Abstract
Dysregulation of the epigenome and constitutional epimutation lead to aberrant expression of the genes, which regulate cancer initiation and progression. Histone deacetylases (HDACs), which are highly conserved in yeast to humans, are known to regulate numerous proteins involved in the transcriptional regulation of chromatin structures, apoptosis, autophagy, and mitophagy. In addition, a non-permissive chromatin conformation is created by HDACs, preventing the transcription of the genes encoding the proteins associated with tumorigenesis. Recently, an expanding perspective has been reported from the clinical trials with HDACis (HDAC inhibitors), which has emerged as a determining target for the study of the detailed mechanisms underlying cancer progression. Therefore, the present review focuses on the comprehensive lucubration of post-translational modifications and the molecular mechanisms through which HDACs alter the ambiguities associated with epigenome, with particular insights into the initiation, progression, and regulation of cancer.
Keywords: Histone deacetylases (HDACs), Cancer, Apoptosis, Autophagy, Mitophagy
Introduction
Epigenetic modification regulates the heritability of gene expression through agile post-translational modifications, which include methylation, phosphorylation, acetylation, ribosylation, action of non-coding RNAs, and ubiquitination, along with the post-translational modifications of the histone proteins and alteration in the nucleosomal organization [1, 2]. These epigenetic modifications together constitute the ‘epigenetic code’, collectively known as the ‘epigenome’, which is responsible for the modulation in different developmental stages and diverse disease states [3]. Cancer has traditionally been viewed as the onset of multiple diseases driven by the accumulation of genetic mutation, along with the disruption of the epigenetic regulatory mechanism that leads to the occurrence of neoplasia [1]. The disruption of epigenome and constitutional epimutations may lead to altered gene expressions and epigenetic abnormality, which promote the malignant transformation of cells, cancer initiation, and cancer progression. Endogenous as well as exogenous stimuli pervert the canalization of cells through the reorganization of chromatin structure and, as a consequence, explicit aberrant gene expression or repression, thereby assigning the cells to achieve the characteristics of cancer [2]. Recent advances in the field of epigenetics have clearly stated that the global epigenetic abnormality, along with numerous genetic alterations in the human cancer cells, is considered the characteristic of cancer [1, 4]. It is believed that epigenetic alteration and the genetic modifications such as the loss or amplification of DNA and the loss of heterozygosity may be serving as the key initiating events in different forms of cancer [5].
Among all the post-translational modifications, there are many extensive studies focusing on histone acetylation and deacetylation [6]. Histone acetylation is responsible for transcriptional activation and maintenance, while histone deacetylation plays an antagonistic role in the deactivation of chromatin through the removal of acetyl groups. The reversible catalytic property of acetylation is known to be regulated by histone acetyltransferases (HATs), whereas histone deacetylases (HDACs) regulate the deacetylation phenomenon, which subsequently serves as transcription co-activators and co-repressors, respectively [6, 7]. HATs, thereby being neutralized and exhibiting a slender competence to bind to the DNA that is negatively charged, acetylate the ε-amino groups of lysine (K) residues in histones. Furthermore, HDACs, which play a prominent role in cell cycle progression, remove these acetyl groups and allow the reformation of the compact chromatin structure. HDACs demonstrate a critical implication in relation to cancer by influencing the cell cycle progression and transcriptional repression. In addition, HDACs exhibit an important role in cancer progression and regulation through involvement in the apoptosis, autophagy, and mitophagy mechanisms. The pharmacological and cytosolic inhibitors of HDACs (e.g., NaB (sodium butyrate), trapoxin, and TSA (trichostatin A)) are responsible for causing cell cycle arrest in mammalian cells [8].
Therefore, exploring the role of epigenetics in cancer initiation and progression may contribute to better understanding the mechanism underlying these processes, as well as to solve the potential reversible epigenetic aberrations for validating its therapeutic effectiveness against cancer. Considering the attention gained by HDACs in recent times, this review focuses on the studies conducted on the role of systematic regulation of homeostasis through acetylation in the regulation of cancer initiation, progression, and regulation.
Histone deacetylases and its inhibitors: an overview
HDACs have remained highly conserved in yeast to humans. After the cloning of HDAC1 and HDAC2, 18 HDACs have been identified and documented in humans so far, and these HDACs are classified based on their structure, function, and subcellular localization, as well as their homology with the yeast HDACs. HDACs are classified into four classes. HDAC1, 2, 3 and 8, analogous to yeast RPD3 are grouped under class I HDACs. HDAC 4, 5, 6, 7, 9, and 10; analogous to yeast HDA1, are grouped under class II HDACs. The sirtuin family (SIRT1–7; an NAD+-dependent stress-responsive protein family that shares a conserved catalytic core domain of 275-amino acid long), homologous to yeast SIR2 family, comprises the class III HDACs. HDAC class IV comprises HDAC11 [9]. The zinc-dependent amidohydrolases classes of HDACs, i.e., class I, II, and IV, are sensitive to hydroxamic acids that chelate Zn2+. On the other hand, HDACs class III, which are resistant to cytosolic as well as hydroxamic acid-derived inhibitors, are inhibited by nicotinamide. A common deacetylase as well as catalytic domain is shared by class I, II, and IV HDACs that catalyzes the deacetylation with the assistance of the histidine–aspartate charge transfer system [6, 9]. A conserved deacetylase domain in the nucleus is possessed by HDACs class I. The HDACs class II governs the acetylation status of the non-histone substrates through shuttling between the nucleus and cytoplasm. Similarly, HDAC6 deacetylates the cellular proteins. Due to the absence of an intrinsic DNA-binding ability, the reinforcement of HDACs to DNA is facilitated through transcription factors and associated protein complexes. HDACs integrate with these transcription factors and protein complexes and reside there to obtain access to DNA. Sin3, mSin3A, N-CoR (nuclear receptor co-repressor), NuRD (nucleosome remodeling and deacetylation), Mi-2/NRD and PRC2, etc., provide shelter to HDAC1 and HDAC2, while SMRT complex performs this role for HDAC3 [6, 10, 11].
Histone deacetylase inhibitors (HDACis) are a new class of cytostatic agents that inhibits proliferation and differentiation of tumor cells through modulating the acetylation and deacetylation status of histones and non-histone proteins such as transcription factors subsequently inducing cell cycle arrest, apoptosis, and autophagy [12]. HDACis can be classified as: (1) hydroxamic acids (hydroxamates); (2) benzamides; (3) cyclic tetrapeptides; (4) short chain fatty (aliphatic) acids, and (5) sirtuin inhibitors. Some examples of hydroxamate class of HDACis are TSA, SAHA (suberoylanilide hydroxamic acid), belinostat, panobinostat, ricolinostat. The benzamide class of HDACis contains entinostat, tacedinaline and mocetinostat. Romidepsin constitutes the cyclic tetrapeptide class of HDACis, while VPA (valproic acid), butyric acid and phenylbutyric acid constitute the short chain fatty acid groups of HDACis. The sirtuin inhibitors include sirtinol, nicotinamide and cambinol [13].
Histone deacetylases beyond histone protein modifications
Apart from histone modification, HDACs also regulate non-histone protein functions through deacetylation [14]. Among these non-histone proteins modulated by HDACs, p53, RUNX3 (Runt-related transcription factor 3), STAT3 (signal transduction and activation of transcription 3), β-catenin, estrogen receptor, Myc (avian myelocytomatosis viral oncogene homolog), GATA family (GATA-binding factors), HIF-1α (hypoxia-inducible factor 1α), NF-κB (nuclear factor κB) and FOXO (Forkhead box protein) play critical role in oncogenesis, cancer progression, and regulation. The role of HDACs in modulation of non-histone targets is elaborated in the preceding texts [13, 15].
Histone deacetylases in non-histone protein modifications
It has been demonstrated that non-histone protein modification through acetylation and deacetylation also exerts a great impact on the epigenetic modulation of the genes that are required to be expressed or suppressed. HDACs (localized to the nucleus) are components of distinct co-repressor complexes, and their deletion or knockdown results in diverse cellular effects such as regulation of histone acetylation and its non-histone targets [11, 16]. Transcription factors are required to facilitate the HDACs to DNA due to the absence of an intrinsic DNA-binding ability, thereby playing an important role in the deacetylation process. The mechanism of active repression of transcription by HDACs is identified by its mode of action on the multiprotein complexes.
In humans, HDAC1 has been implicated in the transcriptional regulation through the formation of the HDAC1/Rpd3 complex with co-repressor Sin3 or Rb, which are channelized toward DNA by the transcription factor mad, E2F, or unliganded nuclear hormone receptors [17, 18]. Similarly, the transcriptional activator and repressor, Myc and Mad or E2F, respectively, bind to the heterodimeric transcription factor Max [19]. Mad–Max heterodimers associated with the mSin3 scaffold proteins bind to the E box-containing DNA sequence that recruits HDAC1 and HDAC2, leading to Snail-mediated repression of EMT (epithelial–mesenchymal transition). In the DNA-bound nuclear receptors, sequential binding of the co-repressors SMRT and N-CoR recruits HDAC3 to the promoter region, causing suppression of transcription. Similarly, active repression of E2F-mediated transcription caused by Rb recruits HDAC1 to the E2F-regulated promoters [20]. Another gene, Muc2, whose loss of function implicates pancreatic and colorectal cancer, plays a significant role in gastrointestinal cell differentiation. Treatment with TSA, an HDACi, characterizes increased acetylation of the histones H3-K9 and H3-K27, and decreased CpG-island methylation at the promoter region of the Muc2 gene, thereby exhibiting a tumor-suppressing ability [21]. On the contrary, in the undifferentiated adenocarcinoma cells, NaB, another HDACi, inhibits the Muc2 expression [22]. Enzymatic activity of HDACs is required for the repression of transcription; therefore, potent HDACis release the HDAC-dependent repression through the reduction of the enzymatic activity of HDACs. In the absence of HDAC1/Rpd3, increased acetylation in the lysine residue on histone H4 has been reported [6, 15].
Histone deacetylases and transcription factor modulation: wielding the master regulator, a special apprehension to p53
The major non-histone target that undergoes constant acetylation and deacetylation is the tumor suppressor p53. The acetylation at the C-terminal lysine residues of p53 by p300/CBP and PCAF (p300-CBP associated factor) enhances the open conformation of p53 and obstructs its DNA-binding specificity, thereby promoting the transcriptional activation of p53 [23]. Co-activators such as p300/CBP and TRRAP (transformation/transcription domain-associated protein) are recruited to the p21 (CIP1/WAF1/CDKN1A) promoter region through the acetylation of p53 at K382 and K380, which in turn increases the histone acetylation post-DNA damage [24–26]. Depsipeptide, an HDAC inhibitor, recruits p300 and p21 through p53 acetylation and has demonstrated therapeutic potency in lung cancer [27, 28]. On the other hand, TSA, another HDACi, stabilizes the acetylation caused by p53 in prostate cancer. Microarray analyses have demonstrated that the acetylation of murine p53 at K317 in mouse knock-in models (exhibiting homology to human K320) promoted induction of the apoptotic genes Pidd (p53-induced protein with a Death Domain) and Noxa [15]. An elevated expression of PUMA (p53-upregulated modulator of apoptosis) in the HCT116 colorectal cells post-DNA damage suggested enhanced apoptosis through p300-mediated p53 acetylation as well as through histone H4 acetylation [29].
