Abstract
Molecular oxygen (O2) is a key player in cell mitochondrial function, redox balance and oxidative stress, normal tissue function and many common disease states. Various chemical, physical and biological methods have been proposed for measurement, real-time monitoring and imaging of O2 concentration, state of decreased O2 (hypoxia) and related parameters in cells and tissue. Here, we review the established and emerging optical microscopy techniques allowing to visualize O2 levels in cells and tissue samples, mostly under in vitro and ex vivo, but also under in vivo settings. Particular examples include fluorescent hypoxia stains, fluorescent protein reporter systems, phosphorescent probes and nanosensors of different types. These techniques allow high-resolution mapping of O2 gradients in live or post-mortem tissue, in 2D or 3D, qualitatively or quantitatively. They enable control and monitoring of oxygenation conditions and their correlation with other biomarkers of cell and tissue function. Comparison of these techniques and corresponding imaging setups, their analytical capabilities and typical applications are given.
Keywords: Fluorescence microscopy, Fluorescence and phosphorescence-based probes, FLIM, Hypoxia, Live cell and tissue imaging, Oxygen microscopy, PLIM
Introduction
Fluorescence microscopy techniques allow visualization of various biological molecules and processes at single-cell and subcellular resolution, in 2D and 3D, in dynamics, multiple colors and with parallel analysis of several imaging biomarkers and functional readouts [1]. Modern microscopy is applicable to the broad range of biological models, as end point or continuous assays (time-lapse monitoring), and becomes recognized as a powerful tool for basic and translational biomedical research. In particular, live fluorescence microscopy allows multi-parametric in vitro studies of normal, diseased or stem cells and complex multicellular structures, providing detailed structural (localization, morphology) and functional (fluxes and dynamics of ions, proteins, other biomolecules and processes) information, and avoiding artifacts associated with cell/tissue fixation and endpoint readouts [2–4]. Currently, this area is experiencing major development, with rapid evolution of the imaging instrumentation and software, introduction of advanced imaging modalities (fluorescence lifetime imaging microscopy (FLIM), super resolution, light sheet), new cell, tissue and disease models (from adherent cells to 3D micro-tissue, ex vivo, and in vivo models), and advanced imaging probes and biosensor constructs providing robust and quantitative readouts.
Rational design and validation of new high-performance functional biosensors and biomarkers is one of the pillars of modern fluorescence microscopy. Although the area is still dominated by the conventional probes, a number of advanced live probes and imaging methodologies have emerged in recent years, which include small molecule probes, bioconjugates, genetically encoded protein biosensors, nanoparticle-based probes and some other structures [5–8].
Being one of the key metabolic markers and environmental parameters of the cell and live tissue, molecular oxygen (O2) is an obvious choice as an analyte and molecular target for fluorescence microscopy of normal and diseased tissue. O2 is essential for aerobic respiration of mammalian cells and is dynamically regulated maintaining the balance between the oxidative stress, hypoxia and anoxia [9]. Analysis of in situ oxygenation and oxygen consumption rate can, therefore, inform on cell viability, metabolic status and (dys)function. These parameters can also be used to study hypoxia-dependent cellular pathways, reactive oxygen species (ROS) signaling, mitochondrial function and cell physiology [10, 11]. As a result, a number of different analytical approaches, probes and detection platforms have been proposed for quantification of cell and tissue oxygen, some of which rely on fluorescence microscopy [10, 12, 13].
The term ‘oxygen microscopy’ has appeared recently [14]. It covers a number of hypoxia-specific stains, endogenously expressed biosensor constructs, phosphorescent oxygen sensing probes and nanoparticle structures, and corresponding imaging techniques for visualization of oxygen distribution in cell and tissue microenvironment. These probes provide indirect or direct, qualitative, semi- or fully quantitative information, endpoint or continuous real-time readouts. They were broadly applied to different experimental models such as 2D and 3D cell cultures, excised slices of live or fixed tissue, or for administration in live animals [10, 12, 13, 15–20]. There is a strong interest in the probes and techniques tailored for in vitro use with a broader range of cell and tissue models, quantitative and real-time oxygen concentration readout. Significant overlaps exist in the uses of different probes and imaging techniques, thus allowing their detailed comparison, benchmarking and defining specific experimental and imaging requirements and application niches for each of them.
Here, we review the recent developments in oxygen microscopy, outlining the differences, merits and limitations of the main techniques, and their ability to serve various biomedical needs. We mainly focus on fluorescence microscopy approaches, without discussing specialized techniques utilizing other measurement settings, such as electron paramagnetic resonance [21], Raman, photoacoustic and transient state microscopies [22]. Comparison with electrochemical (Clark-type electrode) O2 measurements in biomedical applications can be found in recent reviews (e.g., [13]).
Probes and measurement approaches in oxygen microscopy
Contemporary approaches for optical detection and quantification of cell and tissue O2, which can be realized in fluorescence imaging modality, include both indirect and direct probing methods. The first group is represented by the redox-sensitive small molecules coupled with immunofluorescence staining or fluorescent reporter dyes, hypoxia-inducible protein markers and genetically encoded biosensors. Another group of techniques mainly relies on fluorescence/phosphorescence quenching methods and several different types of corresponding probes [10, 12, 13, 19, 20]. A brief summary of these probes (classified as P1-P10), all of which operate under fluorescence microscopy settings, is presented in Table 1.
Table 1.
Main types of probes used in fluorescence-based imaging of cell and tissue O2 and hypoxia
| No. | Probe type | Operation principle | Typical procedure | Advantages/limitations | References |
|---|---|---|---|---|---|
| Indirect probes and techniques for visualization of hypoxia | |||||
| P1 | Redox-sensitive nitroimidazole derivatives | Penetrates cells and tissue and is converted at low O2 by nitroreductase enzyme. Product is detected by antibody staining and fluorescence microscopy | Systemic administration (in blood or medium), accumulation, conversion in cells, tissue sectioning, fixation, immunofluorescence, microscopy imaging |
Small molecule probes, work in cells and animals, use basic fluorescence microscope Endpoint, qualitative (threshold type), dead tissue |
[23–25] |
| P2 | Redox dye-based fluorescent hypoxia-sensitive chemical probes | Cell-permeable precursor dyes, which generate fluorescence upon enzymatic conversion at low O2. Work similar to P1, but do not require fixation and antibody staining | Administration, incubation, (live) fluorescence imaging |
Live imaging of whole animals Indirect, repetitive Complex signal dynamics, qualitative (threshold type), not always reversible |
[20, 26] |
| P3 | Fluorescent protein reporter-based systems | Expression of an endogenous fluorescent protein controlled by a hypoxia-responsive promoter (e.g., HIF). Fluorescence is generated in hypoxic zones | DNA transfection (transient or with generation of stable transgenic cell lines or animals), incubation, fluorescence imaging |
Endogenous probe, live, high-resolution imaging, 2D and 3D. Broadly compatible Qualitative, threshold type. Complex signal dynamics. Not always reversible. Maturation of fluorescent protein can be affected by O2. Complex assay (UnaG-based system) [27] |
[27–31] |
| P4 | Prolyl hydroxylase activity-dependent biosensor constructs | Endogenously expressed FRET biosensor (ProCY) responsive to prolyl hydroxylase activity | DNA transfection (transient or with generation of stable transgenic cells lines or animals), incubation, fluorescence imaging |
Endogenous ratiometric probe, live high-resolution imaging, 3D capabilities Semi-quantitative. Complex signal dynamics |
[32] |
| P5 | O2-dependent fluorescent proteins | Endogenously expressed fluorescent protein (or FRET-based multi-protein biosensor), based on GFP, YFP or DsRed with O2-dependent maturation/folding | DNA transfection (transient or with generation of stable transgenic cells lines or animals), incubation, fluorescence imaging |
Endogenous probe, live and post-mortem imaging, 2D, 3D and whole animals. ‘Memorize’ cellular O2 levels [33] Complex signal dynamics, The effects of protein overproduction on cell function are poorly studied |
[33–35] |
| Direct and reversible optical sensing of O2 concentration/partial pressure | |||||
| P6 | Delayed fluorescence of endogenously overproduced protoporphyrin IX (PPIX) | Dynamic quenching of endogenous PPIX-delayed fluorescence, which is overproduced in mitochondria after 5-aminolevulinic acid (5-ALA) administration | Administration of 5-ALA to cells or tissue, incubation, live phosphorescence lifetime imaging microscopy (PLIM) |
Natural fluorophore with mitochondrial localization, quantitative, real-time, so far used for point measurements Weak signals, long emission lifetimes. 5-ALA effects on cell metabolism are unstudied |
[36–38] |
| P7 | Intravascular (cell-impermeable) phosphorescent probes | Dynamic quenching of an exogenous cell-impermeable phosphor with complex supramolecular (dendrimer) structure and strong two-photon excitability | Administration in blood stream, imaging of vascular and interstitial O2 |
Live, quantitative, real-time two-photon excited in vivo imaging. Stable O2 calibration in serum and some other biological fluids. 3D and combination with other blood flow and metabolism is also possible High probe doses upon systemic administration, short time window. Mostly applicable for imaging blood vessels and microcapillaries. Complex procedure and two-photon PLIM. Limited use with in vitro models |
Reviewed in [12, 39] |
| P8 | Cell-penetrating small molecule phosphors and conjugates | Dynamic quenching of an exogenous phosphor, which has intrinsic ability to efficiently stain cells or 3D tissue models. Various chemical groups such as carbohydrates, mitochondrial targeting groups or cell-penetrating peptides are used as delivery vectors | Probe addition to the growth medium or local administration in animal, incubation (1–16 h), imaging on fluorescence or PLIM microscopes |
Live, quantitative, real-time imaging of intracellular O2 in 2D/3D models. Deep tissue staining, long retention times (days), local administration, different chemistries and spectra Moderate intensity signals. Interaction with biomolecules can alter O2 calibration (cell-specific calibration). Usually require PLIM mode and one-photon excitation. Some probes show toxicity |
Reviewed in [12, 16–18] |
| P9 | Phosphorescent nanosensors | Dynamic quenching of an exogenous phosphor embedded in cell-penetrating polymeric nanoparticles | Probe addition to the growth medium or local administration in animal, incubation (1–16 h), imaging on fluorescence or PLIM microscopes |
Live, real-time, quantitative (ratiometric, PLIM) imaging of intracellular O2 in 2D/3D models. High brightness, also under two-photon excitation, low doses and impact on cell function Cell specificity, slow uptake (12–16 h), less efficient than P8 in 3D tissue staining. Can be toxic/invasive (> 100 nm size of nanosensors) |
Reviewed in [12, 16–18, 40] |
| P10 | Solid-state phosphorescent sensors: coated foils, microparticles and porous scaffolds | Dynamic quenching of the phosphorescent dye molecules embedded in a film coating, microparticles, microporous scaffold or related materials. Measures only peri- and extracellular O2 |
Sensor film or suspension of microparticles is applied or sprayed to cells or tissue surface and imaged In case of hybrid porous O2-sensing polymer scaffolds cells are grown within scaffold and then imaged using fluorescence of PLIM microscopes |
Live, real-time, quantitative 2D or 3D imaging, no staining of the sample. Stable calibration, high-optical signals (intensity, ratiometric or PLIM readouts) Non-invasive, but can only image areas of contact or extracellular O2 (scaffolds) |
[41, 42–44, 10, 19, 45, 46, 47, 48] |
Indirect probes and techniques for cell and tissue hypoxia
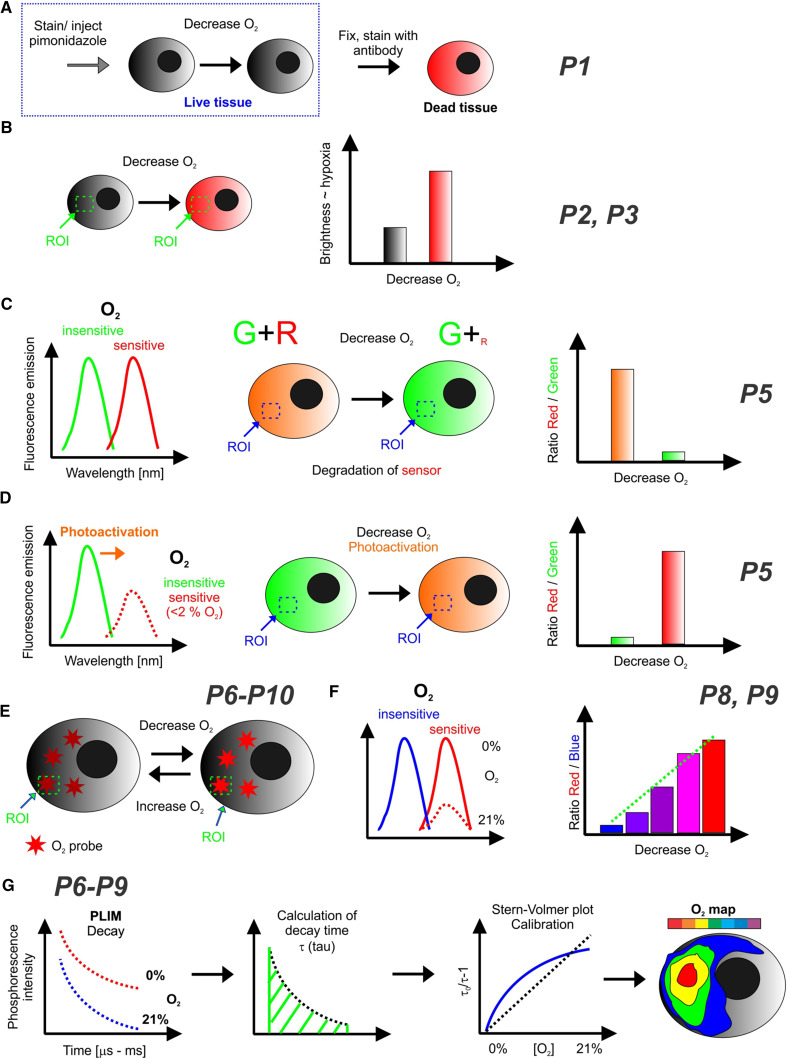
Type P1 probes, used for visualization of hypoxic regions in tissue, mainly in vivo, but also in some in vitro models, comprise redox-sensitive reagents, such as nitroimidazole or related derivatives [20], which are administered systemically to live animals by intravenous injection, or added to culture medium in the case of in vitro models. These small amphiphilic molecules (e.g., for pimonidazole, a trade name for 2-nitroimidazole) readily penetrate through tissue and individual cells. When in cells, they get reduced by cellular reductases in an O2-dependent manner: low reduction at high and physiological O2 levels and high reduction in hypoxic and anoxic zones, with steep threshold transition from one mode to another (see Table 1; Fig. 1a). The resulting chemical product(s) accumulate in cells and interact with cellular components to produce adducts, which are then detected by immunofluorescent staining of thin (1–10 μm) sections of fixed tissue and subsequent imaging on conventional fluorescence microscopes. The advantages of this staining technique is its simplicity and well-established character, applicability to live animals (but not to humans), the ability to ‘freeze and remember’ the state of tissue hypoxia during subsequent sample manipulation, and simple data interpretation. However, the significant disadvantages of this method include indirect, non-quantitative, endpoint threshold hypoxia readout, the need to fix, section tissue samples (complicating, e.g., 3D analysis and reconstruction of ‘hypoxia’ maps) and subsequently probe them with antibodies, high dosage of the probe due to systemic administration and complex dose and time dependence of the optical signal.
