Abstract
Primary cilium is a rod-like plasma membrane protrusion that plays important roles in sensing the cellular environment and initiating corresponding signaling pathways. The sensory functions of the cilium critically depend on the unique enrichment of ciliary residents, which is maintained by the ciliary diffusion barrier. It is still unclear how ciliary cargoes specifically enter the diffusion barrier and accumulate within the cilium. In this review, the organization and trafficking mechanism of the cilium are compared to those of the nucleus, which are much better understood at the moment. Though the cilium differs significantly from the nucleus in terms of molecular and cellular functions, analogous themes and principles in the membrane organization and cargo trafficking are notable between them. Therefore, knowledge in the nuclear trafficking can likely shed light on our understanding of the ciliary trafficking. Here, with a focus on membrane cargoes in mammalian cells, we briefly review various ciliary trafficking pathways from the Golgi to the periciliary membrane. Models for the subsequent import translocation across the diffusion barrier and the enrichment of cargoes within the ciliary membrane are discussed in detail. Based on recent discoveries, we propose a Rab–importin-based model in an attempt to accommodate various observations on ciliary targeting.
Keywords: Primary cilium, Ciliary trafficking, Ciliary diffusion barrier, Ciliary targeting signal, Importin, Transportin1, Rab8
Introduction
The cilium (plural cilia) is a protrusion of the plasma membrane (PM) in eukaryotes that have diverse ranges of cellular functions. There are two types of cilia: motile cilia (or flagella) and immotile cilia (or primary cilia). Motile cilia generate mechanical forces by beating in liquid, while primary cilia are mostly sedentary (reviewed in [1]). The physiological roles of motile cilia have long been recognized for cell locomotion and fluid propulsion. However, it is only during the recent decade that we began to appreciate the biological importance of primary cilia. Primary cilia (hereafter cilia) are now known as signaling antennae for higher eukaryotic cells (reviewed in [2–4]). Cilia are able to sense a diverse range of signals from the cellular environment, including growth factor stimuli, mechanical signals (sound and forces), chemical signals (odorant molecules), and photons.
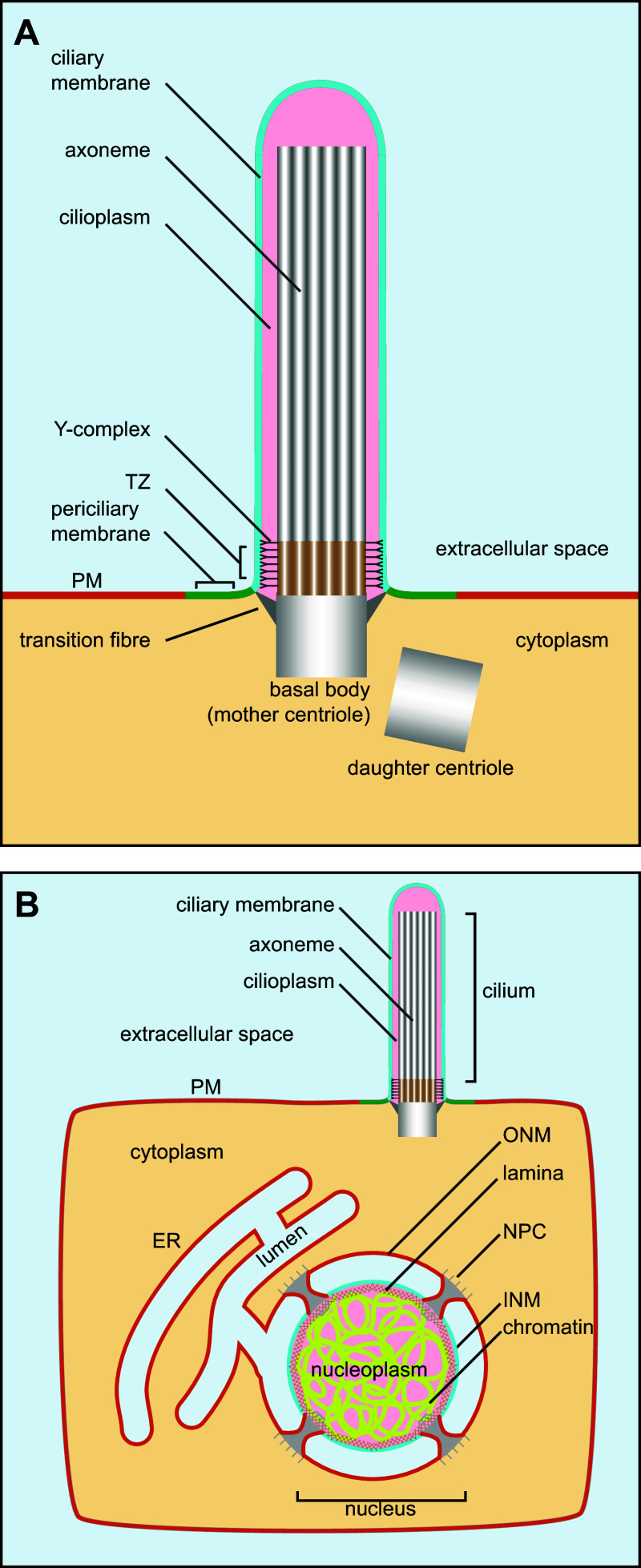
Despite distinct cellular functions, motile and primary cilia have highly conserved structure and organization across species (reviewed in [2, 5]). The ciliary membrane is the extension of the PM. Within the cilium and across its length, lies the axoneme, a bundle of symmetrically arranged microtubule encased by the ciliary membrane (Fig. 1a). The axoneme is the extension of the basal body, which is derived from the mother centriole—one of two centrioles in the centrosome [6]. At the base of the cilium, the distal region of the basal body connects with the ciliary membrane by transition fibers, whose membrane contact sites demark the ciliary membrane from the periciliary membrane, or ciliary pocket, and the PM. The transition zone (TZ) is the base segment of the cilium in between the basal body and the axoneme, where the organization of microtubules changes from nine triplets in the basal body to nine doublets in the axoneme (reviewed in [7]).
Fig. 1.
