Abstract
Statins are potent lipid-lowering drugs. Large prospective clinical trials have shown the anti-thrombotic effect of statins, e.g., preventing deep vein thrombosis. However, the mechanism underlying the beneficial effect of statins in reducing thrombus formation remains to be established. We, thus, conduct this study to investigate the potential molecular mechanisms. The cultured human hepatoma cells (HepG2) were used as the in vitro model. The human protein C gene promoter was cloned into the luciferase reporter to study the transcriptional regulation of human protein C gene. Wistar rats fed with simvastatin (5 mg/kg day) were used as the in vivo model. We found that simvastatin increased the expression of protein C in hepatocytes (361 ± 64% and 313 ± 59% after 2 h and 6 h of stimulation, respectively, both p < 0.01). In the animal study, the serum protein C levels were increased in the simvastatin-treated group (7 ± 2.2 unit/ml vs 23.4 ± 19.3 unit/ml and 23.4 ± 18.2 unit/ml and 1 and 2 weeks of treatment, respectively, both p < 0.05). Regarding the possible molecular mechanism, we found that the level of hepatocyte nuclear factor 1α (HNF1α) was also increased in both the in vivo and in vitro models. We found that the protein C promoter activity was increased by simvastatin, and this effect was inhibited by HNF1α knockdown and constitutively active Rac1. Therefore, stains may modulate protein C expression through small GTPase Rac 1 and HNF1α.
Keywords: Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase, Protein C, Rac1, HNF 1α, Anti-coagulation, Gene
Introduction
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are the most used lipid-lowering drugs, and have been demonstrated to be effective in reducing cardiovascular death in general population, both in primary and in secondary prevention [1, 2]. In addition to their lipid-lowering properties, statins also exert multiple pleiotropic actions [3]. Statins are found to have antioxidant, anti-inflammation, anti-sympathetic activities, and direct antiarrhythmic effects through cell membrane ion channel stabilization [2, 4–7].
Recently, several prospective and observational studies have showed that use of statins was associated with substantial and significant reductions in the risk of venous thromboembolism (VTE) [8–13], e.g., a 50% reduction in the risk among statin users in the Heart and Estrogen/Progestin Replacement Study (HERS) [8]. In the JUPITER Trial [14], relatively healthy people with high levels of C-reactive protein (CRP) and normal low-density lipoprotein cholesterol (LDL-C) levels were given either a placebo or rosuvastatin [14]. It showed an up to 55% reduction of risk of deep vein thrombosis in patients treated with rosuvastatin versus controls [14]. In the Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis (MEGA study) study, a large population-based case–control study including over 4000 patients with venous thrombosis was performed to assess the relationship between statins and thrombosis [15]. Among the 4538 patients who previously experienced a single episode of deep vein thrombosis or pulmonary embolism and 5914 control subjects, 3.3% of patients used statins at the time of their VTE as compared to 5.7% of controls. This yielded a 59% lower risk of VTE with statin use, or an odds ratio of 0.41 [11]. Interestingly, MEGA study also showed that all different types of statins seemed to reduce the risk of venous thrombosis.
Although the clinical beneficial effects of statins to reduce thromboembolism were evident based on the clinical studies, the exact molecular mechanisms remains not completely elucidated. Accordingly, we conducted this study to explore this issue. Specifically, we focused on the effect on anti-thrombotic proteins antithrombin III, protein C, and protein S, which has never been addressed before. We hypothesized that statins might increase the levels of these anti-thrombotic proteins. Understanding the anti-thrombotic properties of statins provides important potential therapeutic implications for clinical practice.
Materials and methods
Culture of HepG2 hepatocytes and transient transfection
Human hepatoma cells HepG2 were cultured at 37 °C in a humidified atmosphere of 95% air and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. After 3 days, cells were transferred to serum-free medium for 24 h. Then, the cells were stimulated with different concentrations of simvastatin for different time intervals. The cells were harvested for subsequent studies. For siRNA inhibition studies, the cells were transfected with HNF1α siRNA or negative control siRNA (Invitrogen) at a final concentration of 100 nM in the presence of LipofectAMINE 2000 (Invitrogen), as per the manufacturer’s instructions. Transient transfection of constitutively active Rac1 (RacV12) and dominant-negative Rac1 (RacN17) was performed as our previously reported method [16].