Moreover, p300 acetylates the RUNX family of transcription factors (RUNX1 as the regulator of hematopoiesis and RUNX3 in gastric cancer) and represses the inappropriate recruitment of HDACs (see Fig. 1). HDAC4 and HDAC5 hinder acetylation of RUNX3 and promote its overexpression in the basal carcinoma cells [15]. Acetylation of p300 also hinders HDAC2 binding with co-repressor BCL6, leading to repressed transcription. In B cell lymphoma, treatment with TSA and nicotinamide inhibits the deacetylation of BCL6 caused by class I/II and class III HDACs [15, 30, 31]. Similarly, another co-repressor, PLZF, is acetylated by p300, resulting in the reduction of the ability of HDAC to bind to DNA, and consequent deactivation of transcription [15, 29–31]. HDAC2 inhibitors also induce apoptosis by causing a decrease in the Bcl-2 expression through the acetylation of Sp1 and C/EBPα, which mechanistically inhibit transcription through decreased promoter-binding ability. Moreover, Tip60/MOF/MOZ acetylates p53, enhancing its stability, and resulting in a low binding affinity, which promotes its tendency toward DNA damage as well as activated oncogenes [32]. Conversely, the inhibition of HDAC1 and SIRT1 causes p53 acetylation, promoting p53-dependent activation of proliferation, cell cycle arrest, DNA repair, senescence, metabolism, apoptosis, and autophagy. HDACis suppress the HDAC1/SIRT1-mediated p53 deacetylation through the inhibition of Mdm2 facilitation [33]. An inhibition of SIRT1-mediated p53 deacetylation is evident through the action of the tumor repressor DBC-1 in breast cancer. On the contrary, AROS, a positive activator of SIRT1, enhances cell survival through the SIRT1-mediated deactivation of p53 via deacetylation [34].
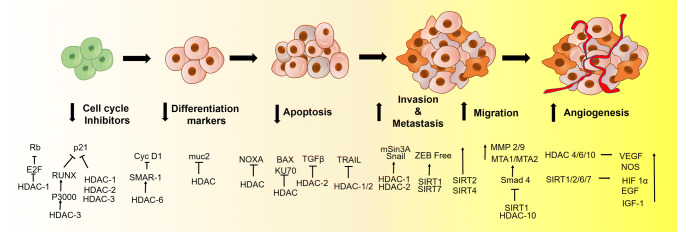
Fig. 1.
HDAC promotes stage-specific progression of cancer: HDACs downregulate cell cycle inhibitors by inhibiting Rb and p21 through E2F and RUNX repression, respectively. In addition, HDAC6 suppresses differentiation marker cyclin D1. HDACs also inhibit Muc2. HDAC inhibits intrinsic apoptotic pathways by inhibiting pro-apoptotic proteins NOXA and BAX by direct acetylation or through non-histone protein KU70 modification. On the other hand, extrinsic apoptosis is inhibited by TRAIL- or TGFβ-mediated pathway. During cancer progression, HDAC1/2 promotes Snail-mediated EMT. Similarly, SIRT1 and HDAC10 promote deacetylation of Smad that subsequently upregulate Snail and MMPs for further EMT upregulation. Similarly, HDAC4, 6, 10, and SIRTs also promotes angiogenesis by promoting VEGF, EGF, and HIF 1α
A small-molecule activator of SIRT1, tenovin-6, causes hyperacetylation of p53 in cancer cells [34]. Deacetylation of p53, along with the mutation in the transactivation domains TAD1 and TAD2, fails to inhibit the transcriptional activation of p53, consequently promoting tumor-cell angiogenesis, migration, and metastasis. CBP/p300 is also responsible for the acetylation of the hematopoietic GATA factors (GATA1–3) and induces their active transcription. Dysregulation of GATA factors through deacetylation by HDACs may contribute to hematopoietic malignancy. HDAC5 represses GATA1-mediated transcription and attenuates the response to cell growth [6, 15]. CBP/p300 and PCAF also acetylate NF-κB, preventing proteosomal degradation by hindering the association of the latter with IkB kinase (IKK) [35]. HDAC3 and SIRT1 have been reported to minimize NF-κB expression by enhancing its ability to bind with IKK. STAT family of transcription regulators have been reported to be activated by NF-κB [35, 36]. CBP/p300-mediated acetylation causes STAT3 dimerization through the inhibition of HDAC3 [37, 38]. In this context, the inhibition of HDACs by pharmacological as well as cellular inhibitors may be able to thrive as an emerging therapeutic approach against cancer (listed in Table 1).
Table 1.
Histone deacetylases and its potential implications in cancer
| Classification (class-wise) | HDAC | Localization | Histone protein/substrate association | Non-histone substrates | Expression in cancer | Stages in cancer/function | Functional involvement | HDACi | References |
|---|---|---|---|---|---|---|---|---|---|
| I | HDAC1 | Nucleus | HDAC2 | RB1, p53, CtIP, CoREST, SHP, BCL6, AMPK, AML1-ETO, Sin3, MyoD, NuRD, PML, PLZF, E2F1, NF-κB, STAT3 | ↑ Gastric; breast; colorectal; lung; HL and liver cancer | Cell proliferation, angiogenesis | Knockdown promotes growth arrest, less viability, induces apoptosis and genomic instability | Tacedinaline, quisinostat, abexinostat, romidepsin, depsipeptide, pracinostat, ricolinostat, entinostat, mocetinostat, tubastatin A | [17, 18, 20, 72] |
| HDAC2 | Nucleus | HDAC1 | CoREST, Sin3, STAT3, AML1-ETO, GCCR, PML, PLZF, BCL6, YY1 | ↑ Gastric; colorectal; HL and prostate cancer | Cell proliferation, angiogenesis, differentiation | Quisinostat, abexinostat, romidepsin, ricolinostat, pracinostat, mocetinostat | [15, 20, 29–31, 72] | ||
| HDAC3 | Nucleus | HDAC4, HDAC5, HDAC7 | NCoR/SMR, SHP, AML1-ETO, NF-κβ GATA1/2, RARα, PML, PLZF, PML–PLZF–RARα, Bcl6, YY1, 2p65, MEF2D STAT1/3 |
↑ Gastric; breast; ALL; HL and colorectal cancer ↓ Liver cancer |
Cell proliferation, angiogenesis, differentiation |
Knockdown helps in relieved transcription repression in APL cells by PML–RARα Induces apoptosis and decreases cell viability while knockdown |
Quisinostat, abexinostat, pracinostat, resminostat, ricolinostat, mocetinostat, entinostat, droxinostat | [20, 35, 37, 38, 73, 74, 132] | |
| HDAC8 | Nucleus | – | SMC3, actin | ↑ Neuroblastoma | Cell proliferation | Reduced proliferation in cervical, lung and colon cancer due to knockdown | Quisinostat, ricolinostat, pracinostat, droxinostat, tubastatin A | [133] | |
| II a | HDAC4 | Nucleus/cytoplasm | HDAC3–NCor | GATA1, HP1 | – | Cell proliferation, angiogenesis, and differentiation |
Induction of apoptosis and decreased cell viability in colon cancer and glioblastoma Increased VEGF expression in chondrosarcoma |
Quisinostat, pracinostat, tasquinimod, CUDC-101/907, TMP269 | [54, 81, 134] |
| HDAC5 | Nucleus/cytoplasm | HDAC3–NCor | GATA1, GATA2, SMAD7, HP1 |
↑ Medulloblastoma ↓ Lung cancer |
Differentiation | Increased cell growth and viability upon overexpression | Quisinostat, pracinostat, LMK-235, TMP269, CUDC-101/907, ricolinostat | [6, 15, 54, 134] | |
| HDAC7 | Nucleus/cytoplasm | HDAC3–NCor | PLAG1, PLAG2, ERα |
↑ ALL ↓ Lung cancer |
Differentiation | Suppresses cell growth arrest in colon and breast cancer | Quisinostat, pracinostat, TMP269, CUDC-101/907, ricolinostat | [133] | |
| HDAC9 | Nucleus/cytoplasm | – | Bcl-2, IL-3, CEBPA, PU.1, c-fms, BPI | ↑ ALL and medulloblastoma | Differentiation | Overexpression decreases sensitivity to DNA damage and increases medulloblastoma cell growth and viability | TMP269, CUDC-101/907, quisinostat, pracinostat | [133] | |
| II b | HDAC6 | Cytoplasm | HDAC11 | α-Tubulin, HSP90, SHP, SMAD |
↑ Breast cancer ↓ Lung cancer |
Differentiation and angiogenesis | Knockdown decreases cell viability and VEGF expression | Tubacin, nexturastat A, TSA, HPOB, resminostat, pracinostat, droxinostat, CAY10603 | [82, 86, 96] |
| HDAC10 | Nucleus | – | – | – | DNA repair |
Overexpression makes less sensitive to DNA damage Overexpression increases VEGF expression |
CUDC-101/907, quisinostat, PCl-24781, HPOB | [70, 97] | |
| III | SIRT1 | Nucleus/cytoplasm | HDAC1, H3K9 H3K14 H3K56 H4K16 H1K26 | p53, β-catenin, Ku70, E2F1, Rb, NF-κB, PGC1α, AKT, PPARγ, MYC, MyoD, PCAF, FOXO3, LXR, HIF1α, ATG5, ATG7, ATG8, SMAD7, FXR, RARβ, SREBP1C/2 |
↓ Lymphoma; colon, liver and lung cancer ↑ Prostate and thyroid cancer |
Chromatin regulation, transcription, DNA repair and metabolism |
Overexpression attenuates apoptosis, promotes DNA repair and genomic stability, suppresses β-catenin-mediated cell proliferation Knockdown induces loss of BRCA1 in breast cancer, inhibits anti-apoptotic genes |
Tenovin-6, BDF4-1, -2a, -2b, -2d, salermide, nicotinamide, JGB-1741, cambinol, EX527, AC-93253, aristoforin, inauhzin, Tenovin-1, Tenovin-6 | [33, 34, 37, 60, 63, 78, 79, 135] |
| SIRT2 | Cytoplasm | H4K16, H3K56, H3K18 | α-Tubulin, HIF1α, PRLR, FOXO1/3A, keratin 8, PAR3, p300, NF-κβ | ↑ Mammary gland; skin and hepatocellular carcinoma | Metabolism, cell cycle and differentiation | Sirt2 knockout results in genomic instability, higher expression of mitotic activators and increased at G2/M expression | Tenovin-6, BDF4-1, -2a, -2b, -2d, salermide, AGK2, aristoforin, nicotinamide, sirtinol, cambinol, AC-93253, AK-7, Tenovin-1, Tenovin-6 | [65, 95, 118, 135] | |
| SIRT3 | Nucleus/mitochondria | H4K16 | IDH2, SDH, CypD, OPA1, FOXO3A, PDH, LCAD, p53, MRPL10, GDH, LKB1, VLCAD, OTC, GOT2, PDP1, Ku70, SKP2, SOD2, NDUFA9, ACECS2, HMGCS2 | ↓ Mammary gland cancer | Metabolism |
Sirt3-null cells exhibit genomic instability Deletion causes breast, ovarian, lung cancer and medulloblastoma Overexpression causes OSCC growth |
AC-93253, Tenovin-6, LC-0296 | [39, 43, 80, 83, 84, 135] | |
| SIRT4 | Mitochondria | SIRT3 | ANT2/3, GDH, MCD, PDH SLC25A5, IDE | ↓ Lung; lymphoma and small epitopic tumors | Metabolism |
