Abstract
Mutations of cystic fibrosis transmembrane conductance regulator (CFTR) cause cystic fibrosis, the most common life-limiting recessive genetic disease among Caucasians. CFTR mutations have also been linked to increased risk of various cancers but remained controversial for a long time. Recent studies have begun to reveal that CFTR is not merely an ion channel but also an important regulator of cancer development and progression with multiple signaling pathways identified. In this review, we will first present clinical findings showing the correlation of genetic mutations or aberrant expression of CFTR with cancer incidence in multiple cancers. We will then focus on the roles of CFTR in fundamental cellular processes including transformation, survival, proliferation, migration, invasion and epithelial–mesenchymal transition in cancer cells, highlighting the signaling pathways involved. Finally, the association of CFTR expression levels with patient prognosis, and the potential of CFTR as a cancer prognosis indicator in human malignancies will be discussed.
Keywords: CFTR, Cancer risk, Cellular processes, Prognosis indicator
Introduction
Cystic fibrosis transmembrane conductance regulator (CFTR) is known as a major anion channel for Cl− and HCO3−, which belongs to the adenosine triphosphate (ATP)-binding cassette (ABC) transporter family [1, 2]. While CFTR was initially found to be expressed in a wide variety of epithelial tissues, subsequent studies indicated that CFTR was expressed in other tissues/cell types as well including the heart [3], smooth muscles [4], neurons [5], endothelial cells [6], sperm [7] and oocytes [8]. Epithelial cells with defective CFTR produce excessive viscous mucus, which consequently accumulates and obstructs the lumen of pancreatic ducts, airway, biliary and reproductive tracts [9], and may result in various clinical manifestations [10]. Thus, mutations of CFTR cause the most common lethal genetic disease in Caucasians—cystic fibrosis (CF) [11, 12]. Although CF is still a disease with relentless morbidity and mortality, more than half of the CF patients can live till age 18 or above in recent years [13], due to an improvement of the treatment strategies. As CF patients now reach adulthood, there is an increasing interest in the association of cancer incidence with the genetic variations of the CFTR gene. In North American and European, large cohort studies have reported increased risk of overall cancers, especially in digestive system, in CF patients [14–18]. Specifically, the mean age at diagnosis of cancer was 40.4 years in CF patients, while 64.2 years in male and 54.8 years in females with one mutated copy of CFTR gene [17]. The risk was even elevated in patients with organ transplantation [18, 19]. Moreover, hyper-methylation of CFTR gene has been reported in several cancer cell lines and primary tumors [20–23]. These studies suggest a potential link between CFTR and the pathogenesis of cancers.
In this review, we will summarize the correlation between the aberrant expression of CFTR, including its expression pattern, genetic mutations/polymorphisms and epigenetic regulation, and cancer incidence in multiple cancers. This review will also discuss various CFTR-regulated signaling pathways involved in the process of cancer development, and the potential implication of CFTR as a regulator of cancer progression and an indicator of cancer prognosis.
Mutations and aberrant expression of CFTR in cancers
CFTR is a 1480 amino acid membrane bound glycoprotein with a molecular mass of 170,000. CFTR protein possesses two membrane-spanning domains (MSDs), each containing six helices and conjoined to a cytoplasmic nucleotide-binding domain (NBD1 and NBD2), where ATP binds to regulate channel gating [24–27]. Over 2000 mutations have been identified in the CFTR gene to date [28], which are situated throughout the entire coding region and the promoter region of the gene. The ΔF508 mutation accounts for approximately 70% of mutations in CF patients. The mutated CFTR anion channel is not fully glycosylated and shows minimal activity in epithelial cells of CF patients. Low temperature or inhibition of histone deacetylases partly rescues ∆F508 CFTR cellular processing defects and function. The majority of the other CFTR mutations are rare, i.e., the four other mutations (G551D, G542X, N1303 K and W1282X) have overall frequencies of above 1%, which are unique to a particular individual or family across the world [29]. In addition, genomic rearrangements, such as large deletions or insertions within the CFTR gene, e.g., Δexons 4–10, Δ95.7 kb starting in intron 1, have also been identified in CF patients, albeit the incidence is low [28].
Since the first report showing increased incidence of gastrointestinal malignancies in CF patients [14], there have been accumulating evidence indicating the association of cancer incidence with the genetic variations of the CFTR gene. Large cohort study in North American and European patients with CF found that there was a significant increase in the risk of cancers affecting the gastrointestinal tract, pancreas and hepatobiliary system [14, 30]. Of note, in a recent study conducted at the University of Minnesota, early colonoscopic screening of adult CF patients revealed a high incidence of colon tumors, especially in males [31]. Conversely, CF gene mutations have also been reported to be associated with a lower risk in several cancers, such as melanoma [32], breast cancer [33], colon cancer [34], prostate cancer [35] and lung cancer [36]. On the other hand, aberrant expression levels of CFTR have been reported by various studies, based on hospital-based case–control studies or laboratory observations. Downregulation of CFTR has been observed in multiple cancers including nasopharyngeal carcinoma (NPC) [37], hepatocellular carcinoma (HCC) [23, 38], intestinal cancer [39, 40], prostate cancer [41], lung cancer [42, 43] and bladder cancer [22, 44]. In contrast, upregulation of CFTR has been found in other types of cancer such as gastric cancer [45], ovarian cancer [46, 47], cervical cancer [48, 49] and endometrial cancer [50, 51].
In our opinion, while numerous genetic variants and aberrant expression levels of CFTR have been frequently identified in different types of cancer, there are considerable contradictory findings regarding the association of CFTR mutations/expression with cancer incidence. Although it is quite clear that different tissue contexts may contribute to the different findings, these discrepancies might also be due to the differences in sample size, study design and various CFTR mutations included in the individual studies. On the other hand, these studies emphasize the importance of understanding the exact physiological role of CFTR in cancer development. Since the association of CFTR mutations or aberrant expression with cancers has been reviewed previously [52, 53], this article will not focus on this aspect and the related information is summarized in Table 1.
Table 1.
The mutations, expression and clinical implication of CFTR in different cancers
| Cancer type | Expression, Methylation and Polymorphisms | Clinical implication | Citations |
|---|---|---|---|
| Reproduction system | |||
| Ovarian cancer | Up-regulation | High CFTR expression is correlated with advanced stage, poor histological grades and high CA-125 | Xu et al. [47] and Zhu et al. [46] |
| Endometrial carcinoma | Up-regulation | Unknown | Xia et al. [50] |
| Cervical cancer | Up-regulation | The combination of CFTR and NF-κB is positively associated with stage, histological grade, lymph node metastasis, and invasive interstitial depth | Peng et al. [49], Wu et al. [143] and Royse et al. [48] |
| Breast cancer |
DeltaF508 carrier related to higher grade III (1) Down-regulation (2) |
Low mRNA expression is correlated with poor prognosis |
(1) Southey et al. [183] (2) Zhang et al. [76] |
| Prostate cancer |
IVS8-5T allele protects against prostate cancer (1) Hyper-methylation (2) Down-regulation (3) |
CFTR methylation indicates the population at higher risk of therapeutic failure |
(1) Qiao et al. [35] (2) Ashour et al. [21] (3) Xie et al. [41] |
| Respiratory system | |||
| Nasopharyngeal carcinoma | Down-regulation | CFTR protein expression is positively correlated with survival | Tu et al. [37] |
| Lung cancer |
DeltaF508 deletion is found in 1/9 lung cancer patients(1) CFTR deletion carrier protects against lung cancer (2) Down-regulation(3) Hyper-methylated(4) |
CFTR methylation is correlated with worse survival in young patients Low mRNA expression is correlated with advanced staging, metastasis and poor prognosis |
(1) Jung et al. [189] (2) Li et al. [36] (3) Son et al. [20], Li et al. [43] and Tian et al. [42] (4) Son et al. [20] |
| Urinary system | |||
| Bladder cancer | Hyper-methylation | Unknown | Yu et al. [22] and Zhao et al. [44] |
| Endocrine system | |||
| Papillary thyroid cancer | Promoter SNP (Rs4148682, rs213950) | Unknown | Oh et al. [190] |
| Digestive system | |||
| Esophageal adenocarcinoma | A risk loci is within or near CFTR | Unknown | Gharahkhani et al. [191] |
| Hepatocellular carcinoma | Hyper-methylation | Unknown | Ding et al. [23] and Moribe et al. [38] |
| Pancreatic adenocarcinoma |
CFTR mutations is not significantly different in patients (1) CFTR mutation is a risk factor for smokers and young patients (2) |
Unknown |
(1) Malats et al. [192], Matsubayashi et al. [193], Piepoli et al. [194] and Schubert et al. [195] (2) McWilliams et al. [30], McWilliams et al. [196] and Hamoir et al. [197] |
| Gastric cancer | Up-regulated in advanced stage | Serum level of CFTR, along with CD199 and CEA, can be diagnosis factors | Suh et al. [45] and Liu et al. [184] |
| Colorectal cancer | Down-regulation |
CFTR mRNA expression is positively correlated with survival Independent prognostic determinant |
Sun et al. [39] and Than et al. [40] |
CFTR regulates multiple malignant phenotypes of cancer cells
Increasing evidence suggest that CFTR plays important roles in modulating multiple cellular processes involved in cancer development, such as transformation, proliferation, survival, migration and invasion (Table 2).
Table 2.
CFTR-related signaling involved in cancer regulation
| Cancer type | Signaling factors | Cancer cell behavior | Citations |
|---|---|---|---|
| Reproduction system | |||
| Endometrial carcinoma | NF-kB/uPAR and mir-125b | Cell viability and invasiveness | Huang et al. [51] and Xia et al. [50] |
| Ovarian cancer | Phosphor-Src | Invasiveness and motility | Zhu et al. [46] and Xu et al. [47] |
| Cervical cancer | Regulated by NF-kB | Unknown | Wu et al. [143] |
| Breast cancer | NF-kB/uPAR | Migration, invasion, EMT and metastasis | Zhang et al. [76] |
| Prostate cancer | miR-193b/uPA | Cell viability, clone formation, migration and invasion | Xie et al. [41] |
| Respiratory system | |||
| Nasopharyngeal carcinoma | Unknown | Migration, invasion and EMT | Tu et al. [37] |
| Lung cancer | uPA/uPAR | Migration, invasion, EMT and metastasis | Li et al. [43] |
| Digestive system | |||
| Esophageal adenocarcinoma | Unknown | Unknown | |
| Hepatocellular carcinoma | IP(3)/G-proteins/PLC/Ca2+ | Apoptosis | Kim et al. [134] |
| Pancreatic adenocarcinoma | MUC4 | Unknown | Singh et al. [142] |
| Gastric cancer | Unknown | Unknown | |
| Intestinal cancer | Wnt/β-catenin pathway; AF-6/MAPK | Tumor formation; cell viability, adhesion, migration, invasion and metastasis | Than et al. [40] and Sun et al. [39] |
| Urinary system | |||
| Bladder cancer | Unknown | Unknown | |
| Endocrine system | |||
| Papillary thyroid cancer | Unknown | Unknown | |
Malignant transformation
While the correlation of CFTR mutations with high incidence of gastrointestinal cancers has been observed for a long time [14], the direct link between CFTR dysfunction and GI cancer has not been established until recently. To investigate the effects of CFTR dysregulation on GI cancer, Than et al. created ApcMin mice that carried an intestinal-specific knockout of Cftr and showed that ApcMin mice with either Cftr heterozygous or homozygous mutants developed more tumors than ApcMin Cftr wild-type mice in the small intestine (SI) and the colon [40]. Strikingly, in Apc+/+ mice aged to one year, Cftr deficiency alone is sufficient to cause the development of intestinal tumors in > 60% of mice. Since both CF patients and Cftr KO mice develop a wide range of dysfunctions in the GI tract, including deficient fluid transport, altered cellular pH, impaired clearance of mucus, abnormal bacterial colonization, microbial dysbiosis and abnormal innate immune responses that lead to chronic inflammation, it is not clear whether all these factors contribute to the development of colon cancer [53]. Importantly, this study firstly revealed that colon organoid formation was significantly increased in Cftr mutant mice compared with wild-type controls, suggesting a role of Cftr in regulating intestinal stem cells (ISC) [40]. Additionally, a number of canonical ISC genes were dysregulated in Cftr KO mouse intestine. In particular, Wnt target genes were aberrantly expressed in Cftr KO tumors which show a significant overlapping with ApcMin Cftr+/+ tumors. These data suggest that Cftr functions as a tumor suppressor gene in the intestinal tract, loss of which may initiate tumor transformation process originated from ISCs. While the question of how Cftr may alter ISC dynamics is unknown, it is well established that the microenvironment is a key regulator of ISCs, including their capacity for malignant transformation. Thus, it is plausible that the inflammatory landscape in the GI tract characterized by chronic inflammation and activation of β-catenin contributes to the malignant transformation of ISCs in CF.
