Abstract
The fetus is shielded from the adverse effects of excessive maternal glucocorticoids by 11β-HSD2, an enzyme which is expressed in the syncytial layer of the placental villi and is capable of converting biologically active cortisol into inactive cortisone. Impairment of this placental glucocorticoid barrier is associated with fetal intrauterine growth restriction (IUGR) and development of chronic diseases in later life. Ontogeny studies show that the expression of 11β-HSD2 is initiated at a very early stage after conception and increases with gestational age but declines around term. The promoter for HSD11B2, the gene encoding 11β-HSD2, has a highly GC-rich core. However, the pattern of methylation on HSD11B2 may have already been set up in the blastocyst when the trophoblast identity is committed. Instead, hCG-initiated signals appear to be responsible for the upsurge of 11β-HSD2 expression during trophoblast syncytialization. By activating the cAMP/PKA pathway, hCG not only alters the modification of histones but also increases the expression of Sp1 which activates the transcription of HSD11B2. Adverse conditions such as stress, hypoxia and nutritional restriction can cause IUGR of the fetus. It appears that different causes of IUGR may attenuate HSD11B2 expression differentially in the placenta. While stress and nutritional restriction may reduce HSD11B2 expression by increasing its methylation, hypoxia may decrease HSD11B2 expression via alternative mechanisms rather than by methylation. Herein, we summarize the advances in the study of mechanisms underlying the establishment of the placental glucocorticoid barrier and the attenuation of this barrier by adverse conditions during pregnancy.
Keywords: Placenta, Cortisol, 11β-hydroxysteroid dehydrogenase 2, Ontogeny, hCG, Histone and epigenetics
Introduction
Glucocorticoids, the end products of the hypothalamus–pituitary–adrenal (HPA) axis, are released in response to stress to regulate a variety of vital functions thereby maintaining homeostasis [1]. In many species, including amphibians, reptiles, rodents and birds, corticosterone is a major biologically active glucocorticoid, while in humans, cortisol is the primary biologically active glucocorticoid. Compelling evidence indicates that glucocorticoids are also important in reproduction [2] and they are not only crucial to the establishment of pregnancy [3], but also pivotal in fetal development [4, 5] and parturition [6, 7]. With regards to fetal development, glucocorticoids act as a double-edged sword depending on exposure time and levels [8]. Towards the end of gestation, appropriate exposure to glucocorticoids are necessary for the maturation of a number of fetal organ systems, particularly the respiratory and digestive systems, to prepare the fetus for extra-uterine life. Thus, synthetic glucocorticoids are widely used in pregnant women with threatened preterm delivery to improve neonatal viability by accelerating lung maturation [5, 9]. Despite of these beneficial effects, excessive glucocorticoid exposure is known to exert a number of adverse effects on the fetus. Accumulating evidence indicates that over-exposure of the fetus to glucocorticoids not only causes fetal growth restriction but also programs the development of chronic diseases such as hypertension, insulin resistance and behavioral abnormalities with the possibility of altered activity of the HAP axis in later life [8, 10]. Therefore, it is essential to control glucocorticoids at optimal levels in the fetal circulation to ensure a safe intrauterine development.
The fetus is shielded by a placental glucocorticoid barrier
The development of the primate fetal adrenal glands is a unique process. The fetal adrenal glands are relative large in comparison with the adult organs. A large fetal zone dominates the cortex throughout gestation. This fetal zone produces dehydroepiandrosterone (DHEA) and its sulfate instead of cortisol [11, 12]. Although a small amount of cortisol can be synthesized from progesterone in the fetal adrenal glands [13], limited de novo cortisol synthesis from cholesterol is not established until the third trimester of pregnancy in the fetal adrenals [11]. In contrast, the maternal adrenal glands gradually become hypertrophic during pregnancy [14]. Although the overall weight and size do not change dramatically, the size of the zona fasciculata which produces cortisol is increased [14]. During pregnancy, there is a three- to eightfold increase in the levels of total cortisol in the maternal circulation [14]. Despite of increased synthesis of corticosteroid binding protein (CBG) by the liver in gestation [15], a two- to fourfold elevation of free cortisol in maternal circulation is still seen from the second–third trimesters [16–18]. Therefore, the amount of cortisol produced by the fetal adrenal glands is minimal compared with that produced by the maternal adrenal glands. However, meet the requirement for fetal organ maturation towards the end of gestation, it is still necessary for the fetus to acquire a sufficient proportion of cortisol from the maternal side. It is estimated that about 40–50% of fetal cortisol is derived from the mother towards the end of gestation [19]. Nonetheless, the majority of maternal cortisol is still blocked from the fetus, despite its lipophilic nature to create a safe environment for development of the fetus since excessive glucocorticoids are detrimental to the fetal development [8, 10]. It is estimated that there is only 15% of maternal cortisol crossing the placenta unmetabolized in normal pregnancy [20].
It is very well recognized that biologically active cortisol or corticosterone is converted into biologically inactive cortisone or 11-dehydrocorticosterone when passing through the placenta in almost all studied placental mammals [20, 21]. It has been reported that the conversion of cortisol into cortisone by homogenized human placental tissue dominates at all gestational ages, albeit a small amount of cortisone can also be converted into cortisol [22]. As such, cortisol levels in the fetal circulation are kept about tenfold lower than those in the maternal circulation [14, 19].
11β-HSD2 acts as the placental glucocorticoid barrier
It is now widely accepted that the glucocorticoid inactivating enzyme, 11β-hydroxysteroid dehydrogenase2 (11β-HSD2), acts as the placental glucocorticoid barrier [20, 23–25]. In addition to 11β-HSD2, a glucocorticoid regenerating enzyme 11β-HSD1 [25, 26] has also been identified. These two glucocorticoid metabolizing enzymes work in opposing ways with differential affinities for their substrates. While 11β-HSD1 is a reductase converting biologically inactive cortisone or 11-dehydrocorticosterone into active cortisol or corticosterone with a Km value in the micromolar range and requiring NADPH as its cofactor, 11β-HSD2 is an exclusive oxidase converting biologically active cortisol or corticosterone into inactive cortisone or 11-dehydrocorticosterone with a Km value in the nanomolar range and requiring NAD+ as its cofactor [25–30].
Table 1 summarizes the main characteristics of 11β-HSD1 and 11β-HSD2. Examination of their distribution in the body revealed that the reductase 11β-HSD1 is distributed widely in the glucocorticoid-target tissues in the body, while the oxidase 11β-HSD2 is distributed mainly in the mineralocorticoid-target organs including the kidney, intestine, salivary glands, exocrine pancreatic gland and sweat glands [25, 26, 29, 30]. The distribution patterns of 11β-HSD1 and 11β-HSD2 are in line with the distribution of glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) in these tissues, respectively. Cortisol binds to GR with a relatively low affinity (Kd: 11 nM), while the MR has equal and high affinities for both cortisol and aldosterone (Kd: 0.5 nM) [31]. The reductase activity of 11β-HSD1 can create more biologically active glucocorticoids in glucocorticoid-target cells, so that adequate amounts of glucocorticoids can be obtained for the low-affinity GR. Therefore, 11β-HSD1 is regarded as a pre-GR amplifier for glucocorticoid actions [32]. Likewise, the specific distribution of 11β-HSD2 in the mineralocorticoid target organs also has its own designated function. Since the MR is bound by cortisol and aldosterone with similar affinities, it is necessary for the mineralocorticoid target cells to express 11β-HSD2 to inactivate cortisol, the concentration of which is about 100- to 1000-fold higher than that of aldosterone in the circulation. This ensures the specificity of MR for aldosterone. Otherwise, the MR would be occupied by the overwhelming glucocorticoid concentration resulting in severe sodium and water retention [31, 33].
Table 1.
Characteristics of 11β-HSD1 and 11β-HSD2 in humans
| 11β-HSD1 | 11β-HSD2 | |
|---|---|---|
| Gene | HSD11B1 | HSD11B2 |
| Chromosome | 1q32 | 16q22 |
| Number of exons | 6 | 5 |
| Promoter | Consensus CAAT box, no TATA box | Rich in CpG, no TATA box |
| Molecular weight | 34 kD | 44 kD |
| Number of amino acids | 292 | 406 |
| Reaction type | Reductase | Oxidase |
| Co-factor | NADPH | NAD+ |
| Affinity | Low (Km: μM) | High (Km: nM) |
| Substrate | Cortisone | Cortisol |
| Product | Cortisol | Cortisone |
| Effect on glucocorticoids | Amplification | Inactivation |
| Distribution in the body | Glucocorticoid-target tissues | Mineralocorticoid-target tissues |
| Co-localization | Glucocorticoid receptor | Mineralocorticoid receptor |
| Distribution in the intrauterine tissues | Amnion and chorion of the fetal membranes; stromal and epithelial cells of the decidua, endothelial cells of the fetal blood vessels in the villous core | Placental villous syncytiotrophoblast, decidual epithelial cells |
The placenta is generally considered a non-classical mineralocorticoid-target tissue, despite the presence of MR in human placenta [34]. Nonetheless, the placenta expresses abundant 11β-HSD2 [25, 35, 36], which is believed to function mainly as a barrier for maternal glucocorticoids [37], although alternative functions such as regulating the local actions of glucocorticoids in the placenta have also been suggested [38]. In addition, the high affinity of 11β-HSD2 for cortisol makes it more suited to serve as a placental glucocorticoid barrier guarding the fetus against maternal glucocorticoids [24, 28].
