Abstract
Three isoforms of plasma membrane Ca2+-ATPase (PMCA) are expressed in the kidney. While PMCA1 and PMCA4 play major role in regulating Ca2+ reabsorption, the role for PMCA2 remains vaguely defined. To define PMCA2 function, PMCA2-interacting complex was characterized by immunoprecipitation followed by nanoLC-ESI-Qq-TripleTOF MS/MS (IP-MS). After subtracting non-specific binders using isotype-controlled IP-MS, 474 proteins were identified as PMCA2-interacting partners. Among these, eight were known and 20 were potential PMCA2-interacting partners based on bioinformatic prediction, whereas other 446 were novel and had not been previously reported/predicted. Quantitative immuno-co-localization assay confirmed the association of PMCA2 with these partners. Gene ontology analysis revealed binding activity as the major molecular function of PMCA2-interacting complex. Functional validation using calcium oxalate monohydrate (COM) crystal-protein binding, crystal-cell adhesion, and crystal internalization assays together with neutralization by anti-PMCA2 antibody compared to isotype-controlled IgG and blank control, revealed a novel role of PMCA2 as a COM crystal-binding protein that was crucial for crystal retention and uptake. In summary, a large number of novel PMCA2-interacting proteins have been defined and a novel function of PMCA2 as a COM crystal-binding protein sheds light onto its involvement, at least in part, in kidney stone pathogenesis.
Electronic supplementary material
The online version of this article (10.1007/s00018-017-2699-2) contains supplementary material, which is available to authorized users.
Keywords: Crystal adhesion, Crystal internalization, Immuno-co-localization, Interactomics, IP-MS, Kidney stone, Renal calculi, Renal tubular cells
Introduction
Plasma membrane Ca2+-ATPase (PMCA) is a P-type ion-transporting ATPase that plays a major role in regulating Ca2+ balance in various types of eukaryotic cells [1]. Activation of this protein requires binding of Ca2+-dependent calmodulin to its C-terminal tail [2], while many other mechanisms, i.e., phosphorylation, phospholipid activation and proteolysis can also affect PMCA activity [3–5]. At present, four PMCA isoforms (PMCA1–4) with more than 30 modified forms generated by alternative RNA splicing have been reported [6, 7]. Such diversity in spliced regions is responsible for their unique membrane localizations and dynamic Ca2+ handling activities. The role of PMCA is becoming more relevant as growing numbers of evidence have demonstrated that PMCA abnormalities can lead to dysfunction of mammalian cells both in vitro [8] and in vivo [9–11].
In the kidney, expression of PMCA1, PMCA2 and PMCA4 has been found at both RNA and protein levels [12, 13]. PMCA1 and PMCA4, which are designated as “housekeeping” PMCA, are highly expressed at basolateral membranes of renal tubular cells, and hence are considered as the major forms responsible for 1/3 of Ca2+ reabsorption along the nephron [whereas the remainders are governed by Na+/Ca2+ exchanger (NCX)] [14]. In contrast, PMCA2 isoform has been found with a much lower level but without restriction to specific membrane compartment [15, 16]. Moreover, two important properties that set apart PMCA2 from the other two isoforms are that the rate of stimulus and Ca2+-binding affinity is considerably much higher [7, 17]. While PMCA1 and PMCA4 play a major role in regulating Ca2+ reabsorption, the role of PMCA2 remains vaguely defined (perhaps due to its low abundant expression). These distinctive features and conservation of PMCA2 in renal cells through evolutionary adaptation have thus come to our attention as PMCA2 may have unique function, rather than functional redundancy in controlling Ca2+ reabsorption [17–19].
To explore functions and regulatory mechanisms of a target protein in a given cell, characterizations of its interacting complex is one of the essential approaches [20]. We, therefore, performed extensive characterizations of PMCA2-interacting partners in renal tubular cells by a combination of immunoprecipitation and mass spectrometry (IP-MS). Quantitative immuno-co-localization assay was performed to confirm the association of PMCA2 with its partners. Finally, functional investigations of PMCA2 were performed using calcium oxalate monohydrate (COM) crystal-protein binding, crystal-cell adhesion, and crystal internalization assays, together with neutralization by specific antibody against PMCA2 compared to isotype-controlled IgG and blank control.
Materials and methods
Cell culture
Madin–Darby canine kidney (MDCK) cell line, which was originally derived from the distal nephron segment [21], was cultivated under standard condition in Eagle’s minimum essential medium (MEM) (Gibco; Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco), 1.2% penicillin G/streptomycin, and 2 mM l-glutamine (Sigma; St. Louis, MO, USA) in a humidified incubator at 37 °C and 5% CO2.
Affinity purification by immunoprecipitation (IP)
MDCK cells were lyzed in a modified RIPA buffer (50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 0.5% Triton X-100, and 1 mM EDTA) and further homogenized by sonication. Cell debris and particulate matters were removed by centrifugation at 10,000×g and 4 °C for 15 min. Prior to IP, 3 mg of cell lysate were pre-cleared with 50 µl of protein G Sepharose beads (50% slurry) at 4 °C on a rotary device for 15 min. Beads with non-specifically bounded proteins were removed by centrifugation at 1500×g and 4 °C for 5 min. Thereafter, the sample was incubated with 1 µg of rabbit polyclonal anti-PMCA2 antibody (Abcam; Cambridge, UK) or 1 µg of isotype-controlled rabbit IgG (Santa Cruz Biotechnology; Santa Cruz, CA, USA) overnight at 4 °C on a rotary device. Protein G Sepharose beads (50 µl) were then added and incubated with each mixture at 4 °C for 4 h. Thereafter, the beads were collected by centrifugation at 1500×g and 4 °C for 5 min and washed five times with 800 µl modified RIPA buffer. The immunoprecipitated proteins were finally eluted from the beads using Laemmli’s buffer and subjected to mass spectrometric identification and SDS-PAGE, in which protein bands were visualized using Oriole fluorescent gel stain (Bio-Rad Laboratories; Hercules, CA, USA). Gel images were acquired with a ChemiDoc MP System (Bio-Rad Laboratories).
In-gel tryptic digestion and identification of proteins by nanoLC-ESI-Qq-TripleTOF MS/MS
Each lane of the SDS-PAGE gel was excised into 20 slices/lane. The gel slices were subjected to in-gel tryptic digestion as described previously [22, 23]. Analysis of the digested peptides was performed using reversed-phase Eksigent Ultra Plus nano-LC 2D HPLC system (Eksigent; Dublin, CA, USA) coupled to the new generation quadrupole time-of-flight (QqTOF) Triple TOF 5600 mass spectrometer (AB SCIEX; Concord, Canada). For LC system, mobile phase A was 2% acetonitrile (ACN)/0.1% formic acid, and mobile phase B was 98% ACN/0.1% formic acid. Samples were loaded using autosampler and desalted using a nanoLC Trap (ChromXP C18-CL, 350 μm I.D. × 0.5 mm, 3 μm particle size, 120 Å pore size) (Eksigent) at a flow rate of 3 μl/min using isocratic 100% mobile phase A for 8 min. After pre-washing, the samples were transferred onto the analytical C18-nanocapillary HPLC column (ChromXP C18-CL, 75 μm I.D. × 15 cm, 3 μm particle size, 120 Å pore size) (Eksigent) and eluted at a flow rate of 300 nl/min. Peptides were separated using a linear and stepwise gradient of 5–40% mobile phase B over 40 min, 40–50% B over 5 min, 60–80% B over 1 min, and 80% B over 10 min, with a total runtime of 70 min including mobile phase equilibration. MS and MS/MS spectra were acquired in positive-ion and high-sensitivity mode with a resolution of ~ 35,000 full width half maximum. The data were acquired using a nanospray needle voltage of 2.4 kV, curtain gas of 30 psi, nebulizer gas of 8 psi, an interface heater temperature of 150 °C. The precursor ions were fragmented in a collision cell using nitrogen as the collision gas with the collision energy setting of 30 ± 13 for induction of CID. Advanced information dependent acquisition (IDA) was used for MS/MS collection on the Triple TOF 5600 to obtain MS/MS spectra for the 20 most abundant precursor ions following each survey MS1 scan. The charge state of + 2 and + 3 of precursor and product ions was collected. Exclusion of former target ions was set for 6 s after 1 occurrence. Raw.wiff file was converted to the searchable.mgf file using MS Data Converter (AB SCIEX) for independent searches using the Mascot software version 2.4.0 (Matrix Science; London, UK) to query against the Uniprot-SwissProt mammalian protein database. Fixed modification was carbamidomethylation at cysteine residues, whereas variable modification was oxidation at methionine residues. Only one missed trypsin cleavage was allowed, and peptide mass tolerances of 50 ppm and 0.4 Da were allowed for MS/MS ions search. The target false discovery rate (FDR) was analyzed by performing a concatenated decoy database search and the identified proteins are reported at FDR < 1%.
Bioinformatics analysis
Proteins that were present exclusively in the anti-PMCA2-IP sample were further analyzed by Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) version 10 (http://www.string-db.org) for their interaction networks. Functional classification was performed using Protein ANalysis THrough Evolutionary Relationships (PANTHER) software (http://pantherdb.org/).
Quantitative immuno-co-localization assay
The cell monolayer was cultivated on coverslips and washed three times with ice-cold membrane preserving buffer (1 mM MgCl2 and 0.1 mM CaCl2 in PBS) prior to fixation with 4% paraformaldehyde at room temperature (set at 25 °C) for 15 min. After washing with PBS, the cells were permeabilized with 0.1% Triton X-100 at room temperature for 15 min and non-specific bindings were blocked with 1% BSA in PBS for 30 min. The cells were then incubated at 37 °C for 1 h with rabbit polyclonal anti-PMCA2 (Abcam) together with each of the following primary antibodies: mouse monoclonal anti-ezrin (Santa Cruz Biotechnology), anti-Na+/K+-ATPase (Santa Cruz Biotechnology), anti-annexin A1 (Chemicon; Temecula, CA, USA), anti-Alix (Santa Cruz Biotechnology), anti-nhRNP K (Santa Cruz Biotechnology), anti-c-Jun (Santa Cruz Biotechnology), anti-SOD-1 (Santa Cruz Biotechnology), and anti-DJ-1 (Santa Cruz Biotechnology) (all were diluted 1:50 in 1% BSA/PBS). For actin staining, Alexa488-conjugated phalloidin (Invitrogen-Molecular Probes; Eugene, OR, USA) was used instead. After rinsing with PBS, the cells were then incubated with Alexa546-conjugated goat anti-rabbit IgG and Alexa488-conjugated goat anti-mouse IgG secondary antibodies (Invitrogen-Molecular Probes) containing 0.1 g/ml Hoechst dye (Sigma) at 37 °C for 1 h. Finally, the cells were washed with PBS and mounted onto slides with 50% glycerol/PBS for subsequent examination under ECLIPSE Ti-Clsi4 Laser Unit (Nikon; Tokyo, Japan).
