Abstract
The estrogen-related receptor γ (ERRγ, NR3B3) is a constitutively active nuclear receptor which has been proposed to act as a mediator of the low-dose effects of a number of environmental endocrine-disrupting chemicals (EDCs) such as the xenoestrogen bisphenol-A (BPA). To better characterize the ability of exogenous compounds to bind and activate ERRγ, we used a combination of cell-based, biochemical, structural and computational approaches. A purposely created stable cell line allowed for the determination of the EC50s for over 30 environmental ERRγ ligands, including previously unknown ones. Interestingly, affinity constants (Kds) of the most potent compounds measured by isothermal titration calorimetry were in the 50–500 nM range, in agreement with their receptor activation potencies. Crystallographic analysis of the interaction between the ERRγ ligand-binding domain (LBD) and compounds of the bisphenol, alkylphenol and naphthol families revealed a partially shared binding mode and minimal alterations of the receptor conformation upon ligand binding. Further biophysical characterizations coupled to molecular dynamics simulations suggested a mechanism through which ERRγ ligands would exhibit their agonistic properties by preserving the transcriptionally active form of the receptor while rigidifying some loop regions with associated functions. This unique mechanism contrasts with the classical one involving a ligand-induced repositioning and stabilization of the C-terminal activation helix H12.
Electronic supplementary material
The online version of this article (10.1007/s00018-019-03129-x) contains supplementary material, which is available to authorized users.
Keywords: Estrogen-related receptor γ, Endocrine disruptors, Xenoestrogens, Protein–ligand interaction
Introduction
Estrogen-related receptors (ERRs) are a family of three nuclear receptors (ERRα, ERRβ and ERRγ) named on the basis of their high level of homology with estrogen receptors α and β (ERα and ERβ). However, despite this particularity, ERRs do not bind to estrogens [1]. Although ERRs are considered as constitutively active orphan receptors, cholesterol has been recently shown to act as an endogenous ERRα agonist [2, 3]. Moreover, some evidence suggests that farnesoids could be generic activators of a subset of nuclear receptors, yet whether ERRs belong to this category remains to be established [4]. In fact, crystal structures of ERRs have shown that these receptors possess a transcriptionally active conformation in the absence of any ligand (apo form) which allows the binding of coactivators [5]. In physiological conditions, the transcriptional activity of these constitutively active receptors is regulated by a number of post-translational modifications (PTMs) such as phosphorylation, acetylation, sumoylation or ubiquitination and the cell- and tissue-specific expression of coregulatory proteins, such as members of the PGC-1 and SRC1-3 families, or the receptor-interacting protein 140 [6, 7]. ERRs are able to recognize estrogen response elements (EREs), making them capable of modulating the ER-mediated response by competing or cooperating with ERs, although the real impact on ER-regulated genes might be less than anticipated [8]. On the other hand, members of the ERR family can also bind to a specific ERR response element (ERRE) and regulate functions and genetic programs that are unique to these receptors [7, 9–11].
The physiological roles of ERRs have been extensively studied in the last few years and it is now well accepted that these receptors are key players in the control of the cellular energy metabolism. This is indicated by their high expression levels in tissues with high energetic demands, and their ability to modulate a very large number of metabolic genes [8]. In particular, ERRs are involved in the regulation of genes implicated in all aspects of mitochondrial functions, including the Krebs cycle and the lipid, carbohydrate, pyruvate, amino acid and nucleic acid metabolisms [6]. In addition to this key role in cellular energy metabolism, it has been shown that ERRs are involved in the regulation of the circadian rhythm, in the heart, kidney and skeletal muscle physiology, and in cell growth and differentiation [12]. It should be noted that despite the fact that all members of the ERR family can recognize the same response elements and theoretically target the same network of genes, they seem to have complementary physiological roles likely due to distinct patterns of expression and distinct activating/deactivating PTMs [6, 13, 14]. ERRα, ERRγ, and to a lesser extent ERRβ, are expressed in estrogen-dependent cancers, and, therefore, their roles in these type of tumors were investigated [15]. ERRα is thought to be involved in tumor growth, with high level of expression correlating with poor prognosis in breast, ovarian and colon cancers [16–18]. In contrast, conflicting results have been reported on the roles of ERRγ and ERRβ in cancer progression. Indeed, some studies have suggested that the proliferation of the MCF-7 breast cancer cell line is stimulated by an exogenous expression of ERRγ and the expression of this receptor in breast cancer is up-regulated [19]. However, other studies have shown that this up-regulation is associated with a favorable outcome and that ERRγ inhibits the growth of breast cancer xenografts [17, 20]. As for ERRβ, contradictory results have attributed it to both proliferative and anti-proliferative effects in breast cancer [12, 15].
It has been shown that the basal activity of ERRγ can be modulated by a number of EDCs, including several phenols, phytoestrogens and pesticides. For instance, bisphenol-A (BPA), an environmental compound whose endocrine-disrupting effects are well documented [21], and bisphenol-E (BPE) were shown to increase ERRγ transcriptional activity [22–24]. On the contrary, the pharmaceutical compounds 4-hydrotamoxifen (4-OHT) and diethylstilbestrol (DES) were found to be inverse agonists of ERRγ, decreasing its basal activity [25, 26]. A previous study using radioligand-binding assays reported that BPA binds to ERRγ with an affinity constant (Kd) of 5 nM [27]. In contrast, reporter gene assays needed a higher concentration to achieve 50% of the maximal transactivation with EC50 values in the low micromolar range [23, 24, 26]. This puzzling discrepancy could reveal a disconnect between the interaction capacity of a compound with the ligand-binding domain (LBD) of ERRγ and its ability to stabilize helix H12 in the active conformation, a key event in ligand-induced nuclear receptor activation. However, another study using isothermal methods showed a Kd of 70 nM for the interaction between ERRγ and BPA [28].
To assess the ability of compounds to bind and modulate ERRγ transcriptional activity, we developed a stable cell line expressing the yeast GAL4 DNA-binding domain (DBD) fused with the human ERRγ LBD, and a luciferase reporter whose expression is driven by GAL4 response elements. Using this cell line to screen compounds, we were able to identify new ERRγ agonists, namely the hydroxyphenyl-trichloroethane (HPTE) and the tetrahydro-2-naphthol (THN). All the EC50 values determined for these molecules were within the high-nanomolar range, in line with their Kds measured by isothermal titration calorimetry (ITC). Further structural and computational analyses showed that ligand binding has no noticeable impact on the protein fold but significantly restrains its structural dynamics. This unusual mechanism likely accounts for the agonistic profile of ERRγ environmental ligands.
Materials and methods
Ligands
Bisphenols, alkylphenols, parabens, 4-hydroxybenzoic acid, HPTE, benzophenones, genistein and tetrahydro-2-naphthol were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France). 4-hydroxytamoxifen (OHT), DY131 and GSK4716 were purchased from bio-techne (Lille, France). All compound stock solutions were prepared at 10 mM in dimethyl sulfoxide (DMSO). The final DMSO concentration during treatment never exceeded 0.1% (v/v) of the culture medium.
Plasmids and transient transactivation experiments
The ERRγ activity was monitored on ERE3-TATA-luciferase and GAL4RE5-βGlobin-Luciferase reporter constructs in U2OS, HeLa and LS-174T cells. The full-length ERRγ (M1-V458) coding sequence was inserted into the EcoRI site of the pSG5puro plasmid kindly provided by Dr. H. Gronemeyer (IGBMC, Illkirch, France). ERE3-TATA-luciferase was a kind gift from Pr J.A. Gustafsson (CNRCS, Houston, Texas). To construct the pSG5-GAL4(DBD)-hERRγ(LBD)-puromycin plasmid, the sequence coding for the hERRγ LBD (L187-V458) was amplified by PCR using primers containing XhoI and BamHI restriction sites and inserted by PCR into the pSG5-GAL4-DBD(M1-S147)-puromycin plasmid [29]. The GAL4RE-x5-βGlobin-Luciferase-neomycin plasmid has been previously described [29]. Transient transfection assays were performed using Jet-PEI (Ozyme, Saint-Quentin-en-Yvelines, France) according to the manufacturer’s instructions. Luciferase assays were performed with the Promega dual reporter kit according to the manufacturer’s instructions. Renilla luciferase encoded by the normalization vector phRLTK (Promega) was used as an internal control for firefly luciferase normalization. Data are expressed as a ratio between firefly luciferase relative light units (RLUs) divided by renilla luciferase RLUs. Tests were performed in duplicate in at least three independent experiments. Data were analyzed for significant differences using Student’s t test (***p < 0.001, **p < 0.01, *p < 0.05). Differences were considered statistically significant at p < 0.05.