Distribution and ontogenesis of 11β-HSD2 in the placenta
Compartmentalized distribution of 11β-HSD2 at the fetal-maternal interface and its implications
The human placenta is classified as hemochorial. This type of placenta is characterized by the direct contact of the maternal blood circulation with the placental villi where the maternofetal nutrients and gas exchanges take place. All villi are covered by two layers of trophoblasts. The outermost layer is the terminally differentiated and continuous multinucleated syncytiotrophoblast, and the inner layer is composed of single and aggregated cytotrophoblasts which are highly proliferative and can differentiate into either villous syncytiotrophoblast or extravillous invasive trophoblasts in the processes of placentation and implantation. Since the syncytiotrophoblast layer lines the intervillous space, it provides the first line of defense for the fetus against any potentially harmful substances from the maternal side.
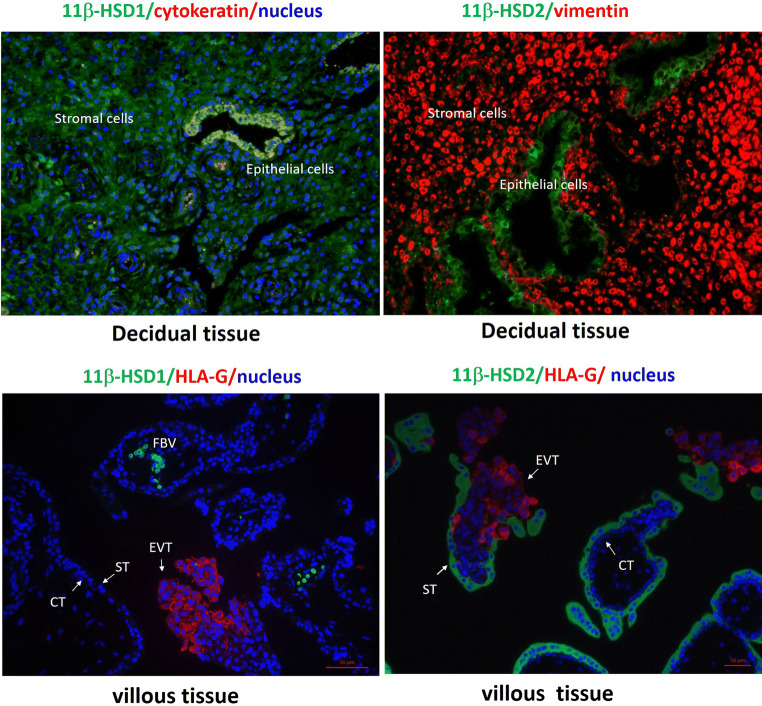
Prior to the acknowledgement of two types of 11β-HSDs in 1990s [25, 26], it was known for around three decades that the human placenta is capable of oxidizing biologically active 11β-hydroxycorticosteroids into inactive 11-oxo-compounds [39]. As described above, the human placenta maintains a high oxidase activity at all gestational ages [20, 40–42], albeit a minimal increase in the reductase activity with increasing gestational age in the placenta [21, 22]. Interestingly, in contrast to the dominant oxidase activity in the placenta, a reductase activity of 11β-HSD has been demonstrated to be dominant in the human fetal membranes [42–44]. Immunohistological staining of placental sections at both term and preterm revealed compartmentalized patterns of 11β-HSD1 and 2 with distinct distributions [25, 35, 45–48]. Specifically, 11β-HSD2 is exclusively localized to the syncytiotrophoblast of the chorionic villi but not to the extravillous trophoblasts, villous cytotrophoblasts and villous core [25, 35, 45–48] (Fig. 1). The absence of 11β-HSD2 in the extravillous trophoblasts suggests that glucocorticoids are required for the invasion of extravillous trophoblasts into the endometrium during implantation.
Fig. 1.
Distribution of 11β-HSD1 and 2 in human decidua and chorionic villous tissues during early gestation. Top panel: Decidual epithelial cells are cytokeratin 7 positive and stromal cells are vimentin positive. Bottom panel: Villous trophoblasts are HLA-G negative while extravillous trophoblasts are HLA-G positive. CT cytotrophoblast; ST syncytiotrophoblast; EVT extravillous trophoblast; FBV fetal blood vessels. Based on the work presented in the Ref. [46]. Human tissue collection was approved by the Ethics Committee of Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine with informed consent
Several studies have shown that glucocorticoids are implicated in a number of events pertinent to the establishment of implantation including inhibition of the maternal immune intolerance of the semi-allograft embryo, enhancement of endometrial stromal cell decidualization and stimulation of human chorionic gonadotropin (hCG) production by the trophoblasts [3]. The specific distribution of 11β-HSD2 in the outer continuous syncytial layer of the chorionic villi can be traced back to as early as the 3rd week post-conception [46, 47], when remodeling of maternal spiral arteries and blood supply to the placenta is fully accomplished and the process of placentation is completed [49]. This early appearance of 11β-HSD2 on the surface of chorionic villi suggests a critical role of 11β-HSD2 in the protection of embryo development even at this very early stage of gestation. Unfortunately, it is unclear whether the expression of 11β-HSD2 is already initiated in the trophoblasts of the trophectoderm of the implanting embryo. If so, the shield against maternal glucocorticoids might already be established even before the completion of placentation. Notably, the formation of the trophoblast plug in the erosive spiral artery can prevent the implanting embryo from direct maternal blood flushing and this plug is not removed until the end of the 6th week after conception [50]. Nonetheless, the implanting embryo is still immersed and nourished by secretions from the endometrial cells [51], which may contain relatively high concentrations of glucocorticoids. Our immunohistochemical staining of the first trimester endometrium shows that abundant 11β-HSD1 is present in the endometrial stromal cells, glandular and endometrial epithelium [46] (Fig. 1), which suggests that, on the one hand, cortisol regeneration is required for the implantation, and that, at the same time, cortisol regeneration may heighten the concentration of cortisol in the endometrial secretion, thus imposing threats to the developing embryo. Therefore, it is very likely that 11β-HSD2 expression is already set up in the trophectoderm layer for the protection of the implanting embryo.
Ontogeny of 11β-HSD2 expression in the placenta
Examination of the ontogeny of placental 11β-HSD2 expression reveals that 11β-HSD2 mRNA and activity increase with gestational age until late gestation [52–55]. McTernan et al. reported 12- and 56-fold increases in 11β-HSD2 mRNA abundance in the villous tissue by early third trimester (27–34 weeks) and term, respectively, in comparison with the levels observed at 4–6 weeks after conception [52]. Schoof et al. reported similar increases in 11β-HSD2 mRNA abundance in the placenta from 16 to 40 weeks of gestation [53]. In addition, Murphy et al. found that there was no further change in 11β-HSD2 mRNA abundance in the placenta around term (36, 37, 38 and > 38 weeks) [56].
11β-HSD2 activity studies reveal a similar pattern of increases across gestational age [54, 55] but there also appeared to be a decline after 36 weeks [56]. Similar declines are observed close to term in other animal species as well, including rats [57], mice [58–60], rabbits [61] and guinea pigs [62]. Therefore, a decline in 11β-HSD2 activity in the placenta around term may be a generalized phenomenon across species and it indicates the requirement of maternal glucocorticoids for fetal organ maturation as well as parturition. In addition, the presence of 11β-HSD1 in the endothelium of the fetal blood vessels in the villous core and in the umbilical cord [63] may also assist in the acquisition of maternal glucocorticoids for this purpose. Indeed, a placental perfusion study shows that a considerable amount of cortisone perfused into the intervillous space on the maternal side is converted into cortisol when passing through the placenta into the fetal circulation [21].
Pathways signaling the establishment of the placental glucocorticoid barrier
Does DNA methylation matter in the setup of placental glucocorticoid barrier?
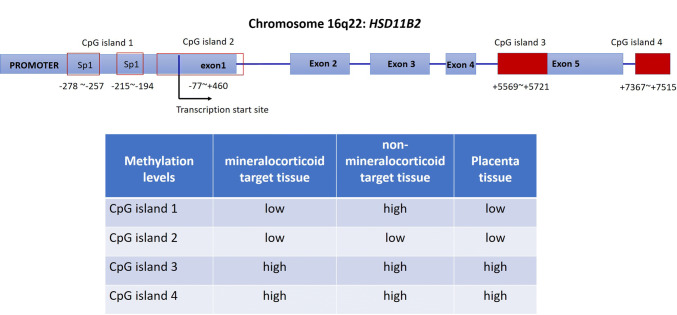
As described above, 11β-HSD2 exhibits cell-specific expression in mineralocorticoid target cells. This cell-specific expression of 11β-HSD2 is now known to be attributed to the epigenetic modification of HSD11B2, the gene encoding 11β-HSD2. The human HSD11B2 gene, which is localized to chromosome 16q22, consists of five exons spreading across 6.2 kb which are separated from each other by small introns [64]. RNase protection assays identified the presence of tissue-specific transcriptional start sites. In the placenta, transcription starts mainly at − 74 nucleotides (nt) and to a lesser extent at − 116 nt. In contrast, the kidney utilizes the − 116 nt almost exclusively [65]. The promoter of the human HSD11B2 gene lacks a TATA box but has a highly enriched GC core, suggesting that the gene may be regulated transcriptionally by factors such as Sp1 which recognize GC-rich sequences [65].
