Abstract
Cancer cells exhibit hallmarks in terms of proliferation, resistance to cell death, angiogenesis, invasion, metastasis, and genomic instability. Despite the progress in cancer research and the comprehension of tumorigenesis mechanisms, cancer remains a major issue in public health. A better understanding of the molecular factors associated with the appearance or progression of cancer may allow the development of therapeutic alternatives. Increasing data highlight the role of long non-coding RNAs in many diseases, including cancer. The long non-coding RNA H19 was the first discovered riboregulator, and it has been shown to be involved at multiple steps of tumorigenesis. Indeed, this lncRNA exert its action at various molecular scales. Understanding the role of H19 in cancer progression may allow to set up therapeutic strategies to prevent tumor expansion and metastatic dissemination. In this review, we will summarize the overexpression of the long non-coding RNA H19 in several types of cancer and the multiple implications of the long non-coding RNA H19 in the different hallmarks that define human cancer.
Keywords: lncRNA, H19, Hallmarks of cancer, Proliferation, Metastasis, miRNA
Introduction
Since it is very beginning, the comprehension of molecular and cellular mechanisms that underpin the apparition and evolution of cancer has considerably evolved, and keeps moving. The study of these physiopathological events has been marked in the 1970s by the discovery of the first ‘‘cancer-triggering’’ genes, called oncogenes, and then by their opposites named tumor suppressor genes. The conceptual framework of tumorigenesis, thus, involves a random intervention of genes that manage essential biological processes such as proliferation, cell death, senescence, motility, and invasion. During cancer development, cells will acquire specific genetic features, increase their proliferative potential, evade cell death control, and immune system vigilance, but also adapt their own metabolism. In 2000, Hanahan and Weinberg enumerated six cancer cell features that would be considered as hallmarks: sustained proliferative signaling, evasion of growth suppressors, replicative immortality, activation of invasion and metastasis, induction of angiogenesis, and resistance to cell death [1]. With recent progress in the field of cancer research came the proposal that tumors are more than masses of proliferating cancer cells, and are a lot more like hierarchical structures composed of multiple cell types that interact with each other. Accordingly, Hanahan and Weinberg added four more hallmarks in 2011: avoiding of immune destruction, tumor-promoting inflammation, genome instability and mutation, and the deregulation of cell energetics [2]. All these hallmarks are considered as distinctive and complementary capabilities that enable tumor growth and metastatic dissemination.
The Encyclopedia of DNA Elements (ENCODE) consortium exposed that nearly 80% of the human genome is transcribed into functional RNAs, but only 2% of the genome codes for proteins [3–5]. From this analysis, it has been highlighted that RNAs that are not translated are called non-coding RNAs (ncRNAs). These ncRNAs are classified according to their length in small ncRNAs (less than 200 nt) and long ncRNAs (more than 200 nt). Small ncRNAs regroup into microRNAs (miRNAs), small interfering RNAs (siRNAs), PIWI-interacting RNAs (piRNAs) that are involved in gene expression regulation, and small nucleolar RNAs (snoRNAs) that act as guides to induce chemical modification of other RNAs [6]. Among long ncRNAs, approximatively 20,000 have been identified as potentially functional and subject to transcriptional regulation by transcription factors and epigenetic modifications [7]. They exhibit classical features of mRNAs such as transcription by RNA polymerase II, 5′ capping, 3′ polyadenylation, and splicing [8, 9]. Long ncRNAs are increasingly described to be involved not only in normal development but also in the development of several pathologies, like neurological disorders [10, 11], diabetes [12], and cancers [13].
Interestingly, embryonic cells and cancer cells share similar features including active proliferation, plasticity, invasive behaviors, and gene expression profiles, all being coordinated by common molecular pathways and epigenetic regulation [14]. One of the common regulators during embryonic development and tumorigenesis is the long non-coding RNA H19, encoded by the H19 gene, which is subjected to genome imprinting and is maternally expressed [15]. The H19 gene is localized near the telomeric region of chromosome 11p15, within a unique locus shared with the IGF2 gene. Alterations of gene expression at the H19/IGF2 locus are associated with malignancies and developmental disorders [16]. The long non-coding RNA H19 of 2.3 kb is transcribed by the RNA polymerase II, polyadenylated, capped, and spliced with conserved secondary RNA structure, and was proposed for the first time by Brannan et al. to act as a riboregulator [17].
The aim of this review is to give an overview of the long non-coding RNA H19 expression in cancer and to highlight its impact on the hallmarks of cancer.
Overexpression of the long non-coding RNA H19 in cancer
H19 is expressed during embryonic development and repressed after birth, excepted for some tissues like mammary gland and uterus [18]. However, it is expressed de novo in cancers of different tissue origins including breast, liver, lung, and esophageal cancers [19–26]. A meta-analysis using ten studies of various solid cancers showed that patients with high expression of H19 have a poor prognosis [27]. More recently, H19 overexpression was reported to be correlated with lower complete remission rate and shorter overall survival of acute myeloid leukemia patients [28]. H19 overexpression and its known biological effects in cancer cells are summarized in Table 1.
Table 1.
Overexpression of the long non-coding RNA H19 in several cancer
| Type of cancer | Known inducers of H19 overexpression | Biological consequences and/or clinical values | References |
|---|---|---|---|
| Glioma | / |
• Increase of cell proliferation • Enhancement of pro-angiogenic factors • Inverse correlation with patient’s survival rate |
[29] [30] [31] |
| Oral squamous cell carcinoma | / |
• Increase of cell proliferation • Promotion of migration and invasion through epithelial-to-mesenchymal (EMT)-associated protein expression regulation • Immune escape through modulation of pro- and anti-immune factors expression |
[32] [35] |
| Lung adenocarcinoma |
• Cisplatin treatment • Benzo[α]pyrene treatment • HGF/SF |
• Acquisition of chemoresistance • Disruption of genomic stability by increased mutation frequency. • Association with patients’ clinical resistance to cisplatin-based chemotherapy • Serological marker for patients’ auxiliary diagnosis • Activation of migration and invasion |
[36] [37] [38] [39] |
| Breast cancer |
• E2F1 • ERα/17β-estradiol • 91H • HGF • TGF- β • Hypoxia |
• Increase of cell proliferation • Paclitaxel resistance through epigenetic silencing of pro-apoptotic genes • Enhancing of cell migratory potential • Potential biomarker for early screening and prognosis monitor • Induction of epithelial-to-mesenchymal transition |
[43] [46] [39] |
| Gastric cancer |
• c-Myc • PEG10 |
• Inhibition of growth suppressors activity • Inhibition of pro-apoptotic genes expression • Promotion of cell migration, invasion, and metastasis • Correlation with poor prognosis and clinical stage • Potential biomarker for diagnosis and early tumor screening |
[49] [50] [51] [52] |
| Liver cancer |
• Cyclin D/CUDR • EGR1/PKM2 • Bcl2 • TNF-α • TGF-β |
• Inhibition of growth suppressors expression • Modulation of telomerase activity • Activation of pro-angiogenic factors expression • Regulation of immunological response • Association with bile acid homeostasis deregulation • Enhancement of obstructive cholestatic liver fibrosis development • Induction of epithelial-to-mesenchymal transition |
[53] [54] [57] [58] [59] [39] |
| Cholangiocarcinoma | • Oxidative stress |
• Activation of invasion and metastasis through regulation of EMT-associated proteins expression • Enhancement of chronic inflammation response to the tumor microenvironment • Correlation with tumor size, TNM stage, post-operative recurrence, and poor prognosis |
[60] [60] [61] |
| Pancreatic cancer | / | • Inhibition of apoptosis through inhibition of caspase 3 cleavage | [62] |
| Renal cell carcinoma | / | • Promotion of migration and invasion | [63] |
| Bladder cancer | • TGF-β |
• Increase of cell proliferation • Increase of blood vessel density • Activation of invasion and metastasis through epigenetic silencing of EMT-associated proteins • Induction of epithelial-to-mesenchymal transition |
[64] [65] [66] [39] |
| Colorectal cancer | / |
• Increase of cell proliferation through enhanced cell cycle progression • Inhibition of growth suppressors expression • Enhancing of tumor migration and invasion |
[67] [68] [69] |
| Cervical cancer | / | • Inhibition of apoptosis | [70] |
| Ovarian cancer | • Cisplatin resistance |
• Inhibition of pro-apoptotic factors • Activation of invasion and metastasis through regulation of EMT-associated proteins expression • Induction of cisplatin resistance through regulation of cell metabolism |
[71] [39] [72] |
| Leukemia |
• Bcr-Abl kinase • c-Myc • ATRA treatment |
• Enhancement of drug resistance • Modulation of telomerase activity |
[73] [74] |
| Osteosarcoma | / | • Promotion of cell migration and invasion | [75] |
| Melanoma | / | • Promotion of glucose metabolism and cell growth | [76] |
H19 expression in cancer cells is modulated by different stimuli including hypoxia, inflammation, cytokines/growth factors, and therapeutic agents (Table 1). H19 exerts its diverse actions by interacting with proteins or with miRNAs as a sponge (Table 2) [77]. H19 is also a precursor of miR-675 [78] that is increasingly found to be involved in physiological processes and cancer development. However, the targets of miR-675 differ according to cancer type, and are listed in Table 3.
