Abstract
Matrix metalloproteinases (MMPs) have been investigated in context of chronic inflammatory diseases and demonstrated to degrade multiple components of the extracellular matrix (ECM). However, following several disappointing MMP clinical trials, recent studies have demonstrated unexpected novel functions of MMPs in viral infections and autoimmune inflammatory diseases in unanticipated locations. Thus, MMPs play additional functions in inflammation than just ECM degradation. They can regulate the activity of chemokines and cytokines of the immune response by precise proteolytic processing resulting in activation or inactivation of signaling pathways. MMPs have been demonstrated to cleave multiple substrates of the central nervous systems (CNS) and contribute to promoting and dampening diseases of the CNS. Initially, believed to be solely promoting pathologies, more than 10 MMPs to date have been shown to have protective functions. Here, we present some of the beneficial and destructive roles of MMPs in CNS pathologies and discuss strategies for the use of MMP inhibitors.
Keywords: Matrix metalloproteinase (MMP), Interferon (IFN), Inflammation, Extracellular matrix (ECM), Virus, Multiple sclerosis (MS)
Introduction
Matrix Metalloproteinases (MMPs) should no longer be regarded as being disease promoting detrimental extracellular proteases—especially given the chequered history of MMP inhibitor drugs [1]. These were developed and trialed at a time when MMPs were few in number, substrates even fewer, and their in vivo roles mostly only deduced from admittedly compelling in vitro studies. While their biological activity is linked to the balance between the levels of MMPs and their inhibitory TIMPs in inflammatory diseases, with a shift in the MMP/TIMP ratio commonly associated with disease [2, 3], the multitude of beneficial disease-dampening functions of MMPs ever increases. So much so, we contend that the major role of MMPs is in fact in temporal modulation of inflammatory and immune processes by precise regulation of the bioactivity of signaling molecules and their pathways. This is to maintain extracellular homeostasis by invoking negative feedback loops to dampen inflammation over time and stimulate tissue resolution. Overall, MMPs are multitasking proteins [4], often with moonlighting functions [5], with additional roles to mere matrix degradation in both the extra- and intra-cellular compartments. Recent evidence further pushes this paradigm shift with critical new roles revealed for MMPs in initiating and then terminating interferon responses and signaling pathways in viral infection and autoimmunity. Here, we describe these in relation to diseases of the central nervous system (CNS).
MMPs: inside the matrix
The CNS is composed of the brain and spinal cord, both structurally and functionally unique organs, and central to life and thought. One arm of neuroscience is to understand the complex neural circuitry of brain and spinal cord. Many studies intend to decipher psychiatric and neurological disorders; however, the underlying molecular mechanisms of various neuropathologies have yet to be elucidated. Nonetheless, given the unimaginable complexity of ~ 100 trillion neurons and their connections, relatively few neurological disorders and disease occur. In the healthy CNS, the microenvironment is a guiding factor that affects neurological development and function [6]. Though not a prominent anatomical feature of the CNS, the regulation and remodeling of the neural extracellular matrix (ECM) are essential to the maintenance of homeostasis in the brain and thought [6–10].
MMPs are key enzymes influencing physiological and pathological processes due to their proteolytic remodeling capabilities [6, 10, 11]. Despite recent advances in understanding that the ECM serves more than a simple role in cell adhesion, structural integrity, and cell signaling [7, 11], the significance of neural ECM signaling and interactions between neural cells remains elusive. MMPs are secreted by many neural cells [12] and contribute to early CNS development as well as synaptic remodeling that continuously shapes the brain throughout adulthood [6, 12–16]. Several studies demonstrate that MMP-mediated proteolysis drives the structural and functional changes that occur during the development and homeostasis of the CNS [17–20].
An important component of embryonic development is the neural stem cell niche that provides a continual supply of new neural cells, including neurons and glia, for the postnatal brain. The maintenance of this stem cell niche is highly dependent on micro-environmental cues and cell to cell interactions [21]. The specific organization of cytoarchitecture and ECM environment delineating the niche guides the fate of neural stem cells and plays a role in regulating their regenerative potential. MMPs have long been ascribed as proteases primarily responsible for the turnover and remodeling of ECM substrates [21], a function that is crucial in the development and maturation of stem cell populations in processes such as neurogenesis. To generate the adult neural stem cell niche, the early postnatal ventricular–subventricular zone (V-SVZ) undergoes rapid and complex reorganization. MMP-12 has been implicated in several aspects of this process. Both intracellular and extracellular MMP-12 is involved in guiding the fate of postnatal stem cell niches in the V-SVZ of the brain [21], owing to its ability to remodel the ECM and inactivate protease inhibitors. Furthermore, elevated MMP-12 expression has been identified in developing ependymal cells (ECs) that line the cerebrospinal fluid filled ventricles in the brain, suggesting another role of MMP-12 in regulating the maturation of ECs [21].
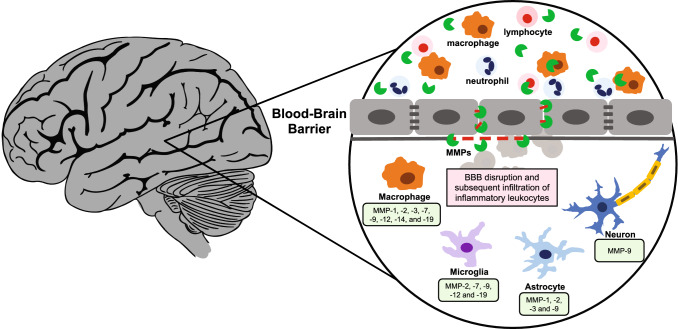
Recently, studies have begun to elucidate the role that MMPs play in regulating neural circuit remodeling [12, 17–21]. MMP-9 has been involved in hippocampal synaptic plasticity and plasticity related processes such as long-term potentiation in murine models [17–19]. Several studies have characterized the expression of MMPs across the brain, with many revealing that the zymogen forms of MMPs are more commonly present in comparison with their active counterparts [6], although there are many stimuli within the neural environment that can cause the activation of MMPs and the precise substrates of these active MMPs remain widely unknown and unexplored [10, 22, 23]. In addition, the localization and function of MMPs vary greatly across differing brain regions [7]. For example, although MMP-9 may support beneficial physiological processes such as the maintenance of synaptic plasticity in the hippocampus [17–19], it has also been suggested to facilitate blood brain barrier (BBB) disruption in neurodegenerative diseases such as multiple sclerosis (Fig. 1) [24–27] and in collagen scanning [28, 29]. It is, therefore, crucial to further characterize the function of individual MMPs and their roles within the Matrix; it is also important to consider that maladaptive remodeling of the neural ECM may contribute to diseases of the CNS.
Fig. 1.
Schematic representation of diverse immune cells secreting proteases and break down the blood brain barrier. Proteases are depicted as green pacmans
MMPs: outside the matrix
Matrix metalloproteinases, as their name suggests, cleave multiple ECM proteins and so remodel the matrix. However, only ~ 27% of MMP substrates are ECM and ECM-associated proteins, whereas 73% of the known MMP substrates are non-ECM proteins [30]. These include chemokines, cytokines, cell-surface receptors, angiogenic factors, aminoacyl transferases, growth factors, and proteins involved in immune signaling [1, 30, 31]. Importantly, MMPs should not be regarded as just detrimental in inflammatory diseases, as multiple beneficial roles for MMPs have been characterized [1, 32]. The tight regulation of MMPs is fundamental to ensure that both their beneficial and detrimental roles are exerted in moderation. Changes in the MMP/TIMP ratios can reveal key biological functions [33, 34]; it is now well characterized that MMPs can be both drug targets and anti-targets depending on the tissue localization, cell types, and stage of the disease [1, 32]. This concept is highlighted by a series of papers describing the roles of MMPs in cleaving and modulating the biological activity of virtually all of the 54 human chemokines [35]. Thus, neutrophils are attracted to sites of injury or infection by 8 CXCL chemokines, one of which, IL8 (CXCL8) is activated by neutrophil-specific MMP-8 in a feedforward mechanism [36], but in vivo, MMP-8’s major role is to inactivate the cognate serpin inhibitor of elastase, alpha 1 antitrypsin [37], the more potent activator of IL8. All eight of the Glu-Leu-Arg (ELR) + CXC chemokine chemoattractants for neutrophils are cleaved and inactivated by macrophage-specific MMP-12 in a feedback loop [38]. Two CCL chemokines, CCL15 and CCL23, are activated by MMP activity to chemoattract macrophages, the most potent being MMP-12 in a feedforward mechanism [39]. Multiple MMPs can cleave and inactivate CCL chemokines, switching these to antagonists, to terminate macrophage infiltration [40, 41]. Other examples include SDF1alpha and beta inactivation by MMP-2 and other MMPs, and the shedding of membrane anchored CX3CL (fractalkine) by MMP-2 which generates a soluble antagonist chemokine [42].