Fluorescence intensity profiles were generated using NIS-Elements D v.4.11 imaging software (Nikon). A linear section of area of interest with a distance of 15 µm was manually drawn across the cell from left to right borders and the intensity profiles were obtained from each color channel. Pixel-to-pixel frequency scatter plots were generated with WCIF ImageJ bundle plugins in ImageJ software (https://imagej.nih.gov/). Pearson’s correlation coefficient (r) values were obtained from the JACoP plugin [24] and r values with p < 0.05 that were considered as valid co-localization of the two signals [25].
Preparation of plain and fluorescence-labeled calcium oxalate monohydrate (COM) crystals
Plain and fluorescence-labeled COM crystals were generated as described previously [26–29]. The plain crystals were prepared by mixing 500 ml of solution A (10 mM CaCl2·2H2O in 10 mM Tris–HCl and 90 mM NaCl, pH 7.4) and 500 ml of solution B (1 mM Na2C2O4 in 10 mM Tris–HCl and 90 mM NaCl, pH 7.4). After an overnight incubation, COM crystals were collected and washed with absolute methanol, left to air dry, and sterilized under UV-light prior to COM crystal-protein binding and crystal-cell adhesion assays. To prepare fluorescence-labeled COM crystals, 0.01 µg/ml fluorescein isothiocyanate (FITC) dye (Thermo Scientific Pierce; Rockford, IL, USA) was added to solution A prior to the addition of solution B as described above. After an overnight incubation in the dark, the FITC-labeled COM crystals were then collected and treated the same way as for the plain crystals prior to crystal internalization assay.
Isolation of apical membranes
Apical membranes were isolated from the polarized MDCK cells using a peeling method as described previously [30, 31]. Briefly, Whatman filter paper (0.18-mm-thick, Whatman International Ltd.; Maidstone, UK) pre-wetted with deionized water was placed onto the cell monolayer. After 5-min incubation, the filter paper was peeled out and the apical membranes retained under the filter paper surface were harvested by rehydration in deionized water and gentle scrapping. The apical membrane-enriched fraction was then lyophilized. Dried apical membranes were solubilized in Laemmli’s buffer and quantitated by Bradford’s method using Bio-Rad Protein Assay. The recovered proteins were then subjected to Western blotting and COM crystal-protein binding assay.
COM crystal-protein binding assay
Apical membrane proteins were dialyzed against deionized water, lyophilized, and then resuspended in 1 ml of protein-free artificial urine, comprising 5 mM CaCl2, 200 mM urea, 4 mM creatinine, 5 mM Na3C6H5O7·2H2O, 54 mM NaCl, 30 mM KCl, 15 mM NH4Cl, 2 mM MgSO4·7H2O, and 9 mM Na2SO4. Then, 5 mg of plain COM crystals were added to the protein solution and allowed binding at 4 °C on a continuous rotator for 16 h. Crystals with bound proteins were collected by centrifugation at 1500×g and 4 °C for 5 min and washed four times with 500 µl PBS and once with 500 µl of 5 mM EDTA prior to elution with Laemmli’s buffer for subsequent Western blotting for PMCA2 (as described below). In parallel, the washed crystals (with proteins bound on the surface) were incubated with rabbit anti-PMCA2 (Abcam), isotype-controlled IgG (Sigma-Aldrich), or rabbit anti-gp135 (Millipore; Billerica MA, USA) antibody (all were diluted 1:100 in 1% BSA) at 37 °C for 1 h. After washing with PBS, the crystals were then incubated with Alexa546-conjugated goat anti-rabbit secondary antibody (1:500 in 1% BSA) at 37 °C for another 1 h. After the final wash with PBS, the presence of PMCA2 on the crystal surface was examined under ECLIPSE 80i fluorescence microscope (Nikon).
Western blotting
Proteins derived from IP (by both isotype-controlled IgG and anti-PMCA2 antibody) or COM crystal-protein binding assay along with positive controls (whole cell lysate and apical membrane protein fraction) were resolved by 10% SDS-PAGE and transferred onto nitrocellulose membrane using TE 77 PWR semi-dry transfer unit (GE Healthcare; Uppsala, Sweden) at 85 mA for 1.5 h. After blocking non-specific bindings with 5% skim milk in PBS at room temperature for 30 min, anti-PMCA2 primary antibody (1:1000 in 1% skim milk/PBS) was incubated with the membrane at 4 °C overnight. The membrane was further incubated with the corresponding secondary antibody conjugated with horseradish peroxidase (1:2000 in 1% skim milk/PBS) (Dako; Glostrup, Denmark) at room temperature for 1 h. The immunoreactive bands were then visualized by SuperSignal West Pico chemiluminescence substrate (Pierce Biotechnology, Inc.; Rockford, IL, USA) and autoradiography.
Neutralization of PMCA2 on the cell surface
MDCK cells were seeded in 6-well culture plate until confluency was reached. Culture medium was then removed and the cells were washed with membrane preserving buffer (1 mM MgCl2 and 0.1 mM CaCl2 in PBS). Non-specific bindings were blocked with 1% BSA in membrane preserving buffer for 15 min. Thereafter, the cells were washed with membrane preserving buffer three times and incubated with 1 µg/ml mouse monoclonal anti-PMCA2 antibody (Santa Cruz Biotechnology) or isotype-controlled IgG (Sigma-Aldrich; St. Louis, MO, USA) at 37 °C for 30 min. After washing with membrane preserving buffer, the cells were subjected to crystal-cell adhesion and crystal internalization assays as described below.
COM crystal-cell adhesion assay
Plain COM crystals (100 µg crystals/ml medium) were added onto the cell monolayer and incubated at 37 °C for 1 h. The unbound crystals were eliminated by five washes with PBS. Finally, the remaining adherent COM crystals on the cell monolayer were counted from 15 random high-power fields (HPFs) under a phase-contrast microscope (Eclipse Ti-S, Nikon; Tokyo, Japan).
COM crystal internalization assay
FITC-labeled COM crystals (1000 µg crystals/ml medium) were added onto the cell monolayer and allowed for internalization at 37 °C for 1 h. The unbound crystals were eliminated by five washes with PBS. Finally, the cells were incubated with 0.1% trypsin/2.5 mM EDTA in PBS to discard adhered but uninternalized crystals. The cells with internalized FITC-labeled COM crystals were then quantified by flow cytometry using BD Accuri™ C6 flow cytometer (Beckman Coulter; Fullerton, CA, USA).
Statistical analysis
All experiments were performed in three biological replicates, unless stated otherwise. Quantitative data are presented as mean ± SEM. One-way ANOVA followed by Tukey’s post hoc test was performed for multiple comparisons of the data among groups. p values less than 0.05 were considered statistically significant.
Results
Analytical methods used in this study are summarized as a schematic in Fig. 1. An IP-MS approach was employed to isolate endogenous PMCA2 and to identify its interacting partners from MDCK cells. SDS-PAGE showed a distinct band at ~ 90 kDa only in the anti-PMCA2-IP samples in all triplicates (Fig. 2a). Western blotting confirmed that PMCA2 band was present only in the anti-PMCA2-IP sample indicating successful PMCA2 pull-down by IP (Fig. 2b). Each lane of the immunoprecipitated proteins derived from anti-PMCA2 or isotype-controlled IgG was excised into 20 gel slices (Fig. 2c) and subjected to in-gel tryptic digestion and identified by nanoLC-ESI-Qq-TripleTOF MS/MS. Initially, IP-MS revealed a total of 1030 proteins in the anti-PMCA2-IP sample (Fig. 2d).
Fig. 1.
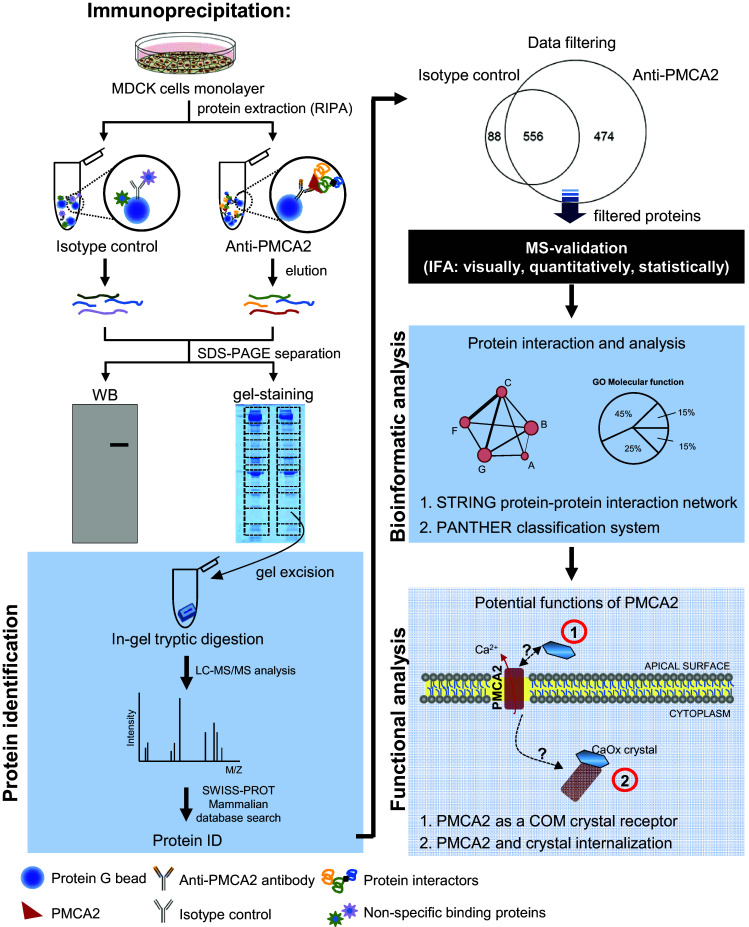
Schematic for characterizations of PMCA2-interacting partners and its function
Fig. 2.
