Abstract
The organization of actin filaments into a wide range of subcellular structures is a defining feature of cell shape and dynamics, important for tissue development and homeostasis. Nervous system function requires morphological and functional plasticity of neurons and glial cells, which is largely determined by the dynamic reorganization of the actin cytoskeleton in response to intrinsic and extracellular signals. Oligodendrocytes are specialized glia that extend multiple actin-based protrusions to form the multilayered myelin membrane that spirally wraps around axons, increasing conduction speed and promoting long-term axonal integrity. Myelination is a remarkable biological paradigm in development, and maintenance of myelin is essential for a healthy adult nervous system. In this review, we discuss how structure and dynamics of the actin cytoskeleton is a defining feature of myelinating oligodendrocytes’ biology and function. We also review “old and new” concepts to reflect on the potential role of the cytoskeleton in balancing life and death of myelin membranes and oligodendrocytes in the aging central nervous system.
Keywords: Glia, Myelin, White matter, Age-associated cognitive decline, Cellular aging, Brain aging, Membrane remodeling
Oligodendrocytes in the developing central nervous system
In eukaryotic cells, control of actin cytoskeleton rearrangements conveys morphological plasticity governing many biological processes in development and organism homeostasis. In the nervous system of vertebrates, actin dynamics is a key feature of neural progenitor migration, specification and axonal function [1]. Glia cells, comprising astrocytes, oligodendroglia and microglia, are the most numerous in the brain and were noticed by Río-Hortega for their complex, polarized morphologies with abundant cytoplasmic “processes”, later characterized as actin-based protrusions.
Tight regulation of actin cytoskeleton rearrangements is critical in oligodendrocytes, specialized cells of the central nervous system (CNS) that produce and deposit myelin around axons to ensure rapid and efficient saltatory conduction of action potentials and provide axonal support. During development, oligodendrocyte progenitor cells (OPCs) migrate from distinct sites in the nervous system toward the vicinity of neurons, where they survey and contact axons that are destined to be myelinated. These pre-myelinating oligodendrocytes will then undergo differentiation to ensheath axons and form the fully mature, multilayered lipid membrane that constitutes myelin. The differentiation program of oligodendrocytes relies on two separate, yet complementary, biological processes: morphological changes, from a simple spindle-like shape characteristic of OPCs to an arborized morphology seen in mature oligodendrocytes, and expression of myelin genes that gives rise to the major structural components of mature sheaths. Furthermore, and unlike Schwann cells that myelinate in a 1:1 relationship with axons in the peripheral nervous system (PNS), a single oligodendrocyte typically contacts and myelinates multiple axons.
Morphological plasticity of oligodendrocytes as a defining feature of their differentiation and maturation
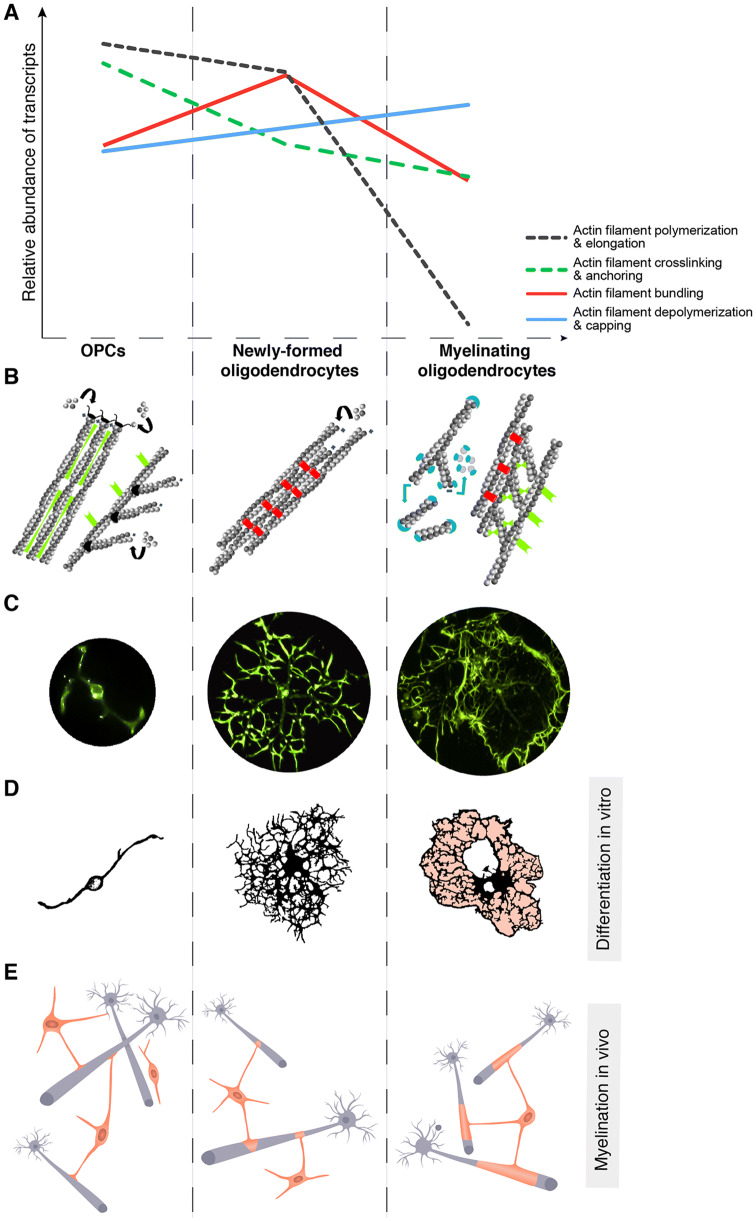
The cytoskeleton of oligodendrocytes is composed of microtubules and actin filaments but devoid of intermediate filaments [2, 3]. These two mechanically distinct cytoskeletal complexes are of specific interest to oligodendrocyte architecture, dynamics and function. The multiple cytoplasmic protrusions that extend from the cell body of oligodendrocytes have growing tips that are highly motile with actin-rich filopodia and lamellipodia-like structures. These protrusions also have long bundles of actin filaments that radiate from the leading edge toward the central area of the lamellipodia, a typical and unique structural organization usually seen in the growth cones of axons [4]. Actin filaments have a higher turnover rate and faster reorganization potential than microtubules, enabling rapid cell shape alterations. It has been long recognized that restructurating of the actin cellular network governs morphological plasticity in oligodendrocytes [5, 6], important for their development and maturation (Fig. 1).
