Abstract
This article reviews the current knowledge on the mechanisms of adaptive response to low doses of ionizing radiation or chemical exposure. A better knowledge of these mechanisms is needed to improve our understanding of health risks at low levels of environmental or occupational exposure and their involvement in cancer or non-cancer diseases. This response is orchestrated through a multifaceted cellular program involving the concerted action of diverse stress response pathways. These evolutionary highly conserved defense mechanisms determine the cellular response to chemical and physical aggression. They include DNA damage repair (p53, ATM, PARP pathways), antioxidant response (Nrf2 pathway), immune/inflammatory response (NF-κB pathway), cell survival/death pathway (apoptosis), endoplasmic response to stress (UPR response), and other cytoprotective processes including autophagy, cell cycle regulation, and the unfolded protein response. The coordinated action of these processes induced by low-dose radiation or chemicals produces biological effects that are currently estimated with the linear non-threshold model. These effects are controversial. They are difficult to detect because of their low magnitude, the scarcity of events in humans, and the difficulty of corroborating associations over the long term. Improving our understanding of these biological consequences should help humans and their environment by enabling better risk estimates, the revision of radiation protection standards, and possible therapeutic advances.
Keywords: Adaptive response, Low-dose, Signaling pathway, Stress response, Epigenetic regulation, Defense mechanism
Introduction
Too little is known about the effects of low levels of exposure to chemicals or radiation, despite the importance of this issue. Because of the varied and polymorphous nature of the various related factors and the consequent difficulty in extrapolating a signal from the diverse background noise, epidemiology alone cannot provide a clear answer. In recent decades, however, the evidence underlying a bundle of presumption appears to show that organisms and cells can adapt to low-dose exposure. Despite discrepancies in some epidemiological and experimental studies, the complementarity of these two approaches should enable advances in answering these questions [1–3].
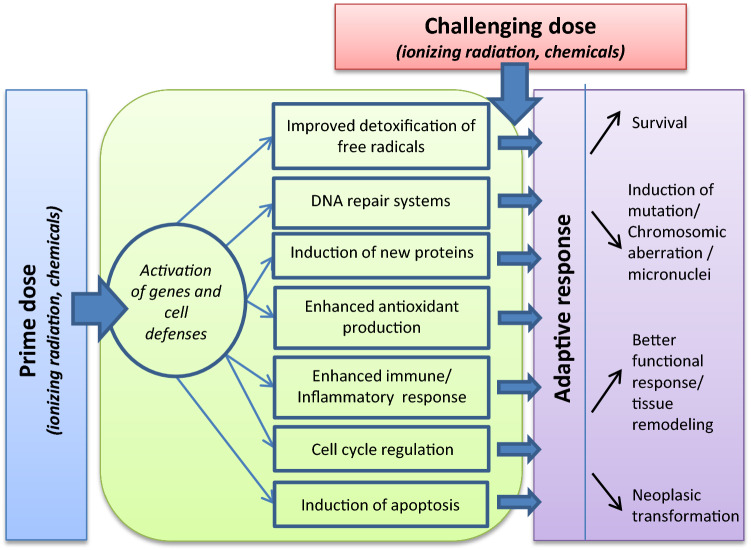
Various physical and chemical toxic agents, some of them natural (ionizing radiation), others released in the environment by human activity (environmental pollutants), affect organisms, which must adapt continuously to these external fluctuations. Human beings are continuously exposed to natural radiation to low doses (average 2.4 mSv) from radon, cosmic rays, terrestrial sources and internal emissions of radioactive isotopes in food and water. People are also exposed to anthropogenic sources of radiation used in medicine, research, and industry which contribute to about 20% of the total radiation exposure. Individual’s exposure could then vary due to medical purposes, occupational exposures and living areas [4]. Cell response to these stresses determines whether the organism can function properly and survive. This response is orchestrated through a multifaceted cellular program involving the concerted action of diverse stress response pathways [5] (Fig. 1). Many studies demonstrate “non-targeted” effects, including adaptive responses, bystander effects, genomic instability, and coordinated response—a suite of effects that could modulate the relation at low doses between cancer induction and increasing doses of both ionizing radiation (IR) and chemicals. This progress in knowledge about the mechanisms of low-dose biological effects calls for the updating of our understanding of them, particularly the adaptive response.
Fig. 1.
Cellular processes of stress response pathways
(adapted from [185])
Two competing hypotheses propose explanations of the effects of exposure to IR or chemicals: the linear no-threshold hypothesis, similar to the basic principle of toxicology first propounded by Paracelsus that “the dose make the poison”, and its no-linear relation alternative offered by threshold models [6, 7], which assume that no increase in risk occurs at doses below a certain level. Another variant of this model is the non-monotonic dose-response model (NMDR) [8], according to which low-level exposures to chemicals or radiation might be beneficial for health, even though larger doses are harmful. The NMDR model is valid for some chemical substances and trace elements that the body needs in small amounts to stay healthy, but which may become deleterious when received in larger doses.
Current conventional models of radiation or chemical dose-response relations are inconsistent with these relatively new findings. As long as the mechanisms of the effects remain unclear, modeling low-dose effects is difficult, and its uncertainties are high. Many experimental studies of species ranging from bacteria to human beings subjected to mild stress induced by low doses of IR or other physical or chemical agents describe NMDR [8–11] that could be considered beneficial [12, 13] or detrimental [14–16]. The low-dose range for chemicals varies substantially according to the substance involved, but for IR UNSCEAR defines low doses as those below 100 mGy, moderate doses as from 0.1 to 1 Gy and high doses as those above 1 Gy [3]. In this review, we focus on the adaptive mechanisms involved after low-dose chemical and radiological exposure at the level of the cell and the organism.
Numerous studies have shown that specific biological mechanisms respond adaptively to low doses of IR or chemicals. Adaptive response can be demonstrated by the ability of a biological system exposed to a small “priming” stress to exhibit a lower detrimental effect on subsequent reception of another higher radiation dose, the “challenge” dose (Fig. 1). Thus several reports and publications define a radioadaptive response as “the ability of low dose radiation to induce cellular changes that alter the level of subsequent radiation induced or spontaneous damage” [9, 17–21]. Low doses of IR, such as low doses of chemical agents, might induce biological mechanisms that make the cells better able to cope with subsequent exposures to high doses. The first adaptive response model of low-dose radiation involved a dose range of 1–100 mGy of γ-rays and postulated that it could be beneficial. Oliveri et al. [9] reported fewer chromatid aberrations for cells previously exposed to a low concentration of [3H]thymidine before acute X-rays than when exposure began only with acute X-rays. The multiple descriptions of low doses stimulating the body’s natural defenses in a wide variety of organisms suggest that this phenomenon is evolutionarily conserved [22]. Nevertheless, adaptation does not occur in every system and with all exposure situations.
Adaptive responses to low doses have been convincingly demonstrated in cultured cells, although doubt remains over how they influence risk in multicellular organisms. Mechanistic studies are thus important for reducing uncertainty in the estimation of both cancer and non-cancer diseases. We will describe several underlying mechanisms at the level of molecules, cells, tissues, and organisms. At the molecular level, key signaling pathways have been identified, by their direct induction by IR or chemicals; these include DNA damage response, as well as reactive oxygen species (ROS) and other signaling molecules involved in cell survival. Adaptive processes also occur at the cellular level due to molecular events: via the elimination of damaged organelles (autophagy) or of damaged cells (through apoptosis or necrosis), or by cell cycle delay or the unfolded protein response. Finally, tissue or organism processes can be implicated in this response by inducing cell proliferation or differentiation, controlling immune/inflammatory reactions, inducing toxic substance excretion through transporters, or activating distant cells through bystander/abscopal effects.
The originality of this review lies in its focus on studies showing an adaptive response to low doses of both IR and chemicals and its updating of the mechanisms thus far identified in other recent reviews [16, 20, 23]. Better knowledge of these mechanisms is needed to improve our understanding of health risks at low levels of environmental or occupational exposure and their involvement in cancer or non-cancer diseases.
Molecular mechanisms of intracellular signaling pathways
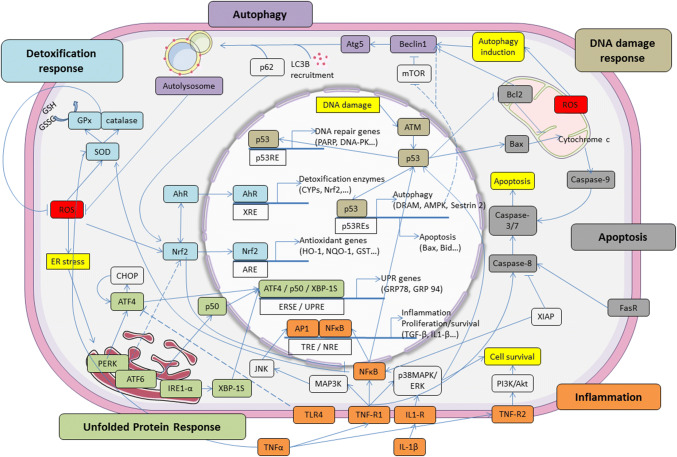
These pathways have evolved to overcome cellular perturbation and promote survival of the organism. Figure 2 and Table 1 summarizes the main data about the different signaling pathways involved in the adaptive response to low doses of radiation and chemicals reported in this review. They include the DNA repair pathway, antioxidant response pathway, immune/inflammatory response, cell survival/death pathway, endoplasmic response to stress, and numerous proteins and transcription factors involved in these stress response pathways [16, 24, 25].
Fig. 2.
Crosstalk of signaling pathways between intracellular molecules involved in the adaptive response. The figure highlights the different pathways and main factors involved in autophagy, apoptosis, inflammation, antioxidant, DNA damage, UPR and detoxification response. AhR aryl hydrocarbon receptor, Akt protein kinase B, AP1 activator protein 1, ARE antioxidant response element, ATF activating transcription factor, ATM ataxia-telangiectasia mutated, Atg autophagy-related, Bax Bcl-2–associated X, Bcl2 B-cell lymphoma 2, CHOP C/EBP homologous protein, ER endoplasmic reticulum, GPx glutathione peroxidase, IRE1 inositol requiring enzyme 1, ERSE ER stress-response element, IL interleukin, JNK c-Jun N-terminal kinases, MAPK mitogen-activated protein kinase, mTOR mechanistic target of rapamycin, LC3B light chain 3β, NF-κB nuclear factor-kappa B, NRE NF-κB response element, Nrf2 nuclear factor erythroid 2 (NFE2)-related factor 2, PERK protein kinase RNA-like ER kinase, PI3K phosphatidylinositol-4,5-bisphosphate 3-kinase, ROS reactive oxygen species, SOD superoxide dismutase, TLR toll-like receptors, TNF tumor necrosis factor, TRE 12-O-tetradecanoylphorbol-13-acetate (TPA) response element, UPR unfolded protein response, UPRE unfolded protein response element, XBP-1S X-box binding protein 1, XIAP X-linked inhibitor of apoptosis protein, XRE xenobiotic response element
Table 1.
Development of adaptation for several signaling pathways induced by low dose irradiation and chemicals (relevant references indicated)
| Signaling pathways | Ionizing radiation types | Chemical compounds |
|---|---|---|
| DNA damage repair (p53, ATM, PARP) | α, β, X, γ [9, 27, 32, 42, 119, 124] | Alkylating agent [29] |
| Nrf2 antioxidant pathway | α, X [54, 68, 146, 149] | Sulforaphane, heavy metals, thiol [45, 46, 66, 145] |
| NF-κB inflammatory pathway | γ, X [61–63, 65, 67, 153] | Thiol [66] |
| MAPK (AKT, ERK, JNK) pathway | γ, X [73–75, 101] | Alkylating agent [75] |
| Autophagy | γ [87, 88] | CBZ, heavy metals [91, 92] |
| CyclinB1/CDK1 | α, X [102, 124, 186] | Not known |
| Apoptosis (Bax/Bcl2, Wnt/β catenin) | γ [103, 104] | Cd [142] |
| UPR response | Not known | Nephrotoxin drugs, Pb [112, 114, 115] |
| Bystander (diffusible factors) | α, γ [119, 126] | Cd [140] |
The enhanced expression of stress proteins is considered to be an important adaptive response to adverse conditions such as exposure to chemicals, heavy metals, or IR. They belong to a wide range of defense mechanisms described in this section but also to the cellular and tissue responses detailed further on.
DNA damage response
To protect the genome integrity, cells possess a sophisticated mechanism of DNA lesions detection and repair, the DNA damage response [26]. Endogenous or exogenous stress can induce several different DNA repair systems, including the mismatch repair, base excision repair, nucleotide excision repair, homologous repair, and non-homologous end joining repair systems.
