Abstract
Low density lipoprotein receptor-related protein (LRP) 1 modulates cell adhesion and motility under normal and pathological conditions. Previous studies documented that LRP1 binds several integrin receptors and mediates their trafficking to the cell surface and endocytosis. However, the mechanism by which LRP1 may regulate integrin activation remains unknown. Here we report that LRP1 promotes the activation and subsequent degradation of β1 integrin and thus supports cell adhesion, spreading, migration and integrin signaling on fibronectin. LRP1 interacts with surface β1 integrin, binds the integrin activator kindlin2 and stimulates β1 integrin–kindlin2 complex formation. Specifically, serine 76 in the LRP1 cytoplasmic tail is crucial for the interaction with kindlin2, β1 integrin activation and cell adhesion. Interestingly, a loss of LRP1 induces the accumulation of several integrin receptors on the cell surface. Following internalization, intracellular trafficking of integrins is driven by LRP1 in a protein kinase C- and class II myosin-dependent manner. Ultimately, LRP1 dictates the fate of endocytosed β1 integrin by directing it down the pathway of lysosomal and proteasomal degradation. We propose that LRP1 mediates cell adhesion by orchestrating a multi-protein pathway to activate, traffic and degrade integrins. Thus, LRP1 may serve as a focal point in the integrin quality control system to ensure a firm connection to the extracellular matrix.
Electronic supplementary material
The online version of this article (10.1007/s00018-017-2707-6) contains supplementary material, which is available to authorized users.
Keywords: Integrins, Adhesion, Lysosomes, LRP1
Introduction
Integrins connect the extracellular matrix (ECM) with the cytoskeleton and are thus essential for cell adhesion and migration. Integrin expression, function and trafficking are tightly controlled and these processes are deregulated in a broad range of human pathologies, including cancer, thrombosis, inflammatory diseases and fibrosis [1, 2]. Although a large number of proteins were identified to modulate integrin activation and subcellular distribution, the mechanisms regulating integrin degradation have only recently gained attention [3, 4]. Low density lipoprotein receptor-related protein (LRP) 1 is a large, cell surface receptor mediating the endocytosis and degradation of a great variety of ligands, including proteinase–inhibitor complexes and ECM proteins [5]. In addition, LRP1 serves as a signaling receptor by recognition of several growth factors, thus modulating the PDGF-BB, TGF-β, BMP, IGF and SHH pathways [6]. LRP1 interacts with integrin αM, αL [7] and β1 [8, 9] subunits and αMβ1 [10] and αMβ2 [7] complexes. Additionally, it facilitates β1 [8], αVβ3, αVβ5 [11] and αM [12] integrin endocytosis as well as β1 integrin maturation and insertion into the plasma membrane [13]. Furthermore, LRP1 was identified as a member of the focal adhesion proteome [14, 15] and it was shown to regulate focal adhesion number, composition and turnover [8, 16, 17]. Given its numerous interactions with structural and regulatory focal adhesion proteins it is, therefore, not surprising that LRP1 was reported to modulate cell adhesion, spreading and migration. For example, LRP1-β2 integrin interplay was found to be critical for leukocyte adhesion to the endothelium [7] and macrophage adhesion to and migration on fibrin [12]. Moreover, LRP1 was described to be required to increase the levels of activated β2 integrin on the neutrophil surface [18]. Nevertheless, despite an evident modulatory role of LRP1 on integrin-mediated processes, a detailed characterization of the underlying mechanism is still missing. In the present study, we systematically investigated LRP1 influence on integrin function and we report that LRP1 promotes β1 integrin activation and subsequently drives its trafficking and degradation.
Materials and methods
Cell isolation and culture
Primary human lung fibroblasts (pHLF) were isolated as previously described [19]. Wild-type and LRP1−/− mouse embryonic fibroblasts (MEF) were a kind gift of Prof. Dr. Thomas Willnow (Max Delbrück Center for Molecular Medicine, Berlin, Germany). pHLF and MEF were maintained in Dulbecco’s modified Eagle medium F-12 (Thermo Fisher Scientific, Darmstadt, Germany) supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin at 37 °C in humidified atmosphere with 5% CO2.
Cell transfection and treatment
Experiments were performed on cells cultured on plastic surfaces coated with 10 µg/ml fibronectin (Sigma-Aldrich, Hamburg, Germany). Cells were transfected with DNA or siRNA at 60–70% confluence using Lipofectamine® 2000 (Thermo Fisher Scientific) or siLentFect™ (Bio-Rad, Munich, Germany), respectively, and analyzed after 24 h. siRNA directed against human LRP1 (sense strand 5′-CCUGUAACCUGCAGUGCUUdTdT-3′) was synthesized by Microsynth AG (Lindau, Germany). Kindlin2-targeting and scrambled siRNA were purchased from Santa Cruz Biotechnology (Heidelberg, Germany) and Ambion (Austin, Texas), respectively. Cells were treated with following reagents: MG132, Blebbistatin, Bafilomycin A1, phorbol 12-myristate 13-acetate (all from Sigma-Aldrich), Ro 32-0432 (Tocris, Bristol, UK), MnCl2, EDTA (both from Roth, Karlsruhe, Germany), purified recombinant RAP (R&D Systems, Wiesbaden, Germany), and native (American Diagnostica, Stamford, Connecticut) or methylamine-activated (BioMac, Leipzig, Germany) α2-macroglobulin.
Mutagenesis
Plasmids encoding LRP1 mini-receptors or the intracellular-transmembrane region of the receptor were a kind gift of Dudley K. Strickland (University of Maryland School of Medicine, Baltimore, MD, USA). Vector encoding GST-fused LRP1 cytoplasmic tail (GST-LRP1cyt) was prepared as described previously [20]. Point mutations were introduced with the QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA). All mutations were confirmed by DNA sequencing.
Flow cytometry
Detached cells were surface-labeled for 1 h at 4 °C with the following antibodies: rat anti-β1 integrin (clone MB1.2, Millipore, Darmstadt, Germany), hamster anti-β3 integrin, rat anti-β1 integrin (clone 9EG7), anti-αv integrin, anti-α5 integrin (all from BD Biosciences, Franklin Lakes, NJ, USA). Flow cytometry was carried out with a FACSCantoTMII cytometer equipped with FACS DiVa software (BD Biosciences) using standard procedures. Data analysis was performed using the FlowJo program (version 9.4.10).
Real-time PCR
RNA isolation, reverse transcription and real-time PCR were conducted as described previously [19]. The following primers were used: α5 integrin forward: 5′-CTGCAAGGTGGTGCTGTCTA-3′ and reverse: 5′-TCCTCTCCCTTGGCACTGTA-3′; β1 integrin forward: 5′-CGATCCTGTGACCCATTGC-3′ and reverse: 5′-TGATGTCGGGACCAGTAGGAC-3′; and β-actin forward: 5′-AGAGGGAAATCGTGCGTGAC-3′; and reverse: 5′-CAATAGTGATGACCTGGCCGT-3′, serving as a reference gene. Changes in gene expression are reported as a ΔCt (Ctβ-actin − CtTarget gene).
Western blotting
Proteins were isolated in a lysis buffer containing 20 mM Tris, pH 7.6, 100 mM NaCl, 2 mM EDTA, 10% glycerol, 1% NP-40, 1 mM Na3VO4, 1 mM PMSF and 1 μg/ml Complete Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN, USA), separated on a 10% SDS polyacrylamide gel and transferred to a PVDF membrane. Proteins were detected with rabbit anti-LRP1 (Epitomics, Burlingame, CA, USA), mouse anti-ubiquitin, mouse anti-kindlin2 (Millipore), mouse anti-β1 integrin (BD Biosciences) and mouse anti-β-actin (Sigma-Aldrich). Rabbit anti-α5 integrin, anti-raf-1, anti-p44/42, anti-src, anti-pak1/2, anti-FAK, anti-paxillin and corresponding phospho-specific rabbit and mouse (p44/42) antibodies were from Cell Signaling Technology (Leiden, The Netherlands).
Surface biotinylation
Cell surface proteins were labeled with EZ-Link™ Sulfo-NHS-SS-Biotin (Thermo Fisher Scientific) for 1 h at 4 °C. After quenching and cell lysis, biotinylated proteins were purified on Pierce™ NeutrAvidin™ Agarose beads (Thermo Fisher Scientific) and analyzed by western blotting.