Fig. 1.
The evolution of actin filament dynamics and expression of actin-binding proteins during oligodendrocyte differentiation and myelination. a Abundance of transcripts encoding proteins that regulate actin dynamics in distinct oligodendrocyte differentiation states. Molecules were grouped into four functional classes according to their effect on actin filament dynamics and/or organization. mRNA expression levels were obtained from http://www.brainrnaseq.org [26]. b Cartoon depicting the predominant actin network structures tied to each developmental stage of oligodendrocyte differentiation. In OPCs, actin filament polymerization and elongation by, for example, formins and the Arp2/3 complex are favored. The second highest represented class of actin-binding proteins in OPCs are crosslinkers and anchoring elements such as filamin, dystrophin and ERM (ezrin, radixin, and moesin) proteins. In newly-formed oligodendrocytes, assembly and elongation of actin filaments predominates, together with an increased abundance of bundling elements (such as coronins and fascin). High expression of filament depolymerizing (e.g., cofilin), severing (e.g., gelsolin), and capping (e.g., capZ) factors greatly favors actin filament disassembly and stabilization in myelinating oligodendrocytes. The second highest represented classes of actin-binding proteins in myelinating oligodendrocytes are crosslinkers (e.g., septins) and bundlers (e.g., anillin and ermin). c F-actin localization in live cultured oligodendrocytes during differentiation (image stills from time lapse microscopy videos [8]). d, e Schematic representation of the morphological transformation-from high protrusion remodeling to membrane-forming oligodendrocytes-characteristic of differentiation in vitro and myelination in vivo, respectively
The use of in vitro differentiation assays from rat primary OPCs, which recapitulate several aspects of differentiation in vivo including the morphological changes and antigenic differentiation [2, 7], has proven essential to dissect intrinsic mechanisms that independently regulate these two processes. A recent study by Azevedo and colleagues applied real-time imaging of living oligodendrocytes cultured with a fluorogenic probe that specifically binds F-actin to show that the subcellular distribution of actin filaments changes drastically during differentiation [8] (depicted in Fig. 1c). Interestingly, extension of myelin-like membranes is accompanied by a change in F-actin content from the rear of the protrusions toward the outermost region of the forming sheets [8]. Perturbation of actin filament assembly, by RNAi mediated knockdown of the actin-regulating protein Jmy in cultured oligodendrocytes, impairs morphological differentiation and disrupts the shift from protrusion remodeling to membrane formation [8]. These observations are in line with studies in live zebrafish, in which establishment of axonal contact is followed by a similar pattern of F-actin delocalization in the oligodendrocyte protrusion [9]. Although some information is available on how actin dynamics is intrinsically regulated in cells of the oligodendrocyte lineage (discussed in the next section), we lack a clear understanding of how actin network organization, central for oligodendrocyte differentiation and maturation, is regulated by axonal and mechanical signals.
Oligodendroglia development in the live CNS also relies on a similar stereotypical “cellular shaping” program that is directed by fast actin turnover, as cells progress through distinct morphological states that are tightly coupled with the successive steps of myelination. Live imaging in zebrafish [10] and mouse [11] showed that in migratory OPCs, highly motile protrusions survey the environment and mediate OPC:OPC interactions. In the early stages of developmental myelination, this “sensing” ability is important for trajectory choice and subsequent establishment of axonal contact, control of OPC density/distribution, and for differentiation [10, 11]. Moreover, the subsequent stages of myelination are also determined by the spatiotemporal interplay between actin-derived protrusion forces and kinetics of actin depolymerization (recently reviewed by Hughes and Appel [12] and Domingues et al. [13]). First, axon contact and ensheathment are driven by Arp2/3-dependent actin polymerization at the leading edge of the lamellipodia-like structures of oligodendrocyte protrusions [14]. Second, actin filament depolymerization by ADF/cofilin1 at the rear of the protrusion decreases membrane surface tension, promoting the lateral spreading of myelin sheets during wrapping and compaction [9].
But how does the molecular spatiotemporal control of actin dynamics during axon wrapping correlate with oligodendrocyte differentiation? MBP has been shown to interact with F-actin in vitro [15] and directly binds to the membrane phospholipid PI(4,5)P2 [16]. In the mature myelin sheath of cultured cells, localization of MBP to the membrane competes with actin disassembly proteins for binding to PI(4,5)P2, triggering their release to the cytosol, which activates actin disassembly and promotes membrane deformation and compaction [14]. This intimate cross talk between regulators of actin dynamics and myelin components is of particular relevance in the context of a cell that has to assemble and maintain specialized domains with a unique composition and mechanical properties far from the cell body. The concept that fast F-actin turnover is tightly controlled in distinct oligodendrocyte domains, generating subcellular tension gradients, fits well with the currently accepted model for myelin biogenesis [17], in which new membrane outgrowth occurs at the front edge of the axon-wrapping protrusion tip, while membrane deformation and compaction is restricted to the rear of the growing sheath. Furthermore, the association of actin filaments or bundles with myosin II motors and crosslinkers may modulate intracellular tension and cell surface area in oligodendrocytes. Actomyosin contractility mediates the spreading of plasma membranes in response to variable physical properties of the supporting matrix in vitro [18] and non-muscle myosin II (NM II) inactivation promotes myelination in the CNS [19]. Expression of the myosin types described in oligodendrocytes to date is developmentally regulated, suggesting that their actin-based motility and contractility functions may have diverse roles in oligodendrocyte biology and myelination. The isoforms NM IIa and b [19] and unconventional myosin Va [20] are predominantly expressed early in differentiation and control cytoplasmic protrusion branching and lamella formation. Unconventional myosin Id on the other hand is mostly expressed in myelinating oligodendrocytes and regulates their maturation and stability of membranes in vitro [21]. However, the subcellular topology of different actin-related processes as well their cooperation and synchronization with the differentiation program in oligodendrocytes remains elusive.
