Abstract
Loss of functional cardiomyocytes is a major underlying mechanism for myocardial remodeling and heart diseases, due to the limited regenerative capacity of adult myocardium. Apoptosis, programmed necrosis, and autophagy contribute to loss of cardiac myocytes that control the balance of cardiac cell death and cell survival through multiple intricate signaling pathways. In recent years, non-coding RNAs (ncRNAs) have received much attention to uncover their roles in cell death of cardiovascular diseases, such as myocardial infarction, cardiac hypertrophy, and heart failure. In addition, based on the view that mitochondrial morphology is linked to three types of cell death, ncRNAs are able to regulate mitochondrial fission/fusion of cardiomyocytes by targeting genes involved in cell death pathways. This review focuses on recent progress regarding the complex relationship between apoptosis/necrosis/autophagy and ncRNAs in the context of myocardial cell death in response to stress. This review also provides insight into the treatment for heart diseases that will guide novel therapies in the future.
Keywords: miRNAs, Cardiomyocyte death, Apoptosis, Necrosis, Autophagy, Heart diseases
Introduction
Heart diseases remain serious problems that lead to increasing morbidity and mortality globally. Given that mammalian heart has limited regenerative capacity, the maintenance of cardiac homeostasis is particularly important. Excessive cell death in the heart has been implicated to be a critical factor resulting in many cardiovascular diseases [1, 2], such as cardiac hypertrophy, coronary artery disease, myocardial infarction (MI), and heart failure. The loss of cardiomyocytes involves a variety of cell death/survival processes, including apoptosis, necrosis, and autophagy, which are regulated by different pathways when the heart faces stress stimuli, intracellular biochemical components, and so on [3]. Over the last few decades, the regulatory mechanisms of cardiomyocyte death have been explored in order to improve the function of the failing myocardium. As a primary source for ATP and reactive oxygen species production in cardiomyocytes [4], the morphology of mitochondria is tightly linked with the cell death processes.
In recent years, growing number of studies indicate that non-coding RNAs (ncRNAs), especially microRNAs (miRNAs), are associated with cell death signaling, and they play essential roles in initiation and progression of heart diseases [5–7]. NcRNAs are a class of RNAs, which originate from genome that generally not translated into proteins. They are classified into two subgroups according to their length: long ncRNAs contain more than 200 nucleotides (lncRNAs); and short ncRNAs (<200 nucleotides), including miRNAs, piwi-interacting RNAs (piRNAs), short interfering RNAs (siRNAs), and others [8]. Among them, miRNAs is a class of small (22–24 nt) and highly conserved ncRNAs, which bind to the 3′ or 5′ untranslated regions (UTRs) of mRNA through complementary base pairing, and thus mediates posttranscriptional gene silencing by inhibiting protein translation or targeting mRNA degradation [9, 10]. The biogenesis of miRNA has been reviewed in detail by Berezikov and Graves et al. [11, 12]. Increasing evidences have widely revealed that many miRNAs are involved in the regulation of cardiomyocyte death pathways during heart diseases progression. Based on these findings, many miRNA-based therapeutic strategies have been directed towards reducing the extent of heart injury and improving the function of failing myocardium. However, the knowledge on contribution of other types of ncRNAs in regulating cell death in cardiovascular diseases remains fairly limited so far. In this review, we highlight the role of ncRNAs in regulating various myocardial cell death pathways that control cardiac physiological and pathological balance, and the potential therapeutic approaches for regulation of ncRNAs in heart diseases are also discussed.
NcRNAs in apoptosis-related cardiovascular diseases
The cell death network comprises of three different modes: apoptosis, autophagy, and necrosis [13, 14]. Among them, apoptosis, also referred as programmed cell death, is a highly conserved and regulated process that is relatively well defined [14]. Apoptosis is activated through two distinct pathways, the extrinsic death receptor pathway and intrinsic mitochondrial pathway, and followed by the structural changes, such as cell shrinkage, plasma membrane blebbing, nuclear condensation, and DNA fragmentation leads to complete demolition of the cell [15].
The extrinsic pathway of apoptosis is mediated via the death receptor (e.g., Fas, TNFR) located on the plasma membrane, where these receptors bind to their ligands (e.g., FasL, TNF-α) to form the death-inducing complex (DISC) that activates caspase-8. The activation of caspase-8 then leads to activation of downstream effector caspase-3 and caspase-7, which degrade proteome and drive cells to death [16]. On the other hand, the intracellular stress signals (e.g., hypoxia, acidosis, oxidative stress, DNA damage) stimulate the pro-apoptotic Bax and Bak in the outer membrane of mitochondria to release cytochrome c into the cytosol [17]. In turn, the downstream caspase-9 and its effector caspase-3 and caspase-7 are activated [18]. In the intrinsic mitochondrial pathway, the changes in the morphology of mitochondria can influence the apoptosis process [19].
Studies have implicated that apoptotic cell death contributes to cardiac pathologies that are associated with disorder of gene expression [20]. In recent years, abundant of miRNAs have been identified as an important contributor in the cell death network during the pathogenesis of myocardial disorders (Table 1). For instance, miR-1 is down-regulated in human infarcted hearts [21], and overexpression of miR-1 in cardiomyocytes can increase apoptosis under oxidative stress by targeting and reducing anti-apoptotic Bcl-2 [22]. In addition, the knockdown of miR-1 can suppress the cardiac arrhythmias [23]. It is well known that miR-133 can antagonize apoptosis triggered by H2O2 by negatively regulating caspase-9 expression [24], while overexpression of miR-133a protects heart against myocardial fibrosis [25]. A recent study found that miR-181c is involved in TNF-α-induced apoptosis and leads to heart failure by targeting Bcl-2 [26]. Besides, miR-21, miR-30, miR-199a, and miR-320 positively modulate the apoptotic program of heart by targeting different apoptosis-related proteins [27–30]. Recently, Wang et al. demonstrated that reactive oxygen species (ROS) can oxidatively modify miR-184, and the modified miR-184 misrecognizes Bcl-xL and Bcl-w, which are not its native targets [31]. Further experiments in mouse model of MI proved that the mismatch of oxidized miR-184 with Bcl-xL and Bcl-w contributes to ROS-induced lesions in myocardial tissue.
Table 1.