Rat model of statin stimulation
Wistar rats (weight 300 ± 20 g) were fed with pure water (vehicle) or simvastatin (5 mg/kg day) via orogastric tube every day for 3 days. Then, the rats were euthanized with intra-peritoneal pentobarbital (200 mg/kg), and the livers were rapidly excised for subsequent molecular studies. The sera were also collected for measurement of protein C, protein S, and anti-thrombin III. The experimental protocol was conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996) and approved by the Institutional Animal Care and Use Committee of the National Taiwan University College of Medicine.
RNA extraction and quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA were isolated and reverse transcribed. The single-stranded cDNA was amplified (ABI-Prism 7900, Applied Biosystems, Foster City, CA, USA), using SYBR Green dye. The primers for PCR amplifications of protein C, protein S, and anti-thrombin III were designed according to the published sequences in Genbank.
The forward and reverse primers were
Protein C
h-PROC-RT-F: GATGGAGAAGAAGCGCAGTC.
h-PROC-RT-R: GTAGTTGGGGTGGACGAAGA.
Product size: 307 bp.
Protein S
h-PROS1-RT-F: GTAAACCAGGTTGGCAAGGA.
h-PROS1-RT-R: TCTTGGGCAAGTTTGAATCC.
Product size: 400 bp.
Antithrombin III
h-antithrombin 3-RT-F: AATAAGACCGAAGGCCGAAT.
h-antithrombin 3-RT-R: CTTTGAAGGGCAACTCAAGC.
Product size: 253 bp.
Cloning of human protein C gene promoter
Promoter cloning of human protein C gene promoter into the pGL3 basic vector and the dual luciferase reporter assay were performed as our previously reported method [17, 18]. Promoter sequencing was verified by direct sequencing. Both TFSEARCH and TRANSFAC were used for in silico analysis to identify the possible putative consensus sequences for transcription factor-binding sites in the promoter. Transient transfection of hepatocytes was carried out using LipofectAMINE 2000 (Invitrogen) according to the manufacturer’s instructions.
Protein extracts and western blot analyses
The cellular and tissue extracts were homogenized in homogenization buffer containing 25 mM Tris (pH 7.5), 0.5 mM EDTA, 0.5 mM EGTA, 1 mM PMSF, 1 mM dithiothreitol, 25 µg/mL leupeptin, 25 mM NaF, and 1 mM Na3VO4. The homogenates were centrifuged at 14,000g for 15 min, and the resulting supernatants were collected for immunoblotting analysis. Protein concentrations were determined by a BCA protein assay reagent kit (Pierce, Rockford, IL, USA).
Proteins were separated by 8% SDS-PAGE and transferred to PVDF membranes (Millipore, Bedford, MA, USA). Membranes are blocked for 1 h at room temperature using non-fat dry milk dissolved in Tris buffer with 0.1% Tween-20. Membranes were incubated with primary antibody in blocking buffer for 12 h at 4 °C. Peroxidase-conjugated secondary antibodies were used for detection of primary antibody. Membranes were incubated in blocking buffer containing secondary antibody for 1 h at room temperature. Signals were detected with an Enhanced Chemiluminescence kit (Amersham Biosciences, Buckinghamshire, UK) and analyzed using Adobe Photoshop 6.0 and Image Gauge V3.12 (Fujifilm, Tokyo, Japan). The primary antibody used in the present study was rabbit polyclonal anti- HNF1α (1:500; Santa Cruz). Rabbit peroxidase-conjugated secondary antibodies (1:4000; Santa Cruz) were used for detection of the primary antibody.
Statistical analysis
All data were presented as means ± standard deviations or median with interquartile range, and analyzed using Mann–Whitney U test for comparison of independent two groups. Kruskal–Wallis test was used for comparison of more than two independent groups, with post hoc test and correction for p values. Data were analyzed using SPSS 15.0 software (SPSS Inc., Chicago, IL, USA).
Results
In vitro and in vivo effect of simvastatin on expressions of antithrombotic proteins
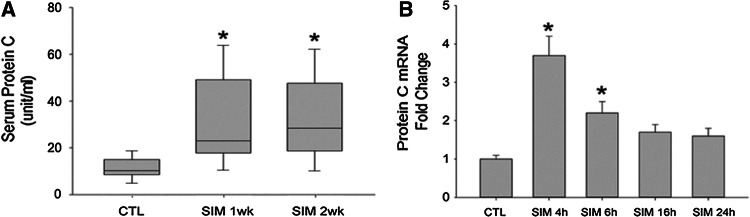
To investigate the underlying mechanisms of statins’ anti-coagulation effect, we first conducted in vivo studies. Wistar rats were fed with pure water (control) or simvastatin (5 mg/kg day) for 1 week and 2 weeks, respectively. We found that serum protein C level was increased in rats after 1 week or 2 weeks of oral simvastatin administration (protein C level 8.4 ± 3.2 unit/ml in 10 control rats vs. 22.7 ± 15.2 unit/ml in 10 simvastatin-fed rats for 1 week [p = 0.012] and 20.3 ± 16.9 unit/ml in 10 simvastatin-fed rats for 2 weeks [p = 0.042]) (Fig. 1a). However, oral simvastatin did not increase serum protein S and anti-thrombin III levels in the in vivo studies.
