Abstract
Hyperlipidemia is a risk factor for atherosclerosis that is characterized by lipid accumulation, inflammatory cell infiltration, and smooth muscle cell proliferation. It is well known that hyperlipidemia is a stimulator for endothelial dysfunction and smooth muscle cell migration during vascular disease development. Recently, it was found that vessel wall contains a variable number of mesenchymal stem cells (MSCs) that are quiescent in physiological conditions, but can be activated by a variety of stimuli, e.g., increased lipid level or hyperlipidemia. Vascular MSCs displayed characteristics of stem cells which can differentiate into several types of cells, e.g., smooth muscle cells, adipocytic, chondrocytic, and osteocytic lineages. In vitro, lipid loading can induce MSC migration and chemokines secretion. After MSC migration into the intima, they play an essential role in inflammatory response and cell accumulation during the initiation and progression of atherosclerosis. In addition, MSC transplantation has been explored as a therapeutic approach to treat atherosclerosis in animal models. In this review, we aim to summarize current progress in characterizing the identity of vascular MSCs and to discuss the mechanisms involved in the response of vascular stem/progenitor cells to lipid loading, as well as to explore therapeutic strategies for vascular diseases and shed new light on regenerative medicine.
Keywords: Stem cells, Progenitor cells, Hyperlipidemia, Atherosclerosis
Introduction
Mesenchymal stem cells (MSCs) are cells with a great potential of differentiation into many types of cells, i.e., chondrocytes, adipocytes, fibroblasts, osteoblasts, smooth muscle cells, etc. MSC was first discovered by Freidenstein in 1968 [1], who proceeded and devoted his efforts in confirming the identity of the cells which were responsible for osteogenesis of transplanted marrow. In a series of studies [2–11], he characterized the development of fibroblast colonies in monolayer cultures of guinea pig bone marrow and confirmed the capability of these cells in bone formation. Since then, many studies demonstrated that MSCs displayed a tri-lineage differentiation potential, i.e., osteogenic, chondrogenic, and adipogenic differentiation capacities, which can be isolated from human tissues, e.g., adipose tissue, skin, cartilage, heart, kidney, liver, lung, muscle, pancreas, spleen, thymus, umbilical cord, and peripheral blood [12–25]. Scientific interest in MSCs is due to their self-renewability and to their potential to differentiate into one or more cell types of the organ from which they originate. Unlike embryonic stem cells, the use of MSCs for therapeutic application does not raise ethical issues, since these cells are derived from adult tissue samples without the need for destroying human embryos. The characteristics of MSCs derived from various tissues are summarized in Table 1. Interestingly, recent studies provided the evidence that vessel wall contains a variable number of MSCs [26]. These cells are quiescent under physiological conditions, and can be activated by a variety of stimuli, e.g., increased lipid level or hyperlipidemia.
Table 1.
Characterisation of MSCs derived from various sources
| Tissue | Literature | Molecular characterisation | Differentiation in vitro | Differentiation in vivo |
|---|---|---|---|---|
| Adipose tissue | Zuk [13] |
FVIII 24.9%, αSMA 29.2% (ECs, SMCs, pericytes) ASO2 85.0%, vimentin 63.2% (mesenchymal markers) (IF) Positive: CD29, CD44, CD71, CD90, CD105, SH3, CD49d Negative: CD31, CD34, CD45 |
Adipogenic Chondrogenic Osteogenic Neurogenic Myogenic |
|
| Umbilical cord | Romanov [14] |
(IF) Positive: αSMA Negative: CD31, vWF, CD34 |
Adipogenic | |
| Synovial membrane | Di Bari [15] |
(RT-PCR) Positive: CD44, etc. Negative: CD31, CD45, CD14, CD20, etc. |
Adipogenic Chondrogenic Osteogenic Myogenic |
|
| Periodontal ligament | Seo [16] |
(IF) Positive: Stro-1, CD146 |
Differentiation potential into cementoblast like cells and collagen forming cells Adipogenic |
Cementum like tissue |
| Tendon | Bi [18], Salingcarnboriboon [17] |
Osteogenic Adipogenic Differentiation potential into tendon like tissues |
Tendon-like tissues formed when cells were implanted into defects made in patella tendon in mice | |
| Skin | Toma [19] |
(IF) Fibronection, vimentin, nestin |
Neurons, Glias, SMCs Cells with the phenotype of peripheral neurons and schwann cells |
|
| Cartilage | Alsalameh [20] |
CD105 + 95% CD166 + 5% CD105 +/CD166 + 3.49% |
Adipogenic Chondrogenic Osteogenic |
|
| Dental pulp | Shi [22] | Stro1 + selected cells: Positive: CD146, αSMA, 3G5 |
Adipocyte Neural cells |
Dental pulp like tissues |
| Spleen, muscle, kidney, lung, liver, brain, thymus, aorta, vein | Da Silva Meirelles [23] |
(FACS) CD29 + CD44 + CD117 − CD49e, CD90.2: expression varied according to tissue origin, Sca-1, CD34: decrease with passage |
Adipogenic, Osteogenic, Chondrogenic |
|
| Blood | Villaron [24] |
(FACS) Positive: CD90, CD106, CD54, CD49b Negative: CD105, CD56, CD34, CD133, CD104, CD62L, HLA-DR |
||
| Cord blood | Campagnoli [25] |
(FACS) Positive: CD29, CD44, CD105, SH3, SH4 Negative: CD45, CD34, CD14, CD68, vWF, HLA-DR |
Adipogenic, Osteogenic, Chondrogenic |
Hyperlipidemia is an established risk factor for the pathogenesis of atherosclerosis [27]. Primary hyperlipidemia occurs either as a result of a single gene defect or multiple subtle genetic defects acting in combination with environmental factors [28]. Familial hypercholesterolemia is caused by a receptor that binds to low-density lipoprotein (LDL), which leads to slowing LDL clearance from blood [29]. Familial defective apoB100 is clinically indistinguishable from hypercholesterolemia, with subjects presenting with xanthomas. Concerning the molecular mechanism of hyperlipidemia, there are different proteins involved, e.g., LDL clearance defect due to mutations in the apoB100 molecule, which is reducing LDL receptor-binding affinity [30]. The most common primary dyslipidemia is familial combined hyperlipidemia, which is thought to account for 10–14% of cases of premature cardiovascular disease [31, 32]. It is also associated with the metabolic syndrome, which may be a subtype of patients at high risk of cardiovascular disease [33]. Familial hypertriglyceridemia is due to hepatic over-production of triglycerides and large very-low-density lipoprotein (VLDL) particles. Some meta-analyses of epidemiological data demonstrate an increased risk of familial hypertriglyceridemia for cardiovascular disease, independent of HDL [34]. Thus, the evidence-linking hyperlipidemia to the pathogenesis of atherosclerosis is so well established.
Atherosclerosis represents one of the greatest threats to human health worldwide. It is a complex chronic inflammatory disease, which affects large- and medium-size arteries. The lesions are characterized by formation of necrotic cores, calcified regions, accumulated modified lipids, inflamed smooth muscle cells (SMCs), endothelial cells, leukocytes, and foam cells [35]. SMCs are the predominant cell type, and their accumulation and proliferation are crucial in determining the severity and characteristics of these advanced lesions [36]. Interestingly, it was found that the vascular adventitia contains stem/progenitor cells, which can differentiate into SMCs and endothelial cells in vitro and in vivo [37]. At the beginning of atherosclerosis, blood leukocytes first attach to the damaged or activated endothelial monolayer, and then migrate into the intima, where they mature into macrophages, which could uptake lipid, yielding foam cells. As the progression goes on, SMCs in the media migrate towards the intima. The resident intimal SMCs (exclusively in human vessel) and media-derived SMCs proliferate and synthesize more extracellular matrix macromolecules such as collagen, elastin, and proteoglycans. However, recent findings indicate the presence of resident stem/progenitor cells or MSCs in the vessel wall, which could participate in the pathogenesis of atherosclerosis (see below). In short, during this process, the role of MSCs might be crucial, because they can migrate from either media or adventitia to the intima where they may differentiate into different cell types, further contributing to atherosclerosis development. This review provides a systematic evaluation of recent preclinical studies to evaluate the use of MSCs to atherosclerosis, especially in response to hyperlipidemia. It will conclude by highlighting the research efforts currently under way to apply MSCs to enhance the process for therapy of atherosclerosis in the near future.