Using luciferase reporter constructs, Agarwal et al. identified that the region from − 2 to − 330 nt relative to the initial ATG codon was an essential region for the basal transcription of the HSD11B2 gene [65]. Foot printing or gel shift experiments show that this region contains two GC-rich domains ( − 278 to − 257 nt and − 215 to − 194 nt) which harbors the canonical binding sites for Sp1 [65]. Apart from the CpG island in the promoter, another three CpG islands are found spanning the promoter and exon 1 ( − 77 to + 460 nt), in exon 5 (+ 5569 to + 5721 nt) and in the downstream region (+ 7367 to + 7515 nt) [66] (Fig. 2). Of these four CpG islands, the two downstream ones are fully methylated in either mineralocorticoid or non-mineralocorticoid target tissues, while the methylation levels of the CpG island in the promoter region are low in mineralocorticoid target tissues, but high in non-mineralocorticoid target tissues [66] (Fig. 2). Of note, the CpG island spanning the promoter and exon 1 is only slightly methylated in both mineralocorticoid or non-mineralocorticoid target tissues [66] (Fig. 2). These data suggest that the methylation level of the CpG island in the promoter is a determining factor as to whether the expression of HSD11B2 is repressed or not, and hypermethylation of this CpG island is responsible for the muted expression in non-mineralocorticoid-target cells [66].
Fig. 2.
Locations of the four CpG islands in HSD11B2 gene and the methylation levels in mineralocorticoid and non-mineralocorticoid target tissues, and in cytotrophoblast and syncytiotrophoblast. Based on the data presented in references 25 and 66. Boxes in red either filled or blank are indications of CpG islands
Of interest, Alikhani-Koopaei et al. found that the methylation levels of the CpG island in HSD11B2 promoter were low in human placental tissue. Consistent with this, a recent study by Hu et al. also reported that almost no 11β-HSD2 methylation was detected in both normal and preeclamptic placental tissues [67]. These findings not only explain why the placental syncytiotrophoblast can maintain such high levels of 11β-HSD2 expression, but also raise an issue as to why 11β-HSD2 is barely expressed in the cytotrophoblasts [46, 47, 63], the progenitor cells for the syncytiotrophoblast. Can DNA methylation be excluded from the mechanisms underlying the silencing of 11β-HSD2 expression before syncytialization?
DNA methylation plays a crucial role in defining cell fate in mammalian development, which provides an epigenetic barrier that reduces developmental potential of a particular cell while helping to establish its distinct cellular identity. The DNA methylation marks in the parental gametes are erased on a global scale in the zygote immediately following fertilization to restore the developmental totipotency [68]. The re-establishment of DNA methylation marks starts during the segregation of the inner cell mass and trophectoderm of the embryo with the commitment towards a distinct cell fate [68, 69]. While the inner cell mass eventually gives rise to the definitive structures of the fetus, the trophectoderm gives rise to the placenta including the cytotrophoblasts, the progenitor cells for both extravillous trophoblasts and villous syncytiotrophoblast. Because low methylation levels of the CpG island in the HSD11B2 promoter are detected in the placenta tissue [66], it is conceivable that the low methylation levels of the CpG island in the HSD11B2 promoter is already set up when the trophoblast fate is determined during the segregation of the trophectoderm and inner cell mass. We compared the methylation levels of the CpG domain (− 244 to + 16) in the HSD11B2 promoter in human placental cytotrophoblasts and syncytiotrophoblasts, and found no differences in the methylation levels in this region between these two types of cells [70]. Although no difference in the methylation level of the CpG island in the HSD11B2 promoter was found between cytotrophoblasts and syncytiotrophoblasts, sporadic methylated cystines were nevertheless observed in this region in both cytotrophoblasts and syncytiotrophoblasts [70]. Currently, we are not clear about the meaning of these sporadic methylations in the regulation of HSD11B2 expression in the trophoblasts.
Our data support the fact that the methylation pattern of the HSD11B2 promoter is already established when the cytotrophoblast fate is determined in the trophectoderm. If it is not a matter of methylation, the question remains as to what is the signaling pathway that underlies the upsurge of HSD11B2 expression during syncytialization. Our studies demonstrate that histone modification and transcription factors activated by hCG during syncytialization might play a critical role in this process [45, 48, 70].
Role of hCG-activated signaling pathways in the setup of the placental glucocorticoid barrier
HCG is the first hormone produced by the trophoblasts of the implanting blastocyst. There are two major forms of hCG in pregnancy: the classical and hyperglycosylated hCG. The classical hCG is produced primarily by differentiated syncytiotrophoblasts, while the hyperglycosylated hCG is produced by the extravillous trophoblasts [71, 72]. The hyperglycosylated hCG is believed to drive the invasion of extravillous trophoblasts into the myometrium possibly through the TGFβ receptor thereby assisting in the implantation process. The best characterized role for the classical hCG is to maintain the function of corpus luteum for the production progesterone and estrogen through the cAMP/PKA pathway coupled with the hCG/LH receptor until the steroidogenic activity of the placenta is fully established around the 3rd and 4th weeks of gestation [72]. Intriguingly, the secretion of hCG continues to increase even after this mission is accomplished and peaks of hCG production are observed around the 10th week of gestation. The concentration of hCG declines after this, but it is still maintained at a high level which is about 18% of the peak value [72]. This pattern of hCG production in gestation strongly indicates a role of hCG beyond the mere regulation of implantation and maintenance of corpus luteum functions in early gestation. Accumulating evidence indicates that hCG is involved in multiple functions that maintain gestation, including promoting angiogenesis in the uterine endothelium, maintaining myometrial quiescence and enhancing syncytialization, a process that continues throughout pregnancy [72, 73].
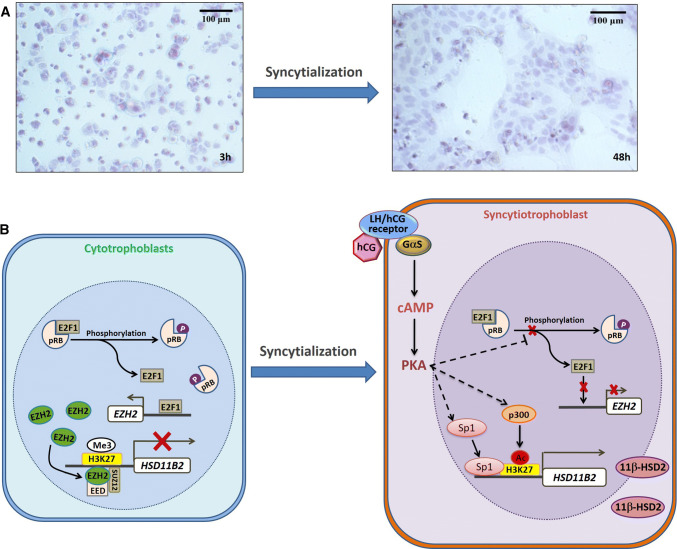
Our study indicates that hCG is also essential in the upsurge of HSD11B2 expression during syncytialization [74]. We found that hCG reduced the phosphorylation of retinoblastoma protein (pRB) via activation of the cAMP/PKA pathway, which sequesters E2F transcription factor 1 (E2F1). This is the transcription factor for EZH2 expression which results in the inactivation of the pRB–E2F1–EZH2 pathway and removal of the repressive marker trimethylation of histone H3 lysine 27 (H3K27me3) at the HSD11B2 promoter [48] (Fig. 3). The removal of H3K27me3 renders H3K27 available for acetylation (H3K27Ac) by p300, which transforms the chromatin from a compact structure into a loose form, allowing access of the HSD11B2 promoter to the transcription factor, Sp1, which enhances its transcription [48] (Fig. 3). Consistently, the hCG-activated cAMP pathway during syncytialization causes a dramatic decrease in EZH2 expression in addition to the reduction in pRB phosphorylation, and increases in Sp1 and p300 along with the robust 11β-HSD2 expression [45, 48, 70]. Concomitantly, the enrichment of H3K27me3 is decreased while the H3K27ac is increased at the HSD11B2 promoter during syncytialization [45, 48]. Consistent with these findings, a recent study demonstrated that inhibition of histone deacetylase can indeed promote 11β-HSD2 expression in JEG3 cells, a choriocarcinoma cell line [75].
Fig. 3.