Generation of the HG5LN GAL4-hERRγ stable reporter cell line
The HG5LN cell line containing the GAL4-responsive reporter gene was obtained by stably expressing the GAL4RE5-βGlob-Luc-SV40-Neo plasmid in HeLa cells as previously described [29]. HG5LN GAL4 cells were generated by transfecting HG5LN cells with the pSG5-GAL4(DBD)puro plasmid. HG5LN GAL4-hERRγ cells were generated by transfecting HG5LN cells with the pSG5-GAL4(DBD)-hERR(LBD)-puro plasmid. Selection of resistant clones was performed by adding puromycin and G418 antibiotics to the medium at 0.5 µg/ml and 1 mg/ml, respectively. Cells were grown in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12) containing phenol red, 1 g/l glucose and supplemented with 10% fetal bovine serum, 100 units/ml of penicillin and 100 µg/ml of streptomycin in a 5% CO2 humidified atmosphere at 37 °C. HG5LN cell medium was supplemented with 1 mg/ml G418. HG5LN GAL4 and HG5LN GAL4-hERRγ cell medium with 1 mg/ml G418 and 0.5 µg/ml puromycin. To test compounds for their ability to activate ERRγ transcriptional activity, cells were grown in DMEM/F-12 without phenol red and supplemented with 5% dextran-coated, charcoal-treated fetal bovine serum (DCC-FBS).
Transactivation assays
HG5LN, HG5LN GAL4 and HG5LN GAL4-hERRγ reporter cell lines were seeded at a density of 25,000 cells per well in 96-well white opaque tissue culture plates (Greiner CellStar). Compounds to be tested were added 24 h later, and cells were incubated at 37 °C for 16 h. At the end of the incubation period, culture medium was replaced with DMEM/F12 medium (Invitrogen) containing 0.3 mM luciferin. Luciferase activity was measured for 2 s in intact living cells using a MicroBeta Wallac luminometer (PerkinElmer). Tests were performed in quadruplicate in at least three independent experiments. Data are expressed as percentage of the basal activity. EC50 values were measured using GraphPad Prism (GraphPad Software Inc). Data were analyzed for significant differences using one-way ANOVA followed by Dunnet’s post-comparison test (vs control) (***p < 0.001, **p < 0.01, *p < 0.05). Differences were considered statistically significant at p < 0.05.
Protein production and purification
The ERRγ-LBD gene was cloned into the pDB-His-GST plasmid. The protein was overproduced in E. coli BL21(DE3) cells for 4 h at 25 °C in LB medium as a N-terminus 6His-GST fusion. After culture, cells were harvested by centrifugation and the pellets re-suspended in lysis buffer (50 mM Tris pH 7.5, 300 mM NaCl, 20 mM Imidazole) supplemented with lysozyme (1 μg/ml) and a protease inhibitor cocktail tablet (complete, EDTA-free, Roche), and then subjected to sonication. The clarified cell lysate was applied onto a Ni2+-affinity column (HisTrap 5 ml; GE Healthcare) equilibrated with lysis buffer. The eluted fusion protein was then dialyzed against 50 mM Tris pH 7.5, 150 mM NaCl, 5% (v/v) glycerol, 1 mM DTT, and cleaved at the same time with the 3C precision protease (homemade), overnight at 4 °C. The sample was then applied onto a Ni2+-affinity column (HisTrap 5 ml; GE Healthcare) to remove the tag. The eluted untagged ERRγ-LBD was further purified onto a gel filtration column (Superdex 75 16/60; GE Healthcare) equilibrated with a buffer containing 50 mM Tris pH 7.5, 150 mM NaCl, 5% (v/v) glycerol, 5 mM DTT. The protein was concentrated (≈ 10 mg/ml) and stored at − 40 °C.
Crystallization
Prior to crystallization assays, purified ERRγ-LBD (4 mg/ml) was mixed with 2 molar equivalent of BPA, BPE, BPB, HPTE, 4-sec-butylphenol, 4-iso-propylphenol, or tetrahydro-2-naphthol for 30 min at 4 °C. Bigger and fewer crystals were obtained using a 100,000× diluted seeding solution prepared with one ERRγ-LBD crystal. The drops contained 1 µl of protein, 1 µl of reservoir, and 0.5 µl of seeding solution. Crystals were obtained in 48 h in 0–100 mM sodium acetate, 100 mM Tris pH 8.5, 23–26% (w/v) PEG 8000.
Data collection and structure determination
For BPE, BPB, HPTE, 4-sec-butylphenol, 4-iso-propylphenol, and tetrahydro-2-naphthol complexes, native data were collected from one crystal on ID30a-1, ID30a-3, or ID30b beamlines at the European synchrotron radiation facilities (λ = 0.8726 Å, 100 K), Grenoble, France. Similar data for the BPA complex were collected on the CBS home source (Rigaku MicroMax 007 HF). Data were processed and scaled with XDS and XSCALE [30]. Crystals belong to space group P 41212. The X-ray structures were solved and refined using Phenix (phenix.refine) [31] and COOT [32]. The percentage of residues located in the favored Ramachandran plot region is 98.65% for all complex structures as calculated with MolProbity [33]. Data collection and refinement statistics are summarized in Table S1. Figures were prepared with PyMOL (http://pymol.org/).
Isothermal titration calorimetry (ITC)
Kds for BPA, BPE, BPB, HPTE, 4-sec-butylphenol, 4-iso-propylphenol, and tetrahydro-2-naphthol were measured by isothermal titration calorimetry. The heat of interaction of ligands with ERRγ was measured using a MicroCal VP-ITC system (Malvern Panalytical) operating at 25 °C. Purified ERRγ (130-434) was first dialyzed for 2 × 2 h against Tris–HCl 50 mM, pH 7.5, NaCl 150 mM, TCEP 1 mM, glycerol 5% (v/v) using a 10 kDa molecular weight cutoff dialysis cassette (Slide-A-Lyzer 0.5 ml 10 K MWCO, Thermo Scientific). Duplicate experiments were carried out in Tris–HCl 50 mM, pH 7.5, NaCl 150 mM, TCEP 1 mM, glycerol 5% (v/v) (syringe and sample cell; the reference cell was filled with pure water). ERRγ (10–20 μM) was disposed in the 2 ml sample cell, and compounds (100–200 µM) were delivered from the 500 μl syringe. Heat exchanges were monitored throughout titrations consisting of 30 injections (one time 1 μl in 2.5 s followed by 29 times 10 μl in 7.1 s, spaced by 300 s) of ligand solutions into the cell containing ERRγ. Data were analyzed with the MicroCal ITC-ORIGIN software (Malvern Panalytical).
Thermal shift assays (Thermofluor®)
This method measured protein unfolding based on fluorescence detection of the denatured form of the protein. Solutions of 15 µL containing 5 µM protein, 15 µM ligand (in 50% DMSO) and 5× Sypro® Orange, in the gel filtration buffer, were added to the wells of a 96-well PCR plate. 50% DMSO was added instead of ligand in the tests for the apo form. The plates were sealed with an optical sealing tape (Bio-Rad) and heated in an Mx3005P Q-PCR system (Stratagene) from 25 to 95 °C at 1 °C intervals. Fluorescence changes in the wells were monitored with a photomultiplier tube. The wavelengths for excitation and emission were 492 nm and 610 nm, respectively. The melting temperatures, Tm, were obtained by fitting the fluorescence data from three independent experiments with a Boltzmann model using the GraphPad Prism software.