The biology of MSCs
As with other stem cell types, MSCs have a high capacity for self-renewal while maintaining multipotency. MSCs acquire display multipotent differentiation potentials in vitro. The criteria of human MSCs are summarized in Table 1. Concerning negative surface markers of the phenotype, CD45 (cluster of differentiation 45), CD34, CD14 or CD11b, and CD79α or CD19 are used to exclude contamination of pan-leukocyte, primitive hematopoietic progenitors, monocytes/macrophages, and B cells in the culture, respectively [26]. Though the recent isolation techniques managed to define MSCs with phenotypic markers and avoid contamination of other cell populations, the criteria still could not uniquely identify all MSCs [38]. Other markers used for MSC characterisation include Stro-1 [39], which can enrich CFU-Fs (colony forming unit fibroblasts) by approximately 100-fold in human MSCs. Recent findings showed that Stro-1 is expressed in endothelial cells, but not MSCs in vivo, which can be induced under MSC culture conditions in vitro [40]. In addition, it is well documented that difference exists across tissue origins and between species [41]. Systemic isolation and evaluation of MSCs from different tissues represents an alternative approach [23]. The similar but not identical phenotype of MSCs originating from different tissues reflects the similar origins but influences of different microenvironments. The proposed hypothesis that MSCs are tissue-resident stem cells led to further investigations of their perivascular origin [42], which has been suggested by other studies, as well [22, 43, 44]. In addition, it is demonstrated recently that MSCs might actually contain tissue-specific progenitors from different mesoderm derivatives [41]. As none of the above approaches provides a definite answer to the question whether MSCs in vivo have a unique identity, more investigation should be carried out to trace the origin during development and to clarify the in vivo function of MSCs.
Vessel wall-resident MSCs
Since Hu et al. [45] reported the presence of vascular stem/progenitor cells (MSC-like cells) in apoE-deficient mice, a large number of publications have been documented for the resident stem cells [46–48]. MSCs have been shown to be present in many different adult tissues that are related to vasculature. For instance, vascular MSCs such as Sca-1 + cells have been shown to be abundant in the adventitia [45]. It was showed that isolated adventitial Sca-1+ cells could differentiate in vitro into SMCs in response to Pdgf-β. In addition, the capacity In vivo of the adventitial Sca-1 + cells to migrate across the vessel wall and subsequently differentiate into SMCs thus contributing to neointima formation was proved when Sca-1 + β-gal− cells carrying the SM22-LacZ gene applied on the adventitial side of vein grafts in ApoE−/− mice were detected after 4 weeks differentiated into SMCs (β-gal+) in the neointima [45]. Furthermore, MSCs have been identified in the human adult vena saphena, which express a panel of MSC markers [49]. These cells displayed a role in promoting angiogenesis in ischemic tissues in a mouse model.
In the vessel wall of small artery in lung tissues, MSCs have also been identified, which can differentiate into SMCs in vitro [50]. Chronic hypoxia resulted in the increase of MSCs in vivo and participated in vascular remodeling [50]. Importantly, these MSCs have a great potential of proliferation in response to inflammatory stimuli that enhance endothelial barrier function after lung injury [51]. In the media of the vessel wall, several reports indicate the presence of MSCs, which can differentiate into endothelial cells, SMCs, chondrocytes, adipocyte, and even neuron cells [52]. Very recently, Roostalu et al. [53] demonstrated that neointimal SMCs of femoral artery after endothelial injury were derived from adventitial MSCs, but not mature SMCs. This study used a combined cell linear tracking system to trace cell fate in vivo in mouse models. Traditionally, it was believed that mature SMCs from media are responsible for SMC accumulation in neointimal lesions; the recent study provides a concept novel role of MSCs within the vessel in vascular repair. Thus, these MSC-like stem cells exert their role in endothelial repair/regeneration (Fig. 1).
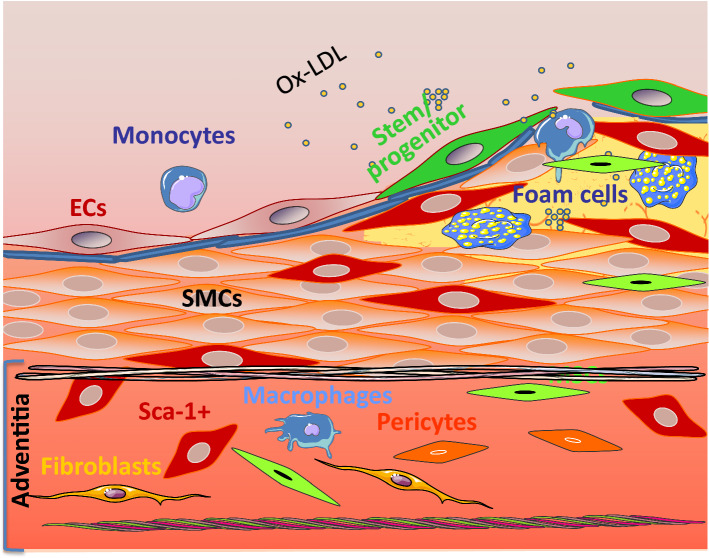
Fig. 1.
Proposed roles for vascular wall resident MSCs in lesion formation. The adventitia is a dynamic layer in active communication with the other vessel wall layers, and it contains various cell types, including Sca1 + cells (red), mesenchymal stem cells (MSCs in green), macrophages (blue), fibroblasts (yellow), and pericytes (orange), amongst others. Vascular resident MSCs have the ability to migrate to the lesions and to differentiate into SMCs and other types of cells
Bone marrow MSCs
With the diffusion chamber system in the 1990s, researchers managed to confirm the differentiation of bone marrow-derived MSCs to bone, cartilage, and fibrous tissue [54]. In the diffusion chamber with standard filters, cells were administered in the chamber remained inside as these cells could not pass through the filter to get mixed with host cells outside. Body fluids (nutrients, salts, and proteins) could pass freely through the filter. After transplantation of the cells into the peritoneal cavity, within diffusion chamber, cells are generated with high capacity for proliferation and differentiation, which serve as an in vivo incubator model [54–56]. Alternatively, Caplan and his group established a method using porous calcium phosphate ceramics loaded with marrow cells and implanted them subcutaneously, with the observation of predominant bone formation, which was slightly different from the mixture of bone, cartilage, and fibrous tissue formation in the diffusion chamber assay [56–58]. By manipulating the culture conditions [59], the cells could be differentiated into either osteoblasts or adipocytes. Thus, it provides preliminary evidence for bone marrow-derived MSCs to differentiate into other cell lineages in vivo and in vitro. Subsequently numerous investigations have been carried out with a variety of differentiation conditions.
MSCs from other stromal tissues
Umbilical cord-derived MSCs have similar immunophenotypic characteristics and functional properties with MSCs derived from other tissues. However, source-dependent differences exist. Compared to MSCs derived from other tissues, umbilical cord-derived MSCs display higher proliferation capacity, which is a significant advantage with regard to their application potential in tissue engineering [60–63]. Umbilical cord-derived MSCs grow faster than bone marrow MSCs at the early passages with a cell population doubling time of 24 h over 40 h of MSCs [62]. Furthermore, umbilical cord-derived MSCs demonstrate a better ability to form colony forming units in vitro, which is also a proof of their relatively better proliferation capacity compared to bone marrow-nucleated cells [63].