Molecular control of actin dynamics in oligodendrocytes during development
The specialized actin-rich cellular protrusions in oligodendrocytes survey the surrounding environment and integrate different extracellular signals necessary for migration and differentiation, in addition to mediating contact with the axons that will be myelinated [22]. So, what are the signaling pathways directing actin remodeling during oligodendrocyte development? The expression of distinct classes of actin regulators is differentially regulated during differentiation (Fig. 1), suggesting that the cell-autonomous transcriptional program that directs oligodendrocyte development and maturation has intrinsic control of the levels, and perhaps also the localization, of actin-binding proteins found in the cell at different developmental stages. Multiple genome-wide expression array analysis revealed that genes functionally linked to cytoskeletal remodeling are among the most heavily regulated during oligodendrocyte differentiation in vitro and myelination [23–26]. Furthermore, at the beginning of protrusion extension in progenitor cells, there is a subcellular enrichment (in protrusions vs soma) of a cell-specific set of transcripts encoding proteins related to actin and microtubule dynamics [8]. This indicates that, in oligodendroglia, fast actin turnover may be spatially regulated as early as in the progenitor state and is probably relevant for differentiation.
Precise regulation of actin filament assembly and dynamics is especially critical for the morphological differentiation of oligodendrocytes and for initial axonal contact and ensheathment during myelination. Expression of genes that encode actin-related proteins that potentiate the nucleation/elongation of actin filaments is higher in progenitor cells and newly formed oligodendrocytes (Fig. 1). Decreasing levels of or inhibiting some of these proteins generally results in arborization defects, with impaired protrusion formation and branching. This is the case for the nucleation-promoting factors: neural Wiskott–Aldrich syndrome protein (N-WASP) [27], WASP-family verprolin homologous protein (WAVE1) [28], Jmy [8] and the Arp2/3 complex, the major actin nucleator found in eukaryote cells [14]. In general, this impairment in morphological differentiation in vivo translates into hypomyelination due to decreased numbers of axons being contacted and ensheathed, but no changes in myelin thickness [14, 28].
Actin depolymerization, on the other hand, results in membrane relaxation that allows for lateral extension of myelin sheaths during wrapping and, later, assembly of structural myelin components during compaction [9, 14]. Concomitantly, genes that encode proteins that block actin filament elongation, or promote filament disassembly, are upregulated in newly formed and mature oligodendrocytes (Fig. 1). Deletion of, for example, ADF/cofilin1 [9] and gelsolin [14] in vivo results in impaired myelin growth and thinner sheaths. Additionally, findings from the Simons lab using interference reflection microscopy (IRM) and atomic force microscopy (AFM) to assess membrane dynamics and measure surface tension in oligodendrocytes in vitro [9] support a model in which spatial restriction of actin turnover (i.e., leveling F- to G-actin ratios) to specific subcellular regions is required for myelin growth and wrapping. Forces derived from actin polymerization promote adhesion-independent expansion of the leading edge in the cytoplasmic protrusions of oligodendrocytes, driving wrapping, while filament depolymerization at the rear results in decreased tension that favors the lateral extension of myelin sheaths [9]. Future studies should address what are the signaling networks that convey spatiotemporal organization of actin turnover in the oligodendrocyte during axon ensheathment and wrapping.
Interestingly, less information is available for the role of bundling proteins and crosslinkers in oligodendrocyte differentiation and myelination during development. Transcript levels of genes encoding actin bundlers peak specifically in newly formed oligodendrocytes. As for actin crosslinkers and anchors, they are hierarchically more represented in OPCs and myelinating oligodendrocytes when compared to other classes of actin-binding proteins (Fig. 1). Ermin, an ERM-like oligodendrocyte-specific protein predicted to bind to actin filaments, is highly expressed in mature oligodendrocytes and found in discrete sites in noncompacted myelin at the abaxonal and paranodal regions [29, 30]. ERM proteins crosslink actin filaments with plasma membranes. The activity of these crosslinkers in organizing actin filaments into complex subcellular scaffolds is important to orchestrate critical mechanical responses. Regulation of cellular stiffness may be a limiting factor for oligodendrocyte differentiation [31], and it is likely that specific actin crosslinkers and bundlers play distinct roles in controlling cytoskeletal changes during late wrapping and/or compaction phases of myelin assembly.
Extracellular factors regulating actin cytoskeleton rearrangements in oligodendrocytes
In addition to an intrinsic program for differentiation, oligodendrocyte development relies on multiple extrinsic signals, including secreted and contact-dependent factors, which can either inhibit or promote survival, proliferation, migration and differentiation. Since this topic was recently reviewed in detail by Mitew et al. [32], for the purpose of this review we focus specifically on those interactions known to exert an effect directly on the actin cytoskeleton. Globally, the sophisticated signaling system that directs OPC migration, differentiation into pro-myelinating oligodendrocytes and, finally, myelination responds either directly to or in an interplay with the extracellular matrix (ECM). ECM-derived signals in turn often activate well-described signaling pathways that induce a reorganization of the actin cytoskeleton. For instance, integrin-linked kinase (Ilk), a focal adhesion adaptor protein that physically links the ECM and integrin receptors to filamentous actin through parvins, regulates protrusion extension and branching during oligodendrocyte differentiation in vitro [33, 34]. Both Ilk and focal adhesion kinase (Fak), which also regulates integrin–ECM signaling, are required for axonal contact and initiation of myelination in vivo [34, 35]. Additionally, proteins that transduce signals in a precise time and location in the cell are important to restrict actin remodeling to specific subcellular regions. This is the case for Rho GTPases [36], key regulators of the actin cytoskeleton in most cell types, which function as molecular switches that integrate ECM-derived signals. As such, Rho GTPase activity regulates various aspects of oligodendrocyte development and myelination in the CNS. For example, integrin-dependent activation of the Src family kinase Fyn, known to play an important role in the myelination of specific CNS regions [37], acts via small Rho GTPase signaling to modulate the morphological differentiation of oligodendrocytes [38]. Additionally, GPR56-a cell surface G protein-coupled receptor that regulates oligodendrocyte development in both zebrafish [39] and mouse [40]-acts via RhoA-dependent cell-autonomous mechanisms to increase OPC proliferation. A Fyn kinase-RhoA signaling axis is also downstream of LINGO1, a transmembrane protein expressed by both neurons and oligodendrocytes that inhibits differentiation by decreasing Fyn kinase activity and activating RhoA [41]. In vitro, constitutively active RhoA inhibits protrusion extension in oligodendrocytes, but its function in vivo remains to be assessed. Curiously, developmental myelination occurs almost normally in the CNS of mice with conditional ablation of Rho GTPases Rac1 and Cdc42 [42]. In these mutants, aberrant accumulation of cytoplasm in the inner tongue of oligodendrocyte processes, resembling myelin outfoldings, is observed, indicating that Rac1 and Cdc42 are necessary for proper myelin sheath formation in later stages of myelination [42]. The spatiotemporal activity of classical Rho GTPases is controlled by other proteins, including guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs) and Rho GDP-dissociation inhibitors. The association with these regulators conveys a coordinated and synergistic activity to Rho GTPases, important, for example, in synaptic development [43] and in other developmental contexts [36] including glial function [44]. Future studies should elucidate how topographical Rho GTPase signaling is coordinated in different cellular domains during oligodendrocyte differentiation.