Immunoprecipitation
Rat anti-β1 integrin (clone MB1.2), mouse anti-kindlin2 (both from Millipore) and rat anti-β1 integrin (clone 9EG7, BD Biosciences) antibodies were incubated with pre-cleared protein lysates at 4 °C overnight. Alternatively, live cells were surface-labeled with an anti-β1 integrin antibody for 1 h at 4 °C, washed with cell culture medium to remove the unbound antibody, subsequently incubated at 37 °C for 16 h and lysed. Immune complexes were captured with Protein G Sepharose™ 4 Fast Flow (GE Healthcare, Munich, Germany) and analyzed by western blotting or MALDI-TOF mass spectrometry.
Silver staining, tryptic in-gel digestion and MALDI-TOF mass spectrometry
Protein identification was performed as previously described [21]. Briefly, proteins were separated on SDS–polyacrylamide gel, detected using a Silver Stain Plus™ Kit (Bio-Rad) and digested with trypsin following reduction and carbamidomethylation. Peptides were analyzed with an Ultraflex TOF/TOF mass spectrometer (Bruker Daltonics, Bremen, Germany). Proteins were identified using obtained mass fingerprints of tryptic digests with Mascot software (http://www.matrixscience.com).
GST and peptide pull-down
GST-LRP1cyt was over-expressed in E. coli strain BL21, purified on Pierce™ Glutathione agarose beads (Thermo Fisher Scientific). N-terminally biotinylated peptides corresponding to the intracellular domain of β1 integrin (HDRREFAKFEKEKMNAKWDTGENPIYKSAVTTVVNPKYEGK) and the scrambled control (EYEFEPDKVDTGAKGTKMAKNEKKFRNYTVHNIWESRKVAP) were synthesized by (ProteoGenix SAS, Schiltigheim, France). Peptides were immobilized on Pierce™ NeutrAvidin™ Agarose beads (Thermo Fisher Scientific). Subsequently, the beads were incubated with protein lysates, washed with lysis buffer and the proteins interacting with LRP1cyt or β1 integrin peptides were determined by western blotting.
Cell adhesion and migration assays
For cell adhesion analysis, 20 × 103 cells were seeded onto 96-well-plate coated with 10 µg/ml collagen I (Corning, Wiesbaden, Germany), fibronectin, fibrinogen, vitronectin or BSA (Sigma-Aldrich). After 15 min incubation at 37 °C, cells were fixed with cold methanol/acetone solution (1:1) for 1 h at 4 °C and subsequently stained with crystal violet (Sigma-Aldrich). The absorbance at 560 nm was measured with a microplate reader. Cell migration was analyzed by a gap closure assay as described previously [22].
Integrin internalization assay
Cells in serum-free medium were allowed to attach to fibronectin (10 µg/ml) for 45 min and subsequently surface-labeled with NHS-SS-biotin (Thermo Fisher Scientific) for 30 min at 4 °C. Labeled surface proteins were internalized in serum-free medium at 37 °C for different time points before removing the remaining surface label with sodium 2-mercaptoethanesulfonate (MesNa, Sigma-Aldrich) for 20 min at 4 °C. After MesNa was quenched with iodoacetamide (Sigma-Aldrich), cells were lysed and protein lysates were added to 96-well plates coated with an anti-β1 integrin antibody (clone MB1.2) and incubated at 4 °C overnight. Subsequently, the plates were incubated with streptavidin-HRP for 1 h at 4 °C. Captured biotinylated β1 integrin was detected using an ELISA plate reader after incubation with ABTS peroxidase substrate (Vector Laboratories, Eching, Germany).
Confocal microscopy
Immunocytochemical analysis was performed on cells adherent to plastic‐immobilized fibronectin (10 µg/ml). Unless indicated otherwise, antibodies were added to the cells after fixation with 4% PFA. The cells were stained with primary antibodies at 4 °C overnight and subsequently incubated with secondary antibodies at room temperature for 1 h. Additionally, cells were stained with Rhodamine Phalloidin (Thermo Fisher Scientific), LysoTracker® (Cambrex, Wiesbaden, Germany) or DAPI (Vector Laboratories). Immunofluorescent images were acquired with a Leica TCS SP5 X confocal microscope using Leica LAS AF software (Leica Microsystems, Wetzlar, Germany).
Live-cell imaging
Bright-field images of live cells maintained at 37 °C in a humidified atmosphere containing 5% CO2 were recorded on a Leica DMI6000 B live imaging microscope (Leica Microsystems) with a DFC 365 FX camera and motorized stage. Time-lapse videos were generated using ImageJ software.
Microscale thermophoresis
The influence of LRP1 on the kindlin2–α5β1 integrin interaction was determined by microscale thermophoresis as described previously [22, 23]. To this end, cells were transfected with GFP-kindlin2-encoding plasmid [3] and lysed after 24 h. A 12-fold titration series of purified recombinant α5β1 integrin (1750 nM to 0.854 nM, R&D Systems) diluted 1:1 with PBS containing 0.05% Tween-20 was prepared, while the lysate concentration was kept constant at 7.25 mg/ml. The mixtures containing diluted integrin and cell lysate (3.5 µl each) were incubated for 30 min to enable binding and subsequently transferred into glass capillaries. The thermophoretic movement was detected using a Monolith NT.115 device (NanoTemper Technologies, Munich, Germany) with the blue laser on for 30 s and off for 5 s at a laser voltage of 50%. Fluorescence was measured before laser on (F Initial) and 30 s after laser-on time (F Hot). The fluorescence was normalized F Norm = F Initial/F Hot and reflects the ratio of labeled molecules. F Norm was plotted directly and multiplied by 10, resulting in a relative change in fluorescence per mill. The determination of a K D value was not possible due to the fact that the concentration of eGFP-labeled kindlin2 was unknown.
Statistical analysis
Analysis of variance followed by Tukey’s post hoc test was used for the comparison of multiple groups. For the comparison of two groups, Student’s t test was used. p < 0.05 was considered statistically significant. Data are expressed as mean ± SD of at least three independent experiments.
Results
LRP1 promotes fibroblast adhesion, spreading and migration
To gain an overview as to how LRP1 influences cell adhesion to various ECM proteins, wild-type and LRP1 knock-out MEF were seeded on collagen-, fibrinogen-, fibronectin- or vitronectin-coated plates. As shown in Fig. 1a and in supplementary Fig. S1a, LRP1 knock-out cells revealed a reduced adhesion to fibronectin and vitronectin by approximately 50% at the 10 min time point after seeding. Both wild-type and LRP1−/− MEF did not adhere to collagen and fibrinogen. Similarly, a siRNA-mediated depletion of LRP1 in primary human lung fibroblasts (pHLF) resulted in the reduced cell adhesion to fibronectin (Fig. 1b and supplementary Fig. S1b). Time-course experiments revealed that the adhesion of LRP1−/− MEF to fibronectin was markedly reduced during the first 25 min period after seeding (Fig. 1c) and that these cells did not spread within 80 min after plating (Fig. 1d and supplementary Movie 1). In addition, we observed a reduced migration on fibronectin (Fig. 1e and supplementary Fig. S1c) and a pronounced cytoskeletal rearrangement characterized by the disruption of F-actin network (supplementary Fig. S1d) in LRP1−/− MEF. LRP1 depletion in MEF and in pHLF was confirmed by western blot (supplementary Fig. S1e and S1f).
Fig. 1.
Fibroblast adhesion and migration depends on LRP1. a Wild-type and LRP1−/− mouse embryonic fibroblasts (MEF) adhesion on plates coated with collagen (COL), fibrinogen (FG), fibronectin (FN), vitronectin (VN) or bovine serum albumin (BSA) as assessed by crystal violet staining. b Adhesion of primary human lung fibroblasts (pHLF) transfected with scrambled (scr) or LRP1-targeting siRNA to FN measured by crystal violet staining. c Time course of wild-type and LRP1−/− MEF adhesion on FN as assessed by crystal violet assay. d Representative bright-field image stills of live-cell imaging of wild-type and LRP1−/− MEF during adhesion to FN. Scale bar: 50 µm (n = 3). e Migration of wild-type and LRP1−/− MEF as assessed by gap closure assay. f Phosphorylation of focal adhesion proteins in wild-type and LRP1−/− MEF during adhesion on FN investigated by western blotting. g Western blot for Paxillin and FAK phosphorylation in scr or anti-LRP1 siRNA-transfected pHLF at 15 min after seeding onto FN-coated plates. Data in a–c, e are mean ± SEM of three to five experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Western blots in f and g are representative of three experiments
Activity of focal adhesion molecules is regulated by LRP1 during cell adhesion
In line with a reduced adhesion and spreading we observed decreased Paxillin and Raf-1 phosphorylation within 20 min of adhesion in LRP1−/− MEF (Fig. 1f and supplementary Fig. S2a and S2b). Furthermore, phosphorylation of FAK and inhibitory phosphorylation of Src at Y527 was reduced at the initial time points of adhesion in LRP1-negative MEF but later normalized to the level observed in wild-type cells (Fig. 1f and supplementary Fig. S2c to S2e). p44/42 and Pak1/2 phosphorylation was not affected by LRP1 depletion (Fig. 1f and supplementary Fig. S2f and S2g). Reduced Paxillin and FAK phosphorylation during adhesion to fibronectin was also observed in pHLF after LRP1 knock-down (Fig. 1g). Fluorescence microscopy showed that Paxillin does not localize to peripheral adhesion structures in LRP1−/− MEF during 40 min of adhesion to fibronectin, while FAK distribution appeared similar in both cell types (supplementary Fig. S2h).