Fig. 1.
Schemes and signal readouts in optical O2 and hypoxia imaging (see also Table 1). a Redox-sensitive indicators (e.g., pimonidazole) coupled with specific antibody staining of fixed cells/tissue. b Redox-sensitive fluorescent hypoxia stains and fluorescent proteins expressed under HRE promoter. Cells and tissues can be imaged live and fluorescence of different regions of interest (ROI) compared to assess the degree of hypoxia. c Fluorescent proteins with maturation rate sensitive to O2, e.g., UnaG-based [27]. Green fluorescent protein has O2-independent maturation, while the red is not produced under hypoxia. Signal ratio can report on hypoxia. d Red-shifted O2-dependent fluorescence of GFP induced by photoactivation [49]. The ratio between red and green fluorescence reports on hypoxia (reversible changes). e–g Quenched phosphorescence and delayed fluorescence-based O2 probes. e O2 probes reversibly change brightness (increases at hypoxia), can be used in semi-quantitative mode shown in b. f Ratiometric O2 probes have two emission bands (e.g., blue and red [40, 50–52]), which allow signal normalization and O2 quantification. g One or two-photon PLIM allows accurate O2 quantification, from measured emission decays and calculated phosphorescence lifetimes (O2 sensitive). Calibration, frequently shown as Stern–Volmer plots, can be linear [53] or non-linear (fitted with the two-site model [54]). PLIM can visualize tissue O2 concentration maps, subcellular gradients, and their dynamics
Some of these limitations can be overcome by designing more complex probe structures, which contain a redox-sensitive chemical moiety (e.g., 2-nitroimidazole, nitroaryl and others) coupled to a fluorophore moiety (fluorescein, indocyanine, cyanine), or its precursor (Table 1; Fig. 1b) [20]. P2 probes eliminate the need for tissue fixation, sectioning and staining with fluorescent antibodies, allowing for direct imaging analysis and sometimes repetitive measurement of live animals, tissue samples and cells. For example, azo-modified Ir(III) complexes exhibit phosphorescence after reduction by azo-reductases in hypoxia and show tunable spectral characteristics [26]. Still, measured optical signals produced by such probes are difficult to relate to specific oxygen concentrations (qualitative, threshold method), they show complex patterns (signal accumulation followed by decline), which are not easy to interpret. The design and optimization of P2 probes is complex, particularly tuning their cell permeability and possible toxic effects on cells and tissue and whole organisms.
Common feature of P1 and P2 probes is that they do not really ‘sense’ the degree of hypoxia but rather inform on the activity of cellular reductases, probe internalization and bioconversion processes. Nonetheless, fluorescent signals generated by these probes can be correlated with tissue hypoxia (frequently detecting O2 levels < 0.3–1%, or < 3–10 μM [20]), which makes them useful for some applications.
Genetically encoded biosensor constructs (P3–P5) based on fluorescent protein reporter moieties provide another way to analyze the tissue hypoxia. Their endogenous nature (produced inside live cells and tissue) has obvious benefits, in addition to their high brightness, tunable spectral characteristics, ease of intracellular targeting, probe biocompatibility and replenishment (degraded and synthesized again). The toolkit of fluorescent proteins also provides multiple options for hypoxia sensing: various hypoxia-responsive promoters, O2-dependent protein maturation or photoactivated fluorescence. Being bulky (> 27 kDa) and overproduced in cells (causing aggregation and other impacts), these biosensors are still regarded safe and non-toxic [55]. P3 type are fluorescent biosensor expression of which is controlled by a hypoxia-responsive element (HRE) promoter, such as HIF-1α gene [28]. In this case, detection of hypoxia can be achieved directly in live cells, similar to fluorescent redox dyes (Fig. 1b), but without the need to introduce exogenous chemicals and/or tissue staining. Easy transfection with plasmid DNA (for transient expression) or stable transformation (biosensor-expressing cell lines or transgenic animals) works well with many cell and tissue models. P3 biosensors can also be expressed specifically in a desired tissue/cell type and their photophysical properties can be tuned to produce bright, photostable and versatile hypoxia sensors [55, 56]. By tuning the degradation of the expressed protein marker and making its turnover faster, one can improve performance and make biosensor response almost real-time and reversible. However, the need to constantly produce such a biosensor in cells complicates its use in some areas such as studies of hypoxia-regulated protein translation [57, 58].
P4 probes include the ProCY [32] and related constructs, which selectively respond to the activity of prolyl hydroxylase enzyme (PHD) towards degradation of HIF-α protein. Complexation of HIF-α and HIF-β subunits, each tagged with a different fluorescent protein, was also used to monitor cellular hypoxia, particularly by live FRET or FLIM–FRET techniques [59]. However, due to overexpression of HIF subunits, such biosensors cannot inform on hypoxia onset and do not allow O2 quantification.
O2-dependent maturation of chromophore moiety in fluorescent proteins is a well-known phenomenon, which has been utilized in genetically encoded biosensors of P5 type. Ratiometric readout can also be achieved by coupling such a sensor with another O2-independent fluorescent protein moiety [27, 35] (Fig. 1c). Some variants of the O2-sensitive (< 2% O2) green fluorescent protein produce red spectral shifts upon photoactivation with intense laser pulse (Fig. 1d) [49]. However, due to high complexity, low sensitivity and efficiency of this process, such biosensors are hard to use in physiological studies [34]. Recently, ratiometric biosensor DsRedFT (Timer) was proposed, which is based on O2-dependent maturation of the different spectral forms of DsRed protein. The ratio between green (low O2) and red (high O2) fluorescence allows simple analysis of hypoxia in tissue and whole animals, on a broad range of fluorescence microscopy platforms [33].
Thus, P1–P5 probes provide indirect and qualitative (threshold) sensing and imaging of cell and tissue hypoxia or oxygenation state, which limits their utility. In contrast, P6–P10 probes, based on dynamic (i.e., collisional) quenching of long-lived photoluminescence of various reporter molecules and materials, provide direct photophysical, reversible and quantitative sensing and imaging of O2 concentration (or partial pressure) [13, 17, 18]. These approaches look more attractive, powerful and versatile, they can be used to quantitatively map oxygen tension and hypoxic regions of live cells over the physiological O2 range (0–20% or 0–200 μM), tissue samples, organs, vasculature and whole organisms, in 2D, 3D and with subcellular spatial resolution.
Direct optical sensing of O2
Endogenous delayed fluorescence technique [60] uses protoporphyrin IX (PPIX), a metal-free precursor of heme, as oxygen-sensing reporter. This reporter (P6, Table 1) is present in most animal cells and tissues. However, brightness of PPIX-delayed fluorescence is low (quantum yield < 1%), so as the physiological PPIX levels in cells. In addition, long lifetime of delayed fluorescence (τo ~ 800 μs) results in its strong quenching by oxygen, even at physiological and hypoxic levels. As a result, optical signals from this reporter are weak, masked by intense short-lived fluorescence of PPIX and tissue autofluorescence in the same spectral region. To enhance the specific signals, test cells, animals or human tissue are usually treated prior to O2 measurements with FDA-approved drug 5-aminolevulinic acid (ALA). Such treatment boosts mitochondrial PPIX levels many-fold, thus increasing specific optical signals and facilitating the measurements. Still, for selective and quantitative, this method requires sensitive phosphorescence lifetime-based O2 detection in time domain (phosphorescence lifetime imaging microscopy, PLIM). While the applicability of this method for high-resolution 2D/3D O2 imaging is still unclear, this is the only technique currently being tested with human patients [60].
Quenched phosphorescence-based probes represent the most advanced means for direct O2 quantification [17]. The major advantages compared to the P1–P5 probes are truly quantitative with real-time readout of O2 concentration, stable O2 calibration. Their large Stokes shifts allow for effective elimination of optical background and sample autofluorescence, and multiplexing with many conventional probes (Fig. 1g). Phosphorescent probes are brighter and more photostable than P6, however, they are exogenous and need to be delivered to cells or tissue. In O2 microscopy applications, they can be applied as intravascular cell-impermeable probes (P7), cell-penetrating small molecule conjugates (P8), nanosensors (P9) and solid-state materials (P10, Table 1; Fig. 1e–g).
The intravascular phosphorescent probes (P7) usually comprise dendrimer-modified metalloporphyrins with protected core and optimized antenna for improved two-photon excitation. They produce stable O2 calibration in blood, serum and other environment [12, 39] (Table 1). These cell-impermeable structures were developed by team of Wilson and Vinogradov initially as Pd-porphyrin and benzoporphyrin phosphors, without and with dendrimeric shielding (trade name Oxyphor) [61]. Later on, improved two-photon excitable probes were produced, and their development continues on [14, 62–64]. However, high analytical performance of P7 probes is downplayed by their complex structure and synthesis procedure, which make them expensive and inaccessible for many users (independent researchers). They also need specialized two-photon PLIM setup for experiments with live animals. Relying on systemic administration in blood/vasculature and use of live animals (cannot be used in humans), P7 share many limitations of the redox probes described above. In addition, the bulk of the animal tissue outside blood vessels and microcapillaries still remains inaccessible for imaging, as it cannot be stained with these probes.