Oligodendrocytes in the adult and aging central nervous system
Myelination is a recent evolutionary acquisition that is central to nervous system function. It comes as no surprise that in adulthood even slight defects in myelin integrity impair performance, correlate with age-associated cognitive decline, and confer an added risk for neurological disorders (see review by Bartzokis on the “myelin model” of brain disease [45]). Advances in the genetic manipulation of rodents and in both electron and optical microscopy have taught us a great deal about oligodendrocyte biology and myelination in development. At the same time, it raised important questions regarding the cell biology of oligodendrocytes and myelin homeostasis in the adult CNS. As we discussed, cells of the oligodendrocyte lineage are capable of great adaptability partly due to an intricate regulation of actin filament reorganization, which acts as an “integrative and responsive mechanical platform” to a plethora of signals. In the next section, we review some recent evidence to theorize that the same sophisticated signaling machinery evolves to fine-tune the actin cytoskeleton in adult-generated and aged oligodendroglia, important for these cells’ survival and function(s) in the altered chemistry and mechanics of the aging CNS. We consider two different aspects. Firstly, OPCs are continuously produced in the adult brain [46, 47]. Why are these cells required and what mechanisms regulate their differentiation? Are they used to replenish pre-existing myelinated tracts and shield them from age-related degeneration? Or are they forming new myelinated tracts? Secondly, recent evidence suggests that in the mouse CNS myelinating oligodendrocytes are remarkably long lived [48] and there is no evidence of retraction of pre-formed internodes under physiological conditions in the live mouse brain cortex [49, 50]. How do these cells remain active and are, together with the myelin membrane itself, protected from the effects of aging?
Adaptive myelination
Evidence for significant de novo myelination in the adult CNS of vertebrates, and especially in the cortex, is robust [51–53]. Since myelination is modulated by neuronal activity [54], the emerging concept is that, in adulthood, activity-dependent regulation of myelination is adaptive and confers plasticity to neural circuits. In fact, experience-mediated control of myelination has been observed in humans and other vertebrates [55–57] and these adaptive changes in myelin are thought to underlie the alterations in brain function and neuronal circuitry architecture that arise from experience in the adult [57, 58]. Active myelination in adults results from continuous birth of oligodendrocytes [50–52] that are integrated and form new internodes on unmyelinated axonal segments [49, 50]. Surprisingly, global myelin homeostasis in the adult CNS most likely does not involve replacement of oligodendrocytes or breakdown of pre-existent internodes. A genetic fate-mapping study in the adult mouse indicates that myelinating oligodendrocytes are long-lived [48], and 14C dating in humans shows remarkably low turnover in brain white matter [52]. Live imaging studies of the mouse cortex also showed that, once integrated, oligodendrocytes are very stable and there are no detectable changes in the number of internodes [49, 50].
Neuronal activity signals oligodendrocyte progenitors to proliferate and myelinate [54]. But on the side of the oligodendrocyte, how is de novo myelination regulated? In the mouse, adult-formed oligodendrocytes are transcriptionally different from development oligodendrocytes and present significant heterogeneity among different brain regions [59]. This suggests some adaptability of oligodendrocytes to distinct neuronal circuits and/or regional mechanical properties in the brain. In the future, it would be interesting to understand how these variable extrinsic signals affect the differentiation program of adult-born oligodendrocytes. ChIP-Seq analysis of Olig2, a critical transcription factor controlling the specification and differentiation of OPCs, showed that in adult spinal cord Olig2 targets genes that belong to the class of cytoskeletal (microtubules and actin) proteins, including Rho GTPases or their regulators, and several actin-binding proteins [60]. These findings indicate that the transcriptional regulation of molecules linked to structural remodeling and cell dynamics remains an important feature of oligodendroglia in the adult CNS.
Myelin maintenance in the healthy aging CNS
In humans, and other mammals, axonal disintegration and white matter changes are the main pathological hallmarks of non-diseased aged brains [61]. This is in stark contrast with neurodegenerative diseases, in which loss of neurons is at the center of pathology [62]. In the adult CNS of humans and non-human primates, structural modifications or changes in myelin integrity/composition severely compromise neural circuit function and are associated with age-related functional decline [63–65]. Loss of myelin and the reduction of internode length are the main age-associated features in adult white matter regions. Curiously, there are also reports of thicker myelin in the adult and aged CNS. In the visual cortex of old monkeys, an increase in the number of lamellae that does not associate with axonal degeneration or loss was observed [66]. In the aging mouse optic nerve, increased myelin thickness (either resulting from additional lamellae or from loss of compaction) is found associated with enlarged axons containing abnormal mitochondria [67]. Although one must consider the possibility that there may be CNS region-specific aging patterns, it is tempting to speculate that formation of additional myelin wraps in pre-existing internodes occurs in response to changes in the metabolic or energetic status of aging axons and may represent one way of neuronal network maintenance in the adult. Globally, these age-related adaptations of myelin composition and structure point to the homeostasis of myelin sheaths being an essential biological process in the functional adult nervous system.