Cell growth and survival
CFTR has been implicated in the regulation of cell growth in various types of cancer [33, 41, 47]. The growth inhibitory effect of CFTR on cancer was first reported in breast cancer cell lines. Abraham et al. [33] showed that CFTR suppressed breast cancer cell growth due to high extracellular ATP concentration. In addition, mouse breast cancer cell line transfected with human CFTR exhibited a slower growth rate in vivo [33]. In our recent study, we evaluated the role of CFTR in prostate cancer development using three prostate cancer cell lines (PC-3, DU-145, LNcap). Our results showed that overexpression of CFTR significantly decreased the growth rate of cancer cells, whereas knockdown of CFTR promoted cancer cell growth. Interestingly, suppression of CFTR function by channel inhibitors exhibited the same effect (data not shown), indicating alternation of the channel function itself affects cancer cell growth. Given that CFTR is an anion channel providing a major pathway for Cl− and HCO3− efflux, which have been demonstrated to be important in keeping characteristics of cancer cells, such as anchorage‐dependent growth and apoptosis [54–56], it is not surprising that CFTR plays essential role in the regulation of cancer cell growth and/or apoptosis. However, it should be noted that knockdown of CFTR in normal prostate epithelial cells does not influence their own growth but rather induces stromal cell proliferation via production of PGE2 [57]. In addition, in contrast to breast cancer and prostate cancer, CFTR has been shown to promote cell growth in ovarian cancer. It was reported that suppression of CFTR inhibited cell proliferation and colony formation in ovarian cancer cell lines, SKOV3 and A2780 [47]. These results suggest that CFTR might play divergent role in regulating normal and malignant cell growth depending on the cellular context and/or their microenvironment. For instance, in cancer cells, pHi is increased compared to normal cells (∼ 7.3–7.6 vs. ∼ 7.2), while extracellular pH (pHe) is decreased (∼ 6.8–7.0 vs. ∼ 7.4). The increased pHi is maintained in cancer cells through the increased expression or activity of plasma membrane ion transporters and pHi regulators [58–60]. This reversed pH gradient in cancer cells is an early event in cancer development and increases during cancer progression [61]. Dysregulated pH in cancer cells enables cellular processes that are sensitive to small changes in pHi, including cell proliferation, migration and metabolism. Interestingly, it was shown recently that crypt epithelial cells in Cftr KO mice maintained an alkaline (pHi) and increased intracellular Cl− concentration [62]. Thus, while there is no direct evidence to date showing the differences in cancer cell growth are due to the channel function of CFTR, it is likely that CFTR channel function related to ion and pHi dynamics contributes to the regulatory role of CFTR in cancer cell growth.
Besides cell proliferation and apoptosis, it has been reported recently that CFTR regulates cancer cell autophagy as well [63]. In CFTR-overexpressing HeLa and A549 cells, the levels of endogenous LC3B-II were enhanced compared with the vector control, whereas CFTRF508Δ overexpression had no obvious effect on cell autophagy. In contrast, rapamycin decreased the expression of CFTR, which could be recovered by E64d plus pepstatin A or MG132, indicating that CFTR degradation involves both proteasome and autophagy pathways [63]. These results suggest that CFTR promotes autophagosome formation both in normal and stress states in HeLa and A549 cells. More interestingly, using protein interaction profiling and global bioinformatics analysis, recent study revealed that ∆F508 was associated with mTOR signaling components, which are closely correlated with autophagy regulation [64]. Inhibition of the PI3K/Akt/mTOR pathway with 6 different inhibitors demonstrated an increase in CFTR stability and expression. In addition, the study identified that CFTR promoted autophagy via Bcl-2-associated athanogene 3 (BAG3), a regulator of autophagy and aggresome clearance [64].
Cell migration and invasion
In carcinomas, acquisition of a mesenchymal-like phenotype that is reminiscent of an oncogenic EMT (epithelium–mesenchymal transition) is associated with pro-metastatic properties, including increased motility, invasion, anoikis resistance, and cancer stem cell characteristics [65, 66]. Several lines of evidence suggest that core polarity proteins are important for the formation and maintenance of the structural organization of epithelial cells, suggesting that their loss could induce or at least contribute to EMT [67, 68]. This notion is further supported by the findings that polarity proteins are cellular targets of an increasing list of oncogenes and tumor suppressors [69–72]. Given the critical role of CFTR in maintaining epithelial cell polarity [73] and cell–cell contact [74, 75], it is foreseeable that dysfunction of CFTR might play a role in the EMT process and cancer progression. Indeed, accumulating evidence has demonstrated that suppression of CFTR expression or activity promotes malignant phenotype of cancer cells, including adhesion, migration and invasion [37, 39, 41, 43, 76]. More importantly, EMT, the key step of cancer progression and metastasis, could be induced by downregulation of CFTR in breast [76], non-small cell lung cancer (NSCLC) [43] and nasopharyngeal carcinoma (NPC) [37] cells. The inhibitory role of CFTR in cancer metastasis has also been demonstrated in mouse model, as loss of CFTR enhances focal invasion of breast cancer cells and NSCLC cells [43, 76]. On the contrary, CFTR appears to promote cell migration, invasion and adhesion in ovarian cancer cells since knockdown of CFTR suppresses the malignant phenotypes [77]. However, whether this effect is related to EMT and whether CFTR exerts the malignancy-promoting effect on ovarian cancer in vivo are not clear.
Taken together, it appears that except for ovarian cancer, CFTR suppresses cancer development via regulating a wide range of cellular processes, supporting its identity as a tumor suppressor [40]. Elevated expression of CFTR has been reported in ovarian cancer tissues and cell lines by two independent studies [46, 47]. In this regard, the promotion of ovarian cancer by CFTR appears to be consistent with the clinical observations. However, it should be noted that while the origin and pathogenesis of ovarian cancer are poorly understood, it is well recognized that ovarian cancer is a heterogeneous disease composed of different types of tumors with widely differing clinicopathologic features and behavior. Thus, a larger sample size and more intensive biological studies are necessary to fully understand the regulatory role of CFTR in ovarian cancer.
Regulators of CFTR
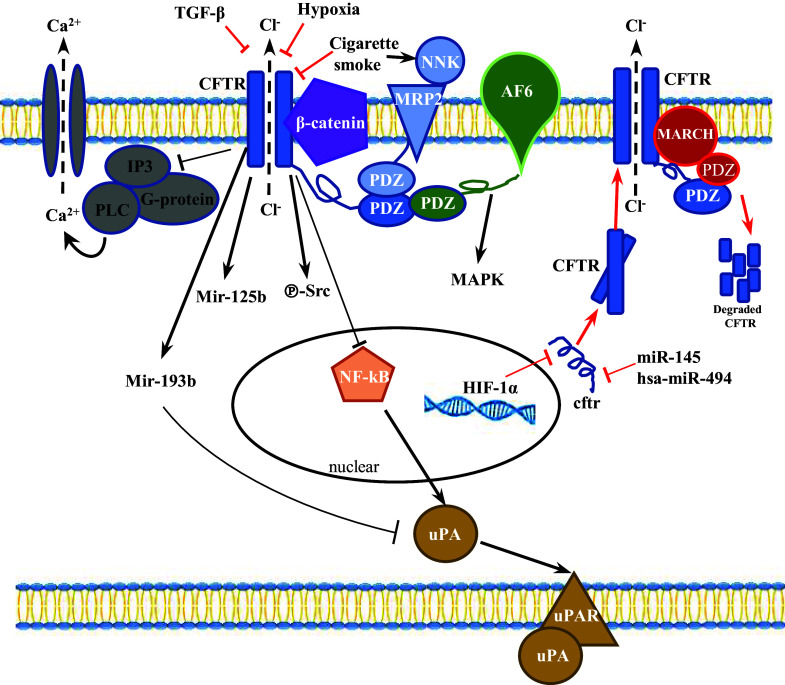
Numerous reports have shown that both genetic and epigenetic abnormalities lead to aberrant expression levels of CFTR in cancer cells. Gene mutations or polymorphisms are the most common genetic changes that cause non-function or low expression allele of CFTR. On the other hand, promoter hyper-methylation of tumor suppressor genes is one of the most well-recognized epigenetic mechanism associated with cancer. In the case of CFTR, hyper-methylation has been observed in prostate cancer [21], NSCLC [20], HCC [23, 38] and bladder cancer [22, 44], supporting CFTR as a tumor suppressor (Table 1). Besides that, the expression levels of CFTR can be regulated by environmental cues, which is of paramount importance for cancer development (Fig. 1).
Fig. 1.
CFTR-mediated signaling pathways in cancer cells. The expression levels of CFTR can be regulated by environmental cues, including hypoxia, TGF-β and cigarette smoke in cancer cells (red arrows), with NF-κB, Wnt/β-catenin, PDZ domain-mediated protein–protein interaction and microRNAs identified as downstream signaling pathways (black arrows)
Hypoxia
Hypoxia, which is closely related to tumor progression, EMT and metastasis [78–80], has been linked to CFTR expression level and its channel-related physiological function such as phosphorylation, ATP hydrolysis and apical membrane recycling [81–84]. Both negative and positive correlation between hypoxia and CFTR have been demonstrated in different tissues, which suggests that the regulation could be tissues specific [81, 83, 84]. For instance, in mice subjected to low oxygen in vivo, CFTR mRNA expression in airways, gastrointestinal tissues, and liver was repressed. In addition, CFTR mRNA expression was diminished in pulmonary human tissues taken from hypoxemic subjects at the time of lung transplantation [81]. The subsequent study revealed that HIF (hypoxia induced factor)-1α was bound to the promoter of CFTR gene, which suppressed both expression and function of CFTR protein in intestinal epithelium and human colon carcinoma cell line—Caco-2 [82] (Fig. 1). This study proposes novel insights into the presence of an HIF-1α-orchestrated mechanism involving CFTR that controls epithelial ion and water transport, supporting the notion that reduced oxygenation of cells caused by chronic hyperventilation plays the crucial role in triggering CFTR abnormalities and the development and pathogenesis of CF. However, the effect of hypoxia on channel function is not specific for CFTR, other studies unrelated to CF showed that low oxygen levels decreased active transport of sodium, chloride and water across primary epithelial cells in a dose-dependent manner [85–88]. In addition, whether CFTR mediates hypoxia-induced downstream pathways and cellular behavior is still an open question.
Transforming growth factor (TGF)-β
Transforming growth factor (TGF)-β, a key inflammatory cytokine, has been found to be negatively correlated with the expression, function and translocation of CFTR in normal epithelial cells [89–91]. TGF-β downregulates CFTR expression and function during chronic inflammation, which may serve as a noxious loop to exaggerate the inflammatory response. During cancer development, TGF-β functions as a tumor suppressor in the early stage of cancer. However, in the later stage, the activation of TGF-β pathway promotes EMT, invasion and metastasis [92, 93]. Interestingly, our recent study found that as a strong EMT activator, TGF-β significantly downregulated the expression of CFTR, and promoted EMT in breast cancer cells [76]. There are no published data on how TGF-β inhibits CFTR mRNA level or whether a putative TGF-β consensus site exists in the CFTR promoter, thus it is unclear whether TGF-β suppresses CFTR expression by transcriptional or post-transctiprional mechanisms [94]. Nevertheless, the complexity and versatility of the TGF-β pathway indicate that several mechanisms, including direct and indirect pathways may play a role in modulating CFTR expression.
Cigarette smoke
Accumulated evidence has demonstrated that cigarette smoke, which is a stimulator of inflammation and oxidative/nitrosative stress that related to a high risk of cancer, induces downregulation and dysfunction of CFTR in epithelial cells [95–98]. Xu et al. observed that the cigarette extract could induce lysosomal degradation of CFTR in bronchial epithelium, which is associated with mitogen-activated protein kinase (MAPK) pathway [99]. Cadmium, a component of cigarette [100], appears to downregulate the expression of the CFTR protein and subsequent chloride transport in human airway epithelial cells [101]. In addition, PDZ domain-mediated interaction between CFTR and MRP2, the tobacco carcinogen NNK transporter, indicates the potential involvement of CFTR in cigarette smoke-mediated transforming of airway epithelial cells [102]. Moreover, it is demonstrated that CFTR, α7 Nicotinic acetylcholine receptor (α7 nAChR) and adenylyl cyclase-1 are physically associated in a macromolecular complex at the apical membrane of surface and glandular airway epithelium [103]. This study establishes the role of α7 nAChR in the pathogenesis of smoking-related lung diseases via interaction with CFTR [103].
Sex hormones
It has been well established that the CFTR expression level in the uterus is dynamically changed during the estrous cycle. Estrogen can stimulate CFTR expression on both mRNA and protein levels in the female reproductive tract [104–106]. As endometrial, ovarian and cervical cancers are known to be estrogen-dependent disorders [107–109], the upregulation of CFTR in these female sex hormone-related cancers is very likely to be related to estrogen accumulation, which possibly explains the distinctive expression pattern of CFTR in cancers originated from the female reproductive tract. In the male reproductive system, we have found that castration results in declined CFTR expression, whereas testosterone treatment upregulates CFTR expression both in cultured primary prostate epithelial cells and in post-castration rat prostate tissue [41]. On the other hand, the mRNA expression level of Cftr is downregulated to about 25% in 23 months aged rat prostate compared to 3 months rat prostate [57], and the protein expression level also downregulated in 30 days rat prostate compared to 3 days rat prostate [41], which is consistent with the age-dependent prevalence of prostate hyperplasia and cancer. Thus, it is likely that in aging prostate, reduced level of testosterone is responsible for the loss of CFTR, which confers cellular susceptibility to malignant transformation.
Altogether, these studies support the notion that CFTR regulation in response to environmental changes contributes to a wide variety of biological processes. In contrast, disturbance of the microenvironment under pathological conditions may lead to dysregulation of CFTR, which is involved in the pathological processes of various diseases, such as cancer. Indeed, it is easy to understand that localized at the apical or basolateral membrane of the cells, ion channels/transporters are in a strategic position to sense and transmit extracellular signals into the intracellular machinery to regulate various cell functions. In this regard, CFTR may function as a key regulator by sensing environmental changes and transducing the micro-environmental signals to intracellular machinery, which subsequently leads to cellular adaptive responses in health and disease.
CFTR regulates cancer cell behavior through both genetic and epigenetic mechanisms
The knowledge base surrounding the structure and function of CFTR that has accumulated in the last 20 years is remarkable. On the other hand, whole-genome and transcriptome sequencing data have shed new light on the molecular mechanisms underlying the function of CFTR. Of note, CFTR has been identified as one of the hub genes that is critical for carcinogenesis [110, 111]. In addition, recent cell functional and animal studies have provided convincing evidence that CFTR is involved in cancer development via regulation of multiple signaling pathways (Fig. 1 and Table 2).