Fig. 1.
Simvastatin increases serum protein C level in rats and increases the expressions of protein C in hepatocytes. a Rats were fed with simvastatin (SIM, 10 mg/kg/day) or vehicles (CTL) for different time intervals and then serum protein C level was measured. b Human hepatic cell line HepG2 cells were stimulated with simvastatin (SIM) (10 μM) or vehicles (CTL) for various time intervals (4 h, 6 h, 16 h, and 24 h) and then total ribonucleic acid (RNA) was extracted and quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR) was performed to quantify the expression level of protein C. N = 10 for each rat group. *p < 0.05 vs. CTL group; N = 6 for each HepG2 group. *p < 0.05 vs. CTL group
We then performed in vitro study to replicate the in vivo results. Human hepatoma cells (HepG2) were cultured and stimulated with simvastatin for different time intervals with various concentrations. Expression of protein C was evaluated by quantitative real-time RT-PCR. We found that simvastatin (10−5 M) increased the levels of protein C mRNAs on a time-depend manner, with the maximal effect at 4 h of stimulation (Fig. 1b).
Since simvastatin showed both in vitro and in vivo effects on the expression of protein C gene, in the next step, we sought to investigate the possible signaling mechanism.
Simvastatin increases protein C gene promoter activity
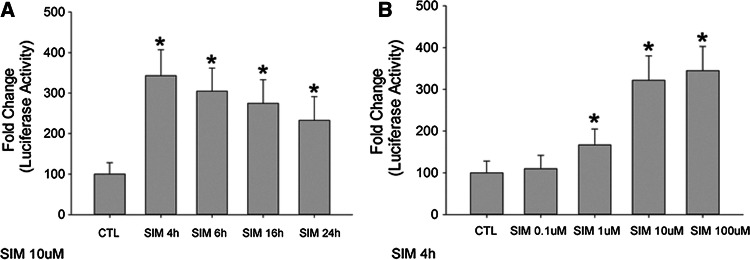
We first investigated whether simvastatin increased the promoter activity of the human protein C gene. The human protein C gene promoter was cloned into the luciferase reporter. We found that simvastatin (10−5 M) increased luciferase activities in HepG2 cells with different time intervals (4 h, 6 h, 16 h, and 24 h) (Fig. 2a). The protein C promoter luciferase activities were also increased after 4 h of simvastatin stimulation with different simvastatin concentrations (10−7–10−4 M) (Fig. 2b). The promoter activity changes were parallel with the mRNA changes as shown in Fig. 1b.
Fig. 2.
Simvastatin increases human protein C gene promoter activity. HepG2 hepatocytes were cultured and transfected with human protein C gene luciferase reporters (pGL3-basic). Simvastatin (SIM) increased human protein C gene promoter activity for different time intervals (4 h, 6 h, 16 h, and 24 h) (stimulated with 10 μM) and with different concentrations (0.1 μM, 1 μM, 10 μM, 100 μM) (stimulated for 4 h), respectively. HepG2 cells transfected with empty vector were served as controls (CTL). N = 6 for each group. *p < 0.05 vs. CTL group
Simvastatin increases protein C gene expression through HNF1α and Rac1
We then investigated the exact signaling mechanism by which simvastatin increased the promoter activity of human protein C gene. It has been shown that hepatic nuclear factors (HNFs) may mediate statins’ pleiotropic effects [19–21]. We first investigated possible transcriptional factor-binding sites in the promoter of the human protein C gene. We found several HNF cognate binding sites that are conservative among species (Fig. 3). Therefore, we hypothesized that simvastatin might modulate protein C gene expression through HNFs.
Fig. 3.