Fluorescence anisotropy
Binding affinity (Kd) measurements of the fluorescein-labeled PGC-1 NR2 peptide for ERRγ in the absence and presence of various ligands were performed using a Safire2 microplate reader (TECAN) with the excitation wavelength set at 470 nm and emission measured at 530 nm. Experiments were performed as previously described [34]. The reported data are the average of three independent experiments. The Kds were obtained by fitting data using the GraphPad Prism software.
Molecular dynamics simulations
The ERRγ LBD structures solved by X-ray crystallography in the presence of BPA and BPE were used to obtain the starting conformations for the corresponding simulations. The initial structure for the unliganded form was obtained by removing the ligand from the BPA-bound ERRγ LBD structure. The three systems were solvated with ~ 23,000 TIP3P water molecules in a dodecahedron box of 725 nm3. Total charges were neutralized with Na+/Cl− ions and additional pairs were added to reach a final physiological concentration of 150 mM.
The Amber ff14SB forcefield [35] was used for describing the protein and GAFF [36] for the ligands. All the simulations were performed with the GROMACS 2018 software [37]. The timestep was set to 2 fs and LINCS constraints were applied to all bonds. Long-range electrostatic interactions were treated with the particle Mesh Ewald method [38] whereas the Van der Walls interactions were implemented with a cutoff of 1.0 nm. The velocity rescaling thermostat [39] and the Parrinello–Rahman barostat [40] were used to maintain constant temperature and pressure at values of 300 K and 1 bar, respectively. A 5000 steps Steepest-Descent energy minimization was first performed for each system, followed by a 500 ps simulation in the canonical ensemble and another 500 ps in the isothermal-isobaric ensemble, both with restraints applied to the protein heavy atom positions. Three independent 500 ns replica (1.5 µs) were produced for each system: apo-ERRγ, ERRγ-BPA, ERRγ-BPE, leading to a total simulation time of 4.5 µs. For analysis purposes, the first 50 ns of each simulation were discarded.
Results
Modulation of the activity of full-length ERRγ and ERRγ-LBD by OHT and BPA in transiently transfected cells
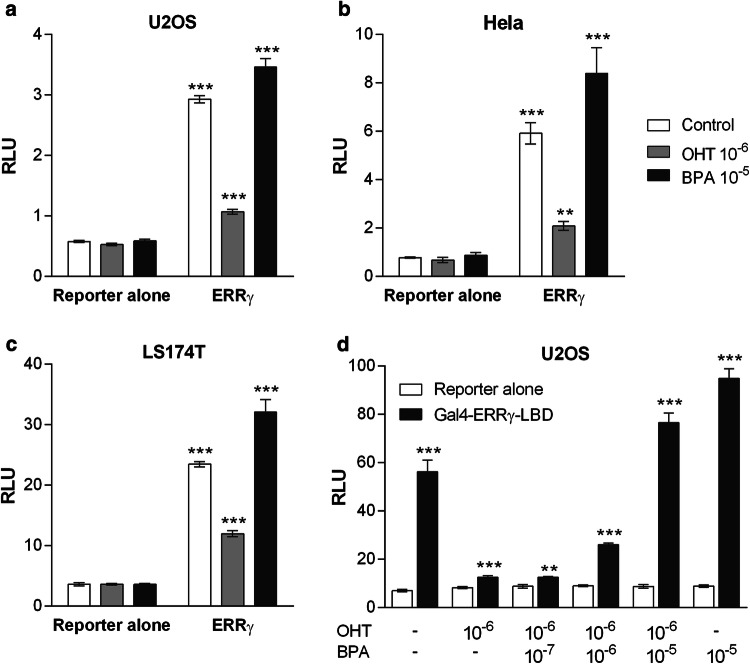
We first transfected U2OS, HeLa and LS174T cell lines with full-length ERRγ and a luciferase reporter gene whose expression is driven by ERRγ, and tested its activity in the presence of the ERRγ antagonist 4-hydroxytamoxifen (OHT) or the agonist BPA (Fig. 1a–c, and supplemental Fig. S1). In those three different cellular contexts, the constitutive activity of ERRγ was visible, increasing the basal luciferase activity compared to cells transfected with the reporter gene alone by about sixfold in the absence of any ligand. In each cell line, OHT (10−6 M) decreased the constitutive activity of ERRγ by two- to threefold, while a treatment with BPA (10−5 M) increased the ERRγ transcriptional activity by 15–25%. We then transiently transfected U2OS cells with a plasmid expressing the GAL4(DBD)-hERRγ(LBD) fusion protein and a luciferase reporter gene whose expression is driven by five GAL4-response elements (Fig. 1d). Those plasmids were subsequently used for the establishment of the HG5LN GAL4-hERRγ stable cell line. Similarly to what we observed with the full-length ERRγ, transfection with ERRγ LBD strongly increased the basal luciferase activity which could be further amplified or strongly reduced by addition of BPA or OHT, respectively. Treatment with BPA was able to reverse the effect of OHT in a dose-dependent manner. The realization that modulation of the constitutive activity of ERRγ by antagonists and agonists could be monitored by a cell-based assay prompted us to establish a new model cell line for the high-throughput screening of ERRγ environmental ligands.
Fig. 1.
Modulation of full-length ERRγ and ERRγ LBD activities by OHT and BPA. Luciferase activity in cells transfected with a–c full-length ERRγ and an ERE-driven luciferase reporter, or a d Gal4-DBD-ERRγ-LBD fusion protein and a GAL4-driven luciferase reporter. a, d Luciferase activity in U2OS, b in HeLa cells, and c in LS174T cells. Data are expressed as a ratio between firefly luciferase relative light units (RLUs) divided by renilla luciferase RLUs. Tests were performed in duplicate in at least three independent experiments. Data were analyzed for significant differences using Student’s t test (***p < 0.001, **p < 0.01, *p < 0.05). Differences were considered statistically significant at p < 0.05
Establishment of the HG5LN GAL4-hERRγ reporter cell line and effect of OHT and BPA
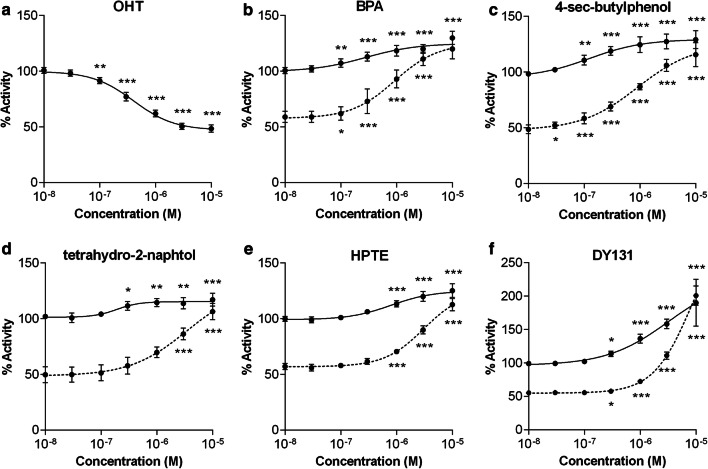
The HG5LN GAL4-hERRγ cell line stably expressing the GAL4-driven reporter gene and the GAL4(DBD)-hERRγ(LBD) fusion protein was obtained as described in the “Materials and methods” section. In this cellular model, OHT was able to decrease the basal luciferase activity in a dose-dependent manner to a maximum of about 50% and displayed an IC50 value of 458 nM (Fig. 2a and Table 1). Conversely, BPA increased the luciferase activity in a dose-dependent manner to a maximum of 125% and exhibited an EC50 value of 174 nM (Fig. 2b and Table 1). When the cells were co-treated with OHT (10−6 M), BPA successfully reversed the effect of the antagonist, indicating that BPA can compete with OHT for binding to ERRγ LBD. Similar dose–response curves in the absence or presence of OHT were obtained for several of the compounds we tested on our cell model, such as the 4-sec-butylphenol (Fig. 2c), the tetrahydro-2-naphthol (Fig. 2d), the HPTE (Fig. 2e) or the DY131 (Fig. 2f). The HG5LN and HG5LN GAL4 cell lines stably expressing the reporter gene alone or the reported gene and GAL4-DBD [29] were used to confirm that OHT, BPA and all other compounds do not increase luciferase gene expression in a non-specific manner at the concentrations used for screening (Supplemental Fig. S2). These data indicate that the HG5LN GAL4-hERRγ cell line stably expressing a GAL4(DBD)-hERRγ(LBD) fusion protein is a powerful tool to identify compounds that are able to modulate ERRγ transcriptional activity. These cells can be used for a standardized high-throughput screening of numerous compounds in 96-well plates. By providing a response based on ERRγ transcriptional activity, our model cell line appears to be highly relevant for the identification of EDCs exerting their adverse effects on the signaling pathways controlled by this receptor.