Ion channel-related signaling
The pancreatic duct epithelium secretes HCO3− at a concentration of around 140 mM by a mechanism that is only partially understood. In the proximal part of pancreatic ducts close to acinar cells, HCO3− secretion across the apical membrane is largely mediated by SLC26A6 CI−/HCO3− exchanger [112–116]. In distal ducts where the luminal HCO3− concentration is already high, most of the HCO3− secretion has been proposed to be mainly mediated by HCO3− conductance of CFTR [117]. However, CFTR Cl− channel has a limited permeability to HCO3− in typical physiologic conditions [118], implying an Cl−/HCO3−-independent pathway. While subsequent studies revealed that CFTR HCO3− permeability was dynamically regulated by intracellular Cl− concentration-sensitive WNK1-SPAK/OSR1 kinase pathway [114], this model is still unable to account for the ability of the ducts to secrete HCO3− at high concentrations in many species, including humans [115]. Despite these confounding factors, the loss of pancreatic bicarbonate secretion in patients with CF [119] indicates that CFTR plays a critical role in bicarbonate secretion. While markedly alteration of HCO3− and pH-regulatory transport proteins have been observed in cancer cells [120], the great majority of research has focused on proton transporters and pH regulators. However, it is interesting to note that at least under some conditions, including during embryonic development or reproduction, alteration of bicarbonate levels triggers the activation of various signaling pathways including miR-125b and CREB [121, 122]. Interestingly, CFTR has been demonstrated to be expressed in the female reproductive tract, including the ovary [123, 124], and involved in uterine HCO3− secretion [7] which is required for sperm capacitation [125, 126]. Of interest, we have found recently that CFTR-mediated HCO3− transport regulates cell proliferation in granulosa cells, which promotes HCO3−/sAC/PKA pathway leading to ERK phosphorylation and cyclin D2 upregulation, defect of which might contribute to the pathogenesis of polycystic ovary syndrome (PCOS) [122, 127]. Of note, PCOS patients show increased risk of ovarian cancer [128]. On the other hand, CFTR is not only a cAMP and PKA-regulated Cl− and HCO3− channel, but also a regulator of other ion channels as well as other signaling [129–132]. It was reported that Glibenclamide, an antidiabetic drug, induced downregulation of CFTR and increased apoptosis in HepG2 human hepatoblastoma cells [133, 134]. Further mechanistic study revealed that downregulation of CFTR triggered intracellular Ca2+ release via regulating formation of inositol 1,4,5-trisphosphate [IP(3)] and PTx-sensitive G-proteins, and induced apoptosis in cancer cells [134].
NF-κB-mediated pathway
As a key transcriptional regulator in inflammation, NF-κB has long been identified as a downstream effector of defective CFTR, which plays essential role in CF pathogenesis [135, 136]. Aberrant expression level, membrane localization and channel functions of CFTR have been shown to induce the expression and activity of NF-κB and the downstream inflammatory signaling [137, 138]. The negative correlation of CFTR with NF-κB-mediated signaling has been demonstrated in various cancers. Loss of CFTR led to NF-κB-mediated activation of urokinase-type plasminogen activator (uPA) expression and activity in breast cancer cells, which is known to be involved in EMT process and metastasis [76]. More importantly, the inhibition of NF-κB activity completely abrogated the CFTR knockdown-induced activation of uPA activity and enhanced cell invasion and migration in breast cancer cells, indicating that the upregulation of NF-κB activity targeting uPA is the major mechanism leading to increased malignancies. The link between CFTR and NF-κB/uPA/uPAR system, was also shown in NSCLC and NPC [37, 43]. In addition to uPA system, our previous studies have demonstrated that CFTR functions as a negative regulator of NF-κB/COX-2/PGE2-mediated pro-inflammatory response in airway and prostate epithelial cells, defective of which results in excessive activation of NF-κB and over production of PGE2 [57, 139]. Given the importance of COX-PGE2 pathway in cancer development, these findings point toward a scenario that defective CFTR leads to exaggerated NF-κB-mediated responses that are likely linked to cancer development. Of interest, CFTR downregulation was also shown to disrupt testicular tight junctions through up-regulation of NF-κB/COX-2/PGE2 in rat model of cryptorchidism [140], which is one of the risk factors for testicular cancer [141].
In pancreatic adenocarcinoma, CFTR was shown to regulate the expression of a tumor-linked mucin MUC4 [142]. The authors identified a NF-κB binding site in the promoter region of MUC4, however, whether NF-κB contributes to the transcriptional regulation of MUC4 is not known. While most of the studies have indicated a negative correlation between CFTR and NF-κB, positive association of CFTR with NF-κB was observed in ovarian cancer cell line and primary tissues [143]. In addition, both CFTR and NF-κB expression levels are positively associated with stage, histological grade, lymph node metastasis, and invasive interstitial depth in ovarian cancer patients. Despite the observed link, it is not clear whether and how CFTR regulates NF-κB expression in ovarian cancer.
It should be noted that while the inverse relationship between CFTR and NF-κB activation has been well established in different experimental settings, the mechanistic link between CFTR dysfunction to aberrant NF-κB activation remains mysteriously missing. It has been reported that Src tyrosine kinase-induced activation of NF-κB is involved in CFTR-mediated inflammation and permeability in biliary epithelium [144]. In addition, a recent study showed that CFTR physically interacted with TRADD, a key adaptor molecule in TNFα signaling, and modulated its degradation, therefore, regulating NF-κB signaling [145] in bronchial epithelial cells. In addition, our recent study has shown that CFTR forms a complex with β-catenin and NF-κB, which regulates NF-κB nuclear translocation and thus NF-κB-mediated inflammatory response in intestinal epithelial cells [146]. However, whether these mechanisms are involved in cancer development awaits further investigation.
Wnt/β-catenin pathway
The Wnt/β-catenin signaling cascade is considered to play a key role in cancer development via modulating numerous cellular processes including cell survival, cancer stem cell activity and EMT process [147]. Interestingly, a recent comprehensive study on human lung epithelial cells has found that the CFTR interactome includes several hundred proteins, with β-catenin listed as a potential interacting protein [148]. Moreover, RNA Seq analysis of adenomas derived from the Cftr knockout (Apc+/+ Cftrfl/fl-Villin-Cre) mouse small intestine and colon reveals the accumulation of Wnt/β-catenin target genes (Ccnd1, CD44, Axin2, Lgr5, Mmp7, Wnt10A and Ptgs2) and intestinal stem cell genes (Lgr5, CD44, Aldh1a1, Aqp4, Ascl2 and Hopx), supporting the notion that loss of Cftr initiates tumor formation via a dysregulated Wnt/β-catenin signaling pathway [40]. Importantly, colon organoid formation is significantly increased in organoids created from Cftr mutant mice compared with wild-type controls, suggesting Cftr plays critical role in regulating the intestinal stem cell compartment and tumor initiation by regulating Wnt/β-catenin pathway. While it is clear that canonical β-catenin pathway is activated in Cftr null small intestine, this study did not provide a mechanical link between Cftr loss and activation of β-catenin. Noticeably, our recent study showed that CFTR co-localized and physically interacted β-catenin in human colon cancer cell line Caco-2, therefore, stabilizing β-catenin [146]. In addition, Cftr mutation or downregulation promoted the degradation of β-catenin and subsequently reduced the nuclear translocation and activity of β-catenin [146]. Indeed, β-catenin activity in the ΔF508 mouse intestine is significantly reduced compared to WT mouse intestine, which is associated with activation of NF-κB and exaggerated inflammatory phenotype. The seemingly contradictory results observed in these two studies can be explained by the following possibilities: first, given the fact that β-catenin plays a central role in maintaining intestinal homeostasis by regulating the self-renewal and differentiation balance between crypt and villi cells, it is plausible that CFTR exerts distinctive regulatory effect on β-catenin activity in different cellular compartments of the intestine. For instance, CFTR negatively regulates β-catenin activity and suppresses ISC growth in the crypt region, whereas positively regulates β-catenin activity by stabilizing it in the villi area. This kind of divergent regulatory role of CFTR might be attributable to the distinctive expression level of CFTR in the different segment and compartment of intestine. It was shown clearly that in the porcine intestine, the pCFTR protein was expressed in the crypt epithelial cells of all intestinal segments with a distinct increase in signal intensities from small to large intestine and a decrease along the crypt–villus axis [149]. Second, CFTR may exert differential effect on Wnt/β-catenin signaling via interacting with different components of the Wnt/β-catenin pathway. The direct interaction between CFTR and β-catenin itself has not only been observed in Caco-2 cells [146], but also in mouse embryonic stem cells (ESC) [150]. Interestingly, CFTR-β-catenin interaction is critical for ESC differentiation, loss of which leads to dramatic defect in mesendoderm differentiation. On the other hand, CFTR has been found to be physically interact with dishevelled segment polarity protein 2 (Dvl2), a key adaptor of Wnt/β-catenin signaling, thereby suppressing the activation of β-catenin in kidney epithelial cells [151]. Over-activation of Wnt/β-catenin pathway due to dysregulated or mutant inhibitors of the pathway has been extensively observed in stem cells and cancer cells. Therefore, it is possible that CFTR plays divergent role on undifferentiated cells and mature cells via its differential regulatory effect on β-catenin signaling, depending on the interacting partners. Finally, ΔF508 protein may have alternative binding partner compared to CFTR WT protein. Up to 90% of ∆F508 CFTR protein is retained in the endoplasmic reticulum (ER) and subsequently targeted for proteolytic degradation by the ER-associated degradation pathway (ERAD) [152]. Therefore, models have been proposed in which differential protein interactions with ∆F508 CFTR contribute to dysregulated signaling pathway and CF pathogenesis [153]. Indeed, a ∆F508 mutation-specific interactome has been identified using a novel deep proteomic analysis method recently [148]. Taken together, despite unanswered questions, in view of the importance of Wnt/β-catenin pathway in embryonic development and tumorigenesis, the demonstrated role of CFTR in regulating β-catenin signaling provides novel insights into the molecular mechanism underlying various physiological and pathological conditions.
Protein–protein interaction
While Ford et al. uncovered the structural features of CFTR indicating a bottom-closed conformation of CFTR in crystals [154], recent studies on CFTR structural determination by single particle electron microscopy revealed an unexpected asymmetric feature of the two NBDs upon binding ATP [27, 155]. These pioneering works reveal major structural rearrangements and conformational changes related to channel activation. Since CF can be defined as a protein misfolding disease, the interactome of CFTR which may affect the protein stability has been characterized by recent studies. Of note, CFTR has been shown to form multiple-protein macromolecular complexes with ion channels, cell adhesion and other signaling molecules [129, 148, 156]. Pankow et al. identified 638 CFTR-interacting proteins which are related to apoptosis (4 proteins, including p53), adhesion (16 proteins, including E-cadherin, Integrins and β-catenin), cell skeleton (56 proteins, including Vimentin) and cellular signaling (22 proteins, including EGFR) [148]. In addition, the authors discovered a deltaF508 deletion-specific interactome, which could be extensively remodeled upon RNAi-mediated rescue [148], indicating the aberrant interactome of CFTR may underlie the pathogenesis of CF. This notion is further supported by several biochemical studies showing physical interaction of CFTR with various functional proteins. For instance, it was reported that CFTR regulated RANTES expression via its PDZ-interacting motif [157].
A recent study using The Cancer Genome Atlas (TCGA) database identified 482 differentially expressed genes (DEGs) in lung squamous cell carcinoma (SCC) with and without metastasis. Among them, 49 DEGs were used to construct a protein–protein interaction network and the results found that CFTR functioned as the hub protein connecting with 182 genes [42]. Cell–cell adhesion via structures such as adherent junctions (AJ) and tight junctions are responsible for establishing the polarity and maintaining the barrier function and strength of the epithelial cells. In colon cancer cells, Sun et al. reported that CFTR interacted with adherent junction molecule AF-6/afadin via PDZ domain, thus regulating epithelial polarity and affecting cancer metastasis [39]. Loss of CFTR resulted in degradation of AF-6/afadin protein and activation of mitogen-activated protein kinase (MAPK), which reduced epithelial tightness and enhanced EMT and malignancies in colon cancer cells [39]. Interestingly, several kinase inhibitors, including MAPK inhibitor, exhibit strong rescue effect on DeltaF508-CFTR function or expression in epithelial cells [158], suggesting a negative regulatory loop between CFTR and MAPK pathway. CFTR has been demonstrated to interact with another tight junction-associated protein ZO-1 as well [159]. Of note, this effect was observed in the absence of cAMP stimulation, a condition when CFTR channel activity is inactivated, suggesting that the channel function of CFTR is not required for its role in tight junction assembly. Since ZO-1/Zonab pathway is implicated in EMT process in different cancers [160, 161], it is very likely that the interaction between CFTR and ZO-1 contributes to cancer development. In addition, the physical interaction between CFTR and heat shock proteins (Hsp), Hsp70 and Hsp90, has long been identified [162, 163]. The Hsp proteins, especially Hsp90, are essential for cancer progression, inhibition of which has been considered as therapeutic targets in multiple cancers [164, 165]. Therefore, the protein–protein interaction between Hsp and CFTR could be another potential mechanism underlying cancer development.
MicroRNAs
Emerging studies have provided evidence indicating the ability of CFTR in linking environmental cues to miRNA changes [166, 167]. MiRNA profiling in wild type and ΔF508-CFTR lung epithelial cell lines revealed 22 differentially expressed miRNAs [168]. In nasal epithelial tissues of CF patients, the expression of miR-145 was elevated with downregulation of its target SMAD3, a key element of the TGF-β1 pathway [169]. In addition, an analysis of CFTR and hypoxia-sensitive miRNAs has revealed common miRNAs that are implicated in cancer development, such as miR-155, miR-21 and miR-23 [170, 171]. These studies suggest the possibility that CFTR regulates cancer development through alteration of miRNAs. Indeed, our recent study has revealed a direct effect of CFTR on miR-193b, which has been proposed to be a putative tumor suppressor targeting uPA in various types of cancers [41]. Interestingly, overexpression of miR-193b was shown to significantly reverse the CFTR knockdown-enhanced proliferation, migration, or invasion and completely abrogated the CFTR knockdown-elevated uPA activity in prostate cancer, indicating that CFTR regulates prostate cancer development via miR-193b/uPA pathway. In another study on endometrial cancer, CFTRinh172, a specific inhibitor of CFTR channel function, reduced the expression of miR-125b and promoted cell viability and invasion in endometrial cancer cell line ISK. Mimic of miR-125b reduced endometrial cancer cell proliferation and progression [50]. While the study did not identify the molecular mechanism underlying the regulatory effect of CFTR on miR-125b or the target of miR-125b in ISK cells, another study has reported that CFTR-mediated HCO3− transport could regulate miR-125b expression, which further modulated the expression level of p53 during embryonic development [121]. It would be of interest to see whether similar CFTR-regulated miRNA mechanism is involved in cancer development.