In silico prediction of transcriptional factor-binding sites in the human protein C gene promoter. Several putative transcriptional factor-binding sites are identified, including the hepatic nuclear factor 1A/1B (HNF 1A/1B), which is evolutionally conservative
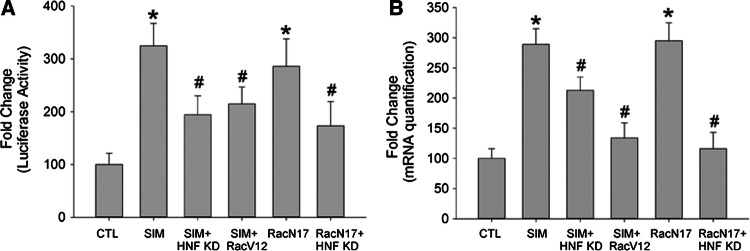
Preliminarily we found that simvastatin only increased the level of HNF1α, but not other HNFs (HNF2, HNF3 and HNF4) in hepatocytes. We found that the level of HNF1α was increased in hepatocytes after simvastatin stimulation (10−5 M) (Fig. 4a), and in rats fed with simvastatin (5 mg/kg day) (Fig. 4b). Interestingly, we found that simvastatin’s effect to increase protein C promoter activity and mRNA level was abolished when HNF1α was knocked down (Fig. 5a, b).
Fig. 4.
Simvastatin increases hepatic nuclear factor 1α (HNF1α) level both in vitro and in vivo. a HepG2 hepatocytes were cultured and stimulated with simvastatin for different time intervals (4 h, 6 h, 16 h, and 24 h). Total protein was extracted and hepatic HNF1α level was measured by western blot. Simvastatin (SIM) (10 μM) increased HNF1α level in HepG2 hepatocytes compared to vehicle-treated cells (CTL). N = 6 for each group. *p < 0.05 vs. CTL group. b, Rats were fed with simvastatin (SIM, 10 mg/kg/day) or vehicles (CTL) for different time intervals and then hepatic HNF1α level was measured by western blot. N = 6 for each group. *p < 0.05 vs. CTL group
Fig. 5.
Simvastatin-induced human protein C gene expression is small. GTPase Rac1 and hepatic nuclear factor 1 dependent. a HepG2 hepatocytes were cultured and transfected with human protein C gene luciferase reporters (pGL3-basic). Simvastatin (SIM) (10 μM) increased protein C promoter activity, which was inhibited by HNF1α knockdown and constitutively active Rac1 (Rac V12). Dominant-negative Rac1 (RacN17) alone increased protein C promoter activity, which was inhibited by HNF1α knockdown. HepG2 cells transfected with empty vector and scramble sequence siRNA were served as controls (CTL). N = 6 for each group. *p < 0.05 vs. CTL group. b Total ribonucleic acid (RNA) was extracted and quantified by reverse transcription–polymerase chain reaction (RT–PCR). Simvastatin (SIM) (10 μM) increased protein C gene messenger RNA (mRNA) level, which was inhibited by HNF1α knockdown and constitutively active Rac1 (Rac V12). Dominant-negative Rac1 (RacN17) alone increased protein C gene mRNA level, which was inhibited by HNF1α knockdown. HepG2 cells transfected with empty vector and scramble sequence siRNA were served as controls (CTL). N = 6 for each group. *p < 0.05 vs. CTL group
Recently, it has been shown that small GTPase Rac1 may also mediate statins’ pleiotropic effect [22–24]. Previously, we also showed that Rac1 plays a pivotal role in the signaling of simvastatin’s pleiotropic effect to inhibit atrial fibrosis [16]. We proved that simvastatin could inhibit Rac1 activity, thus inhibiting Rac1-induced STAT3 activation in atrial myocytes [16]. Accordingly, we found that simvastatin-mediated increase of the protein C gene promoter activity was inhibited by constitutively active Rac1 (Rac V12) (Fig. 5a, b). Interestingly, dominant-negative Rac1 (RacN17) exerted a similar effect as simvastatin to increase protein C gene promoter activity, which was also inhibited by HNF1α knockdown (Fig. 5a, b), suggesting that Rac1 was upstream of HNF1α in the transcriptional regulation of human protein C gene by simvastatin.
Discussion
In the present study, we first show that statins exert its anti-thrombotic effect through increasing the expression of protein C gene, which is dependent on Rac1 and HNF1α. This novel finding has never been addressed before. The results of the present study may provide the rationale to use statins for various clinical conditions that are associated with a hypercoagulability state, such as deep vein thrombosis or pulmonary embolism.