Loss of SIRT4 accelerates MYC induced lymphomas Overexpression induces cell death Downregulation sensitizes toward small cell lung carcinoma, gastric, bladder and breast cancer |
Nicotinamide | [67, 135] | |
| SIRT5 | Mitochondria | – | CPS1, cytochrome c, HMGCS2, PDH, SDH, SOD1, GAPDH | ↑ Non-small cell lung carcinoma and smaller epitopic tumors | Metabolism | Overexpression causes non-small cell lung carcinoma | Nicotinamide, cambinol | [98, 135] | |
| SIRT6 | Nucleus | H3K9, H3K56 | NF-κB, DNA CtBP, HIF1α, PARP1, MYC, TNF, SREBP1, PK SREBP2, CtIP USP10, GCN5, SNF2H, G3BP, FOXO3 | ↓ Larger epitopic tumors; larger intestinal tumors; liver and pancreatic cancer | Chromatin and DNA repair |
Downregulation causes colon, hepatocellular and rectal adenocarcinoma Inhibition of SIRT6 expression regulates anti-apoptotic genes Causes resistance to chemotherapy by enhancing DNA damage in breast cancer Promotes angiogenesis and metastasis in pancreatic cancer |
Nicotinamide, OSS-128167 | [135] | |
| SIRT7 | Nucleolus | H3K18 | p53, mTOR, MYC, PAF53, HIF1α, HIF2α, ELK4, TFIIIC2, RNA Pol I, MYBBP1A | ↓ Smaller ectopic tumors | Transcription and metabolism | Overexpression causes uncontrolled cell growth and loss of contact inhibition in uterine, colon, kidney, ovarian and prostate cancer | – | [135] | |
| IV | HDAC11 | Nucleus/cytoplasm | HDAC6 | – | ↑ Breast; renal and liver cancer | Transcription and cell cycle regulation | Over expression inhibits apoptosis in breast, colon, prostate and ovarian cancer | Mocetinostat, pracinostat, quisinostat, CUDC-907 | [133] |
Histone deacetylases in cancer: unrevealing the crossroad to cancer
Histone deacetylation regulates the expression of numerous genes involved in cancer progression and regulation [15]. Several in vivo and in vitro experiments have been performed to demonstrate the critical role of HDAC in carcinogenesis and its regulation. In addition, the involvement of HDACis has been investigated to put forth a better chemotherapeutic approach against cancer. The following sections indicate the exact involvement of HDACs in subsequent developmental stages of cancer.
Role of histone deacetylases in cellular transformation and cancer initiation
Unlimited proliferation due to lack of proliferation-restraining genes or the inhibition of differentiation may lead to cancer. Remarkably, the mitochondrial sirtuins (mtSIRTs; SIRT-3, 4, and 5) are known to regulate different characteristics of cancer cells. However, the functional characteristics of SIRT3 in cancer are extensively debated due to its dual role as a tumor suppressor as well as a promotor. The in vitro transformation in the SIRT3-knockout mouse embryonic fibroblast cells, post-instigation of a single oncogene (Ras or Myc), validates the tumor-suppressor mechanism exhibited by SIRT3 [39]. An abnormal downregulation of SIRT3 has been reported in gastric cancer (through the upregulation of Notch-1), B cell malignant cells (through hyperacetylation of IDH2 and SOD2), and mantle cell lymphoma tissues [40, 41]. SIRT3 deacetylates and decreases the retention of proto-oncogene product Skp2 (S-phase kinase-associated protein 2) in cytoplasm, which reduces cell proliferation and migration. An exogenous downregulation of SIRT3, mediated through a short hairpin RNA, imparts a decreased proliferative potential to the human melanoma cell—HT294T cells as well as to the immortalized Mel-ST melanocytes [42]. Similarly, inhibition of HCC cell growth due to SIRT3 overexpression has been reported to be accompanied by the reduction of mouse double min 2 (MDM2)-mediated p53 degradation and post-translational regulation [43]. On the other hand, the loss of expression of muc2 gene due to hyperacetylation of the histones H3-K9 and H3-K27 plays an influential role in the differentiation of gastrointestinal epithelial cells in pancreatic and colorectal cancer [21, 22, 44]. Furthermore, the loss of the GATA4 and GATA5 differentiation factors due to promoter hypermethylation is evident in gastric and colorectal carcinoma, while the loss of GATA6 expression in ovarian cancer cells due to hypoacetylation of the histones H3 and H4 has been observed in several cancer types [45]. Treatment with HDACis such as TSA restores the expression of GATA target Dab2, which acts as a tumor suppressor [46].
Recent studies [47] have shown, in ovarian and embryonal carcinoma cells, an inactivation of cellular HDAC1 and HDAC6 by an HDAC1-specific substrate H3K56-derived peptide, disrupting the formation of the HDAC1–LSD1–CoREST complex that subsequently inhibits proliferation and differentiation. Further, knockdown of HDAC8 inhibits transcription of mutant p53 in triple-negative breast cancer (TNBC) cells. From the ChIP analysis, it is revealed that HDACis mask the binding of HDAC8 with transcription factor YY1 that in turn suppresses the p53 transcription signaling a proliferative defect in TNBC cells [48, 49]. Similarly, another HDACi, ZINC24469384, suppresses the proliferation of HepG2 cells by upregulating NR1H4 expression. Subsequent activation of NR1H4 by ZINC24469384 protects p53 from degradation as well as reduces phosphorylation of STAT3. ZINC24469384 also regulates SOCS family genes by upregulating NR1H4 that suppresses the proliferation of hepatocellular carcinoma [50]. Another novel HDACi, CUDC-101, suppresses the proliferation of pancreatic cancer cells by downregulating HDAC1 activity when co-treated with gemcitabine [51].
Role of histone deacetylases in invasion and metastasis
Cancer cells invade through multiple transition states, in which the cells move through epithelial and mesenchymal phenotypes. During the EMT, epithelial cells lose their apical–basal polarity as well as cell–cell adhesion, thereby attaining cell mobility [52]. HDACs have been reported to act as promoters as well as repressors of the EMT. Class I HDACs regulate the extracellular matrix-related genes in humans. Cystatin, a peptidase inhibitor, helps in the suppression of tumor invasion and is known to be suppressed by HDAC1. Therefore, HDAC1, after knocking down or in the presence of its inhibitors, is able to upregulate cystatin and reduce cellular invasion [53]. The downregulation of the expression of HDACs de-represses several genes, including proto-cadherin, RYBP, and STAT6, which in turn inhibits invasion. The expression of the E-cadherin gene is directly regulated by class I HDACs, the loss of which causes an epithelial invasion. Snail, a transcription factor that promotes the repression of E-cadherin, recruits the co-repressor mSin3A along with HDAC1 and HDAC2 to the E-cadherin promoter, where the hyperacetylation of the histones H3 and H4 occurs in correspondence to the elevated levels of methylated histone H3-K9 and diminished levels of methylated histone H3-K4 [54, 55]. In prostate cancer cells, treatment of PPARγ (peroxisome proliferator-activated receptor-γ) in addition to HDAC3 results in its binding to the promoter region of E-cadherin and promotion of invasion, while inhibitors, such as TSA and VPA, reduce invasiveness through the hyperacetylation of histone H4 at the E-cadherin promoter [56]. Interestingly, HDACis such as TSA and SAHA promote nuclear translocation and phosphorylation of Smad2/3 that upregulate Snail at the transcriptional level by binding to its promoter region in hepatocellular carcinoma cells. On the other hand, overexpression of COP9 signalosome 2 (CSN2) inhibits phosphorylation and ubiquitination of Snail to repress its degradation [57]. A reduced migration of the ALL (acute lymphoblastic leukemia) cells to liver, spleen, lymph nodes, and the brain is exhibited as a result of downregulation of the chemokine receptor CXCR4 by the HDACis [58]. HDACis that have been observed to regulate an increased expression of ICAM1 in the tumor-derived endothelial cells enhance the aptness of adherence of lymphocytes to the endothelial cells through hypoacetylation of histone H3 and hypomethylation of histone H3-K4, which enable enhanced tumor infiltration of the lymphocytes [59]. In prostate cancer, transcriptional repression of E-cadherin occurs as a result of the recruitment of SIRT1 to its promoter by the transcription factor ZEB1 and due to the deacetylation of histone H3 that causes hindrance in the RNA polymerase II binding [60]. As a result, diminished prostate cancer cell migration and metastasis are observed in case of SIRT1 inhibition, while the loss of SIRT7 expression furthers a reversal of the EMT phenotype, indicating an interplay between the roles of SIRT1 and SIRT7 in the regulation of EMT [61]. Similarly, upregulation of SIRT1 has been reported to be associated with TGF-β-induced EMT in pancreatic cancer, while the silencing of E-cadherin promotor in pancreatic cancer cells has been demonstrated to be a result of the interaction of SIRT1 with MBD1 (methyl-CpG-binding domain protein-1) and Twist [62]. On the contrary, SIRT1 regulates the EMT process in the ovarian, lung, and oral squamous cell carcinoma through the deacetylation of Smad4 and the repression of TGF-β signaling pathway on MMP-7 [63] (see Fig. 1).