Table 2.
Mediators of lncRNA H19 action in cancer
| Type of cancer | Mediators of H19 action | Impact on hallmarks of cancer | References |
|---|---|---|---|
| Glioma |
• miR-152 • miR-29a |
• Evading growth suppressors • Inducing angiogenesis |
[79] [30] |
| Tongue squamous cell carcinoma | • EZH2 | • Activating invasion and metastasis | [33] |
| Lung adenocarcinoma | • SAHH | • Genomic instability and mutation | [37] |
| Breast cancer |
• E2F1 • ERα • miR-152 |
• Sustaining proliferative signaling |
[40] [41] [29] |
| Gastric cancer |
• p53 • miR-141 • PEG10 |
• Evading growth suppressors • Sustaining proliferative signaling • Activating invasion and metastasis |
[47] [80] [51] |
| Liver cancer | • Telomerase complex | • Enabling replicative immortality | [54] |
| Cholangiocarcinoma | • let-7 | • Tumor-promoting inflammation | [61] |
| Pancreatic cancer | • E2F1 |
• Sustaining proliferative signaling • Resisting cell death |
[62] |
| Renal cell carcinoma | • miR-29b-3p | • Activating invasion and metastasis | [63] |
| Bladder cancer |
• miR-29b-3p • EZH2 |
• Sustaining proliferative signaling • Resisting cell death • Activating invasion and metastasis |
[64] [43] [66] |
| Colorectal cancer |
• eIF4A3 • miR-200a |
• Sustaining proliferative signaling |
[67] [68] |
| Cervical cancer | • miR-138-5p | • Resisting cell death | [70] |
| Leukemia | • Telomerase complex | • Enabling replicative immortality | [74] |
| Osteosarcoma | • miR-200 | • Activating invasion and metastasis | [75] |
| Melanoma | • miR-106a-5p | • Deregulating cell energetics | [76] |
Table 3.
Validated targets of H19-derived miR-675 in cancer
| Type of cancer | Targets of miR-675 | Target function | References |
|---|---|---|---|
| Glioma | • Cadherin 13 | • Atypical cadherin lacking the cytoplasmic domain | [81] |
| Breast cancer | • Cbl-b, c-Cbl | • Ubiquitin ligases E3 | [45, 82] |
| Lung cancer | • GPR55 | • G protein-coupled receptor | [83] |
| Esophageal squamous cell carcinoma | • REPS2 | • Repressor of cell proliferation and migration | [84] |
| Gastric cancer |
• FADD • RUNX1 • CALN1 |
• Apoptotic adaptator that recruits caspase 8 or 10 • Transcription factor • Calcium-binding protein |
[49] [48] [50] |
| Liver cancer |
• HP1 • RB • TWIST1 |
• Heterochromatin assembler and regulator • Tumor suppressor • Transcription factor involved in EMT |
[56] [85] [85] |
| Colorectal cancer |
• RB • DDB2 |
• Tumor suppressor • Transcriptional repressor |
[69] [86] |
| Ovarian cancer | • Slug | • Transcriptional factor involved in EMT | [39] |
| Osteosarcoma | • CALN1 | • Calcium-binding protein | [87] |
Implication of the long non-coding RNA H19 in the hallmarks of cancer
Sustaining proliferative signaling
Proliferation is considered as the most fundamental trait of cancer cells. In normal cells, the regulation of proliferation is stringent, fully controlled, and involved at multiple levels. Production and signaling of growth-promoting factors that modulate entry into and progression through cell division cycles have to be strictly controlled to ensure tissue homeostasis (cell number, tissue architecture, and function). In cancer cells, this control system is altered. H19 is found to sustain cell proliferation in different types of cancers (Table 1). For example, H19 expression is correlated with an increased proliferation of gastric cancer cells, through its association with p53 protein [47]. Moreover, Liu et al. reported that curcumin reduces gastric cancer cells proliferation through inhibiting H19 expression; accordingly, ectopic overexpression of H19 or c-Myc-induced H19 expression impedes the growth inhibitory effect of curcumin [88]. Still in gastric cancer, miR-141 binds to H19 and suppresses its expression, leading to a reduced cell proliferation [80].
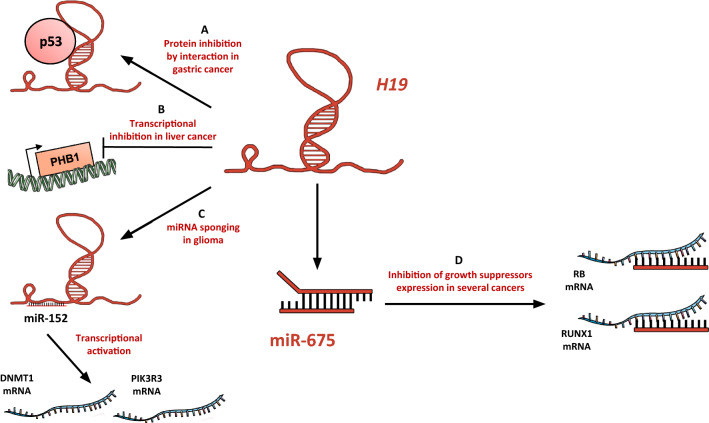
It has been shown that H19 can sustain cancer cell growth through several mechanisms (Fig. 1). H19 can promote cell cycle progression through G1/S transition. Knockdown of H19 in esophageal squamous cell carcinoma results in the inhibition of cell growth in vitro and in vivo by the induction of a G0/G1 arrest [32]. Our team showed that H19, activated by the transcription factor E2F1, promotes cell proliferation by facilitating G1/S transition in breast cancer cells [40] (Fig. 1a). Knockdown of H19 induces cell accumulation in G0/G1 in pancreatic ductal adenocarcinoma, resulting in a slower tumor growth in mice. Interestingly, in this model, H19 knockdown downregulates E2F1 levels, and E2F1 knockdown reduces H19 expression, indicating an activation loop of cell cycle progression [62]. H19 can also stimulate cell cycle progression through the sequestration of eIF4A3, which controls pre-mRNA splicing, in colorectal cancer cells (Fig. 1a). This leads to a reduced expression of cell cycle regulatory genes, including CCND1, CCNE1, and CDK4 [67].
Fig. 1.
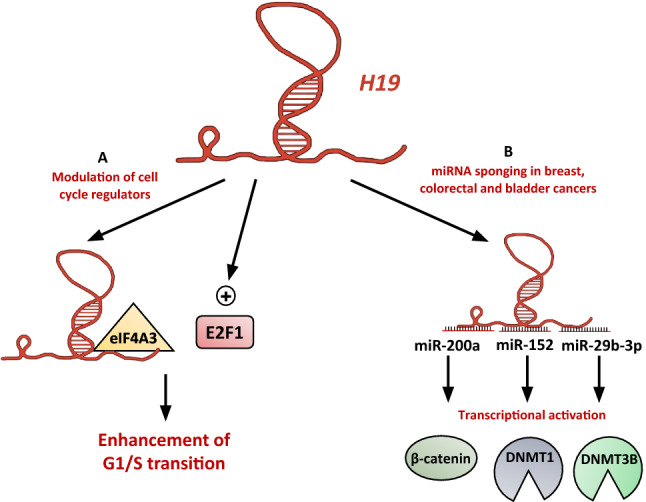
The long non-coding RNA H19 promotes cancer cell proliferation. aH19 promotes the G1/S transition in breast and colorectal cancer cells, in esophageal squamous cell carcinoma and in pancreatic ductal adenocarcinoma [32, 40, 62, 67]. bH19 sponges several miRNAs to allow β-catenin and DNMTs expression in colorectal, breast, and bladder cancer, respectively [29, 64, 68]
H19 can also promote cancer cell proliferation by sponging miRNAs. In colorectal cancer, miR-200a decreases cell proliferation by inhibiting β-catenin expression. H19 is able to sponge miR-200a (Fig. 1b), and thereby enhance β-catenin expression and activity to promote cell proliferation [68]. DNMTs are critical for de novo DNA methylation that ensures a main epigenetic code controlling the transcription profile. Notably, DNMTs allow the expression of growth-promoting genes. DNMT1 is downregulated by miR-152 in breast cancer cells. H19 sponges miR-152 (Fig. 1b), allowing DNMT1 expression and function to enhance cell proliferation [29]. Similarly, DNMT3B is transcriptionally repressed by miR-29b-3p in bladder cancer cells. H19 can sponge miR-29b-3p, leading to the re-expression of DNMT3B and the stimulation of cell proliferation [64].