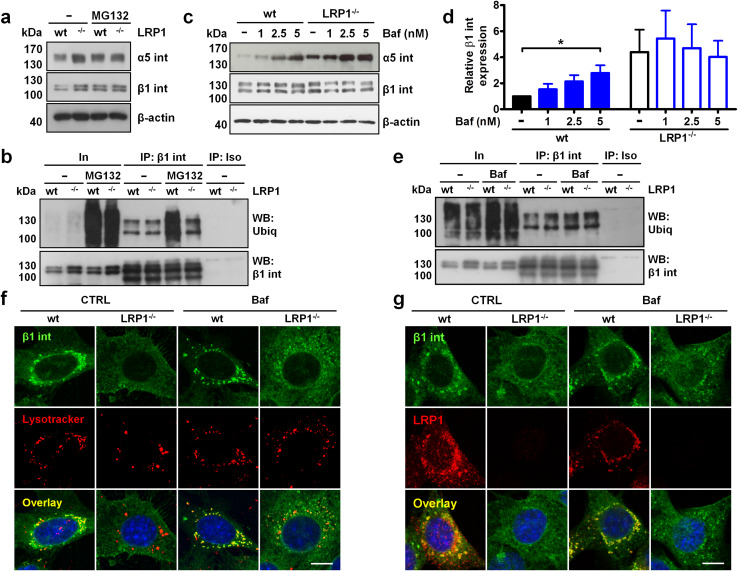
LRP1 stimulates proteasomal and lysosomal degradation of β1 integrin
Since the absence of LRP1 blunted the activation of focal adhesion molecules during cell adhesion to fibronectin, we measured the levels of fibronectin receptor α5β1 integrin. Surprisingly, LRP1−/− cells displayed increased total protein levels of both integrin subunits (Fig. 2a and supplementary Fig. S3a and S3b). This protein accumulation did not result from an increased transcription of α5 and β1 subunit-encoding genes as mRNA levels were unaffected by LRP1 knock-out (supplementary Fig. S3c and S3d, respectively). To gain an insight into the mechanism underlying integrin accumulation, we inhibited the proteasome function with MG132. As seen in Fig. 2a and in supplementary Fig. S3a and S3b, MG132 had no effect on the α5β1 integrin protein expression in LRP1−/− cells but increased β1 and α5 integrin protein levels in wild-type cells, suggesting that LRP1 increases proteasomal degradation of α5β1 integrin. To prove that LRP1 affects β1 integrin degradation in the proteasome, we immunoprecipitated β1 integrin using antibody recognizing the active conformation of this integrin (clone 9EG7) and assessed its ubiquitination levels. Active β1 integrin displayed similar ubiquitination levels in untreated LRP1-negative and -positive cells (Fig. 2b). However, proteasome inhibition with MG132 induced pronounced integrin ubiquitination only in wild-type MEF, confirming that active β1 integrin is degraded in the proteasome in a LRP1-dependent fashion.
Fig. 2.
LRP1 promotes proteasomal and lysosomal degradation of β1 integrin. a Western blot analysis of α5 and β1 integrin (int) expression in wild-type and LRP1−/− MEF treated with 10 µM MG132 for 16 h. b Ubiquitination of active β1 integrin immunoprecipitated with 9EG7 antibody from wild-type or LRP1−/− MEF treated with 10 µM MG132 for 16 h. In input, IP immunoprecipitated material, Iso isotype-matched control antibody. c Expression of α5 and β1 integrin in wild-type and LRP1−/− MEF treated with bafilomycin A1 (Baf) for 16 h. d Densitometric analysis of β1 integrin expression from c. Data are expressed as a ratio of β1 integrin to β-actin band density. e Ubiquitination of active β1 integrin immunoprecipitated with 9EG7 antibody from wild-type or LRP1−/− MEF treated with 2.5 nM Baf for 16 h. f, g Subcellular distribution of β1 integrin and lysosomes labeled with lysotracker (f) or LRP1 (g) in wild-type and LRP1−/− MEF treated with 2.5 nM Baf for 16 h was analyzed by confocal microscopy (middle focal plane). Nuclei were counterstained with DAPI, scale bars: 5 µm (n = 4). Western blots in a–c, e are representative of three to five experiments. β-Actin served as a loading control. Data in d are mean ± SEM of five experiments; *p ≤ 0.05
As inhibition of the proteasome failed to increase β1 integrin levels to those observed in LRP1−/− cells (Fig. 2a, b) we next examined the possibility that LRP1 also modulates lysosomal degradation of integrins [4]. Bafilomycin A1 (Baf), an inhibitor of lysosomal acidification, dose-dependently increased α5 integrin levels in wild-type MEF and to a lesser extent in LRP1−/− cells (Fig. 2c, upper panel). Interestingly, Baf treatment induced β1 integrin accumulation in wild-type cells while β1 integrin levels in LRP1−/− MEF remained unaffected (Fig. 2c, middle panel and Fig. 2d). Since lysosomal degradation of endocytosed β1 integrin driven by an endosomal sorting complex required for the transport (ESCRT) machinery depends on the attachment of ubiquitin moieties [4], we asked whether LRP1 influences β1 integrin ubiquitination after Baf treatment. As seen in Fig. 2e, Baf slightly induced the accumulation of ubiquitinated active β1 integrin in LRP1-positive, but not LRP1-deficient, MEF. To visualize whether LRP1 depletion affects the integrin presence in the lysosomal compartment, we co-stained wild-type and LRP1−/− MEF with Lysotracker and an anti-β1 integrin antibody. In a striking contrast to wild-type MEF, LRP1−/− cells exhibited almost no β1 integrin staining in the lysosomes (Fig. 2f). Baf enhanced β1 integrin accumulation in lysosomes in wild-type MEF and to a lesser extent in LRP1−/− cells (Fig. 2f). When quantified, ≈ 50% LRP1-expressing cells showed β1 integrin localization in lysosomes, whereas such a signal was only observed in 20% LRP1−/− cells (supplementary Fig. S3e). Baf increased vesicular β1 integrin staining in both cell types to 85 and 55%, respectively. Interestingly, β1 integrin colocalized with LRP1 in the same perinuclear vesicles independently of the Baf treatment (Fig. 2g). Collectively, these data suggest that LRP1 exerts a two-layer control of β1 integrin metabolism by promoting proteasomal degradation and integrin trafficking down the lysosomal proteolysis pathway.