Many studies have showed that radiation or chemical-induced adaptive responses involve cell cycle regulation and DNA-dependant protein kinases [27, 28] (Table 1). Mammalian cells can incur DNA damage from endogenous factors such as oxidative metabolism and from exogenous factors such as exposure to IR or to genotoxic chemicals. The induction of an adaptive response through DNA repair pathway was first shown with alkylating agents. Cells chronically exposed to low non-toxic doses were resistant to the induction of sister chromatid exchange by subsequent exposures to high doses of the same chemical [29]. Olivieri et al. [9] further reported the first description of a radioadaptive response in human lymphocytes: a lower frequency of chromosomal aberrations after a high (challenge) dose in cells previously treated with very low (adaptive) doses of IR. A similar induced adaptive response in human lymphocytes was observed in individuals occupationally exposed to x- or γ-rays, who had lower dicentric frequencies than did non-occupationally exposed individuals [30]. Other authors have shown that the induction of DNA damage repair pathways is an important component of the radioadaptive response [31–33], demonstrating that 3-aminobenzamide blocks it in lymphocytes, and thus suggesting the possible role of repair pathways involving poly(ADP-ribose) polymerase.
Experimental studies have reported the induction of DNA repair mechanisms at low doses of radiation [34]. Others have suggested different factors involved in DNA repair, including DNA-dependent protein kinase (DNA-PK) and ERCC5 (XPG) [35, 36]. Moreover, ataxia telangiectasia mutated (ATM) and p53 tumor suppressor proteins are involved in both cell cycle regulation and DNA repair [27, 35, 37] (Fig. 2). The results of these experiments highlight the need for p53 in the induction of adaptive responses; no such response occurred in p53-deficient cells, such as Trp53 knockout cells, or immortalized cells, such as AT or m5S cells [27]. Similarly, reduced p53 function in mice exposed to low-dose radiation administered at a low dose rate accelerated disease in the modified compared to control mice (with full p53 function) exposed to the same radiation [38]. Several authors have proposed schematic representations summarizing p53 involvement in conjunction with the molecular network relevant to signal transfers and double-strand break (DSB) repair [27, 39]. Some suggest that adaptive response to low-dose IR in eukaryotic cells may involve DSB repair by non-homologous end joining repair pathways. Several protein kinases are reported to activate p53; among them, low-dose irradiation efficiently activates p38MAPK, which inversely is downregulated at high doses [40] (Fig. 2).
Borham and Mitchel [41] showed that DNA single-strand breaks are more closely associated with radioresistance induction than the DSBs. Hydroxyl radicals derived from the radiolytic decomposition of H2O or from reactive nitrogen species strongly induce these single-strand breaks. This finding is consistent with the increases in protein levels of AP-endonuclease (a DNA base excision repair protein) that occur after a low dose of α particles [42].
Nrf2 mechanisms dependent on and independent of the Nrf2/Keap1 pathway
Oxidative stress can result in the impairment of DNA and other cell components, such as proteins and lipids. This section develops the major pathway described for the adaptive response to oxidative stress: the Nrf2 pathway. Nrf2 plays a role in controlling the inducible expression of various enzymes responsible for the synthesis of glutathione, direct-acting antioxidants, and reducing equivalents [43].
NF-E2-related 2 (Nrf2) is a transcription factor, and activation of the Nrf2-signaling pathway is an adaptive response to environmental and endogenous stresses. This response renders animals resistant to chemicals and other forms of toxicity by inducing a variety of detoxification or antioxidant enzymes, as reviewed by Osborn and Kensler [44]. This factor belongs to the cap’n collar protein family of basic leucine zipper transcription factors, which bind to the antioxidant responsive element (ARE) and appear essential for maintaining cellular redox balance. Under normal conditions, Keap1 sequesters Nrf2 in the cytoplasm, where Nrf2 then facilitates the degradation of Keap1 via the proteasome.
The Nrf2-antioxidant pathway can be stimulated by irradiation and its preactivation by chemical inducers such as sulforaphane reduce the number of irradiation-induced DNA DSBs [45, 46]. The induction of this protective Nrf2 function by low-dose IR has also been observed in the diabetic mouse model, where it reduces renal inflammation, oxidative damage, remodeling, and dysfunction [47–49]. This group of authors first showed that low-dose radiation prevented diabetic downregulation of both renal Nrf2 expression and the downstream genes NQO1 and HO-1 [47] (Fig. 2, Table 1).
Upregulation of the Nrf2 pathway after low-dose irradiation might also be due to the induction of autophagy. As mention below, low-dose irradiation promotes the autophagy process. A deficiency in autophagy results in competitive inhibition of Nrf2-Keap1 binding by the selective autophagy substrate p62 and stabilizes Nrf2 and its downstream pathway [50]. Other studies have demonstrated that autophagy upregulates Nrf2 by its autophagosomal degradation of Keap1 [51] or by upregulation of the oncogene signaling protein and the subsequent induction of the antioxidant pathway [52]. Nrf2 has also been associated with chemoresistance in cancer cell lines. Overexpression of Nrf2 enhances resistance to chemotherapeutic drugs including cisplatin, doxorubicin, and etoposide in breast carcinoma or neuroblastoma cell lines, and downregulation of Nrf2 makes cancer cells more susceptible to these drugs [53]. In 2015, Chen et al. [54] also demonstrated that exposure to 50 mGy of α-particles induces radioresistance in human lung adenocarcinoma A459 cells after a subsequent exposure to 750 mGy α-particle radiation. The initial exposure to 50 mGy triggers an increase in the expression of Nrf2 and its target HO-1. These authors used short-hairpin RNA (shRNA) of Nrf2 and a chemical inhibitor of HO-1 to suppress the induced radioresistance of the 50 mGy α-particle radiation and thereby demonstrate Nrf2 pathway involvement.
Although Nrf2 is primarily regulated via its interaction with Keap1, studies have begun to show that it can be regulated independently of Keap1. Nrf2 phosphorylation by several signal transduction pathways, the involvement of epigenetic factors such as microRNAs (miRNAs), and Nrf2 interaction with other proteins also play a role in its activation [55]. miRNAs can modulate the Nrf2 signaling network either by their involvement in regulating Nrf2 activity (affectors) or by mediating Nrf2 activity (effectors) [56]. At the post-transcriptional level, components of the Nrf2 pathway can be regulated by several miRNAs (miR-28, 34, 144, 200). For example, Wagner et al. [57] showed that decreased levels of miR-155 is correlated with increased Nrf2 expression and a severe reduction in the expression of the proinflammatory TNFα gene. Nrf2 is also regulated post-transcriptionally by phosphorylation, ubiquitination, and acetylation via a variety of enzymes affecting its interaction with Keap1 (ERK/JNK, PCK, MAPK/ERK, and p38), by its localization (PKC, CK2, and GSK3β/Fyn) in the nucleus or within the proteasome, its degradation (GSK3β/βTrCP), or its binding to DNA binding protein (300/CBP, Maf).
Ligands of AhR (aryl hydrocarbon receptor) activate the XRE (xenobiotic responsive element) to induce the expression of many phase I enzymes, such as the cytochrome P450 s that produce reactive intermediates able to activate the antioxidant pathway via the ARE (Fig. 2). Some of the products of these Nrf2-regulated enzymes can then activate Nrf2 signaling and thereby potentiate the Nrf2 adaptive response. AhR inducers such as TCDD also induce ROS as well as Nrf2 itself and can, therefore, activate both ARE and XRE pathways [58]. Moreover, Nrf2 possesses an ARE sequence within its own promoter region to enhance the adaptive cell defense response. For example, upregulation of miR-125b through Nrf2 expression regulates the expression of the AhR repressor and thus reduces cisplatin-induced injury [59]. Nrf2 knockout mice consistently abrogate the adaptive increase of miR-125b elicited by cisplatin and thus worsen kidney injury.
This items of evidence shows that the Nrf2 pathway is highly regulated and suggests that a better understanding of the interplay between the pathways involved in cellular processes such as apoptosis, survival, differentiation, and inflammation is essential to clarify the coordinated cell protection that produces adaptation against stressful conditions.
NF-κB signaling pathway
The NF-κB (nuclear factor-kappa B) family of transcription factors is a master regulator of the both innate and adaptive immune responses [60]. It also controls the expression of genes involved in the repair of damaged DNA and the regulation of molecules that contribute to the control of cell proliferation, survival, or apoptotic cell death (Fig. 2). Numerous studies have shown that NF-κB, by inducing genes involved in proinflammatory processes, plays a proinflammatory role. Simultaneously, however, reports have described its anti-inflammatory role and the induction of leukocyte apoptosis during the resolution of inflammation. The serine/threonine kinase Akt (protein kinase B) pathway is a central node in cell signaling downstream from inflammatory agents or other cell stimuli and appears to be associated with the adaptive response induced by exposure to low doses of IR (Table 1).
Prasad et al. first reported NF-κB activation after low to moderate radiation (0.25–2 Gy) in lymphoblastoid cells [61]; it has since been reported in endothelial cells as well [62]. Both studies observed that NF-κB binding has a biphasic induction profile and reported transcriptional activities with a first peak at 0.5 Gy and a second at 2 Gy. Rodel et al. further showed that the anti-inflammatory properties of low-dose IR may be due to the interrelations between NF-κB and TGF-β1 (transforming growth factor beta1) in exposed endothelial cells [62]. The induction of X-linked inhibitor of apoptosis (XIAP) expression in the same model demonstrates the role of this protein in modulating NF-κB and TGF-β1 expression; both contribute to anti-inflammatory effects and the modulation of apoptosis after low-dose IR [63]. Consistently with this finding, other works have demonstrated that low doses of IR reduce the IL-1β secretion by macrophages that results from reduced expression of RelA (p65), p38 MAPK, and Akt, which are up- and downstream signaling molecules of NF-κB [64] (Fig. 2). Studies also suggest that Akt activity responds differentially to low-dose IR. Kim et al. [65] showed that a 50-mGy exposure stimulates cell proliferation through the transient activation of Raf and Akt but does not change the cell cycle response-related p53 and p21 levels in lung fibroblasts. A 2-Gy dose of IR, however, induces cell cycle arrest by changing these levels.
As described below, the antioxidant enzyme MnSOD is often cited as an important mechanism of the adaptive response to low doses of IR. Increased NF-κB-mediated SOD2 expression (Fig. 2, Table 1), producing a radioadaptive response, has followed thiol treatment or constitutive activation of Akt [66, 67]. Both classes of agents seem to share the same pathway after activation of NF-κB, which reduces the overall damage induced by the IR challenge dose. Nevertheless, they differ in complexity, with the TNFα signaling pathway implicated in the low-dose radiation-induced response, but not in the thiol-induced pathway [68].
MAPK (ERK/JNK/p38) signaling pathway
The mitogen-activated protein kinase (MAPK) cascade is essential for the transduction of extracellular signals into specific cellular responses such as cell migration and survival and modulate cellular metabolism [69, 70]. Stress-activated MAPK superfamily can be induced by exposure to IR or chemicals and to adapt to these environmental circumstances. These pathways are regulated by a diverse array of intra- and extracellular stressors, including environmental physical/chemical changes and exposure to inflammatory cytokines. Their activation appears to be associated with cell protection against IR and chemicals and with survival of these stressors. Several studies have suggested that extracellular signal-regulating kinase 1/2 (ERK1/2), a member of this superfamily, plays a critical role in determining the cellular outcome—survival or death—after irradiation (Table 1). Low IR doses induce ERK1/2 through ATM, its upstream regulator, whereas high-dose exposure results in ATM-independent dephosphorylation of ERK1/2, and thus shuts down prosurvival signaling [71]. Experiments using siRNA and chemical inhibitors have demonstrated that the cell proliferation stimulated by low-dose IR occurs through ERK1/2 and p38 in normal human fibroblasts, and thus confirmed the activation of the MAPK pathway in lung fibroblasts exposed to 50 mGy radiation [72].
Studies have also reported that low-dose IR (0.01–0.1 Gy) induces ERK activation in immune lymphoblast cells without altering Akt activation and then induces an adaptive response to a subsequent high-dose radiation challenge (2 Gy) [73]. The authors suggest that activation of the ERK pathway in cells lacking Akt activity is compensatory and might explain the differential sensitivity of non-tumor and tumor cells previously observed by others [74].
Bystander cells exposed to conditioned media from IR-exposed cells (1–2 Gy) or to genotoxic chemicals show transient increases in MAPK phosphorylation [75]. ERK, JNK, and p38 are all activated and then decline and are inhibited, in correlation with a decrease in caspase activity. Asur et al. suggest that activation of ERK and p38 results in the cell cycle arrest of bystander cells and then the activation of proliferative pathways to repair damaged components. Cells that escape repair may then undergo apoptosis through MAPK-mediated pathways.
