord SJ, Bernstein L, Johnson KA, Malone KE, McDonald JA, Marchbanks PA, Simon MS, Strom BL, Press MF, Folger SG, Burkman RT, Deapen D, Spirtas R, Ursin G. Breast cancer risk and hormone receptor status in older women by parity, age of first birth, and breastfeeding: a case-control study. Cancer Epidemiol Biomark Prev. 2008;17:1723–1730. doi: 10.1158/1055-9965.EPI-07-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connelly L, Barham W, Pigg R, Saint-Jean L, Sherrill T, Cheng DS, Chodosh LA, Blackwell TS, Yull FE. Activation of nuclear factor kappa B in mammary epithelium promotes milk loss during mammary development and infection. J Cell Physiol. 2010;222:73–81. doi: 10.1002/jcp.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Mobuchon L, Marthey S, Le GS, Laloe D, Le PF, Leroux C. Food deprivation affects the miRNome in the lactating goat mammary gland. PLoS One. 2015;10:e0140111. doi: 10.1371/journal.pone.0140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han LQ, Li HJ, Wang YY, Zhu HS, Wang LF, Guo YJ, Lu WF, Wang YL, Yang GY. mRNA abundance and expression of SLC27A, ACC, SCD, FADS, LPIN, INSIG, and PPARGC1 gene isoforms in mouse mammary glands during the lactation cycle. Genet Mol Res. 2010;9:1250–1257. doi: 10.4238/vol9-2gmr814. [DOI] [PubMed] [Google Scholar]
- 53.Ivanova MM, Radde BN, Son J, Mehta FF, Chung SH, Klinge CM. Estradiol and tamoxifen regulate NRF-1 and mitochondrial function in mouse mammary gland and uterus. J Mol Endocrinol. 2013;51:233–246. doi: 10.1530/JME-13-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma L, Qiu H, Chen Z, Li L, Zeng Y, Luo J, Gou D. miR-25 modulates triacylglycerol and lipid accumulation in goat mammary epithelial cells by repressing PGC-1beta. J Anim Sci Biotechnol. 2018;9:48. doi: 10.1186/s40104-018-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez-Cruz M, Tovar AR, Palacios-Gonzalez B, Del PM, Torres N. Synthesis of long-chain polyunsaturated fatty acids in lactating mammary gland: role of Delta5 and Delta6 desaturases, SREBP-1, PPARalpha, and PGC-1. J Lipid Res. 2006;47:553–560. doi: 10.1194/jlr.M500407-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 PGC-1α overexpression affects pups development. (A) hPGC-1α (white bars) and mPGC-1α (black bar) relative mRNA expression in different tissue specimens isolated from transgenic and wild type mice (n = 6) by real-time qPCR. TBP was used as housekeeping gene to normalize data and wild type mice was used as calibrators. Results are expressed as mean ± SEM. (B) Picture of wild-type and mmtvPGC-1α newborns at 21 days after birth. (C) Picture of wild type and mmtvPGC-1α newborns at 19 days after birth fostered by mmtvPGC-1α and wild-type mother, respectively. (TIFF 5283 kb)