Diagram illustrating the regulation of HSD11B2 expression by human chorionic gonadotropin (hCG)-triggered cAMP/PKA pathway during syncytialization of the trophoblasts. Panel A shows the syncytialization of human primary placental trophoblasts under cultured conditions in DMEM containing 10% fetal calf serum. Panel B illustrates the mechanism underpinning the upregulation of HSD11B2 expression during syncytialization. Before syncytialization, phosphorylation of retinoblastoma protein (pRB) frees the E2F transcription factor 1(E2F1) which drives the expression of the enhancer of zeste homolog 2 (EZH2) in cytotrophoblasts. Subsequently, EZH2 together with cofactors embryonic ectoderm development (EED) and suppressor of zeste 12 homolog (SUZ12) cause trimethylation (Me3) of histone H3 lysine 27 (H3K27) associated with HSD11B2 promoter resulting in diminished expression of HSD11B2. Upon syncytialization, hCG is produced in large quantities and activates the cAMP/PKA pathway, which indirectly blocks the phosphorylation of pRB. Dephosphorylation of pRB sequesters E2F1 thereby reducing the expression of EZH2 and resulting in the attenuation of the down-stream H3K27 methylation. Attenuated H3K27 methylation predisposes H3K27 to acetylation (Ac) by p300, which transforms the condensed chromatin into a more relaxed structure allowing the enrichment of the transcription factor specificity protein 1(SP1) at the HSD11B2 promoter resulting in increased transcription of HSD11B2. This diagram is based on the work described in Refs. [45, 48, 70, 74]
Other signaling pathways involved in the regulation of 11β-HSD2 expression in the placenta
In addition to the above-described hCG-activated signaling pathways and transcription factor, a number of other hormones produced by the syncytialized trophoblasts, signaling pathways and transcription factors may also be involved in the regulation of HSD11B2 expression in placental trophoblasts. For instance, in addition to hCG, corticotropin-releasing hormone (CRH) is also produced in large quantities and activates the cAMP/PKA pathway during syncytialization. It has been shown that CRH indeed increases the abundance of 11β-HSD2 in primary human placental trophoblasts [76]. Of note, binding sites for potential transcription factors including nuclear factor 1 (NF1), Arnt1, Ah-Arnt, AP2, AP4 and Ik2 have been revealed in the methylated CpG sites in the HSD11B2 promoter by in silico analyses [66]. Methylation of these CpG sites is shown to diminish the binding activities of not only Sp1 but also NF1 and Arnt [66]. Moreover, inhibition of the mitogen-activated protein kinases, ERK1/2, increases HSD11B2 expression [77], while suppressing p38 decreases 11β-HSD2 activity [78]. Moreover, activation of both peroxisome proliferator-activated receptor delta (PPARδ) and the hedgehog signaling pathways are associated with increased HSD11B2 expression [79, 80]. Emerging data also indicate that these signaling pathways appear to form a complex network in the regulation of HSD11B2 expression during syncytialization. For example, the cAMP/PKA pathway activated by hCG has been shown to interact with the ERK1/2 and p38 pathways [81], while activation of the PPARγ pathway has been shown to be associated with Sp1 in the regulation of HSD11B2 expression in placental trophoblasts [82]. However, the exact interactions among the complex repertoire of signaling pathways in the regulation of HSD11B2 expression during syncytialization await further investigation.
Effects of adverse conditions on HSD11B2 expression in the placenta
The epigenetic modifications are very susceptible to environmental signals and can be altered at critical periods of development [83]. Accumulating evidence indicates that adverse conditions in pregnancy such as stress and nutritional restriction can result in intrauterine growth restriction which is possibly associated with the increased methylation levels of the CpG island in the promoter of HSD11B2 gene. The increased methylation levels lead to decreased expression of HSD11B2, which can then compromise the placental glucocorticoid barrier, resulting in overexposure of the fetus to maternal glucocorticoids. Overexposure to glucocorticoids causes fetal growth retardation and can cause permanent changes in the expression pattern of the genes associated with the development of chronic diseases in later life.
Intrauterine growth restriction
Intrauterine growth restriction (IUGR) is a condition defined as a fetus being less than 10% of its estimated fetal weight for a particular gestational age. The causes may vary but most often involve nutrition restriction, hypoxia and stress. Epidemiological evidence indicates that IUGR is associated with an increased risk of hypertension, diabetes and obesity in later life, and overexposure of the fetus to maternal glucocorticoids has been identified as a potential underlying mechanism [8, 84, 85]. A considerable body of evidence has been building up in recent years for the correlation between reduced placental 11β-HSD2 expression and IUGR in human pregnancies [52, 54, 86, 87]. Catch-up growth is often seen after IUGR in the first year of extra-uterine life. Interestingly, Tzschoppe et al. showed that the expression of 11β-HSD2 in the placenta correlated not only positively with the birth weight but also inversely with the growth velocity in the first year of extra-uterine life of the IUGR baby [88], further indicating a crucial role of placental 11β-HSD2 in the protection from IUGR. The enhancement of methylation levels in the HSD11B2 promoter is emerging as the major mechanism underlying the reduced 11β-HSD2 abundance in IUGR [89–92]. Marsit et al. reported that the extent of methylation of the HSD11B2 promoter in the placenta was greatest in infants with the lowest birth weights [92]. Unfortunately, there is little information regarding the precise mechanisms underlying the methylation of the HSD11B2 promoter. The mechanisms involved may differ depending on the causes of IUGR.
Stress
Different stressors can be encountered in pregnancy. Notably, both human and animal studies have shown that acute and chronic stress may have differential effects on the expression of 11β-HSD2 in the placenta [93–97]. While acute stress stimulates the expression of 11β-HSD2, chronic stress inhibits the expression of 11β-HSD2 in the placenta [93–97]. In agreement with these findings, acute treatment of cultured placental trophoblasts with glucocorticoids also increased the expression of HSD11B2 [74, 98]. It is likely that the up-regulation of placental 11ß-HSD2 by acute stress may be an immediate protective measure adopted by the fetus against the sudden elevation of maternal glucocorticoids, while the inhibition of placental 11ß-HSD2 by chronic stress may be a strategy adopted by the fetus for its survival because chronic stress is a risk factor for preterm birth [99]. For this reason, the inhibition of placental 11ß-HSD2 by chronic stress enables more cortisol to pass into the fetal circulation thereby promoting the maturation of vital fetal organs for its survival in extra-uterine life. However, this survival strategy occurs at the expense of growth restriction and therefore increases the risk of chronic diseases in later life.
The mechanisms underpinning the differential regulation of placental 11ß-HSD2 expression by acute and chronic stress are not fully understood. However, studies have shown that the stimulation of placental 11ß-HSD2 expression by glucocorticoids occurs at both transcriptional and post-transcriptional levels and is mediated by glucocorticoid receptors [74, 98]. At the transcriptional level, glucocorticoid-stimulated hCG production may account, at least in part, for the stimulation of HSD11B2 expression by these hormones [74]. Accumulating evidence has demonstrated that chronic stress-induced reduction in placental 11ß-HSD2 expression may be associated with the hypermethylation of the HSD11B2 gene promoter [91, 97, 100]. Although the detailed mechanisms underlying the hypermethylation of the HSD11B2 gene promoter by chronic stress is not known, increased expression of the DNA methyltransferases may be involved in this process [97, 101]. Interestingly, chronic stress-induced hypermethylation of the HSD11B2 gene promoter appears to be sexually dimorphic affecting mainly the female offspring [101–104].
Hypoxia
Maternal hypoxia is a common perturbation that can disrupt placental function and slow down fetal development, thus contributing to neonatal impairment. A study in mice showed that maternal hypoxia during mid- to late gestation not only changed the placental morphology but also reduced fetal birth weight with altered gene expressions including reduced MR and GR as well as HSD11B2 expression in the placenta [105]. Human studies also demonstrated that the expression of HSD11B2 was decreased when fetal asphyxia is present in the late phase of IUGR pregnancies [106].
In vitro studies using human placental trophoblasts also showed that when the cells were exposed to hypoxic conditions, both the induction of 11ß-HSD2 and syncytialization of trophoblasts were prevented [107, 108]. A further study revealed that hypoxia may induce an initially rapid down-regulation of 11ß-HSD2 protein synthesis at the translation level, and a later down-regulation of HSD11B2 gene transcription [109]. However, it is not clear whether this transcriptional mechanism involves epigenetic modification of the HSD11B2 gene.
Preeclampsia is characterized by shallow trophoblast invasion with subsequent hypoxemia. Studies in preeclampsia demonstrated no significant change in the methylation level in HSD11B2 gene promoter [67, 110], despite the decrease of 11ß-HSD2 abundance seen in the placental tissue in this condition [53, 67, 82, 111]. These data suggest that hypoxia may affect placental HSD11B2 expression through mechanisms other than modification through methylation.
Nutrition
The effect of nutritional restriction on birth weight and subsequent disease during adulthood is well demonstrated in studies of exposure to famine, most notably seen during the Dutch Hunger Winter [112, 113] as well as by studies in animals [114–116]. Overexposure to glucocorticoids during the critical window of fetal development is believed to be the critical mechanism underlying the early life programming of adult diseases in maternal nutritional restriction [117], suggesting that lack of nutrition can reduce the expression of HSD11B2 in the placenta. Indeed, a number of studies in animals have demonstrated that maternal dietary intake restriction [118, 119] or protein content restriction [120–122] during early- to mid-gestation can result in decreased placental HSD11B2 expression.