Cross-talk between the intracellular pathways
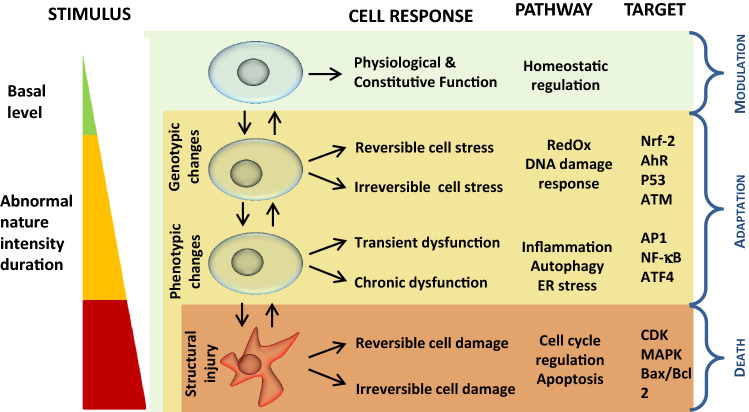
As noted in the previous sections describing molecular activities, several types of relations enable cells to balance between stress responses (e.g., antioxidant, anti-inflammatory, and autophagy) and regulation of either death signals or the cell cycle. Although our understanding about the common hallmarks of these responses remains incomplete at present, they most likely originate from an overlap of several processes that may be initiated at various thresholds (depending on the pathway) and operate in a staggered manner (Fig. 3).
Fig. 3.
Schematic representation of the hierarchical cell stress model in response to chemical or physical stress (adapted from [16, 80]). AhR aryl hydrocarbon receptor, ATM ataxia-telangiectasia mutated, ATF activating transcription factor, Bax Bcl-2–associated X, Bcl2 B-cell lymphoma 2, MAPK mitogen-activated protein kinases, NF-κB nuclear factor-kappa B, Nrf-2 nuclear factor erythroid 2 (NFE2)-related factor 2
Among these pathways, Nrf2 is a central transcription factor responsible for the basal and inducible expression of proteins involved in oxidative stress response, xenobiotic metabolism, and cytoprotection, but it is also linked to differentiation, proliferation, growth, and apoptosis. Nrf2-regulated genes include antioxidant enzymes, molecular chaperones, DNA repair enzymes, and anti-inflammatory response proteins. Nrf2 activation can suppress NF-κB activity and thereby inhibit inflammation. Cross-talk between Nrf2 and AhR or Nrf2 and NF-κB, and the identification of XRE- and NF-κB-binding regions of Nrf2 all suggest that its transcription can be regulated by these pathways, as reported in previous sections (Fig. 2).
The link between the autophagy process and antioxidative response is an important component of the adaptive response. Several laboratories have shown various adaptive mechanisms of human lung adenocarcinoma A549 cells exposed to low-dose IR before high-dose radiation and confirmed the association between p62, an autophagy adaptor protein, and Keap1, the regulator of Nrf2 antioxidant transcription factor [55, 76]. The sequestration of Keap1 by p62 allows Nrf2 to upregulate ARE-regulated genes.
Links between the antioxidative factor Nrf2 and the UPR (unfolded protein response) pathway are also necessary to alleviate ROS production in the endoplasmic reticulum (ER) during UPR-increased protein folding. Cullinan et al. showed that the Nrf2 pathway is induced by ER stress in a manner depending on protein kinase R-like endoplasmic reticulum kinase (PERK) and that it enhances cell survival [77]. Another example is the interaction of the UPR pathway with apoptosis; toll-like receptor 4 (TLR4) inhibits in a TRIF-dependent manner the UPR-induced expression of CHOP [78], a protein that leads to apoptosis after prolonged induction [79]. Nevertheless, these last interrelations as well as many other links between stress response pathways have only been shown in mechanistic molecular studies but have not yet been demonstrated in an adaptive response process induced by low-dose exposure to physical or chemical stress.
Adaptive mechanisms at the cellular level
To maintain a stable intracellular environment, cells can respond to physiological stressors or pathological stimuli in various ways, ranging from the activation of survival pathways to the initiation of cell death. The stress response pathways are evolutionary highly conserved defense mechanisms that determine the cellular response to chemical or physical aggression according to a hierarchical stress model. We present a model adapted from Li et al. [80] (Fig. 3). At the cell level, these cytoprotective processes include autophagy, which enables cell survival by the degradation and recycling of cell components, cell cycle regulation, which orients cells for repair, proliferation, or differentiation, UPR, which allows re-establishment of normal endoplasmic reticulum function, and apoptosis, that is, programmed cell death induced by excess damage. The bystander response is also described in this chapter as it occurs at the cell level and can involve different signaling pathways.
Autophagy
Autophagy is a housekeeping survival mechanism that promotes programmed cell survival, sustaining homeostasis by maintaining cellular integrity and favoring efficient cellular function; it is thus distinct from, even antagonistic to, apoptosis or programmed cell death [81]. Autophagy is strongly induced by starvation and is a key component of the adaptive response of cells and organisms to nutrient deprivation, by promoting survival until nutrients become available again. It is a nonselective bulk degradative pathway, but also a selective pathway mediated by autophagic adaptor proteins in response to stressful conditions [82]. Its induction by various stimuli (xenobiotics, cytokines, endoplasmic reticulum stress, and oxidative stress) suggests that it plays an essential role in cellular homeostasis and adaptation to adverse environments. Collectively, autophagy antagonizes apoptosis and promotes cell survival via a series of responses to damaged organelles, ER stress, and DNA instability. However, massive and persistent autophagy depletes the cell of organelles and critical proteins and can thus kill severely damaged cells by a caspase-independent form of cell death [83]. Cross-talk between autophagy and apoptosis is manifested by regulatory genes shared with common pathways including p53, Atg5, and Bcl-2 [81, 84].
In organs often exposed to stressful conditions, such as the kidney and liver, autophagy promotes cytoprotection and maintains cellular homeostasis in different cell types through a common mechanism aimed at preventing inflammation, oxidative stress, and apoptosis: the clearance of protein aggregates and unwanted organelles. Autophagy is now accepted to be renoprotective. An increased autophagic flux is observed in renal cell lines and murine models exposed to several nephrotoxins including heavy metals and drugs. The sensitization of kidney cells to hypoxic and ischemia-reperfusion injury by blocking autophagy by knocking out the atg gene suggests autophagy is renoprotective, promoting survival of these kidney cells [85]. Autophagy has also been identified as a critical mechanism for glomerular homeostatic maintenance in response to injuries, contributing to the preservation of structure and function [84]. Autophagy is described increasingly often in vascular diseases such as atherosclerosis, where it is required for endothelial cell alignment and atheroprotection under physiological blood flow [86]. Indeed genetic inactivation of autophagy resulted in major atherosclerosis plaque formation only in areas previously resistant to atherosclerosis, but no increase in the normally atheroprone (low shear stress) areas.
Autophagy can be induced by IR [87–90] or by chemical inducers that confer radioprotective effects [91]. The treatment of hematopoietic cells by carbamazepine before irradiation increases both cell survival, as assessed by clonogenic survival, and autophagy, assayed by immunoblot for microtubule-associated protein light chain 3 (LC3) [91]. Paglin et al. [87] showed that the formation of acidic vesicular organelles and autophagy in surviving colonies of cell lines exposed to IR provide long-term protection against damage from low-dose radiation. Exposure to low-dose IR (50 mGy) can also stimulate the autophagy process through the Nrf2 antioxidant pathway. Blockade of the autophagy pathway by an inhibitor suppresses radioresistance and the induction of Nrf2 and HO-1 expression, findings indicating that autophagy mediates Nrf2 upregulation [54].
Exposure to heavy metals at low (subtoxic) doses has also been shown to trigger cell proliferation and autophagy [92]. This study unraveled the unexpected early upregulation of autophagy in 80% of cadmium (Cd)-treated proximal convoluted tubules in vivo (< 10 µM), without induction of apoptosis or tubular dysfunction. Induction of endoplasmic reticulum stress was also observed in this study. The authors concluded that autophagy can serve as an early adaptive mechanism but that continuous activation of this process due to cadmium persistence might impair its efficiency and lead to nephrotoxicity in the long term.
In other diseases, such as neuropathies and ischemic heart diseases, autophagy is more widely accepted as beneficial given its role in eliminating “toxic assets” and promoting cell viability. Thus, autophagy has emerged as a new and potent modulator of disease progression.
Cell cycle regulation and apoptosis
The cellular machinery of the cell cycle is controlled by serine/threonine protein kinases, which are the cyclin-dependent kinases (CDK). They can stop the cell cycle at different steps if the genome is altered and cause either its repair or apoptosis. The Wnt/β-catenin pathway plays a critical role in cellular development, survival, proliferation, etc. [93, 94], but the role of this pathway in adaptive response and particularly in immunity is beginning to become more well-defined [95]. The regulatory function of Wnt/β-catenin signaling is increased after low dose exposure to ionizing radiation. Indeed, it was shown that low level of irradiation promotes the bone marrow stromal cells through the Wnt/β-catenin pathway [96].
The role of cell cycle regulators of the mitochondrial functions enabling radiation-induced adaptive responses at low doses has been reviewed recently [97]. The authors concluded that the Cyclin B1/CDK1 complex, which is the cell cycle G2/M checkpoint regulator, may be considered a key harmonizer for the regulation of mitochondrial functions in cellular adaptive response to genotoxic stress, including low-dose IR. This kinase complex is one of the main components by which cells communicate with mitochondria under stressing conditions; it plays an important role in coordinating mitochondrial energy production related to cell cycle progression and adaptive response to genotoxic stress. Induced radioadaptation has been reported at doses below 0.6 Gy and attributed to a change in the balance of G2/M checkpoint induction that allows time for DNA repair and increases cell survival. Several studies have demonstrated that low-dose radiation induces an adaptive response via stimulation of normal cell proliferation but does not induce a radioadaptive response in tumor cells [73, 74].
Apoptosis is a physiological process of endogenous programmed cell death, mediated by a variety of endogenous and exogenous stimuli including IR. Some miRNAs have been shown to be involved in radioresponsive effects at either high or low doses [98]. These authors suggest a model in which miRNAs may act as “hub” regulators of specific cellular responses, by immediately down-regulating them to stimulate DNA repair mechanisms and then upregulating those involved in suppressing apoptosis for cell survival. They further suggest that among the miRNAs identified miR-608 may contribute to cell cycle arrest. Others have confirmed the decreased apoptosis after low-dose IR and demonstrated it with UV radiation as well [99–101]. Irradiation of human breast epithelial MCF10A cells at 100 mGy produces MnSOD phosphorylation through CyclinB1/CDK1 and then increases resistance to apoptosis induced by a challenge dose of 10 Gy [102]. In human myeloid cells, low-dose irradiation confers some protection against the induction of apoptosis [103]. Microarray analysis of these cells has identified low-dose inducible genes with known roles in apoptosis regulation and cell cycle regulation. Thus, low-dose γ-radiation modifies apoptotic-related gene expression in freshly isolated blood lymphocytes, inducing upregulation of Bcl-2, an anti-apoptotic molecule and down-regulation of Bax, a pro-apoptotic gene [104].
Neurogenesis is another process that may be enhanced by low-dose irradiation [105]. Wei and coauthors related the stimulation of neural stem cell proliferation and reduced neuronal apoptosis in the hippocampus and learning by mice to stimulation of the Wnt/β-catenin signaling pathway by 0.3 Gy radiation, whereas they confirmed deleterious effects after a dose of 3 Gy.
The unfolded protein response (UPR)
The ER is the major cell organelle for the synthesis, folding, and sorting of proteins. The UPR is a protective mechanism for adaptation to environmental stress and recovery of normal ER function. The role of the UPR is to modify cellular functions in response to ER stress and re-establish ER function, by reducing messenger RNA translation, increasing proteasomal degradation, and increasing protein-folding capacity [106, 107]. Under prolonged or unresolvable ER stress, the UPR switches from an adaptive to an apoptotic role. Numerous studies suggest that ER stress and UPR are involved in several pathological situations (ischemia, diabetes, neurodegenerative and renal disorders, and chemical-induced tissue injury) and physiological events (development of different cell types and cytoprotection) [108]. If UPR fails, apoptosis begins. Numerous and widely varied chemicals that perturb calcium signaling, induce ROS production or hypoxia, or deplete amino acids are known to activate the UPR [109, 110]. The ER is one of the main reservoirs of calcium in the cell, and some divalent metals including lead may replace endogenous divalent cations. It has been reported that lead or cadmium induces ER stress and the corresponding GRP94 and GRP78 calcium-binding chaperones [109]. Knockdown of the latter protein results in an increase in both ROS generation and lead cytotoxicity [111]. The authors suggested that these two ER chaperones (GRP94 and GRP78), as well as the UPR response, may function as a defense mechanism against lead toxicity in coordination with antioxidant enzyme induction.