Fig. 2.
ERRγ agonists increase luciferase expression in HG5LN-ERRγ cells in absence or presence of OHT. Dose–response curves of compounds in HG5LN-ERRγ cells. Cells were incubated for 16 h with various concentrations of a OHT, b BPA, c 4-sec-butylphenol, d tetrahydro-2-naphthol, e HPTE, and f DY131. Compounds were incubated alone (line) or in the presence of 10 µM of OHT (dashed line). Tests were performed in quadruplicate in at least three independent experiments. Data are expressed as percentage of the basal activity. Data were analyzed using GraphPad Prism (GraphPad Software Inc) for significant differences using one-way ANOVA followed by Dunnet’s post-comparison test (vs control) (***p < 0.001, **p < 0.01, *p < 0.05). Differences were considered statistically significant at p < 0.05
Table 1.
Functional characteristics of compounds
| Compound | EC50 ± SD (nM) | Type | Kd (nM) | ΔTm (°C) |
|---|---|---|---|---|
| 4-sec-Butylphenol | 109 ± 65 | Alkylphenol | 182.5 ± 17.2 | + 6.3 |
| 4-tert-Butylphenol | 135 ± 79 | Alkylphenol | ND | ND |
| Bisphenol-E | 142 ± 60 | Bisphenol | 48.7 ± 8.2 | + 3.2 |
| Bisphenol-A | 174 ± 56 | Bisphenol | 98.6 ± 21.8 | + 3.3 |
| Tetrahydro-2-naphthol | 260 ± 50 | Naphthol | 201.4 ± 15.5 | + 0.6 |
| 4-iso-Propylphenol | 300 ± 150 | Alkylphenol | 390.4 ± 76.9 | + 1.9 |
| 4α-Cumylphenol | 313 ± 92 | Bisphenol | ND | ND |
| 4-Hydroxytamoxifen | 458 ± 118a | Stilbene | ND | ND |
| GSK4716 | 501 ± 99 | Paraben | ND | ND |
| Bisphenol-B | 528 ± 304 | Bisphenol | 569.0 ± 37.1 | + 2.5 |
| Bisphenol-F | 645 ± 103 | Bisphenol | ND | ND |
| 4-Ethylphenol | 654 ± 311 | Alkylphenol | ND | ND |
| DY131 | 698 ± 62 | Paraben | ND | ND |
| 4-tert-Octylphenol | 771 ± 80 | Alkylphenol | ND | ND |
| Hydroxychlor (HPTE) | 792 ± 243 | Bisphenol | 157.5 ± 3.4 | + 3.1 |
| Bisphenol-C | 805 ± 230 | Bisphenol | ND | ND |
| 2,4-Di-tert-butylphenol | 2090 ± 437 | Alkylphenol | ND | ND |
EC50, half maximal effective concentration; Kd, affinity constant; ΔTm, differential melting temperature; ND, not determined
aIC50 value
Functional characterization of EDCs on ERRγ
The HG5LN GAL4-hERRγ cell line was used for the screening of several compounds known to bind to ERRγ, and compounds for which the affinity for ERRγ had not yet been assessed (Supplemental Fig. S1 and Table 1). Compounds listed in Supplemental Fig. S1 with no data reported in Table 1 are considered as inactive at the tested concentrations. Among all compounds screened on these cells, only OHT displayed antagonistic properties. The BPE was the bisphenol exhibiting the lowest EC50 value (142 nM) whereas BPB and BPA had EC50s of 528 and 174 nM, respectively. BPC, HPTE, a metabolite of the methoxychlor pesticide, and BPF, one of the major BPA substitutes, exhibited EC50 values in the micromolar range whereas that of BPS, another BPA substitute, could not be determined in our screening assay, suggesting a poor binding capacity of this compound. Similarly, all bisphenols with more than two hydroxyphenyl functionalities such as the bisphenols P, M or FL were not able to modify the basal luciferase activity of our cell line. We also tested several members of the alkylphenol family, another class of compounds which has been shown to bind to ERRγ while possessing a single hydroxyphenyl functionality (Supplemental Fig. S1). Among them, the 4-sec-butylphenol exhibited the lowest EC50 value (109 nM). The 4-tert-butylphenol and 4-iso-propylphenol had EC50 values of 135 and 300 nM, respectively. ERRγ activation was lost when the sec group was located closer to the hydroxyl group (2-sec-butylphenol) or when the alkyl chain was shortened (4-ethylphenol), enlarged (4-tert-octylphenol), or elongated (4-butylphenol). Interestingly, the small compound tetrahydro-2-naphthol (Supplemental Fig. S1), belonging to the naphthol family of chemicals whose members have not yet been tested for their ability to modulate ERRγ activity, exhibited an EC50 of 260 nM. Although they were described as ERRγ ligands, all members of benzophenone, paraben and phytoestrogen families that we tested on the HG5LN GAL4-hERRγ cell line failed to modify the basal luciferase activity at the tested concentrations. We then used isothermal titration calorimetry (ITC) to assess the binding characteristics of the most potent ERRγ agonists. BPE was the best binder with a Kd of 48.7 nM, whereas the affinity constant measured for BPA was 98.6 nM (Table 1 and Supplemental Fig. S3), in agreement with a Kd value of 70 nM reported for BPA in a previous study [28]. Other Kds were all comprised between 100 and 500 nM (Table 1). These cell-based and in vitro assays clearly show that within the bisphenol, alkylphenol and naphthol families of compounds, the agonistic potential and affinity of congeners varies greatly according to their chemical structure, and that the most potent ones (e.g., BPA, BPE, 4-sec-butylphenol or tetrahydro-2-naphthol) display correlated EC50 and Kd values in the high-nanomolar range.