Evading growth suppressors
In addition to the presence of positive regulators, cancer cells have to override mechanisms that curb cell proliferation. Many of these control systems are based on molecular actors such as tumor suppressors (like p53 and RB), or other elements with an antiproliferative effect. In this context, H19 can physically interact with p53 protein to suppress its activation (Fig. 2a), thus increasing proliferation of gastric cancer cells [47]. On the other hand, PHB1, a mitochondrial chaperone with diverse functions including cell proliferation, apoptosis, and mitochondrial homeostasis has been reported as a negative regulator of hepatocellular carcinoma [53]. It was found that the expression level of H19 negatively correlates with that of PHB1 in human hepatocellular carcinoma [53]. H19 silencing induces PHB1 expression and prevents PHB1 knockdown-mediated growth, whereas H19 overexpression induces the reverse effect, indicating the ability of H19 to override the tumor suppressing activity of PHB1 (Fig. 2b). In human glioma cell lines, H19 is able to sponge miR-152 (which is known as a tumor suppressor by targeting proliferation and invasion factors such as DNMT1 or PIK3R3 [29, 89]) to downregulate its activity and promote glioma cells proliferation (Fig. 2c) [79].
Fig. 2.
H19 downregulates growth suppressors. a In gastric cancer, H19 interacts physically with p53 to inhibit its antiproliferative activity [47]. bH19 induces the transcriptional inhibition of PHB1 in liver cancer cells [53]. cH19 is able to sponge miR-152 and so enhance glioma cells proliferation [79]. dH19-derived miR-675 inhibits the expression of well-known growth suppressors such as RB and RUNX1 in colorectal and hepatocellular cancers, and in gastric cancer, respectively [48, 69, 85]
H19 can also favor the evasion from growth suppressors via its product miR-675 (Fig. 2d), which downregulates RB protein expression in colorectal cancer cells and hepatocellular carcinoma cells [69, 85]. Moreover, miR-675 is able to negatively regulate RUNX1 expression in gastric cancer cells, leading to the activation of AKT/mTOR pathway and the progression of gastric cancer [48].
Resisting cell death
Programmed cell death by apoptosis is a natural multistep process that plays an important role in the development and life of multicellular organisms, by eliminating damaged cells through a fine-tuned regulatory mechanism. Cancer cells exhibit enhanced tolerance to both environmental and genomic stresses, resulting in resistance to apoptosis and tumor progression.
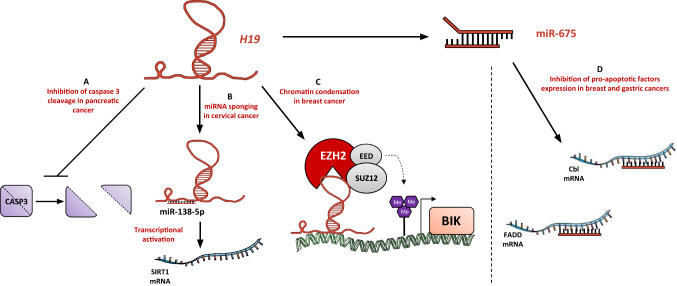
It has been shown that H19 knockdown upregulates expression of pro-apoptotic genes (DDIT3, CASP3) [90], and leads to cholangiocarcinoma cells apoptosis [60]. Furthermore, H19 decreases caspase 3 cleavage in pancreatic ductal carcinoma cell lines (Fig. 3a), thus avoiding apoptosis [62]. SIRT1, the histone deacetylase that mediates heterochromatin formation and reduces tumor suppressors expression, is targeted by miR-138-5p in cervical cancer cells to enhance apoptosis (Fig. 3b). H19 will serve as an endogenous sponge to downregulate miR-138-5p and attenuate its suppressive effect [70]. The expression levels of H19 and miR-675 are negatively correlated with FADD expression level in gastric cancer. miR-675 targets FADD (Fig. 3d) and inhibits caspases 8 and 3 [49].
Fig. 3.
H19 impedes cancer cell death. a In pancreatic cancer cells, H19 prevents caspase 3 cleavage [62]. bH19 sponges miR-138-5p in cervical cancer to allow the transcription of SIRT1 [70]. cH19 is able to physically interact with PRC2 complex to catalyze the trimethylation of H3K27, that will lead to chromatin condensation and repression of pro-apoptotic factor BIK in breast cancer cells [43]. d miR-675 is able to inhibit the expression of pro-apoptotic factors such as Cbl and FADD in breast and gastric cancers, respectively [49, 82]
There are evidences that H19 is involved in drug resistance. Overexpression of H19 in lung adenocarcinoma is associated with acquired resistance to cisplatin and correlated with the patients’ clinical response to cisplatin-based chemotherapy [36]. Knockdown of H19 in glioblastoma leads to increased apoptosis due to temolozomide treatment, suggesting the role of H19 in the anti-apoptotic process of glioblastoma cells [91]. In Bcr-Abl-positive leukemic cells, c-Myc-induced expression of H19 enhances cell survival. Silencing of H19 results in the imatinib-induced apoptosis of leukemic cells and the inhibition of tumor growth induced by Bcr-Abl [73]. H19 silencing in ovarian cancer cell lines induces cell apoptosis characterized by an enhanced expression of the pro-apoptotic protein Bax and a decreased expression of the anti-apoptotic protein Bcl2, as well as the activation of caspases 9 and 3 [71]. In ERα+ breast cancer cells, H19 expression is linked to paclitaxel resistance (Fig. 3c). H19 is involved in the epigenetic silencing of BIK, and contributes to attenuate the apoptosis response [43]. The expression of H19 is suppressed in breast cancer cells by Huaier extract (aqueous extract of Trametes robiniophila murr, used in China for cancer complementary therapy) to promote anti-tumor effects. Interestingly, Huaier extract enhances Cbl expression, that our team previously demonstrated as a miR-675-5p direct target (Fig. 3d) [82].
Enabling replicative immortality
Linked to their proliferative potential, cancer cells require to replicate endlessly to generate macroscopic tumors. This goes against normal cells standards, which divides a limited number of times before undergoing senescence and/or cell death.
It is now known that the protection of chromosomes’ ends by telomeres plays a major role in the unlimited replicative potential [92]. Telomeres are constituted by the repetition of multiple tandem hexamers and have an outward 3′ strand that presents a loop structure to protect them from nucleases. In normal (non-immortalized) cells, telomeres are shortened through cycles of replication. The number of cell generations is thereby dictated by the length of telomeric DNA. Telomeres erosion triggers entrance into replicative senescence and potentially cell death. Telomerase is a RNA-dependent DNA polymerase specific for telomeres. This enzyme adds telomere repeats segments to the ends of telomeric DNA. It is absent from non-immortalized cells but found at significant levels in immortalized cells, including cancer cells. The extension of telomeres length by telomerase is, thus, a ‘‘counter-attack’’ of cancer cells to avoid induction of senescence and/or apoptosis. Telomerase is composed of two major subunits: TERT (telomerase reverse transcriptase) that carries the DNA polymerase activity, and TERC (telomerase RNA component) that serves as a matrix for telomeric sequences.
Recent studies highlighted the role of H19 in the regulation of telomerase activity, but opposite models have been proposed. In liver cancer, the non-coding RNA CUDR (Cancer Up-regulated Drug Resistant) associates with cyclin D1 and PTEN in an inactive trimeric complex. The decrease of PTEN leads to increase the binding capacity of CUDR to cyclin D1, thus, forming an active dimer. CUDR and cyclin D1 accelerate the proliferation of liver cancer stem cells by demethylating H19 promoter. H19 expression allows the enhancement of telomerase activity by promoting TERT/TERC complex formation and inhibiting TERT/TERRA (Telomeric repeat-containing RNA, a telomeric long non-coding RNA) complex formation [54]. El Hajj et al. studied, for their part, the effects of All-trans retinoic acid (ATRA) treatment in acute promyelocytic leukemic cell lines resistant to retinoids. ATRA is associated with TERT repression, and induces H19 expression. H19, thus, induces the inhibition of telomerase activity by disassembling the telomerase complex TERT/TERC. According to El Hajj et al., H19 would function as a molecular chaperone able to promote either the association or the dissociation of TERT to TERC, and thereby telomerase activity, depending on the cellular context [74].