Schematic organization of the cilium and the nucleus. a Diagram showing the structure of the primary cilium. PM plasma membrane, TZ transition zone. b Cilium and nucleus have analogous organization. Both ciliary membrane and cilioplasm have distinct identities from the PM and cytoplasm due to the presence of the diffusion barrier at the cilium base. Similarly, the INM and nucleoplasm have distinct identities from the ER/ONM and the cytoplasm, respectively, due to NPCs. Corresponding or analogous regions are denoted using the same colors. For example, the PM and the ER/ONM are colored orange, the ciliary membrane and INM cyan, the cilioplasm and nucleoplasm pink, the extracellular space, and ER lumen light blue
Ciliary diffusion barrier
The ciliary membrane and the PM are continuous within the same membrane sheet, while the cilioplasm and cytoplasm are connected through the opening at the cilium base. Despite these continuities, both the ciliary membrane and the cilioplasm maintain their unique identities. Diffusion barriers have been proposed to maintain such distinction (reviewed in [2, 5, 7]). Substantial molecular and cellular evidences have demonstrated that membrane diffusion barriers assemble at the TZ or the cilium base to prevent the free mixing of components between the PM and the ciliary membrane. For example, morphologically, the electron microscopy (EM) revealed a narrow space between adjacent transition fibers (alar sheets) at the ciliary opening, which has been hypothesized to preclude the entry of vesicles, particles, or even large proteins [8]; at the TZ, Y-shaped protein complexes have been found to link the axoneme and the ensheathing ciliary membrane [9]; the EM data of Tetrahymena pyriformis revealed a ninefold symmetric “ciliary pore complex” and a connecting membrane ring at the cilium base, possibly serving as soluble and membrane diffusion barriers, respectively [10]. Functionally, consistent with the presence of a diffusion barrier, fluorescence recovery after photobleaching (FRAP) data indicates that ciliary membrane residents can freely move within the cilium, but are prohibited from wandering beyond the cilium base to exchange with the extra-ciliary pool [11–14]; cilium trapping experiments demonstrated that cytoplasmic soluble reporters first appeared at the base of the cilium before gaining access to more distal region [15, 16]. At molecular level, the membrane diffusion barrier is likely formed or contributed by B9/Meckel syndrome/nephronophthisis complex [12, 17, 18], Septin2 complex [11], a sub-set of nucleoporins (Nups) (subunits of the nuclear pore complex) [19, 20] (reviewed in [21]), and densely packed lipids [22]. It is in debate whether Nups can functionally contribute to the ciliary diffusion barrier, as they were found to be negative at the TZ in other studies [16, 23] despite the observation of pericentriolar material localization of Nup188 and Nup93 [23]. We still do not know how these diverse ranges of molecules impose ciliary diffusion barrier. It is expected that some of these macromolecular complexes restrict both membrane and soluble cargoes, while others may be more specific to one type. For example, Septin2-containing barrier probably restricts the entry or exit of membrane cargoes [11]; on the other hand, Nup-based ciliary diffusion barrier primarily restricts the free diffusion of soluble ciliary cargoes, such as KIF17 and Gli2, instead of membrane ones, such as RP2 and Smoothened (reviewed in [21]).
Both soluble cargoes (<40 kDa), such as GFP, dextran and protein A, and membrane cargoes, such as PM-localized membrane proteins and reporters, have been found to possess significant cilium-localized pools [19, 24, 25]. These observations demonstrated that the ciliary diffusion barrier might be leaky and cargoes can passively diffuse through the barrier. Therefore, the ciliary membrane and cilioplasm are expected to share components with the PM and cytoplasm, respectively, at least for molecules of small sizes, such as lipids, ions, and low molecular weight proteins.
The parallel between the cilium and the nucleus in organization and trafficking
The hallmark of eukaryotic cells is the presence of internal organelles. If an organelle is defined as a membrane-enclosed compartment (reviewed in [26]), the cilium is not an organelle by this definition. The cilium has an opening at the base and it shares the same membrane sheet with the PM despite its unique identity. Thus, the cilium can be viewed as a special PM domain which is gated by the diffusion barrier at the cilium base. This topological organization is similar to that of the inner nuclear membrane (INM) (Fig. 1b). The nuclear envelope (NE) consists of two concentric layers of membrane, the INM and the outer nuclear membrane (ONM), which are connected at nuclear pores by short membrane tubes (reviewed in [27–30]). The INM is facing the nucleoplasm and has distinct protein and lipid composition from the ONM or peripheral ER. The unique transmembrane proteins of the INM play key roles in nuclear architecture and function by interacting with chromatin and lamina (reviewed in [31]). The ONM is in continuity and shares essentially the same components with the peripheral ER. The nuclear pore complex (NPC) assembles at the nuclear pore to impose a diffusion barrier for both membrane and soluble cargoes. Therefore, similar to the organization of the cilium, the INM can be regarded as a special membrane domain of the ER which is gated by NPCs.
Small soluble cargoes (<40 kDa) can passively diffuse through the NPC to the nucleus (reviewed in [28]), similar to the cilium diffusion barrier. In the selective entry pathway, the active transport of large soluble cargoes (>40 kDa) across NPCs depends on nuclear localization signals (NLSs) and their cognate nuclear transport receptors, which are karyopherin-β family members (importins) (reviewed in [28, 30, 32]). There are more than 20 importins in the human genome. They facilitate the translocation through the NPC by their weak and transient interaction with Nups. It seems that the translocation of cargo–importin complex is bidirectional random walk and the NPC does not confer the directionality of the transport [33]. Ran-GTPase-activating protein (GAP), which inactivates Ran–GTP to Ran–GDP, localizes to the cytosol, while Ran guanine nucleotide exchange factor (GEF)—RCC1, which regenerates Ran–GTP from Ran–GDP, selectively localizes to the nucleus by binding to the chromatin (reviewed in [28, 30]). The asymmetric localization of Ran GAP and GEF creates a Ran–GTP gradient from the nucleus to the cytosol, which determines the unidirectional nucleocytoplasmic trafficking (reviewed in [28, 30]). Once cargo–receptor complex passes through the NPC and reaches the nucleoplasm, Ran–GTP binds to the receptor and disassembles the complex, therefore, preventing the exit of the cargo through the NPC and enriching it within the nucleus.
Different from soluble cargoes, newly synthesized INM proteins are integrated into the peripheral ER and laterally diffuse to the NPC through the ONM. The subsequent transport through the NPC to the INM seems to depend on the type of cargoes and remains incompletely understood. Two major translocation mechanisms have been proposed (reviewed in [34, 35]). INM proteins possessing NLSs, such as lamin B receptor, can adopt the receptor-mediated selective entry pathway, which operates in a manner similar to the trafficking of soluble cargoes and involves importins and Ran–GTP gradient [36, 37]. Alternatively, in diffusion and retention pathway, the INM protein first passively diffuses through the NPC, a process that is inversely affected by the physical size of its cytosolic domain [38, 39]. Once in the INM, the protein is retained by its nuclear binding partners, such as nuclear lamina or chromatin (reviewed in [31]). It is also conceivable that some cargoes can employ a combination of both pathways.