In human nasopharyngeal carcinoma cells, SAHA and NaB promote EMT by upregulating Snail and vimentin expression, while downregulating E-cadherin expression. Upregulation of Snail expression accompanied by nuclear translocation and cytoplasmic degradation is mediated through GSK3β (glycogen synthase kinase-3β)/β-catenin signaling pathways [64]. In hepatocellular carcinoma, upregulation of SIRT2 causes deacetylation and activation of protein kinase B that targets the Akt/GSK3β/β-catenin signaling pathway to promote EMT. Furthermore, the overexpression of SIRT2 deacetylates Slug proteins and efficiently represses the transcriptional targets of Slug, epithelial cell adhesion, and E-cadherin, leading to EMT regulation, in triple-negative breast cancer cells [65]. In triple-negative breast cancer cells MDA-MB-231 and BT-549, SAHA is reported to promote EMT by downregulating FOXA1. Further, downregulation of FOXA1 by silencing HDAC8 in SAHA treated MDA-MB-231 cells exhibit an alleviated expression of N-cadherin and vimentin [66]. On the other hand, in colorectal cancer, suppression of glutamine metabolism by SIRT4 represses the enzymatic activity of glutamate dehydrogenase through the subsequent repression of α-ketoglutarate, which in turn upregulates the E-cadherin expression and downregulates the vimentin expression [67], while the overexpression of SIRT7 promotes the upregulation of vimentin and downregulates E-cadherin [68]. In recent studies associated with healthy prognosis of HNCC, upregulation of SIRT4 has been reported. In colorectal cancer, an upregulation of the EMT marker E-cadherin as a result of the inhibition of glutamine metabolism suppresses the cancer cell proliferation, migration, and invasion, defining the tumor-suppressive role of SIRT4 [67]. Histone acetylation and deacetylation regulate cancer progression through angiogenesis and metastasis, which permit increased cell migration and tumor growth. Under hypoxia, solid tumors exhibit increased angiogenic and metastatic properties in relation to HDAC expression. Overexpression of HDAC1 under hypoxia induces the hypoxia-responsive genes, HIF-1α and VEGF (vascular endothelial growth factor) and represses the tumor suppressor p53. HDACis such as TSA reverse this effect through the hyperacetylation of histone H4, which leads to the prevention of new vessel formation in the other models of angiogenesis [69]. Elevated expression of HDAC10 in cervical cancer has been reported to be associated with the suppression of metastasis and metalloproteases (MMPs) 2 and 9 [70] (see Fig. 1).
Histone deacetylases in apoptosis and autophagy: a voyage to the therapeutic avenue
Cancer cells attribute a critical regulation of epigenetic regulatory mechanisms by HDACs to maintain a set of genes that are essential for regulation of mechanisms involving apoptosis and autophagy. The cell death mechanism and cross talk with context-dependent autophagy by HDACs uncover novel therapeutic agents and underlying regulatory mechanism in cancer.
Histone deacetylases in apoptosis: a novel insight into cancer regulation
Apoptosis, a coordinated energy-dependent specialized form of cell death mechanism upon the induction of intrinsic or extrinsic stimuli, is modulated by HDACs. TGF-β1 (transforming growth factor-β1)-induced apoptosis is restrained by the deletion of HDAC1, while the overexpression of HDAC1 enhances this apoptosis. On the other hand, HDAC2 functions as a negative regulator of the TGF-β1-induced apoptosis [71]. An elevation in the apoptosis initiation by p53 hyperacetylation is achieved through targeted deletion of both HDAC1 and HDAC2. Similarly, a knockdown of HDAC1 and HDAC2 suppresses proliferation of certain colon carcinoma cells and sensitizes the TRAIL-induced apoptosis in chronic lymphatic leukemia cells [72]. Effective inhibition of growth in colon carcinoma cells is caused as a result of knockdown of HDAC3 [73]. Cytoplasmic accumulation of HDAC3 as a result of caspase-dependent cleavage promotes transcriptional activation of pro-apoptotic genes [74].
SIRT1, a class III HDAC, upregulates the DNA repair mechanism (cyclin D, GADD45, p27/Kip1) and enhances the sensitivity of the cells to attain apoptosis through the deacetylation and activation of the transcription factor FOXO3a upon the induction of genotoxicity, including the detoxification of ROS [75–80]. The transcriptional activity of FOXO3 is regulated by SIRT1, which promotes its ability to cause cell cycle arrest. The higher expression of SIRT1 tips the FOXO-dependent cell cycle arrest and leads to stress resistance [79]. Furthermore, the expression of genes involved in apoptosis (BH-3 only proteins, BIM, Fas ligands, and TNF-related apoptosis-inducing ligands) is also known to be regulated by SIRT1. In NSCLC (non-small cell lung carcinoma), SIRT3 binds with NMNAT2 (nicotinamide mononucleotide adenylyltransferase 2), which in turn regulates cell proliferation and apoptosis. An inhibition of hepatocellular carcinoma through GSK-3β/Bax-dependent apoptotic pathway is regulated by SIRT3 [80]. Caspase-dependent cleavage of HDAC4 releases mitochondrial cytochrome c and assists in the onset of apoptosis [81]. Hyperacetylation of the C-terminal regulatory domain of p53 by SIRT1 inhibitors induces cellular apoptosis.
The higher expression of HDAC6 has been reported to be associated with advanced stages of oral squamous cell carcinoma, which is conversely represented in breast cancer. HDAC6, in association with RUNX2, restrains the pro-apoptotic activity of p53. HDACis such as TSA and butyrate induce apoptosis through caspase-3 activation and Bad upregulation in the tumor cells [82]. Ku70, a multifunctional protein, undergoes acetylation at K539 and K542 by CBP and PCAF, which in turn promotes its binding to the pro-apoptotic protein Bax, leading to its sequestration in the cytoplasm. The treatment with HDAC I/II and III inhibitor (specific to SIRT3) acetylates Ku70 and initiates Bax-dependent apoptosis in neuroblastoma cells [83, 84]. An irregular expression of pro-apoptotic oncoprotein Bcl-6 in several non-Hodgkin’s lymphomas leads to the recruitment of HDACs that repress the target genes involved in cell cycle arrest and apoptosis [85]. HDAC6 binds and deacetylates the molecular chaperon HSP90, which in turn prevents its acetylation at K294, leading to the inhibition of complex formation with its clients and co-chaperons. As a combined therapeutic approach, treatment with HDACis along with HSP90 antagonists may lead to a better therapeutic avenue in cancer treatment [86] (see Fig. 2).
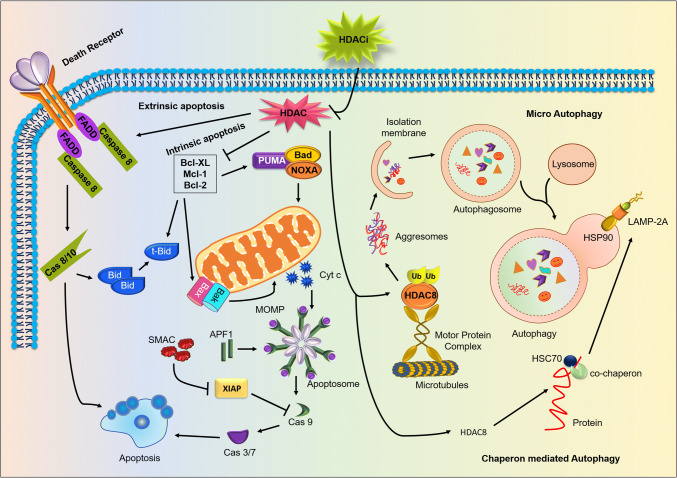
Fig. 2.
HDAC is a potent negative regulator of apoptosis and autophagy in tumorigenesis: permeabilization of HDAC inhibitor inactivates cytosolic HDAC exhibiting activation of the apoptotic and autophagic pathways, leading to negative regulation of cancer progression. HDAC inhibition mainly promotes extrinsic apoptosis by activating caspase 8 through death receptors FADD, TRADD, TRAIL, and TNF. Additionally, HDACs repress the expression of anti-apoptotic proteins like Bcl-2, Bcl-XL and Mcl-1 which in turn stimulates pro-apoptotic proteins like Bax, Bad, Bid, Bak, NOXA and PUMA. Moreover, HDAC inhibition also causes stimulation of both microautophagy and chaperone-mediated autophagy. Chaperon-mediated autophagy is initiated by the inhibition of the HDAC8 and with the help of co-chaperon HSC70. HDACs regulate the LAMP 2A deacetylation to promote autophagosome formation. Furthermore, ubiquitination of misfolded proteins is recognized by HDAC8 that results in the formation of the aggresomes and displace basal interactors of HDACs, in particular Hsp90. HDAC8 targets misfolded proteins to the microtubule-organizing center via microtubules which are degraded by the induction of microautophagy
Histone deacetylases in autophagy: sheathing the double-edged sword
Autophagy is a cytoprotective, tightly regulated intracellular catabolic process that degrades the cytoplasmic constituents, organelles, misfolded proteins, and foreign particles. Autophagy triggered under a variety of internal as well as external stimuli is known to be regulated by HDACs. In Huntington’s disease model, the inhibition of HDAC1 hinders the deacetylation of the mutant Huntington protein (Htt), which is cleared through the induction of autophagy [87]. Class III HDACs (SIRT1), through the deacetylation of the host transcription machinery (which in turn activates the autophagic genes), promotes the expression of various components of autophagy. SIRTs are believed to exhibit direct interactions with the autophagic machinery by deacetylating proteins such as Atg5, Atg7, and Atg8/LC3 in an NAD+-dependent manner [88]. Under nutrient deprivation and oxidative stress, SIRT1 deacetylates and activates nuclear localization of FOXO1 through JNK phosphorylation at Ser27, Ser47, and Thr530, which in turn elevates the Rab7 expression that is responsible for late autophagosome and lysosome fusion [89, 90]. Furthermore, enhanced expression of pro-autophagic BNIP3 is caused as a result of the deacetylation of FOXO3 by SIRT1. FOXO3 is able to upregulate multiple autophagic genes such as ULK2, Beclin 1, LC3, Atg12, Atg4B, VSP34, BNIP3, BNIP3L, and GABARAPL1. Deacetylation of RelA/p65 by SIRT1, in the context of NFқB signaling, during the inflammatory response, and the deacetylation of the IKK (inhibitors of қB kinase) complex modulate autophagy in an NFқB-dependent and NFқB-independent manner, respectively [91–94]. Vorinostat (SAHA), an HDAC inhibitor, induces autophagy through the inactivation of mTOR. Furthermore, induction of oxidative stress and nutrient starvation causes FOXO1 acetylation as well as its dissociation from SIRT2, which leads to its interaction with Atg7 through the acetylated lysine residues Lys262, Lys265, and Lys274. SIRT2 elicits autophagy in cancer cells in an endogenous cytoplasmic FOXO1-dependent manner [95].
HDAC6, a microtubule-associated deacetylase, establishes autophagy and ubiquitin–proteasome link by playing a key role in the autophagosome and lysosome fusion [96]. It has been revealed in recent studies that HDAC10 promotes cell survival through autophagy, while the inhibition of it increases the sensitization of cancer cells to chemotherapy in neuroblastoma and medulloblastoma [97]. In human NSCLC, overexpression of SIRT5 reduces the expression of Nrf2 and its downstream targets, which may facilitate drug resistance. Similarly, in breast cancer cells, SIRT5 regulates ammonia-induced autophagy and mitophagy [98]. HDACs and their inhibitors demonstrate pleiotropic roles in autophagy regulation, which may define a novel therapeutic approach in cancer treatment (see Fig. 2).