Inducing angiogenesis
As long as tumor size remains small, cancer growth does not depend on blood supply, as cells can be sufficiently supplied with oxygen and nutrients via diffusion. However, when tumor grows beyond a few millimeters, it triggers an angiogenic switch to form blood vessels within the tumor, allowing for supplies of nutrients and oxygen, as well as an evacuating arrangement for metabolic wastes. Tumor angiogenesis is driven by numerous cytokines, chemokines, and growth factors such as VASH 2 (vasohibin 2).
Stable overexpression of H19 promotes tumor formation of glioblastoma cells in mice [31]. CD90+ liver cancer cells are cancer stem cells like and show a mesenchymal phenotype. Conigliaro et al. have shown that CD90+ cells express H19 and release it via active production of exosomes. H19 will stimulate angiogenesis and promote cell adhesion to endothelial cell monolayer [55]. H19 can also enhance tumor angiogenesis by sponging miRNAs. H19 sponges miR-29a, resulting in an overexpression of VASH2 in glioma microvessels and glioma-associated endothelial cells, to favor tumor-induced endothelial cell proliferation and tube formation in vitro [30].
Halofuginone, a quinazolinone alkaloid isolated from the plant Dichroa febrifuga, can be used in bladder carcinoma cells to suppress extracellular matrix deposition and cell proliferation. This negative effect is accompanied by a marked decrease in blood vessel density and in H19 gene expression, suggesting a role for H19 in bladder carcinoma angiogenesis [65].
Activating invasion and metastasis
Metastasis is a multi-step process including (i) invasion of tissues surrounding the primary tumor, (ii) escape of the tumor site via the lymphatic circulation of blood vessels, (iii) extravasation from the blood vessels to colonize distant organs, and (iv) formation of secondary tumors. During this process, cancer cells modify their microenvironment to make it permissive and conducive to their growth. In return, tumor microenvironment contributes to cancer cells migration and invasion. Our team previously showed that in response to HGF/SF, H19 is able to modify cell morphology and enhance their migratory potential [93]. H19 expression correlates with metastatic potential of breast cancer cells and is found in common metastatic sites according to primary tumor localization [39]. We also demonstrated that the lncRNA 91H, the H19 antisense RNA, is able to increase oncogenic properties of breast cancer cells by enhancing migration and invasion in vitro and metastasis in xenografted mouse model [44].
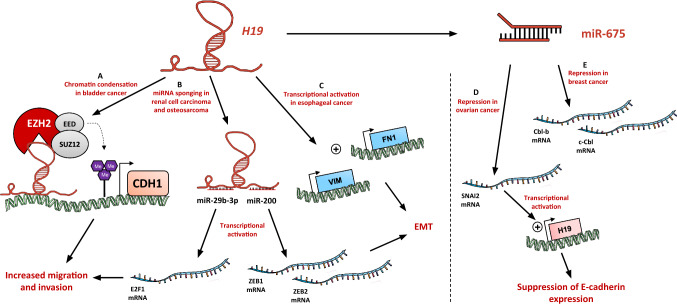
During the first steps of metastasis, carcinoma cells are induced to suppress their epithelial features and upregulate mesenchymal gene expression programs to acquire a new fibroblastic-like phenotype. This phenotypic plasticity, named epithelial-to-mesenchymal transition (EMT), enables cancer cells to invade and disseminate. Several well-known EMT inducers like TGF-β, hypoxia, HGF/SF, or muti-drug resistance were reported to increase H19 gene expression [39, 45, 93]. Xu et al. showed that inhibition of H19 in cholangiocarcinoma reverses EMT and represses cell migration and invasion in vitro [60]. H19 can promote EMT by modulating factors like cell adhesion molecules. In bladder cancer, H19 associates with EZH2, the catalytic subunit of PRC2 complex, to induce chromatin condensation at the promoter of CDH1, and a decrease of E-cadherin expression (Fig. 4a). This leads to the promotion of bladder cancer cell metastasis [66]. Zhang et al. showed that H19 promotes tumor growth and metastasis of tongue squamous cell carcinoma through its association with EZH2. Downregulation of H19 impedes β-catenin/GSK-3β activation, and modulates the expression of mesenchymal/epithelial markers to reverse EMT [33]. H19 also represses E-cadherin expression in human esophageal cancer cell lines, and enhances vimentin and fibronectin expression [34] (Fig. 4c). Knockdown of H19 in this model suppresses cell migration and invasion [32]. H19 acts as a ceRNA in osteosarcoma cells to suppress miR-200 family and increase the expression of ZEB1 and ZEB2 (Fig. 4b) and, thereby, promoting cell migration and invasion [75]. H19 can also regulate expression of DNMT3B and EMT-associated proteins by sponging miR-29b-3p. This repression enhances migration and invasion of bladder cancer cells [64]. miR-29b-3p is also targeted by H19 in clear cell renal cell carcinoma to upregulate E2F1 (Fig. 4b), and thus promoting migration and invasion [63].
Fig. 4.
H19 promotes the migration, invasion, and metastasis of cancer cells. a In bladder cancer, H19 is able to interact with PRC2 complex to induce chromatin condensation at the CDH1 promoter, leading to the repression of E-cadherin [66]. bH19 sponges miR-29b-3p and miR-200 in renal cell carcinoma and osteosarcoma, respectively, to activate the transcription of E2F1, ZEB1, and ZEB2 [63, 75]. cH19 acts as a transcriptional activator in esophageal cancer to enhance the expression of vimentin and fibronectin [34]. d miR-675 represses the expression of SNAI2 in ovarian cancer to create an activation loop of the H19 expression and repress E-cadherin expression [39]. e miR-675 inhibits Cbl-b and c-Cbl expression to induce the activation of AKT and ERK pathways in breast cancer cells [45]
The expression of miR-675 in ovarian carcinoma cells downregulates the slug transcription factor expression (Fig. 4d), creating a H19-slug positive loop involved in the suppression of E-cadherin expression [39]. Similarly, H19/miR-675 upregulation promotes gastric cancer cell migration and invasion in vitro [50]. H19 is also able to cooperate with PEG10 (paternally expressed gene 10) to promote gastric cancer cells transformation, invasion, and anchorage-independent growth [51]. c-Cbl and Cbl-b are involved in the degradation of tyrosine kinase receptors after their activation by growth factors. Our team showed that in breast cancer cells, miR-675 targets c-Cbl and Cbl-b expression to induce the activation of AKT and ERK pathways (Fig. 4e), which in turn increase metastatic potential of breast cancer cells [45].
Interestingly, Wang et al. showed that miR-675 expression could have opposite effects. The miR-675-5p targets miR-200 expression through an increased expression of ZEB1 and ubiquitin-like UBQLN1 protein. This leads to the inhibition of cell migration and invasion in pancreatic ductal adenocarcinoma [94]. According to the authors, contrary findings about H19 and miR-675 mechanisms of action in pancreatic cancer may be due to different cell lines used and the respective levels of H19 expression. However, these results contribute to the complexity and plasticity of H19 mechanisms of action in cancer.
Genomic instability and mutation
Genomic instability and mutation of cancer cells constitute the first of cancer “emerging hallmarks” defined by Hanahan and Weinberg [2]. It is considered as the triggering element of all previous hallmarks: the induction of genome mutations would confer selective advantage to cancer cells and their progeny, allowing them to multiply and takeover normal cells in a local tissue environment. These modulations can be characterized by the inactivation of tumor suppressor genes, or perturbation of epigenetic mechanisms such as DNA methylation. Genomic instability can also concern chromosomal organization. In somatic cells, chromosomal stability and the number of chromosomes pairs are essential features during cell cycle to prevent DNA damages or flawed replication.
In this context, Wolanin et al. showed that the use of curcumin downregulates survivin expression, leading to polyploid formation and defects in chromosome segregation in Bcr-Abl expressing cells. Curcumin disrupts cell cycle regulation and blocks cells in G2/M phase, resulting in the accumulation of abnormalities of mitosis and cytokinesis, and then in apoptosis. [95]. Moreover, curcumin was proved by Kujundžić et al. to downregulate the transcription of both H19 and DNA topoisomerase II alpha (TOPO2A) in several tumor cell lines [96]. Ravid et al. showed that adipose-derived mesenchymal stem cells stably retain their diploid state under various culture conditions. This ability is associated with a reduced H19 expression and a higher basal activity of p53 protein [97]. Shoshani et al. studied, for their part, polyploid mesenchymal stem cells, illustrating that polyploid condition maintains a non-tumorigenic state. They revealed that H19 expression differs between diploid and polyploid cultured mesenchymal stem cells, and that H19 suppression is associated with a tetraploidization of diploid cells and a reduced tumorigenic potential.