The analogy between the cilium and the INM appears to be beyond topology [20, 40] (reviewed and commented in [21] and [41], respectively). NPC and key components of ciliary trafficking machinery, such as the intraflagellar transport (IFT) complex and BBSome, also share similar vesicular coat like structural elements, suggesting a common evolutionary origin of these complexes [42] (reviewed in [43]). As elaborated below, recent development in the field demonstrated that ciliary and nuclear trafficking can similarly operate at molecular level. For example, the conventional nuclear trafficking machinery components, such as Nups, Ran–GTP, importin-β1, TNPO1, and RanBP1, have been observed at the cilium and their proper functions seem essential for ciliogenesis and ciliary import of both membrane and soluble ciliary cargoes [24, 40, 44, 45]. On the other hand, under special pathological conditions or in certain organisms, IFT57, a subunit of the IFT complex, can target to the nucleus and affect gene transcription [46, 47]. Hence, we propose that knowledge in nuclear trafficking can potentially shed light on how ciliary trafficking works.
Ciliary targeting: from the ER to the periciliary membrane
After biosynthesis, membrane proteins and lipids are targeted to specific organelles for their functions. Cells utilize membrane trafficking to move secretory or endocytic cargoes from one compartment to another in bulk quantity. Membrane trafficking entails membrane carriers, such as membrane vesicles or tubules, as vehicles. Much has been known about molecular and cellular mechanisms of the membrane-trafficking processes in secretory and endocytic pathways (reviewed in [26]). In general, it involves the cargo selection and budding from the donor membrane by ARF-family small GTPases and coat proteins, followed by targeting the vesicle toward the acceptor compartment by motor-mediated movement along cytoskeletal tracks. After tethering, the v-SNARE (vesicle-soluble N-ethylmaleimide-sensitive factor attachment protein receptor) on the vesicular membrane pairs with the t-SNARE (target-SNARE) on the acceptor compartment membrane. The formation of the SNARE complex then drives the fusion of the vesicle to the acceptor compartment and cargoes within the vesicle are subsequently discharged to the acceptor compartment.
The identity and sensory functions of the cilium depend on the specific enrichment of receptors and their accessory signaling components within the cilium. Mounting evidences have established that the selective recruitment of proteins to organelles is dependent on targeting signals or sequences which comprise linear arrays of amino acids (reviewed in [26]). The targeting signals usually fall within a certain pattern of consensus that is essential for the recognition by common trafficking receptors. Ciliary targeting signals (CTSs) have been reported for a dozen or so membrane and soluble ciliary residents (reviewed in [2, 48, 49]). However, it is puzzling that these CTSs share neither sequence consensus nor trafficking machinery. After biosynthesis at the ER and the ensuing ER export, ciliary membrane residents, just like general cellular cargoes, follow the secretory pathway by sequentially transiting from the cis to the trans-Golgi. At the trans-Golgi or the trans-Golgi network (TGN), cellular cargoes are packed into membrane carriers destined for the PM by default (reviewed in [26]). CTSs can target ciliary membrane residents to the periciliary membrane either directly or indirectly via the PM. The periciliary membrane is a special membrane region encircling the cilium base. At least in polarized epithelial cells, the periciliary membrane displays distinct identity from both the ciliary membrane and the PM by the enrichment of galectin-3, the exclusion of cortical actin cytoskeleton, and the association with high endocytosis and exocytosis [22, 50–52].
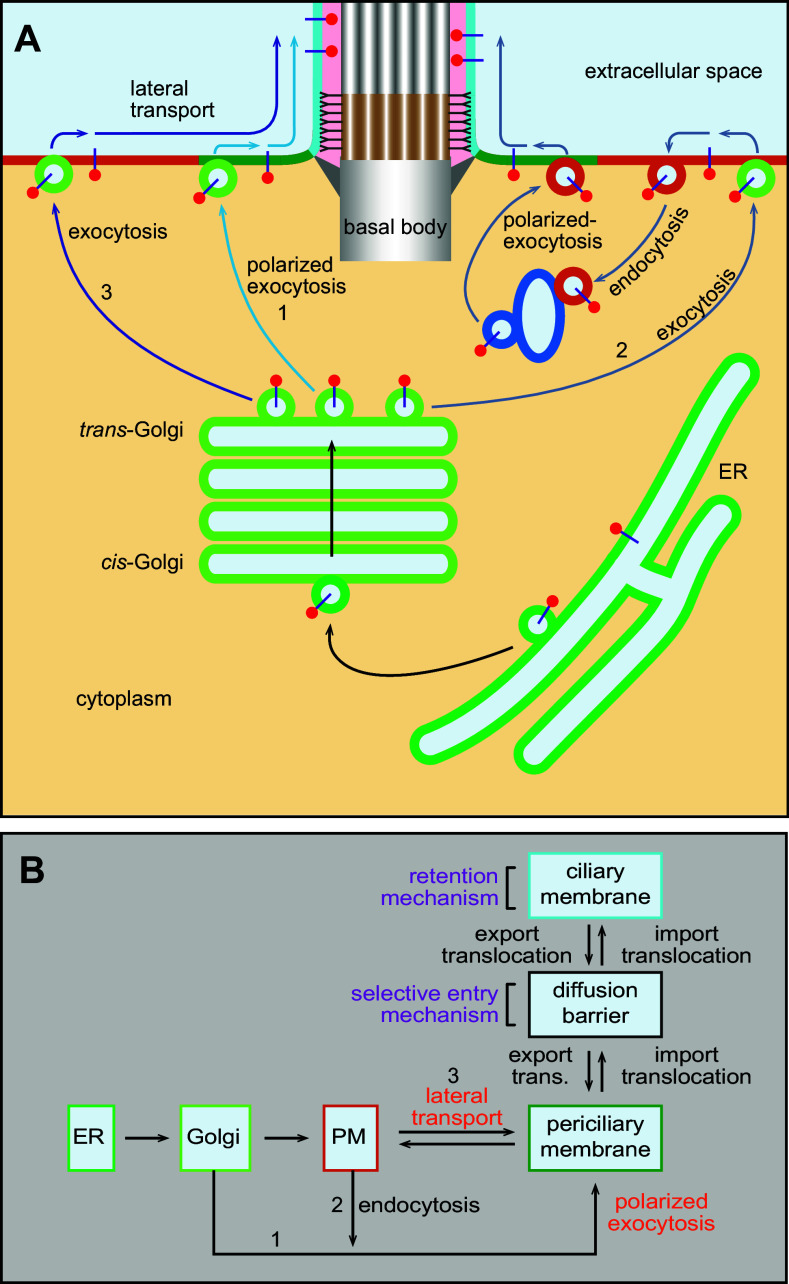
Two major pathways have been proposed for periciliary membrane targeting—polarized exocytosis and lateral transport pathways. The polarized exocytosis pathway is currently more accepted in the ciliary trafficking field. It involves the targeting of cargo-containing membrane carriers or vesicles to the periciliary membrane (reviewed in [2, 5, 49]), which bears molecular and cellular similarity to the well-characterized polarized trafficking to yeast mother–bud neck (reviewed in [53]). Probably, as a result of the polarized exocytosis, the periciliary membrane is known to have a high activity of endocytosis and exocytosis [51, 52]. There are two types of polarized exocytosis pathways. In the direct pathway, the TGN generated carriers with ciliary membrane proteins move directionally to the cilium base and directly fuse with the periciliary membrane [54] (pathway 1, Fig. 2a, b). The CTS of the rhodopsin has been reported to guide the polarized targeting of rhodopsin from the Golgi to presumably the periciliary membrane, by multiple small GTPases including Arf4, Rab11, and Rab8 [55, 56]. In the indirect polarized exocytosis pathway, ciliary cargoes first arrive at the PM by default bulk flow and subsequently undergo endocytosis followed by polarized exocytosis at the periciliary membrane (pathway 2, Fig. 2a, b). For example, the ciliary targeting of the membrane protein, Kim1, involves Rab5-dependent endocytosis from the PM followed by Rab8-dependent polarized exocytosis to the cilium [57]. Microtubule tracks converging at the basal body can facilitate the polarized ciliary targeting. Ciliary cargoes have also been postulated to be first delivered to an intermediated compartment, such as the recycling endosome, before reaching the periciliary membrane (reviewed in [5]).