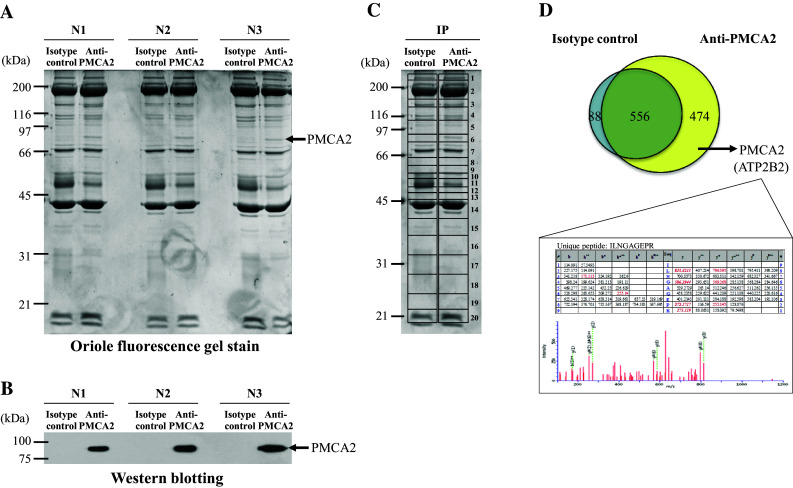
IP-MS analysis of PMCA2-interacting complex. a Consistency of the SDS-PAGE band pattern of immunoprecipitated proteins in three independent experiments using anti-PMCA2 antibody vs. isotype-controlled IgG. b Western blotting to confirm the presence of PMCA2 in the immunoprecipitated samples. c Protein bands were excised to 20 gel slices/lane and subjected to in-gel tryptic digestion and identification by nanoLC-ESI-Qq-TripleTOF MS/MS. d A Venn diagram illustrating number of both specific and non-specific PMCA2 interactors. From a total of 1030 proteins identified in anti-PMCA2-IP sample, subtraction excluded 556 non-specific binders, leaving only 474 proteins to serve as potential PMCA2-interacting partners. The lower panel illustrates MS/MS spectra and fragmented ions of PMCA2 identified from nanoLC-ESI-Qq-TripleTOF MS/MS
By eliminating “background contaminants” caused by non-specific bead/IgG bindings in the isotype-controlled sample, 644 non-specific binding proteins were excluded from the list (556 were common between anti-PMCA2-IP and isotype-controlled samples, whereas 88 were detected only in the isotype-controlled sample) (Fig. 2d). Finally, a total of 474 were defined as the PMCA2-interacting partners (Fig. 2d). Their identities and details of mass spectrometric data are summarized in Supplementary Table S1. As a confirmatory result, PMCA2 was one among those proteins in the PMCA2-interacting complex included in this list (Fig. 2d, Table 1, and Supplementary Table S1). Among 474 PMCA2-interacting proteins identified, eight proteins were the known PMCA2 interactors, of which associations have been confirmed by experimental data [19, 32–34] (Table 1—part I). These included PDZ and LIM domain protein 7 [19], several calcineurin subunits [32], and protein kinase C (PKC) delta [33, 34].
Table 1.
Summary of the PMCA2-interacting proteins identified by IP-MS
| Gene symbol | Protein name | ||
|---|---|---|---|
| I. Known PMCA2-interacting partners (with experimental evidence) | |||
| *Ref. [19] | PDLIM7 | PDZ and LIM domain protein 7 | |
| Ref. [32] | PPP2R2A | Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B alpha isoform (calcineurin) | |
| Ref. [32] | PPP2R1A | Serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A alpha isoform (calcineurin) | |
| Ref. [32] | PPP6C | Serine/threonine-protein phosphatase 6 catalytic subunit (calcineurin) | |
| Ref. [32] | PPP1CA | Serine/threonine-protein phosphatase PP1-alpha catalytic subunit (calcineurin) | |
| Ref. [32] | PPP1CB | Serine/threonine-protein phosphatase PP1-beta catalytic subunit (calcineurin) | |
| Ref. [32] | PPP1CC | Serine/threonine-protein phosphatase PP1-gamma catalytic subunit (calcineurin) | |
| Refs. [33, 34] | PRKCD | Protein kinase C delta type | |
| IIa. Potential PMCA2-interacting partners based on STRING analysis (high confidence: score ≥ 0.70) | |||
| – | – (None detected) | ||
| IIb. Potential PMCA2-interacting partners based on STRING analysis (medium confidence: 0.40 < score < 0.70) | |||
| ATP12A | Potassium-transporting ATPase alpha chain 2 | ||
| ATP1A2 | Sodium/potassium-transporting ATPase subunit alpha-2 | ||
| ATP1A3 | Sodium/potassium-transporting ATPase subunit alpha-3 | ||
| ATP1A4 | Sodium/potassium-transporting ATPase subunit alpha-4 | ||
| IIc. Potential PMCA2-interacting partners based on STRING analysis (low confidence: 0.15 < score ≤ 0.40) | |||
| DAPK1 | Death-associated protein kinase 1 (calcium/calmodulin-dependent serine/threonine kinase) | ||
| DAPK3 | Death-associated protein kinase 3 (calcium/calmodulin-dependent serine/threonine kinase) | ||
| EPHA2 | Ephrin type-A receptor 2 | ||
| #,* | GDI2 | Rab GDP dissociation inhibitor 2 | |
| MCU | Calcium uniporter protein, mitochondrial | ||
| MOCS3 | Adenylyltransferase and sulfurtransferase MOCS3 | ||
| MYL1 | Myosin light chain 1/3, skeletal muscle isoform | ||
| NKRF | NF-kappa-B-repressing factor | ||
| NSF | Vesicle-fusing ATPase | ||
| POTEF | POTE ankyrin domain family member F | ||
| POTFJ | POTE ankyrin domain family member J | ||
| RPS27A | Ubiquitin-40S ribosomal protein S27a | ||
| TRIP13 | Pachytene checkpoint protein 2 homolog | ||
| UBB | Polyubiquitin-B | ||
| UMPS | Uridine 5′-monophosphate synthase | ||
| XPO1 | Exportin-1 | ||
| III. Novel PMCA2-interacting partners | |||
| YWHAQ | 14-3-3 protein theta | ||
| PSMC4 | 26S protease regulatory subunit 6B | ||
| PSMC2 | 26S protease regulatory subunit 7 | ||
| PSMD13 | 26S proteasome non-ATPase regulatory subunit 13 | ||
| PSMD14 | 26S proteasome non-ATPase regulatory subunit 14 | ||
| PSMD3 | 26S proteasome non-ATPase regulatory subunit 3 | ||
| PSMD7 | 26S proteasome non-ATPase regulatory subunit 7 | ||
| OGDH | 2-Oxoglutarate dehydrogenase, mitochondrial | ||
| OGDHL | 2-Oxoglutarate dehydrogenase-like, mitochondrial | ||
| BCKDHB | 2-Oxoisovalerate dehydrogenase subunit beta, mitochondrial | ||
| RPSA | 40S ribosomal protein SA | ||
| PRKAA1 | 5′-AMP-activated protein kinase catalytic subunit alpha-1 | ||
| HSPD1 | 60 kDa heat shock protein, mitochondrial | ||
| RPLP0 | 60S acidic ribosomal protein P0 | ||
| RPL10 | 60S ribosomal protein L10 | ||
| RPL3 | 60S ribosomal protein L3 | ||
| RPL5 | 60S ribosomal protein L5 | ||
| RPL6 | 60S ribosomal protein L6 | ||
| ABI1 | Abl interactor 1 | ||
| ABLIM3 | Actin-binding LIM protein 3 | ||
| TRIP4 | Activating signal cointegrator 1 | ||
| ACAD11 | Acyl-CoA dehydrogenase family member 11 | ||
| SLC25A4 | ADP/ATP translocase 1 | ||
| SLC25A31 | ADP/ATP translocase 4 | ||
| AKAP8 | A-kinase anchor protein 8 | ||
| ALDH7A1 | Alpha-aminoadipic semialdehyde dehydrogenase | ||
| CSN1S2A | Alpha-S2-casein-like A | ||
| ALYREF2 | Aly/REF export factor 2 | ||
| ACE2 | Angiotensin-converting enzyme 2 | ||
| ANKEF1 | Ankyrin repeat and EF-hand domain-containing protein 1 | ||
| ASB7 | Ankyrin repeat and SOCS box protein 7 | ||
| # | ANXA1 | Annexin A1 | |
| AP1B1 | AP-1 complex subunit beta-1 | ||
| AP1M1 | AP-1 complex subunit mu-1 | ||
| AP1M2 | AP-1 complex subunit mu-2 | ||
| AP2B1 | AP-2 complex subunit beta | ||
| AP2M1 | AP-2 complex subunit mu | ||
| A1CF | APOBEC1 complementation factor | ||
| GOT2 | Aspartate aminotransferase, mitochondrial | ||
| DARS | Aspartate–tRNA ligase, cytoplasmic | ||
| ATAD3A | ATPase family AAA domain-containing protein 3A | ||
| ATAD3B | ATPase family AAA domain-containing protein 3B | ||
| ABCE1 | ATP-binding cassette sub-family E member 1 | ||
| ABCF3 | ATP-binding cassette sub-family F member 3 | ||
| ACLY | ATP-citrate synthase | ||
| PFKP | ATP-dependent 6-phosphofructokinase, platelet type | ||
| DDX1 | ATP-dependent RNA helicase DDX1 | ||
| DDX39A | ATP-dependent RNA helicase DDX39A | ||
| DHX8 | ATP-dependent RNA helicase DHX8 | ||
| YME1L1 | ATP-dependent zinc metalloprotease YME1L1 | ||
| EPB41L5 | Band 4.1-like protein 5 | ||
| BZW2 | Basic leucine zipper and W2 domain-containing protein 2 | ||
| ENO3 | Beta-enolase | ||
| EPRS | Bifunctional glutamate/proline–tRNA ligase | ||
| MTHFD2 | Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial | ||
| BRCA2 | Breast cancer type 2 susceptibility protein homolog | ||
| BTBD9 | BTB/POZ domain-containing protein 9 | ||
| BYSL | Bystin | ||
| CAD | CAD protein | ||
| CALU | Calumenin | ||
| CTSZ | Cathepsin Z | ||
| CCAR2 | Cell cycle and apoptosis regulator protein 2 | ||
| CDC45 | Cell division control protein 45 homolog | ||
| CDC5L | Cell division cycle 5-like protein | ||
| CDC123 | Cell division cycle protein 123 homolog | ||
| CENPV | Centromere protein V | ||
| CEP104 | Centrosomal protein of 104 kDa | ||
| CEP164 | Centrosomal protein of 164 kDa | ||
| CLIC1 | Chloride intracellular channel protein 1 | ||
| CHAF1B | Chromatin assembly factor 1 subunit B | ||
| † | SNAP91 | Clathrin coat assembly protein AP180 | |
| CPSF3 | Cleavage and polyadenylation specificity factor subunit 3 | ||
| CLPTM1 | Cleft lip and palate transmembrane protein 1 homolog | ||
| ARCN1 | Coatomer subunit delta | ||
| CCDC28A | Coiled-coil domain-containing protein 28A | ||
| CCDC61 | Coiled-coil domain-containing protein 61 | ||
| CHCHD3 | Coiled-coil-helix-coiled-coil-helix domain-containing protein 3, mitochondrial | ||
| COL4A2 | Collagen alpha-2(IV) chain | ||
| FAM120A | Constitutive coactivator of PPAR-gamma-like protein 1 | ||
| FAM120C | Constitutive coactivator of PPAR-gamma-like protein 2 | ||
| H2AFY | Core histone macro-H2A.1 | ||
| H2AFY2 | Core histone macro-H2A.2 | ||
| CORO6 | Coronin-6 | ||