A number of ‘alternative’ probes for in vivo imaging have been reported in recent years. For example, probes based on phosphorescent Ir(III) complexes [65–67] stain metastatic and solid tumor tissues due to enhanced vascular permeability, and produce steep (almost ‘Off/On’) response to hypoxia. Analytical performance of these probes is usually not as good as for the dendrimeric probes, in terms of brightness, reliability of quantitative measurements, short clearance times and complex distribution in different tissues and organs. They also require more detailed characterization, assessment of toxicity and evaluation of potential side effects, validation in complex physiological and oxygen imaging studies, benchmarking against other probes with relevant disease models. On the other hand, their more simple design and synthesis makes them more accessible, and some are produced commercially and used by external labs.
Over the last decade, cell-penetrating phosphorescent probes have been under active development and application. Unlike the intravascular probes, these probes have the ability to interact with cells, get internalized and retained inside cells for a long time (days) without being toxic to the cells [18]. They allow O2 quantitation directly in cells and tissue samples, quantitatively and with high spatial resolution. While such intracellular probes are not ideal for systemic intravenous administration in live animals, they are excellent tools for in vitro O2 imaging studies with cells and in 3D tissue models (multicellular aggregates, spheroids, organoids or tissue explants), and for local administration in animal tissue.
Small-molecule intracellular oxygen sensing probes (type P8) originated from initial intracellular studies with PdTCPP [68, 69] and RTDP [70, 71] dyes. In these studies, the accumulation of dyes in cells was modest, displayed non-specific binding and interactions with cellular components resulting in prominent, complex distribution patterns in cells, unstable oxygen calibration (heterogeneous lifetime distribution in non-respiring cells) and significant phototoxicity via damage of plasma membrane and DNA [18]. Improvements in intracellular targeting and minimization of other side effects were achieved by conjugation with hydrophilic delivery vectors, such as cell-penetrating peptides, mitochondria targeting groups [16, 18]. Thus, structure–activity analysis of hydrophilic Pt(II) and Ir(III) porphyrins conjugated with various Arg- and Pro-rich cell-penetrating peptides produced a family of probes with different intracellular distribution (e.g., endosomal, lysosomal or whole cell distribution) but moderate photostability and brightness [72–75]. Later on, by modifying the highly photostable but hydrophobic PtTFPP dye with carbohydrate moieties, the Pt-Glc probe was produced [76], which is hydrophilic, uncharged and efficiently stains a broad range of mammalian cells and 3D tissue models without detectable interferences, toxicity or impact on cell function. Pt-Glc was benchmarked against pimonidazole staining in the original study [76] and used in a number of complex physiological studies with cells, ex vivo tissue samples, disease and organoid models helping to reveal interesting new data on intracellular O2 gradients [76–80]. The main drawbacks of this and related probes of this type are low two-photon excitability, compared to P9 type.
Intracellular oxygen nanosensors (P9) are designed to provide: (1) stable incorporation of the phosphorescent reporter and (optionally) secondary antenna dye; (2) formation or stable nanoparticle structures with desired surface functionality (charge, chemical groups, size) for efficient delivery into cells; (3) optimal quenching of the phosphor by O2 while shielding it from potential interferences; (4) high biocompatibility and no significant cyto- and phototoxicity. Compared to P7 and P8, the nanosensors display high intrinsic brightness due to high density of phosphor molecules in each nanoparticle (~ 1% w/w); ease of tuning of nanosensor spectral characteristics by changing the phosphor; biocompatibility and high brightness, including under two-photon excitation [40, 81]. Several generations of nanosensors were described over the years, including the structures that require assisted delivery into the cell [82, 83] and multi-modal, tunable, versatile, two-photon excitable nanoparticles [40, 84, 85]. These O2 probes are compatible with various cell and animal models, provide high brightness, robustness and stable O2 calibration. Their negative sides are the complex design and relatively slow cell internalization process (12–16 h), especially with 3D tissue models [18, 40, 86, 87].
P10 group represents solid-state sensor materials. One example is planar sensor foils, which comprise a phosphorescent thin-film polymeric-coating applied on an oxygen-impermeable substrate, such as polyester film [46, 88]. Such sensors can be placed on tissue surface, such as animal skin, tumor, brain cortex, and then imaged on a camera-based macro-imager [19, 41, 89] (Table 1). Another format is a suspension of sensor microparticle beads applied on tissue surface, allowing multi-point measurement and continuous monitoring of extracellular O2 [43]. Third example is fibrous or microporous polymer sensor materials, which can be used as scaffolds for 3D cell culture and informing on physiological pericellular O2 fluxes [47, 48, 90]. Bandages with sprayable O2-sensitive coatings were also used to monitor healing of injured tissue [41]. The advantages of these materials are that sample does not have to be stained, thus eliminating the problems of probe delivery and cytotoxicity. These sensor materials show high brightness, ease of manufacturing (Fig. 1e), low cost and compatibility with intensity-based detection. Being reusable, they represent an attractive alternative to P1–P9 probes, especially when staining of cells and tissue is not acceptable. However, P10 probes can only report on O2 levels at tissue surface or points of contact between the sensor and sample. P8–P10 probes can also be used on many commercial plate readers with time-resolved fluorescence capability (e.g., BMG, PerkinElmer, Tecan), analysing many biological samples and conditions in parallel [17].
Overall, the group of quenched phosphorescence-based probes and imaging techniques look very attractive and versatile for direct quantification and mapping of cell and tissue O2 concentration in live material (Table 1). However, the other listed approaches can also be appropriate, depending on the biological model, experimental task and available microscope (see “Choice of the microscope and imaging modality”). At the same time, more critical comparison and benchmarking of the different probe types is also necessary.
Choice of the microscope and imaging modality
High analytical power, information content, versatility and broad application base of fluorescence microscopy, have given rise to many modifications of such systems, from conventional one-photon laser-scanning confocal microscope to time-correlated single photon counting (TCSPC)-based FLIM and PLIM platforms. However, only a fraction of these systems are properly configured and suit oxygen microscopy experiments by ordinary users. Although a number of significant advancements in this field have been made and new biological findings reported in the literature, experimental artifacts, technical confusions and failures are still common. Such problems are often associated with lack of knowledge of the probe-sensing principles, unavailability or non-optimal selection of the O2/hypoxia probe, fluorescence microscopy platform or imaging modality, especially when it comes to phosphorescent probes and quantitative O2 imaging. This slows down the adoption this technology and its wider application in physiological studies with complex cell and tissue models.
The range of instruments currently available for fluorescence imaging of cell and tissue hypoxia and oxygenation is presented in Table 2, along with compatible probes and their characteristic features.
Table 2.
Common fluorescence microscopy platforms suitable for oxygen imaging
| Type | Microscope | Compatible probe types | Notes |
|---|---|---|---|
| M1 | Entry-level- wide-field- or confocal fluorescence microscope | P1, P2, P4 | End point, qualitative imaging. 2D, thin tissue sections |
| M2 | Live cell laser-scanning fluorescence microscope, one or two-photon excitation | P1–P5, P8–P10 | Qualitative or semi-quantitative (intensity-based ratiometric mode). 2D, 3D and time-lapse measurements |
| M3 | Live cell camera-based fluorescence microscope: 2D and 3D (Nipkow disk) imaging in intensity mode | P1–P5, P8–P10 | Qualitative or semi-quantitative (intensity-based ratiometric mode). High-resolution, 2D, 3D and time-lapse measurements |
| M4 | Live cell PLIM microscope (wide field or laser scanning, also allows intensity mode) | All probe types, particularly P7–P8 | Quantitative (P6–P10), accurate, high sensitivity and spatial resolution. 2D, 3D and time-lapse measurements |
| M5 | In vivo two-photon imaging system, laser-scanning with PLIM capabilities | P2–P5, P9 | Quantitative, accurate, high sensitivity, deep penetration into tissue (> 300 μm) |
| M6 | Animal macro-imager (fluorescence intensity-based) | P2–P4, P8, P9 | Qualitative (threshold), low resolution, 2D. Imaging animals, large objects (with NIR probes) |
Ex excitation, Em emission, LED light emitting diode, NIR near infrared, PLIM phosphorescence lifetime imaging microscopy
The entry-level fluorescence microscopes (Type M1) are equipped with continuous wave excitation light sources (mercury lamp or LED module) and basic CCD camera. Typically, such systems provide one-color fluorescence intensity readout, no or little automation of sample handling, image acquisition and processing, moderate sensitivity, no control of sample environment and 3D imaging capability. The poorly controlled sample illumination can also cause rapid probe bleaching, making kinetic or any quantitative imaging experiments problematic. Such systems are suitable for snapshot qualitative endpoint imaging of hypoxia in live and fixed samples stained with probes P1–P5 (Table 1). M1 often allow fixed selection of excitation and emission wavelengths and certain fluorophores (DAPI, FITC, rhodamine, GFP).
The live cell imaging laser-scanning microscope (M2) significantly extends the range of available O2 imaging options, allows for prolonged imaging and ratiometric detection [51]. They include incubator on sample stage, which provides control of environment (T, humidity, CO2 and preferably O2), thus allowing prolonged experiments with life cells in physiologically relevant conditions. One-photon systems contain lasers or LEDs providing excitation in the visible range 400–650 nm, pinhole detection optics with two or more photodetectors (PMTs or avalanche photodiodes), multicolor and ratiometric intensity signal readouts. The confocal mode and X–Y–Z scanning capability provide better quality images than on wide-field systems, and allow for analysis of 3D tissue models. Two-photon systems use restricted volume excitation with a NIR femtosecond laser (typically tunable Ti:sapphire), photon-counting detectors with large active area (PMTs); they provide better image quality and light-penetration depth, but are considerably more expensive. M2 systems are suitable for probes P1–P5, P8–P10, but mainly for qualitative or semi-quantitative measurements. Due to their pixel-by-pixel scanning mode, image acquisition is rather slow, especially in 3D imaging mode. Particular examples can be found in [4, 51, 87, 91].
The functionally similar M3 setup is a high-end camera-based microscope for live cell analysis, which has higher sensitivity than M1 and many features of M2, along with fast image acquisition rates. M3 systems are particularly useful for monitoring rapid physiological responses and processes (e.g., brain activity on a sub-second time scale), but usually they are limited to 2D imaging [92]. M3 can operate with the same range of O2 probes as M2, and have similar limitations due to intensity readout mode.
M2 and M3 systems are produced by all major vendors of imaging equipment (Zeiss, Leica, Nikon, Olympus), and by many smaller companies.
M4 group includes live cell PLIM microscopes, based on M2 or M3 platforms and integrated with PLIM (also called μs-FLIM) hardware and software to enable lifetime-based imaging in the microsecond time range. The two main approaches are the time domain and phase domain (frequency modulated) PLIM [93]. The first is better suited for confocal microscopy, while the latter for camera-based systems. PLIM systems can rely on wide-field illumination (e.g., pulsed LED coupled with fast CCD camera [94], wide-field TCSPC [95]) or laser-based scanning [96]. The time-domain PLIM is more stable and accurate, whereas phase measurements can be influenced by scattered light, autofluorescence and photostability [97]. Therefore, it is the method of choice for fully quantitative O2 measurements with phosphorescent probes. Other fluorescent probes can also be imaged using intensity or FLIM mode [98]. These platforms together with P6–P10 O2 probes are most preferred for high-resolution O2 imaging studies with in vitro cell and 3D tissue models. Particular examples can be found in [87, 94, 99, 100]. M4 can be set up by means of a simple upgrade of standard M2 or M3 systems. A number of companies are offering FLIM/PLIM hardware for this, including Becker&Hickl, PicoQuant, LaVision, PCO, Andor and Hamamatsu. Ordinary microscopes can be upgraded with affordable pulsed diode lasers, confocal scanner, FLIM–PLIM compatible hardware (TCSPC or time-gated camera) and software. M4 setups also enable efficient separation of specific phosphorescent signal optical background (scattering and tissue autofluorescence), using spectral (red/NIR emission) and time discrimination (phosphorescence lifetimes are much longer than autofluorescence).