Finally, non-proteolytic roles of MMPs have also been identified and are implicated in cell adhesion, proMMP activation, cell migration, and invasion [43–45]. The hemopexin C-terminal domain [46–48] of MMP-14 binds native type I collagen and opposes MMP cleavage of collagen, whereas the fibronectin triple repeats of MMPs facilitate MMP-1 cleavage of triple helical collagen by opening up the helix [49]. Thus, MMPs are no longer mere matrix degraders but have been widely demonstrated to play key roles in the initiation and resolution of inflammation. It is now time to exit the Matrix and to start characterizing the misunderstood roles of MMPs outside the Matrix.
MMPs are central in the nervous system
MMPs are typically expressed at low levels in the healthy adult CNS. However, following injury or neurological disorders, the protein levels of various MMPs become modulated (Tables 1, 2). Typically, MMP-9 is hardly detectable in the healthy CNS, but is upregulated in diseases such as multiple sclerosis [26, 50]. Although MMP-9 can be expressed in epithelial or endothelial cells, the increase of MMP-9 levels is most likely due to the infiltration of neutrophils, monocytes, and macrophages to the site of injury or inflammation in the brain following disruption of the BBB (Fig. 1) [51–54]. Given its ability to degrade the ECM and tight junction proteins, MMP-9 has been directly implicated in mediating BBB permeability, although this effect could be partially linked to MMP-2 as well [55–58]. In healthy individuals, the highly selective properties of the microvasculature of the CNS allows for the transport of ions, metabolites and cells into the delicate tissues of the brain and spinal cord to be tightly regulated [59]. Damage to this barrier, potentially through the aberrant activity of MMPs and additional proteases, permits the infiltration of inflammatory leukocytes into the CNS that may drastically enhance the neuroinflammatory response, culminating in the onset of CNS disease [60].
Table 1.
Roles and expression of selected MMPs in human CNS diseases
| Human MMPs | Disease | Biological roles and references |
|---|---|---|
| MMP-1 | Alzheimer’s disease | ↑ Levels Alzheimer’s disease cortex [74] |
| MMP-2 | Amyotrophic lateral sclerosis | ↓ During duration of disease [75] |
| HIV/AIDS | ↑ Levels in HIV-associated demented patients [76]; ↑ neuronal apoptosis [77] | |
| Multiple sclerosis | Unchanged mRNA in MS brain lesions [24] | |
| Stroke | ↑ Activity in infarcted cerebral tissue [78] | |
| MMP-3 | Multiple sclerosis | Unchanged mRNA in MS brain lesions [24] |
| MMP-7 | HIV/AIDS | ↑ levels in HIV-associated demented patients [76] |
| Multiple sclerosis | ↑ mRNA levels in MS [24] | |
| MMP-9 | Acute disseminated encephalomyelitis | ↑ Serum levels at acute stage [79] |
| Amyotrophic lateral sclerosis | ↑ levels in CSF in patients with rapid progression of disease [75] | |
| HIV/AIDS | ↑ Levels in HIV-associated demented patients [76] | |
| Multiple sclerosis | ↑ In cerebrospinal fluid (CSF) [50, 80–83]; ↑ mRNA and plasma protein levels in MS patients [24, 26, 84, 85]; ↑ protein levels in serum/leukocytes of MS patients [65, 86] | |
| Seizure | ↑ Levels in seizure patients [87] | |
| Stroke | ↑ Activity [78] and levels [88] in infarcted cerebral tissue | |
| MMP-10 | Stroke | ↑ Levels in infarcted cerebral neurons [88]; proMMP10 as a marker following acute ischemic stroke [89] |
| MMP-12 | Multiple sclerosis | ↑ in active demyelinating lesions [90] |
| MMP-23 | K+ channels regulation | Blocks K+ channels [91] |
| MMP-28 | Multiple sclerosis | ↑ Expression within demyelinated lesions [92] |
| TIMP-1 | Acute disseminated encephalomyelitis | ↑ Serum levels at acute stage [79] |
| Multiple sclerosis | Unchanged mRNA in plasma [26]; ↑ protein levels in serum of MS patients [86]; ↓ protein levels in serum of MS patients [93] | |
| TIMP-2 | Multiple sclerosis | mRNA unchanged but ↑ unbound TIMP2 in plasma of MS patients [26]; Elevated protein levels in serum of MS patients [86] |
| Stroke | ↑ Levels in infarcted cerebral tissue [88] |
Table 2.
Roles and expression of selected MMPs in Mouse and Rat CNS disease models
| Mouse/rat MMPs | Model or disease | Biological roles and references |
|---|---|---|
| MMP-2 | Experimental autoimmune encephalomyelitis | Unchanged mRNA levels during disease course in rats [94] |
| Focal cerebral ischemia | ↑ Expression in rat [57] | |
| Spinal-cord injury | Mmp2−/− mice have ↓ recovery [95] | |
| MMP-3 | Cuprizone model of toxic demyelination | ↑ mRNA expression in the early phases of demyelination and the remyelination phase in mice corpus callosum. ↑ protein levels in astrocytes [96] |
| Experimental autoimmune encephalomyelitis | Unchanged mRNA levels during disease course in rats [94] but elevated in mice [97] | |
| Neuroinflammation | ↓ Neutrophils count in Mmp3−/− in comparison with wild-type mice [98] | |
| MMP-7 | Cuprizone model of toxic demyelination | Unchanged mRNA in this model [96] |
| Experimental autoimmune encephalomyelitis | ↑ Expression in rat CNS during the development of symptoms [94] but not in mice [97]; Mmp7−/− mice are resistant to EAE [99] | |
| MMP-8 | Experimental autoimmune encephalomyelitis | Mmp8−/− mice exhibit ↓ in the number of CNS-infiltrating cells and demyelinating lesions as compared to wild-type counterparts [100] |
| MMP-9 | Alzheimer’s disease | MMP-9 rescued insulin survival signaling in vitro and in early stages in the 5XFAD model of AD [101] |
| Amyotrophic lateral sclerosis | ↑ Motor neuron disease and ↓survival in Mmp9−/− mice [102] | |
| Epilepsy | ↓ Kindled seizure progression in Mmp9−/− mice [103, 104] | |
| Experimental autoimmune encephalomyelitis | ↑Expression in rat and mice CNS during the development of symptoms [94, 97]; ↓ severity in Mmp9−/− mice [105, 106] | |
| Focal cerebral ischemia | ↑ Expression in rat [57]; ↓ ischemic lesion volumes in Mmp9−/− compared with wild type littermates [107] | |
| MMP-10 | Cuprizone model of toxic demyelination | Unchanged mRNA in this model [96] |
| MMP-11 | Cerebral artery occlusion | ↑ Levels following stroke [108] |
| Cuprizone model of toxic demyelination | ↑ mRNA expression in the remyelination phase in mice corpus callosum [96] | |
| Experimental autoimmune encephalomyelitis | Unchanged mRNA levels during disease course in rats [94] | |
| MMP-12 | Aging neuroinflammation | ↑ Cerebral mRNA and protein expression during aging [109] |
| Cuprizone model of toxic demyelination | ↑ mRNA expression in the early phases of demyelination mice cortex and both in the corpus callosum and cortex in the peak of demyelination. ↑ protein levels in microglia, astrocytes and cells of oligodendrocyte lineage [96] | |
| Experimental autoimmune encephalomyelitis |
↑ Expression in rat and mice CNS during the development of symptoms [94, 97] ↑ Severity and disease burden in Mmp12−/− mice as compared to wild-types [110–112] |
|
| Ischemic stroke | ↑ In middle cerebral artery occlusion subjected rats [113] | |
| Spinal cord injury | ↑ Functional recovery of hindlimb strength in Mmp12−/− mice as compared to wild-types [114] | |
| MMP-13 | Cuprizone model of toxic demyelination | Unchanged mRNA in this model [96] |
| Experimental autoimmune encephalomyelitis | Unchanged mRNA levels during disease course in rats [94] | |
| MMP-14/MT1-MMP | Cuprizone model of toxic demyelination | ↑ mRNA expression in the early phases of demyelination and the remyelination phase in mice corpus callosum [96] |
| MMP-15/MT2-MMP | Cuprizone model of toxic demyelination | ↓ mRNA expression in the peak of demyelination in mice [96], |
| MMP-24/MT5-MMP | Cuprizone model of toxic demyelination | ↓ mRNA expression in the peak of demyelination in mice [96], ↑ promotes pro-amyloidogenic regulation of APP metabolism and Mt5-mmp−/− mice rescued amyloid pathology, cognitive decline and inflammation [115]. |
| Sciatic nerve injury | Mt5-mmp−/− mice did not develop neuropathic pain after sciatic nerve injury [116] | |
| Thermal pain stimulation | Mt5-mmp−/− mice displayed ↑ sensitivity to noxious thermal stimuli [117] | |
| MMP-25/MT6-MMP | Experimental autoimmune encephalomyelitis | ↑ Proteolysis inactivates crystallin-αβ that is a suppressor of MS [118] |
| MMP-28 | Experimental autoimmune encephalomyelitis | ↑ Expression within demyelinated lesions [92] |