Moreover, it has been shown that CFTR regulates multiple signaling pathways, such as forkhead box O (FOXO) family via miR-146a, miR-155, miR-370, and miR-708 [172]. In addition, dysfunction of CFTR activates PI3K/AKT-mediated regulation on microRNA-199a-5p, which is implicated in hyper inflammatory response in CF [173]. MiR-155, a target of CFTR, is also been found to be regulated by TGF-β and involved in the regulation of TGF-β/Smads signaling, which may respond to the crosstalk between CFTR and TGF-β/Smads signaling [174, 175]. Given that miRNAs are known to play critical roles in the development of various cancers and a large number of miRNAs are shown to be differentially expressed in CFTR defective cells, we propose that the tumor-regulatory effects of CFTR could be mediated through its action on miRNAs, potentially as a link between environmental cues and epigenetic regulation of cancer progression.
Collectively, emerging evidence has shown that CFTR regulates multiple genetic and epigenetic pathways that are involved in cancer development. However, other important potential contributors that are associated with the channel function itself cannot be underestimated [129, 176]. For instance, while Cl− is essential for maintaining electrolyte fluid balance, HCO3− is critical for mucus formation, epithelial barrier function and luminal pH homeostasis [177, 178]. Thus, CF patients develop a wide range of epithelial dysfunction including deficient fluid transport, altered cellular pH, impaired clearance of mucus, abnormal bacterial colonization, microbial dysbiosis and abnormal innate immune responses that lead to chronic inflammation [10, 52, 53, 179]. Accordingly, a model for tumor development in both humans and mice deficient for CFTR would include long-term chronic inflammation associated with these pathological changes. In particular, chronic upregulation of pro-inflammatory cytokines such as interleukin-6 (IL-6), interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), hypoxia induced factor (HIF)-1α and chemokines in CF has been well established for tumor promotion [180, 181]. In addition, loss of CFTR may cooperate with defects in other ion channels, ultimately leading to activation of β-catenin [182]. Despite these hints, whether CF-related chronic inflammation contributes to the development of cancer is not clear.
Correlation of CFTR expression levels with cancer stage and patient prognosis
A successful therapy for cancer depends heavily on the biomarkers for monitoring the progression of the disease, and predicting the prognosis and survival after clinical intervention. Given the observed alteration in CFTR expression levels in various types of cancer, it is promising that CFTR can be used as a novel cancer biomarker (Table 1).
Colon cancer
The potential of using CFTR as a novel cancer progression indicator has been explored in recent studies. In colon cancer, it has been shown that the TNM (tumor, node and metastasis) classification is correlated with the mRNA expression level of CFTR. CFTR expression is significantly lower in TNM stage 4 patients than TNM stage 2/3 patients. In addition, CFTR expression in patients with metastasis is significantly lower than patients without metastasis. Furthermore, with a median of 120-month follow-up, the authors reveal that patients with low expression of CFTR and its interacting AJ gene AF-6 have significantly shorter overall survival than patients with high CFTR and AF-6 expression [39]. These data indicate that reduced expressions of CFTR and AF-6 are significantly associated with cancer progression and poor prognosis of human colon cancer patients. Besides, Than et al. analyzed disease free survival in a set of stage II colon cancer samples [40]. The Kaplan–Meier analysis and log-rank test revealed that the 3-year relapse free survival was 85% in the CFTR high-expressing group compared with 56% in the CFTR low-expressing group. In addition, the multivariate analysis showed that CFTR expression is an independent prognostic determinant. The identification of CFTR as a prognostic predictor of cancer progression and survival in colon cancer patients is fully consistent with epidemiological and clinical data, indicating that CF patients are at an increased risk for colon cancer [14].
Lung cancer
In a study to investigate the methylation status of CFTR gene in NSCLC, it was found that CFTR methylation was not significantly associated with the prognosis of the patients. However, when patients were categorized by age, CFTR methylation was associated with significantly worse survival among younger patients (≤ 62 years), but not older patients (> 62 years). In a more recent study, CFTR expression in 296 NSCLC tumor samples and 22 normal tissues was evaluated by real-time PCR and correlated with clinical characteristics, staging, and prognosis [43]. The results showed that low CFTR expression was correlated with advanced staging and lymph node metastasis. In addition, low CFTR expression was significantly associated with poor prognosis (overall survival: 45 vs. 36 months; progression-free survival: 41 vs. 30 months). In addition, the data derived from TCGA database show that CFTR has a tendency of down-regulation in lung SCC with metastasis compared to lung SCC without metastasis [42].
Breast cancer
In our recent study, the expression of CFTR was examined in accordance with patients’ Nottingham Prognostic Index (NPI) in breast cancer [76]. The results showed that patients with poor prognosis (NPI3) had significantly lower levels of CFTR transcripts when compared to patients with good prognosis (NPI1). In addition, patients with poor prognosis, including those with metastasis, local recurrence, and death due to breast cancer, had significantly lower levels of CFTR transcripts compared to disease-free patients. Moreover, patients with higher expression levels of CFTR had longer disease-free survival than patients with a lower CFTR transcript level. Thus, reduced expression of CFTR is significantly associated with disease progression and poor prognosis in breast cancer. This is consistent with a report showing that breast cancer in deltaF508 CFTR carriers were poorly differentiated tumors [183].
Prostate cancer
In prostate cancer study, genome-wide methylation analysis was performed on 83 tumors and 10 normal prostate samples [21]. The study identified that CFTR was more frequently methylated in tumor samples along with clinico-pathological indicators of poor prognosis. Particularly, the authors found that simultaneous hypermethylation of CFTR and HTR1B was frequent in patients with a high Gleason score and high Ki-67 levels, suggesting that simultaneous HTR1B and CFTR hypermethylation could help to discriminate advanced prostate cancers [21].
Nasopharyngeal cancer
CFTR is also expressed in nasopharyngeal mucosa. In a recent study, we found that CFTR expression was downregulated in nasopharyngeal cancer [37]. We used a cohort of 225 paraffin-embedded NPC specimens to examine the prognostic value of using CFTR as a novel biomarker of NPC. Our results showed that CFTR expression was significantly correlated with clinical stage and distant metastasis. In addition, patients with high expression levels of CFTR had longer overall survival than patients with lower CFTR expression levels. Besides, we noticed that patients with high CFTR levels had higher 10-year survival rate (41.7%), compared to those with lower CFTR levels (22.6%). Multivariate analysis showed that CFTR expression level was an independent prognostic factor for NPC. Thus, lower expression of CFTR is significantly associated with disease progression and poor prognosis in NPC.
Gastric cancer
In gastric cancer, serum level of CFTR has been reported to be strongly correlated with carbohydrate antigen 199 (CA199), the classical cancer tumor biomarkers in some cases [184]. In addition, the concentrations of CFTR is associated with gastric cancer stage [184]. Combinations of CFTR, CA199, and carcinoembryonic antigen (CEA) yielded the best receiver operating characteristics (ROC) curve, which is used for diagnosis of gastric cancer [184].
Cancers of the female reproductive tract
It has been shown that the expression of CFTR in ovarian cancer is significantly higher than that in benign and normal ovaries. Moreover, CFTR protein level was well-related to advanced clinical stages, poor histological grade and a higher serum Ca-125 level [47]. Similarly, in cervical cancer, the expression of CFTR was reported to be significantly increased in cancer tissues compared to normal cervical tissue. The expression of CFTR and NF-κB activation was positively associated with stage, histological grade, lymph node metastasis, and invasive interstitial depth. Multivariate analysis showed that coexpression of CFTR and NF-κB was an independent prognostic factor for survival [143].
Collectively, these studies indicate that CFTR negatively or positively correlated with cancer stage or patient prognosis, depending on the types of cancer. Furthermore, upregulation of CFTR is correlated with enhanced sensitive to Paclitaxel, a microtubule-stabilizing drug that has been commonly used to treat cancer, in multiple cancer cell lines [185]. In addition, the single nucleotide polymorphisms (SNPs) in CFTR was reported to be associated with the toxicity profile caused by anticancer agent docetaxel [186]. Altogether, these findings suggest the potential role of CFTR as a clinical indicator of diagnosis and/or prognosis in cancer (Table 1).
Concluding remark
Accumulating studies have found a close association of CFTR mutation, hypermethylation or aberrant expression with a wide array of cancers. Both clinical investigation and biological studies have lent support to an important role of CFTR in different stages of cancer development, especially in cancer progression. Compellingly, emerging evidence suggest that CFTR is not merely an ion channel, but also plays a fundamental role in regulating multiple signaling pathways that are involved in a variety of biological processes, pinpointing the potential molecular mechanisms underlying the tumor-regulatory role of CFTR. However, the question of whether channel function is necessary for the regulatory role of CFTR is still unclear. On the one hand, numerous studies have shown that suppression of channel function alters intracellular signaling, suggesting that inactivation of ion channel function itself is sufficient to alter cancer cell behavior [50, 121, 127]. On the other hand, studies from both our group and others have demonstrated that protein–protein interaction via PDZ domain, but not channel function is critical for the tumor-suppressive role of CFTR, indicating that the signal transduction function of CFTR is unlikely to be mediated by its channel function. For instance, various studies have shown that the effect of CFTR knockdown can be mimicked by PDZ binding domain deletion in colon cancer [39], kidney [151], trachea and epididymis [159]. In addition, our recent work reveals that the effect of CFTR knockdown cannot be mimicked by CFTR G551D, a channel function defective mutation with an intact PDZ binding domain (data not shown). Moreover, it should be noted that CFTRinh172 is known to act on the cytoplasmic side of the plasma membrane [187]. Thus, one cannot exclude the possibility that the inhibitor itself disrupts the normal configuration of CFTR and its interaction with other proteins, in addition to the channel blocker function. Despite all these hints, more intensive biochemical studies designed to exclude the gated channel function of CFTR is essential to provide direct evidence for channel-independent role of CFTR. Nevertheless, given the importance of many of the identified CFTR-mediated pathways, such as NF-κB/Cox-2/PGE2 and Wnt/β-catenin pathways, in different cellular processes during cancer development, it is not surprising that CFTR might regulate cancer development in a more complex way than we previously thought.
More importantly, the correlation of CFTR with cancer stages and prognosis may lead to its clinical application as a diagnosis or prognostic predictor in different cancers (Table 1). However, several key issues remain unresolved in this field. For instance, aberrant CFTR expression, either downregulation or upregulation, has been found in different types of cancer, which is considered conflicting results for some time. While it is conceivable that CFTR may be differentially expressed in different types of cancer, the differential microenvironment may affect CFTR expression as well. It should be noted that the expression level and activity of CFTR can be readily modulated by pH, ATP level, hypoxia, sex hormones and cytokines [24–27, 57, 81–84, 104–106, 118]. In addition, though more than 2000 CFTR mutations have been documented so far [188], previous clinical studies have merely focused on several frequent mutations, which may mask the results. This kind of discrepancy also goes to the completely opposite regulatory effects of CFTR on malignant phenotypes, i.e., promoting cell migration and invasion in female reproductive tract cancers but suppressing these malignant phenotypes in other cancers, such as prostate cancer, colon cancer and breast cancer. What determines the functionality of CFTR in different cellular contexts? It is plausible that CFTR reacts differently in response to differential environmental cues. One possibility is that CFTR might have different interacting proteins and that these different interactions might trigger diverse signaling pathways, leading to distinctive effects in different cancer cell types. On the other hand, given the well-established role of CFTR in inflammation, the pathophysiology of interactions between chronic inflammation and malignancy in CF requires focused research. Altogether, future investigation resolving these issues are necessary for the clinical application of CFTR as a diagnostic and prognostic indicator. Given the fact that CFTR is highly accessible cell surface molecule, it may represent an ideal drug target if its exact role in different cancers is identified. Thus, investigation into the role of CFTR in cancer development and the underlying signaling pathways may open up new revenue of prognostic indicators and therapeutic targets for the treatment of cancer.
Abbreviations
- ABC
Adenosine triphosphate (ATP)-binding cassette
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- EMT
Epithelium–mesenchymal transition
- Hsp
Heat shock protein
- HIF-1α
Hypoxia induced factor-1α
- MDR
Multidrug resistance
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NPC
Nasopharyngeal carcinoma
- NSCLC
Non-small cell lung cancer
- PTC
Papillary thyroid cancer
- SCC
Squamous cell carcinoma
- SNPs
Single nucleotide polymorphisms
- TGF-β
Transforming growth factor-β
- uPA
Urokinase-type plasminogen activator
Author contributions
JZ and YW have contributed to data collection and manuscript written. Prof. XJ and Prof. HCC have contributed to write and finalize the paper.
Funding
The work was supported in parts by National 973 Project (2013CB967404, 2013CB967401, 2013CB967403), Research Grant Council of Hong Kong (CUHK 466413, CUHK14119516), National Natural Science Foundation of China (81571390), the Focused Investment Scheme of the Chinese University of Hong Kong and L.K.S Foundation.
Compliance with ethical standards
Conflict of interest
None of the authors have any conflict of interests to declare.
Footnotes
Xiaohua Jiang and Hsiao Chang Chan contributed equally to this work.
Contributor Information
Xiaohua Jiang, Email: xjiang@cuhk.edu.hk.
Hsiao Chang Chan, Email: hsiaocchan@cuhk.edu.hk.