M5 is a system for in vivo tissue imaging set up for experiments with live animals (anesthetized mice and rats [101]). M5 are usually built on the basis of M2–M4 platforms (high-end confocal, two-photon or camera-based systems), which are installed in a dedicated room licensed for animal work with ethical permissions, special licenses for the instrument, animal handling, anesthesia, surgery (implantation of a cranial window), etc. The custom-built two-photon in vivo PLIM platforms have been very successful with intravascular O2 probes, which were specifically tailored to this application and used in a number of high-impact studies of stroke and other pathology models [12, 102–105] [101, 104, 105].
M6 is an animal macro-imager designed for fluorescence and bioluminescent imaging of whole animals, postmortem tissue, organs and other macroscopic objects. Such imagers are usually equipped with wide-field illumination and optics providing multiple excitation and emission wavelengths in the Vis–NIR range, high-sensitivity camera, internal temperature control, local anesthesia equipment and optical insulation (dark box). Due to low-magnification optics, their spatial resolution is relatively low and imaging is only in 2D (signals are averaged across the Z-axis). Such systems work best with red- and infrared probes which reduce strong tissue autofluorescence and light scattering [40, 106, 107]. They are used mostly for qualitative and semi-quantitative endpoint measurements of O2 in live and postmortem tissue samples stained with P1–P5 and P8–P10 probes.
Finally, it is worth noting that imaging systems specially configured for O2 microscopy, especially with probes P6–P10, are not widespread. They are not offered by vendors as standard off-the-shelf products, but rather as advanced customized versions which require certain effort by the user to configure and adapt them for this application [87, 94, 100]. A number of such systems and prototypes tailored to specific applications have been described, including the high-end two-photon PLIM systems for in vivo studies [104, 105, 108, 109]. Another major gap is the lack of dedicated software and signal acquisition settings for O2 imaging, which can provide convenient, robust, automated, high-throughput collection of PLIM images, subsequent fitting, transformation and batch-processing of raw PLIM data (pixel-by-pixel, stack-by stack), determination of lifetime and O2 concentration values, presentation and handling of large sets of imaging data [110, 111]. This situation is beginning to change with several vendors offering integrated solutions for live cell FLIM–PLIM applications including O2 imaging (e.g., Leica, Becker&Hickl, PicoQuant). Additional accessories, such as gas mixer and T-incubator which can ramp gaseous and dissolved O2 concentration, are also highly desirable for such systems, for calibrating the O2 probe and complete imaging setup (probe, microscope and test sample).
Applications of O2 microscopy and general considerations
Fluorescence microscopy and particularly O2 microscopy is applicable to a broad range of biological models: cell lines and primary cells, 2D cultures of adherent cells, multicellular aggregates (spheroids), live tissue explants, cultured tissue, organs-on-a-chip, organoids, and even live animals. However, researchers need to consider specific features of the method and sample (e.g., light scattering, penetration depth) and select the most appropriate O2 probe and microscopy platform (see Tables 1, 2). Thus, probe biodistribution and local concentrations must be considered in the design of O2 imaging experiments. Fluorescent biosensor constructs are expressed endogenously in transfected or transgenic cells and tissues, while the exogenous probes must be delivered to specific locations of the tissue and in reasonable time (e.g., 1–24 h) using appropriate transport mechanisms. Delivery of phosphorescent O2 probes is discussed in detail in recent reviews [18, 85].
Questions such as where, when and how to measure cellular O2 and tissue hypoxia also dictate the choice of the most appropriate biological model, probe and detection equipment. Inherent limitations of optical imaging include the physical dimensions of test objects, light-penetration depth and spatial resolution required for imaging [1, 101, 112–114]), means of probe delivery to the cells and sample manipulation. The biological model also determines maximal duration of imaging experiments. The overall goal of the study and specific analytical and biological questions that researcher wants to address are also important for experimental design.
Visualization of O2 and hypoxia on their own rarely provide complete information on the physiological question. Therefore, it is usually combined with other imaging parameters and functional readouts, which impose additional selection criteria on the O2 imaging probe and method. Compared to the other types, phosphorescent probes and sensors P8–P10 are better suited for multi-parametric imaging. They have very characteristic and well-resolved excitation and emission bands compatible with many conventional probes and fluorescent proteins [33]. They also allow post-processing of the tissue under investigation, including fixation, clearing and immunostaining [98]. Even the O2 probes with two reporter dyes (additional intensity reference or two-photon antenna) leave wide spectral window for 2–3 other fluorophores, they also allow temporal multiplexing (μs PLIM and ns FLIM domains) in each spectral channel [87, 115, 116]. Several recent reviews on optical O2 imaging [10, 12, 13, 16] describe various biological applications of O2 microscopy with mammalian cells and tissue models.
Monitoring of cell and tissue oxygenation upon metabolic stimulation (drugs, cytotoxic agents, and other stimuli) is one of the main foci for oxygen imaging. Indeed, intracellular O2 can inform on the balance between O2 supply to the cells (by diffusion, vascular system and erythrocytes) and its consumption and gradients due to activity of the mitochondria and other oxidative processes [117–119]. Low cellular O2 (hypoxia) induces adaptive responses through several major signaling pathways, which result in major changes in gene expression, proteome, energy-production pathways, cell function, and life/death decisions [120]. Standard 2D cultures of adherent cell lines do not normally display prominent subcellular O2 gradients [14], but the whole monolayer is often hypoxic due to active respiration and its action as O2 sink [117, 121]. These studies also demonstrated that for O2 probes, localisation is not so critical as for many other probes, since O2 gradients between extra- and intracellular space are usually very small, if exist at all, whereas on a larger scale (> 50 μm) O2 gradients in tissue can become very prominent. Therefore, cellular O2 (reported by P8, P9 probes) is informative marker of hypoxia in such models [122], however, this information can also be obtained on more simple instruments such as time-resolved fluorescence plate readers [118, 123, 124]. On the other hand, large and actively respiring cells, such as myocytes or giant umbrella cells of mouse bladder, do show profound intracellular O2 gradients, as revealed with the phosphorescent probes and PLIM [78, 125]. As expected, these gradients also co-localize with active mitochondria [78]. Photoactivated green fluorescent protein (P5 probe) was also used for analysis of subcellular O2 gradients [34], the magnitude of which was determined for adherent Hep3B cells [126].
For larger and more complex models such as multicellular aggregates, spheroids, tissue explants, the presence of spatial O2 gradients is more evident and their roles become significant. Typical spheroids with sizes > 50 μm show clear core to periphery gradients, which depend on the distribution of different cell types and activity of their oxidative phosphorylation machinery [87, 127–129]. One should keep in mind, that under static conditions O2 gradients in such samples also extend into surrounding medium [118], but they might not be visible if the reporter probe is not present in this compartment. On the other hand, dynamics and heterogeneity of O2 in 3D tissue models emphasize the importance of O2 imaging and 3D microscopy as preferred analytical tools. Indeed, mean/averaged values of O2 concentration or O2 consumption rate give little information on the real microenvironment, metabolic heterogeneity of cells and O2 micro-gradients in such micro-tissue models.
Monitoring of O2 in 3D models with phosphorescent probes (types P8, P9) and PLIM method are useful for the analysis of drug action on cells, and effects of their hypoxic status [50, 76, 127] on treatment outcomes. Furthermore, live analysis of O2 by PLIM in neurospheres produced from primary rat neurons was followed by tissue fixation, immunofluorescent staining, cell lysis and Western blot analysis, and this helped to elaborate the effects of sample environment on cell oxygenation, proliferation and distribution of stem cells [87]. O2 PLIM method was also multiplexed with FLIM-based analysis of tissue endogenous autofluorescence [100], temperature [115] and cell proliferation [116], and with conventional imaging readouts and fluorescent probes for cell death during drug treatment [47, 127], changes in intracellular pH, mitochondrial membrane potential, and other biochemical assays such as real-time PCR [130]. Multi-parametric O2 imaging of small intestinal organoid models with P8 probe revealed their high metabolic heterogeneity and emphasized challenges in culturing, standardization and physiological studies with such models [131]. Fluorescent biosensor construct acting under HRE promoter was used to visualize oxygenation state of islets made of MIN6 cells, and correlate it with HIF activity and distribution of dying cells [28–30].
Cells grown in 3D scaffolds and bioengineered tissue samples also show profound O2 gradients. Hybrid polymeric scaffolds modified with an O2-sensitive phosphorescent dye (P10 sensor) provided simple and convenient means to analyze such cultures by PLIM, with single-cell resolution and deep 3D visualization of pericellular O2 concentration, along with the other biomarkers [47].
Using live tissue sections of animal brain and colon mucosa, ex vivo PLIM imaging with phosphorescent probes (P8, P9) helped to analyze in situ oxygenation of cells, informing on tissue viability and metabolic status [50, 80]. Such methodologies substantially extend the capabilities of redox-based hypoxia probes or solid-state sensors in probing hypoxia in colon mucosa [132–134].
In vivo imaging of O2 in brain vasculature is another important application. P7 probes are injected in the tail vein of anesthetized mice (i.e., systemic administration) and analyzed by two-photon PLIM microscopy [135] through implanted cranial window [102, 104]. Similar studies were conducted with bone marrow [136] and tumors [101, 137], which have improved our understanding of oxygenation and blood flow dynamics in these tissues, in norm and under pathological conditions such as stroke or anesthesia [39, 102]. Another approach to functional brain imaging was to apply near-infrared phosphorescent nanoparticles ‘NanO2-IR’ (probe P9 [138]), sensor microparticles (P10 [43]) or planar sensor foils (P10 [46]) on the surface of brain cortex. Although these probes and sensors were imaged in intensity mode, simple experimental settings provided useful data about fast dynamics of neuronal activity, excitation of localized regions of animal brain and associated metabolic responses (relative changes in O2). In these studies, O2 profiles and dynamic changes were also correlated with the other functional readouts generated on the same imaging system, including the fluorescent voltage-sensitive probe and tissue (endogenous FAD+) autofluorescence [43, 46, 138].
Eye tissue, including retina and cornea, is directly accessible for optical microscopy and macroscopy. Thus, Shonat and Kight pioneered one-photon imaging of O2 in mouse retina and compared it with invasive point measurements with microelectrodes [139]. Higher resolution in mapping of retinal vascular oxygenation was achieved in the later studies [140–142]. Cornea cross-linking in the presence of riboflavin is an O2-dependent process, which can benefit from direct oxygen imaging [143, 144]. O2 distribution and diffusion in pig’s eye cornea (ex vivo model) upon such treatment were analyzed with P9 probe and confocal one-photon PLIM [145].
Photodynamic therapy (PDT) uses photosensitizer dyes [146, 147], which upon laser irradiation kill cancer cells in an oxygen-dependent manner [81, 84, 148–150]. Efficiency of PDT can be improved by imaging tissue O2 with phosphorescent probes, which are also good photosensitizers [151].
Imaging skin tissue and transcutaneous O2 is also a useful tool for PDT of skin cancers, diagnostics of metabolic disorders and wound healing. Delayed fluorescence of PPIX (P6 probe) was used to probe O2 gradients between mitochondria and extracellular space [38], in animal tissue and human skin [60, 152], but mainly by point measurements. Phosphorescent sensor foils (P10) were used to visualize transcutaneous O2 gradients, on a wide-field macro-imager [45, 153, 154]. Sprayable phosphorescent bandages (similar to P10) were suggested for monitoring O2 levels in skin and perfused tissue during wound healing by imaging [41, 155].
Future prospects
The above examples demonstrate the high utility and application potential of O2 microscopy, particularly for studies of 3D micro-tissue models, cell metabolism, cancer biology, stem cell research, tissue engineering, regenerative medicine and other areas. More complex models and physiological studies demand high-end customized imaging platforms, such as live cell two-photon FLIM–PLIM systems. At the same time, many existing instruments such as one-photon PLIM, intensity-based and wide-field imagers can also be used in many cases, taking advantage of their suitability for particular models and analytical tasks and considering carefully their inherent limitations. There is a clear trend towards broader use of direct O2 sensing techniques with phosphorescent probes and PLIM, due to their live, real-time, quantitative O2 readout, high-spatial resolution (down to single cell and subcellular level) and suitability for multi-parametric and time-lapse imaging.