Crystal structures of ERRγ in complex with bisphenol, alkylphenol and naphthol derivatives
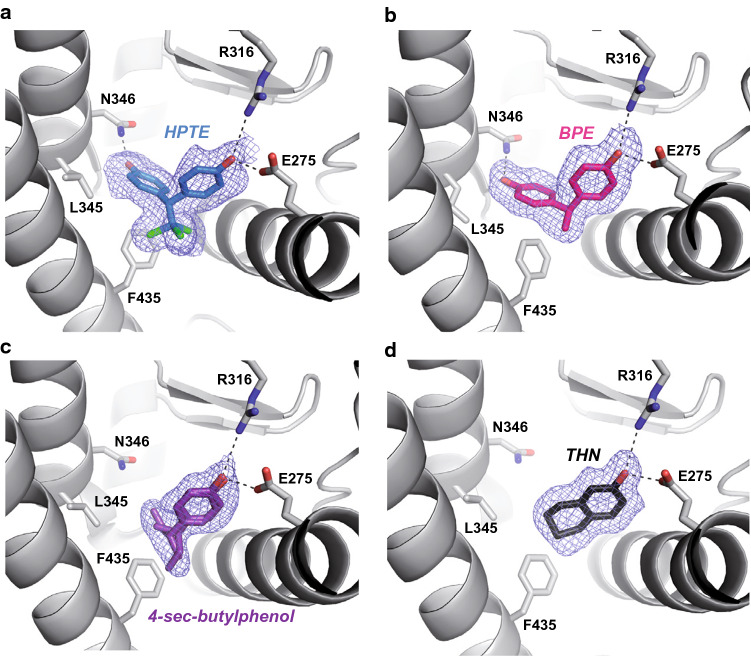
To characterize the mode of binding of structurally different compounds, the ERRγ-LBD was co-crystallized with the bisphenols BPA, BPB, BPE and HPTE, the alkylphenols 4-iso-propylphenol and 4-sec-butylphenol, and the tetrahydro-2-naphthol. The corresponding structures were solved at resolutions ranging from 1.50 Å (4-iso-propylphenol) for the highest to 2.22 Å (BPA) for the lowest. Structure determination and refinement statistics are summarized in Table S1. In all the cases, the overall protein conformation was indistinguishable from that of the unliganded receptor [41], with notably the activation helix H12 in the transcriptionally active position (Supplemental Fig. S4a). The structures with HPTE, BPE, and BPB revealed a binding mode in which one of the two phenol groups interacts with R316 (H5) and E275 (H3) while, on the other side, the second forms a hydrogen bond with N346 (H7). A hydrogen bond between Y326 and N346 holds the latter in position to interact with the second phenol group (Fig. 3a, b and Supplemental Fig. S4b). The remaining contacts involve essentially van der Waals interactions with the hydrophobic residues lining the ligand-binding pocket (LBP). This interaction mechanism is reminiscent of that already reported for BPA (Supplemental Fig. S4c) [27, 28]. Slight differences could nevertheless be observed in ligands and some side chain positioning, particularly in the case of HPTE which displays the bulkiest linker region (Supplemental Fig. S4d). Structures of ERRγ in the presence of 4-sec-butylphenol, tetra-2-hydronaphthol (Fig. 3c, d) and 4-iso-propylphenol (Supplemental Fig. S4e) showed that these small monohydroxylated compounds bind to a sub-pocket overlapping that of bisphenols (Supplemental Fig. S4f) and are delineated by the charge clamp residues (E275 and R316) engaged in a hydrogen bond network with the hydroxyl moiety of the ligands on one side, and F435 from H11 on the other side, forming a C–H/π interaction with the ligands. Minimal rearrangements of the LBP residues were also observed. All compounds but the tetrahydro-2-naphthol were found to form van der Waals contacts of 3.7–4.5 Å in length with F450 from H12. In summary, these data show that ERRγ possesses a LBP to which bisphenol, alkylphenol and naphthol compounds can bind with limited structural impact on the constitutively active conformation of the receptor. Thus, the classical mechanism of nuclear receptor activation involving the re-localization of helix H12 in the active conformation does not apply to this receptor. Instead, the agonistic activity of ligands observed in our cell-based assays could be ascribed to a stabilization of the ERRγ LBD upon ligand binding (e.g., via the interaction with helix H12) rather than to a conformational change.
Fig. 3.
Compounds bind to ERRγ LBD with limited structural impact on its constitutively active conformation. Interactions of a HPTE, b BPE, c 4-sec-butylphenol, and d tetrahydro-2-naphthol with LBP residues reveal two different binding modes of EDCs on ERRγ. Oxygen, nitrogen, and chlorine atoms are colored in red, blue, and green, respectively. Hydrogen bonds are indicated by black dashed lines. The simulated annealing Fo–Fc omit maps are depicted in blue
Thermodynamic stabilization of ERRγ LBD upon ligand binding
We first employed thermal shift assays (ThermoFluor®) to monitor the fraction of unfolded protein as a function of temperature and evaluate the impact of ligand binding on ERRγ LBD stability. Differential melting temperature (ΔTm) measurements for a subset of compounds showed values between + 6.3 °C (4-sec-butylphenol) and + 0.6 °C (tetrahydro-2-naphthol), confirming a stabilization of the domain by ligands (Table 1 and Supplemental Fig. S5a). We next examined the thermodynamic data retrieved from ITC experiments. The results reported in Supplemental Fig. S5b revealed that ligand binding to ERRγ is characterized by a beneficial negative binding enthalpy (e.g., ΔH of − 21.2 and − 19.8 kcal/mol for BPA and BPE, respectively) most likely related to the formation of hydrogen bonds between the phenol groups and the polar residues of ERRγ LBP. However, this favorable enthalpy term is accompanied by a significant loss of entropy (e.g., ΔS of − 38.4 and − 33.5 cal/mol/K for BPA and BPE, respectively), indicative of a gain of order upon ligand binding. Interestingly, inspection of the 1.80 Å resolution structure of ERRγ LBD in the absence of ligand (PDB code 2ZBS) reveals a preformed hydrophobic LBP with only four polar residues forming hydrogen-bonded pairs (Y326–N346 and E275–R316) and no water molecule (Supplemental Fig. S4g), indicating that the entropic penalty cannot be attributed to expulsion of water molecules from the binding pocket. The observed decrease of entropy likely reflects the restrained mobility of ERRγ residues upon ligand binding. This interpretation is supported by structure comparisons showing the stabilization of some residues, such as E275, which are disordered in the apo structure but adopt a unique conformation in the ligand-bound LBD.
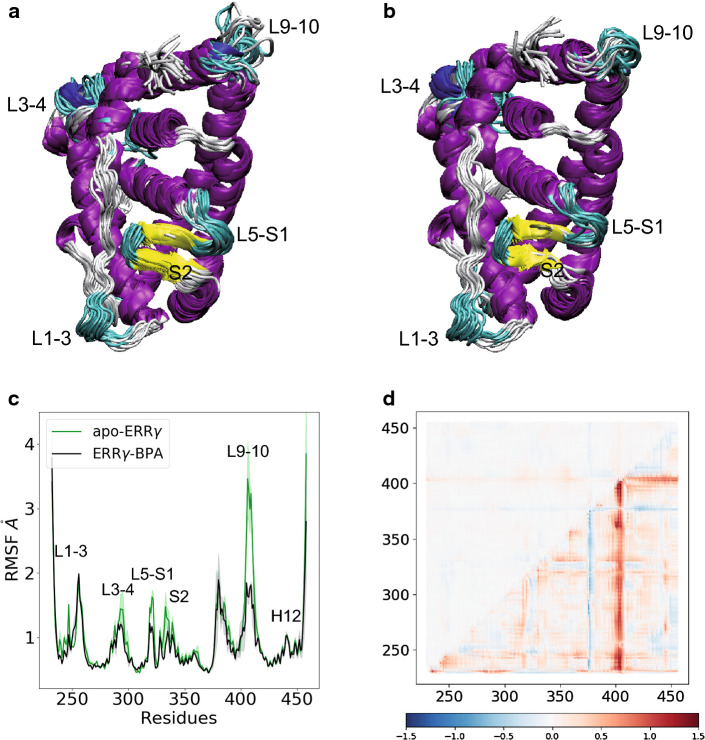
To gain additional insights into the ligand-induced thermodynamic stabilization of ERRγ LBD, we performed molecular dynamics (MD) simulations of ERRγ LBD in its unliganded form, and upon BPA and BPE binding. Multiple MD trajectories based on an explicit-solvent atomistic model revealed that all these systems are stable and exhibit limited structural fluctuations in the sub-μs timescale (Supplemental Fig. S6). Nevertheless, root mean square fluctuation (RMSF) analysis indicated that BPA binding further stabilizes the protein structure by dampening the conformational dynamics in a few LBD regions (Fig. 4a–c). Notably, this effect is particularly significant in loop L9–10, and to a lesser degree in loops L1–3, L3–4, L5–S1 and the β-sheet S1–S2, but it is not noticeable in the H12 region. An equivalent picture is obtained by monitoring the consequences of ligand binding on the distributions of Cα–Cα distances during the simulations (Fig. 4d). Indeed, comparison of apo- and BPA-bound systems indicates that ligand binding perturbs neither the average Cα–Cα distances, nor consequently, the protein fold (upper half of panel d), but overall it rigidifies the system, as indicated by narrower distributions (lower half of panel d). Equivalent results were observed for the BPE-bound system and are reported in Supplemental Fig. S7.