| TIMP-1 | Cuprizone model of toxic demyelination | ↑ mRNA expression in the early phases of demyelination mice cortex and both in the corpus callosum and cortex in the peak of demyelination [96] |
| Epileptic rodent model | ↑ Expression to regulate the nervous system [119] | |
| Experimental autoimmune encephalomyelitis | ↑ Expression in mice CNS during the development of symptoms [97] | |
| TIMP-2 | Cuprizone model of toxic demyelination | ↑ mRNA expression in the peak of demyelination in mice corpus callosum [96] |
| TIMP-3 | Cuprizone model of toxic demyelination | ↑ mRNA expression in the early phases of demyelination in corpus callosum and cortex, and the remyelination phase in mice corpus callosum [96] |
| TIMP-4 | Cuprizone model of toxic demyelination | ↑ mRNA expression in the early phases of demyelination in cortex and the remyelination phase in mice corpus callosum [96] |
Multiple sclerosis (MS) is one of many neuroinflammatory diseases in which aberrant MMP activity has been characterized (Table 1) [61]. Experimental autoimmune encephalomyelitis (EAE) is a widely utilized murine model used to study the pathogenesis of human MS (Table 2) [62]. MMPs have been implicated in the pathogenesis of MS due to their ability to cause loss of BBB integrity and propagate the neuroinflammatory environment [63]. Fragmentation of myelin as a result of MMP-mediated proteolysis has also been implicated in the immunopathogenesis of MS [64], primarily due to the supporting evidence of increased levels of proteases in the brains of MS patients [27, 64, 65] and the ability of these enzymes to enhance the destruction of the myelin sheath and release immunogenic peptides [64, 66]. Most MMPs, including MMP-2, MMP-3, and MMP-9, can cleave myelin basic protein (MBP) to release peptides that contain immunodominant epitopes (Table 3) [64, 66–68]. Interestingly, the charge micro-heterogeneity of MBP may make it more susceptible to MMP cleavage [69]. The previous studies have also suggested that proteolytic cleavage of myelin-derived antigens prior to their ingestion by antigen-presenting cells may influence the strength and specificity of the subsequent immune response [70–72], for example, MT3-MMP via the Nogo-66 receptor cleavage [73]. Thus, classifying the posttranslational modifications that affect the functions, charges, and generation of MBP isoforms that are vulnerable to proteolytic degradation may be a novel approach to gain a better understanding of the underlying biological mechanisms in MS. However, the role of MMPs in regulating neuronal inflammatory cells that then effect destruction remains largely ignored.
Table 3.
Selected substrates of MMPs related to the CNS
| Substrates: gene name | Substrates: protein name | MMPs that can cleave the substrate | References |
|---|---|---|---|
| APP | Amyloid protein precursor | MMP-1, -2, -3, -9, -14, -16, -24 | [120–124] |
| CRYAB | Alpha-crystallin B chain | MMP-9, -25 | [118, 125] |
| DAG1 | Dystroglycan | MMP-2, -9 | [126–128] |
| ENO2 | Gamma-enolase | MMP-1, -2, -8, -9, 14 | [129] |
| GRIN1 | Glutamate receptor ionotropic, NMDA 1/N-methyl-D-aspartate receptor | MMP-7 | [130] |
| IFNA | Interferon alpha | MMP-12 | [131] |
| IFNB | Interferon beta | MMP-9 | [132] |
| IFNG | Interferon gama | MMP-12 | [31] |
| IL1B | Interleukin-1 beta | MMP-1, -2, -3, -9, -14 | [133–135] |
| MAG | Myelin-associated glycoprotein | MMP-2, -7, -9 | [136] |
| MBP | Myelin basic protein | MMP-1, -2, -3, -7, -8, -9, -10, -12, -14, -15, -16, -17, -24, -25 | [64, 66, 67, 69, 118, 137–139] |
| SNAP25 | Synaptosomal-associated protein 25 | MMP-7 | [140] |
| SNCA | Alpha-synuclein | MMP-1, -2, -3, -9, -14 | [141, 142] |
| TAC1 | Substance P of Protachykinin-1 | MMP-8, -9 | [143, 144] |
| TJP1 | Tight junction protein ZO-1 | MMP-9 | [139, 145] |
| TNF | Tumor necrosis factor | MMP-1, -2, -3, -7, -9, -12, -14 | [133, 137, 146–149] |
Hijacking the matrix: link between viral infections, MMPs and CNS pathologies
MMPs have been demonstrated to generate neurotoxic products that lead to neuronal apoptosis in acquired immunodeficiency syndrome (AIDS) [77] and to regulate immune responses during viral infections [150]. Elevated expression of MMP-9 in the CSF of human immunodeficiency virus (HIV)-infected patients has been detected [151], and MMP-2, MMP-7, and MMP-9 have been demonstrated to be elevated in HIV-associated demented AIDS patients [77, 78]. Upon viral entry and replication, the host cell secretes multiple response immune signals including proteases and cytokines. The interactions between viruses, host proteases, cytokine signaling, and CNS pathologies are only starting to be characterized. However, a potentially widespread mechanism was described [77]. In HIV, MMP-14 was induced on neuronal cell surfaces, which activated proMMP-2 secreted from macrophages or microglial cells infected with HIV [77]. The activated MMP-2 then cleaved the chemokine SDF-1 [152], the resulting N-terminally truncated product missing residues 1–4 only, then switched receptor binding specificity [153] from CXCR4 to CXCR3, and was neurotoxic. In human HIV patients cleaved SDF1 was detected in elevated amounts in the CNS [77] and anti-HIV treatment induced beneficial reductions in neuronal autophagy in lentiviral infection [154].
Interferons and MMPs
Cytokines are key players in the regulation of a functioning immune system, but upon dysregulation, they become contributors to multiple pathologies [155]. Interferon-α (IFNα) is a well-studied cytokine that plays critical roles in immunobiology and is implicated in most autoimmune diseases, viral infections, and bacterial infections, and despite its key roles, the extent of its regulation and signaling pathways is not well established [156–158]. In exploring this, Marchant et al. [131]. characterized a novel unexpected function of MMP-12 that translocates to the nucleus during infection by coxsackievirus type B3 (CVB3) or respiratory syncytial virus (RSV). MMP-12 then bound the IκBα promoter upon virus entry into the cells and was essential for antiviral IFNα expression and secretion. In infected mice lacking Mmp12, IFNα is not secreted, resulting in more than 30% death rate in otherwise nonlethal viral infections by CVB3 or RSV. Furthermore, extracellular functions of MMP-12 during viral infections include a negative feedback loop; MMP-12 was demonstrated to cleave IFNα, but not IFNβ, at the C-terminal binding site to its receptor (IFNαR2), leading to the termination of the IFNα pathway and reductions in interferon systemic toxicity [131]. Thus, MMP-12 controls IFNα, but not IFNβ responses, through bona fide intracellular transcription regulation and extracellular proteolytic processing resulting in an effective protective anti-viral IFNα response.
CVB3 infection in Mmp9−/− mice resulted in elevated myocardial injury and foci of infection in comparison with wild-types; in contrast, no difference was observed in Mmp8−/− mice [159]. Elevated immune infiltrate along with increased levels of IFNβ1 and IFNγ was observed in Mmp9−/− mice in comparison with their wild-type counterparts [159]. In addition, Nelissen et al. [132] demonstrated that MMP-9 cleaves and inactivates IFNβ in the context of multiple sclerosis. Minocycline, a tetracycline antibiotic, was demonstrated to reduce the risk of conversion from clinically isolated syndrome to multiple sclerosis [160] through the downregulation of MMP-9 activity [107, 108], which prevented MMP-9 processing of IFNβ in experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis [106, 108]. Alternatively, neutralizing antibodies to IFNβ down-regulated the expression of MMP-9 without affecting TIMPs expression [161]. In an EAE model in Lewis rats, treatment with the broad-spectrum metalloprotease inhibitor BB-1101 reduced the clinical scores through the inhibition of the release of tumor necrosis factor (TNFα) [94], though this was later interpreted to be due to reduced ADAM17 activity, the well characterized TNFα sheddase. Taken together, the combination of MMP-9 inhibitors and lower formulations of IFNβ may indicate a more efficacious way of inhibiting multiple sclerosis and certain viral infections. The precise mechanisms of action have not been fully investigated and will most likely reveal novel roles of MMP-9 in diverse pathologies.