Summary of ncRNAs linked to apoptosis in CVDs
| ncRNAs | Targets/CVDs | Pro- or anti-apoptosis | References |
|---|---|---|---|
| miR-1 | Bcl-2/MI | Promotes apoptosis | [22] |
| miR-133a | Caspase-9/MI | Inhibits apoptosis | [24] |
| miR-17-92 cluster | STAT3/MI, HF | Induces apoptosis | [63, 64] |
| miR-21 | Bcl-2/MI | Anti-apoptotic | [65] |
| miR-30 | P53/MI | Anti-apoptotic | [35] |
| miR-199a | Hif1α, Sirt1/MI | Anti-apoptotic | [29] |
| miR-320 | Hsp20/MI | Pro-apoptotic | [30] |
| miR-149 | Puma/MI | Decreases apoptosis | [66] |
| miR-761 | Mff/MI | Promotes apoptosis | [67] |
| miR-499 | Calcineurin/MI | Inhibits apoptosis | [68] |
| miR-214 | Pten/MI | Anti-apoptotic | [69] |
| miR-145 | Bnip3/MI | Decreases apoptosis | [70] |
| miR-378 | Caspase-3/MI | Anti-apoptotic | [71] |
| miR-195 | Sirt1/lipotoxic cardiomyopathy | Pro-apoptotic | [72] |
| miR-34a | Sirt1/coronary artery disease | Promotes apoptosis | [73] |
| miR-184 | Bcl-xL, Bcl-w/MI | Pro-apoptotic | [31] |
| miR-140 | Mfn1/MI | Induces apoptosis | [38] |
| miR-421 | Pink1/MI | Promotes apoptosis | [4] |
| miR-324-5p | Mtfr 1/MI | Anti-apoptotic | [42] |
| miR-361 | Phb1/MI | Pro-apoptotic | [41] |
| miR-208a | GATA4/DOX cardiotoxicity | Promotes apoptosis | [45] |
| miR-532-3p | ARC/DOX cardiotoxicity | Pro-apoptosis | [46] |
| LncRNA-CARL | miR-539, Phb2/MI | Inhibits apoptosis | [56] |
| CircRNA-HRCR | miR-223, arc/cardiac hypertrophy | Anti-apoptotic | [60] |
The mitochondrial apoptotic pathway is under the control of multiple miRNAs in the heart. It is well known that mitochondria constantly undergo fusion and fission in response to the changes coming from surrounding environments. In fact, mitochondrial fusion and fission are able to inhibit or trigger apoptosis in cardiomyocytes, respectively [32, 33]. Emerging evidences demonstrate that mitochondrial dynamics regulate cardiac function and susceptibility to injury [15]. For instance, the activation of dynamin-related protein-1 (Drp1) is required for mitochondrial fission during apoptosis [34], and knockdown of Drp1 in vivo can reduce infarct size after ischemia/reperfusion injury in adult mice [19]. In addition, miR-30 family regulates mitochondrial apoptotic pathway by suppressing the expression of p53, which targets Drp1 [35]. Wang et al. reported that miR-499 levels are down-regulated in the area at risk for ischemic injury in the heart, and overexpression of miR-499 can decrease the severity of MI through inhibiting the calcineurin-mediated dephosphorylation of Drp1 [36]. Mitofusion1 (Mfn1) is one of the mitochondrial dynamics-related protein that can prevent mitochondrial fission and cell death in cardiomyocytes [37]. In the mouse model of MI, miR-140 suppresses Mfn1 expression by directly binding to its 3′UTR [38]. Pink1 is a Ser/Thr kinase, which is tightly associated with the mitochondrial fusion and fission machinery [39]. Recently, Li and his colleagues exhibited an interplay between E2F1, miR-421, and Pink1, and this network is responsible for the abnormal mitochondrial morphology and myocardial apoptosis [40]. MiR-421 induces mitochondrial fragmentation, apoptosis and myocardial infarction by suppressing Pink1 translation. In the mitochondrial network, another signaling pathway composed of miR-361 and prohibitin 1 (PHB1), initiates mitochondrial fission and apoptosis, and protects heart from ischemia injury [41]. A study found that NFAT4/miR-324-5p/Mtfr 1 (mitochondrial fission regulator 1) axis participates in abnormal mitochondrial function [42]. Overexpression of miR-324-5p leads to reduction of apoptosis and infarct sizes in ischemia/reperfusion (I/R) injury animal model. Despite, many miRNAs have been identified to regulate the balance between cell survival and cell death in cardiomyocytes, the molecular mechanisms of mitochondrial network in heart remain largely explored.
Several miRNAs have been identified to protect heart from the cardiomyocyte death induced by doxorubicin (DOX), a drug widely used in cancer treatment. But this drug is associated risk of congestive heart failure [43, 44]. For instance, antagomir-based silencing of miR-208a or miR-532-3p attenuates DOX-induced myocyte apoptosis and cardiotoxicity [45, 46]. Meanwhile, overexpression of miR-21 or miR-30 inhibits DOX-induced apoptosis in cardiac cells [47, 48]. These studies shed light on new therapeutic strategies to overcome the DOX-induced cardiotoxicity during cancer therapy.
The other classes of ncRNAs such as lncRNAs and circular RNAs (circRNAs) are described as new regulators of the apoptosis pathway in cardiovascular diseases. LncRNAs are a set of RNAs with more than 200 nt in length with rare protein-coding potential, which are located throughout the whole genome [49]. It displays many cellular functions, such as capturing miRNAs, guiding transcription factors (TFs), and affecting the three-dimensional structure of chromatin [50, 51]. LncRNAs participate in various biological processes, including chromatin modification, genomic imprinting, and cell fate determination, and so on [52, 53]. For instance, lncRNA-Braveheart and Fendrr play critical roles during cardiomyocyte differentiation [54, 55]. However, the knowledge of influence of lncRNAs in cardiomyocytes death remains limited. Recently, Wang et al. demonstrated that a cardiac apoptosis-related lncRNA (CARL) acts as an endogenous miR-539 sponge [56]. MiR-539 directly targets prohibitin 2 (PHB2), which is a subunit of the prohibitin complex. The enforced expression of PHB2 blocks mitochondrial fission and apoptosis, as well as reduces infarct sizes in the mice of I/R model [56]. In this context, CARL is able to suppress mitochondrial fission and apoptosis by regulating the miR-539/PHB2 pathway. Another class of ncRNA, circRNAs, was discovered by Hsu et al. decades ago [57] that have 3′ and 5′ ends joined together to form a closed continuous loop. Recent findings reveal that they participate in multiple cellular processes [58]. CircRNA functions as a competing endogenous RNAs to miRNAs through complementary base pairing [59]; however, the functions of circRNAs in the cardiomyocyte death yet to be studied. So far, only one circRNA (heart-related circRNA, HRCR) is identified as a potential modulator of the apoptosis pathway [60]. In this study, HRCR acts as an endogenous miR-223 sponge by directly binding to miR-223 and inhibiting its activity. MiR-223 is known to promote cardiac hypertrophy and heart failure by targeting the anti-apoptotic protein Arc (apoptosis repressor with CARD domain), which is highly expressed in the heart and known to suppress apoptosis in cell [61, 62].
