Abstract
Group 2 innate lymphoid cells (ILC2s) are a subset of innate immune cells that do not express antigen receptors. ILC2-mediated type 2 responses, which are mainly characterized by the production of interleukin (IL)-5 and IL-13, play key roles in inducing inflammation, protecting against infection, and maintaining tissue homeostasis. Although recent years have largely enhanced our understanding of the transcriptional networks and soluble mediators that regulate ILC2 development or function, emerging evidence suggests that ILC2s express a variety of cell-surface molecules and interact with themselves or other immune cells. These cell–cell interactions are essential in the modulation of ILC2 number and their type 2 cytokine production during ILC2-driven allergic inflammation. In this review, we summarize the extensive array of cell-surface molecules on ILC2s that mediate cell–cell interactions and their role in regulating ILC2 generation or function in the context of ILC2-induced allergic inflammation.
Keywords: ILC2, Cell-surface molecules, Allergic inflammation
Introduction
Group 2 innate lymphoid cells (ILC2s) are now recognized as an important subset of innate immune cells that parallel CD4+ Th2 cells in function [1]. In contrast to Th2 cells, ILC2s lack antigen-specific receptors and respond rapidly to local cytokines, such as IL-33, IL-25, and thymic stromal lymphopoietin (TSLP) [2–4]. ILC2s were first found to promote type-2 immunity in gut-associated tissues of mice in 2010 [5–7], and subsequently discovered at other mucosal barrier sites and other sites, including lung, skin, liver, bone marrow, kidney, visceral adipose tissue, and blood. Interestingly, ILC2s are positioned to be tissue-resident cells, as they develop and proliferate locally [8, 9], though ILC2s could migrate from one tissue to another in the context of type 2 inflammation [10].
The development or function of ILC2s has been extensively and thoroughly studied over the past decade. In general, ILC2 develops from common lymphoid progenitors (CLPs) in the fetal liver or adult bone marrow [11, 12], which is followed by early innate lymphocyte precursors (EILPs) that have the potential to differentiate into all ILC subtypes, containing ILC1, ILC2, ILC3, and NK cells [13]. EILPs then develop into a common helper-like ILC progenitors (CHILP) that can further give rise to ILC2 lineage-restricted progenitors, which turn into mature ILC2s. The development of ILC2 is mainly controlled by GATA3 and RORα [4, 12]. Much work has been done in ILC2 regulatory network, including transcriptional factors and soluble mediators, as excellently reviewed elsewhere [4, 14, 15]. Functionally, upon activation, ILC2s can produce a large amount of IL-5, IL-13, IL-9, and amphiregulin (Areg), which play an important role in protecting against helminth infection, allergic inflammation, and tissue repair [4]. Of note, emerging evidence suggests that ILC2s express a variety of cell-surface molecules, through which they can interact with themselves or other immune cells, such as regulatory T cells (Treg) and Th2 cells. This crosstalk is essential in regulating ILC2 responses in the context of lung inflammation. Here, we will focus on the role of cell-surface molecule-mediated cell–cell interactions in the regulation of ILC2 level and function, and address their effect on ILC2-driven allergic inflammation.
Cell-surface molecule-mediated ILC2–cell interactions in the context of allergic inflammation
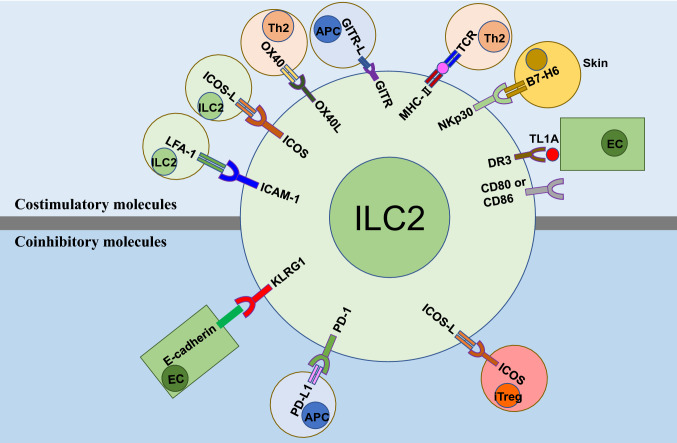
ILC2s act early in immune responses and are key players in the induction of allergic inflammation. Although IL-33, IL-25, and TSLP have been recognized as type 2 alarmins that can activate ILC2s [4, 16, 17], in recent years, various cell-surface molecules that mediate cell–cell interactions have been found to serve as important ILC2 regulators, and play critical roles in ILC2-driven allergic inflammation. Based on their role in ILC2 responses, these cell-surface molecules can be classified into two groups: costimulatory molecules and coinhibitory molecules (Fig. 1).
Fig. 1.