An in vitro study using BeWo cells, a trophoblast cell line, revealed that low levels of amino acids might decrease 11β-HSD2 abundance through leptin-activated JAK-STAT and MAPK signaling pathways [123]. However, an alternative mechanism for this phenomenon involves the possibility of modification of DNA methylation which remains a highly probable option in nutritional restriction because this process depends on the availability of methyl group donors and cofactors produced by methionine and folate metabolism or is provided by dietary-derived vitamins B6 and B12 [124]. However, limited data are available at present regarding the effect of dietary components on the methylation modification of the HSD11B2 gene in the placenta. Of the few studies addressing this issue, investigation of the effect of dietary folic acid on the methylation level of the HSD11B2 gene in the placenta is in favor of the methylation modification of HSD11B2 gene in the placenta by dietary components [104]. In a similar experimental paradigm, folate supplementation during pregnancy was shown to be able to prevent much of the adverse programming effects of maternal protein restriction on the cardiovascular system in the offspring [125], suggesting that folate component in the diet may play a critical role in the modification of HSD11B2 gene methylation in the placenta.
Summary and future perspectives
The placenta is equipped with a glucocorticoid barrier for the protection of the fetus from the adverse effects of excessive maternal glucocorticoids in almost all placental mammals including humans. This barrier is enforced by the expression of 11β-HSD2 in the syncytial layer of the placental villi, which converts biologically active cortisol into inactive cortisone. Impairment of this placental glucocorticoid barrier is associated with fetal IUGR and development of chronic diseases in later life. Ontogeny studies show that the expression of 11β-HSD2 is initiated at a very early stage after conception and increases with gestational age but declines around term. The promoter of HSD11B2, the gene encoding 11β-HSD2, has a highly GC-rich core. However, the pattern of methylation marks on HSD11B2 may have already been set up when the trophoblast identity of the blastocyst is committed. Instead, pregnancy hormones, such as the hCG-initiated signaling pathway, appear to be responsible for the upsurge of 11β-HSD2 expression during trophoblast syncytialization. By activating the cAMP/PKA pathway, hCG not only alters the modification of histones but also increases the expression of Sp1 which activates the transcription of HSD11B2.
Different causes of IUGR such as stress, hypoxia and nutritional restriction can be encountered in pregnancy. Available studies indicate that different causes of IUGR may attenuate HSD11B2 expression in the placenta by differential mechanisms. While stress and nutritional restriction may reduce HSD11B2 expression by increasing its methylation, hypoxia may decrease HSD11B2 expression via an alternative mechanism rather than methylation. However, the detailed mechanisms underlying the attenuation of HSD11B2 expression by adverse conditions have yet to be elucidated. We are unclear about how the methylation pattern of the HSD11B2 promoter is changed by these adverse conditions and neither do we know the exact alternative pathways underpinning the reduction of HSD11B2 expression by other conditions in the placenta. In addition, it still remains a puzzle when and where HSD11B2 expression is initiated in the blastocyst before the 3rd week of gestation. We believe that elucidation of these issues may help to develop strategies to provide a safer intrauterine environment for the development of fetus.
Acknowledgements
This work was supported by National Natural Science Foundation of China (31671566 and 81330018), National Key R&D Program of China (2017YFC1001403) and National Key Basic Research Projects (2014CB943302). The authors would like to thank Dr. Dev Sooranna, Imperial College London, for editing the manuscript.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
References
- 1.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whirledge S, Cidlowski JA. Glucocorticoids and reproduction: traffic control on the road to reproduction. Trends Endocrinol Metabol. 2017;28(6):399–415. doi: 10.1016/j.tem.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michael AE, Papageorghiou AT. Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Hum Reprod Update. 2008;14(5):497–517. doi: 10.1093/humupd/dmn021. [DOI] [PubMed] [Google Scholar]
- 4.Larry CG. Effect of corticosteroids for fetal maturation on perinatal outcomes: NIH consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273(5):413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 5.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525. [PubMed] [Google Scholar]
- 6.Li XQ, Zhu P, Myatt L, Sun K. Roles of glucocorticoids in human parturition: a controversial fact? Placenta. 2014;35(5):291–296. doi: 10.1016/j.placenta.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Chen ZJ, Myatt L, Sun K. 11beta-HSD 1 in human fetal membranes as a potential therapeutic target for preterm birth. Endocr Rev. 2018 doi: 10.1210/er.2017-00188. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds RM. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis—2012 Curt Richter Award Winner. Psychoneuroendocrinology. 2013;38(1):1–11. doi: 10.1016/j.psyneuen.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.cd004454.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds RM. Programming effects of glucocorticoids. Clin Obstet Gynecol. 2013;56(3):602–609. doi: 10.1097/GRF.0b013e31829939f7. [DOI] [PubMed] [Google Scholar]
- 11.Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18(3):378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- 12.Ishimoto H, Jaffe RB. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr Rev. 2011;32(3):317–355. doi: 10.1210/er.2010-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macnaughton MC, Taylor T, McNally EM, Coutts JR. The effect of synthetic ACTH on the metabolism of [4-14C]-progesterone by the previable human fetus. J Steroid Biochem. 1977;8(5):499–504. doi: 10.1016/0022-4731(77)90252-7. [DOI] [PubMed] [Google Scholar]
- 14.Rainey WE, Rehman KS, Carr BR. Fetal and maternal adrenals in human pregnancy. Obstet Gynecol Clin North Am. 2004;31(4):817–835. doi: 10.1016/j.ogc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Scott EM, McGarrigle HH, Lachelin GC. The increase in plasma and saliva cortisol levels in pregnancy is not due to the increase in corticosteroid-binding globulin levels. J Clin Endocrinol Metabol. 1990;71(3):639–644. doi: 10.1210/jcem-71-3-639. [DOI] [PubMed] [Google Scholar]
- 16.Cousins L, Rigg L, Hollingsworth D, Meis P, Halberg F, Brink G, Yen SS. Qualitative and quantitative assessment of the circadian rhythm of cortisol in pregnancy. Am J Obstet Gynecol. 1983;145(4):411–416. doi: 10.1016/0002-9378(83)90309-5. [DOI] [PubMed] [Google Scholar]
- 17.Nolten WE, Rueckert PA. Elevated free cortisol index in pregnancy: possible regulatory mechanisms. Am J Obstet Gynecol. 1981;139(4):492–498. doi: 10.1016/0002-9378(81)90331-8. [DOI] [PubMed] [Google Scholar]
- 18.Burke CW, Roulet F. Increased exposure of tissues to cortisol in late pregnancy. Br Med J. 1970;1(5697):657–659. doi: 10.1136/bmj.1.5697.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gitau R, Adams D, Fisk NM, Glover V. Fetal plasma testosterone correlates positively with cortisol. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F166–F169. doi: 10.1136/adc.2004.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy BE, Clark SJ, Donald IR, Pinsky M, Vedady D. Conversion of maternal cortisol to cortisone during placental transfer to the human fetus. Am J Obstet Gynecol. 1974;118(4):538–541. doi: 10.1016/S0002-9378(16)33697-3. [DOI] [PubMed] [Google Scholar]
- 21.Sun K, Adamson SL, Yang K, Challis JR. Interconversion of cortisol and cortisone by 11beta-hydroxysteroid dehydrogenases type 1 and 2 in the perfused human placenta. Placenta. 1999;20(1):13–19. doi: 10.1053/plac.1998.0352. [DOI] [PubMed] [Google Scholar]
- 22.Giannopoulos G, Jackson K, Tulchinsky D. Glucocorticoid metabolism in human placenta, decidua, myometrium and fetal membranes. J Steroid Biochem. 1982;17(4):371–374. doi: 10.1016/0022-4731(82)90628-8. [DOI] [PubMed] [Google Scholar]
- 23.Beitins IZ, Bayard F, Ances IG, Kowarski A, Migeon CJ. The metabolic clearance rate, blood production, interconversion and transplacental passage of cortisol and cortisone in pregnancy near term. Pediatr Res. 1973;7(5):509–519. doi: 10.1203/00006450-197305000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Seckl JR, Benediktsson R, Lindsay RS, Brown RW. Placental 11 beta-hydroxysteroid dehydrogenase and the programming of hypertension. J Steroid Biochem Mol Biol. 1995;55(5–6):447–455. doi: 10.1016/0960-0760(95)00193-X. [DOI] [PubMed] [Google Scholar]