References
- 1.Gulyas-Kovacs A. Integrated analysis of residue coevolution and protein structure in ABC transporters. PLoS One. 2012;7(5):e36546. doi: 10.1371/journal.pone.0036546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang TC, Kirk KL. The CFTR ion channel: gating, regulation, and anion permeation. Cold Spring Harb Perspect Med. 2013;3(1):a009498. doi: 10.1101/cshperspect.a009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayyid ZN, Sellers ZM. Technological advances shed light on left ventricular cardiac disturbances in cystic fibrosis. J Cyst Fibros. 2017;16(4):454–464. doi: 10.1016/j.jcf.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Adam RJ, Hisert KB, Dodd JD, Grogan B, Launspach JL, Barnes JK, Gallagher CG, Sieren JP, Gross TJ, Fischer AJ, Cavanaugh JE, Hoffman EA, Singh PK, Welsh MJ, McKone EF, Stoltz DA. Acute administration of ivacaftor to people with cystic fibrosis and a G551D-CFTR mutation reveals smooth muscle abnormalities. JCI Insight. 2016;1(4):e86183. doi: 10.1172/jci.insight.86183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcorelles P, Friocourt G, Uguen A, Lede F, Ferec C, Laquerriere A. Cystic fibrosis transmembrane conductance regulator protein (CFTR) expression in the developing human brain: comparative immunohistochemical study between patients with normal and mutated CFTR. J Histochem Cytochem. 2014;62(11):791–801. doi: 10.1369/0022155414546190. [DOI] [PubMed] [Google Scholar]
- 6.Peters W, Kusche-Vihrog K, Oberleithner H, Schillers H. Cystic fibrosis transmembrane conductance regulator is involved in polyphenol-induced swelling of the endothelial glycocalyx. Nanomed Nanotechnol Biol Med. 2015;11(6):1521–1530. doi: 10.1016/j.nano.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Wang XF, Zhou CX, Shi QX, Yuan YY, Yu MK, Ajonuma LC, Ho LS, Lo PS, Tsang LL, Liu Y, Lam SY, Chan LN, Zhao WC, Chung YW, Chan HC. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat Cell Biol. 2003;5(10):902–906. doi: 10.1038/ncb1047. [DOI] [PubMed] [Google Scholar]
- 8.Perniss A, Preiss K, Nier M, Althaus M. Hydrogen sulfide stimulates CFTR in Xenopus oocytes by activation of the cAMP/PKA signalling axis. Sci Rep. 2017;7(1):3517. doi: 10.1038/s41598-017-03742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers. 2015;1:15010. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sloane PA, Rowe SM. Cystic fibrosis transmembrane conductance regulator protein repair as a therapeutic strategy in cystic fibrosis. Curr Opin Pulm Med. 2010;16(6):591–597. doi: 10.1097/MCP.0b013e32833f1d00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79(1 Suppl):S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 12.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73(7):1251–1254. doi: 10.1016/0092-8674(93)90353-R. [DOI] [PubMed] [Google Scholar]
- 13.MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, Knapp EA, Goss CH, Marshall BC. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the cystic fibrosis foundation patient registry. Ann Intern Med. 2014;161(4):233–241. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neglia JP, Fitzsimmons SC, Maisonneuve P, Schoni MH, Schoniaffolter F, Corey M, Lowenfels AB, Boyle P, Dozor AJ, Durie P. The risk of cancer among patients with cystic-fibrosis. N Engl J Med. 1995;332(8):494–499. doi: 10.1056/Nejm199502233320803. [DOI] [PubMed] [Google Scholar]
- 15.Schoni MH, Maisonneuve P, Schoni-Affolter F, Lowenfels AB. Cancer risk in patients with cystic fibrosis: the European data. CF/CSG Group. J R Soc Med. 1996;89(Suppl 27):38–43. [PMC free article] [PubMed] [Google Scholar]
- 16.Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut. 2007;56(8):1153–1163. doi: 10.1136/gut.2004.062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johannesson M, Askling J, Montgomery SM, Ekbom A, Bahmanyar S. Cancer risk among patients with cystic fibrosis and their first-degree relatives. Int J Cancer. 2009;125(12):2953–2956. doi: 10.1002/ijc.24679. [DOI] [PubMed] [Google Scholar]
- 18.Maisonneuve P, Marshall BC, Knapp EA, Lowenfels AB. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst. 2013;105(2):122–129. doi: 10.1093/jnci/djs481. [DOI] [PubMed] [Google Scholar]
- 19.Maisonneuve P, FitzSimmons SC, Neglia JP, Campbell PW, 3rd, Lowenfels AB. Cancer risk in nontransplanted and transplanted cystic fibrosis patients: a 10-year study. J Natl Cancer Inst. 2003;95(5):381–387. doi: 10.1093/jnci/95.5.381. [DOI] [PubMed] [Google Scholar]
- 20.Son JW, Kim YJ, Cho HM, Lee SY, Lee SM, Kang JK, Lee JU, Lee YM, Kwon SJ, Choi E, Na MJ, Park JY, Kim DS. Promoter hypermethylation of the CFTR gene and clinical/pathological features associated with non-small cell lung cancer. Respirology (Carlton, Vic) 2011;16(8):1203–1209. doi: 10.1111/j.1440-1843.2011.01994.x. [DOI] [PubMed] [Google Scholar]
- 21.Ashour N, Angulo JC, Andres G, Alelu R, Gonzalez-Corpas A, Toledo MV, Rodriguez-Barbero JM, Lopez JI, Sanchez-Chapado M, Ropero S. A DNA hypermethylation profile reveals new potential biomarkers for prostate cancer diagnosis and prognosis. Prostate. 2014;74(12):1171–1182. doi: 10.1002/pros.22833. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, Zhu T, Wang Z, Zhang H, Qian Z, Xu H, Gao B, Wang W, Gu L, Meng J, Wang J, Feng X, Li Y, Yao X, Zhu J. A novel set of DNA methylation markers in urine sediments for sensitive/specific detection of bladder cancer. Clin Cancer Res. 2007;13(24):7296–7304. doi: 10.1158/1078-0432.CCR-07-0861. [DOI] [PubMed] [Google Scholar]
- 23.Ding S, Gong BD, Yu J, Gu J, Zhang HY, Shang ZB, Fei Q, Wang P, Zhu JD. Methylation profile of the promoter CpG islands of 14 “drug-resistance” genes in hepatocellular carcinoma. World J Gastroenterol. 2004;10(23):3433–3440. doi: 10.3748/wjg.v10.i23.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK. Identification of the cystic fibrosis gene: genetic analysis. Science (New York, NY) 1989;245((4922)):1073. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 25.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science (New York, NY) 1989;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 26.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science (New York, NY) 1989;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 27.Liu F, Zhang Z, Csanady L, Gadsby DC, Chen J. Molecular structure of the human CFTR ion channel. Cell. 2017;169(1):85–95 e88. doi: 10.1016/j.cell.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 28.CF Genetic Analysis Consortium. http://www.genet.sickkids.on.ca/cftr/. Accessed 25 Apr 2011
- 29.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet. 2003;67(Pt 5):471–485. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 30.McWilliams RR, Petersen GM, Rabe KG, Holtegaard LM, Lynch PJ, Bishop MD, Highsmith WE., Jr Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations and risk for pancreatic adenocarcinoma. Cancer. 2010;116(1):203–209. doi: 10.1002/cncr.24697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Billings JL, Dunitz JM, McAllister S, Herzog T, Bobr A, Khoruts A. Early colon screening of adult patients with cystic fibrosis reveals high incidence of adenomatous colon polyps. J Clin Gastroenterol. 2014;48(9):e85–88. doi: 10.1097/MCG.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 32.Warren N, Holmes JA, Al-Jader L, West RR, Lewis DC, Padua RA. Frequency of carriers of cystic fibrosis gene among patients with myeloid malignancy and melanoma. BMJ. 1991;302(6779):760–761. doi: 10.1136/bmj.302.6779.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham EH, Vos P, Kahn J, Grubman SA, Jefferson DM, Ding I, Okunieff P. Cystic fibrosis hetero- and homozygosity is associated with inhibition of breast cancer growth. Nat Med. 1996;2(5):593–596. doi: 10.1038/nm0596-593. [DOI] [PubMed] [Google Scholar]
- 34.Padua RA, Warren N, Grimshaw D, Smith M, Lewis C, Whittaker J, Laidler P, Wright P, Douglas-Jones A, Fenaux P, Sharma A, Horgan K, West R. The cystic fibrosis delta F508 gene mutation and cancer. Hum Mutat. 1997;10(1):45–48. doi: 10.1002/(SICI)1098-1004(1997)10:1<45::AID-HUMU6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Qiao D, Yi L, Hua L, Xu Z, Ding Y, Shi D, Ni L, Song N, Wang Y, Wu H. Cystic fibrosis transmembrane conductance regulator (CFTR) gene 5T allele may protect against prostate cancer: a case–control study in Chinese Han population. J Cyst Fibros. 2008;7(3):210–214. doi: 10.1016/j.jcf.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Sun Z, Wu Y, Babovic-Vuksanovic D, Cunningham JM, Pankratz VS, Yang P. Cystic fibrosis transmembrane conductance regulator gene mutation and lung cancer risk. Lung Cancer. 2010;70(1):14–21. doi: 10.1016/j.lungcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu Z, Chen Q, Zhang JT, Jiang X, Xia Y, Chan HC. CFTR is a potential marker for nasopharyngeal carcinoma prognosis and metastasis. Oncotarget. 2016;7(47):76955–76965. doi: 10.18632/oncotarget.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moribe T, Iizuka N, Miura T, Kimura N, Tamatsukuri S, Ishitsuka H, Hamamoto Y, Sakamoto K, Tamesa T, Oka M. Methylation of multiple genes as molecular markers for diagnosis of a small, well-differentiated hepatocellular carcinoma. Int J Cancer. 2009;125(2):388–397. doi: 10.1002/ijc.24394. [DOI] [PubMed] [Google Scholar]
- 39.Sun TT, Wang Y, Cheng H, Xiao HZ, Xiang JJ, Zhang JT, Yu SB, Martin TA, Ye L, Tsang LL, Jiang WG, Xiaohua J. Chan HC (2014) Disrupted interaction between CFTR and AF-6/afadin aggravates malignant phenotypes of colon cancer. Biochem Biophys Acta. 1843;3:618–628. doi: 10.1016/j.bbamcr.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Than BLN, Linnekamp JF, Starr TK, Largaespada DA, Rod A, Zhang Y, Bruner V, Abrahante J, Schumann A, Luczak T, Walter J, Niemczyk A, O’Sullivan MG, Medema JP, Fijneman RJA, Meijer GA, Van den Broek E, Hodges CA, Scott PM, Vermeulen L, Cormier RT. CFTR is a tumor suppressor gene in murine and human intestinal cancer. Oncogene. 2017;36(24):3504. doi: 10.1038/onc.2017.3. [DOI] [PubMed] [Google Scholar]
- 41.Xie C, Jiang XH, Zhang JT, Sun TT, Dong JD, Sanders AJ, Diao RY, Wang Y, Fok KL, Tsang LL, Yu MK, Zhang XH, Chung YW, Ye L, Zhao MY, Guo JH, Xiao ZJ, Lan HY, Ng CF, Lau KM, Cai ZM, Jiang WG, Chan HC. CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer. Oncogene. 2013;32(18):2282–2291. doi: 10.1038/onc.2012.251. [DOI] [PubMed] [Google Scholar]
- 42.Tian F, Zhao J, Fan X, Kang Z. Weighted gene co-expression network analysis in identification of metastasis-related genes of lung squamous cell carcinoma based on the Cancer Genome Atlas database. J Thorac Dis. 2017;9(1):42–53. doi: 10.21037/jtd.2017.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Zhang JT, Jiang X, Shi X, Shen J, Feng F, Chen J, Liu G, He P, Jiang J, Tsang LL, Wang Y, Rosell R, Jiang L, He J, Chan HC. The cystic fibrosis transmembrane conductance regulator as a biomarker in non-small cell lung cancer. Int J Oncol. 2015;46(5):2107–2115. doi: 10.3892/ijo.2015.2921. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, Guo S, Sun J, Huang Z, Zhu T, Zhang H, Gu J, He Y, Wang W, Ma K, Wang J, Yu J. Methylcap-seq reveals novel DNA methylation markers for the diagnosis and recurrence prediction of bladder cancer in a Chinese population. PLoS One. 2012;7(4):e35175. doi: 10.1371/journal.pone.0035175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh YS, Yu J, Kim BC, Choi B, Han TS, Ahn HS, Kong SH, Lee HJ, Kim WH, Yang HK. Overexpression of plasminogen activator inhibitor-1 in advanced gastric cancer with aggressive lymph node metastasis. Cancer Res Treat. 2015;47(4):718–726. doi: 10.4143/crt.2014.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu LC, Hu ZH, Liu JJ, Gao J, Lin B. Whole genome expression profiling analysis of metastasis and drug-resistance-related genes in epithelial ovarian cancer cells. Zhongguo yi xue ke xue yuan xue bao Acta Academiae Medicinae Sinicae. 2015;37(6):662–673. doi: 10.3881/j.issn.1000-503X.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Yong M, Li J, Dong X, Yu T, Fu X, Hu L. High level of CFTR expression is associated with tumor aggression and knockdown of CFTR suppresses proliferation of ovarian cancer in vitro and in vivo. Oncol Rep. 2015;33(5):2227–2234. doi: 10.3892/or.2015.3829. [DOI] [PubMed] [Google Scholar]