Microscopes that can perform O2 imaging can be found in many labs, while advanced FLIM and PLIM systems are also spreading rapidly and becoming more user-friendly and popular in related areas (FLIM–FRET). Growing demand for O2 microscopy promotes upgrades of standard imaging systems and makes more affordable hardware and software packages for such applications.
The list of commercially available O2 probes is also growing steadily (Table 3), reflecting the importance of O2 and hypoxia imaging for various experimental models and increasing demand of researchers [9].
Table 3.
Commercially available probes and reagents for optical oxygen microscopy
| Product(s) | Applications | Manufacturer/vendor |
|---|---|---|
| Pimonidazole-based kits for hypoxia detection by immunofluorescence [25] | In vivo labeling, endpoint visualization of hypoxic regions in animal tissue by immunofluorescence. Indirect and qualitative | Hypoxyprobe Inc. |
| Hypoxia detection probe MAR green-emitting redox-sensitive dye [20] | End-point, indirect, live-imaging assay | Goryo Chemical |
| ROS-ID hypoxia/oxidative stress detection kit. Combination of redox-sensitive (red) [20] and ROS-specific (green) turn-on reagents | Endpoint. Combines analysis of hypoxia via cell nitroreductases with overall ROS detection | Enzo Life Sciences |
| HypoxiSense 680-carbonic anhydrase IX-specific near-infrared phosphor | In vivo, endpoint visualization of hypoxic tissue over-expressing CAIX. Indirect and qualitative | PerkinElmer |
| Oxyphors R0, R2 and G2–intravascular phosphorescent probes based on porphyrin dendrimers [61, 156] | Quantitative in vivo PLIM-based O2 imaging | Oxygen Enterprises |
| Image-iT Red Hypoxia reagent (ThermoFisher), LOX-1 Hypoxia probe (Organogenix). Based on phosphorescent iridium complex [67] | Semi-quantitative, real-time O2 imaging. Potentially works in PLIM. 2D and 3D cell cultures. In vivo | ThermoFisher, Organogenix, Invitrogen |
| MitoImage®-NanO2 and MM2 nanoparticle probes based on hydrogel polymer and PtTFPP dye [52, 121] | Direct, real time and quantitative. High-resolution imaging of O2 in 2D and 3D. One- and two-photon PLIM or ratiometric-intensity modes |
Luxcel-Agilent Ibidi |
The traditional, indirectly acting hypoxia probes, such as pimonidazole immunostains, its fluorescent derivatives or protein markers such as HIF, CAIX (top four in Table 3) are still in use. However the direct, reversible and truly quantitative phosphorescent probes (e.g., Oxyphors, MitoImage probes, Table 3) are quickly gaining popularity and frequently used in physiological studies [10, 18].
Many more O2 probes have been described, but could not make their way to dissemination and broader use. This is mainly due to their non-optimal working characteristics, such as low brightness, staining efficiency, cell specificity and biocompatibility; non-optimal sensitivity to O2, stability of O2 calibration, photostability and reproducibility; significant intrinsic and photo-toxicity, impact on cell function; lack of superiority over the existing probes or lack of validation with different cell and tissue models, imaging setups and physiological studies.
Therefore, when choosing an O2 probe for a particular study, user needs to critically review all key application specifications and identify best probe structure, including the type of probe (Table 1), particular commercial product (Table 3) or research-grade probe from developer.
Direct head-to-head comparison of the different probe types is also necessary, as this will allow their more objective and critical evaluation, ranking and defining niche application areas. Ultimately, this will aid the integration of O2 microscopy in the current paradigm of life science and biomedical research. In our view, it should become an integral part of basic tissue culture, complex cell biology studies and particularly advanced 3D cell and tissue models.
Acknowledgements
Financial support of this work by the Science Foundation Ireland (SFI), Grants 13/SIRG/2144 (RID) and 12/RC/2276 (DBP), and the Russian Science Foundation (RSF) Grant 18-15-00407 (RID) is gratefully acknowledged.
Compliance with ethical standards
Conflict of interest
D.B.P. is a former stakeholder of Luxcel Biosciences (now part of Agilent). R.I.D. has no conflicts of interests.
Contributor Information
Dmitri B. Papkovsky, Email: d.papkovsky@ucc.ie
Ruslan I. Dmitriev, Email: r.dmitriev@ucc.ie
References
- 1.James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92(2):897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Almassalha LM, Chandler JE, Zhou X, Stypula-Cyrus YE, Hujsak KA, Roth EW, Bleher R, Subramanian H, Szleifer I, Dravid VP, Backman V. The effects of chemical fixation on the cellular nanostructure. Exp Cell Res. 2017;358(2):253–259. doi: 10.1016/j.yexcr.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnell U, Dijk F, Sjollema KA, Giepmans BN. Immunolabeling artifacts and the need for live-cell imaging. Nat Methods. 2012;9(2):152–158. doi: 10.1038/nmeth.1855. [DOI] [PubMed] [Google Scholar]
- 4.Frigault MM, Lacoste J, Swift JL, Brown CM. Live-cell microscopy–tips and tools. J Cell Sci. 2009;122(6):753–767. doi: 10.1242/jcs.033837. [DOI] [PubMed] [Google Scholar]
- 5.Stich MIJ, Fischer LH, Wolfbeis OS. Multiple fluorescent chemical sensing and imaging. Chem Soc Rev. 2010;39(8):3102–3114. doi: 10.1039/b909635n. [DOI] [PubMed] [Google Scholar]
- 6.Guo Z, Park S, Yoon J, Shin I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem Soc Rev. 2014;43(1):16–29. doi: 10.1039/C3CS60271K. [DOI] [PubMed] [Google Scholar]
- 7.Chinen AB, Guan CM, Ferrer JR, Barnaby SN, Merkel TJ, Mirkin CA. Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem Rev. 2015;115(19):10530–10574. doi: 10.1021/acs.chemrev.5b00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavis LD. Teaching old dyes new tricks: biological probes built from fluoresceins and rhodamines. Annu Rev Biochem. 2017;86:825–843. doi: 10.1146/annurev-biochem-061516-044839. [DOI] [PubMed] [Google Scholar]
- 9.Place TL, Domann FE, Case AJ. Limitations of oxygen delivery to cells in culture: an underappreciated problem in basic and translational research. Free Radic Biol Med. 2017;113:311–322. doi: 10.1016/j.freeradbiomed.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papkovsky DB, Dmitriev RI. Biological detection by optical oxygen sensing. Chem Soc Rev. 2013;42(22):8700–8732. doi: 10.1039/c3cs60131e. [DOI] [PubMed] [Google Scholar]
- 11.Wong H-S, Dighe PA, Mezera V, Monternier P-A, Brand MD. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem. 2017;292(41):16804–16809. doi: 10.1074/jbc.R117.789271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roussakis E, Li Z, Nichols AJ, Evans CL. Oxygen-sensing methods in biomedicine from the macroscale to the microscale. Angew Chem Int Ed. 2015;54(29):8340–8362. doi: 10.1002/anie.201410646. [DOI] [PubMed] [Google Scholar]
- 13.Wolfbeis OS. Luminescent sensing and imaging of oxygen: fierce competition to the Clark electrode. BioEssays. 2015;37(8):921–928. doi: 10.1002/bies.201500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finikova OS, Lebedev AY, Aprelev A, Troxler T, Gao F, Garnacho C, Muro S, Hochstrasser RM, Vinogradov SA. Oxygen microscopy by two-photon-excited phosphorescence. ChemPhysChem. 2008;9(12):1673–1679. doi: 10.1002/cphc.200800296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J-n BuW, Shi J. Chemical design and synthesis of functionalized probes for imaging and treating tumor hypoxia. Chem Rev. 2017;117(9):6160–6224. doi: 10.1021/acs.chemrev.6b00525. [DOI] [PubMed] [Google Scholar]
- 16.Yoshihara T, Hirakawa Y, Hosaka M, Nangaku M, Tobita S. Oxygen imaging of living cells and tissues using luminescent molecular probes. J Photochem Photobiol C. 2017;30:71–95. doi: 10.1016/j.jphotochemrev.2017.01.001. [DOI] [Google Scholar]
- 17.Dmitriev RI, Papkovsky DB. Optical probes and techniques for O2 measurement in live cells and tissue. Cell Mol Life Sci. 2012;69:2025–2039. doi: 10.1007/s00018-011-0914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dmitriev RI, Papkovsky DB. Intracellular probes for imaging oxygen concentration: how good are they? Methods Appl Fluoresc. 2015;3(3):034001. doi: 10.1088/2050-6120/3/3/034001. [DOI] [PubMed] [Google Scholar]
- 19.Quaranta M, Borisov SM, Klimant I. Indicators for optical oxygen sensors. Bioanal Rev. 2012;4:115–157. doi: 10.1007/s12566-012-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elmes RB. Bioreductive fluorescent imaging agents: applications to tumour hypoxia. Chem Commun. 2016;52(58):8935–8956. doi: 10.1039/C6CC01037G. [DOI] [PubMed] [Google Scholar]
- 21.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9(8):1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spielmann T, Xu L, Gad AK, Johansson S, Widengren J. Transient state microscopy probes patterns of altered oxygen consumption in cancer cells. FEBS J. 2014;281(5):1317–1332. doi: 10.1111/febs.12709. [DOI] [PubMed] [Google Scholar]
- 23.Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med. 2008;49(Suppl 2):129S–148S. doi: 10.2967/jnumed.107.045914. [DOI] [PubMed] [Google Scholar]
- 24.Raleigh J, Franko A, Koch C, Born J. Binding of misonidazole to hypoxic cells in monolayer and spheroid culture: evidence that a side-chain label is bound as efficiently as a ring label. Br J Cancer. 1985;51(2):229. doi: 10.1038/bjc.1985.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varia MA, Calkins-Adams DP, Rinker LH, Kennedy AS, Novotny DB, Fowler WC, Raleigh JA. Pimonidazole: a novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinoma. Gynecol Oncol. 1998;71(2):270–277. doi: 10.1006/gyno.1998.5163. [DOI] [PubMed] [Google Scholar]
- 26.Sun L, Li G, Chen X, Chen Y, Jin C, Ji L, Chao H. Azo-based iridium (iii) complexes as multicolor phosphorescent probes to detect hypoxia in 3d multicellular tumor spheroids. Sci Rep. 2015;5:14837. doi: 10.1038/srep14837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erapaneedi R, Belousov VV, Schäfers M, Kiefer F. A novel family of fluorescent hypoxia sensors reveal strong heterogeneity in tumor hypoxia at the cellular level. EMBO J. 2016;35(1):102–113. doi: 10.15252/embj.201592775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skiles ML, Fancy R, Topiwala P, Sahai S, Blanchette JO. Correlating hypoxia with insulin secretion using a fluorescent hypoxia detection system. J Biomed Mater Res B Appl Biomater. 2011;97B(1):148–155. doi: 10.1002/jbm.b.31796. [DOI] [PubMed] [Google Scholar]