- 25.Albiston AL, Obeyesekere VR, Smith RE, Krozowski ZS. Cloning and tissue distribution of the human 11 beta-hydroxysteroid dehydrogenase type 2 enzyme. Mol Cell Endocrinol. 1994;105(2):R11–R17. doi: 10.1016/0303-7207(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 26.Tannin GM, Agarwal AK, Monder C, New MI, White PC. The human gene for 11 beta-hydroxysteroid dehydrogenase. Structure, tissue distribution, and chromosomal localization. J Biol Chem. 1991;266(25):16653–16658. [PubMed] [Google Scholar]
- 27.White PC, Mune T, Agarwal AK. 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev. 1997;18(1):135–156. doi: 10.1210/edrv.18.1.0288. [DOI] [PubMed] [Google Scholar]
- 28.Seckl JR. 11 beta-hydroxysteroid dehydrogenase isoforms and their implications for blood pressure regulation. Eur J Clin Invest. 1993;23(10):589–601. doi: 10.1111/j.1365-2362.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 29.Monder C, Shackleton CH. 11 beta-Hydroxysteroid dehydrogenase: fact or fancy? Steroids. 1984;44(5):383–417. doi: 10.1016/S0039-128X(84)80001-X. [DOI] [PubMed] [Google Scholar]
- 30.Brown RW, Chapman KE, Edwards CR, Seckl JR. Human placental 11 beta-hydroxysteroid dehydrogenase: evidence for and partial purification of a distinct NAD-dependent isoform. Endocrinology. 1993;132(6):2614–2621. doi: 10.1210/endo.132.6.8504762. [DOI] [PubMed] [Google Scholar]
- 31.Hellal-Levy C, Couette B, Fagart J, Souque A, Gomez-Sanchez C, Rafestin-Oblin M. Specific hydroxylations determine selective corticosteroid recognition by human glucocorticoid and mineralocorticoid receptors. FEBS Lett. 1999;464(1–2):9–13. doi: 10.1016/S0014-5793(99)01667-1. [DOI] [PubMed] [Google Scholar]
- 32.Seckl JR, Walker BR. Minireview: 11beta-hydroxysteroid dehydrogenase type 1—a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142(4):1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- 33.Seckl JR, Chapman KE. Medical and physiological aspects of the 11beta-hydroxysteroid dehydrogenase system. Eur J Biochem. 1997;249(2):361–364. doi: 10.1111/j.1432-1033.1997.t01-1-00361.x. [DOI] [PubMed] [Google Scholar]
- 34.Hirasawa G, Takeyama J, Sasano H, Fukushima K, Suzuki T, Muramatu Y, Darnel AD, Kaneko C, Hiwatashi N, Toyota T, Nagura H, Krozowski ZS. 11Beta-hydroxysteroid dehydrogenase type II and mineralocorticoid receptor in human placenta. J Clin Endocrinol Metabol. 2000;85(3):1306–1309. doi: 10.1210/jcem.85.3.6429. [DOI] [PubMed] [Google Scholar]
- 35.Krozowski Z, MaGuire JA, Stein-Oakley AN, Dowling J, Smith RE, Andrews RK. Immunohistochemical localization of the 11 beta-hydroxysteroid dehydrogenase type II enzyme in human kidney and placenta. J Clin Endocrinol Metabol. 1995;80(7):2203–2209. doi: 10.1210/jcem.80.7.7608280. [DOI] [PubMed] [Google Scholar]
- 36.Brown RW, Chapman KE, Murad P, Edwards CR, Seckl JR. Purification of 11 beta-hydroxysteroid dehydrogenase type 2 from human placenta utilizing a novel affinity labelling technique. Biochem J. 1996;313(Pt 3):997–1005. doi: 10.1042/bj3130997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benediktsson R, Calder AA, Edwards CR, Seckl JR. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol. 1997;46(2):161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- 38.Burton PJ, Waddell BJ. Dual function of 11beta-hydroxysteroid dehydrogenase in placenta: modulating placental glucocorticoid passage and local steroid action. Biol Reprod. 1999;60(2):234–240. doi: 10.1095/biolreprod60.2.234. [DOI] [PubMed] [Google Scholar]
- 39.Osinski PA. Steroid 11beta-ol dehydrogenase in human placenta. Nature. 1960;187:777. doi: 10.1038/187777a0. [DOI] [PubMed] [Google Scholar]
- 40.Beitins IZ, Bayard F, Ances IG, Kowarski A, Migeon CJ. The transplacental passage of prednisone and prednisolone in pregnancy near term. J Pediatr. 1972;81(5):936–945. doi: 10.1016/S0022-3476(72)80547-X. [DOI] [PubMed] [Google Scholar]
- 41.Levitz M, Jansen V, Dancis J. The transfer and metabolism of corticosteroids in the perfused human placenta. Am J Obstet Gynecol. 1978;132(4):363–366. doi: 10.1016/0002-9378(78)90768-8. [DOI] [PubMed] [Google Scholar]
- 42.Bernal AL, Flint AP, Anderson AB, Turnbull AC. 11 beta-Hydroxyteroid dehydrogenase activity (E.C. 1.1.1.146) in human placenta and decidua. J Steroid Biochem. 1980;13(9):1081–1087. doi: 10.1016/0022-4731(80)90140-5. [DOI] [PubMed] [Google Scholar]
- 43.Murphy BE. Ontogeny of cortisol-cortisone interconversion in human tissues: a role for cortisone in human fetal development. J Steroid Biochem. 1981;14(9):811–817. doi: 10.1016/0022-4731(81)90226-0. [DOI] [PubMed] [Google Scholar]
- 44.Murphy BE. Chorionic membrane as an extra-adrenal source of foetal cortisol in human amniotic fluid. Nature. 1977;266(5598):179–181. doi: 10.1038/266179a0. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Wang W, Liu C, Wang W, Li W, Shu Q, Chen ZJ, Sun K. Critical role of histone acetylation by p300 in human placental 11beta-HSD2 expression. J Clin Endocrinol Metabol. 2013;98(7):E1189–E1197. doi: 10.1210/jc.2012-4291. [DOI] [PubMed] [Google Scholar]
- 46.Yang Q, Wang W, Liu C, Wang Y, Sun K. Compartmentalized localization of 11beta-HSD 1 and 2 at the feto-maternal interface in the first trimester of human pregnancy. Placenta. 2016;46:63–71. doi: 10.1016/j.placenta.2016.08.079. [DOI] [PubMed] [Google Scholar]
- 47.Salvante KG, Milano K, Kliman HJ, Nepomnaschy PA. Placental 11 beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) expression very early during human pregnancy. J Dev Origins Health Dis. 2017;8(2):149–154. doi: 10.1017/S2040174416000611. [DOI] [PubMed] [Google Scholar]
- 48.Zuo R, Liu X, Wang W, Li W, Ying H, Sun K. A repressive role of enhancer of zeste homolog 2 in 11beta-hydroxysteroid dehydrogenase type 2 expression in the human placenta. J Biol Chem. 2017;292(18):7578–7587. doi: 10.1074/jbc.M116.765800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baergen RN. Mannual of Benerschke and Kaufmann’s pathology of the human placenta. New York: Springer; 2005. [Google Scholar]
- 50.Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol. 1999;181(3):718–724. doi: 10.1016/S0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- 51.Burton GJ, Jauniaux E, Charnock-Jones DS. Human early placental development: potential roles of the endometrial glands. Placenta. 2007;28(Suppl A):S64–S69. doi: 10.1016/j.placenta.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, Kilby MD, Stewart PM. Reduced placental 11beta-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metabol. 2001;86(10):4979–4983. doi: 10.1210/jcem.86.10.7893. [DOI] [PubMed] [Google Scholar]
- 53.Schoof E, Girstl M, Frobenius W, Kirschbaum M, Repp R, Knerr I, Rascher W, Dotsch J. Course of placental 11beta-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase mRNA expression during human gestation. Eur J Endocrinol. 2001;145(2):187–192. doi: 10.1530/eje.0.1450187. [DOI] [PubMed] [Google Scholar]
- 54.Shams M, Kilby MD, Somerset DA, Howie AJ, Gupta A, Wood PJ, Afnan M, Stewart PM. 11Beta-hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Hum Reprod. 1998;13(4):799–804. doi: 10.1093/humrep/13.4.799. [DOI] [PubMed] [Google Scholar]
- 55.Lopez Bernal A, Craft IL. Corticosteroid metabolism in vitro by human placenta, fetal membranes and decidua in early and late gestation. Placenta. 1981;2(4):279–285. doi: 10.1016/S0143-4004(81)80025-2. [DOI] [PubMed] [Google Scholar]
- 56.Murphy VE, Clifton VL. Alterations in human placental 11beta-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta. 2003;24(7):739–744. doi: 10.1016/S0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 57.Mark PJ, Augustus S, Lewis JL, Hewitt DP, Waddell BJ. Changes in the placental glucocorticoid barrier during rat pregnancy: impact on placental corticosterone levels and regulation by progesterone. Biol Reprod. 2009;80(6):1209–1215. doi: 10.1095/biolreprod.108.073650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Condon J, Ricketts ML, Whorwood CB, Stewart PM. Ontogeny and sexual dimorphic expression of mouse type 2 11beta-hydroxysteroid dehydrogenase. Mol Cell Endocrinol. 1997;127(2):121–128. doi: 10.1016/S0303-7207(97)04000-8. [DOI] [PubMed] [Google Scholar]
- 59.Thompson A, Han VK, Yang K. Spatial and temporal patterns of expression of 11beta-hydroxysteroid dehydrogenase types 1 and 2 messenger RNA and glucocorticoid receptor protein in the murine placenta and uterus during late pregnancy. Biol Reprod. 2002;67(6):1708–1718. doi: 10.1095/biolreprod.102.005488. [DOI] [PubMed] [Google Scholar]