Cell-surface molecule-mediated cell–cell interactions regulating ILC2 responses in allergic inflammation. These molecules mainly include costimulatory molecules and coinhibitory molecules. Of note, all the cells that express ILC2 ligands or receptors may bind to their counterparts on ILC2s. EC endothelial cells; APC antigen presenting cells; iTreg induced regulatory T cell
Costimulatory molecules
ICAM-1: LFA-1
Intercellular cell adhesion molecule-1 (ICAM-1 or CD54), which mainly binds to leukocyte function-associated molecule (LFA)-1, is an Ig-like transmembrane protein that is typically expressed on endothelial cells and leukocytes [18]. ICAM-1: LFA-1 is required not only in cell migration, but also in cell–cell interactions and can serve as an important pair of T-cell costimulatory molecules [19, 20]. Notably, they have been shown to play an important role in the pathogenesis of allergic airway inflammation [18, 21, 22]. Interestingly, we and others recently found that ILC2s express both ICAM-1 and LFA-1 [10, 23]. We showed that ICAM-1 is required for the development and function of ILC2, as loss of ICAM-1 in mice results in fewer ILC2s in bone marrow and peripheral tissues and less severe allergic lung inflammation than that of wild-type mice after allergen challenge. ICAM-1-deficient CLPs cannot efficiently differentiate into ILC2s both in vivo and in vitro. Of note, suppressing the interaction of ICAM-1 and LFA-1 using blocking antibodies significantly reduced lung inflammation in mice. We further showed that downregulation of extracellular signal-regulated kinase (ERK) phosphorylation and resultant degradation of GATA3 protein may mediate the effects of ICAM-1 on ILC2 [23]. Besides these effects, one recent study found that β2 integrins (CD18), a subunit of LFA-1 that is highly expressed on ILC2s, is required for Alternaria-induced ILC2 trafficking from the circulation into the lung [10]. This study suggests that ILC2s are not only resident cells, but can also migrate from the circulation to the lung during helminth infection as described [24]. Moreover, several early studies have found that ICAM-1: LFA-1 is also critical for Th2 responses [25–27], which can initiate chronic lung inflammation. Thus, these findings suggest that disruption of ICAM-1 and LFA-1 binding might be a potential strategy to treat asthma.
ICOS: ICOS-L
Inducible costimulator (ICOS), a costimulatory molecule belonging to CD28 superfamily, is typically expressed on activated T cells [28], and is essential for Th2 development and function [29–31]. It has been shown that both human and murine ILC2s express both ICOS and ICOS-ligand (ICOS-L), and their interaction is required for ILC2 survival and efficient function through the activation of STAT5 and Bcl-2, an anti-apoptotic molecule [32]. Therefore, ICOS deficiency, or blocking ICOS caused a sharply decrease in ILC2 number and type 2 cytokine production in IL-33- and Alternaria-induced lung inflammation. Another two studies also found that ICOS signaling regulates ILC2 and loss of ICOS resulted in the amelioration of airway inflammation in mice [33, 34]. In addition, dendritic cells (DC) expressed a high level of ICOS-L [35, 36], suggesting that DC in the lung may also directly interact with ILC2s under inflammatory conditions. Hence, ICOS: ICOS-L signaling pathway is an efficient modulator of ILC2 function and homeostasis, which provides new therapeutic approaches that target ILC2s to treat asthma.
TNFRSF: TNFSF
It is known that several tumor necrosis factor receptor superfamily (TNFRSF) members and their ligands (TNFSF) act as key co-stimulatory signals to T-cell proliferation or survival [37, 38]. Modulation of TNF superfamily signaling pathways is expected to have therapeutic benefit for treating autoimmunity, cancer, and infectious diseases [39, 40]. A number of recent studies showed that several TNFRSF: TNFSF provide co-stimulatory signals to ILC2s, and are discussed as below.
The tumor necrosis factor receptor superfamily, member 4 (TNFRSF4), also known as CD134 or OX40, is an important co-stimulatory molecule in T-cell activation, while its ligand OX40L (TNFSF4) is expressed on many immune cells, especially on DC [41]. OX40: OX40L interaction is crucial for the expansion or survival of Th2 cells [42]. A recent study showed that lung ILC2s expressed high levels of OX40L upon exposure to IL-33 and provided tissue-restricted T-cell co-stimulation that was essential for Th2 and Treg (preferentially GATA3+ Treg) cell responses in the lung; deletion of OX40L on ILC2s abrogated Th2 and Treg cell expansion and allergen-induced lung inflammation [43]. Of note, several earlier studies also suggested that the ILC2 and T-cell crosstalk was indispensable for initiating type 2 immunity [44–48].