Mechanisms of stains related anti-thrombotic prosperities
There are various studies addressing the possible mechanisms of statins’ anti-thrombotic effect. It has been shown that statin therapy decreases platelet activation and aggregation, and thereby may decrease the propensity toward thrombosis [25–28]. In a porcine model of carotid injury, intravenous lovastatin acutely inhibited platelet aggregation and thrombus formation [29]. Some studies also found that fluvastatin reduced platelet aggregation through decreasing soluble P-selectin and ICAM-1 levels [30]. Another study demonstrated that simvastatin inhibited the synthesis of thromboxane A2, together with the reduction of urinary excretion of its metabolite 11-dehydrothromboxane B2 [30], thus inhibiting thromboxane-dependent platelet activation [31, 32]. The recent research also suggested that the antithrombotic effects of statins might be attributed to reduction in platelet membrane cholesterol and reduced isoprostane formation [33, 34].
Finally, because the extrinsic coagulation pathway mediated by tissue factors (TFs) plays a central role in thrombus formation [30], it has been shown that statins decreased tissue factor (TF) expression, which leads to down-regulation of the blood coagulation cascade and reduced thrombin generation [26, 35]. The atorvastatin and thrombogenicity of the carotid atherosclerotic plaque (ATROCAP) study provides definite in vivo evidence that statins decreased TF expression and activity, and macrophage infiltration in human vessels [36]. Another study also showed that statins reduced TF-initiated coagulation, prothrombin activation, factor Va generation, fibrinogen cleavage, factor XIII activation and an increased rate of factor Va inactivation in the patients with coronary artery disease, which was independent of cholesterol reduction [30, 37, 38]. It has been postulated that the effect of statins in TF pathway was mediated by a decrease of Rho activity, which, the same with Rac1 in the present study, also belongs to a family of small GTPase [34, 39].
In addition to inhibition of platelet activity and TF, statins may also exert anti-thrombotic effect through regulating anti-coagulation pathways. It has been shown that oral pravastatin decreased serum plasminogen activator inhibitor-1 [40]. In the present study, we first show that statins exert its anti-thrombotic effect through increasing the expression of protein C gene, which is dependent on Rac1 and HNF1α. This novel finding has never been addressed before. Only one report showed that oral fluvastatin decreased soluble endothelial protein C receptor concentrations, probably implicating a down-regulation of receptor due to elevated protein C level [41].
Protein C is a vitamin K-dependent zymogen of a serine protease that inhibits blood coagulation by proteolytic inactivation of factors Va and VIIIa. When the level of protein C increases, the anti-thrombotic activity also increases. Then, the incidence of systemic thrombosis, such as deep vein thrombosis, pulmonary embolism or thrombus in the heart as seen in patients with atrial fibrillation may decrease. It is the possible molecular mechanism to explain why statins are efficacious in preventing deep vein thrombosis or pulmonary embolism in clinical studies [8–13].
Interaction of statin, Rac 1, HNF1α and protein C gene expression
In the present study, we first show that statins increase the expression of protein C gene through Rac1 and HNF1α. We showed that constitutively active Rac1 (Rac V12) inhibited simvastatin-induced increase of protein C gene promoter activity, and dominant-negative Rac 1 (RacN17) alone was sufficient to decrease protein C gene promoter activity, indicating that RacN17 directly mimics simvastatin and providing strong evidence that the well-known pleiotropic effect of statins to inhibit small GTPase activity directly mediated simvastatin-induced increase of protein C gene promoter activity. Interestingly, we first showed that the effect of the dominant-negative Rac 1 (RacN17) was further abolished when HNF1α was knockdown, indicating that HNF1α was downstream of Rac1 in the statin-mediated modulation of protein C gene expression. This novel signaling cascade was first shown in the transcriptional regulation of human protein C gene.
The HNF transcriptional factor family has been shown to play an important role in the transcriptional regulation of protein C gene. As we have shown (Fig. 3), there are liver-enriched HNF transcription factor-binding sites in the protein C gene promoter [42, 43]. It has also been shown that the close proximity to transcription start site of protein C contains two, partly overlapping, binding sites for HNF1 and HNF3 [42]. Interestingly, individuals who carried a mutation in this region had a higher risk to have protein C deficiency [44]. The results of our study more confirm that HNF is important for the transcriptional regulation of human protein C gene and is the downstream effector of statins to regulate human protein C gene expression.
There are limitations in the present study. First, in the animal study, we did not address the dose–response effect of simvastatin. Second, we also did not test other statins in the animal study. Finally, we did not investigate the detailed signaling mechanism between Rac1 and HNF1 in the cellular study.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan [107-2314-B-002-256].