- 60.Speirs HJ, Seckl JR, Brown RW. Ontogeny of glucocorticoid receptor and 11beta-hydroxysteroid dehydrogenase type-1 gene expression identifies potential critical periods of glucocorticoid susceptibility during development. J Endocrinol. 2004;181(1):105–116. doi: 10.1677/joe.0.1810105. [DOI] [PubMed] [Google Scholar]
- 61.McArdle AM, Denton KM, Maduwegedera D, Moritz K, Flower RL, Roberts CT. Ontogeny of placental structural development and expression of the renin-angiotensin system and 11beta-HSD2 genes in the rabbit. Placenta. 2009;30(7):590–598. doi: 10.1016/j.placenta.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Sampath-Kumar R, Matthews SG, Yang K. 11beta-hydroxysteroid dehydrogenase type 2 is the predominant isozyme in the guinea pig placenta: decreases in messenger ribonucleic acid and activity at term. Biol Reprod. 1998;59(6):1378–1384. doi: 10.1095/biolreprod59.6.1378. [DOI] [PubMed] [Google Scholar]
- 63.Sun K, Yang K, Challis JR. Differential expression of 11 beta-hydroxysteroid dehydrogenase types 1 and 2 in human placenta and fetal membranes. J Clin Endocrinol Metabol. 1997;82(1):300–305. doi: 10.1210/jcem.82.1.3681. [DOI] [PubMed] [Google Scholar]
- 64.Agarwal AK, Rogerson FM, Mune T, White PC. Gene structure and chromosomal localization of the human HSD11 K gene encoding the kidney (type 2) isozyme of 11 beta-hydroxysteroid dehydrogenase. Genomics. 1995;29(1):195–199. doi: 10.1006/geno.1995.1231. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal AK, White PC. Analysis of the promoter of the NAD+ dependent 11 beta-hydroxysteroid dehydrogenase (HSD11 K) gene in JEG-3 human choriocarcinoma cells. Mol Cell Endocrinol. 1996;121(1):93–99. doi: 10.1016/0303-7207(96)03855-5. [DOI] [PubMed] [Google Scholar]
- 66.Alikhani-Koopaei R, Fouladkou F, Frey FJ, Frey BM. Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression. J Clin Investig. 2004;114(8):1146–1157. doi: 10.1172/JCI21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu W, Wang H, Huang H. Analysis of gene expression and preliminary study of methylation about 11beta-HSD2 gene in placentas of Chinese pre-eclampsia patients of Han ethnicity. J Obstetr Gynaecol Res. 2015;41(3):343–349. doi: 10.1111/jog.12555. [DOI] [PubMed] [Google Scholar]
- 68.Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W. Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philos Trans R Soc Lond B Biol Sci. 2013;368(1609):20110330. doi: 10.1098/rstb.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakanishi MO, Hayakawa K, Nakabayashi K, Hata K, Shiota K, Tanaka S. Trophoblast-specific DNA methylation occurs after the segregation of the trophectoderm and inner cell mass in the mouse periimplantation embryo. Epigenetics. 2012;7(2):173–182. doi: 10.4161/epi.7.2.18962. [DOI] [PubMed] [Google Scholar]
- 70.Li JN, Ge YC, Yang Z, Guo CM, Duan T, Myatt L, Guan H, Yang K, Sun K. The Sp1 transcription factor is crucial for the expression of 11beta-hydroxysteroid dehydrogenase type 2 in human placental trophoblasts. J Clin Endocrinol Metabol. 2011;96(6):E899–E907. doi: 10.1210/jc.2010-2852. [DOI] [PubMed] [Google Scholar]
- 71.Cole LA. hCG, the wonder of today’s science. Reprod Biol Endocrinol. 2012;10:24. doi: 10.1186/1477-7827-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nwabuobi C, Arlier S, Schatz F, Guzeloglu-Kayisli O, Lockwood CJ, Kayisli UA. hCG: biological functions and clinical applications. Int J Mol Sci. 2017 doi: 10.3390/ijms18102037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerbaud P, Tasken K, Pidoux G. Spatiotemporal regulation of cAMP signaling controls the human trophoblast fusion. Front Pharmacol. 2015;6:202. doi: 10.3389/fphar.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ni XT, Duan T, Yang Z, Guo CM, Li JN, Sun K. Role of human chorionic gonadotropin in maintaining 11beta-hydroxysteroid dehydrogenase type 2 expression in human placental syncytiotrophoblasts. Placenta. 2009;30(12):1023–1028. doi: 10.1016/j.placenta.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Togher KL, Kenny LC, O’Keeffe GW. Class-specific histone deacetylase inhibitors promote 11-beta hydroxysteroid dehydrogenase type 2 expression in JEG-3 cells. Int J Cell Biol. 2017;2017:6169310. doi: 10.1155/2017/6169310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fahlbusch FB, Ruebner M, Volkert G, Offergeld R, Hartner A, Menendez-Castro C, Strick R, Rauh M, Rascher W, Dotsch J. Corticotropin-releasing hormone stimulates expression of leptin, 11beta-HSD2 and syncytin-1 in primary human trophoblasts. Reprod Biol Endocrinol. 2012;10:80. doi: 10.1186/1477-7827-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guan H, Sun K, Yang K. The ERK1/2 signaling pathway regulates 11beta-hydroxysteroid dehydrogenase type 2 expression in human trophoblast cells through a transcriptional mechanism. Biol Reprod. 2013;89(4):92. doi: 10.1095/biolreprod.113.110924. [DOI] [PubMed] [Google Scholar]
- 78.Sharma A, Guan H, Yang K. The p38 mitogen-activated protein kinase regulates 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) expression in human trophoblast cells through modulation of 11beta-HSD2 messenger ribonucleic acid stability. Endocrinology. 2009;150(9):4278–4286. doi: 10.1210/en.2009-0479. [DOI] [PubMed] [Google Scholar]
- 79.Julan L, Guan H, van Beek JP, Yang K. Peroxisome proliferator-activated receptor delta suppresses 11beta-hydroxysteroid dehydrogenase type 2 gene expression in human placental trophoblast cells. Endocrinology. 2005;146(3):1482–1490. doi: 10.1210/en.2004-1357. [DOI] [PubMed] [Google Scholar]
- 80.Zhu H, Zou C, Fan X, Xiong W, Tang L, Wu X, Tang C. Up-regulation of 11beta-hydroxysteroid dehydrogenase type 2 expression by hedgehog ligand contributes to the conversion of cortisol into cortisone. Endocrinology. 2016;157(9):3529–3539. doi: 10.1210/en.2016-1286. [DOI] [PubMed] [Google Scholar]
- 81.Shu Q, Li W, Li J, Wang W, Liu C, Sun K. Cross-talk between cAMP and MAPK pathways in HSD11B2 induction by hCG in placental trophoblasts. PLoS One. 2014;9(9):e107938. doi: 10.1371/journal.pone.0107938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He P, Chen Z, Sun Q, Li Y, Gu H, Ni X. Reduced expression of 11beta-hydroxysteroid dehydrogenase type 2 in preeclamptic placentas is associated with decreased PPARgamma but increased PPARalpha expression. Endocrinology. 2014;155(1):299–309. doi: 10.1210/en.2013-1350. [DOI] [PubMed] [Google Scholar]
- 83.Robins JC, Marsit CJ, Padbury JF, Sharma SS. Endocrine disruptors, environmental oxygen, epigenetics and pregnancy. Front Biosci. 2011;3:690–700. doi: 10.2741/e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reynolds RM. Corticosteroid-mediated programming and the pathogenesis of obesity and diabetes. J Steroid Biochem Mol Biol. 2010;122(1–3):3–9. doi: 10.1016/j.jsbmb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 85.Nyirenda MJ, Seckl JR. Intrauterine events and the programming of adulthood disease: the role of fetal glucocorticoid exposure (review) Int J Mol Med. 1998;2(5):607–614. doi: 10.3892/ijmm.2.5.607. [DOI] [PubMed] [Google Scholar]
- 86.Gomez-Roig MD, Mazarico E, Cardenas D, Fernandez MT, Diaz M, Ruiz de Gauna B, Vela A, Gratacos E, Figueras F. Placental 11B-hydroxysteroid dehydrogenase type 2 mRNA levels in intrauterine growth restriction versus small-for-gestational-age fetuses. Fetal Diagn Ther. 2016;39(2):147–151. doi: 10.1159/000437139. [DOI] [PubMed] [Google Scholar]
- 87.Dy J, Guan H, Sampath-Kumar R, Richardson BS, Yang K. Placental 11beta-hydroxysteroid dehydrogenase type 2 is reduced in pregnancies complicated with idiopathic intrauterine growth Restriction: evidence that this is associated with an attenuated ratio of cortisone to cortisol in the umbilical artery. Placenta. 2008;29(2):193–200. doi: 10.1016/j.placenta.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 88.Tzschoppe A, Struwe E, Blessing H, Fahlbusch F, Liebhaber G, Dorr HG, Rauh M, Rascher W, Goecke TW, Schild RL, Schleussner E, Scheler C, Hubler A, Dahlem P, Dotsch J. Placental 11beta-HSD2 gene expression at birth is inversely correlated with growth velocity in the first year of life after intrauterine growth restriction. Pediatr Res. 2009;65(6):647–653. doi: 10.1203/PDR.0b013e31819e7337. [DOI] [PubMed] [Google Scholar]