Fig. 2.
Targeting ciliary membrane cargoes to the periciliary membrane by the lateral transport or polarized exocytosis. a Ciliary membrane cargoes are synthesized in the ER and subsequently enter the secretory pathway to the Golgi apparatus. At the TGN, cargoes can take different pathways to the periciliary membrane. In pathway 1, packed into vesicles from the TGN, cargoes can target to the periciliary membrane directly via polarized exocytosis or indirectly, as illustrated by pathway 2, by first to the PM, followed by endocytosis to the endosome and at last to the periciliary membrane by polarized exocytosis. Alternatively, in pathway 3, cargoes are delivered to the PM by constitutive exocytosis from the TGN and they subsequently enter the periciliary membrane by lateral transport. b Flow chart showing the ciliary trafficking pathways and models among various organelles and membrane domains, including the ER, Golgi, PM, periciliary membrane, membrane diffusion barrier, and ciliary membrane. Note that steps marked by two opposite arrows are reversible. 1, 2, and 3 correspond to pathways 1, 2, and 3, respectively, as described in a. export trans. export translocation
In the later stage of the polarized exocytosis, both direct and indirect pathways likely adopt similar tethering and fusion mechanism. The tethering of vesicles with the periciliary membrane can be facilitated by the interaction between Rab8 and the exocyst, an octameric tethering complex (reviewed in [2, 58, 59]). The subsequent fusion of vesicles to the periciliary membrane involves SNAREs and might be utilized to couple to the ensuing translocation across the membrane diffusion barrier. The vesicular membrane contains v-SNAREs, such as Vamp3 and Vamp7 [60, 61], while syntaxin3, SNAP25, and SNAP29 localize to the periciliary membrane to contribute to formation of the t-SNARE complexes [62–64].
On the other hand, in the alternative lateral transport pathway, ciliary cargoes first arrive at the PM following default bulk flow of secretion; they then diffuse within the PM (passively or actively) before reaching the periciliary membrane without membrane fission and fusion (pathway 3, Fig. 2a, b). The lateral transport pathway is perhaps the most straightforward means for transporting ciliary membrane cargoes. Since it does not involve membrane carriers or vesicles, machineries of the conventional membrane trafficking are not expected to participate in this pathway. Ciliary membrane proteins such as agglutinin [65], smoothened [66], D1-type dopaminergic receptor (D1R) [13], and non-ciliary membrane reporter CD8a [24] have been reported to take the lateral transport pathway.
The two pathways differ in the site of membrane fusion or insertion—the periciliary membrane for the direct polarized exocytosis pathway and the PM for lateral transport pathway (Fig. 2a, b). However, cargoes of the two pathways can possibly share the same mechanism for the subsequent import translocation through the diffusion barrier, which is poorly understood at the moment. Previous ultra-structural studies demonstrated that transition fibers cannot allow the passage of the conventional membrane carriers, and furthermore, membrane carriers/vesicles have not been discovered under the EM (reviewed in [2]). Therefore, it seems that membrane-trafficking events, such as endocytosis and exocytosis, do not take place within the cilium. Hence, the import translocation of membrane cargoes is likely by lateral transport through the diffusion barrier without the involvement of membrane carriers.
Import translocation: getting across the diffusion barrier
Similar to the NPC in nucleocytoplasmic trafficking, the ciliary diffusion barrier plays a critical role in ciliary targeting. Although membrane and soluble cargoes, especially small ones, can passively diffuse through the ciliary diffusion barrier, a bona fide ciliary resident probably adopts an active transport mechanism for a more efficient ciliary targeting. The notion is supported by recent FRAP data in which recovery half-lives of ciliary membrane residents, such as fibrocystin, SSTR3, and Arl13b, are much less than those of exogenous reporters, such as CD8a–GFP [24]. Similar to the nucleocytoplasmic trafficking, there are mainly two mechanisms for the enrichment of ciliary residents. In the selective entry mechanism, transport receptors recognize CTSs and facilitate the import translocation of ciliary cargoes through the diffusion barrier. One possible transport receptor is the IFT complex, which localizes at the transition fibers and the base of the TZ. Two types of IFT complexes, IFT-A and IFT-B, loosely associate with each other and can transport cargoes along axoneme through kinesin-2 or dynein motor proteins (reviewed in [1, 67]). The motor protein might pull IFT and its associated cargoes through the diffusion barrier to the cilium. For example, either directly or combined with BBSome complex, IFT can selectively pick up cargoes, such as ciliary G-protein-coupled receptors (GPCRs), including D1R, SSTR3, HTR6, and MCHR1, by binding to their CTSs at the periciliary membrane [13, 42, 68, 69]; IFT subsequently carries these GPCRs through the diffusion barrier at the TZ with the facilitation of kinesin-2 motor protein. At the same time, IFT-binding might also provide cargo retention mechanism inside the cilium (see discussion below).
In addition to their nuclear roles, importins have been recently discovered as cargo receptors for ciliary trafficking. Importins, including importin-β1 and transportin1 (TNPO1, also called importin-β2), act as ciliary transport receptors for Crumbs3, Kif17, and RP2 in Ran-GTPase-regulated manner [40, 44, 45], similar to their roles in the selective entry mechanism of the nucleocytoplasmic trafficking, which is to overcome the diffusion barrier (reviewed in [28, 70–72]). The directional ciliary trafficking and enrichment of these ciliary residents are presumably mediated by ciliary Ran–GTP, which, resembling its function in the nucleocytoplasmic trafficking, dissociates importin and releases cargoes in the ciliary compartment [40, 44, 45]. Consistent with importins’ role as ciliary trafficking receptors, importin-β1 and TNPO1 interaction motifs or domains have been demonstrated to increase the localization of reporters in cilia, possibly by reducing the energy of translocating through the diffusion barrier [24].