Compliance with ethical standards
Conflict of interest
All authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Scarpioni R, Ricardi M, Melfa L, Cristinelli L. Dyslipidemia in chronic kidney disease: are statins still indicated in reduction cardiovascular risk in patients on dialysis treatment? Cardiovasc Ther. 2010;28:361–368. doi: 10.1111/j.1755-5922.2010.00182.x. [DOI] [PubMed] [Google Scholar]
- 2.Humphries KH, Lee M, Sheldon R, Ramanathan K, Dorian P, Green M, Kerr CR. Statin use and recurrence of atrial fibrillation after successful cardioversion. Am Heart J. 2007;154:908–913. doi: 10.1016/j.ahj.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Liakopoulos OJ, Choi YH, Kuhn EW, Wittwer T, Borys M, Madershahian N, Wassmer G, Wahlers T. Statins for prevention of atrial fibrillation after cardiac surgery: a systematic literature review. J Thorac Cardiovasc Surg. 2009;138(678–686):e671. doi: 10.1016/j.jtcvs.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GG, Chaitman BR, Goldberger JJ, Messig M. High-dose atorvastatin and risk of atrial fibrillation in patients with prior stroke or transient ischemic attack: analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Am Heart J. 2011;161:993–999. doi: 10.1016/j.ahj.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Kulik A, Singh JP, Levin R, Avorn J, Choudhry NK. Association between statin use and the incidence of atrial fibrillation following hospitalization for coronary artery disease. Am J Cardiol. 2010;105:1655–1660. doi: 10.1016/j.amjcard.2010.01.341. [DOI] [PubMed] [Google Scholar]
- 6.Shurraw S, Tonelli M. Statins for treatment of dyslipidemia in chronic kidney disease. Perit Dial Int. 2006;26:523–539. [PubMed] [Google Scholar]
- 7.Fauchier L, Pierre B, de Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2008;51:828–835. doi: 10.1016/j.jacc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 8.Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, Vittinghoff E, Hulley S. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132:689–696. doi: 10.7326/0003-4819-132-9-200005020-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ray JG, Mamdani M, Tsuyuki RT, Anderson DR, Yeo EL, Laupacis A. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med. 2001;161:1405–1410. doi: 10.1001/archinte.161.11.1405. [DOI] [PubMed] [Google Scholar]
- 10.Doggen CJ, Lemaitre RN, Smith NL, Heckbert SR, Psaty BM. HMG CoA reductase inhibitors and the risk of venous thrombosis among postmenopausal women. J Thromb Haemost. 2004;2:700–701. doi: 10.1111/j.1538-7836.2004.00696.x. [DOI] [PubMed] [Google Scholar]
- 11.Cushman M. A new indication for statins to prevent venous thromboembolism? Not yet. J Thromb Haemost. 2009;7:511–513. doi: 10.1111/j.1538-7836.2009.03282.x. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Sun T, Zhang P, Tian J, Yang K. Statins for primary prevention of venous thromboembolism. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008203.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal V, Phung OJ, Tongbram V, Bhardwaj A, Coleman CI. Statin use and the prevention of venous thromboembolism: a meta-analysis. Int J Clin Pract. 2010;64:1375–1383. doi: 10.1111/j.1742-1241.2010.02439.x. [DOI] [PubMed] [Google Scholar]
- 14.Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Ridker PM. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramcharan AS, Van Stralen KJ, Snoep JD, Mantel-Teeuwisse AK, Rosendaal FR, Doggen CJ. HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost. 2009;7:514–520. doi: 10.1111/j.1538-7836.2008.03235.x. [DOI] [PubMed] [Google Scholar]