NcRNAs regulate cardiovascular sensitivity to necrosis
Necrosis is morphologically characterized by increased cell volume, organelles swelling, disruption of the plasma membrane, and leakage of intracellular contents [74]. For a long time, necrosis was commonly viewed as a passive, accidental, and unregulated event. In recent years, accumulating evidence indicates that necrosis can also be programmed by cells (Fig. 1). Recently, a relatively new form of necrosis termed programmed necrosis or necroptosis is identified, which exhibits a unique signaling pathway that requires receptor interaction protein kinase 1 and 3 (RIP1 and RIP3) [74, 75]. The necroptosis pathway is induced by a set of death receptors (e.g., TNFR1, TNFR2, and Fas) in certain cell types when apoptotic cell death is blocked [76, 77]. Upon death receptor ligation, RIP1 and RIP3 are catalytically phosphorylated and form a necrosome, which consequently initiates necroptosis, when caspase-8 is inactivated [74]. Necrostatin-1 (Nec-1) has the capability to inhibit necroptosis through disturbing RIP1 and RIP3 interactions [78]. Besides, opening of mitochondrial permeability transition pore (mPTP) in the inner mitochondrial membrane can also contribute to necrotic cell death, which is accompanied by the loss of the electrical potential difference and mitochondrial swelling [79].
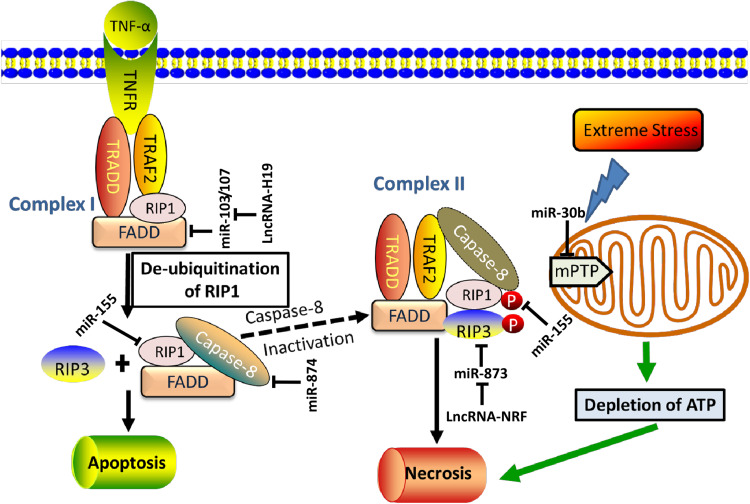
Fig. 1.
Non-coding RNAs regulate necrosis signaling. Currently, necrosis is triggered through two pathways. The first pathway, also named necroptosis, involves death receptors, including TNFR1, TNFR2, and Fas. For example, upon binding its agonist TNF-α, TNFR1 can modulate either apoptotic or necrotic cell death by recruiting complex I. The complex I contains TNFR1, TRADD, RIP1, TRAF2, and FADD. This complex leads to de-ubiquitination of RIP1 releases RIP1 to the cytoplasm, and activates the formation of complex II (composed of TRADD, RIP1, RIP3, TRAF2, FADD, and caspase-8). The caspase-8 activity determines whether cells undergo apoptosis or necroptosis, because caspase-8 is able to cleave RIP1. The activation of caspase-8 induces apoptosis. When caspase-8 is blocked, RIP1 is not cleaved; instead, it recruits RIP3 and leads to the phosphorylation of the two kinases. Subsequently, necroptosis is initiated. On the other hand, excessive stimulation (e.g. Ca2+, oxidative stress) leads to opening of mPTP in the inner mitochondrial membrane. MPTP is regulated by cyclophilin D and its opening results in depletion of ATP, which consequently activates mitochondrial necrosis pathway. The ncRNAs regulating these key components of necrotic/necroptotic are shown in the diagram. TNFR1, TNF-α receptor 1; TRADD, TNFR1-associated death domain protein; TRAF2, TNF receptor-associated factor 2
In cardiomyocyte, the necrosis-induced release of cellular contents triggers inflammatory reaction and pathological consequences, such as MI, heart failure, and stroke [79]. Ischemic injury often leads to hypoxia, increased levels of intracellular calcium and depletion of ATP, which subsequently causes opening of the mPTP. Cyclophilin D, a peptidyl–prolyl isomerase, is a critical regulator of mPTP [80]. Schinzel et al. reported that the suppression of cyclophilin D in mice reduces the infarct size after I/R injury and also promotes resistance to permeability transition in mitochondria [81]. Recent study unveiled that miR-30b inhibits the translation of cyclophilin D and thus it can impair cyclophilin D-induced necrotic cardiomyocyte death [82]. In addition, E2F1 negatively controls the expression of miR-30b in vitro and in vivo [82]. These findings suggest that a novel necrosis-modulating signaling axis is composed of E2F1, miR-30b, and cyclophilin D. Current knowledge about the role of ncRNAs in regulating myocardial necrosis is relatively limited. Cardiomyocyte progenitor cells (CMPCs) provide a potential cell source for the generation of injured myocardium. Liu et al. found that miR-155 expression is increased in CMPCs when they are exposed to H2O2, and overexpression of miR-155 in vitro triggered anti-necrotic responses through blocking RIP1 [83]. This finding revealed a promising therapeutic target for transplantation therapy. In recent years, several research groups provide a new insight into the complex signaling mechanisms that regulated the pathogenesis of cardiomyocyte necrosis. The expression of miR-874 is up-regulated by necrotic damage induced by H2O2 in cardiomyocytes. Under vivo condition, the knockdown of miR-874 decreased the necrotic cells and improved cardiac function following I/R injury to the heart. Further study revealed that miR-874 directly targets caspase-8, which antagonizes necrosis via cleaving the RIP1 and RIP3. In addition, a transcription factor, Foxo3a, can repress miR-874 expression, which is evident from both Foxo3a transgenic and knockout mice studies [84]. FADD (Fas-associated protein with death domain) negatively regulates the formation of RIP1-RIP3 complex and necrosis [85]. MiR-103/107 influences H2O2-induced cell necrosis and myocardial infarct sizes in the heart by interfering the regulation of FADD expression. A bioinformatic study found that lncRNA-H19 contains