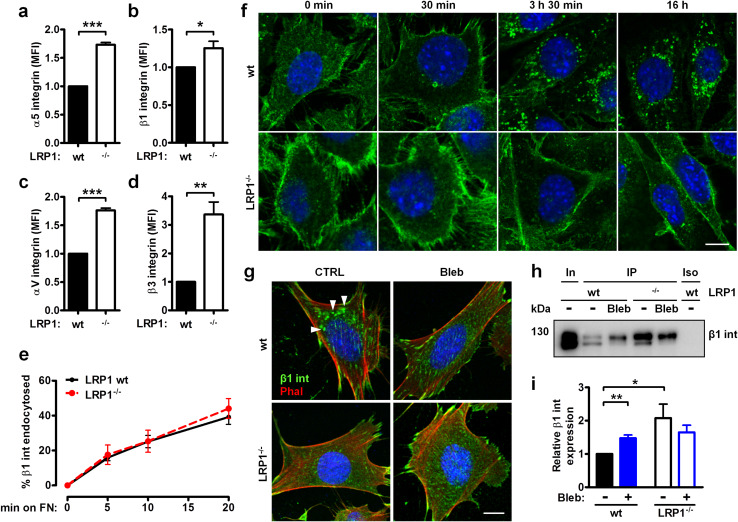
LRP1 reduces integrin abundance at the cell surface and directs intracellular trafficking of endocytosed β1 integrin
Since the loss of LRP1 attenuated integrin degradation, we next profiled the cell surface expression of fibronectin- and vitronectin-binding integrin receptors. Strikingly, all tested integrin subunits, α5, β1, αv and β3, showed a higher abundance on the LRP1−/− MEF cell surface as measured by flow cytometry (Fig. 3a–d) and cell surface biotinylation (supplementary Fig. S4a). However, surface labeling with a cleavable biotin showed that both cell types displayed similar β1 integrin internalization rates (Fig. 3e). As these results indicated that LRP1 is not involved in β1 integrin endocytosis, we speculated that LRP1 affects the intracellular trafficking of integrins. To track the fate of endocytosed integrins, we surface-labeled the cells with an anti-total β1 integrin antibody at 4 °C for 1 h and subsequently shifted the cells back to the 37 °C to allow integrin internalization and subsequent intracellular trafficking. As depicted in Fig. 3f, β1 integrin accumulated in distinct perinuclear vesicles during the 3 h 30 min incubation at 37 °C. Strikingly, β1 integrin did not localize to the similar structures in LRP1−/− cells during the same time period or even during longer (16 h) incubation. Moreover, we found that internalized β1 integrin detected by antibodies recognizing total integrin (supplementary Fig. S4b) or active integrin conformation (supplementary Fig. S4c), colocalized with LRP1 in the same perinuclear vesicles after the cells were shifted to 37 °C for 3 h 30 min. Surface β1 integrin labeled with antibody detecting active conformation did not accumulate in the perinuclear vesicles in LRP1-negative MEF (supplementary Fig. S4c).
Fig. 3.
LRP1 regulates surface levels and intracellular trafficking of endocytosed integrins. a–d Surface expression of α5 (a), β1 (b), αV (c) and β3 (d) integrins on wild-type and LRP1−/− MEF as assessed by flow cytometry. e β1 integrin internalization was analyzed by capture ELISA. f Confocal microscopy analysis of β1 integrin endocytosis. Live cells were surface-labeled with anti-total β1 integrin antibody (clone MB1.2, green) for 1 h at 4 °C and returned to 37 °C for indicated time. g, h Cells were treated with 1 µM blebbistatin (Bleb) for 1 h, surface-labeled with anti-total β1 integrin antibody as in f, and subsequently treated with blebbistatin at 37 °C for 3 h 30 min (g) or 16 h (h). g Distribution of β1 integrin (int, green) and phalloidin-stained F-actin (phal, red) was analyzed by confocal microscopy. Arrows indicate β1 integrin-positive vesicles. h To assess degradation of endocytosed β1 integrin, antibody-integrin complexes were purified from cell lysates. In, input; IP, immunoprecipitated material; Iso, isotype-matched control antibody. i Densitometric analysis of the immunoprecipitated β1 integrin levels from h. Data in a–e and in i are mean ± SEM of three to five experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Nuclei in f and g were counterstained with DAPI, scale bar: 5 µm (n = 3). Western blot in h is representative of three experiments
To understand how LRP1 drives the intracellular trafficking of endocytosed integrins, proteins co-immunoprecipitating with LRP1 were identified by MALDI-TOF mass spectrometry. Using this approach, we determined that the major vault protein and the constituents of the cytoskeleton, namely myosin heavy chain 9 (MYH9), β- and γ-actin and vimentin, bind LRP1 (supplementary Fig. S4d). Given that MYH9 interacts with integrins and mediates de-adhesion [24], we used blebbistatin (Bleb) to test whether the inhibition of class II myosin could affect perinuclear accumulation of β1 integrin. Figure 3g illustrates that Bleb prevented translocation of cell surface-labeled active β1 integrin to perinuclear vesicles in wild-type MEF but not in LRP1-deficient cells. Furthermore, antibody-labeled surface β1 integrin accumulated in LRP1-positive MEF after 16 h of treatment with Bleb (Fig. 3h, i). On the contrary, Bleb did not affect the surface expression of β1 integrin in LRP1−/− cells. In sum, these data indicate that LRP1 regulates intracellular trafficking of integrins by utilizing myosin.
Integrin activation and subsequent degradation is promoted by LRP1
To address the apparent discrepancy between higher integrin levels and lower adhesion of LRP1−/− cells to fibronectin, we measured surface levels of active β1 integrin. The integrin activation index (active-to-total β1 integrin ratio) assessed by flow cytometry using 9EG7 and MB1.2 antibodies, respectively, was lower in LRP1−/− MEF when compared to control cells (Fig. 4a). Activated α2-macroglobulin (α2M), a LRP1 ligand, increased the active-to-total β1 integrin ratio exclusively in wild-type MEF (Fig. 4a). Moreover, activated α2M improved wild-type MEF adhesion to fibronectin in a dose-dependent manner (Fig. 4b). This effect was not observed in LRP1−/− MEF or when the native form of α2M, which is not recognized by LRP1, was used. Since LRP1 ligation improved cell adhesion and β1 integrin activation, we sought to determine the structural elements of LRP1 involved in the regulation of integrin function. Over-expression of recombinant LRP1 mini-receptors containing each of the four ligand-binding clusters fused to the β-chain (miniR-1–4) or the β-chain alone in LRP1−/− MEF revealed that the second ligand-binding cluster is indispensible for the LRP1-mediated adhesion to fibronectin (Fig. 4c).
Fig. 4.
LRP1 promotes integrin activation and subsequent degradation. a Active β1 integrin expression levels on the cell surface of wild-type and LRP1−/− MEF treated with 55.6 nM activated α2-macroglobulin (α2M) for 1 h were measured by flow cytometry. The results are expressed as a mean fluorescence intensity (MFI) ratio of active-to-total integrin detected with 9EG7 and MB1.2 antibodies, respectively. b, c Adhesion of wild-type or LRP1−/− MEF to fibronectin was measured by crystal violet staining. b Cells were pretreated with 55.6 nM native or 13.9–55.6 nM activated α2M for 1 h. c Recombinant LRP1 mini-receptors (miniR) containing each of the four ligand-binding clusters fused to the β-chain (miniR 1–4) or the β-chain alone were over-expressed in LRP1−/− MEF. d Active-to-total β1 integrin ratio on the cell surface of wild-type and LRP1−/− MEF pretreated with 5 mM MnCl2 for 30 min was assessed as described above. e Adhesion to fibronectin of wild-type or LRP1−/− MEF pretreated with 5 mM MnCl2 for 30 min was measured by crystal violet staining. f Analysis of β1 integrin (int) levels in wild-type and LRP1−/− MEF during culture on fibronectin. g Densitometric analysis of β1 integrin expression from f. Data are expressed as a ratio of β1 integrin to β-actin band density. h, i Expression of β1 integrin in wild-type and LRP1−/− MEF treated with 0.1 mM EDTA (h) or 0.2, 1 or 5 mM MnCl2 (i) for 16 h. Data in a–e and in g are mean ± SEM of three to four experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001; ns, not significant. Western blots in f, h and i are representative of three to four experiments. β-Actin served as a loading control
Next, we tested whether a lack of LRP1 prevents integrin activation in the presence of divalent cations. MnCl2 treatment increased the β1 integrin activation index irrespective of LRP1 expression (Fig. 4d). However, adhesion to fibronectin was enhanced only in LRP1-positive MEF (Fig. 4e) indicating that although β1 integrin is susceptible to activation independently of LRP1 presence, the lack of LRP1 functionally impairs integrin-mediated adhesion.
Lastly, we asked whether integrin activation on fibronectin leads to its degradation in a LRP1-dependent manner. In wild-type cells, β1 integrin levels increased during the first 8 h after seeding onto fibronectin and afterwards remained stable for another 24 h (Fig. 4f, g). In stark contrast, β1 integrin expression in LRP1−/− MEF did not change within the first 8 h post seeding. However, these cells displayed continuous β1 integrin accumulation at the 24 and 32 h time points after seeding onto fibronectin suggesting that a lack of LRP1 impairs degradation of activated integrins. Conversely, we observed increased levels of β1 integrin only in wild-type MEF when integrin activation by fibronectin was inhibited by divalent cation chelator EDTA (Fig. 4h). Interestingly, when integrins were forced into high-affinity conformation by MnCl2, we observed a depletion of β1 integrin in both LRP1-positive and -negative cells (Fig. 4i). Collectively, these data suggest that (1) LRP1 supports cell adhesion by mediating β1 integrin activation, (2) the lack of LRP1 does not preclude β1 integrin activation, however, divalent cations-stimulated integrins do not fully rescue cell adhesion in LRP1 absence, and (3) LRP1 connects integrin activation to degradation.