- 29.Skiles ML, Sahai S, Blanchette JO. Tracking hypoxic signaling within encapsulated cell aggregates. J Vis Exp. 2011;58:3521. doi: 10.3791/3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skiles ML, Wilder NB, Sahai S, Blanchette JO. Identifying HIF activity in three-dimensional cultures of islet-like clusters. Int J Artif Organs. 2013;36(3):175–183. doi: 10.5301/ijao.5000193. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Wang H, Li J, Entenberg D, Xue A, Wang W, Condeelis J. Direct visualization of the phenotype of hypoxic tumor cells at single cell resolution in vivo using a new hypoxia probe. Intravital. 2016;5(2):e1187803. doi: 10.1080/21659087.2016.1187803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youssef S, Ren W, H-w Ai. A genetically encoded FRET sensor for hypoxia and prolyl hydroxylases. ACS Chem Biol. 2016;11(9):2492–2498. doi: 10.1021/acschembio.6b00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lidsky PV, Lukyanov KA, Misra T, Handke B, Mishin AS, Lehner CF. Genetically-encoded fluorescent probe for imaging of oxygenation gradients in living Drosophila. Development. 2018 doi: 10.1242/dev.156257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi E, Takano T, Nomura Y, Okano S, Nakajima O, Sato M. In vivo oxygen imaging using green fluorescent protein. Am J Physiol Cell Physiol. 2006;291(4):C781–C787. doi: 10.1152/ajpcell.00067.2006. [DOI] [PubMed] [Google Scholar]
- 35.Potzkei J, Kunze M, Drepper T, Gensch T, Jaeger K-E, Buechs J. Real-time determination of intracellular oxygen in bacteria using a genetically encoded FRET-based biosensor. BMC Biol. 2012;10(1):28. doi: 10.1186/1741-7007-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mik EG, Ince C, Eerbeek O, Heinen A, Stap J, Hooibrink B, Schumacher CA, Balestra GM, Johannes T, Beek JF, Nieuwenhuis AF, van Horssen P, Spaan JA, Zuurbier CJ. Mitochondrial oxygen tension within the heart. J Mol Cell Cardiol. 2009;46(6):943–951. doi: 10.1016/j.yjmcc.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Mik EG, Johannes T, Zuurbier CJ, Heinen A, Houben-Weerts JHPM, Balestra GM, Stap J, Beek JF, Ince C. In vivo mitochondrial oxygen tension measured by a delayed fluorescence lifetime technique. Biophys J. 2008;95(8):3977–3990. doi: 10.1529/biophysj.107.126094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mik EG, Stap J, Sinaasappel M, Beek JF, Aten JA, van Leeuwen TG, Ince C. Mitochondrial PO2 measured by delayed fluorescence of endogenous protoporphyrin IX. Nat Methods. 2006;3(11):939–945. doi: 10.1038/nmeth940. [DOI] [PubMed] [Google Scholar]
- 39.Sakadžić S, Lee J, Boas DA, Ayata C. High-resolution in vivo optical imaging of stroke injury and repair. Brain Res. 2015;1623(Suppl C):174–192. doi: 10.1016/j.brainres.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dmitriev RI, Borisov SM, Düssmann H, Sun S, Müller BJ, Prehn J, Baklaushev VP, Klimant I, Papkovsky DB. Versatile conjugated polymer nanoparticles for high-resolution O2 imaging in cells and 3D tissue models. ACS Nano. 2015;9(5):5275–5288. doi: 10.1021/acsnano.5b00771. [DOI] [PubMed] [Google Scholar]
- 41.Koolen PG, Li Z, Roussakis E, Paul MA, Ibrahim AM, Matyal R, Huang T, Evans CL, Lin SJ. Oxygen-sensing paint-on bandage: calibration of a novel approach in tissue perfusion assessment. Plastic Reconstr Surg. 2017;140(1):89–96. doi: 10.1097/PRS.0000000000003421. [DOI] [PubMed] [Google Scholar]
- 42.Ida KK, Chisholm KI, Malbouisson LMS, Papkovsky DB, Dyson A, Singer M, Duchen MR, Smith KJ (2018) Protection of cerebral microcirculation, mitochondrial function, and electrocortical activity by small-volume resuscitation with terlipressin in a model of haemorrhagic shock. Brit J Anaesth. 10.1016/j.bja.2017.11.074 [DOI] [PubMed]
- 43.Kim IC, Keila KI, Andrew LD, Ilias T, Dmitri BP, Alex D, Mervyn S, Michael RD, Kenneth JS. Hypothermia protects brain mitochondrial function from hypoxemia in a murine model of sepsis. J Cereb Blood Flow Metab. 2015;36(11):1955–1964. doi: 10.1177/0271678X15606457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147(5):1055–1063. e1058. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schreml S, Meier RJ, Wolfbeis OS, Maisch T, Szeimies RM, Landthaler M, Regensburger J, Santarelli F, Klimant I, Babilas P. 2D luminescence imaging of physiological wound oxygenation. Exp Dermatol. 2011;20(7):550–554. doi: 10.1111/j.1600-0625.2011.01263.x. [DOI] [PubMed] [Google Scholar]
- 46.Tsytsarev V, Akkentli F, Pumbo E, Tang Q, Chen Y, Erzurumlu RS, Papkovsky DB. Planar implantable sensor for in vivo measurement of cellular oxygen metabolism in brain tissue. J Neurosci Methods. 2017;281:1–6. doi: 10.1016/j.jneumeth.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jenkins J, Dmitriev RI, Morten K, McDermott KW, Papkovsky DB. Oxygen-sensing scaffolds for 3-dimensional cell and tissue culture. Acta Biomater. 2015;16:126–135. doi: 10.1016/j.actbio.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 48.Yazgan G, Dmitriev RI, Tyagi V, Jenkins J, Rotaru G-M, Rottmar M, Rossi RM, Toncelli C, Papkovsky DB, Maniura-Weber K. Steering surface topographies of electrospun fibers: understanding the mechanisms. Sci Rep. 2017;7:158. doi: 10.1038/s41598-017-00181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elowitz MB, Surette MG, Wolf P-E, Stock J, Leibler S. Photoactivation turns green fluorescent protein red. Curr Biol. 1997;7(10):809–812. doi: 10.1016/S0960-9822(06)00342-3. [DOI] [PubMed] [Google Scholar]
- 50.Dmitriev RI, Borisov SM, Kondrashina AV, Janelle M, Pakan P, Anilkumar U, Jochen H, Prehn M, Zhdanov AV, Mcdermott KW. Imaging oxygen in neural cell and tissue models by means of anionic cell-permeable phosphorescent nanoparticles. Cell Mol Life Sci. 2015;72(2):367. doi: 10.1007/s00018-014-1673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Düssmann H, Perez-Alvarez S, Anilkumar U, Papkovsky DB, Prehn JH. Single-cell time-lapse imaging of intracellular O2 in response to metabolic inhibition and mitochondrial cytochrome-c release. Cell Death Dis. 2017;8(6):e2853. doi: 10.1038/cddis.2017.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kondrashina AV, Dmitriev RI, Borisov SM, Klimant I, O’Brien I, Nolan YM, Zhdanov AV, Papkovsky DB. A phosphorescent nanoparticle-based probe for sensing and imaging of (intra)cellular oxygen in multiple detection modalities. Adv Funct Mater. 2012;22(23):4931–4939. doi: 10.1002/adfm.201201387. [DOI] [Google Scholar]
- 53.Stern O, Volmer M. The fading time of fluorescence. Phys Z. 1919;20:183–188. [Google Scholar]
- 54.Carraway ER, Demas JN, DeGraff BA, Bacon JR. Photophysics and photochemistry of oxygen sensors based on luminescent transition–metal complexes. Anal Chem. 1991;63(4):337–342. doi: 10.1021/ac00004a007. [DOI] [Google Scholar]
- 55.Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol Rev. 2010;90(3):1103–1163. doi: 10.1152/physrev.00038.2009. [DOI] [PubMed] [Google Scholar]
- 56.Mishin AS, Belousov VV, Solntsev KM, Lukyanov KA. Novel uses of fluorescent proteins. Curr Opin Chem Biol. 2015;27:1–9. doi: 10.1016/j.cbpa.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Loenarz C, Thalhammer A, Ge W, Spivakovsky E, Mackeen MM, McDonough MA, Cockman ME, Kessler BM, Ratcliffe PJ, Wolf A. Hydroxylation of the eukaryotic ribosomal decoding center affects translational accuracy. Proc Nat Acad Sci USA. 2014;111(11):4019–4024. doi: 10.1073/pnas.1311750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreev DE, O’Connor PB, Zhdanov AV, Dmitriev RI, Shatsky IN, Papkovsky DB, Baranov PV. Oxygen and glucose deprivation induces widespread alterations in mRNA translation within 20 minutes. Genome Biol. 2015;16(1):90. doi: 10.1186/s13059-015-0651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prost-Fingerle K, Hoffmann MD, Schützhold V, Cantore M, Fandrey J. Optical analysis of cellular oxygen sensing. Exp Cell Res. 2017;356:122–127. doi: 10.1016/j.yexcr.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Mik EG. Measuring mitochondrial oxygen tension: from basic principles to application in humans. Anesth Analg. 2013;117(4):834–46. doi: 10.1213/ANE.0b013e31828f29da. [DOI] [PubMed] [Google Scholar]
- 61.Dunphy I, Vinogradov SA, Wilson DF. Oxyphor R2 and G2: phosphors for measuring oxygen by oxygen-dependent quenching of phosphorescence. Anal Biochem. 2002;310(2):191–198. doi: 10.1016/S0003-2697(02)00384-6. [DOI] [PubMed] [Google Scholar]
- 62.Esipova TV, Karagodov A, Miller J, Wilson DF, Busch TM, Vinogradov SA. Two new “protected” oxyphors for biological oximetry: properties and application in tumor imaging. Anal Chem. 2011;83:8756–8765. doi: 10.1021/ac2022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lebedev AY, Troxler T, Vinogradov SA. Design of metalloporphyrin-based dendritic nanoprobes for two-photon microscopy of oxygen. J Porphyr Phthalocyan. 2008;12(12):1261–1269. doi: 10.1142/S1088424608000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esipova TV, Rivera-Jacquez HJ, Weber B, Masunov AE, Vinogradov SA. Stabilizing g-states in centrosymmetric tetrapyrroles: two-photon-absorbing porphyrins with bright phosphorescence. J Phys Chem A. 2017;121(33):6243–6255. doi: 10.1021/acs.jpca.7b04333. [DOI] [PubMed] [Google Scholar]
- 65.Zheng X, Tang H, Xie C, Zhang J, Wu W, Jiang X. Tracking cancer metastasis in vivo by using an iridium-based hypoxia-activated optical oxygen nanosensor. Angew Chem Int Ed. 2015;54(28):8094–8099. doi: 10.1002/anie.201503067. [DOI] [PubMed] [Google Scholar]
- 66.Hirakawa Y, Yoshihara T, Kamiya M, Mimura I, Fujikura D, Masuda T, Kikuchi R, Takahashi I, Urano Y, Tobita S. Quantitating intracellular oxygen tension in vivo by phosphorescence lifetime measurement. Sci Rep. 2015;5:17838. doi: 10.1038/srep17838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang S, Hosaka M, Yoshihara T, Negishi K, Iida Y, Tobita S, Takeuchi T. Phosphorescent light-emitting iridium complexes serve as a hypoxia-sensing probe for tumor imaging in living animals. Cancer Res. 2010;70(11):4490–4498. doi: 10.1158/0008-5472.CAN-09-3948. [DOI] [PubMed] [Google Scholar]
- 68.Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science. 1988;241(4873):1649–1651. doi: 10.1126/science.3420417. [DOI] [PubMed] [Google Scholar]
- 69.Zheng L, Golub AS, Pittman RN. Determination of PO2 and its heterogeneity in single capillaries. Am J Physiol Heart Circ Physiol. 1996;271(1):H365–H372. doi: 10.1152/ajpheart.1996.271.1.H365. [DOI] [PubMed] [Google Scholar]
- 70.Gerritsen HC, Sanders R, Draaijer A, Ince C, Levine Y. Fluorescence lifetime imaging of oxygen in living cells. J Fluoresc. 1997;7(1):11–15. doi: 10.1007/BF02764572. [DOI] [PubMed] [Google Scholar]
- 71.Sasso MG, Quina FH, Bechara EJ. Ruthenium (II) tris (bipyridyl) ion as a luminescent probe for oxygen uptake. Anal Biochem. 1986;156(1):239–243. doi: 10.1016/0003-2697(86)90178-8. [DOI] [PubMed] [Google Scholar]