| CUL1 | Cullin-1 | ||
| CDK17 | Cyclin-dependent kinase 17 | ||
| CDK20 | Cyclin-dependent kinase 20 | ||
| CLNK | Cytokine-dependent hematopoietic cell linker | ||
| CKAP2 | Cytoskeleton-associated protein 2 | ||
| AGBL1 | Cytosolic carboxypeptidase 4 | ||
| ALDH18A1 | Delta-1-pyrroline-5-carboxylate synthase | ||
| DNASE1L1 | Deoxyribonuclease-1-like 1 | ||
| DGKB | Diacylglycerol kinase beta | ||
| DLST | Dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex, mitochondrial | ||
| DPYS | Dihydropyrimidinase | ||
| CRMP1 | Dihydropyrimidinase-related protein 1 | ||
| DPYSL2 | Dihydropyrimidinase-related protein 2 | ||
| MLH1 | DNA mismatch repair protein Mlh1 | ||
| MSH2 | DNA mismatch repair protein Msh2 | ||
| POLB | DNA polymerase beta | ||
| MCM5 | DNA replication licensing factor MCM5 | ||
| TOP2B | DNA topoisomerase 2-beta | ||
| TOP1MT | DNA topoisomerase I, mitochondrial | ||
| DNAJA1 | DnaJ homolog subfamily A member 1 | ||
| DNAJA2 | DnaJ homolog subfamily A member 2 | ||
| DNAJB12 | DnaJ homolog subfamily B member 12 | ||
| DNAJC10 | DnaJ homolog subfamily C member 10 | ||
| DNAJC11 | DnaJ homolog subfamily C member 11 | ||
| DDOST | Dolichyl-diphosphooligosaccharide–protein glycosyltransferase 48 kDa subunit | ||
| RPN1 | Dolichyl-diphosphooligosaccharide–protein glycosyltransferase subunit 1 | ||
| RPN2 | Dolichyl-diphosphooligosaccharide–protein glycosyltransferase subunit 2 | ||
| STAU1 | Double-stranded RNA-binding protein Staufen homolog 1 | ||
| DBNL | Drebrin-like protein | ||
| DSTYK | Dual serine/threonine and tyrosine protein kinase | ||
| DYRK1A | Dual specificity tyrosine-phosphorylation-regulated kinase 1A | ||
| DYRK1B | Dual specificity tyrosine-phosphorylation-regulated kinase 1B | ||
| † | DNM3 | Dynamin-3 | |
| DST | Dystonin | ||
| DTNA | Dystrobrevin alpha | ||
| DTNB | Dystrobrevin beta | ||
| RANBP2 | E3 SUMO-protein ligase RanBP2 | ||
| TRIM39 | E3 ubiquitin-protein ligase TRIM39 | ||
| TRIM56 | E3 ubiquitin-protein ligase TRIM56 | ||
| UHRF1 | E3 ubiquitin-protein ligase UHRF1 | ||
| EML4 | Echinoderm microtubule-associated protein-like 4 | ||
| † | EHD1 | EH domain-containing protein 1 | |
| † | EHD2 | EH domain-containing protein 2 | |
| † | EHD3 | EH domain-containing protein 3 | |
| † | EHD4 | EH domain-containing protein 4 | |
| ELAVL4 | ELAV-like protein 4 | ||
| ETFA | Electron transfer flavoprotein subunit alpha, mitochondrial | ||
| EEF1A2 | Elongation factor 1-alpha 2 | ||
| EEF1D | Elongation factor 1-delta | ||
| EEF1G | Elongation factor 1-gamma | ||
| ENDOD1 | Endonuclease domain-containing 1 protein | ||
| EPS8 | Epidermal growth factor receptor kinase substrate 8 | ||
| EPS8L2 | Epidermal growth factor receptor kinase substrate 8-like protein 2 | ||
| ERLIN1 | Erlin-1 | ||
| ERLIN2 | Erlin-2 | ||
| EIF4A3 | Eukaryotic initiation factor 4A-III | ||
| GSPT2 | Eukaryotic peptide chain release factor GTP-binding subunit ERF3B | ||
| EIF2S3 | Eukaryotic translation initiation factor 2 subunit 3 | ||
| EIF2S3Y | Eukaryotic translation initiation factor 2 subunit 3, Y-linked | ||
| EIF3B | Eukaryotic translation initiation factor 3 subunit B | ||
| EIF3C | Eukaryotic translation initiation factor 3 subunit C | ||
| EIF3D | Eukaryotic translation initiation factor 3 subunit D | ||
| EIF3E | Eukaryotic translation initiation factor 3 subunit E | ||
| DIS3 | Exosome complex exonuclease RRP44 | ||
| XPO4 | Exportin-4 | ||
| XPO7 | Exportin-7 | ||
| XPOT | Exportin-T | ||
| CAPZA2 | F-actin-capping protein subunit alpha-2 | ||
| KHSRP | Far upstream element-binding protein 2 | ||
| FAF2 | FAS-associated factor 2 | ||
| FAR1 | Fatty acyl-CoA reductase 1 | ||
| FOLR2 | Folate receptor beta | ||
| FHL2 | Four and a half LIM domains protein 2 | ||
| FMR1 | Fragile X mental retardation protein 1 | ||
| FXR2 | Fragile X mental retardation syndrome-related protein 2 | ||
| ALDOA | Fructose-bisphosphate aldolase A | ||
| LGALS8 | Galectin-8 | ||
| GMDS | GDP-mannose 4,6 dehydratase | ||
| GMIP | GEM-interacting protein | ||
| GLUD1 | Glutamate dehydrogenase 1, mitochondrial | ||
| GLUD2 | Glutamate dehydrogenase 2, mitochondrial | ||
| GFPT1 | Glutamine–fructose-6-phosphate aminotransferase [isomerizing] 1 | ||
| GFPT2 | Glutamine–fructose-6-phosphate aminotransferase [isomerizing] 2 | ||
| QARS | Glutamine–tRNA ligase | ||
| AGL | Glycogen debranching enzyme | ||
| GTDC1 | Glycosyltransferase-like domain-containing protein 1 | ||
| GOLGB1 | Golgin subfamily B member 1 | ||
| GBF1 | Golgi-specific brefeldin A-resistance guanine nucleotide exchange factor 1 | ||
| GRN | Granulins | ||
| RAN | GTP-binding nuclear protein Ran | ||
| GNAI1 | Guanine nucleotide-binding protein G(i) subunit alpha-1 | ||
| GNB1 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 | ||
| GNAO1 | Guanine nucleotide-binding protein G(o) subunit alpha | ||
| GNAL | Guanine nucleotide-binding protein G(olf) subunit alpha | ||
| GNAQ | Guanine nucleotide-binding protein G(q) subunit alpha | ||
| GNAT1 | Guanine nucleotide-binding protein G(t) subunit alpha-1 | ||
| GNA11 | Guanine nucleotide-binding protein subunit alpha-11 | ||
| GNA14 | Guanine nucleotide-binding protein subunit alpha-14 | ||
| GNA15 | Guanine nucleotide-binding protein subunit alpha-15 | ||
| CLCN3 | H(+)/Cl(−) exchange transporter 3 | ||
| CLCN5 | H(+)/Cl(−) exchange transporter 5 | ||
| DKC1 | H/ACA ribonucleoprotein complex subunit 4 | ||
| HHIP | Hedgehog-interacting protein | ||
| HNF1B | Hepatocyte nuclear factor 1-beta | ||
| # | HNRNPA1L2 | Heterogeneous nuclear ribonucleoprotein A1-like 2 | |
| HNRNPD | Heterogeneous nuclear ribonucleoprotein D0 | ||
| HNRNPH2 | Heterogeneous nuclear ribonucleoprotein H2 | ||
| HNRNPK | Heterogeneous nuclear ribonucleoprotein K | ||
| HNRNPL | Heterogeneous nuclear ribonucleoprotein L | ||
| HK1 | Hexokinase-1 | ||
| HK2 | Hexokinase-2 | ||
| HK3 | Hexokinase-3 | ||
| KAT2B | Histone acetyltransferase KAT2B | ||
| HDAC1 | Histone deacetylase 1 | ||
| HDAC2 | Histone deacetylase 2 | ||
| HIST1H1A | Histone H1.1 | ||
| HIST1H1C | Histone H1.2 | ||
| HIST1H1D | Histone H1.3 | ||
| HIST1H1T | Histone H1t | ||
| H2AFX | Histone H2AX | ||
| H2BFS | Histone H2B type F-S | ||
| H3F3C | Histone H3.3C | ||
| RBBP4 | Histone-binding protein RBBP4 | ||
| RBBP7 | Histone-binding protein RBBP7 | ||
| KMT2A | Histone-lysine N-methyltransferase 2A | ||
| HMBOX1 | Homeobox-containing protein 1 | ||
| KPNB1 | Importin subunit beta-1 | ||
| IGF2BP1 | Insulin-like growth factor 2 mRNA-binding protein 1 | ||
| IGF2BP2 | Insulin-like growth factor 2 mRNA-binding protein 2 | ||
| IGF2BP3 | Insulin-like growth factor 2 mRNA-binding protein 3 | ||
| ITGA2 | Integrin alpha-2 (fragment) | ||
| ITGB4 | Integrin beta-4 | ||
| IFIT2 | Interferon-induced protein with tetratricopeptide repeats 2 | ||
| IDH3B | Isocitrate dehydrogenase (NAD) subunit beta, mitochondrial | ||
| KLHDC7A | Kelch domain-containing protein 7A | ||
| KEAP1 | Kelch-like ECH-associated protein 1 | ||
| KLHL7 | Kelch-like protein 7 | ||
| KLHL9 | Kelch-like protein 9 | ||
| KHDRBS1 | KH domain-containing, RNA-binding, signal transduction-associated protein 1 | ||
| KIF12 | Kinesin-like protein KIF12 | ||
| KIF13B | Kinesin-like protein KIF13B | ||
| KIF1A | Kinesin-like protein KIF1A | ||
| KIF1B | Kinesin-like protein KIF1B | ||
| KIF1C | Kinesin-like protein KIF1C | ||
| KIF20A | Kinesin-like protein KIF20A | ||
| KIF2B | Kinesin-like protein KIF2B | ||
| KIF2C | Kinesin-like protein KIF2C | ||
| KIFC1 | Kinesin-like protein KIFC1 | ||
| LMNB2 | Lamin-B2 | ||
| LCA5L | Lebercilin-like protein | ||
| LZTS1 | Leucine zipper putative tumor suppressor 1 | ||
| LZTS3 | Leucine zipper putative tumor suppressor 3 | ||
| LRCH1 | Leucine-rich repeat and calponin homology domain-containing protein 1 | ||
| LRCH2 | Leucine-rich repeat and calponin homology domain-containing protein 2 | ||
| LRGUK | Leucine-rich repeat and guanylate kinase domain-containing protein | ||
| LRRC59 | Leucine-rich repeat-containing protein 59 | ||
| LPPR1 | Lipid phosphate phosphatase-related protein type 1 | ||
| ACSL3 | Long-chain-fatty-acid–CoA ligase 3 | ||
| ACSL4 | Long-chain-fatty-acid–CoA ligase 4 | ||
| HELLS | Lymphoid-specific helicase | ||
| KDM7A | Lysine-specific demethylase 7A | ||
| LYG2 | Lysozyme g-like protein 2 | ||
| MVP | Major vault protein | ||
| MARK3 | MAP/microtubule affinity-regulating kinase 3 | ||
| MELK | Maternal embryonic leucine zipper kinase | ||
| PAQR8 | Membrane progestin receptor beta | ||