Regarding the transcriptomic profile of umbilical cord-derived MSCs, most of the surface markers that they express are common mesenchymal markers. However, unlike in bone marrow MSCs, HLA-ABC is weakly expressed in umbilical cord-derived MSCs, indicating that these cells might be less immunogenic than bone marrow MSCs [64]. This immune privilege makes umbilical cord-derived MSCs a good candidate for tissue engineering of vascular grafts, which will be grafted in vivo in the end. Adipose-tissue-derived MSCs present similar properties with MSCs derived from other tissues including bone marrow [23]. The main advantage of adipose-tissue MSCs over bone marrow MSCs is, perhaps, the abundance of adipose tissue which makes it readily available. Arguably, adipose-tissue MSCs are among the most promising MSCs for stem cell-based cell therapeutics and tissue engineering [65].
Hyperlipidemia-induced alterations in MSCs
Lipid loading-induced MSC migration
Atherosclerosis is a chronic inflammatory disease characterized by endothelial dysfunction, lipid deposition, and inflammatory cell accumulation within the arteries. LDL deposited in the intima and cholesterol can be modified or oxidized, which leads to activation of vascular SMCs and macrophages. Modified LDLs have been also known to increase foam cell formation, influencing lesion development; these molecules have been implicated in pro- and anti-atherosclerotic responses [66]. Adventitial progenitor cells, including SCA1 + and MSCs, are believed to be important in vascular remodeling. In a recent study [67], Kokkinopoulos et al. used single-cell gene sequencing techniques to study MSCs derived from wild-type and ApoE-deficient mice. It was found that several genes related to cell migration and matrix protein degradation displayed a significant alteration. Among them, a group of genes have a role in MSC migration; some genes were crucial for endothelial regeneration and five genes were associated with leukocyte adhesion. Results demonstrated that modified LDL and free cholesterol are potent inducers of MSC migration, which inhibits their differentiation. At the same time, ApoE knockout stem cells show a higher migration toward the inner vascular wall in comparison with wild-type counterparts. Furthermore, they found that lipid loading resulted in increase in miRNA-29b production, and led to sirtuin-1 and matrix metalloproteinase-9 expression that enhance MSC migration [67]. All those results provide clarity of the migratory mechanisms of resident AdvSCA-1 + progenitors (Fig. 2).
Fig. 2.
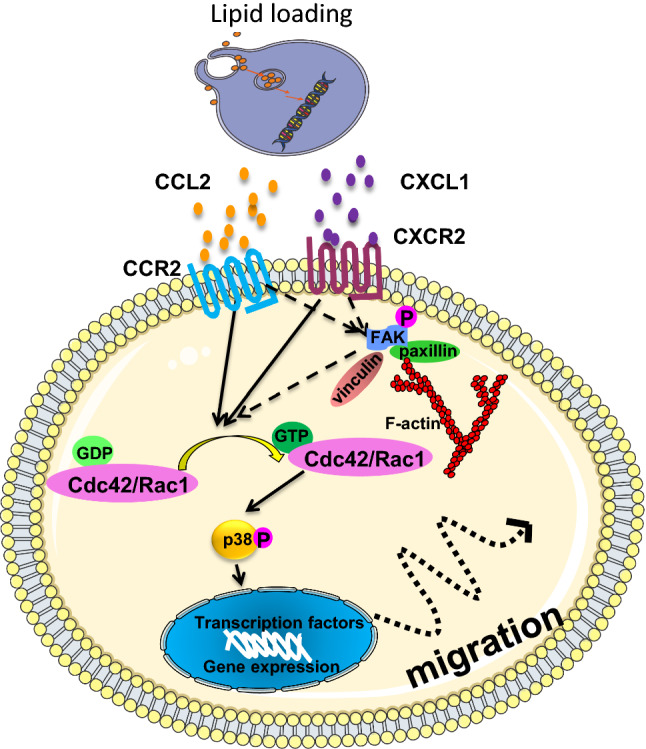
Schematic illustration of the roles of CCL2 and CXCL1 released from lipid loading cells in enhancing MSC chemotaxis. CCL2 and CXCL1 released by lipid loading cells into the medium bind to their corresponding receptors CCR2 and CXCR2 on MSCs. The GTPases Rac1 and Cdc42 become activated and then p38 is phosphorylated via Rac1, finally leading to increased migration, which induces expression of cytoskeleton related proteins paxillin, vinculin and phosphorylated FAK, which may also activate the Rac1 or Cdc42 signalling pathways
It has been shown that ox-LDL induces secretion of chemokines such as monocyte chemoattractant protein-1 (MCP-1) expression in macrophages [68], endothelial cells [69], and vascular SMCs [70]. This ox-LDL-induced MCP-1 plays an important role in monocyte transmigration into the subendothelial space [69, 71]. Study by Zhong et al. [72] indicates the inhibitory effect of curcumin on ox-LDL-induced MCP-1 production, which is mediated by mitogen-activated protein kinase and NF-κB pathways in rat SMCs. Another study [73] demonstrated that ox-LDL induces MCP-1 release in vascular SMCs via urokinase receptor association with CD36 and TLR4. To determine whether hyperlipidemia affects SMC apoptosis, Murray et al. [69] conditionally induce SMC apoptosis in an animal model of SM22α-hDTR/ApoE−/− mice with high-fat diet, which increases serum IL-6, TNFα, and MCP-1. It was found that atractylenolide I inhibited ox-LDL-induced SMC proliferation and migration, and decreased the production of inflammatory cytokines and the expression of MCP-1 [74]. MCP-1 or CCL2 has been detected in macrophage-rich areas bordering the lipid core, while the lesion size and plaque macrophage content were decreased by > 60% in mice-lacking CCL2 [75, 76]. Other studies have demonstrated that free cholesterol prevents macrophages from expressing inflammatory-response genes [77, 78], including Chemokine C-X-C motif Ligand 9, IL-1β, and CXCL 10, and Toll-like receptors [79]. All these findings implicate that lipid accumulation affects the chemokines expression in SMCs and macrophages.
On the other hand, how can adventitial stem/progenitor cells cross the media and migrate to the intima? A recent work provides the first evidence that chemokines released by vascular SMCs play a key role in mediating resident stem cell migration from the adventitia to intima [67]. Sca1 + progenitor cells cultivated from mouse vessels exhibited increased migration when co-cultured with SMCs, which was associated with elevated levels of chemokines, such as CCL2, CXCL1, CCR2, and CXCR2. The GTPases Cdc42 and Rac1 were activated by both CCL2 and CXCL1 stimulation; at the same time, p38 phosphorylation was increased. However, only Rac1 inhibition significantly reduced migration and p38 phosphorylation. Interestingly, migration of MSCs from the adventitia to the neointima can be significantly inhibited or diminished in CCL2-deficient mice after wire injury of arteries. These results provide the evidence that SMC-released chemokines induce MSC migration from the adventitia to the neointima, which involves in signal pathways of CCR2/Rac1/p38 and CXCR2/Rac1/p38 (Fig. 2).
Role of MSCs in atherosclerosis
Atherosclerosis is started from focal intimal influx and accumulation of lipid deposition and vessel stiffness, which result in thickening of the vessel wall and narrowing of the lumen. Modified or oxidized-LDL can be removed by macrophages via scavenger receptors [80]. During this process, foam cells can be formed via the receptor-mediated uptake of ox-LDL by the macrophages. If this process is repeated, free radicals and the excess of oxidized-LDL particles can lead to cell death to form the necrotic core [73]. It indicates that monocyte/macrophage recruitment to the intima is a key event, which is regulated by a multiplicity of endothelial adhesive cytokines and chemokines, e.g., MCP-1, which can be synthesized and released by injured endothelial cells and SMCs [81]. The previous studies have shown that hyperlipidemia result in endothelial damage that leads to inflammatory response [82]. Based on lowering lipid treatment, some endothelial repairing measures have shown some beneficial effects in preventing vascular complications [83, 84]. Schober et al. [85] investigated the function of the CCL2/CCR2 axis in the early monocyte recruitment and macrophage infiltration to injured arteries. In addition, lipid accumulation can regulate commitment of MSCs towards adipogenic fate [86], mechanically involving the heme-oxygenase 1 expression and canonical Wnt signaling cascade.