LRP1 utilizes protein kinase C to regulate cell adhesion and trafficking of active β1 integrin
Results of experiments with manganese described above indicate that LRP1 mediates the pro-adhesive function of activated integrins and that the adhesion defect in LRP1-negative MEF is found downstream of integrin activation. Protein kinase C (PKC) α promotes β1 integrin activation [25] and is required for α5β1 integrin-mediated adhesion to fibronectin [26]. These lines of evidence prompted us to analyze whether LRP1-mediated regulation of integrin function relies on PKC activity. PKC inhibitors Go 6976 and Ro 32-0432 reduced wild-type MEF adhesion to fibronectin to the level observed in LRP1−/− MEF. LRP1-depleted MEF did not react to either PKC inhibitor (Fig. 5a). Conversely, PKC stimulation with Phorbol 12-myristate 13-acetate did not improve the adhesion of LRP1-expressing cells; it, however, enhanced the adhesion of LRP1−/− MEF (Fig. 5b).
Fig. 5.
PKC acts downstream of LRP1 to facilitate cell adhesion and intracellular trafficking of endocytosed β1 integrin. a, b Adhesion of wild-type and LRP1−/− MEF to fibronectin was measured by crystal violet staining. Cells were pretreated with 10 μM Go 6976 (Go) or Ro 32-0432 (Ro) (a) or 100 nM phorbol 12-myristate 13-acetate (PMA) (b) for 1 h. c Subcellular distribution of LRP1 and endocytosed β1 integrin (int) in wild-type and LRP1−/− MEF visualized by confocal microscopy. Cells were treated with Ro or PMA as described above, subsequently incubated with an anti-β1 integrin antibody (clone MB1.2) at 4 °C for 1 h and then shifted to 37 °C for 3 h 30 min. Arrowheads indicate β1 integrin-positive perinuclear vesicles in LRP1−/− MEF stimulated with PMA. Nuclei were counterstained with DAPI, scale bar: 5 µm (n = 3). Data in a and b are mean ± SEM of four experiments; *p ≤ 0.05, **p ≤ 0.01
Next, we tested whether PKC also mediates integrin trafficking downstream of LRP1. Visualization of the cell-surface stained and subsequently internalized activated β1 integrin revealed that PKC inhibition abolishes, whereas activation augments colocalization of LRP1 and β1 integrin in perinuclear vesicles (Fig. 5c). Remarkably, although we observed no effect of PKC inhibitors on β1 integrin subcellular localization in LRP1−/− MEF, PMA induced diffused vesicular staining (Fig. 5c). In sum, these results demonstrate that PKC serves a downstream effector of LRP1 to facilitate cell adhesion and intracellular trafficking of endocytosed activated integrin receptors.
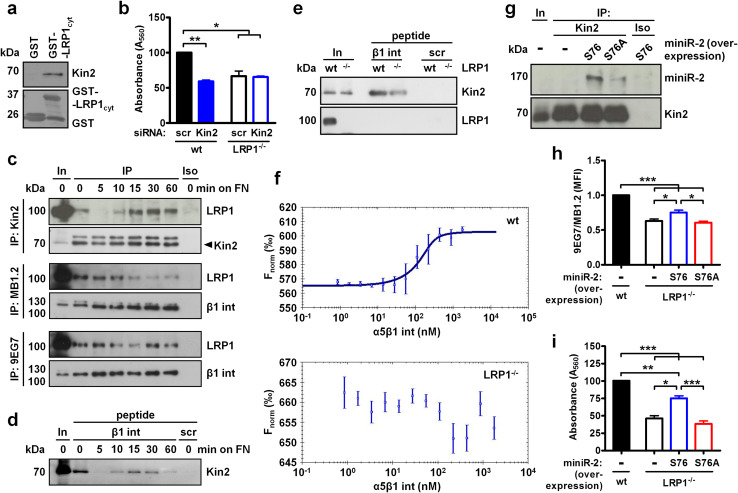
LRP1 binds β1 integrin and integrin activator kindlin2, and enhances β1 integrin–kindlin2 interaction
Since we observed that LRP1−/− MEF display a lower β1 integrin activation index, we next addressed how LRP1 may promote integrin activation. Cytoplasmic tails of β1 integrin and LRP1 contain two NPxY motifs that, in the case of LRP1, are followed by a considerably longer stretch of amino acids [27, 28]. The membrane-distal NPxY motif in β1 integrin sequentially binds the integrin activator kindlin2 and sorting nexin 17 (SNX17) which facilitates integrin recycling [3]. SNX17 also binds the membrane-proximal NPxY sequence in LRP1 [29]; however, the interaction between LRP1 and kindlin2 has not yet been reported. Here, we pulled down kindlin2 from LRP1−/− MEF cell lysate using the GST-fused cytoplasmic tail of LRP1 (Fig. 6a). A LRP1–kindlin2 interaction appears to be relevant for cell adhesion as kindlin2-targeting siRNA reduced the adhesion of wild-type but not LRP1−/− MEF to fibronectin (Fig. 6b).
Fig. 6.
S76 in LRP1 is necessary for kindlin2 binding, integrin activation and cell adhesion. a Pull-down of kindlin2 (Kin2) using GST-fused LRP1 cytoplasmic tail (LRP1cyt). b Adhesion of scrambled (scr) or kindlin2-targeting siRNA-transfected wild-type and LRP1−/− MEF assessed by crystal violet staining. c Co-immunoprecipitation of LRP1 from MEF seeded onto fibronectin for indicated time using anti-kindlin2 antibody mixed with protein extracts (upper panel) or using antibodies directed against total (clone MB1.2, middle panel) or active (clone 9EG7, bottom panel) β1 integrin (int) incubated with live cells at 4 °C for 1 h. In, input; IP, immunoprecipitated material; Iso, isotype-matched control antibody. d, e Kindlin2 pull-down with biotinylated β1 integrin cytoplasmic tail or scrambled (scr) peptides from wild-type MEF adhering to fibronectin (d) or from wild-type vs. LRP1−/− MEF (e). f Binding of kindlin2–GFP fusion protein over-expressed in wild-type or LRP1−/− MEF lysates to recombinant α5β1 integrin as assessed by microscale thermophoresis. g Co-immunoprecipitation of S76 or S76A LRP1 mini-receptor 2 (miniR-2) over-expressed in LRP1−/− MEF with anti-kindlin2 antibody. Ratio of active-to-total β1 integrin (detected with 9EG7 and MB1.2 antibodies, respectively) (h) or adhesion to fibronectin (i) of LRP1−/− MEF after over-expression of S76 or S76A miniR-2. Western blots in a, c–e, g are representative of three to four experiments. Data in b, f, h, i are mean ± SEM of three to four experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
Next, we immunoprecipitated kindlin2 from cells plated on the fibronectin for various time periods. As seen in Fig. 6c, upper panel, LRP1–kindlin2 interaction was dynamically modulated during cell adhesion: both proteins interacted in suspended cells, the interaction was abruptly lost after 5 min of incubation on fibronectin and then gradually restored at the later time points. LRP1 also interacted with cell surface β1 integrin immunoprecipitated with antibodies recognizing total (MB1.2) or active (9EG7) conformation of β1 integrin. Interestingly, LRP1 binding to total β1 integrin was progressively lost (Fig. 6c, middle panel), hence contrasting the LRP1–kindlin2 interaction. The amount of LRP1 co-immunoprecipitated with the anti-active conformation of β1 integrin antibody remained relatively constant throughout the whole cell adhesion period tested (Fig. 6c, bottom panel). These data indicate that (1) the LRP1–kindlin2 interaction is reset after initial contact with fibronectin while integrin remains bound to LRP1, (2) kindlin2 is recruited to LRP1 already occupied with β1 integrin, (3) relatively constant LRP1 binding to the activated fraction of β1 integrin population suggests that the bulk of integrin bound to LRP1 at the initial steps of cell adhesion is not activated, and (4) as kindlin2 is recruited to LRP1, it could activate β1 integrin thus depleting inactive integrin in contact with LRP1.