Basal IFNα and IFNβ production are required for synergistically regulating IFNγ activity. IFNγ can enhance its own expression in natural killer (NK) cells [162], enhance IFNα/IFNβ signaling in a feedback loop through the phosphorylation of STAT1 [163], and work in tandem with TNFα to promote inflammation [164]. In contrast to IFNβ, IFNγ exacerbates multiple sclerosis symptoms in humans [165, 166] and induces CNS demyelination in mice [167]. Dandekar et al. demonstrated a role for IFNγ in demyelination by the activation of macrophages/microglia [168]; however, the post-translational role of IFNγ was not characterized. Dufour et al. recently demonstrated that MMP-12 cleaves the C-terminal end of IFNγ at two sites to remove the IFNγ receptor binding peptide leading to a reduction of the JAK-STAT1 pathway [31]. Processing of both human and murine IFNγ terminated the pSTAT1-Y701 and decreased the total STAT1 levels after 24 h. Genetic ablation of Mmp12 in the mouse led to a general increase of total IFNγ levels and an IFNγ pro-inflammatory protein signature (S100A8, S100A9, iNOS, and STAT1) in a model of acute peritonitis. In two animal models of autoimmunity, Mmp12−/− mice suffered from increased systemic inflammation and elevated IFNγ, iNOS, and MHCII in their joints, lymph nodes, and kidneys. In human lupus nephritis, MMP12 levels were decreased and IFNγ were increased in patients with increasing systemic lupus erythematosus disease activity index (SLEDAI) scores. MMP-12’s proteolytic truncation of IFNγ has a profound effect on the resolution of inflammation and cytokine signaling in autoimmune disease.
MMP therapeutic perspectives: beyond the matrix
Both beneficial and detrimental roles of MMPs have been demonstrated and these physiological functions are disease, tissue, and microenvironment dependent. This duality in their functions may partially explain why so few MMP inhibitors are now used in the clinic. Should we entirely give up on MMP inhibitors, although they have profound impact on most inflammatory diseases? Using unbiased global approaches such as proteomics and N-terminomics, a plethora of novel MMP substrates have recently been identified [4, 126, 138–140]. As an alternative, targeting the substrates of MMPs may be an indirect approach to controlling MMPs’ biological roles without inhibiting their benefits [32, 169]. Considering short-term treatments could be another way to circumvent interfering with the beneficial roles of MMPs as not all MMPs are expressed at the same time and in the same tissue/cells. MMPs play detrimental roles in many more pathologies than cancer and rheumatoid arthritis (e.g., viral infections, MS, SLE) and may still be considered as potential drug targets, although usage of MMP activity as disease indicators seems more realistic in a clinical setting.
In the movie the Matrix, the main character is faced with a dilemma between a red pill that will show him the truth about the Matrix and a blue pill that would return him to his former life. In the MMP field, we are currently facing a similar dilemma and are at a comparable crossroad: do we ‘ingest the red pill’ to adopt a novel view of MMPs’ biological roles that predominantly extend beyond mere matrix degradation or do we ‘choose the blue pill’ and repeat our past mistakes by overlooking that most MMP substrates may not be always associated with the matrix? As demonstrated by Yong, Metz and colleagues [170, 171], ‘choosing the red pill’ can be beneficial; for example, Minocycline, a broad spectrum tetracycline antibiotic, reduced the risk of conversion from a clinically isolated syndrome to multiple sclerosis [171] through the downregulation of MMP-9 activity in an indirect manner [107, 108]. Therefore, controlling MMP activity is feasible through indirect means and should be considered in the context of interferon signaling. Novel inhibitor programs for the control of MMP activity or the regulation of the non-proteolytic roles of MMPs may potentially see the light in the next years, as we are currently changing our initial views of this protease family. In addition, a more profound understanding of the repertoire of MMP substrates might reveal novel functions in immune processes of the CNS. We can benefit from reprogramming our understanding of the roles of MMPs within the Matrix and make the investigation of their functions within the CNS even more fascinating as we are unraveling new connections of biological, metabolic and signaling pathways regulated by MMP activity.
Acknowledgements
We thank Dr. V. Wee Yong, University of Calgary, AB for the help supervising Chopra’s project related to this review. C.M.O holds a Canada Research Chair in Protease Proteomics and Systems Biology. This work was supported by the Southern Alberta Mass Spectrometry (SAMS) Facility, University of Calgary and McCaig Institute and by the Canadian Institutes of Health Research Grants (MOP-37937 to C.M.O.).
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- BBB
Blood brain barrier
- CNS
Central nervous system
- CVB3
Coxsackievirus type B3
- ECM
Extracellular matrix
- ECs
Ependymal cells
- HIV
Human immunodeficiency virus
- IFN
Interferon
- MBP
Myelin basic protein
- MMP
Matrix metalloproteinase
- MS
Multiple Sclerosis
- RSV
Respiratory syncytial virus
- SLE
Systemic lupus erythematosus
- SLEDAI
Systemic lupus erythematosus disease activity index
- TIMP
Tissue inhibitor of metalloprotease
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joint Senior Authors: Christopher M. Overall and Antoine Dufour.
References
- 1.Dufour A, Overall CM. Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol Sci. 2013;34:233–242. doi: 10.1016/j.tips.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol. 2013;13:649–665. doi: 10.1038/nri3499. [DOI] [PubMed] [Google Scholar]
- 3.Hu J, Van den Steen PE, Sang Q-XA, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 4.Butler GS, Overall CM. Proteomic identification of multitasking proteins in unexpected locations complicates drug targeting. Nat Rev Drug Discov. 2009;8:935–948. doi: 10.1038/nrd2945. [DOI] [PubMed] [Google Scholar]
- 5.Butler GS, Overall CM. Updated biological roles for matrix metalloproteinases and new “intracellular” substrates revealed by degradomics. Biochemistry. 2009;48:10830–10845. doi: 10.1021/bi901656f. [DOI] [PubMed] [Google Scholar]
- 6.Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–757. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dauth S, Grevesse T, Pantazopoulos H, et al. Extracellular matrix protein expression is brain region dependent. J Comp Neurol. 2016;524:1309–1336. doi: 10.1002/cne.23965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yong VW, Krekoski CA, Forsyth PA, et al. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/S0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- 9.De Luca C, Papa M. Matrix metalloproteinases, neural extracellular matrix, and central nervous system pathology. Prog Mol Biol Transl Sci. 2017;148:167–202. doi: 10.1016/bs.pmbts.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Small CD, Crawford BD. Matrix metalloproteinases in neural development: a phylogenetically diverse perspective. Neural Regen Res. 2016;11:357–362. doi: 10.4103/1673-5374.179030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonneh-Barkay D, Wiley CA. Brain extracellular matrix in neurodegeneration. Brain Pathol. 2009;19:573–585. doi: 10.1111/j.1750-3639.2008.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujioka H, Dairyo Y, Yasunaga K-I, Emoto K. Neural functions of matrix metalloproteinases: plasticity, neurogenesis, and disease. Biochem Res Int. 2012;2012:789083–789088. doi: 10.1155/2012/789083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agrawal SM, Lau L, Yong VW. MMPs in the central nervous system: where the good guys go bad. Semin Cell Dev Biol. 2008;19:42–51. doi: 10.1016/j.semcdb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzwonek J, Rylski M, Kaczmarek L. Matrix metalloproteinases and their endogenous inhibitors in neuronal physiology of the adult brain. FEBS Lett. 2004;567:129–135. doi: 10.1016/j.febslet.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 16.Iyer RP, Patterson NL, Fields GB, Lindsey ML. The history of matrix metalloproteinases: milestones, myths, and misperceptions. Am J Physiol Heart Circ Physiol. 2012;303:H919–H930. doi: 10.1152/ajpheart.00577.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagy V, Bozdagi O, Matynia A, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorkiewicz T, Balcerzyk M, Kaczmarek L, Knapska E. Matrix metalloproteinase 9 (MMP-9) is indispensable for long term potentiation in the central and basal but not in the lateral nucleus of the amygdala. Front Cell Neurosci. 