MSCs, due to their capabilities of differentiation into multiple cell lineages such as mesodermal lineage and myogenic lineage [87, 88], have been explored as an attractive therapeutic target in various diseases, including myocardial infarction, acute lung injury, acute renal failure, ischemia, etc. [89–95]. Recent studies reveal the anti-inflammatory properties of MSCs as guardians of inflammation [96]. Moreover, the immunomodulatory capacity of MSCs has been increasingly appreciated. Several studies have investigated the capacity of MSCs to modulate both innate and adaptive immune responses [97–103]. For example, MSCs have been shown to reduce monocyte responses after myocardial infarction [101] and to skew macrophages to an anti-inflammatory IL-10-producing phenotype [98–101]. MSCs also inhibit the differentiation and maturation of dendritic cells [97], by reducing the expression of co-stimulatory molecules and pro-inflammatory cytokines (TNF-α and IL-12), while increasing the production of anti-inflammatory cytokines (TGF-β and IL-10) [104, 105], which indirectly suppresses T cell proliferation [104]. However, MSCs can also directly inhibit T-cell proliferation [104–106], by inducing cell cycle arrest in all subsets, resulting in a quiescent state, and decreased proliferation [107], in which MSC treatment not only affects inflammatory responses but also significantly reduces dyslipidaemia in mice. Since the essential role of inflammation and immunomodulatory in the initiation and progression of atherosclerosis, MSC transplantation has been broadly explored as a therapeutic approach to treat atherosclerosis.
MSCs differentiate into SMCs
MSCs derived from different tissues have a potential to differentiate into several cell lineages, including SMCs, as summarized in Table 2. The evidence is accumulating that adult MSCs can differentiate into SMCs in a variety of cardiovascular diseases, including restenosis, transplant arteriosclerosis, and atherosclerosis. A set of in vivo studies demonstrated that the origin of vascular resident MSCs gives rise to SMCs to form neointima, which may contribute to atherosclerosis and restenosis. The differentiation of MSCs towards SMCs is usually achieved through the manipulation of the cell culture condition including the addition of various biochemicals, the concentration of serum, and duration of differentiation. Numerous growth factors like TGFβ1, bone morphogenetic protein 4 (BMP4), angiotensin II [108], sphingosine 1-phosphate [109], and thromboxane A2 [110] could all be utilised to stimulate SMC differentiation [111]. Among these reagents, TGFβ1 is a potent stimulator for SMC differentiation from stem cells both in vitro and in vivo [112–114]. Furthermore, these factors could cooperate or interact with each other. The promotion of SMC differentiation from human MSCs by sphingosine-1-phosphate was dependent on TGFβ1 signaling pathway [115]. Studies also showed that only when TGFβ1 was combined with BMP4 could contractile SMCs be obtained [111, 116].
Table 2.
MSC differentiation into SMCs
| Stem cells | Study | Evidence of SMC differentiation | Result |
|---|---|---|---|
| Bone marrow nucleated cells | Han [120] | Staining of αSMA | Bone marrow-derived cells are recruited in vascular healing as a complementary source of smooth muscle-like cells when the media is severely damaged and few resident SMCs are available to affect repair |
| Bone-marrow transplantation of β-galactosidase expressing cells | Shimizu [121] | Staining of αSMA | Host bone-marrow cells are a source of donor intimal smooth-muscle—like cells in murine aortic transplant arteriopathy |
| Bone marrow purified haematopoietic stem cells | Sata [122] | Staining of αSMA | Haematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis |
| Bone marrow transplantation of SM-LacZ β-gal expressing cells | Hu [123] | SM-LacZ | SMCs in transplant atherosclerotic lesions are originated from recipients, but not bone marrow progenitor cells |
| Sca-1 (+) adventitia cells | Hu [45] | SM-LacZ | When Sca-1 (+) cells carrying the LacZ gene were transferred to the adventitial side of vein grafts in ApoE-deficient mice, β-gal (+) cells were found in atherosclerotic lesions of the intima, and these cells enhanced the development of the lesions |
| Adventitia fibroblast | Li [124] | – | Adventitial fibroblasts seeded on the adventitia side of vascular balloon injury would migrate to neointimal site and take part in neointimal formation |
During the differentiation process, induction of extracellular matrix proteins such as collagen I and elastin by TGFβ1 demonstrates the potential of these differentiated cells to be utilised in vascular graft engineering as production of extracellular matrix proteins would help to increase the mechanical strength and bio-compatibility of the graft. This is consistent with the published results showing elastin matrix enhancement in vascular SMCs upon stimulation of TGFβ1 and hyaluronan [117]. In vitro culture condition with high serum concentration induces SMCs to enter the proliferative synthetic phenotype, which is similar to SMC phenotypic switching during vascular injuries [118, 119]. The synthetic phenotype can be switched back to contractile phenotype upon serum deprivation as indicated by the increases in contractile markers [118]. In SMC differentiation from stem cell sources such as pluripotent stem cells, bone marrow MSCs, and skin-derived precursors, contractile SMCs were acquired with lower serum concentration [125–127]. Although contradictory studies exist demonstrating that serum concentration in the culture medium does not affect SMC marker expression, these reports are few and isolated [128]. Thus, it could be concluded overall that serum concentration reduction or deprivation are in favour of functional SMC differentiation.
The SMC differentiation mechanism is a complex regulatory network. Exploration of novel miRNAs involved in the differentiation network could provide new therapeutic choices. It was confirmed that miR-145, a well-established contractile SMC differentiation enhancer, was also upregulated in a time-dependent manner in the differentiation system in MSCs [129, 130]. Exploration of miR-145-related SMC differentiation mechanism has mainly involved candidates lying upstream and downstream in the pathways. Apart from myocardin and SRF, it was revealed that miR-145 could also be upregulated by TGFβ1 [129, 131, 132]. Smad-binding elements were characterized in an enhancer region of miR-145 and the binding of Smad4 on this region was confirmed by chromatin immunoprecipitation experiments [132]. Jagged 1/Notch signaling pathway could also control the expression of miR-145. Downstream targets of miR-145 include Klf4 (Kruppel-like factor 4), Elk-1 (ELK1, a member of ETS oncogene family), and myocardin [129]. There are also reports in cancer cells showing that miR-145 could target Smad3 to inhibit TGFβ1-induced epithelial–mesenchymal transition and cancer invasion and target ROCK1 to inhibit proliferation and invasion of osteosarcoma cells [133, 134]. As Smad3 and ROCK1 are both components of TGFβ signaling pathways, these imply a complex interaction between miR-145 and TGFβ signaling pathways.
After uncovering novel miRNAs whose expression is changed during MSC–SMC differentiation using miRNA array as mentioned above, miR-503, which is upregulated during the differentiation process and promotes MSC–SMC differentiation, as well as miR-222-5p, which is downregulated in the differentiation process and de-represses MSC–SMC differentiation, emerge to be two promising candidates. To further establish their roles in SMC differentiation, similar approaches to the study of miR-145 involvement were employed placing the exploration focuses on the upstream control of miRNA level and the downstream targets. More importantly, both upstream upregulation of miRNAs and downstream target explorations were tightly oriented within well-established TGFβ1 signaling pathways. Concerning the direct contribution of MSCs in miRNA-mediated SMC differentiation, what is now widely accepted is that the origin and residency of these stem cells could be crucial. Using the models summarized in Table 2, it may be possible to elucidate detailed mechanisms of SMCs differentiation from MSCs. It is well known that the complication of atherosclerosis, e.g., plaque rupture, is a key issue for the death of patients. If we could arbitrarily direct MSCs to differentiate into SMCs to stabilize the plaque, the complication of atherosclerosis might be reduced. Thus, new light might be shed on the pathological features behind differentiation of vascular resident MSCs into SMCs, thus providing more opportunities for new therapeutic choices in cardiovascular diseases.