| # | MTA1 | Metastasis-associated protein MTA1 | |
| MTA2 | Metastasis-associated protein MTA2 | ||
| MTA3 | Metastasis-associated protein MTA3 | ||
| MARS | Methionine–tRNA ligase, cytoplasmic | ||
| MCCC2 | Methylcrotonoyl-CoA carboxylase beta chain, mitochondrial | ||
| MINK1 | Misshapen-like kinase 1 | ||
| #,* | SLC25A22 | Mitochondrial glutamate carrier 1 | |
| #,* | SLC25A18 | Mitochondrial glutamate carrier 2 | |
| † | RHOT1 | Mitochondrial Rho GTPase 1 | |
| MAP4K4 | Mitogen-activated protein kinase kinase kinase kinase 4 | ||
| BUB3 | Mitotic checkpoint protein BUB3 | ||
| MISP | Mitotic interactor and substrate of PLK1 | ||
| NIFK | MKI67 FHA domain-interacting nucleolar phosphoprotein | ||
| SMAD2 | Mothers against decapentaplegic homolog 2 | ||
| SMAD3 | Mothers against decapentaplegic homolog 3 | ||
| SMAD9 | Mothers against decapentaplegic homolog 9 | ||
| MYD88 | Myeloid differentiation primary response protein MyD88 | ||
| NAT10 | N-acetyltransferase 10 | ||
| NCKIPSD | NCK-interacting protein with SH3 domain | ||
| NBEAL2 | Neurobeachin-like protein 2 | ||
| NEFL | Neurofilament light polypeptide | ||
| NAV3 | Neuron navigator 3 | ||
| NPY5R | Neuropeptide Y receptor type 5 | ||
| FAM129B | Niban-like protein 1 | ||
| NCBP1 | Nuclear cap-binding protein subunit 1 | ||
| NUFIP2 | Nuclear fragile X mental retardation-interacting protein 2 | ||
| NUP93 | Nuclear pore complex protein Nup93 | ||
| NCOA5 | Nuclear receptor coactivator 5 | ||
| YBX1 | Nuclease-sensitive element-binding protein 1 | ||
| NOL6 | Nucleolar protein 6 | ||
| UBTF | Nucleolar transcription factor 1 | ||
| TIAL1 | Nucleolysin TIAR | ||
| NPM1 | Nucleophosmin | ||
| OR6N2 | Olfactory receptor 6N2 | ||
| OAT | Ornithine aminotransferase, mitochondrial | ||
| CDC73 | Parafibromin | ||
| PCID2 | PCI domain-containing protein 2 | ||
| GIPC1 | PDZ domain-containing protein GIPC1 | ||
| PCM1 | Pericentriolar material 1 protein | ||
| † | PICALM | Phosphatidylinositol-binding clathrin assembly protein | |
| PGM3 | Phosphoacetylglucosamine mutase | ||
| # | ATP2B2 | Plasma membrane calcium-transporting ATPase 2 | |
| PAPOLA | Poly(A) polymerase alpha | ||
| PAPOLB | Poly(A) polymerase beta | ||
| PCBP3 | Poly(rC)-binding protein 3 | ||
| PABPC1L | Polyadenylate-binding protein 1-like | ||
| PABPC4L | Polyadenylate-binding protein 4-like | ||
| POLDIP3 | Polymerase delta-interacting protein 3 | ||
| PTBP2 | Polypyrimidine tract-binding protein 2 | ||
| PTBP3 | Polypyrimidine tract-binding protein 3 | ||
| PRPF19 | Pre-mRNA-processing factor 19 | ||
| DDX20 | Probable ATP-dependent RNA helicase DDX20 | ||
| DDX4 | Probable ATP-dependent RNA helicase DDX4 | ||
| DDX47 | Probable ATP-dependent RNA helicase DDX47 | ||
| SETX | Probable helicase senataxin | ||
| RBM46 | Probable RNA-binding protein 46 | ||
| † | PDCD6IP | Programmed cell death 6-interacting protein (Alix) | |
| PCNA | Proliferating cell nuclear antigen | ||
| AGO2 | Protein argonaute-2 | ||
| CYR61 | Protein CYR61 | ||
| FAM83B | Protein FAM83B | ||
| PRRC2A | Protein PRRC2A | ||
| RCC2 | Protein RCC2 | ||
| TBRG4 | Protein TBRG4 | ||
| TFG | Protein TFG | ||
| SEC23B | Protein transport protein Sec23B | ||
| ZYG11B | Protein zyg-11 homolog B | ||
| LOX | Protein-lysine 6-oxidase | ||
| NPEPPSL1 | Puromycin-sensitive aminopeptidase-like protein | ||
| HSP90B2P | Putative endoplasmin-like protein | ||
| EIF2S3L | Putative eukaryotic translation initiation factor 2 subunit 3-like protein | ||
| # | HSPA7 | Putative heat shock 70 kDa protein 7 | |
| # | HSP90AA2 | Putative heat shock protein HSP 90-alpha A2 | |
| # | HSP90AA5P | Putative heat shock protein HSP 90-alpha A | |
| # | HSP90AB4P | Putative heat shock protein HSP 90-beta 4 | |
| DHX16 | Putative pre-mRNA-splicing factor ATP-dependent RNA helicase DHX16 | ||
| PDHB | Pyruvate dehydrogenase E1 component subunit beta, mitochondrial | ||
| RAB11FIP1 | Rab11 family-interacting protein 1 | ||
| RACGAP1 | Rac GTPase-activating protein 1 | ||
| RAF1 | RAF proto-oncogene serine/threonine-protein kinase | ||
| RALBP1 | RalA-binding protein 1 | ||
| RANGAP1 | Ran GTPase-activating protein 1 | ||
| G3BP1 | Ras GTPase-activating protein-binding protein 1 | ||
| G3BP2 | Ras GTPase-activating protein-binding protein 2 | ||
| IQGAP1 | Ras GTPase-activating-like protein IQGAP1 | ||
| † | RAC1 | Ras-related C3 botulinum toxin substrate 1 | |
| † | RAC3 | Ras-related C3 botulinum toxin substrate 3 | |
| † | RAB1A | Ras-related protein Rab-1A | |
| RAB26 | Ras-related protein Rab-26 | ||
| RAB37 | Ras-related protein Rab-37 | ||
| RAB39B | Ras-related protein Rab-39B | ||
| #,*,† | RAB5A | Ras-related protein Rab-5A | |
| RCC1 | Regulator of chromosome condensation | ||
| RFC2 | Replication factor C subunit 2 | ||
| RFC4 | Replication factor C subunit 4 | ||
| RFC5 | Replication factor C subunit 5 | ||
| RPA1 | Replication protein A 70 kDa DNA-binding subunit | ||
| RTKN | Rhotekin | ||
| RRM1 | Ribonucleoside-diphosphate reductase large subunit | ||
| BOP1 | Ribosome biogenesis protein BOP1 | ||
| RCL1 | RNA 3′-terminal phosphate cyclase-like protein | ||
| RBM47 | RNA-binding protein 47 | ||
| RALY | RNA-binding protein Raly | ||
| CROCC | Rootletin | ||
| RUVBL1 | RuvB-like 1 | ||
| RUVBL2 | RuvB-like 2 | ||
| MAT1A | S-adenosylmethionine synthase isoform type-1 | ||
| MAT2A | S-adenosylmethionine synthase isoform type-2 | ||
| SEPT10 | Septin-10 | ||
| SEPT11 | Septin-11 | ||
| SEPT14 | Septin-14 | ||
| SEPT6 | Septin-6 | ||
| SEPT7 | Septin-7 | ||
| SEPT8 | Septin-8 | ||
| SEPT9 | Septin-9 | ||
| SQSTM1 | Sequestosome-1 | ||
| SHMT2 | Serine hydroxymethyltransferase, mitochondrial | ||
| SRSF2 | Serine/arginine-rich splicing factor 2 | ||
| SRSF8 | Serine/arginine-rich splicing factor 8 | ||
| ARAF | Serine/threonine-protein kinase A-Raf | ||
| BRAF | Serine/threonine-protein kinase B-raf | ||
| MARK1 | Serine/threonine-protein kinase MARK1 | ||
| MARK2 | Serine/threonine-protein kinase MARK2 | ||
| CDC42BPB | Serine/threonine-protein kinase MRCK beta | ||
| NEK5 | Serine/threonine-protein kinase Nek5 | ||
| PAK2 | Serine/threonine-protein kinase PAK 2 | ||
| PLK1 | Serine/threonine-protein kinase PLK1 | ||
| SH3BP4 | SH3 domain-binding protein 4 | ||
| SNRPN | Small nuclear ribonucleoprotein-associated protein N | ||
| SMTN | Smoothelin | ||
| SNW1 | SNW domain-containing protein 1 | ||
| SLC2A1 | Solute carrier family 2, facilitated glucose transporter member 1 | ||
| SLC22A4 | Solute carrier family 22 member 4 | ||
| † | SNX2 | Sorting nexin-2 | |
| STRBP | Spermatid perinuclear RNA-binding protein | ||
| DDX39B | Spliceosome RNA helicase DDX39B | ||
| SF3B2 | Splicing factor 3B subunit 2 | ||
| SFPQ | Splicing factor, proline- and glutamine-rich | ||
| CTTN | Src substrate cortactin | ||
| SRPK1 | SRSF protein kinase 1 | ||
| STOML3 | Stomatin-like protein 3 | ||
| SMC2 | Structural maintenance of chromosomes protein 2 | ||
| SDHA | Succinate dehydrogenase (ubiquinone) flavoprotein subunit, mitochondrial | ||
| SUN2 | SUN domain-containing protein 2 | ||
| SKIV2L2 | Superkiller viralicidic activity 2-like 2 | ||
| VAT1 | Synaptic vesicle membrane protein VAT-1 homolog | ||
| SYTL3 | Synaptotagmin-like protein 3 | ||
| STXBP2 | Syntaxin-binding protein 2 | ||
| TLN1 | Talin-1 | ||
| TARDBP | TAR DNA-binding protein 43 | ||
| TPX2 | Targeting protein for Xklp2 | ||
| TBPL2 | TATA box-binding protein-like protein 2 | ||
| TAX1BP1 | Tax1-binding protein 1 homolog | ||
| CCT2 | T-complex protein 1 subunit beta | ||
| CCT7 | T-complex protein 1 subunit eta | ||
| CCT6A | T-complex protein 1 subunit zeta | ||
| CCT6B | T-complex protein 1 subunit zeta-2 | ||
| TES | Testin | ||
| † | TOM1L2 | TOM1-like protein 2 | |
| TNIK | TRAF2 and NCK-interacting protein kinase | ||
| TTF2 | Transcription termination factor 2 | ||
| TBL3 | Transducin beta-like protein 3 | ||
| TKTL1 | Transketolase-like protein 1 | ||
| SSR1 | Translocon-associated protein subunit alpha | ||
| TNPO3 | Transportin-3 | ||
| HADHB | Trifunctional enzyme subunit beta, mitochondrial | ||
| RTCB | tRNA-splicing ligase RtcB homolog | ||
| TMOD1 | Tropomodulin-1 | ||
| TMOD2 | Tropomodulin-2 | ||
| TUBAL3 | Tubulin alpha chain-like 3 | ||
| TUBA3A | Tubulin alpha-3 chain | ||
| # | TUBB2A | Tubulin beta-2A chain | |
| # | N/A | Tubulin beta-8 chain-like protein LOC260334 | |
| TUBG1 | Tubulin gamma-1 chain | ||
| TUBG2 | Tubulin gamma-2 chain | ||
| TP53I13 | Tumor protein p53-inducible protein 13 | ||
| HCK | Tyrosine-protein kinase HCK | ||