- 72.Dmitriev RI, Ropiak H, Ponomarev G, Yashunsky DV, Papkovsky DB. Cell-penetrating conjugates of coproporphyrins with oligoarginine peptides: rational design and application to sensing of intracellular O2. Bioconj Chem. 2011;22:2507–2518. doi: 10.1021/bc200324q. [DOI] [PubMed] [Google Scholar]
- 73.Dmitriev RI, Ropiak HM, Yashunsky DV, Ponomarev GV, Zhdanov AV, Papkovsky DB. Bactenecin 7 peptide fragment as a tool for intracellular delivery of a phosphorescent oxygen sensor. FEBS J. 2010;277(22):4651–4661. doi: 10.1111/j.1742-4658.2010.07872.x. [DOI] [PubMed] [Google Scholar]
- 74.Dmitriev RI, Zhdanov AV, Ponomarev GV, Yashunski DV, Papkovsky DB. Intracellular oxygen-sensitive phosphorescent probes based on cell-penetrating peptides. Anal Biochem. 2010;398(1):24–33. doi: 10.1016/j.ab.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 75.Koren K, Dmitriev RI, Borisov SM, Papkovsky DB, Klimant I. Complexes of IrIII-octaethylporphyrin with peptides as probes for sensing cellular O2. ChemBioChem. 2012;13:1184–1190. doi: 10.1002/cbic.201200083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dmitriev RI, Kondrashina AV, Koren K, Klimant I, Zhdanov AV, Pakan JM, McDermott KW, Papkovsky DB. Small molecule phosphorescent probes for O2 imaging in 3D tissue models. Biomater Sci. 2014;2(6):853–866. doi: 10.1039/C3BM60272A. [DOI] [PubMed] [Google Scholar]
- 77.Dmitriev RI, Okkelman IA, Foley T, Papkovsky DB. Live cell microscopy of intestinal organoid oxygenation. FASEB J. 2017;31(1 Suppl):590–591. [Google Scholar]
- 78.Zhdanov AV, Golubeva AV, Okkelman IA, Cryan JF, Papkovsky D. Imaging of oxygen gradients in giant umbrella cells: an ex vivo PLIM study. Am J Physiol Cell Physiol. 2015;309(7):C501–C509. doi: 10.1152/ajpcell.00121.2015. [DOI] [PubMed] [Google Scholar]
- 79.Zhdanov AV, Okkelman IA, Collins FW, Melgar S, Papkovsky DB. A novel effect of DMOG on cell metabolism: direct inhibition of mitochondrial function precedes HIF target gene expression. Biochim Biophys Acta Bioenerg. 2015;1847(10):1254–1266. doi: 10.1016/j.bbabio.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 80.Zhdanov AV, Okkelman IA, Golubeva AV, Doerr B, Hyland NP, Melgar S, Shanahan F, Cryan JF, Papkovsky DB. Quantitative analysis of mucosal oxygenation using ex vivo imaging of healthy and inflamed mammalian colon tissue. Cell Mol Life Sci. 2017;74(1):141–151. doi: 10.1007/s00018-016-2323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi H, Ma X, Zhao Q, Liu B, Qu Q, An Z, Zhao Y, Huang W. Ultrasmall phosphorescent polymer dots for ratiometric oxygen sensing and photodynamic cancer therapy. Adv Funct Mater. 2014;24(30):4823–4830. doi: 10.1002/adfm.201400647. [DOI] [Google Scholar]
- 82.Koo YE, Cao Y, Kopelman R, Koo SM, Brasuel M, Philbert MA. Real-time measurements of dissolved oxygen inside live cells by organically modified silicate fluorescent nanosensors. Anal Chem. 2004;76(9):2498–2505. doi: 10.1021/ac035493f. [DOI] [PubMed] [Google Scholar]
- 83.Xu H, Aylott JW, Kopelman R, Miller TJ, Philbert MA. A real-time ratiometric method for the determination of molecular oxygen inside living cells using sol–gel-based spherical optical nanosensors with applications to rat c6 glioma. Anal Chem. 2001;73(17):4124–4133. doi: 10.1021/ac0102718. [DOI] [PubMed] [Google Scholar]
- 84.Feng Z, Tao P, Zou L, Gao P, Liu Y, Liu X, Wang H, Liu S, Dong Q, Li J, Xu B, Huang W, Wong RWY, Zhao Q. Hyper-branched phosphorescent conjugated polymer dots with iridium(III) complex as the core for hypoxia imaging and photodynamic therapy. ACS App Mater Interf. 2017;9(34):28319–28330. doi: 10.1021/acsami.7b09721. [DOI] [PubMed] [Google Scholar]
- 85.Papkovsky DB, Dmitriev RI. Evolution of cell-penetrating phosphorescent O2 probes. In: Papkovsky DB, Dmitriev RI, editors. Quenched-phosphorescence detection of molecular oxygen: applications in life sciences, Detection Science Series No. 11. London: The Royal Society of Chemistry; 2018. pp. 50–70. [Google Scholar]
- 86.Wang X, Stolwijk JA, Lang T, Sperber M, Meier RJ, Wegener J, Wolfbeis OS. Ultra-small, highly stable, and sensitive dual nanosensors for imaging intracellular oxygen and pH in cytosol. J Amer Chem Soc. 2012;134(41):17011–17014. doi: 10.1021/ja308830e. [DOI] [PubMed] [Google Scholar]
- 87.Dmitriev RI, Zhdanov AV, Nolan YM, Papkovsky DB. Imaging of neurosphere oxygenation with phosphorescent probes. Biomaterials. 2013;34(37):9307–9317. doi: 10.1016/j.biomaterials.2013.08.065. [DOI] [PubMed] [Google Scholar]
- 88.Babilas P, Liebsch G, Schacht V, Klimant I, Wolfbeis OS, Szeimers R, Abels C. In vivo phosphorescence imaging of pO2 using planar oxygen sensors. Microcirculation. 2005;12(6):477–487. doi: 10.1080/10739680591003314. [DOI] [PubMed] [Google Scholar]
- 89.Schreml S, Meier RJ, Kirschbaum M, Kong SC, Gehmert S, Felthaus O, Küchler S, Sharpe JR, Wöltje K, Weiß KT. Luminescent dual sensors reveal extracellular pH-gradients and hypoxia on chronic wounds that disrupt epidermal repair. Theranostics. 2014;4(7):721. doi: 10.7150/thno.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xue R, Nelson MT, Teixeira SA, Viapiano MS, Lannutti JJ. Cancer cell aggregate hypoxia visualized in vitro via biocompatible fiber sensors. Biomaterials. 2016;76:208–217. doi: 10.1016/j.biomaterials.2015.10.055. [DOI] [PubMed] [Google Scholar]
- 91.Müller BJ, Zhdanov AV, Borisov SM, Foley T, Okkelman IA, Tsytsarev V, Tang Q, Erzurumlu RS, Chen Y, Zhang H. Nanoparticle-based fluoroionophore for analysis of potassium ion dynamics in 3D tissue models and in vivo. Adv Funct Mater. 2018 doi: 10.1002/adfm.201704598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oreopoulos J, Berman R, Browne M. Spinning-disk confocal microscopy: present technology and future trends. Methods Cell Biol. 2014;123:153–175. doi: 10.1016/B978-0-12-420138-5.00009-4. [DOI] [PubMed] [Google Scholar]
- 93.Howard SS, Straub A, Horton NG, Kobat D, Xu C. Frequency-multiplexed in vivo multiphoton phosphorescence lifetime microscopy. Nat Photonics. 2013;7(1):33. doi: 10.1038/nphoton.2012.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fercher A, O’Riordan TC, Zhdanov AV, Dmitriev RI, Papkovsky DB. Imaging of cellular oxygen and analysis of metabolic responses of mammalian cells. Methods Mol Biol. 2010;591:257–273. doi: 10.1007/978-1-60761-404-3_16. [DOI] [PubMed] [Google Scholar]
- 95.Hirvonen LM, Festy F, Suhling K. Wide-field time-correlated single-photon counting (TCSPC) lifetime microscopy with microsecond time resolution. Opt Lett. 2014;39(19):5602–5605. doi: 10.1364/OL.39.005602. [DOI] [PubMed] [Google Scholar]
- 96.Becker W. Advanced time-correlated single photon counting applications, Springer Series in Chemical Physics. New York: Springer International Publishing; 2015. [Google Scholar]
- 97.Becker W, Shcheslavskiy V, Rück A. Simultaneous phosphorescence and fluorescence lifetime imaging by multi-dimensional TCSPC and multi-pulse excitation. Adv Exp Med Biol. 2017;1035:19–30. doi: 10.1007/978-3-319-67358-5_2. [DOI] [PubMed] [Google Scholar]
- 98.O’Donnell N, Dmitriev R. Three-dimensional tissue models and available probes for multi-parametric live cell microscopy: a brief overview. Adv Exp Med Biol. 2017;1035:49–67. doi: 10.1007/978-3-319-67358-5_4. [DOI] [PubMed] [Google Scholar]
- 99.Kurokawa H, Ito H, Inoue M, Tabata K, Sato Y, Yamagata K, Kizaka-Kondoh S, Kadonosono T, Yano S, Inoue M. High resolution imaging of intracellular oxygen concentration by phosphorescence lifetime. Sci Rep. 2015;5:10657. doi: 10.1038/srep10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kalinina S, Breymayer J, Schäfer P, Calzia E, Shcheslavskiy V, Becker W, Rück A. Correlative NAD (P) H-FLIM and oxygen sensing-PLIM for metabolic mapping. J Biophotonics. 2016;9(8):800–811. doi: 10.1002/jbio.201500297. [DOI] [PubMed] [Google Scholar]
- 101.Conway JRW, Warren SC, Timpson P. Context-dependent intravital imaging of therapeutic response using intramolecular FRET biosensors. Methods. 2017;128(Suppl C):78–94. doi: 10.1016/j.ymeth.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 102.Devor A, Sakadzic S, Srinivasan VJ, Yaseen MA, Nizar K, Saisan PA, Tian P, Dale AM, Vinogradov SA, Franceschini MA, Boas DA. Frontiers in optical imaging of cerebral blood flow and metabolism. J Cereb Blood Flow Metab. 2012;32(7):1259–1276. doi: 10.1038/jcbfm.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Golub AS, Pittman RN. PO2 measurements in the microcirculation using phosphorescence quenching microscopy at high magnification. Am J Physiol Heart Circ Physiol. 2008;294(6):H2905–H2916. doi: 10.1152/ajpheart.01347.2007. [DOI] [PubMed] [Google Scholar]
- 104.Sakadzic S, Roussakis E, Yaseen MA, Mandeville ET, Srinivasan VJ, Arai K, Ruvinskaya S, Devor A, Lo EH, Vinogradov SA, Boas DA. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat Methods. 2010;7(9):755–759. doi: 10.1038/nmeth.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lecoq J, Parpaleix A, Roussakis E, Ducros M, Houssen YG, Vinogradov SA, Charpak S. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nat Med. 2011;17(7):893–898. doi: 10.1038/nm.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luo S, Zhang E, Su Y, Cheng T, Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32(29):7127–7138. doi: 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 107.Cheong W-F, Prahl SA, Welch AJ. A review of the optical properties of biological tissues. IEEE J Quantum Electron. 1990;26(12):2166–2185. doi: 10.1109/3.64354. [DOI] [Google Scholar]
- 108.Wilson DF, Finikova OS, Lebedev AY, Apreleva S, Pastuszko A, Lee WMF, Vinogradov SA. Oxygen Transp Tissue XXXII, vol 701. Adv Exp Med Biol. New York: Springer; 2011. Measuring oxygen in living tissue: intravascular, interstitial, and “tissue” oxygen measurements; pp. 53–59. [DOI] [PubMed] [Google Scholar]
- 109.Lukina M, Orlova A, Shirmanova M, Shirokov D, Pavlikov A, Neubauer A, Studier H, Becker W, Zagaynova E, Yoshihara T. Interrogation of metabolic and oxygen states of tumors with fiber-based luminescence lifetime spectroscopy. Opt Lett. 2017;42(4):731–734. doi: 10.1364/OL.42.000731. [DOI] [PubMed] [Google Scholar]
- 110.Le Marois A, Suhling K. Quantitative live cell FLIM imaging in three dimensions. Adv Exp Med Biol. 2017;1035:31–48. doi: 10.1007/978-3-319-67358-5_3. [DOI] [PubMed] [Google Scholar]
- 111.Arena ET, Rueden CT, Hiner MC, Wang S, Yuan M, Eliceiri KW. Quantitating the cell: turning images into numbers with ImageJ. Wiley Interdiscip Rev Dev Biol. 2017;6(2):12. doi: 10.1002/wdev.260. [DOI] [PubMed] [Google Scholar]