The seminal work of Alan Peters and colleagues characterized, with unprecedented detail, the age-related alterations in myelin sheath ultrastructure in the normal brain of rhesus monkeys (reviewed in [68]). They described numerous myelin defects, some of which had been previously reported in the mouse CNS by Sturrock [69], including the presence of redundant myelin, splitting of myelin lamellae, and formation of aberrant blisters, presumably containing fluid, adjacent to axons. These pathological findings positively correlate with cognitive decline and were observed in different white matter tracts, such as the prefrontal cortex [70], the corpus callosum [71] and the optic nerve [72]. The generalized pattern of age-associated myelin degeneration throughout the brain and across species suggests that the structural abnormalities may result from intrinsic cellular aging of the myelinating oligodendrocytes.
The cell biology of aged oligodendrocytes
Homeostasis in the adult nervous system must also rely on maintenance of pre-existent myelin sheaths. Myelin is one of the most long-lived CNS structures and probably requires cell-intrinsic biological control of its breakdown and turnover. Preservation of the complex membrane in the aging CNS is a biological process unparalleled in any other living organism. The second most abundant class of proteins present in the myelin membranes (aside from myelin components) is the cytoskeleton, which suggest that regulation of tubulin and actin filament dynamics is important for homeostasis and maintenance of this specialized structure [73, 74]. The protein components of myelin membranes are considerably stable (probably lasting longer than 6 months in the rat brain) [75]. Interestingly, changes in the lipid composition, which most likely affect structural stability, occur in myelin during aging [76].
As an exceptionally long-lived cellular structure that remains metabolically active, myelin membranes are expected to be particularly susceptible to damage. Additionally, cells of the oligodendrocyte lineage are uniquely vulnerable to DNA damage resulting from the global oxidative stress found in the aged brain (reviewed by Tse and Herrup [77]). So, how do oligodendrocytes cope with the biochemical and morphological changes associated with aging? Cellular aging is, to some extent, a cell-autonomous process, and in non-replicative long-lived cells that remain active throughout the organism’s life, such as neurons and oligodendrocytes, identifying molecular pathways involved in resistance to cellular aging would be of particular interest. Biological processes that contribute to the health and longevity of a cell include maintenance of protein quality and mitochondrial function, cytoskeletal integrity, and ECM–cell membrane signaling (reviewed in [78]). Dysregulation of the actin cytoskeleton is a major indicator of cellular aging in yeast [79]. It is possible that the highly dynamic nature of the actin cytoskeleton may simultaneously convey increased vulnerability and an important role as an intermediary structure regulating signaling pathways that, also in mammalian systems, mediate the cellular responses to damage induced by aging (Table 1). Studies in yeast have shown that the actin cytoskeleton endures oxidative damage under different conditions of cellular stress and can directly trigger apoptosis and protein aggregation (reviewed in [79]). Of note is the fact that cellular damage, associated with compromised cellular regeneration or even apoptosis, often appears to associate with increased rates of actin filament depolymerization and/or loss of actin structure integrity (Table 1). Actin is also important for maintenance of cell geometry, a particularly important aspect in aged oligodendrocytes that: must adapt to increasing mechanical stress [80] to sustain specialized cellular compartments away from the cell body; have to maintain proper contact with the axonal membrane; and ensure continuous myelin turnover. In fact, it has been shown that scaffold proteins septin and anillin, which organize the cell’s cytoskeleton, are required in myelinating oligodendrocytes to maintain the structural integrity of myelin sheaths [81]. Genetic ablation of septin/anillin specifically in mouse oligodendroglia leads to the formation of myelin outfoldings that resemble the redundant myelin findings described by Alan Peters in the aged brain of monkeys (reviewed in [82]) and compromises nerve conduction. Interestingly, developmental myelination is unaffected in septin/anillin mutants, supporting the idea that distinct cytoskeletal elements are important for different stages of myelin development and maintenance.
Table 1.
Effects of cellular aging on the actin cytoskeleton
| Age-associated stress or damage | Cell model/organism | Actin cytoskeleton response | Effect on cell fate |
|---|---|---|---|
| Increased and sustained production of reactive oxygen species | Human glial cells [85] | Downregulation of actin polymerization-associated genes | Aging |
| Human myoblasts [86, 87] | Increased actin depolymerization and decreased in cell stiffness | Cell death | |
| Budding yeast [88] | Changes in actin dynamics |
Apoptosis Decreased longevity |
|
| Mammalian cells [89] | |||
| General impaired proteostasis | Worm [90, 91] | Decreased integrity of filamentous actin | Decreased longevity |
| Decreased mitochondrial movement and quality control | Budding yeast [92] | Decreased retrograde actin cable flow | Decreased longevity |
| Loss of actin polarity | |||
| Protein aggregation | Aged human brain [93] | Aberrant actin structures, associated with enhanced actin filament stabilization (Hirano bodies, ADF/cofilin rods) | Unknown (may disrupt actin-dependent processes) |
| Aged rat brain [94] | Cellular dysfunction (impaired intracellular trafficking) | ||
| Lipid peroxidation | Rodent models of CNS injury [95] | Degradation of cytoskeletal components (e.g., spectrin) | Cell death (in neurons) |
Although markers of cellular aging have not been studied in oligodendrocytes, these cells display particular age-related features. These include the appearance of aggregates/dense inclusions, of unknown origin and composition, in the perikaryal cytoplasm, swelling of cytoplasmic processes, and alterations of oligodendrocyte profiles, which are found in pairs, groups, and rows, reflecting an increase in the number of oligodendrocytes [83]. Some of these age-related changes probably result from myelin remodeling during the aging process, including degeneration and turnover of myelin membranes. In the mouse brain, active shedding of myelin fragments from internodes or degenerating myelinating oligodendrocytes is observed in aged brains [84] and may be an oligodendrocyte-autonomous process [50]. Clearance of “old” myelin is done by microglia, and it is likely that the abundant inclusions seen in the microglia of old monkey brains is in fact phagocyted myelin debris. It has recently been proposed that this process of myelin fragment phagocytosis is passive [50], although evidence indicates that it contributes to microglia senescence and dysfunction in aging [84].