- 48.Royse KE, Zhi D, Conner MG, Clodfelder-Miller B, Srinivasasainagendra V, Vaughan LK, Skibola CF, Crossman DK, Levy S, Shrestha S. Differential gene expression landscape of co-existing cervical pre-cancer lesions using RNA-seq. Front Oncol. 2014;4:339. doi: 10.3389/fonc.2014.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng X, Wu Z, Yu L, Li J, Xu W, Chan HC, Zhang Y, Hu L. Overexpression of cystic fibrosis transmembrane conductance regulator (CFTR) is associated with human cervical cancer malignancy, progression and prognosis. Gynecol Oncol. 2012;125(2):470–476. doi: 10.1016/j.ygyno.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Xia X, Wang J, Liu Y, Yue M. Lower cystic fibrosis transmembrane conductance regulator (CFTR) promotes the proliferation and migration of endometrial carcinoma. Med Sci Monit Int Med J Exp Clin Res. 2017;23:966–974. doi: 10.12659/MSM.899341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang W, Jin A, Zhang J, Wang C, Tsang LL, Cai Z, Zhou X, Chen H, Chan HC. Upregulation of CFTR in patients with endometriosis and its involvement in NFkappaB-uPAR dependent cell migration. Oncotarget. 2017;8(40):66951–66959. doi: 10.18632/oncotarget.16441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garg M, Ooi CY. The enigmatic gut in cystic fibrosis: linking inflammation, dysbiosis, and the increased risk of malignancy. Curr Gastroenterol Rep. 2017;19(2):6. doi: 10.1007/s11894-017-0546-0. [DOI] [PubMed] [Google Scholar]
- 53.Hou Y, Guan X, Yang Z, Li C. Emerging role of cystic fibrosis transmembrane conductance regulator—an epithelial chloride channel in gastrointestinal cancers. World J Gastrointest Oncol. 2016;8(3):282–288. doi: 10.4251/wjgo.v8.i3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Nakkash L, Iserovich P, Coca-Prados M, Yang H, Reinach PS. Functional and molecular characterization of a volume-activated chloride channel in rabbit corneal epithelial cells. J Membr Biol. 2004;201(1):41–49. doi: 10.1007/s00232-004-0706-5. [DOI] [PubMed] [Google Scholar]
- 55.Sun X, Chen L, Luo H, Mao J, Zhu L, Nie S, Wang L. Volume-activated chloride currents in fetal human nasopharyngeal epithelial cells. J Membr Biol. 2012;245(2):107–115. doi: 10.1007/s00232-012-9419-5. [DOI] [PubMed] [Google Scholar]
- 56.Voets T, Szucs G, Droogmans G, Nilius B. Blockers of volume-activated Cl− currents inhibit endothelial cell proliferation. Pflug Arch. 1995;431(1):132–134. doi: 10.1007/BF00374387. [DOI] [PubMed] [Google Scholar]
- 57.Xie C, Sun X, Chen J, Ng CF, Lau KM, Cai Z, Jiang X, Chan HC. Down-regulated CFTR during aging contributes to benign prostatic hyperplasia. J Cell Physiol. 2015;230(8):1906–1915. doi: 10.1002/jcp.24921. [DOI] [PubMed] [Google Scholar]
- 58.Gorbatenko A, Olesen CW, Boedtkjer E, Pedersen SF. Regulation and roles of bicarbonate transporters in cancer. Front Physiol. 2014;5:130. doi: 10.3389/fphys.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reshkin SJ, Greco MR, Cardone RA. Role of pHi, and proton transporters in oncogene-driven neoplastic transformation. Philos Trans R Soc Lond B Biol Sci. 2014;369(1638):20130100. doi: 10.1098/rstb.2013.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gallagher FA, Sladen H, Kettunen MI, Serrao EM, Rodrigues TB, Wright A, Gill AB, McGuire S, Booth TC, Boren J, McIntyre A, Miller JL, Lee SH, Honess D, Day SE, Hu DE, Howat WJ, Harris AL, Brindle KM. Carbonic anhydrase activity monitored in vivo by hyperpolarized 13C-magnetic resonance spectroscopy demonstrates its importance for pH regulation in tumors. Can Res. 2015;75(19):4109–4118. doi: 10.1158/0008-5472.CAN-15-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5(10):786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 62.Walker NM, Liu J, Stein SR, Stefanski CD, Strubberg AM, Clarke LL. Cellular chloride and bicarbonate retention alters intracellular pH regulation in Cftr KO crypt epithelium. Am J Physiol Gastrointest Liver Physiol. 2016;310(2):G70–80. doi: 10.1152/ajpgi.00236.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia D, Qu L, Li G, Hongdu B, Xu C, Lin X, Lou Y, He Q, Ma D, Chen Y. MARCH2 regulates autophagy by promoting CFTR ubiquitination and degradation and PIK3CA-AKT-MTOR signaling. Autophagy. 2016;12(9):1614–1630. doi: 10.1080/15548627.2016.1192752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reilly R, Mroz MS, Dempsey E, Wynne K, Keely SJ, McKone EF, Hiebel C, Behl C, Coppinger JA. Targeting the PI3K/Akt/mTOR signalling pathway in cystic fibrosis. Sci Rep. 2017;7(1):7642. doi: 10.1038/s41598-017-06588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hay ED (2005) EMT concept and examples from the vertebrate embryo. In: Savagner P (ed) Rise and fall of epithelial phenotype. Springer, Boston, MA, pp 1–11
- 66.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28(1–2):15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 67.Ozdamar B, Bose R, Barrios-Rodiles M, Wang H-R, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFβ receptors controls epithelial cell plasticity. Science (New York, NY) 2005;307(5715):1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 68.Etienne-Manneville S. Polarity proteins in migration and invasion. Oncogene. 2008;27(55):6970–6980. doi: 10.1038/onc.2008.347. [DOI] [PubMed] [Google Scholar]
- 69.Viloria-Petit AM, David L, Jia JY, Erdemir T, Bane AL, Pinnaduwage D, Roncari L, Narimatsu M, Bose R, Moffat J, Wong JW, Kerbel RS, O’Malley FP, Andrulis IL, Wrana JL. A role for the TGFbeta-Par6 polarity pathway in breast cancer progression. Proc Natl Acad Sci USA. 2009;106(33):14028–14033. doi: 10.1073/pnas.0906796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dow LE, Elsum IA, King CL, Kinross KM, Richardson HE, Humbert PO. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene. 2008;27(46):5988–6001. doi: 10.1038/onc.2008.219. [DOI] [PubMed] [Google Scholar]
- 71.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27(55):6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 72.Ellenbroek SI, Iden S, Collard JG. Cell polarity proteins and cancer. Semin Cancer Biol. 2012;22(3):208–215. doi: 10.1016/j.semcancer.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 73.Moyer BD, Denton J, Karlson KH, Reynolds D, Wang SS, Mickle JE, Milewski H, Cutting GR, Guggino WB, Li M, Stanton BA. A PDZ-interacting domain in CFTR is an apical membrane polarization signal. J Clin Investig. 1999;104(10):1353–1361. doi: 10.1172/Jci7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nilsson HE, Dragomir A, Lazorova L, Johannesson M, Roomans GM. CFTR and tight junctions in cultured bronchial epithelial cells. Exp Mol Pathol. 2010;88(1):118–127. doi: 10.1016/j.yexmp.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 75.Castellani S, Favia M, Guerra L, Carbone A, Abbattiscianni AC, Di Gioia S, Casavola V, Conese M. Emerging relationship between CFTR, actin and tight junction organization in cystic fibrosis airway epithelium. Histol Histopathol. 2017;32(5):445–459. doi: 10.14670/Hh-11-842. [DOI] [PubMed] [Google Scholar]
- 76.Zhang JT, Jiang XH, Xie C, Cheng H, Da Dong J, Wang Y, Fok KL, Zhang XH, Sun TT, Tsang LL, Chen H, Sun XJ, Chung YW, Cai ZM, Jiang WG. Chan HC (2013) Downregulation of CFTR promotes epithelial-to-mesenchymal transition and is associated with poor prognosis of breast cancer. Biochem Biophys Acta. 1833;12:2961–2969. doi: 10.1016/j.bbamcr.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 77.Xu J, Lin L, Yong M, Dong X, Yu T, Hu L. Adenovirusmediated overexpression of cystic fibrosis transmembrane conductance regulator enhances invasiveness and motility of serous ovarian cancer cells. Mol Med Rep. 2016;13(1):265–272. doi: 10.3892/mmr.2015.4509. [DOI] [PubMed] [Google Scholar]
- 78.Ratcliffe P, Koivunen P, Myllyharju J, Ragoussis J, Bovee JV, Batinic-Haberle I, Vinatier C, Trichet V, Robriquet F, Oliver L, Gardie B. Update on hypoxia-inducible factors and hydroxylases in oxygen regulatory pathways: from physiology to therapeutics. Hypoxia (Auckland, NZ) 2017;5:11–20. doi: 10.2147/hp.s127042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kao SH, Wu KJ, Lee WH. Hypoxia, epithelial-mesenchymal transition, and TET-mediated epigenetic changes. J Clin Med. 2016 doi: 10.3390/jcm5020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao T, Li JZ, Lu Y, Zhang CY, Li Q, Mao J, Li LH. The mechanism between epithelial mesenchymal transition in breast cancer and hypoxia microenvironment. Biomed Pharmacother (Biomedecine pharmacotherapie) 2016;80:393–405. doi: 10.1016/j.biopha.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 81.Guimbellot JS, Fortenberry JA, Siegal GP, Moore B, Wen H, Venglarik C, Chen YF, Oparil S, Sorscher EJ, Hong JS. Role of oxygen availability in CFTR expression and function. Am J Respir Cell Mol Biol. 2008;39(5):514–521. doi: 10.1165/rcmb.2007-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng W, Kuhlicke J, Jackel K, Eltzschig HK, Singh A, Sjoblom M, Riederer B, Weinhold C, Seidler U, Colgan SP, Karhausen J. Hypoxia inducible factor-1 (HIF-1)-mediated repression of cystic fibrosis transmembrane conductance regulator (CFTR) in the intestinal epithelium. FASEB J. 2009;23(1):204–213. doi: 10.1096/fj.08-110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaidi T, Mowrey-McKee M, Pier GB. Hypoxia increases corneal cell expression of CFTR leading to increased Pseudomonas aeruginosa binding, internalization, and initiation of inflammation. Investig Ophthalmol Vis Sci. 2004;45(11):4066–4074. doi: 10.1167/iovs.04-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uramoto H, Takahashi N, Dutta AK, Sabirov RZ, Ando-Akatsuka Y, Morishima S, Okada Y. Ischemia-induced enhancement of CFTR expression on the plasma membrane in neonatal rat ventricular myocytes. Jpn J Physiol. 2003;53(5):357–365. doi: 10.2170/jjphysiol.53.357. [DOI] [PubMed] [Google Scholar]
- 85.Mairbaurl H, Wodopia R, Eckes S, Schulz S, Bartsch P. Impairment of cation transport in A549 cells and rat alveolar epithelial cells by hypoxia. Am J Physiol. 1997;273(4 Pt 1):L797–806. doi: 10.1152/ajplung.1997.273.4.L797. [DOI] [PubMed] [Google Scholar]
- 86.Clerici C, Matthay MA. Hypoxia regulates gene expression of alveolar epithelial transport proteins. J Appl Physiol (Bethesda, Md: 1985) 2000;88(5):1890–1896. doi: 10.1152/jappl.2000.88.5.1890. [DOI] [PubMed] [Google Scholar]
- 87.Mairbaurl H, Mayer K, Kim KJ, Borok Z, Bartsch P, Crandall ED. Hypoxia decreases active Na transport across primary rat alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L659–665. doi: 10.1152/ajplung.00355.2001. [DOI] [PubMed] [Google Scholar]
- 88.Karle C, Gehrig T, Wodopia R, Hoschele S, Kreye VA, Katus HA, Bartsch P, Mairbaurl H. Hypoxia-induced inhibition of whole cell membrane currents and ion transport of A549 cells. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1154–1160. doi: 10.1152/ajplung.00403.2002. [DOI] [PubMed] [Google Scholar]
- 89.Howe KL, Wang A, Hunter MM, Stanton BA, McKay DM. TGFbeta down-regulation of the CFTR: a means to limit epithelial chloride secretion. Exp Cell Res. 2004;298(2):473–484. doi: 10.1016/j.yexcr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 90.Prulière-Escabasse V, Fanen P, Dazy AC, Lechapt-Zalcman E, Rideau D, Edelman A, Escudier E, Coste A. TGF-β1 downregulates CFTR expression and function in nasal polyps of non-CF patients. Am J Physiol Lung Cell Mol Physiol. 2005;288(1):L77–L83. doi: 10.1152/ajplung.00048.2004. [DOI] [PubMed] [Google Scholar]
- 91.Gauthier TW, Grunwell JR, Ping XD, Harris FL, Brown LA. Impaired defenses of neonatal mouse alveolar macrophage with cftr deletion are modulated by glutathione and TGFbeta1. Physiol Rep. 2017 doi: 10.14814/phy2.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mishra L, Derynck R, Mishra B. Transforming growth factor-β signaling in stem cells and cancer. Science (New York, NY) 2005;310(5745):68–71. doi: 10.1126/science.1118389. [DOI] [PubMed] [Google Scholar]
- 93.Miyazono K, Suzuki H, Imamura T. Regulation of TGF-beta signaling and its roles in progression of tumors. Cancer Sci. 2003;94(3):230–234. doi: 10.1111/j.1349-7006.2003.tb01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaikuad A, Bullock AN. Structural basis of intracellular TGF-beta signaling: receptors and smads. Cold Spring Harb Perspect Biol. 2016 doi: 10.1101/cshperspect.a022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bodas M, Silverberg D, Walworth K, Brucia K, Vij N. Augmentation of S-nitrosoglutathione controls cigarette smoke-induced inflammatory-oxidative stress and chronic obstructive pulmonary disease-emphysema pathogenesis by restoring cystic fibrosis transmembrane conductance regulator function. Antioxid Redox Signal. 2017;27(7):433–451. doi: 10.1089/ars.2016.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown MB, Hunt WR, Noe JE, Rush NI, Schweitzer KS, Leece TC, Moldobaeva A, Wagner EM, Dudek SM, Poirier C, Presson RG, Jr, Gulbins E, Petrache I. Loss of cystic fibrosis transmembrane conductance regulator impairs lung endothelial cell barrier function and increases susceptibility to microvascular damage from cigarette smoke. Pulm Circ. 2014;4(2):260–268. doi: 10.1086/675989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rab A, Rowe SM, Raju SV, Bebok Z, Matalon S, Collawn JF. Cigarette smoke and CFTR: implications in the pathogenesis of COPD. Am J Physiol Lung Cell Mol Physiol. 2013;305(8):L530–541. doi: 10.1152/ajplung.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, Jones CW, Boydston JA, Clancy JP, Bowen LE, Accurso FJ, Blalock JE, Dransfield MT, Rowe SM. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188(11):1321–1330. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu X, Balsiger R, Tyrrell J, Boyaka PN, Tarran R, Cormet-Boyaka E. Cigarette smoke exposure reveals a novel role for the MEK/ERK1/2 MAPK pathway in regulation of CFTR. Biochem Biophys Acta. 2015;1850(6):1224–1232. doi: 10.1016/j.bbagen.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buser MC, Scinicariello F. Cadmium, lead, and depressive symptoms: analysis of National Health and Nutrition Examination Survey 2011–2012. J Clin Psychiatry. 2017;78(5):e515–e521. doi: 10.4088/JCP.15m10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rennolds J, Butler S, Maloney K, Boyaka PN, Davis IC, Knoell DL, Parinandi NL, Cormet-Boyaka E. Cadmium regulates the expression of the CFTR chloride channel in human airway epithelial cells. Toxicol Sci. 2010;116(1):349–358. doi: 10.1093/toxsci/kfq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li C, Schuetz JD, Naren AP. Tobacco carcinogen NNK transporter MRP2 regulates CFTR function in lung epithelia: implications for lung cancer. Cancer Lett. 2010;292(2):246–253. doi: 10.1016/j.canlet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maouche K, Medjber K, Zahm JM, Delavoie F, Terryn C, Coraux C, Pons S, Cloez-Tayarani I, Maskos U, Birembaut P, Tournier JM. Contribution of alpha7 nicotinic receptor to airway epithelium dysfunction under nicotine exposure. Proc Natl Acad Sci USA. 2013;110(10):4099–4104. doi: 10.1073/pnas.1216939110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rochwerger L, Buchwald M. Stimulation of the cystic fibrosis transmembrane regulator expression by estrogen in vivo. Endocrinology. 1993;133(2):921–930. doi: 10.1210/endo.133.2.7688293. [DOI] [PubMed] [Google Scholar]