| JAK1 | Tyrosine-protein kinase JAK1 | ||
| UTP15 | U3 small nucleolar RNA-associated protein 15 homolog | ||
| UTP18 | U3 small nucleolar RNA-associated protein 18 homolog | ||
| PRPF3 | U4/U6 small nuclear ribonucleoprotein Prp3 | ||
| USP26 | Ubiquitin carboxyl-terminal hydrolase 26 | ||
| USP7 | Ubiquitin carboxyl-terminal hydrolase 7 | ||
| UBA1 | Ubiquitin-like modifier-activating enzyme 1 | ||
| N/A | Uncharacterized protein C2orf57 homolog | ||
| MYO1H | Unconventional myosin-Ih | ||
| UMOD | Uromodulin | ||
| UTS2 | Urotensin-2 | ||
| VPS26A | Vacuolar protein sorting-associated protein 26A | ||
| VPS35 | Vacuolar protein sorting-associated protein 35 | ||
| VASP | Vasodilator-stimulated phosphoprotein | ||
| VTN | Vitronectin | ||
| VDAC1 | Voltage-dependent anion-selective channel protein 1 | ||
| ATP6V1A | V-type proton ATPase catalytic subunit A | ||
| WDR1 | WD repeat-containing protein 1 | ||
| XRCC5 | X-ray repair cross-complementing protein 5 | ||
| YTHDF2 | YTH domain-containing family protein 2 | ||
N/A not applicable
*Different protein isoforms were reported in previous research articles. However, these isoforms exhibit similar function(s)
#Identified as COM crystal-binding proteins in our previous study [31]
†Identified as proteins involved in endocytosis pathway
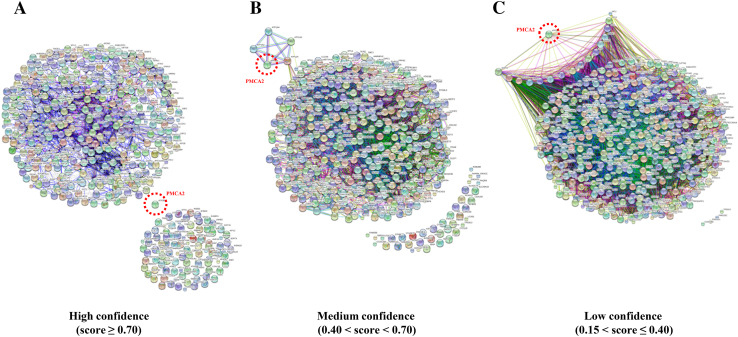
STRING search tool was applied to classify the identified proteins by their likelihoods to serve as the PMCA2-interacting partners. Protein–protein interaction networks with a total input of 466 proteins (excluding eight known PMCA2 interactors as aforementioned) were computed based on the experimental data, literature evidence, and prediction from genomic context analysis [35]. The predicted networks were ranked by three confidence levels, including high confidence (score ≥ 0.70), medium confidence (0.40 < score < 0.70), and low confidence (0.15 < score ≤ 0.4) (Fig. 3). With the high confidence, no protein was predicted to be associated with PMCA2 (Table 1—part IIa). At the medium confidence, the prediction revealed four proteins associated with PMCA2 (Table 1—part IIb). With the low confidence, 16 were predicted to serve as potential PMCA2-interacting partners (Table 1—part IIc). Finally, proteins that were neither known nor potential PMCA2 interactors by such prediction were defined as the “novel PMCA2-interacting partners” (Table 1—part III).
Fig. 3.
Protein–protein interactions networks of PMCA2-interacting partners. Interaction networks of 474 unique proteins associated with PMCA2 were computed by STRING software to predict the likelihood being the PMCA2-interacting partners based on confidence level. a High confidence (score ≥ 0.70). b Medium confidence (0.40 < score < 0.70). c Low confidence (0.15 < score ≤ 0.40). PMCA2 encoded by ATP2B2 gene is highlighted in a red-dotted circle. Only protein nodes that displayed direct interactions to PMCA2 are reported as potential PMCA2-interacting proteins in Table 1 (parts IIa–IIc)
The association of PMCA2 with its partners identified by IP-MS was validated by quantitative immuno-co-localization assay. Using this approach, we successfully confirmed the co-localization of PMCA2 with some of known PMCA2-interacting partners (ezrin and actin, which served as the positive controls) (Fig. 4a), potential PMCA2-interacting partners based on STRING analysis (Na+/K+-ATPase) (Fig. 4b), and novel PMCA2-interacting partners (annexin A1, Alix and hnRNP K) (Fig. 4c). The data showed no association of PMCA2 with non-PMCA2-interacting partners (c-Jun, SOD-1 and DJ-1, which served as the negative controls) (Fig. 4d).
Fig. 4.
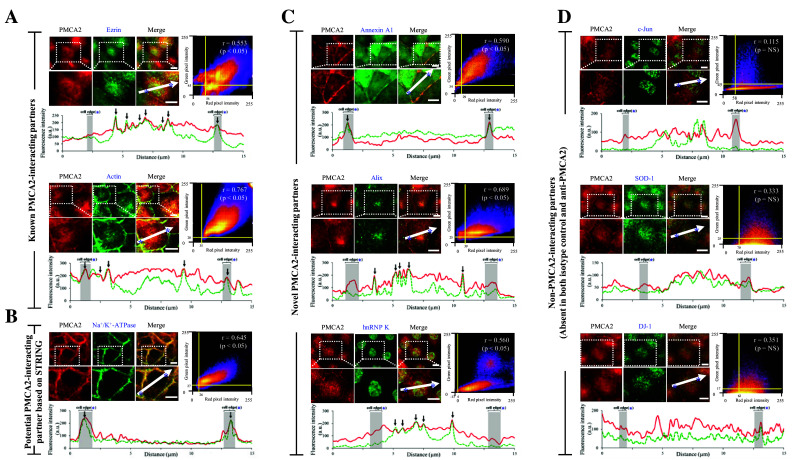
Quantitative immuno-co-localization analysis of PMCA2 and its interacting partners. a PMCA2 vs. known PMCA2-interacting partners (ezrin and actin) (served as the positive controls). b PMCA2 vs. potential PMCA2-interacting partners based on STRING analysis (Na+/K+-ATPase). c PMCA2 vs. novel PMCA2-interacting partners (annexin A1, Alix and hnRNP K). d PMCA2 vs. non-PMCA2-interacting partners (c-Jun, SOD-1 and DJ-1) (served as the negative controls). In each pair, intensity correlation scatter plot estimated the degree of co-localization between red (PMCA2) and green (protein partner of interest) signals. Pixel intensity thresholds are indicated with yellow lines. Pearson’s correlation coefficient (r) of the co-localization is shown in the top-right corner of the plot. Intensity profile of the two immunofluorescence signals along the linear section of area of interest (indicated with white arrow) at a distance of 15 µm across the cell is depicted at the bottom of each pair. PMCA2 is displayed as a red line, whereas the partner protein of interest is displayed in green-dotted line. Area of the cell edge (plasma membrane) is labeled with an asterisk and highlighted in gray. Co-localization of the two probes is indicated with black arrow. A scale bar represents 5-μm-distance
PANTHER analysis revealed eight molecular functions of these PMCA2-interacting partners, including binding (38.9%), catalytic (34.0%), receptor (9.7%), transcription factor (5.6%), transporter (4.2%), enzyme regulator (3.6%), translation regulator (3.4%), and structural molecule (0.6%) activities (Fig. 5a). Further stratification of the binding activity, which was the most prominent function, showed nucleic acid binding (48.9%), protein binding (37.9%), and calcium ion binding (7.8%) as the top-three subgroups. This was consistent with the data reported in our previous large-scale proteomic study demonstrating that PMCA2 isolated from apical membranes of MDCK renal tubular cells was one among the COM crystal-binding proteins [31]. However, such previous proteomic screening had not been validated. This present study thus addressed the potential role of PMCA2 as a COM crystal-binding protein. Expression of PMCA2 in MDCK whole cell as well as apical membranes and COM crystal-binding fraction was confirmed by Western blotting. As shown in Fig. 5b, immunoreactive band of PMCA2 was detectable in whole cell lysate, apical membrane fraction, and COM crystal-bound fraction, confirming the role of PMCA2 as a COM crystal-binding protein. Moreover, immunofluorescence staining clearly showed PMCA2 on the surface of COM crystals after COM crystal-protein binding assay, which further strengthened its role as the COM crystal-binding protein (Fig. 5c).
Fig. 5.
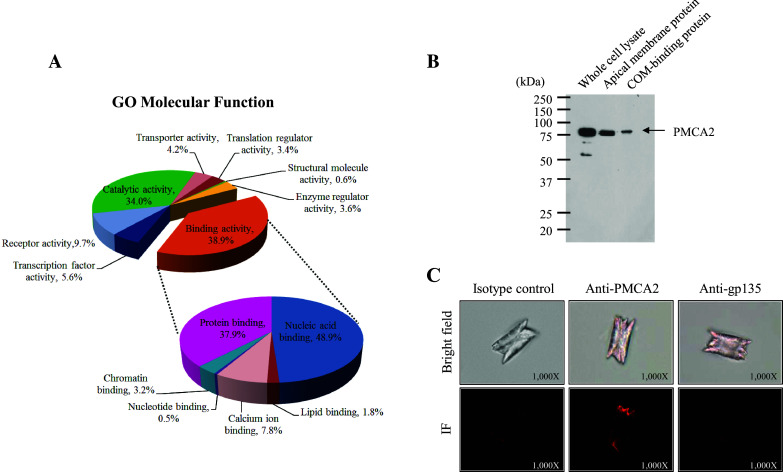
Gene ontology (GO) analysis of PMCA2-interacting partners and a novel role of PMCA2 as a COM crystal-binding protein. a GO classification by molecular function using PANTHER database showed eight potential functions of PMCA2 interactors, especially binding activity, which is the most predominant one (38.9%). Further breakdown of the binding activity is shown as a zoom-in pie chart. b COM crystal-protein binding assay followed by Western blotting to confirm the presence of PMCA2 in whole cell lysate, apical membranes, and COM crystal-bound fraction. c COM crystal-protein binding assay followed by immunofluorescence (IF) staining using anti-PMCA2 primary antibody to confirm the presence of PMCA2 (in red) on the crystal surface, whereas staining with isotype-controlled IgG and anti-gp135 antibody served as the negative controls (original magnification was ×1000)
The functional role of PMCA2 as a potential COM crystal receptor was further validated by crystal-cell adhesion assay on the intact MDCK cells. Analysis of the controlled cells indicated that COM crystals could bind to the cells (Fig. 6a, b). Neutralization of surface PMCA2 expression by a specific anti-PMCA2 antibody dramatically reduced the number of adherent crystals from 28.0 ± 3.4 to 18.1 ± 2.5 (no./HPF) as compared to the blank control, whereas neutralization by the isotype-controlled IgG had no significant effects (Fig. 6a, b). This data confirmed the role of PMCA2 as a potential COM crystal receptor. Moreover, the role of PMCA2 in COM crystal internalization into the cells was investigated using FITC-labeled crystals followed by flow cytometry. While the controlled cells showed the internalized/endocytotic crystals, neutralization of the surface PMCA2 by a specific anti-PMCA2 antibody dramatically reduced the number of internalized crystals from 19.26 ± 0.01 to 13.56 ± 0.02% as compared to the blank control (Fig. 6c, d). There was no significant change observed when the isotype-controlled IgG was used for neutralization (Fig. 6c, d).