Histone deacetylases in mitophagy: controlling the powerhouse
Mitophagy is an intracellular catabolic phenomenon in which an explicit mitochondrial degradation occurs in the autophagosome through the interaction of the outer-mitochondrial membrane (OMM) adaptor molecules (BNIP3, FUNDC1, NIX, and targets of E3 ubiquitin ligases such as Parkin and Mul1) with LC3 [99]. Mitochondrial fragmentation, reduction in the mitochondrial mass, or loss of mitochondrial membrane potential under hypoxia and nutrient starvation leads to mitophagy. Induction of proteolytic cleavage followed by degradation of the fusion protein Opa-1 reduces the size of mitochondria due to membrane depolarization, which favors the uptake of mitochondria by the phagophores [100]. Recent discoveries have explored the role of mitophagy regulators such as Parkin and Pink1, BNIP3/NIX, in addition to FUNDC1 and Mul1, as well as lipid-mediated mitophagy under ceramide stress, and these appear to exhibit a noticeable role in the modulation of mitophagy, subsequently leading to tumorigenesis. A mechanistic detail for the induction of ceramide generation and their pertinent justification as “tumor-suppressor lipid” has been investigated in recent studies using epigenetic biomarkers such as HDACs [101]. It has raised several concerns regarding context-dependent roles of HDACs and their mechanism of action in ceramide-mediated selective removal of mitochondria [102]. The following text describes the role of mitophagy modulators in the regulation of mitophagy in relation to HDACs.
Histone deacetylases in Parkin–Pink1-mediated mitophagy: unraveling the well established
The PARK2 (Parkin) and PARK6 (PINK1) gene products are responsible for promoting mitophagy, thus implicating dysfunctional mitochondria in the etiology of cancer. In lung, breast, ovarian, bladder, and other cancers, Parkin has been frequently observed to be deleted [103, 104]. Parkin enhances mitochondrial integrity by elevating oxidative metabolism and limiting the downstream targets of p53, and by acting as a tumor suppressor [105]. In addition, Parkin as a component of the FBX4 Cullin-ring ligase complex regulates the levels of cyclin D1, cyclin E, and CDK4 in cancers, exhibiting cross talk between mitophagy and apoptosis in the regulation of cancer [104]. In healthy mitochondria, PINK1 recruits Parkin E3 ubiquitin ligase to mitochondria, and itself undergoes proteolysis at the IMM, although in response to mitochondrial depolarization it accumulates at the OMM [106, 107]. PINK1 phosphorylation of ubiquitin at serine 65 is required to recruit Parkin to mitochondria. Parkin substrate proteins on the OMM, including Vdac1, Mfn-2, and Miro, have been reported to be regulated by Parkin activity. Post-ubiquitination, the OMM substrates create a docking site for the LC3-interacting proteins and proceed to Parkin-dependent mitochondrial degradation at the autophagosome. Recruitment of Parkin to the depolarized membranes is promoted by Puma, Noxa, Bim, Bad, BNIP3, Nix, and Beclin1, which facilitate the Parkin–PINK1 interaction and Parkin-dependent ubiquitination of the mitochondrial targets.
Furthermore, the stress caused due to mitochondrial unfolded protein response (UPRmt) upon proteosomal degradation of the Parkin substrate may result in mitophagy [108]. Parkin-dependent turnover of Miro regulates the mitochondrial transport along the microtubules that hobble the microtubule-associated kinesin motor protein complexes to OMM [109]. In addition to Miro, HDAC6 promotes the trafficking of mitochondria along the microtubules to the autophagosomes through the cortactin–actin-dependent remodeling machinery [96, 110, 111]. Finally, the non-mitochondrial substrates of Parkin influence the transcriptional regulator PARIS to direct the PGC-1α expression to inhibit mitochondrial biogenesis by enhancing mitophagy [112]. Disruption in the neuronal metabolism and mitochondrial respiration downregulates the metabolic transcriptional regulator PGC-1α (peroxisome proliferator-activated receptor c coactivator 1α) and multiple PGC-1α target genes that lead to neuroblastoma, in response to abated expression of genes involved in antioxidant defense, oxidative stress-induced neuronal death, and oxidative phosphorylation. In SH-SY5Y neuroblastoma cells, overexpression of PGC-1α results in a remarkable hike in the mRNA expression of the TFAM (mitochondrial transcription factor A) and Mfn-2 [113]. Interestingly, HDACs may regulate PGC-1α, as the HDACis such as VPA and TSA have emerged as potential therapeutic agents against neurological disorders [6]. Both VPA and TSA have been reported to cause an increase in the PGC-1α mRNA expression, leading to the upregulation of Mfn-2, and consequently an elevated mitophagy (see Fig. 3).
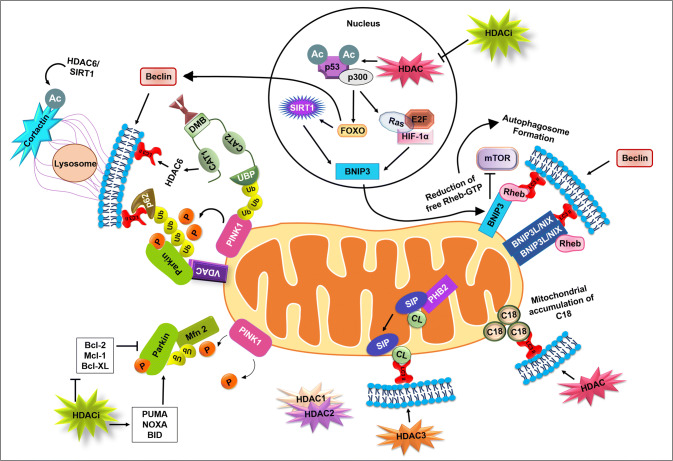
Fig. 3.
HDAC in controlling the powerhouse through mitophagy: HDAC inhibitors (HDACis) deacetylate non-histone target p53 and subsequent downstream effector targets such as FOXO, BNIP3, HIF 1α, E2F, and Ras. The p53 deacetylation stabilizes the PINK1 in the depolarized mitochondrial membrane leading to subsequent recruitment of Parkin. Parkin-mediated ubiquitination of mitochondrial membrane proteins and subsequent interaction with autophagic adaptor molecules such as LC3 and p62 perform PINK–Parkin-mediated mitophagy. In BNIP3/NIX-mediated mitophagy, SIRT1 and HDAC regulate deacetylation of p53 and HIFs. BNIP3 dimers interact with Rheb in a Bcl-2 and Bcl-XL-dependent manner and reduce it. Reduction of free Rheb inactivates mTOR that induces autophagy. The associated downstream molecules promote mitophagosome formation. The involvement of cardiolipins as a direct mitophagic receptor promotes lipid-mediated mitophagy, whereas S1P indirectly regulates cardiolipin-mediated mitophagy. The role of C18 in ceramide-induced mitophagy is not well known; however, the intra-molecular interlink between LC3B and C18-ceramide will put forth the novel mechanism underlying the role of HDACs in ceramide-induced mitophagy
Histone deacetylases in BNIP3/NIX-mediated mitophagy: converging the subordinate
BNIP3 and NIX (BNIP3L) are the two key molecular mediators involved in promoting the hypoxia-induced mitophagy, as these are the target genes for the HIFs [111, 114]. NF-κB [115], FoxO3 [116], RB/E2Fs, oncogenic Ras, and p53 act as transcriptional regulators for BNIP3, while p53 regulates the NIX expression [117]. BNIP3 and NIX, as redox resistance homodimers, integrate into the OMM and protrude out into the cytosol, exposing its LIR domain for direct interaction with processed LC3B-II or GABARAP [118]. Furthermore, the interaction of BNIP3/NIX with Bcl-2 and BclxL may also modulate the binding of these two with LC3. BNIP3-dependent mitophagy is introduced by mitochondrial fragmentation and perinuclear deposition of mitochondria due to the possible interaction of BNIP3 with the fusion protein Opa-1 [119]. Intriguingly, the BNIP3 dimers at the OMM interact with a small GTPase protein Rheb in a Bcl-2 and Bcl-XL-dependent manner and repress its mTOR activation activity. SAHA, an HDAC inhibitor known for mTOR deactivation and induction of autophagy, may also promote mitophagy through BNIP3-dependent Rheb repression [120]. In contrast, NIX interacts with Rheb in an mTOR-independent manner by promoting LC3 processing. Therefore, a NIX/Rheb interaction results in a NIX-dependent and mTOR-independent mitophagy. In DCIS (ductal carcinoma in situ) in human breast cancer, suppressed lymph node metastases and proliferative index have been known to be associated with the upregulation of BNIP3 and NIX [121]. Similarly, in hematological malignancies, and in lung, gastric, pancreatic, and liver cancers, epigenetic silencing of BNIP3 expression by HDACs induces mitophagy and serves as a tumor suppressor. Upregulation of BNIP3 has been known to be associated with the deacetylation of FOXO3 by SIRT1 [116]. Similarly, a FOXO1-dependent elicitation of mitophagy is attributed to the upregulation of BNIP3 in a SIRT2-dependent manner. Deacetylation of FOXO3 by SIRT1 is responsible for the upregulation of GABARAP, which in turn induces mitophagy in a BNIP3- and NIX-dependent manner by stimulating their affinity for direct binding to the LIR region [118] (see Fig. 3).
On the other hand, Mul1, a FOXO1/FOXO3-induced mitochondrial E3 ubiquitin ligase complex, ubiquitinates and targets Mfn-2 for degradation, under serum starvation and other stresses, leading to increased mitochondrial fission and mitophagy. Under hypoxia, the interaction of FUNDC1 (located on the OMM) protein, which is with LC3 at the phagophore, through the LIR motif present on FUNDC1, provides a novel insight into the mitophagy machinery independent of PINK1/PARKIN and BNIP3/NIX. The phosphorylation of FUNDC1 by ULK-1 at serine 17 facilitates mitochondrial turnover by promoting the FUNDC1 and LC3 interaction. The deacetylation of FOXO3 by SIRT1 has been reported to upregulate ULK1/2 and the associated members such as FIP200, ATG13, and ATG101, to promote FUNDC1-dependent mitophagy [118, 122].
Histone deacetylases in lipid-mediated mitophagy: focusing on the expanding horizon
Cardiolipin is a specialized phospholipid present exclusively in the MIM (mitochondrial inner membrane) and assists in the stabilization of cristae and supports the assembly of the function of the different components of the electron transport chain. However, mitochondrial injury, damage signals, and depolarization cause the externalization of cardiolipins to the MOM (mitochondrial outer membrane), where they are exposed to the cytosol [123, 124]. The knockdown of the phospholipid scramblase-3 or NDPK-D (nucleoside diphosphate kinase D) enzyme hinders the translocation of cardiolipins to the MOM [124]. Similarly, the genetic deletion or knockdown of cardiolipin synthase also exhibits the same phenomenon. The onset of these two phenomena causes a sharp decline in the delivery of mitochondria to the autophagosomes. Specific sites identified on the LC3 are required for direct interaction with the cardiolipins present on the MOM for the mitophagic uptake of mitochondria. Similarly, a conserved domain of Beclin-1 possesses higher affinity toward the cardiolipins and its associates for mitophagic engulfment. Therefore, the translocation of cardiolipins from MIM to MOM facilitates the uptake of damaged mitochondria through mitophagy [123].