complementary sites for miR-103/107. The enforced expression and knockdown of H19 in vitro proved the negative correlation between the expression of H19 and sensitivity of cells to necrotic mode of death. These results support a novel model regulating cardiomyocyte necroptosis, which is composed of H19, miR-103/107 and FADD [86]. Another example of miRNAs contributing to necroptosis in the heart is miR-2861 [87]. In this study, miR-2861 is found to inhibit ANT1 and promotes necrotic cell death in cardiomyocytes. ANT1 (adenine nucleotide translocator 1) is a mitochondrial protein involved in cellular energy metabolism [88], which protects cardiomyocyte from necrosis and MI. The level of miR-2861 is increased under severe oxidative stress induced necrosis, and knockdown of miR-286 can protect heart from the progression of myocardial necrosis by blocking the miR-286 induced degrading ANT1 mRNA. Recently, a new p53/lncRNA-NRF/miR-873/RIP pathway draws people’s concern, which provides a new insight into the programmed necrotic cell death during the cardiac pathological process [89]. In this signaling pathway, p53 transcriptionally activates lncRNA-NRF expression. NRF works as an endogenous sponge of miR-873, which suppresses translation of RIP1//RIP3. Thus, the upregulation of lncRNA-NRF leads to increased necrotic cell death in cardiomyocytes and myocardial infarct size upon I/R injury. Experiments carried out in vivo also confirmed this necrotic pathway.
NcRNAs regulate cardiomyocyte autophagy
Autophagy is a conserved intracellular recycling process during which non-functional proteins and organelles are delivered to lysosomes for degradation [90]. This process is mediated by the formation of double-membrane vesicles, termed autophagosomes, which is initiated by vesicle nucleation and elongation involving Beclin-1, Vps34, Atg proteins, etc. [91]. Autophagosomes fuse with the lysosome to form an autolysosome, resulting in breakdown of its contents, after autophagosomes sequester long-lived proteins and damaged organelles such as mitochondria [90, 91]. The produced macromolecules are then recycled and released into the cytosol for reuse in biosynthetic pathways. Autophagy is regulated through two major pathways-mTOR (mammalian target of rapamycin) and Beclin-1 in response to stress signals (e.g., starvation, ROS, and protein aggregates).
Autophagy usually serves to maintain the homeostasis in cells. The dysfunction of autophagy is responsible for cardiovascular disorders. For example, dysfunction of autophagy can influence the renewal of mitochondria in the cardiomyocytes, and that causes deficit in the energy demand of the heart [92]. So far, multiple miRNAs have been linked to the autophagy pathway and cardiac pathologies (Table 2). Inhibition of the mTOR activity is able to prompt autophagy, and its inhibition exhibits a beneficial effect in the animal model of MI [93]. The overexpression of miR-99a attenuated mTOR activity, which led to decreased infarct size and improved cardiac function after MI [94]. Recently, Su et al. reported that miR-221 interferes autophagic balance and cardiac remodeling by modulating the p27/CDK2/mTOR axis in the failing heart [95].
Table 2.
NcRNAs regulating necrosis and autophagy of cardiomyocytes
| ncRNAs | Targets/CVDs | Effects on necrosis/autophagy | References |
|---|---|---|---|
| miR-155 | Rip1/MI | Decreases necrosis | [83] |
| miR-30b | Cyclophilin D/MI | Inhibits necrosis | [82] |
| miR-874 | Caspase-8/MI | Promotes necrosis | [84] |
| miR-2861 | Ant1/MI | Promotes necrosis | [87] |
| lncRNA-H19 | miR-103/107, FADD/MI | Anti-necrosis | [86] |
| lncRNA-NRF | miR-873, RIP/MI | Pro-necrosis | [89] |
| miR-99a | mTOR/MI | Activates beneficial autophagy | [94] |
| miR-22 | PPAR-α/MI | Decreases beneficial autophagy | [101] |
| miR-221 | P27/heart failure | Decreases autophagy | [95] |
| miR-212/132 | Foxo3a/cardiac hypertrophy | Inhibits beneficial autophagy | [99] |
| miR-451 | Tsc1/cardiac hypertrophy | Inhibits harmful autophagy | [100] |
| miR-325 | Arc/MI | Promotes autophagy | [96] |
| miR-30a | Beclin-1/cardiac hypertrophy | Decreases harmful autophagy | [97] |
| miR-34a | Atg9a/cardiac hypertrophy | Inhibits harmful autophagy | [103] |
| miR-497 | Lc3b, Bcl2/MI | Anti-autophagic | [104] |
| lncRNA-APF | miR-188-3p, Atg7/MI | Promotes autophagy | [102] |
On the contrary, activation of autophagy by Beclin-1 was demonstrated to induce cell death and disorder of the heart. For instance, ARC acts as an inhibitor of Beclin-1 and it decreases autophagic cell death and MI size in pressure-overload-induced heart failure [96]. Bioinformatic analysis and further experiments revealed that miR-325 conveyed the autophagic pathway by targeting ARC. Interestingly, miR-325 acts as a downstream of transcription factor E2F1. Another study found that Angiotensin II-induced down-regulation of miR-30a leads to up-regulation of Beclin-1 and excessive autophagy. This consequently results in defective autophagy mediated hypertrophy in cardiomyocytes response to Angiotensin II. Experiments in vitro revealed that miR-30a mimics can reverse the effects of Angiotensin II-induced cardiac hypertrophy by suppressing expression of Beclin-1 [97].