2015;9:73. doi: 10.3389/fncel.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozdagi O, Nagy V, Kwei KT, Huntley GW. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J Neurophysiol. 2007;98:334–344. doi: 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bijata M, Labus J, Guseva D, et al. Synaptic remodeling depends on signaling between serotonin receptors and the extracellular matrix. Cell Rep. 2017;19:1767–1782. doi: 10.1016/j.celrep.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Shan X, Tomlinson L, Yang Q, Colognato H. Distinct requirements for extracellular and intracellular MMP12 in the development of the adult V-SVZ neural stem cell niche. Stem Cell Reports. 2018;10:984–999. doi: 10.1016/j.stemcr.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzniewska B, Rejmak E, Malik AR, et al. Brain-derived neurotrophic factor induces matrix metalloproteinase 9 expression in neurons via the serum response factor/c-Fos pathway. Mol Cell Biol. 2013;33:2149–2162. doi: 10.1128/MCB.00008-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamat PK, Swarnkar S, Rai S, et al. Astrocyte mediated MMP-9 activation in the synapse dysfunction: an implication in Alzheimer disease. Ther Targets Neurol Dis. 2014 doi: 10.14800/ttnd.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindberg RL, De Groot CJ, Montagne L, et al. The expression profile of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in lesions and normal appearing white matter of multiple sclerosis. Brain. 2001;124:1743–1753. doi: 10.1093/brain/124.9.1743. [DOI] [PubMed] [Google Scholar]
- 25.Könnecke H, Bechmann I. The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin Dev Immunol. 2013;2013:914104–914115. doi: 10.1155/2013/914104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtinghagen R, Seifert T, Kracke A, et al. Expression of matrix metalloproteinase-9 and its inhibitors in mononuclear blood cells of patients with multiple sclerosis. J Neuroimmunol. 1999;99:19–26. doi: 10.1016/S0165-5728(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 27.Fainardi E, Castellazzi M, Bellini T, et al. Cerebrospinal fluid and serum levels and intrathecal production of active matrix metalloproteinase-9 (MMP-9) as markers of disease activity in patients with multiple sclerosis. Mult Scler. 2006;12:294–301. doi: 10.1191/135248506ms1274oa. [DOI] [PubMed] [Google Scholar]
- 28.Rosenblum G, Van den Steen PE, Cohen SR, et al. Insights into the structure and domain flexibility of full-length pro-matrix metalloproteinase-9/gelatinase B. Structure. 2007;15:1227–1236. doi: 10.1016/j.str.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Overall CM, Butler GS. Protease yoga: extreme flexibility of a matrix metalloproteinase. Structure. 2007;15:1159–1161. doi: 10.1016/j.str.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Dufour A, Overall CM. Matrix Metalloproteinase Biology. Hoboken: Wiley; 2015. Subtracting Matrix Out of the Equation: New Key Roles of Matrix Metalloproteinases in Innate Immunity and Disease; pp. 131–152. [Google Scholar]
- 31.Dufour A, Bellac CL, Eckhard U, et al. C-terminal truncation of IFN-γ inhibits proinflammatory macrophage responses and is deficient in autoimmune disease. Nat Commun. 2018;9:2416. doi: 10.1038/s41467-018-04717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overall CM, Kleifeld O. Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 33.Overall CM, Sodek J. Concanavalin A produces a matrix-degradative phenotype in human fibroblasts. Induction and endogenous activation of collagenase, 72-kDa gelatinase, and Pump-1 is accompanied by the suppression of the tissue inhibitor of matrix metalloproteinases. J Biol Chem. 1990;265:21141–21151. [PubMed] [Google Scholar]
- 34.Overall CM, Sodek J. Reciprocal regulation of collagenase, 72 kDa-gelatinase, and TIMP gene expression and protein synthesis in human fibroblasts induced by concanavalin A. Matrix Suppl. 1992;1:209–211. [PubMed] [Google Scholar]
- 35.Cox JH, Overall CM. The Cancer Degradome. New York, New York, NY: Springer; 2008. Cytokine substrates: MMP regulation of inflammatory signaling molecules; pp. 519–539. [Google Scholar]
- 36.Tester AM, Cox JH, Connor AR, et al. LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity. PLoS One. 2007;2:e312. doi: 10.1371/journal.pone.0000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortelny N, Cox JH, Kappelhoff R, et al. Network analyses reveal pervasive functional regulation between proteases in the human protease web. PLoS Biol. 2014;12:e1001869. doi: 10.1371/journal.pbio.1001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dean RA, Cox JH, Bellac CL, et al. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR + CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008;112:3455–3464. doi: 10.1182/blood-2007-12-129080. [DOI] [PubMed] [Google Scholar]
- 39.Starr AE, Dufour A, Maier J, Overall CM. Biochemical analysis of matrix metalloproteinase activation of chemokines CCL15 and CCL23 and increased glycosaminoglycan binding of CCL16. J Biol Chem. 2012;287:5848–5860. doi: 10.1074/jbc.M111.314609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McQuibban GA, Gong JH, Tam EM, et al. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 41.McQuibban GA, Gong J-H, Wong JP, et al. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- 42.Dean RA, Overall CM. Proteomics discovery of metalloproteinase substrates in the cellular context by iTRAQ labeling reveals a diverse MMP-2 substrate degradome. Mol Cell Proteomics. 2007;6:611–623. doi: 10.1074/mcp.M600341-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Dufour A, Sampson NS, Zucker S, Cao J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J Cell Physiol. 2008;217:643–651. doi: 10.1002/jcp.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dufour A, Zucker S, Sampson NS, et al. Role of matrix metalloproteinase-9 dimers in cell migration: design of inhibitory peptides. J Biol Chem. 2010;285:35944–35956. doi: 10.1074/jbc.M109.091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dufour A, Sampson NS, Li J, et al. Small-molecule anticancer compounds selectively target the hemopexin domain of matrix metalloproteinase-9. Cancer Res. 2011;71:4977–4988. doi: 10.1158/0008-5472.CAN-10-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Overall CM. Matrix metalloproteinase substrate binding domains, modules and exosites. Overview and experimental strategies. Methods Mol Biol. 2001;151:79–120. [PubMed] [Google Scholar]
- 47.Ellerbroek SM, Wu YI, Overall CM, Stack MS. Functional interplay between type I collagen and cell surface matrix metalloproteinase activity. J Biol Chem. 2001;276:24833–24842. doi: 10.1074/jbc.M005631200. [DOI] [PubMed] [Google Scholar]
- 48.Tam EM, Wu YI, Butler GS, et al. Collagen binding properties of the membrane type-1 matrix metalloproteinase (MT1-MMP) hemopexin C domain. The ectodomain of the 44-kDa autocatalytic product of MT1-MMP inhibits cell invasion by disrupting native type I collagen cleavage. J Biol Chem. 2002;277:39005–39014. doi: 10.1074/jbc.M206874200. [DOI] [PubMed] [Google Scholar]
- 49.Tam EM, Moore TR, Butler GS, Overall CM. Characterization of the distinct collagen binding, helicase and cleavage mechanisms of matrix metalloproteinase 2 and 14 (gelatinase A and MT1-MMP): the differential roles of the MMP hemopexin c domains and the MMP-2 fibronectin type II modules in collagen triple helicase activities. J Biol Chem. 2004;279:43336–43344. doi: 10.1074/jbc.M407186200. [DOI] [PubMed] [Google Scholar]
- 50.Gijbels K, Masure S, Carton H, Opdenakker G. Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J Neuroimmunol. 1992;41:29–34. doi: 10.1016/0165-5728(92)90192-N. [DOI] [PubMed] [Google Scholar]
- 51.Reinhard SM, Razak K, Ethell IM. A delicate balance: role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front Cell Neurosci. 2015;9:280. doi: 10.3389/fncel.2015.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bar-Or A, Nuttall RK, Duddy M, et al. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738–2749. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]