Concluding remarks
The highly dynamic nature of the actin cytoskeleton allows for adaptability and fast-response mechanisms to both intrinsic and extracellular stimuli that are essential for cell biology and tissue development. In oligodendrocytes, the high turnover rate and fast reorganization of actin filaments drive differentiation, an exquisite biological process requiring coordinated activation of an intrinsic transcriptional program and dramatic morphological transformation into highly arborized cells. In the developing CNS, the actin network regulates multiple steps of myelination, from the establishment of axonal contact, to axon wrapping and compaction of fully mature myelin sheath. Several studies from recent years, using complementary approaches to analyze oligodendrocyte differentiation in vitro and myelination in vivo, suggest that evolvability of the actin cytoskeleton and the supramolecular organization of distinct actin structures govern the differentiation process. In our view, collectively these findings pose the question of whether this non-stochastic control of actin-driven processes remains important after developmental myelination: first, for adaptive myelination and internode plasticity in the adult CNS, and secondly in the maintenance of long-lived myelin sheaths. In various eukaryote organisms changes in actin filament dynamics are a common feature of the response to age-associated cellular stress. Considering what we have learned about the structural alterations seen in aged white matter, it is tempting to argue that signaling pathways regulating actin dynamics and sustainability of specific actin structures are important for the maintenance of myelin membranes. In the CNS, white matter is particularly vulnerable to insult (e.g., stroke) and changes in white matter, not associated with axonal loss, correlate with age-associated cognitive impairment. Additionally, the structural deterioration of myelin has an impact on the homeostasis of other glia cells and neurons. Hence, the study of myelin maintenance in the aging CNS is of broader significance in the context of devising strategies to reverse or hamper age-associated functional decline and the increased susceptibility for neurodegenerative diseases.
Acknowledgements
We thank Alexandra Guedes for the illustrations in the article. Work in our laboratories was funded by the project NORTE-01-0145-FEDER-000008-Porto Neurosciences and Neurologic Disease Research Initiative at I3S, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (FEDER). We also acknowledge the financial support of FEDER funds through the COMPETE 2020-Operational Programme for Competitiveness and Internationalization (POCI), Portugal 2020, and by Portuguese funds through FCT (Fundação para a Ciência e a Tecnologia)/MCTES in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274). IMP acknowledges the support of the Marie Curie COFUND Programme “NanoTRAINforGrowth”, the EU FP7 grant agreement number 600375, and the project Nanotechnology-based functional solutions (NORTE-01–0145-FEDER-000019), co-financed by NORTE 2020, under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). MMA (SFRH/BD/90301/2012) and AIS (SFRH/BPD/79417/2011) are recipients of individual fellowships from FCT.
References
- 1.Kevenaar JT, Hoogenraad CC. The axonal cytoskeleton: from organization to function. Front Mol Neurosci. 2015;8:44. doi: 10.3389/fnmol.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kachar B, Behar T, Dubois-Dalcq M. Cell shape and motility of oligodendrocytes cultured without neurons. Cell Tissue Res. 1986;244:27–38. doi: 10.1007/BF00218378. [DOI] [PubMed] [Google Scholar]
- 3.Wilson R, Brophy PJ. Role for the oligodendrocyte cytoskeleton in myelination. J Neurosci Res. 1989;22:439–448. doi: 10.1002/jnr.490220409. [DOI] [PubMed] [Google Scholar]
- 4.Fox MA, Afshari FS, Alexander JK, Colello RJ, Fuss B. Growth cone like sensorimotor structures are characteristic features of postmigratory, premyelinating oligodendrocytes. Glia. 2006;53:563–566. doi: 10.1002/glia.20293. [DOI] [PubMed] [Google Scholar]
- 5.Simpson PB, Armstrong RC. Intracellular signals and cytoskeletal elements involved in oligodendrocyte progenitor migration. Glia. 1999;26:22–35. doi: 10.1002/(SICI)1098-1136(199903)26:1<22::AID-GLIA3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Song J, Goetz BD, Baas PW, Duncan ID. Cytoskeletal reorganization during the formation of oligodendrocyte processes and branches. Mol Cell Neurosci. 2001;17:624–636. doi: 10.1006/mcne.2001.0974. [DOI] [PubMed] [Google Scholar]
- 7.Tang DG, Tokumoto YM, Raff MC. Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months. J Cell Biol. 2000;148:971–984. doi: 10.1083/jcb.148.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azevedo MM, Domingues HS, Cordelieres FP, Sampaio P, Seixas AI, Relvas JB. Jmy regulates oligodendrocyte differentiation via modulation of actin cytoskeleton dynamics. Glia. 2018 doi: 10.1002/glia.23342. [DOI] [PubMed] [Google Scholar]
- 9.Nawaz S, et al. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev Cell. 2015;34:139–151. doi: 10.1016/j.devcel.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- 11.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes EG, Appel B. The cell biology of CNS myelination. Curr Opin Neurobiol. 2016;39:93–100. doi: 10.1016/j.conb.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domingues HS, Cruz A, Chan JR, Relvas JB, Rubinstein B, Pinto IM. Mechanical plasticity during oligodendrocyte differentiation and myelination. Glia. 2018;66:5–14. doi: 10.1002/glia.23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuchero JB, et al. CNS myelin wrapping is driven by actin disassembly. Dev Cell. 2015;34:152–167. doi: 10.1016/j.devcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boggs JM, Rangaraj G. Interaction of lipid-bound myelin basic protein with actin filaments and calmodulin. Biochemistry. 2000;39:7799–7806. doi: 10.1021/bi0002129. [DOI] [PubMed] [Google Scholar]