MSCs and macrophages
Macrophages regulate blood vessel structure and function in healthy and diseased state. The origins of tissue macrophages are diverse [135]. It is known that microglia [136] and Langerhans cells [137] are derived from tissue-resident macrophage stem/progenitors cells. On the other hand, current expert opinion seems also to agree the concept that resident macrophage turnover is owing to the self-renewal and proliferation of mature macrophages via keeping their functional differentiation [138]. Recently, two studies from Simari group have reported that the adventitia of postnatal murine arteries contains a hierarchy of hematopoietic progenitor or stem cells, comprising rare numbers of multilineage progenitor cells and a markedly enriched content of macrophage-dedicated progenitors [139, 140]. They also revealed that Sca1 + CD45 + adventitial macrophage progenitor cells were not replenished via the circulation from bone marrow or spleen; rather, adventitial macrophage progenitor cells were upregulated in hyperlipidemic ApoE−/− and LDL-R−/− mice, indicating that hyperlipidemia influenced progenitor functions. These studies implicate that local MSCs or stem/progenitor cells may be a direct source of macrophages with the vessel wall.
In addition, MSCs also have an ability to influence function and phenotypes of macrophages, in which different biological processes of inflammation and atherosclerosis are crucial. Macrophages, especially M1 subset, are specialized phagocytes that engulf and digest dead cells and invading microbes [141, 142]. Since macrophages have the multifunctional roles in the diseases due to their high plasticity, they display different phenotypes such as a pro-inflammatory M1 and anti-inflammatory M2 phenotype. The early work demonstrated that human MSCs antagonize the M1 phenotype and promote M2 polarization. In cell culture models, MSCs have been shown to influence macrophage phenotype between M1 and M2 lineages, which implicates the presence of soluble, MSC-derived factors that contribute to the cell polarization [142], as indicated by CD206 production, increased IL-10 secretion and phagocytosis, and pro-inflammatory cytokine and nitric oxide release [143]. Furthermore, MSCs can also inhibit MHC class II and CD68 expression of macrophages leading to reducing their stimulatory potency [144]. In vivo mouse model of an excisional wound repair, MSCs derived from human gingiva tissues can migrate to the wound site and polarize M2 for wound healing of the skin [145]. The regulatory effect of MSCs on macrophages is partially mediated through the secretion of different immune modulatory molecules, e.g., PGE2, IL1RA, and IL-6. In addition, the presence of macrophages in damaged tissue and inflammation is essential for MSCs to exert their therapeutic function. One proposed mechanism is that multiple soluble factors are produced for MSCs to elicit M2 polarization. Prostaglandin E2 (PGE2) was found to be constitutively produced by human MSCs at levels able to suppress IL-6 and TNF-α expression in activated macrophages [144]. Furthermore, neutralizing antibodies to GM-CSF and IL-6 revealed that these cytokines synergistically promote MSC-mediated promotion of the M2 phenotype in macrophages [145]. It has also been shown that macrophage colony-stimulating factor-induced differentiation of bone marrow hematopoietic stem cells toward monocytes/macrophages is regulated by TNFα that is released from MSCs under the influence of angiotensin II [146]. These findings suggest that MSCs can directly influence monocyte/macrophage differentiation during the development of atherosclerosis in response to hyperlipidemia. Thus, regulation of MSCs’ functions in situ might be indirectly influence macrophage behaviour and consequent inflammatory response in the vessel wall.
Summary and perspectives
This review provides an updated summary of recent research on MSCs and vascular disease in response to hyperlipidemia. There is a clear evidence that hyperlipidemia not only induces endothelial dysfunction and inflammatory response but also exerts its effect on MSCs. Especially, lipid overloading results in increase in MSC migration, which is a crucial event in atherosclerosis development. MSCs can differentiate into SMCs, macrophages, and possibly endothelial cells that participate in vascular disease. In response to hyperlipidemia, MSCs can release cytokines that influence other cells’ behaviours in vascular diseases. However, there are several questions to be answered regarding the role of MSCs during hyperlipidemia. For example, it is unknown whether hyperlipidemia influences the fate of MSC differentiation toward either endothelial or other cell types. It is also unknown whether hyperlipidemia is essential to activate MSCs in vivo. To answer these questions, further investigation is needed.
Accumulating evidence supports the protective role of MSCs in several models of atherosclerosis and MSC transplantation represents a novel approach for efficient prevention and treatment of atherosclerotic plaque rupture in animal models, because the MSCs can migrate from either media or adventitia to the intima where they may differentiate into different cell types, such as SMC, macrophage, and then secrete a broad range of beneficial factors, which modulate the inflammation status and restore endothelium function. Recent findings elaborated how can adventitial MSCs or stem/progenitor cells cross the media and migrate to the intima and concerning their involvement in hyperlipidemia-induced atherosclerosis. However, most data are derived from animal models or in vitro studies, a large clinic trial on effects of MSCs on atherosclerosis development or treatment is needed.
Funding
This work was supported by British Heart Foundation (RG/14/6/31144) and National Natural Science Foundation of China (91639302, 91339102, and 91539103).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Ting Chen and Yutao Wu have contributed equally.