- 53.Avolio C, Ruggieri M, Giuliani F, et al. Serum MMP-2 and MMP-9 are elevated in different multiple sclerosis subtypes. J Neuroimmunol. 2003;136:46–53. doi: 10.1016/S0165-5728(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 54.Aung LL, Mouradian MM, Dhib-Jalbut S, Balashov KE. MMP-9 expression is increased in B lymphocytes during multiple sclerosis exacerbation and is regulated by microRNA-320a. J Neuroimmunol. 2015;278:185–189. doi: 10.1016/j.jneuroim.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agrawal S, Anderson P, Durbeej M, et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez JI, Teale JM. Multiple expression of matrix metalloproteinases in murine neurocysticercosis: Implications for leukocyte migration through multiple central nervous system barriers. Brain Res. 2008;1214:145–158. doi: 10.1016/j.brainres.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg GA, Navratil M, Barone F, Feuerstein G. Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J Cereb Blood Flow Metab. 1996;16:360–366. doi: 10.1097/00004647-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Brilha S, Ong CWM, Weksler B, et al. Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis. Sci Rep. 2017;7:16031. doi: 10.1038/s41598-017-16250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serlin Y, Shelef I, Knyazer B, Friedman A. Anatomy and physiology of the blood–brain barrier. Semin Cell Dev Biol. 2015;38:2–6. doi: 10.1016/j.semcdb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011;585:3770–3780. doi: 10.1016/j.febslet.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 61.Yong VW, Zabad RK, Agrawal S, et al. Elevation of matrix metalloproteinases (MMPs) in multiple sclerosis and impact of immunomodulators. J Neurol Sci. 2007;259:79–84. doi: 10.1016/j.jns.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 62.Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br J Pharmacol. 2012;164:1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boziki M, Grigoriadis N. An update on the role of matrix metalloproteinases in the pathogenesis of multiple sclerosis. Med Chem. 2018;14:155–169. doi: 10.2174/1573406413666170906122803. [DOI] [PubMed] [Google Scholar]
- 64.Gijbels K, Proost P, Masure S, et al. Gelatinase B is present in the cerebrospinal fluid during experimental autoimmune encephalomyelitis and cleaves myelin basic protein. J Neurosci Res. 1993;36:432–440. doi: 10.1002/jnr.490360409. [DOI] [PubMed] [Google Scholar]
- 65.Kouwenhoven M, Ozenci V, Gomes A, et al. Multiple sclerosis: elevated expression of matrix metalloproteinases in blood monocytes. J Autoimmun. 2001;16:463–470. doi: 10.1006/jaut.2001.0505. [DOI] [PubMed] [Google Scholar]
- 66.Shiryaev SA, Savinov AY, Cieplak P, et al. Matrix metalloproteinase proteolysis of the myelin basic protein isoforms is a source of immunogenic peptides in autoimmune multiple sclerosis. PLoS One. 2009;4:e4952. doi: 10.1371/journal.pone.0004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Proost P, van Damme J, Opdenakker G. Leukocyte gelatinase B cleavage releases encephalitogens from human myelin basic protein. Biochem Biophys Res Commun. 1993;192:1175–1181. doi: 10.1006/bbrc.1993.1540. [DOI] [PubMed] [Google Scholar]
- 68.Starckx S, Van den Steen PE, Verbeek R, et al. A novel rationale for inhibition of gelatinase B in multiple sclerosis: MMP-9 destroys αB-crystallin and generates a promiscuous T cell epitope. J Neuroimmunol. 2003;141:47–57. doi: 10.1016/S0165-5728(03)00217-0. [DOI] [PubMed] [Google Scholar]
- 69.D’Souza CA, Moscarello MA. Differences in susceptibility of MBP charge isomers to digestion by stromelysin-1 (MMP-3) and release of an immunodominant epitope. Neurochem Res. 2006;31:1045–1054. doi: 10.1007/s11064-006-9116-9. [DOI] [PubMed] [Google Scholar]
- 70.Boggs JM, Yip PM, Rangaraj G, Jo E. Effect of posttranslational modifications to myelin basic protein on its ability to aggregate acidic lipid vesicles. Biochemistry. 1997;36:5065–5071. doi: 10.1021/bi962649f. [DOI] [PubMed] [Google Scholar]
- 71.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 72.Lipham WJ, Redmond TM, Takahashi H, et al. Recognition of peptides that are immunopathogenic but cryptic. Mechanisms that allow lymphocytes sensitized against cryptic peptides to initiate pathogenic autoimmune processes. J Immunol. 1991;146:3757–3762. [PubMed] [Google Scholar]
- 73.Ferraro GB, Morrison CJ, Overall CM, et al. Membrane-type matrix metalloproteinase-3 regulates neuronal responsiveness to myelin through Nogo-66 receptor 1 cleavage. J Biol Chem. 2011;286:31418–31424. doi: 10.1074/jbc.M111.249169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leake A, Morris CM, Whateley J. Brain matrix metalloproteinase 1 levels are elevated in Alzheimer’s disease. Neurosci Lett. 2000;291:201–203. doi: 10.1016/S0304-3940(00)01418-X. [DOI] [PubMed] [Google Scholar]
- 75.Fang L, Huber-Abel F, Teuchert M, et al. Linking neuron and skin: matrix metalloproteinases in amyotrophic lateral sclerosis (ALS) J Neurol Sci. 2009;285:62–66. doi: 10.1016/j.jns.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 76.Conant K, McArthur JC, Griffin DE, et al. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 2001;46:391–398. doi: 10.1002/1531-8249(199909)46:3<391::AID-ANA15>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 77.Zhang K, McQuibban GA, Silva C, et al. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003;6:1064–1071. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]
- 78.Clark AW, Krekoski CA, Bou SS, et al. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett. 1997;238:53–56. doi: 10.1016/S0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- 79.Ichiyama T, Kajimoto M, Suenaga N, et al. Serum levels of matrix metalloproteinase-9 and its tissue inhibitor (TIMP-1) in acute disseminated encephalomyelitis. J Neuroimmunol. 2006;172:182–186. doi: 10.1016/j.jneuroim.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 80.Paemen L, Olsson T, Söderström M, et al. Evaluation of gelatinases and IL-6 in the cerebrospinal fluid of patients with optic neuritis, multiple sclerosis and other inflammatory neurological diseases. Eur J Neurol. 1994;1:55–63. doi: 10.1111/j.1468-1331.1994.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 81.Leppert D, Ford J, Stabler G, et al. Matrix metalloproteinase-9 (gelatinase B) is selectively elevated in CSF during relapses and stable phases of multiple sclerosis. Brain. 1998;121(Pt 12):2327–2334. doi: 10.1093/brain/121.12.2327. [DOI] [PubMed] [Google Scholar]
- 82.Sellebjerg F, Madsen HO, Jensen CV, et al. CCR82 delta32, matrix metalloproteinase-9 and disease activity in multiple sclerosis. J Neuroimmunol. 2000;102:98–106. doi: 10.1016/S0165-5728(99)00166-6. [DOI] [PubMed] [Google Scholar]
- 83.Liuzzi GM, Trojano M, Fanelli M, et al. Intrathecal synthesis of matrix metalloproteinase-9 in patients with multiple sclerosis: implication for pathogenesis. Mult Scler. 2002;8:222–228. doi: 10.1191/1352458502ms800oa. [DOI] [PubMed] [Google Scholar]
- 84.Opdenakker G, Nelissen I, Van Damme J. Functional roles and therapeutic targeting of gelatinase B and chemokines in multiple sclerosis. Lancet Neurol. 2003;2:747–756. doi: 10.1016/S1474-4422(03)00587-8. [DOI] [PubMed] [Google Scholar]
- 85.Ozenci V, Rinaldi L, Teleshova N, et al. Metalloproteinases and their tissue inhibitors in multiple sclerosis. J Autoimmun. 1999;12:297–303. doi: 10.1006/jaut.1999.0285. [DOI] [PubMed] [Google Scholar]
- 86.Lee MA, Palace J, Stabler G et al (1999) Serum gelatinase B, TIMP-1 and TIMP-2 levels in multiple sclerosis. A longitudinal clinical and MRI study. Brain 122(Pt 2):191–197 [DOI] [PubMed]
- 87.Li Y-J, Wang Z-H, Zhang B, et al. Disruption of the blood-brain barrier after generalized tonic-clonic seizures correlates with cerebrospinal fluid MMP-9 levels. J Neuroinflammation. 2013;10:80. doi: 10.1186/1742-2094-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cuadrado E, Rosell A, Penalba A, et al. Vascular MMP-9/TIMP-2 and neuronal MMP-10 up-regulation in human brain after stroke: a combined laser microdissection and protein array study. J Proteome Res. 2009;8:3191–3197. doi: 10.1021/pr801012x. [DOI] [PubMed] [Google Scholar]
- 89.Rodríguez JA, Sobrino T, Orbe J, et al. proMetalloproteinase-10 is associated with brain damage and clinical outcome in acute ischemic stroke. J Thromb Haemost. 2013;11:1464–1473. doi: 10.1111/jth.12312. [DOI] [PubMed] [Google Scholar]
- 90.Vos CMP, van Haastert ES, de Groot CJA, et al. Matrix metalloproteinase-12 is expressed in phagocytotic macrophages in active multiple sclerosis lesions. J Neuroimmunol. 2003;138:106–114. doi: 10.1016/S0165-5728(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 91.Rangaraju S, Khoo KK, Feng Z-P, et al. Potassium channel modulation by a toxin domain in matrix metalloprotease 23. J Biol Chem. 2010;285:9124–9136. doi: 10.1074/jbc.M109.071266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Werner SR, Dotzlaf JE, Smith RC. MMP-28 as a regulator of myelination. BMC Neurosci. 2008;9:83. doi: 10.1186/1471-2202-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Waubant E, Goodkin DE, Gee L, et al. Serum MMP-9 and TIMP-1 levels are related to MRI activity in relapsing multiple sclerosis. Neurology. 1999;53:1397–1401. doi: 10.1212/WNL.53.7.1397. [DOI] [PubMed] [Google Scholar]