- 89.Zhao Y, Gong X, Chen L, Li L, Liang Y, Chen S, Zhang Y. Site-specific methylation of placental HSD11B2 gene promoter is related to intrauterine growth restriction. Eur J Hum Genet. 2014;22(6):734–740. doi: 10.1038/ejhg.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lazo-de-la-Vega-Monroy ML, Solis-Martinez MO, Romero-Gutierrez G, Aguirre-Arzola VE, Wrobel K, Wrobel K, Zaina S, Barbosa-Sabanero G. 11 beta-hydroxysteroid dehydrogenase 2 promoter methylation is associated with placental protein expression in small for gestational age newborns. Steroids. 2017;124:60–66. doi: 10.1016/j.steroids.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 91.Togher KL, Togher KL, O’Keeffe MM, O’Keeffe MM, Khashan AS, Khashan AS, Gutierrez H, Gutierrez H, Kenny LC, Kenny LC, O’Keeffe GW, O’Keeffe GW. Epigenetic regulation of the placental HSD11B2 barrier and its role as a critical regulator of fetal development. Epigenetics. 2014;9(6):816–822. doi: 10.4161/epi.28703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS One. 2012;7(3):e33794. doi: 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghaemmaghami P, Dainese SM, La Marca R, Zimmermann R, Ehlert U. The association between the acute psychobiological stress response in second trimester pregnant women, amniotic fluid glucocorticoids, and neonatal birth outcome. Dev Psychobiol. 2014;56(4):734–747. doi: 10.1002/dev.21142. [DOI] [PubMed] [Google Scholar]
- 94.O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology. 2012;37(6):818–826. doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 95.Welberg LA, Thrivikraman KV, Plotsky PM. Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. J Endocrinol. 2005;186(3):R7–R12. doi: 10.1677/joe.1.06374. [DOI] [PubMed] [Google Scholar]
- 96.Cuffe JS, O’Sullivan L, Simmons DG, Anderson ST, Moritz KM. Maternal corticosterone exposure in the mouse has sex-specific effects on placental growth and mRNA expression. Endocrinology. 2012;153(11):5500–5511. doi: 10.1210/en.2012-1479. [DOI] [PubMed] [Google Scholar]
- 97.Jensen Pena C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11beta-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS One. 2012;7(6):e39791. doi: 10.1371/journal.pone.0039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Beek JP, Guan H, Julan L, Yang K. Glucocorticoids stimulate the expression of 11beta-hydroxysteroid dehydrogenase type 2 in cultured human placental trophoblast cells. J Clin Endocrinol Metabol. 2004;89(11):5614–5621. doi: 10.1210/jc.2004-0113. [DOI] [PubMed] [Google Scholar]
- 99.Olson DM, Severson EM, Verstraeten BS, Ng JW, McCreary JK, Metz GA. Allostatic load and preterm birth. Int J Mol Sci. 2015;16(12):29856–29874. doi: 10.3390/ijms161226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ. The roles of DNA methylation of NR3C1 and 11beta-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics. 2013;8(12):1321–1329. doi: 10.4161/epi.26634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Green BB, Armstrong DA, Lesseur C, Paquette AG, Guerin DJ, Kwan LE, Marsit CJ. The role of placental 11-beta hydroxysteroid dehydrogenase type 1 and type 2 methylation on gene expression and infant birth weight. Biol Reprod. 2015;92(6):149. doi: 10.1095/biolreprod.115.128066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mina TH, Raikkonen K, Riley SC, Norman JE, Reynolds RM. Maternal distress associates with placental genes regulating fetal glucocorticoid exposure and IGF2: role of obesity and sex. Psychoneuroendocrinology. 2015;59:112–122. doi: 10.1016/j.psyneuen.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 104.Penailillo R, Guajardo A, Llanos M, Hirsch S, Ronco AM. Folic acid supplementation during pregnancy induces sex-specific changes in methylation and expression of placental 11beta-hydroxysteroid dehydrogenase 2 in rats. PLoS One. 2015;10(3):e0121098. doi: 10.1371/journal.pone.0121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cuffe JS, Walton SL, Singh RR, Spiers JG, Bielefeldt-Ohmann H, Wilkinson L, Little MH, Moritz KM. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J Physiol. 2014;592(14):3127–3141. doi: 10.1113/jphysiol.2014.272856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Borzsonyi B, Demendi C, Pajor A, Rigo J, Jr, Marosi K, Agota A, Nagy ZB, Joo JG. Gene expression patterns of the 11beta-hydroxysteroid dehydrogenase 2 enzyme in human placenta from intrauterine growth restriction: the role of impaired feto-maternal glucocorticoid metabolism. Eur J Obstet Gynecol Reprod Biol. 2012;161(1):12–17. doi: 10.1016/j.ejogrb.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 107.Hardy DB, Yang K. The expression of 11 beta-hydroxysteroid dehydrogenase type 2 is induced during trophoblast differentiation: effects of hypoxia. J Clin Endocrinol Metabol. 2002;87(8):3696–3701. doi: 10.1210/jcem.87.8.8720. [DOI] [PubMed] [Google Scholar]
- 108.Alfaidy N, Gupta S, DeMarco C, Caniggia I, Challis JR. Oxygen regulation of placental 11 beta-hydroxysteroid dehydrogenase 2: physiological and pathological implications. J Clin Endocrinol Metabol. 2002;87(10):4797–4805. doi: 10.1210/jc.2002-020310. [DOI] [PubMed] [Google Scholar]
- 109.Homan A, Guan H, Hardy DB, Gratton RJ, Yang K. Hypoxia blocks 11beta-hydroxysteroid dehydrogenase type 2 induction in human trophoblast cells during differentiation by a time-dependent mechanism that involves both translation and transcription. Placenta. 2006;27(8):832–840. doi: 10.1016/j.placenta.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 110.Hogg K, Blair JD, McFadden DE, von Dadelszen P, Robinson WP. Early onset pre-eclampsia is associated with altered DNA methylation of cortisol-signalling and steroidogenic genes in the placenta. PLoS One. 2013;8(5):e62969. doi: 10.1371/journal.pone.0062969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Causevic M, Mohaupt M. 11beta-Hydroxysteroid dehydrogenase type 2 in pregnancy and preeclampsia. Mol Aspects Med. 2007;28(2):220–226. doi: 10.1016/j.mam.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 112.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70(5):811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 113.Roseboom TJ, van der Meulen JH, Ravelli AC, van Montfrans GA, Osmond C, Barker DJ, Bleker OP. Blood pressure in adults after prenatal exposure to famine. J Hypertens. 1999;17(3):325–330. doi: 10.1097/00004872-199917030-00004. [DOI] [PubMed] [Google Scholar]
- 114.Guo C, Li C, Myatt L, Nathanielsz PW, Sun K. Sexually dimorphic effects of maternal nutrient reduction on expression of genes regulating cortisol metabolism in fetal baboon adipose and liver tissues. Diabetes. 2013;62(4):1175–1185. doi: 10.2337/db12-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Desai M, Crowther NJ, Lucas A, Hales CN. Organ-selective growth in the offspring of protein-restricted mothers. Br J Nutr. 1996;76(4):591–603. doi: 10.1079/BJN19960065. [DOI] [PubMed] [Google Scholar]
- 116.Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R757–R762. doi: 10.1152/ajpregu.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci. 2007;113(5):219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- 118.Belkacemi L, Jelks A, Chen CH, Ross MG, Desai M. Altered placental development in undernourished rats: role of maternal glucocorticoids. Reprod Biol Endocrinol. 2011;9:105. doi: 10.1186/1477-7827-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gnanalingham MG, Williams P, Wilson V, Bispham J, Hyatt MA, Pellicano A, Budge H, Stephenson T, Symonds ME. Nutritional manipulation between early to mid-gestation: effects on uncoupling protein-2, glucocorticoid sensitivity, IGF-I receptor and cell proliferation but not apoptosis in the ovine placenta. Reproduction. 2007;134(4):615–623. doi: 10.1530/REP-06-0369. [DOI] [PubMed] [Google Scholar]
- 120.Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11beta-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142(7):2841–2853. doi: 10.1210/endo.142.7.8238. [DOI] [PubMed] [Google Scholar]
- 121.Kanitz E, Otten W, Tuchscherer M, Grabner M, Brussow KP, Rehfeldt C, Metges CC. High and low proteinratio carbohydrate dietary ratios during gestation alter maternal-fetal cortisol regulation in pigs. PLoS One. 2012;7(12):e52748. doi: 10.1371/journal.pone.0052748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shang Y, Jia Y, Sun Q, Shi W, Li R, Wang S, Sui S, Zhao R. Sexually dimorphic effects of maternal dietary protein restriction on fetal growth and placental expression of 11beta-HSD2 in the pig. Anim Reprod Sci. 2015;160:40–48. doi: 10.1016/j.anireprosci.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 123.Shang Y, Yang X, Zhang R, Zou H, Zhao R. Low amino acids affect expression of 11beta-HSD2 in BeWo cells through leptin-activated JAK-STAT and MAPK pathways. Amino Acids. 2012;42(5):1879–1887. doi: 10.1007/s00726-011-0907-1. [DOI] [PubMed] [Google Scholar]
- 124.West AA, Caudill MA. Applied choline-omics: lessons from human metabolic studies for the integration of genomics research into nutrition practice. J Acad Nutr Diet. 2014;114(8):1242–1250. doi: 10.1016/j.jand.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 125.Torrens C, Brawley L, Anthony FW, Dance CS, Dunn R, Jackson AA, Poston L, Hanson MA. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension. 2006;47(5):982–987. doi: 10.1161/01.HYP.0000215580.43711.d1. [DOI] [PubMed] [Google Scholar]