In addition to its pathological significance, ER stress/UPR also has physiological and protective effects, as shown, for example, by the cytoprotection of renal cell lines after ER stress by clinically relevant nephrotoxins that induce GRP78 and GRP94 [112]. These results have been confirmed in vitro and in vivo by other authors who have demonstrated that some ER stress inducers, such as heavy metals or pharmacological compounds, trigger UPR, upregulate GRP78, and then activate the autophagy pathway [113–115]. In vivo, these actions result in reducing renal ischemia-reperfusion injury through the improvement of renal function and histology [114]. In vitro binding assays investigating the molecular mechanism show that PERK can directly bind Nrf2 and that tunicamycin, a UPR-inducing agent, triggers Nrf2-DNA binding. Nrf2 is phosphorylated during ER stress responses, thus triggering Nrf2 nuclear import in murine fibroblasts, whereas Nrf2 remains cytoplasmic in PERK−/− cells [77].
The bystander effect
The bystander effect is a biological/biochemical change expressed by a cell or a tissue that is not directly targeted by IR or chemicals; it happens to cells neighboring or even distant from targeted cells. It was first demonstrated by Nagasawa and Little [116], who found that although α-particles traversed less than 1% of the nuclei of a monolayer cell culture, 30% of the cells showed increased frequencies of sister chromatid exchanges.
Radiation-induced bystander effects refer to DNA damage-like responses in cells that have not been directly exposed to radiation [117, 118]. Matsumoto reviewed the interrelation between bystander effects and radioadaptive response in 2007 [10]. A more recent finding is that unexposed bystander cells respond to factors released by targeted cells, and thus produce specific responses that can be either deleterious or beneficial [28]. The induction of radioadaptive responses in bystanders depends on the cell type and experimental conditions. Cells that are grown in conditioned medium harvested from cells exposed to low doses of α-particles or γ-rays display increased clonogenic survival after subsequent exposures to radiation [42, 119]. This phenomenon has also been observed in vivo and appears to resemble abscopal effects, defined as radiation responses in tissues “definitively separate” from the irradiated area [120]. Specific irradiation of human tumor cells in vivo produces a bystander effect in subcutaneously growing tumors [121, 122].
The two main types of intercellular signaling pathways identified as producing radiation-induced bystander responses [123] occur through either cell-cell contact (gap-junction intercellular communication) [124, 125] or secreted diffusible factors (e.g., cytokines, ROS, and miRNAs) [126, 127]. Low-dose irradiation can induce the selective removal of precancerous cells via intercellular induction of apoptosis. The use of scavengers and inhibitors confirms the involvement of secreted diffusible factors such as cytokines and reactive oxygen/nitrogen species signaling in the selective removal of transformed cells by non-transformed cells in co-culture [128]. Nitric oxide (NO) from irradiated cells acts as an intercellular signaling molecule to initiate and activate the early steps in the bystander response process after low-dose irradiation [129]. Epigenetic elements such as DNA methylation may also play a role in regulating low-dose radiation effects through the bystander effect [98, 130]. DNA methylation regulates low-dose response through reduction of DNA methyltransferases and global DNA methylation in bystander tissues [131] and may inhibit DNA repair [132]. Several data suggest that miRNA changes may have a protective effect after radiation exposure [133]. Recently studies of their role in the bystander response showed that expression levels of some miRNAs, such as miR-21, are modified by fibroblast irradiation and may act on non-irradiated cells [134, 135].
Until recently it has been difficult to demonstrate a bystander response for chemical exposures because treatments expose all cells simultaneously. Nevertheless, new in vitro experimental models have demonstrated the ability of genotoxic agents or chemotherapeutic drugs to induce bystander effects [136–139]. These effects are, therefore, not specific to IR, and authors have suggested novel mechanisms by which chemotherapeutic agents act on cancer cells. Chemically induced adaptive responses have also been demonstrated in vivo in rats pretreated with low doses of cadmium that induce hepatic metallothionein and produce an adaptive tolerance to lethality from a subsequent high dose [140].
Adaptive mechanisms at the tissue and organism levels
Some biological systems sustain and maintain normal physiology in the organism in response to low or mild-stress. These include antioxidant and immune/inflammatory systems, which are particularly active in detoxifying organs such as the kidneys, lung, and liver as well as the vascular system. They sustain and maintain normal physiology in the body in response to such mild stress and protect against toxicity induced by chemicals or radiation. These systems include the adaptive response provided by transporters, but as this is well described in other studies and is specific to chemical exposure, including endocrine disruptors [141], this review will not describe it specifically.
The antioxidant response
The antioxidant defense system is a key factor that prevents oxidative stress due to by exogenous stress. Mitochondria and ROS appear important to the coordination and regulation of the adaptive response. Because we described the specific role of the Nrf2 pathway above, a more general response by antioxidant enzymes is described here.
The mechanisms of adaptive response have been identified for exposure to heavy metals such as Cd; they involve the induction of metallothioneins (by 10- to 50-fold) after low concentration (< 10 µM) Cd pretreatment of rats [140]. The works of Nair et al. [142] confirmed these results and showed that the chronic exposure of rats to low doses of Cd induces protective mechanisms including the expression of genes for antioxidant enzymes and mitochondrial biogenesis factors. Similarly, we have showed that chronic uranium exposure appears to reinforce the antioxidant system in kidneys, glutathione homeostasis in particular [143]. Uranium is also a heavy metal and a radionuclide known to be nephrotoxic after acute exposure. Chronic exposure at low doses failed to produce nephrotoxic effects but did induce an antioxidative response [143, 144]. These findings suggest that the kidney adapts to uranium during the chronic exposure. Other heavy metals, such as Hg, Cu, or Pb, may produce protective effects at low concentrations by increasing the expression of antioxidant enzymes, according to Korashy and El-Kadi [145], who hypothesize that Pb can replace Zn in the metal-binding site of Keap 1, thereby activating Nrf2 and its downstream proteins HO-1, NQO1, or GSTA1.
High-dose irradiation is particularly known to deactivate antioxidative functions and lead to ROS-induced damage in cells and tissues, while low-dose irradiation or radon inhalation activates antioxidative functions and inhibits ROS-induced damage in in vivo studies [146]: enhanced antioxidative functions have been shown in the brain, lungs, liver and kidneys of mice inhaling radon [146], while low-dose γ-irradiation before or after carbon tetrachloride treatment activates antioxidative functions as an adaptive response in mouse liver and inhibits hepatopathy [147, 148]. The activity of detoxification enzymes such as MnSOD, Catalase, Gpx, and GST has also been reported to rise in cells exposed to low-dose radiation, and thus reduce cell damage [68, 149]. Glutathione content rises after 500 mGy irradiation along with other antioxidants in the liver of mice or in macrophage-like cell lines; this might contribute to a radioadaptive response. Nrf2 plays a role in controlling the inducible expression of various enzymes responsible for the synthesis of glutathione, direct-acting antioxidants, and reducing equivalents.
Immune/inflammatory reactions
Inflammation is a protective response by cells to pathogens, infections, or tissue damage and serves to destroy or wall off both the injurious agent and the injured tissue. It involves the coordinated communication between different immune cells and blood vessels through an intricate cascade of molecular signals designed to remove the stimulus or initiate the healing process. Observations of changes in the immune system after radiation exposure imply that inflammation might also play an essential role in the immunocompetence of living organisms [150]. Recent reviews of several experimental findings reveal that low-dose radiation induces anti-inflammatory properties and may thereby protect against inflammatory diseases [151, 152]. Chronic exposure to low-dose radiation of different wild-type mouse strains may stimulate effects that activate the immunological network of the entire body including cell populations and their surface molecules, together with antibody-producing activity [153].
It has been reported that under protracted chronic exposure to low-dose γ-rays, the normally highly radiosensitive hematopoietic system adapts and becomes radioresistant [150, 153, 154]. Evidence from animal experiments demonstrates that low dose rate irradiation can activate the immune function [155], promoted by enhancement of the proliferative response of splenic and thymic lymphocytes to mitogens, amplification of NK activity, and increased secretion of cytokines that regulate immune cells. Repeated low-dose IR (six fractions of 100 mGy) also enhances other immune cells or animal models. Kojima et al. [147] observed intensified natural killer activity and antibody-dependent cellular cytotoxicity correlated with the increase in the glutathione level and the suppression of tumor growth in Ehrlich-Solid-Tumor-bearing mice. In vitro, low-dose irradiation is described as activating macrophage cells towards a proinflammatory phenotype [156]. Nonetheless, decreased inflammatory cytokine production, reduced migration, and increased chemotaxis in macrophages have also been observed [157]. A recent study by our group showed the importance of dose rate on endothelial cells’ molecular and functional responses; upregulation of antioxidant and anti-inflammatory gene expression after chronic low dose rate exposure (6 mGy/h) produced an adaptive response even when the dose reached 2 Gy [158].
Accordingly, the effect of mild stressors such as low-dose irradiation has modulatory anti-inflammatory properties that have been used in preclinical models of low-dose radiotherapy. In vivo models of induced arthritis treated by fractions of irradiation (1–1.5 Gy) are reported to diminish inflammatory proliferation symptoms, and thus joint swelling [159–161]. In further studies, histological analysis of arthritic joints revealed a reduction in clinical symptoms (cartilage and bone destruction) after local irradiation, apparently related to a reduction of iNOS activity and increased expression of heme oxygenase-1 and Hsp70 [162, 163]. Reduced levels of the inflammatory cytokines TNFα, IFNγ, and IL-6 are associated with an improvement in clinical symptoms in another model of induced arthritis in mice, treated by 0.5-Gy per week for five consecutive weeks [164]. Regulatory T cells (Treg) may thus contribute to reducing clinical symptoms in mice treated with low-dose irradiation [165]. In a human TNF-transgenic mouse model of chronic polyarthritis characterized by synovial inflammation, low-dose irradiation of the mice improves the disease’s clinical progression [166].
The therapeutic effect of low-dose radiation is also observed in diabetic nephropathy. In a type 2 diabetes mouse model, repeated exposure to low-dose radiation at 25 mGy attenuates diabetes-induced higher renal levels of ICAM-1, TNF-α, and PAI-1, three proinflammatory factors involved in the pathogenesis of kidney failure [48]. These results are in line with previous animal studies showing that low-dose radiation modifies the progression of chronic renal failure [167]. As discussed before, the protective mechanisms of enhancing immunity are linked to the antioxidative action of low-dose radiation [168, 169]. Along with inflammation, oxidative stress is a common feature in chronic kidney diseases; in experimental animals, the ablation of the Nrf2 gene, a critical regulator of cytoprotective factors, causes a lupus-like autoimmune nephritis and exacerbates diabetes-induced oxidative stress, inflammation, and nephropathy [170, 171].
The regulation of inflammation in the cells of the vascular tree or other organs often involves an adaptive response by the immune system, associated with an increase in T-reg cells, which control autoreactive T cells and negatively regulate immune response by decreasing proinflammatory lymphocytes and monocytes. Upregulation of T-reg cells is also associated with a decrease in IL-6, which inhibits their production through the action of TGFβ [172]. In fact, increasing the number of T-reg cells is an adaptive response used by different types of tissue as an immune evasion strategy [173]. In this context, repeated exposure to low-dose mild stressors attenuates inflammation, and upregulate the immunosuppressive T-reg cells response [164, 174].
Moreover, inflammation is a hallmark of several cardiovascular disorders, atherosclerosis in particular. It can be described as a chronic inflammatory disease of the arterial wall in which plaque build-up in the intima impairs normal vascular functioning. High doses of IR increase inflammation and atherosclerosis [175–177], while low doses administered at a low dose rate are anti-inflammatory and reduce atherosclerosis in most situations [67, 178–180]. Recent studies demonstrate the importance of dose rate in the inflammatory response: high dose rates are associated with the upregulation of inflammation, whereas low dose rates induce anti-inflammatory responses [175, 178]. The latter study showed that chronic low-dose 137Cs exposure for 6 months reduces levels of the inflammatory mediators CRP, TNFα, MCP-1, and IFN-γ, as well as plaque macrophage content, results that suggest increased plaque stability.
Lung inflammation by benzo[a]pyrene (BaP), a component of cigarette smoke, induces lung tumors in animal models. When human lung fibroblasts were used to study the effect of low-dose radiation on these proinflammatory effects, a single low dose of 90 mGy inhibited the secretion of proinflammatory cytokines (IL-6, CXCL1, and CXCL5), probably due to the suppression of IL-6/IL-6R and CLF1/CNFTR signaling [181]. The authors suggest that low-dose γ-ray exposure suppresses the transformation of human bronchial epithelial cells exposed to BaP by suppressing cytokine secretion. Similarly, the arrest of cancer-facilitating inflammation is a plausible explanation for the reduction of the number of lung tumors by low-dose total body irradiation after their induction by BaP exposure [182].