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. doi: 10.1097/00007890-196803000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Latsinik NV, Luria EA, Friedenstein AJ, Samoylina NL, Chertkov IL. Colony-forming cells in organ cultures of embryonal liver. J Cell Physiol. 1970;75:163–165. doi: 10.1002/jcp.1040750205. [DOI] [PubMed] [Google Scholar]
- 4.Friedenstein A, Kuralesova AI. Osteogenic precursor cells of bone marrow in radiation chimeras. Transplantation. 1971;12:99–108. doi: 10.1097/00007890-197108000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Ivanov-Smolenski AA, Chajlakjan RK, Gorskaya UF, Kuralesova AI, Latzinik NW, Gerasimow UW. Origin of bone marrow stromal mechanocytes in radiochimeras and heterotopic transplants. Exp Hematol. 1978;6:440–444. [PubMed] [Google Scholar]
- 7.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 8.Friedenstein AJ. Stromal mechanisms of bone marrow: cloning in vitro and retransplantation in vivo. Haematol Blood Transfus. 1980;25:19–29. doi: 10.1007/978-3-642-67319-1_3. [DOI] [PubMed] [Google Scholar]
- 9.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 10.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 11.Friedenstein AJ. Marrow stromal fibroblasts. Calcif Tissue Int. 1995;56(Suppl 1):S17. doi: 10.1007/BF03354643. [DOI] [Google Scholar]
- 12.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 13.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.e02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 15.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 16.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 17.Salingcarnboriboon R, Yoshitake H, Tsuji K, Obinata M, Amagasa T, Nifuji A, Noda M. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res. 2003;287:289–300. doi: 10.1016/S0014-4827(03)00107-1. [DOI] [PubMed] [Google Scholar]
- 18.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 19.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 20.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 21.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 22.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res Off J Am Soc Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 23.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 24.Villaron EM, Almeida J, Lopez-Holgado N, Alcoceba M, Sanchez-Abarca LI, Sanchez-Guijo FM, Alberca M, Perez-Simon JA, San Miguel JF, Del Canizo MC. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89:1421–1427. [PubMed] [Google Scholar]
- 25.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.V98.8.2396. [DOI] [PubMed] [Google Scholar]
- 26.Gu W, Hong X, Potter C, Qu A, Xu Q. Mesenchymal stem cells and vascular regeneration. Microcirculation. 2017;24(1):e12324. doi: 10.1111/micc.12324. [DOI] [PubMed] [Google Scholar]
- 27.Tannock LR. Advances in the management of hyperlipidemia-induced atherosclerosis. Expert Rev Cardiovasc Ther. 2008;6:369–383. doi: 10.1586/14779072.6.3.369. [DOI] [PubMed] [Google Scholar]
- 28.Plakkal Ayyappan J, Paul A, Goo YH. Lipid droplet-associated proteins in atherosclerosis. Mol Med Rep. 2016;13:4527–4534. doi: 10.3892/mmr.2016.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein JL, Brown MS. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci USA. 1973;70:2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Innerarity TL, Weisgraber KH, Arnold KS, Mahley RW, Krauss RM, Vega GL, Grundy SM. Familial defective apolipoprotein B-100: low density lipoproteins with abnormal receptor binding. Proc Natl Acad Sci USA. 1987;84:6919–6923. doi: 10.1073/pnas.84.19.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG. Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Investig. 1973;52:1544–1568. doi: 10.1172/JCI107332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genest JJ, Jr, Martin-Munley SS, McNamara JR, Ordovas JM, Jenner J, Myers RH, Silberman SR, Wilson PW, Salem DN, Schaefer EJ. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation. 1992;85:2025–2033. doi: 10.1161/01.CIR.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 33.Carr MC, Brunzell JD. Abdominal obesity and dyslipidemia in the metabolic syndrome: importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk. J Clin Endocrinol Metab. 2004;89:2601–2607. doi: 10.1210/jc.2004-0432. [DOI] [PubMed] [Google Scholar]
- 34.Chin-Dusting JP, Shaw JA. Lipids and atherosclerosis: clinical management of hypercholesterolaemia. Expert Opin Pharmacother. 2001;2:419–430. doi: 10.1517/14656566.2.3.419. [DOI] [PubMed] [Google Scholar]
- 35.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 36.Raines EW, Ross R. Smooth muscle cells and the pathogenesis of the lesions of atherosclerosis. Br Heart J. 1993;69:S30–S37. doi: 10.1136/hrt.69.1_Suppl.S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torsney E, Xu Q. Resident vascular progenitor cells. J Mol Cell Cardiol. 2011;50:304–311. doi: 10.1016/j.yjmcc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroche V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Investig J Tech Methods Pathol. 2005;85:962–971. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 39.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. doi: 10.1182/blood.V78.1.55.55. [DOI] [PubMed] [Google Scholar]
- 40.Ning H, Lin G, Lue TF, Lin CS. Mesenchymal stem cell marker Stro-1 is a 75 kd endothelial antigen. Biochem Biophys Res Commun. 2011;413:353–357. doi: 10.1016/j.bbrc.2011.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, Cossu G, Serafini M, Sampaolesi M, Tagliafico E, Tenedini E, Saggio I, Robey PG, Riminucci M, Bianco P. No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep. 2016;6:897–913. doi: 10.1016/j.stemcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 44.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 45.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Investig. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Q. Progenitor cells in vascular repair. Curr Opin Lipidol. 2007;18:534–539. doi: 10.1097/MOL.0b013e3282a66082. [DOI] [PubMed] [Google Scholar]
- 47.Xu Q. Stem cells and transplant arteriosclerosis. Circ Res. 2008;102:1011–1024. doi: 10.1161/CIRCRESAHA.108.171488. [DOI] [PubMed] [Google Scholar]
- 48.Worsdorfer P, Mekala SR, Bauer J, Edenhofer F, Kuerten S, Ergun S. The vascular adventitia: an endogenous, omnipresent source of stem cells in the body. Pharmacol Ther. 2017;171:13–29. doi: 10.1016/j.pharmthera.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Kränkel N, Katare R, Angelini G, Emanueli C, Madeddu P. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nadaud S, Dierick F, Tiphaine H, Monceau V, Mougenot N, Crisan M, Dorfmuller P, Marodon G, Besson V, Marazzi G. Lung progenitor cells expressing PW1 gene participate in vascular remodeling during pulmonary arterial hypertension. FASEB J. 2015;29:LB569. doi: 10.1096/fasebj.29.1_supplement.lb569. [DOI] [Google Scholar]
- 51.Mao S-Z, Ye X, Liu G, Song D, Liu SF. Resident endothelial cells and endothelial progenitor cells restore endothelial barrier function after inflammatory lung injury. Arterioscler Thromb Vasc Biol. 2015;35:1635–1644. doi: 10.1161/ATVBAHA.115.305519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roostalu U, Aldeiri B, Albertini A, Humphreys NE, Simonsen-Jackson M, Wong JK, Cossu G. Distinct cellular mechanisms underlie smooth muscle turnover in vascular development and repair. Circ Res. 2017;122(2):267–281. doi: 10.1161/CIRCRESAHA.117.312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bab I, Ashton BA, Gazit D, Marx G, Williamson MC, Owen ME. Kinetics and differentiation of marrow stromal cells in diffusion chambers in vivo. J Cell Sci. 1986;84:139–151. doi: 10.1242/jcs.84.1.139. [DOI] [PubMed] [Google Scholar]
- 55.Bennett JH, Joyner CJ, Triffitt JT, Owen ME. Adipocytic cells cultured from marrow have osteogenic potential. J Cell Sci. 1991;99(Pt 1):131–139. doi: 10.1242/jcs.99.1.131. [DOI] [PubMed] [Google Scholar]
- 56.Caplan AI. Mesenchymal stem cells. J Orthopaed Res Off Publ Orthopaed Res Soc. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 57.Ohgushi H, Goldberg VM, Caplan AI. Repair of bone defects with marrow cells and porous ceramic: experiments in rats. Acta Orthopaed Scand. 1989;60:334–339. doi: 10.3109/17453678909149289. [DOI] [PubMed] [Google Scholar]
- 58.Ohgushi H, Goldberg VM, Caplan AI. Heterotopic osteogenesis in porous ceramics induced by marrow cells. J Orthopaed Res Off Publ Orthopaed Res Soc. 1989;7:568–578. doi: 10.1002/jor.1100070415. [DOI] [PubMed] [Google Scholar]