TNFRSF25 (also known as death receptor 3, DR3), a receptor that can engage with its cognate ligand TNFSF15 (also known as TNF-like ligand 1A, TL1A), is constitutively expressed on ILC2s and is involved in ILC2 expansion, survival, and function in the lung [49, 50]. Interestingly, TL1A, which is highly expressed by activated myeloid cells, epithelial and endothelial cells under lung and gut inflammation [51], alone is sufficient to activate ILC2s [50]. TL1A can also synergize with IL-25 and IL-33 to enhance ILC2 effector function [50]. Moreover, a recent study further demonstrates that TL1A enhances the activation of DR3+ skin ILC2s, thus contributing to atopic dermatitis [52]. In addition, lung ILC2s also express a high level of glucocorticoid induced TNFR-related protein (GITR, also known as TNFRSF18) [5, 33], which binds to GITR-L that is mainly expressed on antigen presenting cells (APCs) and endothelial cells [53]. A recent report shows that GITR: GITR-L (DTA-1) signaling in ILC2s promotes autocrine IL-9-induced IL-5 and IL-13 production in the context of papain-induced lung inflammation [54]. Altogether, these findings show that TNFRSF: TNFSF signals are emerging as potent modulators of ILC2 responses to allergens, and suggest that interfering the above pathways may afford clinical benefit in allergic asthma.
NKp30
NKp30 (NCR3, CD337) is an activating type I immunoglobulin-like transmembrane receptor that is mainly expressed on human NK cells, but not present in mice [55, 56]. A subset of ex vivo and cultured ILC2s express NKp30 that engages with its ligand B7-H6, which activates NF-κB signaling in ILC2 to produce type 2 cytokines [57]. Of note, the expression of B7-H6 in lesional skin biopsies of patients with atopic dermatitis was significantly upregulated, suggesting that NKp30: B7-H6 interaction may be involved in ILC2-driven skin inflammation [57].
Other costimulatory molecules
A study reported that ILC2s express MHC-II and can interact with antigen-specific T cells to trigger a dialog in which IL-2 production from T cells enhances ILC2 proliferation and IL-13 production [58]. Moreover, they also found that ILC2s express CD80 and CD86, and blocking antibodies targeting MHC-II or CD80 and CD86 inhibited ILC2 expansion and type-2 cytokine production [58]. Thus, MHC-II and the costimulatory molecules CD80 and CD86 on ILC2s may also play an important role in mediating the crosstalk between ILC2 and T cells in the context of allergic inflammation.
Coinhibitory molecules
KLRG1
Killer cell lectin-like receptor G1 (KLRG1) is typically expressed on NK cells and T cells, and binding to E-cadherin suppresses cytokine production and proliferation of NK and CD8+ T cells [59–61]. Notably, KLRG1 is expressed on mature ILC2s from both mice and humans, and has been identified as a marker of mature tissue ILC2s [16, 62]. KLRG1: E-cadherin interaction inhibits human ILC2 proliferation and IL-5 and IL-13 secretion in response to IL-25 and IL-33 in vitro. Moreover, skin ILC2s from the patients with atopic dermatitis (AD) express higher levels of KLRG1 than that of healthy controls, whereas E-cadherin expression is downregulated in keratinocytes, suggesting that reduced E-cadherin expression may enhance ILC2 responses and lead to disease pathogenesis [16]. In addition, in human asthma, an earlier study found that loss of E-cadherin on lung epithelium associates with asthma severity [63], further suggesting that KLRG1: E-cadherin interaction may be an important negative regulator of ILC2 homeostasis and function. Interestingly, four studies recently showed that sex hormones are important regulators of KLRG1+ ILC2 responses in the context of allergic airway inflammation [64–67]. Of note, lung ILC2s from male mice express higher levels of KLRG1 than that of female mice, though female mice have higher numbers of lung-resident ILC2s [64, 65]. Moreover, a functional subset of KLRG1− ILC2s in females was observed after reproductive age, which contributes to the sex bias in lung ILC2s [65]. However, considering the interaction of KLRG1 and E-cadherin, whether KLRG1− ILC2s from female mice have a more potent function than KLRG1+ subset in male mice requires further investigation. Altogether, these studies suggest that KLRG1 may be an important cell-surface molecule that negatively regulate ILC2 responses and is involved in sex bias in allergic inflammation.
ICOS-L
It is known that ICOS-L on ILC2s can bind with ICOS+ ILC2s in cis or trans formation to promote ILC2 homeostasis and function [32, 34]. Intriguingly, induced Tregs (iTregs) also express ICOS and is necessary to suppress airway hyperreactivity (AHR) [68]. Investigators have shown that direct cell–cell contact is required for the Treg cell–ILC2 interaction [69]. Specifically, ICOS on iTregs can bind with ICOS-L on ILC2s. Their interaction promotes iTregs to produce suppressive cytokines TGF-β and IL-10 that inhibit ILC2 responses in ILC2-dependent asthma [69, 70]. Moreover, the interaction between ICOS-L+ ILC2s and ICOS+ iTregs also restricts the interaction with ICOS on ILC2s, further leading to ILC2 suppression. Therefore, peripheral expansion of iTregs has the potential to become a promising therapeutic target against ILC2-dependent asthma.