Within the diffusion barrier of the NPC, importins alone facilitate the trafficking of cargoes equally well in both import and export translocation [33]. If importins operate similarly at the cilium, cargoes are not significantly enriched within the cilium when the system reaches equilibrium, at which the velocity of import translocation equals that of export. Under this circumstance, the ciliary concentration of a cargo is probably determined by its hydrodynamic volume in a molecularly crowded ciliary environment [25]. Importin binding likely reduces the apparent hydrodynamic volume of a cargo, therefore, resulting in increased ciliary localization. Indeed, fusing importin-binding motif or domain to CD8a significantly increases its ciliary localization [24]. Nonetheless, the boosted ciliary localization is still weak comparing to ciliary membrane residents, suggesting that another mechanism is required to explain the highly selective localization of ciliary residents.
Retention: confinement within the cilium
The retention mechanism prevents cargoes from exiting the cilium, therefore, resulting in the accumulation of cargoes. The mechanism can be implemented by selective binding to cognate ciliary receptors. Being the core structural component of the cilium, the axoneme probably plays an important role in ciliary retention by anchoring cargoes through IFT, kinesin-2, and BBSome [68, 69]. For example, Crumbs3/Par3/Par6/aPKC complex and Smoothened/β-arrestin can accumulate in the cilium by interacting with kinesin-2 [73, 74]. Similarly, the binding between IFT or BBSome and CTSs of GPCRs, which are discussed above, can mediate the ciliary retention of GPCRs [13, 42, 68, 69]. Thus, IFT and BBSome complexes are ciliary targeting machineries that probably employ both selective entry and retention mechanism. In RPE1 cells, Septins 2, 7, and 9 localize along the cilium as a complex [75] probably by interacting with axonemal microtubules (reviewed in [76]); when fused to the microtubule binding protein, tau, a PM-localized reporter has also been demonstrated to localize to the cilium, possibly by tethering to the axoneme [50], highlighting that axoneme binding can be sufficient for ciliary retention.
It seems that, even without an obvious selective entry mechanism, passive diffusion followed by subsequent retention can be sufficient for cilium localization, similar to the diffusion–retention pathway of INM targeting (see above discussion). Supporting this view, artificially forcing exogenous soluble reporters to interact with ciliary residents, by either rapamycin-induced interaction or GBP–GFP-binding system, have been demonstrated to strongly target reporters to the cilium [15, 16]. Furthermore, in photoreceptor cells, the cilium or outer segment localization of arrestin and transducin Gα and Gβγ is also driven by diffusion through the connecting cilium (TZ) and retention by rhodopsin binding (reviewed in [77]). A caveat for the retention mechanism is that tethering by receptor binding is expected to result in slow kinetic movement or immobilization of cargoes. Indeed, INM proteins are largely immobilized by their binding to lamina and/or chromatin [78] (reviewed in [31]) and cilium-associated septins have been observed to be stable and non-dynamic [75]. However, contrary to this prediction, most known ciliary membrane residents are highly mobile within the cilium and their movement is not coupled to the IFT [11, 12, 14, 79]. Furthermore, the diffusion coefficient of SSTR3–GFP at the ciliary membrane is similar to reported values of PM proteins [14]. These evidences imply that ciliary residents are freely diffusible instead of being immobilized or anchored. We, therefore, propose our model below to explain this paradox.
A model based on Rab–importin–CTS ternary complex
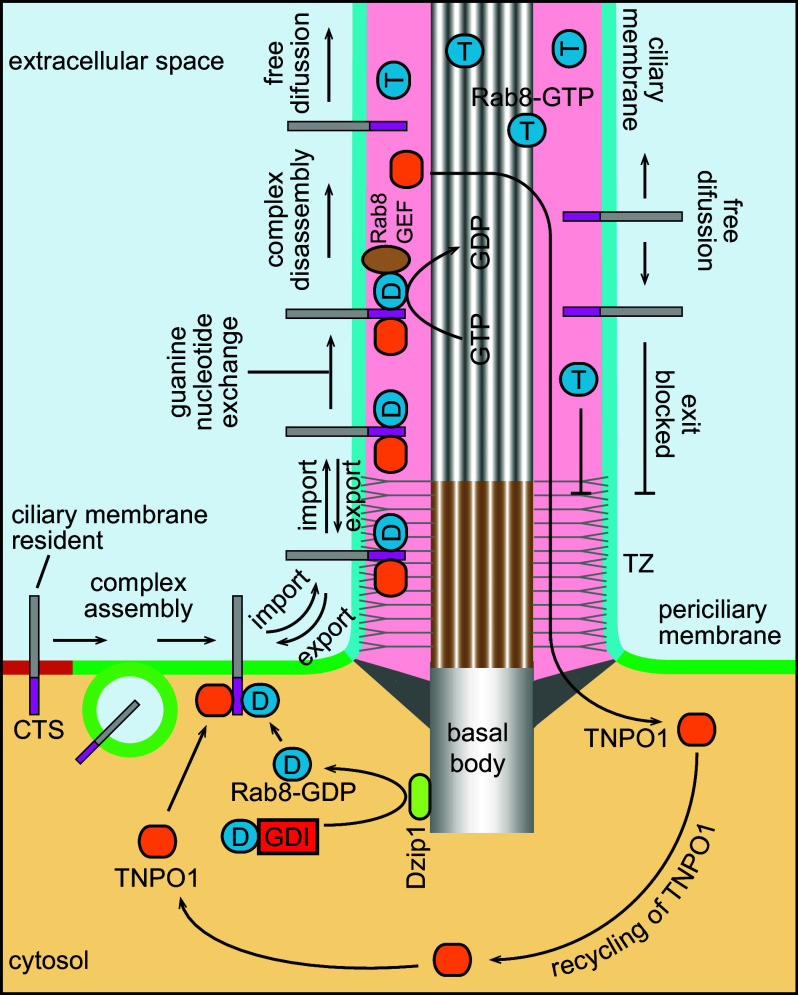
The possible cooperation between Rab GTPases and importins in ciliary targeting has been speculated before [80] (commented in [41]). Rab-family small GTPases specify organelle identities and participate in almost every step of membrane trafficking (reviewed in [81]). Among more than 70 Rabs in the human genome, Rab8 is the most studied cilium-localized Rab and it is a master regulator of ciliogenesis and polarized membrane trafficking to the cilium [55, 82–85]. Compromising the cellular function of Rab8 has also been reported to affect the ciliary entry of membrane residents, such as rhodopsin, fibrocystin, polycystin-1, Smoothened and Kim1, and soluble residents such as EB1 [55–57, 86, 87]. Among these cargoes, fibrocystin and polycystin-1 were demonstrated to directly interact with Rab8 [86, 87]. A recent screen expanded the list of cargoes trafficked by Rab8 and further uncovered that CTSs of fibrocystin, prRDH, rhodopsin, and RP2 can simultaneously interact with both Rab8–GDP and TNPO1 to form a ternary complex [24], demonstrating the cooperation between Rab8 and TNPO1 in ciliary targeting. Though Rab8–GTP but not GDP accumulates in the cilium [84], interestingly, it was found that only Rab8–GDP is involved in the assembly of the ternary complex, suggesting that guanine nucleotide exchange of Rab8–GDP to GTP can disassemble the ternary complex [24, 86].