- 94.Clements JM, Cossins JA, Wells GM, et al. Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-alpha inhibitor. J Neuroimmunol. 1997;74:85–94. doi: 10.1016/S0165-5728(96)00210-X. [DOI] [PubMed] [Google Scholar]
- 95.Hsu J-YC, McKeon R, Goussev S, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 2006;26:9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Skuljec J, Gudi V, Ulrich R, et al. Matrix metalloproteinases and their tissue inhibitors in cuprizone-induced demyelination and remyelination of brain white and gray matter. J Neuropathol Exp Neurol. 2011;70:758–769. doi: 10.1097/NEN.0b013e3182294fad. [DOI] [PubMed] [Google Scholar]
- 97.Pagenstecher A, Stalder AK, Kincaid CL, et al. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- 98.Gurney KJ, Estrada EY, Rosenberg GA. Blood–brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 99.Buhler LA, Samara R, Guzman E, et al. Matrix metalloproteinase-7 facilitates immune access to the CNS in experimental autoimmune encephalomyelitis. BMC Neurosci. 2009;10:17. doi: 10.1186/1471-2202-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Folgueras AR, Fueyo A, García-Suárez O, et al. Collagenase-2 deficiency or inhibition impairs experimental autoimmune encephalomyelitis in mice. J Biol Chem. 2008;283:9465–9474. doi: 10.1074/jbc.M709522200. [DOI] [PubMed] [Google Scholar]
- 101.Kaminari A, Giannakas N, Tzinia A, Tsilibary EC. Overexpression of matrix metalloproteinase-9 (MMP-9) rescues insulin-mediated impairment in the 5XFAD model of Alzheimer’s disease. Sci Rep. 2017;7:683. doi: 10.1038/s41598-017-00794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dewil M, Schurmans C, Starckx S, et al. Role of matrix metalloproteinase-9 in a mouse model for amyotrophic lateral sclerosis. NeuroReport. 2005;16:321–324. doi: 10.1097/00001756-200503150-00003. [DOI] [PubMed] [Google Scholar]
- 103.Mizoguchi H, Nakade J, Tachibana M, et al. Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J Neurosci. 2011;31:12963–12971. doi: 10.1523/JNEUROSCI.3118-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilczynski GM, Konopacki FA, Wilczek E, et al. Important role of matrix metalloproteinase 9 in epileptogenesis. J Cell Biol. 2008;180:1021–1035. doi: 10.1083/jcb.200708213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dubois B, Masure S, Hurtenbach U, et al. Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Investig. 1999;104:1507–1515. doi: 10.1172/JCI6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Larsen PH, Wells JE, Stallcup WB, et al. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J Neurosci. 2003;23:11127–11135. doi: 10.1523/JNEUROSCI.23-35-11127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Asahi M, Asahi K, Jung JC, et al. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 108.Mitsios N, Saka M, Krupinski J, et al. A microarray study of gene and protein regulation in human and rat brain following middle cerebral artery occlusion. BMC Neurosci. 2007;8:93. doi: 10.1186/1471-2202-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y, Zhang M, Hao W, et al. Matrix metalloproteinase-12 contributes to neuroinflammation in the aged brain. Neurobiol Aging. 2013;34:1231–1239. doi: 10.1016/j.neurobiolaging.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 110.Weaver A, Goncalves da Silva A, Nuttall RK, et al. An elevated matrix metalloproteinase (MMP) in an animal model of multiple sclerosis is protective by affecting Th1/Th2 polarization. FASEB J. 2005;19:1668–1670. doi: 10.1096/fj.04-2030fje. [DOI] [PubMed] [Google Scholar]
- 111.Goncalves DaSilva A, Liaw L, Yong VW. Cleavage of osteopontin by matrix metalloproteinase-12 modulates experimental autoimmune encephalomyelitis disease in C57BL/6 mice. Am J Pathol. 2010;177:1448–1458. doi: 10.2353/ajpath.2010.091081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goncalves DaSilva A, Yong VW. Matrix metalloproteinase-12 deficiency worsens relapsing-remitting experimental autoimmune encephalomyelitis in association with cytokine and chemokine dysregulation. Am J Pathol. 2009;174:898–909. doi: 10.2353/ajpath.2009.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chelluboina B, Warhekar A, Dillard M, et al. Post-transcriptional inactivation of matrix metalloproteinase-12 after focal cerebral ischemia attenuates brain damage. Sci Rep. 2015;5:9504. doi: 10.1038/srep09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wells JEA, Rice TK, Nuttall RK, et al. An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci. 2003;23:10107–10115. doi: 10.1523/JNEUROSCI.23-31-10107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baranger K, Marchalant Y, Bonnet AE, et al. MT5-MMP is a new pro-amyloidogenic proteinase that promotes amyloid pathology and cognitive decline in a transgenic mouse model of Alzheimer’s disease. Cell Mol Life Sci. 2016;73:217–236. doi: 10.1007/s00018-015-1992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Komori K, Nonaka T, Okada A, et al. Absence of mechanical allodynia and Abeta-fiber sprouting after sciatic nerve injury in mice lacking membrane-type 5 matrix metalloproteinase. FEBS Lett. 2004;557:125–128. doi: 10.1016/S0014-5793(03)01458-3. [DOI] [PubMed] [Google Scholar]
- 117.Folgueras AR, Valdés-Sánchez T, Llano E, et al. Metalloproteinase MT5-MMP is an essential modulator of neuro-immune interactions in thermal pain stimulation. Proc Natl Acad Sci USA. 2009;106:16451–16456. doi: 10.1073/pnas.0908507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shiryaev SA, Remacle AG, Savinov AY, et al. Inflammatory proprotein convertase-matrix metalloproteinase proteolytic pathway in antigen-presenting cells as a step to autoimmune multiple sclerosis. J Biol Chem. 2009;284:30615–30626. doi: 10.1074/jbc.M109.041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nedivi E, Hevroni D, Naot D, et al. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- 120.LePage RN, Fosang AJ, Fuller SJ, et al. Gelatinase A possesses a beta-secretase-like activity in cleaving the amyloid protein precursor of Alzheimer’s disease. FEBS Lett. 1995;377:267–270. doi: 10.1016/0014-5793(95)01358-X. [DOI] [PubMed] [Google Scholar]
- 121.Ahmad M, Takino T, Miyamori H, et al. Cleavage of amyloid-beta precursor protein (APP) by membrane-type matrix metalloproteinases. J Biochem. 2006;139:517–526. doi: 10.1093/jb/mvj054. [DOI] [PubMed] [Google Scholar]
- 122.Vaisar T, Kassim SY, Gomez IG, et al. MMP-9 sheds the beta2 integrin subunit (CD18) from macrophages. Mol Cell Proteomics. 2009;8:1044–1060. doi: 10.1074/mcp.M800449-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Higashi S, Miyazaki K. Novel processing of beta-amyloid precursor protein catalyzed by membrane type 1 matrix metalloproteinase releases a fragment lacking the inhibitor domain against gelatinase A. Biochemistry. 2003;42:6514–6526. doi: 10.1021/bi020643m. [DOI] [PubMed] [Google Scholar]
- 124.Stix B, Kähne T, Sletten K, et al. Proteolysis of AA amyloid fibril proteins by matrix metalloproteinases-1, -2, and -3. Am J Pathol. 2001;159:561–570. doi: 10.1016/S0002-9440(10)61727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Starckx S, Van den Steen PE, Verbeek R, et al. A novel rationale for inhibition of gelatinase B in multiple sclerosis: MMP-9 destroys alpha B-crystallin and generates a promiscuous T cell epitope. J Neuroimmunol. 2003;141:47–57. doi: 10.1016/S0165-5728(03)00217-0. [DOI] [PubMed] [Google Scholar]
- 126.Zhong D, Saito F, Saito Y, et al. Characterization of the protease activity that cleaves the extracellular domain of beta-dystroglycan. Biochem Biophys Res Commun. 2006;345:867–871. doi: 10.1016/j.bbrc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 127.Yamada H, Saito F, Fukuta-Ohi H, et al. Processing of beta-dystroglycan by matrix metalloproteinase disrupts the link between the extracellular matrix and cell membrane via the dystroglycan complex. Hum Mol Genet. 2001;10:1563–1569. doi: 10.1093/hmg/10.15.1563. [DOI] [PubMed] [Google Scholar]