Fig. 4.
Molecular dynamics analysis of BPA-bound ERRγ LBD. Representative simulated ensembles of the ERRγ LBD in a its apo form, and in b complex with BPA. c The RMSF of the backbone atom positions measured for the apo- (green) and BPA-bound (black) ERRγ LBDs. Average (hard lines) and standard deviation (shades) over the three replica are represented. d Structural and dynamical effects of ligand binding expressed in terms of Cα–Cα distances. Ratio between the averages (upper half) and variances (lower half) of distance distributions in the unliganded and ligand-bound simulations is reported in a logarithmic scale. Red color corresponds to larger structural deviations (upper half) or fluctuations (lower half) in the apo-ERRγ compared to the BPA-bound ERRγ LBDs
Together, our structural, thermodynamic and computational data suggest a global stabilization of the domain upon ligand binding rather than a specific effect of the compound on the dynamics or the repositioning of the activation helix H12 as generally observed in classical nuclear receptors. This view is supported by fluorescence anisotropy measurements showing that while the antagonist OHT can efficiently deactivate ERRγ by dislodging H12 from its active position and preventing coactivator recruitment, all the agonists assayed had little impact on the interaction between the receptor and the coactivator (Supplemental Fig. S8).
Discussion
Many of the environmental compounds investigated in this study are phenolic xenoestrogens that exert their deleterious action by interacting with several identified target receptors including ERα and ERβ, both nuclear and membrane-bound, the G-protein-coupled estrogen receptor (GPER), and ERRγ [42–46]. Using transactivation assays, we previously showed that BPA is a fairly weak ER agonist (EC50 ≈ 0.5 µM) whereas other bisphenols such as BPC or HPTE display much better estrogenic potencies with EC50 values of ≈ 30 nM and 50 nM, respectively [47, 48]. In the present study, we show that BPA is a slightly better ERRγ agonist with Kd and EC50 values of 98.6 ± 21.8 nM and 174 ± 56 nM, respectively. Among the compounds tested, the most potent ones had affinity and activity values in the 50–500 nM range. Together, these studies demonstrate that both ERs and ERRγ can mediate the estrogenic effects of EDCs at doses within the high-nanomolar range and in a receptor-specific fashion depending on the chemical structures of the compounds. Although significant from a toxicological perspective, these interactions are unlikely to account for the low nanomolar effects (0.1–1.0 nM) reported for BPA which might rather be mediated by extranuclear receptors [44]. Taken together, these observations suggest that environmental xenoestrogens can exert their endocrine-disrupting action by interacting with a range of target receptors, the nature of which depends on the chemical structure and the concentration of the compound as well as the cell- and tissue-specific expression of these receptors. In addition to estrogen-responsive genes, ERRγ has been shown to control many specific genetic programs in both normal and cancer cells [7, 8]. ERRγ-regulated genes are involved in oxidative metabolism in skeletal and cardiac muscles [49–51], ion homeostasis [52, 53], insulin signaling [54], or gluconeogenesis [55]. Hence, the relationship that exists between ERRγ and metabolism strongly suggests that this receptor might play a major role in EDC-induced metabolic diseases. In support of this hypothesis, BPA has been shown to have metabolic effects by altering glucose homeostasis and insulin production [56, 57].
ERRγ is a constitutively active receptor exhibiting a robust basal transcriptional activity. In the absence of endogenous ligand, this receptor relies upon other regulatory mechanisms including PTMs [13, 14, 58–61]. As previously observed with other nuclear receptors, ligand binding modulates PTMs and in turn the receptor function via alterations of protein stability, subcellular localization, DNA-binding or transcriptional activity [62–66]. In this regard, reduction in the conformational freedom of L9–10 and other loop regions observed in the liganded ERRγ LBD structures may play a role in the control of the PTM status of the receptor and could account for the H12-independent agonistic activity of ERRγ ligands. Indeed, changes in the dynamics of some LBD regions might alter intra- and/or inter-domain contacts important for PTMs of, not only the LBD itself but also the interconnected N-terminal A/B, DNA-binding (DBD) or hinge domains which have all been shown to be subjected to PTMs [7]. For instance and with respect to the LBD, O-GlcNAcylation at S319 in loop L5–S1 has been shown to increase the transcriptional activity of ERRγ [61] whereas ubiquitination [58] and sumoylation [13] at MTP sites located, respectively, in the hinge region preceding helix H1 or loop L8–9, both in contact with loop L9–10 that undergoes drastic mobility change upon ligand binding, have been reported to modulate ERRγ function. Interestingly, in some other receptors, including the retinoic acid receptor α (RARα), loop L9–10 has been shown to be a regulatory element whose decrease in structural dynamics upon ligand-dependent phosphorylation has been correlated to potentiation of transcriptional activity [63, 67]. Although PTMs have been shown to play a major role in the regulation of ERRγ function, the influence of ligands on these regulatory pathways remains largely uncovered and requires further experimental investigations.
Accession codes
The atomic coordinates and structure factors have been deposited in the Protein Data Bank under accession codes 6I61 (BPB), 6I62 (HPTE), 6I63 (BPA), 6I64 (BPE), 6I65 (4-iso-propylphenol), 6I66 (4-sec-butylphenol), and 6I67 (tetrahydro-2-naphthol).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The CBS is a member of the France-BioImaging (FBI) and the French Infrastructure for Integrated Structural Biology (FRISBI), two national infrastructures supported by the French National Research Agency (ANR-10-INBS-04-01 and ANR-10-INBS-05, respectively). We acknowledge the experimental assistance from the staff of the European Synchrotron Radiation Facility (ESRF, Grenoble, France) during data collection. We would like to acknowledge the financial support from the Plan Cancer Inserm, project CONTERREC C16007FS (PB and WB) and the French Agence Nationale de la Recherche, under grants ANR-13-CESA-0012-04 (PB) and ANR-14-ACHN-0016 (AB).
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Erwan Thouennon and Vanessa Delfosse share the first authorship.
William Bourguet and Patrick Balaguer share the last authorship.
Contributor Information
William Bourguet, Email: william.bourguet@cbs.cnrs.fr.
Patrick Balaguer, Email: patrick.balaguer@inserm.fr.