- 59.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102(Pt 2):341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 60.Hsieh JY, Fu YS, Chang SJ, Tsuang YH, Wang HW. Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton’s jelly of umbilical cord. Stem Cells Dev. 2010;19:1895–1910. doi: 10.1089/scd.2009.0485. [DOI] [PubMed] [Google Scholar]
- 61.Yu S, Long J, Yu J, Du J, Ma P, Ma Y, Yang D, Fan Z. Analysis of differentiation potentials and gene expression profiles of mesenchymal stem cells derived from periodontal ligament and Wharton’s jelly of the umbilical cord. Cells Tissues Organs. 2013;197:209–223. doi: 10.1159/000343740. [DOI] [PubMed] [Google Scholar]
- 62.Abu Kasim NH, Govindasamy V, Gnanasegaran N, Musa S, Pradeep PJ, Srijaya TC, Aziz ZA. Unique molecular signatures influencing the biological function and fate of post-natal stem cells isolated from different sources. J Tissue Eng Regen Med. 2012;9:E252. doi: 10.1002/term.1663. [DOI] [PubMed] [Google Scholar]
- 63.Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han ZB, Xu ZS, Lu YX, Liu D, Chen ZZ, Han ZC. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–1026. [PubMed] [Google Scholar]
- 64.El Omar R, Beroud J, Stoltz JF, Menu P, Velot E, Decot V. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev. 2014;20:523–544. doi: 10.1089/ten.teb.2013.0664. [DOI] [PubMed] [Google Scholar]
- 65.Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells: the great WAT hope. Trends Endocrinol Metab TEM. 2012;23:270–277. doi: 10.1016/j.tem.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cox BE, Griffin EE, Ullery JC, Jerome WG. Effects of cellular cholesterol loading on macrophage foam cell lysosome acidification. J Lipid Res. 2007;48:1012–1021. doi: 10.1194/jlr.M600390-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Kokkinopoulos I, Wong MM, Potter CMF, Xie Y, Yu B, Warren DT, Nowak WN, Le Bras A, Ni Z, Zhou C, Ruan X, Karamariti E, Hu Y, Zhang L, Xu Q. Adventitial SCA-1 + progenitor cell gene sequencing reveals the mechanisms of cell migration in response to hyperlipidemia. Stem Cell Rep. 2017;9:681–696. doi: 10.1016/j.stemcr.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang GP, Deng ZD, Ni J, Qu ZL. Oxidized low density lipoprotein and very low density lipoprotein enhance expression of monocyte chemoattractant protein-1 in rabbit peritoneal exudate macrophages. Atherosclerosis. 1997;133:31–36. doi: 10.1016/S0021-9150(97)00109-3. [DOI] [PubMed] [Google Scholar]
- 69.Clarke MC, Talib S, Figg NL, Bennett MR. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ Res. 2010;106:363–372. doi: 10.1161/CIRCRESAHA.109.208389. [DOI] [PubMed] [Google Scholar]
- 70.Navab M, Imes SS, Hama SY, Hough GP, Ross LA, Bork RW, Valente AJ, Berliner JA, Drinkwater DC, Laks H, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Investig. 1991;88:2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.CIR.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 72.Zhong Y, Liu T, Guo Z. Curcumin inhibits ox-LDL-induced MCP-1 expression by suppressing the p38MAPK and NF-kappaB pathways in rat vascular smooth muscle cells. Inflamm Res Off J Eur Histamine Res Soc. 2012;61:61–67. doi: 10.1007/s00011-011-0389-3. [DOI] [PubMed] [Google Scholar]
- 73.Kiyan Y, Tkachuk S, Hilfiker-Kleiner D, Haller H, Fuhrman B, Dumler I. oxLDL induces inflammatory responses in vascular smooth muscle cells via urokinase receptor association with CD36 and TLR4. J Mol Cell Cardiol. 2014;66:72–82. doi: 10.1016/j.yjmcc.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 74.Li W, Zhi W, Liu F, He Z, Wang X, Niu X. Atractylenolide I restores HO-1 expression and inhibits Ox-LDL-induced VSMCs proliferation, migration and inflammatory responses in vitro. Exp Cell Res. 2017;353:26–34. doi: 10.1016/j.yexcr.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 75.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Investig. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/S1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 77.Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106:1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peled M, Fisher EA. Dynamic aspects of macrophage polarization during atherosclerosis progression and regression. Front Immunol. 2014;5:579. doi: 10.3389/fimmu.2014.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH, Jr, Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boullier A, Gillotte KL, Horkko S, Green SR, Friedman P, Dennis EA, Witztum JL, Steinberg D, Quehenberger O. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J Biol Chem. 2000;275:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 81.Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Nerem RM. The pathogenesis of atherosclerosis: an overview. Clin Cardiol. 1991;14:I1–I16. doi: 10.1002/clc.4960141302. [DOI] [PubMed] [Google Scholar]
- 82.Thanopoulou A, Karamanos B, Archimandritis A. Comment on: McClung JA, Naseer N, Saleem M et al (2005) circulating endothelial cells are elevated in patients with type 2 diabetes mellitus independently of HbA1c. Diabetologia 48:345–350. Diabetologia. 2005;48:2687. doi: 10.1007/s00125-005-0020-7. [DOI] [PubMed] [Google Scholar]
- 83.Jaumdally RJ, Goon PK, Varma C, Blann AD, Lip GY. Effects of atorvastatin on circulating CD34 +/CD133 +/CD45 − progenitor cells and indices of angiogenesis (vascular endothelial growth factor and the angiopoietins 1 and 2) in atherosclerotic vascular disease and diabetes mellitus. J Intern Med. 2010;267:385–393. doi: 10.1111/j.1365-2796.2009.02151.x. [DOI] [PubMed] [Google Scholar]
- 84.Boyle AJ, Whitbourn R, Schlicht S, Krum H, Kocher A, Nandurkar H, Bergmann S, Daniell M, O’Day J, Skerrett D, Haylock D, Gilbert RE, Itescu S. Intra-coronary high-dose CD34 + stem cells in patients with chronic ischemic heart disease: a 12-month follow-up. Int J Cardiol. 2006;109:21–27. doi: 10.1016/j.ijcard.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 85.Schober A, Zernecke A, Liehn EA, von Hundelshausen P, Knarren S, Kuziel WA, Weber C. Crucial role of the CCL2/CCR2 axis in neointimal hyperplasia after arterial injury in hyperlipidemic mice involves early monocyte recruitment and CCL2 presentation on platelets. Circ Res. 2004;95:1125–1133. doi: 10.1161/01.RES.0000149518.86865.3e. [DOI] [PubMed] [Google Scholar]
- 86.Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD, Bellner L, Goldstein D, Peterson SJ, Shapiro JI, Abraham NG. Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther. 2013;4:28. doi: 10.1186/scrt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 88.Short B, Brouard N, Occhiodoro-Scott T, Ramakrishnan A, Simmons PJ. Mesenchymal stem cells. Arch Med Res. 2003;34:565–571. doi: 10.1016/j.arcmed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 89.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med. 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 90.Yao L, Li ZR, Su WR, Li YP, Lin ML, Zhang WX, Liu Y, Wan Q, Liang D. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One. 2012;7:e30842. doi: 10.1371/journal.pone.0030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells. 2010;28:329–343. doi: 10.1002/stem.277. [DOI] [PubMed] [Google Scholar]
- 92.Sheikh AM, Nagai A, Wakabayashi K, Narantuya D, Kobayashi S, Yamaguchi S, Kim SU. Mesenchymal stem cell transplantation modulates neuroinflammation in focal cerebral ischemia: contribution of fractalkine and IL-5. Neurobiol Dis. 2011;41:717–724. doi: 10.1016/j.nbd.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 93.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Ren Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 94.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 96.Karantalis V, Balkan W, Schulman IH, Hatzistergos KE, Hare JM. Cell-based therapy for prevention and reversal of myocardial remodeling. Am J Physiol Heart Circ Physiol. 2012;303:H256–H270. doi: 10.1152/ajpheart.00221.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34 + -derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 98.Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn MJ, Fibbe WE, Roelofs H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- 99.Melief SM, Geutskens SB, Fibbe WE, Roelofs H. Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica. 2013;98:888–895. doi: 10.3324/haematol.2012.078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 102.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 103.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 104.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 105.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 106.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 107.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 108.Kim YM, Jeon ES, Kim MR, Jho SK, Ryu SW, Kim JH. Angiotensin II-induced differentiation of adipose tissue-derived mesenchymal stem cells to smooth muscle-like cells. Int J Biochem Cell Biol. 2008;40:2482–2491. doi: 10.1016/j.biocel.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 109.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- 110.Kim MR, Jeon ES, Kim YM, Lee JS, Kim JH. Thromboxane a(2) induces differentiation of human mesenchymal stem cells to smooth muscle-like cells. Stem Cells. 2009;27:191–199. doi: 10.1634/stemcells.2008-0363. [DOI] [PubMed] [Google Scholar]