All these findings indicate that the immune system is modified after low dose exposure to IR or chemicals; the extent and impact of this modification depend on factors such as the IR dose and its temporal relation to the immune system. At low doses and low dose rates, the effects of IR on the immune system may be suppressive or stimulatory, and their long-term impact may have consequences on various human inflammatory pathologies.
Discussion
The biological effects of low doses of radiation or chemicals are often controversial, in view of the difficulty in detecting noticeable low-level effects or rare events in humans in the short term, despite the great importance of and interest in their long-term biological effects. The development of more sensitive assays is improving the detection of these biological effects at low doses and our understanding of the mechanisms involved. Nevertheless, these effects can include maladaptive responses that may appear beneficial in the short term but may be deleterious over the long term. More specifically, carcinogenesis is an adaptive response process in a given tissue and must thus be understood biologically from its early stages, including DNA repair, tumor promotion, and inflammation. It may, therefore, play an important role in preventing the immortalization of human cells. The study and understanding of the mechanisms involved in the adaptive response are highly complex as they depend on the model (in vitro, in vivo) used, the endpoint, the dose or the dose rate for radiation, and the interval between exposure and the observed biological effects. Additionally, the duration of the adaptive response can also differ strongly: enzymatic or transcription responses act within minutes or hours) to remodeling, epigenetic, or genomic effects that are observed after months or years [22]. Accordingly, the adaptive response fades if the time between the first and second exposure is too long; inversely, if these exposures follow each other too rapidly, adaptive response mechanisms may not have sufficient time to be induced.
Although some recent epidemiological studies have found significantly elevated risks of solid cancers associated with exposure in the 0–100 mGy dose range, their statistical power remains too limited to investigate risks in the low-dose range with sufficient precision [1]. The biological effects of low-dose radiation or chemicals are currently estimated with the linear non-threshold model, but as EJ Calabrese wrote: “Regulators must extrapolate results not only from animal toxicity studies, typically from mice and/or rats to humans, but also from the very high doses usually used in animal experiments to the very low doses that are characteristic of human exposure. These two types of extrapolation are steeped in uncertainty”. Calabrese and Baldwin have proposed a quantitative methodology with scoring criteria for beneficial hormetic effects [183]; it has been applied by others to all NMDR for chemicals [8], modified to include a stepwise decision tree to consider individual NMDR relations in a risk assessment context. This is particularly useful for determining the potential impacts of endocrine disrupting chemicals for which NMDR relations have been experimentally described with relatively higher frequency than for other chemicals [141].
Improving our understanding of these biological consequences is essential for protection of humans and their environment, as it will enable better risk estimates, the revision of standards, and, as some authors suggest, translational applications for new therapies. The neurogenic effects of low-dose radiation have led to the exploration of neurodegenerative disorders. Wei and colleagues, for example, showed that parameters reflecting hippocampal neurogenesis and animal learning both increased in the group exposed to low-dose radiation, but confirmed a detrimental effect on neurogenesis in the high-dose group [105]. An evaluation of the protective mechanisms of low-dose radiation in an animal model of type 2 diabetes-induced kidney injury showed that low-dose radiation attenuates dyslipidemia, inflammation, oxidative stress, and fibrosis—that is, the causes of renal damage in type 2 diabetes [47, 48]. Moreover, the anti-inflammatory effect of low-dose radiation may protect against other inflammatory diseases, such as arthritis symptoms [151]. Single or fractional administration of low-dose radiation also diminishes inflammatory proliferation (cytokines, iNOS) and is associated with improvement in clinical symptoms (cartilage and bone destruction).
Conclusion
This review shows the importance of studying low-dose effects while avoiding extrapolations from high-dose studies, which are not sufficient for estimating the real consequences of low-dose exposures. A better understanding of these low-dose effects is underway, through molecular studies that allow us to identify the pathways responsible for the adaptive mechanisms. DNA repair and antioxidative mechanisms are among the best described pathways involved in adaptive response to exposure to low doses of radiation or chemicals. Their interplay with autophagy, inflammatory responses, and UPR can allow the cells to cope with mild stress through a better functional response leading to a higher likelihood of cell survival. If this protection is insufficient and defense mechanisms are overwhelmed, cell death or neoplastic transformation can be observed. In the coming years, a better understanding of the stress response pathway, molecular pathways of toxicity, and the adverse outcome pathway (AOP) will all be helpful in linking the cellular stress response to organ dysfunction and the risk of an adverse effect on the organism [184].
Acknowledgements
We apologize to the many scientists whose work we were not able to credit due to space restrictions. We thank Joan Francesc Barquinero Estruch (Universitat Autònoma de Barcelona), Klervi Leuraud, Karine Tack and Dominique Laurier (Department of research on the biological and health effects of ionizing radiation, IRSN) for helpful comments and suggestions and Marc Benderitter (Department of research in radiobiology and regenerative medicine, IRSN) for initial discussions on this topic.
Abbreviations
- AhR
Aryl hydrocarbon receptor
- Akt
Protein kinase B
- ARE
Antioxidant responsive element
- ATM
Ataxia telangiectasia mutated
- CDK
Cyclin-dependent kinases
- DSB
Double-strand break
- DNA-PK
DNA-dependent protein kinase
- ER
Endoplasmic reticulum
- ERK
Extracellular signal-regulating kinase
- IR
Ionizing radiation
- JNK
c-Jun N-terminal kinases
- MAPK
Mitogen-activated protein kinase
- miRNA
Micro RNA
- NF-κB
Nuclear factor-kappa B
- Nfr2
NF-E2-related 2 (transcription factor)
- NMDR
Non-monotonic dose-response
- PERK
Protein kinase R-like endoplasmic reticulum kinase
- ROS
Reactive oxygen species
- TGF-β
Transforming growth factor beta
- UPR
Unfolded protein response
- XIAP
X-linked inhibitor of apoptosis
- XRE
Xenobiotic responsive element
Compliance with ethical standards
Conflict of interest
The authors declare they have no conflict of interest.
References
- 1.BEIRVII (2006) Health risks from exposure to low levels of ionizing radiation. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council (U.S.). The National Academies Press [PubMed]
- 2.Acad. Sci. (Paris) (2005) Dose-effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation. Joint Report of the Académie des Sciences (Paris)—Académie Nationale de Médecine [DOI] [PubMed]
- 3.UNSCEAR . Biological mechanisms of radiation actions at low doses—a white paper to guide the Scientific Committee’s future programme of work. New York: United Nations; 2012. [Google Scholar]
- 4.UNSCEAR (2008) United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation. Volume I: Report to the General Assembly, Scientific Annexes A and B; Volume II: Scientific Annexes C, D and E. United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR 2008 Report. United Nations Sales publications E.10.XI.3. United Nations, New York
- 5.Tang FR, Loke WK. Molecular mechanisms of low dose ionizing radiation-induced hormesis, adaptive responses, radioresistance, bystander effects, and genomic instability. Int J Radiat Biol. 2015;91(1):13–27. doi: 10.3109/09553002.2014.937510. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich WF, Paretzke HG, Jacob P. No evidence for increased tumor rates below 200 mSv in the atomic bomb survivors data. Radiat Environ Biophys. 1997;36(3):205–207. doi: 10.1007/s004110050073. [DOI] [PubMed] [Google Scholar]
- 7.Hoel DG, Li P. Threshold models in radiation carcinogenesis. Health Phys. 1998;75(3):241–250. doi: 10.1097/00004032-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Lagarde F, Beausoleil C, Belcher SM, Belzunces LP, Emond C, Guerbet M, Rousselle C. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ Health. 2015;14:13. doi: 10.1186/1476-069X-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223(4636):594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto H, Hamada N, Takahashi A, Kobayashi Y, Ohnishi T. Vanguards of paradigm shift in radiation biology: radiation-induced adaptive and bystander responses. J Radiat Res. 2007;48(2):97–106. doi: 10.1269/jrr.06090. [DOI] [PubMed] [Google Scholar]
- 11.Feinendegen LE, Brooks AL, Morgan WF. Biological consequences and health risks of low-level exposure to ionizing radiation: commentary on the workshop. Health Phys. 2011;100(3):247–259. doi: 10.1097/HP.0b013e31820a83ae. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- 13.Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251(1):13–22. doi: 10.1148/radiol.2511080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pallet N, Anglicheau D, Thervet E. Autophagy is an adaptative mechanism against endoplasmic reticulum stress. Nephrol Dial Transplant. 2009;24(12):3891. doi: 10.1093/ndt/gfp518. [DOI] [PubMed] [Google Scholar]
- 15.Barouki R. Linking long-term toxicity of xeno-chemicals with short-term biological adaptation. Biochimie. 2010;92(9):1222–1226. doi: 10.1016/j.biochi.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Andreau K, Leroux M, Bouharrour A. Health and cellular impacts of air pollutants: from cytoprotection to cytotoxicity. Biochem Res Int. 2012;2012:493894. doi: 10.1155/2012/493894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UNSCEAR (1994) Sources and effects of ionizing radiation, United Nations Scientific Committee on the Effects of Atomic Radiation. Report to the General Assembly, with Scientific Annexes. ANNEX B, Adaptive responses to radiation in cells and organisms. United Nations, New York
- 18.Calabrese EJ, Baldwin LA. The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci. 2001;62(2):330–338. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- 19.Szumiel I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: the pivotal role of mitochondria. Int J Radiat Biol. 2015;91(1):1–12. doi: 10.3109/09553002.2014.934929. [DOI] [PubMed] [Google Scholar]
- 20.Tapio S, Jacob V. Radioadaptive response revisited. Radiat Environ Biophys. 2007;46(1):1–12. doi: 10.1007/s00411-006-0078-8. [DOI] [PubMed] [Google Scholar]
- 21.Rigaud O, Moustacchi E. Radioadaptation for gene mutation and the possible molecular mechanisms of the adaptive response. Mutat Res. 1996;358(2):127–134. doi: 10.1016/S0027-5107(96)00113-3. [DOI] [PubMed] [Google Scholar]
- 22.Sthijns MM, Weseler AR, Bast A, Haenen GR. Time in redox adaptation processes: from evolution to hormesis. Int J Mol Sci. 2016;17(10):1649. doi: 10.3390/ijms17101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabrese EJ. Hormetic mechanisms. Crit Rev Toxicol. 2013;43(7):580–606. doi: 10.3109/10408444.2013.808172. [DOI] [PubMed] [Google Scholar]
- 24.Jennings P, Limonciel A, Felice L, Leonard MO. An overview of transcriptional regulation in response to toxicological insult. Arch Toxicol. 2013;87(1):49–72. doi: 10.1007/s00204-012-0919-y. [DOI] [PubMed] [Google Scholar]
- 25.Wink S, Hiemstra S, Huppelschoten S, Danen E, Niemeijer M, Hendriks G, Vrieling H, Herpers B, van de Water B. Quantitative high content imaging of cellular adaptive stress response pathways in toxicity for chemical safety assessment. Chem Res Toxicol. 2014;27(3):338–355. doi: 10.1021/tx4004038. [DOI] [PubMed] [Google Scholar]