- 128.Michaluk P, Kolodziej L, Mioduszewska B, et al. Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J Biol Chem. 2007;282:16036–16041. doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- 129.Butler GS, Dean RA, Tam EM, Overall CM. Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: dynamics of membrane type 1 matrix metalloproteinase-mediated membrane protein shedding. Mol Cell Biol. 2008;28:4896–4914. doi: 10.1128/MCB.01775-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Szklarczyk A, Ewaleifoh O, Beique J-C, et al. MMP-7 cleaves the NR1 NMDA receptor subunit and modifies NMDA receptor function. FASEB J. 2008;22:3757–3767. doi: 10.1096/fj.07-101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marchant DJ, Bellac CL, Moraes TJ, et al. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat Med. 2014;20:493–502. doi: 10.1038/nm.3508. [DOI] [PubMed] [Google Scholar]
- 132.Nelissen I, Martens E, Van den Steen PE, et al. Gelatinase B/matrix metalloproteinase-9 cleaves interferon-beta and is a target for immunotherapy. Brain. 2003;126:1371–1381. doi: 10.1093/brain/awg129. [DOI] [PubMed] [Google Scholar]
- 133.d’Ortho MP, Will H, Atkinson S, et al. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997;250:751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- 134.Schönbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161:3340–3346. [PubMed] [Google Scholar]
- 135.Ito A, Mukaiyama A, Itoh Y, et al. Degradation of interleukin 1beta by matrix metalloproteinases. J Biol Chem. 1996;271:14657–14660. doi: 10.1074/jbc.271.25.14657. [DOI] [PubMed] [Google Scholar]
- 136.Milward E, Kim KJ, Szklarczyk A, et al. Cleavage of myelin associated glycoprotein by matrix metalloproteinases. J Neuroimmunol. 2008;193:140–148. doi: 10.1016/j.jneuroim.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chandler S, Cossins J, Lury J, Wells G. Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumour necrosis factor-alpha fusion protein. Biochem Biophys Res Commun. 1996;228:421–429. doi: 10.1006/bbrc.1996.1677. [DOI] [PubMed] [Google Scholar]
- 138.Chandler S, Coates R, Gearing A, et al. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201:223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- 139.Asahi M, Wang X, Mori T, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Szklarczyk A, Oyler G, McKay R, et al. Cleavage of neuronal synaptosomal-associated protein of 25 kDa by exogenous matrix metalloproteinase-7. J Neurochem. 2007;102:1256–1263. doi: 10.1111/j.1471-4159.2007.04625.x. [DOI] [PubMed] [Google Scholar]
- 141.Levin J, Giese A, Boetzel K, et al. Increased alpha-synuclein aggregation following limited cleavage by certain matrix metalloproteinases. Exp Neurol. 2009;215:201–208. doi: 10.1016/j.expneurol.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 142.Sung JY, Park SM, Lee C-H, et al. Proteolytic cleavage of extracellular secreted {alpha}-synuclein via matrix metalloproteinases. J Biol Chem. 2005;280:25216–25224. doi: 10.1074/jbc.M503341200. [DOI] [PubMed] [Google Scholar]
- 143.Diekmann O, Tschesche H. Degradation of kinins, angiotensins and substance P by polymorphonuclear matrix metalloproteinases MMP 8 and MMP 9. Braz J Med Biol Res. 1994;27:1865–1876. [PubMed] [Google Scholar]
- 144.Backstrom JR, Tökés ZA. The 84-kDa form of human matrix metalloproteinase-9 degrades substance P and gelatin. J Neurochem. 2002;64:1312–1318. doi: 10.1046/j.1471-4159.1995.64031312.x. [DOI] [PubMed] [Google Scholar]
- 145.Harkness KA, Adamson P, Sussman JD, et al. Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain. 2000;123(Pt 4):698–709. doi: 10.1093/brain/123.4.698. [DOI] [PubMed] [Google Scholar]
- 146.Tam EM, Morrison CJ, Wu YI, et al. Membrane protease proteomics: Isotope-coded affinity tag MS identification of undescribed MT1-matrix metalloproteinase substrates. Proc Natl Acad Sci USA. 2004;101:6917–6922. doi: 10.1073/pnas.0305862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gearing AJ, Beckett P, Christodoulou M, et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 148.Haro H, Crawford HC, Fingleton B, et al. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Investig. 2000;105:143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.English WR, Puente XS, Freije JM, et al. Membrane type 4 matrix metalloproteinase (MMP17) has tumor necrosis factor-alpha convertase activity but does not activate pro-MMP2. J Biol Chem. 2000;275:14046–14055. doi: 10.1074/jbc.275.19.14046. [DOI] [PubMed] [Google Scholar]
- 150.Noorbakhsh F, Overall CM, Power C. Deciphering complex mechanisms in neurodegenerative diseases: the advent of systems biology. Trends Neurosci. 2009;32:88–100. doi: 10.1016/j.tins.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 151.Sporer B, Paul R, Koedel U, et al. Presence of matrix metalloproteinase-9 activity in the cerebrospinal fluid of human immunodeficiency virus-infected patients. J Infect Dis. 1998;178:854–857. doi: 10.1086/515342. [DOI] [PubMed] [Google Scholar]
- 152.McQuibban GA, Butler GS, Gong JH, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 153.Vergote D, Butler GS, Ooms M, et al. Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc Natl Acad Sci USA. 2006;103:19182–19187. doi: 10.1073/pnas.0604678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu Y, Vergote D, Pardo C, et al. CXCR154 activation by lentivirus infection suppresses neuronal autophagy: neuroprotective effects of antiretroviral therapy. FASEB J. 2009;23:2928–2941. doi: 10.1096/fj.08-128819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Baccala R, Kono DH, Theofilopoulos AN. Interferons as pathogenic effectors in autoimmunity. Immunol Rev. 2005;204:9–26. doi: 10.1111/j.0105-2896.2005.00252.x. [DOI] [PubMed] [Google Scholar]
- 156.Ng CT, Mendoza JL, Garcia KC, Oldstone MBA. Alpha and beta type 1 interferon signaling: passage for diverse biologic outcomes. Cell. 2016;164:349–352. doi: 10.1016/j.cell.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Crow MK. Autoimmunity: Interferon α or β: which is the culprit in autoimmune disease? Nat Rev Rheumatol. 2016;12:439–440. doi: 10.1038/nrrheum.2016.117. [DOI] [PubMed] [Google Scholar]
- 159.Cheung C, Marchant D, Walker EK-Y, et al. Ablation of matrix metalloproteinase-9 increases severity of viral myocarditis in mice. Circulation. 2008;117:1574–1582. doi: 10.1161/CIRCULATIONAHA.107.733238. [DOI] [PubMed] [Google Scholar]
- 160.Metz LM, Li DKB, Traboulsee AL, et al. Trial of minocycline in a clinically isolated syndrome of multiple sclerosis. N Engl J Med. 2017;376:2122–2133. doi: 10.1056/NEJMoa1608889. [DOI] [PubMed] [Google Scholar]
- 161.Gilli F, Bertolotto A, Sala A, et al. Neutralizing antibodies against IFN-beta in multiple sclerosis: antagonization of IFN-beta mediated suppression of MMPs. Brain. 2004;127:259–268. doi: 10.1093/brain/awh028. [DOI] [PubMed] [Google Scholar]
- 162.Hardy KJ, Sawada T. Human gamma interferon strongly upregulates its own gene expression in peripheral blood lymphocytes. J Exp Med. 1989;170:1021–1026. doi: 10.1084/jem.170.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tassiulas I, Hu X, Ho H, et al. Amplification of IFN-alpha-induced STAT1 activation and inflammatory function by Syk and ITAM-containing adaptors. Nat Immunol. 2004;5:1181–1189. doi: 10.1038/ni1126. [DOI] [PubMed] [Google Scholar]
- 164.Johnson DR, Pober JS. Tumor necrosis factor and immune interferon synergistically increase transcription of HLA class I heavy- and light-chain genes in vascular endothelium. Proc Natl Acad Sci USA. 1990;87:5183–5187. doi: 10.1073/pnas.87.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–895. doi: 10.1016/S0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 166.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–1102. doi: 10.1212/WNL.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 167.Horwitz MS, Evans CF, Mcgavern DB, et al. Primary demyelination in transgenic mice expressing interferon-gamma. Nat Med. 1997;3:1037–1041. doi: 10.1038/nm0997-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dandekar AA, Anghelina D, Perlman S. Bystander CD8 T-cell-mediated demyelination is interferon-gamma-dependent in a coronavirus model of multiple sclerosis. Am J Pathol. 2004;164:363–369. doi: 10.1016/S0002-9440(10)63126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Overall CM, Kleifeld O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br J Cancer. 2006;94:941–946. doi: 10.1038/sj.bjc.6603043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Faissner S, Mahjoub Y, Mishra M, et al. Unexpected additive effects of minocycline and hydroxychloroquine in models of multiple sclerosis: Prospective combination treatment for progressive disease? Mult Scler. 2017;24:1352458517728811. doi: 10.1177/1352458517728811. [DOI] [PubMed] [Google Scholar]
- 171.Metz LM, Li DKB, Traboulsee AL, et al. Trial of Minocycline in a Clinically Isolated Syndrome of Multiple Sclerosis. N Engl J Med. 2017;376:2122–2133. doi: 10.1056/NEJMoa1608889. [DOI] [PubMed] [Google Scholar]