Benzo[α]pyrene (BαP) is a carcinogen molecule that enhances the interaction between H19 and SAHH (S-adenosyl-l-homocysteine hydrolase, the methyl cycle enzyme). Fu et al. studied the effects of BαP treatment in human lung-derived cells in vitro, and showed that the H19/SAHH interaction following the treatment inhibits the methyltransferase activity of SAHH. This leads to the demethylation of long interspersed element-1 (LINE-1), which has a retrotranscription activity. Hypomethylation of LINE-1 leads to chromosomal instability and in several genomic alterations such as deletion, amplification, and translocation, and has been reported as a marker of poor prognosis in lung cancer [98]. Thus, H19 is able to disrupt the genome stability and induce mutations in cancer cells [37].
In an interesting way, DNA demethylation of rhabdomyosarcoma (RMS) cells due to 5-azacytidine treatment upregulates miR-675 expression. This overexpression has been shown to inhibit cell growth through loss of imprinting at the H19-IGF2 locus: H19 is re-expressed and IGF2 is downregulated. Tarnowski et al. show that miR-675 overexpression in RMS cells impairs insulin signaling in repressing IGF1R and insulin receptor expression [99]. However, the authors highlight the putative role of several other genes demethylation that could contribute to this phenomenon.
Tumor-promoting inflammation
The promotion of inflammation would seem paradoxal for a phenomenon like tumorigenesis, but immune cells have been shown to have functionally important effects on tumor progression [100]. Indeed, an immunosuppressive microenvironment will be generated by the tumor, thereby preventing its infiltration by immune effector cells. However, cancer cells promote the recruitment of macrophages and regulatory T cells that inhibit antitumoral immune response. These cells will release mutagenic factors (such as ROS) or other inflammatory factors (like TNF-α) that enhance the malignancy evolution of surrounding cells [101].
There are few data available about the link between H19 and cancer inflammation. H19 has been shown to influence inflammation-associated pathways initiated by oxidative stress in cholangiocarcinoma cells. Indeed, H19 sponges let-7a/let-7b miRNAs to upregulate IL-6 expression and enhances chronic inflammation response to the tumor microenvironment [61]. However, the existence of inflammation processes involving H19 in other models (pathological or not) can bring us to think that there could exist similar mechanisms in cancer.
Deregulating cellular energetics
In normal cells, the oxygen availability conditions the process of glucose: under aerobic context, glucose is processed via glycolysis in pyruvate and then in carbon dioxide through Krebs cycle, and is dispatched to the respiratory chain in mitochondria. Under anaerobic context, pyruvate is processed in lactate that characterizes fermentation. Cancer cells energy metabolism does not follow that logic: even in presence of oxygen, cancer cells can reprogram their glucose metabolism, and thus their energy production, by favoring glycolysis, in a state called “aerobic glycolysis”. This allows the redirection of glycolysis intermediates into various biosynthetic pathways and facilitates the generation of new cells.
Knockdown of H19 modulates the expression of several genes involved in lipid, carbohydrate, and polyamine metabolisms (such as PLA2G4A, MPI, PYGB) [90]. We previously saw that H19 expression dowregulates PHB1, the chaperone that maintains the functional integrity of the mitochondria [53]. In addition, it has been shown that H19 overexpression in high-grade serous ovarian cancer promotes glutathione metabolism that induces cisplatin resistance [72]. Moreover, H19 enhances pyruvate dehydrogenase kinase 1 (PDK1) expression and, thus, promotes glycolysis in breast cancer stem cells [102].
H19 can also act on miRNAs expression to disrupt cell energetics. H19 sponges miR-106a-5p to upregulate E2F3 expression and to promote glucose metabolism and growth of melanoma cells [76]. H19 also modulates lipid metabolic by sponging miR-130b in ox-LDL-treated macrophages from atherosclerotic patients [59].
Avoiding immune destruction
Immune surveillance is a mechanism in which cells and tissues are checked at any time by an immune system always on alert. It recognizes and eliminates foreign bodies, putative dangers or abnormalities. To keep walking through the multiple steps of tumorigenesis, cancer cells will have to develop strategies that will dissimulate them from the immune system. In that way, solid tumors that could make it through this surveillance have handled to avoid detection or limit immunological elimination and, therefore, prevent cancer destruction. The immunological defecting managed by tumors is observed in some cases of cancer development in immunodeficient individuals [103]. These are generally virus-induced cancers, meaning that this class of cancers can expand depending on the reduction of viral burden in infected individuals, through eliminating virus-infected cells. Cancer cells can also escape to immune surveillance by impeding its mobilization against the tumor. As we previously said in the tumor-promoting inflammation context, tumors will generate an immunosuppressive microenvironment that prevents its infiltration by immune effector cells. However, macrophages and regulatory T cells recruited by cancer cells inhibit antitumoral immune response: those bypassing strategies will, thus, allow tumor growth.
Few are known about the implication of long non-coding RNAs in the invalidation of the immune system. An emerging theory would involve lncRNAs in cases of virus-caused immunodeficiencies [104]. In other pathologies such as laryngeal squamous cell carcinoma, some immunity-associated molecules like FOXP3 or CD274, that are expressed at the surface of lymphocytes and macrophages, respectively, are enhanced. However, other factors that reduce immune response such as IL-10 are upregulated and negatively associated with patient’s survival, meaning that a program leading to the immune response avoidance is set up. Sun et al. showed that a ceRNA network, including several lncRNAs like H19, is involved in the regulation of those molecules, thus developing an immune escape mechanism for LSCC cells [35].
Conclusions
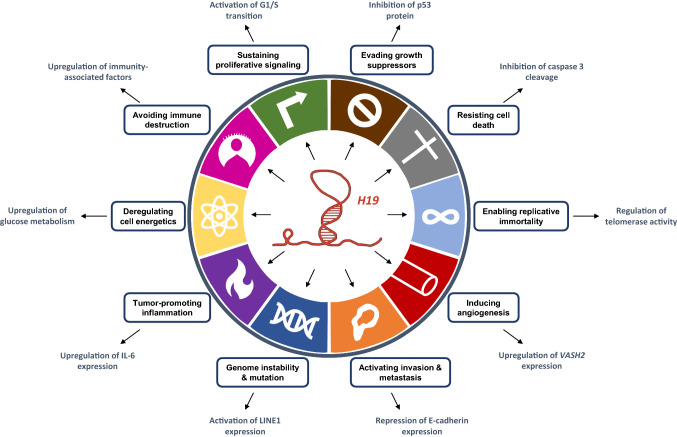
In this review, we highlight the upregulation of the long non-coding RNA H19 and its strong implication in cancer progression. H19 acts through various mechanisms such as interaction with proteins and/or miRNAs or the action of H19-derived miR-675 to sustain the hallmarks of cancer (Fig. 5). The presence of H19 in exosomes, involved in tumor progression, reinforces its importance in this pathology. Although further studies are needed to deepen our knowledge on the function of H19 in a more integrative manner, H19 has already attracted strong interest in terms of clinical application. Thus, plasma levels of H19 have been proposed as a predictive marker for breast, stomach, and lung cancers and as a tool to monitor the evolution of cancer [38, 46, 52]. Moreover, by using a plasmid approach that allows the expression of diphtheria toxin A-chain gene under the control of H19 promoter (DTA-H19/BC-819) to selectively kill H19-expressing cells, Sorin et al. showed a significant reduction of liver metastasis growth in treated animals [105]. This strategy is currently in phase 2b clinical trial for bladder cancer and in phase 1/2a trial for ovarian and pancreatic cancers [106, 107]. Clearly, a better understanding of the mechanisms of action of H19 and the molecular pathways altered by H19 or miR-675 could provide new therapeutic targets and strategies in the development of personalized management of cancer.
Fig. 5.