- 26.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28(5):739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki MS, Ejima Y, Tachibana A, Yamada T, Ishizaki K, Shimizu T, Nomura T. DNA damage response pathway in radioadaptive response. Mutat Res. 2002;504(1–2):101–118. doi: 10.1016/S0027-5107(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 28.Nenoi M, Wang B, Vares G. In vivo radioadaptive response: a review of studies relevant to radiation-induced cancer risk. Hum Exp Toxicol. 2015;34(3):272–283. doi: 10.1177/0960327114537537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samson L, Schwartz JL. Evidence for an adaptive DNA repair pathway in CHO and human skin fibroblast cell lines. Nature. 1980;287(5785):861–863. doi: 10.1038/287861a0. [DOI] [PubMed] [Google Scholar]
- 30.Barquinero JF, Barrios L, Caballin MR, Miro R, Ribas M, Subias A, Egozcue J. Occupational exposure to radiation induces an adaptive response in human lymphocytes. Int J Radiat Biol. 1995;67(2):187–191. doi: 10.1080/09553009514550231. [DOI] [PubMed] [Google Scholar]
- 31.Joiner MC, Lambin P, Marples B. Adaptive response and induced resistance. C R Acad Sci III. 1999;322(2–3):167–175. doi: 10.1016/S0764-4469(99)80040-7. [DOI] [PubMed] [Google Scholar]
- 32.Wiencke JK, Afzal V, Olivieri G, Wolff S. Evidence that the [3H]thymidine-induced adaptive response of human lymphocytes to subsequent doses of X-rays involves the induction of a chromosomal repair mechanism. Mutagenesis. 1986;1(5):375–380. doi: 10.1093/mutage/1.5.375. [DOI] [PubMed] [Google Scholar]
- 33.Wolff S. Failla memorial lecture. Is radiation all bad? The search for adaptation. Radiat Res. 1992;131(2):117–123. doi: 10.2307/3578431. [DOI] [PubMed] [Google Scholar]
- 34.Le XC, Xing JZ, Lee J, Leadon SA, Weinfeld M. Inducible repair of thymine glycol detected by an ultrasensitive assay for DNA damage. Science. 1998;280(5366):1066–1069. doi: 10.1126/science.280.5366.1066. [DOI] [PubMed] [Google Scholar]
- 35.Coleman MA, Yin E, Peterson LE, Nelson D, Sorensen K, Tucker JD, Wyrobek AJ. Low-dose irradiation alters the transcript profiles of human lymphoblastoid cells including genes associated with cytogenetic radioadaptive response. Radiat Res. 2005;164(4 Pt 1):369–382. doi: 10.1667/RR3356.1. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi A, Asakawa I, Yuki K, Matsumoto T, Kumamoto M, Kondo N, Ohnishi K, Tachibana A, Ohnishi T. Radiation-induced apoptosis in the scid mouse spleen after low dose-rate irradiation. Int J Radiat Biol. 2002;78(8):689–693. doi: 10.1080/09553000210132306. [DOI] [PubMed] [Google Scholar]
- 37.Nosel I, Vaurijoux A, Barquinero JF, Gruel G. Characterization of gene expression profiles at low and very low doses of ionizing radiation. DNA Repair (Amst) 2013;12(7):508–517. doi: 10.1016/j.dnarep.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Mitchel RE. Adaption by low dose radiation exposure: a look at scope and limitations for radioprotection. Dose Response. 2015 doi: 10.2203/dose-response.14-025.mitchel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Ohnishi T. p53-dependent signal transduction induced by stress. J Radiat Res. 1997;38(3):179–194. doi: 10.1269/jrr.38.179. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu T, Kato T, Jr, Tachibana A, Sasaki MS. Coordinated regulation of radioadaptive response by protein kinase C and p38 mitogen-activated protein kinase. Exp Cell Res. 1999;251(2):424–432. doi: 10.1006/excr.1999.4582. [DOI] [PubMed] [Google Scholar]
- 41.Boreham DR, Mitchel RE. DNA lesions that signal the induction of radioresistance and DNA repair in yeast. Radiat Res. 1991;128(1):19–28. doi: 10.2307/3578062. [DOI] [PubMed] [Google Scholar]
- 42.Iyer R, Lehnert BE. Alpha-particle-induced increases in the radioresistance of normal human bystander cells. Radiat Res. 2002;157(1):3–7. doi: 10.1667/0033-7587(2002)157[0003:APIIIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 43.Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J. 2005;19(9):1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 44.Osburn WO, Kensler TW. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat Res. 2008;659(1–2):31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan NM, Sandur SK, Checker R, Sharma D, Poduval TB, Sainis KB. Pro-oxidants ameliorate radiation-induced apoptosis through activation of the calcium-ERK1/2-Nrf2 pathway. Free Radic Biol Med. 2011;51(1):115–128. doi: 10.1016/j.freeradbiomed.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 46.Mathew ST, Bergstrom P, Hammarsten O. Repeated Nrf2 stimulation using sulforaphane protects fibroblasts from ionizing radiation. Toxicol Appl Pharmacol. 2014;276(3):188–194. doi: 10.1016/j.taap.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Xing X, Zhang C, Shao M, Tong Q, Zhang G, Li C, Cheng J, Jin S, Ma J, Wang G, Li X, Cai L. Low-dose radiation activates Akt and Nrf2 in the kidney of diabetic mice: a potential mechanism to prevent diabetic nephropathy. Oxid Med Cell Longev. 2012;2012:291087. doi: 10.1155/2012/291087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shao M, Lu X, Cong W, Xing X, Tan Y, Li Y, Li X, Jin L, Wang X, Dong J, Jin S, Zhang C, Cai L. Multiple low-dose radiation prevents type 2 diabetes-induced renal damage through attenuation of dyslipidemia and insulin resistance and subsequent renal inflammation and oxidative stress. PLoS One. 2014;9(3):e92574. doi: 10.1371/journal.pone.0092574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C, Xing X, Zhang F, Shao M, Jin S, Yang H, Wang G, Cui J, Cai L, Li W, Lu X. Low-dose radiation induces renal SOD1 expression and activity in type 1 diabetic mice. Int J Radiat Biol. 2014;90(3):224–230. doi: 10.3109/09553002.2014.877174. [DOI] [PubMed] [Google Scholar]
- 50.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12(3):213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 51.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, Yamamoto M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci USA. 2012;109(34):13561–13566. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, Zhang DD. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29(6):1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen N, Wu L, Yuan H, Wang J. ROS/autophagy/Nrf2 pathway mediated low-dose radiation induced radio-resistance in human lung adenocarcinoma A549 cell. Int J Biol Sci. 2015;11(7):833–844. doi: 10.7150/ijbs.10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85(6):705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 56.Ayers D, Baron B, Hunter T. miRNA influences in NRF2 pathway interactions within cancer models. J Nucleic Acids. 2015;2015:143636. doi: 10.1155/2015/143636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner AE, Boesch-Saadatmandi C, Dose J, Schultheiss G, Rimbach G. Anti-inflammatory potential of allyl-isothiocyanate–role of Nrf2, NF-(kappa) B and microRNA-155. J Cell Mol Med. 2012;16(4):836–843. doi: 10.1111/j.1582-4934.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miao W, Hu L, Scrivens PJ, Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem. 2005;280(21):20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 59.Joo MS, Lee CG, Koo JH, Kim SG. miR-125b transcriptionally increased by Nrf2 inhibits AhR repressor, which protects kidney from cisplatin-induced injury. Cell Death Dis. 2013;4:e899. doi: 10.1038/cddis.2013.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayden MS, Ghosh S. NF-kappaB in immunobiology. Cell Res. 2011;21(2):223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prasad AV, Mohan N, Chandrasekar B, Meltz ML. Activation of nuclear factor kappa B in human lymphoblastoid cells by low-dose ionizing radiation. Radiat Res. 1994;138(3):367–372. doi: 10.2307/3578685. [DOI] [PubMed] [Google Scholar]
- 62.Rodel F, Hantschel M, Hildebrandt G, Schultze-Mosgau S, Rodel C, Herrmann M, Sauer R, Voll RE. Dose-dependent biphasic induction and transcriptional activity of nuclear factor kappa B (NF-kappaB) in EA.hy.926 endothelial cells after low-dose X-irradiation. Int J Radiat Biol. 2004;80(2):115–123. doi: 10.1080/09553000310001654701. [DOI] [PubMed] [Google Scholar]
- 63.Rodel F, Frey B, Capalbo G, Gaipl U, Keilholz L, Voll R, Hildebrandt G, Rodel C. Discontinuous induction of X-linked inhibitor of apoptosis in EA.hy.926 endothelial cells is linked to NF-kappaB activation and mediates the anti-inflammatory properties of low-dose ionising-radiation. Radiother Oncol. 2010;97(2):346–351. doi: 10.1016/j.radonc.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 64.Lodermann B, Wunderlich R, Frey S, Schorn C, Stangl S, Rodel F, Keilholz L, Fietkau R, Gaipl US, Frey B. Low dose ionising radiation leads to a NF-kappaB dependent decreased secretion of active IL-1beta by activated macrophages with a discontinuous dose-dependency. Int J Radiat Biol. 2012;88(10):727–734. doi: 10.3109/09553002.2012.689464. [DOI] [PubMed] [Google Scholar]
- 65.Kim CS, Kim JK, Nam SY, Yang KH, Jeong M, Kim HS, Jin YW, Kim J. Low-dose radiation stimulates the proliferation of normal human lung fibroblasts via a transient activation of Raf and Akt. Mol Cells. 2007;24(3):424–430. [PubMed] [Google Scholar]
- 66.Murley JS, Kataoka Y, Weydert CJ, Oberley LW, Grdina DJ. Delayed radioprotection by nuclear transcription factor kappaB-mediated induction of manganese superoxide dismutase in human microvascular endothelial cells after exposure to the free radical scavenger WR1065. Free Radic Biol Med. 2006;40(6):1004–1016. doi: 10.1016/j.freeradbiomed.2005.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park HS, Seong KM, Kim JY, Kim CS, Yang KH, Jin YW, Nam SY. Chronic low-dose radiation inhibits the cells death by cytotoxic high-dose radiation increasing the level of AKT and acinus proteins via NF-kappaB activation. Int J Radiat Biol. 2013;89(5):371–377. doi: 10.3109/09553002.2013.754560. [DOI] [PubMed] [Google Scholar]
- 68.Murley JS, Baker KL, Miller RC, Darga TE, Weichselbaum RR, Grdina DJ. SOD2-mediated adaptive responses induced by low-dose ionizing radiation via TNF signaling and amifostine. Free Radic Biol Med. 2011;51(10):1918–1925. doi: 10.1016/j.freeradbiomed.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lake D, Correa SA, Muller J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell Mol Life Sci. 2016;73(23):4397–4413. doi: 10.1007/s00018-016-2297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26(22):3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 71.Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22(37):5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- 72.Kim CS, Kim JM, Nam SY, Yang KH, Jeong M, Kim HS, Lim YK, Jin YW, Kim J. Low-dose of ionizing radiation enhances cell proliferation via transient ERK1/2 and p38 activation in normal human lung fibroblasts. J Radiat Res. 2007;48(5):407–415. doi: 10.1269/jrr.07032. [DOI] [PubMed] [Google Scholar]
- 73.Park HS, You GE, Yang KH, Kim JY, An S, Song JY, Lee SJ, Lim YK, Nam SY. Role of AKT and ERK pathways in controlling sensitivity to ionizing radiation and adaptive response induced by low-dose radiation in human immune cells. Eur J Cell Biol. 2015;94(12):653–660. doi: 10.1016/j.ejcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Yu H, Liu N, Wang H, Shang Q, Jiang P, Zhang Y. Different responses of tumor and normal cells to low-dose radiation. Contemp Oncol (Pozn) 2013;17(4):356–362. doi: 10.5114/wo.2013.35289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asur R, Balasubramaniam M, Marples B, Thomas RA, Tucker JD. Involvement of MAPK proteins in bystander effects induced by chemicals and ionizing radiation. Mutat Res. 2010;686(1–2):15–29. doi: 10.1016/j.mrfmmm.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 76.Jiang T, Harder B, Rojo de la Vega M, Wong PK, Chapman E, Zhang DD. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88(Pt B):199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23(20):7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, Ron D, Tabas I. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11(12):1473–1480. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol. 2003;109(3):250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 81.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Q, Kang R, Zeh HJ, 3rd, Lotze MT, Tang D. DAMPs and autophagy: cellular adaptation to injury and unscheduled cell death. Autophagy. 2013;9(4):451–458. doi: 10.4161/auto.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scarlatti F, Granata R, Meijer AJ, Codogno P. Does autophagy have a license to kill mammalian cells? Cell Death Differ. 2009;16(1):12–20. doi: 10.1038/cdd.2008.101. [DOI] [PubMed] [Google Scholar]