In various human diseases including cancer, S1P (sphingosine-1-phosphate) has emerged as a critical regulator of cell proliferation, angiogenesis, migration, and mitogenesis [125]. S1P has been reported to regulate these intracellular processes through the action of HDAC1 and HDAC2. SphK1 and SphK2 (sphingosine kinase isoenzymes) are localized in the nucleus, where they produce S1P. Endogenous SphK2 is mainly associated with isolated chromatin through the core histone proteins. In human breast cancer cells, MCF-7, SphK2 is associated with histone H3. Elevated acetylation status of the H3-K9, H4-K5, and H2B-K12 positions is observed upon the overexpression of SphK2. HATs and HDACs critically maintain a dynamic equilibrium between the acetylation and deacetylation status of the nucleosomal histones at their lysine residues, which regulates the translation mechanism. S1P inhibits the HDAC activity through SphK2, while siSphK2 increases the HDAC activity in HeLa cells. It has also been reported that the overexpression of SphK2 is interlinked with the co-repressor complex of mSin3A and MBD2/3 mediated through HDAC1 and HDAC2. Silencing of SphK2 by HDAC1 and HDAC2 significantly reduces the basal accumulation of p21. HDACis SAHA, and TSA minimize the HDAC1-binding ability of S1P, promoting the cardiolipin shuttling from MIM to MOM [126].
Another class of lipid, ceramides, as central effector molecules in sphingolipid metabolism, induces lipid-mediated cell death which plays tumor-suppressive function in cancer [127]. Ceramides have been reported to regulate apoptosis post the significant localization to mitochondria, forming ceramide channels in the MOM, or by regulating the series of a pro-apoptotic and anti-apoptotic family of proteins [102]. Similarly, ceramides induce autophagy though different signaling pathway during stress. Moreover, endogenous and exogenous C18 ceramide or overexpression of ceramide synthase 1 (CerS1) promotes mitophagy through an intra-molecular interlink between LC3B and C18-ceramide (which functions as a receptor for autophagosomes) [128, 129] (see Fig. 3). Further, expression of mutant LC3B, unable to bind to ceramide, prevented selective mitochondria targeting for mitophagy. C18-ceramide mediated tumor suppression through lethal mitophagy and CerS1 deficiency abolished sodium selenite-induced mitophagy; stable LC3B knockdown prevented CerS1–C18-ceramide-dependent mitophagy and reduced tumor suppression in vivo [128]. Although the role of HDACs in the ceramide-mediated mitophagy has not been explored to a great extent, report showed that ceramide regulates HDACs activity to inhibit cancer growth and metastasis [130]. For example, novel HDACi AR-42 inhibits colon cancer cell growth through ceramide production [131].
Conclusion and future prospective
Histone deacetylases play a crucial role in the regulation of histone and non-histone targets by modulating acetylation status involved in cancer initiation, progression, and metastasis. Studies over the past few decades have revealed that HDACs have been found to dysregulate and/or function incorrectly in cancer, providing a crucial attractive target against cancer. However, the detailed function of HDACs as a central regulator of cellular transformation and carcinogenesis leading to tumor growth still remains unknown. Moreover, dysfunctional apoptosis in cancer contributes through HDACs indicating apoptosis induction by HDACi could have potential cancer therapeutics. In addition, HDACs-dependent autophagy functions in both tumor promotion and suppression and is complex, regulated by more than one signaling pathway. In this setting, more studies are warranted to distinctly analyze the role of individual HDACs in different cancer types at different stages of cancer development. Intriguingly, identification of selective novel HDACi could reveal and explain the function of distinct HDACs which will help in the development of mechanistic-based HDACi antitumor agent for clinical treatment. Till date, most of the HDACi in preclinical and clinical evaluation for cancer therapy are broad spectrum and nonselective. The efficiency of nonselective HDACi for therapeutic use of cancer is limited for toxicity in patients. Hence, the current study aims to develop HDACi with greater target specificity that might be more effective and have less toxicity. In parallel, high-throughput research supports in the development of combination therapy with chemotherapy might be another important direction to enhance the therapeutic efficacy of HDACi. Further detailed elucidation of the mechanisms of HDACs and HDACi could provide better therapeutic effect for selective HDACi against cancer.
Acknowledgements
We convey our thanks to the National Institute of Technology, Rourkela. SP acknowledges DST-INSPIRE, Award reference number (IF180167), Department of Science and Technology, Government of India, for providing fellowship.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nebbioso A, Tambaro FP, Dell’Aversana C, Altucci L. Cancer epigenetics: moving forward. PLoS Genet. 2018;14:e1007362. doi: 10.1371/journal.pgen.1007362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen QW, Zhu XY, Li YY, Meng ZQ. Epigenetic regulation and cancer (review) Oncol Rep. 2014;31:523–532. doi: 10.3892/or.2013.2913. [DOI] [PubMed] [Google Scholar]
- 4.Herceg Z, Ushijima T. Introduction: epigenetics and cancer. Adv Genet. 2010;70:1–23. doi: 10.1016/B978-0-12-380866-0.60001-0. [DOI] [PubMed] [Google Scholar]
- 5.Baxter E, Windloch K, Gannon F, Lee JS. Epigenetic regulation in cancer progression. Cell Biosci. 2014;4:45. doi: 10.1186/2045-3701-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parbin S, Kar S, Shilpi A, Sengupta D, Deb M, Rath SK, Patra SK. Histone deacetylases: a saga of perturbed acetylation homeostasis in cancer. J Histochem Cytochem. 2014;62:11–33. doi: 10.1369/0022155413506582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 8.Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 9.Smith BC, Denu JM. Chemical mechanisms of histone lysine and arginine modifications. Biochim Biophys Acta. 2009;1789:45–57. doi: 10.1016/j.bbagrm.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witt O, Deubzer HE, Milde T, Oehme I. HDAC family: what are the cancer relevant targets? Cancer Lett. 2009;277:8–21. doi: 10.1016/j.canlet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Chueh AC, Tse JW, Togel L, Mariadason JM. Mechanisms of histone deacetylase inhibitor-regulated gene expression in cancer cells. Antioxid Redox Signal. 2015;23:66–84. doi: 10.1089/ars.2014.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017;18:e1414. doi: 10.3390/ijms18071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair SS, Kumar R. Chromatin remodeling in cancer: a gateway to regulate gene transcription. Mol Oncol. 2012;6:611–619. doi: 10.1016/j.molonc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 16.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 17.Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain JP, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601–605. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- 18.Kadosh D, Struhl K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell. 1997;89:365–371. doi: 10.1016/S0092-8674(00)80217-2. [DOI] [PubMed] [Google Scholar]
- 19.Hassig CA, Fleischer TC, Billin AN, Schreiber SL, Ayer DE. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/S0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 20.Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 21.Yamada N, Hamada T, Goto M, Tsutsumida H, Higashi M, Nomoto M, Yonezawa S. MUC2 expression is regulated by histone H3 modification and DNA methylation in pancreatic cancer. Int J Cancer. 2006;119:1850–1857. doi: 10.1002/ijc.22047. [DOI] [PubMed] [Google Scholar]
- 22.Augenlicht L, Shi L, Mariadason J, Laboisse C, Velcich A. Repression of MUC2 gene expression by butyrate, a physiological regulator of intestinal cell maturation. Oncogene. 2003;22:4983–4992. doi: 10.1038/sj.onc.1206521. [DOI] [PubMed] [Google Scholar]
- 23.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/S0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 24.Huang BH, Laban M, Leung CH, Lee L, Lee CK, Salto-Tellez M, Raju GC, Hooi SC. Inhibition of histone deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression, independent of histone deacetylase 1. Cell Death Differ. 2005;12:395–404. doi: 10.1038/sj.cdd.4401567. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Zhao S, Ammanamanchi S, Brattain M, Venkatasubbarao K, Freeman JW. Trichostatin A induces transforming growth factor beta type II receptor promoter activity and acetylation of Sp1 by recruitment of PCAF/p300 to a Sp1.NF-Y complex. J Biol Chem. 2005;280:10047–10054. doi: 10.1074/jbc.M408680200. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Lu S, Wu L, Chai G, Wang H, Chen Y, Sun J, Yu Y, Zhou W, Zheng Q, Wu M, Otterson GA, Zhu WG. Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21(Waf1/Cip1) Mol Cell Biol. 2006;26:2782–2790. doi: 10.1128/MCB.26.7.2782-2790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou W, Zhu WG. The changing face of HDAC inhibitor depsipeptide. Curr Cancer Drug Targets. 2009;9:91–100. doi: 10.2174/156800909787314039. [DOI] [PubMed] [Google Scholar]
- 28.Uo T, Veenstra TD, Morrison RS. Histone deacetylase inhibitors prevent p53-dependent and p53-independent Bax-mediated neuronal apoptosis through two distinct mechanisms. J Neurosci. 2009;29:2824–2832. doi: 10.1523/JNEUROSCI.6186-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi Y, Kurokawa M, Imai Y, Izutsu K, Asai T, Ichikawa M, Yamamoto G, Nitta E, Yamagata T, Sasaki K, Mitani K, Ogawa S, Chiba S, Hirai H. AML1 is functionally regulated through p300-mediated acetylation on specific lysine residues. J Biol Chem. 2004;279:15630–15638. doi: 10.1074/jbc.M400355200. [DOI] [PubMed] [Google Scholar]