- 16.Nawaz S, Kippert A, Saab AS, Werner HB, Lang T, Nave KA, Simons M. Phosphatidylinositol 4,5-bisphosphate-dependent interaction of myelin basic protein with the plasma membrane in oligodendroglial cells and its rapid perturbation by elevated calcium. J Neurosci. 2009;29:4794–4807. doi: 10.1523/JNEUROSCI.3955-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snaidero N, et al. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell. 2014;156:277–290. doi: 10.1016/j.cell.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kippert A, Fitzner D, Helenius J, Simons M. Actomyosin contractility controls cell surface area of oligodendrocytes. BMC Cell Biol. 2009;10:71. doi: 10.1186/1471-2121-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Tewari A, Einheber S, Salzer JL, Melendez-Vasquez CV. Myosin II has distinct functions in PNS and CNS myelin sheath formation. J Cell Biol. 2008;182:1171–1184. doi: 10.1083/jcb.200802091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sloane JA, Vartanian TK. Myosin Va controls oligodendrocyte morphogenesis and myelination. J Neurosci. 2007;27:11366–11375. doi: 10.1523/JNEUROSCI.2326-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamazaki R, Ishibashi T, Baba H, Yamaguchi Y. Knockdown of unconventional myosin ID expression induced morphological change in oligodendrocytes. ASN Neuro. 2016 doi: 10.1177/1759091416669609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buttery PC, ffrench-Constant C. Process extension and myelin sheet formation in maturing oligodendrocytes. Prog Brain Res. 2001;132:115–130. doi: 10.1016/S0079-6123(01)32070-8. [DOI] [PubMed] [Google Scholar]
- 23.Cahoy JD, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dugas JC, Tai YC, Speed TP, Ngai J, Barres BA. Functional genomic analysis of oligodendrocyte differentiation. J Neurosci. 2006;26:10967–10983. doi: 10.1523/JNEUROSCI.2572-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen JA, Maric D, Lau P, Barker JL, Hudson LD. Identification of a novel oligodendrocyte cell adhesion protein using gene expression profiling. J Neurosci. 2006;26:9881–9891. doi: 10.1523/JNEUROSCI.2246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacon C, Lakics V, Machesky L, Rumsby M. N-WASP regulates extension of filopodia and processes by oligodendrocyte progenitors, oligodendrocytes, and Schwann cells-implications for axon ensheathment at myelination. Glia. 2007;55:844–858. doi: 10.1002/glia.20505. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, Vartanian TK. WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J Neurosci. 2006;26:5849–5859. doi: 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brockschnieder D, Sabanay H, Riethmacher D, Peles E. Ermin, a myelinating oligodendrocyte-specific protein that regulates cell morphology. J Neurosci. 2006;26:757–762. doi: 10.1523/JNEUROSCI.4317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B, et al. Juxtanodin: an oligodendroglial protein that promotes cellular arborization and 2′,3′-cyclic nucleotide-3′-phosphodiesterase trafficking. Proc Natl Acad Sci USA. 2005;102:11527–11532. doi: 10.1073/pnas.0500952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lourenco T, Paes de Faria J, Bippes CA, Maia J, Lopes-da-Silva JA, Relvas JB, Graos M. Modulation of oligodendrocyte differentiation and maturation by combined biochemical and mechanical cues. Sci Rep. 2016;6:21563. doi: 10.1038/srep21563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience. 2014;276:29–47. doi: 10.1016/j.neuroscience.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 33.O’Meara RW, Michalski J-P, Anderson C, Bhanot K, Rippstein P, Kothary R. Integrin-linked kinase regulates process extension in oligodendrocytes via control of actin cytoskeletal dynamics. J Neurosci. 2013;33:9781–9793. doi: 10.1523/JNEUROSCI.5582-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michalski JP, Cummings SE, O’Meara RW, Kothary R. Integrin-linked kinase regulates oligodendrocyte cytoskeleton, growth cone, and adhesion dynamics. J Neurochem. 2016;136:536–549. doi: 10.1111/jnc.13446. [DOI] [PubMed] [Google Scholar]
- 35.Forrest AD, Beggs HE, Reichardt LF, Dupree JL, Colello RJ, Fuss B. Focal adhesion kinase (FAK): a regulator of CNS myelination. J Neurosci Res. 2009;87:3456–3464. doi: 10.1002/jnr.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machacek M, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, Scherer SS, McMorris FA. A unique role for Fyn in CNS myelination. J Neurosci. 2001;21:2039–2047. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang X, Draghi NA, Resh MD. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J Neurosci. 2004;24:7140–7149. doi: 10.1523/JNEUROSCI.5319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackerman SD, Garcia C, Piao X, Gutmann DH, Monk KR. The adhesion GPCR Gpr56 regulates oligodendrocyte development via interactions with Galpha12/13 and RhoA. Nat Commun. 2015;6:6122. doi: 10.1038/ncomms7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giera S, et al. The adhesion G protein-coupled receptor GPR56 is a cell-autonomous regulator of oligodendrocyte development. Nat Commun. 2015;6:6121. doi: 10.1038/ncomms7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mi S, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- 42.Thurnherr T, et al. Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. J Neurosci. 2006;26:10110–10119. doi: 10.1523/JNEUROSCI.2158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin-Vilchez S, Whitmore L, Asmussen H, Zareno J, Horwitz R, Newell-Litwa K. RhoGTPase regulators orchestrate distinct stages of synaptic development. PLoS One. 2017;12:e0170464. doi: 10.1371/journal.pone.0170464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feltri LM, Suter U, Relvas JB. The function of RhoGTPases in axon ensheathment and myelination. Glia. 2008;56:1508–1517. doi: 10.1002/glia.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat Rec. 1991;229:384–398. doi: 10.1002/ar.1092290311. [DOI] [PubMed] [Google Scholar]
- 47.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tripathi RB, Jackiewicz M, McKenzie IA, Kougioumtzidou E, Grist M, Richardson WD. Remarkable stability of myelinating oligodendrocytes in mice. Cell Rep. 2017;21:316–323. doi: 10.1016/j.celrep.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat Neurosci. 2018;21:696–706. doi: 10.1038/s41593-018-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill RA, Li AM, Grutzendler J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat Neurosci. 2018;21:683–695. doi: 10.1038/s41593-018-0120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeung MS, et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Xiao L, et al. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci. 2016;19:1210–1217. doi: 10.1038/nn.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibson EM, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 57.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marques S, et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science. 2016;352:1326–1329. doi: 10.1126/science.aaf6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darr AJ, et al. Identification of genome-wide targets of Olig2 in the adult mouse spinal cord using ChIP-Seq. PLoS One. 2017;12:e0186091. doi: 10.1371/journal.pone.0186091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–978. doi: 10.1212/WNL.50.4.972. [DOI] [PubMed] [Google Scholar]