- 112.Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods. 2010;7(8):603–614. doi: 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
- 113.Fischer RS, Wu Y, Kanchanawong P, Shroff H, Waterman CM. Microscopy in 3D: a biologist’s toolbox. Trends Cell Biol. 2011;21(12):682–691. doi: 10.1016/j.tcb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Appel AA, Anastasio MA, Larson JC, Brey EM. Imaging challenges in biomaterials and tissue engineering. Biomaterials. 2013;34(28):6615–6630. doi: 10.1016/j.biomaterials.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jenkins J, Borisov SM, Papkovsky DB, Dmitriev RI. Sulforhodamine nanothermometer for multiparametric fluorescence lifetime imaging microscopy. Anal Chem. 2016;88(21):10566–10572. doi: 10.1021/acs.analchem.6b02675. [DOI] [PubMed] [Google Scholar]
- 116.Okkelman IA, Dmitriev RI, Foley T, Papkovsky DB. Use of fluorescence lifetime imaging microscopy (FLIM) as a timer of cell cycle S phase. PLoS One. 2016;11(12):e0167385. doi: 10.1371/journal.pone.0167385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dmitriev RI, Zhdanov AV, Jasionek G, Papkovsky DB. Assessment of cellular oxygen gradients with a panel of phosphorescent oxygen-sensitive probes. Anal Chem. 2012;84(6):2930–2938. doi: 10.1021/ac3000144. [DOI] [PubMed] [Google Scholar]
- 118.Zhdanov AV, Ogurtsov VI, Taylor CT, Papkovsky DB. Monitoring of cell oxygenation and responses to metabolic stimulation by intracellular oxygen sensing technique. Integr Biol. 2010;2(9):443–451. doi: 10.1039/c0ib00021c. [DOI] [PubMed] [Google Scholar]
- 119.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435(2):297. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fercher A, Borisov SM, Zhdanov AV, Klimant I, Papkovsky DB. Intracellular O2 sensing probe based on cell-penetrating phosphorescent nanoparticles. ACS Nano. 2011;5:5499–5508. doi: 10.1021/nn200807g. [DOI] [PubMed] [Google Scholar]
- 122.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302(5652):1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 123.Zhdanov AV, Dmitriev RI, Hynes J, Papkovsky DB. Kinetic analysis of local oxygenation and respiratory responses of mammalian cells using intracellular oxygen-sensitive probes and time-resolved fluorometry. Methods Enzymol. 2014;542:183–207. doi: 10.1016/B978-0-12-416618-9.00010-8. [DOI] [PubMed] [Google Scholar]
- 124.O’Riordan TC, Zhdanov AV, Ponomarev GV, Papkovsky DB. Analysis of intracellular oxygen and metabolic responses of mammalian cells by time-resolved fluorometry. Anal Chem. 2007;79(24):9414–9419. doi: 10.1021/ac701770b. [DOI] [PubMed] [Google Scholar]
- 125.Hogan MC. Phosphorescence quenching method for measurement of intracellular in isolated skeletal muscle fibers. J Appl Physiol. 1999;86(2):720–724. doi: 10.1152/jappl.1999.86.2.720. [DOI] [PubMed] [Google Scholar]
- 126.Takahashi E, Sato M. Imaging of oxygen gradients in monolayer cultured cells using green fluorescent protein. Am J Physiol Cell Physiol. 2010;299(6):C1318–1323. doi: 10.1152/ajpcell.00254.2010. [DOI] [PubMed] [Google Scholar]
- 127.Dmitriev RI, Borisov SM, Jenkins J, Papkovsky DB. Multi-parametric imaging of tumor spheroids with ultra-bright and tunable nanoparticle O2 probes. Proc SPIE. doi. 2015;10(1117/12):2079604. [Google Scholar]
- 128.Mehta G, Mehta K, Sud D, Song JW, Bersano-Begey T, Futai N, Heo YS, Mycek MA, Linderman JJ, Takayama S. Quantitative measurement and control of oxygen levels in microfluidic poly (dimethylsiloxane) bioreactors during cell culture. Biomed Microdev. 2007;9(2):123–134. doi: 10.1007/s10544-006-9005-7. [DOI] [PubMed] [Google Scholar]
- 129.Leedale J, Herrmann A, Bagnall J, Fercher A, Papkovsky D, Sée V, Bearon RN. Modeling the dynamics of hypoxia inducible factor-1α (HIF-1α) within single cells and 3D cell culture systems. Math Biosci. 2014;258:33–43. doi: 10.1016/j.mbs.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 130.Jenkins J, Papkovsky Dmitri B, Dmitriev Ruslan I. The Ca2+/Mn2+-transporting SPCA2 pump is regulated by oxygen and cell density in colon cancer cells. Biochem J. 2016;473(16):2507–2518. doi: 10.1042/BCJ20160477. [DOI] [PubMed] [Google Scholar]
- 131.Okkelman IA, Foley T, Papkovsky DB, Dmitriev RI. Live cell imaging of mouse intestinal organoids reveals heterogeneity in their oxygenation. Biomaterials. 2017;146:86–96. doi: 10.1016/j.biomaterials.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 132.Colgan SP, Campbell EL, Kominsky DJ. Hypoxia and mucosal inflammation. Annu Rev Pathol. 2016;11:77–100. doi: 10.1146/annurev-pathol-012615-044231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Campbell Eric L, Bruyninckx Walter J, Kelly Caleb J, Glover Louise E, McNamee Eóin N, Bowers Brittelle E, Bayless Amanda J, Scully M, Saeedi Bejan J, Golden-Mason L, Ehrentraut Stefan F, Curtis Valerie F, Burgess A, Garvey John F, Sorensen A, Nemenoff R, Jedlicka P, Taylor Cormac T, Kominsky Douglas J, Colgan Sean P. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40(1):66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17(5):662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sakadzic Sava, Roussakis E, Yaseen MA, Mandeville ET, Srinivasan VJ, Arai K, Ruvinskaya S, Wu W, Devor A, Lo EH, Vinogradov SA, Boas DA. Cerebral blood oxygenation measurement based on oxygen-dependent quenching of phosphorescence. J Vis Exp. 2011;51:e1694. doi: 10.3791/1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oda K, Iwamoto Y, Tsukada K. Simultaneous mapping of unevenly distributed tissue hypoxia and vessel permeability in tumor microenvironment. Biomed Phys Eng Express. 2016;2(6):065017. doi: 10.1088/2057-1976/aa5193. [DOI] [Google Scholar]
- 138.Tsytsarev V, Pumbo E, Borisov S, Arakawa H, Erzurumlu RS, Papkovsky DB. In vivo imaging of brain metabolism activity using a phosphorescent oxygen-sensitive probe. J Neurosci Methods. 2013;216:146–151. doi: 10.1016/j.jneumeth.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shonat RD, Kight AC. Oxygen tension imaging in the mouse retina. Ann Biomed Eng. 2003;31(9):1084–1096. doi: 10.1114/1.1603256. [DOI] [PubMed] [Google Scholar]
- 140.Wilson DF, Vinogradov SA, Grosul P, Sund N, Vacarezza MN, Bennett J. Oxygen Transport to Tissue XXVII, vol 578. Adv Exp Med Biol. New York: Springer; 2006. Imaging oxygen pressure in the rodent retina by phosphorescence lifetime; pp. 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wanek J, P-y Teng, Blair NP, Shahidi M. Inner retinal oxygen delivery and metabolism under normoxia and hypoxia in ratinner retinal oxygen delivery and metabolism. Investig Ophthalmol Vis Sci. 2013;54(7):5012–5019. doi: 10.1167/iovs.13-11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shahidi M, Wanek J, Blair NP, Little DM, Wu T. Retinal tissue oxygen tension imaging in the rat. Investig Ophthalmol Vis Sci. 2010;51(9):4766–4770. doi: 10.1167/iovs.09-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin kinetics of corneal cross-linking. Investig Ophthalmol Vis Sci. 2012;53(4):2360–2367. doi: 10.1167/iovs.11-9385. [DOI] [PubMed] [Google Scholar]
- 144.Richoz O, Hammer A, Tabibian D, Gatzioufas Z, Hafezi F. The biomechanical effect of corneal collagen cross-linking (CXL) with riboflavin and UV-A is oxygen dependent. Transl Vis Sci Technol. 2013;2(7):6–6. doi: 10.1167/tvst.2.7.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McQuaid RM, Mrochen M, Dmitriev R, Papkovski D, Vohnsen B. In-vitro estimation of O2 concentrations during corneal cross-linking (CXL) for porcine corneas and collagen type-I gels. Investig Ophthalmol Vis Sci. 2017;58(8):5674–5674. [Google Scholar]
- 146.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 147.Kennedy J, Pottier R, Pross D. Photodynamic therapy with endogenous protoporphyrin: IX: basic principles and present clinical experience. J Photochem Photobiol B Biol. 1990;6(1–2):143–148. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- 148.Wang X-H, Peng H-S, Yang W, Ren Z-D, Liu Y-A. Mitochondria-targeted theranostic nanoparticles for optical sensing of oxygen, photodynamic cancer therapy, and assessment of therapeutic efficacy. Microchim Acta. 2016;10(183):2723–2731. doi: 10.1007/s00604-016-1917-1. [DOI] [Google Scholar]
- 149.Martin A, Byrne A, Burke CS, Forster RJ, Keyes TE. Peptide-bridged dinuclear Ru (II) complex for mitochondrial targeted monitoring of dynamic changes to oxygen concentration and ROS generation in live mammalian cells. J Am Chem Soc. 2014;136(43):15300–15309. doi: 10.1021/ja508043q. [DOI] [PubMed] [Google Scholar]
- 150.Piffaretti F, Novello AM, Kumar RS, Forte E, Paulou C, Nowak-Sliwinska P, van den Bergh H, Wagnières G. Real-time, in vivo measurement of tissular pO2 through the delayed fluorescence of endogenous protoporphyrin IX during photodynamic therapy. J Biomed Optics. 2012;17(11):115007–115007. doi: 10.1117/1.JBO.17.11.115007. [DOI] [PubMed] [Google Scholar]
- 151.Huntosova V, Gerelli E, Horvath D, Wagnieres G. Measurement of pO2 by luminescence lifetime spectroscopy: a comparative study of the phototoxicity and sensitivity of [Ru (Phen) 3]2+ and PdTCPP in vivo. J Biophotonics. 2017;10(5):708–717. doi: 10.1002/jbio.201600127. [DOI] [PubMed] [Google Scholar]
- 152.Harms FA, Voorbeijtel WJ, Bodmer SI, Raat NJ, Mik EG. Cutaneous respirometry by dynamic measurement of mitochondrial oxygen tension for monitoring mitochondrial function in vivo. Mitochondrion. 2012;13(5):507–514. doi: 10.1016/j.mito.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 153.Babilas P, Lamby P, Prantl L, Schreml S, Jung EM, Liebsch G, Wolfbeis OS, Landthaler M, Szeimies RM, Abels C. Transcutaneous pO2 imaging during tourniquet-induced forearm ischemia using planar optical oxygen sensors. Skin Res Technol. 2008;14(3):304–311. doi: 10.1111/j.1600-0846.2008.00295.x. [DOI] [PubMed] [Google Scholar]
- 154.Schreml S, Szeimies R, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163(2):257–268. doi: 10.1111/j.1365-2133.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 155.Li Z, Roussakis E, Koolen PG, Ibrahim AM, Kim K, Rose LF, Wu J, Nichols AJ, Baek Y, Birngruber R. Non-invasive transdermal two-dimensional mapping of cutaneous oxygenation with a rapid-drying liquid bandage. Biomed Opt Expr. 2014;5(11):3748–3764. doi: 10.1364/BOE.5.003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lo L-W, Koch CJ, Wilson DF. Calibration of oxygen-dependent quenching of the phosphorescence of pd-meso-tetra (4-carboxyphenyl) porphine: a phosphor with general application for measuring oxygen concentration in biological systems. Anal Biochem. 1996;236(1):153–160. doi: 10.1006/abio.1996.0144. [DOI] [PubMed] [Google Scholar]