Fig. 6.
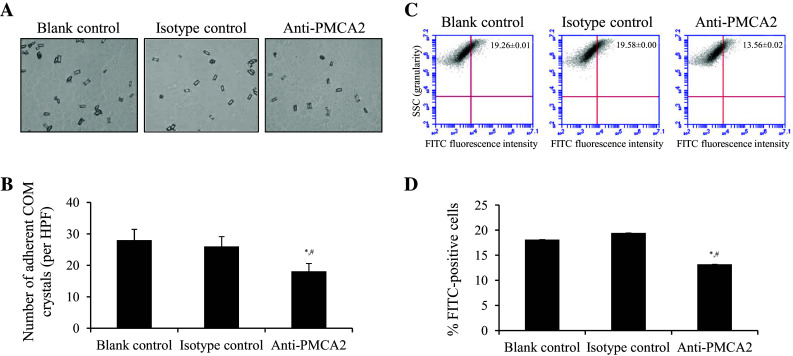
Functional validation of the role of PMCA2 in COM crystal-cell adhesion and crystal internalization. a Phase-contrast microscopic examination after crystal-cell adhesion assay together with neutralization using specific anti-PMCA2 antibody compared to isotype-controlled IgG and blank control (original magnification power was ×400). b Quantitative data were obtained from 15 randomized HPFs and are presented as mean ± SEM of three independent experiments. c Dot plot analysis of side scatter (SSC) or granularity (y-axis) and FITC-fluorescence intensity (x-axis) of the cells after crystal neutralization assay together with neutralization using specific anti-PMCA2 antibody compared to isotype-controlled IgG and blank control. d Quantitative data (percentages of the cells with internalized crystals) were obtained from three independent experiments and are presented as mean ± SEM. *p < 0.05 vs. blank control; # p < 0.05 vs. isotype-controlled IgG
Discussion
The aim of this study was to characterize PMCA2-interacting partners in distal renal tubular cells hoping to gain insights into novel functions of PMCA2. IP-MS was our method of choice to identify affinity-purified proteins because of its capability to detect low abundant proteins and novel protein partners. To eliminate background contaminants caused by non-specific bindings of proteins to IgG or beads that are frequently co-purified in the IP samples, up-front reduction of such contaminants was done during experiments (i.e., by pre-clearing and vigorous washes). Additionally, post-experimental elimination of contaminants (i.e., using highly stringent criteria for MS/MS analysis and subtraction with the isotype-controlled IgG pulled down proteins) was also performed to further discriminate true interactors from non-specific binders.
From a total of 474 proteins identified as the potential PMCA2-interacting proteins, it should be noted that we were unable to detect some of the known PMCA2 interactors. For example, sodium–hydrogen exchange regulatory factor 2 (NHERF2) that has been previously reported as an interactor of PMCA2 in MDCK cells [15, 36] was not found in the present study. This was likely due to the fact that protein–protein interactions naturally do not present in equal stoichiometry [37]. NHERF2 might exhibit a specific but lower abundance and/or lower affinity towards PMCA2, resulting to an increase of risk for protein loss during isolation or purification steps. In addition, interaction between NHERF2 and PMCA2 might be transient (they might be associated only during specific stimuli, cell stage, or signaling events; thereby increasing the difficulty to be isolated and identified by IP-MS) [38]. Another potential factor recognized as experimental limitation that had led to the loss of specific partners was through an over-filtering of the data. Ideally, all proteins presented in the isotype-controlled sample were considered as “background contaminants” and were eventually eliminated. However, some true interactors could, in fact, also bind non-specifically to the beads. This inevitably resulted in the loss of specific binding partners; as evidenced by the removal of ezrin and actin (the known interactors of PMCA2 [15, 39]) from the final list of PMCA2-interacting partners (Table 1). This has been proven by quantitative immuno-co-localization assay (Fig. 4a).
Nevertheless, at least eight genuine PMCA2-associated proteins were identified in this study. These included PDZ and LIM domain protein 7 [19], several calcineurin subunits [32], and PKC delta [33, 34] (Table 1—part I). PDZ/LIM domain protein and PKC are the important activators/modulators that have been found to bind to a consensus sequence at C-terminal tail of all PMCA isoforms [19]. On the other hand, the interaction between calcineurin and PMCA is isoform-specific [32]. Previous evidence have demonstrated that calcineurin interacted very strongly to PMCA2 and only weakly with PMCA4 in human breast adenocarcinoma cells [32]. Moreover, we successfully identified the heterodimerized form of calcineurin, which consisted of catalytic subunit calcineurin A and Ca2+-binding subunit calcineurin B [40], suggesting functionally active form of calcineurin could be also detected by our approach. These supported the validity of the IP-MS data as proteins in the list were likely to be selective towards PMCA2.
To conceptualize these identified PMCA2-interacting complex in a more meaningful manner, STRING software was utilized. The protein–protein interaction networks provided by STRING combined several lines of evidence (through experiments, databases and text mining) to include all possible interactions. Therefore, the interaction networks predicted in the present study provided almost complete overview of these proteins’ associations (Fig. 3). However, it should be kept in mind that these PMCA2-interacting partners might correspond to the ones that interacted directly with PMCA2, as well as those that interacted indirectly via one or more bridging molecules (e.g., other proteins, RNA, etc.) [41].
The most prominent molecular function of all identified proteins was binding activity. Interestingly, a large number of Ca2+-binding proteins were identified in this study (approximately 7.8% of all identified proteins with binding activity; Fig. 5a). Ca2+ homeostasis in distal renal tubular cells is predominantly controlled by two specialized transporters, NCX and PMCA. NCX (with a low Ca2+-binding affinity) can facilitate the removal of a large amount of Ca2+ out of the cells within a short period. This is beneficial when the cells need to get rid of excessive Ca2+ ions after an encounter of a sudden rise of intracellular Ca2+ concentration [42]. In contrast, PMCA is responsible for fine-tuning the intracellular Ca2+ level. It has been recognized as a low-capacity but high-affinity pump that interacts with Ca2+ even when the surrounding concentration of Ca2+ is extremely low [5]. PMCA2, in particular, carries a unique feature in which its activity at the basal level is as high as when its activator (calmodulin) is present [7]. Moreover, PMCA2 has a high Ca2+-binding affinity when compared to other isoforms expressed in MDCK cells [7].
These properties have raised the possibility that PMCA2 may be involved in the pathogenesis of calcium nephrolithiasis. Recent kidney stone research had been intensively conducted to define mechanisms of adhesion of causative crystals on renal cells that subsequently lead to crystal retention/deposition and finally stone formation [43, 44]. Studies of COM crystals, the most common constituent found in human kidney stones have shown that crystal attachment onto renal tubular cells depends largely on charge interaction between cellular surface molecules and the crystals [45, 46]. We thus have postulated that an interaction between COM crystals, on which cationic sites are formed by Ca2+ ions and PMCA2 at apical surface of renal tubular cells, serves as a critical initiating event that promotes crystal retention.
To address this hypothesis, an initial step was taken to find a correlation between PMCA2 and crystal deposition by comparing PMCA2-interacting proteins to a list of COM crystal-binding proteins recently reported [31]. Approximately 22% of COM crystal-binding proteins identified in previous study also served as the PMCA2 interactors (Note that the COM crystal-binding proteins are marked with # in Table 1). Further validation at experimental level by COM crystal-protein binding assay confirmed that PMCA2 served as a COM crystal-binding protein (Fig. 5b, c). We have also shown that PMCA2 is expressed at the apical membranes of MDCK renal tubular cells (Fig. 5b). The localization of PMCA2 at the apical membranes suggested the likelihood of interaction between PMCA2 and COM crystals in physiological condition as crystal deposition occurs inside the tubular lumen where the apical part of epithelial cells is facing. Thus, the role of PMCA2 as a potential receptor on the cell surface to bind with COM crystals was confirmed by crystal-cell adhesion assay together with antibody neutralization (Fig. 6a, b). Our previous studies have also shown that the adherent COM crystals could be internalized into the cells by surface receptors through lipid raft-mediated endocytosis pathway [47, 48]. Similarly, apical membrane localization of PMCA2 is lipid raft-dependent [12], implicating its possible role in mediating COM crystal uptake by endocytosis. The data obtained from IP-MS in the present study supported this hypothesis as there were several proteins involved in vesicle-mediated transport and endocytosis pathway included in the list (marked with † in Table 1). Finally, the role of PMCA2 in crystal internalization into the cells was confirmed experimentally by crystal internalization assay (Fig. 6c, d).
In conclusion, we report herein a large number of PMCA2-interacting proteins, most of which have not been previously reported and can serve as the novel PMCA2-interacting partners. Also, our findings have reinforced the functional versatility of PMCA2 enhanced by different arrays of specific protein interactions and are the first dataset to link PMCA2 to the pathogenesis of kidney stone disease through direct binding to COM crystals as a potential COM crystal receptor that plays role in crystal uptake into renal tubular cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Phornpimon Tipthara and Kedsarin Fong-ngern for their technical assistance. This study was supported by Mahidol University research grant, Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative, and the Thailand Research Fund (IRN60W0004 and IRG5980006). AV is supported by Siriraj Graduate Thesis Scholarship, whereas VT is supported by “Research Staff” Grant.