- 105.Tsang LL, Chan LN, Liu CQ, Chan HC. Effect of phenol red and steroid hormones on cystic fibrosis transmembrane conductance regulator in mouse endometrial epithelial cells. Cell Biol Int. 2001;25(10):1021–1024. doi: 10.1006/cbir.2001.0752. [DOI] [PubMed] [Google Scholar]
- 106.Rochwerger L, Dho S, Parker L, Foskett JK, Buchwald M. Estrogen-dependent expression of the cystic fibrosis transmembrane regulator gene in a novel uterine epithelial cell line. J Cell Sci. 1994;107(Pt 9):2439–2448. doi: 10.1242/jcs.107.9.2439. [DOI] [PubMed] [Google Scholar]
- 107.Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids. 2013;78(8):782–785. doi: 10.1016/j.steroids.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 108.Gibson DA, Saunders PT. Estrogen dependent signaling in reproductive tissues—a role for estrogen receptors and estrogen related receptors. Mol Cell Endocrinol. 2012;348(2):361–372. doi: 10.1016/j.mce.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 109.Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, Linkov F. Factors associated with type I and type II endometrial cancer. Cancer Causes Control. 2010;21(11):1851–1856. doi: 10.1007/s10552-010-9612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo Y, Xing Y. Weighted gene co-expression network analysis of pneumocytes under exposure to a carcinogenic dose of chloroprene. Life Sci. 2016;151:339–347. doi: 10.1016/j.lfs.2016.02.074. [DOI] [PubMed] [Google Scholar]
- 111.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, Maher CA, Fulton R, Fulton L, Wallis J, Chen K, Walker J, McDonald S, Bose R, Ornitz D, Xiong D, You M, Dooling DJ, Watson M, Mardis ER, Wilson RK. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150(6):1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gray MA. Bicarbonate secretion: it takes two to tango. Nat Cell Biol. 2004;6(4):292–294. doi: 10.1038/ncb0404-292. [DOI] [PubMed] [Google Scholar]
- 113.Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO(3)(−) transport in cystic fibrosis. EMBO J. 2002;21(21):5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139(2):620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 115.Saint-Criq V, Gray MA. Role of CFTR in epithelial physiology. Cell Mol Life Sci. 2017;74(1):93–115. doi: 10.1007/s00018-016-2391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. EMBO J. 2006;25(21):5049–5057. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 118.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91(12):5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johansen PG, Anderson CM, Hadorn B. Cystic fibrosis of the pancreas. A generalised disturbance of water and electrolyte movement in exocrine tissues. Lancet (London, England) 1968;1(7540):455–460. doi: 10.1016/S0140-6736(68)92783-9. [DOI] [PubMed] [Google Scholar]
- 120.Patel S. Stressor-driven extracellular acidosis as tumor inducer via aberrant enzyme activation: a review on the mechanisms and possible prophylaxis. Gene. 2017;626:209–214. doi: 10.1016/j.gene.2017.05.043. [DOI] [PubMed] [Google Scholar]
- 121.Lu YC, Chen H, Fok KL, Tsang LL, Yu MK, Zhang XH, Chen J, Jiang X, Chung YW, Ma AC, Leung AY, Huang HF, Chan HC. CFTR mediates bicarbonate-dependent activation of miR-125b in preimplantation embryo development. Cell Res. 2012;22(10):1453–1466. doi: 10.1038/cr.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen H, Guo JH, Zhang XH, Chan HC. Defective CFTR-regulated granulosa cell proliferation in polycystic ovarian syndrome. Reproduction (Cambridge, England) 2015;149(5):393–401. doi: 10.1530/REP-14-0368. [DOI] [PubMed] [Google Scholar]
- 123.Trezise AE, Linder CC, Grieger D, Thompson EW, Meunier H, Griswold MD, Buchwald M. CFTR expression is regulated during both the cycle of the seminiferous epithelium and the oestrous cycle of rodents. Nat Genet. 1993;3(2):157–164. doi: 10.1038/ng0293-157. [DOI] [PubMed] [Google Scholar]
- 124.Chan LN, Tsang LL, Rowlands DK, Rochelle LG, Boucher RC, Liu CQ, Chan HC. Distribution and regulation of ENaC subunit and CFTR mRNA expression in murine female reproductive tract. J Membr Biol. 2002;185(2):165–176. doi: 10.1007/s00232-001-0117-y. [DOI] [PubMed] [Google Scholar]
- 125.Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, Rowlands DK, Liu GY, Zhu H, Ma ZG, Wang XF, Chen ZH, Zhou SC, Dong HS, Zhang XH, Chung YW, Yuan YY, Yang WX, Chan HC. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc Natl Acad Sci USA. 2007;104(23):9816–9821. doi: 10.1073/pnas.0609253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen H, Ruan YC, Xu WM, Chen J, Chan HC. Regulation of male fertility by CFTR and implications in male infertility. Hum Reprod Update. 2012;18(6):703–713. doi: 10.1093/humupd/dms027. [DOI] [PubMed] [Google Scholar]
- 127.Chen H, Chan HC. Amplification of FSH signalling by CFTR and nuclear soluble adenylyl cyclase in the ovary. Clin Exp Pharmacol Physiol. 2017;44(Suppl 1):78–85. doi: 10.1111/1440-1681.12756. [DOI] [PubMed] [Google Scholar]
- 128.Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol. 1996;88(4):554–559. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]
- 129.Kunzelmann K. CFTR: interacting with everything? News Physiol Sci Int J Physiol Prod Jointly Int Union Physiol Sci Am Physiol Soc. 2001;16:167–170. doi: 10.1152/physiologyonline.2001.16.4.167. [DOI] [PubMed] [Google Scholar]
- 130.Gabriel SE, Clarke LL, Boucher RC, Stutts MJ. CFTR and outward rectifying chloride channels are distinct proteins with a regulatory relationship. Nature. 1993;363(6426):263–268. doi: 10.1038/363263a0. [DOI] [PubMed] [Google Scholar]
- 131.Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB. Cftr regulates outwardly rectifying chloride channels through an autocrine mechanism involving Atp. Cell. 1995;81(7):1063–1073. doi: 10.1016/S0092-8674(05)80011-X. [DOI] [PubMed] [Google Scholar]
- 132.Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. Cftr as a camp-dependent regulator of sodium-channels. Science (New York, NY) 1995;269(5225):847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 133.Kim JA, Kang YS, Lee SH, Lee EH, Yoo BH, Lee YS. Glibenclamide induces apoptosis through inhibition of cystic fibrosis transmembrane conductance regulator (CFTR) Cl(−) channels and intracellular Ca(2+) release in HepG2 human hepatoblastoma cells. Biochem Biophys Res Commun. 1999;261(3):682–688. doi: 10.1006/bbrc.1999.1108. [DOI] [PubMed] [Google Scholar]
- 134.Kim JA, Kang YS, Lee SH, Lee EH, Lee YS. Role of pertussis toxin-sensitive G-proteins in intracellular Ca2+ release and apoptosis induced by inhibiting cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channels in HepG2 human hepatoblastoma cells. J Cell Biochem. 2001;81(1):93–101. doi: 10.1002/1097-4644(20010401)81:1<93::AID-JCB1026>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 135.Bodas M, Vij N. The NFκB signaling in cystic fibrosis lung disease: pathophysiology and therapeutic potential. Discov Med. 2010;9(47):346. [PMC free article] [PubMed] [Google Scholar]
- 136.Rottner M, Kunzelmann C, Mergey M, Freyssinet JM, Martinez MC. Exaggerated apoptosis and NF-kappaB activation in pancreatic and tracheal cystic fibrosis cells. FASEB J. 2007;21(11):2939–2948. doi: 10.1096/fj.06-7614com. [DOI] [PubMed] [Google Scholar]
- 137.Hunter MJ, Treharne KJ, Winter AK, Cassidy DM, Land S, Mehta A. Expression of wild-type CFTR suppresses NF-kappaB-driven inflammatory signalling. PLoS One. 2010;5(7):e11598. doi: 10.1371/journal.pone.0011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kowalski MP, Pier GB. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa-induced cellular activation. J Immunol. 2004;172(1):418–425. doi: 10.4049/jimmunol.172.1.418. [DOI] [PubMed] [Google Scholar]
- 139.Chen J, Jiang XH, Chen H, Guo JH, Tsang LL, Yu MK, Xu WM, Chan HC. CFTR negatively regulates cyclooxygenase-2-PGE2 positive feedback loop in inflammation. J Cell Physiol. 2012;227(6):2759–2766. doi: 10.1002/jcp.23020. [DOI] [PubMed] [Google Scholar]
- 140.Chen J, Fok KL, Chen H, Zhang XH, Xu WM, Chan HC. Cryptorchidism-induced CFTR down-regulation results in disruption of testicular tight junctions through up-regulation of NF-κB/COX-2/PGE2. Hum Reprod. 2012;27(9):2585–2597. doi: 10.1093/humrep/des254. [DOI] [PubMed] [Google Scholar]
- 141.Ferguson L, Agoulnik AI. Testicular cancer and cryptorchidism. Front Endocrinol (Lausanne) 2013;4:32. doi: 10.3389/fendo.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Singh AP, Chauhan SC, Andrianifahanana M, Moniaux N, Meza JL, Copin MC, van Seuningen I, Hollingsworth MA, Aubert JP, Batra SK. MUC4 expression is regulated by cystic fibrosis transmembrane conductance regulator in pancreatic adenocarcinoma cells via transcriptional and post-translational mechanisms. Oncogene. 2007;26(1):30–41. doi: 10.1038/sj.onc.1209764. [DOI] [PubMed] [Google Scholar]
- 143.Wu Z, Peng X, Li J, Zhang Y, Hu L. Constitutive activation of nuclear factor kappaB contributes to cystic fibrosis transmembrane conductance regulator expression and promotes human cervical cancer progression and poor prognosis. Int J Gynecol Cancer. 2013;23(5):906–915. doi: 10.1097/IGC.0b013e318292da82. [DOI] [PubMed] [Google Scholar]
- 144.Fiorotto R, Villani A, Kourtidis A, Scirpo R, Amenduni M, Geibel PJ, Cadamuro M, Spirli C, Anastasiadis PZ, Strazzabosco M. The cystic fibrosis transmembrane conductance regulator controls biliary epithelial inflammation and permeability by regulating Src tyrosine kinase activity. Hepatology (Baltimore, Md) 2016;64(6):2118–2134. doi: 10.1002/hep.28817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang H, Cebotaru L, Lee HW, Yang Q, Pollard BS, Pollard HB, Guggino WB. CFTR controls the activity of NF-kappaB by enhancing the degradation of TRADD. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2016;40(5):1063–1078. doi: 10.1159/000453162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu K, Zhang X, Zhang JT, Tsang LL, Jiang X, Chan HC. Defective CFTR-β-catenin interaction promotes NF-κB nuclear translocation and intestinal inflammation in cystic fibrosis. Oncotarget. 2016;7(39):64030–64042. doi: 10.18632/oncotarget.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 148.Pankow S, Bamberger C, Calzolari D, Martinez-Bartolome S, Lavallee-Adam M, Balch WE, Yates JR., 3rd F508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature. 2015;528(7583):510–516. doi: 10.1038/nature15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Plog S, Mundhenk L, Bothe MK, Klymiuk N, Gruber AD. Tissue and cellular expression patterns of porcine CFTR: similarities to and differences from human CFTR. J Histochem Cytochem. 2010;58(9):785–797. doi: 10.1369/jhc.2010.955377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Z, Guo J, Wang Y, Weng Z, Huang B, Yu MK, Zhang X, Yuan P, Zhao H, Chan WY, Jiang X, Chan HC. CFTR-beta-catenin interaction regulates mouse embryonic stem cell differentiation and embryonic development. Cell Death Differ. 2017;24(1):98–110. doi: 10.1038/cdd.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang JT, Wang Y, Chen JJ, Zhang XH, Dong JD, Tsang LL, Huang XR, Cai Z, Lan HY, Jiang XH, Chan HC. Defective CFTR leads to aberrant beta-catenin activation and kidney fibrosis. Sci Rep. 2017;7(1):5233. doi: 10.1038/s41598-017-05435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lukacs GL, Mohamed A, Kartner N, Chang XB, Riordan JR, Grinstein S. Conformational maturation of CFTR but not its mutant counterpart (delta F508) occurs in the endoplasmic reticulum and requires ATP. EMBO J. 1994;13(24):6076–6086. doi: 10.1002/j.1460-2075.1994.tb06954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan JR, Kelly JW, Yates JR, 3rd, Balch WE. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127(4):803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 154.Rosenberg MF, O’Ryan LP, Hughes G, Zhao Z, Aleksandrov LA, Riordan JR, Ford RC. The cystic fibrosis transmembrane conductance regulator (CFTR): three-dimensional structure and localization of a channel gate. J Biol Chem. 2011;286(49):42647–42654. doi: 10.1074/jbc.M111.292268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Z, Chen J. Atomic structure of the cystic fibrosis transmembrane conductance regulator. Cell. 2016;167(6):1586–1597 e1589. doi: 10.1016/j.cell.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 156.Li C, Naren AP. Analysis of CFTR interactome in the macromolecular complexes. Methods Mol Biol (Clifton, NJ) 2011;741:255–270. doi: 10.1007/978-1-61779-117-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Estell K, Braunstein G, Tucker T, Varga K, Collawn JF, Schwiebert LM. Plasma membrane CFTR regulates RANTES expression via its C-terminal PDZ-interacting motif. Mol Cell Biol. 2003;23(2):594–606. doi: 10.1128/MCB.23.2.594-606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Trzcinska-Daneluti AM, Nguyen L, Jiang C, Fladd C, Uehling D, Prakesch M, Al-awar R, Rotin D. Use of kinase inhibitors to correct DeltaF508-CFTR function. Mol Cell Proteom. 2012;11(9):745–757. doi: 10.1074/mcp.M111.016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ruan YC, Wang Y, Da Silva N, Kim B, Diao RY, Hill E, Brown D, Chan HC, Breton S. CFTR interacts with ZO-1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J Cell Sci. 2014;127(20):4396–4408. doi: 10.1242/jcs.148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jayagopal A, Yang JL, Haselton FR, Chang MS. Tight junction-associated signaling pathways modulate cell proliferation in uveal melanoma. Investig Ophthalmol Vis Sci. 2011;52(1):588–593. doi: 10.1167/iovs.10-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jimenez-Salazar JE, Posadas-Rodriguez P, Lazzarini-Lechuga RC, Luna-Lopez A, Zentella-Dehesa A, Gomez-Quiroz LE, Konigsberg M, Dominguez-Gomez G, Damian-Matsumura P. Membrane-initiated estradiol signaling of epithelial-mesenchymal transition-associated mechanisms through regulation of tight junctions in human breast cancer cells. Horm Cancer. 2014;5(3):161–173. doi: 10.1007/s12672-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang Y, Janich S, Cohn JA, Wilson JM. The common variant of cystic fibrosis transmembrane conductance regulator is recognized by hsp70 and degraded in a pre-Golgi nonlysosomal compartment. Proc Natl Acad Sci USA. 1993;90(20):9480–9484. doi: 10.1073/pnas.90.20.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Loo MA, Jensen TJ, Cui L, Hou Y, Chang XB, Riordan JR. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 1998;17(23):6879–6887. doi: 10.1093/emboj/17.23.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gumus M, Ozgur A, Tutar L, Disli A, Koca I, Tutar Y. Design, synthesis, and evaluation of heat shock protein 90 inhibitors in human breast cancer and its metastasis. Curr Pharm Biotechnol. 2016;17(14):1231–1245. doi: 10.2174/1389201017666161031105815. [DOI] [PubMed] [Google Scholar]
- 165.Esfahani K, Cohen V. HSP90 as a novel molecular target in non-small-cell lung cancer. Lung Cancer (Auckland, NZ) 2016;7:11–17. doi: 10.2147/lctt.s60344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jiang X, Zhang JT, Chan HC. Ion channels/transporters as epigenetic regulators?—A microRNA perspective. Sci China Life Sci. 2012;55(9):753–760. doi: 10.1007/s11427-012-4369-9. [DOI] [PubMed] [Google Scholar]
- 167.Xu WM, Hui C, Yu SSB, Jing C, Chan HC. MicroRNAs and cystic fibrosis—an epigenetic perspective. Cell Biol Int. 2011;35(5):463–466. doi: 10.1042/Cbi20100664. [DOI] [PubMed] [Google Scholar]
- 168.Bhattacharyya S, Balakathiresan NS, Dalgard C, Gutti U, Armistead D, Jozwik C, Srivastava M, Pollard HB, Biswas R. Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J Biol Chem. 2011;286(13):11604–11615. doi: 10.1074/jbc.M110.198390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Megiorni F, Cialfi S, Cimino G, De Biase RV, Dominici C, Quattrucci S, Pizzuti A. Elevated levels of miR-145 correlate with SMAD3 down-regulation in cystic fibrosis patients. J Cyst Fibros. 2013;12(6):797–802. doi: 10.1016/j.jcf.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 170.Kimura H, Kawasaki H, Taira K. Mouse microRNA-23b regulates expression of Hes1 gene in P19 cells. Nucleic Acids Symp Ser. 2004;48:213–214. doi: 10.1093/nass/48.1.213. [DOI] [PubMed] [Google Scholar]
- 171.Redova M, Svoboda M, Slaby O. MicroRNAs and their target gene networks in renal cell carcinoma. Biochem Biophys Res Commun. 2011;405(2):153–156. doi: 10.1016/j.bbrc.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 172.Montanini L, Smerieri A, Gulli M, Cirillo F, Pisi G, Sartori C, Amarri S, Bernasconi S, Marmiroli N, Street ME. miR-146a, miR-155, miR-370, and miR-708 Are CFTR-dependent, predicted FOXO1 regulators and change at onset of CFRDs. J Clin Endocrinol Metab. 2016;101(12):4955–4963. doi: 10.1210/jc.2016-2431. [DOI] [PubMed] [Google Scholar]
- 173.Zhang PX, Cheng J, Zou S, D’Souza AD, Koff JL, Lu J, Lee PJ, Krause DS, Egan ME, Bruscia EM. Pharmacological modulation of the AKT/microRNA-199a-5p/CAV1 pathway ameliorates cystic fibrosis lung hyper-inflammation. Nat Commun. 2015;6:6221. doi: 10.1038/ncomms7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhou X, Mao Y, Zhu J, Meng F, Chen Q, Tao L, Li R, Fu F, Liu C, Hu Y, Wang W, Zhang H, Hua D, Chen W, Zhang X. TGF-beta1 promotes colorectal cancer immune escape by elevating B7-H3 and B7-H4 via the miR-155/miR-143 axis. Oncotarget. 2016;7(41):67196–67211. doi: 10.18632/oncotarget.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang D, Cui Y, Li B, Luo X, Li B, Tang Y. miR-155 regulates high glucose-induced cardiac fibrosis via the TGF-beta signaling pathway. Mol BioSyst. 2016;13(1):215–224. doi: 10.1039/c6mb00649c. [DOI] [PubMed] [Google Scholar]
- 176.Gray MA. CFTR is a mechanosensitive anion channel: a real stretch? Cellscience. 2010;7(1):1. [PMC free article] [PubMed] [Google Scholar]
- 177.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Investig. 2009;119(9):2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gelfond D, Heltshe S, Ma C, Rowe SM, Frederick C, Uluer A, Sicilian L, Konstan M, Tullis E, Roach RN, Griffin K, Joseloff E, Borowitz D. Impact of CFTR Modulation on intestinal pH, motility, and clinical outcomes in patients with cystic fibrosis and the G551D mutation. Clin Transl Gastroenterol. 2017;8(3):e81. doi: 10.1038/ctg.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dorsey J, Gonska T. Bacterial overgrowth, dysbiosis, inflammation, and dysmotility in the cystic fibrosis intestine. J Cyst Fibros. 2017;16(Suppl 2):S14–S23. doi: 10.1016/j.jcf.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 180.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (London, England) 2001;357(9255):539–545. doi: 10.1016/s0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 181.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Investig. 2007;117(5):1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Richards J, Greenlee MM, Jeffers LA, Cheng KY, Guo L, Eaton DC, Gumz ML. Inhibition of alphaENaC expression and ENaC activity following blockade of the circadian clock-regulatory kinases CK1delta/epsilon. Am J Physiol Ren Physiol. 2012;303(7):F918–927. doi: 10.1152/ajprenal.00678.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Southey MC, Batten L, Andersen CR, McCredie MR, Giles GG, Dite G, Hopper JL, Venter DJ. CFTR deltaF508 carrier status, risk of breast cancer before the age of 40 and histological grading in a population-based case–control study. Int J Cancer. 1998;79(5):487–489. doi: 10.1002/(SICI)1097-0215(19981023)79:5<487::AID-IJC7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 184.Liu H, Wu W, Liu Y, Zhang C, Zhou Z. Predictive value of cystic fibrosis transmembrane conductance regulator (CFTR) in the diagnosis of gastric cancer. Clin Investig Med. 2014;37(4):E226–232. doi: 10.25011/cim.v37i4.21728. [DOI] [PubMed] [Google Scholar]
- 185.Eng L, Ibrahim-zada I, Jarjanazi H, Savas S, Meschian M, Pritchard KI, Ozcelik H. Bioinformatic analyses identifies novel protein-coding pharmacogenomic markers associated with paclitaxel sensitivity in NCI60 cancer cell lines. BMC Med Genom. 2011;4:18. doi: 10.1186/1755-8794-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Deeken JF, Cormier T, Price DK, Sissung TM, Steinberg SM, Tran K, Liewehr DJ, Dahut WL, Miao X, Figg WD. A pharmacogenetic study of docetaxel and thalidomide in patients with castration-resistant prostate cancer using the DMET genotyping platform. Pharmacogenom J. 2010;10(3):191–199. doi: 10.1038/tpj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Investig. 2002;110(11):1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Claustres M, Theze C, des Georges M, Baux D, Girodon E, Bienvenu T, Audrezet MP, Dugueperoux I, Ferec C, Lalau G, Pagin A, Kitzis A, Thoreau V, Gaston V, Bieth E, Malinge MC, Reboul MP, Fergelot P, Lemonnier L, Mekki C, Fanen P, Bergougnoux A, Sasorith S, Raynal C, Bareil C. CFTR-France, a national relational patient database for sharing genetic and phenotypic data associated with rare CFTR variants. Human mutation. 2017;38(10):1297–1315. doi: 10.1002/humu.23276. [DOI] [PubMed] [Google Scholar]
- 189.Jung Y, Ha H, Jung S-H, Lee MG, Lee H-W, Yoon J, Choi J-W, Yeh B-II. F508 amino acid deletion mutation of CFTR gene in Korean lung cancer patients. Exp Mol Med. 2001;33(1):29–31. doi: 10.1038/emm.2001.6. [DOI] [PubMed] [Google Scholar]
- 190.Oh I-H, Oh C, Yoon T-Y, Choi J-M, Kim SK, Park HJ, Eun YG, Chung DH, Kwon KH, Choe B-K. Association of CFTR gene polymorphisms with papillary thyroid cancer. Oncol Lett. 2012;3(2):455–461. doi: 10.3892/ol.2011.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gharahkhani P, Fitzgerald RC, Vaughan TL, Palles C, Gockel I, Tomlinson I, Buas MF, May A, Gerges C, Anders M, Becker J, Kreuser N, Noder T, Venerito M, Veits L, Schmidt T, Manner H, Schmidt C, Hess T, Böhmer AC, Izbicki JR, Hölscher AH, Lang H, Lorenz D, Schumacher B, Hackelsberger A, Mayershofer R, Pech O, Vashist Y, Ott K, Vieth M, Weismüller J, Nöthen MM, Attwood S, Barr H, Chegwidden L, de Caestecker J, Harrison R, Love SB, MacDonald D, Moayyedi P, Prenen H, Watson RGP, Iyer PG, Anderson LA, Bernstein L, Chow W-H, Hardie LJ, Lagergren J, Liu G, Risch HA, Wu AH, Ye W, Bird NC, Shaheen NJ, Gammon MD, Corley DA, Caldas C, Moebus S, Knapp M, Peters WHM, Neuhaus H, Rösch T, Ell C, MacGregor S, Pharoah P, Whiteman DC, Jankowski J, Schumacher J. Genome-wide association studies in oesophageal adenocarcinoma and Barrett's oesophagus: a large-scale meta-analysis. Lancet Oncol. 2016;17(10):1363–1373. doi: 10.1016/S1470-2045(16)30240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Malats N, Casals T, Porta M, Guarner L, Estivill X, Real FX. Cystic fibrosis transmembrane regulator (CFTR) DeltaF508 mutation and 5T allele in patients with chronic pancreatitis and exocrine pancreatic cancer. PANKRAS II Study Group. Gut. 2001;48(1):70–74. doi: 10.1136/gut.48.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Matsubayashi H, Fukushima N, Sato N, Brune K, Canto M, Yeo CJ, Hruban RH, Kern SE, Goggins M. Polymorphisms of SPINK1 N34S and CFTR in patients with sporadic and familial pancreatic cancer. Cancer Biol Ther. 2003;2(6):652–655. doi: 10.4161/cbt.2.6.530. [DOI] [PubMed] [Google Scholar]
- 194.Piepoli A. Lack of association between and gene polymorphisms and pancreatic cancer in Italian patients. World J Gastroenterol. 2006;12(39):6343. doi: 10.3748/wjg.v12.i39.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schubert S, Traub F, Brakensiek K, von Kopylow K, Marohn B, Maelzer M, Gaedcke J, Kreipe H, Stuhrmann M. CFTR, SPINK1, PRSS1, and CTRC mutations are not associated with pancreatic cancer in German patients. Pancreas. 2014;43(7):1078–1082. doi: 10.1097/MPA.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 196.McWilliams R. Cystic fibrosis transmembrane regulator gene carrier status is a risk factor for young onset pancreatic adenocarcinoma. Gut. 2005;54(11):1661–1662. doi: 10.1136/gut.2005.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hamoir C, Pepermans X, Piessevaux H, Jouret-Mourin A, Weynand B, Habyalimana J-B, Leal T, Geubel A, Gigot J-F, Deprez PH. Clinical and morphological characteristics of sporadic genetically determined pancreatitis as compared to idiopathic pancreatitis: higher risk of pancreatic cancer in CFTR variants. Digestion. 2013;87(4):229–239. doi: 10.1159/000348439. [DOI] [PubMed] [Google Scholar]