- 62.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 63.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037/0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 64.Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55:861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, Wang J, Hu X. Aging of cerebral white matter. Ageing Res Rev. 2017;34:64–76. doi: 10.1016/j.arr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters A, Sethares C, Killiany RJ. Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J Comp Neurol. 2001;435:241–248. doi: 10.1002/cne.1205. [DOI] [PubMed] [Google Scholar]
- 67.Stahon KE, Bastian C, Griffith S, Kidd GJ, Brunet S, Baltan S. Age-related changes in axonal and mitochondrial ultrastructure and function in white matter. J Neurosci. 2016;36:9990–10001. doi: 10.1523/JNEUROSCI.1316-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peters A, Kemper T. A review of the structural alterations in the cerebral hemispheres of the aging rhesus monkey. Neurobiol Aging. 2012;33:2357–2372. doi: 10.1016/j.neurobiolaging.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sturrock RR. Changes in neurologia and myelination in the white matter of aging mice. J Gerontol. 1976;31:513–522. doi: 10.1093/geronj/31.5.513. [DOI] [PubMed] [Google Scholar]
- 70.Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- 71.Bowley MP, Cabral H, Rosene DL, Peters A. Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey. J Comp Neurol. 2010;518:3046–3064. doi: 10.1002/cne.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sandell JH, Peters A. Effects of age on the glial cells in the rhesus monkey optic nerve. J Comp Neurol. 2002;445:13–28. doi: 10.1002/cne.10162. [DOI] [PubMed] [Google Scholar]
- 73.Jahn O, Tenzer S, Werner HB. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol. 2009;40:55–72. doi: 10.1007/s12035-009-8071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishii A, Dutta R, Wark GM, Hwang SI, Han DK, Trapp BD, Pfeiffer SE, Bansal R. Human myelin proteome and comparative analysis with mouse myelin. Proc Natl Acad Sci USA. 2009;106:14605–14610. doi: 10.1073/pnas.0905936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates JR, 3rd, Hetzer MW. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malone MJ, Szoke MC. Neurochemical studies in aging brain. I. Structural changes in myelin lipids. J Gerontol. 1982;37:262–267. doi: 10.1093/geronj/37.3.262. [DOI] [PubMed] [Google Scholar]
- 77.Tse KH, Herrup K. DNA damage in the oligodendrocyte lineage and its role in brain aging. Mech Ageing Dev. 2017;161:37–50. doi: 10.1016/j.mad.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DiLoreto R, Murphy CT. The cell biology of aging. Mol Biol Cell. 2015;26:4524–4531. doi: 10.1091/mbc.E14-06-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amberg D, Leadsham JE, Kotiadis V, Gourlay CW. Cellular ageing and the actin cytoskeleton. Subcell Biochem. 2012;57:331–352. doi: 10.1007/978-94-007-2561-4_15. [DOI] [PubMed] [Google Scholar]
- 80.Arani A, et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage. 2015;111:59–64. doi: 10.1016/j.neuroimage.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patzig J, et al. Septin/anillin filaments scaffold central nervous system myelin to accelerate nerve conduction. Elife. 2016;5:e17119. doi: 10.7554/eLife.17119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peters A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Front Neuroanat. 2009;3:11. doi: 10.3389/neuro.05.011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peters A. Age-related changes in oligodendrocytes in monkey cerebral cortex. J Comp Neurol. 1996;371:153–163. doi: 10.1002/(SICI)1096-9861(19960715)371:1<153::AID-CNE9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 84.Safaiyan S, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci. 2016;19:995–998. doi: 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galatro TF, et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat Neurosci. 2017;20:1162–1171. doi: 10.1038/nn.4597. [DOI] [PubMed] [Google Scholar]
- 86.Yao Y, Lacroix D, Mak AF. Effects of oxidative stress-induced changes in the actin cytoskeletal structure on myoblast damage under compressive stress: confocal-based cell-specific finite element analysis. Biomech Model Mechanobiol. 2016;15:1495–1508. doi: 10.1007/s10237-016-0779-0. [DOI] [PubMed] [Google Scholar]
- 87.Wong SW, Sun S, Cho M, Lee KK, Mak AF. H2O2 exposure affects myotube stiffness and actin filament polymerization. Ann Biomed Eng. 2015;43:1178–1188. doi: 10.1007/s10439-014-1178-2. [DOI] [PubMed] [Google Scholar]
- 88.Gourlay CW, Carpp LN, Timpson P, Winder SJ, Ayscough KR. A role for the actin cytoskeleton in cell death and aging in yeast. J Cell Biol. 2004;164:803–809. doi: 10.1083/jcb.200310148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Celeste Morley S, Sun GP, Bierer BE. Inhibition of actin polymerization enhances commitment to and execution of apoptosis induced by withdrawal of trophic support. J Cell Biochem. 2003;88:1066–1076. doi: 10.1002/jcb.10449. [DOI] [PubMed] [Google Scholar]
- 90.Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci USA. 2009;106:14914–14919. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baird NA, et al. HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science. 2014;346:360–363. doi: 10.1126/science.1253168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Higuchi R, Vevea JD, Swayne TC, Chojnowski R, Hill V, Boldogh IR, Pon LA. Actin dynamics affect mitochondrial quality control and aging in budding yeast. Curr Biol. 2013;23:2417–2422. doi: 10.1016/j.cub.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirano A. Hirano bodies and related neuronal inclusions. Neuropathol Appl Neurobiol. 1994;20:3–11. doi: 10.1111/j.1365-2990.1994.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 94.Cichon J, Sun C, Chen B, Jiang M, Chen XA, Sun Y, Wang Y, Chen G. Cofilin aggregation blocks intracellular trafficking and induces synaptic loss in hippocampal neurons. J Biol Chem. 2012;287:3919–3929. doi: 10.1074/jbc.M111.301911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mustafa AG, Wang JA, Carrico KM, Hall ED. Pharmacological inhibition of lipid peroxidation attenuates calpain-mediated cytoskeletal degradation after traumatic brain injury. J Neurochem. 2011;117:579–588. doi: 10.1111/j.1471-4159.2011.07228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]