Author contributions
AV and VT designed research; AV performed experiments; AV and VT analyzed data; AVand VT wrote the manuscript; all authors reviewed the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Schuh K, Uldrijan S, Gambaryan S, Roethlein N, Neyses L. Interaction of the plasma membrane Ca2+ pump 4b/CI with the Ca2+/calmodulin-dependent membrane-associated kinase CASK. J Biol Chem. 2003;278:9778–9783. doi: 10.1074/jbc.M212507200. [DOI] [PubMed] [Google Scholar]
- 3.Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991;71:129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- 4.Oberleithner H, Westphale HJ, Gassner B. Alkaline stress transforms Madin-Darby canine kidney cells. Pflug Arch. 1991;419:418–420. doi: 10.1007/BF00371126. [DOI] [PubMed] [Google Scholar]
- 5.Strehler EE, Filoteo AG, Penniston JT, Caride AJ. Plasma-membrane Ca(2+) pumps: structural diversity as the basis for functional versatility. Biochem Soc Trans. 2007;35:919–922. doi: 10.1042/BST0350919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strehler EE, Caride AJ, Filoteo AG, Xiong Y, Penniston JT, Enyedi A. Plasma membrane Ca2+ ATPases as dynamic regulators of cellular calcium handling. Ann N Y Acad Sci. 2007;1099:226–236. doi: 10.1196/annals.1387.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domi T, Di Leva F, Fedrizzi L, Rimessi A, Brini M. Functional specificity of PMCA isoforms? Ann N Y Acad Sci. 2007;1099:237–246. doi: 10.1196/annals.1387.043. [DOI] [PubMed] [Google Scholar]
- 8.Lee WJ, Roberts-Thomson SJ, Monteith GR. Plasma membrane calcium-ATPase 2 and 4 in human breast cancer cell lines. Biochem Biophys Res Commun. 2005;337:779–783. doi: 10.1016/j.bbrc.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 9.Spiden SL, Bortolozzi M, Di Leva F, de Angelis MH, Fuchs H, Lim D, Ortolano S, Ingham NJ, Brini M, Carafoli E, Mammano F, Steel KP. The novel mouse mutation Oblivion inactivates the PMCA2 pump and causes progressive hearing loss. PLoS Genet. 2008;4:e1000238. doi: 10.1371/journal.pgen.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz JM, Yang Y, Caride AJ, Filoteo AG, Penheiter AR, Lagziel A, Morell RJ, Mohiddin SA, Fananapazir L, Madeo AC, Penniston JT, Griffith AJ. Modification of human hearing loss by plasma-membrane calcium pump PMCA2. N Engl J Med. 2005;352:1557–1564. doi: 10.1056/NEJMoa043899. [DOI] [PubMed] [Google Scholar]
- 11.Kurnellas MP, Nicot A, Shull GE, Elkabes S. Plasma membrane calcium ATPase deficiency causes neuronal pathology in the spinal cord: a potential mechanism for neurodegeneration in multiple sclerosis and spinal cord injury. FASEB J. 2005;19:298–300. doi: 10.1096/fj.04-2549fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong Y, Antalffy G, Enyedi A, Strehler EE. Apical localization of PMCA2w/b is lipid raft-dependent. Biochem Biophys Res Commun. 2009;384:32–36. doi: 10.1016/j.bbrc.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kip SN, Strehler EE. Vitamin D3 upregulates plasma membrane Ca2+-ATPase expression and potentiates apico-basal Ca2+ flux in MDCK cells. Am J Physiol Ren Physiol. 2004;286:F363–F369. doi: 10.1152/ajprenal.00076.2003. [DOI] [PubMed] [Google Scholar]
- 14.Friedman PA. Mechanisms of renal calcium transport. Exp Nephrol. 2000;8:343–350. doi: 10.1159/000020688. [DOI] [PubMed] [Google Scholar]
- 15.Padanyi R, Xiong Y, Antalffy G, Lor K, Paszty K, Strehler EE, Enyedi A. Apical scaffolding protein NHERF2 modulates the localization of alternatively spliced plasma membrane Ca2+ pump 2B variants in polarized epithelial cells. J Biol Chem. 2010;285:31704–31712. doi: 10.1074/jbc.M110.164137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kip SN, Strehler EE. Characterization of PMCA isoforms and their contribution to transcellular Ca2+ flux in MDCK cells. Am J Physiol Ren Physiol. 2003;284:F122–F132. doi: 10.1152/ajprenal.00161.2002. [DOI] [PubMed] [Google Scholar]
- 17.Lopreiato R, Giacomello M, Carafoli E. The plasma membrane calcium pump: new ways to look at an old enzyme. J Biol Chem. 2014;289:10261–10268. doi: 10.1074/jbc.O114.555565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brini M. Plasma membrane Ca(2+)-ATPase: from a housekeeping function to a versatile signaling role. Pflug Arch. 2009;457:657–664. doi: 10.1007/s00424-008-0505-6. [DOI] [PubMed] [Google Scholar]
- 19.Strehler EE. Plasma membrane calcium ATPases as novel candidates for therapeutic agent development. J Pharm Pharm Sci. 2013;16:190–206. doi: 10.18433/J3Z011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gingras AC, Gstaiger M, Raught B, Aebersold R. Analysis of protein complexes using mass spectrometry. Nat Rev Mol Cell Biol. 2007;8:645–654. doi: 10.1038/nrm2208. [DOI] [PubMed] [Google Scholar]
- 21.Dukes JD, Whitley P, Chalmers AD. The MDCK variety pack: choosing the right strain. BMC Cell Biol. 2011;12:43. doi: 10.1186/1471-2121-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sritippayawan S, Chiangjong W, Semangoen T, Aiyasanon N, Jaetanawanitch P, Sinchaikul S, Chen ST, Vasuvattakul S, Thongboonkerd V. Proteomic analysis of peritoneal dialysate fluid in patients with different types of peritoneal membranes. J Proteome Res. 2007;6:4356–4362. doi: 10.1021/pr0702969. [DOI] [PubMed] [Google Scholar]
- 23.Havanapan PO, Thongboonkerd V. Are protease inhibitors required for gel-based proteomics of kidney and urine? J Proteome Res. 2009;8:3109–3117. doi: 10.1021/pr900015q. [DOI] [PubMed] [Google Scholar]
- 24.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 25.Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol. 2011;300:C723–C742. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thongboonkerd V, Semangoen T, Chutipongtanate S. Factors determining types and morphologies of calcium oxalate crystals: molar concentrations, buffering, pH, stirring and temperature. Clin Chim Acta. 2006;367:120–131. doi: 10.1016/j.cca.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 27.Thongboonkerd V, Semangoen T, Sinchaikul S, Chen ST. Proteomic analysis of calcium oxalate monohydrate crystal-induced cytotoxicity in distal renal tubular cells. J Proteome Res. 2008;7:4689–4700. doi: 10.1021/pr8002408. [DOI] [PubMed] [Google Scholar]
- 28.Chaiyarit S, Mungdee S, Thongboonkerd V. Non-radioactive labelling of calcium oxalate crystals for investigations of crystal-cell interaction and internalization. Anal Methods. 2010;2:1536–1541. doi: 10.1039/C0AY00321B. [DOI] [Google Scholar]
- 29.Chaiyarit S, Singhto N, Thongboonkerd V. Calcium oxalate monohydrate crystals internalized into renal tubular cells are degraded and dissolved by endolysosomes. Chem Biol Interact. 2016;246:30–35. doi: 10.1016/j.cbi.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Fong-ngern K, Chiangjong W, Thongboonkerd V. Peeling as a novel, simple, and effective method for isolation of apical membrane from intact polarized epithelial cells. Anal Biochem. 2009;395:25–32. doi: 10.1016/j.ab.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Fong-ngern K, Peerapen P, Sinchaikul S, Chen ST, Thongboonkerd V. Large-scale identification of calcium oxalate monohydrate crystal-binding proteins on apical membrane of distal renal tubular epithelial cells. J Proteome Res. 2011;10:4463–4477. doi: 10.1021/pr2006878. [DOI] [PubMed] [Google Scholar]
- 32.Holton M, Yang D, Wang W, Mohamed TM, Neyses L, Armesilla AL. The interaction between endogenous calcineurin and the plasma membrane calcium-dependent ATPase is isoform specific in breast cancer cells. FEBS Lett. 2007;581:4115–4119. doi: 10.1016/j.febslet.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 33.Kosk-Kosicka D, Zylinska L. Protein kinase C and calmodulin effects on the plasma membrane Ca2+-ATPase from excitable and nonexcitable cells. Mol Cell Biochem. 1997;173:79–87. doi: 10.1023/A:1006832603134. [DOI] [PubMed] [Google Scholar]
- 34.Usachev YM, DeMarco SJ, Campbell C, Strehler EE, Thayer SA. Bradykinin and ATP accelerate Ca(2+) efflux from rat sensory neurons via protein kinase C and the plasma membrane Ca(2+) pump isoform 4. Neuron. 2002;33:113–122. doi: 10.1016/S0896-6273(01)00557-8. [DOI] [PubMed] [Google Scholar]
- 35.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeMarco SJ, Chicka MC, Strehler EE. Plasma membrane Ca2+ ATPase isoform 2b interacts preferentially with Na+/H+ exchanger regulatory factor 2 in apical plasma membranes. J Biol Chem. 2002;277:10506–10511. doi: 10.1074/jbc.M111616200. [DOI] [PubMed] [Google Scholar]
- 37.Perkins JR, Diboun I, Dessailly BH, Lees JG, Orengo C. Transient protein–protein interactions: structural, functional, and network properties. Structure. 2010;18:1233–1243. doi: 10.1016/j.str.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Boulon S, Ahmad Y, Trinkle-Mulcahy L, Verheggen C, Cobley A, Gregor P, Bertrand E, Whitehorn M, Lamond AI. Establishment of a protein frequency library and its application in the reliable identification of specific protein interaction partners. Mol Cell Proteom. 2010;9:861–879. doi: 10.1074/mcp.M900517-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bozulic LD, Malik MT, Powell DW, Nanez A, Link AJ, Ramos KS, Dean WL. Plasma membrane Ca(2+)-ATPase associates with CLP36, alpha-actinin and actin in human platelets. Thromb Haemost. 2007;97:587–597. [PubMed] [Google Scholar]
- 40.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 41.von Mering C, Krause R, Snel B, Cornell M, Oliver SG, Fields S, Bork P. Comparative assessment of large-scale data sets of protein–protein interactions. Nature. 2002;417:399–403. doi: 10.1038/nature750. [DOI] [PubMed] [Google Scholar]
- 42.Carafoli E, Santella L, Branca D, Brini M. Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 2001;36:107–260. doi: 10.1080/20014091074183. [DOI] [PubMed] [Google Scholar]
- 43.Dardamanis M. Pathomechanisms of nephrolithiasis. Hippokratia. 2013;17:100–107. [PMC free article] [PubMed] [Google Scholar]
- 44.Vinaiphat A, Thongboonkerd V. Prospects for proteomics in kidney stone disease. Expert Rev Proteom. 2017;14:185–187. doi: 10.1080/14789450.2017.1283222. [DOI] [PubMed] [Google Scholar]
- 45.Lieske JC, Leonard R, Toback FG. Adhesion of calcium oxalate monohydrate crystals to renal epithelial cells is inhibited by specific anions. Am J Physiol. 1995;268:F604–F612. doi: 10.1152/ajpcell.1995.268.3.C604. [DOI] [PubMed] [Google Scholar]
- 46.Lieske JC, Leonard R, Swift H, Toback FG. Adhesion of calcium oxalate monohydrate crystals to anionic sites on the surface of renal epithelial cells. Am J Physiol. 1996;270:F192–F199. doi: 10.1152/ajpcell.1996.270.1.C192. [DOI] [PubMed] [Google Scholar]
- 47.Kanlaya R, Sintiprungrat K, Chaiyarit S, Thongboonkerd V. Macropinocytosis is the major mechanism for endocytosis of calcium oxalate crystals into renal tubular cells. Cell Biochem Biophys. 2013;67:1171–1179. doi: 10.1007/s12013-013-9630-8. [DOI] [PubMed] [Google Scholar]
- 48.Fong-ngern K, Sueksakit K, Thongboonkerd V. Surface heat shock protein 90 serves as a potential receptor for calcium oxalate crystal on apical membrane of renal tubular epithelial cells. J Biol Inorg Chem. 2016;21:463–474. doi: 10.1007/s00775-016-1355-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.