The expression of miR-212/132 family is upregulated in failing human hearts [98, 99]. In animal models, the knockout of miR-212/132 protects the cardiac function from pressure-overload-induced heart failure. In contrast, cardiomyocyte-specific overexpression of miR-212/132 impairs normal autophagic response and caused hyperactivation of pro-hypertrophic calcineurin/NFAT pathway through negatively regulating FOXO3 expression. In another study, miRNA microarrays on heart tissue from hypertrophic cardiomyopathy patients and healthy donors revealed that the level of miR-451 is decreased under diseased condition [100]. However, the enforced expression of miR-451 accelerates cardiac hypertrophy via targeting TSC1 (tuberous sclerosis complex 1), which is a known positive regulator of autophagy. Recently, Gupta et al. identified that miR-22 acts as an inhibitor of cardiac autophagy, and miR-22 is considered as a promising prognostic and therapeutic tool for MI patients [101]. The latest evidence shows that lncRNAs also participate in the regulation of cardiomyocyte autophagy. Wang et al. screened in cardiac tissue and found an lncRNA named as lncRNA-APF (autophagy promoting factor) [102]. APF is significantly up-regulated upon anoxia/reoxygenation, and this lncRNA consist of a binding site of miR-188-3p. A series of further experiments revealed that APF, miR-188-3p, and ATG7 constitute a novel regulating axis of autophagic program in the heart and this axis has influence on the autophagic cell death and myocardial infarction.
Crosstalk between three types of cell death
Under different levels of stresses, apoptosis, autophagy, and necrosis occur, respectively. In general, autophagy is induced under condition of mild stress, whereas apoptosis happens under condition of increasing and progressive stress, resulting in release of cytochrome c from mitochondria. Under further extreme stress, necrosis takes place along with ATP depletion [105]. During the cell death processes, mitochondria often functions as a switch between autophagy and apoptosis [105]. Some common cellular stressor factors can trigger signaling pathways that regulate apoptosis and autophagy. For instance, ROS induces autophagy through affecting the activity of Atg4 [106], meanwhile, ROS also plays critical role in apoptosis [107]. In addition, some node genes of signaling pathways, such as Beclin-1/Bcl-2 [108], p53 [109], caspase-8, as well as Atg5/FADD [110], act as switch between apoptosis/autophagy/necroptosis. The case of Bcl-2 family members such as Bax/Bak, the silencing of Bax/Bak causes resistance to mPTP opening and necrosis [111], while Bax and Bak are known to activate apoptosis upon I/R injury. Another study reported that Nix, a Bcl-2 family protein, could mediate apoptosis or necrosis depending on its cellular localization [112].
In the context, miRNAs targeting these node genes or different transcripts sharing a common binding site to the same miRNA are speculated to regulate the crosstalk between apoptosis, autophagy, and necroptosis. However, miRNAs contributed to the crosstalk on cardiac cell survival still remain relatively unknown. For example, Sirt1 is a miR-34a target, and that is able to influence apoptosis via p53 and ROS signaling pathway. In addition, Sirt1 is involved in autophagy via regulating the activity of p53 and FoxO TFs [113]. A recent study found that let-7b inhibits apoptosis and autophagy of human mesenchymal stem cells transplanted into I/R injured heart by suppressing caspase-3 [114]. Li et al. reported that miR-497 can enhance cell apoptosis and reduce autophagy by targeting anti-apoptotic Bcl2 and autophagic gene LC3B [104]. The silencing of miR-497 ameliorated myocardial A/R and I/R injury in vitro and in vivo, respectively. Likewise, miR-153 regulates the survival of cardiomyocytes under oxidative stress condition through modulating apoptosis and autophagy by directly targeting Mcl-1 [115].
Future outlook
Cardiovascular diseases are complex, which involve multiple cellular processes, including cardiomyocyte death, during the disease progression. Based on the studies presented in this review, ncRNAs play essential role in cell survival and death by modulating apoptosis, necrosis, and autophagy processes in cardiomyocytes, and they exhibit enormous potential to treat cardiac diseases in the clinical application (Tables 1, 2; Fig. 1). Experiments on several animal models have validated that blocking of apoptosis or necrosis could be an effective therapeutic strategy for heart diseases. Given that ncRNA mimics or inhibitors can be easily synthesized and transfect cells with low toxicity in vivo, ncRNAs offer promising therapeutic targets in human to treat heart diseases. However, many questions and challenges remain to be solved in the development of ncRNA-based therapy, e.g., the off-target effects. Currently, some of the identified cardiovascular-related miRNAs have been used as diagnostic biomarkers in cardiac pathology [116]. For instance, circulating miR-126 and miR-145 are decreased in patients with coronary artery disease compared to healthy human [117]. However, further studies are still required to explore the complex mechanisms connecting ncRNAs and different mode of cardiac cell death during different myocardial pathological conditions.
Acknowledgements
We thank Chao Chen of the Institute for Translational Medicine, Qingdao University, China for his generous assistance with literature searches.
Compliance with ethical standards
Funding information
This work was supported by the Natural Science Foundation of China (Grant nos: 31430041, 81470522, 81522005), Applied Basic Research Programs of Qingdao, China (Grant no: 17-1-1-46-jch), and Shandong Provincial Natural Science Foundation, China (Grant no: ZR2016CQ31).
Conflict of interest
The authors have declared there are no potential conflicts of interest, funding, acknowledgements.
Footnotes
Yanhan Dong and Cuiyun Liu contributed equally to this work.
Contributor Information
Peifeng Li, Phone: 86-532-82991791, Email: peifli@qdu.edu.cn.
Kun Wang, Phone: 86-532-82991791, Email: wangk696@qdu.edu.cn.