Implication of the long non-coding RNA H19 in the hallmarks of cancer. For each hallmark is figured a representative example of H19 mechanism of action
Acknowledgements
This work was supported by INSERM and grants from “Canceropôle Nord-Ouest (Grant No. 2017)” and “Ligue contre le cancer (Grant No. 2019)”. CL was supported by doctoral fellowships from the University of Lille.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.The ENCODE Project Consortium Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu H, Fang X. A brief review on the human encyclopedia of DNA elements (ENCODE) project. Genom Proteom Bioinform. 2013;11:135–141. doi: 10.1016/j.gpb.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 6.Scott MS, Ono M. From snoRNA to miRNA: dual function regulatory non-coding RNAs. Biochimie. 2011;93:1987–1992. doi: 10.1016/j.biochi.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hon C-C, Ramilowski JA, Harshbarger J, Bertin N, Rackham OJL, Gough J, et al. An atlas of human long non-coding RNAs with accurate 5′ ends. Nature. 2017;543:199–204. doi: 10.1038/nature21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Yang L, Chen L-L. The diversity of long noncoding RNAs and their generation. Trends Genet. 2017;33:540–552. doi: 10.1016/j.tig.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 10.Luo Q, Chen Y. Long noncoding RNAs and Alzheimer’s disease. Clin Investig Aging. 2016;11:867–872. doi: 10.2147/CIA.S107037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Luo Y, Mao Y, Ji J. The link between long noncoding RNAs and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2017;73:73–78. doi: 10.1016/j.pnpbp.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Mirza AH, Kaur S, Pociot F. Long non-coding RNAs as novel players in β cell function and type 1 diabetes. Hum Genom. 2017 doi: 10.1186/s40246-017-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.can-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y, Zhang P, Wang F, Yang J, Yang Z, Qin H. The relationship between early embryo development and tumourigenesis. J Cell Mol Med. 2010;14:2697–2701. doi: 10.1111/j.1582-4934.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature. 1991;351:153. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- 16.Delaval K, Wagschal A, Feil R. Epigenetic deregulation of imprinting in congenital diseases of aberrant growth. BioEssays News Rev Mol Cell Dev Biol. 2006;28:453–459. doi: 10.1002/bies.20407. [DOI] [PubMed] [Google Scholar]
- 17.Brannan CI, Dees EC, Ingram RS, Tilghman SM. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10:28–36. doi: 10.1128/MCB.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vennin C, Dahmani F, Spruyt N, Adriaenssens E. Role of long non-coding RNA in cells: example of the H19/IGF2 locus. Adv Biosci Biotechnol. 2013;04:34–44. doi: 10.4236/abb.2013.45A004. [DOI] [Google Scholar]
- 19.Rainier S, Johnson LA, Dobry CJ, Ping AJ, Grundy PE, Feinberg AP. Relaxation of imprinted genes in human cancer. Nature. 1993;362:747–749. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- 20.Raveh E, Matouk IJ, Gilon M, Hochberg A. The H19 long non-coding RNA in cancer initiation, progression and metastasis—a proposed unifying theory. Mol Cancer. 2015 doi: 10.1186/s12943-015-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adriaenssens E, Dumont L, Lottin S, Bolle D, Leprêtre A, Delobelle A, et al. H19 overexpression in breast adenocarcinoma stromal cells is associated with tumor values and steroid receptor status but independent of p53 and Ki-67 expression. Am J Pathol. 1998;153:1597–1607. doi: 10.1016/S0002-9440(10)65748-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lottin S, Adriaenssens E, Dupressoir T, Berteaux N, Montpellier C, Coll J, et al. Overexpression of an ectopic H19 gene enhances the tumorigenic properties of breast cancer cells. Carcinogenesis. 2002;23:1885–1895. doi: 10.1093/carcin/23.11.1885. [DOI] [PubMed] [Google Scholar]
- 23.Cooper MJ, Fischer M, Komitowski D, Shevelev A, Schulze E, Ariel I, et al. Developmentally imprinted genes as markers for bladder tumor progression. J Urol. 1996;155:2120–2127. doi: 10.1016/S0022-5347(01)66120-2. [DOI] [PubMed] [Google Scholar]
- 24.Ariel I, Miao HQ, Ji XR, Schneider T, Roll D, de Groot N, et al. Imprinted H19 oncofetal RNA is a candidate tumour marker for hepatocellular carcinoma. Mol Pathol. 1998;51:21–25. doi: 10.1136/mp.51.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo M, Suzuki H, Ueda R, Osada H, Takagi K, Takahashi T, et al. Frequent loss of imprinting of the H19 gene is often associated with its overexpression in human lung cancers. Oncogene. 1995;10:1193–1198. [PubMed] [Google Scholar]
- 26.Hibi K, Nakamura H, Hirai A, Fujikake Y, Kasai Y, Akiyama S, et al. Loss of H19 imprinting in esophageal cancer. Cancer Res. 1996;56:480–482. [PubMed] [Google Scholar]
- 27.Liu F, Pan H, Xia G, Qiu C, Zhu Z. Prognostic and clinicopathological significance of long noncoding RNA H19 overexpression in human solid tumors: evidence from a meta-analysis. Oncotarget. 2016;7:83177–83186. doi: 10.18632/oncotarget.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang T, Zhou J, Zhang W, Lin J, Ma J, Wen X, et al. H19 overexpression promotes leukemogenesis and predicts unfavorable prognosis in acute myeloid leukemia. Clin Epigenet. 2018 doi: 10.1186/s13148-018-0486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Li Y, Li Y, Ren K, Li X, Han X, et al. Long non-coding RNA H19 promotes the proliferation and invasion of breast cancer through upregulating DNMT1 expression by sponging miR-152. J Biochem Mol Toxicol. 2017;31:e21933. doi: 10.1002/jbt.21933. [DOI] [PubMed] [Google Scholar]
- 30.Jia P, Cai H, Liu X, Chen J, Ma J, Wang P, et al. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 2016;381:359–369. doi: 10.1016/j.canlet.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Yan Y, Hu M, Chen X, Wang Y, Dai Y, et al. Increased level of H19 long noncoding RNA promotes invasion, angiogenesis, and stemness of glioblastoma cells. J Neurosurg. 2016;124:129–136. doi: 10.3171/2014.12.JNS1426. [DOI] [PubMed] [Google Scholar]
- 32.Tan D, Wu Y, Hu L, He P, Xiong G, Bai Y, et al. Long noncoding RNA H19 is up-regulated in esophageal squamous cell carcinoma and promotes cell proliferation and metastasis. Dis Esophagus. 2017;30:1–9. doi: 10.1111/dote.12481. [DOI] [PubMed] [Google Scholar]
- 33.Zhang D-M, Lin Z-Y, Yang Z-H, Wang Y-Y, Wan D, Zhong J-L, et al. IncRNA H19 promotes tongue squamous cell carcinoma progression through β-catenin/GSK3β/EMT signaling via association with EZH2. Am J Transl Res. 2017;9:3474–3486. [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, Cao L, Qiu L, Dai X, Ma L, Zhou Y, et al. Upregulation of H19 promotes invasion and induces epithelial-to-mesenchymal transition in esophageal cancer. Oncol Lett. 2015;10:291–296. doi: 10.3892/ol.2015.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun J, Lian M, Ma H, Wang R, Ma Z, Wang H, et al. Competing endogenous RNA network analysis of CD274, IL-10 and FOXP3 co-expression in laryngeal squamous cell carcinoma. Mol Med Rep. 2018;17:3859–3869. doi: 10.3892/mmr.2017.8307. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Cheng N, Li X, Pan H, Li C, Ren S, et al. Correlation of long non-coding RNA H19 expression with cisplatin-resistance and clinical outcome in lung adenocarcinoma. Oncotarget. 2016;8:2558–2567. doi: 10.18632/oncotarget.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu Y, Wang W, Li X, Liu Y, Niu Y, Zhang B, et al. LncRNA H19 interacts with S-adenosylhomocysteine hydrolase to regulate LINE-1 methylation in human lung-derived cells exposed to Benzo[a]pyrene. Chemosphere. 2018;207:84–90. doi: 10.1016/j.chemosphere.2018.05.048. [DOI] [PubMed] [Google Scholar]
- 38.Luo J, Li Q, Pan J, Li L, Fang L, Zhang Y. Expression level of long noncoding RNA H19 in plasma of patients with nonsmall cell lung cancer and its clinical significance. J Cancer Res Ther. 2018;14:860–863. doi: 10.4103/jcrt.JCRT_733_17. [DOI] [PubMed] [Google Scholar]