- 16.Tsai CT, Lai LP, Kuo KT, Hwang JJ, Hsieh CS, Hsu KL, Tseng CD, Tseng YZ, Chiang FT, Lin JL. Angiotensin II activates signal transducer and activators of transcription 3 via Rac1 in atrial myocytes and fibroblasts: implication for the therapeutic effect of statin in atrial structural remodeling. Circulation. 2008;117:344–355. doi: 10.1161/CIRCULATIONAHA.107.695346. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CT, Wang DL, Chen WP, Hwang JJ, Hsieh CS, Hsu KL, Tseng CD, Lai LP, Tseng YZ, Chiang FT, Lin JL. Angiotensin II increases expression of alpha1C subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ Res. 2007;100:1476–1485. doi: 10.1161/01.RES.0000268497.93085.e1. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CT, Wu CK, Lee JK, Chang SN, Kuo YM, Wang YC, Lai LP, Chiang FT, Hwang JJ, Lin JL. TNF-alpha down-regulates sarcoplasmic reticulum Ca(2)(+) ATPase expression and leads to left ventricular diastolic dysfunction through binding of NF-kappaB to promoter response element. Cardiovasc Res. 2015;105:318–329. doi: 10.1093/cvr/cvv008. [DOI] [PubMed] [Google Scholar]
- 19.Seo M, Inoue I, Ikeda M, Nakano T, Takahashi S, Katayama S, Komoda T. Statins activate human PPARalpha promoter and increase PPARalpha mRNA expression and activation in HepG2 cells. PPAR Res. 2008;2008:316306. doi: 10.1155/2008/316306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Zhao S. Effect of simvastatin on the expression and regulation mechanism of apolipoprotein M. Int J Mol Med. 2012;29:510–514. doi: 10.3892/ijmm.2011.853. [DOI] [PubMed] [Google Scholar]
- 21.He YJ, Zhang W, Tu JH, Kirchheiner J, Chen Y, Guo D, Li Q, Li ZY, Chen H, Hu DL, Wang D, Zhou HH. Hepatic nuclear factor 1alpha inhibitor ursodeoxycholic acid influences pharmacokinetics of the organic anion transporting polypeptide 1B1 substrate rosuvastatin and bilirubin. Drug Metab Dispos. 2008;36:1453–1456. doi: 10.1124/dmd.108.020503. [DOI] [PubMed] [Google Scholar]
- 22.Bruder-Nascimento T, Callera GE, Montezano AC, He Y, Antunes TT, Cat AN, Tostes RC, Touyz RM. Vascular injury in diabetic db/db mice is ameliorated by atorvastatin: role of Rac1/2-sensitive Nox-dependent pathways. Clin Sci (Lond) 2015;128:411–423. doi: 10.1042/CS20140456. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka S, Fukumoto Y, Nochioka K, Minami T, Kudo S, Shiba N, Takai Y, Williams CL, Liao JK, Shimokawa H. Statins exert the pleiotropic effects through small GTP-binding protein dissociation stimulator upregulation with a resultant Rac1 degradation. Arterioscler Thromb Vasc Biol. 2013;33:1591–1600. doi: 10.1161/ATVBAHA.112.300922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam O, Laufs U. Rac1-mediated effects of HMG-CoA reductase inhibitors (statins) in cardiovascular disease. Antioxid Redox Signal. 2014;20:1238–1250. doi: 10.1089/ars.2013.5526. [DOI] [PubMed] [Google Scholar]
- 25.Vaughan CJ, Delanty N, Basson CT. Statin therapy and stroke prevention. Curr Opin Cardiol. 2001;16:219–224. doi: 10.1097/00001573-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Rosenson RS, Tangney CC. Antiatherothrombotic properties of statins: implications for cardiovascular event reduction. JAMA. 1998;279:1643–1650. doi: 10.1001/jama.279.20.1643. [DOI] [PubMed] [Google Scholar]
- 27.Cipollone F, Mezzetti A, Porreca E, Di Febbo C, Nutini M, Fazia M, Falco A, Cuccurullo F, Davi G. Association between enhanced soluble CD40L and prothrombotic state in hypercholesterolemia: effects of statin therapy. Circulation. 2002;106:399–402. doi: 10.1161/01.CIR.0000025419.95769.F0. [DOI] [PubMed] [Google Scholar]
- 28.Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995;92:3172–3177. doi: 10.1161/01.CIR.92.11.3172. [DOI] [PubMed] [Google Scholar]
- 29.Obi C, Wysokinski W, Karnicki K, Owen WG, McBane RD., 2nd Inhibition of platelet-rich arterial thrombus in vivo: acute antithrombotic effect of intravenous HMG-CoA reductase therapy. Arterioscler Thromb Vasc Biol. 2009;29:1271–1276. doi: 10.1161/ATVBAHA.109.190884. [DOI] [PubMed] [Google Scholar]