References
- 1.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, et al. Apoptosis in the failing human heart. New Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Throm Vasc. 2012;32:1552–1562. doi: 10.1161/ATVBAHA.111.224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K, Zhou LY, Wang JX, Wang Y, Sun T, Zhao B, et al. E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1. Nat Commun. 2015;6:7619. doi: 10.1038/ncomms8619. [DOI] [PubMed] [Google Scholar]
- 5.Small EM, Frost RJA, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:U1022–U1066. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leite-Moreira AM, Lourenco AP, Falcao-Pires I, Leite-Moreira AF. Pivotal role of microRNAs in cardiac physiology and heart failure. Drug Discov Today. 2013;18:1243–1249. doi: 10.1016/j.drudis.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Kumarswamy R, Thum T. Non-coding RNAs in cardiac remodeling and heart failure. Circ Res. 2013;113:676–689. doi: 10.1161/CIRCRESAHA.113.300226. [DOI] [PubMed] [Google Scholar]
- 8.Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol. 2015;12:135–142. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- 9.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 10.Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol. 2009;21:452–460. doi: 10.1016/j.ceb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12:846–860. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- 12.Graves P, Zeng Y. Biogenesis of mammalian microRNAs: a global view. Genom Proteom Bioinform. 2012;10:239–245. doi: 10.1016/j.gpb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orogo AM, Gustafsson AB. Cell death in the myocardium: my heart won’t go on. IUBMB Life. 2013;65:651–656. doi: 10.1002/iub.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skommer J, Rana I, Marques FZ, Zhu W, Du Z, Charchar FJ. Small molecules, big effects: the role of microRNAs in regulation of cardiomyocyte death. Cell Death Dis. 2014;5:e1325. doi: 10.1038/cddis.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao CF, Ren S, Zhang LL, Nakajima T, Ichinose S, Hara T, et al. Caspase-dependent cytosolic release of cytochrome c and membrane translocation of Bax in p53-induced apoptosis. Exp Cell Res. 2001;265:145–151. doi: 10.1006/excr.2001.5171. [DOI] [PubMed] [Google Scholar]
- 18.Jiang XJ, Wang XD. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J Biol Chem. 2000;275:31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- 19.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121(18):2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 20.Clerk A, Cullingford TE, Fuller SJ, Giraldo A, Markou T, Pikkarainen S, et al. Signaling pathways mediating cardiac myocyte gene expression in physiological and stress responses. J Cell Physiol. 2007;212:311–322. doi: 10.1002/jcp.21094. [DOI] [PubMed] [Google Scholar]
- 21.Bostjancic E, Zidar N, Glavac D. MicroRNA microarray expression profiling in human myocardial infarction. Dis Mark. 2009;27:255–268. doi: 10.1155/2009/641082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y, Zheng J, Sun Y, Wu Z, Liu Z, Huang G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J. 2009;50:377–387. doi: 10.1536/ihj.50.377. [DOI] [PubMed] [Google Scholar]
- 23.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H, et al. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci. 2007;120:3045–3052. doi: 10.1242/jcs.010728. [DOI] [PubMed] [Google Scholar]
- 25.Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, et al. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. 2010;106:166–175. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang HJ, Li J, Chi HJ, Zhang F, Zhu XM, Cai J, et al. MicroRNA-181c targets Bcl-2 and regulates mitochondrial morphology in myocardial cells. J Cell Mol Med. 2015;19:2084–2097. doi: 10.1111/jcmm.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47:5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Donath S, Li Y, Qin D, Prabhakar BS, Li P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 2010;6:e1000795. doi: 10.1371/journal.pgen.1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JX, Gao J, Ding SL, Wang K, Jiao JQ, Wang Y, et al. Oxidative modification of miR-184 enables It to target Bcl-xL and Bcl-w. Mol Cell. 2015;59:50–61. doi: 10.1016/j.molcel.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P. MicroRNAs in cardiac apoptosis. J Cardiovasc Transl Res. 2010;3:219–224. doi: 10.1007/s12265-010-9175-9. [DOI] [PubMed] [Google Scholar]
- 34.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/S1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 35.Li JC, Donath S, Li YR, Qin D, Prabhakar BS, Li PF. miR-30 Regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. Plos Genet. 2016;6(1):e1000795. doi: 10.1371/journal.pgen.1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang JX, Jiao JQ, Li QA, Long B, Wang K, Liu JP, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17(1):71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 37.Li JC, Zhou J, Li YR, Qin DN, Li PF. Mitochondrial fission controls DNA fragmentation by regulating endonuclease G. Free Radic Biol Med. 2010;49:622–631. doi: 10.1016/j.freeradbiomed.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 38.Li JC, Li YZ, Jiao JQ, Wang JX, Li YR, Qin DN, et al. Mitofusin 1 is negatively regulated by microRNA 140 in cardiomyocyte apoptosis. Mol Cell Biol. 2014;34:1788–1799. doi: 10.1128/MCB.00774-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dagda RK, Cherra SJ, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang K, Zhou LY, Wang JX, Wang Y, Sun T, Zhao B, et al. E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1. Nat Commun. 2015;6:7619. doi: 10.1038/ncomms8619. [DOI] [PubMed] [Google Scholar]