We propose the following model to explain how the ternary complex works for ciliary trafficking of membrane residents (Fig. 3), reminiscent of the importin–Ran mechanism for nucleocytoplasmic trafficking. (1) The assembly of the ternary complex: for a CTS-containing membrane cargo, Rab8–GDP–TNPO1–CTS ternary complex can assemble at the periciliary membrane regardless of targeting pathways from the Golgi, lateral transport, or polarized exocytosis. Though Rab8–GDP is enriched in the cytosol, it is inhibited by binding to Rab–GDP dissociation inhibitor (Rab–GDI). At the periciliary membrane, Rab8–GDP might be released from the GDI by Dzip1, the basal body localized GDI displacement factor for Rab8 [88]. (2) Import translocation: the ternary complex translocates across the membrane diffusion barrier facilitated by TNPO1. The translocation of the ternary complex is likely reversible, similar to the translocation through the NPC. Therefore, the cilium import and export can quickly reach equilibrium unless the ternary complex is disassembled. (3) Guanine nucleotide exchange: within the cilium, the GDP of Rab8 is exchanged to GTP by cilium-localized Rab8 guanine nucleotide exchange factors (GEFs)—Rabin8 [89] and/or RPGR [90]. (4) Disassembly of the ternary complex and cargo release: as a result of the guanine nucleotide exchange, the ternary complex disassembles, hence releasing the cargo into the ciliary membrane. Though the cargo can freely diffuse throughout the ciliary membrane, the barrier at the base prevents its export from the cilium. The same scenario also applies to Rab8–GTP. Unimpeded by the diffusion barrier, TNPO1 undergoes export translocation and returns to the cytosol for further rounds of transport. Therefore, the net result of trafficking is the accumulation of Rab8–GTP and CTS-containing membrane cargo within the cilium. In the retention mechanism, tethering by receptor binding is expected to cause the reduced-kinetic movement or the immobilization of ciliary cargoes, the prediction which is contradictory to a series of recent findings (see discussion above). The Rab–importin–CTS-based model can easily explain the high mobility of cargoes within the cilium as anchoring mechanism is not required for ciliary localization in this model.
Fig. 3.
Ciliary trafficking model based on Rab–importin–CTS ternary complex. Rab8–GDP and TNPO1 assemble a ternary complex with a CTS-containing ciliary membrane cargo. The complex crosses the diffusion barrier and enters the ciliary membrane (import translocation). At the ciliary membrane, the complex can also cross the diffusion barrier to return to the PM (export translocation). Once Rab8–GDP in the ternary complex is exchanged to Rab8–GTP by cilium-localized Rab8–GEF, the complex disassembles and the ciliary membrane cargo and Rab8–GTP can freely diffuse within the cilium. While TNPO1 is able to cross the diffusion barrier and return to the cytoplasm, the ciliary membrane cargo and Rab8–GTP can no longer exit the cilium, therefore, resulting in their accumulation within the cilium
Rab8 [55, 82–85], Rab10 [91], and Rab23 [13, 80, 92] are currently the only Rabs known to have cilium or flagellum localization. As a member of Rab8 family, Rab10 might similarly assemble a Rab–importin–CTS ternary complex for ciliary trafficking. Supporting this view, it has been suggested that Rab10 might be able to compensate for Rab8 in ciliary trafficking based on the observation that the cilium of Rab8 knockout mice displays normal morphology [93]. Rab23 can functionally antagonize hedgehog signaling [94], the pathway which critically depends on the integrity of the cilium (reviewed in [95]). Disruption of Rab23 can inhibit the ciliary trafficking of Smoothened, D1R and Kif17 [13, 57, 80]. Intriguingly, a recent study demonstrated that TNPO1 and Rab23 can potentially assemble a complex for the ciliary targeting of Kif17 [80], similar to Rab8–TNPO1–CTS ternary complex. Thus, a new concept is emerging that, in addition to Ran, Rabs can cooperate with importins for ciliary targeting. An implication from our model is that CTSs can have importin-binding motifs. Importins are capable of engaging diverse ranges of motifs, which are currently not clearly defined (reviewed in [70, 71]). This is in parallel to the lack of consensus among CTSs. If a CTS-containing ciliary membrane protein interacts with importin, an interesting question arises as why it is not targeted to the INM after its initial synthesis at the peripheral ER, considering that the INM is continuous with the peripheral ER. It is likely that the interaction between CTSs of membrane cargoes and importin is not regulated by Ran, which leads to insignificant accumulation within the INM; alternatively, ciliary membrane cargoes probably possess such strong ER export signals that they are rapidly and efficiently diverted to the secretory pathway en route to the cilium after their biosynthesis. Besides its role in import translocation, it is tempting to further speculate that Rab–importin system might function in the ciliary export of cargoes.
Although the cilium and the nucleus have analogous topology and trafficking mechanism, their trafficking pathways might not be comparable in further molecular details. Nonetheless, an important lesson we can learn from the nuclear trafficking field is that the mechanism of INM targeting is diverse; it can be dependent or independent of one or a combination of following components—Ran, importin, and ATP (reviewed in [35, 96, 97]). There is likely no single unifying mechanism that can account for all the ciliary membrane targeting. However, it is possible that at least a significant number of ciliary cargoes are targeted by the cooperative action of CTSs, importins, and small GTPases, such as Ran and Rabs.
Conclusion
We have a few models or pathways to explain the ciliary targeting at molecular and cellular levels. The targeting of membrane residents to the periciliary membrane is by either the polarized exocytosis or lateral transport pathway. From the periciliary membrane, membrane residents can adopt selective entry mechanism to translocate through the ciliary diffusion barrier and/or retention mechanism to accumulate within the cilium. Increasing evidences have shown that the cilium and nucleus share analogous trafficking mechanisms in addition to the topological organization. Results from recent studies support Rab–importin–CTS-based model as it can account for import translocation across the diffusion barrier and the accumulation of highly mobile ciliary membrane residents. More molecular and cellular roles of Rabs and importins in ciliary targeting are expected to be elucidated in the near future.
Acknowledgements
We apologize to all authors, whose work could not be cited due to space limitations. This work is supported by the following Ministry of Education (Singapore) Grants to L.L.: AcRF Tier 2 MOE2015-T2-2-073 and AcRF Tier1 RG132/15 and AcRF Tier1 RG48/13.