PD-1: PD-L1
Programmed death protein 1 (PD-1), a cell-surface receptor that binds to its ligand PD-L1, is a crucial immune checkpoint that can prevent autoimmune diseases via suppressing inflammatory T-cell responses [71, 72]. However, PD-1 and PD-L1 are a pair of important negative regulators in several cancers and immunotherapies targeting their interaction have been well established [72, 73]. Of note, the expression of PD-1 is enhanced on activated ILC2s [74]. Interestingly, PD-1 has also been proposed to be an important negative regulator of KLRG1+ ILC2 function in both mice and humans [75]. An intrinsic defect in PD-1 signaling increased the number of KLRG1+ ILC2s by promoting STAT5 activation [75]. Whereas a study also found that lung ILC2s also express PD-L1 and upon interaction with PD-1 Th2 cells promotes the expression of GATA3 and production of IL-13 by Th2 cells both in vitro and in vivo [76]. Thus, these studies suggest that PD-1 and PD-L1 are important modulators of ILC2 function and their functions in the context allergic inflammation require further studies.
Concluding remarks
Like Th2 cells, ILC2s can produce copious amounts of type 2 cytokines upon activation, and thus play a pivotal role in inducing allergic inflammation. During the past decade, the transcriptional networks coordinating ILC2 development and function have been well documented using microarrays or RNA-Seq [77–80]. However, treating ILC2-driven allergic diseases by targeting their transcriptional factors is difficult due to their roles in other cells. Noteworthy, increasing evidence shows that ILC2 responses are intricately regulated by multiple factors including cytokines, hormones, lipid mediators, neuropeptides, and nutrients,as discussed [14, 15]. Moreover, similar to T cells, ILC2s express various important cell-surface molecules which mediate the interaction of ILC2s with other immune or stromal cells. Their interactions are crucial for ILC2 survival, proliferation, and cytokine production. These findings provide potential therapeutic strategies for tackling ILC2-induced allergic diseases, such as asthma and AD. Importantly, further studies are required to translate such findings into humans.
The following questions remain to be delineated. First, as shown in the above, ILC2s express both co-stimulatory molecules and co-inhibitory molecules, and how these receptors or ligands integrate to affect ILC2 homeostasis and function in different tissues under physiological and pathological conditions needs to be explored. Second, several studies revealed that ILC2s display heterogeneity and plasticity [81–83]. ILC2s can be divided into conventional ILC2s (mainly stimulated by IL-33), and inflammatory ILC2s (mainly stimulated by IL-25) which have plasticity and can convert into conventional ILC2s [24, 84]. Moreover, two subsets of activated lung ILC2 (one producing IL-10 [85] and the other producing IL-17 [86]) were recently found in the context of allergic airway inflammation. As such, it is important to determine whether ILC2 sub-phenotypes are regulated by the above co-signaling molecules. Third, although ILC2s are considered as resident cells [8, 9], several lines of evidence suggest that ILC2s can be recruited to inflamed tissues via sphingosine 1-phosphate (S1P)-mediated chemotaxis [24], or β2 integrins [10]. The trafficking of ILC2s is of particular interest, as it shows that ILC2s, like adaptive T lymphocytes, can be activated locally and exert their function distantly. Further investigation of ILC2 trafficking will certainly provide novel therapeutic strategies for treating ILC2-driven diseases. Finally, as most data are derived from mouse studies, these findings may be different in humans. A better understanding of the roles of cell-surface molecule-mediated cell–cell interactions in human ILC2s would definitely contribute to the development of novel therapeutic strategies for ILC2-mediated allergic inflammation.
Acknowledgements
This work was supported by the following grants to J. Zhou: National Natural Science Foundation of China (Nos. 91542112; 81571520; 81771665; 81742002), Start-up Fund for High-level Talents of Tianjin Medical University, Science and Technology Program of Guangzhou (No. 201707010452), Natural Science Foundation of Guangdong Province (No. 2017B030311014). It was also supported by National Natural Science Foundation of China (No. 81800031 to A. Lei), Natural Science Foundation of Guangdong Province (No. 2018A030313648 to A. Lei) and Research Foundation of Education Bureau of Human Province, China (No. 18C0458).