- 111.Elcin AE, Parmaksiz M, Dogan A, Seker S, Durkut S, Dalva K, Elcin YM. Differential gene expression profiling of human adipose stem cells differentiating into smooth muscle-like cells by TGFbeta1/BMP4. Exp Cell Res. 2017;352:207–217. doi: 10.1016/j.yexcr.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 112.Guo X, Stice SL, Boyd NL, Chen SY. A novel in vitro model system for smooth muscle differentiation from human embryonic stem cell-derived mesenchymal cells. Am J Physiol Cell Physiol. 2013;304:C289–C298. doi: 10.1152/ajpcell.00298.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grainger DJ, Metcalfe JC, Grace AA, Mosedale DE. Transforming growth factor-beta dynamically regulates vascular smooth muscle differentiation in vivo. J Cell Sci. 1998;111(Pt 19):2977–2988. doi: 10.1242/jcs.111.19.2977. [DOI] [PubMed] [Google Scholar]
- 114.Yamazaki T, Nalbandian A, Uchida Y, Li WL, Arnold TD, Kubota Y, Yamamoto S, Ema M, Mukouyama YS. Tissue myeloid progenitors differentiate into pericytes through TGF-beta signaling in developing skin vasculature. Cell Rep. 2017;18:2991–3004. doi: 10.1016/j.celrep.2017.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Bae YC, Jung JS, Kim JH. Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism. J Cell Sci. 2006;119:4994–5005. doi: 10.1242/jcs.03281. [DOI] [PubMed] [Google Scholar]
- 116.Wang C, Yin S, Cen L, Liu Q, Liu W, Cao Y, Cui L. Differentiation of adipose-derived stem cells into contractile smooth muscle cells induced by transforming growth factor-beta1 and bone morphogenetic protein-4. Tissue Eng Part A. 2010;16:1201–1213. doi: 10.1089/ten.tea.2009.0303. [DOI] [PubMed] [Google Scholar]
- 117.Kothapalli CR, Taylor PM, Smolenski RT, Yacoub MH, Ramamurthi A. Transforming growth factor beta 1 and hyaluronan oligomers synergistically enhance elastin matrix regeneration by vascular smooth muscle cells. Tissue Eng Part A. 2009;15:501–511. doi: 10.1089/ten.tea.2008.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Poliseno L, Cecchettini A, Mariani L, Evangelista M, Ricci F, Giorgi F, Citti L, Rainaldi G. Resting smooth muscle cells as a model for studying vascular cell activation. Tissue Cell. 2006;38:111–120. doi: 10.1016/j.tice.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 119.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Han CI, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. J Vasc Res. 2001;38(2):113–119. doi: 10.1159/000051038. [DOI] [PubMed] [Google Scholar]
- 121.Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN. Host bone-marrow cells are a source of donor intimal smooth- muscle-like cells in murine aortic transplant arteriopathy. Nat Med. 2001;7(6):738–741. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- 122.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8(4):403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 123.Hu Y, Davison F, Ludewig B, Erdel M, Mayr M, Url M, Dietrich H, Xu Q. Smooth muscle cells in transplant atherosclerotic lesions are originated from recipients, but not bone marrow progenitor cells. Circulation. 2002;106(14):1834–1839. doi: 10.1161/01.CIR.0000031333.86845.DD. [DOI] [PubMed] [Google Scholar]
- 124.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101(12):1362–1365. doi: 10.1161/01.CIR.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 125.Wanjare M, Kuo F, Gerecht S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc Res. 2013;97:321–330. doi: 10.1093/cvr/cvs315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Steinbach SK, El-Mounayri O, DaCosta RS, Frontini MJ, Nong Z, Maeda A, Pickering JG, Miller FD, Husain M. Directed differentiation of skin-derived precursors into functional vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:2938–2948. doi: 10.1161/ATVBAHA.111.232975. [DOI] [PubMed] [Google Scholar]
- 127.Tamama K, Sen CK, Wells A. Differentiation of bone marrow mesenchymal stem cells into the smooth muscle lineage by blocking ERK/MAPK signaling pathway. Stem Cells Dev. 2008;17:897–908. doi: 10.1089/scd.2007.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gong Z, Calkins G, Cheng EC, Krause D, Niklason LE. Influence of culture medium on smooth muscle cell differentiation from human bone marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15:319–330. doi: 10.1089/ten.tea.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boucher JM, Peterson SM, Urs S, Zhang C, Liaw L. The miR-143/145 cluster is a novel transcriptional target of Jagged-1/Notch signaling in vascular smooth muscle cells. J Biol Chem. 2011;286:28312–28321. doi: 10.1074/jbc.M111.221945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gays D, Hess C, Camporeale A, Ala U, Provero P, Mosimann C, Santoro MM. An exclusive cellular and molecular network governs intestinal smooth muscle cell differentiation in vertebrates. Development. 2017;144:464–478. doi: 10.1242/dev.133926. [DOI] [PubMed] [Google Scholar]
- 132.Long X, Miano JM. Transforming growth factor-beta1 (TGF-beta1) utilizes distinct pathways for the transcriptional activation of microRNA 143/145 in human coronary artery smooth muscle cells. J Biol Chem. 2011;286:30119–30129. doi: 10.1074/jbc.M111.258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu H, Xu Z, Li C, Xu C, Lei Z, Zhang HT, Zhao J. MiR-145 and miR-203 represses TGF-beta-induced epithelial-mesenchymal transition and invasion by inhibiting SMAD3 in non-small cell lung cancer cells. Lung Cancer. 2016;97:87–94. doi: 10.1016/j.lungcan.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 134.Li E, Zhang J, Yuan T, Ma B. MiR-145 inhibits osteosarcoma cells proliferation and invasion by targeting ROCK1. Tumour Biol. 2014;35:7645–7650. doi: 10.1007/s13277-014-2031-9. [DOI] [PubMed] [Google Scholar]
- 135.Psaltis PJ, Simari RD. Vascular wall progenitor cells in health and disease. Circ Res. 2015;116:1392–1412. doi: 10.1161/CIRCRESAHA.116.305368. [DOI] [PubMed] [Google Scholar]
- 136.Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ghigo C, Mondor I, Jorquera A, Nowak J, Wienert S, Zahner SP, Clausen BE, Luche H, Malissen B, Klauschen F, Bajenoff M. Multicolor fate mapping of Langerhans cell homeostasis. J Exp Med. 2013;210:1657–1664. doi: 10.1084/jem.20130403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gentek R, Molawi K, Sieweke MH. Tissue macrophage identity and self-renewal. Immunol Rev. 2014;262:56–73. doi: 10.1111/imr.12224. [DOI] [PubMed] [Google Scholar]
- 139.Psaltis PJ, Harbuzariu A, Delacroix S, Witt TA, Holroyd EW, Spoon DB, Hoffman SJ, Pan S, Kleppe LS, Mueske CS, Gulati R, Sandhu GS, Simari RD. Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation. 2012;125:592–603. doi: 10.1161/CIRCULATIONAHA.111.059360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Psaltis PJ, Puranik AS, Spoon DB, Chue CD, Hoffman SJ, Witt TA, Delacroix S, Kleppe LS, Mueske CS, Pan S, Gulati R, Simari RD. Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ Res. 2014;115:364–375. doi: 10.1161/CIRCRESAHA.115.303299. [DOI] [PubMed] [Google Scholar]
- 141.Spaggiari GM, Moretta L. Cellular and molecular interactions of mesenchymal stem cells in innate immunity. Immunol Cell Biol. 2013;91:27–31. doi: 10.1038/icb.2012.62. [DOI] [PubMed] [Google Scholar]
- 142.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzon IM, Nepomnaschy I, Costa H, Canones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tsubakimoto Y, Yamada H, Yokoi H, Kishida S, Takata H, Kawahito H, Matsui A, Urao N, Nozawa Y, Hirai H, Imanishi J, Ashihara E, Maekawa T, Takahashi T, Okigaki M, Matsubara H. Bone marrow angiotensin AT1 receptor regulates differentiation of monocyte lineage progenitors from hematopoietic stem cells. Arterioscler Thromb Vasc Biol. 2009;29:1529–1536. doi: 10.1161/ATVBAHA.109.187732. [DOI] [PubMed] [Google Scholar]