References
- 1.Ishikawa H, Marshall WF. Intraflagellar transport and ciliary dynamics. Cold Spring Harb Perspect Biol. 2017;9(3):a021998. doi: 10.1101/cshperspect.a021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nachury MV, et al. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basten SG, Giles RH. Functional aspects of primary cilia in signaling, cell cycle and tumorigenesis. Cilia. 2013;2(1):6. doi: 10.1186/2046-2530-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Gonzalo FR, Reiter JF. Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access. J Cell Biol. 2012;197(6):697–709. doi: 10.1083/jcb.201111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vorobjev IA, Chentsov Yu S. Centrioles in the cell cycle. I. Epithelial cells. J Cell Biol. 1982;93(3):938–949. doi: 10.1083/jcb.93.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiter JF, et al. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012;13(7):608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson RG. The three-dimensional structure of the basal body from the rhesus monkey oviduct. J Cell Biol. 1972;54(2):246–265. doi: 10.1083/jcb.54.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53(2):494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ounjai P, et al. Architectural insights into a ciliary partition. Curr Biol. 2013;23(4):339–344. doi: 10.1016/j.cub.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Q, et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329(5990):436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chih B, et al. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol. 2012;14(1):61–72. doi: 10.1038/ncb2410. [DOI] [PubMed] [Google Scholar]
- 13.Leaf A, Von Zastrow M. Dopamine receptors reveal an essential role of IFT-B, KIF17, and Rab23 in delivering specific receptors to primary cilia. Elife. 2015;4:e06996. doi: 10.7554/eLife.06996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye F, et al. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. Elife. 2013;2:e00654. doi: 10.7554/eLife.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YC, et al. Chemically inducible diffusion trap at cilia reveals molecular sieve-like barrier. Nat Chem Biol. 2013;9(7):437–443. doi: 10.1038/nchembio.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breslow DK, et al. An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J Cell Biol. 2013;203(1):129–147. doi: 10.1083/jcb.201212024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams CL, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192(6):1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambacher NJ, et al. TMEM107 recruits ciliopathy proteins to subdomains of the ciliary transition zone and causes Joubert syndrome. Nat Cell Biol. 2016;18(1):122–131. doi: 10.1038/ncb3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kee HL, et al. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat Cell Biol. 2012;14(4):431–437. doi: 10.1038/ncb2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takao D, et al. An assay for clogging the ciliary pore complex distinguishes mechanisms of cytosolic and membrane protein entry. Curr Biol. 2014;24(19):2288–2294. doi: 10.1016/j.cub.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takao D, Verhey KJ. Gated entry into the ciliary compartment. Cell Mol Life Sci. 2016;73(1):119–127. doi: 10.1007/s00018-015-2058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira OV, et al. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin–Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA. 2006;103(49):18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Viso F, et al. Congenital heart disease genetics uncovers context-dependent organization and function of nucleoporins at cilia. Dev Cell. 2016;38(5):478–492. doi: 10.1016/j.devcel.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madugula V, Lu L. A ternary complex comprising transportin1, Rab8 and the ciliary targeting signal directs proteins to ciliary membranes. J Cell Sci. 2016;129(20):3922–3934. doi: 10.1242/jcs.194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Najafi M, et al. Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc Natl Acad Sci USA. 2012;109(1):203–208. doi: 10.1073/pnas.1115109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberts B, et al., editors. Molecular biology of the cell. 5. New York: Garland Science; 2008. [Google Scholar]
- 27.Hetzer MW. The nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2(3):a000539. doi: 10.1101/cshperspect.a000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8(3):195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]
- 29.Crisp M, Burke B. The nuclear envelope as an integrator of nuclear and cytoplasmic architecture. FEBS Lett. 2008;582(14):2023–2032. doi: 10.1016/j.febslet.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Terry LJ, et al. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318(5855):1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- 31.Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol. 2013;14(1):13–24. doi: 10.1038/nrm3488. [DOI] [PubMed] [Google Scholar]
- 32.Hetzer MW, et al. Pushing the envelope: structure, function, and dynamics of the nuclear periphery. Annu Rev Cell Dev Biol. 2005;21:347–380. doi: 10.1146/annurev.cellbio.21.090704.151152. [DOI] [PubMed] [Google Scholar]
- 33.Yang W, et al. Imaging of single-molecule translocation through nuclear pore complexes. Proc Natl Acad Sci USA. 2004;101(35):12887–12892. doi: 10.1073/pnas.0403675101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonin W, et al. Traversing the NPC along the pore membrane: targeting of membrane proteins to the INM. Nucleus. 2011;2(2):87–91. doi: 10.4161/nucl.2.2.14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katta SS, et al. Destination: inner nuclear membrane. Trends Cell Biol. 2014;24(4):221–229. doi: 10.1016/j.tcb.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Zuleger N, et al. System analysis shows distinct mechanisms and common principles of nuclear envelope protein dynamics. J Cell Biol. 2011;193(1):109–123. doi: 10.1083/jcb.201009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soullam B, Worman HJ. Signals and structural features involved in integral membrane protein targeting to the inner nuclear membrane. J Cell Biol. 1995;130(1):15–27. doi: 10.1083/jcb.130.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boni A, et al. Live imaging and modeling of inner nuclear membrane targeting reveals its molecular requirements in mammalian cells. J Cell Biol. 2015;209(5):705–720. doi: 10.1083/jcb.201409133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ungricht R, et al. Diffusion and retention are major determinants of protein targeting to the inner nuclear membrane. J Cell Biol. 2015;209(5):687–703. doi: 10.1083/jcb.201409127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan S, et al. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. J Cell Biol. 2007;178(3):387–398. doi: 10.1083/jcb.200609096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gruss OJ. Nuclear transport receptor goes moonlighting. Nat Cell Biol. 2010;12(7):640–641. doi: 10.1038/ncb2076. [DOI] [PubMed] [Google Scholar]
- 42.Jin H, et al. The conserved Bardet–Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141(7):1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Field MC, et al. Evolution: on a bender—BARs, ESCRTs, COPs, and finally getting your coat. J Cell Biol. 2011;193(6):963–972. doi: 10.1083/jcb.201102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dishinger JF, et al. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat Cell Biol. 2010;12(7):703–710. doi: 10.1038/ncb2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurd TW, et al. Localization of retinitis pigmentosa 2 to cilia is regulated by Importin beta2. J Cell Sci. 2011;124(Pt 5):718–726. doi: 10.1242/jcs.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Datta M, et al. Genome wide gene expression regulation by HIP1 Protein Interactor, HIPPI: prediction and validation. BMC Genom. 2011;12:463. doi: 10.1186/1471-2164-12-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi L, et al. The ciliary protein IFT57 in the macronucleus of Paramecium . J Eukaryot Microbiol. 2017 doi: 10.1111/jeu.12423. [DOI] [PubMed] [Google Scholar]