Compliance with ethical standards
Conflict of interest
The authors have no financial conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou L. Striking similarity: GATA-3 regulates ILC2 and Th2 cells. Immunity. 2012;37(4):589–591. doi: 10.1016/j.immuni.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14(6):536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 3.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 4.Schuijs MJ, Halim TYF. Group 2 innate lymphocytes at the interface between innate and adaptive immunity. Ann N Y Acad Sci. 2018;1417(1):87–103. doi: 10.1111/nyas.13604. [DOI] [PubMed] [Google Scholar]
- 5.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 6.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107(25):11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350(6263):981–985. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moro K, Kabata H, Tanabe M, Koga S, Takeno N, Mochizuki M, Fukunaga K, Asano K, Betsuyaku T, Koyasu S. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol. 2016;17(1):76–86. doi: 10.1038/ni.3309. [DOI] [PubMed] [Google Scholar]
- 10.Karta MR, Rosenthal PS, Beppu A, Vuong CY, Miller M, Das S, Kurten RC, Doherty TA, Broide DH. β2 integrins rather than β1 integrins mediate Alternaria-induced group 2 innate lymphoid cell trafficking to the lung. J Allergy Clin Immunol. 2018;141(1):329–338. doi: 10.1016/j.jaci.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koga S, Hozumi K, Hirano KI, Yazawa M, Terooatea T, Minoda A, Nagasawa T, Koyasu S, Moro K. Peripheral PDGFRα(+)gp38(+) mesenchymal cells support the differentiation of fetal liver-derived ILC2. J Exp Med. 2018;215(6):1609–1626. doi: 10.1084/jem.20172310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serafini N, Vosshenrich CA, Di Santo JP. Transcriptional regulation of innate lymphoid cell fate. Nat Rev Immunol. 2015;15(7):415–428. doi: 10.1038/nri3855. [DOI] [PubMed] [Google Scholar]
- 13.Yang Q, Li F, Harly C, Xing S, Ye L, Xia X, Wang H, Wang X, Yu S, Zhou X, Cam M, Xue HH, Bhandoola A. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat Immunol. 2015;16(10):1044–1050. doi: 10.1038/ni.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurrell BP, Jahani PS, Akbari O. Social networking of group two innate lymphoid cells in allergy and asthma. Front immunol. 2018;9:2694. doi: 10.3389/fimmu.2018.02694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabata H, Moro K, Koyasu S. The group 2 innate lymphoid cell (ILC 2) regulatory network and its underlying mechanisms. Immunol Rev. 2018;286(1):37–52. doi: 10.1111/imr.12706. [DOI] [PubMed] [Google Scholar]
- 16.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, Fallon PG, Ogg GS. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ealey KN, Moro K, Koyasu S. Are ILC2s Jekyll and Hyde in airway inflammation? Immunol Rev. 2017;278(1):207–218. doi: 10.1111/imr.12547. [DOI] [PubMed] [Google Scholar]
- 18.Stanciu LA, Djukanovic R. The role of ICAM-1 on T-cells in the pathogenesis of asthma. Eur Respir J. 1998;11(4):949–957. doi: 10.1183/09031936.98.11040949. [DOI] [PubMed] [Google Scholar]
- 19.Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144(12):4579–4586. [PubMed] [Google Scholar]
- 20.Xu H, Guan H, Zu G, Bullard D, Hanson J, Slater M, Elmets CA. The role of ICAM-1 molecule in the migration of Langerhans cells in the skin and regional lymph node. Eur J Immunol. 2001;31(10):3085–3093. doi: 10.1002/1521-4141(2001010)31:10<3085::AID-IMMU3085>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukhopadhyay S, Malik P, Arora SK, Mukherjee TK. Intercellular adhesion molecule-1 as a drug target in asthma and rhinitis. Respirology. 2014;19(4):508–513. doi: 10.1111/resp.12285. [DOI] [PubMed] [Google Scholar]
- 22.Grunstein MM, Hakonarson H, Maskeri N, Kim C, Chuang S. Intrinsic ICAM-1/LFA-1 activation mediates altered responsiveness of atopic asthmatic airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2000;278(6):L1154–L1163. doi: 10.1152/ajplung.2000.278.6.L1154. [DOI] [PubMed] [Google Scholar]
- 23.Lei AH, Xiao Q, Liu GY, Shi K, Yang Q, Li X, Liu YF, Wang HK, Cai WP, Guan YJ, Gabrilovich DI, Zhou J. ICAM-1 controls development and function of ILC2. J Exp Med. 2018;215(8):2157–2174. doi: 10.1084/jem.20172359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Mao K, Chen X, Sun MA, Kawabe T, Li W, Usher N, Zhu J, Urban JF, Jr, Paul WE, Germain RN. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science. 2018;359(6371):114–119. doi: 10.1126/science.aam5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meli AP, Fontes G, Avery DT, Leddon SA, Tam M, Elliot M, Ballesteros-Tato A, Miller J, Stevenson MM, Fowell DJ, Tangye SG, King IL. The Integrin LFA-1 controls T follicular helper cell generation and maintenance. Immunity. 2016;45(4):831–846. doi: 10.1016/j.immuni.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salomon B, Bluestone JA. LFA-1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J Immunol. 1998;161(10):5138–5142. [PubMed] [Google Scholar]