References
- 1.Horard B, Vanacker JM. Estrogen receptor-related receptors: orphan receptors desperately seeking a ligand. J Mol Endocrinol. 2003;31(3):349–357. doi: 10.1677/jme.0.0310349. [DOI] [PubMed] [Google Scholar]
- 2.Wei W, Schwaid AG, Wang X, Wang X, Chen S, Chu Q, Saghatelian A, Wan Y. Ligand activation of ERRalpha by cholesterol mediates statin and bisphosphonate effects. Cell Metab. 2016;23(3):479–491. doi: 10.1016/j.cmet.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casaburi I, Chimento A, De Luca A, Nocito M, Sculco S, Avena P, Trotta F, Rago V, Sirianni R, Pezzi V. Cholesterol as an endogenous ERRalpha agonist: a new perspective to cancer treatment. Front Endocrinol. 2018;9:525. doi: 10.3389/fendo.2018.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyanka R, Das S, Samuels HH, Cardozo T. Nuclear receptor engineering based on novel structure activity relationships revealed by farnesyl pyrophosphate. Protein Eng Des Sel. 2010;23(11):809–815. doi: 10.1093/protein/gzq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greschik H, Wurtz JM, Sanglier S, Bourguet W, van Dorsselaer A, Moras D, Renaud JP. Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol Cell. 2002;9(2):303–313. doi: 10.1016/s1097-2765(02)00444-6. [DOI] [PubMed] [Google Scholar]
- 6.Eichner LJ, Giguere V. Estrogen related receptors (ERRs): a new dawn in transcriptional control of mitochondrial gene networks. Mitochondrion. 2011;11(4):544–552. doi: 10.1016/j.mito.2011.03.121. [DOI] [PubMed] [Google Scholar]
- 7.Misra J, Kim DK, Choi HS. ERRgamma: a junior orphan with a senior role in metabolism. Trends Endocrinol Metabol TEM. 2017;28(4):261–272. doi: 10.1016/j.tem.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Deblois G, Giguere V. Oestrogen-related receptors in breast cancer: control of cellular metabolism and beyond. Nat Rev Cancer. 2013;13(1):27–36. doi: 10.1038/nrc3396. [DOI] [PubMed] [Google Scholar]
- 9.Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 2007;5(5):345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Maehara K, Hida T, Abe Y, Koga A, Ota K, Kutoh E. Functional interference between estrogen-related receptor alpha and peroxisome proliferator-activated receptor alpha/9-cis-retinoic acid receptor alpha heterodimer complex in the nuclear receptor response element-1 of the medium chain acyl-coenzyme A dehydrogenase gene. J Mol Endocrinol. 2003;31(1):47–60. doi: 10.1677/jme.0.0310047. [DOI] [PubMed] [Google Scholar]
- 11.Audet-Walsh E, Giguere V. The multiple universes of estrogen-related receptor alpha and gamma in metabolic control and related diseases. Acta Pharmacol Sin. 2015;36(1):51–61. doi: 10.1038/aps.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranhotra HS. The estrogen-related receptors: orphans orchestrating myriad functions. J Recept Signal Transduct Res. 2012;32(2):47–56. doi: 10.3109/10799893.2011.647350. [DOI] [PubMed] [Google Scholar]
- 13.Tremblay AM, Wilson BJ, Yang XJ, Giguere V. Phosphorylation-dependent sumoylation regulates estrogen-related receptor-alpha and -gamma transcriptional activity through a synergy control motif. Mol Endocrinol (Baltim Md) 2008;22(3):570–584. doi: 10.1210/me.2007-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riggins RB. The pERK of being a target: kinase regulation of the orphan nuclear receptor ERRgamma. Receptors Clin Investig. 2014;1:5. [PMC free article] [PubMed] [Google Scholar]
- 15.Misawa A, Inoue S. Estrogen-related receptors in breast cancer and prostate cancer. Front Endocrinol. 2015;6:83. doi: 10.3389/fendo.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariazi EA, Jordan VC. Estrogen-related receptors as emerging targets in cancer and metabolic disorders. Curr Top Med Chem. 2006;6(3):203–215. doi: 10.2174/1568026610606030203. [DOI] [PubMed] [Google Scholar]
- 17.Ariazi EA, Clark GM, Mertz JE. Estrogen-related receptor alpha and estrogen-related receptor gamma associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Can Res. 2002;62(22):6510–6518. [PubMed] [Google Scholar]
- 18.Suzuki T, Miki Y, Moriya T, Shimada N, Ishida T, Hirakawa H, Ohuchi N, Sasano H. Estrogen-related receptor alpha in human breast carcinoma as a potent prognostic factor. Can Res. 2004;64(13):4670–4676. doi: 10.1158/0008-5472.CAN-04-0250. [DOI] [PubMed] [Google Scholar]
- 19.Ijichi N, Shigekawa T, Ikeda K, Horie-Inoue K, Fujimura T, Tsuda H, Osaki A, Saeki T, Inoue S. Estrogen-related receptor gamma modulates cell proliferation and estrogen signaling in breast cancer. J Steroid Biochem Mol Biol. 2011;123(1–2):1–7. doi: 10.1016/j.jsbmb.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Tiraby C, Hazen BC, Gantner ML, Kralli A. Estrogen-related receptor gamma promotes mesenchymal-to-epithelial transition and suppresses breast tumor growth. Can Res. 2011;71(7):2518–2528. doi: 10.1158/0008-5472.CAN-10-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ Health Perspect. 2008;116(1):32–38. doi: 10.1289/ehp.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRgamma) with high constitutive activity. Toxicol Lett. 2006;167(2):95–105. doi: 10.1016/j.toxlet.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Tohme M, Prud’homme SM, Boulahtouf A, Samarut E, Brunet F, Bernard L, Bourguet W, Gibert Y, Balaguer P, Laudet V. Estrogen-related receptor gamma is an in vivo receptor of bisphenol A. FASEB J. 2014;28(7):3124–3133. doi: 10.1096/fj.13-240465. [DOI] [PubMed] [Google Scholar]
- 25.Greschik H, Flaig R, Renaud JP, Moras D. Structural basis for the deactivation of the estrogen-related receptor by diethylstilbestrol or 4-hydroxytamoxifen and determinants of selectivity. J Biol Chem. 2004;279(32):33639–33646. doi: 10.1074/jbc.M402195200. [DOI] [PubMed] [Google Scholar]
- 26.Coward P, Lee D, Hull MV, Lehmann JM. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor gamma. Proc Natl Acad Sci USA. 2001;98(15):8880–8884. doi: 10.1073/pnas.151244398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. J Biochem. 2007;142(4):517–524. doi: 10.1093/jb/mvm158. [DOI] [PubMed] [Google Scholar]
- 28.Abad MC, Askari H, O’Neill J, Klinger AL, Milligan C, Lewandowski F, Springer B, Spurlino J, Rentzeperis D. Structural determination of estrogen-related receptor gamma in the presence of phenol derivative compounds. J steroid Biochem Mol Biol. 2008;108(1–2):44–54. doi: 10.1016/j.jsbmb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Seimandi M, Lemaire G, Pillon A, Perrin A, Carlavan I, Voegel JJ, Vignon F, Nicolas JC, Balaguer P. Differential responses of PPARalpha, PPARdelta, and PPARgamma reporter cell lines to selective PPAR synthetic ligands. Anal Biochem. 2005;344(1):8–15. doi: 10.1016/j.ab.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Kabsch W. Xds. Acta Crystallogr. 2010;66(Pt 2):125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallogr A. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr A. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 33.Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr A. 2010;66(Pt 1):12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pogenberg V, Guichou JF, Vivat-Hannah V, Kammerer S, Perez E, Germain P, de Lera AR, Gronemeyer H, Royer CA, Bourguet W. Characterization of the interaction between retinoic acid receptor/retinoid X receptor (RAR/RXR) heterodimers and transcriptional coactivators through structural and fluorescence anisotropy studies. J Biol Chem. 2005;280(2):1625–1633. doi: 10.1074/jbc.M409302200. [DOI] [PubMed] [Google Scholar]
- 35.Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput. 2015;11:3696–3713. doi: 10.1021/acs.jctc.5b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general AMBER force field. J Comput Chem. 2004;25:1157–1174. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 37.Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. [Google Scholar]