- 41.Wang K, Liu CY, Zhang XJ, Feng C, Zhou LY, Zhao Y, et al. miR-361-regulated prohibitin inhibits mitochondrial fission and apoptosis and protects heart from ischemia injury. Cell Death Differ. 2015;22(6):1058–1068. doi: 10.1038/cdd.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang K, Zhang DL, Long B, An T, Zhang J, Zhou LY, et al. NFAT4-dependent miR-324-5p regulates mitochondrial morphology and cardiomyocyte cell death by targeting Mtfr1. Cell Death Dis. 2015;6:e2007. doi: 10.1038/cddis.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. New Engl J Med. 1998;339(13):900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 44.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Tony H, Yu K, Qiutang Z. MicroRNA-208a silencing attenuates doxorubicin induced myocyte apoptosis and cardiac dysfunction. Oxidative Med Cell Longev. 2015;2015:597032. doi: 10.1155/2015/597032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang JX, Zhang XJ, Feng C, Sun T, Wang K, Wang Y, et al. MicroRNA-532-3p regulates mitochondrial fission through targeting apoptosis repressor with caspase recruitment domain in doxorubicin cardiotoxicity. Cell Death Dis. 2015;6:e1677. doi: 10.1038/cddis.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tong Z, Jiang B, Wu Y, Liu Y, Li Y, Gao M, et al. MiR-21 protected cardiomyocytes against doxorubicin-induced apoptosis by targeting BTG2. Int J Mol Sci. 2015;16(7):14511–14525. doi: 10.3390/ijms160714511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roca-Alonso L, Castellano L, Mills A, Dabrowska AF, Sikkel MB, Pellegrino L, et al. Myocardial MiR-30 downregulation triggered by doxorubicin drives alterations in beta-adrenergic signaling and enhances apoptosis. Cell Death Dis. 2015;6:e1754. doi: 10.1038/cddis.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papait R, Kunderfranco P, Stirparo GG, Latronico MVG, Condorelli G. Long noncoding RNA: a new player of heart failure? J Cardiovasc Transl. 2013;6:876–883. doi: 10.1007/s12265-013-9488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piccoli MT, Gupta SK, Thum T. Noncoding RNAs as regulators of cardiomyocyte proliferation and death. J Mol Cell Cardiol. 2015;89:59–67. doi: 10.1016/j.yjmcc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Ginger MR, Shore AN, Contreras A, Rijnkels M, Miller J, Gonzalez-Rimbau MF, et al. A noncoding RNA is a potential marker of cell fate during mammary gland development. Proc Natl Acad Sci USA. 2006;103:5781–5786. doi: 10.1073/pnas.0600745103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanduri C. Kcnq1ot1: a chromatin regulatory RNA. Semin Cell Dev Biol. 2011;22:343–350. doi: 10.1016/j.semcdb.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 54.Grote P, Wittler L, Hendrix D, Koch F, Wahrisch S, Beisaw A, et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell. 2013;24:206–214. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishii N, Ozaki K, Sato H, Mizuno H, Saito S, Takahashi A, et al. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet. 2006;51:1087–1099. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 56.Wang K, Long B, Zhou LY, Liu F, Zhou QY, Liu CY, et al. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun. 2014;5:3596. doi: 10.1038/ncomms4596. [DOI] [PubMed] [Google Scholar]
- 57.Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280(5720):339–340. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- 58.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 59.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 60.Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016;37(33):2602–2611. doi: 10.1093/eurheartj/ehv713. [DOI] [PubMed] [Google Scholar]
- 61.Koseki T, Inohara N, Chen S, Nunez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci USA. 1998;95(9):5156–5160. doi: 10.1073/pnas.95.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li YZ, Lu DY, Tan WQ, Wang JX, Li PF. p53 initiates apoptosis by transcriptionally targeting the antiapoptotic protein ARC. Mol Cell Biol. 2008;28:564–574. doi: 10.1128/MCB.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du WJ, Pan ZW, Chen X, Wang LM, Zhang Y, Li S, et al. By targeting Stat3 microRNA-17-5p promotes cardiomyocyte apoptosis in response to ischemia followed by reperfusion. Cell Physiol Biochem. 2014;34:955–965. doi: 10.1159/000366312. [DOI] [PubMed] [Google Scholar]
- 64.Danielson LS, Park DS, Rotllan N, Chamorro-Jorganes A, Guijarro MV, Fernandez-Hernando C, et al. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. Faseb J. 2013;27:1460–1467. doi: 10.1096/fj.12-221994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin YJ, Yu YQ, Dong H, Bian XH, Guo X, Dong SM. MicroRNA 21 inhibits left ventricular remodeling in the early phase of rat model with ischemia–reperfusion injury by suppressing cell apoptosis. Int J Med Sci. 2012;9:413–423. doi: 10.7150/ijms.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding SL, Wang JX, Jiao JQ, Tu X, Wang Q, Liu F, et al. A pre-microRNA-149 (miR-149) genetic variation affects miR-149 maturation and its ability to regulate the Puma protein in apoptosis. J Biol Chem. 2013;288:26865–26877. doi: 10.1074/jbc.M112.440453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Long B, Wang K, Li N, Murtaza I, Xiao JY, Fan YY, et al. miR-761 regulates the mitochondrial network by targeting mitochondrial fission factor. Free Radic Biol Med. 2013;65:371–379. doi: 10.1016/j.freeradbiomed.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 69.Lv G, Shao S, Dong H, Bian X, Yang X, Dong S. MicroRNA-214 protects cardiac myocytes against H2O2-induced injury. J Cell Biochem. 2014;115:93–101. doi: 10.1002/jcb.24636. [DOI] [PubMed] [Google Scholar]
- 70.Li R, Yan G, Li Q, Sun H, Hu Y, Sun J, et al. MicroRNA-145 protects cardiomyocytes against hydrogen peroxide (H(2)O(2))-induced apoptosis through targeting the mitochondria apoptotic pathway. PLoS One. 2012;7:e44907. doi: 10.1371/journal.pone.0044907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang J, Song XW, Tian J, Chen HY, Li DF, Wang JF, et al. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis. 2012;17:410–423. doi: 10.1007/s10495-011-0683-0. [DOI] [PubMed] [Google Scholar]
- 72.Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res. 2011;92:75–84. doi: 10.1093/cvr/cvr145. [DOI] [PubMed] [Google Scholar]
- 73.Tabuchi T, Satoh M, Itoh T, Nakamura M. MicroRNA-34a regulates the longevity-associated protein SIRT1 in coronary artery disease: effect of statins on SIRT1 and microRNA-34a expression. Clin Sci (Lond) 2012;123:161–171. doi: 10.1042/CS20110563. [DOI] [PubMed] [Google Scholar]
- 74.Wu W, Liu P, Li J. Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol. 2012;82:249–258. doi: 10.1016/j.critrevonc.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 75.Galluzzi L, Kepp O, Kroemer G. RIP kinases initiate programmed necrosis. J Mol Cell Biol. 2009;1:8–10. doi: 10.1093/jmcb/mjp007. [DOI] [PubMed] [Google Scholar]
- 76.Fiers W, Beyaert R, Boone E, Cornelis S, Declercq W, Decoster E, et al. TNF-induced intracellular signaling leading to gene induction or to cytotoxicity by necrosis or by apoptosis. J Inflamm. 1995;47:67–75. [PubMed] [Google Scholar]