- 27.Jenks SA, Eisfelder BJ, Miller J. LFA-1 co-stimulation inhibits T(h)2 differentiation by down-modulating IL-4 responsiveness. Int Immunol. 2005;17(3):315–323. doi: 10.1093/intimm/dxh211. [DOI] [PubMed] [Google Scholar]
- 28.Wikenheiser DJ, Stumhofer JS. ICOS co-stimulation: friend or foe? Front Immunol. 2016;7:304. doi: 10.3389/fimmu.2016.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalo JA, Tian J, Delaney T, Corcoran J, Rottman JB, Lora J, Al-garawi A, Kroczek R, Gutierrez-Ramos JC, Coyle AJ. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat Immunol. 2001;2(7):597–604. doi: 10.1038/89739. [DOI] [PubMed] [Google Scholar]
- 30.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, Ling V, Collins M, Sharpe AH, Freeman GJ. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165(9):5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 31.Nurieva RI, Duong J, Kishikawa H, Dianzani U, Rojo JM, Ho I, Flavell RA, Dong C. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18(6):801–811. doi: 10.1016/S1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 32.Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, Freeman GJ, Sharpe AH, Akbari O. ICOS: ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42(3):538–551. doi: 10.1016/j.immuni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamachi F, Isshiki T, Harada N, Akiba H, Miyake S. ICOS promotes group 2 innate lymphoid cell activation in lungs. Biochem Biophys Res Commun. 2015;463(4):739–745. doi: 10.1016/j.bbrc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Paclik D, Stehle C, Lahmann A, Hutloff A, Romagnani C. ICOS regulates the pool of group 2 innate lymphoid cells under homeostatic and inflammatory conditions in mice. Eur J Immunol. 2015;45(10):2766–2772. doi: 10.1002/eji.201545635. [DOI] [PubMed] [Google Scholar]
- 35.Hubo M, Trinschek B, Kryczanowsky F, Tuettenberg A, Steinbrink K, Jonuleit H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front Immunol. 2013;4:82. doi: 10.3389/fimmu.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao X, Zhao L, Wang S, Yang J, Yang X. Enhanced inducible costimulator ligand (ICOS-L) expression on dendritic cells in interleukin-10 deficiency and its impact on T cell subsets in respiratory tract infection. Mol Med. 2013;19:346–356. doi: 10.2119/molmed.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croft M. Co-stimulatory members of the TNFR family: keys to effective T cell immunity? Nat Rev Immunol. 2003;3(8):609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 38.Ward-Kavanagh LK, Lin WW, Sedy JR, Ware CF. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity. 2016;44(5):1005–1019. doi: 10.1016/j.immuni.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol Res. 2014;2(2):142–153. doi: 10.1158/2326-6066.CIR-13-0031-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 41.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins SJ, Perona-Wright G, Worsley AG, Ishii N, MacDonald AS. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J Immunol. 2007;179(6):3515–3523. doi: 10.4049/jimmunol.179.6.3515. [DOI] [PubMed] [Google Scholar]
- 43.Halim TYF, Rana BMJ, Walker JA, Kerscher B, Knolle MD, Jolin HE, Serrao EM, Haim-Vilmovsky L, Teichmann SA, Rodewald HR, Botto M, Vyse TJ, Fallon PG, Li Z, Withers DR, McKenzie ANJ. Tissue-restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity. 2018;48(6):1195–1207. doi: 10.1016/j.immuni.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132(1):205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40(3):425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, Salmond RJ, Liew FY. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192(5):2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 47.Liu B, Lee JB, Chen CY, Hershey GK, Wang YH. Collaborative interactions between type 2 innate lymphoid cells and antigen-specific CD4+ Th2 cells exacerbate murine allergic airway diseases with prominent eosinophilia. J Immunol. 2015;194(8):3583–3593. doi: 10.4049/jimmunol.1400951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li BW, de Bruijn MJ, Tindemans I, Lukkes M, KleinJan A, Hoogsteden HC, Hendriks RW. T cells are necessary for ILC2 activation in house dust mite-induced allergic airway inflammation in mice. Eur J Immunol. 2016;46(6):1392–1403. doi: 10.1002/eji.201546119. [DOI] [PubMed] [Google Scholar]