- 38.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. A smooth particle mesh Ewald method. J Chem Phys. 1995;103:8577. [Google Scholar]
- 39.Bussi G, Donadio D, Parrinello M. Canonical sampling through velocity rescaling. J Chem Phys. 2007;126:014101. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 40.Parrinello M, Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys. 1981;52:7182–7190. [Google Scholar]
- 41.Matsushima A, Teramoto T, Okada H, Liu X, Tokunaga T, Kakuta Y, Shimohigashi Y. ERRgamma tethers strongly bisphenol A and 4-alpha-cumylphenol in an induced-fit manner. Biochem Biophys Res Commun. 2008;373(3):408–413. doi: 10.1016/j.bbrc.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 42.Delfosse V, Maire AL, Balaguer P, Bourguet W. A structural perspective on nuclear receptors as targets of environmental compounds. Acta Pharmacol Sin. 2014;36(1):88–101. doi: 10.1038/aps.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levin ER. Extranuclear steroid receptors are essential for steroid hormone actions. Annu Rev Med. 2015;66:271–280. doi: 10.1146/annurev-med-050913-021703. [DOI] [PubMed] [Google Scholar]
- 44.Nadal A, Fuentes E, Ripoll C, Villar-Pazos S, Castellano-Munoz M, Soriano S, Martinez-Pinna J, Quesada I, Alonso-Magdalena P. Extranuclear-initiated estrogenic actions of endocrine disrupting chemicals: is there toxicology beyond paracelsus? J Steroid Biochem Mol Biol. 2018;176:16–22. doi: 10.1016/j.jsbmb.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev. 2011;7(12):715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.le Maire A, Bourguet W, Balaguer P. A structural view of nuclear hormone receptor: endocrine disruptor interactions. Cell Mol Life Sci. 2010;67(8):1219–1237. doi: 10.1007/s00018-009-0249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delfosse V, Grimaldi M, Pons JL, Boulahtouf A, le Maire A, Cavailles V, Labesse G, Bourguet W, Balaguer P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc Natl Acad Sci USA. 2012;109(37):14930–14935. doi: 10.1073/pnas.1203574109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delfosse V, Grimaldi M, Cavailles V, Balaguer P, Bourguet W. Structural and functional profiling of environmental ligands for estrogen receptors. Environ Health Perspect. 2014;122(12):1306–1313. doi: 10.1289/ehp.1408453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6(1):13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Narkar VA, Fan W, Downes M, Yu RT, Jonker JW, Alaynick WA, Banayo E, Karunasiri MS, Lorca S, Evans RM. Exercise and PGC-1alpha-independent synchronization of type I muscle metabolism and vasculature by ERRgamma. Cell Metab. 2011;13(3):283–293. doi: 10.1016/j.cmet.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang T, McDonald C, Petrenko NB, Leblanc M, Wang T, Giguere V, Evans RM, Patel VV, Pei L. Estrogen-related receptor alpha (ERRalpha) and ERRgamma are essential coordinators of cardiac metabolism and function. Mol Cell Biol. 2015;35(7):1281–1298. doi: 10.1128/MCB.01156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alaynick WA, Way JM, Wilson SA, Benson WG, Pei L, Downes M, Yu R, Jonker JW, Holt JA, Rajpal DK, Li H, Stuart J, McPherson R, Remlinger KS, Chang CY, McDonnell DP, Evans RM, Billin AN. ERRgamma regulates cardiac, gastric, and renal potassium homeostasis. Mol Endocrinol (Baltim Md) 2010;24(2):299–309. doi: 10.1210/me.2009-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo Y, Kumar P, Mendelson CR. Estrogen-related receptor gamma (ERRgamma) regulates oxygen-dependent expression of voltage-gated potassium (K+) channels and tissue kallikrein during human trophoblast differentiation. Mol Endocrinol (Baltim Md) 2013;27(6):940–952. doi: 10.1210/me.2013-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim DK, Kim JR, Koh M, Kim YD, Lee JM, Chanda D, Park SB, Min JJ, Lee CH, Park TS, Choi HS. Estrogen-related receptor gamma (ERRgamma) is a novel transcriptional regulator of phosphatidic acid phosphatase, LIPIN1, and inhibits hepatic insulin signaling. J Biol Chem. 2011;286(44):38035–38042. doi: 10.1074/jbc.M111.250613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim DK, Ryu D, Koh M, Lee MW, Lim D, Kim MJ, Kim YH, Cho WJ, Lee CH, Park SB, Koo SH, Choi HS. Orphan nuclear receptor estrogen-related receptor gamma (ERRgamma) is key regulator of hepatic gluconeogenesis. J Biol Chem. 2012;287(26):21628–21639. doi: 10.1074/jbc.M111.315168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menale C, Piccolo MT, Cirillo G, Calogero RA, Papparella A, Mita L, Del Giudice EM, Diano N, Crispi S, Mita DG. Bisphenol A effects on gene expression in adipocytes from children: association with metabolic disorders. J Mol Endocrinol. 2015;54(3):289–303. doi: 10.1530/JME-14-0282. [DOI] [PubMed] [Google Scholar]
- 57.Le Magueresse-Battistoni B, Multigner L, Beausoleil C, Rousselle C. Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol Cell Endocrinol. 2018;475:74–91. doi: 10.1016/j.mce.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 58.Ren Y, Jiang H, Ma D, Nakaso K, Feng J. Parkin degrades estrogen-related receptors to limit the expression of monoamine oxidases. Hum Mol Genet. 2011;20(6):1074–1083. doi: 10.1093/hmg/ddq550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim DK, Kim YH, Hynx D, Wang Y, Yang KJ, Ryu D, Kim KS, Yoo EK, Kim JS, Koo SH, Lee IK, Chae HZ, Park J, Lee CH, Biddinger SB, Hemmings BA, Choi HS. PKB/Akt phosphorylation of ERRgamma contributes to insulin-mediated inhibition of hepatic gluconeogenesis. Diabetologia. 2014;57(12):2576–2585. doi: 10.1007/s00125-014-3366-x. [DOI] [PubMed] [Google Scholar]
- 60.Heckler MM, Thakor H, Schafer CC, Riggins RB. ERK/MAPK regulates ERRgamma expression, transcriptional activity and receptor-mediated tamoxifen resistance in ER + breast cancer. FEBS J. 2014;281(10):2431–2442. doi: 10.1111/febs.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Misra J, Kim DK, Jung YS, Kim HB, Kim YH, Yoo EK, Kim BG, Kim S, Lee IK, Harris RA, Kim JS, Lee CH, Cho JW, Choi HS. O-GlcNAcylation of orphan nuclear receptor estrogen-related receptor gamma promotes hepatic gluconeogenesis. Diabetes. 2016;65(10):2835–2848. doi: 10.2337/db15-1523. [DOI] [PubMed] [Google Scholar]
- 62.Bruck N, Vitoux D, Ferry C, Duong V, Bauer A, de The H, Rochette-Egly C. A coordinated phosphorylation cascade initiated by p38MAPK/MSK1 directs RARalpha to target promoters. EMBO J. 2009;28(1):34–47. doi: 10.1038/emboj.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaillard E, Bruck N, Brelivet Y, Bour G, Lalevee S, Bauer A, Poch O, Moras D, Rochette-Egly C. Phosphorylation by PKA potentiates retinoic acid receptor alpha activity by means of increasing interaction with and phosphorylation by cyclin H/cdk7. Proc Natl Acad Sci USA. 2006;103(25):9548–9553. doi: 10.1073/pnas.0509717103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Goff P, Montano MM, Schodin DJ, Katzenellenbogen BS. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. J Biol Chem. 1994;269(6):4458–4466. [PubMed] [Google Scholar]
- 65.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103(6):843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 66.Xin QL, Qiu JT, Cui S, Xia GL, Wang HB. Transcriptional activation of nuclear estrogen receptor and progesterone receptor and its regulation. Sheng Li Xue Bao. 2016;68(4):435–454. [PubMed] [Google Scholar]
- 67.Chebaro Y, Amal I, Rochel N, Rochette-Egly C, Stote RH, Dejaegere A. Phosphorylation of the retinoic acid receptor alpha induces a mechanical allosteric regulation and changes in internal dynamics. PLoS Comput Biol. 2013;9(4):e1003012. doi: 10.1371/journal.pcbi.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.