- 84.Fougeray S, Pallet N. Mechanisms and biological functions of autophagy in diseased and ageing kidneys. Nat Rev Nephrol. 2015;11(1):34–45. doi: 10.1038/nrneph.2014.201. [DOI] [PubMed] [Google Scholar]
- 85.Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176(3):1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vion AC, Kheloufi M, Hammoutene A, Poisson J, Lasselin J, Devue C, Pic I, Dupont N, Busse J, Stark K, Lafaurie-Janvore J, Barakat AI, Loyer X, Souyri M, Viollet B, Julia P, Tedgui A, Codogno P, Boulanger CM, Rautou PE. Autophagy is required for endothelial cell alignment and atheroprotection under physiological blood flow. Proc Natl Acad Sci USA. 2017;114(41):E8675–E8684. doi: 10.1073/pnas.1702223114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61(2):439–444. [PubMed] [Google Scholar]
- 88.Paglin S, Lee NY, Nakar C, Fitzgerald M, Plotkin J, Deuel B, Hackett N, McMahill M, Sphicas E, Lampen N, Yahalom J. Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res. 2005;65(23):11061–11070. doi: 10.1158/0008-5472.CAN-05-1083. [DOI] [PubMed] [Google Scholar]
- 89.Paglin S, Yahalom J. Pathways that regulate autophagy and their role in mediating tumor response to treatment. Autophagy. 2006;2(4):291–293. doi: 10.4161/auto.2835. [DOI] [PubMed] [Google Scholar]
- 90.Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies? Autophagy. 2009;5(4):442–450. doi: 10.4161/auto.5.4.7667. [DOI] [PubMed] [Google Scholar]
- 91.Kim H, Bernard ME, Flickinger J, Epperly MW, Wang H, Dixon TM, Shields D, Houghton F, Zhang X, Greenberger JS. The autophagy-inducing drug carbamazepine is a radiation protector and mitigator. Int J Radiat Biol. 2011;87(10):1052–1060. doi: 10.3109/09553002.2011.587860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chargui A, Zekri S, Jacquillet G, Rubera I, Ilie M, Belaid A, Duranton C, Tauc M, Hofman P, Poujeol P, El May MV, Mograbi B. Cadmium-induced autophagy in rat kidney: an early biomarker of subtoxic exposure. Toxicol Sci. 2011;121(1):31–42. doi: 10.1093/toxsci/kfr031. [DOI] [PubMed] [Google Scholar]
- 93.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 94.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 95.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8(8):581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 96.Zhang RF, Wang Q, Zhang AA, Xu JG, Zhai LD, Yang XM, Liu XT. Low-level laser irradiation promotes the differentiation of bone marrow stromal cells into osteoblasts through the APN/Wnt/beta-catenin pathway. Eur Rev Med Pharmacol Sci. 2018;22(9):2860–2868. doi: 10.26355/eurrev_201805_14988. [DOI] [PubMed] [Google Scholar]
- 97.Alexandrou AT, Li JJ. Cell cycle regulators guide mitochondrial activity in radiation-induced adaptive response. Antioxid Redox Signal. 2014;20(9):1463–1480. doi: 10.1089/ars.2013.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maes OC, An J, Sarojini H, Wu H, Wang E. Changes in MicroRNA expression patterns in human fibroblasts after low-LET radiation. J Cell Biochem. 2008;105(3):824–834. doi: 10.1002/jcb.21878. [DOI] [PubMed] [Google Scholar]
- 99.Kern P, Keilholz L, Forster C, Seegenschmiedt MH, Sauer R, Herrmann M. In vitro apoptosis in peripheral blood mononuclear cells induced by low-dose radiotherapy displays a discontinuous dose-dependence. Int J Radiat Biol. 1999;75(8):995–1003. doi: 10.1080/095530099139755. [DOI] [PubMed] [Google Scholar]
- 100.Kern PM, Keilholz L, Forster C, Stach C, Beyer TD, Gaipl US, Kalden JR, Hallmann R, Herrmann M. UVB-irradiated T-cells undergoing apoptosis lose L-selectin by metalloprotese-mediated shedding. Int J Radiat Biol. 2000;76(9):1265–1271. doi: 10.1080/09553000050134492. [DOI] [PubMed] [Google Scholar]
- 101.Gaipl US, Meister S, Lodermann B, Rodel F, Fietkau R, Herrmann M, Kern PM, Frey B. Activation-induced cell death and total Akt content of granulocytes show a biphasic course after low-dose radiation. Autoimmunity. 2009;42(4):340–342. doi: 10.1080/08916930902831233. [DOI] [PubMed] [Google Scholar]
- 102.Candas D, Fan M, Nantajit D, Vaughan AT, Murley JS, Woloschak GE, Grdina DJ, Li JJ. CyclinB1/Cdk1 phosphorylates mitochondrial antioxidant MnSOD in cell adaptive response to radiation stress. J Mol Cell Biol. 2013;5(3):166–175. doi: 10.1093/jmcb/mjs062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Amundson SA, Lee RA, Koch-Paiz CA, Bittner ML, Meltzer P, Trent JM, Fornace AJ., Jr Differential responses of stress genes to low dose-rate gamma irradiation. Mol Cancer Res. 2003;1(6):445–452. [PubMed] [Google Scholar]
- 104.Azimian H, Bahreyni-Toossi MT, Rezaei AR, Rafatpanah H, Hamzehloei T, Fardid R. Up-regulation of Bcl-2 expression in cultured human lymphocytes after exposure to low doses of gamma radiation. J Med Phys. 2015;40(1):38–44. doi: 10.4103/0971-6203.152249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei LC, Ding YX, Liu YH, Duan L, Bai Y, Shi M, Chen LW. Low-dose radiation stimulates Wnt/beta-catenin signaling, neural stem cell proliferation and neurogenesis of the mouse hippocampus in vitro and in vivo. Curr Alzheimer Res. 2012;9(3):278–289. doi: 10.2174/156720512800107627. [DOI] [PubMed] [Google Scholar]
- 106.Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006;18(4):444–452. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 107.Pallet N, Fougeray S, Beaune P, Legendre C, Thervet E, Anglicheau D. Endoplasmic reticulum stress: an unrecognized actor in solid organ transplantation. Transplantation. 2009;88(5):605–613. doi: 10.1097/TP.0b013e3181b22cec. [DOI] [PubMed] [Google Scholar]
- 108.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110(10):1389–1398. doi: 10.1172/JCI0216886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kitamura M. Endoplasmic reticulum stress in the kidney. Clin Exp Nephrol. 2008;12(5):317–325. doi: 10.1007/s10157-008-0060-7. [DOI] [PubMed] [Google Scholar]
- 110.La Rovere RM, Roest G, Bultynck G, Parys JB. Intracellular Ca(2+) signaling and Ca(2+) microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium. 2016;60(2):74–87. doi: 10.1016/j.ceca.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 111.Shinkai Y, Kaji T. Cellular defense mechanisms against lead toxicity in the vascular system. Biol Pharm Bull. 2012;35(11):1885–1891. doi: 10.1248/bpb.b212018. [DOI] [PubMed] [Google Scholar]
- 112.Peyrou M, Cribb AE. Effect of endoplasmic reticulum stress preconditioning on cytotoxicity of clinically relevant nephrotoxins in renal cell lines. Toxicol In Vitro. 2007;21(5):878–886. doi: 10.1016/j.tiv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 113.Qian Y, Falahatpisheh MH, Zheng Y, Ramos KS, Tiffany-Castiglioni E. Induction of 78 kD glucose-regulated protein (GRP78) expression and redox-regulated transcription factor activity by lead and mercury in C6 rat glioma cells. Neurotox Res. 2001;3(6):581–589. doi: 10.1007/BF03033212. [DOI] [PubMed] [Google Scholar]
- 114.Chandrika BB, Yang C, Ou Y, Feng X, Muhoza D, Holmes AF, Theus S, Deshmukh S, Haun RS, Kaushal GP. Endoplasmic reticulum stress-induced autophagy provides cytoprotection from chemical hypoxia and oxidant injury and ameliorates renal ischemia-reperfusion injury. PLoS One. 2015;10(10):e0140025. doi: 10.1371/journal.pone.0140025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qian Y, Harris ED, Zheng Y, Tiffany-Castiglioni E. Lead targets GRP78, a molecular chaperone, in C6 rat glioma cells. Toxicol Appl Pharmacol. 2000;163(3):260–266. doi: 10.1006/taap.1999.8878. [DOI] [PubMed] [Google Scholar]
- 116.Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52(22):6394–6396. [PubMed] [Google Scholar]
- 117.Hamada N, Matsumoto H, Hara T, Kobayashi Y. Intercellular and intracellular signaling pathways mediating ionizing radiation-induced bystander effects. J Radiat Res. 2007;48(2):87–95. doi: 10.1269/jrr.06084. [DOI] [PubMed] [Google Scholar]
- 118.Mothersill C, Seymour C. Radiation-induced bystander effects and adaptive responses–the Yin and Yang of low dose radiobiology? Mutat Res. 2004;568(1):121–128. doi: 10.1016/j.mrfmmm.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 119.Iyer R, Lehnert BE. Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat Res. 2002;503(1–2):1–9. doi: 10.1016/S0027-5107(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 120.Nobler MP. The abscopal effect in malignant lymphoma and its relationship to lymphocyte circulation. Radiology. 1969;93(2):410–412. doi: 10.1148/93.2.410. [DOI] [PubMed] [Google Scholar]
- 121.Xue LY, Butler NJ, Makrigiorgos GM, Adelstein SJ, Kassis AI. Bystander effect produced by radiolabeled tumor cells in vivo. Proc Natl Acad Sci USA. 2002;99(21):13765–13770. doi: 10.1073/pnas.182209699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kassis AI. In vivo validation of the bystander effect. Hum Exp Toxicol. 2004;23(2):71–73. doi: 10.1191/0960327104ht420oa. [DOI] [PubMed] [Google Scholar]
- 123.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res. 2003;159(5):581–596. doi: 10.1667/0033-7587(2003)159[0581:NADEOE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 124.Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res. 1998;150(5):497–504. doi: 10.2307/3579865. [DOI] [PubMed] [Google Scholar]
- 125.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA. 2001;98(2):473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iyer R, Lehnert BE, Svensson R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60(5):1290–1298. [PubMed] [Google Scholar]
- 127.Mothersill C, Seymour CB. Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res. 1998;149(3):256–262. doi: 10.2307/3579958. [DOI] [PubMed] [Google Scholar]
- 128.Portess DI, Bauer G, Hill MA, O’Neill P. Low-dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res. 2007;67(3):1246–1253. doi: 10.1158/0008-5472.CAN-06-2985. [DOI] [PubMed] [Google Scholar]
- 129.Han W, Zhu L, Jiang E, Wang J, Chen S, Bao L, Zhao Y, Xu A, Yu Z, Wu L. Elevated sodium chloride concentrations enhance the bystander effects induced by low dose alpha-particle irradiation. Mutat Res. 2007;624(1–2):124–131. doi: 10.1016/j.mrfmmm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 130.Ma S, Liu X, Jiao B, Yang Y. Low-dose radiation-induced responses: focusing on epigenetic regulation. Int J Radiat Biol. 2010;86(7):517–528. doi: 10.3109/09553001003734592. [DOI] [PubMed] [Google Scholar]
- 131.Raiche J, Rodriguez-Juarez R, Pogribny I, Kovalchuk O. Sex- and tissue-specific expression of maintenance and de novo DNA methyltransferases upon low dose X-irradiation in mice. Biochem Biophys Res Commun. 2004;325(1):39–47. doi: 10.1016/j.bbrc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 132.Pogribny I, Raiche J, Slovack M, Kovalchuk O. Dose-dependence, sex- and tissue-specificity, and persistence of radiation-induced genomic DNA methylation changes. Biochem Biophys Res Commun. 2004;320(4):1253–1261. doi: 10.1016/j.bbrc.2004.06.081. [DOI] [PubMed] [Google Scholar]
- 133.Dickey JS, Zemp FJ, Martin OA, Kovalchuk O. The role of miRNA in the direct and indirect effects of ionizing radiation. Radiat Environ Biophys. 2011;50(4):491–499. doi: 10.1007/s00411-011-0386-5. [DOI] [PubMed] [Google Scholar]
- 134.Xu S, Ding N, Pei H, Hu W, Wei W, Zhang X, Zhou G, Wang J. MiR-21 is involved in radiation-induced bystander effects. RNA Biol. 2014;11(9):1161–1170. doi: 10.4161/rna.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu S, Wang J, Ding N, Hu W, Zhang X, Wang B, Hua J, Wei W, Zhu Q. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol. 2015;12(12):1355–1363. doi: 10.1080/15476286.2015.1100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Asur RS, Thomas RA, Tucker JD. Chemical induction of the bystander effect in normal human lymphoblastoid cells. Mutat Res. 2009;676(1–2):11–16. doi: 10.1016/j.mrgentox.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 137.Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67(8):3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 138.Demidem A, Morvan D, Madelmont JC. Bystander effects are induced by CENU treatment and associated with altered protein secretory activity of treated tumor cells: a relay for chemotherapy? Int J Cancer. 2006;119(5):992–1004. doi: 10.1002/ijc.21761. [DOI] [PubMed] [Google Scholar]