- 49.Meylan F, Hawley ET, Barron L, Barlow JL, Penumetcha P, Pelletier M, Sciume G, Richard AC, Hayes ET, Gomez-Rodriguez J, Chen X, Paul WE, Wynn TA, McKenzie AN, Siegel RM. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol. 2014;7(4):958–968. doi: 10.1038/mi.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu X, Pappu R, Ramirez-Carrozzi V, Ota N, Caplazi P, Zhang J, Yan D, Xu M, Lee WP, Grogan JL. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7(3):730–740. doi: 10.1038/mi.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meylan F, Davidson TS, Kahle E, Kinder M, Acharya K, Jankovic D, Bundoc V, Hodges M, Shevach EM, Keane-Myers A, Wang EC, Siegel RM. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008;29(1):79–89. doi: 10.1016/j.immuni.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malhotra N, Leyva-Castillo JM, Jadhav U, Barreiro O, Kam C, O’Neill NK, Meylan F, Chambon P, von Andrian UH, Siegel RM, Wang EC, Shivdasani R, Geha RS. RORα-expressing T regulatory cells restrain allergic skin inflammation. Sci Immunol. 2018;3:21. doi: 10.1126/sciimmunol.aao6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur J Immunol. 2005;35(4):1016–1022. doi: 10.1002/eji.200425818. [DOI] [PubMed] [Google Scholar]
- 54.Nagashima H, Okuyama Y, Fujita T, Takeda T, Motomura Y, Moro K, Hidaka T, Omori K, Sakurai T, Machiyama T, Ndhlovu LC, Riccardi C, So T, Ishii N. GITR cosignal in ILC2s controls allergic lung inflammation. J Allergy Clin Immunol. 2018;141(5):1939–1943. doi: 10.1016/j.jaci.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 55.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, Marcenaro E, Accame L, Malaspina A, Biassoni R, Bottino C, Moretta L, Moretta A. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190(10):1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hollyoake M, Campbell RD, Aguado B. NKp30 (NCR3) is a pseudogene in 12 inbred and wild mouse strains, but an expressed gene in Mus caroli. Mol Biol Evol. 2005;22(8):1661–1672. doi: 10.1093/molbev/msi162. [DOI] [PubMed] [Google Scholar]
- 57.Salimi M, Xue L, Jolin H, Hardman C, Cousins DJ, McKenzie AN, Ogg GS. Group 2 innate lymphoid cells express functional NKp30 receptor inducing type 2 cytokine production. J Immunol. 2016;196(1):45–54. doi: 10.4049/jimmunol.1501102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, Ogg GS, Fallon PG, McKenzie AN. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41(2):283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robbins SH, Nguyen KB, Takahashi N, Mikayama T, Biron CA, Brossay L. Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J Immunol. 2002;168(6):2585–2589. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
- 60.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100(10):3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 61.Rosshart S, Hofmann M, Schweier O, Pfaff AK, Yoshimoto K, Takeuchi T, Molnar E, Schamel WW, Pircher H. Interaction of KLRG1 with E-cadherin: new functional and structural insights. Eur J Immunol. 2008;38(12):3354–3364. doi: 10.1002/eji.200838690. [DOI] [PubMed] [Google Scholar]
- 62.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37(4):634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masuyama K, Morishima Y, Ishii Y, Nomura A, Sakamoto T, Kimura T, Mochizuki M, Uchida Y, Sekizawa K. Sputum E-cadherin and asthma severity. J Allergy Clin Immunol. 2003;112(1):208–209. doi: 10.1067/mai.2003.1526. [DOI] [PubMed] [Google Scholar]
- 64.Laffont S, Blanquart E, Savignac M, Cenac C, Laverny G, Metzger D, Girard JP, Belz GT, Pelletier L, Seillet C, Guery JC. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017;214(6):1581–1592. doi: 10.1084/jem.20161807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kadel S, Ainsua-Enrich E, Hatipoglu I, Turner S, Singh S, Khan S, Kovats S. A major population of functional KLRG1(-) ILC2s in female lungs contributes to a sex bias in ILC2 numbers. Immunohorizons. 2018;2(2):74–86. doi: 10.4049/immunohorizons.1800008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cephus JY, Stier MT, Fuseini H, Yung JA, Toki S, Bloodworth MH, Zhou W, Goleniewska K, Zhang J, Garon SL, Hamilton RG, Poloshukin VV, Boyd KL, Peebles RS, Jr, Newcomb DC. Testosterone attenuates group 2 Innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21(9):2487–2499. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warren KJ, Sweeter JM, Pavlik JA, Nelson AJ, Devasure JM, Dickinson JD, Sisson JH, Wyatt TA, Poole JA. Sex differences in activation of lung-related type 2 innate lymphoid cells in experimental asthma. Ann Allergy Asthma Immunol. 2017;118(2):233–234. doi: 10.1016/j.anai.