As we could not detect kindlin2 binding to endogenous β1 integrin, we performed pull-down experiments with a biotinylated full-length β1 integrin cytoplasmic tail to follow the β1 integrin–kindlin2 interaction during adhesion. Strikingly, the observed binding pattern mimicked that of LRP1 and kindlin2, as the β1 integrin–kindlin2 interaction was interrupted shortly after contact with fibronectin and then reappeared at 10 min post seeding (Fig. 6d). To verify whether LRP1 affects kindlin2–β1 integrin interaction, we incubated β1 integrin peptides with lysates from wild-type or LRP1−/− MEF. As seen in Fig. 6e, the presence of LRP1 in cell lysate enhanced kindlin2 binding to the cytoplasmic tail of β1 integrin, however, an interaction between LRP1 and β1 integrin was not observed.
Next, we examined how LRP1 could promote kindlin2–β1 integrin interaction by employing purified recombinant α5β1 integrin which lacks transmembrane and cytoplasmic domains on both integrin subunits and thus is unable to bind kindlin2. The interaction between recombinant α5β1 integrin and over-expressed GFP-kindlin2 was tested in cell lysates by microscale thermophoresis. As shown in Fig. 6f, a titration series of integrin mixed with wild-type MEF protein extracts showed a sigmoidal binding curve (upper panel) whereas LRP1−/− cells displayed no binding between GFP-kindlin2 and α5β1 integrin (bottom panel). As the α5β1 integrin used for this assay was incapable of direct kindlin2 binding and LRP1 was demonstrated above to interact with kindlin2 and β1 integrin, these data suggest that LRP1 could tether kindlin2 bound to its cytoplasmic tail to β1 integrin associated with its extracellular domains.
S76 in LRP1 cytoplasmic tail mediates the interaction with kindlin2 and is required for integrin activation and cell adhesion
We next focused our efforts on determining the structural features of LRP1 required for kindlin2 binding. Kindlin2 association with integrins requires non-phosphorylated tyrosine residues [30] and several amino acids in LRP1 cytoplasmic tail are required for its interactions with adaptor proteins [20, 27, 31]. Therefore, we mutated Y29, Y63, L66 and S76 to alanine in GST-fused LRP1 cytoplasmic tail and tested the binding of kindlin2 using LRP1−/− MEF lysates. Surprisingly, substitution of Y63, which corresponds to tyrosine in membrane-distal NPxY motif in β1 and β3 integrin preferably recognized by kindlin2 [30], or Y29 and L66 with alanine did not disrupt kindlin2 binding (supplementary Fig. S5a). However, S76A variants of GST-fused LRP1 cytoplasmic tail or over-expressed LRP1 miniR-2 showed a reduction in kindlin2 binding in pull-down (supplementary Fig. S5a) and co-immunoprecipitation experiments (Fig. 6g), respectively. Confocal microscopy revealed that S76A substitution did not affect colocalization of recombinant LRP1 with either active or total β1 integrin following adhesion to fibronectin (supplementary Fig. S5b and S5c, respectively). However, over-expression of S76A miniR-2 variant in LRP1−/− MEF neither improved integrin activation (Fig. 6h) nor restored cell adhesion to fibronectin (Fig. 6i) to the levels observed in cells transfected with unaltered miniR-2. Therefore, S76 in the cytoplasmic tail of LRP1 determines the interaction with kindlin2 and is required for β1 integrin activation and cell adhesion to fibronectin but is dispensable for integrin trafficking. Altogether, these observations indicate that (1) LRP1 binds β1 integrin and kindlin2, (2) these interactions are modulated during cell adhesion to fibronectin, (3) LRP1 enhances kindlin2 binding to the β1 integrin and (4) LRP1 utilizes kindlin2 to promote cell adhesion.
Discussion
Our study identifies LRP1 as a multifunctional integrin regulator, which mediates integrin activation, intracellular trafficking, degradation and downstream signaling. We found that LRP1 relies on PKC and kindlin2 to stimulate integrin function. A complex pattern of association between LRP1, β1 integrin and kindlin2 during cell adhesion suggests that LRP1 acts as a platform for kindlin2 to activate β1 integrin receptors and to stimulate cell adhesion. This notion is supported by the fact that kindlin2–β1 interaction is stronger in cells expressing LRP1 and that S76A LRP1 variant incapable of kindlin2 binding reduces cell adhesion and attenuates integrin activation. Of note, the fact that kindlin2 binds to S76 on LRP1 is a surprising finding considering that this protein was reported to interact with tyrosine residues [30]. Coupling actin to integrins promotes adhesion stability while severing this linkage leads to the disassembly of adhesion complexes [32]. Kindlin2 directly binds actin and the interruption of this interaction prevents cell spreading and disorganizes the actin network [33]. Since we observed that LRP1 depletion exerts similar effects and documented that LRP1 associates with both actin and kindlin2, LRP1 could directly or indirectly (via kindlin2) tether actin filaments to integrins thus regulating focal adhesion properties and turnover.
LRP1−/− cells displayed elevated surface integrin expression suggesting that integrin internalization or trafficking may be impaired in LRP1 absence. Surprisingly, we did not observe any impact of LRP1 on integrin endocytosis. Although integrin recycling assays proved problematic in our model, enhanced re-insertion to cell membrane could be responsible for surface integrin accumulation. Bottcher et al. described an endocytosis-rate independent recycling of β1 integrin in MEF mediated by the substitution of kindlin2 bound to integrin with its competitor SNX17, which directs integrin to the cell surface instead of to the lysosomes [3]. As our results illustrate a dramatic reduction in kindlin2–β1 integrin interaction in LRP1−/− cells, a higher number of integrin subunits occupied by kindlin2 may not be accessible for SNX17 in the presence of LRP1. Moreover, SNX17 interacts with and mediates LRP1 recycling [29], indicating that LRP1 and β1 integrin may compete for SNX17 binding. Therefore, LRP1 could reduce the amount of SNX17 bound to integrin thus essentially preventing access of integrins to the recycling machinery and in consequence limiting their expression at the cell surface.
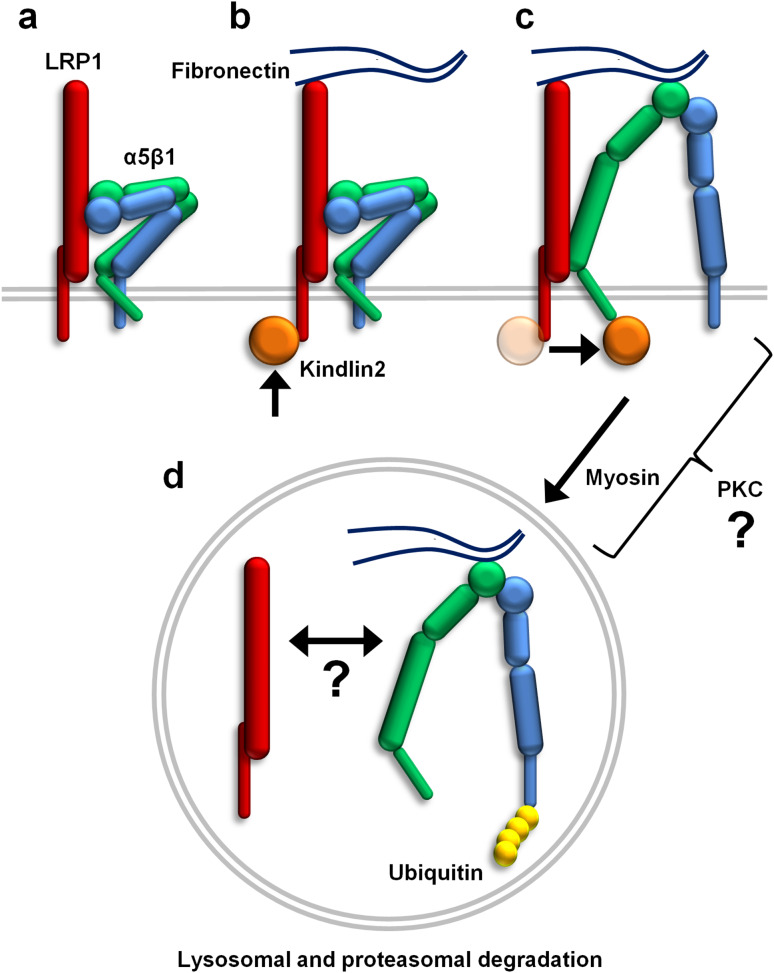
Although the majority of internalized β1 integrin is recycled back to the cell surface, a fraction of the active integrin is ubiquitinated and subsequently degraded together with the fibronectin found in lysosomes [4]. Furthermore, LRP1 binds fibronectin and mediates its degradation [34]. Authors of these studies postulated that degradation is an important mechanism preventing the accumulation of fibronectin and non-functional integrin on the cell surface. Our study reinforces this concept and identifies LRP1 as both an activator and catabolic co-receptor for β1 integrin (summarized in Fig. 7). In fact, we observed localization of active integrin in lysosomes specifically in LRP1-expressing cells. Accordingly, integrin inhibition with EDTA or stimulation by culture on fibronectin increased or decreased, respectively, β1 integrin levels solely in wild-type cells. This indicates that the manipulation of integrin activation exerts a LRP1-dependent effect on integrin stability. Strikingly, forcing integrin into active conformation with Mn2+ stimulated integrin degradation regardless of LRP1 expression, however, only LRP1-positive cells displayed an enhanced adhesion in response to MnCl2. Kindlin2 depletion was reported previously to prevent cell spreading [33] and to reduce cell adhesion [35] to fibronectin in response to MnCl2. Considering our finding that LRP1 binds kindlin2, this interaction seems superfluous for integrin activation and degradation in response to divalent cations but it appears mandatory to induce integrin-mediated cell adhesion on physiological substrates. Furthermore, we observed that LRP1 interacted with MYH9, which was previously reported to assist in the de-adhesion of αLβ2 integrin [24]. Class II myosins regulate focal adhesion maturation and turnover [14] and trafficking of major histocompatibility complex class II and B cell receptors towards lysosomes [36]. As class II myosin inhibition disturbed trafficking and degradation of endocytosed integrin, it appears that LRP1 utilizes class II myosins to direct internalized β1 integrin to lysosomes.