- 39.Matouk IJ, Raveh E, Abu-lail R, Mezan S, Gilon M, Gershtain E, et al. Oncofetal H19 RNA promotes tumor metastasis. Biochim Biophys Acta BBA Mol Cell Res. 2014;1843:1414–1426. doi: 10.1016/j.bbamcr.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Berteaux N, Lottin S, Monté D, Pinte S, Quatannens B, Coll J, et al. H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J Biol Chem. 2005;280:29625–29636. doi: 10.1074/jbc.M504033200. [DOI] [PubMed] [Google Scholar]
- 41.Basak P, Chatterjee S, Weger S, Bruce MC, Murphy LC, Raouf A. Estrogen regulates luminal progenitor cell differentiation through H19 gene expression. Endocr Relat Cancer. 2015;22:505–517. doi: 10.1530/erc-15-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun H, Sun H, Wang G, Wang G, Peng Y, Peng Y, et al. H19 lncRNA mediates 17β-estradiol-induced cell proliferation in MCF-7 breast cancer cells. Oncol Rep. 2015;33:3045–3052. doi: 10.3892/or.2015.3899. [DOI] [PubMed] [Google Scholar]
- 43.Si X, Zang R, Zhang E, Liu Y, Shi X, Zhang E, et al. LncRNA H19 confers chemoresistance in ERα-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. Oncotarget. 2016;7:81452–81462. doi: 10.18632/oncotarget.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vennin C, Spruyt N, Robin Y-M, Chassat T, Le Bourhis X, Adriaenssens E. The long non-coding RNA 91H increases aggressive phenotype of breast cancer cells and up-regulates H19/IGF2 expression through epigenetic modifications. Cancer Lett. 2017;385:198–206. doi: 10.1016/j.canlet.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 45.Vennin C, Spruyt N, Dahmani F, Julien S, Bertucci F, Finetti P, et al. H19 non coding RNA-derived miR-675 enhances tumorigenesis and metastasis of breast cancer cells by downregulating c-Cbl and Cbl-b. Oncotarget. 2015;6:29209–29223. doi: 10.18632/oncotarget.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang K, Luo Z, Zhang Y, Zhang L, Wu L, Liu L, et al. Circulating lncRNA H19 in plasma as a novel biomarker for breast cancer. Cancer Biomark. 2016;17:187–194. doi: 10.3233/CBM-160630. [DOI] [PubMed] [Google Scholar]
- 47.Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J, et al. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012;279:3159–3165. doi: 10.1111/j.1742-4658.2012.08694.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu G, Xiang T, Wu Q-F, Wang W-X. Long noncoding RNA H19-derived miR-675 enhances proliferation and invasion via RUNX1 in Gastric cancer cells. Oncol Res Featur Preclin Clin Cancer Ther. 2016;23:99–107. doi: 10.3727/096504015X14496932933575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan J, Zhang Y, She Q, Li X, Peng L, Wang X, et al. Long noncoding RNA H19/miR-675 axis promotes gastric cancer via FADD/caspase 8/caspase 3 signaling pathway. Cell Physiol Biochem. 2017;42:2364–2376. doi: 10.1159/000480028. [DOI] [PubMed] [Google Scholar]
- 50.Li H, Yu B, Li J, Su L, Yan M, Zhu Z, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishii S, Yamashita K, Harada H, Ushiku H, Tanaka T, Nishizawa N, et al. The H19-PEG10/IGF2BP3 axis promotes gastric cancer progression in patients with high lymph node ratios. Oncotarget. 2017;8:74567–74581. doi: 10.18632/oncotarget.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou X, Yin C, Dang Y, Ye F, Zhang G. Identification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancer. Sci Rep. 2015;5:11516. doi: 10.1038/srep11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramani K, Mavila N, Ko KS, Mato JM, Lu SC. Prohibitin 1 regulates the H19-Igf2 axis and proliferation in hepatocytes. J Biol Chem. 2016;291:24148–24159. doi: 10.1074/jbc.M116.744045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pu H, Zheng Q, Li H, Wu M, An J, Gui X, et al. CUDR promotes liver cancer stem cell growth through upregulating TERT and C-Myc. Oncotarget. 2015;6:40775–40798. doi: 10.18632/oncotarget.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015 doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, Li J, Jia S, Wu M, An J, Zheng Q, et al. miR675 upregulates long noncoding RNA H19 through activating EGR1 in human liver cancer. Oncotarget. 2015;6:31958–31984. doi: 10.18632/oncotarget.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song Y, Liu C, Liu X, Trottier J, Beaudoin M, Zhang L, et al. H19 promotes cholestatic liver fibrosis by preventing ZEB1-mediated inhibition of EpCAM. Hepatol Baltim. 2017;66:1183–1196. doi: 10.1002/hep.29209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Liu C, Barbier O, Smalling R, Tsuchiya H, Lee S, et al. Bcl2 is a critical regulator of bile acid homeostasis by dictating Shp and lncRNA H19 function. Sci Rep. 2016;6:20559. doi: 10.1038/srep20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han Y, Ma J, Wang J, Wang L. Silencing of H19 inhibits the adipogenesis and inflammation response in ox-LDL-treated Raw264.7 cells by up-regulating miR-130b. Mol Immunol. 2018;93:107–114. doi: 10.1016/j.molimm.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 60.Xu Y, Wang Z, Jiang X, Cui Y. Overexpression of long noncoding RNA H19 indicates a poor prognosis for cholangiocarcinoma and promotes cell migration and invasion by affecting epithelial-mesenchymal transition. Biomed Pharmacother. 2017;92:17–23. doi: 10.1016/j.biopha.2017.05.061. [DOI] [PubMed] [Google Scholar]
- 61.Wang W-T, Ye H, Wei P-P, Han B-W, He B, Chen Z-H, et al. LncRNAs H19 and HULC, activated by oxidative stress, promote cell migration and invasion in cholangiocarcinoma through a ceRNA manner. J Hematol Oncol. 2016 doi: 10.1186/s13045-016-0348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma L, Tian X, Wang F, Zhang Z, Du C, Xie X, et al. The long noncoding RNA H19 promotes cell proliferation via E2F-1 in pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2016;17:1051–1061. doi: 10.1080/15384047.2016.1219814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He H, Wang N, Yi X, Tang C, Wang D. Long non-coding RNA H19 regulates E2F1 expression by competitively sponging endogenous miR-29a-3p in clear cell renal cell carcinoma. Cell Biosci. 2017 doi: 10.1186/s13578-017-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lv M, Zhong Z, Huang M, Tian Q, Jiang R, Chen J. lncRNA H19 regulates epithelial–mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim Biophys Acta BBA Mol Cell Res. 2017;1864:1887–1899. doi: 10.1016/j.bbamcr.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Elkin M, Ariel I, Miao H-Q, Nagler A, Pines M, de Groot N, et al. Inhibition of bladder carcinoma angiogenesis, stromal support, and tumor growth by halofuginone. Cancer Res. 1999;59:4111–4118. [PubMed] [Google Scholar]
- 66.Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333:213–221. doi: 10.1016/j.canlet.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 67.Han D, Gao X, Wang M, Qiao Y, Xu Y, Yang J, et al. Long noncoding RNA H19 indicates a poor prognosis of colorectal cancer and promotes tumor growth by recruiting and binding to eIF4A3. Oncotarget. 2016;7:22159–22173. doi: 10.18632/oncotarget.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang W, Ning N, Jin X. The lncRNA H19 Promotes cell proliferation by competitively binding to miR-200a and derepressing β-catenin expression in colorectal cancer. BioMed Res Int. 2017 doi: 10.1155/2017/2767484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsang WP, Ng EKO, Ng SSM, Jin H, Yu J, Sung JJY, et al. Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis. 2010;31:350–358. doi: 10.1093/carcin/bgp181. [DOI] [PubMed] [Google Scholar]
- 70.Ou L, Wang D, Zhang H, Yu Q, Hua F. Decreased expression of miR-138-5p by lncRNA H19 in cervical cancer promotes tumor proliferation. Oncol Res Featur Preclin Clin Cancer Ther. 2018;26:401–410. doi: 10.3727/096504017X15017209042610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu Z, Song L, He J, Sun Y, Liu X, Zou X. Ectopic expressed long non-coding RNA H19 contributes to malignant cell behavior of ovarian cancer. Int J Clin Exp Pathol. 2015;8:10082–10091. [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng Z-G, Xu H, Suo S-S, Xu X-L, Ni M-W, Gu L-H, et al. The essential role of H19 contributing to Cisplatin resistance by regulating glutathione metabolism in high-grade serous ovarian cancer. Sci Rep. 2016;6:26093. doi: 10.1038/srep26093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo G, Kang Q, Chen Q, Chen Z, Wang J, Tan L, et al. High expression of long non-coding RNA H19 is required for efficient tumorigenesis induced by Bcr-Abl oncogene. FEBS Lett. 2014;588:1780–1786. doi: 10.1016/j.febslet.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 74.El Hajj J, Nguyen E, Liu Q, Bouyer C, Adriaenssens E, Hilal G, et al. Telomerase regulation by the long non-coding RNA H19 in human acute promyelocytic leukemia cells. Mol Cancer. 2018 doi: 10.1186/s12943-018-0835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li M, Chen H, Zhao Y, Gao S, Cheng C. H19 functions as a ceRNA in Promoting metastasis through decreasing miR-200s activity in osteosarcoma. DNA Cell Biol. 2016;35:235–240. doi: 10.1089/dna.2015.3171. [DOI] [PubMed] [Google Scholar]