- 30.Guidez F, Howell L, Isalan M, Cebrat M, Alani RM, Ivins S, Hormaeche I, McConnell MJ, Pierce S, Cole PA, Licht J, Zelent A. Histone acetyltransferase activity of p300 is required for transcriptional repression by the promyelocytic leukemia zinc finger protein. Mol Cell Biol. 2005;25:5552–5566. doi: 10.1128/MCB.25.13.5552-5566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melnick AM, Westendorf JJ, Polinger A, Carlile GW, Arai S, Ball HJ, Lutterbach B, Hiebert SW, Licht JD. The ETO protein disrupted in t(8;21)-associated acute myeloid leukemia is a corepressor for the promyelocytic leukemia zinc finger protein. Mol Cell Biol. 2000;20:2075–2086. doi: 10.1128/MCB.20.6.2075-2086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng L, Lin T, Uranishi H, Gu W, Xu Y. Functional analysis of the roles of posttranslational modifications at the p53 C terminus in regulating p53 stability and activity. Mol Cell Biol. 2005;25:5389–5395. doi: 10.1128/MCB.25.13.5389-5395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito A, Lai CH, Zhao X, Saito S, Hamilton MH, Appella E, Yao TP. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 2001;20:1331–1340. doi: 10.1093/emboj/20.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed SM, Quelle DE. p53 acetylation: regulation and consequences. Cancers (Basel) 2014;7:30–69. doi: 10.3390/cancers7010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 36.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nadiminty N, Lou W, Lee SO, Lin X, Trump DL, Gao AC. Stat3 activation of NF-{kappa}B p100 processing involves CBP/p300-mediated acetylation. Proc Natl Acad Sci USA. 2006;103:7264–7269. doi: 10.1073/pnas.0509808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269–273. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 39.Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, Vassilopoulos A, Ozden O, Park SH, Singh KK, Abdulkadir SA, Spitz DR, Deng CX, Gius D. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu W, Denu RA, Krautkramer KA, Grindle KM, Yang DT, Asimakopoulos F, Hematti P, Denu JM. Loss of SIRT3 provides growth advantage for B cell malignancies. J Biol Chem. 2016;291:3268–3279. doi: 10.1074/jbc.M115.702076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Wang WY, Cao LP. SIRT3 inhibits cell proliferation in human gastric cancer through down-regulation of Notch-1. Int J Clin Exp Med. 2015;8:5263–5271. [PMC free article] [PubMed] [Google Scholar]
- 42.George J, Nihal M, Singh CK, Zhong W, Liu X, Ahmad N. Pro-proliferative function of mitochondrial sirtuin deacetylase SIRT3 in human melanoma. J Investig Dermatol. 2016;136:809–818. doi: 10.1016/j.jid.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang YY, Zhou LM. Sirt3 inhibits hepatocellular carcinoma cell growth through reducing Mdm2-mediated p53 degradation. Biochem Biophys Res Commun. 2012;423:26–31. doi: 10.1016/j.bbrc.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 44.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 45.Akiyama Y, Watkins N, Suzuki H, Jair KW, van Engeland M, Esteller M, Sakai H, Ren CY, Yuasa Y, Herman JG, Baylin SB. GATA-4 and GATA-5 transcription factor genes and potential downstream antitumor target genes are epigenetically silenced in colorectal and gastric cancer. Mol Cell Biol. 2003;23:8429–8439. doi: 10.1128/MCB.23.23.8429-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caslini C, Capo-chichi CD, Roland IH, Nicolas E, Yeung AT, Xu XX. Histone modifications silence the GATA transcription factor genes in ovarian cancer. Oncogene. 2006;25:5446–5461. doi: 10.1038/sj.onc.1209533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D, Li W, Zhao R, Chen L, Liu N, Tian Y, Zhao H, Xie M, Lu F, Fang Q, Liang W, Yin F, Li Z. Stabilized peptide HDAC inhibitors derived from HDAC1 substrate H3K56 for the treatment of cancer stem-like cells in vivo. Cancer Res. 2019 doi: 10.1158/0008-5472.CAN-18-1421. [DOI] [PubMed] [Google Scholar]
- 48.Wang ZT, Chen ZJ, Jiang GM, Wu YM, Liu T, Yi YM, Zeng J, Du J, Wang HS. Histone deacetylase inhibitors suppress mutant p53 transcription via HDAC8/YY1 signals in triple negative breast cancer cells. Cell Signal. 2016;28:506–515. doi: 10.1016/j.cellsig.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Yan W, Liu S, Xu E, Zhang J, Zhang Y, Chen X, Chen X. Histone deacetylase inhibitors suppress mutant p53 transcription via histone deacetylase 8. Oncogene. 2013;32:599–609. doi: 10.1038/onc.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song Q, Li M, Fan C, Liu Y, Zheng L, Bao Y, Sun L, Yu C, Song Z, Sun Y, Wang G, Huang Y, Li Y. A novel benzamine lead compound of histone deacetylase inhibitor ZINC24469384 can suppresses HepG2 cells proliferation by upregulating NR1H4. Sci Rep. 2019;9:2350. doi: 10.1038/s41598-019-39487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji M, Li Z, Lin Z, Chen L. Antitumor activity of the novel HDAC inhibitor CUDC-101 combined with gemcitabine in pancreatic cancer. Am J Cancer Res. 2018;8:2402–2418. [PMC free article] [PubMed] [Google Scholar]
- 52.Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 53.Whetstine JR, Ceron J, Ladd B, Dufourcq P, Reinke V, Shi Y. Regulation of tissue-specific and extracellular matrix-related genes by a class I histone deacetylase. Mol Cell. 2005;18:483–490. doi: 10.1016/j.molcel.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Durst KL, Lutterbach B, Kummalue T, Friedman AD, Hiebert SW. The inv(16) fusion protein associates with corepressors via a smooth muscle myosin heavy-chain domain. Mol Cell Biol. 2003;23:607–619. doi: 10.1128/MCB.23.2.607-619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Annicotte JS, Iankova I, Miard S, Fritz V, Sarruf D, Abella A, Berthe ML, Noel D, Pillon A, Iborra F, Dubus P, Maudelonde T, Culine S, Fajas L. Peroxisome proliferator-activated receptor gamma regulates E-cadherin expression and inhibits growth and invasion of prostate cancer. Mol Cell Biol. 2006;26:7561–7574. doi: 10.1128/MCB.00605-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu W, Liu H, Liu ZG, Wang HS, Zhang F, Wang H, Zhang J, Chen JJ, Huang HJ, Tan Y, Cao MT, Du J, Zhang QG, Jiang GM. Histone deacetylase inhibitors upregulate Snail via Smad2/3 phosphorylation and stabilization of Snail to promote metastasis of hepatoma cells. Cancer Lett. 2018;420:1–13. doi: 10.1016/j.canlet.2018.01.068. [DOI] [PubMed] [Google Scholar]
- 58.Crazzolara R, Johrer K, Johnstone RW, Greil R, Kofler R, Meister B, Bernhard D. Histone deacetylase inhibitors potently repress CXCR58 chemokine receptor expression and function in acute lymphoblastic leukaemia. Br J Haematol. 2002;119:965–969. doi: 10.1046/j.1365-2141.2002.03955.x. [DOI] [PubMed] [Google Scholar]
- 59.Hellebrekers DM, Castermans K, Vire E, Dings RP, Hoebers NT, Mayo KH, Oude Egbrink MG, Molema G, Fuks F, van Engeland M, Griffioen AW. Epigenetic regulation of tumor endothelial cell anergy: silencing of intercellular adhesion molecule-1 by histone modifications. Cancer Res. 2006;66:10770–10777. doi: 10.1158/0008-5472.CAN-06-1609. [DOI] [PubMed] [Google Scholar]
- 60.Byles V, Zhu L, Lovaas JD, Chmilewski LK, Wang J, Faller DV, Dai Y. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene. 2012;31:4619–4629. doi: 10.1038/onc.2011.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malik S, Villanova L, Tanaka S, Aonuma M, Roy N, Berber E, Pollack JR, Michishita-Kioi E, Chua KF. SIRT7 inactivation reverses metastatic phenotypes in epithelial and mesenchymal tumors. Sci Rep. 2015;5:9841. doi: 10.1038/srep09841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu J, Zhu W, Xu W, Yao W, Zhang B, Xu Y, Ji S, Liu C, Long J, Ni Q, Yu X. Up-regulation of MBD1 promotes pancreatic cancer cell epithelial-mesenchymal transition and invasion by epigenetic down-regulation of E-cadherin. Curr Mol Med. 2013;13:387–400. [PubMed] [Google Scholar]
- 63.Chen IC, Chiang WF, Huang HH, Chen PF, Shen YY, Chiang HC. Role of SIRT1 in regulation of epithelial-to-mesenchymal transition in oral squamous cell carcinoma metastasis. Mol Cancer. 2014;13:254. doi: 10.1186/1476-4598-13-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang GM, Wang HS, Zhang F, Zhang KS, Liu ZC, Fang R, Wang H, Cai SH, Du J. Histone deacetylase inhibitor induction of epithelial-mesenchymal transitions via up-regulation of Snail facilitates cancer progression. Biochim Biophys Acta. 2013;1833:663–671. doi: 10.1016/j.bbamcr.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Zhou W, Ni TK, Wronski A, Glass B, Skibinski A, Beck A, Kuperwasser C. The SIRT2 deacetylase stabilizes slug to control malignancy of basal-like breast cancer. Cell Rep. 2016;17:1302–1317. doi: 10.1016/j.celrep.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu S, Luo Z, Yu PJ, Xie H, He YW. Suberoylanilide hydroxamic acid (SAHA) promotes the epithelial mesenchymal transition of triple negative breast cancer cells via HDAC8/FOXA1 signals. Biol Chem. 2016;397:75–83. doi: 10.1515/hsz-2015-0215. [DOI] [PubMed] [Google Scholar]
- 67.Miyo M, Yamamoto H, Konno M, Colvin H, Nishida N, Koseki J, Kawamoto K, Ogawa H, Hamabe A, Uemura M, Nishimura J, Hata T, Takemasa I, Mizushima T, Doki Y, Mori M, Ishii H. Tumour-suppressive function of SIRT4 in human colorectal cancer. Br J Cancer. 2015;113:492–499. doi: 10.1038/bjc.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu H, Ye W, Wu J, Meng X, Liu RY, Ying X, Zhou Y, Wang H, Pan C, Huang W. Overexpression of sirt7 exhibits oncogenic property and serves as a prognostic factor in colorectal cancer. Clin Cancer Res. 2014;20:3434–3445. doi: 10.1158/1078-0432.CCR-13-2952. [DOI] [PubMed] [Google Scholar]
- 69.Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, Moon EJ, Kim HS, Lee SK, Chung HY, Kim CW, Kim KW. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 70.Song C, Zhu S, Wu C, Kang J. Histone deacetylase (HDAC) 10 suppresses cervical cancer metastasis through inhibition of matrix metalloproteinase (MMP) 2 and 9 expression. J Biol Chem. 2013;288:28021–28033. doi: 10.1074/jbc.M113.498758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma P, Pan H, Montgomery RL, Olson EN, Schultz RM. Compensatory functions of histone deacetylase 1 (HDAC1) and HDAC2 regulate transcription and apoptosis during mouse oocyte development. Proc Natl Acad Sci USA. 2012;109:E481–E489. doi: 10.1073/pnas.1118403109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inoue S, Mai A, Dyer MJ, Cohen GM. Inhibition of histone deacetylase class I but not class II is critical for the sensitization of leukemic cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Cancer Res. 2006;66:6785–6792. doi: 10.1158/0008-5472.CAN-05-4563. [DOI] [PubMed] [Google Scholar]