- 30.Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203:325–330. doi: 10.1016/j.atherosclerosis.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Puccetti L, Santilli F, Pasqui AL, Lattanzio S, Liani R, Ciani F, Ferrante E, Ciabattoni G, Scarpini F, Ghezzi A, Auteri A, Davi G. Effects of atorvastatin and rosuvastatin on thromboxane-dependent platelet activation and oxidative stress in hypercholesterolemia. Atherosclerosis. 2011;214:122–128. doi: 10.1016/j.atherosclerosis.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Haramaki N, Ikeda H, Takenaka K, Katoh A, Sugano R, Yamagishi S, Matsuoka H, Imaizumi T. Fluvastatin alters platelet aggregability in patients with hypercholesterolemia: possible improvement of intraplatelet redox imbalance via HMG-CoA reductase. Arterioscler Thromb Vasc Biol. 2007;27:1471–1477. doi: 10.1161/ATVBAHA.106.128793. [DOI] [PubMed] [Google Scholar]
- 33.Luzak B, Rywaniak J, Stanczyk L, Watala C. Pravastatin and simvastatin improves acetylsalicylic acid-mediated in vitro blood platelet inhibition. Eur J Clin Invest. 2012;42:864–872. doi: 10.1111/j.1365-2362.2012.02661.x. [DOI] [PubMed] [Google Scholar]
- 34.Perez A, Bartholomew JR. Interpreting the JUPITER trial: statins can prevent VTE, but more study is needed Cleve. Clin J Med. 2010;77:191–194. doi: 10.3949/ccjm.77a.09077. [DOI] [PubMed] [Google Scholar]
- 35.Undas A, Brummel-Ziedins KE, Mann KG. Statins and blood coagulation. Arterioscler Thromb Vasc Biol. 2005;25:287–294. doi: 10.1161/01.ATV.0000151647.14923.ec. [DOI] [PubMed] [Google Scholar]
- 36.Cortellaro M, Cofrancesco E, Arbustini E, Rossi F, Negri A, Tremoli E, Gabrielli L, Camera M. Atorvastatin and thrombogenicity of the carotid atherosclerotic plaque: the ATROCAP study. Thromb Haemost. 2002;88:41–47. doi: 10.1055/s-0037-1613151. [DOI] [PubMed] [Google Scholar]
- 37.Undas A, Brummel KE, Musial J, Mann KG, Szczeklik A. Simvastatin depresses blood clotting by inhibiting activation of prothrombin, factor V, and factor XIII and by enhancing factor Va inactivation. Circulation. 2001;103:2248–2253. doi: 10.1161/01.CIR.103.18.2248. [DOI] [PubMed] [Google Scholar]
- 38.Owens AP, 3rd, Passam FH, Antoniak S, Marshall SM, McDaniel AL, Rudel L, Williams JC, Hubbard BK, Dutton JA, Wang J, Tobias PS, Curtiss LK, Daugherty A, Kirchhofer D, Luyendyk JP, Moriarty PM, Nagarajan S, Furie BC, Furie B, Johns DG, Temel RE, Mackman N. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122:558–568. doi: 10.1172/JCI58969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eto M, Luscher TF. Modulation of coagulation and fibrinolytic pathways by statins. Endothelium. 2003;10:35–41. doi: 10.1080/10623320303359. [DOI] [PubMed] [Google Scholar]
- 40.Dangas G, Badimon JJ, Smith DA, Unger AH, Levine D, Shao JH, Meraj P, Fier C, Fallon JT, Ambrose JA. Pravastatin therapy in hyperlipidemia: effects on thrombus formation and the systemic hemostatic profile. J Am Coll Cardiol. 1999;33:1294–1304. doi: 10.1016/S0735-1097(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 41.Atalar E, Coskun S, Haznedaroglu IC, Yucel N, Ozer N, Sivri B, Aksoyek S, Ovunc K, Ozmen F. Immediate effects of fluvastain on circulating soluble endothelial protein C and free tissue factor pathway inhibitor in acute coronary syndromes. Cardiovasc Drugs Therapy. 2005;19:177–181. doi: 10.1007/s10557-005-2160-x. [DOI] [PubMed] [Google Scholar]
- 42.Spek CA, Bertina RM, Reitsma PH. Unique distance- and DNA-turn-dependent interactions in the human protein C gene promoter confer submaximal transcriptional activity. Biochem J. 1999;340(Pt 2):513–518. doi: 10.1042/bj3400513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spek CA, Bertina RM, Reitsma PH. Identification of evolutionarily invariant sequences in the protein C gene promoter. J Mol Evol. 1998;47:663–669. doi: 10.1007/PL00006424. [DOI] [PubMed] [Google Scholar]
- 44.Spek CA, Lannoy VJ, Lemaigre FP, Rousseau GG, Bertina RM, Reitsma PH. Type I protein C deficiency caused by disruption of a hepatocyte nuclear factor (HNF)-6/HNF-1 binding site in the human protein C gene promoter. J Biol Chem. 1998;273:10168–10173. doi: 10.1074/jbc.273.17.10168. [DOI] [PubMed] [Google Scholar]