- 76.Luan W, Zhou Z, Ni X, Xia Y, Wang J, Yan Y, et al. Long non-coding RNA H19 promotes glucose metabolism and cell growth in malignant melanoma via miR-106a-5p/E2F3 axis. J Cancer Res Clin Oncol. 2018;144:531–542. doi: 10.1007/s00432-018-2582-z. [DOI] [PubMed] [Google Scholar]
- 77.Angrand P-O, Vennin C, Le Bourhis X, Adriaenssens E. The role of long non-coding RNAs in genome formatting and expression. Front Genet. 2015 doi: 10.3389/fgene.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cai X, Cullen BR. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA. 2007;13:313–316. doi: 10.1261/rna.351707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen L, Wang Y, He J, Zhang C, Chen J, Shi D. Long non-coding RNA H19 promotes proliferation and invasion in human glioma cells by downregulating miR-152. Oncol Res Featur Preclin Clin Cancer Ther. 2018 doi: 10.3727/096504018x15178768577951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou X, Ye F, Yin C, Zhuang Y, Yue G, Zhang G. The interaction between MiR-141 and lncRNA-H19 in regulating cell proliferation and migration in gastric cancer. Cell Physiol Biochem. 2015;36:1440–1452. doi: 10.1159/000430309. [DOI] [PubMed] [Google Scholar]
- 81.Shi Y, Wang Y, Luan W, Wang P, Tao T, Zhang J, et al. Long non-coding RNA H19 promotes glioma cell invasion by deriving miR-675. PLoS One. 2014;9:e86295. doi: 10.1371/journal.pone.0086295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Wang X, Chen T, Jiang L, Yang Q. Huaier extract inhibits breast cancer progression through a lncRNA-H19/MiR-675-5p pathway. Cell Physiol Biochem. 2017;44:581–593. doi: 10.1159/000485093. [DOI] [PubMed] [Google Scholar]
- 83.He D, Wang J, Zhang C, Shan B, Deng X, Li B, et al. Down-regulation of miR-675-5p contributes to tumor progression and development by targeting pro-tumorigenic GPR55 in non-small cell lung cancer. Mol Cancer. 2015;14:73. doi: 10.1186/s12943-015-0342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou Y-W, Zhang H, Duan C-J, Gao Y, Cheng Y-D, He D, et al. miR-675-5p enhances tumorigenesis and metastasis of esophageal squamous cell carcinoma by targeting REPS2. Oncotarget. 2016;7:30730–30747. doi: 10.18632/oncotarget.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hernandez JM, Elahi A, Clark CW, Wang J, Humphries LA, Centeno B, et al. miR-675 mediates downregulation of twist1 and Rb in AFP-secreting hepatocellular carcinoma. Ann Surg Oncol. 2013;20:625–635. doi: 10.1245/s10434-013-3106-3. [DOI] [PubMed] [Google Scholar]
- 86.Costa V, Lo Dico A, Rizzo A, Rajata F, Tripodi M, Alessandro R, et al. MiR-675-5p supports hypoxia induced epithelial to mesenchymal transition in colon cancer cells. Oncotarget. 2017;8:24292–24302. doi: 10.18632/oncotarget.14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gong L, Bao Q, Hu C, Wang J, Zhou Q, Wei L, et al. Exosomal miR-675 from metastatic osteosarcoma promotes cell migration and invasion by targeting CALN1. Biochem Biophys Res Commun. 2018;500:170–176. doi: 10.1016/j.bbrc.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 88.Liu G, Xiang T, Wu Q-F, Wang W-X. Curcumin suppresses the proliferation of gastric cancer cells by downregulating H19. Oncol Lett. 2016;12:5156–5162. doi: 10.3892/ol.2016.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li B, Xie Z, Li B. miR-152 functions as a tumor suppressor in colorectal cancer by targeting PIK3R3. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2016;37:10075–10084. doi: 10.1007/s13277-016-4888-2. [DOI] [PubMed] [Google Scholar]
- 90.Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abu-lail R, Hochberg A, et al. The H19 non-coding RNA is essential for human tumor growth. PLoS One. 2007;2:e845. doi: 10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li W, Jiang P, Sun X, Xu S, Ma X, Zhan R. Suppressing H19 modulates tumorigenicity and stemness in U251 and U87MG glioma cells. Cell Mol Neurobiol. 2016;36:1219–1227. doi: 10.1007/s10571-015-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 93.Adriaenssens E, Lottin S, Berteaux N, Hornez L, Fauquette W, Fafeur V, et al. Cross-talk between mesenchyme and epithelium increases H19 gene expression during scattering and morphogenesis of epithelial cells. Exp Cell Res. 2002;275:215–229. doi: 10.1006/excr.2002.5500. [DOI] [PubMed] [Google Scholar]
- 94.Wang J, Zhang Y, Wei H, Zhang X, Wu Y, Gong A, et al. The mir-675-5p regulates the progression and development of pancreatic cancer via the UBQLN1-ZEB1-mir200 axis. Oncotarget. 2017;8:24978–24987. doi: 10.18632/oncotarget.15330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wolanin K, Magalska A, Mosieniak G, Klinger R, McKenna S, Vejda S, et al. Curcumin affects components of the chromosomal passenger complex and induces mitotic catastrophe in apoptosis-resistant Bcr-Abl-expressing cells. Mol Cancer Res. 2006;4:457–469. doi: 10.1158/1541-7786.MCR-05-0172. [DOI] [PubMed] [Google Scholar]
- 96.Kujundžić RN, Grbeša I, Ivkić M, Katdare M, Gall-Trošelj K. Curcumin downregulates H19 gene transcription in tumor cells. J Cell Biochem. 2008;104:1781–1792. doi: 10.1002/jcb.21742. [DOI] [PubMed] [Google Scholar]
- 97.Ravid O, Shoshani O, Sela M, Weinstock A, Sadan T, Gur E, et al. Relative genomic stability of adipose tissue derived mesenchymal stem cells: analysis of ploidy, H19 long non-coding RNA and p53 activity. Stem Cell Res Ther. 2014;5:139. doi: 10.1186/scrt529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saito K, Kawakami K, Matsumoto I, Oda M, Watanabe G, Minamoto T. Long interspersed nuclear element 1 hypomethylation is a marker of poor prognosis in stage IA non-small cell lung cancer. Clin Cancer Res. 2010;16:2418–2426. doi: 10.1158/1078-0432.CCR-09-2819. [DOI] [PubMed] [Google Scholar]
- 99.Tarnowski M, Tarnowski M, Tkacz M, Tkacz M, Czerewaty M, Czerewaty M, et al. 5-Azacytidine inhibits human rhabdomyosarcoma cell growth by downregulating insulin-like growth factor 2 expression and reactivating the H19 gene product miR-675, which negatively affects insulin-like growth factors and insulin signaling. Int J Oncol. 2015;46:2241–2250. doi: 10.3892/ijo.2015.2906. [DOI] [PubMed] [Google Scholar]
- 100.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peng F, Wang J-H, Fan W-J, Meng Y-T, Li M-M, Li T-T, et al. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene. 2018;37:1062–1074. doi: 10.1038/onc.2017.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009;125:1747–1754. doi: 10.1002/ijc.24439. [DOI] [PubMed] [Google Scholar]
- 104.Lazar DC, Morris KV, Saayman SM. The emerging role of long non-coding RNAs in HIV infection. Virus Res. 2016;212:114–126. doi: 10.1016/j.virusres.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sorin V, Ohana P, Mizrahi A, Matouk I, Birman T, Hochberg A, et al. Regional therapy with DTA-H19 vector suppresses growth of colon adenocarcinoma metastases in the rat liver. Int J Oncol. 2011;39:1407–1412. doi: 10.3892/ijo.2011.1171. [DOI] [PubMed] [Google Scholar]
- 106.Gofrit ON, Benjamin S, Halachmi S, Leibovitch I, Dotan Z, Lamm DL, et al. DNA based therapy with diphtheria toxin-A BC-819: a phase 2b marker lesion trial in patients with intermediate risk nonmuscle invasive bladder cancer. J Urol. 2014;191:1697–1702. doi: 10.1016/j.juro.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 107.Lavie O, Edelman D, Levy T, Fishman A, Hubert A, Segev Y, et al. A phase 1/2a, dose-escalation, safety, pharmacokinetic, and preliminary efficacy study of intraperitoneal administration of BC-819 (H19-DTA) in subjects with recurrent ovarian/peritoneal cancer. Arch Gynecol Obstet. 2017;295:751–761. doi: 10.1007/s00404-017-4293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]