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS–ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8(9):1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 69.Rigas D, Lewis G, Aron JL, Wang B, Banie H, Sankaranarayanan I, Galle-Treger L, Maazi H, Lo R, Freeman GJ, Sharpe AH, Soroosh P, Akbari O. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T cell costimulator–inducible T cell costimulator ligand interaction. J Allergy Clin Immunol. 2017;139(5):1468–1477. doi: 10.1016/j.jaci.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aron JL, Akbari O. Regulatory T cells and type 2 innate lymphoid cell-dependent asthma. Allergy. 2017;72(8):1148–1155. doi: 10.1111/all.13139. [DOI] [PubMed] [Google Scholar]
- 71.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chamoto K, Al-Habsi M, Honjo T. Role of PD-1 in immunity and diseases. Curr Top Microbiol Immunol. 2017;410:75–97. doi: 10.1007/82_2017_67. [DOI] [PubMed] [Google Scholar]
- 73.Balar AV, Weber JS. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother. 2017;66(5):551–564. doi: 10.1007/s00262-017-1954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu Y, Tsang JC, Wang C, Clare S, Wang J, Chen X, Brandt C, Kane L, Campos LS, Lu L, Belz GT, McKenzie AN, Teichmann SA, Dougan G, Liu P. Single-cell RNA-seq identifies a PD-1(hi) ILC progenitor and defines its development pathway. Nature. 2016;539(7627):102–106. doi: 10.1038/nature20105. [DOI] [PubMed] [Google Scholar]
- 75.Taylor S, Huang Y, Mallett G, Stathopoulou C, Felizardo TC, Sun MA, Martin EL, Zhu N, Woodward EL, Elias MS, Scott J, Reynolds NJ, Paul WE, Fowler DH, Amarnath S. PD-1 regulates KLRG1(+) group 2 innate lymphoid cells. J Exp Med. 2017;214(6):1663–1678. doi: 10.1084/jem.20161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwartz C, Khan AR, Floudas A, Saunders SP, Hams E, Rodewald HR, McKenzie ANJ, Fallon PG. ILC2s regulate adaptive Th2 cell functions via PD-L1 checkpoint control. J Exp Med. 2017;214(9):2507–2521. doi: 10.1084/jem.20170051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang J, Malhotra N. Transcription factor networks directing the development, function, and evolution of innate lymphoid effectors. Annu Rev Immunol. 2015;33:505–538. doi: 10.1146/annurev-immunol-032414-112025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhong C, Zhu J. Transcriptional regulatory network for the development of innate lymphoid cells. Mediat Inflamm. 2015;2015:264502. doi: 10.1155/2015/264502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seillet C, Mielke LA, Amann-Zalcenstein DB, Su S, Gao J, Almeida FF, Shi W, Ritchie ME, Naik SH, Huntington ND. Deciphering the innate lymphoid cell transcriptional program. Cell Rep. 2016;17(2):436–447. doi: 10.1016/j.celrep.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 80.Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK, Gilfillan S, Colonna M. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16(3):306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lim AI, Menegatti S, Bustamante J, Le Bourhis L, Allez M, Rogge L, Casanova J-L, Yssel H, Di Santo JP. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J Exp Med. 2016;213(4):569–583. doi: 10.1084/jem.20151750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li BW, Stadhouders R, De Bruijn MJ, Lukkes M, Beerens DM, Brem MD, KleinJan A, Bergen I, Vroman H, Kool M. Group 2 innate lymphoid cells exhibit a dynamic phenotype in allergic airway inflammation. Front Immunol. 2017;8:1684. doi: 10.3389/fimmu.2017.01684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour RE, Nyman J, Dionne D, Hofree M, Cuoco MS, Rodman C, Farouq D, Haas BJ, Tickle TL, Trombetta JJ, Baral P, Klose CSN, Mahlakoiv T, Artis D, Rozenblatt-Rosen O, Chiu IM, Levy BD, Kowalczyk MS, Regev A, Kuchroo VK. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature. 2017;549(7672):351–356. doi: 10.1038/nature24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, Williamson PR, Urban JF, Jr, Paul WE. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. 2015;16(2):161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seehus CR, Kadavallore A, Torre B, Yeckes AR, Wang Y, Tang J, Kaye J. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat Commun. 2017;8(1):1900. doi: 10.1038/s41467-017-02023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai T, Qiu J, Ji Y, Li W, Ding Z, Suo C, Chang J, Wang J, He R, Qian Y, Guo X, Zhou L, Sheng H, Shen L. IL-17-producing ST2(+) group 2 innate lymphoid cells play a pathogenic role in lung inflammation. J Allergy Clin Immunol. 2019;143(1):229–244. doi: 10.1016/j.jaci.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]