- 77.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 78.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 80.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 81.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 82.Wang K, An T, Zhou LY, Liu CY, Zhang XJ, Feng C, et al. E2F1-regulated miR-30b suppresses cyclophilin D and protects heart from ischemia/reperfusion injury and necrotic cell death. Cell Death Differ. 2015;22:743–754. doi: 10.1038/cdd.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu J, van Mil A, Vrijsen K, Zhao J, Gao L, Metz CH, et al. MicroRNA-155 prevents necrotic cell death in human cardiomyocyte progenitor cells via targeting RIP1. J Cell Mol Med. 2011;15:1474–1482. doi: 10.1111/j.1582-4934.2010.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang K, Liu F, Zhou LY, Ding SL, Long B, Liu CY, et al. miR-874 regulates myocardial necrosis by targeting caspase-8. Cell Death Dis. 2013;4:e709. doi: 10.1038/cddis.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee EW, Kim JH, Ahn YH, Seo J, Ko A, Jeong M, et al. Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis. Nat Commun. 2012;3:978. doi: 10.1038/ncomms1981. [DOI] [PubMed] [Google Scholar]
- 86.Wang JX, Zhang XJ, Li Q, Wang K, Wang Y, Jiao JQ, et al. MicroRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting FADD. Circ Res. 2015;117:352–363. doi: 10.1161/CIRCRESAHA.117.305781. [DOI] [PubMed] [Google Scholar]
- 87.Wang K, Long B, Li N, Li L, Liu CY, Dong YH, et al. MicroRNA-2861 regulates programmed necrosis in cardiomyocyte by impairing adenine nucleotide translocase 1 expression. Free Radic Biol Med. 2016;91:58–67. doi: 10.1016/j.freeradbiomed.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 88.Walther T, Tschope C, Sterner-Kock A, Westermann D, Heringer-Walther S, Riad A, et al. Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation. 2007;115:333–344. doi: 10.1161/CIRCULATIONAHA.106.643296. [DOI] [PubMed] [Google Scholar]
- 89.Wang K, Liu F, Liu CY, An T, Zhang J, Zhou LY, et al. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ. 2016;23(8):1394–1405. doi: 10.1038/cdd.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He CC, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6(5):600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 93.Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014;114(3):549–564. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Q, Xie J, Li R, Shi J, Sun J, Gu R, et al. Overexpression of microRNA-99a attenuates heart remodelling and improves cardiac performance after myocardial infarction. J Cell Mol Med. 2014;18:919–928. doi: 10.1111/jcmm.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su M, Wang J, Wang C, Wang X, Dong W, Qiu W, et al. MicroRNA-221 inhibits autophagy and promotes heart failure by modulating the p27/CDK2/mTOR axis. Cell Death Differ. 2015;22:986–999. doi: 10.1038/cdd.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bo L, Su-Ling D, Fang L, Lu-Yu Z, Tao A, Stefan D, et al. Autophagic program is regulated by miR-325. Cell Death Differ. 2014;21:967–977. doi: 10.1038/cdd.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pan W, Zhong Y, Cheng C, Liu B, Wang L, Li A, et al. MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy. PLoS One. 2013;8:e53950. doi: 10.1371/journal.pone.0053950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, et al. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 99.Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun. 2012;3:1078. doi: 10.1038/ncomms2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song L, Su M, Wang S, Zou Y, Wang X, Wang Y, et al. MiR-451 is decreased in hypertrophic cardiomyopathy and regulates autophagy by targeting TSC1. J Cell Mol Med. 2014;18:2266–2274. doi: 10.1111/jcmm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C, et al. Preclinical development of a MicroRNA-based therapy for elderly patients with myocardial infarction. J Am Coll Cardiol. 2016;68:1557–1571. doi: 10.1016/j.jacc.2016.07.739. [DOI] [PubMed] [Google Scholar]
- 102.Wang K, Liu CY, Zhou LY, Wang JX, Wang M, Zhao B, et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun. 2015;6:6779. doi: 10.1038/ncomms7779. [DOI] [PubMed] [Google Scholar]
- 103.Huang J, Sun W, Huang H, Ye J, Pan W, Zhong Y, et al. miR-34a modulates angiotensin II-induced myocardial hypertrophy by direct inhibition of ATG9A expression and autophagic activity. PLoS One. 2014;9:e94382. doi: 10.1371/journal.pone.0094382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li XX, Zeng Z, Li Q, Xu QL, Xie JH, Hao HX, et al. Inhibition of microRNA-497 ameliorates anoxia/reoxygenation injury in cardiomyocytes by suppressing cell apoptosis and enhancing autophagy. Oncotarget. 2015;6:18829–18844. doi: 10.18632/oncotarget.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nishida K, Yamaguchi O, Otsu K. Crosstalk between autophagy and apoptosis in heart disease. Circ Res. 2008;103:343–351. doi: 10.1161/CIRCRESAHA.108.175448. [DOI] [PubMed] [Google Scholar]
- 106.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, et al. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA. 2003;100:15883–15888. doi: 10.1073/pnas.2136717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marquez RT, Xu L. Bcl-2: beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res. 2012;2:214–221. [PMC free article] [PubMed] [Google Scholar]
- 109.Crighton D, Wilkinson S, O’Prey J, Syed N, Smith P, Harrison PR, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 110.Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JIL, Woo HN, et al. Essential roles of Atg5 and FADD in autophagic cell death—dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 111.Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci USA. 2012;109:6566–6571. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Diwan A, Matkovich SJ, Yuan QY, Zhao W, Yatani A, Brown JH, et al. Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Investig. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ng F, Tang BL. Sirtuins’ modulation of autophagy. J Cell Physiol. 2013;228:2262–2270. doi: 10.1002/jcp.24399. [DOI] [PubMed] [Google Scholar]
- 114.Ham O, Lee SY, Lee CY, Park JH, Lee J, Seo HH, et al (2015) Let-7b suppresses apoptosis and autophagy of human mesenchymal stem cells transplanted into ischemia/reperfusion injured heart 7 by targeting caspase-3. Stem Cell Res Ther 6 [DOI] [PMC free article] [PubMed]
- 115.Zou Y, Liu W, Zhang J, Xiang D. miR-153 regulates apoptosis and autophagy of cardiomyocytes by targeting Mcl-1. Mol Med Rep. 2016;14:1033–1039. doi: 10.3892/mmr.2016.5309. [DOI] [PubMed] [Google Scholar]
- 116.Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K, et al. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol. 2011;51(5):872–875. doi: 10.1016/j.yjmcc.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 117.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]