- 139.Rugo RE, Almeida KH, Hendricks CA, Jonnalagadda VS, Engelward BP. A single acute exposure to a chemotherapeutic agent induces hyper-recombination in distantly descendant cells and in their neighbors. Oncogene. 2005;24(32):5016–5025. doi: 10.1038/sj.onc.1208690. [DOI] [PubMed] [Google Scholar]
- 140.Klaassen CD, Liu J. Metallothionein transgenic and knock-out mouse models in the study of cadmium toxicity. J Toxicol Sci. 1998;23(Suppl 2):97–102. doi: 10.2131/jts.23.SupplementII_97. [DOI] [PubMed] [Google Scholar]
- 141.Myers JP, Zoeller RT, vom Saal FS. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ Health Perspect. 2009;117(11):1652–1655. doi: 10.1289/ehp.0900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nair AR, Lee WK, Smeets K, Swennen Q, Sanchez A, Thevenod F, Cuypers A. Glutathione and mitochondria determine acute defense responses and adaptive processes in cadmium-induced oxidative stress and toxicity of the kidney. Arch Toxicol. 2015;89(12):2273–2289. doi: 10.1007/s00204-014-1401-9. [DOI] [PubMed] [Google Scholar]
- 143.Poisson C, Stefani J, Manens L, Delissen O, Suhard D, Tessier C, Dublineau I, Gueguen Y. Chronic uranium exposure dose-dependently induces glutathione in rats without any nephrotoxicity. Free Radic Res. 2014;48(10):1218–1231. doi: 10.3109/10715762.2014.945441. [DOI] [PubMed] [Google Scholar]
- 144.Gueguen Y, Rouas C, Monin A, Manens L, Stefani J, Delissen O, Grison S, Dublineau I. Molecular, cellular, and tissue impact of depleted uranium on xenobiotic-metabolizing enzymes. Arch Toxicol. 2014;88(2):227–239. doi: 10.1007/s00204-013-1145-y. [DOI] [PubMed] [Google Scholar]
- 145.Korashy HM, El-Kadi AO. Transcriptional regulation of the NAD(P)H:quinone oxidoreductase 1 and glutathione S-transferase ya genes by mercury, lead, and copper. Drug Metab Dispos. 2006;34(1):152–165. doi: 10.1124/dmd.105.005397. [DOI] [PubMed] [Google Scholar]
- 146.Kataoka T. Study of antioxidative effects and anti-inflammatory effects in mice due to low-dose X-irradiation or radon inhalation. J Radiat Res. 2013;54(4):587–596. doi: 10.1093/jrr/rrs141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kojima S, Nakayama K, Ishida H. Low dose gamma-rays activate immune functions via induction of glutathione and delay tumor growth. J Radiat Res. 2004;45(1):33–39. doi: 10.1269/jrr.45.33. [DOI] [PubMed] [Google Scholar]
- 148.Nomura T, Yamaoka K. Low-dose gamma-ray irradiation reduces oxidative damage induced by CCl4 in mouse liver. Free Radic Biol Med. 1999;27(11–12):1324–1333. doi: 10.1016/S0891-5849(99)00180-X. [DOI] [PubMed] [Google Scholar]
- 149.Bravard A, Luccioni C, Moustacchi E, Rigaud O. Contribution of antioxidant enzymes to the adaptive response to ionizing radiation of human lymphoblasts. Int J Radiat Biol. 1999;75(5):639–645. doi: 10.1080/095530099140285. [DOI] [PubMed] [Google Scholar]
- 150.Cui J, Yang G, Pan Z, Zhao Y, Liang X, Li W, Cai L. Hormetic response to low-dose radiation: focus on the immune system and its clinical implications. Int J Mol Sci. 2017;18(2):280. doi: 10.3390/ijms18020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rodel F, Frey B, Gaipl U, Keilholz L, Fournier C, Manda K, Schollnberger H, Hildebrandt G, Rodel C. Modulation of inflammatory immune reactions by low-dose ionizing radiation: molecular mechanisms and clinical application. Curr Med Chem. 2012;19(12):1741–1750. doi: 10.2174/092986712800099866. [DOI] [PubMed] [Google Scholar]
- 152.Scott BR. Radiation-hormesis phenotypes, the related mechanisms and implications for disease prevention and therapy. J Cell Commun Signal. 2014;8(4):341–352. doi: 10.1007/s12079-014-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ina Y, Sakai K. Activation of immunological network by chronic low-dose-rate irradiation in wild-type mouse strains: analysis of immune cell populations and surface molecules. Int J Radiat Biol. 2005;81(10):721–729. doi: 10.1080/09553000500519808. [DOI] [PubMed] [Google Scholar]
- 154.Luckey TD. Physiological benefits from low levels of ionizing radiation. Health Phys. 1982;43(6):771–789. doi: 10.1097/00004032-198212000-00001. [DOI] [PubMed] [Google Scholar]
- 155.Bogdandi EN, Balogh A, Felgyinszki N, Szatmari T, Persa E, Hildebrandt G, Safrany G, Lumniczky K. Effects of low-dose radiation on the immune system of mice after total-body irradiation. Radiat Res. 2010;174(4):480–489. doi: 10.1667/RR2160.1. [DOI] [PubMed] [Google Scholar]
- 156.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schakel K, Garbi N, Jager D, Weitz J, Schmitz-Winnenthal H, Hammerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 157.Wunderlich R, Ernst A, Rodel F, Fietkau R, Ott O, Lauber K, Frey B, Gaipl US. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin Exp Immunol. 2015;179(1):50–61. doi: 10.1111/cei.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vieira Dias J, Gloaguen C, Kereselidze D, Manens L, Tack K, Ebrahimian TG. Gamma low-dose-rate ionizing radiation stimulates adaptive functional and molecular response in human aortic endothelial cells in a threshold-, dose-, and dose rate-dependent manner. Dose Response. 2018;16(1):1559325818755238. doi: 10.1177/1559325818755238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Budras KD, Hartung K, Munzer BM. Light and electron microscopy studies of the effect of roentgen irradiation on the synovial membrane of the inflamed knee joint. Berl Munch Tierarztl Wochenschr. 1986;99(5):148–152. [PubMed] [Google Scholar]
- 160.Pandey R, Shankar BS, Sharma D, Sainis KB. Low dose radiation induced immunomodulation: effect on macrophages and CD8+ T cells. Int J Radiat Biol. 2005;81(11):801–812. doi: 10.1080/09553000500531886. [DOI] [PubMed] [Google Scholar]
- 161.Trott KR, Parker R, Seed MP. The effect of x-rays on experimental arthritis in the rat. Strahlenther Onkol. 1995;171(9):534–538. [PubMed] [Google Scholar]
- 162.Hildebrandt G, Radlingmayr A, Rosenthal S, Rothe R, Jahns J, Hindemith M, Rodel F, Kamprad F. Low-dose radiotherapy (LD-RT) and the modulation of iNOS expression in adjuvant-induced arthritis in rats. Int J Radiat Biol. 2003;79(12):993–1001. doi: 10.1080/09553000310001636639. [DOI] [PubMed] [Google Scholar]
- 163.Schaue D, Jahns J, Hildebrandt G, Trott KR. Radiation treatment of acute inflammation in mice. Int J Radiat Biol. 2005;81(9):657–667. doi: 10.1080/09553000500385556. [DOI] [PubMed] [Google Scholar]
- 164.Nakatsukasa H, Tsukimoto M, Ohshima Y, Tago F, Masada A, Kojima S. Suppressing effect of low-dose gamma-ray irradiation on collagen-induced arthritis. J Radiat Res. 2008;49(4):381–389. doi: 10.1269/jrr.08002. [DOI] [PubMed] [Google Scholar]
- 165.Zaiss MM, Frey B, Hess A, Zwerina J, Luther J, Nimmerjahn F, Engelke K, Kollias G, Hunig T, Schett G, David JP. Regulatory T cells protect from local and systemic bone destruction in arthritis. J Immunol. 2010;184(12):7238–7246. doi: 10.4049/jimmunol.0903841. [DOI] [PubMed] [Google Scholar]
- 166.Frey B, Gaipl US, Sarter K, Zaiss MM, Stillkrieg W, Rodel F, Schett G, Herrmann M, Fietkau R, Keilholz L. Whole body low dose irradiation improves the course of beginning polyarthritis in human TNF-transgenic mice. Autoimmunity. 2009;42(4):346–348. doi: 10.1080/08916930902831738. [DOI] [PubMed] [Google Scholar]
- 167.Aunapuu M, Pechter U, Gerskevits E, Marjamagi MM, Suuroja S, Arend A, Kolts I, Kuhnel W, Ots M. Low-dose radiation modifies the progression of chronic renal failure. Ann Anat. 2004;186(3):277–282. doi: 10.1016/S0940-9602(04)80017-7. [DOI] [PubMed] [Google Scholar]
- 168.Pathak CM, Avti PK, Kumar S, Khanduja KL, Sharma SC. Whole body exposure to low-dose gamma radiation promotes kidney antioxidant status in Balb/c mice. J Radiat Res. 2007;48(2):113–120. doi: 10.1269/jrr.06063. [DOI] [PubMed] [Google Scholar]
- 169.Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83(6):1029–1041. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yoh K, Itoh K, Enomoto A, Hirayama A, Yamaguchi N, Kobayashi M, Morito N, Koyama A, Yamamoto M, Takahashi S. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60(4):1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 171.Yoh K, Hirayama A, Ishizaki K, Yamada A, Takeuchi M, Yamagishi S, Morito N, Nakano T, Ojima M, Shimohata H, Itoh K, Takahashi S, Yamamoto M. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells. 2008;13(11):1159–1170. doi: 10.1111/j.1365-2443.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 172.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 173.Farooque A, Mathur R, Verma A, Kaul V, Bhatt AN, Adhikari JS, Afrin F, Singh S, Dwarakanath BS. Low-dose radiation therapy of cancer: role of immune enhancement. Expert Rev Anticancer Ther. 2011;11(5):791–802. doi: 10.1586/era.10.217. [DOI] [PubMed] [Google Scholar]
- 174.Weng L, Williams RO, Vieira PL, Screaton G, Feldmann M, Dazzi F. The therapeutic activity of low-dose irradiation on experimental arthritis depends on the induction of endogenous regulatory T cell activity. Ann Rheum Dis. 2010;69(8):1519–1526. doi: 10.1136/ard.2009.121111. [DOI] [PubMed] [Google Scholar]
- 175.Ebrahimian T, Le Gallic C, Stefani J, Dublineau I, Yentrapalli R, Harms-Ringdahl M, Haghdoost S. Chronic gamma-irradiation induces a dose-rate-dependent pro-inflammatory response and associated loss of function in human umbilical vein endothelial cells. Radiat Res. 2015;183(4):447–454. doi: 10.1667/RR13732.1. [DOI] [PubMed] [Google Scholar]
- 176.Hoving S, Heeneman S, Gijbels MJ, te Poele JA, Russell NS, Daemen MJ, Stewart FA. Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE(−/−) mice. Int J Radiat Oncol Biol Phys. 2008;71(3):848–857. doi: 10.1016/j.ijrobp.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 177.AGIR (2010) Report of the independent Advisory Group on Ionising Radiation—Circulatory Disease Risk. Documents of the Health Protection Agency. Radiation, Chemical and Environmental Hazards: Chilton, Doc HPA, RCE-16, 1-116
- 178.Le Gallic C, Phalente Y, Manens L, Dublineau I, Benderitter M, Gueguen Y, Lehoux S, Ebrahimian TG. Chronic internal exposure to low dose 137Cs induces positive impact on the stability of atherosclerotic plaques by reducing inflammation in ApoE−/− mice. PLoS One. 2015;10(6):e0128539. doi: 10.1371/journal.pone.0128539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mitchel RE, Hasu M, Bugden M, Wyatt H, Little MP, Gola A, Hildebrandt G, Priest ND, Whitman SC. Low-dose radiation exposure and atherosclerosis in ApoE(−)/(−) mice. Radiat Res. 2011;175(5):665–676. doi: 10.1667/RR2176.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang C, Jin S, Guo W, Li C, Li X, Rane MJ, Wang G, Cai L. Attenuation of diabetes-induced cardiac inflammation and pathological remodeling by low-dose radiation. Radiat Res. 2011;175(3):307–321. doi: 10.1667/RR1950.1. [DOI] [PubMed] [Google Scholar]
- 181.Chen W, Xu X, Bai L, Padilla MT, Gott KM, Leng S, Tellez CS, Wilder JA, Belinsky SA, Scott BR, Lin Y. Low-dose gamma-irradiation inhibits IL-6 secretion from human lung fibroblasts that promotes bronchial epithelial cell transformation by cigarette-smoke carcinogen. Carcinogenesis. 2012;33(7):1368–1374. doi: 10.1093/carcin/bgs159. [DOI] [PubMed] [Google Scholar]
- 182.Bruce VR, Belinsky SA, Gott K, Liu Y, March T, Scott B, Wilder J. Low-dose gamma-radiation inhibits benzo[a]pyrene-induced lung adenoma development in A/J mice. Dose Response. 2012;10(4):516–526. doi: 10.2203/dose-response.12-040.Bruce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Calabrese EJ, Baldwin LA, Kostecki PT, Potter TL. A toxicologically based weight-of-evidence methodology for the relative ranking of chemicals of endocrine disruption potential. Regul Toxicol Pharmacol. 1997;26(1 Pt 1):36–40. doi: 10.1006/rtph.1997.1115. [DOI] [PubMed] [Google Scholar]
- 184.Miller MF, Goodson WH, Manjili MH, Kleinstreuer N, Bisson WH, Lowe L. Low-dose mixture hypothesis of carcinogenesis workshop: scientific underpinnings and research recommendations. Environ Health Perspect. 2017;125(2):163–169. doi: 10.1289/EHP411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dimova EG, Bryant PE, Chankova SG. Adaptive response: some underlying mechanisms and open questions. Genet Mol Biol. 2008;31:396–408. doi: 10.1590/S1415-47572008000300002. [DOI] [Google Scholar]