- 73.Escaffit F, Vaute O, Chevillard-Briet M, Segui B, Takami Y, Nakayama T, Trouche D. Cleavage and cytoplasmic relocalization of histone deacetylase 3 are important for apoptosis progression. Mol Cell Biol. 2007;27:554–567. doi: 10.1128/MCB.00869-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: role of resveratrol. Biochem Biophys Res Commun. 2010;393:66–72. doi: 10.1016/j.bbrc.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giannakou ME, Partridge L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 2004;14:408–412. doi: 10.1016/j.tcb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 78.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 79.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/S0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 80.Song CL, Tang H, Ran LK, Ko BC, Zhang ZZ, Chen X, Ren JH, Tao NN, Li WY, Huang AL, Chen J. Sirtuin 3 inhibits hepatocellular carcinoma growth through the glycogen synthase kinase-3beta/BCL2-associated X protein-dependent apoptotic pathway. Oncogene. 2016;35:631–641. doi: 10.1038/onc.2015.121. [DOI] [PubMed] [Google Scholar]
- 81.Paroni G, Mizzau M, Henderson C, Del Sal G, Schneider C, Brancolini C. Caspase-dependent regulation of histone deacetylase 4 nuclear-cytoplasmic shuttling promotes apoptosis. Mol Biol Cell. 2004;15:2804–2818. doi: 10.1091/mbc.e03-08-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Medina V, Edmonds B, Young GP, James R, Appleton S, Zalewski PD. Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin A (inhibitors of histone deacetylase): dependence on protein synthesis and synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer Res. 1997;57:3697–3707. [PubMed] [Google Scholar]
- 83.Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627–638. doi: 10.1016/S1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 84.Subramanian C, Opipari AW, Jr, Bian X, Castle VP, Kwok RP. Ku70 acetylation mediates neuroblastoma cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2005;102:4842–4847. doi: 10.1073/pnas.0408351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang GG, Allis CD, Chi P. Chromatin remodeling and cancer, part I: covalent histone modifications. Trends Mol Med. 2007;13:363–372. doi: 10.1016/j.molmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 86.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 87.Jeong H, Then F, Melia TJ, Jr, Mazzulli JR, Cui L, Savas JN, Voisine C, Paganetti P, Tanese N, Hart AC, Yamamoto A, Krainc D. Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell. 2009;137:60–72. doi: 10.1016/j.cell.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R, Bordone L. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009;4:e8414. doi: 10.1371/journal.pone.0008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S, Tailler M, Delahaye N, Tesniere A, De Stefano D, Younes AB, Harper F, Pierron G, Lavandero S, Zitvogel L, Israel A, Baud V, Kroemer G. IKK connects autophagy to major stress pathways. Autophagy. 2010;6:189–191. doi: 10.4161/auto.6.1.10818. [DOI] [PubMed] [Google Scholar]
- 92.Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S, Tailler M, Delahaye N, Tesniere A, De Stefano D, Younes AB, Harper F, Pierron G, Lavandero S, Zitvogel L, Israel A, Baud V, Kroemer G. The IKK complex contributes to the induction of autophagy. EMBO J. 2010;29:619–631. doi: 10.1038/emboj.2009.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Comb WC, Cogswell P, Sitcheran R, Baldwin AS. IKK-dependent, NF-kappaB-independent control of autophagic gene expression. Oncogene. 2011;30:1727–1732. doi: 10.1038/onc.2010.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013;228:2262–2270. doi: 10.1002/jcp.24399. [DOI] [PubMed] [Google Scholar]
- 95.Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12:665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- 96.Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, Pandey UB, Kaushik S, Tresse E, Lu J, Taylor JP, Cuervo AM, Yao TP. HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J. 2010;29:969–980. doi: 10.1038/emboj.2009.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oehme I, Linke JP, Bock BC, Milde T, Lodrini M, Hartenstein B, Wiegand I, Eckert C, Roth W, Kool M, Kaden S, Grone HJ, Schulte JH, Lindner S, Hamacher-Brady A, Brady NR, Deubzer HE, Witt O. Histone deacetylase 10 promotes autophagy-mediated cell survival. Proc Natl Acad Sci USA. 2013;110:E2592–E2601. doi: 10.1073/pnas.1300113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu W, Zuo Y, Feng Y, Zhang M. SIRT5 facilitates cancer cell growth and drug resistance in non-small cell lung cancer. Tumour Biol. 2014;35:10699–10705. doi: 10.1007/s13277-014-2372-4. [DOI] [PubMed] [Google Scholar]
- 99.Polletta L, Vernucci E, Carnevale I, Arcangeli T, Rotili D, Palmerio S, Steegborn C, Nowak T, Schutkowski M, Pellegrini L, Sansone L, Villanova L, Runci A, Pucci B, Morgante E, Fini M, Mai A, Russo MA, Tafani M. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy. 2015;11:253–270. doi: 10.1080/15548627.2015.1009778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tolkovsky AM. Mitophagy. Biochim Biophys Acta. 2009;1793:1508–1515. doi: 10.1016/j.bbamcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 101.Meyers-Needham M, Ponnusamy S, Gencer S, Jiang W, Thomas RJ, Senkal CE, Ogretmen B. Concerted functions of HDAC1 and microRNA-574-5p repress alternatively spliced ceramide synthase 1 expression in human cancer cells. EMBO Mol Med. 2012;4:78–92. doi: 10.1002/emmm.201100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nganga R, Oleinik N, Ogretmen B. Mechanisms of ceramide-dependent cancer cell death. Adv Cancer Res. 2018;140:1–25. doi: 10.1016/bs.acr.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 103.Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187:959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cesari R, Martin ES, Calin GA, Pentimalli F, Bichi R, McAdams H, Trapasso F, Drusco A, Shimizu M, Masciullo V, D’Andrilli G, Scambia G, Picchio MC, Alder H, Godwin AK, Croce CM. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27. Proc Natl Acad Sci USA. 2003;100:5956–5961. doi: 10.1073/pnas.0931262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gong Y, Zack TI, Morris LG, Lin K, Hukkelhoven E, Raheja R, Tan IL, Turcan S, Veeriah S, Meng S, Viale A, Schumacher SE, Palmedo P. Pan-cancer genetic analysis identifies PARK2 as a master regulator of G1/S cyclins. Nat Genet. 2014;46:588–594. doi: 10.1038/ng.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang C, Lin M, Wu R, Wang X, Yang B, Levine AJ, Hu W, Feng Z. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci USA. 2011;108:16259–16264. doi: 10.1073/pnas.1113884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boland ML, Chourasia AH, Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tan Z, Luo X, Xiao L, Tang M, Bode AM, Dong Z, Cao Y. The role of PGC1alpha in cancer metabolism and its therapeutic implications. Mol Cancer Ther. 2016;15:774–782. doi: 10.1158/1535-7163.MCT-15-0621. [DOI] [PubMed] [Google Scholar]
- 114.Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shaw J, Yurkova N, Zhang T, Gang H, Aguilar F, Weidman D, Scramstad C, Weisman H, Kirshenbaum LA. Antagonism of E2F-1 regulated Bnip3 transcription by NF-kappaB is essential for basal cell survival. Proc Natl Acad Sci USA. 2008;105:20734–20739. doi: 10.1073/pnas.0807735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 117.Fei P, Wang W, Kim SH, Wang S, Burns TF, Sax JK, Buzzai M, Dicker DT, McKenna WG, Bernhard EJ, El-Deiry WS. Bnip3L is induced by p53 under hypoxia, and its knockdown promotes tumor growth. Cancer Cell. 2004;6:597–609. doi: 10.1016/j.ccr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 118.Chourasia AH, Boland ML, Macleod KF. Mitophagy and cancer. Cancer Metab. 2015;3:4. doi: 10.1186/s40170-015-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Landes T, Emorine LJ, Courilleau D, Rojo M, Belenguer P, Arnaune-Pelloquin L. The BH3-only Bnip3 binds to the dynamin Opa1 to promote mitochondrial fragmentation and apoptosis by distinct mechanisms. EMBO Rep. 2010;11:459–465. doi: 10.1038/embor.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Melser S, Chatelain EH, Lavie J, Mahfouf W, Jose C, Obre E, Goorden S, Priault M, Elgersma Y, Rezvani HR, Rossignol R, Benard G. Rheb regulates mitophagy induced by mitochondrial energetic status. Cell Metab. 2013;17:719–730. doi: 10.1016/j.cmet.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 121.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001;61:6669–6673. [PubMed] [Google Scholar]
- 122.Wu W, Tian W, Hu Z, Chen G, Huang L, Li W, Zhang X, Xue P, Zhou C, Liu L, Zhu Y, Zhang X, Li L, Zhang L, Sui S, Zhao B, Feng D. ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO Rep. 2014;15:566–575. doi: 10.1002/embr.201438501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Wang KZQ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kagan VE, Jiang J, Huang Z, Tyurina YY, Desbourdes C, Cottet-Rousselle C, Dar HH, Verma M, Tyurin VA, Kapralov AA, Cheikhi A, Mao G, Stolz D, St Croix CM, Watkins S, Shen Z, Li Y, Greenberg ML, Tokarska-Schlattner M, Boissan M, Lacombe ML, Epand RM, Chu CT, Mallampalli RK, Bayir H, Schlattner U. NDPK-D (NM23-H4)-mediated externalization of cardiolipin enables elimination of depolarized mitochondria by mitophagy. Cell Death Differ. 2016;23:1140–1151. doi: 10.1038/cdd.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dany M, Ogretmen B. Ceramide induced mitophagy and tumor suppression. Biochim Biophys Acta. 2015;1853:2834–2845. doi: 10.1016/j.bbamcr.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, Ramshesh VK, Peterson YK, Lemasters JJ, Szulc ZM, Bielawski J, Ogretmen B. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8:831–838. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Praharaj PP, Naik PP, Panigrahi DP, Bhol CS, Mahapatra KK, Patra S, Sethi G, Bhutia SK. Intricate role of mitochondrial lipid in mitophagy and mitochondrial apoptosis: its implication in cancer therapeutics. Cell Mol Life Sci. 2018 doi: 10.1007/s00018-018-2990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 2018;18:33–50. doi: 10.1038/nrc.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu W, Xu B, Yao Y, Yu X, Shen J. The novel HDAC inhibitor AR-42-induced anti-colon cancer cell activity is associated with ceramide production. Biochem Biophys Res Commun. 2015;463:545–550. doi: 10.1016/j.bbrc.2015.05.078. [DOI] [PubMed] [Google Scholar]
- 132.Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. doi: 10.1016/j.jmb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 133.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Investig. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bereshchenko OR, Gu W, Dalla-Favera R. Acetylation inactivates the transcriptional repressor BCL6. Nat Genet. 2002;32:606–613. doi: 10.1038/ng1018. [DOI] [PubMed] [Google Scholar]
- 135.Chalkiadaki A, Guarente L. The multifaceted functions of sirtuins in cancer. Nat Rev Cancer. 2015;15:608–624. doi: 10.1038/nrc3985. [DOI] [PubMed] [Google Scholar]