- 48.Madhivanan K, Aguilar RC. Ciliopathies: the trafficking connection. Traffic. 2014;15(10):1031–1056. doi: 10.1111/tra.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsiao YC, et al. Trafficking in and to the primary cilium. Cilia. 2012;1(1):4. doi: 10.1186/2046-2530-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Francis SS, et al. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J Cell Biol. 2011;193(1):219–233. doi: 10.1083/jcb.201009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molla-Herman A, et al. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123(Pt 10):1785–1795. doi: 10.1242/jcs.059519. [DOI] [PubMed] [Google Scholar]
- 52.Stoops EH, et al. The periciliary ring in polarized epithelial cells is a hot spot for delivery of the apical protein gp135. J Cell Biol. 2015;211(2):287–294. doi: 10.1083/jcb.201502045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84(3):335–344. doi: 10.1016/S0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 54.Papermaster DS, et al. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas . Investig Ophthalmol Vis Sci. 1985;26(10):1386–1404. [PubMed] [Google Scholar]
- 55.Moritz OL, et al. Mutant rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12(8):2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, et al. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J. 2012;31(20):4057–4071. doi: 10.1038/emboj.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boehlke C, et al. Differential role of Rab proteins in ciliary trafficking: Rab23 regulates smoothened levels. J Cell Sci. 2010;123(Pt 9):1460–1467. doi: 10.1242/jcs.058883. [DOI] [PubMed] [Google Scholar]
- 58.Das A, Guo W. Rabs and the exocyst in ciliogenesis, tubulogenesis and beyond. Trends Cell Biol. 2011;21(7):383–386. doi: 10.1016/j.tcb.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emmer BT, et al. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J Cell Sci. 2010;123(Pt 4):529–536. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finetti F, et al. The small GTPase Rab8 interacts with VAMP-3 to regulate the delivery of recycling T-cell receptors to the immune synapse. J Cell Sci. 2015;128(14):2541–2552. doi: 10.1242/jcs.171652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szalinski CM, et al. VAMP7 modulates ciliary biogenesis in kidney cells. PLoS One. 2014;9(1):e86425. doi: 10.1371/journal.pone.0086425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazelova J, et al. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci. 2009;122(Pt 12):2003–2013. doi: 10.1242/jcs.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baker SA, et al. The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol. 2008;183(3):485–498. doi: 10.1083/jcb.200806009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu Q, et al. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol. 2015;17(3):228–240. doi: 10.1038/ncb3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hunnicutt GR, et al. Cell body and flagellar agglutinins in Chlamydomonas reinhardtii: the cell body plasma membrane is a reservoir for agglutinins whose migration to the flagella is regulated by a functional barrier. J Cell Biol. 1990;111(4):1605–1616. doi: 10.1083/jcb.111.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milenkovic L, et al. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187(3):365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3(11):813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 68.Ou G, et al. Sensory ciliogenesis in Caenorhabditis elegans: assignment of IFT components into distinct modules based on transport and phenotypic profiles. Mol Biol Cell. 2007;18(5):1554–1569. doi: 10.1091/mbc.E06-09-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lechtreck KF, et al. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol. 2009;187(7):1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marfori M, et al. Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim Biophys Acta. 2011;9:1562–1577. doi: 10.1016/j.bbamcr.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 71.Twyffels L, et al. Transportin-1 and Transportin-2: protein nuclear import and beyond. FEBS Lett. 2014;588(10):1857–1868. doi: 10.1016/j.febslet.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 72.Soniat M, Chook YM. Nuclear localization signals for four distinct karyopherin-beta nuclear import systems. Biochem J. 2015;468(3):353–362. doi: 10.1042/BJ20150368. [DOI] [PubMed] [Google Scholar]
- 73.Fan S, et al. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol. 2004;14(16):1451–1461. doi: 10.1016/j.cub.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 74.Kovacs JJ, et al. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320(5884):1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghossoub R, et al. Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J Cell Sci. 2013;126(Pt 12):2583–2594. doi: 10.1242/jcs.111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spiliotis ET. Regulation of microtubule organization and functions by septin GTPases. Cytoskeleton (Hoboken) 2010;67(6):339–345. doi: 10.1002/cm.20448. [DOI] [PubMed] [Google Scholar]
- 77.Calvert PD, et al. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16(11):560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Ellenberg J, et al. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J Cell Biol. 1997;138(6):1193–1206. doi: 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Larkins CE, et al. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell. 2011;22(23):4694–4703. doi: 10.1091/mbc.E10-12-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim YS, Tang BL. A role for Rab23 in the trafficking of Kif17 to the primary cilium. J Cell Sci. 2015;128(16):2996–3008. doi: 10.1242/jcs.163964. [DOI] [PubMed] [Google Scholar]
- 81.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 82.Yoshimura S, et al. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178(3):363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knodler A, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA. 2010;107(14):6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129(6):1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 85.Westlake CJ, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci USA. 2011;108(7):2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Follit JA, et al. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol. 2010;188(1):21–28. doi: 10.1083/jcb.200910096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ward HH, et al. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol Biol Cell. 2011;22(18):3289–3305. doi: 10.1091/mbc.E11-01-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang B, et al. GSK3beta-Dzip1-Rab8 cascade regulates ciliogenesis after mitosis. PLoS Biol. 2015;13(4):e1002129. doi: 10.1371/journal.pbio.1002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hattula K, et al. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell. 2002;13(9):3268–3280. doi: 10.1091/mbc.E02-03-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murga-Zamalloa CA, et al. Interaction of retinitis pigmentosa GTPase regulator (RPGR) with RAB8A GTPase: implications for cilia dysfunction and photoreceptor degeneration. Hum Mol Genet. 2010;19(18):3591–3598. doi: 10.1093/hmg/ddq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Babbey CM, et al. Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am J Physiol Renal Physiol. 2010;299(3):F495–F506. doi: 10.1152/ajprenal.00198.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lumb JH, Field MC. Rab23 is a flagellar protein in Trypanosoma brucei . BMC Res Notes. 2011;4:190. doi: 10.1186/1756-0500-4-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sato T, et al. Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis. J Cell Sci. 2014;127(Pt 2):422–431. doi: 10.1242/jcs.136903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eggenschwiler JT, et al. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412(6843):194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- 95.Bangs F, Anderson KV. Primary cilia and mammalian hedgehog signaling. Cold Spring Harb Perspect Biol. 2017;9(5):a028175. doi: 10.1101/cshperspect.a028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ungricht R, Kutay U. Establishment of NE asymmetry-targeting of membrane proteins to the inner nuclear membrane. Curr Opin Cell Biol. 2015;34:135–141. doi: 10.1016/j.ceb.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 97.Burns LT, Wente SR. Trafficking to uncharted territory of the nuclear envelope. Curr Opin Cell Biol. 2012;24(3):341–349. doi: 10.1016/j.ceb.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]