Fig. 7.
Regulation of β1 integrin activation, subcellular trafficking and degradation by LRP1. a Inactive α5β1 integrin is present in the complex with LRP1 on the cell surface. Upon cell contact with fibronectin, kindlin2 is sequentially recruited to LRP1 (b) and to β1 integrin (c) which results in integrin activation. d Following endocytosis, LRP1 employs myosins to direct ubiquitinated integrins toward lysosomal and proteasomal degradation. The molecular mechanism by which PKC mediates LRP1-induced activation and subsequent trafficking of α5β1 integrin and whether LRP1 remains bound to α5β1 integrin during endocytosis, transport and degradation are currently unknown
Regulation of integrin functions by LRP1 might have a pronounced impact on development and homeostasis. β1 integrin activation was shown to be essential for muscle regeneration [37] and vascular morphogenesis [38]. Moreover, macrophage motility depends on a LRP1-dependent shift from cell adhesion facilitated by αMβ2 integrin to cell retraction [12], possibly in a process involving integrin endocytosis [10]. Furthermore, Rabiej et al. showed that integrin endocytosis mediated by LRP1 mediates neuroblast migration in the brain [8]. However, our work defines a new, endocytosis-independent role for LRP1 in the modulation of integrin trafficking which could control retrograde transport of endocytosed β1 integrin to the plasma membrane via the trans-Golgi network in adhering cells during embryogenesis [39].
Modulation of integrins by LRP1 is also of pathological relevance. For instance, homing of hematopoietic stem/progenitor cells to the injured arteries during experimental atherosclerosis requires the stimulation of β2 integrin-dependent adhesion by LRP1 [40]. In kidney fibrosis, β1 integrin–LRP1 interaction induces integrin signaling leading to myofibroblast activation [9]. Interestingly, integrin involvement in fibrogenesis is not limited to the kidney as genetic inactivation or blockage of various integrin subunits attenuates experimental fibrosis in several organs [41]. The underlying mechanism involves recognition and activation of latent transforming growth factor (TGF)-β, a key driver of the fibrotic response, by cell surface-expressed integrins. Whether LRP1 is implicated in this process warrants further investigation, our findings showing augmented surface integrin expression in LRP1-deficient cells suggest that LRP1 loss might potentially enhance integrin-mediated TGF-β activation. Finally, as tumor-associated fibroblasts display deregulated trafficking of active α5β1 integrin [42] and increased LRP1 expression [43], our work may provide a base for future studies addressing LRP1-α5β1 integrin interplay in cancer. In summary, we believe that the novel mechanism of β1 integrin regulation by LRP1 described here may contribute to our understanding of pathways controlling integrin signaling, function and trafficking during development and in disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Yvonne Horn, Markus Schwinn and Horst Thiele for their excellent technical assistance. This work was supported the German Research Foundation (WY119/1-3 to MW; SFB 815, Project A5 and SCHA1082/6 to LS), Excellence Cluster Cardio-Pulmonary System (ECCPS to LW and MW) and the German Center for Lung Research (to MW).
Abbreviations
- α2M
α2-Macroglobulin
- Baf
Bafilomycin A1
- Bleb
Blebbistatin
- ECM
Extracellular matrix
- ESCRT
Endosomal sorting complex required for transport
- FAK
Focal adhesion kinase
- LRP1
Low density lipoprotein receptor-related protein 1
- MEF
Mouse embryonic fibroblasts
- MYH9
Myosin heavy chain 9
- pHLF
Primary human lung fibroblasts
- PKC
Protein kinase C
- PMA
Phorbol 12-myristate 13-acetate
- SNX17
Sorting nexin 17
- TGF-β
Transforming growth factor
Footnotes
Elie El Agha, Saverio Bellusci, Norbert Weissmann and Malgorzata Wygrecka are members of the German Center for Lung Research (DZL).
References
- 1.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9(10):804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 2.Henderson NC, Sheppard D. Integrin-mediated regulation of TGFbeta in fibrosis. Biochim Biophys Acta. 2013;1832(7):891–896. doi: 10.1016/j.bbadis.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottcher RT, Stremmel C, Meves A, Meyer H, Widmaier M, Tseng HY, Fassler R. Sorting nexin 17 prevents lysosomal degradation of beta1 integrins by binding to the beta1-integrin tail. Nat Cell Biol. 2012;14(6):584–592. doi: 10.1038/ncb2501. [DOI] [PubMed] [Google Scholar]
- 4.Lobert VH, Brech A, Pedersen NM, Wesche J, Oppelt A, Malerod L, Stenmark H. Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell. 2010;19(1):148–159. doi: 10.1016/j.devcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88(3):887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strickland DK, Au DT, Cunfer P, Muratoglu SC. Low-density lipoprotein receptor-related protein-1: role in the regulation of vascular integrity. Arterioscler Thromb Vasc Biol. 2014;34(3):487–498. doi: 10.1161/ATVBAHA.113.301924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spijkers PP, da Costa Martins P, Westein E, Gahmberg CG, Zwaginga JJ, Lenting PJ. LDL-receptor-related protein regulates beta2-integrin-mediated leukocyte adhesion. Blood. 2005;105(1):170–177. doi: 10.1182/blood-2004-02-0498. [DOI] [PubMed] [Google Scholar]
- 8.Rabiej VK, Pflanzner T, Wagner T, Goetze K, Storck SE, Eble JA, Weggen S, Mueller-Klieser W, Pietrzik CU. Low density lipoprotein receptor-related protein 1 mediated endocytosis of beta1-integrin influences cell adhesion and cell migration. Exp Cell Res. 2016;340(1):102–115. doi: 10.1016/j.yexcr.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Hu K, Wu C, Mars WM, Liu Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J Clin Investig. 2007;117(12):3821–3832. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranganathan S, Cao C, Catania J, Migliorini M, Zhang L, Strickland DK. Molecular basis for the interaction of low density lipoprotein receptor-related protein 1 (LRP1) with integrin alphaMbeta2: identification of binding sites within alphaMbeta2 for LRP1. J Biol Chem. 2011;286(35):30535–30541. doi: 10.1074/jbc.M111.265413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czekay RP, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160(5):781–791. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao C, Lawrence DA, Li Y, Von Arnim CA, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006;25(9):1860–1870. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salicioni AM, Gaultier A, Brownlee C, Cheezum MK, Gonias SL. Low density lipoprotein receptor-related protein-1 promotes beta1 integrin maturation and transport to the cell surface. J Biol Chem. 2004;279(11):10005–10012. doi: 10.1074/jbc.M306625200. [DOI] [PubMed] [Google Scholar]
- 14.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13(4):383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiller HB, Friedel CC, Boulegue C, Fassler R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011;12(3):259–266. doi: 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dedieu S, Langlois B, Devy J, Sid B, Henriet P, Sartelet H, Bellon G, Emonard H, Martiny L. LRP-1 silencing prevents malignant cell invasion despite increased pericellular proteolytic activities. Mol Cell Biol. 2008;28(9):2980–2995. doi: 10.1128/MCB.02238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orr AW, Pedraza CE, Pallero MA, Elzie CA, Goicoechea S, Strickland DK, Murphy-Ullrich JE. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol. 2003;161(6):1179–1189. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weckbach LT, Gola A, Winkelmann M, Jakob SM, Groesser L, Borgolte J, Pogoda F, Pick R, Pruenster M, Muller-Hocker J, Deindl E, Sperandio M, Walzog B. The cytokine midkine supports neutrophil trafficking during acute inflammation by promoting adhesion via beta2 integrins (CD11/CD18) Blood. 2014;123(12):1887–1896. doi: 10.1182/blood-2013-06-510875. [DOI] [PubMed] [Google Scholar]
- 19.Jablonska E, Markart P, Zakrzewicz D, Preissner KT, Wygrecka M. Transforming growth factor-beta1 induces expression of human coagulation factor XII via Smad3 and JNK signaling pathways in human lung fibroblasts. J Biol Chem. 2010;285(15):11638–11651. doi: 10.1074/jbc.M109.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranganathan S, Liu CX, Migliorini MM, Von Arnim CA, Peltan ID, Mikhailenko I, Hyman BT, Strickland DK. Serine and threonine phosphorylation of the low density lipoprotein receptor-related protein by protein kinase Calpha regulates endocytosis and association with adaptor molecules. J Biol Chem. 2004;279(39):40536–40544. doi: 10.1074/jbc.M407592200. [DOI] [PubMed] [Google Scholar]
- 21.Kwapiszewska G, Wygrecka M, Marsh LM, Schmitt S, Trosser R, Wilhelm J, Helmus K, Eul B, Zakrzewicz A, Ghofrani HA, Schermuly RT, Bohle RM, Grimminger F, Seeger W, Eickelberg O, Fink L, Weissmann N. Fhl-1, a new key protein in pulmonary hypertension. Circulation. 2008;118(11):1183–1194. doi: 10.1161/CIRCULATIONAHA.107.761916. [DOI] [PubMed] [Google Scholar]
- 22.Wujak L, Didiasova M, Zakrzewicz D, Frey H, Schaefer L, Wygrecka M. Heparan sulfate proteoglycans mediate factor XIIa binding to the cell surface. J Biol Chem. 2015;290(11):7027–7039. doi: 10.1074/jbc.M114.606343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreth K, Frey H, Hubo M, Zeng-Brouwers J, Nastase MV, Hsieh LT, Haceni R, Pfeilschifter J, Iozzo RV, Schaefer L. Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol. 2014;35:143–151. doi: 10.1016/j.matbio.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morin NA, Oakes PW, Hyun YM, Lee D, Chin YE, King MR, Springer TA, Shimaoka M, Tang JX, Reichner JS, Kim M. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J Exp Med. 2008;205(1):195–205. doi: 10.1084/jem.20071543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng T, Shima D, Squire A, Bastiaens PI, Gschmeissner S, Humphries MJ, Parker PJ. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18(14):3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vuori K, Ruoslahti E. Activation of protein kinase C precedes alpha 5 beta 1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993;268(29):21459–21462. [PubMed] [Google Scholar]
- 27.Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 2000;275(22):17187–17194. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- 28.Reszka AA, Hayashi Y, Horwitz AF. Identification of amino acid sequences in the integrin beta 1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992;117(6):1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Kerkhof P, Lee J, McCormick L, Tetrault E, Lu W, Schoenfish M, Oorschot V, Strous GJ, Klumperman J, Bu G. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J. 2005;24(16):2851–2861. doi: 10.1038/sj.emboj.7600756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montanez E, Ussar S, Schifferer M, Bosl M, Zent R, Moser M, Fassler R. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22(10):1325–1330. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donoso M, Cancino J, Lee J, van Kerkhof P, Retamal C, Bu G, Gonzalez A, Caceres A, Marzolo MP. Polarized traffic of LRP1 involves AP1B and SNX17 operating on Y-dependent sorting motifs in different pathways. Mol Biol Cell. 2009;20(1):481–497. doi: 10.1091/mbc.E08-08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration–the actin connection. J Cell Sci. 2009;122(Pt 2):199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bledzka K, Bialkowska K, Sossey-Alaoui K, Vaynberg J, Pluskota E, Qin J, Plow EF. Kindlin-2 directly binds actin and regulates integrin outside-in signaling. J Cell Biol. 2016;213(1):97–108. doi: 10.1083/jcb.201501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salicioni AM, Mizelle KS, Loukinova E, Mikhailenko I, Strickland DK, Gonias SL. The low density lipoprotein receptor-related protein mediates fibronectin catabolism and inhibits fibronectin accumulation on cell surfaces. J Biol Chem. 2002;277(18):16160–16166. doi: 10.1074/jbc.M201401200. [DOI] [PubMed] [Google Scholar]
- 35.Theodosiou M, Widmaier M, Bottcher RT, Rognoni E, Veelders M, Bharadwaj M, Lambacher A, Austen K, Muller DJ, Zent R, Fassler R. Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. Elife. 2016;5:e10130. doi: 10.7554/eLife.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vascotto F, Lankar D, Faure-Andre G, Vargas P, Diaz J, Le Roux D, Yuseff MI, Sibarita JB, Boes M, Raposo G, Mougneau E, Glaichenhaus N, Bonnerot C, Manoury B, Lennon-Dumenil AM. The actin-based motor protein myosin II regulates MHC class II trafficking and BCR-driven antigen presentation. J Cell Biol. 2007;176(7):1007–1019. doi: 10.1083/jcb.200611147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rozo M, Li L, Fan CM. Targeting beta1-integrin signaling enhances regeneration in aged and dystrophic muscle in mice. Nat Med. 2016;22(8):889–896. doi: 10.1038/nm.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mana G, Clapero F, Panieri E, Panero V, Bottcher RT, Tseng HY, Saltarin F, Astanina E, Wolanska KI, Morgan MR, Humphries MJ, Santoro MM, Serini G, Valdembri D. PPFIA1 drives active alpha5beta1 integrin recycling and controls fibronectin fibrillogenesis and vascular morphogenesis. Nat Commun. 2016;7:13546. doi: 10.1038/ncomms13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafaq-Zadah M, Gomes-Santos CS, Bardin S, Maiuri P, Maurin M, Iranzo J, Gautreau A, Lamaze C, Caswell P, Goud B, Johannes L. Persistent cell migration and adhesion rely on retrograde transport of beta(1) integrin. Nat Cell Biol. 2016;18(1):54–64. doi: 10.1038/ncb3287. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Gao M, Schouteden S, Roebroek A, Eggermont K, van Veldhoven PP, Liu G, Peters T, Scharffetter-Kochanek K, Verfaillie CM, Feng Y. Hematopoietic stem/progenitor cells directly contribute to arteriosclerotic progression via integrin beta2. Stem Cells. 2015;33(4):1230–1240. doi: 10.1002/stem.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conroy KP, Kitto LJ, Henderson NC. alphav integrins: key regulators of tissue fibrosis. Cell Tissue Res. 2016;365(3):511–519. doi: 10.1007/s00441-016-2407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franco-Barraza J, Francescone R, Luong T, Shah N, Madhani R, Cukierman G, Dulaimi E, Devarajan K, Egleston BL, Nicolas E, Katherine Alpaugh R, Malik R, Uzzo RG, Hoffman JP, Golemis EA, Cukierman E. Matrix-regulated integrin alphavbeta5 maintains alpha5beta1-dependent desmoplastic traits prognostic of neoplastic recurrence. Elife. 2017 doi: 10.7554/eLife.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leca J, Martinez S, Lac S, Nigri J, Secq V, Rubis M, Bressy C, Serge A, Lavaut MN, Dusetti N, Loncle C, Roques J, Pietrasz D, Bousquet C, Garcia S, Granjeaud S, Ouaissi M, Bachet JB, Brun C, Iovanna JL, Zimmermann P, Vasseur S, Tomasini R. Cancer-associated fibroblast-derived annexin A6+ extracellular vesicles support pancreatic cancer aggressiveness. J Clin Investig. 2016;126(11):4140–4156. doi: 10.1172/JCI87734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.